Abstract

St. John’s wort in western Europe has been extensively utilized for the treatment of mild to moderate depression. Hypericin, a red pigment, is found to be responsible for its antidepressant activity. The aim of the current study was to prepare a nanoemulsion (O/W) of hypericin designed for immediate delivery of the drug to the brain for the treatment of depression. The nanoemulsion was prepared by means of a homogenization technique, and that was followed by its physicochemical evaluation. Tween-80, Span-80, β-cyclodextrin, ethanol, and eucalyptus oil were utilized for the manufacturing of the nanoemulsion. Morphological studies have revealed globular structures of nanosize that were confirmed by the zeta analysis. The consistency of particles was revealed by the low polydispersity values. pH values of all formulations lay within the range of nasal pH. The viscosity of the prepared formulations was affected by the increase in concentrations of β-cyclodextrin. After passing from the centrifugation and freeze–thaw studies, the prepared formulations showed good stability. Formulation F2 having a composition of oil phase (0.125 mL), aqueous phase (1.25 mL), and β-cyclodextrin (8%) showed the best results out of all the formulations, and F2 had a pH of 5.7, 5.35 cP viscosity, 1.332 refractive index, 148.8 globule size, and −10.8 zeta potential. The mean percentage drug release and in vitro and ex vivo percentage drug permeations were observed to be 71.75, 76, and 75.07%, respectively. Meanwhile, formulation F2 showed the maximum drug release and permeation. In vivo behavior studies including the open field test, elevated plus maze test, and tail suspension test were conducted to see the antidepressant effect of hypericin along with comparison with a commercially available treatment. In conclusion, the prepared formulation shows good efficacy as an antidepressant and can be considered as a natural alternative over synthetic drugs.
1. Introduction
Depression is a widespread central nervous system (CNS) disease condition. The major clinical symptoms include apathy, loss of interest, appetite loss, etc. The World Health Organization (WHO) estimates that more than 350 million individuals around the world suffer from depression, and many commit suicide each year.1,2 Depression arises majorly because of the turbulences in the brain level of certain neurotransmitters, for example, serotonin, dopamine, and norepinephrine, that help in delivering the messages via the presynaptic neuron to the postsynaptic neuron.3 Various therapies of depression include multiple drug classes such as monoamino oxidase inhibitors, selective serotonin reuptake inhibitors, and tricyclic antidepressants. Such drugs have been effectively absorbed from the gastrointestinal tract (GIT) but undergo an extensive first-pass metabolism that will be a reason for poor oral bioavailability of less than 50%.4−6
Because of the poor central nervous system (CNS) distribution of these antidepressants across the blood–brain barrier, there had been an enhancement in the development of various potential approaches for drug delivery toward the brain. One such approach is to deliver the drug via the intranasal route. Intranasal formulation of the drug increases its bioavailability. As the nasal epithelium is highly permeable, it permits the rapid drug absorption toward the brain primarily because of a high blood flow, large surface area, and avoiding the first-pass metabolism.7,8 The intranasal route can deliver therapeutic effects of drugs to the brain without passing through the blood–brain barrier because of the special connection between the nose and the CNS.9 The ability to target medications to the brain more effectively is made possible by drug absorption across the olfactory area of the nose.10
Nowadays, nanocarriers are considered as novel drug carriers for targeted drug delivery.11−13 Nanoemulsions are colloidal dispersions containing two liquids that are immiscible with each other (most often oil and water) and that are stabilized by a surfactant.14 These are dosage forms developed for carrying the active ingredients to their target sites. It contains an oil phase and an aqueous phase, along with an emulsifier. The size range of nanoemulsion droplets is 10 to 1000 nm.15 The Brownian motion of small droplets is enough to overcome the low gravitational properties, resulting in the appropriate physical stability. Water in oil (W/O) and oil in water (O/W) are two types of emulsion; the latter is more popular in pharmaceutical manufacturing of drugs than the former because of its water compatibility and safety.16,17 For optimal delivery of pharmacologically active lipophilic substances, the composition and structure of nanoemulsions can be modified.18 Nanoemulsions can be used in a wide range of dosage forms such as liquids, aerosols, gels, sprays, and cream. They may also be delivered in a wide range of ways, including topically, intranasally, ophthalmologically, intravenously, and orally.19,20 Intranasal nanoemulsions could be a very likely method for the direct delivery of drugs toward the brain.21
Natural active ingredients are more likely to be used over synthetic ones because of the safety and efficacy of drugs by overcoming the side effects. By using natural sources, various formulations have been designed.22−24 Saint John’s wort, also known as Hypericum, has been among the nine genera that belongs to the family of Clusiaceae Lindl. and is extensively found all over the globe. In contradiction of the many rests of the herbal products, the effectiveness of hypericin, obtained from Hypericum perforatum, had been extensively studied in controlled trials. Few early studies had also revealed the antidepressant activity of hypericin25,26 and had shown that this extract of St John’s wort is much more effective than placebo for the very short-term treatment of moderate to mild depressive conditions. The mechanism of action by which hypericin exerts antidepressant activity is by the reduction in voltage-dependent Ca2+ influx as well as a decrease in the activity of the mitogen-activated protein kinase, depressing the evoked release of glutamate from the cerebrocortical nerve terminals.27,28
The current study aims to prepare a stable oil/water nanoemulsion of hypericin followed by its physicochemical, in vitro drug release, in vitro drug permeation, and ex vivo studies. The prepared formulation has also been evaluated for its antidepressant activities via in vivo behavior studies.
2. Materials and Method
2.1. Materials
The drug hypericin was purchased from Adooq Bioscience LLC, California, USA. Eucalyptus oil was purchased from Flav Chemicals Royal, China. Tween-80, Span-80, and β-cyclodextrin were purchased from Sigma-Aldrich, Shanghai, China. Ethanol and distilled water were purchased from Merck KGaA Darmstadt, Germany. Fluoxetine (Prozac) was purchased from the Clinix Pharmacy, Lahore, Pakistan. All chemicals as well as the solvents used in this experimental work were of analytical grade.
2.2. Method
Using a homogenizer, a high shear mixer, the nanoemulsion was formulated.29 By varying the concentrations of β-cyclodextrin, four formulations were prepared. The 1% alcoholic solution of hypericin was prepared by continuous mixing for 15–20 min. The oil phase was prepared by mixing 1 mL of eucalyptus oil with 0.5 mL of Span-80 in a beaker, and then the hypericin solution was added dropwise with continuous stirring for 30 min. For the preparation of an aqueous solution, initially, different concentrations of β-cyclodextrin were prepared in distilled water. The aqueous phase was finally prepared by the addition of β-cyclodextrin solution in 0.5 mL of Tween-80 under continuous stirring. After that, a stable solution was formulated by gradually adding the oil phase to the aqueous phase under continuous mixing. This mixture was then subjected to homogenization for 30 min at 10,000 rpm using a high shear homogenizer. The compositions of all prepared formulations are shown in Table 1. Figure 1 depicts a graphical representation of the whole process that was adopted to manufacture the nanoemulsion.
