SUMMARY
Inner ear disorders are among the most common congenital abnormalities; however, current tissue culture models lack the cell type diversity to study these disorders and normal otic development. Here, we demonstrate the robustness of human pluripotent stem cell-derived inner ear organoids (IEOs) and evaluate cell type heterogeneity by single-cell transcriptomics. To validate our findings, we construct a single-cell atlas of human fetal and adult inner ear tissue. Our study identifies various cell types in the IEOs including periotic mesenchyme, type I and type II vestibular hair cells, and developing vestibular and cochlear epithelium. Many genes linked to congenital inner ear dysfunction are confirmed to be expressed in these cell types. Additional cell-cell communication analysis within IEOs and fetal tissue highlights the role of endothelial cells on the developing sensory epithelium. These findings provide insights into this organoid model and its potential applications in studying inner ear development and disorders.
In brief
Van der Valk et al. highlight the potential of human stem cell-derived inner ear organoids for studying disorders. Single-cell transcriptomics identified various cell types linked to inner ear dysfunction, which are shared between organoids and the human inner ear. They offer an atlas for studying genetic inner ear disorders with organoids.
Graphical Abstract
INTRODUCTION
The inner ear comprises the cochlea and vestibular organs, which mediate sound perception and balance. Functional impairment of the inner ear is common, affecting more than 5% of the general population with hearing loss or balance disorders.1,2 The etiology of these inner ear disorders varies greatly depending on age, genetics, and environmental factors. Sensorineural hearing loss (SNHL) is one of the most common congenital disorders and affects more than 1 in 1,000 newborns.3,4 Hereditary SNHL can be attributed to either syndromic or nonsyndromic genetic causes.5,6 On the basis of the partial overlap of gene expression between the cochlea and the vestibular organs, it is not surprising that mutations in these deafness genes may also cause vestibular dysfunction, as seen in patients with DNFA9, DFNA11, DFNA15, and Usher syndrome.7
Despite research efforts to understand the mechanisms of genetic SNHL and to identify therapies, a significant challenge is the heterogeneity of SNHL.8 Current animal models are limited to modeling the effect of specific gene mutations. Culture models of differentiated human inner ear cells provide a valuable alternative and offer a way to assess the impact of SNHL genes on the development and function of the human inner ear, as discussed in recent reviews.9–16 Recent progress has been made on human inner ear organoids (IEOs), which can be derived from human pluripotent stem cells (hPSCs), either embryonic (human embryonic stem cells [hESCs]) or induced (human induced pluripotent stem cells [hiPSCs]), using differentiation strategies that result in cultures containing inner ear-like cells.9 In addition, several SNHL gene mutations have been modeled in mouse and hPSC-derived otic culture systems.17 However, most reports focus primarily on hair cells, and it remains unclear to what extend PSC-based systems can be used to model the full range of congenital SNHL disorders.17
A promising system lies in the hPSC-derived IEOs.18 Reports using this model are limited to the use of one hESC and one hiPSC line, and their findings are largely restricted to hair cells and neurons.18–20 However, it is possible that the cellular heterogeneity within these IEOs is more extensive, as recent and ongoing work on the temporal cellular diversity of early IEO aggregates reveals its multilineage character.21 Our goal in the current study was to validate the protocol across multiple hPSC lines and determine the cellular variety and maturity of late-stage hPSC-derived IEOs (differentiation day 75 [D75] and later), focusing on the inner ear cell types. To this end, we provide a tool to determine early differentiation efficiency. We demonstrate on-target induction of inner ear cell types but likewise identify off-target induction of cranial skeletal myocytes, ependymal cells, and vascular endothelial cells. For comparison, we generated an age-matched single-cell transcriptomic atlas of the fetal human inner ear that captures the diversity of the whole membranous labyrinth. In addition, we include single-cell data from adult human vestibular organs to show the extent to which IEOs contain mature cell types of the human inner ear. Our comparative analysis reveals that organoids contain specific sensory and nonsensory vestibular epithelial types, including type I and type II vestibular hair cells, and periotic mesenchyme (POM). Surprisingly, we could identify a subset of cells resembling cochlear nonsensory epithelial cells. By analyzing the cell-cell communication within the organoid model, we found evidence for signaling pathway interactions between hair cells and endothelial cells that could be validated in the human inner ear data. Furthermore, we assigned the expression of known SNHL and balance disorder-associated genes and proteins. These results offer significant understanding into the IEO model system and its possibility for exploring development and disorders of the inner ear.
