Abstract
Extinction learning is tremendously adaptive as it allows an animal to adjust their behavior in a changing environment. Yet, extinction is not without limitations and fear often reemerges over time (i.e. spontaneous recovery). Relative to adults, adolescent rodents and humans are particularly prone to spontaneous recovery following extinction. In this study, we aimed to address whether combining methods of fear regulation (extinction and conditioned inhibition) can facilitate extinction retention. Early adolescent (29 days old, n = 81) and adult (70 days old, n = 80) mice underwent extinction with or without a safety cue present. Safety cue presentations were systematically varied to overlap with or alternate with fear cue presentations. We found that initial safety learning was faster in adolescent mice. In addition, intermixing safety cues into extinction reduced spontaneous recovery during a test two weeks later. The decrease in spontaneous recovery relative to a standard extinction protocol was greater in adolescents than adults. Together, our findings provide initial evidence that safety learning may be inherently stronger during adolescence. These results inform the parameters by which conditioned safety and extinction learning may be merged to augment the inhibition of fear. While methods to enhance fear regulation are valuable for any age, the potential to do so during adolescence is particularly striking.
Keywords: Safety, fear, extinction, spontaneous recovery, conditioned inhibition, adolescence
1. Introduction
Extinction is the process whereby the response to a conditioned stimulus (CS) decreases alongside repeated presentations of the conditioned stimulus in the absence of a previously associated unconditioned stimulus. Extinction learning is tremendously adaptive as it allows an animal to adjust their behavior in a changing environment. In the fear domain, extinction is one of the most commonly studied means of fear regulation. Extinction is also paralleled in the clinic by way of exposure therapy, which promotes the systematic confrontation of stimuli that are perceived as threats and is a common component of treatment for anxiety and fear-related disorders. Yet, extinction is not without limitations. Substantial evidence exists showing that extinction is context-dependent, with fear re-emerging in contexts that differ from the extinction context (a process referred to as renewal; Bouton, 2002; Bouton et al., 2021; Bouton & Bolles, 1979). Fear reduction following extinction is also relatively short-lived, with an extinguished behavioral response re-emerging following the mere passage of time (spontaneous recovery; Bouton, 2002; Quirk, 2002; Rescorla, 2004).
Another factor that has been found to moderate inhibitory learning during extinction is age (cf Bisby et al., 2021; Gerhard et al., 2020). Indeed, relative to adults, adolescent mice and rats show reduced retention of extinction learning as indicated by spontaneous recovery (Kim et al., 2011; Koppensteiner et al., 2019a, 2019b; McCallum et al., 2010; Pattwell et al., 2012), and in some cases poorer extinction learning to begin with (Hefner & Holmes, 2007; Pattwell et al., 2012). Interestingly, the magnitude of spontaneous recovery appears to be greatest when both fear conditioning and extinction have taken place during adolescence, rather than spanning juvenile or adult ages (Baker & Richardson, 2015). Moreover, spontaneous recovery between consecutive days of extinction sessions is markedly high in adolescence, even following robust within-session extinction (Gerhard & Meyer, 2021). Evidence in humans also suggests limitations in extinction learning during adolescence (Ganella et al., 2018; Pattwell et al., 2012).
Increasing the number of extinction trials has garnered some success for enhancing extinction during adolescence. For example, increasing from 30- to 60-tone presentations in adolescent rats was shown to close the age gap, resulting in similar extinction retention between adolescents and adults (Baker et al., 2016; Kim et al., 2011; McCallum et al., 2010). Such an approach may be particularly attractive during adolescent development. Indeed, while adults exhibit greater fear reduction when extinction trials are distributed across multiple training days (Cain et al., 2003; Gerhard & Meyer, 2021), extinction performance during adolescence was shown to be comparable whether 40 extinction trials were massed into one session or spaced across two or four days (Gerhard & Meyer, 2021). Furthermore, massing trials into a single session during adolescence may actually lead to more durable memories, with fear remaining low long-term (Gerhard & Meyer, 2021).
Pharmacological intervention has also been met with success. For example, D-cycloserine (DCS) has been shown to improve extinction retention in adolescent rats, likely by promoting glutamatergic signaling linked to engagement of N-methyl-D-aspartate (NMDA) receptors (Baker et al., 2018; Baker & Richardson, 2017). Enhancing dopaminergic signal is another promising avenue. One study found that administration of quinpirole, an agonist selective for D2 dopamine receptors, into the infralimbic cortex enhanced long-term extinction in adolescents (Zbukvic et al., 2017). Similarly, systemic administration of the D2 partial agonist aripiprazole at the time of extinction also resulted in reduced freezing during a later fear recall test (Ganella et al., 2017).
A third approach to enhancing fear regulation during adolescence circumvents extinction altogether. Explicit safety learning has emerged as a promising method for attenuating fear responding in both adolescent rodents and humans by engaging processes of conditioned inhibition. Conditioned inhibition is an associative learning phenomenon whereby a stimulus that predicts the absence of an outcome comes to control responding. We have previously shown that adolescent mice are able to learn about and effectively use safety cues to inhibit fear (Meyer et al., 2021). Moreover, we have confirmed that through our standard training protocol (Meyer et al., 2021), safety cues pass the summation and retardation of new learning tests, qualifying them as conditioned inhibitors (Rescorla, 1969). Similar findings have been obtained from healthy human children and adolescents (8-18 years old) (Harrewijn et al., 2020). Thus, safety learning may be advantageous to consider by clinicians working with youths (Meyer et al., 2023; Odriozola & Gee, 2020; but see discussion for important clinical considerations when employing a safety learning-based approach).
In this study, we aimed to address whether combining methods of fear regulation (extinction and conditioned inhibition) can facilitate extinction retention. Early adolescent (29 days old) and adult (70 days old) mice underwent extinction with or without the presence of a safety cue. Safety cue presentations were systematically varied to overlap with or alternate with fear cue presentations. Our results inform the parameters by which conditioned safety and extinction learning may be merged to augment the inhibition of fear. While methods to enhance fear regulation are valuable for any age, the potential to do so during adolescence is particularly striking.
2. Material and Methods
2.1. Subjects
Subjects were male mice generated by in-house breedings of C57BL/6N mice obtained from Charles River Laboratories. On postnatal day (PND)21, mice were weaned into group-housed cohorts of 3-5 per cage and randomly assigned (by cage) to undergo behavioral testing during adolescence (PND29, n = 69) or adulthood (PND70, n = 64). An additional cohort of mice (n = 12 adolescents, 16 adults) was included in a follow-up experiment considering the impact of safety cue exposure during fear conditioning. Mice were maintained on a 12-hour light/dark cycle at 18-22°C with food (LabDiet, PicoLab Rodent Diet 20) and water ad libitum. All behavioral tests were conducted during the light cycle. Experiments were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and protocols were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
2.2. Behavioral Training
2.2.1. Discriminative Conditioning
Discriminative conditioning was carried out in standard conditioning chambers (Med Associates) as previously described (Meyer et al., 2019). For four consecutive days, each mouse was placed into the conditioning chamber (Context A) and presented with intermixed trials of fear or safety cues (2.9 and 12.5 kHz tones, counterbalanced, played at 80dB for 20 s) on a variable intertrial interval (ITI; 30-90 s) schedule. Ambient light was provided through an LED Stimulus Light (50 lux) and the chamber was scented with peppermint (1/1000 in 70% ethanol). Delivery of a mild foot shock (1-s, 0.5mA, co-terminating with fear cue presentations) served as the aversive unconditioned stimulus. Mice were exposed to two presentations of the fear cue and 30 presentations of the safety cue each day, with trial order varied daily. Mice were acclimated to the chamber for two minutes prior to any stimulus exposures and remained in the conditioning chamber for one minute after the final stimulus presentation before being returned to their home cages. As a follow-up experiment, to determine the impact of safety cue exposure during conditioning on fear conditioning and extinction, an additional cohort of mice underwent fear conditioning in the absence of safety cues (referred to below as “Fear Conditioned”). These mice were compared to mice in the Standard Extinction group (referred to as “Safety Conditioned” for this analysis only). Session duration, fear cue frequency and intensity, shock intensity, and the timing of fear cue and shock presentations was kept constant between these fear conditioned mice and mice that underwent discriminative conditioning.
