Abstract
Copper is an essential enzyme cofactor in oxidative metabolism, antioxidant defenses and neurotransmitter synthesis. However, intracellular copper, when improperly buffered, can also lead to cell death. Given the growing interest in the use of copper in the presence of the ionophore elesclomol (CuES) for the treatment of gliomas, we investigated the effect of this compound on the surround parenchyma – namely neurons and astrocytes in vitro. Here we show that astrocytes were highly sensitive to CuES toxicity while neurons were surprisingly resistant, a vulnerability profile that is opposite of what has been described for zinc and other toxins. Bolstering these findings, a human astrocytic cell line was similarly sensitive to CuES. Modifications of cellular metabolic pathways implicated in cuproptosis, a form of copper regulated cell death, such as inhibition of mitochondrial respiration or knock-down of ferredoxin 1 (FDX1), did not block CuES toxicity to astrocytes. CuES toxicity was also unaffected by inhibitors of apoptosis, necrosis or ferroptosis. However, we did detect the presence of lipid peroxidation products in CuES-treated astrocytes, indicating that oxidative stress is a mediator of CuES-induced glial toxicity. Indeed, treatment with antioxidants mitigated CuES induced cell death in astrocytes indicating that oxidative stress is a mediator of CuES-induced glial toxicity. Lastly, prior induction of metallothioneins 1 and 2 in astrocytes with zinc plus pyrithione was strikingly protective against CuES toxicity. As neurons express high levels of metallothioneins basally, these results may partially account for their resistance to CuES toxicity. These results demonstrate a unique toxic response to copper in glial cells which contrasts with the cell selectivity profile of zinc, another biologically relevant metal.
Keywords: Metal, Elesclomol, Glia, Cell Death, Free Radicals, Glioma
Graphical Abstract
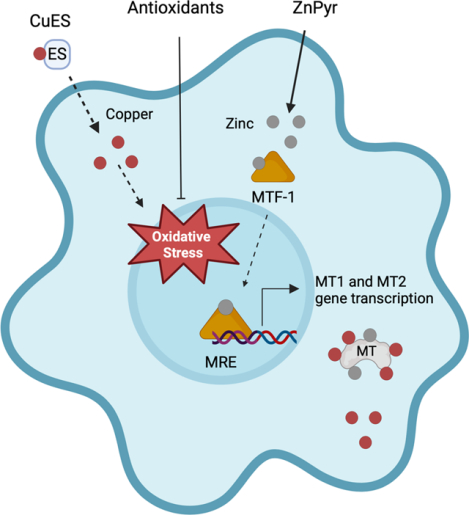
Copper is an essential enzyme cofactor. However, intracellular copper, when improperly buffered, can lead to cell death. Given growing interest in the use of copper in the presence of the ionophore elesclomol (CuES) for the treatment of gliomas, we investigated the effect of this compound on neurons and astrocytes in vitro. Here we show that astrocytes were highly sensitive to CuES toxicity while neurons were surprisingly resistant. We found oxidative stress to be a key mediator of CuES toxicity. Indeed, antioxidant treatment mitigated CuES-induced cell death in astrocytes. We also found that induction of metallothioneins (MTs) with zinc pyrithione (ZnPyr) was protective against CuES toxicity. Created with BioRender.com.
Introduction
Copper is a catalytic and structural cofactor for cuproenzymes involved in oxidative phosphorylation, iron homeostasis, antioxidant defense, as well as neurotransmitter synthesis (Ruiz et al. 2021; Scheiber et al. 2014). Copper also serves important biological functions in the brain, including modulation of synaptic transmission (D’Ambrosi and Rossi 2015). The critical role of copper in key physiological processes is evidenced by the fact that the transition metal is an essential micronutrient in virtually all eukaryotes (Festa and Thiele 2011). Furthermore, genetic mutations that result in copper deficiency lead to human disorders with profound clinical consequences (Jaksch et al. 2000; Garza et al. 2023; Kaler 2013). However, copper is able to redox cycle, allowing it to form toxic free radicals, displace other metals from enzymes, and disrupt proper protein folding (Festa and Thiele 2011). Indeed, the accumulation of intracellular copper is the key driver of pathology in Wilson’s disease (Ala et al. 2007). Moreover, copper dyshomeostasis has been linked to neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease (Gromadzka et al. 2020; Chen et al. 2022; Pal et al. 2021). Indeed, cells in the central nervous system (CNS) appear to be particularly vulnerable to the toxic effects of copper as highlighted by the neurotoxicity/neurodegeneration observed in animal models of chronic copper intoxication (Pal and Prasad 2015). As such, intracellular concentrations of copper are tightly regulated, with the metal being tightly buffered to attomolar concentrations in the cytoplasm by thiol ligands (Morgan et al. 2019), including glutathione and metallothioneins.
A copper-dependent regulated cell death pathway, cuproptosis, was recently described (Tsvetkov et al. 2022). Cuproptosis involves the disruption of mitochondrial respiration through increased lipoylation of tricarboxylic acid (TCA) cycle proteins (Tsvetkov et al. 2022). In this cell death pathway, lipoylation, a post-translational modification of lysine, is mediated by ferredoxin 1 (FDX1), an iron-sulfur cluster protein involved in electron transfer for a variety of biological processes (Schulz et al. 2023). The bulk of research on cuproptosis has involved the copper ionophore elesclomol, which has been previously investigated as a possible anti-neoplastic drug (Yadav et al. 2013). Although it has had limited efficacy in clinical trials thus far (O’Day et al. 2013; Monk et al. 2018), elesclomol is a potent inhibitor of cancer cell proliferation in vitro (Hasinoff et al. 2015; Nagai et al. 2012; Blackman et al. 2012) and, as such, the discovery of cuproptosis has reignited interest in copper’s potential as a treatment strategy for difficult-to-treat cancers. Indeed, cuproptosis-associated genes have been evaluated as predictors of prognosis and treatment response in glioma in silico (Zhang et al. 2022; Zhang et al. 2023a; Ye et al. 2022; Zhu et al. 2022). Gliomas are tumors derived from aberrant glial or glial precursor cells and are amongst the most common and most deadly types of primary brain tumor (Ostrom et al. 2019; Nicholson and Fine 2021). Unfortunately, no novel pharmaceutical treatments that affect patient survival have been successfully developed for this malignancy since temozolomide was introduced over 15 years ago (Stupp et al. 2005; Nicholson and Fine 2021).
While CuES may be effective at targeting glioma and glioblastoma cells, the effect it has on the surrounding parenchyma needs to be considered. An ideal treatment would target tumor cells while leaving the surrounding tissue minimally affected, especially in the brain where treatment such as radiation therapy has been linked to oxidative stress and cognitive dysfunction (Soffietti et al. 2023; Yoo et al. 2023). Gliomas are intermixed with neurons and astrocytes – cell types that have previously been shown to be vulnerable to copper toxicity and dyshomeostasis (Bulcke et al. 2015; Hwang et al. 2014; Gromadzka et al. 2020; Pal and Prasad 2014).
In this study we sought to investigate the effect of copper in the presence of elesclomol (CuES) on the tumor microenvironment using primary rodent cortical cultures and immortalized human astrocytes. To our surprise, we discovered that astrocytes are extremely sensitive to CuEs toxicity while neurons are strongly resistant to this insult. Importantly, our results indicate that CuES toxicity is not mediated via cuproptosis as it lacks dependence on FDX1. To our knowledge, this is one of a few of a biologically relevant compounds that is selectively lethal to astrocytes while leaving neurons unharmed.
Material and Methods
Compounds
Chemicals: elesclomol (cat. no. S1052), Z-VAD-FMK (cat. no. S7023), necrostatin-1s (cat. no. S8641), deferoxamine mesylate (cat. no. S5742), and ferrostain-1 (cat. no. S7243) were purchased from Selleck Chemicals. Cupric chloride dihydrate (cat. no. C3279), Antimycin A (cat. no. A8674), Sodium azide (cat. no. S8032), EUK-134 (cat. no. SML0743) and pyrroloquinoline quinone (PQQ) (cat. no. D7783) were purchased from Sigma. Dizocilpine hydrogen maleate (MK801) (cat. no. M107) was purchased from Milipore. Primary antibodies for immunocytochemistry: chicken anti-MAP2 (Abcam, cat. no. ab5392, RRID:AB_2138153, 1:5000), rabbit anti-GFAP (Abcam, cat. no. ab7260, RRID:AB_305808, 1:1000), mouse anti-4-hydroxynonenal (12F7) (Invitrogen, cat. no. MA5–27570, RRID:AB_2735095, 1:100). Secondary antibodies for immunocytochemistry: donkey anti-rabbit conjugated with Alexa Fluor 488 (Abcam, cat. no. ab150073, RRID:AB_2636877, 1:1000), goat anti-mouse conjugated with Alexa Fluor 568 (Abcam, cat, no. ab175473, RRID:AB_2895153, 1:1000), goat anti-chicken conjugated with Alexa Fluor 647 (Abcam, cat. no. ab150175, RRID:AB_2732800, 1:1000).
Cerebrocortical Cultures
All procedures involving the use of animals were reviewed and approved by the University of Pittsburgh IACUC (Protocol # 21039053). Primary cortical cultures were prepared from embryonic day 16–17 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) as previously described (Hartnett et al. 1997). Briefly, pregnant rats were housed alone in a standard cage in the University central animal facility for a maximum of three days with free access to food and water and were sacrificed humanely and painlessly by CO2 inhalation and further exsanguinated via severing of the jugular vein to assure completion of euthanasia. The animals showed no sign of distress during this procedure and perished quickly and painlessly. Embryos of either sex were removed and quickly decapitated, although they likely had also perished before the dissection was performed. Five-10 embryonic cortices from a single littler were pooled and dissociated with trypsin and cells were plated on 12 mm, poly-L-ornithine (PLO) coated glass coverslips in six well plates at a density of 635,000 cells per well. We consider a single dissociation, from one pregnant animal, a biological replicate. Non-neuronal proliferation was inhibited after 14 days in vitro (DIV) with 2 μM cytosine arabinsoside. Cultures were utilized at 3–4 weeks in vitro. We calculate that approximately 50–70 pregnant rats (approximately 450 embryos) were utilized for these studies as we normally perform one dissociation per week throughout the year. On occasion, a pregnant animal of the wrong gestation age was shipped, and these were excluded from the study (i.e. no cultures were obtained). Otherwise, no cultures were excluded from the study. This primary cortical culture model was chosen as it is a well-validated model in our laboratory and we have previously demonstrated developmental increases in AMPAR and NMDAR subunit expression in these cultures consistent with their in vivo development (Ross and Aizenman 2023; Sinor et al. 2000).
Astrocyte Cultures
To generate astrocyte cultures, mixed cortical cultures were exposed to 1 mM kainate (KA) in HEPES-buffered minimal essential media with 0.01% BSA (MHB) for 18–24 hours to eliminate the neuronal component, which comprise 10–20% of cultures at 3–5 weeks in vitro, while sparing the astrocytes which comprise 80–90% of cells in this preparation (Rosenberg and Aizenman 1989). Following overnight KA treatment, astrocytes were transferred to fresh MHB solution. For experiments utilizing both mixed cultures and astrocyte cultures, coverslips containing neurons and astrocytes were treated in MHB without KA in parallel to control for manipulation of coverslips. We chose to use this model of astrocyte cultures as opposed to pure astrocyte cultures as it has previously been demonstrated that neurons release soluble factors that regulate the expression of astrocytic proteins (Gegelashvili et al. 1997; Swanson et al. 1997; Schlag et al. 1998) and recent evidence strongly suggests that neurons play a key role in the regulation of genes important in astrocyte metabolism and development (Hasel et al. 2017). As such, this model recapitulates key aspects of mature astrocytes in vivo.
C6 glioma cells
Rat C6 glioma cells (ATCC, cat. no. CCl-107; RRID:CVCL_0194) were cultured in Ham’s F12 Nutrient Mixture with GlutaMAX (Gibco) supplemented with 2.5% fetal bovine serum (Thermo Fisher, cat. no. A4766801) 15% horse serum (heat inactivated) (Thermo Fisher, 26050088), and penicillin (100 U/ml), streptomycin (0.1 mg/ml) and maintained in an incubator at 37°C with 5% CO2. Cells were split twice a week. For LDH assays, C6 cells were plated at a 188,000 cells per well in PLO-coated 24 well plates; for CellTiter Glo assays, cells were plated at a density of 32,000 cells per well in PLO-coated 96 well plates. The maximum number of passages utilized for these cells was ~25. C6 cells have been extensively used to study glioma both in vitro and in vivo and are considered by some to be to be “the gold standard” in glioma research [for review, see: (Giakoumettis et al. 2018)]. C6 is not listed as a commonly misidentified cell line by the International Cell Line Authentication Committee (ICLAC). To our knowledge, the C6 cell line has not been further analyzed since karyotype analysis was performed on the initial seed stock at ATCC. However, ATCC performs authentication and quality-control tests on all distribution lots of cell lines.
