Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disease in women of childbearing age and can cause metabolic disorder, infertility, and increased anxiety and depression; as a result, it can seriously affect the physical and mental health of fertile women. PCOS is a highly clinically heterogeneous disease with unclear etiology and pathogenesis, which increases the difficulty of treatment. The thyroid gland has complex regulatory effects on metabolism, reproduction, and emotion, and produces hormones that act on almost all cells of the human body. The clinical manifestations of PCOS are similar to some thyroid diseases. Furthermore, some thyroid diseases, such as subclinical hypothyroidism (SCH), not only increase the incidence rate of PCOS, but also exacerbate its associated metabolic abnormalities and reproductive disorders. Interestingly, PCOS also increases the incidence of some thyroid diseases. However, the role of the thyroid in PCOS remains unclear. This review is intended to thoroughly explore the critical role of the thyroid in PCOS by summarizing the comorbidity of PCOS and thyroid diseases and their combined role in metabolic disorders, related metabolic diseases, and reproductive disorders; and by analyzing the potential mechanism through which the thyroid influences the development and progression of PCOS and its symptoms. We hope this review will provide a valuable reference for the role of the thyroid in PCOS.
Keywords: pcos, thyroid, metabolic abnormalities, reproductive disorders, potential mechanism
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic disease in women of childbearing age, affecting 3–15% of women worldwide (1, 2). PCOS causes reproductive disorders, metabolic disorders, and psychological problems, all of which can seriously affect the physical and mental health of these women (3).
PCOS is a highly clinically heterogeneous disease; there are four specific phenotypes that vary greatly depending on life stage, genotype, race, and environmental factors. The four PCOS phenotypes are classified according to three factors: polycystic ovary morphology, ovulation dysfunction, and hyperandrogenemia (4). The etiology and pathogenesis of PCOS remain unclear, primarily due to the heterogeneity of these phenotypes, which increases the difficulty of treatment of this complex endocrine disease.
The clinical manifestations of PCOS are similar to some thyroid diseases. Increasing evidence shows that PCOS is related to the increased incidence rate of thyroid diseases, such as autoimmune thyroiditis (AIT), and subclinical hypothyroidism (SCH) (5–7). Palomba et al, have made a comprehensive narrative review on PCOS and thyroid disorder recently (8). This review provides us with a comprehensive understanding of the current research between thyroid and PCOS. However, we want to detail and discuss how alteration in thyroid function may influence PCOS to find the role of the thyroid in PCOS. This review intends to explore the important role of the thyroid in PCOS by analyzing the co-occurrence of PCOS and thyroid diseases; summarizing the incidences of metabolic disorders, related metabolic diseases, and reproductive disorders; exploring the possible mechanisms of interaction between PCOS and thyroid diseases; and ultimately providing a valuable reference for the role of the thyroid in PCOS.
2. Co-occurrence of PCOS and thyroid disease
The incidence rate of thyroid diseases is increased in patients with PCOS (9). In 2019, a study from Denmark reported that the risk of thyroid disease in PCOS patients is 2.5 times higher than that in patients without PCOS (10); therefore, we have thoroughly analyzed the comorbidity of several thyroid diseases with PCOS.
2.1. Hyperthyroidism
Graves’ disease (GD) is the primary clinical cause of hyperthyroidism with global incidences reported between 1.5 and 6.5 per 100,000 (11). It tends to increase in frequency with age. It is predominantly considered to be an organ-specific autoimmune disease (11), characterized by infiltration of the thyroid by T and B lymphocytes that react against thyroid antigens and the production of thyroid autoantibodies. The main thyroid autoantibodies are directed against thyroid-stimulating hormone receptors, which can activate the TSH receptor, leading to excessive production of thyroid hormones. Therefore, patients with this disease typically present with hyperthyroidism and diffuse enlargement of the thyroid gland (11).
There are few reports that indicate an association between PCOS and GD. Jung et al. was the first to describe a female patient with PCOS and GD in 2011; she presented with decreased menstruation, low body mass index (BMI: 16.4 kg/m2), mild hirsutism, and thyrotoxicosis ( Table 1 ) (12). In 2012, six female patients with PCOS and GD were identified in a tertiary care center in northern India (13). They presented with goiter based on clinical and ultrasound examination; furthermore, all women were thin, with an average BMI of 22.73 kg/m2, and three of the six women had a waist circumference of < 80 cm. Additionally, UMA Sinha et al. reported two patients from India with both PCOS and GD, who showed elevated anti-thyroid peroxidase (TPO) antibody levels (14). The prevalence of PCOS combined with GD is unclear, because current information is limited to case reports, and extensive epidemiological data is currently lacking. Moreover, the incidence of PCOS combined with GD may differ with race or ethnicity, since the patients referenced in these case reports were all Asian women. The probability of having PCOS combined with dominant or subclinical hyperthyroidism in young female western populations is very low, which may be related to the lower prevalence of hyperthyroidism in the general western population (26).
Table 1.
Comorbidity between PCOS and thyroid disease.
| Participants | Country | Comorbidity between PCOS and thyroid disease | Ref | |
|---|---|---|---|---|
| Study group | Control group | |||
| 18476 women with PCOS | 54757 age-matched control | Danish | The event rate of thyroid disease was 6.0 per 1000 patient-years in PCOS Denmark versus 2.4 per 1000 patient-years in controls. | (10) |
| A 27-year-old female patient | South Korea | She was diagnosed as having PCOS and hyperthyroid Graves’ disease. | (12) | |
| Six women | India | Six women presented with PCOS and Graves’ disease together. | (13) | |
| 80 PCOS patients | 80 age-matched female subjects | India | Statistically significant higher prevalence of autoimmune thyroiditis (22.5% vs. 1.25% of control); high prevalence of goiter among PCOS patients (27.5% vs. 7.5% of control, P value > 0.001); higher percentage of PCOS patients (12.5%; controls 2.5%) had hypoechoic USG pattern. | (14) |
| 5399 patients with GD | 10,798 patients without GD | Taiwan, China | The adjusted hazard ratio for PCOS in patients with GD compared with patients without GD was 1.47 (95%CI = 1.09-1.98). | (15) |
| 168 women diagnosed with PCOS | Brazil | A diagnosis of SCH was established in 11.3% of the women with PCOS. | (16) | |
| 200 females with PCOS | 200 females without PCOS | Pakistan | SCH was found to be more prevalent in participant with PCOS compared to participants without PCOS (43.5% vs. 20.5%; p-value: <0.00001). | (17) |
| 465 women with PCOS | Bangladesh | 10.8% of them had SCH and 18.3% were positive for anti-TPO. | (18) | |
| 4821 participants | 71.31% chinese patients out of the total | 27.0% of them had SCH. | (19) | |
| 175 patients with PCOS | 168 age-matched women without PCOS | Germany | Thyroid function and thyroid-specific antibody tests revealed elevated thyroperoxidase (TPO) or thyroglobulin (TG) antibodies in 14 of 168 controls (8.3%), and in 47 of 175 patients with PCOS (26.9%; P<0.001). | (20) |
| 86 reproductive-age women diagnosed with PCOS | 60 age-BMI matched control women | USA | HT was more common in PCOS patients compared to controls (22.1 and 5%; p = 0.004). | (21) |
| 175 girls with euthyroid CLT (chronic lymphocytic thyroiditis) | 46 age-matched non-CLT girls | India | Significantly higher prevalence of PCOS was noted in girls with euthyroid CLT when compared to their control counterparts (46.8 vs 4.3%, P=0.001). | (22) |
| 97 women with PCOS | 71 healthy female volunteers | Turkey | Twenty-nine patients with PCOS (29/97; 29.9%) had thyroid nodules, whereas only eleven control subjects had thyroid nodules (11/71; 15.5%) (p=.043). | (23) |
| 178 patients with PCOS | 92 age-BMI matched patients with no disease | Turkey | The number of nodules of 1 cm and above was found to be higher only in patients with PCOS compared with the control group. | (24) |
| 6731 patients with AITD | 26,924 controls | Taiwan, China | PCOS risk in patients with AITD was higher than that in the control group (adjusted hazard ratio = 1.39; 95% confidence interval = 1.07-1.71). | (25) |
Recently, Chen et al. compared 5,399 patients with GD and 10,798 patients without GD in the Asian population ( Table 1 ) (15). They found that the cumulative incidence rate of PCOS in patients with GD was significantly higher than that in patients without GD, with an adjusted risk coefficient for PCOS of 1.47 for GD patients compared with those without GD, indicating that women with GD may have an increased risk of developing PCOS. They also analyzed the comorbidities between GD and PCOS, including diabetes, obesity, hypertension, and heart failure. Only the incidence rate of hyperlipidemia was significantly increased in GD patients with PCOS, for the adjusted odds ratio of hyperlipidemia was 1.47 in patients with GD and was 2.18 in patients with GD and PCOS (15).
