Abstract
Background:
Social isolation and connectedness are social determinants of health that have demonstrated impacts on cancer-related outcomes. These constructs have been systematically evaluated among pediatric and older adult cancer populations. In this review, evaluated the prevalence, correlates, and psychosocial implications of social isolation and connectedness among young adult (YA) cancer survivors aged 18-39.
Methods:
Peer-reviewed articles published in English before June 2021 were identified from database searches and included articles’ reference lists according to PRISMA guidelines. Included articles described studies assessing social isolation and/or connectedness among YA cancer survivors.
Results:
In total, 5,094 unique records were identified; 4,143 were excluded after title/abstract screening and 907 were excluded after full-text review. Forty-four articles were included. Few studies used validated measures or directly assessed social isolation or connectedness. Social isolation was similarly prevalent among YAs as older cancer survivors and non-cancer populations. Demographic, clinical, and behavioral risk and protective factors for social isolation were identified. Social isolation was related to worse psychological well-being whereas social connectedness was often, but not always, related to better psychological well-being.
Conclusions:
This growing literature underscores the relevance of social isolation and connectedness as important health determinants among YA cancer survivors. The identified risk and protective factors can identify YAs who may especially benefit from screening for social isolation. Future studies are needed that directly, reliably, and validly evaluate social isolation and connectedness to inform the development of interventions to decrease isolation and increase connectedness.
Keywords: Cancer survivorship, social connectedness, social isolation, systematic review, young adults
More than 80,000 young adults (YAs) aged 18-39 are diagnosed with cancer in the United States annually.1 YAs with cancer face disease- and treatment-related life disruptions that can negatively impact well-being.2–4 In developed countries, young adulthood is often when individuals complete formal education, launch careers, begin living independently, achieve financial independence, establish long-term emotional, romantic, and sexual relationships, and start families.5,6 Core social relationships shift from being primarily with parents to including friends, colleagues, partners, and perhaps children.5 However, YAs who have been diagnosed with cancer can experience delays in these socio-developmental milestones. Thus, the implications of cancer for social well-being may be particularly impactful for YAs.
The construct of social well-being can be viewed through two lenses. According to Holt-Lunstad and colleagues,7 social isolation is a lack of interaction, contact, and/or relationships with other individuals and/or with society at large,8,9 whereas social connection is the structural and functional components of social relationships.7 Broadly conceptualized as umbrella terms, social isolation and connectedness encompass the ways in which individuals connect with others through various channels (e.g., emotional, behavioral, physical).7 Thus, several related constructs are conceptualized within social isolation (e.g., loneliness) and social connectedness (e.g., social support).
Social isolation is an important and well-established determinant of health that is consistently linked to all-cause mortality, morbidity, and negative mental health.10 In cancer, it is associated with low treatment engagement and mortality.11 Although most research on social isolation has focused on older adults, emerging evidence underscores its relevance for YAs. For example, loneliness is more prevalent among YAs than older adults,12,13 and YAs with long-standing illness, such as cancer, have higher incidence and severity of loneliness than healthy peers.14
Social connectedness is another determinant of health that is theorized to mechanistically underlie the health-promoting effects of social relationships.15 It is linked to longer life expectancy, better mental health, better cognitive functioning, and enhanced neuroendocrine and immune regulation.15 In cancer, social connectedness is associated with improved social and emotional well-being,16 and increased social support is associated with decreased mortality.17 Thus, social connectedness and aspects thereof, such as social support and belongingness, could promote well-being for YAs with cancer.
To date, two known systematic reviews have focused on social well-being among YA cancer survivors (i.e., YAs from the point of cancer diagnosis through end of life18). Warner and colleagues19 found that cancer survivors aged 15-39 reported difficulties related to employment, educational attainment, financial stability, interpersonal relationships, and peer support. Although numerous areas of need were identified, the review did not address the prevalence and unique impact of social isolation or connectedness among YA cancer survivors. Pahl and colleagues20 conducted a systematic review of social isolation and connectedness among cancer survivors aged 10-21 diagnosed during childhood or adolescence. However, the normative socio-developmental transitions and cancer-related challenges faced by this younger cohort are different from those faced by YAs. Thus, the prevalence and correlates of social isolation and connectedness in YA cancer survivors remain unclear.
To address this gap, we systematically evaluated the literature regarding social isolation and social connectedness in YA cancer survivors aged 18-39. Within this population we aimed to determine 1) the prevalence of social isolation and connectedness (i.e., how commonly they are experienced), 2) clinical and sociodemographic correlates of social isolation and connectedness, and 3) relationships of social isolation and connectedness with psychological well-being.
Methods
We searched MEDLINE, the Cochrane Database of Systematic Reviews, PROSPERO, and the Joanna Briggs Institute Systematic Review Register and confirmed there were no other systematic reviews on this topic. The protocol was registered on PROSPERO in advance (CRD42021282885).21 The methodology and results are reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)22 guidelines.
Inclusion Criteria
Eligible articles described empirical studies published in English that assessed social isolation and/or connectedness among YAs cancer survivors aged 18-39 at the time of diagnosis and 18-44 at the time of participation. Multiple articles describing the same sample were retained if each presented different relevant results. The age range for participation was extended beyond 39 to ensure that longitudinal studies of YA experiences were included. We focused on YAs aged ≥18 rather than ≥15 to minimize the likelihood that participants transitioned from a pediatric to adult treatment setting during the study. Articles with participants outside this age range were included if they presented results for participants aged 18-39 or a subset thereof separately. Articles that did not report participants’ ages at diagnosis were included if they clearly described YA cancer survivors’ experiences. Consistent with Pahl and colleagues,20 we operationalized social isolation and connectedness according to Holt-Lunstad and colleagues’7 framework. Therefore, studies were included if they assessed social isolation, social connectedness, or a related concept (e.g., loneliness, social support).
Search Strategy
Articles were identified by searching MEDLINE (Ovid), PsycINFO (EBSCO), EMBASE (Elsevier), and CINAHL (EBSCO) in June 2021. A medical librarian developed a search string for EMBASE and subsequently modified it for the remaining systems (Supplemental Appendix 1). Reference lists of included articles were also reviewed. All study designs were included; however, grey literature was excluded. No restrictions were imposed based on publication date, research setting, cancer type, or treatment status.
Study Selection
Identified article citations were uploaded into Rayyan23 and duplicates were removed. To calibrate the screening protocol, three reviewers (GEA, TFDV, JSG) screened the titles and abstracts of the same 77 articles. The reviewers then met as a group with RSF and LBO to resolve conflicts. Reviewer pairs screened the remaining titles and abstracts. The full texts of articles that were not excluded were retrieved and screened. Screening conflicts were resolved through consensus review and discussion.
Assessment of Methodological Quality
We assessed study quality with the Standard Quality Assessment Criteria for Evaluating Primary Research Papers (QualSyst),24 which is appropriate for quantitative (14 criteria) and qualitative (10 criteria) designs. For each study, relevant criteria were scored 0 (not met), 1 (partially met), 2 (met), or not applicable. Criteria scores were summed and divided by the total possible score based on the number of applicable items. Higher scores represented better methodological quality. Scores were categorized as “limited” (<0.50), “adequate” (0.50-0.70), “good” (0.71-0.79), or “strong” (>0.80) quality.25,26
Data Extraction
Data were extracted and recorded in a standardized Excel form. In addition to the extraction plan outlined in the pre-registered protocol, we documented the methods used to assess psychological well-being when available. We defined psychological well-being as any aspect of emotional or affective health; however, we excluded broad conceptualizations of health-related quality of life because these can include components of physical and social health.27 All reviewers pilot tested the extraction form with six articles and met as a group with RSF and LBO to resolve conflicts. Reviewer pairs then extracted data from the remaining articles. Conflicts were resolved through consensus review and discussion. For studies that included broader samples, data were only extracted for participants aged 18-44 at participation.
Results
Search Results
As shown in Figure 1, the initial database search yielded 7,414 titles. After deduplication, 5,050 titles and abstracts were screened. Of these, 4,124 were excluded and 926 full texts were retrieved. Of the full texts reviewed, 887 were excluded and 39 were retained. A search of the included articles’ reference lists identified an additional 44 articles for screening. Of these, 19 were excluded based on title and/or abstract and 20 were excluded after full text review, yielding five additional articles. In total, 44 articles were included.
Figure 1.
