Abstract
Converging, albeit scattered data mainly gathered in animals indicate that the neurotrophin brain-derived neurotrophic factor (BDNF) and the nonapeptide oxytocin (OT) interact in a cooperative way.
Data in humans are really limited and indirect. Therefore, the aim of the present study was to explore the possible existence of a link between OT and BDNF in humans, by means of two peripheral markers, the platelet-poor-plasmatic-BDNF (PPP-BDNF) and the platelet BDNF (PLT-BDNF) and OT levels.
Twenty-six young healthy controls of both sexes who volunteered for the study were included in the study. Fifty ml of peripheral venous blood were drawn from one-night fasting subjects between 8.00 and 9.00 a.m. The BDNF and OT assays were carried out according to common methods. Comparisons for continuous variables were performed by the Student's t-test for variables that follow a normal distribution, and by the Wilcoxon-Mann-Whitney test for variables not normally distributed. The correlations between biological markers were explored by calculating the Pearson's correlation coefficient or Spearman's rank correlation.
The results showed that PLT-BDNF (pg/mg proteins, mean ± SD) and PPP-BDNF (pg/ml, mean ± SD) were 1546 ± 1844 and 10111 ± 1892, respectively. The OT levels (pg/ml, mean ± SD) were 13.92 ± 4.54. The OT levels were significantly higher in women than in men. The Spearman's analysis revealed a statistically significant and negative correlation between OT levels and PLT-BDNF (R = −0.543, p = 0.004).
The findings of this study highlight the presence of a significant and negative correlation between OT and PLT-BDNF in a small group of healthy controls of both sexes. In any case, despite all the limits of peripheral biomarkers, they suggest that this reciprocal influence might have a downstream homeostatic function dampening one activity when the other is activated or no longer necessary, maybe at the level of the stress and/or immune systems.
Keywords: Brain-derived neurotrophic factor, Oxytocin, Biomarkers, Humans plasma, Platelets, Interactions
Highlights
-
•
Converging data mainly gathered in animals indicate that the neurotrophin brain-derived neurotrophic factor (BDNF) and the nonapeptide oxytocin (OT) interact in a cooperative way.
-
•
Data in humans are limited, therefore we explored the possible a link between OT and BDNF in humans, by two peripheral markers, the platelet-poor-plasmatic-BDNF (PPP-BDNF) and the platelet BDNF (PLT-BDNF) and OT levels.
-
•
The results showed that the OT levels were significantly higher in women than men and that there was a statistically significant and negative correlation between OT levels and PLT-BDNF (rho = − 543, p = 0.004).
-
•
These findings suggest that this reciprocal influence might have a downstream homeostatic function dampening one activity when the other is activated or no longer necessary, maybe at the level of the stress and/or immune system.
1. Introduction
During recent years, the scientific community has developed a growing interest towards the possible interaction between the neurotrophins that are involved in synaptic plasticity, branching of dendrites, neural survival, long-term potentiation and memory, and the neuropeptide systems. In particular, the brain-derived neurotrophic factor (BDNF) and the nonapeptide oxytocin (OT) were amongst the most studied compounds [1,2]. The BDNF, along with other neurotrophins (namely, neural growth factor [NGF] and neurotrophins 3 and 4), is virtually ubiquitous within the central nervous system (CNS) and, besides the just-mentioned activities, seems to regulate excitatory/inhibitory neurotransmission, brain development and aging [[2], [3], [4], [5]]. Since in different psychiatric disorders significant changes of BDNF levels have been described, currently, it is considered an interesting biomarker [6,7]. However, there are still controversies on whether BDNF changes might represent the cause or the consequence of psychopathological disorders [2]. To complicate the issue, some authors speculated that BDNF could possibly act as a peripheral transmitter, as it was found in different peripheral organs such as the heart, the gut, the thymus, the spleen and the platelets, albeit its role at this level is still matter of debate [[8], [9], [10], [11]]. Oxytocin is a pleiotropic nonapeptide synthesized in the paraventricular and supraoptic nuclei of the hypothalamus. Besides its classical role in contraction during labor and in milk ejection during nursing [12,13], it is now evident that OT is a modulator of stress and fear responses, social bonding and prosocial emotions, and that it also exerts anti-inflammatory and anti-viral activities [[14], [15], [16], [17], [18], [19]]. Furthermore, OT also seems involved in the pathophysiology of several psychiatric disorders, specifically panic disorder, post-traumatic stress disorder (PTSD), schizophrenia, autism and depression [1,14,20]. Given the importance of those two systems and the shared or overlapped functions, some scholars tried to identify a possible relationship between OT and BDNF. One of the first studies, carried out mainly in animals, demonstrated that OT might increase BDNF and NGF gene expression, as well as their levels in rat hippocampus, while highlighting a potential regulatory role of OT on brain plasticity [21], depending on the developmental stage and the sex [22]. Moreover, the enhancement of BDNF (besides that of aquaporin-4, and of the vascular endothelial growth factor, VEGF) would represent one of the mechanisms supporting the protective role of OT against neuronal damage in the early stages of stroke [23]. A genetic study investigating the BDNF derived from promoter I and II showed that it is highly expressed in the hypothalamus. On the one hand, BDNF loss reduced sexual receptivity and impaired maternal care in female rats, in parallel with a decrease in OT expression over the course of development. On the other hand, it highlighted the contribution to maternal behavior of the BDNF receptor tropomyosin-receptor kinase B (TrkB) signaling in OT neurons [24].