RESULTS
BMP signaling underlies variability in IEO induction across hPSC lines
We aimed to evaluate the robustness of differentiating inner ear epithelium from multiple hPSCs using a streamlined method for IEO induction (Figure 1A).18,21 Eight human healthy-control hPSC lines were used: one hESC line (WA01) and seven hiPSC lines (WTC-SOX2, WTC-GCaMP, LUMC04i10, LUMC44i44, GON0515–03, GON0926–02, and SAH0047–02). In adapting the protocol to a broader cohort of cell lines, we noted that the BMP-4 treatment at D0 was a critical determinant of successful IEO production. In our experience, BMP-4 activity differs between vendors and lots, hampering consistent differentiation efficiency. We sought to determine which morphological features during early differentiation could be used to evaluate IEO induction efficiency objectively. To this end, we treated the cell lines with BMP-4 concentrations ranging from 0 to 20 ng/mL, and we followed morphologic features up to D12 (Figure 1B). The most efficient BMP-4 concentration was determined by the presence of otic vesicles at D21 or the presence of more mature IEOs at D65 and later, using H&E staining or immunohistochemistry (IHC) (Figures 1C and 1D). The otic identity of inner ear vesicles at D21 could be confirmed by the expression of CDH1, TFAP2A, and SOX10,22 with additional SOX2 and SIX1 expression (Figure 1C). The IEOs of D65 and later were CDH1+ SOX10+, and contained MYO7A+ hair cells with TUBB3+ neurons that protrude toward hair cells (Figure 1D). The highest differentiation efficiency was reached at a BMP-4 concentration of 1.25 ng/mL for this set of experiments using BMP-4 from the same lot, with either otic vesicles or epithelial IEOs forming in all eight hPSC lines. We limited the measurement of morphological features following BMP-4 treatment to D3, as during the first 12 days, we noticed a variation in the morphology between cell lines (Figure 1B). LUMC04i10, WTC-SOX2, WTC-GCaMP, and SAH0047–02 aggregates contained an outer epithelial layer, the developing ectoderm,18 which could be clearly monitored over time until D12 (Figure 1B). LUMC44i44, WA01, GON0515–03, and GON0926–02 aggregates, however, became denser after D3, and the outer epithelial layer could not be followed over time (Figure 1B). We measured epithelial thickness, circularity, and area of the D3 aggregates in vitro (Figure S1A). From these morphological features, only epithelial thickness significantly differed between BMP-4 concentrations over all hPSC lines (Figures 1E and S1B–S1C). Thickness decreased with increasing BMP-4 concentrations, with efficient IEO differentiation being achieved with an outer epithelium layer that was on average 35.9 μm thick (SD = 6.2 μm, n = 10 aggregates per cell line, 8 cell lines). Size and circularity of the aggregate were also influenced by the BMP-4 concentration, however less consistent over all cell lines (Figure S1C). Depending on the BMP-4 lot or vendor, however, concentrations up to 20 ng/mL had to be used to reach the same epithelial thickness and subsequent efficient induction of otic vesicles and IEOs (data not shown). In conclusion, optimal BMP-4 concentration at D0 can be inferred from morphological changes as early as D3 and not necessarily by the absolute concentration of BMP-4. A range of 1.25–20 mg/mL is acceptable depending on the vendor, handling, and storage (see STAR Methods). Using aggregates with the optimal outer epithelium thickness, we efficiently induced otic vesicles in all hPSC lines tested.
Figure 1. Otic induction efficiency depends on BMP-4 concentration.
