Abstract
Neuroplasticity in cortico-limbic circuits has been implicated in pain persistence and pain modulation in clinical and preclinical studies. The amygdala has emerged as a key player in the emotional-affective dimension of pain and pain modulation. Reciprocal interactions with medial prefrontal cortical regions undergo changes in pain conditions. Other limbic and paralimbic regions have been implicated in pain modulation as well. The cortico-limbic system is rich in opioids and opioid receptors. Preclinical evidence for their pain modulatory effects in different regions of this highly interactive system, potentially opposing functions of different opioid receptors, and knowledge gaps will be described here. There is little information about cell type- and circuit-specific functions of opioid receptor subtypes related to pain processing and pain-related plasticity in the cortico-limbic system. The important role of anterior cingulate cortex (ACC) and amygdala in MOR-dependent analgesia is most well-established, and MOR actions in the mesolimbic system appear to be similar but remain to be determined in mPFC regions other than ACC. Evidence also suggests that KOR signaling generally serves opposing functions whereas DOR signaling in the ACC has similar, if not synergistic effects, to MOR. A unifying picture of pain-related neuronal mechanisms of opioid signaling in different elements of the cortico-limbic circuitry has yet to emerge.
Keywords: Amygdala, prefrontal cortex, mesolimbic, neuroplasticity, pain, kappa opioid receptor, mu opioid receptor, delta opioid receptor
1. Cortico-limbic plasticity in pain
The limbic system is comprised of cortical and subcortical structures, including medial prefrontal cortical regions (mPFC) with anterior cingulate cortex (ACC), and hippocampus and amygdala. Ventral components of the basal ganglia such as nucleus accumbens (NAc) and paralimbic regions such as the insular cortex (IC) interact closely with these cortico-limbic circuits. The cortico-limbic system plays an important role in pain mechanisms and pain modulation, reward and substance abuse (Navratilova et al., 2015a; Taylor, 2018; Thompson and Neugebauer, 2019). Neuroplasticity defined as a functional and/or structural change that outlasts or becomes independent of the initial event, has been shown in different regions of the mPFC (reviewed in Kummer et al., 2020) and amygdala (reviewed in Neugebauer, 2020) in pain models, and there is some evidence for pain-related neuroplasticity in IC (Qiu et al., 2014) and NAc (see Ren et al., 2021). Interactions of ACC and IC with amygdala are largely facilitatory (Jasmin et al., 2003; Zhuo, 2011) whereas those between infra- and pre-limbic mPFC and amygdala involve powerful feedforward inhibition (Cheriyan et al., 2016; Ji et al., 2010; Kiritoshi and Neugebauer, 2018; Thompson and Neugebauer, 2019). Here we focus on preclinical studies of neuroplasticity in the cortico-limbic system in pain as the basis for understanding opioid system functions in these regions (see Figure 1).
Figure 1.
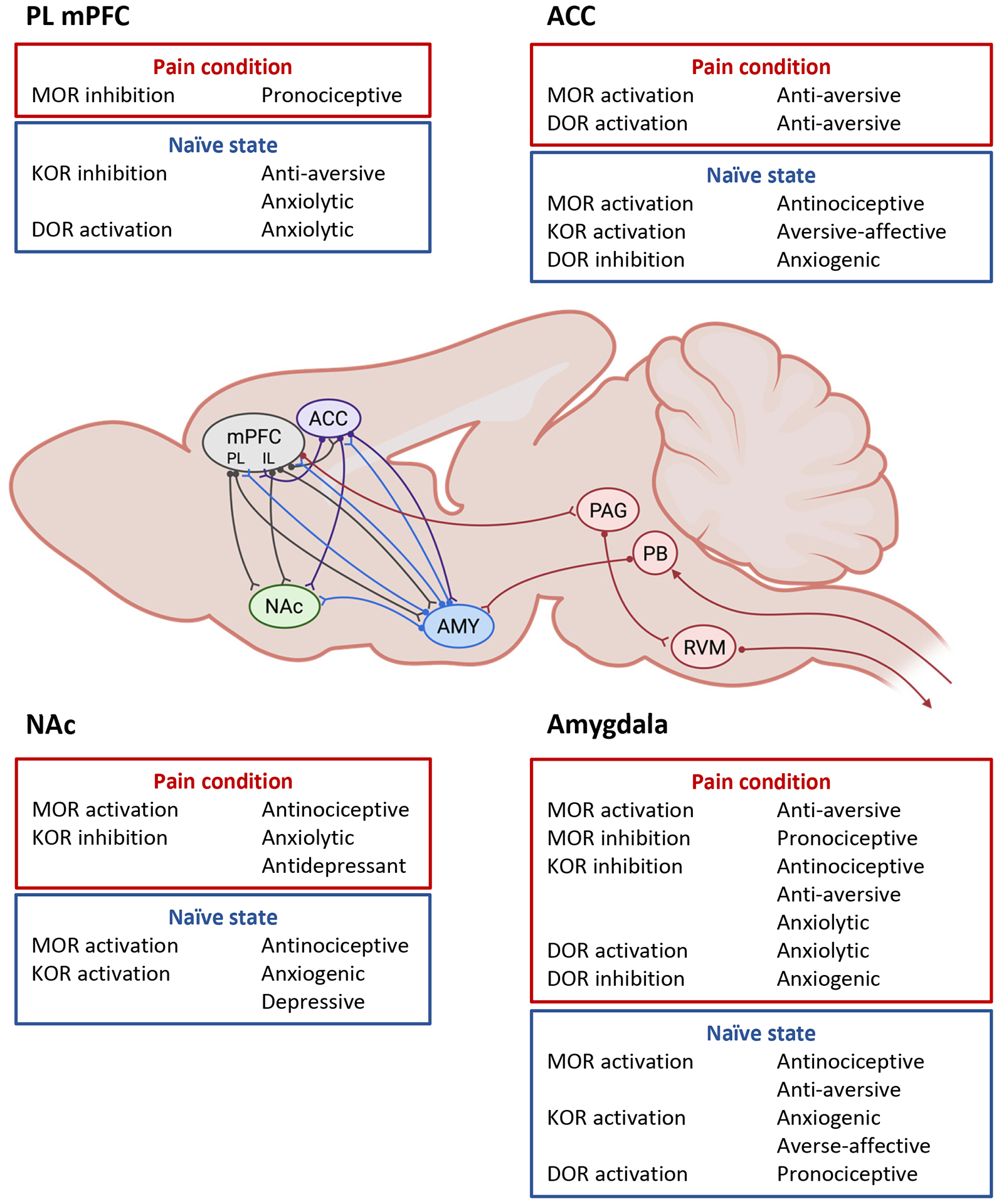
Key effects of MOR, KOR and DOR modulation in different elements of the cortico-limbic system. ACC, anterior cingulate cortex, IL, infralimbic, mPFC, medial prefrontal cortex, NAc, nucleus accumbens, PAG, Periaqueductal gray, PB, Parabrachial nucleus, PL, prelimbic, RVM, Rostral ventromedial medulla. Diagram shows main connections between cortico-limbic areas and brainstem connections.
1.1. Medial prefrontal cortex (mPFC)
The mPFC serves executive functions, decision-making, memory retrieval, and control of emotions and behavior (Bechara and Damasio, 2005; Euston et al., 2012; Wood and Grafman, 2003). Different subregions are infralimbic, prelimbic and anterior cingulate cortices in rodents and Brodmann areas 25, 32 and 24b, respectively, in primates (van Heukelum et al., 2020). The infra- and pre-limbic cortices receive input primarily from limbic rather than somatosensory regions, including pain-related information from thalamus and amygdala; additionally, the ACC receives thalamo-cortical inputs from all sensory modalities (Gabbott et al., 2006; Hoover and Vertes, 2007). Direct inputs from the spinal cord have also been described (Cliffer et al., 1991). In addition to intra- and inter-cortical projections mainly from layer II/III, the different mPFC subregions project to subcortical targets, including mediodorsal thalamus (mostly layer VI), NAc (layer V of IL and PL more so than ACC), lateral hypothalamus (mainly layer V), different amygdala nuclei (layer II and V), nucleus accumbens (ACC and PL to core; IL to shell) periaqueductal gray (PAG, layer V), ventral tegmental area (VTA, layer V of IL and PL but not ACC), parabrachial nucleus (PB, layer V of IL more so than IL but not ACC), nucleus of the solitary tract (NTS, mostly layer V of IL), rostral ventromedial medulla (RVM, layer V of IL and PL and little ACC), and spinal cord (layer V mostly of PL and ACC) (Ding et al., 2001; Gabbott et al., 2005; Gabbott et al., 2006; Vertes, 2004). These targets include regions involved in pain modulation.
1.1.1. Infralimbic and prelimbic mPFC
Il and PL pyramidal cells have large receptive fields and respond to peripheral noxious stimuli (Ji and Neugebauer, 2011, 2014; Ji et al., 2010). Pain-related neuroplasticity has been shown at the BLA-IL and BLA-PL synapses (Cheriyan and Sheets, 2018; Cheriyan and Sheets, 2020; Ji and Neugebauer, 2010; Kiritoshi et al., 2016) and hippocampal-PL and thalamic-PL synapses (Kelly et al., 2016; Kelly and Martina, 2018). Input from the BLA generates monosynaptic excitation and polysynaptic inhibition in IL and PL, including in neurons projecting to PAG and amygdala (Cheriyan et al., 2016). Synaptic feedforward inhibition from BLA is increased in layer V pyramidal cells of IL (Kiritoshi et al., 2016) and PL (Ji et al., 2010) in male rats in a model of arthritic pain and in PL neurons in male mice in a model of neuropathic pain (Zhang et al., 2015b). Excitation-inhibition balance is decreased at the BLA-PL but not BLA-IL synapse (Cheriyan and Sheets, 2020) and excitatory glutamatergic transmission from hippocampus and dorsomedial thalamus to layer V PL neurons is decreased in male rats under neuropathic pain conditions (Kelly et al., 2016; Kelly and Martina, 2018). Excitatory cholinergic modulation of layer V PL is also lost (Radzicki et al., 2017) in male rats in neuropathic pain. As a consequence, decreased background and evoked activity (extracellular recordings in anesthetized rats) was found in IL (Ji and Neugebauer, 2014) and PL (Ji and Neugebauer, 2011; Ji et al., 2010) presumed pyramidal neurons in male rats in an arthritis pain model and reduction of excitability (whole-call patch-clamp in mouse brain slice) of PL but not IL neurons in male mice in a neuropathic pain model (Cheriyan and Sheets, 2018). Accordingly, restoring/increasing activity in IL and PL decreased sensory and affective arthritic (Kiritoshi et al., 2016), inflammatory (Wang et al., 2015), and neuropathic (Lee et al., 2015; Zhang et al., 2015b) pain behaviors in male rodents through downstream inhibition of amygdala output (Ji and Neugebauer, 2014) and activation of nucleus accumbens (Lee et al., 2015). It should be noted that there is evidence for increased intracortical excitatory transmission and hyperexcitability of layer II/III pyramidal cells in the PL in male rats in neuropathic pain (Cordeiro Matos et al., 2015; Metz et al., 2009) and increased local excitatory transmission onto PL layer II/III neurons in male rats in an inflammatory pain model (Wang et al., 2015), and these neurons can engage inhibitory connections with layer V neurons. However, decreased excitatory transmission between PL layer II/III and layer V neurons was reported in a neuropathic pain model (Cheriyan and Sheets, 2018) and decreased excitability of PL layer II/III in an inflammatory pain model (Wang et al., 2015) in male rodents. The IL and PL circuitry is complex, and pain-related changes may be region-, cell type- and projection-specific.
1.1.2. Anterior cingulate cortex (ACC)
Neuroplasticity has been shown in ACC in different pain models (reviewed in Bak et al., 2021; Taylor, 2018; Zhuo, 2011). Like IL and PL neurons (see 1.1.1), ACC neurons have large receptive fields and respond to noxious somatic and visceral stimuli and are activated during pain anticipation or pain avoidance behavior (Gao et al., 2006; Hutchison et al., 1999; Koyama et al., 2000; Koyama et al., 2001; Kuo and Yen, 2005; Sikes and Vogt, 1992; Yamamura et al., 1996). In contrast to IL and PL deactivation in pain conditions (see 1.1.1), ACC pyramidal layer V cells and certain layer II/III neurons (intermediate type), but not interneurons, show increased activity in neuropathic pain models in both sexes as measured with electrophysiology or calcium imaging (Blom et al., 2014; Cao et al., 2009; Santello and Nevian, 2015; Zhao et al., 2018). Increased intracortical excitatory glutamatergic synaptic transmission was found in layer II/III pyramidal cells through a presynaptic mechanism in males in inflammatory pain (Bie et al., 2011; Toyoda et al., 2009; Zhao et al., 2006; Zheng, 2010) and pre- and postsynaptic changes in males in neuropathic pain (Toyoda et al., 2009; Xu et al., 2008) models. In layer V, loss of connectivity between pyramidal cells and interneurons was interpreted to indicate ACC disinhibition in neuropathic male mice (Blom et al., 2014). Changes of several proteins related to synaptic plasticity were also reported in the ACC in male mice under neuropathic pain models, including increases in the expression levels of c-Fos, phosphorylated cyclic-AMP response element binding protein (pCREB), neural cell adhesion molecule 1 (NCAM1), and protein kinase Mzeta (PKMζ) (Bak et al., 2021; Li et al., 2010; Wei et al., 1999; Zhuo, 2011). There is evidence from peripheral nerve block experiments in male rats to suggest that ACC pain-related plasticity persists at least in part independently of primary afferent input (Wei and Zhuo, 2001).
Lesions or functional inactivation, including activation of inhibitory neurons, of the ACC decreases predominantly averse-affective and anxio-depressive aspects of inflammatory and neuropathic pain (Barthas et al., 2015; Chen et al., 2018; Fuchs et al., 2014; LaGraize et al., 2004; Lei et al., 2004; Li et al., 2009; Qu et al., 2011; Ren et al., 2008; Xiao et al., 2013; Xiao and Zhang, 2018), but may also affect the sensory aspects of pain. For example, inhibition of hypersensitivity has been reported with pharmacological or optogenetic inhibition of ACC (Chen et al., 2018; Santello and Nevian, 2015; Tan et al., 2017) or midcingulate cortex (MCC) (Tan et al., 2017) activity in male rodents. Conversely, optogenetic activation of ACC pyramidal neurons in males produces hypersensitivity (Chen et al., 2018) and anxio-depressive behaviors (Barthas et al., 2015); optogenetic activation of MCC pyramidal cells in males induced hypersensitivity but not aversive behavior (Tan et al., 2017). These effects are likely mediated through top-down control systems that involve direct projections to PAG and spinal dorsal horn as well as interactions with IC, thalamus (ventral posterolateral and -medial), amygdala and nucleus accumbens (for recent review see Thompson and Neugebauer, 2019).
1.2. Amygdala
The amygdala is comprised of different nuclei. Lateral, basolateral and central nuclei (LA, BLA, CeA) are of particular importance for sensory and nociceptive processing and pain modulation. A majority of neurons in the lateral and capsular division of the CeA (CeLC) respond to peripheral noxious stimuli primarily with excitation but a population of inhibited neurons has also been found (Bernard et al., 1990, 1992; Goncalves and Dickenson, 2012; Neugebauer and Li, 2002). Nociceptive information reaches the amygdala from the parabrachial nucleus (PB) and from thalamic nuclei (midline, posterior intralaminar/paraventricular, and posterior regions) and cortical regions (mPFC, IC, sensory association cortices) (Neugebauer, 2020). The major if not exclusive source of calcitonin gene-related peptide (CGRP) in the CeA is from the lateral PB (Dobolyi et al., 2005; Harrigan et al., 1994; Palmiter, 2018), which targets almost exclusively the CeLC and delineates what has been termed the “nociceptive amygdala” (Neugebauer et al., 2004). The identification of nociceptive input from the PB to the CeLC as part of a spino-parabrachial-amygdala pain pathway originally triggered the exploration of amygdala processing of nociceptive information and neuroplasticity in pain conditions. Some recent controversy surrounds the relay of spinal nociceptive information from the PB to the CeLC. While there is strong evidence for monosynaptic excitatory projections to the CeLC from neurons in the external lateral PB (Bernard et al., 1989; Bernard et al., 1993; Chiang et al., 2020), including neurons containing calcitonin gene-related peptide (Palmiter, 2018), these PB projection neurons may not receive nociceptive input directly from spinal dorsal horn neurons (Chiang et al., 2019) but rather through intra-PBN connectivity, involving spinal projections to tachykinin receptor 1 positive neurons in the superior lateral PB (Deng et al., 2020; Huang et al., 2019) and dynorphin-expressing neurons in the dorsal lateral PB that send axons to external lateral PB (Chiang et al., 2020). However, older studies have linked spinal input directly to the external lateral PB (Bernard et al., 1989). Thalamic and cortical inputs target primarily the LA-BLA network but also CeA neurons (Fu et al., 2020) and may contain nociceptive information. That information converges with PB input onto CeA neurons to drive a complex set of largely inhibitory GABAergic and peptidergic neurons and connections. This network includes feedforward inhibition of CeLC neurons driven by direct or indirect, via BLA, cortical (IL > PL) projections (Kiritoshi and Neugebauer, 2018).
Increased excitatory transmission at the LA-BLA, BLA-CeLC and PB-CeLC synapses has been shown in different pain models using brain slice electrophysiology, which indicates synaptic plasticity (for review see Neugebauer, 2020). Synaptic plasticity at the PB-CeLC synapse was found at the acute stages of pain models (5–6 hours to about 10 days) such as formalin, arthritis (knee), muscle, visceral (colon), and neuropathic pain. Enhanced excitatory transmission at the LA-BLA and BLA-CeLC synapses was found in an acute arthritis model and at the BLA-CeLC synapse also at the acute stage of a neuropathic pain model (see Neugebauer, 2020). A cluster of inhibitory intercalated cells (ITC) mediate feedforward inhibition of CeA neurons, which is decreased in male rats in acute pain conditions (arthritis model) (Ren et al., 2013; Ren and Neugebauer, 2010). Little is known about synaptic plasticity in the amygdala circuitry in chronic pain conditions, but enhanced excitatory transmission was detected at the BLA-CeA synapse in male rats at the chronic stage (4 weeks) of a neuropathic pain model (Ji et al., 2017). As a result, electrophysiological studies in anesthetized rats recorded increased ongoing and evoked activity of neurons in the CeA and BLA of male rats in acute arthritis (3–6 h) (Ji et al., 2017; Neugebauer and Li, 2003) and acute (2–14 days) (Goncalves and Dickenson, 2012) and chronic (4 weeks) (Ji and Neugebauer, 2019; Ji et al., 2017) neuropathic pain. Increased calcium responses to peripheral stimuli, but not ongoing activity, was also detected in BLA neurons of awake male mice at the acute and chronic stages of neuropathic pain (Corder et al., 2019).