Along this line, recently it has been suggested how the effects of OT on BDNF in the nucleus accumbens might represent one of the underpinnings of individual differences and heterogeneous resilience to the stress of reproduction amongst female mammals [25]. The administration of intranasal OT in a group subjected to an experimental model of chronic stress was found to increase both BDNF and VEGF levels in the hippocampus [26]. Again, OT treatment was shown to increase BDNF levels in the prefrontal cortex of mice of both sexes following maternal deprivation stress [27]. However, the link between neurotrophins and OT is likely more complex than one might hypothesize, since not all studies confirmed the previous findings, as shown in neuroblastoma cell lines widely used in clinical trials [28,29].
The data in humans are still meager. Three genetic studies carried out in adults investigated the DNA methylation of the BDNF and the OT receptor genes in blood cells. A cross-sectional study involving 76 adults, highlighted quantitative DNA methylation before and after an acute psychosocial stress. The authors reported that OT receptor methylation rates were significantly different before and after the acute stress, whereas no significant differences emerged in BDNF methylation rates [30]. In another study, a group of 20 elderly women (≥65 years old) with a positive history of anxiety and/or depression was compared to a similar group of controls. The results showed that the anxious/depressed women showed a significantly higher BDNF DNA methylation rate than the healthy subjects. Again, within this last group, those women who carried a specific OT receptor genotype (namely, the AA genotype) were found to have a greater methylation rate of the sequence [31]. Similarly, higher rates of DNA methylation of BDNF and OT receptor genes in blood cells were described in a cross-sectional study of 89 adults who had previously reported a low maternal care during childhood [32]. More recently, a significant interaction was noted between traumatic experience and epigenetic methylation of BDNF and a possible moderating effect of the OT receptor gene rs53576 in a group of healthy controls, while suggesting that the OT gene may influence epigenetic mechanisms [33].
Recently, Brondino et al. (2018) investigated the role of vasopressin (AVP), BDNF and OT as potential biochemical correlates of subclinical autistic traits in a cohort of healthy young adults. They conducted a multiple regression analysis using the Autism Spectrum Quotient (AQ) score as the dependent variable and age, sex, AVP, BDNF and OT levels as the independent variables. Among the parameters included in the analysis, only BDNF levels were independent predictors of AQ score suggesting that it may be part of a biochemical continuum underlying autistic traits in the general population. However, the correlation calculated between BDNF and OT was not significant (−0.186) [34].
Given the limited information available in humans, the present study aimed to explore the possible existence of a link between OT and BDNF by means of two peripheral markers, the platelet-poor-plasmatic-BDNF and platelet BDNF (PPP-BDNF and PLT-BDNF, respectively) and OT levels, in a group of healthy controls.
2. Material and methods
2.1. Subjects
Twenty-six healthy young (12 men and 14 women, mean age ± SD: 28.5 ± 2.6) volunteers were recruited from March to July 2022 amongst residents, nurses and medical staff attending the Dipartimento di Medicina Clinica e Sperimentale, Section of Psychiatry, University of Pisa, Italy. Three of the total 12 men were single, two married and the remaining had a love relationship. Six women were single, one married and seven had a love relationship (Table 1).
Table 1.
Demographics characteristics of the sample.
| Total sample | Men | Women | |
|---|---|---|---|
| Age | 28.5 ± 3 | 29.9 ± 1.3 | 28.4 ± 2.3 |
| Marital status | |||
| Single | 10 | 3 | 6 |
| Married | 3 | 2 | 1 |
| In love | 13 | 7 | 7 |
| Years of education | 18 + 5 | 17 + 6 | 18 + 9 |
Age and years of education are given as mean ± SD.
Subjects were excluded from the study if they had any history of a chronic disease, current illness, allergy, or regular intake of medications. A one hour-long detailed interview permitted to assess that no subject had a family or personal history of psychiatric disorders. Physical examination and routine laboratory tests were performed and resulted to be within the normative levels. Women were asked about the date of their last menstruation and all agreed to undergo the blood sample in the first and second half of the menstrual cycle (6 were taking contraceptive pills).
All subjects gave their written informed consent prior to enrolment to the study that was approved by the Ethics Committee at the University of Pisa.
2.2. Methods
Fifty ml of peripheral venous blood were drawn from one-night fasting subjects between 8.00 and 9.00 a.m. in order to avoid possible variations related to circadian rhythms. Thirty-five ml of blood for OT assay were collected in vacutainers containing EDTA as anticoagulant, transferred to centrifuge tubes containing aprotinin (Sigma, Milan, Italy) (0.6 TIU/ml of blood) and gently mixed several times to inhibit the activity of proteinases. Blood was then centrifuged at 1,600×g for 15 min at 4 °C and the ensuing plasma was collected and kept at −70 °C until the assay. The remaining 15 ml were collected in vacutainers containing K3-EDTA as anticoagulant, for the determination of PPP- and PLT-BDNF. For this purpose, no longer than 30 min after collection, blood samples were subjected to low-speed centrifuges, 150×g for 15 min at room temperature in order to precipitate erythrocytes and leukocytes. Ensuing supernatants, S1, or the platelet-rich plasma (PRP), were then divided in two 15 ml-Falcon tubes/patient and centrifuged at 1,500×g for 15 min. The resulting two S2 supernatants/patient containing platelet-poor plasma (PPP) and the two P1 pellets/patient containing the whole platelets were taken as separate assay samples: 1) PPP was aliquoted (about 200 μl/tube) in high-quality, low-binding protein Eppendorf test tubes and frozen at −80 °C, until the time of the PPP-BDNF assay; 2) P1 pellets were directly stored at −80 °C, until the analysis of the intra-platelet PLT-BDNF levels.
