Abstract
Background
Otitis media with effusion (OME) is an accumulation of fluid in the middle ear cavity, common amongst young children. The fluid may cause hearing loss. When persistent, it may lead to developmental delay, social difficulty and poor quality of life. Management of OME includes watchful waiting, autoinflation, medical and surgical treatment. Antibiotics are sometimes used to treat any bacteria present in the effusion, or associated biofilms.
Objectives
To assess the effects (benefits and harms) of oral antibiotics for otitis media with effusion (OME) in children.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register, CENTRAL, Ovid MEDLINE, Ovid Embase, Web of Science, ClinicalTrials.gov, ICTRP and additional sources for published and unpublished studies to 20 January 2023.
Selection criteria
We included randomised controlled trials and quasi‐randomised trials in children aged 6 months to 12 years with unilateral or bilateral OME. We included studies that compared oral antibiotics with either placebo or no treatment.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were determined following a multi‐stakeholder prioritisation exercise and were: 1) hearing, 2) otitis media‐specific quality of life and 3) anaphylaxis. Secondary outcomes were: 1) persistence of OME, 2) adverse effects, 3) receptive language skills, 4) speech development, 5) cognitive development, 6) psychosocial skills, 7) listening skills, 8) generic health‐related quality of life, 9) parental stress, 10) vestibular function and 11) episodes of acute otitis media. We used GRADE to assess the certainty of evidence for each outcome.
Although we included all measures of hearing assessment, the proportion of children who returned to normal hearing was our preferred method to assess hearing, due to challenges in interpreting the results of mean hearing thresholds.
Main results
We identified 19 completed studies that met our inclusion criteria (2581 participants). They assessed a variety of oral antibiotics (including penicillins, cephalosporins, macrolides and trimethoprim), with most studies using a 10‐ to 14‐day treatment course. We had some concerns about the risk of bias in all studies included in this review. Here we report our primary outcomes and main secondary outcome, at the longest reported follow‐up time.
Antibiotics versus placebo
We included 11 studies for this comparison, but none reported all of our outcomes of interest and limited meta‐analysis was possible.
Hearing
One study found that more children may return to normal hearing by two months (resolution of the air‐bone gap) after receiving antibiotics as compared with placebo, but the evidence is very uncertain (Peto odds ratio (OR) 9.59, 95% confidence interval (CI) 3.51 to 26.18; 20/49 children who received antibiotics returned to normal hearing versus 0/37 who received placebo; 1 study, 86 participants; very low‐certainty evidence).
Disease‐specific quality of life
No studies assessed this outcome.
Presence/persistence of OME
At 6 to 12 months of follow‐up, the use of antibiotics compared with placebo may slightly reduce the number of children with persistent OME, but the confidence intervals were wide, and the evidence is very uncertain (risk ratio (RR) 0.89, 95% CI 0.68 to 1.17; 48% versus 54%; number needed to treat (NNT) 17; 2 studies, 324 participants; very low‐certainty evidence).
Adverse event: anaphylaxis
No studies provided specific data on anaphylaxis. Three of the included studies (448 children) did report adverse events in sufficient detail to assume that no anaphylactic reactions occurred, but the evidence is very uncertain (very low‐certainty evidence).
Antibiotics versus no treatment
We included eight studies for this comparison, but very limited meta‐analysis was possible.
Hearing
One study found that the use of antibiotics compared to no treatment may result in little to no difference in final hearing threshold at three months (mean difference (MD) ‐5.38 dB HL, 95% CI ‐9.12 to ‐1.64; 1 study, 73 participants; low‐certainty evidence). The only data identified on the return to normal hearing were reported at 10 days of follow‐up, which we considered to be too short to accurately reflect the efficacy of antibiotics.
Disease‐specific quality of life
No studies assessed this outcome.
Presence/persistence of OME
Antibiotics may reduce the proportion of children who have persistent OME at up to three months of follow‐up, when compared with no treatment (RR 0.64, 95% CI 0.50 to 0.80; 6 studies, 542 participants; low‐certainty evidence).
Adverse event: anaphylaxis
No studies provided specific data on anaphylaxis. Two of the included studies (180 children) did report adverse events in sufficient detail to assume that no anaphylactic reactions occurred, but the evidence is very uncertain (very low‐certainty evidence).
Authors' conclusions
The evidence for the use of antibiotics for OME is of low to very low certainty. Although the use of antibiotics compared to no treatment may have a slight beneficial effect on the resolution of OME at up to three months, the overall impact on hearing is very uncertain. The long‐term effects of antibiotics are unclear and few of the studies included in this review reported on potential harms. These important endpoints should be considered when weighing up the potential short‐ and long‐term benefits and harms of antibiotic treatment in a condition with a high spontaneous resolution rate.
Keywords: Child; Child, Preschool; Humans; Anaphylaxis; Anaphylaxis/chemically induced; Anaphylaxis/drug therapy; Anti-Bacterial Agents; Anti-Bacterial Agents/adverse effects; Hearing Loss; Hearing Loss/chemically induced; Hearing Loss/etiology; Otitis Media with Effusion; Otitis Media with Effusion/drug therapy; Quality of Life
Plain language summary
Antibiotics for otitis media with effusion (OME or 'glue ear') in children
Key messages
We are uncertain whether the use of antibiotics improves hearing for children with glue ear, due to a lack of robust evidence.
The use of antibiotics compared to no treatment might slightly reduce the number of children who have glue ear at three months of follow‐up. It is unclear whether this is a long‐lasting effect, as few studies followed up children for more than three months.
The studies included in this review did not report serious harms from treatment with antibiotics. However, there is some suggestion that antibiotics may cause unpleasant side effects such as skin rash.
What is OME?
Glue ear (or 'otitis media with effusion', OME) is a relatively common condition affecting young children. Fluid collects in the middle ear, which may cause hearing impairment. As a result of their poor hearing, children may be behind in their speech and may have difficulties at school.
How is OME treated?
Most of the time OME does not need any treatment and the symptoms will get better with time. In children with persistent OME, different treatments have been used, including medications or surgery (insertion of grommets (ventilation tubes), with or without adenoidectomy). Sometimes, bacteria are present in the fluid that collects in the middle ear. Antibiotics are sometimes used to try and get rid of these bacteria, and improve the symptoms of OME.
What did we want to find out?
We wanted to identify whether antibiotics are better than placebo (sham or dummy treatment), or no treatment, for children with OME.
We also wanted to see whether there are any unwanted effects associated with taking antibiotics for this condition.
What did we do?
We searched for studies that compared oral antibiotic treatment with either placebo or no treatment in children with OME. We compared and summarised the study results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included 19 studies involving over 2500 children. Many different types of oral antibiotics were used and the duration of treatment varied a lot between the studies.
It is unclear whether antibiotics have any effect on hearing, as the evidence was not robust.
When compared to no treatment, antibiotics might slightly reduce the number of children who have OME after three months of follow‐up. Only two studies looked at the number of children with OME after a longer follow‐up time, so we are uncertain whether this is a long‐lasting effect, as OME may recur.
We do not know if treatment with antibiotics has any effect on quality of life as none of the studies included in this review assessed this outcome. We were unable to find much evidence on the occurrence of anaphylaxis ‐ a rare but very serious allergic reaction. None of the studies reported that any children suffered from anaphylaxis, but this may be because no one had a reaction, or simply because the studies did not report this.
What are the limitations of the evidence?
As the evidence included in this Cochrane Review was uncertain, we cannot be sure if treatment with antibiotics gives any benefit to children with OME. As most of the studies were very short in duration, we do not know if any effect of antibiotics would continue over longer time periods ‐ even if OME appears to get better in the short term, it may recur.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to January 2023.
Summary of findings
Summary of findings 1. Antibiotics compared to placebo for otitis media with effusion (OME) in children.
| Antibiotics compared to placebo for otitis media with effusion (OME) in children | ||||||
| Patient or population: children (aged 6 months to 12 years) with otitis media with effusion (OME) Setting: outpatient Intervention: oral antibiotics Comparison: placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| With placebo | With oral antibiotics | Difference | ||||
|
Hearing ‐ return to normal hearing (as complete improvement in air‐bone gap in the worst ear) Follow‐up: 2 months (short‐term) № of participants: 86 (1 RCT) |
Peto OR 9.59 (3.51 to 26.18) | Lower‐risk population* | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of antibiotics on return to normal hearing at 2 months, when compared to placebo. | ||
| 5.0% | 33.5% (15.6 to 57.9) | 28.5% more (10.6 more to 52.9 more) | ||||
| Moderate‐risk population* | ||||||
| 11.0% | 54.2% (30.3 to 76.4) | 43.2% more (19.3 more to 65.4 more) | ||||
| Higher‐risk population* | ||||||
| 15.0% | 62.9% (38.2 to 82.2) | 47.9% more (23.2 more to 67.2 more) | ||||
| Disease‐specific quality of life | No evidence was identified for this outcome. | |||||
|
Presence/persistence of OME Follow‐up: range 6 months to 12 months (medium‐term) № of participants: 324 (2 RCTs) |
RR 0.89 (0.68 to 1.17) | Study population | ⊕⊝⊝⊝ Very low 2 | The evidence is very uncertain about the effect of antibiotics on persistence of OME at 6 to 12 months, when compared to placebo. | ||
| 54.0% | 48.1% (36.7 to 63.2) | 5.9% fewer (17.3 fewer to 9.2 more) | ||||
|
Adverse event: anaphylaxis Follow‐up: range 3 weeks to 12 months № of participants: 244 (3 RCTs) |
Three trials that did not report the incidence of anaphylaxis directly did, however, provide sufficient information to reasonably assume there were no such cases. One trial reported that there were no unwanted side effects from the drug (antibiotic) itself (Thomsen 1989); one trial did not list anaphylaxis amongst adverse events that were reported as "probably or possibly related to active treatment" (Hemlin 1997); and one trial reported that "no infant was withdrawn as a result of direct adverse reaction due to medication" (Leach 2008). | ⊕⊝⊝⊝ Very low 3 | The evidence is very uncertain about the risk of anaphylaxis. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where there were zero events in the comparison group, the assumed risk in the comparison group was imputed from the same outcome comparing antibiotic with no treatment (Table 2). This value was assumed to represent a 'moderate' control group event rate (CER), and values roughly 50% lower and higher are presented as 'lower' and 'higher' CERs respectively. CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by two levels for risk of performance detection and attrition bias. Downgraded by one level for imprecision as the optimal information size was not reached (< 300 events).
2Downgraded by one level for risk of bias, due to the potential for underestimating the prevalence of OME at six months. Participants with two normal examinations at < 6 months were not re‐assessed, but were considered to have resolution of OME. Downgraded by one level for inconsistency, as the studies showed opposite directions of effect. Downgraded by one level for indirectness, as a high‐risk population of children aged < 12 months contributed most of the weight in the analysis. Downgraded by one level for serious imprecision, as the optimal information size was not reached (< 300 events) and one decision threshold was crossed by the confidence interval (RR 0.80).
3Downgraded by three levels for extremely serious imprecision, as this was a narrative synthesis with zero events amongst 244 participants. We are unable to provide an estimate of the effect.
Summary of findings 2. Antibiotics compared to no treatment for otitis media with effusion (OME) in children.
| Antibiotics compared to no treatment for otitis media with effusion (OME) in children | ||||||
| Patient or population: children (aged 6 months to 12 years) with otitis media with effusion (OME) Setting: outpatient Intervention: oral antibiotics Comparison: no treatment | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without oral antibiotic | With oral antibiotics | Difference | ||||
|
Hearing ‐ hearing threshold‡ (3 months; short‐term) № of participants: 73 (1 RCT) |
— | The mean final hearing threshold was 14.1 dB | — | MD 5.38 dB lower (9.12 lower to 1.64 lower) | ⊕⊕⊝⊝ Low 1 | The evidence suggests that antibiotics result in little to no difference in hearing threshold at 3 months when compared with no treatment. |
| Disease‐specific quality of life | No evidence was identified for this outcome. | |||||
|
Presence/persistence of OME (up to 3 months) № of participants: 542 (6 RCTs) |
RR 0.64 (0.50 to 0.80) | 87.4% | 55.9% (43.7 to 69.9) |
31.5% fewer (from 43.7 fewer to 17.5 fewer) | ⊕⊕⊝⊝ Low 2 | Antibiotics may reduce the proportion of children with persistent OME at up to 3 months when compared with no treatment. |
|
Adverse event: anaphylaxis Follow‐up: range 2 months to 3 months № of participants: 88 (2 RCTs) |
Without referring directly to anaphylaxis, two studies reported that no participants experienced adverse effects (Ardehali 2008; Marchisio 1998). It is unlikely, therefore, that any participants experienced anaphylaxis. | ⊕⊝⊝⊝ Very low 3 | The evidence is very uncertain about the risk of anaphylaxis. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
‡Note that additional data were available for the outcome 'Hearing ‐ return to normal hearing'. However, as the follow‐up duration was extremely short (10 days), we did not consider it meaningful to present these data in the summary of findings table.
1Downgraded by one level for a risk of performance bias. Downgraded by one level for serious imprecision as the optimal information size (OIS) was not reached (< 400 participants).
2Downgraded by two levels for risk of performance and detection bias. Not downgraded for imprecision, as there was a common direction of effect, despite some inconsistency (I2 = 72%).
3Downgraded by three levels for extremely serious imprecision, as this was a narrative synthesis with no events reported amongst only 88 children. No estimate of effect size could be calculated. We did not downgrade for risk of bias, as this is an objective outcome, unlikely to be influenced by performance bias.
Background
Description of the condition
Otitis media with effusion (OME) is a common condition in early childhood. The condition, also known as 'glue ear' and serous otitis media, is defined as "the presence of fluid in the middle ear without signs or symptoms of acute infection" (Rosenfeld 2016).
A key clinical feature of OME is hearing loss, due to decreased mobility of the tympanic membrane and consequent loss of sound conduction (Rosenfeld 2016). When hearing loss persists, this may affect speech and language development, and lead to behavioural problems in some children (NICE 2008). Other symptoms that may be attributable to OME include balance (vestibular) problems and ear discomfort (Rosenfeld 2016). When symptoms persist, they may lead to poor school performance and affect a child's daily activities, social interactions and emotions, possibly leading to a poorer quality of life for the child (Rosenfeld 2000).
It is thought that up to 80% of children have had OME by the age of four years, but a decline in prevalence is observed for children beyond six years of age (Williamson 2011). Most episodes of OME in children resolve spontaneously within three months, however approximately 35% of children will have more than one episode of OME and, furthermore, 5% to 10% of episodes will last for more than a year (Rosenfeld 2016). Children with OME following an episode of untreated AOM have a 59% rate of resolution by one month, rising to 74% by three months, while children with newly diagnosed OME of unknown duration demonstrate a resolution rate of 28% by three months and up to 42% by six months (Rosenfeld 2003). The condition is more prevalent in children with Down syndrome or cleft palate (Flynn 2009; Maris 2014). Atopy has been considered a potential risk factor for OME in children (Kreiner‐Møller 2012; Marseglia 2008; Zernotti 2017).
Diagnosis of OME is typically by clinical examination including (pneumatic) otoscopy and/or tympanometry in primary care. Following diagnosis, there will often be a period of active observation, for at least three months. During the observation period the care provider may offer a non‐surgical intervention such as hearing aids or autoinflation. The National Institute for Health and Care Excellence (NICE) and the American Academy of Otolaryngology–Head and Neck Surgery (AAO‐HNS) do not currently recommend the use of antibiotics, antihistamines, decongestants or corticosteroids for OME as there is insufficient evidence to suggest that they are effective treatments (NICE 2008; Rosenfeld 2016). If OME has not resolved within the three‐month observation period, the child may be referred for further management/active intervention. This may include hearing aid provision or review by an ENT surgeon for consideration for myringotomy, ventilation tubes insertion and/or adenoidectomy. The choice of active intervention varies considerably. Earlier active intervention may be considered for children at increased risk of developmental difficulties (see Rosenfeld 2016 for a list of 'at‐risk' factors).
This Cochrane Review focusses on antibiotics as a treatment for OME in children. This review forms part of a suite of five reviews of OME treatment, which will address those interventions identified in a prioritisation exercise as being most important and in need of up‐to‐date Cochrane Reviews, namely ventilation tubes, adenoidectomy with or without ventilation tubes, autoinflation, topical and oral steroids, and antibiotics (Cochrane ENT 2020).
Description of the intervention
The rationale for using antibiotics is to treat the bacteria that are identified in the middle ear fluid of approximately one‐third of children with OME (Park 2004; Poetker 2005), and/or bacterial biofilms that are present even more frequently (Daniel 2012). Studies of oral antibiotics of any type and duration will be included in this review.
How the intervention might work
A bacterial pathogen has been identified in the middle ear fluid of approximately a third of all children with OME (Poetker 2005), and bacterial biofilms have been implicated in the aetiology of OME (Daniel 2012; Seppanen 2020), thus treatment of the infection by antibiotics offers a promising non‐surgical intervention. If antibiotics successfully eliminate the bacteria, this may more speedily resolve the problem of middle ear fluid and its sequelae observed in children with OME (Venekamp 2016). However, not all cases of OME are of bacterial origin and thus the potential benefits of antibiotics must be weighed against the adverse effects of antibiotics and possible risk of bacterial resistance (Venekamp 2016).
Why it is important to do this review
A Cochrane Review assessing the use of antibiotics to treat OME in children was published in 2016 (Venekamp 2016). The review excluded children with pre‐existing or past ventilation tubes, cleft palate or Down syndrome and included 25 randomised controlled trials (RCTs). The Cochrane authors concluded that oral antibiotics are associated with an increased chance of complete resolution of OME at two to three months post‐randomisation (moderate‐certainty evidence). However, there was a higher incidence of adverse effects associated with antibiotics, such as diarrhoea, vomiting or skin rash. The review authors found uncertain evidence for improvements in short‐term hearing, and did not find evidence that children treated with antibiotics had fewer ventilation tube insertions. They found no data on outcomes such as speech, language and cognitive development, or quality of life.
A scoping search undertaken in 2020 identified three abstracts of studies of antibiotics for OME published since the Cochrane Review (Venekamp 2016), although these do not appear to be RCTs. A prioritisation exercise undertaken in 2020 identified a review of antibiotics for OME in children as a top priority (Cochrane ENT 2020). Given the potentially promising findings of the Cochrane Review and the recommendations by international guidelines against the use of antibiotics to treat OME in children, it is timely to update the evidence.
This review has been produced as part of a suite of reviews, which also inform a NICE guideline on the management of OME in children (NICE 2023).
Objectives
To assess the effects (benefits and harms) of oral antibiotics for otitis media with effusion (OME) in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised trials (where trials were designed as RCTs, but the sequence generation for allocation of treatment used methods such as alternative allocation, birth dates or alphabetical order). We included studies that randomised by participant or by cluster.
Types of participants
The population of interest is children aged 6 months to 12 years with unilateral or bilateral otitis media with effusion. If a study included children aged younger than 6 months and older than 12 years, we only included the study if the majority of children fitted our inclusion criteria, or if the authors presented outcome data by age group. We included all children regardless of any comorbidity, such as Down syndrome or cleft palate. The clinical diagnosis of OME was confirmed by oto(micro)scopy or tympanometry, or both.
Types of interventions
Interventions
Oral antibiotics of all types and courses of duration.
Comparators
We were interested in the following two comparisons:
oral antibiotics versus placebo;
oral antibiotics versus no treatment.
If study participants received other treatments, for example intranasal steroids, oral steroids, mucolytics or decongestants, we included these studies if both arms received identical treatment.
We excluded studies in which one antibiotic was compared with another, or studies comparing one dose of an antibiotic to a different dose of the same antibiotic.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies. We assessed all outcomes at very short term (< 6 weeks for adverse events), short term (≤ 3 months), medium term (> 3 months to ≤ 1 year) and long term (> 1 year).
Primary outcomes
-
Hearing:
Proportion of children whose hearing has returned to normal, with normal hearing defined as 20 dB HL or less (assessed using age‐appropriate tests).
Hearing threshold.
It was anticipated that study data for these outcomes may be derived from a variety of assessment methods. To avoid loss of important evidence, we extracted all such data for analysis. However, we gave consideration to the appropriateness of pooling different types of data in meta‐analysis. Our selection of primary outcomes was based principally upon clinical importance, but also permitted applicability across a variety of age‐appropriate assessment methods and considered the types of outcome data that are most likely to be available. Accordingly, we regard the proportion of participants whose hearing has returned to normal as the most important measure of hearing impact. We considered medium‐ and long‐term outcome data as the most clinically important.
-
Disease‐specific quality of life measured using a validated instrument, for example:
OM8‐30 (Haggard 2003);
Otitis Media‐6 (Rosenfeld 1997).
