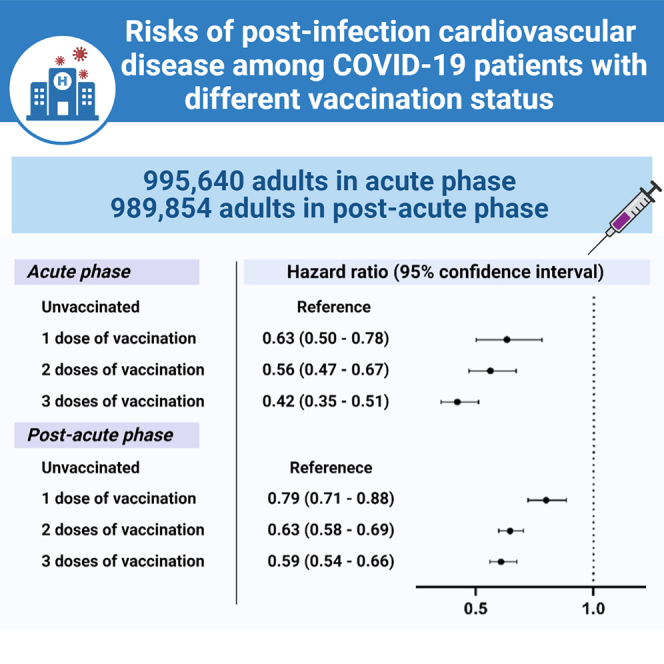
Summary
It is unknown if vaccination affects the risk of post-COVID-19 cardiovascular diseases (CVDs). Therefore, this retrospective cohort study examines the short-term and long-term risks of post-infection CVD among COVID-19 patients with different vaccination status utilizing data from electronic health databases in Hong Kong. Cox proportional hazards regression adjusted with inverse probability of treatment weighting is used to evaluate the risks of incident CVD (coronary heart disease, stroke, heart failure) and all-cause mortality in COVID-19 patients. Compared with unvaccinated patients, vaccinated patients have a lower risk of CVD and all-cause mortality, and the lowest risk is observed in those who completed three doses of vaccine. Similar patterns in the subgroups of different vaccine platforms, age, gender, Charlson comorbidity index, and disease severity are observed. These findings highlight a positive dose-response relationship between overall CVD risk reduction and the number of vaccine doses received.
Keywords: SARS-CoV-2, long COVID, incident CVD, post-COVID CVD, omicron, inactivated vaccine
Graphical abstract
Highlights
-
•
BNT162b2 and CoronaVac are associated with lower risks of post-infection incident CVD
-
•
Positive dose-response relationship between risk reduced and number of vaccine doses
-
•
Vaccine protection persists in the post-acute phase (>28 days after infection)
It is unknown if vaccination affects the risk of post-COVID-19 cardiovascular diseases (CVDs). Therefore, Wan et al. examine the short-term and long-term risks of post-infection CVD among COVID-19 patients with different vaccination status and observe a positive dose-response relationship between overall CVD risk reduction and the number of vaccine doses received.
Introduction
Despite primarily being a respiratory tract infection, coronavirus disease 2019 (COVID-19) can result in systemic inflammation, thereby leading to complications in multiple organ systems, such as cardiovascular and gastrointestinal systems.1 Among an extensive list of possible complications, acute cardiovascular complications have been frequently discussed because of their significant association with COVID-19-related mortality.2 Apart from acute myocardial infarction, previously reported cardiovascular complications of COVID-19 include myocarditis, heart failure, venous thromboembolism, and ischemic stroke.3,4 Higher levels of C-reactive protein and troponin T, and decreased ejection fraction, have been described in patients with severe cardiovascular complications of COVID-19.5 The mechanism of acute post-infection complications is generally regarded as multifactorial, involving systemic inflammatory response, myocardial cell injury, and hypercoagulability.4,6 More recently, it was suggested that COVID-19 may also increase the risk of incident cardiovascular diseases (CVDs) beyond the first 30 days after acute infection,7 and a past history of COVID-19 infection should be regarded as a risk factor for CVD.8,9 The risk was evident even in individuals who had no baseline CVD before contracting COVID-19.7 Some regarded post-acute cardiovascular manifestations as part of “long COVID,”10 which has become a huge burden on healthcare systems in different countries, such as the UK,11 as the reported incidence of major adverse cardiac events at 12 months post COVID-19 infection was as high as 67 per 1,000 persons.7 With respect to the exact mechanism of incident CVD after acute COVID-19 infection, it remains to be elucidated, while some authors postulated that chronic inflammation triggered by persistent virus and autoimmune response that damages the heart due to molecular mimicry might have a role.10
The effectiveness of COVID-19 vaccines in protecting against severe COVID-19 diseases and mortality has been well studied,12,13 yet it is uncertain whether vaccination reduces the risk of acute cardiovascular complications and incident CVD in the post-acute phase. Researchers in Korea pointed out that fully vaccinated individuals (i.e., two doses of mRNA vaccine or viral vector vaccine) had a lower risk of acute myocardial infarction and ischemic stroke that occurred 31 to 120 days after contracting COVID-19,14 thereby supporting the importance of vaccination. Nonetheless, studies regarding other cardiovascular outcomes, such as heart failure and deaths, in people who received inactivated COVID-19 vaccines are lacking. Current evidence mainly focuses on the risk of myocarditis and pericarditis15,16 or immune dysregulation17 after receiving mRNA COVID-19 vaccines. Whether vaccination in fact reduces acute cardiovascular complications of COVID-19, and long-term incident CVD in people with or without baseline cardiovascular risk, deserves our attention.
Long COVID is inevitably associated with a huge economic burden.18 Therefore, it is crucial to evaluate whether vaccination reduces secondary cardiovascular complications in view of the suboptimal COVID-19 vaccine coverage, especially for the booster dose, around the globe. The present study aims to examine the effectiveness of the two COVID-19 vaccines authorized in Hong Kong, namely BNT162b2 from Fosun-BioNTech (Pfizer-BioNTech, mRNA vaccine) and CoronaVac from Sinovac Biotech (HK) Limited (inactivated vaccine) for individuals aged 16 years or above since 23 February 2021 in reducing the risk of acute cardiovascular complications and incident CVD beyond the acute phase of illness. As patients with pre-existing CVD have higher risks of myocardial injury and poor outcome after COVID-19,19 these patients were excluded from the study.
