Abstract
Transient loss of smell is a common symptom of influenza and other upper respiratory infections. Loss of taste is possible but rare with these illnesses, and patient reports of ‘taste loss’ typically arise from a taste / flavor confusion. Thus, initial reports from COVID-19 patients of loss of taste and chemesthesis (i.e., chemical somatosensation like warming or cooling) were met with skepticism until multiple studies confirmed SARS-CoV-2 infections could disrupt these senses. Many studies have been based on self-report or on single time point assessments after acute illness was ended. Here, we describe intensive longitudinal data over 28 days from adults aged 18–45 years recruited in early 2021 (i.e., prior to the Delta and Omicron SARS-CoV-2 waves). These individuals were either COVID-19 positive or close contacts (per U.S. CDC criteria at the time of the study) in the first half of 2021. Upon enrollment, all participants were given nose clips, blinded samples of commercial jellybeans (Sour Cherry and Cinnamon), and scratch-n-sniff odor identification test cards (ScentCheckPro), which they used for daily assessments. In COVID-19 cases who enrolled on or before Day 10 of infection, Gaussian Process Regression showed two distinct measures of function – odor identification and odor intensity – declined relative to controls (exposed individuals who never developed COVID-19). Because enrollment began upon exposure, some participants became ill only after enrollment, which allowed us to capture baseline ratings, onset of loss, and recovery. Data from these four cases and four age- and sex- matched controls were plotted over 28 days to create panel plots. Variables included mean orthonasal intensity of four odors (ScentCheckPro), perceived nasal blockage, oral burn (Cinnamon jellybeans), and sourness and sweetness (Sour Cherry jellybeans). Controls exhibited stable ratings over time. By contrast, COVID-19 cases showed sharp deviations over time. Changes in odor intensity or odor identification were not explained by nasal blockage. No single pattern of taste loss or recovery was apparent, implying different taste qualities might recover at different rates. Oral burn was transiently reduced for some before recovering quickly, suggesting acute loss may be missed in datasets collected only after illness ends. Collectively, intensive daily testing shows orthonasal smell, oral chemesthesis and taste were each altered by acute SARS-CoV-2 infection. This disruption was dyssynchronous for different modalities, with variable loss and recovery rates across both modalities and individuals.
Keywords: gustation, anosmia, trigeminal, recovery, longitudinal, olfaction
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 virus is one of the most devastating infectious disease outbreaks since the H1N1 avian flu of 1918 [1, 2]. By the end of 2021, roughly two years after the start of the pandemic, there were over 281 million cases of COVID-19 globally, resulting in over 5.4 million deaths [3]. Early in the pandemic, SARS-CoV-2 infection was associated with myriad symptoms, one of the most common being anosmia [4–7]. Meta-analysis of dozens of early studies suggested half to three-quarters of COVID-19 patients lost their sense of smell [8]. Further, smell loss was the most predictive symptom of COVID-19 [9] up through the Delta wave; in later waves (i.e., Delta and Omicron), smell loss was still common, albeit with lower incidence than observed in earlier waves [10].
In contrast to other respiratory illnesses that cause acute anosmia – including those caused by rhinoviruses, influenza viruses, and common coronaviruses – both taste and chemesthesis function were reportedly lost in some people with COVID-19 [11–13]. Taste loss following viral illness is possible [14] but had been considered rare [15]. Thus, early in the pandemic, many medical professionals assumed patient reports of taste loss were the result of decreased flavor sensations due to anosmia. However, subsequent work indicated taste loss with COVID-19 was real and not merely the result of a taste flavor confusion. For example, one large crowd-sourced study reported ~60% of COVID-19-positive (COVID-19+) individuals had impaired perception of specific taste qualities (i.e., sweet, salty, sour or bitter tastes) [11], suggesting taste loss in these individuals is distinct from impaired flavor perception accompanying smell loss. The findings of that study and others based on self-reports (e.g., [16–18]), were confirmed by psychophysical tests of taste function, suggesting ~47 to 64% of COVID-19 positive individuals experience taste loss [19, 20]. In another large crowd-sourced study, the taste intensity of putatively pure gustatory stimuli (i.e., salt, sucrose) was reduced in COVID-19+ individuals relative to controls with no symptoms [21]. Collectively, taste dysfunction (distinct from impaired flavor perception due to smell loss) is now also recognized as a common symptom of COVID-19 [22, 23].
Data on disruption of chemesthesis associated with COVID-19 remains quite limited. Consistent with many patient anecdotes that chili and ethanol burn were transiently depressed (e.g., [24]), studies relying on self-report suggested roughly half of individuals with COVID-19 experienced disruptions of chemesthesis [11]. In a small study of Italians with COVID-19-associated smell loss, 57% of patients had reported a severe impairment of nasal chemesthesis at initial diagnosis, but over 90% reported full recovery of chemesthesis six months later [25]. A Swedish study that used “olfactory” stimuli known to concomitantly activate the trigeminal system (e.g., vinegar, chopped garlic, vodka) provided evidence for impairment of nasal chemesthesis [26]. Similarily, Snitz and colleagues found that changes in intensity for odorants with a significant chemesthetic component (e.g., vinegar) were predictive of COVID-19 positivity [27]. Thus, while self-report and clinical assessment both suggest COVID-19 may associate with acute impairment of chemesthesis in many individuals, the time course of loss and recovery is lacking, as is any assessment of the impact on oral chemesthesis. We attempt to fill these knowledge gaps here.
In approximately 85% of COVID-19 cases where chemosensation (smell, taste and/or chemesthesis) has been affected, recovery of chemosensory function is typically seen within ~6 weeks [6, 9, 28]. Unfortunately, some patients do not report appreciable recovery after many months [9, 29–33]. However, without daily chemosensory testing, the precise timing of recovery remains unknown [6, 28, 34]. Because patient anecdotes suggest the timing of recovery of different chemical senses may be dyssynchronous [9], we assessed smell, taste, and chemesthesis function acutely and transiently over 28 days by collecting longitudinal data using commercially available chemosensory stimuli in a cohort of patients diagnosed with COVID-19 and control participants without COVID-19 in early 2021. Here, we present a small case-control series using temporally intensive data collection from remote daily testing. We focus on a handful of COVID-19 cases that allow for visualization of loss and recovery of chemosensory function as well as baseline ratings obtained prior to the onset of illness. The analyses below are largely exploratory; formal hypotheses were not formulated in advance by our research team, as we were unsure about the amount and type of data we would be able to obtain with this design. That said, based on patient anecdotes and preliminary data available at the time (see [35] for context), we anticipated a change in function for smell, taste, and chemesthesis over time.
2. Methods
2.1. Study design and recruitment
This prospective study investigated COVID-19-related chemosensory dysfunction in a community-derived sample of 18- to 45-year-old adults recruited on and around the campus of a large public university in rural central Pennsylvania (i.e., the area surrounding State College, PA). Enrollment using geotargeted ads on social media began in February, 2021, and ended in May, 2021. This period roughly correspond to the rise of the SARS-CoV-2 Alpha wave in the United States. Potentially interested individuals were asked to contact a study team member (author EMW) via email if they believed they qualified for the study, who then emailed them a link to a brief screening questionnaire. The screening questionnaire asked questions about demographics, prior diagnosis of COVID-19, contact with a COVID-19+ individual, and any recent symptoms of COVID-19. Contact with a COVID-19+ individual was defined by the Centers for Disease Control (CDC) screening criteria in use at the time of enrollment (specifically, 15 or more minutes within 6 feet of a confirmed case of COVID-19). Due to the fluidity of the COVID-19 pandemic in early 2021, however, a strict timeline was not rigidly enforced and enrollment occurred on a case-by-case basis. Vaccines were not available to non-health care workers at study initiation in February, 2021, but they became more widely available during the enrollment window, so we added a short retroactive questionnaire to the end of the study to gather self-reported information on vaccination status and date. No attempt was made to confirm these reports against medical records.
Participants with the following conditions were excluded: not diagnosed with COVID-19 or not a close contact of a COVID-19+ individual, pregnant, food allergies (or another reason they could not consume commercial jelly beans), prior history of a disease of the central nervous system (including Alzheimer’s disease, multiple sclerosis, Parkinson’s disease, Huntington’s disease, brain tumor), nasal obstruction (tumor/polyps), a history of nasal surgery, history of a severe head injury/concussion, history of chronic sinus infections, history of radiation therapy to the head or neck (ever), recent chemotherapy (within the last year), a prior diagnosis of smell or taste loss, diabetes, history of lung/pulmonary disease or neurological disease, were unwilling to create a PayPal account for compensation if they did not already have one, or were below 18 years or above 45 years of age.
Data were collected using REDCap, a secure data capture platform for clinical research [36, 37] on a server hosted and maintained by the Penn State College of Medicine in Hershey, PA. The study was performed in compliance with the principles of the Declaration of Helsinki, informed consent was obtained electronically, and the specific protocol was approved by the Institutional Review Board at Penn State (STUDY00016377).
