Abstract
Clinicians need improved prediction models to estimate time to kidney replacement therapy (KRT) for children with chronic kidney disease (CKD). Here, we aimed to develop and validate a prediction tool based on common clinical variables for time to KRT in children using statistical learning methods and design a corresponding online calculator for clinical use. Among 890 children with CKD in the Chronic Kidney Disease in Children (CKiD) study, 172 variables related to sociodemographics, kidney/cardiovascular health, and therapy use, including longitudinal changes over one year were evaluated as candidate predictors in a random survival forest for time to KRT. An elementary model was specified with diagnosis, estimated glomerular filtration rate and proteinuria as predictors and then random survival forest identified nine additional candidate predictors for further evaluation. Best subset selection using these nine additional candidate predictors yielded an enriched model additionally based on blood pressure, change in estimated glomerular filtration rate over one year, anemia, albumin, chloride and bicarbonate. Four additional partially enriched models were constructed for clinical situations with incomplete data. Models performed well in cross-validation and the elementary model was then externally validated using data from a European pediatric CKD cohort. A corresponding user-friendly online tool was developed for clinicians. Thus, our clinical prediction tool for time to KRT in children was developed in a large, representative pediatric CKD cohort with an exhaustive evaluation of potential predictors and supervised statistical learning methods. While our models performed well internally and externally, further external validation of enriched models is needed.
Keywords: Pediatric chronic kidney disease, pediatric nephrology, kidney replacement therapy, end stage kidney disease, prediction, risk stratification
Graphical Abstract
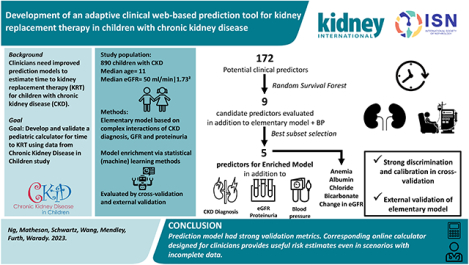
Lay Summary
We aimed to construct a prediction tool for doctors to estimate when children with chronic kidney disease (CKD) might require kidney replacement therapy (KRT). Using statistical (machine) learning methods, we analyzed 172 common clinical variables from 890 children with CKD in North America as predictors of time to KRT to identify the best model. The optimal model included type of CKD, eGFR, proteinuria, blood pressure, anemia, albumin, chloride, and bicarbonate; additional models were constructed to accommodate incomplete data. The tool performed well in testing and the simplest model was externally validated in a European pediatric CKD cohort. By integrating two complementary statistical learning methods with the rich CKiD database, we developed a powerful predictive tool that is adaptive to scenarios with incomplete data. We further designed a corresponding online calculator that is simple to use and provides valid predictions to guide clinical planning and treatment for pediatric CKD patients.
Introduction
Chronic kidney disease (CKD) in children precedes end stage kidney disease (ESKD)1,2, and disease severity and rate of progression are heterogeneous and based on many factors3,4. Identifying clinical profiles associated with varying rates of CKD progression and predicting time to kidney replacement therapy (KRT), defined as dialysis or kidney transplant, is clinically useful for stratifying patient risk, as well as for preparatory efforts by clinicians, patients and their families. Decades of research in children and adults demonstrated that underlying CKD etiology, glomerular filtration rate (GFR) levels at early stages of disease, and proteinuria are strong predictors of progression to ESKD3–8, and these variables were used to construct previously proposed pediatric risk prediction models3,4 using data from the Chronic Kidney Disease in Children (CKiD) study.
We recently demonstrated weak calibration of the adult Kidney Failure Risk Equation (KFRE) when applied to children with CKD which highlighted the need for improved pediatric-specific calculators9. In 2015, we presented a multivariable model to estimate times to first occurrence of KRT or a 50% decline in GFR in children with CKD3. Inclusion of the latter event can weaken inference because it is a surrogate endpoint of disease progression rather than a clinical endpoint. While surrogate endpoints like GFR decline can be useful in randomized clinical trials10, the clinical endpoint of KRT alone is superior. In addition, this model required complete data for 5 or 9 predictors for valid prediction which may not always be clinically available. In 2017, we proposed a simpler model4 based on a larger dataset combining CKiD and European Study Consortium for Chronic Kidney Disorders Affecting Pediatric Patients (ESCAPE) cohorts11. This equation used only underlying CKD etiology (hereafter, referred to as “diagnosis”), estimated GFR (eGFR), and proteinuria but was limited because the prediction was a composite of the surrogate endpoint of 50% eGFR decline or KRT and it did not incorporate BP, a well-recognized risk factor for disease progression.
The availability of new equations to estimate GFR12, updated pediatric blood pressure (BP) guidelines13, more data within CKiD focused on KRT as a clinical endpoint2 and powerful machine learning methods14,15 presented a timely opportunity to enhance KRT-specific risk prediction for children with CKD under treatment by pediatric nephrologists. For improved clinical use, a new proposed model should provide valid estimates even if some predictors are missing or not available and be conveniently accessible online.
The purpose of this analysis was to construct and validate a suite of predictive models for time to KRT for children with CKD based on commonly available clinical data, statistical learning methods, and parametric survival models. Statistical learning methods were used to assess commonly measured candidate predictors and to specify equations to address clinical instances of incomplete data. Lastly, we developed a web-based tool to interpret these complex equations for clinical applications.
Methods
Study population
Initiated in 2005, the CKiD study is a longitudinal cohort of children with CKD and eGFR <90/ml/min|1.73m2 at entry from 56 clinical sites in the United States and Canada. Participants contributed data at annual study visits, including biomarkers of CKD severity, cardiovascular and metabolic health, questionnaires for general health, medical history, and sociodemographic characteristics. All participants and families provided informed consent/assent, and the study protocols were approved by local institutional review boards. A complete description of the study design has been previously published.16
Time scale and outcome
The time origin for this analysis was the second study visit (i.e., Visit 2) in order to evaluate and identify longitudinal predictors of KRT incidence (i.e. risk factor changes over the first study year). For this analysis, 890 of 1098 participants contributed the first two study visits prior to KRT with complete data and were included for analysis (exclusions: 146 had no follow-up data; 47 missed Visit 2 (time origin); 15 initiated KRT prior to time origin; see Supplementary Figure S1). The primary outcome was the time to first occurrence of KRT. Date of dialysis or transplant was extracted from medical records, or participant/family-reported with confirmation. Participants who were event-free at the last available study visit were considered censored. Administrative censoring was performed at 10 years due to limited data availability.