2.2. Subclinical hypothyroidism
The prevalence of SCH is higher in PCOS patients than that in the general population (16). Raj et al. conducted a study of Pakistani women 18–30 years old to determine the incidence of SCH in PCOS patients ( Table 1 ) (17). By comparing 200 PCOS patients with 200 control patients without PCOS, they determined that SCH was more prevalent in PCOS patients (43.5%) than in participants without PCOS (20.5%). They also found that weight gain and BMI of the PCOS patients were significantly higher than those without PCOS (17).
Since hypothyroidism commonly occurs in PCOS patients, this correlation strongly suggests an increased risk of thyroid disease with PCOS; therefore, it is important to explain its clinical impact. SCH may cause mild metabolic abnormalities. For example, a clinical study of 4,065 PCOS patients 12–40 years of age revealed that more patients with SCH were shown to have obesity, central obesity, and goiter compared with the normal thyroid group ( Table 1 ) (18). Furthermore, women with SCH are more likely to have abnormal fasting plasma glucose (FPG) levels and insulin resistance index (HOMA-IR) than women without SCH. Additionally, a study of 4,821 participants, comprised of 1,300 PCOS patients with SCH and 3,521 PCOS patients without SCH, found that the HOMA-IR, triglyceride, serum total cholesterol (TC), low density lipoprotein (LDL), fasting blood glucose (FBG), fasting C-peptide (FCP), and prolactin levels were higher, while high-density lipoprotein cholesterol (HDL), luteinizing hormone (LH), and testosterone levels were lower in the SCH patients ( Table 1 ) (19). Collectively, these results indicate that the incidence of metabolic syndrome is higher in the SCH group, which indicates that SCH may aggravate lipid- and glucose-related metabolic disorders in PCOS patients.
2.3. Thyroiditis
Thyroiditis is a heterogeneous disease of the thyroid gland with various etiologies. Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is a type of AIT and is a common form of thyroiditis in young women (27). HT may occur concurrently with clinical hypothyroidism (the most common), normal thyroid function, or hyperthyroidism.
Janssen et al. were the first to confirm that the prevalence of HT in PCOS patients was higher than that in non-PCOS patients through a systematic prospective study with 175 PCOS patients and 168 healthy controls ( Table 1 ) (20). In 26.9% of PCOS patients and 8.3% of the control group, HT-specific anti-TPO or anti-thyroglobulin (TG) antibody levels were found to be elevated, revealing a threefold increase in the prevalence of HT in PCOS patients relative to controls. Furthermore, thyroid ultrasound examination results revealed that 42.3% of PCOS patients, but only 6.5% of the control group, exhibited a typical HT hypoechoic thyroid ultrasound pattern indicative of mild thyroid damage. Additionally, Arduc et al. conducted a study of women of childbearing age and compared 86 women with PCOS with 60 BMI-matched control women ( Table 1 ) (21). Their results showed that the prevalence of HT in PCOS patients (22.1%) was higher than the control group (5%). Moreover, TSH was elevated in PCOS patients (26.7%) relative to controls (5%), indicating the presence of hypothyroidism in these PCOS patients with HT.
Since HT prevalence is known to be higher in women with PCOS, it is important to understand if the reverse is also true, namely, if the prevalence of PCOS is higher in women with HT than in women without HT. Ganie et al. conducted a prospective case-control study in India on adolescent females 13–18 years old comparing 1,075 HT patients with normal thyroid function and 46 age-matched patients without HT based on negative anti-TPO antibody tests ( Table 1 ) (22). Their results showed that the prevalence of PCOS was significantly higher in HT patients (46.8%) than in non-HT patients (4.3%). Moreover, the BMI, waist circumference, and systolic blood pressure were all higher in HT patients than in controls.
2.4. Thyroid nodule
Thyroid nodules are discrete lesions within the thyroid gland, which can be detected by ultrasonography (28). The incidence rate of thyroid nodules in women is four times that in men, and its incidence rate increases with age and body mass index (29). They are typically benign. But about 5% of these lesions may ultimately be malignant (29). Therefore, the primary goal of thyroid nodule evaluation is to determine whether it is malignant. Generally, only nodules>1 cm should be evaluated as they are more likely to become clinically significant cancers. In rare cases, some nodules<1 cm may lead to future incidence rate and mortality (28). Each thyroid nodule has an independent risk of malignancy, and patients with multiple nodules may need biopsy on multiple nodules. Nodules with highly suspicious ultrasound features should be given priority for biopsy (29, 30).
Karaköse et al. analyzed 97 patients with PCOS and 71 healthy female volunteers as controls; they found that 29 PCOS patients (29.9%) had thyroid nodules, while only 11 control subjects (15.5%) had thyroid nodules ( Table 1 ) (23). Furthermore, participants with thyroid nodules were older with higher fasting blood glucose, BMI, fasting insulin, and HOMA-IR values than participants without thyroid nodules, indicating an increased incidence of nodular goiter in PCOS patients. A retrospective study was conducted on 178 PCOS patients aged 18–45 years and 92 BMI-matched healthy control subjects ( Table 1 ) (24). The PCOS group was higher than the control group in both the presence and number of nodules, including the number of nodules ≥ 1 cm. Further analysis of the PCOS patients showed that, relative to controls, phenotype A PCOS patients had the most prominent characteristics of thyroid dysfunction, such as increased thyroid autoimmunity, thyroid volume, and number of nodules > 1 cm, suggesting that thyroid dysfunction is more common in phenotype A of PCOS.
The incidence rate of thyroid disease is higher in PCOS patients than those without PCOS; conversely, the incidence rate of PCOS is higher in women with thyroid disease than in women with a normal thyroid. These analyses collectively indicate several clear characteristics of patients who have a combination of PCOS with thyroid disease: 1) Patients with combined diseases have more serious clinical manifestations than either disease individually; 2) Thyroid diseases associated with PCOS are primarily clinical hypothyroidism, including SCH, and autoimmune thyroid disease (AITD); and 3) Hyperthyroidism is rare with PCOS. Ho et al. found that the risk of PCOS in HT patients was 1.63 times higher than in those with normal thyroid function, while that in GD patients was 1.24 times higher ( Table 1 ) (25), suggesting that hypothyroidism is more likely than hyperthyroidism to cause PCOS. In conclusion, there is a potential interplay between PCOS and thyroid disease—two common endocrine diseases in females—such that thyroid function affects the clinical and biochemical parameters of PCOS and, in turn, PCOS also affects thyroid function.
3. Thyroid disease exacrerbates metabolic disorders in PCOS patients
Women with PCOS are more likely to suffer from central obesity, T2DM, and dyslipidemia-associated metabolic syndrome than women without PCOS (31, 32).
3.1. Relationship between thyroid function and metabolic disorder in PCOS
The severity of metabolic abnormalities in PCOS patients is related to the degree of thyroid dysfunction. If thyroid hormone levels are too high, hyperthyroidism will appear, accompanied by symptoms such as overeating, hunger, and wasting. Conversely, if thyroid hormone levels are too low, hypothyroidism will occur (33).
Recent studies have reported that the incidence of hypothyroidism is higher in patients diagnosed with PCOS (11–14%) compared with control subjects (1–2%) (34, 35). Metabolic changes observed in both hypothyroidism and PCOS include insulin resistance, dyslipidemia, increased weight, and obesity (36). Compared with PCOS patients with normal thyroid function, women with PCOS and SCH combined have higher triglyceride levels, fasting insulin levels, and HOMA-IR ( Table 2 ) (37). Furthermore, hypothyroidism often occurs in HT patients. Patients with combined PCOS and HT showed more severe metabolic symptoms than patients with PCOS or HT alone (5, 41). Females with combined HT and PCOS had higher BMI, fasting blood glucose, HOMA-IR, and cholesterol compared with the control group or HT group alone (21, 42). These findings suggest that the co-occurrence of PCOS and hypothyroidism is related to more significant metabolic and hormonal changes. The metabolic disorder in PCOS patients with HT-related subclinical and clinical hypothyroidism significantly improved with thyroid supplementation (43, 44), relative to those with normal thyroid function, which provides further evidence that the metabolic abnormalities seen in PCOS patients are related to thyroid dysfunction, which are improved after thyroid function returns to normal.
Table 2.
