Abstract
Background and Objectives
The cerebello-thalamo-cortical circuit plays a critical role in essential tremor (ET). However, abnormalities have been reported in multiple brain regions outside this circuit, leading to inconsistent characterization of ET pathophysiology. Here, we test whether these mixed findings in ET localize to a common functional network and whether this network has therapeutic relevance.
Methods
We conducted a systematic literature search to identify studies reporting structural or metabolic brain abnormalities in ET. We then used ‘coordinate network mapping,’ which leverages a normative connectome (n = 1,000) of resting-state fMRI data to identify regions commonly connected to findings across all studies. To assess whether these regions may be relevant for the treatment of ET, we compared our network with a therapeutic network derived from lesions that relieved ET. Finally, we investigated whether the functional connectivity of this ET symptom network is abnormal in an independent cohort of patients with ET as compared with healthy controls.
Results
Structural and metabolic brain abnormalities in ET were located in heterogeneous regions throughout the brain. However, these coordinates were connected to a common functional brain network, including the cerebellum, thalamus, motor cortex, precuneus, inferior parietal lobe, and insula. The cerebellum was identified as the hub of this network because it was the only brain region that was both functionally connected to the findings of over 90% of studies and significantly different in connectivity compared with a control data set of other movement disorders. This network was strikingly similar to the therapeutic network derived from lesions improving ET, with key regions aligning in the thalamus and cerebellum. Furthermore, positive functional connectivity between the cerebellar network hub and the sensorimotor cortices was significantly reduced in patients with ET compared with healthy controls, and connectivity within this network was correlated with tremor severity and cognitive functioning.
Discussion
These findings suggest that the cerebellum is the central hub of a network commonly connected to structural and metabolic abnormalities in ET. This network may have therapeutic utility in refining and informing new targets for neuromodulation of ET.
Introduction
Essential tremor (ET) is the most common movement disorder in adults and is increasing in prevalence with the aging population.1 The cardinal symptoms of ET are postural and kinetic tremor which predominantly occur in the upper limbs; however, patients may also have additional soft signs and symptoms, such as neuropsychiatric symptoms and cognitive impairments.2,3
It is widely accepted that the cerebello-thalamo-cortical circuit is involved in ET.2,4 Abnormality of this circuit is evidenced by postmortem,5 functional and structural neuroimaging,4,6 and electrophysiologic studies.7 For example, postmortem studies have shown structural changes of Purkinje cells8 and reduced GABA receptors in the cerebellum of patients with ET9 while neuroimaging studies have also shown cerebellar atrophy (for a review see reference 6). In addition, this circuit is causally implicated in ET because deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus (VIM) and zona inserta effectively alleviates symptoms.10 Noninvasive brain stimulation to the motor cortex also elicits some reduction of tremor symptoms, and corticomuscular coherence has demonstrated the intermittent involvement of the motor cortex in tremor.11
Despite many structural neuroimaging studies implicating regions of the cerebello-thalamo-cortical circuit in ET, meta-analyses of these studies seldom converge toward a set of consistent reliable findings and continue to implicate brain regions outside this circuit.12,13 For example, a meta-analysis of gray matter alterations in ET showed significant heterogeneity between studies, and no areas were deemed reliable.12 In addition, Han et al.13 found consistent gray matter alteration in the precuneus of patients with ET in their meta-analysis of voxel-based morphometry studies; however, they showed no consistent abnormalities in the cerebello-thalamo-cortical circuit. Furthermore, many neuroimaging studies have found alterations in various areas outside this circuit, including frontal, visual, and parietal regions.14-16 Such inconsistent evidence has hampered our understanding of the brain regions primarily involved in ET.
Lack of replicability within ET literature has been attributed to clinical heterogeneity in patients with ET and varying methodologies used across studies.2,12 However, an alternative explanation is that the dispersed regions of abnormality in ET are part of a wider functional network, which includes regions both within and outside the cerebello-thalamo-cortical circuit. Therefore, although these neuroimaging findings appear inconsistent, they may simply be localizing different brain regions that form a broader functionally connected network.17
Growing research has demonstrated that many disorders map to broad brain networks more than specific regions.18 Recently, Joutsa et al.19 used lesion network mapping to investigate the functional connectivity of dispersed brain lesions that relieved ET symptoms. All lesion locations were functionally connected to the cerebellum and thalamus, thus revealing a common network mediating tremor relief defined by connectivity to key hubs in these regions.19 This finding suggests that ET may involve a widespread network; however, it remains unknown whether this network mediating tremor relief is the same network underlying ET symptoms. Furthermore, the symptom network cannot be delineated using lesion network mapping because ET, by definition, cannot be caused by lesions.
Thus, recent studies of idiopathic disorders have used a similar method termed ‘coordinate network mapping’ (CNM)17 to map functional connectivity from locations of brain abnormality observed in neurologic disorders to localize the common underlying network.20,21 CNM has successfully mapped the functional networks of Alzheimer disease,17 Parkinson disease dementia,21 and migraine.20
Therefore, the aim of this study was to use structural and metabolic neuroimaging findings to localize the network underlying ET symptoms. First, we will use traditional activation likelihood estimation (ALE) meta-analytic methods to assess whether these findings consistently occur in discrete brain regions; then, we will apply CNM to test whether these findings better localize to a common functional network with connectivity to key network hubs. Next, we will compare the identified ET symptom network to the network mediating tremor relief.19 Finally, we will investigate whether functional connectivity within this ET symptom network is abnormal in an independent cohort of patients with ET and assess whether the dysfunction of this network is related to tremor and cognitive symptoms.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This project used only preexisting, deidentified data and was, therefore, deemed exempt from an ethical review by the Deakin University Human Research Ethics Committee.
Study Selection
A systematic search in the MEDLINE and Embase databases was performed in March 2022. Searches were conducted using search terms relating to ET and structural or metabolic neuroimaging (See eAppendix 1, links.lww.com/WNL/D55 for exact search syntax). The inclusion criteria were as follows: (1) coordinates of significant differences between patients with ET and healthy controls were reported in publications or were provided by the author on request; (2) coordinates of brain abnormalities were identified using T1-weighted MRI, PET, or SPECT at rest; and (3) whole-brain analysis (not region-of-interest analysis) was conducted. Coordinates included in this study were based on the thresholding used in the original publication.
Exclusion criteria were (1) participants had received surgical treatment for ET, (2) use of tracers that do not allow whole-brain analyses (e.g., 123I-FP-CIT SPECT with specific uptake only in the striatum and certain extrastriatal regions), and (3) studies not available in English. If multiple studies evaluated the same participants, only one study was included.
