Abstract
Given evidence pointing toward a role for immune dysregulation in the pathogenesis of schizophrenia, anti-inflammatory agents are promising adjunctive treatments that have potential to support a causal relationship for inflammation and psychopathology and lead to novel treatments for individuals. Indeed, previous meta-analyses have demonstrated small-to-medium effect sizes (ES) in favor of various anti-inflammatory agents, though there is significant heterogeneity and challenges in the interpretation of this literature. Identifying predictors, including sociodemographic variables, trial duration, and/or symptoms themselves, of successful anti-inflammatory trials may help identify which patients who might benefit from these compounds. We performed a meta-regression analysis of 63 adjunctive anti-inflammatory trial arms (2232 patients randomized to adjunctive anti-inflammatory agents and 2207 patients randomized to placebo).Potential predictors of effect size estimates for changes in psychopathology scores from baseline to endpoint included geography, trial duration, sample size, age, sex, race, smoking, body mass index, illness duration, age of onset of psychosis, study quality score and psychopathology scores (total and subscale) at baseline. Geography (β=0.31, p=0.011), smaller sample size (β=0.33, p=0.009), and higher study quality score (β=0.44, p<0.001) were significant predictors of larger ES estimates for change in total psychopathology in favor of anti-inflammatory agents. Smaller sample size (β=0.37, p=0.034) and higher study quality score (β=0.55, p=0.003) were significant predictors of larger ES estimates for change in negative psychopathology in favor of anti-inflammatory agents. Higher study quality score (β=0.46, p=0.019) was a significant predictor of larger ES estimates for change in general psychopathology in favor of anti-inflammatory agents. These findings should be interpreted with caution given concerns of publication bias regarding the geographic differences and small study effects. The lack of an association with other demographic variables should be seen as a primary limitation of the literature that needs to be considered in future studies. The association with study quality score suggests that future anti-inflammatory trials must consider demographic variables known to be associated with inflammation (e.g., BMI and smoking) and evidence of increased baseline inflammation should be incorporated in study design. Moreover, evidence of target engagement and endpoints thoughts to be associated with increased inflammation should be considered as well.
1. Introduction
The immune system has been implicated in the pathogenesis of schizophrenia (Miller and Goldsmith, 2017) with evidence ranging from epidemiological studies (Brown and Derkits, 2010; Khandaker et al., 2013), genetic studies (Hudson and Miller, 2018; Stefansson et al., 2009; Williams et al., 2022), post-mortem studies (North et al., 2022), as well as studies of protein concentrations of cytokines in cerebrospinal fluid (Gallego et al., 2018) and peripheral blood (Goldsmith, 2016; Miller et al., 2011; Upthegrove et al., 2014). Specifically focused on markers of inflammation, inflammatory cytokines have been shown in be elevated during both acute and chronic phases of illness (Goldsmith et al., 2016), which suggests that inflammation could represent a potential treatment target for anti-inflammatory agents in patients with schizophrenia. Inflammation has been associated with both negative and cognitive symptoms in patients with schizophrenia (Goldsmith et al., 2020; Goldsmith and Rapaport, 2020; Sæther et al., 2022), representing two symptom domains that have been shown to be nonresponsive to antipsychotic medications and associated with poor functional outcomes (Davis et al., 2014; Fervaha et al., 2014; Harvey, 2013). Therefore, it seems to reason that anti-inflammatories could be potentially beneficial as adjunctive therapy for these symptoms. Indeed, various anti-inflammatory and potentially anti-inflammatory agents have been studied as add-on treatments with antipsychotic medications in patients with schizophrenia, with a recent meta-analysis suggesting small to medium positive effects for anti-inflammatory agents (Jeppesen et al., 2020).
Previous studies and meta-analyses of medications with anti-inflammatory mechanisms and properties have yielded heterogeneous findings in regard to overall benefit as well as improvement in specific symptom domains (see Jeppesen et al., 2020 for review of these studies). Importantly, there is significant heterogeneity in many of these trials in regard to type of anti-inflammatory medication, dose of medication, length of trial, and baseline symptom severity, etc. For example, many of the medications studied have multiple off-target effects such that it is challenging to interpret the mechanism by which they may or may not be exerting their action (Lucido et al., 2021). Moreover, whereas previous meta-analyses have focused on the effects of anti-inflammatory medications on overall psychopathology as well as specific symptom domains (e.g., positive symptoms, negative symptoms, cognition), no study thus far has investigated specific predictors, including sociodemographic variables, trial duration, and/or symptoms themselves, of successful anti-inflammatory trials in patients with schizophrenia. Indeed, identifying these predictors may allow for future studies with trial designs that enrich their samples for the specific variables that might be expected to improve by targeting inflammatory mechanisms (Miller and Raison, 2023).
As such, we performed a meta-regression analysis of anti-inflammatory drug trials focused on predictors of overall effect size (ES) including sociodemographic variables, trial duration, and overall symptoms and individual symptom domains. Baseline symptom severity is of particular potential relevance, as previous meta-analyses have found significant reductions in pro-inflammatory cytokines following antipsychotic treatment for acute illness exacerbation (i.e., inflammation may be a state marker of acute psychosis). This raises the possibility that anti-inflammatory agents might show greater efficacy in patients with more severe psychopathology at study baseline. A related, yet unresolved question is whether anti-inflammatory agents are associated with a faster rate of improvement but not a greater total improvement by the end of the trial. Therefore, trial duration is another important potential mediator of effect size. Given that no previous studies have investigated whether other sociodemographic variables, such as age, sex, and illness duration, are associated with response to anti-inflammatory medications, we investigated these factors as well. We also seek to provide a critical lens to the anti-inflammatory literature to drive the field forward regarding trial design and interpretation of results.
2. Material and Methods
2.1. Study selection
This systematic review was conducted in accordance with the PRISMA statement. Studies of adjunctive anti-inflammatory agents in patients with schizophrenia were identified from two sources. First, we identified studies from two meta-analyses of trials of adjunctive anti-inflammatory agents in schizophrenia (Cakici et al., 2019; Sommer et al., 2014). Secondly, we systematically searched PubMed, PsycInfo, and Web of Science, and the reference lists of studies that met the inclusion/exclusion criteria for the meta-analysis in October 2022. The inclusion criteria were: 1) randomized placebo-controlled trials of anti-inflammatory agents, in adjunct to antipsychotics, in patients with schizophrenia and other non-affective psychosis; 2) data on the mean and standard deviation (SD) for the change in psychopathology scores (either the Positive and Negative Syndrome Scale [PANSS] or the Brief Psychiatric Rating Scale [BPRS]) from baseline to endpoint were available. The exclusion criteria were: 1) studies without a placebo control group; 2) studies that did not present summary data on the change in psychopathology scores (after attempting to contact the study authors); and 3) trials of monoclonal antibody immunotherapy. We defined non-affective psychosis to include schizophrenia, schizophreniform disorder, brief psychotic disorder, delusional disorder, schizoaffective disorder, and psychotic disorder not otherwise specified. For consistency with the previous meta-analyses (Cakici et al., 2019; Sommer et al., 2014), we defined anti-inflammatory agents to include both primary anti-inflammatory agents (non-steroidal anti-inflammatory drugs: aspirin and COX-2 inhibitors) and agents with anti-inflammatory properties: bexarotene, davenutide, dextromethorphan, estrogen, HMG-CoA reductase inhibitors (i.e., “statins”), melatonin, minocycline, N-acetylcysteine (NAC), omega-3 fatty acids (including eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA], and EPA + DHA), raloxifene, ramelteon, thiazolidinediones (e.g., pioglitazone), varenicline, and Withania somnifera extract. The primary search strategy was: (anti-inflammatory) AND (schizophrenia OR psychosis), limiting to clinical trials in English. Secondary searches were performed for individual anti-inflammatory agents/classes. One author (AC) performed the searches, which were independently verified by another author (BJM). The majority of initial matches were excluded because of the absence of associative data or review articles. After independent searches, review of study methods by two authors (AC and BJM), and attempts to contact other authors, 56 studies, which comprised 65 trial arms, met the inclusion criteria (Akhondzadeh et al., 2003; Akhondzadeh et al., 2004; Baheti et al., 2013; Berk et al., 2008; Breier et al., 2018; Chaudhry et al., 2012; Chen et al., 2012; Chengappa et al., 2018; Deakin et al., 2018; Emsley et al., 2002; Emsley et al., 2002b; Fenton et al., 2001; Ghanizadeh et al., 2014; Ghanizadeh et al., 2014b; Hong et al., 2011; Iranpour et al., 2016; Jamilian e tal., 2014; Javitt et al., 2012; Kelly et al., 2015; Khodaie-Ardakani et al., 2015; Kianimehr et al., 2001; Kulkarni et al., 2008; Kulkarni et al., 2010; Kulkarni et al., 2010b; Kulkarni et al., 2011; Kulkarni et al., 2011b; Kulkarni et al., 2016; Laan et al., 2010; Lee et al., 2015; Lerner et al., 2013; Levkovitz et al., 2010; Liu et al., 2014; Mishra et al., 2020; Modabbernia et al., 2014; Müller et al., 2002; Müller et al., 2010; Pawelczyk et al., 2016; Peet et al., 2001; Peet et al., 2002; Qiao et al., 2018; Rapaport et al., 2005; Rappard and Müller, 2004; Ritsner et al., 2010; Ritsner et al., 2014; Sepehrmanesh et al., 2018; Smith et al., 2016; Sommer et al., 2021; Tajik-Esmaeeli et al., 2017; Usall et al., 2011; Usall et al., 2016; Vincenzi et al., 2014; Wesiser et al., 2017; Wesiser et al., 2019; Wesiser et al., 2021; Zhang et al., 2018). A flowchart summarizing the study selection process is presented in Figure 1. Table 1 presents details of the included studies.