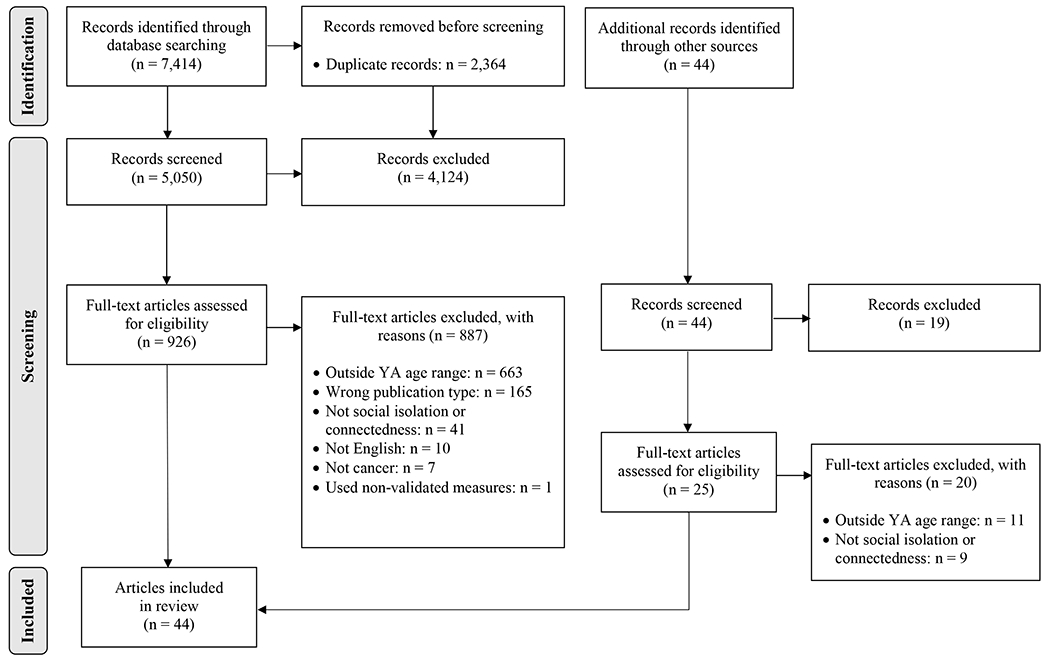
PRISMA Flowchart
Study Characteristics
As outlined in Table 1, most studies used qualitative methods (61.4%) and nearly all were observational (97.7%). Studies were predominantly conducted in the United States (45.5%) or Canada (15.9%), included participants with mixed cancers (72.7%) or breast cancer (20.5%) included participants who were receiving active anti-cancer treatment (36%), and were published since 2010 (81.8%). Supplemental Table 1 (quantitative studies) and Supplemental Table 2 (qualitative studies) show the results of the methodological quality assessments. Overall study quality was strong, with QualSyst scores ranging from 0.75-1.00 (M=0.91) for quantitative studies and 0.65-1.00 (M=0.85) for qualitative studies.
Table 1.
Characteristics of YA participants in the included articles
| First author | Design | Country | N YAs | Age at dx: Mean (SD), range | Age at study: Mean (SD), range | Months since dx | Tx status (time since tx ended) | Race, ethnicity, gender | Cancer types | Assessment of social isolation / social connectedness | Assessment of psychological well-being |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantitative Studies | |||||||||||
| Avis 2012 | Obs | USA | 132 | NR (NR), NR | NR (NR), 25-44 | ≤8 | On | 88% NHW, NR, 100% Female | Breast | • MOS Social Support Survey | • Brief Depression Inventory |
| Bellizzi 2012 | Obs | USA | 440 | NR (NR), 21-39 | NR (NR), NR | <14 | Mixed | 59% NHW, 21% H/L, 63% Female | Mixed | • AYA Hope Patient Survey | • NA |
| Brunet 2014 | Obs | NR | 64 | NR (NR), NR | 28.8 (5.5), 20-39 | Mean = 34.8 | Post (M=2.9 years) | 95% White, NR, 73% Female | Mixed | • Social Provisions Scale | • Perceived Stress Scale-10 |
| Geue 2019a | Obs | Germany | 514 | 29.6 (6.14), 18-39 | <4 years post-diagnosis | ≤48 | On | NR, NR, 75% Female | Mixed | • Illness-specific Social Support Scale-8 | • Hospital Anxiety and Depression Scale • Single item assessing overall life satisfaction |
| Geue 2019b | Obs | Germany | 179 | NR (NR), 18-39 | 27.5 (5.79), 19-42 | 12.1 | On | NR, NR, 62% Female | Hem. | • Illness-specific Social Support Scale-8 | • European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 |
| Hoyt 2013 | Obs | USA | 284 | NR (NR), NR | 25.2 (3.32), 18-29 | 32.4 | On | 46% NHW, 38% H/L, 0% Female | Testicular | • Cancer Assessment for Young Adults-Testicular | • Functional Assessment of Cancer Therapy-General • European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 • Cancer Assessment for Young Adults-Testicular |
| Kaal 2018 | Obs | Netherlan ds | 30 | 25.6 (6.4), 18-35 | 29.8 (5.3), 22-39 | 2.7 | On | NR, NR, NR | Mixed | • 13 items assessing the usefulness of an online AYA community | • NA |
| Naik 2020 | Obs | Canada | 420 | NR (NR), 18-39 | NR (NR), 18-39 | ≤6 | On | NR, NR, 100% Female | Breast | • Canadian Problem Checklist | • NA |
| Okamura 2021 | Obs | Japan | 206 | NR (NR), NR | 33.7 (4.3), 22-39 | <1 to ≥120 | Mixed | NR, NR, 87% Female | Mixed | • Multidimensional Scale of Perceived Social Support short form | • Supportive Care Needs Survey |
| Paskett 2008 | Obs | USA | 11 | NR (NR), NR | NR (NR), 25-44 | Median = 145.2 | Post (NR) | NR, NR, 100% Female | Breast | • MOS Social Support Survey • MOS SF-36 (social functioning scale) |
• NA |
| Roper 2013 | Obs | USA | 40 | NR (NR), NR | 30.41 (5.7), 21-40 | Newly dx | Post (1 to 6 months) | 90% NHW, NR, 60% Female | Lymphoma | • Impact of Cancer (social and relationship scale) | • NA |
| Trevino 2013a | Obs | USA | 71 | NR (NR), NR | 34.0 (5.57), 20-40 | Mean = 40.8 | On | 93% White, 2% H/L, 70% Female | Mixed | • Interpersonal Support Evaluation List-12 | • McGill Quality of Life Questionnaire (psychology and existential subscales) |
| Trevino 2013b | Obs | USA | 95 | NR (NR), NR | 33.4 (5.51), 20-40 | Mean = 43.44 | On | 86% White, 5% H/L, 68% Female | Mixed | • Interpersonal Support Evaluation List-12 | • NA |
| Xie 2017 | Obs | China | 524 | NR (NR), NR | NR (NR), 21-39 | NR | NR | NR, NR, 60% Female | Mixed | • Social Support Rating Scale | • Distress Thermometer • Hospital Anxiety and Depression Scale |
| Zebrack 2017 | Int | USA | 304 | NR (NR), 18-40 | NR (NR), 18-40 | NR | Mixed | 79% White, NR, 82% Female | Mixed | • Duke/UNC Functional Social Support Scale | • Patient Health Questionnaire-4 |
| Qualitative Studies | |||||||||||
| Corbeil 2009 | Obs | Canada | 6 | NR (NR), NR | 25 (NR), 19-30 | Mean = 17.5 | On | NR, NR, 50% Female | Mixed | • Semi-structured interviews | • NA |
| D’Agostino 2013 | Obs | Canada | 9 | 24.7 (3.97), 18-29a | 30.0 (3.16), 23-34a | NR | Post (≥2 years) | NR, NR, 56% Female | Mixed | • Focus groups | • NA |
| Easley 2013 | Obs | Canada | 12 | 32 (3.94), 24-37 | 34 (3.26), 28-38 | NR | Post (1 to 5 years) | 100% White, NR, 92% Female | Thyroid | • Interviews | • Interviews |
| Halliday 2014 | Obs | Australia | 12 | NR (NR), NR | NR (NR), 25-39 | ≥12 | Post (NR) | NR, NR, 100% Female | Hem. | • Interviews | • NA |
| Hamid 2021 | Obs | India | 10 | 31.9 (6.31), 20-38a | 28.6 (6.43) 22-41a | ≤6 | On | NR, NR, 100% Female | Breast | • Semi-structured interviews | • Semi-structured interviews |
| Hanghøj 2021 | Obs | Denmark | 13 | NR (NR), NR | 23.5 (NR), 18-29 | NR | Mixed | NR, NR, 69% Female | Mixed | • Semi-structured interviews | • Semi-structured interviews |
| Hauken 2013 | Obs | Norway | 20 | NR (NR), NR | 31.1 (3.9), 24-35 | Mean = 24.6 | Post (M=16 years) | NR, NR, 75% Female | Mixed | • Semi-structured interviews | • NA |
| Hauken 2019 | Obs | Norway | 20 | NR (NR), NR | 31.1 (3.9), 24-35 | Mean = 24.6 | Post (M=16 years) | NR, NR, 75% Female | Mixed | • Semi-structured Interviews | • NA |
| Kenen 2006 | Obs | UK | 10 | NR (NR), NR | 41.4 (1.51), 39-44a | NR | On | NR, NR, 100% Female | Breast, Ovarian | • Focus groups | • Focus groups |
| Kim 2013 | Obs | Australia | 46 | NR (NR), NR | 31 (NR), 23-38 | NR | On | NR, NR, 74% Female | Mixed | • Online blogs | • NA |
| Knox 2017 | Obs | Canada | 10 | NR (NR), NR | 26 (10.6), 18-35 | NR | Mixed | NR, NR, 70% Female | Mixed | • Semi-structured interviews | • NA |
| Kumar 2013 | Obs | USA | 15 | NR (NR), NR | NR (NR), 18-30 | NR | Mixed (≤5 years) | 87% White, 13% H/L, 67% Female | Mixed | • Semi-structured interviews | • Semi-structured interviews |
| Lidington 2021 | Obs | UK | 65 | 31.7 (NR), 25-39 | 33.6 (NR), 25-42 | Mean = 22.8 | Mixed | 75% White, NR, 60% Female | Mixed | •Semi-structured interviews • Focus groups |
• NA |
| Love 2014 | Obs | USA | 9 | NR (NR), NR | 29.3 (NR), 21-39a | Mean = 85.2 | Mixed | 89% White, NR, 0% Female | Mixed | •Online forum • Focus groups • Semi-structured interviews |
• NA |
| Miedema 2007 | Obs | Canada | 13 | 27.5 (4.7), 20-35a | 30.2 (5.3), 20-37a | NR | Mixed | NR, NR, 60% Female | Mixed | • Interviews | • NA |
| Mikkelsen 2008 | Obs | Denmark | NR | NR (NR), NR | NR (NR), NR | NR | On | NR, NR, NR | Mixed | • Focus groups | • NA |
| Miller 2015 | Obs | USA | 25 | 34 (NR), 26-39 | NR (NR), NR | NR | On | 100% White, NR, 100% Female | Breast | • Interviews | • NA |
| Milosevic 2020 | Obs | Canada | 12 | 34 (3.5), 27-39 | 36 (3.4), 31-41 | ≤60 | NR | 73% White, NR, 100% Female | Breast | • Semi-structured interviews | • NA |
| Mishra 2018 | Obs | USA | 8 | NR (NR), NR | 31.9 (5.2), 23-39a | ≥6 | On | 100% White, 75% H/L, 88% Female | Mixed | • Semi-structured interviews | • Semi-structured interviews |
| Musiello 2014 | Obs | Australia | 7 | NR (NR), NRb | 19.1 (1.6), 18-22a | NR | Mixed (≤6 months) | NR, NR, 71% Female | Mixed | • Focus groups | • Focus groups |
| Nizamli 2011 | Obs | Syria | 17 | NR (NR), NR | 37 (NR), 30-45c | NR | NR | NR, NR, 100% Female | Breast | • Semi-structured interviews | • NA |
| Panjwani 2019 | Obs | USA | 53 | 25.9 (5.3), NR | 28.4 (5.0), 20-39 | Mean = 28 | Mixed | 79% White 8% H/L 68% Female | Hem. | • Semi-structured interviews | • NA |
| Ruddy 2013 | Obs | USA | 36 | NR (NR), NR | 37.8 (4.7), 26-44 | Mean = 22.3 | On | 94% NHW NR, 100% Female | Breast | • Focus groups | • NA |
| Siegel 1999 | Obs | USA | 34 | 30.6 (NR), 22-35 | NR (NR), NR | Mean = 38 | Post (≥6 months) | 85% White, 0% H/L 100% Female | Breast | • Semi-structured interviews | • NA |
