Abstract
Clinical and basic science investigation indicates a link between insulin resistance and anhedonia. Previous results of this laboratory point to impaired nucleus accumbens (NAc) insulin signaling as an underpinning of diet-induced anhedonia, based on use of a glucose lick microstructure assay. The present study evaluated whether advanced glycation end products (AGEs) and their receptor (RAGE), known to mediate obesogenic diet-induced inflammation and pathological metabolic conditions, are involved in this behavioral change. Six weeks maintenance of male and female rats on a high fat-high sugar liquid diet (chocolate Ensure) increased body weight gain, and markedly increased circulating insulin and leptin, but induced anhedonia (decreased first minute lick rate and lick burst size) in males only. In these subjects, anhedonia correlated with plasma concentrations of insulin. Although the diet did not alter plasma or NAc AGEs, or the expression of RAGE in the NAc, marginally significant correlations were seen between anhedonia and plasma content of several AGEs and NAc RAGE. Importantly, a small molecule RAGE antagonist, RAGE229, administered twice daily by oral gavage, prevented diet-induced anhedonia. This beneficial effect was associated with improved adipose function, reflected in the adiponectin/leptin ratio, and increased pCREB/total CREB in the NAc, and a shift in the pCREB correlation with pThr34-DARPP-32 from near-zero to strongly positive, such that both phospho-proteins correlated with the rescued hedonic response. This set of findings suggests that the receptor/signaling pathway and cell type underlying the RAGE229-mediated increase in pCREB may mediate anhedonia and its prevention. The possible role of adipose tissue as a locus of diet-induced RAGE signaling, and source of circulating factors that target NAc to modify hedonic reactivity are discussed.
Keywords: RAGE, AGEs, RAGE229, insulin, nucleus accumbens, lick microstructure, anhedonia
1. Introduction
It is estimated that nearly 10% of the US adult population are affected by type 2 diabetes (T2D) and another 37% have pre-diabetes (U.S. CDC, National Diabetes Statistics Report, 2014). These statistics are alarming not only because of the associated risks of cardiovascular disease, renal disease, retinopathy and neuropathy, but also because of increasing evidence of a link to cognitive impairment (1–3) and depression (4–7). Major depression is at least twice as prevalent among individuals with T2D compared to the general population (8–10), and is associated with increased mortality (9,11).
Epidemiological studies reveal anhedonia as the dimension of depression that is of particular clinical importance in T2D (12). One study of nearly 6000 subjects indicates that among the distinct features of depression, it is specifically anhedonia, and not dysphoria or anxiety, that is strongly associated with T2D and increased mortality (9,13). Moreover, the association between insulin resistance and anhedonia is not limited to depressive illness. Anhedonia is also a cardinal feature of schizophrenia (14,15), where metabolic abnormalities are common, and the prevalence of T2D is two to three times higher than in the general population (16,17). Insulin resistance in this population has been shown to precede the effects of antipsychotic medications and correlate with the severity of negative symptoms (18).
As a core feature of two major psychiatric disorders, typified by diminished subjective and behavioral responses to reward stimuli, anhedonia is associated, in human neuroimaging studies, with decreased ventral striatal activity (19–23). Research in our laboratory, using a rat model of diet-induced insulin resistance, has produced evidence of anhedonia associated with nucleus accumbens (NAc) insulin receptor subsensitivity and suppression of insulin’s facilitatory effect on dopamine release (24–27). A causal connection between NAc insulin resistance and anhedonia is suggested by our finding that the anhedonic effect can be reproduced in chow-fed rats by inactivation of insulin in NAc (26).
A known mechanistic overlap between T2D and depression is increased inflammatory activity. In the case of T2D, a key role has been established for advanced glycation end products (AGEs) (28–30). AGEs form endogenously from the reaction of reducing sugars and reactive carbonyls with free amino groups (31). AGEs also originate exogenously; dietary sugar (32,33) and heat-processed high fat foods (34) are particularly rich sources. In animal models, sugar-derived AGEs have been shown to contribute to the activation of pro-inflammatory signals and development of pathological metabolic conditions (35,36). The receptor for AGEs (RAGE) (37,38), is a cell surface multiligand receptor of the immunoglobulin superfamily that binds AGEs, resulting in pro-inflammatory gene activation (for reviews: 28–30), and has been shown necessary for the induction of diet-induced T2D in mouse models (39). RAGE is expressed by epithelial cells, cardiomyocytes, macrophages, adipocytes, microglia, astrocytes, and neurons (28,30).
The plausible involvement of RAGE in brain pathology induced by high energy diet is supported by findings that, in rats, a high fructose diet induces hippocampal neuroinflammation, hypothalamic NF-κB activation, and suppression of insulin signaling (40). In mice, both HF and HF/high sugar (HS) diets increase neocortical markers of inflammation and oxidative stress (41). Schmidt and coworkers compared HF diet-induced inflammation in wild type and Ager (the gene encoding RAGE) null mice by immunohistochemical detection of Iba1, a marker of activated microglia. Ager null mice displayed fewer diet-induced Iba1-positive cells in hypothalamus than did wild type mice (39). In addition, it was recently shown that a HF/HS, but not HF diet, induces hypothalamic microgliosis that is attenuated in Ager null mice (42). Evidence linking RAGE to anhedonia is demonstration that chronic stress induces anhedonia in wild-type but not Ager null mice (43).
Most of the evidence indicating a link between inflammation and depressive illness is based on high levels of peripheral blood inflammatory markers (44) and neuroimaging that reveals microglial activation (45,46). Two animal studies that traced the link between diet, inflammation, and depression demonstrated that subjects switched from chow to HF or HF/HS displayed increased expression of inflammatory markers in hippocampus (47) and NAc (48), respectively, along with behavioral signs of depression and anxiety. Further, intracerebroventricular administration of the RAGE ligand, HMGB1, upregulated hippocampal TNF-α protein, and induced behavioral signs of depression (49).
In the first experiment of the present study, lick microstructure analysis was combined with analyses of plasma and NAc samples in subjects maintained on HF/HS or control diet in order to test the hypothesis that anhedonia is associated with insulin resistance and altered levels of RAGE and/or AGEs. In the second experiment, a small molecule RAGE antagonist was administered to test the hypothesis that diet-induced effects identified in the first experiment are RAGE-dependent. The method of sugar lick microstructure analysis has been used extensively to differentiate parameters indicative of hedonic impact, motivation to consume, and their underlying mechanisms (50–61). The small molecule RAGE antagonist, RAGE229, shows excellent distribution to brain (brain/plasma ratio = 0.88 at 2 h; based on intraperitoneal administration performed by Contract Research Organization, BioDuro LLC, Irvine, CA) and was previously shown to decrease RAGE ligand–mediated signaling, suppress the acute inflammatory effects of RAGE ligands, improve glucose, insulin, and lipid metabolism, and decrease cardiac and renal pathology in diabetic mice when administered by oral gavage or medicated chow at daily doses of 10–30 mg/kg (62,63).
2. Materials and methods
2.1. Rats
Male and female Sprague–Dawley rats were purchased from Taconic Farms (Germantown, NY) at 10–12 weeks of age. Rats were housed individually in plastic cages with bedding and free access to water. They were maintained on a 12 h light/dark cycle (lights on at 6 am), and allowed at least three days acclimation to vivarium housing prior to initiation of any experimentation. All experimental procedures were approved by the New York University Grossman School of Medicine Institutional Animal Care and Use Committee and were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 85–23). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Diets
All rats in Experiment 1 and Experiment 2 had free access to standard lab pellets (Rodent Diet #5001, Lab Diet, St. Louis, MO) and tap water in the home cage. Half the rats in Experiment 1 and all rats in Experiment 2 also had continuous access to Milk Chocolate Ensure, for which the ratio of % kcal from fat:carb:protein is 4:4:2 with 25% sugar (Abbott,Columbus, OH). We have previously shown that, relative to control, Ensure increases body weight gain and circulating insulin by ~50% over a 4 week period, and induces insulin receptor insensitivity in the NAc (24,25).
2.3. Methods of habituation, preliminary behavioral testing and group assignment
Rats underwent three to five habituation sessions in which they were transported to the laboratory from the vivarium, remained for several hours, and then returned. Upon completion of the habituation series, each rat was placed in a lickometry test chamber and given access to 6% unflavored glucose (Sigma-Aldrich). Sustained licking for a period of 60 seconds (s) was criterion for advancing to the next phase of experimental preparation, and the subject was removed from the chamber. Otherwise, the session continued for up to 30 minutes (min). Any rat that did not meet criterion was retested on up to three additional occasions. All rats met criterion for advancement. Following satisfaction of the lick criterion, subjects underwent a series of three preliminary test sessions of 30 min duration, with access to unflavored 6% glucose. Results of the third test were considered to indicate baseline behavior. The total number of licks/30 min in the baseline session was used to assign subjects to matched groups for experimental testing. Within each sex, two equal sized groups were formed. In Experiment 1, one group was assigned to continue on standard lab chow and one group was supplemented with continuous access to chocolate Ensure. Behavioral testing resumed one week later and was conducted every seven days for a total of six test sessions. Males and females were tested in separate experiments as there were no a priori predictions regarding sex differences.
In Experiment 2, testing was limited to male subjects based on the robust diet-induced behavioral and associated metabolic effects observed in Experiment 1. Rats were assigned to two groups matched for baseline lick parameters and then provided with continuous access to chocolate Ensure for the next seven weeks. During the second week of Ensure access, twice daily oral gavage was initiated (0900 and 1700 h) with one group receiving RAGE229 (10 mg/kg in a volume of 1 ml/kg) and the other receiving vehicle (physiological saline). The RAGE229 dose and route of administration were based on pharmacokinetic studies conducted in mice (62). Behavioral testing was conducted once per week beginning ten days after initiation of gavage treatment, and seventeen days after initiation of Ensure access.
2.4. Apparatus and behavioral measures
Testing was conducted in Med Associates (Georgia, VT) operant chambers with a contact lickometer using Med PC and custom software. The fluid available for consumption in test sessions was 6.1% glucose. Each subject was tested in six (Experiment 1) or five (Experiment 2) 30 min sessions spaced one week apart. The lick parameters recorded included total number of licks per session, a proxy for total consumption, plus multiple measures of lick patterning. These included lick bursts, which are groups of licks separated by an inter-lick interval of > 1 s. This criterion for lick bursts is based on analyses of several laboratories which have concluded that an interval of 1 s is most likely to represent a true pause rather than “missed licks” or lateral tongue protrusions (for review see: 53). The number of bursts emitted per unit time is considered a measure of motivation to consume that is also subject to modulation by satiety. Burst size is the number of licks in a burst, where only bursts of > 3 licks are counted and is considered a measure of reward magnitude or hedonic impact. An additional measure of hedonic reactivity recorded was rate of licking in the first 60 s after the first lick of the session. All behavioral data were automatically collected by computer. 24-h intake of Ensure, chow, and total caloric intake were measured once per week and body weights recorded.
2.5. Plasma and tissue collection
Twenty four to forty eight hours after the final behavioral test session, while still maintained on assigned diets but including a terminal five hour fast, rats were briefly exposed to CO2, and decapitated by guillotine. Trunk blood was collected in BD vacutainer EDTA tubes. The samples were kept on ice for up to 60 min followed by centrifugation at 2,000 g for 20 min at 4°C. The plasma layer was collected and stored at −80°C until assayed. Brains were immediately frozen in powdered dry ice and stored at −80°C. On a future occasion frozen punches (2.0 mm diameter) were collected from three consecutive 500 micron brain sections to obtain samples of NAc that were then stored at −80°C until assay.
2.6. Measurement of plasma glucose, insulin, adiponectin and leptin
Plasma concentrations of glucose were measured using a Accu-Check nano glucose meter. Concentrations of insulin, adiponectin and leptin were determined using rat-specific ELISA kits (Insulin: ALPCO Diagnostics, Cat # 80-INSRT-E01; Adiponectin: R&D R6000B; Leptin: ABCAM, #AB100773) following the manufacturer’s instructions. Absorbance readings were performed using a PowerWave XS2 microplate spectrophotometer (BioTek).
