SUMMARY
Meiotic gene expression in budding yeast is tightly controlled by RNA-binding proteins (RBPs), with the meiosis-specific RBP Rim4 playing a key role in sequestering mid-late meiotic transcripts to prevent premature translation. However, the mechanisms governing assembly and disassembly of the Rim4-mRNA complex, critical for Rim4’s function and stability, remain poorly understood. In this study, we unveil regulation of the Rim4 ribonucleoprotein (RNP) complex by the yeast 14-3-3 proteins Bmh1 and Bmh2. These proteins form a Rim4-Bmh1-Bmh2 heterotrimeric complex that expels mRNAs from Rim4 binding. We identify four Bmh1/2 binding sites (BBSs) on Rim4, with two residing within the RNA recognition motifs (RRMs). Phosphorylation and dephosphorylation of serine/threonine (S/T) residues at these BBSs by PKA kinase and Cdc14 phosphatase activities primarily control formation of Rim4-Bmh1/2, regulating Rim4’s subcellular distribution, function, and stability. These findings shed light on the intricate post-transcriptional regulatory mechanisms governing meiotic gene expression.
Graphical Abstract

In brief
Zhang et al. demonstrate that the spatiotemporally controlled phosphorylation (by PKA) and dephosphorylation (by Cdc14) of Rim4, a meiosis-specific translation suppressor, determine Rim4 distribution, function, and protein stability by regulating the Rim4-Bmh1/2 interaction and, thereby, Rim4-mRNA complex formation.
INTRODUCTION
Meiosis is a crucial process in which haploid gametes are generated for sexual reproduction. It involves DNA replication followed by two chromosome segregations, known as meiosis I and meiosis II. In Saccharomyces cerevisiae (budding yeast), meiosis is orchestrated by a well-characterized transcriptional cascade involving transcription factors such as IME1 and NDT80.1-6 The NDT80 regulon, comprising approximately 300 mid-late meiotic genes, undergoes post-transcriptional regulation to ensure their timely translation and support distinct biological functions during meiosis and sporulation.7,8 RNA-binding proteins (RBPs) play a crucial role in this process, bridging RNA and protein interactions to exert diverse biological functions.9,10 Several RBPs have been identified as meiosis-specific translation suppressors, including RIM4, PES4, and MIP6.7,11 Among these, Rim4 plays a critical role as a master translation suppressor of meiotic transcripts. Deletion of RIM4 results in complete absence of meiotic DNA replication,12 and it is essential for timely translation of key regulators like CLB3 and AMA1,11,13 a CDK1(Cdc28) regulator for meiosis II and an activator of the meiotic anaphase-promoting complex (APC/C), respectively.
Rim4 is characterized by extensive phosphorylation, with about 68% of 57 serine/threonine (S/T) residues showing evidence of phosphorylation in proteomic studies.14 A hypophosphorylated mutant of Rim4, Rim4[47A], displays increased self-assembly, enhanced protein stability, and strengthened binding to CLB3 mRNA,14 suggesting that phosphorylation predominantly regulates Rim4. While Ime2, a CDK2-like kinase, has been reported to phosphorylate Rim4, other kinases and phosphatases may also modify Rim4 because the majority of Rim4 phosphorylation sites identified do not conform to the Ime2 consensus motif. Additionally, while Ime2 stimulates protea-some-mediated Rim4 degradation,14 active autophagy has been observed during meiosis and is required for timely degradation of Rim4 and subsequent translation of CLB3 mRNA.15,16 However, the exact mechanism through which phosphorylation regulates Rim4 ribonucleoprotein (RNP) formation and autophagy-mediated Rim4 degradation remains unknown.
In this study, we aim to identify the specific phosphorylation sites (P-sites) that regulate Rim4 function and distribution. Among these sites, we characterize four RRxS/T sites as common targets of PKA (cyclic AMP [cAMP]-dependent protein kinase) and Cdc14, a cell cycle phosphatase. Our investigation reveals that the activities of PKA and Cdc14 on these P-sites regulate the interaction of Rim4 with 14-3-3 proteins (Bmh1/2 in yeast), which, in turn, exclude mRNAs from binding to Rim4. Additionally, we demonstrate that the subcellular localization of Cdc14, whether in the nucleus or cytosol, determines whether Rim4 binds to mRNAs or undergoes autophagy-mediated degradation.
RESULTS
Site-specific phosphorylation regulates Rim4 subcellular distribution and function
Rim4 functions as an RBP with three RNA recognition motifs (RRMs) (Figures 1A and S1A). Previous proteomics analysis covering 50% of the 114 Rim4 S/T residues identified phosphorylation on 39 of them during meiosis (Figure 1A).14 To investigate the functional relevance of these phosphorylation events, we divided the Rim4 sequence into nine regions (region 1 [R1]–R9) (Figure 1A), and all S/Ts in each region were mutated to either glutamic acid (E) or alanine (A) to mimic the hyper- and hypophosphorylation states, respectively. Remarkably, the E or A mutants in RRM-containing R2 (RRM1), R3 (RRM2), and R5 (RRM3) blocked meiotic DNA replication (Figure 1B) and sporulation almost as effectively as the Drim4 deletion strain (Figure 1C), indicating that all RRMs are essential for Rim4 function, which was previously unknown. Furthermore, R6E, R7E, or R7A mutants showed greatly reduced sporulation efficiency, while R8A slightly influences sporulation (Figure 1C). Among the 19 S/Ts covered by mass spectrometry in R6–R7,15 of them were found to be phosphorylated during meiosis (Figure 1A), indicating that the R6-7 region, which is located next to the C terminus of RRM3 and is intrinsically disordered (Figure 1A), might contain critical P-sites for Rim4 function. In contrast, replacement of S/Ts by E/As in R1, R4 (despite prevalent in vivo phosphorylation), R8, and R9 (despite abundant S/Ts) did not cause obvious functional defects (Figures 1B and 1C). Notably, the R7A and R7E mutants exhibited reduced sporulation, suggesting that phosphorylation regulation of Rim4, at least in R6–R7 region, is dynamic and potentially meiosis stage specific.
Figure 1. Site-specific phosphorylation regulates Rim4 subcellular distribution and function.
(A) Top: Schematic of the Rim4 protein, highlighting RNA recognition motifs (RRMs) and serine/threonine (S/T) residues. Based on a previous proteomics study,14 red lines indicate phosphorylated S/T residues, gray lines represent unphosphorylated S/T residues, and blue lines indicate S/T residues in uncovered regions (gray rectangles). R1–R9 denote the regions used for subsequent phosphorylation analysis. The number of S/T residues in each region is indicated below. Bottom: prediction of Rim4 disordered regions using the DISOPRED3 program.46 The precision score reflects the disorder level of the local area. Unless indicated differently, all Rim4 variants in this study were tagged with EGFP at the N terminus.
(B) Flow cytometry analysis of DNA content (2N versus 4N) in different Rim4 variants. Cells were collected at 12 h in SPM before NDT80 induction. All S/T residues in each region were mutated to alanine (A) or glutamic Acid (E).
(C) Sporulation efficiency (percentage of tetrads) of the indicated Rim4 variants (mean ± standard error [SE]; statistical comparison of Rim4 variants with the wild type [WT] by Welch’s t test), with the number of total cells from 3 independent experiments listed below.
(D) Top: Representative cell images for Rim4 at the indicated time in SPM. Bottom: intracellular distribution of EGFP-Rim4, depicted as fluorescence signals along a line traversing the cell (3× magnification). The brackets indicate the nucleus area based on Nup49-mScarlet signals. Scale bars, 2 μm.
(E) Quantitative analysis of (D), showing the nuclear/cytoplasmic EGFP-Rim4 intensity ratio. The orange dashed line marks a ratio = 1.
(F) Top: FM analysis showing the nuclear/cytoplasmic intensity ratio of EGFP-Rim4 variants in cells at 12 h in SPM, as performed in (E). The orange dashed line represents a value of 1. Center: representative cell images for each Rim4 variant (bottom) with intracellular distribution analysis as performed in (D). Scale bars, 2 μm.
(E and F) Top: data were obtained from three independent experiments. The number of total cells analyzed is listed below. Red bars indicate median ± 95% confidence interval (CI) generated by GraphPad Prism 9 (see details in STAR Methods). Data were analyzed using Dunn’s test. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001.
Next, we investigated how phosphorylation regulates Rim4 function. Using an inducible Gal-NDT80 system (Figure S1B), we synchronized meiotic divisions by inducing NDT80 expression.17 Fluorescence microscopy (FM) analysis revealed that Rim4 was distributed evenly between the nucleus and cytoplasm throughout meiosis (Figures 1D and 1E), with Nup49 (pNUP49:Nup49-mScarlet), a component of the nuclear pore complex (NPC), marking the nuclear envelope. Strikingly, the mRNA-binding-defective Rim4(F139L/F349L) mutant, referred to as Rim4(FLm),18 in which the conserved phenylalanine (F) residues in RRM1 and RRM3 were mutated to leucine (L)19-21 (Figure S1A), exhibited a nuclear retention phenotype (Figures 1F and S1C) and abolished sporulation (Figure S1D). mRNA nuclear export provides the driving force for RBPs. Nuclear retention of Rim4(FLm) suggests that Rim4 loads mRNAs in the nucleus, where transcription occurs.
Our findings support the notion that Rim4 load mRNAs in the nucleus while suppressing translation in the cytosol.13 We observed that aberrant distribution of Rim4, such as nuclear retention, is indicative of Rim4 dysfunction. Among the eight Rim4 mutants with nuclear retention (Figures 1F and S1C), seven of them exhibited suppressed DNA replication and sporulation defects (Figures 1B and 1C). These mutants included RRM-containing R2A, R3A, R5A, R5E, FLm, and LCD (low complexity domain)-located R6E and R7E (Figures 1F, 1B, and 1C). Notably, not all Rim4 phosphorylation mutants displayed nuclear retention or functional defects, suggesting that functional phosphorylation on Rim4 is specific to certain sites. Particularly, R6–R7 of the Rim4 LCD domain (amino acids [aa 424–560) appeared to contain the most crucial P-sites that led to Rim4 nuclear retention, while S/T residues in the RRMs may also be involved in this process.
14-3-3 proteins (Bmh1 and Bmh2) bind and regulate Rim4 function
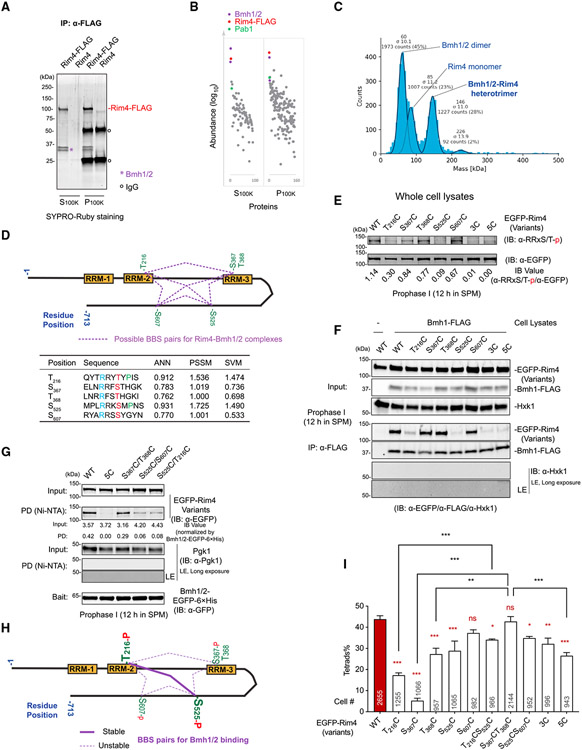
To investigate the potential interaction of Rim4 regulated by phosphorylation, we performed immunoprecipitation (IP) of Rim4-FLAG from cell lysates (12 h in sporulation medium [SPM]) (Figure 2A) and identified its interacting proteins using mass spectrometry (MS). Among the top interactors with Rim4 were Bmh1, Bmh2 (yeast 14-3-3 proteins), and Pab1 (poly(A) binding protein) (Figure 2B). Notably, in the S100k fraction (supernatant, 100,000 × g, 49 min) of the cell lysate, Rim4-FLAG formed a stable heterotrimeric complex with Bmh1 and Bmh2 in a 1:1:1 stoichiometry. This interaction occurred in the absence of RNA binding, a finding validated through mass photometry (MP) (Figure 2C). This suggests that Rim4 can exist in at least two distinct complexes within the cell: one complex containing mRNAs, potentially associated with Pab1, and another complex composed of the Rim4-Bmh1-Bmh2 heterotrimer.
Figure 2. Regulation of Rim4 function by 14-3-3 proteins (Bmh1 and Bmh2).
(A and B) Identification of the Rim4 interactome by immunoprecipitation (IP) and mass spectrometry (MS).
(A) IP of Rim4-FLAG from cell lysates collected at 12 h in SPM, followed by SDS-PAGE and staining with SYPRO-Ruby. Bmh1 and Bmh2 are marked by a purple asterisk.
(B) Abundance of Rim4 interactome proteins identified by MS shown in a graph.