Cell type- and projection-specific amygdala functions in pain that are only beginning to emerge and are the focus of current research efforts (Adke et al., 2021; Hein et al., 2021; Ji and Neugebauer, 2020; Li et al., 2022; Li and Sheets, 2018; Li and Sheets, 2020; Mazzitelli et al., 2022; Wilson et al., 2019; Yakhnitsa et al., 2022). PKCδ, somatostatin (SOM) and corticotropin-releasing factor (CRF) positive neurons form the main CeA cell clusters (Fadok et al., 2017; Kim et al., 2017; McCullough et al., 2018; Pomrenze et al., 2015; Sanford et al., 2017). PKCδ and CRF, but not SOM, neurons express CGRP receptors and receive CGRP input from PB (Harrigan et al., 1994; Kim et al., 2017; Ye and Veinante, 2019). It should be noted that while CRF and SOM neurons have long been recognized as CeA output neurons sending descending and ascending projections, respectively, to brainstem, hypothalamic nuclei and basal forebrain regions (Bartonjo and Lundy, 2022; Fadok et al., 2017; Li et al., 2013; Magableh and Lundy, 2014; Penzo et al., 2014; Pomrenze et al., 2015; Sanford et al., 2017; Ye and Veinante, 2019), PKCδ neurons have recently been shown to project the basal forebrain and some brainstem regions (Singh et al., 2022). PKCδ but not SOM neurons showed increased activity in male mice in a neuropathic pain model (6–14 days) (Adke et al., 2021; Wilson et al., 2019). A subtype of CeM, but not CeL, neurons projecting to the PAG had increased excitability in male and female mice in an acute inflammatory pain model (Li and Sheets, 2018). Analysis of paired-pulse ratio at the PB-CeA synapse indicated decreased synaptic efficacy in SOM positive neurons (capsular and medial CeA) but increased transmission in SOM negative neurons (capsular CeA) in mice of both sexes in a neuropathic pain model (10 days); synaptic efficacy was decreased in CRF positive and CRF negative neurons in the CeL, but increased in CRF positive neurons in CeM (Li and Sheets, 2020).
Studies using various pharmacological or knock-down strategies found that CeA activation generated sensory and emotional affective pain-like behaviors under normal conditions, while CeA inhibition decreased behaviors in models of inflammatory and neuropathic pain (see Neugebauer, 2020). More recently, optogenetic activation of PB input to CeA was shown to generate avoidance behavior (Ito et al., 2021) and to serve as a noxious signal for fear (Sato et al., 2015) in male mice and for avoidance memory in mice of both sexes (Chiang et al., 2020). Aversive learning in male and female mice was also induced by optogenetic activation of calcitonin gene-related peptide (CGRP) containing PB input to CeA (Bowen et al., 2020). Optogenetic activation of CeA-CRF neurons or BLA-CeA produced mechanical hypersensitivity, emotional responses and anxiety-like behaviors and increased activity of nociceptive spinal dorsal horn neurons of male rats under normal conditions (Mazzitelli et al., 2021; Mazzitelli et al., 2022). Conversely, optogenetic inhibition of CeA-CRF neurons or BLA-CeA input decreased emotional responses and spinal dorsal horn activity, but not mechanical hypersensitivity, in male rats in an arthritis pain model (Mazzitelli et al., 2021) and emotional responses and anxiety-like behavior, but not hypersensitivity, in male rats in a neuropathic pain model (Mazzitelli et al., 2022). Chemogenetic activation of CeA-PKCδ neurons increased mechanical hypersensitivity in normal male mice whereas chemogenetic inhibition of CeA-PKCδ neurons inhibited hypersensitivity in a neuropathic pain model. In contrast, chemogenetic inhibition of CeA-SOM neurons produced mechanical hypersensitivity under normal conditions but had no effect in neuropathic male mice (Wilson et al., 2019).
It will be important to determine the cell type- and projection-specific amygdala functions in pain-related neuroplasticity and descending and ascending pain modulation. For example, opposing functions of PKCδ and SOM neurons in the CeLC could reflect a differential pain modulator similar to the ON- and OFF-cells, respectively, in the rostral ventromedial medulla (Chen and Heinricher, 2019). It should be noted, however, that in the context of fear memory formation, PKCδ neurons are considered to act as OFF-cells and SOM as ON-cells, with PKCδ inhibition and SOM activation driving fear responses in mice of both sexes (Ciocchi et al., 2010; Haubensak et al., 2010; Penzo et al., 2014). This complex issue remains to be resolved.
1.3. Mesolimbic dopamine system (nucleus accumbens)
The mesolimbic dopamine system centered on ventral tegmental area (VTA) and nucleus accumbens (NAc) plays a key role in reward mechanisms, and their dysregulation is a critical factor in drug addiction and alcohol use disorder (Koob and Volkow, 2016) and in pain conditions (Borsook et al., 2016; DosSantos et al., 2017; Harris and Peng, 2020; Navratilova and Porreca, 2014). There is strong evidence for decreased dopamine levels in the NAc in pain conditions but increase of dopamine with the alleviation of pain (Navratilova et al., 2015a), although there may be differences between shell and core regions in male mice (Ren et al., 2021). Located in the ventral striatum, the NAc is anatomically and functionally divided into an outer (shell) and central (core) subregion. Principal cells in each region are GABAergic medium spiny neurons that express either D1 or D2 dopamine receptors as part of the direct (D1) or indirect (D2) pathway (Salgado and Kaplitt, 2015). NAc receives dopaminergic projections from VTA (to shell) and substantia nigra (to core) and glutamatergic input from mPFC, IC, ventral hippocampus, midline thalamus, and BLA to shell and core. IL appears to target mainly shell and PL, ACC and IC the core, which also receives CRF input from the CeA (Borrego et al., 2022; Groenewegen et al., 1999; Li et al., 2018; Salgado and Kaplitt, 2015; Stuber et al., 2011; Wright and Groenewegen, 1995). Nociceptive information can reach the NAc through these limbic pathways, but direct input from the spinal cord has also been described (Burstein and Giesler, 1989; Cliffer et al., 1991).
While recordings of nociceptive neurons in NAc appear to be lacking, neuroimaging (fMRI) data in male rats showed decreased activity in NAc core at the onset of a noxious stimulus and signal increase at the offset (Becerra et al., 2013). Preliminary neurochemical and immunohistochemical evidence in transgenic mice points to nociceptive neurons in the NAc shell that show increased responsiveness to touch in a neuropathic pain model (Wojick et al., 2022). Synaptic plasticity in the NAc has been shown in pain models. NMDA receptor-mediated excitatory transmission at the mPFC-NAc (shell) synapse was increased in D1 and D2 neurons in brain slices from neuropathic male mice (7 or 14 days postinduction) (Jing et al., 2022; Wu et al., 2018). Prolonged NMDA receptor kinetics were found in male mice at the acute stage (12 h) of an inflammatory pain model whereas at the later stages (12 days) of inflammatory and neuropathic pain models AMPA receptor-mediated excitatory transmission decreased, resulting in lower AMPA/NMDA ratio in D2 but not D1 NAc (core) neurons (Schwartz et al., 2014). A shift towards calcium permeable AMPA receptors accompanied by decreased excitatory transmission at depolarized potentials was found in the synaptic responses of NAc (core) neurons of male rats in a neuropathic pain model (14 days) (Goffer et al., 2013). Decreased excitatory transmission from IL (and slower NMDA receptor decay time), but increased excitability as a consequence of decreased dopaminergic signaling from VTA, was found in D2 but not D1 NAc (shell) neurons of male mice in a neuropathic pain model (5 days) (Ren et al., 2016). Decreased excitatory transmission from BLA to D2, but not D1, NAc (core) neurons was found in slices from neuropathic male mice (8 days), which was not mediated by dopamine signaling; in contrast, excitatory transmission from PL was increased in D2, but not D1, neurons in the same model (Ren et al., 2021).
Importantly, restoring the observed changes in NMDA receptor function, decreased hypersensitivity and depression-like behaviors in neuropathic male mice (Jing et al., 2022; Wu et al., 2018). Mitigating synaptic changes (decreased excitatory transmission) in D2 NAc neurons prevented the decrease in motivation to earn a natural reward in male mice with inflammatory and neuropathic pain without affecting mechanosensitivity (Schwartz et al., 2014). AMPA potentiators administered into the NAc decreased neuropathic pain-induced depression-like behaviors but not hypersensitivity in male rats (Goffer et al., 2013). Chemogenetic inhibition of hyperexcitable D2 NAc neurons decreased hypersensitivity in neuropathic male mice (Ren et al., 2016). Similarly, optogenetic silencing of D2 or activation of D1 neurons in NAc (shell or core) inhibited thermal hypersensitivity in neuropathic mice (Sato et al., 2022). Inactivation of the NAc (shell) with lidocaine decreased mechanical and thermal hypersensitivity in neuropathic male rats (14 days) (Chang et al., 2014). Interestingly though, optogenetic activation of PL-NAc (core) projections decreased mechanical and thermal hypersensitivity and aversive and depression-like behaviors in male rats in a neuropathic pain model (14 days) (Lee et al., 2015). Similarly, chemogenetic activation of D2 NAc (core) neurons decreased anxiety-like behavior and restored social interaction in neuropathic male mice (8 days; there was no effect, however, on mechanical hypersensitivity (Ren et al., 2021). Increased dopamine release in NAc correlated with pain relief in neuropathic (Kato et al., 2016) and postoperative (Navratilova et al., 2012) male rat pain models, and restoring dopamine levels (with L-DOPA) decreased mechanical hypersensitivity in neuropathic male mice (Ren et al., 2016). Blocking dopaminergic signaling in the NAc with D1 or D2 antagonists attenuated stress-induced analgesia in male rats (Noursadeghi et al., 2022) and the antinociceptive effects of morphine and other interventions in male rats (Altier and Stewart, 1998; Haghparast et al., 2012; Harris and Peng, 2020; Navratilova et al., 2012; see 2.3).
While the important role of the NAc and dopamine signaling in reward and pain relief is now well-established, the functional heterogeneity of this circuitry and cell type- and region-specific changes in pain conditions remain to be explored in terms of behavioral modulation and adaptive or maladaptive functions in pain.
2. Opioid system in cortico-limbic pain plasticity and pain modulation
The focus of this review is on opioid receptor function in pain-related neuroplasticity in corticolimbic circuits discussed in 1. Expression, cellular actions and behavioral effects of Mu, kappa, and delta opioid receptors (MOR, KOR, and DOR) will be described.
2.1. Medial prefrontal cortex (mPFC)
Opioid receptors have been shown to be densely distributed throughout the rodent neocortex, with striking differences in laminar distributions reported by early autoradiographic studies. In the rat, MORs were found to be predominately distributed in laminae I and IV of the cerebral cortex (subregions not specified) whereas DORs were primarily located in laminae II, III, and V, with a relatively equivalent distribution of MORs and DORs in lamina VI (Goodman et al., 1980). In the mouse, MORs are prominent in laminae I, IV, and VI of the frontal cortex (subregions not specified) while DORs are found in all layers (Moskowitz and Goodman, 1984). Laminar distributions of KORs were not investigated in either study. MORs in the mPFC are extensively localized on GABAergic interneurons (Férézou et al., 2007; Taki et al., 2000) to regulate mPFC output that is known to modulate the reward circuitry centered on signaling from the VTA to NAc, where MOR activation facilitates dopamine release through disinhibition (Fields and Margolis, 2015; Trigo et al., 2010). Cellular distributions of KORs within the mPFC have not been well characterized, though KOR are found on VTA dopaminergic neurons (Chefer et al., 2013) and BLA glutamatergic neurons (Tejeda et al., 2015) that project to the mPFC. Though little information is available regarding DOR cellular distributions, conditional knockout of the DOR gene in GABAergic neurons in the forebrain (ACC and unspecified frontal cortical region) of male mice revealed a preservation of some DOR activity, suggesting that DORs may also be located on mPFC excitatory neurons (Chung et al., 2015). Another study found both MORs and DORs to be localized to cell bodies and neurite-like processes in cultured cells (suggested to be both pyramidal and non-pyramidal neurons from morphological analysis) from male mouse frontal cortex brain slices containing the PL, IL, and ACC, with some cells showing co-localization of both receptors predominately in the region of the cell soma (Olianas et al., 2012). The differential cellular distributions may have important functional implications. Opioid receptor location in the mPFC as well as their involvement in adjacent neuronal circuitries support a role in the modulation of pain and pain-related emotional-affective behaviors.
2.1.1. Infralimbic and prelimbic mPFC
Actions in both prelimbic and infralimbic mPFC regions have been implicated in opioid-related processing (Reiner et al., 2019). While there is evidence for opioid receptor functioning in the mPFC, including infra- and prelimbic regions and their connections, in pain modulation, little is known about cell type- and circuit-specific actions of individual opioid receptor types and their role in pain-related neuroplasticity.
2.1.1.1. MOR
Both endogenous and exogenous MOR signaling can be linked to plasticity of interneurons in the mPFC. Chronic systemic administration of morphine (MOR agonist) increased the dendritic branching of SOM interneurons and the dendritic elongation of parvalbumin (PV) interneurons in the mPFC of male mice (Wang et al., 2019). Similarly, endogenous MOR knockdown in the mPFC with a small hairpin RNA (shRNA) viral vector decreased total dendrite length and impaired dendritic complexity of SOM but not PV interneurons, suggesting MOR signaling in SOM interneurons may contribute to their dendritic development (Wang et al., 2019). As PL and IL inhibitory signaling (Jones and Sheets, 2020; Kiritoshi et al., 2016), including SOM and PV interneurons (Jones and Sheets, 2020; Shiers et al., 2018), is involved in cognitive deficits associated with chronic pain, and MORs are expressed in interneurons in this regions (see 2.1), MOR signaling in the mPFC circuitry may influence pain-related processing. One study found that concomitant activation of MOR (with [D-Ala2, N-MePhe4, Gly-ol]-enkephalin, DAMGO) and DOR (with ([D-Pen2,D-Pen5]enkephalin, DPDPE) potentiated dopamine D1-like receptor signaling in male mouse mPFC brain slices that contained the PL, IL, and ACC (Olianas et al., 2012). This finding may suggest that MOR and DOR in these regions can strengthen dopamine D1 receptor modulation of glutamatergic transmission, a function that is critical in neuroplastic processes such as long-term potentiation (Gurden et al., 2000).
Systemic morphine-induced analgesia involved decreased purinergic signaling in the PL in the formalin pain model (Zeng et al., 2021). Intraplantar formalin triggered a slow but sustained increase in adenosine 5’-triphosphate (ATP) in the mouse PL, which was decreased by systemic injection of morphine (Zeng et al. 2021). Furthermore, infusion of ATP into PL weakened antinociceptive effects of morphine, whereas intra-PL delivery of a selective P2X7 receptor antagonist (Brilliant Blue G) partially mimicked morphine’s antinociceptive effects in the formalin test and, like presumed blockade of P2X4 receptors, enhanced morphine analgesia in morphine-tolerant male mice (Zeng et al., 2021). In contrast, systemic injection of a MOR agonist and serotonin-norepinephrine reuptake inhibitor (tramadol) had no acute effect on neuronal firing activity of PL pyramidal neurons in anesthetized male rats (Hasanpour Razmanjani and Reisi, 2022). Systemic application of morphine inhibited the spontaneous firing of the majority (63%) of PL neurons in anesthetized male rats, an effect that was reversed by systemic application of naloxone (Giacchino and Henriksen, 1996). However, electrophoretic application of a MOR agonist (DAMGO) inhibited spontaneous activity in only 38% of neurons, with most showing no change (Giacchino and Henriksen, 1996). Pyramidal cells and interneurons were not distinguished in that study. These findings may suggest the involvement of additional circuitry in mPFC-related MOR signaling.
Descending projections from mPFC to the PAG, where MOR activation inhibits GABAergic transmission to disinhibit output to the RVM to suppress pain behaviors (Vaughan and Christie, 1997; Vaughan et al., 1997), may be involved in the reported potentiation of opioid analgesia by off-label agents. For example, systemic coadministration of morphine and disulfram, an inhibitor of acetaldehyde dehydrogenase that is typically used to promote abstinence in alcohol use disorder, caused an increase in mechanical and thermal withdrawal thresholds that was attributed to an increase in MOR activation efficacy by disulfram in brain systems that include mPFC (unidentified region), PAG and RVM in male rats (de Corde-Skurska et al., 2021). Conversely, blocking MOR signaling in the mPFC has pain facilitating effects. Intra-mPFC (PL) delivery of a MOR antagonist (naloxonazine) increased the intensity of both the first and second phases of formalin-induced orofacial pain behaviors in male rats; this blockade was strong enough to prevent the antinociception by a non-steroidal anti-inflammatory drug (diclofenac) (Tamaddonfard et al., 2020). MOR signaling in PL and descending pathways may also be involved in placebo analgesia. The PL is one of the brain regions activated during placebo analgesia induced by a gabapentin-based conditioning approach in male rats in the spinal nerve ligation (SNL) model of neuropathic pain (Zeng et al., 2018). Microinfusion of a MOR antagonist (naloxone) into PL blocked placebo analgesia in the SNL model and disrupted functional coupling between PL and PAG (Zeng et al., 2018). Chemogenetic and optogenetic inhibition of mPFC (possibly IL) CRF neurons that project to the NAc or a CRF receptor 1 antagonist in the NAc were able to block morphine conditioned place preference (CPP) in neuropathic (chronic constriction injury, CCI) but not sham male mice (Kai et al., 2018), implicating an mPFC to NAc pathway in neuropathic pain-related MOR signaling.
On the input side, the firing rate of dopaminergic VTA neurons projecting to the mPFC (region not specified but possibly IL) decreased in brain slices from male mice with chronic mild stress (CMS)-induced depression (Liu et al., 2018). Intra-VTA morphine significantly increased the firing rate of VTA projection neurons recorded in anesthetized CMS, but not stress-naïve, mice and relieved depressive-like behaviors in the tail suspension, social interaction and sucrose preference tests but increased thermal nociception (decreased paw withdrawal latency) (Liu et al., 2018). Morphine-induced nociceptive responses were prevented by blocking brain-derived neurotrophic factor (BDNF) in VTA but not mPFC, suggesting that MOR in the mesolimbic reward circuitry can differentially regulate depression and nociceptive behaviors and related mPFC functions.
