Abstract
Structural imaging remains an essential component of diagnosis, staging and response assessment in patients with cancer; however, as clinicians increasingly seek to noninvasively investigate tumour phenotypes and evaluate functional and molecular responses to therapy, theranostics — the combination of diagnostic imaging with targeted therapy — is becoming more widely implemented. The field of radiotheranostics, which is the focus of this Review, combines molecular imaging (primarily PET and SPECT) with targeted radionuclide therapy, which involves the use of small molecules, peptides and/or antibodies as carriers for therapeutic radionuclides, typically those emitting α-, β- or auger-radiation. The exponential, global expansion of radiotheranostics in oncology stems from its potential to target and eliminate tumour cells with minimal adverse effects, owing to a mechanism of action that differs distinctly from that of most other systemic therapies. Currently, an enormous opportunity exists to expand the number of patients who can benefit from this technology, to address the urgent needs of many thousands of patients across the world. In this Review, we describe the clinical experience with established radiotheranostics as well as novel areas of research and various barriers to progress.
Radiotheranostics1,2 differs from the vast majority of other cancer therapies in its capacity for simultaneous imaging and therapy. This unique capacity can be exploited clinically in various ways, including by visually assessing the biodistribution of the targeted drug, selecting patients to receive targeted therapies (which can be described as ‘seeing what you treat’) and reducing the high risks of failure associated with drug development by visualizing and quantifying both the presence and engagement of the target, thus offering feedback on pharmacodynamics while also testing candidate radionuclides. In this Review, we describe the clinical successes achieved thus far with radiotheranostic approaches, including differences from other forms of therapy, the current challenges associated with the effective and widespread deployment of radiotheranostic agents, their future potential and emerging opportunities.
What is radiotheranostics?
The selection of patients for targeted therapies is usually based on clinical parameters (such as disease stage), often incorporating information from molecular biomarkers in tissue (such as PD-L1 (REF.3) or HER2 expression4). By contrast, and unlike preceding technologies, radiotheranostic approaches involve the administration of radiolabelled diagnostic forms of targeted compounds (using isotopes such as 99mTc, 18F and 68Ga), enabling expression of the therapeutic target to be visualized in vivo with a companion imaging method before switching to the radiolabelled therapeutic counterpart. Radiotheranostics can also enable visualization of tumour burden, thus allowing clinicians to ‘treat what you see’. Moreover, repeat imaging enables clinicians to assess the effects of therapy on target expression (FIG. 1). Certain radiotheranostics involve radionuclides that, in addition to their therapeutic component (as emitters of either auger-, α- or β-radiation) (TABLES 1 and 2), can visualize the agent in real time (owing to emission of either γ or positron radiation) (FIG. 2). For example, the therapeutic effects of 177Lu-conjugated radiotheranostics are primarily mediated by the emission of β-radiation, while the γ-emissions can be used for imaging, including to confirm the successful localization of the agents and to quantify the radiation dose delivered to both the target lesions and normal organs5,6. The dosimetric potential of personalized radiotheranostics is an underexplored aspect that holds tremendous potential for further optimization of the therapeutic index by informing decisions on the balance between the efficacy and toxicity of these therapies on an individual basis. Unlocking this potential and demonstrating the true utility of dosimetry, however, will require more prospective data from ongoing and future studies with larger groups of patients in clinically relevant settings. Data from prospective trials that demonstrate the utility of dosimetry over the standard approach are finally becoming available7. Efforts to simplify organ dosimetry approaches by involving fewer data points are also underway. In addition, opportunities to combine radiotheranostic approaches with other forms of radiotherapy, such as external-beam radiotherapy (EBRT) in patients with prostate cancer, will also require dosimetry studies to optimize both dose delivery and therapeutic outcomes.
Fig. 1 |. Overview of the concept of radiotheranostics.
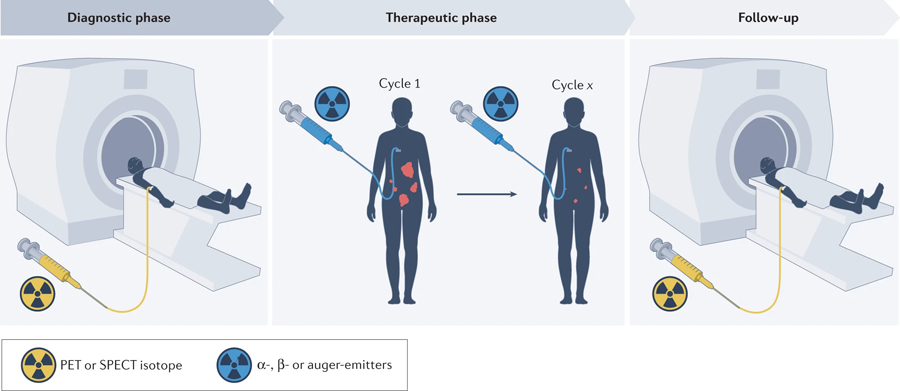
Radiopharmaceuticals are paired with targeted ligands to ‘see with precision’ and then ‘treat with targeting’.
Table 1 |.
Selected isotopes for use in radiotheranostics
| Nuclide | Use | Half-life (t½) | Common production methods | Notes |
|---|---|---|---|---|
| Imaging isotopes | ||||
| 18F | PET | 110 min | Cyclotron: 18O(p,n)18F | Routinely available |
| 43Sc | PET | 3.89 h | Cyclotron: 46Ti(p,α)43Sc; 42Ca(d,n)43Sc; 43Ca(p,n)43Sc | Paired with 47Sc; can be expensive and production is challenging |
| 44gSc | PET | 3.97 h | Cyclotron: 44Ca(p,n)44m/gSc Generator: 44Ti/44Sc | Paired with 47Sc; high γ-emission can negatively affect dosimetry and make handling more challenging; cyclotron produces isomer 44mSc, requires in vivo generator or contaminant |
| 55Co | PET | 17.53 h | Cyclotron: 54Fe(d,n)55Co; 58Ni(p,α)55Co | Paired with 58mCo; production can be challenging |
| 61Cu | PET | 3.34 h | Cyclotron: 61Ni(p,n)61Cu; natNi(d,x)61Cu | Paired with 67Cu; requires local cyclotron access |
| 68Ga | PET | 67.71 min | Cyclotron: 68Zn(p,n)68Ga Generator: 68Ge/68Ga |
Paired with 177Lu |
| 71As, 72As, 74As | PET | 65.30 h, 26.0 h, 17.77 days | Cyclotron: 70Ge(d,n)71As Cyclotron: 72Ge(p,n)72As Generator: 72Se/72As Cyclotron: 74Ge(p,n)74As |
Paired with 77As; can be expensive and production is challenging |
| 76Br | PET | 16.2 h | Cyclotron: 76Se(p,n)76Br | Paired with 77Br and potentially 211At; production can be challenging |
| 86Y | PET | 14.74 h | Cyclotron: 86Sr(p,n)86Y | Paired with 90Y |
| 89Zr | PET | 78.41 h | Cyclotron: 89Y(p,n)89Zr | Readily available; often the optimal isotope for radiolabelling macromolecules with longer in vivo pharmacokinetics |
| 99mTc | SPECT | 6.01 h | Generator: 99Mo/99mTc Cyclotron: 100Mo(p,2n)99mTc |
Readily available; paired with 153Sm, 186Re and 188Re; accelerator-driven fast neutron routes under development |
| 124I | PET | 4.18 days | Cyclotron: 124Te(p,n)124I | Paired with 131I; commercially available but can be expensive; co-emitted high-energy γ-radiation limits handling and clinical use |
| 132La, 133La, 134La | PET | 4.8 h, 3.9 h, 6.45 min | Cyclotron: natBa(p,x)132La Cyclotron: natBa(p,x)132La Generator (in vivo): 134Ce/134La |
Availability is currently limited and production can be challenging, paired with 225Ac or 227Th |
| 152Tb | PET | 17.5 h | Accelerator: tantalum spallation (proton/heavy ion) | Can be paired with 161Tb; 155Tb (t½ 5.32 days) can be used for SPECT; availability is currently limited |
| 203Pb | SPECT (from 203Tl daughter) | 51.92 h | Cyclotron: 205Tl(p,3n)203Pb | Paired with 212Pb; availability is currently limited |
| Imaging/therapeutic isotopes | ||||
| 47Sc | β-Therapy, SPECT | 3.35 days | Generator: 47Ca/47Sc Reactor: 47Ti(n,p)47Sc | Availability currently limited |
| 64Cu | PET, β-therapy | 12.7 h | Cyclotron: 64Ni(p,n)64Cu Reactor: 64Zn(n,p)64Cu |
Readily available |
| 67Cu | β-Therapy, SPECT | 61.83 h | Accelerator: 68Zn(p,2p)67Cu Reactor: 67Zn(n,p)67Cu |
Has the advantages of lower γ-energies being co-emitted; availability currently limited — concerted global efforts to increase supply are ongoing |
| 67Ga | SPECT, auger therapy | 3.26 h | Cyclotron: 68Zn(p,2n)67Ga | Readily available; mainly used for diagnostic purposes |
| 77As | β-Therapy | 38.83 h | Generator: 77Ge/77As | Paired with 72As |
| 111In | SPECT, auger therapy | 2.81 days | Cyclotron: 111Cd(p,n)111In | Readily available; mainly used for diagnostic purposes |
| 117mSn | SPECT, auger therapy | 13.6 days | Cyclotron 114Cd(α,n)117mSn | Limited availability |
| 123I | SPECT, auger therapy | 13.22 h | Cyclotron: 124Xe(p,2n)123I | Readily available; mainly used for diagnostic purposes |
| Imaging/therapeutic isotopes (cont.) | ||||
| 131I | β-Therapy, SPECT | 8.03 days | Reactor: 130Te(n,γ)131I | Readily available; often used as a standalone imaging isotope for thyroid imaging or as 131I-MIBG. Can be paired with 124I |
| 153Sm | β-Therapy, SPECT | 46.28 h | Reactor: 152Sm(n,γ)153Sm | Readily available |
| 161Tb | β-Therapy, SPECT | 6.89 days | Reactor: 160Gd(n,γ)161Gd → 161Tb | Paired with 152Tb but can also be paired with 68Ga; availability currently limited — concerted global efforts to increase supply are ongoing |
| 177Lu | β-Therapy, SPECT | 6.65 days | Reactor: 176Lu(n,γ)177Lu Reactor: 176Yb(n,γ)177Yb → 177Lu |
Readily available; paired with 68Ga |
| 186Re | β-Therapy, SPECT | 3.72 days | Reactor: 185Re(n,γ)186Re | Availability currently limited |
| 188Re | β-Therapy, SPECT | 17.00 h | Generator: 188W/188Re | Availability currently limited |
| Therapeutic isotopes | ||||
| 58mCo | Auger therapy | 9.10 h | Cyclotron: 58Fe(p,n)58mCo; 57Fe(d,n)58mCo; 61Ni(p,α)58mCo | Paired with 55Co; production can be challenging |
| 77Br | Auger therapy | 57.04 h | Cyclotron: 77Se(p,n)77Br | Paired with 76Br |
| 90Y | β-Therapy | 64.05 h | Reactor: 90Zr(n,p)90Y | Readily available, paired with 86Y |
| 149Tb | α-Therapy | 4.12 h | Accelerator: tantalum spallation (proton/heavy ion) Accelerator: 151Eu(3He,5n)149Tb |
Emits γ-, positron- and α-radiation — enabling PET/ SPECT/α-therapy; availability currently limited |
| 211At | α-Therapy | 7.21 h | Cyclotron: 209Bi(α,2n)211At | Availability currently limited — concerted global efforts to increase supply are ongoing; can be paired with diagnostic radioiodine and potentially 76Br |
| 213Bi | α-Therapy | 45.61 min | Generator: 225Ac | Availability currently limited |
| 212Pb/212Bi | α/β-Therapy | 10.6 h/60 min | Generator: 224Ra | Availability currently limited |
| 223Ra | α-Therapy | 11.43 days | Generator: 227Th | Readily available |
| 227Th | α-Therapy | 18.69 days | Generator: 227Ac | Availability currently limited |
| 225Ac | α-Therapy | 9.92 days | Accelerator: 232Th proton spallation Cyclotron: 226Ra(p,2n)225Ac Generator: 229Th generator Light source: 226Ra(γ,2n)225Ra → 225Ac; 226Ra(γ,p)225Fr → 225Ra → 225Ra Reactor: 226Ra(n,2n)225Ra → 225Ac; 226Ra(n,p)225Fr → 225Ra → 225Ra |
Availability currently limited — concerted global efforts to increase supply are ongoing; direct accelerator production is limited owing to contamination with 227Ac |
MIBG, meta-iodobenzylguanidine.