2.2.2. Extinction
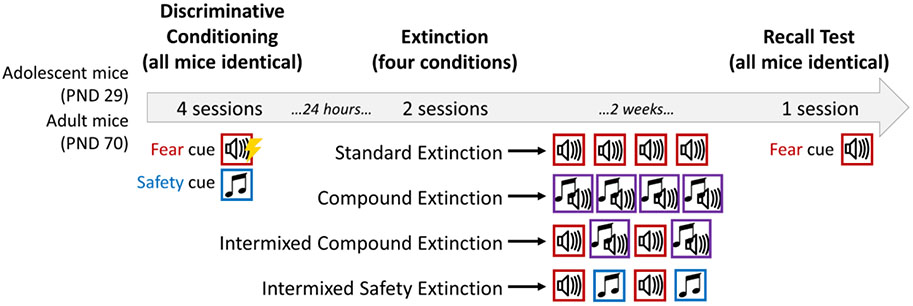
Following discriminative conditioning, cohorts of adolescent and adult mice were each divided into four groups, each of which underwent a different extinction protocol (Figure 1). Extinction took place 24 hours after discriminative conditioning in a new context (Context B) to isolate cued fear from residual contextual fear. Context B was differentiated using a black acrylic A-frame contextual insert, a white acrylic floor cover, and chevron print wallpaper on the back wall of the chambers used for Discriminative Conditioning. Ambient light was provided throughout the session through a ceiling mounted light box (125 lux) and the chamber was scented with (−)-Limonene, 92% (1/1000 in 70% ethanol). After a two-minute baseline, stimulus presentations in the absence of the foot shock began. All groups underwent two sessions of extinction, spaced 24-h apart, consisting of 20 20-s tone presentations. Mice in the “Standard Extinction” group received exclusively fear tone presentations. Mice in the “Compound Extinction” group received exclusively presentations of the fear and safety tones played simultaneously (i.e., as a compound cue). Mice in the “Intermixed Compound Extinction” group received ten fear cues and ten compound cues each session, presented intermixed and pseudorandomly. Mice in the “Intermixed Safety Extinction” group received ten fear cues and ten safety cues each session, presented intermixed and pseudorandomly. For both “Intermixed” groups, each session began and ended with a fear cue trial. In the follow-up experiment designed to determine the impact of safety cue exposure during conditioning on fear conditioning and extinction, the cohort of Fear Conditioned mice also received extinction, during which they received exclusively fear tone presentations (i.e., identical to the extinction protocol for the “Standard Extinction” / “Safety Conditioned” group). Tone presentations occurred on a variable ITI schedule (average 60 s, range 40-80 s) with ITI order consistent across all groups. For all groups, mice remained in the chamber for one minute after the final tone before being returned to their home cages.
Figure 1. Schematic of Experimental Design.
All mice underwent four sessions of discriminative conditioning, receiving two fear cues (paired with a footshock) and 28 safety cues pseudorandomly presented each day. Following the fourth day of conditioning, mice were assigned to one of four extinction conditions. For each of two sessions, mice received 20 tone presentations, consisting of fear cues and/or safety cues. Red boxes indicate fear cues, blue boxes indicate safety cues, purple boxes indicate the simultaneous presentation of a fear and safety cue. Two weeks after extinction, all mice received one presentation of a fear cue. One additional cohort (not pictured) underwent fear conditioning in the absence of safety cues, followed by exposure to standard extinction. PND = postnatal day.
2.2.3. Fear Test
To provide a measure of extinction retention, all mice underwent a fear test two weeks following extinction. The test took place in Context B and consisted of a two-minute baseline period followed by a single 20-s fear tone presentation (no shock) after which mice remained in the testing chamber for one minute after the tone before being returned to their home cages.
2.3. Statistics
All behavioral sessions were recorded and analyzed using Video Freeze® software (Med Associates). Video Freeze® software was set at a motion threshold of 18 Units for automatic scoring of freezing behavior. Average cued freezing was determined by dividing the cumulative time freezing during the cue by the cue duration (20 s) followed by conversion to percentage. Discriminative Conditioning and Extinction data were averaged across all cues of the same type within each session. Discrimination index was determined for each day of Discriminative Conditioning by subtracting average cued freezing during safety cues from freezing during fear cues. Spontaneous Recovery was determined by evaluating average cued freezing relative to levels attained at the end of the previous session to account for baseline differences in freezing between groups following exposure to different extinction protocols. For between-session spontaneous recovery, freezing during Tone 1 of Extinction Day 2 was compared to freezing during Tone 20 of Extinction Day 1. For long-term spontaneous recovery, freezing during the fear cue presentation was compared to freezing during Tone 20 of Extinction Day 2. Difference from Standard was determined by normalizing spontaneous recovery data from the Compound Extinction, Intermixed Compound Extinction, and Intermixed Safety Extinction to data in the Standard Extinction group. While within-subject normalization is a more common statistical approach (e.g., Tipps et al., 2014) than between-subject, our goal was to pursue a complementary analysis that would inform age differences in the magnitude of the efficacy for extinction modifications to attenuate spontaneous recovery.
Statistical analyses were performed in R Studio. Multifactor Analysis of Variance (ANOVAs) with the Greenhouse-Geisser correction for non-spherical data (when applicable) were used to evaluate behavioral data. Significant interactions were decomposed using post-hoc Bonferroni-corrected multiple comparisons. For discriminative conditioning, Stimulus Type (fear, safety) served as a within-subjects measure and Session (discriminative conditioning, Day 1-4) served as a repeated measure. Age (adolescent, adult) served as a between-subjects measure. A similar model was used to analyze discrimination magnitude, with the term for Stimulus Type removed. For extinction, the value of freezing averaged across 10 cue presentations was calculated within each day. Stimulus Type (fear, safety, compound – as relevant) served as a within-subjects measure and Session (Day 1-2) served as a repeated measure. Age (adolescent, adult) and Extinction Condition (standard, compound, intermixed compound, intermixed safety – or as relevant, safety conditioned, fear conditioned) served as between-subjects measures. For fear test, Age (adolescent, adult) and Extinction Condition (standard, compound, intermixed compound, intermixed safety – or as relevant, safety conditioned, fear conditioned) served as between-subjects measures. For comparisons of spontaneous recovery normalized to the Standard Extinction group, Age (adolescent, adult) and Extinction Condition (compound, intermixed compound, intermixed safety) served as between-subjects measures. Across all analyses, differences were considered significant for P values less than 0.05. Differences were considered trending for P values less than 0.07. Where appropriate, eta squared values were considered as a measure of effect size.
3. Results
3.1. Adolescents exhibit greater discrimination between fear and safety cues
3.1.1. Cue-elicited freezing
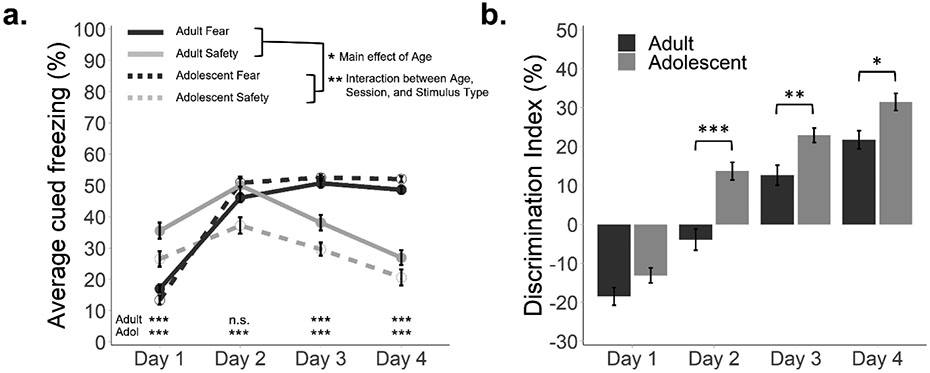
As shown previously using a similar training protocol (Meyer et al., 2019, 2021), both adolescent and adult mice learned to discriminate between fear and safety cues across the four conditioning sessions, freezing more to the fear than the safety cues (Figure 2a). This was supported by a main effect of Stimulus Type (F(1, 125) = 53.17, p < 0.001, η2 = 0.30) and an interaction between Session and Stimulus Type (F(3, 375) = 211.25, p < 0.001, η2 = 0.63). Age differences in discriminative conditioning were also observed (main effect of Age, F(1, 125) = 5.16, p = 0.025, η2 = 0.04; interaction between Age, Session, and Stimulus Type, F(3, 375) = 3.89, p = 0.009, η2 = 0.03) suggesting a larger discrimination magnitude in the adolescent group.
Figure 2. Adolescents exhibit greater discrimination between fear and safety cues.