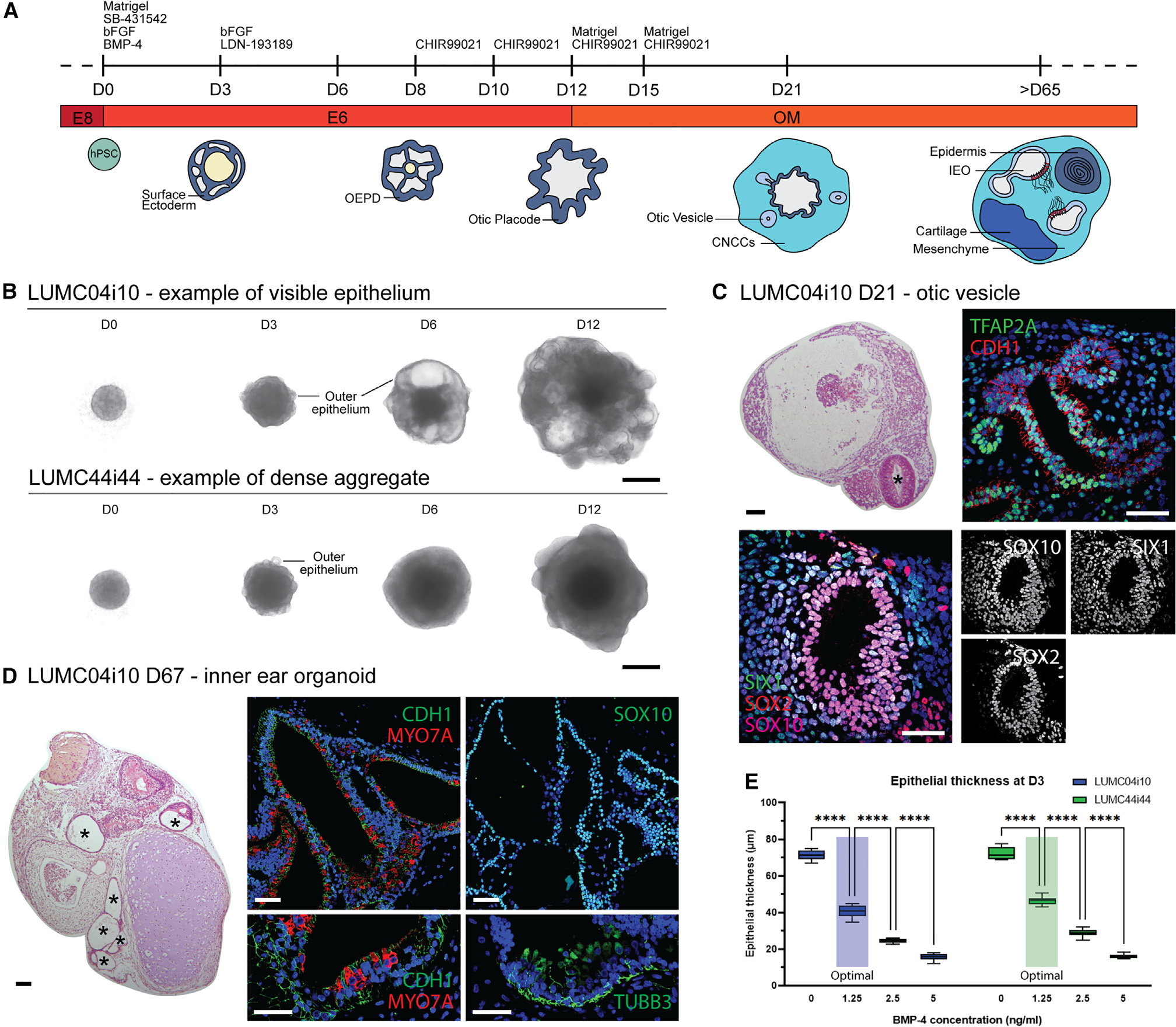
(A) Overview of inner ear organoid differentiation.
(B) Bright-field images of cell aggregates at different time points for two hiPSC lines showing morphological differences. Representative images from ≥8 aggregates (≥3 experiments). Scale bars, 500 μm.
(C) Representative H&E and IHC images of D21 aggregates containing otic vesicles (asterisk) expressing TFAP2A, CDH1, SOX10, SIX1, and SOX2. Scale bars: H&E, 100 μm; IHC, 50 μm.
