Abstract
Background:
Extracellular vesicles (EVs), membrane-bound particles containing a variety of RNA types, DNA, proteins, and other macromolecules, are now appreciated as an important means of communication between cells and tissues, both in normal cellular physiology and as a potential indicator of cellular stress, environmental exposures, and early disease pathogenesis. Extracellular signaling through EVs is a growing field of research for understanding fundamental mechanisms of health and disease and for the potential for biomarker discovery and therapy development. EVs are also known to play important roles in mediating the effects of exposure to environmental stress.
Objectives:
This seminar addresses the application of new tools and approaches for EV research, developed in part through the National Institutes of Health (NIH) Extracellular RNA Communication Program, and reflects presentations and discussions from a workshop held 27–28 September 2021 by the National Institute of Environmental Health Sciences (NIEHS) and the National Center for Advancing Translational Sciences (NCATS) on “Extracellular Vesicles, Exosomes, and Cell-Cell Signaling in Response to Environmental Stress.” The panel of experts discussed current research on EVs and environmental exposures, highlighted recent advances in EV isolation and characterization, and considered research gaps and opportunities toward identifying and characterizing the roles for EVs in environmentally related diseases, as well as the current challenges and opportunities in this field.
Discussion:
The authors discuss the application of new experimental models, particularly organ-on-chip (OOC) systems and in vitro approaches and how these have the potential to extend findings in population-based studies of EVs in exposure-related diseases. Given the complex challenges of identifying cell-specific EVs related to environmental exposures, as well as the general heterogeneity and variability in EVs in blood and other accessible biological samples, there is a critical need for rigorous reporting of experimental methods and validation studies. The authors note that these efforts, combined with cross-disciplinary approaches, would ensure that future research efforts in environmental health studies on EV biomarkers are rigorous and reproducible. https://doi.org/10.1289/EHP12980
Introduction
Extracellular vesicles (EVs) play key roles in intercellular communication, in both normal cell physiology and in disease processes, including immune suppression, inflammation, and oncogenic signaling.1 The term EVs encompasses a number of membrane-bound particles—such as exosomes, microvesicles, ectosomes, and apoptotic bodies—that are found in a variety of human biofluids. Nomenclature for EVs varies according to the cell, tissue of origin, size, and subcellular origin (see van der Pol et al., Table 1).1 The biogenesis and transport of different EVs is covered in several reviews,2–4 and EV cargo consists of a variety of RNAs, including small nuclear RNA (snRNA), microRNA (miRNA), transfer RNA (tRNA), Y RNAs, and specific fragments from long transcripts bound to RNA-binding proteins, as well as DNA, proteins, and lipids that are involved in intercellular signaling and cellular processes.2 Together, EVs and extracellular signaling, make up a significant focus of current investigation for fundamental biological research, discovery of new biomarkers, and therapeutic applications. There is also growing interest in the roles for EVs in altering cell–cell communication in response to hazards from the environment,4 in identifying their role in environmentally related diseases (including pro-inflammatory signaling related to air pollution exposures), in the transport of proteins involved in neurodegenerative diseases, and in characterizing EV signaling in other environmentally related health conditions.
EV cargo comprises a range of proteins, lipids, DNA, and RNA involved in cell–cell signaling in health and disease. To accelerate progress in the field of extracellular RNA (exRNA) biology, including extracellular vesicle carriers, and to generate foundational knowledge in terms of the basic biology, as well as potential applications toward prognosis, diagnosis, and intervention of many diseases, the National Institutes of Health (NIH) launched the Common Fund’s Extracellular RNA Communication Program in 2013.5,6 In stage 1 of that program (ERCC1), the Extracellular RNA Communication Program Consortium addressed five major challenges in the field: a) mechanisms of exRNA biogenesis, export, and secretion from the cell of origin; b) reference profiles for exRNA species from healthy human biofluids; c) utility of exRNA for biomarker development; d) utility of exRNA for therapeutic development; and e) community-wide resources for exRNA standards, protocols, and data. The ERCC Portal (https://exrna.org/) is the gateway for all ERCC-developed data and resources, which include the exRNA Atlas,7,8 ExceRpt exRNA processing pipeline (https://exrna.shinyapps.io/mirdar/), and miRDaR software application for selection of extracellular miRNA isolation kits.9,10 The exRNA Atlas is a data resource that includes small and long RNA sequencing and real-time polymerase chain reaction (RT-PCR)–derived exRNA profiles from human biofluids (https://exrna-atlas.org).7–10 Data from 9,987 samples are currently available in the exRNA Atlas across both healthy and several disease conditions, biofluids, RNA sources, and different RNA isolation protocols.
In 2019, ExRNA Communication Program stage 2 (ERCC2) was launched to address challenges due to the inherent heterogeneity of exRNA carriers and the lack of technology to accurately assess exRNA carriers.11 Therefore, ERCC2 focused on developing novel tools and technologies to isolate different exRNA carriers and analyze their cargo. With the fundamental knowledge gained from these technologies, the remaining challenges in the EV field—such as identifying EV cell/tissue of origin and the functional effects of EV signaling, particularly in response to environmental exposures—can begin to be addressed.
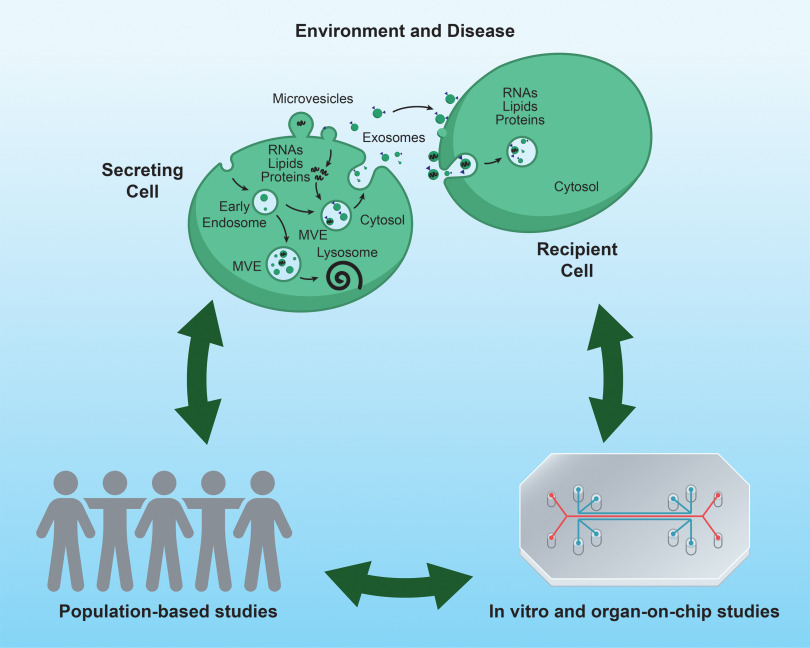
This seminar reflects the presentations and discussions from the Environmental Health Sciences (NIEHS) and the National Center for Advancing Translational Sciences (NCATS) workshop on “Extracellular Vesicles, Exosomes, and Cell-Cell Signaling in Response to Environmental Stress.” We use selected examples of EVs and environmental exposures in neurodegenerative diseases, lung and cardiopulmonary diseases, and reproductive outcomes to discuss the current state-of-the science and future directions of this emerging and important field. Although environmental factors described in these examples focus on chemical and other toxicant exposures, EVs released from microbes also affect signaling, for example, with indoor dust in inflammatory pulmonary disease.12 There are many experimental models for characterizing the effects of environmental stressors on EV-mediated extracellular signaling, but in this seminar we focus on the potential of organ-on-chip (OOC) platforms to complement findings from population-based studies (Figure 1).
