Abstract
Background
Prediabetes is an intermediate state of hyperglycemia, which acts as a precursor to Diabetes mellitus if left untreated. Nisha (Curcuma longa) and Amalaki (Emblica officinalis) combination has been advocated as drugs of choice to treat the early manifestations of Diabetes mellitus.
Objective
This prospective, randomized, single-blind, placebo-controlled, comparative study was planned to assess the efficacy and safety of Nisha-Amalaki capsules in preventing progression to Diabetes mellitus in prediabetic patients when administered for 6 months.
Methods
The study was conducted on prediabetic participants randomized to receive either Nisha-Amalaki (500 mg) or placebo one capsule twice a day for six months. The effect of study medications on IDRS (Indian Diabetes Risk Score), BMI (Body Mass Index), blood sugar, serum insulin, HOMA-IR (Homeostasis Model Assessment-Estimated Insulin Resistance), HbA1c (glycated hemoglobin), oxidative markers, Ayurvedic symptoms and Quality of Life (QoL) scores was assessed at regular intervals.
Results
58 of the 62 participants enrolled completed the study. Significant fall in IDRS score [p < 0.001], BMI [p < 0.001], fasting, and 2 h post-OGTT sugar, insulin, HbA1c, HOMA-IR, and oxidative stress markers [p < 0.001] was observed in patients receiving Nisha-Amalaki at 6 months. Ayurvedic symptoms and QoL scores also improved at 6 months in the treatment group.
Conclusion
Treatment with Nisha-Amalaki capsules improved all study parameters including insulin sensitivity at 6 months as compared to placebo in prediabetic patients. Thus Nisha-Amalaki should be considered as prophylactic therapy in prediabetics to delay progression to diabetes.
Keywords: Prameha, Prophylactic, IDRS, HOMA-IR, Ayurvedic symptoms score, Quality of life score
1. Introduction
Prediabetes is defined as a clinical metabolic state in which the level of blood sugar is higher than the normal range although it has not yet crossed the threshold for diabetes. If left untreated, however, there is a high risk of progression to the diabetic state [1]. The criteria to diagnose prediabetes varies between different organizations. As per the World Health Organization (WHO), it is a state of intermediate hyperglycemia as determined by two main parameters; impaired fasting glucose (IFG) wherein the fasting plasma glucose (FPG) level is between 110 to 125 mg/dL and impaired glucose tolerance (IGT) wherein the 2 h plasma glucose following ingestion of a glucose load (75 g) ranges between 140 to 200 mg/dL [2]. Although the IGT threshold value of the American Diabetes Association (ADA) is the same as that of WHO (140–200 mg/dL) for the diagnosis of prediabetes, the threshold value is lower with regard to IFG (100–125 mg/dL) with an additional diagnostic parameter viz. hemoglobin A1c (HbA1c) value ranging between 5.7 and 6.4% [3].
An increase in the global prevalence of prediabetes has been reported with 7.3% of the adult population having IGT in 2017 [approximately 352.1 million people]. It has been estimated that this figure will increase to 8.3% of the world's adult population by 2045 indicating 587 million prediabetic individuals [4]. As per the National Diabetes Statistics Report, January 2022 CDC, USA, 96 million American adults (38%) are prediabetic [5]. In the Indian adult population, the prevalence of diabetes was found to be 6.65% and prediabetes was 5.57% as per the report of the Demographic and Health Survey 2015–16 (also known as the National Family Health Survey-4) [6].
The pathophysiology of prediabetes is mainly due to pancreatic β-cell dysfunction and impaired insulin sensitivity which occur much before alterations in glucose levels are detected. Individuals suffering from prediabetes may often also have co-morbidities like hypertension, obesity, and raised lipid levels, all of which increase the risk of adverse cardiac events [7]. Observational evidence has also shown an association of prediabetes with early forms of micro and macrovascular diabetic complications like altered kidney function, autonomic and sensorimotor neuropathy, early retinopathy, cardiovascular and cerebrovascular disease [8].
Various diabetic scoring systems have been formulated that predict the risk of occurrence of diabetes using both non-invasive variables like age, sex, family history of diabetes, BMI etc. and laboratory variables like blood glucose and HbA1c values. One such score is the Indian Diabetes Risk Score (IDRS). IDRS score was developed using four basic variables viz. age, abdominal obesity, family history of diabetes, and physical activity, thus making it a cost-effective scoring system to predict prediabetes in a resource poor setting like India. A high IDRS score has been shown to be associated with a higher risk of prediabetes, metabolic syndrome, cardiovascular disease, etc [9]. The IDRS scale uses two modifiable risk factors (waist circumference and physical inactivity) and two non-modifiable risk factors (age and family history of diabetes), explaining a fact that if modifiable risk factors get improved, the risk score can be considerably reduced [10,11].
Prediabetes or Prameha poorva-rupa as described in Ayurveda is the precursor stage to Diabetes mellitus or Prameha with erratic lifestyle and poor dietary habits listed as the two main causes for progression to Prameha [12,13]. A careful look at the prediabetic features described in Ayurvedic literature shows that all these features are the pathological consequences of raised blood sugar levels with an inability to reach the cell to produce adequate energy [14]. Prediabetes, if left uncontrolled for a prolonged duration, leads to the diabetic state [15].
The combination of 2 Indian medicinal plants viz. Nisha/Tumeric (Curcuma longa) and Amalaki (Emblica officinalis) selected for this study is a combination that has been advocated in Ayurveda by Vagbhata as a drug of choice for the treatment of Prameha [[16], [17], [18], [19], [20]].
Nisha/Tumeric (C. longa) is a perennial herbaceous rhizome, belonging to the Zingiberaceae family, that is native to Southeast Asia and the Indian subcontinent. It is commonly used to treat various medical conditions since centuries [21]. Curcumin, a polyphenol, is the main active constituent present in the rhizome and constitutes 2–8% of the rhizome [22]. It has been shown to reduce hyperglycemia and hyperlipidemia in rodent models of diabetes. Its action similar that of thiazolidinedione group of antihyperglycemic drugs through activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) [23]. Thus, curcumin may help improve both carbohydrate and lipid metabolism which is important in the management of diabetes [24]. However, clinical studies using Curcumin in prediabetic and type 2 diabetic patients have thrown up conflicting results. Yang et al. in their randomized study in patients with metabolic syndrome, demonstrated that administration of curcumin extract (1890 mg/day) for 12 weeks was associated with lipid-lowering effect but did not improve weight and glucose homeostasis in patients with metabolic syndrome [25]. Adab Z et al. conducted a randomized double-blind clinical study to assess the effect of turmeric supplementation on glycemic status, lipid profile, hs-CRP and total antioxidant capacity in hyperlipidemic type 2 diabetic patients for 8 weeks. They found that although turmeric improved some fractions of the lipid profile and decreased body weight in these patients, it had no significant effect on the glycemic status, hs-CRP, and total antioxidant capacity [26]. Na et al.'s results of their randomized, double-blind, placebo-controlled trial showed that curcuminoids supplementation significantly decreased fasting blood glucose, HbA1c and insulin resistance index (HOMA-IR) in type 2 diabetic patients [27]. A 6-month randomized, double-blinded, placebo-controlled clinical trial in patients diagnosed with type 2 diabetes conducted by Chuengsamarn et al. showed that curcumin intervention significantly reduced pulse wave velocity, increased serum adiponectin and decreased serum leptin levels, lowered abdominal obesity and improved BMI. In addition, curcumin treatment appeared to slightly lower fasting glucose, HbA1c, total cholesterol and LDL-C and slightly elevate HDL-C over time, these results were not statistically significant [28].
Amalaki(E. officinalis) or Indian gooseberry, belonging to the family Euphorbeaceae, has an important role in the traditional systems of Indian medicine. Although all the parts of the plant are of medicinal value, its fruit possesses majority of its pharmacological properties viz. antioxidant, anti-inflammatory, cytoprotective and immunomodulatory activity etc. which are responsible for its therapeutic effects [29]. It has been used in the treatment of type 2 Diabetes mellitus (T2DM) due to its hypoglycemic and hypolipidemic effects [[30], [31], [32]]. Pre-clinical and clinical studies have shown that prolonged treatment with the fruit can significantly improve symptoms associated with diabetic complications [33,34].
