Abstract
Objective
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death worldwide. Telehealth rehabilitation may offer new opportunities in patient therapy. This systematic review aimed to evaluate the effects of internet-mediated telerehabilitation and compare them with the outcomes of conventional pulmonary rehabilitation in COPD patients.
Methods
Electronic databases PubMed, Prospero, Scopus, and Cochrane were searched for randomized controlled trials from January 2005 to December 2021. Two investigators reviewed studies for relevance and extracted study population, methods, and results data.
Results
Ten studies were eligible for systematic review from the initial selection (n = 1492). There was considerable heterogeneity in telerehabilitation approaches. Functional exercise capacity and quality of life were assessed in all studies. None of the results were inferior to conventional care. High adherence and high levels of safety were observed.
Conclusion
Telerehabilitation in COPD patients is a safe therapy approach that increases and maintains functional exercise capacity and quality of life, making it an equivalent option to conventional outpatient rehabilitation. However, there is currently a lack of a unified approach to the composition of therapy and the use of technology, which needs to be addressed in the future.
Keywords: telehealth, telerehabilitation, pulmonary rehabilitation, pulmonary disease, exercise
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide.1 The overall prevalence is approximately 14.3% in men and 7.6% in women over 30, with an increasing incidence.2 The high number of cases, the rapidly increasing incidence of COPD, and the associated severe socio-economic impacts require more effective and affordable treatment options.3 The essential element of non-pharmacological treatment of patients with COPD is pulmonary rehabilitation (PR), which represents an established and proven intervention. PR is a highly effective therapeutic approach to improve exercise tolerance, shortness of breath, and health status.4 However, several limitations in the provision of PR exist, including geographical constraints due to inaccessibility, comorbidities, excessive centralization in specialized workplaces, and thus relatively low usability for a wide range of patients who could otherwise benefit from this intervention.5,6 Alternative home-delivered PR models using telemedicine, allowing remote monitoring of clinical indicators or even PR itself, could be a potential solution to the shortcomings mentioned above.7,8 In a recent Cochrane analysis, a positive effect of PR on the patient’s functional capacity and overall psychological well-being was described, with simultaneous relief of symptoms (shortness of breath, fatigue).9 In addition, telemedicine is cost-effective and is considered a valuable means of reducing the costs associated with COPD treatment. All the benefits described above have the potential to provide a higher standard of care for patients with COPD, primarily by increasing the availability of effective treatment.10,11
Several meta-analyses focused on the feasibility and comparison of telerehabilitation (TR) clinical outcomes in patients with COPD.12,13 However, this systematic review focuses only on those studies that use advanced telehealth technologies to monitor PR in COPD. The main criterion of advanced telehealth from our point of view was whether the results of the intervention were reported to the health center via the Internet. For example, a mobile app that sends the collected data to health professionals or a web interface where the collected data is stored. We do not consider a phone or video call to be advanced telehealth. Studies that used these approaches were included in the previously mentioned meta-analyses.
The main goal of this systematic review is to evaluate the effects of PR in patients with COPD using various Internet platforms for data transmission, which have then been compared to regular outpatient PR programs.
Materials and Methods
This prospectively registered protocol for this systematic review was designed according to the International prospective register of systematic reviews (PROSPERO registration number: CRD42022304699).
Search Strategy
Electronic databases were searched for relevant studies published between 2005 and December 2021, including PubMed, Prospero, Scopus, and Cochrane Library. Gray literature and reference lists from relevant articles were also reviewed. The search was limited to publications in English, in adults, and to clinical randomized trials. The sensitivity-maximizing strategy combined terms related to COPD; telehealth or telerehabilitation; home care; technology; PR, or exercise training. The selection process involved a keyword search that is summarized in Supplementary Table 1.
The titles and abstracts of the included articles were screened independently by two reviewers (MH and FD) to identify relevant studies. Discrepancies were resolved after a discussion between the two reviewers. The unresolved disagreements were settled by a third reviewer LB. The full text of relevant papers was searched and checked by 2 reviewers according to inclusion and exclusion criteria. In case of missing or ambiguous data, authors of relevant papers were contacted to ascertain eligibility status and to retrieve missing data. Data related to study design, number of subjects, study population, study outcomes, and a description of the intervention and technology used in the delivery of telerehabilitation were extracted for analysis. This data extraction process was repeated after seven days by another independent reviewer.
Inclusion and Exclusion Criteria
Randomized controlled trials (RCT) were included if they assessed the effects of home-based exercise (eg, PR or another exercise such as aerobic or resistance training) delivered using advanced telehealth technology in adult patients with COPD.
The main criterion of advanced telehealth was whether the intervention results were reported to the health center via the Internet, regardless of how the exercise data were sent. Studies using only telephone or video calls were not considered advanced telehealth interventions and therefore were not included. We included studies published in English from 2005 to 2021. At least one of the following parameters had to be reported: functional exercise capacity, quality of life, and dyspnoea.
Studies related to other pulmonary diseases or including patients with severe comorbidities (cardiologic, neurologic, oncologic, or other diseases that significantly impacted the patient’s clinical condition) were excluded. Systematic reviews, meta-analyses, prospective and retrospective observational cohort studies, expert comments, letters, and case reports were not included.
Data Extraction and Quality Assessment
Data extraction was conducted independently by two authors (MH & FD), and any disagreement in the interpretation of data was resolved by a third author (LB). The authors used an Excel file to extract data, including (a) the origin of the papers: authors, year, and country; (b) participants’ characteristics: sample, age, sex, and baseline pulmonary function; (c): study design: summary of intervention and control, intervention dosage, and study duration (d) data-collection time point, (e) measurements, (f) the method of sending training data and (g) adverse events.