Activation Likelihood Estimation Meta-analysis
We performed an ALE meta-analysis of neuroimaging findings in ET using GingerALE 3.0.2. This analysis was conducted using validated methods22 to identify spatial convergence of foci from different studies (eAppendix 2, links.lww.com/WNL/D55).
Coordinate Network Mapping
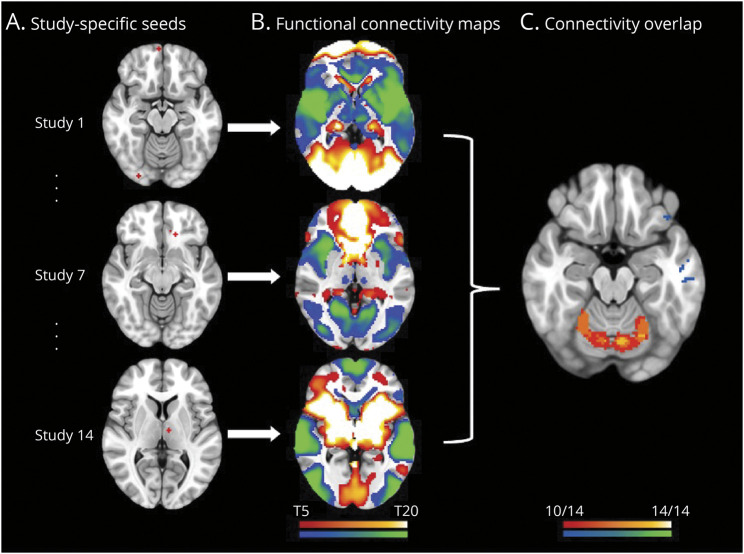
CNM was used to assess whether brain abnormalities in ET map to a functionally connected brain network, rather than to particular brain regions.17 First, coordinates reported in the included studies were converted to MNI152 space, and 4-mm spherical seeds were created on a standard brain template for each MNI coordinate. These seeds were then combined into a single map for each study (referred to as a ‘study-specific seed’ hereafter; Figure 1A). Second, a normative connectome of resting-state fMRI (rs-fMRI) scans from 1,000 healthy adults23 was used to identify the brain regions that are functionally connected to each study-specific seed (Figure 1B). Separate positive and negative functional connectivity maps were generated for each study-specific seed. Third, these connectivity maps were thresholded at t ≥ ± 7, corresponding to whole-brain family-wise error (FWE) corrected p < 10−6,20,21,24 and binarised. To ensure that our results were not driven by thresholds, we also generated maps thresholded at t ≥ ± 5 and t ≥ ± 9. The binarised functional connectivity maps were then overlaid to identify the brain regions that are significantly functionally connected to all, or most, of the study-specific seeds17 (Figure 1C; see eAppendix 3, eFigure 1, links.lww.com/WNL/D55 for threshold selection).
Figure 1. Coordinate Network Mapping Method.
(A) Study-specific seeds: 4-mm seeds were created at every coordinate reported as significantly abnormal in essential tremor for each included study. (B) Functional connectivity maps: Maps of voxels that were functionally connected to the study-specific seed were generated using a normative human connectome (n = 1,000). (C) Connectivity overlap: Each study's functional connectivity map was thresholded, binarised, and overlaid to identify the regions that are functionally connected with the most study-specific seeds. Cool colors = negative connectivity; warm colors = positive connectivity.
Relevance to Tremor Relief Networks
It is unknown whether the network mediating ET symptom improvement, previously defined using lesions relieving tremor,19 is the same network that is driving ET symptoms. Therefore, we investigated the similarity of these networks visually, and also statistically by assessing the spatial correlations between the functional connectivity maps of our CNM and the lesion network mapping analysis.19,25 Both the CNM and lesion network mapping analyses generate functional connectivity maps of t-values, which represent the degree of functional connectivity to each voxel in the brain from the study-specific seed or lesion, respectively (See CNM methods above; Figure 1B). Therefore, spatial correlations were run between each study-specific seed functional connectivity map and each lesion functional connectivity map to generate an average Pearson r value for each pair of functional connectivity maps.25 An ANOVA with planned contrasts evaluated whether the spatial correlation between our ET CNM network and the ET lesion network19 was significantly greater (i.e., the networks were more similar) than the spatial correlations between our ET network and lesion networks of a data set of control disorders. To avoid bias in control cohort selection, this data set included all other movement disorders on which our laboratory has performed lesion network mapping: Holmes tremor (n = 36),26 cervical dystonia (n = 25),24 tics (n = 19), and parkinsonism (n = 29).27 Significance level was set at p < 0.05.
Hub of the Essential Tremor Network
Our CNM analysis revealed a brain network sensitive to ET by mapping functional connectivity from brain abnormalities found in patients with ET. In addition to this, we sought to define a brain network specific to ET, by identifying connectivity patterns that were unique to ET compared with other movement disorders. To do this, we compared our CNM results with a control data set comprising all CNM analyses that our laboratory has conducted in other movement disorders, namely cervical dystonia, Tourette syndrome, and parkinsonian disorders. An ANOVA with planned contrasts was used to identify voxels at which functional connectivity from ET study-specific seeds were significantly different from that of the control disorders.26 Threshold-free cluster enhancement was used to correct for multiple comparisons (using FSL Randomise), with the significance level set at p < 0.05 (corrected).28
Next, to identify the brain regions that were both sensitive and specific to ET, we performed a conjunction analysis of the above maps resulting from the CNM and specificity analyses. This conjunction identified regions comprising voxels that were both connected to over 90% of study-specific seeds20 and specifically connected to ET compared with other movement disorders. The voxels surviving both analyses were defined as the ‘hub’ of our ET network.
Validation in an Independent Cohort of Patients With ET
The above analyses identified the hub of the ET network, which was then used in a seed-to-voxel analysis to test whether the functional connectivity of this network hub was abnormal in an independent data set of patients with ET. This data set comprised 48 patients with ET and 49 healthy controls, collated from 2 published rs-fMRI studies.16,29 All images were preprocessed and analyzed using CONN, Statistical Parametric Mapping toolbox (SPM12) and Matlab (version R2020b) (preprocessing methods are detailed in eAppendix 4, links.lww.com/WNL/D55).
Functional connectivity from the network hub to the rest of the voxels in the brain was compared between ET and healthy controls using a nonparametric permutation-based inference with threshold-free cluster enhancement. ‘Scanning site’ was entered as a covariate of no interest to control for any differences across study sites. Clusters with a FWE rate corrected p < 0.05 were considered significant.