Figure 1.
Intercellular communication and extracellular vesicles (EVs) in environmental disease research. EVs enable communication between cells through transfer of EV cargo (e.g., RNA, proteins, and lipids). Understanding the role for EVs in environmentally related disease will benefit from combining population-based research with research in experimental models. Note: MVE, multivesicular endosome.
Discussion
EVs and Environmentally Related Diseases
EVs and neurodegenerative diseases.
Emerging evidence has suggested that EVs may be critically involved in the etiology and progression of many neurodegenerative conditions that have environmental risk factors, including Parkinson’s disease (PD),13 Alzheimer’s disease (AD),14 and amyotrophic lateral sclerosis (ALS).15 With the portion of the world’s population years of age projected to nearly double by the year 2050,16 the anticipated increased prevalence of age-related neurodegenerative diseases has created an urgent need to better understand risk, as well as underlying pathogenetic mechanisms. To this end, EVs have garnered considerable attention for their diverse functions within the central nervous system (CNS), including their critical role in intercellular communication between neuronal and glial cell populations during normal physiological conditions and disease states. As examples, EVs are involved in regulation of neuronal firing, myelin homeostasis, immune responses, synaptic plasticity and blood–brain barrier integrity.17 It is well known that for several neurodegenerative diseases, including sporadic and familial PD, a hallmark pathological feature is the aggregation of alpha-synuclein () in the CNS.18 However, accumulating studies have shown that EVs are implicated in the spreading of this neuronal protein, as well as other proteins, such as amyloid beta () and phosphorylated tau (p-tau), which can correlate with symptom severity of neurodegenerative diseases.19–21 For example, individuals with elevated amyloid beta/tau accumulation showed faster cognitive decline in a longitudinal study of preclinical AD.22 It has also been reported that CNS EVs transported and secreted the TAR DNA-binding protein 43 (TDP-43) in tissues of patients with ALS, which may explain the early progression of the disease.23 Data obtained from animal and in vitro models have also provided new insight into links between EVs and neurological diseases. For example, EVs isolated from transgenic mice overexpressing the P301L mutation of tau, a protein implicated in the pathogenesis of AD, provided evidence for how tau phosphorylation can be accelerated during disease progression.24 Furthermore, Kanthasamy et al. have shown in an dopaminergic neuronal cell model that misfolded was secreted through exosomes following manganese exposure, providing in vitro evidence linking the potential importance of EVs in mediating the neurotoxic response to metals.25 An important area of future exploration will be the identification of the many potential environmental factors that can directly impact EV function and increase the risk for developing neurological disorders, factors which, in addition to heavy metals, may include air pollutants,26 viral particles,27 and tobacco smoke.28 A challenge in the field will be how to integrate the study of EVs and their role in disease risk with exposomic approaches that consider the cumulative measure of environmental influences throughout the life span.29
Although EVs offer a promising gateway into understanding, treating, and possibly delaying neurodegenerative disorders,30 there is still a vast uncharted territory to navigate. The seminar authors note that future research must continue to unravel the complex roles of EVs in disease etiology and progression. One particular direction is to scrutinize the exact mechanisms by which EVs contribute to protein misfolding and spread, which could lead to breakthroughs in targeting these processes therapeutically. Moreover, leveraging EVs for noninvasive disease diagnostics is another fascinating prospect. The development of sensitive and specific biomarkers from neuronal EVs, such as , , and p-tau, could potentially revolutionize early detection and intervention of neurodegenerative diseases.31 However, these efforts are not without challenges. The heterogeneous nature of EVs—coupled with the technical difficulties associated with their isolation, characterization, and analysis—necessitates continued methodological advancements and standardization.
EVs, environmental exposure, and lung and cardiopulmonary diseases.
Several studies have implicated EVs in pulmonary development, function, and disease.32 Mechanistically, it has been demonstrated that EVs secreted in lung airways affect the pathophysiology of lung diseases and open paths to new therapies.33 For instance, EVs from cultured stem cells have been reported to ameliorate airway inflammation34 and rescue fetal lung underdevelopment in animal models.35 Protein cargo in EVs from patient-derived cell lines has been associated with idiopathic pulmonary fibrosis.36 Human studies have shown that changes in EVs can anticipate and help to predict loss of lung function over time. In a prospective study of patients with chronic obstructive pulmonary disease (COPD), the number of circulating EVs were early predictors of lung function decline 1 y later.37 In cross-sectional studies, patients with COPD consistently showed higher numbers of blood EVs compared with control patients without COPD.38–40 A recent longitudinal study also showed that profiling the miRNA cargo in circulating EVs can identify individuals with subclinical lung injury along with eventual age-related, steeper decline in lung function.41 Thus, EVs and their cargo may be useful biomarkers of age-related pulmonary dysfunction and lung disease.
EVs may also mediate the effects of exposures on the respiratory system by modulating inflammation, thrombosis, endothelial dysfunction, tissue remodeling, and angiogenesis.42 For instance, rats exposed to tobacco smoke showed specific changes in blood EV biomarkers after 2 months of exposure that the investigators had anticipated to elicit lung function decline after 4 and 6 months.43 Consistent with animal data, smokers with early preclinical loss of lung function were shown to have a higher circulating level of EVs than smokers with normal lung function, and both groups of smokers had more EVs than healthy nonsmokers.44 In cell culture, alveolar macrophages exposed to cigarette smoke extract had higher levels of suppressor of cytokine signaling 3 (SOCS3), a homeostatic protein carried by EVs between alveolar cells.45 The same study also found that enrichment of SOCS3 was stimulated by increased reactive oxygen species levels, resulting in inactivation of the proteasome.46 Cigarette smoke condensate also affected several small noncoding RNAs (sncRNAs) in airway epithelial cells.46 Thus, there is evidence that exposure to tobacco and cigarette smoke is associated with altered EV release and EV cargo, and in vitro experiments may provide potential mechanisms through which these exposures induce toxicity.
The lungs are highly vulnerable to the effects of air pollutants.32 Growing evidence also indicates that EVs are part of the lung responses to air pollution exposure. By leveraging both in vivo and in vitro studies, Wang et al.47 found that exposure to particulate matter in diameter () increased the number of small EVs. In that study, EV-encapsulated miR-421 contributed to cardiac dysfunction following exposure by suppressing cardiac angiotensin converting enzyme 2.47 In another example of complementary in vivo and in vitro analyses, Bollati et al.48 evaluated the expression of EV-encapsulated blood miRNAs upon short-term PM exposure. The in vivo study isolated circulating EVs from healthy steel plant workers before and after workplace PM exposure. miRNA profiling of circulating EVs after 3 d of workplace PM exposure showed increased expression of miR-128 and miR-302c, two miRNAs with known inflammatory functions. When lung alveolar cells were exposed to PM, a time-dependent release of miR-128 encapsulated in EVs was observed.48 These data demonstrate that exposure to PM results in the release of specific EV-encapsulated miRNAs from lung epithelial cells that can be detected in human blood. Thus, observational and in vivo findings can be pursued to determine the biological feasibility of the role of EVs and potential mechanisms through which EVs and their cargo may explain observed associations.