Table 1. Composition and Percentage of βeta-Cyclodextrin in Formulations.
| formulation | oil phase (mL) | water phase (mL) | beta-cyclodextrin (%) |
|---|---|---|---|
| F1 | 0.125 | 1.25 | 4 |
| F2 | 0.125 | 1.25 | 8 |
| F3 | 0.125 | 1.25 | 12 |
| F4 | 0.125 | 1.25 | 16 |
Figure 1.
Graphical representation of the method of preparation.
3. Characterization of the Nanoemulsion
3.1. pH, Viscosity, and Refractive index (RI)
To check the pH of the formulations, an ADWA pH meter (AD1050) was used, and all the readings were recorded at room temperature (25 °C). The viscosity of the nanoemulsion is a very essential property that affects drug absorption and pourability.30 By using a viscometer (Brookfield-model RV-III, New Castle, DE, USA), the viscosity of the produced formulations was determined at 25 °C using spindle no.4, and the readings were recorded. The refractive index (which can be used to show how the drug interacts chemically with excipients) was measured to evaluate the nanoisotropic emulsion’s character. Using a refractometer (ATAGO, Tokyo, Japan), the refractive index of the formulations was measured.
3.2. Scanning Electron Microscopy (SEM)
The morphology and structure of nanoemulsion droplets were examined using a scanning electron microscope (EVO LS10 Zeiss Germany) at a point-to-point resolution.31 A drop of the nanoemulsion was placed to the grid of the holey film, and the images were taken after drying.32
3.3. Globule Size, Polydispersity Index (PDI), and Zeta Potential Analysis
The size of globules of the nanoemulsion plays a very substantial part in the permeation of drugs via the nasal cavity.33 The typical diameter of an olfactory axon is around 200 nm in various species; nonetheless, in the case of humans, it varies from 100 to 700 nm.34 Therefore, the size of globules of these novel formulations ought to be lower than 200 nm for effective permeation of the drug. Globule sizes of the prepared formulations were determined by using a Malvern Zetasizer (Malvern Instruments Ltd., Worcestershire, UK). The sample was diluted with distilled water and then placed in a cuvette and analyzed.
The proportion of the standard deviation to the mean droplet size is known as the polydispersity index.35,36 This reveals the constancy of the droplet size throughout the formulation. The higher the polydispersity value is, the lower is the homogeneity of the size of droplet in the formulation. The polydispersity index (PDI) was analyzed using a Malvern Zetasizer (Malvern Instruments Ltd., Worcestershire, UK). All samples were analyzed at 25 °C.
The zeta potential is the magnitude of electrostatic repulsion or attraction among particles. This is a crucial parameter that affects the nanoemulsion stability. This confirms that flocculation or aggregation is not present; thus, it leads to a very stable formulation with a better shelf life. The surface charge of droplets was determined by the zeta potential, which is majorly accountable for the stability of the colloidal dispersions.4 The zeta potential was evaluated by using a Malvern Zetasizer (Malvern Instruments Ltd., Worcestershire, UK). Zeta potential measurements were made by diluting nanoemulsion formulations, and their values were then determined by the electrophoretic mobility of the oil droplets.
3.4. Dilution Test
The idea behind this test is that a nanoemulsion can have a higher proportion of continuous phase added to it without any stability issues.37 To check this, a 1 mL sample of the nanoemulsion was taken in a test tube, 4 mL of water was added in it, and then it was observed.
3.5. Dye Soluble Test
A water-soluble dye is dispersible inside the O/W globule but solubilized in the aqueous phase of the W/O globule.38 To observe this, 1 mL of the nanoemulsion along with some droplets of the hydrophilic color (amaranth) was thoroughly mixed in an Eppendorf tube. The formulation was evaluated using a fluorescence inverted microscope (DFC310 FX, Leica, Solms, Germany).
3.6. Fourier Transform Infrared Spectrophotometer (FTIR)
The most influential instrument for recognizing the various kinds of chemical bonds or functional groups is a Fourier transform infrared spectrophotometer (FTIR). Spectra were recorded in the FTIR instrument (PerkinElmer Spectrum Two, USA) by means of PC-based software-controlled instrument operation and processing of the data. The wavelength of light absorbed is a prominent feature of the chemical bond, as could be seen in an annotated spectrum. By interpretation of the infrared absorption spectrum, multiple chemical bonds in a compound could be determined. For FTIR analysis, a small quantity of all samples as well as all of the formulations was used. Data of infrared transmittance were screened over a wavenumber between 600 and 3800 cm–1.
3.7. Stability Studies
3.7.1. Centrifugation
To check the stability of nanoemulsions, the prepared formulations were centrifuged at 5000 rpm for 30 min in a Sorvall Legend RT centrifuge (US),39 and then these formulations were observed for any phase separation, creaming, or cracking.
3.7.2. Freeze–Thaw Method
The stability of the nanoemulsions at high and low temperatures was assessed. Prepared formulations were stored at a low temperature (−21 °C) for 24 h and then were directly thawed at a relatively high temperature (40 °C) for 1 h.39 After that, they were examined for any phase separation and cracking/creaming.
3.8. InVitro Drug Release Studies
By using the USP Rotating Paddle Dissolution Apparatus (USP II Sotax Smart AT7 Dissolution Tester) at 37 °C and 50 rpm, drug release tests were carried out. The phosphate-buffered solution (pH 6.8) was used to fill the apparatus' vessels. Each dialysis membrane was filled with 1 mL of each formulation and tied at both ends. Then these membranes were hung by a thread to the paddles of each vessel that had been already filled with the buffer solution.40 The samples (1 mL) were taken out from each vessel, suitably diluted, and then subjected to UV analysis.
3.9. In Vitro Permeation Studies
The Franz diffusion cell (Variomag Telesystem, H+P Labortechnik, Oberschleißheim, Germany) was used to perform the permeation studies. In the recipient compartment, 7 mL of the buffer was added, and 1 mL of the sample was then added in the donor compartment. A cellophane membrane was placed between the donor and the recipient compartment. The drug was allowed to permeate across the cellophane membrane. The samples, each 1 mL in volume, were collected at various intervals of time and sufficiently diluted before being subjected to UV analysis.40
3.10. Ex Vivo Permeation Studies
For the ex vivo permeation studies, goat nasal mucosa was selected. The mucosal layer of the nasal cavity was separated on the day of the experiment from the goat head, which was obtained from a local slaughterhouse. To be more precise, the nasal membrane was separated by using a surgical scissor. Throughout the isolation, the mucosal layer was preserved and hydrated by using the saline solution. The receptor compartment (7 mL) of the Franz cell was filled with phosphate buffer (pH 6.8). The mucosal layer was placed between the donor and receptor compartments, with the mucosal side toward the donor with a 0.63 cm2 diffusion area. The medium temperature was maintained at 37 ± 0.5 °C. One milliliter of the sample to be studied was poured on the donor compartment. Samples were withdrawn from the receptor compartment after 5, 10, 20, 30, and 60 min. The receptor compartment volume was substituted by an equivalent volume of a fresh medium of buffer. To calculate the percent drug release, samples withdrawn were suitably diluted and then analyzed by a UV spectrophotometer.
