Summary
The increasing prevalence of diabetes, high avoidable morbidity and mortality due to diabetes and diabetic complications, and related substantial economic burden make diabetes a significant health challenge worldwide. A shortage of diabetes specialists, uneven distribution of medical resources, low adherence to medications, and improper self-management contribute to poor glycemic control in patients with diabetes. Recent advancements in digital health technologies, especially artificial intelligence (AI), provide a significant opportunity to achieve better efficiency in diabetes care, which may diminish the increase in diabetes-related health-care expenditures. Here, we review the recent progress in the application of AI in the management of diabetes and then discuss the opportunities and challenges of AI application in clinical practice. Furthermore, we explore the possibility of combining and expanding upon existing digital health technologies to develop an AI-assisted digital health-care ecosystem that includes the prevention and management of diabetes.
Keywords: diabetes, artificial intelligence, digital health, management
Graphical abstract
Guan et al. review the recent progress in the application of AI in diabetes care and then discuss the opportunities for and challenges of AI application in clinical practice. Furthermore, they explore the possibility of combining and expanding upon existing digital health technologies to develop an AI-assisted digital health-care ecosystem.
Introduction
The increasing prevalence of diabetes has become a global public health concern in the 21st century. Previously, diabetes was prevalent in affluent “Western” countries; however, currently, diabetes occurs worldwide, catalyzed by the consumption of nutrient-poor and calorie-rich foods and an increasingly sedentary lifestyle.1 Diabetes has already become a critical public health concern in China, with a rapid increase in prevalence from 0.67% in 1980 to 9.7% in 2007 and then slightly increasing to 11.2% in 2017.2 According to the 2021 IDF Diabetes Atlas, the prevalence of diabetes in adults 20–79 years of age (95% confidence interval [CI]) has reached 13.0% (11.3%–14.7%) in China, with an estimate of 72,839.5 (95% CI 62,926.1–81,980.7; in thousands) adults undiagnosed.3 In 2019, approximately 824,000 adults were estimated to die due to diabetes and related complications, with the estimated diabetes-related health expenditures ranking second to that of the United States.2 The increasing prevalence of diabetes, high avoidable morbidity and mortality due to diabetes and diabetic complications, and related substantial economic burden make diabetes a significant health challenge.4
Several challenges exist in preventing and managing diabetes in traditional face-to-face medical practices. First, prevention and early diagnosis of diabetes remain significant obstacles since many cases of diabetes remain undiagnosed for many years.5 Second, the management of patients with diabetes involves regular follow-up of thorough examinations of blood glucose control and diabetic complications. In addition, integrated diabetes management requires collaboration among endocrinology, podiatry, nutrition, nephrology, and ophthalmology. These factors result in an uneven distribution of medical resources, with a lack of high-quality human resources and an insufficient capacity of primary health care. Third, diabetes is perhaps the most prominent example of a highly prevalent chronic disease that demands a patient’s active continuous role in its management due to its dependence on diet and exercise, its wide array of complications across the body’s physiological systems, and its need for self-monitoring.6,7
The emergence of digital health technologies (DHTs), especially artificial intelligence (AI), may help address these obstacles and alleviate the disease burden of diabetes in the future, because AI-based DHTs in diabetes care could help implement better prevention strategies for high-risk populations, manage diabetic patients who are unable to attend physician appointments in person, deliver real-time health and metabolic information, promote better self-management of patients, and save time and money by reducing travel to in-person appointments.8 First introduced in 2000 by Seth Frank,9 digital health broadly encompasses internet-focused applications and media to improve medical content, commerce, and connectivity. The term “digital health” has expanded to encompass a much broader set of scientific concepts and technologies, including genomics, AI, analytics, wearables, mobile applications, and telemedicine.10 AI is a broad branch of computer science that develops theories, methods, technologies, and application systems to simulate, extend, and expand human intelligence in machines.11 Machine learning (ML)12 is a subcategory of AI that uses statistical techniques to build intelligent systems. Using a supervised or unsupervised approach, an intelligent system can automatically learn and improve its performance, such as accuracy, without being explicitly programmed. Deep learning (DL),13 which uses advanced ML techniques, has achieved significant success in computer vision and natural language processing tasks, primarily attributed to its excellent feature extraction and pattern recognition capabilities, which use multiple processing layers (artificial neurons) to learn representations of data with different levels of abstraction such that it associates the input with a diagnostic output.
Since the mid-20th century, researchers have proposed and developed AI-based clinical decision-support systems. Rule-based approaches were shown to diagnose diabetes, choose appropriate treatments, and provide interpretations of clinical reasoning in the 1970s.14 However, rule-based systems are expensive to build and brittle. Furthermore, it is challenging to encode higher-order interactions between different pieces of knowledge provided by different specialists, with system effectiveness restricted by the comprehensiveness of pre-existing medical knowledge.12 Hence, recent AI research has leveraged ML methods, which can account for complex interactions, to identify patterns from data.15
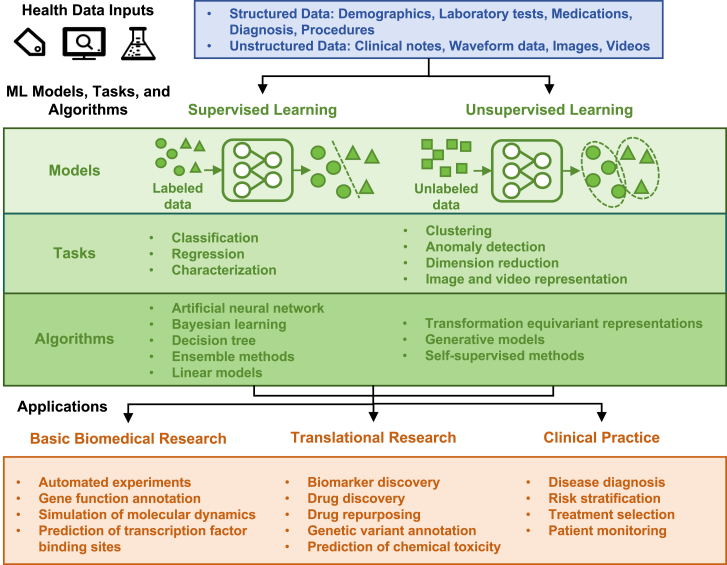
The application of ML in diabetes care and research has been widely explored in basic biomedical research,16 translational research,14 and clinical practice17 (Figure 1). Basic ML algorithms can be roughly classified into two categories according to the type of tasks to be solved: supervised and unsupervised.18 Supervised ML methods involve collecting several “training” cases, which contain inputs (e.g., fundus photographs) and the desired output labels (e.g., the presence or absence of diabetic retinopathy). By analyzing the patterns in all the labeled input-output pairs, the algorithm learns to produce the correct output for a given input in new cases.19 Unsupervised ML methods infer the underlying patterns in unlabeled data to find subclusters of the original data (e.g., subclusters of diabetes20,21), identify outliers in the data, produce low-dimensional representations of the data, or represent images and videos. In addition, there are other types of ML, such as semi-supervised learning22 and reinforcement learning.23,24 Semi-supervised learning is a branch of ML that uses labeled and unlabeled data to perform certain learning tasks22 and permits the harnessing of large amounts of unlabeled data available in combination with typically smaller sets of labeled data. Considering that a large body of health-care data in diabetes management lacks supervised information (e.g., annotations of retinal lesions in fundus photographs), which requires expensive human effort in labeling or scoring, semi-supervised learning could utilize unlabeled or unscored data together with only a small amount of supervised data to improve the performance of AI models.25,26 Reinforcement learning is a process that can be used to learn optimal actions from data and is designed to learn an optimal strategy that maximizes the overall rewards.24 It has been utilized to develop dynamic treatment regimens and provide a precise insulin dosage to react to the immediate needs of patients with diabetes.23 Despite the rapid progress of ML methods, there are several potential flaws, including data bias,27 overfitting,28 resource-intensive training,29 and limited transfer learning.30
Figure 1.
Current applications of machine intelligence in diabetes care and research
Common algorithms used in supervised learning include31 (1) artificial neural networks, such as Boltzmann machines, restricted Boltzmann machines, multi-layer perceptron, radial basis function networks, recurrent neural networks, Hopfield networks, convolutional neural networks, and spiking neural networks; (2) Bayesian learning, such as naive Bayes, Gaussian naive Bayes, multiple naive Bayes, average one-dependence estimators, Bayesian belief networks, and Bayesian networks; (3) decision trees, such as classification and regression tree, Iterative Dichotomiser 3, C4.5 algorithm, C5tree.0 algorithm, chi-squared automatic interaction detection, decision stump, and supervised learning in quest; (4) ensemble methods, such as random forest, bagging, boosting, AdaBoost, and XGBoost; and (5) linear models, such as linear regression, logistic regression, generalized linear models, Fisher linear discriminant analysis, quadratic discriminant analysis, least absolute shrinkage and selection operator regression, multi-modal logistic regression, naive Bayes classifier, and perceptron and linear support vector machine. Common algorithms used in unsupervised learning include32,33 (1) transformation equivariant representations, such as group equivariant convolutions and autoencoding transformations; (2) generative models, such as flow-based generative models, generative adversarial networks, autoencoders, and disentangled representations; and (3) self-supervised methods, such as autoregressive and self-attention models.
In summary, ML methods enable the development of AI applications that facilitate the discovery of previously unrecognized patterns in the data without the need to specify decision rules for each specific task or to account for complex interactions among input features. Therefore, ML has become the preferred framework for building AI utilities.34 Based on the present background, we review the recent advancements in the application of AI in the clinical practice of diabetes management and then discuss the opportunities and challenges of AI applications. Furthermore, we explore the possibility of combining and expanding upon existing DHTs to develop an AI-assisted digital health-care ecosystem that includes the prevention and management of diabetes as a promising vision for the future of diabetes care.
Application of AI in the prediction and prevention of diabetes
Prediction of diabetes onset
The prediction of diabetes onset is a part of pre-emptive medicine, accurately identifying individuals highly likely to develop diabetes at the pre-illness stage. Thus, this technology could eventually decrease the incidence of diabetes by implementing medical interventions for these people at a very early stage. Predicting the onset of diabetes did not occur with the advent of ML technology. Abbasi et al. reported the usefulness of statistical models, such as logistic regression, Cox proportional hazard model, or Weibull distribution analysis, to predict the onset of diabetes in non-diabetic individuals within 5 to 10 years, with concordance index (C index) ranging from 0.74 to 0.94.35
However, ML is a promising tool that can maximize predictive performance compared with conventional statistical models. Choi et al. reported that the area under the curve (AUC) of predicting new-onset diabetes within 5 years for hospitalized patients was 0.78, derived from ML-based logistic regression.36 Ravaut et al. recently reported that an ML model using administrative health data could predict diabetes onset within 5 years with an AUC of 0.80.37 Similarly, Nomura et al. developed an ML-based prediction model to identify diabetes signatures before the onset of diabetes using the gradient-boosting decision tree method, with an AUC and overall accuracy of 0.71 and 94.9%, respectively.38 Recently, a DL system developed by Zhang et al. could predict disease development and perform risk stratification for type 2 diabetes (T2D) using retinal images and clinical risk factors.39
Management of modifiable risk factors for diabetes
AI could be employed in understanding risk factors for diabetes onset owing to human limitations and biases when dealing with large spaces of risk factors. Based on these recognizable modifiable risk factors, precise interventions for preventing diabetes can be implemented in different individuals. Genetic, clinical, anthropometric, demographic, and behavioral risk factors have been recognized in previous research on normal glucose hemostasis (NGH)-type 1 diabetes (T1D), NGH-T2D, NGH-gestational diabetes (GD), and GD-T2D progression.40,41,42,43 Modifiable factors identified using the AI method increase the risk of developing diabetes, including high blood pressure, high blood cholesterol, tobacco smoking, insufficient physical activity, poor diet, and overweight or obesity.44,45,46
Application of AI in the screening and classification of diabetes
Screening of diabetes
Current diagnostic guidelines for diabetes47 are driven by invasive measurements in clinics, possibly influenced by behavioral and ethnic factors. Considering that the early stages of T2D are often asymptomatic, individuals can carry the disease for years undiagnosed.48 Unfortunately, late-stage diagnosis can lead to health complications and lower life expectancy. To prevent this pattern, researchers have prioritized T2D diagnosis, attempting to develop accurate diagnostic methods from readily available data and non-invasive, inexpensive tests.49 These limitations have motivated the use of AI-based diagnostics that have high classification accuracy and leverage large datasets (including data from wearable and continuous monitoring devices) that are not easily interpreted. These AI-based models are less invasive and highly accessible, which could effectively improve the willingness of the general population to screen and provide personalized screening plans for high-risk populations.