Predictors
Supplementary Table S1 presents all candidate predictors evaluated for the prediction model. These included sociodemographic (sex, age, maternal education, household income, family history of kidney disease), CKD severity (U25 eGFR, urine protein:creatinine ratio [UPCR], years with CKD, anemia, BP stage based on 2017 American Academy of Pediatrics [AAP] guidelines13), birth history (prematurity, low birth weight, small for gestational age), laboratory markers (metabolic, blood and lipid panels), and medication use (antihypertensive therapy, alkali therapy, growth hormone, among many others). For time-varying variables, we also investigated change over approximately 1 year from Visit 1 to Visit 2 (i.e., divided by the duration in years for annualized change) for continuous variables and for binary variables (i.e., diagnoses, therapy use), the four different possible responses between two visits (no/no; no/yes; yes/no; yes/yes).
CKD diagnosis type (glomerular or non-glomerular), U25 eGFR12, and UPCR were considered essential variables a priori for the simplest “elementary” model2–4. A full list of diagnoses is presented in Supplementary Table S2. All serum and urine biomarkers were measured centrally (University of Rochester, PI: GJ Schwartz), except for bicarbonate which was measured locally.
Statistical methods
Prediction model development process
Briefly, the development of the clinical KRT prediction models comprised three steps, followed by a cross-validation step. The first step was to develop an elementary model using the most fundamental established predictors of KRT: eGFR, UPCR and diagnosis. Various functional forms of these variables were tested in a parametric survival model. The second step was to identify the best additional candidate predictors out of 172 selected CKiD variables using a random survival forest (RSF). The third step brought the first two together, exploring an enriched set of models based on adding the important variables identified from the RSF to the elementary model. Lastly, the entire three-step process was validated using 10-fold cross-validation.
Parametric survival models
Elementary and enriched parametric survival models used the generalized gamma (GG) family and maximum likelihood methods to estimate predicted time to KRT17. The GG distribution is defined by 3 parameters: location (β; linked to the median), scale (σ; linked to the interquartile ratio) and shape (κ; linked to the positions of the first and third quartiles relative to the median18), and is denoted as GG(β, σ, κ). All predictors were allowed to modify the location parameter; type of CKD diagnosis (described below) was allowed to additionally modify the scale and shape. These models were used to estimate the time at which the 10th, 25th and 50th percentiles for a given profile are expected to experience KRT.
Functional form of CKD diagnosis, eGFR, and UPCR for development of the elementary prediction model
To develop the elementary model, we first investigated classification of diagnoses. We previously reported distinct risk functions for those with glomerular non-hemolytic uremic syndrome (HUS) diagnoses, HUS diagnoses, and non-glomerular diagnoses2. Since we did not have sufficient data for HUS-specific prediction (n=48), we fit two GG models with HUS combined with glomerular diagnoses and then non-glomerular diagnoses. This approach determined which diagnostic group those with HUS resembled in terms of time to KRT. The Akaike’s Information Criterion (AIC) values were 1490.287 and 1479.916, respectively, indicating that HUS disease should be grouped together with non-glomerular diagnoses.
To flexibly model eGFR and UPCR as continuous variables (natural log-transformed) and overcome limitations associated with broad groupings of GFR and UPCR by category for predictive modeling, we investigated three continuous functional forms of each variable (linear, natural cubic spline, and linear spline, with a single knot at 45 ml/min|1.73m2 for eGFR or 0.5 mg/mgCr for UPCR), along with interactions of diagnosis with eGFR and/or UPCR. This analysis compared 36 models (=32 functional forms × 22 interaction possibilities); the model with the lowest AIC was selected as the elementary model, enabling prediction with the minimum number of variables and serving as the foundation for subsequent enriched models incorporating more predictors.
Random survival forest to identify candidate predictors
To broadly evaluate a pool of variables for enhancement of the elementary model, we used RSF, a supervised statistical learning method designed to classify outcomes (with censoring) using regression trees19 (randomForestSRC package in R). In brief, RSF is a bootstrapped regression tree method to identify variables most closely associated with progression to KRT as candidate predictors, including potentially identifying predictors not previously known or suspected to be important. Two key metrics assessed the value of each predictor: variable importance (VIMP), which evaluates how much classification error would be introduced if the predictor were unavailable; and minimal depth of the maximal subtree, which evaluates how early in the branching process a variable is generally selected. We used 1000 trees in each forest and the number of variables explored per node was , or 14 variables.
Development of enriched models
To build upon the elementary model which included diagnosis, eGFR, and UPCR as predictors, we a priori decided to include blood pressure (categorized as a binary variable defined as normal vs. elevated/Stage 1/Stage 2 according to AAP guidelines13) in all enriched models as a modifier of the location parameter, since it was previously identified as a key clinical variable associated with CKD progression20–22. Beyond these four variables, we investigated 9 additional candidate predictors (modifying the location parameter) using best subset selection, a supervised statistical learning method that evaluates all possible combinations of predictors to identify sets of variables that yield the lowest AIC (a measure of training error and goodness-of-fit)15. A total of 512 (= 29) models were evaluated including the null (elementary + BP) model in this procedure. The model with the lowest AIC in the best subset analysis was selected for use as the fully enriched model.
Evaluation of models
For internal model validation, we also calculated optimism-corrected c-statistic for discrimination23 and the Greenwood-Nam-D’Agostino (GND) goodness-of-fit test24,25 for calibration comparing 2-year and 5-year risk of KRT (with at least 5 events per bin25 and the χ2 critical value has n bins −1 degrees of freedom). The GND null hypothesis is that the observed risk equals the predicted risk; lower values of the test statistic indicate improved calibration. These metrics were compared across models from elementary to fully enriched in which we expected validation measures to improve with additional predictors. We also compared model fit for nested models relative to the elementary model and enriched model using likelihood ratio tests (LRTs).
Cross-validation and external validation
We conducted 10-fold cross-validation of the entire model-building process26,27. In this procedure, 10 random samples comprising 90% of the data were used to develop the elementary and enriched model with the concatenation of the remaining 10% per fold used to evaluate model fit. In the model development stage of the cross-validation, we executed the same three-step process described above so the results from the functional forms of elementary model predictors, the RSF, and best subset selection were allowed to vary. For the concatenation of the excluded data, standardized residual times from the model were compared to the standard exponential distribution to evaluate over-fitting (full details in Supplement)2 and calibration curves with slope (based on a regression without an intercept) using the GND method. Significance testing was not included because cross-validation sets are not independent.
External validation of the elementary model used data from the ESCAPE cohort which was part of Furth et al.4 To account for regional differences in KRT initiation28,29, the outcome of KRT or eGFR<20ml/min|1.73m2 was used (which is about the GFR at which KRT is initiated in this North American population). We assessed standardized residual times, calculated Harrell’s c-statistic and performed the GND test (full description in the Supplement) estimated the calibration slope (based on a regression without an intercept). Statistical significance was assessed at p<0.05.
All analyses and graphs were conducted in R 3.6.3 (R Core Team, Vienna, Austria).
