Abstract
Environmental factors and host microbiota strongly influence Type-1 Diabetes (T1D) progression. We report here that neonatal immunization with Group-A Streptococcus suppresses T1D development in non-obese diabetic (NOD) mice by promoting clonal expansion of N-acetyl-D-glucosamine (GlcNAc)-specific B-1 B cells that recognize pancreatic β-cell-derived antigens bearing GlcNAc-containing post-translational modifications. Early exposure to Lancefield Group-A cell-wall carbohydrate-antigens increased production of GlcNAc-reactive serum antibodies and enhanced localization of innate-like GlcNAc-specific B cells to pancreatic tissue during T1D pathogenesis. We show that B-1 B cell-derived GlcNAc-specific IgM engages apoptosis-associated β-cell antigens, thereby suppressing diabetogenic T-cell activation. Likewise, adoptively transferring GlcNAc-reactive B-1 B cells significantly delayed T1D development in naïve recipients. Collectively, these data underscore potentially protective involvement of innate-like B cells and natural antibodies in T1D progression. These findings suggest that previously reported associations of reduced T1D risk following GAS infection are B cell-dependent and demonstrate the potential for targeting the natural-antibody repertoire in considering therapeutic strategies for T1D.
Keywords: Natural antibody, Type-1 Diabetes, Group-A Streptococcus, N-acetyl-D-glucosamine
Introduction
Although the notion that environmental factors contribute to precipitation of Type-1 Diabetes (T1D) in genetically susceptible individuals is a widely cited paradigm, mechanistic understanding of this phenomenon is limited. One intriguing possibility, outlined by the ‘hygiene hypothesis’, suggests that exposure to environmental antigens and microorganisms during critical stages of immune-system development suppresses the development of autoimmunity (1). Consistent with this possibility, housing cleanliness and the commensal microbiota exhibit a well-documented impact on T1D penetrance in the commonly studied non-obese diabetic (NOD) mouse model (2). Multiple prior studies additionally reported decreased T1D incidence in diabetes-prone rodents following treatment with Streptococcus pyogenes (Group-A Streptococcus, GAS) (3-5), supporting specific involvement of particular infectious microorganisms on influencing T1D onset in these models. Although mechanistic understanding of these observations are limited, retrospective epidemiological studies have similarly reported a reduced risk for T1D in humans following childhood cases of scarlet fever, highlighting the potential relevance of host-GAS interactions on human T1D pathogenesis (6, 7).
The group-specific cell-wall carbohydrate expressed by S. pyogenes Lancefield Group-A Carbohydrate (GAC), elicits clonally restricted N-acetyl-D-glucosamine (GlcNAc)-specific B-lymphocyte responses in both mice and humans (8, 9). Although not previously examined in the context of T1D protection, GlcNAc-specific antibodies induced by GAS exhibit reactivity for carbohydrate epitopes generated through post-translational glycosylation of host proteins (9), including the dynamic O-GlcNAc modifications abundant in pancreatic β cells where they are vital in nutrient sensing (10). Although post-translational modification (PTM)-associated neo-epitopes have been suggested to drive loss of self-tolerance in T1D and other autoimmune diseases (11), we asked whether GlcNAc-specific B cell responses are involved in delaying development of T1D in NOD mice following exposure to GAS.
Methods
Mice:
C57BL/6J, B6.129S7-Rag1tm1Mom/J (C57BL/6J Rag1 KO), NOD/ShiLtJ, and NOD/ShiLtJ.BDC2.5 TCR transgenic mice were purchased from Jackson Laboratories, and B6-H2g7 congenic mice were generously provided by Dr. Hubert Tse (UAB, Birmingham, AL). All animal use was in-accordance with IACUC protocols; mice were given standard chow and acidified water ad libitum. Heat-killed, pepsin-treated vaccine stocks prepared as previously described (12) and 5x107 bacteria or 50 ug OK-432, a lyophilizate of penicillin-treated Streptococcus pyogenes A3su (Picibanil, Chugai Pharmaceutical Co. Ltd., Japan) were administered intraperitoneally (i.p.) to NOD mice at 14 days of age. Litters derived from individual dams were randomized with respect to immunization to reduce litter-dependent effects during T1D incidence studies. Diabetes incidence was monitored in female NOD mice from 10- to 30-weeks-of-age by weekly measurements of glycosuria (Bayer Diastix); mice were classified as diabetic and sacrificed following two consecutive urine glucose measurements of ≥1/4% and a blood glucose measurement of ≥250mg/dL measured with an Accu-Chek Comfort Curve glucose meter (Bayer). HGAC78, HGAC41 and HGAC39 (17,21) were generously provided by Dr. Moon Nahm (UAB), CTD110.6 (22) was provided by Drs. Gerald Hart (Johns Hopkins University) and Mary Ann Accavitti (Hybridoma Core Facility, UAB), RL-2 (23) was provided by Dr. Larry Gerace (Johns Hopkins University) and Dr. James Thomas (Vanderbilt University) provided the insulin-specific hybridoma (clone 7CD9). Hybridomas were grown in serum-free media (Hybridoma SF media, Gibco) and culture supernatants were passed over protein G-Sepharose or GlcNAc-sepharose columns for purification of monoclonal antibodies (MAbs) and concentrated using AmiconUltra centrifugal filters (Ultracel-10K, Millipore). Appropriate molecular weight, antigen-binding, and endotoxin levels were confirmed for purified MAbs by SDS-PAGE, ELISA and limulus assay (Limulus Amebocyte Lysate Pyrogent, Lonza), respectively, and concentrations of MAb preparations were determined by spectrophotometric analysis (ND-1000, NanoDrop Technologies) prior to storage at 4°C. In some cases, MAbs were directly conjugated to Alexa Fluor dyes (Life Technologies). C57BL/6J-derived MIN6 insulinoma cells were obtained from Dr. Hubert Tse, and maintained in Dulbecco’s modified Eagles medium supplemented with 10% fetal-calf serum. MIN6 cells are not represented in the database of commonly misidentified cell lines maintained by ICLAC, were authenticated through analysis of insulin expression, and were free of Mycoplasma contamination as determined through luminescent mycoplasma detection (MycoAlert, Lonza).
Enzyme-linked immunosorbent assay (ELISA) and ELISPOT:
Half-area (ELISA) or full area (ELISPOT) 96-well flat-bottom EIA/RIA plates (Costar) were coated overnight at 4°C with 2 μg/mL of Group A Carbohydrate (5S PG-PS, BD Lee Labs), GlcNAc35BSA (Pyxis Laboratories), or immunoglobulin isotype-specific capture antibodies (goat anti-mouse, Southern Biotechnology). Plates were blocked with PBS+2%BSA and, following adsorption of diluted sera, serum immunoglobulin was detected by alkaline-phosphatase conjugated mouse immunoglobulin isotype-specific secondary antibodies (goat, Southern Biotechnology) and phosphatase substrate reactions. Purified monoclonal antibodies were used as standards for quantitation of serum immunoglobulin, and plate absorbance was read at 405nm (SPECTROstar Omega, BMG Labtech). ELISPOT assays to enumerate ASCs were blocked with PBS+2% gelatin prior to incubation of spleen and BM cells overnight at 37°C in RPMI+10% fetal calf serum. Spot detection was facilitated by alkaline-phosphatase conjugated mouse immunoglobulin isotype-specific secondary antibodies (goat, Southern Biotechnology) and 5-bromo-4-chloro-3-indolyl phosphate substrate reactions.
Histology:
For whole-pancreas histology, pancreata were dissected from the abdominal cavity following euthanization and whole-body perfusion with PBS+1x Heparin, flash frozen in optimal cutting temperature medium (Tissue-Tek, Sakura Finetek), and stored at −80°C until sectioning. Six μm sections were cut using a cryostat at −20°C, (Leica CM1850, Laboratory of Dr. Frances Lund UAB) and air dried prior to storage at −80°C. Tissue sections were fixed with either PBS+0.5% paraformaldehyde and 0.5% Triton X-100 or ice-cold acetone, and non-specific binding was blocked using PBS+5% normal horse serum or PBS+2% BSA. Spontaneous insulitis was examined at 10- to 12-week-old pre-diabetic time points, and histological scoring of islet lesions was completed by a third-party in a blind manner on a scale of 0-3 (0: no infiltrate; 1: peri-insulitis; 2: progressed peri-insulitis with breakdown of peri-islet membrane; 3: advanced insulitis) following immunofluorescence labeling of laminin (rabbit polyclonal, Novus Biologicals), CD4 (clone RM4-5, BioLegend) and IgM (goat polyclonal, ThermoFisher). Distribution of GlcNAc epitopes was evaluated using various GlcNAc-specific MAbs, wherein islets were differentiated from exocrine tissue by insulin (MAb 7CD9) and laminin staining. Detection of passively administered MAbs during high-dose STZ treatments (single 200mg/kg dose i.p.) was accomplished with the Igha allotype-specific MAb RS-3.1. Complement deposition was measured with C1q-, C4- (Hycult Biotechnology) and C3b- (Cedar Lanes Laboratories) specific Abs, and signals were later quantitated using ImageJ analysis software. Mouse islets were purified as described in (13), whereas islets from other species were kindly provided by Drs. Anthony Thompson and Luke Cui (Islet Procurement Facility, UAB). Purified islets were cytospun prior to staining as described above. Coverslips were mounted using Fluoromount, which in some cases, contained DAPI for nuclear staining (Southern Biotechnology), and prepared tissues were imaged using a Leica Leitz DMRB fluorescence microscope.