Although the efficacy of both these plants when given in combination with other plants has been evaluated in T2DM in experimental and clinical studies, the combination of these plants has been tested mainly in experimental studies [[35], [36], [37]]. Additionally, the traditional dose of Nisha-Amalaki powder is 5 gm/day. The high dose and unpleasant taste of the Nisha-Amalaki powder makes it difficult for patients to consume daily. To reduce its daily dose and to mask its unpleasant taste, a standardized Ayurvedic proprietary medicine named ‘EmbliQur’ was prepared in capsule form, which is commercially available in the Indian market.
This comparative study was thus planned to assess the efficacy and safety of this Ayurvedic proprietary medicine, viz. Nisha-Amalaki capsules as compared to placebo in preventing disease progression in patients with Prediabetes when given for 6 months. Placebo was used as the comparator mainly because the first mode of management of prediabetics as per the American Diabetes Association and International Diabetic Federation is diet and lifestyle modification which was implemented amongst all the study participants [38,39]. Additionally, as per the article by Jose and Thomas, metformin is recommended for prediabetics when the HbA1c levels are above 6.9% [40]. Further, this being a pilot study, we wished to initially evaluate its efficacy in improving the glycemic status in prediabetic patients. Hence, Nisha-Amalaki capsules were given as add-on therapy to one group and to keep the blinding, the second group was given placebo capsules. Use of the placebo is ethically justified as per the ICMR ‘Ethical Guidelines for Biomedical Research on Human Subjects’ (2017)] wherein it is stated that placebo may be given in addition to standard of care (in this case diet and lifestyle modification as per ADA & IDF) and the use of placebo would not add any additional risk of serious or irreversible harm to the participants [41].
The primary objective was to assess the change in the Indian Diabetes Risk Score (IDRS) score between the two study groups at the end of treatment. The secondary objectives were to assess the change in the fasting and 2-h blood sugar and insulin levels after the Oral Glucose Tolerance Test (OGTT), glycated hemoglobin levels (HbA1c), oxidative markers, ayurvedic symptoms and Quality of Life (QoL) scores between the two study groups at the end of 6 months.
2. Materials and methods
This single-blind, randomized, comparative, placebo-controlled, interventional study was conducted in a allopathic tertiary care public hospital following Institutional Ethics Committee approval [Approval Number: ECARP/2017/121]. The study was registered in Clinical Trial Registry of India - CTRI number CTRI/2018/08/015,503. The study was conducted in compliance with the various clinical research guidelines [Indian Good Clinical Practice (2001), Declaration of Helsinki (2013) and ICMR ‘Ethical Guidelines for Biomedical Research on Human Subjects’ (2017)] and also with the CONSORT (2010) reporting guidelines.
2.1. Selection of study participants
Individuals of either sex aged between 18 and 65 years who fulfilled at least two of the following criteria as per the American Diabetes Association viz. fasting blood glucose between 100 and 125 mg/dL, 2-h blood glucose between 140 and 199 mg/dL post OGTT and/or glycated hemoglobin between 5.7% and 6.5% were requested to participate in the study. Individuals with an IDRS ≥ 60 and ready to give written informed consent for the study were enrolled. Individuals with chronic history of smoking, alcohol or tobacco addiction, history of any major illness like uncontrolled hypertension, CVS disorders like IHD, heart failure, renal disorders, tuberculosis, epilepsy, cancer, neuropathies and psychiatric disorders, those on medications known to affect glucose tolerance e.g., glucocorticoids, anti-psychotic drugs, anti-depressants, anti-retroviral therapy, etc., those consuming other herbal medications and pregnant and lactating women were excluded from the study.
2.2. Study procedures
Enrolled participants were randomized in 1:1 ratio to either one of the two study groups. Treatment group received Nisha-Amalaki capsules (500 mg) twice a day. The capsules used in the study were EmbliQur® extract comprising E. officinalis fruit extract and C. longa rhizome extract (2:1) received from Pharmanza Herbal Pvt. Ltd., Anand, India. Although ayurvedic texts specify the proportion of Nisha-Amalaki to be 1:1, however a study by Yadav RK et al. revealed that an equal composition of E. officinalis and C. longa was not very effective in diabetes management when given alone, especially when the fasting blood sugar levels were above 200 mg% [42]. Additionally, compounds of E. officinalis such as gallic acid, and ellagic acid work by upregulation of pAkt, PPAR-gamma, and GLUT 4 while, curcuminoids act via PI3K-AKT-GSK3B signal pathway [43]. Hence, a higher content of E. officinalis was used in this proprietary Ayurvedic formulation to enhance the PPAR-gamma agonistic effect. The contents of the formulation used in the study were: C. longa alcohol extract (28.1%), C. longa water extract (1.76%), C. longa oil (1.27%), Phosphatidylcholine (12.65%), E. officinalis extract (40.41%) and E. officinalis juice powder (15.81%). The formulation had phosphatidylcholine as one of the excipients for bioavailability enhancement. This extract was standardized to contain total curcuminoids (<20.0%) and gallic acid (<5.0%) with total tannins (<20.0%) [36].
The Placebo Group received capsules, consisting of only 500 mg of maltodextrin, in a dose of one capsule twice a day. Participants from both groups also received counselling regarding their diet and exercises to be done during the study period. They were requested to consume their study medications after food with lukewarm water for the duration of the study i.e. 6 months.
2.3. Study assessment variables
The efficacy variables assessed during the study included body weight and BMI measured at monthly intervals, fasting and 2 h blood sugar post OGTT (baseline, 3 and 6 months post treatment), IDRS scoring, serum fasting and post OGTT insulin (baseline and 6 months), HOMA-IR for insulin resistance, HbA1c, lipid profile, oxidative stress markers (MDA and SOD) and C-reactive protein for inflammation (baseline and 6 months). An Ayurvedic symptom score which assessed the presence or absence of symptoms related to the diabetic state as mentioned in Ayurvedic texts [44,45] viz. Atikshudha (polyphagia), Atimutrata (polyuria), Atitrishna (polydipsia), Atisveda (excessive sweating), Hastha Pada Daha (burning sensation in peripheral limbs), Alasya (laziness), Atinidra (excessive sleep), Shithilangata (flabbiness of the body), Hastha-Pada Shunyata (feeling of numbness in peripheral limbs), Mukha Shosha (dryness of mouth) and Sheeta Priyata (preference for cold items) was performed at monthly intervals while Quality of life was assessed using the Health related quality of life, SF-36 at baseline and end of therapy. Prakriti of the study participants was clinically assessed at baseline prior to initiation of therapy using a validated questionnaire [46].
Safety parameters evaluated during the study were clinical assessment, any adverse reactions reported by the study patients at every study visit and laboratory investigations viz. hematological (hemoglobin, complete blood count) and biochemical tests like liver function and renal function tests which were performed at baseline, 3 and 6 months post treatment.
2.4. Statistical considerations
2.4.1. Sample size
As this was a Proof-of-Concept Study, with no previous clinical results available with this formulation, a sample size of 60 participants (30 in each group) was considered adequate to address the study objectives [[47], [48], [49]].
2.5. Statistical analysis
Analysis was done using Student's unpaired ‘t’ test for parametric data or Mann–Whitney test in case of non-parametric data for between group comparison and Student's paired ‘t’ test or Repeated Measures Analysis of Variance with Tukey–Kramer multiple comparisons test in case of parametric data or Wilcoxon matched-pairs signed-ranks test in case of non-parametric data in case of within group comparison. p ≤ 0.05 was considered as the cutoff for statistical significance.
