Abstract
BACKGROUND & AIMS:
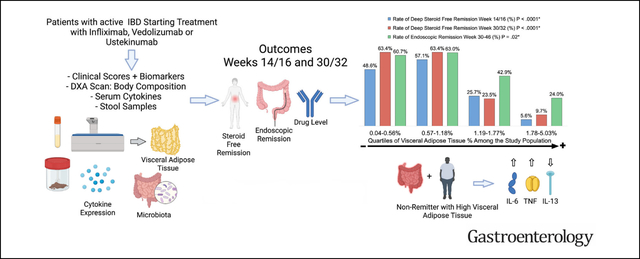
We sought to assess the association between intra-abdominal visceral adipose tissue (IA-VAT) and response to 3 different biologic drugs in patients with inflammatory bowel disease (IBD) and to investigate its effects on inflammatory cytokine expression, pharmacokinetics, and intestinal microbiota.
METHODS:
We prospectively enrolled subjects with active IBD initiating infliximab, vedolizumab, or ustekinumab and a healthy control group. Baseline body composition (including IA-VAT as percent of total body mass [IA-VAT%]) was measured using GE iDXA scan. Primary outcome was corticosteroid-free deep remission at weeks 14–16, defined as Harvey Bradshaw Index <5 for Crohn’s disease and partial Mayo score <2 for ulcerative colitis, with a normal C-reactive protein and fecal calprotectin. Secondary outcomes were corticosteroid-free deep remission and endoscopic remission (Endoscopic Mayo Score ≤1 in ulcerative colitis or Simple Endoscopic Score for Crohn’s disease ≤2) at weeks 30–46.
RESULTS:
A total of 141 patients with IBD and 51 healthy controls were included. No differences in body composition parameters were seen between the IBD and healthy control cohorts. Patients with higher IA-VAT% were less likely to achieve corticosteroid-free deep remission (P < .001) or endoscopic remission (P = .02) vs those with lower IA-VAT%. Furthermore, nonresponders with high IA-VAT% had significantly higher serum interleukin-6 and tumor necrosis factor at baseline compared with responders and patients with low IA-VAT%. Drug pharmacokinetic properties and microbiota diversity were similar when comparing high and low IA-VAT% groups.
CONCLUSIONS:
Higher IA-VAT% was independently associated with worse outcomes. This association could be driven at least partially by discrete differences in inflammatory cytokine expression.
Keywords: Inflammatory Bowel Diseases, Visceral Adipose Tissue, Infliximab, Vedolizumab, Ustekinumab
Graphical Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are immune-mediated diseases that fall into a spectrum of conditions known as inflammatory bowel diseases (IBDs). Although biologic agents have improved outcomes substantially, a considerable number of patients do not respond to therapy.1–3 There are a few well-known mechanisms that explain nonresponse to biologics, and others remain unknown. Identifying these mechanisms can potentially lead to interventions to improve the effectiveness of currently available treatment options and help to better position them in personalized treatment algorithms.
Some studies have described an association between obesity, high intra-abdominal visceral adipose tissue (IA-VAT) mass, and worse outcomes in patients with IBDs.4–6 However, these observations have been limited by their methodology and retrospective or post-hoc nature4–6 Moreover, these studies have been restricted to patients receiving anti-tumor necrosis factor (TNF)-α agents and it is unclear whether a similar association exists between IA-VAT mass and other biologics with a different mechanism of action, such as vedolizumab and ustekinumab. Furthermore, the mechanisms underlying the putative influence of IA-VAT on medication response are not known.
The aim of this study was to assess whether body composition, and IA-VAT in particular, correlates with response to treatment to 3 different biologics used in the treatment of IBDs. We also sought to explore potential mechanisms for how IA-VAT can negatively affect outcomes, including its correlation with pharmacokinetics, overexpression of inflammatory cytokines, and/or changes in the gastrointestinal microbiota.
Methods
Design and Patients
The CONSTELLATION study was a prospective, observational cohort study performed at Froedtert and The Medical College of Wisconsin (Milwaukee, WI). The study was reviewed and approved by the local Institutional Review Board (PRO00027334) and all patients signed informed consent. The study enrolled subjects 18 years or older with a confirmed diagnosis of CD or UC or healthy, age- and gender-matched controls between May 2017 and September 2021. Patients with IBD were screened for inclusion in the study at the time of initiating treatment with standard dosing of infliximab, vedolizumab, or ustekinumab. The patients had to meet the following 2 additional criteria:
Moderate to severe active endoscopic disease within 90 days before start of the biologic. Moderate to severe active endoscopic disease was defined as Simple Endoscopic Score for CD ≥7 in CD (or ≥4 if isolated ileal disease) or an Endoscopic Mayo Score (EMS) ≥2 in UC.
Either on oral corticosteroids or with clinically active disease defined as a Harvey Bradshaw Index ≥5 in CD or a partial Mayo score ≥2 in UC. Healthy controls without IBD were enrolled in parallel to those with IBD (matched to age and gender to assess in a 1:3 ratio) to assess differences in body composition, inflammatory cytokine concentrations, and microbiota with the IBD cohort.
We excluded patients with ileostomy or colostomy, short gut, impending need for surgery; those on total parenteral nutrition; and those with comorbid celiac disease, ischemic, or microscopic colitis. Pregnant women were excluded, and all female patients were required to have a negative urine pregnancy test performed at screening. Patients that met inclusion criteria and were willing to perform all study procedures were invited to participate.
Procedures and Collected Data
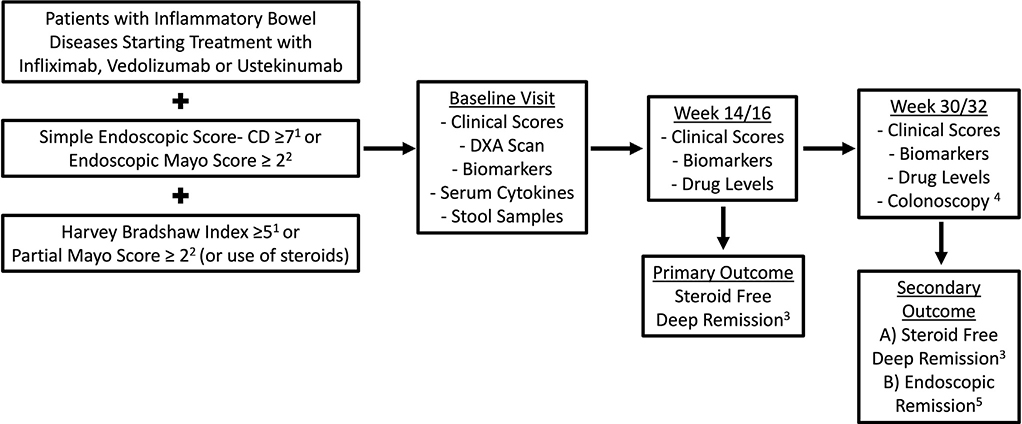
Patients were started on biologic therapy per standard of care. There were 3 study visits: baseline (week 0), post-induction (week 14 for infliximab and vedolizumab and week 16 for ustekinumab), and a third visit during maintenance at week 30 (infliximab and vedolizumab) or week 32 (ustekinumab). The timing for study visits was selected based on the dosing schedule for each drug to obtain trough drug levels at the time of the visit. Blood and stool samples were obtained at each visit per protocol. The study design is summarized in Figure 1.
Figure 1.
Study design. 1Patients with Crohn’s disease. 2Patients with ulcerative colitis. 3Defined as a Harvey Bradshaw Index <5 or partial Mayo score <2 and normal C-reactive protein/fecal calprotectin while off steroids. 4When done between weeks 30 and 46 of therapy. 5Defined as a simple endoscopic score-CD ≤2 in Crohn’s disease and endoscopic Mayo score ≤1 in ulcerative colitis. DXA, dual-energy x-ray absorptiometry.