(D) H&E and IHC images of D67 aggregates showing an IEO with epithelial vesicles (asterisks, CDH1+, SOX10+), containing hair cells (MYO7A+) and neurons (TUBB3+). Scale bars: H&E, 100 μm; IHC, 50 μm.
(E) Epithelial thickness measurements of D3 aggregates treated with different BMP-4 concentrations at D0 for two cell lines. Efficient IEO induction achieved at 1.25 ng/mL BMP-4 (“Optimal”). Statistical significance analyzed using 2-way ANOVA with Šidák correction. Data considered significant if p < 0.05. n = 10 per datapoint shown in a min-max boxplot. Representative graph from two experiments.
CNCCs, cranial neural crest cells; D, differentiation day; IEO, inner ear organoid; OEPD, otic-epibranchial placode domain. ****p ≤ 0.0001. H&E and IHC images (C and D) are representative of ≥6 aggregates (≥2 experiments). See also Figure S1.
Single-cell and single-nucleus RNA sequencing unravel the cellular diversity of IEOs
To comprehensively characterize the cell types that arise in the efficiently differentiated aggregates, we performed single-cell RNA sequencing (scRNA-seq) as well as single-nucleus RNA sequencing (snRNA-seq) on D75–D110 aggregates derived from four hPSC lines (WA01, WTC-SOX2, WTC-GCaMP, and LUMC04i10). The WTC-SOX2 and WTC-GCaMP lines are commonly used for our experiments and therefore included, although their reporter functions are beyond the scope of this paper. We decided to include snRNA-seq in addition to scRNA-seq as certain cell types, such as epithelial cells, might be better presented by the single nucleus approach.23,24 More important, both methods allow similar discrimination of cell types when intronic sequences are included in snRNA-seq analyses.25,26 In addition to dissociation of whole aggregates into single cells, we used another approach by which aggregates were first cut in slices using a vibratome. This approach allowed us to select sections that contain IEO-like vesicles by bright-field evaluation before dissociation into single cells.
Using differentially expressed genes and cell type-specific marker genes for annotation, we captured a similar cell type heterogeneity in the scRNA-seq (Figure S2) and snRNA-seq data (Figure S3). The vibratome approach, however, did not result in a relatively larger number of otic cell types (Figure S2B). We identified some variation between the scRNA-seq and snRNA-seq approach: using scRNA-seq of the WTC-SOX2 and WTC-GCaMP hiPSC lines, clusters of cycling cells and cells with a high ribosomal content were identified that could not be identified using the snRNA-seq approach in the WA01 and LUMC04i10 hiPSC lines. Similarly, adipocytes could not be identified using scRNA-seq of the WTC-SOX2 and WTC-GCaMP hiPSC lines but were seen in the snRNA-seq approach.