Metabolic profile in PCOS with thyroid disease.
| Participants | Metabolic profile | Ref | |
|---|---|---|---|
| Study group | Control group | ||
| 114 PCOS patients with SCH | 253 PCOS patients without SCH | Exacerbated the metabolic disorders (insulin resistance and dyslipidemia) in PCOS patients with SCH | (35) |
| 20 patients with PCOS and SCH | 39 patients with PCOS and normal thyroid function and 53 healthy women with normal thyroid function | Dyslipidemia with higher triglyceride levels and insulin resistance in group with PCOS and subclinical hypothyroidism | (37) |
| 148 women with PCOS, without Type 2 diabetes mellitus (T2DM) and CVD present at baseline | At baseline, prevalent prediabetes was present in 18 (12%) of PCOS cases and it progressed to T2DM in 5 (3%) of the cases. Incident prediabetes during the follow-up was noted in 47 (32%) women or 4.7 per 1000 persons/year. cardiovascular risk in PCOS women with prediabetes was high. | (38) | |
| 100 females with PCOS | 100 normal controls | Significantly higher HOMA-IR and frequency of subjects with dyslipidemia in SCH PCOS subjects | (39) |
| 583 women with PCOS | Patients with elevated TSH levels had significantly increased fasting insulin and total cholesterol (TC)/high-density lipoprotein cholesterol (HDL) ratio. | (40) | |
In conclusion, thyroid function status directly affects metabolic disorders in PCOS patients. Generally, hypothyroidism will increase the metabolic burden of the body, leading to obesity and insulin resistance. Therefore, the thyroid function in PCOS patients must not be ignored, and efforts should be made to strengthen the detection of thyroid function.
3.2. Increased risk of T2DM complications with thyroid dysfunction in PCOS
The association between PCOS and T2DM has been fully confirmed. For example, a study monitored 148 PCOS patients for three years; initially, none of the patients had diabetes and 18 (12%) exhibited pre-diabetes symptoms ( Table 2 ) (38). Over the course of the study, they found that 5 (3%) patients developed T2DM and 47 (32%) women developed pre-diabetes symptoms. This association is important to monitor, because the BMI of patients with pre-diabetes tends to increase, and the deterioration of glucose tolerance in PCOS patients may be accelerated.
Increasing studies have shown that hypothyroidism is associated with T2DM. Brenta et al. found that the prevalence of hypothyroidism in patients with T2DM was higher than in non-diabetic patients, and that diabetes complications in SCH patients were more common than those without SCH (45), which indicates hypothyroidism may be associated with T2DM. For every doubling of TSH, the incidence rate of T2DM increased 1.09 times. A large study of Danish PCOS patients (n = 19,199) showed that the prevalence of diabetes in Danish PCOS patients was higher than that in the control group (46). Moreover, compared with the control group, the incidence rate of thyroid disease was higher in the PCOS group, which indicates that thyroid disease may affect the incidence rate of diabetes complications in PCOS patients.
3.3. Dyslipidemia and risk of cardiovascular disease
Approximately 70% of PCOS patients exhibit a lipid metabolism disorder, including quantitative and qualitative changes in lipid mass spectra and lipoprotein parameters (16, 47). Compared with a PCOS group with normal thyroid function, the blood lipid profile of a PCOS group with SCH was significantly altered with a higher incidence of dyslipidemia (35, 39). SCH has been shown to adversely affect the lipid parameters of PCOS. In PCOS patients with SCH, lipid profiles are readily altered, resulting in increased LDL cholesterol (LDL-C) and triglyceride, and decreased HDL cholesterol (HDL-C) levels ( Table 2 ) (40). Moreover, even in patients with PCOS and SCH with normal TSH levels, studies have still shown increased serum TC, LDL-C, non–HDL-C, and triglyceride levels with an associated decrease in HDL-C levels (48, 49), which indicates that SCH may increase the risk of cardiovascular disease in PCOS patients.
Women with PCOS are prone to dyslipidemia. The most common dyslipidemia features are hypertriglyceridemia, decreased HDL-cholesterol concentrations and the presence of small, dense LDL particles, which is characteristic of the atherogenic lipoprotein phenotype (50). Therefore, it is speculated that PCOS patients have a higher tendency to develop atherosclerosis.
In conclusion, patients with a combination of PCOS and thyroid disease have more severe metabolic abnormalities and may greatly increase risk of T2DM and cardiovascular disease than those with PCOS alone. The progression of metabolic disorders in PCOS can be mitigated to a certain extent by improving thyroid function.
4. Reproductive health disorders
Fertility disorders are one of the main characteristics of PCOS. The conception time of PCOS patients is significantly longer than that of the general population, and 40–70% of them are diagnosed with infertility, which is defined as the lack of conception after one year of routine unprotected sexual intercourse (51). Thyroid dysfunction also affects female reproduction; therefore, fertility problems may be more frequent and severe in patients with both PCOS and thyroid diseases, than in patients with thyroid diseases or PCOS alone (52).
4.1. Ovulation disorder, menstrual disorder, and fertility decline
Lack of ovulation is the most common type of PCOS-induced female infertility, and ovulation disorders are diagnosed in 75–85% of PCOS patients; only approximately 30% experience periodic ovulation (7, 53). Anovulatory infertility in women with PCOS is often associated with irregular menstruation (54). In adult women, both hypothyroidism and hyperthyroidism can cause menstrual disorder and fertility decline (7). Krassas et al. found that the levels of sex hormone binding globulin (SHBG), estradiol (E2), testosterone, androstenedione, LH, and follicle stimulating hormone (FSH) were higher in hyperthyroid patients than in those with normal thyroid function (55). Furthermore, the menstrual cycle of women with hyperthyroidism was irregular with abnormal ovulation relative to healthy women in the control group (56). Patients with hypothyroidism tend to have lower levels of SHBG, E2, testosterone, and androstenedione, and higher levels of prolactin than those with normal thyroid function (55, 56). In the pre-puberty stage, hypothyroidism may lead to delayed puberty (57); it may also lead to irregular menstruation, breakthrough bleeding, low endometrial thickness, ovulation dysfunction, and non-hyperplasia of endometrium due to anovulation (58).
All receptors in the thyroid hormone function signaling pathway, including thyrotropin releasing hormone receptor (TRHR), thyrotropin receptor (TSHR), and thyroid hormone receptors (TRs), have been detected in the monkey uterus and have been shown to be affected by steroid hormones (59), suggesting that the thyroid plays a regulatory role in female fertility. Some studies have shown that hypothyroidism may be related to the formation of ovarian cysts (60, 61).
4.2. Fertility risk
Compared with pregnant women with normal thyroid function, the spontaneous abortion rate of untreated SCH patients was higher than pregnant women with normal thyroid function (relative risk ratio (RR) = 1.90) (62). There was no significant difference between treated SCH patients and women with normal thyroid function (RR = 1.14), which indicates that hypothyroidism can increase the risk of spontaneous abortion.
Autoimmunity increases non-fertility risk. Antithyroid antibody is the most common autoimmune factor in infertile couples who have failed in vitro fertilization at least twice (63). The pooled results of multiple studies indicate that infertile women are more likely to develop AITD than the controls (64). Some studies have shown that AITD may lead to a 3–5-fold increase in the total abortion rate (65). Among the 438 women who received assisted reproductive technology, conception rates were similar between women with and without AITD, while women with AITD had significantly higher spontaneous abortion rates than women without AITD (53% and 23%, respectively) (66). Furthermore, SCH patients with thyroid autoimmunity have a significantly higher risk of miscarriage than other SCH patients (67, 68).
4.3. Maternal and infant health during pregnancy
PCOS and thyroid insufficiency also increase maternal and infant health risks during pregnancy, leading to pregnancy-related diseases. Compared with healthy controls, PCOS patients had an increased risk of maternal complications during pregnancy, including gestational diabetes, pregnancy induced hypertension syndrome (PIH), preeclampsia, premature birth, and increased need for a cesarean section during delivery (69, 70).
The risk of premature birth is closely related to PIH and obesity. Obesity also increases the mean time to conception (71). Elevated anti-TPO antibodies and the presence of both subclinical and dominant hypothyroidism can lead to infertility and adverse pregnancy outcomes, such as spontaneous abortion, preterm birth, small for gestational age (SGA), preeclampsia, stillbirth, cesarean section, and impaired fetal neurointellectual development (72). Therefore, women with combined PCOS and thyroid disease have a higher risk of infertility and pregnancy complications.
In conclusion, thyroid diseases, especially hypothyroidism and AITDs, exacerbate PCOS-related reproductive problems.
5. Analysis of potential thyroid-related etiologies of PCOS
Iodinated TG synthesized in follicular epithelial cells of the thyroid gland is decomposed by hydrolase to primarily form tetraiodothyronine, also known as thyroxine (T4), and a small amount of triiodothyronine (T3) (73–75). Maintaining homeostasis of circulating THs is the main function of the hypothalamic-pituitary-thyroid (HPT) axis (76); it is controlled by a highly specific system, in which iodothyronine deiodinases (DIOs) play a key role in tissue-specific hormone regulation (77, 78). Since the thyroid plays a key role in human metabolism, we specifically analyzed the possible role of the thyroid in PCOS development.