Body Composition Assessment
At baseline, all patients underwent a body composition assessment. Whole-body scans were conducted with a Lunar iDXA (GE Healthcare) dual-energy x-ray absorptiometry scanner. Body composition parameters were analyzed using enCORETM (version 14.10.022), and IA-VAT was measured with the CoreScan (GE Healthcare). Dual-energy x-ray absorptiometry is a 3-compartment method considered as a reference technique for measuring body composition (ie, bone, lean, and fat mass) due to its high precision, safety, and accuracy compared with other body composition assessments.7 Parameters measured included lean mass, total adipose tissue, and IA-VAT. The percentage of IA-VAT mass from the total body mass (IA-VAT%) was used for the analysis, which aids in the interpretation of IA-VAT burden, as is not confounded by the total body mass of the patient. IA-VAT% values were used as a continuous variable or stratified as “high” or “low” based on the median IA-VAT% of the study population, as no reference values for the IBD population are available.
Clinical and Laboratory Variables
At baseline, we collected demographic characteristics, a complete medical and surgical history. Phenotype of disease was classified according to the Montreal classification.8 At every visit, medication history and disease activity were recorded. We collected the following biomarkers of disease activity: C-reactive protein and fecal calprotectin. Furthermore, serum drug trough concentrations and anti-drug antibodies were obtained at the post-induction and maintenance visits, per protocol. The results were not available to the managing physician, but the patient could have had drug levels measured as standard of care in parallel. Timing of collection was based on the drug: weeks 14 and 30 for infliximab and vedolizumab and weeks 16 and 32 for ustekinumab (if the patient was still on the drug). Drug levels and anti-drug antibodies were measured using a drug-tolerant, homogeneous mobility assay for all drugs.
At baseline, serum cytokines that are known to be highly expressed in patients with a high IA-VAT burden and reported to be dysregulated in patients with IBD, were measured.9,10 Plasma concentrations of interferon-gamma, interleukin (IL)-1β, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-15, IL-22, and TNFα were measured in a sub-group (selected randomly) of patients with IBD and in healthy controls, using a high-sensitivity assay with planar-array technology, on a Quanterix HD-X and SP-X analyzers using Simoa assay kits according to manufacturer’s instructions. Fecal microbiota, in a sub-group of patients with IBD and healthy controls with available baseline fecal samples, were measured through 16S sequencing. Full methodology, including cytokine measurement, microbiome analysis, and bioinformatics are provided in the Supplementary Material.
Outcomes
The primary outcome was corticosteroid-free deep remission (SFDR) at weeks 14–16, which was a composite outcome defined as a Harvey Bradshaw Index <5 in CD or a partial Mayo score <2 in UC, in combination with a serum C-reactive protein ≤0.5 mg/dL and a fecal calprotectin ≤150 μg/mg of stool, while off corticosteroids. Steroid tapering was done based on the primary gastroenterologist’s decision. Biologic dose escalation was monitored and accounted throughout the study follow-up. A modified intention-to-treat analysis was used for SFDR; patients who discontinued the drug due to ineffectiveness or who required surgical treatment for IBD before weeks 14–16 were considered to have failed to meet the primary outcome. Dose escalation was not considered as a failure of primary outcome. Secondary outcomes were SFDR at weeks 30–32 and endoscopic remission at weeks 30–46 (when colonoscopy or sigmoidoscopy was performed as standard of care). We defined endoscopic remission as a Simple Endoscopic Score for CD ≤2 in CD or an EMS ≤1 in UC. Patients who did not undergo a standard-of-care follow-up colonoscopy were not included in the endoscopic remission outcome analysis.
Statistical Analysis
All analyses were conducted using R, version 4.0.3 and JMP, version 15.1.0. Descriptive statistics were used to examine the baseline characteristics of the cohorts. Continuous variables were compared using Student t test, Mann Whitney U test, or Kruskal-Wallis test (for non-normally distributed variables). Normality of continuous variables was evaluated using the Shapiro-Wilk W test. The χ2 test was used to evaluate distributions of categorical variables. Logistic regression modeling was performed for each outcome. The first set of models were unadjusted, followed by stepwise multiple regression models constructed with those variables found significant in the univariate analysis (P < .05). Because it would be expected to find a high collinearity between some anthropometric variables (eg, subcutaneous and visceral adipose tissue), variance inflation factor was examined for each variable in the model to identify multicollinearity among body composition parameters and IA-VAT%. If the variance inflation factor was >10, these variables were not included together in the models with IA-VAT%. All of the analyses were conducted again on subgroups stratified by IBD type (CD or UC) and index drug (infliximab, vedolizumab, and ustekinumab). A P value <.05 was considered statistically significant.
Results
Patient Characteristics and Body Composition
A total of 192 patients were recruited (141 with IBD and 51 matched controls) between May 2017 and September 2021; 79 had CD and 62 had UC. Within the IBD cohort, 52, 46, and 43 patients initiated infliximab, vedolizumab, and ustekinumab, respectively. All patients had a post-induction evaluation, although 128 were evaluated at weeks 30–32 (13 patients were lost to follow-up). The baseline characteristics of the IBD population are shown in Table 1. No differences in body composition parameters were found between the IBD and the healthy control groups, between the CD and UC cohorts, or between those that had been on or off steroids at baseline (Supplementary Tables 1, 2, and 3, respectively).