| Snyder 2010 | Obs | USA | 70 | 32.9 (NR), 23-39 | 35.2 (NR), NR | 85.7% of sample: <36 | Mixed | 61% NHW 4% H/L, 100% Female | Breast | • Semi-structured interviews | • Semi-structured interviews |
| Victorson 2019 | Obs | USA | 16 | NR (NR), NR | 33.1 (3.7), 28-39 | NR | Post (≤5 years) | 50% White, 6% H/L, 75% Female | Mixed | • Focus groups | • NA |
| Zebrack 2010 | Obs | USA | 17 | NR (NR), NR | NR (NR), 18-35 | NR | Post (NR) | NR, NR, NR | Mixed | • Focus groups | • NA |
| Mixed Methods Studies | |||||||||||
| Darabos 2022 | Obs | USA | 59 | NR (NR), NR | 35.1 (4.84), 24-42 | <60 | Mixed | 93% White, NR, 97% Female | Mixed | • PROMIS Social Isolation 8-item short form • Two open ended questions about social support preferences |
• NA |
| Munoz 2016 | Obs | USA | 31 | NR (NR), NR | 33.2 (5.1), 21-39 | NR | Post (≤5 years) | 29% Black, 42% H/L, 65% Female | Mixed | • Semi-structured interviews | • NA |
Note. Participants in Geue 2019b were a subset of those in Geue 2019a. Participants in Trevino 2013a were a subset of those in Trevino 2013b. Participants in Hauken 2019 were redundant with those in Hauken 2013.
SD=Standard Deviation; NR=Not Reported; NA=Not Applicable; Obs=Observational; Int=Interventional; Hem=Hematological; H/L=Hispanic/Latinx
Calculated by the review authors from participant-level data provided in the manuscript
all participants had been diagnosed within the prior 12 months, and therefore were no more than one year older than their age at diagnosis
no quotes from participants older than 44 were provided in the paper
Prevalence of Social Isolation and Social Connectedness among YA Cancer Survivors
Most articles (n=37, 86.4%) reported prevalence of social isolation and connectedness (Table 2). Within those, 11 (29.7%) used quantitative methods, 25 (67.6%) used qualitative methods, and 1 (2.7%) used mixed methods. Quantitative studies found that, relative to older cancer survivors and cancer-free individuals, YAs reported similar levels of social isolation/connectedness and related constructs (e.g., loneliness28 and social support29–31). For some YAs, cancer positively impacted their relationships with family and friends32 and increased the value they placed on those relationships.33 In qualitative studies, YAs identified family, friends, neighbors, colleagues, other cancer survivors, and healthcare professionals as key sources of connectedness.34–36
Table 2.
Results related to the prevalence of social isolation and connectedness
| First author | Results | Meta-inferences |
|---|---|---|
| Quantitative Studies | ||
| Avis et al. (2012) | YAs reported average levels of social support on the RAND Social Support Scale (M=4.3, SD=0.7, range: 1-5) relative to older adults with cancer. | • In general, YAs reported average-to-elevated levels of social isolation, although there is also evidence that cancer can positively impact important relationships. • Engagement with supportive interventions such as online communities were related to decreased reports of social isolation and increased social connectedness. • Few quantitative studies assessed social isolation and/or connectedness directly (i.e., with a baseline measure of social isolation/connectedness), and often assessed prevalence through indirect means (i.e., social problems, social support, general questions about social relationships). |
| Bellizzi et al. (2012) | On average, 11% of YAs reported that cancer negatively impacted their relationships with family and friends, while 63% reported that cancer had a positive impact on these relationships. | |
| Brunet et al. (2014) | YAs reported slightly diminished levels of perceived social support on the Social Provisions Scale (M=3.33, SD=0.47, range: 1-4) relative to a sample of college students, public school teachers, and military nurses.37 Half of respondents reported they had previously been involved in a support group designed for people with cancer. | |
| Geue et al. (2019b) | YAs reported elevated levels of detrimental interactions (M=4.2, SD=3.1, range: 0-16) and comparable levels of positive support (M=13.4, SD=2.8, range: 0-16) as measured by the Illness-specific Social Support Scale relative to older adult participants. Only 12.3% (n=22) of YAs compared reported having no detrimental interactions and 29.1% (n=52) had positive support scores at the maximum value of 16. | |
| Kaal et al. (2018) | After engaging with an online YA support community, 13 YAs (48%) reported not feeling lonely anymore; 17 (63%) reported having good contact with peers; 12 (44%) reported they had made new friends; and 17 (63%) reported they felt supported. | |
| Naik et al. (2020) | On the Canadian Problem Checklist, 10.5% of YAs (n=44) reported feeling alone, comparable to older adults with cancer. | |
| Paskett et al. (2008) | YAs aged 35-44 reported lower social functioning as measured by the MOS SF-36 scale (M=73.75, SD=28.53) than the general US female population within the same age group (M=83.07, SD=23.27), and relative to older breast cancer survivors. | |
| Roper et al. (2013) | At the conclusion of treatment through 6 months post-treatment, YAs reported placing higher value on relationships with family and friends than before having been diagnosed with cancer (Ms = 3.9 to 4.1, range: 1-5) as well as feeling a special bond with people with cancer (Ms=3.6 to 4.0, range: 1-5). | |
| Trevino et al. (2013b) | Relative to patients with arthritis or at risk for cardiovascular disease and healthy adults, 38 YAs reported decreased availability of overall social resources (M=42.38, SD=4.39, range: 12-48) as well as a diminished sense of having someone available to talk to about problems (M=13.97, SD=1.89, range: 4-16), decreased tangible or material aid (M=14.57, SD=1.66, range: 4-16), and a decreased sense of belonging or of having someone to engage in activity with (M= 13.85, SD=2.11, range: 4-16) as measured by 12 items from the Interpersonal Support Evaluation List. | |
| Xie et al. (2017) | YAs reported moderate levels of social support according to established cut points as measured by the Chinese version of the Social Support Rating Scale (M=40.58, SD=6.96, range: 12-66). | |
| Zebrack et al. (2017) | YAs reported average levels of social support as measured by the Duke-UNC Functional Social Support Questionnaire (M=4.06, SD=0.81, range: 1-5) relative to older patients with advanced cancer.39 | |
|
| ||
| Qualitative Studies | ||
| Corbeil et al. (2009) | Nearly all YAs reported that they relied heavily on family for support throughout their cancer experience. | • Social isolation-related themes emerged in multiple studies. Some of these studies focused on topics closely related to isolation, such as social support, while others targeted more distant concepts, such as fertility, identity formation, physical activity, nutrition, weight management, and the overall experience of being a YA with cancer. • YAs frequently described feeling different from peers, lacking support from peers and family, and feeling concerned about social relationships • Multiple studies identified feeling isolated from other YAs experiencing cancer as a major concern • Among those who reported feeling socially connected, important sources of support included family, friends, neighbors, colleagues, other cancer survivors, and healthcare professionals. |
| D’Agostino et al. (2013) | YAs with cancer expressed concerns about relationships with peers and family, including feelings of social isolation. | |
| Easley et al. (2013) | YAs with thyroid cancer expressed feeling isolated in three ways: 1) from other cancer survivors due to having a “good” cancer (e.g., high chance of survival, no chemotherapy), 2) due to mandatory physical isolation periods following radioactive iodine treatments, and 3) from peers without cancer. | |
| Halliday et al. (2014) | YAs with uncertain fertility described feeling different and disconnected from peers with no cancer history. | |
| Hamid et al. (2021) | Kashmiri YAs with breast cancer in the study reported receiving support from parents, siblings, other family members. Other common sources of support included friends, neighbors, colleagues, other women with breast cancer, and healthcare professionals. | |
| Hauken et al. (2019) | Most YAs reported lacking peer support during cancer treatment. | |