Insulin Granule Purification:
Approximately 109 MIN6 insulinoma cells were grown in culture with Dulbecco’s modified Eagles Medium (Gibco) +10% fetal calf serum (Hyclone) and post-nuclear supernatant (PNS) prepared as described in (14), PNS was resuspended in isotonic media, loaded onto a 8%-30% continuous Nycodenz (Sigma-Aldrich) gradient and centrifuged at 107,000g, (Optima XPN 80k-IVD and SW 41 Ti rotor (Beckman Coulter), UAB Microbiology Department). The resulting gradient was subsequently fractionated using an automatic fractionator (Frac-920, Amersham Biosciences), and fraction activity was assessed as described previously (14), in addition to binding assays with antigen-specific MAbs.
Flow cytometry:
For analysis of low-frequency antigen-specific B cells, ~107 splenocytes were pelleted in 96-well round bottom plates by centrifugation at 1200rpm for 2 minutes and blocked with PBS+2.5% fetal calf serum and 2ug/mL anti-CD16/32 (Ab93) (15). Non-B cells were gated out using a dump cocktail containing biotinylated MAbs against CD3, CD4, CD8, CD11c and F4/80, and Streptavidin PerCP. Dead cells were excluded from analysis by propidium iodide (Sigma-Aldrich). B-cell phenotypes were subsequently identified using various fluorescence-conjugated antibodies. Antigen-specific B cells were detect using GAC (BD Lee Labs) for single epitope multiple staining of GAC-binding B cells (16), and were further interrogated with GAC-associated IdI-3a idiotope-specific MAb IA.1 (17). During analysis of pancreas-infiltrating B cells pancreas tissue was digested in HBSS+10mM HEPES (Gibco) supplemented with 1mg/mL Collagenase Type-IV and 10U/mL DNAse1 (Sigma Aldrich) following whole-body perfusion and dissection of pancreatic lymph nodes and omentum. Liberated lymphocytes were isolated with lymphocyte separation media (Cellgro). For flow cytometric experiments involving MIN6 insulinoma cells, apoptotic MIN6 cells were generated by x-ray irradiation (30Gy) or treatment with 25uM STZ and assessed for apoptosis with Annexin-V and 7AAD, or active Caspase-3 (all from BD Biosciences). In some cases, apoptotic MIN6 cells were labeled with a lipophilic membrane tracker (10−6 M PKH26, Sigma), and their uptake by BMDC was evaluated by flow cytometry following a 30-minute co-culture at 37°C with apoptotic MIN6 cells at 1:1 cell ratios. Data were acquired on a LSRII or FACSAria (Becton Dickinson) and subsequently analyzed using FlowJo software (Treestar).
Adoptive transfer of PerC B cells to naïve NOD recipients:
Bulk CD19 positive PerC B cells were FACS-sorted from adult naïve NOD mice and adult NOD mice that had been immunized with J17A4 at day 14 at after birth. 2X105 CD19 positive B cells from each source were administered i.p. to non-diabetic naïve adult NOD mice. The mice receiving transfers together with a group of non-transferred NOD mice were monitored for 30wks for the emergence of diabetes.
Immunoglobulin Heavy Chain Sequencing:
Single GAC+ B cells were sorted directly into 384-well plates containing 3ul 10mM Tris and 0.75 units/ml of RNAsinPlus (Promega). Resulting lysates were used to generate cDNA using the High Capacity cDNA synthesis kit (Applied Biosystems) following manufacture’s protocol. Following the generation of cDNA, rearranged B cell receptor genes were amplified by nested PCR using IGHV family- and constant region-specific primers as previously described (18). The Resulting amplicons were subsequently sequenced by Sangers sequencing at the Heflin Center Genomics Core (UAB). Sequence fasta files were submitted to the IGMT server for IGH gene identification and analyzed using scripts modified from ImmuneDiversity (19), VDJ tools (20), and R. Clonal profiles were rendered using Circos software package (21), wherein pairing (edges) of IGHV (right vertex) and IGHD (left vertex) genes, as well as IGHJ gene identities (left vertex inlay) and mutation number (right vertex inlay) were depicted.
In vitro T-cell stimulation assays:
T lymphocytes from the T cell receptor transgenic NOD.BDC2.5 mice (22) were used to evaluate presentation of islet cell-derived antigens by BMDC (generation described in (23)). Briefly, peripheral T cells were collected from secondary lymphoid tissues, purified using a negative naive T cell isolation kit (Miltenyi Biotechnology), and labeled with CellTrace CFSE (LifeTechnologies). 5X104 purified T cells were subsequently co-cultured with C57BL/6 H-2g7 BMDCs at a 10:1 ratio and stimulated with purified islet cells, the insulin-derived peptide (InsB9-23) as a negative control, and the BDC2.5 mimotope. T cell activation was evaluated after 5 days of co-culture and following 6-hour treatment with Brefeldin A, via the dilution of CFSE and intracellular staining for TNF-α (BD Biosciences.
Statistical Analysis:
For each experiment, the mean and s.e.m. were determined for the respective measured parameter, and statistical analyses were performed using Student’s t-test (single-factorial comparisons) or one-way or two-way ANOVA when appropriate (multifactorial comparisons), with Tukey’s and Bonferroni post hoc tests, respectively. For mouse experiments, sample sizes were not predetermined through statistical methods, but instead chosen based on pilot studies and previously reported results such that appropriate statistical testing could yield significant results. In all cases, samples were tested for similar variance to ensure the assumptions of the statistical comparisons used were met, blinding was not done in allocation of animals to experimental groups, but was done for histological scoring of insulitis, which was completed by a third-party. No specific randomization or exclusion criteria were applied to mouse samples due to using inbred mouse strains. Analysis was completed using GraphPad Prism software (GraphPad Software).
Code Availability:
Program scripts used during the analysis of Immunoglobulin sequencing data are available through previously published analysis software, described in the Immunoglobulin Heavy Chain Sequencing subsection.
Data Availability:
GAC+ B cell immunoglobulin heavy chain nucleotide sequence data are available through GenBank. Sequences for naïve and d14 GAS immunized NOD mice may be found at the accession numbers KY210724 - KY210801 and KY210802 - KY210877, respectively Mammalian glycan microarray data is publicly available through the Consortium for Functional Glycomics gateway. (http://www.functionalglycomics.org/static/index.shtml). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Neonatal GAS exposure protects NOD mice from spontaneous T1D
Previous studies reported that repeated administration of the GAS-preparation OK-432 to NOD mice suppressed spontaneous T1D development (3, 4). Similar to these findings, we found that a single immunization with OK-432 at 14 days of age (d14) reduced T1D penetrance in female NOD mice relative to naive controls (Figure 1a). We further found that immunization with a heat-killed, pepsin-treated vaccine GAS preparation (Strain J17A4) also protected NOD mice from T1D. Conversely, similar immunization with Group C Streptococci (Strain C74), which expresses a cell-wall bearing N-acetyl-galactosamine epitopes, did not impact T1D penetrance, (Figure 1b). Unlike neonatal GAS exposure, immunizing NOD mice with GAS as adults did not protect mice from T1D (Figure 1c), suggesting that the timing of exposure was central to GAS related efficacy in delaying T1D.
Figure 1. Neonatal immunization with GAS suppresses spontaneous T1D in NOD mice and produces GlcNAc-reactive antibody responses.