Adverse events ‐ anaphylactic reaction.
Secondary outcomes
Presence/persistence of OME.
-
Adverse events ‐ measured by the number of participants affected.
-
Tympanic membrane changes, such as:
atrophy;
atelectasis or retraction;
persistent perforation;
myringosclerosis;
tympanosclerosis.
-
Patient‐related, such as:
vomiting;
diarrhoea;
dry throat;
nasal stinging;
cough;
long‐term hearing loss;
postsurgical haemorrhage;
pain.
-
-
Receptive language skills, measured using a validated scale, for example:
Peabody Picture Vocabulary Test ‐ Revised (Dunn 2007);
relevant domains of the Reynell Developmental Language Scales (Reynell 1985);
relevant domains of the Preschool Language Scale (PLS) (Zimmerman 1992);
relevant domains of the Sequenced Inventory of Communication (SCID) (Hedrick 1984).
-
Speech development, or expressive language skills, measured using a validated scale, for example:
Schlichting test (Schlichting 2010);
Lexi list (Schlichting 2007);
relevant domains of the Reynell Developmental Language Scales (Reynell 1985);
relevant domains of the PLS (Zimmerman 1992);
relevant domains of the SCID (Hedrick 1984).
-
Cognitive development, measured using a validated scale, for example:
Griffiths Mental Development Scales (Griffiths 1996);
McCarthy General Cognitive Index (McCarthy 1972);
Bayley Scales of Infant and Toddler Development (Bayley 2006).
-
Psychosocial outcomes, measured using a validated scale, for example:
the Social Skills Scale of the Social Skills Rating System (Gresham 1990);
Child Behavior Checklist (Achenbach 2011);
Strengths and Difficulties Questionnaire (Goodman 1997);
Pediatric Symptom Checklist (Jellinek 1988).
Listening skills, for example listening to stories and instructions effectively. Given that there are few validated scales to assess listening skills in children with OME, we will include any methods used by trialists.
-
Generic health‐related quality of life assessed using a validated instrument, for example:
EQ‐5D (Rabin 2001);
TNO AZL Children’s QoL (TACQOL) (Verrips 1998);
TNO AZL Pre‐school children QoL (TAPQOL) (Fekkes 2000);
TNO AZL Infant Quality of Life (TAIQOL) (TNO 1997);
Infant Toddler Quality of Life Questionnaire (ITQOL) (Landgraf 1994);
Child Health Questionnaire (CHQ) (Landgraf 1996).
-
Parental stress, measured using a validated scale, for example:
Parenting Stress Index (Abidin 1995).
-
Vestibular function:
balance;
co‐ordination.
Number of doctor‐diagnosed AOM episodes within a specified time frame.
These outcomes were identified as the most important in two studies that aimed to develop a core outcome set for children with OME (Bruce 2015; Liu 2020). As this review forms part of a suite of reviews of interventions for OME, not all outcomes are relevant for all reviews.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. We contacted original authors for clarification and further data if trial reports were unclear, and we arranged translations of papers where necessary. The date of the search was 20 January 2023.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 20 January 2023);
the Cochrane Central Register of Controlled Trials (CENTRAL 2023, Issue 1), searched via the Cochrane Register of Studies to 20 January 2023;
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 20 January 2023);
Ovid EMBASE (1974 to 20 January 2023);
Web of Science, Web of Science (1945 to 20 January 2023);
-
ClinicalTrials.gov, www.clinicaltrials.gov:
searched via the Cochrane Register of Studies to 20 January 2023;
searched via www.clinicaltrials.gov to 20 January 2023;
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), https://apps.who.int/trialsearch/:
searched via the Cochrane Register of Studies to 20 January 2023;
searched via https://apps.who.int/trialsearch/ to 20 January 2023.
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for otitis media with effusion. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Technical Supplement to Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions version 6.1) (Lefebvre 2020). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
The Cochrane ENT Information Specialist used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components:
Known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'a RCT' or as 'not a RCT'.
The machine learning classifier (RCT model) (Wallace 2017), available in the Cochrane Register of Studies (CRS‐Web), which assigns a probability of being a true RCT (from 0 to 100) to each citation. We assumed citations assigned a probability score below the cut‐point at a recall of 99% to be non‐RCTs. For those that scored on or above the cut‐point we either manually dual screened these results or sent them to Cochrane Crowd for screening.
Cochrane Crowd is Cochrane's citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me website on the Cochrane Information Specialist's portal and see Marshall 2018; McDonald 2017; Noel‐Storr 2018; Thomas 2017.
Two review authors (KG, CM) independently screened titles and abstracts retrieved by the search to identify potentially relevant studies. At least two review authors (KG, CM, SM) then independently evaluated the full text of each potentially relevant study to determine whether it met the inclusion/exclusion criteria for this review. Any differences were resolved by discussion and consensus, with the involvement of a third author (KW) where necessary.
Screening eligible studies for trustworthiness
Two review authors (KG, CM, MR, KW) appraised all studies meeting our inclusion criteria for trustworthiness using a screening tool developed by Cochrane Pregnancy and Childbirth. This tool includes specified criteria to identify studies that are considered sufficiently trustworthy to be included in the review (see Appendix 2 and Figure 1). For any studies assessed as being potentially 'high risk', we attempted to contact the study authors to obtain further information or address any concerns. We had planned to exclude these studies from the review if we were unable to contact the authors, or there was persisting uncertainty about the study. However, when using the trustworthiness tool, there were only four studies where we had no concerns (Leach 2008; Mandel 1987; Mandel 1991; van Balen 1996).
1.

The Cochrane Pregnancy and Childbirth Trustworthiness Screening Tool
All the remaining studies had at least some concerns ‐ although this was often due to a paucity of information, rather than a specific concern over trustworthiness:
Balle 1990; Endo 1997; Ernston 1985; Hemlin 1997; Karlidag 2002; Manrique 1987; Marchisio 1998; Møller 1990; Podoshin 1990; Puhakka 1985; Sundberg 1984 and Thomsen 1989 all reported few (or no) baseline characteristics for the participants included in the study. We were therefore unable to assess whether there was excessive similarity between the randomised groups.
Three studies recruited identical numbers of participants to each group, without a description of blocked randomisation (Ardehali 2008; Chen 2013; Healy 1984), and two studies did not clearly report the numbers allocated to each group (Møller 1990; Podoshin 1990).
The number of participants lost to follow‐up was known (or appeared) to be zero for four trials, without adequate explanation (Ardehali 2008; Endo 1997; Karlidag 2002; Puhakka 1985).
Finally, we were unable to identify a prospective trial registration for one recently published study (Chen 2013).
We were unsure whether this high level of studies with concerns reflected a genuine problem with the data from these studies, or whether the assessment tool was perhaps too sensitive. We note that this tool ‐ and others used for the same purpose ‐ has not yet been validated.
Consequently, we decided to include all studies in the main analyses of this review, but we did investigate the effect of excluding studies with concerns over trustworthiness on the overall results (see Sensitivity analysis).
Data extraction and management
At least two review authors (KG, CM, MR, RV, KW) independently extracted outcome data from each study using a standardised data collection form. Where a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Any discrepancies in the data extracted by the two authors were checked against the original reports, and differences were resolved through discussion and consensus, with recourse to a third author (KG or CM) where necessary. If required, we contacted the study authors for clarification. We included key characteristics of the studies, such as the study design, setting, sample size, population and the methods for defining or collecting outcome data in the studies.
We extracted data on study findings according to treatment assignment, irrespective of whether study participants complied with treatment or received treatment to which they were randomised.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each study and outcome:
For continuous data: the mean values, standard deviation and number of patients for each treatment group at the different time points for outcome measurement. Where endpoint data were not available, we extracted the values for change‐from‐baseline data instead. If values for the individual treatment groups were not reported, where possible we extracted summary statistics (e.g. mean difference) from the studies.
For binary data: we extracted information on the number of participants experiencing an event, and the number of participants assessed at that time point. If values for the individual treatment groups were not reported, where possible we extracted summary statistics (e.g. risk ratio) from the studies.
For ordinal scale data: we did not include any data from an ordinal scale in this review.
We pre‐specified time points of interest for the outcomes in this review. Where studies reported data at multiple time points, we took the longest available follow‐up point within each of the specific time frames. For example, if a study reported an outcome at 4 months, 8 months and 12 months of follow‐up, then the 12‐month data are included for the time point > 3 months to ≤ 1 year.
Assessment of risk of bias in included studies
Two authors (KG, CM, MR, RV, KW) undertook assessment of the risk of bias of the included studies independently, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane risk of bias tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the study and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We summarised dichotomous data ‐ such as presence of OME ‐ as risk ratios (RR) and 95% confidence intervals (CI), and we summarised continuous data as a mean difference (MD) and 95% CI. For the outcomes presented in the summary of findings tables, we also provide both the relative and absolute measures of effect.
Unit of analysis issues
For this review we anticipated that the unit of analysis would be the child. However, some studies reported findings by ear, and therefore we have used both the child and ear as the unit of analysis.
All studies randomised participants to antibiotics or no treatment/placebo at the level of the child ‐ as this is an intervention that affects both ears. Some studies in this review included children with bilateral OME ‐ either exclusively (Endo 1997; Møller 1990), or as a proportion of included participants (Balle 1990; Ernston 1985; Hemlin 1997; Manrique 1987; Sundberg 1984; Thomsen 1989). This gave rise to a number of issues regarding the unit of analysis, as some studies reported outcomes (particularly the persistence of OME) for each ear.
We considered that outcomes for ears within the same individual were likely to be correlated ‐ for example, if a child had resolution of OME in one ear, they may be more likely to experience resolution in the contralateral ear. Ears of the same individual are not independent. Standard meta‐analysis techniques assume that all data are independent. Therefore, inclusion of the raw data (for the number of ears) is likely to overestimate the precision of any effect, and result in an excessively narrow confidence interval.
To account for this correlation, we used the suggested methods in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), which are more commonly employed in the analysis of cluster‐randomised trials. We treated individuals who contributed two ears to the analysis (all of those with bilateral disease) as a 'cluster' of two data points. We then attempted to account for the correlation in these clusters, by assuming a certain correlation between ears of the same individual. We could not identify a figure for this correlation in the published literature, so we used an estimated correlation of 0.5 in the main analysis, but conducted sensitivity analyses using correlations of 0 and 1, to test the limits of this assumption. We then reduced the effective size of the trials by the 'design effect' ‐ which accounts for correlation between ears, and the average cluster size (which would be 2 for trials where all children had bilateral disease, and less than 2 if trials included a mixture of children with bilateral and unilateral disease).
If we had identified cluster‐randomised trials, we would have assumed that the data from participants was no longer independent and adjusted our analyses accordingly, using the design effect approach as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). If we had identified cross‐over RCTs then we would have included data from the first phase of the trial only. However, this was not necessary for the review. We did identify one study with multiple arms (Mandel 1991); in this instance, we pooled data from the three relevant arms for this review (all groups that received antibiotics) and compared them to the control group.
Dealing with missing data
We attempted to contact study authors by email where data on an outcome of interest to the review were not reported, but the methods described in the paper suggested that the outcome was assessed. We did the same if not all data required for meta‐analysis were reported. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs (if these were reported), as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the included studies for potential differences between them in the types of participants recruited, interventions or controls used, and the outcomes measured. We assessed statistical heterogeneity by considering both the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance (with values over 50% suggesting substantial heterogeneity), and the P value from the Chi² test (Higgins 2021).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol or trial registry, whenever this could be obtained. If the protocol or trial registry entry was not available, we compared the outcomes reported to those listed in the methods section. If results are mentioned but not reported adequately in a way that allows analysis (e.g. the report only mentions whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We then sought further information from the study authors. If no further information could be found, we noted this as being a 'high' risk of bias when the risk of bias tool was used. If there was insufficient information to judge the risk of bias we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to produce a funnel plot to explore possible publication biases, if we were able to pool 10 or more studies in a single analysis. However, this was not possible, as too few studies were included in the meta‐analyses.
Data synthesis
Where two or more studies reported the same outcome we performed a meta‐analysis using Review Manager 5 (RevMan 2014). We report pooled effect measures for dichotomous outcomes as a risk ratio (RR) using the Mantel‐Haenszel methods. For continuous outcomes measured using the same scales we report the mean difference (MD). We used a random‐effects model.
Where it was not possible to pool the findings from studies in a meta‐analysis, we have presented the results of each study and provide a narrative synthesis of findings.
Subgroup analysis and investigation of heterogeneity
We proposed the following subgroup analyses if sufficient data were available in study reports:
children with mild hearing loss versus moderate or worse;
children with allergy versus those without (using the trialists' own definition);
children aged up to four years versus children aged four years and over;
children with previous ventilation tubes versus those without ventilation tubes;
children with cleft palate versus children without;
children with Down syndrome versus children without.
However, we did not find any data suitable for conducting these subgroup analyses. No studies provided subgroup data for children with different features (for example, for those with mild hearing loss, compared to those with moderate or worse hearing loss). Many of the trials did not provide sufficient background information (for example on hearing level) for us to conduct subgroup analysis at the level of the individual study. Where data were provided, trials often recruited a mixed population that encompassed all subgroups (for example, most trials recruited children aged 1 to 12 years, not specifically children aged < 4 years, or ≥ 4 years).
Sensitivity analysis
According to our protocol, we carried out sensitivity analyses to assess whether the results of a fixed‐effect model were notably different to those from a random‐effects model.
We also planned to conduct a sensitivity analysis to exclude studies at high risk of bias (with four or more domains rated as high risk, using the risk of bias tool). This applied to a single study (Ernston 1985).
Where possible, we also carried out sensitivity analyses to assess the impact of excluding studies that had any concerns when using the Trustworthiness Screening tool.
Two studies reported hearing data separately for right and left ears. We pooled these data for analysis, and made adjustments to account for the correlation between ears of the same individual. We were unable to identify a published correlation coefficient, therefore for the main analysis we assumed correlation of 0.5 between ears of the same individual, but tested this assumption using correlation coefficients of 0.3 and 0.7 in a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
Two independent authors (KG, CM) used the GRADE approach to rate the overall certainty of evidence using GRADEpro GDT (https://gradepro.org/). The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct, and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
When assessing imprecision, we used a minimally important difference of a risk ratio (or odds ratio) of 0.8 or 1.25 for dichotomous outcomes. For most continuous data we considered a minimally important difference to be half of the standard deviation for the control/comparator group. The exception to this was hearing thresholds, where a difference of 10 dB HL was used as the minimally important difference.
We included a summary of findings table, constructed according to the recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), for the following comparisons:
oral antibiotics versus placebo;
oral antibiotics versus no treatment.
We prioritised the following four outcomes for the summary of findings tables:
hearing;
disease‐specific quality of life;
presence/persistence of OME;
adverse events ‐ anaphylactic reaction.
Results
Description of studies
Results of the search
The searches (September 2021 and January 2023) retrieved a total of 7441 records. This reduced to 4157 after the removal of duplicates. The Cochrane ENT Information Specialist sent all 4157 records to the Screen4Me workflow. The Screen4Me workflow identified 84 records as having previously been assessed: 50 had been rejected as not RCTs and 34 had been assessed as possible RCTs. The remaining 4073 references were sent to the RCT classifier, which rejected an additional 1514 records as not RCTs (with 99% sensitivity) and 116 records as possible RCTs. The Cochrane Crowd assessed 2443 of the remaining references, rejecting 1313 as not RCTs and identifying 1130 as possible RCTs. Following this process, the Screen4Me workflow rejected a total of 2877 records and identified 1280 possible RCTs for title and abstract screening (see Table 3).
1. RCTs identified through Cochrane Crowd and the RCT classifier.
| Possible RCTs | Rejected | |
| Known assessments | 34 | 50 |
| RCT classifier | 116 | 1514 |
| Cochrane Crowd | 1130 | 1313 |
| Total | 1280 | 2877 |
RCT: randomised controlled trial
We excluded 76 additional duplicates. We screened the titles and abstracts of the remaining 1204 records. We discarded 886 records and retrieved 318 full‐text records. We subsequently discarded an additional 239 irrelevant records and removed an additional five duplicates.
We excluded 49 records (linked to 41 studies) with reasons recorded in the review (see Excluded studies).
We included 19 studies (23 records) where results were available (Ardehali 2008; Balle 1990; Chen 2013; Endo 1997; Ernston 1985; Healy 1984; Hemlin 1997; Karlidag 2002; Leach 2008; Mandel 1987; Mandel 1991; Manrique 1987; Marchisio 1998; Møller 1990; Podoshin 1990; Puhakka 1985; Sundberg 1984; Thomsen 1989; van Balen 1996).
We identified two studies that are awaiting assessment because we did not have enough information to determine eligibility (Koay 1998; Tawfik 2002). See Characteristics of studies awaiting classification. We did not identify any ongoing studies.
A flow chart of study retrieval and selection is provided in Figure 2.
2.
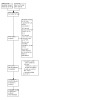
A flow chart of study retrieval and selection.
Included studies
A full description of each included study is given in Characteristics of included studies, and a summary of the main features of all studies is shown in Table 4.