The methodological quality of the included studies was assessed using the TESTEX tool.14 The TESTEX tool was chosen for its reliability and suitability for exercise researchers as it facilitates a comprehensive review of exercise-based studies. The advantage of this tool is that it considers the criteria for blinding participants or study investigators, which are very difficult to implement in exercise-based studies. TESTEX contains 12 criteria, some of which can be evaluated with more than 1 point, allowing one study to obtain a maximum of 15 points. A total of 5 points can be obtained for the study’s methodological quality, while 10 points can be obtained for the reporting of the study. Higher scores signify better quality of the study and study reporting. The studies were classified according to their scores as follows: high quality as 12 points or above, good quality as 7 to 11 points, and low quality as 6 points or below. High-quality interventions were defined as highly relevant, reproducible, and very well methodologically described, with excellent reporting outcomes. Good quality interventions were defined as moderately clinically relevant, with limitations in results and good reproducibility for further experiments. Low-quality interventions showed substantial limitations regarding relevance and the method used with low reproducibility. This individual approach was chosen because validated TESTEX cut-off scales have not yet been recommended.
Results
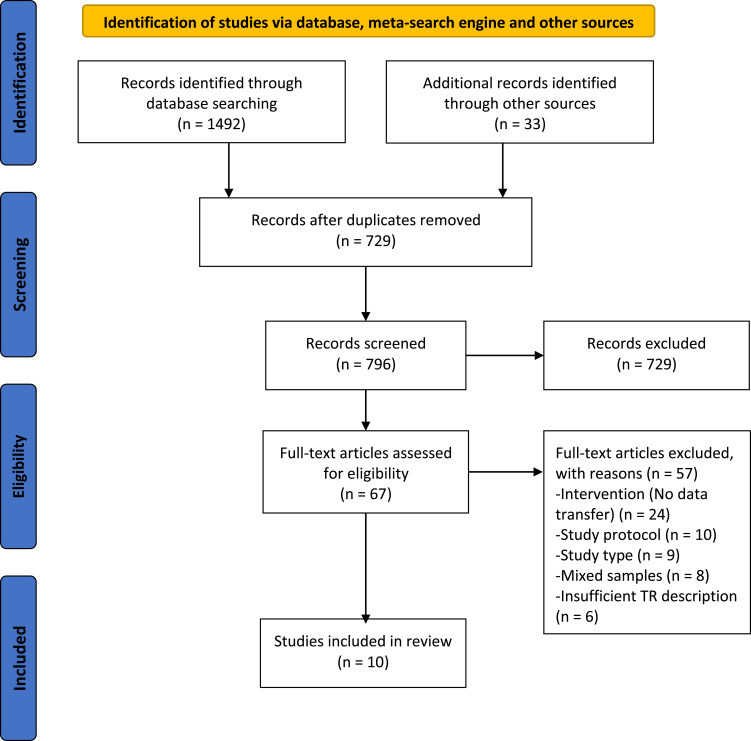
We identified 1525 potentially relevant records. After removing duplicates from screening the title and abstract for irrelevant material, 67 full-text papers met the entry criteria and were selected to be further assessed for inclusion. After a detailed review of the selected papers, 56 were excluded because of the study protocol (n = 10), lacked data transfer functionality in the intervention (n = 24), not a RCT (n = 9), mixed samples (included patients with different pulmonary disease – COPD, Cystic fibrosis, Asthma) (n = 8), insufficient pulmonary telerehabilitation description for eligibility evaluation (n = 6). Thus, a total of 10 studies were found to be eligible for inclusion in the systematic review. The PRISMA diagram is shown in Figure 1.
Figure 1.
Flow diagram detailing the search strategy.
Studies Included/Study Selection
The characteristics and findings of the ten included studies15–24 are listed in Table 1. Methodological quality, incidence of adverse events, and adherence to exercise prescription are provided in Table 2. Two of the ten studies were conducted in the Netherlands,15,20 two in the USA,21,22 and two others were conducted in Taiwan.23,24 One study was conducted in each of the following countries, including the United Kingdom,17 Spain,16 and Greece.19 One study was multicentric, with centers in Belgium, Greece, the United Kingdom, Switzerland, and the Netherlands.18 Three studies were published between 2008 and 2014,15,23,24 and seven were published from 2015 to 2021.16–22
Table 1.