(a) Spontaneous T1D incidence in female NOD mice following intraperitoneal GAS preparation OK-432 administration at d14 (blue) relative to sham-immunized, naïve mice (white). (b) Spontaneous T1D incidence in female NOD mice following d14 immunization with heat-killed, pepsin-treated vaccine preparations of GAS strain J17A4 (red) or Group C Streptococcus strain C74 (grey), relative to sham-immunized, naïve mice (white). (c) Spontaneous T1D incidence in female NOD mice following intraperitoneal immunization of eight-week-old adults with heat-killed, pepsin-treated vaccine preparation of GAS strain J17A4 (black). Data are pooled from 2-3 independent experiments; *p<0.05, **p<0.01 compared to C74, by Mantel-Cox Log-rank Test. (d) (Left) Representative pancreatic-tissue histology from 10-12 week old naïve and J17A4-immunized NOD mice showing IgM+ (red) and CD4+ (green) lymphocyte infiltration, and peri-islet membrane integrity (pan-laminin staining, white); scale bar = 100μm. (Right) Mean insulitis scores, in 10-12 week-old naïve and J17A4-immunized NOD mice. Data are mean ± s.e.m. from one representative experiment of two, with n=3/ group. (e) Flow-cytometric enumeration of pancreas-infiltrating CD19+B lymphocytes (left), CD4+ T lymphocytes (center) and CD8+ T lymphocytes (right) in 10-12 week old pre-diabetic naïve (n=12) and J17A4-immunized (n=11) NOD mice. Data are mean ± s.e.m. pooled from two independent experiments; analysis of variance though one-tail Mann Whitney test **p<0.01, ***p<0.001. (f) Longitudinal analysis of serum antibody responses to Group-A Carbohydrate and GlcNAc35BSA in NOD mice following d14 immunization with OK-432 (blue, n=7), J17A4 (red, n=6) or GCS (grey, n=5) and unimmunized controls (black, n=6). Data are mean ± s.e.m. from one representative experiment of three independent experiments. (g) ELISPOT analysis of spleen- and bone marrow-localized GAC-specific IgM antibody-secreting cell (ASC) numbers in 10-12 week old naïve or J17A4-immunized NOD mice. Data are mean ± s.e.m. pooled from two independent experiments with n=10 per group; **p<0.01, ***p<0.001, by two-way ANOVA.
Neonatal immunization with GAS drives expansion of Group-A Carbohydrate-reactive B-1 B cells. (h) Representative flow-cytometry profiles and mean ± s.e.m. frequencies of Group-A Carbohydrate-binding (GAC+) PerC B cells in naïve (n=10) and J17A4-immunized (n=9) 10–12 week-old NOD mice (left), and examination of B-1a (CD5+CD43+CD23−), B-1b (CD5−CD43+CD23−) + B-2 (CD5−CD43−CD23+) surface phenotypes (middle), and CD43− as well as CD11b-expression profiles (right) on GAC+ B cells. (i) Absolute numbers of PerC-, Spleen- and ILN-localized GAC+ B cells in 10-12 week-old naïve (white, n=10) and J17A4-immunized (red, n=9) NOD mice, and (j) numbers of B-1a, B-1b and B-2 or IgD+ and IgD−/lo GAC+ B cells in the PerC and spleen, respectively. (k) Representative flow cytometric analyses comparing expression of IgM and IgD B-cell receptors, innate-like B-cell surface markers CD36 and CD9, and the IdI-1 idiotype associated with a subset of VH6 (J606)-expressing GlcNAc-specific hybridomas. Data are mean ± s.e.m. pooled from two independent experiments; ns (not significant), **p<0.01, ***p<0.001, by two-way ANOVA. (l) Volcano plot depicting identification of statistically significant changes in GAC+ B cell V(D)J clonotype frequencies following d14 immunization in NOD mice determined by two-way ANOVA with Bonferroni correction. (m) Circos plots, described in Methods section, depicting GAC+ B-cell IGH sequences derived from four naïve (n=85) and four J17A4-immunized (n=83) mice.
Consistent with T1D pathology, histological examination of pancreata from naive 10- to 12-week-old pre-diabetic NOD mice revealed significant B- and T-lymphocyte infiltration as well as a breakdown of the peri-islet membrane in most islets examined (24, 25). In marked contrast, islets of NOD mice immunized with GAS as neonates were predominantly free of lymphocytic infiltration and perturbations to the peri-islet membrane (Figure 1d). Flow-cytometric analysis of pancreatic-tissue digests similarly revealed that fewer J17A4-immunized NOD mice developed significant lymphocytic infiltrates, relative to age-matched naive controls (Figure 1e).
Serum ELISAs revealed that NOD mice immunized with J17A4 and OK-432 as neonates produced IgM antibodies that bound both GAC- and GlcNAcylated-BSA (GlcNAc35BSA) evident by four-weeks-of-age and remained elevated relative to control mice for >16 weeks (Figure 1f), whereas no significant increases in GlcNAc-reactive IgG antibodies were observed (data not shown). ELISPOT analysis further showed that GAC-reactive IgM antibody-secreting cell (ASC) numbers were increased in the spleen and bone marrow of d14 J17A4-immunized mice relative to unimmunized controls (Figure 1g). Collectively, these findings indicate specific involvement of GAC-specific B-cell responses during early life in delaying T1D development.
We therefore investigated a role for B cells in dampening diabetogenesis by neonatal immunization with GAS. Like we showed previously in C57Bl/6 mice (35), GAC-binding (GAC+) B cells were enriched in the peritoneal cavity (PerC) of 10- to 12-week-old naive NOD mice and exhibited predominantly B220loCD5+CD43+CD23− (B-1a), B220loCD5−CD43+CD23− (B-1b), and to a lesser extent B220+CD5−CD43−CD23+ (B-2) B-cell phenotypes (Figure 1h). The numbers of PerC localized GAC+ B cells were increased five-fold in mice immunized with GAS (J17A4) as neonates (Figure 1i) and were skewed towards a B-1b phenotype that exhibited increased expression of CD11b (Figure 1j). Likewise, innate-like (B-1 and MZ) GAC+ B cells, typified by increased CD36 and CD9 expression were increased in spleens of d14 J17A4-immunized mice (Figure 1k).
GAC+ B-1b B cell expansion in NOD mice immunized with GAS as neonates was accompanied by expansion of B cell clones expressing the IdI-1 idiotope (Figure 1k), previously associated with a subset of GlcNAc-specific IGHV6 (VH-J606) encoded antibodies (17, 35, 36). Sequencing the immunoglobulin heavy-chain (IGH) genes of single FACS-sorted GAC+ B cells, isolated from 10- to 12-week-old naïve and d14 J17A4-immunized NOD mice, revealed that similar to other mouse strains (30, 37), GAC+ B cells in NOD mice were pauciclonal, >80% expressed IGHV6–3 genes, and GAC+ B-cell IGH gene sequences were predominantly germline with little somatic mutation, consistent with their early emergence as B-1 B cells in ontogeny (Figure 1m) (35). Relative to GAC+ B cells of naïve mice, which expressed predominantly IGHV6-3 D1-1 J2 and IGHV6-3 D3-3 J2 rearrangements, several notable V(D)J configurations, including IGHV6-3 D2-5 J3 and IGHV6-3 D2-4 J3 BCR, were enriched in and conserved between individual d14 J17A4-immunized NOD mice (Figure 1l). Collectively, these data indicate that neonatal immunization with GAS leads to altered clonal selection in the GAC-reactive B-cell repertoire and enhances clonal expansion of otherwise low-frequency GAC-reactive B-1b B-cell clonotypes.
GAC-reactive monoclonal antibodies (MAbs) recognize secretory granule-associated GlcNAc epitopes in pancreatic β cells
Considering involvement of self-reactive IgM antibodies in regulating autoimmunity (26-28), and the potential for GAS-specific antibodies to engage endogenous GlcNAc-containing PTMs (10), we initially explored the potential for a panel of hybridoma-derived GlcNAc-specific MAbs with distinct IG-gene composition and GAC reactivity profiles to bind GlcNAc PTMs in β cells (29-32). Intriguingly, we found that the GAC-reactive MAb HGAC78 (μ, IGHV6-3) exhibited robust reactivity for islets purified from human and mouse pancreata (Figure 2a). We used a mammalian glycan microarray to further probe the endogenous glycan specificity of the GAC-reactive MAbs HGAC78 and HGAC41, relative to the O-GlcNAc-specific MAbs CTD110.6 and RL2 (γ1, IGHV1-54). Each MAb exhibited exquisite specificity for GlcNAc-terminating carbohydrate structures and were particularly reactive with monomeric GlcNAc as well as GlcNAc-β-1,4- and GlcNAc-β-1,6-terminating glycans (Figure 2b), Importantly, most structures bound in microarray analysis represented cryptic epitopes, generally contained on the interior of mature carbohydrates. Further, there were subtle differences in the binding profiles of individual MAbs suggesting heterogenous fine epitope specificity amongst the B cell clonotypes which comprise the GlcNAc-specific B cell repertoire. GlcNAc-specific antibodies appeared to bind a subset of insulin+ granule-like puncta (Figure 2a), in a manner strongly inhibited by adding soluble GlcNAc monomers (data not shown). Further analysis of fractionated MIN6 post-nuclear supernatants confirmed HGAC78 reactivity correlated closely with insulin secretory granule (ISG) abundance, as measured by ELISA with anti-insulin (clone 7CD9) and anti-glutamic acid decarboxylase 65 (GAD65) MAbs (Figure 2c). Further supporting this notion, ELISA and SPR analysis revealed the O-GlcNAc-specific MAb CTD110.6 did not react with GAC-associated GlcNAc epitopes, despite exhibiting a higher affinity for GlcNAc-haptenated BSA antigens than anti-GAC clones. (Figure 2d). Interestingly lack of apparent ISG reactivity exhibited by CTD110.6 correlated with lack of binding to GAC.
