Key Points
Question
How many cancers were attributable to infections in the US in 2017?
Findings
In this meta-analysis, population attributable fractions for 31 infection-cancer pairs were calculated and 4.3% of the cancers diagnosed among adults in 2017 were found to be attributable to 8 infections; approximately half the cancers due to infections were attributable to the human papillomavirus. Among children, Epstein-Barr virus was responsible for 2.2% of cancers.
Meaning
Infections continue to represent important targets for cancer prevention efforts.
Abstract
Importance
Infections are largely modifiable causes of cancer. However, there remains untapped potential for preventing and treating carcinogenic infections in the US.
Objective
To estimate the percentage and number of incident cancers attributable to infections in the US among adults and children for the most recent year cancer incidence data were available (2017).
Data Sources
A literature search from 1946 onward was performed in MEDLINE on January 6, 2023, to obtain the data required to calculate population attributable fractions for 31 infection-cancer pairs. National Health and Nutrition Examination Survey data were used to estimate the population prevalence of hepatitis B and C viruses and Helicobacter pylori.
Study Selection
Studies conducted in the US or other Western countries were selected according to specific infection-cancer criteria.
Data Extraction and Synthesis
Data from 128 studies were meta-analyzed to obtain the magnitude of an infection-cancer association or prevalence of the infection within cancer cells.
Main Outcomes and Measures
The proportion of cancer incidence attributable to 8 infections.
Results
Of the 1 666 102 cancers diagnosed in 2017 among individuals aged 20 years or older in the US, 71 485 (4.3%; 95% CI, 3.1%-5.3%) were attributable to infections. Human papillomavirus (n = 38 230) was responsible for the most cancers, followed by H pylori (n = 10 624), hepatitis C virus (n = 9006), Epstein-Barr virus (n = 7581), hepatitis B virus (n = 2310), Merkel cell polyomavirus (n = 2000), Kaposi sarcoma–associated herpesvirus (n = 1075), and human T-cell lymphotropic virus type 1 (n = 659). Cancers with the most infection-attributable cases were cervical (human papillomavirus; n = 12 829), gastric (H pylori and Epstein-Barr virus; n = 12 565), oropharynx (human papillomavirus; n = 12 430), and hepatocellular carcinoma (hepatitis B and C viruses; n = 10 017). The burden of infection-attributable cancers as a proportion of total cancer incidence ranged from 9.6% (95% CI, 9.2%-10.0%) for women aged 20 to 34 years to 3.2% (95% CI, 2.4%-3.8%) for women aged 65 years or older and from 6.1% (95% CI, 5.2%-7.0%) for men aged 20 to 34 years to 3.3% (95% CI, 1.9%-4.4%) for men aged 65 years or older. Among those aged 19 years or younger, 2.2% (95% CI, 1.3%-3.0%) of cancers diagnosed in 2017 were attributable to Epstein-Barr virus.
Conclusions and Relevance
Infections were estimated to be responsible for 4.3% of cancers diagnosed among adults in the US in 2017 and, therefore, represent an important target for cancer prevention efforts.
This meta-analysis estimates the percentage and number of incident cancers attributable to infections in the US among adults and children for the most recent year cancer incidence data were available (2017).
Introduction
Public awareness of the association between infections and cancer is low.1 However, the strongest associations in cancer etiology involve infections.2,3 Infections associated with the most cancers globally are preventable (vaccination for human papillomavirus [HPV] and hepatitis B virus [HBV]) or treatable (direct-acting antivirals [DAAs] for hepatitis C virus [HCV] and antibiotic therapy for Helicobacter pylori).4 Although efforts to reduce the prevalence of these infections in the US are ongoing,5 there remains considerable untapped potential to prevent and treat carcinogenic infections.
Islami and colleagues6 estimated that 3.3% of cancers diagnosed in the US in 2014 among those aged 30 years or older were attributable to infections. However, this study did not include all carcinogenic infections or infection-associated cancers and excluded cancers diagnosed among those younger than 30 years, thereby providing an incomplete portrait of the infection-associated cancer burden in the US. Furthermore, in the more than 10 years since the International Agency for Research on Cancer (IARC) updated its assessment of infections,7 evidence has accumulated on the role of carcinogenic infections in additional cancers.8,9,10 Hence, the burden of infection-attributable cancer in the US is likely greater than previously estimated.6
Given the considerable potential for the prevention and treatment of carcinogenic infections and the lack of comprehensive estimates of the impact of infections on cancer incidence in the US, quantifying the infection-attributable cancer burden is a priority. In this study, we estimated the percentage and number of incident cancers attributable to infections among adults and children in the US for the most recent year cancer incidence data were available (2017).
Methods
Selection of Infections and Cancers
We included carcinogenic and probably carcinogenic infections and associated cancers with sufficient evidence according to the IARC, with several exceptions. First, we excluded parasitic infections (ie, Opisthorchis viverrini, Clonorchis sinensis, and Schistosoma hematobium) because they do not occur in endemic form in the US. Second, because only some non-Hodgkin lymphoma (NHL) subtypes demonstrate a relationship with HCV,11,12,13,14 NHL cases were restricted to those subtypes and not aggregated as a single entity. Third, we did not attribute cancers directly with HIV because HIV, through immunosuppression, amplifies the carcinogenic effects of infections (Epstein-Barr virus [EBV], HPV, and Kaposi sarcoma–associated herpesvirus) already included in this analysis.14 Fourth, we did not consider the role of HIV in cancer of the conjunctiva (a cancer associated with HIV but no other infection) because that cancer is very rare in the US, and we lacked the data required to examine that association.15 Fifth, we analyzed 6 additional infection-cancer pairs. We included intrahepatic bile duct cancer due to increased risks associated with HBV and HCV.16,17,18 We included 2 cancers in which EBV is believed to play an etiologic role: diffuse large B-cell lymphoma (DLBCL) and gastric cancer.10,19 We added H pylori and esophageal adenocarcinoma because several meta-analyses have reported an inverse association between that infection and cancer.20,21,22,23,24,25 Accounting for the protective effect of H pylori provides a more accurate estimate of the association of H pylori with cancer incidence. We included cancer of the larynx because there is broad support for the etiologic role of HPV in a small fraction of laryngeal cancers.26,27 Because EBV is associated with a proportion of lymphomas arising in children, the role of EBV in cancers diagnosed among children (aged ≤19 years) was analyzed.14 Because this study used publicly available and secondary data, it was exempt from research ethics board review.
Population Attributable Fractions
Population attributable fractions (PAFs) represent the proportion of cancer incidence associated with the exposure. Formula 1 requires the prevalence of the infection in the general population (Pe) and its relative risk (RR) associated with the cancer28; formula 2 can estimate PAFs using prevalence in cases (Pc) instead of Pe29; and formula 3 can be used when the attributable fraction in the exposed group approaches 1.0 (ie, when RRs or odds ratios [ORs] are high), such that the Pc ≈ PAF, and/or when mechanistic evidence exists for the role of the infection in cancer, thereby allowing the Pc to approximate PAF.14,30,31 We estimated PAFs for HBV, HCV, and H pylori via formula 1 and the remaining infections via formula 3. We report PAFs for 31 infection-cancer pairs.
The formulas for calculating PAFs for binary exposures are as follows:
PAF = Pe(RR − 1)/(1 + Pe[RR − 1]) (formula 1);
PAF = Pc([RR − 1]/RR) (formula 2); and
PAF = Pc (formula 3).
