Abstract
Background and Objectives
Elevations in circulating glial fibrillary acidic protein (GFAP), a putative marker of reactive astrocytosis, have been found to associate with cognitive decline and dementia status. Further validation in diverse cohorts and evaluation of potential health disparities are necessary for broader generalization. The goal of this study was to examine the associations between demographics, cardiovascular risk factors, and APOE ε4 status with serum GFAP levels among Mexican American and non-Hispanic White older adults across the continuum from cognitively unimpaired to Alzheimer disease dementia.
Methods
Serum GFAP levels were assayed using a Simoa HD-1 analyzer in older adults enrolled in the observational Texas Alzheimer Research and Care Consortium. Associations between demographic and clinical characteristics with serum GFAP levels were evaluated using linear regression. The diagnostic accuracy of serum GFAP was further examined using area under the receiver operating characteristic curves (AUROC) in univariate and adjusted models, and optimal cut points were derived using the maximum Kolmogorov-Smirnov metric. All models were also stratified by ethnicity and disease stage.
Results
A total of 1,156 Mexican American and 587 non-Hispanic White participants were included (mean age = 68 years, standard deviation = 10; 65% female). Older age (β = 0.562 (95% CI 0.515–0.609), p < 0.001), apolipoprotein ε4 status (β = 0.139 (95% CI 0.092–0.186), p < 0.001), and cognitive impairment (β = 0.150 (95% CI 0.103–0.197), p < 0.001) were positively associated with serum GFAP. By contrast, higher body mass index (β = −0.181 (95% CI -0.228 to −0.134), p < 0.001), diabetes (β = −0.065 (95% CI -0.112 to −0.018), p < 0.001), and tobacco use (β = −0.059 (95% CI -0.106 to −0.012), p < 0.001) were inversely associated with serum GFAP. AUROC values were generally comparable across ethnicities and model fit improved with inclusion of additional covariates. However, optimal cut-off values were consistently lower in Mexican Americans relative to non-Hispanic White participants.
Discussion
The study results highlight the importance of understanding the role of broader demographic and clinical factors on circulating GFAP levels within diverse cohorts to enhance precision across clinical, research, and community settings.
Introduction
The establishment of a biological definition for Alzheimer disease (AD), characterized by the presence of amyloid beta (aβ), tau, and neurodegeneration, has afforded new opportunities for early diagnosis and interventions targeting core pathologic features.1 Neuroimaging and CSF measures are considered the gold standard diagnostics; however, there is now significant momentum to validate blood-based biomarkers in an effort to increase accessibility, scalability and affordability.2,3 Blood-based biomarkers further offer the potential to evaluate multiple pathophysiologic features of AD and related dementias (ADRD), such as neuroinflammation and glial cell activation.4 In particular, circulating levels of glial fibrillary acidic protein (GFAP), an intermediate filament protein of the astrocytic cytoskeleton and putative marker of reactive astrocytosis,5 have been shown to increase with cerebral aβ burden6-8 and associate with the risk of all-cause and AD dementia in population-based studies.9,10 In our prior work examining the serum levels of GFAP, total tau, neurofilament light (NFL), ubiquitin carboxyl-terminal hydrolase LI, soluble CD14, and YKL-40 in Mexican American (MA) older adults, GFAP alone was associated with incident dementia over a four-year follow-up period.11
Although circulating levels of GFAP and other proteins have displayed the potential to improve ADRD risk stratification,5-11 previous studies have been largely limited to homogenous research cohorts, and additional validation in diverse ethnic and racial cohorts reflective of the broader population is critical for wide scale implementation.3,12 There is growing recognition in the field that structural and social determinants of health, such as access to health care, education, and built environment, have an important role in explaining variance in ADRD outcomes.13 Work by our team and others has demonstrated ethnic and racial differences in blood biomarker levels in minoritized groups that have higher average exposure to factors linked with health inequities.12,14-16 For example, among Black and non-Hispanic White (NHW) participants matched for age, APOE ε4 carriage, and cognitive status, Schindler et al. reported lower accuracy of plasma phosphorylated tau (p-tau) 181, p-tau 231, and NFL for CSF aβ42/aβ40 positivity among Black adults.17 In the HABLE study of MA and NHW adults, O'Bryant et al. reported lower plasma aβ40 and NFL and higher total tau levels among MA participants.18,19 Given the significant heterogeneity across and within diverse groups,12,17 it is important to understand the underlying factors that contribute to observed differences. Previous research has indicated that circulating ADRD biomarker levels are associated with cardiovascular and broader medical comorbidities, including obesity, hypertension, and chronic kidney disease,3,15,20-22 which disproportionately affect minoritized communities.12,23 Therefore, improved understanding of the role of common comorbidities on ADRD blood biomarker levels within diverse cohorts may enhance their validity and generalizability.12,17
The goal of this study was to examine the association between circulating GFAP with demographics, cardiovascular risk factors, and APOE ε4 status among MA and NHW older adults across the continuum from cognitively unimpaired (CU) to dementia due to AD. Based on previous research,23 we hypothesized that MA older adults would present with increased cardiovascular risk burden relative to NHW participants, which would be associated with serum GFAP levels. In addition, we further aimed to evaluate whether adjustment for pertinent demographic and clinical factors improved the diagnostic accuracy of serum GFAP for mild cognitive impairment (MCI) and dementia among MA and NHW older adults.
Methods
Sample
Assays were conducted on blood specimens obtained at the baseline visit of the Texas Alzheimer Research and Care Consortium (TARCC) study, which is a collaborative effort across 10 academic medical centers in Texas with data collection occurring between 2005 and 2017.24 Participating sites enrolled and longitudinally followed adults 50 years and older at the time of recruitment. For the current analyses, inclusion criteria included MA or NHW ethnicity and available data on serum GFAP. This study included participants with unimpaired cognition, MCI, and dementia due to AD. Individuals with a diagnosis of dementia not due to possible or probable Alzheimer disease and/or a history of major stroke were excluded.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the institutional review board at each institution and was conducted in adherence with The Code of Ethics of the World Medical Association. Participants provided written informed consent before enrollment with appropriate legal representation for individuals lacking capacity to consent. University of Texas Health at San Antonio Institutional Review Board approval was obtained to process and analyze deidentified samples and clinical/demographic data.
