Abstract
Heat shock protein 90 (HSP90) is a chaperone with vital roles in regulating proteostasis, long recognized for its function in protein folding and maturation. A view is emerging that identifies HSP90 not as one protein that is structurally and functionally homogeneous but, rather, as a protein that is shaped by its environment. In this Review, we discuss evidence of multiple structural forms of HSP90 in health and disease, including homo-oligomers and hetero-oligomers, also termed epichaperomes, and examine the impact of stress, post-translational modifications and co-chaperones on their formation. We describe how these variations influence context-dependent functions of HSP90 as well as its interaction with other chaperones, co-chaperones and proteins, and how this structural complexity of HSP90 impacts and is impacted by its interaction with small molecule modulators. We close by discussing recent developments regarding the use of HSP90 inhibitors in cancer and how our new appreciation of the structural and functional heterogeneity of HSP90 invites a re-evaluation of how we discover and implement HSP90 therapeutics for disease treatment.
Introduction
Recent developments in understanding the complexity of factors that change protein structure, interaction and function1-8 have greatly reshaped our reductionist approach to proteins rooted in the one gene–one enzyme–one function concept framed in 1941 by Beadle and Tatum9. Heat shock protein 90 (HSP90), a molecular chaperone that assists the conformational folding or unfolding of large proteins or macromolecular protein complexes, is no exception. Viewed as a centrepiece in the cellular folding machinery composed of chaperones and co-chaperones (that is, proteins that assist chaperones in their function), eukaryotic HSP90 has been widely studied as a highly dynamic interactor with co-chaperones and ‘client’ proteins that executes ubiquitous folding or degradation decisions, to regulate binding of ligands to their receptors and to facilitate assembly of multiprotein complexes10-13. These transient protein–protein interactions (PPIs) are regulated by ATP binding and hydrolysis, shaped by unique co-chaperone interactions and occur in a cyclic conformational manner. The PPIs mediated by HSP90 within the context of the proteostasis network, that is, the cellular machineries that regulate the fate of proteins within the cell from synthesis to function to degradation, are critical for maintaining the cellular proteome.
In this Review we concentrate on advances in understanding HSP90 as a protein whose conformation, structure, protein interaction profile, function and cellular location are strongly influenced by and highly responsive to the cellular environment. We start with a brief introduction of HSP90 and then discuss numerous factors that can influence HSP90 conformation and assembly, and in turn function. We next describe homo-oligomeric and hetero-oligomeric HSP90 forms identified in the context of cells and tissues, both in health and disease. We discuss how these HSP90 forms are shaped by post-translational modifications (PTMs), co-chaperones and other factors, and present evidence for HSP90 oligomer formation being a gain-of-function state of HSP90, expanding HSP90 versatility from protein folding to protein holding and scaffolding. In this context we discuss epichaperomes, hetero-oligomeric HSP90 forms composed of tightly bound chaperones, co-chaperones and other factors. Epichaperomes are prevalent in disease states, and act as scaffolds that pathologically rewire PPIs (as opposed to folding chaperones). We conclude by discussing how these insights into the complexity of HSP90 in health and disease have a major impact on how we discover and develop HSP90 therapeutics.
HSP90 structure, function and conformational cycle
The structure, function and conformational cycle of HSP90 have been discussed in several excellent reviews12,14,15. Briefly, in mammalian cells, HSP90 is a family of four paralogues, that is, homologous genes present in the genome of the same species that arise by duplication events and code for proteins with similar but not identical functions: HSP90α, HSP90β, tumour necrosis factor receptor-associated protein 1 (TRAP1; also known as HSP75) and glucose regulated protein 94 (GRP94; also known as endoplasmin and gp96)13,16-18. In normal cells, HSP90 is primarily a cytoplasmic protein with a small nuclear pool, whereas TRAP1 is mainly found in mitochondria and GRP94 in the endoplasmic reticulum13,16-18.
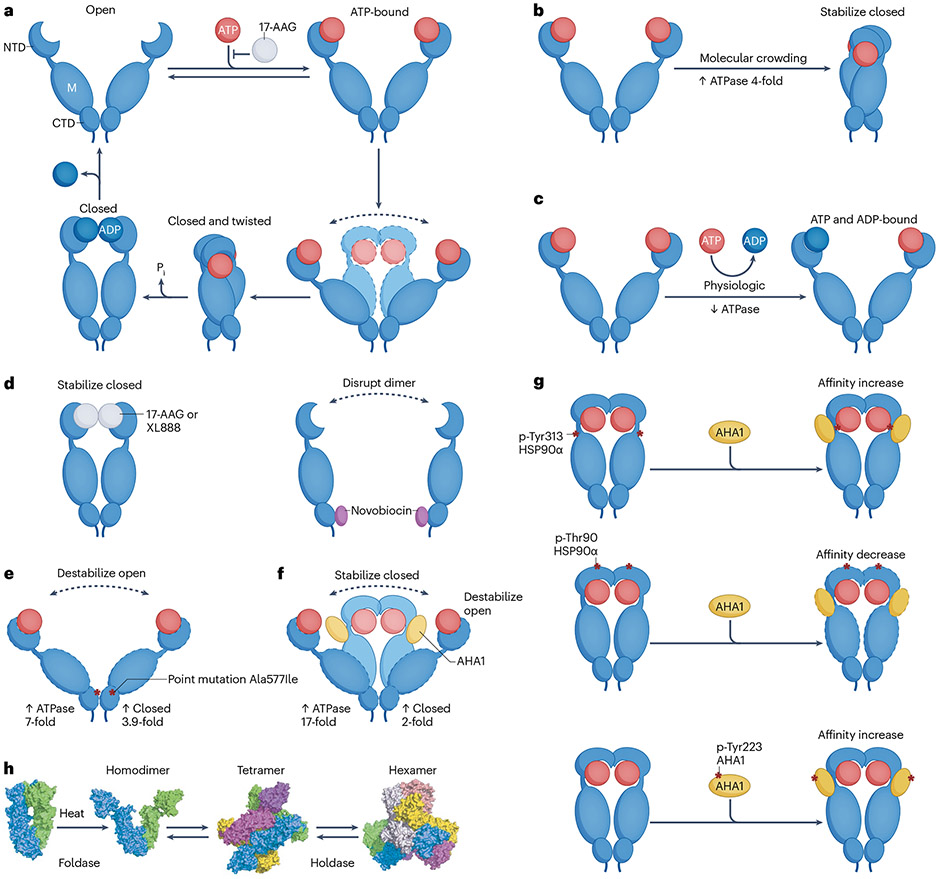
Functional recombinant HSP90 in solution consists of a dimer of two HSP90 protomers. HSP90 protomers comprise three domains (Fig. 1a): an amino-terminal domain (NTD), containing an ATP and drug binding site19-21, as well as co-chaperone-interacting motifs22; a middle domain (designated ‘M’ in Fig. 1a) that provides docking sites for client proteins and co-chaperones23-25 and which participates in forming the active ATPase with the NTD26,27; and a carboxy-terminal domain (CTD) that contains the protomer dimerization motif, a second drug binding region and interaction sites for additional co-chaperones22,28,29.
Fig. 1 ∣. Factors that can alter the balance of putative HSP90 conformations and HSP90 oligomerization state.
a, Heat shock protein 90 (HSP90) consists of three regions: an amino-terminal domain (NTD), the middle domain (‘M’) and the carboxy-terminal domain (CTD), connected by flexible linker regions. The NTD contains the ATP/ADP binding site, which is also bound by several inhibitors such as 17-AAG, which impact HSP90 function by competitively binding to the ATP pocket. HSP90 alternates constantly between open and closed conformations. These conformational changes of the HSP90 dimer accompany ATP binding (in the open conformation), hydrolysis of ATP (in the closed conformation) and subsequent release of ADP. b–g, Examples of factors that may impact in cellulo HSP90 activity by shifting the pool of available conformations. Molecular crowding increases HSP90 ATPase activity by favouring the closed conformation which occupies less space in a crowded environment (panel b). An ATP and ADP-bound HSP90 heterodimer, representing an ATPase-incompetent form, may represent the predominant dimer conformation state under physiological nucleotide conditions (panel c). Small molecule interactors may perturb the natural equilibrium of HSP90 conformers, selecting for or enriching specific HSP90 conformations; 17-AAG or XL888 binding to the ATP binding site stabilizes an extended conformation similar to ADP binding, whereas novobiocin binding of the CTD disrupts dimer formation (panel d). Numerous specific point mutations have been found to impact HSP90 conformation; Ala577Ile, a non-naturally occurring mutant chosen to exemplify the impact of site-specific alterations on the conformation and activity of HSP90, acts by destabilizing the open conformation and increasing the closed conformation, and thus increases HSP90 ATPase activity (panel e). Co-chaperones such as AHA1 bind to HSP90 to promote and stabilize its closed conformation, increasing HSP90 ATPase activity (panel f). Post-translational modifications (PTMs) of specific HSP90 residues (here shown for the HSP90α isoform) or of HSP90 co-chaperones (here shown for AHA1) can alter the interaction between HSP90 and AHA1, in turn impacting available HSP90 conformations, and thus ATPase activity (panel g). h, Heat or other factors may impact HSP90 conformation and induce HSP90 oligomerization. Structural map shows the proposed structure and function of HSP90 tetramers and hexamers that may form under heat shock. Individual HSP90 units are coloured green, cyan, magenta, yellow, orange and grey. Panel h adapted from ref. 49 under a Creative Commons licence CC BY 4.0. The sum of these in cellulo impacts on HSP90 ultimately determine its functional status. 17-AAG, 17-allylamino-17-demethoxy-geldanamycin; AHA1, activator of HSP90 ATPase homologue 1; XL888, a tropane-scaffold amino-terminal binder of HSP90 (see Supplementary Table 1).
HSP90 is best known as a foldase, that is, a protein that guides a set of ‘client’ proteins by ensuring that their folding and assembly occur correctly10-13. The foldase function of HSP90 is dependent on its ability to cycle through several conformations leading to acquisition of ATPase activity, mediated by nucleotide binding. ATP binding to the open state of each protomer (in the open state, the NTDs of each protomer do not interact) initially promotes NTD–NTD association (closed conformation) followed by NTD–middle domain interaction (closed and twisted conformation), generating an ATPase-competent HSP90 dimer (Fig. 1a). Upon ATP hydrolysis, ADP remains bound to the NTD of each protomer, leading to dissociation of the two NTDs and regeneration of the open conformation30,31.
There are hundreds of reported HSP90 regulated proteins, referred to as clients, which have been summarized in a HSP90 molecular chaperone complex database32. As discussed above, HSP90 binds to clients in different conformations and establishes extensive contacts with these proteins rather than through specific motifs23,33-36. HSP90 may aid client proteins by facilitating the formation of an active conformation of a protein such as for kinases37, by assembling them into multiprotein complexes as described for the kinetochore38 or by promoting the binding of ligands to proteins such as in the binding of a steroid hormone to its receptor39. Many HSP90 clients have important functions in diseases, such as cancer, and HSP90 inhibition has been pursued as a therapeutic avenue (reviewed elsewhere11,40,41). Small molecules designed to either bind HSP90 directly through pockets identified in the NTD, middle domain and CTD or, alternatively, interfere with HSP90 binding to co-chaperones have been reported by several groups, from both academia and industry. Excellent review articles describe these reagents42-45. Paralogue-specific binders46-48 and allosteric HSP90 inhibitors49 are also in preclinical development. A summary of HSP90-targeting molecules, including those discussed throughout this Review, is presented in Supplementary Table 1.
Studies of small molecule HSP90 modulators have so far viewed HSP90 mostly as a protein that is structurally and functionally homogeneous but shows altered expression in disease. Based on this paradigm, HSP90 has been targeted for its function as a foldase of client proteins that themselves have an important biological function in the specific disease context examined (reviewed elsewhere11,41,50). For example, HER2-positive breast cancers are targetable through HSP90 inhibition because HER2 is a client protein of HSP90 which becomes degraded upon HSP90 inhibition. However, not all HER2-expressing tumours are sensitive to HSP90 inhibitors and neither is HER2 degradation sufficient to make HSP90 inhibition therapeutically curative in most tumours, suggesting that HSP90 and the impact of HSP90 modulators are more complex than previously thought51,52.
The cellular environment shapes HSP90
Unlike studies using recombinant proteins to develop models of HSP90 conformational dynamics, the unique large-scale conformational landscape (that is, the population of possible interconverting conformers of a protein, such as NTD open or closed) that HSP90 can occupy in cells affects HSP90 and its activity in ways not readily predicted from solution-based studies. Here, we describe some of these factors that can alter the balance of putative HSP90 conformers, including molecular crowding (that is, an environment where the properties of molecules change due to high concentrations of macromolecules), cellular nucleotide concentration, small molecule modulators and PTMs of HSP90, and co-chaperones (also discussed in detail in Box 1).
