Abstract
Wide differences exist among the polyene antibiotics, nystatin, rimocidin, filipin, pimaricin, and amphotericin B, with reference to steroid interference with their antifungal activities against Candida albicans. Of the numerous steroids tested, ergosterol was the only one which effectively antagonized the antifungal activity of all five polyene antibiotics. The antifungal activities of nystatin and amphotericin B were the least subject to vitiation by the addition of steroids other than ergosterol, and those of filipin, rimocidin, and pimaricin were the most sensitive to interference. Attempts to delineate the structural requirements of steroids possessing polyene-neutralizing activity in growing cultures of C. albicans are discussed. The ultraviolet absorbance of certain antibiotic steroid combinations was also studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GHOSH B. K., CHATTERJEE A. N. Leishmanicidal activity of nystatin, a polyene antifungal antibiotic. I. The probable mechanism of action of nystatin on Leishmania donovani. Antibiot Chemother (Northfield) 1962 Mar;12:204–206. [PubMed] [Google Scholar]
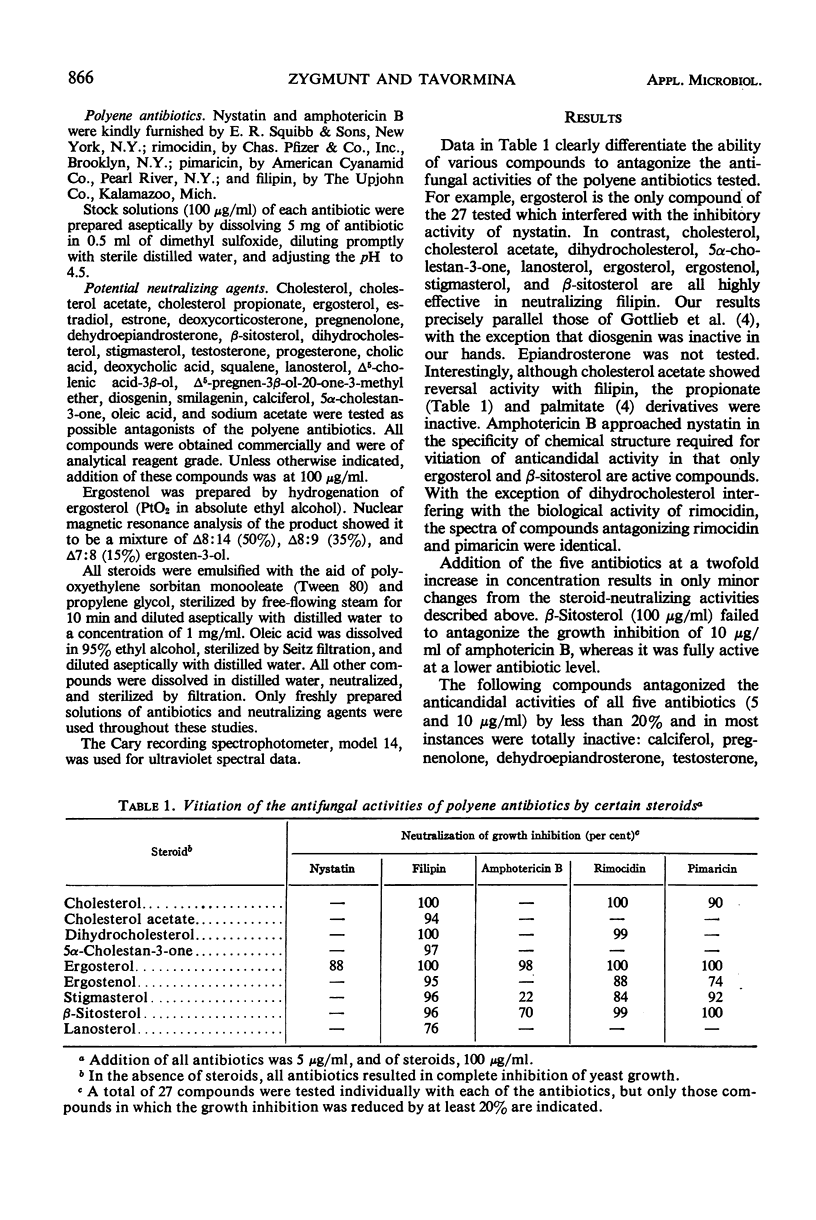
- GOTTLIEB D., CARTER H. E., SLONEKER J. H., AMMANN A. Protection of fungi against polyene antibiotics by sterols. Science. 1958 Aug 15;128(3320):361–361. doi: 10.1126/science.128.3320.361. [DOI] [PubMed] [Google Scholar]