Table 1.
Baseline Characteristics of the Study Population With Inflammatory Bowel Diseases
| Characteristics | Total cohort (n = 141) | CD (n = 79) | UC (n = 62) |
|---|---|---|---|
|
| |||
| Female gender, n (%) | 79 (56.0) | 49 (62.0) | 30 (48.4) |
| Age, y, mean (SD) | 40.19 (16.9) | 38.71 (14.8) | 42.08 (19.1) |
| Hispanic ethnicity, n (%) | 6 (4.3) | 2 (2.5) | 4 (6.5) |
| Race, n (%) | 0.12 | ||
| White | 129 (91.5) | 69 (87.3) | NA |
| African American | 10 (7.1) | 8 (10.1) | NA |
| Asian | 2 (1.4) | 2 (2.5) | NA |
| Other | 0 (0) | 0 (0) | NA |
| Active smoker, n (%) | 14 (9.9) | 13 (16.5) | 1 (1.6) |
| Age of diagnosis, n (%) | |||
| Younger than 40 y | 99 (70.2) | 56 (70.9) | 43 (69.4) |
| 40 y or older | 42 (29.8) | 23 (29.1) | 19 (30.7) |
| Years with IBD, median (IQR) | 6 (2–13) | 8 (2–14) | 5 (1–10) |
| History of bowel resection, n (%) | 20 (14.18) | 20 (25.32) | 0 (0) |
| No. of bowel resections (if any), n (%) | |||
| 1 | 11 (5) | 11 (55) | 0 (0) |
| 2 | 5 (25) | 5 (25) | 0 (0) |
| 3 | 2 (10) | 2(10) | 0 (0) |
| 4 | 1 (5) | 1 (5) | 0 (0) |
| Unknown (≥1)a | 1 (5) | 1 (5) | 0 (0) |
| Phenotype of CD | |||
| Location, n (%) | |||
| L1: Ileal | 19 (24.1) | 19 (24.1) | NA |
| L2: Colonic | 14 (17.7) | 14 (17.7) | NA |
| L3: Ileocolonic | 46 (58.2) | 46 (58.2) | NA |
| L4: Upper gastrointestinal tract involvement, n (%) | 6 (7.6) | 6 (7.6) | NA |
| Peri-anal disease, n (%) | 3 (3.8) | 3 (3.8) | NA |
| B1: Not stricturing, nonpenetrating, n (%) | 32 (40.5) | 32 (40.5) | NA |
| B2: Stricturing, n (%) | 30 (38.0) | 30 (38.0) | NA |
| B3: Penetrating, n (%) | NA | ||
| Yes | 17 (21.5) | 17 (21.5) | NA |
| No | 62 (78.5) | 62 (78.5) | NA |
| Phenotype of UC | |||
| UC extension, n (%) | |||
| Proctitis | 2 (3.2) | NA | 2 (3.2) |
| Left-sided colitis | 18 (29.0) | NA | 18 (29.0) |
| Pan-colitis | 42 (67.7) | NA | 42 (67.7) |
| Baseline body composition parameters | |||
| Total mass, kg, mean (SD) | 81.61 (20.9) | 82.15 (22.4) | 80.93 (18.9) |
| Body mass index, kg/m2, mean (SD) | 28.29 (6.9) | 28.74 (7.6) | 27.70 (5.8) |
| Percentage of body fat, mean (SD) | 35.01 (10.5) | 35.80 (10.4) | 34.00 (10.6) |
| Total IA-VAT mass, kg, mean (SD) | 1.20 (1.1) | 1.24 (1.1) | 1.15 (1.1) |
| Total fat mass, kg, mean (SD) | 29.64 (14.4) | 30.67 (15.5) | 28.31 (12.9) |
| IA-VAT percentage of total body mass, mean (SD) | 1.32 (0.98) | 1.36 (0.98) | 1.27 (0.99) |
| Total lean mass, kg, mean (SD) | 48.51 (10.9) | 48.04 (10.4) | 49.12 (11.5) |
| Medications at baseline and previous exposure | |||
| Previous use of biologics, n (%) | 87 (61.7) | 54 (68.4) | 33 (53.2) |
| No. of previous biologics, n (%) | |||
| 1 | 34 (39.1) | 18 (33.3) | 16 (48.5) |
| 2 | 30 (34.5) | 20 (37.0) | 10 (30.3) |
| 3 | 15 (17.2) | 10 (18.5) | 5 (15.2) |
| 5 | 2 (2.3) | 2 (3.7) | 0 (0) |
| Unknown (≥1)b | 6 (6.9) | 4 (7.4) | 2 (6.1) |
| Biologic started, n (%) | |||
| Infliximab | 52 (36.9) | 33 (41.8) | 19 (30.7) |
| Ustekinumab | 43 (30.5) | 33 (41.8) | 10 (16.1) |
| Vedolizumab | 46 (32.6) | 13 (16.5) | 33 (53.2) |
| Steroids at baseline, n (%) | |||
| Budesonide | 37 (26.2) | 29 (36.7) | 8 (12.9) |
| Prednisone | 57 (40.4) | 23 (29.1) | 34 (54.8) |
| None | 47 (33.3) | 27 (34.2) | 20 (32.3) |
| Dose of budesonide,c n (%) | |||
| 6 mg | 4 (10.8) | 4 (13.8) | 0 (0) |
| 9 mg | 33 (89.2) | 25 (86.2) | 8 (100) |
| Dose of prednisone,c mg, mean (SD) | 31.93 (10.4) | 29.57 (11.4) | 33.53 (9.5) |
| Months the patient had been on steroids,c median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| Use of mesalamine, n (%) | 13 (9.2) | 3 (3.8) | 10 (16.1) |
| Receiving combination therapy with immunomodulator, n (%) | 63 (44.7) | 41 (51.9) | 22 (35.5) |
| Receiving a thiopurine for combination therapy, n (%) | 51 (36.2) | 32 (40.5) | 19 (30.7) |
| Dose of thiopurine,c mg, mean (SD) | 101.0 (49.5) | 96.9 (42.0) | 107.9 (60.7) |
| Receiving a methotrexate for combination therapy, n (%) | 15 (10.6) | 12 (15.1) | 3 (4.8) |
| Dose of methotrexate,c n (%) | |||
| 12.5 mg | 13 (86.7) | 10 (83.3) | 3 (100) |
| 15 mg | 2 (13.3) | 2 (16.7) | 0 (0) |
| Baseline disease activity | |||
| Simple Endoscopic Score for CD,d median (IQR) | 9 (7–15) | 9 (7–15) | NA |
| Harvey Bradshaw Index,d median (IQR) | 5 (3–8) | 5 (3–8) | NA |
| Partial Mayo Score,e median (IQR) | 5 (4–6) | NA | 5 (4–6) |
| EMS,e n (%) | |||
| 2 | 36 (58.1) | NA | 36 (58.1) |
| 3 | 26 (41.9) | NA | 26 (41.9) |
| C-reactive protein, mg/dL, median (IQR) | 0.5 (0.25–1.3) | 0.5 (0.3–1.6) | 0.34 (0.2–1.0) |
| Albumin, mg/dL, mean (SD) | 4.17 (0.5) | 4.19 (0.5) | 4.15 (0.5) |
| Fecal calprotectin,f μg/mg, median (IQR) | 675 (285–1250) | 485 (182–1373) | 829 (323–1250) |
| Simple Inflammatory Bowel Disease Questionnaire, mean (SD) | 47.96 (11.1) | 46.95 (12.0) | 49.26 (9.7) |
IQR, interquartile range; NA, not applicable.
Patients with a history of resection but unknown number.
Patients with previous exposure to at least 1 biologic, but unknown how many.
As applicable.
Patients with CD.
Patients with UC.
70 patients in the cohort had baseline fecal calprotectin performed.
Rates and Predictors of Remission
Overall, 48 patients (34.0%) and 51 patients (40.0%) had achieved SFDR at weeks 14–16 and 30–32, respectively. Rates of SFDR post induction (weeks 14–16) and during maintenance (weeks 30–32) and endoscopic remission were significantly lower among patients in the 2 highest IA-VAT% quartiles (Figure 2). Differences in patient characteristics between those that did and did not achieve SFDR at weeks 14–16 and 30–32 are shown in Tables 2 and Supplementary Table 4, respectively. With the exception of total lean mass, all baseline body composition parameters were significantly lower among those who achieved SFDR (Table 2 and Supplementary Table 4).
Figure 2.