2.3. BDNF assay
On the day of BDNF assay, the soluble, cytosolic fractions of whole platelet pellets (P1) were prepared. One P1 aliquot/control subject was removed from −80 °C, thawed and immediately placed on ice. Each sample was homogenized by an osmotic-mechanical technique, followed by subcellular fractionation and then suspended in 6 ml of ice-cold lysis buffer solution, 10 mM Tris-HCl, pH = 7.9, containing a mixture of protease inhibitors (Protease Inhibitor Cocktail, Sigma Aldrich, code: P8340), diluted 1: 500 (v/v), and homogenized by sonication on ice for 60” using a cell sonicator (Vibra-Cell Ultrasonic Liquid Processor, Sonics & Materials). The homogenate was subsequently transferred into Eppendorf tubes and centrifuged at 15,000×g for 5 min at 4 °C by a microfuge. The resulting supernatant, or soluble fraction of platelets, was used for the BDNF assay. The assay ranges from 7.8 to 500 pg/ml with intra-assay and inter-assay variations of ≤7.3% and ≤7.6%, respectively, and pro-BDNF cross-reactivity of approximately 5.3%; no cross-reactivity with other tested neurotrophins. BDNF levels below the quantitation limit of 156.25 pg/ml were given a value of 78.125 pg/ml.
2.3.1. Preparation of platelet soluble fractions
The day of the BDNF assay, whole-platelet pellets were thawed and homogenized to obtain platelets’ soluble fractions: platelets were immediately placed on ice and homogenized in 6 ml ice-cold lysis buffer containing protease inhibitors (1:500, v:v), by means of an ultrasound mechanical device, and centrifuged as previously described. The resulting supernatant was used for the PLT-BDNF assay.
2.3.2. BDNF sandwich-ELISA assay
A sandwich-ELISA kit, developed for the determination of the free-mature form of BDNF, was employed (Biosensis, mature BDNF RapidTM, Thebarton, Australia). The day of assay, one aliquot/patient of thawed PPP and freshly prepared platelet soluble fractions were properly diluted in the assay buffer, according to the kit guidelines. After incubation at RT and repeated washing steps, BDNF was revealed by adding a biotinylated secondary anti-BDNF antibody and a horseradish-peroxidase enzyme (HRP) bound to a streptavidin complex. The revelation reaction was started by the addition of tetramethylbenzidine (TMB), a HRP substrate, and halted by a concentrated strong acid. Absorbance was read at 450 nm by the MultiSkan spectrophotometer.
For interpolating BDNF unknowns, a calibration curve was built using a 4 PL nonlinear regression equation, obtaining PPP-BDNF and PLT-BDNF concentrations as ng/ml, after correction for the dilution factor.
Due to the high inter- and intra-individual variability of platelet counts, as well as to take into account possible different yields of platelets and proteins after the centrifugation steps and platelet extraction procedures, intra-platelet BDNF contents (pg/ml) were normalized for the amount of total proteins present in each final platelet soluble fraction. Proteins were measured by Bradford's method using γ-globulins as the standard [35]. This method is also easy to adapt to very low protein concentrations (<25 μg/ml; 1–20 μg total) as well as for rapid determinations in 96-well microplates.
2.3.3. Oxytocin radioimmunoassay
On the day of the assay, 6 ml of each sample of plasma were acidified with 6 ml of buffer A (1% trifluoroacetic acid in H2O) and centrifuged at 17,000×g for 20 min at 4 °C; after this centrifugation, the supernatant was collected. C-18 sep-columns were equilibrated by washing them with 1 ml of buffer B (60% acetonitrile in 1% trifluoroacetic acid) followed by buffer A (3 ml, 3 times). Acidified plasma solution was loaded into the pre-treated C-18 Sep-column; the column was washed slowly with buffer A (3 ml, twice) and the washing liquid was discarded. Oxytocin was then eluted with buffer B (3 ml, once) and collected into a polystyrene tube. The eluate was evaporated in a centrifugal concentrator (Speedvac), and the remaining product was lyophilized by a freeze dryer.
Radioimmunoassay was performed by a Phoenix Pharmaceuticals Oxytocin RIA kit (Belmont, California, USA) according to a method we had previously developed [36]. The cross-reactivity of the OT antibody was 100% with OT and null with Lys-vasopressin, Arg-vasopressin, GH, alpha-ANP, Met-Enkephalin, GRF, somatostatin, TRH, VIP, Pacap 27- NH2. The sensitivity of the assay, measured as IC50, was 10–30 pg/tube. The intra-assay and inter-assay values were 9% and 11%, respectively. Lyophilized samples and standard OT were rehydrated with RIA buffer, and dilutions of standard oxytocin were prepared (from 1 to 128 pg/tube). Primary rabbit anti-OT antibodies were added to each sample and each standard, except for the non-specific binding tubes, and then the mixtures were stored for 24 h at 4 °C. 125I-Oxytocin was added to mixtures which were subsequently stored for 24 h at 4 °C. Goat anti-rabbit IgG serum and normal rabbit serum were added to each tube; subsequently, tubes were centrifuged at 1,700×g for 20 min at 4 °C. All the supernatant was carefully aspirated and pellets were counted by a gamma-counter (Wizard, PerkinElmer, Milan, Italy). All samples were assayed in duplicate. Standard curves and calculations of unknown samples were performed by means of Graphpad Prism3 software.