AI-based screening for diabetes primarily focuses on the following two aspects. First, AI has been broadly utilized for screening purposes because it enables the identification and use of predictors that lack apparent relationships with diabetes. Tapak et al. implemented artificial neural networks, support vector machine, fuzzy c-means, random forest, logistic regression, and linear discriminant analysis for a dataset of 6,500 subjects in Iran.50 Ten risk factors were used as predictors (blood glucose-related information was not included). The study demonstrated that support vector machine had a superior AUC compared with logistic regression and linear discriminant analysis. Similarly, Maniruzzaman et al. compared Gaussian process-based techniques with different kernels (linear polynomial and radial basis) against linear discriminant analysis, quadratic discriminant analysis, and naive Bayes.51 The highest accuracy was achieved using the Gaussian process method with a radial kernel. Second, the development of wide-ranging sensing technologies and the generation of associated novel datasets are opening increasing avenues for AI screening for diabetes. Shu et al. extensively studied the effects of texture features extracted from facial-specific regions on diabetes detection using eight texture extractors.52 The best texture feature extractor for diabetes mellitus detection could achieve an accuracy of 99.02%, a sensitivity of 99.64%, and a specificity of 98.26% using support vector machine. Li et al. established a non-invasive diabetes risk prediction model based on tongue feature fusion and predicted the risk of pre-diabetes and diabetes using ML techniques.53 The best performance of their model had an average accuracy of 0.821 and an average area under the receiver operating characteristic curve (AUROC) of 0.924. Zhang et al. demonstrated that DL models could be used to detect T2D solely from fundus images or in combination with clinical metadata with AUROCs of 0.85–0.93.39
General classification of diabetes based on existing clinical guidelines
AI can predict the risk of diabetes onset and classify diabetes. Linear discriminant analysis, quadratic discriminant analysis, naive Bayesian method, Gaussian process classification, and other technologies can help classify the four types of diabetes and guide the selection of follow-up treatment plans for different types of diabetes. This is helpful to primary medical institutes that cannot conduct islet function or other antibody tests. Although AI can classify different types of diabetes more accurately,51,54,55 further algorithm upgrading is required to meet the clinical treatment needs.
Refined, precise classification of diabetes
Existing treatment strategies for diabetes cannot stop the progressive course of the disease and prevent the development of chronic diabetic complications. One explanation for these shortcomings is that the diagnosis of diabetes is based on the measurement of only one metabolite, glucose; however, the disease is heterogeneous in terms of clinical presentation and progression. A refined classification system could provide a powerful tool to individualize treatment regimens and identify individuals with an increased risk of complications at diagnosis. Using data-driven cluster analysis (k-means and hierarchical clustering) in European patients with newly diagnosed diabetes (n = 8,980), Ahlqvist et al. identified five replicable clusters of patients with diabetes with significantly different patient characteristics and risk of diabetic complications.20 Clusters were based on six variables (glutamate decarboxylase antibodies, age at diagnosis, body mass index, HbA1c, and homeostatic model assessment of two estimates of β cell function and insulin resistance). Zou et al. tested whether this novel diabetes clustering applies to Chinese and US participants in two cross-sectional population-based datasets21 and confirmed the novel diabetes subgroups proposed by Ahlqvist et al., suggesting possible generalizability of this European-oriented diabetes classification in different ethnicities and populations.
Application of AI in the comprehensive management of diabetes
Health education
Health education aims to empower diabetic patients with increased knowledge and awareness of diabetes, which can further facilitate better disease self-management. Alotaibi et al. designed an intelligent mobile diabetes management system (SAED),56 whose pilot study showed that it could significantly decrease HbA1c levels and enhance the awareness of basic knowledge regarding diabetes among participants. Hamon and Gagnayre applied natural language processing methods to web forms to identify patients’ knowledge gaps and recommend tailored educational strategies.57 Recently, Chen et al. evaluated the effect of an intelligent mobile health technology-based diabetes education program on glucose control in patients with T2D initiating a pre-mixed insulin.58 The 12-week education program and the initiation of insulin therapy significantly decreased the HbA1c levels in patients with T2D.
Medical nutrition therapy
AI-based automatic diet monitoring
Dietary monitoring is critical in patients with diabetes, especially as self-reporting of food intake is inaccurate and often impractical59; therefore, it is necessary to develop an automated solution for dietary monitoring. A diet-monitoring system can be divided into two categories based on the degree of automation. The semi-automatic system requires users to mark the approximate position of the food in the picture. In automated dietary monitoring systems, users send food pictures to the server, which analyzes the images and estimates the nutritional characteristics of the food.60 Thus, a food image analysis system must solve several problems, including image segmentation, food recognition and classification, food volume estimation, and calorie intake conversion.
Recent research has shown increasing accuracy in estimating energy intake based on food images. Vasiloglou et al. designed a smartphone system (GoCARB), which is specially designed for patients with T1D and can estimate the carbohydrate content in meals.61 No differences were found between the estimations of dietitians and those of GoCARB, regardless of the meal size. Zhang et al. developed a mobile food identification system that automatically identifies food and estimates its caloric and nutritional content without user intervention.62 In this experiment, the accuracy of detecting 15 different foods was greater than 85%. To model the characteristics of food energy distribution in an eating scene, Fang et al. introduced the new concept of “food energy distribution.”63 The mapping of a food image to its energy distribution is learned using a generative adversarial network architecture. The food energy was estimated from the image based on the energy distribution image predicted by the generative adversarial network. The average error in the estimated energy consumption was 209 kcal per eating occasion.
AI-based diet recommendations
A proper diet is indispensable in managing diabetic patients by maintaining normal blood sugar levels and reducing the burden of pancreatic islet β cells.64 A targeted and reasonable diet can control blood sugar and lipids and supplement protein and other nutrients.65 The diet recommendation system for patients with diabetes should be based on their knowledge of medical nutrition, considering their eating patterns and helping them develop good dietary habits. Several diet recommendation systems for patients with diabetes have been developed with an accuracy comparable to that of dietitians. Chen et al. built a diet recommendation system for chronic diseases with expert knowledge and provided chronic diseases with more convenient and precise dietary recommendations.66 The dietary recommendations result from the assessment of dietitians, and the verification accuracy is 100%. Zeevi et al. discovered that people eating identical meals showed high variability in the post-meal blood glucose response. Based on ML algorithms, personalized diets created with the help of an accurate predictor of blood glucose response that integrates parameters, such as dietary habits, physical activity, and gut microbiota, may successfully lower post-meal blood glucose and its long-term metabolic consequences.67
Physical therapy
A scientific, personalized, and quantitative exercise prescription that can potentially be an essential therapeutic agent for patients with diabetes is highly recommended. However, it is often poorly implemented, as clinicians lack the necessary knowledge and skills, while participants have low adherence due to design defects (e.g., prescriptions fail to take individual willingness, the appeal of exercise, and complex physical conditions into account). Thus, intelligent, personalized prescriptions are worth investigating. Everett et al. presented a coaching application that delivers specific physical activity advice to contextual information gathered in real time (e.g., if location data indicate that the patient is in a park, then suggest an activity to perform there).68 Sun et al. investigated whether a year-long cloud platform-based and intelligent, personalized exercise prescription intervention could improve the health outcomes of Chinese middle-aged and older adult community dwellers.69 These observations suggest that this exercise prescription intervention program might promote certain health outcomes, such as cardiovascular function and body composition, in middle-aged and older adult Chinese community dwellers.
Blood glucose (BG) monitoring
BG prediction
It is usual for people with diabetes to experience BG variation throughout the day owing to carbohydrate ingestion and insulin action. The ability to anticipate BG excursions could provide an early warning regarding ineffective or poor treatment. Thus, information collected from new technologies for diabetes management, such as continuous glucose monitoring (CGM) devices, could lead to the real-time prediction of future glucose levels. Predicting near-future BG on a minute, hour, or overnight timescale enables better diabetes management and therefore remains an active research topic. However, the prediction of BG levels is challenging, because of the number of physiological factors involved, such as delays associated with the absorption of food and insulin and the lag associated with measurements in the interstitial tissue. Errors in CGM also increase the difficulty of predicting BG values (approximately 9% of the mean absolute relative difference for the best sensors70). The prediction horizon range previously explored ranged between 5 and 180 min. Short-term predictions were the most frequently explored, as most studies used prediction horizons below 60 min.71 Artificial neural network approaches are the most widely applied methodology, but other ML methodologies, such as random forest and support vector machine, are being adopted with increasing frequency. Kodama et al. performed a meta-analysis to assess the current ability of ML algorithms to predict hypoglycemia (i.e., alert hypoglycemia possibility before its symptoms occur),72 revealing that the pooled estimates were 0.80 for sensitivity, 0.92 for specificity, 10.42 for positive likelihood ratio, and 0.22 for negative likelihood ratio. Particularly, it is worth highlighting that AI algorithms could be quite useful in times when it is difficult to manage diabetes. For example, Elhadd et al. developed a machine-based algorithm from clinical and demographic data, physical activity, and glucose variability to predict hyperglycemic and hypoglycemic excursions in patients with T2D on multiple glucose-lowering therapies who fast during Ramadan. This model accurately estimated normal glucose levels in 95.2% readings and hyperglycemic events in 82.6% readings, but fewer hypoglycemic events (27.9%).73
Detection of adverse glycemic events
As with BG prediction, glycemic episode detection encompasses a set of tools that address the complexity of effective BG control. These tools enable us to detect the occurrence of glycemic episodes and provide us with the opportunity to respond promptly to their effects. In contrast to BG prediction, most of the reviewed studies on this topic focus on detecting hyperglycemia or hypoglycemia in situations where it is not possible to monitor BG effectively. Therefore, most of these studies dealt with real-time approaches rather than predictions of future events. These studies utilized various types of input data, including electroencephalogram, electrocardiogram, self-monitoring BG (SMBG), and electronic health records (EHRs).74,75,76,77,78 For detecting hypoglycemia, Kodama et al. summarized that the pooled estimates of sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were 0.79, 0.80, 8.05, and 0.18, respectively.72 In particular, the clinical applicability of these AI algorithms should be evaluated according to patients’ risk profiles, such as for hypoglycemia and its associated complications (e.g., arrhythmia and neuroglycopenia), as well as the average ability of the AI algorithms. Continued research is required to develop more accurate AI algorithms than those currently available and to enhance the feasibility of applying AI in clinical settings.