Results
The analysis in the post-infection acute phase involved 90,607 unvaccinated and 905,033 vaccinated patients (85,703 with one dose, 426,948 with two doses, 392,382 with three doses). After the exclusion of deaths that occurred within the first 28 days post infection, the analysis in the post-acute phase included 87,253 unvaccinated and 902,601 vaccinated patients (84,534 with one dose, 425,965 with two doses, 392,102 with three doses). Table 1 describes the baseline characteristics with standardized mean difference (SMD) before and after weighting. The SMD for all characteristics among the groups was <0.2, indicating a good balance in all characteristics between groups.
Table 1.
Baseline characteristics before and after weighting among individuals with different vaccination status
| (A) | Before weighting | After weighting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rowhead | Unvaccinated | One dose | Two doses | Three doses | SMD | Unvaccinated | One dose | Two doses | Three doses | SMD |
| N | 90,607 | 85,703 | 426,948 | 392,382 | – | 1,010,821 | 1,005,271 | 995,933 | 995,907 | – |
| Age, years | 56.4 (21.6) | 56.8 (19.5) | 48.8 (17.1) | 50.3 (15.8) | 0.269 | 49.0 (20.4) | 49.7 (19.3) | 50.8 (17.5) | 50.8 (16.2) | 0.059 |
| Gender, male | 37,731 (41.6) | 362,11 (42.3) | 184,189 (43.1) | 174,584 (44.5) | 0.032 | 443,191 (43.8) | 442,844 (44.1) | 432,482 (43.4) | 433,763 (43.6) | 0.007 |
| CCI | 0.5 (1.3) | 0.4 (1.1) | 0.2 (0.7) | 0.2 (0.6) | 0.201 | 0.2 (0.8) | 0.2 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.008 |
| Cancer | 5,403 (6.0) | 4,086 (4.8) | 1,0167 (2.4) | 7,919 (2.0) | 0.124 | 27,693 (2.7) | 27,982 (2.8) | 27,834 (2.8) | 28,433 (2.9) | 0.004 |
| Chronic kidney disease | 2,329 (2.6) | 1,591 (1.9) | 2,939 (0.7) | 2,236 (0.6) | 0.099 | 9,213 (0.9) | 9,181 (0.9) | 9,267 (0.9) | 9,893 (1.0) | 0.005 |
| Respiratory disease | 3,193 (3.5) | 2,473 (2.9) | 6,570 (1.5) | 5,434 (1.4) | 0.085 | 17,120 (1.7) | 17,601 (1.8) | 17,747 (1.8) | 17,991 (1.8) | 0.005 |
| Diabetes | 12,386 (13.7) | 13,181 (15.4) | 40,224 (9.4) | 33,243 (8.5) | 0.130 | 96,731 (9.6) | 98,648 (9.8) | 99,072 (9.9) | 100,844 (10.1) | 0.010 |
| Dementia | 1,289 (1.4) | 636 (0.7) | 722 (0.2) | 261 (0.1) | 0.098 | 2,990 (0.3) | 2,952 (0.3) | 3,078 (0.3) | 3,380 (0.3) | 0.004 |
| Renin-angiotensin-system agents | 14,167 (15.6) | 15,049 (17.6) | 45,383 (10.6) | 39,505 (10.1) | 0.134 | 111,621 (11.0) | 113,673 (11.3) | 114,305 (11.5) | 115,978 (11.6) | 0.010 |
| Beta blockers | 9,887 (10.9) | 9,631 (11.2) | 26,006 (6.1) | 20,587 (5.2) | 0.139 | 66,847 (6.6) | 67,088 (6.7) | 66,324 (6.7) | 67,744 (6.8) | 0.004 |
| Calcium channel blockers | 21,728 (24.0) | 22,938 (26.8) | 69,729 (16.3) | 60,054 (15.3) | 0.174 | 166,207 (16.4) | 171,977 (17.1) | 174,305 (17.5) | 176,088 (17.7) | 0.018 |
| Diuretics | 4,813 (5.3) | 3,491 (4.1) | 7,551 (1.8) | 5,981 (1.5) | 0.129 | 21,522 (2.1) | 21,992 (2.2) | 22,015 (2.2) | 22,951 (2.3) | 0.006 |
| Nitrates | 1,620 (1.8) | 1,189 (1.4) | 2,513 (0.6) | 2,124 (0.5) | 0.072 | 7,602 (0.8) | 7,605 (0.8) | 7,526 (0.8) | 7,677 (0.8) | 0.001 |
| Lipid-lowering agents | ||||||||||
| Statin | 16,715 (18.4) | 18,758 (21.9) | 57,877 (13.6) | 54,142 (13.8) | 0.131 | 144,288 (14.3) | 145,719 (14.5) | 147,781 (14.8) | 149,105 (15.0) | 0.011 |
| Fibrate | 552 (0.6) | 605 (0.7) | 2,091 (0.5) | 1,834 (0.5) | 0.018 | 5,299 (0.5) | 5,002 (0.5) | 5,093 (0.5) | 5,138 (0.5) | 0.002 |
| PCSK9 inhibitors | 3 (0.0) | 9 (0.0) | 14 (0.0) | 36 (0.0) | 0.006 | 64 (0.0) | 62 (0.0) | 63 (0.0) | 62 (0.0) | <0.001 |
| Insulins | 3,122 (3.4) | 2,275 (2.7) | 5,424 (1.3) | 3,836 (1.0) | 0.102 | 15,424 (1.5) | 14,922 (1.5) | 14,846 (1.5) | 15,791 (1.6) | 0.005 |
| Antidiabetic drugs | ||||||||||