There is evidence to implicate hereditary factors, non-neuronal mechanisms, and sexual dimorphism in the role of mPFC in MOR signaling. Parental morphine exposure in male and female rats prior to mating resulted in reduced spontaneous activity of unidentified PL neurons and in decreased thermal (hot plate test) and visceral (acetic acid writhing test) nociception and decreased nocifensive behavior in the first and second phase of the formalin test in male offspring of morphine-abstinent rats compared to those from naïve parents (Ashabi et al., 2018). Significantly enhanced antinociceptive effects of systemic morphine and upregulation of MOR signaling in NAc but not mPFC were also found in the offspring of morphine-abstinent rats (Ashabi et al., 2018). The data implicate hereditary factors in altered mPFC function in pain-related MOR signaling. Proteomic analysis suggests that glial cells may also be linked to MOR signaling in the PL mPFC. In the PL of male rats with mechanical and thermal hyperalgesia induced by a MOR agonist (fentanyl), downregulation of five types of myelin-related proteins was found, and blockade of oligodendrocyte apoptosis with a caspase 3 inhibitor (z-DEVD-fmk) in the PL mPFC prevented fentanyl-induced hyperalgesia (Wang et al., 2022). Considering that oligodendrocytes (and other glia) express MOR (Stiene-Martin et al., 2001), it is reasonable to conclude that PL glial cells may contribute to MOR-related signaling. Finally, it is important to note that MOR signaling mechanisms in the mPFC may differ with regard to sex, leading to potential sexual dimorphisms in pain processing in this region. Intravenous self-administration with a potent MOR agonist (remifentanil) led to sex-specific differences in the excitability and synaptic modulation of PL layer 5/6 pyramidal neurons recorded in brain slices. Whereas remifentanil treatment caused a long-lasting hypoexcitable state in males and females, decreased excitability occurred on a faster timeline in females while hypoexcitability was preceded by hyperexcitability in males (Anderson et al., 2021). Different synaptic mechanisms were involved in hypoexcitability of PL pyramidal cells in males and females. Decreased excitatory synaptic transmission mediated by AMPA-type glutamate receptors was recorded in females, whereas the hyper- and hypoactive states in males were driven by decreased and increased GABAB receptor signaling, respectively (Anderson et al., 2021). No significant effects on neuronal excitability were observed in IL pyramidal cells, suggesting region-specific differences in MOR signaling (Anderson et al., 2021). Chemogenetic activation of PL pyramidal cells mitigated remifentanil-induced cognitive deficits in an extradimensional shift test and restored neuronal excitability and synaptic regulation in PL pyramidal neurons (Anderson et al., 2021). Together, the data may suggest sex-specific MOR-related pain modulatory mechanisms involving mPFC, explain previously reported sex differences in response to opioid-related pain management (Huhn et al., 2018, 2019), and provide support for tailoring opioid-related therapeutic strategies to biological sex in chronic pain patients.
2.1.1.2. KOR
KOR signaling in the mPFC involves presynaptic inhibition of glutamatergic transmission including onto NMDA receptor-expressing neurons. In the PL mPFC (laminae V and VI of the cingulate cortex, area 3) of male rats, KORs were found to be primarily localized on axons and axon terminals, with a small proportion associated with dendritic shafts and glial processes, and KOR-labeled axons contacted NMDA NR1 subunit-immunoreactive postsynaptic targets (Svingos and Colago, 2002). KOR activation with a selective agonist (U69,593) decreased frequency, but not amplitude, of glutamatergic miniature excitatory postsynaptic potentials (mEPSP) in layer V pyramidal neurons (likely PL and ACC) recorded in brain slices from male rats (Tejeda et al., 2013). However, this modulation may be input-specific as systemic U69,593 decreased monosynaptic field excitatory postsynaptic potentials (fEPSPs) in mPFC neurons recorded in anesthetized male rats when evoked by BLA but not hippocampal (fornix) stimulation (Tejeda et al., 2015). Intra-mPFC application of U69,593 reduced both electrical and optogenetic BLA-evoked glutamatergic fEPSPs in mPFC neurons, an effect that was blocked by systemic administration of a KOR antagonist (nor-binaltorphimine, nor-BNI) (Tejeda et al., 2015). Intra-mPFC (PL/ACC) administration of U69,593 also decreased extracellular dopamine levels in male rats, whereas administration of nor-BNI increased extracellular dopamine levels; these effects were not observed in mice line lacking KOR in dopaminergic neurons, suggesting an action of KOR on mesocortical dopaminergic terminals (Tejeda et al., 2013). Therefore, KOR signaling in the mPFC may regulate glutamatergic and dopaminergic inputs including through a tonically active system, though constitutive mPFC KOR activity may decline with age (Sirohi and Walker, 2015).
In pain conditions, alterations in mPFC KOR signaling may influence neuroplasticity related to opioid reward. mRNA levels of dynorphin, an endogenous ligand for KOR, were increased in the mPFC (subregion not specified) of male mice with morphine conditioning and a hindpaw incision pain model compared to that of sham mice, while no differences were present after recovery from incisional injury (Nwaneshiudu et al., 2020). In this study, blockade of KOR with systemic nor-BNI also led to an enhancement of morphine conditioning behavior in injured animals; together, this may suggest a potential protective role for mPFC KOR activation in acute pain when primed for morphine reward. U69,593-stimulated [35S]GTPγS binding increased in the mPFC (subregions not differentiated) in brain slices from male rats 7 days after CCI induction; however, no significant differences in binding were found between 30 day-CCI and sham animals (Llorca-Torralba et al., 2020), suggesting that KOR-related neuroplasticity in the mPFC may play a more critical role at the acute stage of neuropathic pain. Anxiolytic effects of mPFC KOR blockade have also been reported, as intra-mPFC (likely PL) infusion of nor-BNI in naïve male mice increased time spent in the center of the open field test (OFT), whereas systemic nor-BNI administration did not have a significant effect on anxiety-like behavior (Tejeda et al., 2015). Intra-mPFC nor-BNI microinjection in male rats also blocked conditioned place aversion (CPA) produced by systemic U69,593 (Tejeda et al., 2013). Since pain has an averse-affective component and is often comorbid with anxiety, shared neurobiological mechanisms in the mPFC may contribute to pain modulation and emotional network neuroplasticity (Vachon-Presseau et al., 2016), which could be regulated by blockade of mPFC KOR signaling. However, the role of KOR signaling in mPFC subregions in pain-related behaviors and neuroplasticity remains to be determined, and data suggest rather complex KOR actions in the mPFC related to pain, anxiety, and brain reward processing.
As with MOR, potential sexual dimorphism has been reported for KOR-related nociceptive mechanisms in the mPFC. In a mouse model of Fabry disease, a condition commonly associated with painful neuropathy (Burand and Stucky, 2021), downregulation of prodynorphin and KOR mRNA levels in the mPFC (region not specified) of males but upregulation in females was measured, and males, but not females, displayed mechanical and thermal hypersensitivity in the plantar aesthesiometer and Hargreaves tests, respectively, compared to wild type (Rullo et al., 2021). Interestingly, the same trend was not seen at the protein level – females displayed an increase but males showed no difference in mPFC KOR protein levels in the disease model compared to wild type (Rullo et al., 2021). The data suggest that early dysregulation of the dynorphinergic KOR system may contribute to the nociceptive symptoms of Fabry disease, possibly in a sex-dependent way. Overall, it is not clear if KOR signaling in mPFC is protective or harmful with regard to pain and pain-related neuroplasticity.
2.1.1.3. DOR
The DOR system has been implicated in sensory and affective pain regulation (Cahill et al., 2022; Gavériaux-Ruff and Kieffer, 2011; Nadal et al., 2006) but pain-related neuronal and behavioral effects of DOR signaling in the mPFC remain to be determined.
Like MOR signaling, DOR-related mechanisms in the mPFC may affect interneuron plasticity. Following DOR downregulation with shRNA, SOM but not PV mPFC (likely PL) interneurons demonstrated decreased total dendrite length and dendritic complexity; however, DOR but not MOR knockdown impaired dendritic morphology in SOM interneurons following a single morphine injection in male mice (Wang et al., 2019). These results suggest that while both MOR and DOR are critically involved in mPFC dendrite development, the endogenous signaling mechanisms may have differential roles regarding dendritic remodeling in response to exogenous activation. DORs in the mPFC may also have associations with MORs with regard to dopaminergic transmission, as one study reported that concomitant activation of DOR (with [D-Pen2,D-Pen5]enkephalin, DPDPE) and MOR (DAMGO), but not KOR (U50,488), in the male mouse mPFC enhanced dopamine D1-like receptor signaling in brain slices that included IL, PL and ACC, and a large percentage of dopamine D1 receptor positive cells expressed DOR and/or MOR immunoreactivity in neuronal cell bodies and processes of mPFC primary cell cultures with substantial co-localization of DORs and MORs (Olianas et al., 2012). As these two receptors have been shown to form heterodimers with novel functional properties (George et al., 2000), potential DOR-MOR interactions may influence opioid-related neurotransmission in the mPFC.
Little has been investigated with regard to DOR signal transduction in the mPFC under pain conditions. A DOR agonist (DPDPE) decreased [35S]GTPγS binding significantly in the mPFC (subregions not differentiated) in brain slices from male rats 30 days after CCI induction relative to 7 days after induction after a non-significant increase at 7 days compared to sham controls (Llorca-Torralba et al., 2020); this may suggest that DOR-related activity changes in the mPFC are associated with early rather than chronic stages of the neuropathic condition. Neuronal effects of DOR signaling in the mPFC have been studied under normal conditions but not in pain models. In brain slices from male mice, a DOR agonist (KNT-127) significantly decreased the frequency, but not amplitude, rise time, or decay time, of spontaneous and miniature excitatory postsynaptic currents (EPSCs) in PL pyramidal cells, and this effect was blocked by a DOR antagonist (naltrindole, NTI) (Yamada et al., 2021). KNT-127 also significantly increased the paired-pulse ratio, suggesting that DOR activation decreases glutamate transmission through a presynaptic site of action. KNT-127 decreased excitability of PL pyramidal neurons in the PL mPFC measured as the reduction in the number of action potentials and firing threshold (Yamada et al., 2021). Administration of KNT-127 into the PL mPFC of male mice decreased anxiety-like behavior (OFT) and glutamate, but not GABA, release induced by a sodium channel activator (veratrine) (Saitoh et al., 2018). KNT-127 administration into PL mPFC also decreased veratrine-induced c-Fos expression in the amygdala (LA, BLA and CeA) (Saitoh et al., 2018). KNT-127 alone had no effect. Inhibitory effects of DOR activation in the PL mPFC may therefore contribute to pain modulation but this remains to be determined.
2.1.2. Anterior cingulate cortex (ACC)
Endogenous opioid neurotransmission in the ACC plays an important role in pain modulation. The ACC has one of the highest levels of opiate ligand binding in the CNS, and MOR and DOR, but less so KOR, are localized in the ACC (Mansour et al., 1987; Tempel and Zukin, 1987; Vogt et al., 2001; Zubieta et al., 2001). Neuroimaging (PET) studies in humans found evidence for increased release of endogenous opioids in the ACC during acute experimental pain in healthy subjects (muscle pain induced by hypertonic saline (Scott et al., 2007) and heat pain (Sprenger et al., 2006)) and in patients with peripheral or central neuropathic pain (Maarrawi et al., 2007), fibromyalgia (Harris et al., 2007) and migraine during a spontaneous migraine attack (DaSilva et al., 2014), supporting a role of endogenous opioid function in the modulation of pain. Preclinical studies also found that release of endogenous opioids in the rodent ACC is necessary for the relief of the aversiveness of ongoing pain (Navratilova et al., 2015a).
2.1.2.1. MOR
MOR is expressed at high levels in superficial and deep layers of the ACC though differences are noted between studies (Mansour et al., 1987; Tempel and Zukin, 1987; Vogt et al., 2001; Wang et al., 2018; Zubieta et al., 2001). A greater number of functional DAMGO receptor sites was reported on axons than on somata and proximal dendrites (Vogt et al., 2001). Converging evidence from clinical PET studies measuring binding of MOR radiotracers suggests increased opioid release and MOR activation in the ACC of healthy subjects undergoing sustained experimental muscle pain (Scott et al., 2007; Zubieta et al., 2001) and in patients diagnosed with a variety of pain conditions such as fibromyalgia (Harris et al., 2007; Schrepf et al., 2016), migraine (DaSilva et al., 2014), and post-stroke pain (Willoch et al., 2004). Activation of the MOR system on the ACC was associated with reductions in affective ratings of the pain experience (McGill Pain Questionnaire affective scores) (Zubieta et al., 2001). Neuroimaging PET studies also found that increased ACC activity (regional blood flow) correlated with MOR-mediated antinociceptive effects of exogenous opioids (remifentanil) and placebo analgesia during noxious heat stimulation in normal subjects (Petrovic et al., 2002; Wager et al., 2007).
Evidence from preclinical studies also implicates endogenous opioids and MOR signaling in the ACC in affective rather than sensory pain relief (Navratilova et al., 2015a). In male rats with postsurgical pain (incision model) or neuropathic pain (SNL model), blockade of ACC opioid signaling with naloxone or ablation of ACC MOR expressing neurons with the cytotoxic ribosome inhibitor dermorphin-saporin inhibited conditioned place preference (CPP) and NAc dopamine release resulting from non-opioid pain-relieving treatments such as peripheral nerve block or intrathecal administration of an α2-adrenergic agonist (clonidine), but had no effect on mechanical and thermal hypersensitivity (Navratilova et al., 2015b). Thus, ACC opioid and MOR signaling is required for pain relief by non-opioid treatments through the activation of reward circuits. MOR blockade with β-funaltrexamine (β-FNA) in the ACC also prevented CPP and NAc dopamine release induced by morphine administration into the amygdala (CeA) in neuropathic male rats (SNL model). Therefore, endogenous MOR signaling in the ACC is involved in affective pain relief by MOR activation in the CeA through a functional connection from CeA to ACC (Navratilova et al., 2020).
Injection of morphine into the ACC decreased aversive but not sensory pain behaviors in models of neuropathic (SNL model) and postsurgical pain (Gomtsian et al., 2018; LaGraize et al., 2006; Navratilova et al., 2015a; Navratilova et al., 2015b). Morphine in the ACC decreased the aversiveness of noxious cutaneous stimulation in male SNL rats measured as escape/avoidance behavior in the light-dark test (LaGraize et al., 2006) and produced CPP and dopamine release in the NAc in male rats with postinjury and SNL pain but not in uninjured rats (Navratilova et al., 2015b). ACC morphine-induced CPP in SNL rats was blocked by pretreatment of NAc with a dopamine receptor blocker (α-flupenthixol). Morphine in the ACC did not affect mechanical (von Frey or Randall-Selitto) or thermal (Hargreaves or hot plate) hypersensitivity (Gomtsian et al., 2018; LaGraize et al., 2006; Navratilova et al., 2015a; Navratilova et al., 2015b). However, mechanical (Randall-Selitto) and thermal (hot plate) antinociceptive effects of ACC morphine have also been reported; they were blocked by β-FNA and were decreased in a neuropathic pain model (CCI) compared to normal male rats, possibly due to the downregulation of MOR expression measured at the mRNA level (Wang et al., 2020). Still, evidence suggests that MOR signaling in the ACC relieves predominantly the aversiveness of pain and activates reward/motivation circuits.
At the cellular level, systemic administration of morphine inhibited laser-heat stimulus evoked activity of ACC neurons in awake male and female rats measured with single-unit multi-array recordings (Kuo and Yen, 2005; Wang et al., 2009). In brain slices from male rats with complete Freund’s adjuvant (CFA)-induced inflammatory pain, a MOR agonist (DAMGO) decreased evoked glutamatergic transmission in layer II/III neurons, and this effect was blocked by a MOR antagonist (CTAP). The effect was due to a presynaptic action because DAMGO increased paired-pulse ratio and decreased frequency, but not amplitude, of mEPSCs (Zheng, 2010).
2.1.2.2. KOR/DOR
KOR expression in ACC is more uniformly distributed across different layers but at a lower level than MOR whereas DOR expression in ACC is strong in superficial and deep layers (Mansour et al., 1987; Tempel and Zukin, 1987) though segregation of DOR and MOR neurons in different ACC layers of the ACC has been reported with DOR expression prominent in layer II/III and MOR in layer V (Wang et al., 2018). PET studies in humans showed that KOR binding is greater in men than women in multiple brain regions, including ACC (Vijay et al., 2016). There is also evidence for lateralization of the endogenous opioid system in the human ACC. In radioimmunoassays, levels of opioid peptides Leu-enkephalin-Arg (LER, DOR/MOR agonist) and Met-enkephalin-Arg-Phe (MEAP, KOR/MOR agonist) were lateralized to the left and right ACC, respectively (Watanabe et al., 2015), which may be linked to the lateralization of higher functions and their modulation, such as positive and negative emotions and pain (Brügger et al., 2011; Kim et al., 2012; Knoll and Carlezon, 2010; Shippenberg, 2009; Symonds et al., 2006).
Pain-related functions and cellular actions of KOR signaling in the ACC are largely unknown. Administration of a KOR agonist (U69,593) into the ACC of naïve male mice and rats by microdialysis decreased dopamine, glutamate and GABA levels and induce conditioned place aversion whereas a KOR antagonist (nor-BNI) enhanced dopamine release and blocked U69,593-mediated conditioned place aversion (Tejeda et al., 2013). Consistent with presynaptic KOR actions, U69,593 decreased the mEPSP frequency, but not amplitude, in ACC layer V pyramidal cells in brain slices (Tejeda et al., 2013).
