Abstract
Drug-induced immune hemolytic anemia (DIIHA) is a relatively uncommon cause of anemia, and its diagnosis can be challenging. Although beta-lactam antimicrobial agents are often associated with DIIHA, any medication can potentially cause it. We describe a patient presenting with yellow skin discoloration and orange-colored urine after starting metaxalone for treatment of lumbosacral sprain. Laboratory studies were consistent with warm hemolytic anemia. Symptoms improved remarkably after discontinuation of metaxalone, coupled with initiation of glucocorticoids and rituximab.
Keywords: Metaxalone, Drug induced immune hemolytic anemia, Hemolysis
1. Introduction
Autoimmune hemolytic anemia (AIHA) is a rare autoantibody-mediated immune disorder with an incidence of 1–3 per 100 000 individuals per year.1–3 Warm autoimmune hemolytic anemia (w-AIHA) is the most common type of AIHA, comprising approximately 70%–80% of all adult and about 50% of pediatrics cases.1,2 About 50% of w-AIHA cases are primary (or idiopathic) whereas the remainder are considered secondary to lymphoproliferative syndromes; malignant diseases (chronic lymphoblastic leukemia, non-Hodgkin’s lymphoma, solid organ tumors); rheumatologic diseases (systemic lupus erythematosus); infections, drugs or previous transfusion or transplantation.4,5
In 1962, the United States Food and Drug Administration (US FDA) approved metaxalone for use in the US.7 Since then, it has been a widely used muscle relaxant.8,9 It acts centrally as a skeletal muscle relaxant, but its exact mechanism of action has not yet been established.
We describe a 35-year-old man with no significant past medical history presenting with warm autoimmune hemolytic anemia due to metaxalone use. While the FDA label for metaxalone notes hemolytic anemia as an adverse effect (likely due to evidence in animal models), human reports are largely missing from the literature.
2. Case
A 35-year-old man without significant past medical history presented with complaints of 1 week history of yellow discoloration of skin and eyes. A week prior to presentation, he was seen at an urgent care facility for evaluation of low back pain, where he was prescribed methylprednisolone (Medrol) and metaxalone (Skelaxin). About 72-h after he had taken a total of eight doses of 800 mg metaxalone, he noticed yellow discoloration of his skin and eyes, accompanied by orange-colored micturition. He denied any associated fever, night sweats, weight loss or early satiety.
On admission, vital signs were stable, and Body Mass Index (BMI) was 34.02 kg/m2. Physical examination revealed jaundice, scleral icterus, and a 14 cm palpable splenomegaly. There was no hepatomegaly or lymphadenopathy. The patient works as a paralegal, and denied using over-the-counter herbal products or medications, recreational drugs, tobacco, but drinks approximately two beers per week. He reported no known family history of anemia.
Initial laboratory findings were remarkable for hemolytic anemia as shown by low hemoglobin, indirect hyperbilirubinemia, increased reticulocyte count, low haptoglobin and elevated lactate dehydrogenase (LDH) (Table 1). For hyperbilirubinemia workup, we obtained serologies for viral hepatitis (A, B, C) which were all negative. To rule out other causes of hemolysis, we obtained flow cytometry for Paroxysmal Nocturnal Hemoglobinuria (PNH), Disseminated Intravascular Coagulation (DIC) panel, Epstein–Barr virus (EBV) and Cytomegalovirus(CMV) PCR, as well as HIV-1/HIV-2 antigen/antibody and Antinuclear antibodies (ANA) blood screening, which were all negative. Peripheral blood smear (PBS) revealed red blood cell anisocytosis and spherocytes, placing hemolytic anemia higher on the differential diagnosis. Direct antiglobulin test (DAT) was positive for IgG (4+) and negative for C3 which is consistent with a warm-reactive autoantibody. Immunohematology serology was also reactive for warm autoantibodies. Computerized Tomography (CT) of the abdomen with contrast revealed splenomegaly measuring 14 cm in the Anterior-Posterior (AP) dimension, and flow cytometry ruled out non-Hodgkin’s lymphoma or high-grade myeloid neoplasm.