To assess whether significant group differences in functional connectivity were exclusive to our ET network hub, we performed a control analysis that repeated the seed-to-voxel analysis using hubs derived from other disorders analyzed with CNM by our laboratory (i.e., cervical dystonia, Tourette syndrome, parkinsonian disorders).
The seed-to-voxel analysis revealed a significant group difference in functional connectivity from the ET network hub to bilateral clusters in the sensorimotor cortices. To assess whether this abnormal functional connectivity correlated with clinical scores in these patients with ET, the functional connectivity values between the ET network hub and the bilateral sensorimotor cluster in patients with ET were calculated in CONN, and then extracted. Correlations were then conducted in Jamovi (version 2.3.13.0) between these values and the clinical scores of patients with ET, including tremor severity (Fahn-Tolosa-Marin tremor rating scale; FTM), cognitive functioning (Frontal assessment battery; FAB), patient age, and disease duration (see eAppendix 5, links.lww.com/WNL/D55 for details).
The ET network hub identified by the above analyses fell predominantly within cerebellar lobule VI and lobules VIIB/VIII, which closely aligned with cerebellar motor representation 1 (Lobule I-VI) and motor representation 2 (Lobule VIII).30,31 Previous work has indicated that these regions of the cerebellum have differential involvement in ET and has recommended that they be assessed separately.32 Therefore, a post hoc analysis was conducted in which the network hub was divided into 2 ROIs along the fissure between lobules VI and VII using the “cerebellum atlas in MNI space after normalization with FLIRT”33 in FSL (for visualization refer to Results). These ROIs are henceforth referred to as (1) Lobule VI Cluster and (2) Lobule VIIB/VIII Cluster. To investigate whether these separate portions of the network hub have distinct involvement in ET, the functional connectivity from each ROI to the sensorimotor cortices in patients with ET was correlated with their clinical scores.
To further examine the relevance of our ET network to therapeutic interventions, we also investigated whether functional connectivity from our ROIs to the established clinical target for deep brain stimulation (MNI coordinates: x = ±13, y = −18, z = −234) was correlated with patient clinical scores.
Visual inspection of the correlations between functional connectivity and clinical scores using scatterplots showed a number of possible outliers. To assess this objectively, data points with a Cook distance of >0.5 were considered outliers and removed from the model35 (see eAppendix 6, eFigure 2, links.lww.com/WNL/D55 for outlier removal).
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Included Studies
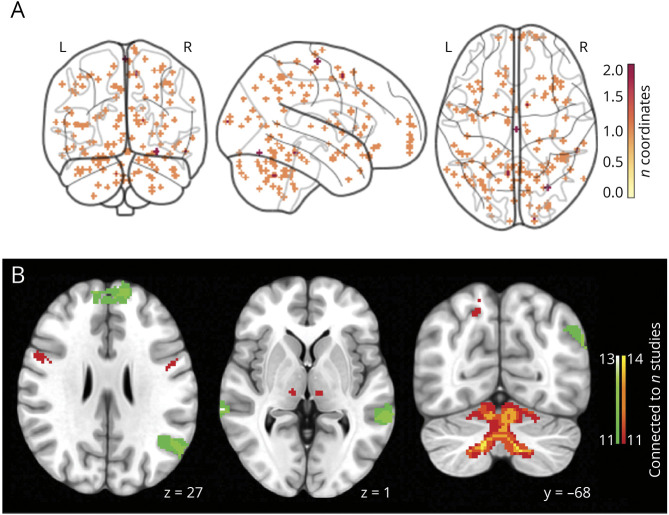
A total of 2,558 records were identified in the systematic search. After screening, 14 studies met inclusion criteria and were included in the analyses (See eFigure 3, links.lww.com/WNL/D55 for PRISMA flowchart). Overall, these studies included 219 participants diagnosed with ET and 221 healthy control participants. Of the included studies, brain abnormalities in ET were identified utilizing T1-weighted MRI in 8 studies, PET in 4 studies (H215O: n = 2; C15O2: n = 1; F-18-FDG: n = 1), and 2 studies used SPECT (Tc-99mHMPAO: n = 1; 99mTc-Ethyl cysteinate dimer: n = 1) (See the eTable for a summary of included studies). The coordinates of brain abnormalities reported in these studies were widespread across various brain regions (Figure 2A).
Figure 2. Coordinate Network Mapping Results.
(A) Locations of structural or metabolic abnormality in essential tremor. 150 coordinates of significant structural or metabolic differences between patients with ET and controls, extracted from 14 neuroimaging studies. Reported areas of abnormality were widely dispersed across the brain. (B) The network of essential tremor. The coordinate network mapping analysis revealed a network of regions sensitive to essential tremor (connected to 11/14 study-specific seeds). Warm colors = positive connectivity; cool colors = negative connectivity.
Activation Likelihood Estimation Meta-analysis
There were no significant clusters using the recommended meta-analytic methods.22 An exploratory analysis using a less stringent threshold (p < 0.001 uncorrected) showed 8 significant clusters spread across various brain regions (eFigure 4, links.lww.com/WNL/D55).
Coordinate Network Mapping
Despite the heterogeneity of coordinate locations (Figure 2A), CNM demonstrated that study-specific seeds were functionally connected to a common brain network encompassing the cerebellum, thalamus, and cortical regions (Figure 2B; for all brain slices see eFigure 5A, links.lww.com/WNL/D55).
Positive connectivity from 11 of 14 study-specific seeds converged on the bilateral precentral gyri, thalamus, inferior parietal lobes, right precuneus, and left insula, and all 14 of 14 study-specific seeds were functionally connected to the cerebellum (Figure 2B). In addition, negative connectivity from over 11 of 14 study-specific seeds converged on the superior and inferior frontal gyri and the superior and middle temporal gyri. These results were robust across thresholds (t ≥ ± 7, 5, 9; eFigure 5A–C, links.lww.com/WNL/D55).
To evaluate whether this result was driven by local connectivity of coordinates located in the cerebellum, we also performed the CNM analysis excluding all cerebellar coordinates. The functional connectivity from distal coordinates strongly resembled the main results, with 8 of 12 (2 studies had cerebellum coordinates only) study-specific seeds still positively connected to the cerebellum (eFigure 6, links.lww.com/WNL/D55).