(C) MP analysis of the cytosolic Rim4-FLAG complex from (A), displaying the number of contrast events related to molecular mass. The solid lines are Gaussian fits to the peaks. The mean and standard deviation (σ) of each Gaussian curve is shown above it, as is the number of counts accounting for the peak.
(D) Schematic of 14-3-3 protein (Bmh1/2) binding sites (BBSs) on Rim4 predicted by the 14-3-3-Pred program.24 The prediction was made with three methods: artificial neural network (ANN; cutoff = 0.55), position-specific scoring matrix (PSSM; cutoff = 0.8), and support vector machine (SVM; cutoff = 0.25). Consensus binding site sequence R(blue)XXS/T(red)-XP(green) is indicated, with phosphorylated S/T residues (red) required for Bmh1/2 binding. Proline (P) at the +2 position is optional. Purple dashed lines represent possible Bmh1/2-Rim4 complexes based on phosphorylation states of the BBSs.
(E) Immunoblotting (IB) of the indicated EGFP-Rim4 variants with S/T residues mutated into cysteine (C). Whole-cell lysates from prophase I were analyzed using the indicated antibodies. The numbers below the IB image quantify the level of phosphorylated Rim4 BBSs normalized by the level of Rim4, based on IB (α-RRxS/T-p/α-EGFP).
(F) CoIP of the indicated EGFP-Rim4 variants with Bmh1-FLAG (pBMH1: Bmh1-FLAG) in prophase I cell lysates.
(G) Ni-NTA (nickel-nitrilotriacetic acid) pulldown of EGFP-Rim4 variants with Bmh1/2-EGFP-His6 from prophase I cell lysates.
(F) and (G) LE: long exposure.
(H) Model depicting the possible phosphorylation status of the binding sites and Bmh1/2-Rim4 interaction in prophase I cells. BBS-T216 and BBS-S525 are the primary P-sites for stable Rim4-Bmh1/2 interaction (solid line), while contributions from other BBSs are weak or transient (dashed line).
(I) Sporulation (tetrad formation) efficiency of EGFP-Rim4 variants and statistical analysis compared with WT Rim4, as described (Figure 1C). The asterisks in red indicate comparison with the WT. *p < 0.05, **p < 0.01, ***p < 0.001.
Bmh1 and Bmh2 are known to interact with phosphorylated S/T residues in a consensus sequence, RXXS/T-pXP, where proline (P) at the +2 position is optional.22,23 Rim4 contains five predicted Bmh binding sites (BBSs) (Figure 2D),24 which are likely accessible for Bmh binding (Figure S2A). Four of these BBSs, BBS-T216, BBS-S367, BBS-S525, and BBS-S607, conform to the RRxS/T-p consensus, while BBS-T368 (-RFST368-) is different and largely overlaps with BBS-S367 (Figure 2D). Bmh1 and Bmh2 form heterodimers or homodimers, with each monomer consisting of nine α helices that create an amphipathic groove, serving as the phosphorylation-binding pocket.25 These dimers can accommodate two BBSs simultaneously,25 allowing various combinations that may regulate Rim4 in distinct ways (Figure 2D).
In a previous proteomics analysis, phosphorylation of S525 on Rim4 was detected during meiosis.14 To determine the specific BBS(s) on Rim4 that are phosphorylated, we systematically substituted the S/T residues within the BBSs with cysteine (C) and analyzed their phosphorylation status at prophase I. Immunoblotting (IB) using an antibody specific to RRxS/T-p (phosphorylated RRxS/T motif) suggested that multiple BBSs are phosphorylated. Among them, S525-p showed the strongest contribution to the IB signal, followed by T216-p, while other sites contribute mildly (Figure 2E). Consistently, the 3C mutant (T216C/S525C/S607C) and 5C mutant (T216C/S367C/T368C/S525C/S607C) completely abolished detection of Rim4 phosphorylation by the α-RRxS/T-p antibody (Figure 2E). Furthermore, Bmh1-FLAG IP pulled down wild-type Rim4 but not the 5C or 3C variants (Figure 2F). Among the single BBS mutants, only S525C and T216C significantly reduced the interaction between Rim4 and Bmh1 (Figure 2F). Additionally, we demonstrated that the bead-immobilized recombinant Bmh1/2 (Figure S2B) dimer pulled down less of the S525C-containing mutants (S525C/T216C and S525C/S607C) compared with wild-type Rim4 and S367C/T368C from prophase I cell lysate but more than the 5C mutant, which almost completely abolished the Rim4-Bmh1/2 interaction (Figure 2G). These findings indicate that phosphorylation of S525 and T216 primarily mediate the binding of Bmh1/2 to Rim4, at least in prophase I cells, while other BBSs contribute to the interaction (Figure 2H).
Notably, only BBS-T216 and BBS-S525 contain a proline (P) at the +2 position. This might enable them to bind Bmh1/2 with higher affinity. Nonetheless, except for S607C, the single BBS mutations led to a reduction in sporulation efficiency (Figure 2I) not strictly correlated with their contribution to stable Rim4-Bmh1/2 interaction at prophase I. For example, S367C and T368C did not affect stable Rim4-Bmh1 interaction (Figure 2F) but significantly reduced sporulation (Figure 2I). It is possible that phosphorylation of S367 and T368 occurs at different meiotic stages or as highly transient and dynamic events. Interestingly, the sporulation defect in S367C and T216C mutants could be partially rescued by T368C and S525C mutations, respectively. Additionally, the sporulation efficiency in Rim4(5C) cells was significantly higher than that in T216C and S367C mutants (Figure 2I). These findings suggest that binding of Bmh1/2 to different pairs of BBSs regulates Rim4 in distinct ways. While completely eliminating Bmh binding from Rim4 (5C) is detrimental, it is less toxic than trapping Rim4 in specific binding state(s); e.g., caused by S367C or T216C. This scenario is consistent with our flow cytometry analysis showing that single mutations (e.g., S367C or T216C) are less functional than Rim4 (5C) in supporting meiotic DNA replication (Figure S2C).
PKA stimulates Rim4-Bmh1/2 interaction by phosphorylating Rim4 at BBSs
The S/T residues within the RRxS/T consensus sequence, which corresponds to the four BBSs (T216, S367, S525, and S607) on Rim4, are canonical targets of PKA kinase. In budding yeast, there are three partially redundant PKA homologs: TPK1, TPK2, and TPK3. To investigate the role of PKA in meiosis and sporulation, we utilized a Tpk1(M164G)/Tpk2(M147G)/Tpk3(M165G) (tpk-as) system, which allows conditional and acute inhibition of PKA activity using 1NM-PP126 (Figure S3A). Tpk-as slightly affected PKA function in vegetative cell proliferation, while adding 1NM-PP1 abolished it (Figure S3B), because PKA is essential for cell survival; remarkably, inhibiting PKA led to a significant reduction in sporulation efficiency (Figure 3A) without affecting DNA replication (Figure S3C), indicating that PKA is mainly involved in regulating meiosis and sporulation after S phase.
Figure 3. PKA stimulates Rim4-Bmh1/2 interaction by phosphorylating Rim4 at BBSs.
(A) Sporulation (tetrad formation) efficiency of Tpk-as and WT strains, analyzed as in Figure 1C. The 1NM-PP1 treatment (10 μM) at multiple time points, which inhibits Tpk-as activity, is indicated in the top schematic.
(B) Top: experimental design showing 1NM-PP1 treatment (10 μM, red rod) and sample collection (blue rod) at the indicated time points. Bottom: IB analysis of Tpk-as cell lysates at prophase I (12 h in SPM) or during meiotic divisions (14 h in SPM) with 1NM-PP1 or mock treatment. IB results show phosphorylated EGFP-Rim4 (EGFP-Rim4-p) detected by α-RRxS/T-p in red, total EGFP-Rim4 detected by α-EGFP in green, and the merged image. Pgk1 served as a loading control.
(C) Quantification of EGFP-Rim4-p signals from (B) normalized to EGFP-Rim4 signals (IB, α-RRxS/T-p/α-EGFP).
(D) IB of whole-cell lysates with α-RRxS/T-p antibody and Ponceau S staining under the indicated conditions, as in (B), but showing the whole IB images. Red asterisks indicate EGFP-Rim4-p positions.
(E) Quantification of IB signals between 20 and 150 kDa from (D), normalized by Ponceau S signals. Data are compared with the reference group (Pro-I without 1NM-PP1) and presented as fold change.
(F) In vitro phosphorylation of recombinant Rim4-His6 by PKA (catalytic subunit), detected by IB with the α-RRxS/T-p antibody. Rim4(5C)-His6 served as a negative control. Ponceau S staining shows protein levels.
(G and H) Representative SYPRO-Ruby image of a pull-down assays using bead-immobilized Bmh1/2-EGFP-His6 (bait) and recombinant Rim4-His6 variants (input) that are PKA or mock treated (G) and quantification of (G) showing the mole ratio between pull-down Rim4 and immobilized Bmh1/2-EGFP (H) from 3 independent experiments (mean ± SD).
(I) Model illustrating PKA-mediated phosphorylation at Rim4 BBSs stimulating Rim4 interaction with Bmh1/2. Created with BioRender.
In (A), (C), and (E), data are shown as mean ± SE (three independent experiments, unpaired Welch’s t test). *p < 0.05, **p < 0.01, ***p < 0.001.
Strikingly, IB using the α-RRxS/T-p antibody revealed that inhibiting PKA activity eliminated RRxS/T phosphorylation in Rim4 (Figures 3B and 3C) and resulted in an approximately 50% decrease in total intracellular RRxS/T-p levels at prophase I (Figures 3D and 3E), suggesting that PKA regulates meiosis and sporulation by phosphorylating its substrates, including Rim4.
To demonstrate direct phosphorylation of Rim4 by PKA, we performed in vitro phosphorylation assays. PKA was able to phosphorylate recombinant Rim4, but not Rim4(5C), where the BBSs were mutated, at RRxS/T sites (IB, α-RRxS/T-p) (Figure 3F). Moreover, bead-immobilized EGFP-tagged recombinant Bmh1, Bmh2, or Bmh1/2 pulled down PKA-treated Rim4 (Rim4-BBS-p) with a ratio of approximately 2.5:1 but not untreated Rim4. In contrast, Rim4(5C) did not interact with Bmh1/2 even after PKA treatment (Figures 3G and 3H). These results demonstrate that PKA-mediated phosphorylation of Rim4 at BBSs promotes formation of the Rim4-Bmh1/2 complex. While Rim4 primarily forms a complex with the Bmh1/2 heterodimer in vivo, in vitro experiments showed that Bmh1 and Bmh2 homodimers efficiently interacted with Rim4-BBS-p (Figure 3G and 3H), consistent with their redundant roles in supporting meiosis and sporulation.
In contrast to PKA, previous studies have identified Ime2 as a kinase that phosphorylates Rim4 during meiosis.11 However, in vitro experiments showed that Ime2 treatment did not lead to apparent phosphorylation of recombinant Rim4 at the BBSs (Figure S3D). Additionally, acute inhibition of PKA by 1NM-PP1 abolished Rim4 phosphorylation within the BBSs in cells during prophase I and meiotic divisions (Figures 3B and 3C). Therefore, it can be concluded that PKA is primarily responsible for Rim4 phosphorylation at the BBSs, facilitating Rim4-Bmh1/2 complex formation (Figure 3I).
Cdc14 binds and dephosphorylates Rim4 on BBSs
The P-sites on Rim4 BBSs can be completely dephosphorylated within approximately 2 h upon Tpk-as inhibition (Figures 3B and 3C), indicating the presence of phosphatases that actively antagonize PKA during meiosis. Rim4 contains a PxL site (P454E455L456) in its LCD region, which is predicted to interact with Cdc14,27 an essential cell cycle phosphatase.28 To investigate whether Cdc14 dephosphorylates Rim4, a temperature-sensitive cdc14-1 allele29 was used to analyze Rim4 RRxS/T-p. Interestingly, 30°C allows normal vegetative proliferation (mitosis) in cdc14-1 cells (Figure S3B) but abolished sporulation (Figure S4A). Remarkably, inactivation of cdc14-1 (30°C) led to a 3-fold increase in Rim4 RRxS/T-p levels in cells arrested in prophase I (Figures 4A and 4B). Additionally, Rim4 was among the many proteins with enhanced RRxS/T phosphorylation in cdc14-1 cells under non-permissive temperature (Figures 4C and 4D), indicating that Cdc14 actively dephosphorylates RRxS/T-p sites in cells, including those on Rim4, even before meiotic divisions.
Figure 4. Cdc14 binds and dephosphorylates Rim4 on BBSs.
(A) IB analysis of cell lysates from cdc14-1 or Cdc14 (WT) strains at prophase I under permissive (23°C) or restrictive (30°C) conditions. Pgk1 served as a loading control.
(B) Quantification of EGFP-Rim4-p signals from (A) normalized to EGFP-Rim4 signals (IB, α-RRxS/T-p/α-EGFP).
(C) IB of whole-cell lysates with α-RRxS/T-p antibody and Ponceau S staining under the indicated conditions as in (A) but showing the whole gel images. Red asterisks indicate EGFP-Rim4-p positions.