Figure 2. GAS-induced antibodies bind to β-cell GlcNAc-PTMs but do not delay T1D development.
(a) Representative immunofluorescence image depicting MAb HGAC78 (green) reactivity with a subset of insulin-positive (7CD9, red) granule like structures in human islets; scale bar = 50μm. (b) Mammalian glycan microarray analysis reveals reactivity for β-GlcNAc-terminating glycans exhibited by GAS-induced MAbs HGAC78,HGAC41, and non-GAC binding CTD110.6 and RL2); data are means from triplicate experiments, column dendrograms illustrate similarity in MAb binding profiles. (c) Insulin secretory granules (ISGs) were isolated from MIN6 insulinoma cell post-nuclear supernatant by ultracentrifugation and fractionated with an automated fraction collector. Fractions were assayed by ELISA for (Top) ISGs using antibodies specific for insulin and glutamic acid decarboxylase 65 (GAD65) and (Bottom) GlcNAc post-translational modifications using GlcNAc-specific MAbs HGAC78 and CTD110.6. (d) Surface plasmon resonance analysis of HGAC78 and CTD110.6 MAb affinity for GlcNAc35BSA and GlcNAc-containing GAC. (e) Schematic depicting the model utilized to examine islet-localization of passively administered MAbs in STZ-treated mice. (f) Representative immunofluorescence images depicting IgMa-staining in insulin+ islets, and colocalization of IgMa with complement components C1q and C3b in control IgM-treated (top) and HGAC78-treated (bottom) C57BL/6 Rag1 KO mice; scale bar denotes 50μm. (g) Quantification of IF-signal intensities of from control IgM− or HGAC78-treated following STZ treatment. Data are mean pixel intensity ± s.e.m. from one-representative experiment, repeated twice, with n=3 mice/group, and 3–4 islets/mouse, ns (not significant), *p<0.05, **p<0.01, ***p<0.001, by two-tailed students t test. (h) Schematic depicting the production protocol used to generate anti- GAC-sera. (i) Quantitation of donor GAC-reactive sera in hyperimmunized mice. (j) Schematic depicting the administration protocol of anti-sera or monoclonal MAb HGAC78 to naïve NOD mice to examine the impact on spontaneous T1D until 30-weeks of age. (k) T1D incidence in antisera-treated mouse cohorts; (Upper left) T1D incidence in mice treated with sera pooled from naïve NOD mice (white) is plotted relative to untreated naïve mice (black), (Upper right) T1D incidence in mice treated with pooled J17A4 antisera (red, filled), and GlcNAc-adsorbed J17A4 antisera (red, empty) is plotted relative to mice treated with sera pooled from naïve NOD mice (white), (Lower left) T1D incidence in mice treated with pooled OK-432 antisera (blue) is plotted relative to mice treated with sera pooled from naïve NOD mice (white), (Lower right) Analysis of spontaneous T1D incidence in monoclonal antibody-treated mouse cohorts; incidence in mice treated with GlcNAc-reactive IgM MAb HGAC78 (green) is plotted relative to mice treated with isotype control MAb A16 (specificity, Dextran) (purple) and untreated naïve mice (white). Data are pooled from 2-3 independent experiments; statistical comparison by Mantel-Cox Log-rank test.
In summary, GlcNAc-specific antibodies elicited by GAS immunization bound epitopes associated with ISG that most-likely represented cryptic epitopes generated during autophagosomal degradation of mature carbohydrates.
We next investigated whether GlcNAc-specific antibodies elicited by GAS immunization may serve to promote clearance of apoptotic β cells during pancreatic remodeling similar to previously reported natural antibody activity (33). We first explored this possibility by evaluating GlcNAc-specific antibody binding to MIN6 insulinoma cells that were treated with apoptosis-inducing agents, relative to live cells. HGAC78 exhibited no surface reactivity with live MIN6 cells detectable by flow cytometry, despite exhibiting robust binding to intracellular antigens following cell permeabilization. (Supplement Figure 1a). Conversely, we observed dramatic increases in GlcNAc epitope accessibility on apoptotic MIN6 cells following irradiation (Supplement Figure 1b). Binding of GAC-specific MAbs HGAC41 and HGAC78 to irradiated MIN6 cells coincided with Annexin-V and activated caspase-3 staining, was inhibited by addition of soluble GlcNAc but not GalNac, and was superior to that of the O-GlcNAc-specific MAb CTD110.6 (Supplement Figure 1d).
We next examined whether anti-GlcNAc antibodies engaged apoptotic β cells in vivo using a protocol of high-dose STZ treatment to synchronously induce β-cell apoptosis in C57BL/6 Rag1 KO mice following passive administration of allotype-marked HGAC78 or control IgMa MAb (Figure 2e). Similar to our in-vitro analyses, we detected robust IgM deposition in pancreatic islets of STZ-treated mice administered HGAC78, but not in HGAC78+vehicle control treated mice, nor in mice administered a control IgM antibody (Figure 2f,g). Moreover, IgMa deposition in HGAC78+STZ treated mice coincided with staining for early complement components C1q and C3b, indicating HGAC78 mediated classical complement pathway activation on apoptotic β cells (Figure 2f). Although islet-localized C1q was clearly increased in the presence of HGAC78, C3b staining intensity did not differ between STZ-treated mice, suggesting that innate pathways mediated complement activation on apoptotic β cells in the absence of serum antibodies. In further support of this possibility, flow-cytometric analysis of irradiated MIN6 cells incubated in 10% C57BL/6 Rag1 KO mouse serum revealed robust deposition of complement component C3b in the absence of detectable C1q binding. Chelation of either Ca2+ or Mg2+ abrogated C3b deposition specifically implicating the lectin-dependent and alternative complement pathways, respectively, in antibody-independent opsonization of apoptotic β cells (Supplement Figure 1f). Surprisingly, pre-opsonization of irradiated MIN6 cells with HGAC78 did not affect C1q binding (Supplement Figure 1g) but diminished detectable C3b deposition compared to MIN6 cells pre-treated with control IgM, in which complement activity presumably stemmed from innate pathways (Supplement Figure 1g,h). We reasoned that serum IgM may compete with GlcNAc-reactive serum lectin opsonins for apoptotic β cell-associated substrates, leading to differential production of C3-derived ligands. Indeed, pre-incubation of MIN6 cells with 10% normal mouse serum partially inhibited HGAC78 binding to apoptosis-associated β-cell epitopes, an effect that was recapitulated with recombinant GlcNAc-reactive innate serum lectins human ficolin-3 (rHuFic-3) and mouse mannose binding lectin-2 (rMoMBL-2) (Supplement Figure 1i).
We next used PKH-26 membrane-tracking dye to label apoptotic MIN6 cells and analyze the effect of HGAC78 opsonization on their uptake by bone marrow-derived dendritic cells (BMDCs) in the presence or absence of complement. These results clearly demonstrate that pre-opsonization by HGAC78 promotes significant complement-dependent increases in PKH-26+ MIN6 cell-bearing BMDCs, relative to pre-treatment with control IgM (Supplement Figure 1j,k Moreover, these data imply that classical complement pathway activation by HGAC78 mediates more-efficient uptake of apoptotic β cells than does C3b deposited via innate pathways.
Lastly, we sought to investigate whether passive administration of GlcNAc-specific antibodies to NOD mice was able to suppress spontaneous T1D in vivo. Antisera was collected from NOD mice following a neonatal prime-adult boost immunization strategy with either GAS J17A4 or OK-432 vaccine preparations, as well as serum from naïve NOD donors (Figure 2h). Serum collected from GAS-immunized mice contained GAC-specific IgM at concentrations increased >300-fold over that of naïve serum (Figure 2i). We pooled serum from each donor group and normalized these preparations by their total IgM content, before administering 6 independent doses of each IgM pool to NOD pups between 7-21 days of age (Figure 2j). Based on quantified concentrations of GAC-specific IgM present in these preparations, we estimated that serum preparations from naïve, GAS J17A4 and GAS OK-432 immunized mice provided 0.001ug, 7.5ug, and 12ug of GAC-reactive antibodies per treatment, respectively. However, we were unable to reproducibly demonstrate that passive administration of polyclonal serum Ab enriched for GlcNAc-specific IgM reduced T1D penetrance (Figure 2k). Similarly, administration of the GlcNAc-specific MAb HGAC78 to naïve NOD pups also did not appear to affect penetrance of T1D relative to mice treated with control IgM (Figure 2j, lower right). Thus, although GlcNAc-reactive B-cell responses to GAS appear to significantly delay T1D protection potentially by facilitating efficacious clearance of apoptotic β cells and thereby limiting T-cell activation, we could not demonstrate that passive serum anti-GlcNAc antibodies were sufficient to dampen spontaneous T1D in NOD mice.