Relevance to Tremor Relief Network
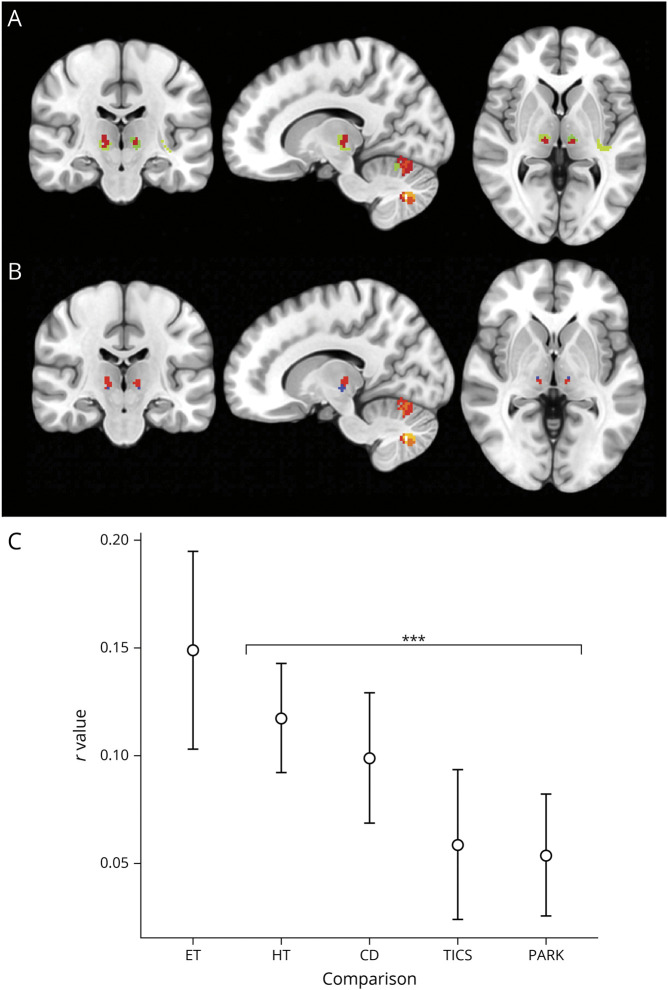
To assess whether our ET network is similar to the network mediating tremor relief,19 we first overlaid the networks visually and found striking similarities in the thalamus and cerebellum (Figure 3A). We also visually compared our ET network with the established DBS target site,34 which overlapped almost exactly in the VIM (Figure 3B). In assessing the networks' similarity statistically, the spatial correlation analysis demonstrated that our ET network was significantly more correlated with the tremor relief network19 than lesion networks of control disorders (F = 5.16, df = 4, p < 0.001; Figure 3C). Of the control disorders, the network of Holmes tremor, which is characterized by resting and intention tremor,3 was the most similar to our ET network.
Figure 3. Relevance of the Essential Tremor Network to Tremor Relief.
(A) Our essential tremor symptom network (red) aligned closely with the tremor relief network (green; Joutsa et al., 2018) in the thalamus and cerebellum. (B) The established deep brain stimulation target (blue) also overlapped with our essential tremor network (red) in the ventral intermediate nucleus of the thalamus. (C) Spatial correlations revealed that our essential tremor symptom network was more similar to the tremor relief network (Joutsa et al., 2018) than networks of a control group of all control disorders. CD = cervical dystonia; ET = essential tremor; HT = Holmes tremor; PARK = Parkinsonism; TICS = Tics, ***p < 0.001.
Hub of the Essential Tremor Network
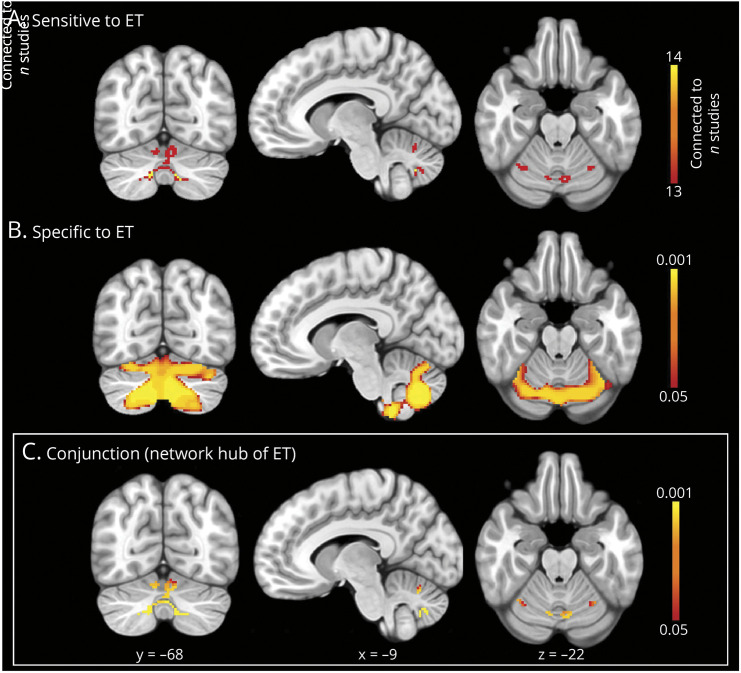
The hub of the ET network was defined by the conjunction of voxels that were sensitive and specific to ET. The CNM analysis localized the network sensitive to ET and found that the cerebellum was the only brain region positively connected to over 90% of study-specific seeds (Figure 4A). The cerebellum was also the only brain region with significantly greater positive functional connectivity from study-specific seeds in ET compared with control disorders (Figure 4B). Therefore, a conjunction analysis of these sensitive and specific voxels defined the hub of the ET network in cerebellar lobules VI and VIIB/VIII (Figure 4C).
Figure 4. Defining the Hub of the Essential Tremor Network.
(A) Sensitive to ET: Coordinate network mapping found the cerebellum to be most strongly connected to coordinates of brain abnormality in ET (thresholded at 90%, connected to 13/14 study-specific seeds); (B) Specific to ET: A specificity analysis revealed voxels with significantly greater functional connectivity to coordinates of abnormality in essential tremor than control disorders (p < 0.05). (C) Conjunction (network hub of ET): A conjunction analysis identified a network hub that was both sensitive and specific to essential tremor.
