Abstract
Background
Alcohol dependence is a major public health problem that is characterised by recidivism and a host of medical and psychosocial complications. Besides psychosocial interventions, different pharmacological interventions have been or currently are under investigation through Cochrane systematic reviews.
Objectives
The primary aim of the review is to assess the benefits/risks of anticonvulsants for the treatment of alcohol dependence.
Search methods
We searched the Cochrane Drugs and Alcohol Group Trials Register (October 2013), PubMed (1966 to October 2013), EMBASE (1974 to October 2013) and CINAHL (1982 to October 2013).
Selection criteria
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) comparing anticonvulsants alone or in association with other drugs and/or psychosocial interventions versus placebo, no treatment and other pharmacological or psychosocial interventions.
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration.
Main results
A total of 25 studies were included in the review (2641 participants). Most participants were male, with an average age of 44 years. Anticonvulsants were compared with placebo (17 studies), other medications (seven studies) and no medication (two studies). The mean duration of the trials was 17 weeks (range four to 52 weeks). The studies took place in the USA, Europe, South America, India and Thailand. Variation was reported in the characteristics of the studies, including their design and the rating instruments used. For many key outcomes, the risk of bias associated with unclear or unconcealed allocation and lack of blinding affected the quality of the evidence.
Anticonvulsants versus placebo: For dropouts (16 studies, 1675 participants, risk ratio (RR) 0.94, 95% confidence interval (Cl) 0.74 to 1.19, moderate‐quality evidence) and continuous abstinence (eight studies, 634 participants, RR 1.21, 95% Cl 95% 0.97 to 1.52, moderate‐quality evidence), results showed no evidence of differences. Moderate‐quality evidence suggested that anticonvulsants reduced drinks/drinking days (11 studies, 1126 participants, mean difference (MD) ‐1.49, 95% Cl ‐2.32 to ‐0.65) and heavy drinking (12 studies, 1129 participants, standardised mean difference (SMD) ‐0.35, 95% Cl ‐0.51 to ‐0.19). Moreover, withdrawal for medical reasons (12 studies, 1410 participants, RR 1.22, 95% Cl 0.58 to 2.56, moderate‐quality evidence) showed no evidence of difference, but for specific adverse effects (nine studies, 1164 participants), two of 18 adverse event outcomes favoured placebo. The direction of results was confirmed by subgroup analyses for topiramate and partially for gabapentin and valproate.
Anticonvulsants versus naltrexone: No evidence of difference was shown in dropout rates (five studies, 528 participants, RR 0.74, 95% CI 0.52 to 1.06), severe relapse rates (four studies, 427 participants, RR 0.69, 95% Cl 0.44 to 1.07) and continuous abstinence rates (five studies, 528 participants, RR 1.21, 95% Cl 0.99 to 1.49); anticonvulsants were associated with fewer heavy drinking days (three studies, 308 participants, MD ‐5.21, 95% Cl ‐8.58 to ‐1.83), more days to severe relapse (three studies, 244 participants, MD 11.88, 95% Cl 3.29 to 20.46) and lower withdrawal for medical reasons (three studies, 245 participants, RR 0.13, 95% Cl 0.03 to 0.58).
Authors' conclusions
At the current stage of research, randomised evidence supporting the clinical use of anticonvulsants to treat alcohol dependence is insufficient. Results are conditioned by heterogeneity and by the low number and quality of studies comparing anticonvulsants with other medications. The uncertainty associated with these results leaves to clinicians the need to balance possible benefits/risks of treatment with anticonvulsants versus other medications as supported by evidence of efficacy.
Plain language summary
Anticonvulsants for alcohol dependence
Review question
This review looked at evidence on the efficacy and acceptability of anticonvulsants alone or in combination with another medication or a psychosocial intervention for the treatment of alcohol dependence.
Background
Alcohol dependence is a major public health problem characterised by recidivism and a host of medical and psychosocial complications. Together with psychosocial interventions, different pharmacological interventions have been tested in trials and systematic reviews. In this review, we wanted to discover whether anticonvulsants are better than placebo or are better than other medications, psychosocial interventions or no intervention.
Study characteristics
In October 2013, we used electronic medical databases to find all published and unpublished medical trials that compared anticonvulsants with placebo or other interventions. We also used other sources, such as conference proceedings, likely to contain trials relevant to the review. To be included in the review, medical trials had to have a randomised design and had to include adult participants (older than 18 years of age) with a diagnosis of alcohol dependence.
We identified 25 medical trials involving a total of 2641 participants. 80% of participants in these trials were male; mean age was 44 years. Most studies compared anticonvulsants versus placebo (17 studies), but some researchers compared anticonvulsants versus other medications (seven studies) or no medication (two studies). The mean duration of the trials was 17 weeks (range four to 52 weeks). Half of the trials took place in the USA, the other half in Spain, Brasil, Germany, Greece, Italy, India and Thailand. The anticonvulsant included in most of the trials was topiramate; other medications were gabapentin, valproate, levetiracetam, oxcarbazepine, zonisamide, carbamazepine, pregabalin and tiagabine. Included studies used 73 different rating instruments and differed in design, quality, characteristics of patients, tested medications, services provided and treatments delivered.
Key results
In 17 studies versus placebo, anticonvulsants were shown to be more effective than placebo in terms of number of drinks per drinking day and average heavy drinking. However, we found no clear evidence that anticonvulsants led to more participants abstaining from alcohol, fewer participants drinking heavily or fewer participants leaving treatment (dropouts). In terms of safety issues, the rate of withdrawal from treatment due to adverse effects was not lower or higher in participants treated with anticonvulsants than in those treated with placebo. Moreover, for two of 18 specific side effects (dizziness and paraesthesia), anticonvulsants were worse than placebo. Other major known side effects, such as those affecting cognitive functioning (attention, confusion, speech problems), were insufficiently explored by primary studies. For single medications, results were globally confirmed for topiramate and partially for gabapentin and valproate.
In the five studies in which anticonvulsants were compared with naltrexone, a medication considered efficacious for the treatment of alcohol dependence, anticonvulsants were associated with a lower number of heavy drinking days, with a higher number of days before a severe relapse occurred and with a lower rate of patient withdrawal for medical reasons. However, anticonvulsants were not more or less effective than naltrexone in affecting the rate of participants who showed severe relapse, who were not drinking during the trial or who left treatment (dropouts).
Quality of evidence
In looking at primary outcomes (dropouts, abstinence from alcohol during the trial, number of drinks per drinking day, heavy drinking, rate of patient withdrawal for medical reasons), the quality of the included studies was considered moderate. However, moving to subgroup analysis, as in the case of single types of medications, as well as to comparisons versus other medications, the finding of the review is limited by the small number of available studies.
Authors' conclusions
At the current stage of research, evidence supporting the clinical use of anticonvulsants to treat alcohol dependence is insufficient. Results are conditioned by heterogeneity and by the low number and quality of studies comparing anticonvulsants versus other medications. The uncertainty associated with these results leaves to clinicians the need to balance the possible benefits/risks of treatment with anticonvulsants versus other medications as supported by evidence of efficacy.
Summary of findings
Summary of findings for the main comparison. Anticonvulsants versus placebo for alcohol dependence.
| Anticonvulsants versus placebo for alcohol dependence | ||||||
| Patient or population: patients with alcohol dependence Settings: Intervention: anticonvulsants versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anticonvulsants versus placebo | |||||
| Dropouts Follow‐up: mean 12 weeks | Study population | RR 0.94 (0.74 to 1.19) | 1675 (16 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 329 per 1000 | 309 per 1000 (243 to 391) | |||||
| Moderate | ||||||
| 245 per 1000 | 230 per 1000 (181 to 292) | |||||
| Continuous abstinence Follow‐up: mean 15.5 weeks | Study population | RR 1.21 (0.97 to 1.52) | 634 (eight studies) | ⊕⊕⊕⊝ moderate2 | ||
| 299 per 1000 | 362 per 1000 (290 to 455) | |||||
| Moderate | ||||||
| 309 per 1000 | 374 per 1000 (300 to 470) | |||||
| Drinks/drinking day Follow‐up: mean 11.9 weeks | Mean drinks/drinking day in the intervention groups was 1.49 lower (2.32 to 0.65 lower) | 1126 (11 studies) | ⊕⊕⊕⊝ moderate3 | |||
| Heavy drinking Follow‐up: mean 11.2 weeks | Mean heavy drinking in the intervention groups was 0.35 standard deviations lower (0.51 to 0.19 lower) | 1129 (12 studies) | ⊕⊕⊕⊝ moderate4 | |||
| Adverse events—withdrawn for medical reasons Follow‐up: mean 16 weeks | Study population | RR 1.22 (0.58 to 2.56) | 1410 (12 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 52 per 1000 | 64 per 1000 (30 to 134) | |||||
| Moderate | ||||||
| 67 per 1000 | 82 per 1000 (39 to 172) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Heterogeneity. 2206 events. 3Six/11 studies with unclear allocation concealment and/or blinding or incomplete outcome data. 4Five/12 studies with unclear allocation concealment and/or blinding or incomplete outcome data.
Background
Description of the condition
Alcohol dependence is a major public health problem that is characterised by recidivism and a host of medical and psychosocial complications (Anderson 2006; APA 2000; Rehm 2011; WHO 2004). A wide and well‐documented range of consequences are associated with acute and chronic use of alcohol, such as medical, psychological and social problems, as well as crime, violence and neonatal drug exposure (Chase 2005; Mannelli 2007; Rehm 2011). Abuse of/dependence on alcohol has become a persistent health problem worldwide, and this significantly contributes to the global burden of disease (WHO 2011). According to recent population surveys, between 1% and 10% of the population, depending on the countries considered, is affected (lifetime prevalence) by alcohol dependence. One‐year prevalence of alcohol use disorders in people between 15 and 64 years of age is estimated at 5.2% in the American region, 5.5% in European countries and over 10% in Eastern European countries (Rehm 2009).
Description of the intervention
Together with psychosocial interventions, such as Alcoholics Anonymous, disulfiram treatment represents the most traditional intervention proposed for the treatment of alcohol abuse and dependence. In past decades, other pharmacological interventions, such as acamprosate, benzodiazepines, naltrexone, gamma‐hydroxybutyrate and anticonvulsants, have been developed and tested in trials and systematic reviews (Amato 2010; Rösner 2010; Rösner 2010 a; Leone 2010; Minozzi 2010). Among anticonvulsants, medications such as carbamazepine, topiramate and valproate have been evaluated in clinical trials for their efficacy in alcohol withdrawal, as well as for their effects on alcohol dependence, seen in outcomes such as retention in treatment, use of alcohol and time to relapse (Brady 2002; Johnson 2003; Johnson 2007; Mueller 1997; Salloum 2005).
How the intervention might work
The tendency toward alcohol addiction seems to depend on the effect of alcohol in increasing the availability of dopamine in specific areas of the brain mesolimbic system. This action, which is shared by other drugs, such as heroin, cocaine, cannabis and nicotine, has been involved in the rewarding effects of alcohol and self administration behaviour in animals and humans (DiChiara 1988; Drevets 2001; Volkow 2003). Anticonvulsants have been proposed in the treatment of alcohol dependence based on mechanisms of action such as strengthening of gamma‐aminobutyric acid (GABA)‐mediated neurotransmission (Czapinski 2005; Landmark 2007), inhibition of glutamate activity (achieved by antagonising the excitatory effects of glutamate receptors and suppressing glutamate release) and blocking of calcium channels, decreasing in this way alcohol‐induced dopamine release within the nucleus accumbens (Czapinski 2005; Kenna 2009; Landmark 2007). On the basis of these effects, anticonvulsant drugs may modulate the craving associated with alcoholism or may alter the subjective effects of alcohol (Koob 1997; Miranda 2008), thereby reducing the risk of relapse.
Why it is important to do this review
Although the efficacy of disulfiram, acamprosate, naltrexone and gamma‐hydroxybutyrate in treating alcohol dependence has been or is currently under investigation through Cochrane systematic reviews (Fox 2003; Rösner 2010; Rösner 2010 a; Leone 2010; Srisurapanont 2005), this is not the case with anticonvulsants. In fact, the only Cochrane review on anticonvulsants for the treatment of alcohol disorders refers to alcohol withdrawal, which represents only the first step in the treatment of addiction (Minozzi 2010). Therefore, despite the existence of neurobiological plausibility and clinical trials supporting their efficacy, anticonvulsants have not been evaluated by a systematic Cochrane review.
Objectives
The primary aim of the review is to assess the benefits/risks of anticonvulsants for the treatment of alcohol dependence.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and clinical controlled trials (CCTs).
Types of participants
Alcohol dependence diagnosed using standardised criteria (Diagnostic and Statistical Manual of Mental Disorders (DSM) or equivalent) (APA 2000) or by specialists. However, we had to also accept trials that did not employ explicit diagnostic criteria. According to the protocol, we had to examine the effects of including in the sensitivity analyses participants with uncertain diagnoses. Trials that included participants with additional diagnoses of substance abuse or dependence were also eligible. People with other co‐morbid mental health conditions had to be included and considered in the subgroup analysis.
People younger than 18 years of age and pregnant women were excluded because of the substantially different approach required for clinical management of these individuals.
Types of interventions
Experimental intervention
Anticonvulsant drugs alone, in combination with other drugs or in combination with any psychosocial intervention.
Control intervention
Placebo.
No intervention.
Other pharmacological interventions.
Any psychosocial intervention.
Furthermore, we had to consider different factors as confounders/moderators and take them into account in the analysis whenever possible.
Setting (inpatient or outpatient treatment).
Starting dose/rate and pattern of dose reduction.
Scheduled duration of treatment.
Severity of dependence (quantity and frequency of intake).
Use of drugs.
Health status.
Psychiatric co‐morbidity.
Other pharmacological treatment offered.
Other psychosocial treatment offered.
Social status.
Environmental conditions and interaction.
Number of previous treatment attempts and number of previous treatment outcomes.
Types of outcome measures
Primary outcomes
Efficacy: use of alcohol as number of participants who reported use during treatment and/or number of participants with positive breath alcohol analysis.
Safety: number and type of adverse events experienced during treatment.
Acceptability: dropouts from treatment as number of participants who did not complete treatment.
Results at follow‐up as number of participants using alcohol at follow‐up.
Secondary outcomes
Use of other substances as number of participants who reported use of other substances of abuse during treatment and/or number of participants with positive urine analyses for substances of abuse.
Craving as measured by validated scales (e.g. Brief Substance Craving Scale (BSCS), Visual Analog Scale (VAS), Obsessive‐Compulsive Drinking Scale (OCDS)).
Severity of dependence as measured by validated scales (e.g. Addiction Severity Index (ASI), Clinical Global Impression scale (CGI), Severity of Dependence Scale (SDS), Drinker Inventory of Consequences scale (DIC)).
Psychiatric symptoms/psychological distress diagnosed using standard criteria (e.g. Diagnostic and Statistical Manual of Mental Disorders (DSM)) or measured by validated scales (e.g. Hamilton Depression Rating Scale, Profile of Mood States Scale (POMSS), Positive and Negative Syndrome Scale (PANSS)).
Liver enzyme levels (alanine aminotransferase, aspartate aminotransferase, γ‐glutamyltransferase).
Search methods for identification of studies
The search incorporated several methods to identify completed or ongoing studies.
Electronic searches
Relevant trials were obtained from the following sources.
Cochrane Drugs and Alcohol Group Specialised Register (searched 29 October 2013).
Cochrane Central Register of Controlled Trials (CENTRAL; 2012, issue 8).
MEDLINE (PubMed) (from 1966 to October 2013).
EMBASE (embase.com) (1974 to October 2013).
CINAHL (EBSCO) (1982 to October 2013).
Databases were searched using a strategy developed by incorporating the filter for identification of RCTs (Lefebvre 2011) combined with selected MeSH terms and free‐text terms related to alcohol dependence. The search strategies for CENTRAL, PubMed, EMBASE and CINHAL can be found in Appendix 1; Appendix 2; Appendix 3; and Appendix 4, respectively.
We also searched some of the main electronic sources of ongoing trials.
Current Controlled Trials (www.controlled‐trials.com/).
Clinical Trials.gov (www.clinicaltrials.gov/).
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en).
Osservatorio Nazionale sulla Sperimentazione Clinica dei Medicinali (https://oss‐sper‐clin.agenziafarmaco.it/).
Trialsjournal.com.
Searching other resources
We searched the reference lists of all relevant papers to identify further studies, as well as conference proceedings likely to contain trials relevant to the review. We contacted investigators to ask for information about incomplete trials.
All searches included non–English language literature, and studies with English language abstracts were assessed for inclusion. When considered likely to meet inclusion criteria, studies had to be translated.
Data collection and analysis
Selection of studies
Two review authors (PPP, ET) inspected the search hits by reading titles and abstracts. Each potentially relevant study located in the search was obtained in full text and was assessed for inclusion independently by two review authors (PPP, ET). Doubts were resolved by discussion among all review authors.
Data extraction and management
Data were extracted independently by two review authors (ET, PPP). Information on methodology, participants (sociodemographic and clinical information relevant to the review aims) and interventions (medications and non‐pharmacological interventions), as well as primary and secondary outcomes, was collected. Any disagreement was discussed, and persisting disagreement had to be dealt with by a third review author, who acted as a mediator.
Assessment of risk of bias in included studies
Two review authors (ET, PPP) assessed study quality according to the criteria indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane review involves use of a two‐part tool to address five specific domains (namely, sequence generation, allocation concealment, blinding, incomplete outcome data and other issues). The first step in using this tool involves describing what was reported to have happened in the study. The second step in using the tool involves assigning a judgement related to the risk of bias for that entry: low risk; high risk; unclear risk. To make these judgements, we used the criteria indicated by the handbook adapted to the field of addiction.
The domains of sequence generation and allocation concealment (avoidance of selection bias) were addressed by the tool by a single entry for each study.
Blinding of participants and personnel was judged to interfere with both subjective and objective outcomes pertaining to the behaviour of participants (such as retention in treatment) and was addressed by a single entry for each study; blinding of outcome assessors was considered separately for objective outcomes (e.g. retention in treatment, use of substance of abuse as measured by breath or urine analysis) and subjective outcomes (e.g. craving, severity of dependence, psychiatric symptoms/psychological distress).
Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except for dropout from treatment, which very often is the primary outcome measure in trials on addiction. See Appendix 5 for a detailed description of the assessment of risk of bias in included studies.
Measures of treatment effect
Dichotomous outcomes (dropouts, use of alcohol, adverse events) were analysed by calculating the risk ratio (RR) for each trial, with uncertainty in each result expressed by 95% confidence intervals (CIs).
Continuous outcomes (alcohol use, craving, liver enzyme levels) were analysed by calculating the mean difference (MD) with 95% CI. Weighted mean differences and 95% CIs were calculated by comparing and pooling mean score differences from the end of treatment to baseline for each group. In case of missing data on the standard deviation (SD) of the changes, this measure was imputed by using the SD at the end of treatment for each group.
When all studies assessed the same outcome but measured it using different scales, the standardised mean difference had to be applied as a summary statistic to standardise the results to a uniform scale (according to suggested procedures of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
We did not have to use data presented as number of positive urine tests or alcohol breath tests over the total number of tests in experimental and control groups as measures of substance use. This decision was made because using number of tests instead of number of participants as the unit of analysis violates the hypothesis of independence among observations. In fact, the results of tests done for each participant are not independent.
Meta‐analysis was performed only when results from at least two studies were available.
Unit of analysis issues
If all arms of a multi‐arm trial had to be included in the meta‐analysis and one arm had to be included in more than one of the treatment comparisons, we divided the number of events and the number of participants in that arm by the number of comparisons made. Such methods avoid the multiple use of participants in the pooled estimate of treatment effect while retaining information from each arm of the trial. The precision of the pooled estimate results was slightly compromised.
Dealing with missing data
Information on missing data was collected from the studies and was requested of the original investigators. In the absence of supplemental data from the study authors, when needed measures were available from primary studies, and missing data were obtained from available values according to suggested procedures of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When the assumption that data are missing at random was supported by available information, only available data had to be analysed; if this was not the case, other approaches, such as the last observation carried forward option or the assumption that missing data correspond to poor outcomes, had to be pursued. To assess how results are sensitive to changes made in the assumptions, sensitivity analysis had to be performed. Moreover, the potential impact of missing data on the findings of the review had to be addressed in the Discussion section.
Assessment of heterogeneity
The presence of heterogeneity between trials was tested by using the I‐squared (I2) statistic. A P value of the Chi2 test less than 0.05 indicates significant heterogeneity.
Assessment of reporting biases
A funnel plot (plot of the effect estimate from each study against the sample size or the effect standard error) had to be used to assess the potential for bias related to the size of the trials.
Data synthesis
Outcomes from individual trials, when possible, were combined through meta‐analysis (comparability of intervention and outcomes between trials). Given the expected heterogeneity of results among studies due to differences in populations and in types of interventions, we planned to use the random‐effects model.
Subgroup analysis and investigation of heterogeneity
Analysis of subgroups was performed according to type of anticonvulsant, length of the trial, associated interventions and psychiatric co‐morbidity.
Sensitivity analysis
To incorporate assessment into the review process, we planned to first plot intervention effect estimates stratified for risk of bias for each relevant domain. If differences in results among studies were identified at different risks of bias, we planned to perform sensitivity analysis by excluding from the analysis studies with high risk of bias.
Results
Description of studies
For substantive descriptions of studies, see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
We identified 2727 records from the literature search. After we removed duplicates, 2092 records remained, including 22 ongoing trials and six unpublished studies presented at conferences. 2055 were excluded on the basis of title and abstract; 37 articles were retrieved for more detailed evaluation, eight of which were excluded after the full text was read. The other 22 ongoing trials and the six unpublished studies had insufficient information to be included in the analysis. Moreover, multiple reports referred to the same study (three reports for Johnson 2003, two for Johnson 2007 and two for Martinotti 2007). Therefore, 25 studies satisfied all criteria for inclusion in the review. See Figure 1.
1.
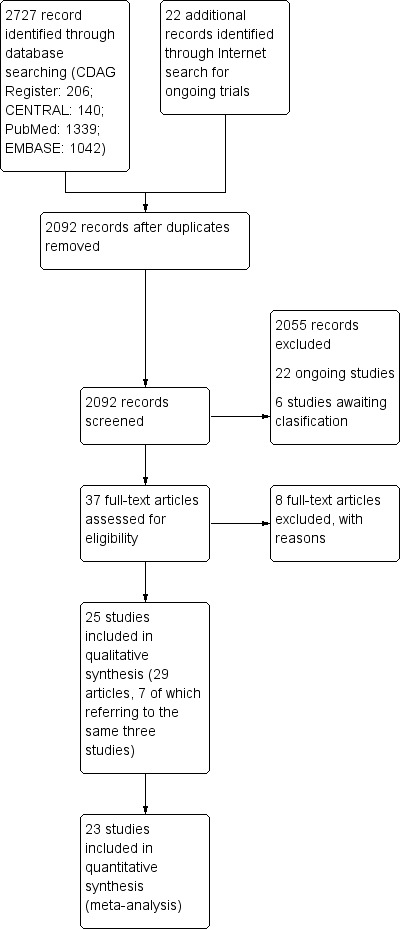
Study flow diagram.
Included studies
25 studies with 2641 participants met the inclusion criteria for this review (for details, see Characteristics of included studies).
23 studies were included in the quantitative synthesis.
Duration of trials
The mean duration of the trials was 17 weeks (range four to 52 weeks). The median duration was 12 weeks.
Treatment regimens and setting
The anticonvulsants studied in the included studies were topiramate (10 trials), gabapentin (five trials), valproate (three trials), levetiracetam and oxcarbazepine (two trials each), zonisamide, carbamazepine, pregabalin and tiagabine (one trial each). For more information, see Appendix 6.
In four trials, participants were concomitantly treated for alcohol withdrawal with hydroxyzine (Anton 2009) or benzodiazepines (Paparrigopoulos 2010; Paparrigopoulos 2011; Trevisan 2008). In one trial, participants were concomitantly treated with lithium for bipolar mood disorder (Salloum 2005). In another trial, participants were concomitantly treated with naltrexone (Anton 2011). In another trial, participants randomly assigned to the anticonvulsant gabapentin also received flumazenil on days one and two (Anton 2009).
21 trials were conducted with outpatients at the community level or at mental health centres. In four trials, participants were hospitalised at the beginning of the study.
12 trials were conducted in the USA, three in Spain, two each in Brasil, Germany, Greece and Italy, one in India and one in Thailand.
Psychosocial treatments concomitantly given with antidepressants were:
motivational, cognitive‐behavioural or relapse prevention psychotherapy (Anton 2009; Anton 2011; Baltieri 2008; Brady 2002; Brower 2008; Florez 2008; Florez 2011; Kampman 2013; Likhitsathian 2013; Paparrigopoulos 2010; Paparrigopoulos 2011);
supportive psychotherapy (De Sousa 2008; Rubio 2009); and
counselling, self help and/or compliance enhancement interventions (Arias 2010; Fertig 2012;Furieri 2007; Johnson 2003; Johnson 2007;Martinotti 2007; Martinotti 2010; Salloum 2005);
Croissant 2006; Mueller 1997;Richter 2012; and Trevisan 2008 did not specify the availability of psychotherapy.
Rating instruments utilised in the included studies
The 25 included studies utilised 73 different rating instruments; to see a list of them, see Appendix 7.
Outcomes
For some outcomes reported in the included studies, it was difficult to make comparisons and to pool results because of the different ways used to measure them, the cutoff chosen and the availability of data from the study or from the primary investigator. This was particularly the case with the use of alcohol and with alcohol abstinence, which were expressed in various ways (drinking days, drinks per drinking day, heavy drinking days, days abstinent, continuous abstinence, time to first drinking, time to heavy drinking) using dichotomous or continuous measures. The cutoff chosen for the definition of heavy drinking was also different, with most studies adopting a cutoff value of five (four) standard drink units for men (women), while others considered six (five) drinks as a critical threshold value for heavy drinking in men (women) or five or more drinks for both men and women, or used a weight measure (such as 60 (48) grams of alcohol per day for men (women) or more than 90 grams per week). Moreover, some studies additionally assigned participants to the heavy drinking category if they presented to any study visit with a blood alcohol level > 0.08%, or if drinking occurred on five or more days a week.In light of the heterogeneity of measures adopted, comparisons versus placebo on heavy drinking as continuous outcomes were carried out using the standardised mean difference as the measure of effect (12 studies); for comparisons versus naltrexone, the number of heavy drinking days was used (three studies). In looking at other measures of alcohol use, the number of drinks per drinking day was available from 13 studies; the percentage of participants not drinking during the trial, indicated as "continuous abstinence", was available from 14 studies; the mean percentage of days of abstinence from alcohol was available from 11 studies; and the time to first relapse, expressed in weeks, was available from 11 studies. In terms of the other primary outcomes, the dropout rate was available from 22 studies; adverse events, expressed as withdrawal for medical reasons, were available from 17 studies. Regarding secondary outcomes, measures of craving were available from 13 studies; given the heterogeneity of these measures, in trials versus placebo this outcome was evaluated as standardised mean difference. Liver enzyme levels (GGT) were available from 10 studies. To see a list of outcomes, see Appendix 8.
Participants
2641 alcohol dependents according to DSM‐III‐R, DSM‐IV or ICD‐10 criteria. 80% were males; mean age was 44 years.
Comparisons
Anticonvulsants versus placebo: 17 studies, 1765 participants.
-
Specific anticonvulsants versus placebo.
Topiramate versus placebo: six studies, 979 participants.
Gabapentin versus placebo: five studies, 269 participants.
Valproate versus placebo: three studies, 126 participants.
Levetiracetam versus placebo: two studies, 331 participants.
-
Anticonvulsants versus placebo according to length of trial.
Up to six weeks: four studies, 198 participants.
Over six weeks: 12 studies, 1477 participants.
-
Anticonvulsants versus placebo according to psychosocial interventions.
Associate psychotherapy: seven studies, 578 participants.
Associated other interventions: six studies, 810 participants.
-
Anticonvulsants versus placebo according to psychiatric comorbidity.
Excluding psychiatric comorbidity: eight studies, 1203 participants.
Not excluding psychiatric comorbidity: eight studies, 472 participants.
-
Anticonvulsants versus other medications: seven studies, 658 participants.
Anticonvulsants versus naltrexone: five studies, 528 participants.
Anticonvulsants versus disulfiram: one study, 100 participants.
Anticonvulsants versus acamprosate: one study, 30 participants.
-
Specific anticonvulsants versus other specific medications: seven studies, 658 participants.
Topiramate versus naltrexone: three studies, 385 participants.
Topiramate versus disulfiram: one study, 100 participants.
Oxacarbazepine versus acamprosate: one study, 30 participants.
Oxacarbazepine versus naltrexone: one study, 84 participants.
Pregabalin versus naltrexone: one study, 59 participants.
-
Anticonvulsants versus no medication: two studies, 210 participants.
Tiagabine versus no pharmacological treatment: one study, 120 participants.
Topiramate versus no pharmacological treatment: one study, 90 participants.
-
Anticonvulsants versus anticonvulsants.
Valproic acid versus gabapentin: one study, 38 participants.
Excluded studies
Eight studies did not meet the criteria for inclusion in this review. The grounds for exclusion were study design not in the inclusion criteria: five studies (Karam‐Hage 2003; Knapp 2010; Le Strat 2012; Narayana 2008; Schacht 2013); study population not in the inclusion criteria: two studies (Miranda 2008; Mitchell 2012) and outcome measures not in the inclusion criteria: three studies (Miranda 2008; Myrick 2007; Schacht 2013). See Characteristics of excluded studies.
Risk of bias in included studies
All studies were randomised controlled trials.
Allocation
The random sequence generation was judged adequate (low risk of bias) in 17 studies and inadequate in one study. In the other remaining studies, details provided did not allow specific evaluation of this criterion. Allocation concealment was judged adequate in 12 studies and inadequate in seven studies. For the other six studies, details provided did not allow a specific evaluation of the procedures adopted to prevent participants and investigators from foreseeing assignment.
Blinding
Knowledge of allocated interventions during the study by participants and personnel was judged to be adequately prevented in 15 studies. Seven studies were unblinded and were judged as inadequate (high risk of bias) for potential interference with the willingness of staying in treatment or drinking. The remaining studies did not provide sufficient information to allow a specific evaluation of this criterion. Blinding of outcome assessment for subjective outcomes was judged adequate in 14 studies and inadequate in seven studies. The remaining studies did not provide sufficient information to allow a specific evaluation of this criterion. Blinding of outcome assessment for objective outcomes was judged adequate in all 25 studies.
Incomplete outcome data
In 19 studies, missing data on participants were considered by using appropriate methods (low risk of bias); in three studies, this issue was not appropriately addressed (high risk of bias); in all other studies, provided information did not allow a specific evaluation of incomplete outcome data addressed (unclear risk).
Other potential sources of bias
No other potential threats to validity were detected.
See Figure 2 and Figure 3 for a summary of these results.
2.
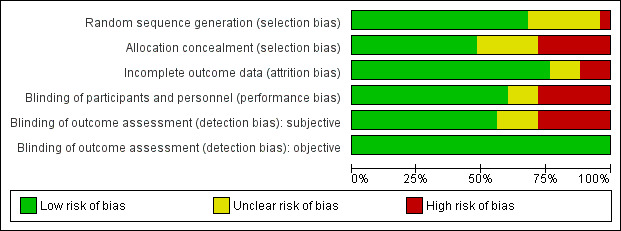
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
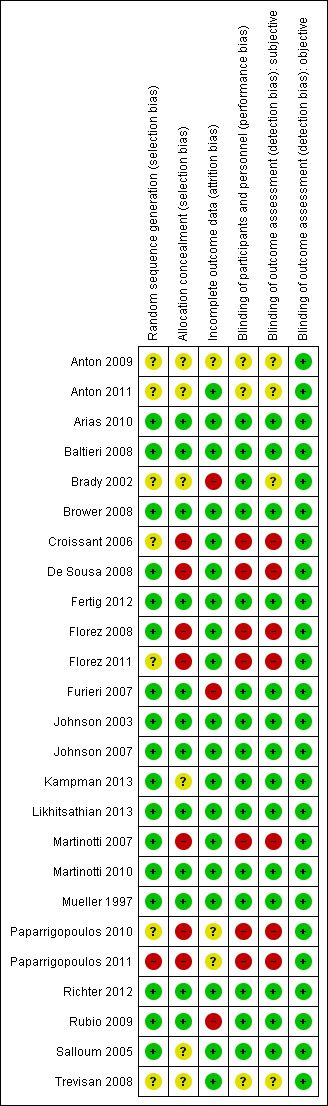
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
1. Anticonvulsants versus placebo
1.1 Dropouts
16 studies (Anton 2009; Arias 2010; Baltieri 2008; Brady 2002; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Mueller 1997; Richter 2012; Rubio 2009; Salloum 2005; Trevisan 2008), 1675 participants, RR 0.94 (95% Cl 0.74 to 1.19); the difference was not statistically significant, and substantial heterogeneity was found (Chi² = 38.35). No studies with high risk of bias were found for this outcome (see Figure 4 or Analysis 1.1).
4.
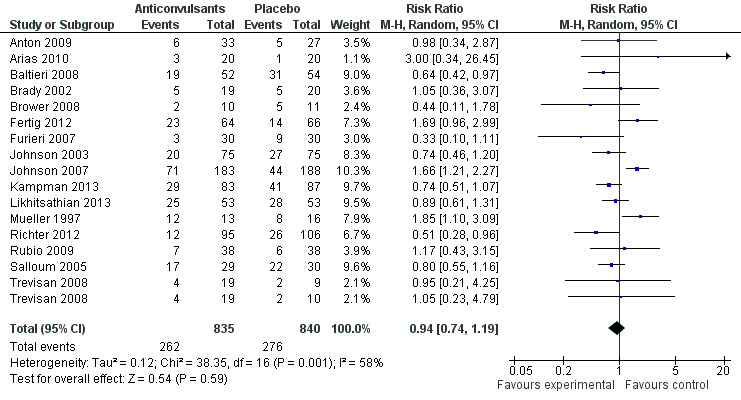
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.1 Dropouts.
1.1. Analysis.
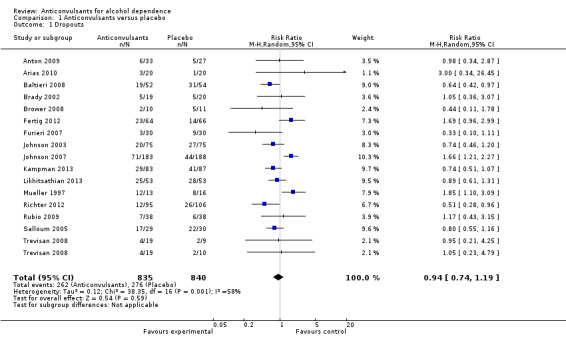
Comparison 1 Anticonvulsants versus placebo, Outcome 1 Dropouts.
1.2 Heavy drinking, dichotomous outcome
Five studies (Brady 2002; Brower 2008; Mueller 1997; Richter 2012; Salloum 2005), 330 participants, RR 0.84 (95% CI 0.57 to 1.22); the difference was not statistically significant, and high heterogeneity was found (Chi² = 9.88). When studies with high risk of bias were excluded, four studies remained (Brower 2008; Mueller 1997; Richter 2012; Salloum 2005), 301 participants, RR 0.88 (95% CI 0.58 to 1.34); the result remained not statistically significant, and again substantial heterogeneity was found (Chi² = 8.48) (see Analysis 1.2).
1.2. Analysis.
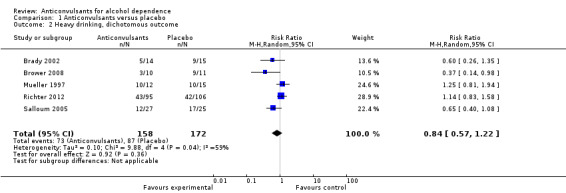
Comparison 1 Anticonvulsants versus placebo, Outcome 2 Heavy drinking, dichotomous outcome.
1.3 Alcohol use, continuous outcomes: drinks/drinking days
11 studies (Anton 2011; Brady 2002; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009; Salloum 2005), 1126 participants, MD ‐1.49 (95% CI ‐2.32 to ‐0.65); the difference was statistically significant in favour of anticonvulsants. When studies with high risk of bias were excluded, eight studies remained (Anton 2011; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Salloum 2005), 991 participants, MD ‐1.33 (95% CI ‐2.37 to ‐0.29); the result did not change (see Figure 5 or Analysis 1.3.).
5.
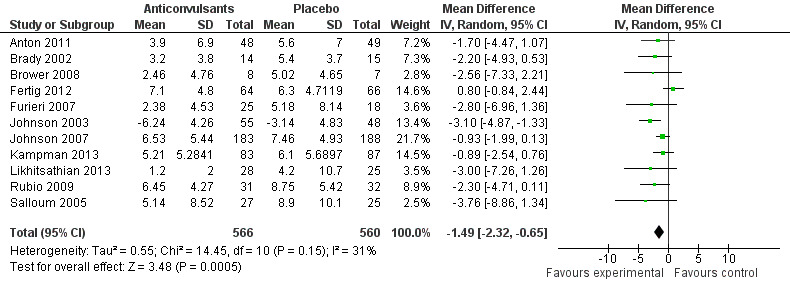
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.3 Alcohol use, continuous outcome: drinks/drinking day.