Examination Procedures
Standardized medical histories, physical and neurologic examinations, and cognitive assessments were conducted in accordance with National Alzheimer Coordinate Center Uniform Data Set Version 2.25 Race and ethnicity were identified by self-report. Response options for race included (1) White, (2) Black or African American, (3) American Indian or Alaskan Native, (4) Native Hawaiian or other Pacific Islander, (5) Asian, (6) other, or (7) unknown. Participants were also asked whether they identified as Hispanic and in response to an affirmative answer were provided with the following response options: (1) Mexican/Chicano/Mexican American, (2) Puerto Rican, (3) Cuban, (4) Dominican, (5) Central American, (6) South American, (7) other, or (8) unknown. Weight in kilograms and height in centimeters were obtained for calculation of body mass index (BMI). Systolic blood pressure (SBP) was evaluated using an automated brachial cuff in the supine position. Diabetes was assessed by self-report, medical records, and use of glucose-lowering medications. Current tobacco use was assessed by self-reported tobacco use in the past 30 days.
Clinical Diagnoses
Classifications of CU, MCI, and dementia were assigned based on the review of clinical and cognitive assessments at the annual visits. Diagnoses were adjudicated in multidisciplinary consensus review conferences comprised at least one physician, neuropsychologist, and research coordinator. Possible or probable diagnoses for Alzheimer disease were assigned based on NINCDS-ADRDA criteria.26 MCI subtypes (amnestic vs nonamnestic) were determined using established criteria defined by Petersen et al.27
Blood Draw and Storage
Blood was collected, processed, and stored in TARCC in alignment with established guidelines.28 In brief, nonfasting blood was collected by venipuncture in the morning. Serum tubes were allowed to clot for 30–60 minutes. After centrifugation at 2000×g for 10 minutes at room temperature, serum was aliquoted into polypropylene tubes and specimens were stored in −80°C freezers within 2 hours of collection. Apolipoprotein E (APOE) genotyping was performed with PCR as previously described,24 and APOE ε4 carrier status was defined by the presence of at least one ε4 allele.
Quantification of Serum Biomarker Levels
GFAP levels were quantified in serum using the Neurology 4-Plex A Kit on a Simoa HD-1 Analyzer (Quanterix, Lexington, MA) at the University of Vermont Laboratory for Clinical Biochemistry. The analytical range was 0.8—3,420 pg/mL, and the interassay coefficient of variance was 13.9%. A certified laboratory technician, blinded to clinical and demographic information, performed all assays between November and December 2019 using a single batch of reagents.
Statistical Analyses
Serum GFAP levels had a left-skewed, non-normal distribution and were natural log-transformed and standardized to z-scores within the cohort before analyses. Demographic and clinical variables were compared across diagnostic groups and ethnicities using the chi-squared statistic for categorical variables or with independent t tests or Mann-Whitney U tests for continuous variables. For all subsequent analyses, continuous variables were transformed to z-scores to enable comparisons across outcomes. The associations between demographic (age, sex) and clinical (BMI, SBP, diabetes, tobacco use, APOE ε4 status, diagnostic group) characteristics with serum GFAP were evaluated using linear regression across the whole sample and then with stratification by diagnostic group and ethnicity. Associations with age and sex were examined with adjustment for site. All other models were adjusted for age, sex, and site. In exploratory analyses, interaction terms between ethnicity and demographic and clinical characteristics were added. The diagnostic accuracy of serum GFAP was examined using area under the receiver operating characteristic curves (AUROC) from pairwise logistic regression models for the whole sample and then with stratification by ethnic group and sex. Model 1 included standardized log-transformed GFAP and adjustment for site. Model 2 also included adjustment for age, sex, and site, and Model 3 also included adjustment for age, sex, site, BMI, diabetes, tobacco use, and APOE ε4 status. Comparisons of the models were performed using the test by Delong et al.29 In supplementary analyses, AUROC was performed for amnestic MCI rather than all subtypes of MCI. Optimal cut points and their sensitivity and specificity were derived using the maximum Kolmogorov-Smirnov metric.30 Statistical tests were 2-sided, and statistical significance was set at p < 0.05. For all models evaluating associations with demographic and clinical variables, p values were Bonferroni-corrected for the number of independent variables, which placed the raw p value for statistical significance at p < 0.006. Analyses were conducted on participants with available data, and participants with missing values were excluded from analyses (missing age N = 5, BMI N = 31, missing SBP N = 48, missing ethnicity N = 16). Statistical analyses were performed using SPSS version 28.
Data Availability
All data that support our findings, which we can legally share, are accessible by request through TARCC: ais.swmed.edu/redcap/surveys/?s=CX8MJ4Y7XD. This study is reported in accordance with the STROBE checklist (eAppendix 1, links.lww.com/WNL/D50).
Results
Participant Characteristics
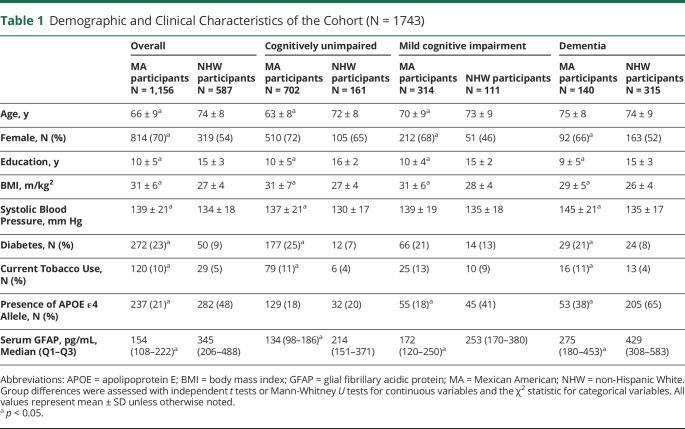
A total of 1,743 participants (65% female; mean age 69 ± 10) were included in this study, of which 1,156 self-identified as MA ethnicity and 587 self-identified as NHW ethnicity. As compared with the NHW participants, MA participants were younger, more commonly female, had less education, and had higher BMI, SBP, diabetes prevalence, and tobacco use (Table 1). APOE ε4 carrier status was lower in the MA participants. Serum GFAP values increased with advancing disease severity across the sample. However, in unadjusted models, MA participants had lower serum GFAP values relative to NHW participants across the whole sample and with stratification by the diagnostic group.
Table 1.
