Abstract
BACKGROUND:
Diffuse idiopathic skeletal hyperostosis (DISH) is an incompletely defined disease process with no known unifying pathophysiological mechanism.
OBJECTIVE:
To our knowledge, no genetic studies have been performed in a North American population. To summarize genetic findings from previous studies and to comprehensively test for these associations in a novel and diverse, multi-institutional population.
METHODS:
Cross-sectional, single nucleotide polymorphism (SNP) analysis was performed in 55 of 121 enrolled patients with DISH. Baseline demographic data were available on 100 patients. Based on allele selection from previous studies and related disease conditions, sequencing was performed on COL11A2, COL6A6, fibroblast growth factor 2 gene, LEMD3, TGFB1, and TLR1 genes and compared with global haplotype rates.
RESULTS:
Consistent with previous studies, older age (mean 71 years), male sex predominance (80%), a high frequency of type 2 diabetes (54%), and renal disease (17%) were observed. Unique findings included high rates of tobacco use (11% currently smoking, 55% former smoker), a higher predominance of cervical DISH (70%) relative to other locations (30%), and an especially high rate of type 2 diabetes in patients with DISH and ossification of the posterior longitudinal ligament (100%) relative to DISH alone (100% vs 47%, P < .001). Compared with global allele rates, we found higher rates of SNPs in 5 of 9 tested genes (P < .05).
CONCLUSION:
We identified 5 SNPs in patients with DISH that occurred more frequently than a global reference. We also identified novel environmental associations. We hypothesize that DISH represents a heterogeneous condition with both multiple genetic and environmental influences.
KEY WORDS: Diffuse idiopathic skeletal hyperostosis, DISH, Forestier disease, Ossification of the posterior longitudinal ligament, OPLL, North American, Single nucleotide polymorphism analysis
ABBREVIATIONS:
- DISH
diffuse idiopathic skeletal hyperostosis
- FGF2
fibroblast growth factor 2 gene
- OPLL
ossification of the posterior longitudinal ligament
- SNPs
single nucleotide polymorphisms
- UTR
untranslated region.
Diffuse idiopathic skeletal hyperostosis (DISH), also referred to as Forestier disease among over 27 other synonyms,1 is a systemic condition that most notably results in exuberant growth of flowing osteophytes along the anterior portion of the vertebral column. This can result in dysphagia when occurring cervically and/or alterations in spinal biomechanics resulting in increased vulnerability to traumatic injuries or accelerated degenerative changes at unfused segments resulting in pain or nerve impingement (Figure 1).
FIGURE 1.
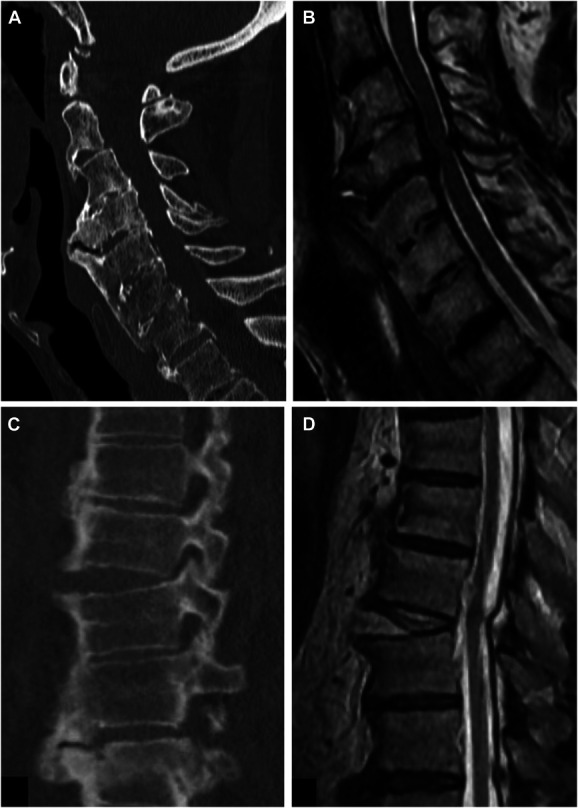
Consequences of DISH. Three of the most common and severe clinical consequences are illustrated through case examples from this study. A, and B, are from the same patient who developed dysphagia and progressive myelopathy from spinal cord compression that ultimately required surgical intervention. C, and D, show a patient with DISH who experienced an unstable 3-column hyperextension injury requiring surgical intervention. DISH, diffuse idiopathic skeletal hyperostosis.
DISH prevalence has been studied in different global populations and ranges between 2.9% and 42% depending on population genetics, classification criteria, and possibly environmental influences.1 The pathophysiology is poorly understood, with varying evidence suggesting genetic, metabolic, and/or signaling pathway aberrations, without a known unifying mechanism.2 It is suspected that the genetic underpinning for DISH overlaps with morphologically similar conditions such as ossification of the posterior longitudinal ligament (OPLL).3-5 Currently, only 1 polymorphism has been associated with DISH; however, this was limited to a sample number of 3 patients with coexisting ossification of the OPLL.6
The absence of a known unifying mechanism for the genetic, metabolic, and underpinnings for DISH may stem from the presence of several different haplotypes resulting in a predisposition toward a phenotypically similar disease state or a polygenic etiology.7 Similarly, considerable variation in the radiographic morphology is observed (Figure 2). Previous studies on genetics have been performed on Portuguese, Czech, and Japanese populations, most of which have been limited to single gene analysis or phenotypically homogenous familial clusters.5-7 Our objective was to perform genetic susceptibility testing in a novel and diverse patient population in the United States with a predominance of cervical DISH.
FIGURE 2.