Figure 1.
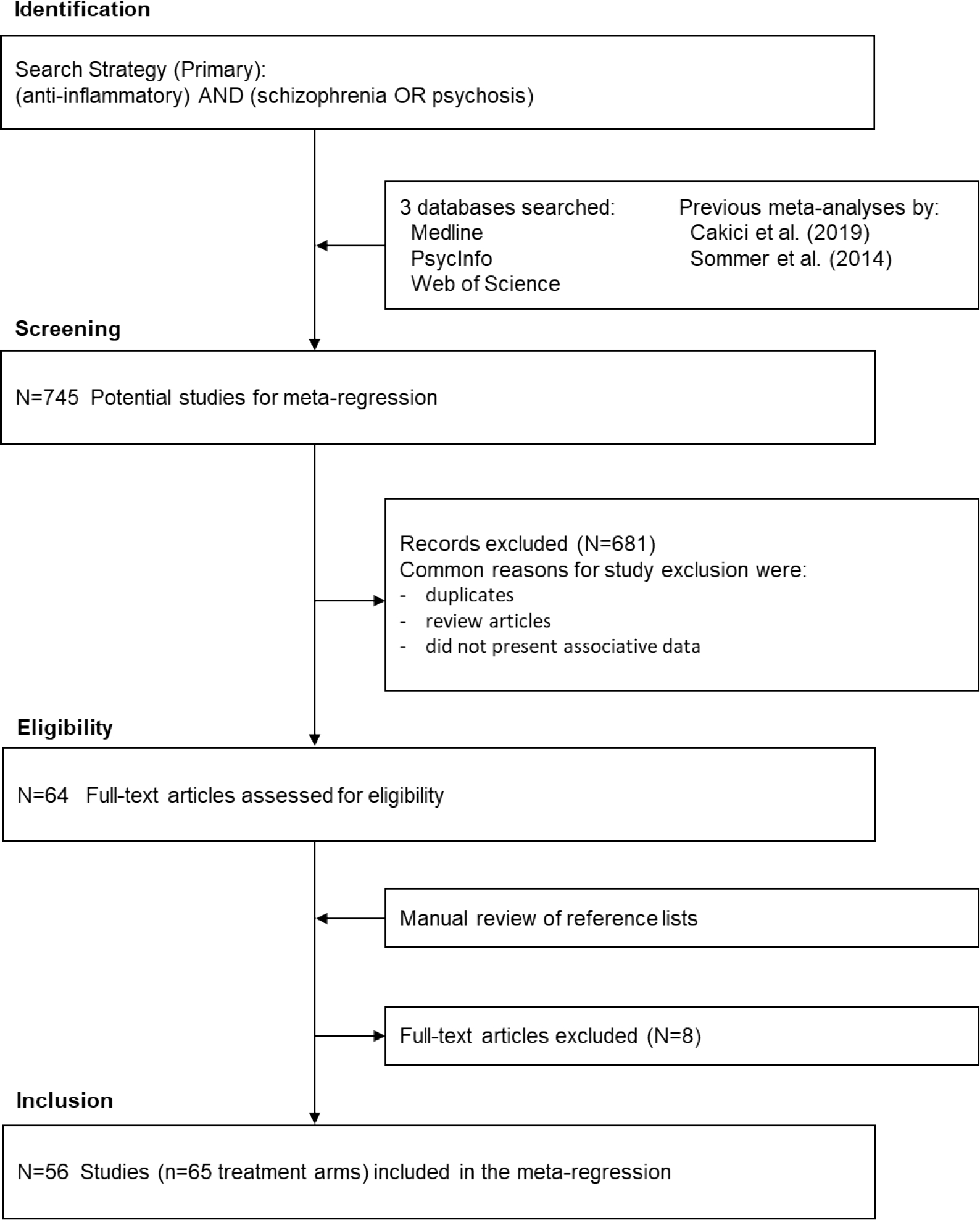
Flow Chart of the Study Selection Process
Details of study selection according to the PRISMA flow diagram template.
Table 1.
Studies of Adjunctive Anti-Inflammatory Agents in Schizophrenia
| Author | Year | Country | Agent | Trial Duration | N | Age | Sex | Smoking | BMI | Illness Duration | Age of Onset | Study Quality | PANSS Total | ES Total | PANSS Positive | ES Positive | PANSS Negative | ES Negative | PANSS General | ES General |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akhondzadeh | 2003 | Iran | Estrogen | 8 | 16 | 32.7 | 0 | 6 | 113.0 | −1.74 | −0.99 | −0.02 | −1.83 | |||||||
| Akhondzadeh | 2007 | Iran | Celecoxib | 6 | 30 | 33.7 | 58 | 7.9 | 25.2 | 6 | 92.0 | −0.93 | ||||||||
| Baheti | 2013 | US | Celecoxib | 6 | 31 | 1 | 92.6 | −0.30 | 29.0 | −0.86 | 25.7 | −0.51 | 37.9 | −0.51 | ||||||
| Berk | 2008 | Australia | NAC | 24 | 69 | 36.6 | 70 | 12.2 | 6 | 64.0 | −0.57 | 16.4 | −0.12 | 15.1 | −.0.52 | 32.5 | −0.46 | |||
| Breier | 2018 | US | NAC | 52 | 30 | 23.6 | 50 | 1.4 | 4 | 56.2 | −0.75 | 17.0 | −0.21 | 13.9 | −0.45 | |||||
| Chaudhry | 2012 | Pakistan | Minocycline | 52 | 56 | 26.2 | 60 | 3 | 82.2 | −0.20 | 19.0 | −0.21 | 22.3 | −0.40 | 41.0 | −0.02 | ||||
| Chaudhry | 2012 | Brazil | Minocycline | 52 | 15 | 26.2 | 60 | 4 | 63.0 | −1.77 | 11.4 | −1.04 | 23.1 | −1.38 | 28.5 | −1.73 | ||||
| Chengappa | 2014 | US | WSE | 14 | 34 | 46.3 | 51 | 30.2 | 22.1 | 24.2 | 6 | 69.9 | −0.75 | 19.6 | −0.27 | 16.5 | −0.43 | 33.8 | −0.75 | |
| Deakin | 2018 | UK | Minocycline | 52 | 103 | 25.6 | 72 | 27.9 | 5 | 67.1 | 0.58 | 16.3 | 0.19 | 17.7 | 0.19 | |||||
| Emsley | 2002 | South Africa | EPA | 12 | 20 | 44.9 | 72 | 5 | 76.2 | −0.70 | 18.5 | 24.6 | 33.2 | |||||||
| Emsley | 2006 | South Africa | EPA | 12 | 39 | 42.9 | 67 | 5 | 59.2 | 0.03 | ||||||||||
| Farokhnia | 2013 | Iran | NAC | 8 | 21 | 32.8 | 48 | 81 | 7.2 | 7 | 113.4 | −1.11 | 30.2 | 0.22 | 27.4 | −1.27 | 55.8 | −0.77 | ||
| Fenton | 2001 | US | EPA | 16 | 43 | 40.0 | 61 | 5 | 74.0 | 0.06 | ||||||||||
| Ghanizadeh | 2014 | Iran | Lovastatin | 8 | 20 | 30.5 | 69 | 19 | 21.9 | 0.7 | 6 | 129.9 | −0.17 | 30.8 | −0.02 | 26.9 | 0.17 | 70.6 | 0.00 | |
| Ghanizadeh | 2014 | Iran | Minocycline | 8 | 15 | 30.6 | 17 | 3 | 43.9 | −0.25 | 11.7 | 0.21 | 5.6 | −0.43 | 18.9 | −0.20 | ||||
| Hong | 2011 | US | Varenicline | 8 | 32 | 42.8 | 66 | 63 | 3 | 61.0 | −0.45 | |||||||||
| Iranpour | 2016 | Iran | Pioglitazone | 8 | 21 | 37.5 | 69 | 15.0 | 6 | 70.5 | −1.08 | 17.9 | −0.53 | 17.4 | −1.31 | 34.4 | −0.49 | |||
| Jamilian | 2014 | Iran | Omega-3 | 8 | 30 | 31.5 | 52 | 56 | 9.7 | 4 | 96.1 | −0.08 | 26.7 | 0.07 | 23.8 | 0.02 | 45.9 | −0.27 | ||
| Javitt | 2012 | US | Davenutide | 12 | 19 | 42.3 | 65 | 3 | 54.0 | 0.40 | ||||||||||
| Javitt | 2012 | US | Davenutide | 12 | 19 | 43.3 | 65 | 3 | 52.0 | 0.54 | ||||||||||
| Kelly | 2015 | US | Minocycline | 10 | 28 | 42.6 | 74 | 23.8 | 18.9 | 7 | 80.0 | −0.56 | ||||||||
| Khodaie | 2014 | Iran | Minocycline | 8 | 20 | 40.1 | 72 | 19.9 | 7 | 71.4 | −1.56 | 16.3 | −0.36 | 17.5 | −1.80 | 37.5 | −0.52 | |||
| Khodaie | 2015 | Iran | Raloxifene | 8 | 21 | 31.9 | 100 | 31.9 | 7 | 100.7 | −1.56 | 30.3 | −0.20 | 24.1 | −1.36 | 46.3 | −1.02 | |||
| Kianimehr | 2014 | Iran | Raloxifene | 8 | 25 | 61.2 | 0 | 15.4 | 32.2 | 5 | 105.5 | −0.75 | 25.8 | −0.89 | 28.0 | −0.08 | 50.4 | −0.74 | ||
| Kulkarni | 2001 | Australia | Estrogen | 4 | 12 | 34.5 | 0 | 7.9 | 4 | 68.2 | −0.42 | 16.3 | −0.09 | 14.0 | 0.32 | 35.3 | −0.25 | |||
| Kulkarni | 2001 | Australia | Estrogen | 4 | 12 | 33.9 | 0 | 8.9 | 4 | 75.8 | −0.64 | 18.3 | −0.42 | 16.6 | 0.45 | 41.0 | −0.76 | |||
| Kulkarni | 2010 | Australia | Raloxifene | 12 | 9 | 52.4 | 0 | 33.0 | 19.5 | 4 | 69.4 | −0.07 | 18.1 | 0.44 | 15.7 | −0.38 | 35.7 | 0.00 | ||
| Kulkarni | 2010 | Australia | Raloxifene | 12 | 13 | 52.1 | 0 | 26.8 | 25.3 | 4 | 83.5 | −1.39 | 19.8 | −0.67 | 21.6 | −0.73 | 42.1 | −1.21 | ||
| Kulkarni | 2008 | Australia | Estrogen | 4 | 56 | 33.6 | 0 | 10.9 | 22.8 | 4 | 77.5 | −0.59 | 19.8 | 17.1 | 40.6 | |||||
| Kulkarni | 2011 | Australia | Estrogen | 2 | 26 | 32.0 | 100 | 6.8 | 4 | 72.6 | 0.20 | 20.0 | 0.28 | 16.4 | −0.03 | 36.2 | 0.10 | |||
| Kulkarni | 2015 | Australia | Estrogen | 8 | 56 | 35.1 | 0 | 12.2 | 22.9 | 4 | 75.0 | −0.18 | 19.2 | −0.18 | 17.8 | 0.01 | 38.0 | −0.18 | ||
| Kulkarni | 2015 | Australia | Estrogen | 8 | 62 | 35.4 | 0 | 11.3 | 24.0 | 4 | 74.4 | −0.44 | 18.2 | −0.43 | 19.0 | −0.33 | 37.2 | −0.34 | ||
| Kulkarni | 2016 | Australia | Raloxifene | 12 | 26 | 53.0 | 0 | 30.0 | 24.7 | 28.3 | 6 | 80.0 | −0.63 | 18.4 | −0.30 | −0.63 | ||||