(a) Average percentage of time spent freezing during fear and safety cues across discriminative conditioning sessions. Adults exhibited overall higher freezing than adolescents and required one additional day to discriminate between fear and safety cues. (b) Average percentage difference between freezing to fear versus safety cues (discrimination index). Adolescents exhibited an overall greater magnitude of discrimination than adults, with discrimination index reaching significance on Days 2, 3, and 4. n = 64 adult/69 adolescent. Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
To determine the number of sessions required to attain significant discrimination (i.e., greater freezing during the fear cue than the safety cue, Figure 2a), post-hoc t-tests were performed between fear and safety cues for each session, by age group. Both adult (t(63) = 8.20, padj < 0.001) and adolescent (t(68) = 6.80, padj < 0.001) mice exhibited greater freezing during the safety cue than the fear cue during the first conditioning session. While unexpected, this was likely attributable to the stimulus presentation order, as the first cue of the session was a fear stimulus, leading to generalization to subsequent safety stimuli before discrimination was attained. During the second conditioning day, adolescent (t(68) = 6.05, padj < 0.001), but not adult (t(63) = 1.43, padj > 0.999) mice discriminated, exhibiting greater freezing during the fear cue than the safety cue. All mice discriminated during the third (adult: t(63) = 4.91, padj < 0.001; adolescent: t(68) = 12.30, padj < 0.001) and fourth (adult: t(63) = 9.22, padj < 0.001; adolescent: t(68) = 14.30, padj < 0.001) conditioning days. These data indicate that fear and safety discrimination is faster in adolescent mice.
3.1.2. Discrimination index
The impression that fear and safety discrimination is faster in adolescent mice was confirmed by an examination of discrimination index (Figure 2b), which revealed a main effect of Session (F(3, 393) = 207.62, p < 0.001, η2 = 0.61), Age (F(1, 131) = 21.19, p < 0.001, η2 = 0.14) and interaction between Session and Age (F(3, 393) = 3.94, p = 0.009, η2 = 0.03), driven by a greater discrimination index in adolescents relative to adults during DC2 (t(131) = 4.98, padj < 0.001), DC3 (t(131) = 3.28, padj = 0.005), and DC4 (t(131) = 4.98, padj = 0.012). This work largely replicates our previous findings that adolescent and adult mice can learn to discriminate between fear and safety cues (Meyer et al., 2021), while also providing a novel direct comparison between the two age groups.
3.2. Intermixing safety cues into extinction does not alter extinction performance
Following discriminative conditioning, mice were randomly assigned to one of four extinction conditions: Standard Extinction (n = 17 adults, 17 adolescents), Compound Extinction (n = 13 adults, 16 adolescents), Intermixed Compound Extinction (n = 17 adults, 20 adolescents), or Intermixed Safety Extinction (n = 17 adults, 16 adolescents). Extinction training began 24 hours after the fourth day of discriminative conditioning. Analyses were carried out to confirm that no differences in discriminative conditioning were apparent between subsequently assigned extinction conditions (no main effect of Condition and interactions between Condition and Age, Session, and Epoch all p > 0.079). Consistency among groups was further established through an analysis of freezing during the first fear cue presentation of the first extinction session. Freezing did not differ by age or by condition (all p > 0.075; Adolescent fear by condition ranged from 58.10 ± 5.30 to 72.24 ± 2.40; Adult fear by condition ranged from 50.43 ± 5.83 to 66.48 ± 4.59).
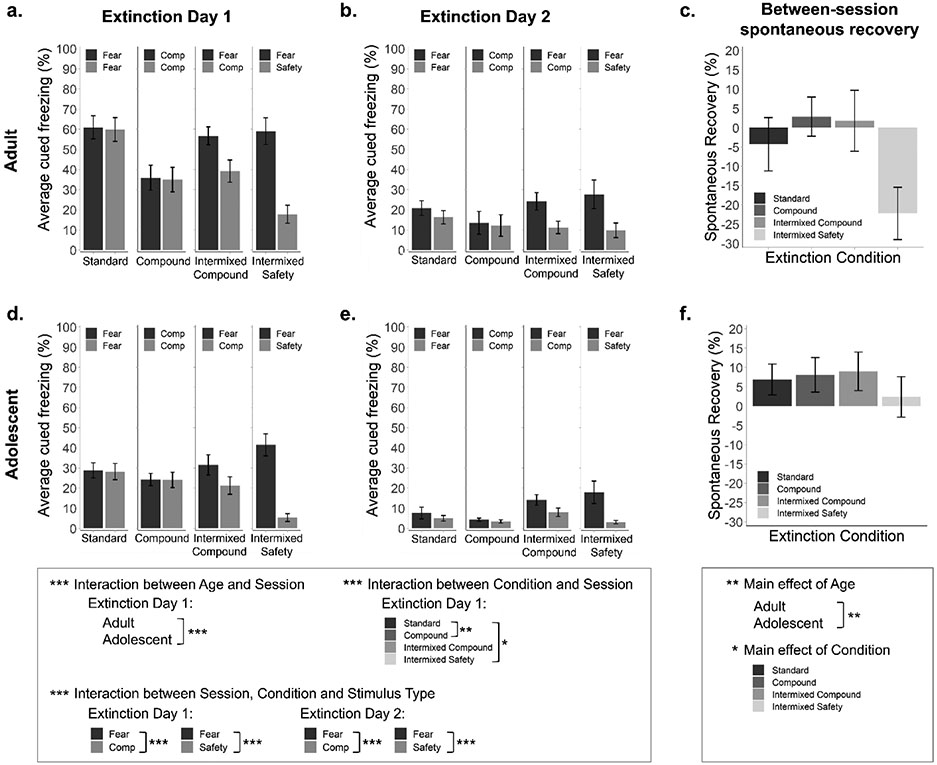
Extinction data are presented in Figure 3. Overall, freezing decreased across the two extinction sessions (main effect of Session, F(1, 125) = 328.97, p < 0.001, η2 = 0.73). By age, average freezing across the session was higher in adults than adolescents (main effect of Age, F(1, 125) = 31.24, p < 0.001, η2 = 0.20), an effect likely driven by higher freezing in adults during the first session of extinction (despite initial consistency in freezing during the first tone) which rapidly reduced to comparable levels between ages during the second session (interaction between Age and Session, F(1, 125) = 18.25, p < 0.001, η2 = 0.13). This data is consistent with our previous work indicating that adults exhibit greater extinction gains when trials are spaced across multiple sessions, providing the opportunity for consolidation of extinction learning, while adolescents show no notable differences in extinction learning between massed or spaced extinction session designs (Gerhard & Meyer, 2021).
Figure 3. Intermixing safety cues into extinction does not alter extinction performance.
(a,d) Average percentage of time spent freezing during fear, safety, or compound (Comp) cues across extinction day 1 for adult and adolescent mice (bar height reflects an average across 10 cue presentations and across all mice in a given group). Fear and Compound (Comp) cue presentations in the Standard and Compound extinction groups are qualitatively identical and presented in separate bars of 10-tone averages for consistency with Intermixed Extinction groups. Adults exhibited overall higher freezing than adolescents. Freezing was higher during fear cue presentations than both safety and compound cue presentations. (b,e) Average percentage of time spent freezing during fear, safety, or compound cues across extinction day 2 for adult and adolescent mice. Freezing did not differ between ages but was higher during fear cue presentations than both safety and compound cue presentations. (c,f) Average percentage of time spent freezing during the first trial of extinction day 2 relative to the final trial of extinction day 1 (between-session spontaneous recovery) for adult and adolescent mice. Adults exhibited overall lower spontaneous recovery than adolescents. Spontaneous recovery differed between extinction conditions, though individual comparisons did not reveal significant effects. Standard Extinction (n = 17 adult/17 adolescent), Compound Extinction (n = 13 adult/16 adolescent), Intermixed Compound Extinction (n = 17 adult/20 adolescent), Intermixed Safety Extinction (n = 17 adult/16 adolescent). Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
An interaction between Condition and Session was also observed (F(3, 125) = 6.72, p < 0.001, η2 = 0.14) driven by differences in freezing between conditions on Day 1 (F(3, 262) = 4.92, p = 0.002, η2 = 0.05) but not Day 2 (p = 0.086). Specifically, on Day 1, freezing in the Standard Extinction group was greater than the Compound Extinction group (t(124) = 3.77, padj = 0.002) and the Intermixed Safety Extinction group (t(132) = 2.82, padj = 0.030), with no other differences observed (padj’s > 0.324).