Drug therapy
Optimal dosing strategies of insulins for patients and clinicians
Unlike many other medications, insulin must be titrated, which means that the dose will change in response to real-time indicators such as current BG or based on what the patient has recently eaten. This process complicates insulin dosing and requires providers to perform complex calculations to determine the best dose. Errors in insulin dosing can be dangerous, as incorrect dosages can lead to serious adverse effects, such as hypoglycemia, which can cause death. Previous studies have focused on optimizing the insulin dose for both patients and clinicians. For patients with T1D, Tyler et al. utilized k-nearest-neighbor methods to generate recommendations for optimal insulin dosing in the context of a quality control algorithm.79 Pesl et al. used case-based reasoning in the ABC4D (short for Advanced Bolus Calculator for Diabetes) bolus calculator for meal-time dosing advice.80 For patients with T2D, Bergenstal et al. demonstrated that the combination of automated insulin titration guidance with support from health-care professionals offers superior glycemic control compared with support from health-care professionals alone in a multi-center randomized controlled trial.81 For clinicians, early AI-enabled decision support systems were generally designed to assist clinicians who were authoritative in the insulin dosing strategies for their patients. This treatment model is still worth discussing today, since most patients with diabetes do not utilize automated insulin delivery systems on account of personal preferences or relatively high costs. In the context of T1D, Fong et al. predicted future BG as lying within pre-defined ranges (rather than exact values) and generated advice based on the prediction.82 With the inputs of SMBG and non-rapid insulin, this work is worth discussing today because SMBG and non-rapid insulin formulations are still used in many parts of the world. In the context of T2D, Wang et al. developed insulin dosing guidance for insulin therapy as a constrained optimization problem.83 For both T1D and T2D, Nguyen et al. leveraged tree-based methods to predict whether patients would require more than 6 units of total daily dosing, which achieved an AUROC of 0.85.84 A challenging aspect of measuring the agreement between human expert- and AI-generated recommendations is that, even when they disagree, both can be correct, because humans (including AI developers) often express a wide variety of priorities and risk preferences. Thus, it may be helpful to frame AI recommendation tools as a support for human decision-making rather than as a replacement for human decision-making.
Optimal dosing strategies for insulin in the closed-loop automated insulin-delivery system
The closed-loop automated insulin-delivery system for diabetes treatment consists of a CGM system, insulin pump, and control algorithm.85 The control algorithm acts as a "brain" in the closed-loop system, analyzing the data fed back by the CGM system and automatically adjusting the insulin infusion rate accordingly. Therefore, an accurate control algorithm is crucial for a closed-loop system. The algorithms used in closed-loop systems primarily include model predictive control, proportional-integral-derivative, fuzzy logic, and learning algorithms.86 MD Logic is a wireless, fully automated, closed-loop system that uses an algorithm based on fuzzy-logic theory (a form of probabilistic logic), a learning algorithm,16 and an alert module and personalized system setting.87 Phillip et al. found that patients with T1D at a diabetes camp treated with an artificial pancreas system based on MD Logic had less nocturnal hypoglycemia and tighter glucose control than those treated with a sensor-augmented insulin pump.87 Nimri et al. showed that the MD Logic system demonstrated a safe and efficient profile during overnight use by children, adolescents, and adults with T1D and provides an effective means of mitigating the risk of nocturnal hypoglycemia.88
Optimal strategy for anti-diabetic drug therapy
Clinicians provide therapeutic advice beyond insulin dosing guidance (particularly T2D), as diabetes is often associated with multiple comorbidities, leading to complex guidelines for personalized treatment, with gaps often present in which no recommendations are available. Toussi et al. designed a decision tree model from an EHR to identify gaps in T2D guidelines and generated therapeutic rules based on clinicians’ past actions.89 Wright et al. discovered that sequential pattern mining effectively identifies temporal relationships between medications and accurately predicts the following medication likely to be prescribed for a patient with diabetes.90 Treatment escalation of diabetes is another critical aspect of drug therapy strategies, which is required as a patient’s disease progresses, and multiple studies have aimed to identify optimal escalation points as an aid to clinicians. Murphree et al. employed several AI methods to identify and predict patients with T2D for whom metformin monotherapy is likely to fail, requiring therapy escalation.91 They leveraged data regarding HbA1c, demographics, and comorbidities to train their models. Similarly, Fiorini et al. trained an artificial neural network to predict when patients would need to escalate from metformin monotherapy, using health-care visit records.92 Recently, Tarumi et al. deployed an EHR-integrated system for predicting changes in HbA1c associated with alternative treatment options in T2D, demonstrating the feasibility of AI-based tools in clinical practice.93
Application of AI in the prediction, screening, and management of diabetic complications
Regarding the management of diabetic complications, the primary task of endocrinologists is to control modifiable risk factors for developing these complications and identify referable patients with severe complications as early as possible. Thus, the optimization of the management algorithms could be based on the prediction of diabetic complications and more convenient screening methods (Table 1).
Table 1.
Application of AI in predicting and screening diabetic complications
| Reference | AI method | Inputs | Description | Performance |
|---|---|---|---|---|
| Objective: Predict the development of diabetic complications | ||||
| Lagani et al.94 | ANN | risk factors | predicts development of diabetes-related complications | the results of the internal validation (T1D) reported a C index between 0.66 and 0.833, all statistically significantly different from 0.5 (p ≤ 0.0001); these results were further corroborated by the external validation (49 patients with T1D), where all models obtained similarly high and statistically significant C indexes (p < 0.05 except for the hypoglycemia and CVD model, with p = 0.0584 and 0.0932, respectively); quite surprisingly, the models obtained good performance also on the T2D external cohorts, with only the neuropathy and retinopathy models achieving close to uninformative results |
| Marini et al.95 | BN | risk factors | estimates long-term development and progression of complications (T1D) | the population predicted in the wrong state was below 10% on both DDO-DBN and EI-DBN |
| Armengol et al.96 | case-based reasoning | risk factors | estimates risk of complications | the results were 100% correct in determining the kind of risk (progression or development) and the risk of stroke, 90% correct in determining amputation risk, and 72.45% correct in determining the global risk and the risk of infarct |
| Dagliati et al.97 | RF, SVM, and LR | risk factors | predicts development of diabetes-related complications | final models, tailored in accordance with the complications, provided an accuracy up to 0.838 |
| Khan et al.98 | network analysis | longitudinal data | quantifies progression of comorbidities | they presented a research framework based on network theory to understand chronic disease progression along with associated comorbidities that manifest over time |
| Ljubic et al.99 | RNN | hospitalization longitudinal data | predicts development of 10 selected complications (T2D) | the prediction accuracy was between 73% (myocardial infarction) and 83% (chronic ischemic heart disease), while the accuracy of traditional models was between 66% and 76% |
| Yang et al.100 | LASSO regression | risk factors | estimates the risk of amputation in patients treated with canagliflozin | LASSO produced the best prediction, yielding a C index of 0.81 |
| Zhang et al.39 | CNN | retinal images and risk factors | predicts development of chronic kidney disease (CKD) | the combined model could achieve a C index of 0.845 on the internal test set and 0.719 on the external test set |
| Arcadu et al.101 | CNN | retinal images | predicts the future threat of significant diabetic retinopathy (DR) worsening at a patient level within 2 years | the proposed DL models were designed to predict future DR progression and were trained against DR severity scores assessed after 6, 12, and 24 months from the baseline visit; the performance of one of these models (prediction at month 12) resulted in an AUC equal to 0.79 |
| Bora et al.102 | CNN | retinal images and risk factors | predicts the risk of developing DR within 2 years | the three-field DL system had an AUC of 0.79 in the internal validation set; assessment of the external validation set, which contained only one-field color fundus photographs, with the one-field DL system gave an AUC of 0.70 |
| Objective: Screen and evaluate diabetic complications | ||||
| Dai et al.103 | DeepDR (CNN) | retinal images | presents AI systems for DR screening | the grading of DR as mild, moderate, severe, and proliferative achieves AUCs of 0.943, 0.955, 0.960, and 0.972, respectively (internal validation); in external validations, AUCs for grading range from 0.916 to 0.970 |
| Abramoff et al.104 | IDx-DR (CNN) | retinal images | presents AI systems for DR screening | the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% (>85%), specificity of 90.7% (>82.5%), and imageability rate of 96.1% for detecting the presence of more than mild DR |
| Bhaskaranand et al.105 | EyeArt (CNN) | retinal images | presents AI systems for DR screening | the system could achieve 91.3% sensitivity and 91.1% specificity for detecting referral-warranted DR (more than mild non-proliferative DR) |
| Gulshan et al.106 | CNN | retinal images | presents AI systems for DR screening | for detecting referable DR, the algorithm had an AUC of 0.991 for EyePACS-1 and 0.990 for Messidor-2 |
| Ting et al.107 | CNN | retinal images | presents AI systems for DR screening | the AUC of the system for referable DR was 0.936, sensitivity was 90.5%, and specificity was 91.6%; for vision-threatening DR, AUC was 0.958, sensitivity was 100%, and specificity was 91.1% |
| Acharya et al.108 | morphological image processing and SVM | retinal images | presents AI systems for DR screening | the system could identify different stages of DR with an average accuracy of more than 85%, a sensitivity of 82%, and a specificity of 86% |
| Saleh et al.109 | fuzzy RF | retinal images | presents AI systems for DR screening | the best results yielded an accuracy of 77.32%, sensitivity of 76.89% and specificity of 77.43% for detecting the presence of DR |
| Huang et al.110 | ML classifiers | metabolic biomarkers | presents AI systems for CKD screening | two metabolites in combination with five clinical variables were identified as the best set of predictors, and their predictive performance yielded a mean AUC of 0.857 |
| Sabanayagam et al.111 | CNN | retinal images and risk factors | presents AI systems for CKD screening | in participants with diabetes, the system could achieve an AUC of 0.889 using fundus images only |
| Zhang et al.39 | CNN | retinal images and risk factors | presents AI systems for CKD screening | the system could be used to identify CKD solely from fundus images or in combination with clinical metadata with AUCs of 0.85–0.93 |
| Huang et al.112 | DT, RF, SVM, BN | clinical and genotyping data | presents AI systems for diabetic nephropathy (DN) screening | the proposed method yielded accuracy, specificity, and sensitivity of 85.27%, 83.32, and 85.24%, respectively (internal testing dataset); on a separate testing dataset, the classification accuracy, specificity, and sensitivity were 78.50%, 80.64, and 81.40%, respectively |
| Zhang et al.113 | RF, SVM | clinical data | predicts and differentiates DN and non-diabetic renal disease | the AUCs for the RF and SVM methods were 0.953 and 0.947, respectively (internal validation); the AUCs for the external validation of the RF and SVM methods were 0.920 and 0.911, respectively |
| Khandakar et al.114 | CNN and k-means clustering technique | thermogram images | classifies thermograms based on the severity of diabetic foot complications | it was found that the popular VGG 19 CNN model showed an accuracy, precision, sensitivity, F1 score, and specificity of 95.08%, 95.08%, 95.09%, 95.08%, and 97.2%, respectively, in the stratification of severity |
| Kim et al.115 | ANN, RF, and SVM | smartphone images | estimates the likelihood of healing of diabetes-related foot ulcers | the model could achieve AUC of 0.734, accuracy of 0.811, precision of 0.828, recall of 0.923, and F1 score of 0.873 |
| Metsker et al.116 | ANN, SVM, DT, linear regression, and logistic regression | electronic health record (EHR) | presents AI systems for polyneuropathy screening based on EHR | it could achieve 0.80 precision, 0.82 recall, 0.81 F1 score, 0.83 accuracy, and 0.90 AUC |
| Williams et al.117 | CNN | corneal confocal microscopy images | identify diabetic peripheral neuropathy using corneal confocal microscopy images | the AUC of the system was 0.83, sensitivity was 67.7%, and specificity was 86.7% |
| Salahouddin et al.118 | CNN | corneal confocal microscopy images | identify diabetic peripheral neuropathy using corneal confocal microscopy images | the AUC of the system was 0.95, sensitivity was 92%, and specificity was 80% |
| Preston et al.119 | CNN | corneal confocal microscopy images | identify diabetic peripheral neuropathy using corneal confocal microscopy images | the system could achieve recall of 0.83, precision of 1.0, and F1 score of 0.91 |
AI, artificial intelligence; ANN, artificial neural network, BN, Bayesian network; RF, random forest; SVM, support vector machine; RNN, recurrent neural network; CNN, convolutional neural network; ML, machine learning; LASSO, least absolute shrinkage and selection operator; DT, decision tree; NB, naive Bayes; DBN, dynamic Bayesian networks; DDO-DBN, data-driven only network; EI-DBN, network designed with expert intervention; AUC, the area under the curve; EHR, electronic health record; DN, diabetic nephropathy; DR, diabetic retinopathy; T1D, type 1 diabetes; T2D, type 2 diabetes; C index, concordance index.
