Abstract
Background:
Transcranial electrical stimulation (tES) may improve psychosis symptoms, but few investigations have targeted brain regions causally linked to psychosis symptoms. We implemented a novel montage targeting the extrastriate visual cortex (eVC) previously identified by lesion network mapping in the manifestation of visual hallucinations.
Objective:
To determine if lesion network guided High Definition-tES (HD-tES) to the eVC is safe and efficacious in reducing symptoms related to psychosis.
Methods:
We conducted a single-blind crossover pilot study (NCT04870710) in patients with psychosis spectrum disorders. Participants first received HD-tDCS (direct current), followed by 4 weeks of wash out, then 2Hz HD-tACS (alternating current). Participants received 5 days of daily (2 × 20min) stimulation bilaterally to the eVC. Primary outcomes included the Positive and Negative Syndrome Scale (PANSS), biological motion task, and Event Related Potentials (ERP) from a steady state visual evoked potential (SSVEP) paradigm. Secondary outcomes included the Montgomery-Asperg Depression Rating Scale, Global Assessment of Functioning (GAF), velocity discrimination and visual working memory task, and emotional ERP.
Results:
HD-tDCS improved PANSS general psychopathology in the short-term (d=0.47; pfdr=0.03), with long-term improvements in general psychopathology (d=0.62; pfdr=0.05) and GAF (d=−0.56; pfdr=0.04) with HD-tACS. HD-tDCS reduced SSVEP P1 (d=0.25; pfdr=0.005), which correlated with general psychopathology (β=0.274, t=3.59, p=0.04). No significant differences in safety or tolerability measures were identified.
Conclusion:
Lesion network guided HD-tES to the eVC is a safe, efficacious, and promising approach for reducing general psychopathology via changes in neuroplasticity. These results highlight the need for larger clinical trials implementing novel targeting methodologies for the treatments of psychosis.
Keywords: Positive Symptoms, Transcranial Electrical Stimulation, Lesion Network Mapping
Introduction
Transcranial electrical stimulation (tES) modulates cortical activity and influences cognition1, perception2, and positive symptoms in psychosis3. Few researchers have integrated recent neuroimaging findings to identify optimal stimulation targets, such as location, frequency, and circuits4. Innovations in tES hardware and software now allows for more focal stimulation (using high definition tES, HD-tES) compared to sponge montages5 and greater spatial target engagement using current flow models6. While HD-tES advances have been effective for the treatment of neuropsychiatric disorders7 few studies have used HD-tES in psychosis1,4,8,9.
Psychotic disorders consist of negative symptoms10, positive symptoms11, cognitive deficits12 and disorganized thoughts and/or behavior13. Positive symptoms, such as hallucinations are often debilitating with visual hallucinations (VH) associated with more severe morbidity, delusions, suicidal behavior, and catatonia14. Estimations related to the prevalence of VH in psychosis have been reported to be upwards of 27% in individuals diagnosed with schizophrenia, 15% in affective psychosis and roughly 7% in the general population15. In addition, others have shown that the prevalence of VH can be as high as 33% in first-episode of psychosis16. Lifetime prevalence rates have been estimated to be between 23–31%17. While antipsychotics treat positive symptoms, ~30% of individuals are treatment resistant18, which may result in metabolic dysregulation19, agranulocytosis, and risk of seizures20. Thus, there is a critical need for novel, neurobiologically informed, non-invasive, and safe treatments for psychosis symptom management, such as HD-tES.
To optimize tES parameters we used a combination of neuroimaging, neurophysiological, and cause-effect studies. The extrastriate visual cortex (eVC) was of particular importance due to its role in motion perception, neurocognition, and social cognition21,22. For instance, in a large cross-sectional neuroimaging study we identified thinning of the eVC (V5/MT) across the psychosis spectrum compared to controls, which correlated with poor cognition and response inhibition23. In fMRI studies examining active visual and/or auditory hallucinations in drug-free adolescents with brief psychotic disorders or adults with psychosis spectrum disorders, the authors found activation of the primary and secondary visual cortices24,25. Results from a lesion networking mapping (LNM) study, a powerful tool used to make causal inferences from lesions causally linked to symptoms26, identified the eVC to be implicated in VH27. Pathologically elevated eVC activity has also been demonstrated in psychosis28. Lastly, a study examining the neural basis of motion perception in schizophrenia found that reduced V5/MT activation was associated with lower delta (2Hz) evoked amplitude during motion related tasks and poorer cognitive performance29. While brain frequency specific characteristics have not been utilized in past tES targeting of the visual cortex, results such as those from Martinez et al. 201829 highlight the importance of oscillatory mechanisms in the eVC. This convergent body of work highlights the importance of the eVC and delta frequency in psychosis and provides a framework for neurobiologically informed treatment with HD-tES.
To examine the translational value of the eVC in psychosis, we conducted a proof-of-concept single blind crossover study at a single site to characterize the efficacy and safety of using cathodal HD-tDCS (transcranial direct current stimulation) or delta frequency (2hz) HD-tACS (transcranial alternating current stimulation) in improving psychosis symptoms, visual processing, and visual evoked potentials.
Methods
Participants
This study enrolled outpatients beginning October 1, 2020 with the final study visit completed on January 2, 2022. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center, Massachusetts. Participants signed written informed consent and were compensated for their participation (see trial protocol in Supplement 1).
We intended to recruit 10 individuals (5 sham and 5 HD-tDCS) between the ages of 18 to 55 years with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder using the Structured Clinical Interview for DSM-V, and with a lifetime history of VH and/or experiencing mild to moderate symptoms of VH. Since recruitment efforts were hindered due to institutional restrictions during the COVID-19 pandemic, we removed the VH requirement and sham condition. Instead, the study was transitioned to a crossover design using HD-tDCS followed by 2Hz HD-tACS.
Participants had no antipsychotic medication change in the month prior to participation. Participants were excluded if they had an intelligence quotient <60, any major medical or neurologic condition, a diagnosis of substance abuse or positive urine drug screen, history of moderate-to-severe visual impairment secondary to glaucoma, cataract or macular degeneration, serious medical illness or instability requiring hospitalization within the last year, relevant skin allergies, metallic or electronic implants, or if they were pregnant or breastfeeding.
Procedure
This proof-of-concept study used a between-participants, single blind, non-randomized, crossover design, with two tES treatment conditions. Participants first received HD-tDCS, followed by 4 weeks of wash out (beginning the following week after day 5 of HD-tDCS treatment), then received 2Hz HD-tACS (Figure 1A). Clinical assessments were performed by a psychiatrist at baseline, day 5 and 1-month. Participants arrived at the hospital on a Monday, were briefed on study procedures by a research assistant, followed by electroencephalography (EEG) including a steady-state visual evoked potential (SSVEP) task, and emotional scene processing task (International Affective Picture System; IAPS). Visual processing tasks were conducted while seated in a dark room under the supervision of study staff (Figure 1B). Then, 2 sessions of 20 min HD-tDCS was administered daily for 5 days while the participant sat comfortably, quietly and without disruption. A 15–20 min break was provided between the 2 sessions and participants were asked to complete a brief sensation questionnaire related to sensations felt during the administration of tES. On a Friday, and after 5 days of treatment, baseline assessments were repeated. These assessments were performed again after 1-month. Participants then received HD-tACS, which consisted of the same study procedures as HD-tDCS.