Table 1.
Initial laboratory results.
| Lab | Value | Reference |
|---|---|---|
| Hemoglobin (g/dL) | 8.1 | 12.0–15.6 |
| MCV (fl) | 96.1 | 80.7–98.3 |
| Platelet count (E9/L) | 314 | 153–416 |
| Total bilirubin (mg/dL) | 6.6 | 0.2–1.2 |
| Direct bilirubin (mg/dL) | 1.05 | 0.1–0.5 |
| Haptoglobin (mg/dL) | 6 | 30–200 |
| Reticulocyte count (%) | 18.54 | 0.5–1.7 |
| LDH (U/L) | 294 | 125–220 |
| Prothrombin time (seconds) | 13.0 | 12.1–14.8 |
| International normalized ratio (INR) | 1.0 | 0.9–1.1 |
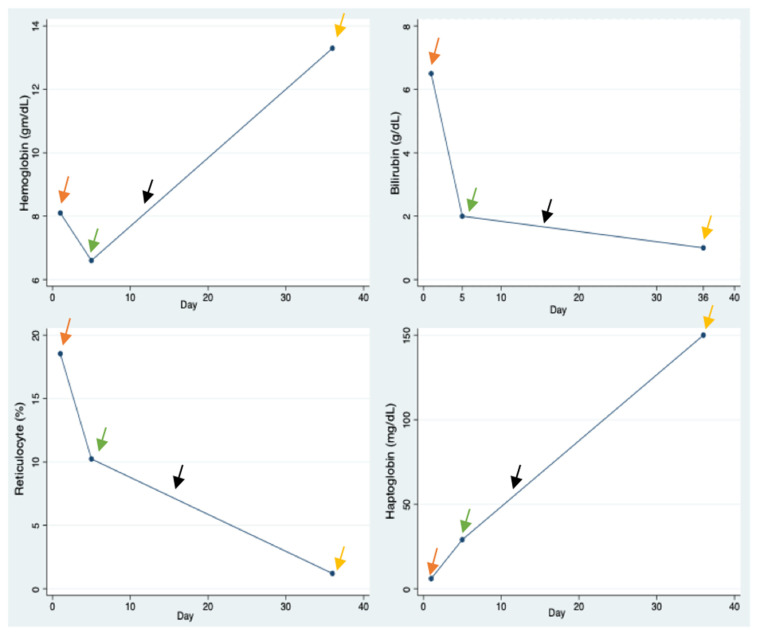
DIIHA secondary to Metaxalone was diagnosed and prednisone 120 mg once daily was initiated on day 1. Due to lack of significant improvement on hemolysis-associated laboratory parameters (hemoglobin, haptoglobin, reticulocyte count and bilirubin), a weekly infusion of Rituximab 900 mg for 4 weeks was started on day 5. Both clinical and laboratory parameters improvement ensued afterwards, and patient was discharged home (Fig. 1).
Fig. 1.
Lab values. Orange arrows – Initiation of prednisone. Green arrows – Initiation of rituximab therapy. Black arrows – Initiation of prednisone taper. Yellow arrows – Completion of rituximab therapy.
After 14 days of steroid use, prednisone was slowly tapered off based on a protocol of 10 mg weekly until a daily dose of 20 mg, followed by 5 mg every week until a dose of 15 mg, and subsequently by 2.5 mg every two weeks with the aim of withdrawing the drug.10 During follow up, drug-dependent Red Blood Cell (RBC) antibody testing to evaluate metaxalone-induced immune hemolysis was completed (Blood Center of Wisconsin test code 3110) but resulted as negative. Hemolysis laboratory indices were also monitored and had remarkably improved.