3. Results
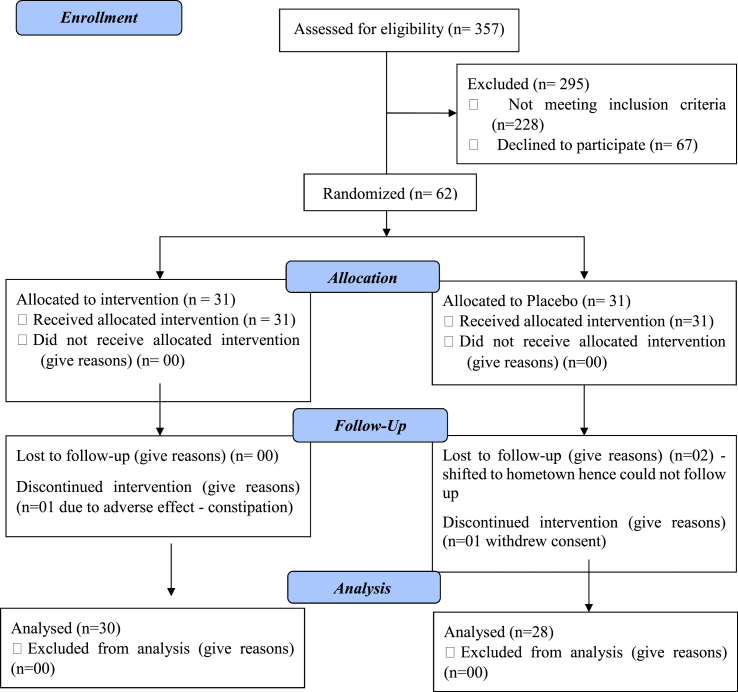
A total of 357 participants were screened for their pre-diabetic status, of which 62 participants with Pre-diabetes were recruited over a period of 2 years (2018–2020). Of these, 58 patients completed the study with 30 patients in the Nisha-Amalaki treatment group & 28 patients in the Placebo group. Fig. 1 is the CONSORT 2010 Flow chart showing the status of patients enrolled in the study.
Fig. 1.
CONSORT 2010 Flow chart showing the status of patients enrolled in the study.
3.1. Demographics of the study participants
A look at the demographics of the study participants showed that most participants aged between 43 and 60 years with equal distribution of men and women in both the study groups (Table 1). A look at the prakriti of the study participants showed that majority of the patients were of the Kaphapradhan prakriti (Table 2).
Table 1.
Demographic details of the study patients.
| Total (n = 62) | Treatment Group (n = 31) | Placebo Group (n = 31) | P value | |
|---|---|---|---|---|
| Gender (Female) (n, %) | 36 (58.06%) | 17 (27.41%) | 19 (30.65%) | 0.796 |
| Gender (Male) (n, %) | 26 (41.94%) | 14 (22.58%) | 12 (19.35%) | |
| Age (mean ± SD) | 62 (51.45 ± 8.79) | 48.50 ± 9.55 | 54.61 ± 6.70 |
Results expressed as Number (%) for gender and (mean ± SD) for age.
p values calculated using Chi-square test for gender difference in both the groups.
Table 2.
Distribution of patients according to Prakriti.
| Treatment Group (n = 31) (%) | Placebo Group (n = 31) (%) | |
|---|---|---|
| Kaphapradhan | 13 (41.96%) | 19 (61.29%) |
| Pittapradhan | 9 (29.03%) | 8 (25.80%) |
| Vatapradhan | 9 (29.03%) | 4 (12.90%) |
3.2. Effect on efficacy variables
3.2.1. IDRS score
Treatment with Nisha-Amalaki capsules improved the IDRS score significantly from the baseline values and also the Placebo group at 6 months (Table 3). Sub-analysis of the 2 modifiable risk factors viz. waist circumference and physical inactivity showed that there was a significant improvement in the scores of both these variables in the Treatment group which was not seen in the Placebo group at the end of the study (Table 3A, Table 3BA and 3B).
Table 3.
Effect on IDRS score.
| Sr. No. | Group | Day 0 | Day 180 | P value |
|---|---|---|---|---|
| IDRS score [Median (Range)] | ||||
| 1 | Treatment Group (n = 30) | 70 (60–80) | 60 (50–80) @@,## | 0.0026, 0.0016 |
| 2 | Placebo Group (n = 28) | 70 (60–90) | 70 (50–90) | 0.11 |
Result expressed as Median (Range).
@@p < 0.01 as compared to Day 0 using Wilcoxon matched-pairs signed-ranks test.
##p < 0.01 as compared to Group B (Placebo) using Mann–Whitney test.
Table 3A.
Effect on Waist Circumference score.
| Sr. No. | Group | Day 0 | Day 180 | P value |
|---|---|---|---|---|
| Waist Circumference [Median (Range)] | ||||
| 1 | Treatment Group (n = 30) | 10 (0–20) | 0 (0–20) @@@,### | 0.0016, 0.0004 |
| 2 | Placebo Group (n = 28) | 10 (0–20) | 10 (0–20) | 0.999 |
Result expressed as Median (range).
@@@p < 0.001 as compared to Day 0 using Wilcoxon matched-pairs signed-ranks test.
###p < 0.001 as compared to Group B (Placebo) using Mann–Whitney test.
Table 3B.
Effect on Physical activity.
| Sr. No. | Group | Day 0 | Day 180 | P value |
|---|---|---|---|---|
| Physical activity [Median (Range)] | ||||
| 1 | Treatment Group (n = 30) | 20 (10–30) | 20 (0–20) @@@,# | <0.001, 0.0318 |
| 2 | Placebo Group (n = 28) | 20 (10–30) | 20 (10–30) | 0.125 |
Result expressed as Median (range).
@@@p < 0.001 as compared to Day 0 using Wilcoxon matched-pairs signed-ranks test.
#p < 0.05 as compared to Group B (Placebo) using Mann–Whitney test.
3.2.2. Body weight and BMI
Significant reduction in body weight and BMI was observed in the Treatment group although it was not statistically significant with respect to the Placebo group (Table 4).
Table 4.
Effect on body weight & BMI.
| Group | Day 0 | Day 180 | P value |
|---|---|---|---|
| Body weight [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 69.45 ± 12.52 (64.85–74.04) | 67.87 ± 12.40 (63.24–72.45)### | 0.0003 0.83 |
| Placebo Group (n = 28) | 68.21 ± 8.59 (64.88–71.55) | 68.46 ± 8.54 (65.15–7177) | 0.53 |
| BMI [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 28.61 ± 4.62 (26.92–30.31) | 27.14 ± 6.71 (24.67–29.60)### | 0.0006 0.97 |
| Placebo Group (n = 28) | 27.99 ± 4.20 (26.36–29.61) | 28.08 ± 4.08 (26.45–29.66) | 0.54 |
Results are expressed as Mean ± SD (95% Confidence Interval).
###p < 0.001 as compared to Day 0 using paired Student's ‘t’.
3.2.3. Laboratory variables
Significant improvement in the laboratory-based efficacy variables was observed in the Treatment group with respect to their baseline values. A significant fall in fasting blood glucose was observed in the Treatment group as compared to their baseline values at Day 90 & Day 180 and in comparison to the Placebo group. A fall in the 2 h post OGTT blood glucose values was seen at Day 180 in the Treatment group as compared to the baseline values & Placebo group, although the fall was significant only at Day 180. Similarly, a significant lowering of fasting and post 2-h OGTT insulin levels was observed in the Treatment group at Day 180 with respect to the baseline values and the Placebo group. Fasting and 2 h post OGTT insulin values in the Placebo group in fact showed a rise in the insulin values. HbA1c values also showed a significant decrease in patients receiving the study treatment as compared to their baseline values whereas HbA1c values in the Placebo group showed a rise at 6 months. The effect of the formulation on insulin resistance was also assessed using the HOMA-IR ratio. It was observed that there was a significant improvement in the HOMA-IR ratio post treatment with Nisha-Amalaki capsules which statistically lower than that seen in the Placebo group.
Improvement in the lipid profile was seen in patients receiving the study treatment as compared to their baseline values with special reference to Total Cholesterol & HDL-Cholesterol. However, this improvement was not statistically significant.
Treatment with Nisha-Amalaki modulated the values of the oxidative markers, wherein a significant fall in the pro-oxidant marker MDA and similar improvement in the antioxidant marker, SOD was seen in the Treatment group as compared to their baseline values & the Placebo group. Similarly, improvement in the inflammatory C-reactive protein values was observed in the Treatment group which was significantly greater than that seen in the Placebo group. The results are summarized in Table 5.
Table 5.