2.4. Statistical analyses
All demographic, clinical and laboratory data were presented for continuous variable in terms of mean ± standard deviation (SD) and variation range (min and max values), when required. Categorical variables were expressed as frequencies (number) and percentages.
The Kolmogorov-Smirnov test was used to determine normality of distribution of the variables. Oxytocin and PPP-BDNF were normally distributed, while PLT-BDNF data were not. Comparisons for continuous variables were performed with the independent-sample Student's t-test for variables that follow a normal distribution, Wilcoxon-Mann-Whitney test for variables not normally distributed.
The correlations between biological markers were explored by calculating the Pearson's correlation coefficient or Spearman's rank correlation. Pearson's r correlation is used to measure the degree of the relationship between linearly related variables. For the Pearson's r correlation, both variables should be normally distributed (normally distributed variables have a bell-shaped curve). Other assumptions include linearity and homoscedasticity. Linearity assumes a straight-line relationship between each of the two variables and homoscedasticity assumes that data is equally distributed about the regression line. Spearman's rank correlation is a non-parametric test that is used to measure the degree of association between two variables. The Spearman rank correlation test does not carry any assumptions about the distribution of the data and is the appropriate correlation analysis when the variables are measured on a scale that is at least ordinal. The assumptions of the Spearman correlation are that data must be at least ordinal and the scores on one variable must be monotonically related to the other variable.
The major limitation of the present study is the low number of subjects selected. Therefore, since the high possibility of both type-I and type-II errors our results should be considered preliminary. In this study, p values lower than .05 were considered statistically significant.
Statistical analyses were performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA).
For the calculation of the calibration curves and the "best-fit" regression analysis for each quantitative assay used in the study, the GraphPad Prism® software was used (Version 7.0.2, San Diego, USA).
3. Results
The results showed that PLT-BDNF (pg/mg proteins, mean ± SD) and PPP-BDNF (pg/ml, mean ± SD) were, respectively, 1546.03 ± 1844.28 vs 10110.78 ± 1892.01. The OT levels (pg/ml, mean ± SD) were 13.92 + 4.54 (Table 2, panel a).
Table 2.
Oxytocin (OT, pg/ml), platelet BDNF (PLT-BDNF, pg/mg proteins) and plasma BDNF (PPP-BDNF, pg/ml) levels in the total sample (panel a) and according to sex (panel b) with statistical analyses (Student's t-test or Mann-Whitney test*).
| Panel a. | |||||
|---|---|---|---|---|---|
| N | Min | Max | Mean | Std. Deviation | |
| OT | 26 | 6.70 | 22.40 | 13.92 | 4.54 |
| PLT-BDNF | 26 | 142.88 | 9372.27 | 1546.03 | 1844.28 |
| PPP-BDNF | 26 | 6854.75 | 13780.07 | 10110.78 | 1892.01 |
| Panel b. | ||||||
|---|---|---|---|---|---|---|
| Gender | N | Mean value | Std. Deviation | t/Z | p | |
| OT | Men | 12 | 12.02 | 4.36 | 2.106 | 0.0046 |
| Women | 14 | 15.55 | 4.17 | |||
| PPP-BDNF | Men | 12 | 10849.50 | 1932.12 | 1.943 | 0.64 |
| Women | 14 | 9477.59 | 1670.27 | |||
| PLT-BDNF* | Men | 12 | 2052.12 | 2516.85 | 1.132 | . 258 |
| Women | 14 | 1112.21 | 861.42 | |||
No sex-related differences were noted in both PPP-BDNF and PLT-BDN, while OT levels were higher in women than in men (15.55 ± 4.1 vs 12.02 ± 4.36, p = 0.0046) (Table 2, panel b).
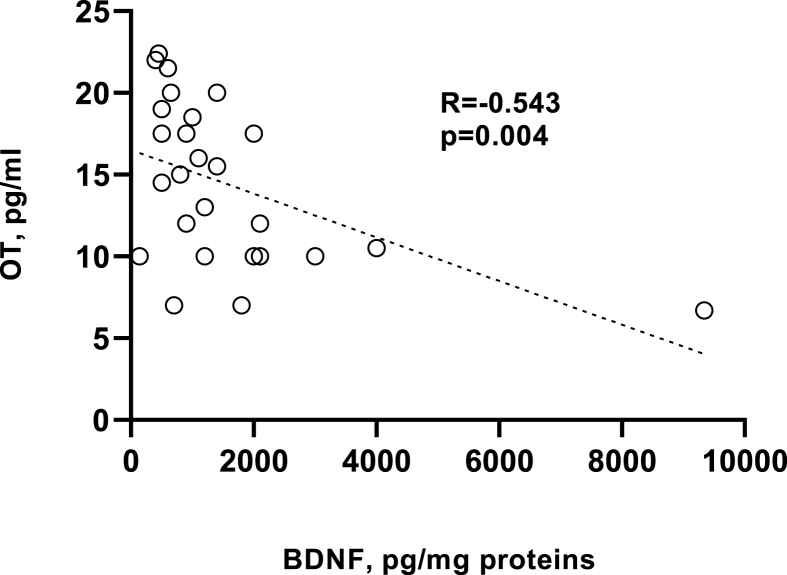
The Spearman's analyses revealed a statistically significant and negative correlation between OT levels and PLT-BDNF (R = −0.543, p = 0.004) (Fig. 1).