Validation in an Independent Cohort of Patients With ET
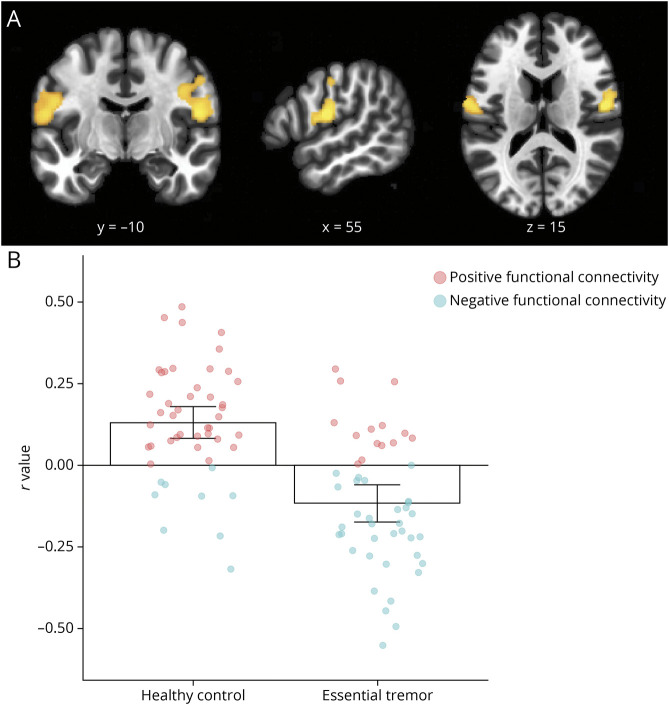
Functional connectivity from the ET network hub (Figure 4C) was investigated in rs-fMRI data from an independent cohort of patients with ET compared with healthy controls. Patients with ET had significantly different functional connectivity between the network hub and bilateral clusters in the sensorimotor cortex compared with healthy controls (Figure 5A), with functional connectivity, on average, positively correlated in healthy controls, yet anticorrelated in patients with ET (Figure 5B). This abnormal functional connectivity was exclusive to our ET network hub, with no significant group differences observed using hubs of control disorders.
Figure 5. Abnormal Functional Connectivity in Essential Tremor.
(A) Functional connectivity between the network hub in the cerebellum and the sensorimotor cortices was significantly different in patients with essential tremor compared with healthy controls. (B) Bar charts (mean ± 95% CI for each group) and individual participant connectivity values show that, on average, these regions were positively connected in healthy controls (left) yet negatively functionally connected in patients with essential tremor (right).
Functional connectivity between the network hub and bilateral sensorimotor cluster did not significantly correlate with clinical scores. However, the network hub spans across both cerebellar motor representation 1 (Lobule I-VI; Figure 6, blue) and motor representation 2 (Lobule VIII; Figure 6, green),30,31 which may have distinct involvement in ET symptoms.32 Therefore, we divided the network hub accordingly33 and investigated the functional connectivity of the 2 separate hub clusters: (1) Lobule VI cluster and (2) Lobule VIIB/VIII cluster (Figure 6).
Figure 6. Hub of the Essential Tremor Network: Division of Distinct Functional Representations.
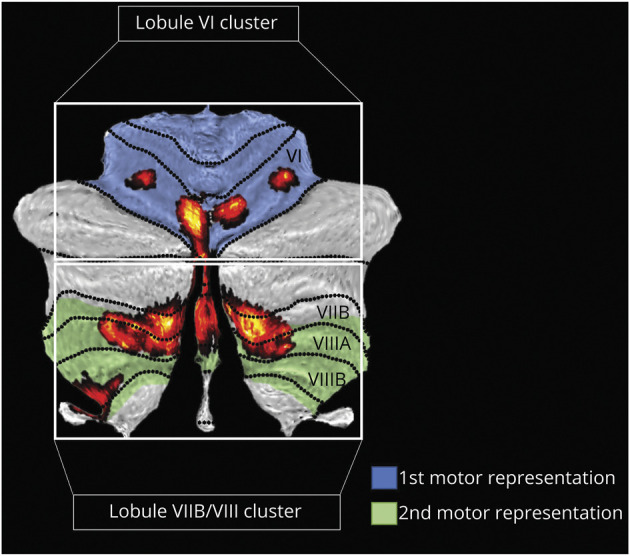
The cerebellum can be organized based on functional gradients, which includes 2 functionally distinct motor representations: motor representation 1 (shaded blue) and motor representation 2 (shaded green).30,31 When overlaid onto these functional gradients, our network hub of ET (red) falls largely within the 2 motor representations. Therefore, we split the hub into 2 separate clusters along the fissure between lobule VI and VII to investigate whether these portions of the hub may have differential involvement in ET. These 2 hub clusters (red) are referred to as (1) Lobule VI Cluster, and (2) Lobule VIIB/VIII Cluster.
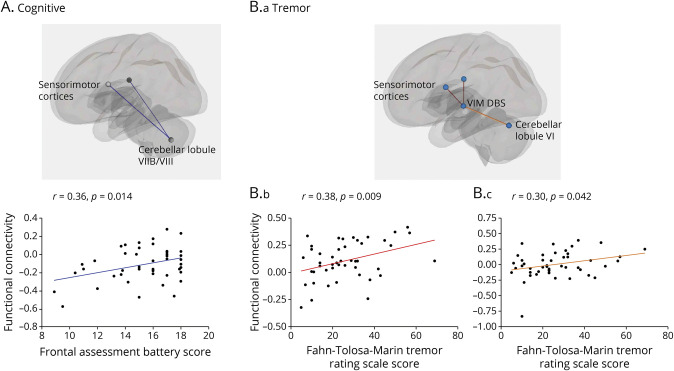
In this post hoc analysis, cognitive scores (FAB) were positively correlated with functional connectivity between the lobule VIIB/VIII cluster and bilateral sensorimotor cluster (r = 0.36, p = 0.014) (Figure 7A), suggesting that less positive connectivity between these regions relates to lower cognitive functioning. In addition, tremor (FTM) scores positively correlated with functional connectivity from the VIM DBS site to both the bilateral sensorimotor cluster (r = 0.38, p = 0.009) (Figure 7B.b) and the lobule VI cluster (r = 0.30, p = 0.042) (Figure 7B.c), indicating that greater positive functional connectivity between these clusters and the VIM relates to worse tremor severity. Patient age and disease duration were not significantly correlated with functional connectivity.
Figure 7. Correlations Between Functional Connectivity and Clinical Symptoms.
(A) Cognitive: Functional connectivity between cerebellar lobule VIIB/VIII cluster and sensorimotor cortices was positively correlated with Frontal Assessment Battery scores in patients with essential tremor. (B) Tremor: Fahn-Tolosa-Marin tremor rating scale scores were positively correlated with functional connectivity between the VIM DBS site and the sensorimotor cortices (B.b, red) and with functional connectivity between the VIM DBS site and cerebellar lobule VI cluster (B.c, orange). Visual inspection of the rightmost scatterplot (Figure 7B.c) indicates the presence of a possible outlier with low functional connectivity between lobule VI and the VIM DBS site. The removal of this data point changed the correlation from r = 0.30; p = 0.042 to r = 0.26; p = 0.089. However, this data point fell well below our objective Cook distance cutoff for data point removal (>0.5) (Cook distance = 0.19) and was therefore retained. DBS = deep brain stimulation; VIM = ventral intermediate nucleus of the thalamus.