Table 2 |.
Radiopharmaceuticals approved for radionuclide therapy in oncology indications
| Agent | Approval (FDA, EMA) | Companion diagnostics | Indication | Efficacy data |
|---|---|---|---|---|
| [131I]sodium iodide | 1971a,118 | [131I]sodium iodide | Treatment of selected patients with differentiated thyroid carcinoma | Enables complete thyroid ablation in 92% of patients with low-risk thyroid cancer; 98% were free of disease at 5 years146; uptake observed in 59% of patients with metastatic thyroid cancer147,148 |
| 153Sm-EDTMP | 1997, 1998 |
99mTc-bisphosphonates, including 99mTc-medronate; 99mTc-oxidronate; 99mTc-pyrophosphate |
Palliation of bone pain in patients with multiple painful skeletal metastases | 62–72% had pain relief at 4 weeks following a single dose of 153Sm-EDTMP, including complete pain relief in 31%; pain relief persisted for up to 16 weeks in 43% of patients149; improvements in 4-week pain scores and opioid use vs controls26,149 |
| 90Y-ibritumomab tiuxetanb | 2002, 2004 | 111In-ibritumomab | R/R low-grade or follicular B cell NHL; previously untreated patients with follicular NHL who achieve a partial or complete response to first-line chemotherapy | ORR 80% vs 56%, P = 0.02 for rituximab alone (CR in 30% vs 16%); median DOR 14.2 vs 12.1 months (P = 0.64), durable responses ≥6 months. in 64% vs 47% (P = 0.03)17; clinical benefit was also seen in various combination settings150–154 |
| 131I-tositumomabc | 2003, 2003 | 131I-tositumomab | CD20+ R/R low-grade, follicular or transformed NHL following disease progression during or after rituximab | ORR 49–64%; median DOR 6.5–16 months in single-arm studies155,156 |
| 131I-iobenguane (or MIBG) | 2018a,157 | 123I-iobenguane | Noradrenaline-positive pheochromocytomas or paragangliomas | ORR 25%, DCR 92%; 53% of responders had tumour responses lasting ≥6 months, median OS 36.7 months158 |
| 223Ra-dichloride | 2013, 2013 | – | CRPC with symptomatic bone metastases and no known visceral metastases | Median OS 14.9 vs 11.3 months with placebo, HR 0.70, 95% CI 0.58–0.83, P < 0.001; time to first SSE 15.6 vs 9.8 months, HR 0.66, 95% CI 0.52–0.83, P < 0.001; increase in FACT-P QOL score ≥10 points in 25% vs 16% of patients, P = 0.02 (REFs24,159,160) |
| 177Lu-DOTATATE | 2018, 2017 |
68Ga-DOTATATE (USA); 64Cu-DOTATATE (USA); 68Ga-DOTATOC (EU) |
Somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumours | Median OS 48.0 vs 36.3 monthsd, HR 0.84, 95% CI 0.60–1.17, P = 0.30; increase in QOL37 |
| 177Lu-PSMA-617 | 2022, pending | 68Ga-PSMA-11, FDA approved in 2021 and 2022 | Treatment of metastatic CRPC following disease progression on AR inhibitors and taxane-based chemotherapy | Median PFS 8.7 vs 3.4 months; median OS 15.3 vs 11.3 months in the 177Lu-PSMA-617 and control groups; time to first SSE 11.5 vs 6.8 months HR 0.50, 95% CI 0.40–0.62, P < 0.001 (REF.43) |
EMA nationally authorized medicinal product.
Discontinued in the USA in 2021.
Approval withdrawn and discontinued in 2014.
Improvement in median overall survival (OS) likely underestimated owing to 36% crossover from control arm. AR, androgen receptor; CI, confidence interval; CR, complete response; CRPC, castration-resistant prostate cancer; DCR, disease control rate; DOR, duration of response; DOTATATE, DOTA0, Tyr3-octreotate; EDTMP, ethylenediamine tetramethylene phosphonate; HR, hazard ratio; MIBG, meta-iodobenzylguanidine; NHL, non-Hodgkin lymphoma; OR, objective response; ORR, objective response rate; PFS, progression-free survival; PSMA, prostate-specific membrane antigen; QOL, quality of life; R/R, relapsed and/or refractory; SSE, symptomatic skeletal event.
Fig. 2 |. Responses to approved theranostics, as demonstrated using their imaging counterpart.
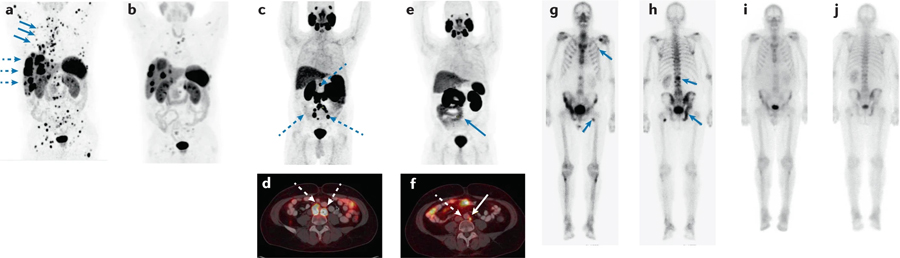
a | Coronal PET 68Ga-DOTATATE maximum-intensity projection (MIP) depicting a patient with an atypical bronchial carcinoma, with disease progression on everolimus with extensive osseous (blue solid arrows) and hepatic (dashed arrows) metastases. b | After four cycles of 177Lu-DOTATATE, a marked reduction in both the number and extent of bone and liver lesions can be observed. c,d | Coronal PET 68Ga-PSMA-11 MIP (panel c) and fused axial PET–CT images (panel d) depicting a patient with Gleason grade 9 prostate cancer with extensive retroperitoneal and pelvic nodal metastases following radical prostatectomy, androgen-deprivation therapy and abiraterone. e | After five cycles of 177Lu-PSMA-617, the adenopathy is markedly decreased in size. f | Fused axial PET–CT images provide a more detailed view of the pelvic nodal metastases after 177Lu-PSMA, with several nodes that are visible in panel d no longer present in panel f. g,h | Anterior (panel g) and posterior (panel h) coronal PET 99mTc-MDP MIPs depicting a patient with de novo metastatic Gleason grade 9 prostate cancer with metastatic lesions located in the spine, ribs, pelvis and femur. i,j | After six cycles of 223Ra-dichloride, a decreased intensity of uptake can be observed at all metastatic sites.
Radiotheranostic agents that specifically direct a lethal payload of α-, β- or to a lesser extent, auger-emitting radioisotopes8–10 — such as 223Ra, 177Lu or 111In — have proved very effective as anticancer treatments (TABLE 2). Importantly, radiotheranostic approaches address the common challenges posed by heterogeneous target expression in two important ways. First, the diagnostic aspect allows clinicians to evaluate the extent of heterogeneous target expression between different lesions before therapy. Second, the therapeutic aspect offers the potential for ‘crossfire’ radiation with cytotoxic bystander effects on the adjacent, target-negative tumour cells when radioisotopes with longer path length (such as those emitting β-radiation) are used. This effect, which is limited to high-energy β-particles (emitted by 90Y and 177Lu, among others), might offer a distinct advantage against tumours with microscopic variations in target expression, and an absence of this form of radiation might limit the efficacy of α-emitting and auger-emitting radioisotopes of short path length. As a result of this effective combination of diagnosis and therapy, the delineation of targets with limited or non-uniform expression does not necessarily limit their utility as targets for radionuclide therapy. Heterogeneous target expression is one of the main limitations of the effectiveness of traditional cancer therapies11; therefore, the ability to deposit lethal ionization below the required target saturation levels of even the lowest-availability antigens is a major advantage of radionuclide therapies12. Moreover, drug development, especially in the field of oncology, is associated with failure rates of around 90%, earning the transition from preclinical research to clinical implementation the moniker ‘the valley of death’13. Nevertheless, the development processes for targeted imaging agents enables early assessment of the biodistribution of both the intended target (across a range of patients and tumour sites) and the radioactively labelled ligand — ameliorating the risk of failure, as the development of a potential lead ligand could be quickly halted, adapted or accelerated on the basis of data from early biodistribution studies, thereby increasing the success rates of radionuclide therapies over those of conventional oncological therapies. The potential for conjugation of a radioisotope onto a ligand with established pharmacokinetic and targeting properties, as seen with 177Lu-PSMA-617, offers another method of minimizing the risk of failed clinical translation.
Established radiotheranostics
The long history of using radiotheranostics to target the same structure for both diagnostic imaging and radionuclide therapy dates back to the 1930s, when Hertz et al.14 first presented the concept, followed by the use of radioactive iodine in patients with hyperthyroidism14,15. The successful clinical application of radioactive iodine in a patient with thyroid cancer was initially reported by Seidlin et al. in 1946 (REF.16). Diagnostic imaging and treatment of both benign (for example, Graves’ disease and goitre) and malignant (differentiated thyroid cancer) thyroid diseases is based on selective uptake via the sodium–iodine symporter, which is predominantly expressed in thyroid tissue. Accordingly, radioactivity — especially during treatment — is selectively deposited in tissues that express the sodium–iodine symporter, largely sparing other organs and tissues11. Despite these distant origins, radioactive iodine remains an important treatment for patients with either benign or malignant thyroid diseases (TABLE 1).
Anti-CD20 radioimmunotherapies
Several decades after the global expansion in the use of radioactive iodine, the completely new concept of radiotheranostics slowly found its way into the clinic. The beginning of the twenty-first century saw the rapid development of radioimmunotherapy for patients with lymphoma. Radioactively labelled mouse monoclonal antibodies targeting the CD20 antigen expressed on the surface of all B cells were explored using two separate approaches17 and, accordingly, provided a novel therapy for patients with B cell lymphomas. In 2002, the FDA approved 90Y-ibritumomab tiuxetan, the first radioimmunotherapy, for patients with relapsed and/or refractory, low-grade or follicular B cell non-Hodgkin lymphoma (NHL)18 (TABLE 2). Another anti-CD20-binding mouse antibody, 131I-tositumomab, received FDA approval in 2003 for the treatment of patients with relapsed and/or refractory NHL19 (TABLE 2). Regrettably, despite very good clinical performance and a limited toxicity profile in several clinical trials of conventional radioimmunotherapies plus high-dose myeloablative conditioning chemotherapy, both drugs were commercially unsuccessful, leading to the discontinuation of 131I-tositumomab in 2014 (REFS17,20–22) (TABLE 2). This commercial failure has been attributed to various factors, including physicians’ reluctance to refer patients owing to the availability of alternative non-radioactive therapies, the scarcity of plans for logistical co-operation between nuclear medicine and oncology clinics, educational issues and, in the USA, medical reimbursement concerns23. The market’s rejection of these two radioimmunotherapies temporarily caused a setback to the field and curtailed further investments in the development of other radiotheranostic agents.