(D) Quantification of IB signals between 20 and 150 kDa from (C), normalized by Ponceau S signals. Data are compared with the reference group (Cdc14 [WT] at 30°C) and presented as fold change.
(E) Representative IB image with the indicated antibodies, showing coIP of EGFP-Rim4 with Cdc14(C283S)-FLAG in prophase I cell lysates. Empty protein G beads served as a negative control.
(F) IB analysis showing recombinant FLAG-Cdc14 pulled down EGFP-Rim4 (pRIM4, EGFP-Rim4) from prophase I cell lysates, controlled by empty α-FLAG beads.
(G) Schematics of Rim4 variants with a WT or modified Cdc14 docking site (PxLm and PxLSic1) and Rim4 with the C-terminal 289 residues truncated (Rim4 [ΔC289]).
(H) Recombinant Cdc14-FLAG pulled down recombinant Rim4 variants using α-FLAG beads. Rim4-EGFP-His6 serves as a competitor. Red asterisk: Rim4(PxLm)-His6, black asterisk: Rim4(ΔC289)-His6. SDS-page with Cdc14-FLAG further separated from Rim4(ΔC289)-His6 is shown in (Figure S4E).
(I) Sporulation efficiency of the indicated Rim4 variants presented as tetrad percentage as in Figure 1C.
(J) IB analysis of prophase I cell lysates derived from the indicated EGFP-Rim4 variants using the indicated antibodies. Asterisks indicate non-specific bands.
(K) Quantification of EGFP-Rim4-p signals from (J) normalized to EGFP-Rim4 signals (IB, α-RRxS/T-p/α-EGFP).
(L) IB analysis of recombinant Bmh1/2-EGFP-His6 pulled down endogenous EGFP-Rim4 from prophase I cell lysates pre-incubated (30 min at 23°C) with Cdc14 (0.1 μg/μL) or mock treatment. The numbers listed below the images are the EGFP-Rim4-p signal normalized by the EGFP-Rim4 signal.
In (B), (D), (I), and (K), data are shown as mean ± SE (three independent experiments, unpaired Welch’s t test). *p < 0.05, **p < 0.01.
Release of Cdc14 from the nucleolus during anaphases marks the peak of Cdc14 activity throughout the cell cycle. However, our finding suggests that partial release of Cdc14 from the nucleolus, which is not detectable by FM because of the low intracellular level of Cdc14 (Figure S4B), might occur before meiotic divisions; e.g., during prophase I. To investigate this, ultracentrifugation (100,000 × g) was applied to detergent-free lysates of Cdc14-FLAG cells to remove the nucleolus from the supernatant (S100k), followed by IP using α-FLAG antibodies to enrich Cdc14-FLAG for IB detection. The results showed that more Cdc14-FLAG was released from the nucleolus in meiotic prophase I cells compared with cells at stationary phase or logarithmic growth phase (Figure S4C), suggesting that some Cdc14 is available to act on its substrates during meiotic prophase I.
Besides being expressed at a low level, Cdc14 only transiently binds to its substrates.30,31 To identify bona fide Cdc14 substrates, an enzymatically dead mutant of Cdc14, Cdc14(C283S), which stabilizes the Cdc14-substrate interaction, has been used in IP-based Cdc14 proteomics studies.32,33 CoIP experiments using Cdc14(C283S)-FLAG under the Cdc14 promoter revealed robust binding between Cdc14(C283S)-FLAG and Rim4 in prophase I cell lysates (Figure 4E). Although the endogenous wild-type Cdc14-Rim4 interaction was undetectable by coIP (Figure S4D), addition of bead-immobilized recombinant Cdc14-FLAG to prophase I cell lysates efficiently pulled down Rim4 at a concentration of 0.1 μg/μL (Figure 4F). Thus, Cdc14 binds Rim4 in prophase I cells.
To investigate the role of the P454E455L456 sequence in R6 of Rim4 as a Cdc14 docking site, two mutants were generated: Rim4(ΔC289), which lacks 289 residues from the C terminus, including the PxL site, and Rim4(PxLm), in which the PxL site was mutated to A454E455G456 (Figure 4G). Both mutants showed significantly reduced interaction with bead-immobilized recombinant Cdc14 (Cdc14-FLAG) compared with wild-type Rim4 and GFP-Rim4 (Figures 4H and S4E) when analyzed as recombinant proteins. Rim4(PxLm) impaired sporulation (Figure 4I) while still supporting normal meiotic DNA replication (Figure S4F), partially copying the behavior of the cdc14-1 mutant at non-permissive temperature (Figures S4A and S4G). Notably, replacing the mutated Rim4 PxL site and its flanking residues (TFTGPELNL) with a Cdc14 docking site derived from Sic1 (FKNAPLLAP) (Figure 4G) partially but significantly restored sporulation (Figure 4I). Sic1 is a known Cdc14 substrate,34 suggesting that Cdc14 docks on the P454E455L456 site of Rim4.
In cells arrested at prophase I, the level of Rim4 RRxS/T phosphorylation was significantly increased in Rim4(PxLm) mutants compared with Rim4(5C) and Rim4(PxLm+5C) mutants (Figures 4J and 4K). Conversely, treating cell lysates with recombinant Cdc14 led to reduced Rim4 phosphorylation at RRxS/T sites and decreased interaction between Rim4 and Bmh1/2 (detected by α-EGFP IB) (Figure 4L). This suggests that Cdc14 docks on the P454E455L456 site to dephosphorylate Rim4 RRxS/T-p sites, leading to disassembly of the Rim4-Bmh1/2 complex. Notably, RRxS/T-p signals unrelated to Rim4 also exhibited reduced interaction with Bmh1/2 after Cdc14 treatment (Figure S4H), indicating that Cdc14 may regulate the global interaction between Bmh1/2 and RRxS/T-p sites.
Cdc14 antagonizes PKA to safeguard intracellular Rim4 distribution via Bmh1/2
Rim4 is normally distributed evenly between the cytoplasm and nucleus, and an aberrant shift in this equilibrium indicates Rim4 dysfunction (Figures 1B, 1C, and 1F). Intriguingly, inactivation of cdc14-1 by shifting the temperature to 30°C increased the nuclear signal of Rim4 (Figures 5A and 5B), while inhibiting Tpk- as with 1NM-PP1 had the opposite effect (Figures 5C and 5D). Remarkably, the Cdc14-binding-defective Rim4(PxLm) mutant showed severe nuclear retention, while the Rim4(5C) mutant, which is defective in Bmh1/2 binding by mutating PKA-targeted BBSs, exhibited reduced nuclear localization (Figures 5E and 5F), largely reminiscent of the effects observed upon inactivation of Tpk-as and Cdc14-1, respectively. Strikingly, introduction of the 5C mutation rescued the distribution of Rim4(PxLm), and vice versa (Figures 5E and 5F), as effective as Rim4(PxLSic1) (Figures S5A and S5B). Therefore, it can be concluded that Bmh1/2 binding inhibits Rim4 nuclear export through the RRxS/T sites, which are the primary targets for PKA and Cdc14.
Figure 5. Cdc14 antagonizes PKA to safeguard intracellular Rim4 distribution via Bmh1/2.
(A) Representative FM images of EGFP-Rim4 intracellular distribution in Cdc14 (WT) or cdc14-1 strains at prophase I, 23°C (permissive condition) or 30°C (restrictive condition). Nup49-mScarlet marks the nuclear membrane. Right, quantification of the fluorescence signals along the yellow dashed lines or the red dashed arc, as in 3× magnified images. Strains were grown in YPA (2% potassium acetate, 2% peptone, and 1% yeast extract) at 30°C for 14 h before transferring into SPM. Scale bars, 5 μm.
(B) Quantitative and statistical analysis of (A), showing the nuclear/cytoplasmic EGFP-Rim4 intensity ratio, with the numbers of analyzed cells listed below, as described (Figure 1E).
(C) Representative FM images of EGFP-Rim4, showing intracellular distribution in Tpk-as strains at prophase I, with (+1NM-PP1) or without (no treatment, NT) 10 μM 1NM-PP1 treatment at 0 h, 2 h, 4 h, 6 h, and 8 h in SPM. Scale bar, 5 μm.
(D) Quantification of (C) as in (B).
(E) Representative FM images of the indicated EGFP-Rim4 variants in prophase I cells, showing their intracellular distribution. Scale bars, 5 μm.
(F) Quantification and statistical analysis of (E) as in Figure 1E. The asterisks in blue, magenta, olive, and green indicate comparisons with Rim4 (WT), Rim4(PxLm), Rim4(FLm), and Rim4(5C), respectively. The blue line and magenta line indicate a ratio of 1 and the median value of Rim4(PxLm), respectively.
(G and H) Recombinant Bmh1/2-GFP (1:1) pulled down recombinant Rim4-His6 under the indicated conditions, including PKA treatment and with total yeast RNAs.
(G) SYPRO-Ruby staining of input and pull-down samples resolved by SDS-PAGE.
(H) Quantification of (G) from three independent experiments, shown as mean ± SD, unpaired t test. **p < 0.01.
In (B), (D), and (F), data from three independent experiments were analyzed. Red bars indicate median ± 95% CI. Statistical analysis was performed using Dunn’s test (B and F) and Mann-Whitney test (D). *p < 0.05, **p < 0.01, ***p < 0.001.
To understand how Bmh1/2 binding affects Rim4 distribution, we have shown previously that Rim4 variants with mutated RRMs, such as Rim4(FLm), Rim4(R2E), Rim4(R3E), and Rim4(R5E/A), exhibited nuclear retention (Figure 1F). This led to the proposal that Bmh1/2 binding interferes with Rim4-mRNA complex formation. Several pieces of evidence support this hypothesis. First, the 5C mutation rescued the distribution of Rim4(PxLm) but not that of Rim4(FLm), indicating that functional Rim4 RRMs are required for Bmh1/2-regulated Rim4 distribution (Figures 5E and 5F). Second, BBS-T216, BBS-S367 and BBS-T368 are located within the RRMs (RRM2 and RRM3); moreover, T216 phosphorylation is required for stable Rim4-Bmh1/2 interaction at prophase I (Figure 2F), while T216C, S367C, and T368C reduced sporulation efficiency (Figure 2I), suggesting that Bmh1/2 binding at these BBSs could affect Rim4-mRNA interaction. Third, analysis of the Rim4-Bmh1/2 complex purified by IP from prophase I cell lysates showed no detectable mRNAs, indicating that Bmh1/2 binding excludes mRNAs from the complex (Figure 2C). Last, and most importantly, using PKA-treated Rim4, Rim4(BBS-p), it was confirmed that total yeast mRNAs compete with Bmh1/2 for binding to Rim4(BBS-p) in vitro (Figures 5G and 5H), indicating that Rim4 interaction with Bmh1/2 prevents Rim4-mRNA complex formation and vice versa.
Determining whether PKA phosphorylates Rim4 outside of RRxS/T sites has proven to be challenging. However, the Cdc14 docking site, P454E455L456, located in R6 of Rim4, provides a convenient position for Cdc14 to target S/T residues in R5, R6, and R7 (Figure S5C). Previous findings showed that mutations in R5-E, R6-E, and R7-E resulted in nuclear retention of Rim4 (Figure 1E). While the BBS-S367 and BBS-T368 are present in R5 and BBS-S525 is present in R7, the mechanism by which R6-E leads to nuclear retention of Rim4 remains unknown. Interestingly, introducing the R6-A mutation partially alleviated nuclear retention of Rim4(PxLm) (Figures S5D and S5E), although it was not as effective as the 5C mutation (Figure 5F), indicating that R6 harbors P-sites targeted by Cdc14. Furthermore, Cdc14 exhibits a preference for Serine-Proline (SP) target motifs, particularly in intrinsically disordered regions (IDRs).35,36 Rim4 contains two SP motifs in its LCD: S532P533 (SP1) in R7 and S658P659 (SP2) in R8. Mutating S532 and S658 to alanine (S532A/S658A, i.e., SA12) (Figure S5C) partially reduced nuclear retention of Rim4(PxLm) (Figures S5D and S5E). This suggests that Cdc14 targets Rim4 P-sites beyond the BBSs.
As expected, the 5C mutation, SA12 mutation, or R6-A mutation did not rescue sporulation in Rim4(PxLm) cells (Figure S5F). This indicates that Cdc14 regulates Rim4 function through multiple P-sites, including the BBSs, which will be further discussed later.
Bmh1/2 temporally protects Rim4 from autophagy
The timing of Rim4 degradation is tightly regulated and occurs specifically during meiotic divisions to ensure timely translation of its sequestered mRNAs. Before Rim4 degradation, formation of cytosolic puncta by Rim4, which co-localizes with Atg8 during meiotic divisions, was observed.18 This suggests that Rim4 is selectively enriched in forming or enclosed autophagosomes. Interestingly, despite being highly abundant, Bmh1/2 (Bmh1/2-EGFP) does not co-localize with Rim4 puncta (mScarlet-Rim4) (Figure S6A), indicating that Bmh1/2 dissociates from Rim4 prior to selective autophagic Rim4 degradation.