Demographic and Clinical Characteristics of the Cohort (N = 1743)
| Overall | Cognitively unimpaired | Mild cognitive impairment | Dementia | |||||
| MA participants N = 1,156 |
NHW participants N = 587 |
MA participants N = 702 |
NHW participants N = 161 |
MA participants N = 314 |
NHW participants N = 111 | MA participants N = 140 |
NHW participants N = 315 |
|
| Age, y | 66 ± 9a | 74 ± 8 | 63 ± 8a | 72 ± 8 | 70 ± 9a | 73 ± 9 | 75 ± 8 | 74 ± 9 |
| Female, N (%) | 814 (70)a | 319 (54) | 510 (72) | 105 (65) | 212 (68)a | 51 (46) | 92 (66)a | 163 (52) |
| Education, y | 10 ± 5a | 15 ± 3 | 10 ± 5a | 16 ± 2 | 10 ± 4a | 15 ± 2 | 9 ± 5a | 15 ± 3 |
| BMI, m/kg2 | 31 ± 6a | 27 ± 4 | 31 ± 7a | 27 ± 4 | 31 ± 6a | 28 ± 4 | 29 ± 5a | 26 ± 4 |
| Systolic Blood Pressure, mm Hg | 139 ± 21a | 134 ± 18 | 137 ± 21a | 130 ± 17 | 139 ± 19 | 135 ± 18 | 145 ± 21a | 135 ± 17 |
| Diabetes, N (%) | 272 (23)a | 50 (9) | 177 (25)a | 12 (7) | 66 (21) | 14 (13) | 29 (21)a | 24 (8) |
| Current Tobacco Use, N (%) | 120 (10)a | 29 (5) | 79 (11)a | 6 (4) | 25 (13) | 10 (9) | 16 (11)a | 13 (4) |
| Presence of APOE ε4 Allele, N (%) | 237 (21)a | 282 (48) | 129 (18) | 32 (20) | 55 (18)a | 45 (41) | 53 (38)a | 205 (65) |
| Serum GFAP, pg/mL, Median (Q1–Q3) | 154 (108–222)a | 345 (206–488) | 134 (98–186)a | 214 (151–371) | 172 (120–250)a | 253 (170–380) | 275 (180–453)a | 429 (308–583) |
Abbreviations: APOE = apolipoprotein E; BMI = body mass index; GFAP = glial fibrillary acidic protein; MA = Mexican American; NHW = non-Hispanic White.
Group differences were assessed with independent t tests or Mann-Whitney U tests for continuous variables and the χ2 statistic for categorical variables. All values represent mean ± SD unless otherwise noted.
p < 0.05.
Associations Between Demographic and Clinical Variables With Serum GFAP
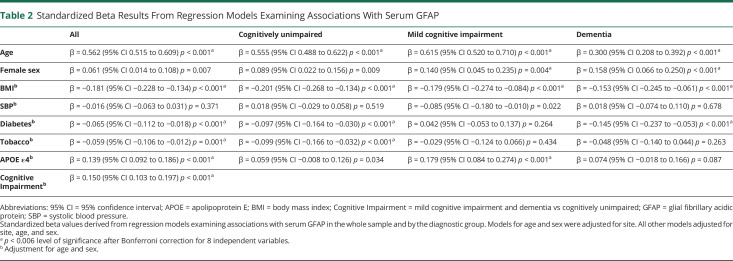
In models adjusted for site, older age was associated with higher GFAP levels, and there were also strong trends toward a positive association with female sex (Table 2). All subsequent models were adjusted for age, sex, and site. Across the sample, APOE ε4 carriage and the presence of cognitive impairment (MCI or dementia) were associated with higher GFAP levels. By contrast, higher BMI, diabetes, and tobacco use were associated with lower GFAP levels. SBP was not significantly associated with serum GFAP. The pattern of results was largely consistent in models stratified by the diagnostic group. However, sex only reached statistical significance with Bonferroni adjustment in the MCI and dementia groups, and diabetes only reached significance in the CU and dementia groups. Tobacco use was only significant in the CU group, and APOE ε4 status was only significant in the MCI group.
Table 2.
Standardized Beta Results From Regression Models Examining Associations With Serum GFAP
| All | Cognitively unimpaired | Mild cognitive impairment | Dementia | |
| Age | β = 0.562 (95% CI 0.515 to 0.609) p < 0.001a | β = 0.555 (95% CI 0.488 to 0.622) p < 0.001a | β = 0.615 (95% CI 0.520 to 0.710) p < 0.001a | β = 0.300 (95% CI 0.208 to 0.392) p < 0.001a |
| Female sex | β = 0.061 (95% CI 0.014 to 0.108) p = 0.007 | β = 0.089 (95% CI 0.022 to 0.156) p = 0.009 | β = 0.140 (95% CI 0.045 to 0.235) p = 0.004a | β = 0.158 (95% CI 0.066 to 0.250) p < 0.001a |
| BMIb | β = −0.181 (95% CI −0.228 to −0.134) p < 0.001a | β = −0.201 (95% CI −0.268 to −0.134) p < 0.001a | β = −0.179 (95% CI −0.274 to −0.084) p < 0.001a | β = −0.153 (95% CI −0.245 to −0.061) p < 0.001a |
| SBPb | β = −0.016 (95% CI −0.063 to 0.031) p = 0.371 | β = 0.018 (95% CI −0.029 to 0.058) p = 0.519 | β = −0.085 (95% CI −0.180 to −0.010) p = 0.022 | β = 0.018 (95% CI −0.074 to 0.110) p = 0.678 |
| Diabetesb | β = −0.065 (95% CI −0.112 to −0.018) p < 0.001a | β = −0.097 (95% CI −0.164 to −0.030) p < 0.001a | β = 0.042 (95% CI −0.053 to 0.137) p = 0.264 | β = −0.145 (95% CI −0.237 to −0.053) p < 0.001a |
| Tobaccob | β = −0.059 (95% CI −0.106 to −0.012) p = 0.001a | β = −0.099 (95% CI −0.166 to −0.032) p < 0.001a | β = −0.029 (95% CI −0.124 to 0.066) p = 0.434 | β = −0.048 (95% CI −0.140 to 0.044) p = 0.263 |
| APOE ε4b | β = 0.139 (95% CI 0.092 to 0.186) p < 0.001a | β = 0.059 (95% CI −0.008 to 0.126) p = 0.034 | β = 0.179 (95% CI 0.084 to 0.274) p < 0.001a | β = 0.074 (95% CI −0.018 to 0.166) p = 0.087 |
| Cognitive Impairmentb | β = 0.150 (95% CI 0.103 to 0.197) p < 0.001a |
Abbreviations: 95% CI = 95% confidence interval; APOE = apolipoprotein E; BMI = body mass index; Cognitive Impairment = mild cognitive impairment and dementia vs cognitively unimpaired; GFAP = glial fibrillary acidic protein; SBP = systolic blood pressure.
Standardized beta values derived from regression models examining associations with serum GFAP in the whole sample and by the diagnostic group. Models for age and sex were adjusted for site. All other models adjusted for site, age, and sex.
p < 0.006 level of significance after Bonferroni correction for 8 independent variables.