The role of EVs in lung and cardiopulmonary diseases, in addition to their interactions with environmental exposures, constitutes an intriguing and still relatively untapped area of research. Future research could focus on comprehending the specific ways in which EVs contribute to the onset and progression of various pulmonary diseases, including the role they play in mediating the effects of different environmental exposures. Given that current studies suggest EVs can mediate responses to harmful substances, such as tobacco smoke and air pollutants, we must delve deeper into understanding these interactions and the specific molecular mechanisms involved. Moreover, there is growing evidence suggesting that EVs can serve as predictive biomarkers for lung function decline.41,49 The development of sensitive and reliable assays for EV-based biomarkers that reflect exposures and predict disease could revolutionize early detection of lung disease and lead to improved therapeutic interventions. However, this ambition is challenged by the inherent complexity and diversity of EVs, and the technical issues related to their isolation and characterization. Efforts to overcome these hurdles will require the development of robust and standardized methodologies.50 The exploration of therapeutic potential of EVs in pulmonary diseases, such as stem cell–derived EVs in alleviating airway inflammation and rescuing fetal lung underdevelopment, is an exciting prospect.51 However, the effective and safe use of EVs in therapeutic applications will require a deeper understanding of their biogenesis, cargo selection, and intercellular trafficking, in both healthy and disease states, as well as refinement of techniques to engineer and administer EVs.52,53
EVs, environment, and reproductive outcomes.
Exosomes (a subset of EVs that are in size) function as a communication channel between the mother and the fetus during human pregnancy and parturition.54 Both animal model studies55 and human trials56 have shown that fetal exosomes constitute of total exosomes in maternal blood at various stages of pregnancy.56 Information gathered from studies suggests the following: a) Feto-maternal exosomes can be communication channels, and they can propagate through the placenta54–56; b) fetal exosomes carry a balanced inflammatory cargo (both pro- and anti-inflammatory mediators) during early stages of gestation, and the cargo profile changes to pro-inflammatory (pro-parturition) prior to delivery57; c) inflammatory cargo-enriched fetal exosomes are high in maternal uterine tissues (decidua, myometrium, and cervix) at term58 and are capable of inducing uterotonic changes58; and d) proteomic56 and miRNA cargo59 of fetal exosomes are unique at different stages of human gestation and are different in term subjects compared with women delivering preterm, suggestive of the biomarker potential of exosomes.56,60
The biomarker potential of exosomes to predict preterm birth and other adverse pregnancy outcomes has been explored and reported, specifically in response to environmental exposures. Cigarette smoking was shown to be a major risk for preterm birth and preterm premature rupture of the fetal membranes (pPROM).61 Exposure to cigarette smoke in vitro led to human fetal membrane (amniochorion) senescence, which leads to the release of exosomes that carry labor-triggering inflammatory signaling molecules in humans.61 These data suggest that exosomes coming from prematurely aged or inflamed fetal cells can activate the labor process and cause preterm birth.57,60
Like cigarette smoke, other environmental toxicants, including flame retardants [polybrominated diphenyl ethers (PBDEs), bisphenol-A (BPA), and bisphenol-S (BPS)] were linked to preterm birth and pPROM.62 A recent study demonstrated that when exposed to PBDE or BPA/BPS, placental cells released exosomes containing cargo indicative of underlying inflammation.63 These exosomes caused paracrine signaling to generate an inflammatory process in the recipient cells, often resulting in pro-parturition signaling.60 These results suggest that exosomes isolated from circulating maternal blood samples from exposed women can be developed as biomarkers to identify the underlying risk of adverse pregnancies.
Other potential exosomal biomarkers for underlying pathology during pregnancy are changes in miRNAs and mRNAs. In placental tissue, miRNA expression was increased with preeclampsia (PE)64–72 and was associated with birth weight (including in mothers without PE).67 In maternal circulation, miRNA levels increased during pregnancy compared with the nonpregnant state.64,73,74 Circulating miRNAs have been demonstrated to be useful clinical biomarkers for several diseases, including endometriosis,75 autoimmune diseases,76 and cancer.77–80 miRNAs can be indicators of normal or pathological physiological processes, such as adaptive and innate immunity,81,82 angiogenesis,83,84 inflammation,85 hypoxia,70,86,87 and blood pressure regulation,88 as well as cell differentiation, apoptosis, migration and remodeling.89–91 High levels of miR-210 (a response to hypoxia) have been associated with reduced trophoblast invasion,68,71,92 reduced mitochondrial respiration in trophoblast cells,72 and defective placentation.68 The role of miR-210 in modulating fetal sex-dependent inflammatory activation has also been reported in placental cells.93 In addition, analysis of plasma exosomal mRNA from normotensive and preeclamptic mothers revealed a panel of 18 transcripts that could identify mothers at risk of preeclampsia before clinical symptoms develop.94 Further studies on cell-free exosomal RNA from diverse pregnancy cohorts of pregnant women suggested successful tracking of pregnancy progression for either normal or potentially preeclampsia outcomes.95 Certainly, all of these biological pathways have relevance for pregnancy health and development and may result in alteration in exosomal miRNA and mRNA expression that can be detected in maternal circulation as early as the first trimester of pregnancy.59 In summary, exosomes carry miRNAs and mRNAs that can be isolated from the maternal circulation as early as the first trimester and may be indicators of underlying physiology and pathology that lead to clinical biomarkers and indicate targets for intervention.
In perinatal medicine, a major challenge is to monitor the exposure of the fetus to pollutants. Given that clinical sampling from the fetus is impractical during normal pregnancies, fetal EVs circulating in the maternal systems can be an ideal biomarker indicative of fetal well-being and placental health. Future research may focus on identifying fetal-specific EVs in minimally invasive maternal biospecimens during pregnancy and characterizing their cargo (e.g., miRNA, the proteome) to develop exposure risk-associated changes in the fetus during its developmental stages.
Studying EV Biology Using OOCs
Overview of OOC.
Environmental toxicant exposure and cellular response are often reflected in EVs; however, their biomarker potential and functional capability need more exploration. The true reflection of a tissue response is not often captured in traditional two-dimensional (2D) cell cultures after specific exposures and EV analysis. Animal models are also not often suitable, for example, because pregnancy tissue architecture and exposure-associated endocrine responses are vastly different in experimental animal models currently used in reproductive biology experiments compared with humans.
Limits in studying complex human organ systems have encouraged interdisciplinary collaborations to develop advanced humanized platforms that better mimic the structure and functions of human organ systems. The combination of microfabrication, microfluidics, and well-characterized cell lines or the availability of induced pluripotent stem cells has provided many physiological models that more accurately mimic human anatomy, functions, and in vivo responses. These platforms, termed OOCs or microphysiological systems (MPS), provide compartments (or chambers) enabling culturing and organizing cellular, extracellular matrices (ECMs), and other microenvironmental layers within these compartments while still providing cellular signals, and sometimes even cells themselves, to propagate between the compartments through interconnected fluid paths.96 These systems allow researchers to test many different biomolecular factors under more physiologically relevant in vitro environments, for better understanding of human physiology through more data much faster and potentially much more cost effectively.