Some evidence suggests that DOR signaling in the ACC has inhibitory effects on neuronal activity and behaviors in pain models. In neuropathic male mice with sciatic nerve ligation, DOR dysfunction measured by agonist-induced G protein activation and astrogliosis due to impaired DOR-mediated astrocyte differentiation was found in the ACC, and these changes were linked to anxiety-like behaviors (EPM and light-dark test) (Narita et al., 2006b). Administration of a DOR agonist ([D-Ala2, D-Leu5]-enkephalin, DADLE) into ACC reversed CFA-induced conditioned place avoidance, but not thermal hypersensitivity, in male rats and decreased phosphorylation of NMDA receptor subunits GluN1, GluN2A, GluN2B, NMDA, but not AMPA, receptor-mediated currents and discharge frequency of ACC pyramidal cells in rat brain slices, suggesting that DOR activation alleviates affective pain by decreasing activity of ACC pyramidal cells (Ma et al., 2022). In brain slices from normal male and female mice, DPDPE inhibited local inhibitory transmission from PV interneurons to ACC layer V pyramidal cells through an action on DOR on PV interneurons without affecting excitatory inputs from medial thalamus, and this disinhibition resulted in increased excitability (action potential firing) of ACC pyramidal cells. MOR activation with DAMGO decreased excitatory thalamic inputs and ACC pyramidal excitability (Birdsong et al., 2019). Neuronal mechanisms of DOR signaling in the ACC and potential changes and behavioral consequences in pain conditions and pain-related neuroplasticity remain to be determined.
2.2. Amygdala
The amygdala plays an important role in opioid analgesia manning (Bagley and Ingram, 2020; Helmstetter et al., 1998; Manning, 1998; Manning and Mayer, 1995a, b; McGaraughty et al., 2004; McGaraughty and Heinricher, 2002). MOR, DOR and KOR that are all expressed at various levels in the amygdala but may have different functions. MOR signaling in the amygdala is linked to analgesia, reward and the regulation of fear, anxiety and related behaviors (Bagley and Ingram, 2020; Bodnar, 2022; Tershner and Helmstetter, 2000; Wilson and Junor, 2008; Zhang et al., 2013). Amygdala DOR is also involved in anxiolysis (Klenowski et al., 2015). In contrast, amygdala KOR signaling has anxiogenic and aversive behavioral effects (Cahill et al., 2014; Limoges et al., 2022).
2.2.1. MOR
MORs are expressed in all nuclei of the amygdala and at high levels in the BLA (Mansour et al., 1987) and on ITC cells (Wang et al., 2018; Winters et al., 2017). MOR immunoreactivity in the BLA is found on dendritic spines and axon terminals and also in Golgi apparatus (Zhang et al., 2015a). MOR can act presynaptically at excitatory synapses in CeM neurons (Zhu and Pan, 2005) and CeLC neurons (Kissiwaa et al., 2020) but also on terminals of inhibitory synapses in CeL and on postsynaptic sites where there was some co-expression with CRF receptors in male mice (Jaferi and Pickel, 2009). Given the opposite intracellular signal mechanisms of MOR and CRF-receptors through Gi and Gs proteins, respectively, MOR signaling including through endogenous opioids may counterbalance the excitatory effects of CRF as has been shown for stress responses in male rats in the locus coeruleus (Curtis et al., 2001; Valentino and Van Bockstaele, 2001). In the BLA, MOR expression is lower and has been found on pyramidal cells and interneurons as well as on excitatory synaptic terminals in male rats, and as a result there could be inhibitory and dis-inhibitory effects of MOR on BLA output neurons (Zhang et al., 2015a).
Neuronal effects of MOR signaling in the amygdala have been studied under normal conditions but not in pain models. Brain slice electrophysiology showed hyperpolarization and decreased input resistance mostly in interneurons in the rat LA with methionine-enkephalin (ME) or DAMGO (Sugita et al., 1993), and these effects were blocked with naloxone and CTOP (Sugita and North, 1993). DAMGO increased spike adaptation in rat LA pyramidal neurons by potentiating Kv1.2-mediated voltage-dependent potassium currents through the activation of the phospholipase A2-arachidonic acid-lipoxygenases signaling pathway (Faber and Sah, 2004). ME or endogenous opioids also produced an outward current in male rat ITC cells by activating a potassium conductance, which was blocked by a MOR antagonist (CTAP) (Winters et al., 2017). Likewise, DAMGO hyperpolarized ITC cells from male mice (Blaesse et al., 2015). DAMGO also inhibited CeL and CeM neurons, including those projecting to PB, by inducing postsynaptic potassium currents in male rat brain slices (Chieng et al., 2006).
MOR can also regulate excitatory and inhibitory transmission in the amygdala. DAMGO decreased excitatory inputs from dorsal midline thalamus to BLA pyramidal cells and CeL neurons as well as feedforward excitation of CeM neurons in mice brain slices (Goedecke et al., 2019). DAMGO inhibited BLA-evoked excitatory transmission in ITC cells (Winters et al., 2017), CeLC neurons (Kissiwaa et al., 2020) and in CeM neurons (Zhu and Pan, 2005), and also PB-evoked excitatory transmission in CeLC neurons (Kissiwaa et al., 2020) through a presynaptic action in male rat brain slices. DAMGO induced long-term depression in dorsomedial striatum neurons by activating MOR on excitatory inputs from BLA in male mice brain slices (Muñoz et al., 2020). In male rat brain slices, DAMGO decreased inhibitory GABAergic, but not excitatory glutamatergic, transmission (mIPSCs and evoked IPSCs) in BLA neurons projecting to the CeA through a presynaptic action that involved Kv1.1 and Kv1.2 potassium channels, and this effect was blocked by a MOR antagonist (CTAP) (Finnegan et al., 2006). DAMGO also decreased GABAergic transmission between ITC and CeM neurons by hyperpolarizing ITC cells in male mouse brain slices without affecting excitatory transmission from BLA to ITC or CeM (Blaesse et al. 2015), although another study in male rat brain slices reported presynaptic inhibition of excitatory transmission from BLA to ITC with DAMGO that was blocked by CTAP (Winters et al., 2017). DAMGO or morphine decreased evoked GABAergic transmission (IPSPs) in CeA neurons in male rat brain slices through a presynaptic mechanism without affecting membrane properties (Bajo et al., 2011). A MOR antagonist (CTOP) increased inhibitory transmission suggesting tonic activation of MOR. The different pre- and postsynaptic MOR actions on excitatory and inhibitory elements of the complex amygdala circuitry do not yield a clear picture yet and remain to be explored in pain conditions.
This is important because of evidence for decreased MOR signaling measured as DAMGO-stimulated [35S]GTPγS binding to amygdala cell membranes in mouse models of inflammatory (CFA) and neuropathic (sciatic nerve ligation) pain in males (Narita et al., 2006a). MOR expression in the amygdala was downregulated in males in a rat surgical (incision) pain model combined with perioperative stress through microRNA (miRNA-339-5p)-mediated posttranscriptional regulation (Zhu et al., 2022). In addition to pain-related changes in MOR signaling there is also evidence for MOR-induced amygdala neuroplasticity. Prenatal (days 11–18 post-conception) systemic morphine application decreased and increased MOR density expression in the BLA and CeA, respectively, in male but not female rats (Vathy et al., 2003). Systemic morphine treatment of newborn rats on postnatal days 1–4, but not after day 22, decreased MOR binding in the BLA (Tempel, 1991). Intermittent systemic morphine treatment induced FosB/DeltaFosB transcription factor expression in limbic brain regions of male mice, including BLA and CeA (Kaplan et al., 2011). Chronic escalating, but not single, morphine application in male mice significantly altered expression of genes involved in neuroplasticity, including neurogenesis, cell growth, and signaling proteins such as G protein-coupled receptors, scaffolding and signaling proteins, and neuropeptides (Befort et al., 2008).
MOR signaling in the amygdala is antinociceptive and inhibits averse-affective behaviors through a mechanism that involves at least in part descending modulation of the PAG-RVM circuitry. Morphine injections into BLA decreased thermal nociception (increased tail-flick latency) (Helmstetter et al., 1995; McGaraughty and Heinricher, 2002) and increased activity of RVM OFF-cells while decreasing RVM-ON cell activity in naïve male rats (McGaraughty and Heinricher, 2002). The antinociceptive effects (tail-flick test) of DAMGO injected into BLA of male rats were decreased by lidocaine injection into PAG or RVM and blocked by lesion of PAG or RVM (Helmstetter and Tershner, 1994; Helmstetter et al., 1998). Morphine or β-endorphin injected into BLA or CeA increased tail-flick latencies and shock-evoked jump thresholds in male rats and this effect was blocked by blocking opioid receptors in the PAG (Pavlovic et al., 1996). MOR activation was more effective on the jump test than tail-flick test, suggesting modulation of the affective pain component, which is also supported by a study showing that DAMGO injection into CeA inhibited conditioned place aversion induced in male rats by an inflammatory pain condition (CFA) but did not affect heat hyperalgesia (paw withdrawal latency) in this pain model (Zhang et al., 2013). Interestingly, administration of morphine into right but not left CeA produced conditioned place preference (CPP) in neuropathic male rats (SNL model) without affecting mechanical hypersensitivity (Navratilova et al., 2020), suggesting lateralized modulation consistent with pain-related neuroplasticity in the right but not left CeA (Allen et al., 2020; Neugebauer, 2020). Bilateral lesion of the CeA, but not BLA, abolished systemic morphine-induced antinociception in the tail-flick and formalin tests in male rats (Manning and Mayer, 1995a, b), suggesting that the CeA is required for morphine-induced antinociception including the suppression of spinally mediated nociceptive reflexes. Conversely, systemic naltrexone increased Fos expression in the CeA (especially CeLC) in naïve mice and the effect was greater in a model of latent sensitization, and CTAP injection into the CeA precipitated mechanical hypersensitivity in male and female mice (Cooper et al., 2022). Chemogenetic silencing of MOR expressing PB neurons that project to CeA decreased nociceptive behaviors in male and female mice in the formalin test, jumps in the hotplate test, and anxiety-like behaviors in the EPM, suggesting that MOR on excitatory nociceptive inputs could modulate affective pain behaviors through effects on CeA (Liu et al., 2022).
In healthy humans, a PET study using a MOR radiotracer ([11C] carfentanil) found that sustained masseter muscle pain induced the release of endogenous opioids in the amygdala to activate MOR, which was associated with decreased sensory and affective pain ratings (Zubieta et al., 2001). Using a similar approach, placebo analgesia was shown to increase heat pain-induced MOR activation in limbic regions including the right amygdala whereas placebo-induced anticipatory opioid decrease was detected in the left amygdala (Wager et al., 2007). Placebo analgesia involving MOR activation may be linked to neuroimmune mechanisms. Using [11C] carfentanil, a PET study in healthy humans subjected to experimental masseter muscle pain found that placebo analgesia-induced MOR activation in the left amygdala and NAc correlated with decreased plasma levels of a pro-inflammatory cytokine (IL-18) (Prossin et al., 2022).
2.2.2. KOR
KOR expressing cells are located in CeA while little expression is observed in BLA or ITC (Fallon and Leslie, 1986; Gomes et al., 2020; Hurd, 1996; Le Merrer et al., 2009; Mansour et al., 1996; Marchant et al., 2007; Peckys and Landwehrmeyer, 1999). KORs expression in CeA can be pre- and post-synaptic (Chieng et al., 2006; Gilpin et al., 2014). KOR activation with U69,593 increased or decreased GABAergic transmission (spontaneous IPSCs) in different sets of BLA neurons in brain slices from adolescent (30–40 days) but not adult (>60 days) male rats through a presynaptic action but had no effect on excitatory transmission (Przybysz et al., 2017; Varlinskaya et al., 2020). This effect was blocked in the presence of TTX suggesting a network action of KOR signaling. Another KOR agonist (U50,488H) decreased excitatory synaptic transmission and blocked long-term potentiation (field potentials) evoked in BLA neurons by stimulation of LA afferents in male mouse brain slices (Huge et al., 2009). In CeM neurons from male rats, U69,593 decreased inhibitory transmission presynaptically (Gilpin et al., 2014; Przybysz et al., 2017), and this effect was not age-dependent (Przybysz et al., 2017). Similarly, U69,593 increased excitability of CRF neurons in the CeL in male rat brain slices through synaptic disinhibition, i.e., inhibition of feedforward inhibition driven by optical activation of PB input (Hein et al., 2021). U69,593 had no effect on PB-evoked excitatory synaptic transmission on CeA-CRF neurons (Hein et al., 2021) and on unidentified CeLC neurons (Kissiwaa et al., 2020). Single-unit recordings in anesthetized transgenic male rats showed that stereotaxic administration of U69,593 increased the responsiveness of CeA neurons to noxious mechanical stimuli and this effect was reversed by optogenetic silencing of CRF neurons in the CeA (Ji and Neugebauer, 2020). As a consequence of CeA neuronal activation by U69,593, activity of spinal dorsal horn neurons to innocuous and noxious mechanical stimuli increased (Ji and Neugebauer, 2020), suggesting that descending facilitation from CeA-CRF neurons is under the control of KOR signaling.
In models of neuropathic (SNL) (Navratilova et al., 2019) or functional (morphine priming followed by a stressor) (Yakhnitsa et al., 2022) pain, a KOR antagonist (nor-BNI) decreased synaptically evoked spiking of CeL neurons and excitability of CeL-CRF neurons in rat brain slices from SNL but not control male rats. This effect was mediated through a presynaptic action to increase feedforward inhibition driven by optogenetic and electrical stimulation of PB input. There was no effect on excitatory synaptic transmission. The data suggest tonic KOR-mediated disinhibition of CeA (including CRF) neurons in pain conditions. In anesthetized male rats, nor-BNI administered into CeA decreased ongoing activity and responses of CeA neurons to peripheral mechanical stimulation (Yakhnitsa et al., 2022).
Administration of U69,593 into the right CeA in naïve male rats increased vocalizations to noxious stimuli, and induced anxiety-like behaviors in the open field test and avoidance in the conditioned place “preference” test, but had no effect on mechanical thresholds in the paw pressure test (Hein et al., 2021). CRF neurons were involved in the KOR mediated behavioral effects because they were blocked by optogenetic silencing of CeA-CRF neurons. In neuropathic male rats (SNL model), administration of nor-BNI into the right CeA eliminated aversiveness and therefore blocked conditioned place preference to intravenous gabapentin but had no effect on mechanosensitivity in the von Frey test (Navratilova et al., 2019). Nor-BNI administered into right CeA also decreased vocalizations evoked by noxious stimuli and anxiety-like behavior in the elevated plus maze in males in a rat functional pain model (morphine priming plus stressor) but had mixed effects on mechanical hypersensitivity, showing inhibitory effects in the von Frey test but not paw pressure test (Yakhnitsa et al., 2022). Nor-BNI administration into the right but not left CeA prevented mechanical hypersensitivity (von Frey test) in males in an injury-free rat model of medication overuse (sumatriptan priming with stressor) (Xie et al., 2017) and also decreased mechanical hypersensitivity (von Frey test) in males in a rat model of trigeminal neuropathic pain (infraorbital CCI) but had no significant effect on anxiety-like behavior (elevated plus maze) (Turnes et al., 2022). The role and mechanisms of KOR signaling in the amygdala in sensory aspects of pain remain to be determined.
Nor-BNI administration into the right but not left CeA of male rats restored impaired diffuse noxious inhibitory controls (DNIC) induced by capsaicin injected into the forepaw as a conditioning stimulus in models of neuropathic (SNL) (Phelps et al., 2019) and functional (morphine priming with subsequent stressor) pain where dynorphin A levels were increased (Nation et al., 2018). Outcome measures for DNIC modulation included mechanosensitivity (paw pressure test) and spinal nociceptive processing (responses of dorsal horn neurons to mechanical stimuli).
There is evidence for pain-related changes in KOR signaling in the amygdala. In males in the rat model of medication overuse (sumatriptan priming with stressor), stress-evoked increases of dynorphin levels and phosphorylation of KOR were detected in both the left and right CeA (Xie et al., 2017). Phosphorylation could involve MAP kinase activation because of the inhibitory effects of a MAP kinase inhibitor (U0126) injected into the CeA (Xie et al., 2017), and increased dynorphin release is likely downstream of CRF activation (Bruchas et al., 2009). In a transgenic mouse model of Fabry disease, male but not female mice showed mechanical (plantar aesthesiometer) and thermal (Hargreaves test) hypersensitivity and there was a decrease of prodynorphin mRNA levels in the amygdala of males but an increase in females, suggesting sex-specific adaptation to pain (Rullo et al., 2021). In a mouse model of inflammatory pain (CFA), a significant increase of [35S]GTPγS binding to amygdala cell membranes stimulated by a KOR agonist (ICI199,441) was found in males (Narita et al., 2006a). Increased KOR signaling and activation found in different pain models and inhibitory effects of KOR blockade in the amygdala point to the amygdala KOR system as an important pain mechanism and potentially useful target.
2.2.3. DOR
DOR expression is high in many brain areas including the amygdala (Mansour et al., 1987; Peckys and Landwehrmeyer, 1999). DOR neurons are mainly found in the BLA (Wang et al., 2018) whereas DOR expression in the CeA is mostly on axon terminals (Wilson et al., 2002) but has also been identified on dendrites, mainly on CRF neurons (Reyes et al., 2017). Neuronal DOR expression is also supported by immunohistochemistry and in situ hybridization of enkephalin mRNA in BLA and CeA (Zhang and McDonald, 2016). A DOR agonist (ICI174864) or endogenously released opioids acting on DOR inhibited glutamatergic transmission from BLA to ITC through a presynaptic action in male rats (Winters et al., 2017). A DOR1 agonist (DPDPE), but not DOR2 agonist (deltorphin II), inhibited excitatory transmission from BLA and PB to CeL neurons through a presynaptic action in male mouse brain slices (Zhou et al., 2021). Deltorphin II inhibited excitatory transmission evoked from BLA, but not PB, in a subset of CeLC neurons from male rats (Kissiwaa et al., 2020). In males in a mouse model of inflammatory pain (CFA), DPDPE decreased the increased excitatory transmission onto CeL neurons at 4 days whereas deltorphin II had inhibitory effects on PB-CeL transmission at 7 days, suggesting a functional switch from D1 to D2 (Zhou et al., 2021). Neither agonist had an effect at 21 days. Consistent with deceased DOR function in pain, [35S]GTPγS binding to amygdala cell membranes stimulated by a DOR agonist (SNC80) was suppressed in mouse models of inflammatory (CFA) and neuropathic (sciatic nerve ligation) pain at the 4 week time point, and this was linked to anxiety-like behaviors (light-dark test and elevated plus maze) in males in these pain models because injection of a DOR antagonist (naltrindole) had anxiogenic effects (Narita et al., 2006a). On the other hand, administration of DPDPE into the CeA had pronociceptive effects (decreasing mechanical thresholds) in normal male mice but not in the pain model and had no effect on anxiety-like behaviors (open field and elevated plus maze tests) in either condition (Zhou et al., 2021). Deltorphin II administration into CeA had no effect on mechanical nociception in either condition but had anxiolytic effects in the CFA model (7 days but not 4 days) whereas a DOR2 antagonist (naltriben), but not a DOR1 antagonist (BNTX), had anxiogenic effects at that stage (Zhou et al., 2021). The data suggest a link of DOR signaling in the amygdala to anxiety-like behaviors in pain, but the modulation of pain-related amygdala processing, plasticity and behaviors remains to be determined.