While mice in the Standard and Compound Extinction groups experienced only Fear cues or Compound cues (respectively), data in Figure 3 (a, b, d, and e) is presented across two bars for visualization purposes. The distribution of cues between these bars was matched to the Intermixed Compound and Intermixed Safety groups, in which cue presentations could be divided by differential stimulus types. Freezing differed between Stimulus Types (main effect, F(1, 125) = 119.27, p < 0.001, η2 = 0.49), driven by discrimination between the Fear and Safety cues as well as Fear and Compound cues. This was further supported by an interaction between Condition and Stimulus Type (F(3, 125) = 39.54, p < 0.001, η2 = 0.49), suggesting that dissimilar stimuli, (e.g., Fear-Safety or Fear-Compound) differentiate responding more than similar stimuli, (e.g., Fear-Fear or Compound-Compound). In other words, these data confirm that behavioral differences are driven by animal responding to distinct cue types. As freezing reduced across extinction, stimulus discrimination decreased (interaction between Session, Condition, and Stimulus Type, F(3, 125) = 31.02, p < 0.001, η2 = 0.43), though the same pattern was present on both days: Freezing did not differ between the two groupings of fear cues (Standard Extinction; padj’s > 0.150) or compound cues (Compound Extinction; padj’s > 0.999) on either day, but freezing was higher during fear cues than compound cues (Intermixed Compound Extinction, Day 1: t(36) = 5.45, padj < 0.001; Day 2: t(36) = 5.52, padj < 0.001) and higher during fear cues than safety cues (Intermixed Safety Extinction, Day 1: t(32) = 11.10, padj < 0.001; Day 2: t(32) = 5.19, padj < 0.001) on both days. These data parallel previously reported findings that the safety cue attains conditioned inhibitor properties through discriminative conditioning allowing it to directly inhibit freezing when the fear and safety cues are played as a compound (Meyer et al., 2019, 2021).
Outside of differential freezing driven by Stimulus Type, overall freezing did not differ between conditions (no main effect of Condition, p = 0.478). Furthermore, no additional impact of age on condition or extinction between conditions was observed (all p > 0.285). Together, these data suggest that all extinction training protocols result in similarly low levels of fear by the end of extinction training.
3.3. Intermixing safety cues into extinction improves long-term extinction retention
Following a delay, conditioned responses will often re-emerge through a process known as spontaneous recovery (Bouton, 2002; Quirk, 2002; Rescorla, 2004). Therefore, to examine the longevity of extinction learning, we considered relative levels of freezing between extinction sessions (the final tone on Day 1 and the first tone on Day 2) as a measure of between-session spontaneous recovery (Figure 3c, f) and relative levels of freezing between the end of extinction (the final tone on Day 2) and a cue presentation two weeks later as a measure of long-term spontaneous recovery (Figure 4).
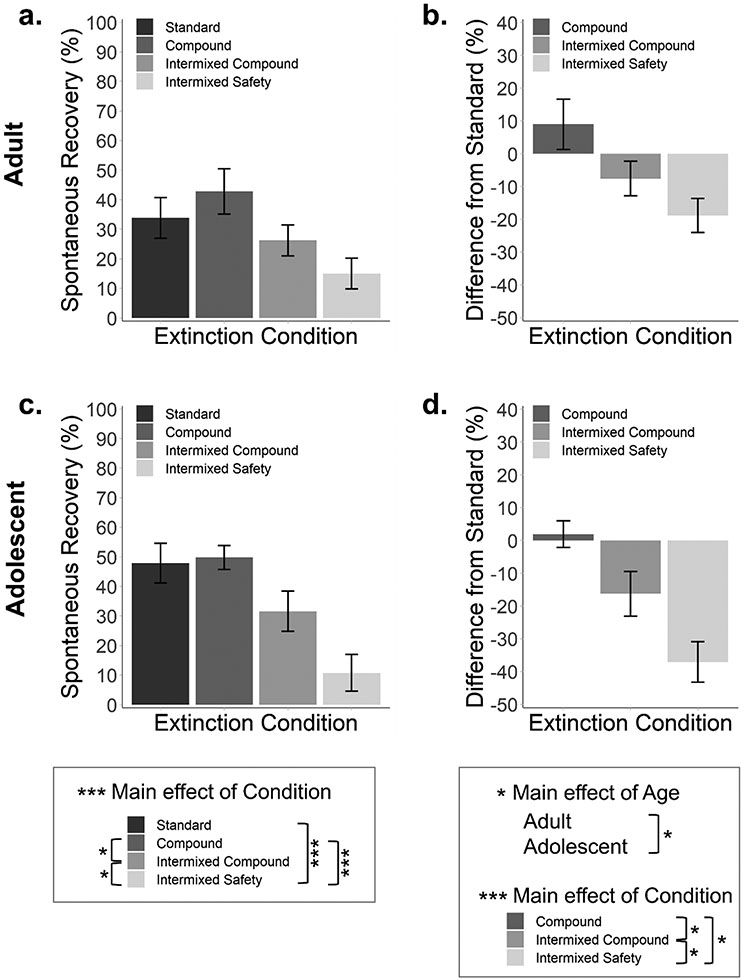
Figure 4. Intermixing safety cues into extinction improves long-term extinction retention.
(a,c) Average percentage of time spent freezing during a fear cue presentation relative to the final trial of extinction day 2 (long-term spontaneous recovery) for adult and adolescent mice. Spontaneous recovery did not differ between ages but was attenuated in mice for whom a safety cue had been intermixed into extinction. (b,d) Average percentage spontaneous recovery normalized to mice in the Standard Extinction condition for adult and adolescent mice. Relative to adults, adolescents exhibited an overall greater attenuation of spontaneous recovery across all modified extinction protocols normalized to Standard Extinction. Relative to Standard Extinction, intermixing a safety cue was more effective than all other conditions for attenuating spontaneous recovery. Standard Extinction (n = 17 adult/16 adolescent), Compound Extinction (n = 13 adult/16 adolescent), Intermixed Compound Extinction (n = 17 adult/20 adolescent), Intermixed Safety Extinction (n = 17 adult/16 adolescent). Error bars represent the mean ± SEM. *p < 0.05, ***p < 0.001.
3.3.1. Between-session spontaneous recovery
Examination of fear at the beginning of Day 2 relative to the end of extinction Day 1 (Figure 3c, f) revealed a main effect of Age (F(1, 125) = 8.31, p = 0.005, η2 = 0.06), driven by greater spontaneous recovery in adolescent mice. There was also a main effect of Condition (F(3, 125) = 3.022, p = 0.032, η2 = 0.07), though with corrections for multiple comparisons the only observed difference was marginally greater spontaneous recovery in the Compound Extinction groups relative to the Intermixed Safety Extinction groups (t(60) = 2.69, padj = 0.054), with no other comparisons reaching significance (padj’s > 0.102). There was no interaction between Age and Condition (p = 0.362).
3.3.2. Long term spontaneous recovery
We examined cued fear two weeks after extinction to measure longevity of the extinction memory (Figure 4). This time point corresponded to late adolescence in the adolescent groups. Mice were returned to the extinction context and presented with a single fear stimulus presentation. One mouse died due to a health condition unrelated to the experiment prior to the fear test resulting in n = 16 for the adolescent Standard Extinction group.
Analysis of spontaneous recovery during the long-term fear test revealed a main effect of Condition (F(3, 124) = 10.99, p < 0.001, η2 = 0.21), though no effect of Age or interaction between Age and Condition (p’s > 0.211). Collapsing across age, post-hoc t-tests indicated that spontaneous recovery was significantly lower for mice that underwent Intermixed Safety Extinction than Standard Extinction (t(64) = 4.39, padj < 0.001), Compound Extinction (t(60) = 5.91, padj < 0.001), or Intermixed Compound Extinction (t(68) = 2.72, padj = 0.048). In addition, spontaneous recovery was lower for mice that underwent Intermixed Compound Extinction relative to those that underwent Compound Extinction (t(64) = 2.87, padj = 0.030). No other differences were observed (padj’s > 0.498).
To allow direct comparison of how developmental factors influence learning in the three manipulated extinction protocols used in this study, spontaneous recovery data from the Compound Extinction, Intermixed Compound Extinction, and Intermixed Safety Extinction was normalized to data in the Standard Extinction group. There was a main effect of Condition (F(2, 93) = 14.58, p < 0.001, η2 = 0.24), with all three conditions differing from one another. Relative to Standard Extinction, spontaneous recovery was reduced to a greater extent following Intermixed Compound Extinction than Compound Extinction (t(64) = 2.83, padj = 0.018), Intermixed Safety Extinction than Compound Extinction (t(60) = 5.51, padj < 0.001), and Intermixed Safety Extinction relative to Intermixed Compound Extinction (t(68) = 2.50, padj = 0.045). In other words, presenting a non-fear stimulus between presentations of fear cues during extinction leads to stronger extinction retention, particularly if that stimulus is a safety cue. This analysis also revealed a main effect of Age (F(1, 93) = 5.31, p = 0.023, η2 = 0.05), driven by overall greater attenuation of spontaneous recovery in adolescents undergoing modified extinction. The interaction between Age and Condition was not significant (p = 0.607).