223Ra-dichloride
The next major milestone was the publication of data from the ALSYMPCA trial, a prospective randomized phase III study, which demonstrated for the first time that 223Ra-dichloride, delivered over several cycles, significantly prolongs both the median overall survival (OS) and time to skeletal complications of men with castration-resistant prostate cancer (CRPC) and bone metastases24. The reported median OS benefit of more than 3 months and the corresponding hazard ratio (HR) of 0.70, both relative to placebo, were perceived as practice-changing, leading to FDA approval in 2013 (TABLE 2). Before the introduction of 223Ra-dichloride, bone-seeking radiopharmaceuticals used for the treatment of bone metastases were typically β-emitters, such as 89Sr-chloride and 153Sm-ethylenediamine tetramethylene phosphonate (EDTMP). These agents were usually administered as a single infusion, leading to palliation of pain symptoms and an improved quality of life (QOL); nonetheless, concerns regarding the risks of haematological toxicities precluded repeat administration25–27. The success of 223Ra-dichloride led to a rapid decline in the use of 89Sr-chloride and 153Sm-EDTMP in the USA, although both are still used clinically in many countries in which 223Ra-dichloride is not routinely available. The mechanism of action of 223Ra-dichloride differs from that of other radiotheranostic agents that directly target tumour cells: taking advantage of the similarity of radium to calcium, 223Ra predominantly localizes to areas of increased bone turnover, which is a characteristic feature of bone metastases, with the α-emitting 223Ra-dichloride then irradiating the surrounding cells, including tumour cells. Despite this apparent effectiveness, the widespread clinical use of 223Ra-dichloride had to be modified when a life cycle management phase III study (ERA 223) combining 223Ra-dichloride with the novel androgen-receptor signalling inhibitor (ARSI) abiraterone revealed an increased rate of symptomatic skeletal events, leading to premature unblinding28. 223Ra-dichloride is currently still considered to be a potent bone-metastasis-directed treatment, although this agent is now typically administered alongside bone-protective agents such as zoledronic acid and/or denosumab.
Somatostatin analogues
The next major step in the field of radiotheranostics began with the development of somatostatin-receptor-targeting agents for patients with neuroendocrine tumours (NETs). In parallel with development of the imaging agent 111In-pentetreotide, which was approved for clinical use by the FDA in 1994, the internalizing properties of somatostatin analogue peptides that specifically target somatostatin receptor 2 enable targeted delivery of 111In to NET cells29. This treatment was named peptide receptor radionuclide therapy (PRRT). Despite involving the administration of high levels of radioactivity (up to 18.5 GBq of 111In per cycle and, cumulatively, 160 GBq), 111In-pentetreotide had only modest levels of efficacy (partial response rate of 8%, related to the lack of intercalation of the electrons in the DNA helix30,31), with high costs. This limited efficacy led to the progressive abandonment of auger-emitters in favour of β-emitters32,33 (initially 90Y and, more recently, 177Lu) linked to the targeting molecule by macrocyclic chelators, such as DOTA (instead of DTPA). Several non-controlled, retrospective studies and a few prospective trials initially demonstrated excellent responses (objective response rate (ORR) 18–60% for 177Lu-DOTATATE with median progression-free survival (PFS) of 20–36 months) and mild to moderate toxicities (mainly involving the bone marrow and kidneys) in patients with NETs receiving these therapies34. Among the various ligands (DOTATOC, DOTATATE, DOTANOC), 177Lu-labelled compounds quickly gained widespread use owing to the high response rates and advantages over 90Y peptides including the possibility of γ-imaging and reduced toxicities. The year 2012 saw the launch of the prospective, randomized phase III NETTER-1 trial, which in 2017 revealed a significant improvement in PFS in patients with somatostatin-receptor-positive midgut carcinoid tumours who received four cycles of 177Lu-DOTATATE, compared with non-reactive high-dose octreotide35. The corresponding HR of 0.21 reflected an almost five times greater risk of disease progression for patients who did not receive 177Lu-DOTATATE. Given the potential risks of haematological toxicities associated with radiotheranostics, in addition to the mild to moderate severity of most toxicities, QOL evaluations have provided additional evidence of the tolerability and efficacy of this therapeutic approach — which are important criteria for regulatory authorities. Indeed, the NETTER-1 trial demonstrated significantly improved health-related QOL in patients receiving 177Lu-DOTATATE compared with the control group (including a mean time to deterioration of overall health status of 28.8 months versus 6.1 months)36. These ground-breaking results led to the FDA approval of 177Lu-DOTATATE and an improvement in the standard of care for patients with locally advanced and/or inoperable somatostatin-receptor-positive gastroenteropancreatic NETs (GEP-NETs) almost 25 years after the introduction of PRRT; they also attracted the attention of major pharmaceutical companies. Long-term follow-up data from NETTER-1 confirm the significant improvement in median PFS and the excellent tolerability seen at earlier time points37. Importantly, the FDA also approved 68Ga-DOTATATE (TABLE 2), a one-pot, simple kit-based preparation, for PET-based imaging of tumours in both adult and paediatric patients with somatostatin-receptor-positive NETs. The radiotheranostic partnering of 68Ga-DOTATATE (USA) or 68Ga-DOTATOC (DOTA-(d-Phe1, Tyr3)-octreotide) (EU) and 177Lu-DOTATATE has made the ‘treat what you see’ paradigm into a reality.
The design of the ALSYMPCA24 and NETTER-1 (REF.35) trials provides a model for the development of new radiotheranostic agents and for expanding the use of existing radionuclide therapies. Current phase III trials testing the efficacy of somatostatin-based radiotheranostics in patients with GEP-NETs include COMPETE (NCT03049189), COMPOSE (NCT04919226) and NETTER-2 (NCT03972488), all of which are designed to expand the application of somatostatin-receptor-directed radiotheranostics to patients with more aggressive tumours. Additional studies are currently exploring the possibility of using 177Lu-DOTATATE for the treatment of other somatostatin-receptor-expressing tumours beyond NETs, such as small-cell lung cancer and meningioma (NCT05142696, NCT03971461).
PSMA-based agents
NETs are rare tumours that affect a limited number of patients, who often receive treatment at a few specialized centres, although the expansion of radiotheranostics to relatively common malignancies such as prostate, breast or lung cancer is transforming both the field and its perception. Accordingly, advocates of radiotheranostic approaches have responded quickly to the introduction of a highly specific peptide ligand capable of binding to the prostate-specific membrane antigen (PSMA)38, which is overexpressed in most prostate cancers. Initial reports on imaging39 and radionuclide therapy40 with PSMA-targeted radiotheranostics have resulted in the development of multiple PSMA binding ligands. The past few years have seen the FDA approval of two PSMA PET agents, 18F-DCFPyL and 68Ga-PSMA-11 (TABLE 2) for patients with prostate cancer with suspected metastases who are candidates for initial definitive therapy and for those with suspected disease recurrence based on an elevated serum prostate-specific antigen (PSA) level. PSMA PET is now also included in clinical practice guidelines41, the 2022 NCCN guidelines42 for primary disease staging, for the detection and localization of disease recurrence or persistence in patients with sustained high serum PSA levels after radical prostatectomy or radiotherapy, for documenting disease progression, and in the selection of patients for application of 177Lu-PSMA-617 as in the phase III VISION study. Data from this trial were published in 2021 and indicate a statistically significant improvement in OS for patients with metastatic CRPC who received up to six cycles of 177Lu-PSMA-617 plus standard-of-care therapy (typically ARSIs with or without GNrH agonists or glucocorticoids) compared with standard-of-care therapy43 (TABLE 2). In March 2022, the FDA approved 177Lu-PSMA-617 for men with PSMA-positive metastatic CRPC as determined by 68Ga-PSMA-11 PET imaging. Data from the VISION trial also indicate that 177Lu-PSMA-617 plus standard-of-care therapy delays the time to worsening of health-related QOL, the onset of pain and the time to first symptomatic skeletal event versus standard-of-care therapy alone43 (TABLE 2). The case for FDA and EMA approval of 177Lu-PSMA-617 in 2022 was supported by additional data from the TheraP trial, a randomized phase II study that compared the efficacy of 177Lu-PSMA-617 with that of cabazitaxel in men with metastatic CRPC with disease progression on docetaxel (12-month PFS 19% versus 3%), thus indicating the superiority of 177Lu-PSMA-617 over second-line chemotherapy44. Patient-reported outcomes (PROs) were also improved with 177Lu-PSMA-617 in the TheraP trial44. Prospective trials are increasingly incorporating PRO-based QOL assessments in an attempt to provide a more holistic evaluation of the effects of radiotheranostics. Building on data from the TheraP and VISION trials, several ongoing phase III trials are expected to provide data on the efficacy of PSMA-based radiotheranostics earlier in the course of metastatic CRPC, including PSMAfore (NCT04689828) and PSMAddition (NCT04720157); on the performance of alternative peptide ligands, including in SPLASH (NCT04647526) and ECLIPSE (NCT05204927); and on antibody-based PSMA-targeted radiotheranostics in PROSTACT (NCT04876651). Other trials are exploring the efficacy of α-emitters such as 225Ac-PSMA (NCT05219500, NCT04597411). New additional diagnostic agents that could be combined with approved therapeutic agents are also currently in development, such as 64Cu-SAR-bisPSMA45, which contains two PSMA binding motifs, thus offering a ‘dual-targeting’ approach that can be adapted according to availability and logistics, and is currently being tested in the phase I PROPELLER study (NCT04839367). SAR-bisPSMA could potentially also be deployed with the therapeutic 67Cu isotope.
Other agents
Another important therapeutic concept fitting beneath the umbrella of radiotheranostics is the use of radioactive microspheres (small, injectable 25–32 μm diameter particles typically made from glass, resin or poly-lactic acid)46 for the delivery of selective intra-arterial radiotherapy (SIRT), which is often referred to as transarterial radioembolization (TARE). Three distinct types of microsphere are currently available, including glass or resin microspheres bound to 90Y and a poly-lactic acid labelled with 166Ho (REF.47). All three types of microsphere can be used to treat patients with primary liver cancers or liver metastases, for example, from primary colorectal cancer, NETs or breast cancer47–49. Despite initially encouraging clinical results, data from a successful phase III study involving TARE were only reported in December 2021 (REF.48). The EPOCH study (NCT01483027) demonstrated a significant improvement in both median PFS (8.0 months versus 7.2 months; HR 0.69, 95% CI 0.54–0.88; P = 0.0013) and hepatic PFS (9.1 months versus 7.2 months; HR 0.59, 95% CI 0.46–0.77; P < 0.0001) in patients who received radioembolization with 90Y-glass microspheres plus chemotherapy compared with those who received chemotherapy only; however, median OS was not significantly different48. Personalized dosing seems to improve the response rates to this approach, although these promising phase II data need to be confirmed in a randomized phase III study7,50.
Promises and challenges for radiotheranostics
Promises
Rapid growth and future demand.
Radiotheranostic applications are gaining prominence in both cancer imaging and cancer therapy51. This increase in interest largely relates to the advent of diagnostic and therapeutic compounds that have fundamentally changed the way we manage cancer. Some of these procedures are now widely available clinically, and others will likely soon follow. For example, although the NETTER-1 and VISION trials both used standard doses for therapy, the therapeutic index can potentially be improved by optimizing the amount of injected radioactivity, optimizing treatment regimens including their time intervals and the number of treatment cycles, and by personalizing treatment based on dosimetry and early imaging-based response assessments.