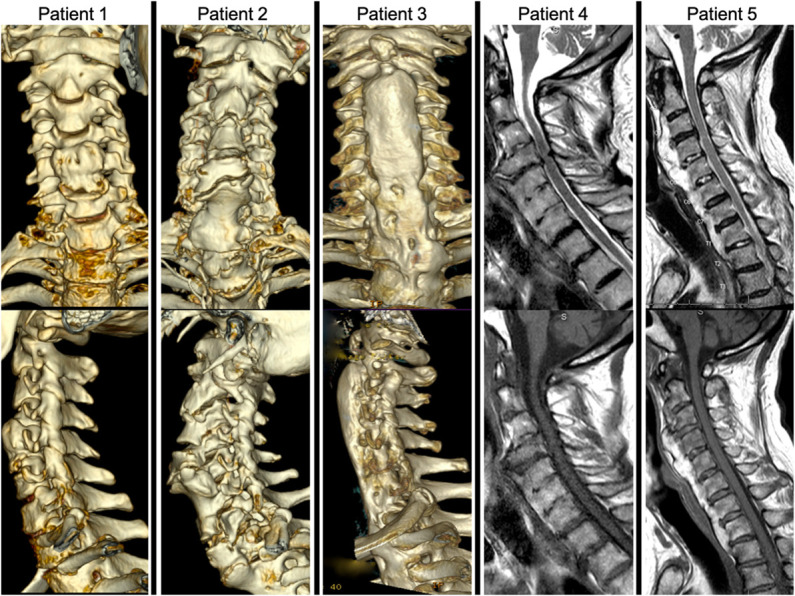
Phenotypic variability. Panel of 5 patients from our cohort highlighting the heterogeneity of cervical DISH. 3-dimensional reconstructed computed tomography anterior-posterior and lateral views illustrate variability in disease severity. Patient 1 demonstrates mild DISH with osteophytes bridging from C4 to C6. Patient 2 has a discontinuous DISH pattern through the cervical spine with breaking at C5-6 resulting in esophageal compression and dysphagia. Patient 3 shows a more laminar, continuous pattern. T1-weighted and T2-weighted MRI images demonstrate variability in T2 hyperintensity of the ectopic bone mass. Patient 4 with DISH that is homogenous in intensity to native bone, whereas patient 5 has a remarkable amount of T2 hyperintensity in the ectopic bone mass in comparison with patient 4 and the patient's native bone. DISH, diffuse idiopathic skeletal hyperostosis.
METHODS
Institutional review board approval was obtained from both collaborating institutions (Henry Ford Health—#10912 and University of Alabama—#150708005). Written consent was obtained when appropriate. During a study period from 2016 to 2022, retrospective chart review, whole blood collection, and genetic analysis were performed.
Patients with DISH were defined as having extensive flowing osteophytes on the anterior aspect of the vertebral column with relative intervertebral disk preservation. Diagnosis was made based on computed tomography scan and/or MRI, with agreement between a radiologist and a member of the research team. Patient demographic data were collected by retrospective chart review (Table 1). Patients were contacted for blood donation consent and to complete incomplete data. No patients were excluded; however, whole blood collection and complete demographic data acquisition were not possible on all patients (Figure 3).
TABLE 1.
Demographics
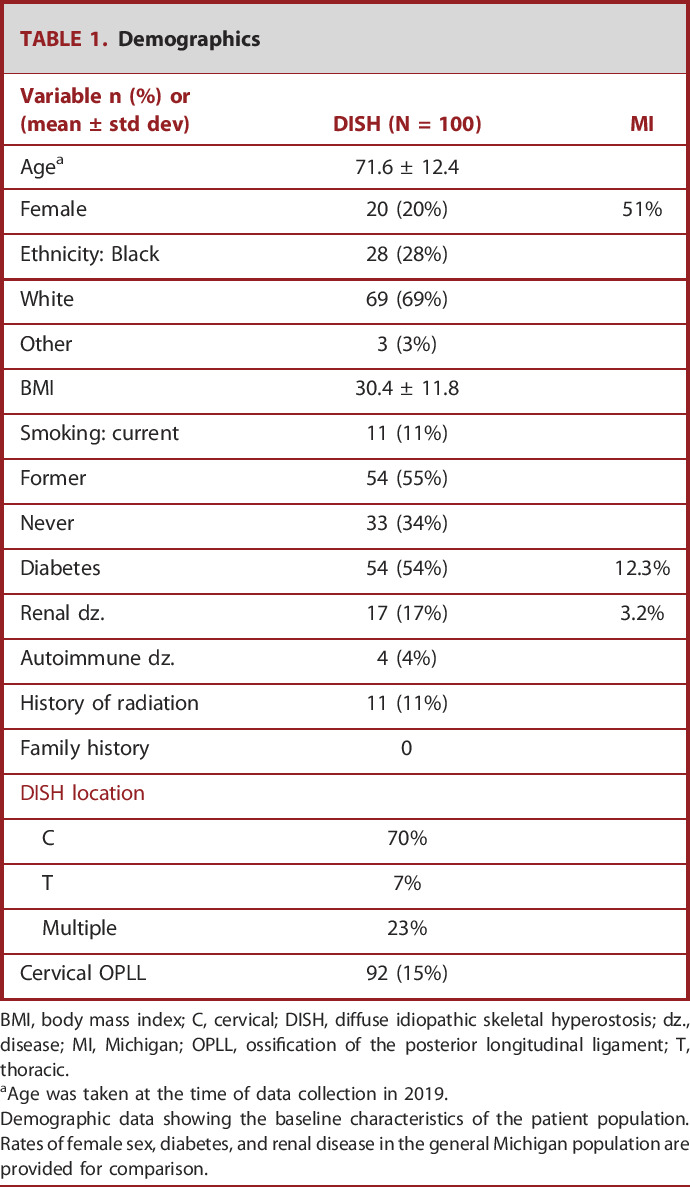
| Variable n (%) or (mean ± std dev) | DISH (N = 100) | MI |
|---|---|---|
| Agea | 71.6 ± 12.4 | |
| Female | 20 (20%) | 51% |
| Ethnicity: Black | 28 (28%) | |
| White | 69 (69%) | |
| Other | 3 (3%) | |
| BMI | 30.4 ± 11.8 | |
| Smoking: current | 11 (11%) | |
| Former | 54 (55%) | |
| Never | 33 (34%) | |
| Diabetes | 54 (54%) | 12.3% |
| Renal dz. | 17 (17%) | 3.2% |
| Autoimmune dz. | 4 (4%) | |
| History of radiation | 11 (11%) | |
| Family history | 0 | |
| DISH location | ||
| C | 70% | |
| T | 7% | |
| Multiple | 23% | |
| Cervical OPLL | 92 (15%) |
BMI, body mass index; C, cervical; DISH, diffuse idiopathic skeletal hyperostosis; dz., disease; MI, Michigan; OPLL, ossification of the posterior longitudinal ligament; T, thoracic.