| Laan | 2010 | Netherlands | Aspirin | 12 | 33 | 31.1 | 83 | 3.7 | 4 | 71.1 | −0.37 | 16.5 | −0.23 | 18.4 | −0.17 | 36.3 | −0.32 | |||
| Lee | 2015 | Taiwan | DM | 11 | 74 | 30.4 | 60 | 5 | 86.3 | 0.21 | 20.3 | 0.30 | 22.0 | 0.05 | 39.0 | 0.26 | ||||
| Lerner | 2013 | Israel | Bexarotene | 6 | 45 | 41.5 | 90 | 27.1 | 14.4 | 27.1 | 6 | 73.2 | −0.16 | 15.5 | −0.58 | 18.6 | 0.17 | |||
| Levkovitz | 2010 | Israel | Minocycline | 22 | 36 | 25.0 | 74 | 3.8 | 21.2 | 4 | 80.4 | 0.47 | 14.5 | 0.08 | 22.3 | −0.49 | 43.6 | 0.26 | ||
| Liu | 2014 | China | Minocycline | 16 | 39 | 27.4 | 62 | 2.0 | 5 | 81.3 | −0.51 | 16.9 | −0.17 | 25.0 | −0.86 | 39.9 | −0.37 | |||
| Mishra | 2020 | India | Ramelteon | 4 | 25 | 37.8 | 59 | 69 | 22.2 | 5 | 97.5 | −0.34 | 29.7 | −0.13 | 20.4 | 47.4 | ||||
| Mishra | 2020 | India | Ramelteon | 4 | 24 | 36.4 | 60 | 75 | 23.2 | 5 | 88.5 | −0.22 | 19.5 | 26.7 | −0.23 | 42.7 | ||||
| Modabbernia | 2014 | Iran | Melatonin | 8 | 18 | 32.8 | 70 | 56 | 7 | 113.5 | −0.89 | 28.4 | −0.53 | 26.9 | −0.56 | 58.2 | −0.97 | |||
| Muller | 2002 | Germany | Celecoxib | 5 | 25 | 35.7 | 50 | 4 | 71.8 | −0.54 | 19.0 | 18.7 | ||||||||
| Muller | 2010 | Germany | Celecoxib | 6 | 25 | 28.6 | 54 | 1.3 | 4 | 94.5 | −0.55 | 25.5 | −0.53 | 21.5 | −0.38 | 47.5 | −0.49 | |||
| Pawelczyk | 2016 | Poland | EPA+DHA | 26 | 36 | 23.2 | 59 | 41 | 6 | 98.4 | −0.59 | |||||||||
| Peet | 2001 | UK | DHA | 12 | 16 | 42.8 | 67 | 2 | 73.4 | 0.12 | 17.8 | 0.33 | ||||||||
| Peet | 2001 | UK | EPA | 12 | 15 | 44.0 | 62 | 2 | 69.9 | −0.27 | 18.9 | −0.25 | ||||||||
| Peet | 2002 | UK | EPA | 12 | 9 | 5 | 88.4 | −1.08 | ||||||||||||
| Qiao | 2017 | China | EPA+DHA | 12 | 28 | 32.0 | 60 | 10.8 | 21.1 | 4 | 87.8 | 0.04 | 25.9 | 19.4 | 42.5 | |||||
| Rapaport | 2005 | US | Celecoxib | 9 | 18 | 45.7 | 83 | 29.0 | 23.9 | 21.8 | 6 | 84.1 | 0.64 | 16.8 | 0.82 | 27.1 | 0.34 | 40.1 | 0.50 | |
| Rappard | 2004 | US | Celecoxib | 11 | 138 | 2 | 78.6 | 0.08 | ||||||||||||
| Ritsner | 2010 | Israel | Pregnenolone | 8 | 14 | 34.4 | 68 | 13.3 | 23.6 | 5 | 17.6 | −0.07 | 23.4 | 0.05 | 39.9 | 0.03 | ||||
| Ritsner | 2010 | Israel | Pregnenolone | 8 | 6 | 35.9 | 77 | 26.6 | 11.3 | 23.2 | 5 | 20.5 | −0.25 | 25.8 | 0.11 | 47.7 | −0.28 | |||
| Ritsner | 2014 | Israel | Pregnenolone | 8 | 25 | 27.4 | 87.0 | 2.7 | 24.30 | 4 | −0.52 | 0.04 | −0.79 | 0.07 | ||||||
| Sepehrmanesh | 2018 | Iran | NAC | 12 | 12 | 39.1 | 48 | 15.4 | 5 | 104.0 | −0.72 | 22.9 | −0.08 | 30.4 | −1.07 | 50.5 | −0.64 | |||
| Smith | 2016 | Netherlands | Varenicline | 8 | 42 | 45.0 | 84 | 5 | 56.2 | −0.23 | ||||||||||
| Sommer | 2021 | Netherlands | Simvastatin | 104 | 58 | 4 | 58.9 | 14.1 | 14.8 | 35.2 | −0.87 | |||||||||
| Tajik-Esmaeeli | 2017 | Iran | Simvastatin | 8 | 33 | 43.9 | 89 | 58 | 20.1 | 7 | 47.1 | −0.95 | 9.2 | 0.00 | 18.7 | −0.83 | 19.2 | −0.45 | ||
| Usall | 2011 | Spain | Raloxifene | 12 | 16 | 61.4 | 0 | 35.0 | 26.4 | 4 | 62.6 | −0.57 | 10.6 | −1.04 | 22.5 | −0.70 | 30.8 | −0.77 | ||
| Usall | 2016 | Spain | Raloxifene | 12 | 38 | 61.7 | 0 | 35.1 | 26.6 | 4 | 80.5 | −0.67 | 17.1 | −0.35 | 24.4 | −0.53 | 39.0 | −0.74 | ||
| Vincenzi | 2014 | US | Pravastatin | 12 | 30 | 43.6 | 63 | 40 | 31.4 | 21.7 | 21.6 | 8 | 75.2 | −0.17 | 17.0 | −0.15 | 20.5 | 0.05 | 37.6 | −0.13 |
| Weiser | 2012 | Israel | Aspirin | 16 | 100 | 42.2 | 50 | 13.0 | 3 | 92.0 | −0.28 | |||||||||
| Weiser | 2017 | Israel | Raloxifene | 16 | 100 | 56.2 | 0 | 24.7 | 31.6 | 3 | 101.7 | 0.24 | 23.6 | 0.11 | 27.0 | 0.26 | 51.1 | 0.21 | ||
| Weiser | 2019 | Israel | Minocycline | 16 | 100 | 43.5 | 56 | 26.3 | 3 | 94.6 | 0.14 | 23.9 | 24.5 | 46.1 | ||||||
| Zhang | 2018 | China | Minocycline | 12 | 25 | 33.4 | 50 | 6.1 | 5 | 79.0 | −0.29 | 15.2 | −0.20 | 26.3 | −0.28 | 37.3 | −0.22 | |||
| Zhang | 2018 | China | Minocycline | 12 | 25 | 33.5 | 48 | 6.1 | 5 | 78.5 | −0.32 | 14.9 | 0.31 | 26.4 | −0.85 | 37.4 | −0.25 |
Selected sociodemographic and clinical characteristics of included trials. Trial duration is in weeks. Age, illness duration, and age of onset are the means in years for study subject. BMI and PANSS scores are the means for study subjects. Sex is the percentage of males and smoking is the percentage of smokers in the study.
Each of the included studies was assessed and assigned a “Quality Score” by one author (BJM), which was independently verified by another author (DRG). Quality scores for studies of anti-inflammatory agents were based on the sum of the presence or absence of ten factors (one point for each): whether the study considered potential effects of age, sex, race/ethnicity, body mass index (BMI), smoking, socioeconomic status (SES), alcohol/illicit drugs, illness duration, antipsychotic medications, and diet/exercise(including exercise by either 1) matching anti-inflammatory and placebo groups, or 2) controlling for these variables in the analysis.