Figure 1: Study Design, Timeline and Transcranial Electrical Stimulation (tES) Montage:
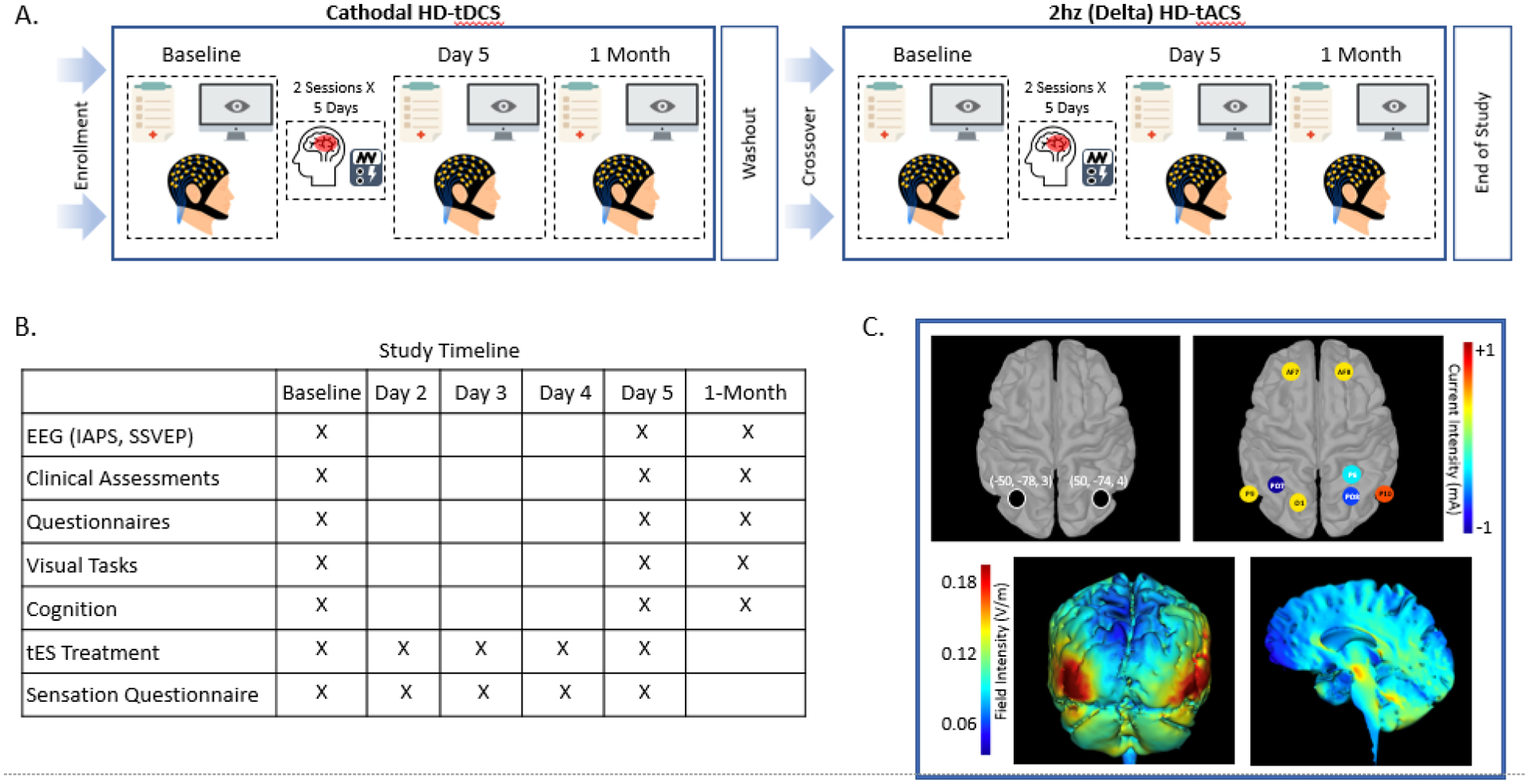
A. Depicts the experimental crossover study design. B. Demonstrates the study timeline showing when the primary and secondary outcomes were collected, as well as the days participants received electrical stimulation. C. Shows the stimulation coordinates in Montreal Neurologic Institute (MNI) space for the bilateral extrastriate visual cortex target, stimulation electrode montage (current intensity depicted in heatmap), and the current flow modeling (field intensity depicted in heatmap). Note: HD-tDCS, High-Definition Transcranial Direct Current Stimulation; HD-tACS, HHD-Transcranial Alternating Current Stimulation; EEG, Electroencephalogram; IAPS, International Affective Picture System; SSVEP, Steady State Visual Evoked Potential;
Treatment
HD-tDCS and HD-tACS was delivered by a Soterix MXN-9 High Definition-Transcranial Electrical Current Stimulator, Model 9002A (Supplement 2). The stimulation montage was designed to target the lesion network mapping findings associated with VH, which identified the bilateral eVC27 (Figure 1C). The delta (2Hz) frequency peak for this study was extracted from the Maritnez et al 2018 paper, which conducted a time-frequency analysis of a motion processing task in patients with schizophrenia (Supplement 3). Electrical current field modeling6 using HD-Explore and HD-Targets (Soterix Medical) guided decision-making about where to place electrodes, with the goal of delivering focalized current to the bilateral eVC. The montage consisted of cathodal PO7 and anodal P9, O1, AF7 on the left, and cathodal P6, P08 and anodal P10, AF8 on the right according to the International 10-10 System. HD-tACS used the same montage but with 2hz in-phase alternating current being delivered (Figure 1C).
Outcome Measures
The North-East Visual Hallucination Interview (NEVHI) was employed to establish participants with a past history of VH30,31. The questionnaire includes 3 binary responses related to VH. If answered ‘yes’ to one of these questions, the participant is identified as having VH. See Table 1 for count of participants with past VH. It is important to note, that no individuals were experiencing active VH.
Table 1.
Baseline Demographic Characteristics
| HD-tDCS | HD-tACS | |
|---|---|---|
| Sex (M/F) | 3/3 (N=6) | 2/2 (N=4) |
| Race/Ethnicity | ||
| Black | 2 | 2 |
| White | 3 | 2 |
| Other | 1 | 0 |
| Age, mean (SD) | 29.7 (2.6) | 29.8 (3.1) |
| Schizophrenia | 3 | 2 |
| Schizoaffective | 1 | 1 |
| Bipolar | 2 | 1 |
| NEVHI Q1–3: VH+/VH− | 4/2 | 2/2 |
| CPZ Equivalence, Mean (SD) | 260.9 (269.6) | 314.4 (279.0) |
| Illness Duration in Years, Mean (SD) | 11.8 (3.7) | 9.5 (1.0) |
Notes: HD-tDCS, High-Definition Transcranial Direct Current Stimulation; HD-tACS, High-Definition Transcranial Alternating Current Stimulation; NEVHI, North-East Visual Hallucination Interview; VH+, visual hallucinations present; VH−, no visual hallucinations; CPZ, chlorpromazine; SD, Standard Deviation
The primary outcomes examined were the Positive and Negative Syndrome Scale (PANSS), biological motion detection, and SSVEP between timepoints and stimulation montages. PANSS total, positive, negative, and general scores were used. Visual processing outcomes were obtained by a biological motion task to assess the accuracy for determining the direction of motion32 (Supplement 4). Event Related Potential (ERP) measures were obtained through a SSVEP task to assess changes in biomarkers of the early visual response, the P1 and N1 (Supplement 5).