5.1. Elevated insulin resistance in the development of PCOS
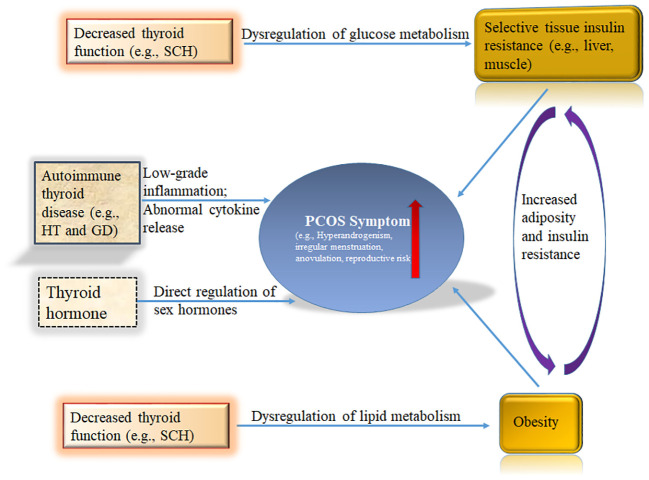
Insulin resistance and hyperinsulinemia play a key role in the development of PCOS, which in turn increases the risk of developing T2DM, irregular menstruation, and reproductive difficulties in PCOS patients ( Figure 1 ) (79, 80). Regardless of BMI, the proportion of insulin resistance in PCOS patients is highly elevated compared with the general population. PCOS-related insulin resistance is characterized by decreased sensitivity and reactivity to insulin-mediated glucose utilization, primarily in skeletal muscle and adipose tissue (81). Significant internal factors are involved in the development of PCOS-related insulin resistance, although in some cases, it may only be acquired due to exogenous obesity. The root cause of PCOS is related to defects in intracellular insulin signaling downstream of the insulin receptor, which includes the hyperphosphorylation of serine residues on the insulin receptor substrate 1 (IRS-1) protein, which then binds to the insulin receptor and initiates insulin-specific intracellular responses (82–84). Insulin resistance has been observed not only in peripheral tissues of PCOS patients, such as adipocytes and skeletal muscle, but also in fibroblasts and ovarian granulosa and theca cells.
Figure 1.
Schematic summary of the role of the thyroid in polycystic ovary syndrome (PCOS). Hyperinsulinism and obesity are necessary for full-blown pathogenesis of PCOS. Thyroid hormones participate in the regulation of blood glucose at the central and peripheral levels. Hypothyroidism has been shown to cause insulin resistance. THs regulate lipid metabolism. Decreased thyroid function readily leads to lipid metabolism disorders, including obesity. The immune state of the body also plays a key role in PCOS. HT and GD are common AITDs that have been repeatedly shown to increase PCOS risk in women. Sex hormones are regulated by Thyroid signal pathway. THs, Thyroid hormones; HT, Hashimoto’s thyroiditis; GD, Graves’ disease; AITD, autoimmune thyroid disease; SCH, subclinical hypothyroidism.
A study of PCOS patients compared BMI-matched PCOS patients with SCH (SCH-PCOS group) and PCOS patients with normal thyroid function, which revealed that the HOMA-IR of the SCH-PCOS group was significantly higher than that of the normal PCOS group (19). Additionally, the HOMA-IR in PCOS patients with normal thyroid function was significantly higher than that in the normal thyroid control group (85). Furthermore, it was found that the HOMA-IR of PCOS patients with hypothyroidism was the highest among all groups, and after receiving thyroid treatment, the HOMA-IR of PCOS patients with hypothyroidism decreased (86). These reports confirm the effect of hypothyroidism on carbohydrate metabolism parameters.
Thyroid hormones participate in the regulation of blood glucose at the central and peripheral levels ( Figure 1 ). Centrally, T3 regulates glucose synthesis in the liver through the sympathetic nervous system (87). Peripherally, T3 enhances skeletal muscle glucose transporter 4 (GLUT-4) expression and downstream signaling cascade proteins responsible for glucose transport, which increases the insulin-dependent transmembrane glucose transport (88). Mueller et al. discovered a significant correlation between TSH—the most sensitive indicator of thyroid function—and insulin resistance, but not between insulin resistance and the patient’s age or BMI (89). Since hypothyroidism has been shown to cause insulin resistance, this relationship may explain how hypothyroidism could be an underlying factor for PCOS.
The compensatory hyperinsulinemia seen in insulin resistance is closely related to the anovulation seen in PCOS. For instance, the insulin resistance of ovulating PCOS patients is lower than that of PCOS patients with anovulation (90). All insulin level reduction treatments have been shown to improve ovarian dysfunction and ovulation (57, 91, 92). Significant insulin resistance is believed to be associated with hyperandrogenism and acanthosis nigricans (93). The compensatory hyperinsulinemia of insulin resistance may allow the ovaries to partially escape LH desensitization, resulting in increased responsiveness of ovarian steroids to LH (94, 95). Therefore, these studies raise the possibility that hyperinsulinemia may lead to ovarian androgen excess.
5.2. Lipid metabolism disorders and obesity in PCOS
THs regulate lipid metabolism. Decreased thyroid function readily leads to lipid metabolism disorders, including obesity ( Figure 1 ) (33). Thyroid status is inversely related to blood lipid concentration. T3 regulates the activities of several key enzymes involved in lipoprotein transport, such as cholesterol ester transfer protein (CETP) and hepatic lipase (HL), thus regulating the distribution of HDL-C (96–98); furthermore, T3 may affect the synthesis and degradation of LDL-C. The LDL receptor gene promoter contains a T3 response element, which regulates the gene expression of LDL receptor C, thereby increasing the clearance rate of LDL-C (99). This thyroid-controlled regulation may explain why PCOS patients with hypothyroidism are prone to atherosclerotic cardiovascular disease.
PCOS patients are more prone to SCH; since obesity commonly occurs in PCOS patients with SCH, obesity may be a causative factor in the relationship between PCOS and SCH ( Figure 1 ) (18). Since obesity leads to increased levels of leptin, this increased leptin could stimulate the hypothalamus and lead to increased TRH secretion (100). Additionally, DIO2 activity may be reduced through related proinflammatory conditions and insulin resistance, resulting in relatively low T3 and high TSH levels (101, 102). These possibilities could explain the high incidence of SCH in PCOS.
The influence of SCH on PCOS metabolic parameters is regulated by BMI. Mild thyroid hormone deficiency has no significant clinical impact on women with PCOS and low to normal BMI (103). However, overweight or obese PCOS patients will develop clinical symptoms when coupled with existing diseases, even if thyroid hormone deficiency is mild (103). Since PCOS symptoms may improve with weight loss, moderate weight loss (defined as a 5–10% reduction in initial weight) can improve many characteristics of PCOS, such as the insulin sensitivity index, and the hyperandrogenism (104, 105).
Recent evidence shows that excess adipose tissue is an important cause of excess androgen and estrogen production (106, 107). SHBG, also known as testosterone estradiol binding globulin, can regulate and control the concentration of active androgen, namely, free testosterone, thus causing hyperandrogenism symptoms (107). SHBG primarily binds to testosterone, but it also binds to dihydrotestosterone (DHT), androstenediol, estradiol, and estrone. Zhu et al. found a significant correlation between TSH and insulin sensitivity, insulin secretion, and SHBG, but only in the high BMI group (108).
In conclusion, PCOS is the most common obesity-related endocrine syndrome in women. The prevalence of obesity in PCOS case studies have been associated with race (109). At least one-third of normal weight PCOS patients exhibit abdominal obesity, while obese PCOS patients exhibit a more widespread distribution of adipose tissue (110, 111). Obesity produces testosterone through insulin resistance and circulating androstenedione, and inhibits the production of gonadotropin, which plays a role in PCOS (112).
5.3. Immune status in PCOS during some thyroid diseases
The immune state of the body also plays a key role in PCOS ( Figure 1 ). In an Italian study, the prevalence of AITD in PCOS patients was significantly higher (26.03%) than in PCOS patients without other autoimmune diseases (9.72%) (113). Compared with Europeans and South Americans, the risk of AITD in women with PCOS is significantly higher among Asians (113). HT and GD are common AITDs that have been repeatedly shown to increase PCOS risk in women ( Figure 1 ). HT, also known as chronic lymphoid thyroiditis and autoimmune thyroiditis, is an autoimmune disease characterized by increased serum gamma globulin and detection of thyroid autoantibodies in patients (27). Additionally, the thyroid tissue of HT patients exhibits lymphocyte infiltration, fibrosis, interstitial atrophy, and eosinophilic change of acinar cells. GD is also an autoimmune disease caused by sensitization of thyroid antigens by T lymphocytes, which stimulate B lymphocytes to synthesize antibodies against these antigens, thereby causing diffuse goiter with thyroid hyperfunction.
Sex hormones have immunomodulatory effects both in vivo and in vitro. In animal models, estrogen is associated with increased B cell activity and decreased T cell activity (114). The production of autoantibodies in female mice was higher than that in male mice. Estrogen can reduce the activity of suppressor T cells, increase the activity of B cells, increase the secretion of Th2 cytokine IL6, and guide the immune response to Th2 and the formation of antibodies (114). Compared with men, women have a higher CD4+/CD8+ ratio, CD4+ level, and antibody level (115). Androgen reduces most components of the immune system and enhances the activity of suppressor T cells, the Th1 response, and CD8+ activation (116). The above results indicate that estrogen in PCOS may exacerbate the production of autoantibodies caused by autoimmune thyroid diseases. In addition, progesterone can reduce the proliferation of macrophages, the synthesis of IL-6, and the production of peripheral antibodies (116). The fluctuation of progesterone concentration during the ovulation cycle and pregnancy is most likely associated with reversible changes of the immune system (117).