Adjustment for age and sex.
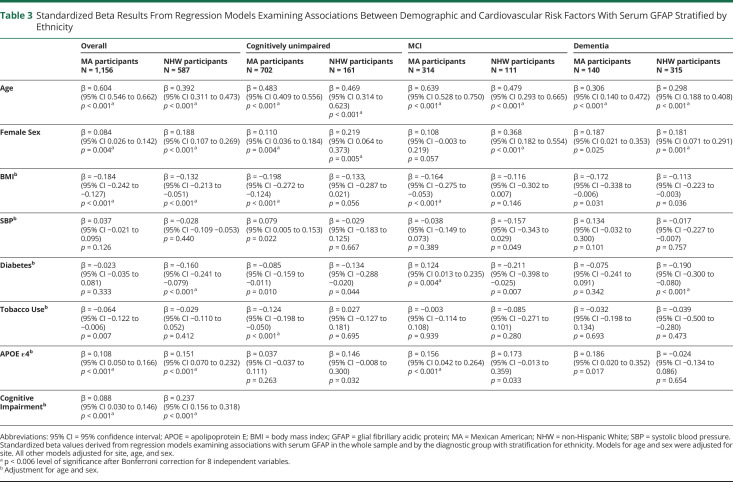
Associations Between Demographic and Clinical Variables With Serum GFAP With Stratification for Ethnicity
The associations between demographic and clinical factors with serum GFAP were typically consistent between ethnicities across the sample and by the diagnostic group (Table 3). However, sex was not significantly associated with serum GFAP in the MCI or dementia groups among MA participants. Diabetes displayed more consistent negative associations with GFAP across diagnostic groups among NHW participants relative to MA participants, whereas BMI was more consistently associated with lower GFAP across diagnostic groups in MA participants relative to NHW participants. Tobacco use only reached statistical significance in the CU group, and APOE ε4 was only significant in the MCI group among MA participants. In both MA and NHW participants, the presence of cognitive impairment was significantly associated with serum GFAP; however, the standardized beta was almost 3 times larger among NHW participants. Exploratory analyses (eTable 1, links.lww.com/WNL/D51) indicated significant interactions between ethnicity with age and diabetes, alongside a strong trend for cognitive impairment.
Table 3.
Standardized Beta Results From Regression Models Examining Associations Between Demographic and Cardiovascular Risk Factors With Serum GFAP Stratified by Ethnicity
| Overall | Cognitively unimpaired | MCI | Dementia | |||||
| MA participants N = 1,156 |
NHW participants N = 587 |
MA participants N = 702 |
NHW participants N = 161 | MA participants N = 314 |
NHW participants N = 111 | MA participants N = 140 |
NHW participants N = 315 |
|
| Age | β = 0.604 (95% CI 0.546 to 0.662) p < 0.001a | β = 0.392 (95% CI 0.311 to 0.473) p < 0.001a | β = 0.483 (95% CI 0.409 to 0.556) p < 0.001a | β = 0.469 (95% CI 0.314 to 0.623) p < 0.001a | β = 0.639 (95% CI 0.528 to 0.750) p < 0.001a | β = 0.479 (95% CI 0.293 to 0.665) p < 0.001a | β = 0.306 (95% CI 0.140 to 0.472) p < 0.001a | β = 0.298 (95% CI 0.188 to 0.408) p < 0.001a |
| Female Sex | β = 0.084 (95% CI 0.026 to 0.142) p = 0.004a | β = 0.188 (95% CI 0.107 to 0.269) p < 0.001a | β = 0.110 (95% CI 0.036 to 0.184) p = 0.004a | β = 0.219 (95% CI 0.064 to 0.373) p = 0.005a | β = 0.108 (95% CI −0.003 to 0.219) p = 0.057 | β = 0.368 (95% CI 0.182 to 0.554) p < 0.001a | β = 0.187 (95% CI 0.021 to 0.353) p = 0.025 | β = 0.181 (95% CI 0.071 to 0.291) p = 0.001a |
| BMIb | β = −0.184 (95% CI −0.242 to −0.127) p < 0.001a | β = −0.132 (95% CI −0.213 to −0.051) p < 0.001a | β = −0.198 (95% CI −0.272 to −0.124) p < 0.001a | β = −0.133, (95% CI −0.287 to 0.021) p = 0.056 | β = −0.164 (95% CI −0.275 to −0.053) p < 0.001a | β = −0.116 (95% CI −0.302 to 0.007) p = 0.146 | β = −0.172 (95% CI −0.338 to −0.006) p = 0.031 | β = −0.113 (95% CI −0.223 to −0.003) p = 0.036 |
| SBPb | β = 0.037 (95% CI −0.021 to 0.095) p = 0.126 | β = −0.028 (95% CI −0.109 −0.053) p = 0.440 | β = 0.079 (95% CI 0.005 to 0.153) p = 0.022 | β = −0.029 (95% CI −0.183 to 0.125) p = 0.667 | β = −0.038 (95% CI −0.149 to 0.073) p = 0.389 | β = −0.157 (95% CI −0.343 to 0.029) p = 0.049 | β = 0.134 (95% CI −0.032 to 0.300) p = 0.101 | β = −0.017 (95% CI −0.227 to −0.007) p = 0.757 |
| Diabetesb | β = −0.023 (95% CI −0.035 to 0.081) p = 0.333 | β = −0.160 (95% CI −0.241 to −0.079) p < 0.001a | β = −0.085 (95% CI −0.159 to −0.011) p = 0.010 | β = −0.134 (95% CI −0.288 −0.020) p = 0.044 | β = 0.124 (95% CI 0.013 to 0.235) p = 0.004a | β = −0.211 (95% CI −0.398 to −0.025) p = 0.007 | β = −0.075 (95% CI −0.241 to 0.091) p = 0.342 | β = −0.190 (95% CI −0.300 to −0.080) p < 0.001a |
| Tobacco Useb | β = −0.064 (95% CI −0.122 to −0.006) p = 0.007 | β = −0.029 (95% CI −0.110 to 0.052) p = 0.412 | β = −0.124 (95% CI −0.198 to −0.050) p < 0.001a | β = 0.027 (95% CI −0.127 to 0.181) p = 0.695 | β = −0.003 (95% CI −0.114 to 0.108) p = 0.939 | β = −0.085 (95% CI −0.271 to 0.101) p = 0.280 | β = −0.032 (95% CI −0.198 to 0.134) p = 0.693 | β = −0.039 (95% CI −0.500 to −0.280) p = 0.473 |
| APOE ε4b | β = 0.108 (95% CI 0.050 to 0.166) p < 0.001a | β = 0.151 (95% CI 0.070 to 0.232) p < 0.001a | β = 0.037 (95% CI −0.037 to 0.111) p = 0.263 | β = 0.146 (95% CI −0.008 to 0.300) p = 0.032 | β = 0.156 (95% CI 0.042 to 0.264) p < 0.001a | β = 0.173 (95% CI −0.013 to 0.359) p = 0.033 | β = 0.186 (95% CI 0.020 to 0.352) p = 0.017 | β = −0.024 (95% CI −0.134 to 0.086) p = 0.654 |
| Cognitive Impairmentb | β = 0.088 (95% CI 0.030 to 0.146) p < 0.001a | β = 0.237 (95% CI 0.156 to 0.318) p < 0.001a | ||||||
Abbreviations: 95% CI = 95% confidence interval; APOE = apolipoprotein E; BMI = body mass index; GFAP = glial fibrillary acidic protein; MA = Mexican American; NHW = non-Hispanic White; SBP = systolic blood pressure.