The secondary outcomes examined included the Montgomery-Asberg Depression Rating Scale (MADRS), Global Assessment of Functioning (GAF), visual processing behavioral tasks, and emotional processing ERPs. Visual processing measures were obtained through a velocity discrimination and a visuospatial working memory task to assess accuracy of speed detection and visual working memory, respectively32 (Supplementary 4). Emotional ERP measures were obtained using the IAPS, which consists of unpleasant, pleasant, and neutral scene stimuli, to assess changes in a motivationally-relevant early visual biomarker, the early posterior negativity (EPN)33 (Supplementary 5).
Exploratory analyses included determining whether significant (p<0.1) target engagement of EEG measures using tES would be correlated with significant (p<0.1) changes in clinical or behavioral measures.
Statistical Analysis
All statistics were performed using R software (v4.1.2) and RStudio. For individuals missing 1-month assessments (HD-tDCS n=1, HD-tACS n=1), values were imputed using the Amelia package34 while accounting for scores across sessions. Modeling constraints were considered for imputation and implemented using the polynomial to account for the effect of time. One imputation model was run to obtain imputed values. The “ggstatsplot” package was used for statistical analysis and plots35. The “WRS2” package was used for two-way ANOVA36. Chlorpromazine equivalents was calculated using “chlorpromazineR” and the Leucht et al methodology37. We used non-parametric tests consisting of the Friedman and Durbin Conover tests to examine within group differences. Trimmed means (20 percent) two-way ANOVA models were used to examine group (HD-tDCS, HD-tACS) by session (baseline, day 5 & 1-month) interactions. To assess the relationship between changes (follow up - baseline) in clinical and EEG measurements, rank-based estimation regression while controlling for skewness38 was used with baseline clinical measurements used as a covariate. An alpha value of 0.10 was set for significance due to the sample size of the study and to help identify effect sizes to power future large scale trials39. An alpha value of 0.10 was used to determine significance throughout the analysis for this study in order to achieve a balance between the probabilities of committing Type I and II errors when working with small sample sizes, which in turn substantially increases the power of the effect39. Kendall (W) and Rank Biserial Effect Size (RBES) was calculated. False discovery rate (FDR) corrected p-values are reported for pairwise comparisons. To confirm significant results, analyses were re-run using the non-imputed dataset and are reported in the supplement.
Results
A total of 6 participants with a psychosis spectrum disorder were enrolled in the study. All 6 received HD-tDCS and 4 received 2Hz HD-tACS (Figure 2). Baseline demographic and clinical characteristics are summarized in Table 1.
Figure 2.
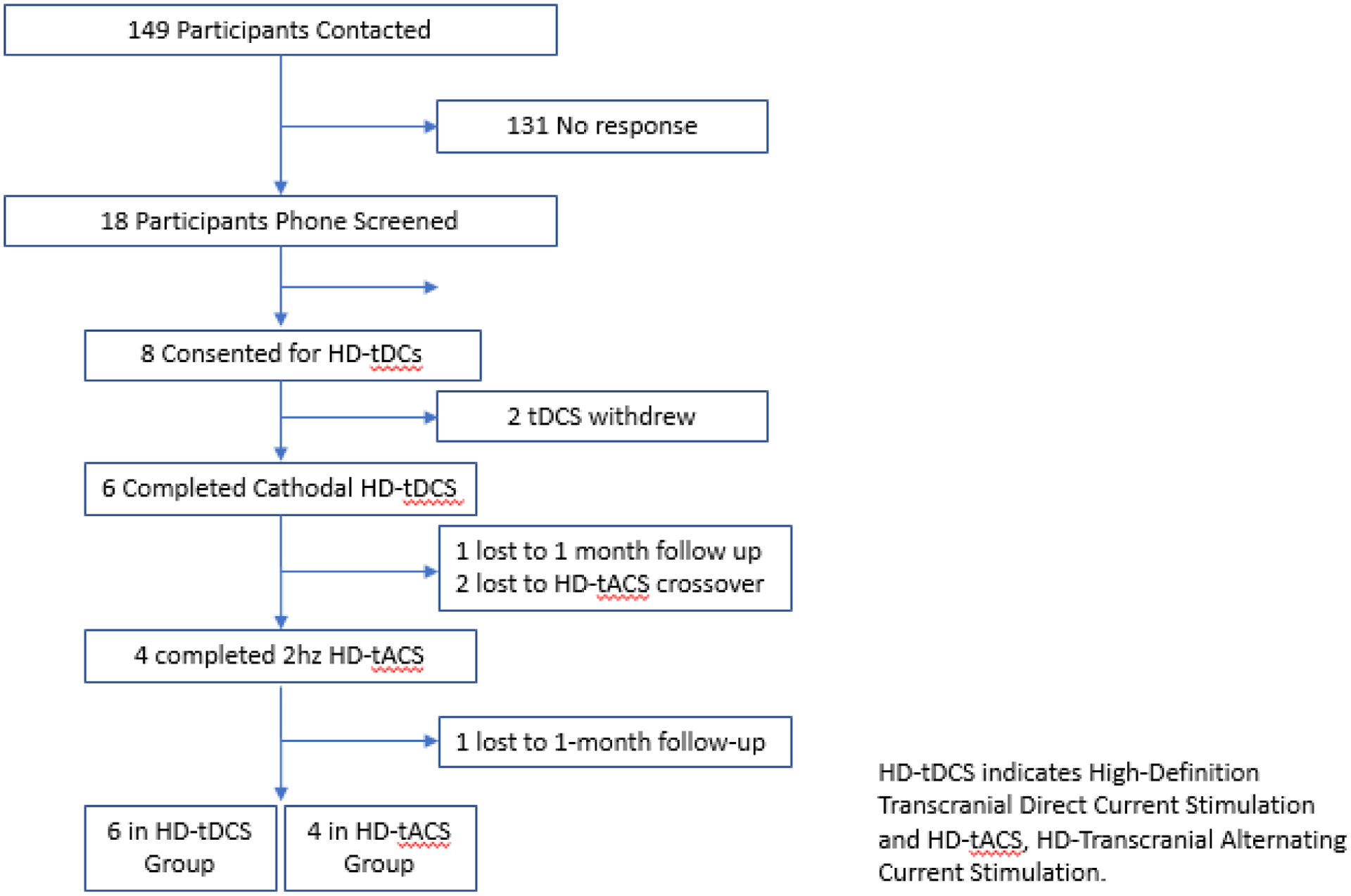
CONSORT Flow Diagram
Primary Outcomes
There were significant differences across sessions for PANSS general symptoms in the HD-tDCS (W=0.42; p=0.04) and HD-tACS condition (W=0.58; p=0.07), but not for total, positive or negative symptoms (Table 2A, Figure 3A). Post hoc comparisons in the HD-tDCS showed a significant reduction from baseline to day 5 for PANSS general scores (RBES=0.47; pfdr=0.03) and significant increase from day 5 to 1-month (RBES=−0.50; pfdr=0.03). For HD-tACS, significant reductions in PANSS general score between day 5 and 1-month (RBES=0.69; pfdr=0.05) and from baseline to 1-month (RBES=0.62; pfdr=0.05) was observed. There were no significant differences between HD-tDCS 1-month and HD-tACS baseline nor between HD-tDCS baseline and HD-tACS 1-month (eFigure 1). These analyses were repeated without imputed data and results were similar for the HD-tDCS and HD-tACS findings (Supplement 6). An exploratory analysis was conducted for PANSS P3 Hallucination score despite these participants not having acute hallucinatory symptoms, but there were no significant difference noted in either the HD-tDCS or HD-tACS group. Post hoc analysis showed a significant group by session interaction (F=12.42, p=0.02) between HD-tDCS and HD-tACS (eTable 1, Figure 3B).