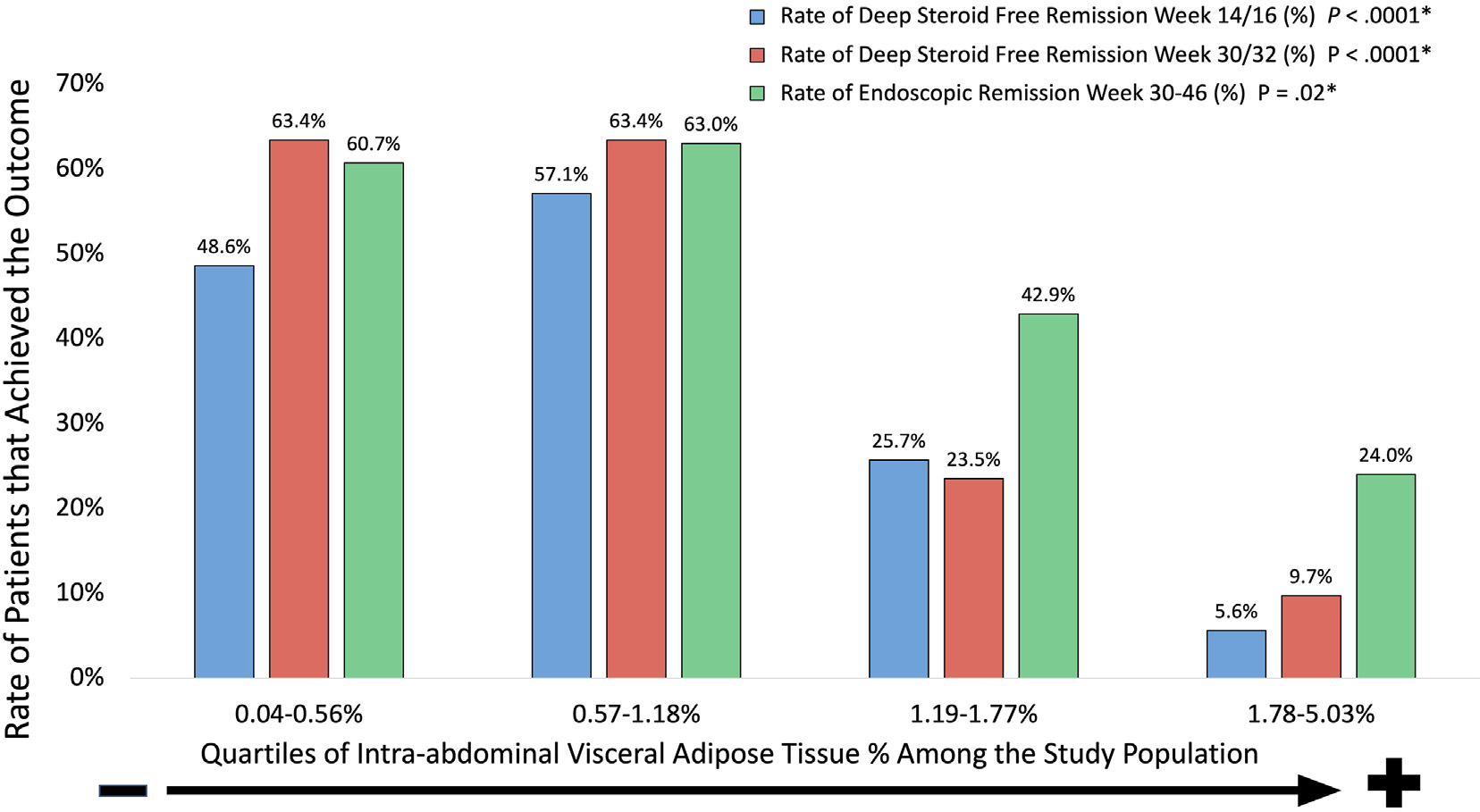
Patients in the higher IA-VAT quartiles had lower rates of steroid-free remission and endoscopic remission. Outcomes for infliximab and vedolizumab are at weeks 14 and 30 and outcomes for ustekinumab are for weeks 16 and 32. *P value for differences among groups (Kruskal–Wallis Test).
Table 2.
Differences in Baseline Characteristics Between Patients Who Did and Did Not Achieve Steroid-Free Remission at Week 14 (Infliximab or Vedolizumab) or Week 16 (Ustekinumab) of Therapy
| Characteristic | SFDR at week 14 (n = 48) | No SFDR at week 14 (n = 93) | P value |
|---|---|---|---|
|
| |||
| Female gender, n (%) | 28 (58.3) | 51 (54.8) | .69 |
| Age, y, mean (SD) | 34.94 (12.4) | 42.90 (18.3) | .007a |
| Hispanic ethnicity, n (%) | 2 (4.2) | 4 (4.3) | .97 |
| Race, n (%) | .56 | ||
| White | 45 (93.8) | 84 (90.3) | |
| African American | 2 (4.2) | 8 (8.6) | |
| Asian | 1 (2.1) | 1 (1.1) | |
| Other | 0 (0) | 0 (0) | |
| Disease type, n (%) | .69 | ||
| CD | 28 (58.3) | 51 (54.8) | |
| UC | 20 (41.7) | 42 (45.2) | |
| Active smoker at baseline, n (%) | 5 (10.4) | 9 (9.7) | .89 |
| Years with IBD, median (IQR) | 5 (1–14) | 6 (2–13) | .63 |
| History of bowel resection,b n (%) | 5 (10.4) | 15 (16.1) | .36 |
| Phenotype of CDb | |||
| Location, n (%) | .32 | ||
| L1: Ileal | 4 (14.3) | 15 (29.4) | |
| L2: Colonic | 6 (21.4) | 8 (15.7) | |
| L3: Ileocolonic | 18 (64.3) | 28 (54.9) | |
| L4: Upper gastrointestinal tract involvement, n (%) | 2 (7.1) | 4 (7.8) | 1.00 |
| B1: Not stricturing, nonpenetrating, n (%) | 14 (50.0) | 20 (39.2) | .35 |
| B2: Stricturing, n (%) | 7 (25.0) | 23 (45.1) | .08 |
| B3: Penetrating, n (%) | 5 (17.9) | 12 (23.5) | .56 |
| Phenotype of UCc | |||
| UC extension, n (%) | .51 | ||
| Proctitis | 1 (5.0) | 1 (2.38) | |
| Left-sided colitis | 7 (35.0) | 11 (26.19) | |
| Pan-colitis | 12 (60.0) | 30 (71.43) | |
| Body composition parameters at baseline | |||
| Total mass, kg, mean (SD) | 72.6 (16.3) | 86.3 (21.5) | <.001a |
| Body mass index, kg/m2, mean (SD) | 25.1 (4.2) | 29.9 (7.4) | <.001a |
| Percentage of body fat, mean (SD) | 30.7 (9.5) | 37.2 (10.3) | <.001a |
| Total IA-VATc mass, kg, mean (SD) | 0.7 (0.5) | 1.5 (1.2) | <.001a |
| Total fat mass, kg, mean (SD) | 22.7 (10.0) | 33.2 (15.1) | <.001a |
| IA-VATd percentage of total body mass, mean (SD) | 0.8 (0.5) | 1.6 (1.1) | <.001a |
| Total lean mass, kg, mean (SD) | 46.8 (11.0) | 49.4 (10.7) | .17 |
| Medications at baseline and previous exposure | |||
| Previous use of biologic, n (%) | 20 (41.7) | 67 (72.0) | <.01a |
| Biologic started, n (%) | .65 | ||
| Infliximab | 17 (35.4) | 35 (37.6) | |
| Ustekinumab | 13 (27.1) | 30 (32.3) | |
| Vedolizumab | 18 (37.5) | 28 (30.1) | |
| Receiving steroids at baseline, n (%) | 27 (56.3) | 67 (72.0) | .06 |
| Use of mesalamine, n (%) | 6 (12.5) | 7 (7.53) | .33 |
| Receiving combination therapy with immunomodulator, n (%) | 19 (39.6) | 44 (47.3) | .38 |
| Receiving a thiopurine for combination therapy, n (%) | 16 (33.3) | 35 (37.6) | .61 |
| Receiving a methotrexate for combination therapy, n (%) | 5 (10.4) | 10 (10.8) | .95 |
| Baseline disease activity | |||
| Simple Endoscopic Score for CD,b median (IQR) | 11 (7.3–16) | 8 (7–14) | .46 |
| Harvey Bradshaw Index,b median (IQR) | 5 (2–7) | 6 (3–8) | .14 |
| Partial Mayo Score,c median (IQR) | 6 (4–7) | 5 (4–6) | .27 |
| Endoscopic Mayo Score,c n (%) | .32 | ||
| 2 | 10 (50) | 10 (50) | |
| 3 | 10 (50) | 10 (50) | |
| Baseline C-reactive protein, mg/dL, median (IQR) | 0.3 (0.1–0.6) | 0.7 (0.3–1.6) | .006a |
| Baseline albumin, mg/dL, mean (SD) | 4.28 (0.4) | 4.11 (0.5) | .043a |
| Baseline fecal calprotectin,e μg/mg, median (IQR) | 553.5 (274–980) | 851 (291 –2208) | .146 |
| Drug level at weeks 14–16 in the higher 2 quartilesf n (%) | 33 (73.3) | 31 (35.6) | <.0001 |
| Infliximab pharmacokineticsg | |||
| Infliximab drug levels week 14, mg/dL, median (IQR) | 11.4 (9.1–18.9) | 3.7 (0.5–13.0) | .03a |
| Detectable anti-infliximab antibodies, n (%) | 5 (15.2) | 1 (5.9) | .31 |
| Vedolizumab pharmacokineticsg | |||
| Vedolizumab drug levels week 14, mg/dL, median (IQR) | 14.3 (11.3–23.6) | 9.0 (5.6–14.0) | .01a |
| Detectable anti-vedolizumab antibodies, n (%) | 1 (3.9) | 0 (0) | .40 |
| Ustekinumab pharmacokineticsg | |||
| Ustekinumab drug levels week 16, mg/dL, median (IQR) | 7.8 (6.5–10.3) | 3.6 (2.3–5.2) | .003a |
| Detectable anti-ustekinumab antibodies, n (%) | 0 (0) | 0 (0) | NA |
| Dose escalated before weeks 14–16, n (%) | 2 (2.2)g | 0 (0) | .31 |
IQR, interquartile range; NA, not applicable.