The number of new clinical trials exploring radiotheranostic approaches continues to increase substantially. This development is driven by several factors, including the growing availability of novel hybrid imaging technologies for better cancer detection and monitoring and the increasing application of nuclear medicine in oncology, including the approvals of several new radiotheranostic agents for cancer diagnosis and therapy. For example, according to estimates from an editorial published in September 2019, the number of US patients eligible each year for novel radiotheranostic agents52 includes 20,000 with NETs for diagnostic 68Ga-DOTATATE or 64Cu-DOTATATE PET imaging, and 7,500 for 177Lu-DOTATATE therapy, as well as 160,000 patients with prostate cancer who are eligible for diagnostic PSMA imaging and 40,000 for therapy with 177Lu-PSMA. The accuracy of these estimates remains to be established, although these numbers suggest that radiotheranostic procedures are becoming a relevant option for an increasing number of patients. Additional factors driving the expansion of radiotheranostic applications include: first, the availability of data from several pivotal clinical trials that demonstrate the clinical benefits of novel diagnostic and therapeutic radiopharmaceuticals such as NETTER-1 (REFS35,36), OSPREY53, 68Ga-PSMA-11 (REF.54), VISION43 and TheraP44; second, the 2022 FDA approval of 177Lu-PSMA-617 for the treatment of metastatic prostate cancer; third, the adoption of new technologies for early cancer detection55–58 and the availability of better therapies that improve both the response rates and OS of patients with cancer59–62; fourth, the rising prevalence of cancer worldwide (19.3 million new cases were registered worldwide in 2020, and this number is expected to increase to 28.4 million in 2040, with the largest increase occurring in developing countries by 2040)63; fifth, the increasing global life expectancy60–62,64; and sixth, technological advances, such as expected improvements in small-molecule, peptide and antibody technologies, the identification of new targets and delivery mechanisms for radionuclide-based imaging and therapy (such as cell-surface molecules and targets located in the tumour microenvironment), and the emergence of novel light-based probes and combination therapies, such as those involving antibody–drug conjugates (ADCs).
Market valuation.
A general consensus exists that radiotheranostics has a promising future, although estimates of market value and predictions of growth vary. In part, this variation relates to how the market is defined: for example, narrowly as the market for radiopharmaceuticals; more broadly as the market for nuclear medicine; or, as the overall theranostics market that also includes optical probes and in vitro testing and the range of radiopharmaceuticals considered relevant to theranostic approaches. Accordingly, estimated market valuations for 2021 range from $1.7 billion to ~$6.0 billion65–67, with estimated compound annual growth rates (CAGRs) ranging from 4.7–10.7% to as high as 19.6% between 2022 and 2029–2031 (FIG. 3). In an attempt to increase both efficiency and profitability, the radiopharmaceutical industry has seen several mergers and acquisitions over the past years. Nevertheless, start-up companies continue to enter this growing market in an attempt to address previously unmet needs, such as the poor prognoses of patients with certain cancers (despite considerable general progress with non-radiolabelled targeted therapies), and to develop novel methods of killing cancer cells using targeted radiation.
Fig. 3 |. The predicted global nuclear medicine market 2013–2026.
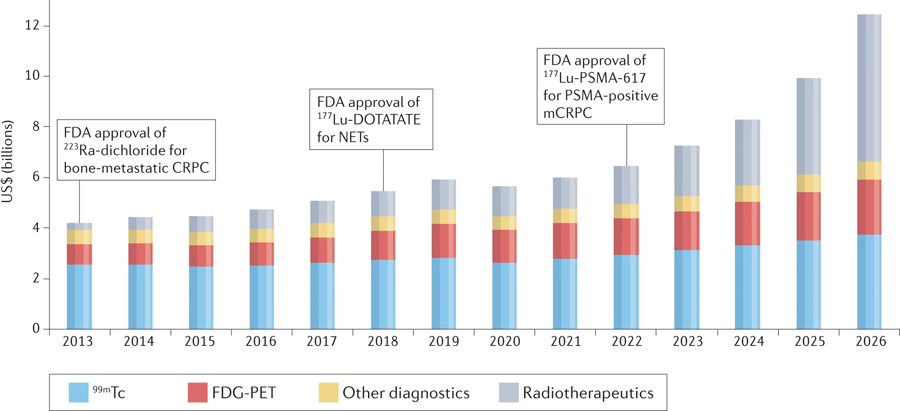
This projected market growth likely reflects the availability of a greater number of agents, implementation at an increasing number of centres and projected increases in the numbers of patients with cancer globally. ©MEDraysintell Nuclear Medicine Report C Directory, Edition 2021. CRPC, castration-resistant prostate cancer; mCRPC, metastatic CRPC; NET, neuroendocrine tumour; PSMA, prostate-specific membrane antigen.
North America will remain the dominant region for radiotheranostic applications, with ~45% of market value, followed by Europe (led by Germany, the UK and France) and the Asia Pacific region (led by China, Japan and India). Substantial growth is also expected both in South America and in parts of Asia over the coming decade. A substantial unmet need for radiotheranostics also exists in low and middle income countries (LMICs)51,65–67.
Assuming that the current promise of radiotheranostics holds up, justifying the continued large investments in research and development (R&D) and the clinical introduction of new agents that improve patient outcomes, the greatest improvements in outcomes are likely to be achieved in cancers with the highest incidence and mortality rates (such as lung cancer, with 235,760 new cases and 131,880 deaths in the USA in 2021) as well as in certain malignancies with a generally lower incidence that also have very high mortality rates, such as pancreatic, ovarian, small-cell lung and hepatobiliary cancers. However, the expansion of radiotheranostics also faces numerous challenges.
Challenges
Production, distribution and storage.
Both within and across countries, wide variations exist in the availability of medical cyclotrons, good manufacturing practice (GMP)-compliant production facilities (which can lead to substantial differences in costs between commercial suppliers and ‘in-house’ producers) for radiotheranostic agents, and dedicated theranostic treatment centres that meet the relevant radiation safety standards. Thus, reliable distribution networks capable of ensuring both the safe and timely delivery of these agents must be established to meet the rapid increase in demand. All radiotheranostic agents have a limited shelf-life, mainly owing to the radioactive half-lives of the radionuclides (TABLE 1). In contrast to conventional cancer therapies, both manufacturing (central versus local) and logistics (delivery, application and waste management) must be adjusted to compensate for the much shorter shelf-lives of radiotheranostic agents and the resulting limitations in the number of patients who can receive treatment per production cycle. However, as previously mentioned, such challenges have already been successfully addressed for radioiodine, 90Y radioembolization and even for relatively short-lived diagnostic radioisotopes such as 18F and 68Ga. The limited global supply of rare earth radioisotopes, which are frequently used for radiotheranostic applications, also poses challenges.
Workforce and equipment.
A Lancet Oncology Commission report in 2021 (REF.68) revealed a serious international shortage of physicians specializing in nuclear medicine and the use of the advanced imaging equipment (such as PET–CT or SPECT–CT) needed for therapy planning, dosimetry and response assessment. The current number of nuclear medicine physicians and treatment facilities might suffice in some western countries, although the commission noted significant shortages of both staff and equipment in many other countries across the globe, especially in LMICs.
Access to radiotheranostics.
To bring the benefits of radiotheranostics to patients worldwide and also overcome inequities in access to health care, these agents must be made accessible in all countries with appropriate nuclear medicine facilities. For example, an analysis by the Society of Nuclear Medicine and Molecular Imaging Global Initiative published in September 2020 (REF.69) showed that agents that were introduced clinically many years ago, such as 131I for the treatment of hyperthyroidism, were available in 94% of the 35 queried countries (including several LMICs), whereas other agents (which are used widely in routine clinical practice in the USA and parts of western Europe) such as 153Sm-EDTMP were only used in 51% of countries. 177Lu-DOTATATE and 177Lu-PSMA were rarely available, likely reflecting the fact that several of these agents were only approved by the FDA in the past few years and have not been approved in many countries outside of Europe and the USA.
Training of expert personnel.
The size of the existing workforce and the number of sites capable of preparing and administering radiotheranostic agents in both the USA and many other countries is currently simply not sufficient to meet the growing demand — hence the need for training programmes (at all levels) and site upgrades and/or the establishment of truly multidisciplinary care centres capable of providing adequate medical physics and clinical and nursing care in the same location. A crucial need exists for a new generation of radiochemists and radiopharmacists to safely design, manufacture and produce diagnostic and therapeutic radiopharmaceuticals on an industrial scale.
Radionuclide-based therapies must be administered by properly trained physicians, generally nuclear medicine specialists, and in certain scenarios by radiologists with additional training in nuclear medicine. Historically, many nuclear medicine training programmes have placed less of an emphasis on therapeutics than on diagnostic procedures. This emphasis largely reflects the distribution of procedural volumes (roughly 90% diagnostic versus 10% therapeutic in many larger institutions) and, increasingly since 2001, the widespread use of hybrid imaging techniques, necessitating additional training in structural imaging techniques. However, with the increasing availability of radiotheranostic agents, training programmes must now place a greater emphasis on the administration of radionuclide-based therapies with adequate training in the principles of internal medicine. Beyond technical expertise in the safe handling of these agents, along with expertise in dosimetry and radiation safety, this training also requires greater engagement with patient management, including a deep understanding of disease processes, pathology, pharmacology and treatment algorithms, to enable physicians to apply radiotheranostics in the overall context of the patient’s disease management. Such training will probably require dedicated subspecialty or fellowship training pathways70–72.
Regulation.
Before marketing approval, national or international regulatory bodies (such as the FDA in the USA or EMA for the EU) must review the safety and efficacy of any proposed new drugs or imaging agents. Novel agents can be administered to patients before marketing approval under the auspices of a clinical trial; however, full regulatory approval generally forms the basis for the initiation of widespread clinical use and the initiation of reimbursement systems by both government agencies and private insurers. A lack of co-ordination of approval processes between various regulatory agencies can delay drug availability: for example, the FDA does not recognize the approval decisions of other national agencies or the EMA, and independent FDA review is required before marketing approval in the USA, sometimes leading to delays and/or additional administrative costs. These considerations might explain why 177Lu-DOTATATE received FDA approval in February 2018, approximately 4 months after EMA approval. Conversely, other national regulatory agencies might not necessarily make the same decisions as the FDA, owing to the application of different thresholds for clinical benefit and/or cost effectiveness73.
Clinical trials and the real world.
To date, the development of radionuclide therapies has been largely enabled by compassionate use provision available at academic centres and single-centre trials. However, robust data from multicentre trials are required for marketing approval of new drugs, including radiopharmaceuticals. These trials are generally conducted under very specific conditions (including in carefully selected patient populations with highly specific inclusion and exclusion criteria and timings of procedures); data from such trials might not always accurately reflect outcomes in ‘real-world’ conditions when these treatments are administered less selectively to patients with differing disease burdens, comorbidities, ages and/or ethnicities74–76. Interest in investigating how promising clinical trial data can be reproduced in the real world has therefore increased. The collection of real-world evidence (RWE) is one option, and this approach is increasingly being used by the FDA77. However, obtaining a statistically robust amount of RWE often takes several years, is associated with additional costs and remains far from perfect as a confirmatory tool78.
Managing the expectations of physicians and patients.
The VISION and TheraP trials have provided evidence to support the utility of 177Lu-PSMA-617 in patients with metastatic CRPC, providing a justification for approval, which was announced in March 2022. However, comprehensive data on the utility of 177Lu-PSMA-617 in patients with early-stage prostate cancer, or in those with other malignancies, are still being generated. Moreover, the gap between the development of new radiotheranostic probes and their successful clinical translation and regulatory approval is growing. Nevertheless, anecdotally, patients are already enquiring about this therapy for non-approved conditions, reflecting sometimes unrealistic expectations, in part related to press releases and based on opinions expressed by various online media79.