2.7. NAc sample preparation
Tissue samples were placed in 2 mL reinforced plastic tubes with RIPA buffer (Cell Signaling Technology; Cat # 9806) containing Pierce™ phosphatase and protease inhibitors (ThermoFisher Scientific; Cat # A32957 and A32953, respectively) and 0.5 mM PMSF Protease Inhibitor (ThermoFisher Scientific; Cat # 36978). Tissue homogenization was performed using a Bead Ruptor 12 bead mill (Omni International). Samples were homogenized for 3 min at 3.1 m/s, followed by incubation in ice for 10 min. The homogenization/incubation cycle was repeated an additional 2–3 times. NAc protein extracts were subjected to centrifugation at 4°C, 20,000 g for 15 min and the soluble homogenate was collected.
2.8. Immunoblotting
Plasma AGEs, carboxymethyl lysine (CML), a specific AGE ligand of RAGE, and NAc RAGE, CML, AGEs, pCREB/CREB, and pDARPP-32(Thr34)/DARPP-32 were measured by immunoblotting. Sample protein concentration was quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Samples were diluted to 1–6 μg/μL, mixed with sample reducing agent and loading dye (Thermo Scientfic; # NP0009 and NP0007, respectively), boiled, and separated in 10% Protean TGX gels (Bio-Rad). Individual gels contained at least 4 samples from each diet group, all of the same sex. The amount of total protein loaded ranged between 10 and 50 μg depending on the target protein. The separated proteins were blotted onto nitrocellulose membranes (Bio-Rad). Membranes were incubated in blocking buffer (5% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 (TBST) for p-DARPP32, Odyssey LI-COR Blocking Buffer for all other proteins) for 1 h at room temperature, followed by overnight incubation at 4°C with primary antibodies against the specific target protein (RAGE: R&D Systems, #AF1179; CML: R&D Systems, #MAB3247; AGEs: Abcam, #23722; CREB: Cell Signaling Technology, #9197; p-CREB: Cell Signaling Technology, #9196; DARPP-32: Cell Signaling Technology, #mAb2306; pDARPP-32 (Thr34): Cell Signaling Technology, #mAb12438) in 5% BSA TBST(p-DARPP32) or in a 1:1 LI-COR Blocking Buffer/TBST solution (all other proteins). Membranes were washed 3 times for 5 min in 0.1% TBS-T and incubated with secondary antibody (LI-COR #925–32214, #925–32214, #925–32214) in 1% BSA TBST (p-DARPP32) or TBST (all other proteins) for 1 h at room temperature. Membranes were washed twice in TBST and scanned with a LI-COR Odyssey Classic imaging system. Antibodies were stripped by incubating the membrane in a 0.2 M NaOH solution for 15 min at room temperature followed by 3 washes in TBST. The membrane was blocked and incubated with a second primary antibody against a specific target and/or a reference protein (Transferrin: GeneTex, #GTX112729; b-Actin: Santa Cruz, #sc-69879) as described above, washed, incubated with secondary antibody and scanned. Target and reference protein bands were quantified and target protein fluorescence signal for each lane was normalized to the respective reference protein signal. Target protein levels from each individual blot were further normalized by the average target protein level of the control group samples.
3. Results
3.1. Experiment 1
Males: intake and body weight.
On the day of diet assignment, body weights of male subjects in the control and Ensure-supplemented groups were 440.7 (± 10.7) g and 443.7 (± 9.9) g, respectively. During the six week period of testing, which began one week after diet initiation, the mean 24 hour caloric intake of subjects in the control and Ensure-supplemented groups was 105 (± 6.7) and 141.7 (± 7.1) kcal, respectively (t(22)=3.1, p<.01; Table 1), yielding a higher terminal body weight (t(22)=3.2, p<.01; Table 1) and percentage increase in body weight (t(22)=8.1, p<.01; Fig 1) in the Ensure-supplemented group.
Table 1.
Intakes and Body Weights
| Diet | Initial BW | Final BW | Mean 24 h Chow | Mean 24 h Ensure | Mean 24 h Calories |
|---|---|---|---|---|---|
| Male | |||||
| Chow | 440.7 (±10.7) g | 522 (±14.8) g | 31.5 (±1.8) g | 105.0 (±6.7) kcal | |
| Chow + Ensure | 443.7 (±9.9) g | 590 (±15.2) g * | 11.8 (±1.2) g | 94.5 (±2.6) ml | 141.7 (±7.1) kcal * |
| Female | |||||
| Chow | 289 (±3.1) g | 323 (±4.3) g | 23.9 (±1.5) g | 79.8 (±4.8) kcal | |
| Chow + Ensure | 289 (±3.7) g | 387 (±10.7) g * | 8.2 (±0.8) g | 71.5 (±5.0) ml | 103.1 (±2.9) kcal * |
p <.01
Figure 1.
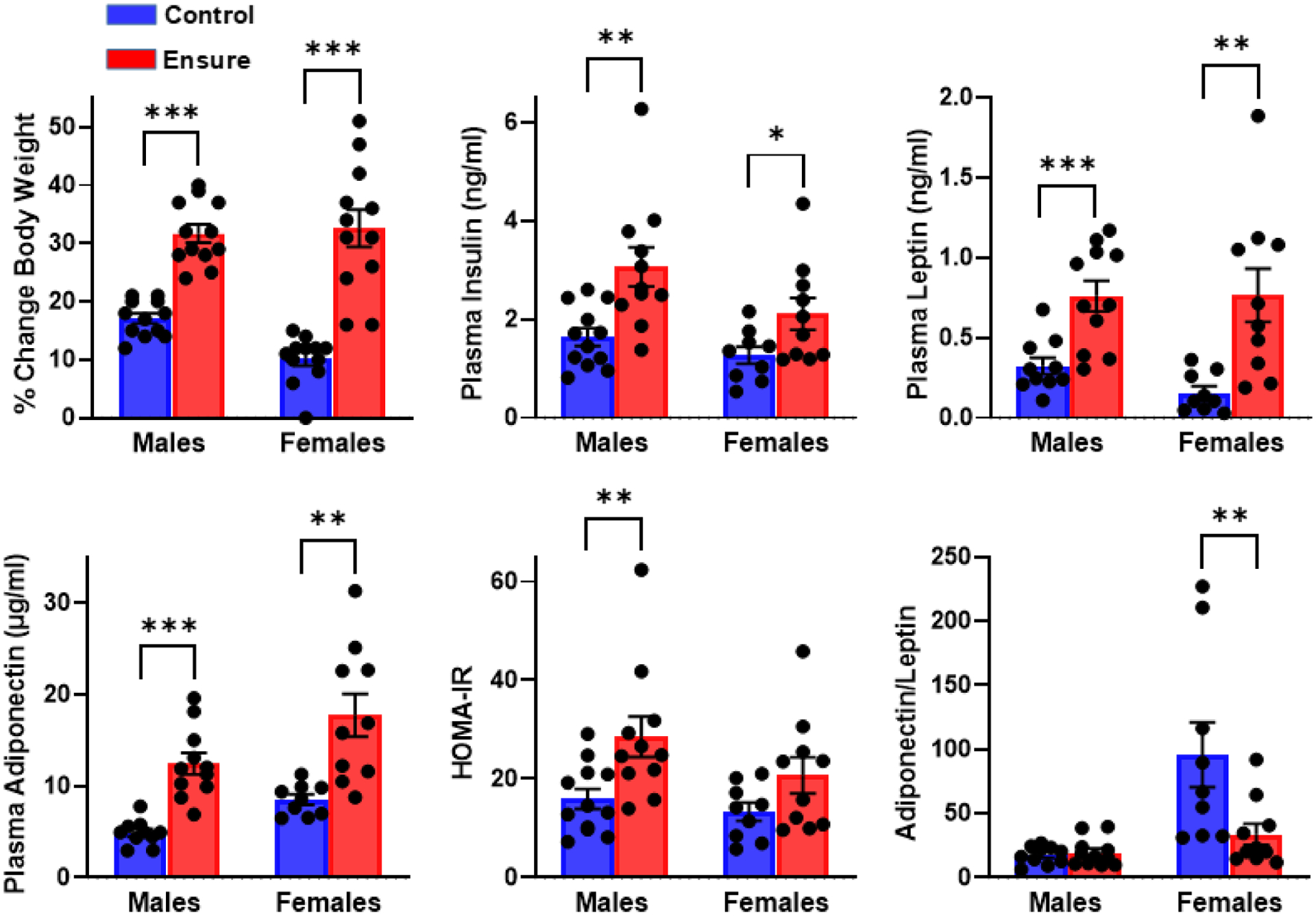
Effects of six weeks of diet supplementation with chocolate Ensure, relative to a chow control diet, on percentage body weight gain in male and female rats shown along with effects on plasma concentrations of insulin, leptin, and adiponectin. Insulin resistance, estimated by HOMA-IR and based on plasma glucose and insulin concentrations, is shown, as is the adiponectin/leptin ratio, a measure of adipose health. n= 12 males, 12 females per diet; ***p<.001, **p<.01, *p<.05
Females: intake and body weight.
On the day of diet assignment, body weights of female subjects in the control and Ensure-supplemented groups were 289 (± 3.1) g and 289 (± 3.7) g, respectively. During the six week period of testing, the mean 24 h caloric intake of subjects in the control and Ensure-supplemented groups was 79.8 (± 4.8) and 103.1 (± 2.9) kcal, respectively (t(22)=3.9, p<.001; Table 1), yielding a higher terminal body weight (t(22)=5.5, p<.001; Table 1) and percentage increase in body weight (t(22)=6.6, p<.001; Fig 1) in the Ensure-supplemented group.
Males: plasma.
Subjects in the Ensure-supplemented group showed elevated plasma insulin (t(21)=3.36, p<.005), leptin (t(19)=3.9, p<.001) and adiponectin (t(19)=5.86, p<.001) when compared to subjects maintained on the control diet (Fig 1). Although fasting glucose did not differ between diet groups (156 (±6.7) vs 162 (±4.7) mg/dl, t(22)=.75, p>.05), insulin resistance, estimated from fasting glucose and insulin concentrations, using homeostatic model assessment (HOMA-ir; 64,65), was suggested (t(21)=2.83, p<.01) in the group consuming Ensure (Fig 1). The adiponectin/leptin ratio, which when decreased, suggests adipose tissue dysfunction, was unaffected by diet (t(19)=0.45).
Nε-carboxy-methyl-lysine (CML) is a major AGE and ligand of RAGE, found in tissue and blood, and is a key modulator of adipokine expression and insulin resistance (66). Western blot analyses probing for CML modifications produced multiple bands, suggesting that these post-translational modifications occurred on multiple different proteins. There were no diet-induced changes in CML modifications on plasma proteins (57 kDa: t(21)=1.05, 50 kDa: t(21)=0.3; 25 kDa: t(21)=0.98); nor was there a difference in general AGE adducts (t(21)=.33) (Fig 2).
Figure 2.