3. Discussion
AIHA is a broad term used for hemolysis caused by autoantibodies. This can be further sub-classified into warm AIHA (hemolysis due to autoantibodies binding at body temperature), Cold Agglutin Disease (hemolysis occurring at colder temperatures) or DIIHA which occurs after a drug triggers the immune system to produce autoantibodies against red blood cells. DIIHA is rare and accounts for 16 to 18 percent of cases of secondary w-AIHA.6 It can present as mild or severe AIHA and can manifest as-soon-as hours to as-long-as months after initial drug intake. Most cases of DIIHA are thought to be attributable to drug-dependent antibodies in which the immune response is caused by a bond between the drug and the red blood cell (RBC) membrane. In other cases, DIIHA is caused by drug-independent antibodies where drugs are thought to induce red blood cell autoantibodies that coat the red blood cells and are subsequently destroyed by macrophages. 11 A relatively newer mechanism of DIIHA is non-immune protein adsorption in which drugs modify the RBC membrane enabling non-immunologic adsorption12 (Table 2).
Table 2.
Drugs causing autoimmune hemolytic anemia grouped by mechanism of action.
| Drug-independent antibodies | Drug dependent antibodies | Nonimmunologic protein adsorption |
|---|---|---|
| Fludarabine | Penicillin | Cephalothin |
| Levodopa | Cefotetan | Cisplatin |
| Methyldopa | Ceftriaxone | Carboplatin |
| Procainamide | Quinidine | Sulbactam |
| Phenacetin | Clavulanate | |
| Tazobactam |
Common adverse effects reported with metaxalone include dizziness, headache, and gastrointestinal disturbance such as nausea and vomiting. Hemolytic anemia, jaundice and leukopenia have been listed as rare side effects, however the mechanism by which these side effects occur remains unclear.
The clinical presentation of DIIHA is often ambiguous and mostly linked to the underlying anemia. Symptoms such as jaundice, dark discoloration of urine and fatigue are often reported. Pallor, jaundice, hepatomegaly, splenomegaly, or lymphadenopathy may be noted during physical examination.13,14 The temporal relationship between the use of metaxalone and development of symptoms is evident in our case as jaundice occurred after exposure to the medication.13
Laboratory findings suggestive of hemolytic anemia include elevated LDH, reticulocyte count and indirect bilirubin, and low hemoglobin and haptoglobin. PBS may show poikilocytosis, schistocytes, spherocytes, anisocytosis, or polychromasia.14 The patient in this case had laboratory evidence of hemolytic anemia and his PBS revealed anisocytosis and spherocytes. Of importance, a comprehensive medical and medication history is very important in any patient presenting with signs and symptoms of hemolytic anemia.
The diagnosis of DIIHA is established by a positive DAT. In rare cases where patients have received transfusions or have had significant intravascular hemolysis, the DAT may be negative. If IgG alone as seen in this patient, or IgG with C3 are positive, WAIHA and DIIHA should be taken into consideration, and further evaluation should be carried out to evaluate whether an autoantibody is present.14 The mainstay of treatment is identification and withdrawal of the offending agent. Previous publications have suggested that corticosteroids may play a role in the management of DIIHA, however these case reports are often confounded by simultaneous administration of corticosteroid therapy alongside discontinuation of the offending drug. Regardless, corticosteroids are generally regarded as the first-line treatment for autoimmune hemolytic anemia. Patients are usually managed with Prednisone at 1 mg/kg/day for 2–4 weeks followed by a steroid taper.15
Rituximab, a monoclonal IgG antibody that targets CD20 can also be used in DIIHA refractory to steroids, as seen in our patient whose hemoglobin level continued to drop despite being on prednisone. Rituximab is commonly used in the treatment of chronic lymphocytic leukemia, Non-Hodgkin’s Lymphoma, and rheumatoid arthritis, however off-label, it is used in the management of AIHA.16 The use of Rituximab for treatment of AIHA has been studied both as monotherapy and as combination therapy with steroids. A Danish phase III randomized clinical trial compared corticosteroid monotherapy (n = 32) with corticosteroid plus Rituximab therapy (n = 32) in 64 participants with newly diagnosed AIHA. A satisfactory response (interpreted as normalization of hemoglobin with no evidence of ongoing hemolytic activity) was seen in 75% of the group treated with combination therapy versus 36% in the group treated with monotherapy.17 Other combinations such as Cyclophosphamide, Dexamethasone and Rituximab therapy have also been studied.