Although unrealistic expectations must be tempered, unfounded fears must also be addressed. Referring physicians, patients, their families and the general public might harbour misgivings about the safe use of radiopharmaceuticals. Such ‘radiophobia’ is a real effect80, and it is for health-care providers, hospitals, treating physicians and patient support groups to fully educate the public on both the advantages and limitations of radiotheranostics.
Financial viability.
The development and clinical application of radiotheranostics is associated with high costs. The reimbursement processes for radiotheranostics are often complex and vary across different national health-care systems according to whether reimbursement is government funded or insurance based, the mechanisms by which reimbursement costs are calculated and the availability of radiotheranostics. Owing to these various aspects, reimbursement might not always fully cover the true costs of delivery of this therapy. This could introduce barriers to both access and availability, particularly in LMICs. The high costs of newly approved therapeutic radiopharmaceuticals mirrors that seen with other newly approved cancer drugs81, whose cost effectiveness and sometimes marginal benefits have been criticized73,76.
The high costs of novel radiotheranostic agents partly reflect the R&D costs of radiotheranostics. In 2019, the pharmaceutical industry overall spent $83 billion on R&D. This was ten times more than in the 1980s when adjusted for inflation, increasing from historic rates of around 12–15% to up to 25% of net revenues. A similar level of investment in R&D of around 15% can only be observed in certain other innovation-driven industries such as software development and the manufacture of semiconductors82. Novartis, the provider of 177Lu-DOTATATE and 177Lu-PSMA-617, spent US$8.98 billion (18.4% of net revenue) on R&D in 2020 (REF.83). Nevertheless, high R&D expenditure is at best only one contributing factor to high drug prices. Although pricing in the UK and other countries may be based on health technology assessments of the expected benefit and willingness to pay for such benefit, in the USA and in many other countries with multiple private insurance providers, the pricing of novel drugs is based on market considerations and closely linked to the price point of other drugs that are approved for the same or similar indications. Thus, drug pricing is somewhat arbitrary and not necessarily linked either to R&D costs or to the extent of clinical benefit. Moreover, owing to differences in the approach to medical reimbursement, market prices often vary substantially from country to country. In the USA, drug prices are currently not regulated or negotiated by government agencies, such as the Center for Medicare and Medicaid services (CMS), which contributes to higher health-care costs compared with those seen in many other countries84–86. The dominant positions of a few pharmaceutical companies are another factor that contributes to high prices. For example, Novartis, the manufacturer of 177Lu-DOTATATE and 177Lu-PSMA-617 currently dominates the market for theranostic agents, although this situation might change as other companies begin to develop alternative PSMA-targeted theranostics.
The high and rising costs of modern anticancer drugs and (now increasingly) radiopharmaceuticals poses considerable challenges to both health-care systems and patients worldwide. High drug prices are particularly problematic in the USA: US health-care spending currently accounts for 17.7% of gross domestic product (GDP) and has continued to increase (in 2019 by 4.6%, reaching US$11.6 trillion). During the next decade, national health-care expenditure is projected to increase by 5.4% annually, to 19.7%, a growth rate that is 1.1% faster than projected GDP growth87. Economically developed countries generally tend to spend a larger proportion of GDP on health care; nonetheless, the trajectory of US health-care spending might not be sustainable and is beginning to constrain investment in other sectors of society, such as R&D investments by companies that provide employer-sponsored health insurance and investment into education and infrastructure by the federal government88. Although radiotheranostics is currently only a very small fraction of health-care expenditure, this situation could change rapidly with the advent of new agents marketed at similar prices to 177Lu-DOTATATE and 223Ra-dichloride. Despite these agents often becoming available at lower market prices in LMICs, their costs relative to GDP renders these agents essentially unaffordable in many of these countries. Thus, even when accounting for the fact that the market prices of most drugs and radiopharmaceuticals are generally lower outside the USA, costs are a major challenge to the worldwide89 adoption of radiotheranostics and will need to be addressed in the coming years.
Biological challenges.
Currently, therapeutic strategies involving radiotheranostics lead to objective responses in only 30–60% of patients. Moreover, median PFS is only in the region of 28–36 months for patients with indolent NETs5 and ~9 months for those with aggressive metastatic CRPCs43.
The reasons for the lack of consistent tumour control include suboptimal drug delivery owing to insufficient tumour perfusion, heterogeneous expression of receptors and/or target antigens on the tumour cell surface, and the type of radiation delivered. Most current radiopharmaceutical therapies use radionuclides that emit β-radiation, which confers the advantage of a greater radiation coverage area; however, β-emitters mainly induce single-strand breaks in DNA, as opposed to α-emitters, which mainly induce double-strand DNA breaks90. A lack of retention of radiopharmaceuticals at the target site (owing to rapid dissociation of the targeted probe) might also limit efficacy. Finally, some tumours are inherently radioresistant or might acquire radioresistance following irradiation. This resistance arises from the diverse range of genomic alterations present in cancer cells and their microenvironment, which, under the selective pressures of toxic radiation, can become radioresistant via cellular senescence, hypoxia, metabolic alterations and/or an increased capacity for DNA damage repair91.
Tumour dedifferentiation, which is associated with the loss of specific cell-surface receptors or antigens, is an important aspect of radioresistance, particularly when applied to targeted radiotheranostics. Relevant examples of tumour dedifferentiation include the loss of sodium–iodine symporter expression in dedifferentiated thyroid cancer and of somatostatin receptor expression in high-grade NETs92,93. The relative paucity of specific targets for clinically aggressive cancers, which confer a particularly poor prognosis, is an additional challenge.
Balancing efficacy and toxicity.
Therapeutic efficacy requires the delivery of a certain target dose of radiation to tumour cells. This consideration is particularly relevant for β-emitters, which induce mainly single-strand breaks and scattered double-strand breaks, which can be less cytotoxic than α-radiation11,94. Unfortunately, improving efficacy by augmenting the amount of radiation administered also increases the risks of toxicity to nonmalignant tissues95. This trade-off between efficacy and toxicity is a crucial determinant of the amount of radioactivity that can be safely administered and needs to be studied specifically for each therapeutic radiopharmaceutical.
Most contemporary therapeutic radiopharmaceuticals administered at predefined tolerated doses confer mild to moderate toxicities according to NCI criteria96. Such events are broadly characterized as either acute, subacute or chronic, but require better biological characterization. Transient subacute bone marrow compromise (transient anaemia and decreased white cell and platelet counts) is an adverse event that is common to many radiotheranostic agents97. Chronic adverse events are clinically most concerning and are typically permanent. Most of these events can be ascribed to two mechanisms: inflammation and/or fibrosis, resulting in reduced organ function; and radiation-induced clonal selection, leading to uninhibited proliferation94. Xerostomia, the chronic salivary gland toxicity resulting from exposure to high-dose radioiodine or PSMA-targeted radiotheranostic agents involving 177Lu, is an example of a chronic fibrotic adverse effect. This effect can lead to mild xerostomia in 8% of patients, and this risk increases to 89% with use of 225Ac-PSMA, becoming severe in 10% of patients treated with α-emitters92–94,98 Another example is the chronic renal damage associated with 90Y-labelled PRRT, which occurs in 2.8% of patients94. Long-term bone marrow toxicities might also be seen in patients receiving radionuclide therapies, such as the rare but almost invariably fatal therapy-related myeloproliferative syndrome. The lifetime incidence of therapy-related myeloproliferative syndrome in patients with metastatic thyroid cancers or those with NETs who receive repeated high doses of radioiodine or 90Y-PRRT is around 1.5–2.3%97. Some of the established chronic adverse events (such as renal failure or salivary gland impairment) are dose dependent, while others (such as therapy-related myeloproliferative syndrome) are stochastic and have an indeterminate relationship with the extent of radiation exposure. By definition, a stochastic event is random and not directly related to the administered dose; however, the probability of such events increases at higher doses. The current interpretation is that these events have a very high threshold and are related to individual susceptibility97. Classical risk factors (such as previous treatment with myelotoxic chemotherapy or extensive bone marrow irradiation) and broad clinical characteristics (such as thrombocytopenia) explain only a minority of the adverse effects of radiotheranostics94.
Bone marrow.
Bone marrow is a crucial dose-limiting organ for most systemic therapies, such as radioiodine, radioligand therapy (such as 177Lu-DOTATATE or 177Lu-PSMA-617) and radioimmunotherapy. In the case of PRRT, cumulative doses of 177Lu-DOTATATE of around 30 GBq can be safely administered to most individuals, even in the absence of dosimetric assessments. Data on re-treatment, resulting in lifetime exposures of up to 60 GBq of 177Lu or higher has been reported from a few individuals with very advanced-stage disease99,100, in whom concerns of possible myelodysplasia were tempered by the expected improvements in survival duration. Of note, clinical characteristics and prior treatments received are both only partially predictive of adverse events in a given individual. This lack of any notable correlation has led to the concept of individual, possibly genomics-based, susceptibility94.
To address these safety issues, several strategies have been proposed101, such as the use of α-emitter theranostics (which seem to be effective even in patients with cancers that are refractory to 177Lu-PSMA-617 or 177Lu-DOTATATE102,103), development of innovative agents for current targets, targeting of alternative receptors and/or antigens, use of combination therapies and/or sensitization techniques, locoregional administration of therapy, use of free-radical scavenging therapies36,104–106 and individualization of therapy8,107,108. Further assessments regarding how variances in host genomic profiles influence a patient’s response to various cancer drugs might prove crucial for the development of novel individualized treatment strategies109.
Future developments
Most multicentre clinical trials of radiotheranostics involve patients with metastatic NETs or prostate cancer. However, an increasing number of novel targeted radiotheranostic agents are being explored in patients with a range of advanced-stage and/or metastatic cancer types (Supplementary Table 1). Radiotheranostic approaches have shown efficacy in many cancers, nonetheless, combination therapies hold the potential to further improve clinical outcomes and are currently being evaluated in clinical trials.
Combination therapies
Combination with established systemic therapies is an emerging, exciting approach with the potential to improve the outcomes of patients receiving radiotheranostic agents (FIG. 4). Approaches involving targeted radionuclides currently under evaluation, either in preclinical studies or in early-phase trials, include combinations with chemotherapy, radiosensitizers, EBRT and immunotherapies (NCT04343885, NCT04419402, NCT05146973, NCT03874884, NCT05109728, NCT03805594 and NCT03658447)110–125.
Fig. 4 |. Therapeutic approaches involving radiotheranostics.