Data Acquisition and Selection
To obtain data for the PAF calculations, we searched IARC monographs14,32,33,34,35,36 and other PAF analyses,4,6,37,38,39,40 contacted experts, and performed a literature search to identify knowledge syntheses from which we could identify individual studies. The search was conducted in MEDLINE (1946-onward) on January 6, 2023 (eTable 1 in Supplement 1). For each included record, K.D.V.-A. or S.M. reviewed its references and the records obtained from a forward citation search. For EBV-attributable cancers among children, studies were identified from an ongoing systematic review by our team (PROSPERO protocol: CRD42021269730). For all infections, the cancer cases had to be primary, invasive, and not yet treated. We opted to select studies conducted in North America; however, obtaining relevant data for several infection-cancer associations (eg, H pylori, noncardia gastric cancer [NCGC], EBV, and DLBCL) necessitated the inclusion of studies conducted in other Western countries. The specific inclusion criteria for each infection-cancer pair are reported in the footnotes of eTables 4, 6, 7, and 9 to 22 in Supplement 1. Two of us (K.D.V.-A. and S.M.) extracted data and verified each other’s extractions. We contacted the authors of studies that met the inclusion criteria but that did not report the required data.
The National Health and Nutrition Examination Survey (NHANES) was the source of the HBV, HCV, and H pylori prevalence estimates because, when weighted, it is representative of the resident civilian noninstitutionalized US population.41 NHANES tested participants’ (aged ≥6 years) serum samples for hepatitis B surface antigen and anti-HCV antibodies. Samples testing positive or indeterminate for anti-HCV antibodies were tested for HCV RNA.42,43,44 To assess the prevalance of HBV and HCV with greater precision, we combined data from 6 cross-sectional NHANES cycles conducted from 1999 to 2010. H pylori serologic status was assessed via enzyme-linked immunosorbent assay in the 1999-2000 NHANES cycle. To minimize possible bias and maximize the available data, we performed multiple imputations with chained equations (25 imputed databases); the variables used in the imputation model are listed in eAppendix 5 in Supplement 1. For additional background and a methodological description of other infection-cancer pairs, please refer to eAppendix 2 and eAppendices 6 to 28 in Supplement 1.
Statistical Analysis
We used meta-analytic techniques to summarize the measure of association between a given infection and its cancer and the prevalence of an infection in a cancer. A fixed-effect model was adopted if the index of consistency (I2) was less than 25%. Data on the number of individuals testing positive and the number with valid results were used to calculate pooled prevalence estimates. Pooled prevalence estimates and exact 95% CIs were calculated via random effects with the DerSimonian and Laird method, in which the Freeman-Tukey double arcsine transformation was used to stabilize variance.45,46 When study authors did not provide the RR, OR, or 95% CIs for Pc, OpenEpi, version 3.0147 was used to calculate these estimates. Analyses of NHANES data and meta-analyses were conducted in Stata/SE, version 17 (StataCorp LLC). PAF calculations and corresponding 95% CIs48 for formula 1 were performed in R, version 3.6.3 (R Group for Statistical Computing).49 For 2 infections associated with the same cancer, we summed their attributable cases. However, for HBV and HCV in hepatocellular carcinoma (HCC) and intrahepatic bile duct cancer and for EBV and H pylori in NCGC, PAFs were combined with the following equation: 1 – (1 – HBV PAF) × (1 – HCV PAF),29 then partitioned for reporting.
To obtain the number of attributable cases, we multiplied PAF by cancer incidence counts. We used the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program Incidence–US Cancer Statistic Public Use Database with Puerto Rico, 2019 submission to obtain the incidence of malignant cancers (excluding nonmelanoma skin cancers).50 The International Classification of Diseases for Oncology, Third edition codes associated with each cancer analyzed are provided in eAppendices 3 and 4 in Supplement 1. The results are presented for males and females aged 9 years or younger, 10 to 19 years, 20 to 34 years, 35 to 49 years, 50 to 64 years, and 65 years or older. To adjust the number of cases of Hodgkin lymphoma and DLBCL attributable to EBV, we applied separate PAFs to cancer incidence partitioned by HIV status. To partition cancer incidence by HIV status, we applied the proportions of Hodgkin lymphoma and DLBCL cases occurring in people with HIV in the US available in the literature (see eAppendix 16 and eAppendix 19 in Supplement 1 for details).51,52
Results
A summary of PAF inputs and findings for HBV, HCV, and H pylori is provided in Table 1 and for the remaining infections in Table 2.14,36,53,54,55 Across all infections and cancers, 128 studies were meta-analyzed. The characteristics of individual studies can be found in eTables 4, 6, 7, and 9 to 22 in Supplement 1. The forest plots displaying the pooled measure of associations and prevalence of the infection in cancer can be found in eFigures 1 to 17 in Supplement 1.
Table 1. Infections Where PAFs Were Estimated Using the Prevalence of Infection in the Population and Measures of Association.
| Infection, cancer(s) | Data used to estimate measure of associationa | OR (95% CI) | Source of prevalence data | Range of prevalence estimates by sex and age group, y | PAF, % (95% CI)b |
|---|---|---|---|---|---|
| Hepatitis B virus | |||||
| Hepatocellular carcinoma | Pooled ORs from 4 US case-control studies | 24.2 (14.5 to 40.3) | NHANES data collected, 1999-2010 |
|
9.4 (2.9 to 14.5) |
| Intrahepatic bile duct | Pooled ORs from 4 US case-control studies | 3.4 (1.2 to 9.4) | 0.9 (0.0 to 1.8) | ||
| HCV | |||||
| Hepatocellular carcinoma | Pooled ORs from 5 US case-control studies | 29.8 (11.9 to 74.6) | NHANES data collected, 1999-2010 |
|
32.1 (9.0 to 45.2) |
| Intrahepatic bile duct | Pooled ORs from 4 US case-control studies | 4.5 (3.5 to 5.7) | 4.9 (2.2 to 7.4) | ||
| Burkitt lymphoma (age, ≥50 y only) | ORs from 5 studies (from Australia, Canada, Europe, and the US) assessing HCV seropositivity in the InterLymph Non-Hodgkin Lymphoma Subtypes Project | 4.1 (1.1 to 15.4) | 3.7 (0.0 to 7.1) | ||
| Chronic lymphocytic leukemia or small vessel lymphoma | 2.08 (1.23 to 3.49) | 1.9 (0.0 to 3.7) | |||
| DLBCL |
|
1.5 (0.2 to 2.8) | |||
| Lymphoplasmacytic lymphoma | 2.51 (1.03 to 6.17) | 2.0 (0.0 to 3.9) | |||
| Marginal zone lymphoma | 3.04 (1.65 to 5.60) | 2.7 (0.1 to 5.2) | |||
| Helicobacter pylori | |||||
| Gastric, noncardia | Pooled ORs from nested case-control studies from the US, Europe, and Australia: 5 studies that used ELISA or EIA corrected for measurement error and 3 studies that used immunoblot | 12.8 (8.5 to 19.2) | NHANES data collected, 1999-2000 |
|
75.4 (67.8 to 78.8) |
| Gastric, MALT, and DLBCL | One US study of 20 cases matched to 82 controls | 7.9 (1.6 to 38.1) | 70.8 (0.8 to 90.5) | ||
| Esophageal adenocarcinoma, protective effect | Pooled ORs from US case-control and nested case-control studies: 3 studies used ELISA and 1 study used immunoblot | 0.73 (0.55 to 0.95) | −10.9 (−1.8 to −20.8)c |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus; MALT, mucosa-associated lymphoid tissue; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; PAF, population attributable fraction.