Standardized beta values derived from regression models examining associations with serum GFAP in the whole sample and by the diagnostic group with stratification for ethnicity. Models for age and sex were adjusted for site. All other models adjusted for site, age, and sex.
p < 0.006 level of significance after Bonferroni correction for 8 independent variables.
Adjustment for age and sex.
In models stratified by the diagnostic group, there was a significant interaction between ethnicity and diabetes for the MCI group and a significant interaction between ethnicity and APOE ε4 carriage for the dementia group.
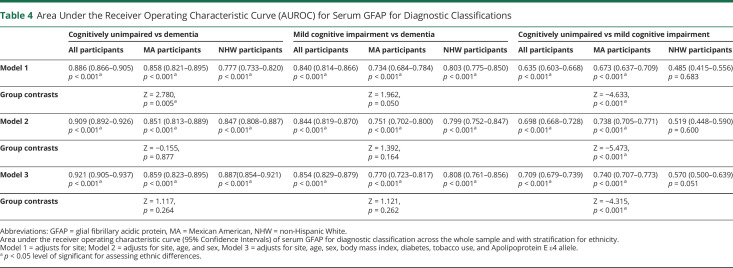
Serum GFAP and Diagnostic Classifications Across the Whole Sample and With Stratification by Ethnicity
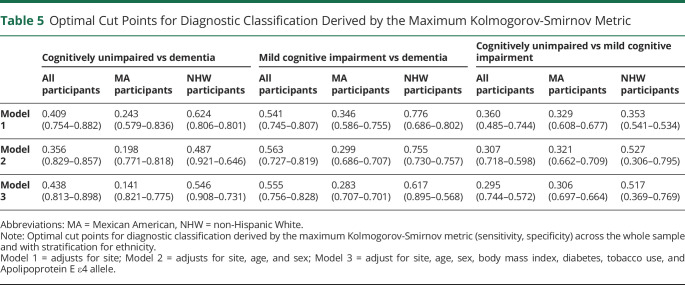
As presented in Table 4, the AUROC was highest for discriminations between dementia and CU with better performance among MA participants relative to NHW participants. Across the whole sample and by ethnicity, the AUROC was improved with additional adjustment for BMI, diabetes, tobacco use, and APOE ε4 status. For predicting the odds of dementia vs MCI, the highest AUROC was found in the fully adjusted models, and no significant differences were observed between ethnicities. For predicting odds of MCI vs CU, inclusion of additional covariates improved the AUROC with stronger discriminability observed among MA participants relative to NHW participants. In supplementary analyses restricting the MCI sample to the amnestic subtype (aMCI) (eTable 2, links.lww.com/WNL/D52), the discriminability of dementia relative to aMCI was stronger among NHW participants than MA participants when adjusting only for site, which was attenuated by inclusion of additional covariates. The discriminability of aMCI relative to CU was higher in MA participants than NHW participants with adjustment for age, sex, and site. Although overall AUROCs were typically similar between ethnicities, cut-off values differed (Table 5). Across pairwise comparisons and models, lower optimal cut-off values were derived for MA participants relative to NHW participants.
Table 4.
Area Under the Receiver Operating Characteristic Curve (AUROC) for Serum GFAP for Diagnostic Classifications
| Cognitively unimpaired vs dementia | Mild cognitive impairment vs dementia | Cognitively unimpaired vs mild cognitive impairment | |||||||
| All participants | MA participants | NHW participants | All participants | MA participants | NHW participants | All participants | MA participants | NHW participants | |
| Model 1 | 0.886 (0.866–0.905) p < 0.001a | 0.858 (0.821–0.895) p < 0.001a | 0.777 (0.733–0.820) p < 0.001a | 0.840 (0.814–0.866) p < 0.001a | 0.734 (0.684–0.784) p < 0.001a | 0.803 (0.775–0.850) p < 0.001a | 0.635 (0.603–0.668) p < 0.001a | 0.673 (0.637–0.709) p < 0.001a | 0.485 (0.415–0.556) p = 0.683 |
| Group contrasts | Z = 2.780, p = 0.005a | Z = 1.962, p = 0.050 | Z = −4.633, p < 0.001a | ||||||
| Model 2 | 0.909 (0.892–0.926) p < 0.001a | 0.851 (0.813–0.889) p < 0.001a | 0.847 (0.808–0.887) p < 0.001a | 0.844 (0.819–0.870) p < 0.001a | 0.751 (0.702–0.800) p < 0.001a | 0.799 (0.752–0.847) p < 0.001a | 0.698 (0.668–0.728) p < 0.001a | 0.738 (0.705–0.771) p < 0.001a | 0.519 (0.448–0.590) p = 0.600 |
| Group contrasts | Z = −0.155, p = 0.877 | Z = 1.392, p = 0.164 | Z = −5.473, p < 0.001a | ||||||
| Model 3 | 0.921 (0.905–0.937) p < 0.001a | 0.859 (0.823–0.895) p < 0.001a | 0.887(0.854–0.921) p < 0.001a | 0.854 (0.829–0.879) p < 0.001a | 0.770 (0.723–0.817) p < 0.001a | 0.808 (0.761–0.856) p < 0.001a | 0.709 (0.679–0.739) p < 0.001a | 0.740 (0.707–0.773) p < 0.001a | 0.570 (0.500–0.639) p = 0.051 |
| Group contrasts | Z = 1.117, p = 0.264 | Z = 1.121, p = 0.262 | Z = −4.315, p < 0.001a | ||||||
Abbreviations: GFAP = glial fibrillary acidic protein, MA = Mexican American, NHW = non-Hispanic White.