Fig. 1.
Correlation between oxytocin (OT, pg/mL) levels and platelet BDNF (PLT-BDNF, pg/mg proteins) by means of the Spearman's analyses (R = −0.543, p = 0.004). The dotted line is a graphical representation of the correlation trend, obtained from the best linear fit of data.
4. Discussion
To our knowledge, this is one of the first studies carried out in healthy subjects exploring the possible correlations between peripheral BDNF and OT levels. The BDNF was assayed in both plasma and platelets. The values we observed are consistent with those reported in the literature, with BDNF being 10-fold higher in platelets than in plasma [9]. Indeed, PLT-BDNF represents the most relevant peripheral component of this neurotrophin, since more than 90% of circulating BDNF is stored in platelets and their α-granules [37,38]. A close relationship has been indeed found between BDNF and platelets under physiological conditions. It has been demonstrated that the amount of BDNF in serum is nearly identical to the amount of BDNF found in washed platelet lysates [37], while there is an approximately 100- to 200-fold estimated difference between the plasma and serum levels of BDNF, as platelets release BDNF during the clotting process [9]. Therefore the difference between the serum and the plasma BDNF levels seems to reflect the amount of BDNF stored in circulating platelets, and the BDNF in platelets might serve as a reservoir for circulating BDNF [39].
The OT levels were also consistent with previous data [40]. In addition, present findings support our previous observation, in a larger sample, of statistically significant higher OT levels in women than in men [40].
More interestingly, the present study revealed the presence of a significant and negative correlation between PLT-BDNF and OT levels, that is to say, the lower the neurotrophin the higher the neuropeptide and vice versa. According to our knowledge, this is the first study to report this kind of correlation in healthy human subjects, as previously, mainly observations in animals or genetic studies in humans reported a positive association or no correlation between BDNF and OT [21,26,27,34]. Indeed, it should be underlined that this was also our original hypothesis, given available data highlighting overlapping involvement of both BDNF and OT in neuroplasticity, stress response and inflammation. Preclinical studies suggest that reduced BDNF activity might increase apoptosis and decreased hippocampal neurogenesis that might be associated with chronic mild stress typical of depression [41]. Furthermore, several studies showed that depressed patients have lower BDNF plasma levels than controls [[42], [43], [44], [45]]. Although decreased plasma BDNF was proposed as a potential diagnostic, prognostic and therapeutic biomarker of mood disorders, particularly associated with disease severity and response to antidepressant (AD) treatments [46], this matter still remains controversial, More correctly, the impaired BDNF signaling might be interpreted as a vulnerability factor towards development of mood disorders as it impacts neuroplasticity, inflammation patterns or hypothalamic–pituitary–adrenal axis /HPA) action [47].
Some activities of OT overlap with those of BDNF. It is now evident that OT acts as a potent modulator of the immune system, even during development, and it plays a prominent anti-inflammatory role in the context of immune system function and homeostatic cellular processes. Many OT anti-inflammatory effects appear to be, at least in part, exerted through the attenuation of inflammatory processes mediated by microglia and macrophages [[48], [49], [50], [51]]. Oxytocin is released during the stress response and it is an important modulator of anxiety and fear response, mainly with anxiolytic effects [36,[52], [53], [54]]. Elevated plasma OT was significantly associated with gaps in social relationships, with less positive relationships with a primary partner, with anxiety due to a romantic relationship [36] or anxiety traits in women who are in love [36], as well as with elevated cortisol levels [55]. Therefore, given its particular modulatory functions, OT is supposed to link social behaviors and experiences together with the capacity to heal in the face of stress or trauma.
However, emerging evidence seems to broaden the spectrum of activities of both BDNF and OT. Indeed, it is increasingly reported that psychedelic drugs may exert some of their long-lasting therapeutic effects by inducing structural and functional neural plasticity, and that both ADs and LSD or psilocin bind the TrkB [56,57]. As far as OT is concerned, some scholars for the first time demonstrated that patients with central diabetes insipidus, after a pharmacological challenge with 3,4-methylenedioxymethamphetamine (MDMA, also known as "ecstasy") to provoke OT release, showed a small increase in plasma OT, a condition named “OT deficiency” [58.].
Obviously, these are preliminary, albeit intriguing data that might open new horizons to both research and therapeutic strategies.
The findings of the present study would indicate that the PLT-BDNF and OT are negatively related: this reciprocal influence might have a downstream homeostatic function dampening one activity when the other is activated or vice versa. This would be true if both PLT-BDNF and OT would exert their functions on the same targets, such as those at the level of the immune system or of the HPA axis that would be shared or complementary. Alternatively, the situation in both healthy and pathological conditions might be different and the relations between OT and BDNF might also diverge, when necessary. Interestingly, in a recent study BDNF serum levels were decreased in malnourished patients with anorexia nervosa and normalized after partial weight recovery, while OT levels were basically increased and did not change [59]. Again some authors investigated the correlation between plasma BDNF and OT and autistic traits in the general population, without detecting any relationship between the two biomarkers [60].