3.4. Fear memories are stronger following simultaneous fear and safety conditioning relative to fear conditioning alone
The findings outlined above suggest that the introduction of a safety cue alongside the fear cue during extinction significantly impacts extinction retention. Thus, an experiment was designed to determine the impact of safety cue exposure during initial fear conditioning. An additional cohort of mice underwent fear conditioning, receiving tone-footshock pairings across four sessions in the absence of safety cues (n = 16 adults, 12 adolescents). We then evaluated extinction retention for mice in this Fear Conditioned group relative to the cohort of mice described above that underwent discriminative conditioning followed by the Standard extinction protocol (referred to as “Safety Conditioned” for this analysis only) (Figure 5).
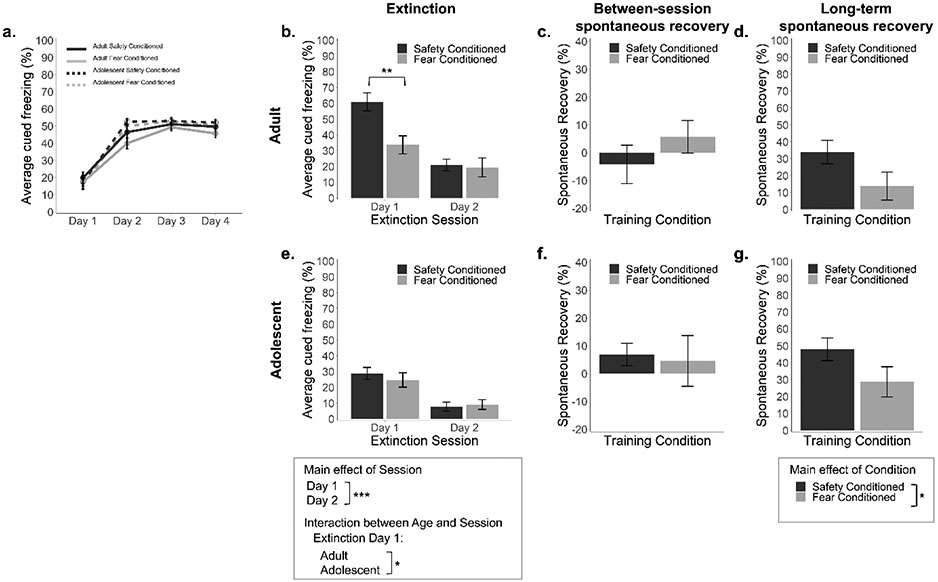
Figure 5. Fear memories are stronger following simultaneous fear and safety conditioning relative to fear conditioning alone.
(a) Average percentage of time spent freezing during fear cues across fear/discriminative conditioning sessions. Adults exhibited marginally lower freezing than adolescents. (b,e) Average percentage of time spent freezing during fear cues across extinction day 1 (left) and day 2 (right) for adult and adolescent mice with a history of either safety conditioning or fear conditioning. Adults exhibited overall higher freezing than adolescents on day 1. Freezing was higher for adults previously exposed to safety conditioning than adults exposed to fear conditioning on day 1. (c,f) Average percentage of time spent freezing during the first trial of extinction day 2 relative to the final trial of extinction day 1 (between-session spontaneous recovery) for adult and adolescent mice. No differences between ages or groups were observed. (d,g) Average percentage of time spent freezing during a fear cue presentation relative to the final trial of extinction day 2 (long-term spontaneous recovery) for adult and adolescent mice. Spontaneous recovery was marginally greater for adolescents. Spontaneous recovery was significantly greater in mice previously exposed to safety conditioning. Safety Conditioned (n = 17 adult/16-17 adolescent; this cohort of mice is shown above as “Standard Extinction”), Fear Conditioned (n = 16 adult/12 adolescent). Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Freezing increased in all groups across conditioning days (Figure 5a), supported by a main effect of Session (Greenhouse-Geisser (ε = 0.82) corrected values, F(2.46, 142.41) = 159.93, p < 0.001, η2 = 0.73). Freezing did not differ between conditions and only marginally differed between ages (trending interaction between Age and Session, Greenhouse-Geisser (ε = 0.82) corrected values, F(2.46, 142.41) = 2.68, p = 0.060, η2 = 0.04), driven by slightly higher rates by which freezing increased across sessions for adolescent mice.
During extinction (Figure 5b, e), there was a main effect of Age (F(1, 58) = 14.27, p < 0.001, η2 = 0.20) and interaction between Age and Session (F(1, 58) = 5.02, p = 0.029, η2 = 0.08), driven by lower freezing in adolescents than adults on Day 1. Though, paralleling the above, freezing during the first fear cue presentation of the first extinction session did not differ by age (all p > 0.719; Adolescent fear by condition ranged from 52.70 ± 6.14 to 63.70 ± 5.96; Adult fear by condition ranged from 54.24 ± 6.87 to 66.48 ± 4.59). There was a main effect of Session (F(1, 58) = 134.176, p < 0.001, η2 = 0.70), indicative of successful extinction performance across all groups. Notably, there was also an interaction between Condition and Session (F(1, 58) = 15.91, p < 0.001, η2 = 0.22) and Condition, Age, and Session (F(1, 58) = 6.56, p = 0.013, η2 = 0.10). Decomposition of these effects revealed that while freezing between Conditions did not differ for adolescents on either day or adults on day two (padj’s > 0.999), freezing was greater during the first extinction day in adult mice that previously underwent safety conditioning relative to mice that underwent fear conditioning only (t(31) = 3.39, p = 0.008).
Analyses of spontaneous recovery (Figure 5c, f) revealed no differences between ages or conditions for spontaneous recovery between extinction sessions (all p’s > 0.345). In contrast, two weeks following extinction (Figure 5d, g) there was a main effect of Condition (F(1, 57) = 6.53, p = 0.013, η2 = 0.10), driven by greater spontaneous recovery in mice that underwent safety conditioning relative to mice that underwent fear conditioning only. While this effect did not differ by age (no interaction, p = 0.952), freezing was marginally higher in adolescents than adults (F(1, 57) = 3.55, p = 0.065, η2 = 0.059).
4. Discussion
Shortcomings in extinction retention during adolescence have been widely observed and serve as a possible mechanism for limitations in the efficacy of current treatments for anxiety. In this study, we found that combining methods of fear regulation (extinction and conditioned inhibition) can facilitate extinction retention. Specifically, intermixing safety cues into extinction appears to facilitate the strength of a long-term extinction memory, despite animals receiving half as many fear cue presentations during extinction. While both adolescents and adults benefit from the presence of safety cues, the decrease in spontaneous recovery relative to a standard extinction protocol was greater in adolescents than adults. We also found that initial safety learning (as indicated by rate of discrimination magnitude increase) was faster in adolescent mice. Taken together, our data provide initial evidence that safety learning may be inherently stronger during adolescence. In line with a myriad of studies showing that adolescents are highly responsive to cues and experiences related to reward, threat, or social interaction (Galván, 2013; Gerhard et al., 2020; Pattwell et al., 2013; Somerville, 2013), we posit that safety cues may be another class of affective cue to which adolescents are highly tuned.
Another interesting finding was that all extinction protocols used in this study resulted in similarly low levels of fear by the completion of the two extinction training sessions. This occurred despite both intermixed conditions including half as many standalone fear cue presentations as the standard and compound extinction conditions. Thus, while intermixing safety cues into extinction does not necessarily increase the rate of within session extinction, it does allow for a reduced number of fear cue exposures to produce the same within session efficacy (in addition to enhanced retention, as noted above).
Consideration of findings between ages showed that freezing was reliably lower for adolescents relative to adults during the first extinction session. This reflects a marked difference from several studies suggesting that freezing during extinction learning, particularly in mice, is often higher for adolescents than adults (Pattwell et al., 2012; Hefner & Holmes, 2007) though is consistent with one report in which faster within-session extinction during adolescence in mice was observed (Lawson et al., 2022). Nonetheless, spontaneous recovery was either equal between ages or greater in adolescents across all experiments, suggesting limited retention across time, consistent with many other reports (Kim et al., 2011; Koppensteiner et al., 2019a, 2019b; McCallum et al., 2010; Pattwell et al., 2012). Thus, while adolescents appear to exhibit substantial within-session extinction, the strength of the extinction memory formed may be weak. One possible explanation is that reductions in adolescent freezing across extinction sessions are at least partially due to habituation, rather than inhibitory learning. Though the present data cannot distinguish between these possibilities, clinical studies have suggested that reductions in fear responding due to mere exposure (habituation) are not predictive of maintained reductions in fear responding over time (e.g., Craske et al., 2008, 2014; Rowe & Craske, 1998). If this is the case, intermixing safety cues may have facilitated extinction learning by providing a more dynamic affective structure to the learning environment and reducing the total number of repetitive exposures to the fear cue, preventing habituation.