Box 1. Post-translational modifications of HSP90 and co-chaperones.
Heat shock protein 90 (HSP90) contains numerous residues that can be post-translationally modified, and context-dependent post-translational modifications (PTMs) such as phosphorylation, acetylation, sumoylation, nitrosylation, methylation, O-linked N-acetylglucosaminylation (O-GlcNAcylation) and ubiquitination have been identified (see Fig. 1 for HSP90 domain organization and impact of PTMs on co-chaperone association). Indeed, a role for context-dependent HSP90 phosphorylation in modulating client protein interaction was identified nearly 30 years ago137. For an in-depth analysis of HSP90 family and co-chaperone PTMs, their functional consequences and the allosteric interactions between PTM sites, the reader is referred to several excellent reviews1,2,14,97,138-142. A curated and continuously updated list of HSP90 and co-chaperone PTM sites can be found on the PhosphoSitePlus website. Several HSP90 PTMs occur at ‘hot spots’ that are predicted by molecular dynamics simulations and confirmed by various biophysical and biochemical techniques to have long-range conformational effects on HSP90 (refs. 143-145). Some PTMs act by orchestrating allosteric movements in HSP90 (ref. 145), whereas others act as transmitters of conformational changes2,138. By impacting conformational dynamics, both HSP90 and co-chaperone PTMs can either promote or inhibit HSP90 client binding and folding activity146-148. Importantly, most HSP90 family and HSP90 co-chaperone PTMs have been identified indirectly by mutation of specific amino acids, but only few have been validated in cells using PTM-specific antibodies. Thus, analysis of the impact of PTMs in vitro may not fully reflect impact in cells. In cellulo studies using an antibody able to identify phosphorylation of HSP90-Tyr313 demonstrated more than a 7-fold increase in activator of HSP90 ATPase homologue 1 (AHA1) binding to this PTM-modified HSP90 (refs. 26, 63). These results were confirmed by observing a similar impact on AHA1 association with site-specific phosphomimetic HSP90 mutants, confirming the important contribution of specific HSP90 PTMs in regulating this protein–protein interaction (PPI). Furthermore, site-specific phosphorylation of AHA1 itself has been shown to impact its interaction with HSP90 (ref. 146). In cells it is very likely that multiple PTMs interact with or compete with each other, or independently overlap in their impact on HSP90 function.
HSP90 PTMs can alter one or more HSP90 conformations, and in turn impact binding to several, or only a specific subset, of co-chaperones (reviewed elsewhere13,14,149) and client proteins. Co-chaperones may bind distinct HSP90 conformations, remodel HSP90 structure differently and elicit diverse impacts on function. For example, HSP90 ATPase activity is inhibited in vitro while bound to the co-chaperone HSP70/HSP90 organizing protein (HOP)150 but is activated by AHA1 binding151. The co-chaperone p23 binds to and stabilizes the closed ATP-bound conformation152 and weakly inhibits HSP90 ATPase activity153. In vitro, HOP also blocks amino-terminal domain (NTD) dimerization, whereas AHA1 accelerates structural rearrangements in HSP90 that facilitate NTD dimerization14.
Acetylation of HSP90α on Lys294 in SKBr3 breast cancer cells was one of the earliest specific PTM sites identified149. Introduction of acetylation-mimetic variants into these cells demonstrated that acetylation at Lys294 reduced the interaction of HSP90 with several co-chaperones (p23, CDC37, FK506-binding protein 4 (FKBP4; also known as FKBP52), AHA1, HOP, carboxy terminus of Hsp70-interacting protein (CHIP) and HSP70) and with a diverse set of client proteins (for example, kinases, including HER2 and v-Src) and transcription factors (including androgen receptor (AR), mutant p53 and hypoxia-inducible factor 1α (HIF1α)). This PTM did not affect ATP binding to HSP90. Introduction of an unacetylated Lys mimetic (Lys294Arg) restored client and co-chaperone interaction levels to those seen for wild-type HSP90. In contrast, phosphorylation of Thr90 in HSP90α, a PTM that is significantly elevated in proliferating cells, decreased HSP90α affinity for ATP154. Phosphomimetic Thr90Glu mutation of HSP90α enhanced binding to the co-chaperones AHA1, p23, protein phosphatase 5 (PP5) and CHIP, whereas it diminished binding to HSP70, CDC37 and HOP. This mutation had little impact on FKBP4 interaction. The ability of the HSP90 Thr90Glu mutant to form complexes with client proteins such as proto-oncogene tyrosine-protein kinase SRC, RAC-α serine/threonine-protein kinase AKT or protein kinase Cγ (PKCγ) was impaired, indicating that phosphorylation of Thr90 affects HSP90 chaperoning of these clients. Similarly, phosphorylation of Thr115 in HSP90α reduced ATPase activity, stabilized interactions with p23 and abolished binding to AHA1, but had no effect on HOP binding, suggesting stabilization of the open HSP90 conformation141,155. Sumoylation of HSP90α at Lys191 or HSP90 phosphorylation at Tyr313 independently increases the binding affinity of AHA1 for HSP90 (refs. 26,63,156). HSP90 phosphorylation by the mitotic checkpoint kinase MPS1 is essential for mitotic arrest whereas CDC14-mediated HSP90 dephosphorylation is required for exit from mitosis141,155. MPS1 phosphorylation of HSP90 also sensitizes cancer cells to HSP90 inhibitors74,97, as does HSP90 sumoylation and inhibition of Wee1-mediated HSP90 phosphorylation156,157. Thus, various PTMs can have overlapping but independent effects on HSP90 function. Specific PTMs can also impact the intracellular location of an individual co-chaperone. For example, Thr198 phosphorylation of HOP modulates its distribution between the cytosol and the nucleus158.

Finally, specific HSP90 and co-chaperone assemblies may form to regulate specific needs of different proteins159. For example, HSP90–CDC37–PP5 complexes form a structural platform for kinase dephosphorylation25 and HSP90–FKBP5 assemblies form complexes with hTERT to enhance telomerase activity in cancer cells160. In addition, the HSP70–HOP–HSP90 ternary complex is required for proteasomal assembly rather than folding of its individual components, and is functionally required for proteasomal activity in cancer cells102. Given the complexity of PTM modulation of HSP90 conformational dynamics and co-chaperone interactions, a full elucidation of the PTM code remains an ongoing and difficult challenge161,162. (‘C’, ‘M’ and ‘N’ in the figure designate amino-terminal, middle and carboxy-terminal domain, respectively.)
Molecular crowding
The cellular protein concentration is surprisingly high and similar among bacteria, yeast and mammalian cells and tissues, ranging from 230 mg ml−1 in Escherichia coli to 280 mg ml−1 in yeast and from 160 mg ml−1 to 310 mg ml−1 in various mammalian tissues53-55. As such, proteins comprise 20–30% of the cellular volume, which limits the available intracellular physical space56. In such crowded environments, the space available for the movement of molecules is limited. This phenomenon is referred to as the excluded volume (that is, the volume occupied by macromolecules and unavailable to others). One study demonstrated that HSP90 conformation is significantly impacted by molecular crowding57. The data are consistent with a model in which excluded volume provides a driving force that favours the closed active state of HSP90 as opposed to the open state that is incapable of ATP hydrolysis. Thus, by favouring the closed state, crowding may increase the ATP-dependent steady-state HSP90 ATPase activity in cellulo (Fig. 1b). GRP94, the HSP90 paralogue in the endoplasmic reticulum, responds similarly to molecular crowding, but this is not the case for two other molecular chaperones, HSP60 and HSP70 (ref. 57). The structural basis for these differences is not yet understood.
Impact of nucleotide binding
An additional influence on in cellulo HSP90 conformational states is the regulatory impact of the physiologic ATP to ADP ratio (cells contain approximately five times more ATP than ADP58) and the relative affinity of HSP90 open and closed states for these nucleotides. To that end, it should be noted that the HSP90 open state displays a significantly stronger affinity for ADP compared with ATP. Indeed, binding of ADP at its physiologic concentration to the open state of human HSP90 inhibits progression to the ATPase-competent closed conformation59. An HSP90 dimer where two ADPs simultaneously bind to both HSP90 protomers or a dimer where ADP binds to one protomer and ATP to the other protomer may represent predominant dimer conformers under physiological in cellulo nucleotide conditions59 (Fig. 1c). Furthermore, nucleotide binding and its conformational impact on HSP90 may differentially affect client protein interactions. For example, ATP binding favours HSP90 complex formation with the glucocorticoid receptor but has no effect on the binding to p53 and tau in vitro60. Importantly, the ability of HSP90 PTMs to modify the impact of nucleotide affinity on HSP90 conformational dynamics must also be considered (see below and Box 1).
Impact of inhibitors on HSP90 conformation
Similar to the endogenous HSP90 ligands (ATP and ADP), naturally occurring or synthetic HSP90 inhibitors also alter HSP90 conformational states. In vitro, NTD inhibitors, such as 17-allylamino-17-demethoxy-geldanamycin (17-AAG), impact the conformational dynamics of recombinant HSP90 by competitively binding to the NTD ATP pocket of each protomer resulting in stabilization of the HSP90 open conformation (Fig. 1a). In contrast, in cellulo binding of two structurally distinct HSP90-NTD inhibitors, 17-AAG and a tropane-scaffold amino-terminal binder of HSP90 (XL888), induced and stabilized a compact and extended HSP90 conformation, similar to what has been proposed for ADP binding61 (Fig. 1d). The HSP90-CTD inhibitor novobiocin specifically disrupted HSP90β homodimers with little impact on HSP90α homodimers61. Intriguingly, in cells, HSP90-NTD inhibitors also promoted an increase in HSP90α/β heterodimers61. The distinct impact of inhibitors on HSP90 in vitro as compared with in cellulo supports the notion that in cells, intrinsic or extrinsic factors may perturb the natural equilibrium of populated conformers, selecting for or enriching specific HSP90 conformations.
Influence of post-translational modifications
A recent in vitro study examined two additional HSP90-specific mechanisms, besides crowding, that alter the proportion of open to closed HSP90 conformational states and compared the impact of these modifications on HSP90 ATPase activity62. A single point mutation, Ala577Ile, in the HSP90 CTD destabilized the open conformation and increased the closed conformation by 3.9-fold (Fig. 1e). Association with activator of HSP90 ATPase homologue 1 (AHA1), the activator of HSP90 ATPase, resulted in a 2-fold increase in the HSP90 closed state (Fig. 1f). Surprisingly, this study demonstrated that achieving a greater percentage of the HSP90 closed state does not correlate with increased HSP90 ATPase activity. Thus, crowding stimulated ATPase activity by 4-fold (Fig. 1b), the Ala577Ile point mutation increased HSP90 ATPase activity by 7-fold, whereas AHA1 addition increased HSP90 ATPase activity by 17-fold, even though it caused the smallest increase in ratio of closed versus open conformation. Furthermore, the modest fold increase in the unmodified HSP90 closed state induced by AHA1 in vitro is likely an underestimate of what can occur in cells. In cellulo phosphorylation of a specific tyrosine (Tyr313) in the HSP90 middle domain led to more than a 7-fold increase in AHA1 binding26,63 (Fig. 1g). These results were confirmed by observing a similar impact on AHA1 association with site-specific phosphomimetic HSP90 mutants, identifying the important contribution of specific HSP90 PTMs in regulating its PPIs. We describe further details concerning the impact of PTMs on HSP90 and co-chaperone interactions and on HSP90 activity in Box 1.
Oligomerization of HSP90
Although HSP90 is widely studied in the context of its dimeric structure, similar to some other chaperones64 it can form higher-order oligomers (Fig. 1h). One study purified HSP90 from porcine brains and investigated the functional impact of heat-induced HSP90 oligomerization65. They found that acquisition of heat-induced oligomeric states was antagonized by ATP or ATP-competitive HSP90 inhibitors65, suggesting that HSP90 conformation is an important determinant of oligomerization potential. As the occupation of the amino-terminal ATP binding site by either ATP or an inhibitor induces a more compact conformation of the chaperone as discussed above61, it is likely that a more open conformation is required for oligomers to form at high temperature. A later study purified HSP90 from porcine brains66 and confirmed that HSP90 is able to self-oligomerize upon heat shock or in the presence of divalent cations such as Mg2+ and Ca2+. The authors were able to identify tetramers, hexamers and dodecamers. Interestingly, they found that the stability of HSP90 oligomers varied66. HSP90 homo-oligomers that formed upon heat shock were retained and thus stable on native polyacrylamide gel electrophoresis (PAGE), consistent with prior results67, but this was not the case for HSP90 oligomers induced by divalent cations, which disassembled when run on native PAGE66,68. As cations increase the hydrophobic surface of HSP90 dimers69, leading to an auto-association process, these data suggest that several distinct mechanisms may favour oligomerization, with the resulting HSP90 oligomers possibly varying in organization and architecture.