To further investigate whether release of Bmh1/2 triggers Rim4 degradation through selective autophagy, the activity of Atg1-as (Atg1[M102G]) was measured in prophase I cell lysates using a chemical genetic approach (Figure 6A) with recombinant Rim4 supplementation. A substrate of selective autophagy can stimulate Atg1 activity.37 It was observed that recombinant Rim4 stimulated Atg1-as activity at concentrations ranging from 0.1–0.4 μM (Figures 6B and 6C), which falls within the physiological range of intracellular Rim4 levels (Figure S6B; ~0.2–1.2 μM). Remarkably, PKA-treated Rim4, Rim4-BBS-p, was less efficient than Rim4 in activating Atg1 (Figures 6B and 6C). It is likely due to the presence of endogenous free Bmh1/2 proteins in the cell lysate that form complex with Rim4-BBS-p. Consistently, supplementing Bmh1/2 into the cell lysates further suppressed Atg1-as activation triggered by Rim4BBS-p (Figures 6B and 6C). Because Cdc14 is responsible for driving Rim4-Bmh1/2 disassembly, the immunoprecipitated Rim4-FLAG complex (IP, α-FLAG) from prophase I cell lysates (Figure 2A, lane 1) was treated with recombinant Cdc14 (0.1 μg/mL) or mock treated, followed by an Atg1-as kinase assay. As shown in Figure S6C, the Rim4-Bmh1/2 complex suppressed Atg1-as activity and only stimulated Atg1-as activity after Cdc14 treatment. These findings lead to the conclusion that the binding and release of Bmh1/2 during meiosis play a crucial role in determining the timing of Rim4 degradation through selective autophagy.
Figure 6. Bmh1/2 temporally protects Rim4 from autophagy.
(A) Schematic of the chemical-genetic strategy for monitoring Atg1-as kinase activity in vitro. Atg1-as thiophosphorylates substrates using a bulky ATPγS analog (N6-PhEt-ATP-γ-S). Thiophosphorylated substrates can be alkylated with para-nitrobenzyl mesylate (PNBM) and detected by IB using anti-thiophosphate ester (α-thioP) antibodies.
(B and C) Atg1-as kinase assay in synchronized meiotic cell lysates (collected at prophase I, 12 h in SPM) supplemented with the indicated amounts of Rim4, PKA-phosphorylated Rim4 (Rim4-p), or a premixed combination of Rim4-p and Bmh1/2-EGFP.
(B) IB with the indicated antibodies shows Atg1-as kinase activity (Atg1-as-p), indicated by Atg1-as autophosphorylation. Pgk1 serves as a loading control.
(C) Quantification as sums of anti-ThioP signal intensity, shown as mean ± SD from three independent experiments (unpaired t test; *p ≤ 0.05, **p ≤ 0.01).
(D) CoIP of EGFP-Rim4 or EGFP-Rim4(5C) with Bmh1-FLAG (IP, α-FLAG) in cells collected at the indicated time (6 h in SPM, early meiotic stage; 12 h in SPM, prophase I without NDT80 induction; 16 h in SPM, meiotic divisions 4 h after NDT80 expression).
The immunoprecipitated Rim4-FLAG complex is predominantly found in association with Bmh1/2 in cells during prophase I (12 h in SPM) (Figures 2A and 2C). Consistent with this observation, the intracellular level of Rim4 phosphorylated at RRxS/T sites reaches its peak around prophase I, accompanied by the strongest interaction with Bmh1, as determined by IB(α-EGFP) and Bmh1-FLAG IP (α-FLAG) (Figures 6D and S6D). These results indicate a shift in equilibrium toward formation of the Rim4-Bmh1/2 complex around prophase I, which presumably facilitates release of mRNAs from the existing Rim4-mRNA complex.
It has been shown previously (see the related paper18 in this issue of Cell Reports) that mRNAs protect Rim4 from selective autophagy until they are released.18 Therefore, our findings indicate that Bmh1/2 replaces the role of mRNAs as the primary protector of Rim4 around prophase I, likely driven by increased PKA activity, reduced Cdc14 activity, or both. Importantly, during meiotic divisions, the level of Rim4 phosphorylated at RRxS/T sites decreased significantly (Figures 3B, 3C, and 6D), in line with the dramatically decreased interaction between Rim4 and Bmh1 (16 h in SPM) (Figure 6D), coinciding with a decrease in Rim4 protein level (Figure 6D). In contrast, Rim4(5C), which is defective in binding to Bmh1/2 (Figures 2F and 2G), is more stable than Rim4 at the end of meiotic divisions (16 h in SPM) (Figure 6D), likely because of being trapped in a complex with mRNAs. These findings suggest that, while formation of the Rim4-Bmh1/2 complex protects Rim4 from selective autophagy, it seems to facilitate its degradation during meiotic divisions because of subsequent Rim4 dephosphorylation.
Cdc14 orchestrates autophagic Rim4 degradation
The level of Rim4 phosphorylated at RRxS/T sites and the interaction between Rim4 and Bmh1 dramatically decrease during meiotic divisions (Figure 6D), coinciding with upregulation of Cdc14 activity and its relocation from the nucleus to the cytosol (during anaphase). We hypothesize that cytosolic Cdc14 is responsible for the massive release of Bmh1/2 from Rim4 after Bmh1/2 stimulated Rim4-mRNA disassembly, allowing autophagic Rim4 degradation in the cytosol. Indeed, through IB analysis, we observed that the intracellular level of Rim4(PxLm) is higher than wild-type Rim4 throughout meiosis (Figures 7A-7C), presumably because of enhanced Rim4(PxLm)-Bmh1/2 interaction. In line with this reasoning, introduction of 5C abolishes the stabilizing effect of PxLm on Rim4, resulting in autophagy-mediated degradation of Rim4 (Rim4[PxLm+5C]) during meiotic divisions (Figures 7A-7C). These observations indicate that Cdc14 releases Bmh1/2 from Rim4 during meiotic divisions to enable programmed degradation of Rim4. Notably, the intracellular level of Rim4(PxLm+5C) at prophase I is lower than that of wild-type Rim4 (Figures 7A and 7C), indicating that lack of Bmh1/2 binding and Cdc14 regulation caused premature Rim4 degradation; consistently, we observed slightly increased autophagic Rim4(PxLm+5C) degradation compared with Rim4 at prophase I (Figure 7C). Interestingly, Rim4(PxLm) exhibited slightly enhanced autophagic degradation at prophase I, in contrast to meiotic cell divisions (Figure 7C). This finding provides additional support for our hypothesis that relocation of Cdc14 into the cytosol during meiotic divisions leads to a shift in its role. Specifically, instead of facilitating formation of the autophagy-resistant Rim4-mRNA complex, cytosolic Cdc14 now stimulates autophagic degradation of Rim4.
Figure 7. Cdc14 orchestrates autophagic Rim4 degradation.
(A) IB of cell lysates from EGFP-Rim4 variant strains collected at prophase I (12 h in SPM) or post-meiosis stage (20 h in SPM) using the indicated antibodies. Pgk1 serves as a loading control.
(B) Quantitative analysis of (A), measuring the ratio of free EGFP to total EGFP signal, which indicates autophagic degradation of EGFP-tagged Rim4 variants.
(C) Quantitative analysis of(A), measuring full-length EGFP-Rim4 intracellular level normalized by Pgk1. (B) and (C) Data from three independent experiments are presented as mean ± SE and analyzed using unpaired Welch’s t test. *p < 0.05, **p < 0.01, ***p < 0.001.
(D) Quantitative analysis of Figure S7A, measuring the ratio of nuclear versus cytoplasmic EGFP-Rim4 variant intensity at different time points in SPM, with 1NM-PP1 to inhibit autophagy (12 h in SPM) or mock treatment. β-Estradiol was added to the cells at 12 h in SPM to induce Ndt80 expression. Data are shown as mean ± SE from three independent experiments. More than 300 cells of each strain were analyzed for every indicated time point. Šidák’s multiple-comparisons test after one-way ANOVA was performed to compare different strains between the 0.5 h and 12 h time points or 14 and 22 h time points. The p values are shown in the table on the right. White, p = 0.05; yellow, p < 0.05 (significant; *p < 0.05, **p < 0.01); blue, p > 0.05.
(E) Model illustrating the spatiotemporal regulation of Rim4 function and stability by PKA and Cdc14. Left: Before meiotic divisions, Cdc14 primarily promotes Rim4-mRNA assembly in the nucleus because of its nuclear localization. Right: during meiotic anaphase, increased PKA activity facilitates Rim4 to release bound mRNAs, resulting in Rim4-Bmh1/2 complex formation. Next, relocation of Cdc14 from the nucleus to the cytoplasm during anaphase drives Rim4-Bmh1/2 disassembly and therefore determines the timing of Rim4 degradation by autophagy and translation of Rim4-sequestered mRNAs. Created with BioRender.
Last, we performed FM analysis of EGFP-tagged Rim4, Rim4(PxLm), and Rim4(PxLm+5C) during meiosis progression. The temporal profile of the intracellular EGFP signal from EGFP-Rim4 cells closely matches that of EGFP-Rim4 (PxLm+5C) cells, while the signal of EGFP-Rim4(PxLm) cells is significantly higher (Figures S7A and S7B). Notably, this analysis cannot discriminate EGFP-Rim4 variants from free GFP; nonetheless, the result is consistent with our IB analysis (Figures 7A and 7C). Furthermore, the nuclear retention phenotype of Rim4(PxLm) is observed at the meiosis initiation stage (0.5 h in SPM) and increases as meiosis progresses, reaching its peak at prophase I (12 h in SPM) (Figure 7D), in correlation with the level of Rim4 phosphorylated at RRxS/T sites (Figure 6D). Remarkably, the nuclear retention phenotype of Rim4(PxLm) gradually disappears during meiotic divisions, approaching the wild-type level (Figures 7D and S7A), possibly because of relocation of Cdc14 into the cytosol, which will be discussed later. Strikingly, inhibition of autophagy during meiotic divisions preserves nuclear retention of Rim4(PxLm) (Figure 7B), indicating that upregulated autophagic Rim4 degradation in the cytosol by cytosolic Cdc14 may prevent Rim4(PxLm) from entering the nucleus.
In summary, spatiotemporally controlled phosphorylation (by PKA) and dephosphorylation (by Cdc14) of Rim4, particularly within RRxS/T sites, determine Rim4 distribution, function, and protein stability by regulating the interaction between Rim4 and Bmh1/2 and, thereby, Rim4-mRNA complex formation (Figure 7E).
DISCUSSION
The roles of Bmh1/2 interaction with Rim4
Bmh1 and Bmh2 are 14-3-3 proteins abundantly present in the nucleus and cytoplasm of yeast cells, and 14-3-3 proteins are known to be involved in a wide range of biological processes.22,38-43 The rigid α-helical backbone of 14-3-3 proteins allows them to modulate the structure of their binding partners.44 In the soluble heterotrimeric Rim4-Bmh1/2 complex purified from cell lysates, no other macromolecular components, such as RNAs or proteins, were detected (Figure 2C), indicating that Bmh1/2 sequester and isolate Rim4 from interactions.
Rim4 contains five BBSs. Our data demonstrate that Bmh1/2 and mRNA compete for binding to Rim4 (Figures 5G and 5H), likely through RRM2 (BBS-T216) and RRM3 (BBS-S367 and BBS-T368). This competition may also involve unstructured segments that regulate Rim4-mRNA interaction, such as R6, as computationally predicted (Figure S1E). Alternatively, phosphorylation in R6 might affect BBSs phosphorylation, thereby indirectly regulating Rim4-mRNA interaction. Thus, depending on the binding affinities of each BBS to Bmh1/2 and their phosphorylation status, by docking on different pairs of the BBSs, Bmh1/2 can either keep mRNAs away from Rim4 or establish a threshold for Rim4-mRNA complex formation. We speculate that this mechanism ensures that Rim4 interacts only with its specific mRNA substrates that tightly associate with the three RRMs of Rim4. Intriguingly, sporulation in S367C/T368C cells was higher than that in the 5C (T216C/S367C/T368C/S525C/S607C), in which sporulation is higher than the individual S367C or T368C mutants (Figure 2I), indicating that different BBSs might carry counteracting effects on Bmh1/2-regulated Rim4 function.
Some state(s) of the Rim4-Bmh1/2 complex might regulate Rim4 beyond mRNA interaction. Particularly, occupation of BBS-S607 by Bmh1/2 masks a nearby nuclear localization signal (NLS) of Rim4, which contains K600R601, as identified in our accompanying manuscript.18 This could directly regulate the nuclear import of Rim4 in an mRNA-independent manner under certain conditions. In addition, Rim4 self-assembly into aggregates has been reported; we found that Rim4-Bmh1/2 can exist as a stable heterotrimer (Figure 2C), therefore, Bmh1/2 might inhibit Rim4 self-assembly. Consistently, BBS-S525 and BBS-S607 reside in the LCD region that mediates Rim4 self-assembly.13
PKA-mediated site-specific phosphorylation on Rim4 versus Ime2-mediated cumulative phosphorylation
Ime2 phosphorylates Rim4 extensively during meiotic divisions,14 whereas PKA-mediated phosphorylation of Rim4 within the BBSs (RRxS/T) peaks around prophase I (Figure 6D). PKA phosphorylates the BBSs (RRxS/T) of Rim4, facilitating its interaction with Bmh1/2, which negatively regulates Rim4-mRNA complex formation and protects Rim4 from premature degradation by autophagy. On the other hand, Ime2 mediates cumulative phosphorylation of Rim4, leading to its degradation via the proteasome.14 Therefore, PKA and Ime2 regulate Rim4 through distinct mechanisms.