Table 2A.
Primary Outcome Results
| PANSS Total | ||||||||
| Baseline | 49.50 [43.50–59.25] | 59.50 [54.50–67.50] | ||||||
| Day 5 | 44.00 [40.50–49.75] | 0.11 | 0.34 | [0.15,1.00] | 56.50 [50.50–64.75] | 0.47 | 0.19 | [0.00,1.00] |
| 1 Month | 50.00 [48.25–54.75] | 47.50 [43.75–52.00] | ||||||
| PANSS Positive | ||||||||
| Baseline | 14.50 [11.75–16.50] | 13.50 [11.0018.00] | ||||||
| Day 5 | 12.50 [8.75–15.50] | 0.17 | 0.26 | [0.08,1.00] | 14.50 [11.75–17.25] | 0.53 | 0.14 | [0.00,1.00] |
| 1 Month | 10.00 [9.25–13.75] | 13.00 [10.00–16.25] | ||||||
| PANSS Negative | ||||||||
| Baseline | 11.00 [8.50–13.50] | 19.50 [13.00–24.00] | ||||||
| Day 5 | 11.00 [8.5013.50] | 0.17 | 0.19 | [0.03,1.00] | 20.00 [13.00–26.00] | 0.53 | 0.11 | [0.02,1.00] |
| 1 Month | 14.00 [13.00–18.00] | 11.50 [10.25–12.75] | ||||||
| PANSS General | ||||||||
| Baseline | 25.00 [22.25–29.25] | 28.50 [26.50–31.50] | ||||||
| Day 5 | 20.50 [18.50–23.25] | 0.04 | 0.42 | [0.19, 1.00] | 27.50 [25.25–30.00] | 0.07 | 0.58 | [0.44,1.00] |
| 1 Month | 25.50 [23.50–27.50] | 22.50 [21.75–24.25] | ||||||
| SSVEP P100 Voltage | ||||||||
| Baseline | 1.725 [0.910–3.035] | 1.160 [0.480–02.933] | ||||||
| Day 5 | 1.180 [0.503–2.053] | 0.02 | 0.65 | [0.51,1.00] | 1.005 [0.483–3.238] | 0.78 | 0.06 | [0.06,1.00] |
| 1 Month | 1.160 [0.218–2.860] | 2.560 [1.035–4.412] | ||||||
| SSVEP N100 Voltage | ||||||||
| Baseline | −2.240[−3.710–−1.055] | −1.275[−1.900–−0.855] | ||||||
| Day 5 | −0.600[−1.135–−0.478] | 0.02 | 0.69 | [0.53,1.00] | −1.760[−2.277–−1.433] | 0.82 | 0.05 | [0.05,1.00] |
| 1 Month | −1.090[−2.000–−0.630] | −2.050[−2.353–−1.545] | ||||||
Figure 3: Primary Outcome Results:
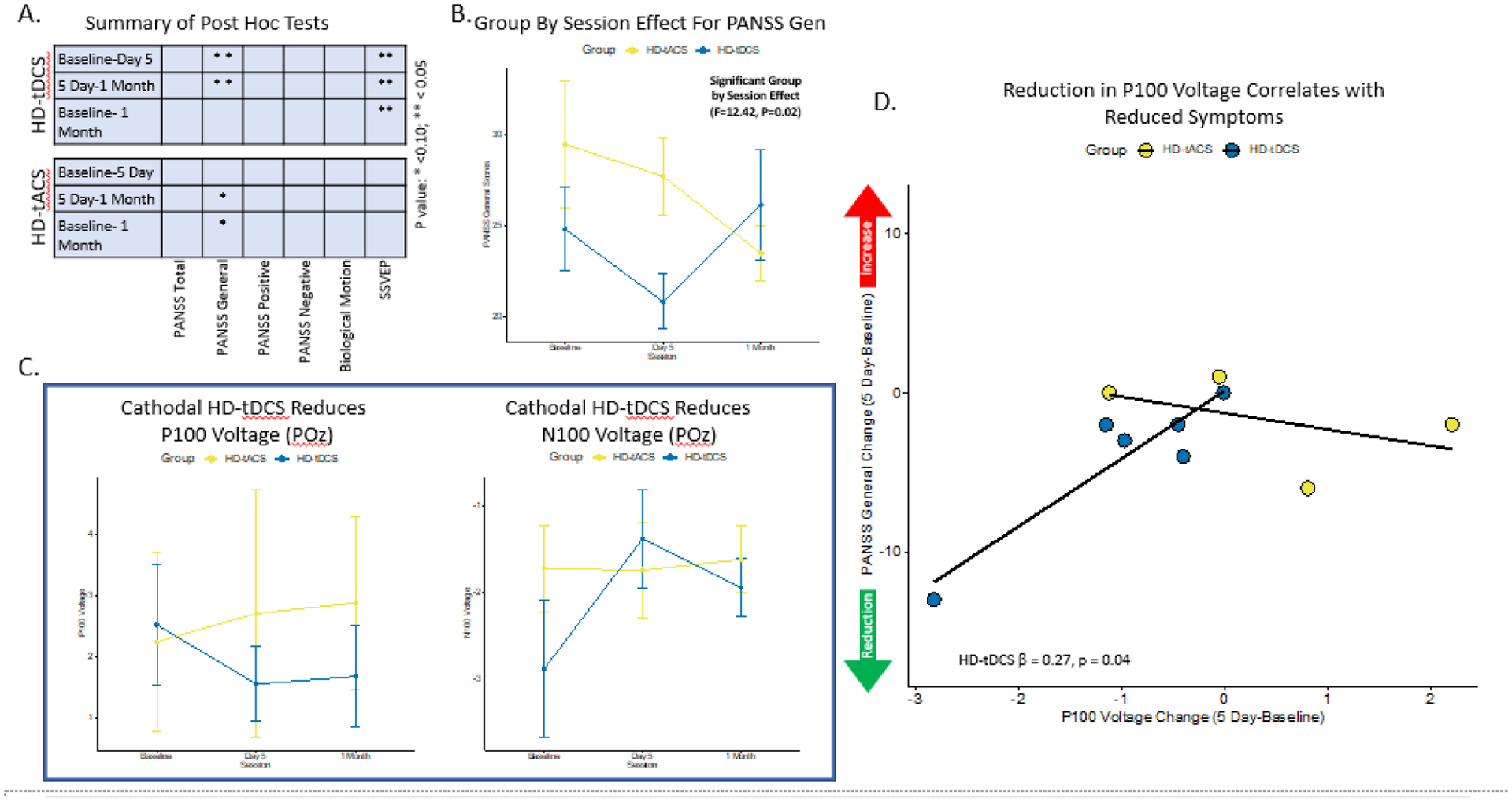
A. Demonstrates the summary of post-hoc pairwise comparisons by session contrasts for both HD-tDCS and HD-tACS. B. Depicts the group by session interaction effect for the PANSS General score. C. Shows the SSVEP P100 and N100 results at the POz sensor across sessions. D. Demonstrates the regression results between change scores (5 Day-Baseline) for P100 Voltage and PANSS General score with a significant result in the HD-tDCS condition. Notes: High-Definition Transcranial Current Stimulation; HD-tACS, High-Definition Transcranial Alternating Current Stimulation; PANSS, Positive and Negative Syndrome Scale; SSVEP, Steady State Visual Evoked Potential
There were significant differences across sessions for the SSVEP P1 voltage in the HD-tDCS group for bilateral trials at POz (W=0.65; p=0.02) (Table 2A, Figure 3A,C). HD-tDCS post hoc analyses showed a significant decrease in voltage for P1 from baseline to 5 day (RBES=0.25; pfdr=0.005) and baseline to 1-month (RBES=0.33; pfdr=0.008). The SSVEP N1 voltage was significantly different across sessions in the HD-tDCS group for bilateral POz (W=0.69; p=0.02). HD-tDCS post hoc analyses showed a significant increase in voltage for N1 from baseline to 5 day (RBES=−0.56; pfdr=0.002) and baseline to 1-month (RBES=−0.28; pfdr=0.04), as well as a significant decrease from 5 day to 1-month (RBES=0.39; pfdr=0.04). There were no significant session differences noted for P1 and N1 in the HD-tACS group. There was no significant group by session effect noted for P1 or N1 (eTable 1, Figure 3C). These results were repeated without imputed values and the results were similar (Supplement 6).