1.3. Analysis.
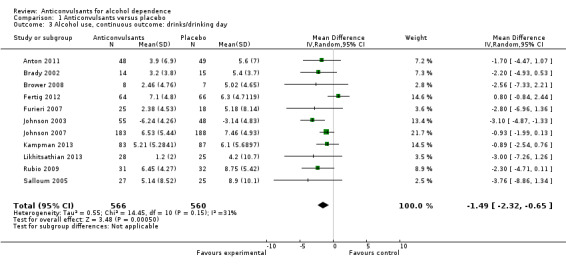
Comparison 1 Anticonvulsants versus placebo, Outcome 3 Alcohol use, continuous outcome: drinks/drinking day.
1.4 Alcohol use, continuous outcomes: heavy drinking
Because heavy drinking was expressed in different ways (percentage of heavy drinking days; percentage of heavy drinking days per week; mean heavy drinking weeks, etc.) in this analysis, the standardised mean difference was applied.
12 studies (Anton 2011; Arias 2010; Baltieri 2008; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Likhitsathian 2013; Rubio 2009; Salloum 2005; Trevisan 2008), 1129 participants, SMD ‐0.35 (95% CI ‐0.51 to ‐0.19); the difference was statistically significant in favour of anticonvulsants. When studies with high risk of bias were excluded, 10 studies remained (Anton 2011; Arias 2010; Baltieri 2008; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Likhitsathian 2013; Salloum 2005; Trevisan 2008), 1023 participants, SMD ‐0.30 (95% CI ‐0.47 to ‐0.13); the result did not change.
See Figure 6 or Analysis 1.4
6.
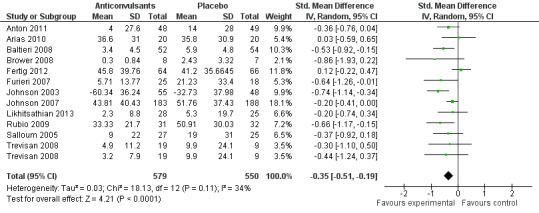
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.4 Alcohol use, continuous outcome: heavy drinking.
1.4. Analysis.
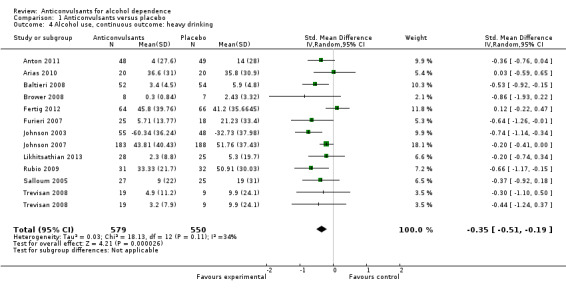
Comparison 1 Anticonvulsants versus placebo, Outcome 4 Alcohol use, continuous outcome: heavy drinking.
1.5 Continuous abstinence, dichotomous outcomes
Data regarding the numbers of participants not drinking during the trial were available from eight studies (Anton 2009; Baltieri 2008; Brady 2002; Brower 2008; Fertig 2012; Furieri 2007; Mueller 1997; Richter 2012), 634 participants, RR 1.21 (95% CI 0.97 to 1.52); the result was not statistically significant. When studies with high risk of bias were excluded, six studies remained (Anton 2009; Baltieri 2008; Brower 2008; Fertig 2012; Mueller 1997; Richter 2012), 545 participants, RR 1.15 (95% CI 0.86 to 1.53); the difference was not statistically significant (see Figure 7 or Analysis 1.5).
7.
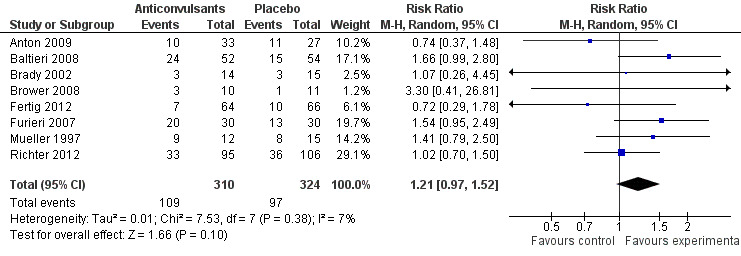
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.5 Continuous abstinence, dichotomous outcome.
1.5. Analysis.
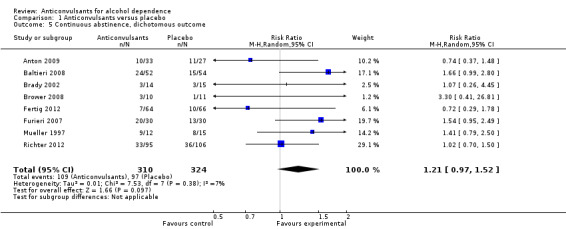
Comparison 1 Anticonvulsants versus placebo, Outcome 5 Continuous abstinence, dichotomous outcome.
1.6 Abstinence, continuous outcomes
1.6.1 Time to first relapse (weeks)
Four studies (Baltieri 2008; Richter 2012; Salloum 2005; Trevisan 2008), 415 participants, MD 0.83 (95% CI ‐0.28 to 1.95); the difference was not statistically significant.
1.6.2 Days abstinent (%)
Eight studies (Anton 2009; Arias 2010; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Rubio 2009), 814 participants, MD 8.47 (95% CI 1.57 to 15.38); the difference was statistically significant in favour of anticonvulsants, and statistically significant heterogeneity was found (Chi² = 15.69). When studies with high risk of bias were excluded, six studies remained (Anton 2009; Arias 2010; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007), 708 participants, MD 6.23 (95% CI ‐1.79 to 14.25); the result was not statistically significant, and substantial heterogeneity was shown (Chi² = 12.98).
For both, see Figure 8 or Analysis 1.6.
8.
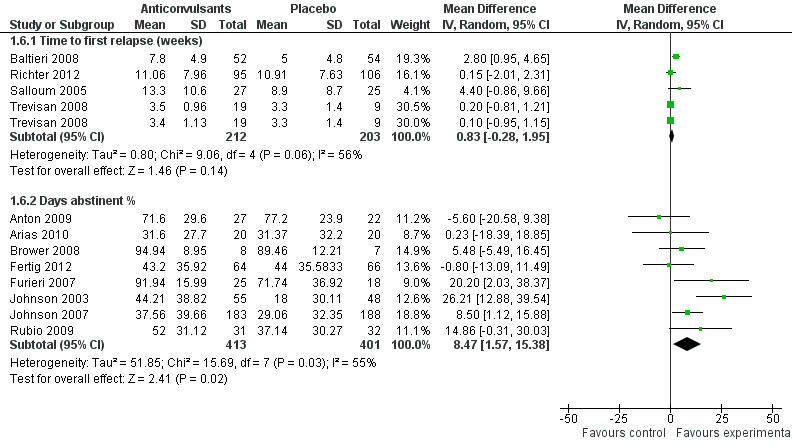
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.6 Abstinence, continuous outcome.
1.6. Analysis.
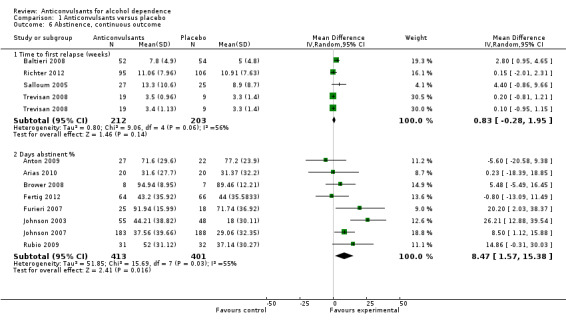
Comparison 1 Anticonvulsants versus placebo, Outcome 6 Abstinence, continuous outcome.
1.7 Adverse events
1.7.1 Withdrawal for medical reasons
12 studies (Arias 2010; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Mueller 1997; Richter 2012; Rubio 2009; Salloum 2005; Trevisan 2008), 1410 participants, RR 1.22 (95% CI 0.58 to 2.56); the difference was not statistically significant, and moderate and statistically significant results for heterogeneity were shown (Chi² = 23.55). No studies with high risk of bias were present for this outcome.
1.7.2 Other adverse events
Nine studies (Baltieri 2008; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Salloum 2005), 1164 participants, considered several single different adverse events. Of 18 adverse event outcomes including more than one study, 16 showed no statistically significant difference between anticonvulsants and placebo, and for two adverse events, the result was statistically significant in favour of placebo.
Dizziness: six studies (Baltieri 2008; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Likhitsathian 2013), 882 participants, RR 1.98 (95% CI 1.28 to 3.06).
Paraesthesia: seven studies (Baltieri 2008; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013), 1052 participants, RR 2.67 (95% CI 1.53 to 4.65).
When studies with high risk of bias were excluded, eight studies remained (Baltieri 2008; Brower 2008; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Salloum 2005), 1104 participants; the result did not change.
For all, see Figure 9 or Analysis 1.7.
9.
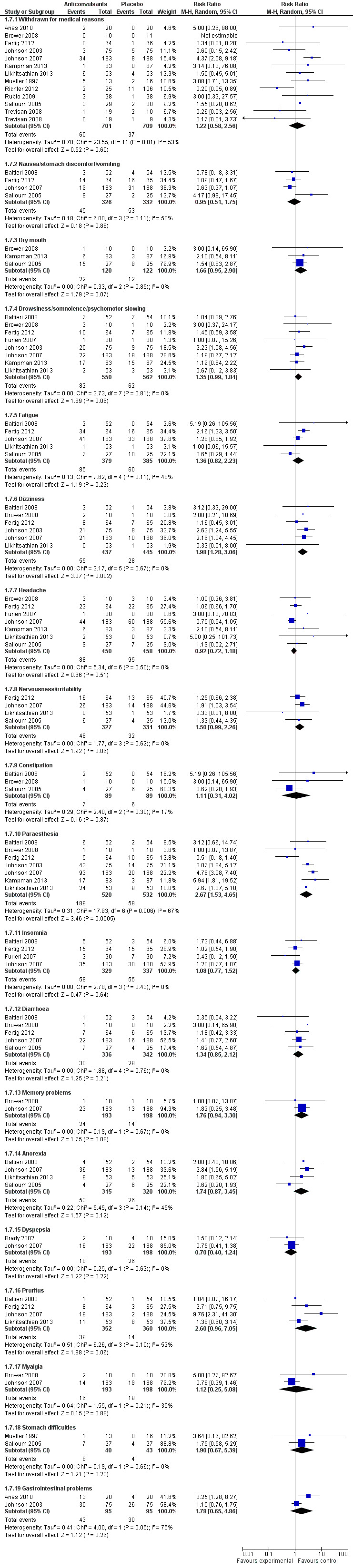
Forest plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.7 Adverse events.
1.7. Analysis.
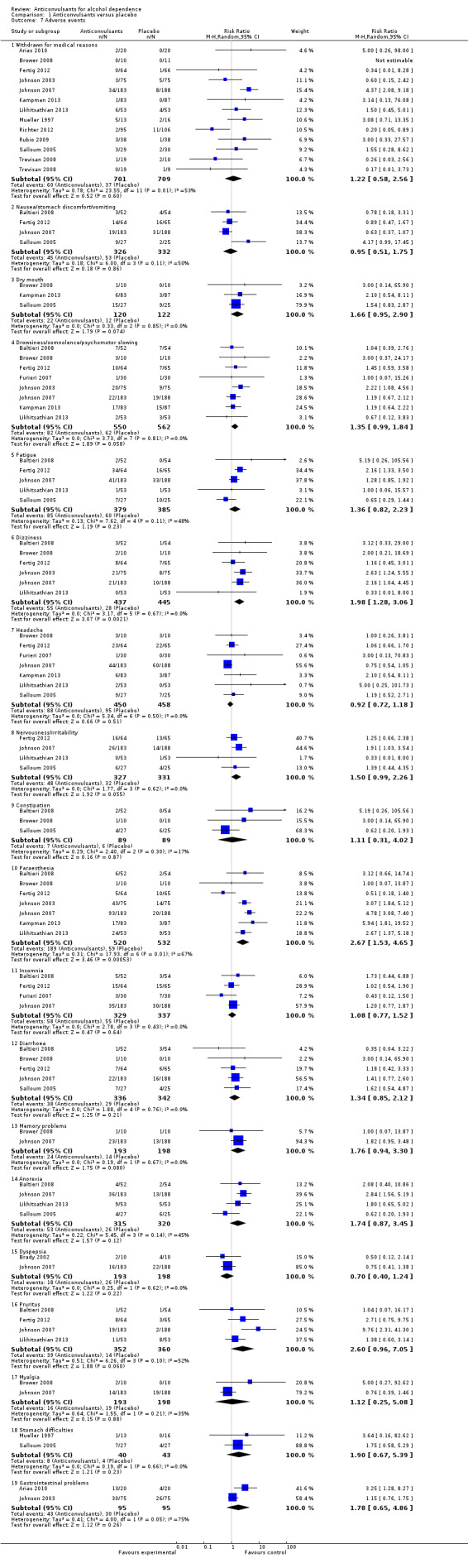
Comparison 1 Anticonvulsants versus placebo, Outcome 7 Adverse events.
It was not possible to investigate other known adverse effects of antiepileptics, such as cognitive dysfunction, because of the lack of data from primary studies.
1.8 Craving
Craving was measured with OCDS or other analogue scales; therefore in this analysis, the standardised mean difference was applied.
Six studies (Baltieri 2008; Brady 2002; Furieri 2007; Johnson 2007; Rubio 2009; Trevisan 2008), 553 participants, SMD ‐0.35 (95% CI ‐0.60 to ‐0.09); the difference was statistically significant in favour of anticonvulsants. When studies with high risk of bias were excluded, three studies remained (Baltieri 2008; Johnson 2007; Trevisan 2008), 418 participants, SMD ‐0.20 (95% CI ‐0.59 to 0.19); the result was not statistically significant, and substantial heterogeneity was found (Chi² = 7.82) (see Analysis 1.8).
1.8. Analysis.
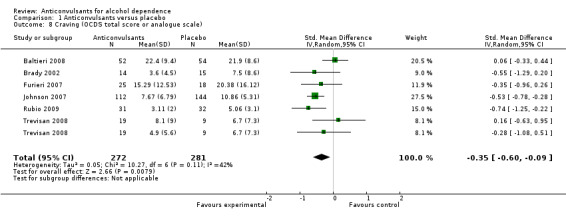
Comparison 1 Anticonvulsants versus placebo, Outcome 8 Craving (OCDS total score or analogue scale).
1.9 Liver enzyme levels
Results for enzyme levels (γ‐glutamyltransferase (GGT)), seven studies, 405 participants, MD 0.07 (95% CI ‐8.51 to 8.65); no statistically significant differences shown (see Analysis 1.9).
1.9. Analysis.
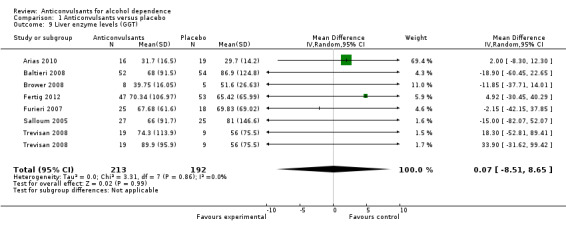
Comparison 1 Anticonvulsants versus placebo, Outcome 9 Liver enzyme levels (GGT).
1.10 Mood
Regarding mood, the heterogeneity of instruments used and lack of available data did not allow us to pool results together. Singular studies revealed the following.
Johnson 2007, using the Profile of Mood States (POMS), 256 participants; imputing the baseline value for all dropouts and calculating the mean difference in treatment effect between topiramate and placebo on the basis of least‐squares mean estimators, while improvement with time was observed, did not reveal evidence of differences between groups (MD ‐0.58 (95% CI ‐3.31 to 2.15)).
Mueller 1997, 29 participants, using MANOVA on Global Assessment of Functioning score, Beck's Depression Inventory, Spielberger State Anxiety Score and positive and negative subscales of the Revised Profile of Mood States; significant time effects seen, with improvement in scores over follow‐up times, but no significant treatment group effect or time by treatment group interaction.
Salloum 2005, 59 participants, considered mood bipolar disorder among inclusion criteria. In this study, overall, average Bech‐Rafaelsen Mania Scale scores for mania decreased by approximately 60% during double‐blind therapy, with final scores of 5.6 (SD 7.7) and 6.1 (SD 7.8) for the valproate and placebo groups, respectively. Depressive symptom levels, however, remained at relatively high levels for both groups, and final mean Hamilton Depression Rating Scale‐25 scores were 16.3 (SD 10.2) and 14.4 (SD 9.7) for the valproate and placebo groups, respectively.
Trevisan 2008, 57 participants, depression Profile of Mood States subscale score with random‐effects regression models showed no evidence of differences between gabapentin or divalproex and placebo, although an effect of time on this measure was evident.
Fertig 2012, 130 participants, scores of MADRS (3.2 leviteracetam group vs 3.8 placebo group), HARS (2.2 leviteracetam group vs 2.9 placebo group) and POMS (53.6 leviteracetam group vs 59.1 placebo group) and S12‐mental aggregate score (51.0 leviteracetam group vs 48.7 placebo group) revealed no statistically significant differences between the two groups.
2. Specific anticonvulsants versus placebo
2.1 Dropouts
2.1.1 Topiramate
Six studies (Baltieri 2008; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009), 979 participants, RR 0.91 (95% CI 0.65 to 1.28); the difference was not statistically significant.
2.1.2 Gabapentin
Four studies (Anton 2009; Brower 2008; Furieri 2007; Trevisan 2008), 169 participants, RR 0.62 (95% CI 0.33 to 1.16); the difference was not statistically significant.
2.1.3 Valproate
Three studies (Brady 2002; Salloum 2005; Trevisan 2008), 126 participants, RR 0.83 (95% CI 0.59 to 1.17); the difference was not statistically significant.
2.1.4 Levetiracetam
Two studies (Fertig 2012; Richter 2012), 331 participants, RR 0.94 (95% CI 0.29 to 3.04); the difference was not statistically significant.
For all, see Analysis 2.1
2.1. Analysis.
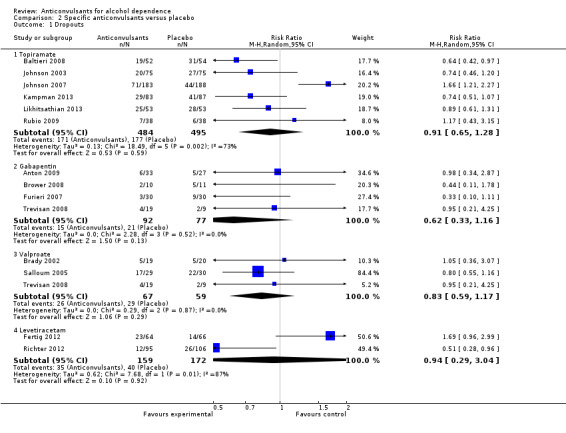
Comparison 2 Specific anticonvulsants versus placebo, Outcome 1 Dropouts.
2.2 Heavy drinking, dichotomous outcomes: valproate
Two studies (Brady 2002; Salloum 2005), 81 participants, RR 0.64 (95% CI 0.42 to 0.98); the difference was statistically significant in favour of valproate (see Analysis 2.2).
2.2. Analysis.
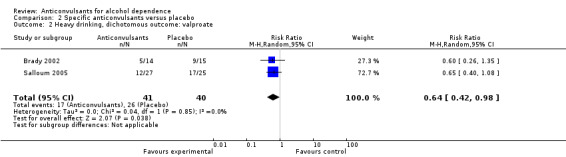
Comparison 2 Specific anticonvulsants versus placebo, Outcome 2 Heavy drinking, dichotomous outcome: valproate.
2.3 Alcohol use, continuous outcomes: drinks/drinking days
2.3.1 Topiramate
Five studies (Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009), 760 participants, MD ‐1.55 (95% CI ‐2.56 to ‐0.53); the difference was statistically significant in favour of topiramate. When studies with high risk of bias were excluded, four studies remained (Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013), 697 participants, MD ‐1.61 (95% CI ‐2.77 to ‐0.44); the difference remained statistically significant.
2.3.2 Gabapentin
Three studies (Anton 2011; Brower 2008; Furieri 2007), 155 participants, MD ‐2.14 (95% CI ‐4.21 to ‐0.06); the difference was statistically significant in favour of gabapentin. When studies with high risk of bias were excluded, two studies remained (Anton 2009; Brower 2008), 112 participants, MD ‐1.92 (95% CI ‐4.31 to 0.48); the difference became not statistically significant.
2.3.3 Valproate
Two studies (Brady 2002; Salloum 2005), 81 participants, MD ‐2.55 (95% CI ‐4.96 to ‐0.14); the difference was statistically significant in favour of valproate.
For all, see Analysis 2.3.
2.3. Analysis.
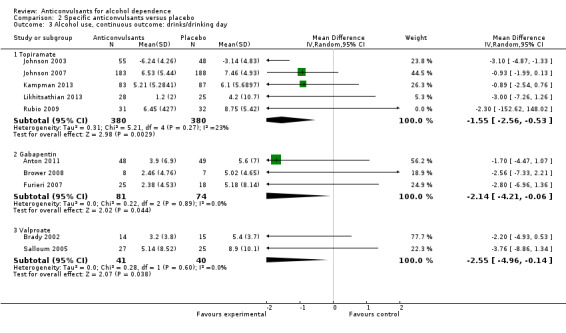
Comparison 2 Specific anticonvulsants versus placebo, Outcome 3 Alcohol use, continuous outcome: drinks/drinking day.
2.4 Alcohol use, continuous outcomes: heavy drinking
2.4.1 Topiramate
Five studies (Baltieri 2008; Johnson 2003; Johnson 2007; Likhitsathian 2013; Rubio 2009), 696 participants, SMD ‐0.44 (95% CI ‐0.69 to ‐0.20); the difference was statistically significant in favour of topiramate. When studies with high risk of bias were excluded, four studies remained (Baltieri 2008; Johnson 2003; Johnson 2007; Likhitsathian 2013), 633 participants; the difference remained statistically significant.
2.4.2 Gabapentin
Four studies (Anton 2011; Brower 2008; Furieri 2007; Trevisan 2008), 183 participants, SMD ‐0.45 (95% CI ‐0.75 to ‐0.15); the difference was statistically significant in favour of gabapentin. When studies with high risk of bias were excluded, three studies remained (Anton 2009; Brower 2008; Trevisan 2008), 140 participants, SMD ‐0.40 (95% CI ‐0.74 to ‐0.06); the result did not change.
2.4.3 Valproate
Two studies (Salloum 2005; Trevisan 2008), 80 participants, SMD ‐0.39 (95% CI ‐0.84 to 0.06); the difference was not statistically significant.
For all, see Analysis 2.4.
2.4. Analysis.
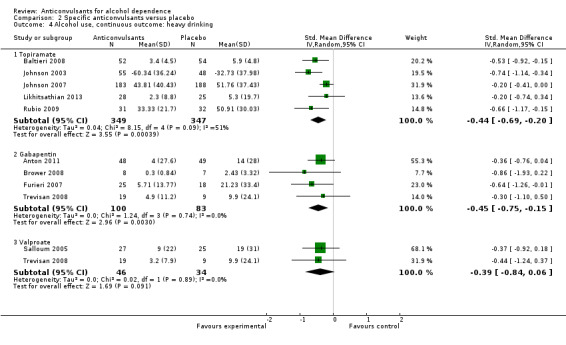
Comparison 2 Specific anticonvulsants versus placebo, Outcome 4 Alcohol use, continuous outcome: heavy drinking.
2.5 Continuous abstinence, dichotomous outcomes
2.5.1 Gabapentin
Two studies (Anton 2009; Brower 2008), 81 participants, RR 1.12 (95% CI 0.30 to 4.24); the difference was not statistically significant.
2.5.2 Levetiracetam
Two studies (Fertig 2012; Richter 2012), 331 participants, RR 0.97 (95% CI 0.68 to 1.38); the difference was not statistically significant.
For both, see Analysis 2.6.
2.6. Analysis.
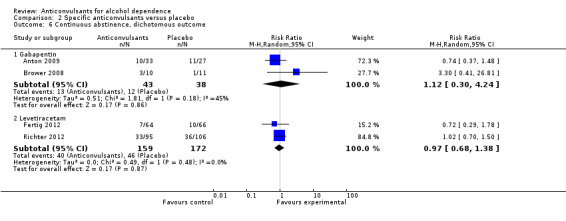
Comparison 2 Specific anticonvulsants versus placebo, Outcome 6 Continuous abstinence, dichotomous outcome.
2.6 Abstinence, continuous outcomes
2.6.1 Time to first relapse (weeks): valproate
Two studies (Salloum 2005; Trevisan 2008), 80 participants, MD 1.45 (95% CI ‐2.46 to 5.36); the difference was not statistically significant.
2.6.2 Days abstinent (%): topiramate
Three studies (Johnson 2003; Johnson 2007; Rubio 2009), 537 participants, MD 15.51 (95% CI 4.55 to 26.47); the difference was statistically significant in favour of topiramate. When studies with high risk of bias were excluded, two studies remained (Johnson 2003; Johnson 2007), 474 participants, MD 16.45 (95% CI ‐0.82 to 33.71); the difference was no longer statistically significant.
2.6.3 Days abstinent (%): gabapentin
Three studies (Anton 2009; Brower 2008; Furieri 2007), 107 participants, MD 5.82 (95% CI ‐6.87 to 18.51); the difference was not statistically significant. When studies with high risk of bias were excluded, two studies remained (Anton 2009; Brower 2008), 64 participants, MD 1.16 (95% CI ‐9.43 to 11.75); the result did not change.
For all, see Analysis 2.5.
2.5. Analysis.
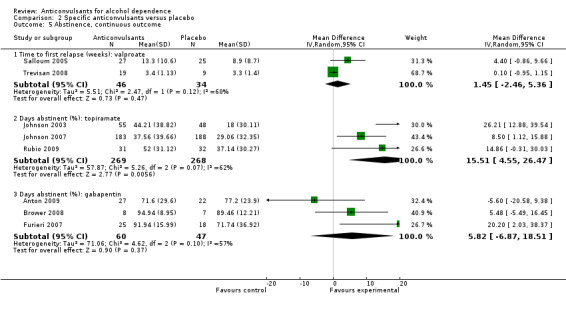
Comparison 2 Specific anticonvulsants versus placebo, Outcome 5 Abstinence, continuous outcome.
2.7 Adverse events
2.7.1 Withdrawn for medical reasons: topiramate
Five studies (Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009), 873 participants, RR 2.06 (95% CI 0.88 to 4.80); the difference was not statistically significant.
2.7.2 Withdrawn for medical reasons: valproate
Two studies (Salloum 2005; Trevisan 2008), 87 participants, RR 0.75 (95% CI 0.10 to 5.87); the difference was not statistically significant.
2.7.3 Withdrawn for medical reasons: levetiracetam
Two studies (Fertig 2012; Richter 2012), 331 participants, RR 0.22 (95% CI 0.06 to 0.85); the difference was statistically significant in favour of levetiracetam.
For all, see Analysis 2.7.
2.7. Analysis.
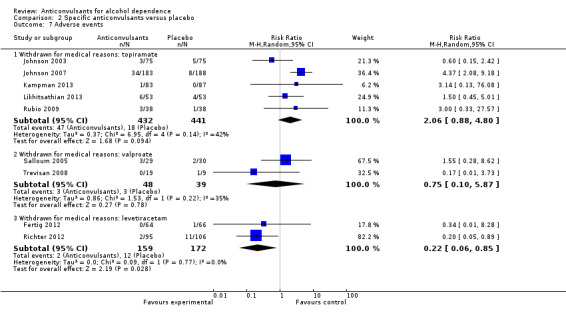
Comparison 2 Specific anticonvulsants versus placebo, Outcome 7 Adverse events.
2.7.4 Other adverse events: topiramate
Five studies (Baltieri 2008; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013), 903 participants, considered single different adverse events. Three of 11 comparisons showed evidence of differences in favour of placebo.
Dizziness: RR 2.29 (95% CI 1.39 to 3.78).
Paraesthesia: RR 3.74 (95% CI 2.82 to 4.97).
Anorexia: RR 2.49 (95% CI 1.52 to 4.08).
Other adverse events: gabapentin
Two studies (Brower 2008; Furieri 2007), 80 participants; two different adverse events reported (drowsiness and headache), and the difference was not statistically significant.
2.8 Craving: OCDS total score or analogue scale
2.8.1 Topiramate
Three studies (Baltieri 2008; Johnson 2007; Rubio 2009), 425 participants, SMD ‐0.39 (95% CI ‐0.82 to 0.04); the difference was not statistically significant. When studies with high risk of bias were excluded, two studies remained (Baltieri 2008; Johnson 2007), 362 participants, SMD ‐0.26 (95% CI ‐0.83 to 0.32); the result did not change.
2.8.2 Gabapentin
Two studies (Furieri 2007; Trevisan 2008), 71 participants, SMD ‐0.33 (95% CI ‐0.81 to 0.16); the difference was not statistically significant.
2.8.3 Valproate
Two studies (Brady 2002; Trevisan 2008), 57 participants, SMD ‐0.21 (95% CI ‐0.90 to 0.48); the difference was not statistically significant.
For all, see Analysis 2.8.
2.8. Analysis.
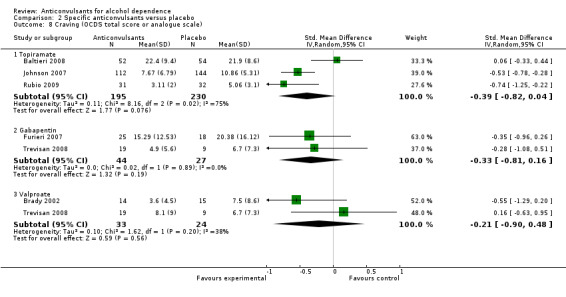
Comparison 2 Specific anticonvulsants versus placebo, Outcome 8 Craving (OCDS total score or analogue scale).
2.9 Liver enzyme levels
2.9.1 GGT: Gabapentin
Three studies (Brower 2008; Furieri 2007Trevisan 2008), 84 participants, MD ‐4.75 (95% CI ‐25.36 to 15.87); the difference was not statistically significant. When studies with high risk of bias were excluded, two studies remained (Brower 2008; Trevisan 2008), 41 participants, MD 0.71 (95% CI ‐39.31 to 40.74); the result did not change.
2.9.2 GGT: Valproate
Two studies (Salloum 2005Trevisan 2008), 80 participants, MD 0.68 (95% CI ‐48.11 to 49.47); the difference was not statistically significant.
For all, see Analysis 2.9.
2.9. Analysis.
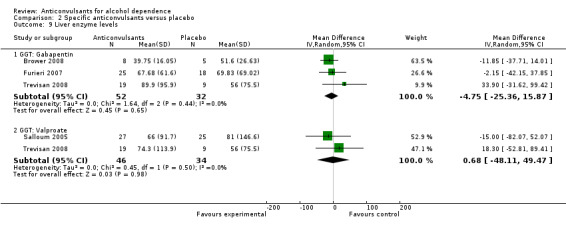
Comparison 2 Specific anticonvulsants versus placebo, Outcome 9 Liver enzyme levels.
3. Anticonvulsants versus placebo according to length of trial
3.1 Dropouts
3.1.1 Up to six weeks
Four studies (Anton 2009; Brower 2008; Furieri 2007; Trevisan 2008), 198 participants, RR 0.67 (95% CI 0.37 to 1.19); the difference was not statistically significant.
3.1.2 Over six weeks
12 studies (Arias 2010; Baltieri 2008; Brady 2002; Fertig 2012; Johnson 2003; Johnson 2007; Mueller 1997; Kampman 2013; Likhitsathian 2013; Richter 2012; Rubio 2009; Salloum 2005), 1477 participants, RR 0.99 (95% CI 0.76 to 1.29); the difference was not statistically significant.
For both, see Analysis 3.1.
3.1. Analysis.
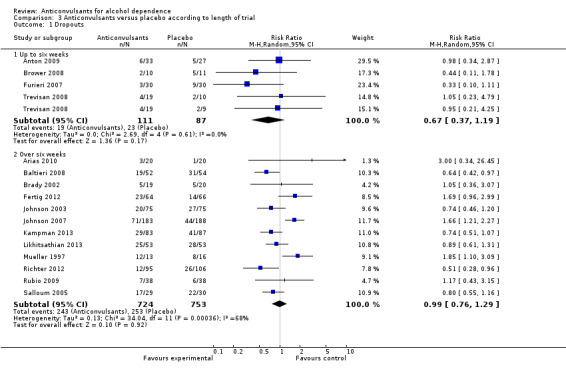
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 1 Dropouts.
3.2 Heavy drinking, dichotomous outcomes: over six weeks
Four studies (Brady 2002; Mueller 1997; Richter 2012; Salloum 2005), 309 participants, RR 0.94 (95% CI 0.67 to 1.32); the difference was not statistically significant.
See Analysis 3.2.
3.2. Analysis.
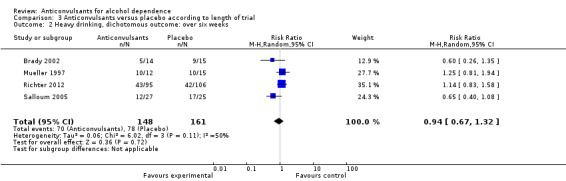
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 2 Heavy drinking, dichotomous outcome: over six weeks.
3.3 Alcohol use, continuous outcome: drinks/drinking days
3.3.1 Up to six weeks
Three studies (Anton 2011; Brower 2008; Furieri 2007), 155 participants, MD ‐2.14 (95% CI ‐4.21 to ‐0.06); the difference was statistically significant in favour of anticonvulsants.
3.3.2 Over six weeks
Eight studies (Brady 2002; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009; Salloum 2005), 971 participants, MD ‐1.44 (95% CI ‐2.46 to ‐0.42); the difference was statistically significant in favour of anticonvulsants.
For both, see Analysis 3.3.
3.3. Analysis.
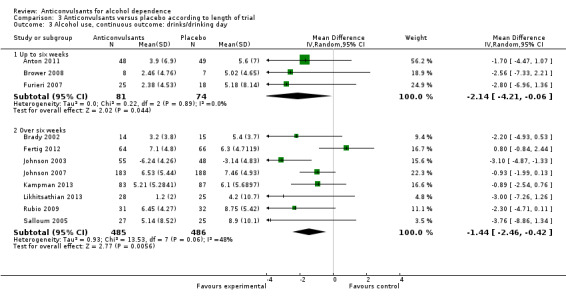
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 3 Alcohol use, continuous outcome: drinks/drinking day.
3.4 Alcohol use, continuous outcomes: heavy drinking
3.4.1 Up to six weeks
Four studies (Anton 2011 ; Brower 2008; Furieri 2007; Trevisan 2008), 211 participants, SMD ‐0.45 (95% CI ‐0.73 to ‐0.17); the difference was statistically significant in favour of anticonvulsants.
3.4.2 Over six weeks
Eight studies (Arias 2010; Baltieri 2008; Fertig 2012; Johnson 2003; Johnson 2007; Likhitsathian 2013; Rubio 2009; Salloum 2005), 918 participants, SMD ‐0.32 (95% CI ‐0.53 to ‐0.10); the difference was statistically significant in favour of anticonvulsants. However, substantial heterogeneity was found (Chi² = 15.73).
For both, see Analysis 3.4.
3.4. Analysis.
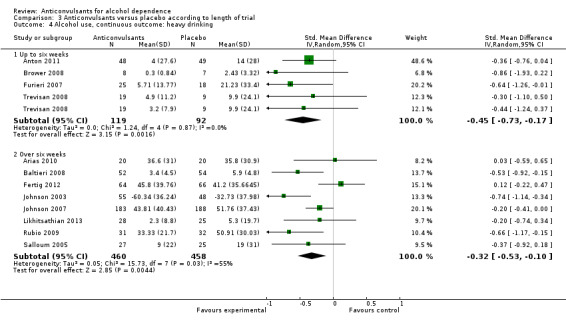
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 4 Alcohol use, continuous outcome: heavy drinking.
3.5 Continuous abstinence, dichotomous outcomes
3.5.1 Up to six weeks
Three studies (Anton 2009; Brower 2008; Furieri 2007), 141 participants, RR 1.23 (95% CI 0.65 to 2.32); the difference was not statistically significant.
3.5.2 Over six weeks
Five studies (Baltieri 2008; Brady 2002; Fertig 2012; Mueller 1997; Richter 2012), 493 participants, RR 1.19 (95% CI 0.92 to 1.54); the difference was not statistically significant.
For both, see Analysis 3.5.
3.5. Analysis.
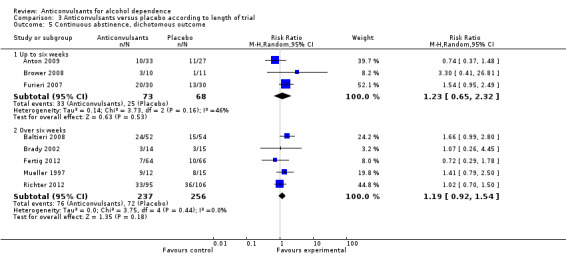
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 5 Continuous abstinence, dichotomous outcome.
3.6 Abstinence, continuous outcomes
3.6.1 Days abstinent (%): up to six weeks
Three studies (Anton 2009; Brower 2008; Furieri 2007), 107 participants, MD 5.82 (95% CI ‐6.87 to 18.51); the difference was not statistically significant.