Results
Cohort description
Table 1 describes the demographic and clinical characteristics of the cohort at the time origin, approximately 1 year after the baseline visit. In this sample, the median age was 11.4 years [interquartile range (IQR): 6.7, 15.3] years, 63% were boys, and 21% were of self- or parentalreported Black race. Only 48 participants (5%) had a diagnosis of HUS, and 21% had glomerular, non-HUS diagnoses; the remainder had non-glomerular diagnoses (74%). The median eGFR at the time origin was 50.4 ml/min|1.73m2 [IQR: 35.0, 64.6] and the median UPCR was 0.32 mg/mgCr [IQR: 0.13, 1.00]. There was substantial heterogeneity in change of these biomarkers in the year between study entry and the time origin: the median change per year was 1.3% decline in eGFR and 1.3% increase in UPCR, but the IQR for eGFR was −10.3% to +7.8% per year, and was −32.6% to +51.4% per year for UPCR. The median duration of follow-up was 5.2 [IQR: 2.2, 7.9] years and 29% initiated KRT (56% dialysis and 44% kidney transplant). The cumulative incidence function of KRT is presented in Supplementary Figure S2.
Table 1.
Clinical and demographic characteristics of 890 CKiD participants at the time origin (i.e., second study visit). Longitudinal data of progression was characterized by the annualized percent change in GFR and proteinuria between the first and second study visit. Median [IQR] or n (%).
| Characteristics | Median [IQR] or n (%) n= 890 |
|---|---|
| Demographics and clinical history | |
| Age, years | 11.4 [6.7, 15.3] |
| Male sex | 557 (63%) |
| Self- or parental reported Black race | 187 (21%) |
| Hispanic ethnicity | 121 (14%) |
| Abnormal birth historya | 261 (30%) |
| Kidney disease characteristics, severity and progression | |
| CKD diagnosis | |
| Glomerular, hemolytic uremic syndrome (HUS) | 48 (5%) |
| Glomerular, non-HUS | 186 (21%) |
| Non-glomerular, non-CAKUT | 159 (18%) |
| Non-glomerular, CAKUT | 497 (56%) |
| Age at disease onset | |
| Present at birth | 605 (69%) |
| <1 year old | 41 (5%) |
| 1–5 years old | 73 (8%) |
| 6–10 years old | 73 (8%) |
| ≥11 years old | 89 (10%) |
| Years with CKD | 7.9 [4.5, 12.4] |
| U25eGFR, ml/min|1.73m2 | 50.4 [35.0, 64.6] |
| 1-year annualized % change in U25eGFR | −1.3% [−10.3%, +7.8%] |
| Urine protein/creatinine (UPCR), mg/mgCr | 0.32 [0.13, 1.00] |
| 1-year annualized % change in UPCR | + 1.3% [−32.6%, +51.4%] |
| Elevated blood pressure, Stage 1 or 2 hypertension | 331 (37%) |
| Laboratory measures | |
| Serum albumin, g/dL | 4.4 [4.2, 4.6] |
| Serum potassium, mmol/L | 4.4 [4.1, 4.7] |
| Serum phosphate, mg/dL | 4.6 [4.0, 5.0] |
| Serum chloride, mmol/L | 104 [102, 107] |
| Serum bicarbonate, mmol/L | 23 [21, 25] |
| Total cholesterol, mg/dL | 169 [147, 191] |
| Anemia | 228 (27%) |
| Follow-up and outcomes | |
| Duration of follow-up, years | 5.2 [2.2, 7.9] |
| Total duration of follow-up, years | 4596.8 |
| Any kidney replacement therapy | 261 (29%) |
| Dialysis | 147 (17%) |
| Transplant | 114 (13%) |
Defined as premature birth or small for gestational age or low birthweight (birthweight < 2500g)
Development of the elementary parametric survival model for prediction
Supplementary Table S3 presents the AIC for the parametric models investigating the functional forms of diagnosis, eGFR, and UPCR. The best model included eGFR with a linear spline at 45 ml/min|1.73m2 with an interaction with diagnosis, UPCR with a linear spline at 0.5 mg/mgCr, and diagnosis modifying the location parameter, as well as diagnosis modifying both the scale and shape parameters (AIC= 1476.477). This elementary model represents the fewest variables required to predict time to KRT and was substantially lower than the null model with no covariates (AIC= 2021.164).
Random survival forest and development of enriched models
Figure 1 depicts the most important variables by VIMP and minimal depth of the maximal subtree from the RSF analysis; variables towards the top and right are stronger predictors. The results identified eGFR and UPCR as the most important predictors of KRT, affirming our prior decision to build the elementary model around these variables. The next set of important variables included annual change in UPCR, albumin, phosphate, potassium, and chloride. The third set of important variables included annual change in eGFR, bicarbonate, calcium, hematocrit, red blood cell count, anemia, and initiation of erythropoietin stimulating agent (ESA). These were conceptually grouped as markers of CKD progression (change in eGFR and proteinuria over the past year), markers of CKD comorbidities: anemia (since hematocrit, erythrocyte count, present anemia, persistent anemia, and ESA use may be summarized by a single diagnosis); hypoalbuminemia (serum albumin), and metabolic derangement (bicarbonate, chloride, phosphate, potassium, calcium); for a total of 9 additional variables. Supplementary Table S4 presents the full rankings of the top variables.
Figure 1.