3.11. In Vivo Behavioral Study
The Institutional Animal Ethical Committee, University of Central Punjab, Lahore, Pakistan, authorized the experimental protocols for conducting the animal studies. Animals (Swiss albino rats) weighing 100–150 g were obtained from the animal facility of the University of Central Punjab, Lahore, Pakistan. All animals (equal number of males and females; n = 8) were divided into four main groups. The social isolation model and tail hanging model were used for induction of depression for up to 21 days. Groups II to IV were subjected to a depression model. Food and water supplies were maintained with controlled temperature and humidity. Groups were as follows: group I, normal rats (−ve control); group II, animals exposed to a depressive environment (+ve control); group III: depressed rats treated with hypericin; and group IV, depressed rats treated with the marketed formulation (fluoxetine).41,42
3.11.1. Tail Suspension Test (TST)
In this test, the isolated rats were suspended with adhesive tape in each chamber. The mouse should not touch the walls and is placed in the middle of the compartment.43 The animal was said to be immobile if its limbs were not moving when it was passively hanging. For the tail suspension test, specially constructed wooden tail suspension boxes (60 cm height, 40 cm width, and 30 cm depth) were employed. Every rat was placed inside a separate three-walled rectangular enclosure to prevent the animals from watching or interacting with one another. The top of each compartment box had a 1 cm wide × 60 cm long aluminum suspension bar that was utilized to suspend the tail of each mouse.
3.11.2. Open Field Test
Stressed rats exhibit a decrease in open-field activity and an increase in stereotyped behavior.44,45 Thigmotaxis (moving toward that solid object) is a characteristic of rats who like to stay close to walls and move more on the periphery, and it is more apparent in rats who exhibit depressive symptoms. Rats with less sadness are more likely to spend time in the open, middle part of the box.46,47 For conducting the open field test, the open-field apparatus consisted of a wooden box with dimensions of 105 cm length, 60 cm width, and 40 cm depth.48 Rats were randomly placed in one corner of the behavioral box and allowed to explore freely for 3 min. Activities in the open field were recorded for 3 min including number of circular motion, number of head raise, and number of times rat had come in the center of the box as well as in the corner of the box, and the number of times rats climbed was also recorded.
3.11.3. Elevated Plus Maze Test (EPM)
This test was carried out in accordance with the previously conducted research studies.49,50 To conduct this test, an elevated plus maze apparatus was employed. This apparatus was made up of two opposed open arms that measured 48 cm long by 10 cm wide and two enclosed arms that measured 48 cm long by 10 cm wide and 60 cm tall, all linked by a central platform to form the appearance of a plus sign. The middle field unites both the open and closed arms. Each rat was positioned individually in the apparatus’ center field, facing one of its closed arms. Animal behavior was recorded for 3 min. After every rat, the device was cleaned with a disinfection solution with no odor. The frequency of entrances into open and closed arms as well as the length of time spent in open arms was counted.51
4. Results and Discussion
4.1. pH, Viscosity, and Refractive Index
The pH of all the prepared formulations lay in the nasal pH range (4.5–6.4),52−54 signifying its nonirritant nature. Because the prepared formulations were oil in water (O/W) nanoemulsions, the viscosity was expected to be lower, favoring the administration of nanoemulsions via the nasal cavity.55 The viscosity of prepared nanoemulsion formulations ranges from 3.57 to 8.43. The refractive index of nanoemulsions shows a mean value of 1.329, signifying the clear isotropic nature of the formulations. Table 2 shows all the pH, viscosity, and refractive index values of the prepared formulations.
Table 2. pH, Viscosity, and Refractive Index Readings of the Prepared Formulations.
| formulations | pH | viscosity (cP) | refractive index |
|---|---|---|---|
| F1 | 5.2 | 3.57 | 1.327 |
| F2 | 5.7 | 5.35 | 1.332 |
| F3 | 6.1 | 6.44 | 1.329 |
| F4 | 5.9 | 8.43 | 1.331 |
4.2. Scanning Electron Microscopy (SEM)
Scanning electron microscopy was performed to examine the surface morphology of the particulate carriers. The outcomes (Figure 2) revealed nanosized spherical-shaped globules. Moreover, particles are evenly distributed and well separated from each other, indicating a stable formulation.
Figure 2.
Scanning electron microscopy indicating nanosized particles with suitably spherical oil globules of prepared formulations.
4.3. Globule Size, Polydispersity Index (PDI), and Zeta Potential Analysis
Globule size is a crucial parameter that is of very high importance when formulating a nanoemulsion. Table 3 indicates that the globule size of all the prepared formulations ranges between 93.42 and 236 nm. The smaller globule size indicates that the formulation is more stable. A recent study had stated that nanoemulsions with an average globule size of 200 nm showed a much larger rate as well as extent of drug absorption as compared to the average globule size of 700 nm via the olfactory pathway.34 Further, Dv (intensity-weighted distribution of particles) or Dz (volume-weighted distribution of particles) mentioned in the Table 3 after size analysis of nanoemulsion F2 is shown in Figure 3.
Table 3. Globule Size, Polydispersity Index (PDI), and Zeta Potential of Prepared Formulations.
| formulations | Z-average (nm) | Dv (nm) | Dz (nm) | %age | polydispersity index (PDI) | zeta potential (mV) |
|---|---|---|---|---|---|---|
| F1 | 93.42 | 110 | 97 | 66 | 0.320 | –11.5 |
| F2 | 148.8 | 160 | 154 | 84 | 0.445 | –10.8 |
| F3 | 117.2 | 128 | 122 | 72 | 0.398 | –12.0 |
| F4 | 236 | 244 | 239 | 74 | 0.531 | –12.8 |
Figure 3.
Globule size analysis of nanoemulsion F2.
PDI generally ranges from 0 to 1, where 0 (zero) stands for a monodisperse system and 1 for a polydisperse particle dispersion.56 The formulations’ low polydispersity values (0.320–0.531) in Table 3 show the consistency of particle diameter within the formulations. The zeta potential values of formulations were measured and ranged between −10.8 and −12.8 mV, indicating the formation of a stable nanoemulsion. The zeta potential of nanoemulsion F2 is shown in Figure 4.
Figure 4.
Zeta potential of nanoemulsion F2.
4.4. Dilution Test
O/W nanoemulsions with a polarity of 2:1 can be diluted with water, whereas W/O could not be diluted with water and undergo phase inversion. The prepared nanoemulsions were diluted with water, and no phase separation was observed, indicating that the nanoemulsions were O/W nanoemulsions.
4.5. Dye Solubility Test
When a water-soluble dye is added in an O/W nanoemulsion, the aqueous phase of the nanoemulsion takes up that color.57Figure 5 clearly shows the uniform distribution of amaranth dye in the aqueous phase indicating that the prepared nanoemulsions are O/W nanoemulsions.
Figure 5.
Dye solubility test (F1–F4) indicating oil-in-water (O/W) nanoemulsions.