In our patient, drug-antibody to metaxalone was negative likely due to prior immunosuppressive therapies. Given the temporal sequence of metaxalone drug intake followed by symptom onset and findings consistent with w-AIHA, coupled with the absence of other offending agents or triggers, we can conclude that there was a causal relationship. Hemolysis-associated laboratory parameters normalized with treatment and remained normal after completion of corticosteroid taper.
In conclusion, DIIHA should be a top differential diagnosis in patients with newly diagnosed hemolytic anemia and recent metaxalone exposure. It is important to diagnose DIIHA as soon as possible because it can be lethal. A detailed history and serological workup should be conducted because DIIHA can be challenging to distinguish from other autoimmune anemias. In most situations, stopping Metaxalone is curative however, some patients may require steroids and Rituximab.
Footnotes
Conflict of interests
None.
Funding
None.
References
- 1. Kalfa TA. Warm antibody autoimmune hemolytic anemia. Hematology. 2016;2016(1):690–697. doi: 10.1182/asheducation-2016.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4(6):607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- 3. Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. BMJ. 1981;282(6281):2023–2027. doi: 10.1136/bmj.282.6281.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aladjidi N, Leverger G, Leblanc T, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica. 2011;96(5):655–663. doi: 10.3324/haematol.2010.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokol RJ, Hewitt S, Stamps BK, Hitchen PA. Autoimmune haemolysis in childhood and adolescence. Acta Haematol. 1984;72(4):245–257. doi: 10.1159/000206397. [DOI] [PubMed] [Google Scholar]
- 6. Garratty G, Petz LD. Drug-induced immune hemolytic anemia. Am J Med. 1975;58(3):398–407. doi: 10.1016/0002-9343(75)90606-3. [DOI] [PubMed] [Google Scholar]
- 7.Full prescribing information - accessdata. fda.gov. [Accessed May 18, 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022503s001lbl.pdf .
- 8. Barcellini W. New insights in the pathogenesis of autoimmune hemolytic anemia. Transfus Med Hemotherapy. 2015;42(5):287–293. doi: 10.1159/000439002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toth PP, Urtis J. Commonly used muscle relaxant therapies for acute low back pain: a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone. Clin Therapeut. 2004;26(9):1355–1367. doi: 10.1016/j.clinthera.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10. Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemias. Haematologica. 2014;99(10):1547–1554. doi: 10.3324/haematol.2014.114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24(4–5):143–150. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 12. Garratty G. Drug-induced immune hemolytic anemia. Hematology. 2009;2009(1):73–79. doi: 10.1182/asheducation-2009.1.73. [DOI] [PubMed] [Google Scholar]
- 13. Packer CD, Hornick TR, Augustine SA. Fatal hemolytic anemia associated with metformin: a case report. J Med Case Rep. 2008;2(1) doi: 10.1186/1752-1947-2-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan Gomez J, Saleem T, Snyder S, Joseph M, Kanderi T.Drug-induced immune hemolytic anemia due to amoxicillin-Clavulanate: a case report and review. Cureus. Published online. 2020. doi: [DOI] [PMC free article] [PubMed]
- 15. Kuter DJ. Warm autoimmune hemolytic anemia and the best treatment strategies. Hematology. 2022;2022(1):105–113. doi: 10.1182/hematology.2022000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodrigo C, Rajapakse S, Gooneratne L. Rituximab in the treatment of autoimmune haemolytic anaemia. Br J Clin Pharmacol. 2015;79(5):709–719. doi: 10.1111/bcp.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163(3):393–399. doi: 10.1111/bjh.12541. [DOI] [PubMed] [Google Scholar]