Statistically significant.
Applies to patients with CD.
Applies to patients with UC.
70 patients in the cohort had baseline fecal calprotectin performed.
Stratified by each biologic and done at week 14 (infliximab or vedolizumab) or week 16 (ustekinumab)
As applies based on the drug the patient was taking.
Both patients who received dose escalation were on infliximab.
Multivariable models were developed to assess the relationship between IA-VAT% (primary parameter of interest) and SFDR at weeks 14–16, considering those variables that were significant in the univariate analysis. Due to the high collinearity among the various body composition readings, those with a variance inflation factor ≥10 were excluded from the multivariable models: total body mass, body mass index, and total body fat. Among the body composition parameters, only IA-VAT and total body fat percentages were included. Higher IA-VAT% (odds ratio [OR] per percent increase, 0.4; 95% CI, 0.16–0.98), previous exposure to biologics (OR, 3.499; 95% CI, 1.43–8.53), and drug levels in the 2 highest quartiles for each biologic (OR, 2.97; 95% CI, 1.20–7.32) were independently associated with failure to achieve SFDR at weeks 14–16. Age, total body fat percentage, baseline C-reactive protein, and albumin became nonsignificant (Table 3).
Table 3.
Multivariable Analysis Showing Those Baseline Factors Independently Associated With Achievement of Deep Steroid-Free Remission at Week 14 (Infliximab and Vedolizumab) or Week 16 (Ustekinumab)
| Baseline variable | Adjusted OR | 95% CI | P value |
|---|---|---|---|
|
| |||
| Previous exposure to a biologic | 3.49 | 1.43–8.53 | <.01a |
| Age (OR per year) | 1.01 | 1.04–0.97 | .76 |
| Visceral adipose tissue (OR per IA-VAT% of body mass) | 0.40 | 0.16–0.98 | .03a |
| Total body fat (OR per % of body mass) | 0.97 | 0.92–0.98 | .19 |
| Baseline C-reactive protein (OR per mg/dL) | 0.72 | 1.02–1.09 | .03a |
| Baseline albumin (OR per g/dL) | 0.58 | 0.05–6.71 | .67 |
| Drug level higher than the median within the populationbc | 2.97 | 1.20–7.32 | .02a |
Statistically significant.
Stratified by each drug group: infliximab, vedolizumab, and ustekinumab.
Median drug levels were 9.8, 11.1, and 4.9 mg/dL for infliximab, vedolizumab, and ustekinumab, respectively.
When adjusting for factors significantly associated with SFDR at weeks 30–32 in the univariate analysis (Supplementary Table 4), drug levels at weeks 30–32 in the 2 lowest quartiles for each biologic and high IA-VAT% were independently associated with failure to achieve SFDR at weeks 30–32 (OR, 0.26; 95% CI, 0.10–0.68 and OR, 0.25; 95% CI, 0.09–0.64, respectively). Previous use of biologics, age, and total body fat percentage were not associated with achievement of SFDR at weeks 30–32.
Sub-Group Analysis by Index Biologic and Disease Phenotype
Infliximab.
Within the infliximab cohort, 17 patients (32.7%) and 41 patients (46.3%) achieved SFDR at weeks 14 and 30, respectively, and 16 (50%) of those with an endoscopic assessment achieved endoscopic remission. Rates of SFDR and endoscopic remission in infliximab patients were significantly higher among subjects in the lower 2 IA-VAT% quartiles (Supplementary Figure 1). Differences in infliximab patient’s characteristics between those who did and did not achieve SFDR at week 14 are shown in Supplementary Table 5. There was a poor correlation between IA-VAT% at baseline and week 14 and 30 levels (ρ = −.07 [P = .58] and ρ = −.19 [P = .23], respectively). Patients with high baseline IA-VAT% quartiles had similar infliximab drug levels at week 14 compared with those with low IA-VAT% (8.9 μg/mL [IQR, 2.6–17.6 μg/mL] vs 10.4 μg/mL [IQR, 1.3–14.0 μg/mL]; P = .41) (Figure 3A). Concomitantly, week 14 levels were significantly higher in patients who achieved SFDR at that time point (Supplementary Table 5).
Figure 3.
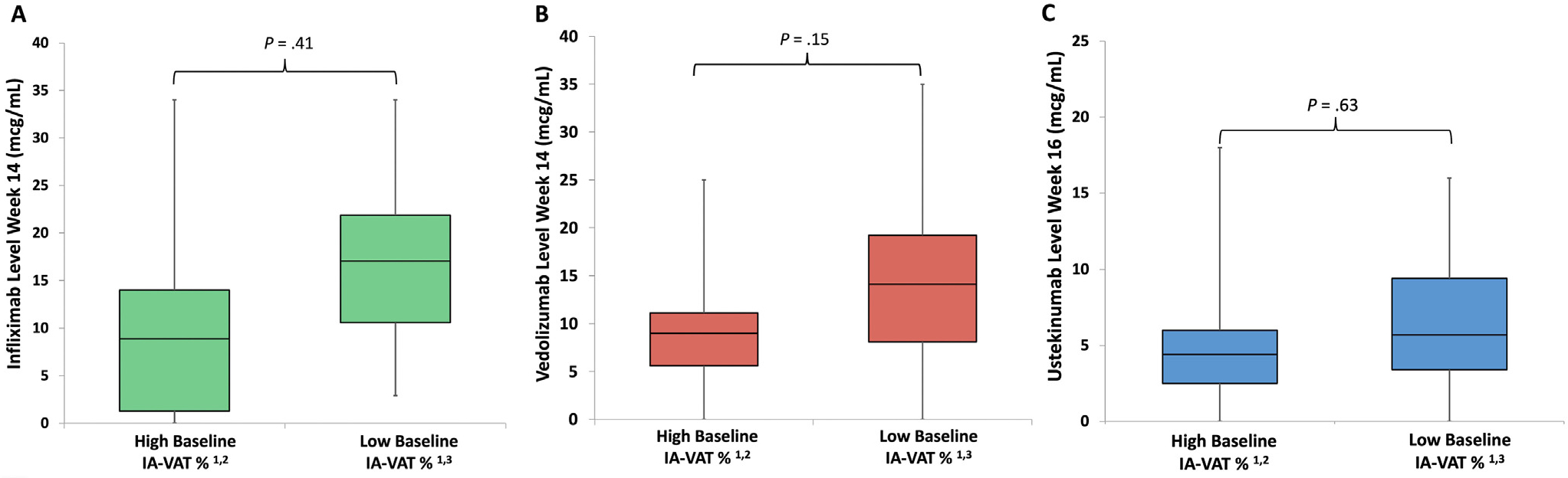
No significant differences in infliximab (A), vedolizumab (B), or ustekinumab (C) drug concentrations were seen when comparing patients with high and low visceral adipose tissue percenetage. 1IA-VAT%, visceral adipose tissue. 2Patients with high IA-VAT% were those on the highest 2 quartiles of the cohort (≥1% of total body mass). 3Patients with low IA-VAT% were those on the lower 2 quartiles of the cohort (<1% of total body mass).
Vedolizumab.