Taken together, these studies demonstrate that numerous factors can influence the conformation of HSP90 in cells to either inhibit or activate its folding activity, affect specific client protein interactions or affect the sensitivity to HSP90 inhibitors either directly or indirectly. These factors can also affect the ability of HSP90 to acquire novel structures and, as described below, novel functions through oligomerization.
Native HSP90 multimers in stressor and non-stressor conditions
With HSP90 sensitive to the impact of many factors that alter its conformation and assembly, it is intuitive that modification of such factors across cell types and tissues, in both stressor conditions and non-stressor conditions, would have a dramatic impact on HSP90, both structurally and functionally. It is likely that an interplay between these factors contributes to the formation of context-specific pools of HSP90 conformations, each endowed with distinct interactors and, in turn, functions. We will highlight alterations in HSP90 structure identified in the context of cells below and analyse the effects of the cellular environment on HSP90 (that is, in normal cellular states, or following exposure to acute or chronic stressors, as in disease). In vivo, PTM modifications as well as changes in the expression and localization of HSP90 and co-chaperones may not only alter the structure of HSP90, thereby favouring the formation of multimeric (homo-oligomeric or hetero-oligomeric) HSP90 assemblies, but also reshape its interactors and function. Such changes can transform HSP90 from a folding protein into a holdase (that is, a protein that assists others by protecting them from aggregation and subsequent degradation without directly refolding them) or into a scaffold (that is, a protein that mediates protein interactions or alters how proteins interact) (Figs. 2 and 3). We will differentiate, where known, the effect of acute and chronic stressors on HSP90, and whether HSP90 modification by these stressors is an adaptive or maladaptive response. Adaptive response refers to successful coping with stressors and implies that the stressor response is activated quickly when needed but also efficiently terminated when the stressor condition is removed70. An example for an adaptive response is recovery of a cell from heat shock. In contrast, maladaptive responses represent a failure to cope with stressors and return to normalcy, leading to vulnerability to stressor-associated pathology70. An example is the establishment of a pathologic state, such as a cell becoming cancerous.
Fig. 2 ∣. HSP90 oligomers.
a, Forms of heat shock protein 90 (HSP90) in normal cells and in cells under a pathologic condition. HSP90 may homo-oligomerize or hetero-oligomerize in cellulo, in both non-stressor and stressor conditions, to form dynamic or long-lived higher-order structures. In healthy cells and tissues, HSP90 predominantly forms dynamic, short-lived homo-oligomers with low-affinity interactions between oligomer components. Native forms of HSP90 isolated from pig brain are shown below. Conversely, HSP90 in diseased cells and tissues exists as long-lived hetero-oligomers due to high-affinity interactions among oligomer components. Native forms of HSP90 isolated from cancer cells are shown below. b, In addition to being structurally distinct from dimeric HSP90, which is predominant in the absence of stressors and primarily involved in folding of client proteins, oligomeric forms may acquire new functions. These gain-of-function states of HSP90 may act as holdases that protect client proteins from denaturation under conditions of acute stress (that is, adaptive forms) or may acquire pathologic scaffolding functions under conditions of chronic stress (that is, maladaptive forms). c, Examples of specific disease-related stressors and their influence on the nature, structure and function of context-dependent HSP90 oligomers. Under conditions of genetic or environmental stress, such as stress associated with malignant transformation, HSP90 forms epichaperomes, long-lived hetero-oligomeric HSP90 structures composed of HSP90, heat shock cognate 70 (HSC70) and other co-chaperones. These hetero-oligomers act as scaffolding platforms to aberrantly rewire the interaction of thousands of proteins, enhancing the activity of protein pathways that facilitate a malignant phenotype and activate survival pathways51,80. In midbrain dopaminergic neurons exposed to genetic stressors (for example, PARKIN mutations), HSP90 recruits HSC70, HSP70/HSP90 organizing protein (HOP), HSP40 and several other co-chaperones to form epichaperomes, to rewire the interaction of numerous proteins involved in inflammatory signalling pathways75. Following exposure of dopaminergic neurons to toxic stressors (for example, rotenone), HSP90 recruits HSP60 into epichaperomes which act to aberrantly rewire the interaction of proteins involved in dopamine synthesis pathways75. In brains of patients with human sporadic Alzheimer disease (that is, where the phenotype is caused by a complex interplay of proteotoxic, genetic and environmental stressors), HSP90 was found to recruit HSC70 and other co-chaperones into epichaperomes, which negatively impacts the interaction of proteins important for synaptic plasticity76. AHA1, activator of HSP90 ATPase homologue 1.
Fig. 3 ∣. Unique post-translational modifications affect the interaction of HSP90 with co-chaperones and client proteins in disease.
In disease, the context-specific repertoire of co-chaperones and the post-translational modifications (PTMs) of both heat shock protein 90 (HSP90) and its co-chaperones may become altered, favouring the selection of distinct HSP90 functional states over others. By changing the abundance of specific HSP90 conformations, these factors may have a dramatic impact on cellular networks, as distinct conformers may engage in distinct interactions with co-chaperones and client proteins, and in turn remodel protein–protein interaction networks, through shifting the assembly of multiprotein complexes required to regulate biomolecular pathways. a, impact of specific HSP90 PTMs on the abundance of specific HSP90–co-chaperone complexes, and in turn on cellular phenotype, in the context of cancer cells. Phosphorylation of the HSP90 carboxy-terminal domain (CTD) at the indicated sites regulates binding to co-chaperones and enhances the tumour-promoting activity of HSP90. Phosphorylation at these sites was shown to facilitate HSP70/HSP90 organizing protein (HOP) binding, which in turn promoted cell proliferation and p53 stabilization. Non-phosphorylated HSP90, in contrast, favoured C-terminus of Hsp70-interacting protein (CHIP) binding, decreased proliferation of cells and p53 destabilization. b, Changes in the expression levels of co-chaperones and their impact on HSP90 and client protein binding, and in turn on cellular phenotype and brain function, in the context of neurodegenerative diseases. Transgenic (Tg) mouse models for frontotemporal dementia (top), Parkinson disease (middle) and Alzheimer disease (bottom) are shown. rTg4510 mice express a repressible form of human tau protein containing the Pro301Leu mutation that has been linked to familial frontotemporal dementia. in these mice, activator of HSP90 ATPase homologue 1 (AHA1) overexpression enhances tau fibril formation and neurotoxicity, a pathologic effect that is dependent on and mediated through its interaction with HSP90 (ref. 105). HOP overexpression facilitates α-synuclein toxicity in mouse models of Parkinson disease (M83/STi1 Tg mice)103, whereas peptidyl-prolyl cis–trans isomerase (FK506-binding protein 5 (FKBP5)) overexpression alters association of HSP90 with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which results in an accelerated rate of AMPA recycling107, a process detrimental to synaptic plasticity (that is, learning and memory). c, impact of HSP90 PTMs on its cellular location and interaction with client proteins, as found in MDA-MB-231 triple-negative breast cancer cells, a cell line used to model invasive disease. Acetylation of HSP90 at the indicated sites was associated with HSP90 translocation to the cell surface and with its extracellular secretion, and promoted the interaction of HSP90α with matrix metalloproteinase 2 (MMP2), enhancing the invasiveness of breast cancer cells. ‘C’, ‘M’ and ‘N’ designate amino-terminal, middle and carboxy-terminal domain, respectively. PFF, pre-formed fibril.
Homo-oligomerization and hetero-oligomerization of HSP90
HSP90 is thought to act as a dimer to execute its folding functions but several studies propose many other forms exist in cells and tissues, with the nature and prevalence of these forms dictated by the cellular environment, including by the type and nature of potential stressors (Fig. 2a). Early studies biochemically characterized the HSP90 protein isolated from mammalian tissues and organs as well as from cancer cells to find it is indistinguishable when analysed by PAGE under denaturing conditions (SDS-PAGE), but distinct under non-denaturing conditions (native PAGE)65,71. Native forms of HSP90 isolated from rat liver and other normal, unstressed tissues predominantly existed as homo-oligomeric structures that dissociated into smaller dimer and monomer species when subjected to electrophoresis, implying dynamic, low-affinity interactions between oligomer components under normal conditions67. Conversely, in cancer cells, homo-oligomeric HSP90 forms were a minor component of the total HSP90 fraction. Instead, in L5178Y murine lymphoma cell extracts, HSP90 was found to form hetero-oligomers, observed as a smear under non-denaturing conditions, rather than discrete bands as observed for homo-oligomeric forms65. Similar observations were made later in human cancer cells and primary tumour specimens51, where, along with dimers, a population of stable HSP90 hetero-oligomers were noted. These hetero-oligomeric forms of HSP90 were dubbed epichaperomes. Independent of tissue of origin, tumour subtype or genetic background, approximately 50–60% of tumours were found to contain variable HSP90 epichaperome levels, with ~10–15% showing high epichaperome levels51,72-74. Similar long-lived HSP90 hetero-oligomers were identified in Parkinson disease models (for example, induced pluripotent stem cell (iPSC)-derived dopaminergic neurons expressing mutant PARKIN, where the phenotype is caused by a genetic stressor) but not in wild-type iPSC-derived dopaminergic neurons75. Likewise, epichaperomes were present in post-mortem human sporadic Alzheimer disease brain (that is, where the phenotype is caused by a complex interplay of proteotoxic, genetic and environmental stressors) but not in brains from age-matched non-cognitively impaired subjects76. Further, epichaperomes were observed in iPSC-derived neurons from a patient with a familial form of Alzheimer disease (that is, where the phenotype is caused by a genetic stressor such as duplication in the amyloid precursor protein (APP) gene locus) but not in wild-type iPSC-derived neurons76. Additionally, PS19 transgenic (Tg) mouse brains (that is, a model of frontotemporal dementia, a neurodegenerative disease where the phenotype is caused by mutant tau overexpression, a proteotoxic stressor) but not the brains of wild-type littermates contained epichaperomes76, together supporting the preferential presence of epichaperomes in disease.
Thus, HSP90 homo-oligomers and epichaperome hetero-oligomers, which can be either short-lived or long-lived, form in cellulo (Fig. 2b). The prevalence of these forms is dictated by the environment, with different co-mixed HSP90 pools found in cells in non-stressor conditions or under acute or chronic stressor conditions. The formation of such diverse multimeric HSP90 forms may provide a template for the acquisition of new structures and stabilities, which in turn may increase the fitness and functionality of HSP90 (ref. 77). Below we review factors associated with HSP90 homo-oligomerization and hetero-oligomerization and provide evidence for the function of these multimeric HSP90 forms in disease.
Composition of HSP90 multimers
HSP90 dimer formation, a proposed prerequisite for HSP90 ATPase function, is dependent on the identity of the HSP90 isoform. HSP90α homodimers form more readily and are markedly more stable compared with HSP90β homodimers16. This is due to a difference in two amino acids in the CTD of HSP90β compared with HSP90α. Using recombinant proteins, HSP90α/β heterodimers could not be identified by mass spectrometry analysis and HSP90 heterodimers could not be isolated using Sepharose-immobilized HSP90β (ref. 16). Nonetheless, both HSP90α and HSP90β can form homo-oligomers71 or participate in hetero-oligomers in cellulo, as in epichaperomes51,76. In porcine brains, where HSP90α is six times more abundant than HSP90β, a study detected predominantly HSP90α homodimers and homo-oligomers78. Another study demonstrated the presence of HSP90α and HSP90β homodimers and of HSP90α–HSP90β heterodimers in HeLa cancer cells61. Higher-order homo-oligomers were not reported in this study. The composition of epichaperomes is also context-dependent (Fig. 2c). For example, in cancer cells HSP90α and HSP90β were shown to recruit heat shock cognate 70 (HSC70), HOP (also known as STI1), HSP40, HSP110, FKBP4, AHA1, CDC37 and other co-chaperones and factors to form epichaperomes51. As detailed below, HSP90 in these epichaperomes takes on scaffolding functions not found in normal cells, altering the assembly and connectivity of proteins important for maintaining a malignant phenotype and enhancing their activity, which provides a survival advantage to cancer cells and tumour-supporting cells in the tumour microenvironment51,72,79,80. In midbrain dopaminergic neurons exposed to toxic stressors (such as rotenone, a pesticide that impairs mitochondrial function and is associated with Parkinson disease), HSP90 recruited HSP60 into epichaperomes75. These epichaperomes act to aberrantly rewire the interaction of proteins involved in dopamine synthesis pathways75. In neurons exposed to genetic stressors (such as PARKIN mutation), HSP90 recruited HSC70, HOP, HSP40 and several other co-chaperones to epichaperomes. These disease-specific epichaperomes alter the interaction of numerous proteins involved in inflammatory signalling pathways75. HSP90, HSC70 and HOP are epichaperome constituents in Alzheimer disease where epichaperomes rewire the connectivity of, and thus negatively impact, proteins integral for synaptic plasticity, brain energetics and immune response76.