Technological advancements in the past decade have allowed the development of OOCs and 3D bio-printed tissue constructs that have provided a better platform to test organ response and the functional and biomarker potentials of EVs.97 OOCs are typically made from polydimethylsiloxane (PDMS) by soft lithographic and photolithographic approaches. OOCs are often interconnected through microchannels to maintain intercellular interactions.96,97 Three-dimensional bioprinting of tissues is a recent development and involves a method using hydrogel “bioinks” (materials suitable for biofabrication) loaded with cells of interest to construct engineered tissue-like structures.
Case study: OOCs and EVs in Fetal–Maternal Interactions.
OOCs were recently used to determine the propagation (feto-maternal) and function of EVs released by human fetal membrane cells and their impact on the maternal uterine environment. The signature of the cargo signals carried by fetal cell–derived EVs suggests a major role in determining maternal tissue fate and, therefore, pregnancy outcome.55 Release of EVs from the fetal cells and their importance in implantation, embryogenesis, pregnancy maintenance, and parturition have been demonstrated in several studies.98–103 Pregnancy homeostasis requires EV-mediated paracrine communication, which is normal and physiologic where EV cargo is likely to influence uterine cellular functions.104 However, changes in communication signals (based on the cargo contents of EVs) can be pathologic and disrupt the homeostasis of reproductive tissues. Thus, OOCs provide a suitable platform to study EV propagation.
Data also suggest that OOCs are an ideal platform to determine EV trafficking, function, and alterations related to various toxicants. Reports have shown that cigarette smoke exposure,61 as well as accumulation of other toxicants (e.g., PBDE, BPA), in the intra-amniotic cavity cause fetal membrane (amniochorion) cellular senescence.62 Senescent cell–derived EVs carry a unique set of inflammatory molecules: senescence-associated secretory proteins (SASPs), cellular oxidative injury markers, and damage-associated molecular pattern markers (DAMPs).104 SASPs and DAMP signaling via EVs are needed to promote term parturition; however, their arrival prior to term triggers premature disruption of reproductive tissue that is absent or very low in normal fetal cell–derived EVs.105 Thus, uptake of these EVs by the maternal tissues triggers a pro-inflammatory change to promote preterm labor leading to preterm births. A functional study using feto-maternal interface OOCs that used four cell types from both fetal and maternal sides interconnected through microchannels showed that EVs could propagate through different cell layers.98 Propagation of SASP- and DAMP-carrying EVs caused inflammation in feto-maternal interface cells. These data have been physiologically validated in mouse models where injection of EVs containing DAMPs caused preterm birth and caused inflammatory changes similar to those seen in cells of the OOC.60
Future directions for OOCs in EV research.
Researchers expect a substantial role for EVs in response to chemical toxicant exposures and environmental stressors. Insights into chemical effects on EVs includes the application of OOCs and MPS under controlled conditions with mixed cell types to mimic tissues capable of additional features, such as immune tolerance, which can help to characterize EVs associated with different exposures.96,106,107 Absorption, distribution, metabolism, and excretion (ADME) characteristics, metabolizing enzymes, transporters, and cellular polarity are among the end points for integration into exposure-related EVs,108 such as a human kidney MPS modeling ochratoxin A toxicity.109 Research consortiums are examining OOC and MPS systems using various cell types to mimic kidney, liver, gut, and lung, as well as the blood–brain barrier, with future objectives for incorporating toxicological effects on EVs.110
Challenges for Studying EVs in Population-Based Studies
Barriers to using EVs as biomarkers in population-based studies.
One of the barriers to be considered when using EVs as biomarkers in population-based studies is the heterogeneity and variability in EVs given that EVs can vary in size, source, and cargo.8,111 Several factors impact the intra-individual variability, including physiological changes, infections, diet, and medication use, all of which are difficult to control for in population-based studies.112 Moreover, in population-based studies, there is typically access only to samples that are easy to collect, such as saliva, blood, and urine, and not necessarily the specific tissue of interest.113 Thus, sample type is both a contributor to heterogeneity in detected EVs and typically only a proxy for the tissue of interest. Heterogeneity and variability in methods for isolation and characterization of EVs lead to challenges with reproducibility.9,114
Another barrier to using EVs is the variety of methods available for separating and isolating EVs from biofluids.114 Although absolute purification of EVs may be unattainable, methods for separation of EVs vary in the levels of non-EV components co-isolated with EVs, and methods should be chosen based on the research question of interest.50 Identifying and selecting EVs based on their tissue of origin is complicated.115 However, because EVs carry surface markers derived from their cells of origin, antibodies against cell-type–specific surface markers can be used to isolate cell-specific EVs from plasma or serum.116 Through this technique, EVs from neurons, astrocytes, oligodendrocytes, and hepatocytes can be identified.117–119 Isolation of EVs with these antibodies can enrich EVs for those cell type sources by 50- to 100-fold, thereby amplifying “their voices.”
Need for standardization.
Ideally, protocols and methods developed for isolating EVs should be rapid, reproducible, have a high-throughput capacity, and be specific in separating EVs.12,120 When surface markers are used to enrich for source-cell-type–specific EVs, the sensitivity varies across different antibodies used, which can lead to different results. The choice of EV isolation and analysis techniques can affect the sensitivity of EV detection and cargo identification.113 The tools for accurately assessing EV characteristics need further standardization.120 The board members of the International Society for Extracellular Vesicles have published editorials that outline the minimum information for studies of EVs (MISEV), which provide guidance for standardization of EV research.121,122 The authors of MISEV2018 note that there is no one-size-fits-all approach to EV separation and concentration, but detailed reporting of the method should be made to ensure reproducibility. Because it can be difficult to quantify the total number of EVs, studies should report the starting volumes of biofluids, justify the method used for normalization, and discuss alternative strategies that were considered.50,120 In general, the MISEV recommends reporting as many analytical parameters as possible.50
Cost and throughput considerations.
Once EVs are isolated, investigators are often interested in sequencing their content to obtain, for instance, RNA expression or genetic data. However, sequencing presents further challenges when performed on EVs enriched for specific cell types. First, because the EVs of interest are only a subset of the total EV population, the genetic material that can be isolated is often in very low concentrations, typically lower than the standards used by sequencing core facilities.111 Therefore, sequencing RNA or DNA in EVs isolated based on their cell type of origin requires specific sequencing protocols adapted for low RNA or DNA concentrations. Furthermore, tissues are not composed of only one cell type. For instance, the brain is composed of four main cell types, namely, neurons, oligodendrocytes, microglia, and astrocytes. Sequencing each cell type would require performing four parallel isolations to obtain EV preparations enriched for each cell type, which would then all be sequenced. Hence, the number of EV biospecimens would be four times greater than that of the original sample size, and the laboratory costs for isolation and sequencing would be proportionally higher.123 This issue could be resolved by using deconvolution analyses.8,123,124 Deconvolution has also been used to identify exRNA cargo type in various biofluids8 and to map extracellular RNA-binding proteins and their associated RNAs across major human biofluids and carriers.124 Although methods for deconvolution are still under development, they offer a way to reduce cost by removing the need to perform EV separation and source-cell-type enrichment before sequencing.
Practical considerations for applying EV biology to population-based studies.