U138-MG glioma cells
Human U138-MG glioma cells (ATCC, cat. no. HTB-16; RRID: CVCL_0020) and were cultured in Eagle’s Minimum Essential Medium (ATCC, cat. no. 30-2003) supplemented with 10% fetal bovine serum (Thermo Fisher, cat. no. A4766801), penicillin (100 U/ml), streptomycin (0.1 mg/ml) and maintained in an incubator at 37°C with 5% CO2. Cells were split one to two times a week. For LDH assays, U138-MG cells were plated at a density of 80,000 cells per well poly-d-lysine (Gibco, cat. no. A3890401) -coated 24 well plates. The maximum number of passages utilized for these cells was ~25. These cells were derived from an individual with grade IV glioblastoma and were included for additional verification of the vulnerability of glioma cells to copper-elesclomol treatment in vitro Authentication using short tandem repeat profiling was performed in 2012 and 2016 (Allen et al. 2016; Bady et al. 2012). Although similarity was reported between U138-MG and U118-MG, U138-MG is not listed as a commonly misidentified cell line by the ICLAC and ATCC notes that U138-MG and U118-MG are reportedly derived from two separate individuals with similar STR patterns.
Immortalized Human Astrocytes
Immortalized human fetal astrocytes (hTert) (Applied Biological Materials, cat. no. T0281) were cultured on poly-D-lysine (PDL) (Gibco, cat. no. A3890401) coated flasks in Prigrow IV medium (Applied Biological Materials, cat. no. TM004) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 ng/mL human epidermal growth factor (EGF) (Gibco, cat. no. PHG0323), and 100 U/mL penicillin, 100 μg/mnL streptomycin (Sigma). Cells were split twice a week and seeded at a density of 40,000 cells per well onto PDL-coated 24 well plates for LDH assays and 14,000 cells per well onto PDL-coated 96-well plates for CellTiter Glo assays. The maximum number of passages utilized for these cells was ~20. This immortalized astrocyte cell line was generated from primary human astrocytes that are representative of cortical astrocytes and have been previously used as a cell model of normal human astrocytes in multiple studies investigating glioma and glioblastoma (Agnihotri et al. 2019; Erkan et al. 2014; Baskin et al. 2015). These immortalized human astrocytes are not listed as a commonly misidentified cell line by the ICLAC. STR analysis was performed by Applied Biological Materials on this immortalized cell line for comparison to its parental primary cells, however it is not clear what year this analysis was completed.
Cell Treatments
All experimental paradigms were performed in MHB unless otherwise stated. Cells were exposed to indicated concentrations of copper elesclomol (CuES) overnight or for a two-hour pulse. For CuES pulse experiments, cells were exposed to CuES for 2 hours and then rinsed with MHB. Cells were then incubated overnight in fresh MHB before toxicity/viability measurements. All experiments using drugs to modify CuES toxicity, except for zinc pyrithione (ZnPyr), included a one-hour pre-incubation period before the addition of CuES. Drugs remained in their respective treatment groups overnight before toxicity or viability assays were conducted. For zinc pre-conditioning experiments, cortical cultures were simultaneously exposed to 1 mM KA and 20 μM zinc in the presence of 250 nM pyrithione. Following overnight incubation with KA and ZnPyr, coverslips were moved to fresh MHB before treatment with CuES.
siRNA Treatment
Mixed cortical cultures were treated with 1 μM Acell rat FDX1 SMARTPool siRNA (Horizon, cat. no. E-089545-00-0020) for 48 hours in DMEM-Glutamax supplemented with 2% FBS and 31 mM HEPES. Sister cultures were treated in parallel with 1 μM Acell non-targeting control siRNA (Horizon, cat. no. D-001910-10-20). For initial confirmation of FDX1 knockdown, RNA was collected after 48 hours. For CuES toxicity experiments, siRNA treated coverslips were transferred to MHB after 48 hours and exposed to CuEs overnight. RNA was collected from sister cultures treated identically to confirm the persistence of knockdown.
Toxicity Assays
The toxicity of treatment conditions was assessed using a using a commercially available colorimetric lactate dehydrogenase (LDH) activity assay kit (Sigma, cat. no. MAK066). Following experimental treatment, medium was collected for LDH assays as this enzyme is rapidly released from cells following damage to the plasma membrane (Aras et al. 2008). Toxicity is represented by increased OD450 values. A minimum of three experiments from separate culture dates were performed, each in triplicate.
Viability Assays
The CellTiterGlo Luminescent Cell Viability Assay (Promega, cat. no. G7570) was used to assess viability following copper-ionophore treatment in experiments utilizing EUK-134 and PQQ, as these compounds interfered with the enzymatic reaction utilized in the LDH assay. Experiments were performed according to the manufacturer’s directions. Viability was calculated as percent of control luminescence values due to variability between experiments in absolute luminescence values. A minimum of three experiments from separate culture dates were performed, each in triplicate, for primary cultures and from three separate split dates for cell lines.
Immunocytochemistry
Coverslips were fixed with 4% paraformaldehyde/4% sucrose for 20 minutes, washed three times with PBS, and were then blocked and permeabilized with 0.05% Triton-X with 10% normal goat serum in PBS for 45 minutes. Coverslips were then incubated with primary antibodies at 4°C overnight. The following day, cells were incubated with secondary antibodies for one hour at room temperature before mounting with Fluoromount-G mounting medium (Thermo Fisher, cat. no. 00-4958-02). Three to four random fields of view were imaged from each coverslip (60x) on a Nikon A1R laser scanning confocal.
Quantitative Polymerase Chain Reaction
Total RNA was isolated from cortical cultures using the TRIzol Plus RNA Purification Kit (Invitrogen, cat. no., 12183555). RNA samples were eluted in RNAse free H2O and approximately 500 nanograms of RNA from each sample was reverse transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, cat. no. 1708891). Quantitative PCR (qPCR) was performed using the following primers: β-actin sense (FW): 5′-ACTCTTCCAGCCTTCCTTC-3′; β-actin antisense (RV): 5′- ATCTCCTTCTGCATCCTGTC-3′; FDX1 sense (FW): 5’-TCGATGGATTTGGTGCGTGT-3’; FDX1 antisense (RV): 5’- CAGGCACACGGACAGTCATA; MT1 sense (FW): 5’-CACCGTTGCTCCAGATTCAC-3’; MT1 antisense (RV): 5’- GCAGCAGCACTGTTCGTCAC-3’; MT2 sense (FW): 5’- GCAGCAGCACTGTTCGTCAC-3’; MT2 antisense (RV): TGCACTTGTCCGAAGCCTCT-3’. qPCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, cat. no. 1725271) and a CFX96 Touch Real-Time PCR detection system (Bio-Rad). The qPCR reactions for each sample were run in triplicate and all targets were normalized to β-actin.
Statistics
Data are presented as means ± SEM. The number of experiments noted for each experiment represents biological replicate. All statistical analyses were performed using GraphPad Prism 9 (GraphPad, RRID:SCR_002798). Normality was assessed by the Shapiro-Wilk test. The ROUT method was used to identify outliers. This method uses nonlinear regression and an adapted false discovery rate to identify multiple outliers by comparing whether data points are far enough from the predicted model to be considered outliers (Motulsky and Brown 2006),. No outliers were identified. For comparison of two sample means, a two-tailed t-test was used. A one sample t-test was used for analysis of normalized values (control matched to 1). For comparison of more than two sample means, a one-way analysis of variance (ANOVA) with Sidak’s or Dunnet’s test for multiple comparisons was used (specified in figure legends). For analysis of LDH values in experiments comparing cultures with neurons and astrocytes to astrocytes alone, a two-way ANOVA with Sidak’s test for multiple comparisons was used to compare the effect of drug treatments between sample groups. Although no formal power analysis was performed, samples sizes were determined a priori based on a large number of previous studies utilizing our cell culture models to examine cell death (Aizenman et al. 2000; Hartnett et al. 1997; Aras et al. 2008). No blinding was performed.
Results
Primary rodent neurons and astrocytes are differentially vulnerable to copper-elesclomol induced toxicity
A growing number of in silico studies have found that gliomas may be susceptible to cuproptosis (Zhang et al. 2023b; Ye et al. 2022; Zhu et al. 2022). In support of these results, we found that that glioma cell lines are sensitive to copper in the presence of elesclomol (CuES) in vitro (Fig. S1). As gliomas are generated within the brain parenchyma, an optimal treatment would selectively target glioma cells while leaving the surrounding tissue uninjured. However, no investigations to date have examined the effect of CuES on healthy neurons and astrocytes. Therefore, we assessed the effect of CuES on the glioma microenvironment, using mixed cerebrocortical cultures containing neurons and astrocytes to evaluate the susceptibility of these cell types to this treatment. We found that overnight treatment with concentrations at or above 2 μM copper in the presence of 1 μM elesclomol resulted in significant cytotoxicity as evidenced by increased LDH release (Fig. 1A). Strikingly, phase contrast microscopy revealed the presence of healthy-appearing neurons surrounded by swollen astrocytes in CuES-treated cultures (Fig. 1B). This finding was surprising in light of the significant body of evidence demonstrating that neurons are exquisitely sensitive to toxicity induced by transition metals, particularly zinc, while astrocytes are generally resistant to this cell stress (Dineley et al. 2000; Choi et al. 1988). Indeed, exposing the cerebrocortical cultures to overnight treatment with 10–30 μM zinc along with 250 nM of the zinc ionophore pyrithione (ZnPyr) resulted in a significant elevation in LDH release (Fig. 1C) and phase contrast microscopy confirmed that neurons were primarily affected by this treatment, while astrocyte morphology remained intact (Fig. 1D).
Figure 1. Copper and zinc toxicity appear to target different cell populations in mixed neuronal glial cultures.
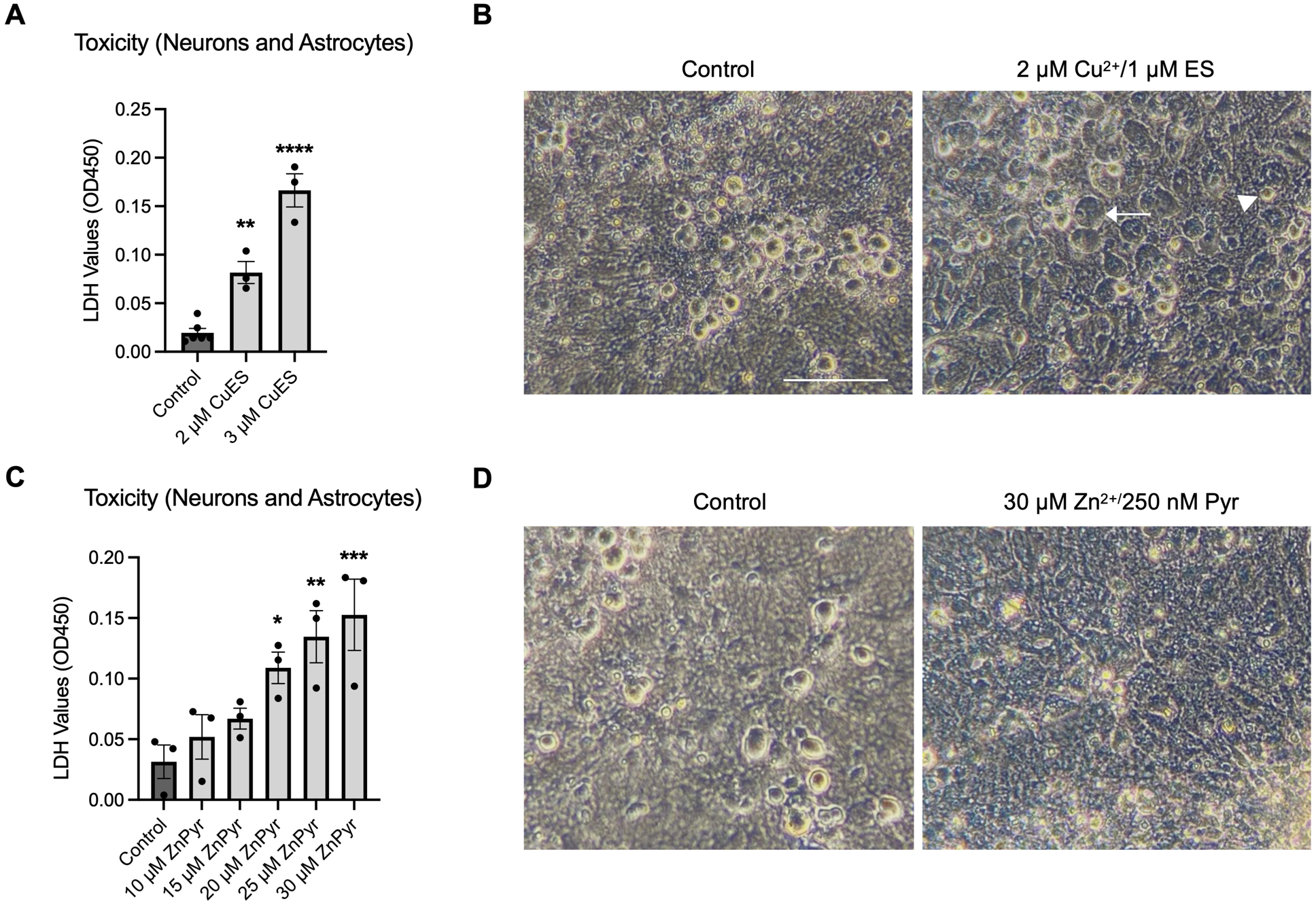
(A) LDH assay showing concentration-dependent copper toxicity in the presence of 1 μM elesclomol in cerebrocortical cultures (F(2,9)=62.82, one-way ANOVA, p<0.0001, Dunnet post-hoc, control vs 2 μM CuES, p=0.0021; control vs 3 μM CuES, p<0.0001, n=3 biological replicates). (B) Phase contrast images of cerebrocortical cultures following overnight treatment with CuES, demonstrating swollen glia (arrow), but seemingly healthy-appearing, phase-bright neurons (arrowhead). C. LDH assay showing concentration-dependent zinc toxicity in the presence of 250 nM of the zinc ionophore, pyrithione (F(5,12)=6.672, one-way ANOVA, p=0.0034, Dunnet post-hoc, control vs 20 μM ZnPyr, p=0.0477; control vs 25 μM ZnPyr, p=0.0085, control vs 30 μM ZnPyr, p=0.0026, n=3 biological replicates). D. Phase contrast images of cerebrocortical cultures following overnight treatment with ZnPyr showing a lack of phase bright neurons, but seemingly healthy glia. Scale bar is 100 μM.