Discussion
There are several important findings in this study. First, brain abnormalities underlying ET occur in various brain regions but are part of a functionally connected brain network defined by common connectivity to a network hub located in the cerebellum. Second, this connectivity profile underlying ET symptoms is similar to the connectivity profile of lesions that mediate tremor relief19 and aligns with the established DBS site in the VIM. Finally, functional connectivity within our ET network was abnormal in an independent cohort of patients with ET compared with healthy controls, and connectivity from the network hub was also significantly correlated with both tremor and cognitive symptoms.
The cerebello-thalamo-cortical circuit is widely thought to be involved in ET, yet neuroimaging studies have shown brain abnormalities in a variety of regions both within and outside this circuit, and previous meta-analyses have failed to show robust findings.12,13 This study used ALE meta-analysis to assess the convergence of previously reported structural and metabolic brain abnormalities in ET and found no brain areas were consistent across studies. Therefore, we used CNM to reconcile these seemingly inconsistent neuroimaging findings and showed that they localize to a single functionally connected network, which included the regions of the cerebello-thalamo-cortical circuit and further extended to a small number of key nonmotor regions (inferior parietal lobes, right precuneus and left insula; Figure 2B).
The involvement of this network in ET is strongly supported by previous evidence. For example, the thalamus acts as a relay station with connections to both the cerebellum and the cortex and is an effective target site for deep brain stimulation to modulate this network and relieve tremor.36 In addition, research using magnetoencephalography demonstrated altered communication of a network including the motor cortex, thalamus, and cerebellum during tremor in patients with ET.7 Furthermore, regions of our ET network outside of the cerebello-thalamo-cortical circuit have also been implicated in ET for their potential contribution to tremor severity or cognitive symptoms. The inferior parietal lobes are involved in integrating visual and sensorimotor information, with significant functional connections to the sensorimotor cortices, cerebellar lobule VI (both part of our ET network), and the visual cortex.37 A number of studies have identified the involvement of the parietal lobes in ET14,15,38 and have suggested that activity in this area is associated with tremor severity increasing with visual feedback.14,38
Consistent with our findings, the precuneus has also been consistently identified as abnormal in ET13 and may be associated with cognitive impairments in patients with ET.39 Interestingly, Passamonti et al.39 showed increased functional connectivity between cerebellar lobule VI (the hub of our network) and the precuneus in patients with ET with low cognitive functioning and suggested that cognitive symptoms in ET may involve a dysfunction of the precuneus, possibly driven by the cerebellum. In line with this hypothesis, the left insula was also identified as part of our ET network, which is thought to be involved in switching between the default mode network and task-positive networks,40 and may also be implicated in cognitive symptoms of ET.41
The cerebellum has been strongly implicated as the driver of symptom generation in ET, with studies showing morphological changes and loss of Purkinje cells,8 GABAergic dysfunction,9 and abnormal cerebellar connectivity.6 In this study, the cerebellum was found to be the hub of our ET network, connected to all 14 study-specific seeds, and the only brain region with positive connectivity specific to ET compared with other movement disorders. This network hub localized to cerebellar lobules VI and VIIB/VIII, which aligned with the cerebellar motor representations (Figure 7).30,31 Notably, this pattern of connectivity was still present even when all cerebellar coordinates were removed from the analysis (eFigure 6, links.lww.com/WNL/D55), demonstrating that dispersed brain abnormalities seen in ET may be distal nodes of the ET network influenced by dysfunction of the cerebellar hub.
A loss of positive functional connectivity between the network hub in the cerebellum and the sensorimotor cortices bilaterally was demonstrated in an independent cohort of patients with ET, which is consistent with previous functional neuroimaging studies that have also shown reduced connectivity between these regions in ET in both resting-state29,42 and task-based32 paradigms. More specifically, this study showed the network hub and the sensorimotor cortices were anticorrelated in patients with ET, whereas these regions were positively correlated in healthy controls; these results support the hypothesis that the cerebellum may be “disconnected” from the motor network in ET.43 As such, the decoupling of the cerebellum with other regions of this network has been suggested to impede communication between the cerebellum and motor cortex during action, which may give rise to tremor.43
The cerebellum has a complex functional neuroanatomy which subserves a myriad of motor and nonmotor processes,30 yet because of challenges of resolution and segmentation, cerebellar lobules have rarely been investigated separately in the ET literature.44,45 Recent studies30,31 have established that the organization of the cerebellum involves 2 motor representations (lobules I-VI and VIII; Figure 6), each of which has different contributions to motor processing. This may be reflected in ET pathophysiology as Buijink et al.32 used functional connectivity analyses to demonstrate the differential involvement of the anterior (lobules I – V/VI) and posterior (lobules VI/VII – X) cerebellum in ET. The results of this study provide further support for this distinction, showing that functional connectivity of the separate hub clusters in lobule VI and lobules VIIB/VIII correlated with tremor and cognitive functioning, respectively.
Here, reduced positive connectivity between lobules VIIB/VIII and the sensorimotor cortices was related to worse cognitive performance in patients with ET, which is consistent with previous evidence from cerebellar strokes whereby lesions to lobules VIIB/VIII were associated with cognitive performance46 and functional neuroimaging studies which have also shown these regions to be involved with working memory and executive functions.47
Interestingly, tremor severity was not correlated with functional connectivity between the cerebellum and sensorimotor cortices directly but was positively correlated with functional connectivity between cerebellar lobule VI and the VIM and connectivity between the VIM and the sensorimotor cortices (Figure 7B). This finding supports the hypothesis that abnormal oscillations in the cerebello-thalamo-cortical circuit underlie tremor, with the thalamus mediating abnormal connectivity between the cerebellum and sensorimotor cortices.2,4 Consistently, dysfunctional connectivity of the cerebello-thalamo-cortical circuit has been shown to be associated with tremor in a number of previous functional neuroimaging studies.42 Furthermore, stimulation and ablation of the VIM effectively alleviates tremor by modulating activity within this circuit, thereby causally demonstrating the intermediary role of the thalamus within this perturbed network of ET.10,48
A major finding of this study was that our symptom network mapped from structural and metabolic brain abnormalities in ET aligned with the therapeutic network mapped from lesions that relieved tremor in patients with ET.19 These networks each showed converging connectivity to key sites in the VIM and cerebellar lobule VI, indicating that these regions may be involved in both ET symptom generation and relief.