GAC-reactive monoclonal antibodies (MAbs) inhibit T cell activation ex vivo
To determine whether antibody opsonization impacted subsequent presentation of β cell-derived antigens we co-cultured antigen-loaded BMDCs with CFSE-labeled diabetogenic NOD.BDC2.5 T cell receptor transgenic T cells (22, 34). Islet cell-loaded BMDCs stimulated moderate BDC2.5 T cell activation, however, pre-opsonization of islet cells with HGAC78 and other anti-GlcNAc mAbs significantly decreased frequencies of CFSEloBDC2.5 T cells (Figure 3a,b). As expected, BMDCs loaded with mimotope potently stimulated proliferation and TNFα production by BDC2.5 T cells relative to those loaded with an irrelevant control peptide. By contrast, HGAC78 did not affect activation of T cells stimulated by BMDC loaded with the BDC2.5 TCR mimotope (Figure 3c), suggesting that HGAC78 specifically modulated the uptake and/or processing of islet cell antigens by APC.
Figure 3. GAC-reactive MAbs inhibit T cell activation ex vivo.
(a) Representative flow-cytometric profiles of TNF-α staining and CFSE-dilution in NOD.BDC2.5 T cells following stimulation with an irrelevant control peptide (InsB9-23) or primary islet cells, in the presence of control IgM or HGAC78 IgM. (b) Fold-change in BDC2.5 T-cell activation under GlcNAc-specific MAb pre-opsonized conditions relative to control Ig conditions; data are means± s.e.m of four replicates, from one-representative experiment repeated three times; *p<0.05, **p<0.01, ***p<0.001, by one-way ANOVA. (c) Representative flow-cytometric profiles of intracellular staining for TNF-α production and cellular cell division by CFSE-dilution in NOD.BDC2.5 T cells following stimulation by BMDCs loaded with an irrelevant control peptide (InsB9-23) or the BDC2.5 T-cell mimotope in the presence of control IgM or HGAC78 IgM. (d) Representative flow-cytometric profiles depicting analysis of CFSE-dilution and TNFα production by NOD.BDC2.5 T cells following stimulation with BMDCs loaded with islet cells under opsonizing conditions with the indicated GlcNAc-specific MAb and appropriate isotype-control antibodies. (e) Frequency of TNF-α+CFSElo activated BDC2.5 T cells under the indicated conditions; data are means± s.e.m. (f) (Top) Representative flow-cytometric analysis of CD25+Foxp3+ CD4+ T regulatory cells (Treg) in spleen, inguinal lymph node (ILN), pancreatic lymph node (PanLN) and pancreatic-tissue digests of Helios+ natural Tregs. Enumeration of (g) total Treg cell and (h) percentage of Helios+ natural Tregs in each tissue of naïve (n=8) and d14 GAS-immunized (n=8) 10-week-old NOD mice; data are mean ± s.d. pooled from two independent experiments, with individual data points overlaid. Statistical analysis by two-way ANOVA.
Other GlcNAc-specific MAbs, including HGAC41, CTD110.6, and RL2 similarly suppressed BDC2.5 T-cell activation and TNFα production. In contrast HGAC39 (γ3, IGHV6-6), which did not interact with apoptosis-associated β-cells, was unable to mediate suppression of T cells towards their islet antigenic cargo HGAC39 (γ3, IGHV6-6) (Figure 3b), associating suppressive activity with specific antigen reactivity (Figures 3b,d,e)
The abundance of Foxp3+CD25+ regulatory T cells (Treg) as well as the frequency of Treg cells expressing Helios in pancreatic tissue and draining pancreatic lymph nodes was not influenced by neonatal immunization with GAS, indicating that neonatal immunization with GAS did not suppress T1D pathogenesis through the induction of Treg cells Figures 3f,g,h. Further analysis of 12 immunomodulating cytokines revealed no significant differences in serum concentrations between mice immunized with GAS as neonates, and GCS-immunized or naïve control mice (Supplement Figure 2).
Thus, delayed development of T1D in NOD mice following neonatal immunization with GAS is associated with the enhanced production of GlcNAc-reactive B cells and little or no apparent direct T cell mediated suppression.
GAC-reactive B-1 B cells infiltrate the pancreas during T1D
When we directly examined pancreas-infiltrating B cells in 10- to 12-week-old pre-diabetic NOD mice, we found that the numbers of GAC+ B cells were dramatically increased in pancreatic-tissue digests and pancreatic lymph nodes (PanLN) when NOD mice were immunized with GAS J17A4 as d14 neonates relative to the ILN’s (Figure 4a). By contrast, phosphorylcholine-binding B cells, a specificity largely restricted to B-1 B cells, were not enriched in the pancreas from naive NOD mice), nor were GAC+ B cells detected in non-diabetic C57BL/6 mouse pancreas (Figure 4g). Collectively, these results demonstrate that specific recruitment of GlcNAc-reactive B-1 B cells to the pancreas was associated with diabetogenesis initiation.
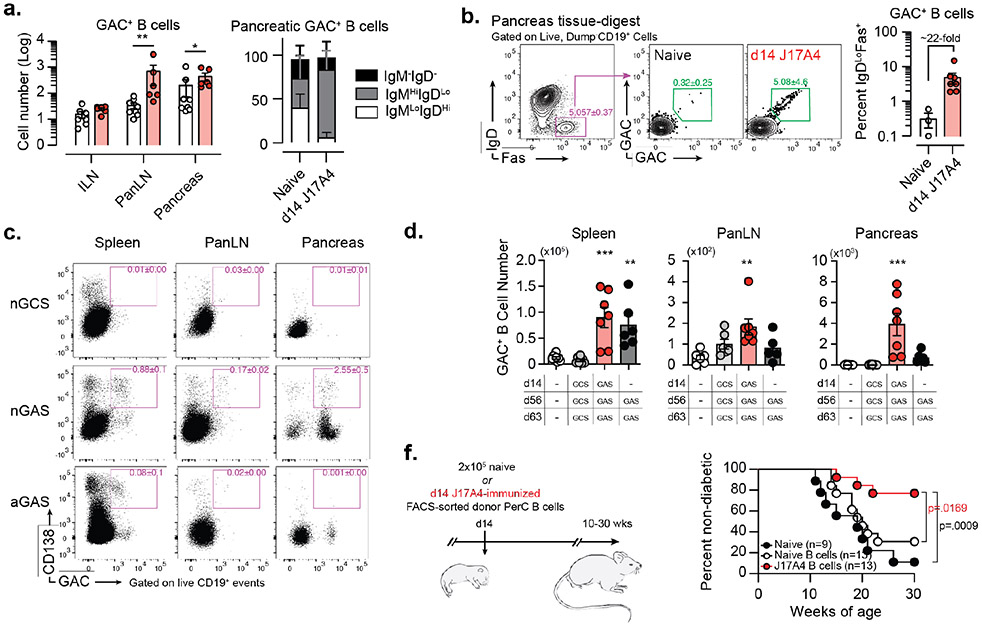
Figure 4. GAC-reactive B-1 B cells localize to the pancreas during spontaneous T1D pathogenesis and inhibit T1D progression in naïve NOD mice.
(a) GAC+ B-cell numbers from ILN, pancreatic LN (PanLN) and pancreatic-tissue digests (left), + surface Ig-isotype distribution of pancreatic GAC+ B cells (right) in pre-diabetic 10-12-week-old naïve (white, n=9) and J17A4 (red, n=6) NOD mice. Data are mean ± s.e.m. pooled from two independent experiments; *p<0.05, **p<0.01, by one-tailed Mann-Whitney test. (b) Representative flow-cytometry profiles depicting GAC+ B cell staining of IgDloFas+-activated B cells in pancreas tissue digests and mean ± s.e.m from naïve (n=3) and d14 J17A4-immunized (n=7) mice. Data are pooled from two independent experiments. (c) Representative flow-cytometry profiles depicting GAC+ plasmablasts (CD138+) staining in spleen, pancreatic lymph node (PanLN), and pancreas-tissue digests in mice hyperimmunized with GCS (nGCS) or GAS (nGAS) following neonatal priming at d14, and mice hyperimmunized with GAS in the absence of neonatal priming (aGAS). (d) Total GAC+ B-cell numbers in spleen, PanLN, and Pancreas of hyperimmunized NOD mice; data are mean ± s.e.m. pooled from two independent experiments; **p<0.01, ***p<0.001, by one-way ANOVA with Tukey’s multiple comparisons. (e) (Left) Schematic depicting strategy to adoptively transfer PerC B cells to naïve NOD recipients, and (Right) spontaneous T1D incidence in adult NOD mice following neonatal engraftment of naïve adult NOD B cells (n=13), or d14 GAS-immunized adult NOD B cells (n=13), relative to untreated NOD mice (n=9); data are pooled from three independent experiments, exact p values determined by log-rank mantel-cox test are shown.