The LDH values reported in the previous experiments represent the total LDH released by both neurons and astrocytes present in our cultures. Therefore, to determine whether astrocytes and neurons were differentially vulnerable to CuES and ZnPyr treatment, we next assessed the relative contributions of each cell type to CuES- and ZnPyr-induced LDH release by comparing cultures containing both neurons and astrocytes to those containing astrocytes alone. To achieve astrocyte cultures lacking neurons (astrocyte cultures), mixed cerebrocortical cultures were treated overnight in media containing 1 mM kainate, as previously described (Aizenman et al. 2000), which causes widespread neuronal death without affecting the viability of astrocytes (Prieto and Alonso 1999; Qu et al. 2003; Aizenman et al. 2000). Following kainate or vehicle treatment, cultures were treated with increasing concentrations of CuES or ZnPyr overnight. As suggested by our observations noted above, astrocytes in the mixed cultures contributed the majority of the LDH signal following CuES treatment. In cultures containing neurons and astrocytes, significant toxicity was seen at concentrations at or above 3 μM CuES while in cultures containing astrocytes alone, significant toxicity was observed at concentrations at or above 2 μM CuES (Fig. 2A). These data demonstrate that astrocytes are the primary cell type affected by CuES in the mixed cultures. To confirm the differential sensitivity of neurons and astrocytes to zinc and copper toxicity, we used the same experimental paradigm described above to assess the relative contributions of neurons and astrocytes to zinc-ionophore induced LDH release. These experiments confirmed that astrocytes are relatively resistant to zinc-induced toxicity while neurons remain highly sensitive (Fig. 2B). Indeed, while mixed cultures showed significant toxicity at or above concentrations of 20 μM Zn2+ in the presence of 250 nM pyrithione, no concentration of ZnPyr tested elicited significant LDH release in astrocyte cultures lacking neurons, underscoring the differential vulnerability of neurons and astrocytes to zinc ionophore and copper ionophore induced toxicity.
Figure 2. Astrocytes are selectively vulnerable to CuES toxicity and neurons in co-culture provide some protection against this insult.
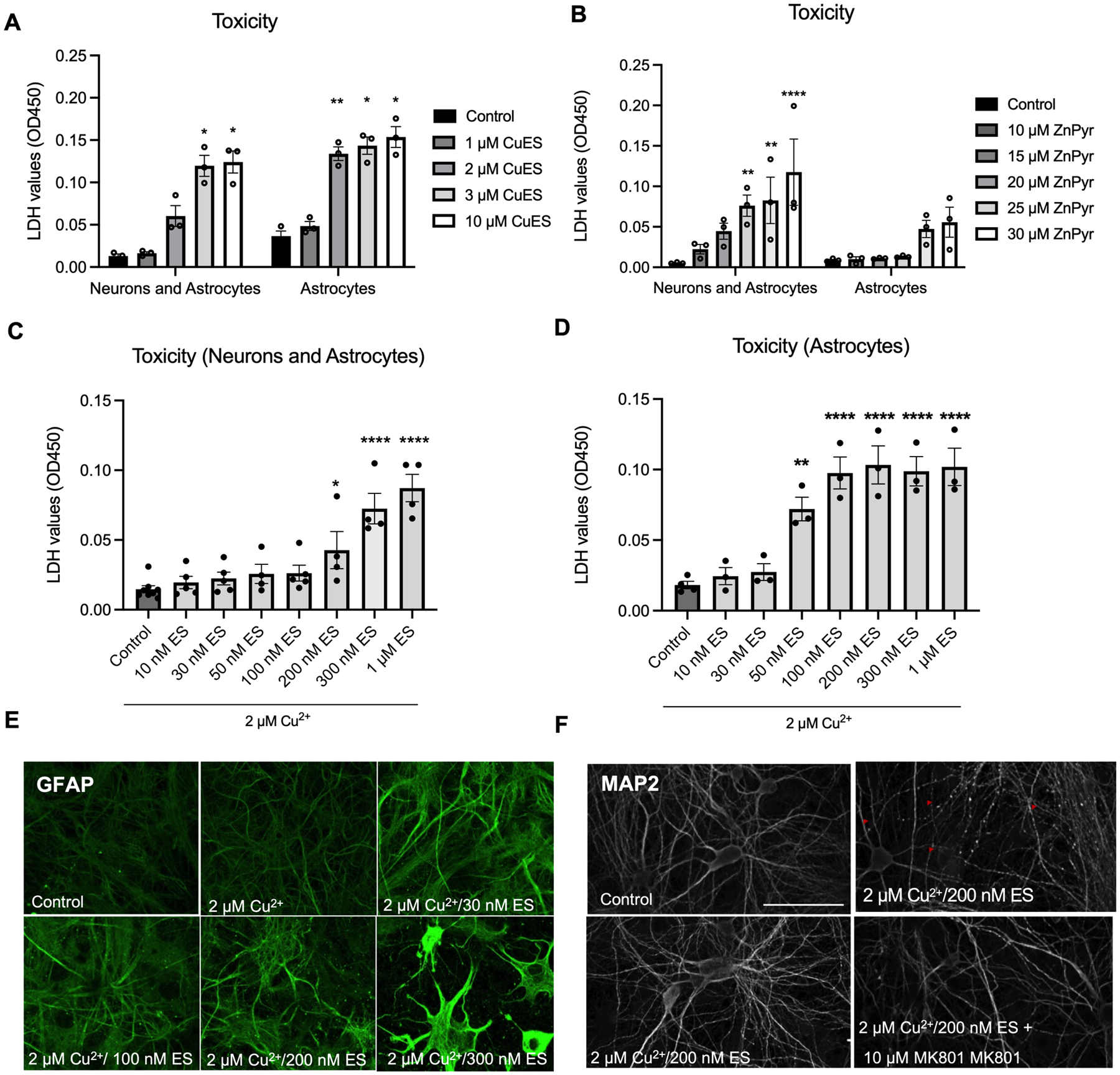
Mixed cortical cultures were first exposed to MHB in the absence or presence of 1 mM kainate (KA) overnight, with the treatment generating neuron-deprived, astrocyte cultures. Cultures-containing coverslips were then transferred to plates containing fresh MHB with various concentrations of CuES or ZnPyr. (A) LDH assay comparing toxicity of CuES (1 μM elesclomol) in the presence and absence of neurons. Note that in the presence of neurons, CuES does not induce a significant elevation in LDH until 3 μM copper while in the absence of neurons, CuES induces significant toxicity at 2 μM CuES (F(4,16)=4.953, two-way ANOVA, p=0.00223 for KA; p<0.0001 for Cu2+ treatment; p=0.0086 for interaction of KA and Cu2+ treatments; Sidak’s post hoc test; Neurons and astrocytes within group comparisons: control vs 1 μM CuES, p=0.5596; control vs. 2 μM CuES, p=0.1699; control vs 3 μM CuES, p=0.0337; control vs 10 μM CuES, p=0.0372; Astrocytes within group comparisons: control vs 1 μM CuES, p=0.1661, control vs 2 μM CuES, p=0.0040, control vs 3 μM CuES, p=0.0227; control vs 10 μM CuES, p=0.0457, n=3 biological replicates). (B) LDH assay comparing the toxicity of ZnPyr (250 nM elesclomol) in the presence and absence of neurons. When neurons are present, significant LDH is observed beginning at 20 μM ZnPyr while no concentration of ZnPyr tested was toxic in the absence of neurons (F(5,20)=2.044, repeated measures two-way ANOVA, p=0.1047 for KA, p<0.0001, p=0.1158 for interaction; Neurons and astrocytes within group comparison: control vs 10 μM ZnPyr, p=0.8978; control vs 15 μM ZnPyr, p=0.2089; control vs 20 μM ZnPyr, p=0.0052; control vs 25 μM ZnPyr, p=0.0023; control vs 30 μM ZnPyr, p<0.0001; Astrocytes within group comparison: control vs 10 μM ZnPyr, p>0.9999; control vs 15 μM ZnPyr, p>0.9999; control vs 20 μM ZnPyr, p=9998; control vs 25 μM ZnPyr, p=0.2274; control vs 30 μM ZnPyr, p=0.0946, n=3 biological replicates). (C) LDH assay of elesclomol concentration response curve using mixed cerebral cultures demonstrates significant copper (2 μM) toxicity at concentrations at or above 200 nM elesclomol (F(7,31)=14.25, one-way ANOVA, p<0.0001, Dunnet post hoc, control vs 10 nM ES, p=0.9939; control vs 30 nM ES, p=0.9327; control vs 50 nM, p=0.7880; control vs 100 nM ES, p=0.6836; control vs 200 nM ES, p=0.0295; control vs 300 nM ES, p<0.0001; control vs 1 μM ES, p<0.0001, n=4–5 biological replicates) while an (D) LDH assay of the same elesclomol concentration curve in astrocyte cultures shows significant copper (2 μM) toxicity beginning at 50 nM elesclomol (F(7,17)=17.90, one-way ANOVA, p<0.0001, Dunnet post hoc, control vs 10 nM ES, p=0.9948; control vs 30 nM ES, p=0.9650; control vs 50 nM ES, p=0.0027; control vs 200 nM ES, p<0.0001; control vs 300 nM ES, p<0.0001; control vs 1 μM ES, p<0.0001, n=3 biological replicates). (E) GFAP staining of mixed cortical cultures treated with CuES shows changes in astrocyte morphology beginning at 30 nM ES and becoming more pronounced at higher concentrations of the ionophore while (F) neuronal architecture is preserved, even at much higher ES concentrations. Rarely, dendritic beading was observed (red arrow heads) however this phenotype could be rescued with co-treatment with the NMDA antagonist MK801 suggesting that this neuronal injury was due to a failure of astrocytes to buffer excess glutamate. Scale bars are 100 μM.
Intriguingly, our results suggested that not only are neurons more resistant to CuES toxicity but that they may protect astrocytes against CuES mediated injury. Elesclomol concentration response experiments demonstrated that in cultures containing both neurons and astrocytes in the presence of 2 μM Cu2+, 200–300 nM ES was necessary to induce significant elevations in LDH signal (Fig. 2C.). However, as little as 50 nM elesclomol was sufficient to induce significant cytotoxicity in astrocyte cultures (Fig. 2D), demonstrating increased sensitivity in the absence of neurons. Moreover, in our initial experiment comparing the vulnerability of neurons and astrocytes to CuES toxicity, described above, (Fig. 2A), a two-way repeated measures ANOVA revealed an interaction between the kainate treatment and CuES (F(4,16), p=0.0086), reflecting the possibility that astrocyte cultures may be more sensitive to CuES toxicity in the absence of neurons.
Given that a growing body of literature suggests that changes in astrocyte cytoarchitecture are reflective of astrocyte dysfunction and may contribute to brain pathology (Lazic et al. 2022; Villablanca et al. 2023), we examined astrocyte morphology following CuES using glial fibrillary acid (GFAP) staining. We found that astrocytic architecture was disrupted at concentrations as low as 2 μM Cu2+ in the presence of elesclomol concentrations as low as 30 nM, even when neurons were present in culture (Fig. 2E), further emphasizing the exquisite sensitivity of astrocytes to CuES. In contrast, most neurons had preserved architecture at 2 μM Cu2+/200 nM ES, as revealed by microtubule-associated protein 2 (MAP2) staining (Fig. 2F). Very infrequently (<10% of fields viewed), a few neurons were observed to have dendritic beading following CuES treatment – a phenotype often observed with excitotoxicity (Olney et al. 1979; Hasbani et al. 1998; Weilinger et al. 2016). As dendritic beading was only observed at the highest concentrations of CuES utilized and appeared to correspond with areas of substantial astrocyte damage, we hypothesized that this phenotype was a result of a failure of damaged astrocytes to buffer excess glutamate in the cultures. Indeed, extracellular glutamate has been shown to accumulate in astrocyte-poor neuronal cultures resulting in neuronal injury (Rosenberg 1991) and impairment of the glutamate transporter GLT-1, predominantly, but not exclusively, expressed in astrocytes (Petr et al., 2015) leads to neuronal death that can be rescued by NMDA receptor antagonism (Kawahara et al. 2002; Wang et al. 1998). To test whether the observed dendritic beading was caused by excitotoxicity or by CuES toxicity, cultures were treated with the NMDA receptor antagonist MK801. Co-treatment with 10 μM MK801 in the presence of CuES abrogated the observed beading suggesting that this form of neuronal injury was a result of excitotoxicity rather than a direct effect of CuES treatment.