Age was taken at the time of data collection in 2019.
Demographic data showing the baseline characteristics of the patient population. Rates of female sex, diabetes, and renal disease in the general Michigan population are provided for comparison.
FIGURE 3.
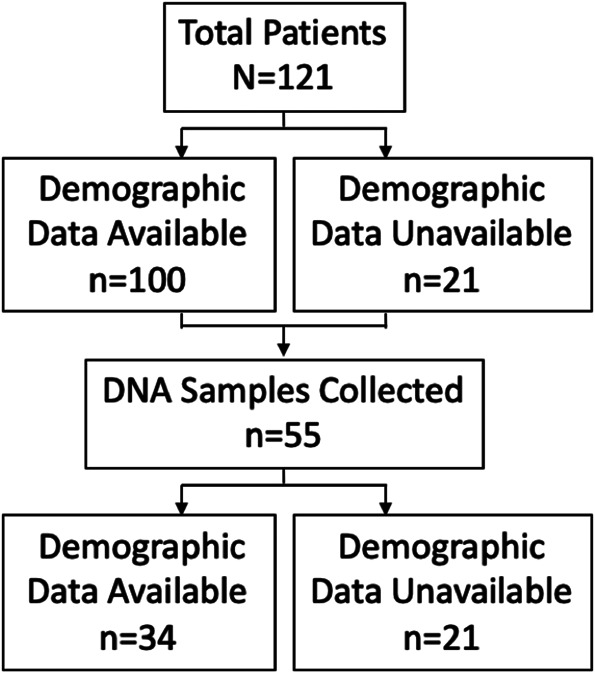
Patient accountability. A flow diagram representing the number of patients enrolled, the number with available demographic data, and the number with sequencing data.
DNA Isolation
Whole blood samples were stored in ethylenediaminetetraacetic acid at −80°C. Total DNA was isolated from frozen whole blood using DNeasy Blood & Tissue Kits (QIAGEN) adapted from the manufacturer's protocol. DNA was concentrated, and purity was confirmed using a Nanodrop Spectrometer.
Single Nucleotide Polymorphism Genotyping
Single nucleotide polymorphism (SNP) variants were detected by real-time polymerase chain reaction using TaqMan SNP Genotyping Assay (Thermo Fisher Scientific). One allele was labeled with FAM dye, and the other was labeled with VIC fluorescent dye. Thermal cycling DNA was performed on the Bio-Rad CFX-96 real-time system using the following protocol: enzyme activation at 95.0°C for 10 minutes followed by a denaturing step of 95.0°C for 0:15 seconds, terminating in an anneal/extended step 60.0°C for 1:00 minutes for 40 cycles.
Allelic discrimination was determined by instrument software Bio-Rad CFX manager determined by FAM and VIC labeling of alleles. SNP variants were selected based on literature review for relevant SNPs related to DISH and OPLL susceptibility (Figure 4). SNPs of interest included rs2071025, rs200963433, rs1476217, rs3747676, rs201930700, rs2241716, rs145135062, rs145135062, rs200212492, and rs1800469. The following cell DNA samples were obtained from the National Institute of General Medical Sciences Human Genetic Cell Repository at the Coriell Institute for Medical Research: HG01058, HG00551, HG00553, HG00554, HG00640, HG00637, HG01377, HG00115, NA19681, and NA19670. These samples were used as positive and negative controls for SNP genotyping (Supplemental Table 1, http://links.lww.com/NEU/D677, Supplemental Table 2, http://links.lww.com/NEU/D678, Supplemental Table 3, http://links.lww.com/NEU/D679). Each individual SNP was input into National Center for Biotechnology Information 1000 Genome Browser to find its corresponding control. See supplemental tables (Supplemental Table 1, http://links.lww.com/NEU/D677, Supplemental Table 2, http://links.lww.com/NEU/D678, Supplemental Table 3, http://links.lww.com/NEU/D679) for detailed SNP genotyping probe description with assay ID and accompanying positive and negative controls. Global reference for allelic frequency was obtained from http://www.ensembl.org.
FIGURE 4.