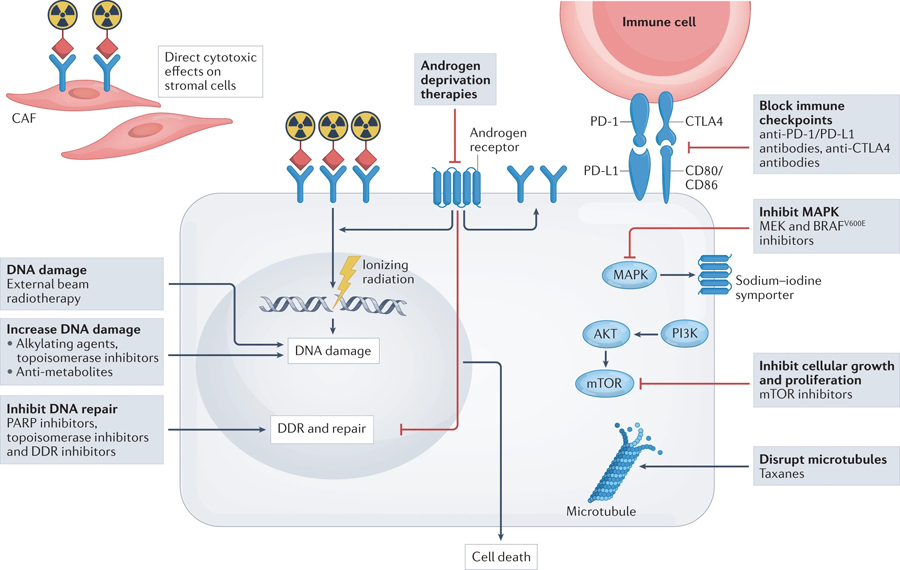
Therapeutic effects on cancer cells caused by DNA damage induced by either α-, β- or auger-emitting radionuclides can be enhanced via combination with drugs that either cause direct damage to DNA (such as chemotherapies) or inhibit DNA damage repair directly (such as PARP inhibitors) or through modulation of the associated signalling pathways (such as novel androgen-deprivation therapies). Radiotheranostics can also target the tumour microenvironment (such as cancer-associated fibroblasts (CAFs)) and kill stromal cells, which can indirectly lead to tumour regression. Bystander effects, owing to use of β-emitters, on the DNA of cancer cells that do not express radiotheranostic target proteins can nonetheless lead to tumour cell death. Targeted radionuclide therapies might also induce antigen presentation following cancer cell death and, when combined with immune-checkpoint inhibitors, lead to enhanced antitumour activity. DDR, DNA damage response.
The antitumour activity of targeted radionuclide therapy is based on the induction of DNA damage, suggesting the potential for synergistic therapeutic effects through combination with conventional chemotherapies, including the antimetabolite capecitabine, the alkylating agent temozolamide or the topoisomerase inhibitor topotecan (NCT02358356, NCT02736500). Enhanced effects have been demonstrated in several preclinical models and have been explored in early-phase clinical trials of radiolabelled peptides and antibodies in patients across several cancers including NETs, colorectal cancer and neuroblastoma126–129. For example, the randomized phase II CONTROL NET study, in which patients with pancreatic or midgut NETs (pNETs and mNETs, respectively) received 177Lu-octreotate plus capecitabine and temozolamide versus 177Lu-octreotate monotherapy met the target landmark PFS in patients who received combination therapy (12-month PFS 76% for patients with pNETs, 15-month PFS 90% for patients with mNETs). Numerically greater ORRs were also observed in patients with either mNETs or pNETs who receiving combination therapy (ORR 31% versus 15% and 68% versus 33%, respectively), albeit with a greater incidence of grade 3 (mostly haematological) adverse events in patients with mNETs (at least one event in 75% versus 28%). Long-term follow-up will be required to determine any significant differences in PFS129. Further studies involving combination therapies, such as a phase I study demonstrating that 177Lu-J591 plus docetaxel is feasible and safe in patients with metastatic CRPC130 and an ongoing phase II study comparing 177Lu-PSMA-617 plus docetaxel against docetaxel in patients with newly diagnosed metastatic CPRC (NCT04343885), have either been completed or are currently ongoing.
Activation of the mTOR and PI3K signalling pathways has been shown to induce radioresistance. Therefore, mTOR inhibitors might have a role in enhancing responsiveness to radionuclide therapy. Data from preclinical studies provide contrasting results110,131,132; nonetheless, data from the single-arm, phase I NETTLE study demonstrate tolerability and clinical responses in patents with GEP-NETs who received 177Lu-DOTATATE and everolimus126,133.
DNA damage induced by radionuclide therapy might also be enhanced by combination therapy with agents that inhibit DNA repair. PARP proteins, for example, are involved in the repair of both single-strand and double-strand DNA breaks, and PARP inhibitors have been shown to sensitize various preclinical models to radionuclide therapy112,113. This approach is currently being investigated in academic clinical trials testing olaparib in combination with 177Lu-DOTATATE in patients with GEP-NETs (NCT04086485) and with 177Lu-PSMA-617 in patients with metastatic CRPC (NCT03874884).
The DNA damage response (DDR) pathway also includes ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs); inhibitors of these proteins have therefore been explored as radiosensitizers114. Most clinical studies investigating combinations of these agents with radiotherapy have focused on EBRT, while combinations with radionuclide therapy have so far been explored mainly in preclinical models, with improvements in antitumour activity observed with each modality relative to monotherapy116,117. Similarly, inhibitors of other molecules that might modulate the function of DDR proteins (such as EGFR or HSP90) have been shown to enhance the efficacy of radiolabelled antibodies and peptides in preclinical models115. Synergy between radionuclide therapy and the DDR pathway is an important area of potential exploration in future clinical trials (for example, NCT04750954).
The ability of EBRT to enhance antitumour immunity has been established mechanistically, and clinical trials combining radiotherapy with immune-checkpoint inhibitors have provided some encouraging results118,119,134,135. In contrast to focal EBRT, radionuclide therapies are administered systemically and can therefore target more-widespread disease. Nonetheless, similar to EBRT, enhancement of therapeutic efficacy has been observed when these agents are combined with immune-checkpoint inhibitors in preclinical studies120,121. Clinical trials combining 177Lu-PSMA-617 with the anti-PD-1 antibody pembrolizumab in patients with metastatic CRPC are ongoing (NCT03658447, NCT03805594). This approach appears to be well tolerated, with response rates suggesting that radioligand therapy leads to improved antitumour immunity (ORR 44–78%)122,123. Radionuclide therapy might also have synergistic effects when combined with EBRT. An example of this effect is provided by the administration of the LAT-1-targeting radionuclide [131I]iodo-l-phenylalanine (131I-IPA) in a rat model of glioma124; this combination is currently being evaluated in IPAX-1, a phase I/II clinical trial involving patients with recurrent glioblastoma (NCT03849105).
Combination therapy might also have a role in promoting the upregulation of the targets of radionuclide therapy. For example, in patients with radioiodine-refractory thyroid cancer, expression of the sodium–iodine symporter can be upregulated by treatment with MAP kinase inhibitors such as selumetinib, thereby restoring radioiodine avidity and therapeutic responsiveness62. Several multicentre trials are currently exploring the use of tyrosine kinase inhibitors that target MEK or BRAFV600E in patients with NRAS or BRAFV600E-mutant radioiodine-refractory thyroid cancer (IRAS 183600, MERAIODE, NCT03244956). Conversely, in one study, blockade of the androgen receptor (AR) has been shown to upregulate PSMA expression in patients with CRPC, although downregulation of PSMA expression was also observed after androgen deprivation in patients with hormone-sensitive disease in the same study125. In addition to effects on PSMA expression, AR blockade has been demonstrated to delay the repair of DNA damage and sensitize cells to radiation, which further supports the rationale for its use in combination with 177Lu-PSMA-617. The efficacy of 177Lu-PSMA-617 plus enzalutamide versus enzalutamide alone is currently being investigated in patients with metastatic CRPC who have progressed on docetaxel but have not received prior novel anti-androgen therapy in the phase II Enza-P study (NCT04419402).
Novel targets and approaches
The development of radiotheranostics has focused principally on targeting emitters of either α- or β-radiation to the surfaces of tumour cells followed by intracellular trafficking and retention, resulting in DNA damage2. Novel and potentially clinically important radiotheranostic approaches are expanding the range of targets to include those present in the tumour microenvironment, such as blood vessels, cancer-associated fibroblasts (CAFs), the stromal matrix and immune cells136,137. The stromal cells located in the tumour microenvironment are generally more genetically stable than tumour cells, which might downregulate or entirely lose expression of certain targets; stromal cells might also contribute to the development of an immunosuppressive microenvironment and to drug resistance138. Prolyl endopeptidase FAP (FAP), expressed on CAFs, has emerged as a target of novel radiotheranostics that is broadly expressed by the fibroblasts present in most adenocarcinomas and is being evaluated in several clinical trials supported by both industry and academia (NCT04571086, NCT04621435, NCT04849247 and NCT04939610; Supplementary information)101,139,140. The current generation of FAP-targeted theranostic agents includes various ligands with biological half-lives that range from short (FAPI-46) to substantially longer (FAP-2286)139. Identifying the optimal combination of FAP ligand and radionuclide will be an important stage in the development of these agents that will likely depend on the intended clinical use, for example, diagnosis versus therapy, including use as monotherapy versus combination therapy.
Pairing α- and β-emitting radiotheranostic agents might provide another potentially fruitful approach, taking advantage of the different radiation path lengths and cell-kill mechanisms. In addition, damage to cells that are not directly targeted can arise from irradiation of nearby target-null cells (crossfire effects) or the release of biologically active factors (bystander effects), such as free radicals or immune system factors, resulting in cytotoxicity far away from the irradiated cells141. Finally, pretargeting approaches can potentially enhance the uptake of radiolabelled proteins and peptides by tumour cells and thus reduce the risk of haematological toxicities142–145.
Conclusions
The careful deployment of radiotheranostics in patients with cancer has the potential to considerably improve treatment outcomes. However, several major challenges remain to be addressed. Generating evidence to enable a wider range of radiotheranostic agents to receive regulatory approval and rapidly reach the market is imperative. Moreover, strategies are needed to improve the availability of radiotheranostics globally. The current success of radiotheranostics will likely attract increasing interest from both academia and industry in identifying and developing novel targeted agents, which is expected to generate earlier and better methods of cancer detection, individualized treatments and improved outcomes for patients.
Supplementary Material
Key points.
Radiotheranostics combines molecular imaging (primarily PET and SPECT) with targeted radionuclide therapy, typically with radionuclides that emit α-, β- or auger-radiation.
The exponential, global expansion of radiotheranostics in oncology stems from the potential to target and eliminate tumour cells with minimal adverse effects owing to a mechanism of action that is distinctly different from that of most other systemic therapies.
Approvals of new radiotheranostic agents such as 177Lu-DOTATATE and 177Lu-PSMA-617 alongside the availability of companion diagnostic agents (such as 68Ga-DOTATATE and 68Ga-PSMA-11, respectively) have driven a resurgence of interest in the field that is driving numerous clinical trials testing novel radiotheranostics.
Novel and potentially clinically important radiotheranostic approaches are expanding the range of targets to include those present in the tumour microenvironment, such as blood vessels, cancer-associated fibroblasts, the stromal matrix and immune cells.
Although access to radiotheranostics is expanding, challenges such as lack of isotope availability, shortages of trained personnel, regulatory burdens and costs might all limit the extent of global dissemination.
Acknowledgements
The authors thank G. Scott for the careful editing of this manuscript and S. Lapi (University of Alabama) and J. Engle (University of Wisconsin-Madison) for help with Table 1. This work was supported in part by NIH grants R35 CA232130 (J.S.L.) and P30 CA008748 (J.S.L., L.B., H.S.), and NHMRC Investigator grant 1177837 (A.M.S.).
Footnotes
Competing interests
L.B. has acted as a consultant and/or speaker for AAA-Novartis, Clovis Oncology, Iba, ITM and MTTI and received research funding from AAA-Novartis. K.H. has received personal fees from Adacap, Aktis Oncology, Amgen, Bayer, BTG, Curium, Endocyte, GE Healthcare, IPSEN, Pharma15, Novartis, Siemens Healthineers, SIRTEX, Theragnostics and YMabs; has received non-financial support from ABX and Sofie Biosciences and has received research funding from BTG. A.M.S. has acted as a consultant of Imagion Bio and ImmunOs; has received research funding from AbbVie, AVID, Cyclotek, Curis; has recevied research funding from AVID, Adalta, EMD Serono, Fusion, Humanigen, ITM, Merck, Medimmune, Telix Pharmaceuticals and Theramyc, and is a co-founder of Certis Therapeutics and Paracrine Therapeutics. J.S.L. has acted as an adviser of Boxer, Clarity Pharmaceuticals, Curie Therapeutics, Earli, Evergreen Theragnostics, Telix Pharmaceuticals, TPG Capital and Varian Medical Systems; is a co-inventor on technologies licensed to Diaprost, Daiichi Sankyo, Elucida Oncology, Macrocyclics and Samus Therapeutics; and is the co-founder of, and holds equity in, pHLIP and Sharp RTx. H.S. declares no competing interests.