5.4. Sex hormones in PCOS patients may be misregulated by thyroid signal pathway
fT3 has been detected in follicular fluid (118, 119). The transcripts of TSH and its receptors not only exist in ovarian structures, such as oocytes, cumulus cells, granulosa cells, and the ovarian epithelium, but also in syncytiotrophoblast villi (120). Thyroid signal pathway receptors, including TRHR, TSHR, and TRs, have been identified in the monkey uterus, and estrogen and progesterone have been shown to affect their expression (59). TSH, TR α 1, and TR β 1 receptor expression have also been detected in human endometrium (121). The expression of TR α 1 and TR β were highest in the endometrium before ovulation. T3 regulates the synthesis of endometrial protein mRNAs, including leukemia inhibitory factor (LIF), which is important in the implantation process, and glucose transporter 1 (GLUT-1) (122). In addition, the placenta has T3 and T4 membrane transporters, DIO2 and DIO3 enzymes that regulate thyroid hormones (123). Oocytes and embryos cultured in a thyroid hormone-rich medium showed improved blastocyst formation, implantation ability, apoptosis rate, and vitality after cryopreservation (124). Therefore, the thyroid plays an important role in the reproductive system ( Figure 1 ).
Significant hypothyroidism accompanied by elevated TRH led to hyperprolactinemia, interruption of pulsatile LH secretion, decreased SHBG synthesis, interruption of peripheral estrogen metabolism, and increased ovarian androgen production (125). In a study of female pigs, hypothyroidism led to increased gonadotropin receptor sensitivity in the ovaries, which ultimately promoted ovarian hypertrophy and the formation of multiple ovarian cysts (126). Moreover, hypothyroidism may lead to severe and irregular menstrual bleeding, spotting during the menstrual cycle, insufficient endometrial thickness, ovulation disorders, and eventually endometrial hyperplasia disorders (127, 128).
6. Conclusions and expectations
The clinical manifestations of PCOS are similar to some thyroid diseases. The thyroid diseases associated with PCOS are primarily SCH and AITDs; the co-occurrence of PCOS with hyperthyroidism is relatively rare. This association suggests that the thyroid may affect the clinical manifestations of PCOS by influencing multiple systems including metabolism and immunity. Insulin resistance is a widely recognized cause of PCOS, and thyroid function affects the degree of insulin resistance; specifically, hypothyroidism leads to more serious insulin resistance than hyperthyroidism. Obesity has seriously affected the health of modern people, and patients with hypothyroidism are prone to obesity. The patients with PCOS accompanied by hypothyroidism tend to have high BMI with a corresponding increase in metabolic disease burden. When the thyroid function of patients with SCH or clinical hypothyroidism is restored through thyroid treatment, their metabolic abnormalities improve (103). In addition, the thyroid also plays an important role in PCOS-related reproductive disorders. In conclusion, the role of the thyroid in PCOS is complex and involves multiple pathways. Thyroid function directly affects the clinical manifestations of PCOS, which increases the heterogeneity of the clinical PCOS phenotype. In fact, clinical reports have shown that some PCOS symptoms have been alleviated or even eliminated by restoring thyroid function (129). This evidence strongly suggests that the thyroid plays a crucial role in the pathogenesis, development, and progression of PCOS. Therefore, patients with PCOS require rigorous thyroid function detection, monitoring, and correction over time, which will mitigate or perhaps fully prevent the further deterioration of PCOS symptoms.
6.1. Limitation
Because the goal of this review is to find the role of the thyroid in PCOS, we focus on positive results between thyroid and PCOS. But there are quite a few negative data between thyroids and PCOS. Many factors are related to the above negative data, including the size of study populations, anthropometric parameters and follow-up period. For example, the diagnosis of PCOS has not yet been unified. Different studies have different diagnostic criteria for PCOS. Some studies require to exclude thyroid abnormalities, when PCOS is suspected. This will bring a wide variation in findings.
Author contributions
All authors participated in the research and preparation of the manuscript. HF and QR contributed with the first draft of the manuscript, and actively participated in subsequent editing of the manuscript. ZS, GD and LL contributed with writing of subsequent versions and editing. LL and HF also did the final review and submission. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by Diabetes Talent Research Project of China International Medical Foundation (2018-N-01-26), the Fundamental Research Funds for the Central Universities (1507219059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers (2016) 2:16057. doi: 10.1038/nrdp.2016.57 [DOI] [PubMed] [Google Scholar]
- 2. Vause TDR, Cheung AP, Reproductive E, Infertility C. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can (2010) 32:495–502. doi: 10.1016/S1701-2163(16)34504-2 [DOI] [PubMed] [Google Scholar]
- 3. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev (2015) 36:487–525. doi: 10.1210/er.2015-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotterdam EA-SPCWG . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 5. Gaberscek S, Zaletel K, Schwetz V, Pieber T, Obermayer-Pietsch B, Lerchbaum E. Mechanisms in endocrinology: thyroid and polycystic ovary syndrome. Eur J Endocrinol (2015) 172:R9–21. doi: 10.1530/EJE-14-0295 [DOI] [PubMed] [Google Scholar]
- 6. Zhao H, Zhang Y, Ye J, Wei H, Huang Z, Ning X, et al. A comparative study on insulin secretion, insulin resistance and thyroid function in patients with polycystic ovary syndrome with and without hashimoto's thyroiditis. Diabetes Metab Syndr Obes (2021) 14:1817–21. doi: 10.2147/DMSO.S300015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kowalczyk K, Franik G, Kowalczyk D, Pluta D, Blukacz L, Madej P. Thyroid disorders in polycystic ovary syndrome. Eur Rev Med Pharmacol Sci (2017) 21:346–60. [PubMed] [Google Scholar]
- 8. Palomba S, Colombo C, Busnelli A, Caserta D, Vitale G. Polycystic ovary syndrome and thyroid disorder: a comprehensive narrative review of the literature. Front Endocrinol (Lausanne) (2023) 14:1251866. doi: 10.3389/fendo.2023.1251866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anaforoglu I, Topbas M, Algun E. Relative associations of polycystic ovarian syndrome vs metabolic syndrome with thyroid function, volume, nodularity and autoimmunity. J Endocrinol Invest (2011) 34:e259–264. doi: 10.3275/7681 [DOI] [PubMed] [Google Scholar]
- 10. Glintborg D, Rubin KH, Nybo M, Abrahamsen B, Andersen M. Increased risk of thyroid disease in Danish women with polycystic ovary syndrome: a cohort study. Endocr Connect (2019) 8:1405–15. doi: 10.1530/EC-19-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wemeau JL, Klein M, Sadoul JL, Briet C, Velayoudom-Cephise FL. Graves' disease: Introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol (Paris) (2018) 79:599–607. doi: 10.1016/j.ando.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Jung JH, Hahm JR, Jung TS, Kim HJ, Kim HS, Kim S, et al. A 27-year-old woman diagnosed as polycystic ovary syndrome associated with Graves' disease. Intern Med (2011) 50:2185–9. doi: 10.2169/internalmedicine.50.5475 [DOI] [PubMed] [Google Scholar]
- 13. Nisar S, Shah PA, Kuchay MS, Bhat MA, Rashid A, Ahmed S, et al. Association of polycystic ovary syndrome and Graves' disease: Is autoimmunity the link between the two diseases. Indian J Endocrinol Metab (2012) 16:982–6. doi: 10.4103/2230-8210.103006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha U, Sinharay K, Saha S, Longkumer TA, Baul SN, Pal SK. Thyroid disorders in polycystic ovarian syndrome subjects: A tertiary hospital based cross-sectional study from Eastern India. Indian J Endocrinol Metab (2013) 17:304–9. doi: 10.4103/2230-8210.109714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen HH, Chen CC, Hsieh MC, Ho CW, Hsu SP, Yip HT, et al. Graves' disease could increase polycystic ovary syndrome and comorbidities in Taiwan. Curr Med Res Opin (2020) 36(6):1063–7. doi: 10.1080/03007995.2020.1756235 [DOI] [PubMed] [Google Scholar]