Factors facilitating HSP90 multimer formation
Homo-oligomerization and hetero-oligomerization are driven by factors that favour the specific HSP90 conformation that enables oligomer formation, rather than by the HSP90 concentration itself51,69,81. For example, studies in cancer51,72,81 and neurodegenerative diseases75,76 have found epichaperome formation to be independent of HSP90 expression levels. Although specific mechanisms for in cellulo HSP90 oligomerization are not fully understood, PTMs may play an important role in stabilizing specific HSP90 conformations that facilitate multimer formation81-83. These multimer-facilitating conformations may become stabilized by PTMs within constituent chaperones, interacting proteins or both. For example, a study reported that N-glycosylation on Asn62, but not on Asn217, promoted a conformational state in the HSP90 paralogue GRP94 that favoured epichaperome formation and allowed GRP94 to re-localize from the endoplasmic reticulum to the plasma membrane and form stable interactions with plasma membrane proteins in breast cancer cells81. As a result, the functions of these plasma membrane proteins were enhanced and downstream cellular protein networks were aberrantly remodelled81. Computational methods rationalized the impact of N-glycosylation on Asn62 (pathologic) compared with Asn217 (physiologic) on GRP94 to show that each PTM induces distinct states of GRP94, which interact with distinct pools of proteins83. For homo-oligomerization of HSP90, the CTD and the residue Trp320 in the HSP90 middle domain are important84, suggesting that specific PTMs have evolved to fine-tune and stabilize specific HSP90 conformations, with each individual PTM or combination of PTMs thus enabling the formation of a specific HSP90 multimeric state.
Multimerization affects affinity of HSP90 for interactors
The affinity of HSP90 for specific co-chaperones depends on its multi-merization state. For example, the co-chaperone p23 predominantly binds HSP90 dimers, but can also interact with HSP90 homo-oligomers in vitro, shifting the HSP90 dimer–oligomer equilibrium towards the dimer85. AHA1 prefers binding to homo-oligomeric HSP90 compared with the dimer, and binding of AHA1 does not interfere with HSP90 oligomerization in vitro86. The significance of these findings in cells remains to be fully elucidated, although studies have found that AHA1 binding to HSP90 is enhanced whereas p23 binding is decreased in the context of cancer epichaperomes51. As noted above, HSP90 in epichaperomes is tightly bound to other chaperones and co-chaperones, as evidenced by native PAGE, affinity purifications and other techniques51,72,75,76,81,87. Changes in the affinity of proteins are important drivers in disease and have been studied in the context of many disorders, including cancer, neurodegenerative diseases and others88-90. Whether for HSP90 these changes in affinity are modulated by selection of specific PTM-driven HSP90 (and co-chaperone) conformations or by changes in co-chaperone concentration, or both, is yet to be determined.
Function of HSP90 multimers
HSP90 homo-oligomers and hetero-oligomers are both gain-of-function HSP90 forms. For example, the oligomerized state of HSP90 interacted in vitro with atypical substrates that are not clients of the HSP90 dimers with foldase function to maintain some (for example, dihydrofolate reductase) in a folding-competent state, while preventing others (for example, firefly luciferase) from irreversible thermal denaturation, enabling substrate refolding upon removal of the heat stress65,91. Acute stress, such as a heat shock, appears to increase the proportion of HSP90 oligomers, presumably to increase the holdase function of HSP90 to protect proteins from aggregation or degradation. Whether the HSP90 oligomers observed in cells act as ‘amped-up’ foldases, as proposed92, or whether they acquire new functions as holdases remains to be validated (Fig. 2c).
In contrast to HSP90 homo-oligomers, HSP90 in epichaperomes acts as a specialized holdase (that is, a scaffolding platform). HSP90 in epichaperomes does not bind unfolded proteins but, rather, participates in the binding and assembly of already active proteins and protein complexes70,77. This gain-of-function scaffolding activity of HSP90 is not protective but, rather, maladaptive, as by aberrantly rewiring the assembly and interaction of proteins, it enables a pathologic phenotype. Systems-level investigations of proteins impacted by HSP90 epichaperomes indicate that epichaperome formation alters the interaction network of individual proteins crucial for maintaining context-dependent disease phenotypes5,70,75-77. For example, in neurodegenerative disorders, HSP90 epichaperome formation impacts proteins important for neuronal function, such as proteins involved in synaptic plasticity, cell-to-cell communication, protein translation, cell cycle re-entry, axon guidance, metabolic processes and inflammation, leading to neuronal dysfunction and cognitive decline5,70,76,93. In cancer, epichaperome formation impacts proteins crucial for maintaining context-dependent malignant phenotypes, such as proteins involved in signalling, metabolic or immunoregulatory pathways, among others51,77,87. For example, a recent study found that epichaperomes have emerged as a mechanism of adaptation of mitosis in the context of cancer cells80. Key processes including localization of nuclear mitotic apparatus protein 1 (NuMA) in mitotic cells, or forming and tethering of mitotic spindles onto centrosomes and to the cell cortex by NuMA (alone or as part of mitotic complexes with regulatory proteins), were dependent on epichaperome formation, and these functions could not effectively occur in the absence of epichaperome activity80. It is important to note that not all cancer cells contain epichaperomes and, as such, this is a context-dependent mechanism of adaptation. As normal cells lack epichaperomes, this mechanism is a cancer phenomenon, rather than a general mechanism of mitosis regulation.
Both loss and gain in PPIs are mediated through epichaperome formation82. For example, examining post-mortem human brains of patients with sporadic Alzheimer disease and age-matched counterparts with no cognitive impairment, one study identified 942 proteins that lost interactions with at least 1 partner whereas 1,191 proteins formed new interactions through HSP90 epichaperomes76. Functional reversal of phenotypes to pre-stressor states by HSP90 epichaperome disruptors supports epichaperomes as key orchestrators of context-dependent PPI network dysfunctions70,72,75-77,87,93.
In summary, HSP90 and its paralogues (Box 2) may homo-oligomerize or hetero-oligomerize under stressor conditions to acquire high binding affinity for proteins (both co-chaperones and clients in the case of epichaperomes), as opposed to the low-affinity interactions observed under normal conditions. HSP90 may undergo self-oligomerization, especially when alone, but in the presence of other proteins, such as are present in the cellular environment of cancer cells, HSP90 may instead form high-affinity oligomeric or hetero-oligomeric complexes. In addition to being structurally distinct from dimeric HSP90, which is primarily involved in folding of client proteins, the stressor-induced oligomeric forms may acquire new functions. Higher-order HSP90 oligomers may act as holdases that protect client proteins from denaturation at elevated temperature or may acquire pathologic scaffolding functions. As discussed here, hetero-oligomeric HSP90 structures (that is, epichaperomes) act as scaffolding platforms to aberrantly rewire the interaction of thousands of proteins in diseased cells. These gain-of-function states of HSP90 are in contrast to the more commonly observed dimeric state of HSP90, predominant in the absence of stressors, that participates in the folding of its clients.
Box 2. HSP90 paralogues also form multimers.
Homo-oligomerization and hetero-oligomerization of heat shock protein 90 (HSP90) paralogues have also been observed. Glucose regulated protein 94 (GRP94) expressed as a recombinant protein has been shown to homo-oligomerize at elevated temperature and, similar to oligomerized HSP90, can prevent heat-induced denaturation of the non-client proteins glutathione S-transferase and citrate synthase91. Similar to HSP90, GRP94 self-oligomerization at elevated temperatures is markedly inhibited by ATP and ADP as well as by the adenosine analogue 5′-N-ethylcarboxamidoadenosine (NECA), which binds to the amino-terminal domain (NTD) nucleotide binding pocket of GRP94 with 10-fold greater affinity than ATP. Whereas ATP-mediated inhibition of GRP94 requires divalent cations (divalent cations bind to the tertiary phosphate of ATP to stabilize its binding to GRP94), NECA is able to prevent GRP94 oligomerization in the absence of divalent cations due to its lack of phosphate groups163.
GRP94 multimers were also noted in vivo. Early studies found that GRP94 is present in several forms in cellulo, implying that GRP94 conformational heterogeneity is the cause of such complexity164. GRP94 multimers were observed when GRP94 purified from rat hepatocyte microsomes was run on native polyacrylamide gel electrophoresis (PAGE)165. In experimental conditions that mimic human plasma, these GRP94 multimers formed stable high molecular weight complexes with human IgG. These GRP94 multimers may be of biological significance, as similar complexes with pathologic function were identified in the plasma of patients with diabetes but not that of non-diabetic subjects165. One study used native gel techniques and chemical biology probes to test 64 cancer cell lines encompassing 11 distinct tumour types and identified GRP94 epichaperomes in a subset of those cell lines, especially in breast, pancreatic, lung and renal cancers81. In breast cancer, GRP94 epichaperome formation was associated with the receptor tyrosine kinase status (that is, HER2 or EGFR) of the cells81. GRP94 epichaperomes may form under the influence of various stressor conditions (for example, alcohol, pathogens and others), and thus may occur in several distinct disease states. For example, such epichaperomes may form in alcohol-induced liver damage, where GRP94 epichaperome disruptors alleviated inflammatory responses in primary macrophages166, and have been found in influenza A virus infection with secondary bacterial pneumonia, where GRP94 epichaperome disruptors enhanced pneumococcal clearance from lung tissues and ameliorated lung pathology in a mouse model167. The significance of epichaperome formation was also shown in inflammation, where GRP94 epichaperome disruptors reduced the pro-inflammatory profile of M2 macrophages in disease models168, and in viral infection, where GRP94 epichaperome disruptors showed antiviral activity against dengue virus serotypes and Zika virus strains in multiple human cell lines in viral replication models169.
In the case of tumour necrosis factor receptor-associated protein 1 (TRAP1; the predominant mitochondrial HSP90 paralogue), dimers and homo-oligomeric tetramers have been observed by native PAGE analysis from cell extracts, where the tetrameric form appears responsive to an imbalance in ATP levels170. Hetero-oligomeric TRAP1 species have yet to be identified. However, a recent study used cryogenic electron microscopy and native gel techniques to identify TRAP1 tetramers in numerous cancer-derived cell lines and in HEK293 cells170. Based on their data, the authors proposed that these oligomers are sensitive to the oxidative phosphorylation state of mitochondria as their abundance changed in response to both decreased (that is, by inhibition of ATP synthase with oligomycin or during hypoxia) and increased (that is, by inhibition of lactate dehydrogenase to force cells to rely on mitochondria to produce ATP) oxidative phosphorylation. Intriguingly, mitochondria have been found to have steady-state temperatures at or above those that induce heat shock in cells171. As TRAP1 is a mitochondrial protein it will be interesting to understand how TRAP1 tetramers respond to alterations in oxidative phosphorylation in this high-temperature environment.
Co-chaperones and PTMs in health and disease
Whereas core proteostasis components (that is, chaperones) including HSP90 and a selection of HSP90 co-chaperones (including, but not limited to, HOP, HSP40, HSP60, HSP10, HSP70 and small HSPs, a class of chaperones with low molecular masses of 12–43 kDa) (see ref. 94 for further details) are abundantly expressed across both non-stressed and stressed tissues, tissue-specific and disease-specific (that is, stressor-specific) functional chaperone networks form only under specific conditions94-96. Not surprisingly, given their general importance for organismal survival, only 3 of 32 core chaperones contain known heritable disease-causing mutations. However, comparison between the core chaperone subset of 32 proteins94 and a cancer epichaperome51 identified an overlap of 9 core chaperones, 6 of which are associated with signalling and cell cycle progression. This is consistent with the concept that dysregulation of core chaperones can support pathological states including cancer and neurodegeneration94. in addition to a context-specific repertoire of co-chaperones, both HSP90 and its co-chaperones may be modified in a cell-specific, tissue-specific and disease-specific manner through PTMs and altered cellular localization97-99. Below we highlight studies where the impact of PTMs and co-chaperones on HSP90 function and cellular localization is revealed in the context of a specific biological system, with the focus on disease states.