Group A Carbohydrate-reactive B cells are specifically recruited to pancreatic tissues and activated by beta cell antigens. (f) Frequency of GAC+ B cells represented in the CD19+ B cell compartment of Inguinal lymph node (ILN), Pancreatic lymph node (PanLN) and Pancreas of 10-12 week old naïve (n=8) and J17A4-immunized (n=6) NOD mice. Data are mean ± s.e.m. pooled from two independent experiments; **p<0.01, by two-way ANOVA. (g) Numbers of pancreas infiltrating phosphorylcholine-reactive (PC+) and GAC+ B cells in 10-12 week old naïve C57BL/6 (black) and NOD (white) mice; data are mean ± s.e.m. from one representative experiment, repeated twice, with n=3 per group. (h) (Left) Representative flow cytometric analysis of IL-10 production by total CD19+ B cells isolated from the Spleen, PerC, ILN, PanLN or pancreatic tissue digests of naïve (top) and d14 GAS-immunized (bottom) 10-12-week-old female NOD mice. (Right) Quantification of mean frequencies of IL-10 producing B cells represented in each tissue of naïve (n=4, white) and d14 GAS-immunized (n=3, red); data are mean ± s.e.m. with individual data points overlaid from one representative experiment completed twice.
Unlike the pancreas-infiltrating GAC+ B cells present in naïve NOD mice that exhibited predominantly IgD+ phenotypes, those present in neonatally J17A4-immunized mice were almost exclusively IgD−/loIgM+ (Figure 4a) and highly enriched in a population of IgD−IgM+Fas+ GAC-binding B cells (Figure 4b,left), indicating in-situ activation. The frequency of these activated GAC+ B cells, uniquely localized to the pancreas, was increased >20-fold by prior neonatal d14 immunization (Figure 4b right). Although GAC+ B cells in d14 GAS-immunized mice exhibited signs of activation in pancreatic tissue, we did not detect significant differentiation of pancreas-localized GAC+ B cells into CD138-expressing antibody secreting cells.
We sought to understand the mechanisms by which GAC+ B cells modulated diabetogenesis in pancreatic tissue. Relative to mice immunized with GCS via the neonatal prime:adult boost regimen, GAS-immunized mice exhibited increased GAC+ B-cell numbers in the spleen, moderate increases in the PanLNs, and a 20-fold increase in pancreatic-tissue digests. Additionally, more GAC+ ASCs were found in both PanLN and pancreas relative to control mice. By contrast, secondary and tertiary GAS vaccinations in the absence of perinatal priming gave rise to many fewer GAC+ B cells and GAC+ plasmablasts in the pancreas and PanLN, whereas ASC abundance within the spleens was equivalent between each mouse group (Figures 4c-d).. These results suggest that the decrease in diabetogenesis of NOD mice immunized with GAS as neonates correlated with the striking propensity of GAC+ B cells generated by neonatal B-cell responses to localize in NOD mouse pancreatic tissue.
To examine this possibility further we adoptively transferred FACS-sorted PerC B cells from d14 GAS immunized or from naïve adult NOD mice donors i.p. to neonatal NOD recipient mice (Figure 4e). Mice that were adoptively transferred with B cells from naïve donors exhibited T1D incidence that did not differ significantly from that of unmanipulated NOD mice. However, mice that received B cells from d14 GAS immunized donors exhibited a significantly reduced incidence of T1D by 30 weeks-of-age (Figure 4e). Collectively these results indicate that the delay in T1D development following neonatal immunization with GAS is linked to enhanced localization of GAC binding B cells in the pancreas of NOD mice. Because B-1 B cells have been shown to suppress the development of autoimmune disease through the production of immunosuppressive cytokines, such as IL-10, we sought to investigate whether neonatal immunization with GAS modulated the capacity of GAC+ B cells to produce IL-10. Interestingly, the overall GAC+ B cell compartment was enriched for IL-10-producing cells relative to the bulk CD19+ B cell compartment, with ~50% of GAC+ B cells staining for intracellular IL-10 following ex vivo stimulation. GAC+ B cells represented in the spleen and PerC were similar in their ability to produce IL-10. However, when we compared naïve mice with those immunized with GAS as neonates, the relative frequency of IL-10-producing GAC+ B cells was not significantly affected by immunization (Figure 4h). (Nevertheless, because GAC+ B cells were expanded following neonatal immunization it stands to reason that the overall numbers of Il10 producing GAC+ B cells is increased. By extension one could reason that because of their increased propensity to localize to the to the pancreas during diabetogenesis that there is a greater likelihood that they will produce IL10 in situ.
Discussion
Observations presented herein portray a novel scenario in which exposure to exogenous microbial antigens during perinatal life stimulates T1D-inhibitory B cell clonotype development. T1D protection following early immunization with GAS was associated with significant increases in GlcNAc-reactive serum antibody levels and B-1 B cell numbers, as well as more efficient localization of GlcNAc-reactive B cells to pancreatic tissue during T1D pathogenesis. B lymphocytes play a critical role in the pathogenesis of T1D through their antigen-presenting capabilities (38, 39). While this function is likely key to the formation of pancreas-localized ectopic lymphoid follicles (25) and the priming of T cells that ultimately kill β cells (40), our data indicate that PTM-reactive innate-like B cells may subvert this processes during the early phases of T1D initiation.
Formation of immunologically relevant PTM-associated epitopes driven by constitutively high levels of ER stress in β cells may contribute to failure of peripheral tolerance during T1D pathogenesis (41). O-GlcNAc protein-modifications are not only represented at uniquely high levels in β cells relative to other tissues (42), but aberrant O-GlcNAcylation is associated with β cell stress and dysfunction during T2D (43, 44). Our findings reveal a potential interplay between innate pattern recognition receptors and B cell clonotypes during immune recognition of GlcNAc modifications represented on a subset of insulin secretory granules and apoptosis-associated β cell epitopes.
Indeed, GlcNAc epitopes are known to serve as damage-associated molecular patterns to facilitate recognition of apoptotic cells by macrophages during efferocytosis (45). Perhaps of significance in this context, NOD mouse macrophages, exhibit defective efferocytosis, leading to reduced clearance of senescent cells and potentiating induction of adaptive immune responses towards β cell-associated antigens (46, 47). Although waves of β cell apoptosis occurring through pancreatic remodeling during early life have long been considered an initiation point for T1D pathogenesis (40), recent reports describing early type-1 interferon signatures in T1D patients, similar to those observed during apoptotic cell-specific immune responses of SLE patients, may support this concept (48). Furthermore, Batf3-dependent DCs that drive potent antigen cross-presentation and are essential to T1D pathogenesis express a diverse array of glycan-specific innate receptors (49-51). Effective clearance of glycosylated autoantigens may therefore represent a key step in subverting T1D pathogenesis and maintaining tolerance towards β cell antigens.
Our studies indicate that GlcNAc-specific antibodies act analogously to natural IgM to promote maintenance of tolerance to β cells. Although present in naïve mice in the absence of deliberate immunization, early immunization with GlcNAc-bearing Group A Streptococcus leads to long-lasting increases in the serum concentrations of GlcNAc-specific antibodies. Similar to previously reported natural IgM antibodies (33), certain GlcNAc-reactive IgM antibody clonotypes engage neoepitopes generated during β-cell apoptosis and initiate classical complement pathways, leading to significant increases in the phagocytosis of apoptotic β-cell fragments by dendritic cells.
Apoptosis-associated carbohydrate epitopes additionally are substrates for innate complement activation on β cells, and GlcNAc-reactive serum antibodies may exist in competition with the GlcNAc-reactive serum opsonins ficolin and mannose-binding lectin. Although the differences in classical versus innate complement activation on the immunological outcome of β cell engagement remain unclear, we find that, relative to the classical complement pathway, C3b-deposition driven by innate pathways is inefficient at promoting β-cell uptake by BMDC. Differential processing of C3b can drastically affect trafficking of opsonized antigens, with generation of C3dg/C3d opsonins facilitating adaptive immune responses by targeting antigens to follicular dendritic-cell networks (52).
The role of complement activation in T1D therefore requires further investigation. Although often ignored due to the absence of functional C5 genes in NOD mice (53), early components of the complement system remain relevant to T1D initiation, and expression of the complement receptor from the Immunoglobulin super-family (CRIg), as well as the polysaccharide-specific C4 opsonin and other complement system genes by intra-islet macrophage was recently correlated with slowing of T1D development in NOD mice (54). Human T1D patients similarly exhibit dysregulated serum concentrations of early complement components, and post-mortem tissue specimens from T1D patients demonstrate clear signs of islet-localized complement activation (55, 56). Although our studies indicate that glycan epitopes serve as important ligands for innate immune system engagement, a better understanding of the driving forces and outcome of β cell-targeted complement activity is needed.