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
Pure hypericin, ethanol, β-cyclodextrin, Tween-80, Span-80, eucalyptus oil, and all the nanoemulsion formulations (F1–F4) were subjected to FTIR as demonstrated in Figure 6. The FTIR spectrum of eucalyptus oil showed peaks at 2924.05 cm–1 due to alkane C–H stretching vibrations, at 1465.13 cm–1 due to alkane C–H bending bands, and at 1375.31 cm–1 due to phenol O–H bending bands. Peaks from 1167.25 to 1214.24 cm–1 showed sulfone S=O stretching bands. Alcohol C–O stretching bands were from 1053.23 to 1079.15 cm–1, and alkene C=C bending bands were from 842.39 to 983.78 cm–1. A previously conducted research58 had shown similar peaks of the FTIR spectrum of eucalyptus oil. The FTIR spectrum of β-CD exhibited intense absorption peaks at 3305 (O–H stretching vibrations), 2929 (C–H stretching vibrations), 1641 (HO-H bending vibration), and 1151 and 1020 cm–1 (C–C, C–O–C stretching vibration). These results are comparable with an earlier study.59 The FTIR spectrum of Tween-80 showed a broader peak at 3368.80 cm–1 due to aliphatic primary amine N–H stretching bands. A sharp peak was present due to secondary alcohol C–O stretching bands at 1121.58 cm–1, anhydride CO-O–CO stretching bands at 1042.09 cm–1, and 1–4 substituted C–H bending bands at 821.55 cm–1, comparable with the study previously performed. The FTIR spectrum of formulations (F1–F4) showed similar peaks to those of the reported spectrum of pure drug and other excipients used, confirming the stability of the formulations.
Figure 6.
FTIR spectrum of (A) hypericin drug, (B) beta-cyclodextrin, (C) Span 80, (D) eucalyptus oil, (E) Tween 80, (G) ethanol, nanonemulsion F1, nanoemulsion F2, nanoemulsion F3, and nanoemulsion F4.
4.7. Stability Studies
4.7.1. Centrifugation
After centrifugation for 30 min, it was observed that there were no signs of phase separation, cracking, or creaming. Recent studies had suggested that in both industrial and scientific contexts, the process of centrifugation involves using a centrifuge to use the centrifugal force to separate heterogeneous mixtures. However, after the test was performed, no sedimentation of the layers or particles has been observed.
4.7.2. Freeze–Thaw Method
After the freezing (24 h) and thawing (1 h) processes, the stability of formulations was observed. No phase separation, creaming, or cracking was observed after this whole process, indicating that it is a stable nanoemulsion.
4.8. In Vitro Drug Release Studies
The cellophane membrane was used for the drug release studies. The samples were drawn out after 5, 10, 20, 30, and 60 min and evaluated spectrophotometrically at a wavelength of 590 nm, and the absorbance of each sample was recorded. Figure 7 shows the percentage drug release of the formulations. F2 (79%) had been shown to have the highest drug release among all the formulations, with an average percent drug release of all the formulations calculated of about 71.75%.
Figure 7.
Graphical representation of drug release studies of the pure drug (hypericin) and prepared formulations. One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
4.9. In Vitro Permeation Studies
Samples were drawn out after 5, 10, 20, 30, and 60 min from the start of the process, and the absorbance were recorded at a wavelength of 590 nm. The graph between the concentration and absorbance was plotted. Figure 8 shows the graphical representation of percentage drug permeation of the formulations through the membrane. F2 (86%) had shown the highest drug permeation among all the formulations, with the average drug permeation of all the formulations calculated as 76%.
Figure 8.
In vitro permeation release of the pure drug (hypericin) and prepared formulations. One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
4.10. Ex Vivo Permeation studies
Samples were analyzed at 590 nm after 5, 10, 20, 30, and 60 min, and percentage drug release was calculated for each formulation. Figure 9 shows the percentage drug release of the formulations from the nasal mucosa. F2 (84.06%) was shown to have the highest drug permeation among all the formulations, with the average drug permeation of all the formulations calculated to be about 75.07%.
Figure 9.
Ex vivo percentage drug release of pure drug (hypericin) and prepared formulations. One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
4.11. In Vivo Behavioral Studies
4.11.1. Tail Suspension Test (TST)
Comparison of the antidepressant potency of the prepared formulation with that of a commercially available antidepressant was carried out effectively using the tail suspension test. Figure 10 shows an exceedingly intriguing outcome of the current investigation, where hypericin administration increased mobility under stress in the tail suspension test when compared with the marketed formulation (MF).
Figure 10.
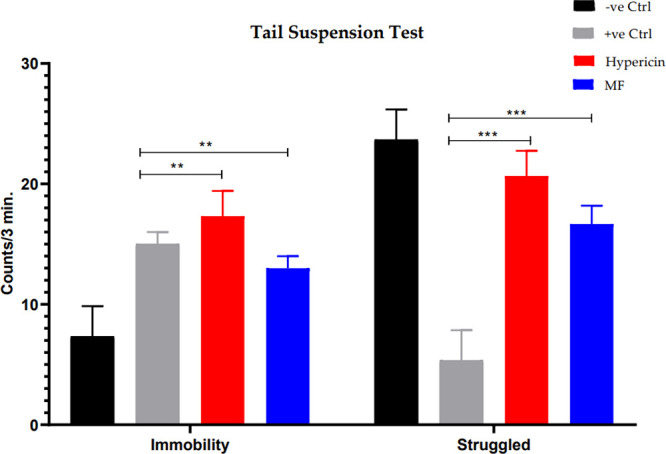
Tail suspension test indicating immobility and struggle of −ve ctrl, +ve ctrl, hypericin, and marketed formulation (MF). One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 8 per group).
4.11.2. Open Field Test
An open field test was also conducted. A number of important conventional and ethological parameters can be collected and analyzed.60,61 Parameters such as circular motion, head raise, climbing, corner, and center were analyzed before inducing depression, after inducing depression, and after administering hypericin via the intranasal route and the marketed formulation (MF) orally. Compared with the healthy rats, when depression was induced, all of the parameters had shown a significant reduction, indicating the decreased autonomous exploration and spontaneous activities and increased depression. Results demonstrated in Figure 11 clearly depict the rapid therapeutic effects of hypericin.
Figure 11.

Open field test indicating circular motion, head raise, climbing, corner, and center of −ve ctrl, +ve ctrl, hypericin, and marketed formulation (MF). One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 00.01; ***P < 0.001 (n = 8 per group).
4.11.3. Elevated Plus Maze Test (EPM)
In the EPM test, a number of entries were observed during the study. At first, the entry of normal animals was observed in both open and closed arms. Afterward, the depression induced entries were observed in both open and closed arms, and lastly, hypericin and marketed formulation (MF) induced activities were observed and compared. The significant difference in the entries of animals in the open arms and closed arms suggested that hypericin had shown to be eliminating the depression from the animals when compared to the +ve control group as shown in Figure 12A,B.
Figure 12.

Elevated plus maze test open arm (A) and closed arm (B). One-way ANOVA was applied to check statistical significance. Each bar in the graph represents means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (n = 8 per group).
5. Discussion
Currently, depression is considered as a global disorder, and the number of people suffering from depression is increasing day by day. Unfortunately, many antidepressant drugs have shown undesirable side effects, and about 30% of the patients have not responded to these drugs.62 Therefore, seeking safe and effective antidepressant drugs from natural and traditional herbs might aid us in discovering a novel approach for the treatment of depression. Hypericin, commonly found in the St. John’s wort, has been utilized in various countries for treating depression.63,64
Several studies have reported that the intranasal route can be a good option as a nose to brain targeting approach for treating the depressive disorders.21,65−68 Trigeminal and olfactory pathways have been considered to be merely the routes via which the brain is linked to the outside environment [69,70]. Drugs with limited oral bioavailability because of substantial metabolism have been studied using this route as a viable alternative.71−73 Additionally, the utilization of this nasal route of administration of medication allows the various therapeutics to avoid the first-pass effect. So, intranasal doses are frequently considered to be 2 to 10 times lower than doses that are administered orally.74 To deliver the medication directly toward the brain through trigeminal, olfactory, and neuronal routes, a very large number of novel formulations are currently being thoroughly explored.