Characteristics of Studies That Evaluated the Effects of Internet Mediated Rehabilitation in COPD Patients
| Study | Study Type | N | Sex | Age | Duration/Frequency | Technology | Exercise Program | Data Collection | Monitoring/Feedback | Outcomes Primary/Secondary |
|---|---|---|---|---|---|---|---|---|---|---|
| Galdiz (2020)16 | Multicentre RCT (1:1) | 94 | M,W | 63 | 48 weeks 3/week |
Smartphone, delivering data to web-based platform | Aerobic training - 30 min of leg cycle ergometry. Strength training - 30 min of weight lifting. | Uploaded by patient after every session | Mobile application with exercise diary, pulse oximeter | 6MWT, HRQoL (SF-36, CRQ)/BODE |
| Chaplin (2017)17 | RCT (1:1) | 103 | M,W | 66 | 8 weeks 7/week |
Interactive web-based PR programme | Aerobic training - walking at 85% intensity of baseline ESWT. Strength training - upper and lower extremity resistance training. | Recorded by patient after each training | Online application - questionnaires, self-reporting, exercise diary | ISWT, ESWT, HADS, CAT, PRAISE, BCKQ, HRQoL (Euro QOL, CRQ) |
| Demeyer (2017)18 | Multicentre RCT (1:1) | 343 | M,W | 67 | 12 weeks 4–7/week |
Smartphone application, step counter with daily activity goal, text messages | Aerobic training - walking/steps per day. If participant meet the goals, next week daily goal increase by 500 steps. | NR | Step counter. Automated coaching in application - activity goal, graphical representation of performance. Weekly text message with activity proposal. Telephone contact in case of non-compliance. | Physical activity (steps per day)/6MWT, isometric quadriceps force, CAT, CCQ, mMRC |
| Vasilopoulou (2016)19 | RCT (1:1:1) | 150 | M,W | 67 (64) | 48 weeks 3/week |
Tablet application - individualized training plan. Spirometer. Pedometer. Data stored on the web-based platform | Aerobic training – Individual walking drills Strength training - Arm and leg exercise |
Uploaded by patient on specific days every week | Pedometer, Spirometer, Oximeter, Questionnaires, Training log. Phone or video call once a week | Lung function, 6MWT, Peak Work Rate, HRQoL (SGRQ, CAT, mMRC) |
| Vorrink (2016)20 | Multicentre RCT (1:1) | 157 | M,W | 63 | 24 weeks 7/week |
Smartphone application for patients, website for physiotherapists | Aerobic training - walking - 1) steps per day 2) 30min of intensive walking per day; Intensity: baseline + 20% | Automatically | Accelerometer embedded in smartphone. Application showing physical activity in quantitative and qualitative form | Steps per day/6MWT, HRQoL (CRQ), BMI |
| Moy (2015)21 | RCT (2:1) | 239 | M,W | 67 | 16 weeks 5–7/week |
Website with individualized goal setting, iterative feedback, educational and motivational content, online community forum | Aerobic training - Walking/daily step-count. | Uploaded by patient at least once a week | Pedometer | HRQoL (SGRQ)/daily step count |
| Moy (2016)22 | RCT (2:1) | 239 | M,W | 67 | 32 weeks 5–7/week |
Website with individualized goal setting, iterative feedback, motivational content, online community forum | Aerobic training - Walking/daily step-count. | Uploaded by patient at least once a week | Pedometer | HRQoL (SGRQ)/daily step count |
| Wang (2014)23 | RCT (1:1) | 30 | M | 72 | 24 weeks 4–6/week |
Mobile application - music with preset walking tempo, website for collecting data | Aerobic training - Walking at constant speed with individualised tempo. Intensity: 80% of maximal capacity predicted from ISWT |
Automatically | Mobile application - time of walking at preset speed. Phonecall when patient missed one day of training | ISWT, muscle strength |
| Tabak (2014)15 | RCT (1:1) | 34 | M,W | 65 | 4 weeks 4–7/week |
Smartphone application, 3D accelerometer for activity registration. Web portal with a symptom diary for self-treatment of exacerbations | Aerobic training - Walking/daily step-count. | Data from accelerometer automatically, diary filled by patient | Pedometer, symptom diary. Automatic feedback messages - summary of activity behaviour and advice how to improve or maintain it. | Number of steps per day/CCQ, MRC, MFI-20 |
| Liu (2008)24 | RCT (1:1) | 60 | M | 72 | 48 weeks 7/week |
Mobile application - music with preset walking tempo, questionnaires for respiratory symptoms | Aerobic training - Endurance walking at constant speed with individualised tempo. | Automatically | Website - monitoring frequency of performance and the duration of the endurance walking. Phonecall when patient missed one day of training | ISWT, Spirometry, Short Form-12 |
Abbreviations: 6MWT, 6 minute walking test; CCQ, Clinical COPD Questionnaire; CRQ, Chronic Respiratory Questionnaire; mMRC, modified British Medical Research Council; CAT, COPD Assessment Test; HADS, Hospital Anxiety and Depression Scale; HRQoL, Health Related Quality of Life; SGRQ, St. George’s Respiratory Questionnaire; CG, control group; IG, intervention group; PR, PR; ISWT, Incremental Shuttle Walking Test; ESWT, Endurance Shuttle Walking test; PRAISE, PR Adapted Index of Self-Efficacy; BCKQ, Bristol COPD Knowledge Questionnaire; VAS, Visual Analogue Score; MFI-20, Multidimensional Fatigue Inventory; NR, not reported.
Table 2.
Methodological Quality of Studies That Have Evaluated the Effects of Internet Mediated Rehabilitation in COPD Patients
| Study | Randomization Process | Blinding of Assessors | Intention-to-Treat Analysis | Adverse Events | Adherence/Compliance |
|---|---|---|---|---|---|
| Galdiz (2020)16 | Stratified by centre | No | Yes | 56 acute exacerbations (26 patients) in IG vs 47 in CG (21 patients) | Adherence to scheduled appointments 92.4% in IG and 84.4% in CG |
| Chaplin (2017)17 | Simple randomization | Yes | No - 41 dropouts | No | NR |
| Demeyer (2017)18 | Stratified by centre | Yes | No - 15 dropouts | 48 patients (30%) from CG and 43 patients (27%) from IG experienced at least one exacerbation. 11 musculoskeletal events in IG, 2 in CG | Patients used step counter >6 days out of 7. Minimum was 4 days out of 7 |
| Vasilopoulou (2016)19 | Simple randomization | No | No - 3 dropouts | NR | Overall compliance to the different components of home-based maintenance tele-rehabilitation was 93.5% |
| Vorrink (2016)20 | Simple randomization, no stratification | Yes | No - 36 dropouts | NR | Adherence in wearing the device 89%. On average, physical activity goal obtained only in 34% of days |
| Moy (2015)21 | Stratified by modified Medical Research Council dyspnea score and urban versus rural status | Yes | Yes | Minor musculoskeletal adverse events significantly more in IG (26,6%, 41/154) compared to CG (4,8%, 4/84) | Adherence in IG: 94% of participants completed the survey and 86% uploaded valid data. In CG: 95% completed the survey and 81% uploaded valid data |
| Moy (2016)22 | Stratified by modified Medical Research Council dyspnea score and urban versus rural status | Yes | Yes | Minor musculoskeletal adverse events significantly more in IG (27,9%, 43/154) compared to CG (10%, 8/84) in addition in IG:6 pulmonary, 3 cardiac, 5 others. CG: 1 pulmonary, 1 cardiac, 3 other | Adherence significantly higher in IG with mean 76.7% of the 366 days having valid data versus CG mean 63.7%. Number of log-ins to the website in IG decreased significantly (mean 6.8 at month 1 declined to mean 3 at month 12) |
| Wang (2014)23 | Simple randomization | No | No - 6 dropouts | NR | NR |
| Tabak (2014)15 | Stratified by gender | No | No - 4 dropouts | NR | On average was the device worn in 109% of prescribed sessions. Overall compliance with activity coach (monitoring device) was 86%. Patients made only 58% of prescribed reports |
| Liu (2008)24 | Simple randomization | No | No - 12 dropouts | NR | 100% adherence during supervised period. 50% adherence in self-managed period |
Abbreviations: NR, not reported; IG, intervention group; CG, control group.