2.2. Data extraction and meta-regression
Data were extracted on study year, geography (categorized as the Middle East, Australia, Europe, Asia, and the United States), anti-inflammatory agent, trial duration (in weeks), sample size, mean age, sex (% male), race (% Caucasian), smoking (% yes), mean body mass index (BMI), mean illness duration, mean age of onset of psychosis, mean psychopathology scores (total and subscale) at baseline, and mean and SD for the change in psychopathology scores (total and subscale) from baseline to endpoint for each study that satisfied the inclusion and exclusion criteria. One author (AC) extracted the data and the other author (BJM) independently verified the data. We then calculated ES estimates for changes in psychopathology scores (total and subscale) from baseline to endpoint using Cohens d. We also calculated ES estimates for changes in psychopathology scores from baseline to week 4 and/or week 8 when such data were available. Negative ES estimates denote greater reduction in psychopathology scores for adjunctive anti-inflammatory treatment versus placebo. An effect size of 0.2 is considered small, 0.5 medium, and 0.8 large. The majority of studies used the PANSS for psychopathology scores. For studies using the BPRS, we used BPRS data in the calculation of ES estimates, but for correlative and regression analyses (see below), we converted mean BPRS scores to PANSS scores using the method of Leucht et al. (2006).
A one-sample Kolmogorov-Smirnov test was used to examine demographic and clinical variables for normality. Although ES estimates were normally distributed, two extreme outliers (Akhondzadeh et al., 2003; Chaudhry et al., 2012 [Brazil])—defined as and ES >3 times the interquartile range—were subsequently excluded from the analyses. Age, race, smoking, BMI, illness duration, age of onset, and total, negative, and general symptoms were all normally distributed. Trial duration, sample size, sex, and positive symptoms were not normally distributed. We then compared clinical and sociodemographic variables based on whether the ES estimate for change in total psychopathology was <0 or >0 using Student’s t-test (2-sided), Mann-Whitney U test, or Chi-square test. We then calculated bivariate correlations (Spearman’s rho) between ES estimates for changes in psychopathology scores and other clinical and sociodemographic variables. We also performed four, separate linear meta-regression models for ES estimates for changes in total, positive, negative, and general psychopathology, each including geography, sample size, study quality score, trial duration, age, sex, and baseline psychopathology scores as covariates. Data were analyzed with SPSS version 27 (SPSS, Inc.; Chicago, Illinois) and p-values were considered statistically significant at the 0.05 level.
3. Results
A total of 56 studies, which comprised 65 trial arms, met the inclusion criteria. After exclusion of two extreme outliers, 63 trial arms were included in the analyses. This comprised 2232 patients randomized to adjunctive anti-inflammatory agents and 2207 patients randomized to placebo. The most common anti-inflammatory agents studied were minocycline (n=10 trials), omega-3 fatty acids (n=9), raloxifene (n=8), estrogen (n=6), celecoxib (n=6), and NAC (n=4). The geographical distribution of trials was the Middle East (n=21), Europe (n=12), the United States (n=11), Australia (n=10), Asia (n=7), and South Africa (n=2). Data on year, trial duration and sample size were available for all studies. Data on mean age, sex, and total psychopathology scores were available for n=59 trial arms, but significantly fewer for illness duration (n=40), age of onset (n=22), BMI (n=10), and smoking (N=10). Data on positive, negative, and general psychopathology scores were available for n=49, n=46, and n=43 trial arms, respectively. The mean (SD) study quality score was 4.6 (1.4).
The baseline clinical and demographic characteristics of positive or negative anti-inflammatory trials (n=60 trials with ES estimates for change in total psychopathology) are presented in Table 2. “Positive” trials (ES <0) had a significantly lower proportion of males (46.7% versus 65.6%) and higher study quality scores (4.9 versus 3.9), but otherwise there were no significant differences in clinical or demographic characteristics.