As described by the MISEV2018 guidelines, several technical factors can affect EVs in a collected sample. It is important to consider processing to ensure that cells do not become apoptotic or lysed.50 Samples containing cells should be processed quickly, because if samples are kept outside their natural condition (i.e., ex vivo) for long periods of time, they will be less representative of what was present at the moment of blood or urine sampling.50 In addition, it is not clear whether samples are affected by long periods of storage. Although one study reported no significant effect of storage for 1 y at on EVs in plasma, the effect of storage for longer periods has not been studied.112 The same study reported that a single freeze–thaw cycle did not affect EV concentration or size in plasma, but another study reported that the presence of platelets can affect the number of EVs measured after a freeze–thaw.125 In addition, freeze–thaw of separated EVs can affect the quality of EVs because they can adhere to the surface of the storage container, differentially leak protein cargo at different temperatures,126 and degrade RNA.127 Methods used for protection of EVs in storage, such as cryoprotection, lyophilization, and spray drying, have been reported.128 These should be used consistently and any effects of these methods on EV characteristics should be tested. It will be important to perform technical quality control steps for determining sample quality before reporting any results, as outlined in the recommendations set by the MISEV50,120,121,129 in determining the appropriate controls to test the quality of samples for their research. Given the challenges and costs of population-based studies of environmentally related diseases, combining multiple approaches, such as conducting parallel human and experimental studies, might be especially fruitful in environmental health sciences, where clinical trials are rarely feasible.
Future Directions
Population studies are useful in identifying associations and developing biomarkers, but they are limited in examining mechanisms. Indeed, mechanistic studies typically require that the investigators control and assign the exposure(s) or directly modulate the mechanistic pathways to demonstrate their roles. For instance, investigators may use antisense RNA to silence EV-encapsulated miRNAs in in vitro studies to understand their functions. Moreover, in population studies the exact source of the EV and its uptake tissue are often unknown.
Single EV analysis provides a way to overcome such issues that are intrinsic to bulk EV analysis. Methods for single EV analysis have been reviewed elsewhere,130,131 but briefly, they involve different methodologies that allow for the analysis of differential surface markers or cargo on a single EV level. These methods tend to rely on universal markers of EVs, antibodies with high affinity and low nonspecific binding, and stringent purification methods that remove background interference.132 Some methods that have been employed include digital droplet PCR, digital enzyme-linked immunosorbent assays, flow cytometry (modified to work on the nanoscale using optimized optics and fluidics),133 nanoparticle tracking analysis, Raman spectroscopy, and microscopy.130,131 Although recent advancements in the field have shown promise, especially in early detection of cancer,132 most methods are burdened by high cost, complexity, and sometimes low throughput,133 making it difficult for adoption in large population studies at present. Technological advancements in the future may make these methods more accessible.
In this seminar, we have provided examples of experimental models that reveal mechanisms through which EVs may play a role in environment-related diseases, and when paired with population studies, experimental models are useful in providing meaning behind observed associations. For example, exposure to manganese led to accumulation of in EVs in cultured dopaminergic neuronal cells expressing , providing a mechanism through which exposure to manganese can lead to Parkinsonism.25 As another example, associations between exposure to PM in an occupational setting and circulating EV miRNA were confirmed through exposures in alveolar cells grown in culture.48 We also provide an overview of OOC systems as useful experimental models to confirm observational associations, given that they allow for more intercellular interactions compared with 2D cell culture systems. As evidenced by examples cited in this seminar, the authors note that EV research would be greatly advanced by cross-disciplinary collaborations and communication between researchers conducting population-based studies and experimental in vitro and animal studies. Population studies can provide real-life data on the influences of exposures on EVs and generate hypothesis on their roles on the paths leading to human disease. Experimental studies can help isolate the impact of specific exposures and pinpoint mechanistic roles of EVs. Together, cross-disciplinary research has the potential to accelerate knowledge on the functions of EVs in environmental health sciences.
References
- 1.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. 2012. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64(3):676–705, PMID: , 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 2.Pegtel DM, Gould SJ. 2019. Exosomes. Annu Rev Biochem 88:487–514, PMID: , 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 3.van Niel G, D’Angelo G, Raposo G. 2018. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4):213–228, PMID: , 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 4.Carberry CK, Keshava D, Payton A, Smith GJ, Rager JE. 2022. Approaches to incorporate extracellular vesicles into exposure science, toxicology, and public health research. J Expo Sci Environ Epidemiol 32(5):647–659, PMID: , 10.1038/s41370-022-00417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ainsztein AM, Brooks PJ, Dugan VG, Ganguly A, Guo M, Howcroft TK, et al. 2015. The NIH extracellular RNA communication consortium. J Extracell Vesicles 4:27493, PMID: , 10.3402/jev.v4.27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Extracellular RNA Communication Consortium, Ansel KM, Bitzer M, Breakefield XO, Charest A, et al. 2019. Extracellular RNA Communication Consortium: establishing foundational knowledge and technologies for extracellular RNA research. Cell 177(2):231–242, PMID: , 10.1016/j.cell.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bioinformatics Research Laboratory. 2016. eRNA Atlas: Data, Tools & Computable Knowledge. https://exrna-atlas.org [accessed 4 August 2023].
- 8.Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, et al. 2019. exRNA Atlas analysis reveals distinct extracellular RNA cargo types and their carriers present across human biofluids. Cell 177(2):463–477.e15, PMID: , 10.1016/j.cell.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozowsky J, Kitchen RR, Park JJ, Galeev TR, Diao J, Warrell J, et al. 2019. exceRpt: a comprehensive analytic platform for extracellular RNA profiling. Cell Syst 8(4):352–357.e3, PMID: , 10.1016/j.cels.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan S, Yeri A, Cheah PS, Chung A, Danielson K, De Hoff P, et al. 2019. Small RNA sequencing across diverse biofluids identifies optimal methods for exRNA isolation. Cell 177(2):446–462.e16, PMID: , 10.1016/j.cell.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateescu B, Jones JC, Alexander RP, Alsop E, An JY, Asghari M, et al. 2022. Phase 2 of extracellular RNA communication consortium charts next-generation approaches for extracellular RNA research. iScience 25(8):104653, PMID: , 10.1016/j.isci.2022.104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, et al. 2013. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy 43(4):443–454, PMID: , 10.1111/cea.12085. [DOI] [PubMed] [Google Scholar]
- 13.Si X, Tian J, Chen Y, Yan Y, Pu J, Zhang B. 2019. Central nervous system-derived exosomal alpha-synuclein in serum may be a biomarker in Parkinson’s disease. Neuroscience 413:308–316, PMID: , 10.1016/j.neuroscience.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Eren E, Leoutsakos JM, Troncoso J, Lyketsos CG, Oh ES, Kapogiannis D. 2022. Neuronal-derived EV biomarkers track cognitive decline in Alzheimer’s disease. Cells 11(3):436, PMID: , 10.3390/cells11030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsu M, Hama Y, Utsumi J, Takashina K, Yasumatsu H, Mori F, et al. 2019. MicroRNA expression profiles of neuron-derived extracellular vesicles in plasma from patients with amyotrophic lateral sclerosis. Neurosci Lett 708:134176, PMID: , 10.1016/j.neulet.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 16.U.N. Department of Economic and Social Affairs, Population Division. 2020. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons. ST/ESA/SER.A/451. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd-2020_world_population_ageing_highlights.pdf [accessed 4 August 2023].