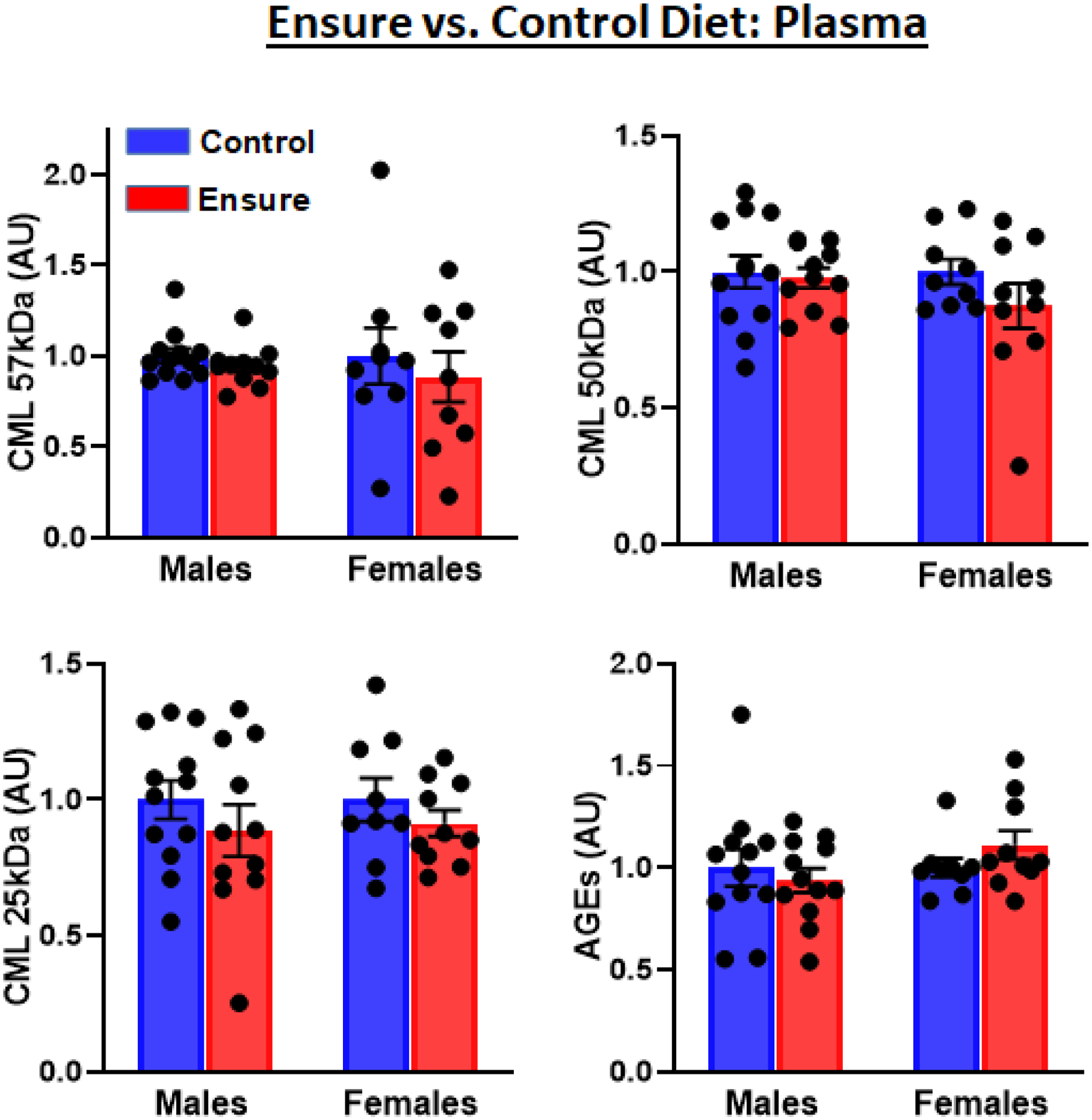
Plasma CML modifications (three different molecular weights) and AGE adducts in male and female subjects consuming the Ensure-supplemented versus chow control diet were detected by Western blotting.
Females:plasma.
Subjects in the Ensure-supplemented group showed elevated plasma concentrations of insulin (t(17)=2.21, p<.05), leptin (t(18)=3.4, p<.01), and adiponectin (t(17)=3.65, p<.01) compared to subjects maintained on the control diet (Fig 1). Fasting glucose did not differ between groups (177 (±6.7) vs 161 (±6.1) mg/dl, t(17)=1.8, p>.05), and, as estimated from HOMA-ir, there was no evidence of insulin resistance (t(17)=1.74, p>.05). However, the adiponectin/leptin ratio was lower in the group consuming Ensure (t(17)=2.45, p<.05).
Western blot analyses indicated no diet-induced changes in plasma CML modifications (57 kDa: t(16)=0.55, 50 kDa: t(17)=1.2; 25 kDa: t(17)=0.95); nor was there a difference in AGE adducts (t(17)=1.55, p>.05) (Fig 2).
Males: nucleus accumbens.
Western analyses probing for CML produced two bands. A marginally significant decrease in the 50kDA band was observed in the Ensure-consuming group (t(22)=1.98, p=.059). This decrease was not seen in the 45kDA band (t(22)=0.15); nor were there diet-related differences in AGE adducts (t(20)=0.9) or RAGE (t(21)=1.08) (Fig 3).
Figure 3.
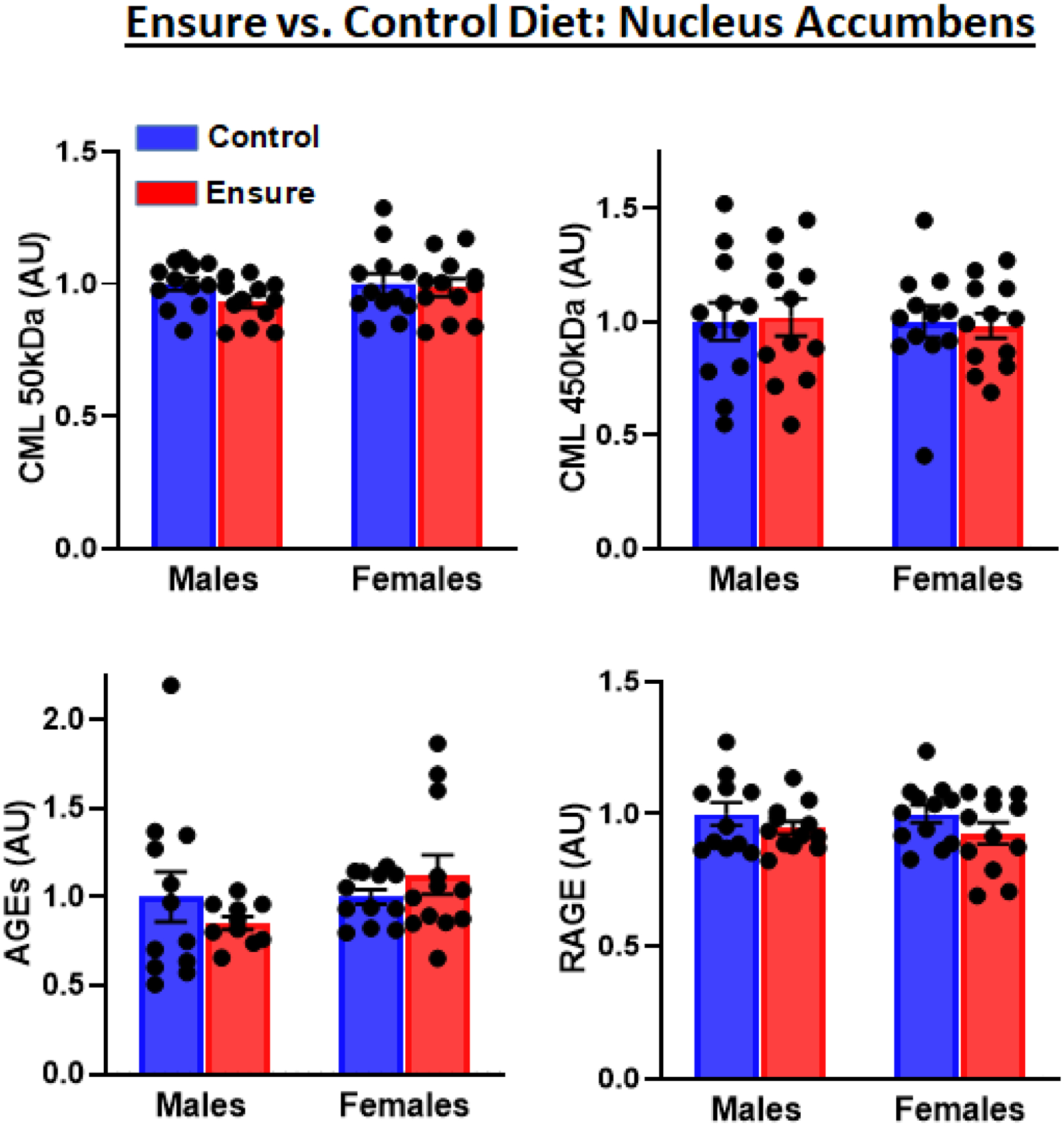
Nucleus accumbens levels of CML modifications (two different molecular weights), AGE adducts, and expression of RAGE protein were detected by Western blotting in male and female subjects consuming the Ensure-supplemented versus chow control diet.
Females: nucleus acumbens.
There were no diet-related differences in the 50kDA CML band (t(22)=0.27), the 45kDA CML band (t(22)=0.21), AGE adducts (t(22)=1.06), or RAGE (t(22)=1.38) (Fig 3).
Males: lick microstructure and correlations with measures taken in plasma and nucleus accumbens.
Males with access to Ensure displayed a decrease in all three lick parameters across the six weeks of testing. Specifically, first minute lick rate (F(1,22)=4.8, p<.05), lick burst size F(1,22)=4.24, p=.05), and the number of lick bursts emitted per session (F(1,22)=4.48, p<.05) were all lower in the Ensure-consuming relative to control group (Fig 4). However, in the final test session, which preceded blood and brain collection by 24–48 h, only lick burst size, the measure of hedonic impact, was significantly decreased (t(22)=2.57, p<.025; Fig 5).
Figure 4.
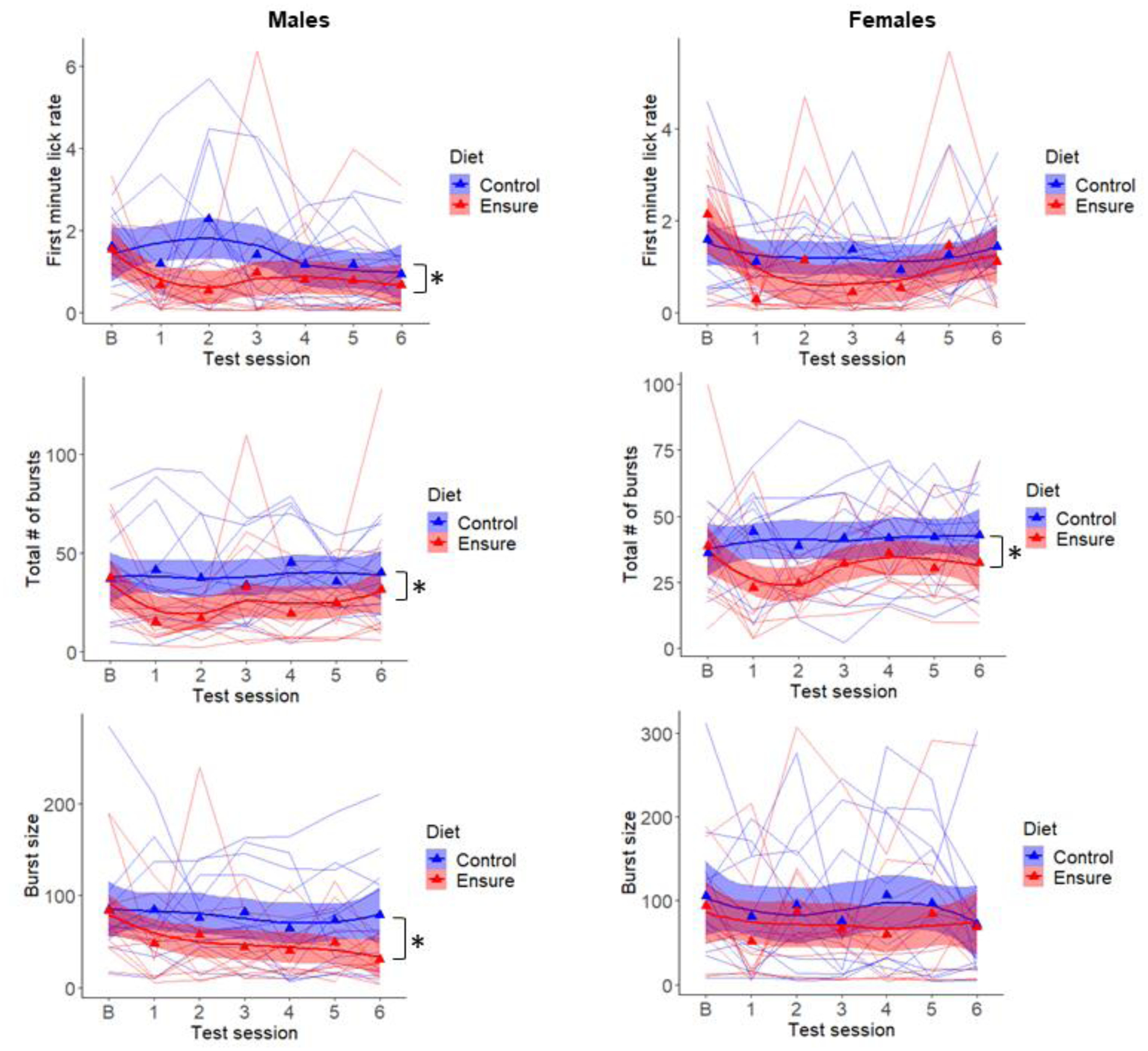
Lick microstructure for 6% glucose was tested in male and female subjects under baseline conditions (b), prior to diet assignment, and once each week during consumption of the Ensure-supplemented or chow control diet. Measures of hedonic impact, first minute lick rate (top) and average lick burst size (bottom), and motivation to consume, number of lick bursts emitted per session (middle), are shown. Triangles are mean values and lines represent individual subjects. *p<.05
Figure 5.