PTMs in disease
One study found that phosphorylation of specific CTD residues of HSP90 (Thr725 and Ser726 in HSP90α and Ser718 in of HSP90β) regulates binding to co-chaperones and enhances the tumour-promoting activity of HSP90 (ref. 100) (Fig. 3a). C-terminal phosphorylation of HSP90 was higher in proliferating A375 cancer cells compared with conditions of serum starvation and was enhanced in human breast tumour specimens when compared with paired normal tissue100, indicating that these HSP90 residues are sensitive to the cellular environment. Phosphorylation at these sites favoured HOP binding in vitro and in A375 cancer cells, whereas non-phosphorylated HSP90 favoured CHIP binding100. HSP90-CTD phosphorylation led to an increased proliferation rate in cells100. Enhanced binding of HOP by HSP90 in the cellular environment may force HSP90 to act as a holdase by preventing its transition to a conformation required for the release of the bound interactor protein101, which is crucial for its foldase function. This may endow HSP90 with new scaffolding functions, as was observed in cancer51,102 and neurodegenerative diseases76,103. For example, the aforementioned study100 showed that cells transfected with a HSP90α mutant in which both phosphorylation residues were mutated to aspartic acid contained higher levels of mutant p53 protein, an HSP90 client, compared with cells expressing an HSP90 variant with two alanine substitutions. it remains unclear whether p53 stabilization is due to direct binding of p53 by dimeric HSP90 or indirectly through HSP90 oligomer regulation of p53 transcription, or both.
Co-chaperone imbalance in disease
Imbalances in co-chaperone levels in diseased cells are hypothesized to result in a defect in the folding capacity of the cell (that is, increase in cancer and decrease in neurodegeneration)104, but it is important to investigate how these imbalances also relate to changes in the conformation, assembly and function of HSP90 across cells and tissues. indeed, several studies support upregulation in the levels of several HSP90 co-chaperones, including AHA1, the peptidyl-prolyl cis–trans isomerase FKBP5 and HOP, as pathologic in the context of neurodegenerative diseases (Fig. 3b). For example, in rTg4510 mice, which express a repressible form of human tau containing the Pro301Leu mutation that has been linked with familial frontotemporal dementia, overexpression of AHA1, an activator of HSP90 ATPase activity and presumably of the folding capacity of HSP90, contributes to tau fibril formation and neurotoxicity, in a process requiring HSP90 (ref. 105). Similarly, HOP overexpression facilitates α-synuclein toxicity in mouse models of Parkinson disease103 and amyloid toxicity in a mouse model of Alzheimer disease106. FKBP5 overexpression alters association of HSP90 with αamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which results in an accelerated rate of AMPA recycling107, a process detrimental to synaptic plasticity (that is, learning and memory). in cancer models, HOP knockout rendered cells more resistant, rather than more sensitive, to killing by heat shock or azetidine-2-carboxylic acid, both stressors that induce protein misfolding and aggregation102. These results are counterintuitive in the context of a folding HSP90 function but explained by participation of these co-chaperones in epichaperomes. Supportive of this assumption, recent structural data of human full-length HSP90 in complex with FKBP5 found HSP90 to adopt an extended conformation of decreased ATPase activity but not of impaired nucleotide binding. As part of this complex and when binding to the Alzheimer disease protein tau, HSP90 serves as a scaffold to trap FKBP5 and nucleate multiple conformations of tau108. in vitro studies support a holding function of HSP90 when bound to tau, α-synuclein and TDP-43 (ref. 109). Finally, HSP90-containing epichaperomes were found to both promote tau toxicity76,93 and alter AMPA receptor clustering and synaptic plasticity5,70,76.
HSP90 intracellular and extracellular location
Numerous studies have reported a change in the cellular localization of HSP90 and its paralogues under stressor conditions (reviewed elsewhere99,110-112). For example, HSP90α is secreted and found on the surface of cancer cells, and stress conditions such as serum starvation, hypoxia, high concentration of glucose or oxidative stress promote the export and extracellular localization of HSP90. Normal cells secrete HSP90α following extracellular environmental stress, whereas many tumour cells constitutively secrete it (see several reviews on extracellular HSP90 in refs. 110,113-115). A majority of the tumour-secreted HSP90α is exosome-bound and presented on the cell’s surface116. One study found lysine acetylation of HSP90 at Lys69, Lys100 and Lys558 to be associated with HSP90 translocation to the surface of breast cancer cells and with its extracellular secretion117 (Fig. 3c). Acetylation also promoted the interaction of HSP90α with matrix metalloproteinase 2 (MMP2), an extracellular HSP90 binder, and enhanced invasiveness of breast cancer cells117. Treatment with an antibody targeting acetylated HSP90α-Lys69 markedly inhibited the invasiveness of MDA-MB-231 breast cancer cells. In contrast, phosphorylation of the HSP90β residue Ser255 and, to a lesser extent, Ser226 inhibited HSP90 secretion from chronic myeloid leukaemia K562 cells, and the non-phosphorylatable Ala226–Ala255 mutant was secreted more efficiently than the wild-type protein118. This exemplifies that changes in the conformation of HSP90, mediated by phosphorylation within the structurally disordered charged linker connecting the HSP90 NTD and middle domain, alter HSP90 secretion. The function of extracellular HSP90 remains incompletely understood but both ATPase-dependent (that is, foldase) and ATPase-independent (that is, holdase and scaffolding) functions have been proposed (reviewed elsewhere110,113,115). For example, an HSP90 construct retaining only the charged linker and part of the middle domain (residues 236–350, thus lacking the N-terminal, ATP binding region) was able to promote cell migration119, suggesting an ATPase-independent function of this HSP90 construct. The ATPase-defective mutant HSP90α-Asp93Asn was as capable of promoting tumour formation and metastasis as wild-type HSP90α in mice, further supporting the importance of the ATPase-independent function of HSP90 in cancer119. Interestingly, these functions are modulated by co-chaperones in the extracellular milieu, the presence of which is context-dependent. For example, one study found that co-chaperones, such as AHA1, protein phosphatase 5 (PP5), metalloproteinase inhibitor 2 (TIMP2) and HOP, were secreted from cells under stressor conditions, such as in cells under heat shock and in fibrosarcoma cells, whereas p23 and CDC37 were not120. In the extracellular milieu, the co-chaperone TIMP2 inhibited the ATPase activity of HSP90 and acted to scaffold MMP2 onto HSP90 (ref. 120).
In summary, cells utilize the ‘shape-shifting’ ability of HSP90 rather than regulation of HSP90 expression as a mechanism of stress adaptation, especially maladaptation. In this context, HSP90 conformational regulation, through the factors we discussed above, has evolved as an adaptive or maladaptive mechanism to favour specific protein ensembles by stabilizing or destabilizing conformations with dedicated functions. This paradigm agrees with systems-level studies that conclude alterations in the strength of interactions and in cellular mislocalization of proteins, which in turn can be influenced by alterations in PTMs, stabilization of disease-enriched protein conformations and other protein-modifying mechanisms, are used by cells to remodel their proteomes in response to stressors3,7,82. These protein modifications support a quick repurposing of proteins with new features while using relatively few cellular resources.
Implications for HSP90-based therapy
Given that disease-promoting HSP90 forms are distinct both structurally and functionally from HSP90 in normal cells, it is relevant to re-evaluate HSP90 as a target for therapy, including cancer therapy. Whereas the antitumour activity of HSP90-targeting agents has long been recognized (see reviews11,41,50), their clinical development was hampered by lack of understanding about how to differentiate HSP90 in normal cells from HSP90 expressed by tumours and tumour-supporting cells. As HSP90 in epichaperomes is a conformational mutant, this disease-related variant can be targeted specifically to inhibit pathologic HSP90 forms while sparing HSP90 functions in normal cells. Our growing appreciation of the diversity of HSP90 forms will guide HSP90 inhibitor design (Box 3), biomarker discovery and therapy implementation (Box 4).
Box 3. HSP90 conformer heterogeneity impacts HSP90 inhibitor binding.
Several reports suggest that cellular conditions, which influence the formation of distinct heat shock protein 90 (HSP90) conformers, enhance the sensitivity of cells to HSP90 inhibitors (reviewed elsewhere97). For example, introduction of v-Src or a MET kinase mutant (that is, oncogene stressors) into a mouse embryonal fibroblast cell line resulted in increased HSP90 sumoylation and a greater sensitivity to ganetespib, an HSP90-amino-terminal domain (NTD) inhibitor156. Phosphorylation of HSP90α on Thr115, which is elevated in certain cancers, conferred sensitivity to SNX-2112 and ganetespib, both HSP90-NTD inhibitors141. Interestingly, acetylation of HSP90, which diminishes ATP binding and HSP90 chaperone function, increased sensitivity to certain HSP90 inhibitors172,173. Glycosylation of glucose regulated protein 94 (GRP94) on Asn62 enhanced GRP94 incorporation into epichaperomes and sensitized cancer cells to PU-WS13, a GRP94-NTD binder81. In contrast, changes in Asn217 glycosylation, which is modified in normal cells and, presumably, necessary for the foldase functions of GRP94, had no effect on cellular sensitivity to PU-WS13.
In this context, it is important to consider that not all HSP90 inhibitors act equally well or equally selectively on specific disease-promoting HSP90 conformations or disease-associated HSP90 assemblies in comparison with HSP90 conformers found in normal cells. The first feature determines drug efficacy, whereas the latter influences the therapeutic index (that is, the safety profile during administration). For example, a study compared the efficacy of PU-H71 and the structurally similar compound Debio-0932 (also called CUDC-305), and demonstrated that only PU-H71 suppressed HSP90 epichaperomes (and thus cancer cell growth) effectively, whereas partial epichaperome suppression by Debio-0932 resulted in cancer cell re-growth87. Both PU-H71 and Debio-0932 bind to the same NTD pocket of HSP90 and have comparable binding affinity. Thus, it is important to appreciate that HSP90 inhibitor binding does not necessarily equate with engagement of pathologic HSP90 conformers in cellulo and in vivo131.
Another important aspect to consider is that each small molecule may impact HSP90 conformation differently upon binding, which in turn may translate into distinct biological activity in cells. Although 17-allylamino-17-demethoxy-geldanamycin (17-AAG) (a derivative of geldanamycin) and a tropane-scaffold amino-terminal binder of HSP90 (XL888) in cells induced similar conformational changes in HSP90, as noted earlier, not all HSP90 conformations were identically impacted by the two NTD inhibitors61. PU-AD and PU-H71, two structurally related NTD interactors, trap HSP90 in a co-chaperone-bound and client protein-bound conformation, whereas few client proteins are isolated in the geldanamycin-bound HSP90 conformation76,131,174. The HSP90-carboxy-terminal domain (CTD) inhibitor novobiocin behaves quite differently from the NTD inhibitors 17-AAG and XL888 in cells, in that it seems to be relatively specific for disrupting HSP90β dimerization. Allosteric CTD binders, such as derrubone, chlorobiocin and coumermycin A1, increase oligomerization of dimeric HSP90 into its hexameric state in vitro49. The impact of these distinct HSP90 inhibitors on epichaperomes remains to be investigated. At present it is known that PU-AD and PU-H71 stabilize HSP90 epichaperomes upon binding, and this ′trapped′ drug–epichaperome intermediate promotes disassembly and functional inhibition of epichaperomes76,131.
Given the complexity of HSP90 small molecule interactions and mechanisms of action, with these compounds acting either by inhibiting the ATPase activity of HSP90 or by disrupting oligomers, or both, referring to such agents with the general term of HSP90 inhibitors is far too simplistic. For this reason, agents such as PU-H71 are currently referred to as epichaperome disrupters, rather than HSP90 inhibitors82.
Box 4. Current status of HSP90 therapies.
More than 20 heat shock protein 90 (HSP90) inhibitors have been investigated in clinical studies targeting cancer, but their development as therapeutics has been impeded by several factors including poor understanding of unique HSP90 forms in disease, limited appreciation of how HSP90 conformers in health and disease can be therapeutically differentiated, and no clear biomarker and diagnostic assay for patient stratification77,175. In this context, the functional switch of HSP90 from a folder of individual proteins to a scaffolder of aberrant protein–protein interaction (PPI) network rewiring in disease has important therapeutic ramifications. Agents that act specifically on epichaperomes, either directly though HSP90 (refs. 51,72) or indirectly through heat shock cognate 70 (HSC70) (ref. 80), have been reported. PU-H71 (zelavespib) used in cancer applications79,176,177 and PU-AD (icapamespib) used for diseases of the central nervous system such as glioblastoma and neurodegenerative diseases178 are epichaperome-targeting small molecules72,76,131. PU-H71 and PU-AD become kinetically trapped within epichaperome-bound HSP90, whereas these inhibitors rapidly dissociate from dimeric HSP90 in normal tissues, thus discriminating between pathologic and physiologic HSP90 conformers72,76,131. These data suggest that treatment schedules should be based on target engagement with the pathologic pool of HSP90 specifically, rather than using the maximal tolerated dose, which will also inhibit HSP90 pools in normal cells79,131,176.