- 17.Jin T, Gu J, Li Z, Xu Z, Gui Y. 2021. Recent advances on extracellular vesicles in central nervous system diseases. Clin Interv Aging 16:257–274, PMID: , 10.2147/CIA.S288415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennet MC. 2005. The role of α-synuclein in neurodegenerative diseases. Pharmacol Ther 105(3):311–331, PMID: , 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. 2008. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J 37(3):323–332, PMID: , 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 20.Lin CH, Yang SY, Horng HE, Yang CC, Chieh JJ, Chen HH, et al. 2017. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J Neurol Neurosurg Psychiatry 88(10):818–824, PMID: , 10.1136/jnnp-2016-314857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritz B, Rhodes SL, Bordelon Y, Bronstein J. 2012. α-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PLoS One 7(5):e36199, PMID: , 10.1371/journal.pone.0036199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. 2019. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol 76(8):915–924, PMID: , 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, et al. 2016. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain 139(pt 12):3187–3201, PMID: , 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker S, Polanco JC, Götz J. 2016. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. J Alzheimers Dis 54(3):1207–1217, PMID: , 10.3233/JAD-160371. [DOI] [PubMed] [Google Scholar]
- 25.Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, et al. 2019. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci Signal 12(572):eaau4543, PMID: , 10.1126/scisignal.aau4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson S, Baccarelli A, Prada D. 2022. Role of brain extracellular vesicles in air pollution-related cognitive impairment and neurodegeneration. Environ Res 204(pt C):112316, PMID: , 10.1016/j.envres.2021.112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutchy NA, Peeples ES, Sil S, Liao K, Chivero ET, Hu G, et al. 2020. Extracellular vesicles in viral infections of the nervous system. Viruses 12(7):700, PMID: , 10.3390/v12070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garza AP, Morton L, Pállinger É, Buzás EI, Schreiber S, Schott BH, et al. 2023. Initial and ongoing tobacco smoking elicits vascular damage and distinct inflammatory response linked to neurodegeneration. Brain Behav Immun Health 28:100597, PMID: , 10.1016/j.bbih.2023.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller GW, Jones DP. 2014. The nature of nurture: refining the definition of the exposome. Toxicol Sci 137(1):1–2, PMID: , 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dar GH, Badierah R, Nathan EG, Bhat MA, Dar AH, Redwan EM, et al. 2022. Extracellular vesicles: a new paradigm in understanding, diagnosing and treating neurodegenerative disease. Front Aging Neurosci 14:967231, PMID: , 10.3389/fnagi.2022.967231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagey DW, El Andaloussi S. 2023. The promise and challenges of extracellular vesicles in the diagnosis of neurodegenerative diseases. Handb Clin Neurol 193:227–241, PMID: , 10.1016/B978-0-323-85555-6.00014-X. [DOI] [PubMed] [Google Scholar]
- 32.Kurt OK, Zhang J, Pinkerton KE. 2016. Pulmonary health effects of air pollution. Curr Opin Pulm Med 22(2):138–143, PMID: , 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastor L, Vera E, Marin JM, Sanz-Rubio D. 2021. Extracellular vesicles from airway secretions: new insights in lung diseases. Int J Mol Sci 22(2):583, PMID: , 10.3390/ijms22020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong L, Wang Y, Zheng T, Pu Y, Ma Y, Qi X, et al. 2021. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Res Ther 12(1):4, PMID: , 10.1186/s13287-020-02072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antounians L, Catania VD, Montalva L, Liu BD, Hou H, Chan C, et al. 2021. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med 13(590):eaax5941, PMID: , 10.1126/scitranslmed.aax5941. [DOI] [PubMed] [Google Scholar]
- 36.Velázquez-Enríquez JM, Santos-Álvarez JC, Ramírez-Hernández AA, Reyes-Jiménez E, López-Martínez A, Pina-Canseco S, et al. 2021. Proteomic analysis reveals key proteins in extracellular vesicles cargo associated with idiopathic pulmonary fibrosis in vitro. Biomedicines 9(8):1058, PMID: , 10.3390/biomedicines9081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, Tando Y, et al. 2014. Annual FEV1 changes and numbers of circulating endothelial microparticles in patients with COPD: a prospective study. BMJ Open 4(3):e004571, PMID: , 10.1136/bmjopen-2013-004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, He M, et al. 2012. Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax 67(12):1067–1074, PMID: , 10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- 39.Buzas EI, György B, Nagy G, Falus A, Gay S. 2014. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10(6):356–364, PMID: , 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 40.Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, et al. 2013. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med 188(1):60–68, PMID: , 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckhardt CM, Gambazza S, Bloomquist TR, De Hoff P, Vuppala A, Vokonas PS, et al. 2023. Extracellular vesicle-encapsulated microRNAs as novel biomarkers of lung health. Am J Respir Crit Care Med 207(1):50–59, PMID: , 10.1164/rccm.202109-2208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedikter BJ, Wouters EFM, Savelkoul PHM, Rohde GGU, Stassen FRM. 2018. Extracellular vesicles released in response to respiratory exposures: implications for chronic disease. J Toxicol Environ Health B Crit Rev 21(3):142–160, PMID: , 10.1080/10937404.2018.1466380. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Ding L, Zhang Y, Ni S. 2014. Circulating endothelial microparticles involved in lung function decline in a rat exposed in cigarette smoke maybe from apoptotic pulmonary capillary endothelial cells. J Thorac Dis 6(6):649–655, PMID: , 10.3978/j.issn.2072-1439.2014.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, et al. 2011. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med 184(2):224–232, PMID: , 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haggadone MD, Mancuso P, Peters-Golden M. 2020. Oxidative inactivation of the proteasome augments alveolar macrophage secretion of vesicular SOCS3. Cells 9(7):E1589, PMID: , 10.3390/cells9071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corsello T, Kudlicki AS, Garofalo RP, Casola A. 2019. Cigarette smoke condensate exposure changes RNA content of extracellular vesicles released from small airway epithelial cells. Cells 8(12):1652, PMID: , 10.3390/cells8121652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Wang T, Rui W, Xie J, Xie Y, Zhang X, et al. 2022. Extracellular vesicles enclosed-miR-421 suppresses air pollution (PM2.5)-induced cardiac dysfunction via ACE2 signalling. J Extracell Vesicles 11(5):e12222, PMID: , 10.1002/jev2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, et al. 2015. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol 35(1):59–67, PMID: , 10.1002/jat.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Ma Y, Chen Y. 2023. Extracellular vesicles and COPD: foe or friend? J Nanobiotechnology 21(1):147, PMID: , 10.1186/s12951-023-01911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750, PMID: , 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azhdari MH, Goodarzi N, Doroudian M, MacLoughlin R. 2022. Molecular insight into the therapeutic effects of stem cell-derived exosomes in respiratory diseases and the potential for pulmonary delivery. Int J Mol Sci 23(11):6273, PMID: , 10.3390/ijms23116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann IK, Wood MJA, Fuhrmann G. 2021. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 16(7):748–759, PMID: , 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 53.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. 2019. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med 51(3):1–12, PMID: , 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon R, Shahin H. 2021. Extracellular vesicles in spontaneous preterm birth. Am J Reprod Immunol 85(2):e13353, PMID: , 10.1111/aji.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheller-Miller S, Choi K, Choi C, Menon R. 2019. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol 221(5):502.e1–502.e12, PMID: , 10.1016/j.ajog.