Among patients who started vedolizumab, 18 (39.1%) and 17 (41.4%) had achieved SFDR at weeks 14 and 30, respectively, and 20 of those with endoscopic assessment (62.5%) had achieved endoscopic remission. Rates of SFDR and endoscopic remission in vedolizumab patients were significantly higher among those patients with lower IA-VAT% (Supplementary Figure 2). Differences in vedolizumab patient characteristics between those who did and did not achieve SFDR at week 14 are shown in Supplementary Table 6. A fair correlation was seen between baseline IA-VAT% and weeks 14 and 30 vedolizumab levels (ρ = −.46 [P = .002] and ρ = −.31 [P = .063], respectively). Patients with a high IA-VAT% did not have higher vedolizumab drug level at week 14 compared with those with low IA-VAT% (9.0 μg/mL [IQR, 5.6–11.1 μg/mL] vs 14.1 μg/mL [IQR, 8.1–19.2 μg/mL]; P = .15) (Figure 3B). Concurrently, week 14 vedolizumab levels were significantly higher in patients who achieved week 14 SFDR compared with those who did not (Supplementary Table 6).
Ustekinumab.
Of the 43 patients starting ustekinumab, 13 (30.2%) and 12 (28.5%) were in SFDR at weeks 16 and 32, respectively. Nine of the 34 (26.5%) with endoscopic assessment had achieved endoscopic remission. Rates of SFDR and endoscopic remission in ustekinumab patients were significantly higher in the lower IA-VAT% quartiles (Supplementary Figure 3). Differences between those that did and did not achieve SFDR with ustekinumab at week 16 are shown in Supplementary Table 6. Fair and poor correlations were seen between baseline IA-VAT% and ustekinumab drug levels at weeks 16 and 32, respectively (ρ = −.31 [P = .06]) and ρ = −.042 [P = .82], respectively). Patients with high IA-VAT% quartiles had a nonsignificantly higher ustekinumab drug level at week 14 compared with those in the low IA-VAT % (5.7 μg/mL [IQR, 3.4–9.4 μg/mL] vs 4.4 μg/mL [IQR, 2.5–6.0 μg/mL]; P = .19) (Figure 3C). Ustekinumab drug levels at week 16 were significantly higher in those patients who achieved SFDR at week 16 (Supplementary Table 7).
Disease Type: Crohn’s Disease vs Ulcerative Colitis
On stratifying the analysis by disease sub-type (ie, CD and UC), results were similar to the overall study population. Rates of SFDR at weeks 14–16 and 30–32, and rates of endoscopic remission were significantly higher among those patients with lower IA-VAT% for both CD and UC (Supplementary Figures 4 and 5, respectively).
Baseline Cytokine Profile, Body Composition, and Drug Efficacy
Among patients in the study cohort, 45 with IBD and 50 controls had a complete serum cytokine profile performed at baseline. These patients were selected at random and no differences in patient characteristics were seen between groups (data now shown). Patients with IBD had significantly higher baseline serum levels of IL-6, IL-10, IL-15, IL-22, and TNFα, and when compared with the control group (Supplementary Table 8). IA-VAT% was positively correlated with IL-6 and TNFα (ρ = −.0.37 [P = .01] and ρ = .53 [P < .001], respectively), but negatively correlated with IL-13 (ρ = −.37 [P = .01]) (Supplementary Table 9). When stratifying patients that did and did not achieve SFDR at weeks 14–16, nonresponders with high IA-VAT% had significantly higher serum levels of IL-6 and TNFα at baseline compared with responders, as well as with all patients with lower IA-VAT% (Figure 4 and Supplementary Table 10).
Figure 4.
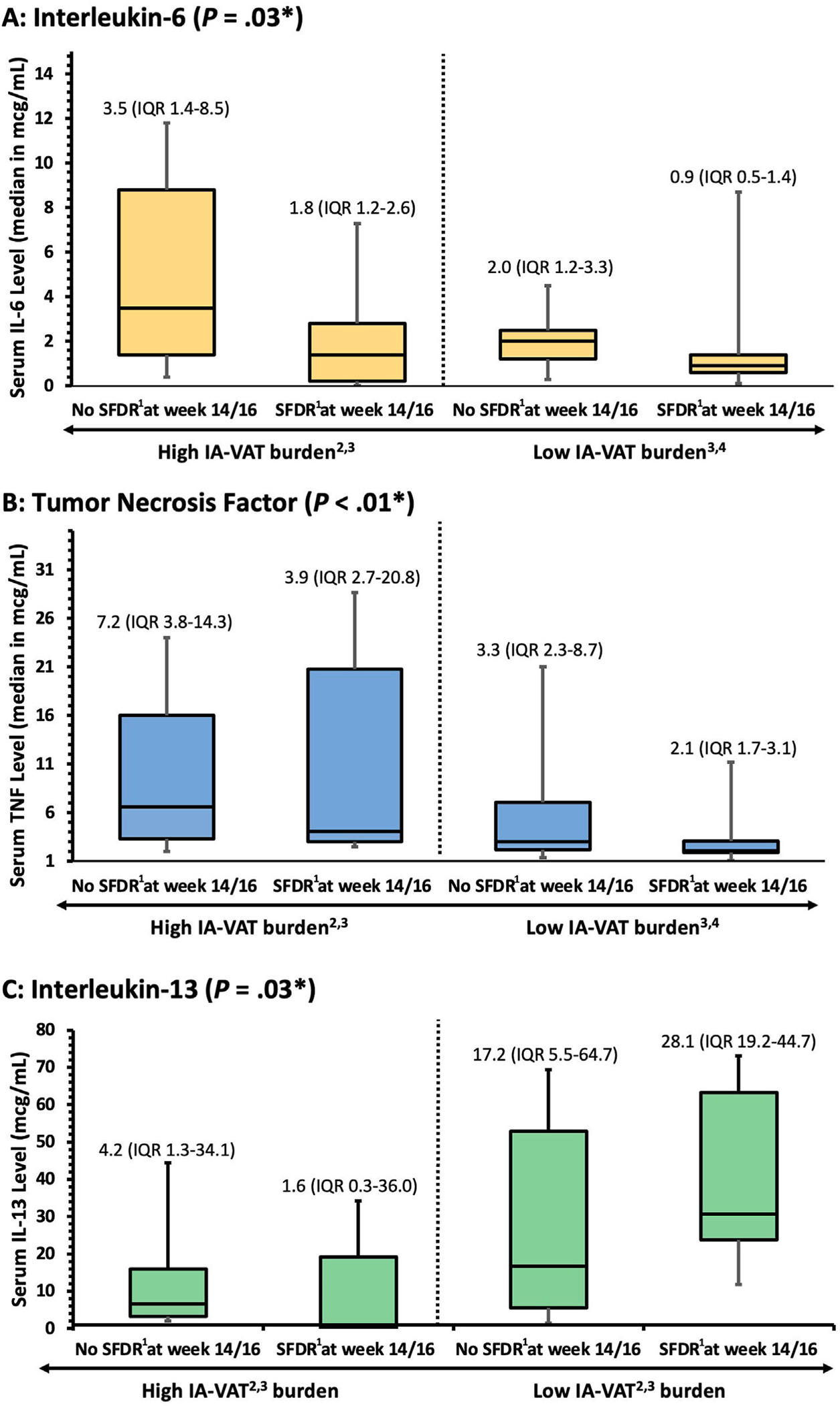
Significant differences in serum interleukin 6 (A), TNFα (B), and IL-13 (C) were seen when stratifying patients by high or low visceral adipose tissue (IA-VAT) burden. 1SFDR is defined as a Harvey Bradshaw Index <5 or partial Mayo score <2 and normal C-reactive protein/fecal calprotectin while off steroids. 2IA-VAT, intra-abdominal visceral adipose tissue. 3High IA-VAT burden was defined as equal or higher to median IA-VAT of the population (≥1% of the total body mass). 4High IA-VAT burden was defined as less than the median IA-VAT of the population (<1% of the total body mass). *P value for differences among groups (Kruskal–Wallis Test).