Area under the receiver operating characteristic curve (95% Confidence Intervals) of serum GFAP for diagnostic classification across the whole sample and with stratification for ethnicity.
Model 1 = adjusts for site; Model 2 = adjusts for site, age, and sex, Model 3 = adjusts for site, age, sex, body mass index, diabetes, tobacco use, and Apolipoprotein E ε4 allele.
p < 0.05 level of significant for assessing ethnic differences.
Table 5.
Optimal Cut Points for Diagnostic Classification Derived by the Maximum Kolmogorov-Smirnov Metric
| Cognitively unimpaired vs dementia | Mild cognitive impairment vs dementia | Cognitively unimpaired vs mild cognitive impairment | |||||||
| All participants | MA participants | NHW participants | All participants | MA participants | NHW participants | All participants | MA participants | NHW participants | |
| Model 1 | 0.409 (0.754–0.882) | 0.243 (0.579–0.836) | 0.624 (0.806–0.801) | 0.541 (0.745–0.807) | 0.346 (0.586–0.755) | 0.776 (0.686–0.802) | 0.360 (0.485–0.744) | 0.329 (0.608–0.677) | 0.353 (0.541–0.534) |
| Model 2 | 0.356 (0.829–0.857) | 0.198 (0.771–0.818) | 0.487 (0.921–0.646) | 0.563 (0.727–0.819) | 0.299 (0.686–0.707) | 0.755 (0.730–0.757) | 0.307 (0.718–0.598) | 0.321 (0.662–0.709) | 0.527 (0.306–0.795) |
| Model 3 | 0.438 (0.813–0.898) | 0.141 (0.821–0.775) | 0.546 (0.908–0.731) | 0.555 (0.756–0.828) | 0.283 (0.707–0.701) | 0.617 (0.895–0.568) | 0.295 (0.744–0.572) | 0.306 (0.697–0.664) | 0.517 (0.369–0.769) |
Abbreviations: MA = Mexican American, NHW = non-Hispanic White.
Note: Optimal cut points for diagnostic classification derived by the maximum Kolmogorov-Smirnov metric (sensitivity, specificity) across the whole sample and with stratification for ethnicity.
Model 1 = adjusts for site; Model 2 = adjusts for site, age, and sex; Model 3 = adjust for site, age, sex, body mass index, diabetes, tobacco use, and Apolipoprotein E ε4 allele.
In exploratory analyses assessing AUROCs with stratification by sex and ethnicity (eTable 3, links.lww.com/WNL/D53), there were generally no significant differences by the group. However, the discrimination of MCI relative to CU was stronger for female participants relative to male participants in the whole sample and within MA participants.
Discussion
In our study of MA and NHW older adults across the continuum of CU to AD dementia, BMI, diabetes, and tobacco use were significantly associated with lower serum GFAP levels. These findings directly contrasted with the associations of traditional AD risk factors, including older age and APOE ε4 carrier status, which positively associated with circulating GFAP levels as previously reported.31,32 Associations between demographic and clinical factors with serum GFAP levels were generally consistent across ethnicities and diagnostic groups. However, in models only adjusted for age and sex, the standardized beta for the presence of cognitive impairment in association with serum GFAP was roughly 3 times larger among the NHW participants relative to MA participants. Inclusion of additional covariates, including age, sex, BMI, tobacco use, and APOE ε4 carrier status, improved discriminability of dementia relative to CU with no significant differences observed across ethnic groups. Nonetheless, the optimal cut points for diagnostic discrimination differed between ethnic groups, which persisted with adjustment for covariates. Overall, the results highlight the importance of understanding the role of broader demographic and clinical factors on circulating GFAP levels within diverse cohorts in effort to enhance precision for neurodegenerative disease.
Across ethnic groups, older age and APOE ε4 carrier status were related to higher GFAP levels, which is consistent with previous reports31,32 and may reflect their well-established associations with neurodegenerative disease risk.33 However, similar findings have also been reported among CU middle-aged adults,34 suggesting that they may also be partially attributable to differences in protein generation and/or turnover.3 Cardiovascular risk factors, including higher BMI, diabetes, and tobacco use, were in contrast associated with lower serum GFAP levels. The inverse relationships with serum GFAP are interesting considering these factors have been associated with increased risk of ADRD,35 although the directionality is less consistent in late life.36,37 Previous research has demonstrated negative associations between circulating ADRD biomarkers and cardiovascular risk factors with some variability across the samples and biomarkers examined.3,12 For example, higher BMI was associated with lower plasma p-tau 181, p-tau 217, and NFL levels but was unrelated to plasma aβ42/aβ40 and total tau in the multiethnic WHICAP study.38 In the BIOFINDER studies, higher creatinine and lower BMI were associated with increased plasma GFAP and NFL levels.21 However, there were no consistent associations with hypertension, hyperlipidemia, diabetes, ischemic heart disease, or prior stroke.21 The results highlight the complex relationship between ADRD blood biomarkers and broader medical comorbidities, which may vary based on disease burden, the degree of comorbidity management, and distinct constellations of risk factors present in the cohorts examined.
Encouragingly, AUROC analyses generally demonstrated similar diagnostic accuracy of serum GFAP in MA and NHW participants in our study. Not surprisingly, the AUROC was the highest for the pairwise comparison of dementia vs CU and poorest for MCI vs CU. In models only adjusted for site, the discrimination of both dementia and MCI relative to CU was higher in MA relative to NHW participants, which may be related in part to the larger MA participant sample size. Further expansion of the models to include additional adjustment for BMI, diabetes, tobacco use, and APOE ε4 status optimized model fit across the sample and with stratification by ethnic group. AUROCs were generally consistent across ethnicities with the exception of stronger discriminability of MCI vs CU among MA participants relative to NHW participants.