As we were able to identify similar cell types with both approaches, we integrated the data to a combined dataset of sequenced cells and nuclei for further analyses (Figures 2A–2C and S4). Of the cell types, 8.3% (3.7%–11.1% between cell lines) assigned to an otic identity that includes otic epithelium, hair cells, or POM (Figure 2C). The unique transcriptomic signature of hair cells in the otic epithelium is demonstrated by their distinct clustering from the epithelial cells, similar to single-cell studies of the mouse and human inner ear.27,28 We wondered if the other populations contained cells reminiscent of the inner ear spatial environment, thus we set out to analyze these populations. Most of these cell types were of mesenchymal origin (68.0%; Figure 2B). This group contained a cluster of myocytes (TNNC1, TPM3, SRL, CKM, and LDB3)29 (Figure 2D). The presence of these myocytes corresponds with our observations of irregular contractions in later stage aggregates in vitro (data not shown). This mesenchymal population expressed skeletal myocyte-specific genes (TNNT1, MYBPC1, MYOT, RYR1, and JPH1) (Figure S5A),29 with a marked lack of expression of marker genes for either smooth muscle cells (ACTA2, MYOCD, and MYH11)30 or cardiomyocytes (MYH6, MYL7, TNNI3, MYBPC3, and CACNA1C).29 In line with the fetal skeletal myocyte gene expression of MYL4, ACTC1, and TNNT2 (Figure S5A),31–33 we could identify different transcriptional stages of skeletal myogenic differentiation within this population (Figure S5B): myocyte precursors (PAX7), myoblasts/myocytes (MYOD1, MYOG), and myotubes (MYL2, MYH2, MYH7, ITBG1).34 Interestingly, when identifying upstream genetic regulators that control cranial myogenesis, we observed TBX1 and PITX2 gene expression, with a relative absence of PAX3 (Figure S5C), limiting the possible identity to that of cranial skeletal myocytes, as reviewed by A. Grimaldi and colleague.35 Moreover, moderate expression of NKX2–5 could be seen in this cluster (data not shown). Although NKX2–5 expression is well established in cardiomyocyte development, expression on the protein and mRNA level has been described in developing cranial skeletal myocytes.36 Using IHC, we confirmed the presence of ACTC1+ TNNT1+ MYOG+ skeletal myocytes in our aggregates (Figure 2E). The mesenchymal population consists also of a chondrocyte population (MATN4, COL9A1, SOX6, SOX9, COL2A1) which contains an early mature signature (CNMD, EPYC, FRZB) (Figure S5D) with more mature genes such as ISG15, IFI6, and MX1 expressed only in a fraction of the cells37 (Figure S5E). Additionally, we identified fibroblasts (COL1A1, PDGFRA, TWIST2, APCDD1, DPT) and pericytes (CSPG4, PDGFRB, RGS5, KCNJ8, ABCC9) forming distinct clusters within the mesenchymal cell types (Figures S5F and S5G).
Figure 2. Single-cell transcriptomic analysis reveals IEO cellular diversity.
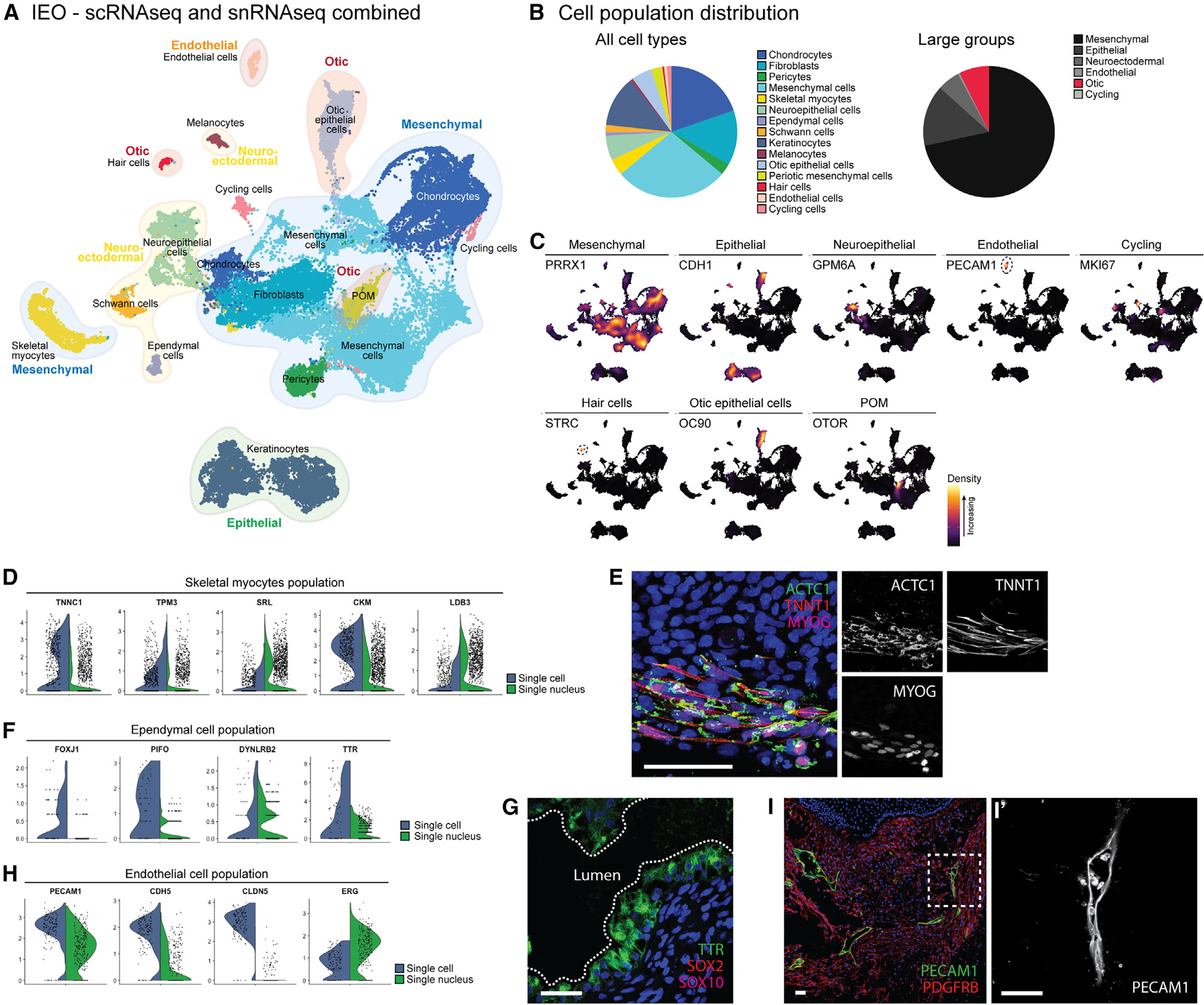
(A) Uniform manifold approximation and projection (UMAP) plot showing scRNA-seq and snRNA-seq data of D75-D110 IEOs with cell type annotations.
(B) Relative contribution of cell types and large groups.
(C) Marker genes for cell type annotation.
(D) Skeletal myocyte cluster highlighted by skeletal myocyte marker genes.
(E) ACTC1+ TNNT1+ MYOG+ skeletal myocytes in a D75 aggregate. Scale bar, 50 μm.
(F) Ependymal cell cluster with marker gene expression.
(G) TTR+ SOX2− SOX10− ependymal cells in a D75 aggregate. Scale bar, 50 μm.
(H) Endothelial cluster with marker genes.
(I) PECAM1+ endothelial cells forming luminal-like structures in a D75 aggregate (I′). Scale bars, 50 μm.