However, we speculate that Ime2 might also regulate Rim4-mRNA complex formation, although the specific S/T residues targeted by Ime2 remain to be identified. Notably, mutations mimicking hyperphosphorylation and hypophosphorylation in the RRM1 (R2) and RRM2 (R3) domains of Rim4 result in distinct subcellular distribution patterns (Figure 1F), both leading to loss of Rim4 function (Figures 1B and 1C). Additionally, R6 of Rim4 contains P-sites that regulate Rim4 distribution and likely play a role in mRNA binding. Although some of these P-sites may be targets of Cdc14-mediated dephosphorylation (Figures S5E and S5F), the kinase responsible for their phosphorylation remains elusive. It is plausible that Ime2 phosphorylates the P-sites in the RRMs and their flanking sequences (e.g., R6), thereby modulating Rim4-mRNA complex assembly.
Interestingly, Ime2-mediated phosphorylation also influences Rim4 self-assembly, promoting enhanced interaction with CLB3. The significance of PKA and Ime2 phosphorylation-regulated Rim4 self-assembly in the context of Rim4-mRNA interaction warrants further investigation, particularly concerning its impact on selection of meiotic transcripts.
The dual role of Cdc14 in Rim4 regulation
Our study reveals that Rim4 undergoes dynamic shuttling between the nucleus and cytoplasm,18 reaching an equilibrium between the two compartments (Figures 1D and 1E). Before meiotic divisions, Cdc14 resides primarily in the nucleus. Our data indicate that Cdc14 plays a role in facilitating Rim4 nuclear export by dissociating Bmh1/2 from Rim4. As mRNAs are transcribed in the nucleus, releasing Bmh1/2 from Rim4 in the nucleus ensures efficient Rim4-mRNA complex formation, which drives Rim4 nuclear export.
In the cytoplasm, we speculate that the Rim4-mRNA complex remains relatively stable and inactive. However, disassembly of a small portion of the Rim4-mRNA complex might occur, driven by a low level of PKA-mediated phosphorylation at the BBSs (RRxS/T) sites or by spontaneous mRNA release. The resulting Rim4-Bmh1/2 complex is then transported back to the nucleus to load mRNAs again. Bmh1/2 and mRNAs play a protective role by preventing Rim4 degradation through selective autophagy. Therefore, although autophagy is active throughout meiosis, Rim4, whether in complex with Bmh1/2 or mRNAs, remains largely stable before meiotic divisions (Figure 7E, left).
Around prophase I and meiosis I, when translation is scheduled for Rim4-bound mRNAs, Rim4 is intensively phosphorylated by PKA. This phosphorylation enables Bmh1/2, as indicated by the peak of Rim4-Bmh1/2 interaction (Figure 6D), to facilitate timely release of Rim4-sequestered mRNAs. Subsequently, during anaphase I, Cdc14 activity is upregulated and relocates to the cytoplasm, where Cdc14 counteracts PKA to release Bmh1/2 from Rim4. Likely because of the low concentration of cytosolic mRNAs or their sequestration by ribosomes, Rim4 cannot bind mRNAs efficiently in the cytosol to prevent its degradation by autophagy (Figure 7E, right). It is important to note that, when Cdc14 in the cytosol removes Bmh1/2 from protecting Rim4, Cdc14 upregulates autophagy by stimulating Atg1 activity.45 Thus, in the cytoplasm, Cdc14 sensitizes Rim4 degradation while upregulating autophagy.
Our data indicate that Cdc14 is partially active before meiotic divisions. Under 30°C, mitosis in cdc14-1 cells was normal, but sporulation was zero, which could be rescued by lowering the temperature to 23°C (Figure S4A); in contrast, mitosis was blocked by increasing the temperature to 35°C (Figure S4A). Presumably, the activity of Cdc14-1 is higher when the temperature is lower. We speculate that meiotic Cdc14 activity is regulated or used differently from mitosis; e.g., phosphorylating the meiosis-specific RBP Rim4. Notably, at prophase I, Rim4 is among many proteins with phosphorylation affected by cdc14-1 inactivation (Figures 4C and 4D), indicating that the substrates and their regulation by Cdc14 before meiotic divisions are yet to be explored.
Limitations of the study
The present study has several limitations that should be acknowledged. First, characterization of BBSs using IB, IP, and FM analyses suggests that BBS-T216 and BBS-S525 are the primary sites for stable Rim4-Bmh1/2 interaction during prophase I. However, this pattern may change at different meiotic stages, and further investigation is required to elucidate these variations. Additionally, the RRxS/T-p antibody employed in this study was unable to detect BBS-T368 phosphorylation (RFST-p), leaving this aspect unclear and requiring future research. Secondly, the Rim4 sequence was divided into regions (R1–R9), and all S/Ts in each region were mutated to analyze their contribution to phosphorylation-mediated Rim4 regulation. However, it is worth noting that there is little in vivo evidence of phosphorylation of some S/Ts in certain regions, such as R2, R3, and R5 (Figure 1A). Nonetheless, it is possible that certain phosphorylation events could destabilize Rim4, rendering them undetectable using the employed methods. Nonetheless, this strategy allowed us to focus on promising S/Ts among the 114 total sites screened. For example, we discovered that BBS-T216 (in R3) is phosphorylated and plays a critical role in stable Rim4-Bmh1/2 interaction despite previous proteomics studies failing to detect in vivo BBS-T216 phosphorylation.14
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Fei Wang (Fei.Wang@UTSouthwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
S. cerevisiae strain and manipulation
Saccharomyces cerevisiae strains were derivatives of W303 (ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100) (Table S1). Unless otherwise indicated, the analog sensitive version of ATG1(Atg1-as) was created by gatekeeper residue change (M102G) as described earlier37,47 and introduced into the background of listed strains to allow conditional autophagy inhibition by 1NM-PP1.
Tpk-as (Tpk1[M164G]/Tpk2[M147G]/Tpk3[M165G]) strain were created by similar strategy as described earlier.26 The PKA activity in this strain can be conditional inhibited by 1NM-PP1. For long term inhibition, 10 μM 1NM-PP1 was added to the sporulation medium every 2 h from 0 h, until 8 h (Figure 3A). For acute inhibition, 10 μM 1NM-PP1 was added to the pre-meiosis cells at 8 h in SPM, and harvest the prophase I cells at 12 h in SPM; or to the prophase I cells at 12 h in SPM, and harvest the meiotic division cells at 14 h in SPM.
Deletion strains were constructed in a parent background by PCR-mediated knock-out with one of the following drug resistance or prototrophic marker cassettes: pFA6a-kanMX6/pFA6a-NatMX48/pKlURA/pCgHIS.49 C- terminal tagging at the endogenous genomic locus was introduced by PCR-mediated epitope tagging as previously described.50
Specifically, strains with pGAL1:Ndt80 GAL4(848)-ER were constructed by replacing the endogenous NDT80 promoter with the inducible GAL1,10 promoter as described.17
The N terminus EGFP tagged Rim4 strains were created by the following steps: the endogenous RIM4 ORF was deleted by URA3 as described above. Then the pRS303 backbone plasmids carrying pRIM4:EGFP-Rim4 variants (See Table S2) were linearized and integrated into the Δrim4 yeast genome at his3 locus and selected by histone prototrophy, respectively.
CDC14 temperature-sensitive mutant (cdc14-1) was constructed in W303 haploid by introducing a single nucleotide mutation (G918 to A) in the genomic CDC14 open reading frame region. The temperature-sensitive strain was isolated by replica plating and restrictive temperature lethality. The colonies that lost viability at 37°C were selected after plates grown in 23°C were replicated to plates in 37°C and cultured for 2 days. The mutation site in the selected colony was confirmed by sanger sequencing. The temperature-sensitive mutant verified was then mated to a CDC14 wild type haploid, and the diploid was induced sporulation at 23°C and dissected through yeast spore dissection microscope. haploid spores with both mating type a and a that lost viability at 37°C were selected after replica plating from 23°C to 37°C.
The pNUP49:Nup49-mScarlet fragment was carried on a pRS304 backbone plasmid (pFW338). The plasmid was linearized and integrated to the yeast genome at TRP1 locus, and selected by tryptophan prototrophy.
All strains used in this study were diploids mated by the haploids harboring the needed genotype (Table S1).
Media
The following media were used in this study. YPD (2% peptone, 1% yeast extract, 2% glucose), YPA (2% peptone, 1% yeast extract, 2% potassium acetate), SD (0.67% yeast nitrogen base, 2% glucose, auxotrophic amino acids and vitamins), and standard sporulation medium SPM (0.6% potassium acetate, pH 8.5). The SD-dropout medium was made with dropout stock powder lacking histidine (-His), tryptophan (-Trp), leucine (-Leu) and/or uracil (-Ura).
METHOD DETAILS
Sporulation and Synchronized Ndt80 Arrest/Release
A single colony of yeast strains from YPD plate were picked, spread on YPG (3% glycerol) plates and grown at 30°C for 2 days. Colonies grown out were spread to YPD plates and grown until cells formed a lawn (~24 h). Cells on plates were collected and suspended in YPA liquid medium (OD600 = 0.3) and grown for 14-16 h at 30°C. Cells were then pelleted, washed with water twice and resuspended in SPM to final OD600 = 2. For M-phase synchronization, following incubation in SPM for 12 h, the synchronized strains containing pGAL1:Ndt80 and GAL4(848)-ER recombinant transcription regulator were released from the prophase I arrest by addition of 1 μM β-estradiol to induce Ndt80 expression. To assess the sporulation efficiency, percentage of tetrads were counted after 48 h in SPM at room temperature. At least 300 cells for each strain were counted under bright field with Olympus microscope (BX40×, 40× objective).
Vegetative growth and growth curve determination
The cells were inoculated from a single colony of a fresh YPD streak to YPD medium, and cultured at 30°C with 250 rpm shaking for overnight. Back diluted the strain to fresh YPD medium to concentration of 0.2 OD600. The cells were then growing at 30°C with 250 rpm shaking. The cells reached to about 0.8 OD600 after 4 h culture, and the cells were collected for transforming.
For growth curve determination, the overnight culture was back diluted to 0.025 OD600 to fresh YPD medium. The cells were then growing at indicated condition, with 250 rpm shaking. After 4 h culture, start to check the OD600 value every 90 min until 960 min.
The exponential growth part of the curve (from the beginning to OD600 ≈ 1.6) was used to do the non-linear regression with Malthusian equation with GraphPad Prism 10:
In which, is the population at minutes; is the start population; is the rate constant; is the time in minute. Then apply , and resolve the doubling time ():
DNA replication analysis with flow cytometry
Cells were collected by 12 h and fixed in 70% ethanol at 4°C for overnight. Afterward, the cells were pelleted and resuspended in 50 mM Sodium Citrate (pH 7.0) followed by sonication at 30% power for 15 s. Cells were pelleted again and resuspended in the same solution. The cells were treated with 0.25 mg/mL RNase A at 37°C for overnight. Next, the cells were incubated in 1 μM SYTOX Green dye at RT in the dark for at least 1 h before analyzed on flow cytometer (BD FACSCalibur). The forward scatter (FSC) and side scatter (SSC) were used to gate the living cells. The cell counts (histogram) of green (530/30 nm) signal in gate were collected and analyzed by Flowing Software 2.5.1 (https://bioscience.fi/services/cell-imaging/flowing-software/).
Plasmid construction
Plasmids used in this study are listed in Table S2. pRS303-pRIM4:EGFP-Rim4 (pFW208) was constructed by subcloning genomic Rim4 with its promoter and terminator amplified by PCR into SalI/NotI restriction sites of pRS303, followed by inserting EGFP with a linker containing a PacI restriction site amplified by PCR between the promoter and start codon of Rim4 by Gibson Assembly.17 The mutagenesis of Rim4 as shown in Table S2 were introduced by overlap extension PCR to amplify the mutant Rim4 CDS and terminator and cloned into PacI and NotI of pFW208.
The plasmids for Rim4 variants expression were constructed by subcloning PCR-amplified Rim4 or Rim4 mutants CDS into the NdeI and KpnI restriction site of pET29b(+) by Gibson Assembly. The plasmids for Bmh1/2-GFP and Bmh1-3FLAG expression were constructed by subcloning overlap extension PCR-amplified Bmh1/2-GFP or Bmh1-3FLAG into NdeI and KpnI restriction sites of pET29b(+).