Immortalized human astrocytes are also sensitive to CuES treatment
Given the unexpected finding that primary rat cortical astrocytes are selectively vulnerable to CuES-induced toxicity, we next validated these findings in an additional astrocyte model. For these experiments, we utilized an immortalized human astrocyte cell line which allowed us not only to test another cellular model of astrocytes, but also determine whether human astrocytes are also sensitive to copper ionophore toxicity. Indeed, a concentration response curve demonstrated that human astrocytes are also exquisitely sensitive to copper ionophore toxicity showing a significant increase in LDH signal at 2 μM Cu2+ in the presence of 100 nM elesclomol (Fig. 3A). Much like we observed in the C6 glioma cells (Fig. S1), sublethal concentrations of CuES induced a dramatic change in morphology in our immortalized human astrocyte, with a similar loss of spindle-like appearance and the rounding of cells (Fig. 3B). Thus, human astrocytes are also sensitive to copper ionophore induced cell death suggesting that susceptibility to CuES toxicity is not restricted to primary rat cortical astrocytes.
Figure 3. Human immortalized astrocytes are sensitive to copper ionophore induced cell death.
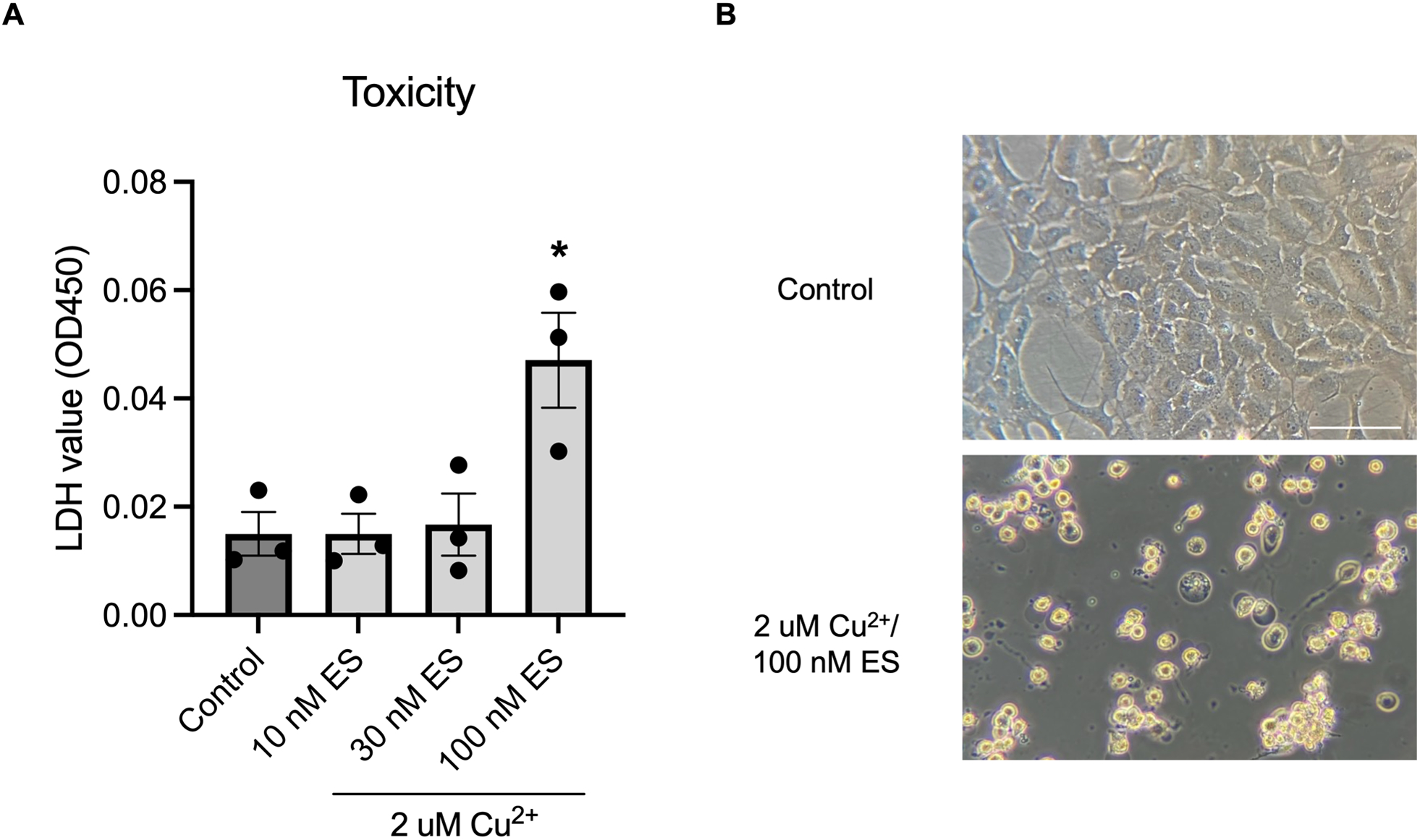
(A) LDH assay showing concentration-dependent copper (2 μM) toxicity with increasing elesclomol concentrations (F(3, 8)=7.102, one-way ANOVA, p=0.0121, Dunnet post-hoc, control vs 10 nM ES, p>0.9999; control vs 30 nM ES, p=0.9937; control vs 100 nM ES, p=0.0127, n=3 biological replicates). (B) Phase contrast images of immortalized astrocytes after overnight treatment with CuES showing substantially altered morphology. Scale bar is 100 μM.
CuES-induced astrocyte death is not mediated via cuproptosis
Having established that astrocytes are vulnerable to CuES treatment, we next aimed to determine whether the selective astrocyte cell death was mediated by the recently described cuproptosis pathway, which involves FDX1-mediated lipoylation and subsequent dysfunction of TCA cycle proteins, (Tsvetkov et al. 2022; Li et al. 2022a). As a previous study has shown that shifting metabolism away from mitochondrial oxidative respiration and towards glycolysis by inhibiting oxidative phosphorylation is protective against cuproptosis (Tsvetkov et al. 2022), we first treated astrocyte cultures with the complex III inhibitor, antimycin A. Astrocytes were pre-treated for one hour with 300 nM antimycin A before the addition of CuES and then incubated overnight with both drugs. LDH assays revealed that inhibition of complex III was not protective against CuES induced cell death (Fig. 4A). As sodium azide, a complex IV inhibitor, has been shown to be a potent inhibitor of mitochondrial respiration, we also tested whether this drug could attenuate CuES-induced cell death. Using the same treatment paradigm as described for antimycin A, we found that 300 μM sodium azide was also ineffective at rescuing astrocytes from CuES toxicity (Fig. 4B). Thus, inhibition of mitochondrial respiration, an important target of the cuproptosis pathway, is ineffective at attenuating CuES-induced death in astrocytes.
Figure 4. CuES induced astrocyte toxicity is not mediated by cuproptosis.
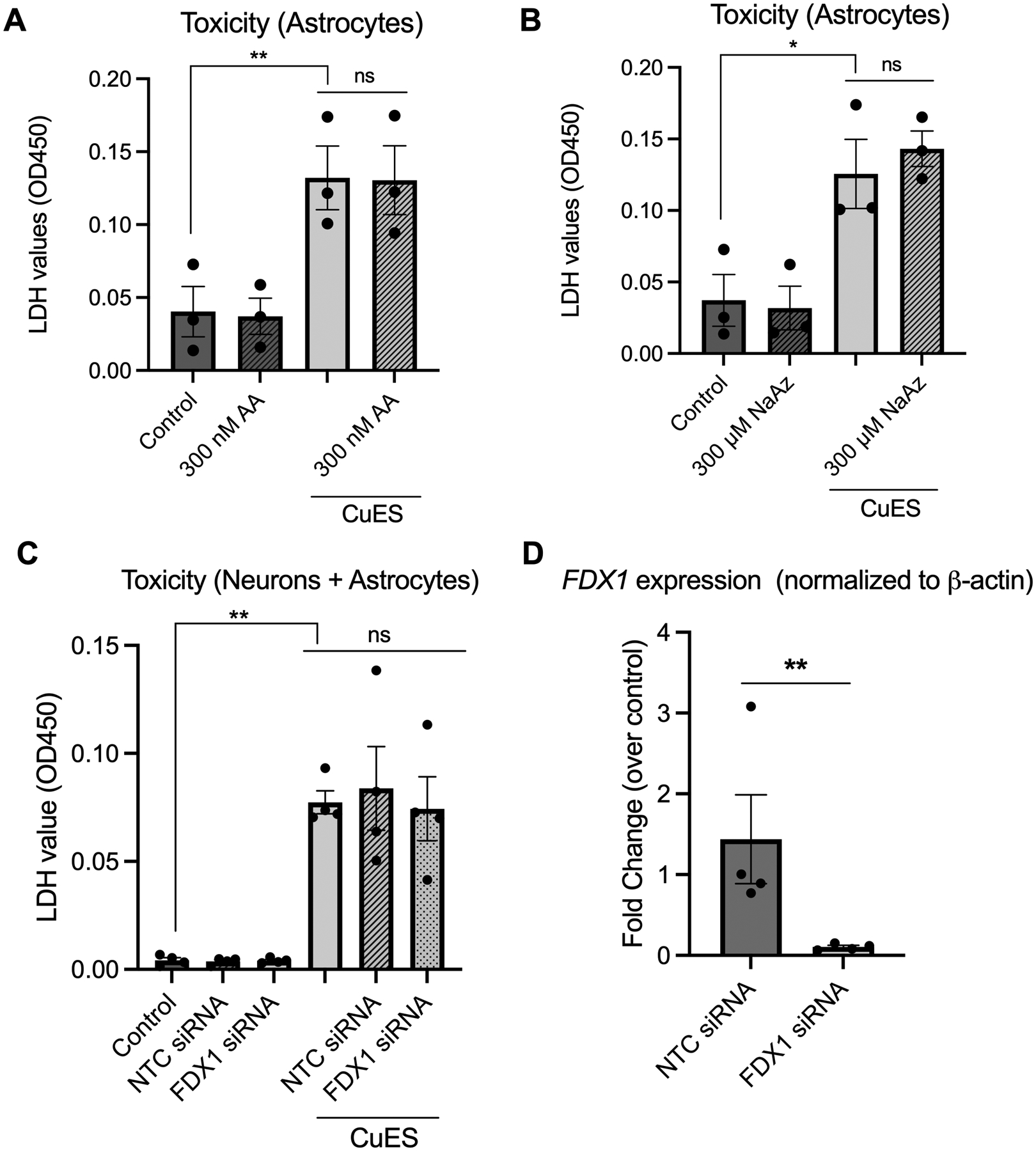
All experiments utilized 2 μM copper. Experiments conducted in astrocyte cultures utilized 200 nM ES while those conducted in cultures containing neurons and astrocytes utilized 300 nM ES. Inhibition of mitochondrial respiration with (A) 300 nM antimycin A (AA), a complex III inhibitor, is insufficient to rescue CuES toxicity in primary astrocytes (F(1.052, 2.105)=103.1, repeated measures one-way ANOVA, p=0.0080, Sidak’s post hoc, control vs CuES, p=0.0054; CuES vs CuES + AA, p=08162, n=3 biological replicates) as is (B) inhibition with 300 μM of the complex IV inhibitor sodium azide (NaAz) (F(1.418, 2.83)=75.11, repeated measures one-way ANOVA, p=0.0037, Sidak post-hoc, control vs CuES, p=0.0128; CuES vs CuES + NaAz, p=0.5748, n=3 biological replicates). (C) Treatment with FDX1 siRNA does not rescue cultures from CuES toxicity as evidenced by significant LDH release (F(1.244, 3.733)= 20.55, repeated measures one-way ANOVA, p=0.0114, Sidak post-hoc, control vs CuES, p=0.0041; CuES vs CuES + NTC siRNA, p=0.9713; CuES vs CuES + CuES + FDX1 siRNA, p=0.9914, n=4 biological replicates), which (D) cannot be explained by a lack of knockdown in siRNA treated cultures (t(3)=6.143, ratio paired two-tailed t-test, p=0.0087, n=4 biological replicates).
Having determined that inhibiting oxidative phosphorylation was insufficient to rescue astrocytes from CuES, we next turned our attention to FDX1, an iron sulfur protein that is a key modulator of cuproptosis (Tsvetkov et al. 2022) and asked whether knockdown of this protein would be protective against CuES toxicity as previous experiments demonstrated that FDX1 knockdown was sufficient to confer resistance against cuproptosis (Tsvetkov et al. 2022). Given that our knockdown protocol required us to use cultures containing both neurons and astrocytes to avoid an extended incubation period in serum-free media, we first determined via qPCR whether FDX1 was abundant in both cell cultures. To do so, we compared FDX1 expression in cultures containing neurons and astrocytes to those containing astrocytes alone. We found no significant difference between FDX1 expression between these cultures (Fig. S2A), consistent with the fact that neurons make up a relatively small percentage of cells in our mixed cerebrocortical cultures. These data suggested that if knockdown (KD) were successful in mixed cultures, we would have achieved KD largely in the astrocytic component. We next tested whether we could KD FDX1 in our cultures using siRNA. Indeed, 48 hours following siRNA treatment, we observed a significant reduction in FDX1 expression compared to the non-targeting control (NTC) siRNA (Fig. S2B). Having demonstrated that FDX1 was expressed in our primary cultures and could be knocked down, we treated cultures with siRNA for 48 hours after which time cells were treated overnight with CuES. LDH results demonstrated that FDX1 KD did not attenuate CuES-induced toxicity (Fig. 4C). Of note, qPCR was also performed on sister cultures which demonstrated that this lack of rescue could not be attributed to a lack of KD as cultures treated with FDX1 siRNA showed greater than a 90% reduction in FDX1 expression compared to the NTC (Fig. 4D). These experiments demonstrate that FDX1 is not a modulator of copper ionophore-induced toxicity in our cultures and that, importantly, cuproptosis does not mediate the observed astrocyte cell death.