2.2. Chemosensory stimuli and assessment
The longitudinal design consisted of brief daily assessment every day for 28 days, followed by four additional follow-up sessions every 2 weeks, for a total of 32 sessions over a 12-week period. This analysis only looks at data from the first 4 weeks (data are available for secondary analysis at [DOI to be provided upon acceptance]). In each daily session, participants were asked to complete questions on COVID-19 status or symptoms that had changed since the last session, as well as self-administered psychophysical smell and taste tests. They were instructed to minimize any distracting smells or odors before any sensory testing and were also asked not to eat or drink (anything other than water) or smoke for at least 30 minutes prior to testing.
Upon enrollment, the first author arranged contactless delivery of all research materials in a large plastic zip-top bag. Specifically, participants were given 32 ScentCheckPro cards (Item #098515, Lot #0821) from Taylor Corp (North Mankato, MN). Each ScentCheckPro card consisted of 4 microencapsulated scents in a scratch-n-sniff format, with one scent located near each corner of the postcard sized card Specifically, for each corner of the card, participants were given 4 options: one correct answer and three distractors drawn from the following list: coconut, grape, coffee, lemon, bubble gum, popcorn, pine, cinnamon, flowers, banana, or none of these.
Participants were also given 36 lidded plastic 2 oz souffle cups (Solo P200N; Lake Forest IL) labeled with blinding codes. Individual cups contained one of two kinds of red colored jellybeans (Jelly Belly, Fairfield, CA): either Sour Cherry (Lot #20200601) or Cinnamon (Lot #200731). Each cup contained three jellybeans of a single flavor. Jellybeans are a highly familiar confection in the United States that are made with sweeteners (sugar, and/or corn syrup), corn starch, confectioners glaze, added color and natural or artificial flavors. They have a shiny candy shell and a soft gel center, and come in many different colors and flavors including fruit or spice flavors. With the nose pinched closed, the Sour Cherry jellybean used here evoke both sweetness and sourness, while the Cinnamon jellybean elicits sweetness and a mild warming/burning sensation. Because of the glazed outer shell, jellybeans have little to no orthonasal smell, and both jellybeans had a similar red color, so there were no obvious cues of the specific flavor in each cup. Across days, jellybean presentation order was counterbalanced with pairwise randomization, so that a participant who was presented with Sour Cherry on Day 1 would get Cinnamon on Day 2, while the next participant would start with Cinnamon on Day 1 before receiving Sour Cherry on Day 2. This procedure was used to maximize the range of chemosensory stimuli used in the study (i.e., taste, smell, and chemesthesis) while minimizing participant burden on any given day of the study (i.e., a very brief test time to enhance compliance). Individual lidded cups were labeled with random 3-digit blinding codes, and these codes were programmed into REDCap prompts for each session to help ensure participants sampled the correct jellybean on the correct day. We also provided a disposable foam padded nose clip (A-M Systems; Sequim WA; Model #166500, Lot #189615) to allow ratings of oral sensation to be collected with occluded nostrils to minimize olfactory input.
Once a day, participants were asked to rate how blocked their nose was using a horizontal 0–100 visual analog scale (VAS) scale anchored with ‘Not blocked at all’ to ‘Completely blocked’. Participants were then asked to scratch the spot containing the encapsulated odorant on each postcard sized smell card with a coin or fingernail for 5–10 seconds before sniffing; they were then asked to bring the card one inch from their nose and sniff the odor. For each odor spot, participants were asked to first identify the odor they smelled from four multiple choice options presented in REDCap before rating the perceived intensity of the odor on a 0–100 VAS anchored with labels of ‘None’ to ‘Very intense’. This process was repeated for the four different odorants on a given card.
Next, participants were asked to pinch their nose closed using the provided nose clip and put all three jellybeans from the cup into their mouth. With their nose pinched closed, they were asked to chew the jellybeans slowly, and rate the perceived intensity of various qualities on five different horizontal 0–100 VAS scales labeled ‘None’ to ‘Very intense’. These qualities included: sourness, sweetness, warming/burning, cherry flavor, and cinnamon flavor. Participants were then asked to unpinch their nose and exhale (while still chewing the jellybeans) and rate the same five intensity scales with their nose unpinched (data available at [DOI to be added if accepted]). All five scales were presented in both conditions (nose closed / nose open) to minimize any “dumping” artifacts [38]. To decrease daily test time and participant burden on days 1 through 28, participants assessed only one jellybean flavor per day (either Sour Cherry or Cinnamon, counterbalanced as described above). In four follow up sessions at weeks 6, 8, 10 and 12, a longer assessment was deemed reasonable, so participants were presented with two cups of jellybeans, one with each flavor. These data are not reported here.
2.3. Categorization of participants as COVID-19 cases versus controls
For the purposes of this study, a COVID-19 close contact was defined as a participant who had been exposed to a COVID-19+ individual (e.g., 15 or more minutes within 6 feet of a confirmed case of COVID-19, per US CDC guidelines at the time), but never developed any symptoms or received a positive diagnosis in subsequent testing. Conversely, a COVID-19 case was defined as a participant who was either formally diagnosed with COVID-19 or began having symptoms while enrolled in the study following their recent exposure. By studying close contacts who later became cases while enrolled in our study, we were able to observe acute changes in chemosensation using controlled stimuli from the earliest days of their infection. When COVID-19 symptoms started prior to the participant receiving a positive COVID-19 diagnosis via clinical testing (typically a positive PCR test), an estimated day of infection (Day 0) was defined as the first day of symptoms. When a positive test preceded COVID-19 symptoms, the date of the positive test was used as Day 0 of infection. Data from controls were not centered on the day of infection, as these participants enrolled at their discretion. For these individuals, the day of rating (Day 0) is defined as the first day of their participation in the study.
2.4. Data analysis overview
A total of 55 participants were enrolled in the study between February, 2021, and May, 2021, a period roughly corresponding to the rise and peak of the Alpha wave in North America, and prior to the Delta and Omicron wave. (Specifically, North American data from the GISAID database (gisaid.org) indicates the Delta variant was less that 0.1% of sequences at the end of Feburary, 2021, and ~4.7% at the end of May, 2021, before climbing to ~80% of sequences in North America by the end of July, 2021). For this analysis, 39 participants with confirmed COVID-19 infection were identified as COVID-19 cases (Figure 1). Of these 39, 15 participants were identified as having an active COVID-19 infection and enrolled in the study prior to or during the first 10 days of infection. Of these 15 participants, four COVID-19 cases entered the study on or before day 1 of their infection, allowing us to capture acute changes in their symptoms throughout their entire infectious period, including early onset of symptoms. As shown in Figure 1, three more cases enrolled on days 2–4 of infection, and nine enrolled on day 5–10 of their infection. An additional 24 participants were identified as COVID-19 cases, but only after their initial 10-days of infection had passed; because we did not enroll these subjects early enough to capture potential changes in chemosensory function during acute illness, their data were not included in these analyses. (These data are available for secondary analysis at [DOI to be provided upon acceptance]. Finally, for the analyses summarized here, 15 participants were identified as controls, as they did not develop COVID-19 during the study despite being a close contact of a COVID-19+ individual. Demographically, the two groups were fairly similar: most self-identified as Non-Hispanic White, and the cases consisted of 5 males and 10 females with a mean age of 22.5 years (range 18–43 years) while the controls consisted of 2 males and 13 females with a mean age 23.9 years (range 18–44 years).