In recent years, nanoemulsions (NEs) have been given considerable attention for intranasal drug delivery, and this has been attributed to their benefits including the ability to safeguard the drug from chemical or biological degradation, the ability to improve drug absorption by inducing mucoadhesion, and the capacity to form a positive electrical surface for enhancing nasal residence time.75 NEs could be utilized for solving the issues of stability and solubility of the drug.76,77 Drugs that are hydrophobic are dissolved in the oily phase, and when the drug is released from the nanoemulsion and comes in contact with the nearby environment that is aqueous, nanoprecipitation occurs. This phenomenon governs the particle formation with an extremely high surface as well as significant enhancement of dissolution rate of drugs according to the Noyes–Whitney equation.78 Nanoemulsions could also be utilized for carrying products from natural origins.79,80
In a recent study,4 paroxetine O/W nanoemulsions have been prepared for nose to brain targeting by utilizing the spontaneous emulsification method. The ex vivo studies have shown a threefold improvement in comparison to the paroxetine suspensions. Moreover, in the in vivo studies, NEs had expressively enhanced the behavioral activities of rats. In another study, kaempferol-loaded nanoemulsions had been made for direct brain targeting.81,82
In the current study, different formulations of nanoemulsions were prepared by varying the β-cyclodextrin concentrations. β-Cyclodextrin is frequently used owing to its appropriate size of cavity for interacting with a wide variety of hydrophobic guest particles, particularly phytochemicals and drugs. Furthermore, a few more studies have described that production of an inclusion complex with polyphenols enhances its effect as an antioxidant, which might result in increased solubility of the biological component.83−86 Ethanol, Tween-80, Span-80, and distilled water were the other chemicals that were utilized for the manufacturing of nanoemulsions.
The prepared formulations had shown nanosized droplet size (Table 3), indicating greater surface area and thus favoring the transit through the nasal membrane leading to better hypericin release from nanoemulsions, therefore increasing the bioavailability of drugs.87 The small droplet size can also result in uniform spreading due to low interfacial tension and improved wettability. The PDI value indicates the uniformity of droplet size within the nanoemulsion. PDI values of the prepared formulations were mostly found to be less than 0.50, indicating a narrow size distribution.88 Uniform-sized droplets in our formulation ensure the stability of our formulations.
The pH of all prepared formulations lies within the range of nasal pH (3.5–6.4),52 which indicates their nonirritancy. The low viscosity confirms the rapid drug release from nanoemulsions, therefore making sure of fast absorption as well as quick onset of action. Generally, a low viscosity also indicates small size of the droplets and higher aqueous content.89 A recent study had also documented that the viscosity of the nanoemulsions had been affected by the size of droplets.90 A lower viscosity is an ideal characteristic of the O/W nanoemulsion.91 The refractive index also indicates the clear isotropic nature of all of the formulations. SEM has indicated the clear distribution of oil droplets in the aqueous phase. It has also revealed the presence of nanosized droplets in the external phase of all the formulations.
For a physically stable nanoemulsion, a charge value of ±30 mV is required.92 All the prepared formulations have shown zeta potential values that lie within this range, suggesting that the prepared formulations were very much stable. In the literature, it was reported that a high PDI indicates the formation of a bimodal distribution, with one population of small droplets of around 15.3, 15, and 17 nm for nanoemulsions and another population of large droplets of around 465, 517, and 534 nm, respectively, as reported by Mayer et al.93 On the other hand, the full particle size distribution measurements indicated that some droplet aggregation had occurred in case of emulsion, resulting in bimodal distribution.94 The FTIR spectrum of all the prepared formulations (F1–F4) had shown peaks very similar to those of the reported spectrum of pure drug and all of the other chemicals used. Stability studies indicated no phase separation or other instability in the formulations.
Drug release studies were performed using dissolution studies, and permeation studies were performed via Franz cell studies. It was observed that all the formulations had shown slightly different patterns of the release and permeation of the drug that are linked to concentration of beta-cyclodextrin. But overall, better results were seen with F2.
Animal behavior studies were used to evaluate the antidepressant effect of prepared formulations,95,96 and upon comparison with control groups as well as with the marketed formulation, it was found that all the behavioral parameters had shown a significant decrease in depression after the administration of hypericin (Figure 11).
6. Conclusions
The intranasal route is extensively used to exploit target drugs for the treatment of depression. Exploration of the olfactory region of the nasal mucosa can further add fuel to this research. The stable and suitable formulation of the nanoemulsion has been developed and evaluated successfully. pH values lie within the range of the nasal pH. The low viscosity indicates that the prepared formulations have nanosized droplets. The globule size indicates the formation of the nanosized droplets in the prepared formulations. Stability studies have shown the formation of a stable formulation. Results of permeation studies confirm the rapid hypericin permeation as well as prominent percentage drug release, indicating a possibility to provide a rapid antidepressant effect. The in vivo studies prove that hypericin could be an interesting candidate of natural source for the treatment of depression over available synthetic formulations.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R457), King Saud University, Riyadh, Saudi Arabia. The authors are very thankful to the Faculty of Pharmaceutical Sciences, University of Central Punjab, Lahore-54000, Pakistan, for the facilitation of this research work;
Data Availability Statement
All the data are contained in the manuscript.
This research is supported by the Researchers Supporting Project Number (RSP2023R457), King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Notes
All the experiments were approved by the Ethical Review Committee (ERC) of the Faculty of Pharmaceutical Sciences, University of Central Punjab Lahore, Pakistan, under vide reference number (UCP) UCP/FOPS/ERC/App#01/2022.