| Hauken et al. (2013) | Upon re-entry into everyday life after cancer treatment, YAs reported increased social isolation and a lack of social connectedness with families and friends, employers, colleagues, and healthcare providers even when surrounded by family and friends and enrolled in regular follow-ups. Conversely, those who had met other YAs reported feeling greater social connectedness. | |
| Kenen et al. (2006) | YAs with breast cancer carrying a BRCA1 or BRCA2 mutation reported feeling isolated, separate, and alone, and identified instances of social separation initiated by both themselves and others even in the presence of social support. | |
| Kim et al. (2013) | Isolation was one of the primary themes identified in analysis of online blogs written by YAs. Conversely, engaging with a social network on the internet promoted feelings of social connectedness from both new and existing relationships. | |
| Knox et al. (2017) | An advanced cancer diagnosis was universally experienced as isolating and unexpected. All YAs reported feeling that they did not belong and that others could not understand them, particularly peers with no cancer history or with localized cancer. | |
| Kumar et al. (2013) | Most, but not all, YAs reported having steady emotional support from family and friends. Another important source of support was clinical providers, although a poor clinical alliance was related to increased isolation. | |
| Lidington et al. (2021) | “Facing isolation” emerged as a major theme among YAs, comprised of subthemes including feeling distant from friends, feeling younger than other cancer patients, and feeling concerned about missing out. | |
| Love et al. (2014) | Young men reported feeling unsupported by and isolated from other males. | |
| Miedema et al. (2007) | Some YAs mentioned losing friends since their cancer diagnosis, as well as difficulty socializing and making new friends due to decreased energy. | |
| Mikkelsen et al. (2008) | “Needs for social support” emerged as a major theme among YAs after hospital discharge and after completing treatment. Some YAs spoke about a need for connection with same-aged peers with cancer. | |
| Miller (2015) | Many YA women with breast cancer expressed feeling uncertain and socially isolated, especially from same-age peers with no cancer history and older women with breast cancer. | |
| Milosevic et al. (2020) | YAs with breast cancer reported feeling distanced from peers with no cancer history with respect to physical activity and eating patterns. | |
| Mishra et al. (2018) | YAs reported feelings of isolation. | |
| Musiello et al. (2014) | “Isolation” emerged as a key theme among YAs treated in adult hospital settings, primary related to the age difference relative to older cancer patients. YAs reported feeling isolated and unable to connect with similarly aged peers going through similar experiences. | |
| Nizamli et al. (2011) | “Social isolation” emerged as a subtheme of “Social dysfunction” among Female-identifying Syrian YAs with breast cancer. YAs reported that Syrian society has a negative perception of cancer patients, associating them with death. | |
| Panjwani et al. (2019) | One third (36%) of YAs spontaneously reported uncertainty related to peers and social life. | |
| Ruddy et al. (2013) | YAs reported that social support decreased during the transition from active treatment to longer-term survivorship. Many YAs also wished they could connect with other patients in a similar life stage. | |
| Siegel et al. (1999) | A sense of isolation and of being different from other women was a prominent theme among YAs with breast cancer. | |
| Snyder et al. (2010) | YAs with breast cancer expressed feeling isolated in support groups due to a sense that support services and organizations are designed to target the needs of older people with breast cancer. | |
| Victorson et al. (2019) | YAs reported feeling misunderstood, isolated, and alone, or incapable of actively engaging in and being open to new relationships because of cancer. | |
|
| ||
| Mixed methods Studies | ||
| Munoz et al. (2016) | Asian/Pacific Islander, Black non-Hispanic, and Hispanic YAs all reported obtaining social support by connecting to friends and strangers using social networking sites, connecting in-person with family and friends, and connecting with others by participating in religious or spiritual activities such as attending worship services. | • Racial/ethnic minority YAs identified numerous strategies and avenues for maintaining social connection |
Note. Trevino et al. (2013a) was excluded from this table because participants were a subset of those in Trevino et al. (2013b) and all relevant variables were redundant across the two publications.
Conversely, some studies found that YAs had elevated levels of social isolation. For example, Paskett and colleagues37 found that YA breast cancer survivors reported worse social functioning than cancer-free peers and older survivors. Similarly, quantitative studies found that, relative to other populations, YAs expressed more detrimental interactions,30 diminished social support,38,39 and lower social functioning.28 Themes related to social isolation emerged in numerous qualitative studies. Some focused on topics closely related to isolation and connectedness.36,40–46 However, others focused on more distant concepts, such as coping,47 uncertainty,34,48,49 fertility,50 physical activity, nutrition, and weight,51 and the general lived experience YA cancer survivors.52–59 In some studies, isolation was directly related to the disease experience. For example, Halliday and colleagues50 found that young female hematological cancer survivors described a sense of “otherness” associated with having uncertain fertility. Kenen and colleagues42 found that social separation was exacerbated by carrying a BRCA mutation. Knox and colleagues52 identified advanced cancer as particularly isolating, as YAs reported feeling poorly understood by both cancer-free peers and YAs with localized disease.
Two studies identified the transition off treatment as particularly isolating,60,61 and four identified isolation from other YA cancer survivors as a major concern.44,54,56,61 Conversely, in three studies, YAs who connected with peers experienced greater social connectedness.53,59,62 Kaal and colleagues62 found lower social isolation and greater connectedness among YAs engaged with an online YA support community. Kim and colleagues53 found that online communities helped YAs befriend other YA cancer survivors, increasing their sense of connectedness. Finally, Hauken and colleagues59 found that YAs who met other YA cancer survivors expressed greater feelings of connectedness.
Clinical and Sociodemographic Correlates of Social Isolation and Social Connectedness
Clinical and sociodemographic correlates of social isolation and connectedness were identified in 18 articles (40.9%), although few correlates were explored in more than one study (Table 3). Within these articles, 6 (33.3%) used quantitative methods, 11 (61.1%) used qualitative methods, and 1 (5.6%) used mixed methods. Quantitative studies found that YAs reporting greater social support were more likely to be physically active,38 White, female, married, and diagnosed with breast (versus another) cancer,63 employed or in school,64 and have a stronger alliance with their oncologist.39 In qualitative studies, greater feelings of connectedness were associated with receiving positive comments or actions from others65 and being a male with a partner who viewed cancer as a joint undertaking.43 Three quantitative studies explored age at study participation among YAs, with one finding that older YAs demonstrated greater social connectedness than younger YAs,63 one finding the opposite,64 and one finding a non-linear relationship.31 Specifically, Xie and colleagues31 found that YAs aged 21-30 at the time of participation reported lower social support than YAs aged <20 or 31-39. In addition, one quantitative study explored age at diagnosis. Bellizzi and colleagues32 found that a smaller proportion of YAs aged 21-29 at diagnosis reported cancer-associated improvements in their relationships with siblings relative to YAs younger than 20, and in their relationships with spouses/significant others relative to YAs aged 30-39.32
Table 3.