| Sulfonylurea | 5,632 (6.2) | 6,455 (7.5) | 18,728 (4.4) | 14,699 (3.7) | 0.096 | 45,436 (4.5) | 45,617 (4.5) | 45,555 (4.6) | 46,425 (4.7) | 0.004 |
| Metformin | 9,842 (10.9) | 11,520 (13.4) | 35,950 (8.4) | 29,734 (7.6) | 0.110 | 86,960 (8.6) | 86,933 (8.6) | 87,057 (8.7) | 88,364 (8.9) | 0.005 |
| DPP-4 inhibitors | 2,793 (3.1) | 2,500 (2.9) | 6,282 (1.5) | 4,948 (1.3) | 0.079 | 16,860 (1.7) | 16,637 (1.7) | 16,632 (1.7) | 17,431 (1.8) | 0.004 |
| SGLT-2 inhibitors | 1,013 (1.1) | 1,168 (1.4) | 3,909 (0.9) | 3,607 (0.9) | 0.024 | 10,771 (1.1) | 9,749 (1.0) | 9,705 (1.0) | 9,933 (1.0) | 0.005 |
| GLP-1 agonists | 131 (0.1) | 140 (0.2) | 544 (0.1) | 546 (0.1) | 0.005 | 1,800 (0.2) | 1,355 (0.1) | 1,357 (0.1) | 1,388 (0.1) | 0.006 |
| Oral anticoagulants | 1,589 (1.8) | 1,127 (1.3) | 2,044 (0.5) | 1,669 (0.4) | 0.080 | 6,639 (0.7) | 6,549 (0.7) | 6,467 (0.6) | 6,913 (0.7) | 0.003 |
| Antiplatelets | 6,399 (7.1) | 4,858 (5.7) | 10,409 (2.4) | 8,717 (2.2) | 0.144 | 30,318 (3.0) | 30,276 (3.0) | 30,719 (3.1) | 31,496 (3.2) | 0.005 |
| Immunosuppressants | 1,007 (1.1) | 703 (0.8) | 1,853 (0.4) | 1,744 (0.4) | 0.047 | 5,849 (0.6) | 5,684 (0.6) | 5,488 (0.6) | 5,813 (0.6) | 0.002 |
| Severe COVID-19 | 584 (0.6) | 353 (0.4) | 456 (0.1) | 302 (0.1) | 0.059 | 1,786 (0.2) | 1,730 (0.2) | 1,852 (0.2) | 2,211 (0.2) | 0.006 |
| BNT162b2 recipients | 0 (0.0) | 30,269 (35.3) | 247,465 (58.0) | 218,405 (55.7) | NA | 0 (0.0) | 436,293 (43.4) | 556,019 (55.8) | 548,554 (55.1) | NA |
| CoronaVac recipients | 0 (0.0) | 55,434 (64.7) | 179,483 (42.0) | 173,977 (44.3) | NA | 0 (0.0) | 568,978 (56.6) | 439,914 (44.2) | 447,353 (44.9) | NA |
| (B) |
Before weighting |
After weighting |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | One dose | Two doses | Three doses | SMD | Unvaccinated | One dose | Two doses | Three doses | SMD | ||
| N | 87,253 | 84,534 | 425,965 | 392,102 | – | 999,740 | 998,762 | 990,070 | 990,046 | – | |
| Age, years | 55.4 (21.1) | 56.4 (19.3) | 48.8 (17.1) | 50.3 (15.8) | 0.254 | 49.2 (20.2) | 49.5 (19.2) | 50.6 (17.3) | 50.6 (16.1) | 0.050 | |
| Gender, male | 35,886 (41.1) | 35,561 (42.1) | 183,620 (43.1) | 174,405 (44.5) | 0.037 | 438,139 (43.8) | 439,332 (44.0) | 429,225 (43.4) | 430,408 (43.5) | 0.008 | |
| CCI | 0.5 (1.2) | 0.4 (1.0) | 0.2 (0.7) | 0.2 (0.6) | 0.188 | 0.2 (0.8) | 0.2 (0.8) | 0.2 (0.8) | 0.3 (0.8) | 0.006 | |
| Cancer | 4,910 (5.6) | 3,982 (4.7) | 10,002 (2.3) | 7,865 (2.0) | 0.117 | 26,941 (2.7) | 27,128.5 (2.7) | 26,945.8 (2.7) | 27,527.9 (2.8) | 0.003 | |
| Chronic kidney disease | 2,013 (2.3) | 1,490 (1.8) | 2,858 (0.7) | 2,215 (0.6) | 0.091 | 8,730 (0.9) | 8,659.3 (0.9) | 8,691.1 (0.9) | 9,238.1 (0.9) | 0.004 | |
| Respiratory disease | 2,863 (3.3) | 2,365 (2.8) | 6,457 (1.5) | 5,414 (1.4) | 0.078 | 16,687 (1.7) | 17,025.7 (1.7) | 17,123.5 (1.7) | 17,369.3 (1.8) | 0.004 | |
| Diabetes | 11,589 (13.3) | 12,893 (15.3) | 40,000 (9.4) | 33,173 (8.5) | 0.126 | 95,750 (9.6) | 97,334.8 (9.7) | 97,677.4 (9.9) | 99,217.4 (10.0) | 0.008 | |
| Dementia | 952 (1.1) | 532 (0.6) | 638 (0.1) | 249 (0.1) | 0.084 | 2,442 (0.2) | 2,403.8 (0.2) | 2,435.1 (0.2) | 2,697.7 (0.3) | 0.003 | |
| Renin-angiotensin-system agents | 13,355 (15.3) | 14,756 (17.5) | 45,127 (10.6) | 39,433 (10.1) | 0.132 | 110,382 (11.0) | 112,263 (11.2) | 112,787 (11.4) | 11,4308 (11.5) | 0.009 | |
| Beta blockers | 9,278 (10.6) | 9,435 (11.2) | 25,832 (6.1) | 20,540 (5.2) | 0.136 | 65,791 (6.6) | 66,040 (6.6) | 65,225 (6.6) | 66,471 (6.7) | 0.003 | |
| Calcium channel blockers | 20,257 (23.2) | 22,439 (26.5) | 69,318 (16.3) | 59,941 (15.3) | 0.169 | 164,969 (16.5) | 169,507 (17.0) | 171,767 (17.3) | 173,382 (17.5) | 0.015 | |
| Diuretics | 4,133 (4.7) | 3,305 (3.9) | 7,378 (1.7) | 5,935 (1.5) | 0.116 | 20,595 (2.1) | 20,913 (2.1) | 20,860 (2.1) | 21,668 (2.2) | 0.005 | |