There were no significant differences observed on the biological motion task for either treatment condition (eTable 2).
In exploratory analyses, a significant relationship was identified between the improvement in PANSS general score and the reduction in P1 observed between day 5 and baseline (β=0.274, t=3.59, p=0.04) (eTable 3, Figure 3D).
Secondary Outcomes
There were significant differences across sessions for GAF scores in the HD-tACS condition (W=0.44; p=0.06) (Table 2B, Figure 4A). Post hoc comparisons in the HD-tACS showed a significant increase in GAF from day 5 to 1-month (RBES=−0.56; pfdr=0.05) and baseline to 1-month (RBES=−0.56; pfdr=0.04). These analyses were repeated without imputed data and results were similar for the HD-tACS findings (Supplement 6). There was no group by session effect observed for GAF (eTable 1, Figure 4B). There were no significant differences noted for MADRS within or between conditions (Table 2B, eTable 1).
Table 2B.
Secondary Outcome Results
| GAF | |||||||||
| Baseline | 70.00 [62.00–78.75] | 65.00 [60.00–66.25] | |||||||
| Day 5 | 68.50 [61.25–78.75] | 0.93 | 0.006 | [0.006,1.00] | 65.00 [61.75–66.25] | 0.06 | 0.44 | [0.19,1.00] | |
| 1 Month | 63.00 [53.50–65.00] | 68.00 [65.75–72.50] | |||||||
| MADRS | |||||||||
| Baseline | 6.00 [4.25–15.25] | 5.50 [3.75–10.00] | |||||||
| Day 5 | 3.50 [3.00–5.50] | 0.38 | 0.15 | [0.02,1.00] | 4.00 [2.25–8.00] | 0.53 | 0.11 | [0.02,1.00] | |
| 1 Month | 6.00 [2.00–7.75] | 2.50 [0.00–5.50] | |||||||
| IAPS Unpleasant EPN | |||||||||
| Baseline | 6.525 [6.348–6.787] | ||||||||
| Day 5 | 5.751 [5.139–5.856] | 0.01 | 0.84 | [0.76,1.00] | |||||
| 1 Month | 3.25 [3.247–4.348] | ||||||||
| IAPS Pleasant EPN | |||||||||
| Baseline | 6.127 [5.946–6.298] | ||||||||
| Day 5 | 5.19 [5.087–5.229] | 0.25 | 0.28 | [0.04,1.00] | |||||
| 1 Month | 5.300 [3.685–5.731] | ||||||||
| IAPS Neutral EPN | |||||||||
| Baseline | 6.216 [5.740–6.319] | ||||||||
| Day 5 | 6.164 [4.529–6.823] | 0.07 | 0.52 | [0.36, 1.00] | |||||
| 1 Month | 4.052 [3.74–55.002] | ||||||||
Notes: HD-tDCS, High-Definition Transcranial Current Stimulation; HD-tACS, HD Transcranial Alternating Current Stimulation; PANSS, Positive and Negative Syndrome Scale; SSVEP, Steady State Evoked Potential; GAF, Global Assessment of Functioning; MADRS, Montgomery–Åsberg Depression Rating Scale; IAPS, International Affective Picture System; EPN, Early Posterior Negativity; IQR, Interquartile Range. Statistics reported here include individuals with imputed values for follow-up visits
Figure 4: Secondary Outcome Results:
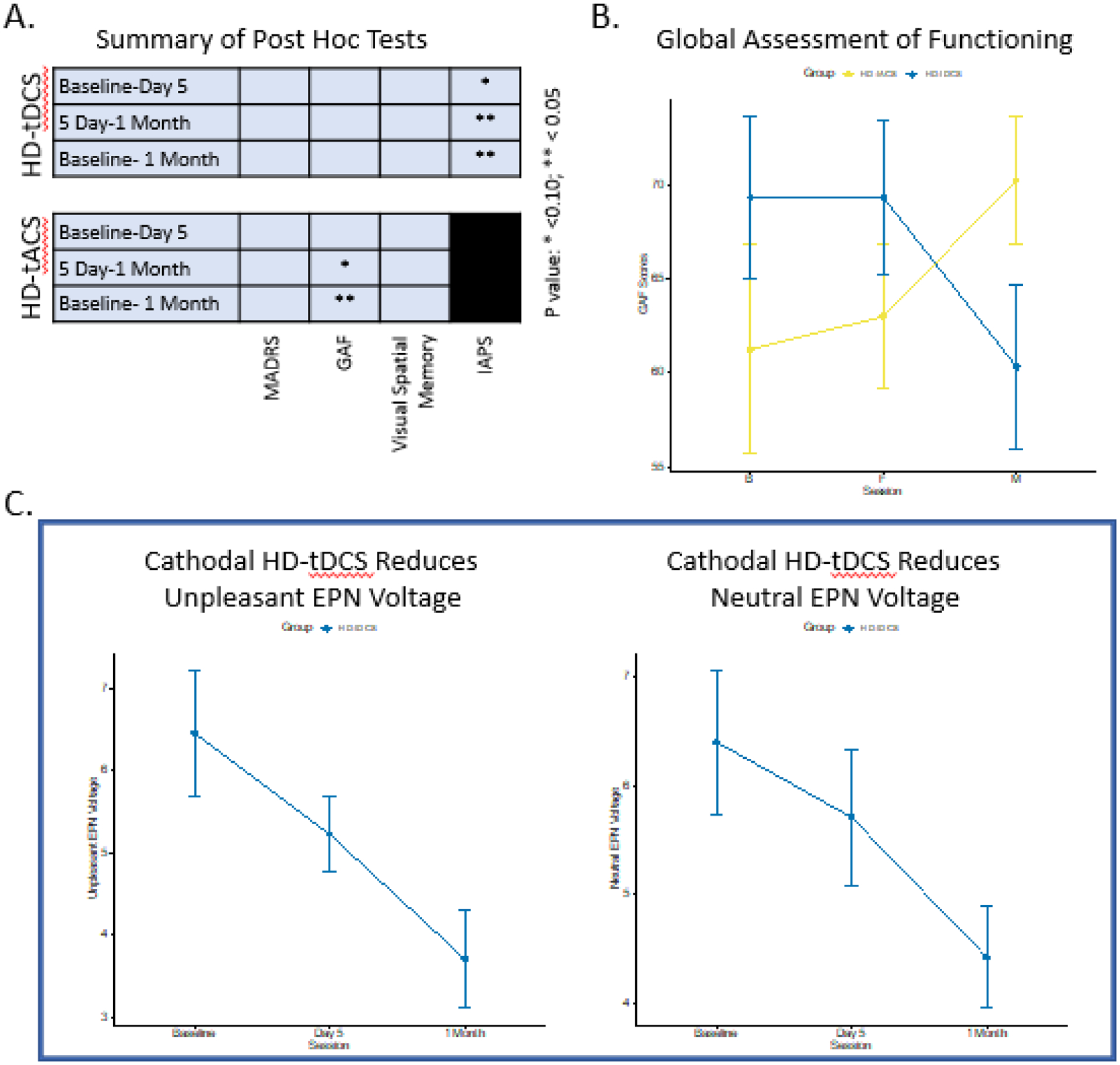
A. Demonstrates the summary of post-hoc pairwise comparisons by session contrasts for both HD-tDCS and HD-tACS. B. Depicts the results for GAF scores across sessions for both HD-tDCS and HD-tACS with a significant reduction in the HD-tACS group at 1 Month. C. Shows the IAPS EPN Voltage for Unpleasant and Neutral stimuli at P6, P7, PO6, PO7, O1, and O2 sensors across sessions. Notes: HD-tDCS, High-Definition Transcranial Current Stimulation; HD-tACS, HD Transcranial Alternating Current Stimulation; GAF, Global Assessment of Functioning; IAPS, International Affective Picture System; EPN, Early Posterior Negativity. 1 participant in the HD-tDCS condition was not able to complete IAPS assessments
There were significant differences across sessions for the IAPS EPN voltages in the HD-tDCS condition for both unpleasant (W=0.84; p=0.01) and neutral (W=0.52; p=0.07) stimuli, but not for pleasant (Table 2B, Figure 4C). Pairwise comparisons in the HD-tDCS condition showed a significant decrease in response amplitude to unpleasant stimuli from baseline to day 5 (RBES=−0.68; pfdr=0.07), day 5 to 1-month (RBES=0.76; pfdr=0.004) and baseline to 1-month (RBES=0.84; pfdr=0.0007). Pairwise comparisons showed that response amplitudes to neutral stimuli decreased from baseline to 1-month (RBES=0.76; pfdr=0.06). These analyses were repeated without imputed IAPS data and results were similar for the HD-tDCS findings in the unpleasant stimuli, but not significant for neutral stimuli (Supplement 6).
There were no significant differences observed on the visual spatial working memory or velocity discrimination task for either treatment condition (eTable 2).
In exploratory analyses, no significant relationship was identified between the improvement in PANSS general score and the reduction in unpleasant (β=0.529, t=2.18, p=0.16) or neutral (β=0.173, t=0.37, p=0.75) stimuli observed between day 5 and baseline (eTable 3).
There were no serious adverse events reported in either stimulation condition and no participant withdrew from the study due to side effects. The stimulation montage was well tolerated and no participant reported above a moderate sensation on the sensation scale (eFigure 2).