Results related to clinical and sociodemographic correlates of social isolation and social connectedness
| First author | Results | Meta-inferences |
|---|---|---|
| Quantitative Studies | ||
| Bellizzi et al. (2012) | Age was associated with positive cancer-related changes to relationships. Compared to YAs aged 21-29, a greater proportion of YAs aged 15 to 20 reported more improvements in relationships with siblings (79.7% vs 61.3%, p<0.05) and YAs aged 30-39 reported greater improvements in relationships with spouses/significant others (69.2% vs 58.8%, p<0.05). | • Social isolation and social connectedness were related to multiple demographic characteristics, clinical variables, and social determinants of health • Greater social connectedness was associated with: • Increased physical activity • White (vs. non-White) race • Being married/partnered • Breast (vs. other site) cancer • Female sex • Having a stronger alliance with an oncologist • Being employed or in school • The relationship of age to social isolation/connectedness was inconsistent across studies |
| Brunet et al. (2014) | Perceived social support was positively related to physical activity (rpb=0.28, p<0.05). | |
| Trevino et al. (2013a) | White participants (M=42.66, SD=4.44) reported higher levels of total social support as measured by 12 items from the ISEL compared to non-White participants (M=37.50, SD=4.04, t(62)=−2.26, p<0.05). Additionally, feeling a sense of belonging or of having someone to engage in activity with was higher among female (M=14.09, SD=2.14) versus male participants (M=12.95, SD=1.98, t(62)=−2.02, p<0.05), White (M=13.88, SD=2.09) versus non-White participants (M= 11.50, SD=1.91, t(62)=−2.22, p<0.05), married (M=14.21, SD= 1.93) versus nonmarried participants (M=13.04, SD=2.28, t(62)=−2.21, p<0.05), and breast cancer survivors (M= 14.59, SD=1.97) versus survivors with other cancer diagnoses (M=13.29, SD=2.12, t(62)=−2.40, p<0.05). Older age was also associated with a greater sense of belonging (r=0.29, p<0.05). | |
| Trevino et al. (2013b) | A greater reported level of appraisal support, or having someone to talk to about problems, was associated with having a stronger alliance with one’s oncologist among YAs with advanced cancer (r[88]=0.32; p=0.002). | |
| Xie et al., (2017) | YAs aged 36-39 (M=43.40, SD= 6.36) reported the highest levels of social support while those aged 21-25 (M=37.21, SD=7.51) reported the lowest levels (F=21.74, p<0.001). | |
| Zebrack et al. (2017) | YAs aged 30-39 at diagnosis reported lower levels of social support than those aged 18-29 at diagnosis (β=−0.27, p=0.009) and YAs employed or in school reported higher levels of social support than those not employed or in school (β=0.34, p=0.001). | |
|
| ||
| Qualitative Studies | ||
| Hanghøj et al. (2021) | YAs in active treatment and those with impaired immune systems reported particularly elevated loneliness during the first lockdown period of the COVID-19 pandemic. | • Patients qualitatively generated numerous correlates of greater social isolation, including: • Cancer type • Being in active treatment • Being immune suppressed • Having cancer-related changes to appearance • Having increased physical late effects of disease • Having a cancer-related genetic mutation • Having young children • Being in medical care settings • Cultural background |
| Hauken et al. (2019) | YAs with changes to appearance (e.g., hair loss) and other treatment side effects (e.g., fatigue) reported greater social isolation. | |
| Hauken et al. (2013) | YAs who experienced late effects of cancer expressed increased feelings of social isolation. | |
| Kenen et al. (2006) | Having a genetic mutation was related to increased isolation among YAs with breast cancer carrying a BRCA1 or BRCA2 mutation. | |
| Knox et al. (2017) | Having small children increased feelings of isolation among YAs with advanced cancer. | |
| Lidington et al. (2021) | YAs with young children reported feeling particularly isolated and as if they missed out on important developmental changes. | |
| Love et al. (2014) | For YA males, having a spouse/romantic partner who viewed the cancer experience as a joint effort was associated with receiving more effective social support. | |
| Miller (2015) | YAs with breast cancer expressed that social isolation was particularly salient in the context of medical settings and encounters due to limited presence of other YA survivors. | |
| Nizamli et al. (2011) | Social isolation was exacerbated by a negative societal view of Syrian women with breast cancer, related to a cultural understanding that cancer means death. | • YAs reported feeling less isolated when they: • Had a supportive spouse/romantic partner (YA men only) • Received positive comments or actions from others |
| Siegel et al. (1999) | Many YAs who attended a patient support group reported feeling increased isolation due to a lack of connection with older attendees. | |
| Zebrack et al. (2010) | YA survivors expressed feeling less isolated when they received positive comments or actions from others. | |
|
| ||
| Mixed Methods Studies | ||
| Darabos et al. (2022) | YAs with lower levels of perceived isolation preferred to receive support through conversations that were not minimizing and that did not contain expressions of pity/worry (OR = 0.88, 95% CI: 0.78–1.00). | • Social isolation is associated with the type and content of supportive conversations |
Multiple cancer-related variables were associated with social isolation in qualitative studies. These included greater symptom burden,41,59 being in active treatment and/or immunosuppressed,66 having a genetic mutation,42 and physically being in healthcare settings.48 Siegel and colleagues67 found that YA breast cancer survivors who attended age-inclusive support groups reported feeling more isolated due to difficulties connecting with older attendees. Non-disease-related correlates also emerged. Two studies52,54 found that having small children was associated with social isolation. Lidington and colleagues54 elucidated that YAs expressed concern about missing their children’s developmental milestones. Culture was also relevant, with Nizamli and colleagues57 reporting that isolation was exacerbated by the cultural understanding that cancer implies death in their study of Syrian YA breast cancer survivors. Finally, social isolation had implications for communication preferences. Darabos and colleagues68 found that YAs reporting less social isolation preferred receiving support through conversations that avoided minimizing cancer and expressing pity or worry.
Psychological Implications of Social Isolation and Social Connectedness
The relationships of social isolation and connectedness to psychological well-being were explored in 17 articles (38.6%) (Table 4). Within these articles, 9 (52.9%) used quantitative methods and 8 (47.1%) used qualitative methods. None used mixed methods. Greater social isolation was generally associated with worse psychological well-being. Brunet and colleagues38 found that social support was lower among YAs reporting more stress, and Okamura and colleagues69 found that poor social support was associated with greater unmet psychological needs. Conversely, greater social connectedness was generally associated with better psychological well-being. Multiple studies found that greater social support was related to fewer symptoms of depression29,31,70 and anxiety.31,70 Similarly, Xie and colleagues31 and Zebrack and colleagues64 found that greater support was associated with less distress. Finally, social support was related to improved psychological and existential health-related quality of life,63 better emotional functioning,30 and better cognitive-emotional regulation.71
Table 4.
Results related to relationships of social isolation and social connectedness to psychological well-being
| First author | Results | Meta-inferences |
|---|---|---|
| Quantitative Studies | ||
| Avis et al. (2012) | Reported social support was lower among YAs diagnosed with breast cancer who also reported more depressive symptomatology compared to those who did not report depressive symptomatology (M=4.1 vs. 4.4, p<0.001). | • In general, greater social isolation was associated with worse psychological well-being, including: • Greater stress • Greater unmet psychological needs • Conversely, greater social connectedness was associated with better psychological well-being, including: • Less depression • Less anxiety • Less severe grief • Less distress • Better emotional functioning |
| Brunet et al. (2014) | Both lower perceived social support (r=−0.68, p<0.05) and not having previously been involved with a support group designed for people with cancer (rpb=−0.25, p<0.05) were associated with greater stress. | |
| Geue et al. (2019a) | Positive social support was negatively associated with anxiety and depression at both baseline (anxiety: β=−0.09, p=0.008, depression: (β=−0.10, p=0.003) and at 12-month follow-up (anxiety: β=−0.09, p=0.003, depression: (β=−0.14, p=0.001). Detrimental interactions were also positively associated with anxiety at baseline only (β=0.07, p=0.042). | |
| Geue et al. (2019b) | Positive social support was positively associated (r=0.16, p<0.05) and detrimental interactions were negatively associated (r=−0.17, p<0.05) with emotional functioning. | |
| Hoyt et al. (2013) | Social connectedness was significantly associated with better cognitive-emotional regulation (rs ranged from 0.67 to 0.72, all ps <0.001). | |
| Okamura et al. (2021) | Poor social support was associated with having greater unmet psychological needs (β=−0.15, p=0.02). | |
| Trevino et al. (2013a) | Among YAs with advanced cancer, greater overall social support was associated with greater psychological HRQOL (β=0.43, p<0.001), greater existential HRQOL (β=0.43, p<0.001), and less severe grief (β=−0.28, p<0.05). Greater reported appraisal support, or having someone to talk to about problems, was also associated with greater psychological HRQOL (β=0.37, p<0.01) and greater existential HRQOL (β=0.30, p<0.05) adjusting for metastatic disease, as well as less severe grief (β=−0.29, p<0.05). Greater reported tangible support, or material aid, was associated only with greater psychological (β=0.47, p<0.01) and existential (β=0.31, p<0.05) HRQOL. Finally, greater belonging support, or the sense of having someone to engage in activity with, was associated only with greater existential HRQOL (β=0.34, p<0.01). |
|
| Xie et al. (2017) | Greater social support was associated with less distress (r=−0.81, p<0.001) as well as less anxiety and depression (r=−0.84, p<0.001). | |
| Zebrack et al. (2017) | Reported social support was lower among distressed YAs (M=3.53, SD=0.89, range: 1-5) than non-distressed YAs (M=4.13, SD=0.78, range: 1-5; β=−0.54, p=0.001). | |
|
| ||
| Qualitative Studies | ||
| Easley et al. (2013) | One YA with thyroid cancer discussed the psychological effects of mandatory physical isolation following radioactive iodine treatment. They described the treatment as “traumatic” and that it made them feel like they “had a plague or something.” | • Social isolation was related to worse psychological well-being, including feelings of: • Distress • Trauma • Anxiety • Depression • Emotional instability • Specifically in the context of the COVID-19 pandemic, for some YAs increased social isolation enabled time off for recovery, reflection, peace, and quiet. • Increased social connectedness was sometimes related to worse psychological well-being, including feelings of worry and guilt related to feeling responsible for loved ones emotions, as well as trauma related to hearing other survivors cancer stories. |
| Hamid et al. (2021) | Social support from healthcare professionals was associated with decreased negative feelings, increased positive coping, and decreased fears related to disease, treatment, results, and recovery. | |
| Hanghøj et al. (2021) | Isolation from others was related to feelings of being alone with negative thoughts, increased anxiety, emotional instability, and symptoms related to depression. Conversely, increased isolation during the COVID-19 pandemic made it easier for some YAs to have time off for reflection and find peace and quiet. | |
| Kenen et al. (2006) | YA breast cancer survivors with strong social support expressed feelings of worry and guilt related to feeling responsible for the emotional well-being of their loved ones. Greater connectedness with other cancer survivors sometimes contributed to “trauma” related to hearing other people’s cancer stories. | |
| Kumar et al. (2013) | Lack of family and social support was associated with emotional distress and feeling “dejected and frustrated.” | |
| Mishra et al. (2018) | One YA explained that being around family and friends was associated with increased feelings of happiness. | |
| Musiello et al. (2014) | Feeling isolated in the treatment environment was associated with feelings of distress. | |
| Snyder et al. (2010) | YAs reported that support received from family improved emotional well-being. | |
Qualitative studies identified psychosocially relevant sources of social connectedness. Hamid and colleagues36 found that social support from healthcare professionals was related to decreased negative affect, increased positive coping, and reduced fear. Kumar and colleagues35 found that lack of family support was related to emotional distress. A participant in Mishra and colleagues’55 study reported feeling happier around family and friends, and Snyder and colleagues44 found that family support improved emotional well-being. Specific contexts were also explored. Musiello and colleagues56 found that feeling isolated while physically in healthcare settings was related to distress, and a participant with thyroid cancer in Easley and colleagues’45 study described physical isolation following radioactive iodine treatments as “traumatic.”