3.6.2 Days abstinent (%): over six weeks
Five studies (Arias 2010; Fertig 2012Johnson 2003; Johnson 2007; Rubio 2009), 707 participants, MD 9.98 (95% CI 0.95 to 19.01); the difference was statistically significant in favour of anticonvulsants. However, a statistically significant result for substantial heterogeneity was found (Chi² = 10.23).
For both, see Analysis 3.6.
3.6. Analysis.
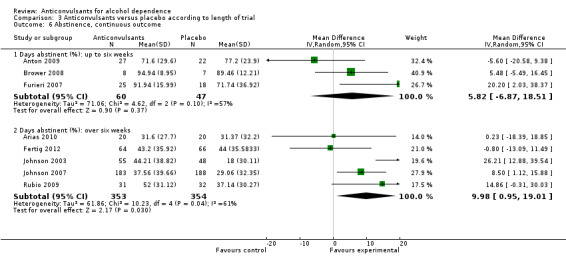
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 6 Abstinence, continuous outcome.
3.7 Adverse events
3.7.1 Withdrawal for medical reasons: up to six weeks
One study, two arms (Trevisan 2008), 57 participants, RR 0.22 (95% CI 0.04 to 1.41); the difference was not statistically significant.
3.7.2 Withdrawal for medical reasons: over six weeks
10 studies (Arias 2010; Fertig 2012; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Mueller 1997; Richter 2012; Rubio 2009; Salloum 2005), 1332 participants, RR 1.52 (95% CI 0.72 to 3.20); the difference was not statistically significant.
For both, see Analysis 3.7.
3.7. Analysis.
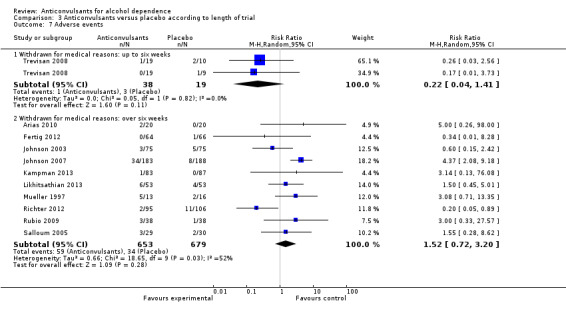
Comparison 3 Anticonvulsants versus placebo according to length of trial, Outcome 7 Adverse events.
4. Anticonvulsants versus placebo according to psychosocial interventions
In this analysis, studies were considered according to the presence of associated psychotherapy or other psychosocial intervention (counselling, self help, compliance enhancement interventions).
4.1 Dropouts
4.1.1 Associated psychotherapy
Seven studies (Anton 2009; Baltieri 2008; Brady 2002; Brower 2008; Kampman 2013; Likhitsathian 2013; Rubio 2009), 578 participants, RR 0.78 (95% CI 0.63 to 0.96); the difference was statistically significant in favour of anticonvulsants.
4.1.2 Associated other interventions
Six studies (Arias 2010; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Salloum 2005), 810 participants, RR 1.05 (95% CI 0.67 to 1.65); the difference was not statistically significant.
For both, see Analysis 4.1.
4.1. Analysis.
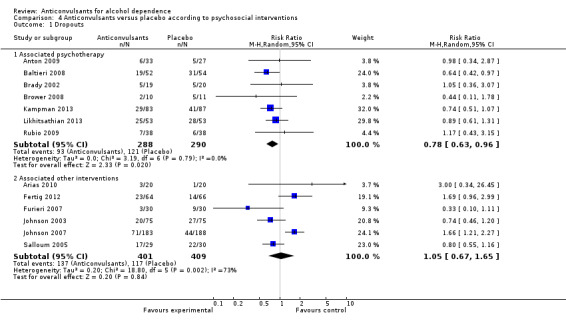
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 1 Dropouts.
4.2 Heavy drinking, dichotomous outcomes: associated psychotherapy
Two studies (Brady 2002; Brower 2008), 50 participants, RR 0.49 (95% CI 0.26 to 0.92); the difference was statistically significant in favour of anticonvulsants (see Analysis 4.2).
4.2. Analysis.
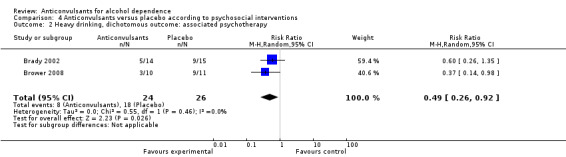
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 2 Heavy drinking, dichotomous outcome: associated psychotherapy.
4.3 Alcohol use, continuous outcomes: drinks/drinking days
4.3.1 Associated psychotherapy
Six studies (Anton 2011; Brady 2002; Brower 2008; Kampman 2013; Likhitsathian 2013; Rubio 2009), 427 participants, MD ‐1.68 (95% CI ‐2.73 to ‐0.63); the difference was statistically significant in favour of anticonvulsants.
4.3.2 Associated other interventions
Five studies (Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Salloum 2005), 699 participants, MD ‐1.44 (95% CI ‐3.06 to 0.18); the difference was not statistically significant.
For both, see Analysis 4.3.
4.3. Analysis.
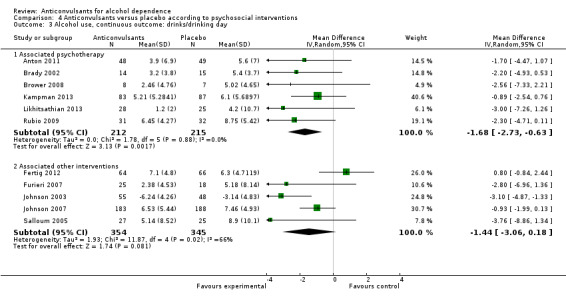
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 3 Alcohol use, continuous outcome: drinks/drinking day.
4.4 Alcohol use, continuous outcomes: heavy drinking
4.4.1 Associated psychotherapy
Five studies (Anton 2011; Baltieri 2008; Brower 2008; Likhitsathian 2013; Rubio 2009), 334 participants, SMD ‐0.46 (95% CI ‐0.68 to ‐0.25); the difference was statistically significant in favour of anticonvulsants.
4.4.2 Associated other interventions
Six studies (Arias 2010; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Salloum 2005), 739 participants, SMD ‐0.28 (95% CI ‐0.55 to 0.01); the difference was statistically significant in favour of anticonvulsants, and statistically significant substantial heterogeneity was found (Chi² = 12.80).
For both, see Analysis 4.4.
4.4. Analysis.
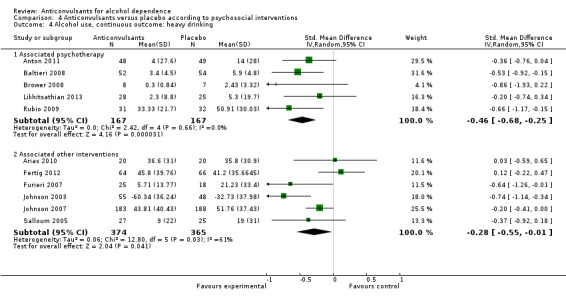
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 4 Alcohol use, continuous outcome: heavy drinking.
4.5 Continuous abstinence, dichotomous outcomes
4.5.1 Associated psychotherapy
Four studies (Anton 2009; Baltieri 2008; Brady 2002; Brower 2008), 216 participants, RR 1.24 (95% CI 0.73 to 2.10); the difference was not statistically significant.
4.5.2 Associated other interventions
Two studies (Fertig 2012; Furieri 2007), 190 participants, RR 1.16 (95% CI 0.55 to 2.45); the difference was not statistically significant.
For both, see Analysis 4.5.
4.5. Analysis.
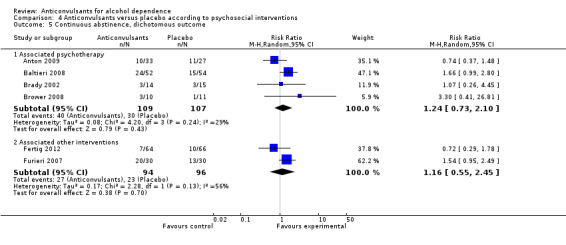
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 5 Continuous abstinence, dichotomous outcome.
4.6 Abstinence, continuous outcomes, days abstinent (%)
4.6.1 Associated psychotherapy
Three studies (Anton 2009; Brower 2008; Rubio 2009), 127 participants, MD 4.94 (95% CI ‐5.50 to 15.37); the difference was not statistically significant.
4.6.2 Associated other interventions
Four studies (Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007), 647 participants, MD 12.57 (95% CI 1.51 to 23.64); the difference was statistically significant in favour of anticonvulsants, and statistically significant substantial heterogeneity was found (Chi² = 9.95).
For both, see Analysis 4.6
4.6. Analysis.
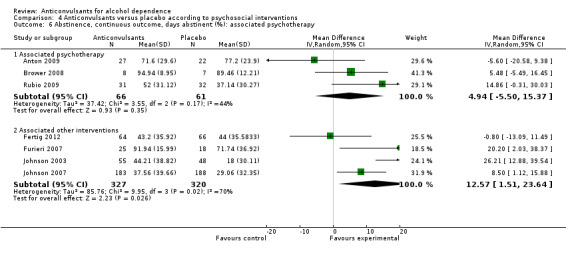
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 6 Abstinence, continuous outcome, days abstinent (%): associated psychotherapy.
4.7 Adverse events
4.7.1 Withdrawal for medical reasons: associated psychotherapy
Four studies (Brower 2008; Kampman 2013; Likhitsathian 2013; Rubio 2009), 373 participants, RR 1.86 (95% CI 0.68 to 5.09); the difference was not statistically significant.
4.7.2 Withdrawal for medical reasons: associated other interventions
Five studies (Arias 2010; Fertig 2012; Johnson 2003; Johnson 2007; Salloum 2005), 750 participants, RR 1.74 (95% CI 0.60 to 5.06); the difference was not statistically significant.
For both, see Analysis 4.7.
4.7. Analysis.
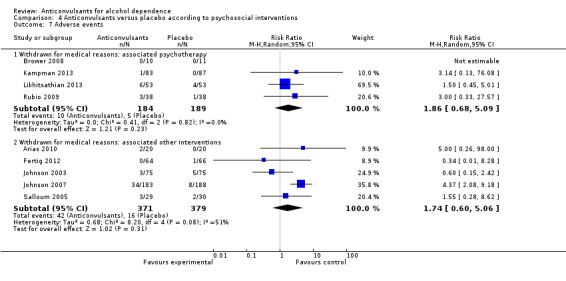
Comparison 4 Anticonvulsants versus placebo according to psychosocial interventions, Outcome 7 Adverse events.
5. Anticonvulsants versus placebo according to psychiatric co‐morbidity
In this analysis, studies were considered according to the presence of the exclusion criteria of major psychiatric conditions and/or use of psychotropic medications.
5.1 Dropouts
5.1.1 Excluding psychiatric co‐morbidity
Eight studies (Anton 2009; Baltieri 2008; Brady 2002; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Richter 2012), 1203 participants, RR 0.85 (95% CI 0.63 to 1.16); the difference was not statistically significant.
5.1.2 Not excluding psychiatric co‐morbidity
Eight studies (Arias 2010; Brower 2008; Fertig 2012; Furieri 2007; Mueller 1997; Rubio 2009; Salloum 2005; Trevisan 2008), 472 participants, RR 1.08 (95% CI 0.72 to 1.61); the difference was not statistically significant.
For both, see Analysis 5.1.
5.1. Analysis.
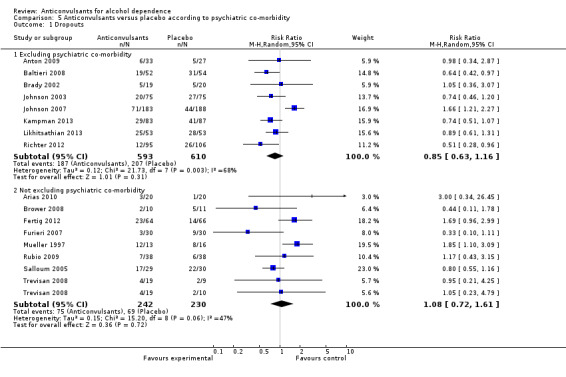
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 1 Dropouts.
5.2 Heavy drinking, dichotomous outcomes
5.2.1 Excluding psychiatric co‐morbidity
Two studies (Brady 2002; Richter 2012), 230 participants, RR 0.92 (95% CI 0.51 to 1.68); the difference was not statistically significant.
5.2.2 Not excluding psychiatric co‐morbidity
Three studies (Brower 2008; Mueller 1997; Salloum 2005), 100 participants, RR 0.74 (95% CI 0.37 to 1.45); the difference was not statistically significant.
For both, see Analysis 5.2.
5.2. Analysis.
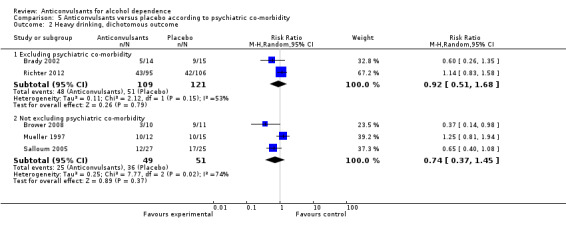
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 2 Heavy drinking, dichotomous outcome.
5.3 Alcohol use, continuous outcomes: drinks/drinking days
5.3.1 Excluding psychiatric co‐morbidity
Six studies (Anton 2011; Brady 2002; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013), 823 participants, MD ‐1.55 (95% CI ‐2.35 to ‐0.75); the difference was statistically significant in favour of anticonvulsants.
5.3.2 Not excluding psychiatric co‐morbidity
Five studies (Brower 2008; Fertig 2012; Furieri 2007; Rubio 2009; Salloum 2005), 303 participants, MD ‐1.52 (95% CI ‐3.51 to 0.48); the difference was not statistically significant.
For both, see Analysis 5.3.
5.3. Analysis.
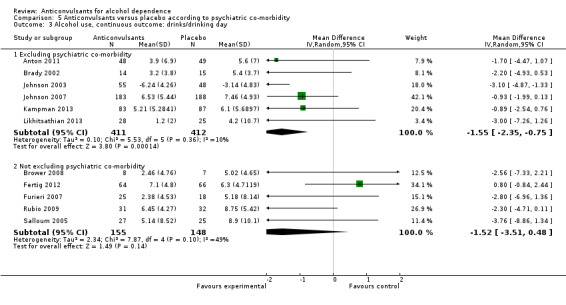
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 3 Alcohol use, continuous outcome: drinks/drinking day.
5.4 Alcohol use, continuous outcomes: drinks/drinking days
5.4.1 Excluding psychiatric co‐morbidity
Five studies (Anton 2011; Baltieri 2008; Johnson 2003; Johnson 2007; Likhitsathian 2013), 730 participants, SMD ‐0.39 (95% CI ‐0.60 to ‐0.18); the difference was statistically significant in favour of anticonvulsants.
5.4.2 Heavy drinking: not excluding psychiatric co‐morbidity
Seven studies (Arias 2010; Brower 2008; Fertig 2012; Furieri 2007; Rubio 2009; Salloum 2005; Trevisan 2008), 399 participants, SMD ‐0.31 (95% CI ‐0.58 to ‐0.05); the difference was statistically significant in favour of anticonvulsants.
For both, see Analysis 5.4.
5.4. Analysis.
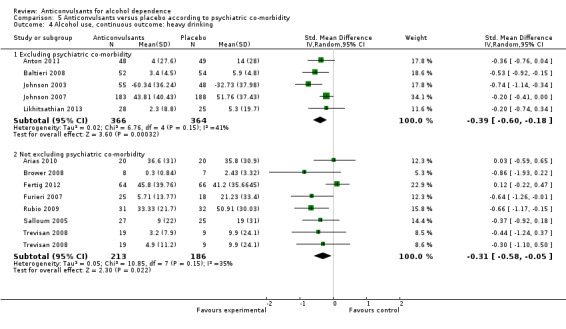
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 4 Alcohol use, continuous outcome: heavy drinking.
5.5 Continuous abstinence, dichotomous outcomes
5.5.1 Excluding psychiatric co‐morbidity
Four studies (Anton 2009; Baltieri 2008; Brady 2002; Richter 2012), 396 participants, RR 1.12 (95% CI 0.80 to 1.55); the difference was not statistically significant.
5.5.2 Not excluding psychiatric co‐morbidity
Four studies (Brower 2008; Fertig 2012; Furieri 2007; Mueller 1997), 238 participants, RR 1.37 (95% CI 0.98 to 1.92); the difference was not statistically significant.
For both, see Analysis 5.5.
5.5. Analysis.
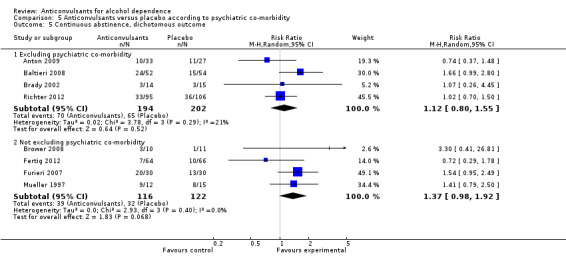
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 5 Continuous abstinence, dichotomous outcome.
5.6 Abstinence, continuous outcomes
5.6.1 Time to first relapse (weeks): not excluding psychiatric co‐morbidity
Two studies (Salloum 2005; Trevisan 2008), 108 participants, MD 0.26 (95% CI ‐0.60 to 1.13); the difference was not statistically significant.
5.6.2 Days abstinent (%): excluding psychiatric co‐morbidity
Three studies (Anton 2009; Johnson 2003; Johnson 2007), 523 participants, MD 9.92 (95% CI ‐5.19 to 25.03); the difference was not statistically significant.
5.6.3 Days abstinent (%): not excluding psychiatric co‐morbidity
Five studies (Arias 2010; Brower 2008; Fertig 2012; Furieri 2007; Rubio 2009), 291 participants, MD 6.95 (95% CI ‐0.37 to 14.26); the difference was not statistically significant.
For all, see Analysis 5.6.
5.6. Analysis.
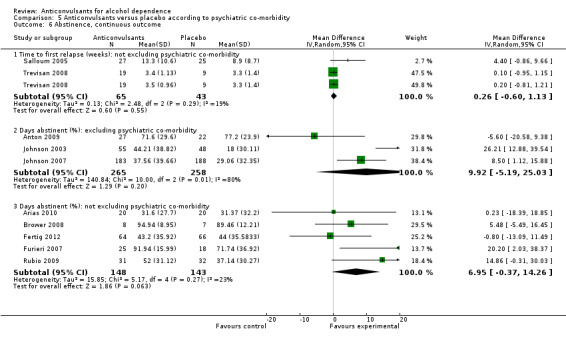
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 6 Abstinence, continuous outcome.
5.7 Adverse events
5.7.1 Withdrawal for medical reasons: excluding psychiatric co‐morbidity
Five studies (Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Richter 2012), 998 participants, RR 1.17 (95% CI 0.34 to 4.00); the difference was not statistically significant.
5.7.2 Withdrawal for medical reasons: not excluding psychiatric co‐morbidity
Six studies (Arias 2010; Fertig 2012; Mueller 1997; Rubio 2009; Salloum 2005; Trevisan 2008), 391 participants, RR 1.35 (95% CI 0.55 to 3.29); the difference was not statistically significant.
For both, see Analysis 5.7.
5.7. Analysis.
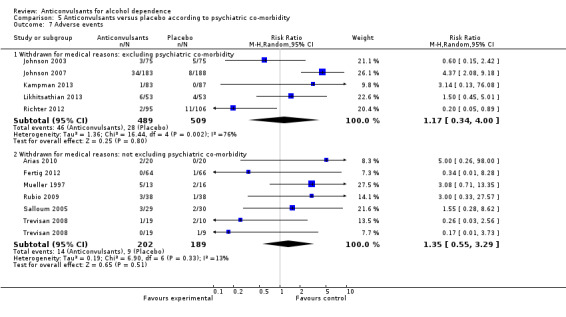
Comparison 5 Anticonvulsants versus placebo according to psychiatric co‐morbidity, Outcome 7 Adverse events.
Anticonvulsants versus other medication
For comparisons between anticonvulsants and other medications, only naltrexone was involved in more than one study. The following results were obtained.
Dropouts
It was possible to pool data from five studies (Baltieri 2008; Florez 2008; Florez 2011; Martinotti 2007; Martinotti 2010), 528 participants, RR 0.74 (95% CI 0.52 to 1.06). The results of meta‐analysis showed no evidence of differences between anticonvulsants and naltrexone. When studies with high risk of bias were excluded, two studies remained (Baltieri 2008; Martinotti 2010), 160 participants, RR 0.82 (95% CI 0.52 to 1.28); the result did not change when specific anticonvulsants (topiramate) were studied: three studies (Baltieri 2008; Florez 2008; Florez 2011), 385 participants, RR 0.79 (95% CI 0.52 to 1.19).
See Analysis 6.1 and Analysis 7.1.
6.1. Analysis.
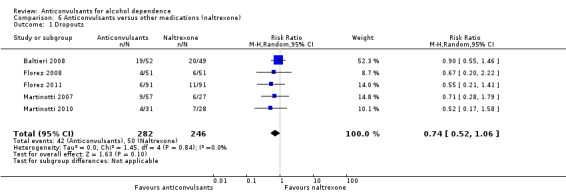
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 1 Dropouts.
7.1. Analysis.
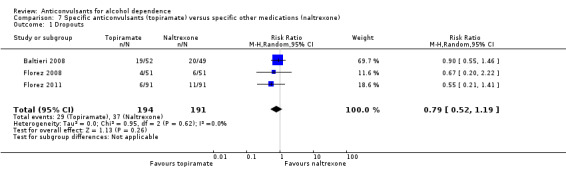
Comparison 7 Specific anticonvulsants (topiramate) versus specific other medications (naltrexone), Outcome 1 Dropouts.
Use of alcohol
With regard to the use of alcohol during the trial, when heavy drinking rate was examined (severe relapse), four studies were identified (Florez 2008; Florez 2011; Martinotti 2007; Martinotti 2010), 427 participants, RR 0.69 (95% CI 0.44 to 1.07); the difference was not statistically significant. When specific anticonvulsants were examined (topiramate), two studies (Florez 2008; Florez 2011), 284 participants, RR 0.54 (95% CI 0.36 to 0.81), the difference was statistically significant in favour of the anticonvulsant; when the number of heavy drinking days was examined, three studies (Florez 2011; Martinotti 2007; Martinotti 2010), 308 participants, MD ‐5.21 (95% CI ‐8.58 to ‐1.83), the evidence favoured anticonvulsants.
See Analysis 6.2; Analysis 6.3; and Analysis 7.2.
6.2. Analysis.
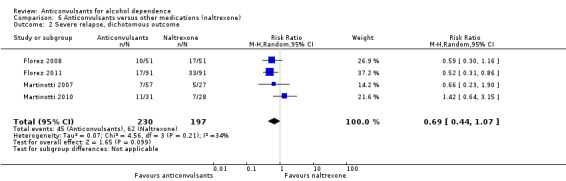
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 2 Severe relapse, dichotomous outcome.
6.3. Analysis.
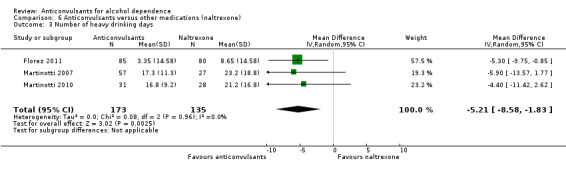
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 3 Number of heavy drinking days.
7.2. Analysis.
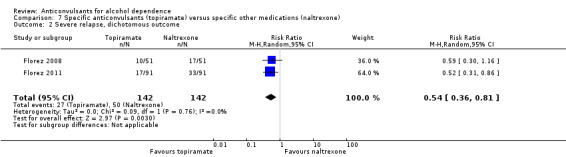
Comparison 7 Specific anticonvulsants (topiramate) versus specific other medications (naltrexone), Outcome 2 Severe relapse, dichotomous outcome.
Abstinence from alcohol
For rate of continuous abstinence (number of participants not drinking during the trial), five studies (Baltieri 2008; Florez 2008; Florez 2011; Martinotti 2007; Martinotti 2010), 528 participants, RR 1.21 (95% CI 0.99 to 1.49), results of meta‐analysis showed no evidence of differences between anticonvulsants and naltrexone, although a trend for statistical significance favouring anticonvulsants was seen. When studies with high risk of bias were excluded, two studies remained (Baltieri 2008; Martinotti 2010), 160 participants, RR 1.43 (95% CI 0.96 to 2.12); no evidence of difference was shown. For specific anticonvulsants (topiramate), three studies (Baltieri 2008; Florez 2008; Florez 2011), 385 participants, RR 1.18 (95% CI 0.94 to 1.49); the result did not substantially change. For number of days to severe relapse, three studies (Baltieri 2008; Martinotti 2007; Martinotti 2010), 244 participants, MD 11.88 (95% CI 3.29 to 20.46); the evidence favoured anticonvulsants. When studies with high risk of bias were excluded, two studies remained (Baltieri 2008; Martinotti 2010), 160 participants, RR 16.32 (95% CI 6.96 to 25.68); the result did not change.
See Analysis 6.4; Analysis 6.5; and Analysis 7.3.
6.4. Analysis.
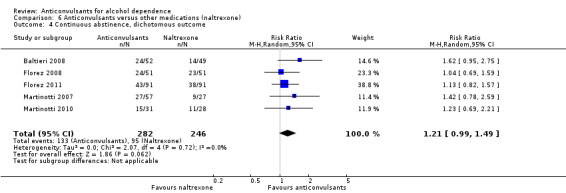
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 4 Continuous abstinence, dichotomous outcome.
6.5. Analysis.
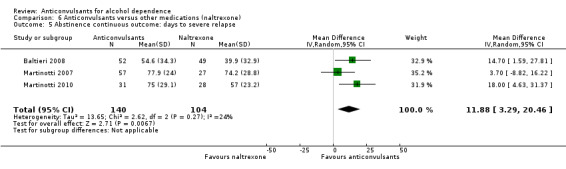
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 5 Abstinence continuous outcome: days to severe relapse.
7.3. Analysis.
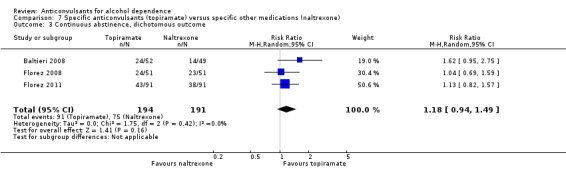
Comparison 7 Specific anticonvulsants (topiramate) versus specific other medications (naltrexone), Outcome 3 Continuous abstinence, dichotomous outcome.
Adverse events
For safety issues, studies investigating the rate of withdrawal from the study for medical reasons: three studies (Florez 2008; Martinotti 2007; Martinotti 2010), 245 participants, RR 0.13 (95% CI 0.03 to 0.58); evidence of a lower dropout rate favoured anticonvulsants. Four studies (Baltieri 2008; Florez 2008; Martinotti 2007; Martinotti 2010), 346 participants, considered several single different adverse events. Of six adverse event outcomes including more than one study, four showed no statistically significant difference between anticonvulsants and naltrexone, but in two the result was statistically significant.
Hypotension: two studies (Martinotti 2007; Martinotti 2010), 143 participants, RR 0.09 (95% CI 0.01 to 0.75), favouring anticonvulsants.
Paraesthesia: two studies (Baltieri 2008; Florez 2008), 203 participants, RR 6.07 (95% CI 1.11 to 33.17), favouring naltrexone.
See Analysis 6.6.
6.6. Analysis.
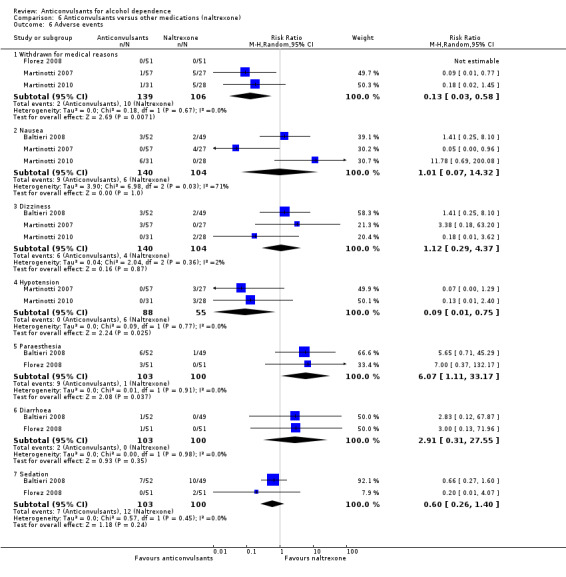
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 6 Adverse events.
Craving
For meta‐analysis carried out on craving scores (OCDS total score), four studies (Florez 2008; Florez 2011; Martinotti 2007; Martinotti 2010), 385 participants, MD ‐2.25 (95% CI ‐3.58 to ‐0.93); the evidence favoured anticonvulsants. This result was confirmed by the comparison of topiramate versus naltrexone: two studies (Florez 2008; Florez 2011), 257 participants, MD ‐2.74 (95% CI ‐4.28 to ‐1.21).
See Analysis 6.7 and Analysis 7.4.
6.7. Analysis.
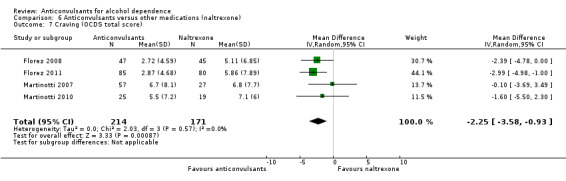
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 7 Craving (OCDS total score).
7.4. Analysis.
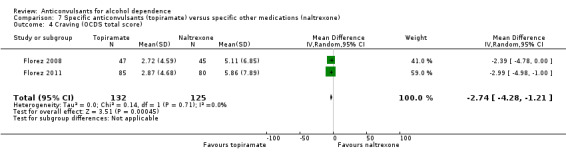
Comparison 7 Specific anticonvulsants (topiramate) versus specific other medications (naltrexone), Outcome 4 Craving (OCDS total score).
Liver enzyme levels (GGT)
Three studies (Baltieri 2008; Florez 2008; Florez 2011), 358 participants, MD ‐1.08 (95% CI ‐10.82 to 8.66); the difference was not statistically significant.
Anticonvulsants versus no medication
Meta‐analysis carried out on two open studies (Paparrigopoulos 2010; Paparrigopoulos 2011) comparing anticonvulsants (tiagabine and topiramate) versus no medication with 205 participants found evidence of differences favouring anticonvulsants for relapse rate (205 participants, RR 0.76 (95% CI 0.58 to 1.00)), mood measures (HDRS, 198 participants, MD ‐3.80 (95% CI ‐5.19 to ‐2.41); HARS, 198 participants, MD ‐3.26 (95% CI ‐4.64 to ‐1.88)) and craving (198 participants, MD ‐4.97 (95% CI ‐8.97 to ‐1.15)), although for this last comparison, significant and considerable heterogeneity was found (Chi² = 19.97).
See Analysis 8.1; Analysis 8.2; and Analysis 8.3.
8.1. Analysis.
Comparison 8 Anticonvulsants versus no medication, Outcome 1 Alcohol use dichotomous: relapse.
8.2. Analysis.
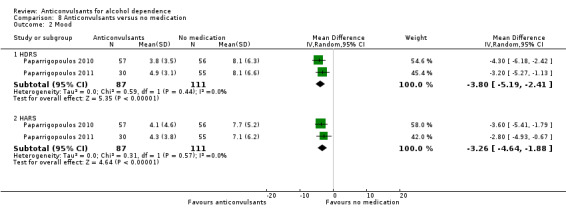
Comparison 8 Anticonvulsants versus no medication, Outcome 2 Mood.
8.3. Analysis.
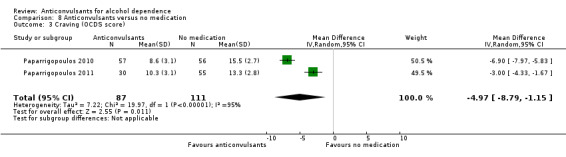
Comparison 8 Anticonvulsants versus no medication, Outcome 3 Craving (OCDS score).
Anticonvulsants versus anticonvulsants
Only one study (Trevisan 2008), 38 participants, included comparisons between anticonvulsants (valproic acid and gabapentin). This study found no evidence of differences in dropout, alcohol use, abstinence, craving or adverse events.
Discussion
Summary of main results
Although all selected studies are RCTs comparing anticonvulsants versus placebo, no medication or other medications, they differ in terms of design, quality, characteristics of participants, medications tested and services and treatments delivered.
The length of studies varied, with two studies lasting 52 weeks, one 39 weeks, four 26 weeks, one 24 weeks, two 16 weeks, one 14 weeks, one 13 weeks, eight 12 weeks, three six weeks and two four weeks.
All included studies were based on formal diagnostic criteria for alcohol dependence (19 according to DSM‐IV; one according to DSM‐III‐R; three according to ICD‐10 and two according to both DSM‐IV and ICD‐10); no studies were based on specialist evaluation, and none employed explicit diagnostic criteria for alcohol dependence.
The anticonvulsant evaluated in the 17 placebo‐controlled studies was topiramate in six trials, gabapentin in five trials, valproate in three trials, levetiracetam in two trials and zonisamide and carbamazepine in one trial each.
The anticonvulsant evaluated in the seven trials comparing anticonvulsants versus other medications was topiramate in four trials, oxcarbazepine in two trials and pregabalin in one trial; in these trials, anticonvulsants were compared with naltrexone in five trials, acamprosate in one trial and disulfiram in one trial.
Two trials compared, respectively, tiagabine and topiramate versus no medication.
In one study, participants were concomitantly treated with hydroxyzine HCL (50 mg) for seven days and with multi‐vitamins for 30 days; moreover, participants randomly assigned to gabapentin received flumazenil for two days; in one study, participants were concomitantly treated with naltrexone; in another study, participants were concomitantly treated with lithium for bipolar disorder.
Five studies did not include requirements on abstinence to enter the study; however, two of these excluded participants reporting withdrawal symptoms, and one excluded those who were unable to be safely withdrawn from alcohol on an outpatient basis and those who had undergone medical detoxification during the screening phase; three studies required abstinence, respectively, for one night and for two and four days; one study excluded those showing withdrawal during one week of placebo‐led‐in‐fase preceding randomisation; 13 included assisted detoxification provided before randomisation, and three studies provided detoxification after randomisation.
12 studies assessed medication compliance through pill count (return of unused medication or use of devices such as electronic pill bottle caps); eight studies through return of unused medications; one study by adding riboflavine to medication and placebo; three studies through supervision by a family member; 10 studies through self report and five studies by monitoring plasma concentrations of medications; one study did not assess adherence to medication, and for four studies, this information was not available. In 11 of the included studies, more than one procedure (such as self report and family member involvement or pill count and plasma concentration of medication) was applied.
-
Four studies did not report on the presence of concomitant associated psychosocial treatment; the remaining were associated with the following.
Motivational, cognitive‐behavioural psychotherapy or relapse prevention therapy (11 trials).
Supportive psychotherapy (two trials).
Counselling, self help and/or compliance enhancement interventions (eight trials).
12 studies were conducted in the USA, three in Spain; two in Brasil, Italy, Germany and Greece, one in India and one in Thailand.
For the effects of interventions, it has to be considered that studies differed also in outcome variables and their definitions, resulting in the possibility of pooling together data and carrying out meta‐analyses.
Anticonvulsants versus placebo
For comparisons between anticonvulsants and placebo, results of the pooled studies regarding dropout rate and continuous abstinence rate revealed no evidence of differences. For other comparisons, anticonvulsants were shown to be associated with 1.49 fewer drinks/drinking days (1.33 when studies with high risk of bias were excluded) and with 0.35 standard deviations lower (0.30 when studies with high risk of bias were excluded) heavy drinking than placebo. Other results regarding primary outcomes were not statistically significant, conditioned by the presence of heterogeneity and/or not replicated after exclusion of studies with high risk of bias. Regarding safety issues, no evidence of differences was seen between anticonvulsant‐treated and placebo‐treated participants in rate of dropout for medical reasons, although two of 18 adverse event outcomes favoured placebo. However, the insufficiency of data from primary studies did not allow specific investigation of other adverse effects, such as cognitive functioning (besides psychomotor retardation and memory impairment), which with some antiepileptics, such as topiramate, may have a serious impact on treatment (Cavanna 2010). See Table 1 for an overall synthesis of the most relevant outcomes.
The direction of these results was globally confirmed by analyses comparing topiramate versus placebo, and partially by those comparing gabapentin and valproate versus placebo; the corresponding study base of fewer than 200 participants for most comparisons is too sparse to permit any conclusions. When the effects of confounders/moderators were examined, inconsistent differential impact on primary outcome was observed globally, although important limits, such as low numbers of studies and of participants in some subgroups and the presence of heterogeneity, must be noticed.
Anticonvulsants versus other drugs
Anticonvulsants were not shown to differ from naltrexone in dropout rate, in rate of severe relapse or in rate of continuous abstinence, although on average, they were associated with fewer days of heavy drinking, more days to severe relapse and lower rate of withdrawal for medical reasons when compared with naltrexone. These results were confirmed after studies with high risk of bias were excluded, although a specific look at the comparison of topiramate versus naltrexone revealed that the rate of severe relapse also favoured topiramate.
Anticonvulsants versus no medication
Meta‐analysis carried out on two open studies comparing anticonvulsants (tiagabine and topiramate) versus no medication, 205 participants, found evidence of differences in relapse rate, craving and mood measures favouring anticonvulsants.