Figure 1:
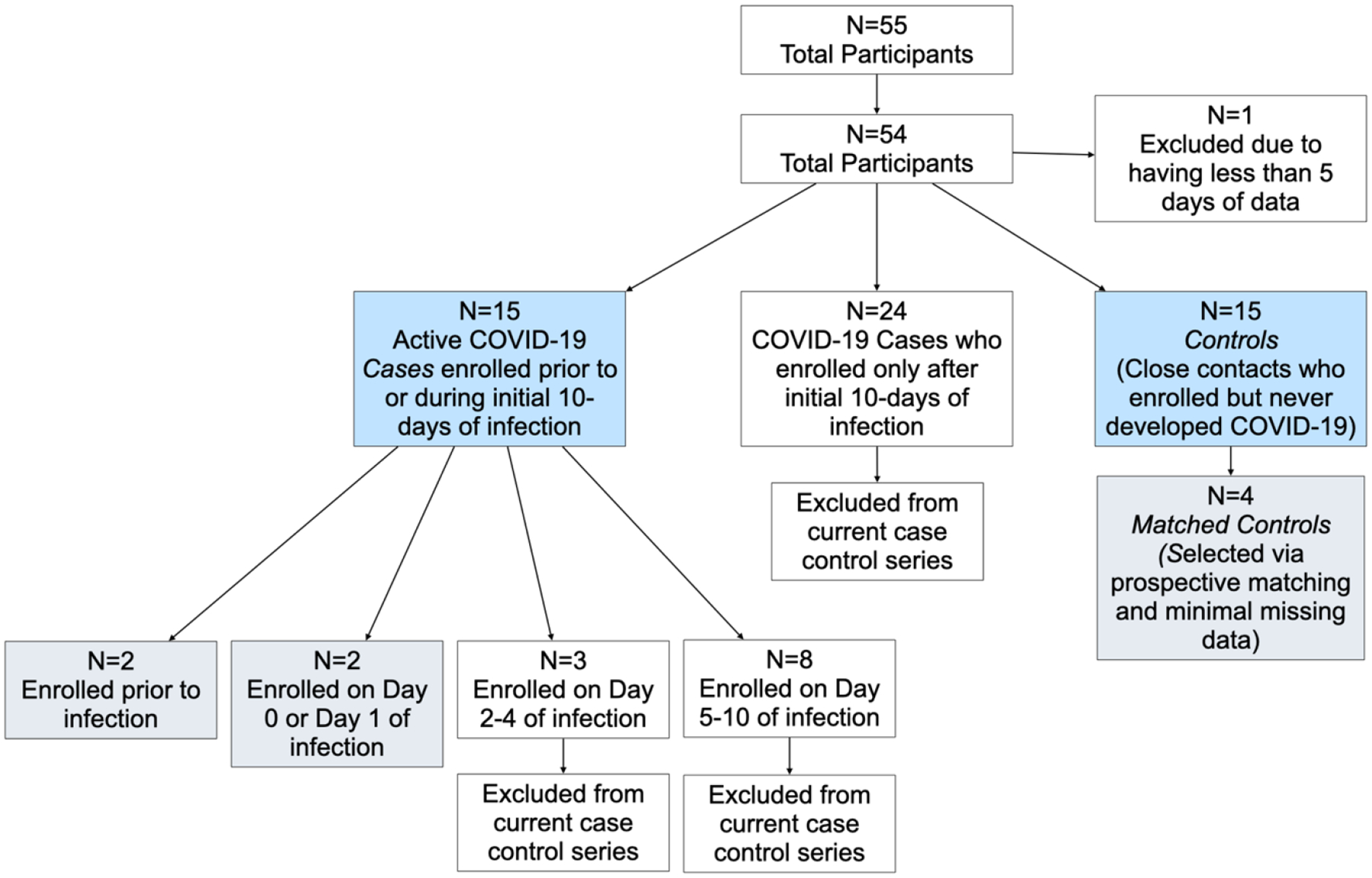
Flow diagram summarizing selection of cases and controls for this case series. Blue boxes indicate the 30 participants included in the Gaussian Process model regression, and gray boxes indicate the 8 individuals shown in the in-depth panel plots.
2.4.1. Gaussian Process Regression of 15 cases and 15 controls
To assess whether deviation of smell intensity in the cases differed from controls, so we identified the fifteen COVID-19 cases in our study who had enrolled on or before day 10 of either infection and tested if their average deviation in smell intensity over time differed from the 15 participants who were controls. To do this, a grand mean of smell intensity was calculated for the controls across all days and individuals. Deviation scores (deltas) for the 15 COVID-19 cases were then calculated by subtracting that individual’s ratings from the grand mean of the 15 controls, resulting in a deviation score for smell intensity. Similarly, for the controls, we subtracted each control’s individual rating from the grand mean of all controls, to get a deviation score for that rating relative to performance of the group. We used a Gaussian Process Regression model to analyze the deviation (delta) scores for cases and controls over time (both individually and as a group). A Gaussian Process model is a probabilistic unsupervised machine learning concept used for regressions in which the model makes predictions by utilizing prior knowledge about the smoothness of plausible time series and provides uncertainty measures for such predictions [39]. COVID-19 cases were normalized on Day 0 of infection and missing values were extrapolated via the Gaussian Process Regression model. Controls were normalized on Day 0 of rating. To avoid overfitting sparse data, ratings of the two jellybean flavors were not modeled via Gaussian Process Regressions, as the counterbalancing of flavors across days meant only half as many data points were available for analysis. Analyses and data visualization were conducted using SAS software (Version 9.4), R using RStudio software (Version 2021.09.0), Python software (Version 3.9.10), or DataGraph version 4.7.1 (Visual Data Tools, Inc; Arlington TX).
Elsewhere, it has been suggested smell and taste changes occur within the first four days of disease onset [31], and the median incubation period for symptom onset is approximately five days [40]. Likewise, data from human challenge trials indicates viral load peaks ~5 days after inoculation [41]. We observed the same overall pattern within our data. As reported below, it became clear the confidence interval of cases did not include zero in the first week of infection. Given the unique opportunity to explore acute and early changes in chemosensation in a small number of participants who had been enrolled prior to acute illness, we also performed an in-depth analysis of these individuals, in hopes of better understanding the initial trajectory of changes in chemosensation.
2.4. Panel Plots of an in-depth case control series with 4 cases and 4 matched controls
To complement the GPR model above, we also present a very small case series restricted to four specific cases who enrolled in the study prior to or on Day 1 of infection (Figure 1). Studying these four participants in detail allowed us to capture changes in symptoms throughout their entire infectious period, including early onset of symptoms. This prospective approach maximizes our potential to capture baseline ratings, loss, and recovery of chemosensory function that would not be possible with patients recruited only after they were ill. This is a distinct and unique feature of this case series, as most other studies have assessed chemosensory function multiple days into a participant’s isolation period, which does not allow for visualization of initial loss [32, 42–47]. For the other 12 participants who entered on or before Day 10 of infection (see Figure 1), we did not capture their initial loss, so they are not included in this analysis.
From the 15 controls used in the GRP model, four were selected as matched controls for the four COVID-19 cases, based on age, gender, and race (Figure 1). Matching was performed manually by an experienced epidemiologist (author CE). To be considered for matching with a specific COVID-19 case, potential candidates were required to (a) provide data on a minimum of 80% of days, and (b) remain active for the full 28-day data collection period (to avoid bias from dropout over time). If more than one potential candidate met the age, gender, and race criteria to be included in a matched pair, the control used here was randomly selected. Three of the four cases showed similar levels of compliance. One case (Sub 35) only had data for 18 days, but this was still deemed sufficient given that we had pre-illness ratings from this individual. This process resulted in a final analysis of four cases (mean 26 years; range 21–43) and four matched controls (mean 25.5 years; range 22–38 years) (Figure 1; Table 1).
Table 1:
Participant demographics from COVID-19 case-control series identifying cases and matched controls.
| ID | Status | Race (Self-Identified) | Gender (Self-Identified) | Age | Estimated Day of Infection relative to Day 0 of the Study |
|---|---|---|---|---|---|
| 35 | Case | White/Caucasian | Female | 21 | −4 |
| 3 | Control | White/Caucasian | Female | 22 | n/a |
| 45 | Case | White/Caucasian | Female | 19 | 0 |
| 10 | Control | White/Caucasian | Female | 19 | n/a |
| 62 | Case | White/Caucasian | Male | 21 | 1 |
| 1 | Control | White/Caucasian | Male | 23 | n/a |
| 63 | Case | White/Caucasian | Female | 43 | −14* |
| 22 | Control | White/Caucasian | Female | 38 | n/a |
Follow up via email revealed this participant was exposed twice. Based on symptoms, the first exposure resulted in enrollment as a close contact, but not infection. The participant only became ill following the second exposure.
For these four cases and their matched controls, we plotted six key variables related to smell, taste, and chemesthesis. Specific outcomes were selected a priori by two authors (EMW and JEH) as being the most salient and theoretically interesting variables. These were: (1) perceived nasal blockage, (2) mean orthonasal smell intensity of the four odorants on a given ScentCheckPro card, (3) sourness and (4) sweetness from the Sour Cherry jellybeans, and (5) oral burn (from the Cinnamon jellybeans). Also, we included (6) oral burn from the Sour Cherry jellybeans (as a negative control) and (7) number correct on the daily odor identification task from the ScentCheckPro card. These seven variables were then plotted across all 28 days to create a series of panel plots for all eight individuals.
In summary, ratings were collected on 101 point VAS, and they included: daily ratings of nasal blockage; a daily measure of orthonasal smell intensity derived from the mean of four scratch-n-sniff spots on a ScentCheckPro card for a given day; oral burn ratings collected every other day from a Cinnamon jellybean; and sweetness, sourness, and burn ratings collected every other day from a Sour Cherry jellybean.
3. Results and Discussion
3.1. Odor identification scores and ratings of orthonasal intensity from a commercial scratch-n-sniff card (n=30)
For controls, the grand mean correct on the daily odor identification (OdorID) across the entire study period was 3.32 out of 4 possible (Figure 2, Supplemental Figures 1, 2). There was some evidence of a learning effect, as controls got slightly better at the task over time (see upward slope, Figure 2A and 2B). For OdorID, the delta score for cases deviates from zero, with a maximal dip occurring around days 5 to 8 (Figure 2A and 2B). Conversely, this dip was not observed in the controls, indicating that the drop in OdorID performance was limited to COVID-19 cases.