A recent study on depression by Siddiqi et al.25 showed functional connectivity from lesions associated with depression mapped to the same functional network that is modulated by effective treatment sites of both DBS and transcranial magnetic stimulation. Our results suggest that this principle may also be true in ET and may further extend to functional networks mapped from brain abnormalities using CNM.
For example, the specific site in the VIM that was localized by both the symptomatic and therapeutic networks is also the precise site currently targeted with DBS, which is extremely effective in alleviating tremor and is a clinically approved treatment for ET.48 Furthermore, noninvasive brain stimulation methods targeting other aspects of our network have also shown some efficacy in reducing ET symptoms, such as the motor cortex and cerebellum.49 Particularly, a recent study showed effective tremor reduction using phase-locked transcranial alternating current stimulation over cerebellar lobule VIII,50 which is within the hub of the network identified in this study.
Therefore, our localization of the symptomatic network of ET may have implications for refining therapeutic targets for ET treatment or potentially identifying new target sites that modulate this network. Furthermore, these findings demonstrate that networks identified with CNM are potentially relevant for clinical treatments, which may be applied in a wide range of other disorders. This approach may be a particularly important advantage for idiopathic conditions where networks cannot be derived from causal or beneficial brain lesions.
However, this study's results should be interpreted in light of its limitations. First, a systematic search of the literature was used to identify the studies/coordinates used in our analyses and therefore may be influenced by publication bias.13 Second, the CNM analysis leveraged a large normative connectome, rather than disease-specific or age-specific data; however, this has been investigated in previous studies and showed minimal effect on the results.17 Finally, coordinates evaluated in our CNM analyses relied on the original study author's judgment of patient inclusion and study design/analysis; therefore, these studies consisted of heterogeneous methodologies and participants with ET. However, this should have biased us away from these results, but instead our findings showed that despite clinical heterogeneity, structural and metabolic abnormalities were still connected to a common functional network.
This study defined a brain network underlying ET, with the central hub of this network located in distinct regions of cerebellar lobules VI and VIIB/VIII. This network closely aligned with the therapeutic network derived from lesions relieving tremor,19 encompassed effective treatment sites for brain stimulation, and was significantly abnormal in an independent cohort of patients with ET. These findings may help to refine current targets and inform new testable targets for therapeutic neuromodulation in ET.
Acknowledgment
We would like to thank Jordan Morrison-Ham for her help drafting this manuscript, and Stephan Palm and William Drew for their technical neuroimaging advice.
Glossary
- ALE
activation likelihood estimation
- CNM
coordinate network mapping
- DBS
deep brain stimulation
- ET
essential tremor
- FWE
family-wise error
- VIM
ventral intermediate nucleus of the thalamus
Appendix. Authors
Footnotes
Editorial, page 639
Study Funding
The authors report no targeted funding.
Disclosure
E.F.P. Younger, E.G. Ellis and N. Parsons are funded by the Deakin University Postgraduate Research Scholarship; P. Pantano has received funding for travel from Novartis, Genzyme, and Bracco and a speaking honorarium from Biogen, research support from Italian Ministry of Foreign Affairs and Fondazione Italiana Sclerosi Multipla; K. Caeyenberghs is supported by a Veski Fellowship, the Victorian Near-miss Award Pilot is administered by Veski for the Victorian Health and Medical Research Workforce Project on behalf of the Victorian Government and the Association of Australian Medical Research Institutes, funding for the Pilot has been provided by the Victorian Department of Jobs, Precincts and Regions; J. Benito-Leon is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451); J.P. Romero was funded by the Spanish Ministry of Science and Innovation grant (PID2020-113222RB- C21/AEI/10.13039/501100011033); J. Joutsa has received research grants from the Finnish Medical Foundation, Instrumentarium Research Foundation, Sigrid Juselius Foundation, Finnish Foundation for Alcohol Studies, University of Turku (private donation, Sigrid Juselius), Turku University Hospital (ERVA funds), and conference travel support from Abbvie and Abbott, and lecturer honoraria from Lundbeck; D.T. Corp and S. Tommasin report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Louis ED, McCreary M. How common is essential tremor? Update on the worldwide prevalence of essential tremor. Tremor Other Hyperkinet Mov (N Y). 2021;11(1):28. doi: 10.5334/tohm.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welton T, Cardoso F, Carr JA, et al. . Essential tremor. Nat Rev Dis Primers. 2021;7(1):83. doi: 10.1038/s41572-021-00314-w [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Ad Hoc Scientific Committee. Mov Disord. 2008;13(S3):2-23. doi: 10.1002/mds.870131303 [DOI] [PubMed] [Google Scholar]
- 4.Raethjen J, Deuschl G. The oscillating central network of essential tremor. Clin Neurophysiol. 2012;123(1):61-64. doi: 10.1016/j.clinph.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Faust PL. Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol. 2020;16(2):69-83. doi: 10.1038/s41582-019-0302-1 [DOI] [PubMed] [Google Scholar]
- 6.Cerasa A, Quattrone A. Linking essential tremor to the cerebellum—neuroimaging evidence. Cerebellum. 2016;15(3):263-275. doi: 10.1007/s12311-015-0739-8 [DOI] [PubMed] [Google Scholar]
- 7.Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. 2009;24(11):1629-1635. doi: 10.1002/mds.22633 [DOI] [PubMed] [Google Scholar]
- 8.Louis ED. Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum. 2016;15(3):235-242. doi: 10.1007/s12311-015-0692-6 [DOI] [PubMed] [Google Scholar]
- 9.Paris-Robidas S, Brochu E, Sintes M, et al. . Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135(1):105-116. doi: 10.1093/brain/awr301 [DOI] [PubMed] [Google Scholar]
- 10.Dallapiazza RF, Lee DJ, De Vloo P, et al. . Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90(4):474-482. doi: 10.1136/jnnp-2018-318240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raethjen J, Govindan R, Kopper F, Muthuraman M, Deuschl G. Cortical involvement in the generation of essential tremor. J Neurophysiol. 2007;97(5):3219-3228. doi: 10.1152/jn.00477.2006 [DOI] [PubMed] [Google Scholar]
- 12.Luo R, Pan P, Xu Y, Chen L. No reliable gray matter changes in essential tremor. Neurol Sci. 2019;40(10):2051-2063. doi: 10.1007/s10072-019-03933-0 [DOI] [PubMed] [Google Scholar]