- 16. Benetti-Pinto CL, Berini Piccolo VR, Garmes HM, Teatin Juliato CR. Subclinical hypothyroidism in young women with polycystic ovary syndrome: an analysis of clinical, hormonal, and metabolic parameters. Fertil Steril (2013) 99(2):588–92. doi: 10.1016/j.fertnstert.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 17. Raj D, Pooja F, Chhabria P, Kalpana F, Lohana S, Lal K, et al. Frequency of subclinical hypothyroidism in women with polycystic ovary syndrome. Cureus (2021) 13(9):e17722. doi: 10.7759/cureus.17722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamrul-Hasan A, Aalpona FTZ, Selim S. Impact of subclinical hypothyroidism on reproductive and metabolic parameters in polycystic ovary syndrome - A cross-sectional study from Bangladesh. Eur Endocrinol (2020) 16(2):156–60. doi: 10.17925/EE.2020.16.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xing Y, Chen J, Liu J, Ma H. The impact of subclinical hypothyroidism on patients with polycystic ovary syndrome: A meta-analysis. Horm Metab Res (2021) 53(6):382–90. doi: 10.1055/a-1463-3198 [DOI] [PubMed] [Google Scholar]
- 20. Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol (2004) 150(3):363–9. doi: 10.1530/eje.0.1500363 [DOI] [PubMed] [Google Scholar]
- 21. Arduc A, Aycicek Dogan B, Bilmez S, Imga Nasiroglu N, Tuna MM, Isik S, et al. High prevalence of Hashimoto's thyroiditis in patients with polycystic ovary syndrome: does the imbalance between estradiol and progesterone play a role? Endocr Res (2015) 40(4):204–10. doi: 10.3109/07435800.2015.1015730 [DOI] [PubMed] [Google Scholar]
- 22. Ganie MA, Marwaha RK, Aggarwal R, Singh S. High prevalence of polycystic ovary syndrome characteristics in girls with euthyroid chronic lymphocytic thyroiditis: a case-control study. Eur J Endocrinol (2010) 162(6):1117–22. doi: 10.1530/EJE-09-1012 [DOI] [PubMed] [Google Scholar]
- 23. Karakose M, Hepsen S, Cakal E, Sayki Arslan M, Tutal E, Akin S, et al. Frequency of nodular goiter and autoimmune thyroid disease and association of these disorders with insulin resistance in polycystic ovary syndrome. J Turk Ger Gynecol Assoc (2017) 18(2):85–9. doi: 10.4274/jtgga.2016.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altuntas SC, Gunes M. Investigation of the relationship between autoimmune and nodular goiter in patients with euthyroid polycystic ovary syndrome and their phenotypes. Horm Metab Res (2022) 54(6):396–406. doi: 10.1055/a-1825-0316 [DOI] [PubMed] [Google Scholar]
- 25. Ho CW, Chen HH, Hsieh MC, Chen CC, Hsu SP, Yip HT, et al. Increased risk of polycystic ovary syndrome and it's comorbidities in women with autoimmune thyroid disease. Int J Environ Res Public Health (2020) 17(7):2422. doi: 10.3390/ijerph17072422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH. T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 27. Zeber-Lubecka N, Hennig EE. Genetic susceptibility to joint occurrence of polycystic ovary syndrome and Hashimoto's thyroiditis: how far is our understanding? Front Immunol (2021) 12:606620. doi: 10.3389/fimmu.2021.606620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JS, Kim BG, Stybayeva G, Hwang SH. Diagnostic performance of various ultrasound risk stratification systems for benign and Malignant thyroid nodules: A meta-analysis. Cancers (Basel) (2023) 15(2):424. doi: 10.3390/cancers15020424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uppal N, Collins R, James B. Thyroid nodules: Global, economic, and personal burdens. Front Endocrinol (Lausanne) (2023) 14:1113977. doi: 10.3389/fendo.2023.1113977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anil G, Hegde A, Chong FH. Thyroid nodules: risk stratification for Malignancy with ultrasound and guided biopsy. Cancer Imaging (2011) 11(1):209–23. doi: 10.1102/1470-7330.2011.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cobin RH. Cardiovascular and metabolic risks associated with PCOS. Intern Emerg Med (2013) 8 Suppl 1:S61–64. doi: 10.1007/s11739-013-0924-z [DOI] [PubMed] [Google Scholar]
- 32. Krentz AJ, von Muhlen D, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause (2007) 14(2):284–92. doi: 10.1097/GME.0b013e31802cc7ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev (2014) 94(2):355–82. doi: 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding X, Yang L, Wang J, Tang R, Chen Q, Pan J, et al. Subclinical hypothyroidism in polycystic ovary syndrome: A systematic review and meta-analysis. Front Endocrinol (Lausanne) (2018) 9:700. doi: 10.3389/fendo.2018.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gawron IM, Baran R, Derbisz K, Jach R. Association of subclinical hypothyroidism with present and absent anti-thyroid antibodies with PCOS phenotypes and metabolic profile. J Clin Med (2022) 11(6):1547. doi: 10.3390/jcm11061547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 37. Celik C, Abali R, Tasdemir N, Guzel S, Yuksel A, Aksu E, et al. Is subclinical hypothyroidism contributing dyslipidemia and insulin resistance in women with polycystic ovary syndrome? Gynecol Endocrinol (2012) 28(8):615–8. doi: 10.3109/09513590.2011.650765 [DOI] [PubMed] [Google Scholar]
- 38. Velija-Asimi Z, Burekovic A, Dujic T, Dizdarevic-Bostandzic A, Semiz S. Incidence of prediabetes and risk of developing cardiovascular disease in women with polycystic ovary syndrome. Bosn J Basic Med Sci (2016) 16(4):298–306. doi: 10.17305/bjbms.2016.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu Q, Wang JB. Subclinical hypothyroidism in PCOS: impact on presentation, insulin resistance, and cardiovascular risk. BioMed Res Int (2016) 2016:2067087. doi: 10.1155/2016/2067087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trummer C, Schwetz V, Giuliani A, Obermayer-Pietsch B, Lerchbaum E. Impact of elevated thyroid-stimulating hormone levels in polycystic ovary syndrome. Gynecol Endocrinol (2015) 31(10):819–23. doi: 10.3109/09513590.2015.1062864 [DOI] [PubMed] [Google Scholar]
- 41. Hama M, Abe M, Kawaguchi T, Ishida Y, Nosaka M, Kuninaka Y, et al. A case of myocardial infarction in a young female with subclinical hyperthyroidism. Int J Cardiol (2012) 158(2):e23–25. doi: 10.1016/j.ijcard.2011.10.038 [DOI] [PubMed] [Google Scholar]
- 42. Newman CB. Effects of endocrine disorders on lipids and lipoproteins. Best Pract Res Clin Endocrinol Metab (2023) 37(3):101667. doi: 10.1016/j.beem.2022.101667 [DOI] [PubMed] [Google Scholar]
- 43. Ghosh H, Rai S, Manzar MD, Pandi-Perumal SR, Brown GM, Reiter RJ, et al. Differential expression and interaction of melatonin and thyroid hormone receptors with estrogen receptor α improve ovarian functions in letrozole-induced rat polycystic ovary syndrome. Life Sci (2022) 295:120086. doi: 10.1016/j.lfs.2021.120086 [DOI] [PubMed] [Google Scholar]
- 44. Selen Alpergin ES, Bolandnazar Z, Sabatini M, Rogowski M, Chiellini G, Zucchi R, et al. Metabolic profiling reveals reprogramming of lipid metabolic pathways in treatment of polycystic ovary syndrome with 3-iodothyronamine. Physiol Rep (2017) 5(1):e13097. doi: 10.14814/phy2.13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brenta G, Caballero AS, Nunes MT. Case finding for hypothyroidism should include type 2 diabetes and metabolic syndrome patients: A Latin American thyroid society (Lats) position statement. Endocr Pract (2019) 25(1):101–5. doi: 10.4158/EP-2018-0317 [DOI] [PubMed] [Google Scholar]
- 46. Glintborg D, Hass Rubin K, Nybo M, Abrahamsen B, Andersen M. Morbidity and medicine prescriptions in a nationwide Danish population of patients diagnosed with polycystic ovary syndrome. Eur J Endocrinol (2015) 172(5):627–38. doi: 10.1530/EJE-14-1108 [DOI] [PubMed] [Google Scholar]
- 47. Guo F, Gong Z, Fernando T, Zhang L, Zhu X, Shi Y. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: A retrospective study in Chinese population. Front Endocrinol (Lausanne) (2022) 13:892125. doi: 10.3389/fendo.2022.892125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. HUNT Study Eur J Endocrinol (2007) 156(2):181–6. doi: 10.1530/eje.1.02333 [DOI] [PubMed] [Google Scholar]
- 49. Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, et al. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas (2009) 62(3):301–5. doi: 10.1016/j.maturitas.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 50. Adamska A, Krentowska A, Lebkowska A, Hryniewicka J, Lesniewska M, Adamski M, et al. Decreased deiodinase activity after glucose load could lead to atherosclerosis in euthyroid women with polycystic ovary syndrome. Endocrine (2019) 65(1):184–91. doi: 10.1007/s12020-019-01913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lauritsen MP, Pinborg A, Loft A, Petersen JH, Mikkelsen AL, Bjerge MR, et al. Revised criteria for PCOS in WHO Group II anovulatory infertility - a revival of hypothalamic amenorrhoea? Clin Endocrinol (Oxf) (2015) 82(4):584–91. doi: 10.1111/cen.12621 [DOI] [PubMed] [Google Scholar]