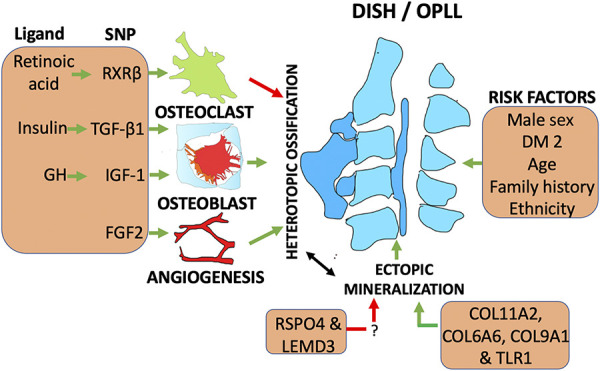
Known DISH/OPLL associations. This figure summarizes previously hypothesized associations with DISH and the related condition, ossification of the posterior longitudinal ligament. These previously explored genetic associations are comprehensively reviewed in our study in a novel North American population. One possible mechanism illustrated here is the interaction of ectopic mineralization using altered collagen regulation providing a nidus for heterotopic ossification through altered osteoblast/osteoclast activity. Red lines indicate factors that are protective against DISH: RSPO and LEMD3 have been proposed protective. FGF2 has diverse functions including repair after injury by promotion of angiogenesis. While there is no known unifying mechanism for DISH predisposition, this figure best approximates what can be found in the existing body of peer-reviewed literature. DISH, diffuse idiopathic skeletal hyperostosis; FGF2, fibroblast growth factor 2 gene; GH, growth hormone; OPLL, ossification of the posterior longitudinal ligament.
Osteophyte Measurement
Osteophyte width was approximated by measuring the maximum osteophyte extension perpendicular to a line drawn along the anterior extent of 2 disk spaces.
Statistics
For this retrospective study, χ2 tests, 2 sample t-tests, and Wilcoxon 2 sample tests were used to compare demographic and comorbidity information for patients with DISH with and without presence of cervical OPLL. For the continuous variables, Shapiro-Wilk tests for normality were performed, and if significant, the nonparametric Wilcoxon tests were used to compare the 2 groups. Similar analyses were performed to assess differences between patients with DISH with and without genetic testing. The Fisher exact test was performed to assess the associations of DISH with genetic haplotypes using global frequencies as the references. Among the patients with DISH with genetic results available, Fisher exact tests were also performed to test for associations between OPLL and the genetic test results. All testing was performed at the 0.05 level. Statistical analyses were performed using SAS version 9.4.
RESULTS
Population Characteristics
Patient demographics are summarized in Table 1, and a Michigan population disease prevalence8 is provided for comparison. Consistent with prior literature, patients with DISH are older (mean 71.6 ± 12.4 years), predominantly men (80%), and have a high rate of coexisting diabetes (54%, none with type 1 diabetes). Considering the mean and standard deviation of the patients' ages, most of the women in the study were presumably postmenopausal; however, this was not specifically asked. There were low rates of autoimmune disease (4%), moderate rates of renal disease (17%), and no patients with a known family history. Patients self-identified as White (69%), Black (28%), and Other (3%).
Of 121 patients, 91 had information regarding the presence of cervical OPLL: 14 (15%) had coexisting OPLL and 77 (85%) did not. Interestingly, all 14 of the patients with DISH with coexisting OPLL had diabetes, whereas 45% of the patients with DISH alone had diabetes (P < .001). No other comparisons reached statistical significance (Table 2).
TABLE 2.
Comparing Patients With and Without OPLL
| Variable | Response | No OPLL (N = 77) | OPLL (N = 14) | P value |
|---|---|---|---|---|
| Age | Mean ± Std Dev | 71.6 ± 12.5 | 73.2 ± 9.6 | .653 |
| Sex | Female | 16 (21%) | 3 (21%) | .956 |
| Ethnicity | Black | 22 (29%) | 4 (29%) | .751 |
| White | 52 (68%) | 10 (71%) | ||
| Other | 3 (4%) | 0 (0%) | ||
| Race | Non-Black | 55 (71%) | 10 (71%) | >.99 |
| Black | 22 (29%) | 4 (29%) | ||
| BMI | Mean ± std dev | 29.6 ± 12.0 | 29.6 ± 5.0 | .470 |
| Median (IQR) | 27.9 (24, 33) | 32 (24, 34) | ||
| Smoking | Current | 10 (13%) | 0 (0%) | .354 |
| Former | 43 (57%) | 9 (64%) | ||
| Never | 23 (30%) | 5 (36%) | ||
| Renal disease | 13 (17%) | 3 (21%) | .681 | |
| Autoimmune disease | 2 (3%) | 1 (7%) | .381 | |
| Diabetes | 35 (45%) | 14 (100%) | <.001 | |
| History of radiation | 8 (10%) | 3 (21%) | .244 |
BMI, body mass index; DISH, diffuse idiopathic skeletal hyperostosis; OPLL, ossification of the posterior longitudinal ligament.
Comparison between patients with DISH alone vs patients with DISH and OPLL. An important finding is that 100% of patients with DISH and OPLL also had diabetes.
When comparing diabetic (mean = 8.8, SD = 4.7, median = 8, quartile range = 5, 12) vs nondiabetic (mean = 8.1, SD = 3.6, median = 8, IQR = 5, 10) patients with DISH, there was no difference in maximum osteophyte width (P = .686).
Patients with genetic results were compared with the group where DNA samples were not collected (Supplemental Table 1, http://links.lww.com/NEU/D677, Supplemental Table 2, http://links.lww.com/NEU/D678, Supplemental Table 3, http://links.lww.com/NEU/D679). The genetic testing group had higher rates of Black patients (41% vs 21%, P = .035) and a different distribution of smoking status (21% current, 59% former and 21% never vs 6% current, 53% former, and 41% never, P = .033) (Supplemental Table 1, http://links.lww.com/NEU/D677, Supplemental Table 2, http://links.lww.com/NEU/D678, Supplemental Table 3, http://links.lww.com/NEU/D679). The reasons for these differences are unclear; however, many of the samples were obtained from hospitalized patients. Therefore, the differences may relate to a patient's likelihood of hospitalization or willingness to consent. No differences were observed between patients with and without genetic testing for age, sex, body mass index, renal disease, autoimmune disease, diabetes, and history of radiation.