2.3. Mesolimbic dopamine system (nucleus accumbens)
Pain relief depends heavily on the reward system (Fields and Margolis, 2015; Leknes and Tracey, 2008; Porreca and Navratilova, 2017). Analgesic effects of opioids are strongly related to dopamine signaling in the mesolimbic NAc system. Dopaminergic signaling and the NAc play an important role in drug addiction and modulation of opioid-induced analgesia (Apkarian et al., 2013; Harris and Peng, 2020; Navratilova et al., 2015a). Blockade of dopamine D2 receptors in the NAc shell reduced the analgesic efficacy of morphine and substance P analogues administered in the ventral tegmental area in experimental male rats (Altier and Stewart, 1998). Conversely, dopamine signaling in NAc can be modulated by opioids. All opioid receptor subtypes (MOR, DOR and KOR) are expressed in NAc and MOR and DOR can colocalize on dendrites of NAc neurons (Gerfen et al., 1990; Meng et al., 1993; Meshul and McGinty, 2000; Svingos et al., 1998; Svingos et al., 1999; Svingos et al., 1997). There is evidence for differential functions of different NAc opioid receptor subtypes in pain processing as described below (2.3.1 and 2.3.2).
2.3.1. MOR and DOR
Opioid signaling in NAc is crucial for dopamine turnover. Stimulation of MOR and DOR with agonists in NAc provokes rapid extracellular dopamine release in the NAc measured by in vivo microdialysis (Di Chiara and Imperato, 1988; Fusa et al., 2005; Hipolito et al., 2008; Saigusa et al., 2017; Spanagel et al., 1992; Yokoo et al., 1994). Dopamine increase following activation of MOR and DOR is subregion-specific as it was observed in the core but not shell subregion in male rats (Hipolito et al., 2008). Administration of agonists for MOR (DAMGO), DOR1 (DPDPE) and DOR2 (DSLET) into NAc significantly increased the extracellular levels of dopamine in a dose-related manner that was partially naloxone-sensitive. An interaction between two DORs subtypes, DOR1 and DOR2, and MOR was also suggested. MOR activation engaged DOR1 to activate DOR2 and DOR1, but not DOR2, activated MOR causing the rapid increase in extracellular dopamine in male rats (Hirose et al., 2005).
Accumulating evidence points to the importance of NAc in opioid-mediated antinociception. Intra-NAc activation of MOR with microinjection of morphine induced sensory antinociception (Dill and Costa, 1977; Yu and Han, 1990), and the analgesic effects of morphine given systemically or into the habenula were reversed by intra-NAc administration of an opioid receptor antagonist (naloxone) in male animals (Dill and Costa, 1977; Ma et al., 1992), suggesting that habenula and NAc do not act independently in the antinociceptive circuitry. Co-activation, but not individual activation, of MOR and DOR in NAc with DAMGO and DPDPE, respectively, produced antinociception measured as attenuation of the jaw-opening reflex in male rats (Schmidt et al., 2002). In males in a rat model of neuropathic pain (sciatic nerve ligation), intra-NAc administration of morphine markedly reduced hypersensitivity to mechanical and thermal stimuli as reflected in the increased latency of paw withdrawal reflexes (Bian and Yu, 2015). Interestingly, in males in a mouse model of neuropathic pain (CCI), mRNA levels of DOR, but not MOR, markedly increased in the ipsilateral NAc, and levels of proenkephalin, a ligand for MOR and DOR, were also elevated, suggesting pain-related plasticity in opioid NAc signaling (Wawrzczak-Bargiela et al., 2020). Conversely, blocking MOR and DOR in NAc produced pronociceptive effects. Either a specific MOR antagonist (CTOP) or a DOR antagonist (naltrindole) injected bilaterally into NAc blocked antinociception measured as attenuation of the jaw-opening reflex that was induced by intrathecally delivered DAMGO into the lumbar region of male rats (Gear and Levine, 2011) or by subdermal capsaicin injection into the plantar surface of the male rat paw (Schmidt et al., 2002). Antinociceptive effects of MORs and DORs can be attributed to dopamine receptor activation in NAc. Subdermal capsaicin evoked heterosegmental pain-induced antinociception in male rats that was reduced by bilateral intra-NAc pretreatment with either naloxone or a dopamine receptor antagonist (flupentixol) (Gear et al., 1999).
Pain, particularly chronic pain, is associated with negative affective states such as anxiety and depression. Activation of MOR and DOR in NAc with a high affinity agonist (UFP-512) administered into NAc of naïve male rats produced antidepressant-like behavior in the forced swim test and anxiolytic like behavior in the elevated plus maze and novelty-induced hypophagia tests (Kabli et al., 2014). Bilateral intra-NAc pretreatment with a MOR antagonist (CTOP) and a DOR antagonist (naltrindole) abolished antidepressant- and anxiolytic-like effects of UFP-512 (Kabli et al., 2014).
2.3.2. KOR
The mesolimbic KOR system has been implicated in reward, stress and nociception. Activation of KOR in NAc by pharmacological agents or by the endogenous ligand dynorphin, reduces motivational value of rewards and provokes aversion and depression-like behaviors that can be attributed to inhibition of dopamine release. Intra NAc microdialysis application of a KOR agonist (U69,593) decreased dopamine efflux in the dialysate by 50% whereas administration of a KOR antagonist (nor-BNI) increased dopamine levels in male rats dose-dependently (Spanagel et al., 1992). KORs on dopaminergic neurons are necessary for the development of aversive behaviors. Transgenic male mice with deletion of KOR on dopaminergic neurons did not develop conditioned place aversion (CPA) to systemically applied U69,593, and dopamine levels collected by microdialysis in the NAc were reduced (Chefer et al., 2013). In male rats in the formalin pain model, systemic morphine-induced dopamine release in the microdialysis probe in NAc was significantly decreased and this decrease was KOR dependent since intra-NAc microinjection of an antibody against dynorphin A restored the morphine effect on dopamine levels (Narita et al., 2005).
KOR activation in NAc produces negative affective anxiety- and depression like behaviors. A KOR agonist (U50,488) microinjected into NAc had anxiogenic effects in male mice in elevated place maze and light-dark tests. Anxiogenic effects were prevented by intra-NAc pretreatment with a KOR antagonist (nor-BNI) (Wang et al., 2016). Local intra-NAc microinjections of U50,488 or dynorphin derivative E-2078 produced strong conditioned place aversion in male rats (Bals-Kubik et al., 1993; Wee and Koob, 2010). Dynorphin can also be released by optical stimulation of dynorphin-containing neurons in the NAc shell region of transgenic male mice (Al-Hasani et al., 2015). Region-specific behavioral effects were found and could be observed in the same animal. Photoactivation of dynorphin neurons in the ventral NAc shell provoked real-time place aversion whereas photoactivation of dorsal NAc shell neurons evoked real-time place preference behavior, while neither intervention induced anxiety-like behavior in the open field test (Al-Hasani et al., 2015). Optical activation of dynorphin-containing neurons in the ventral NAc of transgenic male and female mice also decreased motivation to self-administer sucrose while driving real-time place aversion (Massaly et al., 2019). Chemogenetic silencing of dynorphin containing neurons in male rats restored motivation for sucrose reward, and intra-NAc administration of a low dose of nor-BNI prevented aversive behavior induced by stimulation of dynorphin neurons, implicating KOR signaling (Massaly et al., 2019).
In an inflammatory pain condition (intradermal CFA injection) in mice of both sexes, optical stimulation of dynorphin neurons in the NAc shell region produced similar real-time place aversion as under normal conditions but required a higher dose of nor-BNI to block the effect, which suggests enhanced KOR signaling in the NAc in pain conditions by activation of dynorphin neurons to increase dynorphin levels and drive negative affective pain behaviors (Massaly et al., 2019). Activation of KOR signaling in NAc has pronociceptive effects. Antinociception (jaw-opening reflex) induced by subdermal capsaicin injection in the rat hind paw was blocked with intra-NAc administration of U69,593 but not nor-BNI. U69,593 also blocked the antinociceptive effects of DAMGO and DPDPE in male rats, suggesting opposing interactions between KOR and MOR/DOR signaling (Schmidt et al., 2002). In male mice in neuropathic pain (CCI model), mRNA expression of KOR and DOR as well as their endogenous ligands, prodynorphin and proenkephalin, was significantly upregulated, suggesting plasticity of the NAc opioidergic system in pain (Wawrzczak-Bargiela et al., 2020).
KOR signaling in the mesolimbic dopamine circuit has also been implicated in stress and depression. A single immobilization stress or forced swim test resulted in increased immunoreactivity of dynorphin (A and B) in NAc of male rats (Shirayama et al., 2004). Microinjections of nor-BNI into NAc prevented the depressive phenotype in the learned helplessness model, and these effects were stronger after infusion into NAc shell rather than core (Nestler and Carlezon, 2006; Shirayama et al., 2004).
Little is known about cell type and specific circuitry of opioid receptor subtypes in NAc related to pain processing but in slices containing NAc from naïve male and female mice, differential regulation by KOR of dopamine D1 and D2 medium spiny neurons (MSNs) has been demonstrated. U69,593 inhibited D1 MSNs more strongly than D2 MSNs. Excitatory drive from amygdala, but not hippocampus, to NAc was reduced in D1 MSN by KOR activation whereas presynaptic KOR inhibited inhibitory inputs (disinhibition) from both amygdala and hippocampus to D2 MSN (Tejeda et al., 2017). The relevance of these actions for pain processing in the mesolimbic system remain to be determined.
3. Conclusions
While the cortico-limbic system is an important target for opioid actions in pain modulation, reward mechanisms and related functions, the cellular and circuit actions of different opioids receptors and behavioral consequences of opioid receptor signaling in different elements of the cortico-limbic system are not fully understood. Data discussed in this review are summarized in Table 1. The ACC has consistently been implicated in the affective component of pain, and endogenous opioid signaling in the ACC plays a critical role in pain modulation. High levels of MOR and DOR rather than KOR are found in the ACC. MOR activation mitigates averse-affective pain behaviors and similar if not synergistic effects have been reported for DOR whereas the role of KOR in this area is largely unknown. The picture is less clear for the PL and IL regions of the mPFC in part because of the complexity of their functions in pain. The amygdala plays an important role in averse-affective behaviors and pain modulation and is rich in opioid receptors and endogenous ligands. MOR and KOR serve opposing functions whereas DOR signaling is less clear and appears to undergo a change from normal to pain conditions. Data are not quite clear about the role of amygdala MOR and KOR signaling in sensory compared to averse-affective pain behaviors. In the NAc, a key element of the central reward system, opioid signaling interacts with the dopaminergic system in addiction control and analgesia. All opioid receptor subtypes are expressed in NAc and MOR and DOR can colocalize on dendrites of NAc neurons. MOR and DOR have opposing functions to KOR on dopamine signaling and behaviors in pain models.
Table 1.
Behavioral effects of MOR, KOR, and DOR drugs in preclinical studies
| Receptor | Drug Type | Compound | Route of Administration | Subregion | Species | Sex | Pain Model | Behavioral Effect | Behavioral Test | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||||
| MOR | Agonist | Morphine | i.p. | - | Mouse | Male | Formalin | Antinociceptive | Licking and biting time | Zeng 2021 |
| i.p. | Mouse | Male | CCI | Increased preference behavior | CPP | Kai 2018 | ||||
| Oral | Rat | Both | Naïve | Antinociceptive (in male offspring) | Hot plate test, acetic acid writhing test | Ashabi 2018 | ||||
| Formalin | Licking time | |||||||||
| s.c. | Mouse | Male | Hindpaw incision | Increased preference behavior | CPP | Nwaneshiudu 2020 | ||||
| i.v. | Rat | Male | SNL | Antinociceptive | von Frey test | Navratilova 2015b | ||||
| Increased preference behavior | CPP | |||||||||
| i.p. | Rat | Male | SNL | Antinociceptive | von Frey test | LaGraize 2006 | ||||
| Decreased avoidance behavior | LDB | |||||||||
| i.p. | Rat | Male | NMDA-induced CeA lesion | No effect | Formalin test | Manning 1995a | ||||
| NMDA-induced BLA lesion | Antinociceptive | |||||||||
| i.p. | Rat | Male | NMDA-induced CeA lesion | No effect | Tail flick test | Manning 1995b | ||||
| Fentanyl (long-term) | s.c. | Rat | Male | Naïve | Pronociceptive | von Frey test, Hargreaves test | Wang 2022 | |||
| Remifentanil (long-term) | i.v. | Mouse | Female | Naïve | Increased cognitive deficits | ED shift task | Anderson 2021 | |||
| Male | No effect | |||||||||
| Female | Naïve | Increased depressive-like behavior | FST | |||||||
| Male | No effect | |||||||||
| Agonist + antagonist | Morphine + naloxone | s.c. + intra-NAc microinjection | Rat | Male | Naïve | Pronociceptive | Hot plate test | Dill 1977 | ||
| s.c. + intra-CN microinjection | No effect | |||||||||
| s.c. + intra-GP microinjection | No effect | |||||||||
| Agonist + acetaldehyde dehydrogenase inhibitor | Morphine + disulfram | Oral | Rat | Male | Naïve | Antinociceptive | Randall-Selitto test, tail flick test | de Corde-Skurska 2021 | ||
| Antagonist + agonist | Naloxone + morphine | i.p. + intra-ACC microinjection | Rat | Male | SNL | No effect | von Frey test | LaGraize 2006 | ||
| No effect | LDB | |||||||||
| KOR | Agonist | U69,593 (long-term) | s.c. | Rat | Male | Naïve | Increased aversion behavior | CPA | Tejeda 2013 | |
| U69,593 | s.c. | Mouse | Male | KOR KO on dopaminergic neurons | Decreased aversive behavior | CPA | Chefer 2013 | |||
| Antagonist | nor-BNI | s.c. | Rat | Male | Naïve | No effect | OFT | Tejeda 2015 | ||
| DOR | Antagonist | NTI | s.c. | Mouse | Male | Naïve | Increased anxiety-like behavior | LDB, EPM | Narita 2006b | |
| mPFC | ||||||||||
| MOR | Agonist | Morphine | Microinjection | ACC | Rat | Male | Incision model | Increased preference behavior | CPP | Navratilova 2015b |
| No effect | von Frey test | |||||||||
| SNL | Increased preference behavior | CPP | ||||||||
| No effect | von Frey test | |||||||||
| Microinjection | ACC | Rat | Male | SNL | No effect | von Frey test | LaGraize 2006 | |||
| Decreased avoidance behavior | LDB | |||||||||
| Microinjection | rostral ACC, caudal ACC | Rat | Male | SNL | No effect | Tail flick assay, von Frey test, Randall-Selitto test, Hargreaves test | Gomtsian 2018 | |||
| rostral ACC | Increased preference behavior | CPP | ||||||||
| caudal ACC | No effect | |||||||||
| Microinjection | ACC | Rat | Male | CCI | Antinociceptive | Randall-Selitto test, hot plate test | Wang 2020 | |||
| Agonist + antagonist | Morphine + naloxone | No effect | ||||||||
| Morphine + β-FNA | No effect | |||||||||
| Antagonist | Naloxonazine | Microinjection | PL | Rat | Male | Orofacial formalin | Pronociceptive | Face rubbing | Tamaddonfard 2020 | |
| Naloxone | Microinfusion | PL | Rat | Male | SNL | Pronociceptive | von Frey test | Zeng 2018 | ||
| Microinjection | ACC | Rat | Male | Incision model | Decreased preference behavior | CPP | Navratilova 2015b | |||
| No effect | von Frey test | |||||||||
| SNL | Decreased preference behavior | CPP | ||||||||
| No effect | von Frey test | |||||||||
| Antagonist + agonist | β-FNA + morphine | Microinjection + i.v. | Antinociceptive | von Frey test | ||||||
| Decreased preference behavior | CPP | |||||||||
| Selective ablation of MOR-expressing neurons | Derm-SAP | Microinjection | Decreased preference behavior | CPP | ||||||
| No effect | Hargreaves test | |||||||||
| Antagonist + agonist | β-FNA + morphine | Microinjection | right ACC + right CeA | Rat | Male | SNL | Decreased preference behavior | CPP | Navratilova 2020 | |
| KOR | Antagonist | nor-BNI | Microinfusion | PL | Rat | Male | U69,593-conditioned | Decreased aversion behavior | CPA | Tejeda 2013 |
| Microinfusion | PL | Rat | Male | Naïve | Decreased anxiety-like behavior | OFT | Tejeda 2015 | |||
| DOR | Agonist | DADLE | Microinjection | rostral ACC | Rat | Male | CFA | Decreased aversion behavior | CPA | Ma 2022 |
| No effect | Hot plate test | |||||||||
| KNT-127 | Microinfusion | PL | Mouse | Male | Naïve | No effect | OFT | Saitoh 2018 | ||
| Agonist + sodium channel activator | KNT-127 + veratrine | Decreased anxiety-like behavior | ||||||||
| Antagonist | Naltrindole | Microinjection | ACC | Mouse | Male | Naïve | Increased anxiety-like behavior | LDB, EPM | Narita 2006b | |
| Amygdala | ||||||||||
| MOR | Agonist | Morphine | Microinjection | right CeA | Rat | Male | SNL | No effect | von Frey test, Randall-Selitto test | Navratilova 2020 |
| Increased preference behavior | CPP | |||||||||
| left CeA | No effect | von Frey test, Randall-Selitto test, CPP | ||||||||
| Microinjection | BLA | Rat | Male | Naïve | Antinociceptive | Tail flick test | Helmstetter 1995 | |||
| Microinjection | BLA | Rat | Male | Naïve | Antinociceptive | Tail flick test, jump test | Pavlovic 1996 | |||
| CeA | ||||||||||
| Microinfusion | BLA | Rat | Male | Naïve | Antinociceptive | Tail flick test | McGaraughty 2002 | |||
| DAMGO | Microinjection | BLA | Rat | Male | Naïve | Antinociceptive | Tail flick test | Helmstetter 1998 | ||
| Microinjection | CeA | Rat | Male | CFA | Decreased aversion behavior | CPA | Zhang 2013 | |||
| No effect | Hargreaves test | |||||||||
| β-endorphin | Microinjection | BLA | Rat | Male | Naïve | Antinociceptive | Tail flick test, jump test | Pavlovic 1996 | ||
| CeA | ||||||||||
| Agonist + antagonist | Morphine + naltrexone | Microinjection | BLA + PAG | No effect | ||||||
| β-endorphin + naltrexone | ||||||||||
| Antagonist | CTAP | Microinjection | CeA | Mouse | Both | Plantar incision model | Pronociceptive | von Frey test | Cooper 2022 | |
| Chemogenetic inhibition of MOR-expressing PB input neurons | AAVDJ-EF1a-DIO-hM4D(Gi)-mCherry | Microinjection | CeA | Mouse | Both | Formalin | Antinociceptive | Hot plate test | Liu 2022 | |
| Decreased anxiety-like behavior | EPM | |||||||||
| KOR | Agonist | U69,593 | Microinfusion | right CeA | Rat | Male | Naïve | Increased emotional-affective responses | Vocalizations to noxious stimulus | Hein 2021 |
| Increased anxiety-like behavior | OFT | |||||||||
| Decreased preference behavior | CPP | |||||||||
| No effect | Paw pressure test | |||||||||
| Antagonist | nor-BNI | Microinfusion | right CeA | Rat | Male | SNL | Decreased gabapentin-induced preference behavior | CPP | Navratilova 2019 | |
| No effect | von Frey test | |||||||||
| Microinfusion | right CeA | Rat | Male | Functional pain model | Decreased emotional-affective responses | Vocalizations to noxious stimulus | Yakhnitsa 2022 | |||
| Decreased anxiety-like behavior | EPM | |||||||||
| Antinociceptive | von Frey test | |||||||||
| No effects | Paw pressure test | |||||||||
| Microinfusion | right CeA | Rat | Male | Medication overuse model | Antinociceptive | von Frey test | Xie 2017 | |||
| left CeA | No effect | |||||||||
| Microinfusion | right CeA | Rat | Male | Infraorbital CCI | Antinociceptive | von Frey test | Turnes 2022 | |||
| No effect | EPM | |||||||||
| left CeA | No effect | von Frey test, EPM | ||||||||
| Microinjection | right CeA | Rat | Male | SNL + capsaicin | Antinociceptive | Randall-Selitto test | Phelps 2019 | |||
| left CeA | No effect | |||||||||
| Microinjection | right CeA | Rat | Male | Functional pain model | Antinociceptive | Randall-Selitto test | Nation 2018 | |||
| left CeA | No effect | |||||||||
| DOR | Agonist | DPDPE | Microinfusion | CeA | Mouse | Male | Naïve | Pronociceptive | von Frey test | Zhou 2021 |
| No effect | OFT, EPM | |||||||||
| CFA | No effect | von Frey test, OFT, EPM | ||||||||
| Deltorphin II | Naïve | No effect | von Frey test, OFT, EPM | |||||||
| CFA | No effect | Von Frey test | ||||||||
| Decreased anxiety-like behavior | OFT, EPM | |||||||||
| DOR1 antagonist | BNTX | No effect | ||||||||
| DOR2 antagonist | Naltriben | Increased anxiety-like behavior | ||||||||
| Antagonist | Naltrindole | Microinjection | BLA | Mouse | Male | CFA | Increased anxiety-like behavior | LDB, EPM | Narita 2006a | |
| CCI | ||||||||||
| NAc | ||||||||||
| MOR | Agonist | Morphine | Microinjection | n.s. | Rat | Male | Naïve | Antinociceptive | Hot plate test | Dill 1977 |
| Microinfusion | n.s. | Rat | Male | SNL | Antinociceptive | Hot plate test, Randall-Selitto test | Bian 2015 | |||