In the prediction of diabetic complications, several studies tried to utilize risk factors (mainly from EHRs) to develop AI algorithms to predict various types of diabetes-related complications with favorable predictive performance.94,95,96,97,98,99,100 This precise prediction could enable health-care professionals to implement intensive management for patients at high risk. Recently, retinal images were used to predict the incidence and progression of chronic kidney disease (CKD)39 and diabetic retinopathy (DR).101,102 Since retinal examinations are routinely performed for patients with diabetes and the retina is a convenient window into the homeostasis of the body, it is feasible for the integration of such algorithms into DR screening programs.
In the screening and evaluation of diabetic complications, the screening of DR using retinal images was studied well in multi-ethnic cohorts, with comparable accuracy compared with professional graders (discussed in detail in the conclusion and perspective).103,104,105,106,107,108,109 For CKD39,110,111 and diabetic nephropathy112,113 screening, studies utilized clinical data, metabolic biomarkers, genotyping data, and/or retinal images to achieve AUCs generally over 0.8. For evaluation of diabetic foot, one study classified thermogram images based on the severity of diabetic foot complications (95.08% accuracy)114 and the other study used smartphone images to estimate the likelihood of healing of diabetes-related foot ulcers (AUC = 0.73).115 For neuropathy screening, previous studies explored the development of AI systems to identify diabetic neuropathy based on EHRs116 or corneal confocal microscopy images,117,118,119 with AUCs ranging from 0.83 to 0.95.
Opportunities and challenges of AI applications in the clinical practice of diabetes care
Opportunities for AI application in the clinical practice of diabetes care
Precision
As described by the National Institutes of Health, precision medicine is an emerging disease treatment and prevention approach that considers individual variability in genes, environment, and lifestyle for each person. This approach allows doctors and researchers to more accurately predict treatment and prevention strategies for a specific disease and target them toward particular groups of people.120 It requires significant computing power (supercomputers), algorithms that can learn by themselves at an unprecedented rate (DL), and, generally, an approach that uses the cognitive capabilities of physicians on a new scale (AI). AI-based diabetes management has the potential to precisely predict, classify, and treat diabetes and diabetes-related complications for a specific patient based on individual variability.
Penetration
Many people in remote or low-income settings are not well supported by conventional health care.121 These individuals often have the poorest understanding of the disease, low use of maintenance medications, and poor health outcomes.122 The reach of AI-based DHTs, coupled with the relative reduction in cost and increased capability of mobile communications, has led to its greater penetration into traditionally difficult-to-reach communities compared with robust conventional health care.121 For example, with the growing burden of DR, AI technology can increase the productivity of existing DR tele-ophthalmology screening programs and lower unit economic costs within the community.123 This will enable providers to project expertise to geographically remote locations, improve the productivity of existing resources, and optimize the flow of patients within entire health systems.124
Prediction
Two important mechanisms of the AI domain can be used to develop decisional systems for medical predictions: a knowledge-based system that involves logic and a system based on probabilistic reasoning.125 The benefits of these systems are significant. They can simplify the physicians’ work, saving their time and energy (which otherwise would be wasted on too many things they have to do). However, an automated system can detect imperceptible things (things hardly noticed or resulting from complex computation and reasoning, things not evident, or the effects of too many factors involved). In diabetes management, the prediction of the onset of diabetes and diabetic complications would eventually decrease the incidence of diabetes and diabetic complications by implementing appropriate medical interventions for those at high risk at a very early stage.
Personalization
The essence of practicing medicine is to obtain as much data as possible regarding the patient’s health or disease and make decisions based on the data. Instead of making the same medical decisions based on a few similar physical characteristics, medicine has shifted toward personalization and precision. In addition to many disruptive technologies, AI has the biggest potential to support this transition by analyzing the vast amounts of data patients and health-care institutions record at every moment. Removing the repetitive parts of a physician’s job might lead to them spending more precious time with patients with diabetes, improving the human touch and promoting personalized diabetes care.120
Furthermore, patients with diabetes have an increasing need for personalized management as medical technology advances. AI could potentially provide personalized health education, diet recommendations, physical therapy, BG monitoring, and treatment regimens for individual patients based on their unique characteristics, needs, and preferences.
Challenges of AI application in the clinical practice of diabetes care
Data quality control
Since clinical AI systems are developed on a considerable amount of real-world health data, the corresponding labels and data quality will directly determine model performance. Data quality may have the following problems: (1) poor quality of the data themselves, such as unfair and blurred images; (2) poor quality of the data labels, such as incorrect labels; and (3) insufficient data, where only a small portion of the data has been labeled. Moreover, AI-enabled DHTs can amplify implicit bias and discrimination if trained on data that reflect the health-care disparities experienced by groups defined by race, ethnicity, gender, sexual orientation, socioeconomic status, or geographic location.126 To mitigate these pitfalls, AI algorithms must be trained on fair datasets that include and accurately represent social, environmental, and economic factors that influence health.127
Poor technology design
AI-based DHTs need to be developed through a constant process of refinement and iterative development to address users’ demands. The initial versions of most DHTs are always challenging to navigate, which requires the adoption of user-centered design principles. User-centered design principles involve an iterative procedure of analyzing the possible problems encountered by users, developing mock-ups of a solution, testing solutions, and reevaluating whether it tackles the problem.128 However, a previous study demonstrated that many EHR vendors did not follow basic usability principles.129 Of 41 vendors assessed, 14 (34%) had not met the ONC certification requirement of stating their user-centered design process (ONC is short for the US Department of Health and Human Services’ Office of the National Coordinator for Health Information Technology). Another study chose 11 commercially available mobile applications (4 for diabetes, 4 for depression, and 3 for caring for the elderly) through expert review of commercially available applications, then invited 26 participants (10 with diabetes) to investigate their usability. The results showed that participants could complete only 43% of the tasks without assistance. Although participants had interest in having technology to support self-management, they reported a lack of confidence with technology, as well as frustration with design features and navigation.130 Thus, improper or user-unfriendly design of AI-based DHTs will probably give rise to non-adoption or early abandonment of the technology.
Lack of clinical integration
With the maturation of AI systems, many AI researchers envision drastic changes in clinical practice as their clinical deployment increases. Cutting-edge AI systems cannot realize their full potential unless they are integrated into clinical and digital workflows. However, while a growing number of AI systems have been deployed in clinical settings with the promise of improving patient care, many have struggled to gain adoption and realize this promise. Application of AI systems in the real world may lead to many unintended outcomes, including alert fatigue,131 additional burdens for clinicians, disruption of interpersonal communications, and generation of specific threats requiring increased vigilance to recognize.132 In addition, two main barriers have been identified that may undermine clinicians’ enthusiasm to integrate AI systems into clinical workflows.133 First, experts may struggle to develop trust with these systems because of the numerous inputs and complex data integration involved, which can make it challenging or impossible to convey the specific logic behind an alert or recommendation. Second, some evidence suggests that AI systems could also be perceived as encroaching on clinicians’ professional role by making a competing diagnosis, presenting a “threat to autonomy” that may make clinicians reluctant to use, rely on, and trust them.
Privacy concerns
An overriding issue for the future of AI in medicine is implementing data privacy and security assurances. Due to the pervasive problems of hacking worldwide, there is no room for algorithms that possibly risk revealing the patients’ medical history in clinical practice.134 Moreover, there are potentially fatal risks for patients if certain types of AI algorithms are hacked, such as overdosing insulin for diabetic patients in closed-loop automated insulin-delivery systems. It is also becoming increasingly possible for an individual’s identity to be determined by facial recognition or genomic sequences from massive databases, which further impedes privacy protection.
The development of robust AI systems relies on large training and validation datasets. However, the volume of local data is often insufficient, which could be addressed by the centralization of data. There exist inherent disadvantages in centralized solutions, including increased data traffic and concerns about data ownership, confidentiality, privacy, and security and creating data monopolies that favor data aggregators.135 Federated computing approaches have further been developed, wherein dedicated parameter servers are responsible for aggregating and distributing local learning; however, a remainder of a central structure is kept.136 As an alternative, Warnat-Herresthal et al. introduced a decentralized ML approach named swarm learning,136 which dispenses with a dedicated server, shares the parameters via the swarm network, and builds the models independently on private data at the individual sites. Swarm learning provides security measures to support data sovereignty, security, and confidentiality by recognizing the network participants.
Non-adherence
Non-adherence has been proposed as one of the leading causes of delays in adopting DHTs in clinical practice.137 User adherence is crucial to the effectiveness of DHT applications in the real world, which can be affected by convenience, user experience, and true benefits brought by this technology (for example, helping them decide when to contact their provider, being more aware of their symptoms, and feeling more connected to their provider).138,139 Thus, the potential ways to promote user adherence include smart design, visible EHRs, integration of electronic patient-reported outcomes in clinics, and voice enablement.140
Imperfection of laws and regulations
AI in medicine inevitably results in legal challenges regarding medical negligence attributed to complex decision-support systems. When malpractice cases involving medical AI applications arise, the legal system must provide clear guidance on which entity holds liability.141 Medical professional malpractice insurance must be clear about coverage when health-care decisions are made in part by an AI system. With the deployment of automated AI for specific clinical tasks, the credentials needed for diagnostic, therapeutic, supportive, and paramedical tasks need to be updated, and the roles of health-care professionals will continue to evolve as various AI modules are incorporated into the standard of care.14
Conclusion and perspective
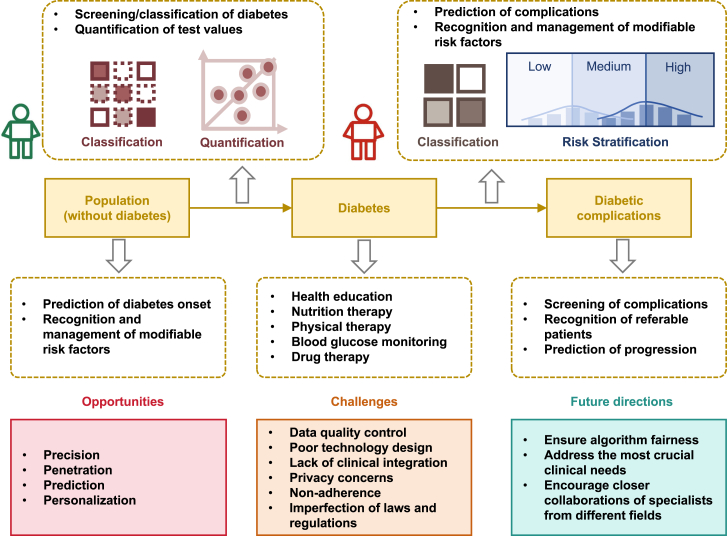
The increasing prevalence of diabetes has become a global public health concern in the 21st century, fueled by the increasing consumption of calorie-rich foods and sedentary lifestyles. Several challenges exist in managing diabetes in traditional face-to-face medical practices, such as ineffective prevention systems, uneven distribution of medical resources, and improper self-management. The emergence of novel DHTs, especially AI, may help address these obstacles and alleviate the disease burden of diabetes in the future. Previous studies have shown that applying AI in diabetes management involves all aspects of disease control, including prediction, prevention, screening, diagnosis, and treatment. Integrating AI into clinical practice care could shift diabetes care toward precision, penetration, prediction, and personalization. However, several obstacles remain in the research and application of AI in diabetes management, including data quality control, poor technology design, privacy concerns, lack of clinical integration, non-adherence, and the establishment of laws and regulations (Figure 2).
Figure 2.