Discussion
This is the first tES intervention for psychosis to precisely target the eVC, guided by lesion network mapping and HD-tES current flow models. We demonstrated that stimulating this region using HD-tDCS may improve general psychopathology in the short-term (5 days), with longer-term (1-month) improvements in general psychopathology and functioning noted with HD-tACS. Furthermore, eVC stimulation with HD-tDCS may induce a sustained reduction in early visual ERPs from visual steady-state and emotional scene paradigms, but this effect was not observed using HD-tACS. Regression analysis in the HD-tDCS condition indicates that general psychopathology and electrophysiological reductions are linked, suggesting that engaging the eVC with HD-tES may play a role in the alleviation of psychosis symptoms. Lastly, both HD-tES montages used in this study were well tolerated (eFigure 2).
The HD-tDCS general psychopathology results are consistent with findings in the literature from randomized control trials with 8 studies demonstrating short-term improvements (SMD=0.31), while 4 studies did not show longer-term benefits at 4–12 weeks (SMD=0.15)40. These studies used 2mA stimulation intensity, anodal to the left dorsolateral prefrontal cortex (F3) and cathodal to right frontal (F4) or left temporoparietal junction (T3, P3), stimulation area ranged from 25–35cm2, and sessions ranged from 5–10 sessions. Further support comes from a case report of a patient with treatment resistant auditory hallucinations and VH who underwent cathodal tDCS to Oz for 10 sessions and then the temporoparietal area for 10 sessions, and they experienced a 29% reduction in general psychopathology symptoms at 1-month41. The HD-tACS general psychopathology findings are also consistent with a case series of 3 clozapine resistant patients with schizophrenia receiving theta (4.5 Hz) tACS demonstrating an 18% improvement in symptoms42. This study used 2 mA stimulation intensity, F3 and F4 electrode placement, 25cm2 area, for 20 sessions over 4 weeks. While these studies are promising they were conducted using sponge montages, which decrease the focality of stimulation, and traditional montages were used targeting primarily frontal, temporal, and parietal regions, which don’t specifically target networks associated with behavior or psychosis symptomatology. Our study expands on this literature by demonstrating that HD-tDCS to the eVC which is causally linked to VH27 and motion processing29, resulted in a larger short-term effects size change (RBES=0.47) for general psychopathology than has been reported previously. We are also the first to demonstrate that 2Hz tACS to the eVC can result in a long-term moderate effect size (RBES=0.62) improvement at 1-month, which may be due to neuroplastic changes induced by phase locking of intrinsic brain rhythms43, but further work is needed in this area.
The mechanism through which HD-tDCS or HD-tACS decreases general psychopathology is not fully understood. However, the findings of the present study suggest that HD-tDCS to the eVC induces a neuroplastic change to the SSVEP P1 and IAPS EPN ERPs with the former being correlated with a change in general psychopathology, however, this effect was not observed with HD-tACS. This observation may be explained by the fact that tDCS can modulate cortical excitability using anodal stimulation which tends to increase (i.e. the resting potential becomes less negative), while cathodal stimulation tends to decrease the underlying membrane potential (i.e. the resting potential becomes more negative)44,45. Furthermore, studies have demonstrated that tDCS can modulate visual cortical function in a polarity-dependent manner, where anodal stimulation can increase and cathodal stimulation can decrease the amplitude of the N70 component from the visual-evoked potential46. While there is no study to date examining the relationship between P1 and general psychopathology, a study using dynamic facial expressions to examine ERP responses in schizophrenia, found that greater N200 latency was associated with lower general psychopathology scores47. Different from tDCS, tACS is known to modulate endogenous neural oscillations by applying oscillating electrical current with a periodic waveform to the brain48. Using tACS to target the occipital cortex, it was demonstrated that different stimulation frequencies can interact with endogenous rhythmic activities in a frequency-specific manner to induce phosphenes49. While these studies are informative, more research is needed to better understand the mechanisms underlying the improvement in general psychopathology.