POM, periotic mesenchyme. IHC images (E, G, and I) are representative of n ≥ 6 aggregates (≥2 experiments). See also Figures S2–S5 and S8.
The second largest group within the aggregate are the epithelial cells consisting of keratinocytes and otic epithelial cells (12.6%). The keratinocytes within the aggregate showed a similar cell type-diversity to the skin organoids.38 Within this population expressing CDH1, KRT5, and KRT19, we could identify basal (CXCL14), intermediate (KRT1), and peridermal (KRT4) keratinocytes (Figure S5H).
Neuroectodermal cell types are involved in certain types of SNHL, such as Waardenburg syndrome affecting the melanocyte lineage, but also in neurofibromatosis type II, which is characterized by neoplastic Schwann cells.39 To get a better understanding of these populations, we set out to determine how mature these cell types are in our IEOs. The neuroectodermal group (9.4%) consisted of neuroepithelial cells, ependymal cells as well as Schwann cells and melanocytes. The neuroepithelial population could be divided into a group of neuroepithelial progenitor cells (SOX2 and SNHG11) and more mature central (GPM6A, MATK, and RASFRF1) and peripheral (PRPH and PIRT) neurons (Figure S5I).40 These latter expressed genes for ion channels known to be enriched in the peripheral nervous system, specifically in sensory neurons (P2RX3, SCN7A, and SCN10A).41 When analyzing semi-thin sections, we identified occasional groups of neurons in the stroma surrounding the IEOs. They contain a distinct cell body with a large, round nucleus and a single dendritic process, suggestive for neurons (Figure S5J). The dendritic processes were surrounded by cells with elongated, flat nuclei with morphologies similar to Schwann cells. Using transmission electron microscopy (TEM), we observed evidence of Schwann cells associated with these neurons near the otic vesicles (Figure S5K). The presence of these Schwann cells was confirmed by the transcriptomics data, with a Schwann cell population expressing marker genes (SOX10, ERBB3, S100B, and PLP1), as well as genes for immature (NGFR, NCAM1, L1CAM, and CDH2) and (pro)-myelinating Schwann cells (POU3F1, CDKN1C, MPZ, PTN, and CRYAB) (Figure S5L).42 We also discovered a population of ependymal cells that expressed FOXJ1, PIFO, DYNLRB2, and TTR (Figure 2F).43 We confirmed the presence of TTR+ SOX2− SOX10− ependymal cells forming vesicle-like structures (Figure 2G), in contrast to the TTR− SOX2+ SOX10+ otic vesicles. The last neuroectodermal population was composed of melanocytes, which expressed genes of various stages of melanocyte differentiation, including marker genes for melanoblasts (SOX10, EDNRB, DCT, and MITF), melanocytes (KIT, PAX3, and EDNRB) and that of differentiated fully pigmented melanocytes (TYRP1, TYR, and GPR143) (Figure S5M).44,45 Although melanocytes are generally known for their role in skin pigmentation,38 a subset of melanocytes incorporates into the ionic regulatory epithelium in the inner ear46,47; hence, we sought to locate this population in IEOs. Interestingly, melanocytes (MLANA+) were found within or closely related to the SOX10+ IEOs (Figure S5N), similar to the developing human fetal vestibular organs (around fetal week 10) and cochlea (around fetal week 11).46,47