The characteristics of included studies are reported in Supplement 1 under their respective infection and cancers.
The PAF was calculated by summing the number of attributable cases obtained after applying sex- and age-specific PAFs, then dividing the sum by the associated cancer cases.
To account for H pylori’s protective effect in esophageal adenocarcinoma, cases attributable to esophageal adenocarcinoma were subtracted from the total cases attributable to H pylori.
Table 2. Infections Where PAFs Were Estimated Using the Prevalence of Infection in Cancer Tissue.
| Source of prevalence estimatesa | No. of cases used to estimate PAF | Sex, age, or HIV group | PAF, % (95% CI) |
|---|---|---|---|
| EBV, detected via EBER ISH or LMP-1 (Hodgkin lymphoma only) | |||
| Burkitt lymphoma | |||
| 7 Studies | 397 | ≤19 y | 15.5 (8.1-23.0) |
| 1 Study | 51 | ≥20 y | 33.8 (19.8-58.0) |
| Hodgkin lymphoma | |||
| 4 Studies | 148 | ≤9 y | 62.2 (41.8-82.5) |
| 7 Studies | 443 | 10-19 y | 22.3 (13.3-32.7) |
| 4 Studies | 983 | 15-44 y | 20.5 (18.0-23.1) |
| 3 Studies | 369 | ≥45 y | 42.5 (33.0-52.1) |
| 6 Studies | 282 | HIV-positive adults | 92.9 (89.9-95.9) |
| Nasopharyngeal carcinoma | |||
| 2 Studies | 16 | ≤19 y | 100.0 (63.1-100.0) |
| 7 Studies | 629 | ≥20 y | 61.2 (45.1-77.2) |
| ENKTL, nasal type | |||
| Universally associated with EBV53 | NA | ≥20 y | 100.0 |
| DLBCL | |||
| 14 Studiesb | 5164 | HIV-negative adults | 4.9 (3.3-6.5) |
| 5 Studies | 264 | HIV-positive adults | 45.7 (27.9-63.6) |
| Gastric carcinoma | |||
| 7 Studies | 541 | Male | 12.8 (8.3-17.8) |
| 321 | Female | 1.8 (0.3-4.1) | |
| HPV, high-risk types, anogenital tract cancers, detected via PCRc | |||
| Anal squamous cell carcinoma | |||
| 5 Studies | 175 | Male | 90.2 (80.2-97.3) |
| 260 | Female | 96.3 (90.0-99.8) | |
| Cervix | |||
| Necessary cause54 | NA | Female | 100.0 |
| Penis | |||
| 5 Studies | 269 | Male | 38.6 (17.9-59.4) |
| Vagina | |||
| 2 Studies | 85 | Female | 72.2 (62.8-81.7) |
| Vulva | |||
| 6 Studies | 53 | <50 y | 74.4 (62.7-86.0) |
| 230 | ≥50 y | 45.7 (21.9-69.4) | |
| HPV, type 16, head and neck cancers, detected via PCR with E6 and/or E7 | |||
| Oropharynx | |||
| 17 Studies | 1905 | ≥20 y | 60.3 (51.2-69.1) |
| Oral cavity | |||
| 7 Studies | 683 | ≥20 y | 7.9 (3.3-14.0) |
| Larynx | |||
| 5 Studies | 194 | ≥20 y | 12.7 (3.7-25.4) |
| KSHV | |||
| Kaposi sarcoma | |||
| Necessary cause14 | NA | ≥20 y | 100.0 |
| Primary effusion lymphoma | |||
| Universally associated with KSHV55 | NA | ≥20 y | 100.0 |
| Human T-cell lymphotropic virus, type 1 | |||
| Adult T-cell leukemia or lymphoma | |||
| Necessary cause36 | NA | ≥20 | 100.0 |
| Merkel cell polyomavirus, detected via PCR, IHC, or ISH | |||
| Merkel cell carcinoma | |||
| 11 Studies | 779 | ≥20 | 70.3 (57.3-82.0) |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; EBER, Epstein-Barr virus–encoded RNA; EBV, Epstein-Barr virus; ENKTL, extranodal natural killer T-cell lymphoma; HPV, human papillomavirus; IHC, immunohistochemistry; ISH, in situ hybridization; KSHV, Kaposi sarcoma–associated herpesvirus; LMP-1, latent membrane protein 1; NA, not applicable; PAF, population attributable fraction; PCR, polymerase chain reaction.
The characteristics of included studies are reported in Supplement 1 under their respective infection and cancers.
Included 13 studies in which study authors reported that the study population was HIV negative and/or immunocompetent and 1 study with 567 DLBCL cases that did not report HIV status.
High-risk HPV types include types classified by the International Agency for Research on Cancer as group 1 (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), group 2A (68), and group 2B (26, 30, 34, 53, 66, 67, 70, 73, 82, 85, and 97) carcinogens.
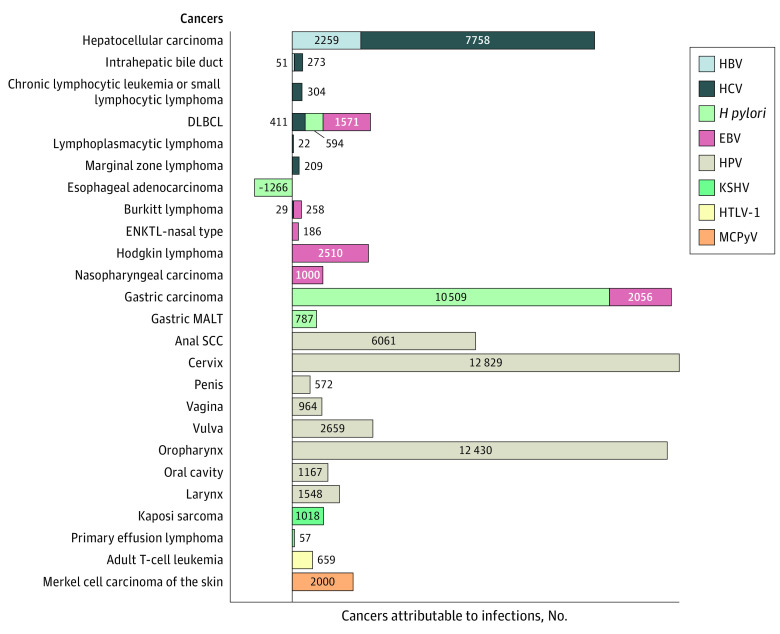
We estimated that infections were responsible for 4.3% (95% CI, 3.1%-5.3%) of the 1 666 102 cancers diagnosed among adults in the US in 2017—translating to 71 485 infection-attributable cancers. Of the 1 666 102 cancers diagnosed, 2.3% were attributable to HPV (n = 38 230), 0.6% to H pylori (n = 10 624), 0.5% to HCV (n = 9006), 0.5% to EBV (n = 7581), 0.1% to HBV (n = 2310), 0.1% to Merkel cell polyomavirus (n = 2000), 0.06% to Kaposi sarcoma–associated herpesvirus (n = 1075), and 0.04% to adult T-cell leukemia or lymphoma (human T-cell lymphotropic virus type 1 [HTLV-1]; n = 659). The cancers with the highest number of infection-attributable cases were cervical (HPV; n = 12 829), gastric (H pylori and Epstein-Barr virus; n = 12 565), oropharynx (HPV; n = 12 430), and HCC (HBV and HCV; n = 10 017) (Figure 1).