An open question remains regarding what types of stimuli may be introduced into extinction to facilitate retention. Here we showed evidence that intermixing a safety cue enhances retention. Our previous work has shown that a safety cue trained via the methods used here gains conditioned inhibitor properties. One possibility is that retention may only benefit from intermixing a strong indicator of the absence of threat. Interestingly, intermixing a compound (simultaneous fear and safety cue presentation) nominally reduces spontaneous recovery relative to standard extinction, but impacts on retention are not significant. Thus, engaging processes of conditioned inhibition (i.e., inhibiting the fear elicited by the fear cue) does not appear to be as efficacious as merely processing a conditioned inhibitor. While studies of extinction following discriminative conditioning in rodents are rare, studies of fear learning and extinction in humans often include both CS+ and CS− presentations, to provide a direct comparison of cue-directed fear within subjects. However, the number of presentations is often limited. Our training protocol is designed to over-train a CS−, allowing it to develop into a conditioned inhibitor. Indeed, while most mice freeze more to the fear cue than the safety cue within two sessions, we have found four sessions to be necessary to see reduced freezing to the compound (i.e., summation test). This may explain why other studies using a CS+/CS− design (e.g., Ganella et al., 2018; Pattwell et al., 2012) find evidence of diminished extinction learning and retention during adolescence. Nonetheless, our study cannot fully distinguish what qualities the intermixed cue must possess in order to enhance extinction retention.
Previously, Dunsmoor and colleagues discovered that introducing a novel stimulus to coterminate with a fear cue during extinction (i.e., replacing the unconditioned stimulus that was present during fear conditioning) also facilitates extinction retention in both adult rodents and humans (Dunsmoor et al., 2015). The same group found that aversive-to-appetitive counterconditioning (i.e., replacing the unconditioned stimulus present during fear conditioning with a positive outcome) similarly reduces later threat arousal in adult humans (Keller et al., 2020, 2023). Newall and colleagues provided an important developmental extension of these findings, showing that 7-12 year old children also exhibit reduced fear following counterconditioning than standard extinction (Newall et al., 2017). Across these studies, the authors posited that omission of the unconditioned stimulus alone may not be sufficient and that introducing surprise into the structure of an extinction protocol signals change and encourages new learning. Similar mechanisms may be at play for the present findings.
An unexpected finding in the present study was an apparent strengthening of a fear memory when initial conditioning occurred alongside a safety cue, relative to conditioning that included exclusively a fear cue, on both short timescales (in adults) and long timescales (in both ages). This data highlights the importance of considering the timing of when a safety cue is presented in the learning process, as this may impact the capacity for fear regulation. It may be that safety cue presence ubiquitously enhances learning about cues presented in close temporal proximity, impacting both aversive and inhibitory responding, though through differing mechanisms. Indeed, while a safety cue may add a dynamic structure to the learning environment during extinction and prevent habituation, the outcome of the safety cue does not change between conditioning and extinction. On the other hand, the strengthening of initial fear learning following discriminative conditioning relative to fear conditioning that our data suggests may result from safety cues increasing prediction error (a term describing the difference between the expectation of an outcome and reality of what occurs; McNally et al., 2011). By serving as a strong contrast to the aversive association formed with the fear cue during conditioning, the presence of safety cues may reallocate attention to fear cues in a way that strengthens excitatory fear learning. Moreover, as both cues are in the same modality (tone) the mouse must learn to direct attention towards the differing pitches of the tones, driving prediction error until this discrimination can be accomplished.
Our group and others have put forth a case for the benefits of safety learning during adolescence to augment fear regulation and inform age-tailored treatments for anxiety (Meyer et al., 2023; Odriozola & Gee, 2020). Building on this, the present study has important translational implications, informing the feasibility and parameters of using safety cues to enhance extinction learning. Extinction learning serves as the basis for exposure therapy, a key component of cognitive behavioral therapy, a frontline treatment for anxiety disorders (Abramowitz et al., 2019; Craske et al., 2014; Lissek et al., 2005). While exposure therapy is a highly efficacious treatment approach for both adults (Abramowitz et al., 2019; Hipol & Deacon, 2013; Olatunji et al., 2010) and pediatric populations (Reid et al., 2017; Whiteside et al., 2016), there remain concerns about patient attrition (Kehle-Forbes et al., 2016) and ability to tolerate exposures (Keleher et al., 2020). The acceptability of a given treatment strongly predicts patient engagement in the therapeutic process, as well as treatment success (Calvert & Johnston, 1990). Safety cues may encourage treatment initiation and adherence as well as mitigate patient attrition by serving as a source of temporary relief when distress is at its highest (Milosevic & Radomsky, 2008, 2013; Rachman et al., 2008). Furthermore, while many pediatric patients show symptom reduction at the time of treatment, the long-term efficacy of Cognitive Behavioral Therapy may be limited. One study considering multi-year prospective data from patients receiving treatment for anxiety during adolescence estimated that only ~20% of treated youth exhibit an absence of all anxiety disorder symptoms at long-term follow-up (Ginsburg et al., 2018). Additional work establishing the dosage, timing, and duration of administration, for incorporating safety cues into existing treatments, particularly those tailored for adolescents, will be integral to extending the translational potential of this basic behavioral neuroscience work.
Clinical translation must be done with caution for the given findings, and we acknowledge many limitations of our study in mice. For example, the safety cue was trained through direct contrast with a fear cue in the discriminative conditioning procedure. This may result in an associative link being formed between the fear and safety cue. For safety learning to have translational value, it would be important to show that a safety cue capable of fear inhibition could be acquired independently of a threat exposure and that inhibitory properties could generalize across a variety of fear-provoking stimuli and environments. Regarding the former, others have suggested that a social support figure may develop as a safety cue through positive interactions over time, even in the absence of overt training alongside a threat (Eisenberger, 2013; Hornstein, Fanselow, & Eisenberger, 2016; Hornstein et al., 2022). While the authors posit that social support figures are a special class of safety cues that may activate alternative neural circuitry to inhibit fear, a similar progressive development of a safety cue may be possible. In this way, application in a clinical setting may be less focused on developing a safety cue, but rather identifying and appropriately engaging with individualized safety cues that already exist for the patient. Follow up studies to empirically test this possibility and identify whether some modalities of safety cues are more effective than others will be crucial. Regarding the question of generalizability, while the current study cannot speak to this, we direct readers to literature suggesting that conditioned inhibition should transfer to reduce responding to a variety of independently acquired conditioned stimuli (Holland, 1989).
Controversy remains regarding when and to what extent safety cues can be used to mitigate fear. In the laboratory, studies investigating extinction of a fear cue in the presence of a conditioned inhibitor (i.e., the compound used here) consistently reveal limited extinction learning and retention, with fear remaining high at later tests (Lovibond et al., 2000; McConnell & Miller, 2010; Pearce & Wilson, 1991; Pineño et al., 2008; Thomas & Ayres, 2004). This outcome is often referred to as the “protection from extinction” effect (Rescorla, 2003; Soltysik et al., 1983). This concept is paralleled in the clinic by the consensus that defaulting to safety cues to attenuate fear can lead to avoidance, minimize processing of the actual threat value of a fear cue, and limit inhibitory learning. Our work is in part consistent with this, such that mice in the Compound Extinction group exhibited high spontaneous recovery. However, we also present evidence that intermittent exposure to either a compound or a safety cue (with the latter being the most efficacious) may overcome limitations traditionally associated with applying conditioned inhibition in an extinction setting.
One limitation of the present study is that tests for extinction retention were carried out in the same context as extinction (ABB context design). While our findings provide a valuable addition to the literature regarding spontaneous recovery, further examination of the extent to which introducing safety cues into extinction impacts renewal, by considering fear recall in the conditioning context (ABA context design), or a novel context (ABC context design) would provide an important complement. Although, experimental designs of this nature can be difficult to interpret during adolescence, as fear relapse may be similarly high (i.e., a ceiling effect) resulting from both spontaneous recovery and renewal (e.g., Kim et al., 2011). Nonetheless, others have shown that counterconditioning mitigates renewal in a novel context relative to standard extinction in adult humans, though not in the original fear conditioning context (Keller et al., 2023). In contrast, in adult rats it was observed that counterconditioning led to greater renewal in both a novel context and the original fear conditioning context relative to extinction (Holmes et al., 2016). Unpacking the context dependency of safety cues for enhancing extinction retention will be necessary to inform the generalizability and translational potential of the present findings.
Another limitation is the use of exclusively male mice. Fear regulation processes have been shown to differ between males and females (Gruene et al., 2015; Marusak et al., 2021; Milad et al., 2009; Perry et al., 2020; Trask et al., 2020). Moreover, marked sex differences exist in the maturational trajectories of fear circuitry (Gerhard et al., 2020). Additional research in this area that considers both developmental age and sex will be critical for understanding and treating anxiety disorders across the lifespan.