Figure 2.
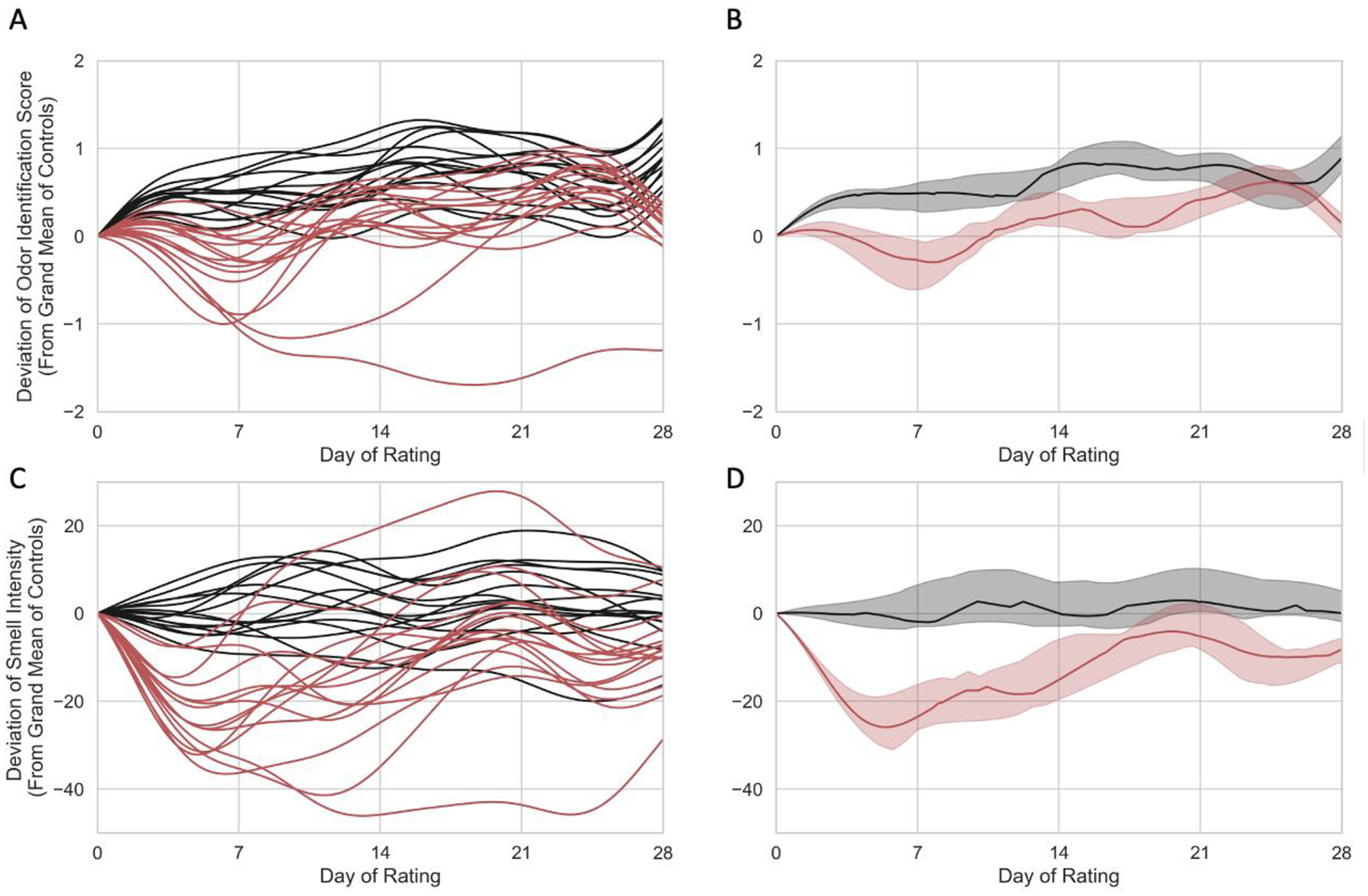
Individual (left) and group level (right) Gaussian Process Regression models for 15 controls (black) and 15 COVID-19 cases (red) who entered the study on or before day 10 of their infection. For cases, Day of Rating is centered on estimated day of infection. Odor identification scores (top) and odor intensity ratings (bottom) are shown as deviation scores calculated from a grand mean of controls across time. (A) Individual odor identification data in a Gaussian Process Regression model of deviation scores from 15 COVID-19 cases (red) and 15 controls (black). (B) Group level odor identification data in a Gaussian Process Regression model for COVID-19 cases (red) and controls (black). (C) Individual deviation scores for smell intensity ratings for 15 COVID-19 cases (red) and 15 controls. (D) Group level smell intensity data in a Gaussian Process Regression model for 15 COVID-19 cases (red) and 15 controls (black). In panels B and D, the solid line represents the group median, and the shaded region shows the interquartile range. Panels A and C show maximal loss roughly around day. Panel B shows some evidence of a learning effect for the OdorID task in the controls; no evidence of learning is seen for controls in the intensity task shown in panel D.
After performing the OdorID task for 4 days, the median number correct for controls increased by ~0.5, and after 14 days (two weeks), the median number correct for controls increased by ~0.7–0.8 above the grand mean; that is, there was nearly perfect performance on a 4 item OdorID task. Based on odor intensity ratings (discussed below), smell loss for cases appears to be maximal near Day 5 (see Figure 2C and 2D). Accordingly, we would also expect OdorID performance to be lowest on Day 5. However, this is just when the first increase due to the learning effect appears to occur (Figure 2A and 2B). If we assume cases (at least those who are not totally anosmic) show similar ability to learn as the controls, this offsetting bump upward would minimize the apparent dip in OdorID performance seen around Day 5. Thus, the COVID-19-associated drop in identification performance may appear smaller than it actually is due to a simultaneous offsetting increase in performance due to learning (or practice). Evidence of such learning is also seen in Supplemental Figures 1 and 2. Regarding suprathreshold odor intensity, the grand mean of perceived intensity ratings for controls across time was 59.47 on 0 to 100 VAS. The mean on Day 0 was used to calculate daily deviation scores for cases and controls (Figure 2C and 2D). Data were centered using Day 0 means so the delta could be more easily compared across the data set. For the cases (Figure 2C and 2D), the delta score of smell intensity clearly deviates from zero, and this difference was maximal in the first week of infection, as noted above. In sharp contrast, this pattern is not seen in the controls, with a score of zero falling inside the interquartile range across the entire study period (Figure 2D). This indicates there was a significant drop in smell intensity for cases but not controls for ratings of the scratch-n-sniff spots on commercial ScentCheckPro cards. In contrast with the learning seen for the OdorID data, we failed to observe any evidence of a learning effect for smell intensity ratings. Collectively, these data suggest OdorID tasks may be less sensitive to acute changes in smell with COVID-19, relative to odor intensity ratings, at least in repeated testing situations that encourage learning or practice effects.
3.2. Case series controls with early onset data (n=4)
Given the maximal deviation in smell early in acute illness, we chose to explore the specific changes in multiple chemosensory modalities in the handful of cases who enrolled prior to onset of acute illness in greater detail.
Throughout the course of the study, controls exhibited normal function for smell, taste, and chemesthesis (Figure 3). Specifically, mean scores on odor identification, orthonasal smell intensity ratings, and ratings of perceived nasal blockage were relatively constant across days, although some participants were more variable than others. A few patterns deserve comment. For example, with Subject 1 we see a clear learning effect for odor identification where they became better at the task over time (Figure 3). Separately, controls may vary in perceived nasal blockage from day to day. For example, with Subject 3, we see a slight decrease in orthonasal smell intensity and a slight increase in nasal blockage in the last four days of testing (Figure 3). Such mild transient hyposmia would be wholly consistent with conductive smell loss due to nasal blockage typically seen with allergies or the common cold. Similarly, for taste, ratings of sweetness and sourness remained relatively constant throughout the course of the study for controls, although ratings were noisier for some participants than others. One rating for Subject 22 deserves comment: in the third week of testing, we observed a sharp drop in sour taste intensity and sharp increase in burn intensity, but only for a single day (Figure 3). While we cannot be sure, we suspect they simply picked the wrong cup from the bag of samples, as Sour Cherry jellybeans should not burn and should be sour. Subject 22 may have misread the 3-digit blinding code, or our research team may have mislabeled the cup. Subject 1 also shows one aberrant burn rating near the very end of the study. Still, these single point deviations do not alter their overall patterns. Separately, we note Subject 22 tends to give Cinnamon jellybeans a relatively high amount of oral burn relative to the other participants; we can speculate they may eat spicy food infrequently, as large variation in burn ratings due to dietary exposure is very common (e.g., [48–51]).