| DAMGO | Microinjection | n.s. | Rat | Male | Naïve | No effect | Jaw-opening reflex | Schmidt 2002 | ||
| Antagonist | CTOP | Microinjection | n.s. | Rat | Male | DAMGO-induced antinociception | Pronociceptive | Jaw-opening reflex | Gear 2011 | |
| Microinjection | n.s. | Rat | Male | Capsaicin-induced antinociception | Pronociceptive | Jaw-opening reflex | Schmidt 2022 | |||
| Microinjection | n.s. | Rat | Male | UFP-512-induced anxiolytic and antidepressant effects | Increased anxiety- and depressive-like behavior | EPM, novelty-induced hypophagia test | Kabli 2014 | |||
| Naloxone | Microinjection | n.s. | Rat | Male | Capsaicin-induced antinociception | Pronociceptive | Jaw-opening reflex | Gear 1999 | ||
| Dopamine receptor antagonist + agonist | α-flupenthixol + morphine | Microinjection | NAc + rostral ACC | Rat | Male | SNL | Decreased preference behavior | CPP | Navratilova 2015b | |
| MOR + DOR | Agonist + agonist | DAMGO + DPDPE | Microinjection | n.s. | Rat | Male | Naïve | Antinociceptive | Jaw-opening reflex | Schmidt 2002 |
| UFP-512 | Microinjection | n.s. | Rat | Male | Naïve | Decreased anxiety- and depressive-like behavior | EPM, novelty-induced hypophagia test | Kabli 2014 | ||
| KOR | Photoactivation of dynorphin neurons | - | Optical activation | ventral NAc shell | Mouse | Both | Naïve | Decreased motivation | Sucrose self-administration | Massaly 2019 |
| Increased aversion behavior | Real-time place aversion | |||||||||
| NAc shell cold spot | Rat | Male | CFA | Increased aversion behavior | ||||||
| Photostimulation | ventral NAc shell | Mouse | Male | Naïve | Increased aversion behavior | Real-time place aversion | Al-Hasani 2015 | |||
| No effect | OFT | |||||||||
| dorsal NAc shell | Increased preference behavior | Real-time place preference | ||||||||
| No effect | OFT | |||||||||
| Photoactivation of dynorphin neurons + antagonist | nor-BNI | Microinjection | NAc shell cold spot | Rat | Male | CFA | Decreased aversion behavior | Real-time place aversion | Massaly 2019 | |
| Agonist | U69,593 | Microinjection | n.s. | Rat | Male | Capsaicin-induced antinociception | Pronociceptive | Jaw-opening reflex | Schmidt 2022 | |
| DAMGO-induced antinociception | ||||||||||
| DPDPE-induced antinociception | ||||||||||
| E-2078 | Microinjection | n.s. | Rat | Male | Naïve | Increased aversion behavior | CPA | Bals-Kubik 1993 | ||
| U50,488 | ||||||||||
| Microinjection | n.s. | Mouse | Male | Naïve | Increased anxiety-like behavior | EPM, LDB | Wang 2016 | |||
| Antagonist | nor-BNI | U50,488-induced anxiety-like behavior | Decreased anxiety-like behavior | EPM | ||||||
| Microinfusion | NAc shell | Rat | Male | Learned helplessness model | Decreased depressive-like behavior | Conditioned avoidance test | Shirayama 2004 | |||
| Microinjection | ventral NAc shell | Mouse | Both | Dynorphin neuron stimulation | Decreased aversive behavior | Real-time place aversion | Massaly 2019 | |||
| Chemogenetic silencing of dynorphin neurons | Dyn2.0-hM4Di-IRES-mCherry | Microinjection | NAc shell cold spot | Rat | Male | Naïve | Increased motivation | Sucrose self-administration | ||
| DOR | Agonist | DPDPE | Microinjection | n.s. | Rat | Male | Naïve | No effect | Jaw-opening reflex | Schmidt 2002 |
| Antagonist | Naltrindole | Microinjection | n.s. | Rat | Male | DAMGO-induced antinociception | Pronociceptive | Jaw-opening reflex | Gear 2011 | |
| Microinjection | n.s. | Rat | Male | Capsaicin-induced antinociception | Pronociceptive | Jaw-opening reflex | Schmidt 2002 | |||
| Microinjection | n.s. | Rat | Male | UFP-512-induced anxiolytic and antidepressant effects | Increased anxiety- and depressive-like behavior | EPM, novelty-induced hypophagia test | Kabli 2014 | |||
β-FNA, β-funaltrexamine; BNTX, 7-benzylidenenaltrexone; CCI, chronic constriction injury; CeA, central nucleus of the amygdala; CFA, complete Freund’s adjuvant; CMS, chronic mild stress; CN, caudate nucleus; CPA, conditioned place aversion; CPP, conditioned place preference; CTOP, [Cys2-Tyr3-Orn5-Pen7]-amide; DADLE, [D-Ala2, D-Leu5]-enkephalin; DAMGO, D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin; Derm-SAP, dermorphin-saporin; DPDPE, D-Pen2,5 enkephalin D-Pen2,5-enkephalin; ED, extradimensional shift task; EPM, elevated plus maze; FST, forced swim test; GP, globus pallidus; i.p., intraperitoneal; i.v., intravenous; KO, knock out; LDB, light/dark box; mPFC, medial prefrontal cortex; NMDA, N-methyl-D-aspartate; nor-BNI, nor-binaltorphimine; n.s., not specified; NTI, naltrindole; OFT, open field test; PB, parabrachial nucleus; PL, prelimbic; RVM, rostral ventromedial medulla; s.c., subcutaneous; SNL, spinal nerve ligation; TST, tail suspension test; UFP-512, (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid); vlPAG, ventrolateral periaqueductal gray
In conclusion, modulation of pain-related neuroplasticity by opioid signaling in cortico-limbic circuits and underlying synaptic and cellular mechanisms are largely understudied areas, though MOR and DOR signaling in the ACC and KOR signaling in the amygdala have been linked to inhibitory and disinhibitory neuronal effects, respectively. Since evidence suggests that changes in cortico-limbic circuits contribute to emotional affective and cognitive aspects of pain and possibly sensory pain modulation, it is important to determine opioid receptor subtype functions and pain-related changes in these brain regions to provide the knowledge basis for the development of novel and improved therapeutic strategies.
Acknowledgments
Work in the authors’ lab is supported by National Institute of Health (NIH) grants R01 NS038261 (VN), R01 NS106902 (VN), R01 NS109255 (GJ), R01 NS118731 (VN), R01 NS120395 (VN), R01 NS129552 (VN), Garrison Family Foundation and Giles McCrary Endowed Chair in Addiction Medicine (VN).
Footnotes
Credit authorship contribution statement
Volker Neugebauer: Conceptualization, Methodology, Investigation, Writing - original draft, review & editing, Supervision, Project administration, Funding acquisition. Guangchen Ji, Vadim Yakhnitsa, Peyton Presto, Nico Antonucci: Methodology, Investigation, Writing - original draft, review and editing. Brianna Mendoza: Methodology, Investigation, Writing - original draft.
Declarations of interest: none
Bibliography
- Adke AP, Khan A, Ahn HS, Becker JJ, Wilson TD, Valdivia S, Sugimura YK, Martinez Gonzalez S, Carrasquillo Y, 2021. Cell-Type Specificity of Neuronal Excitability and Morphology in the Central Amygdala. eNeuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, 2015. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen HN, Bobnar HJ, Kolber BJ, 2020. Left and right hemispheric lateralization of the amygdala in pain. Prog Neurobiol, 101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier N, Stewart J, 1998. Dopamine receptor antagonists in the nucleus accumbens attenuate analgesia induced by ventral tegmental area substance P or morphine and by nucleus accumbens amphetamine. J Pharmacol Exp Ther 285, 208–215. [PubMed] [Google Scholar]
- Anderson EM, Engelhardt A, Demis S, Porath E, Hearing MC, 2021. Remifentanil self-administration in mice promotes sex-specific prefrontal cortex dysfunction underlying deficits in cognitive flexibility. Neuropsychopharmacology 46, 1734–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S, 2013. Neural mechanisms of pain and alcohol dependence. Pharmacol. Biochem. Behav 112C, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G, Sadat-Shirazi MS, Akbarabadi A, Vousooghi N, Kheiri Z, Toolee H, Khalifeh S, Zarrindast MR, 2018. Is the Nociception Mechanism Altered in Offspring of Morphine-Abstinent Rats? J Pain 19, 529–541. [DOI] [PubMed] [Google Scholar]
- Bagley EE, Ingram SL, 2020. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 173, 108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Madamba SG, Siggins GR, 2011. Neuroadaptation of GABAergic transmission in the central amygdala during chronic morphine treatment. Addict Biol 16, 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak MS, Park H, Kim SK, 2021. Neural Plasticity in the Brain during Neuropathic Pain. Biomedicines 9, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS, 1993. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264, 489–495. [PubMed] [Google Scholar]
- Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I, 2015. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 77, 236–245. [DOI] [PubMed] [Google Scholar]
- Bartonjo JJ, Lundy RF, 2022. Target-specific projections of amygdala somatostatin-expressing neurons to the hypothalamus and brainstem. Chem Senses 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Navratilova E, Porreca F, Borsook D, 2013. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J. Neurophysiol 110, 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, 2005. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior 52, 336–372. [Google Scholar]
- Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, Lardenois A, Thibault C, Dembele D, Le Merrer J, Becker JA, Poch O, Kieffer BL, 2008. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci 27, 2973–2984. [DOI] [PubMed] [Google Scholar]
- Bernard J-F, Huang GF, Besson JM, 1990. Effect of noxious somesthetic stimulation on the activity of neurons of the nucleus centralis of the amygdala. Brain Res 523, 347–350. [DOI] [PubMed] [Google Scholar]
- Bernard J-F, Huang GF, Besson JM, 1992. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol 68, 551–569. [DOI] [PubMed] [Google Scholar]
- Bernard J-F, Peschanski M, Besson JM, 1989. A possible spino (trigemino)-ponto-amygdaloid pathway for pain. Neurosci. Letts 100, 83–88. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM, 1993. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329, 201–229. [DOI] [PubMed] [Google Scholar]
- Bian H, Yu LC, 2015. Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor AIP induced antinociception in rats with mononeuropathy. Neurosci Lett 599, 129–132. [DOI] [PubMed] [Google Scholar]
- Bie B, Brown DL, Naguib M, 2011. Increased synaptic GluR1 subunits in the anterior cingulate cortex of rats with peripheral inflammation. Eur J Pharmacol 653, 26–31. [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Jongbloets BC, Engeln KA, Wang D, Scherrer G, Mao T, 2019. Synapse-specific opioid modulation of thalamo-cortico-striatal circuits. Elife 8, e45146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Goedecke L, Bazelot M, Capogna M, Pape HC, Jungling K, 2015. mu-Opioid Receptor-Mediated Inhibition of Intercalated Neurons and Effect on Synaptic Transmission to the Central Amygdala. J. Neurosci 35, 7317–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom SM, Pfister J-P, Santello M, Senn W, Nevian T, 2014. Nerve Injury-Induced Neuropathic Pain Causes Disinhibition of the Anterior Cingulate Cortex. The Journal of Neuroscience 34, 5754–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, 2022. Endogenous opiates and behavior: 2020. Peptides 151, 170752. [DOI] [PubMed] [Google Scholar]
- Borrego MB, Grigsby KB, Townsley KG, Chan A, Firsick EJ, Tran A, Savarese A, Ozburn AR, 2022. Central nucleus of the amygdala projections onto the nucleus accumbens core regulate binge-like alcohol drinking in a CRF-dependent manner. Neuropharmacology 203, 108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I, 2016. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68, 282–297. [DOI] [PubMed] [Google Scholar]
- Bowen AJ, Chen JY, Huang YW, Baertsch NA, Park S, Palmiter RD, 2020. Dissociable control of unconditioned responses and associative fear learning by parabrachial CGRP neurons. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C, 2009. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS. One 4, e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger M, Ettlin DA, Meier M, Keller T, Luechinger R, Barlow A, Palla S, Jäncke L, Lutz K, 2011. Taking Sides with Pain - Lateralization aspects Related to Cerebral Processing of Dental Pain. Front Hum Neurosci 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burand AJ Jr., Stucky CL, 2021. Fabry disease pain: patient and preclinical parallels. Pain 162, 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Giesler GJ Jr., 1989. Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res 497, 149–154. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Liu SS, Xue L, Magnussen C, Ong E, Grenier P, Sutherland A, Olmstead MC, 2022. Delta opioid receptor activation modulates affective pain and modality-specific pain hypersensitivity associated with chronic neuropathic pain. J Neurosci Res 100, 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AM, Cook C, Ong E, Moron JA, Evans CJ, 2014. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol 5, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XY, Xu H, Wu LJ, Li XY, Chen T, Zhuo M, 2009. Characterization of intrinsic properties of cingulate pyramidal neurons in adult mice after nerve injury. Mol Pain 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV, 2014. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 155, 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Bäckman CM, Gigante ED, Shippenberg TS, 2013. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacology 38, 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Heinricher MM, 2019. Descending Control Mechanisms and Chronic Pain. Curr Rheumatol Rep 21, 13. [DOI] [PubMed] [Google Scholar]
- Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li ZH, Lu YC, Inoue K, Tsuda M, Li YQ, Nakatsuka T, Zhuo M, 2018. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun 9, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, Kaushik MK, Ferreira AN, Sheets PL, 2016. Specific Targeting of the Basolateral Amygdala to Projectionally Defined Pyramidal Neurons in Prelimbic and Infralimbic Cortex. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, Sheets PL, 2018. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J. Neurosci 38, 4829–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, Sheets PL, 2020. Peripheral nerve injury reduces the excitation-inhibition balance of basolateral amygdala inputs to prelimbic pyramidal neurons projecting to the periaqueductal gray. Mol Brain 13, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM, 2019. Parabrachial Complex: A Hub for Pain and Aversion. J Neurosci 39, 8225–8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE, 2020. Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron 106, 927–939 e925. [DOI] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB, 2006. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 497, 910–927. [DOI] [PubMed] [Google Scholar]
- Chung PC, Boehrer A, Stephan A, Matifas A, Scherrer G, Darcq E, Befort K, Kieffer BL, 2015. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res 278, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A, 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ Jr., 1991. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 11, 852–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AH, Hedden NS, Corder G, Lamerand SR, Donahue RR, Morales-Medina JC, Selan L, Prasoon P, Taylor BK, 2022. Endogenous μ-opioid receptor activity in the lateral and capsular subdivisions of the right central nucleus of the amygdala prevents chronic postoperative pain. J Neurosci Res 100, 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro Matos S, Zhang Z, Seguela P, 2015. Peripheral Neuropathy Induces HCN Channel Dysfunction in Pyramidal Neurons of the Medial Prefrontal Cortex. J Neurosci 35, 13244–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G, 2019. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363, 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ, 2001. Evidence for functional release of endogenous opioids in the locus ceruleus during stress termination. J Neurosci 21, Rc152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Nascimento TD, DosSantos MF, Zubieta JK, 2014. Migraine and the Mu-opioidergic system-Can we directly modulate it? Evidence from neuroimaging studies. Curr Pain Headache Rep 18, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Corde-Skurska A, Krzascik P, Lesniak A, Sacharczuk M, Nagraba L, Bujalska-Zadrozny M, 2021. Disulfiram Abrogates Morphine Tolerance-A Possible Role of μ-Opioid Receptor-Related G-Protein Activation in the Striatum. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Zhou H, Lin JK, Shen ZX, Chen WZ, Wang LH, Li Q, Mu D, Wei YC, Xu XH, Sun YG, 2020. The Parabrachial Nucleus Directly Channels Spinal Nociceptive Signals to the Intralaminar Thalamic Nuclei, but Not the Amygdala. Neuron 107, 909–923 e906. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244, 1067–1080. [PubMed] [Google Scholar]
- Dill RE, Costa E, 1977. Behavioural dissociation of the enkephalinergic systems of nucleus accumbens and nucleus caudatus. Neuropharmacology 16, 323–326. [DOI] [PubMed] [Google Scholar]
- Ding DC, Gabbott PL, Totterdell S, 2001. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res 917, 81–89. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M, 2005. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol 489, 92–119. [DOI] [PubMed] [Google Scholar]
- DosSantos MF, Moura B. d. S., DaSilva AF, 2017. Reward Circuitry Plasticity in Pain Perception and Modulation. Frontiers in Pharmacology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL, 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Sah P, 2004. Opioids Inhibit Lateral Amygdala Pyramidal Neurons by Enhancing a Dendritic Potassium Current. Journal of Neuroscience 24, 3031–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Muller C, Kovacevic A, Tovote P, Luthi A, 2017. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM, 1986. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 249, 293–336. [DOI] [PubMed] [Google Scholar]
- Férézou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B, 2007. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex 17, 1948–1957. [DOI] [PubMed] [Google Scholar]
- Fields HL, Margolis EB, 2015. Understanding opioid reward. Trends Neurosci 38, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL, 2006. {micro} Opioid Receptor Activation Inhibits GABAergic Inputs to Basolateral Amygdala Neurons Through Kv1.1/1.2 Channels. Journal of Neurophysiology 95, 2032–2041. [DOI] [PubMed] [Google Scholar]
- Fu J-Y, Yu X-D, Zhu Y, Xie S-Z, Tang M-Y, Yu B, Li X-M, 2020. Whole-Brain Map of Long-Range Monosynaptic Inputs to Different Cell Types in the Amygdala of the Mouse. Neuroscience Bulletin 36, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML, 2014. The anterior cingulate cortex and pain processing. Front Integr Neurosci 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusa K, Takahashi I, Watanabe S, Aono Y, Ikeda H, Saigusa T, Nagase H, Suzuki T, Koshikawa N, Cools AR, 2005. The non-peptidic delta opioid receptor agonist TAN-67 enhances dopamine efflux in the nucleus accumbens of freely moving rats via a mechanism that involves both glutamate and free radicals. Neuroscience 130, 745–755. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492, 145–177. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Busby SJ, 2006. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Gao J, Wu X, Owyang C, Li Y, 2006. Enhanced responses of the anterior cingulate cortex neurones to colonic distension in viscerally hypersensitive rats. J Physiol 570, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gavériaux-Ruff C, Kieffer BL, 2011. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol 22, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear RW, Aley KO, Levine JD, 1999. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci 19, 7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear RW, Levine JD, 2011. Nucleus accumbens facilitates nociception. Exp Neurol 229, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF, 2000. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 275, 26128–26135. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr., Sibley DR, 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Giacchino JL, Henriksen SJ, 1996. Systemic morphine and local opioid effects on neuronal activity in the medial prefrontal cortex. Neuroscience 70, 941–949. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M, Koob GF, Schweitzer P, 2014. Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology 77, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedecke L, Bengoetxea X, Blaesse P, Pape HC, Jüngling K, 2019. μ-opioid receptor-mediated downregulation of midline thalamic pathways to basal and central amygdala. Sci Rep 9, 17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffer Y, Xu D, Eberle SE, D’Amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J, 2013. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci 33, 19034–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, Devi LA, 2020. Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci U S A 117, 11820–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, Navratilova E, 2018. Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 159, 2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves L, Dickenson AH, 2012. Asymmetric time-dependent activation of right central amygdala neurones in rats with peripheral neuropathy and pregabalin modulation. Eur. J. Neurosci 36, 3204–3213. [DOI] [PubMed] [Google Scholar]