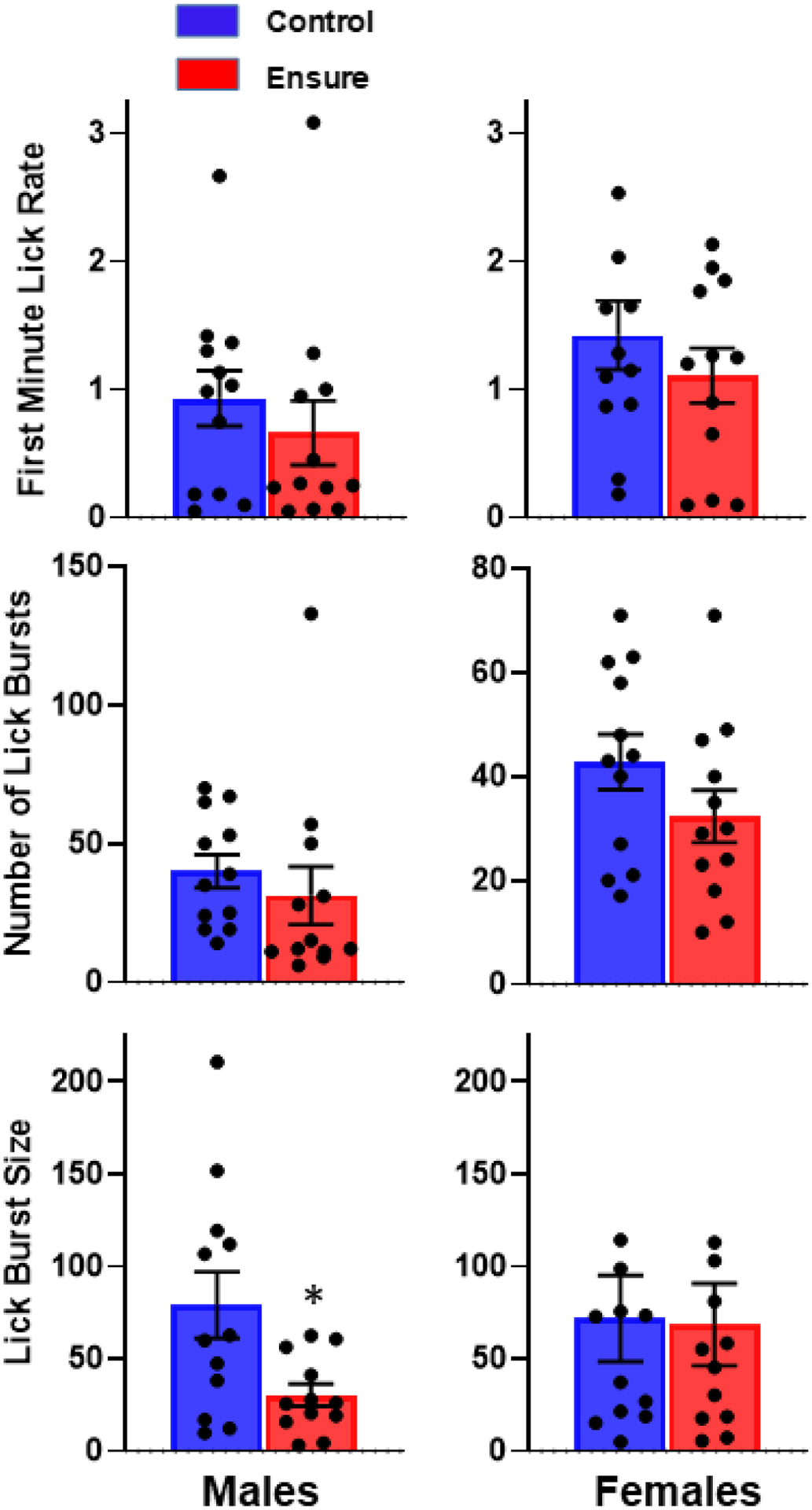
Lick microstructure results in the final test (session #6 in Figure 4) prior to collection of blood and brain. *p<.05
Spearman correlation coefficients were calculated to assess monotonic association between lick microstructure parameters and measures taken in plasma and NAc (Table 2). This assessment was confined to the final behavioral test that preceded plasma and brain sample collection in the Ensure-consuming group. Association was seen between anhedonia, as reflected in lick burst size, and plasma insulin (rs= −0.62, t(9)= 2.36, p<.05), as well as a trend toward association with NAc RAGE (rs= −0.53, t(10)= 1.98, p=.075). Marginally significant negative associations were also seen between first minute lick rate and plasma CML (50kDa; rs= −0.56, t(9)= 2.04, p=.071) and AGEs (rs= −0.54, t(10)= 2.05, p=.067).
Table 2.
Rank Order Correlations
| Behavior | Insulin | HOMA-ir | CML57kDa | CML50kDa | CML25kDa | AGEs | A/L | NAcRAGE | NAcCML50kDa | NAcCML45kDa | NAcAGEs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||||
| 1st m lick rate | +.02 | -.07 | -.17 | -.56† | -.43 | -.54† | -.15 | -.34 | +.17 | -.07 | +.15 |
| # lick bursts | +.32 | +.30 | 0 | -.44 | -.43 | -.25 | -.27 | -.45 | -.02 | -.54† | -.04 |
| licks/burst | -.62* | -.51 | -.06 | -.41 | −45 | -.2 | +.13 | -.53† | +.07 | +.05 | +.05 |
| Female | |||||||||||
| 1st m lick rate | +0.3 | +.43 | -.20 | +.03 | +.65* | -.36 | -.09 | -.04 | -.14 | -.10 | -.31 |
| # lick bursts | +.72* | +.85* | +.43 | -.44 | +.59† | -.71* | -.54 | -.33 | -.14 | -.13 | -.16 |
| licks/burst | -.26 | -.35 | -.62† | +0.3 | -.09 | +.22 | +.10 | +.44 | -.21 | +.30 | -.23 |
p at least <.05
marginally significant difference (p≤ .075)
There were no indications of association between any behavioral measure and adiponectin/leptin ratio.
Females: lick microstructure and correlations with measures taken in plasma and nucleus accumbens.
Females displayed no diet-induced change in the two measures of hedonic impact. Specifically, first minute lick rate (F(1,22)=3.0, p>.05) and lick burst size (F(1,22)=0.5) were unchanged by Ensure consumption. Females did, however, show a diet-induced decrease in the motivational measure, number of lick bursts emitted (F(1,22)=5.77, p<.05) (Fig 4). However, in the final test session, which preceded blood and brain collection, neither the number of lick bursts (t(22)=1.43, p>.10; Fig 5) nor any other measure was significantly decreased in the Ensure-consuming group relative to control.
Positive associations were seen between motivation to consume, as reflected in number of lick bursts, and plasma insulin (rs= +.72, t(8)= 2.85, p<.05), HOMA-ir (rs= +.85, t(8)= 4.65, p<.01) and plasma CML (25kDA; rs= +.59, t(8)= 2.06, p=.073), but a negative association was seen with plasma AGEs (rs= −.71, t(8)= 2.84, p<.05) (Table 2).
There were no indications of association between any behavioral measure and adiponectin/leptin ratio or measures taken in nucleus accumbens.
Summary and additional probes.
In contrast to females, male subjects displayed the predicted diet-induced anhedonia, robust increases in plasma insulin and HOMA-ir, and multiple associations between anhedonia and plasma and NAc levels of AGEs and/or RAGE. Their NAc samples were therefore also probed for two intracellular proteins, pCREB and pDARPP-32 (Thr34), which are known to be responsive to diverse reward stimuli and, in particular, palatable food. Past studies indicate that acute consumption increases phosphorylation of DARPP-32 (Thr34), and chronic consumption decreases phosphorylation of CREB (67–72). Results for tissue samples obtained in Experiment 1 are presented together with results obtained in Experiment 2 below.
3.2. Experiment 2
This experiment was designed to test the potential effects of a small molecule antagonist of RAGE, RAGE229 in the above studies in male rats. Male rats were selected for study in this experiment, since only the male subjects displayed evidence of anhedonia. At the start of Experiment 2, male subjects assigned to vehicle-treatment weighed an average of 460 ± 9.2 grams, and subjects assigned to RAGE229-treatment weighed an average of 475 1± 7.2 grams. RAGE229 vs. vehicle gavage was instituted one week after the beginning of the Ensure consumption. During the six week period of combined Ensure consumption and daily gavage treatment, the mean 24 h intake of chow by vehicle-and RAGE229-treated subjects was 7.8 (±1.8) g, yielding 26.1 kcal, and 6.6 (±1.2) g, yielding 22.2 kcal, respectively. The mean 24 hour intakes of Ensure were 73.6 (±2.8) ml, yielding an additional 78 kcal, and 69 (±3.0) ml, yielding an additional 73.1 kcal, respectively. The percentage body weight gain of the two groups during this period did not differ significantly, with the vehicle group gaining 22 (±1.7)% and the RAGE229 group gaining 18.6 (±0.9)% (t(36)=1.82, p>.05).
Treatment with RAGE229 had no effect on plasma glucose (t(36)=0.1), insulin (t(36)=.6) or adiponectin (t(36)=.0), but did result in lower leptin (t(36)=2.35, p<.05) and higher adiponectin/leptin ratio (t(36)=3.01, p<.01; Fig 6). AGEs and RAGE were not assayed in these subjects.
Figure 6.