2. Comparison of studies.
| Study | Participants | Setting | Intervention | Comparator | Concomitant treatment | Follow‐up (main outcomes reported at this time) | Notes |
| Ardehali 2008 | Children aged 2 to 12 years with chronic OME (≥ 3 months), without response to 3 courses of antibiotics (n = 60) | Single‐centre study from Iran | Co‐amoxiclav, 40 mg/kg/day in 3 divided doses for 3 months | No treatment | None reported | 3 months | A third arm received anti‐reflux medication, but this was not relevant to this review, therefore data were not extracted |
| Balle 1990 | Children aged 1 to 10 years with at least 3 months duration of uni‐ or bilateral OME (n = 264) | Study conducted in Denmark. No details on participant recruitment. | 4 weeks treatment with amoxicillin and clavulanate potassium. Concentration not stated. Children aged 1 to 5 had 5 mL 3 times daily, children aged 6 to 10 had 7.5 mL 3 times daily. | Placebo | None reported | 12 months | Note that this study did not provide any data for outcomes of interest in this review |
| Chen 2013 | Children aged 3 to 14 years with OME for less than 3 months (n = 84) | Single‐centre, university ENT department in China | Clarithromycin 15 mg/kg/day in 2 divided doses for 1 week, then 5 to 8 mg/kg/day until the tympanogram was type A (range of treatment 5 to 12 weeks) | No treatment | Topical glucocorticoid spray was given to all participants for 12 weeks It appears that all participants underwent tympanocentesis at the start of the trial |
12 weeks | Two children in the intervention group actually received azithromycin, instead of clarithromycin |
| Endo 1997 | Children with bilateral OME, aged less than 2 years (n = 26) | Single‐centre study from Brazil | Sulfamethoxazole‐trimethoprim 20 mg/kg/day in a single night‐time dose for 30 days | Stated to be placebo, but no information on the nature of the placebo is provided | None reported | 4 weeks | Note that this trial also included older children, but they received a different intervention, not relevant for this review |
| Ernston 1985 | Children aged < 12 years with uni‐ or bilateral OME for at least 3 months (n = 91) | Single‐centre study from Sweden | Cefaclor 20 mg/kg twice daily for 10 days | No treatment | None reported | 10 days | — |
| Healy 1984 | Children aged 2 to 5 years with OME for ≤ 6 weeks (n = 200) | Single‐centre, university hospital study from USA | Trimethoprim‐sulfamethoxazole (8 mg and 40 mg/kg/day respectively) in 2 divided doses for 4 weeks | No treatment | None reported | 4 weeks | — |
| Hemlin 1997 | Children aged 2 to 12 years with uni‐ or bilateral OME for at least 3 months (n = 81) | Single‐centre study from Sweden | Cefixime 8 mg/kg per day in 2 divided doses for 10 days | Placebo suspension of similar appearance | None reported | 12 to 21 days | Note that a third arm received antibiotics and steroids. Data are not relevant for this review, so have not been included here. |
| Karlidag 2002 | Children aged 2 to 12 years with uni‐ or bilateral OME (n = 42) | Single‐centre study from Turkey | Ampicillin/sulbactam 25 mg/kg/day, in 2 divided doses for 8 weeks | No treatment | None reported | 8 weeks | Note that a third arm received antibiotics and steroids. Data are not relevant for this review, so have not been included here. |
| Leach 2008 | Aboriginal infants aged < 12 months with OME (n = 103) | Recruited from 3 Aboriginal communities in Australia | Amoxicillin 50 mg/kg/day for 24 weeks, or until bilateral normal middle ear status | Placebo suspension of equivalent volume | None reported | 24 weeks | — |
| Mandel 1987 | Children aged 7 months to 12 years with OME (n = 340) | Single‐centre study from the USA | Amoxicillin 40 mg/kg per day in 3 divided doses for 2 weeks | Placebo | Both groups also received an additional placebo for the third arm of this study | 4 weeks | A third treatment arm received antibiotics and antihistamine. Data were not relevant for this review, therefore not included. |
| Mandel 1991 | Children aged 7 months to 12 years with OME (n = 331) | Single‐centre study from the USA | Erythromycin and sulfisoxazole (50 mg and 150 mg/kg/day respectively) in 4 divided doses for 2 weeks or Cefaclor 40 mg/kg/day in 3 divided doses for 2 weeks or Amoxicillin 40 mg/kg/day in 3 divided doses for 2 weeks |
Placebo | None reported | 4 weeks | Note that data from all of the arms receiving antibiotics have been pooled for the purposes of analysis |
| Manrique 1987 | Children aged 8 months to 13 years with unilateral or bilateral OME for at least 3 months (52 ears assessed, number of children unclear) | Single‐centre study from Spain | Amoxicillin 50 mg/kg/day for 8 days | No treatment | Participants in both groups received a daily dose of a decongestant syrup (chlorhydrate ambroxol, dose dependent on age) for the duration of the trial | 3 months | — |
| Marchisio 1998 | Children aged 5 to 7 years with OME for at least 3 months (n = 120) | Multicentre study from Italy | Ceftibuten 9 mg/kg/day in 1 daily dose for 14 days | Placebo (nasal saline drops) | None reported | 8 weeks | — |
| Møller 1990 | Children aged 1 to 15 years with bilateral OME for at least 3 months, awaiting ventilation tube insertion (n = 147) | Single‐centre study from Norway | Erythromycin 50 mg/kg/day in 2 divided doses for 14 days | Placebo | None reported | 1 month | — |
| Podoshin 1990 | Children aged 4 to 8 years with OME for ≥ 2 months, who had received no previous treatment (n = 86) | Single‐centre study from Israel | Amoxicillin 50 mg/kg/day for 14 days | Lactose powder placebo | Both groups received an additional placebo to account for the third intervention in this study (antibiotics plus prednisone) | 2 months | Note that there was a third arm in this study, where participants received both antibiotics and steroids. Data have not been extracted for this review. |
| Puhakka 1985 | Children aged 7 months to 11 years with OME (n = 46) | Single‐centre study from Finland | 6 mg trimethoprim and 18.5 mg sulfadiazine/kg/day in 2 divided doses for 10 days | Placebo | Both groups received an additional placebo to account for the third intervention in this study (antibiotics plus prednisone) | 8 weeks | Note that there was a third arm in this study, where participants received both antibiotics and steroids. Data have not been extracted for this review. |
| Sundberg 1984 | Children aged 1.5 to 11 years with unilateral or bilateral OME for at least 3 months (n = 75) | Single‐centre study from Sweden | Erythromycin ethylsuccinate 20 to 30 mg/kg twice daily for 10 days | No treatment | None reported | 10 days | Note that this study did not provide any data for outcomes of interest in this review |
| Thomsen 1989 | Children aged 1 to 10 years with at least 3 months of unilateral or bilateral OME (n = 264) | Single‐centre study from Denmark | Amoxicillin and clavulanate potassium with 125 mg/31.25 mg 3 times daily (if aged 1 to 5) or 187 mg/46.9 mg 3 times daily (if aged 6 to 10) for 1 month | Placebo | In patients with bilateral disease, a ventilation tube was inserted in the right ear, and the left ear was included in the study | 3 months | — |
| van Balen 1996 | Children aged ≤ 6 years with at least 3 months of OME (n = 162) | Single‐centre study from the Netherlands | Amoxicillin 20 mg/kg/day plus 5 mg/kg/day clavulanic acid in 3 divided doses for 14 days | Placebo | One drop of decongestant (oxymetazoline 0.25%) 3 times daily | 2 weeks | — |
OME: otitis media with effusion
Study design
All the included studies were described as randomised controlled trials.
Participants
Most of the studies recruited children aged from approximately 2 to 12 years, with bilateral or unilateral OME. One study included participants of mixed ages, but we were only able to use the data for children aged < 2 years (as older children received a different intervention; Endo 1997).
Many, but not all, studies required participants to have a diagnosis of OME that had persisted for at least three months (Ardehali 2008; Balle 1990; Ernston 1985; Hemlin 1997; Manrique 1987; Marchisio 1998; Møller 1990; Sundberg 1984; Thomsen 1989; van Balen 1996). One study required the persistence of OME for at least six weeks (Healy 1984), and another for two months (Podoshin 1990). A single study specifically recruited individuals with short duration of symptoms (< 3 months; Chen 2013), whilst the remainder did not specify the duration of OME.
Few studies provided information on the extent of hearing impairment at baseline.
Interventions and comparisons
Comparison 1: Oral antibiotics versus placebo
We identified 11 studies for this comparison (Balle 1990; Endo 1997; Hemlin 1997; Leach 2008; Mandel 1987; Mandel 1991; Møller 1990; Podoshin 1990; Puhakka 1985; Thomsen 1989; van Balen 1996). However, Balle 1990 does not provide data for any outcomes of interest to this review.
A number of different antibiotics were used, including:
-
Penicillins
Amoxicillin (Leach 2008; Mandel 1987; Mandel 1991; Podoshin 1990).
Co‐amoxiclav (Balle 1990; Thomsen 1989; van Balen 1996).
-
2nd or 3rd generation cephalosporins
Cefaclor (Mandel 1991).
Cefixime (Hemlin 1997).
Ceftibuten (Marchisio 1998).
-
Macrolides
Erythromycin (Møller 1990).
Erythromycin and sulfisoxazole (Mandel 1991).
Trimethoprim and sulfamethoxazole (Endo 1997), or trimethoprim and sulfadiazine (Puhakka 1985).
Most studies provided antibiotic treatment for 10 to 14 days, although some required a longer course (28 to 30 days: Balle 1990; Endo 1997; Thomsen 1989) and one used treatment for 24 weeks (Leach 2008). Three studies assessed outcomes as soon as the antibiotics were stopped (Endo 1997; Leach 2008; van Balen 1996). Four studies assessed outcomes approximately two weeks after stopping antibiotics (Hemlin 1997; Mandel 1987; Mandel 1991; Møller 1990), and the remaining four studies had a delay of approximately six to eight weeks before assessing outcomes (Marchisio 1998; Podoshin 1990; Puhakka 1985; Thomsen 1989).
Comparison 2: Oral antibiotics versus no treatment
We identified eight studies for this comparison (Ardehali 2008; Chen 2013; Ernston 1985; Healy 1984; Karlidag 2002; Manrique 1987; Marchisio 1998; Sundberg 1984). However, Sundberg 1984 does not provide data for any outcomes of interest to this review.
A number of different antibiotics were used, including:
-
Penicillins
Amoxicillin (Manrique 1987).
Co‐amoxiclav (Ardehali 2008).
Ampicillin and sulbactam (Karlidag 2002).
-
2nd or 3rd generation cephalosporins
Cefaclor (Ernston 1985).
-
Macrolides
Erythromycin (Sundberg 1984).
Clarithromycin (Chen 2013).
Trimethoprim and sulfamethoxazole (Healy 1984).
The duration of treatment varied from a minimum of 8 or 10 days (Ernston 1985; Manrique 1987; Sundberg 1984) to a maximum of three months (Ardehali 2008). Follow‐up was typically at the end of the treatment (immediately after antibiotics were discontinued), except for Chen 2013 (5 to 12 weeks of treatment, follow‐up at 12 weeks) and Manrique 1987 (eight days of treatment, follow‐up at three months).
Outcomes
Hearing
Return to normal hearing
As with other reviews in this suite, few studies reported our preferred outcome measure for hearing ‐ the proportion of children in whom hearing returns to normal. This outcome was only measured by two studies. Podoshin 1990 reported the proportion of children in whom there was complete resolution of the air‐bone gap in the worst affected ear, after two months of follow‐up. Ernston 1985 reported the proportion of children in whom "hearing thresholds returned to normal", but did not provide a definition of normal hearing.
Final hearing thresholds or change in hearing threshold
Two studies assessed speech reception thresholds (Mandel 1987; Mandel 1991); one of these also assessed speech awareness thresholds for younger children (Mandel 1987). One study reported the mean air‐bone gap after three months of follow‐up (Chen 2013).
Disease‐specific quality of life
We did not identify any studies that assessed disease‐specific quality of life.
Adverse events: anaphylaxis
None of the studies included in this review specifically reported on the occurrence of anaphylaxis. However, five studies did provide some information, which suggested that no anaphylactic reactions had occurred (Ardehali 2008; Hemlin 1997; Leach 2008; Marchisio 1998; Thomsen 1989).
Presence/persistence of OME
We noted that there was some variation in how the outcome 'presence or persistence of OME' was assessed and reported. Most studies reported on clearance or resolution of OME, i.e. the number of participants with no evidence of OME in either ear at follow‐up. Many studies included participants with both bilateral and unilateral diseases. Consequently, for those with bilateral disease, this would only include children in whom both ears had resolved.
For the majority of studies we were therefore able to identify the proportion of participants in each in whom at least one ear had persistent OME at the follow‐up time ‐ both ears were assessed in every participant. However, some studies reported this outcome slightly differently:
Two studies only assessed the ear(s) that had been affected at the start of the trial (Healy 1984; Marchisio 1998). Both ears were assessed for those with bilateral disease, but for those with unilateral disease only one ear was checked at follow‐up ‐ the ear affected by OME at the start of the study.
One study only classed OME as persistent if any ear affected at baseline was still affected at follow‐up (Hemlin 1997). For those with bilateral disease, if one ear had resolved at follow‐up then the child was not considered to have "persistent OME".
One study assessed only one ear in each participant: for those with bilateral disease, the "worst affected ear" was assessed at follow‐up (Podoshin 1990).
Some studies assessed and reported the outcome (presence of OME) at the level of the individual ear, rather than at the level of the participant. Children with bilateral disease therefore contributed two data points to the outcome measure. We are aware that the outcomes for ears within the same individual are likely to be strongly correlated, and these data are clustered. We therefore adjusted these data according to the methods in the Cochrane Handbook for Systematic Reviews of Interventions, using an estimated intra‐cluster correlation coefficient of 0.5. However, we also conducted a sensitivity analysis to assess the impact of this adjustment, using an ICC of zero (no correlation between the ears of a given individual) and one (complete correlation between the ears of a given individual).
The underlying approach in all the studies was to assess the difference in persistence of OME, albeit with slightly different methods of measuring this effect. We therefore considered that it was reasonable to pool the data in a meta‐analysis. However, we also undertook a subgroup analysis to identify whether there may be differences in the effect estimates depending on which method of outcome assessment was used.
Adverse events
Adverse events were inconsistently reported across the studies. Data were frequently presented for only one group ‐ those who received the intervention. It was not clear whether this was because no events occurred in the placebo arm, or whether adverse events were not assessed in this group.
Number of doctor‐diagnosed episodes of acute otitis media
This outcome was only assessed by three studies (Healy 1984; Mandel 1987; Mandel 1991), and was reported as the proportion of participants who experienced at least one episode of acute otitis media during a four‐week follow‐up period.
We did not identify any data for our other secondary outcomes of interest (Secondary outcomes), including expressive and receptive language skills, cognitive development, psychosocial skills, listening skills, generic health‐related quality of life, parental stress and vestibular function.
Excluded studies
We excluded 41 studies (linked to 49 records) from this review. The main reason for exclusion of each study is listed below:
Nineteen articles were not randomised controlled trials (de Castro 1982; Eiden 1997; Fujita 1994; Gasper 2003; Gibson 1996; Hozawa 2001; Iino 1989; Iino 2001; Kobayashi 2001; Kuriyama 1980; Leonetti 1988; Paradise 1997; Parlea 2012; Persico 1978; Shubich 1996; Smales 1992; Stenstrom 2005; van Balen 1997; Zocconi 1994).
-
Twelve articles considered an incorrect population, enroling participants who did not have OME, including:
children with acute otitis media (Perrin 1974);
children with a persistent effusion after a recent episode of acute otitis media (Corwin 1986; Giebink 1990; Schloss 1988);
children with recurrent episodes of acute otitis media (Ferrara 2005; Gaskins 1982; Principi 1989; Roark 1997; Schwartz 1982; Schwartz 1982a; Tracy 1995; Varsano 1985).
Six articles used an intervention other than antibiotics (Berman 1990; Bernard 1991; Choung 2008; Daly 1991; Rohail 2006; Velepic 2011).
-
Three studies compared the use of antibiotics to a different, active intervention (not to placebo or no treatment), including:
different doses of the same antibiotic (Donaldson 1990);
a decongestant (Marks 1981);
a different antibiotic (Yin 2002).
Yeldandi 2001 did use a relevant comparator. However, both groups received co‐interventions, and the nature and frequency of these was not balanced across the groups. This rendered the comparison inaccurate, therefore we excluded this study.
Risk of bias in included studies
See Figure 3 for a summary of the risk of bias across all included studies, and Figure 4 for details of the risk of bias for individual studies.
3.

Risk of bias graph (our judgements about each risk of bias item presented as percentages across all included studies).
4.

Risk of bias summary (our judgements about each risk of bias item for each included study).
Allocation
We rated the risk of selection bias as unclear for almost all the studies included in this review. This was due to insufficient detail describing the methods for random allocation to the study groups, and/or a lack of detail regarding methods used to conceal allocation. Only four studies provided a description of adequate randomisation methods (Ardehali 2008; Healy 1984; Leach 2008; van Balen 1996). Only one of these studies also described appropriate methods to conceal allocation (Leach 2008).
Blinding
The assessment of performance and detection bias varied across the different studies.
We rated a number of studies at high risk of performance bias, as participants would have been aware if they were receiving the active intervention or were in the control group. This included all of the studies for the comparison 'antibiotics versus no treatment' (Ardehali 2008; Chen 2013; Ernston 1985; Healy 1984; Karlidag 2002; Manrique 1987; Marchisio 1998; Sundberg 1984). We rated one further study at high risk of performance bias, as we had concerns over the adequacy of blinding, despite the use of placebo (Podoshin 1990). We rated a number of studies as at unclear risk of performance bias ‐ although participants appeared to be blinded to group allocation, it was not clear whether this also extended to study personnel (Balle 1990; Mandel 1987; Puhakka 1985; Thomsen 1989). We assessed five studies as having a low risk of performance bias, due to blinding of participants and study personnel (Hemlin 1997; Leach 2008; Mandel 1991; Møller 1990; van Balen 1996).
Only seven studies indicated that outcome data were collected by blinded assessors, or the outcomes were sufficiently objective that blinding was considered unlikely to impact on the results (Ardehali 2008; Balle 1990; Leach 2008; Mandel 1991; Marchisio 1998; Sundberg 1984; van Balen 1996). We rated five studies at high risk of detection bias, as outcome assessors were aware of group allocation (Endo 1997; Ernston 1985; Healy 1984; Karlidag 2002; Mandel 1987; Puhakka 1985), and we rated a number of studies at unclear risk for this domain, due to a lack of information on blinding (Chen 2013; Hemlin 1997; Manrique 1987; Møller 1990; Podoshin 1990; Thomsen 1989).
Incomplete outcome data
Most of the studies had complete outcome data for most or all randomised participants, and we therefore considered them at low risk of bias for this domain (Ardehali 2008; Balle 1990; Chen 2013; Ernston 1985; Healy 1984; Hemlin 1997; Karlidag 2002; Leach 2008; Mandel 1987; Mandel 1991; Marchisio 1998; Møller 1990; Puhakka 1985; Sundberg 1984; Thomsen 1989; van Balen 1996).
Endo 1997 and Manrique 1987 did not provide details on loss to follow‐up, therefore it was unclear whether there was a risk of bias for this domain. We noted very substantial dropout in the placebo group for the study Podoshin 1990, leading to imbalance in the groups and the potential for bias.
Selective reporting
We rated almost all of the included studies at unclear risk of selective reporting bias, as we were unable to identify a published protocol for the studies and could therefore not compare the reported results to the intended analysis plan. We had specific concerns about three of the included studies, which we rated at high risk of selective reporting. The study Balle 1990 included a narrative report of improvement in OME, but did not provide any data to support this claim. Data included in the study Mandel 1987 were subsequently reported in a second paper (Cantekin 1991), which identified a different rate of 'cure' for OME, suggesting that there may be a risk of reporting bias. Finally, Møller 1990 assessed hearing outcomes, but presented very limited data, which precluded comparison across the two groups.
Other potential sources of bias
We considered that an additional source of bias for many studies was the short duration of follow‐up (three months or less, sometimes as short as 10 days). This would likely be insufficient to expect natural resolution of OME for those who received no treatment or placebo. Consequently, there is a risk that any treatment effect seen in favour of antibiotics may be overestimated.
Effects of interventions
Oral antibiotics versus placebo
We identified 11 studies for this comparison (Balle 1990; Endo 1997; Hemlin 1997; Leach 2008; Mandel 1987; Mandel 1991; Møller 1990; Podoshin 1990; Puhakka 1985; Thomsen 1989; van Balen 1996). However, Balle 1990 did not provide data for any outcomes of interest to this review.
Hearing ‐ return to normal hearing
Short‐term follow‐up
A single study considered the number of participants in whom hearing returned to normal after two months of follow‐up. This was reported as the proportion of children in whom the air‐bone gap completely resolved over the follow‐up period. The Peto odds ratio for complete resolution of the air‐bone gap was 9.59 for those receiving antibiotics compared to placebo (95% confidence interval (CI) 3.51 to 26.18; 41% versus 0%; 1 study, 86 participants; Analysis 1.1; very low‐certainty evidence).
1.1. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 1: Normal hearing (as complete improvement in air‐bone gap in worst ear): short‐term
Hearing ‐ hearing threshold
Short‐term follow‐up
This was assessed by two studies at four weeks, using the speech reception threshold. The mean difference was ‐2.58 dB HL in favour of antibiotics (95% CI ‐4.52 to ‐0.65; 2 studies, 499 participants; I2 = 0%; Analysis 1.2; low‐certainty evidence). One study presented data from both ears separately for this analysis. We pooled the data, assuming a correlation between ears of 0.5. However, varying this correlation coefficient made very little difference to the overall effect estimates (Analysis 1.3; Analysis 1.4).
1.2. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 2: Hearing threshold: speech reception threshold (short‐term). Correction of variance assuming correlation coefficient of 0.5
1.3. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 3: Sensitivity analysis: speech reception threshold: assuming correlation coefficient of 0.3
1.4. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 4: Sensitivity analysis: speech reception threshold: assuming correlation coefficient of 0.7
One study also presented data on speech awareness thresholds, for those children aged under two years, or in whom hearing could not be assessed in other ways. The results were very similar, with a mean difference in hearing level of ‐2.14 dB HL (95% CI ‐5.10 to 0.82; 1 study, 102 participants; Analysis 1.5; very low‐certainty evidence).
1.5. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 5: Hearing threshold: speech awareness threshold (short‐term)
Disease‐specific quality of life
This outcome was not assessed by any of the included studies.
Adverse events ‐ anaphylactic reaction
This outcome was not specifically reported by any of the included studies. See details below regarding generic adverse event reporting.
Presence/persistence of OME
Short‐term follow‐up
Nine studies assessed the presence of OME at up to three months follow‐up. The risk ratio (RR) for the persistence of OME in those who received antibiotics was 0.88 (95% CI 0.78 to 1.00; 66% versus 76%; 9 studies, 1375 participants; I2 = 69%; Analysis 1.6; low‐certainty evidence).
1.6. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 6: Persistence of OME (short‐term)
We conducted some sensitivity analyses for this result. One study also reported the number of participants with acute otitis (in whom persistence of OME would be difficult to assess). Assuming that these participants also had persistent OME made little difference to the overall result (RR 0.87, 95% CI 0.79 to 0.96; Analysis 1.7). Using different correlation coefficients between ears of the same individual also had almost no impact on the overall results (Analysis 1.8; Analysis 1.9).
1.7. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 7: Sensitivity analysis: persistence (short‐term) including cases of acute otitis (Mandel 1987)
1.8. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 8: Sensitivity analysis: persistence (short‐term) assuming ICC of 1.0 (complete correlation between ears) (Puhakka 1985)
1.9. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 9: Sensitivity analysis: persistence (short‐term) assuming ICC of zero (no correlation between ears) (Puhakka 1985)
Medium‐term follow‐up
The remaining two studies assessed the presence of OME at 6 to 12 months of follow‐up. The risk ratio for persistence in those receiving antibiotics was 0.89 (95% CI 0.68 to 1.17; 48% versus 54%; 2 studies, 324 participants; I2 = 32%; Analysis 1.10; very low‐certainty evidence).