Sample Size and Recruitment
Only two studies16,19 provided information on the sample size calculation. The total number of participants from the included studies was 1210. Among these, 645 participants were in the intervention group (IG), and 565 were in the control group (CG). The sample size of the studies ranged from 30 to 343 participants. None of the included studies reported any barriers to patient recruitment.
Participants
All participants in the included studies were adult patients with COPD with various disease severity, but GOLD COPD stages were not reported. Almost 80% of participants were males, with one study enrolling only male participants.24 The mean age of participants ranged from 63 to 72 years. The mean percentage of the predicted normal for forced expiratory volume in one second (FEV1) was between 42% predicted and 80% predicted.
Control Groups
In six studies,15–20 patients received usual care: medications, physiotherapy, exercise recommendation, leaflet information, and/or group sessions at local physiotherapy practices. Patients in two studies23,24 underwent the same training protocol as the intervention group but without a telerehabilitation approach. In two studies,21,22 participants received pedometers as an addition to usual care, but no further information was given regarding their use.
Interventions
Training interventions varied among trials. Seven studies were unimodal based only on aerobic exercise,15,18,20–24 and three used other modalities such as resistance training or chest physiotherapy.16,17,19 Three trials were designed as maintenance programs after completing an 8-week16,19 or 12-week20 outpatient hospital PR program.
In most studies, exercise training frequency, intensity, and duration varied, making a comparison among studies difficult. The most commonly used aerobic modality was walking, which was included in all trials except one16 in which cycle ergometry was used. Three studies included upper and lower extremity resistance exercises.16,17,19 One study16 included chest physiotherapy to aerobic and resistance exercise. No study specified whether they followed local or international guidelines when developing the exercise program.
To promote motivation, automated coaching and motivational or educational tips were sent to patients weekly17,21,22 or daily.15,18,20 In three studies, patients were contacted by phone if they missed a preset count of exercise sessions.18,23,24
The intervention period ranged from 1 to 12 months. The number of exercise sessions per week was between 3 and 7, but in most studies, the frequency of exercise prescription was 4–7 times a week.15,17,18,20–24 The duration of one exercise session was individualized, with the goal being a prescribed daily step count15,18,20–22 or measured time of walking ability at a prescribed pace.23,24 Only one study provided 30 minutes of aerobic and 30 minutes of resistance exercise.16 In two studies, the duration of the exercise was not specified.17,19
One trial used Endurance Shuttle Walking Test (ESWT) to set the training intensity, and the intensity was set at 85% of the baseline ESWT.17 In two studies, the exercise intensity for aerobic training was based on the initial outcome of the Incremental Shuttle Walking Test (ISWT)23,24 and was set at 80% of maximal capacity. Three studies used baseline mean activity level in the initial week represented with steps per day.20–22 One study15 set reference activity for intensity based on the difference between the baseline activity of the participant (measured during 1st week) and a social activity norm, and patients were encouraged to be as or more active than the reference activity. One study’s exercise intensity of resistance training was set using a visual analog scale (VAS).17 Other authors did not report intensity setting for resistance training. Exercise progression was described in seven studies. Weekly automatic (software) assessment and adjustment were conducted in three trials.18,21,22 In another three studies, exercise progression was made by a specialist, either regularly or on prescribed visits23,24 or irregularly based on supervisor consideration.20 In the last study,17 the research team instructed patients to increase the walking time and resistance training intensity progressively, maintaining a Visual Analog Scale (VAS) in the 4–7 range.17
Monitoring and Training Data Transfer
No study incorporated real-time PR monitoring, all studies used an asynchronous model of patient monitoring. Four studies collected data using web-based platforms in which patients essentially self-reported and manually input data about their training and health status17 and/or uploaded data from a pedometer or accelerometer.15,21,22 The other six studies used mobile phone or tablet applications to monitor patients or used other devices, such as accelerometers connected to phone applications. Five out of six trials transferred training data automatically,18–20,23,24 and in the other study, patients delivered collected data to a web-based platform “by hand” after each session.16 One study monitored vital signs using oximetry16 and one oximetry combined with a portable spirometer.19
Adherence and Compliance
Adherence or compliance was assessed and reported in almost all of the studies, with only two17,23 studies not reporting this information. Adherence in trials was defined as a percentage of the prescribed exercise sessions, including attendance to scheduled appointments, uploading valid data, or wearing dedicated devices. Five studies reported adherence,16,20–22,24 and the adherence rate was moderate to high. The intervention group ranged from 76.7% to 100%, and CG ranged from 63.7% to 100%. Compliance was reported in four studies15,16,18,19 and was mainly assessed as a count or percentage of completed prescribed exercise sessions. In three studies,15,18,19 high compliance with prescribed frequency was reported at 82–93%. One study16 showed moderate compliance with the performance of scheduled exercise with 60%. One study team assessed patients’ training group preferences regardless of the randomization. Before the randomization, 38% of patients wanted to be in the telerehabilitation group, the most significant portion of patients with the statement. After finishing the program, 54% of patients in the conventional PR group preferred to would have done web program, and only 14% of patients in the telerehabilitation group would instead attend regular PR sessions.17
Safety and Adverse Events
Reporting adverse events was lacking since only 416,18,21,22 of 10 studies provided data about safety with variable reporting. In two trials,18,22 authors assessed the number of participants affected by adverse events. Demeyer et al18 reported patients with at least one exacerbation in IG (27%, 43/172) and in CG (30%, 48/171), with no statistical difference (p = 0.54). In the other trial,22 there were significantly more patients with adverse events in IG (27.9%, 43/154) compared to CG (10%, 8/84; p < 0.001). Two trials assessed several adverse events. One study16 registered episodes of acute exacerbation of COPD with no significant difference between groups, with 56 exacerbations in IG affecting 57% of participants in the group versus 47 in CG in 44% of participants. Another work21 reported a significant difference between groups. There were 41 adverse events in the intervention group and 4 in the control group (p = 0.003). The previous study17 only stated no severe adverse events, but no statistical data were presented.