We discovered a population of endothelial cells in the IEOs using the transcriptomic data that could be reminiscent of the blood-labyrinth barrier of the inner ear. This population (0.7%) expressed known endothelial markers PECAM1, CDH5, CLDN5, and ERG (Figure 2H),48 with concomitant expression of vascular marker genes (FLT1, VWF, NOTCH4, and NRP1)48,49 and relative absence of lymphatic marker genes (PROX1 and PDPN) (Figure S5O).50 LYVE1 and FLT4, both expressed in this population, are known lymphatic-specific genes, although these genes have been observed during fetal vascular endothelial development.51,52 We could confirm the presence of PECAM1+ endothelial cells within the aggregate forming lumen-containing structures throughout the aggregate (Figure 2I).
In summary, using different hPSC lines, we could robustly induce multilineage cell types within the IEOs. The cell types we identified are known to be expressed in the environment of the inner ear, although we could not grant all of them with an “inner ear identity.” In addition to the putative cranial identity of the skeletal myocytes, we identified gene expression profiles that match embryonic or fetal development, for instance for the skeletal myocytes, chondrocytes, and vascular endothelial cells. WTC-GCaMP and WTC-SOX2 aggregates (which share a parental genome) showed a similar cell type distribution based on scRNA-seq data (Figure S4B). However, this distribution differed from the LUMC04i10 and WA01 cell line that are used for snRNA-seq. This could be due to variability in dissociation method (either single cell or single nucleus), or cell line variation. The latter is supported by the difference we observe between the WA01 and LUMC04i10 cell line, notably the large neuroepithelial cell type cluster found in the WA01 cell line and a relatively smaller mesenchymal cluster. In this study, we did not perform thorough batch-to-batch variation tests to rule out the role of cell lines or the transcriptomics method used.
Generation of a single-cell atlas of fetal and adult human inner ear tissues
To compare cell type diversity within IEOs to those found in the native human inner ear, we carried out snRNA-seq on fresh inner ear tissue collected at different stages of human inner ear development. We collected one fetal inner ear of fetal age week 7.5 (FW7.5), one fetal inner ear of FW9.2, and an ampulla and utricle from a 47-year-old donor (Figure 3A). At FW9.2, we would expect that the mature cochlea is still in development and the epithelial populations could be separated out in different domains (Figure 3B). The cochlear duct floor at this stage divides in a medial, prosensory, and lateral domain, giving rise to the future Kölliker’s organ, organ of Corti, and outer sulcus, respectively. In addition, the roof of the cochlear duct will give rise to Reissner’s membrane and the stria vascularis, as reviewed by E.C. Driver and colleague.53 Vestibular development, which precedes that of the cochlea, has a more mature phenotype with distinct epithelial cell types at FW9.2.46,54
Figure 3. Generation of the human fetal and adult inner ear single-cell atlas.