Interestingly, Hanghøj and colleagues66 found that increased social isolation during the COVID-19 pandemic was related to better psychological well-being, as it created time for reflection, peace, and quiet. Additionally, Kenen and colleagues42 found that increased social connectedness was related to worse psychological health. They reported that YA breast cancer survivors with strong social support expressed worry and guilt about loved ones’ emotional well-being, and that connectedness with other cancer survivors sometimes contributed to “trauma” from hearing others’ stories.
Discussion
This systematic review summarized results from 44 articles reflecting 42 unique samples, most of which were published in the past 10 years. This growing literature highlights the increased attention being paid to YA cancer survivors as an age-defined population and the importance of social isolation and connectedness, broadly conceptualized by Holt-Lunstad and colleagues7 as the ways in which individuals socially connect, as health determinants. Results across several qualitative studies established the scope of social isolation and connectedness among YA cancer survivors, and descriptive quantitative studies supported the generation of evidence-based hypotheses. However, only one study was interventional,64 none used a randomized controlled trial design, and few used validated measures or assessed social isolation or connectedness directly. There is a clear need for future research to fill these gaps.
In several studies, YA cancer survivors reported levels of social isolation and connectedness similar to older survivors and non-cancer populations. However, while prevalence may be similar, the risk factors and psychological consequences of social isolation and connectedness are likely different for YA cancer survivors given the unique socio-developmental context of young adulthood. We identified several risk factors for greater social isolation among YAs (e.g., having young children,52,54 greater symptom burden,41,59 being in healthcare settings48,67), and several protective factors (e.g., being married/partnered,63 female sex,63 being employed or in school64). These factors may identify YA survivors most in need of screening and intervention. Interestingly, the impact of age was not clear. Research specifically designed to test this relationship could elucidate the impact of age within young adulthood on the risk of social isolation. Additionally, exploring the impact of developmental stage within the YA age range (e.g., emerging adulthood, young adulthood) could also be important. These life stages can have different developmental goals, which in turn could have different implications for the social impact of cancer.
Results also identified important social contexts for minimizing social isolation and increasing connectedness, such as fostering peer connection among YA cancer survivors. In multiple studies, YAs reported difficulty connecting with peer survivors,44,45,48,52,56,59–61 which sometimes led to increased isolation in environments specifically designed to foster connectedness, such as age-inclusive cancer support groups. Although the need for peer connection has been well established,72 optimal strategies for facilitating such connection remain unclear. For example, social media is a promising source of social support for YAs,73 and two included studies found that YAs who met other YA cancer survivors through online communities reported lower social isolation and greater connectedness.53,62 However, recent evidence has also linked high social media use to increased isolation among younger generations.74,75 There may be a tipping point after which social media stops being helpful, or it may be that the impact of social media varies depending on the purpose of its use. Future research is needed to explore how best to foster peer-to-peer support and balance online and in-person interactions for YA cancer survivors.
Generally, social isolation was related to worse psychological well-being while social connectedness was often but not always related to better psychological well-being. While there are psychometrically strong measures available to directly assess social isolation (e.g., Patient Reported Outcomes Measurement Information System [PROMIS] Social Isolation item bank,76 Lubben Social Network Scale77) and connectedness (e.g., Social Connectedness Scale78), almost no studies used these measures. Rather, studies used qualitative methods or assessed related constructs (e.g., loneliness, social support) that may not provide a complete picture. Future research is needed that directly assesses social isolation and connectedness using validated measures, in addition to the related constructs already being captured per Holt-Lunstad and colleagues’ framework, to yield a more comprehensive understanding of social well-being among YA cancer survivors.
Results of this review may be particularly relevant in the aftermath of the COVID-19 pandemic. During the height of the pandemic, social isolation and loneliness increased for society at large79–83 and for cancer survivors in particular.84 Emerging research suggests that the negative social implications of the pandemic may be long-lasting.85 In one study conducted during the early stages of the pandemic, some YAs found that increased social isolation enabled time for recovery, reflection, peace, and quiet.66 It is possible that the adjusted social expectations during this period may have reduced pressure to gather with friends and the cancer experience was less isolating when more people, regardless of health status, were isolated. However, this may no longer be the case following society’s establishment of post-pandemic norms. Clinical providers may benefit from assessing social isolation and connectedness as part of YA cancer care in the aftermath of the pandemic.
Limitations
We may have missed eligible articles for this review despite using a comprehensive search strategy. Many included articles did not report participants’ ages at diagnosis, and age ranges were often suggested by eligibility criteria. Some articles had age-related eligibility criteria that were broader than those targeted by this review (e.g., 15-39 at diagnosis). In these instances, we considered the average years since diagnosis to determine if most-to-all participants were likely within our target age range. For qualitative studies, we only extracted quotes from participants within the target age range. However, some data provided by participants outside our target age range may have been considered. In the future, authors should report both age at diagnosis and age at participation to better distinguish research focused on YA survivors of pediatric cancers from individuals diagnosed during young adulthood. We attempted to identify when multiple articles presented results from a single sample; however, we may have missed instances. Most had limited racial/ethnic diversity (excepting Munoz and colleagues46), minimal consideration of other indicators of marginalization (e.g., rurality, sexual orientation, gender identity), and small sample sizes, limiting the generalizability of results. Finally, the heterogeneity of methods made it challenging to compare results across studies or draw definitive conclusions, and many studies were cross-sectional.
Conclusions
Social isolation and social connectedness are gaining attention as important health determinants among YA cancer survivors. However, measures to directly assess these constructs are underused and the impact of interventions on these outcomes remains underexplored. Preliminary qualitative work has established the definition and scope of the problem and descriptive quantitative exploration supports the generation of evidence-based hypotheses. Research is now needed that assesses social isolation and social connectedness directly, reliably, and validly to support the development and evaluation of translational behavioral interventions.
Supplementary Material
Funding:
RSF was supported by the National Cancer Institute (#K08CA247973).
Conflicts of interest:
Damon R. Reed reports personal fees from SpringWorks and Eisai Inc. for service on Data and Safety Monitoring Boards outside the submitted work. Brian D. Gonzales reports personal fees from SureMed Compliance and Elly Health, Inc., outside the submitted work. Heather S. L. Jim reports grant funding from Kite Pharma and personal fees from RedHill BioPharma, Janssen Scientific Affairs, and Merck outside the submitted work.The remaining authors disclosed no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures 2020 Special Section: Cancer in Adolescents and Young Adults. 2020. [Google Scholar]
- 2.Kwak M, Zebrack BJ, Meeske KA, et al. Trajectories of Psychological Distress in Adolescent and Young Adult Patients With Cancer: A 1-Year Longitudinal Study. Journal of Clinical Oncology. Jun 10 2013;31(17):2160–2166. doi: 10.1200/Jco.2012.45.9222 [DOI] [PubMed] [Google Scholar]
- 3.Patterson P, McDonald FEJ, Zebrack B, Medlow S. Emerging Issues among Adolescent and Young Adult Cancer Survivors. Seminars in Oncology Nursing. Feb 2015;31(1):53–59. doi: 10.1016/j.soncn.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 4.Quinn GP, Gonçalves V, Sehovic I, Bowman ML, Reed DR. Quality of life in adolescent and young adult cancer patients: a systematic review of the literature. Patient related outcome measures. 2015;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. May 2000;55(5):469–80. [PubMed] [Google Scholar]