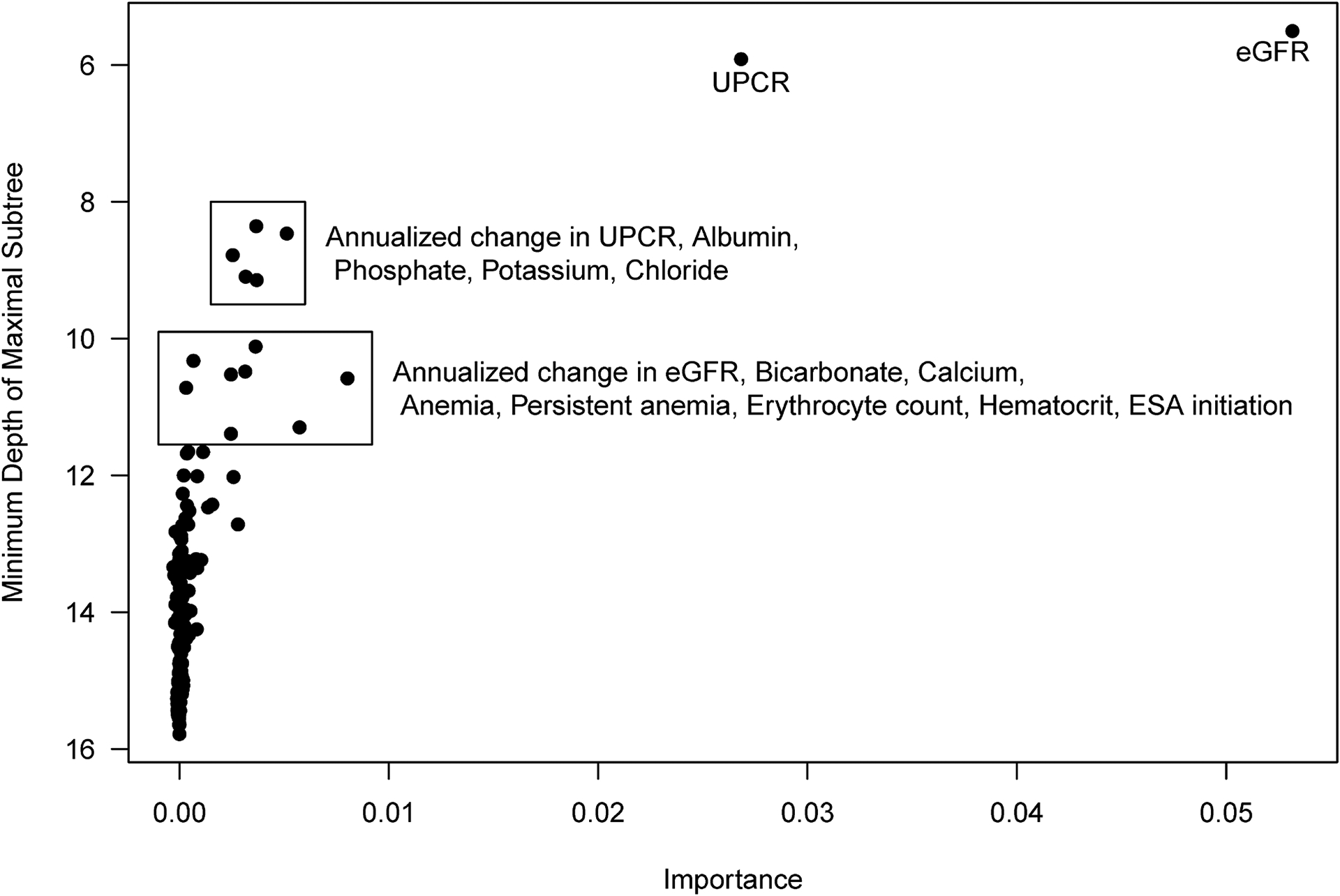
Results from random survival forest plotting metrics of predictive value (minimum depth of maximal subtree versus variable importance) to identify candidate predictors for parametric survival models. Minimum depth of maximal subtree indicates how early in the branching process a predictor is selected, on average; smaller values correspond to earlier selection and thus greater predictive value (y-axis is descending). Importance measures how much prediction error is introduced by randomly permuting the values of a predictor within the dataset; larger values indicate more such error, attributing greater importance to the correct ordering of the values. Groups of variables are denoted and variables listed were included in best subset selection methods of parametric survival models.
Best subset selection was used to evaluate which combination of these 9 additional candidate variables yielded the best predictive model by AIC. Supplementary Figure S3 presents AIC values for the 512 (= 29) models evaluated. The elementary model was the base model (AIC= 1476.477); adding BP as a binary variable (i.e., normal vs. elevated, Stage 1 or Stage 2 hypertension) yielded an AIC of 1471.702. Substantial improvements in minimum AIC were observed as models included up to 5 additional variables, while models with combinations of 6 or more additional variables yielded higher (worse) AIC than the best model with 5 additional variables. This enriched model included annual change in eGFR, anemia, albumin, chloride and bicarbonate in addition to eGFR, UPCR, diagnosis, and BP stage.
Table 2 presents the coefficients for the GG model parameters and model fit statistics and calibration for the elementary model, the enriched model, and four partially enriched models, for circumstances in which data may be unavailable. The evaluation of each model was the test error quantified by AIC and this decreased with the inclusion of additional variables. Enriched models had significantly better fit relative to the elementary model by LRT; the fully enriched model had significantly better fit than less enriched models, with the exception of Partially Enriched Model 4 (p= 0.080). There was improved discrimination in the enriched models (c-statistic= 0.868 for the elementary model to 0.880 for Partially Enriched Model 4 and the enriched model). The GND test statistic for 2-year risk decreased from 2.201 (elementary) to 1.526 (Partially Enriched Model 4) and for 5-year risk decreased from 4.123 (elementary) to 2.643 (enriched) and there were no significant differences between predicted and observed risk. The integration of these models into a unified adaptive tool is presented as a decision tree in Figure 2, describing logic based on available data that leads to the model with the lowest AIC.
Table 2.
Summary of parametric survival generalized gamma models for elementary, partially enriched and fully enriched models, including coefficients and model fit statistics. The unit of analysis were models with model fit and calibration described at the bottom.
| Partially enriched models | ||||||
|---|---|---|---|---|---|---|
| Parameter | Elementary (eGFR, UPCR, diagnosis only) | PE Model 1 (Elementary + BP + ΔGFR) | PE Model 2 (Elementary + BP + Anemia) | PE Model 3 (Elementary + BP + lab values) | PE Model 4 (Elementary + BP + Anemia + lab values) | Enriched (All predictors) |
| β | ||||||