1.10. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 10: Persistence of OME (medium‐term)
Again, one study also reported the number of children with acute otitis media without perforation, in whom a diagnosis of persistent OME would have been difficult to assess. Inclusion of these participants in the analysis made a very slight difference to the overall effect estimate (RR 0.93, 95% CI 0.69 to 1.25; Analysis 1.11).
1.11. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 11: Sensitivity analysis: persistence of OME (medium‐term); defined as 'OME' or 'AOM without perforation' (Leach 2008)
Adverse events
Adverse events were inconsistently reported across the studies. Data were frequently presented for only one group ‐ those who received the intervention. It was not clear whether this was because no events occurred in the placebo arm, or whether adverse events were not assessed in this group. We have therefore provided a narrative summary of the adverse events that were reported (including gastrointestinal disturbance and rash) in Table 5.
3. Adverse effects.
| Adverse event | Trial(s) | Reported data |
| Anaphylaxis | Ardehali 2008; Hemlin 1997; Leach 2008; Marchisio 1998; Thomsen 1989 | Without referring directly to anaphylaxis, 5 trials provided sufficient information to reasonably assume there were no such cases.
|
| Gastrointestinal upset | Chen 2013; Hemlin 1997; Mandel 1987; Mandel 1991; Thomsen 1989; van Balen 1996 |
|
| Skin rash or irritation | Mandel 1987; Mandel 1991; Thomsen 1989; van Balen 1996 |
|
| Mild sedation, irritability or both | Mandel 1987 |
|
| Ear drum perforation | Leach 2008 |
|
CI: confidence interval; RR: risk ratio
Two studies indicated that no adverse events occurred:
Møller 1990 reported that "no adverse events were reported".
Thomsen 1989 reported that "no unwanted side effects from the drug" were experienced.
Endo 1997, Podoshin 1990 and Puhakka 1985 did not report on adverse events. It is not clear whether this is because none occurred, or simply because they were not assessed and reported.
Receptive language skills
This outcome was not reported by any of the included studies.
Speech development or expressive language skills
This outcome was not reported by any of the included studies.
Cognitive development
This outcome was not reported by any of the included studies.
Psychosocial outcomes
This outcome was not reported by any of the included studies.
Listening skills
This outcome was not reported by any of the included studies.
Generic health‐related quality of life
This outcome was not reported by any of the included studies.
Parental stress
This outcome was not reported by any of the included studies.
Vestibular function
This outcome was not reported by any of the included studies.
Number of doctor‐diagnosed episodes of acute otitis media
Short‐term follow‐up (up to three months)
Two studies provided information on the number of participants who experienced at least one episode of acute otitis media over four weeks of follow‐up. The risk ratio for acute otitis media was 0.68 for those receiving antibiotics (95% CI 0.42 to 1.10; 9% versus 13%; 2 studies, 615 participants; I2 = 0%; Analysis 1.14; very low‐certainty evidence).
1.14. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 14: Episodes of acute otitis media
Oral antibiotics versus no treatment
Eight studies were included in this comparison (Ardehali 2008; Chen 2013; Ernston 1985; Healy 1984; Karlidag 2002; Manrique 1987; Marchisio 1998; Sundberg 1984). However, very limited data were available for our outcomes of interest.
Hearing ‐ return to normal hearing
Very short‐term follow‐up (< 6 weeks)
A single study assessed the proportion of children whose hearing had "returned to normal thresholds" after 10 days of antibiotic treatment. No definition of normal hearing was provided. The risk ratio was 4.70 in favour of antibiotics (95% CI 1.96 to 11.22; 52% versus 11%; 1 study, 91 participants; Analysis 2.1; very low‐certainty evidence).
2.1. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 1: Hearing returned to normal (very short‐term)
This outcome was not assessed at later time points.
Hearing ‐ hearing threshold
Short‐term follow‐up (up to three months)
Again, this outcome was assessed by a single study. The mean final hearing threshold after three months of follow‐up was ‐5.38 dB HL lower (better) for those who received antibiotics (95% CI ‐9.12 to ‐1.64; 1 study, 73 participants; Analysis 2.2; low‐certainty evidence).
2.2. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 2: Final hearing threshold (short‐term)
This outcome was not assessed at any other time points.
Disease‐specific quality of life
This outcome was not assessed by any of the included studies.
Adverse events ‐ anaphylactic reaction
This outcome was not specifically reported by any of the included studies. See details below regarding generic adverse event reporting.
Presence/persistence of OME
Short‐term follow‐up (up to three months)
Five studies provided data at this time point. Overall, the risk ratio for persistence of OME at up to three months was 0.64 for those receiving antibiotics (95% CI 0.50 to 0.80; 56% versus 87%; 6 studies, 542 participants; I2 = 72%; Analysis 2.3; low‐certainty evidence). As some data were adjusted to account for potential correlation between ears of the same individual, we conducted a sensitivity analysis to ensure that this did not affect the results. However, the overall effect estimates were similar when using different assumed correlation between the ears of the same individual (Analysis 2.4; Analysis 2.5).
2.3. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 3: Persistence of OME (short‐term)
2.4. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 4: Sensitivity analysis 1: persistence of OME (short‐term). ICC = zero
2.5. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 5: Sensitivity analysis 2: persistence of OME (short‐term). ICC = 1.0
No data are available for later time points.
Adverse events
We were unable to carry out any meta‐analysis of adverse events, as these were inconsistently reported across the studies. Data from Chen 2013 on the occurrence of vomiting are presented in Table 5 ‐ this was the only study to report any adverse effects.
Two studies indicated that no adverse events occurred:
Ardehali 2008 stated that "No subjects experienced complications during or after the study".
Marchisio 1998 stated, "No medication side effects were reported in any subject".
Five studies did not report adverse events (Ernston 1985; Healy 1984; Manrique 1987; Karlidag 2002; Sundberg 1984). It is not clear whether this is because none occurred, or simply because they were not assessed and reported.
Receptive language skills
This outcome was not reported by any of the included studies.
Speech development or expressive language skills
This outcome was not reported by any of the included studies.
Cognitive development
This outcome was not reported by any of the included studies.
Psychosocial outcomes
This outcome was not reported by any of the included studies.
Listening skills
This outcome was not reported by any of the included studies.
Generic health‐related quality of life
This outcome was not reported by any of the included studies.
Parental stress
This outcome was not reported by any of the included studies.
Vestibular function
This outcome was not reported by any of the included studies.
Number of doctor‐diagnosed episodes of acute otitis media
Short‐term follow‐up (up to three months)
One study assessed this outcome. The proportion of children who experienced one or more episodes of acute otitis media during four weeks of follow‐up was lower in the group who received antibiotics, but the confidence intervals were very wide, and the absolute effect was small (RR 0.40, 95% CI 0.08 to 2.01; 2% versus 5%; 1 study, 196 participants; Analysis 2.6; very low‐certainty evidence).
2.6. Analysis.

Comparison 2: Antibiotic versus no treatment, Outcome 6: Episodes of acute otitis media (short‐term)
All pre‐specified sensitivity analyses are reported in Table 6.
4. Sensitivity analyses.
| Outcome | Main analysis result (95% CI) | Sensitivity analysis | Sensitivity analysis result (95% CI) |
| Antibiotics versus placebo | |||
| 1.2 Hearing threshold: speech reception threshold (short‐term) | MD ‐2.58 (‐4.52 to ‐0.65) | Fixed‐effect model | MD ‐2.58 (‐4.52 to ‐0.65) |
| 1.6 Persistence of OME (short‐term) | RR 0.88 (0.78 to 1.00) | Exclusion of studies with any concerns over trustworthiness | RR 0.92 (0.85 to 1.00) |
| 1.6 Persistence of OME (short‐term) | RR 0.88 (0.78 to 1.00) | Fixed‐effect model | RR 0.90 (0.84 to 0.96) |
| 1.10 Persistence of OME (medium‐term) | RR 0.89 (0.68 to 1.17) | Exclusion of studies with any concerns over trustworthiness | RR 1.06 (0.73 to 1.53) |
| 1.10 Persistence of OME (medium‐term) | RR 0.89 (0.68 to 1.17) | Fixed‐effect model | RR 0.87 (0.70 to 1.09) |
| 1.14 Episodes of acute otitis media | RR 0.68 (0.42 to 1.10) | Fixed‐effect model | RR 0.67 (0.42 to 1.09) |
| Antibiotics versus no treatment | |||
| 2.3 Persistence of OME (short‐term) | RR 0.64 (0.50 to 0.80) | Fixed‐effect model | RR 0.60 (0.53 to 0.68) |
| 2.3 Persistence of OME (short‐term) | RR 0.64 (0.50 to 0.80) | Exclusion of studies at high risk of bias | RR 0.66 (0.50 to 0.86) |
CI: confidence interval; MD: mean difference; OME: otitis media with effusion; RR: risk ratio
Discussion
Summary of main results
Antibiotics compared to placebo
One study provided very low‐certainty evidence of an increase in the proportion of children with normal hearing at two months following antibiotic treatment. Two studies indicated that there may be a small difference in mean final hearing thresholds between those who received antibiotics or placebo, after four weeks of follow‐up. However, we have some concerns regarding the use of mean hearing thresholds to assess hearing in this condition (see below).
Persistence of OME after three months of follow‐up may be reduced for those who received antibiotics, compared to placebo. A similar effect size was seen after 6 to 12 months of follow‐up, but the confidence intervals were very wide and included the possibility of no effect. The evidence at this later time point was very uncertain.
The evidence on anaphylaxis was very uncertain, as few studies reported specifically on this outcome. However, antibiotics probably increase the risk of gastrointestinal disturbance, and may increase the risk of vomiting, abdominal pain and itching or rash. The evidence was very uncertain for other adverse effects (including diarrhoea and sedation or irritability).
The effect of antibiotics compared to placebo on episodes of acute otitis media was very uncertain.
Antibiotics compared to no treatment
Similar effects were seen when antibiotics were compared to no treatment. A single study indicated that there may be improvement in the number of children whose hearing returns to normal levels after using antibiotics, but the follow‐up was extremely short (10 days), and the evidence was very uncertain. One other study considered final hearing thresholds after three months of follow‐up, and found that there may be a small mean difference between those who received and did not receive antibiotics.
Antibiotics may reduce the proportion of children who have persistent OME after up to three months of follow‐up. It is very uncertain whether the use of antibiotics has an impact on the number of episodes of acute otitis media during three months of follow‐up. The evidence on adverse effects was also very uncertain. We did not identify any evidence on quality of life or developmental outcomes.
Overall completeness and applicability of evidence
Many of the studies included in this review specifically enrolled children who had symptoms of OME for at least three months. Therefore, we do not know whether this treatment may have similar effects in children with a shorter duration of disease. However, this is likely to be the appropriate population, as many practitioners would recommend a period of watchful waiting (to see if the symptoms of OME resolve) before considering any treatment.
A wide range of antibiotics were included across the studies in this review. The duration of treatment was also extremely varied, ranging from a 10‐day course of antibiotics up to six months of continuous treatment. We did not have sufficient data to determine if the efficacy (and harms) of different antibiotics or treatment strategies varied.
In keeping with other reviews in this suite, we noted that few studies reported our preferred outcome measure for hearing ‐ the number of children who returned to normal hearing. We have concerns that assessment of hearing using the mean difference in final hearing threshold (or mean change in hearing threshold) may not be the most appropriate way to assess hearing. OME has a high spontaneous resolution rate. Consequently, we would anticipate that the change in hearing threshold for most children will be similar across the groups, as many children will improve with or without treatment. Therefore, even if a subset of children had substantial benefit from the intervention, the overall mean difference between the two groups would appear to be small. When assessed using the mean difference, the marked benefit seen in a subgroup of participants is ‘diluted’ by the children who get better regardless of treatment. Therefore, an apparently small mean difference between the two groups may actually be consistent with a substantial change in the number of children in whom hearing returns to normal.
Most of the studies included in this review assessed outcomes at the end of the treatment period. However, it is not clear whether any effects seen immediately after discontinuation of antibiotics will persist in the longer term. Importantly, we did not assess whether the use of antibiotics has any impact on the need for further medical treatment, or the requirement for surgery in children with OME.
We intended to include studies where children had craniofacial anomalies, or conditions such as Down syndrome. However, the majority of studies specifically excluded children with these conditions (Ardehali 2008; Ernston 1985; Healy 1984; Hemlin 1997; Leach 2008; Mandel 1987; Mandel 1991; Marchisio 1998; Møller 1990; Podoshin 1990; van Balen 1996). The remaining studies did not state that children with these conditions were excluded, but none specifically recruited children with these high‐risk conditions. Therefore, we do not know whether the efficacy of antibiotics may differ for these children.
We included all studies, regardless of whether participants had previously received other treatment for OME, including ventilation tube insertion. However, we recognise that those who have received additional treatment may represent a subset of children with persistent OME, and that results may not be applicable to all children.
Quality of the evidence
We considered the certainty of all the evidence to be low or very low. Many of the studies had significant concerns or lack of clarity regarding the risk of bias. The majority of studies failed to give sufficient information on their randomisation and allocation concealment, therefore we had concerns over the possibility of differences in confounding variables between the two groups. We also had concerns about some studies regarding the potential for performance or detection bias, as participants, study personnel and outcome assessors were not always blinded to the group allocation.
Although we included 19 studies, many considered only a few of our pre‐specified outcomes of interest. Outcomes were also reported at a variety of time points, precluding meaningful meta‐analysis. Consequently, the number of participants included in many of the analyses was small, leading to wide confidence intervals and imprecision in the estimated effects.
Potential biases in the review process
We have attempted to minimise the potential for bias during the review process by adhering to the Cochrane Handbook for Systematic Reviews of Interventions throughout the conduct of this review (Higgins 2021). We conducted comprehensive searches, and ensured that study selection, data extraction and GRADE assessment were carried out by at least two independent authors, to ensure reproducibility of findings.
We acknowledge that there is little consensus on the definition of 'normal hearing'. Consequently, our selection of a hearing threshold of ≤ 20 dB HL as 'normal' was based on discussion between the author team, review of earlier studies and a pragmatic choice of outcome measure. However, we were as inclusive as possible with this outcome measure, and have included data where authors provided an alternative definition of normal hearing. If we had rigidly used a definition of ≤ 20 dB HL then the data included in this review would have been even more sparse.
Agreements and disagreements with other studies or reviews
The previous Cochrane Review on this topic came to similar conclusions ‐ that antibiotics may increase the resolution of OME, but the impact on hearing was uncertain, and they can be associated with adverse effects (Venekamp 2016). It should be noted that the previous review also included a number of trials that compared antibiotics to medical interventions (including antihistamines, decongestants, mucolytics and intranasal corticosteroids).
Authors' conclusions
Implications for practice.
The use of antibiotics compared to no treatment may slightly reduce the proportion of children with otitis media with effusion (OME) at up to three months of follow‐up. However, the overall impact of antibiotics on hearing is very uncertain. Adverse effects were poorly reported in the studies included in this review; however, it is worth noting that antibiotics may be associated with potentially harmful effects (Venekamp 2016). In addition, consideration should be given to antibiotic stewardship ‐ particularly where benefits from treatment are uncertain.
Implications for research.
This review forms part of a suite of five reviews that consider interventions for OME (Galbraith 2022; MacKeith 2022a; MacKeith 2022b; Mulvaney 2022a; Mulvaney 2022b). Here we present implications for research in this field, which are shared across the suite of reviews:
As OME is a fluctuating condition with high rates of resolution and recurrence, and a highly variable impact on children, clinical trials (and, in particular, randomised controlled trials) may not be the research design of choice. Instead, evidence may be better obtained from surgical or clinical registries (for example, see Schmalbach 2021) or prospective cohort studies, with the use of 'big data'. These data sets may also be used to help identify subgroups of children who are at greater risk of persistent disease or long‐term consequences of OME. A clearer understanding of possible subgroups of children is needed to better target interventions to those who need them most, whilst avoiding over‐treatment for those in whom spontaneous resolution is anticipated.
Adverse effects of interventions are important and should always be assessed. However, randomised controlled trials are also not the best method to consider these, especially when events are rare. Observational studies with longer follow‐up and larger numbers of participants are needed to provide more robust evidence on the frequency of side effects. It is important to note that the protocol, inclusion criteria and search strategy used for this review would have excluded these types of studies. It is therefore possible that evidence of this type may exist. With this in mind, we would advocate a review of observational data, to assess whether evidence regarding longer‐term outcomes and adverse events is already available. This may be particularly important when assessing harms from serious but rare adverse events.
It is encouraging that a core outcome set has been developed in this field (Bruce 2015; Liu 2020). Guidance on how to measure the different outcomes would also be helpful for future research.
Comparison of mean hearing thresholds is widely used in research to assess the impact of different interventions on hearing. However, this outcome measure risks underestimating the potential impact of interventions on hearing. Small changes in mean hearing thresholds may be consistent with a substantial improvement in the number of children whose hearing returns to normal ‐ particularly in a condition with a high spontaneous resolution rate. We would encourage researchers to assess hearing with the proportion of children in whom hearing returns to normal, in preference to mean hearing thresholds.
History
Protocol first published: Issue 4, 2022
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane ENT (closed in March 2023). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We are grateful to the 20 Cochrane Crowd screeners for screening 2443 records to identify 1130 possible RCTs, and reject 1313 references as not RCTs. We are particularly grateful to Bernardo Costa, Stefanie Rosumeck, Nikolaos Sideris, Susanna Wisniewski, Anna Resolver, Lai Ogunsola, Shammas Mohammed, Sarah Moore, Brian Duncan, Mohammad Aloulou, Ana‐Marija Ljubenković, Vighnesh D, Ahlam Jamal Alhemedi, Neetu Bhadra, Amin Sharifan, Abu Emmil Qawarizmi Bin Abu Sofian, Helen Ramsay, Dinah Amoah, Maike Scherhans and Natalya Clark for screening more than 200 records each.
Editorial and peer reviewer contributions
Cochrane ENT supported the authors in the development of this review prior to its closure in March 2023. Our grateful thanks to Jenny Bellorini, Managing Editor, and Samantha Cox, Information Specialist, from Cochrane ENT, without whom the development of these reviews would not have been possible.
The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Richard Rosenfeld, Department of Otolaryngology‐Head and Neck Surgery, State University of New York Downstate Health Sciences University, USA.
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service.
Editorial Assistant (conducted editorial policy checks, supported the editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service.
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service.