Protein expression and purification
Recombinant protein purification
pET-based protein expression in BL21 (DE3) E. coli cells was induced by IPTG as described previously.51 RIM4 variants-6× His/6× His-3Flag-Cdc14 were expressed at 16°C overnight. Cells were collected by centrifugation, resuspended in bacteria lysate buffer (BLB: 50 mM Tris-HCl pH 8.0, 300 mM NaCl, 10 mM MgCl2,10 mM Imidazole, 10% glycerol, 5 mM 2-ME, 1 mM PMSF, 1× cOmplete protease inhibitor cocktail [Roche, 11873580001] and 1 ng/μL Pepstatin), and lysed using a High-Pressure Cell Press Homogenizer (Avestin Emulsiflex-C5). The lysate was supplemented with 0.1 mg/mL DNase I, 0.1 mg/mL RNase A, and 0.1% Triton X-100 and cleared by centrifugation at 30,000g for 1 h at 4°C. The supernatant was applied to an NTA-Ni column (Qiagen, 30230), and washed three times with BLB supplied with gradually ascent concentration of imidazole (10 mM, 25 mM, 50 mM) and gradually descent concentration of Sodium Chloride (500 mM, 300 mM, 150 mM). The proteins with 6× His were eluted with 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 250 mM Imidazole, 10% glycerol and 5 mM 2-ME. The eluted proteins were separated by Superdex 200 Increase 10/300 GL column (GE Healthcare) equilibrated in size exclusive buffer (SEC: 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 10% glycerol, 2mM 2-ME). Finally, purified proteins were concentrated in SEC and frozen in liquid nitrogen. The concentration of the proteins were determined by Bradford Assay52 and the purity of the proteins were qualified by SDS-PAGE and Coomassie Bright Blue R-250 staining (Figure S3C).
IP of proteins expressed in yeast
To isolate the Rim4-FLAG complex, frozen cell lysate powder (~500 OD600 units) was thawed in 2 mL 1 × IP buffer (50 mM HEPES-KOH [pH 6.8], 150 mM KOAc, 2 mM Mg(OAc)2, 1 mM CaCl2, 15% glycerol, 1% NP-40, 1× cOmplete protease inhibitor cocktail [Roche, 11873580001], 1× PhosStop phosphatase inhibitors[Roche, 4906845001]) and cleared twice by centrifugation at 1,000 × g for 5 min at 4°C. 10 μL of Protein G Dynabeads (Invitrogen) that were loaded with mouse anti-FLAG M2 antibody (Sigma, F3165) were then added to the cell extract, incubated for 3 h at 4°C with constant agitation. The beads were collected, washed 5 times with IP buffer, and bound proteins were eluted with 25 μL 1 mg/mL 3 × FLAG peptide (Sigma, F4799) at 4°C. Eluates were aliquoted, frozen in liquid nitrogen, and stored at −80°C. The purified Rim4-FLAG complex was resolved by SDS-PAGE and analyzed by SYPRO Ruby staining (Thermo-Fisher, S12000).
Cell collection for co-immunoprecipitation, immunoblotting and ATG1 kinase activity assay
Yeast cells grown in SPM or YPD medium were pelleted at 3,000 × g for 5 min, 4°C, washed with ice-cold distilled water containing 10 mM PMSF. The pellets were resuspended in ice-cold yeast lysis buffer (YLB: 50mM HEPES-KOH, pH 6.8, 150 mM KOAc, 2 mM MgCl2, 1 mM CaCl2, 0.2 M sorbitol, 10mM PMSF, 2× protease inhibitor cocktails [Roche, 11873580001]), dropped into liquid nitrogen, ground using a Retsch ball mill (PM100 or MM400) and stored at −80°C.
Co-IP and pull-down assays
Weigh 200 mg of cell powder from the ball milling. Thaw in 1 mL 1 × IP buffer (50 mM HEPES-KOH [pH 6.8], 150 mM KOAc, 2 mM Mg(OAc)2, 1 mM CaCl2, 15% glycerol, 1% NP-40, 1× cOmplete protease inhibitor cocktail [Roche, 11873580001], 1× PhosStop phosphatase inhibitors[Roche, 4906845001]) on ice for 5 min and cleared twice by centrifugation at 1,000 × g for 5 min at 4°C. Incubate the supernatant (input) with 5 μL ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich, A2220) (Figures 2A, 2F, 6D, S4C, and S6D) or Invitrogen Protein G Dynabeads (Fisher Scientific, 10004D) incorporating Mouse-anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, F3165) (Figures 4E and S4D) at 4°C for 30 min. The beads were collected, washed 5 times with IP buffer, and bound proteins were eluted with 25 μL 1 mg/mL 3 × FLAG peptide (Sigma, F4799) at 4°C. The eluates were analyzed by immunoblotting.
The recombinant proteins were incubated with 5 μL of ANTI-FLAG M2 Affinity Gel (Sigma-Aldrich, A2220), Invitrogen His-Tag Isolation and Pull Down Dynabeads (Fisher Scientific, 10103D) or Invitrogen Protein G Dynabeads (Fisher Scientific, 10004D) incorporating Mouse-anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, F3165) or Mouse-anti-GFP monoclonal antibody (Roche, 11814460001) at 4°C for 30 min. For FLAG pull down (Figures 4F, 4H, and S4E), the proteins on beads were eluted by 25 μL 1 mg/mL 3 × FLAG peptide (Sigma, F4799); for His-tag pull down (Figures 2G and 4L), the proteins on beads were eluted by 25 μL 250 mM imidazole; for GFP pull down (Figures 3G and 5G), the proteins on beads were eluted by heating at 70°C for 10 min in 2× SDS loading buffer. The eluates were analyzed by immunoblotting.
Immunoblotting
For cell lysate immunoblotting, weigh a portion of cell powder from ball milling, add 1 mL/mg cell powder volume of 1 × IP buffer (50 mM HEPES-KOH [pH 6.8], 150 mM KOAc, 2 mM Mg(OAc)2, 1 mM CaCl2, 15% glycerol, 1% NP-40, 1 × cOmplete protease inhibitor cocktail [Roche, 11873580001], 1 × PhosStop phosphatase inhibitors[Roche, 4906845001]), and thaw on ice for 5 min cleared twice by centrifugation at 1,000 × g for 5 min at 4°C. 2× SDS Loading Buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 0.1% BPB, 20% Glycerol, 10% 2-ME) was added to the cell lysate supernatant or the co-IP/pull down assay eluates, to the final concentration of 1 ×. Heated at 70°C for 5 min. The supernatants were separated by SDS-PAGE (30 min at 200 V) using the 4–20% gradient PAGE gels (26-well: Criterion Tris-Glycine [TGX] Stain-Free gels [Bio-Rad, 5678095]; 15-well: SuperPAGE Bis-Tris gels [GenScript, M00657]). Subsequentially, the protein samples in the gels were electroblotted onto nitrocellulose membranes (Bio-Rad, 1620115) using a Trans-Blot semi-dry transferring cell (Bio-Rad, 1703940).
Transferred membranes were stained with 0.1% Ponceau S solution. After that, block the membrane in 5% skim milk or 1% BSA in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Tween 20). Next, incubate sequentially in primary antibodies and HRP-, StarBright B700- or Alexa Fluor 488-conjugated secondary antibodies. Ponceau S staining and IB images were captured by ChemiDoc MP imaging system (Bio-Rad, 12003154).
ATG1 kinase activity assay
The ATG1 kinase activity assay was described in our previous study.45 Briefly, the cell lysate was mixed with 1 × kinase buffer (150 mM KOAc, 10 mM Mg[OAc]2, 0.5 mM EGTA, 5 mM NaCl, 20 mM HEPES-KOH [pH 7.3], 5% glycerol) in equal volume (w/v) on ice and thawed by pipetting. After spin at 1000 ×g for 5 min twice, supernatants were mixed with different concentration of recombinant Rim4 and equal volumes of 2 × kinase mix (kinase buffer, energy mix [90 mM creatine phosphate, 2.2 mM ATP, 0.45 mg/mL creatine kinase] and 0.2 mM N6-phenylethyl-ATPγS [N6-PhEt-ATPγS, Axxora, BLG-P026-05]) and incubated for 1.5 h at room temperature. Reactions were quenched with 20 mM EDTA and then alkylated with 2.5 mM p-Nitrobenzyl Mesylate (PNBM, Abcam, ab138910) for 45 min at room temperature, heated in sample loading buffer, and analyzed by SDS-PAGE and following immunoblotting assay. Thiophosphorylated substrates were identified by immunoblotting with a rabbit anti-thiophosphate ester primary antibody [51-8] (Abcam, ab92570) and StarBright B700 labeled goat anti-Rabbit IgG secondary antibody. Blot imaging was done using a Bio-Rad ChemiDoc MP Imaging System.
Endogenous Rim4 concentration determination
1.4 OD600 yeast cells harboring Rim4 with C-terminal tagged EGFP (Rim4-EGFP) in SPM were collected at 0.5 h, 10 h, 12 h, 14 h and 16 h, treat with PMSF as described above.
Then, we expressed and purified recombinant Rim4-EGFP-6× His with the same linker used in the genomic version of Rim4-EGFP between the Rim4 EGFP. Dilute the protein into 500 nM, and do a serial half dilution for 9 times, until the last concentration is 500/29 nM (0.9765625 nM). Discard a half volume of the last dilution to maintain the volumes in the 10 dilution gradients were the same.
Next, add 100 μL 1 × SDS loading buffer to the cell samples. And supply the recombinant protein samples with identical volume of the proteins of 2× SDS Loading buffer. Load 10 μL of both cell and protein samples on the same PAGE gel. Thus, in each well, there were cell samples equivalent to 0.14 OD600; and 2.5, 1.25, 0.625, 0.3125 … 0.00488 pmol recombinant protein in each well. Blot to the same NC membrane after gel running. Detect the endogenous Rim4-EGFP and recombinant Rim4-GFP-6×His on the membrane by Western Blot.
Quantify the bands according to the quantification and statistics section below. Plot the band volumes vs. amount (pmol) of the recombinant proteins in each lane on X-Y coordinate. Do a simple linear regression with GraphPad Prism 10, and the fit equation is:
Here, presents the band volumes and presents the amount (pmol) of the recombinant proteins in each lane. Do a transpose for later conversion of band volume to amount:
Here, presents the amount (pmol) of the recombinant proteins in each lane and x presents the band volumes.
Based on the equation and the band volumes of the endogenous Rim4-GFP bands, calculate the concentration range of Rim4-GFP in each lane was: 0.05–0.41 pmol.
It was reported that the average volume of diploid yeast cells was 8.2 × 10−8 μL53 while the cell density of 1 OD600 yeast cell was 3×107 cell/mL.54 Therefore, the volume of yeast cell content in each lane (10 μL sample) was 1.4 OD600 × 3 × 107 cell/mL/OD600 × 8.2 × 10−8 μL/cell × 1000 μL/mL = 0.34 μL.
Hence, based on our calculation and knowledge, we finally calculated the average concentration of endogenous Rim4-GFP in one cell ranged from 0.23 to 1.21 μM.
Fluorescence microscopy imaging
200 μL cells growing in SPM were concentrated to 10 μL. Drop 5 μL to the glass slides (Corning, 2975-246). The cells were then covered by a piece of agar containing the same media and immediately observed under Spinning disk Confocal (Yokogawa Spinning Disk Confocal CSU-W1) Zeiss Axio Observer microscope supplied with Hamamatsu Orca-Fusion sCMOS camera and a Zeiss Plan Apochromat 63×/0.9-NA oil-immersion objective. Exposure time was 200 ms for mScarlet (561–617/71 nm), EGFP (473–525/50 nm), mNeonGreen (515–542/27 nm) or mTFP1 (473–488/10 nm) channels (laser power 100%). Images were captured with SlideBook 6 software. z stack of 5 planes, 1 μm/plane, were taken for each field (202.44 μm × 202.44 μm), each channel. 3 fields containing more than 300 cells were taken for each sample. The images were split for the best focus, pseudo-colored, analyzed by ImageJ (version 1.53u).
Mass spectrometry
The cells harboring pZEV:Rim4-FLAG were driven to sporulation, β-estradiol, the inducer of ZEV promoter was added at 0 h (immediate after cells transferred into SPM). Cells were harvested at 12 h and snap frozen to liquid nitrogen. Smash the cells by ball milling. Next, the cell lysate powder was dissolved in 1 × IP buffer (50 mM HEPES-KOH, pH 6.8, 150 mM KOAc, 2 mM Mg(OAc)2, 1 mM CaCl2, 15% glycerol, 1% NP-40, 1× cOmplete protease inhibitor cocktail [Roche, 11873580001], 1× PhosStop phosphatase inhibitors [Roche, 4906845001]), followed by centrifugation at 100,000 × g for 49 min, 4°C, to separate the cytosolic fraction (supernatant, S100) and membrane fraction (pellet, including nuclear, organelles and membranes, P100). The cytosolic fraction (S100) and detergent dissolved membrane fraction (P100) were incubated with the anti-FLAG M2 monoclonal antibody bound Protein G resin on ice for 30 min, respectively. The beads were then collected and washed 5 times with IP buffer. The eluents by 25 μL 1 mg/mL 3×FLAG peptides were sent to UT Southwestern Medical Center Proteomics Core Facility for mass spectrometry analysis.