| Nitrates | 1,465 (1.7) | 1,139 (1.3) | 2,473 (0.6) | 2,116 (0.5) | 0.068 | 7,359 (0.7) | 7,362 (0.7) | 7,247 (0.7) | 7,395 (0.7) | 0.001 | |
| Lipid-lowering agents | |||||||||||
| Statin | 15,836 (18.1) | 18,404 (21.8) | 57,586 (13.5) | 54,066 (13.8) | 0.129 | 142,941 (14.3) | 144,204 (14.4) | 146,082 (14.8) | 147,301 (14.9) | 0.010 | |
| Fibrate | 532 (0.6) | 597 (0.7) | 2,082 (0.5) | 1,832 (0.5) | 0.018 | 5,213 (0.5) | 4,965 (0.5) | 5,054 (0.5) | 5,084 (0.5) | 0.002 | |
| PCSK9 inhibitors | 3 (0.0) | 9 (0.0) | 14 (0.0) | 36 (0.0) | 0.006 | 63 (0.0) | 62 (0.0) | 63 (0.0) | 62 (0.0) | <0.001 | |
| Insulins | 2,770 (3.2) | 2,180 (2.6) | 5,314 (1.2) | 3,808 (1.0) | 0.095 | 14,827 (1.5) | 14,314 (1.4) | 14,183 (1.4) | 14,993 (1.5) | 0.004 | |
| Antidiabetic drugs | |||||||||||
| Sulfonylurea | 5,343 (6.1) | 6,347 (7.5) | 18,640 (4.4) | 14,669 (3.7) | 0.095 | 44,878 (4.5) | 45,129 (4.5) | 45,010 (4.5) | 45,794 (4.6) | 0.003 | |
| Metformin | 9,416 (10.8) | 11,317 (13.4) | 35,795 (8.4) | 29,692 (7.6) | 0.109 | 86,058 (8.6) | 86,164 (8.6) | 86,189 (8.7) | 87,388 (8.8) | 0.004 | |
| DPP-4 inhibitors | 2,554 (2.9) | 2,423 (2.9) | 6,212 (1.5) | 4,927 (1.3) | 0.075 | 16,427 (1.6) | 16,212 (1.6) | 16,179 (1.6) | 16,842 (1.7) | 0.003 | |
| SGLT-2 inhibitors | 985 (1.1) | 1,153 (1.4) | 3,897 (0.9) | 3,601 (0.9) | 0.025 | 10,507 (1.1) | 9,698 (1.0) | 9,643 (1.0) | 9,835 (1.0) | 0.004 | |
| GLP-1 agonists | 129 (0.1) | 138 (0.2) | 544 (0.1) | 545 (0.1) | 0.005 | 1,728 (0.2) | 1,352 (0.1) | 1,353 (0.1) | 1,379 (0.1) | 0.005 | |
| Oral anticoagulants | 1,463 (1.7) | 1,067 (1.3) | 2,014 (0.5) | 1,657 (0.4) | 0.077 | 6,400 (0.6) | 6,325 (0.6) | 6,225 (0.6) | 6,635 (0.7) | 0.003 | |
| Antiplatelets | 5,681 (6.5) | 4,620 (5.5) | 10,219 (2.4) | 8,661 (2.2) | 0.133 | 29,231 (2.9) | 29,108 (2.9) | 29,391 (3.0) | 30,121 (3.0) | 0.004 | |
| Immunosuppressants | 948 (1.1) | 690 (0.8) | 1,837 (0.4) | 1,739 (0.4) | 0.046 | 5,692 (0.6) | 5,584 (0.6) | 5,362 (0.5) | 5,623(0.6) | 0.002 | |
| Severe COVID-19 | 328 (0.4) | 235 (0.3) | 336 (0.1) | 254 (0.1) | 0.042 | 1,249 (0.1) | 1,196 (0.1) | 1,222 (0.1) | 1,426 (0.1) | 0.003 | |
| BNT162b2 recipients | 0 (0.0) | 30,158 (35.7) | 247,274 (58.1) | 218,344 (55.7) | NA | 0 (0.0) | 434,877 (43.5) | 554,848 (56.0) | 547,149 (55.3) | NA | |
| CoronaVac recipients | 0 (0.0) | 54,376 (64.3) | 178,691 (41.9) | 173,758 (44.3) | NA | 0 (0.0) | 563,885 (56.5) | 435,222 (44.0) | 442,897 (44.7) | NA | |
(A) Within 28 days since COVID-19 infection; (B) after 28 days since COVID-19 infection
All parameters are expressed in either frequency (percentage) or mean (SD). PCSK9, proprotein convertase subtilisin/kexin type 9; DPP-4, dipeptidyl peptidase-4; SGLT-2, sodium/glucose cotransporter 2; GLP-1, glucagon-like peptide-1; NA, not applicable.
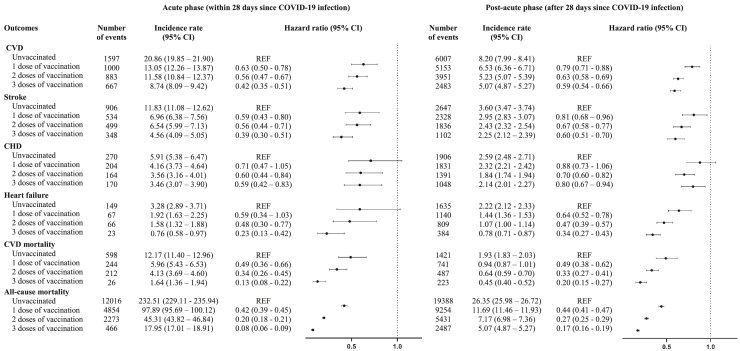
The incidence rates (IRs) and the risks of composite CVD, stroke, coronary heart disease, heart failure, cardiovascular mortality, and all-cause mortality among individuals with different vaccination status at acute and post-acute phases are depicted in Figure 1. During the acute phase, the IRs (95% confidence intervals [Cis]) of outcome events were the highest in unvaccinated patients compared to vaccinated patients, and they decrease by number of doses. A similar pattern was observed in the post-acute phase, with higher Irs recorded in unvaccinated patients and lowest at third dose.
Figure 1.
Incidence rate and risk of cardiovascular outcomes and mortality among individuals with different vaccination status at acute and post-acute phases after weighting
Incidence rate (cases/1,000 person-years) with 95% CI based on Poisson distribution. Hazard ratio with 95% CI was obtained by Cox regression adjusted with weighting. REF, reference level; CVD, cardiovascular disease; CHD, coronary heart disease.