Overview of AI application in diabetes management
Good examples of AI applications in clinical practice
AI-based detection of diabetic retinopathy
Automatic retinal screening, an AI technology that automatically interprets the presence of DR from fundus images, has been widely integrated into diabetes care worldwide. The first well-established device was IDx-DR, approved by the FDA in 2018 for its high diagnostic performance in clinical trials.104,142 This device facilitates DR screening, especially in rural communities where patients have difficulty accessing an ophthalmologist.143 Several DL algorithms with high sensitivity have recently been developed for DR screening, predominantly focusing on identifying referable DR or vision-threatening DR. However, the importance of identifying early-stage DR should not be neglected. Evidence suggests that proper intervention at an early stage could significantly delay the progression of DR and even reverse mild non-proliferative DR to a DR-free stage. To overcome this issue, Dai et al. developed an automated, interpretable, and validated system (named DeepDR) that performs real-time image quality feedback, retinal lesion detection, and early- to late-stage DR grading with high sensitivity and specificity.103
Self-management tools for blood glucose monitoring
Self-management tools are familiar to some diabetes patients because they have already self-checked various biomedical data, such as actively measuring BG levels through SMBG. With patients’ self-management tools, AI technology interprets their biomedical data and alerts like a diabetologist to improve the patient’s BG control. The Guardian Connect System (manufactured by Medtronic) is an example of an AI system with this functionality. This system is based on CGM, has an accompanying smartphone application,144 and was certified by the FDA in 2018. It is characterized by using AI to predict a hypoglycemic attack an hour in advance based on the CGM data and alerts the patient. According to the product data, the accuracy of the alert was 98.5% only 30 min before the onset of hypoglycemia. AI issues alert patients to hypoglycemia from their biomedical data in this system, which is sometimes challenging to understand. The patient can then take glucose tablets to prevent hypoglycemia and associated complications.
Recommendations for future directions
Although numerous studies have focused on AI applications in diabetes management, several factors hinder the integration of AI-based DHTs into clinical practice (Table 2). For future research, the multiple etiologies of bias in AI decision support require a comprehensive, multi-faceted approach to ensure algorithm fairness across the phases of algorithm design, training and development, and assessment and deployment to mitigate potential harms of algorithm bias.127 Moreover, future studies should prioritize applications that address the most crucial clinical demands. For the integration of AI-based DHTs into diabetes care, closer collaboration between AI specialists and endocrinologists should be encouraged to explore and innovate clinically significant AI-based systems, which could be incorporated into daily clinical practice. Hospital administrators would have to evaluate and mitigate clinical workflow disruption when introducing innovative AI applications. Companies will have to determine the appropriate framework within which they can conduct prospective clinical trials to evaluate the performance of AI systems in a clinical setting. In addition, insurers should assess the value created by medical AI systems and revise their reimbursement policy to reduce the cost of health care while improving its quality.14
Table 2.
Challenges for AI application in diabetes care and how they may be overcome with future development
| Challenge | Description | Mitigating strategies |
|---|---|---|
| Data quality control | data quality may have the following problems: (1) poor quality of the data themselves, (2) poor quality of the data labels, and (3) insufficient data. | ensure the quality of data used in the training process |
| AI may amplify implicit bias and discrimination if trained on data reflecting the health-care disparities | train AI algorithms on fair datasets that include and accurately represent social, environmental, and economic factors that influence health | |
| Poor technology design | the initial versions of most AI systems are always challenging to navigate | understand the needs of the end user (for example, patients and providers) |
| many EHR vendors did not follow basic usability principles | develop software and applications with input from end users | |
| patients reported lack of confidence with technology, as well as frustration with design features and navigation of commercially available mobile applications | utilize iterative design process | |
| Lack of clinical integration | application of AI systems in the real world may lead to many unintended outcomes | develop AI algorithms that could be integrated into current clinical and digital workflows |
| experts may struggle to develop trust with AI systems | demonstrate explainability analysis of AI systems | |
| AI systems could also be perceived as encroaching on clinicians’ professional role | support the clinical decision-making of clinicians instead of making solely a competing diagnosis | |
| Privacy concerns | implementing data privacy and security assurances is an overriding issue for the future of AI in medicine, since there are pervasive problems of hacking worldwide |
|
| Non-adherence | user adherence is crucial to the effectiveness of AI applications in the real world, which can be affected by convenience, user experience, and true benefits brought by this technology |
|
| Imperfection of laws and regulations | AI in medicine results in legal and regulatory challenges regarding medical negligence attributed to complex decision-support systems |
|
AI, artificial intelligence; EHR, electronic health record.
Prior AI models in diabetes management offered several advantages, such as data analysis, pattern recognition, decision support, and patient education, but faced limitations in natural language understanding, personalized treatment, accuracy, and potential biases.71 However, large language models (LLMs), which could accept image and text inputs and produce text outputs, have shown promise in various aspects145 of medical care. LLMs are built using natural language processing techniques and designed to recognize and understand the structure and meaning of human language, classify texts according to their content or purpose, and generate responses that are appropriate and coherent.146 The transition from previous AI technology to the current LLMs in diabetes management represents a significant leap in the capabilities of AI-driven health-care solutions. LLMs, with advanced natural language processing abilities, offer a more comprehensive understanding of the medical literature, patient data, and individualized care requirements.146,147 However, LLMs may have risks in terms of inconsistency or misleading information.148 Despite these risks, LLMs have the potential to revolutionize patient monitoring, treatment personalization, and patient education, leading to improved outcomes and better quality of life for patients with diabetes.149,150
Construction of an AI-assisted digital health-care ecosystem for diabetes management
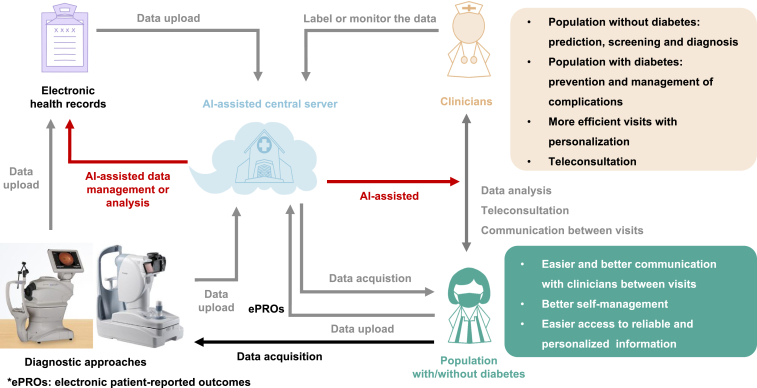
Integrating AI-based DHTs will probably become increasingly feasible in the future as technology improves, and such integration will enable new models of diabetes care. Here, we propose the construction of an AI-assisted digital health-care ecosystem for diabetes management (Figure 3). This ecosystem consists of several essential sessions enabled by AI: (1) Recognize the risk factors of diabetes and predict the risk of diabetes onset in the general public. Based on the risk and modifiable risk factors, the system can provide personalized suggestions and continuous monitoring to control these contributing factors. (2) Implement diabetes screening in high-risk populations. (3) Assist medical practitioners and patients in the basic management of diabetes: health education, medical nutrition therapy, physical therapy, and drug therapy. (4) Provide the prediction, screening, and management of diabetic complications.
Figure 3.
Proposed AI-assisted digital health-care ecosystem for diabetes management
Population with or without diabetes will use digital platforms to report electronic patient-reported outcomes (ePROs) to their electronic health record (EHR) and/or their clinicians. The information from the EHR and other diagnostic approaches will be integrated in a centralized and secure server environment. AI algorithms will be running on the data to enable the integration of all aspects of diabetes control, more effective visits with easier and better communication between visits, and better self-management through easier access to reliable and personalized health information.
In conclusion, AI has the potential to optimize diabetes care by providing personalized, precise, and data-driven support to patients and health-care professionals. By addressing the challenges and capitalizing on the opportunities, AI could play a pivotal role in transforming diabetes care and improving the lives of millions of people worldwide.
Acknowledgments
This study was supported by the Shanghai Research Center for Endocrine and Metabolic Diseases (2022ZZ01002) (W.J.), the Shanghai Municipal Key Clinical Specialty (W.J.), the National Key Research and Development Program of China (2018YFA0800402) (W.J.), the General Program of NSFC (81870598) (H.L.), the Excellent Young Scholars of NSFC (82022012) (H.L.), the Two Hundred Program from Shanghai Jiao Tong University School of Medicine (20221830) (H.L.), the College-level Project Fund of Shanghai Sixth People's Hospital (ynlc201909) (X.W.), the Interdisciplinary Program of Shanghai Jiao Tong University (YG2022QN089) (X.W.), and the General Program of NSFC (62272298) (B.S.).
Author contributions
W.J., H.L., R.L., B.S., and Z.G. conceived and designed this review article. Z.G., R.L., L.W., D.L., S.Y., Z.W., J.S., C.C., Y.L., J.L., X.W., and S.H. wrote the initial draft of the manuscript. Z.G. and R.L. conceived and designed the figures. W.J., H.L., B.S., X.Y., and X.H. reviewed and edited the manuscript. All authors have read and approved the contents of this article.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Weiping Jia, Email: wpjia@sjtu.edu.cn.
Bin Sheng, Email: shengbin@sjtu.edu.cn.