Figure 1. Infection-Attributable Cancers Among Adults in the US in 2017, by Cancer.
This figure shows the number of cases attributable to specific infections by cancer site. Due to Helicobacter pylori’s protective effect in esophageal adenocarcinoma, the 1266 cases attributable to esophageal adenocarcinoma get subtracted from the total cases associated with H pylori. DLBCL indicates diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; ENKTL, extranodal natural killer T-cell lymphoma; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; HTLV-1, human T-cell lymphotropic virus type 1; KSHV, Kaposi sarcoma–associated herpesvirus; MALT, mucosa-associated lymphoid tissue; MCPyV, Merkel cell polyomavirus; and SCC, squamous cell carcinoma.
Weighted and imputed sex-age group–specific HBV and HCV prevalence estimates based on NHANES data were higher than estimates that were only weighted; however, imputed H pylori prevalence estimates were higher or lower depending on the sex-age group and, overall, did not differ (eTables 2, 3, and 8 in Supplement 1). If we had not imputed missing data, 115 fewer HCC cases would be attributed to HBV and 1238 fewer cases to HCV. The combined PAFs for HBV and HCV in HCC ranged from 4.6% for women aged 20 to 39 years to 63.2% for men aged 60 to 64 years (eTable 5 in Supplement 1). PAFs for H pylori in NCGC steadily increased with age from 63.3% for men aged 20 to 24 years to 83.8% for men aged 85 years or older and from 52.2% for women aged 20 to 24 years to 85.4% for women aged 80 to 84 years (eTable 8 in Supplement 1).
The burden of infection-attributable cancers as a proportion of total cancer incidence ranged from 9.6% (95% CI, 9.2%-10.0%) for women aged 20 to 34 years to 3.2% (95% CI, 2.4%-3.8%) for women aged 65 years or older and from 6.1% (95% CI, 5.2%-7.0%) for men aged 20 to 34 years to 3.3% (95% CI, 1.9%-4.4%) for men aged 65 years or older. The proportion of cancers attributable to infections was slightly higher in males (4.4%; 95% CI, 3.6%-5.7%) than females (4.2%; 95% CI, 3.4%-4.8%). Although younger age groups had a higher proportion of infection-attributable cancers, the absolute number of cancers attributable to infections increased with age (Figure 2). The importance of HCV with cancer varied by age group and sex: 2 cancers among women aged 20 to 34 years and 4750 cancers among men aged 50 to 64 years were attributable to HCV. Epstein-Barr virus had a smaller role in producing cancer, but among men aged 20 to 34 years, it was responsible for almost half of all infection-attributable cancers. The role of HPV in anogenital cancers was pronounced for women aged 20 to 49 years. Human papillomavirus was responsible for 53.5% of the infection-attributable cancer burden; HPV in head and neck cancers accounted for 32.7% of the burden among males, while among females, HPV in anogenital cancers made up 60.0% of the burden. Among children, 332 cancers (2.2% of all cancers [95% CI, 1.3%-3.0%]) were attributable to EBV, with 77.7% of these being Hodgkin lymphomas (Table 3).
Figure 2. Infection-Attributable Cancers in the US in 2017, by Sex and Age Group (in Years).
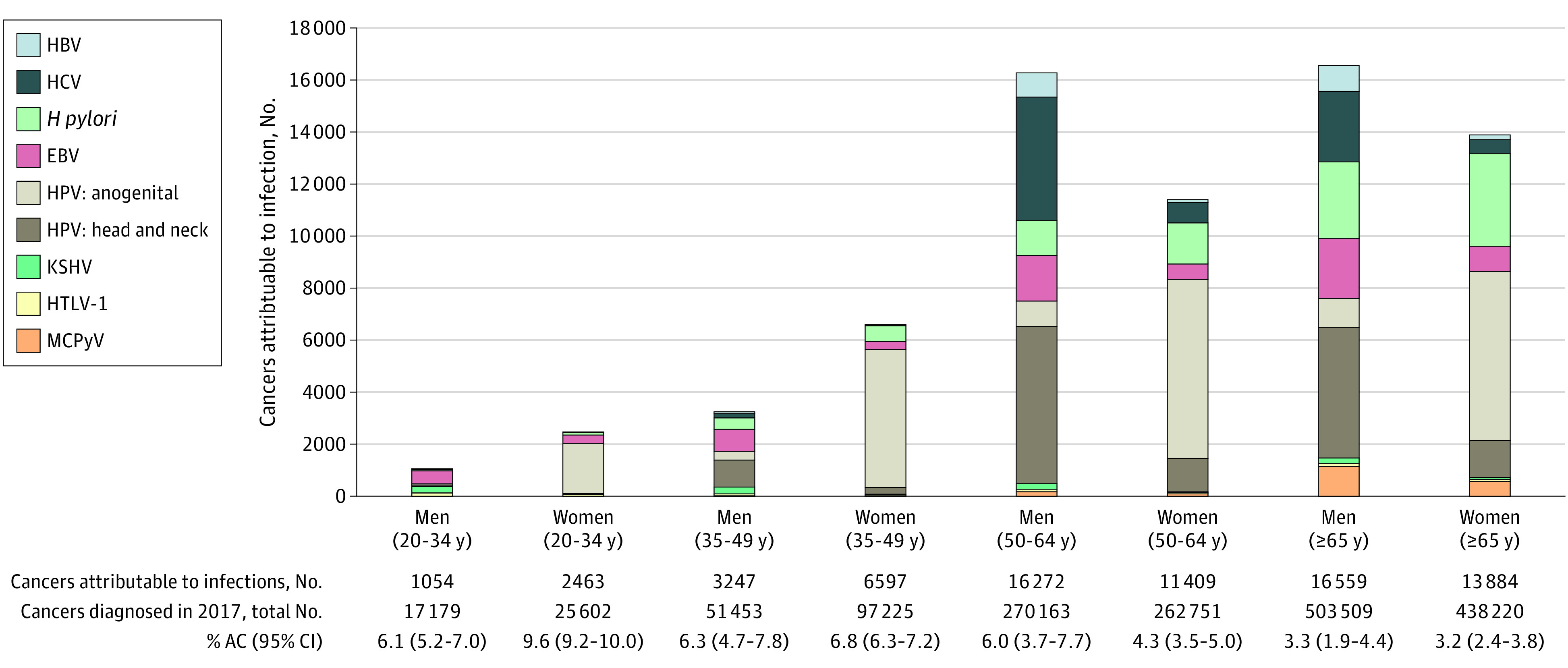
% AC indicates the proportion of the number of incident cancers diagnosed in 2017 attributable to infections; EBV, Epstein-Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; H pylori, Helicobacter pylori; HPV, human papillomavirus; HTLV-1, human T-cell lymphotropic virus type 1; KSHV, Kaposi sarcoma–associated herpesvirus; and MCPyV, Merkel cell polyomavirus.