Anticonvulsants versus anticonvulsants
One study, 38 participants, found no evidence of differences in dropout, alcohol use, abstinence, craving or adverse events between gabapentin and valproate. Given the limited number of participants, this evaluation of the head‐to‐head comparative efficacy of anticonvulsants cannot be conclusive at all.
Overall completeness and applicability of evidence
Completeness of the database
For primary outcomes, in the comparison with placebo, rates of completeness vary widely: from 94% (16 RCTs) for dropouts to 47% (eight RCTs) for continuous abstinence from alcohol, and to 71% (12 RCTs) for withdrawal for medical reasons. In the comparison with other medications (naltrexone), rates of completeness were 100% (five RCTs) for both dropouts and continuous abstinence from alcohol and 60% for withdrawal for medical reasons (three RCTs).
Applicability of the results
Beside the limits in external validity due to the general requirements of RCTs in terms of strict inclusion criteria, highly homogenous study groups, limitations in dose adjustment, etc., the types of participants (adults dependent on alcohol) are quite representative of the general population of alcohol dependents. Moreover, interventions (anticonvulsant dosages), settings (prevailing outpatient treatment) and outcomes investigated (dropouts, alcohol use, adverse events) are important to populations, practitioners and decision makers and are relevant to the context of current practice. Different from other reviews in the field of addiction, for which a large majority of studies were conducted in the USA, half of the studies included in this review were conducted in other countries. This is an important issue in terms of generalisability of the evidence, because different social contests can influence differently the severity of dependence and availability to enter an experimental design; also, different clinical contexts can influence differently the selection of participants to trials and the results of treatment, acting as an effect modifier in the estimation of efficacy of treatment.
Quality of the evidence
For evaluation of the quality of evidence, supplementary information was collected from the authors of the primary studies, mainly because some features of the study design were omitted from trial reports. The poor reporting of the study design mainly concerns the methods used in generating random sequences, the specification of person groups included in the blinding process and the methods applied for allocation concealment. From a methodological perspective, the overall quality of the included studies was considered moderate. Of 25 studies with 2641 participants, 17 studies compared anticonvulsants versus placebo (1765 participants), seven versus other medications (658 participants) and two versus no medication (210 participants). For comparisons versus placebo, participants and operators were blind to allocation of the interventions in 14 studies, and outcome assessors were judged to be blind to the allocation of interventions in 13 studies for subjective outcomes and in all 17 studies for objective outcomes; 13 studies were judged to have low risk of bias for sequence generation, 13 for outcome data addressed and 11 for allocation concealment. For comparisons versus other medications, although five of seven studies were judged to have low risk of bias for sequence generation and all seven for outcome data addressed, only two were judged to have low risk of bias for allocation concealment. Moreover, for performance bias, only two were blind, and for detection bias, two studies were judged to be at low risk of bias for measurement of subjective outcomes and all seven for objective outcomes (judged as not influenced by lack of blinding). The two studies versus no medication were shown to be at high or unclear risk of bias in all domains considered, with the exception of objective outcomes in detection bias, for which lack of blinding was judged to not influence the results. Moreover, four studies (three vs placebo and one vs other medication) did not specify how compliance with medication intake was monitored, and one (vs placebo) did not assess adherence to medication. However, when studies at high risk of bias were excluded from the analysis comparing anticonvulsants versus placebo, the numbers of studies and of participants did not change too much without substantially changing the results. Therefore, the overall quality of evidence for the efficacy of anticonvulsants versus placebo evaluated using primary outcomes may be judged as moderate (Table 1). However, moving to subgroup analysis, as in the case of single classes of anticonvulsants, single types of medications and confounder/moderator evaluation, as well as to comparisons versus other medications, the finding of the review is limited by the small number of studies included in the meta‐analysis of study outcomes. Therefore the precision of the calculated effects is low. Finally, the great heterogeneity of the scales used in the primary studies and the way in which results were reported often made it impossible for review authors to undertake a cumulative analysis.
Potential biases in the review process
Reporting bias can jeopardise the validity of any meta‐analysis. We have tried to limit the influence of reporting bias by screening several data sets and by requesting unpublished results from the contact authors. This approach has resulted in a substantial increase in available data.
Given the number of studies included in meta‐analyses, the only funnel plot considered was carried out on dropout. However, in light of the low number of included studies and their heterogeneity, it does not seem to provide support for any conclusions on the presence of publication bias (see Figure 10).
10.
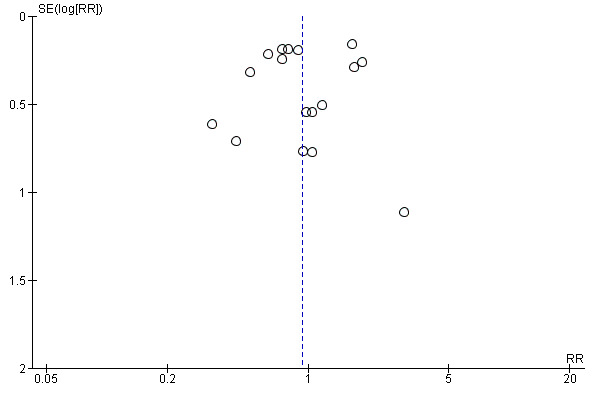
Funnel plot of comparison: 1 Anticonvulsants versus placebo, outcome: 1.1 Dropouts.
Agreements and disagreements with other studies or reviews
Over past years, interest in the use of anticonvulsants for the treatment of alcohol dependence has substantially increased. Both preclinical and clinical studies have investigated the potential involvement of this class of medication in the treatment of substance use disorders, and clinical trials specifically designed for evaluation of the efficacy and safety of anticonvulsants in alcohol dependence have been performed. However, reviews of the literature on the subject (Olsmed 2008; De Sousa 2010; Shinn 2010; Arbaizar 2010) are almost exclusively focused on the efficacy of topiramate. Moreover, among these reviews, only one was characterised by a meta‐analytical approach (Arbaizar 2010). It included three randomised studies with a total of 635 participants and found topiramate to be better than placebo in reducing the percentage of days of elevated intake of alcohol and in increasing the numbers of abstinence days, although it was worse than placebo in terms of frequency of adverse effects. Our review, besides applying Cochrane methodology, extends the evaluations to different classes of anticonvulsants and to specific medications; moreover, it considers a wide range of primary and secondary outcomes, including dropout, which is a very common event in substance dependence treatment (Daley 1993) and represents a relevant methodological problem to be managed in clinical trials (Carroll 1987; Nich 2002). On the whole, results obtained for this outcome show no evidence of differences between anticonvulsants and placebo. Results obtained for other outcomes (alcohol use/abstinence and adverse effects) are substantially consistent with those obtained in previous reviews (Olsmed 2008; De Sousa 2010; Shinn 2010; Arbaizar 2010).
The presence of psychiatric co‐morbidity, depression in particular, and the association of psychosocial interventions may have a robust impact on the outcome of alcohol dependence (Lubman 2007; Davis 2008; Swendsen 1998; Weiss 2006; Project MATCH Research Group 1997; Anton 2006). However, because of the limited numbers of studies and participants involved in specific subgroup analyses regarding these conditions, we cannot be confident in attributing any meaning other than chance to the small differential level of evidence associated with these potential confounders/moderators.
Authors' conclusions
Implications for practice.
This review provides insufficient randomised evidence supporting the effectiveness of anticonvulsants for the treatment of alcohol dependence. According to the results of the review, anticonvulsants were found to be associated with a statistically significant lower number of drinks per drinking days and lower average heavy drinking than placebo (GRADE quality of evidence: moderate), while not showing evidence of differences in comparison with placebo in dropout rate of participants, continuous abstinence rate and withdrawal for medical reasons (GRADE quality of evidence: moderate). For other relevant measures of alcohol use (heavy drinking rate, percentage of days of abstinence), the results were inconclusive. Moreover, safety issues, particularly those regarding cognitive functioning, should be deeply explored.
The specific medication most supported by evidence of efficacy was topiramate, with a study base of more than 970 participants in RCTs versus placebo (six RCTs) and 380 participants in RCTs versus naltrexone (three RCTs), although for gabapentin, valproate and levetiracetam, the corresponding study base of fewer than 200 participants for most comparisons is still too small to permit any conclusions.
Finally, because of the low number and low quality of RCTs exploring the comparative effectiveness of different anticonvulsants versus other medications supported by evidence of efficacy, no conclusions can be drawn on this point.
The overall uncertainty associated with these results leaves to clinicians the need to balance possible benefits/risks of treatment with anticonvulsants versus those expected from other medications supported by evidence of efficacy for the treatment of alcohol dependence.
Implications for research.
Much research is required to strengthen evidence on the efficacy of anticonvulsants in the treatment of alcohol dependence. In implementing new trials on the topic, specific attention should be paid to the main methodological challenges associated with addiction research, particularly the rate of dropouts and related handling of missing data, and the validation of self report measures by objective measures, the choice of outcomes and related measures to allow comparison of results between studies and, more generally, the stricter adherence to methodological standards of reporting, as outlined in the CONSORT statement (Moher 2001). Some new trials are ongoing and will be added to the review as soon as results become available. Moreover, in addition to comparison with placebo, future researchers should address questions regarding head‐to‐head comparisons among anticonvulsants and versus other medications supported by evidence of efficacy for the treatment of alcohol dependence, such as acamprosate, naltrexone and GHB (Leone 2010; Rösner 2010; Rösner 2010 a).
Acknowledgements
We thank the team of the Cochrane Drugs and Alcohol Group (CDAG, Rome, Italy) for their support in the preparation and conduct of this review, especially Suzana Mitrova for help with the search strategy and Laura Amato for comments and suggestions. We also thank Arias AJ and Feinn R (Alcohol Research Center, Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT), Brower KJ (University of Michigan, Addiction Research Center and Substance Abuse Section, Departments of Psychiatry, Medical School, Ann Arbor, MI), De Sousa A (Consultant Psychiatrist and Psychotherapist, Founder Trustee—Desousa Foundation, Mumbai, State Maharashtra, India), Fertig JB (Division of Treatment and Recovery Research, Medications Development Team, National Institute on Alcohol Abuse and Alcoholism NIH/DHHS, 5635 Fishers Lane, Bethesda, MD), Florez G (Unidad Asistencial "As Burgas", Ourense, Spain), Furieri FA and Nakamura‐Palacios EM (Vitòria Municipal Addiction Treatment Center and Department of Phisiological Sciences, Health Science Center, Federal University of Espirito Santo, Vitòria, Espirito Santo, Brazil), Johnson BA (Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville), Likhitsathian S (Department of Psychiatry, Faculty of Medicine, Chiang Mai University, Muang, Chiang Mai, Thailand), MacKillop J (Department of Psychology, University of Georgia, Athens, GA), Martinotti G (Department of Psychiatry, Catholic University Medical School, Rome, Italy), Mueller TI (Brown University School of Medicine, Department of Psychiatry and Human Behavior, Butler Hospital, Providence, RI), Rubio G (Servicio de Psiquiatría, Laboratorio de Psicofisiología Clínica, Hospital Universitario 12 de Octubre, Madrid) for the disposal of unreported data and information*. *Authors of primary studies not mentioned in this section were not contacted, as all relevant data and information were available from the trial publication, or they were unable to provide requested data or information.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Alcohol‐Related Disorders] explode all trees
((alcohol*) near (abuse* or addict* or dependen* or disorder* or drink* or consumption)):ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Drinking Behavior] explode all trees
#1 or #2 or #3
MeSH descriptor: [Anticonvulsants] explode all trees
anticonvulsant* or Acetazolamide or bromide* or Carbamazepine or Chlormethiazole or Clorazepate or depakote or depakene or depakine or divalproex or Ethosuximide or felbamate or fosphenytoin or gabapentin or lamotrigine or Levetiracetam or Metaclazepam or lidocaine or “magnesium sulphate” or mephobarbital or lignocaine or memantine or methsuximide or mysoline or mizodin or oxcarbazepine or paraldehyde or Phenobarbita or pentobarbital or phenytoin or primidone or promazine or sertan or tetrabamate or tiagabine or topamax or topiramate or valproic or valproate or vigabatrin or zonisamide or zonegran (Word variations have been searched)
#5 or #6
#4 and #7
Appendix 2. PubMed search strategy
alcohol‐related disorders[MeSH]
((alcohol[tiab]) AND (abuse*[tiab] OR addict*[tiab] OR dependen*[tiab] OR disorder*[tiab] OR drink*[tiab] OR consumption[tiab]))
Drinking behaviour[MeSH]
#1 OR #2 OR #3
Anticonvulsants[MeSH]
anticonvulsant* OR acetazolamide OR bromide* OR carbamazepine OR chlormethiazole OR clorazepate OR depakote OR depakene OR depakine OR divalproex OR ethosuximide OR felbamate OR fosphenytoin OR gabapentin OR lamotrigine OR levetiracetam OR metaclazepam OR lidocaine OR "magnesium sulphate" OR mephobarbital OR lignocaine OR memantine OR methsuximide OR mysoline OR mizodin OR oxcarbazepine OR paraldehyde OR phenobarbital OR pentobarbital OR phenytoin OR primidone OR promazine OR sertan OR tetrabamate OR tiagabine OR topamax OR topiramate OR valproic OR valproate OR vigabatrin OR zonisamide OR zonegran
#5 OR #6
randomized controlled trial [pt]
controlled clinical trial [pt]
placebo [tiab]
drug therapy [sh]
trial [tiab]
randomized [tiab]
randomly [tiab]
groups [tiab]
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
animals [mh] NOT humans [mh]
16 NOT 17
4 AND 7 AND 18
Appendix 3. EMBASE search strategy
'alcoholism'/exp
(alcohol*:ab,ti AND (abuse*:ab,ti OR addict*:ab,ti OR dependen*:ab,ti OR disorder*:ab,ti OR drink*:ab,ti OR consum*:ab,ti))
'drinking behavior'/exp
#1 or #2 or #3
'anticonvulsive agent'/exp
anticonvulsant*:de,ab,ti OR acetazolamide:de,ab,ti OR bromide*:de,ab,ti OR carbamazepine:de,ab,ti OR chlormethiazole:de,ab,ti OR clorazepate:de,ab,ti OR depakote:de,ab,ti OR depakene:de,ab,ti OR depakine:de,ab,ti OR divalproex:de,ab,ti OR ethosuximide:de,ab,ti OR felbamate:de,ab,ti OR fosphenytoin:de,ab,ti OR gabapentin:de,ab,ti OR lamotrigine:de,ab,ti OR levetiracetam:de,ab,ti OR metaclazepam:de,ab,ti OR lidocaine:de,ab,ti OR 'magnesium sulphate':de,ab,ti OR mephobarbital:de,ab,ti OR lignocaine:de,ab,ti OR memantine:de,ab,ti OR methsuximide:de,ab,ti OR mysoline:de,ab,ti OR mizodin:de,ab,ti OR oxcarbazepine:de,ab,ti OR paraldehyde:de,ab,ti OR phenobarbital:de,ab,ti OR pentobarbital:de,ab,ti OR phenytoin:de,ab,ti OR primidone:de,ab,ti OR promazine:de,ab,ti OR sertan:de,ab,ti OR tetrabamate:de,ab,ti OR tiagabine:de,ab,ti OR topamax:de,ab,ti OR topiramate:de,ab,ti OR valproic:de,ab,ti OR valproate:de,ab,ti OR vigabatrin:de,ab,ti OR zonisamide:de,ab,ti OR zonegran:de,ab,ti
#5 OR #6
'crossover procedure'/exp
'double blind procedure'/exp
'single blind procedure'/exp
'controlled clinical trial'/exp
'clinical trial'/exp
placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)
'randomized controlled trial'/exp)
#8 OR #9 OR 10 #11 OR #12 OR #13 OR #14
#4 AND #7 AND #15 AND [humans]/lim AND [embase]/lim
Appendix 4. CINAHL search strategy
(MH "Alcohol‐Related Disorders+")
TX (alcohol* and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy) )
S1 or S2
(MH "Anticonvulsants+")
(MH "Valproic Acid")
TX (Acetazolamide or carbamazepine or Chlormethiazole or Clorazepate or divalproex or Ethosuximide or felbamate or foshenytoin or gabapentin or lamotrigine or levetiracetam or metaclazepam or lidocaine or mephobarbital or lignocaine or methsuximide or oxacarbazepine or paraldehyde or penthobarbital or phenytoin or primidone or tiagabine or topiramate or valproate or vigabatrin or zonisamide)
S4 or S5 or S6
(MH "Random Assignment")
(MH "Clinical Trials+")
TX random*
TX placebo*
TX group*
TX ((singl* or doubl* or tripl* or trebl*) and (mask* or blind*))
MH "Crossover Design"
TX crossover*
TX allocate*
TX assign*
S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17
S3 and S7 and S18
Appendix 5. Criteria for risk of bias in RCTs and CCTs
|
Item |
Judgement |
Description |
|
| 1 | Was the method of randomisation adequate? | Low risk | Investigators describe a random component in the sequence generation process, such as random number table; a computer random number generator; coin tossing; shuffling of cards or envelopes; throwing of dice; drawing of lots; minimisation |
| High risk | Investigators describe a non‐random component in the sequence generation process, such as odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | ||
| Unclear risk | Insufficient information about the sequence generation process to permit judgement | ||
| 2 | Was the treatment allocation concealed? | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed or nonopaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure Observational prospective study |
||
| Unclear risk | Insufficient information to permit judgement. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement | ||
| 3 | Was knowledge of the allocated interventions adequately prevented during the study? (blinding of participants and provider) | Low risk | Blinding of participants and providers and unlikely that the blinding could have been broken No blinding, but outcome measurements not likely to be influenced by lack of blinding |
| High risk | No blinding or incomplete blinding; outcome or outcome measurement likely to be influenced by lack of blinding Blinding of key study participants and personnel attempted but likely that blinding could have been broken |
||
| Unclear risk | Insufficient information to permit judgement | ||
| 4 | Was knowledge of the allocated interventions adequately prevented during the study? (blinding of outcome assessor) Objective outcomes Subjective outcomes |
Low risk |
Blinding of outcome assessor; unlikely that the blinding could have been broken No blinding but objective outcome measurement not likely to be influenced by lack of blinding |
| High risk | No blinding or incomplete blinding; outcome or outcome measurement likely to be influenced by lack of blinding | ||
| Unclear risk | Insufficient information to permit judgement | ||
| 5 | Were incomplete outcome data adequately addressed? For all outcomes except retention in treatment or dropout |
Yes |
No missing outcome data Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups For dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size Missing data imputed by appropriate methods All randomly assigned participants reported/analysed in the group to which they were allocated by randomization, irrespective of non‐compliance and co‐interventions (intention‐to‐treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups For dichotomous outcome data, proportions of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size As‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation |
||
| Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement (e.g. number randomly assigned not stated, no reasons for missing data provided; number of dropouts not reported for each group) |
Appendix 6. Treatment regimens in included studies
Topiramate: used in 10 trials (Baltieri 2008; De Sousa 2008; Florez 2008; Florez 2011; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Paparrigopoulos 2011; Rubio 2009). Dose ranged from 150 to 400 mg/d.
Gabapentin: used in five trials (Anton 2009; Anton 2011; Brower 2008; Furieri 2007; Trevisan 2008). Doses ranged from 600 to 1500 mg/d.
Valproate: used in three trials (Brady 2002; Salloum 2005; Trevisan 2008) (up to 1500 mg/d).
Levetiracetam: used in two trials (Fertig 2012; Richter 2012) (500 to 2000 mg/d).
Oxcarbazepine: used in two trials (Croissant 2006; Martinotti 2007). Dose ranged from 600 to 1800 mg/d. Two distinct ranges of doses (600 to 900 and 1500 to 1800 mg/d) corresponding to different arms were adopted in Martinotti 2007.
One trial each:
Zonisamide (up to 500 mg/d) (Arias 2010).
Carbamazepine (600 mg/d) (Mueller 1997).
Pregabalin (150 to 450 mg/d) (Martinotti 2010).
Tiagabine (15 to 20 mg/d) (Paparrigopoulos 2010).
Appendix 7. Rating instruments utilised in included studies
Addiction Severity Index (McLellan 1980; McLellan 1985; Kokkevi 1995; McLellan 1992), utilised in Brady 2002; De Sousa 2008; Florez 2008; Florez 2011; Johnson 2003; Kampman 2013; Mueller 1997; Salloum 2005.
Timeline follow‐back method (TLFB) (Sobell 1979; Sobell 1988; Sobell 1992), utilised in Anton 2009; Arias 2010; Brady 2002; Brower 2008; Croissant 2006; Fertig 2012; Florez 2011; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Mueller 1997; Paparrigopoulos 2010; Richter 2012; Rubio 2009; Salloum 2005; Trevisan 2008.
Form 90 for documenting alcohol consumption (Miller 1996), utilised in Anton 2011; Rubio 2009.
Pattern of alcohol use (Hughes 1980), utilised in Paparrigopoulos 2010.
Alcohol Withdrawal Rating Scale, utilised in Martinotti 2007.
Alcohol Dependence Scale (ADS) (Skinner 1982; Stockwell 1983; Skinner 1984), utilised in Anton 2009; Croissant 2006; De Sousa 2008.
Alcohol Use Disorders Identification Test (AUDIT) (Bohn 1995 a), utilized in Likhitsathian 2013.
Short Alcohol Dependence Data Questionnaire (SADD) (Davidson 1989), utilised in Mueller 1997.
Comprehensive Drinking Profile (Miller 1984), utilised in Johnson 2003.
Alcohol Use Disorders Identification Test (Bohn 1995 a), utilised in Johnson 2003; Johnson 2007.
Short Alcohol Dependence Data (SADD) (Raistrick 1983), utilised in Baltieri 2008.
Questionnaire on sociodemographic characteristics and alcohol and drug consumption, utilised in Baltieri 2008.
Alcohol Use Inventory (Horn 1983), utilised in Salloum 2005.
Drinker Inventory of Consequences (DrInC) (Miller 1995), utilised in Fertig 2012.
Modified Quantitative Alcohol Inventory/Craving Scales (Weiss 2003), utilised in Salloum 2005.
Alcohol Urge Questionnaire (AUQ) (Bohn 1995 b), utilised in Arias 2010.
Composite International Diagnostic Interview (CIDI) (Robins 1988), utilised in Paparrigopoulos 2011.
Clinical Institute Withdrawal Assessment for Alcohol‐revised (CIWA‐Ar) (Sullivan 1989), utilised in Anton 2009; Anton 2011; Brower 2008; Fertig 2012; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Martinotti 2010; Mueller 1997; Paparrigopoulos 2010; Paparrigopoulos 2011; Trevisan 2008.
Obsessive‐Compulsive Drinking Scale (OCDS) (Anton 1995; Moak 1998; Roberts 1999), utilised in Anton 2009; Anton 2011; Baltieri 2008; Brady 2002; Brower 2008; Croissant 2006; Florez 2008; Florez 2011; Johnson 2003; Martinotti 2007; Martinotti 2010; Paparrigopoulos 2010; Paparrigopoulos 2011; Richter 2012; Trevisan 2008.
Penn Alcohol Craving Scale (PACS) (Flannery 1999), utilised in Kampman 2013.
Craving Analogue Scale (Mottola 1993), utilised in Brady 2002; Martinotti 2007; Martinotti 2010.
Frequency, duration and intensity of craving (Anton 1995), utilised in De Sousa 2008.
Fagerstrom Test for nicotine dependence (Fangeström 1978), utilised in Fertig 2012; Florez 2008; Florez 2011.
Minnesota Cocaine Craving Scale (MCCS) (Halikas 1991), utilised in Kampman 2013.
Cocaine Selective Severity Assessment (CSSA) (Kampman 1998), utilised in Kampman 2013.
Epworth Sleepiness Scale (Johns 1991), utilised in Anton 2009; Anton 2011.
Pittsburgh Sleep Quality Inventory (PSQI) (Buysse 1989), utilised in Anton 2009; Trevisan 2008.
Insomnia Severity Index (Bastien 2001), utilised in Anton 2009; Anton 2011.
Sleep Problems Questionnaire (SPQ) (Jenkins 1988), utilised in Brower 2008.
Sleep Diaries (Conroy 2006), utilised in Brower 2008.
Polysomnography (PSG), utilised in Brower 2008.
Insomnia Interview Schedule (Morin 1993), utilised in Brower 2008.
Structured Clinical Interview for DSM‐IV (SCID) (First 1994; First 1995; First 1997; Spitzer 1990; Spitzer 1992), utilised in Anton 2009; Anton 2011; Arias 2010; Brady 2002; Brower 2008; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Martinotti 2007; Martinotti 2010; Mueller 1997; Rubio 2009; Salloum 2005.
Composite International Diagnostic Interview (CIDI) (WHO), utilised in Paparrigopoulos 2010.
Schedules for Clinical Assessment in Neuropsychiatry (SCAN) (Wing 1990), utilised in Paparrigopoulos 2011.
Mini International Neuropsychiatric Interview (MINI) (Sheehan 1998), utilised in Kampman 2013; Likhitsathian 2013.
Symptom Checklist‐90 Revised (SCL90‐R; Derogatis 1977), utilised in Brady 2002; Martinotti 2007; Martinotti 2010.
WHO Psychiatric Disability Assessment Schedule (WHODAS) (Janca 1996), utilised in Florez 2008; Florez 2011.
Short Index of Problems (Feinn 2003), utilised in Brower 2008.
Global Assessment Scale (GAS) (Endicott 1976), utilised in Paparrigopoulos 2010; Paparrigopoulos 2011; Salloum 2005.
California Personality Inventory–Socialization Subscale (CPI‐SO) (Cooney 1990), utilised in Mueller 1997.
International Personality Disorder Examination (IPDE) (Loranger 1994), utilised in Florez 2008; Florez 2011.
Mini‐Mental State Exam (Folstein 1975), utilised in Brower 2008; Furieri 2007.
Clinical Global Impression (CGI) (Guy 1976), utilised in Kampman 2013; Martinotti 2007; Martinotti 2010.
Global Assessment of Function (GAF) (APA 1987), utilised in Mueller 1997.
Profile of Mood States (POMS) (McNair 1971; Guadagnoli 1989), utilised in Anton 2009; Anton 2011; Fertig 2012; Mueller 1997; Trevisan 2008.
Life‐Time Charting of Bipolar Episodes (Post 1986), utilised in Salloum 2005.
Bech‐Rafaelsen Mania Scale (BRMS) (Bech 1979), utilised in Salloum 2005.
Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery 1979), utilised in Fertig 2012; Johnson 2007.
Hamilton Depression Rating Scale (HAM‐D) (Hamilton 1960; Williams 1988; Thase 1983), utilised in Baltieri 2008; Brower 2008; Paparrigopoulos 2010; Paparrigopoulos 2011; Rubio 2009; Salloum 2005.
Hamilton Anxiety Rating Scale (HARS) (Hamilton 1959), utilised in Brower 2008; Fertig 2012; Paparrigopoulos 2010; Paparrigopoulos 2011.
Beck Depression Inventory (BDI) (Beck 1961), utilised in Arias 2010; Anton 2011; Brady 2002; Croissant 2006; Mueller 1997.
Beck Anxiety Inventory (BAI) (Beck 1988), utilised in Brady 2002.
State‐Trait Anxiety Inventory (STAI) (Spielberger 1970; Spielberger 1983), utilised in Croissant 2006; Mueller 1997.
Continuous Performance Test (Conners 1995), utilised in Rubio 2009.
Stop‐Signal Task (Logan 1994), utilised in Rubio 2009.
Differential Reinforcement of Low‐Rate Responding (DRLR) (Gordon 1988), utilised in Rubio 2009.
Barratt Impulsivity Scale (Patton 1995), utilised in Brady 2002; Rubio 2009,
Buss‐Durkee Hostility Index (Buss 1957), utilised in Brady 2002.
Anger, Irritability, Aggression Scale (Coccaro 1991), utilised in Brady 2002.
Readiness to Change Questionnaire (RCQ) (Rollnik 1992), utilised in Florez 2008; Florez 2011.
European Quality of Life Questionnaire (EQ‐5D) (EuroQuoL 1990), utilised in Florez 2008; Florez 2011.
Quality of Life (QOL) Index (Spitzer 1981), utilised in Martinotti 2010.
Quality‐of‐Life Short Form 12 (SF‐12) (Szabo 1996), utilised in Fertig 2012.
Medical Outcomes Study Short Form 36‐item questionnaire (SF‐36) (Kongsakon 2000), utilised in Likhitsathian 2013.
Weekly Self‐Help Activity Questionnaire, utilised in Salloum 2005.
Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale (Lingjaerde 1987), utilised in Baltieri 2008; Florez 2008; Florez 2011; Furieri 2007.
Morisky‐Green test for medication compliance (Morisky 1986), utilised in Florez 2008; Florez 2011.
Systematic assessment for treatment‐emergent events questionnaire (Levine 1986), utilised in Johnson 2003.
Somatic Symptoms Checklist and Medication Adherence Form, utilised in Salloum 2005,
COMBINE SAFTEE (Johnson 2005), utilised in Paparrigopoulos 2011; Rubio 2009.
Reasons for Early Termination Form (Crits‐Christoph 1999), utilised in Salloum 2005.
Self report symptom inventory, utilised in Trevisan 2008.
Appendix 8. Outcomes
Dropout (dropout rate): Anton 2009; Arias 2010; Baltieri 2008; Brady 2002; Brower 2008; De Sousa 2008; Fertig 2012; Florez 2008; Florez 2011; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Martinotti 2007; Martinotti 2010; Mueller 1997; Paparrigopoulos 2011; Richter 2012; Rubio 2009; Salloum 2005; Trevisan 2008.
Heavy drinking, dichotomous outcome (participants with heavy drinking during the trial): Brady 2002; Brower 2008; Florez 2008; Florez 2011; Likhitsathian 2013; Mueller 1997; Richter 2012; Salloum 2005.
Drinks/drinking day: Anton 2011; Brady 2002; Brower 2008; Croissant 2006; Fertig 2012; Florez 2011; Furieri 2007; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Rubio 2009; Salloum 2005.
Heavy drinking, continuous outcome: Anton 2011; Arias 2010; Baltieri 2008; Brower 2008; De Sousa 2008; Fertig 2012; ; Florez 2011; Furieri 2007; Johnson 2003; Johnson 2007; Martinotti 2007; Martinotti 2010; Rubio 2009; Salloum 2005; Trevisan 2008.
Continuous abstinence, dichotomous outcome (participants abstinent during the trial): Anton 2009; Baltieri 2008; Brady 2002; Brower 2008; Croissant 2006; De Sousa 2008; Fertig 2012; Florez 2008; Florez 2011; Furieri 2007; Martinotti 2007; Martinotti 2010; Mueller 1997; Richter 2012.
Time to first relapse: Anton 2009; Baltieri 2008; Brower 2008; Croissant 2006; De Sousa 2008; Martinotti 2007; Martinotti 2010; Mueller 1997; Richter 2012; Salloum 2005; Trevisan 2008.
Percentage of days of abstinence: Anton 2009; Arias 2010; Brower 2008; Croissant 2006; De Sousa 2008; Fertig 2012; Florez 2011; Furieri 2007; Johnson 2003; Johnson 2007; Rubio 2009.
Adverse events (withdrawal for medical reasons): Arias 2010; Brower 2008; Fertig 2012; Florez 2008; Johnson 2003; Johnson 2007; Kampman 2013; Likhitsathian 2013; Martinotti 2007; Martinotti 2010; Mueller 1997; Paparrigopoulos 2010; Paparrigopoulos 2011; Richter 2012; Rubio 2009; Salloum 2005; Trevisan 2008.
Craving: Baltieri 2008; Brady 2002; De Sousa 2008; Florez 2008; Florez 2011; Furieri 2007; Johnson 2007; Martinotti 2007; Martinotti 2010; Paparrigopoulos 2010; Paparrigopoulos 2011; Rubio 2009; Trevisan 2008.
Liver enzyme levels (GGT): Arias 2010; Baltieri 2008; Brower 2008; Fertig 2012; Florez 2008; Florez 2011; Furieri 2007; Paparrigopoulos 2010; Salloum 2005; Trevisan 2008.