A positive dose-response relationship between the magnitude of risk reduction and the number of vaccine doses received was observed. During the acute phase, hazard ratios (HRs) (95% CI) of outcome in first-dose recipients (CVD, 0.63 [0.50–0.78]; stroke, 0.59 [0.43–0.80]; cardiovascular mortality, 0.49 [0.36–0.66]; all-cause mortality, 0.42 [0.39–0.45)) was higher than that in third-dose recipients (CVD, 0.42 [0.35–0.51]; stroke, 0.39 [0.30–0.51); cardiovascular mortality, 0.13 (0.08–0.22); all-cause mortality, 0.08 (0.06–0.09)) compared to unvaccinated patients. A similar pattern was also observed in the individual outcome of coronary heart disease (first-dose recipient, 0.71 [0.47–1.05]; third-dose recipient, 0.59 [0.42–0.83)) and heart failure (first-dose recipient, 0.59 [0.34–1.03]; third-dose recipient, 0.23 [0.13–0.42)) in which insignificant findings were observed. During the post-acute phase, HRs of outcome in first-dose recipients (CVD, 0.79 [0.71–0.88]; stroke, 0.81 [0.68–0.96]; heart failure, 0.64 [0.52–0.78); cardiovascular mortality, 0.49 [0.38–0.62]; all-cause mortality, 0.44 [0.41–0.47)) were also higher than that in third-dose recipients (CVD, 0.59 [0.54–0.66); stroke, 0.60 [0.51–0.70]; heart failure, 0.34 [0.27–0.43]; cardiovascular mortality, 0.20 [0.15–0.27); all-cause mortality, 0.17 [0.16–0.19)) compared to unvaccinated patients. Tables S1–S5 show similar results from five sensitivity analyses.
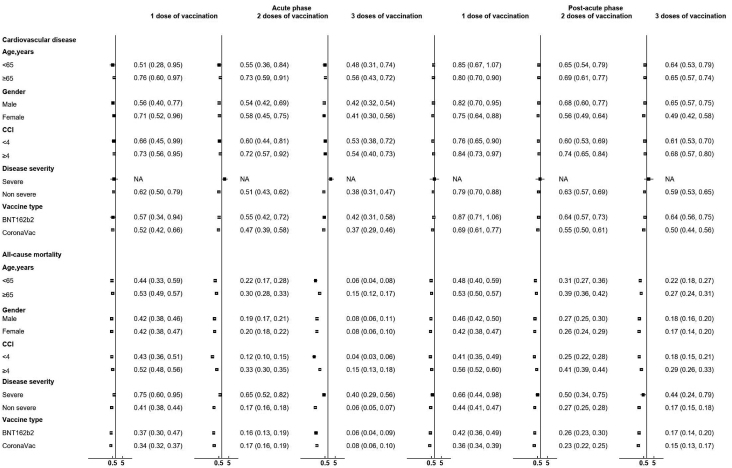
The results of the subgroup analyses stratified by age, gender, Charlson Comorbidity Index (CCI), and disease severity are reported in Figures 2 and S1. Similar to the main analysis, larger risk reductions in terms of all-cause mortality were observed in all subgroups of patients who received three doses of vaccines when compared to those who received fewer doses in both the acute and post-acute phase. This trend was not observed for the outcome of CVD, possibly due to a small number of outcome events recorded, especially in the subgroup of patients with severe COVID-19 who were vaccinated.
Figure 2.
Risk of CVD and mortality among individuals with different vaccination status compared with unvaccinated individuals at acute and post-acute phases by age, gender, Charlson index, disease severity, and vaccine type
HR with 95% CI was obtained by Cox regression adjusted with weighting. NA, not available due to insufficient number of events.
Discussion
This study evaluated the association between BNT162b2 or CoronaVac and the risk of CVD and mortality in the acute and post-acute phase after a COVID-19 infection. Both BNT162b2 and CoronaVac were associated with lower risks of post-infection incident CVD. A trend of positive dose-response relationship between the magnitude of overall CVD risk reduction and the number of vaccine doses received was observed, and thus the public were encouraged to get vaccinated and boosted to reduce the burden of CVD and mortality after COVID-19 infection.
Our study produced similar findings to a previous study that demonstrated vaccine effectiveness in lowering the risk of incident CVD within 28 days after COVID-19 infection among a cohort of diabetes patients.20 At present, similar studies in the COVID-19 setting are scarce, and whether COVID-19 vaccines improve cardiovascular outcome is an important clinical question to be addressed.21 In terms of the risk reduction of post-acute incident CVD, our findings are consistent with a Korean cohort study that reported reduced risks of acute myocardial infarction and ischemic stroke beyond 30 days after COVID-19 infection in fully vaccinated people who received mRNA or viral vector vaccines during the pre-Omicron era.14 In addition to their findings, we recruited patients in the period of February 2021 to May 2022, which overlaps with the Omicron-dominant wave, and observed a positive dose-response relationship between vaccine effectiveness against post-infection incident CVD and the number of vaccine doses administered. We noted the strongest protection in those who completed three doses of COVID-19 vaccine. Apart from acute myocardial infarction and ischemic stroke, we also noticed risk reduction in terms of heart failure, cardiovascular deaths, and all-cause mortality. To the best of our knowledge, no study that specifically addresses the effectiveness of inactivated vaccines against incident CVD has been published so far. Although waning effectiveness against COVID-19 infection has been observed in vaccine recipients,22 the present study reinforces the importance of vaccination, given the possible benefits of reducing cardiovascular complications after COVID-19 infection in the long run. Even though Omicron is associated with less severe disease compared to the earlier delta variant,23,24 it is unknown whether Omicron causes more cardiovascular complications at the moment. As previous research demonstrated a substantial risk of CVD in people previously infected with COVID-19 even after 1 year,7 vaccine uptake during the current Omicron outbreak should be promoted.
Our finding of COVID-19 vaccines reducing acute cardiovascular complications is in analogy to the case of influenza vaccine, in which a meta-analysis revealed that preventing influenza infection with vaccination improves cardiovascular outcome in patients with coronary heart disease because influenza infection is considered a risk factor and trigger for acute coronary syndrome.25 Similarly, with proven effectiveness against COVID-19 infection and severe disease,20,26,27,28 COVID-19 vaccines reduce COVID-19-induced myocardial injury, venous thromboembolism, and acute coronary syndrome.29 Intriguingly, the reduction in major CVD events demonstrated in this study was not mainly contributed by the reduction in coronary heart diseases (including acute coronary syndrome) but rather stroke and heart failure. Indeed, more investigation regarding this topic is needed. On the other hand, cardiovascular complications beyond the acute phase of COVID-19 infection have been inadequately explored, and current studies mainly focused on patients with risk factors and more severe COVID-19 who were hospitalized.7 A US study reported an increased risk of incident CVD beyond the first 30 days after COVID-19 infection in a cohort of veterans, of whom more than 50% were obese.7 It was uncertain whether younger people without cardiovascular risk factors are subject to a similar extent of increased CVD risks after contracting COVID-19. In the present study, vaccinated individuals without established CVD had a substantially lower risk of incident CVD, thereby supporting the role of vaccination. It is unclear how COVID-19 is linked to heightened cardiovascular risks in the post-acute phase, and so is the mechanism of vaccination in protecting against longer-term CVD. It was hypothesized that chronic inflammation triggered by continuous viral reservoirs in the heart as post-acute sequelae and autoimmune response as a result of molecular mimicry might have contributed to the development of CVD in the chronic phase.10 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells elicited by vaccination, which were shown to be strongly associated with COVID-19 disease severity30 and longer lasting compared to antibody response,31 might have potentially played a role in protecting against incident CVD in the post-acute phase, yet further studies are warranted to fill the research gap.