Figure 3:
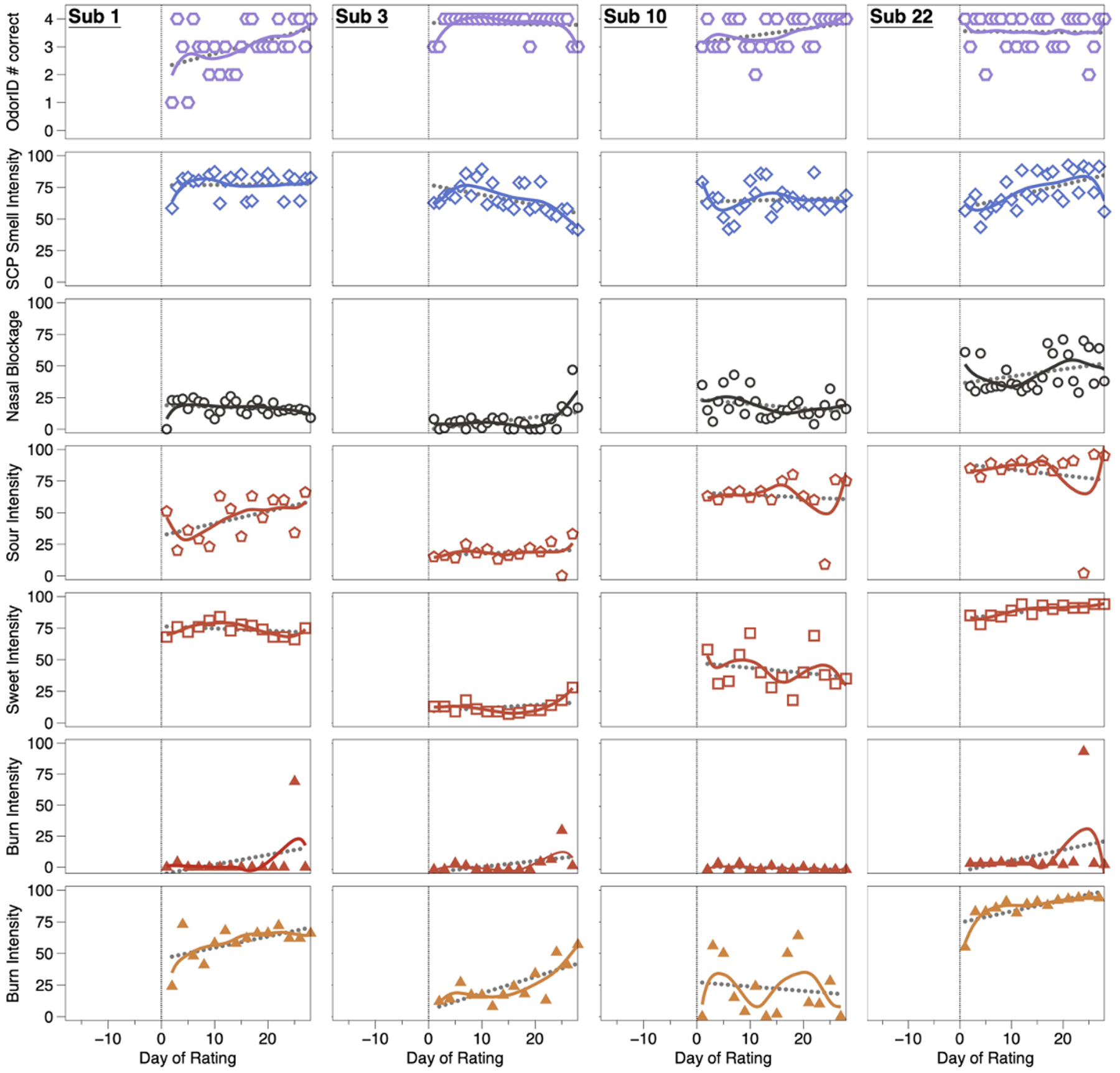
OdorID scores, and intensity ratings from matched controls over time. These participants (Subjects 1, 3, 10, and 22) show generally consistent ratings across the study. To help illustrate uniformity across the observation period, solid (colored) lines were fit via LOESS regression and dotted lines (gray) were fit via linear regression. A vertical line on Day 0 highlights the start of the 28-day study. Open hexagons (1st row) are the number correct on a ScentCheckPro card, while open diamonds (2nd row) are the mean daily smell intensity ratings from the same card. Open circles (3rd row) reflect ratings of perceived nasal blockage. Red symbols (rows 4, 5, and 6) reflect specific quality ratings from Sour Cherry jellybeans collected with a pinched nose. Orange triangles (row 7) indicate burn ratings from Cinnamon jellybeans collected with a pinched nose.
In summary, daily data from these four controls suggest individuals without COVID-19 are able to correctly identify the odors from the ScentCheckPro cards, and to consistently rate the various attributes from the cards (orthonasal intensity) and the jellybeans (taste, burn). While some minor variation is observed over time and across participants, the ratings are generally stable over the study period, in sharp contrast to the COVID-19+ cases (Figure 4).
Figure 4:
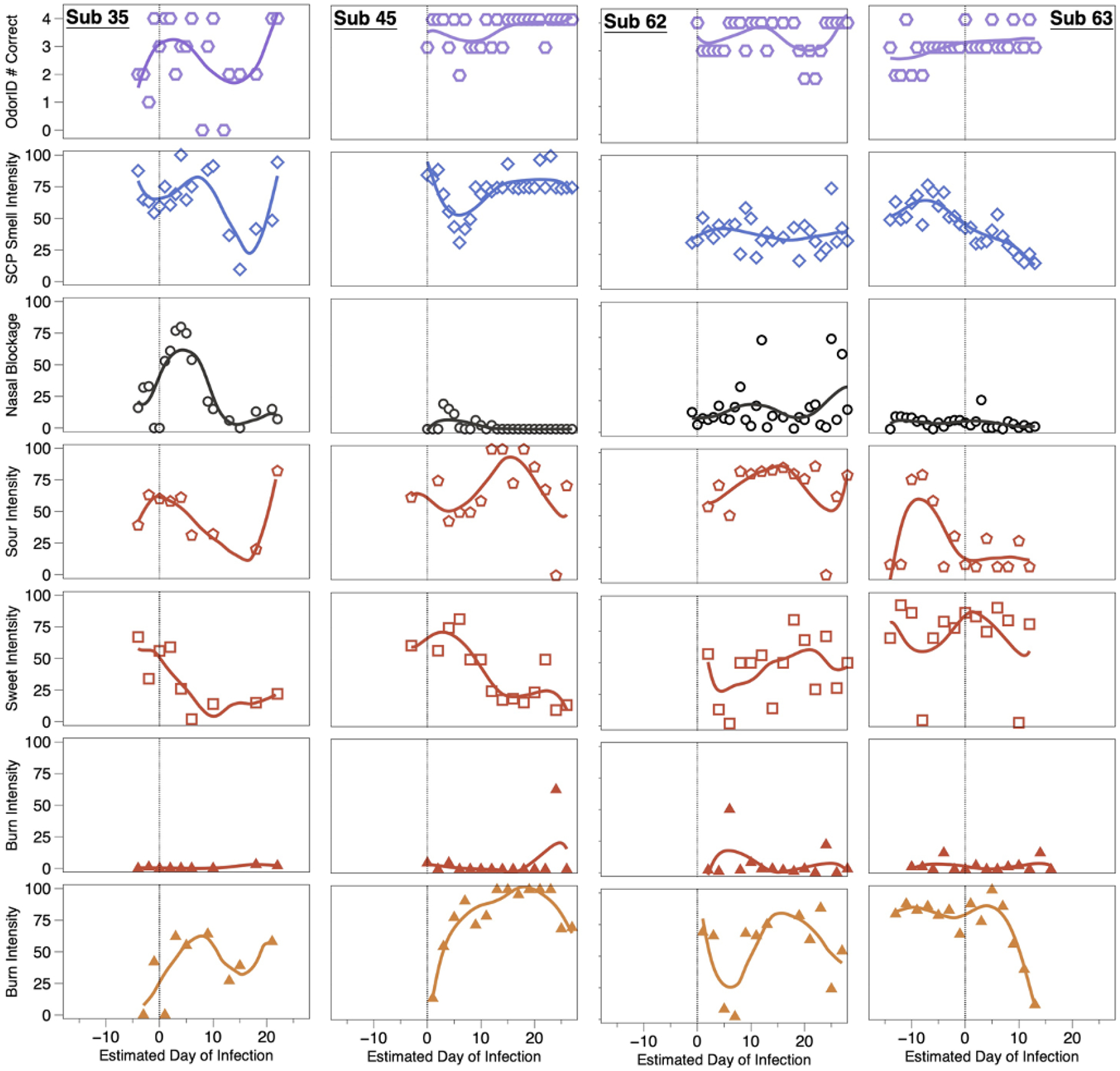
OdorID scores and VAS ratings from four COVID-19 positive individuals. COVID-19 cases (subjects 35, 45, 62, and 63) tended to show transient alterations of smell, taste, and/or chemesthesis during the observation period. To help illustrate uniformity across the study days, solid lines were fit via LOESS regression. A vertical line at Day 0 was added to highlight the estimated day of infection. Symbols and rows match those used in Figure 3: row 1 is daily number correct, and row 2 is orthonasal intensity of four scratch-n-sniff patches from a ScentCheckPro card, row 3 is ratings of perceived nasal blockage, rows 4–6 are sour, sweet, and burn ratings for Sour Cherry jellybeans with the nose pinched, and row 7 is burn for Cinnamon jellybeans with the nose pinched.