The present study suffers from some limitations that should be acknowledged. First, the sample size was small, however it included a similar number of healthy men and women of almost the same age who had a similar lifestyle. Second, it is still unclear how peripheral BDNF and OT might reflect those present in the CNS. If it is evident that the relationships between neuronal BDNF and its peripheral counterpart still require further investigation, the link between neuroendocrine systems, HPA axis and the immune/inflammatory response can be a key aspect to understand the regulation of BDNF release and storage into the bloodstream. This hypothesis deserves confirmation, in order to clarify if the BDNF platelet reservoir and BDNF extracellular levels might reflect, at different levels, the functionality of brain-periphery cross-talks activities in adaptation to stressors. Although controversies do exist, neurotrophins can cross the blood-brain barrier, and the CNS and serum levels of these proteins have been found to be positively interrelated [61]. Moreover, it seems that peripheral and central OT are synthesized by different cells [62], although parallel changes in OT levels have been observed in plasma and in the brain supporting the use of the peripheral OT as a biomarker of that present in the CNS [63,64].
5. Conclusions
To sum up, our study indicates that the PLT-BDNF and OT are negatively related, at least in healthy controls of both sexes and with similar lifestyles.
This relationship between OT and neurotrophins might hypothetically represent one of the components of the reparative, antistress, prosocial and promoting sense of safety activities of OT. Therefore, it would be interesting to investigate whether the observed relationship is present or reverted in different stressful situations or psychopathological conditions requiring a cooperative rather than a different activity. Currently, we are assessing BDNF, OT and a battery of personality, temperament questionnaires and rating scales in healthy subjects and in some psychiatric conditions to investigate if the combinations of the two biomarkers might be related to specific individual traits, and psychological or psychopathologic dimensions.
Declaration of competing interest
None.
References
- 1.Marazziti D., Catena Dell'Osso M. The role of oxytocin in neuropsychiatric disorders. Curr. Med. Chem. 2008;15:698–704. doi: 10.2174/092986708783885291. [DOI] [PubMed] [Google Scholar]
- 2.Lima Giacobbo B., Doorduin J., Klein H.C., Dierckx R.A.J.O., Bromberg E., de Vries E.F.J. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 4.Bramham C.R., Panja D. BDNF regulation of synaptic structure, function, and plasticity. Neuropharmacology. 2014;76:601–602. doi: 10.1016/j.neuropharm.2013.08.012. Pt C. [DOI] [PubMed] [Google Scholar]
- 5.Edelmann E., Lessmann V., Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology. 2014;76 Pt C:610–627. doi: 10.1016/j.neuropharm.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 6.Autry A.E., Monteggia L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Jiang H., Liu Y., Hou Z., Yue Y., Zhang Y., Zhao F., Xu Z., Li Y., Mou X., Li L., Wang T., Zhao J., Han C., Sui Y., Wang M., Yang Z., Lu Y., Zhu Y., Li J., Shen X., Sun F., Chen Q., Chen H., Yuan Y. Combined serum levels of multiple proteins in tPA-BDNF pathway may aid the diagnosis of five mental disorders. Sci. Rep. 2017;7:6871. doi: 10.1038/s41598-017-06832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lommatzsch M., Braun A., Mannsfeldt A., Botchkarev V.A., Botchkareva N.V., Paus R., Fischer A., Lewin G.R., Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived neurotrophic functions. Am. J. Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., Virchow J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Raznahan A., Toro R., Proitsi P., Powell J., Paus T., Bolton P.F., Murphy D.G. A functional polymorphism of the brain-derived neurotrophic factor gene and cortical anatomy in autism spectrum disorder. J. Neurodev. Disord. 2009;1:215–223. doi: 10.1007/s11689-009-9012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakerley J.B., Lincoln D.W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J. Endocrinol. 1973;57:477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- 13.Windle R.J., Forsling M.L. Renal responses to oxytocin during the 4 days of the oestrous cycle in the rat. J. Endocrinol. 1997;154:347–353. doi: 10.1677/joe.0.1540347. [DOI] [PubMed] [Google Scholar]
- 14.Amico J.A., Mantella R.C., Vollmerm R.R., Li X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- 15.Keverne E.B., Curley J.P. Vasopressin, oxytocin and social behaviour. Curr. Opin. Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Young LJ L.J., Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 17.Ebner K., Bosch O.J., Krömer S.A., Singewald N., Neumann I.D. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- 18.Kemp A.H., Guastella A.J. Oxytocin: prosocial behavior, social salience, or approach-related behavior? Biol. Psychiatr. 2010;67 doi: 10.1016/j.biopsych.2009.11.019. e33–35. [DOI] [PubMed] [Google Scholar]
- 19.Carter C.S. Oxytocin, love and the COVID-19 crisis. Clin. Neuropsychiatr. 2020;17:195. [PMC free article] [PubMed] [Google Scholar]
- 20.Scantamburlo G., Hansenne M., Fuchs S., Pitchot W., Maréchal P., Pequeux C., Ansseau M., Legros J.J. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Havranek T., Zatkova M., Lestanova Z., Bacova Z., Mravec B., Hodosy J., Strbak V., Bakos J. Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J. Neurosci. Res. 2015;93:893–901. doi: 10.1002/jnr.23559. [DOI] [PubMed] [Google Scholar]