Vulnerability of tumours to PU-H71 in cellulo, in vivo and in patients with cancer was found to be directly proportional to HSP90 epichaperome levels, with most PU-H71-sensitive tumours exhibiting greater epichaperome levels than those not responsive to PU-H71 (refs. 51,72-74,176). This approach thus lends itself to precision medicine using intrinsic epichaperome levels as a biomarker for patient selection82. Diagnostic assays for epichaperome quantification and precise measurements of target engagement are readily available for use in the clinic82. For example, in a human clinical trial conducted in patients with metastatic breast cancer, predominantly triple-negative breast cancer176, a significant positive correlation was found between baseline epichaperome levels (determined by an epichaperome PET imaging diagnostic assay)131 and time to progression on epichaperome therapy. Tumour regression was observed in epichaperome-high tumours upon treatment79,176. In a patient with refractory acute myeloid leukaemia transformed from myeloproliferative neoplasms, which usually is associated with a poor prognosis, complete remission retained beyond 4 years was reported79. In this patient, high baseline epichaperome levels were detected in both abnormal immature white blood cells (called blasts) and granulocytes but not in normal (that is, healthy donor-derived) lymphocytes79. An immediate decrease in the number of blasts present in peripheral blood and a decrease in the total white blood cell count to normal levels was observed, paralleled by decreased HSP90 epichaperome levels and inhibition of oncogenic signalling pathways, in both blasts and in leukaemia progenitor and stem cell populations, resulting in normalization of peripheral blood counts and resolution of splenomegaly and constitutional symptoms79. PU-H71 was administered in these clinical studies at a dose determined to maximally engage the target in epichaperome-positive tumours131. It remains to be determined whether these observations are unique to these purine-derived amino-terminal domain (NTD) inhibitors, or whether other structurally distinct HSP90-NTD inhibitors may have similar activity.
The novel NTD-engaging HSP90 inhibitor pimitespib was investigated in a recently completed phase III clinical trial in Japanese patients with gastrointestinal stromal tumours who were refractory to standard treatment179, and this inhibitor has now been approved for use by the Japanese Food and Drug Administration (FDA) in patients with gastrointestinal stromal tumours whose disease has progressed after a series of tyrosine kinase inhibitors. To our knowledge, pimitespib has not been evaluated in the context of epichaperomes, although similar to other ATP-competitive HSP90-NTD inhibitors, its binding to HSP90 is retained for extended periods in tumour compared with normal tissues — a characteristic of NTD inhibitor binding to HSP90 in epichaperomes.
Inhibition of HSP90 and its impact on therapy
In cancer, the impact of therapeutics on immune cell populations and vice versa is now widely recognized as key to the success of such therapies. Initial preclinical studies identified a potential negative effect of HSP90 inhibitors on immune cell populations121. For example, one study showed that the HSP90-NTD inhibitor geldanamycin inhibited the presentation of MHC molecules that enable a robust T cell-based response in OVA models when applied in high concentrations122. This negative effect was likely a consequence of high levels of geldanamycin targeting all forms of HSP90. HSP90 knockdown, which also eliminates all HSP90 forms, mimicked these effects123. These and other studies, using either high HSP90 inhibitor concentrations or genetic HSP90 ablation, led to the conclusion that HSP90 folding activity is important for the presentation of both MHC class I and class II antigens (reviewed elsewhere121).
More recently, studies in cellulo and in vivo proposed that HSP90 inhibitors, when used at biologically active rather than excessive concentrations, can positively affect the tumour immunopeptidome and stimulate the antitumour immune response124-126. One study proposed that use of low-dose daily administration of HSP90 inhibitor is sufficient to impact pathologic HSP90 functions125. Using this treatment paradigm the authors showed that HSP90 therapy with the NTD inhibitor NVP-HSP9090 stimulated, rather than inhibited, antigen presentation on tumour cells, and in vivo the efficacy of this treatment paradigm was dependent on MHC class I presentation125. This approach was also efficacious in potentiating the antitumour efficacy of non-specific immune adjuvants125. Importantly, the goal of low-dose daily administration of the HSP90 inhibitor was to avoid upregulation of the molecular chaperone HSP70. HSP70 induction is a component of the heat shock response that is stimulated by high-dose HSP90 inhibitor and is protective for both normal and cancer cells127. Unfortunately, given the difficulty in obtaining tumour biopsies for pharmacodynamic assessment of on-target activity, most clinical trials to date have relied on assessing HSP70 upregulation in readily available peripheral blood leukocytes128-130.
Other researchers proposed a model of intermittent HSP90 inhibitor dosing to impact disease HSP90 forms with limited physiologic HSP90 inhibition131. Using this approach, HSP90 inhibitors have been shown to increase antitumour immunity by activating type I interferon response genes132, by enhancing antigen expression on the surface of tumour cells126, by reducing production of metabolites (for example, inosine) that create an immunosuppressing microenvironment133 and by reducing expression of multiple immune checkpoint proteins, including PDL1 and PDL2 (ref. 134). For example, ganetespib (100 mg kg−1 intravenous dose once every 3 days) showed combinatorial efficacy with anti-PDL1 antibody in the MC-38 syngeneic mouse model134. Both reduction of PDL1 surface expression on tumour cells and an increase in the number of activated CD8+ T cells within the tumour microenvironment were observed. Ganetespib given once a week in combination with an anti-CTL4 antibody promoted CD8+ T cell killing of tumours132. PU-H71, which is an epichaperome disrupter (see Box 3), was tested in cellular and murine models using intermittent doses resulting in augmented antigen-specific T cell responses during priming, including CAR-T cell cytotoxicity, with little effect on ongoing immune responses, including graft versus host disease135. PU-H71 also improved the in vivo efficiency of CAR-T cells in a B-ALL mouse model135. In a patient with an unclassified myeloproliferative neoplasm who progressed to acute myeloid leukaemia despite allogeneic stem cell transplantation, PU-H71 rebalanced immune cell populations and appeared to decrease the immunosuppressive microenvironment, thus improving the efficacy of cellular immunotherapy by allowing donor-derived T lymphocytes to exert immune surveillance79. No negative effects were observed for PU-H71 on the immune system in this study during 4 years of treatment79.
In summary, these new studies strongly suggest a re-evaluation of how we develop HSP90 therapies in disease (Box 4). A judicious selection of the HSP90 inhibitor and the implementation of a dose and schedule that maximizes target engagement restricted to pathologic HSP90 conformer pools while minimizing engagement of normal HSP90 may be used to better harness both the immunostimulatory and anticancer effects of HSP90 and expand the translation of these inhibitors beyond cancer treatment.
Conclusions and perspectives
Stressors associated with disease (that is, genotoxic, proteotoxic and environmental), as well as the cellular environment itself, remodel HSP90 conformation and function, suggesting these multiple conformation and activation states of HSP90 may have evolved as a mechanism of stressor regulation. In this context, rewiring of the physiological complement of chaperone complexes into pathologic oligomeric structures, characterized by both altered composition and strength of interaction, may be a driving mechanism behind their disease-associated activity. By altering HSP90 structurally, stressors redirect HSP90 to function as a holdase or scaffolding protein, creating structurally and functionally heterogeneous HSP90 pools that may coexist in individual cells. This complexity, although it might appear daunting for therapeutics discovery, in fact creates new avenues for the discovery of HSP90 therapeutics, whereby targeting of HSP90 in disease is possible while sparing normal cell HSP90 forms and functions.
Treating cancer with HSP90 drugs might inhibit tumour growth and at the same time make the tumour more susceptible to the patient’s immune system or to cancer immunotherapies. However, to harness this potential for HSP90 inhibitors requires a personalized medicine approach where the complexities of HSP90, as well as the impact of individual HSP90 binders on both the tumour and the immune system, are reflected in the drug discovery and development stages. In this context it is important to understand that inhibition of all HSP90 pools, including the normal folding functions of HSP90, is unnecessary and likely detrimental for optimal biological activity in cancer and other diseases.
As with any discovery, much remains unknown for these stressor-induced HSP90 forms. A conformational switch drives chaperone incorporation into homo-oligomers and hetero-oligomers, but the mechanisms underlying context-dependent pathologic structural changes remain largely unknown. The organization and architecture of HSP90, co-chaperones and interactors in these multimers remain to be elucidated. The nature and identity of epichaperome component chaperones and co-chaperones, and their organization, may dictate function by conferring an epichaperome structure with specificity for individual protein interactors. Expanding mechanistic insights into context-dependent HSP90 multimer composition, structure and function will be necessary to move this field forward. Future investigations into the abundance, composition and function of HSP90 epichaperomes in diseases other than cancer and neurodegeneration, such as in chronic disorders (for example, addiction, pain and inflammation), is warranted. It will also be key to understand the complexity of intracellular and extracellular HSP90 forms on a systems level. As cells do not function in isolation, the crosstalk between diseased cells, tissues and organs and the immune system will be important to elucidate mechanistically136.
Supplementary Material
Acknowledgements
This work was supported by the National Cancer Institute (NCI) Intramural Program and the National Institutes of Health (NIH) Extramural Program (R01 CA172546, P01 CA186866, R56 AG061869, R01 AG067598, P01 AG014449, P01 AG017617, R01 AG074004, R56 AG072599, RF1 AG071805 and P30 CA08748), The Breast Cancer Fund, Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (MSKCC).
Glossary
- α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors
Receptors integral to plasticity and synaptic transmission at many postsynaptic membranes. Their modulation is the ultimate mechanism that underlies much of the plasticity of excitatory transmission that is expressed in the brain.
- CAR-T cell
(Chimeric antigen receptor (CAR)-T cell). CAR-T cell therapy is a type of cancer immunotherapy treatment that uses T cells that are genetically altered to enable them to locate and destroy cancer cells more effectively. CARs recognize and bind to specific proteins, or antigens, on the surface of cancer cells.
- Graft versus host disease
A condition in which immune cells from a donor may view the recipient’s body as foreign, and the donated cells may attack the recipient’s body.
- OVA models
Tumour models that express the immunogen ovalbumin (OVA). They usually develop into highly immunogenic tumours for which the transfer of OVA-specific T cells confers tumour rejection.
- Tumour microenvironment
An ecosystem that surrounds a tumour. It includes immune cells, the extracellular matrix, blood vessels and other cells, such as fibroblasts.
Related links
- HSP90 molecular chaperone complex database: https://www.picard.ch/downloads/
- PhosphoSitePlus: https://www.phosphosite.org/homeAction.action
Footnotes
Competing interests
Memorial Sloan Kettering Cancer Center (MSKCC) holds the intellectual rights to the epichaperome portfolio. G.C. and C.S.D. are inventors on patents related to heat shock protein 90 (HSP90) composition of matter and methods of use. All other authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41580-023-00640-9.