Methodological Evaluation and Study Quality Results
Study quality and reporting results using the TESTEX scale (Supplementary Table 2) showed that the overall quality was good, with an average score of 9.4 points (range: 6–13). Study quality was assessed with an average score of 3.3 points (range: 2–4 points), and study reporting was assessed with an average score of 6.1 points (range: 4–9 points).
The study quality assessment showed an above-average level of study design and reporting. Three of the ten studies were below average, indicating that this set is a good sample with strong research foundations and is likely to be reproduced well. Only one study was rated high quality, and only one was rated low quality.
Outcomes
Functional Exercise Capacity
Seven studies assessed functional capacity with a walking test, although various types were used. Several authors used the 6MWT.16,18–20 Another evaluation method was Incremental Shuttle Walk Test,17,23,24 respectively ISWT, and Endurance Shuttle Walk Test.17
In four of the ten studies, significant improvement in functional capacity was reported in the IG exercise group.17,18,23,24 In other studies, there was no significant difference in functional capacity between baseline and end of training programs in IG or CG.16,19,20
One study19 compared a control group with two intervention groups: telerehabilitation and a Hospital-Based PR program. A total of 6 studies compared the results of functional capacity between one intervention and one control group,16–18,20,23,24 and three of them reported significant improvement in functional capacity in the intervention group over the control group.18,23,24 The remaining three studies found non-significant differences between groups.16,17,20 One study did not report statistical differences between groups in functional capacity.19
Physical Activity - Steps per Day
Five trials assessed daily physical activity with the number of steps per day.15,18,20–22 The physical activity results were compared between one intervention and one control group in three studies.18,20,21 Two reported significant improvements in physical activity in the intervention group over the control group.18,21 On the other hand, other authors20 reported that physical activity assessed by steps per day significantly decreased in both IG and CG. However, there was no significant difference between groups, and at the same time, there was no significant difference in the 6MWT results compared with the baseline. Another study22 reported that after eight months of follow-up, the primary significant difference between groups (gained during the supervised program) was again non-significant. One study did not report statistical differences between groups in physical activity.15 Only a non-significant increase in IG and a non-significant decrease in CG were present compared with baseline physical activity.
Dyspnoea and Respiratory Symptoms
There was no significant difference in dyspnea evaluation between groups in any reviewed study, for assessment authors used Medical Research Council (MRC),15 Modified Medical Research Council (mMRC),18,19 and dyspnoea domain from Chronic Respiratory Disease Questionnaire (CRQ).16,17,20 Significant improvement of dyspnoea in the intervention group (p < 0.001) was reported in one trial.17 Respiratory symptoms were assessed using CAT in three works,17–19 but no significant outcomes were found.
Quality of Life
Only one of 10 studies23 did not use any questionnaire to assess the quality of life (QoL). The QoL measures included the St. George’s Respiratory Questionnaire (SGRQ),19,21,22 Clinical COPD Questionnaire (CCQ),15,18 CRQ,15–17,20 Short Form-3616 and Short Form-12.24 Two studies reported significant differences between groups in the evaluation of the quality of life domains, Liu et al24 found significantly greater values in Short Form-12 physical of the IG compared to CG (45.4 ± 1.1 versus 34.3 ± 1.5; p < 0.001). In one trial18 significant difference between groups in CCQ – functional state (p < 0.026) was reported.18 Other authors did not find a significant difference between IG and CG. Nevertheless, there was a significantly better outcome in the intervention group in some works.15–18,21 One team reported the capability of their telerehabilitation program to preserve the benefits of the primary PR program over 12 months when evaluating the quality of life using SGRQ, compared to deterioration in CG. However, patients in the control group did not undergo primary PR.19
Discussion
This systematic review provides an overview of internet-mediated rehabilitation’s primary effects on patients with COPD. Recently, several papers dealing with this topic have been published. However, this systematic review has aimed to evaluate the effect and describe the telerehabilitation methodologies provided exclusively using various Internet platforms for data transmission in COPD patients. Only ten clinical randomized controlled trials were included in this review,15–24 which indicates the newness of this type of work. Studies that only used a phone or video for communication or included patients with other pulmonary diseases and/or comorbidities were not included. This is the first review article to focus on this specific type of intervention in COPD patients.
Despite the recommendations for PR application,25 we can find many differences in various traditional PR programs in training modalities, frequency, duration, and intensity,26 and the same situation is present in telehealth PR interventions. Given these differences, comparing the training in this review is difficult, but we can compare the expected endpoint. All studies assessed functional exercise capacity, and in all of them, outcomes in telehealth intervention were not inferior to usual care. Results in functional capacity between IG and CG were either equal or even statistically better in IG in four trials.18,21,23,24 These results support the usability of telehealth PR as an equivalent substitute to traditional outpatient PR in this area. In addition, all trials used an asynchronous model, giving health specialists more flexibility and might allow them to treat more patients in a given time.