Effect on laboratory efficacy variables
| Fasting Blood Sugar [Mean ± SD (CI)] | ||||
|---|---|---|---|---|
| Day 0 | Day 90 | Day 180 | P values | |
| Treatment Group (n = 30) | 121.37 ± 13.13 (116.47–126.28) | 115.22 ± 12.03 (110.73-119.71)∗∗ | 109.88 ± 12.06 (105.34-114.34) ∗∗∗,@@@ | 0.001, 0.0001 |
| Placebo Group (n = 28) | 116.74 ± 6.29 (115.90–127.80) | 121.34 ± 12.32(116.56-126.11)∗ | 123.83 ± 11.24 (119.47-128.19)∗∗ | 0.05,0.001 |
| Post 2 h OGTT Blood Sugar [Mean ± SD (CI)] | ||||
| Day 0 | Day 90 | Day 180 | ||
| Treatment Group (n = 30) | 164.82 ± 41.96 | 163.04 ± 43.89 | 150.12 ± 31.87 | 0.04 |
| (149.15–180.48) | (146.66–179.42) | (138.22–162.01)∗ | 0.13 | |
| Placebo Group (n = 28) | 159.69 ± 19.51 | 157.33 ± 20.87 | 161.39 ± 23.0 | 0.43 |
| (152.12–167.26) | (149.24–165.42) | (152.47–170.31) | ||
| Fasting Insulin [Mean ± SD (CI)] | ||||
| Day 0 | Day 180 | |||
| Treatment Group (n = 30) | 18.38 ± 8.38 (15.25–21.51) | 14.09 ± 6.77 (11.57–16.62)###, @@ | 0.005 | 0.01 |
| Placebo Group (n = 28) | 15.11 ± 3.77 (13.64–16.56) | 17.42 ± 3.99 (15.88–18.97)### | 0.0002 | |
| Post 2 h OGTT Insulin [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 45.96 ± 18.29 (39.13–52.78) | 35.75 ± 19.15 (28.6–42.9)### | 0.0008 | 0.25 |
| Placebo Group (n = 28) | 41.14 ± 12.51 (36.28–45.98) | 40.72 ± 12.66 (35.81–45.63) | 0.88 | |
| HOMA-IR [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 5.70 ± 3.06 (4.56–6.85) | 3.88 ± 2.06 (3.11–4.65)###,@@ | 0.009 | |
| 0.003 | ||||
| Placebo Group (n = 28) | 4.38 ± 1.24 (3.90–4.87) | 5.39 ± 1.56 (4.78–5.99)# | 0.01 | |
| HbA1c [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 6.14 ± 0.37 (5.99–6.27) | 6.10 ± 0.35 (5.96–6.22)# | 0.04 | |
| 0.5 | ||||
| Placebo Group (n = 28) | 6.11 ± 0.23 (6.02–6.20) | 6.15 ± 0.29 (6.04–6.27) | 0.12 | |
| Total Cholesterol [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 176.65 ± 34.65 (163.72–189.59) | 165.95 ± 28.16 (155.44–176.46) | 0.194 | |
| Placebo Group (n = 28) | 191.61 ± 30.54 (179.77–203.45) | 190.23 ± 25.52 (180.33–200.12) | 0.855 | |
| Triglycerides [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 128.62 ± 60.24 (106.13–151.12) | 119.23 ± 54.54 (98.87–139.59) | 0.529 | |
| Placebo Group (n = 28) | 132.78 ± 30.39 (121–144.56) | 133.31 ± 28.01 (122.44–144.17) | 0.946 | |
| HDL Cholesterol [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 42.81 ± 8.56 (39.61–46.01) | 46.09 ± 7.79 (43.19–49) | 0.126 | |
| Placebo Group (n = 28) | 41.08 ± 8.08 (37.95–44.21) | 42.16 ± 7.73 (39.16–45.16) | 0.612 | |
| LDL Cholesterol [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 118.43 ± 27.56 (108.14–128.72) | 113.10 ± 26.94 (103.04–123.16) | 0.452 | |
| Placebo Group (n = 28) | 121.66 ± 24.41 (112.19–131.12) | 122.19 ± 21.47 (113.57–130.52) | 0.931 | |
| VLDL Cholesterol [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 25.73 ± 12.05 (21.23–30.22) | 23.85 ± 10.91 (19.77–27.92) | 0.529 | |
| Placebo Group (n = 28) | 26.56 ± 6.08 (24.20–28.91) | 26.66 ± 5.60 (24.49–28.83) | 0.946 | |
| Oxidative stress marker – MDA [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 3.81 ± 1.08 (3.41–4.22) | 2.4 ± 1.05 (2.01–2.79)###,@@@ | 0.0001 | |
| 0.0001 | ||||
| Placebo Group (n = 28) | 3.85 ± 1.49 (3.27–4.42) | 3.77 ± 1.18 (3.31–4.23) | 0.66 | |
| Oxidative stress marker – SOD [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 0.95 ± 0.45 (0.79–1.12) | 2.31 ± 1.12 (1.89–2.73)###,@@@ | 0.0001, 0.0001 | |
| Placebo Group (n = 28) | 0.91 ± 0.43 (0.74–1.08) | 1.11 ± 0.52 (0.91–1.32)## | 0.001 | |
| C-reactive protein (CRP) [Mean ± SD (CI)] | ||||
| Treatment Group (n = 30) | 3.79 ± 3.27 (2.57–5.01) | 3.35 ± 3.16 (2.18–4.53)#,@@ | 0.08 | |
| 0.003 | ||||
| Placebo Group (n = 28) | 5.33 ± 2.72 (4.28–6.39) | 5.29 ± 3.15 (4.07–6.52) | 0.86 | |
Results are expressed as Mean ± SD (95% Confidence Interval).
∗p < 0.05, ∗∗p < 0.01 & ∗∗∗p < 0.001 as compared to Day 0 using Repeated Measures Analysis of Variance with Tukey–Kramer Multiple Comparisons test.
#p < 0.05, ##p < 0.01 & ###p < 0.001 as compared to Day 0 using paired Student's ‘t’ test in case of parametric data or Wilcoxon matched-pairs signed-ranks test in case of non-parametric data.
@p < 0.05, @@ p < 0.01 & @@@p < 0.001 as compared to Placebo Group using unpaired Student's ‘t’ test in case of parametric data or Mann–Whitney test in case of non-parametric data.
3.2.4. Ayurvedic symptom score
Significant improvement was also noted in the various ayurvedic symptoms as compared to the baseline score in patients receiving the study medications. This improvement was significantly better than that seen in patients receiving Placebo with respect to certain parameters viz. excessive thirst, burning sensation, excessive sweating, laziness, flabbiness and polyuria.
3.2.5. Quality-of-life score (SF-36)
Statistically significant improvement in the Quality-of-Life scores was seen in patients who received Nisha-Amalaki capsules compared to those who received placebo with respect to the overall Quality of Life, energy levels, emotional well-being, social functioning, improvement in pain score and General Health aspects (refer Table 6).
Table 6.
Effect on QOL (SF-36).