Despite these limitations, the present study adds to a growing body of work highlighting the efficacy of safety learning as a means to augment fear regulation during adolescence. Moreover, our findings show that alternating between states of fear and conditioned inhibition during extinction, while not remaining in a state of constant fear inhibition, facilitates extinction memory retention. While much remains to be done, establishing a course for increasing the longevity of extinction, particularly during adolescence, is an important area of study.
Highlights.
Fear and safety discrimination is more robust in adolescents than adults
Introducing safety cues into extinction augments retention in adolescents and adults
Modified extinction reduces spontaneous recovery more for adolescents than adults
Compound extinction does not reduce spontaneous recovery in adolescents or adults
Acknowledgements
This work was supported by the National Institutes of Mental Health (NIMH) Pathway to Independence Award (K99MH119320) and the National Institutes of Health (NIH) National Center for Advancing Translational Science (TL1TR002386) to H.C.M., and R01 MH123154 to F.S.L, as well as the Pritzker Neuropsychiatric Disorders Research Consortium (F.S.L.). We gratefully acknowledge Gabrielle Magalhães and Sara Yazdi for useful comments and suggestions on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Deacon BJ, & Whiteside SPH (2019). Exposure therapy for anxiety: Principles and practice, 2nd ed (pp. xvi, 459). The Guilford Press. [Google Scholar]
- Baker KD, Bisby MA, & Richardson R (2016). Impaired fear extinction in adolescent rodents: Behavioural and neural analyses. Neuroscience & Biobehavioral Reviews, 70, 59–73. 10.1016/j.neubiorev.2016.05.019 [DOI] [PubMed] [Google Scholar]
- Baker KD, McNally GP, & Richardson R (2018). D-Cycloserine facilitates fear extinction in adolescent rats and differentially affects medial and lateral prefrontal cortex activation. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 86, 262–269. 10.1016/j.pnpbp.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Baker KD, & Richardson R (2015). Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learning & Memory, 22(11), 537–543. 10.1101/lm.039487.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, & Richardson R (2017). Pharmacological evidence that a failure to recruit NMDA receptors contributes to impaired fear extinction retention in adolescent rats. Neurobiology of Learning and Memory, 143, 18–26. 10.1016/j.nlm.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Bisby MA, Stylianakis AA, Baker KD, & Richardson R (2021). Fear extinction learning and retention during adolescence in rats and mice: A systematic review. Neuroscience & Biobehavioral Reviews, 131, 1264–1274. 10.1016/j.neubiorev.2021.10.044 [DOI] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52(10), 976–986. 10.1016/S0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Contextual control of the extinction of conditioned fear. Learning and Motivation, 10(4), 445–466. 10.1016/0023-9690(79)90057-2 [DOI] [Google Scholar]
- Bouton ME, Maren S, & McNally GP (2021). Behavioral and neurobiological mechanisms of pavlovian and instrumental extinction learning. Physiological Reviews, 101(2), 611–681. 10.1152/physrev.00016.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, & Barad M (2003). Temporally massed CS presentations generate more fear extinction than spaced presentations. Journal of Experimental Psychology: Animal Behavior Processes, 29(4), 323–333. 10.1037/0097-7403.29.4.323 [DOI] [PubMed] [Google Scholar]
- Calvert SC, & Johnston C (1990). Acceptability of treatments for child behavior problems: Issues and implications for future research. Journal of Clinical Child Psychology, 19, 61–74. 10.1207/s15374424jccp1901_8 [DOI] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, & Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46(1), 5–27. 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway C, Zbozinek T, & Vervliet B (2014). Maximizing Exposure Therapy: An Inhibitory Learning Approach. Behaviour Research and Therapy, 58, 10–23. 10.1016/j.brat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Campese VD, Ceceli AO, LeDoux JE, & Phelps EA (2015). Novelty-Facilitated Extinction: Providing a Novel Outcome in Place of an Expected Threat Diminishes Recovery of Defensive Responses. Biological Psychiatry, 78(3), 203–209. 10.1016/j.biopsych.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI (2013). An empirical review of the neural underpinnings of receiving and giving social support: implications for health. Psychosomatic medicine, 75(6), 545–556. 10.1097/PSY.0b013e31829de2e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A (2013). The Teenage Brain: Sensitivity to Rewards. Current Directions in Psychological Science, 22(2), 88–93. 10.1177/0963721413480859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Drummond KD, Ganella EP, Whittle S, & Kim JH (2018). Extinction of Conditioned Fear in Adolescents and Adults: A Human fMRI Study. Frontiers in Human Neuroscience, 11. https://www.frontiersin.org/articles/10.3389/fnhum.2017.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Lee-Kardashyan L, Luikinga SJ, Nguyen DLD, Madsen HB, Zbukvic IC, Coulthard R, Lawrence AJ, & Kim JH (2017). Aripiprazole Facilitates Extinction of Conditioned Fear in Adolescent Rats. Frontiers in Behavioral Neuroscience, 11. https://www.frontiersin.org/articles/10.3389/fnbeh.2017.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard DM, & Meyer HC (2021). Extinction trial spacing across days differentially impacts fear regulation in adult and adolescent male mice. Neurobiology of Learning and Memory, 186, 107543. 10.1016/j.nlm.2021.107543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard DM, Meyer HC, & Lee FS (2020). An Adolescent Sensitive Period for Threat Responding: Impacts of Stress and Sex. Biological Psychiatry, 0(0). 10.1016/j.biopsych.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Becker-Haimes EM, Keeton C, Kendall PC, Iyengar S, Sakolsky D, Albano AM, Peris T, Compton SN, & Piacentini J (2018). Results From the Child/Adolescent Anxiety Multimodal Extended Long-Term Study (CAMELS): Primary Anxiety Outcomes. Journal of the American Academy of Child & Adolescent Psychiatry, 57(7), 471–480. 10.1016/j.jaac.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, & Shansky RM (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. ELife, 4, e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn A, Kitt ER, Abend R, Matsumoto C, Odriozola P, Winkler AM, Leibenluft E, Pine DS, & Gee DG (2020). Comparing neural correlates of conditioned inhibition between children with and without anxiety disorders – A preliminary study. Behavioural Brain Research, 112994. 10.1016/j.bbr.2020.112994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, & Holmes A (2007). Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behavioural Brain Research, 176(2), 210–215. 10.1016/j.bbr.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipol LJ, & Deacon BJ (2013). Dissemination of evidence-based practices for anxiety disorders in Wyoming: A survey of practicing psychotherapists. Behavior Modification, 37(2), 170–188. 10.1177/0145445512458794 [DOI] [PubMed] [Google Scholar]
- Holland PC (1989). Transfer of negative occasion setting and conditioned inhibition across conditioned and unconditioned stimuli. Journal of experimental psychology: Animal behavior processes, 15(4), 311–328. 10.1037/0097-7403.15.4.311 [DOI] [PubMed] [Google Scholar]
- Holmes NM, Leung HT, & Westbrook RF (2016). Counterconditioned fear responses exhibit greater renewal than extinguished fear responses. Learning & Memory (Cold Spring Harbor, N.Y.), 23(4), 141–150. 10.1101/lm.040659.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein EA, Craske MG, Fanselow MS, & Eisenberger NI (2022). Reclassifying the Unique Inhibitory Properties of Social Support Figures: A Roadmap for Exploring Prepared Fear Suppression. Biological psychiatry, 91(9), 778–785. 10.1016/j.biopsych.2021.11.017 [DOI] [PubMed] [Google Scholar]
- Hornstein EA, Fanselow MS, & Eisenberger NI (2016). A Safe Haven: Investigating Social-Support Figures as Prepared Safety Stimuli. Psychological science, 27(8), 1051–1060. 10.1177/0956797616646580 [DOI] [PubMed] [Google Scholar]
- Kehle-Forbes SM, Meis LA, Spoont MR, & Polusny MA (2016). Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychological Trauma: Theory, Research, Practice, and Policy, 8(1), 107–114. 10.1037/tra0000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher J, Jassi A, & Krebs G (2020). Clinician-reported barriers to using exposure with response prevention in the treatment of paediatric obsessive-compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders, 24, 100498. 10.1016/j.jocrd.2019.100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NE, Cooper SE, McClay M, & Dunsmoor JE (2023). Counterconditioning reduces contextual renewal in a novel context but not in the acquisition context. Neurobiology of Learning and Memory, 201, 107749. 10.1016/j.nlm.2023.107749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NE, Hennings AC, & Dunsmoor JE (2020). Behavioral and neural processes in counterconditioning: Past and future directions. Behaviour Research and Therapy, 125, 103532. 10.1016/j.brat.2019.103532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, & Richardson R (2011). Immunohistochemical Analyses of Long-Term Extinction of Conditioned Fear in Adolescent Rats. Cerebral Cortex, 21(3), 530–538. 10.1093/cercor/bhq116 [DOI] [PubMed] [Google Scholar]