- 22.Bakos J., Lestanova Z., Strbak V., Havranek T., Bacova Z. Neonatal manipulation of oxytocin prevents lipopolysaccharide-induced decrease in gene expression of growth factors in two developmental stages of the female rat. Neuropeptides. 2014;48:281–286. doi: 10.1016/j.npep.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Momenabadi S., Vafaei A.A., Bandegi A.R., Zahedi-Khorasani M., Mazaheri Z., Vakili A. Oxytocin reduces brain injury and maintains blood-brain barrier integrity after ischemic stroke in mice. NeuroMolecular Med. 2020;22:557–571. doi: 10.1007/s12017-020-08613-3. [DOI] [PubMed] [Google Scholar]
- 24.Maynard K.R., Hobbs J.W., Phan B.N., Gupta A., Rajpurohit S., Williams C., Rajpurohit A., Shin J.H., Jaffe A.E., Martinowich K. BDNF-TrkB signaling in oxytocin neurons contributes to maternal behavior. Elife. 2018;7 doi: 10.7554/eLife.33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T.Y., Shahrokh D., Hellstrom I.C., Wen X., Diorio J., Breuillaud L., Caldji C., Meaney M.J. Brain-derived neurotrophic factor in the nucleus accumbens mediates individual differences in behavioral responses to a natural, social reward. Mol. Neurobiol. 2020;57:290–301. doi: 10.1007/s12035-019-01699-2. [DOI] [PubMed] [Google Scholar]
- 26.Dayi A., Cetin F., Sisman A.R., Aksu I., Tas A., Gönenc S., Uysal N. The effects of oxytocin on cognitive defect caused by chronic restraint stress applied to adolescent rats and on hippocampal VEGF and BDNF levels. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015;21:69–75. doi: 10.12659/MSM.893159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dayi A., Kiray M., Sisman A., Ozbal S., Baykara B., Aksu I., Uysal N. Dose dependent effects of oxytocin on cognitive defects and anxiety disorders in adult rats following acute infantile maternal deprivation stress. Biotech. Histochem. 2019;94:469–480. doi: 10.1080/10520295.2018.1528384. [DOI] [PubMed] [Google Scholar]
- 28.Bakos J., Strbak V., Ratulovska N., Bacova Z. Effect of oxytocin on neuroblastoma cell viability and growth. Cell. Mol. Neurobiol. 2012;32:891–896. doi: 10.1007/s10571-012-9799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakos J., Strbak V., Paulikova H., Krajnakova L., Lestanova Z., Bacova Z. Oxytocin receptor ligands induce changes in cytoskeleton in neuroblastoma cells. J. Mol. Neurosci. 2013;50:462–468. doi: 10.1007/s12031-013-9960-4. [DOI] [PubMed] [Google Scholar]
- 30.Unternaehrer E., Luers P., Mill J., Dempster E., Meyer A.H., Staehli S., Lieb R., Hellhammer D.H., Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress, Transl. Psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chagnon Y.C., Potvin O., Hudon C., Préville M. DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. Front. Genet. 2015;6:230. doi: 10.3389/fgene.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unternaehrer E E., Meyer A.H., Burkhardt S.C., Dempster E., Staehli S., Theill N., Lieb R., Meinlschmidt G. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015;18:451–461. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- 33.Lee H.S., Kwon A., Lee S.H. Oxytocin receptor genes moderate BDNF epigenetic methylation by childhood trauma. J. Affect. Disord. 2022;306:167–173. doi: 10.1016/j.jad.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 34.[a] Minutillo A., Panza G., Mauri M.C. Musical practice and BDNF plasma levels as a potential marker of synaptic plasticity: an instrument of rehabilitative processes. Neurol. Sci. 2021;42:1861–1867. doi: 10.1007/s10072-020-04715-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; [b] Brondino N., Fusar-Poli L., Rocchetti M., Bertoglio F., Bloise N., Visai L., Politi P. BDNF levels are associated with autistic traits in the general population. Psychoneuroendocrinology. 2018;89:131–133. doi: 10.1016/j.psyneuen.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 36.Marazziti D D., Dell'Osso B., Baroni S., Mungai F., Catena M., Rucci P., Albanese F., Giannaccini G., Betti L., Fabbrini L., Italiani P., Del Debbio A., Lucacchini A., Dell'Osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin. Pract. Epidemiol. Ment. Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimura H., Altar C.A., Chen R., Nakamura T., Nakahashi T., Kambayashi J., Sun B., Tandon N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation, Thromb. Haemostasis. 2002;87:728–734. doi: 10.1055/s-0037-1613072. [DOI] [PubMed] [Google Scholar]
- 38.Bus B.A., Molendijk M.L., Penninx B.J., Buitelaar J.K., Kenis G., Prickaerts J., Elzinga B.M., Voshaar R.C. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Radka S.F., Holst P.A., Fritsche M., Altar C.A. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–301. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- 40.Marazziti D., Baroni S., Mucci F., Piccinni A., Moroni I., Giannaccini G., Carmassi C., Massimetti E., Dell'Osso L. Sex-related differences in plasma oxytocin levels in humans. Clin. Pract. Epidemiol. Ment. Health. 2019;15:58–63. doi: 10.2174/1745017901915010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H., Chen Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dell'Osso L., Del Debbio A., Veltri A., Bianchi C., Roncaglia I., Carlini M., Massimetti G., Catena Dell'Osso M., Vizzaccaro C., Marazziti D., Piccinni A. Associations between brain-derived neurotrophic factor plasma levels and severity of the illness, recurrence and symptoms in depressed patients. Neuropsychobiology. 2010;62:207–212. doi: 10.1159/000319946. [DOI] [PubMed] [Google Scholar]