Genetic Testing Results
When compared with global haplotype frequencies (Table 3), there were higher rates of SNPs in COL6A6 (rs200963433, P < .001), fibroblast growth factor 2 gene (FGF2) (rs3747676, P = .039), LEMD3 (rs201930700, P < .001), TGFB1 (rs2241716, P = .002), and TLR1 (rs145135062, P < .001). Differences did not reach statistical significance for COL11A2 (rs2071025, P = .083), FGF2 (rs1476217, P = .521), TGFB1 (rs1800469, P = .093), and TLR1 (rs200212492, P = .237).
TABLE 3.
Genotype and Allelic Distribution
| Gene symbol SNP | Genotype | DISH n (%) | Global n (%) | P | Consequence |
|---|---|---|---|---|---|
| COL11A2 rs2071025 | A|G | 20 (36.4) | 1055 (42) | .083 | Variable base on genomic location |
| G|G | 6 (10.9) | 477 (19) | |||
| A|A | 29 (52.7) | 972 (39) | |||
| COL6A6 rs200963433 | C|T | 7 (12.7) | 4 (0.2) | <.001 | Missense variant |
| C|C | 48 (87.3) | 2500 (99.8) | |||
| T|T | 0 | 0 | |||
| FGF2 rs1476217 | A|C | 27 (49.1) | 1208 (48) | .521 | 3′ UTR variant |
| A|A | 18 (32.7) | 692 (28) | |||
| C|C | 10 (18.2) | 604 (24) | |||
| FGF2 rs3747676 | T|C | 30 (54.5) | 945 (38) | .039 | 3′ UTR variant |
| T|T | 19 (34.5) | 1159 (46) | |||
| C|C | 6 (11.0) | 400 (16) | |||
| LEMD3 rs201930700 | C|T | 6 (11.2) | 0 | <.001 | Missense variant |
| C|C | 48 (88.8) | 251 188 (100) | |||
| T|T | 0 | 0 | |||
| TFGB1 rs2241716 | C|T | 1 (1.8) | 460 (18) | .002 | Intron variant |
| C|C | 54 (98.2) | 1985 (79) | |||
| T|T | 0 | 59 (2) | |||
| TGFB1 rs1800469 | G|A | 18 (32.7) | 1075 (43) | .093 | Intron variant |
| A|A | 6 (11.0) | 384 (15) | |||
| G|G | 31 (56.3) | 1045 (42) | |||
| TLR1 rs145135062 | T|C | 2 (3.6) | 6 (0.2) | <.001 | Missense variant |
| T|T | 53 (96.4) | 2498 (99.8) | |||
| C|C | 0 | 0 | |||
| TLR1 rs200212492 | A|G | 2 (3.6) | 217 (1) | .237 | Missense variant |
| A|A | 53 (96.4) | 2503 (99) | |||
| G|G | 0 | 0 |
DISH, diffuse idiopathic skeletal hyperostosis; FGF2, fibroblast growth factor 2 gene; SNP, single nucleotide polymorphism; UTR, untranslated region.
Compares the allelic frequencies between patients with DISH in this study and globally observed frequencies. SNP consequences have been noted to result in a range of genomic consequences as denoted in the table. Comparison data and consequences are provided by http://www.ensembl.org.
Of the 34 patients with genetic testing and demographic information, 5 (17%) had OPLL. In contrast to a previous study,6 no differences were observed between the DISH alone vs patients with DISH and OPLL for any of the genetic test results (Supplemental Table 1, http://links.lww.com/NEU/D677, Supplemental Table 2, http://links.lww.com/NEU/D678, Supplemental Table 3, http://links.lww.com/NEU/D679). No differences in haplotypes were observed when comparing diabetic vs nondiabetic patients with DISH, P > .05 (range 0.196->0.99).
DISCUSSION
Clinical Attributes
DISH is a poorly defined and incompletely understood condition with limited studies restricted to genetically homogenous populations. Owing to a commonly asymptomatic occurrence, lack of pathophysiological understanding, slow progression, and variable phenotypical patterns, there is a lack of consensus on the diagnostic criteria for DISH.1 The most frequently used definition is the radiographic presence of flowing ossification along the anterior aspect of the vertebral column, relative intervertebral disk preservation and the absence of apophyseal ankylosis and inflammatory changes of the sacroiliac joint.1,9 Others have further attempted to differentiate DISH from ankylosing spondylitis, stating that the presence of HLA B27 is an exclusion criterion for DISH; however, this concept has been challenged because the 2 conditions may at times coexist.10 Patients with DISH often have abundant ossification throughout the body and not limited to the spine; however, the role of peripheral enthesopathies in the diagnosis and pathogenesis is uncertain.
The thoracic spine is more commonly affected than the cervical spine,11 with resultant symptoms rarely occurring before the age 40 years.12,13 However, in our population we found a higher prevalence of cervical DISH (70%, Table 1) than previously reported. The reason for this discrepancy compared with prior literature is unclear but may be related to the acquisition of data from neurosurgical departments to which patients have been referred for treatment at both institutions.
Mechanical compression of the esophagus by anterior osteophytes can result in dysphagia (Figure 1), in some cases requiring gastrostomy tube placement. Anecdotally, when many of the patients in this study were interviewed, they reported dysphagia. Resection of symptomatic osteophytes is considered a morbid procedure with often ungratifying results.
In earlier stages, abnormal biomechanical movement of the cervical spine results in chronic neck pain. Distraction extension injuries result in a transverse shift of the fractured segment with an increased risk of neurological compromise because of lack of dissipation seen in a normal spine (Figure 1).11 No surgical intervention to reverse the autofusion process has been described. There are no medical therapies or disease-modifying pharmacotherapeutics with known efficacy.