| Intercept | 2.8624 | 2.8993 | 2.9967 | 3.7450 | 4.4095 | 4.4493 |
| Per 1 log increase in (eGFR/45) | 2.0868 | 1.9511 | 1.9769 | 2.1789 | 2.0944 | 1.9837 |
| Per 1 log increase in (eGFR/45)×(eGFR ≥ 45) | −1.0758 | −1.0091 | −1.1370 | −1.3874 | −1.4511 | −1.4158 |
| Glomerular non-HUS vs. Non-glom/HUS | −0.1532 | −0.0718 | −0.0511 | −0.1071 | −0.0231 | 0.0268 |
| Per 1 log increase in (eGFR/45) × Glom non-HUS | 1.0386 | 0.8132 | 0.9807 | 0.6812 | 0.7360 | 0.6275 |
| Per 1 log increase in (eGFR/45)×(eGFR ≥ 45) × Glom non-HUS | −1.2154 | −1.0010 | −1.0714 | −0.5795 | −0.6282 | −0.5253 |
| Per 1 log increase in (UPCR/0.5) | −0.1959 | −0.2123 | −0.2127 | −0.1720 | −0.1984 | −0.1968 |
| Per 1 log increase in (UPCR/0.5) × (UPCR ≥ 0.5) | −0.3146 | −0.2721 | −0.2566 | −0.2102 | −0.1803 | −0.1759 |
| Elevated BP/hypertension vs. normal BP | −0.2229 | −0.2337 | −0.2242 | −0.2339 | −0.2282 | |
| Per 1 increase in Δ( log(eGFR)) per year | 0.4349 | 0.3934 | ||||
| Anemia vs. no anemiaa | −0.3617 | −0.3162 | −0.3033 | |||
| Per 1 unit increase in albumin, mg/dLb | 0.3802 | 0.3168 | 0.3172 | |||
| Per 1 unit increase in chloride, mmol/Lb | −0.0235 | −0.0248 | −0.0255 | |||
| Per 1 unit increase in CO2, mmol/Lb | 0.0005 | −0.0074 | −0.0071 | |||
| σ | ||||||
| Intercept | 0.7440 | 0.7454 | 0.7441 | 0.7204 | 0.7242 | 0.7262 |
| Glomerular non-HUS vs. Non-glom/HUS | 0.2627 | 0.1744 | 0.2521 | 0.2426 | 0.2172 | 0.1390 |
| K | ||||||
| Intercept | 0.4253 | 0.3888 | 0.3513 | 0.4467 | 0.4163 | 0.3914 |
| Glomerular non-HUS vs. Non-glom/HUS | −0.0005 | 0.2204 | 0.1023 | 0.0768 | 0.1367 | 0.2787 |
| Model fit and calibration | ||||||
| AIC | 1476.48 | 1470.09 | 1457.68 | 1456.72 | 1446.15 | 1445.06 |
| Likelihood ratio test | ||||||
| Compared to elementary | Ref | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 |
| Compared to enriched | <0.001 | <0.001 | <0.001 | <0.001 | 0.080 | Ref |
| Optimism-corrected c-statistic | 0.865 | 0.866 | 0.874 | 0.871 | 0.875 | 0.875 |
| Greenwood-Nam-D’Agostino test statistic | ||||||
| At 2 years with 4 bins () | 2.201 | 2.213 | 2.237 | 2.318 | 1.526 | 1.903 |
| p-value | 0.53 | 0.53 | 0.52 | 0.51 | 0.68 | 0.59 |
| At 5 years with 6 bins () | 4.123 | 5.022 | 4.827 | 4.231 | 3.518 | 2.643 |
| p-value | 0.53 | 0.41 | 0.44 | 0.52 | 0.62 | 0.75 |
Anemia defined as current low hemoglobin.
Laboratory-measured serum biomarkers from renal panel.
Figure 2.
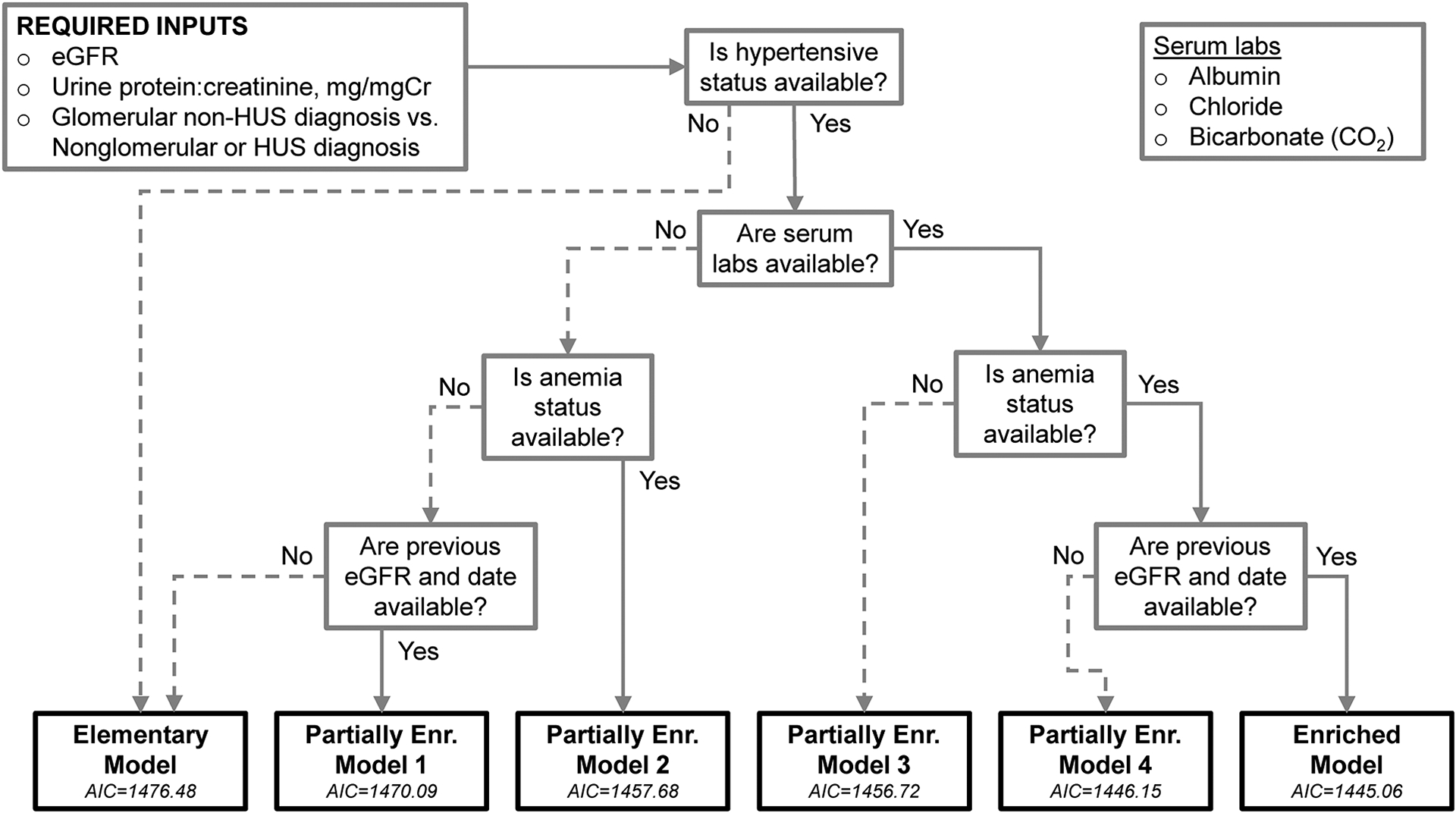
Decision tree describing adaptive approach for model based on availability of data for use in the online calculator. Akaike’s Information Criterion (AIC) statistics for each model are presented and correspond to improved penalized model fit (i.e., lower corresponds to lower error).
Cross-validation and external validation results
Figure 3 presents results from the cross-validation of the fully enriched model. Figure 3a presents the calibration plot for bins of predicted 5-year estimated risk which was very close to the observed risk (i.e., 1- KM(t)) and the c-statistic for the cross-validation sample was 0.868. The calibration slope was 1.019 (slope for perfect agreement= 1) and was very close to the average calibration slopes over the 10-fold calibration (1.017). The survival function of expected standardized residual times closely aligned with the standard exponential distribution (Figure 3b). Cross-validation of the elementary model was similar in terms of calibration, discrimination (c-statistic= 0.861) and standardized residual times.