Peer reviewers (provided comments and recommended an editorial decision): David E Tunkel, Pediatric Otolaryngology, Johns Hopkins Medical Institutions, Baltimore, MD, USA (clinical/content review); Kelvin Kwofie, UPMC (clinical/content review); Jessica Scaife, Surgical Intervention Trials Unit, Nuffield Department of Surgical Sciences, University of Oxford (consumer review); Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review); Jo Platt, Cochrane Evidence Production and Methods Directorate (search review). One additional peer reviewer provided clinical/content peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
The search strategies were designed to identify all relevant studies for a suite of reviews on various interventions for otitis media with effusion.
| CENTRAL (CRS) | Cochrane ENT Register (CRS) | MEDLINE (Ovid) |
| 1 MESH DESCRIPTOR Otitis Media with Effusion EXPLODE ALL AND CENTRAL:TARGET 2 ("otitis media" adj6 effusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 3 (OME):TI,TO AND CENTRAL:TARGET 4 (Secretory otitis media):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 5 (Serous otitis media):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 6 (Middle‐ear effusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 7 (glue ear):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 8 (middle‐ear perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 9 MESH DESCRIPTOR Otitis Media AND CENTRAL:TARGET 10 (otitis media):TI,TO AND CENTRAL:TARGET 11 #9 OR #10 AND CENTRAL:TARGET 12 (((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis)):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 13 #11 AND #12 AND CENTRAL:TARGET 14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #13 AND CENTRAL:TARGET |
1 MESH DESCRIPTOR Otitis Media EXPLODE ALL AND INREGISTER 2 ("otitis media" OR OME OR "glue ear" OR middle‐ear effusion OR middle‐ear perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 3 #1 OR #2 4 (effusion or Recurrent or persistent or serous or secretory or perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND INREGISTER 5 #3 AND #4 |
1 exp Otitis Media with Effusion/ 2 ("otitis media" adj6 effusion).ab,ti. 3 OME.ti. 4 Secretory otitis media.ab,ti. 5 Serous otitis media.ab,ti. 6 Middle‐ear effusion.ab,ti. 7 Glue ear.ab,ti. 8 middle‐ear perfusion.ab,ti. 9 Otitis Media/ 10 otitis media.ti. 11 9 or 10 12 ((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis).ab,ti. 13 11 and 12 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 drug therapy.fs. 20 randomly.ab. 21 trial.ab. 22 groups.ab. 23 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24 exp animals/ not humans.sh. 25 23 not 24 26 14 and 25 |
| Embase (Ovid) | Web of Science (Web of knowledge) | Trial registries (CRS) |
| 1 exp secretory otitis media/ 2 ("otitis media" adj6 effusion).ab,ti. 3 OME.ti. 4 Secretory otitis media.ab,ti. 5 Serous otitis media.ab,ti. 6 Middle‐ear effusion.ab,ti. 7 glue ear.ab,ti. 8 middle‐ear perfusion.ab,ti. 9 otitis media/ 10 otitis media.ti. 11 9 or 10 12 ((effusion or Recurrent or persistent or serous or secretory or perfusion) adj3 otitis).ab,ti. 13 11 and 12 14 1 or 2 or 4 or 5 or 6 or 7 or 8 or 13 15 (random* or factorial* or placebo* or assign* or allocat* or crossover*).tw. 16 (control* adj group*).tw. 17 (trial* and (control* or comparative)).tw. 18 ((blind* or mask*) and (single or double or triple or treble)).tw. 19 (treatment adj arm*).tw. 20 (control* adj group*).tw. 21 (phase adj (III or three)).tw. 22 (versus or vs).tw. 23 rct.tw. 24 crossover procedure/ 25 double blind procedure/ 26 single blind procedure/ 27 randomization/ 28 placebo/ 29 exp clinical trial/ 30 parallel design/ 31 Latin square design/ 32 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33 exp ANIMAL/ or exp NONHUMAN/ or exp ANIMAL EXPERIMENT/ or exp ANIMAL MODEL/ 34 exp human/ 35 33 not 34 36 32 not 35 37 14 and 36 |
11 #10 AND #9 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 10 #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 9 TS=(randomised OR randomized OR randomisation OR randomisation OR placebo* OR (random* AND (allocat* OR assign*) ) OR (blind* AND (single OR double OR treble OR triple) )) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 8 (TI=(otitis media) ) AND TS=((effusion or Recurrent or persistent or serous or secretory or perfusion) NEAR/3 otitis) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 7 TOPIC: ((middle‐ear perfusion) ) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 6 TOPIC: ((glue ear) ) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 5 TOPIC: ((Middle‐ear effusion) ) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 4 TOPIC: ((Serous otitis media) ) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 3 TOPIC: ((Secretory otitis media) ) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 2 TITLE: (OME) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years 1 TOPIC: ("otitis media" NEAR/6 effusion) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
1 ("otitis media" OR OME OR "glue ear" OR middle‐ear effusion OR middle‐ear perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 2 (effusion or Recurrent or persistent or serous or secretory or perfusion):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 3 #1 AND #2 4 http*:SO AND CENTRAL:TARGET 5 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET 6 #4 OR #5 7 #3 AND #6 |
| ClinicalTrials.gov | ICTRP | |
| (EXPAND[Concept] "otitis media" OR EXPAND[Concept] "glue ear" OR middle‐ear ) AND (effusion OR Recurrent OR persistent OR serous OR secretory OR perfusion ) | Interventional Studies | (otitis media AND effusion) OR glue ear OR middle‐ear effusion OR middle‐ear perfusion |
Appendix 2. Tool for screening eligible studies for scientific integrity/trustworthiness
This screening tool has been developed by Cochrane Pregnancy and Childbirth. It includes a set of predefined criteria to select studies that, based on available information, are deemed to be sufficiently trustworthy to be included in the analysis.
| Criteria questions | Assessment | Comments and concerns | |
| High risk | Low risk | ||
| Research governance | |||
| Are there any retraction notices or expressions of concern listed on the Retraction Watch Database relating to this study? | Yes | No | |
| Was the study prospectively registered (for those studies published after 2010) If not, was there a plausible reason? | No | Yes | |
| When requested, did the trial authors provide/share the protocol and/or ethics approval letter? | No | Yes | |
| Did the trial authors engage in communication with the Cochrane Review authors within the agreed timelines? | No | Yes | |
| Did the trial authors provide IPD data upon request? If not, was there a plausible reason? | No | Yes | |
| Baseline characteristics | |||
| Is the study free from characteristics of the study participants that appear too similar? (e.g. distribution of the mean (SD) excessively narrow or excessively wide, as noted by Carlisle 2017) |
No | Yes | |
| Feasibility | |||
| Is the study free from characteristics that could be implausible? (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months) | No | Yes | |
| In cases with (close to) zero losses to follow‐up, is there a plausible explanation? | No | Yes | |
| Results | |||
| Is the study free from results that could be implausible? (e.g. massive risk reduction for main outcomes with small sample size)? | No | Yes | |
| Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)? | No | Yes | |
| For abstracts only: | |||
| Have the study authors confirmed in writing that the data to be included in the review have come from the final analysis and will not change? | No | Yes | |
Data and analyses
Comparison 1. Antibiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Normal hearing (as complete improvement in air‐bone gap in worst ear): short‐term | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.59 [3.51, 26.18] |
| 1.2 Hearing threshold: speech reception threshold (short‐term). Correction of variance assuming correlation coefficient of 0.5 | 2 | 499 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐4.52, ‐0.65] |
| 1.3 Sensitivity analysis: speech reception threshold: assuming correlation coefficient of 0.3 | 2 | 499 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐4.38, ‐0.78] |
| 1.4 Sensitivity analysis: speech reception threshold: assuming correlation coefficient of 0.7 | 2 | 499 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐4.64, ‐0.53] |
| 1.5 Hearing threshold: speech awareness threshold (short‐term) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6 Persistence of OME (short‐term) | 9 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.00] |
| 1.6.1 Persistence defined as effusion in one ear or both | 7 | 1208 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 1.6.2 Persistence defined as effusion both ears if bilateral at baseline, and in one/both ears if unilateral | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 1.6.3 Persistence defined as effusion in the worst ear | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 1.7 Sensitivity analysis: persistence (short‐term) including cases of acute otitis (Mandel 1987) | 9 | 1456 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.79, 0.96] |
| 1.7.1 Persistence defined as effusion in one ear or both | 8 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 1.7.2 Persistence defined as effusion in both ears (if bilateral at baseline) and in one or both ears (if unilateral) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 1.7.3 Persistence defined as effusion in the worst ear | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 1.8 Sensitivity analysis: persistence (short‐term) assuming ICC of 1.0 (complete correlation between ears) (Puhakka 1985) | 9 | 1445 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.97] |
| 1.8.1 Persistence defined as effusion in one ear or both | 8 | 1278 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 1.8.2 Persistence defined as effusion in both ears (if bilateral at baseline) and in one or both ears (if unilateral) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 1.8.3 Persistence defined as effusion in the worst ear | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 1.9 Sensitivity analysis: persistence (short‐term) assuming ICC of zero (no correlation between ears) (Puhakka 1985) | 9 | 1473 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 1.9.1 Persistence defined as effusion in one ear or both | 8 | 1306 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.82, 1.03] |
| 1.9.2 Persistence defined as effusion in both ears (if bilateral at baseline) and in one or both ears (if unilateral) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 1.9.3 Persistence defined as effusion in the worst ear | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.84] |
| 1.10 Persistence of OME (medium‐term) | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 1.10.1 Persistence defined as 'OME' in one or both affected ears | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.73, 1.53] |
| 1.10.2 Persistence defined as effusion in affected ear | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.61, 1.04] |
| 1.11 Sensitivity analysis: persistence of OME (medium‐term); defined as 'OME' or 'AOM without perforation' (Leach 2008) | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.25] |
| 1.11.1 Persistence defined as 'OME or AOM without perforation' in one or both affected ears | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.33] |
| 1.11.2 Persistence defined as effusion in affected ear | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.61, 1.04] |
| 1.12 Adverse event: eardrum perforation | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.18, 1.01] |
| 1.13 Adverse event: 'gastrointestinal' | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.14 Episodes of acute otitis media | 2 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.42, 1.10] |
1.12. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 12: Adverse event: eardrum perforation
1.13. Analysis.

Comparison 1: Antibiotic versus placebo, Outcome 13: Adverse event: 'gastrointestinal'
Comparison 2. Antibiotic versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Hearing returned to normal (very short‐term) | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [1.96, 11.22] |
| 2.2 Final hearing threshold (short‐term) | 1 | 73 | Mean Difference (IV, Random, 95% CI) | ‐5.38 [‐9.12, ‐1.64] |
| 2.3 Persistence of OME (short‐term) | 6 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.80] |
| 2.3.1 Analysis by child: persistence in any ear | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.75] |
| 2.3.2 Analysis by child: persistence in any affected ear | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.03] |
| 2.3.3 Analysis by ear. Adjusted for non‐independence, assuming ICC of 0.5 | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.64, 0.98] |
| 2.4 Sensitivity analysis 1: persistence of OME (short‐term). ICC = zero | 6 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.80] |
| 2.4.1 Analysis by child: persistence in any ear | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.75] |
| 2.4.2 Analysis by child: persistence in any affected ear | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.03] |
| 2.4.3 Analysis by ear. Adjusted for non‐independence, assuming ICC of 0 | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 2.5 Sensitivity analysis 2: persistence of OME (short‐term). ICC = 1.0 | 6 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.50, 0.79] |
| 2.5.1 Analysis by child: persistence in any ear | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.75] |
| 2.5.2 Analysis by child: persistence in any affected ear | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.03] |
| 2.5.3 Analysis by ear. Adjusted for non‐independence, assuming ICC of 1 | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.59, 0.99] |
| 2.6 Episodes of acute otitis media (short‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ardehali 2008.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 3 months of treatment and follow‐up An additional group received anti‐reflux medication. Data for this group were not extracted, as the intervention was not of relevance to this review. |
|
| Participants |
Setting: Conducted in Iran. No further details provided. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 2 to 12 years. Children with chronic otitis media with effusion which lasted 3 months or more. Refractory to 3 periods of antibacterial treatment. Confirmed by physical examination and type B tympanogram in at least 1 ear without clinical signs and signs of active infection. Exclusion criteria:
|
|
| Interventions |
Antibiotic group Co‐amoxiclav 40 mg/kg/day in 3 divided doses, max 750 mg/day Comparator No treatment |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: No other results assessed at 2‐week follow‐up |
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: “In a prospective randomized clinical trial study…” “The patients were randomly allocated to receive…” "...according to a computer‐generated randomization schedule" Comment: computer‐generated randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants were not blinded to their intervention; no placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “by two unique independent ENT surgeons blinded to subject group assignment.” Comment: outcome assessors were blinded to the interventions received by participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: full follow‐up is reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration is available for assessment. |
| Other bias | Low risk | Comment: no other concerns were noted. |
Balle 1990.
| Study characteristics | ||
| Methods | Parallel‐group, double‐blind randomised controlled trial with 4 weeks of treatment and a total of 12 months of follow‐up | |
| Participants |
Setting: Study conducted in Denmark. No details regarding recruitment. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: At least 3 months of type C2 or B tympanometry curves uni‐ or bilaterally, and not allergic to penicillin Exclusion criteria: None reported |
|
| Interventions |
Antibiotic group: 4 weeks treatment with amoxicillin and clavulanate potassium. Concentration not stated. Children aged 1 to 5 had 5 mL 3 times daily, children aged 6 to 10 had 7.5 mL 3 times daily. Comparator: Placebo was used, but no details on the nature of this were provided |
|
| Outcomes | No data are reported for any of the outcomes included in this review The only outcome described is the specific type of bacteria cultured from the nasopharynx |
|
| Funding sources | Quote: “We thank Astra Medical company for supplying the antibiotic and their kind support.” Pharmaceutical company funding |
|
| Declarations of interest | No declaration is made | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided on the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | No information is provided on any methods used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study states that this trial was "double blind" but there is no information on how blinding of study personnel was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For the outcomes in this paper, outcome measurement is unlikely to be influenced by the (possible) lack of blinding for study personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively few dropouts across the trial, and balanced between groups. Likely insufficient to cause bias in the reported results. |
| Selective reporting (reporting bias) | High risk | No protocol is available. Authors allude to an improvement in OME during the initial treatment phase, but do not report any data to support this claim. |
| Other bias | Low risk | No other concerns were identified. |
Chen 2013.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 8 to 12 weeks of treatment and follow‐up | |
| Participants |
Setting: Single‐centre, conducted in a university ENT Department in China between June 2009 and March 2011 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 3 to 14 years. Otitis media with effusion less than 3 months. Aural fullness, hearing loss or tinnitus; integrity, invagination or fluid level of tympanic membrane; type B or C2 tympanogram Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 42 randomised, n = 36 completed) Clarithromycin 15 mg/kg/day in 2 divided doses for 1 week, followed by low‐dose clarithromycin (5 to 8 mg/kg/day) in 4 divided doses until the tympanogram was type A. The low‐dose antibiotic was continued for 1 week after the effusion resolved, then stopped. The entire course was less than 12 weeks duration (range 5 to 12 weeks, mean 9.91 (SD 2.41) weeks). Two children in this group received azithromycin instead Comparator (n = 42 randomised, n = 37 completed) No treatment Background interventions administered to all participants Both groups received a topical glucocorticoid nasal spray for 12 weeks Treatment administered before entry into the trial All children appear to have undergone tympanocentesis at the start of the trial for study of bacterial colonisation in the ear |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: Detection of bacterial biofilm in the middle ear |
|
| Funding sources | The study was supported by grants from National Basic Research Program of China (2011CB504502), National Natural Science fund of China (30973306) and Key Nature Fund of Guangdong Province (8251008901000016) | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "involved subjects were randomly divided into two subgroups" Comment: no information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding methods used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: this was an open‐label study and participants were aware of their group allocation. No placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: there is no information to describe whether outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there are few dropouts and the numbers are relatively balanced between the 2 groups. This is probably insufficient to change the direction of effect seen in the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration is available with which to compare the reported outcomes. |
| Other bias | Low risk | Comment: no other concerns identified. |
Endo 1997.
| Study characteristics | ||
| Methods | Three‐arm, parallel‐group, randomised controlled trial with 8‐month follow‐up | |
| Participants |
Setting: Study conducted in Brazil. No further details provided. Sample size:
Unclear whether this is the number randomised or the number completed.
Participant (baseline) characteristics:
Inclusion criteria: Children with bilateral secretory otitis media. Diagnostic criteria, based on 3 parameters: clinical picture; otoscopy; and pure tone audiometry and/or tympanometry Exclusion criteria: not reported |
|
| Interventions |
Antibiotic group Sulfamethoxazole‐trimethoprim 20 mg/kg/day in a single night‐time dose for 30 days Placebo group No information is provided on the nature of the placebo |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
|
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes | Children over 2 years of age received antibiotics AND prednisolone, therefore we can only use the data for those aged ≤ 2 years in analysis Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “with 40 children being randomly chosen to enter the placebo group and 108 receiving clinical treatment”. Comment: no information is provided regarding the process for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details are reported on methods used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: participants were blinded as they received either active drug or placebo. There was no report, however, regarding whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: it was not reported whether outcome assessments were carried out blind to treatment assignment. A lack of blinding could affect the interpretation of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no details are provided regarding loss to follow‐up. It is unclear whether the authors are reporting full follow‐up, or whether they simply do not report details of those who failed to return for follow‐up, as the total number randomised to each intervention is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: very limited information on how the outcome 'resolution' of OME was assessed. Follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Ernston 1985.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 10 days of treatment and follow‐up | |
| Participants |
Setting: Single‐centre, conducted in a hospital setting in Sweden. Study dates not reported. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged less than 12 years. OME in one or both ears, diagnosed by otomicroscopy, showing fluid behind an intact ear drum and tympanometry disclosing a type B curve Unhealed at several examinations during a more than 3‐month period Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 46 randomised, n = 46 completed) Cefaclor 20 mg/kg twice daily for 10 days Watchful waiting (n = 45 randomised, n = 45 completed) Remained untreated awaiting surgery Treatment administered before entry into the trial At least 3 months of watchful waiting |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: Data are reported for long‐term relapse after treatment, but this is only available for children who initially ‘healed’, as other children received myringotomy ± VT insertion |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The children were randomly divided into two groups”. Comment: no information about random sequence generation. However, we note an unusual discrepancy in the gender balance between the 2 groups, with 28 boys and 18 girls in one group, and 28 girls and 17 boys in the other group. This seems unlikely to have occurred by chance alone, therefore may suggest a problem with randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding any methods used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo was used, this was an open‐label trial therefore participants were aware of their group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: there is no statement to suggest that outcome assessors were blinded. As no placebo was used we presume that this was an open trial, and outcome assessors were aware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: full follow‐up is reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available for comparison. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Healy 1984.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 4 weeks of treatment and follow‐up | |
| Participants |
Setting: Single‐centre, conducted in a university hospital in Boston, USA between September 1981 and August 1982 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 2 to 5 years with otitis media with effusion, present for more than 6 weeks. Defined as a positive pneumatic otoscopic exam and/or type B, C1 or C2 tympanogram. Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 100 randomised, n = 96 completed) Trimethoprim‐sulfamethoxazole (8 mg and 40 mg/kg/day respectively) in 2 divided doses. Administered in liquid preparation for 4 weeks. No treatment group (n = 100 randomised, n = 93 completed) Observation |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
|
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The author would simply call a disinterested person who would pull a previously randomly arranged card which would show the word either 'control' or 'antibiotic'". Comment: simple drawing of lots ‐ adequate method for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on whether or how group allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and study personnel were aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome assessors were aware of group allocation. Tympanometry and otoscopy were used for outcome assessment, and there may be some subjectivity in interpretation of these results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: limited number of dropouts, insufficient to cause a risk of bias in the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration is available for comparison. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Hemlin 1997.
| Study characteristics | ||
| Methods | Three‐arm, double‐blind, parallel‐group, randomised controlled trial with 10 days of treatment and 12 to 21 days follow‐up For this review, we compared those who received antibiotics (alone), to those with no treatment. Data on steroids are relevant for a separate review in this suite (Mulvaney 2022a). |
|
| Participants |
Setting: Single‐centre, conducted in a hospital ENT department in Sweden. No study dates reported. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 2 to 12 years. Unilateral or bilateral secretory otitis media of at least 3 months duration, confirmed by otomicroscopy and tympanometry. Immobile and pale eardrum on otomicroscopy and a type B tympanogram in at least one of the ears. Exclusion criteria:
|
|
| Interventions |
Antibiotics group (n = 61 completed) Liquid suspension of cefixime 20 mg/mL, given at a dose of 8 mg/kg per day in 2 divided doses for 10 days Steroids and antibiotics group (n = 59 completed) Received antibiotic as above, plus 6 mg betamethasone tablets given in a single dose on the morning of day 10 Placebo group (n = 20 completed) Placebo suspension and tablets of similar appearance |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: Some longer‐term outcomes reported, but only for those who were healed at early follow‐up |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment with cefixime or cefixime plus betamethasone or placebo was allocated at random with a ratio of 3:3:1." Comment: no information on generation of random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided regarding how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The drugs were dispensed double‐blind by a double‐dummy technique" Comment: participants and study personnel were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information regarding whether outcome assessors were blinded. Tympanometry and otomicroscopy were both used in assessment of the outcome, and may involve some subjectivity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: for the data used in this review, dropout was low and unlikely to affect the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration was available to compare. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Karlidag 2002.
| Study characteristics | ||
| Methods | Unblinded, parallel‐group randomised controlled trial with 8 weeks of treatment and follow‐up For this review, we compared those who received antibiotics (alone) to those with no treatment. Data on steroids are relevant for a separate review in this suite (Mulvaney 2022a). |
|
| Participants |
Setting: Single‐centre, conducted in Turkey between January and December 2001 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 2 to 12 years. Diagnosed with otitis media with effusion based on:
Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 20 randomised, n = 20 completed) Ampicillin/sulbactam 25 mg/kg/day, administered in 2 divided doses, orally for 8 weeks Steroids and antibiotics group (n = 20 randomised, n = 20 completed) Antibiotic as above, plus budesonide intranasal spray, 200 µg/day administered in 2 divided doses for 8 weeks No treatment group (n = 22 randomised, n = 22 completed) Active monitoring |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
|
|
| Funding sources | None reported | |
| Declarations of interest | Not reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: participants were "randomly allocated" into 3 groups. No information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided on any methods used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants were aware of their treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was an unblinded trial. We presume that the outcome assessors were also aware of treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: full follow‐up is reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Leach 2008.