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal P, et al. 2020. Protein profile changes in circulating placental extracellular vesicles in term and preterm births: a longitudinal study. Endocrinology 161(4):bqaa009, PMID: , 10.1210/endocr/bqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheller-Miller S, Trivedi J, Yellon SM, Menon R. 2019. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci Rep 9(1):608, PMID: , 10.1038/s41598-018-37002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hadley EE, Sheller-Miller S, Saade G, Salomon C, Mesiano S, Taylor RN, et al. 2018. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol 219(5):478.e1–478.e21, PMID: , 10.1016/j.ajog.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menon R, Debnath C, Lai A, Guanzon D, Bhatnagar S, Kshetrapal PK, et al. 2019. Circulating exosomal miRNA profile during term and preterm birth pregnancies: a longitudinal study. Endocrinology 160(2):249–275, PMID: , 10.1210/en.2018-00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menon R. 2022. Fetal inflammatory response at the fetomaternal interface: a requirement for labor at term and preterm. Immunol Rev 308(1):149–167, PMID: , 10.1111/imr.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, et al. 2014. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 184(6):1740–1751, PMID: , 10.1016/j.ajpath.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Behnia F, Peltier MR, Saade GR, Menon R. 2015. Environmental pollutant polybrominated diphenyl ether, a flame retardant, induces primary amnion cell senescence. Am J Reprod Immunol 74(5):398–406, PMID: , 10.1111/aji.12414. [DOI] [PubMed] [Google Scholar]
- 63.Sheller-Miller S, Radnaa E, Arita Y, Getahun D, Jones RJ, Peltier MR, et al. 2020. Environmental pollutant induced cellular injury is reflected in exosomes from placental explants. Placenta 89:42–49, PMID: , 10.1016/j.placenta.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betoni JS, Derr K, Pahl MC, Rogers L, Muller CL, Packard RE, et al. 2013. MicroRNA analysis in placentas from patients with preeclampsia: comparison of new and published results. Hypertens Pregnancy 32(4):321–339, PMID: , 10.3109/10641955.2013.807819. [DOI] [PubMed] [Google Scholar]
- 65.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. 2011. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 204(2):178.e12–178.e121, PMID: , 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, et al. 2012. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension 59(2):265–273, PMID: , 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 67.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, et al. 2011. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol 179(2):590–602, PMID: , 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. 2007. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196(3):261.e1–261.e6, PMID: , 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li YX, et al. 2014. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 63(6):1276–1284, PMID: , 10.1161/HYPERTENSIONAHA.113.02647. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, et al. 2012. Elevated levels of hypoxia‐inducible microRNA‐210 in pre‐eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med 16(2):249–259, PMID: , 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. 2009. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200(6):661.e1–661.e7, PMID: , 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 72.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. 2012. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 33(10):816–823, PMID: , 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. 2010. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta 31(9):781–784, PMID: , 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subramanian A, Weiss D, Nyhan K, Dewan A, Jukic AMZ. 2023. Circulating miRNAs in the first trimester and pregnancy complications: a systematic review. Epigenetics Dec 18(1):2152615, PMID: , 10.1080/15592294.2022.2152615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. 2013. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab 98(1):281–289, PMID: , 10.1210/jc.2012-2415. [DOI] [PubMed] [Google Scholar]
- 76.Zeng L, Cui J, Wu H, Lu Q. 2014. The emerging role of circulating microRNAs as biomarkers in autoimmune diseases. Autoimmunity 47(7):419–429, PMID: , 10.3109/08916934.2014.929667. [DOI] [PubMed] [Google Scholar]
- 77.Kosaka N, Iguchi H, Ochiya T. 2010. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101(10):2087–2092, PMID: , 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M. 2011. Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol 45(4):355–360, PMID: , 10.1097/MCG.0b013e3181f18ac2. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Hou J, Jin W, Li J, Yue Y, Jin H, et al. 2014. Increased circulating microRNA-155 as a potential biomarker for breast cancer screening: a meta-analysis. Molecules 19(5):6282–6293, PMID: , 10.3390/molecules19056282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu W, Wang Z, Shen LI, Wei Q. 2016. Circulating microRNA-21 as a potential diagnostic marker for colorectal cancer: a meta-analysis. Mol Clin Oncol 4(2):237–244, PMID: , 10.3892/mco.2015.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gracias DT, Katsikis PD. 2011. MicroRNAs: key components of immune regulation. Adv Exp Med Biol 780:15–26, PMID: , 10.1007/978-1-4419-5632-3_2. [DOI] [PubMed] [Google Scholar]
- 82.Singh RP, Massachi I, Manickavel S, Singh S, Rao NP, Hasan S, et al. 2013. The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 12(12):1160–1165, PMID: , 10.1016/j.autrev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. 2006. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108(9):3068–3071, PMID: , 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 84.Suárez Y, Sessa WC. 2009. MicroRNAs as novel regulators of angiogenesis. Circ Res 104(4):442–454, PMID: , 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chou WW, Wang YT, Liao YC, Chuang SC, Wang SN, Juo SHH. 2013. Decreased microRNA-221 is associated with high levels of TNF-α in human adipose tissue-derived mesenchymal stem cells from obese woman. Cell Physiol Biochem 32(1):127–137, PMID: , 10.1159/000350131. [DOI] [PubMed] [Google Scholar]
- 86.Ivan M, Harris AL, Martelli F, Kulshreshtha R. 2008. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med 12(5A):1426–1431, PMID: , 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan YC, Banerjee J, Choi SY, Sen CK. 2012. miR-210: the master hypoxamir. Microcirculation 19(3):215–223, PMID: , 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. 2011. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet 4(2):197–205, PMID: , 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 89.Nagpal N, Kulshreshtha R. 2014. miR-191: an emerging player in disease biology. Front Genet 5:99, PMID: , 10.3389/fgene.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seabrook JL, Cantlon JD, Cooney AJ, McWhorter EE, Fromme BA, Bouma GJ, et al. 2013. Role of LIN28A in mouse and human trophoblast cell differentiation. Biol Reprod 89(4):95, PMID: , 10.1095/biolreprod.113.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, Zuo Z, Lu X, Wang L, Wang H, Zhu Z. 2012. MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol Rep 27(2):594–598, PMID: , 10.3892/or.2011.1530. [DOI] [PubMed] [Google Scholar]