References
- 1.Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai C., Liu Y., Li Y., Shi Y., Zou H., Bao Y., Shen Y., Cui X., Fu C., Jia W., SIM Study Group Effectiveness of quality of care for patients with type 2 diabetes in China: findings from the Shanghai Integration Model (SIM) Front. Med. 2022;16:126–138. doi: 10.1007/s11684-021-0897-7. [DOI] [PubMed] [Google Scholar]
- 3.Magliano D.J., Boyko E.J., International Diabetes Federation . Idf diabetes atlas. International Diabetes Federation; 2021. IDF Diabetes Atlas. [Google Scholar]
- 4.Jia W. Diabetes care in China: Innovations and implications. J. Diabetes Investig. 2022;13:1795–1797. doi: 10.1111/jdi.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Prevention of Diabetes Mellitus. JAMA. 2021;325:190. doi: 10.1001/jama.2020.17738. [DOI] [PubMed] [Google Scholar]
- 6.Hermanns N., Ehrmann D., Shapira A., Kulzer B., Schmitt A., Laffel L. Coordination of glucose monitoring, self-care behaviour and mental health: achieving precision monitoring in diabetes. Diabetologia. 2022;65:1883–1894. doi: 10.1007/s00125-022-05685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., Collins B.S., Hilliard M.E., Isaacs D., Johnson E.L., et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S68–S96. doi: 10.2337/dc23-S005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashrafzadeh S., Hamdy O. Patient-Driven Diabetes Care of the Future in the Technology Era. Cell Metabol. 2019;29:564–575. doi: 10.1016/j.cmet.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Frank S.R. Digital health care--the convergence of health care and the Internet. J. Ambul. Care Manag. 2000;23:8–17. doi: 10.1097/00004479-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Mathews S.C., McShea M.J., Hanley C.L., Ravitz A., Labrique A.B., Cohen A.B. Digital health: a path to validation. NPJ Digit. Med. 2019;2:38. doi: 10.1038/s41746-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy J., Minsky M.L., Shannon C.E. A proposal for the Dartmouth summer research project on artificial intelligence - August 31, 1955. AI Mag. 2006;27:12–14. [Google Scholar]
- 12.Samuel A.L. Some studies in machine learning using the game of checkers (Reprinted from Journal of Research and Development, vol 3, 1959. IBM J. Res. Dev. 2000;44:206–226. doi: 10.1147/rd.441.0206. [DOI] [Google Scholar]
- 13.LeCun Y., Bengio Y., Hinton G. Deep learning. Nature. 2015;521:436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 14.Yu K.-H., Beam A.L., Kohane I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 15.Jordan M.I., Mitchell T.M. Machine learning: Trends, perspectives, and prospects. Science. 2015;349:255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 16.Check Hayden E. The automated lab. Nature. 2014;516:131–132. doi: 10.1038/516131a. [DOI] [PubMed] [Google Scholar]
- 17.Klonoff A.N., Andy Lee W.-A., Xu N.Y., Nguyen K.T., DuBord A., Kerr D. Six Digital Health Technologies That Will Transform Diabetes. J. Diabetes Sci. Technol. 2023;17:239–249. doi: 10.1177/19322968211043498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deo R.C. Machine Learning in Medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu K.-H., Snyder M. Omics Profiling in Precision Oncology. Mol. Cell. Proteomics. 2016;15:2525–2536. doi: 10.1074/mcp.O116.059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A., Vikman P., Prasad R.B., Aly D.M., Almgren P., et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 21.Zou X., Zhou X., Zhu Z., Ji L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 2019;7:9–11. doi: 10.1016/S2213-8587(18)30316-4. [DOI] [PubMed] [Google Scholar]
- 22.van Engelen J.E., Hoos H.H. A survey on semi-supervised learning. Mach. Learn. 2020;109:373–440. doi: 10.1007/s10994-019-05855-6. [DOI] [Google Scholar]
- 23.Coronato A., Naeem M., De Pietro G., Paragliola G. Reinforcement learning for intelligent healthcare applications: A survey. Artif. Intell. Med. 2020;109 doi: 10.1016/j.artmed.2020.101964. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman O., Johansson F., Komorowski M., Faisal A., Sontag D., Doshi-Velez F., Celi L.A. Guidelines for reinforcement learning in healthcare. Nat. Med. 2019;25:16–18. doi: 10.1038/s41591-018-0310-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G., Ou S.X., Huang Y.H., Wang C.R. Semi-supervised learning methods for large scale healthcare data analysis. Int. J. Comput. Healthc. 2015;2:98–110. doi: 10.1504/ijcih.2015.069788. [DOI] [Google Scholar]
- 26.Ramos J.M.A., Perdómo O., González F.A. Deep Semi-Supervised and Self-Supervised Learning for Diabetic Retinopathy Detection. arXiv. 2022 doi: 10.48550/arXiv.2208.02408. Preprint at. [DOI] [Google Scholar]
- 27.Gianfrancesco M.A., Tamang S., Yazdany J., Schmajuk G. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern. Med. 2018;178:1544–1547. doi: 10.1001/jamainternmed.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ying X. An Overview of Overfitting and its Solutions. J. Phys, Conf. Ser. 2019;1168:022022. [Google Scholar]
- 29.Gómez-Carmona O., Casado-Mansilla D., López-de-Ipiña D., García-Zubia J. Optimizing computational resources for edge intelligence through model cascade strategies. IEEE Internet Things J. 2022;9:7404–7417. [Google Scholar]
- 30.Pan S.J., Yang Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 2010;22:1345–1359. [Google Scholar]
- 31.Johnson K.W., Torres Soto J., Glicksberg B.S., Shameer K., Miotto R., Ali M., Ashley E., Dudley J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018;71:2668–2679. doi: 10.1016/j.jacc.2018.03.521. [DOI] [PubMed] [Google Scholar]
- 32.Qi G.J., Luo J. Small Data Challenges in Big Data Era: A Survey of Recent Progress on Unsupervised and Semi-Supervised Methods. IEEE Trans. Pattern Anal. Mach. Intell. 2022;44:2168–2187. doi: 10.1109/TPAMI.2020.3031898. [DOI] [PubMed] [Google Scholar]
- 33.Jing L., Tian Y. Self-Supervised Visual Feature Learning With Deep Neural Networks: A Survey. IEEE Trans. Pattern Anal. Mach. Intell. 2021;43:4037–4058. doi: 10.1109/TPAMI.2020.2992393. [DOI] [PubMed] [Google Scholar]
- 34.Roberts K., Boland M.R., Pruinelli L., Dcruz J., Berry A., Georgsson M., Hazen R., Sarmiento R.F., Backonja U., Yu K.-H., et al. Biomedical informatics advancing the national health agenda: the AMIA 2015 year-in-review in clinical and consumer informatics. J. Am. Med. Inf. Assoc. 2017;24:e185–e190. doi: 10.1093/jamia/ocw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbasi A., Peelen L.M., Corpeleijn E., van der Schouw Y.T., Stolk R.P., Spijkerman A.M.W., van der A D.L., Moons K.G.M., Navis G., Bakker S.J.L., Beulens J.W.J. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ. 2012;345 doi: 10.1136/bmj.e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi B.G., Rha S.W., Kim S.W., Kang J.H., Park J.Y., Noh Y.K. Machine Learning for the Prediction of New-Onset Diabetes Mellitus during 5-Year Follow-up in Non-Diabetic Patients with Cardiovascular Risks. Yonsei Med. J. 2019;60:191–199. doi: 10.3349/ymj.2019.60.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravaut M., Harish V., Sadeghi H., Leung K.K., Volkovs M., Kornas K., Watson T., Poutanen T., Rosella L.C. Development and Validation of a Machine Learning Model Using Administrative Health Data to Predict Onset of Type 2 Diabetes. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomura A., Yamamoto S., Hayakawa Y., Taniguchi K., Higashitani T., Aono D., Kometani M., Yoneda T. SAT-LB121 Development of a Machine-Learning Method for Predicting New Onset of Diabetes Mellitus: A Retrospective Analysis of 509,153 Annual Specific Health Checkup Records. J. Endocr. Soc. 2020;4 doi: 10.1210/jendso/bvaa046.2194. [DOI] [Google Scholar]
- 39.Zhang K., Liu X., Xu J., Yuan J., Cai W., Chen T., Wang K., Gao Y., Nie S., Xu X., et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat. Biomed. Eng. 2021;5:533–545. doi: 10.1038/s41551-021-00745-6. [DOI] [PubMed] [Google Scholar]
- 40.López B., Torrent-Fontbona F., Viñas R., Fernández-Real J.M. Single Nucleotide Polymorphism relevance learning with Random Forests for Type 2 diabetes risk prediction. Artif. Intell. Med. 2018;85:43–49. doi: 10.1016/j.artmed.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Peddinti G., Cobb J., Yengo L., Froguel P., Kravić J., Balkau B., Tuomi T., Aittokallio T., Groop L. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia. 2017;60:1740–1750. doi: 10.1007/s00125-017-4325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin H.-C., Su C.-T., Wang P.-C. An Application of Artificial Immune Recognition System for Prediction of Diabetes Following Gestational Diabetes. J. Med. Syst. 2011;35:283–289. doi: 10.1007/s10916-009-9364-8. [DOI] [PubMed] [Google Scholar]
- 43.Allalou A., Nalla A., Prentice K.J., Liu Y., Zhang M., Dai F.F., Ning X., Osborne L.R., Cox B.J., Gunderson E.P., Wheeler M.B. A Predictive Metabolic Signature for the Transition From Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes. 2016;65:2529–2539. doi: 10.2337/db15-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C., Li L., Wang L., Ping Z., Flory M.T., Wang G., Xi Y., Li W. Evaluating the risk of type 2 diabetes mellitus using artificial neural network: An effective classification approach. Diabetes Res. Clin. Pract. 2013;100:111–118. doi: 10.1016/j.diabres.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 45.Gholipour K., Asghari-Jafarabadi M., Iezadi S., Jannati A., Keshavarz S. Modelling the prevalence of diabetes mellitus risk factors based on artificial neural network and multiple regression. East. Mediterr. Health J. 2018;24:770–777. doi: 10.26719/emhj.18.012. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L., Shang X., Sreedharan S., Yan X., Liu J., Keel S., Wu J., Peng W., He M. Predicting the Development of Type 2 Diabetes in a Large Australian Cohort Using Machine-Learning Techniques: Longitudinal Survey Study. JMIR Med. Inform. 2020;8 doi: 10.2196/16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 48.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 49.Abhari S., Niakan Kalhori S.R., Ebrahimi M., Hasannejadasl H., Garavand A. Artificial Intelligence Applications in Type 2 Diabetes Mellitus Care: Focus on Machine Learning Methods. Healthc. Inform. Res. 2019;25:248–261. doi: 10.4258/hir.2019.25.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapak L., Mahjub H., Hamidi O., Poorolajal J. Real-data comparison of data mining methods in prediction of diabetes in iran. Healthc. Inform. Res. 2013;19:177–185. doi: 10.4258/hir.2013.19.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maniruzzaman M., Kumar N., Menhazul Abedin M., Shaykhul Islam M., Suri H.S., El-Baz A.S., Suri J.S. Comparative approaches for classification of diabetes mellitus data: Machine learning paradigm. Comput. Methods Progr. Biomed. 2017;152:23–34. doi: 10.1016/j.cmpb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Shu T., Zhang B., Yan Tang Y. An extensive analysis of various texture feature extractors to detect Diabetes Mellitus using facial specific regions. Comput. Biol. Med. 2017;83:69–83. doi: 10.1016/j.compbiomed.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Yuan P., Hu X., Huang J., Cui L., Cui J., Ma X., Jiang T., Yao X., Li J., et al. A tongue features fusion approach to predicting prediabetes and diabetes with machine learning. J. Biomed. Inf. 2021;115 doi: 10.1016/j.jbi.2021.103693. [DOI] [PubMed] [Google Scholar]
- 54.Barakat N.H., Bradley A.P., Barakat M.N.H. Intelligible Support Vector Machines for Diagnosis of Diabetes Mellitus. Ieee T Inf Technol B. 2010;14:1114–1120. doi: 10.1109/Titb.2009.2039485. [DOI] [PubMed] [Google Scholar]
- 55.Amit kumar D., Pragati A. Classification of Diabetes Mellitus Using Machine Learning Techniques. Int. J. Eng. Appl. Sci. 2015;2 [Google Scholar]
- 56.Alotaibi M.M., Istepanian R., Philip N. A mobile diabetes management and educational system for type-2 diabetics in Saudi Arabia (SAED) mHealth. 2016;2:33. doi: 10.21037/mhealth.2016.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamon T., Gagnayre R. Improving knowledge of patient skills thanks to automatic analysis of online discussions. Patient Educ. Counsel. 2013;92:197–204. doi: 10.1016/j.pec.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Chen S., Lu J., Peng D., Liu F., Lu W., Zhu W., Zhou J., Jia W. Effect of a Mobile Health Technology–Based Diabetes Education Program on Glucose Control in Patients With Type 2 Diabetes Initiating Premixed Insulin: A Prospective, Multicenter, Observational Study. Diabetes Care. 2023;46:e6–e7. doi: 10.2337/dc22-0510. [DOI] [PubMed] [Google Scholar]