| Group | Day 0 | Day 180 | P value |
|---|---|---|---|
| Overall Quality of Life [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 2206 ± 584.93 (1987.6–2424.4) | 2527 ± 611.69 (2298.6–2755.4)∗, @@@ | 0.042 0.0011 |
| Placebo Group (n = 28) | 2162.14 ± 436.75 (1992.8–2331.5) | 2050 ± 423.58 (1885.7–2214.3) | 0.33 |
| Physical function [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 67.33 ± 22.70 (58.86–75.81) | 75.83 ± 21.41 (67.94–83.72) ∗∗∗ | 0.0003 0.07 |
| Placebo Group (n = 28) | 69.64 ± 19.15 (62.22–77.07) | 65.18 ± 23.63 (56.07–74.34)∗ | 0.03 |
| Role limitations due to physical health [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 53.89 ± 39.86 (39.01–68.77) | 63.33 ± 38.13 (49.10–77.57) ∗ | 0.05 0.23 |
| Placebo Group (n = 28) | 53.57 ± 37.09 (39.19–67.96) | 51.79 ± 35.96 (37.84–65.73) | 0.57 |
| Role limitations due to emotional problems [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 58.89 ± 40.76 (43.67–74.11) | 66.67 ± 38.16 (52.42–80.91) | 0.16 0.18 |
| Placebo Group (n = 28) | 50 ± 37.95 (35.28–64.72) | 53.57 ± 36.67 (39.35–67.79) | 0.38 |
| Energy [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 61.22 ± 22.28 (52.90–69.54) | 66.67 ± 20.14 (59.15–74.19) ∗∗∗,@ | 0.009 0.02 |
| Placebo Group (n = 28) | 59.64 ± 15.57 (53.61–65.68) | 56.07 ± 15.60 (50.02–62.12)∗ | 0.05 |
| Emotional well-being [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 61.07 ± 21.55 (53.02–69.11) | 69.60 ± 18.09 (62.85–76.36) ∗∗∗,@@@ | 0.0003 0.0003 |
| Placebo Group (n = 28) | 56.86 ± 17.77 (49.97–63.75) | 51.43 ± 17.48 (44.65–58.21)∗∗∗ | 0.001 |
| Social Functioning [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 68.17 ± 20.08 (60.67–75.66) | 73.58 ± 23.16 (64.94–82.23) @@@ | 0.10 0.0006 |
| Placebo Group (n = 28) | 60.71 ± 16.91 (54.16–67.27) | 56.25 ± 16.84 (49.72–62.78) | 0.07 |
| Pain [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 73.25 ± 24.12 (64.25–82.25) | 80.5 ± 19.98 (73.04–87.96) ∗∗,@@@ | 0.01 0.0001 |
| Placebo Group (n = 28) | 58.93 ± 19.38 (51.41–66.45) | 56.52 ± 17.56 (49.71–63.33) | 0.39 |
| General health [Mean ± SD (CI)] | |||
| Treatment Group (n = 30) | 53.33 ± 18.82 (46.31–60.36) | 64.5 ± 20.31 (56.92–72.09)∗∗∗,@ | 0.001 0.02 |
| Placebo Group (n = 28) | 56.07 ± 15.95 (49.89–62.26) | 52.86 ± 17.82 (45.95–59.77) | 0.18 |
Results are expressed as Mean ± SD (95% Confidence Interval).
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as compared to Day 0 using paired Student's ‘t’ test in case of parametric data or Wilcoxon's matched pairs test in case of non-parametric data.
@p < 0.05, @@p < 0.01, @@@p < 0.001 as compared to Placebo Group using unpaired Student's ‘t’ test in case of parametric data or Mann–Whitney test in case of non-parametric data.
3.3. Safety assessment
Patients' safety was assessed using medical history, clinical examination and laboratory reports. One patient from the Treatment Group refused to further continue in the study due to complaints of constipation after consuming the study medication for 7 days. There were no significant alterations in the laboratory safety parameters in both study groups.
4. Discussion
Prediabetes, the asymptomatic precursor to the diabetic condition is not without its own problems. The annual conversion rate from prediabetes to diabetes risk is as high as 5%–10% making it a high-risk condition [50]. It is postulated that 1 in 3 American adults have prediabetes but nearly 80% of these individuals are not be aware of it putting them at high risk of progressing to the diabetic state [5]. National Family Health Survey-4 (NFHS-4) carried out from 2015 to 2016 showed that the prevalence of prediabetes among Indian was 5.57% [51]. People with prediabetes are at increased risk of developing many of the diabetic complications viz. diabetic retinopathy, neuropathy, nephropathy and macrovascular complications if the condition is left untreated [52]. The main approaches to prevent progression to T2DM is through lifestyle interventions, physical activity, weight loss and lastly, pharmacotherapy [53].
This proprietary Ayurvedic formulation, Nisha-Amalaki capsules (EmbliQur) is a combination of two Ayurvedic botanicals- Amalaki and Turmeric in a ratio of 2:1 respectively. Both Nisha and Amalaki, individually or in combination with other plants, have been shown to have antihyperglycemic, antihyperlipidemic, anti-inflammatory and antioxidant properties [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37],54,55].
As per Ayurvedic literature, Nisha (C. longa) is Laghu and Ruksha in nature, Tikta (bitter), Katu (pungent) in taste, Katu vipaka and Ushna veerya. These attributes help decrease the kapha and kleda in the body and improves glucose metabolism. Amalaki also has anti-diabetic properties. The combination of Nisha and Amalaki has tridoshahara property which helps normalize the altered kapha, meda and kleda. Due to deepana and pachana properties of the combination, it improves the digestive capacity of the body (Agni), which helps improve altered glucose metabolism. Due to its varied properties, this formulation is used in the P. poorva-rupa stage of the disease for Samprapti Bhanga as it helps in protecting against progression to Prameha [16].
This study was conducted to see if Nisha-Amalaki capsules would help delay prediabetes from turning into diabetes. Of the 62 patients enrolled in the study, 58 completed the study, 30 in the Nisha-Amalaki group & 28 in the Placebo group.
As per Ayurvedic texts, individuals are susceptible to different diseases based on their prakriti. We assessed the prakriti of the study participants to evaluate the prakriti-wise predominance in the prediabetic condition. We observed that of the 62 recruited participants, 32 were from Kaphapradhan prakriti, 17 were from Pittapradhan prakriti and 13 were from Vatapradhan Prakriti. Our results were similar to that reported by Gupta et al. [56].
The IDRS scale is a non-invasive scoring method that is used as a primary screening tool for diabetes. It consists of 2 non-modifiable risk factors like age and family history and 2 modifiable ones like abdominal obesity and physical activity to assess the risk. A score of 60 and above indicates high risk for diabetes [9]. In our study, all the patients had IDRS scores above 60, however following treatment with Nisha-Amalaki capsules, a fall in the IDRS score was noted in these patients at 6 months which was highly significant as compared to the scores noted in patients who received Placebo. It was noted that reduction in the scores of the 2 non-modifiable risk factors was the main driving factors in the overall reduction of the IDRS score. Although the mean IDRS score did not fall below the cutoff score of 60 which would have indicated a lowering of the risk for diabetes from high to medium risk, this was possibly due to the short duration of the study i.e. 6 months. A longer duration of treatment with the study medications would probably lower the risk further. This lowering of the IDRS score with the study medication is efficacious as the same degree of fall of the IDRS score was not observed in the Comparator group.
Significant improvement was also observed in the laboratory parameters in the treatment group on Day 180 viz. fasting and post 2 h OGTT blood sugar, fasting and post 2 h OGTT insulin, HbA1c, oxidative stress markers (MDA & SOD) and C-reactive protein (CRP) levels.
The HOMA-IR ratio is used to estimate the level of insulin resistance by applying fasting plasma insulin and fasting plasma glucose values in a formula ((FPI × FPG)/22.5) [56]. Insulin secretion is dependent on the pancreatic β-cell response to blood glucose concentrations. This in turn is regulated by insulin-mediated glucose production in the liver. Thus, altered functioning of the β-cells will lead to decreased insulin secretion when stimulated by blood glucose levels. Improvement in the HOMA-IR ratio indicates a decrease in insulin resistance which in turn indicates a slowing in the progression to the diabetic state. In our study, a significant improvement in the HOMA-IR ratio in the Nisha-Amalki group was observed, which was better than the Placebo group at 6 months.
Thus, the study results confirm that the formulation does have anti-hyperglycemic effect with insulin sensitizing effect. C. longa and curcumin have been shown to lower the inflammatory response to hyperglycemia, improve pancreatic β-cell function resulting in an increase in pancreatic secretion of insulin, thus improving the pre-diabetic state [57]. E. officinalis fruit has demonstrated its efficacy in lowering hyperglycemia and decreasing oxidative stress in both preclinical and clinical studies [58]. The anti-diabetic property of Amalaki has been attributed to its constituents viz. ellagic acid which stimulates the pancreatic β-cells to secrete insulin [59] and gallic acid, which has been shown to up-regulate pAkt, PPAR-γ and Glut4 pathways thus improving insulin sensitivity [43]. Both these plants have also shown to have anti-oxidant and anti-inflammatory properties, which explains the improvement in the oxidative stress markers and CRP levels [21,29].
Improvement in the various parameters of the Ayurveda symptom score was also observed following treatment with Nisha-Amalaki capsules which was significantly better in comparison to Placebo. Symptoms like Atitrishna, Atimutrata, Hasta-Pada Daha, Atisveda, Alasya and Shithilangata showed greater improvement. These symptoms are similar to the diabetic symptoms described in modern medicine and improvement in these symptoms corelated with improvement seen in HbA1c, glucose and insulin levels in patients receiving Nisha-Amalaki capsules. Significant improvement in the Quality-of-Life scores was also observed in the Nisha-Amalaki group indicating that treatment with the study medications helped improve the overall Quality of Life in these patients.