- Goodman RR, Snyder SH, Kuhar MJ, Young WS 3rd, 1980. Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A 77, 6239–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P, 1999. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877, 49–63. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM, 2000. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci 20, Rc106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghparast A, Ghalandari-Shamami M, Hassanpour-Ezatti M, 2012. Blockade of D1/D2 dopamine receptors within the nucleus accumbens attenuated the antinociceptive effect of cannabinoid receptor agonist in the basolateral amygdala. Brain Res 1471, 23–32. [DOI] [PubMed] [Google Scholar]
- Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS, 1994. Corticotropin releasing factor neurons are innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res. Bull 33, 529–534. [DOI] [PubMed] [Google Scholar]
- Harris HN, Peng YB, 2020. Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regen Res 15, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK, 2007. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 27, 10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanpour Razmanjani N, Reisi P, 2022. The Acute Effects of Different doses of Tramadol on Neuronal Activity of Medial Prefrontal Cortex in Rats. Adv Biomed Res 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ, 2010. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein M, Ji G, Tidwell D, D’Souza P, Kiritoshi T, Yakhnitsa V, Navratilova E, Porreca F, Neugebauer V, 2021. Kappa opioid receptor activation in the amygdala disinhibits CRF neurons to generate pain-like behaviors. Neuropharmacology 185, 108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PSF, Poore LH, 1995. Microinfusion of Mu but not Delta or Kappa opioid agonists into the basolateral amygdala results in inhibition of the tail flick reflex in pentobarbital-anesthetized rats. J. Pharm. Exp. Thera 275, 381–388. [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA, 1994. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. J. Neurosci 14, 7099–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PSF, 1998. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res 779, 104–118. [DOI] [PubMed] [Google Scholar]
- Hipolito L, Sanchez-Catalan MJ, Zanolini I, Polache A, Granero L, 2008. Shell/core differences in mu- and delta-opioid receptor modulation of dopamine efflux in nucleus accumbens. Neuropharmacology 55, 183–189. [DOI] [PubMed] [Google Scholar]
- Hirose N, Murakawa K, Takada K, Oi Y, Suzuki T, Nagase H, Cools AR, Koshikawa N, 2005. Interactions among mu- and delta-opioid receptors, especially putative delta1- and delta2-opioid receptors, promote dopamine release in the nucleus accumbens. Neuroscience 135, 213–225. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP, 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212, 149–179. [DOI] [PubMed] [Google Scholar]
- Huang T, Lin SH, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q, 2019. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huge V, Rammes G, Beyer A, Zieglgänsberger W, Azad SC, 2009. Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur J Pain 13, 124–129. [DOI] [PubMed] [Google Scholar]
- Huhn AS, Berry MS, Dunn KE, 2018. Systematic review of sex-based differences in opioid-based effects. Int Rev Psychiatry 30, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Berry MS, Dunn KE, 2019. Review: Sex-Based Differences in Treatment Outcomes for Persons With Opioid Use Disorder. Am J Addict 28, 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, 1996. Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience 72, 767–783. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO, 1999. Pain-related neurons in the human cingulate cortex. Nat. Neurosci 2, 403–405. [DOI] [PubMed] [Google Scholar]
- Ito M, Nagase M, Tohyama S, Mikami K, Kato F, Watabe AM, 2021. The parabrachial-to-amygdala pathway provides aversive information to induce avoidance behavior in mice. Mol Brain 14, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Pickel VM, 2009. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience 159, 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT, 2003. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature 424, 316–320. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V, 2010. Pain-related deactivation of mPFC neurons involves mGluR1 and GABAA receptors. Soc. Neurosci. Abstr 40, 680.683. [Google Scholar]
- Ji G, Neugebauer V, 2011. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. J Neurophysiol 106, 2642–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V, 2014. CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur. J. Neurosci 39, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V, 2019. Contribution of Corticotropin-Releasing Factor Receptor 1 (CRF1) to Serotonin Receptor 5-HT2CR Function in Amygdala Neurons in a Neuropathic Pain Model. Int. J. Mol. Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V, 2020. Kappa opioid receptors in the central amygdala modulate spinal nociceptive processing through an action on amygdala CRF neurons. Mol Brain 13, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V, 2010. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci 30, 5451–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Zhang w., Mahimainathan L, Narasimhan M, Kiritoshi T, Fan X, Wang J, Green TA, Neugebauer V, 2017. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci 37, 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing PB, Chen XH, Lu HJ, Gao YJ, Wu XB, 2022. Enhanced function of NR2C/2D-containing NMDA receptor in the nucleus accumbens contributes to peripheral nerve injury-induced neuropathic pain and depression in mice. Mol Pain 18, 17448069211053255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AF, Sheets PL, 2020. Sex-Specific Disruption of Distinct mPFC Inhibitory Neurons in Spared-Nerve Injury Model of Neuropathic Pain. Cell Rep 31, 107729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Nguyen T, Balboni G, O’Dowd BF, George SR, 2014. Antidepressant-like and anxiolytic-like effects following activation of the mu-delta opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry 19, 986–994. [DOI] [PubMed] [Google Scholar]
- Kai Y, Li Y, Sun T, Yin W, Mao Y, Li J, Xie W, Chen S, Wang L, Li J, Zhang Z, Tao W, 2018. A medial prefrontal cortex-nucleus acumens corticotropin-releasing factor circuitry for neuropathic pain-increased susceptibility to opioid reward. Transl Psychiatry 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Fan W, Young AJ, Guy MD, 2011. Opiate sensitization induces FosB/ΔFosB expression in prefrontal cortical, striatal and amygdala brain regions. PLoS One 6, e23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Ide S, Minami M, 2016. Pain relief induces dopamine release in the rat nucleus accumbens during the early but not late phase of neuropathic pain. Neurosci Lett 629, 73–78. [DOI] [PubMed] [Google Scholar]
- Kelly CJ, Huang M, Meltzer H, Martina M, 2016. Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Front Cell Neurosci 10, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Martina M, 2018. Circuit-selective properties of glutamatergic inputs to the rat prelimbic cortex and their alterations in neuropathic pain. Brain Struct. Funct 223, 2627–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, 2017. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mátyás F, Lee S, Acsády L, Shin HS, 2012. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc Natl Acad Sci U S A 109, 15497–15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi T, Ji G, Neugebauer V, 2016. Rescue of Impaired mGluR5-Driven Endocannabinoid Signaling Restores Prefrontal Cortical Output to Inhibit Pain in Arthritic Rats. J. Neurosci 36, 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi T, Neugebauer V, 2018. Pathway-Specific Alterations of Cortico-Amygdala Transmission in an Arthritis Pain Model. ACS Chem. Neurosci 9, 2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissiwaa SA, Patel SD, Winters BL, Bagley EE, 2020. Opioids differentially modulate two synapses important for pain processing in the amygdala. Br J Pharmacol 177, 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski P, Morgan M, Bartlett SE, 2015. The role of δ-opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol 172, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr., 2010. Dynorphin, stress, and depression. Brain Res 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Kato K, Mikami A, 2000. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett 283, 17–20. [DOI] [PubMed] [Google Scholar]
- Koyama T, Kato K, Tanaka YZ, Mikami A, 2001. Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res 39, 421–430. [DOI] [PubMed] [Google Scholar]
- Kummer KK, Mitrić M, Kalpachidou T, Kress M, 2020. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Yen CT, 2005. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J Neurophysiol 94, 1825–1836. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Borzan J, Peng YB, Fuchs PN, 2006. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol 197, 22–30. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN, 2004. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol 188, 139–148. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL, 2009. Reward processing by the opioid system in the brain. Physiol Rev 89, 1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J, 2015. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci 35, 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei LG, Sun S, Gao YJ, Zhao ZQ, Zhang YQ, 2004. NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp Neurol 189, 413–421. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I, 2008. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci 9, 314–320. [DOI] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B, 2013. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Chen K, Sheets PL, 2022. Topographic organization underlies intrinsic and morphological heterogeneity of central amygdala neurons expressing corticotropin-releasing hormone. J Comp Neurol 530, 2286–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Sheets PL, 2018. The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J. Physiol 596, 6289–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, Sheets PL, 2020. Spared nerve injury differentially alters parabrachial monosynaptic excitatory inputs to molecularly specific neurons in distinct subregions of the central amygdala. Pain 161, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TT, Ren WH, Xiao X, Nan J, Cheng LZ, Zhang XH, Zhao ZQ, Zhang YQ, 2009. NMDA NR2A and NR2B receptors in the rostral anterior cingulate cortex contribute to pain-related aversion in male rats. Pain 146, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M, 2010. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330, 1400–1404. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen Z, Fan G, Li A, Yuan J, Xu T, 2018. Cell-Type-Specific Afferent Innervation of the Nucleus Accumbens Core and Shell. Front Neuroanat 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges A, Yarur HE, Tejeda HA, 2022. Dynorphin/kappa opioid receptor system regulation on amygdaloid circuitry: Implications for neuropsychiatric disorders. Front Syst Neurosci 16, 963691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Tang QQ, Yin C, Song Y, Liu Y, Yang JX, Liu H, Zhang YM, Wu SY, Song Y, Juarez B, Ding HL, Han MH, Zhang H, Cao JL, 2018. Brain-derived neurotrophic factor-mediated projection-specific regulation of depressive-like and nociceptive behaviors in the mesolimbic reward circuitry. Pain 159, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ye M, Pao GM, Song SM, Jhang J, Jiang H, Kim JH, Kang SJ, Kim DI, Han S, 2022. Divergent brainstem opioidergic pathways that coordinate breathing with pain and emotions. Neuron 110, 857–873.e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Torralba M, Pilar-Cuéllar F, da Silva Borges G, Mico JA, Berrocoso E, 2020. Opioid receptors mRNAs expression and opioids agonist-dependent G-protein activation in the rat brain following neuropathy. Prog Neuropsychopharmacol Biol Psychiatry 99, 109857. [DOI] [PubMed] [Google Scholar]
- Ma QP, Shi YS, Han JS, 1992. Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res 583, 292–295. [DOI] [PubMed] [Google Scholar]
- Ma Y, Qin GH, Guo X, Hao N, Shi Y, Li HF, Zhao X, Li JG, Zhang C, Zhang Y, 2022. Activation of δ-opioid Receptors in Anterior Cingulate Cortex Alleviates Affective Pain in Rats. Neuroscience 494, 152–166. [DOI] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L, 2007. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain 127, 183–194. [DOI] [PubMed] [Google Scholar]
- Magableh A, Lundy R, 2014. Somatostatin and corticotrophin releasing hormone cell types are a major source of descending input from the forebrain to the parabrachial nucleus in mice. Chem Senses 39, 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, 1998. A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J. Neurosci 18, 9453–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Mayer DJ, 1995a. The central nucleus of the amygdala contributes to the production of morphine antinociception in the formalin test. Pain 63, 141–152. [DOI] [PubMed] [Google Scholar]
- Manning BH, Mayer DJ, 1995b. The central nucleus of the amygdala contributes to the production of morphine antinociception in the rat tail-flick test. J. Neurosci 15, 8199–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ, 1996. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience 71, 671–690. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ, 1987. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7, 2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB, 2007. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J. Comp Neurol 504, 702–715. [DOI] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau R. W. t., McCall JG, Al-Hasani R, Bruchas MR, Moron JA, 2019. Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 102, 564–573 e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitelli M, Marshall K, Pham A, Ji G, Neugebauer V, 2021. Optogenetic Manipulations of Amygdala Neurons Modulate Spinal Nociceptive Processing and Behavior Under Normal Conditions and in an Arthritis Pain Model. Front Pharmacol 12, 668337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitelli M, Yakhnitsa V, Neugebauer B, Neugebauer V, 2022. Optogenetic manipulations of CeA-CRF neurons modulate pain- and anxiety-like behaviors in neuropathic pain and control rats. Neuropharmacology 210, 109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Hartmann J, Carlezon WA Jr., Ressler KJ, 2018. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Farr DA, Heinricher MM, 2004. Lesions of the periaqueductal gray disrupt input to the rostral ventromedial medulla following microinjections of morphine into the medial or basolateral nuclei of the amygdala. Brain Research 1009, 223–227. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Heinricher MM, 2002. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain 96, 153–162. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H, 1993. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A 90, 9954–9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF, 2000. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience 96, 91–99. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M, 2009. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad Sci U. S. A 106, 2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz AS, Goodman RR, 1984. Light microscopic autoradiographic localization of mu and delta opioid binding sites in the mouse central nervous system. J Neurosci 4, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz B, Haggerty DL, Atwood BK, 2020. Synapse-specific expression of mu opioid receptor long-term depression in the dorsomedial striatum. Sci Rep 10, 7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal X, Baños JE, Kieffer BL, Maldonado R, 2006. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci 23, 830–834. [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T, 2006a. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31, 739–750. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T, 2005. Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology 30, 111–118. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Narita M, Kaneko C, Hareyama N, Miyatake M, Shindo K, Miyoshi K, Nakajima M, Nagumo Y, Sato F, Wachi H, Seyama Y, Suzuki T, 2006b. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem 97, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Nation KM, De FM, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, Porreca F, 2018. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain 159, 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Atcherley CW, Porreca F, 2015a. Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci 38, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, Neugebauer V, Porreca F, 2019. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain 160, 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Nation K, Remeniuk B, Neugebauer V, Bannister K, Dickenson AH, Porreca F, 2020. Selective modulation of tonic aversive qualities of neuropathic pain by morphine in the central nucleus of the amygdala requires endogenous opioid signaling in the anterior cingulate cortex. Pain 161, 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Porreca F, 2014. Reward and motivation in pain and pain relief. Nat. Neurosci 17, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F, 2015b. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 35, 7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F, 2012. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. U. S. A 109, 20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr., 2006. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, 2020. Amygdala physiology in pain. Handb Behav Neurosci 26, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, 2002. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J. Neurophysiol 87, 103–112. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, 2003. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol 89, 716–727. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS, 2004. The amygdala and persistent pain. Neuroscientist 10, 221–234. [DOI] [PubMed] [Google Scholar]
- Noursadeghi E, Rashvand M, Haghparast A, 2022. Nucleus accumbens dopamine receptors mediate the stress-induced analgesia in an animal model of acute pain. Brain Res 1784, 147887. [DOI] [PubMed] [Google Scholar]
- Nwaneshiudu CA, Shi XY, Clark JD, 2020. Incisional Injury Modulates Morphine Reward and Morphine-Primed Reinstatement: A Role of Kappa Opioid Receptor Activation. Anesth Analg 130, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P, 2012. Potentiation of dopamine D1-like receptor signaling by concomitant activation of δ- and μ-opioid receptors in mouse medial prefrontal cortex. Neurochem Int 61, 1404–1416. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, 2018. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci 41, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic ZW, Cooper ML, Bodnar RJ, 1996. Opioid antagonists in the periaqueductal gray inhibit morphine and β-endorphin analgesia elicited from the amygdala of rats. Brain Res 741, 13–26. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB, 1999. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88, 1093–1135. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B, 2014. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci 34, 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M, 2002. Placebo and opioid analgesia-- imaging a shared neuronal network. Science 295, 1737–1740. [DOI] [PubMed] [Google Scholar]
- Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K, 2019. Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain 160, 1614–1621. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De GG, Crawford E, Janak PH, George O, Rice KC, Messing RO, 2015. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front Neurosci 9, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Navratilova E, 2017. Reward, motivation, and emotion of pain and its relief. Pain 158 Suppl 1, S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin A, Koch A, Campbell P, Laumet G, Stohler CS, Dantzer R, Zubieta JK, 2022. Effects of placebo administration on immune mechanisms and relationships with central endogenous opioid neurotransmission. Mol Psychiatry 27, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybysz KR, Werner DF, Diaz MR, 2017. Age-dependent regulation of GABA transmission by kappa opioid receptors in the basolateral amygdala of Sprague-Dawley rats. Neuropharmacology 117, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, Zhao M, Huganir RL, Luo J, Xu H, Zhuo M, 2014. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci 34, 13505–13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F, 2011. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 152, 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzicki D, Pollema-Mays SL, Sanz-Clemente A, Martina M, 2017. Loss of M1 Receptor Dependent Cholinergic Excitation Contributes to mPFC Deactivation in Neuropathic Pain. J. Neurosci 37, 2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y, 2019. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology 44, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, Chen J, 2008. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 153, 268–278. [DOI] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ, 2016. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 19, 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Wei X, Wickersham I, Martina M, Apkarian AV, Surmeier DJ, 2021. Adaptive alterations in the mesoaccumbal network after peripheral nerve injury. Pain 162, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Kiritoshi T, Gregoire S, Ji G, Guerrini R, Calo G, Neugebauer V, 2013. Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors. J. Neurophysiol 110, 1765–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Neugebauer V, 2010. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol. Pain 6, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Kravets JL, Connelly KL, Unterwald EM, van Bockstaele EJ, 2017. Localization of the delta opioid receptor and corticotropin-releasing factor in the amygdalar complex: role in anxiety. Brain Struct. Funct 222, 1007–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rullo L, Posa L, Caputi FF, Stamatakos S, Formaggio F, Caprini M, Liguori R, Candeletti S, Romualdi P, 2021. Nociceptive behavior and central neuropeptidergic dysregulations in male and female mice of a Fabry disease animal model. Brain Res Bull 175, 158–167. [DOI] [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Waddington JL, 2017. Mechanisms underlying delta- and mu-opioid receptor agonist-induced increases in extracellular dopamine level in the nucleus accumbens of freely moving rats. J Oral Sci 59, 195–200. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Suzuki S, Soda A, Ohashi M, Yamada M, Oka JI, Nagase H, Yamada M, 2018. The delta opioid receptor agonist KNT-127 in the prelimbic medial prefrontal cortex attenuates veratrine-induced anxiety-like behaviors in mice. Behav Brain Res 336, 77–84. [DOI] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG, 2015. The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg 93, 75–93. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS, 2017. A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 93, 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Nevian T, 2015. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 86, 233–246. [DOI] [PubMed] [Google Scholar]
- Sato D, Narita M, Hamada Y, Mori T, Tanaka K, Tamura H, Yamanaka A, Matsui R, Watanabe D, Suda Y, Senba E, Watanabe M, Navratilova E, Porreca F, Kuzumaki N, Narita M, 2022. Relief of neuropathic pain by cell-specific manipulation of nucleus accumbens dopamine D1- and D2-receptor-expressing neurons. Mol Brain 15, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ito M, Nagase M, Sugimura YK, Takahashi Y, Watabe AM, Kato F, 2015. The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Mol Brain 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL, Tambeli CH, Levine JD, Gear RW, 2002. mu/delta Cooperativity and opposing kappa-opioid effects in nucleus accumbens-mediated antinociception in the rat. Eur J Neurosci 15, 861–868. [DOI] [PubMed] [Google Scholar]
- Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE, 2016. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 157, 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC, 2014. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Koeppe RA, Zubieta JK, 2007. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse 61, 707–714. [DOI] [PubMed] [Google Scholar]
- Shiers S, Pradhan G, Mwirigi J, Mejia G, Ahmad A, Kroener S, Price T, 2018. Neuropathic Pain Creates an Enduring Prefrontal Cortex Dysfunction Corrected by the Type II Diabetic Drug Metformin But Not by Gabapentin. J Neurosci 38, 7337–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, 2009. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology 34, 247. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS, 2004. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem 90, 1258–1268. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA, 1992. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol 68, 1720–1732. [DOI] [PubMed] [Google Scholar]
- Singh S, Wilson TD, Valdivia S, Benowitz B, Chaudhry S, Ma J, Adke AP, Soler-Cedeño O, Velasquez D, Penzo MA, Carrasquillo Y, 2022. An inhibitory circuit from central amygdala to zona incerta drives pain-related behaviors in mice. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Walker BM, 2015. Maturational alterations in constitutive activity of medial prefrontal cortex kappa-opioid receptors in Wistar rats. J Neurochem 135, 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS, 1992. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89, 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tölle TR, 2006. Opioidergic activation in the medial pain system after heat pain. Pain 122, 63–67. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF, 2001. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia 36, 78–88. [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A, 2011. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, North RA, 1993. Opioid actions on neurons of rat lateral amygdala in vitro. Brain Res 612, 151–155. [DOI] [PubMed] [Google Scholar]
- Sugita S, Tanaka E, North RA, 1993. Membrane properties and synaptic potentials of three types of neurone in rat lateral amygdala. J. Physiol 460, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM, 1998. Cellular sites for activation of delta-opioid receptors in the rat nucleus accumbens shell: relationship with Met5-enkephalin. J Neurosci 18, 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, 2002. Kappa-Opioid and NMDA glutamate receptors are differentially targeted within rat medial prefrontal cortex. Brain Res 946, 262–271. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM, 1999. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci 19, 1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM, 1997. mu-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci 17, 2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds LL, Gordon NS, Bixby JC, Mande MM, 2006. Right-lateralized pain processing in the human cortex: an FMRI study. J Neurophysiol 95, 3823–3830. [DOI] [PubMed] [Google Scholar]
- Taki K, Kaneko T, Mizuno N, 2000. A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience 98, 221–231. [DOI] [PubMed] [Google Scholar]
- Tamaddonfard E, Erfanparast A, Salighedar R, Tamaddonfard S, 2020. Medial prefrontal cortex diclofenac-induced antinociception is mediated through GPR55, cannabinoid CB1, and mu-opioid receptors of this area and periaqueductal gray. Naunyn-Schmiedeberg’s Archives of Pharmacology 393, 371–379. [DOI] [PubMed] [Google Scholar]
- Tan LL, Pelzer P, Heinl C, Tang W, Gangadharan V, Flor H, Sprengel R, Kuner T, Kuner R, 2017. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat Neurosci 20, 1591–1601. [DOI] [PubMed] [Google Scholar]
- Taylor AMW, 2018. Corticolimbic circuitry in the modulation of chronic pain and substance abuse. Prog Neuropsychopharmacol Biol Psychiatry 87, 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, Chefer V, O’Donnell P, Shippenberg TS, 2013. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38, 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P, 2015. Prefrontal Cortical Kappa Opioid Receptors Attenuate Responses to Amygdala Inputs. Neuropsychopharmacology 40, 2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Wu J, Kornspun AR, Pignatelli M, Kashtelyan V, Krashes MJ, Lowell BB, Carlezon WA Jr., Bonci A, 2017. Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 93, 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel A, 1991. Visualization of mu opiate receptor downregulation following morphine treatment in neonatal rat brain. Brain Res Dev Brain Res 64, 19–26. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS, 1987. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A 84, 4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershner SA, Helmstetter FJ, 2000. Antinociceptioon produced by mu opioid receptor activation in the amygdala is partly dependent on activation of mu opioid and neurotensin receptors in the ventral periaqueductal gray. Brain Res 865, 17–26. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Neugebauer V, 2019. Cortico-limbic pain mechanisms. Neurosci Lett 702, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Zhuo M, 2009. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R, 2010. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend 108, 183–194. [DOI] [PubMed] [Google Scholar]
- Turnes JM, Araya EI, Barroso AR, Baggio DF, Koren LO, Zanoveli JM, Chichorro JG, 2022. Blockade of kappa opioid receptors reduces mechanical hyperalgesia and anxiety-like behavior in a rat model of trigeminal neuropathic pain. Behav Brain Res 417, 113595. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV, 2016. The Emotional Brain as a Predictor and Amplifier of Chronic Pain. J. Dent. Res 95, 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, 2001. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 158, 331–342. [DOI] [PubMed] [Google Scholar]
- van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, Vogt BA, Glennon JC, Havenith MN, 2020. Where is Cingulate Cortex? A Cross-Species View. Trends Neurosci 43, 285–299. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Johnson JM, Przybysz KR, Deak T, Diaz MR, 2020. Adolescent forced swim stress increases social anxiety-like behaviors and alters kappa opioid receptor function in the basolateral amygdala of male rats. Prog Neuropsychopharmacol Biol Psychiatry 98, 109812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathy I, Slamberová R, Rimanóczy A, Riley MA, Bar N, 2003. Autoradiographic evidence that prenatal morphine exposure sex-dependently alters mu-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog Neuropsychopharmacol Biol Psychiatry 27, 381–393. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ, 1997. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 498 ( Pt 2), 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ, 1997. How opioids inhibit GABA-mediated neurotransmission. Nature 390, 611–614. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Vijay A, Wang S, Worhunsky P, Zheng MQ, Nabulsi N, Ropchan J, Krishnan-Sarin S, Huang Y, Morris ED, 2016. PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 6, 205–214. [PMC free article] [PubMed] [Google Scholar]
- Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RG, Vogt BA, 2001. Colocalization of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J Pharmacol Exp Ther 299, 840–848. [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK, 2007. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A 104, 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G, 2018. Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 98, 90–108.e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J, Wang LP, Wang Y, 2015. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat. Commun 6, 7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Huang J, Chang JY, Woodward DJ, Luo F, 2009. Morphine modulation of pain processing in medial and lateral pain pathways. Mol Pain 5, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hou K, Wang H, Fu F, Yu L, 2020. Role of mu-opioid receptor in nociceptive modulation in anterior cingulate cortex of rats. Mol Pain 16, 1744806920966144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu P, Ma L, Wang F, 2019. Opioid signal transduction regulates the dendritic morphology of somatostatin and parvalbumin interneurons in the medial prefrontal cortex. NeuroReport 30, 592–599. [DOI] [PubMed] [Google Scholar]
- Wang XX, Cui LL, Gan SF, Zhang ZR, Xiao J, Li CH, Luo F, 2022. Inhibition of Oligodendrocyte Apoptosis in the Prelimbic Medial Prefrontal Cortex Prevents Fentanyl-induced Hyperalgesia in Rats. J Pain 23, 1035–1050. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Hang A, Lu YC, Long Y, Zan GY, Li XP, Wang Q, Zhao ZX, He L, Chi ZQ, Liu JG, 2016. kappa Opioid receptor activation in different brain regions differentially modulates anxiety-related behaviors in mice. Neuropharmacology 110, 92–101. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Fitting S, Hussain MZ, Kononenko O, Iatsyshyna A, Yoshitake T, Kehr J, Alkass K, Druid H, Wadensten H, Andren PE, Nylander I, Wedell DH, Krishtal O, Hauser KF, Nyberg F, Karpyak VM, Yakovleva T, Bakalkin G, 2015. Asymmetry of the endogenous opioid system in the human anterior cingulate: a putative molecular basis for lateralization of emotions and pain. Cereb Cortex 25, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzczak-Bargiela A, Ziolkowska B, Piotrowska A, Starnowska-Sokol J, Rojewska E, Mika J, Przewlocka B, Przewlocki R, 2020. Neuropathic Pain Dysregulates Gene Expression of the Forebrain Opioid and Dopamine Systems. Neurotox Res 37, 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF, 2010. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 210, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Li P, Zhuo M, 1999. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 19, 9346–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhuo M, 2001. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 532, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tölle TR, 2004. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain 108, 213–220. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Junor L, 2008. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology 33, 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, McDonald AJ, 2002. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett 328, 160–164. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Martinez GS, Sugimura YK, Carrasquillo Y, 2019. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep 29, 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BL, Gregoriou GC, Kissiwaa SA, Wells OA, Medagoda DI, Hermes SM, Burford NT, Alt A, Aicher SA, Bagley EE, 2017. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat. Commun 8, 14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojick JA, McCall NM, James JG, Corder G, 2022. Characterization of a Nociceptive Neural Ensemble in the Nucleus Accumbens Shell. The Journal of Pain 23, 17–18. [Google Scholar]
- Wood JN, Grafman J, 2003. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci 4, 139–147. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ, 1995. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol 361, 383–403. [DOI] [PubMed] [Google Scholar]
- Wu XB, Jing PB, Zhang ZJ, Cao DL, Gao MH, Jiang BC, Gao YJ, 2018. Chemokine receptor CCR2 contributes to neuropathic pain and the associated depression via increasing NR2B-mediated currents in both D1 and D2 dopamine receptor-containing medium spiny neurons in the nucleus accumbens shell. Neuropsychopharmacology 43, 2320–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Yang Y, Zhang Y, Zhang XM, Zhao ZQ, Zhang YQ, 2013. Estrogen in the anterior cingulate cortex contributes to pain-related aversion. Cereb Cortex 23, 2190–2203. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zhang YQ, 2018. A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev 90, 200–211. [DOI] [PubMed] [Google Scholar]
- Xie JY, De FM, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F, 2017. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia 37, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M, 2008. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28, 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhnitsa V, Ji G, Hein M, Presto P, Griffin Z, Ponomareva O, Navratilova E, Porreca F, Neugebauer V, 2022. Kappa Opioid Receptor Blockade in the Amygdala Mitigates Pain Like-Behaviors by Inhibiting Corticotropin Releasing Factor Neurons in a Rat Model of Functional Pain. Front Pharmacol 13, 903978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Takahashi J, Iio K, Nagase H, Saitoh A, 2021. Modulation of glutamatergic synaptic transmission and neuronal excitability in the prelimbic medial prefrontal cortex via delta-opioid receptors in mice. Biochem Biophys Res Commun 560, 192–198. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R, 1996. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res 735, 83–92. [DOI] [PubMed] [Google Scholar]
- Ye J, Veinante P, 2019. Cell-type specific parallel circuits in the bed nucleus of the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct. Funct 224, 1067–1095. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Yamada S, Yoshida M, Tanaka T, Mizoguchi K, Emoto H, Koga C, Ishii H, Ishikawa M, Kurasaki N, et al. , 1994. Effect of opioid peptides on dopamine release from nucleus accumbens after repeated treatment with methamphetamine. Eur J Pharmacol 256, 335–338. [DOI] [PubMed] [Google Scholar]
- Yu LC, Han JS, 1990. Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. Int J Neurosci 54, 245–251. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Hu D, Yang W, Hayashinaka E, Wada Y, Watanabe Y, Zeng Q, Cui Y, 2018. A voxel-based analysis of neurobiological mechanisms in placebo analgesia in rats. Neuroimage 178, 602–612. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Luo H, Gao Z, Zhu X, Shen Y, Li Y, Hu J, Yang J, 2021. Reduction of prefrontal purinergic signaling is necessary for the analgesic effect of morphine. iScience 24, 102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, McDonald AJ, 2016. Light and electron microscopic analysis of enkephalin-like immunoreactivity in the basolateral amygdala, including evidence for convergence of enkephalin-containing axon terminals and norepinephrine transporter-containing axon terminals onto common targets. Brain Res 1636, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ, 2015a. Mu opioid receptor localization in the basolateral amygdala: An ultrastructural analysis. Neuroscience 303, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Zhang M, Li A, Pan L, Berman BM, Ren K, Lao L, 2013. DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience 252, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW, 2015b. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep 12, 752–759. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M, 2006. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J. Neurosci 26, 8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Zhou H, Huang L, Xie Z, Wang J, Gan W-B, Yang G, 2018. Neuropathic Pain Causes Pyramidal Neuronal Hyperactivity in the Anterior Cingulate Cortex. Frontiers in Cellular Neuroscience 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, 2010. Activation of mu opioid receptor inhibits the excitatory glutamatergic transmission in the anterior cingulate cortex of the rats with peripheral inflammation. Eur J Pharmacol 628, 91–95. [DOI] [PubMed] [Google Scholar]
- Zhou W, Li Y, Meng X, Liu A, Mao Y, Zhu X, Meng Q, Jin Y, Zhang Z, Tao W, 2021. Switching of delta opioid receptor subtypes in central amygdala microcircuits is associated with anxiety states in pain. J Biol Chem 296, 100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ, 2005. Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 133, 97–103. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Sun M, Liu P, Shao W, Xiong M, Xu B, 2022. Perioperative stress prolong post-surgical pain via miR-339–5p targeting oprm1 in the amygdala. Korean J Pain 35, 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, 2011. Cortical plasticity as a new endpoint measurement for chronic pain. Mol. Pain 7, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS, 2001. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293, 311–315. [DOI] [PubMed] [Google Scholar]