Limitations
We acknowledge several important limitations in understanding our results. First, due to institutional restrictions surrounding the COVID-19, recruitment efforts were significantly hindered and thus a sham condition was not conducted. However, there is significant power in this cross-over design, which demonstrated differential effects on symptoms and electrophysiology. Additionally, due to our small sample size we were forced to allocate treatment protocols in one order (HD-tDCS and then HD-tACS). While this was not ideal, we believe that stimulation effects from HD-tDCS and HD-tACS are still apparent since we implemented a stringent washout period of 4 weeks and implemented an electrophysiological readout at 5 days and 1 month. Moreover, our results suggested that the effects from HD-tDCS were no longer significantly related to our variables of interest at the 1-month follow up. Second, our single blind design may have introduced a potential bias in clinical measures; however, the combination of objective markers such as EEG and behavioral tasks can be seen as control measures for this phenomenon. Third, imputed data was used for 1-month assessments, but the results were similar when repeated using unimputed data. Fourth, subjects were stable outpatients not experiencing clinically significant symptoms and future studies should be performed in an acute population. Furthermore, future studies should employ and validate a wide range of clinical assessments such as the NEVHI or University of Miami Parkinson’s Disease Hallucinations Questionnaire (UM-PDHQ) to ensure they are capturing key features of symptoms31,50. Fifth, velocity discrimination is likely a better behavioral target than biological motion when stimulating the eVC51, but future studies should conduct brain stimulation online while the patient is performing the task as compared to offline, which is how it was conducted in the current study. Additionally, the lack of change in biological motion scores from the two stimulations arms suggest that this task may be a reliable way to measure the absence of off target effects. Fifth, the lack of positive psychosis symptom findings may be due to a lack of self-reported psychosis symptoms scales, which may be a more accurate measure of predicting outcomes52,53. Lastly, we did not use each individuals structural MRI, which would have allowed us to personalize the stimulation location and current flow54,55, as well as maximize the effects of HD-tES. Despite these limitations, this is an important proof of concept study that lays the foundation for future studies investigating the treatment of positive and general symptoms of psychosis with HD-tES.
Conclusions
Findings from the present study suggest that lesion network guided HD-tES to the eVC is a safe, efficacious, and promising approach for reducing general psychopathology via changes in neuroplasticity. These results highlight the need for larger clinical trials implementing novel targeting methodologies and montages with the hopes of identifying effective future treatments for psychosis.
Supplementary Material
Highlights.
Casual lesion network targeting of the extrastriate visual cortex (eVC) with tES may be a promising approach.
Short-term improvement was observed in general psychopathology with HD-tDCS.
Long-term improvement was observed in general psychopathology with HD-tACS.
HD-tDCS reduced early visual evoked responses which linked to general psychopathology improvements.
Both HD-tDCS and HD-tACS stimulation to the eVC was well tolerated.
Acknowledgements
The authors would like to thank the individuals who participated in this research and for their willingness to complete study visits during the COVID-19 pandemic. In addition, we would like to thank all study team members for their supporting roles in this research.
Funding
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflicts of interest
References:
- 1.Sun CH, Jiang WL, Cai DB, et al. Adjunctive multi-session transcranial direct current stimulation for neurocognitive dysfunction in schizophrenia: A meta-analysis. Asian Journal of Psychiatry. 2021;66:102887. doi: 10.1016/j.ajp.2021.102887 [DOI] [PubMed] [Google Scholar]
- 2.Schülke R, Straube B. Transcranial Direct Current Stimulation Improves Semantic Speech–Gesture Matching in Patients With Schizophrenia Spectrum Disorder. Schizophrenia Bulletin. 2019;45(3):522–530. doi: 10.1093/schbul/sby144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta T, Kelley NJ, Pelletier-Baldelli A, Mittal VA. Transcranial Direct Current Stimulation, Symptomatology, and Cognition in Psychosis: A Qualitative Review. Front Behav Neurosci. 2018;12:94. doi: 10.3389/fnbeh.2018.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond N, Reinhart RMG, Keshavan M, Lizano P. An Integrated Neuroimaging Approach to Inform Transcranial Electrical Stimulation Targeting in Visual Hallucinations. Harv Rev Psychiatry. 2022;30(3):181–190. doi: 10.1097/HRP.0000000000000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomons CD, Shanmugasundaram V. Transcranial direct current stimulation: A review of electrode characteristics and materials. Medical Engineering & Physics. 2020;85:63–74. doi: 10.1016/j.medengphy.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS. NeuroImage. 2013;74:266–275. doi: 10.1016/j.neuroimage.2013.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parlikar R, Vanteemar SS, Shivakumar V, Narayanaswamy CJ, Rao PN, Ganesan V High definition transcranial direct current stimulation (HD-tDCS): A systematic review on the treatment of neuropsychiatric disorders. Asian Journal of Psychiatry. 2021;56:102542. doi: 10.1016/j.ajp.2020.102542 [DOI] [PubMed] [Google Scholar]
- 8.Nayok SB, Pathak H, Suhas S, et al. Concurrent conventional & high-definition transcranial direct current stimulation for treatment of schizophrenia with co-morbid obsessive-compulsive disorder: A case report. Brain Stimul. 2021;14(6):1483–1485. doi: 10.1016/j.brs.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose A, Shivakumar V, Chhabra H, et al. Feasibility and Clinical Utility of High-definition Transcranial Direct Current Stimulation in the Treatment of Persistent Hallucinations in Schizophrenia. East Asian Arch Psychiatry. 2017;27(4):162–164. [PubMed] [Google Scholar]
- 10.Correll CU, Schooler NR. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. NDT. 2020;Volume 16:519–534. doi: 10.2147/NDT.S225643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pienkos E, Giersch A, Hansen M, et al. Hallucinations Beyond Voices: A Conceptual Review of the Phenomenology of Altered Perception in Psychosis. Schizophrenia Bulletin. 2019;45(Supplement_1):S67–S77. doi: 10.1093/schbul/sby057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fett AKJ, Velthorst E, Reichenberg A, et al. Long-term Changes in Cognitive Functioning in Individuals With Psychotic Disorders: Findings From the Suffolk County Mental Health Project. JAMA Psychiatry. 2020;77(4):387. doi: 10.1001/jamapsychiatry.2019.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventura J, Thames AD, Wood RC, Guzik LH, Hellemann GS. Disorganization and reality distortion in schizophrenia: A meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophrenia Research. 2010;121(1–3):1–14. doi: 10.1016/j.schres.2010.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chouinard VA, Shinn AK, Valeri L, et al. Visual hallucinations associated with multimodal hallucinations, suicide attempts and morbidity of illness in psychotic disorders. Schizophrenia Research. 2019;208:196–201. doi: 10.1016/j.schres.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40 Suppl 4:S233–245. doi: 10.1093/schbul/sbu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen S, Goodall T, Jones C, James R, Surtees A. What Is the Prevalence of Visual Hallucinations in a First-Episode Psychosis Population? A Systematic Review and Meta-analysis of the Literature. Schizophrenia Bulletin Open. 2023;4(1):sgad002. doi: 10.1093/schizbullopen/sgad002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy-Jones S, Smailes D, Corvin A, et al. Occurrence and co-occurrence of hallucinations by modality in schizophrenia-spectrum disorders. Psychiatry Research. 2017;252:154–160. doi: 10.1016/j.psychres.2017.01.102 [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, Davidson M, Tamminga CA. Treatment-refractory schizophrenia. Dialogues in Clinical Neuroscience. 2004;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. The Lancet Psychiatry. 2020;7(1):64–77. doi: 10.1016/S2215-0366(19)30416-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molden E Therapeutic drug monitoring of clozapine in adults with schizophrenia: a review of challenges and strategies. Expert Opinion on Drug Metabolism & Toxicology. 2021;17(10):1211–1221. doi: 10.1080/17425255.2021.1974400 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y Abnormal Visual Motion Processing in Schizophrenia: A Review of Research Progress. Schizophrenia Bulletin. 2011;37(4):709–715. doi: 10.1093/schbul/sbr020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong F Primary visual cortex and visual awareness. Nat Rev Neurosci. 2003;4(3):219–229. doi: 10.1038/nrn1055 [DOI] [PubMed] [Google Scholar]