Table 2.
Clinical and Demographic Characteristics of Included Trials
| Variable | Trial Result | p-value+ | |
|---|---|---|---|
| Positive | Negative | ||
| (ES<0) | (ES>0) | ||
| N=45 | N=15 | ||
|
| |||
| Trial Duration (weeks) | 11.8 (9.9) | 14.8 (11.2) | 0.06 |
| Sample size (anti-inflammatory group) | 30 (18) | 52 (40) | 0.09 |
| Age (years) | 38.7 (9.5) | 37.8 (9.1) | 0.75 |
| Sex (% male) | 46.7 (31.0) | 65.6 (22.4) | 0.02 |
| Smoking (% yes) | 55.6 (18.2) | N/A | |
| BMI | 26.5 (4.0) | 28.4 (0.8) | 0.28 |
| Illness duration (years) | 14.9 (10.1) | 12.1 (9.8) | 0.54 |
| Age of Onset (years) | 24.6 (3.5) | 24.4 (4.1) | 0.88 |
| PANSS Total | 80.7 (17.5) | 76.1 (14.6) | 0.38 |
| PANSS Positive | 19.8 (5.6) | 19.9 (3.9) | 0.83 |
| PANSS Negative | 21.0 (5.2) | 22.1 (4.0) | 0.59 |
| PANSS General | 40.4 (9.9) | 42.6 (4.9) | 0.56 |
| Study Quality Score | 4.9 (1.4) | 3.9 (1.2) | 0.03 |
| n (%) | n (%) | ||
|
| |||
| Geography | 0.44 | ||
| Middle East | 15 (33.3) | 4 (26.7) | |
| Europe | 9 (20.0) | 2 (13.3) | |
| United States | 6 (13.3) | 5 (11.1) | |
| Australia | 9 (20.0) | 1 (6.7) | |
| Asia | 5 (11.1) | 2 (13.3) | |
| South Africa | 1 (2.2) | 1 (6.7) | |
Selected sociodemographic and clinical characteristics of included trials, stratified based on whether the overall effect size favored anti-inflammatory (ES<0) or placebo (ES>0).
When considered as a continuous variable, ES estimates for change in total psychopathology were significantly correlated with geography (ρ=0.27, p=0.034),sample size (ρ=0.30, p=0.020), and study quality score (ρ=−0.45, p<0.001) meaning smaller studies and studies with higher quality scores were associated with greater ES estimates in favor of anti-inflammatory agents (Figure 2). The mean ES was −0.55 for studies in the Middle East, −0.47 in Australia, −0.38 in Europe, −0.33 in South Africa, −0.20 in Asia, and −0.11 in the United States. In a linear meta-regression model, geography (β=0.31, p=0.011), smaller sample size (β=0.33, p=0.009), and higher study quality score (β=0.44, p<0.001) were significant predictors of larger ES estimates for change in total psychopathology in favor of anti-inflammatory agents.
Figure 2.
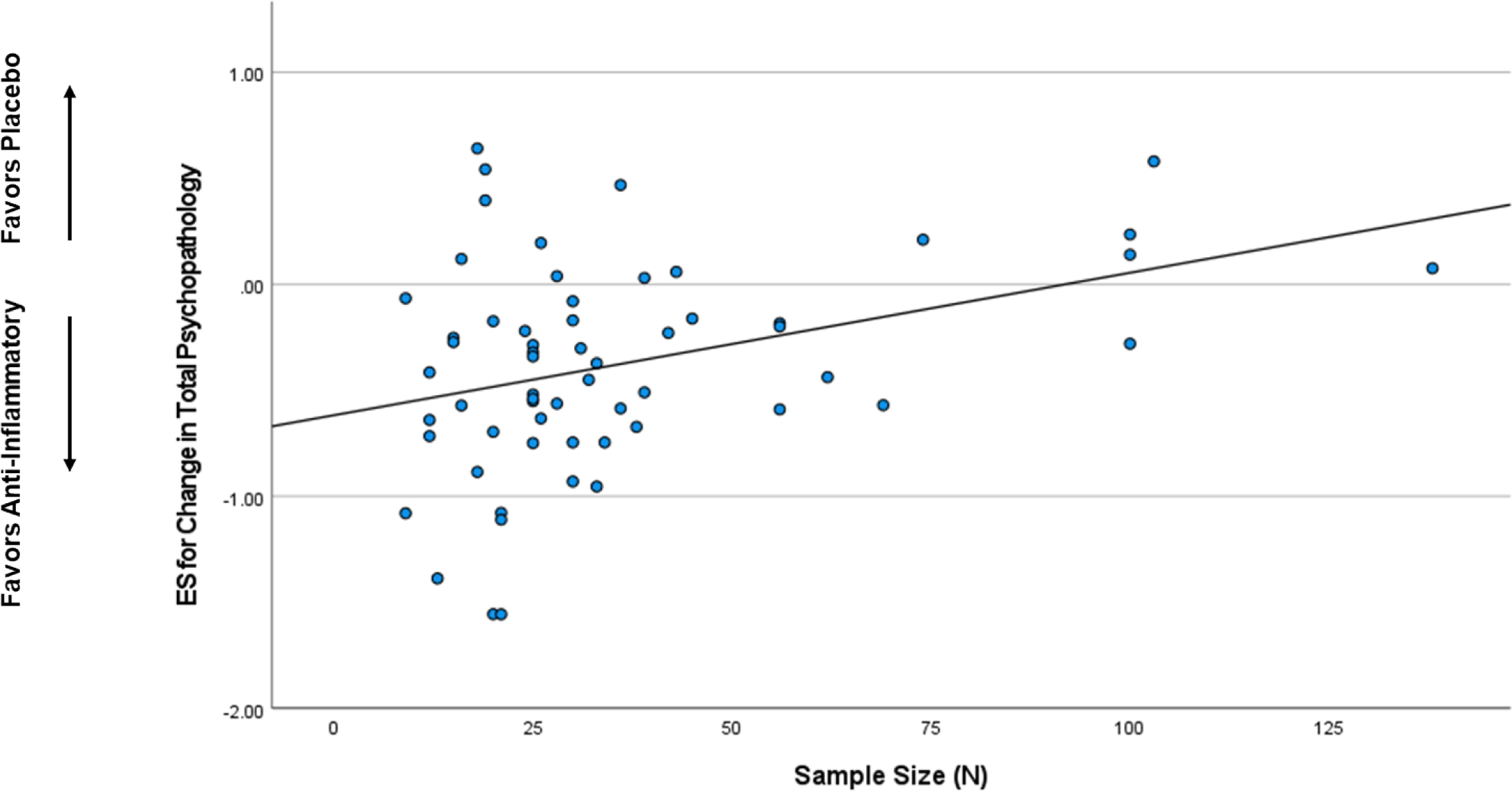
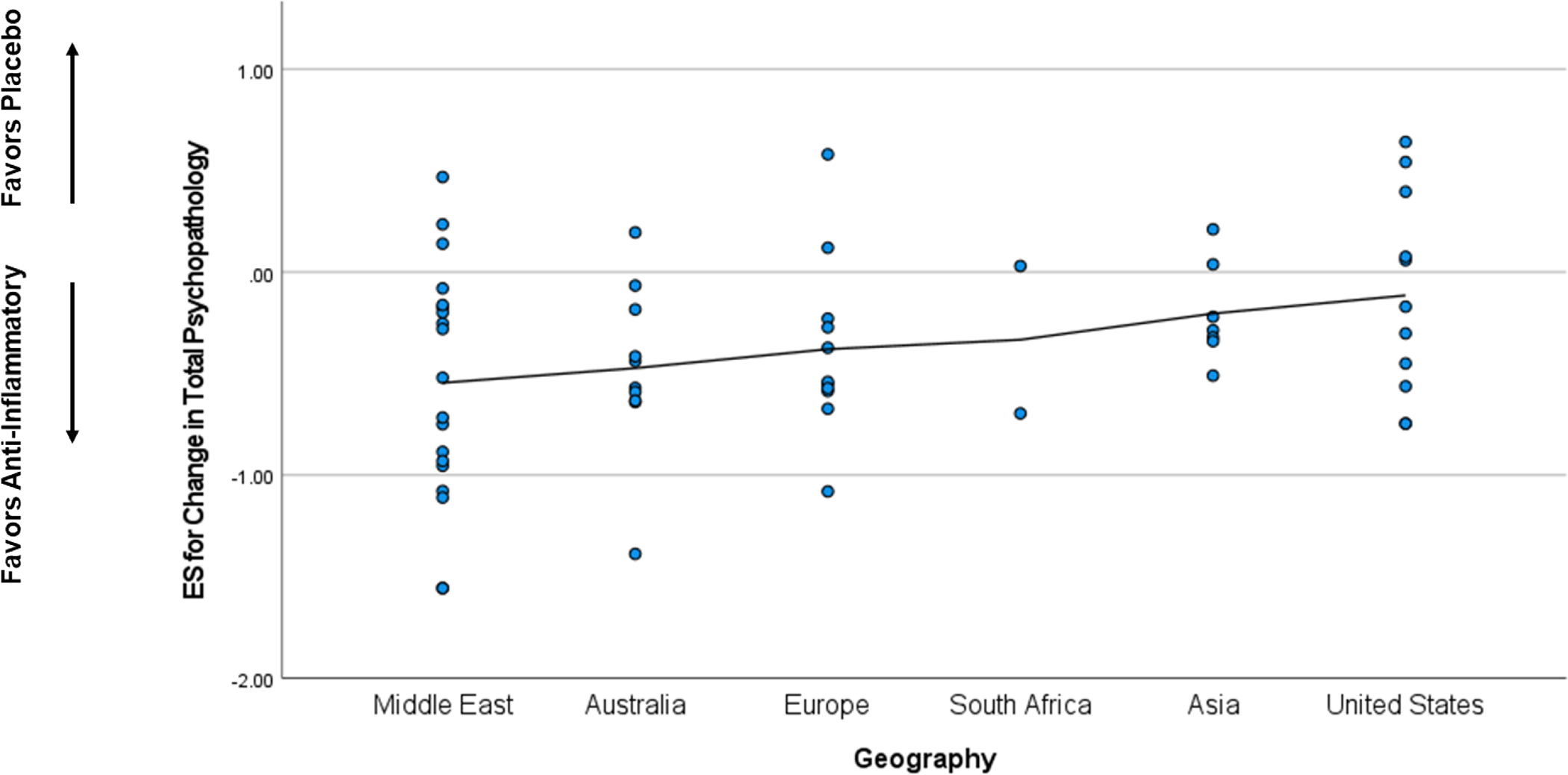
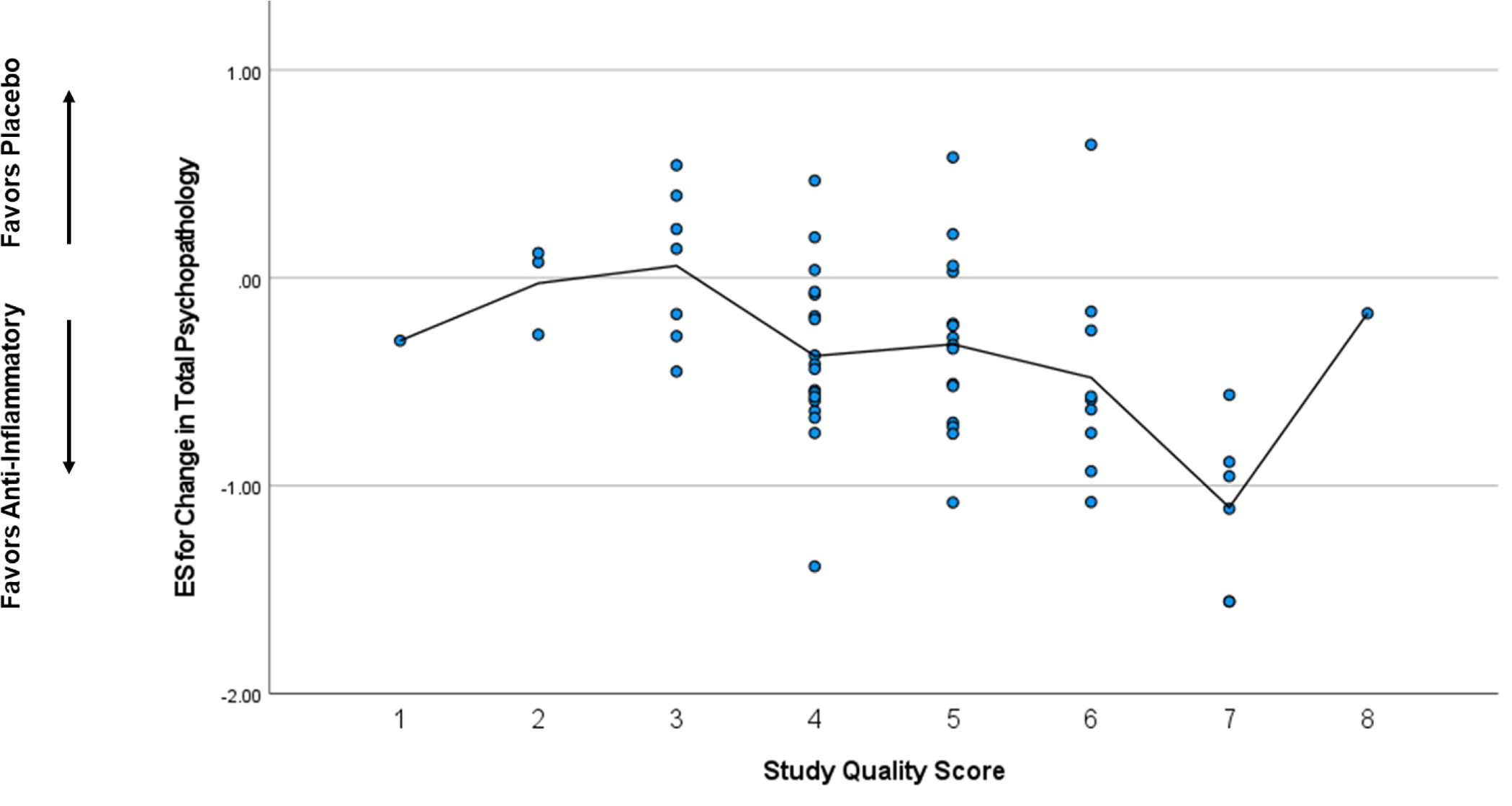
Correlation between Selected Characteristics and Effect Size Estimates
2a. Sample Size
Smaller sample sizes were associated with larger effect sizes in favor of anti-inflammatory agents.
2b. Geography
Geography was associated with outcome, with the largest effect sizes in favor of anti-inflammatory agents seen in trials performed in the Middle East and Australia.