Pathogenesis
The etiology and pathogenesis of DISH is yet to be fully understood.2 Multiple hypotheses exist based on metabolic characteristics of cohorts,14-16 familial clustering of early progression,13,17 canine studies on breeds with a high rate of DISH18 and studies on OPLL, a seemingly related condition.3 These associations drive the search for a unifying genetic underpinning as summarized in Figure 4.
Metabolic risk factors have also been linked to the development of DISH. These include obesity, high waist-to-hip circumference ratio, dyslipidemia, hypertension, glucose intolerance, type 2 diabetes, hyperuricemia, hyperinsulinemia, and elevated IGF-1 and growth hormone levels.14-16,19-22 Consistent with previous studies, we did observe 51% of patients having coexisting diabetes and separately, 17% had renal disease. A novel finding in our study cohort was that 100% of patients with DISH and OPLL had diabetes, strengthening the hypothesized metabolic association.
Another proposed mechanism is that the DISH phenotype may result from dysregulation of systemic factors related to bone turnover in an inflammatory state.23 This proposed mechanism may explain associations with elevated IGF1 and low levels of Dickkopf-1, an inhibitor of the Wnt/β-catenin–mediated osteoblastogenesis pathway that has been associated with DISH.24 However, the universality of this association has been challenged,25 and further studies are needed to validate these findings.23 A proinflammatory state resulting in the formation of DISH is an attractive hypothesis because it may lead to the possibility of a therapeutic target.
Associations of DISH with metabolic aberrations have been found. These include hyperinsulinemia,16 reduced pyrophosphate breakdown,26 dyslipidemia,22 hyperuricemia,22 increased vitamin A levels,22 and elevated IGF-1 and growth hormone levels.19 These aberrations are substantiated by the association with obesity, high waist-to-hip circumference ratio, hypertension, glucose intolerance, and type 2 diabetes.2,22 The genetic and environmental influences on these observations are uncertain, with advanced age as a significant confounder without any prior robust case-control studies to limit this interaction.
Changes in signaling pathways may be the unifying links between genetic aberrations and metabolic and inflammatory derangements. Proposed mediators include upregulation of osteoblastic differentiation by growth hormone/IGF-1, Wnt-β catenin, TGF-β, platelet-derived growth factor subunit BB, PGI2, fibroblast growth factor, and ET1; chondrogenesis by hyperinsulinemia; promotion of mesenchymal cell differentiation by BMP2; and polymorphisms in structural proteins encoded by genes such as COL6A1.2,27,28 However, further confirmatory studies are needed. In our cohort, rates of SNPs within several separate regulatory genes that were higher than a global reference supported a hypothesized polygenic etiology.
Genetics
The strongest prior evidence for the genetic inheritance of DISH comes from familial clustering, despite the fact we did not see this in our patient population. In other studies, 103 individuals from 12 unrelated families in the Azores revealed an associated pyrophosphate arthropathy with coexisting peripheral and axial enthesopathic calcifications that was inherited in an autosomal dominant fashion.17 However, a whole-genome, microsatellite, linkage analysis followed by “identity-by-state/identity-by-descent” genetic analysis in a cohort of 92 individuals from 12 pedigrees with early onset DISH did not reveal—or confirm—an associated haplotype.7 This study did identify SNPs within RSPO4 and LEMD3 genes, as possibly possessing a small protective role.7 They hypothesize that a haplotype reducing Wnt activation would potentially protect against new bone formation.
Genomic analysis of patients with DISH has produced inconsistent results possibly reflecting the presence of multiple susceptibility haplotypes. In Japan, nucleotide variants in the collagen 6A1 gene were associated with DISH; however, this was not seen in Czech patients.5 In a Korean cohort, polymorphisms in the FGF2 were associated with DISH.6 These polymorphisms are located in noncoding regions, suggesting a minor effect on DISH susceptibility.7 However, the results from our study did not confirm this prior finding.
OPLL is a related condition that has been more extensively studied in a Japanese population where it is a common cause of compressive myelopathy. SNPs in several different loci of the COL6A1 gene have been robustly established as susceptibility polymorphisms in OPLL.29 COL6A1 encodes type VI collagen α chain that might serve as an extracellular scaffold for osteoblasts or chondrocytes that subsequently proceed to membranous or endochondral ossification.5 Further studies showed that SNPs of intron 32 of the COL6A1 gene were found in Japanese patients with DISH who did not have coexisting OPLL.5,6 However, this mutation was not found in Czech patients.5 Overall, variation in over 30 different genes and micro-RNA profiles have been established in association with OPLL, most notably in genes encoding interleukin, retinoic X, and toll-like receptors, transforming growth factor β, nucleotide pyrophosphatase, and bone morphogenic protein.30
In our study, we looked for previously tested metabolic and genetic hypotheses in a novel population (Table 4). This is the first study to assess for a broad array of previously hypothesized alleles in a diverse, North American population.
TABLE 4.
Tested Genes and Source Literature
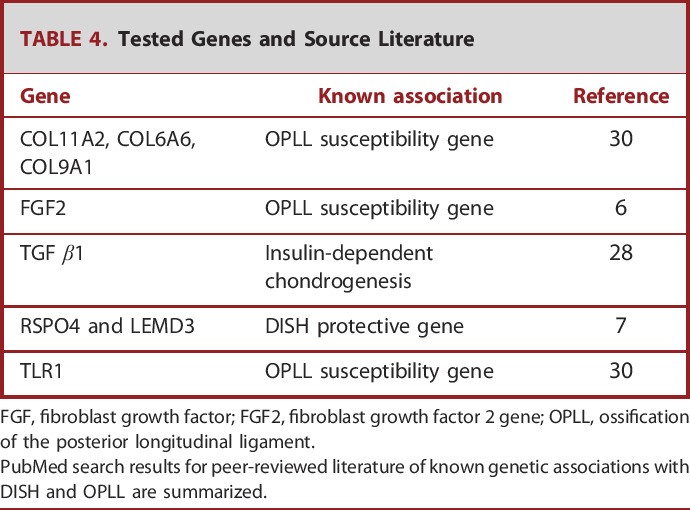
| Gene | Known association | Reference |
|---|---|---|
| COL11A2, COL6A6, COL9A1 | OPLL susceptibility gene | 30 |
| FGF2 | OPLL susceptibility gene | 6 |
| TGF β1 | Insulin-dependent chondrogenesis | 28 |
| RSPO4 and LEMD3 | DISH protective gene | 7 |
| TLR1 | OPLL susceptibility gene | 30 |
FGF, fibroblast growth factor; FGF2, fibroblast growth factor 2 gene; OPLL, ossification of the posterior longitudinal ligament.