3.3. Cases with early onset data (n=4)
All four cases converted from being close contacts to being COVID-19+ while enrolled in the study, enabling visualization of changes in their responses over time (Figure 4). For brevity, we only highlight a few notable points here and a more detailed account is provided in the supplemental materials. When smell loss was observed, it was largely unrelated to nasal blockage, consistent with prior reports [4–7, 11] and the idea that COVID-19-associated smell loss arises from ACE2 receptor-mediated disruption of the olfactory epithelium, rather than the conductive losses typically seen with the common cold. Regarding chemesthesis, the lack of burn from the Sour Cherry jellybeans served as a negative control, suggesting participants were successful in discriminating between burn from a Cinnamon jellybean and a lack of burn from a Sour Cherry jellybean. From this, we can assume changes in burn observed here for the Cinnamon jellybeans was not merely a failure to understand the task.
3.3.1. Subject 35
Symptoms for Subject 35 included cough, runny nose/congestion, sore throat, and headache. Odor intensity ratings dropped through day 15 before recovering; her OdorID scores showed a similar pattern, but her data were noisier given the learning effect noted previously. Her nasal blockage resolved around Day 8, but she still showed impaired smell. Sourness ratings declined until ~Day 15 while sweetness declined until ~Day 6, before each began to recover. This was not merely a taste/flavor semantic confusion, as ratings were obtained while wearing nose clips. Her data also suggest sweet and sour taste are each transiently affected with an active COVID-19 infection, and loss and recovery may be dyssynchronous (i.e., sweetness did not recover as swiftly as sourness). Separately, she showed large changes in burn from Cinnamon jellybeans, suggesting oral chemesthesis is affected by COVID-19 infection, and this may be dyssynchronous from altered taste or smell function.
3.3.2. Subject 45
Subject 45 reported no symptoms despite becoming an active COVID-19 case while enrolled. Notably, despite being nominally asymptomatic, she clearly showed altered smell function that was reflected in both in OdorID performance and orthonasal intensity ratings, with maximal loss around Day 5. This highlights that some COVID-19+ individuals may be unaware of altered smell function, consistent with meta-analysis by Hannum and colleagues [8, 52]. As above, this transient disruption could not be attributed to nasal blockage. Regarding taste ratings, she also exhibited temporal dyssynchrony for different qualities. For the Cinnamon jellybeans, she showed a monotonic increase in burn over ~3 weeks, before showing a small drop at the end of the study. Elsewhere, some patients reported an increase in the ability to feel sensations in the mouth (including burning) during recovery from COVID-19 [53], so her temporal pattern may potentially reflect acute hypoalgesia, followed by hyperalgesia, before eventually returning to normal. In any case, her data support the idea that oral chemesthesis can be affected acutely by SARS-CoV-2 infection.
3.3.3. Subject 62
Like Subject 45, Subject 62 failed to report any symptoms, but unlike the prior cases, his orthonasal intensity ratings and OdorID performance remained relatively constant over time, and nasal blockage was generally low. This highlights that while many individuals with COVID-19 experience smell loss, some do not (e.g., [8, 52]). Regarding taste, noisy data preclude any strong conclusions, but tentatively, it seems he may have experienced large changes in both sweetness and sourness. That said, there is a sharp drop in sour taste intensity and sharp increase in burn intensity on two separate days (Figure 4). We suspect Subject 62 may have simply tasted the wrong sample on these days, as Sour Cherry jellybeans should be sour without any burn. Still, despite these caveats, his panel plots also suggest he experienced acute changes in oral chemesthesis without concomitant smell loss. If true, this would highlight that mechanisms of loss across all three chemosensory modalities are likely to be distinct.
3.3.3. Subject 63
Subject 63 enrolled 2 weeks before becoming a case. This greatly exceeds the expected incubation period (5 to 7 days) [40, 54], so we contacted her via email and she reported a second exposure to a COVID-19+ individual. Thus, we assume she became ill upon her second exposure rather than the initial exposure that caused her to enroll. Her data reveal changes in smell, taste, and chemesthesis. However, the 28-day observation period only captures initial illness without recovery, as she initially enrolled after an exposure that did not cause infection. Consistent with this interpretation, she did not report any symptoms for the first 2 weeks, before reporting many symptoms (sore throat, fever or chills, dry cough, body aches, fatigue, diarrhea, nausea or vomiting, headache, and dry cough). Notably, her mean orthonasal ratings began to decline somewhat a few days before the estimated day of infection, in the absence of nasal blockage. Further, her intensity data suggest she experienced hyposmia, rather than full anosmia, so it is not surprising that her OdorID performance remained relatively constant across the study period. This suggests rated smell intensity might provide more nuanced assessment of smell function versus odor identification (as discussed above). Her taste data were somewhat noisy, but it seems sourness may have been more affected than sweetness. Tentatively, her plots suggest she lost taste function in a quality specific manner, along with partial smell loss and loss of oral chemesthesis, with staggered timing of each.
4. General Discussion
By using intensive longitudinal data collected daily for 28 days, we were able to assess COVID-19-associated changes over time. Further, by leveraging enrollment of individuals upon exposure rather than waiting until they were already ill, we were able to create a small case control series that captured both initial loss and recovery. We can draw several conclusions from the results described here. First, number correct on a short odor identification task may potentially miss dysfunction in hyposmic individuals who have clearly depressed perceived intensity, but who still retain enough function to successfully complete an identification task. Longer tests, such as the 40-question University of Pennsylvania Smell Identification Test (Sensonics), may be better at differentiating these levels of smell loss, though their greater cost and time-to-complete make them impractical for this type of intensive longitudinal study. Second, we find smell loss appears maximal around Day 5, but this varies somewhat across participants. Third, COVID-19 related chemosensory dysfunction can manifest as reduced oral chemesthesis, reduced taste, and/or reduced orthonasal smell with little to no nasal blockage, with temporally staggered onset and time course. Collectively, these results, although limited in scope, extend prior work by providing direct assessment of multiple sensory modalities repeatedly over time using stable, commercially-available products as stimuli.
A strength of this study involved the use of commercial stimuli like jellybeans to collect ratings while the nose was blocked with nose clips. First, because of their glazed candy shell, jellybeans have little to no orthonasal scent and the odorant is only released from the food matrix upon chewing. For our purpose, this gave us a convenience way to deliver consistent shelf stable stimuli safely during a pandemic. All jellybeans of the same flavor came from the same lot; given routine quality control measures in commercial manufacturing, we are confident participants received consistent stimuli even if we do not know the exact formulation of the jellybeans. Second, our use of similarly colored jellybeans with different flavors minimizes potential biases participants may have from prior experience or knowledge with other foods (e.g., this is a lemon, and I know lemons tend to be sour [55]). When this lack of expectation is coupled with the use of nose clips, we believe the data shown here reflect true differences in taste and chemesthesis and not merely they result of a flavor taste confusion. Other studies have also reported loss in function when using taste stimuli that do not have an olfactory component [21].
Currently, there is disagreement regarding whether different types of taste cells are differentially affected by SARS-CoV-2 infection. Indeed, several studies have showed quality-specific differences (i.e., [47, 56–60]), while others have not (i.e., [19, 44–46, 61–65]). Here, sourness and sweetness from consistent stimuli were lost and recovered at similar rates for some participants, but this was not uniformly true. This implies specific taste qualities may recover at different rates, although additional work is needed to confirm this. Also, our data indicate taste qualities may be differentially affected, consistent with other reports [20, 66]. While the specific mechanisms underlying taste dysfunction with SARS-CoV-2 infection remain unclear, several mechanisms have be proposed. For example, ACE2 could allow for the infection of Type 2 (sweet, bitter and umami) taste receptor cells by SARS-CoV-2. Saliva could affect gustation as salivary glands express high levels of ACE2 and TMPRSS2. SARS-CoV-2 may even affect the central nervous system, as the virus has been detected in the cerebrospinal fluid [67–71].