- 6.Itzep N, Roth M. Psychosocial Distress Due to Interference of Normal Developmental Milestones in AYAs with Cancer. Children. 2022;9(3):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt-Lunstad J, Robles TF, Sbarra DA. Advancing social connection as a public health priority in the United States. American Psychologist. 2017;72(6):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleisch Marcus A, Illescas AH, Hohl BC, Llanos AA. Relationships between social isolation, neighborhood poverty, and cancer mortality in a population-based study of US adults. PLoS One. 2017;12(3):e0173370. doi: 10.1371/journal.pone.0173370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavaleta D, Samuel K, Mills CT. Measures of Social Isolation. Social Indicators Research. 2017/03/01 2017;131(1):367–391. doi: 10.1007/s11205-016-1252-2 [DOI] [Google Scholar]
- 10.Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 2017/11/01/ 2017;152:157–171. doi: 10.1016/j.puhe.2017.07.035 [DOI] [PubMed] [Google Scholar]
- 11.Moore S, Leung B, Bates A, Ho C. Social isolation: Impact on treatment and survival in patients with advanced cancer. Journal of Clinical Oncology. 2018;36(34_suppl):156–156. doi: 10.1200/JCO.2018.36.34_suppl.156 [DOI] [Google Scholar]
- 12.Child ST, Lawton L. Loneliness and social isolation among young and late middle-age adults: Associations with personal networks and social participation. Aging & Mental Health. 2019/02/01 2019;23(2):196–204. doi: 10.1080/13607863.2017.1399345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beam CR, Kim AJ. Psychological sequelae of social isolation and loneliness might be a larger problem in young adults than older adults. Psychological Trauma: Theory, Research, Practice, and Policy. 2020;12(S1):S58–S60. doi: 10.1037/tra0000774 [DOI] [PubMed] [Google Scholar]
- 14.McGlone M, Long E. Are young adults with long-standing illness or disability at increased risk of loneliness? Evidence from the UK Longitudinal Household Study. J Public Health Res. Oct 14 2020;9(4):1861. doi: 10.4081/jphr.2020.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haslam C, Cruwys T, Haslam SA, Jetten J. Social connectedness and health. Encyclopaedia of geropsychology. 2015;2015:46–1. [Google Scholar]
- 16.Salazar SMDC, Dino MJS, Macindo JRB. Social connectedness and health-related quality of life among patients with cancer undergoing chemotherapy: A mixed method approach using structural equation modelling and photo-elicitation. Journal of clinical nursing. n/a(n/a)doi: 10.1111/jocn.16675 [DOI] [PubMed] [Google Scholar]
- 17.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast cancer research and treatment. 2019/10/01 2019;177(3):537–548. doi: 10.1007/s10549-019-05340-7 [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Dictionary of Cancer Terms. Accessed May 7, 2023. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivor
- 19.Warner EL, Kent EE, Trevino KM, Parsons HM, Zebrack BJ, Kirchhoff AC. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer. Apr 1 2016;122(7):1029–37. doi: 10.1002/cncr.29866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahl DA, Wieder MS, Steinberg DM. Social isolation and connection in adolescents with cancer and survivors of childhood cancer: A systematic review. Journal of Adolescence. 2021/02/01/ 2021;87:15–27. doi: 10.1016/i.adolescence.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong G, Fox R, Vigoreux T, et al. Social Isolation and Connection among young adult cancer survivors: A systematic review. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021282885 [DOI] [PMC free article] [PubMed]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews. 2016/12/05 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kmet LM, Lee RC, Cook LS. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. 2004. https://www.ihe.ca/advanced-search/standard-quality-assessment-criteria-for-evaluating-primary-research-papers-from-a-variety-of-fields
- 25.Lee L, Packer TL, Tang SH, Girdler S. Self-management education programs for age-related macular degeneration: A systematic review. Australasian journal on ageing. 2008;27(4):170–176. [DOI] [PubMed] [Google Scholar]
- 26.Maharaj S, Harding R. The needs, models of care, interventions and outcomes of palliative care in the Caribbean: a systematic review of the evidence. BMC Palliative Care. 2016/01/22 2016;15(1):9. doi: 10.1186/s12904-016-0079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revicki DA, Kleinman L, Cella D. A history of health-related quality of life outcomes in psychiatry. Dialogues Clin Neurosci. Jun 2014;16(2):127–35. doi: 10.31887/DCNS.2014.16.2/drevicki [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik H, Leung B, Laskin J, et al. Emotional distress and psychosocial needs in patients with breast cancer in British Columbia: younger versus older adults. Breast cancer research and treatment. Jan 2020;179(2):471–477. doi: 10.1007/s10549-019-05468-6 [DOI] [PubMed] [Google Scholar]
- 29.Avis NE, Levine B, Naughton MJ, Case DL, Naftalis E, Van Zee KJ. Explaining age-related differences in depression following breast cancer diagnosis and treatment. Breast cancer research and treatment. Nov 2012;136(2):581–91. doi: 10.1007/s10549-012-2277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geue K, Götze H, Friedrich M, et al. Perceived social support and associations with health-related quality of life in young versus older adult patients with haematological malignancies. Health and Quality of Life Outcomes. Aug 22 2019;17(1):145. doi: 10.1186/s12955-019-1202-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Ding S, He S, Duan Y, Yi K, Zhou J. A Prevalence Study of Psychosocial Distress in Adolescents and Young Adults With Cancer. Cancer nursing. May/Jun 2017;40(3):217–223. doi: 10.1097/ncc.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 32.Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. Oct 15 2012;118(20):5155–62. doi: 10.1002/cncr.27512 [DOI] [PubMed] [Google Scholar]
- 33.Roper K, Cooley ME, McDermott K, Fawcett J. Health-related quality of life after treatment of Hodgkin lymphoma in young adults. Oncol Nurs Forum. Jul 2013;40(4):349–60. doi: 10.1188/13.Onf.349-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbeil A, Laizner AM, Hunter P, Hutchison N. The experience of uncertainty in young adults with cancer. Cancer nursing. Sep-Oct 2009;32(5):E17–27. doi: 10.1097/NCC.0b013e3181a5690d [DOI] [PubMed] [Google Scholar]
- 35.Kumar AR, Schapira L. The impact of intrapersonal, interpersonal, and community factors on the identity formation of young adults with cancer: a qualitative study. Psycho-oncology. Aug 2013;22(8):1753–8. doi: 10.1002/pon.3207 [DOI] [PubMed] [Google Scholar]
- 36.Hamid W, Khan TA. Experiences of social support among Kashmiri women with breast cancer. Health, Risk & Society. 2021/02/17 2021;23(1-2):52–72. doi: 10.1080/13698575.2021.1901858 [DOI] [Google Scholar]
- 37.Paskett ED, Herndon JE 2nd, Day JM, et al. Applying a conceptual model for examining health-related quality of life in long-term breast cancer survivors: CALGB study 79804. Psycho-oncology. Nov 2008;17(11):1108–20. doi: 10.1002/pon.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunet J, Love C, Ramphal R, Sabiston CM. Stress and physical activity in young adults treated for cancer: the moderating role of social support. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. Mar 2014;22(3):689–95. doi: 10.1007/s00520-013-2023-0 [DOI] [PubMed] [Google Scholar]
- 39.Trevino KM, Fasciano K, Prigerson HG. Patient-oncologist alliance, psychosocial well-being, and treatment adherence among young adults with advanced cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. May 1 2013;31(13):1683–9. doi: 10.1200/jco.2012.46.7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Agostino NM, Edelstein K. Psychosocial challenges and resource needs of young adult cancer survivors: implications for program development. J Psychosoc Oncol. 2013;31(6):585–600. doi: 10.1080/07347332.2013.835018 [DOI] [PubMed] [Google Scholar]
- 41.Hauken MA, Larsen TMB. Young adult cancer patients’ experiences of private social network support during cancer treatment. Journal of clinical nursing. Aug 2019;28(15-16):2953–2965. doi: 10.11n/jocn.14899 [DOI] [PubMed] [Google Scholar]
- 42.Kenen R, Ardern-Jones A, Eeles R. “Social separation” among women under 40 years of age diagnosed with breast cancer and carrying a BRCA1 or BRCA2 mutation. J Genet Couns. Jun 2006;15(3):149–62. doi: 10.1007/s10897-005-9015-2 [DOI] [PubMed] [Google Scholar]
- 43.Love B, Thompson CM, Knapp J. The need to be Superman: the psychosocial support challenges of young men affected by cancer. Oncol Nurs Forum. Jan 1 2014;41(1):E21–7. doi: 10.1188/14.Onf.E21-e27 [DOI] [PubMed] [Google Scholar]
- 44.Snyder KA, Pearse W. Crisis, social support, and the family response: exploring the narratives of young breast cancer survivors. J Psychosoc Oncol. 2010;28(4):413–31. doi: 10.1080/07347332.2010.484830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Easley J, Miedema B, Robinson L. It’s the “good” cancer, so who cares? Perceived lack of support among young thyroid cancer survivors. Oncol Nurs Forum. Nov 2013;40(6):596–600. doi: 10.1188/13.Onf.596-600 [DOI] [PubMed] [Google Scholar]
- 46.Munoz AR, Kaiser K, Yanez B, et al. Cancer experiences and health-related quality of life among racial and ethnic minority survivors of young adult cancer: a mixed methods study. Support Care Cancer. Dec 2016;24(12):4861–4870. doi: 10.1007/s00520-016-3340-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miedema B, Hamilton R, Easley J. From “invincibility” to “normalcy”: coping strategies of young adults during the cancer journey. Palliat Support Care. Mar 2007;5(1):41–9. doi: 10.1017/s147895150707006x [DOI] [PubMed] [Google Scholar]
- 48.Miller LE. Young Breast Cancer Survivors’ Experiences of Uncertainty. Journal of Applied Communication Research. 2015/10/02 2015;43(4):429–449. doi: 10.1080/00909882.2015.1083600 [DOI] [Google Scholar]