- 59.Schoeller D.A. Limitations in the assessment of dietary energy intake by self-report. Metabolism. 1995;44:18–22. doi: 10.1016/0026-0495(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 60.Zhu F., Bosch M., Boushey C.J., Delp E.J. An Image Analysis System for Dietary Assessment and Evaluation. Proc. Int. Conf. Image Proc. 2010:1853–1856. doi: 10.1109/ICIP.2010.5650848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasiloglou M.F., Mougiakakou S., Aubry E., Bokelmann A., Fricker R., Gomes F., Guntermann C., Meyer A., Studerus D., Stanga Z. A Comparative Study on Carbohydrate Estimation: GoCARB vs. Nutrients. 2018;10 doi: 10.3390/nu10060741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Yu Q., Siddiquie B., Divakaran A., Sawhney H. "Snap-n-Eat": Food Recognition and Nutrition Estimation on a Smartphone. J. Diabetes Sci. Technol. 2015;9:525–533. doi: 10.1177/1932296815582222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang S., Shao Z., Kerr D.A., Boushey C.J., Zhu F. An End-to-End Image-Based Automatic Food Energy Estimation Technique Based on Learned Energy Distribution Images: Protocol and Methodology. Nutrients. 2019;11 doi: 10.3390/nu11040877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitagishi Y., Nakanishi A., Minami A., Asai Y., Yasui M., Iwaizako A., Suzuki M., Ono Y., Ogura Y., Matsuda S. Certain Diet and Lifestyle May Contribute to Islet beta-cells Protection in Type-2 Diabetes via the Modulation of Cellular PI3K/AKT Pathway. Open Biochem. J. 2014;8:74–82. doi: 10.2174/1874091X01408010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sami W., Ansari T., Butt N.S., Ab Hamid M.R. Effect of diet on type 2 diabetes mellitus: A review. Int J Health Sci-Ijh. 2017;11 doi: 10.1002/dmrr.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen R.C., Ting Y.H., Chen J.K., Lo Y.W. Taai; 2015. The Nutrients of Chronic Diet Recommended Based on Domain Ontology and Decision Tree. 2015 Conference on Technologies and Applications of Artificial Intelligence; pp. 289–295. [Google Scholar]
- 67.Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M., et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Everett E., Kane B., Yoo A., Dobs A., Mathioudakis N. A Novel Approach for Fully Automated, Personalized Health Coaching for Adults with Prediabetes: Pilot Clinical Trial. J. Med. Internet Res. 2018;20:e72. doi: 10.2196/jmir.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun T., Xu Y., Xie H., Ma Z., Wang Y. Intelligent Personalized Exercise Prescription Based on an eHealth Promotion System to Improve Health Outcomes of Middle-Aged and Older Adult Community Dwellers: Pretest-Posttest Study. J. Med. Internet Res. 2021;23 doi: 10.2196/28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey T.S., Chang A., Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J. Diabetes Sci. Technol. 2015;9:209–214. doi: 10.1177/1932296814559746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Contreras I., Vehi J. Artificial Intelligence for Diabetes Management and Decision Support: Literature Review. J. Med. Internet Res. 2018;20 doi: 10.2196/10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kodama S., Fujihara K., Shiozaki H., Horikawa C., Yamada M.H., Sato T., Yaguchi Y., Yamamoto M., Kitazawa M., Iwanaga M., et al. Ability of Current Machine Learning Algorithms to Predict and Detect Hypoglycemia in Patients With Diabetes Mellitus: Meta-analysis. JMIR Diabetes. 2021;6 doi: 10.2196/22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elhadd T., Mall R., Bashir M., Palotti J., Fernandez-Luque L., Farooq F., Mohanadi D.A., Dabbous Z., Malik R.A., Abou-Samra A.B., for PROFAST-Ramadan Study Group Artificial Intelligence (AI) based machine learning models predict glucose variability and hypoglycaemia risk in patients with type 2 diabetes on a multiple drug regimen who fast during ramadan (The PROFAST - IT Ramadan study) Diabetes Res. Clin. Pract. 2020;169 doi: 10.1016/j.diabres.2020.108388. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen H.T., Jones T.W. Detection of nocturnal hypoglycemic episodes using EEG signals. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010;2010:4930–4933. doi: 10.1109/IEMBS.2010.5627233. [DOI] [PubMed] [Google Scholar]
- 75.Skrøvseth S.O., Årsand E., Godtliebsen F., Hartvigsen G. Mobile phone-based pattern recognition and data analysis for patients with type 1 diabetes. Diabetes Technol. Therapeut. 2012;14:1098–1104. doi: 10.1089/dia.2012.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sudharsan B., Peeples M., Shomali M. Hypoglycemia prediction using machine learning models for patients with type 2 diabetes. J. Diabetes Sci. Technol. 2015;9:86–90. doi: 10.1177/1932296814554260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otto E.A., Tannan V. Evaluation of the utility of a glycemic pattern identification system. J. Diabetes Sci. Technol. 2014;8:830–838. doi: 10.1177/1932296814532210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Zhu J.M., Liang Y.B., Chen H.B., Yin S.M., Chen Z.C. Non-invasive blood glucose detection system based on conservation of energy method. Physiol. Meas. 2017;38:325–342. doi: 10.1088/1361-6579/aa50cf. [DOI] [PubMed] [Google Scholar]
- 79.Tyler N.S., Mosquera-Lopez C.M., Wilson L.M., Dodier R.H., Branigan D.L., Gabo V.B., Guillot F.H., Hilts W.W., El Youssef J., Castle J.R., Jacobs P.G. An artificial intelligence decision support system for the management of type 1 diabetes. Nat. Metab. 2020;2:612–619. doi: 10.1038/s42255-020-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pesl P., Herrero P., Reddy M., Oliver N., Johnston D.G., Toumazou C., Georgiou P. Case-Based Reasoning for Insulin Bolus Advice. J. Diabetes Sci. Technol. 2017;11:37–42. doi: 10.1177/1932296816629986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bergenstal R.M., Johnson M., Passi R., Bhargava A., Young N., Kruger D.F., Bashan E., Bisgaier S.G., Isaman D.J.M., Hodish I. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet. 2019;393:1138–1148. doi: 10.1016/S0140-6736(19)30368-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fong S., Zhang Y., Fiaidhi J., Mohammed O., Mohammed S. Evaluation of stream mining classifiers for real-time clinical decision support system: A case study of blood glucose prediction in diabetes therapy. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/274193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Fu H., Zeng D. Learning Optimal Personalized Treatment Rules in Consideration of Benefit and Risk: with an Application to Treating Type 2 Diabetes Patients with Insulin Therapies. J. Am. Stat. Assoc. 2018;113:1–13. doi: 10.1080/01621459.2017.1303386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen M., Jankovic I., Kalesinskas L., Baiocchi M., Chen J.H. Machine learning for initial insulin estimation in hospitalized patients. J. Am. Med. Inf. Assoc. 2021;28:2212–2219. doi: 10.1093/jamia/ocab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cobelli C., Renard E., Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60:2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rigla M., García-Sáez G., Pons B., Hernando M.E. Artificial Intelligence Methodologies and Their Application to Diabetes. J. Diabetes Sci. Technol. 2018;12:303–310. doi: 10.1177/1932296817710475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillip M., Battelino T., Atlas E., Kordonouri O., Bratina N., Miller S., Biester T., Stefanija M.A., Muller I., Nimri R., Danne T. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N. Engl. J. Med. 2013;368:824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 88.Nimri R., Bratina N., Kordonouri O., Avbelj Stefanija M., Fath M., Biester T., Muller I., Atlas E., Miller S., Fogel A., et al. MD-Logic overnight type 1 diabetes control in home settings: A multicentre, multinational, single blind randomized trial. Diabetes Obes. Metabol. 2017;19:553–561. doi: 10.1111/dom.12852. [DOI] [PubMed] [Google Scholar]
- 89.Toussi M., Lamy J.B., Le Toumelin P., Venot A. Using data mining techniques to explore physicians' therapeutic decisions when clinical guidelines do not provide recommendations: Methods and example for type 2 diabetes. BMC Med. Inf. Decis. Making. 2009;9:28. doi: 10.1186/1472-6947-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright A.P., Wright A.T., McCoy A.B., Sittig D.F. The use of sequential pattern mining to predict next prescribed medications. J. Biomed. Inf. 2015;53:73–80. doi: 10.1016/j.jbi.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Murphree D.H., Arabmakki E., Ngufor C., Storlie C.B., McCoy R.G. Stacked classifiers for individualized prediction of glycemic control following initiation of metformin therapy in type 2 diabetes. Comput. Biol. Med. 2018;103:109–115. doi: 10.1016/j.compbiomed.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fiorini S., Hajati F., Barla A., Girosi F. Predicting diabetes second-line therapy initiation in the Australian population via time span-guided neural attention network. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tarumi S., Takeuchi W., Chalkidis G., Rodriguez-Loya S., Kuwata J., Flynn M., Turner K.M., Sakaguchi F.H., Weir C., Kramer H., et al. Leveraging Artificial Intelligence to Improve Chronic Disease Care: Methods and Application to Pharmacotherapy Decision Support for Type-2 Diabetes Mellitus. Methods Inf. Med. 2021;60:e32–e43. doi: 10.1055/s-0041-1728757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lagani V., Chiarugi F., Manousos D., Verma V., Fursse J., Marias K., Tsamardinos I. Realization of a service for the long-term risk assessment of diabetes-related complications. J. Diabet. Complicat. 2015;29:691–698. doi: 10.1016/j.jdiacomp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Marini S., Trifoglio E., Barbarini N., Sambo F., Di Camillo B., Malovini A., Manfrini M., Cobelli C., Bellazzi R. A Dynamic Bayesian Network model for long-term simulation of clinical complications in type 1 diabetes. J. Biomed. Inf. 2015;57:369–376. doi: 10.1016/j.jbi.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 96.Armengol E., Palaudàries A., Plaza E. Individual prognosis of diabetes long-term risks: a CBR approach. Methods Inf. Med. 2001;40:46–51. [PubMed] [Google Scholar]
- 97.Dagliati A., Marini S., Sacchi L., Cogni G., Teliti M., Tibollo V., De Cata P., Chiovato L., Bellazzi R. Machine Learning Methods to Predict Diabetes Complications. J. Diabetes Sci. Technol. 2018;12:295–302. doi: 10.1177/1932296817706375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan A., Uddin S., Srinivasan U. Comorbidity network for chronic disease: A novel approach to understand type 2 diabetes progression. Int. J. Med. Inf. 2018;115:1–9. doi: 10.1016/j.ijmedinf.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Ljubic B., Hai A.A., Stanojevic M., Diaz W., Polimac D., Pavlovski M., Obradovic Z. Predicting complications of diabetes mellitus using advanced machine learning algorithms. J. Am. Med. Inf. Assoc. 2020;27:1343–1351. doi: 10.1093/jamia/ocaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang L., Gabriel N., Hernandez I., Winterstein A.G., Guo J. Using machine learning to identify diabetes patients with canagliflozin prescriptions at high-risk of lower extremity amputation using real-world data. Pharmacoepidemiol. Drug Saf. 2021;30:644–651. doi: 10.1002/pds.5206. [DOI] [PubMed] [Google Scholar]
- 101.Arcadu F., Benmansour F., Maunz A., Willis J., Haskova Z., Prunotto M. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. NPJ Digit. Med. 2019;2:92. doi: 10.1038/s41746-019-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bora A., Balasubramanian S., Babenko B., Virmani S., Venugopalan S., Mitani A., de Oliveira Marinho G., Cuadros J., Ruamviboonsuk P., Corrado G.S., et al. Predicting the risk of developing diabetic retinopathy using deep learning. Lancet. Digit. Health. 2021;3:e10–e19. doi: 10.1016/S2589-7500(20)30250-8. [DOI] [PubMed] [Google Scholar]