That GlcNAc-specific IgM mediated suppression of diabetogenic CD4+ T cell activation in the absence of complement activity indicates an additional mechanism of immune modulation likely occurs at the point of DC antigen engagement. NAbs can attenuate inflammatory signals driven by innate receptors in DCs (57), and glycan-bearing antigens can elicit potent cell-mediated immunity through engagement of various lectin receptors on DCs (58, 59). Thus, the natural antibody repertoire content during early life may significantly impact immune system responsiveness to PTMs presented on T1D-associated autoantigen.
B-1 cell numbers induced by neonatal immunization were increased in the pancreas and PLN and were sufficient to inhibit progression of T1D upon adoptive transfer to naive NOD mice. However, neither passively administered immune sera nor anti-GlcNAc mAb were found to inhibit the progression of T1D. How can these findings be reconciled with the clear demonstration of passively administered anti-GlcNAc mAb binding to β cells in vivo following streptozotocin treatment? Interpretation of these results may be impacted by the ability of streptozotocin to induce islet blood hyperperfusion and vascular permeability (60, 61), thereby permitting access of passively administered IgM antibody to islets which may not occur during spontaneous diabetogenesis of NOD mice.
Further, we have shown that passively administered mAb HGAC78 is rapidly depleted from circulation in mice, presumably due to target-mediated disposition resulting from accessible GlcNAc epitopes, which likely precluded reaching sufficient local concentrations in pancreatic tissue to dampen T1D. On the other hand, GlcNAc-specific B cells bearing IgDlo Fashi phenotypes that enrich in pancreatic tissue may mediate the immunosuppressive effect via in situ secretion of IgM. Although we were unable to demonstrate expression of the canonical plasma cell marker CD138 by these cells, recent demonstration of important protective roles for CD138-negative non-proliferating B-1 cells in lung in mouse models of influenza virus infection expression via their secretion of IgM highlight the likelihood that as of yet poorly understood subsets of plasma cells contribute to immunological outcomes in vivo (62). It remains possible that B-1 B cells delay development of T1D in NOD mice through other mechanisms. For example, receptors for the metabolite γ-aminobutyric acid (GABA) expressed by T cells are implicated in T1D progression in the T1D-prone BB rat (63), and more-recent studies have reported that B cell- or plasma cell-derived GABA can dampen CD8+ and CD4+ T cells inflammatory responses in mouse models of cancer (64).
Thus, it is possible that soluble mediators beyond antibodies are produced by GlcNAc positive B-1 bells and are implicated in dampening of T1D in NOD mice and warrant further investigation.
Collectively, our observations indicate a significant involvement of cryptic glycan epitopes in immunological recognition of senescent β cells, and that clonal composition of the GlcNAc-specific B cell repertoire during early life can significantly influence immune responsiveness to T1D autoantigens. Early exposure to GlcNAc-bearing microorganisms may represent an important environmental factor that influences T1D progression in humans. Although maturation of GlcNAc-specific B cell responses in humans is delayed relative to initiation of T1D pathogenesis, we postulate that NAb repertoire defects precede emergence of β cell-specific autoantibodies associated with and currently used to determine the risk of T1D progression.
Supplementary Material
Key Points.
Neonatal but not adult immunization with Group-A Streptococcus delays T1D in NOD mice
Immunization enrichs B1 cells reactive to apoptotic β cells in the pancreas
These expanded B1 cells delay T1D progression by multiple immunological mechanisms
Acknowledgements
We would like to extend a special thanks to Lisa Jia and Dr. Jeffrey Sides for technical assistance and Enid Keyser (UAB consolidated flow cytometry core (CFCC) for her assistance and expertise in cell sorting. Special thanks to Dr. Denise Kaminski for expert editing. Glycan microarray analysis was completed through the Consortium for Functional Glycomics (Protein-Glycan Interaction Core, Emory University), This research is part of the doctoral dissertation research conducted by J.S.N. and B.L.P.D. in the Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294.
This work was supported by US National Institutes of Health (NIH) grants R01 AI4782 (J.F.K.), F31 AI120500 and T32 A1007051 (J.S.N.), and F30 DK082277 and T32 GM008861 (B.L.P.D), and Juvenile Diabetes Research Foundation grants 2-SRA-2014-300-Q-R and 17-2012-123 (J.F.K.). NIH P30 AR048311 and P30 AI027767 provided support for the UAB CFCC, and G20 RR022807-01 provided support for the UAB Animal Resources Program X-irradiator.
Footnotes
Competing financial interests
The authors have no financial conflict of interest to disclose.
References
- 1.Bach JF, and Chatenoud L. 2012. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb Perspect Med 2: a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathis D, and Benoist C. 2012. The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol Rev 245: 239–249. [DOI] [PubMed] [Google Scholar]
- 3.Satoh J, Shintani S, Oya K, Tanaka S, Nobunaga T, Toyota T, and Goto Y. 1988. Treatment with streptococcal preparation (OK-432) suppresses anti-islet autoimmunity and prevents diabetes in BB rats. Diabetes 37: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 4.Toyota T, Satoh J, Oya K, Shintani S, and Okano T. 1986. Streptococcal preparation (OK-432) inhibits development of type I diabetes in NOD mice. Diabetes 35: 496–499. [DOI] [PubMed] [Google Scholar]
- 5.Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, Normier G, Chatenoud L, Thieblemont N, and Bach JF. 2006. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55: 179–185. [PubMed] [Google Scholar]
- 6.Tenconi MT, Devoti G, Comelli M, Pinon M, Capocchiano A, Calcaterra V, Pretti G, and Pavia TDMRG. 2007. Major childhood infectious diseases and other determinants associated with type 1 diabetes: a case-control study. Acta Diabetol 44: 14–19. [DOI] [PubMed] [Google Scholar]
- 7.Uloha AI, and Lialikau SA. 2003. [The relationship between insulin-dependent diabetes mellitus and acute infections in children]. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw 9: 73–76. [PubMed] [Google Scholar]
- 8.Emmrich F, Schilling B, and Eichmann K. 1985. Human immune response to group A streptococcal carbohydrate (A-CHO). I. Quantitative and qualitative analysis of the A-CHO-specific B cell population responding in vitro to polyclonal and specific activation. The Journal of experimental medicine 161: 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner JR, Tartakoff AM, and Greenspan NS. 1990. Cytologic assessment of nuclear and cytoplasmic O-linked N-acetylglucosamine distribution by using anti-streptococcal monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America 87: 5608–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akimoto Y, Kreppel LK, Hirano H, and Hart GW. 1999. Localization of the O-linked N-acetylglucosamine transferase in rat pancreas. Diabetes 48: 2407–2413. [DOI] [PubMed] [Google Scholar]
- 11.Mowen KA, and David M. 2014. Unconventional post-translational modifications in immunological signaling. Nat Immunol 15: 512–520. [DOI] [PubMed] [Google Scholar]
- 12.Mc CM, and Lancefield RC. 1955. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. The Journal of experimental medicine 102: 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiter EH 1997. The NOD Mouse: A Model For Insulin-Dependent Diabetes Mellitus. Curr. Protoc. Immunol 24:15.9.1–15.9.23. [DOI] [PubMed] [Google Scholar]
- 14.Hutton JC, Wong R, and Davidson HW. 2009. Isolation of Dense Core Secretory Vesicles from Pancreatic Endocrine Cells by Differential and Density Gradient Centrifugation. Curr. Protoc. Cell Biol 42:3.32.1–3.32.20. [DOI] [PubMed] [Google Scholar]
- 15.Oliver AM, Grimaldi JC, Howard MC, and Kearney JF. 1999. Independently ligating CD38 and Fc gammaRIIB relays a dominant negative signal to B cells. Hybridoma 18: 113–119. [DOI] [PubMed] [Google Scholar]
- 16.Townsend SE, Goodnow CC, and Cornall RJ. 2001. Single epitope multiple staining to detect ultralow frequency B cells. J Immunol Methods 249: 137–146. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan NS, and Davie JM. 1985. Serologic and topographic characterization of idiotopes on murine monoclonal anti-streptococcal group A carbohydrate antibodies. Journal of immunology (Baltimore, Md. : 1950) 134: 1065–1072. [PubMed] [Google Scholar]
- 18.Tiller T, Busse CE, and Wardemann H. 2009. Cloning and expression of murine Ig genes from single B cells. J Immunol Methods 350: 183–193. [DOI] [PubMed] [Google Scholar]
- 19.Cortina-Ceballos B, Godoy-Lozano EE, Samano-Sanchez H, Aguilar-Salgado A, Velasco-Herrera Mdel C, Vargas-Chavez C, Velazquez-Ramirez D, Romero G, Moreno J, Tellez-Sosa J, and Martinez-Barnetche J. 2015. Reconstructing and mining the B cell repertoire with ImmunediveRsity. MAbs 7: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shugay M, Bagaev DV, Turchaninova MA, Bolotin DA, Britanova OV, Putintseva EV, Pogorelyy MV, Nazarov VI, Zvyagin IV, Kirgizova VI, Kirgizov KI, Skorobogatova EV, and Chudakov DM. 2015. VDJtools: Unifying Post-analysis of T Cell Receptor Repertoires. PLoS Comput Biol 11: e1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, and Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz JD, Wang B, Haskins K, Benoist C, and Mathis D. 1993. Following a diabetogenic T cell from genesis through pathogenesis. Cell 74: 1089–1100. [DOI] [PubMed] [Google Scholar]