COPD is a heavy burden for patients, as the illness follows an inevitable trajectory of progressive worsening of lung function, accompanied by symptoms (eg, dyspnoea, fatigue). Advancing decline in physical, emotional, and social functioning is typically seen, resulting in reduced quality of life.27,28 Concerning the quality of life, any of the authors in this review reported significant differences between groups. There were statistically significant improvements in the two trials only in specific domains of QoL assessments.18,24 These outcomes show that telehealth PR can be as beneficial as usual care. However, this area deserves more aimed research because we assume telehealth can bring more benefits in QoL once the programs are well established.
Adherence to PR is a common problem in managing patients with COPD. Predictors of non-adherence include smoking, advanced age, limited functional capacity, social situation, and traveling distance.29 To overcome these different barriers, an individual approach is needed for each patient.30 Nevertheless, overall adherence was reported to be moderate to high in the compared studies. Although the data are not extensive and adherence in the control groups was not significantly lower, motivation and control played a major role. In some studies, patients received motivational messages regularly; in others, they were warned if they did not perform the assigned tasks. Moreover, it is in the ability to motivate and alert the patient, ideally by automated mechanisms, that we see great benefit and promise for future good adherence to telehealth PR.
On the other hand, technology brings the additional obstacle of the need to master the control of it. Bentley et al31 reported that 47% of participants in their randomized controlled feasibility study withdrew mainly because of difficulty using the technology and that simplicity and usability were more critical for engagement than personalization. Therefore, the intervention should be simplified for future use. This and the mentioned need for an individual approach suggests that work will have to be done in both individualization and simplification to improve and support adherence.
Long-term maintenance of improvements achieved after the rehabilitation intervention phase would benefit patients with COPD. Continued supervised maintenance programs can reduce healthcare use in patients with COPD32 and improve health-related quality of life and exercise capacity at 6 to 12 months.33 Three studies were designed as a 12-month maintenance program after an initial supervised hospital-based program. While Vorrink et al20 reported that their telehealth intervention did not improve or maintain physical activity nor had an effect on functional exercise capacity or HRQoL, the other two trials16,19 reported a good effect in preserving the initial clinically meaningful improvement in observed outcomes with significant differences between IG and CG, meaning TR was equally effective as usual care16 or hospital-based maintenance rehabilitation.19 Tabak et al15 reassessed outcomes after a 9-months self-management period following three months of supervised TR and found that initial improvement lasted in participants in the intervention group. We cannot conclude any outcome from this limited information and more evidence is still needed to support the capability of long-term maintenance. However, at least the data seems promising, indicating that telehealth PR can help maintain primary intervention improvements.
Impact on the reduction of the risk for acute exacerbations, visits to the emergency department, and hospitalizations was monitored by Vasilopoulou et al.19 In multivariate analysis, they reported that home-based telerehabilitation and hospital-based, outpatient rehabilitation were in 12-month follow-up an independent predictor of a lower risk for acute exacerbation of COPD, incidence rate ratio (IRR) was 0.517; p < 0.001 for home-based TR and IRR 0.635; p = 0.003 for the home-based PR group. Both also remained independent predictors of a lower risk for hospitalizations for acute exacerbation of COPD (IRR 0.189; p < 0.001) and (IRR 0.375; p = 0.001) for home-based TR and hospital-based PR, respectively. Similar outcomes were reported in other studies with initial PR programs with follow-up home maintenance training.34,35 Vasilopoulou et al19 also calculated cost savings for delivering their home-based telerehabilitation and reported that the total cost per patient in the TR program is equivalent to 60% of the total estimated cost36 per patient and year. Thus, a decrease in exacerbations and hospitalizations can bring down expenses for COPD patients’ treatment, and some lately published works support this.37,38 Unfortunately, such information was rare among the studies evaluated. In the future, more emphasis should be placed on this topic to justify the financial efficiency of telehealth PR.
Adverse events associated with TR were reported only in 5 studies,16–18,21,22 and no participant suffered any severe complication associated with the telerehabilitation intervention. From our perspective, reporting adverse events seems insufficient when only 50% of studies reported them. Since patients rehabilitate on their own, without any supervision, and in some cases, the training same as an in- or outpatient hospital PR program, the question of safety and adverse events management should be analyzed more, even though classic home-based PR is considered as safe,39–41 more confirmative data of internet-mediated telerehabilitation safety would be beneficial.
Insufficient PR availability is challenging. However, the Covid pandemic has shown us the need for readiness for unexpected situations that can cause interruption or impossibility to initiating PR in indicated patients. In addition, fear and social distance at this time could lead to a deterioration in the psychological condition of patients with COPD and, thus, a reduction in motivation to movement therapy.42,43 Considering this, the possibilities of telehealth in the field of PR should pay due attention to how it integrates into the care of patients with COPD.44
After evaluating study quality and reporting of exercise, only one study in ten achieved a high-quality assessment.21 Although the quality of most of the other studies can be assessed as average, the amount of information reported for each study needs to be increased. Most often, the recruitment conditions were not specified, information on the blinding of the data assessor and/or intention-to-treat analysis was missing, and exercise intensity was not adjusted during the treatment. Despite the higher quality of some studies, the overall quality of a systematic review could be better, leading to misinterpretation of results. However, this area will continue to develop, and we can expect an improvement in the methodological quality and the possibility of better outcomes in future systematic reviews on this topic.