Figure 3.

Model validation assessment based on 10-fold cross-validation of model building process for the Enriched Model including calibration plot depicting observed risk on predicted risk from at 5-year risk of kidney replacement therapy (3a) and survival function of standardized residual times for participants (3b). The calibration plot demonstrates close correspondence between observed and predicted 5-year risk and the survival function aligns closely with the expected standard exponential for strong model fit.
To externally validate the elementary model using European ESCAPE data (descriptive statistics in Supplementary Table S5 and cumulative incidence in Supplementary Figure S4), the GND test for 5-year risk did not demonstrate significant differences between the predicted risk and observed risk ( = 6.926; p= 0.140), the calibration slope was 0.996 (95%CI: 0.903, 1.088, p= 0.90), and the c-statistic was 0.854 (95%CI: 0.835, 0.873) indicating strong discrimination. The standardized residual times were congruent with the standard exponential distribution. Full results and further interpretation are in Supplementary Figure S5.
Enhancement of risk prediction by model enrichment
Figure 4 presents a visual comparison of the Elementary Model (based on diagnosis, GFR and UPCR) and Partially Enriched Model 1 (Elementary Model with current BP stage and GFR from the previous year) of predicted median times to KRT to demonstrate the enhanced risk prediction provided by blood pressure and longitudinal GFR as additional predictors. This hypothetical profile is a patient with non-glomerular or HUS diagnosis, current GFR= 30 ml/min|1.73m2 and UPCR= 2mg/mgCr corresponding to the elementary model variables: the median predicted time to KRT (y-axis) is 3.3 years (black horizontal line); this estimate does not incorporate GFR from 1 year ago (x-axis) and is thus invariant to it. The light and dark grey lines describe the predicted median time to KRT using Partially Enriched Model 1 across a range of GFR values from 1 year ago, if the patient had normal BP or elevated BP/hypertension , respectively. The elementary model prediction was in between the lines contrasting BP categories, which is reasonable when BP data is not available. The predicted time to KRT was longer for normal BP compared to elevated BP/hypertension reflecting the well-established relationship of accelerated CKD progression with higher BP. In addition, for Partially Enriched Model 1, the time to KRT was shorter when the annual GFR decline was faster. This reflects higher risk associated with accelerated progression over the course of 1 year.
Figure 4.

Comparison of Elementary Model with Partially Enriched Model 1 including blood pressure and longitudinal GFR. The example profile is a child with non-glomerular or HUS diagnosis, a current GFR of 30 ml/min|1.73m2 and a urine protein:creatinine ratio (UPCR) of 2 mg/mgCr. The y-axis represents the median predicted time to KRT and the x-axis represents GFR from one year ago with the dashed line indicating no change. The three lines represent different models: the elementary model (light grey) is constant across previous GFR, and the partially enriched model 1 (dark grey) is depicted by blood pressure status (normal or elevated).
Online tool for clinical use
Because individual predictions are not easily derived without specialized software, we developed an accompanying accessible online clinical tool to estimate times to KRT for individual patients. This tool requires input of U25 eGFR, UPCR, and diagnosis but additional optional variables can be entered (if available) for improved estimates. The best model (i.e., lowest AIC) based on available data computes the estimated time to KRT following the logic described in Figure 2. Estimated times are provided for the 10th, 25th and 50th percentiles in years rounded to the nearest month, with an upper limit of 10 years. The output provides the time by which the pth percentile of children with the same profile will experience KRT. For instance, if a patient has a U25 eGFR of 60, UPCR of 0.8 mg/mgCr, glomerular diagnosis, hypertension, anemia, albumin level of 4.5, chloride level of 105, CO2 level of 22, and experiences a 10% annual decline in eGFR, the output would state that 10% of children with this profile are expected to initiate KRT within 2.5 years, 25% within 5.1 years, and 50% within 10.1 years (>10 years). R code of all models for this hypothetical profile is provided (Supplementary Appendix 1). The online calculator is available at: https://ckid-gfrcalculator.shinyapps.io/CKiD_KRT_Risk/.
Discussion
In this paper, we propose a predictive tool to estimate time to KRT initiation for children with CKD. This model is based on the largest observational cohort of children with CKD in North America and predicts the clinically meaningful outcome of KRT initiation rather than a composite outcome including KRT and accelerated disease progression. The predictors evaluated are commonly measured clinical variables, and multiple models allow for an adaptive prediction of risk depending on availability of patient data. Cross-validation of the model-building process and the external validation in a European cohort of the elementary model demonstrated strong discrimination and calibration and gives confidence that this suite of six unified models (presented in a corresponding online tool) will help with risk stratification as well as for dialysis and transplant planning for children.
The model development capitalized on statistical learning methods: the RSF agnostically evaluated candidate predictors and best subset selection evaluated all possible combinations to specify the optimal models. Crucially, our models were also informed by clinical insight, previous literature, and the culmination of numerous CKiD-specific risk factor studies. It was somewhat surprising that BP was not identified as a key predictor in the RSF analysis, but because of its established importance in CKD progression11,20–22,30 it was included in the enriched models. BP is routinely assessed as part of clinic care so its inclusion is broadly applicable, although we encourage careful measurement for valid estimates of BP percentiles and classification of BP13.
Major strengths of the study included a population with a wide spectrum of kidney function at study entry and centrally measured biomarkers as predictors. Furthermore, CKiD collected data from 2005 and there are now enough observed KRT events to develop a robust predictive model for a clinical endpoint, which overcomes previous limitations of models based on a weaker surrogate endpoint of GFR decline. In addition, this analysis used the latest U25 GFR estimating equations12 and updated AAP BP guidelines13. The longitudinal data collection offered an opportunity to explore the predictive value of annual measurements (specifically, between the first and second visit). The rich longitudinal data was a major feature of this analysis, although only annual GFR change was identified in the RSF and included in two enriched models.
Using an adaptive approach based on six models overcame a limitation of previous risk calculators requiring complete data3. Even if data are limited, clinicians should be confident of a good prediction of KRT risk using only elementary predictors. Additional data will enhance risk prediction, as evidenced by improvement in training error rate for partially and fully enriched models. The custom-designed online calculator translates multiple underlying GG models into a simple user interface. This adaptive tool provides an interpretable output of estimated median times to KRT initiation: in the absence of other data, a reasonable estimate for individual patients is the 50th (average) percentile. To provide estimated variability, we report the 10th and 25th percentiles. For many profiles, there could be substantial variability in the percentiles and we note that the risk prediction is not deterministic.