Table 3. Estimates of the Percentage and Number of Cancers Attributable to EBV Among Individuals Aged 19 Years or Younger in the US in 2017.
| Sex and age group | All cancers | Burkitt lymphoma | Hodgkin lymphoma | Nasopharyngeal carcinoma | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of incident cancers diagnosed | No. of ACs | PAF, % (95% CI) | No. of incident cancers diagnosed | No. of ACs | No. of incident cancers diagnosed | No. of ACs | No. of incident cancers diagnosed | No. of ACs | |
| Males | |||||||||
| ≤9 y | 3836 | 54 | 1.4 (0.9-1.9) | 68 | 11 | 70 | 44 | 0 | 0 |
| 10-19 y | 4172 | 130 | 3.1 (1.9-4.3) | 76 | 12 | 427 | 95 | 23 | 23 |
| Total | 8008 | 184 | 2.3 (1.4-3.2) | 144 | 23 | 497 | 139 | 23 | 23 |
| Females | |||||||||
| ≤9 y | 3236 | 17 | 0.5 (0.3-0.7) | 25 | 4 | 21 | 13 | 0 | 0 |
| 10-19 y | 3808 | 131 | 3.4 (2.1-4.8) | 28 | 4 | 477 | 107 | 20 | 20 |
| Total | 7044 | 148 | 2.1 (1.3-2.9) | 53 | 8 | 498 | 120 | 20 | 20 |
| Overall | 15 052 | 332 | 2.2 (1.3-3.0) | 197 | 31 | 995 | 258 | 43 | 43 |
Abbreviations: ACs, attributable cases; EBV, Epstein Barr virus; PAF, population attributable fraction.
We performed several additional analyses. By using an overall NHL OR of 1.81 rather than NHL subtype–specific ORs for HCV, we found that 403 fewer NHLs were attributable to HCV. When PAFs for H pylori and NCGC were applied to NCGC incidence (not including reclassified overlapping lesions and not otherwise specified gastric sites), the number of cases of NCGC attributable to H pylori decreased by 3009 cases. If we attributed 100% of anal squamous cell carcinomas to HPV (as done in a global analysis),4 364 additional cases would be attributable to HPV.
Discussion
In this study, we found that in 2017, 4.3% of cancers diagnosed among US adults were attributable to 8 infections. In contrast, Islami and colleagues6 estimated that 3.3% of cancers diagnosed among US adults in 2014 were due to infections. Our higher estimate is largely due to the inclusion of intrahepatic bile duct cancers (HBV and HCV), gastric mucosa–associated lymphoid tissue and DLBCL (H pylori), EBV-associated cancers, adult T-cell leukemia or lymphoma (HTLV-1), and Merkel cell polyomavirus; if we excluded these infections or cancers, our overall PAF estimate would be 3.6%. On the other hand, our estimate is lower than that reported in a global analysis that found that 4.8% of cancers diagnosed in the US in 2018 were attributable to infections.4 Because our analysis included more infection-cancer pairs, we believe the difference is due to PAFs in the global analysis combining infection prevalence for regions composed of several countries, some of which may have higher infection prevalence than the US.
Many studies have used NHANES data to estimate the prevalence of HBV and HCV in the US.56,57,58,59 However, some groups with the highest burden of these infections, especially HCV, are excluded from the NHANES sampling frame.60 To address this limitation, Edlin and colleagues60 conducted a systematic literature search for HCV RNA prevalence among NHANES-excluded groups, such as incarcerated and homeless individuals and nursing home residents. They estimated that 23% of HCV RNA–positive individuals were missing from the NHANES sampling frame. Our imputed estimates of HCV prevalence were 28.6% higher than the nonimputed data for males (1.8% vs 1.4%) and 33.3% higher for females (0.8% vs 0.6%). Although we did not directly account for individuals outside the NHANES sampling frame, our imputed HCV RNA estimates were somewhat comparable to those reported by Edlin and colleagues.60 Furthermore, by performing imputations, we retained the desired sex and age group granularity (the estimate from Edlin et al60 is for the entire US population).
There may be more EBV-attributable cases of cancer than generally recognized. We included EBV’s role in DLBCLs and gastric cancer, leading to an additional approximately 3600 cancers being attributed to EBV. Diffuse large B-cell lymphoma is the most frequently diagnosed type of NHL,61 and gastric cancer is among the 15 most commonly diagnosed cancers in the US.62 Moreover, more than 300 cancer cases among children were attributed to EBV. We used an equation to combine the PAFs for EBV and H pylori in NCGC; however, the independence of EBV in producing gastric carcinoma remains unclear.
The importance of HPV relative to other infections is consistent with PAF analyses conducted in higher-income countries, such as Australia, Canada, France, and the UK.40,63,64,65 Women aged 20 to 34 years had the highest proportion of infection-attributable cancers, largely due to cervical cancer comprising 7.1% of all cancers diagnosed in this group. Although HPV vaccination efforts have been under way since 2006, females vaccinated at 11 to 12 years of age in 2006 would be, at the most, 23 years of age in 2017. For this reason, the cervical cancer burden remained high in 2017 but is expected to decrease in subsequent years. Although HPV prevention efforts often focus on females, we found that 1.7% (n = 14 618) of all cancers diagnosed among males in 2017 were due to HPV. In North America, HPV prevalence in head and neck cancers is higher than in other continents.66 To estimate HPV prevalence in head and neck cancers, we included only studies using E6 and/or E7 messenger RNA for HPV-16 detection; however, by restricting to HPV-16 (the most prevalent HPV type found in head and neck cancers),66 a small proportion of cancers may have been missed.
Limitations
Although we sought to provide comprehensive estimates of the role of infections in cancer incidence in the US by (1) correcting for measurement error (H pylori and NCGC association), (2) imputing missing HBV, HCV, and H pylori prevalence data, (3) including additional infection-related cancers, (4) using different PAF estimates for people with HIV, and (5) defining the distribution of infection-attributable cancers by sex and age, several limitations must be mentioned. First, some PAF inputs were based on sparse data, particularly for H pylori and gastric mucosa–associated lymphoid tissue and DLBCL (1 study)67 and EBV and Burkitt lymphoma in adults (1 study).68 Second, although most studies originated in the US, for certain infection-cancer pairs, we relied on studies conducted in other Western countries. Third, PAF formula 1 assumes no confounding of the exposure-disease association.28 We selected studies that matched and/or adjusted on strong confounders, and residual confounding cannot be ruled out entirely. Although residual confounding cannot explain the strong associations included in this analysis, it could have a minor effect on the magnitude of those associations. Fourth, our analysis could not account for the possible impact that curative DAAs had on HCV prevalence. With approximately 2 years between their introduction and 2017, we believe that the possible impact would be minor, but we recognize that DAAs are effective for patients with advanced disease, thereby impacting short-term HCC risk. We also did not account for long-term HBV treatments introduced in the mid-2000s. Although not a limitation per se, the burden of infection-attributable cancers is higher in certain groups, such as people with HIV and organ transplant recipients.
We have, in effect, quantified the opportunity that exists to reduce the burden of infection-attributable cancers in the US. A total of 84.2% of infection-attributable cancers (n = 60 170) were due to infections that can be prevented or treated effectively (HBV, HCV, H pylori, and HPV). Specifically, prophylactic vaccination for HBV and HPV confers more than 95% efficacy,69,70 more than 90% of HCV infections are curable with DAAs,71,72 and H pylori is treatable with antibiotic therapy.73 In addition, antiretroviral therapy for HIV can reduce the risk of HIV-associated cancers, such as cervical cancer, Kaposi sarcoma, and lymphomas. However, vaccine hesitancy, gaps in vaccine coverage, less than optimal uptake of DAAs and antiretroviral therapies, and antibiotic resistance (H pylori) pose significant challenges to progress. There is currently no way to prevent or treat EBV infection, but there are promising efforts to develop an EBV vaccine.74
Conclusion
Infections were estimated to be responsible for 4.3% of cancers diagnosed in the US in 2017 and, therefore, represent an important target for the development of prevention efforts (for EBV) and the continuation of current approaches (for HBV, HCV, H pylori, and HPV) to reduce their prevalence and associated disease burden.