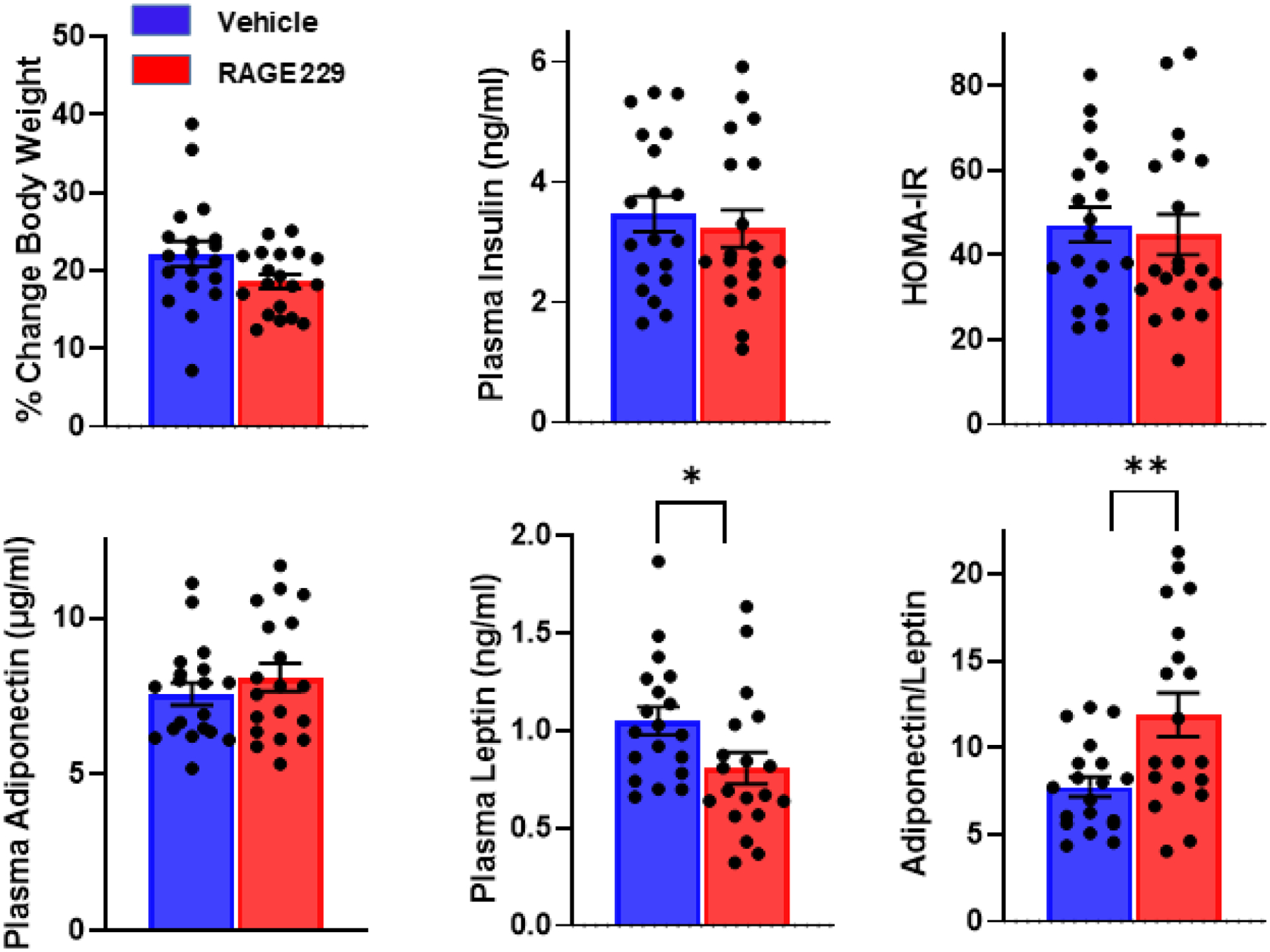
Male subjects were maintained on the Ensure-supplemented diet for six weeks with twice daily oral gavage of RAGE229 (10 mg/kg) or saline vehicle beginning in week two and continuing through the end of the experiment. Percentage increase in body weight is shown along with plasma concentrations of insulin, leptin, and adiponectin. Insulin resistance, estimated by HOMA-IR and based on plasma glucose and insulin, is shown, as is the adiponectin/leptin ratio. n= 19/group; **p<.01, *p<.05
Across the five weekly tests of lick microstructure, all lick parameters were decreased compared to pre-diet baseline measures (see Fig 7). However, the two measures of hedonic impact, first minute lick rate (F(1,36)=4.97, p<.05) and lick burst size (F(1,36)=4.63, p<.05) were higher in the group treated with RAGE229, while the measure of motivation to consume, lick burst number, was not different (F(1,36)=1.85, p>.10; Fig 7). In the final test session, which preceded blood and brain collection by 24 h, first minute lick rate (t(36)=2.02, p=.05) and lick burst size (t(36)=2.24, p<.05) were greater in the RAGE229 treated group, but number of lick bursts (t(36)=0.7) was not (Fig 8). There were no significant correlations between plasma hormone levels and behavioral measures.
Figure 7.
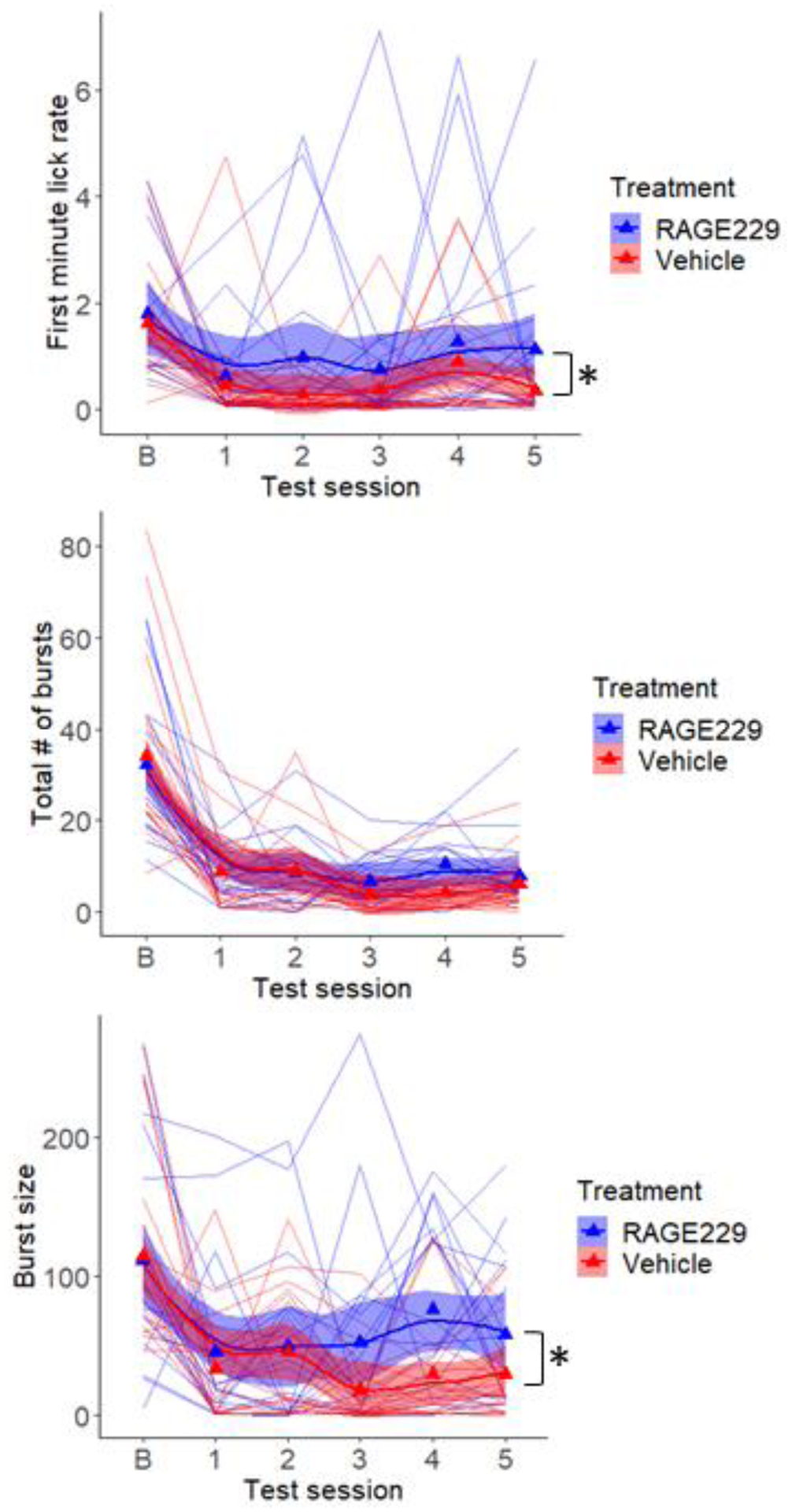
Lick microstructure for 6% glucose was tested under baseline conditions (b), prior to diet implementation, and once each week during consumption of the Ensure-supplemented diet. Beginning in the second week of diet, one group received twice daily oral gavage of RAGE229 (10 mg/kg), and the other received saline vehicle. Measures of hedonic impact, first minute lick rate (top) and average lick burst size (bottom), and motivation to consume, number of lick bursts emitted per session (middle), are shown. Triangles are mean values and lines represent individual subjects. *p<.05
Figure 8.
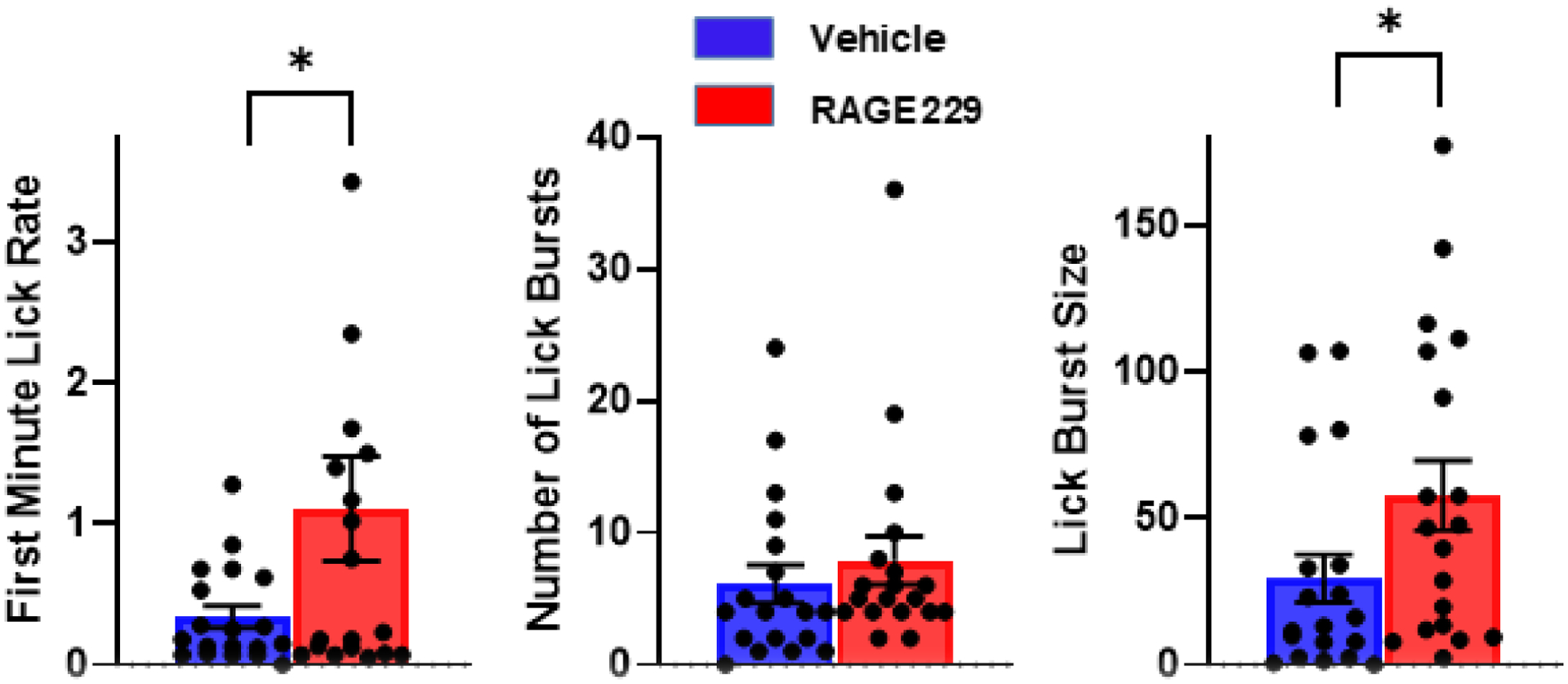
Lick microstructure results in the final test (session #5 in Figure 7) prior to collection of blood and brain. *p<.05
In Experiment 1, there was no diet-related difference in pThr34-DARPP-32/total DARPP (Fig 9, left) and there were no differences observed in rats treated with RAGE229 vs. vehicle (Fig 9, right) (diet: t(22)=1.5, p>.05); RAGE229: t(20)=1.06, p>.05). With respect to pCREB, in Experiment 1, no diet-related difference was observed in pCREB in the NAc (t(18)=0.68). However, in Experiment 2, where both groups consumed Ensure, RAGE229 increased pCREB (t(17)=4.62, p<.001; Fig 10). Although neither diet nor RAGE229 affected the NAc level of p34Thr-DARPP-32, when considered at the level of the individual rat, pThr34-DARPP-32 correlated with lick burst size regardless of treatment (Fig 11, left panel; vehicle: rs= +.69, p<.05; RAGE229: rs= +.69, p<.05). Moreover, RAGE229 shifted the correlation between pCREB and pThr34-DARPP-32 from near-zero under vehicle treatment to +.75 (p<.025) under RAGE229 treatment (Fig 11, middle panel), and increased the correlation between pCREB and lick burst size from near-zero to +.57 (p = ~.10) (Fig 11, right panel).