Although AUROC values for serum GFAP were generally similar between the ethnic groups, the optimal cut-off values derived from these models differed. Regardless of the pairwise contrasts examined, the optimal cut points were lower among MA participants relative to NHW participants, and the findings persisted with adjustment for additional demographic and clinical characteristics. While we fully concede that further replication is necessary and that these values should not be adopted in clinical or research settings, the findings illuminate the importance of further validation of ADRD blood biomarkers in diverse cohorts. As the pattern of results remained with adjustment for pertinent demographic and clinical characteristics in our study, the results also highlight the need to examine a broader array of variables that may contribute to disparities. For example, O'Bryant et al. previously reported associations between physical functioning, dyslipidemia, hypertension, diabetes, and chronic kidney disease with plasma levels of aβ40, aβ42, NFL, and total tau.15,39 In addition, factors such as access to medical care, environmental pollutants, and exposure to chronic stress secondary to discrimination require further examination.12
The findings of this study must be considered in the context of its limitations. First, our sample lacked neuroimaging and CSF outcomes, and diagnostic classifications were determined by consensus review. In light of the potential influence of culture, education, and literacy on clinical decision-making,12 the underlying neurodegenerative disease burden may have differed between the ethnic groups. In the IDEAS Cohort Study, individuals of Hispanic ethnicity diagnosed with MCI or dementia had lower likelihood of amyloid PET positivity relative to NHW participants,40 suggesting the importance of further evaluations in cohorts with additional ADRD biomarker data available. In addition, we are unable to examine whether the associations of serum GFAP with demographic and clinical factors persist with adjustment for neuropathologic burden. Finally, our study cohort, TARCC, was conducted across several academic institutions over a number of years.24 Differences in storage time and/or collection parameters may contribute to variability; however, serum GFAP levels have been shown to be fairly robust to freeze-thaw cycles.41
In summary, serum GFAP levels were associated with age, sex, BMI, diabetes, tobacco use, and APOE ε4 carrier status in our cohort of MA and NHW older adults across the continuum from CU to AD dementia. The diagnostic accuracy of serum GFAP was generally consistent between ethnicities, and model fit improved with adjustment for demographic and clinical variables. Notably, the optimized cut-off values for log-transformed standardized serum GFAP derived from these models differed between ethnicities with lower cut points in MA participants relative to NHW participants. Overall, the findings highlight the importance of further validation of ADRD blood biomarkers in diverse cohorts and command a call for broader examinations of the multidimensional determinants of health that may contribute to underlying disparities.12
Acknowledgment
The authors thank the TARCC participants, study team, and investigators.
Glossary
- AD
Alzheimer disease
- APOE
apolipoprotein E
- AUROC
area under the receiver operating characteristic curves
- BMI
body mass index
- CU
cognitively unimpaired
- GFAP
glial fibrillary acidic protein
- MA
Mexican American
- MCI
mild cognitive impairment
- NFL
neurofilament light
- NHW
non-Hispanic White
- SBP
systolic blood pressure
- TARCC
Texas Alzheimer Research and Care Consortium
Appendix. Authors
| Name | Location | Contribution |
| Mitzi M. Gonzales, PhD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases; Department of Neurology, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Gabriel Vela, BS | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Vinu Philip, MBBS | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Hector Trevino, BS, MPH | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Ashley LaRoche, BS | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Chen-Pin Wang, PhD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases; Department of Population Health Sciences, University of Texas Health Science Center, San Antonio; South Texas Veterans Health Care System, Geriatric Research, Education and Clinical Center, San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Danielle M. Parent, BS | Departments of Pathology and Laboratory Medicine, and Biochemistry, Larner College of Medicine, University of Vermont, Burlington | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Tiffany Kautz, PhD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases; Department of Medicine, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content |
| Claudia L. Satizabal, PhD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio; Department of Population Health Sciences, University of Texas Health Science Center, San Antonio; Department of Neurology, Boston University School of Medicine, MA, | Drafting/revision of the manuscript for content, including medical writing for content |
| Jeremy Tanner, MD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases; Department of Neurology, University of Texas Health Science Center at San Antonio | Drafting/revision of the manuscript for content, including medical writing for content |
| Sid O'Bryant, PhD | Institute for Translational Research and Department of Family Medicine, University of North Texas Health Science Center, Fort Worth | Drafting/revision of the manuscript for content, including medical writing for content |
| Gladys Maestre, MD, PhD | Neurosciences Laboratory, Biological Research Institute and Research Institute of Cardiovascular Diseases, Faculty of Medicine, Universidad del Zulia, Maracaibo, Venezuela; Department of Biomedical Sciences, Division of Neurosciences, University of Texas Rio Grande Valley School of Medicine, Brownsville | Drafting/revision of the manuscript for content, including medical writing for content |
| Russell P. Tracy, PhD | Departments of Pathology & Laboratory Medicine, and Biochemistry, Larner College of Medicine, University of Vermont, Burlington | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sudha Seshadri, MD | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases; Department of Neurology, University of Texas Health Science Center at San Antonio; Department of Neurology, Boston University School of Medicine, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The Texas Alzheimer Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer Disease and Related Disorders, Additional funding was provided by grants through TARCC [2018-28-81-JI], the National Institute on Aging [R01 AG077472, AG054076, AG059421, P30AG066546], the National Institute of Neurologic Disorders and Stroke [NS100605, UF1NS125513] and the Alzheimer Drug Discovery Foundation [GDAPB-202010-2020940].