It is important to note that our treatment paradigm differed from the paradigm used in the characterization of cuproptosis by Tsvetkov et al. (2022) as we exposed cells to CuES overnight rather than a much shorter, 2-hour pulse. To pursue the possibility that treatment duration might influence mechanism, we next treated astrocytes for 2 hours with CuES and then incubated them in fresh media overnight. Similar to what we observed with the overnight exposure, pulsed CuES treatment led to cell death as evidenced by increased LDH release (Fig. S3A). Phase contrast microscopy revealed once again the presence of healthy appearing neurons surrounded by swollen astrocytes (Fig. S3B). To determine whether cell death induced by this shorter CuES exposure was mediated by cuproptosis, we treated astrocytes with the complex III inhibitor antimycin A and the complex IV inhibitor sodium azide, as described above. Once again, we found no difference in toxicity between groups exposed to CuES in the presence or absence of inhibitors of oxidative phosphorylation (Figs. S3C, S3D). These results suggest that both short and long exposure to CuES triggers a cell death pathway in astrocytes but not neurons that is distinct from cuproptosis.
Having established that astrocytes undergo copper-ionophore triggered cell death in a manner distinct from cuproptosis, we next turned our attention to other known, regulated cell death pathways such as apoptosis, necroptosis, and ferroptosis. Although there are some reports that elesclomol induces apoptosis in cancer cells and that copper and/or elesclomol can trigger ferroptosis (Xue et al. 2023; Gao et al. 2021; Hasinoff et al. 2015), we found that the pan-caspase inhibitor Z-VAD-FMK (100 μM), the necroptosis inhibitor necrostatin-1s (Nec-1s) (20 μM), and the iron chelator deferoxamine (DFO) (50 μM) all failed to abrogate cell death in astrocytes exposed to CuES both overnight (F(1.316, 3.949)=17.26, repeated measures one-way ANOVA, p=0.0131, Šídák post-hoc, CuES vs Z-VAD-FMK, p=0.5793; CuES vs Nec-1s, p=0.5840; CuES vs DFO, p=0.1559, n=4 biological replicates) and for a 2-hour pulse (F(1.125, 3.374)=9.081, repeated measures one-way ANOVA, p=0.0482, Šídák post-hoc, CuES vs Z-VAD-FMK, p=0.9686; CuES vs Nec-1s, p=0.7346; CuES vs DFO, p>0.9999, n=4 biological replicates). Given recent evidence that copper can contribute to ferroptosis independent of iron accumulation (Xue et al. 2023), we also tested the ferroptosis inhibitor ferrostatin-1 (Fer-1) (1 μM) on astrocytes exposed to CuES overnight. As we observed with DFO treatment, 1 μM Fer-1 failed to rescue cells (F(3,9)=4.547, repeated-measures one-way ANOVA, p=0.0013, Šídák post-hoc, control vs CuES, p=0.0170; CuES vs CuES + Fer-1, p=0.8260, n=4 biological replicates). Concentrations as high as 30 μM Fer-1 also did not rescue astrocytes from CuES toxicity, strongly suggesting that ferroptosis is not the cell death pathway mediating CuES induced death in astrocytes.
Copper-ionophore treatment induces oxidative stress in astrocytes
Given that neither cuproptosis nor other canonical regulated cell death pathways appear to mediate CuES-induced death in astrocytes, we evaluated whether oxidative stress may be involved in CuES toxicity due to copper’s ability to redox cycle between the Cu2+ and Cu+ state and induce reactive oxygen species (Valko et al. 2016). In cancer cells, elesclomol has been shown to transport copper to the mitochondria where redox cycling of copper results in the production of mitochondrial reactive oxygen species (ROS) (Nagai et al. 2012). Additionally, there is evidence that copper is able to bind to and form a complex with glutathione, and that this complex is capable of generating superoxide radicals (Speisky et al. 2009). Therefore, we assessed whether CuES treatment resulted in lipid peroxidation, an oxidative protein modification that can be triggered by reactive oxygen and nitrogen species (Li et al. 2022b; Murphy et al. 2022). We chose to look at 4-hydoxy-2-nonenal (4-HNE) as it is both an indicator of oxidative damage as well as a mediator of oxidative stress in cells due to its reactive nature (Li et al. 2022b). An increase in 4-HNE staining in glial fibrillary acid protein (GFAP)-stained astrocytes was observed when cultures containing neurons and astrocytes were exposed to 2 μM Cu in the presence of 200 nM elsesclomol, consistent with an increase in oxidative stress. (Fig. 5A).
Figure 5. CuES mediates toxicity, in part, through induction of oxidative stress in astrocytes and antioxidant treatment improves viability.
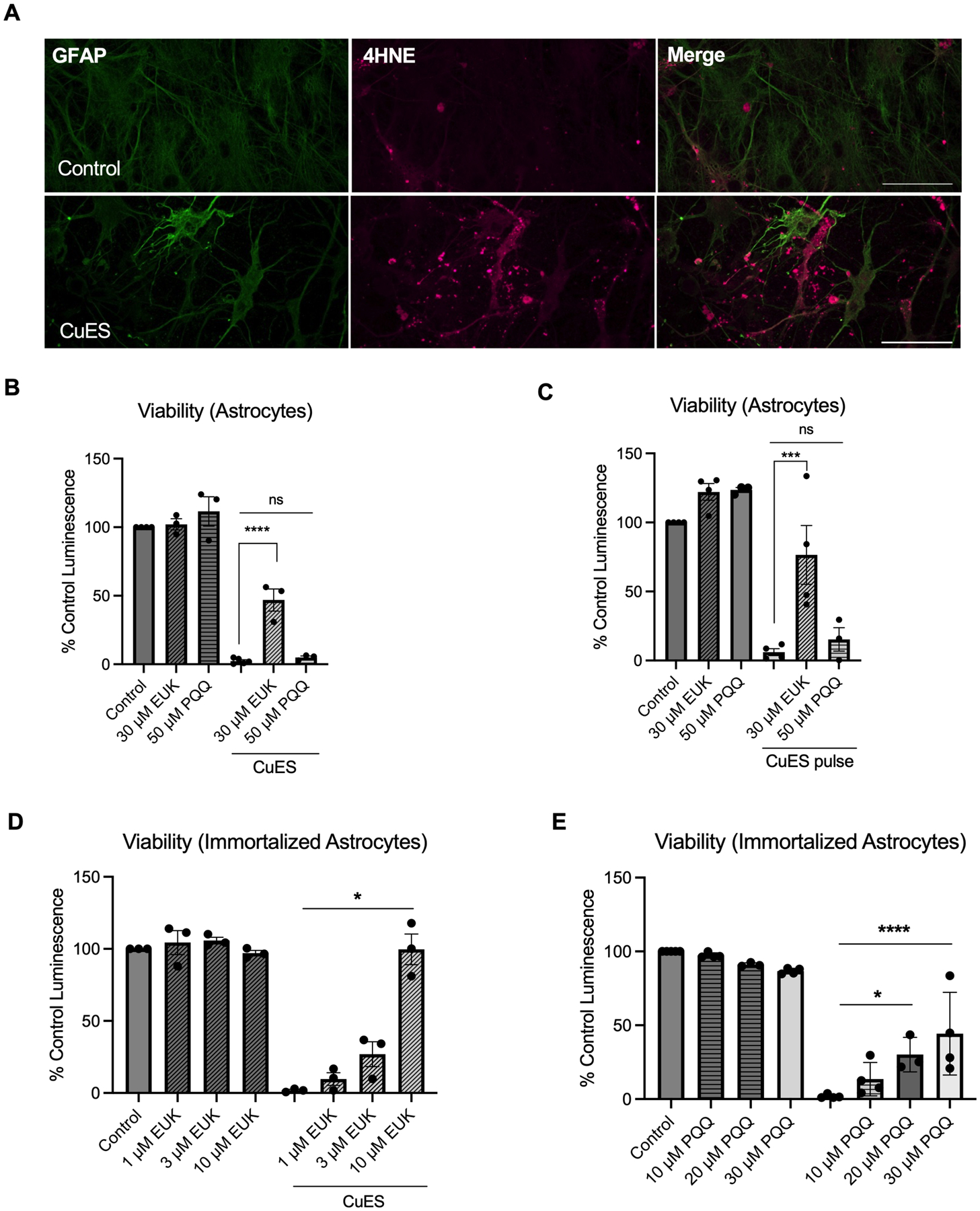
Cultures containing neurons and astrocytes were treated with 2 μM Cu/200 nM ES overnight and were subsequently stained with 4-HNE, a marker of lipid peroxidation. (A) Confocal imaging revealed an increase in 4-HNE signal in CuES treated astrocytes as compared to controls strongly indicating increased oxidative stress following copper ionophore treatment. Treatment with the antioxidant EUK134 but not PQQ significantly increased viability of astrocyte cultures exposed to 2 μM Cu/200 nM ES both (B) overnight (F(2,8)=40.29, one-way ANOVA, p<0.0001, Sidak post hoc, CuES vs CuES + 30 μM EUK134, p<0.0001; CuES vs 50 μM PQQ, p=0.84894, n=3 biological replicates) and (C) for a 2-hour pulse (F(5,16)=24.9, one-way ANOVA, p<0.0001, Sidak post-hoc, CuES vs CuES + 30 μM EUK134, p=0.0003; CuES vs CuES + 50 μM PQQ, p=0.8007, n=4 biological replicates). In contrast, immortalized human astrocytes exposed to 2 μM Cu/100 nM ES overnight were rescued by treatment with both (D) EUK134 (F(1.338, 2.667)=108.6, repeated measures one-way ANOVA, p=0.0029, CuES vs CuES + 1 μM EUK134, p=0.4179; CuES vs CuES + 3 μM EUK134, p=0.2406; CuES vs CuES vs CuES + 10 μM EUK134, p=0.0300, n=3 biological replicates) and (E) PQQ (F(7,23)=48.71, one-way ANOVA, p<0.0001, CuES vs CuES + 10 μM PQQ, p=0.4184; CuEs vs CuES + 20 μM PQQ, p=0.0113; CuES vs CuES + 30 μM PQQ, p<0.0001, n=4 biological replicates). Scale bar is 50 μm.
While these findings strongly suggested that CuES treatment was sufficient to induce oxidative stress in astrocytes, whether this stress mediated CuES-induced cell death was still unknown. Therefore, to determine whether attenuation of oxidative stress would also attenuate CuES-induced cell death, we treated astrocytes with the superoxide dismutase (SOD)-catalase mimic, EUK-134, which has been shown to catalytically eliminate both superoxide and hydrogen peroxide (Doctrow et al. 2002; Sharpe et al. 2002), before and during exposure to CuES. Remarkably, 30 μM EUK-134 was able to restore viability by over 40% in astrocytes treated overnight with CuES and by approximately 70% in astrocytes exposed to a CuES pulse (Fig. 5B). Thus, reactive species are a significant mediator of CuES-induced astrocyte cell death. Given evidence that redox cycling of copper may contribute to CuES-induced ROS generation (Nagai et al. 2012), we next investigated whether pyrroloquinoline quinone (PQQ), a naturally occurring redox cofactor that has been shown to act as an antioxidant through suppression of reactive oxygen species (Zhang and Rosenberg 2002), could also rescue astrocytes from CuES-induced death. In contrast to our findings with EUK-134, we found that 50 μM PQQ failed to protect astrocytes from CuES treatment (Fig. 5C). Given evidence that manganese-salen compounds, such as EUK-134, protect against mitochondrial oxidative stress (Melov et al. 2001; Hinerfeld et al. 2004), these results suggest that CuES induced oxidative stress may be mitochondrially generated.
As our prior experiments confirmed that immortalized human astrocytes are also sensitive to CuES toxicity, we next assessed whether oxidative stress mediated their susceptibility to copper ionophore treatment, as it did in primary rodent astrocytes. Indeed, treatment with EUK-134 rescued viability of immortalized human astrocytes in a concentration dependent manner with 10 μM EUK-134 restoring viability to control levels (Fig. 5D). Unlike what we observed in primary cultures, however, treatment with PQQ also restored viability of CuES treated immortalized astrocytes (Fig. 5E). Together, these results suggest that oxidative stress is a common mechanism underlying astrocyte cell death in response to CuES treatment.
Given that antioxidant treatment protected both primary rodent astrocytes and immortalized human astrocytes from CuES-induced death, we tested whether treatment with EUK-134 or PQQ attenuated the sensitivity of C6 glioma cells against CuES toxicity to determine whether antioxidant treatment could serve as a glioprotective strategy without inhibiting the potential effectiveness of this therapeutic treatment. However, both compounds rescued C6 glioma cells from CuES mediated cell death in a concentration dependent manner (Figs. S4A, 4B) Moreover, 10 μM EUK-134 was able to restore the viability of C6 glioma cells to close to 100% (Fig. S4A) of control cells while 50 μM PQQ restored viability to approximately 70% of control cells (Fig S4B). Thus, oxidative stress appears to be a major factor in CuES-induced glioma cell death as well as in healthy astrocytes.