Mass photometry
The cytosolic fraction of the samples sent for mass spectrometry was sent to the UT Southwestern Medical Center Macromolecular Biophysics Resource for mass photometry analysis. The result of the counts of particles were plotted on histogram by the molecular mass. The peaks were Gaussian fitted, and the mean molecular mass and standard deviation (σ) were calculated.
In vitro phosphorylation and dephosphorylation of recombinant proteins
To conduct the in vitro phosphorylation of Rim4, 6 μM recombinant Rim4-6×His was incubated with 0.5 μg/μL PKA catalytic subunit (Sigma, P2645) or 0.6 μM Ime2st (ΔC241) (purified in Wang Lab) in reaction buffer (50 mM HEPES-NaOH, pH 6.8, 150 mM KOAc, 2 mM Mg[OAc]2, 1 mM CaCl2, 1% NP-40, 5 mM NaF, 50 mM β-Glycerophosphate, 10 mM Na3VO4, 50 mM Na2PPi [Disodium pyrophosphate], 1 ng/μL Pepstatin, 1× cOmplete Protease Inhibitor Cocktail [Roche, 11873580001], 1 mM PMSF, 15% Glycerol) at RT for 1 h.
Rim4-p in the cell lysate were first immobilized on beads, either by pulling down via Bmh1/2-GFP-6His immobilized on Ni-NTA resin (EGFP-Rim4) or by directly immobilizing on anti-FLAG M2 monoclonal antibody bound Protein G dynabeads (Rim4-3FLAG). Then the Rim4-p proteins on beads were de-phosphorylated by incubating with 1.5 μM 3FLAG-Cdc14 in reaction buffer (50 mM HEPES-NaOH, pH 6.8, 150 mM KOAc, 2 mM Mg[OAc]2, 1 mM CaCl2, 0.075% NP-40, 1 mM DTT, 1 ng/μL Pepstatin, 1 × cOmplete Protease Inhibitor Cocktail [Roche, 11697498001], 1 mM PMSF, 15% Glycerol) RT for 30 min.
In silico predictions
The functional domains of Rim4 were mapped by Simple Modular Architecture Research Tool (SMART, http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1).
The disordered regions of Rim4 were predicted by DISOPRED3 program (http://bioinf.cs.ucl.ac.uk/psipred/, check the DISOPRED3 analysis). The PBDATA file were downloaded and the scores of each position were extracted and plot on a Cartesian coordinate system.
The RNA binding sites in the C terminus LCD areas were predicted by ProNA2020 program (https://predictprotein.org/).
The 14-3-3 binding sites were predicted by 14-3-3-pred program (http://www.compbio.dundee.ac.uk/1433pred/). The prediction was done with 3 methods: Artificial Neural Network (ANN, cut-off = 0.55), Position-Specific Scoring Matrix (PSSM, cut-off = 0.8) and Support Vector Machine (SVM, cut-off = 0.25). The position of all S and T residues were calculated and scored. The 5 sites with all three method scores above the cut-off were selected as positive sites.
The full length Rim4 protein (entry: P38741) structure was predicted by AlphaFold (https://www.alphafold.ebi.ac.uk/). The structure of RRMs of Rim4 were predicted by Robetta program with the RoseTTAFold method (https://robetta.bakerlab.org/). All the predicted structures were downloaded as PDB files, visualized and analyzed by Chimera software (version 1.16) (https://www.cgl.ucsf.edu/chimera/).
QUANTIFICATION AND STATISTICAL ANALYSIS
Immunoblotting quantification
The single band volumes of the immunoblotting were analyzed by Image Lab software (Bio-Rad, version 6.1). Background of each band volume was subtracted by local background subtraction method provided by the software. All band volumes of the protein of interest were normalized by that of the internal reference protein Pgk1 or Hxk1. The EGFP-Rim4 variants autophagic degradation were calculated by free EGFP signals divided by total EGFP signals, including full length EGFP-Rim4 variants and the free EGFP signals. Phosphorylated EGFP-Rim4 (EGFP-Rim4-p) levels were calculated by EGFP-Rim4-p signal (detected by α-RRxS/T-p antibody) divided by EGFP-Rim4 signal (detected by α-EGFP antibody).
The overall area volumes of the immunoblotting were analyzed by ImageJ (1.53u). Global background was subtracted by rolling ball method with radius of 50 pixels. The overall phosphorylation levels were calculated by the sum of the phosphorylation signal (detected by α-RRxS/T-p antibody) between 20 kDa and 150 kDa marker divided by the Ponceau S signal of the same blot, between 20 kDa and 150 kDa. Because of technical difficulties, the Ponceau S signals were not consistent between experiments, so for the analysis of each blot, the ratio between phosphorylation and Ponceau S signal was further normalized by one chosen reference group which was set as value 1.
Statistics were performed by GraphPad Prism 10. Data from three independent experiments were pooled in a Column table, plot as mean ± SE and analyzed by unpaired Welch’s t test for independent pairwise comparison or Dunnet T3 test after Brown-Forsythe Welch ANOVA test for multiple comparison.
Sporulation efficiency calculation
At 60 h after transferring into SPM, cells were collected and diluted accordingly. Added the samples into a hemocytometer chamber (Hausser Scientific, 3110) and count the total cell numbers as well as the numbers of the cells with matured spores (tetrads) in the central squares from the corners under bright field with Olympus microscope (BX40×, 40 × objective). When the total cell number reached to 300, continue to count all cells in the same central square to avoid bias. The sporulation efficiency is presented by the tetrad percentage, i.e., tetrads number divided by total cell number. Statistics were performed by GraphPad Prism 10. Data from at least three independent experiments were pooled in a Column table, plot as mean ± SE and analyzed by unpaired Welch’s t test for independent pairwise comparison or Dunnet T3 test after Brown-Forsythe Welch ANOVA test for multiple comparison.
FM analysis
Image processing
FM images were analyzed by ImageJ (1.53u). The Min and Max Display Value of the mScarlet channel were set as (0, 300); that of the EGFP channel were set as (0, 500). The layer best fit the focus were split and pseudo-colored with the LUT indicated in the figure legend.
Whole cell signal analysis
In a duplicated EGFP channel, the cells were labeled by threshold of (15, 65535). Then, fill the holes and separate the cells with Watershed algorithm. The cell areas are stored in ROI manager. Back to the original EGFP channel, the intensities in the labeled cells were measured. Statistics were performed by GraphPad Prism 10. The data were input into X-Y table, with Time as the row (X) title, and Strain and Treatment as the column (Y) title. Then the values were plotted to the Points & Connection lines graph as mean ± SE. Three independent experiments were repeated. More than 300 cells were measured for each time point, each strain, each treatment. The data of the last time point were analyzed by uncorrected Dunn’s test after an Kruskal-Wallis ANOVA.
Nuclear versus cytoplasmic signal analysis
First, the cell nucleus is delineated based on the Nup49-mScarlet signal and the cytoplasm is delineated based on both the Nup49-mScarlet and EGFP-Rim4 signals. Measure the intensity in both portions, respectively. Then, the ratio of nuclear intensity to cytoplasmic intensity is calculated. Statistics were performed by GraphPad Prism 10. In all figures except for Figure 7D, the data from more than 300 cells were input into a column table and plotted into a scatterplot, as median ±95% Confidence Interval. The data were analyzed by Uncorrected Dunn’s test after a Kruskal-Willis ANOVA test for independent pairwise comparison or Dunn’s multiple comparison test after a Kruskal-Willis ANOVA test for comparison between multiple strains. For Figure 7D, the data from more than 300 cells were input into an X-Y table, with Time as the row (X) title, and Strain and Treatment as the column (Y) title. Then the values were plotted to the Points & Connection lines graph as mean ± SE. Three independent experiments were repeated. More than 300 cells were measured for each time point, each strain, each treatment. The first 3 time points (0.5, 10, 12 h) before Ndt80 inducing and the last 2 time points (14, 22 h) after Ndt80 inducing were spilt into two tables for independent analysis. They were separately analyzed by two-way ANOVA with column (Strain and Treatment) main effect.
Gray value analysis
A presentative cell image was picked. Draw a line through interested structure (e.g., the nucleus based on Nup49-mScarlet; or the Cdc14-mNeonGreen puncta) and the cytoplasm (based on fluorescent protein tagged Rim4 signal). Then the gray values along the line were measured and plotted by the distance with GraphPad Prism 10.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Monoclonal anti-EGFP mouse IgG1κ [7.1 and 13.1] (1:10,000) | Roche | Cat# 11814460001; RRID: AB_390913 |
| Monoclonal anti-FLAG® tag mouse IgG1 [M2] (1:5,000) | Sigma-Aldrich | Cat# F3165; RRID: AB_259529 |
| Monoclonal anti-Phospho-PKA Substrate (RRxS*/T*) rabbit IgG [100G7E] (1:1,000) | Cell Signaling Technologies | Cat# 9624S; RRID: AB_331817 |
| Recombinant anti-Thiophosphate ester (thioP) Rabbit IgG [51-8] (1:3,000) | Abcam | Cat# ab92570; RRID: AB_10562142 |
| Recombinant Anti-Histone H2B (yeast Htb2) Rabbit IgG [EPR18094] (1:2,500) | Abcam | Cat# ab188291 |
| Polyclonal anti-hexokinase 1 (Hxk1) Rabbit IgG (1:10,000) | United States Biological | Cat# 169073 |
| Polyclonal anti-PGK1 Rabbit IgG (1:10,000) | Antibodies Online Inc. | Cat# ABIN568371; RRID: AB_2924789 |
| StarBright® B700 labeled goat antimouse IgG secondary antibody (1:10,000) | Bio-Rad | Cat# 12004158; RRID: AB_2884948 |
| Alexa Fluor® 488 labeled goat antirabbit IgG secondary (1:10,000) | Invitrogen | Cat# A11034; RRID: AB_2576217 |
| ECL-anti-rabbit HRP IgG (1:10,000) | Cytiva | Cat# NA9340; RRID: AB_772191 |
| Chemicals, peptides, and recombinant proteins | ||
| SYTOX Green | Thermo-Fisher | Cat# S7020 |
| β-estradiol | Sigma-Aldrich | Cat# E8875 |
| 1NM-PP1 | APExBIO | Cat# B1299 |
| Ni-NTA agarose | Qiagen | Cat# 30230 |
| Imidazole | Fisher Scientific | Cat# ICN1020335 |
| Dynabeads Protein G | Fisher Scientific | Cat# 10004D |
| PhosStop | Roche | Cat# 4906845001 |
| Pepstatin | Roche | Cat# 11359053001 |
| cOmplete Protease Inhibitor Cocktail | Roche | Cat# 11873580001 |
| PMSF | Sigma-Aldrich | Cat# 78830 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat# A2153 |
| 4-20% Criterion™ TGX Stain-Free™ Gel | Bio-Rad | Cat# 5678095 |
| SurePAGE™ 4–20% Bis-Tris Gel | GenScript | Cat# M00657 |
| Anti-FLAG® M2 Affinity Gel | Sigma-Aldrich | Cat# A2220 |
| 3 × FLAG peptides | Sigma-Aldrich | Cat# F4799 |
| λ-Phosphatase | NEB | Cat# P0753S |
| N6-PhET-ATPγS | Axxora | Cat# BLG-P026-05 |
| p-Nitrobenzyl mesylate (PNBM) | Abcam | Cat# Ab138910 |
| Zymolyse (20T) | AMS Bio | Cat# 120491-1 |
| Protein Kinase A Catalytic Subunit from bovine heart | Sigma-Aldrich | Cat# P2645 |
| Rim4-6His | This paper | NA |
| Rim4-GFP-6His | This paper | NA |
| Rim4(5C)-6His | This paper | NA |
| Rim4(PxLm)-6His | This paper | NA |
| Bmh1-GFP-6His | This paper | NA |
| Bmh2-GFP-6His | This paper | NA |
| Bmh1-3FLAG-6His | This paper | NA |
| 6His-3FLAG-Cdc14 | (Feng et al.)45 | NA |
| Critical commercial assays | ||
| Yeast Transformation Kit | Sigma-Aldrich | Cat# YEAST1 |
| GenCatch™ Plasmid DNA Mini-Prep Kit | Epoch | Cat# 2160250 |
| GenCatch™ Gel Extraction Kit | Epoch | Cat# 2260250 |
| Experimental models: Organisms/strains | ||
| This paper | Table S1 | |
| Recombinant DNA | ||
| This paper | Table S2 | |
| Software and algorithms | ||
| Image Lab™ (version 6.1) | Bio-Rad | https://www.bio-rad.com/en-us/product/image-lab-software |
| ImageJ (version 1.53u) | National Institutes of Health (Public Domain) | https://imagej-nih-gov.foyer.swmed.edu/ij/ |
| SlideBook 6 | Intelligent imaging | https://www.intelligent-imaging.com/slidebook |
| Flowing Software (version 2.5.1) | Turku Bioscience Center | https://bioscience.fi/services/cell-imaging/flowing-software/ |
| SnapGene (version 7.0.2) | Dotmatic | https://www.snapgene.com/ |
| GraphPad Prism (version 10.0.1) | Dotmatic | https://www.graphpad.com/ |
| AlphaFold | DeepMind Technologies | https://alphafold.com/ |
| Robetta | Baker Lab, HHMI’s Janelia Research Campus | http://www.robetta.org/ |
Highlights.