References
- Singh A. R.; Singh S.A. Towards a suicide free society: identify suicide prevention as public health policy. Mens Sana Monographs 2004, 2 (1), 21. [PMC free article] [PubMed] [Google Scholar]
- Maria Michel T.; Pulschen D.; Thome J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18 (36), 5890–5899. 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- Salim S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12 (2), 140–147. 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey Y. R.; Kumar S.; Gupta B. K.; Ali J.; Baboota S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology 2015, 27 (2), 025102 10.1088/0957-4484/27/2/025102. [DOI] [PubMed] [Google Scholar]
- Pires P. C.; Paiva-Santos A. C.; Veiga F. Nano and Microemulsions for the Treatment of Depressive and Anxiety Disorders: An Efficient Approach to Improve Solubility, Brain Bioavailability and Therapeutic Efficacy. Pharmaceutics 2022, 14 (12), 2825. 10.3390/pharmaceutics14122825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Yang C.; Wang L.; Xiang Y.; Zhang W.; Li Y.; Zhuang X. Comparable intestinal and hepatic first-pass effect of YL-IPA08 on the bioavailability and effective brain exposure, a rapid anti-PTSD and anti-depression compound. Front. Pharm. 2020, 11, 588127 10.3389/fphar.2020.588127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D. S.; Guastella A. J.; Westlye L. T.; Andreassen O. A. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol. Psych. 2016, 21 (1), 29–38. 10.1038/mp.2015.166. [DOI] [PubMed] [Google Scholar]
- Wu H.; Hu K.; Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin. Drug Delivery 2008, 5 (10), 1159–1168. 10.1517/17425247.5.10.1159. [DOI] [PubMed] [Google Scholar]
- Khan A. R.; Liu M.; Khan M. W.; Zhai G. Progress in brain targeting drug delivery system by nasal route. J. Controlled Release 2017, 268, 364–389. 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Trevino J. Non-invasive strategies for nose-to-brain drug delivery. J. Clin. Trials 2020, 10, 7. [PMC free article] [PubMed] [Google Scholar]
- Akram Ghumman S.; Mahmood A.; Noreen S.; Aslam A.; Ijaz B.; Amanat A.; Kausar R.; Rana M.; Hameed H. Chitosan-Linseed mucilage polyelectrolyte complex nanoparticles of Methotrexate: In vitro cytotoxic efficacy and toxicological studies. Arab. J. Chem. 2023, 16 (2), 104463 10.1016/j.arabjc.2022.104463. [DOI] [Google Scholar]
- Noreen S.; Hasan S.; Ghumman S. A.; Bukhari S. N. A.; Ijaz B.; Hameed H.; Iqbal H.; Aslam A.; Elsherif M. A. M.; Noureen S.; Ejaz H. pH Responsive Abelmoschus esculentus Mucilage and Administration of Methotrexate: In-Vitro Antitumor and In-Vivo Toxicity Evaluation. Int. J. Mol. Sci. 2022, 23 (5), 2725. 10.3390/ijms23052725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabha B.; Bharadwaj K. K.; Pati S.; Choudhury B. K.; Sarkar T.; Kari Z. A.; Edinur H. A.; Baishya D.; Atanase L. I. Development of polymer-based nanoformulations for glioblastoma brain cancer therapy and diagnosis: An update. Polymers 2021, 13 (23), 4114. 10.3390/polym13234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozogul Y.; Karsli G. T.; Durmuş M.; Yazgan H.; Oztop H. M.; McClements D. J.; Ozogul F. Recent developments in industrial applications of nanoemulsions. Adv. Colloid Interface Sci. 2022, 102685 10.1016/j.cis.2022.102685. [DOI] [PubMed] [Google Scholar]
- Patel R. P.; Joshi J. R. An overview on nanoemulsion: a novel approachInt. J. Pharm. Sci. Res. 2012, 3 (12), 4640. [Google Scholar]
- Hosseini S. F.; Ramezanzade L.; McClements D. J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021, 109, 322–339. 10.1016/j.tifs.2021.01.045. [DOI] [Google Scholar]
- Atanase L. I.; Larraya C.; Tranchant J. F.; Save M. Rational design of tetrahydrogeraniol-based hydrophobically modified poly (acrylic acid) as emulsifier of terpene-in-water transparent nanoemulsions. Eur. Polym. J. 2017, 94, 248–258. 10.1016/j.eurpolymj.2017.07.011. [DOI] [Google Scholar]
- Mahmood Z. Potential of nano-emulsions as phytochemical delivery system for food preservation. Pak. J. Pharm. Sci. 2017, 30 (6), 2259. [PubMed] [Google Scholar]
- Tan S.; Stanslas J.; Basri M.; Karjiban R. A. A.; Kirby B.; Sani D.; Basri H.; et al. Nanoemulsion-based Parenteral Drug Delivery System of Carbamazepine: Preparation, Characterization, Stability Evaluation and Blood-Brain Pharmacokinetics. Curr. Drug Delivery 2015, 12 (6), 795–804. 10.2174/1567201812666150901112544. [DOI] [PubMed] [Google Scholar]
- Tayeb H. H.; Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine 2018, 13 (19), 2507–2525. 10.2217/nnm-2018-0088. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Xiong G.; Tsang W. C.; Schätzlein A. G.; Uchegbu I. F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370 (3), 593–601. 10.1124/jpet.119.258152. [DOI] [PubMed] [Google Scholar]
- Ghumman S. A. Formulation and evaluation of quince seeds mucilage–sodium alginate microspheres for sustained delivery of cefixime and its toxicological studies. Arab. J. Chem. 2022, 15 (6), 103811 10.1016/j.arabjc.2022.103811. [DOI] [Google Scholar]
- Ghumman S. A.; Noreen S.; Hameed H.; Elsherif M. A.; Shabbir R.; Rana M.; Junaid K.; Bukhari S. N. A. Synthesis of pH-Sensitive Cross-Linked Basil Seed Gum/Acrylic Acid Hydrogels by Free Radical Copolymerization Technique for Sustained Delivery of Captopril. Gels 2022, 8 (5), 291. 10.3390/gels8050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureen S.; Noreen S.; Ghumman S. A.; Batool F.; Hameed H.; Hasan S.; Noreen F.; Elsherif M. A.; Bukhari S. N. A. Prunus armeniaca Gum-Alginate Polymeric Microspheres to Enhance the Bioavailability of Tramadol Hydrochloride: Formulation and Evaluation. Pharmaceutics 2022, 14 (5), 916. 10.3390/pharmaceutics14050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki O.; Katsumata Y.; Oya M.; Bladt S.; Wagner H. Inhibition of monoamine oxidase by hypercin. Planta medica 1984, 50 (03), 272–274. 10.1055/s-2007-969700. [DOI] [PubMed] [Google Scholar]
- Ahsan Waqar M.; Riaz T.; Zaman M.; Majeed I.; Nadeem Alvi M.; Ishaque A.; Tabassam N.; Mehboob T.; Waqas M.; Munir M.; Tayyab S. Origin, Synthesis and Various Mechanisms of Hypericin as Antidepressant, Photosensitizer and Antiviral: Hypericin as Antidepressant, Photosensitizer and Antiviral. Pak. J. Health Sci. 2022, 07–12. 10.54393/pjhs.v3i06.321. [DOI] [Google Scholar]
- Lei C.; Li N.; Chen J.; Wang Q. Hypericin Ameliorates Depression-like Behaviors via Neurotrophin Signaling Pathway Mediating m6A Epitranscriptome Modification. Molecules 2023, 28 (9), 3859. 10.3390/molecules28093859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholghi G.; Arjmandi-Rad S.; Zarrindast M. R.; Vaseghi S. St. John’s wort (Hypericum perforatum) and depression: what happens to the neurotransmitter systems?. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395 (6), 629–642. 10.1007/s00210-022-02229-z. [DOI] [PubMed] [Google Scholar]