References
- 1.Duan G & Walther D The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol 11, e1004049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stetz G, Tse A & Verkhivker GM Dissecting structure-encoded determinants of allosteric cross-talk between post-translational modification sites in the Hsp90 chaperones. Sci. Rep 8, 6899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Miller D, Li F, Liu X & Levy SF A large accessory protein interactome is rewired across environments. eLife 9, e62365 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussinov R, Tsai CJ & Jang H Protein ensembles link genotype to phenotype. PLoS Comput. Biol 15, e1006648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsberg SD et al. Disease-specific interactome alterations via epichaperomics: the case for Alzheimer’s disease. FEBS J. 289, 2047–2066 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bludau I & Aebersold R Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat. Rev. Mol. Cell Biol 21, 327–340 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Sui X et al. Widespread remodeling of proteome solubility in response to different protein homeostasis stresses. Proc. Natl Acad. Sci. USA 117, 2422–2431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steurer B et al. DNA damage-induced transcription stress triggers the genome-wide degradation of promoter-bound Pol II. Nat. Commun 13, 3624 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beadle GW & Tatum EL Genetic control of biochemical reactions in neurospora. Proc. Natl Acad. Sci. USA 27, 499–506 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitesell L & Lindquist SL HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Trepel J, Mollapour M, Giaccone G & Neckers L Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schopf FH, Biebl MM & Buchner J The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol 18, 345–360 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Biebl MM & Buchner J Structure, function, and regulation of the hsp90 machinery. Cold Spring Harb. Perspect. Biol 11, a034017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prodromou C Mechanisms of Hsp90 regulation. Biochem. J 473, 2439–2452 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearl LH Review: the HSP90 molecular chaperone—an enigmatic ATPase. Biopolymers 105, 594–607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiti S & Picard D Cytosolic Hsp90 isoform-specific functions and clinical significance. Biomolecules 12, 1166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altieri DC, Stein GS, Lian JB & Languino LR TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta 1823, 767–773 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzec M, Eletto D & Argon Y GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 1823, 774–787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prodromou C et al. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90, 65–75 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Stebbins CE et al. Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89, 239–250 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Chiosis G et al. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol 8, 289–299 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Zuehlke A & Johnson JL Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 93, 211–217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang RY et al. Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism. Nature 601, 460–464 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verba KA et al. Atomic structure of Hsp90–Cdc37–Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberoi J et al. HSP90–CDC37–PP5 forms a structural platform for kinase dephosphorylation. Nat. Commun 13, 7343 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W et al. Hsp90 middle domain phosphorylation initiates a complex conformational program to recruit the ATPase-stimulating cochaperone Aha1. Nat. Commun 10, 2574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolmarans A, Lee B, Spyracopoulos L & LaPointe P The mechanism of Hsp90 ATPase stimulation by Aha1. Sci. Rep 6, 33179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcu MG, Chadli A, Bouhouche I, Catelli M & Neckers LM The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem 275, 37181–37186 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Allan RK, Mok D, Ward BK & Ratajczak T Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J. Biol. Chem 281, 7161–7171 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Prodromou C et al. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 19, 4383–4392 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wayne N & Bolon DN Dimerization of Hsp90 is required for in vivo function. design and analysis of monomers and dimers. J. Biol. Chem 282, 35386–35395 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Echeverria PC, Bernthaler A, Dupuis P, Mayer B & Picard D An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PLoS ONE 6, e26044 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southworth DR & Agard DA Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol. Cell 42, 771–781 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K et al. The structure of an Hsp90–immunophilin complex reveals cochaperone recognition of the client maturation state. Mol. Cell 81, 3496–3508 e3495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czemeres J, Buse K & Verkhivker GM Atomistic simulations and network-based modeling of the Hsp90–Cdc37 chaperone binding with Cdk4 client protein: a mechanism of chaperoning kinase clients by exploiting weak spots of intrinsically dynamic kinase domains. PLoS ONE 12, e0190267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karagoz GE et al. Hsp90–Tau complex reveals molecular basis for specificity in chaperone action. Cell 156, 963–974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN & Cochran BH p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell Biol 19, 1661–1672 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makhnevych T & Houry WA The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta 1823, 674–682 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Pratt WB & Dittmar KD Studies with purified chaperones advance the understanding of the mechanism of glucocorticoid receptor–hsp90 heterocomplex assembly. Trends Endocrinol. Metab 9, 244–252 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Jhaveri K, Taldone T, Modi S & Chiosis G Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta 1823, 742–755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neckers L & Workman P Hsp90 molecular chaperone inhibitors: are we there yet? Clin. Cancer Res 18, 64–76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraro M et al. Allosteric modulators of HSP90 and HSP70: dynamics meets function through structure-based drug design. J. Med. Chem 62, 60–87 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Shrestha L, Patel HJ & Chiosis G Chemical tools to investigate mechanisms associated with HSP90 and HSP70 in disease. Cell Chem. Biol 23, 158–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Wang L, You QD & Xu XL Heat shock protein 90 inhibitors: an update on achievements, challenges, and future directions. J. Med. Chem 63, 1798–1822 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Bickel D & Gohlke H C-terminal modulators of heat shock protein of 90 kDa (HSP90): state of development and modes of action. Bioorg. Med. Chem 27, 115080 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Yang S et al. Design and synthesis of TRAP1 selective inhibitors: H-bonding with Asn171 residue in TRAP1 increases paralog selectivity. ACS Med. Chem. Lett 12, 1173–1180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel PD et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol 9, 677–684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugh KW, Alnaed M, Brackett CM & Blagg BSJ The biology and inhibition of glucose-regulated protein 94/gp96. Med. Res. Rev 42, 2007–2024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng S, Woodruff J, Pathak PK, Matts RL & Deng J Crystal structure of the middle and C-terminal domains of Hsp90α labeled with a coumarin derivative reveals a potential allosteric binding site as a drug target. Acta Crystallogr. D. Struct. Biol 78, 571–585 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaeger AM & Whitesell L HSP90: enabler of cancer adaptation. Annu. Rev. Cancer Biol 3, 275–297 (2019). [Google Scholar]
- 51. Rodina A et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 538, 397–401 (2016). This study describes the discovery of epichaperomes, reports on molecular factors that drive epichaperome formation in cancer and discusses the implications of epichaperomes in cancer for rationalizing the vulnerability of tumours to HSP90 therapy.
- 52.Pastorek M, Muller P, Coates PJ & Vojtesek B Intrinsic proteotoxic stress levels vary and act as a predictive marker for sensitivity of cancer cells to Hsp90 inhibition. PLoS ONE 13, e0202758 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown GC Total cell protein concentration as an evolutionary constraint on the metabolic control distribution in cells. J. Theor. Biol 153, 195–203 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Srivastava DK & Bernhard SA Enzyme–enzyme interactions and the regulation of metabolic reaction pathways. Curr. Top. Cell. Regul 28, 1–68 (1986). [DOI] [PubMed] [Google Scholar]
- 55.Albe KR, Butler MH & Wright BE Cellular concentrations of enzymes and their substrates. J. Theor. Biol 143, 163–195 (1990). [DOI] [PubMed] [Google Scholar]
- 56.Ellis RJ & Minton AP Cell biology: join the crowd. Nature 425, 27–28 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Halpin JC, Huang B, Sun M & Street TO Crowding activates heat shock protein 90. J. Biol. Chem 291, 6447–6455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maldonado EN & Lemasters JJ ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19PA, 78–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Halpin JC & Street TO Hsp90 sensitivity to ADP reveals hidden regulation mechanisms. J. Mol. Biol 429, 2918–2930 (2017). This report is the first to suggest that an ATP–ADP heterodimer of cytosolic HSP90 may be the predominant conformer under physiologic conditions and suggests that altering ADP inhibition of HSP90 may provide a mechanism of HSP90 regulation in cells.
- 60.Lopez A et al. Client binding shifts the populations of dynamic Hsp90 conformations through an allosteric network. Sci. Adv 7, eabl7295 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chavez JD, Schweppe DK, Eng JK & Bruce JE In vivo conformational dynamics of Hsp90 and its interactors. Cell Chem. Biol 23, 716–726 (2016). This study is the first to describe a mass spectrometry-based approach to allow quantitative measurements of in vitro and in cellulo effects of small molecule inhibitors on HSP90 conformation, and interaction with co-chaperones and client proteins. The technique allows the derivation of structural models explaining how HSP90 engages its interaction partners in cellulo.
- 62.Schmid S & Hugel T Controlling protein function by fine-tuning conformational flexibility. eLife 9, e57180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu W et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90–P50CDC37–AHA1 chaperone machine. Mol. Cell 47, 434–443 (2012). This study is the first to demonstrate that Yes kinase-mediated phosphorylation of HSP90α-Tyr313 in cells results in a 7-fold enrichment of chaperone–AHA1 complexes. Further, the affinity of AHA1 for the phosphomimetic HSP90-Tyr313Glu is increased 3.5-fold compared with AHA1 affinity for wild-type HSP90.
- 64.Takakuwa JE, Nitika, Knighton LE & Truman AW Oligomerization of Hsp70: current perspectives on regulation and function. Front. Mol. Biosci 6, 81 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yonehara M, Minami Y, Kawata Y, Nagai J & Yahara I Heat-induced chaperone activity of HSP90. J. Biol. Chem 271, 2641–2645 (1996). This report is one of the first on HSP90 oligomer formation induced by heat, noting that following oligomerization under heat HSP90 acquires a distinct activity, that is, binding substrates and preventing their irreversible aggregation.
- 66.Moullintraffort L et al. Biochemical and biophysical characterization of the Mg2+-induced 90-kDa heat shock protein oligomers. J. Biol. Chem 285, 15100–15110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nemoto T & Sato N Oligomeric forms of the 90-kDa heat shock protein. Biochem. J 330, 989–995 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jakob U et al. Structural organization of procaryotic and eucaryotic Hsp90. Influence of divalent cations on structure and function. J. Biol. Chem 270, 14412–14419 (1995). [DOI] [PubMed] [Google Scholar]
- 69.Chadli A, Ladjimi MM, Baulieu EE & Catelli MG Heat-induced oligomerization of the molecular chaperone Hsp90. Inhibition by ATP and geldanamycin and activation by transition metal oxyanions. J. Biol. Chem 274, 4133–4139 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Ginsberg SD et al. The penalty of stress—epichaperomes negatively reshaping the brain in neurodegenerative disorders. J. Neurochem 159, 958–979 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minami Y, Kawasaki H, Miyata Y, Suzuki K & Yahara I Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J. Biol. Chem 266, 10099–10103 (1991). [PubMed] [Google Scholar]
- 72.Bolaender A et al. Chemical tools for epichaperome-mediated interactome dysfunctions of the central nervous system. Nat. Commun 12, 4669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kourtis N et al. Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat. Med 24, 1157–1166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zong H et al. A hyperactive signalosome in acute myeloid leukemia drives addiction to a tumor-specific Hsp90 species. Cell Rep. 13, 2159–2173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kishinevsky S et al. HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nat. Commun 9, 4345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Inda MC et al. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat. Commun 11, 319 (2020). This study demonstrates the impact of epichaperome formation on brain activity in Alzheimer disease; reports epichaperome formation in the human brain and the proteins and protein networks that become dysregulated through epichaperomes in Alzheimer disease; and shows how these networks, and their function, can be restored to pre-stressor levels through pharmacologic epichaperome disruption.
- 77.Joshi S et al. Adapting to stress—chaperome networks in cancer. Nat. Rev. Cancer 18, 562–575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garnier C et al. Phosphorylation and oligomerization states of native pig brain HSP90 studied by mass spectrometry. Eur. J. Biochem 268, 2402–2407 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Sugita M et al. Targeting the epichaperome as an effective precision medicine approach in a novel PML-SYK fusion acute myeloid leukemia. NPJ Precis. Oncol 5, 44 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodina A et al. Systems-level analyses of protein–protein interaction network dysfunctions via epichaperomics identify cancer-specific mechanisms of stress adaptation. Nat. Commun 14, 3742 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yan P et al. Molecular stressors engender protein connectivity dysfunction through aberrant N-glycosylation of a chaperone. Cell Rep. 31, 107840 (2020). This report is the first to show that PTMs may promote HSP90 incorporation into epichaperomes.
- 82.Ginsberg SD, Sharma S, Norton L & Chiosis G Targeting stressor-induced dysfunctions in protein–protein interaction networks via epichaperomes. Trends Pharmacol. Sci 44, 20–33 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castelli M et al. How aberrant N-glycosylation can alter protein functionality and ligand binding: an atomistic view. Structure 10.1016/j.str.2023.05.017 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee CC, Lin TW, Ko TP & Wang AH The hexameric structures of human heat shock protein 90. PLoS ONE 6, e19961 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lepvrier E et al. Hsp90 oligomerization process: how can p23 drive the chaperone machineries? Biochim. Biophys. Acta 1854, 1412–1424 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Lepvrier E et al. Hsp90 oligomers interacting with the Aha1 cochaperone: an outlook for the Hsp90 chaperone machineries. Anal. Chem 87, 7043–7051 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Joshi S et al. Pharmacologically controlling protein–protein interactions through epichaperomes for therapeutic vulnerability in cancer. Commun. Biol 4, 1333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muda K et al. Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14–3-3. Proc. Natl Acad. Sci. USA 111, E34–E43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kato H et al. Cancer-derived UTX TPR mutations G137V and D336G impair interaction with MLL3/4 complexes and affect UTX subcellular localization. Oncogene 39, 3322–3335 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Yan W et al. Structural insights into the SPRED1–Neurofibromin–KRAS complex and disruption of SPRED1–neurofibromin interaction by oncogenic EGFR. Cell Rep. 32, 107909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nemoto TK, Ono T & Tanaka K Substrate-binding characteristics of proteins in the 90 kDa heat shock protein family. Biochem. J 354, 663–670 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lepvrier E, Thomas D & Garnier C Hsp90 quaternary structures and the chaperone cycle: highly flexible dimeric and oligomeric structures and their regulation by co-chaperones. Curr. Proteom 16, 5–11 (2019). [Google Scholar]
- 93.Rickner HD et al. Single cell transcriptomic profiling of a neuron-astrocyte assembloid tauopathy model. Nat. Commun 13, 6275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shemesh N et al. The landscape of molecular chaperones across human tissues reveals a layered architecture of core and variable chaperones. Nat. Commun 12, 2180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hadizadeh Esfahani A, Sverchkova A, Saez-Rodriguez J, Schuppert AA & Brehme M A systematic atlas of chaperome deregulation topologies across the human cancer landscape. PLoS Comput. Biol 14, e1005890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brehme M et al. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 9, 1135–1150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Backe SJ, Sager RA, Woodford MR, Makedon AM & Mollapour M Post-translational modifications of Hsp90 and translating the chaperone code. J. Biol. Chem 295, 11099–11117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rein T Post-translational modifications and stress adaptation: the paradigm of FKBP51. Biochem. Soc. Trans 48, 441–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shevtsov M et al. Membrane-associated heat shock proteins in oncology: from basic research to new theranostic targets. Cells 9, 1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller P et al. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene 32, 3101–3110 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Richter K, Muschler P, Hainzl O, Reinstein J & Buchner J Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the ATPase cycle. J. Biol. Chem 278, 10328–10333 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Bhattacharya K et al. The Hsp70–Hsp90 co-chaperone Hop/Stip1 shifts the proteostatic balance from folding towards degradation. Nat. Commun 11, 5975 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lackie RE et al. Stress-inducible phosphoprotein 1 (HOP/STI1/STIP1) regulates the accumulation and toxicity of α-synuclein in vivo. Acta Neuropathol. 144, 881–910 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shelton LB, Koren J 3rd & Blair LJ Imbalances in the Hsp90 chaperone machinery: implications for tauopathies. Front. Neurosci 11, 724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shelton LB et al. Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc. Natl Acad. Sci. USA 114, 9707–9712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lackie RE et al. Increased levels of stress-inducible phosphoprotein-1 accelerates amyloid-β deposition in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun 8, 143 (2020). This study is the first to show that despite HOP overexpression being protective in Caenorhabditis elegans, the principal effect of elevated HOP in mouse models of neurodegeneration is deleterious.