| Study characteristics | ||
| Methods | Two‐arm, double‐blind, parallel‐group, randomised controlled trial with 24 weeks of treatment and follow‐up | |
| Participants |
Setting: Three Aboriginal communities in tropical northern Australia; recruitment between 1996 and 2001 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aboriginal infants aged less than 12 months ‐ enrolled as soon as possible after birth. OME diagnosed with:
Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 52 randomised, n = 51 completed) Amoxicillin 50 mg/kg/day in 2 divided doses. Administered for 24 weeks, or until bilateral normal middle ear status was detected at 2 consecutive monthly examinations (i.e. success). Mean duration of treatment 5.7 months. Placebo group (n = 52 randomised, n = 51 completed) Placebo of equivalent volume. Mean duration of treatment 5.2 months. |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study:
|
|
| Funding sources | The NHMRC and the Menzies School of Health Research | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated random number series was stratified by age at randomization (less than 6 months versus greater than 6 months) and allocation was randomized within blocks of 7 subjects”; “Participants were consecutively allocated a random number according to the sequence provided by the systems manager.” Comment: computerised randomisation method. |
| Allocation concealment (selection bias) | Low risk | Quote: “The use and size of block randomization was concealed from investigators until data collection was completed” Comment: adequate attempts to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Placebo was designed, manufactured and packaged by Institute of Drug Technology, Melbourne. Bottles were provided by the manufacturers of amoxicillin. Original amoxicillin labels were removed before applying the study label. Senior staff at the community clinics had access to the allocation via a locked box containing stapled double envelopes. Clinic staff were not involved in data collection. The biostatistician was provided with the codes and the allocation to either ‘A’ or ‘B’, but was unaware of whether ‘A’ or ‘B’ was amoxicillin or placebo.” Comment: participants and study personnel were unaware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Placebo was designed, manufactured and packaged by Institute of Drug Technology, Melbourne. Bottles were provided by the manufacturers of amoxicillin. Original amoxicillin labels were removed before applying the study label. Senior staff at the community clinics had access to the allocation via a locked box containing stapled double envelopes. Clinic staff were not involved in data collection. The biostatistician was provided with the codes and the allocation to either ‘A’ or ‘B’, but was unaware of whether ‘A’ or ‘B’ was amoxicillin or placebo.” “Allocation to placebo or amoxicillin, and the use and size of block randomization was concealed from investigators until data collection was completed. “ Comment: outcome assessors were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: limited dropout (2 in antibiotics group and 7 in the placebo group). This is unlikely to substantially impact on the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: a trial registration was identified for this study, although we note that this was retrospectively registered, after completion of the study. Therefore, we are unable to compare the reported outcomes with the pre‐specified analysis plan. |
| Other bias | High risk | Comment: during follow‐up, children with 2 consecutive normal examinations were discharged, and did not continue follow‐up for the full 6 months. Outcome data at 6 months may therefore underestimate the occurrence of OME ‐ as children may have relapsed during the follow‐up period, but investigators would have been unaware of this. |
Mandel 1987.
| Study characteristics | ||
| Methods | Three‐arm, double‐blind, parallel‐group, randomised controlled trial with 2 weeks of treatment, and main follow‐up at 2 and 4 weeks. Additional follow‐up for up to 3 months. One study arm received antibiotics plus antihistamine – data were not included in this extraction, as they are not of relevance to this review. |
|
| Participants |
Setting: Single‐centre, conducted in an outpatient setting at the Children's Hospital of Pittsburgh between July 1981 and October 1984 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Diagnosis of otitis media with effusion. Based on a decision tree algorithm, combining findings of a validated otoscopist with results of tympanometry and middle ear muscle reflex testing (Cantekin 1983). If tympanometry or middle ear muscle reflex testing could not be used then otoscopy alone was used. Exclusion criteria:
|
|
| Interventions |
Antibiotic and placebo group (n = 168 randomised, n = 155 completed) Liquid suspension of amoxicillin, 40 mg/kg per day in 3 divided doses, and a placebo of similar appearance and taste to decongestant‐antihistamine Placebo group (n = 172 randomised, n = 150 completed) Two placebos similar in appearance and taste to amoxicillin and decongestant‐antihistamine respectively, and containing the same inert ingredients There was another intervention group, antibiotics plus antihistamines; these data were not used in this review Background interventions administered to all participants If an acute symptomatic episode (i.e. one accompanied by fever, otalgia, or both) occurred, an antimicrobial agent other than amoxicillin (e.g. cefaclor or erythromycin‐sulfisoxazole) was administered for 10 days concurrently with the originally assigned decongestant‐antihistamine or its placebo. |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study:
|
|
| Funding sources | Funding from a national grant. Methods state that study drugs were “supplied by” Beecham Laboratories, but it is unclear whether this was a funding source for the trial, or simply where the medication was obtained | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were then grouped according to age (7 to 23 months, 2 to 5 years, or 6 to 12 years), duration of otitis media with effusion (less than four weeks, four to eight weeks, more than eight weeks, or unknown), and whether an antimicrobial agent had been administered during the preceding two months for the otitis media present at entry. The stratification scheme thus resulted in 24 subgroups. Within each subgroup, subjects were randomly assigned in a double‐blind fashion (in blocks of three) to one of the following three groups…” Comment: actual method of randomisation is not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information regarding how or whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: authors report that participants were blinded. Unclear whether study personnel were all blinded to group allocations, and some study visits were conducted separately (and prior) to outcome assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Ninety‐two percent of the observations were made by one of us (E.M.M.), who was blinded to the subjects’ treatment groups.” “At 4 weeks, coherence [between tympanometry and otoscopy findings] for the placebo‐treated group was 88%, which was significantly higher than coherence values for the two antibiotic‐treated groups (P= .012). Although the observers were blinded to treatment assignments, some unknown factors such as reported side effects or conversations with parents might have induced clues about the assignment, thus influencing the observer.” Comment: although blinding was reported, concerns have been raised over the adequacy of blinding, due to differences in outcome data between the intervention and comparator arms. Therefore, we considered that this trial may be at risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 6% loss to follow‐up, balanced across groups (from data in Cantekin 1991). Unlikely to significantly affect results. |
| Selective reporting (reporting bias) | High risk | Comment: no protocol available to assess. Cantekin 1991 reports a different cure rate using the same data as Mandel 1987, raising concerns that data were selectively reported. |
| Other bias | High risk | Comment: potential detection bias through a very short period of follow‐up and through the use of a diagnostic algorithm that relied mainly on otoscopic rather than more objective tympanometric measurements. |
Mandel 1991.
| Study characteristics | ||
| Methods | Four‐arm, double‐blind, parallel‐group, randomised controlled trial with 2 weeks of treatment and 4 weeks of follow‐up | |
| Participants |
Setting: Conducted in the USA between July 1984 and December 1987 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 7 months to 12 years. Otitis media with effusion and no symptoms of AOM (e.g. otalgia or fever). Based on a decision tree algorithm, combining findings of a validated otoscopist with results of tympanometry and middle ear muscle reflex testing (Cantekin 1983). If tympanometry or middle ear muscle reflex testing could not be used then otoscopy alone was used. Exclusion criteria:
|
|
| Interventions |
Erythromycin‐sulfisoxazole group (n = 84 randomised) 50 mg/kg/day erythromycin and 150 mg/kg/day sulfisoxazole in 4 divided doses for 2 weeks Cefaclor group (n = 83 randomised) 40 mg/kg/day in 3 divided doses for 2 weeks Amoxicillin group (n = 83 randomised) 40 mg/kg/day in 3 divided doses for 2 weeks Placebo group (n = 81 randomised) Three placebos were prepared, colour matched to the different drugs. Participants in this group received one of the 3 placebos. |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: No other results assessed at 2‐week follow‐up |
|
| Funding sources | NIH, Eli Lilly (pharmaceutical funding) and Ross Laboratories (industry funded, in part) | |
| Declarations of interest | Not reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Within each subgroup, subjects were randomly assigned, in blocks of four…” Comment: no information on generation of random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding whether or how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The medication assigned was unknown to the study physician and to the parent” Comment: placebo was used to maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The medication assigned was unknown to the study physician and to the parent” Comment: outcomes were assessed by blinded physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: few dropouts, which we consider insufficient to introduce significant bias in the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Manrique 1987.
| Study characteristics | ||
| Methods | Four‐arm, parallel‐group, randomised controlled trial with 3 months of treatment and follow‐up Note: data were only extracted for the 2 groups that provided a relevant comparison for this review. Additional groups received either no treatment, or antibiotics plus topical nasal antiseptic/decongestant. |
|
| Participants |
Setting: Conducted in Spain Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Children with unilateral or bilateral OME for at least 3 months Exclusion criteria: None stated |
|
| Interventions |
Antibiotic group: Amoxicillin 50 mg/kg/day for 8 days (n = 31 ears) Comparator: No treatment (n = 21 ears) Background interventions given to all participants: Participants in both groups received a daily dose of a decongestant syrup (chlorhydrate ambroxol, dose‐dependent on age) for the duration of the trial |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: No other outcomes were assessed |
|
| Funding sources | None reported | |
| Declarations of interest | No declaration is made | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No report of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was used for the amoxicillin, therefore we presume that participants were aware of their treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no report of whether blinding was used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition was reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | From the translation, the trial does not appear to be reported in great detail. Therefore, we are unable to assess additional risks of bias. |
Marchisio 1998.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 2 weeks of treatment and 8 weeks of follow‐up | |
| Participants |
Setting: Multicentre study conducted in 11 primary schools in Italy, during the winter months (October to January), from 1993 to 1995 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged 5 to 7 years. Children attending the first year of primary school diagnosed with otitis media with effusion, which persisted for at least 3 months. Diagnosed with otoscopy and tympanometry. OME was defined as asymptomatic middle ear effusion, demonstrated by an abnormal appearance of the tympanic membrane, diffusely opaque, with impaired mobility or presence of air‐fluid levels associated with a flat, type B tympanometric curve. Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 58 randomised, n = 52 completed) Ceftibuten 9 mg/kg/day in one daily dose for 14 days Comparator (n = 62 randomised, n = 59 completed) Nasal saline drops (no information on duration or frequency of use) |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
|
|
| Funding sources | Work was supported in part by Recordati SpA, Italy, which supplied Ceftibuten Isocef | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “One hundred and twenty children were randomised” Comment: no information on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided on how or whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants were aware of their allocated intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Investigators were blinded to treatment assignments and patients and parents were asked not to discuss medications or duration of treatment with investigators” Comment: outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: relatively few dropouts, probably insufficient to introduce bias in the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Møller 1990.
| Study characteristics | ||
| Methods | Two‐arm, double‐blind, parallel‐group randomised controlled trial with 14 days of treatment and 1 month of follow‐up | |
| Participants |
Setting: Single‐centre, conducted in an outpatient setting in a hospital in Norway. Study dates not reported. Sample size:
Participant (baseline) characteristics:
Inclusion criteria:
Diagnosed by otomicroscopy, tympanometry and pure tone hearing tests Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 69 completed) Erythromycin 50 mg/kg/day in 2 divided doses for 14 days Placebo group (n = 72 completed) Placebo treatment |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: Pure tone hearing tests were performed, but not fully reported, and only reported according to ears with OME and ears without. No comparison of hearing in groups with and without antibiotics. |
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information is provided regarding randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding whether or how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The drugs were administered double blind… dispensed by the hospital pharmacist in two daily doses…” Comment: participants were blinded to their allocated intervention. As the only interaction with study personnel (prior to outcome assessment) was at the point of treatment allocation, we also consider the study personnel to have been blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information is provided regarding whether outcome assessors were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: relatively low dropout rate, likely insufficient to introduce bias in the results. |
| Selective reporting (reporting bias) | High risk | Comment: no protocol is available with which to compare the reported outcomes. Limited data are reported on hearing tests, precluding comparison of the 2 groups, despite this outcome being assessed and recorded. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Podoshin 1990.
| Study characteristics | ||
| Methods | Three‐arm, double‐blind, parallel‐group, randomised controlled trial with 2 weeks of treatment and 2 months follow‐up | |
| Participants |
Setting: Single‐centre, conducted in an ENT department in Israel between September 1987 and December 1988 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Aged greater than 4 years. OME of at least 2 month duration, who had received no previous treatment for OME. Diagnosis made by pneumo‐otoscopy using a Welch Allyn halogen illuminated otoscope plus presence of a flat tympanogram (type B). Exclusion criteria:
|
|
| Interventions |
Antibiotic plus placebo group (n = 49 completed) Amoxicillin 50 mg/kg/day plus placebo Antibiotics plus steroids group (n = 50 completed) As above plus 1 mg/kg prednisolone, reduced by 5 mg every 2 days, therefore tapering course for a total of 14 days. Tablets of prednisolone were pulverised and placed in unmarked gelatin capsules. Placebo group (n = 37 completed) Two placebos of lactose powder placed in capsules that were identical to those containing the pulverised prednisolone |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
|
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were treated randomly by our directions.” Comment: no further information about generation of a random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided regarding how or whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants stated to be blinded. However, no placebo seems to have been used for the antibiotic (only for the prednisolone). The attrition rate was much higher (13%) in the placebo‐only group compared with either the antibiotic and placebo group (2%) or the prednisolone and placebo group (0%). This raises the possibility that participants may have been aware that they were not taking any active treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information is provided regarding whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: substantial dropout in the placebo group, which may be sufficient to introduce bias in the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
Puhakka 1985.
| Study characteristics | ||
| Methods | Three‐arm, parallel‐group, randomised controlled trial with 6 or 10 days of treatment and 8 weeks of follow‐up | |
| Participants |
Setting: Single‐centre, conducted in an ENT department in a hospital in Finland. Study dates not reported. Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Children suffering from otitis media with effusion. Diagnosed by examination and if necessary otomicroscopy. Exclusion criteria: Acute otitis media within the preceding 3 months |
|
| Interventions |
Antibiotic group (n = 22 children (35 ears) randomised) 6 mg trimethoprim and 18.5 mg sulfadiazine/kg/day in 2 divided doses for 10 days plus placebo Antibiotics and steroids group (n = 29 children (47 ears) randomised) As above plus 1 mg/kg/day of oral prednisolone, divided into 3 doses and given as a decreasing dose for 6 days Comparator: (n = 24 children (40 ears) randomised) Two placebo tablets Background interventions administered to all participants Myringotomy was conducted on all affected ears at the first visit |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study: Bacterial cultures of middle ear fluid |
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Children were randomly allocate (sic) to three therapy groups” Comment: no information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information regarding whether or how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this is described as a double‐blind study, but it is not clear who was blinded. It is possible that participants were blinded, but that study personnel were aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Follow‐up examinations were always carried out by the ENT specialist who had examined the child initially”. Comment: there is no description of outcome assessors being blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available for comparison. |
| Other bias | High risk | Comment: randomisation occurred at the level of the child, but results are reported at the level of the individual ear. No description of correlation between ears, and we cannot determine whether an individual child had cure in both ears or only one ear. |
Sundberg 1984.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group randomised controlled trial | |
| Participants |
Setting: Single‐centre study from Sweden Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Children aged from 1.5 to 11 years with unilateral or bilateral OME for at least 3 months. OME was diagnosed in the presence of a non‐purulent effusion behind an intact tympanic membrane on otomicroscopy, and the presence of a type B curve at tympanometry. All children were awaiting surgery (myringotomy) for OME Exclusion criteria: None reported |
|
| Interventions |
Antibiotics: Erythromycin ethylsuccinate 20 to 30 mg/kg twice daily for 10 days Control group: No treatment |
|
| Outcomes | No data are reported for any of the outcomes included in this review The only outcome described is the specific type of bacteria cultured from the nasopharynx |
|
| Funding sources | No funding was reported | |
| Declarations of interest | No declaration was made | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "At this stage every second consecutive child in the care of each otologist separately was allotted [sic] to the test group". Comment: quasi‐randomisation. |
| Allocation concealment (selection bias) | High risk | Quote: "At this stage every second consecutive child in the care of each otologist separately was allotted [sic] to the test group". Comment: allocation was entirely predictable, due to alternate allocation to each group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no placebo was used. Participants would have been aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: for the outcomes included in this study (identification of nasopharyngeal pathogens), risk of detection bias is likely to be low, despite the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: full follow‐up is reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was identified. |
| Other bias | Low risk | Comment: no other concerns were identified. |
Thomsen 1989.
| Study characteristics | ||
| Methods | Two‐arm, double‐blind, parallel‐group, randomised controlled trial with 1 month of treatment and 12 months of follow‐up | |
| Participants |
Setting: Single‐centre, conducted in a hospital in Denmark between June 1984 and June 1986 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: At least 3 months of unilateral or bilateral otitis media with effusion. Type C2 or B tympanometry curves. Exclusion criteria: Allergy to penicillin |
|
| Interventions |
Antibiotic group (n = 131 randomised, n = 109 completed) Amoxicillin and clavulanate potassium with 125 mg amoxicillin and 31.25 mg clavulanate potassium per 5 mL. Dose 5 mL 3 times daily (if aged 1 to 5) or 7.5 mL 3 times daily (if aged 6 to 10). Administered for 1 month. Placebo group (n = 133 randomised, n = 111 completed) No further details provided Background interventions administered to all participants In patients with bilateral disease, a ventilation tube was inserted in the right ear, and the left ear was included in the study |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study:
|
|
| Funding sources | Not reported | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “a double‐blind, randomized, placebo controlled clinical trial…” Comment: no further details on methods used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details are provided regarding whether or how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: stated to be double‐blind and placebo used. Participants were presumably blinded to intervention, but unclear if this extends to study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: limited dropout, balanced across groups, and insufficient to introduce bias in the results. Some concern that dropout may have been related to intervention (9 in placebo had concomitant infection, compared to 3 in intervention group), but not a large number of participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol or trial registration is available with which to compare the reported outcomes. |
| Other bias | Low risk | Comment: no other concerns identified. |
van Balen 1996.