The effectiveness of vaccine in reducing post-infection cardiovascular risks was consistently demonstrated across subgroups of different age, gender, and CCI. The HR of the severe COVID-19 subgroup was apparently high with a large CI compared to patients with mild disease, possibly due to a small number of severe COVID-19 cases recorded among vaccinated patients. Further studies are warranted to confirm our findings. There seemed to be a difference in the post-acute phase between HRs of people receiving one dose of BNT162b2 versus one dose of CoronaVac, but the difference was no longer evident among third-dose recipients, thereby reinforcing the importance of booster shots in protecting against post-infection CVD, irrespective of the type of vaccination.
Strengths of the study
This study enrolled COVID-19 patients with different vaccination status and is one of the studies that provide real-world evidence on the association between mRNA (BNT162b2) and inactivated virus (CoronaVac) vaccine and the risk of incident CVD after acute COVID-19 infection. Our findings highlighted the importance of optimizing vaccine coverage and promoting booster uptake in order to reduce the burden of cardiovascular complications. Another advantage of this study is that we demonstrated both vaccines were associated with lower risks of post-infection incident CVD in individuals without established CVD at baseline.
Limitations of the study
Several limitations were present in this study. First, positive cases in this study were defined by either a positive PCR test or a positive rapid antigen test (RAT) result reported to the Department of Health of Hong Kong. It is possible that some asymptomatic COVID-19 infections were not captured in the current study. Second, the outcome events were solely defined by the diagnosis codes recorded in the electronic database, and, therefore, we could not rule out the possibility of misclassification or underdiagnosis. In the present study, severe COVID-19 was determined by intensive care unit (ICU) admission or the use of ventilatory support only. Both conditions depend on the availability of resources and clinical judgment, and, ideally, more objective parameters should be utilized to determine disease severity. Third, the number of outcome events recorded was small, especially among fully vaccinated patients. Therefore, some HRs could not be estimated in the subgroup analyses. Moreover, the comparison between people who received different numbers of vaccine doses was unavailable due to insufficient events detected within the subgroups. Further study on this comparison is warranted. Theoretically, we were unable to differentiate possible cardiovascular side effects of previous COVID-19 vaccination and the CVD complications of COVID-19. However, most cardiac events related to the vaccine were reported within 1 week of vaccination.32,33,34 Hence, the likelihood of side effects of vaccination misinterpreted as COVID-19 complications was minimal. Furthermore, although higher body mass index (BMI) was reported as an independent indicator of the risk of long COVID,35 data on BMI were not available in the current study. Last, there might be residual confounding, such as lifestyle factors, socioeconomic status, health literacy, and concomitant flu vaccination, which may affect the intention to get vaccinated and the risk of developing CVD.
Conclusions
The risk of CVD and mortality in both the acute and post-acute phase after COVID-19 infection was lower among recipients of BNT162b2 and CoronaVac. There was a positive dose-response relationship between the magnitude of overall risk reduction of CVD and the number of vaccine doses received.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| original code | This paper | github (https://github.com/kcyan96/hope_vaccine_cvd) |
| R Version 4.0.3 | R Foundation for Statistical Computing | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact Esther Chan (ewchan@hku.hk).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant Details
Study period and population
This territory-wide retrospective cohort study enrolled patients aged 18 years or above who have a documented COVID-19 infection between 23 February 2021 and 31 October 2022, defined by either a positive Polymerase Chain Reaction (PCR) result or a positive Rapid Antigen Test (RAT). The date of documented COVID infection was defined as the index date. Patients with a history of CVD, including stroke, coronary heart disease, or heart failure, before the index date were excluded from the cohort. Patients were followed up from the index date until the occurrence of study outcome or the end of the study (23 January 2023), whichever occurred first. The baseline characteristics of study participants, including their health status, disease and medication history, are presented in Table 1.
Study design
At the time of publication, two COVID-19 vaccines were provided by the Hong Kong Government, namely BNT162b2 and CoronaVac, and they have been in use since 23 February 2021. Patients in this study were classified into four mutually exclusive groups based on their vaccination status: (i) unvaccinated (reference group); (ii) received one dose of BNT162b2 or CoronaVac; (iii) received two doses of BNT162b2 or CoronaVac; and (iv) received three doses of BNT162b2 or CoronaVac. Since the choice of either heterologous or homologous COVID-19 booster shots were only made available from 11 November 2021 onwards for priority groups and 1 January 2022 for the general population, 36,37a small proportion of the local population received a heterologous booster after the primary series, and therefore they were excluded from the analysis.
Ethics approval
This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the DH Ethics Committee (LM171/2021). Anonymous data was extracted from the Hospital Authority Clinical Management System, and hence, consent from participants was not required.
Method details
Outcome
The primary outcome of this study was a composite of coronary heart disease, stroke, and heart failure, determined by the primary diagnosis code at hospitalization based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (coronary heart disease: 410.x-414.x, 36.0, 36.1, V45.81; stroke: 430.x-438.x; heart failure: 428.x, 398.91, 402.01, 402.11, 402.91, 404.01–404.03, 404.11–404.13, 404.91–404.93) The secondary outcomes were cardiovascular mortality (International Classification of Diseases, Tenth Revision: I00-99) and all-cause mortality. Information regarding all-cause mortality was extracted from the Hong Kong Death Registry, which is an official registry that records all registered deaths in Hong Kong.
Covariates
Baseline characteristics included age, gender, Charlson Comorbidity Index (CCI),38 comorbidities (chronic kidney disease, respiratory disease, diabetes and dementia), drug usage (renin-angiotensin-system agents, beta blockers, calcium channel blockers, diuretics, nitrates, lipid lowering agents [statin, fibrate, PCSK9 inhibitor], insulins, antidiabetic drugs [sulphonylurea, metformin, DPP-4 inhibitor, SGLT-2 inhibitor, GLP-1 agonist], oral anticoagulants, antiplatelets and immunosuppressants), and the severity of COVID-19. Severe COVID-19 was defined by ICU admission or the use of ventilatory support within seven days of infection, identified by the ICD-9-CM procedure codes (39.65, 89.18, 93.90, 93.95, 93.96, 96.04, 96.7x).