- 43.Klein A.B., Williamson R., Santini M.A., Clemmensen C., Ettrup A., Rios M., Knudsen G.M., Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 44.Molendijk M.L., Spinhoven P., Polak M., Bus B.A., Penninx B.W., Elzinga B.M. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol. Psychiatr. 2014;19:791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 45.Rana T., Behl T., Sehgal A., Srivastava P., Bungau S. Unfolding the role of BDNF as a biomarker for treatment of depression. J. Mol. Neurosci. 2021;71:2008–2021. doi: 10.1007/s12031-020-01754-x. [DOI] [PubMed] [Google Scholar]
- 46.Dimitriadis M., van den Brink R.H.S., Comijs H.C., Oude Voshaar R.C. Prognostic effect of serum BDNF levels in late-life depression: moderated by childhood trauma and SSRI usage? Psychoneuroendocrinology. 2019;103:276–283. doi: 10.1016/j.psyneuen.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Cattaneo A., Cattane N., Begni V., Pariante C.M., Riva M.A. The human BDNF gene: peripheral gene expression and protein levels as biomarkers for psychiatric disorders, Transl. Psychiatry. 2016;6:e958. doi: 10.1038/tp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karelina K., Stuller K.A., Jarrett B., Zhang N., Wells J., Norman G.J., De Vries A.C. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42:3606–3611. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szeto A., Sun-Suslow N., Mendez A.J., Hernandez R.I., Wagner K.V., McCabe P.M. Regulation of the macrophage oxytocin receptor in response to inflammation. Am. J. Physiol. Endocrinol. Metab. 2017;312:E183–E189. doi: 10.1152/ajpendo.00346.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mairesse J., Zinni M., Pansiot J., Hassan-Abdi R., Demene C., Colella M., Charriaut-MaraMarlangue C., Rideau Batista Novais A., Tanter M., Maccari S., Gressens P., Vaiman D., Soussi-Yanicostas N., Baud O. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia. 2019;67:345–359. doi: 10.1002/glia.23546. [DOI] [PubMed] [Google Scholar]
- 51.Smith C.J., Bilbo S.D. Sickness and the social brain: love in the time of COVID. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.633664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy M.M., McDonald C.H., Brooks P.J., Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 53.Uvnas-Moberg K., Petersson M. Oxytocin, ein Vermittler von Antistress, Wohlbefinden, sozialer Interaktion, Wachstum und Heilung [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing] Z. Psychosom. Med. Psychother. 2005;51:57–80. doi: 10.13109/zptm.2005.51.1.57. [DOI] [PubMed] [Google Scholar]
- 54.Carter C.S. The role of oxytocin and vasopressin in attachment, Psychodyn. Psychiatry. 2017;45:499–517. doi: 10.1521/pdps.2017.45.4.499. [DOI] [PubMed] [Google Scholar]
- 55.Taylor S.E., Gonzaga G.C., Klein L.C., Hu P., Greendale G.A., Seeman T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- 56.Grieco S.F., Castren E., Knudsen G.M., Kwan A.C., Olson D.E., Zuo Y., Holmes T.C., Xu X. Psychedelics and neural plasticity: therapeutic implications. J. Neurosci. 2022;42(45):8439–8449. doi: 10.1523/JNEUROSCI.1121-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moliner R R., Girych M., Brunello C.A., et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 2023;26:1032–1041. doi: 10.1038/s41593-023-01316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atila C., Holze F., Murugesu R., Rommers N., Hutter N., Varghese N., O'Sailer C., Eckert A., Heinrichs M., Liechti M.E., Christ-Crain M. Oxytocin in response to MDMA provocation test in patients with arginine vasopressin deficiency (central diabetes insipidus): a single-centre, case-control study with nested, randomised, double-blind, placebo-controlled crossover trial. Lancet Diabetes Endocrinol. 2023;11 doi: 10.1016/S2213-8587(23)00120-1. 454-446. [DOI] [PubMed] [Google Scholar]
- 59.Tyszkiewicz-Nwafor M., Rybakowski F., Dmitrzak-Weglarz M., Skibinska M., Paszynska E., Dutkiewicz A., Słopien A. Brain-derived neurotrophic factor and oxytocin signaling in association with clinical symptoms in adolescent inpatients with anorexia nervosa - a longitudinal study. Front. Psychiatr. 2020;28:1032. doi: 10.3389/fpsyt.2019.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brondino N., Fusar-Poli L., Rocchetti M M., Bertoglio F., Bloise N., Visai L., P Politi P. BDNF levels are associated with autistic traits in the general population. Psychoneuroendocrinology. 2018;89:131–133. doi: 10.1016/j.psyneuen.2018.01.008. 2018. [DOI] [PubMed] [Google Scholar]
- 61.Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 62.Uvnås-Moberg K., Widstrom A.M., Nissen E., Björvell H. Personality traits in women 4 days postpartum and their correlation with plasma levels of oxytocin and prolactin. J. Psychosom. Obstet. Gynaecol. 1990;11:261–273. doi: 10.3109/01674829009084422. [DOI] [Google Scholar]
- 63.Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R., Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 64.Marazziti D., Baroni S., Giannaccini G., Betti L., Massimetti G., Carmassi C., Catena-Dell'Osso M. A link between oxytocin and serotonin in humans: supporting evidence from peripheral markers. Eur. Neuropsychopharmacol. 2012;22:578–583. doi: 10.1016/j.euroneuro.2011.12.010. [DOI] [PubMed] [Google Scholar]