| Study characteristics | ||
| Methods | Two‐arm, parallel‐group, randomised controlled trial with 2 weeks of treatment and follow‐up | |
| Participants |
Setting: Conducted in a general practice setting in the Netherlands between December 1992 and August 1994 Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Children with bilateral otitis media with effusion for at least 3 months. Presence of fluid in the middle ear cavity behind an intact tympanic membrane, based on tympanometry findings by the GP with type B or C2 curves Exclusion criteria:
|
|
| Interventions |
Antibiotic group (n = 82 randomised, n = 79 completed) Suspension of co‐amoxiclav 20 mg/kg/day amoxicillin plus 5 mg/kg/day clavulanic acid in 3 divided doses for 14 days Placebo group (n = 80 randomised, n = 74 completed) Placebo suspension with same colour and taste Treatment administered before entry into the trial Watchful waiting for 3 months to ensure OME persistence Background interventions administered to all participants One drop of oxymetazoline 0.25% (decongestant) 3 times daily |
|
| Outcomes |
Primary outcomes relevant to this review:
Secondary outcomes relevant to this review:
Other outcomes reported in the study:
|
|
| Funding sources | Study drug and placebo were supplied by SmithKline Beecham | |
| Declarations of interest | None reported | |
| Notes |
Research integrity checklist:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The suspensions were dispensed to participating general practitioners in a double‐blind fashion with computerised four‐block randomisation". Comment: adequate method of randomisation reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on allocation concealment to make a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study groups were randomised to treatment with a suspension of co‐amoxiclav, at a daily dose of 20 mg/kg amoxicillin with 5 mg/kg clavulanate potassium, or a placebo suspension with the same colour and taste”. “The suspensions were dispensed to participating general practitioners in a double‐blind fashion with computerised four‐block randomisation. Throughout the study, doctor and patient remained blinded.” Comment: adequate methods to ensure blinding of participants and study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All tympanograms were also classified by a second well‐trained general practitioner (FvB). In cases of disagreement between the study doctor and FvB, a team of experts was consulted.” “Throughout the study, doctor and patient remained blinded.” Comment: outcome assessors were blinded to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 9 of 162 children did not return for follow‐up (5%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol is available with which to compare the reported outcomes. |
| Other bias | High risk | Comment: follow‐up is inadequate to allow appropriate comparison of antibiotics and no intervention. Outcomes reported at this stage may be more likely to favour the active intervention, as insufficient time has elapsed to allow for spontaneous resolution. |
ENT: ear, nose and throat; NIH: National Institutes of Health; OME: otitis media with effusion; PTA: pure tone average; SD: standard deviation; URTI: upper respiratory tract infection; VT: ventilation tube
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berman 1990 | INTERVENTION: treatment with steroids and is relevant for another review in this suite (Mulvaney 2022a) |
| Bernard 1991 | INTERVENTION: treatment with ventilation tubes and is relevant for another review in this suite (MacKeith 2022b) |
| Choung 2008 | INTERVENTION: treatment with steroids, and is relevant for another review in this suite (Mulvaney 2022a) |
| Corwin 1986 | POPULATION: participants were children who had a persistent effusion, 1 month after an isolated episode of acute otitis media, not children with OME |
| Daly 1991 | INTERVENTION: participants received a combined intervention of antibiotics and steroids |
| de Castro 1982 | ALLOCATION: not randomised |
| Donaldson 1990 | COMPARATOR: both groups received different doses of the same antibiotic |
| Eiden 1997 | STUDY DESIGN: commentary article, not an RCT |
| Ferrara 2005 | PARTICIPANTS: had recurrent acute otitis media not OME |
| Fujita 1994 | ALLOCATION: not randomised |
| Gaskins 1982 | PARTICIPANTS: wrong patient population (children with recurrent acute otitis media) |
| Gasper 2003 | STUDY DESIGN: narrative review, not an RCT |
| Gibson 1996 | ALLOCATION: not randomised |
| Giebink 1990 | PARTICIPANTS: children with effusion after an episode of acute otitis media, not OME |
| Hozawa 2001 | ALLOCATION: not randomised |
| Iino 1989 | ALLOCATION: not randomised |
| Iino 2001 | ALLOCATION: not randomised |
| Kobayashi 2001 | ALLOCATION: not randomised |
| Kuriyama 1980 | ALLOCATION: not randomised |
| Leonetti 1988 | STUDY DESIGN: commentary article, not an RCT |
| Marks 1981 | COMPARATOR: antibiotics are compared to a decongestant preparation containing brompheniramine maleate, dextromethorphan and phenylephrine |
| Paradise 1997 | ALLOCATION: not randomised |
| Parlea 2012 | ALLOCATION: not randomised |
| Perrin 1974 | PARTICIPANTS: had acute otitis media, not OME |
| Persico 1978 | ALLOCATION: not randomised |
| Principi 1989 | PARTICIPANTS: wrong patient population |
| Roark 1997 | PARTICIPANTS: wrong patient population |
| Rohail 2006 | INTERVENTION: a variety of different interventions were used in this trial, including some antibiotics, decongestants, steroids and antihistamines |
| Schloss 1988 | PARTICIPANTS: effusion persisting after acute otitis media, not OME |
| Schwartz 1982 | PARTICIPANTS: had recurrent acute otitis media, not OME |
| Schwartz 1982a | PARTICIPANTS: had recurrent acute otitis media, not OME |
| Shubich 1996 | ALLOCATION: not randomised |
| Smales 1992 | ALLOCATION: not randomised |
| Stenstrom 2005 | ALLOCATION: not randomised |
| Tracy 1995 | PARTICIPANTS: had recurrent acute otitis media, not OME |
| van Balen 1997 | STUDY DESIGN: commentary article, not an RCT |
| Varsano 1985 | PARTICIPANTS: had recurrent acute otitis media, not OME |
| Velepic 2011 | INTERVENTION: treatment with ventilation tubes and is relevant for another review in this suite (MacKeith 2022b) |
| Yeldandi 2001 | COMPARISON: co‐interventions were not identical across the 2 study arms |
| Yin 2002 | COMPARISON: wrong comparator |
| Zocconi 1994 | ALLOCATION: not randomised |
OME: otitis media with effusion; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Koay 1998.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Unable to obtain full text |
Tawfik 2002.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Unable to obtain full text |
Differences between protocol and review
In our protocol we planned to use the Trustworthiness Tool developed by Cochrane Pregnancy and Childbirth to determine which studies would be included in the main analyses (Mulvaney 2022b). As described in the text, we used this tool to assess the studies, but did not use it to determine whether a study should be included in the main analysis.
Contributions of authors
Caroline A Mulvaney: drafted the protocol. Screened the search results and selected studies, conducted data extraction, carried out statistical analyses and GRADE assessment. Drafted the text of the review.
Kevin Galbraith: drafted the protocol. Screened the search results and selected studies, conducted data extraction, carried out statistical analyses and GRADE assessment. Drafted the text of the review.
Katie Webster: screened the search results and selected studies, conducted data extraction and carried out statistical analyses. Drafted the text of the review.
Mridul Rana: screened the search results, conducted data extraction, and reviewed and edited the text of the review.
Rachel Connolly: conducted data extraction and reviewed and edited the text of the review.
Tal Marom: reviewed the protocol, reviewed the analyses, and reviewed and edited the text of the review.
Mat Daniel: reviewed the protocol, reviewed the analyses, and reviewed and edited the text of the review.
Roderick P Venekamp: co‐wrote and edited the protocol, conducted data extraction, reviewed the analyses, and reviewed and edited the text of the review.
Anne GM Schilder: co‐wrote and edited the protocol. Reviewed the analyses and reviewed and edited the text of the review.
Samuel MacKeith: drafted the protocol. Screened the search results and selected studies. Provided clinical guidance throughout the review process. Reviewed the analyses and reviewed and edited the text of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
Declarations of interest
Caroline A Mulvaney: none known.
Kevin Galbraith: none known.
Katie Webster: none known.
Mridul Rana: no relevant interests; core surgical trainee, Oxford University Hospitals.
Rachel Connolly: National Institute for Health and Care Excellence (employment: systematic reviewer on the upcoming NICE guideline on otitis media with effusion in under 12s).
Tal Marom: no relevant interests; attending otolaryngologist.
Mat Daniel: Aventa Med (stock; consultant); Nottingham University Hospitals NHS Trust (employment: ENT consultant); has published research papers relevant to the interventions in the work; co‐author of the TARGET trial.
Roderick P Venekamp: no relevant interests; works as a GP; editorial board member of Cochrane ARI and Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Joint Co‐ordinating Editor of Cochrane ENT until April 2020, but had no role in the editorial process for this review; treats patients with OME in her NHS practice; her evidENT team at the UCL Ear Institute is supported by the National Institute of Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC), with research projects being supported by the NIHR, Wellcome Trust, RNiD, ENT UK and industry; National Specialty Lead for the NIHR Clinical Research Network ENT and Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Research Initiative; in her role as Director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, she advises CRO, biotech and pharma companies in the hearing field on clinical trial design and delivery.
Samuel MacKeith: ENT private practice (employment); sees patients with general ENT problems in NHS and private practice; Assistant Co‐ordinating Editor of Cochrane ENT 2020 to 2023, but had no role in the editorial process for this review.
New
References
References to studies included in this review
Ardehali 2008 {published data only}
- Ardehali MM, Seraj JM, Asiabar MK, Adibi H. The possible role of gastroesophageal reflux disease in children suffering from chronic otitis media with effusion. Acta Medica Iranica 2008;46(1):33-7. [CENTRAL: CN-00708224] [EMBASE: 351792703] [Google Scholar]
Balle 1990 {published data only}
- Balle VH, Stangerup SE, Sederberg-Olsen J, Thomsen J, Vejlsgaard R. Amoxicillin/clavulanate treatment in secretory otitis media. Bacteriological findings in the nasopharynx. Acta Oto-laryngologica 1990;110(3-4):274-8. [CENTRAL: CN-00071274] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen K, Wu X, Jiang G, Du J, Jiang H. Low dose macrolide administration for long term is effective for otitis media with effusion in children. Auris, Nasus, Larynx 2013;40(1):46-50. [CENTRAL: CN-00880075] [PMID: ] [DOI] [PubMed] [Google Scholar]
Endo 1997 {published data only}
- Endo LH, Antunes AB, Vidolin C, Bilecki MM, Magalhaes KVB. Secretory media otitis: clinical treatment vs placebo [Otite media secretora: tratamento clinco versus placebo]. Revista Brasileira de Otorrinolaringologia 1997;63(2):116-9. [CENTRAL: CN-00316174] [Google Scholar]
Ernston 1985 {published data only}
- Ernstson S, Anari M. Cefaclor in the treatment of otitis media with effusion. Acta Oto-laryngologica. Supplementum 1985;424:17-21. [CENTRAL: CN-00040675] [PMID: ] [DOI] [PubMed] [Google Scholar]
Healy 1984 {published data only}
- Healy GB. Antimicrobial therapy of chronic otitis media with effusion. International Journal of Pediatric Otorhinolaryngology 1984;8(1):13-7. [CENTRAL: CN-00036115] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hemlin 1997 {published data only}
- Hemlin C, Carenfelt C, Papatziamos G. Single dose of betamethasone in combined medical treatment of secretory otitis media. Annals of Otology, Rhinology, and Laryngology 1997;106(5):359-63. [CENTRAL: CN-00139646] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Papatziamos G, Hemlin C, Carenfelt C. Recent Advances in Otitis Media. Kugler Publications, Amsterdam/New York, 1994. [Google Scholar]
Karlidag 2002 {published data only}
- Karlidag T, Kaygusuz I, Gok U, Yalcin S, Keles E, Ozturk L. The efficacy of combining antibiotic treatment with topical intranasal steroid administration in the treatment of chronic otitis media with effusion [Efuzyonlu otitis media tedavisinde antibiyotik ile birlikte intranazal steroid kullaniminin etkinligi]. Kulak Burun Bogaz Ihtisas Dergisi: KBB [Journal of Ear, Nose, and Throat] 2002;9(4):257-62. [CENTRAL: CN-00411239] [PMID: ] [PubMed] [Google Scholar]
Leach 2008 {published data only}
- Leach AJ, Morris PS, Mathews JD, Hayhurst B, Koops H, Melder A, et al. Compared to placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatrics 2008;8:23. [CENTRAL: CN-00639992] [EMBASE: 2008359321] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00539149. Long-term antibiotics for treatment and prevention of otitis media in aborignal children [Amoxycillin versus placebo for resolution of otitis media with effusion and prevention of acute otitis media with perforation in aboriginal infants: a randomised controlled trial]. https://clinicaltrials.gov/show/NCT00539149 (first received 4 October 2007). [CENTRAL: CN-00652606]
Mandel 1987 {published data only}
- Cantekin EI, McGuire TW, Griffith TL. Antimicrobial therapy for otitis media with effusion ('secretory' otitis media). JAMA 1991;266(23):3309-17. [CENTRAL: CN-00079882] [PMID: ] [PubMed] [Google Scholar]
- Mandel EM, Rockette HE, Bluestone CD, Paradise JL, Nozza RJ. Efficacy of amoxicillin with and without decongestant-antihistamine for otitis media with effusion in children. Results of a double-blind, randomized trial. New England Journal of Medicine 1987;316(8):432-7. [CENTRAL: CN-00046384] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mandel 1991 {published data only}
- Mandel EM, Rockette HE, Paradise JL, Bluestone CD, Nozza RJ. Comparative efficacy of erythromycin-sulfisoxazole, cefaclor, amoxicillin or placebo for otitis media with effusion in children. Pediatric Infectious Disease Journal 1991;10(12):899-906. [CENTRAL: CN-00080817] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manrique 1987 {published data only}
- Manrique MJ, Hernández J, Huarte A, Rivera R, García-Tapia R. Treatment of secretory otitis media with Ambroxol [Tratamiento de la otitis serosa con Ambroxol]. Acta Pediatrica Española 1987;45(1):17-20. [Google Scholar]
Marchisio 1998 {published data only}
- Marchisio P, Principi N, Passali D, Salpietro DC, Boschi G, Chetrì G, et al. Epidemiology and treatment of otitis media with effusion in children in the first year of primary school. Acta Oto-Laryngologica 1998;118(4):557-62. [CENTRAL: CN-00154484] [EMBASE: 1998254893] [PMID: ] [DOI] [PubMed] [Google Scholar]
Møller 1990 {published data only}
- Moller P, Dingsor G. Otitis media with effusion: can erythromycin reduce the need for ventilating tubes? Journal of Laryngology and Otology 1990;104(3):200-2. [CENTRAL: CN-00067688] [PMID: ] [DOI] [PubMed] [Google Scholar]
Podoshin 1990 {published data only}
- Podoshin L, Fradis M, Ben-David Y, Faraggi D. The efficacy of oral steroids in the treatment of persistent otitis media with effusion. Archives of Otolaryngology-Head & Neck Surgery 1990;116(12):1404-6. [CENTRAL: CN-00071613] [PMID: ] [DOI] [PubMed] [Google Scholar]
Puhakka 1985 {published data only}
- Puhakka H, Haapaniemi J, Tuohimaa P, Ruuskanen O, Eskola J. Peroral prednisolone in the treatment of middle-ear effusion in children: a double-blind study. Auris, Nasus, Larynx 1985;12 Suppl 1:S268-71. [CENTRAL: CN-00043250] [EMBASE: 16731329] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sundberg 1984 {published data only}
- Sundberg L, Cederberg A, Eden T, Ernstson S. The effect of erythromycin on the nasopharyngeal pathogens in children with secretory otitis media. Acta Oto-Laryngologica 1984;97(3-4):379-83. [DOI] [PubMed] [Google Scholar]
Thomsen 1989 {published data only}
- Thomsen J, Sederberg-Olsen J, Balle V, Vejlsgaard R, Stangerup SE, Bondesson G. Antibiotic treatment of children with secretory otitis media. A randomized, double-blind, placebo-controlled study. Archives of Otolaryngology-Head & Neck Surgery 1989;115(4):447-51. [CENTRAL: CN-00058572] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Thomsen J, Sederberg-Olsen J, Stangerup SE, Balle V, Vejlsgaard R. Long-term antibiotic treatment of children with secretory otitis media: a double-blind placebo-controlled study. Acta Oto-Laryngologica. Supplementum 1988;449:49-50. [CENTRAL: CN-00056944] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Balen 1996 {published data only}
- Balen FA, Melker RA, Touw-Otten FW. Double-blind randomised trial of co-amoxiclav versus placebo for persistent otitis media with effusion in general practice. Lancet 1996;348(9029):713-6. [CENTRAL: CN-00129985] [EMBASE: 1996280110] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berman 1990 {published data only}
- Berman S, Grose K, Nuss R, Huber-Navin C, Roark R, Gabbard SA, et al. Management of chronic middle ear effusion with prednisone combined with trimethoprim-sulfamethoxazole. Pediatric Infectious Disease Journal 1990;9(8):533-8. [CENTRAL: CN-00071176] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernard 1991 {published data only}
- Bernard PA, Stenstrom RJ, Feldman W, Durieux-Smith A. Randomized, controlled trial comparing long-term sulfonamide therapy to ventilation tubes for otitis media with effusion. Pediatrics 1991;88(2):215-22. [CENTRAL: CN-00077175] [PMID: ] [PubMed] [Google Scholar]
- Feldman W, Bernard P, Smith A, Stenstrom R. Sulfonamide prophylaxis vs ventilation tubes in hearing-loss due to recurrent otitis-media with effusion (Rome) - preliminary results of a randomized controlled trial. Clinical and Investigative Medicine [Medecine Clinique et Experimentale] 1987;4:A33. [CENTRAL: CN-02494379] [Google Scholar]
- Feldman W, Bernard P, Smith A, Stenstrom R. Sulfonamide prophylaxis vs ventilation tubes in hearing-loss due to recurrent otitis-media with effusion - preliminary results of a randomized controlled trial. American Journal of Diseases of Children 1987;4:389-9. [CENTRAL: CN-02494378] [Google Scholar]
- Feldman W, Bernard P, Smith A, Stenstrom R. Ventilation tubes vs sulfonamide prophylaxis for recurrent acute otitis-media or serious otitis-media - follow-up on a randomized, controlled trial. American Journal of Diseases of Children 1988;4(4):407-7. [CENTRAL: CN-02494377] [Google Scholar]
Choung 2008 {published data only}
- Choung YH, Shin YR, Choi SJ, Park K, Park HY, Lee JB, et al. Management for the children with otitis media with effusion in the tertiary hospital. Clinical and Experimental Otorhinolaryngology 2008;1(4):201-5. [CENTRAL: CN-00671567] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corwin 1986 {published data only}
- Corwin MJ, Weiner LB, Daniels D. Efficacy of oral antibiotics for the treatment of persistent otitis media with effusion. International Journal of Pediatric Otorhinolaryngology 1986;11(2):109-12. [CENTRAL: CN-00044237] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Corwin MJ, Weiner LB, Daniels DA, Daniels DA. Effects of oral antibiotics on the outcome of serous otitis media. Pediatric Research 1982;16:238A. [CENTRAL: CN-00449256] [Google Scholar]
Daly 1991 {published data only}
- Daly K, Giebink GS, Batalden PB, Anderson RS, Le CT, Lindgren B, et al. Resolution of otitis media with effusion with the use of a stepped treatment regimen of trimethoprim-sulfamethoxazole and prednisone. Pediatric Infectious Disease Journal 1991;10(7):500-6. [CENTRAL: CN-00077629] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Daly K, Giebink GS, Lindgren B, Anderson RS. Controlled clinical trial for prevention of chronic otitis media with effusion. In: Lim DJ, Bluestone CD, Klein JO , editors(s). Recent Advances on Otitis Media: Proceedings of the 4th International Symposium; 1987 Jun 1-4; Toronto (ON). 1987:247-50. [CENTRAL: CN-00452502]
de Castro 1982 {published data only}
- Castro FJ, Jaeger RW, Martin L, Temeck JW, Tournour B. Serous otitis media. A double-blind trial with sulfisoxazole. Missouri Medicine 1982;79(9):629-30. [CENTRAL: CN-00029746] [EMBASE: 13245698] [PMID: ] [PubMed] [Google Scholar]
Donaldson 1990 {published data only}
- Donaldson JD, Martin GF, Maltby CC, Seywerd EB. The efficacy of pulse-dosed antibiotic therapy in the management of persistent otitis media with effusion. Journal of Otolaryngology 1990;19(3):175-8. [CENTRAL: CN-00068309] [PMID: ] [PubMed] [Google Scholar]
Eiden 1997 {published data only}
- Eiden P. Antibiotic therapy for otitis media infection with effusion in children can make surgery unnecessary. Deutsche Apotheker Zeitung 1997;137(8):41-2. [Google Scholar]
Ferrara 2005 {published data only}
- Ferrara S, Sammartano D, Ferrara P. Long-term management of recurrent otitis media with effusion in children. In: XVIII IFOS World Congress; 2005 Jun 25-30; Rome (Italy). 2005. [CENTRAL: CN-00526409]
Fujita 1994 {published data only}
- Fujita A, Kurata K, Takahashi H, Takagita S. Clinical efficacy of clarithromycin treatment of refractory otitis media with effusion. Practica Otologica 1994;87(9):1287-91. [Google Scholar]