eAppendix 1. Acknowledgements
eAppendix 2. Acronyms and Abbreviations
eAppendix 3. Cancers and Associated ICD-O-3 Codes
eTable 1. Search Performed in MEDLINE 1946–January 6, 2023
eAppendix 4. Cancer Incidence
eAppendix 5. Multiple Imputation
eAppendix 6. Hepatitis B and C Viruses
eTable 2. Estimated Prevalence of HBsAg Infection in the US, NHANES Data Collected 1999–2010
eTable 3. Estimated Prevalence of HCV-RNA Infection in the US, NHANES Data Collected 1999–2010
eAppendix 7. Hepatocellular Carcinoma
eTable 4. Characteristics of Case-Control Studies on the Association Between HBV or HCV Infection and HCC
eFigure 1. Pooled ORs for the Association Between Each (1) HBV and (2) HCV and HCC
eTable 5. HBV and HCV Associated PAFs (%) for HCC, by Age Group and Sex
eAppendix 8. Non-Hodgkin Lymphoma
eTable 6. The Association Between HCV Infection and NHL Subtypes as Reported in the InterLymph Study
eAppendix 9. Intrahepatic Bile Duct Cancer
eTable 7. Characteristics of Case-Control Studies on the Association Between HBV or HCV Infection and Intrahepatic Bile Duct Cancer
eFigure 2. Pooled ORs for the Association Between Each (1) HBV and (2) HCV and Intrahepatic Bile Duct Cancer
eAppendix 10. Helicobacter pylori
eTable 8. Estimated H. pylori Prevalence in the US and PAFs for NCGC
eAppendix 11. Gastric Cancer (Non-Cardia)
eTable 9. Characteristics of Studies on the Association Between H. pylori Infection Detected Using ELISA or EIA and NCGC
eTable 10. Characteristics of Studies on the Association Between H. pylori Infection Detected Using Immunoblot and NCGC
eFigure 3. Pooled Corrected (1) and Uncorrected (2) ORs for the Association Between H. pylori and NCGC
eAppendix 12. Gastric MALT and DLBCL
eAppendix 13. Esophageal Adenocarcinoma
eTable 11. Characteristics of Studies on the Association Between H. pylori Infection and Esophageal Adenocarcinoma
eFigure 4. Forest Plot of the Association Between H. pylori Infection and Esophageal Adenocarcinoma (Fixed Effects)
eAppendix 14. Epstein-Barr Virus
eAppendix 15. Burkitt Lymphoma
eTable 12. Characteristics of Studies on EBV Prevalence in BLs From Individuals Aged 0-19
eFigure 5. Forest Plot of EBV Prevalence (%) in BL Tumor Tissues Collected From Individuals Aged 0-19
eAppendix 16. Hodgkin Lymphoma
eTable 13. Characteristics of Studies Reporting on EBV Prevalence in HLs
eFigure 6. Forest Plot of EBV Prevalence (%) in HL Tumor Tissues Collected From Individuals Aged 0–19
eFigure 7. Forest Plot of EBV Prevalence (%) in HL Tumor Tissues
eAppendix 17. Nasopharyngeal Carcinoma
eTable 14. Characteristics of Studies Reporting on EBV Prevalence in NPC Cases
eFigure 8. Forest plot of EBV Prevalence (%) in NPC Tumor Tissues Collected From Adults
eAppendix 18. Extranodal Natural Killer T-Cell Lymphoma – Nasal Type
eAppendix 19. Diffuse Large B-Cell Lymphoma
eTable 15. Characteristics of Studies Reporting on EBV Prevalence in DLBCL Cases
eFigure 9. Forest Plot of EBV Prevalence (%) in DLBCL Tumor Tissues
eAppendix 20. Gastric Carcinoma
eTable 16. Characteristics of Studies Reporting on EBV Prevalence in GC Cases
eFigure 10. Forest Plot of EBV Prevalence (%) in GC, by Sex
eAppendix 21. Human Papillomavirus
eAppendix 22. Anal SCC
eTable 17. Characteristics of Studies Reporting on HR-HPV Prevalence in Invasive Anal SCCs, by Sex and HIV Status
eFigure 11. Forest Plot of the Prevalence (%) of HR-HPV in Anal SCC, by Sex
eAppendix 23. Penile Cancer
eFigure 12. Forest Plot of HR-HPV Prevalence (%) in Penile Cancer
eTable 18. Characteristics of Studies Reporting on HR-HPV Prevalence in Penile Cancers
eAppendix 24. Vaginal Cancer
eTable 19. Characteristics of Studies Reporting on HR-HPVa Prevalence in Vaginal Cancers
eAppendix 25. Vulvar Cancer
eTable 20. Characteristics of Studies Reporting on the Prevalence of HR- HPV in Vulvar Cancer Cases, by Age-Group
eFigure 13. Forest Plot for HR-HPV Prevalence (%) in Vulvar Cancer, by Age Group
eAppendix 26. Head and Neck Cancers
eTable 21. Characteristics of Studies Reporting on HPV16 Prevalence Detected via E6 and/or E7 in HNCs
eFigure 14. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Oropharynx
eFigure 15. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Oral Cavity
eFigure 16. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Larynx
eAppendix 27. Merkel Cell Polyomavirus
eAppendix 28. Merkel Cell Carcinoma of the Skin
eTable 22. Characteristics of Studies Reporting on MCPyV Prevalence in Merkel Cell Carcinoma of the Skin
eFigure 17. Forest Plot of MCPyV Prevalence (%) in Merkel Cell Carcinoma of the Skin
eReferences.