- 92.Anton L, Olarerin-George AO, Schwartz N, Srinivas S, Bastek J, Hogenesch JB, et al. 2013. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol 183(5):1437–1445, PMID: , 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muralimanoharan S, Guo C, Myatt L, Maloyan A. 2015. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond) 39(8):1274–1281, PMID: , 10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moufarrej MN, Vorperian SK, Wong RJ, Campos AA, Quaintance CC, Sit RV, et al. 2022. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 602(7898):689–694, PMID: , 10.1038/s41586-022-04410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasmussen M, Reddy M, Nolan R, Camunas-Soler J, Khodursky A, Scheller NM, et al. 2022. RNA profiles reveal signatures of future health and disease in pregnancy. Nature 601(7893):422–427, PMID: , 10.1038/s41586-021-04249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowers EC, Hassanin AAI, Ramos KS. 2020. In vitro models of exosome biology and toxicology: new frontiers in biomedical research. Toxicol In Vitro 64:104462, PMID: , 10.1016/j.tiv.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richardson L, Kim S, Menon R, Han A. 2020. Organ-on-chip technology: the future of feto-maternal interface research? Front Physiol 11:715, PMID: , 10.3389/fphys.2020.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radnaa E, Richardson LS, Sheller-Miller S, Baljinnyam T, de Castro Silva M, Kumar Kammala A, et al. 2021. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab Chip 21(10):1956–1973, PMID: , 10.1039/d0lc01323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tantengco OAG, Richardson LS, Medina PMB, Han A, Menon R. 2021. Organ-on-chip of the cervical epithelial layer: a platform to study normal and pathological cellular remodeling of the cervix. FASEB J 35(4):e21463, PMID: , 10.1096/fj.202002590RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson LS, Kim S, Han A, Menon R. 2020. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip 20(23):4486–4501, PMID: , 10.1039/d0lc00875c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen K, Liang J, Qin T, Zhang Y, Chen X, Wang Z. 2022. The role of extracellular vesicles in embryo implantation. Front Endocrinol (Lausanne) 13:809596, PMID: , 10.3389/fendo.2022.809596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi S, Tan Q, Liang J, Cao D, Wang S, Liang J, et al. 2021. Placental trophoblast cell-derived exosomal microRNA-1290 promotes the interaction between endometrium and embryo by targeting LHX6. Mol Ther Nucleic Acids 26:760–772, PMID: , 10.1016/j.omtn.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghafourian M, Mahdavi R, Akbari Jonoush Z, Sadeghi M, Ghadiri N, Farzaneh M, et al. 2022. The implications of exosomes in pregnancy: emerging as new diagnostic markers and therapeutics targets. Cell Commun Signal 20(1):51, PMID: , 10.1186/s12964-022-00853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menon R, Mesiano S, Taylor RN. 2017. Programmed fetal membrane senescence and exosome-mediated signaling: a mechanism associated with timing of human parturition. Front Endocrinol (Lausanne) 8:196, PMID: , 10.3389/fendo.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shahin HI, Radnaa E, Tantengco OAG, Kechichian T, Kammala AK, Sheller-Miller S, et al. 2021. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cells. Biol Reprod 105(2):464–480, PMID: , 10.1093/biolre/ioab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin Y, Long L, Huang Q. 2020. Extracellular vesicles in toxicological studies: key roles in communication between environmental stress and adverse outcomes. J Appl Toxicol 40(9):1166–1182, PMID: , 10.1002/jat.3963. [DOI] [PubMed] [Google Scholar]
- 107.Holman NS, Church RJ, Nautiyal M, Rose KA, Thacker SE, Otieno MA, et al. 2019. Hepatocyte-derived exosomes promote liver immune tolerance: possible implications for idiosyncratic drug-induced liver injury. Toxicol Sci 170(2):499–508, PMID: , 10.1093/toxsci/kfz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Ness KP, Cesar F, Yeung CK, Himmelfarb J, Kelly EJ. 2022. Microphysiological systems in absorption, distribution, metabolism, and elimination sciences. Clin Transl Sci 15(1):9–42, PMID: , 10.1111/cts.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Imaoka T, Yang J, Wang L, McDonald MG, Afsharinejad Z, Bammler TK, et al. 2020. Microphysiological system modeling of ochratoxin A-associated nephrotoxicity. Toxicology 444:152582, PMID: , 10.1016/j.tox.2020.152582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rusyn I, Chiu WA, Wright FA. 2022. Model systems and organisms for addressing inter- and intra-species variability in risk assessment. Regul Toxicol Pharmacol 132:105197, PMID: , 10.1016/j.yrtph.2022.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. 2018. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:738, PMID: , 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuana Y, Böing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, et al. 2015. Handling and storage of human body fluids for analysis of extracellular vesicles. J Extracell Vesicles 4:29260, PMID: , 10.3402/jev.v4.29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. 2015. Isolation of extracellular vesicles: determining the correct approach (review). Int J Mol Med 36(1):11–17, PMID: , 10.3892/ijmm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Allelein S, Medina-Perez P, Lopes ALH, Rau S, Hause G, Kölsch A, et al. 2021. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci Rep 11(1):11585, PMID: , 10.1038/s41598-021-91129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mustapic M, Eitan E, Werner JK Jr, Berkowitz ST, Lazaropoulos MP, Tran J, et al. 2017. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci 11:278, PMID: , 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y, He X, Li Q, Lai H, Zhang H, Hu Z, et al. 2020. EV-origin: enumerating the tissue-cellular origin of circulating extracellular vesicles using exLR profile. Comput Struct Biotechnol J 18:2851–2859, PMID: , 10.1016/j.csbj.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pistono C, Bister N, Stanová I, Malm T. 2021. Glia-derived extracellular vesicles: role in central nervous system communication in health and disease. Front Cell Dev Biol 8:623771, PMID: , 10.3389/fcell.2020.623771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa LLW, et al. 2020. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med 12(568):eaba0599, PMID: , 10.1126/scitranslmed.aba0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Royo F, Moreno L, Mleczko J, Palomo L, Gonzalez E, Cabrera D, et al. 2017. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci Rep 7:42798, PMID: , 10.1038/srep42798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. 2013. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2:20360, PMID: , 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. 2014. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913, PMID: , 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doyle LM, Wang MZ. 2019. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7):727, PMID: , 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alexander RP, Kitchen RR, Tosar JP, Roth M, Mestdagh P, Max KEA, et al. 2022. Open problems in extracellular RNA data analysis: insights from an ERCC online workshop. Front Genet 12:778416, PMID: , 10.3389/fgene.2021.778416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.LaPlante EL, Stürchler A, Fullem R, Chen D, Starner AC, Esquivel E, et al. 2023. exRNA-eCLIP intersection analysis reveals a map of extracellular RNA binding proteins and associated RNAs across major human biofluids and carriers. Cell Genom 3(5):100303, PMID: , 10.1016/j.xgen.2023.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. 2017. Methodological guidelines to study extracellular vesicles. Circ Res 120(10):1632–1648, PMID: , 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 126.Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. 2017. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles 6(1):1359478, PMID: , 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu Y, Deng W, Klinke DJ II.. 2015. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 140(19):6631–6642, PMID: , 10.1039/c5an00688k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. 2018. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol 9:1199, PMID: , 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snyder OL, Campbell AW, Christenson LK, Weiss ML. 2021. Improving reproducibility to meet Minimal Information for Studies of Extracellular Vesicles 2018 guidelines in nanoparticle tracking analysis. J Vis Exp 177:10.3791/63059, PMID: , 10.3791/63059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwon Y, Park J. 2022. Methods to analyze extracellular vesicles at single particle level. Micro Nano Syst Lett 10(1):14, 10.1186/s40486-022-00156-5. [DOI] [Google Scholar]
- 131.Hilton SH, White IM. 2021. Advances in the analysis of single extracellular vesicles: a critical review. Sens Actuators Rep 3:100052, PMID: , 10.1016/j.snr.2021.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferguson S, Yang KS, Zelga P, Liss AS, Carlson JCT, Del Castillo CF, et al. 2022. Single-EV analysis (sEVA) of mutated proteins allows detection of stage 1 pancreatic cancer. Sci Adv 8(16):eabm3453, PMID: , 10.1126/sciadv.abm3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arce JE, Welsh JA, Cook S, Tigges J, Ghiran I, Jones JC, et al. 2023. The NanoFlow repository. Bioinformatics 39(6):btad368, PMID: , 10.1093/bioinformatics/btad368. [DOI] [PMC free article] [PubMed] [Google Scholar]