Data and analyses
Comparison 1. Anticonvulsants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 16 | 1675 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.19] |
| 2 Heavy drinking, dichotomous outcome | 5 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.22] |
| 3 Alcohol use, continuous outcome: drinks/drinking day | 11 | 1126 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐2.32, ‐0.65] |
| 4 Alcohol use, continuous outcome: heavy drinking | 12 | 1129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.51, ‐0.19] |
| 5 Continuous abstinence, dichotomous outcome | 8 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.97, 1.52] |
| 6 Abstinence, continuous outcome | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Time to first relapse (weeks) | 4 | 415 | Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.28, 1.95] |
| 6.2 Days abstinent % | 8 | 814 | Mean Difference (IV, Random, 95% CI) | 8.47 [1.57, 15.38] |
| 7 Adverse events | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Withdrawn for medical reasons | 12 | 1410 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.58, 2.56] |
| 7.2 Nausea/stomach discomfort/vomiting | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.51, 1.75] |
| 7.3 Dry mouth | 3 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.95, 2.90] |
| 7.4 Drowsiness/somnolence/psychomotor slowing | 8 | 1112 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.99, 1.84] |
| 7.5 Fatigue | 5 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.82, 2.23] |
| 7.6 Dizziness | 6 | 882 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.28, 3.06] |
| 7.7 Headache | 7 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.72, 1.18] |
| 7.8 Nervousness/irritability | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.99, 2.26] |
| 7.9 Constipation | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.31, 4.02] |
| 7.10 Paraesthesia | 7 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.53, 4.65] |
| 7.11 Insomnia | 4 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.52] |
| 7.12 Diarrhoea | 5 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.85, 2.12] |
| 7.13 Memory problems | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.94, 3.30] |
| 7.14 Anorexia | 4 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.87, 3.45] |
| 7.15 Dyspepsia | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.24] |
| 7.16 Pruritus | 4 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [0.96, 7.05] |
| 7.17 Myalgia | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.25, 5.08] |
| 7.18 Stomach difficulties | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.67, 5.39] |
| 7.19 Gastrointestinal problems | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.65, 4.86] |
| 8 Craving (OCDS total score or analogue scale) | 6 | 553 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.60, ‐0.09] |
| 9 Liver enzyme levels (GGT) | 7 | 405 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐8.51, 8.65] |
Comparison 2. Specific anticonvulsants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate | 6 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.28] |
| 1.2 Gabapentin | 4 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 1.3 Valproate | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 1.4 Levetiracetam | 2 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.29, 3.04] |
| 2 Heavy drinking, dichotomous outcome: valproate | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.42, 0.98] |
| 3 Alcohol use, continuous outcome: drinks/drinking day | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate | 5 | 760 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐2.56, ‐0.53] |
| 3.2 Gabapentin | 3 | 155 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐4.21, ‐0.06] |
| 3.3 Valproate | 2 | 81 | Mean Difference (IV, Random, 95% CI) | ‐2.55 [‐4.96, ‐0.14] |
| 4 Alcohol use, continuous outcome: heavy drinking | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate | 5 | 696 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.69, ‐0.20] |
| 4.2 Gabapentin | 4 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.75, ‐0.15] |
| 4.3 Valproate | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.84, 0.06] |
| 5 Abstinence, continuous outcome | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Time to first relapse (weeks): valproate | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐2.46, 5.36] |
| 5.2 Days abstinent (%): topiramate | 3 | 537 | Mean Difference (IV, Random, 95% CI) | 15.51 [4.55, 26.47] |
| 5.3 Days abstinent (%): gabapentin | 3 | 107 | Mean Difference (IV, Random, 95% CI) | 5.82 [‐6.87, 18.51] |
| 6 Continuous abstinence, dichotomous outcome | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Gabapentin | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.30, 4.24] |
| 6.2 Levetiracetam | 2 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.38] |
| 7 Adverse events | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Withdrawn for medical reasons: topiramate | 5 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.88, 4.80] |
| 7.2 Withdrawn for medical reasons: valproate | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.10, 5.87] |
| 7.3 Withdrawn for medical reasons: levetiracetam | 2 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.85] |
| 8 Craving (OCDS total score or analogue scale) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.82, 0.04] |
| 8.2 Gabapentin | 2 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.81, 0.16] |
| 8.3 Valproate | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.90, 0.48] |
| 9 Liver enzyme levels | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 GGT: Gabapentin | 3 | 84 | Mean Difference (IV, Random, 95% CI) | ‐4.75 [‐25.36, 15.87] |
| 9.2 GGT: Valproate | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.68 [‐48.11, 49.47] |
Comparison 3. Anticonvulsants versus placebo according to length of trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to six weeks | 4 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.19] |
| 1.2 Over six weeks | 12 | 1477 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.29] |
| 2 Heavy drinking, dichotomous outcome: over six weeks | 4 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.32] |
| 3 Alcohol use, continuous outcome: drinks/drinking day | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Up to six weeks | 3 | 155 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐4.21, ‐0.06] |
| 3.2 Over six weeks | 8 | 971 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.46, ‐0.42] |
| 4 Alcohol use, continuous outcome: heavy drinking | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Up to six weeks | 4 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.73, ‐0.17] |
| 4.2 Over six weeks | 8 | 918 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.10] |
| 5 Continuous abstinence, dichotomous outcome | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Up to six weeks | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.65, 2.32] |
| 5.2 Over six weeks | 5 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.92, 1.54] |
| 6 Abstinence, continuous outcome | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Days abstinent (%): up to six weeks | 3 | 107 | Mean Difference (IV, Random, 95% CI) | 5.82 [‐6.87, 18.51] |
| 6.2 Days abstinent (%): over six weeks | 5 | 707 | Mean Difference (IV, Random, 95% CI) | 9.98 [0.95, 19.01] |
| 7 Adverse events | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Withdrawn for medical reasons: up to six weeks | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.04, 1.41] |
| 7.2 Withdrawn for medical reasons: over six weeks | 10 | 1332 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.72, 3.20] |
Comparison 4. Anticonvulsants versus placebo according to psychosocial interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Associated psychotherapy | 7 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.96] |
| 1.2 Associated other interventions | 6 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.67, 1.65] |
| 2 Heavy drinking, dichotomous outcome: associated psychotherapy | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.26, 0.92] |
| 3 Alcohol use, continuous outcome: drinks/drinking day | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Associated psychotherapy | 6 | 427 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.73, ‐0.63] |
| 3.2 Associated other interventions | 5 | 699 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐3.06, 0.18] |
| 4 Alcohol use, continuous outcome: heavy drinking | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Associated psychotherapy | 5 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.68, ‐0.25] |
| 4.2 Associated other interventions | 6 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.55, ‐0.01] |
| 5 Continuous abstinence, dichotomous outcome | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Associated psychotherapy | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.73, 2.10] |
| 5.2 Associated other interventions | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.55, 2.45] |
| 6 Abstinence, continuous outcome, days abstinent (%): associated psychotherapy | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Associated psychotherapy | 3 | 127 | Mean Difference (IV, Random, 95% CI) | 4.94 [‐5.50, 15.37] |
| 6.2 Associated other interventions | 4 | 647 | Mean Difference (IV, Random, 95% CI) | 12.57 [1.51, 23.64] |
| 7 Adverse events | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Withdrawn for medical reasons: associated psychotherapy | 4 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.68, 5.09] |
| 7.2 Withdrawn for medical reasons: associated other interventions | 5 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.60, 5.06] |
Comparison 5. Anticonvulsants versus placebo according to psychiatric co‐morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding psychiatric co‐morbidity | 8 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.16] |
| 1.2 Not excluding psychiatric co‐morbidity | 8 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.61] |
| 2 Heavy drinking, dichotomous outcome | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding psychiatric co‐morbidity | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.68] |
| 2.2 Not excluding psychiatric co‐morbidity | 3 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.37, 1.45] |
| 3 Alcohol use, continuous outcome: drinks/drinking day | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Excluding psychiatric co‐morbidity | 6 | 823 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐2.35, ‐0.75] |
| 3.2 Not excluding psychiatric co‐morbidity | 5 | 303 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.51, 0.48] |
| 4 Alcohol use, continuous outcome: heavy drinking | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Excluding psychiatric co‐morbidity | 5 | 730 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.60, ‐0.18] |
| 4.2 Not excluding psychiatric co‐morbidity | 7 | 399 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.58, ‐0.05] |
| 5 Continuous abstinence, dichotomous outcome | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Excluding psychiatric co‐morbidity | 4 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.80, 1.55] |
| 5.2 Not excluding psychiatric co‐morbidity | 4 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.98, 1.92] |
| 6 Abstinence, continuous outcome | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Time to first relapse (weeks): not excluding psychiatric co‐morbidity | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.60, 1.13] |
| 6.2 Days abstinent (%): excluding psychiatric co‐morbidity | 3 | 523 | Mean Difference (IV, Random, 95% CI) | 9.92 [‐5.19, 25.03] |
| 6.3 Days abstinent (%): not excluding psychiatric co‐morbidity | 5 | 291 | Mean Difference (IV, Random, 95% CI) | 6.95 [‐0.37, 14.26] |
| 7 Adverse events | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Withdrawn for medical reasons: excluding psychiatric co‐morbidity | 5 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.34, 4.00] |
| 7.2 Withdrawn for medical reasons: not excluding psychiatric co‐morbidity | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.55, 3.29] |
Comparison 6. Anticonvulsants versus other medications (naltrexone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 5 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.06] |
| 2 Severe relapse, dichotomous outcome | 4 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.07] |
| 3 Number of heavy drinking days | 3 | 308 | Mean Difference (IV, Random, 95% CI) | ‐5.21 [‐8.58, ‐1.83] |
| 4 Continuous abstinence, dichotomous outcome | 5 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.99, 1.49] |
| 5 Abstinence continuous outcome: days to severe relapse | 3 | 244 | Mean Difference (IV, Random, 95% CI) | 11.88 [3.29, 20.46] |
| 6 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Withdrawn for medical reasons | 3 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.58] |
| 6.2 Nausea | 3 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.07, 14.32] |
| 6.3 Dizziness | 3 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.29, 4.37] |
| 6.4 Hypotension | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.75] |
| 6.5 Paraesthesia | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 6.07 [1.11, 33.17] |
| 6.6 Diarrhoea | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.31, 27.55] |
| 6.7 Sedation | 2 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.26, 1.40] |
| 7 Craving (OCDS total score) | 4 | 385 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐3.58, ‐0.93] |
| 8 Liver enzyme levels (GGT) | 3 | 358 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐10.82, 8.66] |
6.8. Analysis.
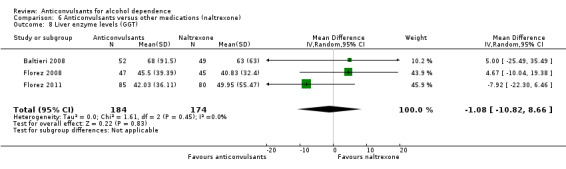
Comparison 6 Anticonvulsants versus other medications (naltrexone), Outcome 8 Liver enzyme levels (GGT).
Comparison 7. Specific anticonvulsants (topiramate) versus specific other medications (naltrexone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 3 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.52, 1.19] |
| 2 Severe relapse, dichotomous outcome | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 3 Continuous abstinence, dichotomous outcome | 3 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.49] |
| 4 Craving (OCDS total score) | 2 | 257 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐4.28, ‐1.21] |
Comparison 8. Anticonvulsants versus no medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol use dichotomous: relapse | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 2 Mood | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 HDRS | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐5.19, ‐2.41] |
| 2.2 HARS | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐3.26 [‐4.64, ‐1.88] |
| 3 Craving (OCDS score) | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐4.97 [‐8.79, ‐1.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anton 2009.
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | 60 participants; mean age 46.3 years; 76.7% male; 88% Caucasian; 14 years of education on average; 52% married; 87.4% heavy drinking days on average Inclusion criteria: age between 18 and 70 years; meeting DSM‐IV criteria for alcohol dependence by SCID; drinking at least five drinks per day on 70% of the days in the last month; having a last drink no more than 72 hours before randomisation; having stable housing for at least three months Exclusion criteria: having other major psychiatric conditions or meeting criteria for other substance abuse or dependence except for marijuana or nicotine; taking any psychoactive medications in the last two weeks and/or benzodiazepines in the urine or use of zolpidem or zalepon in the past two weeks; history of alcohol withdrawal seizures or delirium tremens; current suicidal ideation; use of disulfiram, naltrexone, acamprosate or anticonvulsants; having clinically significant medical problems that would impair participation; being a sexually active female of child‐bearing potential, pregnant, breast‐feeding or not using a reliable form of birth control; having pending current charges for a violent crime; taking gabapentin or flumazenil in the last month or experiencing adverse effects from either at any time in the past; having elevations of ALT and AST greater than three times normal |
|
| Interventions | (1) Gabapentin plus flumazenil, 33 participants; (2) placebo, 27 participants Drug dose: gabapentin up to 1200 mg/d; flumazenil 2 mg/d Associated medications: hydroxyzine HCl (50 mg) was given on days one through seven; thiamine (200 mg) and multivitamins were also to be taken for 30 days Participants were involved in weekly manualised behavioural intervention (Miller 2004) Setting: outpatient Duration: 39 days (flumazenil was administered for two days). Country of origin: USA |
|
| Outcomes | Primary drinking outcomes were percentage of days abstinent during the trial and time to first heavy drinking day after randomisation Secondary endpoints were number of subjects completely abstinent during the study and change in %CDT levels as a biomarker of heavy alcohol consumption |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods applied to account for missing data. Intent‐to‐treat approach not clearly reported |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blindness of outcome assessors |
| Blinding of outcome assessment (detection bias) objective | Low risk | Double‐blind stated. Medication and placebo prepared to appear identical Outcome measurement not likely to be influenced by lack of blinding |
Anton 2011.
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | 150 participants; mean age 44.5 years; 82% male; 88% Caucasian; 23% heavy drinking days on average Inclusion criteria: meeting DSM‐IV criteria for alcohol dependence; consuming on average five or more standard drinks per day for men or four or more drinks per day for women; being able to maintain sobriety for four days before randomisation, living near the study site in a stable living situation Exclusion criteria: meeting DSM‐IV criteria for other substance dependence (except nicotine); using illicit drugs in the past 30 days or having a positive urine drug screen; meeting DSM‐IV criteria for an Axis I disorder; having current suicidal or homicidal ideation; needing maintenance with psychotropic or anticonvulsant medication; having unstable medical conditions; having liver enzyme (ALT and AST) levels greater than three times normal; using disulfiram, acamprosate or either of the study medications within the past 30 days; taking an opioid medication on a routine basis; having legal charges pending; having undergone more than one previous inpatient medical detoxification treatment |
|
| Interventions | (1) Naltrexone plus gabapentin, 50 participants; (2) naltrexone plus placebo, 50 participants; (3) placebo plus placebo, 50 participants Drug dose: naltrexone 50 mg/d; gabapentin up to 1200 mg/d Participants provided sessions of combined behavioural intervention therapy using the behavioural intervention treatment manual of the COMBINE study (Miller 2004), which combined cognitive‐behavioural therapy, motivation interviewing and 12‐step facilitation techniques in a client needs–based approach Setting: outpatient Duration: 16 weeks (gabapentin added for first six weeks). Country of origin: USA |
|
| Outcomes | Primary outcome measures: time to relapse to drinking; symptoms such as difficulty falling asleep and/or staying asleep, irritability and nervousness, as measured by a symptom checklist and specific scales Secondary outcome measures: percentage of days drinking; drinks per drinking day; retention in the protocol; craving for alcohol; CDT and GGT measured as change from baseline; psychological and general health functioning as measured by Beck Anxiety and Depression scales; liver function tests (ALT and AST) |
|
| Notes | This study has been included for the only six weeks of gabapentin use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Methods applied to account for missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Double‐dummy placebo‐controlled medication design applied. Medication dispensed in identically packaged blister cards. No specific reference made to blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | Double‐dummy placebo‐controlled medication design applied. Medication dispensed in identically packaged blister cards. No specific reference made to blindness of outcome assessors |
| Blinding of outcome assessment (detection bias) objective | Low risk | Double‐dummy placebo‐controlled medication design applied. Medication dispensed in identically packaged blister cards. No further details provided Outcome measurement not likely to be influenced by lack of blinding |
Arias 2010.
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | 40 participants; mean age 49.2 years; 57.5% male; 90% Caucasian; 43% college education; 65% married; 80% employed; 53 drinking days (past 60 days) on average; 45 heavy drinking days on average; age of onset of alcohol dependence 37 years on average; AUQ score 18 on average; BDI score 10 on average; drug use disorder 40%; concomitant antidepressant 37% Inclusion criteria: meeting DSM‐IV Alcohol Dependence criteria (past month) and at least two heavy drinking days (men, 94 standard drinks per day; women, 93 standard drinks per day) per week between screening and baseline; being able to read at an eighth grade or higher level, with no gross cognitive impairment; women of child‐bearing potential, non‐lactating and practising a reliable method of birth control, with negative serum pregnancy test before treatment Exclusion criteria: having a current, clinically significant disease or abnormality, having participated in a pharmacotherapy study in the preceding 30 days or being currently dependent on drugs other than nicotine; having a history of hypersensitivity to any sulfonamide, a penicillin allergy, a severe allergic reaction to any drug or a systemic autoimmune; having a history of renal calculi |
|
| Interventions | (1) Zonisamide, 20 participants; (2) placebo, 20 participants Drug dose: zonisamide up to 500 mg/d All participants received six biweekly sessions of brief (20‐minute) cognitive‐behavioural counselling and psychoeducation Setting: outpatient Duration: 12 weeks. Country of origin: USA |
|
| Outcomes | Primary outcomes were abstinent days per week and heavy drinking days per week Secondary outcomes were drinks per week, alcohol urge score and GGT concentration; adverse effects were also considered. Alcohol consumption was assessed using TLFB (Sobell 1992); desire (urge/craving) to drink was assessed with the AUQ (Bohn 1995 b) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation in groups of ten using a specific software |
| Allocation concealment (selection bias) | Low risk | Block randomisation was implemented by an investigational pharmacy to ensure that investigators and staff were blind to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All efficacy analyses were by intention‐to‐treat, with all available data included |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Zonisamide capsules were over‐encapsulated to appear identical to a placebo capsule filled with lactose. Investigators and staff were blind to study group assignment |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Zonisamide capsules were over‐encapsulated to appear identical to a placebo capsule filled with lactose. Investigators and staff were blind to study group assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Zonisamide capsules were over‐encapsulated to appear identical to a placebo capsule filled with lactose. Investigators and staff were blind to study group assignment |
Baltieri 2008.
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | 155 participants; mean age 44.3 years; 100% male; 71% Caucasian; 36% high school graduate; 51.6% married; 10 years on average since alcohol‐related problems occurred; 301 grams of alcohol used per day on average; mean score on SADD was 29; HDRS score was 10 on average Inclusion criteria: being 18 to 60 years of age with an ICD‐10 (WHO 1992) diagnosis of alcohol dependence Exclusion criteria: younger than 18 years or older than 65 years of age; current diagnosis of dependence or abuse of other substances except, nicotine; serious clinical co‐existing diseases (e.g. inadequately controlled diabetes, cardiac failure, alcoholic cirrhosis); previous treatment with naltrexone or topiramate within six months of randomisation; concomitant psychiatric disorders that might require specific drug treatment; inability to give full informed consent; clinical history of mental retardation |
|
| Interventions | (1) Topiramate, 52 participants; (2) naltrexone, 49 participants; (3) placebo, 54 participants Drug dose: topiramate up to 300 mg/d; naltrexone, 50 mg/d All participants received standardised brief cognitive‐behavioural interventions. Relapse prevention counselling was also offered Setting: outpatient Duration: 12 weeks. Country of origin: Brazil |
|
| Outcomes | Time to first relapse (consumption of > 60 g ethyl alcohol); cumulative abstinence duration; weeks of heavy drinking; alcohol abuse hepatic indices (GGT, AST, ALT) and MCV; side effects | |
| Notes | All participants received one‐week detoxification before initiation of double‐blind treatment. Participants who manifested withdrawal symptoms were given medications such as lorazepam and B1 vitamin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number list was used |
| Allocation concealment (selection bias) | Low risk | Only two pharmacists from the pharmacy sector knew which medication corresponded to the specific code. Packages containing the capsules were distributed to participants by two blinded research assistants. Medication codes were revealed to researchers only after all participants had completed the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Criteria followed intention‐to‐treat principle. Any randomly assigned participant who took at least one dose was included in the evaluation. Participants who missed a visit or withdrew from the study were deemed to be non‐abstinent at the time of missed visits |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | All capsules in each treatment group had identical appearance and size and were manufactured by the pharmacy. Doctors were blind to medication condition |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Medication codes were revealed to researchers only after all participants had completed the study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Medication codes were revealed to researchers only after all participants had completed the study |
Brady 2002.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 39 participants; mean age 40.1 years; 39% male; 46% Caucasian; BDI (Beck 1961), 11 on average; education 14 years on average; ASI (McLellan 1985) composite score 0.77 on average Inclusion criteria: meeting DSM‐IV criteria for alcohol dependence according to the SCID (First 1997) Exclusion criteria: any other substance dependence diagnosis (except nicotine and caffeine); court commitment to treatment; current use of any antidepressant, antipsychotic, anticonvulsant, anxiolytic agent or pharmacological treatment for alcoholism (disulfiram or naltrexone); severe medical illness; platelet count < 100 000/mm3 or ALT, AST > three times normal; bipolar disorder, psychotic disorder, major depressive disorder; other psychiatric disorders severe enough to interfere with study participation |
|
| Interventions | (1) Divalproex, 19 participants; (2) placebo, 20 participants Drug dose: divalproex, up to 1500 mg/d All participants received one hour per week of manualised, alcohol‐directed CBT (Kadden 1995; Monti 1989) Setting: outpatient Duration: 12 weeks. Country of origin: USA |
|
| Outcomes | Drinks per drinking day; % of days drinking, % of days heavy drinking (five or more drinks in 24‐hour period), measured using TLFB (Sobell 1992) and breath alcohol level; alcohol craving measured with the OCDS (Anton 1996) and the Craving Analogue Scale; impulsivity measured with the Barratt Impulsivity Scale (Patton 1995); irritability and aggression evaluated with the Buss‐Durkee Hostility Index (Buss 1957) and the Anger, Irritability, Aggression Scale (Coccaro 1991); depression measured with the BDI (Beck 1961) and anxiety measured with the BAI(Beck 1988); global psychological symptoms measured with the SCL90‐R (Derogatis 1977); illicit drug use evaluated with urine drug screen. Adverse effects included platelet count and liver function evaluation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Because of participant attrition and missing data, analyses conducted on a final sample of 29 participants. Group means and last‐point‐carried‐forward analysis used for estimate of missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Manufacturer provided divalproex and matching placebo tablets. Unblinded investigator monitored laboratory reports |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | Information insufficient to permit judgement |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Brower 2008.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 21 participants: mean age 45 years; 52% male; 9.5% Afro‐American; 14 years education on average; 38% married; 65% employed; 24 years of age on average at onset of problem drinking; 93.5% drinking days in past 42 days Inclusion criteria: 18 years and older; meeting DSM‐IV criteria for current alcohol dependence according to SCID (First 1997); meeting study criteria for insomnia, evaluated by Insomnia Interview Schedule (Morin 1993); insomnia persisting during placebo lead‐in period for at least one week of abstinence in the absence of withdrawal symptoms as determined by the CIWA‐Ar (Sullivan 1989); expressing desire or willingness to abstain from alcohol and other drugs (except nicotine) during the study. Women eligible if they were not nursing, tested negative for pregnancy and used reliable contraception if pregnancy was possible Exclusion criteria: insomnia due to medications; requiring treatment with medications known to affect sleep; taking medications known to influence drinking outcomes such as naltrexone, disulfiram or acamprosate; insomnia due to medical illness, chronic pain, a non‐alcohol substance use disorder (except nicotine dependence); DSM‐IV panic disorder, social phobia, generalised anxiety disorder, post‐traumatic stress disorder, major depression, anorexia nervosa or bulimia nervosa in the past month; lifetime history of psychosis, bipolar disorder or obsessive‐compulsive disorder; danger to self or others; unstable or distant housing; illiteracy; cognitive impairment (Mini‐Mental State Exam < 27; Folstein 1975); personality disorders judged likely to interfere with compliance; known allergy or hypersensitivity to gabapentin; impaired renal function; evidence of a primary sleep disorder including sleep apnoea or periodic leg movement disorder |
|
| Interventions | (1) Gabapentin, 10 participants; (2) placebo, 11 participants Drug dose: gabapentin up to 1500 mg/d Participants also received up to six 30‐minute sessions of behavioural therapy as outlined in a treatment manual with focus to enhance adherence to study medication (Carroll 1996 a) Setting: outpatient Duration: six weeks. Country of origin: USA |
|
| Outcomes | Survival in days to first episode of heavy drinking; frequency and quantity of self‐reported drinking using the TLFB (Sobell 1988), using breath tests for alcohol at each study visit and a blood level for GGT; craving measured by the OCDS (Anton 1996; Moak 1998); consequences of drinking measured with the Short Index of Problems (Feinn 2003); subjective sleep using the Sleep Problems Questionnaire (Jenkins 1988) and sleep diaries (Conroy 2006); objective sleep using polysomnography; anxiety and depression, evaluated with the HDRS (Williams 1988) and the HARS (Hamilton 1959) | |
| Notes | Study included one to two weeks of a single‐blind placebo lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation balanced for gender (Stout 1994). Randomisation schedule generated by a statistician |
| Allocation concealment (selection bias) | Low risk | Randomisation implemented by a non‐blinded research pharmacist. Study investigators, raters and participants blinded to treatment assignment until all study visits completed and data set cleaned and locked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used; any participants lost to follow‐up were categorised as relapsed to heavy drinking at the time of last assessment |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Study investigators and raters blinded to treatment assignment. Gabapentin and placebo capsules identical in appearance |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Study investigators and raters blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Study investigators and raters blinded to treatment assignment |
Croissant 2006.
| Methods | Randomised controlled open study | |
| Participants | 30 participants; mean age 45.6 years; 75% male; 25% more than 12 years of education; 50% married; 50% employed; 11.5 years average duration of alcohol dependence; 12 drinks/d on average of ethanol consumption; 17.30 drinks per drinking day on average; OCDS score 16 on average Inclusion criteria: alcohol dependence according to both ICD‐10 and DSM‐IV; age between 18 and 65 years; knowing enough German to fill in the questionnaires provided; being committed to the goal of total abstinence; participants required to have a fixed residence Exclusion criteria: positive screening results for opiates, amphetamine and cocaine; severe somatic, psychiatric or terminal disease; pregnancy, lactation period, suicidal tendencies and legal or illegal drug addiction (except nicotine dependence and infrequent THC consumption); participating in other clinical trials; taking antipsychotic drugs or antidepressants, carbamazepine or benzodiazepines; participants for whom oxcarbazepine or acamprosate was contraindicated; participants unable to give full informed consent |
|
| Interventions | (1) Oxcarbazepine, 15 participants; (2) acamprosate, 15 participants Drug dose: oxcarbazepine up to 1200 mg/d; acamprosate 1998 mg/d Setting: outpatient Duration: 12 weeks of treatment plus 12 weeks of follow‐up. Country of origin: Germany |
|
| Outcomes | Primary endpoint: time to first severe relapse, based on TLFB or failure to attend clinical examinations with no reliable information on drinking status available Secondary endpoints: time to first consumption of any ethanol; percentage of days abstinent; abstinence rates; drinks per drinking day; craving measured by total score on OCDS‐G and SCL90‐R Evaluation of safety and tolerability: safety evaluated by biological markers of heavy drinking (GGT, MCV) and markers of possible toxicity (AST, ALT, number of thrombocytes, sodium); tolerability assessed by recording side effects using questionnaires |
|
| Notes | Participants enrolled after completion of detoxification | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated; no further information available from the study |
| Allocation concealment (selection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat basis approach followed, including all participants who received at least one dose of medication. Last observation carried forward also applied. Missing data not replaced |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study. Data management and statistics performed by a biometric section, which was blind to drug assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
De Sousa 2008.
| Methods | Randomised controlled open study | |
| Participants | 100 participants; mean age 43.4 years; 100% male; 98% secondary education; 98% married; 72% employed; 27 on average on Severity of Alcohol Dependence Scale; 0.71 on average on ASI severity score; 54 on average on craving score; 84 days of drinking in the last month on average; 10 drinks per day on average Inclusion criteria: age between 18 and 65 years; alcohol dependence according to DSM‐IV criteria; having a stable family environment so that the family could ensure maximisation of treatment compliance and could provide regular follow‐up information Exclusion criteria: presence of other substance use disorders (excluding nicotine dependence); presence of any co‐morbid psychiatric disorder; any medical condition present that would interfere with treatment compliance or would be a contraindication to drugs in the study; any routine liver function test values greater than three times above normal value; previous treatment with either of the two drugs in the study |
|
| Interventions | (1) Topiramate, 50 participants; (2) disulfiram, 50 participants Drug dose: topiramate 150 mg/d; disulfiram 250 mg/d Participants involved in weekly supportive group psychotherapy. Participants also received symptomatic treatment for depression (duloxetine 20 to 40 mg/d) and/or insomnia (zolpidem 5 to 10 mg at night) when needed Setting: outpatient Duration: nine months. Country of origin: India |
|
| Outcomes | Accumulated reported days of abstinence; days until first relapse (defined as consumption of more than five alcoholic drinks/40 g alcohol in 24 hours); number of drinks consumed per typical week; number of drinks consumed per typical occasion; craving measures; GGT measured every three months; discontinuation of treatment; drop out from the study | |
| Notes | Participants enrolled after completion of detoxification | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a qualified statistician". A computer programme was used |
| Allocation concealment (selection bias) | High risk | Unblinded study: treatment allocated by clinic staff according to serial number on the list |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome analyses conducted on an intention‐to‐treat principle. Dropouts considered as relapses |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Fertig 2012.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 130 participants; mean age 45 years; 76% male; 32% African American; 60% high school education; 36.7% married; 64% employed; 14.5 drinks/d on average; 19 years on average age of onset of regular drinking Inclusion criteria: alcohol dependence determined by DSM‐IV criteria; age 18 or older; drinking very heavily (10 or more drinks/drinking days for men; eight or more drinks/drinking days for women), with at least 40% of the days during any consecutive 60‐day interval during the 90‐day period before the clinic screening visit and at least one HDD (five or more drinks/ drinking days for men; four or more drinks/days for women) occurring within 14 days before randomisation Exclusion criteria: DSM‐IV dependence on any psychoactive substances other than alcohol and nicotine; other psychiatric illnesses; inability to be safely withdrawn from alcohol on an outpatient basis or having undergone medical detoxification during screening phase; pharmacotherapy for alcohol dependence within one month before randomisation; current psychotherapy for alcohol problems; abnormal creatinine clearance; non‐stable use of a selective serotonin reuptake inhibitor (SSRI); current use of a dual uptake inhibitor, serotonin–norepinephrine reuptake inhibitor (SNRI), tricyclic antidepressant or monoamine oxidase inhibitor antidepressant; use of anticonvulsants, hypnotics, antipsychotics, psychomotor stimulants or antianxiety agents |
|
| Interventions | (1) Levetiracetam extended release, 64 participants; (2) placebo, 66 participants Drug dose: levetiracetam 500 to 2000 mg/d All participants received a manualised Brief Behavioral Compliance Enhancement Treatment (BBCET) Setting: outpatient Duration: 16 weeks. Country of origin: USA |
|
| Outcomes | Primary outcome: percentage of participants with no heavy drinking days; weekly percentage of heavy drinking days Secondary outcomes: drinks per day; drinks per drinking day; percentage of days abstinent; percentage of participants abstinent; alcohol‐related consequences; mood; quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted stratified block randomisation procedure applied |
| Allocation concealment (selection bias) | Low risk | Assignment of treatment condition made via web‐ or telephone‐based system according to certain predetermined stratification parameters |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat approach applied, including all randomly assigned participants Continuous outcomes analysed using a repeated‐measures mixed effects model |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Participant, caregiver, investigator, outcomes assessor blinded to treatment assignment. Levetiracetam supplied in over‐encapsulated tablets with identical matching placebos |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Participant, caregiver, investigator, outcomes assessor blinded to treatment assignment. Levetiracetam supplied in over‐encapsulated tablets with identical matching placebos |
| Blinding of outcome assessment (detection bias) objective | Low risk | Participant, caregiver, investigator, outcomes assessor blinded to treatment assignment. Levetiracetam supplied in over‐encapsulated tablets with identical matching placebos |
Florez 2008.
| Methods | Randomised controlled open study | |
| Participants | 102 participants; mean age 46.7 years; 85% male; 82% only elementary school education; 69% married; 48% employed; 73% reporting more than 700 ethanol grams per week; OCDS score 17 on average Inclusion criteria: meeting ICD‐10 criteria for alcohol dependence (WHO 1992); having an ethanol intake of at least 210 grams per week for men and 140 grams per week for women assessed with the EuropASI (Kokkevi 1995) Exclusion criteria: younger than 18 or older than 65 years of age; current diagnosis of dependence or abuse of other substances except nicotine; current psychiatric diagnosis other than personality disorders; any clinically significant medical condition that in the opinion of the researchers would adversely affect safety or study participation; inability to give full informed consent; not speaking Spanish or Galician; clinical history of mental retardation; pregnancy or breast‐feeding; not having a significant other to provide accurate daily alcohol‐related information to researchers |
|
| Interventions | (1) Topiramate, 51 participants; (2) naltrexone, 51 participants Drug dose: topiramate up to 200 to 400 mg/d; naltrexone 50 mg/d All participants received individualised psychological therapy based on Relapse Prevention Model (Carroll 1996 b) Setting: outpatient Duration: six months. Country of origin: Spain |
|
| Outcomes | Efficacy was defined according to the following categories:
Participants in groups three, four and five and dropouts were considered as relapsed |
|
| Notes | For those who needed detoxification, treatment with clorazepate was used. Treatment with naltrexone or topiramate was started after detoxification was completed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out through a computer programme |
| Allocation concealment (selection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants analysed on an intent–to‐treat basis. Dropouts assumed to have resumed heavy drinking on the day after their last contact |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Florez 2011.
| Methods | Randomised controlled open study | |
| Participants | 182 participants; mean age 47.7 years; 85% male; 87% only elementary school education; 62% married; 46% employed; 74% reporting more than 700 ethanol grams per week; OCDS score 18 on average Inclusion criteria: meeting ICD‐10 criteria for alcohol dependence; having an ethanol intake, during the past six months before detoxification, of at least 210 g/wk for men and 140 g/wk for women, assessed with the EuropASI and the Alcohol Timeline Followback; expressing a desire to stop drinking alcohol Exclusion criteria: younger than 18 or older than 65 years of age; current diagnosis of dependence or abuse of other substances except nicotine assessed with the EuropASI and a urine drug screen; current psychiatric diagnosis other than personality disorders assessed with the EuropASI; any clinically significant medical condition that in the opinion of the researchers would adversely affect safety or study participation assessed with the EuropASI; inability to give full informed consent; clinical history of mental retardation; pregnancy or breast‐feeding; not having a significant other to provide accurate daily alcohol‐related information to researchers |
|
| Interventions | (1) Topiramate, 91 participants; (2) naltrexone, 91 participants Drug dose: topiramate up to 200 to 400 mg/d; naltrexone 50 mg/d All participants received weekly psychological therapy based on BRENDA model (Volpicelli 2001) Setting: outpatient Duration: six months. Country of origin: Spain |
|
| Outcomes | Efficacy was defined according to the following categories:
The following variables were also used: number of heavy drinking days; percentage of days abstinent during previous three months; total drinking days during previous three months; days to first drink; drinks per drinking day Participants in groups three, four and five and dropouts were considered relapsed |
|
| Notes | All participants were detoxified before starting treatment with naltrexone or topiramate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stated. No further information available from the study |
| Allocation concealment (selection bias) | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants analysed on an intent–to‐treat basis. Dropouts assumed to have resumed heavy drinking on the day after their last contact |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Furieri 2007.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 60 participants; mean age 44.2 years; 100% male; 6.6 years on average of education; 37% married; 17% employed; 16 years on average age of alcohol use onset; 27 years of drinking on average; 17 drinks per day on average in the past 90 days; CIWA‐Ar score 25 on average Inclusion criteria: 18 to 65 years old; consuming at least 35 drinks per week during the past year and the past 90 days; being abstinent from alcohol from no longer than 14 days before baseline; meeting DSM‐IV criteria for alcohol dependence (First 1994); being in stable clinical condition; having normal serum liver transaminases; having a plasma GGT level less than 800 U/L; being diagnosed as non‐demented with Mini Mental State Examination (Folstein 1975); having no severe withdrawal signs or symptoms; scoring less than 15 on the CIWA‐Ar (Sullivan 1989); having completed seven‐day treatment for acute alcohol withdrawal Exclusion criteria: meeting diagnostic criteria for other substance intoxication or withdrawal or unstable medical or mental disorder other than alcohol dependence, except nicotine and/or caffeine; having convulsions or delirium during abstinence from alcohol; having used pharmacological agents known to reduce the convulsive threshold or to alter alcohol withdrawal or craving during the past 30 days; having a previous history of drug hypersensitivity or adverse reactions to gabapentin, diazepam or other benzodiazepines and haloperidol |
|
| Interventions | (1) Gabapentin, 30 participants; (2) placebo, 30 participants Drug dose: gabapentin up to 600 mg/d Participants involved in weekly BBCET (Johnson 2003b) Setting: outpatient Duration: four weeks. Country of origin: Brazil |
|
| Outcomes | Primary outcomes: alcohol use with the TLFB (Sobell 1992) as follows: drinks per day, drinks per drinking day, vital signs, adverse events, percentage of heavy drinking days, percentage of days of abstinence; alcohol craving using the OCDS (Bohn 1996): haematological and biochemical measurements, including transaminases and plasma GGT | |
| Notes | All participants received one‐week detoxification before initiation of double‐blind treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out by a computer programme |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was held by a research supervisor, to be broken only in case of emergency". Study medication dispensed in medication containers, each labelled with participant code |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only completers analysis performed |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Double‐blind stated. Medication and matching placebo prepared by pharmaceutical company. Participants and psychiatrist blind to treatment condition |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Investigator and supervisor blind to treatment condition |
| Blinding of outcome assessment (detection bias) objective | Low risk | Investigator and supervisor blind to treatment condition |
Johnson 2003.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 150 participants; mean age 41.5 years; 71% male; 64% Caucasian; 34 on average age of alcoholism onset; nine drinks per day on average; 0.68 on average ASI Alcohol composite score (McLellan 1985) Inclusion criteria: 21 to 65 years old; meeting DSM‐IV criteria for alcohol dependence according to the SCID (First 1994); scoring eight or greater on the alcohol use disorders identification test (Bohn 1995 a); drinking on average at least 21 standard drinks per week for women and at least 35 per week for men during the 90 days before enrolment; having a negative urine toxicological screen for narcotics, amphetamines or sedative‐hypnotics Exclusion criteria: having a current Axis I psychiatric diagnosis other than alcohol or nicotine dependence; having important alcohol withdrawal symptoms (CIWA‐Ar score > 15) (Sullivan 1989); having clinically significant physical abnormalities; having a history of or current renal impairment, renal stones, seizures or unstable hypertension; being pregnant or lactating; taking medications with a potential effect on alcohol consumption or a carbonic anhydrase inhibitor; being compelled to receive treatment for alcohol dependence to avoid imprisonment or loss of employment; receiving treatment for alcohol dependence within 30 days before enrolment |
|
| Interventions | (1) Topiramate, 75 participants; (2) placebo, 75 participants Drug dose: topiramate up to 300 mg/d Participants involved in BBCET intervention (Johnson 2003b) Setting: outpatient Duration: 12 weeks. Country of origin: USA |
|
| Outcomes | Primary outcomes: self‐reported drinking behaviour using TLFB method (Sobell 1992) and breath alcohol concentration: drinks per day, drinks per drinking day, percentage of heavy drinking days, percentage of days abstinent; plasma GGT concentration Secondary outcomes: self‐reported craving measured on 14‐item OCDS (Bohn 1996); "safe" drinking days Other outcomes: adverse event profile (gathered systematically by trained practitioners using a modified version of the systematic assessment for treatment‐emergent events questionnaire (Levine 1986)); illicit drug use evaluated with urine drug screens |
|
| Notes | Although abstinence at study entry was not an enrolment criterion, participants were instructed to attempt drinking cessation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data randomly assigned through an urn randomisation procedure (Stout 1994), balancing on sex, average drinks per day and age of onset |
| Allocation concealment (selection bias) | Low risk | All participants, those administering the interventions and those assessing outcomes unaware of group assignment. Medication dispensed in blister packs labelled with identification, study and visit numbers and date |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed by intention‐to‐treat. Methods applied to account for missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Blinding stated for participants and personnel. Topiramate and matching placebo tablets provided by the pharmaceutical company |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Blinding stated for personnel assessing outcomes |
| Blinding of outcome assessment (detection bias) objective | Low risk | Blinding stated for personnel assessing outcomes |
Johnson 2007.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 371 participants; mean age 47.3 years; 73% male; 33.4 years on average age of alcoholism onset; 19.4% heavy drinking days in previous 28 days; 4.5 drinks/drinking days; 1.4 CIWA‐Ar score on average (Sullivan 1989) Inclusion criteria: between 18 and 65 years old; fulfilling DSM‐IV (APA 1994) diagnostic criteria for alcohol dependence using SCID (First 1997); drinking 35 or more (men) and 28 or more (women) standard drinks per week as measured by TLFB (Sobell 1992); scoring eight or higher on Alcohol Use Disorders Identification Test (Bohn 1995 a); having a body mass index higher than 18; having a negative urine toxicological screening result for opioids, cocaine, amphetamines, antidepressants, propoxyphene, barbiturate, tetrahydrocannabinol and benzodiazepines; expressing a desire to stop or reduce consumption of alcohol, with the possible long‐term goal of abstinence Exclusion criteria: having a current Axis I psychiatric diagnosis on DSM‐IV other than alcohol, nicotine or caffeine dependence; having a history of substance abuse or dependence excluding dependence on alcohol, nicotine or caffeine; having clinically significant alcohol withdrawal symptoms (CIWA‐Ar score > 10; Sullivan 1989); having made more than four unsuccessful formal inpatient treatment attempts to curb alcohol dependence; having received formal psychotherapy for a psychiatric disorder other than alcohol dependence; taking antipsychotics, antiepileptics, mood stabilisers, carbonic anhydrase inhibitors, opioid analgesics or systemic steroids; having clinically significant depression; having suicidal ideation or having attempted suicide; receiving treatment for alcohol dependence other than Alcoholics Anonymous; having clinically significant medical condition (i.e. on physical examination, electrocardiogram recording, haematological assessment, biochemistry including bilirubin concentration and urinalysis); having a history of or current renal impairment (i.e. creatinine clearance 60 mL/min), renal stones, seizures or unstable hypertension; having progressive neurodegenerative disorders or clinically significant neurological disorders including seizures; being pregnant or lactating; taking medications that could affect alcohol consumption or a carbonic anhydrase inhibitor; having been compelled to receive treatment for alcohol dependence to avoid imprisonment, parole, probation or loss of employment; being from the same household as another study participant |
|
| Interventions | (1) Topiramate, 183 participants; (2) placebo, 188 participants Drug dose: topiramate up to 300 mg/d Participants involved in weekly BBCET (Johnson 2003b) Setting: outpatient Duration: 14 weeks. Country of origin: USA |
|
| Outcomes | Primary outcome: percentage of heavy drinking days (number of days on which men consumed five standard drinks per day and women consumed four standard drinks per day divided by the number of study days) Secondary outcome: percentage of days abstinent; drinks per drinking day; laboratory measure of alcohol consumption (plasma GGT) Safety outcome: vital signs; haematological and biochemical tests (including liver function tests, bicarbonate and pH level); depressed mood (Montgomery‐Asberg Depression Rating Scale) (Montgomery 1979); withdrawal symptoms (CIWA‐Ar) (Sullivan 1989); concomitant medications; adherence with taking medication; dose‐serum topiramate level concordance; retention; breath alcohol concentration; adverse events |
|
| Notes | To be enrolled, participants had to express a desire to stop or reduce their consumption of alcohol, with the possible long‐term goal of abstinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: randomly assigned "in a 1:1 ratio to topiramate or placebo according to a computer‐generated code. Randomization was balanced using permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Participants and investigators blinded to treatment assignment. To maintain the blind, sealed envelopes containing study medication identification provided to investigators, who were instructed that this envelope could be opened only if specific emergency treatment would be dictated by knowing participants’ treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Inferential testing conducted on all randomly assigned participants returning for at least one double‐blind visit and receiving at least one medication dose. Methods applied to account for missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Participants and investigators blinded to treatment assignment. Topiramate and matching placebo tablets used |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Outcome assessors blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome assessors blinded to treatment assignment |
Kampman 2013.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 170 participants; mean age 45 years; 79% male; 83% African American; 12.7 years of education on average; 49.4% heavy drinking days at baseline; 12.7 days of cocaine use in past 30 days Inclusion criteria: both genders, 18 years of age or older; meeting DSM‐IV criteria for cocaine and alcohol dependence determined by the SCID‐IV; using no less than $200 worth of cocaine within the past 30 days and meeting the following drinking criteria as measured by the TLFB (Sobell 1995):
Two consecutive days of abstinence from cocaine and alcohol; negative breathalyzer tests; CIWA‐Ar (Sullivan 1989) score below eight; living a commutable distance and agreeing to attend all research visits including follow‐up visits; speaking, understanding and printing in English Exclusion criteria: abstinent from cocaine or alcohol for 30 consecutive days; meeting DSM‐IV criteria for dependence on any substance other than cocaine and alcohol (except for nicotine and cannabis); needing treatment with any psychoactive medications; current use of phenytoin or any drug of similar class; meeting DSM‐IV criteria for schizophrenia, organic mental disorder or any other clinically significant psychiatric disorder that will interfere with study participation; significant haematological, pulmonary, endocrine, cardiovascular, renal or gastrointestinal disease; severe physical or medical illness such as AIDS, active hepatitis, hepatocellular injury as evidenced by elevated bilirubin levels (> 1.3) or elevated levels (> 3.5× normal) of AST and serum ALT, severe renal disease, severe respiratory disease or severe diarrhoea with resulting metabolic acidosis, serum bicarbonate < 20 mEq/L; history of epilepsy or seizure disorder; use of an investigational medication in the 30 days before randomisation; history of nephrolithiasis; history of hypersensitivity to topiramate; female positive on a pregnancy test, contemplating pregnancy in the next six months, nursing or not using an effective contraceptive method; current use of a carbonic anhydrase inhibitor; history of glaucoma |
|
| Interventions | (1) Topiramate, 83 participants; (2) placebo, 87 participants Drug dose: topiramate 300 mg/d Participants received weekly individual cognitive‐behavioural relapse prevention therapy utilising Cognitive‐Behavioural Coping Skills Therapy (CBT) manual (Project MATCH; Kadden 1992) Setting: outpatient Duration: 13 weeks. Country of origin: USA |
|
| Outcomes | Primary outcomes: days abstinent from drinking and frequency of heavy drinking days; days of cocaine use Secondary outcomes: days abstinent from drinking and frequency of heavy drinking days during follow‐up period after discontinuation of medication; days of cocaine use in the follow‐up period after discontinuation of medication; measures of alcohol and cocaine craving during medication treatment phase | |
| Notes | Medication adherence was measured by pill count | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stated; randomisation balance across two treatment groups assessed by comparing baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Methods applied to account for missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Participants and care providers blinded to assigned treatment |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Outcome assessors blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Outcome assessors blinded to treatment assignment |
Likhitsathian 2013.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 106 participants; mean age 41.5 years; 100% male; 8.5 years of education on average; 29.3 years on average the age of alcoholism onset; 15.4 mean drinks per day; CIWA‐Ar score 1.2 on average (Sullivan 1989) Inclusion criteria: between 18 and 60 years old; fulfilling DSM‐IV (APA 1994) diagnostic criteria for alcohol dependence using MINI (Sheehan 1998); > one week of ≥ 35 standard drinks in men or ≥ 28 standard drinks in women, during four‐week period before admission; Alcohol Use Disorders Identification Test (AUDIT) score of eight or higher (Bohn 1995 a); mild or no alcohol withdrawal; likely to be discharged within 14 days; body mass index ≥ 18 kg/m2; intention to decrease or stop drinking (Sullivan 1989) Exclusion criteria: previous or current cognitive disorder, schizophrenia and other psychotic disorders, bipolar disorder or antisocial personality disorder; other substance dependence, except nicotine and caffeine dependence; treated with antipsychotics, mood stabilisers, anticonvulsants, opioid analgesics, systemic steroids, carbonic anhydrase inhibitors, hydrochlorothiazide, metformin, pioglitazone or disulfiram; risk of suicide; physical illness, including narrow‐angle glaucoma, renal impairment, urinary stone and epilepsy; unstable medical conditions; pregnancy and breast‐feeding; receiving medication for 14 days or longer while inpatient |
|
| Interventions | (1) Topiramate, 53 participants; (2) placebo, 53 participants Drug dose: topiramate up to 300 mg/d During residential phase, participants were involved in one or two sessions of individual motivational enhancement therapy (MET), individual counseling for alcohol and drug use, group therapy and family counseling. After discharge, participants received two or three sessions of individual MET Setting: inpatient up to 14 days, then outpatient Duration: 12 weeks. Country of origin: Thailand |
|
| Outcomes | Primary outcomes: heavy drinking days (numbers of days on which men consumed ≥ five standard drinks per day or women consumed ≥ four standard drinks per day divided by the number of study days) and time to first day of heavy drinking. Drinking characteristics were assessed using timeline follow‐back (Sobell 1992) Secondary outcomes: participants with heavy‐drinking relapses; drinking days; drinks per day; drinks per drinking day; alcohol craving, assessed by an 11‐point Likert‐type questionnaire; plasma gamma glutamyltransferase (GGT); HRQoL measured using the Medical Outcomes Study Short Form 36‐item questionnaire (SF‐36), Thai version (Kongsakon 2000); side effects measured by a six‐point Likert‐type questionnaire (Johnson 2007) |
|
| Notes | Participants were enrolled at the end of a two‐ to four‐week residential treatment programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation balanced using permuted blocks of six. Random allocation sequences generated by computer |
| Allocation concealment (selection bias) | Low risk | Topiramate and matching placebo capsules provided by Department of Pharmaceutical Sciences. A random number indicating intervention or control treatment kept in an opaque and sealed envelope. Envelope opened after baseline assessment of each participant had been completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed by intention‐to‐treat. Methods applied to account for missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Participants and care providers blinded to assigned treatment |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Outcome assessors blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Topiramate supplied in over‐encapsulated tablets with identical matching placebos. Outcome assessors blinded to treatment assignment |
Martinotti 2007.