2c. Study Quality
Higher study quality scores were associated with larger effect sizes in favor of anti-inflammatory agents.
In a linear meta-regression model, there were no significant predictors of ES estimates for change in positive psychopathology. By contrast, smaller sample size (β=0.37, p=0.034) and higher study quality score (β=0.55, p=0.003) were significant predictors of larger ES estimates for change in negative psychopathology in favor of anti-inflammatory agents. In a linear meta-regression model, only higher study quality score (β=0.46, p=0.019) was a significant predictor of larger ES estimates for change in general psychopathology in favor of anti-inflammatory agents. only sample size was a significant predictor of ES estimates for change in negative psychopathology (β=0.34, p=0.030). (See also Supplementary Table.)
Given the significant difference in sex for trials with ES<0 versus ES>0 regarding total psychopathology), in a post-hoc analysis, we investigated predictors of ES estimates in studies of women only. There were n=12 such trials, all either estrogen (n=5) or the selective estrogen receptor modulator raloxifene (n=7). In a linear meta-regression model, there were no significant predictors of ES estimates for change in total psychopathology.
4. Discussion
This study is the first meta-regression analysis investigating multiple predictors of successful anti-inflammatory trials, including sociodemographic variables, trial duration and domains of psychopathology. We examined effect size estimates across 63 trial arms that included 2232 patients with schizophrenia (or related non-affective psychoses) and 2207 healthy controls. We found that geography, smaller sample size, and higher study quality scores were the only significant predictors of larger ES estimates for change in total psychopathology in favor of anti-inflammatory agents. In other words, the smaller the sample size, and the higher the study quality score, the larger the effect size in favor of anti-inflammatory medications compared to placebo. Regarding geography, the largest effect sizes were found in studies conducted in the Middle East and the smallest effect sizes in studies conducted in the United States. Though there were no significant difference in predictors of change in positive symptoms, smaller sample size was a significant predictor of change in negative psychopathology and higher study quality score was a significant predictor of change in negative and general symptoms in favor of anti-inflammatory medications.