Data Sharing Statement
References
- 1.National Cancer Opinion Survey prepared for American Society of Clinical Oncology. Harris Insights & Analytics. Accessed April 4, 2023. https://www.asco.org/sites/new-www.asco.org/files/content-files/research-and-progress/documents/2017-ASCO-National-Cancer-Opinion-Survey-Results.pdf
- 2.Helicobacter and Cancer Collaborative Group . Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347-353. doi: 10.1136/gut.49.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128(1):176-184. doi: 10.1002/ijc.25321 [DOI] [PubMed] [Google Scholar]
- 4.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180-e190. doi: 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 5.Viruses that can lead to cancer. American Cancer Society. Accessed February 1, 2023. https://www.cancer.org/cancer/cancer-causes/infectious-agents/infections-that-can-lead-to-cancer/viruses.html
- 6.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V, Baan R, Straif K, et al. ; WHO International Agency for Research on Cancer Monograph Working Group . A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10(4):321-322. doi: 10.1016/S1470-2045(09)70096-8 [DOI] [PubMed] [Google Scholar]
- 8.Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore). 2015;94(20):e792. doi: 10.1097/MD.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae JM, Kim EH. Epstein-Barr virus and gastric cancer risk: a meta-analysis with meta-regression of case-control studies. J Prev Med Public Health. 2016;49(2):97-107. doi: 10.3961/jpmph.15.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang J, Suh CH, Won Kim K, et al. The incidence of Epstein-Barr virus–positive diffuse large B-cell lymphoma: a systematic review and meta-analysis. Cancers (Basel). 2021;13(8):1785. doi: 10.3390/cancers13081785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3069-3075. doi: 10.1158/1055-9965.EPI-08-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2078-2085. doi: 10.1158/1055-9965.EPI-06-0308 [DOI] [PubMed] [Google Scholar]
- 13.Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014(48):130-144. doi: 10.1093/jncimonographs/lgu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer . Biological Agents: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 100B. International Agency for Research on Cancer; 2012. [Google Scholar]
- 15.Hassan WM, Bakry MS, Hassan HM, Alfaar AS. Incidence of orbital, conjunctival and lacrimal gland malignant tumors in USA from Surveillance, Epidemiology and End Results, 1973-2009. Int J Ophthalmol. 2016;9(12):1808-1813. doi: 10.18240/ijo.2016.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Zhao Y, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12:289. doi: 10.1186/1471-2407-12-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2012;27(10):1561-1568. doi: 10.1111/j.1440-1746.2012.07207.x [DOI] [PubMed] [Google Scholar]
- 18.Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13:161. doi: 10.1186/s12957-015-0583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavakoli A, Monavari SH, Solaymani Mohammadi F, Kiani SJ, Armat S, Farahmand M. Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):493. doi: 10.1186/s12885-020-07013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5(12):1413-1417, 1417.e1-1417.e2. doi: 10.1016/j.cgh.2007.08.010 [DOI] [PubMed] [Google Scholar]
- 21.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila). 2008;1(5):329-338. doi: 10.1158/1940-6207.CAPR-08-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuo X, Zhang Y, Wang Y, Zhuo W, Zhu Y, Zhang X. Helicobacter pylori infection and oesophageal cancer risk: association studies via evidence-based meta-analyses. Clin Oncol (R Coll Radiol). 2008;20(10):757-762. doi: 10.1016/j.clon.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 23.Nie S, Chen T, Yang X, Huai P, Lu M. Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus. 2014;27(7):645-653. doi: 10.1111/dote.12194 [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Li L, Zhang C, et al. Systematic review with meta-analysis: association of Helicobacter pylori infection with esophageal cancer. Gastroenterol Res Pract. 2019;2019:1953497. doi: 10.1155/2019/1953497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie FJ, Zhang YP, Zheng QQ, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19(36):6098-6107. doi: 10.3748/wjg.v19.i36.6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Gao L, Li H, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis. 2013;207(3):479-488. doi: 10.1093/infdis/jis698 [DOI] [PubMed] [Google Scholar]
- 27.Torrente MC, Rodrigo JP, Haigentz M Jr, et al. Human papillomavirus infections in laryngeal cancer. Head Neck. 2011;33(4):581-586. doi: 10.1002/hed.21421 [DOI] [PubMed] [Google Scholar]
- 28.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531-541. [PubMed] [Google Scholar]
- 29.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325-332. doi: 10.1093/oxfordjournals.aje.a121617 [DOI] [PubMed] [Google Scholar]
- 30.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609-e616. doi: 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- 31.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 32.International Agency for Research on Cancer . Human Papillomavirus: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 90. International Agency for Research on Cancer; 2007. [Google Scholar]
- 33.International Agency for Research on Cancer . Malaria and Some Polyomaviruses (SV40, BK, JC, and Merkel Cell Viruses): Volume 104. International Agency for Research on Cancer; 2014. [Google Scholar]
- 34.International Agency for Research on Cancer . Epstein-Barr Virus and Kaposi’s Sarcoma Herpesvirus/Human Herpesvirus 8: Volume 70. International Agency for Research on Cancer; 1997. [Google Scholar]
- 35.International Agency for Research on Cancer . Hepatitis Viruses: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 59. International Agency for Research on Cancer; 1994. [Google Scholar]
- 36.International Agency for Research on Cancer . Human Immunodeficiency Viruses and Human T-Cell Lymphotropic Viruses: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 67. International Agency for Research on Cancer; 1996. [PMC free article] [PubMed] [Google Scholar]
- 37.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607-615. doi: 10.1016/S1470-2045(12)70137-7 [DOI] [PubMed] [Google Scholar]
- 38.de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29(16):2173-2181. doi: 10.1097/QAD.0000000000000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136(2):487-490. doi: 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- 40.Volesky KD, El-Zein M, Franco EL, Brenner DR, Friedenreich CM, Ruan Y; ComPARe Study Team . Cancers attributable to infections in Canada. Prev Med. 2019;122:109-117. doi: 10.1016/j.ypmed.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 41.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat 2. 2013;(161):1-24. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention . NHANES 1999-2000 Laboratory Data Overview. Accessed October 1, 2021. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=1999
- 43.Centers for Disease Control and Prevention . NHANES 2007-2008 Laboratory Data Overview. Accessed October 1, 2021. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2007
- 44.Centers for Disease Control and Prevention . NHANES 2009-2010 Laboratory Data Overview. Accessed October 1, 2021. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2009
- 45.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404-413. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 47.Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health. 2013. Accessed April 2, 2022. http://www.OpenEpi.com
- 48.Walter SD. Local estimates of population attributable risk. J Clin Epidemiol. 2010;63(1):85-93. doi: 10.1016/j.jclinepi.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 49.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing. Accessed January 30, 2022. https://www.R-project.org/
- 50.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence - U.S. Cancer Statistics 2001-2017 Public Use Research Database, 2019 Submission (2001-2017), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2020. Accessed September 19, 2023. https://www.cdc.gov/cancer/uscs/public-use/
- 51.Shiels MS, Koritzinsky EH, Clarke CA, Suneja G, Morton LM, Engels EA. Prevalence of HIV infection among U.S. Hodgkin lymphoma cases. Cancer Epidemiol Biomarkers Prev. 2014;23(2):274-281. doi: 10.1158/1055-9965.EPI-13-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 2011;305(14):1450-1459. doi: 10.1001/jama.2011.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haverkos BM, Pan Z, Gru AA, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11(6):514-527. doi: 10.1007/s11899-016-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-19. doi: [DOI] [PubMed] [Google Scholar]