A clinical study conducted by Damle et al to assess the efficacy of Nisha-Amalaki churna in newly diagnosed patients of T2DM showed significant improvement in subjective parameters like polyuria, polydipsia, polyphagia and fatigue following therapy for 3 months [60]. Significant improvement in fasting and post prandial blood glucose levels and HBA1c values was observed following treatment with Nisha-Amalaki churna. Tarpe et al. reported that giving Nisha-Amalaki after Vaman karma provided significant relief in the signs and symptoms associated with diabetes and also a decrease in both fasting and post prandial blood sugar levels [61]. A case report by Wale et al. documented the significant lowering of fasting blood and post prandial blood sugar levels and HbA1c levels in a patient with uncontrolled diabetes who was administered Nisha-Amalaki churna for 8 weeks [62]. A randomized placebo controlled clinical trial of ‘Nisha Amalaki’ conducted on 50 patients (25 in each group) for a period of 6 weeks wherein patients were administered Nisha Amalaki in a dose of one gram twice daily along with diet control. These patients showed significant improvement in their symptoms along with lowering of blood glucose levels as compared to the Placebo group [63]. GC Nanda et al. demonstrated that Nisha Amalaki when given in a dose of 1 gm twice a day for 6 weeks in 100 diabetic patients (Madhumeha) in an open labeled clinical trial showed moderate hypoglycemic effect. There was a reduction in fasting blood sugar level and clinical symptoms of Madhumeha [64]. Samagandhi et al. conducted a clinical study comparing the hypoglycemic effect of wheatgrass juice, Nisha Amalaki and their combination in 30 diabetic patients over a period of 2 months. It was seen that Nisha-Amalaki significantly relieved the ayurvedic symptoms and fasting and post prandial blood sugarswhen given per se and in combination with wheatgrass juice [65]. A case series by Bhat et al. has shown that Nisha Amalaki given to 3 pregnant women diagnosed with gestational diabetes along with a diabetic diet and Asnadi Gana Kashaya helped control the blood sugar levels during the gestation period and preventing any diabetic complication both in mother and baby [66].
A comparison with similar other studies done in prediabetic patients showed that lifestyle modification (LSM) did cause weight reduction and lowering of fasting blood sugar and HbA1c levels. Addition of metformin however significantly lowered the HbA1c levels compared to the LSM arm [67]. A study by Ramachandran et al. showed that it was possible to prevent diabetes in native Asian Indian patients with IGT using lifestyle modification, despite their relatively low BMI and highly insulin-resistant characteristics. Metformin was also effective when given in at a low dose (500 mg/day). The results also showed there was no additional benefit seen by combining metformin with LSM [68]. A systematic review by AK Jenum et al. showed a 35% relative reduction and 7.4% absolute reduction in the incidence of diabetes among individuals of South Asian origin receiving lifestyle modification interventions [69].
Chinese herbal medicines (CHMs) have been shown to be effective in the treatment of prediabetes. A systematic review by Song et al. has shown that CHMs reduced the incidence of diabetes, increase the incidence of normalization of prediabetes, and lowered BMI, FPG, 2hPG, triglycerides & cholesterol levels, however there was no significant lowering of HbA1c [70].
Although different ayurvedic plants/formulations have been used in the management of type 2 Diabetes mellitus, there is limited literature on their potential in the prediabetic condition. Gangwar & Pandey studied the efficacy of Darvyadi Ghana Vati, a classical Ayurvedic formulation in 60 prediabetic patients in comparison with Metformin for a period of 3 months. They found a significant reduction in fasting and postprandial sugar levels & HbA1c in both groups post treatment with comparable results in both groups, indicating that this formulation can be used as an adjuvant to Metformin [71]. Gaddam et al. demonstrated that dietary supplementation with Fenugreek powder, 5 g twice a day before meals, given to prediabetic patients for 3 years resulted in significant reduction in fasting plasma glucose (FPG), postprandial plasma glucose (PPPG) and LDL cholesterol with a significant increase in serum insulin. It was observed that controls had 4.2 times higher chance of developing diabetes compared to those in the Fenugreek group. The outcome of diabetes in Fenugreek group was positively associated with serum insulin and negatively associated with insulin resistance (HOMA IR) [72]. Pickering et al. conducted an exploratory placebo-controlled comparative clinical study to assess the safety and efficacy of a specialized Trigonella foenum-graceum L. seed extract in supporting blood glucose metabolism in a pre-diabetic cohort of 54 participants when given for 12 weeks. There was a significant difference in FBG, PPBG and triglycerides between the 2 treatment groups, with no changes in HbA1c, fasting and PP Insulin or C-peptide. There was no difference in total cholesterol, LDL & HDL cholesterol or CRP [73]. A clinical trial found that a mix of polyherbal combination (Tinospora cordifolia, Pterocarpus marsupium, Gymnema sylvestre, Zingiber officinale and Momordica charantia) and lifestyle changes helped prevent prediabetes from turning into diabetes, better than lifestyle changes alone [74]. A case report by Mishra & Tomar showed that administration of Darviyadi capsules (containing the aqueous extracts of Devdaru, Daru haldi, Nagarmotha and Triphala) for 90 days in a 40-year-old lady with prediabetes resulted in weight reduction, improvement of BMI and fasting and postprandial blood sugar levels [75].
Our study has its limitations, mainly the small sample size and short study duration. However, this being a proof of concept study, we aimed at explore the efficacy of this formulation and the feasibility of performing a long-term study. A longer duration study would probably help understand the sustained effect of this formulation in lowering the risk score and preventing progression from prediabetes to diabetes.
5. Conclusion
Thus, the study medication viz. Nisha-Amalaki capsules were effective in improving the clinical status as well as the laboratory-based parameters viz. HbA1c, fasting and post OGTT glucose as well as insulin levels of pre-diabetic patients. Improvement in the insulin sensitivity was also seen as also the antioxidant and anti-inflammatory status. Treatment with this medication also improved the clinical symptoms as per the Ayurvedic symptoms score and quality of life over a period of 6 months indicating that Nisha-Amalaki may play a role in delaying the progression to the diabetic state. Thus, Nisha-Amalaki capsules can be used as prophylactic therapy in prediabetics as an adjuvant to lifestyle modification strategies.
CRediT author statement
RM: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing original draft, Writing - Review & Editing, Visualization, Project Supervision, Project administration. SK: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing - Review & Editing, Visualization, Project Supervision. DK: Methodology, Formal analysis, Investigation, Writing original draft, Visualization, Project administration. AD: Conceptualization, Funding acquisition. LH: Conceptualization, Funding acquisition.
Funding
This study was funded by Pharmanza Herbal Pvt Ltd.
Declaration of competing interest
The authors declare conflict of interest with regard to financial support received for the study.