Anecdotal reports and preliminary psychophysics suggest loss of chemesthesis with SARS-CoV-2 infection is real [11, 24, 25, 32], We extend prior reports here by showing oral chemesthesis, not just nasal chemesthesis, may be altered by COVID-19. This effect appears to be highly transitory, which could cause underreporting, especially when assessment occurs multiple days after illness has started. Definitionally, chemesthesis includes both thermal and tactile percepts like warming, cooling, and buzzing, and these sensations occur via distinct and specialized receptors. Even if focusing solely on burn, multiple receptors like TRPV1 and TRPA1 are involved [72]. Despite multiple advantages of commercial stimuli (high consistency, low cost, shelf stability, etc.), use of commercial jellybeans here limits interpretation somewhat. That is, cinnamon flavored candies presumably contain cinnamaldehyde, a well-known TRPA1 agonist [73, 74]However, we cannot rule out whether they contain capsaicin (or another TRPV1 agonist), as food labeling laws in the United States allow manufacturers to declare such ingredients as natural or artificial flavors on the package without being more specific, so we cannot make strong inferences about which specific chemesthetic mechanisms might be affected by SARS-CoV-2 infection. Nonetheless, present data extend prior work by clearly showing oral burn can be transiently affected by COVID-19.
4. Limitations and Conclusions
Our data suggest intensive cohort study designs are imperative for understanding and tracking symptoms of COVID-19 patients. Through intensive daily ratings we were able to examine and follow participants from initial exposure to catch symptoms as the emerged, allowing for the visualization of symptom onset, not just recovery. Thus, a strength of this study is the nature of the cohort examined, and it exemplifies a need for more cohort studies to catch patients before and during the most infectious period of their illness. A few limitations should be noted. First, all sensory testing in this study was performed remotely at home, due to pandemic related safety restrictions meant to protect both participants and our research team. Because participants made daily ratings at their leisure without direct supervision, we cannot obtain the same level of stimulus control we would have with an in-person lab-based study. Also, while all study materials were clearly labeled, we cannot preclude whether participants may have occasionally chosen the incorrect blinding codes on some days or that our staff might have mislabeled these samples. Further, we should note the commercial ScentCheckPro scratch-n-sniff cards used here were not validated as a clinical smell test; also, they were originally designed as an odor identification task, rather than a smell intensity task, so we cannot assume all stimulus concentrations were precisely matched for intensity. This concern is partially offset however by the randomization of odorants on any given card, and the use of daily means. Finally, we fully acknowledge this study had a very small number of participants (albeit with many data points per participant), so present findings should be taken as tentative until confirmed. Attempts to generalize the incidence or prevalence of distinct types of loss or dysfunction should not be made from this small case-control series. Despite these limitations, this dataset is highly unique in that it captures changes very early in COVID-19 illness with intensive daily sampling.
Here, we extend current knowledge by showing oral chemesthesis, taste, and/or orthonasal smell function can each be acutely affected by COVID-19. Further, we find such disruption may be dyssynchronous for the different chemical senses, with differing rates of loss and recovery across modalities and individuals. Also, odor intensity ratings revealed potentially hyposmic individuals who might be missed if smell function is only assessed via odor identification scores. Finally, disrupted chemosensation, especially for chemesthesis, appears to be highly transient, suggesting studies that collect a single snapshot in time, often retrospectively, may underestimate the true prevalence of loss.
Supplementary Material
Weir et al Highlights.
We tracked taste, chemesthesis, and smell over 28 days in cases and controls
Controls were generally stable while COVID-19 cases had sharp deviations over time
We observed acute loss of oral chemesthesis, which has not be shown previously
Different taste qualities may decline and recover at different rates
Acknowledgements
The authors wish to thank our participants for their time and critical contribution to this work.
Funding / COI disclosure
EMW, SM, JEH and RCG each receive partial salary support from a competitive grant [1U01DC019573] from the National Institutes of Deafness and Communications Disorders (NIDCD); JEH also receives salary support from the United States Department of Agriculture (USDA) via the National Institute of Food and Agriculture (NIFA) Hatch Act Appropriations [Project PEN04708 and Accession # 1019852]. Drs. Munger, Hayes, and Gerkin each hold equity in Redolynt, LLC, which they co-founded in 2021. This financial interest has been reviewed by the Individual Conflict of Interest Committee at each of their respective universities and is being actively being managed by each university. None of the other authors have any conflicts to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The data gathered for this program are available at: [DOI to be added upon publication].
References
- 1.Morens DM, Taubenberger JK, and Fauci AS, A centenary tale of two pandemics: the 1918 influenza pandemic and COVID-19, part I. American journal of public health, 2021. 111(6): p. 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Costa VG, Saivish MV, Santos DER, de Lima Silva RF, and Moreli ML, Comparative epidemiology between the 2009 H1N1 influenza and COVID-19 pandemics. Journal of infection and public health, 2020. 13(12): p. 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. World Health Organization (COVID-19) Dashboard. 2022; Available from: https://covid19.who.int.
- 4.Passarelli PC, Lopez MA, Bonaviri GM, Garcia-Godoy F, and D’Addona A, Taste and smell as chemosensory dysfunctions in COVID-19 infection. Am J Dent, 2020. 33(3): p. 135–137. [PubMed] [Google Scholar]
- 5.Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, and Parma V, Coronaviruses and the chemical senses: past, present, and future. Chemical senses, 2020. 45(6): p. 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Printza A, Katotomichelakis M, Valsamidis K, Metallidis S, Panagopoulos P, Panopoulou M, et al. , Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. Journal of Clinical Medicine, 2021. 10(5): p. 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. , Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature medicine, 2020: p. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannum ME, Ramirez VA, Lipson SJ, Herriman RD, Toskala AK, Lin C, et al. , Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. medRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, et al. , Recent Smell Loss Is the Best Predictor of COVID-19 Among Individuals With Recent Respiratory Symptoms. Chem Senses, 2021. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Bartheld CS and Wang L, Prevalence of Olfactory Dysfunction with the Omicron Variant of SARS-CoV-2: A Systematic Review and Meta-Analysis. Cells, 2023. 12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, et al. , More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chemical Senses, 2020. 45(7): p. 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karni N, Klein H, Asseo K, Benjamini Y, Israel S, Nammary M, et al. , Self-Rated Smell Ability Enables Highly Specific Predictors of COVID-19 Status: A Case-Control Study in Israel. Open Forum Infect Dis, 2021. 8(2): p. ofaa589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huart C, Philpott C, Konstantinidis I, Altundag A, Trecca EMC, Cassano M, et al. , Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Henkin RI, Larson AL, and Powell RD, Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Otol Rhinol Laryngol, 1975. 84(5 Pt 1): p. 672–82. [DOI] [PubMed] [Google Scholar]
- 15.Hummel T, Landis BN, and Huttenbrink KB, Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg, 2011. 10: p. Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsetto D, Hopkins C, Philips V, Obholzer R, Tirelli G, Polesel J, et al. , Self-reported alteration of sense of smell or taste in patients with COVID-19: a systematic review and meta-analysis on 3563 patients. Rhinology, 2020. 58(5): p. 430–436. [DOI] [PubMed] [Google Scholar]
- 17.Dawson P, Rabold EM, Laws RL, Conners EE, Gharpure R, Yin S, et al. , Loss of taste and smell as distinguishing symptoms of COVID-19. Clinical Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Printza A and Constantinidis J, The role of self-reported smell and taste disorders in suspected COVID-19. European Archives of Oto-Rhino-Laryngology, 2020. 277: p. 2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzatenta A, Neri G, D’Ardes D, De Luca C, Marinari S, Porreca E, et al. , Smell and taste in severe CoViD-19: self-reported vs. testing. Frontiers in Medicine, 2020. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstantinidis I, Delides A, Tsakiropoulou E, Maragoudakis P, Sapounas S, and Tsiodras S, Short-Term Follow-Up of Self-Isolated COVID-19 Patients with Smell and Taste Dysfunction in Greece: Two Phenotypes of Recovery. ORL, 2020. 82(6): p. 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen H, Albayay J, Höchenberger R, Bhutani S, Boesveldt S, Busch NA, et al. , Covid-19 affects taste independently of smell: results from a combined chemosensory home test and online survey from a global cohort (N=10,953). medRxiv, 2023: p. 2023.01.16.23284630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callejon-Leblic MA, Moreno-Luna R, Del Cuvillo A, Reyes-Tejero IM, Garcia-Villaran MA, Santos-Peña M, et al. , Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. Journal of clinical medicine, 2021. 10(4): p. 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liou J-M, Chen M-J, Hong T-C, and Wu M-S, Alteration of taste or smell as a predictor of COVID-19. Gut, 2021. 70(4): p. 806–807. [DOI] [PubMed] [Google Scholar]
- 24.Hayes JEP, Valentina. COVID-19, smell and taste – how is COVID-19 different from other respiratory diseases? 2020; Available from: https://theconversation.com/covid-19-smell-and-taste-how-is-covid-19-different-from-other-respiratory-diseases-139543.