- 107.Blair LJ et al. The disease-associated chaperone FKBP51 impairs cognitive function by accelerating AMPA receptor recycling. eNeuro 6, 242–318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oroz J et al. Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex. Nat. Commun 9, 4532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rutledge BS, Choy WY & Duennwald ML Folding or holding?—Hsp70 and Hsp90 chaperoning of misfolded proteins in neurodegenerative disease. J. Biol. Chem 298, 101905 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sager RA et al. Targeting extracellular Hsp90: a unique frontier against cancer. Front. Mol. Biosci 9, 982593 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee AS Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 14, 263–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amoroso MR et al. TRAP1 revisited: novel localizations and functions of a ‘next-generation’ biomarker (review). Int. J. Oncol 45, 969–977 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Jay D, Luo Y & Li W Extracellular heat shock protein-90 (eHsp90): everything you need to know. Biomolecules 12, 911 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Secli L, Fusella F, Avalle L & Brancaccio M The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell. Mol. Life Sci 78, 4069–4083 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poggio P, Sorge M, Secli L & Brancaccio M Extracellular HSP90 machineries build tumor microenvironment and boost cancer progression. Front. Cell Dev. Biol 9, 735529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tang X et al. Tumour-secreted Hsp90α on external surface of exosomes mediates tumour–stromal cell communication via autocrine and paracrine mechanisms. Sci. Rep 9, 15108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang Y et al. Role of acetylation and extracellular location of heat shock protein 90α in tumor cell invasion. Cancer Res. 68, 4833–4842 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weidenauer L & Quadroni M Phosphorylation in the charged linker modulates interactions and secretion of Hsp90β. Cells 10, 1701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zou M et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90α in tumour progression. Oncogene 36, 2160–2171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baker-Williams AJ et al. Co-chaperones TIMP2 and AHA1 competitively regulate extracellular HSP90:client MMP2 activity and matrix proteolysis. Cell Rep. 28, 1894–1906.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Graner MW HSP90 and immune modulation in cancer. Adv. Cancer Res 129, 191–224 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Yamano T et al. Two distinct pathways mediated by PA28 and hsp90 in major histocompatibility complex class I antigen processing. J. Exp. Med 196, 185–196 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kunisawa J & Shastri N Hsp90α chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity 24, 523–534 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Jaeger AM et al. Deciphering the immunopeptidome in vivo reveals new tumour antigens. Nature 607, 149–155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jaeger AM et al. Rebalancing protein homeostasis enhances tumor antigen presentation. Clin. Cancer Res 25, 6392–6405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haggerty TJ et al. Heat shock protein-90 inhibitors enhance antigen expression on melanomas and increase T cell recognition of tumor cells. PLoS ONE 9, e114506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rerole AL, Jego G & Garrido C Hsp70: anti-apoptotic and tumorigenic protein. Methods Mol. Biol 787, 205–230 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Yuno A et al. Clinical evaluation and biomarker profiling of Hsp90 inhibitors. Methods Mol. Biol 1709, 423–441 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Do K et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest. N. Drugs 33, 921–930 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajan A et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin. Cancer Res 17, 6831–6839 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pillarsetty N et al. Paradigms for precision medicine in epichaperome cancer therapy. Cancer Cell 36, 559–573.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mbofung RM et al. HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat. Commun 8, 451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Calvo-Vidal MN et al. Oncogenic HSP90 facilitates metabolic alterations in aggressive B-cell lymphomas. Cancer Res. 81, 5202–5216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zavareh RB, Spangenberg SH, Woods A, Martinez-Pena F & Lairson LL HSP90 inhibition enhances cancer immunotherapy by modulating the surface expression of multiple immune checkpoint proteins. Cell Chem. Biol 28, 158–168.e5 (2021). [DOI] [PubMed] [Google Scholar]
- 135.Martinet J et al. Impact of epichaperome inhibitor PU-H71 on anti-tumor T cell responses and its implications for immune and cellular therapy. Blood 140, 1171–1172 (2022). [Google Scholar]
- 136.Miles J, Scherz-Shouval R & van Oosten-Hawle P Expanding the organismal proteostasis network: linking systemic stress signaling with the innate immune response. Trends Biochem. Sci 44, 927–942 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Mimnaugh EG, Worland PJ, Whitesell L & Neckers LM Possible role for serine/ threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J. Biol. Chem 270, 28654–28659 (1995). [DOI] [PubMed] [Google Scholar]
- 138.Soroka J et al. Conformational switching of the molecular chaperone Hsp90 via regulated phosphorylation. Mol. Cell 45, 517–528 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Mollapour M & Neckers L Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta 1823, 648–655 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cloutier P & Coulombe B Regulation of molecular chaperones through post-translational modifications: decrypting the chaperone code. Biochim. Biophys. Acta 1829, 443–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woodford MR et al. Mps1 mediated phosphorylation of Hsp90 confers renal cell carcinoma sensitivity and selectivity to Hsp90 inhibitors. Cell Rep. 14, 872–884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wengert LA, Backe SJ, Bourboulia D, Mollapour M & Woodford MR TRAP1 chaperones the metabolic switch in cancer. Biomolecules 12, 786 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuehlke AD et al. An Hsp90 co-chaperone protein in yeast is functionally replaced by site-specific posttranslational modification in humans. Nat. Commun 8, 15328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rehn A et al. A methylated lysine is a switch point for conformational communication in the chaperone Hsp90. Nat. Commun 11, 1219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rehn A et al. Allosteric regulation points control the conformational dynamics of the molecular chaperone Hsp90. J. Mol. Biol 428, 4559–4571 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Wolfgeher D et al. The dynamic interactome of human Aha1 upon Y223 phosphorylation. Data Brief. 5, 752–755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dunn DM et al. c-Abl mediated tyrosine phosphorylation of Aha1 activates its co-chaperone function in cancer cells. Cell Rep. 12, 1006–1018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bachman AB et al. Phosphorylation induced cochaperone unfolding promotes kinase recruitment and client class-specific Hsp90 phosphorylation. Nat. Commun 9, 265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scroggins BT et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol. Cell 25, 151–159 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schmid AB et al. The architecture of functional modules in the Hsp90 co-chaperone Sti1/Hop. EMBO J. 31, 1506–1517 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Panaretou B et al. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol. Cell 10, 1307–1318 (2002). [DOI] [PubMed] [Google Scholar]
- 152.Ali MM et al. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McLaughlin SH et al. The co-chaperone p23 arrests the Hsp90 ATPase cycle trap client proteins. J. Mol. Biol 356, 746–758 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Wang X et al. Thr90 phosphorylation of Hsp90α by protein kinase A regulates its chaperone machinery. Biochem. J 441, 387–397 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Woodford MR et al. Hsp90 chaperone code and the tumor suppressor VHL cooperatively regulate the mitotic checkpoint. Cell Stress. Chaperones 26, 965–971 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mollapour M et al. Asymmetric Hsp90 N domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Mol. Cell 53, 317–329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mollapour M, Tsutsumi S & Neckers L Hsp90 phosphorylation, Wee1 and the cell cycle. Cell Cycle 9, 2310–2316 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Longshaw VM, Chapple JP, Balda MS, Cheetham ME & Blatch GL Nuclear translocation of the Hsp70/Hsp90 organizing protein mSTI1 is regulated by cell cycle kinases. J. Cell Sci 117, 701–710 (2004). [DOI] [PubMed] [Google Scholar]
- 159.Biebl MM, Riedl M & Buchner J Hsp90 co-chaperones form plastic genetic networks adapted to client maturation. Cell Rep. 32, 108063 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Lagadari M, Zgajnar NR, Gallo LI & Galigniana MD Hsp90-binding immunophilin FKBP51 forms complexes with hTERT enhancing telomerase activity. Mol. Oncol 10, 1086–1098 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 161.Mayer MP & Le Breton L Hsp90: breaking the symmetry. Mol. Cell 58, 8–20 (2015). [DOI] [PubMed] [Google Scholar]
- 162.Nguyen MTN et al. Isoform-specific phosphorylation in human Hsp90β affects interaction with clients and the cochaperone Cdc37. J. Mol. Biol 429, 732–752 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Thorne ME & McQuade KL Heat-induced oligomerization of gp96 occurs via a site distinct from substrate binding and is regulated by ATP. Biochem. Biophys. Res. Commun 323, 1163–1171 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Feldweg AM & Srivastava PK Molecular heterogeneity of tumor rejection antigen/heat shock protein GP96. Int. J. Cancer 63, 310–314 (1995). [DOI] [PubMed] [Google Scholar]
- 165.Pagetta A et al. Structural insights into complexes of glucose-regulated protein 94 (Grp94) with human immunoglobulin G. Relevance for Grp94–IgG complexes that form in vivo in pathological conditions. PLoS ONE 9, e86198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ratna A et al. Myeloid endoplasmic reticulum resident chaperone GP96 facilitates inflammation and steatosis in alcohol-associated liver disease. Hepatol. Commun 5, 1165–1182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sumitomo T et al. GP96 drives exacerbation of secondary bacterial pneumonia following influenza A virus infection. mBio 12, e0326920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chaumonnot K et al. The HSP GRP94 interacts with macrophage intracellular complement C3 and impacts M2 profile during ER stress. Cell Death Dis. 12, 114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rothan HA et al. Small molecule grp94 inhibitors block dengue and Zika virus replication. Antivir. Res 171, 104590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Joshi A et al. The mitochondrial HSP90 paralog TRAP1 forms an OXPHOS-regulated tetramer and is involved in mitochondrial metabolic homeostasis. BMC Biol. 18, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chretien D et al. Mitochondria are physiologically maintained at close to 50°C. PLoS Biol. 16, e2003992 (2018). This study is the first to clearly demonstrate that mitochondria are more than 10°C warmer than adjacent cytoplasm when the respiratory chain is fully functional, establishing their physiologic temperature under these conditions at 45–47°C, equivalent to what is considered a heat shock temperature range.
- 172.Woodford MR et al. Mutation of the co-chaperone Tsc1 in bladder cancer diminishes Hsp90 acetylation and reduces drug sensitivity and selectivity. Oncotarget 10, 5824–5834 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rao R et al. HDAC6 inhibition enhances 17-AAG-mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood 112, 1886–1893 (2008). [DOI] [PubMed] [Google Scholar]
- 174.Moulick K et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat. Chem. Biol 7, 818–826 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM & Workman P Maximizing the therapeutic potential of HSP90 inhibitors. Mol. Cancer Res 13, 1445–1451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jhaveri KL et al. Measuring tumor epichaperome expression using [124I] PU-H71 positron emission tomography as a biomarker of response for PU-H71 plus nab-paclitaxel in HER2-negative metastatic breast cancer. JCO Precis. Oncol 4, PO.20.00273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Speranza G et al. First-in-human study of the epichaperome inhibitor PU-H71: clinical results and metabolic profile. Invest. N. Drugs 36, 230–239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Silverman M, Wallner B, Key C, Duggan SM & Reynolds L A single- and multiple-ascending dose study to evaluate the safety and pharmacokinetics of oral PU-AD, an epichaperome inhibitor to treat Alzheimer’s disease. Alzheimers Dement. 16, e041144 (2020). [Google Scholar]
- 179.Kurokawa Y et al. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Ann. Oncol 33, 959–967 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.