Zinc preconditioning is protective against CuES toxicity
Although our experiments established that CuES-induced oxidative stress mediates cell death in astrocytes, they did not reveal why neurons are able to survive this insult – especially since astrocytes are thought to have more robust systems for buffering oxidative stress as compared to neurons (Bell et al. 2015; Muyderman et al. 2007; Dringen et al. 2015). However, neurons are highly enriched in metallothionein 3 (MT3) (Aschner et al. 1997), which can bind copper with high affinity (Calvo et al. 2018; Mehlenbacher et al. 2022). Moreover, previous research has shown most of MT3 in our neuronal preparation exists in its apo-form (Aras et al. 2009; Aras and Aizenman 2011) allowing it to bind excess metal. Although there is some data to support low levels of MT3 expression in astrocytes (Hidalgo et al. 2001), these cells primarily express metallothionein 1 (MT1) and metallothionein 2 (MT2) (Aschner et al. 1997). Importantly, MT1 and MT2 are expressed at low levels basally but their expression can be substantially induced by zinc (Silva et al. 2023). Therefore, we hypothesized that MT expression may buffer excess cytosolic copper and partially account for the differential sensitivity of neurons and astrocytes to CuES mediated injury.
To test whether the induction of MTs could protect astrocytes from CuES toxicity, we preconditioned astrocyte cultures with 20 μM Zn2+ in the presence of 250 nM Pyr during kainate treatment, conditions that are not injurious to our astrocyte cultures (Fig. 3B). We first confirmed that overnight treatment with 20 μM ZnPyr was sufficient to increase expression of MT1 and MT2 in astrocyte cultures via qPCR, which was indeed the case (Fig. 6A). Having established that this concentration of ZnPyr induced a significant increase in MT1 and MT2 gene expression, we next treated astrocytes with CuES following an overnight exposure to ZnPyr. Remarkably, ZnPyr preconditioning completely abrogated CuES-induced toxicity and restored the LDH signal to that of the control group (Fig. 6B). Together these data suggest that upregulation of MTs are protective against CuES induced cell death and injury and that the differing basal levels of MTs in neurons and astrocytes may explain their differential sensitivity to CuES toxicity. It is important to note that a higher concentration of zinc is needed to induce neuronal death than the concentration of copper used here to induce astrocyte death (Fig. 2A, 2B), likely due to the need to overcome the buffering actions provided by MT3.
Figure 6. Preconditioning of astrocytes with ZnPyr is protective against CuES induced toxicity.
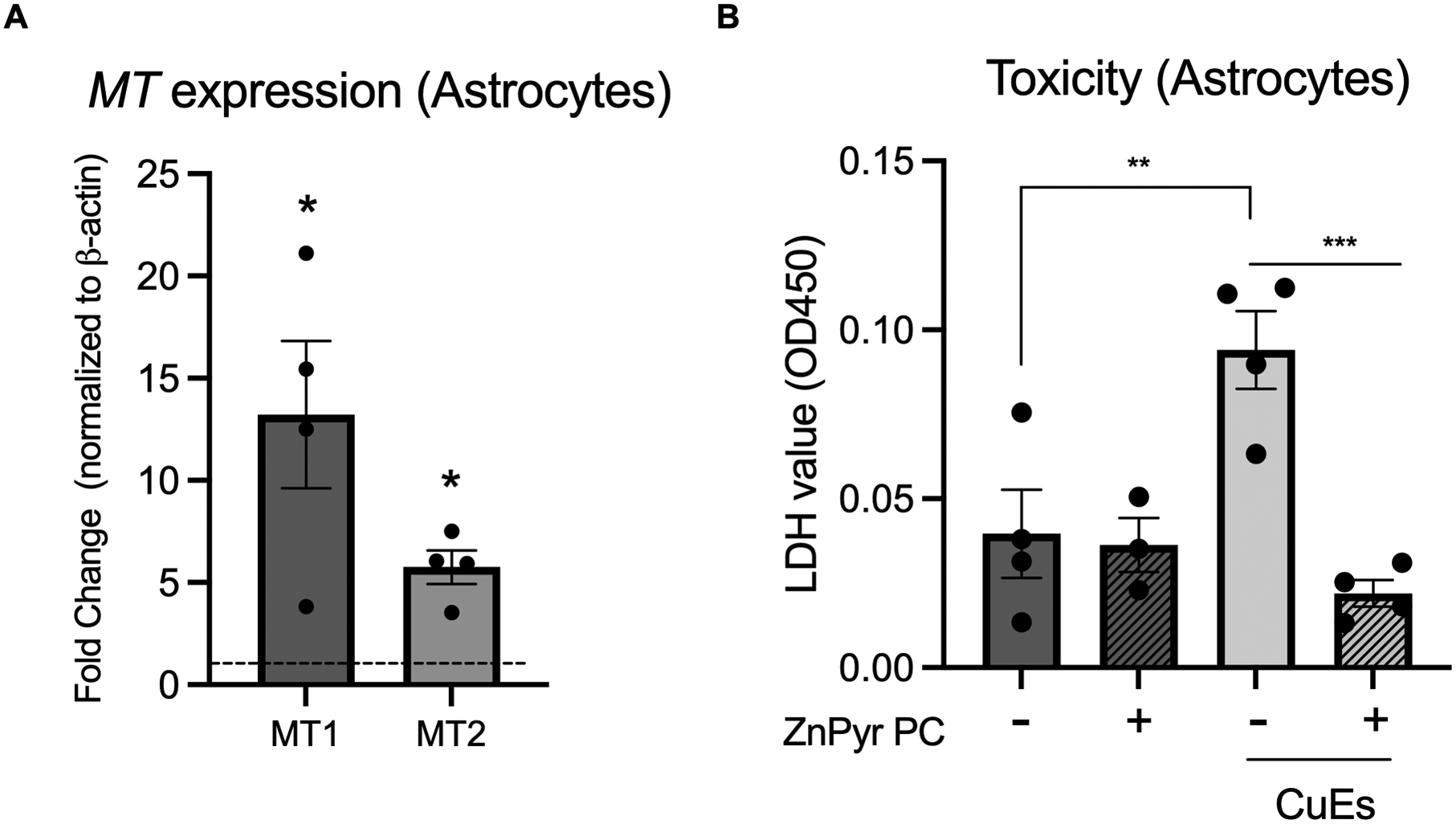
Prior to exposure to 2 μM Cu2+/200 nM ES, astrocytes were treated overnight with 20 μM Zn2+/250 nM Pyr (ZnPyr PC= ZnPyr preconditioning), (A) which significantly increased expression of MT1 and MT2 (t(3.387,3), MT1 fold change vs 1, p=0.0429; t(5.796,3), MT2 fold change vs 1, p=0.0102, one sample t-test, n=4 biological replicates) and (B) completely abrogated CuES induced LDH release (F(3,11)=10.39, one-way ANOVA, p=0.0015, Sidak post hoc, control vs CuES, p=0.0046; CuES vs CuES + ZnPyr PC, p=0.0006, n=3–4 biological replicates).
Discussion
In this report we demonstrate that primary rodent and immortalized human astrocytes are sensitive to cell death induced by CuES treatment. Surprisingly, neurons in primary culture were highly resistant to this insult. This finding is in striking contrast to the selective vulnerability of neurons to the redox-inert metal zinc, when compared to astrocytes (Dineley et al. 2000). An extensive investigation into pathways underlying copper ionophore induced toxicity in astrocytes revealed that, in contrast to the recently described cuproptosis pathway (Tsvetkov et al. 2022), CuES mediated toxicity to astrocytes in mixed cultures is FDX1-independent and is not attenuated by treatment with inhibitors of oxidative phosphorylation. Furthermore, astrocytes cannot be rescued by established inhibitors of apoptosis, ferroptosis, or necroptosis. However, consistent with previous research that has found that treatment with elesclomol, likely complexed with endogenous copper, can result in the generation of ROS (Kirshner et al. 2008; Nagai et al. 2012; Blackman et al. 2012), we have demonstrated that CuES treatment results in oxidative stress in primary rodent astrocytes and that antioxidant treatment rescues primary astrocytes, immortalized human astrocytes, and C6 glioma cells from CuES induced cell death. Importantly, we discovered that primary astrocytes can also be rescued following zinc pyrithione preconditioning, likely via the induction of metallothioneins 1 and 2, which could buffer excess intracellular copper. Interestingly, the presence of neurons in the cultures seemed to exert a protective effect on surrounding astrocytes.
One possible explanation for the divergence between Tsvetkov et al.’s (2022) findings and the results presented here is that cells that are heavily reliant on mitochondrial respiration for energy production are most vulnerable to cuproptosis (Tsvetkov et al. 2022). Astrocytes, however, are more metabolically flexible, utilizing oxidative phosphorylation, glycolysis, and β-oxidation of fatty acids, amongst other pathways, for energy production (Hertz et al. 2007; Harders et al. 2023; Juaristi et al. 2019; Arend et al. 2019). Of note, a recent study found proliferating astrocytes in culture are not dependent on oxidative phosphorylation for growth or survival (Silva et al. 2023). Moreover, respiration deficient astrocytes can survive by glycolysis in vivo (Supplie et al. 2017). Therefore, astrocytes may naturally shift to glycolysis in the presence of stimuli that compromise mitochondrial respiration. Furthermore, copper itself has been shown to stimulate glycolytic flux in astrocytes (Bulcke and Dringen 2015; Scheiber and Dringen 2011). Thus, it is possible that CuES treatment drives astrocytes further towards glycolysis rendering them less reliant on mitochondrial energy production and therefore less likely to be susceptible to cuproptotic cell death mediated by FDX1-dependent TCA cycle dysfunction, which would account for the failure of complex III and IV inhibitors to ameliorate astrocyte death in our cultures. Additionally, this lack of reliance on TCA cycle function would partially account for the lack of attenuation of CuES toxicity we observed with FDX1 knockdown. It is important to note that copper was also found to bind to lipoylated TCA cycle proteins resulting in their oligomerization and proteotoxic stress, which was reversed by FDX1 knockout (Tsvetkov et al. 2022; Tsvetkov et al. 2019). The lack of rescue by FDX1 knockdown in our cultures thus indicates that proteotoxic stress may not be a major contributor to CuES induced cell death in astrocytes. Finally, it is important to consider that the concentration of elesclomol found to elicit cuproptosis in various cell lines was 40 nM (Tsvetkov et al. 2022; Tsvetkov et al. 2019) whereas this concentration of the copper ionophore was insufficient to induce cell death in our cell cultures. Therefore, the higher concentrations of elesclomol needed in our investigation may trigger the alternative cell death-inducing pathway described here.
We found that oxidative stress, rather than perturbed mitochondrial metabolism, is a primary mediator of copper ionophore induced cell death in astrocytes. Not only did astrocytes in primary culture show increased levels of 4-HNE, an end product of lipid peroxidation (Murphy et al. 2022), but antioxidant treatment attenuated CuES-induced cell death in primary astrocytes, human immortalized astrocytes, and C6 glioma cells. The antioxidant EUK-134, a SOD-catalase mimic, rescued all three cell types from CuES mediated cell death, demonstrating that oxidative stress is a common mechanism underlying CuES-induced cytoxicity in astrocyte-like cells in our studies. To our surprise, PQQ, an essential nutrient and free radical scavenger due its ability to redox cycle (Stites et al. 2000), rescued human immortalized astrocytes and C6 glioma cells from CuES mediated cell death, but had no effect on the viability of primary astrocyte cultures. It is worth noting that while EUK-134 restored viability to nearly control levels in CuES treated cell lines, it had a less dramatic effect in primary cultured astrocytes. Thus, there may be an additional mechanism mediating CuES induced death in primary cultures that EUK-134 but not PQQ was able to partially overcome. Although the mechanism underlying the differential rescue of PQQ in these cell types is unclear, it is worth further investigation as potential differences in the type or location of reactive species production could be exploited for targeted treatment of gliomas while leaving healthy tissue unperturbed. For example, catalase has been shown to have the ability to scavenge peroxynitrite (Gebicka and Didik 2009), a reactive nitrogen species, while PQQ can prevent the formation of peroxynitrite but cannot protect against its toxicity once formed (Zhang and Rosenberg 2002). Despite the evidence presented here that oxidative stress mediates CuES induced cell death in astrocytes, an important limitation of this study is that the types of reactive species induced by CuES treatment and where within the cell the formation of these species occurs are still open questions.