- 25.Ferreli F, Di Bari M, Gaino F, Albanese A, Politi LS, Spriano G, et al. Trigeminal features in COVID-19 patients with smell impairment. in International Forum of Allergy & Rhinology. 2021: Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iravani B, Arshamian A, Ravia A, Mishor E, Snitz K, Shushan S, et al. , Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chemical senses, 2020. 45(6): p. 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snitz K, Honigstein D, Weissgross R, Ravia A, Mishor E, Perl O, et al. , An olfactory self-test effectively screens for COVID-19. Communications Medicine, 2022. 2(1): p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E, Tomasoni M, et al. , Olfactory and gustatory outcomes in COVID-19: A prospective evaluation in nonhospitalized subjects. Otolaryngology–Head and Neck Surgery, 2020. 163(6): p. 1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC, et al. , COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. Laryngoscope, 2021. 131(5): p. 1095–1100. [DOI] [PubMed] [Google Scholar]
- 30.Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. , Long COVID in a prospective cohort of home-isolated patients. Nature Medicine, 2021: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein H, Asseo K, Karni N, Benjamini Y, Nir-Paz R, Muszkat M, et al. , Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clinical Microbiology and Infection, 2021. 27(5): p. 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otte MS, Bork M-L, Zimmermann PH, Klußmann JP, and Lüers J-C, Patients with COVID-19-associated olfactory impairment also show impaired trigeminal function. Auris Nasus Larynx, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein ST, et al. , A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology, 2022. 60(3): p. 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Antonucci P, Capriotti V, et al. , Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. European Archives of Oto-Rhino-Laryngology, 2022. 279(1): p. 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir EM, Reed DR, Pepino MY, Veldhuizen MG, and Hayes JE, Massively collaborative crowdsourced research on COVID19 and the chemical senses: Insights and outcomes. Food Quality and Preference, 2022. 97: p. 104483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG, Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 2009. 42(2): p. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. , The REDCap consortium: Building an international community of software platform partners. Journal of biomedical informatics, 2019. 95: p. 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark CC and Lawless HT, Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chemical senses, 1994. 19(6): p. 583–594. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, An intuitive tutorial to Gaussian processes regression. arXiv preprint arXiv:2009.10862, 2020. [Google Scholar]
- 40.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. , The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Annals of Internal Medicine, 2020. 172(9): p. 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Killingley B, Mann AJ, Kalinova M, Boyers A, Goonawardane N, Zhou J, et al. , Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nature Medicine, 2022. 28(5): p. 1031–1041. [DOI] [PubMed] [Google Scholar]
- 42.Printza A and Constantinidis J, The role of self-reported smell and taste disorders in suspected COVID-19. European Archives of Oto-Rhino-Laryngology, 2020. 277(9): p. 2625–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cecchetto C, Di Pizio A, Genovese F, Calcinoni O, Macchi A, Dunkel A, et al. , Assessing the extent and timing of chemosensory impairments during COVID-19 pandemic. Scientific Reports, 2021. 11(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaira LA, Lechien JR, Salzano G, Salzano FA, Maglitto F, Saussez S, et al. , Gustatory Dysfunction: A Highly Specific and Smell-Independent Symptom of COVID-19. Indian Journal of Otolaryngology and Head & Neck Surgery, 2020: p. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. , Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head & Neck, 2020. 42(7): p. 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hintschich CA, Wenzel JJ, Hummel T, Hankir MK, Kühnel T, Vielsmeier V, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. in International forum of allergy & rhinology. 2020: Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer-Cornelius T, Cornelius J, Oberle M, Metternich FU, and Brockmeier SJ, Objective gustatory and olfactory dysfunction in COVID-19 patients: a prospective cross-sectional study. European Archives of Oto-Rhino-Laryngology, 2021: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyu C, Schijvens D, Hayes JE, and Stieger M, Capsaicin burn increases thickness discrimination thresholds independently of chronic chili intake. Food Research International, 2021. 149: p. 110702. [DOI] [PubMed] [Google Scholar]
- 49.Nolden AA and Hayes JE, Perceptual and affective responses to sampled capsaicin differ by reported intake. Food quality and preference, 2017. 55: p. 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawless H, Oral chemical irritation: Psychophysical properties. Chemical Senses, 1984. 9(2): p. 143–155. [Google Scholar]
- 51.Lawless H, Rozin P, and Shenker J, Effects of oral capsaicin on gustatory, olfactory and irritant sensations and flavor identification in humans who regularly or rarely consume chili pepper. Chemical senses, 1985. 10(4): p. 579–589. [Google Scholar]
- 52.Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, et al. , Taste loss as a distinct symptom of COVID-19: A systematic review and meta-analysis. medRxiv, 2021: p. 2021.10.09.21264771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Rawi NH, Sammouda AR, AlRahin EA, Al Ali FA, Al Arayedh GS, Daryanavard HA, et al. , Prevalence of Anosmia or Ageusia in COVID-19 Patients Among UAE Population. International Dental Journal, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cimolai N, In pursuit of the right tail for the COVID-19 incubation period. Public Health, 2021. 194: p. 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hintschich CA, Niv MY, and Hummel T. The taste of the pandemic—contemporary review on the current state of research on gustation in coronavirus disease 2019 (COVID-19). in International Forum of Allergy & Rhinology: Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC, et al. , COVID-19: Recovery from chemosensory dysfunction. A multicentre study on smell and taste. The Laryngoscope, 2021. 131(5): p. 1095–1100. [DOI] [PubMed] [Google Scholar]
- 57.Salcan İ, Karakeçili F, Salcan S, Ünver E, Akyüz S, Seçkin E, et al. , Is taste and smell impairment irreversible in COVID-19 patients? European Archives of Oto-Rhino-Laryngology, 2021. 278(2): p. 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altin F, Cingi C, Uzun T, and Bal C, Olfactory and gustatory abnormalities in COVID-19 cases. European Archives of Oto-Rhino-Laryngology, 2020. 277(10): p. 2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bidkar V, Mishra M, Selvaraj K, Joshi P, Shrikrishna B, Dabhekar S, et al. , Testing Olfactory and Gustatory Dysfunctions among Quarantine COVID-19 Suspects. Indian Journal of Otolaryngology and Head & Neck Surgery, 2020: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adamczyk K, Herman M, Fraczek J, Piec R, Szykula-Piec B, Zaczynski A, et al. , Sensitivity and specifity of prediction models based on gustatory disorders in diagnosing COVID-19 patients: a case-control study. medRxiv, 2020. [Google Scholar]
- 61.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. , Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head & Neck, 2020. 42(6): p. 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaira LA, Hopkins C, Petrocelli M, Lechien J, Chiesa-Estomba C, Salzano G, et al. , Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. The Journal of Laryngology & Otology, 2020. 134(8): p. 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Soma D, Giovanditto F, et al. , Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. Journal of Otolaryngology-Head & Neck Surgery, 2020. 49(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, and De Riu G, Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head & neck, 2020. 42(7): p. 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrocelli M, Cutrupi S, Salzano G, Maglitto F, Salzano F, Lechien J, et al. , Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. The Journal of Laryngology & Otology, 2021: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burges Watson DL, Campbell M, Hopkins C, Smith B, Kelly C, and Deary V, Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PloS one, 2021. 16(9): p. e0256998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doyle ME, Appleton A, Liu Q-R, Yao Q, Mazucanti CH, and Egan JM, Human Taste Cells Express ACE2: a Portal for SARS-CoV-2 Infection. bioRxiv, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. , SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell, 2020. 181(2): p. 271–280. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. , High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International journal of oral science, 2020. 12(1): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis A, Frontera J, Placantonakis DG, Lighter J, Galetta S, Balcer L, et al. , Cerebrospinal fluid in COVID-19: A systematic review of the literature. Journal of the neurological sciences, 2021: p. 117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. , Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. The Lancet Neurology, 2020. 19(11): p. 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald ST, Bolliet D, and Hayes J, Chemesthesis : chemical touch in food and eating. 2016, Chichester, West Sussex: John Wiley & Sons, Inc. pages cm. [Google Scholar]
- 73.Alpizar YA, Voets T, and Talavera K, Molecular mechanisms underlying the role of TRP channels in chemesthesis. Chemesthesis: Chemical Touch in Food and Eating, 2016: p. 48. [Google Scholar]
- 74.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. , Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron, 2004. 41(6): p. 849–857. [DOI] [PubMed] [Google Scholar]
- 75.Chiesa-Estomba CM, Lechien JR, Radulesco T, Michel J, Sowerby LJ, Hopkins C, et al. , Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. European journal of neurology, 2020. 27(11): p. 2318–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiter ER, Coelho DH, Kons ZA, and Costanzo RM, Subjective smell and taste changes during the COVID-19 pandemic: Short term recovery. American Journal of Otolaryngology, 2020. 41(6): p. 102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaaban N, Høier ATZB, and Andersen BV, A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease. Foods, 2021. 10(4): p. 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data gathered for this program are available at: [DOI to be added upon publication].