These results should be interpreted with caution, especially as larger effects sizes for smaller studies suggests the possibility of publication bias and/or overestimation of treatment effects. We initially dichotomized studies based on ES<0 and ES>0, which likely overestimates the number of truly “positive” studies. Similarly, the significant geographic variation may pose concern for publication bias, though further work should determine whether there may be true differences in geographic response to anti-inflammatory agents. Interestingly, when the studies from the Middle East were excluded in a sensitivity analysis, geography and sample size were no longer significant predictors of effect size. The association with study quality score suggests that future anti-inflammatory trials must consider demographic variables known to be associated with inflammation (e.g., BMI and smoking). These variables were not considered in the majority of included trials, despite the extensive literature on the association of elevated weight and adiposity on increased inflammation (Borst, 2004; Hotamisligil et al., 1994; Nieto-Vazquez et al., 2008; Park et al., 2005; Popa et al., 2007; Weisberg et al., 2003). Moreover, variables such as illness duration has been shown to be associated with worse psychopathology and treatment resistance (Griffiths et al., 2021; Murru and Carpiniello, 2018; Zoghbi et al., 2023), which may in turn, have implications for inflammation (Jiao et al., 2022; Labonté et al., 2022; Leboyer et al., 2021). As such, the lack of an association with other sociodemographic variables in our analyses and others (Çakici et al., 2019; Jeppesen et al., 2020) is a potentially important negative finding and this should be seen as a primary limitation of the literature that needs to be considered in future studies.
Despite these limitations, the findings of an association between sample size and negative psychopathology but not positive symptoms, may be of some interest. Indeed, there is a potential association between inflammation and negative symptoms (Boozalis et al., 2017; Garcia-Rizo et al., 2012; Goldsmith et al., 2019; Goldsmith et al., 2018; Goldsmith et al., 2021; Goldsmith and Rapaport, 2020; Stojanovic et al., 2014; Zhu et al., 2018), which suggests that we might expect that anti-inflammatory agents would have an effect on these specific symptoms. Similar associations have been found for cognition (Adamowicz et al., 2022; Goldsmith et al., 2020; Miller et al., 2018; Miller et al., 2021; Patlola et al., 2023; Sæther et al., 2022), whereas studies of inflammation and positive symptoms have not been as robust Though this may be considered a time when anti-inflammatory agents may be of potential benefit, the literature on the effect of inflammation on the brain has largely come from studies of chronic, low-grade elevated inflammation. Studies of the impact of inflammation on the brain in healthy individuals, non-human primates, and individuals with depression support a role for inflammation impacting the basal ganglia and associated reward circuitry in the brain that may underlie anhedonia and motivational deficits (Brydon et al., 2008; Eisenberger et al., 2010; Eisenberger et al., 2009; Felger et al., 2007; Felger and Miller, 2012; Felger et al., 2013; Harrison et al., 2015; Miller et al., 2009; Miller and Raison, 2015). These symptoms encompass deficits in motivation and pleasure, which are negative symptoms that are known to involve signaling in the ventral striatum (Juckel et al., 2012; Juckel et al., 2006; Kirschner et al., 2016; Prettyman et al., 2021; Radua et al., 2015) and may be particularly sensitive to the effects of anti-inflammatories, especially in individuals with evidence of increased inflammation.
A primary limitation of the anti-inflammatory literature is the lack of data on baseline inflammatory markers. This is of great importance for two reasons. First, inflammation is likely only elevated in a subgroup of individuals with psychiatric illness, including schizophrenia. Similar to individuals with depression (Osimo et al., 2019), inflammatory markers are likely only elevated in approximately 30% of patients with schizophrenia (Miller et al., 2014). Therefore, in the absence of inflammation, one would likely not expect an adjunct anti-inflammatory to have an effect. This is certainly the case in a trial of a cytokine antagonist in depression that showed benefit only in individuals with high CRP (Raison et al., 2013) in addition to other trials of anti-inflammatories in patients with depression (Nettis et al., 2021; Rapaport et al., 2016; Savitz et al., 2018). This may partially explain the negative findings in the largest monoclonal antibody trial in schizophrenia (Girgis et al., 2018), in which the mean baseline CRP was not considered high per American Heart Association/Center for Disease Control and Prevention guidelines (CRP>3 mg/L) (Pearson et al., 2003). Future studies of anti-inflammatory agents should measure and report baseline inflammatory markers and enrich samples for those individuals with evidence of increased inflammation.
Second, without evidence that anti-inflammatories decrease inflammation by reporting pre/post inflammatory marker concentrations, it is difficult to interpret whether an improvement in symptoms is actually driven by the anti-inflammatory effect of the medication. Similarly, many of the medications included in this meta-regression analysis have multiple off-target effects besides inflammation. For example, minocycline, one of the most frequently studied anti-inflammatory agent included herein, may also work via antioxidant, anti-apoptotic, anti-bacterial, and via neurotrophic factors (Dean et al., 2012).
Anti-inflammatory clinical trial design incorporating these considerations should drive the field forward and allow for the identification of predictors of response and patient stratification to support precision medicine (Miller and Raison, 2023). Moreover, choosing the appropriate outcomes for these trials (e.g., negative symptoms and/or brain-related neuroimaging measures associated with inflammation) may also lead to more successful trials that are adequately powered to detect differences between active medication versus placebo or between groups expected to respond (high inflammation) versus those not expected to respond (low inflammation).
5. Conclusions
Findings from this meta-regression analysis suggest that that sample size, geography, and study quality are important predictors of response to anti-inflammatory treatment in schizophrenia. Future anti-inflammatory trials must be rigorously designed and consider demographic variables known to be associated with inflammation (e.g., BMI and smoking), and evidence of increased baseline inflammation should also be incorporated in study design. Rationale study design that also includes measurement of inflammatory markers (perhaps both peripheral and CSF) to stratify patients and demonstrate target engagement, and the incorporation of appropriate study outcomes may support the use of these medications in clinical settings to improve the lives of patients with schizophrenia.
Supplementary Material
Highlights.
Anti-inflammatories have potential benefit in patients with schizophrenia.
This meta-analysis investigated potential predictors of successful trials.
Small sample size and geographic variation correlated with greater effect sizes.
Baseline variables, including inflammation, should be considered in future trial designs.
Funding
The work was supported by the National Institutes of Health (NIH) K23 MH114037 to DRG.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial or personal interests to report.