| Methods | Randomised controlled open study | |
| Participants | 84 participants; mean age 40.3; 81% male; 39.3% secondary school of education; 16.4 years on average the duration of alcohol misuse; 21 on average the OCDS score; 8.5 daily drinks on average; 14.8 mean years of addiction Inclusion criteria: detoxified alcohol dependent with history of alcohol use disorders of at least three years, currently meeting clinical criteria for alcohol dependence (DSM‐IV) using SCID interview, who declared their commitment to the goal of total abstinence Exclusion criteria: severe physical illness; evidence of mental disorders severely interfering with their cognitive capacity or reality test; individuals regularly taking anticonvulsants, antidepressants or antipsychotics; pregnant or lactating participants; history of severe adverse events or well‐known hypersensitivity to oxcarbazepine or naltrexone; previous treatment with oxcarbazepine or naltrexone |
|
| Interventions | (1) Oxcarbazepine low dosage, 28 participants; (2) oxcarbazepine full dosage, 29 participants; (3) naltrexone, 27 participants Drug dose: oxcarbazepine low dosage, 600 to 900 mg/d; oxcarbazepine full dosage, 1500 to 1800 mg/d; naltrexone 50 mg/d All participants were offered a supportive self‐help group alternatively run by counsellors and psychologists, with a frequency of two days per week for the duration of the study Setting: outpatient Duration: 12 weeks. Country of origin: Italy |
|
| Outcomes | Primary outcome was divided into four different variables (alcohol free, minor relapse, major relapse, dropout); craving and psychiatric symptoms were considered as secondary endpoints | |
| Notes | Participants were required to be detoxified before randomisation and declaring their commitment to the goal of total abstinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated system used. Random assignment achieved in a non–centre‐specific manner with an interactive voice‐response central randomisation service. Random assignment stratified according to the presence of a co‐morbid psychiatric diagnosis |
| Allocation concealment (selection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Primary and secondary efficacy analyses were performed on the intent‐to‐treat population, which included all randomly assigned patients who took at least one dose of study medication." To take care of missing data, the last observation carried forward method was also applied |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Martinotti 2010.
| Methods | Randomised controlled double‐blind study | |
| Participants | 59 participants; mean age 40.3 years; 80% males; 8.5 daily drinks on average; 14.8 mean years of addiction Inclusion criteria: history of alcohol use disorders of at least three years; daily alcohol intake of at least six units; currently meeting clinical criteria for alcohol dependence (DSM‐IV; APA 2000); commitment to the goal of total abstinence Exclusion criteria: having a severe physical illness or evidence of mental disorder severely interfering with cognitive capacity; regularly taking anticonvulsants, antidepressants or antipsychotics; pregnancy or lactation; history of severe adverse reaction, well‐known hypersensitivity to or previous treatment with pregabalin or naltrexone |
|
| Interventions | (1) Pregabalin, 31 participants; (2) naltrexone, 28 participants Drug dose: pregabalin, 150 to 450 mg/d; naltrexone, 50 mg/d All participants were offered a supportive self‐help group alternatively run by counsellors and psychologists two days per week for the duration of the study Setting: outpatient Duration: 12 weeks. Country of origin: Italy |
|
| Outcomes | Primary outcomes: maintenance of abstinence and relapse to drinking Secondary outcomes: number of abstinent days; number of heavy drinking days; time to first drink (duration of abstinence); alcohol craving; safety (electrocardiogram, urinalysis, haematological and clinical chemical analyses of blood samples) |
|
| Notes | Participants were required to be detoxified before randomisation and declaring their commitment to the goal of total abstinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated system used. Random assignment stratified according to the presence of a co‐morbid psychiatric diagnosis |
| Allocation concealment (selection bias) | Low risk | Quote: "All study personnel in contact with the participants were unaware of the randomisation sequence" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses performed on intent‐to‐treat population. Last observation carried forward (LOCF) method also applied to take into account missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Tablets identical in appearance. Quote: "All study personnel in contact with the participants were unaware of the randomisation sequence" |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Quote: "All study personnel in contact with the participants were unaware of the randomisation sequence" |
| Blinding of outcome assessment (detection bias) objective | Low risk | Quote: "All study personnel in contact with the participants were unaware of the randomisation sequence" |
Mueller 1997.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 29 participants; mean age 38.7 years; 60% male; 90% Caucasian; 50% married; 24 years on average age of alcoholism onset; all alcohol dependents (16 drinks/drinking day on average) Inclusion criteria: adults with alcohol abuse or dependence as defined in the DSM‐III‐R (APA 1987) admitted for treatment of alcohol withdrawal Exclusion criteria: younger than 18 years old; prior or current history of epilepsy (excluding alcohol withdrawal seizures); existing pregnancy or consideration thereof within the next year; major cognitive limitations that would impair consent; psychosis; allergy to carbamazepine; cirrhosis; liver function tests elevated more than 2.5 times the upper limits of normal; cardiomyopathy or arrhythmia; history of immune compromise; medical conditions requiring active medical pharmacological management; current use of or withdrawal from opiates, benzodiazepines or barbiturates; lack of a significant other who could corroborate the participant's self‐report |
|
| Interventions | (1) Carbamazepine, 13 participants; (2) placebo, 16 participants Drug dose: carbamazepine 600 mg/d verifying blood concentration to be close to 6 mg/L. Setting: inpatient for around five days, then outpatient Duration: 12 months. Country of origin: USA |
|
| Outcomes | Retention in treatment; drinking behaviour (mean time to first drink, mean time to return to heavy drinking, drinks per drinking day and maximum number of heavy drinking days); mood and functioning; side effects | |
| Notes | Treatment started after alcohol detoxification | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned by a biostatistician (urn randomisation) |
| Allocation concealment (selection bias) | Low risk | Dispensing pharmacist aware of medication group assignment; however did not interact with participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis and last point carried forward analysis applied |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Double‐blind stated; measures adopted to protect blindness of participants and personnel. Dispensing pharmacist aware of medication group assignment but did not interact with participants |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Double‐blind stated; measures adopted to protect blindness of outcome assessors |
| Blinding of outcome assessment (detection bias) objective | Low risk | Double‐blind stated; measures adopted to protect blindness of outcome assessors |
Paparrigopoulos 2010.
| Methods | Randomised controlled open study | |
| Participants | 120 participants; mean age 46.8 years; 74.2% male; 90% Caucasian; 31% with more than 12 years of education; 57% married; 248 gr/d alcohol consumption on average Inclusion criteria: age 18 to 70 years; alcohol dependence; absence of serious physical illness; absence of another preexisting or co‐existing major psychiatric disorder on DSM‐IV Axis I; absence of abuse of another drug; participants with affective or anxiety symptoms were not excluded from the study if concurrent with an alcohol‐abusing period; however, individuals who fulfilled a DSM‐IV diagnosis of depressive or anxiety disorder were excluded from the study if such symptoms had been recorded before onset of alcoholism or during periods of abstinence |
|
| Interventions | (1) Tiagabine, 60 participants; (2) no pharmacological treatment, 60 participants Drug dose: tiagabine up to 15 to 20 mg/d Participants were involved in short‐term psychotherapy (four to six weeks) of cognitive‐behavioural orientation consisting of both individual sessions (twice a week) and family interventions (once every two weeks) Setting: inpatient for four to six weeks, then outpatient Duration: six months. Country of origin: Greece |
|
| Outcomes | Retention in treatment; abstinence from alcohol; MCV and liver enzymes; alcohol craving; mood and functioning; side effects | |
| Notes | All participants underwent a detoxification protocol that included vitamin replacement and administration of diazepam. Tiagabine was added to the last part of the detoxification period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Paparrigopoulos 2011.
| Methods | Randomised controlled open study | |
| Participants | 90 participants; mean age 45 years; 88.2% male; 90% Caucasian; eight years of education on average; 57% married; 26 years of age at onset of alcohol dependence on average; 280 gr/d of alcohol consumption on average Inclusion criteria: age 18 to 65 years; fulfilling DSM‐IV‐TR diagnostic criteria for alcohol abuse/dependence; absence of a serious physical illness; absence of another major psychiatric disorder on DSM‐IV‐TR Axis I assessed through the SCAN (Wing 1990); absence of other drug abuse |
|
| Interventions | (1) Topiramate, 30 participants; (2) no pharmacological treatment, 55 participants Drug dose: topiramate up to 75 mg/d Participants were involved in cognitive‐behavioural short‐term psychotherapy of four‐ to six‐week duration Setting: inpatient for four to six weeks, then outpatient Duration: six months. Country of origin: Greece |
|
| Outcomes | Abstinence from alcohol, based on self reports (cross‐checked with a family member) and on alcohol breath test and GGT evaluation; symptoms of depression and anxiety assessed by the HDRS and the HARS; craving assessed by OCDS; overall functioning assessed using the Global Assessment Scale (GAS) | |
| Notes | Participants underwent a detoxification protocol that included vitamin replacement and administration of diazepam. Tiagabine was added for the last part of the detoxification period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assignment to topiramate augmentation group made on a 2:1 ratio; thus, every third intake assigned to the topiramate group |
| Allocation concealment (selection bias) | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) all outcomes | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) subjective | High risk | Unblinded study |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
Richter 2012.
| Methods | Randomised placebo‐controlled double‐blind multi‐centre study | |
| Participants | 201 participants; mean age 47.7 years; 72% male; 16.8 years on average duration of alcohol consumption Inclusion criteria: patients aged 18 and 65 years who fulfilled the criteria of alcohol dependence according to both DSM‐IV and ICD‐10, and who were recently (three to 14 days before) inpatients detoxified from alcohol Exclusion criteria: positive drug urine for benzodiazepines or other sedative‐hypnotics; positive breath alcohol test; current diagnosis of any other psychiatric illness according to DSM‐IV by Mini International Neuropsychiatric Interview (Sheehan 1998); pregnancy or lactation period; suicidal tendencies; legal or illegal drug addiction (except nicotine dependence and infrequent, not current, consumption of cannabinoids); history of epilepsy; hallucinatory alcoholic state, Korsakoff’s syndrome, Wernicke encephalopathy and decompensated liver cirrhosis, as well as suspected cirrhosis or other severe medical disorders |
|
| Interventions | (1) Levetiracetam, 95 participants; (2) placebo, 106 participants Drug dose: levetiracetam up to 2.000 mg/d Setting: outpatient Duration: 16 weeks. Country of origin: Germany |
|
| Outcomes | Primary outcome: time to first severe relapse Secondary outcomes: overall abstinence rates; time to first consumption of any ethanol; drinks per drinking day; adherence to treatment; safety and tolerability; craving |
|
| Notes | All participants were detoxified before they were enrolled in the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out by a random generator (computerised) |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared for each centre a separate randomisation list and packed blinded medication boxes; randomisation central and independent of the centre and blinded for physician and participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data analysed on an intention‐to‐treat (ITT) basis, including all participants who were randomly assigned and received at least one dose of medication. Last observation carried forward also applied |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Pharmacy prepared for each centre packed blinded medication boxes. Measures adopted to protect blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Pharmacy prepared for each centre packed blinded medication boxes. Measures adopted to protect blindness of outcome assessors |
| Blinding of outcome assessment (detection bias) objective | Low risk | Pharmacy prepared for each centre packed blinded medication boxes. Measures adopted to protect blindness of outcome assessors |
Rubio 2009.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 76 participants; mean age 42 years; 100% male; 90% Caucasian; 40% with more than 12 years of education; 16% married; 16% employed; 37 years on average age at onset of alcohol dependence; 16.3 on average CIWA score before detoxification Inclusion criteria: alcohol dependence (DSM‐IV criteria) using the SCID (First 1995); age range 18 to 50; male Exclusion criteria: current psychiatric or neurological disorders associated with impulsivity (personality disorders, bipolar disorders, schizophrenia or epilepsy) |
|
| Interventions | (1) Topiramate, 38 participants; (2) placebo, 38 participants Drug dose: topiramate up to 250 mg/d Both groups of participants were offered supportive group therapy once a week throughout the study period. Basic low structured relapse prevention was tackled Setting: outpatient Duration: 12 weeks. Country of origin: Spain |
|
| Outcomes | Alcohol use assessed using TLFB (Sobell 1979) with heavy drinking days defined as five standard drinks a day (Flannery 2002); average craving (frequency, duration and intensity of craving) (Miller 1996); adverse effects rated for severity (Johnson 2005) and with open‐ended questions; impulsive behavior evaluated through use of the Continuous Performance Test (AX version) (Conners 1995), the Stop‐Signal Task (Logan 1994), the Differential Reinforcement of Low‐Rate Responding (Gordon 1988), the Barratt Impulsiveness Scale, version 11 (Patton 1995); severity of anxiety and depression symptoms using HDRS (Hamilton 1960) and HARS (Hamilton 1959). Concentration of CDT was used as an indirect measure of alcohol consumption | |
| Notes | All participants were detoxified with diazepam for two weeks before randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Capsules individually labelled and sealed in plastic wrappers by a local pharmacy. Each participant identified by a number and a letter. Only one person on the staff of the pharmacy knew the code |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only completers analysis performed |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Double‐blind stated. Capsules identical in appearance across all doses and conditions. Measures adopted to protect blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Double‐blind stated. Capsules identical in appearance across all doses and conditions. Measures adopted to protect blindness of outcome assessors |
| Blinding of outcome assessment (detection bias) objective | Low risk | Double‐blind stated. Capsules identical in appearance across all doses and conditions. Measures adopted to protect blindness of outcome assessors |
Salloum 2005.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 59 participants; mean age 38 years; 71% male; 15% married; 58% employed; 58% met criteria for mixed bipolar subtype, 21% were manic and 21% were depressed; half of participants had other substance use disorders (cannabis abuse or dependence and cocaine abuse were the most frequent diagnoses); 16 years on average duration of heavy drinking (up to intoxication); 96 on average the number of drinks per week; 20.8 average score at 25‐item HDRS; 14 years on average duration of bipolar disorder Inclusion criteria: men and non‐pregnant, non‐nursing women aged 18 to 65 years; meeting four of the seven DSM‐IV alcohol dependence criteria; actively drinking alcohol in the past month; having a concurrent acute episode of bipolar I disorder (manic, mixed or depressed) evaluated with the SCID for DSM‐IV (First 1994) Exclusion criteria: schizophrenia, schizoaffective disorder, any non‐bipolar psychotic disorder, mental retardation or signs of impaired cognitive functioning; current DSM‐IV diagnosis of opioid or cocaine dependence or current use of intravenous drugs; epilepsy, history of brain injury or any organic brain syndrome; severe cardiac, liver, kidney, endocrine, haematological or other unstable medical condition; persistent elevation of liver function enzyme levels greater than three‐fold above reference range of AST, ALT, GGT and alkaline phosphatase; inability or unwillingness to use contraception; inability to read or understand study forms and to agree to informed consent. Participants were not excluded for other DSM‐IV substance use disorders such as cannabis abuse or dependence, nicotine dependence or other substance abuse disorders |
|
| Interventions | (1) Valproate plus lithium, 29 participants; (2) placebo plus lithium, 30 participants Drug dose: valproate serum concentration 50 to 100 μg/mL; lithium serum concentration 0.7 to 1.2 mEq/L Psychosocial associated intervention: dual diagnosis recovery counselling, consisting of weekly individual sessions that integrate psychoeducational and cognitive‐behavioural principles (Daley 1994) Setting: outpatient Duration: 24 weeks. Country of origin: USA |
|
| Outcomes | Primary outcome: proportion of heavy drinking days (defined as four drinks per day for women and five drinks per day for men) and number of drinks per heavy drinking day
Secondary outcomes: proportion of any drinking days, number of drinks per any drinking day, time to relapse to sustained heavy drinking (defined as three consecutive heavy drinking days) Alcohol use was measured with the TLFB (Sobell 1992) and breath alcohol concentration; use of other drugs was measured with the urine drug screen Mood outcomes: remission of mania (defined as a score of seven on the Bech‐Rafaelsen Mania Scale) (Bech 1979) and remission of depression (defined as a score of seven on the HRSD‐25 (Thase 1983)). For psychiatric evaluation, Charting of Bipolar Episodes (Post 1986), BRMS, HRSD‐25 and the Global Assessment Scale (Endicott 1976) were used Adverse effects were measured with the Somatic Symptoms Checklist and the Medication Adherence Form |
|
| Notes | All participants were recruited for the study after acute withdrawal symptoms cleared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced coin randomisation method used to stratify groups (Efron 1971) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Statistical analyses completed on a
modified intent‐to‐treat study group, as defined by completion of at least one assessment while participant was receiving double‐blind therapy Mixed model with restricted maximum likelihood estimation method and unrestricted covariance matrix allowed to handle missing data |
| Blinding of participants and personnel (performance bias) all outcomes | Low risk | Valproate and placebo identical‐appearing. Procedures adopted to ensure double‐blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Evaluators blind to group intervention assignment |
| Blinding of outcome assessment (detection bias) objective | Low risk | Evaluators blind to group intervention assignment |
Trevisan 2008.
| Methods | Randomised placebo‐controlled double‐blind study | |
| Participants | 57 participants; mean age 47.7 years; 100% males; 72% white; 36.8% with high school education level; 35.1% unemployed; 14% married; 17.5% with cocaine use disorder; 19.7 heavy drinking days in the last 30 days on average Inclusion criteria: meeting DSM‐IV criteria for alcohol dependence (Spitzer 1992); requiring a detoxification intervention; being abstinent for no longer than one week; having a breathalyzer reading < 0.02 gr/dL Exclusion criteria: current DSM‐IV opiate dependence or benzodiazepine abuse or dependence; serious current psychiatric symptoms, such as suicidal or homicidal ideation; taking anticonvulsant medication, including carbamazepine, phenytoin, valproic acid or gabapentin; having medical problems that preclude safe entry into the study, including liver function tests over three times normal level, seizure disorder and pancreatitis; requiring inpatient detoxification, including participants with history of delirium tremens, cardiac disease or unstable psychiatric illness |
|
| Interventions | (1) Valproic acid, 19 participants; (2) gabapentin, 19 participants; (3) placebo, 19 participants Drug dose: valproic acid, up to 1500 mg/d; gabapentin, 1200 mg/d Associated medication: lorazepam based on assessment of withdrawal symptoms using CIWA‐Ar Setting: inpatient for a few days, then outpatient Duration: four weeks. Country of origin: USA |
|
| Outcomes | Primary outcome: time to relapse; number of drinking days; number of heavy drinking days; percentage of heavy drinking days Secondary outcomes: CIWA‐Ar withdrawal; alcohol craving; psychiatric stress; serum GGT; side effects |
|
| Notes | Participants requiring a detoxification intervention were included. They could also receive lorazepam based on assessment of their withdrawal symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Random effects regression models applied |
| Blinding of participants and personnel (performance bias) all outcomes | Unclear risk | Tablets identical in appearance. No other details given on blindness of participants and personnel |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome measurement not likely to be influenced by lack of blinding |
ALT: Alanine aminotransferase.
ASI: Addiction Severity Index.
AST: Aspartate aminotransferase.
AUQ: Alcohol Urge Questionnaire.
BAI: Beck Anxiety Inventory.
BBCET: Brief Behavioral Compliance Enhancement Treatment.
BDI: Beck Depression Inventory.
CBT: Cognitive‐behavioural therapy.
CDT: Carbohydrate‐deficient transferrin.
CIWA‐Ar: Clinical Institute Withdrawal Assessment for Alcohol scale, Revised.
COMBINE: Combining Medications and Behavioral Interventions.
DSM‐IV: Diagnostic and Statistic Manual of Mental Disorders (American Psychiatric Association), Fourth Edition.
EuropASI: European Addiction Severity Index.
GGT: γ‐Glutamyltransferase.
HARS: Hamilton Anxiety Rating Scale.
HDRS: Hamilton Depression Rating Scale.
ICD: International Classification of Disease.
MCV: Mean cellular volume.
MINI: Mini International Neuropsychiatric Interview.
OCDS: Obsessive Compulsive Drinking Scale.
SADD: Short Alcohol Dependence Data.
SCAN: Schedules for Clinical Assessment in Neuropsychiatry.
SCID: Structured Clinical Interview for DSM.
SCL90‐R: Symptom Checklist‐90 Revised.
TLFB: Timeline follow‐back.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Karam‐Hage 2003 | Study design not in the inclusion criteria: open study, not randomised |
| Knapp 2010 | Study design not in the inclusion criteria: no control group |
| Le Strat 2012 | Study design not in the inclusion criteria: review on the use of levetiracetam in alcohol dependence |
| Miranda 2008 | Study population not in the inclusion criteria: laboratory study looking at the cue‐elicited craving of topiramate‐ versus placebo‐treated heavy alcohol drinkers. Presence of alcohol dependence not an inclusion criterion: only 60% of participants had alcohol use disorder |
| Mitchell 2012 | Study population not in the inclusion criteria. Presence of alcohol dependence not an inclusion criterion: participants were non–treatment‐seeking "heavy social drinkers" |
| Myrick 2007 | Outcome measures not in the inclusion criteria of the review: gives indication about gabapentin safety by investigating interactions between gabapentin and alcohol |
| Narayana 2008 | Study design not in the inclusion criteria: open study, not randomised |
| Schacht 2013 | Study design not in the inclusion criteria; outcome measures not in the inclusion criteria of the review: analyzes functional magnetic resonance imaging data from alcohol‐dependent participants enrolled in another included trial (Anton 2009) |
Characteristics of studies awaiting assessment [ordered by study ID]
Bajovic 2012.
| Methods | Randomised open‐label trial |
| Participants | 182 patients with ICD‐10 criteria for alcohol dependence |
| Interventions | (1) Oxcarbazepine, 51 participants; (2) topiramate, 42 participants; (3) lamotrigine, 49 participants; (4) symptomatic therapy, 40 participants Drug dose: oxcarbazepine 600 to 1200 mg/d; topiramate 100 to 200 mg/d); lamotrigine 75 to 200 mg/d Setting: inpatient and outpatient Duration: six months. Country of origin: Serbia |
| Outcomes | Alcohol relapse; alcohol craving; side effects |
| Notes | Conference proceedings |
De Vita 2012.
| Methods | Randomised, parallel, placebo‐controlled trial |
| Participants | 60 detoxified alcohol‐dependent (DSM‐IV‐TR) outpatients |
| Interventions | (1) Topiramate low dosage (not specified), 30 participants; (2) placebo, 30 participants Setting: outpatient Duration: six months. Country of origin: Italy |
| Outcomes | Relapse; craving for alcohol; psychiatric symptoms evaluated with the Symptom Check List 90‐Revised (SCL‐90‐R); quality of life |
| Notes | Conference proceedings |
MacKillop 2012.
| Methods | Randomised placebo‐controlled trial |
| Participants | 99 non–treatment‐seeking heavy drinkers |
| Interventions | (1) Topiramate; (2) placebo Drug dose: topiramate up to 200 mg/d Setting: outpatient Duration: five weeks. Country of origin: USA |
| Outcomes | Alcohol consumption |
| Notes | Conference proceedings |
Mason 2010.
| Methods | Randomised placebo‐controlled double‐blind study |
| Participants | 150 participants; mean age 43.9; 55% male Inclusion criteria: both genders over age 18 with alcohol dependence Exclusion criteria: meeting DSM‐IV‐TR criteria for dependence on illicit substances; significant medical disorders that will increase potential risk or interfere with study participation; women with child‐bearing potential who are pregnant or nursing or who refuse to use a reliable method of birth control; treatment with an investigational drug in the last month |
| Interventions | (1) Gabapentin low dosage; (2) gabapentin high dosage; (3) placebo Drug dose: gabapentin 900 mg/d; gabapentin 1800 mg/d Standardised behavioural therapy Duration: 12 weeks; Country of origin: USA |
| Outcomes | Primary outcomes: drinking quantity and frequency Secondary outcomes: mood, sleep, craving |
| Notes | Information from conference proceedings and ClinicalTrials.gov |
Miranda 2011.
| Methods | Randomised placebo‐controlled trial |
| Participants | 91 non–treatment‐seeking heavy drinkers; mean age 35.9; 58% male; 19% African American |
| Interventions | (1) Topiramate; (2) placebo Drug dose: topiramate up to 200 mg/d Setting: outpatient Duration: five weeks. Country of origin: USA |
| Outcomes | Number of drinking days; percentage of heavy drinking days; average number of drinks per drinking day; craving |
| Notes | Conference proceedings |
Rubio 2002.
| Methods | Randomised open‐label trial |
| Participants | 20 participants |
| Interventions | (1) Gabapentin, 10 participants; (2) acamprosate, 10 participants Drug dose: gabapentin 800 to 1600 mg/d; acamprosate 1998 mg/d Setting: outpatient Duration: 12 weeks. Country of origin: Spain |
| Outcomes | Alcohol consumption; craving; depression measured with HDRS |
| Notes | Conference proceedings. Full data are not yet available |
HDRS: Hamilton Depression Rating Scale.