Baseline Fecal Microbiota and Body Composition
Fecal microbiome was analyzed on 93 subjects (41 patients with IBD selected at random and 51 controls). Patients with IBD had significantly lower α- (Shannon) and β-diversity compared with healthy controls (Supplementary Figure 6). Conversely, within the IBD cohort, there were no significant differences in α- (Shannon) or β-diversity among patients with high or low IA-VAT% (Supplementary Figure 7). However, patients with a higher IA-VAT% had an enrichment of Eubacterium (hallii group), Bacteroides, and Blautia (Supplementary Figure 8).
Discussion
Predicting nonresponse to biologic therapy and understanding its mechanisms in patients with IBD remains an important unmet need. In this study, we assessed how body composition (particularly IA-VAT%) correlates with biologic drug effectiveness, pharmacokinetics, systemic cytokine profiles, and the microbiome. We found that although body composition parameters in patients with IBD were similar to healthy controls, patients with higher IA-VAT% were less likely to achieve SFDR and endoscopic remission compared with patients with lower IA-VAT%. These findings remained true even when stratified by specific biologic and disease types. Moreover, these results were not explained by confounding factors known to influence response, such as drug pharmacokinetics.
Previous data have shown an association between IA-VAT measured by cross-sectional imaging and response to anti-TNFα agents.5,6,11 These studies have found conflicting results, which may be due to the inherent limitations seen in retrospective studies and methodology to measure IA-VAT. Another study looking into pooled data from infliximab clinical trials found that obesity (defined as a body mass index ≥30) was not associated with lower rates of response.12 We used standardized tools to assess disease activity at predetermined time points. Furthermore, we used dual-energy x-ray absorptiometry scans, capable of measuring both total and regional visceral fat with the ability to calculate ratios of complete body fat and lean mass. This allowed the interpretation of body composition without confounding by other parameters.13 In fact, we found that lean mass was not associated with SFDR.
This analysis also adds to the body of literature by including patients with UC (not just CD) and those starting biologic agents with diverse mechanisms of action beyond anti-TNFα (vedolizumab and ustekinumab). Our findings that IA-VAT% is also relevant to patients with UC is important, as it has been postulated that the “creeping fat” traditionally seen in CD maybe be the main driver of cytokine expression. However, our findings that IA-VAT% is important in UC as well as CD may suggest that “creeping fat” may be a result of, and not the driver of, gut inflammation.14
We also tested the hypothesis that patients with IBD with higher IA-VAT% had higher levels of systemic inflammatory cytokines. Using a high-sensitivity assay, we found that baseline levels (treatment start) of both IL-6 and TNFα were significantly higher in nonresponders with high IA-VAT% compared with remitters or even nonresponders with low IA-VAT%. These results may suggest that the lack of effectiveness seen in these patients may be at least partially driven by higher IA-VAT% and potential differences in inflammatory pathways. The role of TNFα in the pathogenesis of IBD has been well described and high baseline TNFα levels have been associated with nonresponse to therapy.15–17 Furthermore, mesenteric adipose tissue produces a high number of inflammatory cytokines, especially TNFα and IL-6.14 Concomitantly, patients with high IA-VAT% (especially those who achieved SFDR) had lower IL-13 serum concentrations compared with the low IA-VAT burden group (in particular, those who achieved SFDR). The role of IL-13 in IBD has been debated and may have anti-inflammatory and pro-tissue repair functions.18 More research looking into the role that IL-13 has in IBD pathogenesis, obesity, and IA-VAT is warranted. In our study, the number of patients who had baseline cytokines levels did not allow for sub-group analysis accounting for IBD phenotype or the individual biologics, but future studies (in serum and tissue) should investigate whether cytokine expression varies among patients starting biologics with different mechanisms of action stratified by IA-VAT%. It is critical to highlight that that these discrete serum cytokine profiles may be different in the actual tissue. Studies looking into cytokine expression in tissue from visceral fat across different anatomic locations and how they relate with response to therapy in IBD are warranted. Perhaps some cytokines may act in a paracrine manner, and others may exert their effect systemically.
Interestingly, there was no correlation between baseline IA-VAT% and drug levels, despite the positive correlation between drug levels and efficacy seen in all drugs. These findings may imply that the lower rates of effectiveness seen in patients with higher IA-VAT% may not be explained by a higher drug clearance or differences in volume distribution, and that adjusting biologic drug levels based on the patient’s IA-VAT burden may not overcome the lower effectiveness. However, more comprehensive population pharmacokinetic analyses exploring the link between IA-VAT burden with volume distribution and biologic drug clearance are warranted. Overall, there was an independent association between drug concentrations and efficacy, although the effect size was not as strong as the relationship with IA-VAT burden. When studying the relationships between both IA-VAT% and total IA-VAT mass, we did find a negative correlation between these parameters and vedolizumab levels at week 14 only. These results could be explained by the weight-based dosing that patients receive when starting infliximab or ustekinumab. Of note, this correlation was not only seen with IA-VAT% (which considers total body mass), but also with the absolute IA-VAT mass of the patient.
Although, and as expected, patients with IBD presented with intestinal dysbiosis, there were no differences in diversity between patients with high and low IA-VAT%, despite some taxa being differentially enriched between these 2 groups. Eubacterium is known to be enriched in patients with a higher IA-VAT burden, which matches the results of this study.19 Conversely, in the general population, Blautia has been negatively associated with IA-VAT.20 Although patients with obesity have been found to have an altered microbiome,21 these differences may not apply to patients with active IBD who are known to present with dysbiosis.22 Another area that needs to be further analyzed is the role of gut bacterial translocation into the mesenteric adipose tissue, and vice versa.23 Overall, more research looking into luminal and transmesenteric metagenomics, and the relationship with clinical observations are warranted.
Strengths of this study include the prospective study design, specific inclusion of patients with objective active inflammation, standardized follow-up, and testing using a high-sensitivity cytokine assay. Important limitations include the noninterventional nature of the study and the lack of a standardized corticosteroid taper. Moreover, the definition of “high” and “low” IA-VAT% was based on the study population, as there is no standardized and widely accepted definition for these parameters. This may challenge how we can extrapolate the results to other populations, including patients with different racial and ethnic backgrounds. Another limitation related to the method used to measure IA-VAT is the inability to differentiate among different types of IA-VAT (eg, adipose tissue surrounding the bowel vs perirenal adipose tissue). This may be important, as mesenteric adipose tissue may be metabolically dissimilar vs perirenal fat. Nonetheless, this may not impact the findings because the retroperitoneal perirenal adipose tissue, like the mesenteric adipose tissue, has been shown to be metabolically active.24–26
In conclusion, a higher IA-VAT% burden is associated with lower response to therapy with infliximab, vedolizumab, or ustekinumab in both CD and UC. Although the exact mechanisms of these findings warrant further investigation, the overexpression of certain cytokines may play an important role. Future studies looking into interventions to lower IA-VAT burden in this population are needed. Moreover, studying these observations in patients starting IBD therapy with small molecules are also needed.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Identifying and understanding mechanisms of nonresponse to biologic therapy in patients with inflammatory bowel diseases is critical to plan interventions aiming to improve outcomes.
NEW FINDINGS
High intra-abdominal visceral adipose tissue burden is significantly associated with nonresponse to infliximab, vedolizumab, or ustekinumab therapy. These findings may be explained by differences in inflammatory cytokine expression, but do not seem related with disparities in drug pharmacokinetics or microbiota.
LIMITATIONS
This was a noninterventional study and the results may not apply to other patient populations.
CLINICAL RESEARCH RELEVANCE
Interventions aiming to decrease intra-abdominal visceral adipose tissue burden in patients with IBD may help improve rates of response to biologic therapy. Studies assessing rates of response to novel small molecule drugs available for IBD in patients with a high intra-abdominal visceral adipose tissue burden are warranted and may help to better position therapies.