- Salawi A.; Almoshari Y.; Sultan M. H.; Madkhali O. A.; Bakkari M. A.; Alshamrani M.; Safhi A. Y.; Sabei F. Y.; Al Hagbani T.; Ali M. S.; Alam M. S.; et al. Production, Characterization, and In Vitro and In Vivo Studies of Nanoemulsions Containing St. John’s Wort Plant Constituents and Their Potential for the Treatment of Depression. Pharmaceuticals 2023, 16 (4), 490. 10.3390/ph16040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.; Nair A. B.; Jacob S.; Patel R. K.; Shah H.; Shehata T. M.; Morsy M. A. Nanoemulsion based vehicle for effective ocular delivery of moxifloxacin using experimental design and pharmacokinetic study in rabbits. Pharmaceutics 2019, 11 (5), 230. 10.3390/pharmaceutics11050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawi A.; Khan A.; Zaman M.; Riaz T.; Ihsan H.; Butt M. H.; Aman W.; Khan R.; Majeed I.; Almoshari Y.; Alshamrani M. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14 (12), 2369. 10.3390/polym14122369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.; Zaman M.; Salawi A.; Khan M. A.; Iqbal M. O.; Riaz R.; Ahmed M. M.; Butt M. H.; Alvi M. N.; Almoshari Y.; Alshamrani M. Synthesis of Chemically Cross-Linked PH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8 (5), 281. 10.3390/gels8050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee B.; Gorain B.; Mohananaidu K.; Sengupta P.; Mandal U. K.; Choudhury H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 2019, 565, 258–268. 10.1016/j.ijpharm.2019.05.032. [DOI] [PubMed] [Google Scholar]
- Morrison E. E.; Costanzo R. M. Morphology of olfactory epithelium in humans and other vertebrates. Microsc. Res. Tech 1992, 23 (1), 49–61. 10.1002/jemt.1070230105. [DOI] [PubMed] [Google Scholar]
- Farshbaf-Sadigh A.; Jafarizadeh-Malmiri H.; Anarjan N.; Najian Y. Preparation of ginger oil in water nanoemulsion using phase inversion composition technique: Effects of stirring and water addition rates on their Physico-chemical properties and stability. Z. Phys. Chem. 2021, 235 (3), 295–314. 10.1515/zpch-2019-1427. [DOI] [Google Scholar]
- Hussain A.; Mahdi W. A.; Alshehri S.; Bukhari S. I.; Almaniea M. A.; et al. Application of green nanoemulsion for elimination of rifampicin from a bulk aqueous solution. International Journal of Environmental Research and Public Health 2021, 18 (11), 5835. 10.3390/ijerph18115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale R. R.; Osmani R. A.; Ghodake P. P.; Shaikh S. M.; Chavan S. R. Nanoemulsion: A review on novel profusion in advanced drug delivery. Ind. J. Pharm. Biol. Res. 2014, 2 (1), 122. 10.30750/ijpbr.2.1.19. [DOI] [Google Scholar]
- Rao S. V. R.; Shao J. J. I. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: I. Formulation development. Int. J. Pharm. 2008, 362 (1–2), 2–9. 10.1016/j.ijpharm.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Shafiq S.; Shakeel F.; Talegaonkar S.; Ahmad F. J.; Khar R. K.; Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66 (2), 227–243. 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zaman M.; Hanif M. In vitro and ex vivo assessment of hydrophilic polymer- and plasticizer-based thin buccal films designed by using central composite rotatable design for the delivery of meloxicam. Adv. Polym. Technol. 2018, 37 (6), 1823–1836. 10.1002/adv.21841. [DOI] [Google Scholar]
- Wang Y.; Tian A.; Zhang F.; Yu J.; Ling J. Inflammatory Immune Process and Depression-like Behavior in Hypothyroid Rats: A [18F] DPA-714 Micro Positron Emission Tomography Study. Pharmaceuticals 2023, 16 (2), 279. 10.3390/ph16020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumman S. A.; Hameed H.; Noreen S.; Al-Hussain S. A.; Kausar R.; Irfan A.; Shabbir R.; Rana M.; Amanat A.; Zaki M. E. A. In Vitro/In Vivo Evaluation of Clomipramine Orodispersible Tablets for the Treatment of Depression and Obsessive-Compulsive Disorder. Pharmaceuticals 2023, 16 (2), 265. 10.3390/ph16020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.; Lee B.; Kim M.; Lee H.; Park H. J.; Hahm D. H. Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34 (2), 265–270. 10.1016/j.pnpbp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V.; Tuohimaa P. Measuring grooming in stress and comfort. Proc. Meas. Behav 2000, 3, 148–149. [Google Scholar]
- Dunn A. J.; Berridge C. W.; Lai Y. I.; Yachabach T. L. CRF-induced excessive grooming behavior in rats and mice. Peptides 1987, 8 (5), 841–844. 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Crawley J. N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985, 9 (1), 37–44. 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Bale T. L.; Contarino A.; Smith G. W.; Chan R.; Gold L. H.; Sawchenko P. E.; Koob G. F.; Vale W. W.; Lee K. F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nature Genetics 2000, 24 (4), 410–414. 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Kraeuter A.-K.; Guest P. C.; Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Pre-Clin. Models 2019, 99–103. 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- Casey A. B.; Cui M.; Booth R. G.; Canal C. E. Selective” serotonin 5-HT2A receptor antagonists. Biochem. Pharm. 2022, 115028 10.1016/j.bcp.2022.115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo R.; Hasegawa T.; Fukuyama K.; Shiroyama T.; Okada M. Current limitations and candidate potential of 5-HT7 receptor antagonism in psychiatric pharmacotherapy. Front. Psych. 2021, 12, 623684 10.3389/fpsyt.2021.623684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żmudzka E.; Lustyk K.; Głuch-Lutwin M.; Wolak M.; Jaśkowska J.; Kołaczkowski M.; Sapa J.; Pytka K. Novel Multimodal Salicylamide Derivative with Antidepressant-like, Anxiolytic-like, Antipsychotic-like, and Anti-Amnesic Activity in Mice. Pharmaceuticals 2023, 16 (2), 175. 10.3390/ph16020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Misra A.; Babbar A. K.; Mishra A. P.; Mishra P.; Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Phamr. 2008, 358 (1–2), 285–291. 10.1016/j.ijpharm.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Hehar S. S.; Mason J. D. T.; Stephen A. B.; Washington N.; Jones N. S.; Jackson S. J.; Bush D. Twenty-four hour ambulatory nasal pH monitoring. Clinic. Otolaryngol. Allied Sci. 2001, 24 (1), 24–25. 10.1046/j.1365-2273.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- Gu F.; Fan H.; Cong Z.; Li S.; Wang Y.; Wu C. Preparation, characterization, and in vivo pharmacokinetics of thermosensitive s nasal gel of donepezil hydrochloride. Acta Pharm. 2020, 70 (3), 411–422. 10.2478/acph-2020-0032. [DOI] [PubMed] [Google Scholar]
- Pangeni R.; Sharma S.; Mustafa G.; Ali J.; Baboota S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25 (48), 485102 10.1088/0957-4484/25/48/485102. [DOI] [PubMed] [Google Scholar]
- Baboota S.; Shakeel F.; Ahuja A.; Ali J.; Shafiq S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57 (3), 315. 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- Kader N.; et al. Novel Approaches for Colloidal Drug Delivery System: Nanoemulsion. Res. J. Pharm. Dosage Forms Technol. 2018, 10 (4), 253–258. 10.5958/0975-4377.2018.00037.X. [DOI] [Google Scholar]
- Pant M.; et al. Insecticidal activity of eucalyptus oil nanoemulsion with karanja and jatropha aqueous filtrates. Int. Biodeterior. Biodegrad. 2014, 91, 119–127. 10.1016/j.ibiod.2013.11.019. [DOI] [Google Scholar]