- 49.Panjwani AA, Marín-Chollom AM, Pervil IZ, et al. Illness Uncertainties Tied to Developmental Tasks Among Young Adult Survivors of Hematologic Cancers. J Adolesc Young Adult Oncol. Apr 2019;8(2):149–156. doi: 10.1089/jayao.2018.0024 [DOI] [PubMed] [Google Scholar]
- 50.Halliday LE, Boughton MA, Kerridge I. Mothering and self-othering: the impact of uncertain reproductive capability in young women after hematological malignancy. Health Care Women Int. 2014;35(3):249–65. doi: 10.1080/07399332.2013.770005 [DOI] [PubMed] [Google Scholar]
- 51.Milosevic E, Brunet J, Campbell KL. Exploring tensions within young breast cancer survivors’ physical activity, nutrition and weight management beliefs and practices. Disabil Rehabil. Mar 2020;42(5):685–691. doi: 10.1080/09638288.2018.1506512 [DOI] [PubMed] [Google Scholar]
- 52.Knox MK, Hales S, Nissim R, et al. Lost and stranded: the experience of younger adults with advanced cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. Feb 2017;25(2):399–407. doi: 10.1007/s00520-016-3415-8 [DOI] [PubMed] [Google Scholar]
- 53.Kim B, Gillham DM. The experience of young adult cancer patients described through online narratives. Cancer nursing. Sep-Oct 2013;36(5):377–84. doi: 10.1097/NCC.0b013e318291b4e9 [DOI] [PubMed] [Google Scholar]
- 54.Lidington E, Vlooswijk C, Stallard K, et al. ‘This is not part of my life plan’: A qualitative study on the psychosocial experiences and practical challenges in young adults with cancer age 25 to 39 years at diagnosis. Eur J Cancer Care (Engl). Sep 2021;30(5):e13458. doi: 10.1111/ecc.13458 [DOI] [PubMed] [Google Scholar]
- 55.Mishra SI, Rishel Brakey H, Kano M, Nedjat-Haiem FR, Sussman AL. Health related quality of life during cancer treatment: Perspectives of young adult (23-39 years) cancer survivors and primary informal caregivers. Eur J Oncol Nurs. Feb 2018;32:48–54. doi: 10.1016/j.ejon.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 56.Musiello T, Platt V, Plaster M, Haddow L, Ives A. Dealing with Cancer: The Experiences of Adolescents and Young Adults Treated in Adult Hospitals in Western Australia. Journal of Adolescent and Young Adult Oncology. 2014;3(1):42–46. doi: 10.1089/jayao.2013.0025 [DOI] [Google Scholar]
- 57.Nizamli F, Anoosheh M, Mohammadi E. Experiences of Syrian women with breast cancer regarding chemotherapy: a qualitative study. Nurs Health Sci. Dec 2011;13(4):481–7. doi: 10.nn/j.1442-2018.2011.00644.x [DOI] [PubMed] [Google Scholar]
- 58.Victorson D, Garcia SF, Sanford S, Snyder MA, Lampert S, Salsman JM. A Qualitative Focus Group Study to Illuminate the Lived Emotional and Social Impacts of Cancer and Its Treatment on Young Adults. J Adolesc Young Adult Oncol. Dec 2019;8(6):649–659. doi: 10.1089/jayao.2019.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauken MA, Larsen TM, Holsen I. Meeting reality: young adult cancer survivors’ experiences of reentering everyday life after cancer treatment. Cancer nursing. Sep-Oct 2013;36(5):E17–26. doi: 10.1097/NCC.0b013e318278d4fc [DOI] [PubMed] [Google Scholar]
- 60.Mikkelsen TH, Søndergaard J, Jensen AB, Olesen F. Cancer rehabilitation: psychosocial rehabilitation needs after discharge from hospital? Scand J Prim Health Care. 2008;26(4):216–21. doi: 10.1080/02813430802295610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruddy KJ, Greaney ML, Sprunck-Harrild K, Meyer ME, Emmons KM, Partridge AH. Young Women with Breast Cancer: A Focus Group Study of Unmet Needs. J Adolesc Young Adult Oncol. Dec 1 2013;2(4):153–160. doi: 10.1089/jayao.2013.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaal SE, Husson O, van Dartel F, et al. Online support community for adolescents and young adults (AYAs) with cancer: user statistics, evaluation, and content analysis. Patient Prefer Adherence. 2018;12:2615–2622. doi: 10.2147/ppa.S171892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trevino KM, Fasciano K, Block S, Prigerson HG. Correlates of social support in young adults with advanced cancer. Supportive Care in Cancer. 2013/02/01 2013;21(2):421–429. doi: 10.1007/s00520-012-1536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zebrack B, Kwak M, Sundstrom L. First Descents, an adventure program for young adults with cancer: who benefits? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. Dec 2017;25(12):3665–3673. doi: 10.1007/s00520-017-3792-7 [DOI] [PubMed] [Google Scholar]
- 65.Zebrack B, Chesler MA, Kaplan S. To foster healing among adolescents and young adults with cancer: What helps? What hurts? Supportive Care in Cancer. 2010/01/01 2010;18(1):131–135. doi: 10.1007/s00520-009-0719-y [DOI] [PubMed] [Google Scholar]
- 66.Hanghøj S, Pappot N, Hjerming M, Taarnhøj GA, Boisen KA, Pappot H. Experiences of Social Isolation During the COVID-19 Lockdown Among Adolescents and Young Adult Cancer Patients and Survivors. J Adolesc Young Adult Oncol. Apr 2021;10(2):142–147. doi: 10.1089/jayao.2020.0202 [DOI] [PubMed] [Google Scholar]
- 67.Siegel K, Gluhoski V, Gorey E. Age-Related Distress Among Young Women with Breast Cancer. Journal of Psychosocial Oncology. 1999/07/29 1999;17(1):1–20. doi: 10.1300/J077v17n01_01 [DOI] [Google Scholar]
- 68.Darabos K, Berger AJ, Ford JS. “Empathy without sympathy”: An analysis of support-related preferences among young adult cancer survivors. J Psychosoc Oncol. 2022;40(4):457–472. doi: 10.1080/07347332.2021.1914271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okamura M, Fujimori M, Sato A, Uchitomi Y. Unmet supportive care needs and associated factors among young adult cancer patients in Japan. BMC Cancer. 2021/01/05 2021;21(1):17. doi: 10.1186/s12885-020-07721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geue K, Göbel P, Leuteritz K, et al. Anxiety and depression in young adult German cancer patients: Time course and associated factors. Psycho-oncology. 2019;28(10):2083–2090. doi: 10.1002/pon.5197 [DOI] [PubMed] [Google Scholar]
- 71.Hoyt MA, Cano SJ, Saigal CS, Stanton AL. Health-related quality of life in young men with testicular cancer: validation of the Cancer Assessment for Young Adults (CAYA). Journal of cancer survivorship : research and practice. Dec 2013;7(4):630–40. doi: 10.1007/s11764-013-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kent EE, Smith AW, Keegan TH, et al. Talking About Cancer and Meeting Peer Survivors: Social Information Needs of Adolescents and Young Adults Diagnosed with Cancer. J Adolesc Young Adult Oncol. Jun 2013;2(2):44–52. doi: 10.1089/jayao.2012.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazard AJ, Collins MKR, Hedrick A, et al. Initiation and changes in use of social media for peer support among young adult cancer patients and survivors. Psycho-oncology. 2021;30(11):1859–1865. doi: 10.1002/pon.5758 [DOI] [PubMed] [Google Scholar]
- 74.Primack BA, Shensa A, Sidani JE, et al. Social Media Use and Perceived Social Isolation Among Young Adults in the U.S. Am J Prev Med. Jul 2017;53(1):1–8. doi: 10.1016/j.amepre.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonsaksen T, Ruffolo M, Leung J, et al. Loneliness and its association with social media use during the COVID-19 outbreak. Social Media+ Society. 2021;7(3):20563051211033821. [Google Scholar]
- 76.Hahn EA, DeWalt DA, Bode RK, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. May 2014;33(5):490–9. doi: 10.1037/hea0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. Aug 2006;46(4):503–13. doi: 10.1093/geront/46.4.503 [DOI] [PubMed] [Google Scholar]
- 78.Lee RM, Robbins SB. Measuring belongingness: The social connectedness and the social assurance scales. Journal of counseling psychology. 1995;42(2):232. [Google Scholar]
- 79.Sayin Kasar K, Karaman E. Life in lockdown: Social isolation, loneliness and quality of life in the elderly during the COVID-19 pandemic: A scoping review. Geriatric Nursing. 2021/09/01/ 2021;42(5):1222–1229. doi: 10.1016/j.gerinurse.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorenko JA, Moran C, Flynn M, Dobson K, Konnert C. Social Isolation and Psychological Distress Among Older Adults Related to COVID-19: A Narrative Review of Remotely-Delivered Interventions and Recommendations. Journal of Applied Gerontology. 2021;40(1):3–13. doi: 10.1177/0733464820958550 [DOI] [PubMed] [Google Scholar]
- 81.Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The Impact of Social Isolation and Loneliness on the Mental Health of Children and Adolescents in the Context of COVID-19. Journal of the American Academy of Child & Adolescent Psychiatry. 2020/11/01/ 2020;59(11):1218–1239.e3. doi: 10.1016/i.iaac.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arora T, Grey I, Östlundh L, Lam KBH, Omar OM, Arnone D. The prevalence of psychological consequences of COVID-19: A systematic review and meta-analysis of observational studies. Journal of Health Psychology. 2022;27(4):805–824. doi: 10.1177/1359105320966639 [DOI] [PubMed] [Google Scholar]
- 83.Ernst M, Niederer D, Werner AM, et al. Loneliness before and during the COVID-19 pandemic: A systematic review with meta-analysis. Am Psychol. Jul-Aug 2022;77(5):660–677. doi: 10.1037/amp0001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miaskowski C, Paul SM, Snowberg K, et al. Loneliness and symptom burden in oncology patients during the COVID-19 pandemic. Cancer. 2021;127(17):3246–3253. doi: 10.1002/cncr.33603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patulny R, Bower M. Beware the “loneliness gap”? Examining emerging inequalities and long-term risks of loneliness and isolation emerging from COVID-19. Australian Journal of Social Issues. 2022;57(3):562–583. doi: 10.1002/ais4.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