Disclosure
M. M. Gonzales has received funding from the Texas Alzheimer Research and Care Consortium, the National Institute on Aging, the Alzheimer Association, Alzheimer Drug Discovery Foundation, and institutional pilot awards, as well as a travel award from the Alzheimer Association International Conference. M. M. Gonzales also owns stock in Abbvie. C. Wang has received support from the NIH. C. Satizabal has received support from the NIH. J. Tanner has received support from the NIH. S. O'Bryant received funding from the National Institute on Aging and has a sponsored research agreement from Cx Precision Medicine, where he serves as the founding scientist and owns stock. S. O'Bryant also reports having multiple patents issued or pending on precision medicine for neurodegenerative diseases. G. Maestre has received funding from the National Institute on Aging, the National Eye Institute, and the Texas Alzheimer Research and Care Consortium. G. Maestre serves on the committees for FundaConCiencia Inc and APPREMED. R. Tracy has received funding from the NIH. S. Seshadri has received funding from the National Institute on Aging, the National Institute of Neurologic Disorders and Stroke, and the Alzheimer Association, as well as consulting fees from Biogen. G. A. Vela, V. Philip, H. Trevino, A. LaRoche, C. Wang, D. M. Parent, and T. F. Kautz report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Jack CR, Bennett DA, Blennow K, et al. . NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampel H, O'Bryant SE, Molinuevo JL, et al. . Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14(11):639-652. doi: 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson O, Edelmayer RM, Boxer AL, et al. . The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan AR, Touchard S, Leckey C, et al. . Inflammatory biomarkers in Alzheimer's disease plasma. Alzheimers Dement. 2019;15(6):776-787. doi: 10.1016/j.jalz.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121-130. doi: 10.1016/j.ceb.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Asken BM, Elahi FM, La Joie R, et al. . Plasma glial fibrillary acidic protein levels differ along the spectra of amyloid burden and clinical disease stage. J Alzheimers Dis. 2020;78(1):265-276. doi: 10.3233/jad-200755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedet AL, Milà-Alomà M, Vrillon A, et al. . Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471-1483. doi: 10.1001/jamaneurol.2021.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shir D, Graff-Radford J, Hofrenning EI, et al. . Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer's disease and vascular pathology. Alzheimers Dement (Amst). 2022;14(1):e12291. doi: 10.1002/dad2.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajan KB, Aggarwal NT, McAninch EA, et al. . Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065-1076. doi: 10.1002/ana.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales MM, Wiedner C, Wang C-P, et al. . A population-based meta-analysis of circulating GFAP for cognition and dementia risk. Ann Clin Transl Neurol. 2022;9(10):1574-1585. doi: 10.1002/acn3.51652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzales MM, Wang C-P, Short MI, et al. . Blood biomarkers for cognitive decline and clinical progression in a Mexican American cohort. Alzheimers Dement (Amst). 2022;14(1):e12298. doi: 10.1002/dad2.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babulal GM, Quiroz YT, Albensi BC, et al. . Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292-312. doi: 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stites SD, Midgett S, Mechanic-Hamilton D, et al. . Establishing a framework for gathering structural and social determinants of health in Alzheimer's disease research centers. Gerontologist. 2022;62(5):694-703. doi: 10.1093/geront/gnab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales MM, Short MI, Satizabal CL, et al. . Blood biomarkers for dementia in Hispanic and non-Hispanic White adults. Alzheimers Dement (N Y). 2021;7(1):e12164. doi: 10.1002/trc2.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Bryant SE, Petersen M, Hall J, Johnson LA, Team HHS. Medical comorbidities and ethnicity impact plasma Alzheimer's disease biomarkers: important considerations for clinical trials and practice. Alzheimer's Demen. 2023;19(1):36-43. doi: 10.1002/alz.12647 [DOI] [PubMed] [Google Scholar]
- 16.Khan MJ, Desaire H, Lopez OL, Kamboh MI, Robinson RAS. Why inclusion matters for Alzheimer's disease biomarker discovery in plasma. J Alzheimers Dis. 2021;79(3):1327-1344. doi: 10.3233/jad-201318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler SE, Karikari TK, Ashton NJ, et al. . Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245–e257. doi: 10.1212/wnl.0000000000200358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Bryant S, Petersen M, Hall J, et al. . Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: results from the HABLE study. Alzheimers Dement. 2022;18(2):240-250. doi: 10.1002/alz.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Bryant SE, Johnson LA, Barber RC, et al. . The health & aging brain among latino elders (HABLE) study methods and participant characteristics. Alzheimers Dement (Amst). 2021;13(1):e12202. doi: 10.1002/dad2.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. . Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128-1140. doi: 10.1002/alz.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichet Binette A, Janelidze S, Cullen N, et al. . Confounding factors of Alzheimer's disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement. 2022;19(4):1403-1414. doi: 10.1002/alz.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielke MM, Dage JL, Frank RD, et al. . Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398-1405. doi: 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minas TZ, Kiely M, Ajao A, Ambs S. An overview of cancer health disparities: new approaches and insights and why they matter. Carcinogenesis. 2020;42(1):2-13. doi: 10.1093/carcin/bgaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waring S, O'Bryant SE, Reisch JS, Diaz-Arrastia R, Knebl J, Doody R. The Texas Alzheimer's Research Consortium longitudinal research cohort: study design and baseline characteristics. Tex Public Health J. 2008;60:9-13. [Google Scholar]
- 25.Beekly DL, Ramos EM, Lee WW, et al. . The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249-258. doi: 10.1097/wad.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group* under the auspices of Department of health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939-944. doi: 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC. Mild Cognitive Impairment: Aging to Alzheimer's Disease. Oxford University Press; 2003. [Google Scholar]
- 28.O'Bryant SE, Gupta V, Henriksen K, et al. . Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11(5):549-560. doi: 10.1016/j.jalz.2014.08.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 30.Bradley AP. ROC curve equivalence using the Kolmogorov–Smirnov test. Pattern Recognition Lett. 2013;34(5):470-475. doi: 10.1016/j.patrec.2012.12.021 [DOI] [Google Scholar]
- 31.Abdelhak A, Foschi M, Abu-Rumeileh S, et al. . Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158-172. doi: 10.1038/s41582-021-00616-3 [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee P, Pedrini S, Stoops E, et al. . Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021;11(1):27. doi: 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134-147. doi: 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milà-Alomà M, Brinkmalm A, Rodriguez JL, et al. . Distinctive effect of biological sex in AD-related CSF and plasma biomarkers. Alzheimers Dement. 2021;17(S5):e052959. doi: 10.1002/alz.052959 [DOI] [Google Scholar]
- 35.Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath ER, Beiser AS, O'Donnell A, et al. . Determining vascular risk factors for dementia and dementia risk prediction across mid- to later life. Neurology. 2022;99(2):e142-e153. doi: 10.1212/wnl.0000000000200521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Brok MGHE, Eggink E, Hoevenaar-Blom MP, et al. . Low values for blood pressure, BMI, and Non-HDL cholesterol and the risk of late-life dementia. Neurology. 2022;99(15):e1630-e1639. doi: 10.1212/wnl.0000000000200954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickman AM, Manly JJ, Honig LS, et al. . Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353-1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Bryant SE, Petersen M, Hall JR, et al. . Plasma biomarkers of Alzheimer's disease are associated with physical functioning outcomes among cognitively normal adults in the multiethnic HABS-HD cohort. J Gerontol A Biol Sci Med Sci. 2023;78(1):9-15. doi: 10.1093/gerona/glac169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkins CH, Windon CC, Dilworth-Anderson P, et al. . Racial and ethnic differences in amyloid PET positivity in individuals with mild cognitive impairment or dementia: a secondary analysis of the imaging dementia–evidence for amyloid scanning (IDEAS) cohort study. JAMA Neurol. 2022;79(11):1139-1147. doi: 10.1001/jamaneurol.2022.3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simrén J, Weninger H, Brum WS, et al. . Differences between blood and cerebrospinal fluid glial fibrillary acidic protein levels: the effect of sample stability. Alzheimers Dement. 2022;18(10):1988-1992. doi: 10.1002/alz.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support our findings, which we can legally share, are accessible by request through TARCC: ais.swmed.edu/redcap/surveys/?s=CX8MJ4Y7XD. This study is reported in accordance with the STROBE checklist (eAppendix 1, links.lww.com/WNL/D50).