PubMed search results for peer-reviewed literature of known genetic associations with DISH and OPLL are summarized.
Limitations
A major limitation in this study is the lack of a control group of patients without the presence of DISH for comparison. Instead, we used the global allele rates as a comparison, which might also include patients in the control population that have DISH, depending on the disease prevalence. The use of a global control is additionally problematic due to population heterogeneity, regional variation, and methodology differences in the groups that submitted data to create a global database.
Another limitation is that we were unable to obtain samples from all patients enrolled in the study, and most of the samples were obtained from hospitalized patients, which may lead to sample bias. When the demographics of patients with genetic testing were compared with those without testing, there were significant differences in race and smoking status. These issues likely limit the generalizability of these results.
CONCLUSION
This study highlights the need for a better definition and understanding of DISH. Ideally, this would include a genetically based classification system and/or a better radiographic definition. Our results support the hypothesis that DISH has either a polygenic predisposition or multiple distinct genotypic haplotypes converging to produce a similar phenotype. Strong evidence exists to support a component of environmental influence, especially in diabetic patients. The high rates of diabetes, especially in patients with DISH and OPLL, raise questions, such as what are the possible effects of medication vs the importance of insulin as a growth promoter? To fully address this issue, and better understand whether diabetes is an environmental promotor rather than a confounding variable, a study comparing patients with and without DISH as well as look at patients with isolated OPLL would be needed. The high prevalence of postmenopausal women and older men raises the question of an unidentified hormonal influence.
The genetic results of our study support the need for a globally coordinated consortium with the use of whole-genome sequencing. This would also help guide translational research that could help to pin down discrete and pharmacologically targetable pathophysiological mechanisms. There are currently no targeted treatments for ectopic bone formation related to the SNPs suggested by this study. The goal of further research would be to develop mouse knockdown models of suspected genes to better understand their function. Ultimately, further understanding of the pathogenesis of DISH may be translated into improved early diagnosis and treatment of this condition.
Acknowledgments
We would like to recognize Regan Gaskin, MPA, and Kelly Tundo RN, BSN, CCRP, for their assistance in research coordination and Lonni Schultz, PhD, for biostatistical expertise and Susan MacPhee for editorial assistance.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Silvienne Sint Jago, Email: sintjago@uab.edu.
Matthew S. Erwood, Email: merwood.me@gmail.com.
Azam Basheer, Email: abashee1@hfhs.org.
Ian Y. Lee, Email: ilee1@hfhs.org.
Farah D. Lubin, Email: flubin@uab.edu.
Lonni Schultz, Email: lschult1@hfhs.org.
Beverly C. Walters, Email: bcwmd@bcwmd.com.
Funding
This study did not receive any funding or financial support. Thomas Zervos received funding from the Henry Ford Health Graduate Medical Education Research Grant (1702). Matthew S. Erwood received funding from the University of Alabama North Family Clinical Scholar award. Farah E. Lubin received funding from NIH/NINDS R21 NS116937.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
SUPPLEMENTAL DIGITAL CONTENT
Supplemental Table 1. Comparing patients included and not included in genetic testing. Patients included with DNA available for testing were compared with patients where samples were not available. Notable differences between the groups include race and smoking status.
Supplemental Table 2. Associations between OPLL and genetic results. A previous publication suggested that there is a unique FGF 2 allele that occurs more frequently in patients with DISH and OPLL vs DISH alone.6 Our results did not corroborate this finding.
Supplemental Table 3. SNP sequence data and control IDs. Information needed to replicate the results from this study is provided for reference.