While we dealt with independent variables from a purely predictive framework, rather than etiologic or causal perspective, many predictors are established etiologic risk factors for pediatric CKD progression, including diagnosis7, eGFR3,4, proteinuria6,31,32, blood pressure20–22,30,33, anemia34–36 and bicarbonate37. Chloride, which was included in two partially enriched models and the fully enriched model, represents a metabolic derangement that is likely linked to bicarbonate, but future work should investigate chloride as a risk factor for CKD progression.
We caution about interpreting the Table 2 coefficients directly. In general, negative values indicate shorter time to KRT (increased risk) and positive values denote longer time to KRT (protective). However, when looking at the GFR coefficient, the intertwined relationship between GFR and diagnosis (which also modifies the scale and shape parameters) obfuscates direct interpretation of any one individual coefficient. In addition to interaction, linear splines for GFR and proteinuria further complicate coefficient interpretation. We provide R code for readers wishing to explore these models further (Supplementary Appendix 1), but we emphasize that the goal was to develop the best prediction tool that offered the lowest test error quantified by AIC. While it may be useful to inspect the coefficients for face validity, the primary unit of evaluation was the prediction model itself.
There are important limitations to this analysis. We were not able to estimate diagnosis-specific risk beyond two broad diagnostic categories, nor could we incorporate response to specific therapies, for example treatments for glomerular diseases like FSGS, lupus nephritis or atypical HUS. Second, while CKiD offers broad representation of a pediatric CKD population, extreme patient characteristics (compared to Table 1) may yield invalid estimates. Third, we were only able to externally validate the elementary models but cross-validation methods demonstrated sound calibration, discrimination and model fitting for the enriched models. Other potentially important predictors, such as ambulatory blood pressure22,30 and uric acid38, did not have complete data for analysis; though these may also not be as readily available clinically.
In summary, using data from the CKiD study, we developed a predictive tool for time to KRT to aid clinical decision making and KRT planning in children and adolescents with CKD. We presented a series of equations to predict time to KRT based on routinely measured data in an adaptive way and designed a website for applied use by clinicians managing children with kidney diseases. While this predictive model was internally validated using cross-validation methods and with an external validation of the elementary model, future studies are necessary for additional external validation of the enriched models in other pediatric CKD cohorts.
Supplementary Material
Description of cross-validation and external validation methods.
Supplementary Table S1. List of variables evaluated in random survival forest analysis to identify potential predictive candidates in subsequent parametric survival models to predict time to kidney replacement therapy.
Supplementary Table S2. Distribution of specific chronic kidney disease diagnoses represented in the study population (n= 890).
Supplementary Table S3. Akaike Information Criterion (AIC) values as a measure of test error from different functional forms of GFR, proteinuria and interactions with diagnosis as modifiers of the location parameter (β) in generalized gamma parametric survival model.
Supplementary Table S4. Ranking of variables from random survival forest according to top 20 minimal depth of maximal subtree classification and top 20 variable importance.
Supplementary Table S5. Descriptive characteristics of the ESCAPE cohort used for external validation.
Supplementary Figure S1. Cohort flow diagram of those with no follow-up time, prevalent kidney replacement therapy event prior to time origin, and those with missed visits at the time origin.
Supplementary Figure S2. Kaplan-Meier estimates of cumulative incidence of kidney replacement therapy in the CKiD cohort.
Supplementary Figure S3. Results from best subset selection of top 9 variables identified from random survival forest analysis presenting Akaike information criterion (AIC) on best subset models with additional variables (29= 512, including the elementary model as the null model).
Supplementary Figure S4. Kaplan-Meier estimates of cumulative incidence of ESKD in the ESCAPE cohort.
Supplementary Figure S5. Results and interpretation from external validation of elementary model using data from the ESCAPE study including calibration plot depicting observed risk on predicted risk from at 5-year risk of kidney replacement therapy and survival function of standardized times of residuals for participants.
Supplementary Appendix 1. R Code of models presented in Table 2 and used in the online clinical calculator for a hypothetical patient profile.
Supplementary Appendix 2. Content from online clinical calculator describing model purpose, use, strengths and limitations designed for clinicians.
Supplementary Table S6. List of principal site investigators of the Chronic Kidney Disease in Children (CKiD) cohort study.
Acknowledgements
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U24-DK-082194, U24-DK-66116). F.W. received support from the NIH T32 institutional training grant (T32 HL007024). The CKID website is located at https://www.statepi.jhsph.edu/ckid and a list of CKiD collaborators can be found at https://statepi.jhsph.edu/ckid/site-investigators/ and is presented in Supplementary Table S6. The authors acknowledge Drs. Alvaro Muñoz and Christopher Cox for critical insight and feedback on cross-validation methods. The authors deeply appreciate Dr. Franz Schaefer for providing permission to use ESCAPE study data used in Furth et al.4 for the external validation analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None to disclose.
Data sharing statement
CKiD data is available upon request at the NIDDK Repository: https://repository.niddk.nih.gov/studies/ckid/
Analytic datasets and R programs for this analysis is available upon request to the corresponding author (dng@jhu.edu).