Peer review information
Nature Reviews Clinical Oncology thanks Jeremie Calais, Jacek Capala, Kazuma Ogawa, Rodney Hicks and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41571-022-00652-y.
References
- 1.Jadvar H, Chen X, Cai W & Mahmood U Radiotheranostics in cancer diagnosis and management. Radiology 286, 388–400 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann K et al. Radiotheranostics: a roadmap for future development. Lancet Oncol 21, e146–e156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA. VENTANA PD-L1 (SP142) assay - P160002/ S009, https://www.fda.gov/medical-devices/recently-approved-devices/ventana-pd-l1-sp142-assay-p160002s009 (2020)
- 4.FDA. HERCEPTIN (trastuzumab) label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf (2010)
- 5.Park S et al. Somatostatin receptor imaging and theranostics: current practice and future prospects. J. Nucl. Med 62, 1323–1329 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN. NCCN Guidelines, https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1448 (2021).
- 7.Garin E et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol 6, 17–29 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Sgouros G, Bodei L, McDevitt MR & Nedrow JR Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov 19, 589–608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ku A, Facca VJ, Cai Z & Reilly RM Auger electrons for cancer therapy - a review. EJNMMI Radiopharm. Chem 4, 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poty S, Francesconi LC, McDevitt MR, Morris MJ & Lewis JS Alpha-emitters for radiotherapy: from basic radiochemistry to clinical studies-part 1. J. Nucl. Med 59, 878–884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouget JP, Catherine L, Deshayes E, Boudousq V & Navarro-Teulon I Introduction to radiobiology of targeted radionuclide therapy. Front. Med 2, 12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pouget JP et al. From the target cell theory to a more integrated view of radiobiology in targeted radionuclide therapy: the Montpellier group’s experience. Nucl. Med/ Biol 104–105, 53–64 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Adams DJ The valley of death in anticancer drug development: a reassessment. Trends Pharmacol. Sci 33, 173–180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertz S, Roberts A & Evans RD Radioactive iodine as an indicator in the study of thyroid physiology. Proc. Soc. Exp. Biol. Med 38, 510–513 (1938). [Google Scholar]
- 15.Chapman EM & Evans RD The treatment of hyperthyroidism with radioactive iodine. J. Am. Med. Assoc 131, 86–91 (1946). [DOI] [PubMed] [Google Scholar]
- 16.Seidlin SM, Marinelli LD & Oshry E Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J. Am. Med. Assoc 132, 838–847 (1946). [DOI] [PubMed] [Google Scholar]
- 17.Witzig TE et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol 15, 2453–2463 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Grillo-Lopez AJ Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert. Rev. Anticancer. Ther 2, 485–493 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Friedberg JW & Fisher RI Iodine-131 tositumomab (Bexxar): radioimmunoconjugate therapy for indolent and transformed B-cell non-Hodgkin’s lymphoma. Expert. Rev. Anticancer. Ther 4, 18–26 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Leahy MF, Seymour JF, Hicks RJ & Rurner JH Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphoma. J. Clin. Oncol 20, 4418–4425 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci PF et al. High activity 90Y-ibritumomab tiuxetan (Zevalin) with peripheral blood progenitor cells support in patients with refractory/resistant B-cell non-Hodgkin lymphomas. Br. J. Haematol 139, 590–599 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Timmerman L Why Good Drugs Sometimes Fail: The Bexxar Story, https://xconomy.com/national/2013/08/26/why-good-drugs-sometimes-fail-in-the-market-the-bexxar-story/ (2013).
- 23.Schaefer NG, Huang P, Buchanan JW & Wahl RL Radioimmunotherapy in non-Hodgkin lymphoma: opinions of nuclear medicine physicians and radiation oncologists. J. Nucl. Med 52, 830–838 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med 369, 213–223 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Porter AT & McEwan AJ Strontium-89 as an adjuvant to external beam radiation improves pain relief and delays disease progression in advanced prostate cancer: results of a randomized controlled trial. Semin. Oncol 20, 38–43 (1993). [PubMed] [Google Scholar]
- 26.Sartor O, Quadramet 424Sm10/11 Study Group. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 63, 940–945 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Handkiewicz-Junak D et al. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur. J. Nucl. Med. Mol. Imaging 45, 846–859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith M et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20, 408–419 (2019). [DOI] [PubMed] [Google Scholar]
- 29.de Herder WW et al. Neuroendocrine tumors and somatostatin: imaging techniques. J. Endocrinol. Invest 28, 132–136 (2005). [PubMed] [Google Scholar]
- 30.De Jong M et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin. Nucl. Med 32, 133–140 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Bodei L, Kassi A, Adelstein SJ & Mariani G Radionuclide therapy with iodine-125 and other auger-electron-emitting radionuclides: experimental models and clinical applications. Cancer Biother. Radiopharm 18, 861–877 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Anthony LB et al. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin. Nucl. Med 32, 123–132 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Smit Duijzentkunst DA, Kwekkeboom DJ & Bodei L Somatostatin receptor 2-targeting compounds. J. Nucl. Med 58, 54S–60S (2017). [DOI] [PubMed] [Google Scholar]
- 34.Bodei L, Kwekkeboom DJ, Kidd M, Modlin IM & Krenning EP Radiolabeled somatostatin analogue therapy of gastroenteropancreatic cancer. Semin. Nucl. Med 46, 225–238 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Strosberg J et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med 376, 125–135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strosberg J et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J. Clin. Oncol 36, 2578–2584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strosberg JR et al. NETTER-1 investigators. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22, 1752–1763 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Eder M et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem 23, 688–697 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M & Zechmann CM [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 39, 1085–1086 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Kratochwil C et al. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 987–988 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Mottet N, et al. EAU Guidelines: Prostate Cancer, https://uroweb.org/guideline/prostate-cancer/ (2001).
- 42.NCCN. Recently Updated Guidelines, https://www.nccn.org/guidelines/recently-published-guidelines (2022).
- 43.Sartor O, VISION Investigators. Lutetium-177- PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med 16, 1091–1103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofman MS et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021). [DOI] [PubMed] [Google Scholar]
- 45.McInnes LE et al. Therapeutic efficacy of a bivalent inhibitor of prostate-specific membrane antigen labeled with 67Cu. J. Nucl. Med 1, 8290832 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.d’Abadie P et al. Microspheres used in liver radioembolization: from conception to clinical effects. Molecules 26, 3966 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber M et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 49, 1682–1699 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulcahy MF et al. Radioembolization with chemotherapy for colorectal liver metastases: a randomized, open-label, international, multicenter, phase III trial. J. Clin. Oncol 39, 3897–3907 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braat AJA et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol 21, 561–570 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Garin E, Tselikas L, Guiu B & Campillo-Gimenez B Personalised dosimetry for SIRT: new standard or bridge to surgical resection? - Authors’ reply. Lancet Gastroenterol. Hepatol 6, 162 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Arnold C Theranostics could be big business in precision oncology. Nat. Med 28, 606–608 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Czernin J, Sonni I, Razmaria A & Calais J The future of nuclear medicine as an independent specialty. J. Nucl. Med 60, 3S–12S (2019). [DOI] [PubMed] [Google Scholar]
- 53.Pienta KJ et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J. Urol 206, 52–61 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fendler WP et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 5, 856–863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phallen J et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med 9, eaan2415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein EA et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol 32, 1167–1177 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Bredno J, Lipson J, Venn O, Aravanis AM & Jamshidi A Clinical correlates of circulating cell-free DNA tumor fraction. PLoS ONE 16, 0256436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chabon JJ et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 580, 245–251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markham MJ et al. Clinical cancer advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. J. Clin. Oncol 38, 1081 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Allemani C et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashim D et al. The global decrease in cancer mortality: trends and disparities. Ann. Oncol 27, 926–933 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Howlader N et al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med 383, 640–649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung H et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Tamura R et al. Alterations of the tumor microenvironment in glioblastoma following radiation and temozolomide with or without bevacizumab. Ann. Transl. Med 8, 297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Future Market Insights. Radiopharmaceuticals Market By Radioisotope Type (Technetium-99, Fluorine-18, Iodine-131, Leutetium-177), Application (Oncology, Cardiology, Gastroenterology, Neuroendocrinology, Neurology, Nephrology), Source (Cyclotrons, Nuclear Reactors) & Region – Forecast 2021–2031 https://www.futuremarketinsights.com/reports/radiopharmaceuticals-market (2021).
- 66.Fortune Business Insights. Nuclear Medicine Market Size, Share & COVID-19 Impact Analysis, By Type (Diagnostic Radiopharmaceuticals and Therapeutic Radiopharmaceticals), By Application (Neurology, Cardiology, Oncology, and Others), By End-user (Hopsitals & Clinics, Diagnostic Centers, and Others), and Regional Forecast, 2021–2028 Market Research Report. https://www.fortunebusinessinsights.com/industry-reports/nuclear-medicine-radiopharmaceuticals-market-101812 (2022).
- 67.DataM Intelligence. Global Theranostics Market: Market Size, Share and Forecast; Market Outlook, Opportunity and Data Analysis 2022–2029 https://www.datamintelligence.com/research-report/theranostics-market (2021).
- 68.Hricak H et al. Medical imaging and nuclear medicine: a Lancet Oncology Commission. Lancet Oncol 22, e136–e172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cutler CS et al. Global issues of radiopharmaceutical access and availability: a Nuclear Medicine Global Initiative project. J. Nucl. Med 62, 422–430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bodei L, Chiti A, Modlin IM, Scott AM & Schoder H The path to the future: education of nuclear medicine therapeutic specialists as responsible physicians. J. Nucl. Med 60, 1663–1664 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Herrmann K et al. Joint EANM, SNMMI and IAEA enabling guide: how to set up a theranostics centre. J. Nucl. Med 10.2967/jnumed.122.264321 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee ST et al. The importance of training, accreditation and guidelines for the practice of theranostics: the australian perspective. J. Nucl. Med 10.2967/jnumed.122.263996 (2022). [DOI] [PubMed] [Google Scholar]
- 73.Cherla A, Naci H, Kesselheim AS, Gyawali B & Mossialos E Assessment of coverage in england of cancer drugs qualifying for US food and drug administration accelerated approval. JAMA Intern. Med 181, 490–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elimova E et al. Updating reports of phase 3 clinical trials for cancer. JAMA Oncol 7, 593–596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebrahim S et al. Reanalyses of randomized clinical trial data. JAMA 312, 1024–1032 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Schnog JB, Samson MJ, Gans ROB & Duits AJ An urgent call to raise the bar in oncology. Br. J. Cancer 125, 1477–1485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahn HJ et al. Radiation-induced CXCL12 upregulation via histone modification at the promoter in the tumor microenvironment of hepatocellular carcinoma. Mol. Cell 42, 530–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.US Food and Drug Administration. Real-World Evidence, https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (2020).
- 79.Johnson SB et al. Cancer misinformation and harmful information on facebook and other social media: a brief report. J. Natl Cancer Inst 10.1093/jnci/djab141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindberg JCH ‘J’accuse.!’: the continuous failure to address radiophobia and placing radiation in perspective. J. Radiol. Prot 41, 459 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Rajkumar SV The high cost of insulin in the United States: an urgent call to action. Mayo Clin. Proc 95, 22–28 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Takakusagi Y et al. Multimodal molecular imaging study evaluates pharmacological alteration of the tumor microenvironment to improve radiation response. Cancer Res 78, 6828–6837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Congressional Budget Office. Research and Development in the Pharmaceutical Industry, https://www.cbo.gov/publication/57126 (2021).