REFERENCES
- 1.Kuperus JS, de Gendt EEA, Oner FC, et al. Classification criteria for diffuse idiopathic skeletal hyperostosis: a lack of consensus. Rheumatology. 2017;56(7):1123-1134. [DOI] [PubMed] [Google Scholar]
- 2.Mader R, Verlaan JJ, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol. 2013;9(12):741-750. [DOI] [PubMed] [Google Scholar]
- 3.Havelka S, Veselá M, Pavelková A, et al. Are DISH and OPLL genetically related? Ann Rheum Dis. 2001;60(9):902-903. [PMC free article] [PubMed] [Google Scholar]
- 4.Niu CC, Lin SS, Yuan LJ, et al. Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC Musculoskelet Disord. 2017;18(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukahara S, Miyazawa N, Akagawa H, et al. COL6A1, the candidate gene for ossification of the posterior longitudinal ligament, is associated with diffuse idiopathic skeletal hyperostosis in Japanese. Spine. 2005;30(20):2321-2324. [DOI] [PubMed] [Google Scholar]
- 6.Jun JK, Kim SM. Association study of fibroblast growth factor 2 and fibroblast growth factor receptors gene polymorphism in Korean ossification of the posterior longitudinal ligament patients. J Korean Neurosurg Soc. 2012;52(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couto AR, Parreira B, Thomson R, et al. Combined approach for finding susceptibility genes in DISH/chondrocalcinosis families: whole-genome-wide linkage and IBS/IBD studies. Hum Genome Var. 2017;4(1):17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmer G, Hertel E, Travis A, Lyon-Callo S, McKane P, Tian Y. Health Risk Behaviors Within the State of Michigan. 2020. https://www.michigan.gov/-/media/Project/Websites/mdhhs/Folder4/Folder30/Folder3/Folder130/Folder2/Folder230/Folder1/Folder330/MiBRFS_Annual_Report_2019.pdf?rev=3eccf33253e142c5849c74e4bc967d9b [Google Scholar]
- 9.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology. 1976;119(3):559-568. [DOI] [PubMed] [Google Scholar]
- 10.Kuperus JS, Waalwijk JF, Regan EA, et al. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: a systematic review. Rheumatology. 2018;57(12):2120-2128. [DOI] [PubMed] [Google Scholar]
- 11.Katoh H, Okada E, Yoshii T, et al. A comparison of cervical and thoracolumbar fractures associated with diffuse idiopathic skeletal hyperostosis—a nationwide multicenter study. J Clin Med. 2020;9(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitten JJ, James B. Diffuse idiopathic skeletal hyperostosis in a 33-year-old woman with PCOS and metabolic syndrome: a rare scenario. BMJ Case Rep. 2019;12(10):e223740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorman C, Jawad ASM, Chikanza I. A family with diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis. 2005;64(12):1794-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denko CW, Malemud CJ. Body mass index and blood glucose: correlations with serum insulin, growth hormone, and insulin-like growth factor-1 levels in patients with diffuse idiopathic skeletal hyperostosis (DISH). Rheumatol Int. 2006;26(4):292-297. [DOI] [PubMed] [Google Scholar]
- 15.Eckertova M, Krskova K, Penesova A, et al. Impaired insulin secretion and uptake in patients with diffuse idiopathic skeletal hyperostosis. Endocr Regul. 2009;43(4):149-155. [PubMed] [Google Scholar]
- 16.Littlejohn GO, Smythe HA. Marked hyperinsulinemia after glucose challenge in patients with diffuse idiopathic skeletal hyperostosis. J Rheumatol. 1981;8(6):965-968. [PubMed] [Google Scholar]
- 17.Bruges-Armas J, Couto AR, Timms A, et al. Ectopic calcification among families in the Azores: clinical and radiologic manifestations in families with diffuse idiopathic skeletal hyperostosis and chondrocalcinosis. Arthritis Rheum. 2006;54(4):1340-1349. [DOI] [PubMed] [Google Scholar]
- 18.Woodard JC, Poulos PW, Parker RB, Jackson RI, Eurell JC. Canine diffuse idiopathic skeletal hyperostosis. Vet Pathol. 1985;22(4):317-326. [DOI] [PubMed] [Google Scholar]
- 19.Denko CW, Boja B, Moskowitz RW. Growth promoting peptides in osteoarthritis and diffuse idiopathic skeletal hyperostosis—insulin, insulin-like growth factor-I, growth hormone. J Rheumatol. 1994;21(9):1725-1730. [PubMed] [Google Scholar]
- 20.Kiss C, Szilágyi M, Paksy A, Poór G. Risk factors for diffuse idiopathic skeletal hyperostosis: a case-control study. Rheumatology. 2002;41(1):27-30. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa N, Akiyama I. Diffuse idiopathic skeletal hyperostosis associated with risk factors for stroke: a case-control study. Spine. 2006;31(8):e225-229; discussion e230. [DOI] [PubMed] [Google Scholar]
- 22.Vezyroglou G, Mitropoulos A, Antoniadis C. A metabolic syndrome in diffuse idiopathic skeletal hyperostosis. A controlled study. J Rheumatol. 1996;23(4):672-676. [PubMed] [Google Scholar]
- 23.Mader R, Verlaan JJ. Bone: exploring factors responsible for bone formation in DISH. Nat Rev Rheumatol. 2011;8(1):10-12. [DOI] [PubMed] [Google Scholar]
- 24.Senolt L, Hulejova H, Krystufkova O, et al. Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis. 2012;71(1):71-74. [DOI] [PubMed] [Google Scholar]
- 25.Aeberli D, Schett G, Eser P, Seitz M, Villiger PM. Serum Dkk-1 levels of DISH patients are not different from healthy controls. Joint Bone Spine. 2011;78(4):422-423. [DOI] [PubMed] [Google Scholar]
- 26.Timms AE, Zhang Y, Russell RGG, Brown MA. Genetic studies of disorders of calcium crystal deposition. Rheumatology. 2002;41(7):725-729. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka T, Imakiire A, Mizuno F, Yamamoto K. Activation of nuclear factor κB at the onset of ossification of the spinal ligaments. J Orthop Sci. 2000;5(6):572-578. [DOI] [PubMed] [Google Scholar]
- 28.Mueller MB, Blunk T, Appel B, et al. Insulin is essential for in vitro chondrogenesis of mesenchymal progenitor cells and influences chondrogenesis in a dose-dependent manner. Int Orthop. 2013;37(1):153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka T, Ikari K, Furushima K, et al. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 2003;73(4):812-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang C, Wang P, Liu X, et al. Whole-genome sequencing reveals novel genes in ossification of the posterior longitudinal ligament of the thoracic spine in the Chinese population. J Orthop Surg. 2018;13(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparing patients included and not included in genetic testing. Patients included with DNA available for testing were compared with patients where samples were not available. Notable differences between the groups include race and smoking status.
Supplemental Table 2. Associations between OPLL and genetic results. A previous publication suggested that there is a unique FGF 2 allele that occurs more frequently in patients with DISH and OPLL vs DISH alone.6 Our results did not corroborate this finding.
Supplemental Table 3. SNP sequence data and control IDs. Information needed to replicate the results from this study is provided for reference.