Figure 9.
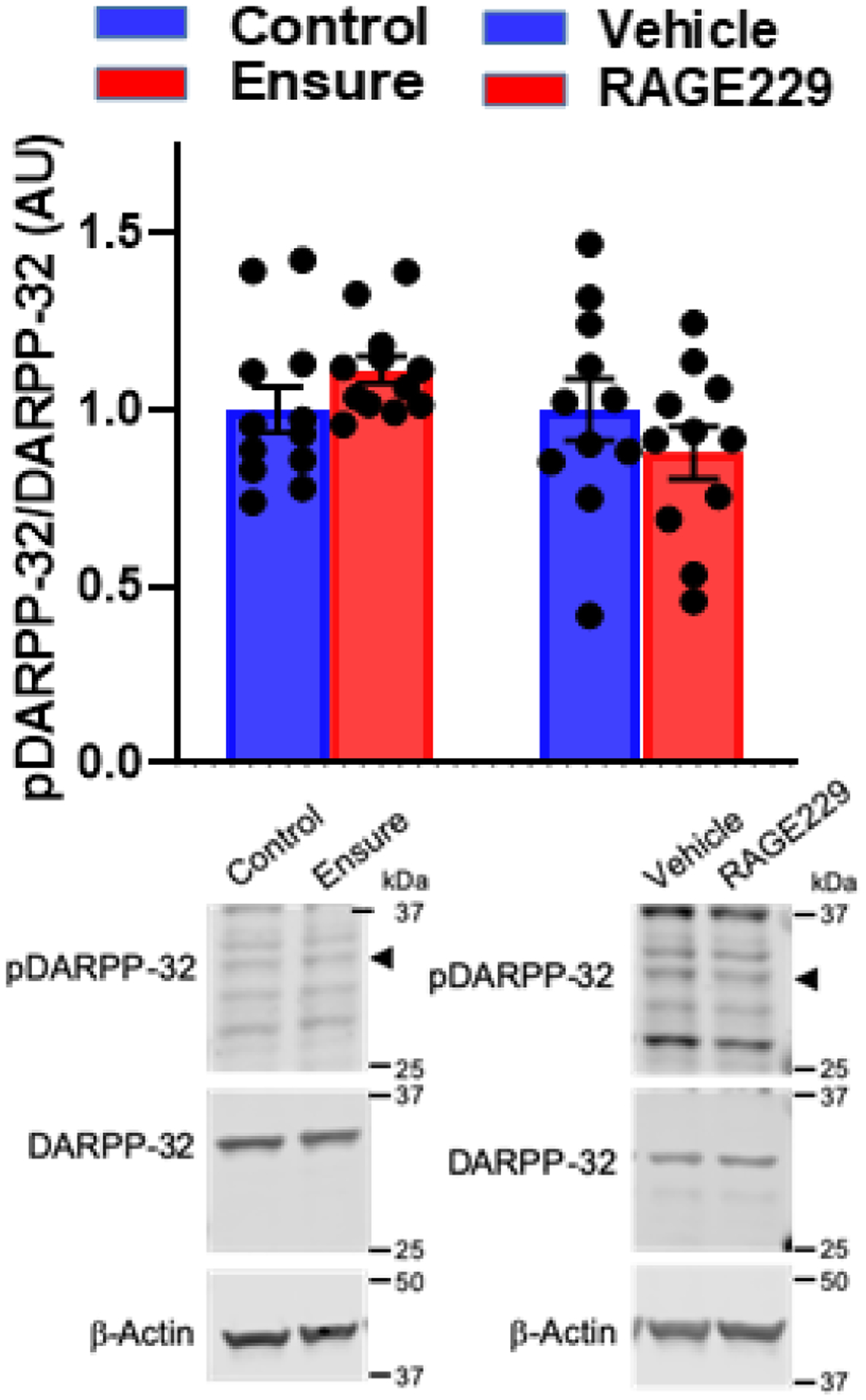
Western blotting of pThr34-DARPP-32/DARPP-32 in NAc of male subjects maintained on control chow versus the Ensure-supplemented diet in Experiment 1 (left). and Experiment 2 (right). In experiment 2, male subjects were maintained on the Ensure-supplemented diet and treated twice-daily with oral gavage of saline vehicle versus RAGE 229 (10 mg/kg) (right). Representative blots included.
Figure 10.
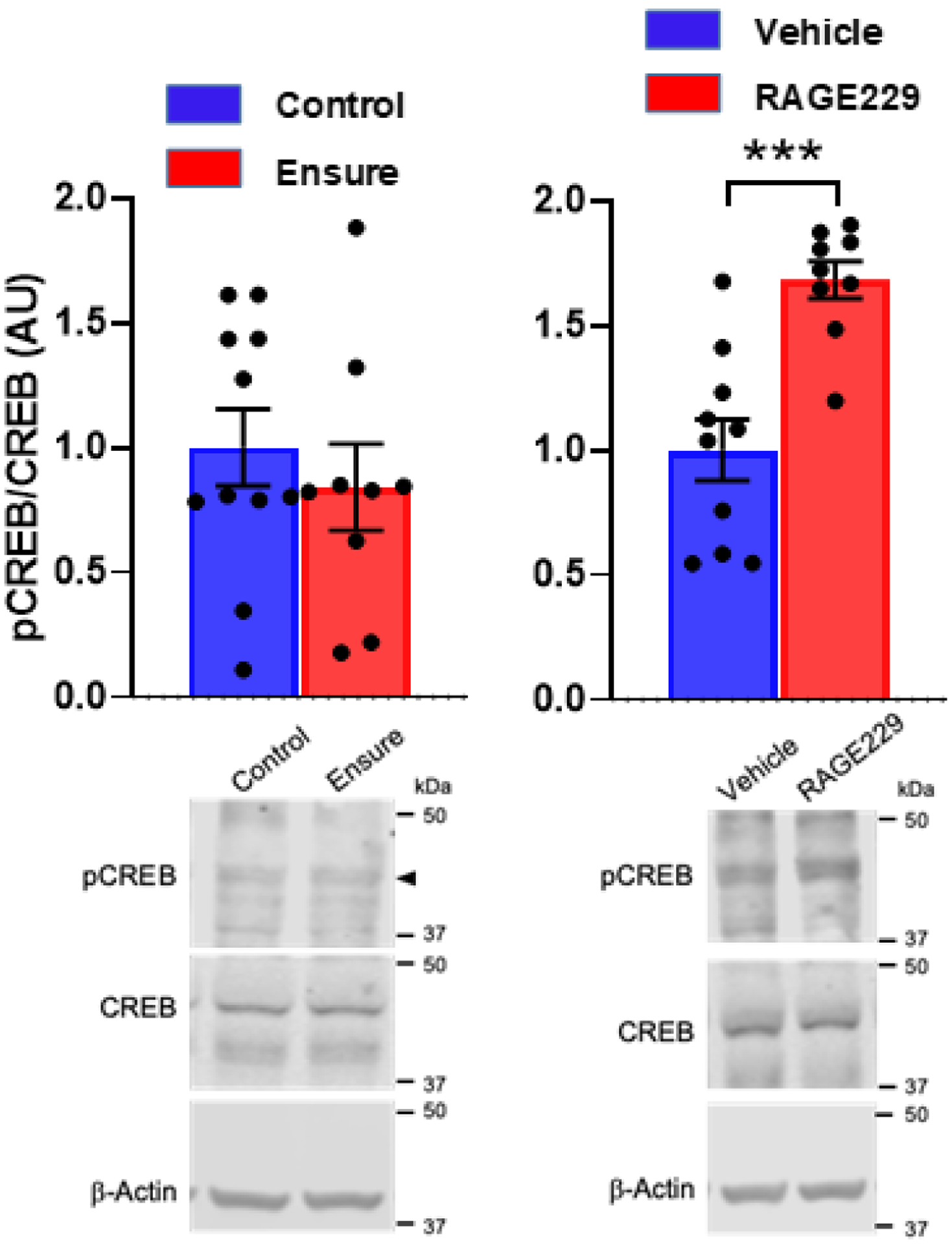
pCREB/CREB in NAc of male subjects maintained on control chow versus the Ensure-supplemented diet in Experiment 1 (left), and pCREB/CREB in NAc of male subjects maintained on the Ensure-supplemented diet and treated twice-daily with oral gavage of saline vehicle versus RAGE 229 (10 mg/kg) in Experiment 2 (right). Representative blots included. ***p<.001
Figure 11.
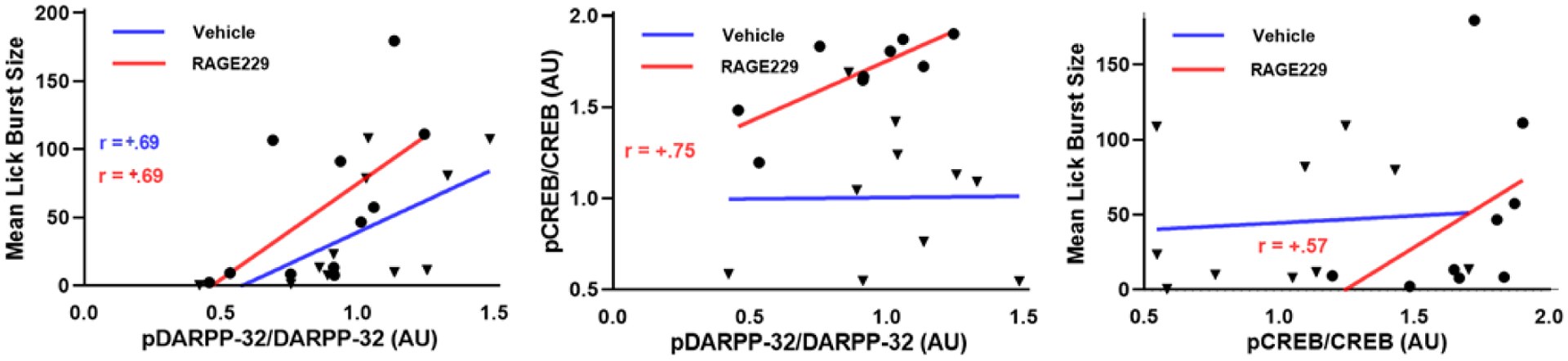
Spearman correlations between mean lick burst size and NAc pThr34-DARPP-32 (left), pCREB (right), and between pThr34-DARPP-32 and pCREB (middle) in vehicle-treated and RAGE229-treated male rats consuming Ensure. ▼=vehicle subject; ●=RAGE229 subject; correlation coefficients are shown.
4. Discussion
In previous studies in male rats, Ensure supplementation increased the rate of body weight gain, fasting plasma insulin, and induced NAc insulin insensitivity relative to chow-fed subjects (24). In a glucose lick microstructure study that included both sexes, acute inactivation of NAc insulin markedly decreased hedonic impact (lick burst size) with no effect on motivation to consume (number of lick bursts emitted). However, when effects of a solid HF/HS diet were evaluated, males displayed a decrease in hedonic impact and females displayed an increase. The purpose of the present study was therefore several-fold: First, to determine how Ensure supplementation, which produces a greater increase in the rate of body weight gain than the solid HF/HS diet (73,74), affects lick microstructure for glucose in male and female rats; second, to determine whether AGEs and their receptor, RAGE, are associated with anhedonia in the lick microstructure protocol; and third, to test for causal involvement of RAGE in behavioral and metabolic effects of Ensure supplementation using a novel small molecule RAGE antagonist, RAGE 229.
In the present study, Ensure supplementation was found to produce similar increases in body weight gain, fasting plasma insulin, leptin, and adiponectin in males and females. Insulin resistance, however, as estimated using the HOMA-ir method, was only confirmed in males. Behaviorally, males and females diverged as well, although not in the same manner seen previously in response to the solid HF/HS diet. Here, males displayed the predicted decrease in hedonic impact of glucose licking, reflected in both the first minute lick rate and mean burst size measures, while females did not; females displayed a decrease in motivation to consume, reflected in the number of lick bursts emitted per session. However, there was no effect of diet on burst number emitted by females in the final test preceding blood collection, though burst number showed a strong positive correlation with plasma insulin and HOMA-ir. This finding, and the female response to solid HF/HS diet (i.e., increased hedonic impact) suggest that diet-induced hyperinsulinemia and IR may drive increased sugar consumption in females, possibly in search of the nutritive signal that is blunted by NAc insulin receptor subsensitivity (24,25).