- 84.Vokinger KN et al. Price changes and within-class competition of cancer drugs in the USA and Europe: a comparative analysis. Lancet Oncol 23, 514–520 (2022). [DOI] [PubMed] [Google Scholar]
- 85.Himmelstein DU, Campbell T & Woolhandler S Health care administrative costs in the United States and Canada. Ann. Intern. Med 21, 134–142 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Pozen A & Cutler DM Medical spending differences in the United States and Canada: the role of prices, procedures, and administrative expenses. Inquiry 47, 124–134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.CMS.gov: Centers for Medicare and Medicaid Services. NHE Fact Sheet, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet (2021).
- 88.Institute of Medicine. Delivering Affordable Cancer Care in the 21st Century: Workshop Summary (National Academies Press, 2013). [PubMed] [Google Scholar]
- 89.Dusetzia SB Your money or your life - the high cost of cancer drugs under medicare part D. N. Engl. J. Med 10.1056/NEJMp2202726. (2022). [DOI] [PubMed] [Google Scholar]
- 90.Pouget J-P & Constanzo J Revisiting the radiobiology of targeted alpha therapy. Front. Med 8, 692436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang L et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res 37, 87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De la Vieja A & Riesco-Eizaguirre G Radio-iodide treatment: from molecular aspects to the clinical view. Cancers 13, 995 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waseem N, Aparici CM & Kunz PL Evaluating the role of theranostics in grade 3 neuroendocrine neoplasms. J. Nucl. Med 60, 882–891 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Bodei L et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 42, 5–19 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Sarnelli A et al. Therapeutic schemes in 177Lu and 90Y-PRRT: radiobiological considerations. Q. J. Nucl. Med. Mol. Imaging 61, 216–231 (2017). [DOI] [PubMed] [Google Scholar]
- 96.National Cancer Institute, Division of Cancer Treatment & Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE) Last Updated: 09/21/20, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (2022).
- 97.Bodei L et al. Myeloid neoplasms after chemotherapy and PRRT: myth and reality. Endocr. Relat. Cancer 23, C1–C7 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Ma J, Li L, Liao T, Gong W & Zhang C Efficacy and safety of 225Ac-PSMA-617-targeted alpha therapy in metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Front. Oncol 12, 796657 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yordanova A et al. Safety of multiple repeated cycles of 177Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur. J. Nucl. Med. Mol. Imaging 44, 1207–1214 (2017). [DOI] [PubMed] [Google Scholar]
- 100.van der Zwan WA et al. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)] octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 46, 704–717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kratochwil C et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med 59, 795–802 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Kratochwil C, Haberkorn U & Giesel FL (225)Ac-PSMA-617 for therapy of prostate cancer. Semin. Nucl. Med 50, 133–140 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Ballal S, Yadav MP, Bal C, Sahoo RK & Tripathi M Broadening horizons with (225) Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 47, 934–946 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Kristiansson A et al. Kidney protection with the radical scavenger α1-microglobulin (A1M) during peptide receptor radionuclide and radioligand therapy. Antioxidants 10, 1271 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bohuslavizki KH et al. Salivary gland protection by amifostine in high-dose radioiodine therapy of differentiated thyroid cancer. Strahlenther. Onkol 175, 57–61 (1999). [DOI] [PubMed] [Google Scholar]
- 106.Crumbaker M et al. Phase I/II trial of the combination of 177Lutetium prostate specific membrane antigen 617 and idronoxil (NOX66) in men with end-stage metastatic castration-resistant prostate cancer (LuPIN). Eur. Urol. Oncol 4, 963–970 (2021). [DOI] [PubMed] [Google Scholar]
- 107.Bodei L et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol 21, e431–e443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Giorgi U et al. Circulating androgen receptor gene amplification and resistance to 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: results of a phase 2 trial. Br. J. Cancer 125, 1226–1232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scott AM & Bodei L Pharmacogenomics in radionuclide therapy: impact on response to theranostics. J. Nucl. Med 1, 884–885 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan TG, O’Neill E, Habjan C & Cornelissen B Combination strategies to improve targeted radionuclide therapy. J. Nucl. Med 61, 1544–1552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Satapathy S et al. 177Lu-DOTATATE plus radiosensitizing capecitabine versus octreotide long-acting release as first-line systemic therapy in advanced grade 1 or 2 gastroenteropancreatic neuroendocrine tumors: a single-institution experience. JCO Glob. Oncol 7, 1167–1175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nonnekens J et al. Potentiation of peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics 18, 1821–1832 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cullinane C et al. Enhancing the anti-tumour activity of 177Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci. Rep 10, 10196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang R-X & Zhou P-K DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal. Transduct. Target Ther 1, 60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelly MP et al. Therapeutic efficacy of 177Lu-CHX-A″-DTPA-hu3S193 radioimmunotherapy in prostate cancer is enhanced by EGFR inhibition or docetaxel chemotherapy. Prostate 1, 92–104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wickstroem K et al. Synergistic effect of a mesothelin-targeted 227Th conjugate in combination with DNA damage response inhibitors in ovarian cancer xenograft models. J. Nucl. Med 60, 1293–1300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lundsten S, Spiegelberg D, Raval NR & Nestor M The radiosensitizer Onalespib increases complete remission in 177Lu-DOTATATE-treated mice bearing neuroendocrine tumor xenografts. Eur. J. Nucl. Med. Mol. Imaging 47, 980–990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weichselbaum RR, Liang H, Deng L & Fu Y-X Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol 14, 365–379 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Parikh AR et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat. Cancer 2, 1124–1135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen H et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 9, 7948–7960 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czernin J et al. Immune-checkpoint blockade enhances 225Ac-PSMA617 efficacy in a mouse model of prostate cancer. J. Nucl. Med 62, 228–231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aggarwal RS et al. Immunogenic priming with 177Lu-PSMA-617 plus pembrolizumab in metastatic castration resistant prostate cancer (mCRPC): a phase 1b study. J. Clin. Oncol 39, 5053 (2021). [Google Scholar]
- 123.Sandu SK et al. PRINCE: Interim analysis of the phase Ib study of 177Lu-PSMA-617 in combination with pembrolizumab for metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol 32, S626–S677 (2021). [Google Scholar]
- 124.Samnick S et al. Efficacy of systemic radionuclide therapy with p-131I-iodo-L-phenylalanine combined with external beam photon irradiation in treating malignant gliomas. J. Nucl. Med 50, 2025–2032 (2009). [DOI] [PubMed] [Google Scholar]
- 125.Emmett L et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68 Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J. Nucl. Med 60, 950–954 (2019). [DOI] [PubMed] [Google Scholar]
- 126.Claringbold PG, Brayshaw PA, Price RA & Turner JH Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 38, 302–311 (2011). [DOI] [PubMed] [Google Scholar]
- 127.Herbertson RA et al. Targeted chemoradiation in metastatic colorectal cancer: a phase I trial of 131I-huA33 with concurrent capecitabine. J. Nucl. Med 55, 534–539 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Genolla J et al. high-activity therapy with 131I-metaiodobenzylguanidine (131I-mIBG) and topotecan for the treatment of high-risk refractory neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 46, 1567–1575 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Pavlakis N et al. Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET Study: Phase II study evaluating the activity of 177Lu-Octreotate peptide receptor radionuclide therapy (LuTate PRRT) and capecitabine, temozolomide CAPTEM) — First results for pancreas and updated midgut neuroendocrine tumors (pNETS, mNETS). J. Clin. Oncol 38, Abstr. 4608 (2020). [Google Scholar]
- 130.Batra JS et al. Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urol. Oncol 38, 848.e9–848.e16 (2020). [DOI] [PubMed] [Google Scholar]
- 131.Pool SE et al. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res 73, 12–18 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Johnbeck CB, Nielsen CK, Knigge U & Kjaer A Synergistic effect of combined treatment with 177Lu-DOTATATE and Everolimus in neuroendocrine tumors as monitored by 18F-FDG-PET: studies in human neuroendocrine xenografts [abstract]. J. Nucl. Med 53, 57 (2012). [Google Scholar]
- 133.Claringbold PG & Turner JH NeuroEndocrine tumor therapy with Lutetium-177-octreotate and everolimus (NETTLE): a phase I study. Cancer Biother. Radiopharm 30, 261–269 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Schoenfeld JD et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 23, 279–291 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Theelen WSME et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med 9, 467–475 (2021). [DOI] [PubMed] [Google Scholar]
- 136.Scott AM, Wolchok JD & Old LJ Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Lee ST, Buarvenich I & Scott AM Novel target selection for nuclear medicine studies. Semin. Nucl. Med 49, 357–368 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Valkenburg KC, De Groot AE & Pienta KJ Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol 15, 366–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baum RP et al. Feasibility, biodistribution and preliminary dosimetry in peptide-targeted radionuclide therapy (PTRT) of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-Human Results. J. Nucl. Med 63, 415–423 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lindner T et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med 59, 1415–1422 (2018). [DOI] [PubMed] [Google Scholar]
- 141.Widel M Radionuclides in radiation-induced bystander effect; may it share in radionuclide therapy? Neoplasma 64, 641–654 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Altai M, Membreno R, Cook B, Tolmachev V & Zeglis BM Pretargeted imaging and therapy. J. Nucl. Med 58, 1553–1559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheal SM et al. Curative multicycle radioimmunotherapy monitored by quantitative SPECT/CT-based theranostics, using bispecific antibody pretargeting strategy in colorectal cancer. J. Nucl. Med 58, 1735–1742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keinänen O et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl Acad. Sci. USA 10, 28316–28327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zeglis BM et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med 54, 1389–1396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schlumberger M et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol 6, 618–626 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Ho AL et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med 14, 623–632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Keizer B et al. Efficacy of high therapeutic doses of iodine-131 in patients with differentiated thyroid cancer and detectable serum thyroglobulin. Eur. J. Nucl. Med. Mol. Imaging 28, 198–202 (2001). [DOI] [PubMed] [Google Scholar]
- 149.Serafini AN et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J. Clin. Oncol 16, 1574–1581 (1998). [DOI] [PubMed] [Google Scholar]
- 150.Gopal AK et al. 90Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood 28, 1132–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith MR et al. Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium-90-ibritumomab tiuxetan in untreated mantle-cell lymphoma: Eastern Cooperative Oncology Group Study E1499. J. Clin. Oncol 30, 3119–3126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morschhauser F et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol 1, 1977–1983 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Lugtenburg PJ et al. Rituximab-PECC induction followed by 90 Y-ibritumomab tiuxetan consolidation in relapsed or refractory DLBCL patients who are ineligible for or have failed ASCT: results from a phase II HOVON study. Br. J. Haematol 187, 347–355 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Shimoni A et al. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer 1, 4706–4714 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Davies AJ et al. Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol 15, 1469–1479 (2004). [DOI] [PubMed] [Google Scholar]
- 156.Kaminski MS et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J. Clin. Oncol 1, 3918–3928 (2001). [DOI] [PubMed] [Google Scholar]
- 157.EMA. European Medicines Agency: Science Medicines Health. List of nationally authorised medicinal products, https://www.ema.europa.eu/en/documents/psusa/iodine-131i-iobenguane-list-nationally-authorised-medicinal-products-psusa/00001764/201505_en.pdf (2016).
- 158.Pryma DA et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J. Nucl. Med 60, 623–630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sartor O et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15, 738–746 (2014). [DOI] [PubMed] [Google Scholar]
- 160.Nilsson S et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann. Oncol 27, 868–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