References
- 1.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22(12):1999–2009. doi: 10.1007/s00467-006-0410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng DK, Matheson MB, Warady BA, Mendley SR, Furth SL, Muñoz A. Incidence of Initial Renal Replacement Therapy Over the Course of Kidney Disease in Children. Am J Epidemiol. 2019;188(12):2156–2164. doi: 10.1093/aje/kwz220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015;65(6):878–888. doi: 10.1053/j.ajkd.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furth SL, Pierce C, Hui WF, et al. Estimating Time to ESRD in Children With CKD. Am J Kidney Dis. 2018;71(6):783–792. doi: 10.1053/j.ajkd.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123(10):754–762. [DOI] [PubMed] [Google Scholar]
- 6.Ardissino G, Testa S, Daccò V, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol. 2004;19(2):172–177. doi: 10.1007/s00467-003-1268-0 [DOI] [PubMed] [Google Scholar]
- 7.Pierce CB, Cox C, Saland JM, Furth SL, Muñoz A. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol. 2011;174(5):604–612. doi: 10.1093/aje/kwr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23(2):351–359. doi: 10.1681/ASN.2011070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon G, Pierce CB, Ng DK, CKiD Study Investigators. Revisiting the Application of an Adult Kidney Failure Risk Prediction Equation to Children With CKD. Am J Kidney Dis. Published online December 28, 2022:S0272–6386(22)01088–5. doi: 10.1053/j.ajkd.2022.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Gansevoort RT, Coresh J, et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104. doi: 10.1053/j.ajkd.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 11.ESCAPE Trial Group, Wühl E, Trivelli A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066 [DOI] [PubMed] [Google Scholar]
- 12.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 14.Lemley KV. Machine Learning Comes to Nephrology. J Am Soc Nephrol. 2019;30(10):1780–1781. doi: 10.1681/ASN.2019070664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning: With Applications in R. Springer Nature; 2021. [Google Scholar]
- 16.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox C, Chu H, Schneider MF, Muñoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. doi: 10.1002/sim.2836 [DOI] [PubMed] [Google Scholar]
- 18.Matheson M, Muñoz A, Cox C. Describing the Flexibility of the Generalized Gamma and Related Distributions. J Stat Distrib App. 2017;4(1):15. doi: 10.1186/s40488-017-0072-5 [DOI] [Google Scholar]
- 19.Ishwaran Hemant, Kogalur Udaya B., Blackstone Eugene H., Lauer Michael S.. Random survival forests. The Annals of Applied Statistics. 2008;2(3):841–860. doi: 10.1214/08-AOAS169 [DOI] [Google Scholar]
- 20.Reynolds BC, Roem JL, Ng DKS, et al. Association of Time-Varying Blood Pressure With Chronic Kidney Disease Progression in Children. JAMA Netw Open. 2020;3(2):e1921213–e1921213. doi: 10.1001/jamanetworkopen.2019.21213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JT, Carroll MK, Ng DK, Furth SL, Warady BA. Achieved clinic blood pressure level and chronic kidney disease progression in children: a report from the Chronic Kidney Disease in Children cohort. Pediatr Nephrol. Published online November 16, 2020. doi: 10.1007/s00467-020-04833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionne JM, Jiang S, Ng DK, et al. Mean Arterial Pressure and Chronic Kidney Disease Progression in the CKiD Cohort. Hypertension. 2021;78(1):65–73. doi: 10.1161/HYPERTENSIONAHA.120.16692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–2546. [PubMed] [Google Scholar]
- 24.D’Agostino RB, Nam BH. Evaluation of the Performance of Survival Analysis Models: Discrimination and Calibration Measures. In: Handbook of Statistics. Vol 23. Advances in Survival Analysis. Elsevier; 2003:1–25. doi: 10.1016/S0169-7161(03)23001-7 [DOI] [Google Scholar]
- 25.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med. 2015;34(10):1659–1680. doi: 10.1002/sim.6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453–473. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. [DOI] [PubMed] [Google Scholar]
- 28.van Stralen KJ, Tizard EJ, Jager KJ, et al. Determinants of eGFR at start of renal replacement therapy in paediatric patients. Nephrol Dial Transplant. 2010;25(10):3325–3332. doi: 10.1093/ndt/gfq215 [DOI] [PubMed] [Google Scholar]
- 29.Atkinson MA, Roem JL, Gajjar A, Warady BA, Furth SL, Muñoz A. Mode of Initial Renal Replacement Therapy and Transplant Outcomes in the Chronic Kidney Disease in Children (CKiD) Study. Pediatr Nephrol. 2020;35(6):1015–1021. doi: 10.1007/s00467-019-04416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuels J, Ng D, Flynn JT, et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60(1):43–50. doi: 10.1161/HYPERTENSIONAHA.111.189266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong CS, Pierce CB, Cole SR, et al. Association of Proteinuria with Race, Cause of Chronic Kidney Disease, and Glomerular Filtration Rate in the Chronic Kidney Disease in Children Study. Clin J Am Soc Nephrol. 2009;4(4):812–819. doi: 10.2215/CJN.01780408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuhrman DY, Schneider MF, Dell KM, et al. Albuminuria, Proteinuria, and Renal Disease Progression in Children with CKD. Clin J Am Soc Nephrol. 2017;12(6):912–920. doi: 10.2215/CJN.11971116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn JT, Mitsnefes M, Pierce C, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52(4):631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koshy SM, Geary DF. Anemia in children with chronic kidney disease. Pediatr Nephrol. 2008;23(2):209–219. doi: 10.1007/s00467-006-0381-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson MA, Kim JY, Roy CN, Warady BA, White CT, Furth SL. Hepcidin and Risk for Anemia in CKD: A Cross-sectional and Longitudinal Analysis in the CKiD Cohort. Pediatr Nephrol. 2015;30(4):635–643. doi: 10.1007/s00467-014-2991-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altemose KE, Kumar J, Portale AA, et al. Vitamin D Insufficiency, Hemoglobin and Anemia in Children with Chronic Kidney Disease. Pediatr Nephrol. 2018;33(11):2131–2136. doi: 10.1007/s00467-018-4020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown DD, Roem J, Ng DK, et al. Low Serum Bicarbonate and CKD Progression in Children. CJASN. 2020;15(6):755–765. doi: 10.2215/CJN.07060619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodenbach KE, Schneider MF, Furth SL, et al. Hyperuricemia and Progression of CKD in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort Study. Am J Kidney Dis. 2015;66(6):984–992. doi: 10.1053/j.ajkd.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of cross-validation and external validation methods.
Supplementary Table S1. List of variables evaluated in random survival forest analysis to identify potential predictive candidates in subsequent parametric survival models to predict time to kidney replacement therapy.
Supplementary Table S2. Distribution of specific chronic kidney disease diagnoses represented in the study population (n= 890).
Supplementary Table S3. Akaike Information Criterion (AIC) values as a measure of test error from different functional forms of GFR, proteinuria and interactions with diagnosis as modifiers of the location parameter (β) in generalized gamma parametric survival model.
Supplementary Table S4. Ranking of variables from random survival forest according to top 20 minimal depth of maximal subtree classification and top 20 variable importance.
Supplementary Table S5. Descriptive characteristics of the ESCAPE cohort used for external validation.
Supplementary Figure S1. Cohort flow diagram of those with no follow-up time, prevalent kidney replacement therapy event prior to time origin, and those with missed visits at the time origin.
Supplementary Figure S2. Kaplan-Meier estimates of cumulative incidence of kidney replacement therapy in the CKiD cohort.
Supplementary Figure S3. Results from best subset selection of top 9 variables identified from random survival forest analysis presenting Akaike information criterion (AIC) on best subset models with additional variables (29= 512, including the elementary model as the null model).
Supplementary Figure S4. Kaplan-Meier estimates of cumulative incidence of ESKD in the ESCAPE cohort.
Supplementary Figure S5. Results and interpretation from external validation of elementary model using data from the ESCAPE study including calibration plot depicting observed risk on predicted risk from at 5-year risk of kidney replacement therapy and survival function of standardized times of residuals for participants.
Supplementary Appendix 1. R Code of models presented in Table 2 and used in the online clinical calculator for a hypothetical patient profile.
Supplementary Appendix 2. Content from online clinical calculator describing model purpose, use, strengths and limitations designed for clinicians.
Supplementary Table S6. List of principal site investigators of the Chronic Kidney Disease in Children (CKiD) cohort study.
Data Availability Statement
CKiD data is available upon request at the NIDDK Repository: https://repository.niddk.nih.gov/studies/ckid/
Analytic datasets and R programs for this analysis is available upon request to the corresponding author (dng@jhu.edu).