- 52. Rushing JS, Santoro N. Fertility issues in polycystic ovarian disease: A systematic approach. Endocrinol Metab Clin North Am (2021) 50(1):43–55. doi: 10.1016/j.ecl.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 53. Plowden TC, Schisterman EF, Sjaarda LA, Zarek SM, Perkins NJ, Silver R, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab (2016) 101(6):2358–65. doi: 10.1210/jc.2016-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract (2015) 21(11):1291–300. doi: 10.4158/EP15748.DSC [DOI] [PubMed] [Google Scholar]
- 55. Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev (2010) 31(5):702–55. doi: 10.1210/er.2009-0041 [DOI] [PubMed] [Google Scholar]
- 56. Unuane D, Tournaye H, Velkeniers B, Poppe K. Endocrine disorders & female infertility. Best Pract Res Clin Endocrinol Metab (2011) 25(6):861–73. doi: 10.1016/j.beem.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 57. Shpakov AO. Improvement effect of metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharm (Basel) (2021) 14(1):42. doi: 10.3390/ph14010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, et al. Thyroid hormone receptors and reproduction. J Reprod Immunol (2011) 90(1):58–66. doi: 10.1016/j.jri.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 59. Hulchiy M, Zhang H, Cline JM, Hirschberg AL, Sahlin L. Receptors for thyrotropin-releasing hormone, thyroid-stimulating hormone, and thyroid hormones in the macaque uterus: effects of long-term sex hormone treatment. Menopause (2012) 19(11):1253–9. doi: 10.1097/gme.0b013e318252e450 [DOI] [PubMed] [Google Scholar]
- 60. Hansen KA, Tho SP, Hanly M, Moretuzzo RW, McDonough PG. Massive ovarian enlargement in primary hypothyroidism. Fertil Steril (1997) 67(1):169–71. doi: 10.1016/s0015-0282(97)81876-6 [DOI] [PubMed] [Google Scholar]
- 61. Panico A, Lupoli GA, Fonderico F, Colarusso S, Marciello F, Poggiano MR, et al. Multiple ovarian cysts in a young girl with severe hypothyroidism. Thyroid (2007) 17(12):1289–93. doi: 10.1089/thy.2007.0056 [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Wang H, Pan X, Teng W, Shan Z. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: A systematic review and meta-analysis. PloS One (2017) 12(4):e0175708. doi: 10.1371/journal.pone.0175708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lambert M, Hocke C, Jimenez C, Frantz S, Papaxanthos A, Creux H. [Repeated in vitro fertilization failure: Abnormalities identified in the diagnostic assessment]. Gynecol Obstet Fertil (2016) 44(10):565–71. doi: 10.1016/j.gyobfe.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 64. Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf) (2007) 66(3):309–21. doi: 10.1111/j.1365-2265.2007.02752.x [DOI] [PubMed] [Google Scholar]
- 65. Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol (2004) 150(6):751–5. doi: 10.1530/eje.0.1500751 [DOI] [PubMed] [Google Scholar]
- 66. Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab (2003) 88(9):4149–52. doi: 10.1210/jc.2003-030268 [DOI] [PubMed] [Google Scholar]
- 67. Bucci I, Giuliani C, Di Dalmazi G, Formoso G, Napolitano G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front Endocrinol (Lausanne) (2022) 13:768363. doi: 10.3389/fendo.2022.768363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid (2014) 24(11):1642–9. doi: 10.1089/thy.2014.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update (2015) 21(5):575–92. doi: 10.1093/humupd/dmv029 [DOI] [PubMed] [Google Scholar]
- 70. Chen X, Gissler M, Lavebratt C. Association of maternal polycystic ovary syndrome and diabetes with preterm birth and offspring birth size: a population-based cohort study. Hum Reprod (2022) 37(6):1311–23. doi: 10.1093/humrep/deac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Frene V, Vansteelandt S, T'Sjoen G, Gerris J, Somers S, Vercruysse L, et al. A retrospective study of the pregnancy, delivery and neonatal outcome in overweight versus normal weight women with polycystic ovary syndrome. Hum Reprod (2014) 29(10):2333–8. doi: 10.1093/humrep/deu154 [DOI] [PubMed] [Google Scholar]
- 72. Feigl S, Obermayer-Pietsch B, Klaritsch P, Pregartner G, Herzog SA, Lerchbaum E, et al. Impact of thyroid function on pregnancy and neonatal outcome in women with and without PCOS. Biomedicines (2022) 10(4):750. doi: 10.3390/biomedicines10040750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Allelein S, Schott M. [Thyroid dysfunction]. MMW Fortschr Med (2016) 158 Spec No 1:45–52. doi: 10.1007/s15006-016-7652-z [DOI] [PubMed] [Google Scholar]
- 74. Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther (2017) 173:135–45. doi: 10.1016/j.pharmthera.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Flamant F, Cheng SY, Hollenberg AN, Moeller LC, Samarut J, Wondisford FE, et al. Thyroid hormone signaling pathways: time for a more precise nomenclature. Endocrinology (2017) 158(7):2052–7. doi: 10.1210/en.2017-00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol (2016) 6(3):1387–428. doi: 10.1002/cphy.c150027 [DOI] [PubMed] [Google Scholar]
- 77. Russo SC, Salas-Lucia F, Bianco AC. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology (2021) 162(8):bqab059. doi: 10.1210/endocr/bqab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Akarsu E, Korkmaz H, Oguzkan Balci S, Borazan E, Korkmaz S, Tarakcioglu M. Subcutaneous adipose tissue type II deiodinase gene expression reduced in obese individuals with metabolic syndrome. Exp Clin Endocrinol Diabetes (2016) 124(1):11–5. doi: 10.1055/s-0035-1564129 [DOI] [PubMed] [Google Scholar]
- 79. Wawrzkiewicz-Jalowiecka A, Kowalczyk K, Trybek P, Jarosz T, Radosz P, Setlak M, et al. In search of new therapeutics-molecular aspects of the PCOS pathophysiology: genetics, hormones, metabolism and beyond. Int J Mol Sci (2020) 21(19):7054. doi: 10.3390/ijms21197054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest (2017) 40(1):1–8. doi: 10.1007/s40618-016-0523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rasool SUA, Nabi M, Ashraf S, Amin S. Insulin receptor substrate 1 Gly972Arg (rs1801278) polymorphism is associated with obesity and insulin resistance in Kashmiri women with polycystic ovary syndrome. Genes (Basel) (2022) 13(8):1463. doi: 10.3390/genes13081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rasool SUA, Ashraf S, Nabi M, Masoodi SR, Fazili KM, Amin S. Clinical manifestations of hyperandrogenism and ovulatory dysfunction are not associated with his1058 C/T SNP (rs1799817) polymorphism of insulin receptor gene tyrosine kinase domain in Kashmiri women with PCOS. Int J Endocrinol (2021) 2021:7522487. doi: 10.1155/2021/7522487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Daghestani MH. RS1799817 in INSR associates with susceptibility to polycystic ovary syndrome. J Med Biochem (2020) 39(2):149–59. doi: 10.2478/jomb-2019-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Y, Wang Y, Liu H, Zhang S, Zhang C. Association between HOMA-IR and ovarian sensitivity index in women with PCOS undergoing ART: A retrospective cohort study. Front Endocrinol (Lausanne) (2023) 14:1117996. doi: 10.3389/fendo.2023.1117996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Trouva A, Alvarsson M, Calissendorff J, Asvold BO, Vanky E, Hirschberg AL. Thyroid status during pregnancy in women with polycystic ovary syndrome and the effect of metformin. Front Endocrinol (Lausanne) (2022) 13:772801. doi: 10.3389/fendo.2022.772801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, et al. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci U.S.A. (2009) 106(14):5966–71. doi: 10.1073/pnas.0805355106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. El Agaty SM. Triiodothyronine improves age-induced glucose intolerance and increases the expression of sirtuin-1 and glucose transporter-4 in skeletal muscle of aged rats. Gen Physiol Biophys (2018) 37(6):677–86. doi: 10.4149/gpb_2018026 [DOI] [PubMed] [Google Scholar]
- 89. Mueller A, Schofl C, Dittrich R, Cupisti S, Oppelt PG, Schild RL, et al. Thyroid-stimulating hormone is associated with insulin resistance independently of body mass index and age in women with polycystic ovary syndrome. Hum Reprod (2009) 24(11):2924–30. doi: 10.1093/humrep/dep285 [DOI] [PubMed] [Google Scholar]
- 90. Cho LW, Kilpatrick ES, Keevil BG, Jayagopal V, Coady AM, Rigby AS, et al. Insulin resistance variability in women with anovulatory and ovulatory polycystic ovary syndrome, and normal controls. Horm Metab Res (2011) 43(2):141–5. doi: 10.1055/s-0030-1270450 [DOI] [PubMed] [Google Scholar]