BASIC RESEARCH RELEVANCE
Tumor necrosis factor–α and interleukin-6 pathways may be linked with nonresponse to treatment and the pathogenesis of inflammatory bowel diseases. Further studies looking into the role of intra-abdominal visceral adipose tissue as a metabolic and pro-inflammatory organ are needed.
Funding
The authors acknowledge support from the Digestive Disease Center at the Medical College of Wisconsin and National Institutes of Health Clinical and Translation Science Awards: UL1TR001436, TL1TR001437, and KL2TR001438.
Abbreviations used in this paper:
- CD
Crohn’s disease
- EMS
Endoscopic Mayo Score
- IBD
inflammatory bowel disease
- IL
interleukin
- IA-VAT
intra-abdominal visceral adipose tissue
- IQR
interquartile range
- OR
odds ratio
- SFDR
corticosteroid-free deep remission
- TNF
tumor necrosis factor
- UC
ulcerative colitis
Footnotes
Conflicts of interest
These authors disclose the following: Andres J. Yarur is a consultant for Takeda Pharmaceuticals USA, Procise Inc, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, and Arena Pharmaceuticals. Gil Y. Melmed is a consultant to Abbvie, Arena, Boehringer-Ingelheim, Bristol-Meyer Squibb/Celgene, Entasis, Janssen, Medtronic, Pfizer, Samsung Bioepis, Shionogi, Takeda, and TechLab, and received research funding from Pfizer. Jean F. Colombel has research grants from AbbVie, Janssen Pharmaceuticals, Takeda, and Bristol Myers Squibb; is receiving payment for lectures from AbbVie, and Takeda; is receiving consulting fees from AbbVie, Amgen, AnaptysBio, Allergan, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Genentech (Roche), Janssen Pharmaceuticals, Kaleido Biosciences, Immunic, Iterative Scopes, Merck, Landos, Microba Life Science, Novartis, Otsuka Pharmaceutical, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, Vifor; and hold stock options in Intestinal Biotech Development. Maria T. Abreu is a consultant for AbbVie Inc, Arena Pharmaceuticals, Bristol Myers Squibb, Eli Lilly Pharmaceuticals, Gilead, Janssen Ortho, LLC, Janssen Global Services, LLC, Janssen Biotech NART, Janssen Biotech, Inc (non-branded), Janssen Biotech, Inc. (Women in GI advisory board), Microba, Prometheus Biosciences, UCB Biopharma, and WebMD Global LLC. Teaching, lecturing, or speaking for Alimentiv, Janssen, Prime CME, Takeda Pharmaceuticals, Intellisphere LLC (HCP Live Institutional Perspectives in GI). Grants Prometheus, Takeda, and Pfizer. Poonam Beniwal-Patel receives speaker fees from Takeda. Parakkal Deepak: consultant or on an advisory board for Janssen, Pfizer, Prometheus Biosciences, Boehringer Ingelheim, AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, CorEvitas LLC, and Scipher Medicine Corporation. He has also received funding under a sponsored research agreement unrelated to the data in the article from Takeda Pharmaceutical, Arena Pharmaceuticals, Bristol Myers Squibb-Celgene, and Boehringer Ingelheim. Stephan R. Targan is a stockholder, founder, and consultant for Prometheus Biosciences. The remaining authors disclose no conflicts.
CrediT Authorship Contributions
Andres J. Yarur, MD (Conceptualization: Lead; Data curation: Equal; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Equal; Methodology: Lead; Project administration: Supporting; Software: Equal; Supervision: Equal; Validation: Equal; Visualization: Equal; Writing – original draft: Lead).
Alexandra Bruss, BS (Data curation: Equal; Funding acquisition: Supporting; Investigation: Equal; Project administration: Lead; Supervision: Lead; Writing – review & editing: Supporting).
Andrea Moosreiner, MS, RD (Conceptualization: Equal; Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Project administration: Supporting; Resources: Equal; Software: Equal; Visualization: Equal; Writing – review & editing: Supporting).
Poonam Beniwal-Patel, MD (Conceptualization: Equal; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Equal; Methodology: Supporting; Writing – review & editing: Equal).
Lizbeth Nunez, BS (Data curation: Equal; Investigation: Equal; Project administration: Equal; Supervision: Equal; Writing – review & editing: Supporting).
Brandon Berens, BS (Data curation: Equal; Investigation: Equal; Project administration: Supporting; Supervision: Supporting; Writing – review & editing: Equal).
Jean F. Colombel, MD (Conceptualization: Equal; Investigation: Supporting; Methodology: Equal; Visualization: Equal; Writing – review & editing: Equal).
Stephan Targan, MD (Formal analysis: Equal; Methodology: Equal; Validation: Equal; Visualization: Equal; Writing – review & editing: Equal).
Caroline Rupcich, PA-C (Conceptualization: Supporting; Data curation: Supporting; Investigation: Equal; Supervision: Supporting; Writing – review & editing: Supporting).
Gil Y. Melmed, MD, MS (Formal analysis: Equal; Methodology: Equal; Visualization: Equal; Writing – review & editing: Equal).
Maria T. Abreu, MD (Conceptualization: Equal; Methodology: Equal; Visualization: Supporting; Writing – review & editing: Equal).
Parakkal Deepak, MD (Conceptualization: Equal; Formal analysis: Equal; Investigation: Supporting; Methodology: Supporting; Validation: Equal; Visualization: Equal; Writing – review & editing: Lead).
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2023.06.036.
Data Availability
Data available upon reasonable request.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 3.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005;352:2499–2507. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Proudfoot J, Xu R, et al. Impact of obesity on short- and intermediate-term outcomes in inflammatory bowel diseases: pooled analysis of placebo arms of infliximab clinical trials. Inflamm Bowel Dis 2018;24:2278–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding NS, Malietzis G, Lung PFC, et al. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther 2017;46:883–891. [DOI] [PubMed] [Google Scholar]
- 6.Gu P, Chhabra A, Chittajallu P, et al. Visceral adipose tissue volumetrics inform odds of treatment response and risk of subsequent surgery in IBD patients starting antitumor necrosis factor therapy. Inflamm Bowel Dis 2022;28:657–666. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd JA, Fan B, Lu Y, et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012;27:2208–2216. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5–36. [DOI] [PubMed] [Google Scholar]
- 9.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–342. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Zhu W, Gong J, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis 2015;17:225–234. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Proudfoot J, Xu R, et al. Obesity and response to infliximab in patients with inflammatory bowel diseases: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol 2018;113:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imboden MT, Welch WA, Swartz AM, et al. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS One 2017;12:e0175110–e0175112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hall-mark or an innocent bystander? Gut 2007;56:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murch SH, Lamkin VA, Savage MO, et al. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut 1991;32:913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murch SH, Braegger CP, Walker-Smith JA, et al. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut 1993;34:1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez KB, Leone V, Chang EB. Microbial metabolites in health and disease: navigating the unknown in search of function. J Biol Chem 2017;292:8553–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biancheri P, Sabatino AD, Ammoscato F, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol 2014;44:370–385. [DOI] [PubMed] [Google Scholar]
- 19.Yan H, Qin Q, Chen J, et al. Gut microbiome alterations in patients with visceral obesity based on quantitative computed tomography. Front Cell Infect Mi 2022;11:823262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozato N, Saito S, Yamaguchi T, et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. Npj Biofilms Microbiomes 2019;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 22.Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011;60:631. [DOI] [PubMed] [Google Scholar]
- 23.Ha CWY, Martin A, Sepich-Poore GD, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 2020; 183:666–683.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigoraș A, Balan RA, Caruntu I-D, et al. Perirenal adipose tissue—current knowledge and future opportunities. J Clin Med 2021;10:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baer PC, Koch B, Hickmann E, et al. Isolation, characterization, differentiation and immunomodulatory capacity of mesenchymal stromal/stem cells from human perirenal adipose tissue. Cells 2019;8:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynes MD, Tseng Y. Deciphering adipose tissue heterogeneity. Ann N York Acad Sci 2018;1411:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request.