Characteristics of ongoing studies [ordered by study ID]
Ait‐Daoud 2010.
| Trial name or title | New pharmacotherapy for alcohol and co‐morbid disorders |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 294 participants Inclusion criteria: over the age of 18; DSM‐IV diagnosis of alcohol and nicotine dependence; stable residence; negative pregnancy test at intake and using an acceptable form of contraception; literate in English and able to follow instructions and make use of behavioural treatments; expressing a wish to stop drinking; willing to participate in a treatment programme for nicotine dependence Exclusion criteria: current Axis I DSM‐IV psychiatric disorder that warrants treatment or would preclude safe participation in the protocol; other neurological or psychiatric disorders, such as dependence on any substances except nicotine, caffeine and marijuana; seizure disorders or epilepsy; history of suicide attempts and/or current suicidal ideation/plan as assessed by SCID; serious medical illnesses; severe or adverse reactions to medications (including topiramate); currently receiving active treatment with topiramate; receipt of a drug with known potential for toxicity to a major organ system; female participants pregnant, lactating or not adhering to an acceptable form of contraception; concomitant pharmacotherapy with psychotropics; current use of nicotine replacement treatment or participation in any other treatment for nicotine dependence; clinically significant haematological or biochemical test results requiring urgent treatment; electroconvulsive therapy within the three months preceding screening; members of the same household |
| Interventions | (1) Topiramate high dose, 98 participants; (2) topiramate low dose, 98 participants; placebo, 90 participants Drug dose: topiramate high dose, up to 250 mg/d; topiramate low dose, up to 125 mg/d Alcohol‐dependent smokers will receive brief behavioural compliance enhancement treatment (BBCET) plus a smoking self help manual as their psychosocial treatment Duration: 18 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: percentage of heavy drinking days (TLFB method of measuring alcohol consumption will be used); continuous abstinence rate for smoking (determined by a combination of self report and CO monitoring after the quit date) Secondary outcomes: quality of life (assessed using the Quality of Life Enjoyment and Satisfaction Questionnaire); craving for alcohol and nicotine (using craving scales) |
| Starting date | September 2010 |
| Contact information | Ann Richards, BS, 434 243 0570, AER7G@virginia.edu; Eva Jenkins‐Mendoza, BS, 434 243 0549, EMJ9C@virginia.edu, University of Virginia Center for Addiction Research & Education, Charlottesville, Virginia, United States 22911 |
| Notes |
Anthenelli 2008.
| Trial name or title | Topiramate to aid smoking cessation in recovering alcohol‐dependent men |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 180 participants Inclusion criteria: 18 to 65 years of age; male outpatients with a diagnosis of DSM‐IV‐TR nicotine dependence and alcohol dependence in early full remission; current tobacco smokers; motivated to try to quit smoking and maintain abstinence from alcohol and other illicit drugs Exclusion criteria: any clinically significant laboratory evidence of haematological, hepatic, cardiovascular, renal, pulmonary or thyroid disease; current significant neurological, hepatic, renal, gastrointestinal, pulmonary, metabolic, cardiovascular, infectious or endocrine disease; a history of known hypersensitivity to topiramate; current suicidal or homicidal risk; any investigational drug taken within 30 days of baseline; current seizure disorder or history of severe alcohol withdrawal |
| Interventions | (1) Topiramate, 90 participants; (2) placebo, 90 participants Dose: topiramate 200 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: Four‐week continuous abstinence from smoking Secondary outcomes: percentage relapsing to any drinking |
| Starting date | January 2009 |
| Contact information | Stephanie Nolting, MEd, BA, +151 386 13100 ext 5507, stephanie.nolting@va.gov; Robert Anthenelli, MD, +151 386 13100, ext 4914, robert.anthenelli@va.gov, VA Medical Center, Cincinnati, Ohio, United States 45220 |
| Notes |
Batki 2010.
| Trial name or title | Topiramate treatment of alcohol use disorders in veterans with post‐traumatic stress disorder (PTSD): a pilot controlled trial of augmentation therapy |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 30 participants Inclusion criteria: 18 to 65 years old; both genders; current DSM‐IV diagnosis of PTSD; current DSM‐IV diagnosis of an alcohol use disorder; meeting criteria for "heavy" or "at‐risk" drinking by NIAAA thresholds; receiving treatment for PTSD; expressing desire to reduce alcohol consumption; female must have negative urine pregnancy test and must be postmenopausal or practising an effective method of birth control; having a BAC less than 0.02% Exclusion criteria: psychotic disorders, bipolar disorder, dementia or other unstable psychiatric disorders; clinically significant unstable medical conditions, including renal disease, seizure disorders, glaucoma, history of kidney stones; concurrent participation in another treatment study; female pregnant or lactating; current or within four weeks of topiramate use; current or within four weeks of medications for alcohol dependence; needing acute medical detoxification from alcohol based on a score of 12 or higher on the Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA‐AD); legally mandated to participate in an alcohol treatment programme; suicide attempt or suicidal ideation in the six months before enrolment; adverse event or hypersensitivity reaction to topiramate; currently treated with another anticonvulsant; individuals who in the opinion of the investigator should not be enrolled in the study because of precautions, warnings or contraindications outlined in the topiramate package insert |
| Interventions | (1) Topiramate; (2) placebo Dose: topiramate 300 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: change in percentage of days abstinent from alcohol Secondary outcomes: percentage of days abstinent from alcohol; changes in PTSD measures |
| Starting date | April 2010 |
| Contact information | Brooke Lasher, BA, 415‐221‐4810, ext 4954, brooke.lasher@va.gov; Steve L Batki, MD, 415‐221‐4810, ext 3671, steven.batki@ucsf.edu, University of California, San Francisco; Department of Veterans Affairs, VA Medical Center, San Francisco, California, United States 94121 |
| Notes |
Beresford 2011.
| Trial name or title | A double‐blind trial of divalproex sodium for affective lability and alcohol use following traumatic brain injury (TBI) |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | Inclusion criteria: 18 to 65 years old; both genders; history of remote (one year before study enrolment) non‐penetrating TBI; currently using alcohol; symptoms of affective lability: mood swings, irritability, frustration Exclusion criteria: history of bipolar disorder or anxiety disorder before any head injury; history of head injury in which the cranium was opened traumatically or surgically; history of stroke; history of seizure disorder other than those caused by ethanol withdrawal; evidence of active liver disease; current diagnosis or past history of major psychosis, alcohol amnesic syndrome, dementia; current suicidal/homicidal ideations; medical conditions that would constitute contraindications to treatment with divalproex sodium; taking medications known to affect metabolism of divalproex sodium |
| Interventions | (1) Valproic acid; (2) placebo Duration: 14 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: affective lability (presence or extent of symptoms yielded by the Neurobehavioral Rating Scale‐Revised as well as by the Agitated Behavior Scale Secondary outcomes: alcohol use measured using the TLFB for Drugs and Alcohol method (Sobell 1979) |
| Starting date | 2011 |
| Contact information | Brandon Schmidt, MA, +172 085 44200; Thomas Beresford, MD, +130 331 59130, Denver Research Institute, Veterans Affairs Medical Center, Denver, Colorado, United States 80220 |
| Notes |
Ciraulo 2009.
| Trial name or title | A double‐blind, placebo‐controlled, parallel‐group design trial of: levetiracetam, zonisamide, topiramate and placebo control for the treatment of alcohol‐dependent subjects |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | Inclusion criteria: 21 to 65 years of age; both genders; DSM‐IV‐TR diagnosis of alcohol dependence; at least 28 standard drinks per week for women or 35 drinks per week for men; able to provide informed consent, comprehend and follow study procedures; negative urine toxicological screen for opioids, cocaine, amphetamines, methamphetamine and benzodiazepines; score > eight on the Alcohol Use Disorder Identification Test (AUDIT); suitable for outpatient management of alcoholism; expressing desire to stop drinking or reduce alcohol consumption; women must be postmenopausal or must be using an effective method of birth control Exclusion criteria: dependent on DSM IV‐TR drugs or substances other than ethanol, nicotine or caffeine; DSM IV‐TR diagnosis of any current Axis I diagnosis other than alcohol, nicotine or caffeine dependence requiring intervention interfering with the course of the study; receiving inpatient treatment for alcohol dependence, other than alcohol detoxification; score of 10 or greater on the CIWA‐Ar; being treated with acamprosate, disulfiram, naltrexone, antipsychotic, anticonvulsant, sedative‐hypnotics, opioids, psychomotor stimulant‐amphetamine derivatives, methylphenidate; legally mandated to participate in an alcohol treatment programme; use of any medication known to inhibit or induce cytochrome P450 3A4 enzymes; has attempted suicide or has had suicidal ideation; renal disease or history of kidney stones; AST or ALT > three times upper limit of normal range; history of significant neurological disorder; pregnant or lactating; clinically significant medical conditions precluding administration of study medications or limiting participation in the clinical trial; history of treatment with levetiracetam, topiramate or zonisamide; score of 25 or less on the Folstein Mini Mental Examination; history of anticonvulsant‐induced rash; taking drugs such as sulfonamides, sulfonylureas, carbonic anhydrase inhibitors, thiazides and loop diuretics; reporting average drinks per day within the guidelines for safe levels of alcohol consumption; having a "sulfa" allergy |
| Interventions | (1) Levetiracetam; (2) zonisamide, (3) topiramate, (4) placebo Drug dose: leviracetam 2000 mg/d; zonisamide 2000 to 4000 mg/d; topiramate 300 mg/d Duration: 14 weeks. Country of origin: USA |
| Outcomes | Primary outcome: mean number of drinks consumed per day Secondary outcomes: mean levels of attention and verbal fluency and composite measure of neurotoxicity |
| Starting date | May 2009 |
| Contact information | Megan Putnam, 617‐414‐1990, megan.putnam@bmc.org, Boston University School of Medicine, Massachusetts, United States 02118 |
| Notes |
Del Bello 2007.
| Trial name or title | Quetiapine plus topiramate or placebo for bipolar mania and alcohol use in adolescents and young adults |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 50 participants Inclusion criteria: 12 to 25 years old; both genders; meeting DSM‐IV‐TR criteria for bipolar disorder, type I, manic or mixed episode; Young Mania Rating Scale score > 16; meeting DSM‐IV‐TR criteria for current alcohol abuse or dependence; drinking > eight drinks in 30 days within the previous six months; fluent in English; If female and of child‐bearing potential, using one method of birth control Exclusion criteria: manic symptoms resulting from acute medical illness; intoxication or withdrawal from drugs or alcohol; unstable medical illness or laboratory abnormalities > three times upper limits of normal; documented history of mental retardation; use of substance other than alcohol, nicotine or cannabis; positive urine pregnancy test or lactating; history of nephrolithiasis; treatment with concurrent mood stabilisers, antipsychotics or antidepressants; treatment with antipsychotics or other mood stabilisers and antidepressants; treatment with fluoxetine; history of non‐response or hypersensitivity to quetiapine or topiramate; serious suicidal ideation or any serious suicide attempt; treatment for substance use; court‐ordered to substance use treatment; history of a medication change during the prior 30 days precipitating manic symptoms; history of a partial response to any existing medications |
| Interventions | (1) Quetiapine plus topiramate; (2) quetiapine plus placebo Dose: quetiapine 400 mg/d; topiramate 300 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: number of drinks per day; number of drinks per drinking day; number of heavy drinking days; percentage of days abstinent Secondary outcomes: YMRS scores |
| Starting date | April 2008 |
| Contact information | Jennifer Beavers, 513‐558‐6195, jennifer.beavers@uc.edu, University of Cincinnati Medical Center, Cincinnati, Ohio, United States 45227 |
| Notes |
Fischer 2011.
| Trial name or title | A 14‐week randomised, placebo‐controlled study of topiramate for alcohol use disorders in veterans with post‐traumatic stress disorder |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 30 participants Inclusion criteria: male; ages 21 to 64 years; diagnosis of PTSD with a score of 50 or higher on the Clinician Administered PTSD scale (CAPS); alcohol abuse or dependence diagnosis in the medical record or by consuming more than 35 standard drinks per week over the previous four weeks as measured by the TLFB interview; a desire to reduce drinking behaviour Exclusion criteria: taking a carbonic anhydrase inhibitor (e.g. zonisamide, acetazolamide, dichlorphenamide); taking or having recently taken acamprosate, naltrexone, disulfiram, topiramate; change in benzodiazepine or other medication dose within the past four weeks; seizure disorder; head trauma with loss of consciousness or a diagnosis of postconcussive syndrome; suicide attempt or suicidal ideation; history of kidney stones; history of glaucoma; ALT or AST liver enzymes elevated more than twice the upper limit of normal; more than four unsuccessful attempts at inpatient alcohol treatment; medically unstable; a history of delirium tremens or alcohol withdrawal seizure; compulsory treatment to avoid legal consequences; currently in a setting without access to alcohol |
| Interventions | (1) Topiramate; (2) placebo Dose: topiramate up to 400 mg/d Duration: 14 weeks. Country of origin: USA |
| Outcomes | Primary outcome: number of days of heavy drinking Secondary outcomes: number of days abstinent; number of PTSD symptoms; number of memory/cognitive complaints |
| Starting date | August 2011 |
| Contact information | Jennifer Duncan, (410) 605‐7000, ext 4738, Jennifer.Duncan5@va.gov, Department of Veterans Affairs, University of Maryland School of Medicine, Maryland Health Care System, Baltimore, Maryland, United States 21201 |
| Notes |
Frye 2005.
| Trial name or title | An evaluation of divalproex versus olanzapine for alcohol abuse relapse prevention in patients with bipolar disorder |
| Methods | Randomised single‐blind trial |
| Participants | Inclusion criteria: age 18 to 65 years; meeting DSM‐IV criteria for manic episode based on the SCID; meeting DSM‐IV criteria for alcohol dependence or abuse based on the SCID; negative urine pregnancy test Exclusion criteria: inability to give informed consent; liver function tests greater than three times upper limit of normal; history of adverse reaction to divalproex sodium or olanzapine; history of seizure; history of major head trauma; history of hypertension, neurological illness, active hepatitis, hepatic encephalopathy, pancreatitis; not practising a reliable form of birth control |
| Interventions | (1) Divalproex sodium; (2) olanzapine Dose: divalproex sodium up to 2500 mg/d; olanzapine up to 20 mg/d Duration: 46 weeks. Country of origin: USA |
| Outcomes | Primary outcome: alcohol abuse relapse Secondary outcomes: number of drinking days, percentage of drinking days per month, standard drinks per drinking occasion, craving (assessed through the TLFB method); major mood relapse and adjunctive medication (assessed by prospective life charting) |
| Starting date | September 2005 |
| Contact information | Frye Mark A, UCLA Neuropsychiatric Institute, Los Angeles, California, United States 90095 |
| Notes | Study completed |
Johnson 2000.
| Trial name or title | Combining medication treatments for alcoholism |
| Methods | Randomised controlled double‐blind trial |
| Participants | 320 participants Inclusion criteria: 18 years and older; both genders; diagnosis of alcohol dependence and drinking greater than or equal to 14 alcohol drinks/wk for women and 21 alcohol drinks/wk for men; providing written informed consent; good physical health; literate in English and able to follow instructions and to complete questionnaires accurately; willingness to participate in behavioural treatments for alcoholism; providing evidence of stable residence in the study Exclusion criteria: psychiatric disorder other than alcohol or nicotine dependence; liver enzymes greater than four times upper limit of the normal range or elevated direct bilirubin; serious medical co‐morbidity or any condition that can interfere with the receipt of ondansetron; severe or life‐threatening adverse reactions to medications; females pregnant, nursing or not using an acceptable form of contraception; having received treatment for alcohol dependence within the past 30 days; members of the same household; treated with medications having a potential effect on alcohol consumption or mood; urine must be free of opiates, cocaine, amphetamines, barbiturates, benzodiazepines and other prescription and non‐prescription drugs; severe alcohol withdrawal symptoms that require inpatient treatment; mandatory participation in an alcohol treatment programme; pyrexia of unknown origin; history of seizures; history of kidney stones; history of glaucoma |
| Interventions | (1) Ondansetron; (2) topiramate; (3) ondansetron plus topiramate; (4) placebo Associated behavioural therapy Duration: 13 weeks. Country of origin: USA |
| Outcomes | Primary outcome: reduction of alcohol consumption Secondary outcome: abstinence from alcohol consumption |
| Starting date | March 2005 |
| Contact information | Mindy Borszich, BA, 434 243 0549, uvacare@virginia.edu; Eva Jenkins‐Mendoza, BS, 434 243‐0562, uvacare@virginia.edu; University of Virginia Center for Addiction Research and Education, Charlottesville, Virginia, United States 22911 |
| Notes |
Johnson 2007 b.
| Trial name or title | Novel pharmacotherapy for dual dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 180 participants Inclusion criteria: both genders; 18 years and older; meeting at least three of the DSM‐IV diagnostic criteria for alcohol and cocaine dependence; expressing a desire for treatment; literacy in English and able to follow study rules, instruction and treatment; reporting cocaine use of at least once per month and alcohol consumption of ≥ 21 drinks/wk and ≥ 14 drinks/wk for men and women, respectively, during the past 30 days; showing at least one positive urine drug screen for cocaine at screen or baseline before randomisation Exclusion criteria: individuals likely to require hospitalisation for severe medical complications or surgery, or those whose severity of illness precludes utilisation of behavioural treatments; women pregnant, breast‐feeding or unwilling to use effective birth control; physical or psychiatric conditions; dependence, defined by DSM‐IV criteria, on any psychoactive substance other than cocaine, alcohol, nicotine, caffeine or marijuana or physiological dependence on alcohol requiring medical detoxification; mandated by court to obtain treatment for alcohol and/or cocaine dependence; elevation of liver enzymes (AST, ALT), blood urea nitrogen (BUN) or lactate dehydrogenase (LDH) greater than four times upper limit of the normal range or elevated direct bilirubin; not expected to complete the study protocol because of probable incarceration or relocation from the clinic area; severe or life‐threatening adverse reactions to topiramate in the past or during this clinical trial; AIDS or HIV with CD4 positive T cell counts < 500 mm3; receiving pharmacotherapy for treatment of AIDS or HIV; active syphilis that has not been treated; currently receiving active treatment with topiramate |
| Interventions | (1) Topiramate; (2) placebo Dose: topiramate up to 300 mg/d Participants will be offered weekly cognitive‐behavioural therapy Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: weekly mean proportion of cocaine‐free days and self reported drinking Secondary outcomes: psychosocial functioning as exemplified by improved general well‐being, social functioning and quality of life |
| Starting date | March 2007 |
| Contact information | Mindy Borszich, BA, 1‐888‐882‐2345, uvacare@virginia.edu; University of Virginia Center for Addiction Research and Education, Charlottesville, Virginia, United States 22911 |
| Notes |
Jorge 2011.
| Trial name or title | Treatment strategy for alcohol use disorders in veterans with TBI |
| Methods | Randomised controlled double‐blind trial |
| Participants | 90 participants Inclusion criteria: veterans attending alcohol use disorder rehabilitation treatment; 18 to 60 years old; all male; alcohol dependence according to DSM‐IV criteria; history of heavy drinking; absence of withdrawal symptoms Exclusion criteria: other substance abuse different from nicotine or cannabis (DSM‐IV criteria); unstable medical conditions such as severe heart disease, liver or renal failure or evidence of neoplasia; liver enzymes (ALT, AST) serum levels > three times upper limit of normal; unstable psychiatric conditions; diagnosis of schizophrenia or schizoaffective disorder; requiring therapy with valproate or naltrexone or history of significant adverse effects from either study drug; requiring therapy with topiramate, lamotrigine or carbamazepine; requiring long‐term treatment with opioid analgesics for refractory pain; having failed three previous intensive alcohol rehabilitation programmes in the past two years; females |
| Interventions | (1) Sodium valproate; (2) naltrexone Drug dose: sodium valproate up to 2000 mg/d; naltrexone 50 mg/d Duration: eight weeks. Country of origin: USA |
| Outcomes | Primary outcome: time to relapse to heavy drinking |
| Starting date | June 2011 |
| Contact information | Kyla D Kennedy, BA, (319) 353‐4600, kyla‐kennedy@uiowa.edu, Iowa City VA Medical Center, Iowa City, IA, Iowa, United States 52246‐2208 |
| Notes |
Kampman 2011.
| Trial name or title | A phase II, double‐blind, placebo‐controlled, pilot trial of vigabatrin for the treatment of cocaine and alcohol dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 60 participants Inclusion criteria: both genders; 18 years of age or older; meeting DSM‐IV criteria for current diagnoses of cocaine and alcohol dependence; three consecutive days of abstinence from alcohol directly before the day of randomisation; verifiable address of principal residence and agreeing to attend all research visits including follow‐up visits; speaking, understanding and printing in English; able to give informed consent Exclusion criteria: meets DSM‐IV criteria for dependence on any substance other than cocaine and alcohol (except nicotine and cannabis); needing treatment with any psychoactive medications; meeting current or lifetime DSM‐IV criteria for schizophrenia or organic mental disorder or meeting current DSM‐IV diagnosis of any other clinically significant psychiatric disorder that will interfere with study participation; evidence of history of significant haematological, pulmonary, endocrine, cardiovascular, renal or gastrointestinal disease; severe physical or medical illness such as AIDS, active hepatitis, significant hepatocellular injury; use of an investigational medication in the 30 days before randomisation; history of prior treatment with vigabatrin; history of prior treatment with drugs with known retinotoxicity; history of visual field defects or predisposing factors; female positive on a pregnancy test, contemplating pregnancy in the next six months, nursing or not using an effective contraceptive method |
| Interventions | (1) Vigabatrin; (2) placebo Drug dose: vigabatrin up to 3000 mg/d Duration: eight weeks. Country of origin: USA |
| Outcomes | Primary outcomes: reduction in cocaine use (number of benzoylecgonine (BE) negative urine samples); alcohol abstinent days and heavy drinking days Secondary outcomes: measures of cocaine and alcohol craving; addiction severity (ASI); disease severity and improvement; alcohol and cocaine withdrawal severity (CIWA and Cocaine Selective Severity Assessment); depression and anxiety (HDRS and HARS) |
| Starting date | April 2011 |
| Contact information | Donna Simpson, +121 524 39959, addicted@med.upenn.edu, University of Pennsylvania, Treatment Research Center, Philadelphia, Pennsylvania, United States 19104 |
| Notes |
Kranzler 2008.
| Trial name or title | Topiramate treatment of problem drinkers |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 160 participants Inclusion criteria: both genders; age 18 to 65 years; average weekly ethanol consumption of >= 24 standard drinks for men or >= 18 standard drinks for women; able to read English at the eighth grade or higher level with no evidence of significant cognitive impairment; willing to nominate an individual who will know the participant's whereabouts to facilitate follow‐up during the study; if a woman of child‐bearing potential, must be non‐lactating, practicing a reliable method of birth control and having a negative serum pregnancy test; if applicable, individuals treated with a single antidepressant that has been stable in dosage for a minimum of four weeks; willing to provide signed informed consent to participate in the study Exclusion criteria: current, clinically significant physical disease or abnormality, including direct bilirubin elevations > 110% or transaminase elevations > 300% normal; history of nephrolithiasis, glaucoma, serious psychiatric illness (i.e. schizophrenia, bipolar disorder, severe or psychotic major depression, panic disorder, borderline or antisocial personality disorder, organic mood or mental disorders, eating disorder or substantial suicide or violence risk); current DSM‐IV diagnosis of drug dependence (other than nicotine dependence); current DSM‐IV diagnosis of alcohol dependence that is clinically moderate or severe; history of hypersensitivity to topiramate; currently taking any tricyclic antidepressant |
| Interventions | (1) Topiramate; (2) placebo Dose: topiramate up to 200 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: drinking days and heavy drinking days Secondary outcomes: mean daily alcohol consumption; change in gamma‐glutamyl transferase (GGT) or CDT levels; severity of alcohol‐related problems |
| Starting date | February 2008 |
| Contact information | Timothy S Pond, BA, 215‐222‐3200, ext 241, pond_t@mail.trc.upenn.edu, University of Pennsylvania, Philadelphia, Pennsylvania, United States 19104 |
| Notes |
Mariani 2010.
| Trial name or title | Gabapentin for abstinence initiation in alcohol dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 60 participants Inclusion criteria: between the ages of 18 and 65; meeting DSM‐IV criteria for current alcohol dependence; seeking treatment for alcohol dependence; drinking a minimum of five standard drinks for men or four standard drinks for women at least four days per week over the past 28 days; able to provide informed consent and to comply with study procedures Exclusion criteria: history of DSM‐IV diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder; diagnosis of current major depressive disorder or any other current Axis I psychiatric disorder as defined by DSM‐IV‐TR, other than alcohol dependence, that in the investigator's judgement might require intervention over the course of the study; receiving prescribed psychotropic medication; evidence of moderate to severe alcohol withdrawal (CIWA‐Ar > 13); history of allergic reaction to candidate medication; history of alcohol withdrawal seizures or alcohol withdrawal delirium; pregnancy, lactation or failure to use adequate contraceptive methods in female participants; unstable medical conditions, such as poorly controlled diabetes or hypertension (> 140/90 mmHg); current DSM‐IV‐TR diagnosis of other substance dependence, with the exception of nicotine and caffeine dependence; legally mandated to participate in an alcohol use disorder treatment programme; significant risk for suicide; participants likely, based on history, to place themselves in danger (e.g. driving while intoxicated or otherwise unwilling to follow safety precautions); renal insufficiency or abnormal renal function |
| Interventions | (1) Gabapentin; (2) placebo Drug dose: gabapentin up to 1200 mg/d Duration: eight weeks. Country of origin: USA |
| Outcomes | Primary outcome: number of days of abstinence |
| Starting date | August 2010 |
| Contact information | John J. Mariani, MD, 212‐543‐5987, jm2330@columbia.edu, New York State Psychiatric Institute, New York, New York, United States 10032 |
| Notes |
Mason 2009.
| Trial name or title | Duloxetine versus pregabalin for alcohol dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 150 participants Inclusion criteria: both genders; 18 years of age and older; meeting DSM‐IV criteria for alcohol dependence and drinking an average of ≥ 21 drinks weekly for males, ≥ 14 for females, with at least one heavy drinking day (≥ five males, ≥ four females) per week; seeking research‐based outpatient treatment for alcohol problems; willing to participate in abstinence‐oriented individual counselling sessions; normal bilirubin, and ALT, AST and GGT values no greater than three times the ULN, with no evidence of hepatic insufficiency; in good physical health; not using any medications, supplements or herbs that could potentially increase risk of hepatotoxicity Exclusion criteria: active suicidal ideation; medical disorders that will increase potential risk or interfere with study participation; sexually active female individuals who are pregnant or nursing or refuse to use a reliable method of birth control; males who refuse to use a reliable method of birth control; meeting DSM‐IV criteria for current anxiety or affective disorders, current or lifetime bipolar disorder, other substance use disorders or any current major Axis I disorder other than alcohol or nicotine dependence; inability to understand and/or comply with the provisions of the protocol and consent form; treatment with an investigational drug during the previous month; prior treatment with pregabalin or duloxetine; treatment with an antidepressant medication; sensitivity to study drugs; ongoing treatment with disulfiram, naltrexone, acamprosate or other medications that may affect study outcomes; ongoing treatment with drugs that may increase potential risk; abstinence longer than one month before randomisation; individuals who require medicated detoxification; individuals for whom treatment of alcoholism is mandated by a legal authority; inability to identify at least one collateral informant to verify drinking status at baseline and monthly during study and to assist in tracking participant for follow‐up assessments |
| Interventions | (1) Duloxetine; (2) pregabalin; (3) placebo Drug dose: duloxetine up to 60 mg/d; pregabalin up to 600 mg/d Behavioural: standardised behavioural therapy Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: drinking quantity and frequency; affective state Secondary outcomes: craving; mood; sleep; adverse events; physiological reactivity |
| Starting date | July 2009 |
| Contact information | Susan B Quello, BA, BS, 858‐784‐7327, squello@scripps.edu; Barbara J. Mason, PhD, The Scripps Research Institute Pearson Center for Alcoholism and Addiction Research, La Jolla, California, United States 92037 |
| Notes |
Messing 2008.
| Trial name or title | Placebo‐controlled cross‐over trial of levetiracetam on ethanol intake |
| Methods | Randomised placebo‐controlled double‐blind cross‐over trial |
| Participants | 46 participants Inclusion criteria: healthy adults who are social drinkers 21 to 50 years of age; moderate to heavy social drinkers (women seven to 21 drinks/wk, men seven to 25 drinks/wk); body mass index > 18 and < 30; If female, must be non‐lactating, not pregnant and using a reliable contraceptive method; able and willing to provide written informed consent to understand and follow the instructions of the investigator and to understand all rating scales; negative urine drug screen at all visits, with the exception of cannabinoids. Exclusion criteria: positive urine drug screen, except cannabinoids; use of cocaine, amphetamines or other stimulants, hallucinogens, ecstasy or other psychoactive drugs; use of PCP or ketamine; history of abusing inhalants; current or past dependence on any psychoactive drug (except nicotine or caffeine), including alcohol; current or prior enrolment in an alcohol or other drug treatment programme, or current legal problems related to alcohol or other drug use; binge drinking more than three times per week; alcohol consumption > 21 drinks/wk for women and > 25 drinks/wk for men; currently trying to quit alcohol and/or recreational drug use; positive for lifetime abnormal opioid use or prescription drug abuse; clinically significant medical or psychiatric illness; bilirubin more than two times normal upper limit; AST (SGOT), ALT (SGPT) or alkaline phosphatase more than two times normal upper limit; body mass index > 30 or < 18; pregnant woman or woman of child‐bearing potential not currently using an adequate means of contraception; currently taking any medication other than some over‐the‐counter products; BAC level greater than 0.02%; estimated creatinine clearance < 50 mL/min; chronic pain condition requiring treatment; neurological dysfunction or psychiatric disorder severe enough to interfere with assessment; allergy to levetiracetam; cardiac pathology or abnormal initial EKG; having received an investigational drug within 30 days before the study; individuals unable to read or speak English or unable to adhere to scheduled appointments, unlikely to comply with the study protocol or unsuitable for any other reason |
| Interventions | (1) Levetiracetam; (2) placebo Drug dose: levetiracetam 1000 mg/d Duration: six weeks. Country of origin: USA |
| Outcomes | Primary outcomes: number of drinks/wk consumed while taking study drug and placebo |
| Starting date | November 2008 |
| Contact information | Robert O. Messing, MD, UCSF, Department of Neurology, Ernest Gallo Clinic and Research Center, Children's Hospital Oakland Research Institute‐CRC, Berkeley, California, United States 94705 |
| Notes |
Ostacher 2007.
| Trial name or title | Adjunctive topiramate for treatment of alcohol dependence in patients with bipolar disorder |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 80 participants Inclusion criteria: age 18 years and older; meeting DSM‐IV criteria for alcohol dependence; meeting DSM‐IV criteria for bipolar disorder; ≥ eight heavy drinking days (defined as ≥ five standard drinks per day for men, ≥ four standard drinks per day for women) in the prior four weeks; receiving a stable dose of accepted maintenance treatment for bipolar disorder for the past four weeks; If participant is taking more than one agent, at least one agent must be adequately dosed; antidepressant treatment is permitted if the dose has been stable for the past four weeks Exclusion criteria: pregnant women or women of child‐bearing potential who are not using a medically accepted means of contraception; women who are lactating; important alcohol withdrawal symptoms; urine toxicological screen positive for amphetamines or cocaine; meeting DSM‐IV criteria for current substance dependence other than cannabis or nicotine; meeting current full DSM‐IV criteria for manic, hypomanic or mixed episode; serious suicide or homicide risk; unstable medical illness, including cardiovascular, hepatic, renal, respiratory, endocrine, neurological or haematological disease or uncontrolled seizure disorder; history of nephrolithiasis or treatment with any drug associated with nephrolithiasis; current treatment with zonisamide; current treatment with any carbonic anhydrase inhibitors; current treatment with any drug known to decrease drinking; individuals who have begun a new psychosocial treatment within 12 weeks of study enrolment; any psychotic disorder, including schizoaffective disorder (current or past); clinical or laboratory evidence of untreated hypothyroidism; diagnosis or history of glaucoma; past intolerance to topiramate; any use of topiramate in the past 12 months; any investigational psychotropic drug within the last three months |
| Interventions | (1) Topiramate; (2) placebo Dose: topiramate 300 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcome: amount of alcohol consumed Secondary outcomes: safety and tolerability of topiramate; effect of decreased drinking on mood symptoms |
| Starting date | August 2007 |
| Contact information | Michael J. Ostacher, MD, MPH, 617‐726‐5258, mostacher@partners.org ; Andrew Peckham, 617‐724‐6545, apeckham@partners.org, Massachusetts General Hospital Bipolar Clinic and Research Program, Boston, Massachusetts, United States 02114 |
| Notes |
Petrakis 2007.
| Trial name or title | The use of anticonvulsants for treatment of patients with alcohol dependence and post‐traumatic stress disorder |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 90 participants Inclusion criteria: both genders; between the ages of 18 and 60 years; current alcohol abuse or dependence; current PTSD; receiving a stable dose of antidepressant medication; medically and neurologically healthy; for women, negative pregnancy test and use of acceptable method of contraception Exclusion criteria: females who are pregnant or lactating; individuals with a current unstable medical condition such as neurological, cardiovascular, endocrine, renal, liver or thyroid pathology; individuals who meet current SCID criteria for a major Axis I diagnosis; history of substance dependence (other than alcohol, tobacco or cannabis) by DSM‐IV criteria; individuals taking mood stabilisers and antipsychotic medications; individuals with a history of allergy to topiramate or lamotrigine |
| Interventions | (1) Lamotrigine; (2) topiramate; (3) placebo Dose: lamotrigine 250 mg/d; topiramate 250 mg/d Duration: 16 weeks. Country of origin: USA |
| Outcomes | Primary outcomes: drinking; craving; PTSD symptoms |
| Starting date | June 2009 |
| Contact information | Elizabeth Ralevski, PhD, 203‐932‐5711, ext 4282, elizabeth.ralevski@yale.edu, VA Connecticut Healthcare System, West Haven, Connecticut, United States 06516 |
| Notes |
Ralevski 2007.
| Trial name or title | Topiramate for treatment of patients with borderline personality disorder and alcohol dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 30 participants Inclusion criteria: 21 to 60 years old; both genders; diagnosis of alcohol dependence; diagnosis of borderline personality disorder Exclusion criteria: serious or unstable medical condition; opiate dependence; major Axis I disorder; taking mood stabilisers and antipsychotic medications; liver functional test abnormalities that do not exceed three times normal values |
| Interventions | (1) Topiramate plus SSRI; (2) placebo plus SSRI Drug dose: topiramate 250 mg/d Duration: eight weeks. Country of origin: USA |
| Outcomes | Primary outcomes: drinking; craving; aggression Secondary outcomes: affect; adverse effects |
| Starting date | March 2007 |
| Contact information | Elizabeth Ralevski, PhD, +120 393 25711, ext 4282, elizabeth.ralevski@yale.edu, Yale University, VA Connecticut Healthcare System, West Haven, Connecticut, United States 06516 |
| Notes |
Schaefer 2008.
| Trial name or title | Efficacy and safety of levetiracetam in prevention of alcohol relapse in recently detoxified alcohol‐dependent patients |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 201 participants Inclusion criteria: at least 18 years of age and not older than 70 years; good knowledge of German language; alcohol dependence according to DSM‐IV and ICD‐10; having understood the meaning and consequences of the study; negative drug screening regarding benzodiazepines and opiates; for women, negative pregnancy test and use of acceptable method of contraception Exclusion criteria: alcohol withdrawal syndrome beginning or existing; simultaneous ambulatory or stationary curing therapy; any further substance dependence except nicotine and/or caffeine dependence; idiopathic epilepsy; anamnesis of heavy cerebral traumas or other heavy neurological illnesses; current medication that can affect significantly withdrawal symptoms or craving or can promote abstinence or further substances that can affect glutamatergic, dopaminergic, cholinergic, serotonergic or opioid system; contraindications or heavy adverse effects in relation to the study medication; hypersensitivity opposite pyrrolidonderivate; pregnancy or quiet time or insufficient contraception; acute severe psychiatric disturbance in need of treatment; acute suicidality; severe internal illness; simultaneous participation at another clinical study |
| Interventions | (1) Levetiracetam; (2) placebo Drug dose: levetiracetam 1500 to 2000 mg/d Duration: not specified. Country of origin: Germany |
| Outcomes | Primary outcome: comparison alcohol free "surviving" (heavy alcohol relapse) Secondary outcomes: time up to first drinking; cumulative time of not drinking over the study duration; frequency of lapses; tolerability of the study medication; dropout rate; adverse effects; changes with neuropsychological testing, HDRS, HARS, OCDS, TLFB, SCL‐90; quality of life |
| Starting date | May 2007 |
| Contact information | Martin Schaefer, MD, Department of Psychiarty, Charite Campus Mitte, Berlin, and Department of Psychiatry, Kliniken Essen‐Mitte, Essen, Germany |
| Notes |
South Carolina 2001.
| Trial name or title | Alcohol Research Center—treatment and implications |
| Methods | Randomised controlled double‐blind trial |
| Participants | 160 participants Inclusion criteria: meeting criteria for alcohol dependence and uncomplicated alcohol withdrawal syndrome; medically stable (not likely to require hospitalisation for medical complications within 10 days); clinical withdrawal assessment before the study; medically acceptable for study treatment; able to read, write and speak English; negative urine drug screen for benzodiazepines or other sedative‐hypnotics, opiates and stimulant Exclusion criteria: diagnosis of any substance dependence syndrome other than alcohol dependence (excluding nicotine and caffeine); use of pharmacological agents known to lower the seizure threshold or to augment or decrease alcohol withdrawal syndrome; history of alcohol withdrawal seizures, epilepsy or delirium tremens; diagnosis of schizophrenia, bipolar disorder or dementia; liver function tests higher than normal; history of hepatic encephalopathy, jaundice, ascites, diabetes or renal disease; females who are pregnant or nursing; individuals with known sensitivity of previous adverse reaction to gabapentin, lorazepam or other benzodiazepines; history of severe gastrointestinal disease that might render absorption of the medication difficult or might produce medical instability of the patient during detoxification; unable to provide informed consent |
| Interventions | (1) Gabapentin; (2) lorazepam Drug dose: not specified Duration: not specified. Country of origin: USA |
| Outcomes | Not reported |
| Starting date | Not reported |
| Contact information | Medical University of South Carolina, Charleston, South Carolina, United States 29425 |
| Notes |
Tolliver 2009.
| Trial name or title | A double‐blind, placebo‐controlled trial of lamotrigine in individuals with bipolar disorder and co‐morbid alcohol dependence |
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | 60 participants Inclusion criteria: age 18 to 65; meeting DSM‐IV‐TR criteria for alcohol dependence with active alcohol use in the past 30 days; meeting DSM‐IV‐TR criteria for bipolar I or bipolar II disorder; average alcohol consumption of at least 35 drinks/wk for men, 28 drinks/wk for women in the last four weeks of active drinking before enrolment; able to provide informed consent and to function at an intellectual level sufficient to allow accurate completion of assessment instruments; currently under the care of a psychiatrist; currently taking a therapeutic dosage of one or more mood‐stabilising medications; stable psychiatric symptoms; agreeing to identify collateral individuals for contact to facilitate follow‐up appointments Exclusion criteria: primary psychiatric diagnosis other than bipolar disorder; any uncontrolled neurological condition; any history of Stevens‐Johnson syndrome or other severe rash requiring hospitalisation; any history of head injury with loss of consciousness; any history of learning disability, alcoholic dementia or electroconvulsive therapy in the past three months; any uncontrolled medical condition; plasma levels of liver transaminases (AST, ALT) greater than three times normal range; concomitant use of valproic acid, carbamazepine, oxcarbazepine, phenytoin, primidone, phenobarbital, disulfiram, naltrexone, acamprosate, topiramate, benzodiazepines or any other medications not allowed per the protocol; women of child‐bearing potential who are pregnant or lactating or who refuse adequate forms of contraception; current suicidal or homicidal risk; baseline scores greater than 35 on the Montgomery‐Asberg Depression Rating Scale or greater than 16 on the Young Mania Rating Scale |
| Interventions | (1) Lamotrigine; (2) placebo Dose: lamotrigine 200 mg/d Duration: 12 weeks. Country of origin: USA |
| Outcomes | Primary outcome: percentage of days of abstinence from alcohol. Secondary outcomes: drinks per week, drinking days per week, heavy drinking days per week; biomarkers of alcohol use; depression; mania/hypomania symptoms; neurocognitive performance |
| Starting date | February 2010 |
| Contact information | Bryan K Tolliver, MD, PhD (843) 792‐5215 tollive@musc.edu; Delisa G Brown (843) 792‐0572, browndg@musc.edu, Clinical Neuroscience Division, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina Recruiting Charleston, South Carolina, United States 29425 |
| Notes |
ALT: Alanine aminotransferase.
ASI: Addiction Severity Index.
AST: Aspartate aminotransferase.
CIWA‐Ar: Clinical Institute Withdrawal Assessment for Alcohol scale, Revised.
DSM‐IV: Diagnostic and Statistic Manual of Mental Disorders (American Psychiatric Association), Fourth Edition.
GGT: γ‐Glutamyltransferase.
HARS: Hamilton Anxiety Rating Scale.
HDRS: Hamilton Depression Rating Scale.
OCDS: Obsessive‐Compulsive Drinking Scale.
PTSD: Post‐traumatic stress disorder.
SCID: Structured Clinical Interview for DSM.
SCL90‐R: Symptom Checklist‐90 Revised.
TLFB: Timeline follow‐back.