Given neurons’ reliance on mitochondrial metabolism (Zheng et al. 2016; Wong-Riley 1989; Halim et al. 2010), it is all the more surprising that neurons are not vulnerable to cuproptosis, and that it is astrocytes rather than neurons that are selectively vulnerable to injury induced copper. One clue to a potential mechanism underlying the resistance of neurons to CuES toxicity comes from our ZnPyr preconditioning experiments, which showed that ZnPyr treatment before CuES exposure completely protected astrocytes from copper ionophore induced cell death. Importantly, overnight exposure significantly upregulated MT1 and MT2 expression, consistent with a large body of data demonstrating that increases in intracellular free zinc drive metallothionein upregulation (Andrews 2000; Heuchel et al. 1994; Radtke et al. 1993). As noted previously, astrocytes primarily express low levels of the MT1 and MT2 while neurons are highly enriched in MT3. Importantly, studies have demonstrated that MT3 may exist predominantly in its apo – or non-metal bound – form (Yang et al. 2001; Aras et al. 2009) and is able to bind redox active free Cu2+ (Meloni et al. 2007). Thus, high levels of non-metal bound MT3 in neurons may buffer the increase in intracellular copper facilitated by elesclomol and prevent the metal from redox cycling, protecting neurons from copper-induced increases in oxidative species. Although MT3 has been shown to have a high affinity for copper (Calvo et al. 2018; Artells et al. 2014; Mehlenbacher et al. 2022), it is worth noting that in in vitro preparations, when apo-MT2 is added to solutions containing copper-bound proteins, it is able to extract Cu+ from them, indicating a high affinity for the metal (Banci et al. 2010). Additionally, previous research has shown that induction of MT1 and MT2 via zinc treatment protects astrocytes from methylmercury-induced cytotoxicity (Aschner et al. 1998; Rising et al. 1995), indicating that increased MT expression is protective against a variety of metals. Although we posit that the protective effect of MTs is mediated through its metal binding functions, research also suggests that these proteins are protective against, and can even scavenge, free radicals such as superoxide and hydroxyl radicals (Thornalley and Vašák 1985; Ruttkay-Nedecky et al. 2013; Chubatsu and Meneghini 1993; Uchida et al. 2002). Therefore, it is also possible that MT-facilitated rescue of astrocytes is mediated through their ability to mitigate oxidative stress, which we found to drive CuES induced cell death.
The findings described here could have significant implications for the potential treatment of gliomas with copper elesclomol therapy. Firstly, we have demonstrated in our proof-of-concept experiments that glioma cells are sensitive to CuES induced cell death although this treatment results in oxidative stress induced cell death in healthy astrocytes as well. Although proliferating glioma cells are often highly glycolytic, a number of studies have found that the TCA cycle is intact in some tumor cells and cancer stem cells (Dekker et al. 2020; Datta et al. 2021; DeBerardinis et al. 2007). Therefore, metabolic profiling of gliomas may allow for targeted therapy of tumors most susceptible to this treatment modality. The range of metabolic phenotypes of cancer cells, and glioma cells in particular, may explain the large number of in silico studies linking cuproptosis-related gene expression to glioma prognosis (Zhang et al. 2022; Zhang et al. 2023b; Ye et al. 2022; Zhu et al. 2022). Higher FDX1 expression has been linked to a higher pathological tumor grade as well as overall poorer prognosis in multiple in silico investigations of glioma and glioblastoma (Zhang et al. 2023b; Guowei et al. 2023). However, given the importance of FDX1 in the regulation of mitochondrial metabolism and cuproptosis, these tumors may be most susceptible to CuES treatment. Together, these studies highlight the importance of combining molecular studies with in silico investigations to improve treatment outcomes.
The finding that metallothionein induction is protective against copper ionophore induced cytotoxicity may also have important implications for the treatment of gliomas. Malignant gliomas have been found to express higher levels of MT genes and higher expression has been associated with a more aggressive tumor type and poorer patient prognosis (Masiulionytė et al. 2019; Mehrian-Shai et al. 2015). In vitro, loss of MT1E and MT2A expression sensitized human glioblastoma cells to disulfiram treatment (Corsello et al. 2020), which is particularly relevant to the data presented here as disulfiram is another copper ionophore (Kannappan et al. 2021). Therefore, gliomas with lower MT expression are more likely to respond favorably to CuES treatment and MTs may represent a new target for anti-neoplastic therapeutics. In conclusion, we have demonstrated that glioma cells and astrocytes are highly susceptible to CuES induced cell death while neurons are resistant to this insult. We have also demonstrated that this cytoxicity is FDX1-independent and mediated by oxidative stress. Additionally, we have shown that induction of metallothionein gene expression may protect against CuES toxicity.
Identifying methods of glioprotection is likely a critical step in order to move CuES treatment from in vitro studies to pre-clinical models of glioma as well as to clinical studies. While the finding that neurons are spared from CuES toxicity is promising, the detrimental effect of this compound on astrocytes should not be overlooked. Although astrocytes, unlike neurons, are mitotic and theoretically could recover from this insult, oxidative stress has been associated with astrocyte senescence and reduced proliferation (Bitto et al. 2010; Cohen and Torres 2019). These findings may have negative therapeutic implications for the potential use of CuES in the treatment of gliomas. In addition to reducing proliferation, astrocyte senescence has been associated with an increase in neurotoxic secretions which could damage surrounding neurons unaffected by CuES treatment (Limbad et al. 2020; Pertusa et al. 2007). In fact, we observed limited excitotoxic injury to neurons as a result of astrocytic damage following CuES treatment. In addition to oxidative stress, aging has been found to contribute to astrocyte senescence (Cohen and Torres 2019) – a point that is of particular importance in glioblastoma, a very aggressive glioma, as the incidence of this tumor type increases with age (Ostrom et al. 2019). Therefore, damage by CuES to healthy astrocytes may have detrimental and long-lasting consequences. Learning how to protect astrocytes from CuES, or how to increase the selectivity of copper toxicity for glioma cells may have important therapeutic benefits and improve long-term positive patient outcomes.
Supplementary Material
Funding:
This work was supported by NIH grants NS043277 (EA), 5T32AG021885 (JG) and EY024481 (PAR).
List of Abbreviations
- 4-HNE
4-hydoxy-2-nonenal
- AA
Antimycin A
- BSA
Bovine serum albumin
- CuES
Copper-elesclomol
- MK801
Dizocilpine hydrogen maleate
- DIV
Days in vitro
- DFO
Deferoxamine mesylate
- ES
Elesclomol
- Fer-1
Ferrostatin-1
- FDX1
Ferredoxin-1
- FBS
Fetal Bovine Serum
- GFAP
Glial fibrillary acid protein
- ICLAC
International Cell Line Authentication Committee
- LDH
Lactate Dehydrogenase
- KA
Kainate
- MAP2
Microtubule-associated protein 2
- MT
Metallothionein
- Nec-1s
Necrostatin-1s
- NTC siRNA
Non-targeting control siRNA
- OD
Optical density
- PLO
Poly-L-ornithine
- PQQ
Pyrroloquinoline quinone
- ROS
Reactive oxygen species
- RRIDs
Research Resource Identifiers
- NaAz
Sodium Azide
- TCA cycle
Tricarboxylic acid cycle
- qPCR
Quantitative polymerase chain reaction
- ZnPyr
Zinc pyrithione
Footnotes
Ethics Statement and Consent to Participate: Animals procedures were approved by the IACUC of the University of Pittsburgh (Protocol # 21039053).
Conflict of Interest Statement: The authors declare no competing financial interests.
Data Availability:
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
- Agnihotri S, Mansouri S, Burrell K, Li M, Mamatjan Y, Liu J, Nejad R, et al. (2019) Ketoconazole and Posaconazole Selectively Target HK2-expressing Glioblastoma Cells. Clin. Cancer Res 25, 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ (2000) Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem 75, 1878–1888. [DOI] [PubMed] [Google Scholar]
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (2007) Wilson’s disease. Lancet 369, 397–408. [DOI] [PubMed] [Google Scholar]
- Allen M, Bjerke M, Edlund H, Nelander S, Westermark B (2016) Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med 8, 354re3. [DOI] [PubMed] [Google Scholar]
- Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol 59, 95–104. [DOI] [PubMed] [Google Scholar]
- Aras MA, Aizenman E (2011) Redox regulation of intracellular zinc: molecular signaling in the life and death of neurons. Antioxid. Redox Signal 15, 2249–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MA, Hara H, Hartnett KA, Kandler K, Aizenman E (2009) Protein kinase C regulation of neuronal zinc signaling mediates survival during preconditioning. J. Neurochem 110, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MA, Hartnett KA, Aizenman E (2008) Assessment of cell viability in primary neuronal cultures. Curr Protoc Neurosci Chapter 7, Unit 7.18. [DOI] [PubMed] [Google Scholar]
- Arend C, Ehrke E, Dringen R (2019) Consequences of a Metabolic Glucose-Depletion on the Survival and the Metabolism of Cultured Rat Astrocytes. Neurochem. Res 44, 2288–2300. [DOI] [PubMed] [Google Scholar]
- Artells E, Palacios O, Capdevila M, Atrian S (2014) In vivo-folded metal-metallothionein 3 complexes reveal the Cu-thionein rather than Zn-thionein character of this brain-specific mammalian metallothionein. FEBS J. 281, 1659–1678. [DOI] [PubMed] [Google Scholar]
- Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, Bush AI (1997) Metallothioneins in brain--the role in physiology and pathology. Toxicol. Appl. Pharmacol 142, 229–242. [DOI] [PubMed] [Google Scholar]
- Aschner M, Conklin DR, Yao CP, Allen JW, Tan KH (1998) Induction of astrocyte metallothioneins (MTs) by zinc confers resistance against the acute cytotoxic effects of methylmercury on cell swelling, Na+ uptake, and K+ release. Brain Res. 813, 254–261. [DOI] [PubMed] [Google Scholar]
- Bady P, Diserens A-C, Castella V, Kalt S, Heinimann K, Hamou M-F, Delorenzi M, Hegi ME (2012) DNA fingerprinting of glioma cell lines and considerations on similarity measurements. Neuro. Oncol 14, 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P (2010) Affinity gradients drive copper to cellular destinations. Nature 465, 645–648. [DOI] [PubMed] [Google Scholar]
- Baskin R, Woods NT, Mendoza-Fandiño G, Forsyth P, Egan KM, Monteiro ANA (2015) Functional analysis of the 11q23.3 glioma susceptibility locus implicates PHLDB1 and DDX6 in glioma susceptibility. Sci. Rep 5, 17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KFS, Al-Mubarak B, Martel M-A, McKay S, Wheelan N, Hasel P, Márkus NM, et al. (2015) Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun 6, 7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Sell C, Crowe E, Lorenzini A, Malaguti M, Hrelia S, Torres C (2010) Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res 316, 2961–2968. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Cheung-Ong K, Gebbia M, Proia DA, He S, Kepros J, Jonneaux A, et al. (2012) Mitochondrial electron transport is the cellular target of the oncology drug elesclomol. PLoS One 7, e29798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulcke F, Dringen R (2015) Copper oxide nanoparticles stimulate glycolytic flux and increase the cellular contents of glutathione and metallothioneins in cultured astrocytes. Neurochem. Res 40, 15–26. [DOI] [PubMed] [Google Scholar]
- Bulcke F, Santofimia-Castaño P, Gonzalez-Mateos A, Dringen R (2015) Modulation of copper accumulation and copper-induced toxicity by antioxidants and copper chelators in cultured primary brain astrocytes. J Trace Elem Med Biol 32, 168–176. [DOI] [PubMed] [Google Scholar]
- Calvo JS, Lopez VM, Meloni G (2018) Non-coordinative metal selectivity bias in human metallothioneins metal-thiolate clusters. Metallomics 10, 1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Min J, Wang F (2022) Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther 7, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Yokoyama M, Koh J (1988) Zinc neurotoxicity in cortical cell culture. Neuroscience 24, 67–79. [DOI] [PubMed] [Google Scholar]
- Chubatsu LS, Meneghini R (1993) Metallothionein protects DNA from oxidative damage. Biochem. J 291 ( Pt 1), 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Torres C (2019) Astrocyte senescence: Evidence and significance. Aging Cell 18, e12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG, Humeidi R, et al. (2020) Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 1, 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosi N, Rossi L (2015) Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int 90, 36–45. [DOI] [PubMed] [Google Scholar]
- Datta S, Sears T, Cortopassi G, Woolard K, Angelastro JM (2021) Repurposing FDA approved drugs inhibiting mitochondrial function for targeting glioma-stem like cells. Biomed. Pharmacother 133, 111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 104, 19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LJM, Wu S, Jurriëns C, Mustafa DAN, Grevers F, Burgers PC, Sillevis Smitt PAE, Kros JM, Luider TM (2020) Metabolic changes related to the IDH1 mutation in gliomas preserve TCA-cycle activity: An investigation at the protein level. FASEB J. 34, 3646–3657. [DOI] [PubMed] [Google Scholar]
- Dineley KE, Scanlon JM, Kress GJ, Stout AK, Reynolds IJ (2000) Astrocytes are more resistant than neurons to the cytotoxic effects of increased [Zn(2+)](i). Neurobiol. Dis 7, 310–320. [DOI] [PubMed] [Google Scholar]
- Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, et al. (2002) Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J. Med. Chem 45, 4549–4558. [DOI] [PubMed] [Google Scholar]
- Dringen R, Brandmann M, Hohnholt MC, Blumrich E-M (2015) Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res 40, 2570–2582. [DOI] [PubMed] [Google Scholar]
- Erkan EP, Ströbel T, Lewandrowski G, Tannous B, Madlener S, Czech T, Saydam N, Saydam O (2014) Depletion of minichromosome maintenance protein 7 inhibits glioblastoma multiforme tumor growth in vivo. Oncogene 33, 4778–4785. [DOI] [PubMed] [Google Scholar]
- Festa RA, Thiele DJ (2011) Copper: an essential metal in biology. Curr. Biol 21, R877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C (2021) Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol. Oncol 15, 3527–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza NM, Swaminathan AB, Maremanda KP, Zulkifli M, Gohil VM (2023) Mitochondrial copper in human genetic disorders. Trends Endocrinol. Metab 34, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicka L, Didik J (2009) Catalytic scavenging of peroxynitrite by catalase. J Inorg Biochem 103, 1375–1379. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A (1997) Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J. Neurochem 69, 2612–2615. [DOI] [PubMed] [Google Scholar]
- Giakoumettis D, Kritis A, Foroglou N (2018) C6 cell line: the gold standard in glioma research. Hippokratia 22, 105–112. [PMC free article] [PubMed] [Google Scholar]