- Koppensteiner P, Galvin C, & Ninan I (2019a). Lack of experience-dependent intrinsic plasticity in the adolescent infralimbic medial prefrontal cortex. Synapse, 73(6), e22090. 10.1002/syn.22090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppensteiner P, Itter RV, Melani R, Galvin C, Lee FS, & Ninan I (2019b). Diminished Fear Extinction in Adolescents Is Associated With an Altered Somatostatin Interneuron–Mediated Inhibition in the Infralimbic Cortex. Biological Psychiatry, 86(9), 682–692. 10.1016/j.biopsych.2019.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K, Scarlata MJ, Cho WC, Mangan C, Petersen D, Thompson HM, Ehnstrom S, Mousley AL, Bezek JL, & Bergstrom HC (2022). Adolescence alcohol exposure impairs fear extinction and alters medial prefrontal cortex plasticity. Neuropharmacology, 211, 109048. 10.1016/j.neuropharm.2022.109048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, & Pine DS (2005). Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy, 43(11), 1391–1424. 10.1016/j.brat.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Davis NR, & O’Flaherty AS (2000). Protection from extinction in human fear conditioning. Behaviour Research and Therapy, 38(10), 967–983. 10.1016/s0005-7967(99)00121-7 [DOI] [PubMed] [Google Scholar]
- Marusak H, Peters C, Iadipaolo A, & Rabinak C (2021). Are There Sex Differences in Fear Conditioning and Extinction Before Puberty? A Preliminary Study in Pre-Adolescent Children. Biological Psychiatry, 89(9), S109–S110. 10.1016/j.biopsych.2021.02.283 [DOI] [Google Scholar]
- McCallum J, Kim JH, & Richardson R (2010). Impaired Extinction Retention in Adolescent Rats: Effects of D-Cycloserine. Neuropsychopharmacology, 35(10), 2134–2142. 10.1038/npp.2010.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BL, & Miller RR (2010). Protection from extinction provided by a conditioned inhibitor. Learning & Behavior, 38(1), 68–79. 10.3758/LB.38.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, & Blair HT (2011). Placing prediction into the fear circuit. Trends in Neurosciences, 34(6), 283–292. 10.1016/j.tins.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Fields A, Vannucci A, Gerhard DM, Bloom PA, Heleniak C, Opendak M, Sullivan R, Tottenham N, Callaghan BL, & Lee FS (2023). The Added Value of Crosstalk Between Developmental Circuit Neuroscience and Clinical Practice to Inform the Treatment of Adolescent Anxiety. Biological Psychiatry Global Open Science, 3(2), 169–178. 10.1016/j.bpsgos.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Gerhard DM, Amelio PA, & Lee FS (2021). Pre-adolescent stress disrupts adult, but not adolescent, safety learning. Behavioural Brain Research, 400, 113005. 10.1016/j.bbr.2020.113005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, & Gee DG (2019). Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proceedings of the National Academy of Sciences, 116(52), 26970–26979. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, & Novales JE (2009). Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience, 164(3), 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, & Radomsky AS (2008). Safety behaviour does not necessarily interfere with exposure therapy. Behaviour Research and Therapy, 46(10), 1111–1118. 10.1016/j.brat.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Milosevic I, & Radomsky AS (2013). Incorporating the Judicious Use of Safety Behavior Into Exposure-Based Treatments for Anxiety Disorders: A Study of Treatment Acceptability. Journal of Cognitive Psychotherapy, 27(2), 155–174. 10.1891/0889-8391.27.2.155 [DOI] [PubMed] [Google Scholar]
- Newall C, Watson T, Grant K-A, & Richardson R (2017). The relative effectiveness of extinction and counter-conditioning in diminishing children’s fear. Behaviour Research and Therapy, 95, 42–49. 10.1016/j.brat.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Odriozola P, & Gee DG (2020). Learning About Safety: Conditioned Inhibition as a Novel Approach to Fear Reduction Targeting the Developing Brain. American Journal of Psychiatry, appi.ajp.2020.20020232. 10.1176/appi.ajp.2020.20020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, & Deacon BJ (2010). Efficacy of cognitive behavioral therapy for anxiety disorders: A review of meta-analytic findings. The Psychiatric Clinics of North America, 33(3), 557–577. 10.1016/j.psc.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, & Lee FS (2012). Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences, 109(40), 16318–16323. 10.1073/pnas.1206834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Lee FS, & Casey BJ (2013). Fear learning and memory across adolescent development: Hormones and Behavior Special Issue: Puberty and Adolescence. Hormones and Behavior, 64(2), 380–389. 10.1016/j.yhbeh.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, & Wilson PN (1991). Effects of extinction with a compound conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 17, 151–162. 10.1037/0097-7403.17.2.151 [DOI] [PubMed] [Google Scholar]
- Perry CJ, Ganella DE, Nguyen LD, Du X, Drummond KD, Whittle S, Pang TY, & Kim JH (2020). Assessment of conditioned fear extinction in male and female adolescent rats. Psychoneuroendocrinology, 116, 104670. 10.1016/j.psyneuen.2020.104670 [DOI] [PubMed] [Google Scholar]
- Pineño O, Zilski-Pineno JM, & Miller RR (2008). Habituation of unconditioned fear can be attenuated by the presence of a safe stimulus: Assessment using the neophobic response of the rat. Behavioural Processes, 77(1), 55–60. 10.1016/j.beproc.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ (2002). Memory for Extinction of Conditioned Fear Is Long-lasting and Persists Following Spontaneous Recovery. Learning & Memory, 9(6), 402–407. 10.1101/lm.49602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S, Radomsky AS, & Shafran R (2008). Safety behaviour: A reconsideration. Behaviour Research and Therapy, 46(2), 163–173. 10.1016/j.brat.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Reid AM, Bolshakova MI, Guzick AG, Fernandez AG, Striley CW, Geffken GR, & McNamara JP (2017). Common Barriers to the Dissemination of Exposure Therapy for Youth with Anxiety Disorders. Community Mental Health Journal, 53(4), 432–437. 10.1007/s10597-017-0108-9 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1969). Pavlovian conditioned inhibition. Psychological Bulletin, 72(2), 77–94. 10.1037/h0027760 [DOI] [Google Scholar]
- Rescorla RA (2003). Protection from extinction. Learning & Behavior, 31(2), 124–132. 10.3758/bf03195975 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (2004). Spontaneous Recovery. Learning & Memory, 11(5), 501–509. 10.1101/lm.77504 [DOI] [PubMed] [Google Scholar]
- Rowe MK, & Craske MG (1998). Effects of varied-stimulus exposure training on fear reduction and return of fear. Behaviour Research and Therapy, 36(7–8), 719–734. 10.1016/s0005-7967(97)10017-1 [DOI] [PubMed] [Google Scholar]
- Soltysik SS, Wolfe GE, Nicholas T, Wilson WJ, & Garcia-Sanchez JL (1983). Blocking of inhibitory conditioning within a serial conditioned stimulus-conditioned inhibitor compound: Maintenance of acquired behavior without an unconditioned stimulus. Learning and Motivation, 14, 1–29. 10.1016/0023-9690(83)90010-3 [DOI] [Google Scholar]
- Somerville LH (2013). Special issue on the teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–127. 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BL, & Ayres JJB (2004). Use of the ABA fear renewal paradigm to assess the effects of extinction with co-present fear inhibitors or excitors: Implications for theories of extinction and for treating human fears and phobias. Learning and Motivation, 35, 22–52. 10.1016/S0023-9690(03)00040-7 [DOI] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, & Lattal KM (2014). Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learning & Memory, 21(8), 380–393. 10.1101/lm.035261.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Reis DS, Ferrara NC, & Helmstetter FJ (2020). Decreased cued fear discrimination learning in female rats as a function of estrous phase. Learning & Memory, 27(6), 254–257. 10.1101/lm.051185.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SPH, Deacon BJ, Benito K, & Stewart E (2016). Factors associated with practitioners’ use of exposure therapy for childhood anxiety disorders. Journal of Anxiety Disorders, 40, 29–36. 10.1016/j.janxdis.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbukvic IC, Park CHJ, Ganella DE, Lawrence AJ, & Kim JH (2017). Prefrontal Dopaminergic Mechanisms of Extinction in Adolescence Compared to Adulthood in Rats. Frontiers in Behavioral Neuroscience, 11. https://www.frontiersin.org/articles/10.3389/fnbeh.2017.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]