- 55.Surveillance, Epidemiology, and End Results Program . Primary effusion lymphoma. Accessed April 4, 2023. https://seer.cancer.gov/seertools/hemelymph/51f6cf57e3e27c3994bd5378/
- 56.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. doi: 10.7326/M13-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388-397. doi: 10.1002/hep.28109 [DOI] [PubMed] [Google Scholar]
- 58.Roberts H, Ly KN, Yin S, Hughes E, Teshale E, Jiles R. Prevalence of HBV infection, vaccine-induced immunity, and susceptibility among at-risk populations: US households, 2013-2018. Hepatology. 2021;74(5):2353-2365. doi: 10.1002/hep.31991 [DOI] [PubMed] [Google Scholar]
- 59.Watts T, Lauver D, Sethi AK, Snedden T, Zahner S. Hepatitis C virus infections among people aged 15-44, United States, 2009-2018. Public Health Nurs. 2021;38(2):167-175. doi: 10.1111/phn.12808 [DOI] [PubMed] [Google Scholar]
- 60.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363. doi: 10.1002/hep.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Types of B-cell lymphoma. American Cancer Society. Accessed April 4, 2023. https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/b-cell-lymphoma.html
- 62.Cancer stat facts: stomach cancer. National Cancer Institute. Accessed April 4, 2023. https://seer.cancer.gov/statfacts/html/stomach.html
- 63.Parkin DM. 11: Cancers attributable to infection in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S49-S56. doi: 10.1038/bjc.2011.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shield KD, Marant Micallef C, de Martel C, et al. New cancer cases in France in 2015 attributable to infectious agents: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(3):263-274. doi: 10.1007/s10654-017-0334-z [DOI] [PubMed] [Google Scholar]
- 65.Antonsson A, Wilson LF, Kendall BJ, Bain CJ, Whiteman DC, Neale RE. Cancers in Australia in 2010 attributable to infectious agents. Aust N Z J Public Health. 2015;39(5):446-451. doi: 10.1111/1753-6405.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319-1331. doi: 10.1016/S1470-2045(14)70471-1 [DOI] [PubMed] [Google Scholar]
- 67.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330(18):1267-1271. doi: 10.1056/NEJM199405053301803 [DOI] [PubMed] [Google Scholar]
- 68.Mbulaiteye SM, Pullarkat ST, Nathwani BN, et al. Epstein-Barr virus patterns in US Burkitt lymphoma tumors from the SEER residual tissue repository during 1979-2009. APMIS. 2014;122(1):5-15. doi: 10.1111/apm.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927. doi: 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- 70.Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143-2159. doi: 10.1016/S0140-6736(17)31821-4 [DOI] [PubMed] [Google Scholar]
- 71.Wedemeyer H, Craxí A, Zuckerman E, et al. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir ± dasabuvir ±ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: a meta-analysis. J Viral Hepat. 2017;24(11):936-943. doi: 10.1111/jvh.12722 [DOI] [PubMed] [Google Scholar]
- 72.Patel SV, Jayaweera DT, Althoff KN, et al. Real-world efficacy of direct acting antiviral therapies in patients with HIV/HCV. PLoS One. 2020;15(2):e0228847. doi: 10.1371/journal.pone.0228847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunt R, Fallone C, Veldhuyzan van Zanten S, et al. ; CHSG 2004 participants . Canadian Helicobacter Study Group Consensus Conference: update on the management of Helicobacter pylori—an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18(9):547-554. doi: 10.1155/2004/326767 [DOI] [PubMed] [Google Scholar]
- 74.Balfour HH Jr, Schmeling DO, Grimm-Geris JM. The promise of a prophylactic Epstein-Barr virus vaccine. Pediatr Res. 2020;87(2):345-352. doi: 10.1038/s41390-019-0591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Acknowledgements
eAppendix 2. Acronyms and Abbreviations
eAppendix 3. Cancers and Associated ICD-O-3 Codes
eTable 1. Search Performed in MEDLINE 1946–January 6, 2023
eAppendix 4. Cancer Incidence
eAppendix 5. Multiple Imputation
eAppendix 6. Hepatitis B and C Viruses
eTable 2. Estimated Prevalence of HBsAg Infection in the US, NHANES Data Collected 1999–2010
eTable 3. Estimated Prevalence of HCV-RNA Infection in the US, NHANES Data Collected 1999–2010
eAppendix 7. Hepatocellular Carcinoma
eTable 4. Characteristics of Case-Control Studies on the Association Between HBV or HCV Infection and HCC
eFigure 1. Pooled ORs for the Association Between Each (1) HBV and (2) HCV and HCC
eTable 5. HBV and HCV Associated PAFs (%) for HCC, by Age Group and Sex
eAppendix 8. Non-Hodgkin Lymphoma
eTable 6. The Association Between HCV Infection and NHL Subtypes as Reported in the InterLymph Study
eAppendix 9. Intrahepatic Bile Duct Cancer
eTable 7. Characteristics of Case-Control Studies on the Association Between HBV or HCV Infection and Intrahepatic Bile Duct Cancer
eFigure 2. Pooled ORs for the Association Between Each (1) HBV and (2) HCV and Intrahepatic Bile Duct Cancer
eAppendix 10. Helicobacter pylori
eTable 8. Estimated H. pylori Prevalence in the US and PAFs for NCGC
eAppendix 11. Gastric Cancer (Non-Cardia)
eTable 9. Characteristics of Studies on the Association Between H. pylori Infection Detected Using ELISA or EIA and NCGC
eTable 10. Characteristics of Studies on the Association Between H. pylori Infection Detected Using Immunoblot and NCGC
eFigure 3. Pooled Corrected (1) and Uncorrected (2) ORs for the Association Between H. pylori and NCGC
eAppendix 12. Gastric MALT and DLBCL
eAppendix 13. Esophageal Adenocarcinoma
eTable 11. Characteristics of Studies on the Association Between H. pylori Infection and Esophageal Adenocarcinoma
eFigure 4. Forest Plot of the Association Between H. pylori Infection and Esophageal Adenocarcinoma (Fixed Effects)
eAppendix 14. Epstein-Barr Virus
eAppendix 15. Burkitt Lymphoma
eTable 12. Characteristics of Studies on EBV Prevalence in BLs From Individuals Aged 0-19
eFigure 5. Forest Plot of EBV Prevalence (%) in BL Tumor Tissues Collected From Individuals Aged 0-19
eAppendix 16. Hodgkin Lymphoma
eTable 13. Characteristics of Studies Reporting on EBV Prevalence in HLs
eFigure 6. Forest Plot of EBV Prevalence (%) in HL Tumor Tissues Collected From Individuals Aged 0–19
eFigure 7. Forest Plot of EBV Prevalence (%) in HL Tumor Tissues
eAppendix 17. Nasopharyngeal Carcinoma
eTable 14. Characteristics of Studies Reporting on EBV Prevalence in NPC Cases
eFigure 8. Forest plot of EBV Prevalence (%) in NPC Tumor Tissues Collected From Adults
eAppendix 18. Extranodal Natural Killer T-Cell Lymphoma – Nasal Type
eAppendix 19. Diffuse Large B-Cell Lymphoma
eTable 15. Characteristics of Studies Reporting on EBV Prevalence in DLBCL Cases
eFigure 9. Forest Plot of EBV Prevalence (%) in DLBCL Tumor Tissues
eAppendix 20. Gastric Carcinoma
eTable 16. Characteristics of Studies Reporting on EBV Prevalence in GC Cases
eFigure 10. Forest Plot of EBV Prevalence (%) in GC, by Sex
eAppendix 21. Human Papillomavirus
eAppendix 22. Anal SCC
eTable 17. Characteristics of Studies Reporting on HR-HPV Prevalence in Invasive Anal SCCs, by Sex and HIV Status
eFigure 11. Forest Plot of the Prevalence (%) of HR-HPV in Anal SCC, by Sex
eAppendix 23. Penile Cancer
eFigure 12. Forest Plot of HR-HPV Prevalence (%) in Penile Cancer
eTable 18. Characteristics of Studies Reporting on HR-HPV Prevalence in Penile Cancers
eAppendix 24. Vaginal Cancer
eTable 19. Characteristics of Studies Reporting on HR-HPVa Prevalence in Vaginal Cancers
eAppendix 25. Vulvar Cancer
eTable 20. Characteristics of Studies Reporting on the Prevalence of HR- HPV in Vulvar Cancer Cases, by Age-Group
eFigure 13. Forest Plot for HR-HPV Prevalence (%) in Vulvar Cancer, by Age Group
eAppendix 26. Head and Neck Cancers
eTable 21. Characteristics of Studies Reporting on HPV16 Prevalence Detected via E6 and/or E7 in HNCs
eFigure 14. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Oropharynx
eFigure 15. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Oral Cavity
eFigure 16. Forest Plot of HPV16 E6/E7 Prevalence (%) in Cancer of the Larynx
eAppendix 27. Merkel Cell Polyomavirus
eAppendix 28. Merkel Cell Carcinoma of the Skin
eTable 22. Characteristics of Studies Reporting on MCPyV Prevalence in Merkel Cell Carcinoma of the Skin
eFigure 17. Forest Plot of MCPyV Prevalence (%) in Merkel Cell Carcinoma of the Skin
eReferences.
Data Sharing Statement