- Gromadzka G, Tarnacka B, Flaga A, Adamczyk A (2020) Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guowei L, Xiufang L, Qianqian X, Yanping J (2023) The FDX1 methylation regulatory mechanism in the malignant phenotype of glioma. Genomics 115, 110601. [DOI] [PubMed] [Google Scholar]
- Halim ND, Mcfate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58, 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harders AR, Arend C, Denieffe SC, Berger J, Dringen R (2023) Endogenous Energy Stores Maintain a High ATP Concentration for Hours in Glucose-Depleted Cultured Primary Rat Astrocytes. Neurochem. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E (1997) NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+-free solutions in rat cortical neurons in vitro. J. Neurochem 68, 1836–1845. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP (1998) Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp. Neurol 154, 241–258. [DOI] [PubMed] [Google Scholar]
- Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Márkus NM, et al. (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun 8, 15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff BB, Wu X, Yadav AA, Patel D, Zhang H, Wang D-S, Chen Z-S, Yalowich JC (2015) Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II). Biochem. Pharmacol 93, 266–276. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab 27, 219–249. [DOI] [PubMed] [Google Scholar]
- Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 13, 2870–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J, Aschner M, Zatta P, Vasák M (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull 55, 133–145. [DOI] [PubMed] [Google Scholar]
- Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S (2004) Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem 88, 657–667. [DOI] [PubMed] [Google Scholar]
- Hwang JEC, Bruyne M. de, Warr CG, Burke R (2014) Copper overload and deficiency both adversely affect the central nervous system of Drosophila. Metallomics 6, 2223–2229. [DOI] [PubMed] [Google Scholar]
- Jaksch M, Ogilvie I, Yao J, Kortenhaus G, Bresser HG, Gerbitz KD, Shoubridge EA (2000) Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet 9, 795–801. [DOI] [PubMed] [Google Scholar]
- Juaristi I, Llorente-Folch I, Satrústegui J, Del Arco A (2019) Extracellular ATP and glutamate drive pyruvate production and energy demand to regulate mitochondrial respiration in astrocytes. Glia 67, 759–774. [DOI] [PubMed] [Google Scholar]
- Kaler SG (2013) Inborn errors of copper metabolism. Handb Clin Neurol 113, 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannappan V, Ali M, Small B, Rajendran G, Elzhenni S, Taj H, Wang W, Dou QP (2021) Recent Advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents. Front. Mol. Biosci 8, 741316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Hosoya R, Sato H, Tanaka M, Nakajima T, Iwabuchi S (2002) Selective blockade of astrocytic glutamate transporter GLT-1 with dihydrokainate prevents neuronal death during ouabain treatment of astrocyte/neuron cocultures. Glia 40, 337–349. [DOI] [PubMed] [Google Scholar]
- Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang C-Y, Zhang M, Du Z, Barsoum J, Bertin J (2008) Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther 7, 2319–2327. [DOI] [PubMed] [Google Scholar]
- Lazic A, Balint V, Stanisavljevic Ninkovic D, Peric M, Stevanovic M (2022) Reactive and senescent astroglial phenotypes as hallmarks of brain pathologies. Int. J. Mol. Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-R, Bu L-L, Cai L (2022a) Cuproptosis: lipoylated TCA cycle proteins-mediated novel cell death pathway. Signal Transduct. Target. Ther 7, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao T, Li J, Xia M, Li Y, Wang X, Liu C, et al. (2022b) Oxidative Stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-related Diseases. J. Immunol. Res 2022, 2233906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbad C, Oron TR, Alimirah F, Davalos AR, Tracy TE, Gan L, Desprez P-Y, Campisi J (2020) Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS One 15, e0227887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulionytė B, Valiulytė I, Tamašauskas A, Skiriutė D (2019) Metallothionein Genes are Highly Expressed in Malignant Astrocytomas and Associated with Patient Survival. Sci. Rep 9, 5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlenbacher MR, Elsiesy R, Lakha R, Villones RLE, Orman M, Vizcarra CL, Meloni G, Wilcox DE, Austin RN (2022) Metal binding and interdomain thermodynamics of mammalian metallothionein-3: enthalpically favoured Cu+ supplants entropically favoured Zn2+ to form Cu4+ clusters under physiological conditions. Chem. Sci 13, 5289–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrian-Shai R, Yalon M, Simon AJ, Eyal E, Pismenyuk T, Moshe I, Constantini S, Toren A (2015) High metallothionein predicts poor survival in glioblastoma multiforme. BMC Med. Genomics 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni G, Faller P, Vasák M (2007) Redox silencing of copper in metal-linked neurodegenerative disorders: reaction of Zn7metallothionein-3 with Cu2+ ions. J. Biol. Chem 282, 16068–16078. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B (2001) Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci 21, 8348–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BJ, Kauderer JT, Moxley KM, Bonebrake AJ, Dewdney SB, Secord AA, Ueland FR, Johnston CM, Aghajanian C (2018) A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: An NRG oncology/gynecologic oncology group study. Gynecol. Oncol 151, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MT, Bourassa D, Harankhedkar S, McCallum AM, Zlatic SA, Calvo JS, Meloni G, Faundez V, Fahrni CJ (2019) Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl. Acad. Sci. USA 116, 12167–12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky HJ, Brown RE (2006) Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Bayir H, Belousov V, Chang CJ, Davies KJA, Davies MJ, Dick TP, et al. (2022) Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab 4, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyderman H, Wadey AL, Nilsson M, Sims NR (2007) Mitochondrial glutathione protects against cell death induced by oxidative and nitrative stress in astrocytes. J. Neurochem 102, 1369–1382. [DOI] [PubMed] [Google Scholar]
- Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, Chu J, Beaudette-Zlatanova BC, et al. (2012) The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic. Biol. Med 52, 2142–2150. [DOI] [PubMed] [Google Scholar]
- Nicholson JG, Fine HA (2021) Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 11, 575–590. [DOI] [PubMed] [Google Scholar]
- O’Day SJ, Eggermont AMM, Chiarion-Sileni V, Kefford R, Grob JJ, Mortier L, Robert C, et al. (2013) Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol 31, 1211–1218. [DOI] [PubMed] [Google Scholar]
- Olney JW, Fuller T, Gubareff T. de (1979) Acute dendrotoxic changes in the hippocampus of kainate treated rats. Brain Res. 176, 91–100. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro. Oncol 21, v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Prasad R (2014) Recent discoveries on the functions of astrocytes in the copper homeostasis of the brain: a brief update. Neurotox Res 26, 78–84. [DOI] [PubMed] [Google Scholar]
- Pal A, Prasad R (2015) An overview of various mammalian models to study chronic copper intoxication associated Alzheimer’s disease like pathology. Biometals 28, 1–9. [DOI] [PubMed] [Google Scholar]
- Pal A, Rani I, Pawar A, Picozza M, Rongioletti M, Squitti R (2021) Microglia and astrocytes in alzheimer’s disease in the context of the aberrant copper homeostasis hypothesis. Biomolecules 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa M, García-Matas S, Rodríguez-Farré E, Sanfeliu C, Cristòfol R (2007) Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem 101, 794–805. [DOI] [PubMed] [Google Scholar]
- Prieto M, Alonso G (1999) Differential sensitivity of cultured tanycytes and astrocytes to hydrogen peroxide toxicity. Exp. Neurol 155, 118–127. [DOI] [PubMed] [Google Scholar]
- Qu Y, Vadivelu S, Choi L, Liu S, Lu A, Lewis B, Girgis R, et al. (2003) Neurons derived from embryonic stem (ES) cells resemble normal neurons in their vulnerability to excitotoxic death. Exp. Neurol 184, 326–336. [DOI] [PubMed] [Google Scholar]
- Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W (1993) Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 12, 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rising L, Vitarella D, Kimelberg HK, Aschner M (1995) Metallothionein induction in neonatal rat primary astrocyte cultures protects against methylmercury cytotoxicity. J. Neurochem 65, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA (1991) Accumulation of extracellular glutamate and neuronal death in astrocyte-poor cortical cultures exposed to glutamine. Glia 4, 91–100. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Aizenman E (1989) Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett 103, 162–168. [DOI] [PubMed] [Google Scholar]
- Ross MM, Aizenman E (2023) GluA1-Shank3 interaction decreases in response to chronic neuronal depolarization. Neurosci. Lett 809, 137305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz LM, Libedinsky A, Elorza AA (2021) Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci 8, 711227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int. J. Mol. Sci 14, 6044–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber IF, Dringen R (2011) Copper accelerates glycolytic flux in cultured astrocytes. Neurochem. Res 36, 894–903. [DOI] [PubMed] [Google Scholar]
- Scheiber IF, Mercer JFB, Dringen R (2014) Metabolism and functions of copper in brain. Prog. Neurobiol 116, 33–57. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB (1998) Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol. Pharmacol 53, 355–369. [DOI] [PubMed] [Google Scholar]
- Schulz V, Basu S, Freibert S-A, Webert H, Boss L, Mühlenhoff U, Pierrel F, et al. (2023) Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2. Nat. Chem. Biol 19, 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MA, Ollosson R, Stewart VC, Clark JB (2002) Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J 366, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EA, Dalla Costa AP, Ruas JS, Siqueira-Santos ES, Francisco A, Castilho RF (2023) Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation. Cells 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor JD, Du S, Venneti S, Blitzblau RC, Leszkiewicz DN, Rosenberg PA, Aizenman E (2000) NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro. J. Neurosci 20, 8831–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, Pellerino A, Bruno F, Mauro A, Rudà R (2023) Neurotoxicity from Old and New Radiation Treatments for Brain Tumors. Int. J. Mol. Sci 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisky H, Gómez M, Burgos-Bravo F, López-Alarcón C, Jullian C, Olea-Azar C, Aliaga ME (2009) Generation of superoxide radicals by copper-glutathione complexes: redox-consequences associated with their interaction with reduced glutathione. Bioorg. Med. Chem 17, 1803–1810. [DOI] [PubMed] [Google Scholar]
- Stites TE, Mitchell AE, Rucker RB (2000) Physiological importance of quinoenzymes and the O-quinone family of cofactors. J. Nutr 130, 719–727. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, Bent M. J. van den, Weller M, Fisher B, Taphoorn MJB, Belanger K, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC (1997) Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci 17, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Vašák M (1985) Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 827, 36–44. [DOI] [PubMed] [Google Scholar]
- Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, et al. (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov P, Detappe A, Cai K, Keys HR, Brune Z, Ying W, Thiru P, et al. (2019) Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol 15, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Gomi F, Masumizu T, Miura Y (2002) Growth inhibitory factor prevents neurite extension and the death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J. Biol. Chem 277, 32353–32359. [DOI] [PubMed] [Google Scholar]
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol 90, 1–37. [DOI] [PubMed] [Google Scholar]
- Villablanca C, Vidal R, Gonzalez-Billault C (2023) Are cytoskeleton changes observed in astrocytes functionally linked to aging? Brain Res. Bull 196, 59–67. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chung HJ, Schnuer J, Lea E, Robinson MB, Potthoff WK, Aizenman E, Rosenberg PA (1998) Dihydrokainate-sensitive neuronal glutamate transport is required for protection of rat cortical neurons in culture against synaptically released glutamate. Eur. J. Neurosci 10, 2523–2531. [DOI] [PubMed] [Google Scholar]
- Weilinger NL, Lohman AW, Rakai BD, Ma EMM, Bialecki J, Maslieieva V, Rilea T, et al. (2016) Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci 19, 432–442. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT (1989) Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 12, 94–101. [DOI] [PubMed] [Google Scholar]
- Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, Kroemer G, Chen X, Tang D, Liu J (2023) Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AA, Patel D, Wu X, Hasinoff BB (2013) Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J Inorg Biochem 126, 1–6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Maret W, Vallee BL (2001) Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc. Natl. Acad. Sci. USA 98, 5556–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Zhang S, Cai J, Ye L, Gao L, Wang Y, Tong S, et al. (2022) Development and validation of cuproptosis-associated prognostic signatures in WHO 2/3 glioma. Front. Oncol 12, 967159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J-Y, Lee Y-J, Kim Y-J, Baik T-K, Lee J-H, Lee M-J, Woo R-S (2023) Multiple low-dose radiation-induced neuronal cysteine transporter expression and oxidative stress are rescued by N-acetylcysteine in neuronal SH-SY5Y cells. Neurotoxicology 95, 205–217. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xie L, Liu J, Liu A, He M (2023a) Construction and validation of a cuproptosis-related prognostic model for glioblastoma. Front. Immunol 14, 1082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu X, Wang D, Ruan X, Wang P, Liu L, Xue Y (2023b) A novel cuproptosis-related gene signature to predict prognosis in Glioma. BMC Cancer 23, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dai X, Li Z (2022) Molecular subtypes of cuproptosis regulators and their correlation with clinical prognosis and immune response in glioma. Am J Transl Res 14, 8085–8102. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rosenberg PA (2002) The essential nutrient pyrroloquinoline quinone may act as a neuroprotectant by suppressing peroxynitrite formation. Eur. J. Neurosci 16, 1015–1024. [DOI] [PubMed] [Google Scholar]
- Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T (2016) Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wan Q, Tan J, Ouyang H, Pan X, Li M, Zhao Y (2022) A novel prognostic signature of cuproptosis-related genes and the prognostic value of FDX1 in gliomas. Front. Genet 13, 992995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