- Yuan C.; Jin Z.; Xu X. J. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89 (2), 492–496. 10.1016/j.carbpol.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Walsh R. N.; Cummins R. A. The open-field test: a critical review. Psych. Bull. 1976, 83 (3), 482. 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- Choleris E.; et al. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25 (3), 235–260. 10.1016/S0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Millan M. J. J. P. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol. Ther. 2006, 110 (2), 135–370. 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ng Q. X.; Venkatanarayanan N.; Ho C.Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affective Disord. 2017, 210, 211–221. 10.1016/j.jad.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Whiskey E.; Werneke U.; Taylor D. J. A systematic review and meta-analysis of Hypericum perforatum in depression: a comprehensive clinical review. Int. Clin. Psychopharmacol. 2001, 16 (5), 239–252. 10.1097/00004850-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Mittal D.; et al. Insights into direct nose to brain delivery: current status and future perspective. Drug Delivery 2014, 21 (2), 75–86. 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- Md S.; et al. Donepezil nanosuspension intended for nose to brain targeting: in vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. 10.1016/j.ijbiomac.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Antunes J. L.; et al. Nanosystems, Drug Molecule Functionalization and Intranasal Delivery: An Update on the Most Promising Strategies for Increasing the Therapeutic Efficacy of Antidepressant and Anxiolytic Drugs. Pharmaceutics 2023, 15 (3), 998. 10.3390/pharmaceutics15030998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S. K.; Pathak K. Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence. Colloids Interfaces 2023, 7 (1), 23. 10.3390/colloids7010023. [DOI] [Google Scholar]
- Thorne R.; et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127 (2), 481–496. 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Mistry A.; Stolnik S.; Illum L. J. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379 (1), 146–157. 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Alam M. I.; et al. Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Delivery 2013, 20 (6), 247–251. 10.3109/10717544.2013.822945. [DOI] [PubMed] [Google Scholar]
- Mustafa G.; et al. Nano-ropinirole for the management of Parkinsonism: blood–brain pharmacokinetics and carrier localization. Expert Rev. Neurother. 2015, 15 (6), 695–710. 10.1586/14737175.2015.1036743. [DOI] [PubMed] [Google Scholar]
- Alam M. I.; et al. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470 (1–2), 99–106. 10.1016/j.ijpharm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Ugwoke M. I.; et al. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53 (1), 3–22. 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- Pires P. C.; Paiva-Santos A. C.; Veiga F.J.P. Antipsychotics-Loaded Nanometric Emulsions for Brain Delivery. Pharmaceutics 2022, 14 (10), 2174. 10.3390/pharmaceutics14102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort C.; et al. Opportunities and challenges for the nasal administration of nanoemulsions. Curr. Top. Med. Chem. 2015, 15 (4), 356–368. 10.2174/1568026615666150108144655. [DOI] [PubMed] [Google Scholar]
- Mahajan H. S.; et al. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Delivery 2014, 21 (2), 148–154. 10.3109/10717544.2013.838014. [DOI] [PubMed] [Google Scholar]
- Singh Y.; et al. Nanoemulsion: Concepts, development and applications in drug delivery. J. Controlled Release 2017, 252, 28–49. 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Bonferoni M. C.; et al. A novel ionic amphiphilic chitosan derivative as a stabilizer of nanoemulsions: Improvement of antimicrobial activity of Cymbopogon citratus essential oil. Colloids Surf., B 2017, 152, 385–392. 10.1016/j.colsurfb.2017.01.043. [DOI] [PubMed] [Google Scholar]
- Bonferoni M.; et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur. J. Pharm. Biopharm. 2018, 123, 31–41. 10.1016/j.ejpb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Colombo M.; et al. Validation of an HPLC-UV method for analysis of Kaempferol-loaded nanoemulsion and its application to in vitro and in vivo tests. J. Pharm. Biomed. Anal. 2017, 145, 831–837. 10.1016/j.jpba.2017.07.046. [DOI] [PubMed] [Google Scholar]
- Colombo M.; et al. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543 (1–2), 214–223. 10.1016/j.ijpharm.2018.03.055. [DOI] [PubMed] [Google Scholar]
- Fang Z.; Bhandari B. J. Encapsulation of polyphenols–a review. Trends Food Sci. Technol. 2010, 21 (10), 510–523. 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- Mercader-Ros M.a.T.; et al. Kaempferol complexation in cyclodextrins at basic pH. J. Agric. Food Chem. 2010, 58 (8), 4675–4680. 10.1021/jf904218j. [DOI] [PubMed] [Google Scholar]
- Batool N.; et al. Orally Administered, Biodegradable and Biocompatible Hydroxypropyl−β–Cyclodextrin Grafted Poly (methacrylic acid) Hydrogel for pH Sensitive Sustained Anticancer. Drug Delivery. 2022, 8 (3), 190. 10.3390/gels8030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M.; et al. Beta-Cyclodextrin and Maltodextrin Based Solid Dispersion of Meloxicam to Enhance the Solubility of Meloxicam; Formulation and In-vitro Evaluation. J. Chem. Soc. Pak. 2019, 41 (1), 133–133. [Google Scholar]
- Zhang R.; et al. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Delivery 2019, 26 (1), 328–342. 10.1080/10717544.2019.1582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preetz C.; et al. Application of atomic force microscopy and ultrasonic resonator technology on nanoscale: Distinction of nanoemulsions from nanocapsules. Eur. J. Pharm. Sci. 2010, 39 (1–3), 141–151. 10.1016/j.ejps.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Dapčević Hadnađev T.; et al. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115 (3), 313–321. 10.1002/ejlt.201100321. [DOI] [Google Scholar]
- Demisli S.; et al. Development and study of nanoemulsions and nanoemulsion-based hydrogels for the encapsulation of lipophilic compounds. Nanomaterials 2020, 10 (12), 2464. 10.3390/nano10122464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath S.; et al. Design, Development and Evaluation of Novel Nanoemulsion of Terbinafine HCl. Acta Pharm. 2012, 5 (10), 7. [Google Scholar]
- Gurpreet K., Singh S., Review of nanoemulsion formulation and characterization techniques. Indian Journal of Pharmaceutical Sciences, 2018. 80( (5), ).
- Mayer S.; Weiss J.; McClements D. J. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: factors influencing droplet size and stability. J. Colloid Interface Sci. 2013, 402, 122–130. 10.1016/j.jcis.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Mun S.; Decker E. A.; McClements D. J. Influence of droplet characteristics on the formation of oil-in-water emulsions stabilized by surfactant– chitosan layers. Langmuir 2005, 21 (14), 6228–6234. 10.1021/la050502w. [DOI] [PubMed] [Google Scholar]
- Kobayashi T.; Hayashi E.; Shimamura M.; Kinoshita M.; Murphy N. P.; et al. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology 2008, 197 (4), 567–580. 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Wang Z.; Pan X.; Yang J.; Wu C.; et al. Antidepressant-like effects of Xiaochaihutang in perimenopausal mice. J. Ethnopharmacol. 2020, 248, 112318 10.1016/j.jep.2019.112318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are contained in the manuscript.