Dr. Miller has no disclosures relevant to the present work. In the past year, Dr. Miller received research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Augusta University; and Honoraria from Atheneum, Carlat Psychiatry, ClearView Healthcare Partners, Psychopharmacology Institute, and Psychiatric Times.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamowicz DH, Shilling PD, Palmer BW, Nguyen TT, Wang E, Liu C, Tu X, Jeste DV, Irwin MR, Lee EE, 2022. Associations between inflammatory marker profiles and neurocognitive functioning in people with schizophrenia and non-psychiatric comparison subjects. Journal of psychiatric research 149, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boozalis T, Teixeira AL, Cho RY, Okusaga O, 2017. C-Reactive Protein Correlates with Negative Symptoms in Patients with Schizophrenia. Front Public Health 5, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst SE, 2004. The role of TNF-alpha in insulin resistance. Endocrine 23, 177–182. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ, 2010. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. The American journal of psychiatry 167, 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD, 2008. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry 63, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC, 2019. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychological medicine 49, 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Horan WP, Marder SR, 2014. Psychopharmacology of the negative symptoms: current status and prospects for progress. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24, 788–799. [DOI] [PubMed] [Google Scholar]
- Dean OM, Data-Franco J, Giorlando F, Berk M, 2012. Minocycline: therapeutic potential in psychiatry. CNS Drugs 26, 391–401. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2009. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage 47, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH, 2007. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biological psychiatry 62, 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH, 2012. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology 33, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH, 2013. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G, 2014. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta psychiatrica Scandinavica 130, 290–299. [DOI] [PubMed] [Google Scholar]
- Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jiménez JA, Ahmed A, Rothstein TL, Lencz T, Malhotra AK, 2018. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: New data and an updated meta-analysis. Schizophrenia research 202, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Bernardo M, Kirkpatrick B, 2012. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry research 198, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Ciarleglio A, Choo T, Haynes G, Bathon JM, Cremers S, Kantrowitz JT, Lieberman JA, Brown AS, 2018. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Tocilizumab, An Interleukin-6 Receptor Antibody, For Residual Symptoms in Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Addington J, Bearden C, Cadenhead K, Cannon T, Cornblatt B, Mathalon D, McGlashan T, Seidman L, Tsuang M, Woods SW, Walker EF, Perkins DO, 2019. Association of baseline inflammatory markers and the development of negative symptoms in individuals at clinical high risk for psychosis. Brain, behavior, and immunity 76, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ, 2018. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophrenia research 199, 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Massa N, Miller BJ, Miller AH, Duncan E, 2021. The interaction of lipids and inflammatory markers predict negative symptom severity in patients with schizophrenia. NPJ Schizophr 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Massa N, Pearce BD, Wommack EC, Alrohaibani A, Goel N, Cuthbert B, Fargotstein M, Felger JC, Haroon E, Miller AH, Duncan E, 2020. Inflammatory markers are associated with psychomotor slowing in patients with schizophrenia compared to healthy controls. NPJ Schizophr 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ, 2016. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, 2020. Inflammation and Negative Symptoms of Schizophrenia: Implications for Reward Processing and Motivational Deficits. Front Psychiatry 11, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths K, Millgate E, Egerton A, MacCabe JH, 2021. Demographic and clinical variables associated with response to clozapine in schizophrenia: a systematic review and meta-analysis. Psychological medicine 51, 376–386. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD, 2015. A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, 2013. Assessment of everyday functioning in schizophrenia: implications for treatments aimed at negative symptoms. Schizophrenia research 150, 353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM, 1994. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America 91, 4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson ZD, Miller BJ, 2018. Meta-Analysis of Cytokine and Chemokine Genes in Schizophrenia. Clinical schizophrenia & related psychoses 12, 121–129b. [DOI] [PubMed] [Google Scholar]
- Jeppesen R, Christensen RHB, Pedersen EMJ, Nordentoft M, Hjorthøj C, Köhler-Forsberg O, Benros ME, 2020. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders - A comprehensive systematic review and meta-analysis. Brain, behavior, and immunity 90, 364–380. [DOI] [PubMed] [Google Scholar]
- Jiao S, Cao T, Cai H, 2022. Peripheral biomarkers of treatment-resistant schizophrenia: Genetic, inflammation and stress perspectives. Front Pharmacol 13, 1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Knutson B, Wrase J, Brune M, Heinz A, Schlagenhauf F, 2012. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology 66, 50–56. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A, 2006. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage 29, 409–416. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB, 2013. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychological medicine 43, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Hager OM, Bischof M, Hartmann MN, Kluge A, Seifritz E, Tobler PN, Kaiser S, 2016. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. Journal of psychiatry & neuroscience : JPN 41, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté C, Zhand N, Park A, Harvey PD, 2022. Complete blood count inflammatory markers in treatment-resistant schizophrenia: Evidence of association between treatment responsiveness and levels of inflammation. Psychiatry research 308, 114382. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Godin O, Terro E, Boukouaci W, Lu CL, Andre M, Aouizerate B, Berna F, Barau C, Capdevielle D, Clauss-Kobayashi J, Chereau I, T DA, Dubertret C, Dubreucq J, Fond G, Laouamri H, Leignier S, Lancon C, Llorca PM, Mallet J, Le Corvoisier P, Misdrahi D, Passerieux C, Rey R, Pignon B, Urbach M, Szoke A, Schürhoff F, Tamouza R, 2021. Immune Signatures of Treatment-Resistant Schizophrenia: A FondaMental Academic Centers of Expertise for Schizophrenia (FACE-SZ) Study. Schizophr Bull Open 2, sgab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucido MJ, Bekhbat M, Goldsmith DR, Treadway MT, Haroon E, Felger JC, Miller AH, 2021. Aiding and Abetting Anhedonia: Impact of Inflammation on the Brain and Pharmacological Implications. Pharmacol Rev 73, 1084–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2015. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2023. Burning down the house: reinventing drug discovery in psychiatry for the development of targeted therapies. Molecular psychiatry 28, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B, 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley PF, McEvoy JP, 2018. Inflammation, substance use, psychopathology, and cognition in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Schizophrenia research 195, 275–282. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH, 2014. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clinical schizophrenia & related psychoses 7, 223–230. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Goldsmith DR, 2017. Towards an Immunophenotype of Schizophrenia: Progress, Potential Mechanisms, and Future Directions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Herzig KH, Jokelainen J, Karhu T, Keinänen-Kiukaanniemi S, Järvelin MR, Veijola J, Viinamäki H, Päivikki T, Jääskeläinen E, Isohanni M, Timonen M, 2021. Inflammation, hippocampal volume, and cognition in schizophrenia: results from the Northern Finland Birth Cohort 1966. Eur Arch Psychiatry Clin Neurosci 271, 609–622. [DOI] [PubMed] [Google Scholar]
- Murru A, Carpiniello B, 2018. Duration of untreated illness as a key to early intervention in schizophrenia: A review. Neurosci Lett 669, 59–67. [DOI] [PubMed] [Google Scholar]
- Nettis MA, Lombardo G, Hastings C, Zajkowska Z, Mariani N, Nikkheslat N, Worrell C, Enache D, McLaughlin A, Kose M, Sforzini L, Bogdanova A, Cleare A, Young AH, Pariante CM, Mondelli V, 2021. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 46, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M, 2008. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 114, 183–194. [DOI] [PubMed] [Google Scholar]
- North HF, Weissleder C, Fullerton JM, Webster MJ, Weickert CS, 2022. Increased immune cell and altered microglia and neurogenesis transcripts in an Australian schizophrenia subgroup with elevated inflammation. Schizophrenia research 248, 208–218. [DOI] [PubMed] [Google Scholar]
- Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM, 2019. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychological medicine 49, 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R, 2005. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69, 29–35. [DOI] [PubMed] [Google Scholar]
- Patlola SR, Donohoe G, McKernan DP, 2023. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: A systematic review and meta-analysis. Progress in neuro-psychopharmacology & biological psychiatry 121, 110668. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr., Taubert K, Tracy RP, Vinicor F, 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511. [DOI] [PubMed] [Google Scholar]
- Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF, 2007. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48, 751–762. [DOI] [PubMed] [Google Scholar]
- Prettyman GE, Kable JW, Didier P, Shankar S, Satterthwaite TD, Davatzikos C, Bilker WB, Elliott MA, Ruparel K, Wolf DH, 2021. Relationship of ventral striatum activation during effort discounting to clinical amotivation severity in schizophrenia. NPJ Schizophr 7, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, Fusar-Poli P, 2015. Ventral Striatal Activation During Reward Processing in Psychosis: A Neurofunctional Meta-Analysis. JAMA Psychiatry 72, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D, 2016. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular psychiatry 21, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sæther LS, Ueland T, Haatveit B, Maglanoc LA, Szabo A, Djurovic S, Aukrust P, Roelfs D, Mohn C, Ormerod M, Lagerberg TV, Steen NE, Melle I, Andreassen OA, Ueland T, 2022. Inflammation and cognition in severe mental illness: patterns of covariation and subgroups. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, Drevets D, Yates W, Gleason O, Drevets WC, Preskorn SH, 2018. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 8, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA, 2009. Common variants conferring risk of schizophrenia. Nature 460, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, Labad J, 2014. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41, 23–32. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM, 2014. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophrenia research 155, 101–108. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr., 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, Palmer E, Karwath A, Barsky A, Gkoutos GV, Wood S, Barnes NM, David AS, Donohoe G, Neill JC, Deakin B, Khandaker GM, Upthegrove R, 2022. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry 79, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang L, Liu F, Wu R, Guo W, Ou J, Zhang X, Zhao J, 2018. Altered Serum Tumor Necrosis Factor and Interleukin-1β in First-Episode Drug-Naive and Chronic Schizophrenia. Frontiers in neuroscience 12, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi AW, Lieberman JA, Girgis RR, 2023. The neurobiology of duration of untreated psychosis: a comprehensive review. Molecular psychiatry 28, 168–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