Whether the sex differences in lick microstructure induced by HF/HS diet in these studies are anomalous is difficult to assess given that induction of a depression-like phenotype by obesogenic diets has generally been only tested in male rats and mice (47,75–81). The observed sex differences do, however, lay the groundwork for future distinct studies testing the effect of estrous and estrogen-related hormones (vs. androgens) in consummatory anhedonia. Given that males have consistently met the behavioral criterion of diet-induced anhedonia, the remainder of the present study, including association between anhedonia and metabolic responses to diet, was focused on males.
Anhedonia correlated with the concentrations of plasma insulin. This association is consistent with past observations that hyperinsulinemia and NAc insulin resistance decrease the rewarding and reinforcing effects of glucose in male rats (24–26). There were also several associations suggestive of a connection between anhedonia, AGEs and RAGE. For example, decreases in all lick microstructure parameters were associated with increased plasma CML modifications (25kDa and 50kDA), though only (marginally) significant for the 50kDA variant, and only in relation to the first minute lick rate. These trends were not seen in females. Also unlike females, Ensure-supplemented males displayed associations between decreased lick parameters and NAc level of RAGE, though only (marginally) significant in relation to lick burst size.
The present findings that HF/HS diet did not alter AGEs or RAGE cannot be considered a comprehensive assessment. AGEs are not the only endogenous ligands for RAGE. RAGE binds other ligands linked to inflammation such as HMGB1 and multiple members of the S100/calgranulin family, resulting in pro-inflammatory gene activation (28–30); none of which were assayed here but represent an avenue for future investigations. An additional caveat is in the emergence of the hypothesis that elevated AGEs in obese subjects are trapped in adipose, and contribute to adipose dysfunction and adipokine signaling. In that model, it is suggested that this may account for lower plasma AGE content (66). Specifically, it has been shown that there is CML accumulation, increased expression of Hmgb1 and increased expression of RAGE in adipose tissue of obese mice (39), which is associated with dysregulated inflammatory adipokines in adipocytes via a RAGE-dependent pathway. Moreover, mice with adipocyte-specific deletion of Ager (82), the gene that codes for RAGE, were protected from HF feeding in terms of adiposity and metabolic parameters, such as glucose and insulin tolerance (82). The increased adiponectin/leptin ratio observed in response to RAGE229 in the present study is indicative of improved adipose function (83,84), and draws attention to the possibility that the critical depot for AGEs and RAGE in the Ensure-consuming subjects may dwell, at least in part, in the visceral adipose tissue. If one of the initiating signal(s) for anhedonia is emitted from the adipocyte, one possibility is that exosomes derived from the adipose tissue cross the blood-brain barrier, as recently shown (85), and through their cargo, transport signals that affect NAc and/or other reward-related brain regions (86). Alternatively, circulating factors that cross the blood-brain barrier may initiate these events.
Twice daily administration of RAGE229 did not alter 24 h Ensure consumption, total caloric intake, body weight gain, or fasting plasma insulin. However, both behavioral measures of anhedonia were less affected by Ensure-diet in the RAGE229-treated subjects than in vehicle-treated subjects. The behavioral measure of motivation to consume was unaffected by RAGE229. These results are consistent with previous findings indicating that the mechanistic underpinnings of lick burst size and lick burst number are dissociable (87). Moreover, they support two important conclusions. First, restoration of lick burst size and first minute lick rate by RAGE229 with no change in mean 24 h Ensure consumption suggests that the effect of Ensure on these measures reflects anhedonia rather than alternatives such as taste contrast or sensory-specific satiety. Second, these results tie RAGE to anhedonia. Despite the fact that sugar preference is a standard assay for anhedonia in rodent models of depression (88), it will be important to evaluate effects of the current HF/HS diet and RAGE229 on other domains of hedonic reactivity to determine whether the present diet- and RAGE229induced effects extend beyond consummatory behavior to hedonic reactivity in general, consistent with other models of obesogenic diet-induced ‘depression’ (47,75–81).
In an initial probe of potential mechanistic underpinnings, effects of diet and RAGE229 on pThr34-DARPP-32, a marker of dopamine (D)1R signaling, and pCREB, a transcription factor that is activated by several receptor-activated protein kinases including Protein Kinase A (PKA) downstream of the D1R (70, 89–91), were determined. Despite the fact that Ensure-diet had no effect on levels of pThr34-DARPP-32 or pCREB, RAGE229 markedly increased the NAc level of pCREB, indicating that RAGE signaling directly or indirectly affects NAc function. pThr34-DARPP-32, which was unchanged by RAGE229, correlated with lick burst size regardless of gavage treatment. Interestingly, RAGE229 not only increased pCREB but shifted its’ correlation with pThr34-DARPP-32 from near-zero to +.75, as well as increasing its correlation with lick burst size. This set of findings suggests that the receptor/signaling pathway and cell type underlying the RAGE229-mediated increase in pCREB may mediate anhedonia and its prevention. Importantly, a limitation of the present study is that the entire NAc was profiled; therefore, future studies must investigate specific effects in neurons vs. non-neuronal cells, such as microglia, astrocytes or endothelial cells, as examples.
In summary, the present study has produced several findings suggesting a role for RAGE in HF/HS diet-induced anhedonia, and has pointed to several questions for continuing investigation. These questions center on (i) the sex difference in behavioral response to the liquid HF/HS diet despite similar weight gain and hyperinsulinemia, (ii) whether anhedonia extends to behavioral domains beyond consummatory, (iii) the possible involvement of RAGE ligands, other than AGEs, not assayed in the present study, (iv) adipose tissue as a depot for accumulation of RAGE ligands and signaling, (v) cell-type specific effects of RAGE229 on pCREB and pThr34-dDARPP-32 in the NAc, and (vi) whether circulating factors of adipose origin signal to NAc, target a subset of non-D1Rexpressing neurons, and modify hedonic reactivity.
Highlights.
Six weeks of continuous access to a liquid high fat-high sugar diet, known to induce nucleus accumbens insulin resistance, induced anhedonia in a glucose lick microstructure assay in male, but not female, rats
Anhedonia correlated with plasma concentrations of insulin and displayed marginally significant correlations with plasma advanced glycation end products and the expression of their receptor (RAGE) in nucleus accumbens.
Diet-induced anhedonia was prevented by concurrent treatment with the small molecule RAGE antagonist, RAGE229.
Treatment with RAGE229 increased pCREB/CREB in the nucleus accumbens and shifted its’ correlation with pThr34-DARPP-32/DARPP-32 from near-zero to strongly positive, such that both phospho-proteins correlated with the rescued hedonic response.
Acknowledgements
This research was supported by R21MH121239 from NIMH/NIH and R01 DA050165 from NIDA/NIH to KDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biessels GJ, Reagan LP (2015) Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16:660–671. [DOI] [PubMed] [Google Scholar]
- 2.Bruehl H, Sweat V, Hassenstab J, Polyakov V, Convit A (2010) Cognitive impairment in nondiabetic middle- aged and older adults is associated with insulin resistance. J Clin Exp Neuropsychol 32:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW (2016) Diabetes and cognitive impairment. Curr Diab Rep 16:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghasemi R, Dargahi L, Haeri A, Moosavi M, Mohamed Z, Ahmadiani A (2013) Brain insulin dysregulation: implication for neurological and neuropsychiatric disorders. Mol Neurobiol 47:1045–1065. [DOI] [PubMed] [Google Scholar]
- 5.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP (2015). Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 64: 3927–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenkovich K, Brown ME, Svrakic DM, Lustman PJ (2015) Depression in type 2 diabetes mellitus: prevalence, impact, and treatment, Drugs 75: 577–587. [DOI] [PubMed] [Google Scholar]
- 7.Watson KT, Simard JF, Henderson VW, Nutkiewicz L, Femke Lamers BA, Nasca C, Rasgon N, Penninx BWJH (2021) Incident major depressive disorder predicted by three measures of insulin resistance: A Dutch cohort study. Am J Psychiatry 178: 914–920. [DOI] [PubMed] [Google Scholar]
- 8.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. (2006) The prevalence of comorbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 23:1165–1173. [DOI] [PubMed] [Google Scholar]
- 9.Nefs G, Pouwer F, Denollet J, Kramer H, Wijnands-van Gent CJ, Pop VJ. (2012) Suboptimal glycemic control in type 2 diabetes: a key role for anhedonia? J Psychiatr Res 46:549–54. [DOI] [PubMed] [Google Scholar]
- 10.Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, Pouwer F (2010) Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 53:2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dooren FEP, Nefs G, Schram MT, Verhey FRJ, Denollet J, Pouwer F (2013). Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS One 8: e57058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter J, Swardfager W (2016) Mood and metabolism: Anhedonia as a clinical target in Type 2 diabetes Psychoneuroendocrinol 69: 123–132. [DOI] [PubMed] [Google Scholar]
- 13.Nefs G, Pop VJ, Denollet J, Pouwer F (2016) Depressive symptoms and all-cause mortality in people with type 2 diabetes: a focus on potential mechanisms. Br J Psychiat 209:142–149. [DOI] [PubMed] [Google Scholar]
- 14.Horan WP, Kring AM, Blanchard JL (2006) Anhedonia in schizophrenia: A review of assessment strategies. Schiz Bulletin 32: 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran EK, Culbreth AJ, Barch DM (2022) Anhedonia in schizophrenia. Curr Top Behav Neurosci 58: 129–145. [DOI] [PubMed] [Google Scholar]
- 16.Perry BI, Salimkumar D, Green D, Meakin A, Gibson A, Mahajan D, Tahird T, Singh SP (2017) Associated illness severity in schizophrenia and diabetes mellitus: A systematic review. Psychiat Res 256: 102–110. [DOI] [PubMed] [Google Scholar]
- 17.Mizuki Y, Sakamoto S, Okahisa Y, Yada Y, Hashimoto N, Takaki M, Yamada N (2021) Mechanisms underlying the comorbidity of schizophrenia and Type 2 Diabetes Mellitus. Internat J Neuropsychopharmacol 24: 367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soontornniyomkij V, Lee EE, Jin H, Martin AS, Daly RE, Liu J, Tu XM, Todd Eyler L, Jeste DV (2019) Clinical correlates of insulin resistance in chronic schizophrenia: Relationship to negative symptoms. Front Psychiat 10: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowd EC, Barch DM (2010) Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry 67:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA (2012) Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163:1784–1790. [DOI] [PubMed] [Google Scholar]
- 21.Harvey PO, Pruessner J, Czechowska Y, Lepage M (2007) Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in nonclinical subjects. Mol Psychiatry 12:767–775. [DOI] [PubMed] [Google Scholar]
- 22.Keedwell PA, Williams SC, Brammer MJ, Phillips ML (2005) The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58:843–853. [DOI] [PubMed] [Google Scholar]
- 23.Wacker J, Dillon DG, Pizzagalli DA (2009) The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG fMRI, and volumetric techniques. Neuroimage 46:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold R, Jones KT, Cabeza de Vaca S, Reith MEA, Carr KD, Rice ME (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nature Comm 6 DOI: 10.1038/ncomms9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods CS, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, Cabeza de Vaca S, Sclafani A, Carr KD (2016) Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol Behav 159: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr KD, Weiner SP (2022) Effects of nucleus accumbens insulin inactivation on microstructure of licking for glucose and saccharin in male and female rats. Physiol Behav 249:113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel JC, Stouffer MA, Mancini M, Nicholson C, Carr KD, Rice ME (2019) Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur J Neurosci 49:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM (2013) Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 14:19891–18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litwinoff E, Hurtado Del Pozo C, Ramasamy R, Schmidt AM (2015) Emerging targets for therapeutic development in diabetes and its complications: The RAGE signaling pathway. Clin Pharmacol Ther 98:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramasamy R, Shekhtman A, Schmidt AM (2016) The multiple faces of RAGE--opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targets 20:431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycoxylation end products: a review. Diabetologia 44:129–146. [DOI] [PubMed] [Google Scholar]