Quantification and statistical analysis
Weighting
Inverse probability of treatment weighting (IPTW) using propensity score was employed to minimise confounding across comparison groups. The propensity score model included all baseline characteristics mentioned above as covariates. A standardised mean difference (SMD) of less than 0.2 between comparison groups post-weighting was balanced between groups.39
Statistical analysis
In order to examine short-term and long-term effects, the observation period was divided into the acute phase (<28 days post-infection) and the post-acute phase (≥28 days post-infection). This cut-off has been used in previous studies.40,41 The incidence rates of outcome events and their corresponding 95% confidence intervals (CIs) were assessed based on their Poisson distribution. The risks of outcomes were compared between groups using IPTW-weighted Cox proportional hazards regression. Hazard ratios (HRs) and their corresponding 95% CIs were reported. The proportional hazard assumption of the models for CVD outcomes were evaluated using the interaction with time based on Schoenfeld residuals. There was no significant interaction, indicating that the models satisfied the proportional hazard assumption. Five sensitivity analyses were conducted. The first sensitivity analysis only included patients with positive PCR test results as confirmed COVID-19 infection cases. The second sensitivity analysis excluded patients who contracted COVID-19 more than 180 days since the last dose of vaccination, since waning immunity has been described in prior studies.42 The third sensitivity analysis excluded patients who received additional vaccine doses within 28 days after COVID-19 infection. The fourth sensitivity analysis was adjusted for mortality as a competing risk while evaluating associations. A competing risk Cox regression using the cause-specific cox regression method43 was conducted. The fifth sensitivity analysis further divided the post-acute phase into two periods (28–89 days and ≥90 days). Subgroup analyses stratified by age (<65, ≥65 years), gender (male, female), Charlson Comorbidity index (<4, ≥4) and disease severity (mild, severe) were conducted.
Two-tailed tests were used when analysing results from this study and a pP-value less than 0.05 was inferred as statistically significant. All statistical analyses were conducted using R version 4.0.3. At least two investigators conducted the statistical analyses independently for quality assurance. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists were followed to guide transparent reporting of the cohort study.
Acknowledgments
We gratefully acknowledge the Centre for Health Protection, the Department of Health, and the Hospital Authority for facilitating data access.
This work was supported by funding from HMRF Research on COVID-19, The Hong Kong Special Administrative Region (HKSAR) Government (principal investigator, E.W.C.; ref. no. COVID1903011); Collaborative Research Fund, University Grants Committee, the HKSAR Government (principal investigator, I.C.K.W.; ref. no. C7154-20GF); and a research grant from the Health Bureau, the HKSAR Government (principal investigator, I.C.K.W.; ref. no. COVID19F01). I.C.K.W. and F.T.T.L. are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by the AIR@InnoHK administered by Innovation and Technology Commission.
Author contributions
Concept and design, E.Y.F.W., A.H.Y.M., V.K.C.Y., I.C.K.W., and E.W.C.; acquisition, analysis, or interpretation of data, E.Y.F.W., A.H.Y.M., V.K.C.Y., C.I.Y.C., B.W., F.T.T.L., C.S.L.C., X.L., C.K.H.W., I.C.K.W., and E.W.C.; drafting of the manuscript, E.Y.F.W., A.H.Y.M., V.K.C.Y., and C.I.Y.C.; critical revision of the manuscript for important intellectual content, all authors; statistical analysis, E.Y.F.W., V.K.C.Y., C.I.Y.C., and B.W.; administrative, technical, or material support, I.C.K.W. and E.W.C.; supervision, I.C.K.W. and E.W.C.
Declaration of interests
E.Y.F.W. has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region and the Hong Kong Research Grants Council (RGC) outside the submitted work. F.T.T.L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong RGC and has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region outside the submitted work. C.S.L.C. has received grants from the Health Bureau of the Hong Kong Government, Hong Kong RGC, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen and personal fees from PrimeVigilance outside the submitted work. X.L. has received research grants from the Health Bureau of the Government of the Hong Kong Special Administrative Region, research and educational grants from Janssen and Pfizer, internal funding from the University of Hong Kong, and consultancy fees from Merck Sharp & Dohme unrelated to this work. I.C.K.W. reports grants from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, the Hong Kong Health and Medical Research Fund in Hong Kong, National Institute for Health Research in the United Kingdom, the European Commission, and the National Health and Medical Research Council in Australia; consulting fees from IQVIA and World Health Organization; payment for expert testimony for Appeal Court of Hong Kong; and is a non-executive director of Jacobson Medical in Hong Kong and Therakind in the United Kingdom outside of the submitted work. E.W.C. reports grants from RGC (Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and the Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region and a honorarium from Hospital Authority outside the submitted work.
Published: September 15, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101195.
Contributor Information
Ian Chi Kei Wong, Email: wongick@hku.hk.
Esther Wai Yin Chan, Email: ewchan@hku.hk.