- 23.Inaba K, Swiggard WJ, Steinman RM, Romani N, and Schuler G. 1998. Isolation of Dendritic Cells. Curr. Protoc. Immunol 3.7.1–3.7.15. [DOI] [PubMed] [Google Scholar]
- 24.Korpos E, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, Holmberg D, Cardell S, and Sorokin L. 2013. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 62: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendall PL, Yu G, Woodward EJ, and Thomas JW. 2007. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. Journal of immunology (Baltimore, Md. : 1950) 178: 5643–5651. [DOI] [PubMed] [Google Scholar]
- 26.Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology 11: 34–46. [DOI] [PubMed] [Google Scholar]
- 27.Bendelac A, Bonneville M, and Kearney JF. 2001. Autoreactivity by design: innate B and T lymphocytes. Nature reviews. Immunology 1: 177–186. [DOI] [PubMed] [Google Scholar]
- 28.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, and Silverman GJ. 2003. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 9: 736–743. [DOI] [PubMed] [Google Scholar]
- 29.Shikhman AR, Greenspan NS, and Cunningham MW. 1994. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. Journal of immunology (Baltimore, Md. : 1950) 153: 5593–5606. [PubMed] [Google Scholar]
- 30.Nahm MH, Clevinger BL, and Davie JM. 1982. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on Vk. Journal of immunology (Baltimore, Md. : 1950) 129: 1513–1518. [PubMed] [Google Scholar]
- 31.Comer FI, Vosseller K, Wells L, Accavitti MA, and Hart GW. 2001. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 293: 169–177. [DOI] [PubMed] [Google Scholar]
- 32.Snow CM, Senior A, and Gerace L. 1987. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 104: 1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrenstein MR, and Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nature reviews. Immunology 10: 778–786. [DOI] [PubMed] [Google Scholar]
- 34.Haskins K, Portas M, Bradley B, Wegmann D, and Lafferty K. 1988. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes 37: 1444–1448. [DOI] [PubMed] [Google Scholar]
- 35.New JS, Dizon BLP, Fucile CF, Rosenberg AF, Kearney JF, and King RG. 2020. Neonatal Exposure to Commensal-Bacteria-Derived Antigens Directs Polysaccharide-Specific B-1 B Cell Repertoire Development. Immunity 53: 172–186 e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz CT, Bartholow TL, Greenspan NS, Fulton RJ, Monafo WJ, Perlmutter RM, Huang HV, and Davie JM. 1987. Molecular dissection of the murine antibody response to streptococcal group A carbohydrate. The Journal of experimental medicine 165: 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenspan NS, Fulton RJ, and Davie JM. 1986. Analysis of anti-streptococcal group A carbohydrate idiotope levels in sera: correlation of magnitude of expression with idiotope position and VK haplotype. Journal of immunology (Baltimore, Md. : 1950) 137: 228–233. [PubMed] [Google Scholar]
- 38.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, and Tisch RM. 1998. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. Journal of immunology (Baltimore, Md. : 1950) 161: 3912–3918. [PubMed] [Google Scholar]
- 39.Marino E, Tan B, Binge L, Mackay CR, and Grey ST. 2012. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 61: 2893–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathis D, Vence L, and Benoist C. 2001. beta-Cell death during progression to diabetes. Nature 414: 792–798. [DOI] [PubMed] [Google Scholar]
- 41.van Lummel M, Zaldumbide A, and Roep BO. 2013. Changing faces, unmasking the beta-cell: post-translational modification of antigens in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 20: 299–306. [DOI] [PubMed] [Google Scholar]
- 42.Lubas WA, Frank DW, Krause M, and Hanover JA. 1997. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. The Journal of biological chemistry 272: 9316–9324. [DOI] [PubMed] [Google Scholar]
- 43.Hart GW, Slawson C, Ramirez-Correa G, and Lagerlof O. 2011. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80: 825–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardiville S, and Hart GW. 2014. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab 20: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duvall E, Wyllie AH, and Morris RG. 1985. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56: 351–358. [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien BA, Huang Y, Geng X, Dutz JP, and Finegood DT. 2002. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes 51: 2481–2488. [DOI] [PubMed] [Google Scholar]
- 47.Stoffels K, Overbergh L, Giulietti A, Kasran A, Bouillon R, Gysemans C, and Mathieu C. 2004. NOD macrophages produce high levels of inflammatory cytokines upon encounter of apoptotic or necrotic cells. J Autoimmun 23: 9–15. [DOI] [PubMed] [Google Scholar]
- 48.Planas R, Carrillo J, Sanchez A, de Villa MC, Nunez F, Verdaguer J, James RF, Pujol-Borrell R, and Vives-Pi M. 2010. Gene expression profiles for the human pancreas and purified islets in type 1 diabetes: new findings at clinical onset and in long-standing diabetes. Clin Exp Immunol 159: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, and Unanue ER. 2014. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 41: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, and Murphy KM. 2008. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shortman K., and Heath WR. 2010. The CD8+ dendritic cell subset. Immunol Rev 234: 18–31. [DOI] [PubMed] [Google Scholar]
- 52.Carroll MC 2004. The complement system in regulation of adaptive immunity. Nat Immunol 5: 981–986. [DOI] [PubMed] [Google Scholar]
- 53.Baxter AG, and Cooke A. 1993. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes 42: 1574–1578. [DOI] [PubMed] [Google Scholar]
- 54.Fu W, Wojtkiewicz G, Weissleder R, Benoist C, and Mathis D. 2012. Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nat Immunol 13: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Fillmore TL, Schepmoes AA, Clauss TR, Gritsenko MA, Mueller PW, Rewers M, Atkinson MA, Smith RD, and Metz TO. 2013. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. The Journal of experimental medicine 210: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowe P, Wasserfall C, Croker B, Campbell-Thompson M, Pugliese A, Atkinson M, and Schatz D. 2013. Increased complement activation in human type 1 diabetes pancreata. Diabetes Care 36: 3815–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Khanna S, Goodyear CS, Park YB, Raz E, Thiel S, Gronwall C, Vas J, Boyle DL, Corr M, Kono DH, and Silverman GJ. 2009. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. Journal of immunology (Baltimore, Md. : 1950) 183: 1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams EW, Ratner DM, Seeberger PH, and Hacohen N. 2008. Carbohydrate-mediated targeting of antigen to dendritic cells leads to enhanced presentation of antigen to T cells. Chembiochem 9: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh SK, Stephani J, Schaefer M, Kalay H, Garcia-Vallejo JJ, den Haan J, Saeland E, Sparwasser T, and van Kooyk Y. 2009. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Molecular immunology 47: 164–174. [DOI] [PubMed] [Google Scholar]
- 60.Carlsson PO, Flodstrom M, and Sandler S. 2000. Islet blood flow in multiple low dose streptozotocin-treated wild-type and inducible nitric oxide synthase-deficient mice. Endocrinology 141: 2752–2757. [DOI] [PubMed] [Google Scholar]
- 61.Beppu H, Maruta K, Kurner T, and Kolb H. 1987. Diabetogenic action of streptozotocin: essential role of membrane permeability. Acta Endocrinol (Copenh) 114: 90–95. [DOI] [PubMed] [Google Scholar]
- 62.Choi YS, and Baumgarth N. 2008. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med 205: 3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendu SK, Akesson L, Jin Z, Edlund A, Cilio C, Lernmark A, and Birnir B. 2011. Increased GABA(A) channel subunits expression in CD8(+) but not in CD4(+) T cells in BB rats developing diabetes compared to their congenic littermates. Mol Immunol 48: 399–407. [DOI] [PubMed] [Google Scholar]
- 64.Zhang B, Vogelzang A, Miyajima M, Sugiura Y, Wu Y, Chamoto K, Nakano R, Hatae R, Menzies RJ, Sonomura K, Hojo N, Ogawa T, Kobayashi W, Tsutsui Y, Yamamoto S, Maruya M, Narushima S, Suzuki K, Sugiya H, Murakami K, Hashimoto M, Ueno H, Kobayashi T, Ito K, Hirano T, Shiroguchi K, Matsuda F, Suematsu M, Honjo T, and Fagarasan S. 2021. B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature 599: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GAC+ B cell immunoglobulin heavy chain nucleotide sequence data are available through GenBank. Sequences for naïve and d14 GAS immunized NOD mice may be found at the accession numbers KY210724 - KY210801 and KY210802 - KY210877, respectively Mammalian glycan microarray data is publicly available through the Consortium for Functional Glycomics gateway. (http://www.functionalglycomics.org/static/index.shtml). The data that support the findings of this study are available from the corresponding author upon reasonable request.