Acknowledgement
Dr Harsha Khodke & Dr Prerna Hosmani for the participant enrollment and study conduct.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2023.100806.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tabak A.G., Herder C., Rathmann W., Brunner E.J., Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization/International Diabetes Federation Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of the WHO/IDF consultation. http://www.who.int/diabetes/publications/ Definition and diagnosis of diabetes_new.pdf Available at:
- 3.American Diabetes A2 Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 4.Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. doi: 10.1186/s40842-019-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention National diabetes Statistics report website. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 6.Chandrupatla S.G., Khalid I., Muthuluri T., Dantala S., Tavares M. Diabetes and prediabetes prevalence among young and middle-aged adults in India, with an analysis of geographic differences: findings from the National Family Health Survey. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020065. https://doi:10.4178/epih.e2020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannick B., Wynn A., Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med. 2016;241(12):1323–1331. doi: 10.1177/1535370216654227. https://doi:10.1177/1535370216654227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards C.M., Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin N Am. 2016;45(4):751–764. doi: 10.1016/j.ecl.2016.06.007. https://doi:10.1016/j.ecl.2016.06.007 PMID: 27823603. [DOI] [PubMed] [Google Scholar]
- 9.Dudeja P., Singh G., Gadekar T., Mukherji S. Performance of Indian Diabetes Risk Score (IDRS) as screening tool for diabetes in an urban slum. Med J Armed Forces India. 2017;73(2):123–128. doi: 10.1016/j.mjafi.2016.08.007. http://doi:10.1016/j.mjafi.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V., Deepa R., Deepa M., Somannavar S., Datta M. A simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J Assoc Phys India. 2005;53:759–763. [PubMed] [Google Scholar]
- 11.Dugg P., Cherian V., Upadhyay M.K. Opportunistic screening for diabetes using Indian diabetes risk Score among patients aged 30 years and above attending rural health training center in Delhi. Int J Med Sci Publ Health. 2019;8(4):264–269. [Google Scholar]
- 12.Garde G.K. Chapter 10, Verse 2-3. Chaukhamba Surabharati Prakashan; Varanasi, Revised edition: 2012. Sarth vagbhat of maharshi vagbhat, sutrasathana; pramehanidanam; p. 190. [Google Scholar]
- 13.Sharma P. Chapter 6, Verse 4. Chaukhamba Orientalia; Varanasi, Reprint edition: 2011. Charaksamhita Part II, chikitsasathana; Prameha chikitsa; p. 117. [Google Scholar]
- 14.Rastogi S., Singh N., Gutch M., Bhattacharya A. Predicting and preventing diabetes: translational potential of Ayurveda information on pre-diabetes. J Ayurveda Integr Med. 2021 Oct-Dec;12(4):733–738. doi: 10.1016/j.jaim.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta Shastri Ambika. 13th ed. Chaukhamba Sanskrit Publication Varanasi; 2002. Sushrut samhita of sushrut, sootra sthana; p. 94. [Chapter 21; Verse 37] [Google Scholar]
- 16.Garde G.K. Chapter 12, Verse 5. Chaukhamba Surabharati Prakashan; Varanasi, Revised edition: 2012. Sarth vagbhat of maharshi vagbhat, chikitsa sthana; pramehachikitsitam; p. 287. [Google Scholar]
- 17.Gogte V.M. Ch.118, 1st ed. Bhartiya Vidya Bhavan; Mumbai: 2000. Ayurvedic pharmacology and therapeutic uses of medicinal plants (dravyagunavignyan), Part II; p. 514. [Google Scholar]
- 18.Chunekar K.C., Pandye G.S. 1st ed. Chukhambha Bharti Academy; Varanasi: 2002. Bhavprakash nighantu of sri bhavamishra, haritakyadi varga (shlok 196-197) p. 114. [Google Scholar]
- 19.Gogte V.M. Ch.12, 1st ed. Bhartiya Vidya Bhavan; Mumbai: 2000. Ayurvedic pharmacology and therapeutic uses of medicinal plants (dravyagunavignyan), Part II; p. 311. [Google Scholar]
- 20.Chunekar K.C., Pandye G.S. 1st ed. Chukhambha Bharti Academy; Varanasi: 2002. Bhavprakash nighantu of sri bhavamishra, haritakyadi varga, (shlok 39) p. 10. [Google Scholar]
- 21.Srivastava B.B.L., Ripanda A.S., Mwanga H.M. Ethnomedicinal, phytochemistry and antiviral potential of turmeric (Curcuma longa) Compounds. 2022;2:200–221. doi: 10.3390/compounds2030017. [DOI] [Google Scholar]
- 22.Zhang D.W., Fu M., Gao S.H., Liu J.L. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/636053. https://doi:10.1155/2013/636053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama T., Mae T., Kishida H., Tsukagawa M., Mimaki Y., Kuroda M., et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53(4):959–963. doi: 10.1021/jf0483873. http://doi:10.1021/jf0483873 [DOI] [PubMed] [Google Scholar]
- 24.Kim H.S., Hwang Y.C., Koo S.H., Park K.S., Lee M.S., Kim K.W., et al. PPAR-gamma activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic beta-cells. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y.S., Su Y.F., Yang H.W., Lee Y.H., Chou J.I., Ueng K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother Res. 2014;28(12):1770–1777. doi: 10.1002/ptr.5197. https://doi:10.1002/ptr.5197 [DOI] [PubMed] [Google Scholar]
- 26.Adab Z., Eghtesadi S., Vafa M., Heydari I., Shojaei A., Haqqani H., et al. Effect of turmeric on body measurement indices, glycemic condition, and lipid profile in hyperlipidemic patients with type 2 diabetes. Iran J Nutr Sci Food Technol. 2013;8(3):217–227. http://nsft.sbmu.ac.ir/article-1-1440-en.html URL. [Google Scholar]
- 27.Na L.X., Li Y., Pan H.Z., Zhou X.L., Sun D.J., Meng M., et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. 2013;57(9):1569–1577. doi: 10.1002/mnfr.201200131. https://doi:10.1002/mnfr.201200131 [DOI] [PubMed] [Google Scholar]
- 28.Chuengsamarn S., Rattanamongkolgul S., Phonrat B., Tungtrongchitr R., Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25(2):144–150. doi: 10.1016/j.jnutbio.2013.09.013. http://doi:10.1016/j.jnutbio.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 29.Variya B.C., Bakrania A.K., Patel S.S. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res. 2016;111:180–200. doi: 10.1016/j.phrs.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Yadav S.S., Singh M.K., Singh P.K., Kumar V. Traditional knowledge to clinical trials: a review on therapeutic actions of Emblica officinalis. Biomed Pharmacother. 2017;93:1292–1302. doi: 10.1016/j.biopha.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar M.S., Ramzan A., Ali A., Ahmad M. Effect of Amla fruit (Emblica ofcinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int J Food Sci Nutr. 2011;62(6):609–616. doi: 10.3109/09637486.2011.560565. http://doi:10.3109/09637486.2011.560565 [DOI] [PubMed] [Google Scholar]
- 32.Upadya H., Prabhu S., Prasad A., Subramanian D., Gupta S., Goel A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia BMC Compl Alternative. Med. 2019;19:27. doi: 10.1186/s12906-019-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. http://doi:10.1016/s0378-8741(02)00059-4 [DOI] [PubMed] [Google Scholar]
- 34.Huang H.Z., Qiu M., Lin J.Z., Li M.Q., Ma X.T., Ran F., et al. Potential effect of tropical fruits Phyllanthus emblica L. for the prevention and management of type 2 diabetic complications: a systematic review of recent advances. Eur J Nutr. 2021;60(7):3525–3542. doi: 10.1007/s00394-020-02471-2. [DOI] [PubMed] [Google Scholar]
- 35.Dawane J.S., Pandit V.A., Deshpande S.S., Mandpe A.S. Preventive and protective effect of Nishamalaki in STZ induced diabetic complications in wistar rats. J Clin Diagn Res. 2016;10:FF01. doi: 10.7860/JCDR/2016/17940.7912. http://doi:10.7860/JCDR/2016/17940.7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panda V., Deshmukh A., Singh S., Shah T., Hingorani L. An Ayurvedic formulation of Emblica officinalis and Curcuma longa alleviates insulin resistance in diabetic rats: involvement of curcuminoids and polyphenolics. J Ayurveda Integr Med. 2021;12(3):506–513. doi: 10.1016/j.jaim.2021.05.005. http://doi:10.1016/j.jaim.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao G., Bhat S., Rao G.S., Bhat G.P. Antidiabetic and antioxidant efficacy of a powdered mixture of Curcuma longa and emblica officinalis in diabetic rats in comparison with glyburide. Webmed Central Diabetes. 2013;4(2):WMC004065. http://doi:10.9754/journal.wmc.2013.004065 [Google Scholar]
- 38.American Diabetes Association - Prediabetes https://diabetes.org/diabetes/prediabetes
- 39.International Diabetes Federation – Diabetes Prevention https://idf.org/about-diabetes/diabetes-prevention