Our findings hold substantial significance within the context of modern healthcare delivery and patient-centered care. The confirmation that both center-based and telehealth PR are equally effective provides healthcare practitioners with the confidence to embrace technology-enabled care without compromising patient outcomes. This finding reinforces the principle that quality care can be delivered through various modes, enabling healthcare systems to leverage innovative approaches that address diverse patient needs and preferences. For patients who face geographical, mobility, or scheduling constraints, the availability of telehealth-based PR offers a convenient and accessible alternative.45 This finding thus promotes inclusivity by ensuring that patients who may have previously faced barriers to traditional center-based rehabilitation can now benefit similarly from effective remote interventions. In conclusion, the equivalency of outcomes between center-based and telehealth PR carries profound implications for patient-centered care, resource allocation, and the evolution of healthcare practices.
Future Directions
Future studies reporting telehealth PR training interventions for patients with COPD are recommended to report:
limitations on recruitment flow and exercise intervention implementation
between-group statistical comparisons
exercise intervention specification (modality, duration, frequency, intensity)
activity monitoring in control groups
adherence to exercise intervention and compliance with exercise prescription
possibility of real-time assessment of patients if necessary (eg, if a deterioration of the parameters has been detected) or at regular intervals (to ensure correct performance of the training).
For sufficient evidence of feasibility in the future methodological design of clinical trials is recommended:
unbiased randomization with description of the randomization method
specified eligibility criteria
allocation concealment
blinding of assessors
intention-to-treat analysis.
Limitations
Due to the increasing number of new publications on the topic of telerehabilitation in COPD patients and despite our constantly ongoing literature searches, it is possible that some eligible studies were not included in this systematic review. Although only those studies that specifically use interventions based on various internet platforms for telerehabilitation in COPD are included in this systematic review, the primary limitation of most of the included studies is the high heterogeneity of the interventions used and the low sample size. However, these limitations are balanced by similarly defined endpoints. It is, therefore, necessary in the future to create a uniform intervention procedure that will be applicable in several different ways depending on the possibilities and habits of individual telerehabilitation centers. It is the use of different training modalities in combination with the different duration of the intervention that may have led to a reduction in the overall quality of the generalizable findings. Based on the findings, we were unable to draw a conclusion as to which protocol is better for increasing endurance, improving strength or BODE index. Further investigation on the effectiveness of different protocols is therefore warranted. Secondary limitations include the impossibility of assessing usual care in most control groups, as it needs to be identifiable what this care includes.
Another potential limitation of our study is the restriction to English-language publications. While we acknowledge that this choice may introduce language bias and limit the generalizability of our findings, it was made to ensure the feasibility of comprehensive data extraction and synthesis within the scope of this review.
Final limitation may be a potential bias in selecting patients suitable for inclusion. Despite the relatively higher mean age, mainly motivated and technically able participants could have been selected. Therefore, the resulting sample may not represent the general COPD population.
Conclusion
In conclusion, this review shows that telehealth PR can increase or maintain functional exercise capacity and quality of life. It may also provide tools to intensify motivation and thus promote adherence to physical therapy in patients with COPD. Therefore, it can be used as an adequate substitute for conventional PR in indicated cases. Currently, there is a lack of consensus on the composition of PR and the technology and procedure used, making comparisons very difficult. Efforts in this area should focus on the development of at least a framework of uniform home training procedures, which would allow comparison of different technological approaches and also improvement in methodological quality.
Acknowledgments
Supported by Ministry of Health of the Czech Republic (University Hospital Brno, 65269705), No. NU21J-09-00004.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2). doi: 10.7189/jogh.05.020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J COPD. 2016;11(1):2805–2812. doi: 10.2147/COPD.S119992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mccarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. PR for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochester CL, Vogiatzis I, Holland AE, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of PR. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 6.Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of PR programmes. Eur Respir J. 2014;43(5):1326–1337. doi: 10.1183/09031936.00145613 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Granero MA, Sanchez-Morillo D, Leon-Jimenez A. Computerised analysis of telemonitored respiratory sounds for predicting acute exacerbations of COPD. Sensors. 2015;15(10):26978–26996. doi: 10.3390/s151026978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie MA, Okubo Y, Annegarn J, Wieching R, Lord SR, Delbaere K. Disentangling the health benefits of walking from increased exposure to falls in older people using remote gait monitoring and multi-dimensional analysis. Physiol Meas. 2017;38(1):45. doi: 10.1088/1361-6579/38/1/45 [DOI] [PubMed] [Google Scholar]
- 9.Cox NS, Dal Corso S, Hansen H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;2021(1). doi: 10.1002/14651858.CD013040.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De La Torre-Diéz I, López-Coronado M, Vaca C, Aguado JS, De Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed e-Health. 2015;21(2):81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoli L, Zanaboni P, Masella C, Ursini N. Systematic review of telemedicine services for patients affected by chronic obstructive pulmonary disease (COPD). Telemed e-Health. 2009;15(9):877–883. doi: 10.1089/tmj.2009.0044 [DOI] [PubMed] [Google Scholar]