Differences between protocol and review
The risk of bias assessment was updated according to the update from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Moreover, although in the protocol we chose to apply a meta‐analysis fixed‐effect model (unless significant heterogeneity was noted), the heterogeneity of populations and types of interventions resulting from the studies included in the review convinced us to apply the more appropriate random‐effects model.
Contributions of authors
Two review authors (PPP, ET) inspected the search hits by reading titles and abstracts. Each potentially relevant study located by the search was obtained in full text and was assessed for inclusion independently by two review authors (PPP, ET). Doubts were resolved by discussion between the review authors. Two review authors (PPP, ET) assessed study quality. Data were extracted independently by two review authors (PPP, ET). Disagreements were discussed among all review authors.
Sources of support
Internal sources
New source of support, Not specified.
External sources
No sources of support supplied
Declarations of interest
None
New
References
References to studies included in this review
Anton 2009 {published data only}
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. Journal of Clinical Psychopharmacology 2009;29:334‐42. [DOI] [PubMed] [Google Scholar]
Anton 2011 {published data only}
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. American Journal of Psychiatry 2011;168:709‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arias 2010 {published data only}
- Arias AJ, Feinn R, Oncken C, Covault J, Kranzler HR. Placebo‐controlled trial of zonisamide for the treatment of alcohol dependence. Journal of Clinical Psychopharmacology 2010;30:318‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baltieri 2008 {published data only}
- Baltieri DA, Daró FR, Ribeiro PL, Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction 2008;103:2035‐44. [DOI] [PubMed] [Google Scholar]
Brady 2002 {published data only}
- Brady KT, Myrick H, Henderson S, Coffey SF. The use of divalproex in alcohol relapse prevention: a pilot study. Drug and Alcohol Dependence 2002;67:323‐30. [DOI] [PubMed] [Google Scholar]
Brower 2008 {published data only}
- Brower KJ, Myra Kim H, Strobbe S, Karam‐Hage MA, Consens F, Zucker RA. A randomized double‐blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcoholism: Clinical and Experimental Research 2008;32:1429‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Croissant 2006 {published data only}
- Croissant B, Diehl A, Klein O, Zambrano S, Nakovics H, Heinz A, et al. A pilot study of oxcarbazepine versus acamprosate in alcohol‐dependent patients. Alcoholism: Clinical and Experimental Research 2006;30:630‐5. [DOI] [PubMed] [Google Scholar]
De Sousa 2008 {published data only}
- Sousa AA, Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. Journal of Substance Abuse Treatment 2008;34:460‐3. [DOI] [PubMed] [Google Scholar]
Fertig 2012 {published data only}
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, et al. NCIG 002 Study Group. A double‐blind, placebo‐controlled trial assessing the efficacy of levetiracetam extended‐release in very heavy drinking alcohol‐dependent patients. Alcoholism: Clinical and Experimental Research 2012;36:1421‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Florez 2008 {published data only}
- Flórez G, García‐Portilla P, Alvarez S, Saiz PA, Nogueiras L, Bobes J. Using topiramate or naltrexone for the treatment of alcohol‐dependent patients. Alcoholism: Clinical and Experimental Research 2008;32:1251‐9. [DOI] [PubMed] [Google Scholar]
Florez 2011 {published data only}
- Florez G, Saiz PA, Garcia‐Portilla P, Alvarez S, Nogueiras L, Bobes J. Topiramate for the treatment of alcohol dependence: comparison with naltrexone. European Addiction Research 2011;17:29‐36. [DOI] [PubMed] [Google Scholar]
Furieri 2007 {published data only}
- Furieri FA, Nakamura‐Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2007;68:1691‐700. [DOI] [PubMed] [Google Scholar]
Johnson 2003 {published data only}
- Johnson BA, Ait‐Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol‐dependent individuals: a randomized controlled trial. Archives of General Psychiatry 2004;61:905‐12. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait‐Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 2003;361::1677‐85. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Ait‐Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction 2006;101:1561‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2007 {published data only}
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for Alcoholism Advisory Board, Topiramate for Alcoholism Study Group. Improvement of physical health and quality of life of alcohol‐dependent individuals with topiramate treatment: US multisite randomized controlled trial. Archives of Internal Medicine 2008;168:1188‐99. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for Alcoholism Advisory Board, Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA 2007;298:1641‐51. [DOI] [PubMed] [Google Scholar]
Kampman 2013 {published and unpublished data}
- Kampman KM. A Phase II, Randomized, Double‐blind, Placebo‐Controlled, Pilot Trial of Topiramate for Alcohol and Comorbid Cocaine Dependence. ClinicalTrials.gov 2005.
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O'Brien CP. A double‐blind, placebo‐controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug and Alcohol Dependence 2013;133:94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Likhitsathian 2013 {published data only}
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12‐week, randomized, placebo‐controlled trial. Drug and Alcohol Dependence 2013;133:440‐6. [DOI] [PubMed] [Google Scholar]
Martinotti 2007 {published data only}
- Martinotti G, Nicola M, Romanelli R, Andreoli S, Pozzi G, Moroni N, et al. High and low dosage oxcarbazepine versus naltrexone for the prevention of relapse in alcohol‐dependent patients. Human Psychopharmacology 2007;22:149‐56. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Romanelli R, Nicola M, Reina D, Mazza M, Janiri L. Oxcarbazepine at high dosages for the treatment of alcohol dependence. American Journal of Addiction 2007;16:247‐8. [DOI] [PubMed] [Google Scholar]
Martinotti 2010 {published data only}
- Martinotti G, Nicola M, Tedeschi D, Andreoli S, Reina D, Pomponi M, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double‐blind, comparison trial. Journal of Psychopharmacology 2010;24:1367‐74. [DOI] [PubMed] [Google Scholar]
Mueller 1997 {published data only}
- Mueller TI, Stout RL, Rudden S, Brown RA, Gordon A, Solomon DA, et al. A double‐blind, placebo‐controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcoholism: Clinical and Experimental Research 1997;21:86‐92. [PubMed] [Google Scholar]
Paparrigopoulos 2010 {published data only}
- Paparrigopoulos T, Tzavellas E, Karaiskos D, Malitas P, Liappas I. An open pilot study of tiagabine in alcohol dependence: tolerability and clinical effects. Journal of Psychopharmacology 2010;24:1375‐80. [DOI] [PubMed] [Google Scholar]
Paparrigopoulos 2011 {published data only}
- Paparrigopoulos T, Tzavellas E, Karaiskos D, Kourlaba G, Liappas I. Treatment of alcohol dependence with low‐dose topiramate: an open‐label controlled study. BMC Psychiatry 2011;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richter 2012 {published data only}
- Richter C, Effenberger S, Bschor T, Bonnet U, Haasen C, Preuss UW, et al. Efficacy and safety of levetiracetam for the prevention of alcohol relapse in recently detoxified alcohol‐dependent patients: a randomized trial. Journal of Clinical Psychopharmacology 2012;32:558‐62. [DOI] [PubMed] [Google Scholar]
Rubio 2009 {published data only}
- Rubio G, Martínez‐Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. Journal of Clinical Psychopharmacology 2009;29:584‐9. [DOI] [PubMed] [Google Scholar]
Salloum 2005 {published data only}
- Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double‐blind placebo‐controlled study. Archives of General Psychiatry 2005;62:37‐45. [DOI] [PubMed] [Google Scholar]
Trevisan 2008 {published data only}
- Trevisan LA, Ralevski E, Keegan K, Oville A, Vuppalapati D, Gonzalez G, et al. Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol‐dependent patients. Addictive Disorders and Their Treatment 2008;7:119. [Google Scholar]
References to studies excluded from this review
Karam‐Hage 2003 {published data only}
- Karam‐Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Journal of Psychiatry and Clinical Neuroscience 2003;57:542‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knapp 2010 {published data only}
- Knapp CM, Sarid‐Segal O, Richardson MA, Colaneri LS, Afshar M, Devine E, et al. Open label trial of the tolerability and efficacy of zonisamide in the treatment of alcohol dependence. American Journal of Drug and Alcohol Abuse 2010;36:102‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Strat 2012 {published data only}
- Strat Y. Levetiracetam in the treatment of alcohol dependence: toward the end of the story?. Alcoholism: Clinical and Experimental Research 2012;36:1309‐10. [DOI] [PubMed] [Google Scholar]
Miranda 2008 {published data only}
- Miranda R Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcoholism: Clinical and Experimental Research 2008;32:489‐97. [DOI] [PubMed] [Google Scholar]
Mitchell 2012 {published data only}
- Mitchell JM, Grossman LE, Coker AR, Messing RO. The anticonvulsant levetiracetam potentiates alcohol consumption in non‐treatment seeking alcohol abusers. Journal of Clinical Psychopharmacology 2012;32:269‐72. [DOI] [PubMed] [Google Scholar]
Myrick 2007 {published data only}
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double‐blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcoholism: Clinical and Experimental Research 2007;31:221‐7. [DOI] [PubMed] [Google Scholar]
Narayana 2008 {published data only}
- Narayana PL, Gupta AK, Sharma PK. Use of anti‐craving agents in soldiers with alcohol dependence syndrome. Medical Journal of Armed Forces India 2008;64:320‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schacht 2013 {published data only}
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Effects of a GABA‐ergic medication combination and initial alcohol withdrawal severity on cue‐elicited brain activation among treatment‐seeking alcoholics. Psychopharmacology 2013;227:627‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bajovic 2012 {published data only}
- Bajovic B, Zivkovic N, Djokic G, Pavicevic D. Oxcarbazepine, topiramate and lamotrigine in the treatment of alcohol craving. European Neuropsychopharmacology 2012;22:S65‐6. [Google Scholar]
De Vita 2012 {published data only}
- Vita O, Martinotti G, Nicola M, Tedeschi D, Hatzigiakoumis DS, Monetta M, et al. Topiramate at low dosage vs. placebo in alcohol dependence. European Psychiatry 2012;27:1. [Google Scholar]
MacKillop 2012 {published data only}
- MacKillop J, Monti PM, Tidey J, Swift RM, Ray LA, Miranda R. Behavioral economic analysis of topirmate's effects on motivation for alcohol. Alcoholism: Clinical and Experimental Research 2012;36:247A. [Google Scholar]
Mason 2010 {published and unpublished data}
- Mason BJ. Gabapentin Treatment of Alcohol Dependence. ClinicalTrials.gov 2006.
- Mason BJ. Gabapentin in alcoholism: from laboratory study to clinical trial. Basic Clinical Pharmacology and Toxicology 2010:107. [Google Scholar]
- Mason BJ. Human laboratory and clinical trial evidence for gabapentin treatment of alcohol dependence. Alcoholism: Clinical and Experimental Research 2010;34:64A. [Google Scholar]
Miranda 2011 {published data only (unpublished sought but not used)}
- Miranda R, MacKillop J, Meehan J, Justus A, Tidey J, Ramirez J, et al. Biobehavioral effects of topiramate among heavy drinkers. Alcoholism: Clinical and Experimental Research. 2011; Vol. 35:21A.
Rubio 2002 {published data only}
- Rubio G, Ponce G, Ortiz S, Oliva JM, Manzanares J, Jimenez‐Arriero MA, et al. Comparison between gabapentine and acamprosate in the treatment of alcohol dependent patients. European Neuropsychopharmacology 2002;12:398. [Google Scholar]
References to ongoing studies
Ait‐Daoud 2010 {unpublished data only}
- Ait‐Daoud N, Anthenelli RM, Cinciripini PM, Johnson BA. New Pharmacotherapy for Alcohol and Co‐morbid Disorders. ClinicalTrials.gov 2010.
Anthenelli 2008 {unpublished data only}
- Anthenelli R, Heffner JL, Nolting S. Topiramate to Aid Smoking Cessation in Recovering Alcohol Dependent Men. ClinicalTrials.gov 2008.
Batki 2010 {unpublished data only}
- Batki LS. Topiramate Treatment of Alcohol Use Disorders in Veterans With Post Traumatic Stress Disorder (PTSD): A Pilot Controlled Trial of Augmentation Therapy. ClinicalTrials.gov 2010.
Beresford 2011 {unpublished data only}
- Beresford TP. A Double Blind Trial Of Divalproex Sodium For Affective Lability And Alcohol Use Following Traumatic Brain Injury. ClinicalTrials.gov 2011.
Ciraulo 2009 {unpublished data only}
- Ciraulo DA, Sarid‐Segal O, Afshar M. A Double‐Blind, Placebo‐Controlled, Parallel Group Design Trial of: Levetiracetam, Zonisamide, Topiramate, and Placebo Control for the Treatment of Alcohol Dependent Subjects. ClinicalTrials.gov 2009.
Del Bello 2007 {unpublished data only}
- DelBello M. Quetiapine Plus Topiramate or Placebo for Bipolar Mania and Alcohol Use in Adolescents & Young Adults. ClinicalTrials.gov 2007.
Fischer 2011 {unpublished data only}
- Fischer BA. A 14‐week Randomized, Placebo‐controlled Study of Topiramate for Alcohol Use Disorders in Veterans With Posttraumatic Stress Disorder. ClinicalTrials.gov 2011.
Frye 2005 {unpublished data only}
- Frye MA. An Evaluation of Divalproex vs. Olanzapine for Alcohol Abuse Relapse Prevention in Patients With Bipolar Disorder. ClinicalTrials.gov 2005.
Johnson 2000 {unpublished data only}
- Johnson BA, Ait‐Daoud N. Combining Medications: Treatment for Alcoholism. ClinicalTrials.gov 2000.
Johnson 2007 b {unpublished data only}
- Johnson B, Ait‐Daoud N. Novel Pharmacotherapy for Dual Dependence. ClinicalTrials.gov 2007.
Jorge 2011 {unpublished data only}
- Jorge RE. Treatment Strategy for Alcohol Use Disorders in Veterans With TBI. ClinicalTrials.gov 2011.
Kampman 2011 {unpublished data only}
- Kampman KM. A Phase II, Double‐Blind, Placebo‐Controlled, Pilot Trial of Vigabatrin for the Treatment of Cocaine and Alcohol Dependence. ClinicalTrials.gov 2011.
Kranzler 2008 {unpublished data only}
- Kranzler HR, Covault J, Arias A, Oncken C, Tennen H, Gelernter J, Armeli S, Drazinic C, Chamberlain S, Levine E, Jensen K, Lieberman R. Topiramate Treatment of Problem Drinkers. ClinicalTrials.gov 2008.
Mariani 2010 {unpublished data only}
- Mariani J. Gabapentin for Abstinence Initiation in Alcohol Dependence. ClinicalTrials.gov 2010.
Mason 2009 {unpublished data only}
- Mason BJ. Duloxetine Versus Pregabalin for Alcohol Dependence. ClinicalTrials.gov 2009.
Messing 2008 {unpublished data only}
- Messing RO, Mitchell JM. Placebo‐Controlled Crossover Trial of Levetiracetam on Ethanol Intake. ClinicalTrials.gov 2008.
Ostacher 2007 {unpublished data only}
- Ostacher MJ. Adjunctive Topiramate for Treatment of Alcohol Dependence in Patients With Bipolar Disorder. ClinicalTrials.gov 2007.
Petrakis 2007 {unpublished data only}
- Petrakis IL. The Use of Anticonvulsants for Treatment of Patients With Alcohol Dependence and Post Traumatic Stress Disorder. ClinicalTrials.gov 2007.
Ralevski 2007 {unpublished data only}
- Ralevski E. Topiramate for Treatment of Patients With Borderline Personality Disorder and Alcohol Dependence. ClinicalTrials.gov 2007.
Schaefer 2008 {unpublished data only}
South Carolina 2001 {unpublished data only}
- Alcohol Research Center—Treatment and Implications. ClinicalTrials.gov 2001.
Tolliver 2009 {unpublished data only}
- Tolliver BK, Brady KT. A Double‐Blind, Placebo‐Controlled Trial of Lamotrigine In Individuals With Bipolar Disorder and Comorbid Alcohol Dependence. ClinicalTrials.gov 2009.
Additional references
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005063.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2006
- Anderson P, Baumberg B. Alcohol in Europe: A Report for the European Commission. London: Institute of Alcohol Studies, 2006. [Google Scholar]
Anton 1995
- Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self‐rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research 1995;19:92‐9. [DOI] [PubMed] [Google Scholar]
Anton 1996
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Archives of General Psychiatry 1996;53:225‐31. [DOI] [PubMed] [Google Scholar]
Anton 2006
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Journal of the American Medical Association 2006;295:2003‐17. [DOI] [PubMed] [Google Scholar]
APA 1987
- American Psychiatric Association. DSM‐III‐R Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Arbaizar 2010
- Arbaizar B, Diersen‐Sotos T, Gómez‐Acebo I, Llorca J. Topiramate in the treatment of alcohol dependence: a meta‐analysis. Actas Espanolas de Psiquiatria 2010;38:8‐12. [PubMed] [Google Scholar]
Bastien 2001
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2001;2:297‐307. [DOI] [PubMed] [Google Scholar]
Bech 1979
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech‐Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatrica Scandinavica 1979;59:420‐30. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56:893‐7. [DOI] [PubMed] [Google Scholar]
Bohn 1995 a
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol and Drugs 1995;56:423‐32. [DOI] [PubMed] [Google Scholar]
Bohn 1995 b
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research 1995;19:600‐6. [DOI] [PubMed] [Google Scholar]
Bohn 1996
- Bohn MJ, Barton BA, Barron KE. Psychometric properties and validity of the obsessive‐compulsive drinking scale. Alcoholism: Clinical and Experimental Research 1996;20:817‐23. [DOI] [PubMed] [Google Scholar]
Buss 1957
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting and Clinical Psychology 1957;21:343‐9. [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
Carroll 1987
- Carroll KM. Enhancing retention in clinical trials of psychosocial treatments: practical strategies. Beyond the therapeutic alliance: keeping the drug‐dependent individual in treatment. NIDA Research Monograph. Vol. 165, National Institute on Drug Abuse (NIDA), 1997. [PubMed] [Google Scholar]
Carroll 1996 a
- Carroll K, O’Malley S. Compliance Enhancement: A Manual for the Psychopharmacotherapy of Alcohol Dependence. New Haven, CT: Yale University, 1996. [Google Scholar]
Carroll 1996 b
- Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Experimental and Clinical Psychopharmacology 1996;4:46‐54. [Google Scholar]
Cavanna 2010
- Cavanna AE, Ali F, Rickards HE, McCorry D. Behavioral and cognitive effects of anti‐epileptic drugs. Discovery Medicine 2010;9:138‐44. [PubMed] [Google Scholar]
Chase 2005
- Chase V, Neild R, Sadler CW, Batey RG. The medical complications of alcohol use: understanding mechanisms to improve management. Drug and Alcohol Review 2005;24:253‐65. [DOI] [PubMed] [Google Scholar]
Coccaro 1991
- Coccaro EF, Harvey PD, Kupsaw‐Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry and Clinical Neuroscience 1991;3:S44‐51. [PubMed] [Google Scholar]
Conners 1995 [Computer program]
- Conners C. Continuous Performance Test Computerprogram 3.0: User's Manual.. Toronto, Ontario, Canada: Multi‐Health Systems, Inc, 1995.
Conroy 2006
- Conroy DA, Todd Arnedt J, Brower KJ, Strobbe S, Consens F, Hoffmann R, et al. Perception of sleep in recovering alcohol‐dependent patients with insomnia: relationship with future drinking. Alcoholism: Clinical and Experimental Research 2006;30:1992‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooney 1990
- Cooney NL, Kadden RM, Litt MD. A comparison of methods for assessing sociopathy in male and female alcoholics. Journal of Studies on Alcohol and Drugs 1990;51:42‐8. [DOI] [PubMed] [Google Scholar]
Crits‐Christoph 1999
- Crits‐Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Archives of General Psychiatry 1999;56:493‐502. [DOI] [PubMed] [Google Scholar]
Czapinski 2005
- Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Current Topics in Medicinal Chemistry 2005;5:3‐14. [DOI] [PubMed] [Google Scholar]
Daley 1993
- Daley DC, Marlatt GA. Relapse prevention: cognitive and behavioral interventions. In: Lowinson JH, Ruiz P, Millman BR, Langrod JC editor(s). Substance Abuse, A Comprehensive Textbook. Baltimore: Williams & Wilkins, 1993:533‐42. [Google Scholar]
Daley 1994
- Daley DC, Thase ME. Dual Disorders Recovery Counseling. Independence, Mo: Independence Press, 1994. [Google Scholar]
Davidson 1989
- Davidson R, Bunting B, Raistrick D. The homogeneity of the alcohol dependence syndrome: a factorial analysis of the SADD questionnaire. British Journal of Addiction 1989;84:907‐15. [DOI] [PubMed] [Google Scholar]
Davis 2008
- Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Current Opinion on Psychiatry 2008;21:14‐8. [DOI] [PubMed] [Google Scholar]
De Sousa 2010
- Sousa A. The role of topiramate and other anticonvulsants in the treatment of alcohol dependence: a clinical review. CNS and Neurological Disorders Drug Targets 2010;9:45‐9. [DOI] [PubMed] [Google Scholar]
Derogatis 1977
- Derogatis LR. SCL‐90‐R: Administration, Scoring, and Procedure Manual I. Baltimore: Johns Hopkins University Clinical Psychometrics Research Unit, 1977. [Google Scholar]
DiChiara 1988
- Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 1988;85:5274‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drevets 2001
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine‐induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry 2001;49:81‐96. [DOI] [PubMed] [Google Scholar]
Efron 1971
- Efron B. Forcing a sequential experiment to be balanced. Biometrika 1971;58:403‐17. [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33:766‐71. [DOI] [PubMed] [Google Scholar]
EuroQuoL 1990
- EuroQoL Group. EuroQoL—a new facility for the measurement of health–related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
Fangeström 1978
- Fagerström KO. Measuring degrees of physical dependence to tobacco smoking with reference to individualization of treatment. Addiction Behavior 1978;3:235‐41. [DOI] [PubMed] [Google Scholar]
Feinn 2003
- Feinn R, Tennen H, Kranzler HR. Psychometric properties of the short index of problems as a measure of recent alcohol‐related problems. Alcoholism: Clinical and Experimental Research 2003;27:1436‐41. [DOI] [PubMed] [Google Scholar]
First 1994
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM‐IV axis I disorders—patient edition (SCID‐I/P,Version 2.0). Structured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition (SCID‐I/P, Version 2.0). New York: New York State Psychiatric Institute, Biometrics Research Department, 1994. [Google Scholar]
First 1995
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV. Patient. Washington, DC: American Psychiatric Press, 1995. [Google Scholar]
First 1997
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition (SCID‐I/P, Version 2.0). Structured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition (SCID‐I/P, Version 2.0). New York: New York State Psychiatric Institute, Biometrics Research Department, 1997. [Google Scholar]
Flannery 1999
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of thePenn Alcohol Craving Scale. Alcoholism: Clinical and Experimental Research 1999;23:1289‐95. [PubMed] [Google Scholar]
Flannery 2002
- Flannery BA, Allen JP, Pettinati HM, et al. Using acquired knowledge and new technologies in alcoholism treatment trials. Alcoholism: Clinical and Experimental Research 2002;26:423‐9. [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Fox 2003
- Fox C, Loughlin P, Cook C. Disulfiram for alcohol dependence. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004331] [DOI] [Google Scholar]
Gordon 1988
- Gordon M, Mettelman BB. The assessment of attention: I.Standardization and reliability of a behavior‐based measure. Journal of Clinical Psychology 1988;44:682‐90. [DOI] [PubMed] [Google Scholar]
Guadagnoli 1989
- Guadagnoli E, Mor V. Measuring cancer patients’ affect: revision and psychometric properties of the profile of mood states (POMS). Journal of Consulting and Clinical Psychology 1989;11:150‐4. [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. DHEW pub. No. (ADM) 76‐338. Rockville, MD: National Institute of Mental Health, 1976.
Halikas 1991
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving in cocaine patients using the Minnesota Cocaine Craving Scale. Comprehensive Psychiatry 1991;32:22‐7. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Psychiatry 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. www.cochrane‐handbook.org 2011.
Horn 1983
- Horn JL, Wanberg KW, Foster FM. The Alcohol Use Inventory. Baltimore, MD: Psych Systems, 1983. [Google Scholar]
Hughes 1980
- Hughes PH, Venulet J, Khant U, Medina Mora ME, Navaratnam V, Poshyachinda V, et al. Core Data for Epidemiological Studies on Non Medical Drug Users. Geneva: WHO Offset Publication, 1980. [PubMed] [Google Scholar]
Janca 1996
- Janca A, Kastrup M, Katschnig H, López–Ibor JJ, Mezzich JE, Sartorius N. The World Health Organization Short Disability Assessment Schedule (WHO DAS–S): a tool for the assessment of difficulties in selected areas of functioning of patients with mental disorders. Social Psychiatry and Psychiatric Epidemiology 1996;31:349‐54. [DOI] [PubMed] [Google Scholar]
Jenkins 1988
- Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. Journal of Clinical Epidemiology 1988;41:313‐21. [DOI] [PubMed] [Google Scholar]
Johns 1991
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540‐5. [DOI] [PubMed] [Google Scholar]
Johnson 2003b
- Johnson BA, DiClemente CC, Ait‐Daoud N, Stoks SM. Brief Behavioral Compliance Enhancement Treatment (BBCET) manual. In: Johnson BA, Ruiz P, Galanter M editor(s). Handbook of Clinical Alcoholism Treatment. Baltimore, MD: Lippincott Williams & Wilkins, 2003:282‐301. [Google Scholar]
Johnson 2005
- Johnson BA, Ait‐Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. Journal of Studies on Alcohol and Drugs 2005;15 Suppl:157‐67. [DOI] [PubMed] [Google Scholar]
Kadden 1992
- Kadden R, Carroll KM, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive Behavioral Coping Skills Manual. Washington, DC: U.S. Governmmet Printing Office, 1992. [Google Scholar]
Kadden 1995
- Kadden R, Carroll K, Donovan D, Cooney M, Monti P, Abrams D, et al. Cognitive‐Behavioral Coping Skill Therapy Manual. Project MATCH Monograph Series. National Institute on Alcohol Abuse and Alcoholism, 1995. [Google Scholar]
Kampman 1998
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinieb RM, D’Angelo LD, et al. Reliability and validity of the cocaine selective severity assessment. Addiction Behavior 1998;23:449‐61. [DOI] [PubMed] [Google Scholar]
Kenna 2009
- Kenna GA, Lomastro TL, Schiesl A, Leggio L, Swift RM. Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Current Drug Abuse Review 2009;2:135‐42. [DOI] [PubMed] [Google Scholar]
Kokkevi 1995
- Kokkevi A, Hartgers C. European adaptation of a multidimensional assessment instrument for drug and alcohol dependence. European Addiction Research 1995;1:208‐10. [Google Scholar]
Kongsakon 2000
- Kongsakon, Silpakit C. Thai version of the medical outcome study 36 items short form health survey: an instrument for measuring clinical results in mental disorder patients. Ramathibodi Medical Journal 2000;23:8‐19. [Google Scholar]
Koob 1997
- Koob GF, Nestler EJ. The neurobiology of drug addiction. Journal of Neuropsychiatry and Clinical Neurosciences 1997;9:482‐97. [DOI] [PubMed] [Google Scholar]
Landmark 2007
- Landmark CJ. Targets for antiepileptic drugs in the synapse. Medical Science Monitor 2007;13:RA 1‐7. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Leone 2010
- Leone MA, Vigna‐Taglianti F, Avanzi G, Brambilla R, Faggiano F. Gamma‐hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD006266.pub2] [DOI] [PubMed] [Google Scholar]
Levine 1986
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin 1986;22:343‐81. [PubMed] [Google Scholar]
Lingjaerde 1987
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatrica Scandinavica 1987;334 Suppl:1‐100. [DOI] [PubMed] [Google Scholar]
Logan 1994
- Logan J. On the ability to inhibit thought and action: a user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH editor(s). Inhibitory Processes in Attention, Memory and Language. San Diego: Academic Press, 1994. [Google Scholar]
Loranger 1994
- Loranger AW, Sartorius N, Andreoli P, Berger P, Buchheim SM, Channabasavanna SM, et al. The international personality disorder examination. Archives of General Psychiatry 1994;51:215‐24. [DOI] [PubMed] [Google Scholar]
Lubman 2007
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co‐occurring mood and anxiety disorders among substance‐abusing youth. Journal of Affective Disorders 2007;103:105‐12. [DOI] [PubMed] [Google Scholar]
Mannelli 2007
- Mannelli P, Pae CU. Medical comorbidity and alcohol dependence. Current Psychiatry Reports 2007;9:217‐24. [DOI] [PubMed] [Google Scholar]
McLellan 1980
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. Journal of Nervous and Mental Disease 1980;168:26‐33. [DOI] [PubMed] [Google Scholar]
McLellan 1985
- McLellan AT, Luborsky L, Cacciola J, Griffith JE, McGahan P, O’Brien CP. Guide to the Addiction Severity Index: Background Administration and Field Testing Results. Rockville, MD: US Department of Health and Human Services, 1985. [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment 1992;9:199‐213. [DOI] [PubMed] [Google Scholar]
McNair 1971
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational & Industrial Testing Service, 1971. [Google Scholar]
Miller 1984
- Miller WR, Marlatt GA. Manual for the Comprehensive Drinker Profile. Odessa, FL: Psychological Assessment Resources, 1984. [Google Scholar]
Miller 1995
- Miller W. The Drinker Inventory of Consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse. In: Mattson M, Marshall LA editor(s). NIAAA Project MATCH Monograph Series. Vol. 4, Bethesda, MD : National Institute on Alcohol Abuse and Alcoholism, 1995:1‐94. [Google Scholar]
Miller 1996
- Miller WR. Form 90: A structured assessment interview for drinking and related behaviors test manual. In: Mattson ME, Marshall LA editor(s). NIAAA Project Match Monograph Series. Vol. 5, Rockville, MD: National Institute on Alcoholism and Alcohol Abuse, 1996. [Google Scholar]
Miller 2004
- Miller WR. Combined Behavioral Intervention Manual: A Clinical Research Guide for Therapists Treating People With Alcohol Abuse and Dependence. Vol. 1, Bethesda, MD: National Institute of Health, 2004. [Google Scholar]
Minozzi 2010
- Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005064.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moak 1998
- Moak DH, Anton RF, Latham PK. Further validation of the obsessive‐compulsive drinking scale (OCDS): relationship to alcoholism severity. American Journal of Addiction 1998;7:14‐23. [PubMed] [Google Scholar]
Moher 2001
- Moher D, Jones A, Lepage L, CONSORT Group (Consolidated Standards for Reporting of Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Journal of the American Medical Association 2001;285:1992‐5. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Monti 1989
- Monti PM, Abrams DB, Kadden RM, Cooney NL. Treating Alcohol Dependence: A Coping Skills Training Guide. New York: Guildford Press, 1989. [Google Scholar]
Morin 1993
- Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford, 1993. [Google Scholar]
Morisky 1986
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self–reported measure of medication adherence. Medical Care 1986;24:67‐74. [DOI] [PubMed] [Google Scholar]
Mottola 1993
- Mottola CA. Measurement strategies: the visual analogue scale. Decubitus 1993;6:56‐8. [PubMed] [Google Scholar]
Nich 2002
- Nich C, Carroll KM. Intention‐to‐treat meets missing data: implications of alternate strategies for analyzing clinical trials data . Drug and Alcohol Dependence 2002;68:121‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsmed 2008
- Olmsted CL, Kockler DR. Topiramate for alcohol dependence. Annals of Pharmacotherapy 2008;42:1475‐80. [DOI] [PubMed] [Google Scholar]
Patton 1995
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsivity Scale. Journal of Clinical Psychology 1995;51:768‐74. [DOI] [PubMed] [Google Scholar]
Post 1986
- Post RM, Rubinow DR, Ballenger JC. Conditioning and sensitisation in the longitudinal course of affective illness. British Journal of Psychiatry 1986;149:191‐201. [DOI] [PubMed] [Google Scholar]
Project MATCH Research Group 1997
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol and Drugs 1997;58:7‐29. [PubMed] [Google Scholar]
Raistrick 1983
- Raistrick D, Dunbar G, Davidson R. Development of a questionnaire to measure alcohol dependence. British Journal of Addiction 1983;78:89‐95. [DOI] [PubMed] [Google Scholar]
Rehm 2009
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol‐use disorders. Lancet 2009;373:2223‐33. [DOI] [PubMed] [Google Scholar]
Rehm 2011
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol and Research Health 2011;34:135‐43. [PMC free article] [PubMed] [Google Scholar]
Roberts 1999
- Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcoholism: Clinical and Experimental Research 1999;23:1484‐91. [PubMed] [Google Scholar]
Robins 1988
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry 1988;45:1069‐77. [DOI] [PubMed] [Google Scholar]
Rollnik 1992
- Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. British Journal of Addiction 1992;87:743‐54. [DOI] [PubMed] [Google Scholar]
Rösner 2010
- Rösner S, Hackl‐Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD004332.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rösner 2010 a
- Rösner S, Hackl‐Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD001867.pub3] [DOI] [PubMed] [Google Scholar]
Sheehan 1998
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry 1998;59(Suppl 20):22‐33. [PubMed] [Google Scholar]
Shinn 2010
- Shinn AK, Greenfield SF. Topiramate in the treatment of substance‐related disorders: a critical review of the literature. Journal of Clinical Psychiatry 2010;71:634‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 1982
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology 1982;91:199‐209. [DOI] [PubMed] [Google Scholar]
Skinner 1984
- Skinner HA, Horn JL. Alcohol Dependence Scale: Users Guide. Toronto, Canada: Addiction Research Foundation, 1984. [Google Scholar]
Sobell 1979
- Sobell LC, Maisto SA, Sobell MB, et al. Reliability of alcohol abusers’ self‐reports of drinking behavior. Behavioral Research and Therapy 1979;17:157‐60. [DOI] [PubMed] [Google Scholar]
Sobell 1988
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction 1988;83:393‐402. [DOI] [PubMed] [Google Scholar]
Sobell 1992
- Sobell LC, Sobell MB. Timeline follow‐back: a technique for assessing self‐reported alcohol consumption. In: Litten RZ, Allen JP editor(s). Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press Inc, 1992:41‐72. [Google Scholar]
Sobell 1995
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M editor(s). Assessing Alcohol Problems: A Guide for Clinicians and Researchers. Rockville MD: National Institute on Alcohol Abuse and Alcoholism, 1995:55‐73. [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch R, Lushene RE. Manual for the Trait‐State Anxiety Inventory. Palo Alto, CA: Consulting Psychlogists, 1970. [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, LuShene R, Vagg PR, Jacobs GA. Manual for The State‐Trait Anxiety Inventory STAI (Form Y). Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
Spitzer 1981
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a conciseQL‐index for use by physicians. Journal of Chronic Diseases 1981;34:585‐97. [DOI] [PubMed] [Google Scholar]
Spitzer 1990
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM‐111‐R (SCID). Washington, DC: American PsychiatricAssociation, 1990. [Google Scholar]
Spitzer 1992
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM‐III‐R (SCID) I. History, rationales and description. Archives of General Psychiatry 1992;49:624‐9. [DOI] [PubMed] [Google Scholar]
Srisurapanont 2005
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001867.pub2] [DOI] [PubMed] [Google Scholar]
Stockwell 1983
- Stockwell T, Murphy D, Hodgson R. The severity of alcohol dependence questionnaire—Its use reliability and validity. British Journal of Addiction 1983;78:145‐55. [DOI] [PubMed] [Google Scholar]
Stout 1994
- Stout RL, Wirtz PW, Carbonari JP, Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol and Drugs 1994;S 12:70‐5. [DOI] [PubMed] [Google Scholar]
Sullivan 1989
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA‐Ar). British Journal of Addiction 1989;84:1353‐7. [DOI] [PubMed] [Google Scholar]
Swendsen 1998
- Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio‐Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Comprehensive Psychiatry 1998;39:176‐84. [DOI] [PubMed] [Google Scholar]
Szabo 1996
- Szabo S. The World Health Organization Quality of Life (WHOQOL). In: Spiker B editor(s). Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia: Lippincott‐Raven Publishers, 1996:355–362.. [Google Scholar]
Thase 1983
- Thase ME, Hersen M, Bellack AS, Himmelhoch JM, Kupfer DJ. Validation of a Hamilton subscale for endogenomorphic depression. Journal of Affective Disorders 1983;5:267‐78. [DOI] [PubMed] [Google Scholar]
Volkow 2003
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights for imaging studies. Journal of Clinical Investigation 2003;111:1444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpicelli 2001
- Volpicelli JR, Pettinati HM, McLellan AT, McLellan T, O’Brien CHP. Enhanced medication and treatment adherence for addiction treatment: the BRENDA model. New York: Guilford Press, 2001. [Google Scholar]
Weiss 2003
- Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, et al. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. American Journal of Psychiatry 2003;160:1320‐5. [DOI] [PubMed] [Google Scholar]
Weiss 2006
- Weiss RD, Kueppenbender KD. Combining psychosocial treatment with pharmacotherapy for alcohol dependence. Journal of Clinical Psychopharmacology 2006;26(Suppl 1):S37‐S42. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]
WHO 2004
- World Health Organization. Global Status Report on Alcohol. Geneva: WHO, 2004. [Google Scholar]
WHO 2011
- World Health Organization. Global Status Report on Alcohol and Health. Geneva: WHO, 2011. [Google Scholar]
Williams 1988
- Williams JBW. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry 1988;45:742‐7. [DOI] [PubMed] [Google Scholar]
Wing 1990
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry 1990;47:589‐93. [DOI] [PubMed] [Google Scholar]