- 23.Türközer HB, Lizano P, Adhan I, et al. Regional and Sex-Specific Alterations in the Visual Cortex of Individuals With Psychosis Spectrum Disorders. Biological Psychiatry. 2022;92(5):396–406. doi: 10.1016/j.biopsych.2022.03.023 [DOI] [PubMed] [Google Scholar]
- 24.van Ommen MM, van Laar T, Renken R, Cornelissen FW, Bruggeman R. Visual Hallucinations in Psychosis: The Curious Absence of the Primary Visual Cortex. Schizophrenia Bulletin. 2023;49(Supplement_1):S68–S81. doi: 10.1093/schbul/sbac140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The Neurodynamic Organization of Modality-Dependent Hallucinations. Cerebral Cortex. 2013;23(5):1108–1117. doi: 10.1093/cercor/bhs082 [DOI] [PubMed] [Google Scholar]
- 26.Fox MD. Mapping Symptoms to Brain Networks with the Human Connectome. N Engl J Med. 2018;379(23):2237–2245. doi: 10.1056/NEJMra1706158 [DOI] [PubMed] [Google Scholar]
- 27.Kim NY, Hsu J, Talmasov D, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2021;26(4):1299–1309. doi: 10.1038/s41380-019-0565-3 [DOI] [PubMed] [Google Scholar]
- 28.Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Research. 2001;41(10–11):1459–1474. doi: 10.1016/S0042-6989(01)00069-4 [DOI] [PubMed] [Google Scholar]
- 29.Martínez A, Gaspar PA, Hillyard SA, et al. Impaired Motion Processing in Schizophrenia and the Attenuated Psychosis Syndrome: Etiological and Clinical Implications. AJP. 2018;175(12):1243–1254. doi: 10.1176/appi.ajp.2018.18010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holiday KA, Pirogovsky-Turk E, Malcarne VL, et al. Psychometric Properties and Characteristics of the North-East Visual Hallucinations Interview in Parkinson’s Disease. Mov Disord Clin Pract. 2017;4(5):717–723. doi: 10.1002/mdc3.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosimann UP, Collerton D, Dudley R, et al. A semi-structured interview to assess visual hallucinations in older people. Int J Geriat Psychiatry. 2008;23(7):712–718. doi: 10.1002/gps.1965 [DOI] [PubMed] [Google Scholar]
- 32.Türközer HB, Hasoğlu T, Chen Y, et al. Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychol Med. 2019;49(10):1740–1748. doi: 10.1017/S0033291718002477 [DOI] [PubMed] [Google Scholar]
- 33.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention. Published online 1997:39–58. [Google Scholar]
- 34.Honaker J, King G, Blackwell M. Amelia II: A Program for Missing Data. J Stat Soft. 2011;45(7). doi: 10.18637/jss.v045.i07 [DOI] [Google Scholar]
- 35.Patil I Visualizations with statistical details: The “ggstatsplot” approach. JOSS. 2021;6(61):3167. doi: 10.21105/joss.03167 [DOI] [Google Scholar]
- 36.Mair P, Wilcox R. Robust statistical methods in R using the WRS2 package. Behav Res. 2020;52(2):464–488. doi: 10.3758/s13428-019-01246-w [DOI] [PubMed] [Google Scholar]
- 37.Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-Response Meta-Analysis of Antipsychotic Drugs for Acute Schizophrenia. AJP. 2020;177(4):342–353. doi: 10.1176/appi.ajp.2019.19010034 [DOI] [PubMed] [Google Scholar]
- 38.Kloke JD, McKean JW Rfit: Rank-based Estimation for Linear Models. The R Journal. 2012;4(2):57. doi: 10.32614/RJ-2012-014 [DOI] [Google Scholar]
- 39.Kim JH, Choi I. Choosing the Level of Significance: A Decision‐theoretic Approach. Abacus. 2021;57(1):27–71. doi: 10.1111/abac.12172 [DOI] [Google Scholar]
- 40.Lee HS, Rast C, Shenoy S, Dean D, Woodman GF, Park S. A meta-analytic review of transcranial direct current stimulation (tDCS) on general psychopathology symptoms of schizophrenia; immediate improvement followed by a return to baseline. Psychiatry Research. 2022;310:114471. doi: 10.1016/j.psychres.2022.114471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiozawa P, da Silva ME, Cordeiro Q, Fregni F, Brunoni AR. Transcranial Direct Current Stimulation (tDCS) for the Treatment of Persistent Visual and Auditory Hallucinations in Schizophrenia: A Case Study. Brain Stimulation. 2013;6(5):831–833. doi: 10.1016/j.brs.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 42.Kallel L, Mondino M, Brunelin J. Effects of theta-rhythm transcranial alternating current stimulation (4.5 Hz-tACS) in patients with clozapine-resistant negative symptoms of schizophrenia: a case series. J Neural Transm. 2016;123(10):1213–1217. doi: 10.1007/s00702-016-1574-x [DOI] [PubMed] [Google Scholar]
- 43.Krause MR, Vieira PG, Csorba BA, Pilly PK, Pack CC. Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc Natl Acad Sci USA. 2019;116(12):5747–5755. doi: 10.1073/pnas.1815958116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg CJ, Nitsche MA. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614 [DOI] [PubMed] [Google Scholar]
- 45.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0 [DOI] [PubMed] [Google Scholar]
- 46.Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability Changes Induced in the Human Primary Visual Cortex by Transcranial Direct Current Stimulation: Direct Electrophysiological Evidence. Invest Ophthalmol Vis Sci. 2004;45(2):702. doi: 10.1167/iovs.03-0688 [DOI] [PubMed] [Google Scholar]
- 47.Fukuta M, Kirino E, Inoue R, Arai H Response of Schizophrenic Patients to Dynamic Facial Expressions: An Event-Related Potentials Study. Neuropsychobiology. 2014;70(1):10–22. doi: 10.1159/000363339 [DOI] [PubMed] [Google Scholar]
- 48.Elyamany O, Leicht G, Herrmann CS, Mulert C. Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. 2021;271(1):135–156. doi: 10.1007/s00406-020-01209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-Dependent Electrical Stimulation of the Visual Cortex. Current Biology. 2008;18(23):1839–1843. doi: 10.1016/j.cub.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 50.Papapetropoulos S, Katzen H, Schrag A, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson’s disease. BMC Neurol. 2008;8(1):21. doi: 10.1186/1471-2377-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaina LM, Gross CG. Perceptual deficits in patients with impaired recognition of biological motion after temporal lobe lesions. Proc Natl Acad Sci USA. 2004;101(48):16947–16951. doi: 10.1073/pnas.0407668101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biancosino B, Barbui C, Marmai L, Fagioli F, Sabatelli R, Grassi L. Relationship between Self-Reported and Observer-Reported Ratings for Psychopathology in Psychiatric Inpatients. Psychopathology. 2007;40(6):418–423. doi: 10.1159/000106472 [DOI] [PubMed] [Google Scholar]
- 53.Kaiser C, Oswald AJ. The scientific value of numerical measures of human feelings. Proc Natl Acad Sci USA. 2022;119(42):e2210412119. doi: 10.1073/pnas.2210412119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-Individual Variation during Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front Psychiatry. 2012;3. doi: 10.3389/fpsyt.2012.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thair H, Holloway AL, Newport R, Smith AD. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front Neurosci. 2017;11:641. doi: 10.3389/fnins.2017.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