- HICKEY R. J. The antagonism between the antifungal antibiotic, ascosin, and some long-chain, unsaturated fatty acids. Arch Biochem Biophys. 1953 Oct;46(2):331–336. doi: 10.1016/0003-9861(53)90205-7. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J Bacteriol. 1961 Dec;82:889–897. doi: 10.1128/jb.82.6.889-897.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., BOROWSKA Z., LASKIN A. I. Location and role of sterol at nystatin-binding sites. J Bacteriol. 1962 Dec;84:1152–1160. doi: 10.1128/jb.84.6.1152-1160.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
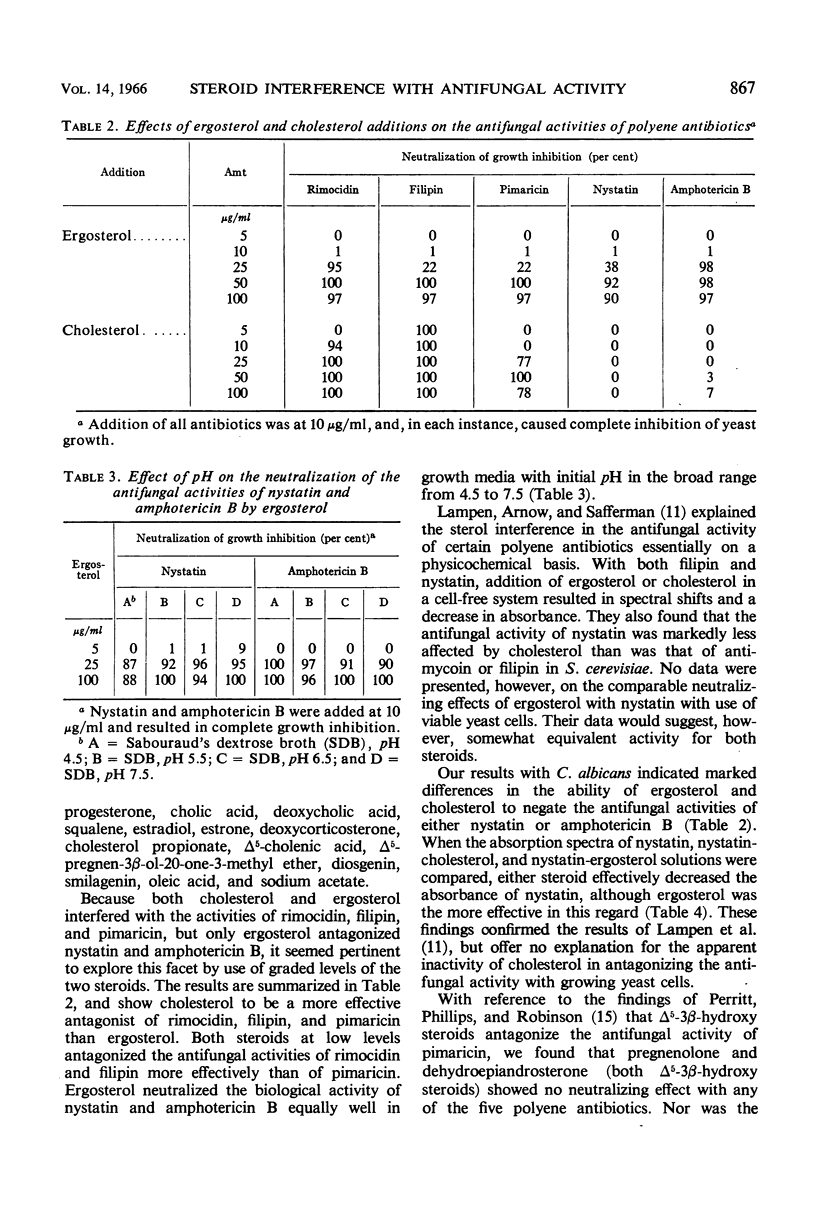
- LAMPEN J. O., ARNOW P. M., SAFFERMAN R. S. Mechanism of protection by sterols against polyene antibiotics. J Bacteriol. 1960 Aug;80:200–206. doi: 10.1128/jb.80.2.200-206.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. Inhibition of algae by nystatin. J Bacteriol. 1961 Aug;82:247–251. doi: 10.1128/jb.82.2.247-251.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINI F., ARNOW P., LAMPEN J. O. The effect of monovalent cations on the inhibition of yeast metabolism by nystatin. J Gen Microbiol. 1961 Jan;24:51–62. doi: 10.1099/00221287-24-1-51. [DOI] [PubMed] [Google Scholar]
- SUTTON D. D., ARNOW P. M., LAMPEN J. O. Effect of high concentrations of nystatin upon glycolysis and cellular permeability in yeast. Proc Soc Exp Biol Med. 1961 Oct;108:170–175. doi: 10.3181/00379727-108-26882. [DOI] [PubMed] [Google Scholar]