14-3-3 proteins (Bmh1 and Bmh2) bind and regulate Rim4 function
Bmh1/2 temporally protect Rim4 from autophagy
Cdc14 antagonizes PKA to safeguard intracellular Rim4 distribution via Bmh1/2
Cdc14 orchestrates autophagic Rim4 degradation during meiotic divisions
ACKNOWLEDGMENTS
We thank Beth Levine and the University of Texas Southwestern Medical Center for Autophagy Research for initial project discussion. The authors would like to acknowledge assistance from the University of Texas Southwestern Quantitative Light Microscopy Core, a Shared Resource of the Harold C. Simmons Cancer Center, supported in part by an NCI Cancer Center Support Grant, 1P30 CA142543-01, and an NIH Shared Instrumentation Award to Dr. K. Luby-Phelps (1S10OD028630-01). We thank Vladimir Denic (Harvard University, Boston, MA, USA) for reagents and protocols. We thank J. Seemann, J. Friedman, M. Henne, and members of the Wang lab for comments on the manuscript. This work was supported by a grant from the National Institutes of Health (R01GM133899 to F.W.) and from the Welch Foundation (I-2019-20190330 to F.W.), as well as funding from Nancy Cain and Jeffrey A. Marcus Scholar in Medical Research in Honor of Dr. Bill S. Vowell (to F.W.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113052.
REFERENCES
- 1.Kassir Y, Granot D, and Simchen G (1988). IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell 52, 853–862. 10.1016/0092-8674(88)90427-8. [DOI] [PubMed] [Google Scholar]
- 2.Neiman AM (2011). Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189, 737–765. 10.1534/genetics.111.127126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AP (1994). Control of meiotic gene expression in Saccharomyces cerevisiae. Microbiol. Rev 58, 56–70. 10.1128/mr.58.1.56-70.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu S, and Herskowitz I (1998). Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell 1, 685–696. 10.1016/s1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- 5.Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, and Herskowitz I (1998). The transcriptional program of sporulation in budding yeast. Science 282, 699–705. 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Ajimura M, Padmore R, Klein C, and Kleckner N (1995). NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell Biol 15, 6572–6581. 10.1128/MCB.15.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin L, Zhang K, Sternglanz R, and Neiman AM (2017). Predicted RNA Binding Proteins Pes4 and Mip6 Regulate mRNA Levels, Translation, and Localization during Sporulation in Budding Yeast. Mol. Cell Biol 37, e00408–16. 10.1128/MCB.00408-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, and Weissman JS (2012). High-resolution view of the yeast meiotic program revealed by ribosome profiling. Science 335, 552–557. 10.1126/science.1215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebauer F, Schwarzl T, Valcárcel J, and Hentze MW (2021). RNA-binding proteins in human genetic disease. Nat. Rev. Genet 22, 185–198. 10.1038/s41576-020-00302-y. [DOI] [PubMed] [Google Scholar]
- 10.Dreyfuss G, Kim VN, and Kataoka N (2002). Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol 3, 195–205. 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 11.Berchowitz LE, Gajadhar AS, van Werven FJ, De Rosa AA, Samoylova ML, Brar GA, Xu Y, Xiao C, Futcher B, Weissman JS, et al. (2013). A developmentally regulated translational control pathway establishes the meiotic chromosome segregation pattern. Genes Dev. 27, 2147–2163. 10.1101/gad.224253.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enyenihi AH, and Saunders WS (2003). Large-scale functional genomic analysis of sporulation and meiosis in Saccharomyces cerevisiae. Genetics 163, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berchowitz LE, Kabachinski G, Walker MR, Carlile TM, Gilbert WV, Schwartz TU, and Amon A (2015). Regulated Formation of an Amyloid-like Translational Repressor Governs Gametogenesis. Cell 163, 406–418. 10.1016/j.cell.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter K, Bell RB, Yunus J, Amon A, and Berchowitz LE (2018). Phosphorylation-Mediated Clearance of Amyloid-like Assemblies in Meiosis. Dev. Cell 45, 392–405.e6. 10.1016/j.devcel.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Zhang R, Feng W, Tsuchiya D, Ballew O, Li J, Denic V, and Lacefield S (2020). Autophagy of an Amyloid-like Translational Repressor Regulates Meiotic Exit. Dev. Cell 52, 141–151.e5. 10.1016/j.devcel.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Denic V, and Lacefield S (2020). Autophagy prevents runaway meiotic divisions. Autophagy 16, 969–970. 10.1080/15548627.2020.1739449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlile TM, and Amon A (2008). Meiosis I is established through division-specific translational control of a cyclin. Cell 133, 280–291. 10.1016/j.cell.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Feng W, Qian S, Li S, and Wang F (2022). Autophagy inspects Rim4-mRNA interaction to safeguard programmed meiotic translation. Preprint at bioRxiv. 10.1101/2022.12.22.521672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soushko M, and Mitchell AP (2000). An RNA-binding protein homologue that promotes sporulation-specific gene expression in Saccharomyces cerevisiae. Yeast 16, 631–639. . [DOI] [PubMed] [Google Scholar]
- 20.Hoffman DW, Query CC, Golden BL, White SW, and Keene JD (1991). RNA-binding domain of the A protein component of the U1 small nuclear ribonucleoprotein analyzed by NMR spectroscopy is structurally similar to ribosomal proteins. Proc. Natl. Acad. Sci. USA 88, 2495–2499. 10.1073/pnas.88.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai K, Oubridge C, Ito N, Avis J, and Evans P (1995). The RNP domain: a sequence-specific RNA-binding domain involved in processing and transport of RNA. Trends Biochem. Sci 20, 235–240. 10.1016/s0968-0004(00)89024-6. [DOI] [PubMed] [Google Scholar]
- 22.van Hemert MJ, van Heusden GP, and Steensma HY (2001). Yeast 14-3-3 proteins. Yeast 18, 889–895. 10.1002/yea.739. [DOI] [PubMed] [Google Scholar]
- 23.Johnson C, Crowther S, Stafford MJ, Campbell DG, Toth R, and MacKintosh C (2010). Bioinformatic and experimental survey of 14-3-3-binding sites. Biochem. J 427, 69–78. 10.1042/BJ20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madeira F, Tinti M, Murugesan G, Berrett E, Stafford M, Toth R, Cole C, MacKintosh C, and Barton GJ (2015). 14-3-3-Pred: improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics 31, 2276–2283. 10.1093/bioinformatics/btv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu H, Subramanian RR, and Masters SC (2000). 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol 40, 617–647. 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 26.Yorimitsu T, Zaman S, Broach JR, and Klionsky DJ (2007). Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 18, 4180–4189. 10.1091/mbc.e07-05-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataria M, Mouilleron S, Seo MH, Corbi-Verge C, Kim PM, and Uhlmann F (2018). A PxL motif promotes timely cell cycle substrate dephosphorylation by the Cdc14 phosphatase. Nat. Struct. Mol. Biol 25, 1093–1102. 10.1038/s41594-018-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzano-López J, and Monje-Casas F (2020). The Multiple Roles of the Cdc14 Phosphatase in Cell Cycle Control. Int. J. Mol. Sci 21, 709. 10.3390/ijms21030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culotti J, and Hartwell LH (1971). Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp. Cell Res 67, 389–401. 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- 30.Flint AJ, Tiganis T, Barford D, and Tonks NK (1997). Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94, 1680–1685. 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradshaw RA, and Dennis EA (2009). Handbook of Cell Signaling (Academic press; ). [Google Scholar]
- 32.Bloom J, Cristea IM, Procko AL, Lubkov V, Chait BT, Snyder M, and Cross FR (2011). Global analysis of Cdc14 phosphatase reveals diverse roles in mitotic processes. J. Biol. Chem 286, 5434–5445. 10.1074/jbc.M110.205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JS, Broadus MR, McLean JR, Feoktistova A, Ren L, and Gould KL (2013). Comprehensive proteomics analysis reveals new substrates and regulators of the fission yeast clp1/cdc14 phosphatase. Mol. Cell. Proteomics 12, 1074–1086. 10.1074/mcp.M112.025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, and Amon A (1998). The Phosphatase Cdc14 Triggers Mitotic Exit by Reversal of Cdk-Dependent Phosphorylation. Mol. Cell 2, 709–718. 10.1016/S1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 35.Gray CH, Good VM, Tonks NK, and Barford D (2003). The structure of the cell cycle protein Cdc14 reveals a proline-directed protein phosphatase. EMBO J. 22, 3524–3535. 10.1093/emboj/cdg348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremmer SC, Hall H, Martinez JS, Eissler CL, Hinrichsen TH, Rossie S, Parker LL, Hall MC, and Charbonneau H (2012). Cdc14 phosphatases preferentially dephosphorylate a subset of cyclin-dependent kinase (Cdk) sites containing phosphoserine. J. Biol. Chem 287, 1662–1669. 10.1074/jbc.M111.281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamber RA, Shoemaker CJ, and Denic V (2015). Receptor-Bound Targets of Selective Autophagy Use a Scaffold Protein to Activate the Atg1 Kinase. Mol. Cell 59, 372–381. 10.1016/j.molcel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trembley MA, Berrus HL, Whicher JR, and Humphrey-Dixon EL (2014). The yeast 14-3-3 proteins BMH1 and BMH2 differentially regulate rapamycin-mediated transcription. Biosci. Rep 34, e00099. 10.1042/BSR20130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slubowski CJ, Paulissen SM, and Huang LS (2014). The GCKIII kinase Sps1 and the 14-3-3 isoforms, Bmh1 and Bmh2, cooperate to ensure proper sporulation in Saccharomyces cerevisiae. PLoS One 9, e113528. 10.1371/journal.pone.0113528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callejo M, Alvarez D, Price GB, and Zannis-Hadjopoulos M (2002). The 14-3-3 protein homologues from Saccharomyces cerevisiae, Bmh1p and Bmh2p, have cruciform DNA-binding activity and associate in vivo with ARS307. J. Biol. Chem 277, 38416–38423. 10.1074/jbc.M202050200. [DOI] [PubMed] [Google Scholar]
- 41.Tkach JM, Yimit A, Lee AY, Riffle M, Costanzo M, Jaschob D, Hendry JA, Ou J, Moffat J, Boone C, et al. (2012). Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol 14, 966–976. 10.1038/ncb2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavade JN, Puccia CM, Herod SG, Trinidad JC, Berchowitz LE, and Lacefield S (2022). Identification of 14-3-3 proteins, Polo kinase, and RNA-binding protein Pes4 as key regulators of meiotic commitment in budding yeast. Curr. Biol 32, 1534–1547.e9. 10.1016/j.cub.2022.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parua PK, Dombek KM, and Young ET (2014). Yeast 14-3-3 protein functions as a comodulator of transcription by inhibiting coactivator functions. J. Biol. Chem 289, 35542–35560. 10.1074/jbc.M114.592287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennington KL, Chan TY, Torres MP, and Andersen JL (2018). The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 37, 5587–5604. 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng W, Argöello-Miranda O, Qian S, and Wang F (2022). Cdc14 spatiotemporally dephosphorylates Atg13 to activate autophagy during meiotic divisions. J. Cell Biol 221, e202107151. 10.1083/jcb.202107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones DT, and Cozzetto D (2015). DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics 31, 857–863. 10.1093/bioinformatics/btu744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blethrow J, Zhang C, Shokat KM, and Weiss EL (2004). Design and use of analog-sensitive protein kinases. Curr Protoc Mol Biol 18, Unit 18 11. 10.1002/0471142727.mb1811s66. [DOI] [PubMed] [Google Scholar]
- 48.Longtine MS, McKenzie A 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, and Pringle JR (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. . [DOI] [PubMed] [Google Scholar]
- 49.Goldstein AL, and McCusker JH (1999). Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553. . [DOI] [PubMed] [Google Scholar]
- 50.Denic V, and Weissman JS (2007). A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 130, 663–677. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Brown EC, Mak G, Zhuang J, and Denic V (2010). A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol. Cell 40, 159–171. 10.1016/j.molcel.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kielkopf CL, Bauer W, and Urbatsch IL (2020). Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc 2020, 102269. 10.1101/pdb.prot102269. [DOI] [PubMed] [Google Scholar]
- 53.Jorgensen P, Nishikawa JL, Breitkreutz BJ, and Tyers M (2002). Systematic identification of pathways that couple cell growth and division in yeast. Science 297, 395–400. 10.1126/science.1070850. [DOI] [PubMed] [Google Scholar]
- 54.Day A, Schneider C, and Schneider BL (2004). Yeast cell synchronization. Methods Mol. Biol 241, 55–76. 10.1385/1-59259-646-0:55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.