- 40.Jose J., Thomas N. How should one tackle prediabetes in India? Indian J Med Res. 2018 Dec;148(6):675–676. doi: 10.4103/ijmr.IJMR_1785_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Indian Council of Medical Research. National ethical guidelines for biomedical and health research involving human participants. Indian Council of Medical Research; New Delhi: 2017. Section 7 - clinical trials of drugs and other interventions.https://www.icmr.nic.in/sites/default/files/guidelines/ICMR_Ethical_Guidelines_2017.pdf Available from: [Google Scholar]
- 42.Yadav R.K., Mishra R., Chhipa R.P., Audichya K.C. Clinical trial of an indigenous compound drug Nishaamalaki in the management of Madhumeha vis-à-vis diabetes mellitus. Ancient Sci Life. 2001;XXI(1):18–24. [PMC free article] [PubMed] [Google Scholar]
- 43.Variya B.C., Bakrania A.K., Patel S.S. Antidiabetic potential of gallic acid from Emblica officinalis: improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine. 2020;73 doi: 10.1016/j.phymed.2019.152906. [DOI] [PubMed] [Google Scholar]
- 44.Garde G.K. Chapter 11, Verse 38-39. Chaukhamba Surabharati Prakashan; Varanasi, Revised edition: 2012. Sarth vagbhat of maharshi vagbhat, nidan sthana; pramehanidanm; p. 193. [Google Scholar]
- 45.Yadunandana Upadhyaya. Chapter 33, Verse 5. Chaukhamba Publications; New Delhi: 2002. Madhav nidanam of sri madhavakara, Part II, pramehanidanm; pp. 7–8. 32nd edition. [Google Scholar]
- 46.Bhalerao S., Deshpande T., Thatte U. Prakriti (Ayurvedic concept of constitution) and variations in platelet aggregation. BMC Compl Alternative Med. 2012;12:248. doi: 10.1186/1472-6882-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitehead A.L., Julious S.A., Cooper C.L., Campbell M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057–1073. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat. 2005;4(4):287–291. [Google Scholar]
- 49.Bell M.L., Whitehead A.L., Julious S.A. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol. 2018;10:153–157. doi: 10.2147/CLEP.S146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerstein H.C., Santaguida P., Raina P., Morrison K.M., Balion C., Hunt D., et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007 Dec;78(3):305–312. doi: 10.1016/j.diabres.2007.05.004. Epub 2007 Jun 29. PMID: 17601626. [DOI] [PubMed] [Google Scholar]
- 51.Chandrupatla S.G., Khalid I., Muthuluri T., Dantala S., Tavares M. Diabetes and prediabetes prevalence among young and middle-aged adults in India, with an analysis of geographic differences: findings from the National Family Health Survey. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020065. Epub 2020 Sep 18. PMID: 32972049; PMCID: PMC7871157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsilingiris D., Vallianou N.G., Dalamaga M. Prediabetes screening: questionable benefits in the golden years. Metabol Open. 2021; Apr 7;10 doi: 10.1016/j.metop.2021.100091. http://doi:10.1016/j.metop.2021.100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galaviz K.I., Narayan K.M.V., Lobelo F., Weber M.B. Lifestyle and the prevention of type 2 diabetes: a status report. Am J Lifestyle Med. 2015;;12(1):4–20. doi: 10.1177/1559827615619159. http://doi:10.1177/1559827615619159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antony B., Benny M., Kaimal T.N.B. A pilot clinical study to evaluate the effect of Emblica officinalis extract (Amlamax™) on markers of systemic inflammation and dyslipidemia. Indian J Clin Biochem. 2008;23(4):378–381. doi: 10.1007/s12291-008-0083-6. http://doi.10.1007/s12291-008-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marton L.T., Pescinini-E-Salzedas L.M., Camargo M.E.C., Barbalho S.M., Haber J.F.D.S., Sinatora R.V., et al. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front Endocrinol (Lausanne) 2021;12:669448. doi: 10.3389/fendo.2021.669448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. http://doi:10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 57.Ghorbani Z., Hekmatdoost A., Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metabol. 2014;12(4) doi: 10.5812/ijem.18081. http://doi:10.5812/ijem.18081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ansari A., Shahriar M.S., Hassan M.M., Das S.R., Rokeya B., Haque M.A., et al. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac J Tropical Med. 2014:21–25. doi: 10.1016/S1995-7645(13)60185-6. [DOI] [PubMed] [Google Scholar]
- 59.Fatima N., Hafizur R.M., Hameed A., Ahmed S., Nisar M., Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur J Nutr. 2017;56(2):591–601. doi: 10.1007/s00394-015-1103-y. http://doi:10.1007/s00394-015-1103-y [DOI] [PubMed] [Google Scholar]
- 60.Damle N. Dr. Clinical study to evaluate efficacy of Nisha Amalaki Churna in newly diagnosed prameha type ii Diabetes Mellitus. Indian J Appl Res. 2021;11(3):42–43. http://doi:10.36106/ijar [Google Scholar]
- 61.Tarpe S., Tarpe R., Bapardekar D. Clinical efficacy of vamana karma followed by nisha-amalaki yoga in sthula pramehi W.S.R. To type II diabetes mellitus. World J Pharmaceut Res. 2017;6(8):2504–2517. http://10.20959/wjpr20178-9222 [Google Scholar]
- 62.Wale Savita Arjun. Rashmi Patekar, Mohammed Nazir K.K. Nisha- amalaki in uncontrolled type 2 diabetes mellitus- A case report. Int J Ayurveda Pharma Res. 2022;10(3):56–61. [Google Scholar]
- 63.Yadav R.K., Mishra R., Chhipa R.P., Audichya K.C. Clinical trial of an indgenous compound drug nishaamalki in the management of madhumeha vis-à-vis diabetes mellitus. Ancient Sci Life. 2001;21(1):18–24. [PMC free article] [PubMed] [Google Scholar]
- 64.Nanda G.C., Chopra K.K., Sahu D.P., Padhi M.M. Nisha amalaki in madhumeha (niddm): a clinical study. Journal of Research in Ayurveda and Siddha. 1998;19(1–2):34–40. [Google Scholar]
- 65.Samagandi K., Sharma J., Kumar S., Tripathy T.B. A clinical study on the efficacy of wheat grass juice in Prameha. IRJP. 2012;3(4):194–199. [Google Scholar]
- 66.Bhat Gayathri N.V., Deepthi G.B. A rationale approach to gestational diabetes mellitus through Ayurveda - case series. J Ayurveda Integr Med Sci. 2021;6(2):257–264. [Google Scholar]
- 67.Kulkarni S., Xavier D., George B., Umesh S., Fathima S., Bantwal G. Effect of intensive lifestyle modification & metformin on cardiovascular risk in prediabetes: a pilot randomized control trial. Indian J Med Res. 2018 Dec;148(6):705–712. doi: 10.4103/ijmr.IJMR_1201_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V., et al. The Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 69.Jenum A.K., Brekke I., Mdala I., Muilwijk M., Ramachandran A., Kjøllesdal M., et al. Effects of dietary and physical activity interventions on the risk of type 2 diabetes in South Asians: meta-analysis of individual participant data from randomised controlled trials. Diabetologia. 2019 Aug;62(8):1337–1348. doi: 10.1007/s00125-019-4905-2. [DOI] [PubMed] [Google Scholar]
- 70.Song Y., Wang H., Qin L., Li M., Gao S., Wu L., et al. Efficiency and safety of Chinese herbal medicine in the treatment of prediabetes: a systemic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2020 Oct 14;2020 doi: 10.1155/2020/3628036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gangwar Anshu, Kr Ajai. Pandey, A clinical evaluation of Darvyadi Ghana Vati in the cases of prediabetes. International Journal Of Research In Medical Sciences And Technology (IJRMST) 2018;6:192–234. [Google Scholar]
- 72.Gaddam A., Galla C., Thummisetti S., Marikanty R.K., Palanisamy U.D., Rao P.V. Role of Fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J Diabetes Metab Disord. 2015;14:74. doi: 10.1186/s40200-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pickering E., Steels E., Rao A., Steadman K.J. An exploratory study of the safety and efficacy of a Trigonella foenum-graecum seed extract in early glucose dysregulation: a double-blind randomized placebo-controlled trial. Pharmaceutics. 2022 Nov 14;14(11):2453. doi: 10.3390/pharmaceutics14112453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakanekar A., Kohli K., Tatke P. Ayurvedic polyherbal combination (PDBT) for prediabetes: a randomized double blind placebo controlled study. J Ayurveda Integr Med. 2019;10(4):284–289. doi: 10.1016/j.jaim.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mishra Ratnapravha, Tomar Prajakta. An ayurvedic approach for management of pre diabetes: a case study. Annals of the Romanian Society for Cell Biology. 2021:2606. https://www.annalsofrscb.ro/index.php/journal/article/view/2798 Retrieved from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.