- 12.Stafinski T, Nagase FI, Avdagovska M, Stickland MK, Menon D. Effectiveness of home-based PR programs for patients with chronic obstructive pulmonary disease (COPD): systematic review. BMC Health Serv Res. 2022;22(1). doi: 10.1186/s12913-022-07779-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Hu D, Xu Y, Wu L, Lou L. Effect of PR in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2022;54(1):262–273. doi: 10.1080/07853890.2021.1999494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13(1):9–18. doi: 10.1097/XEB.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 15.Tabak M, Vollenbroek-Hutten MMR, Van Der Valk PDLPM, Van Der Palen J, Hermens HJ. A telerehabilitation intervention for patients with chronic obstructive pulmonary disease: a randomized controlled pilot trial. Clin Rehabil. 2014;28(6):582–591. doi: 10.1177/0269215513512495 [DOI] [PubMed] [Google Scholar]
- 16.Galdiz JB, Gómez A, Rodriguez D, et al. Telerehabilitation programme as a maintenance strategy for COPD Patients: a 12-month randomized clinical trial. Arch Bronconeumol. 2021;57(3):195–204. doi: 10.1016/j.arbres.2020.03.034 [DOI] [PubMed] [Google Scholar]
- 17.Chaplin E, Hewitt S, Apps L, et al. Interactive web-based PR programme: a randomised controlled feasibility trial. BMJ open. 2017;7(3):e013682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demeyer H, Louvaris Z, Frei A, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017;72(5):415–423. doi: 10.1136/thoraxjnl-2016-209026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasilopoulou M, Papaioannou AI, Kaltsakas G, et al. Home-based maintenance telerehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J. 2017;49(5):1602129. [DOI] [PubMed] [Google Scholar]
- 20.Vorrink SNW, Kort HSM, Troosters T, Zanen P, Lammers JWJ. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after PR. Eur Respir J. 2016;48(4):1019–1029. doi: 10.1183/13993003.00083-2016 [DOI] [PubMed] [Google Scholar]
- 21.Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest. 2015;148(1):128–137. doi: 10.1378/chest.14-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moy ML, Martinez CH, Kadri R, et al. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2016;18(8):e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CH, Chou P-C, Joa W-C, et al. Mobile-phone-based home exercise training program decreases systemic inflammation in COPD: a pilot study. BMC Pulm Med. 2014;14(1). doi: 10.1186/1471-2466-14-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu WT, Wang C-H, Lin H-C, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J. 2008;32(3):651–659. doi: 10.1183/09031936.00104407 [DOI] [PubMed] [Google Scholar]
- 25.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in PR. (Erratum appears in Am J Respir Crit Care Med. 2014 Jun 15;189(12):1570). Am J Respir Crit Care Med. 2013;188(8). doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 26.Burge AT, Cox NS, Abramson MJ, Holland AE. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2020;2020(4). doi: 10.1002/14651858.CD012626.pub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68(3):369–378. doi: 10.1111/ijcp.12345 [DOI] [PubMed] [Google Scholar]
- 28.Gregersen TL, Green A, Frausing E, Ringbæk T, Brøndum E, Ulrik CS. Do telemedical interventions improve quality of life in patients with COPD? A systematic review. Int J COPD. 2016;11(1):809–822. doi: 10.2147/COPD.S96079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayton C, Clark A, Olive S, et al. Barriers to PR: characteristics that predict patient attendance and adherence. Respir Med. 2013;107(3):401–407. doi: 10.1016/j.rmed.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 30.Oates GR, Hamby BW, Stepanikova I, et al. Social determinants of adherence to PR for chronic obstructive pulmonary disease. COPD. 2017;14(6):610–617. doi: 10.1080/15412555.2017.1379070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth. 2020;8(6):e16203. doi: 10.2196/16203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins AR, Gowler H, Curtis F, Holden NS, Bridle C, Jones AW. Efficacy of supervised maintenance exercise following PR on health care use: a systematic review and meta-analysis. Int J COPD. 2018;13:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malaguti C, Dal Corso S, Janjua S, Holland AE. Supervised maintenance programmes following PR compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;2021(8):CD013569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117(4):976–983. doi: 10.1378/chest.117.4.976 [DOI] [PubMed] [Google Scholar]
- 35.Behnke M, Jörres RA, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home-based exercise programme over 18 months in patients with severe COPD. Monaldi Arch Chest Dis. 2003;59(1):44–51. [PubMed] [Google Scholar]
- 36.Geitona M, Hatzikou M, Steiropoulos P, Alexopoulos EC, Bouros D. The cost of COPD exacerbations: a university hospital - Based study in Greece. Respir Med. 2011;105(3):402–409. doi: 10.1016/j.rmed.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 37.Zhang A, Wang L, Long L, et al. Effectiveness and economic evaluation of hospital-outreach PR for patients with chronic obstructive pulmonary disease. Int J COPD. 2020;15:1071–1083. doi: 10.2147/COPD.S239841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Zhao Q, Li W, Zhao X, Li K. The cost-effectiveness of PR for COPD in different settings: a systematic review. Appl Health Econ Health Policy. 2021;19(3):313–324. doi: 10.1007/s40258-020-00613-5 [DOI] [PubMed] [Google Scholar]
- 39.Kovelis D, Gomes ARS, Mazzarin C, Biazim SK, Pitta F, Valderramas S. Effectiveness and safety of supervised home-based physical training in patients with COPD on long-term home oxygen therapy: a randomized trial. Chest. 2020;158(3):965–972. doi: 10.1016/j.chest.2020.02.063 [DOI] [PubMed] [Google Scholar]
- 40.Dushianthan A. Safety and effectiveness of home-based PR in COPD. Thorax. 2009;64(7):619. [Google Scholar]
- 41.Gigliotti F, Romano EM, Vulpio E, et al. Assessment of PR (PR) safety in patients with chronic respiratory diseases. Eur Respir J. 2017;50:PA756. doi: 10.1183/1393003.congress-2017.PA756 [DOI] [Google Scholar]
- 42.Mousing CA, Sørensen D. Living with the risk of being infected: COPD patients’ experiences during the coronavirus pandemic. J Clin Nurs. 2021;30(11–12):1719–1729. doi: 10.1111/jocn.15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpato E, Centanni S, Banfi P, et al. Narrative analysis of the impact of covid-19 on patients with chronic obstructive pulmonary disease, their caregivers, and healthcare professionals in Italy. Int J COPD. 2021;16:2181–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutkowski S. Management challenges in chronic obstructive pulmonary disease in the covid-19 pandemic: telehealth and virtual reality. J Clin Med. 2021;10(6):1–11. doi: 10.3390/jcm10061261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Formiga MF, Dosbaba F, Hartman M, et al. Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in COPD: protocol for a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:2147–2155. doi: 10.2147/COPD.S266234 [DOI] [PMC free article] [PubMed] [Google Scholar]