Gaskins 1982 {published data only}
- Gaskins JD, Holt RJ, Kyong CU, Weart CW, Ward J. Chemoprophylaxis of recurrent otitis media using trimethoprim/sulfamethoxazole. Drug Intelligence & Clinical Pharmacy 1982;16(5):387-90. [CENTRAL: CN-00028043] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gasper 2003 {published data only}
- Gasper K, St Anna L, Montgomery L. Are antibiotics effective for otitis media with effusion? Journal of Family Practice 2003;52(4):321-3. [EMBASE: 36427691] [PubMed] [Google Scholar]
Gibson 1996 {published data only}
- Gibson PG, Stuart JE, Wlodarczyk J, Olson LG, Hensley MJ. Nasal inflammation and chronic ear disease in Australian Aboriginal children. Journal of Paediatrics and Child Health 1996;32(2):143-7. [CENTRAL: CN-00131589] [PMID: ] [DOI] [PubMed] [Google Scholar]
Giebink 1990 {published data only}
- Giebink GC, Batalden PB, Le CT, Russ JN, Knox JK, Anderson RS. Randomized controlled trial comparing trimethoprim-sulfamethoxazole, prednisone, ibuprofen, and no treatment in chronic otitis media with effusion. In: Lim DJ, Bluestone CD, Klein JO , editors(s). Recent Advances Otitis Media: Proceedings of the Fourth International Symposium; 1988 Jun 1-4 Toronto (Canada). 1988:240-4. [CENTRAL: CN-00452593]
- Giebink GS, Batalden PB, Le CT, Lassman FM, Buran DJ, Seltz AE. A controlled trial comparing three treatments for chronic otitis media with effusion. Pediatric Infectious Disease Journal 1990;9(1):33-40. [CENTRAL: CN-00065358] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hozawa 2001 {published data only}
- Hozawa T. Evidence-based macrolide therapy for children with serous otitis media. Japanese Journal of Antibiotics 2001;54(Suppl C):30-2. [EMBASE: 36495134] [PubMed] [Google Scholar]
Iino 1989 {published data only}
- Iino Y, Ishitoya J, Ikeda M, Ito Y, Usami M, Kawashiro N, et al. Factors on delayed recovery of otitis media with effusion in children--clinical and bacteriological study. Nihon Jibiinkoka Gakkai Kaiho 1989;92(8):1183-91. [CENTRAL: CN-00063873] [PMID: ] [DOI] [PubMed] [Google Scholar]
Iino 2001 {published data only}
- Iino Y. Efficacy of macrolide therapy for children with serous otitis media. Japanese Journal of Antibiotics 2001;54 Suppl C:23-5. [EMBASE: 36495133] [PMID: ] [PubMed] [Google Scholar]
Kobayashi 2001 {published data only}
- Kobayashi K. Applicability of long-term low-dosage macrolide therapy for children with serous otitis media. Japanese Journal of Antibiotics 2001;54 Suppl C:26-9. [PMID: ] [PubMed] [Google Scholar]
Kuriyama 1980 {published data only}
- Kuriyama K. Synergistic effect of antibiotics and Chinese drug on recurrent otitis media. Otolaryngology 1980;52(9):617-22. [EMBASE: 11228874] [Google Scholar]
Leonetti 1988 {published data only}
- Leonetti JP, Stankiewicz JA. Antimicrobial prophylaxis for recurrent otitis media. Otolaryngology - Head & Neck Surgery 1988;99(1):81-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marks 1981 {published data only}
- Marks NJ, Mills RP, Shaheen OH. A controlled trial of cotrimoxazole therapy in serous otitis media. Journal of Laryngology and Otology 1981;95(10):1003-9. [CENTRAL: CN-00026372] [EMBASE: 1982075258] [PMID: ] [DOI] [PubMed] [Google Scholar]
Paradise 1997 {published data only}
- Paradise J, Campbell T, Dollaghan C, Feldman H, Bernard B, Colborn K, et al. Receptive vocabulary, cognition, and parent-rated behavior at age 3 years in relation to otitis media in the first 3 years of life. In: Abstract Book of the Association of Health Service Research. Vol. 14. 1997:350-1. [CENTRAL: CN-00452820]
Parlea 2012 {published data only}
- Parlea E, Georgescu M, Calarasu R. Tympanometry as a predictor factor in the evolution of otitis media with effusion. Journal of Medicine and Life 2012;5(4):452-4. [CENTRAL: CN-00850240] [EMBASE: 369159817] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Perrin 1974 {published data only}
- Perrin JM, Charney E, MacWhinney JBJ, McInerny TK, Miller RL, Nazarian LF. Sulfisoxazole as chemoprophylaxis for recurrent otitis media. A double-blind crossover study in pediatric practice. New England Journal of Medicine 1974;291(13):664-7. [CENTRAL: CN-00010786] [PMID: ] [DOI] [PubMed] [Google Scholar]
Persico 1978 {published data only}
- Persico M, Podoshin L, Fradis M, Fradis M. Otitis media with effusion: a steroid and antibiotic therapeutic trial before surgery. Annals of Otology, Rhinology, and Laryngology 1978;87:191-5. [CENTRAL: CN-00449363] [PMID: ] [DOI] [PubMed] [Google Scholar]
Principi 1989 {published data only}
- Principi N, Marchisio P, Massironi E, Grasso RM, Filiberti G. Prophylaxis of recurrent acute otitis media and middle-ear effusion. Comparison of amoxicillin with sulfamethoxazole and trimethoprim. American Journal of Diseases of Children 1989;143(12):1414-8. [CENTRAL: CN-00063984] [PMID: ] [DOI] [PubMed] [Google Scholar]
Roark 1997 {published data only}
- Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatric Infectious Disease Journal 1997;16(4):376-81. [CENTRAL: CN-00138754] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rohail 2006 {published data only}
- Rohail A, Gill ZI, Butt MR. A comparison of medical treatment versus surgical treatment for the management of otitis media with effusion. Annals of King Edward Medical College 2006;12(1):64-7. [CENTRAL: CN-00597368] [Google Scholar]
Schloss 1988 {published data only}
- Schloss MD, Dempsey EE, Rishikof E, Sorga S, Grace MGA. Double blind study comparing erythromycin-sulfisoxazole (Pediazole) TID to placebo in chronic otitis media with effusion. In: Lim DJ, Bluestone CD, Klein JO , editors(s). Recent Advances on Otitis Media: Proceedings of the 4th International Symposium; 1987 Jun 1-4; Toronto (ON). 1988:261-3. [CENTRAL: CN-00452893]
- Schloss MD, Rishikof E, Sorger S, Dempsey EE, Grace M. A double-blind study comparing erythromycin-sulfisoxazole (Pediazole (R)) t.i.d. with placebo in chronic otitis media with effusion. Today's Therapeutic Trends 1988;5(4):43-56. [CENTRAL: CN-00192403] [EMBASE: 1988100915] [Google Scholar]
Schwartz 1982 {published data only}
- Schwartz RH, Rodriguez WJ. Trimethoprim-sulfamethoxazole treatment of persistent otitis media with effusion. Pediatric Infectious Disease Journal 1982;1(5):333-5. [CENTRAL: CN-00030082] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schwartz 1982a {published data only}
- Schwartz RH, Puglise J, Rodriguez WJ. Sulphamethoxazole prophylaxis in the otitis-prone child. Archives of Disease in Childhood 1982;57(8):590-3. [CENTRAL: CN-00028780] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shubich 1996 {published data only}
- Shubich I. Otitis media with effusion and allergy control in children: a prospective study. In: Sixth International Symposium on Otitis Media; 1996; Fort Lauderdale (FL). 1996:173-4. [CENTRAL: CN-00452904]
Smales 1992 {published data only}
- Smales O. Antibiotics for otitis media with effusion. New Zealand Medical Journal 1992;105(927):42. [PMID: ] [PubMed] [Google Scholar]
Stenstrom 2005 {published data only}
- Stenstrom R, Pless IB, Bernard P. Hearing thresholds and tympanic membrane sequelae in children managed medically or surgically for otitis media with effusion. Archives of Pediatrics & Adolescent Medicine 2005;159(12):1151-6. [CENTRAL: CN-00532329] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tracy 1995 {published data only}
- Tracy JM, Demain JG, Hoffman K, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Annals of Allergy, Asthma & Immunology 1995;74:59. [CENTRAL: CN-00285148] [DOI] [PubMed] [Google Scholar]
- Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Annals of Allergy, Asthma & Immunology 1998;80(2):198-206. [CENTRAL: CN-00148310] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Balen 1997 {published data only}
- Balen FA. Antibiotics in otitis media with effusion [Antibiotica bij otitis media met effusie]. Nederlands Tijdschrift voor Geneeskunde 1997;141(8):401-2. [PMID: ] [PubMed] [Google Scholar]
Varsano 1985 {published data only}
- Varsano I, Volovitz B, Mimouni F. Sulfisoxazole prophylaxis of middle ear effusion and recurrent acute otitis media. American Journal of Diseases of Children 1985;139(6):632-5. [CENTRAL: CN-00038335] [PMID: ] [DOI] [PubMed] [Google Scholar]
Velepic 2011 {published data only}
- Velepic M, Starcevic R, Bonifacic M, Ticac R, Kujundzic M, Udovic DS, et al. The clinical status of the eardrum: an inclusion criterion for the treatment of chronic secretory otitis media in children. International Journal of Pediatric Otorhinolaryngology 2011;75(5):686-90. [CENTRAL: CN-00784332] [EMBASE: 51315541] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeldandi 2001 {published data only}
- Yeldandi V, MacLeod C, Mulvaney AM. Open-label, randomised, comparative study of usual care with or without clarithromycin suspension in serous otitis media with inflammation. In: 22nd International Congress of Chemotherapy; 2001 Jun 30-Jul 3; Amsterdam (the Netherlands). 2001. [ABSTRACT NO.: P10.009] [CENTRAL: CN-00453008]
Yin 2002 {published data only}
- Yin TF, Tang QL, Xie DH, Lu YD. Efficacy of cefaclor in treating children with secretory otitis media. Shanghai Medicine 2002;23(2):65-6. [CENTRAL: CN-00416980] [Google Scholar]
Zocconi 1994 {published data only}
- Zocconi E. Antibiotics and oral steroids in the treatment of otitis media with effusion. Pediatria Medica e Chirurgica 1994;16(3):273-5. [PubMed] [Google Scholar]
References to studies awaiting assessment
Koay 1998 {published data only}
- Koay B, Commins DJ, Bates S, Mitchell B, Moore A, Bates G, et al. In search of a medical treatment for otitis media with effusion (OME): a randomised double-blind controlled trial (RCT). In: 7th International Congress of Pediatric Otorhinolaryngology. Helsinki, Finland, 7-10 June, 1998. 1998:64. [ABSTRACT NUMBER: 289] [CENTRAL: CN-00292550]
Tawfik 2002 {published data only}
- Tawfik S, Belal A, Sorour W. A comparative study of the different treatment modalities of otitis media with effusion in children. In: 8th International Congress of Paediatric Otorhinolaryngology (ESPO). Oxford, UK, 11-14 September, 2002. 2002:151, Abstract No. P2.26. [CENTRAL: CN-00508402]
Additional references
Abidin 1995
- Abidin RR. Parenting Stress Index: Professional Manual. Odessa, FL: Psychological Assessment Resources, 1995. [Google Scholar]
Achenbach 2011
- Achenbach T M. Child Behavior Checklist. In: Kreutzer JS, DeLuca J, Caplan B, editors(s). Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 2011. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: Harcourt Assessment, Inc, 2006. [Google Scholar]
Bruce 2015
- Bruce I, Harman N, Williamson P, Tierney S, Callery P, Mohiuddin S, et al. The management of Otitis Media with Effusion in children with cleft palate (mOMEnt): a feasibility study and economic evaluation. Health Technology Assessment 2015;19(68):1-374. [DOI: 10.3310/hta19680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantekin 1983
- Cantekin EI. Algorithm for diagnosis of otitis media with effusion. Annals of Otology, Rhinology & Laryngology 1983;92(6 Suppl):6-6. [DOI: 10.1177/00034894830920S603] [DOI] [Google Scholar]
Cochrane ENT 2020
- Cochrane ENT. Otitis media with effusion: a project to prioritise Cochrane systematic reviews. https://ent.cochrane.org/otitis-media-effusion-ome-glue-ear 2020 (accessed 3 November 2021).
Daniel 2012
- Daniel M, Imtiaz-Umer S, Fergie N, Birchall JP, Bayston R. Bacterial involvement in otitis media with effusion. International Journal of Pediatric Otorhinolaryngology 2012;76:1416-22. [DOI: 10.1016/j.ijporl.2012.06.013] [DOI] [PubMed] [Google Scholar]
Dunn 2007
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test (PPVT™-4). 4th edition. Pearson Education, 2007. [Google Scholar]
Fekkes 2000
- Fekkes M, Theunissen NC, Brugman E, Veen S, Verrips EG, Koopman HM, et al. Development and psychometric evaluation of the TAPQOL: a health-related quality of life instrument for 1–5-year-old children. Quality of Life Research 2000;9:961-72. [DOI] [PubMed] [Google Scholar]
Flynn 2009
- Flynn T, Möller C, Jönsson R, Lohmander A. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. International Journal of Pediatric Otorhinolaryngology 2009;73:1441-6. [DOI: 10.1016/j.ijporl.2009.07.015] [DOI] [PubMed] [Google Scholar]
Galbraith 2022
- Galbraith K, Mulvaney CA, MacKeith S, Marom T, Daniel M, Venekamp RP, et al. Autoinflation for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015253. [DOI: 10.1002/14651858.CD015253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1997
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry 1997;38:581-6. [DOI] [PubMed] [Google Scholar]
Gresham 1990
- Gresham FM, Elliott SN. Social Skills Rating System. Circle Pines, MN: American Guidance Service, 1990. [Google Scholar]
Griffiths 1996
- Griffiths R. The Griffiths Mental Development Scales from Birth to Two Years, Manual, the 1996 revision. Henley: Association for Research in Infant and Child Development, Test Agency, 1996. [Google Scholar]
Haggard 2003
- Haggard MP, Smith SC, Nicholls EE. Quality of life and child behaviour. In: Rosenfeld RM, Bluestone CD, editors(s). Evidence-Based Otitis Media. 2nd edition. Hamilton, Ontario: BC Decker Inc, 2003:401-29. [https://researchonline.lshtm.ac.uk/id/eprint/15108] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hedrick 1984
- Hedrick DL, Prather EM, Tobin AR. Sequenced Inventory of Communication Development. Seattle, WA: University of Washington Press, 1984. [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Jellinek 1988
- Jellinek MS, Murphy JM, Robinson J, Feins A, Lamb S, Fenton T. Pediatric Symptom Checklist: screening school-age children for psychosocial dysfunction. Journal of Pediatrics 1988;112(2):201-9. [DOI: 10.1016/s0022-3476(88)80056-8] [DOI] [PubMed] [Google Scholar]
Kreiner‐Møller 2012
- Kreiner-Møller E, Chawes BL, Caye-Thomasen P, Bønnelykke K, Bisgaard H. Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clinical and Experimental Allergy 2012;42(11):1615-20. [DOI: 10.1111/j.1365-2222.2012.04038.x] [DOI] [PubMed] [Google Scholar]
Landgraf 1994
- Landgraf JM. The Infant/Toddler Child Health Questionnaire: conceptual framework, logic content, and preliminary psychometric results. Boston: Health Act, 1994. [Google Scholar]
Landgraf 1996
- Landgraf JL, Abetz L, Ware JE. The CHQ User’s Manual. Boston: The Health Institute, New England Medical Center, 1996. [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liu 2020
- Liu PZ, Ismail-Koch H, Stephenson K, Donne AJ, Fergie N, Derry J, et al. A core outcome set for research on the management of otitis media with effusion in otherwise-healthy children. International Journal of Pediatric Otorhinolaryngology 2020;134:110029. [DOI: 10.1016/j.ijporl.2020.110029] [DOI] [PubMed] [Google Scholar]
MacKeith 2022a
- MacKeith S, Mulvaney CA, Galbraith K, Marom T, Daniel M, Venekamp RP, et al. Adenoidectomy for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015252. [DOI: 10.1002/14651858.CD015252] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKeith 2022b
- MacKeith S, Mulvaney CA, Galbraith K, Marom T, Daniel M, Venekamp RP, et al. Ventilation tubes (grommets) for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD015215. [DOI: 10.1002/14651858.CD015215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maris 2014
- Maris M, Wojciechowski M, Van de Heyning P, Boudewyns A. A cross-sectional analysis of otitis media with effusion in children with Down syndrome. European Journal of Pediatrics 2014;173:1319-25. [DOI: 10.1007/s00431-014-2323-5] [DOI] [PubMed] [Google Scholar]
Marseglia 2008
- Marseglia GL, Pagella F, Caimmi D, Caimmi S, Castellazzi AM, Poddighe D, et al. Increased risk of otitis media with effusion in allergic children presenting with adenoiditis. Otolaryngology – Head and Neck Surgery 2008;138(5):572-5. [DOI: 10.1016/j.otohns.2008.01.020] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall J, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 1972
- McCarthy D. Manual for the McCarthy Scales of Children's Abilities. New York: Psychological Corp, 1972. [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-17; Cape Town, South Africa. 2017.
Mulvaney 2022a
- Mulvaney CA, Galbraith K, MacKeith S, Marom T, Daniel M, Venekamp RP, et al. Topical and oral steroids for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015255. [DOI: 10.1002/14651858.CD015255] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence. Otitis media with effusion in under 12s: surgery. Clinical guideline [CG60]. Published: 27 February 2008. https://www.nice.org.uk/guidance/cg60. [PubMed]
NICE 2023
- National Institute for Health and Care Excellence. Otitis media with effusion in under 12s. NICE guideline [NG233]. Published: 30 August 2023. https://www.nice.org.uk/guidance/ng233. [PubMed]
Noel‐Storr 2018
- Noel-Storr AH. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford, UK. 2018.
Park 2004
- Park C-W, Han J-H, Jeong J-H, Cho S-H, Kang M-J, Tae K, et al. Detection rates of bacteria in chronic otitis media with effusion in children. Journal of Korean Medical Science 2004;19:735-8. [DOI: 10.3346/jkms.2004.19.5.735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poetker 2005
- Poetker DM, Lindstrom DR, Edmiston CE, Krepel CJ, Link TR, Kerschner JE. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. International Journal of Pediatric Otorhinolaryngology 2005;69(6):799-804. [DOI: 10.1016/j.ijporl.2005.01.012] [DOI] [PubMed] [Google Scholar]
Rabin 2001
- Rabin R, Charro F. EQ-5D: a measure of health status from the EuroQol Group. Annals of Medicine 2001;33(5):337-43. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynell 1985
- Reynell JH. Reynell Development Language Scales Manual. Windsor, UK: NFER-NELSON, 1985. [Google Scholar]
Rosenfeld 1997
- Rosenfeld RM, Goldsmith AJ, Tetlus L, Balzano A. Quality of life for children with otitis media. Archives of Otolaryngology--Head & Neck Surgery 1997;123:1049-54. [DOI: 10.1001/archotol.1997.01900100019002] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2000
- Rosenfeld RM, Bhaya MH, Bower CM, Brookhouser PE, Casselbrant ML, Chan KH, et al. Impact of tympanostomy tubes on child quality of life. Archives of Otolaryngology--Head & Neck Surgery 2000;126:585-92. [DOI] [PubMed] [Google Scholar]
Rosenfeld 2003
- Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope 2003;113:1645-57. [DOI: 10.1097/00005537-200310000-00004] [DOI] [PubMed] [Google Scholar]
Rosenfeld 2016
- Rosenfeld RM, Shin JJ, Schwartz SR, Coggins R, Gagnon L, Hackell JM, et al. Clinical practice guideline: otitis media with effusion (update). Otolaryngology - Head & Neck Surgery 2016;154(1 Suppl):S1-S41. [DOI] [PubMed] [Google Scholar]
Schlichting 2007
- Schlichting JEPT, Lutje Spelberg HC. Lexilijst Begrip: an instrument to investigate language comprehension in children aged 15-25 months in the context of early identification. Amsterdam: Pearson Assessment & Information BV, 2007. [Google Scholar]
Schlichting 2010
- Schlichting JEPT, Lutje Spelberg HC. Schlichting Test for Language Comprehension; Instruction Manual. Woooden: Bohn Stafleu van Loghum, 2010. [Google Scholar]
Schmalbach 2021
- Schmalbach CE, Brereton J, Bowman C, Denneny III JC. American Academy of Otolaryngology–Head and Neck Surgery/Foundation Reg-ent Registry: Purpose, Properties, and Priorities. Otolaryngology–Head and Neck Surgery 2021;164(5):964-71. [DOI] [PubMed] [Google Scholar]
Seppanen 2020
- Seppanen E, Thornton R, North H, Corscadden KJ, Wiertsema S, Vijayasekaran S, et al. Bacterial reservoirs in the middle ear of otitis-prone children are associated with repeat ventilation tube insertion. Pediatric Infectious Disease Journal 2020;39(2):91-6. [DOI: 10.1097/INF.0000000000002541] [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al, Living Systematic Review Network. Living systematic reviews 2: combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
TNO 1997
- TNO—Prevention and Health/LUMC. TAIQOL—Questionnaire for parents of children aged 1—5 years. Leiden, The Netherlands: Leiden University Medical Center, 1997. [Google Scholar]
Verrips 1998
- Verrips GH, Vogels AGC, Verloove-Vanhorick SP, Fekkes M, Koopman HM, Kamphuis RP, et al. Health-related quality of life measure for children - the TACQOL. Journal of Applied Therapeutics 1998;1(4):357-60. [Google Scholar]
Wallace 2017
- Wallace BC, Noel-Storr AH, Marshall IJ, Cohen AM, Smalheiser NR, Thomas J. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;24(6):1165-8. [DOI: 10.1093/jamia/ocx053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2011
- Williamson I. Otitis media with effusion in children. BMJ Clinical Evidence 2011;2011:0502. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Zernotti 2017
- Zernotti ME, Pawankar R, Ansotegui I, Badellino H, Croce JS, Hossny E, et al. Otitis media with effusion and atopy: is there a causal relationship? World Allergy Organization Journal 2017;10(1):37. [DOI: 10.1186/s40413-017-0168-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmerman 1992
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-3. San Antonio, TX: The Psychological Corporation, 1992. [Google Scholar]
References to other published versions of this review
Mulvaney 2022b
- Mulvaney CA, Galbraith K, MacKeith S, Marom T, Daniel M, Venekamp RP, et al. Antibiotics for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015254. [DOI: 10.1002/14651858.CD015254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venekamp 2016
- Venekamp RP, Burton MJ, Dongen TMA, Heijden GJ, Zon A, Schilder AGM. Antibiotics for otitis media with effusion in children. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD009163. [DOI: 10.1002/14651858.CD009163.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