- 103.Dai L., Wu L., Li H., Cai C., Wu Q., Kong H., Liu R., Wang X., Hou X., Liu Y., et al. A deep learning system for detecting diabetic retinopathy across the disease spectrum. Nat. Commun. 2021;12:3242. doi: 10.1038/s41467-021-23458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abràmoff M.D., Lou Y., Erginay A., Clarida W., Amelon R., Folk J.C., Niemeijer M. Improved Automated Detection of Diabetic Retinopathy on a Publicly Available Dataset Through Integration of Deep Learning. Invest. Ophthalmol. Vis. Sci. 2016;57:5200–5206. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- 105.Bhaskaranand M., Ramachandra C., Bhat S., Cuadros J., Nittala M.G., Sadda S.R., Solanki K. The Value of Automated Diabetic Retinopathy Screening with the EyeArt System: A Study of More Than 100,000 Consecutive Encounters from People with Diabetes. Diabetes Technol. Therapeut. 2019;21:635–643. doi: 10.1089/dia.2019.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gulshan V., Peng L., Coram M., Stumpe M.C., Wu D., Narayanaswamy A., Venugopalan S., Widner K., Madams T., Cuadros J., et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316:2402–2410. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 107.Ting D.S.W., Cheung C.Y.-L., Lim G., Tan G.S.W., Quang N.D., Gan A., Hamzah H., Garcia-Franco R., San Yeo I.Y., Lee S.Y., et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images From Multiethnic Populations With Diabetes. JAMA. 2017;318:2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acharya U.R., Lim C.M., Ng E.Y.K., Chee C., Tamura T. Computer-based detection of diabetes retinopathy stages using digital fundus images. Proc. Inst. Mech. Eng. H. 2009;223:545–553. doi: 10.1243/09544119jeim486. [DOI] [PubMed] [Google Scholar]
- 109.Saleh E., Błaszczyński J., Moreno A., Valls A., Romero-Aroca P., de la Riva-Fernández S., Słowiński R. Learning ensemble classifiers for diabetic retinopathy assessment. Artif. Intell. Med. 2018;85:50–63. doi: 10.1016/j.artmed.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Huang J., Huth C., Covic M., Troll M., Adam J., Zukunft S., Prehn C., Wang L., Nano J., Scheerer M.F., et al. Machine Learning Approaches Reveal Metabolic Signatures of Incident Chronic Kidney Disease in Individuals With Prediabetes and Type 2 Diabetes. Diabetes. 2020;69:2756–2765. doi: 10.2337/db20-0586. [DOI] [PubMed] [Google Scholar]
- 111.Sabanayagam C., Xu D., Ting D.S.W., Nusinovici S., Banu R., Hamzah H., Lim C., Tham Y.-C., Cheung C.Y., Tai E.S., et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet. Digit. Health. 2020;2:e295–e302. doi: 10.1016/S2589-7500(20)30063-7. [DOI] [PubMed] [Google Scholar]
- 112.Huang G.M., Huang K.Y., Lee T.Y., Weng J. An interpretable rule-based diagnostic classification of diabetic nephropathy among type 2 diabetes patients. BMC Bioinf. 2015;16(Suppl 1):S5. doi: 10.1186/1471-2105-16-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang W., Liu X., Dong Z., Wang Q., Pei Z., Chen Y., Zheng Y., Wang Y., Chen P., Feng Z., et al. New Diagnostic Model for the Differentiation of Diabetic Nephropathy From Non-Diabetic Nephropathy in Chinese Patients. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.913021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khandakar A., Chowdhury M.E.H., Reaz M.B.I., Ali S.H.M., Kiranyaz S., Rahman T., Chowdhury M.H., Ayari M.A., Alfkey R., Bakar A.A.A., et al. A Novel Machine Learning Approach for Severity Classification of Diabetic Foot Complications Using Thermogram Images. Sensors. 2022;22 doi: 10.3390/s22114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim R.B., Gryak J., Mishra A., Cui C., Soroushmehr S.M.R., Najarian K., Wrobel J.S. Utilization of smartphone and tablet camera photographs to predict healing of diabetes-related foot ulcers. Comput. Biol. Med. 2020;126 doi: 10.1016/j.compbiomed.2020.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Metsker O., Magoev K., Yakovlev A., Yanishevskiy S., Kopanitsa G., Kovalchuk S., Krzhizhanovskaya V.V. Identification of risk factors for patients with diabetes: diabetic polyneuropathy case study. BMC Med. Inf. Decis. Making. 2020;20:201. doi: 10.1186/s12911-020-01215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams B.M., Borroni D., Liu R., Zhao Y., Zhang J., Lim J., Ma B., Romano V., Qi H., Ferdousi M., et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: a development and validation study. Diabetologia. 2020;63:419–430. doi: 10.1007/s00125-019-05023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salahouddin T., Petropoulos I.N., Ferdousi M., Ponirakis G., Asghar O., Alam U., Kamran S., Mahfoud Z.R., Efron N., Malik R.A., Qidwai U.A. Artificial Intelligence-Based Classification of Diabetic Peripheral Neuropathy From Corneal Confocal Microscopy Images. Diabetes Care. 2021;44:e151–e153. doi: 10.2337/dc20-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Preston F.G., Meng Y., Burgess J., Ferdousi M., Azmi S., Petropoulos I.N., Kaye S., Malik R.A., Zheng Y., Alam U. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia. 2022;65:457–466. doi: 10.1007/s00125-021-05617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mesko B. The role of artificial intelligence in precision medicine. Expert Review of Precision Medicine and Drug Development. 2017;2:239–241. doi: 10.1080/23808993.2017.1380516. [DOI] [Google Scholar]
- 121.Graham G.N., Ostrowski M., Sabina A.B. Population health-based approaches to utilizing digital technology: a strategy for equity. J. Publ. Health Pol. 2016;37:154–166. doi: 10.1057/s41271-016-0012-5. [DOI] [PubMed] [Google Scholar]
- 122.Himes B.E., Weitzman E.R. Innovations in health information technologies for chronic pulmonary diseases. Respir. Res. 2016;17:38. doi: 10.1186/s12931-016-0354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie Y., Nguyen Q.D., Hamzah H., Lim G., Bellemo V., Gunasekeran D.V., Yip M.Y.T., Qi Lee X., Hsu W., Li Lee M., et al. Artificial intelligence for teleophthalmology-based diabetic retinopathy screening in a national programme: an economic analysis modelling study. Lancet. Digit. Health. 2020;2:e240–e249. doi: 10.1016/S2589-7500(20)30060-1. [DOI] [PubMed] [Google Scholar]
- 124.Gunasekeran D.V., Wong T.Y. Artificial Intelligence in Ophthalmology in 2020: A Technology on the Cusp for Translation and Implementation. Asia. Pac. J. Ophthalmol. 2020;9:61–66. doi: 10.1097/01.APO.0000656984.56467.2c. [DOI] [PubMed] [Google Scholar]
- 125.Albu A., Stanciu L. 19–21. IEEE; 2015. Benefits of Using Artificial Intelligence in Medical Predictions; pp. 1–4. [Google Scholar]
- 126.Johnson-Mann C.N., Loftus T.J., Bihorac A. Equity and Artificial Intelligence in Surgical Care. JAMA Surg. 2021;156:509–510. doi: 10.1001/jamasurg.2020.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loftus T.J., Shickel B., Ozrazgat-Baslanti T., Ren Y., Glicksberg B.S., Cao J., Singh K., Chan L., Nadkarni G.N., Bihorac A. Artificial intelligence-enabled decision support in nephrology. Nat. Rev. Nephrol. 2022;18:452–465. doi: 10.1038/s41581-022-00562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schnall R., Rojas M., Bakken S., Brown W., Carballo-Dieguez A., Carry M., Gelaude D., Mosley J.P., Travers J. A user-centered model for designing consumer mobile health (mHealth) applications (apps) J. Biomed. Inf. 2016;60:243–251. doi: 10.1016/j.jbi.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ratwani R.M., Benda N.C., Hettinger A.Z., Fairbanks R.J. Electronic Health Record Vendor Adherence to Usability Certification Requirements and Testing Standards. JAMA. 2015;314:1070–1071. doi: 10.1001/jama.2015.8372. [DOI] [PubMed] [Google Scholar]
- 130.Sarkar U., Gourley G.I., Lyles C.R., Tieu L., Clarity C., Newmark L., Singh K., Bates D.W. Usability of Commercially Available Mobile Applications for Diverse Patients. J. Gen. Intern. Med. 2016;31:1417–1426. doi: 10.1007/s11606-016-3771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carspecken C.W., Sharek P.J., Longhurst C., Pageler N.M. A clinical case of electronic health record drug alert fatigue: consequences for patient outcome. Pediatrics. 2013;131:e1970–e1973. doi: 10.1542/peds.2012-3252. [DOI] [PubMed] [Google Scholar]
- 132.Ash J.S., Berg M., Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J. Am. Med. Inf. Assoc. 2004;11:104–112. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Henry K.E., Kornfield R., Sridharan A., Linton R.C., Groh C., Wang T., Wu A., Mutlu B., Saria S. Human-machine teaming is key to AI adoption: clinicians' experiences with a deployed machine learning system. NPJ Digit. Med. 2022;5:97. doi: 10.1038/s41746-022-00597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 135.Kaissis G.A., Makowski M.R., Rückert D., Braren R.F. Secure, privacy-preserving and federated machine learning in medical imaging. Nat. Mach. Intell. 2020;2:305–311. doi: 10.1038/s42256-020-0186-1. [DOI] [Google Scholar]
- 136.Warnat-Herresthal S., Schultze H., Shastry K.L., Manamohan S., Mukherjee S., Garg V., Sarveswara R., Händler K., Pickkers P., Aziz N.A., et al. Swarm Learning for decentralized and confidential clinical machine learning. Nature. 2021;594:265–270. doi: 10.1038/s41586-021-03583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Solomon D.H., Rudin R.S. Digital health technologies: opportunities and challenges in rheumatology. Nat. Rev. Rheumatol. 2020;16:525–535. doi: 10.1038/s41584-020-0461-x. [DOI] [PubMed] [Google Scholar]
- 138.Blakey J.D., Bender B.G., Dima A.L., Weinman J., Safioti G., Costello R.W. Digital technologies and adherence in respiratory diseases: the road ahead. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.01147-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rudin R.S., Fanta C.H., Qureshi N., Duffy E., Edelen M.O., Dalal A.K., Bates D.W. A Clinically Integrated mHealth App and Practice Model for Collecting Patient-Reported Outcomes between Visits for Asthma Patients: Implementation and Feasibility. Appl. Clin. Inf. 2019;10:783–793. doi: 10.1055/s-0039-1697597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Navarro-Millán I., Zinski A., Shurbaji S., Johnson B., Fraenkel L., Willig J., Danila M.I., Yun H., Curtis J.R., Safford M.M. Perspectives of Rheumatoid Arthritis Patients on Electronic Communication and Patient-Reported Outcome Data Collection: A Qualitative Study. Arthritis Care Res. 2019;71:80–87. doi: 10.1002/acr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Computer programs to support clinical decision making. JAMA. 1987;258:2374–2376. [PubMed] [Google Scholar]
- 142.Abràmoff M.D., Lavin P.T., Birch M., Shah N., Folk J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit. Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nomura A., Noguchi M., Kometani M., Furukawa K., Yoneda T. Artificial Intelligence in Current Diabetes Management and Prediction. Curr. Diabetes Rep. 2021;21:61. doi: 10.1007/s11892-021-01423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soupal J., Haskova A., Prazny M. Real-time CGM Is Superior to Flash Glucose Monitoring for Glucose Control in Type 1 Diabetes: The CORRIDA Randomized Controlled Trial. Diabetes Care. 2021;43:2744–2750. doi: 10.2337/dci20-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.OpenAI GPT-4 Technical Report. arXiv. 2023 doi: 10.48550/arXiv.2303.08774. Preprint at. [DOI] [Google Scholar]
- 146.Ali S.R., Dobbs T.D., Hutchings H.A., Whitaker I.S. Using ChatGPT to write patient clinic letters. Lancet. Digit. Health. 2023;5:e179–e181. doi: 10.1016/S2589-7500(23)00048-1. [DOI] [PubMed] [Google Scholar]
- 147.Patel S.B., Lam K. ChatGPT: the future of discharge summaries? Lancet. Digit. Health. 2023;5:e107–e108. doi: 10.1016/S2589-7500(23)00021-3. [DOI] [PubMed] [Google Scholar]
- 148.Ji Z., Lee N., Frieske R., Yu T., Su D., Xu Y., Ishii E., Bang Y.J., Madotto A., Fung P. Survey of Hallucination in Natural Language Generation. ACM Comput. Surv. 2023;55:1–38. doi: 10.1145/3571730. Article 248. [DOI] [Google Scholar]
- 149.Stokel-Walker C., Van Noorden R. What ChatGPT and generative AI mean for science. Nature. 2023;614:214–216. doi: 10.1038/d41586-023-00340-6. [DOI] [PubMed] [Google Scholar]
- 150.Howard A., Hope W., Gerada A. ChatGPT and antimicrobial advice: the end of the consulting infection doctor? Lancet Infect. Dis. 2023;23:405–406. doi: 10.1016/S1473-3099(23)00113-5. [DOI] [PubMed] [Google Scholar]