- 13.Han Q, Hou Y, Shang H. A voxel-wise meta-analysis of gray matter abnormalities in essential tremor. Front Neurol. 2018;9:495. doi: 10.3389/fneur.2018.00495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer DB, Coombes SA, Chu WT, et al. . A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain. 2018;141(2):472-485. doi: 10.1093/brain/awx338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang W, Chen H, Wang H, et al. . Multiple resting‐state networks are associated with tremors and cognitive features in essential tremor. Mov Disord. 2015;30(14):1926-1936. doi: 10.1002/mds.26375 [DOI] [PubMed] [Google Scholar]
- 16.Benito-León J, Louis ED, Romero JP, et al. . Altered functional connectivity in essential tremor: a resting-state fMRI study. Medicine (Baltimore). 2015;94(49):e1936. doi: 10.1097/md.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darby RR, Joutsa J, Fox MD. Network localization of heterogeneous neuroimaging findings. Brain. 2019;142(1):70-79. doi: 10.1093/brain/awy292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joutsa J, Corp DT, Fox MD. Lesion network mapping for symptom localization: recent developments and future directions. Curr Opin Neurol. 2022;35(4):453-459. doi: 10.1097/wco.0000000000001085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joutsa J, Shih LC, Horn A, et al. . Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 2018;84(1):153-157. doi: 10.1002/ana.25285 [DOI] [PubMed] [Google Scholar]
- 20.Burke MJ, Joutsa J, Cohen AL, et al. . Mapping migraine to a common brain network. Brain. 2020;143(2):541-553. doi: 10.1093/brain/awz405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weil RS, Hsu JK, Darby RR, Soussand L, Fox MD. Neuroimaging in Parkinson's disease dementia: connecting the dots. Brain Commun. 2019;1(1):fcz006. doi: 10.1093/braincomms/fcz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eickhoff SB, Nichols TE, Laird AR, et al. . Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70-85. doi: 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A, Soussand L, McManus P, Fox M. GSP1000 preprocessed connectome. Harvard Dataverse, V3. November 17, 2020. Updated April 17, 2021. 10.7910/DVN/ILXIKS [DOI] [Google Scholar]
- 24.Corp DT, Joutsa J, Darby RR, et al. . Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142(6):1660-1674. doi: 10.1093/brain/awz112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi SH, Schaper FL, Horn A, et al. . Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. 2021;5(12):1707-1716. doi: 10.1038/s41562-021-01161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joutsa J, Shih LC, Fox MD. Mapping holmes tremor circuit using the human brain connectome. Ann Neurol. 2019;86(6):812-820. doi: 10.1002/ana.25618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain. 2018;141(8):2445-2456. doi: 10.1093/brain/awy161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 29.Tikoo S, Pietracupa S, Tommasin S, et al. . Functional disconnection of the dentate nucleus in essential tremor. J Neurol. 2020;267(5):1358-1367. doi: 10.1007/s00415-020-09711-9 [DOI] [PubMed] [Google Scholar]
- 30.Guell X, Schmahmann J. Cerebellar Functional Anatomy: A Didactic Summary Based on Human fMRI Evidence. Springer; 2020:1-5. [DOI] [PubMed] [Google Scholar]
- 31.Guell X, Schmahmann JD, Gabrieli JD, Ghosh SS. Functional gradients of the cerebellum. Elife. 2018;7:e36652. doi: 10.7554/elife.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buijink AW, van der Stouwe AM, Broersma M, et al. . Motor network disruption in essential tremor: a functional and effective connectivity study. Brain. 2015;138(10):2934-2947. doi: 10.1093/brain/awv225 [DOI] [PubMed] [Google Scholar]
- 33.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;47(1):S122-S146. doi: 10.1016/s1053-8119(09)71166-8 [DOI] [PubMed] [Google Scholar]
- 34.Horn A, Kühn AA, Merkl A, Shih L, Alterman R, Fox M. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. Neuroimage. 2017;150:395-404. doi: 10.1016/j.neuroimage.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook RD, Weisberg S. Residuals and Influence in Regression. Chapman and Hall; 1982. [Google Scholar]
- 36.Hopfner F, Deuschl G. Managing essential tremor. Neurotherapeutics. 2020;17(4):1603-1621. doi: 10.1007/s13311-020-00899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Li C-SR. Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect. 2014;4(1):53-69. doi: 10.1089/brain.2013.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy A, Coombes SA, Chung JW, et al. . Cortical dynamics within and between parietal and motor cortex in essential tremor. Mov Disord. 2019;34(1):95-104. doi: 10.1002/mds.27522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Passamonti L, Novellino F, Cerasa A, et al. . Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain. 2011;134(8):2274-2286. doi: 10.1093/brain/awr164 [DOI] [PubMed] [Google Scholar]
- 40.Molnar-Szakacs I, Uddin LQ. Anterior insula as a gatekeeper of executive control. Neurosci Biobehavioral Rev. 2022;139:104736. doi: 10.1016/j.neubiorev.2022.104736 [DOI] [PubMed] [Google Scholar]
- 41.Lan H, Suo X, Li W, et al. . Abnormalities of intrinsic brain activity in essential tremor: a meta‐analysis of resting‐state functional imaging. Hum Brain Mapp. 2021;42(10):3156-3167. doi: 10.1002/hbm.25425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenka A, Bhalsing KS, Panda R, et al. . Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology 2017;59(2):157-168. doi: 10.1007/s00234-016-1771-1 [DOI] [PubMed] [Google Scholar]
- 43.Sharifi S, Buijink AW, Luft F, et al. . Differences in olivo-cerebellar circuit and cerebellar network connectivity in essential tremor: a resting state fMRI study. Cerebellum. 2022. doi: 10.1007/s12311-022-01486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hett K, Lyu I, Trujillo P, et al. . Anatomical texture patterns identify cerebellar distinctions between essential tremor and Parkinson's disease. Hum Brain Mapp. 2021;42(8):2322-2331. doi: 10.1002/hbm.25331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez AM, Trujillo P, Hernandez AB, et al. . Structural correlates of the sensorimotor cerebellum in Parkinson's disease and essential tremor. Mov Disord. 2020;35(7):1181-1188. doi: 10.1002/mds.28044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoodley CJ, MacMore JP, Makris N, Sherman JC, Schmahmann JD. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin. 2016;12:765-775. doi: 10.1016/j.nicl.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23(1-2):65-79. doi: 10.1155/2010/840942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10(2):148-161. doi: 10.1016/s1474-4422(10)70322-7 [DOI] [PubMed] [Google Scholar]
- 49.Shih LC, Pascual-Leone A. Non-invasive brain stimulation for essential tremor. Tremor Other Hyperkinet Mov (N Y). 2017;7(0):458. doi: 10.5334/tohm.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreglmann SR, Wang D, Peach RL, et al. . Non-invasive suppression of essential tremor via phase-locked disruption of its temporal coherence. Nat Commun. 2021;12(1):363-415. doi: 10.1038/s41467-020-20581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.