- 91. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol (2018) 14(5):270–84. doi: 10.1038/nrendo.2018.24 [DOI] [PubMed] [Google Scholar]
- 92. Wang R, Li W, Bordewijk EM, Legro RS, Zhang H, Wu X, et al. First-line ovulation induction for polycystic ovary syndrome: an individual participant data meta-analysis. Hum Reprod Update (2019) 25(6):717–32. doi: 10.1093/humupd/dmz029 [DOI] [PubMed] [Google Scholar]
- 93. Agrawal K, Mathur R, Purwar N, Mathur SK, Mathur DK. Hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN) syndrome reflects adipose tissue dysfunction ("Adiposopathy" or "Sick fat") in asian Indian girls. Dermatology (2021) 237(5):797–805. doi: 10.1159/000512918 [DOI] [PubMed] [Google Scholar]
- 94. Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab (1996) 81(1):302–9. doi: 10.1210/jcem.81.1.8550768 [DOI] [PubMed] [Google Scholar]
- 95. Zhao H, Zhang J, Cheng X, Nie X, He B. evaluation, and treatment. J Ovarian Res (2023) 16(1):9. doi: 10.1186/s13048-022-01091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tan KC, Shiu SW, Kung AW. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J Clin Endocrinol Metab (1998) 83(1):140–3. doi: 10.1210/jcem.83.1.4491 [DOI] [PubMed] [Google Scholar]
- 97. Tan KC, Shiu SW, Kung AW. Effect of thyroid dysfunction on high-density lipoprotein subfraction metabolism: roles of hepatic lipase and cholesteryl ester transfer protein. J Clin Endocrinol Metab (1998) 83(8):2921–4. doi: 10.1210/jcem.83.8.4938 [DOI] [PubMed] [Google Scholar]
- 98. Jung KY, Ahn HY, Han SK, Park YJ, Cho BY, Moon MK. Association between thyroid function and lipid profiles, apolipoproteins, and high-density lipoprotein function. J Clin Lipidol (2017) 11(6):1347–53. doi: 10.1016/j.jacl.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 99. Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta (2007) 1771(9):1216–25. doi: 10.1016/j.bbalip.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 100. Pekary AE, Sattin A, Blood J. Rapid modulation of TRH and TRH-like peptide release in rat brain and peripheral tissues by leptin. Brain Res (2010) 1345:9–18. doi: 10.1016/j.brainres.2010.05.039 [DOI] [PubMed] [Google Scholar]
- 101. Oliveira TS, Shimabukuro MK, Monteiro VRS, Andrade CBV, Boelen A, Wajner SM, et al. Low inflammatory stimulus increases D2 activity and modulates thyroid hormone metabolism during myogenesis in vitro . Metabolites (2022) 12(5):416. doi: 10.3390/metabo12050416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Okada J, Isoda A, Hoshi H, Watanabe T, Yamada E, Saito T, et al. Free triiodothyronine /free thyroxine ratio as an index of deiodinase type 1 and 2 activities negatively correlates with casual serum insulin levels in patients with type 2 diabetes mellitus. Endocr J (2021) 68(10):1237–40. doi: 10.1507/endocrj.EJ21-0169 [DOI] [PubMed] [Google Scholar]
- 103. Kowalczyk K, Radosz P, Baranski K, Pluta D, Kowalczyk D, Franik G, et al. The influence of treated and untreated subclinical hypothyroidism on metabolic profile in women with polycystic ovary syndrome. Int J Endocrinol (2021) 2021:8427150. doi: 10.1155/2021/8427150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev (2011) 7):CD007506. doi: 10.1002/14651858.CD007506.pub2 [DOI] [PubMed] [Google Scholar]
- 105. Dietz de Loos ALP, Jiskoot G, Timman R, Beerthuizen A, Busschbach JJV, Laven JSE. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod BioMed Online (2021) 43(2):298–309. doi: 10.1016/j.rbmo.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 106. Yuxin L, Chen L, Xiaoxia L, Yue L, Junjie L, Youzhu L, et al. Research progress on the relationship between obesity-inflammation-aromatase axis and male infertility. Oxid Med Cell Longev (2021) 2021:6612796. doi: 10.1155/2021/6612796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Torres Irizarry VC, Jiang Y, He Y, Xu P. Hypothalamic estrogen signaling and adipose tissue metabolism in energy homeostasis. Front Endocrinol (Lausanne) (2022) 13:898139. doi: 10.3389/fendo.2022.898139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta (2019) 499:142–8. doi: 10.1016/j.cca.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 109. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA (2012) 307(5):491–7. doi: 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 110. Echiburu B, Perez-Bravo F, Galgani JE, Sandoval D, Saldias C, Crisosto N, et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids (2018) 130:15–21. doi: 10.1016/j.steroids.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 111. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 112. Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes (2014) 63(4):1270–82. doi: 10.2337/db13-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM. Association between PCOS and autoimmune thyroid disease: a systematic review and meta-analysis. Endocr Connect (2018) 7(11):1158–67. doi: 10.1530/EC-18-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Canellada A, Alvarez I, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J Steroid Biochem Mol Biol (2008) 111(3-5):255–61. doi: 10.1016/j.jsbmb.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 115. He S, Mao X, Lei H, Dong B, Guo D, Zheng B, et al. Peripheral blood inflammatory-immune cells as a predictor of infertility in women with polycystic ovary syndrome. J Inflammation Res (2020) 13:441–50. doi: 10.2147/JIR.S260770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature (2022) 606(7915):791–6. doi: 10.1038/s41586-022-04522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang CY, McDonnell DP. Estrogen receptor signaling in the immune system. Endocr Rev (2023) 44(1):117–41. doi: 10.1210/endrev/bnac017 [DOI] [PubMed] [Google Scholar]
- 118. Ashkar FA, Bartlewski PM, Singh J, Malhi PS, Yates KM, Singh T, et al. Thyroid hormone concentrations in systemic circulation and ovarian follicular fluid of cows. Exp Biol Med (Maywood) (2010) 235(2):215–21. doi: 10.1258/ebm.2009.009185 [DOI] [PubMed] [Google Scholar]
- 119. Medenica S, Garalejic E, Arsic B, Medjo B, Bojovic Jovic D, Abazovic D, et al. Follicular fluid thyroid autoantibodies, thyrotropin, free thyroxine levels and assisted reproductive technology outcome. PloS One (2018) 13(10):e0206652. doi: 10.1371/journal.pone.0206652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update (2014) 20(6):884–904. doi: 10.1093/humupd/dmu028 [DOI] [PubMed] [Google Scholar]
- 121. Kirkland JL, Mukku V, Hardy M, Young R. Evidence for triiodothyronine receptors in human endometrium and myometrium. Am J Obstet Gynecol (1983) 146(4):380–3. doi: 10.1016/0002-9378(83)90817-7 [DOI] [PubMed] [Google Scholar]
- 122. Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril (2011) 95(1):230–7. doi: 10.1016/j.fertnstert.2010.06.079 [DOI] [PubMed] [Google Scholar]
- 123. Zuniga LFF, Munoz YS, Pustovrh MC. Thyroid hormones: Metabolism and transportation in the fetoplacental unit. Mol Reprod Dev (2022) 89(11):526–39. doi: 10.1002/mrd.23647 [DOI] [PubMed] [Google Scholar]
- 124. Ashkar FA, Semple E, Schmidt CH, St John E, Bartlewski PM, King WA. Thyroid hormone supplementation improves bovine embryo development in vitro . Hum Reprod (2010) 25(2):334–44. doi: 10.1093/humrep/dep394 [DOI] [PubMed] [Google Scholar]
- 125. Escobar-Morreale HF, Asuncion M, Calvo RM, Sancho J, San Millan JL. Receiver operating characteristic analysis of the performance of basal serum hormone profiles for the diagnosis of polycystic ovary syndrome in epidemiological studies. Eur J Endocrinol (2001) 145(5):619–24. doi: 10.1530/eje.0.1450619 [DOI] [PubMed] [Google Scholar]
- 126. Ison EK, Kent-Dennis CE, Fazioli J, Mulligan MK, Pham A, Pasternak JA. Compensatory mechanisms in response to induced hypothyroidism in the late gestation pig fetus. Biol Reprod (2023) 108(5):731–43. doi: 10.1093/biolre/ioad024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Koutras DA. Disturbances of menstruation in thyroid disease. Ann N Y Acad Sci (1997) 816:280–4. doi: 10.1111/j.1749-6632.1997.tb52152.x [DOI] [PubMed] [Google Scholar]
- 128. Barbero A, Pagano M, Tuli G, Buganza R, de Sanctis L, Bondone C. Menorrhagia as main presentation sign of severe hypothyroidism in a pediatric patient: a case report. Ital J Pediatr (2022) 48(1):171. doi: 10.1186/s13052-022-01363-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, et al. Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci (2022) 23(2):583. doi: 10.3390/ijms23020583 [DOI] [PMC free article] [PubMed] [Google Scholar]