Supplemental information
Data and code availability
The original code for the main analysis has been deposited on github (https://github.com/kcyan96/hope_vaccine_cvd) and is publicly available as of the date of publication. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
The electronic health records, vaccination records and COVID-19 infection records datasets cannot be deposited in a public repository because these are confidential medical records and the data custodians (the Hospital Authority and the Department of Health of the Hong Kong Special Administrative Region) have not given permission for sharing due to patient confidentiality and privacy concerns. For access, please approach the lead contact to direct your request to the Hospital Authority’s data sharing portal. In addition, processed datasets derived from these data which were used in the analyses reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., Fralick M., Schou M., Lamberts M., Gerds T., et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewek J., Jatczak-Pawlik I., Maciejewski M., Jankowski P., Banach M. COVID-19 and cardiovascular complications–preliminary results of the LATE-COVID study. Arch. Med. Sci. 2021;17:818–822. doi: 10.5114/aoms/134211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranard L.S., Fried J.A., Abdalla M., Anstey D.E., Givens R.C., Kumaraiah D., Kodali S.K., Takeda K., Karmpaliotis D., Rabbani L.E., et al. Approach to acute cardiovascular complications in COVID-19 infection. Circ. Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbasi J. The COVID Heart—One Year After SARS-CoV-2 Infection, Patients Have an Array of Increased Cardiovascular Risks. JAMA. 2022;327:1113–1114. doi: 10.1001/jama.2022.2411. [DOI] [PubMed] [Google Scholar]
- 9.Wang W., Wang C.-Y., Wang S.-I., Wei J.C.-C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022;43:1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed M.O., Banerjee A. Long COVID and cardiovascular disease: a learning health system approach. Nat. Rev. Cardiol. 2022;19:287–288. doi: 10.1038/s41569-022-00697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Raddad L.J., Chemaitelly H., Butt A.A., National Study Group for COVID-19 Vaccination Effectiveness of the BNT162b2 Covid-19 Vaccine against the B. 1.1. 7 and B. 1.351 Variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.-E., Huh K., Park Y.-J., Peck K.R., Jung J. Association Between Vaccination and Acute Myocardial Infarction and Ischemic Stroke After COVID-19 Infection. JAMA. 2022;328:887–889. doi: 10.1001/jama.2022.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., Watkinson P., Khunti K., Harnden A., Coupland C.A.C., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ali D., Elshafeey A., Mushannen M., Kawas H., Shafiq A., Mhaimeed N., Mhaimeed O., Mhaimeed N., Zeghlache R., Salameh M., et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell Mol. Med. 2022;26:636–653. doi: 10.1111/jcmm.17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh S.Y., Chen H.M., Hsu C.Y. Prolonged peripheral seronegative spondyloarthritis following BioNTech coronavirus disease 2019 vaccination: A case report. Int. J. Rheum. Dis. 2023;26:774–777. doi: 10.1111/1756-185X.14512. [DOI] [PubMed] [Google Scholar]
- 18.Cutler D.M. American Medical Association; 2022. p. e221809. (The Costs of Long COVID. In 5). [DOI] [PubMed] [Google Scholar]
- 19.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., Rajagopal S., Pai A.R., Kutty S. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front. Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan E.Y.F., Mok A.H.Y., Yan V.K.C., Wang B., Zhang R., Hong S.N., Chui C.S.L., Li X., Wong C.K.H., Lai F.T.T., et al. Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA. 2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: A case control study. J. Infect. 2022;85:e140–e144. doi: 10.1016/j.jinf.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kow C.S., Ramachandram D.S., Hasan S.S. Can COVID-19 vaccines improve cardiovascular outcomes? Trav. Med. Infect. Dis. 2022;48 doi: 10.1016/j.tmaid.2022.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D. Clinical Severity of, and Effectiveness of mRNA Vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. bmj. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayr F.B., Talisa V.B., Castro A.D., Shaikh O.S., Omer S.B., Butt A.A. COVID-19 disease severity in US Veterans infected during Omicron and Delta variant predominant periods. Nat. Commun. 2022;13:3647–3656. doi: 10.1038/s41467-022-31402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Arocutipa C., Saucedo-Chinchay J., Mamas M.A., Vicent L. Travel Medicine and Infectious Disease; 2022. Influenza Vaccine Improves Cardiovascular Outcomes in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 26.Yan V.K.C., Wan E.Y.F., Ye X., Mok A.H.Y., Lai F.T.T., Chui C.S.L., Li X., Wong C.K.H., Li P.H., Ma T. Emerging Microbes & Infections; 2022. Effectiveness of BNT162b2 and CoronaVac Vaccinations against Mortality and Severe Complications after SARS-CoV-2 Omicron BA. 2 Infection: A Case-Control Study; pp. 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung H., He S., Nasreen S., Sundaram M.E., Buchan S.A., Wilson S.E., Chen B., Calzavara A., Fell D.B., Austin P.C. Effectiveness of BNT162b2 and mRNA-1273 Covid-19 Vaccines against Symptomatic SARS-CoV-2 Infection and Severe Covid-19 Outcomes in Ontario, Canada: Test Negative Design Study. bmj. 2021;374 doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burckhardt R.M., Dennehy J.J., Poon L.L.M., Saif L.J., Enquist L.W. Are COVID-19 Vaccine Boosters Needed? The Science behind Boosters. J. Virol. 2022;96:01973211–e201921. doi: 10.1128/jvi.01973-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., Castellanos D.A., Saleeb S.F., de Ferranti S.D., Newburger J.W., Friedman K.G. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park D.Y., An S., Kaur A., Malhotra S., Vij A. Myocarditis after COVID-19 mRNA vaccination: A systematic review of case reports and case series. Clin. Cardiol. 2022;45:691–700. doi: 10.1002/clc.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., Chui C.S.L., Wan E.Y.F., Wong C.K.H., Chan E.W.Y., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case–control study. Ann. Intern. Med. 2022;175:362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chudzik M., Lewek J., Kapusta J., Banach M., Jankowski P., Bielecka-Dabrowa A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2022;11:4980. doi: 10.3390/jcm11174980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.HKSAR Government . 2021. Third Dose COVID-19 Vaccination Arrangements for Persons under Certain Groups.https://www.info.gov.hk/gia/general/202111/03/P2021110300536.htm [Google Scholar]
- 37.HKSAR Government . 2021. Further Expansion of COVID-19 Vaccination Arrangements from January 1.https://www.info.gov.hk/gia/general/202112/24/P2021122400509.htm [Google Scholar]
- 38.Glasheen W.P., Cordier T., Gumpina R., Haugh G., Davis J., Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits. 2019;12:188–197. [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart E.A., Lee B.K., Leacy F.P. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J. Clin. Epidemiol. 2013;66:S84–S90.e1. doi: 10.1016/j.jclinepi.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen K., Ren S., Heath K., Dasmariñas M.C., Jubilo K.G., Guo Y., Lipsitch M., Daugherty S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. Bmj. 2022;376 doi: 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., Lipsitch M., Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. Bmj. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., Groome M.J., Huppert A., O'Brien K.L., Smith P.G., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozenne B., Sørensen A., Scheike T., Torp-Pedersen C., Gerds T. riskRegression: Predicting the Risk of an Event using Cox Regression Models. R J. 2017;9:440–460. doi: 10.32614/RJ-2017-062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original code for the main analysis has been deposited on github (https://github.com/kcyan96/hope_vaccine_cvd) and is publicly available as of the date of publication. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
The electronic health records, vaccination records and COVID-19 infection records datasets cannot be deposited in a public repository because these are confidential medical records and the data custodians (the Hospital Authority and the Department of Health of the Hong Kong Special Administrative Region) have not given permission for sharing due to patient confidentiality and privacy concerns. For access, please approach the lead contact to direct your request to the Hospital Authority’s data sharing portal. In addition, processed datasets derived from these data which were used in the analyses reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.