- 32.Cannizzaro L, Rossoni G, Savi F, Altomare A, Marinello C, Saethang T, Carini M, Payne DM, Pisitkun T, Aldini G, Leelahavanichkul A. (2017) Regulatory landscape of AGE-RAGE-oxidative stress axis and its modulation by PPARγ activation in high fructose diet-induced metabolic syndrome. Nutr Metab 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzmán-Ruiz M, Layritz C, Legutko B, Zinser E, García-Cáceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschöp MH, Yi CX. (2017) Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg T, Cai W, Peppa M, Dardaine V, Uribarri J, Vlassara H (2004) Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104:1287–1291. [DOI] [PubMed] [Google Scholar]
- 35.Aragno M, Mastrocola R (2017) Dietary sugars and endogenous formation of advanced glycation end products: Emerging mechanisms of disease. Nutrients 14;9 (4). pii: E385. doi: 10.3390/nu9040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigro D, Menotti F, Cento A, Serpe L, Chiazza F, Dal Bello F, Romaniello F, Medana C, Collino M, Aragno M, Mastrocola R (2017) Chronic administration of saturated fats and fructose differently affect SREBP activity resulting in different modulation of Nrf2 and Nlrp3 inflammasome pathways in mice liver. J Nutr Biochem 42: 160–171. [DOI] [PubMed] [Google Scholar]
- 37.Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Prysiecki C, Shaw A (1993) Survey of the distribution of a newly characterized receptor for advanced glycation end products of tissues. Am J. Pathol 143: 1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, Wang F, Pan Y-C, Tsang TC, Stern D (1992) Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung, which are present on the endothelial cell surface. J. Biol. Chem 267:14987–14997 [PubMed] [Google Scholar]
- 39.Song F, Hurtado del Pozo C, Rosario R, Shan Zou Y, Ananthakrishnan R, Xu X, Patel PR, Benoit VM, Yan SF, Li H, Friedman RA, Kim JK, Ramasamy R, Ferrante AW Jr, Schmidt AM (2014) RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes 63:1948–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, Kanoski SE (2015) Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 25:227–239. [DOI] [PubMed] [Google Scholar]
- 41.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ (2010) Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzmán-Ruiz M, Layritz C, Legutko B, Zinser E, García-Cáceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschöp MH, Yi C-X (2017) Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6: 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franklin TC, Wohleb ES, Zhang Y, Fogaça M, Hare B, Duman RS (2018) Persistent increase in microglial RAGE contributes to chronic stress-induced priming of depressive-like behavior Biol Psychiat 83:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS (2016) Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neurosci Biobehav Rev 64:148–166. [DOI] [PubMed] [Google Scholar]
- 45.Drake C, Boutin H, Jones MS, Denes A, Mccoll BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A., Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM (2011) Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 25: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G,Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. (2016) High-Fat diet induced anxiety and anhedonia: Impact on brain homeostasis and inflammation. Neuropsychopharmacol 41:1874–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Décarie-Spain L, Sharma S, Hryhorczuk C, Issa-Garcia V, Barker PA, Arbour N, Alquier T, Fulton S (2018) Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Molec Metab 10: 1e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, Peng W, Zhang T, Zhou JR, Jiang CL, Wang YX (2017) Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid- HMGB1. Brain Behav Immun 59:322–332. [DOI] [PubMed] [Google Scholar]
- 50.Davis JD, Smith GP (1992) Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228. [PubMed] [Google Scholar]
- 51.Spector AC, Klumpp PA, Kaplan JM (1998) Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694. [DOI] [PubMed] [Google Scholar]
- 52.Lardeaux S, Kim JJ, Nicola SM (2013) Intermittent access to sweet high-fat liquid induces increased palatability and motivation to consume in a rat model of binge consumption. Physiol Behav 10: 114–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naneix F, Peters KZ, McCutcheon JE (2020) Investigating the effect of physiological need states on palatability and motivation using microstructural analysis of licking. Neurosci 447: 155–166. [DOI] [PubMed] [Google Scholar]
- 54.Davis JD (1973) The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39–45. [DOI] [PubMed] [Google Scholar]
- 55.Davis JD, Levine MW (1977) A model for the control of ingestion. Psychol Rev 84: 379–412. [PubMed] [Google Scholar]
- 56.Dwyer DM, Lydall ES, Hayward AJ (2011) Simultaneous contrast: Evidence from licking microstructure and cross-solution comparisons. J Exper Psychol: Anim Behav Proc 37: 200–210. [DOI] [PubMed] [Google Scholar]
- 57.Hsiao S, Fan RJ (1993) Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behav Neurosci 107: 317–326. [DOI] [PubMed] [Google Scholar]
- 58.Spector AC, St. John SJ (1998) Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol 274: R1687–R1703. [DOI] [PubMed] [Google Scholar]
- 59.Davis JD, Perez MC (1993) Food deprivation- and palatability induced microstructural changes in ingestive behavior, Am J Physiol 264: R97–R103. [DOI] [PubMed] [Google Scholar]
- 60.Eisen S, Davis JD, Rauhofer E, Smith GP (2001) Gastric negative feedback produced by volume and nutrient during a meal in rats. Am J Physiol Regul Integr Comp Physiol 281: R1201–R1214. [DOI] [PubMed] [Google Scholar]
- 61.Davis JD, Smith GP, Kung TM (1995) Cholecystokinin changes the duration but not the rate of licking in vagotomized rats. Behav Neurosci 109: 991–996. [DOI] [PubMed] [Google Scholar]
- 62.Manigrasso MB, Rabbani P, Egaña-Gorroño L, Quadri N, Frye L, Zhou B, Reverdatto S, Ramirez LS, Dansereau S, Pan J, Li H, D’Agati VD, Ramasamy R, DeVita RJ, Shekhtman A, Schmidt AM (2021) Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci Transl Med 13(621):eabf7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson RA, Arivazhagan L, Ruiz HH, Zhou B, Qian K, Manigrasso MB, Bernadin R, Mangar K, Shekhtman K, Li H, Ramasamy R, Schmidt AM (2023) Pharmacological antagonism of receptor for advanced glycation end products signaling promotes thermogenesis, healthful body mass and composition, and metabolism in mice. Obesity 31:1825–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC (2016) Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab 60:138–142. [DOI] [PubMed] [Google Scholar]
- 65.Mather K (2009) Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 296: E398–E399 [DOI] [PubMed] [Google Scholar]
- 66.Gaens KHJ, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJH, Niessen HW, Rensen SS, Buurman WA, Greve JWM, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CDA, Schalkwijk CG (2014) Nε-(Carboxymethyl)lysinereceptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol 34:1199–1208. [DOI] [PubMed] [Google Scholar]
- 67.Bocarsly ME, Avena NM (2013) A high-fat diet or galanin in the PVN decreases phosphorylation of CREB in the nucleus accumbens. J Neurosci 248: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adams WK, Sussman JL, Kaur S, D’Souza AM, Kieffer TJ, Winstanley CA (2015) Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signaling – two markers of addiction vulnerability. Eur J Neurosci 42: 3095–3104. [DOI] [PubMed] [Google Scholar]
- 69.Kim S, Shou J, Abera S, Ziff EB (2018) Sucrose withdrawal induces depression and anxiety-like behavior by Kir2.1 upregulation in the nucleus accumbens. Neuropharmacol 130: 10e17. [DOI] [PubMed] [Google Scholar]
- 70.Shiflett MW, Mauna JC, Chipman AM, Peet C, Thiels E (2009) Appetitive Pavlovian conditioned stimuli increase CREB phosphorylation in the nucleus accumbens. 92: 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rauggi R, Scheggi S, Cassanelli A, De Montis MG, Tagliamonte A, Gambarana C (2005) The mesolimbic dopaminergic response to novel palatable food consumption increases dopamine-D1 receptor-mediated signaling with complex modifications of the DARPP-32 phosphorylation pattern. J Neurochem 92: 867–877. [DOI] [PubMed] [Google Scholar]
- 72.Nishi A, Kuroiwa M, Shuto T (2011) Mechanisms for the modulation of dopamine D1 receptor signaling in striatal neurons. Front Neuroanat 5: Article 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramirez I (1987) Feeding a liquid diet increases energy intake, weight gain and body fat in rats. J Nutr 117:2127–2134. [DOI] [PubMed] [Google Scholar]
- 74.Sclafani A (1987) Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form and taste. Neurosci Biobehav Rev 11:155–162. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Cheng Y, Zhou Y, Du H, Zhang C, Zhao Z, Chen Y, Zhou Z, Mei J, Wu W, Chen M (2022) High fat diet-induced obesity leads to depressive and anxiety-like behaviors in mice via AMPK/mTOR-mediated autophagy. Exp Neurol 348:113949. [DOI] [PubMed] [Google Scholar]
- 76.Hassan AM, Mancano G, Kashofer K, Fröhlich EE, Matak A, Mayerhofer R, Reichmann F, Olivares M, Neyrinck AM, Delzenne NM, Claus SP, Holzer P (2019) High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome, Nutritional Neurosci 22:877–893 [DOI] [PubMed] [Google Scholar]
- 77.Tsai SF, Hsu P-L, Chen Y-W, Hossain MS, Chen P-C, Tzeng S-F, Chen P-S, Kuo Y-M (2022) High-fat diet induces depression-like phenotype via astrocyte-mediated hyperactivation of ventral hippocampal glutamatergic afferents to the nucleus accumbens. Molec Psychiat 27:4372–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vagena E, Ryu JK, Baeza-Raja B, Walsh NM, Syme C, Day JP, Houslay MD, Baillie GS (2019) A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl Psychiat 9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abildgaard A, Solskov L, Volke V, Harvey BH, Lund S, Wegener G (2011) A high-fat diet exacerbates depressive-like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinol 36:623–633. [DOI] [PubMed] [Google Scholar]
- 80.Pawar GR, Agrawal YO, Nakhate KT, Patil CR, Sharma C, Ojha S, Mahajan UB, Goyal SN (2022) Ghrelin alleviates depression-like behaviour in rats subjected to high-fat diet and diurnal rhythm disturbance. Am J Transl Res 15:7098–7108. [PMC free article] [PubMed] [Google Scholar]
- 81.Deal AW, Seshie O, Lenzo A, Cooper N, Ozimek N, Solberg Woods LC (2020) High-fat diet negatively impacts both metabolic and behavioral health in outbred heterogeneous stock rats Physiol Genomics 52:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurtado Del Pozo C, Ruiz HH, Arivazhagan L, Aranda JF, Shim C, Daya P, Derk J, MacLean M, He M, Frye L, Friedline RH, Noh HL, Kim JK, Friedman RA, Ramasamy R, Schmidt AM (2019) A receptor of the immunoglobulin superfamily regulates adaptive thermogenesis. Cell Rep 28: 773–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J (2017) Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesityassociated cardiometabolic risk. Adipocyte 7:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vega GL, Grundy SM (2013) Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes 2013:409679. doi: 10.1155/2013/409679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL (2020) Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int J Mol Sci 21:4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C, Bi Y (2022) Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab 34:1264–1279. [DOI] [PubMed] [Google Scholar]
- 87.Johnson AW (2018) Characterizing ingestive behavior through licking microstructure: Underlying neurobiology and its use in the study of obesity in animal models Int J Dev Neurosci 64: 38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheggi S, Graziella De Montis M, Gambarana C (2018) Making sense of rodent models of anhedonia. Int J Neuropsychopharmacol 21: 1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436–445 [DOI] [PubMed] [Google Scholar]
- 90.Teegarden SL, Nestler EJ, Bale TL (2008) ΔFosB-mediated alterations in dopamine signaling are normalized by a palatable high fat diet. Biol Psychiat 64: 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haberny SL, Berman Y, Meller E, Carr KD (2010) Chronic food restriction increases D-1 dopamine receptor agonist-induced phosphorylation of extracellular signalregulated kinase 1/2 and cyclic AMP response element-binding protein in caudateputamen and nucleus accumbens. Neurosci 125: 289–298. [DOI] [PubMed] [Google Scholar]