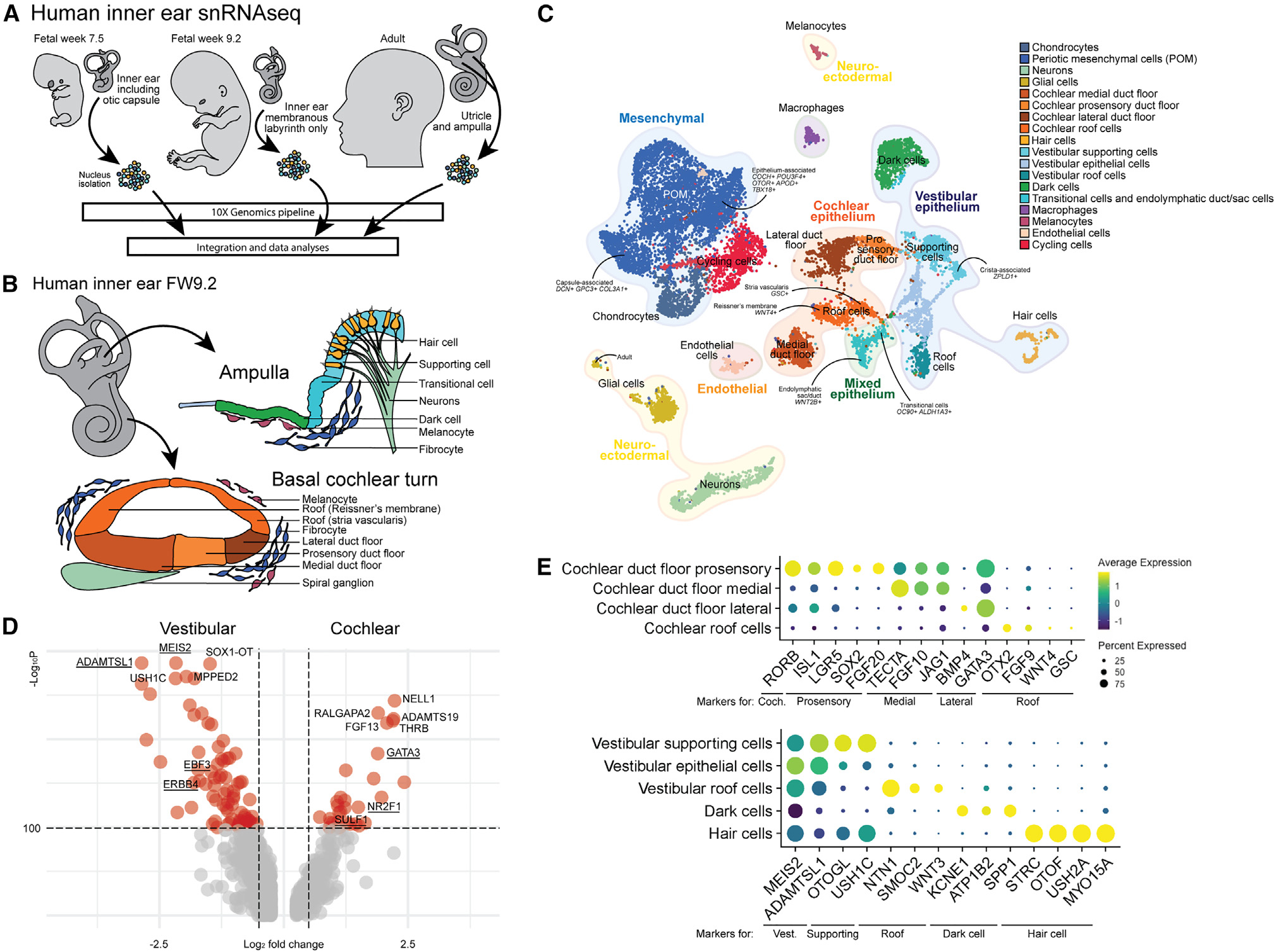
(A) Experimental overview of fresh human inner ear tissue collection from FW7.5 (n = 1), FW9.2 (n = 1), and adult donor (n = 1).
(B) The human inner ear at FW9.2, illustrating cellular diversity in the vestibular ampulla and basal turn of the cochlea.
(C) UMAP plot of combined human inner ear datasets with cell type annotations.
(D) Volcano plot showing differentially expressed genes between vestibular supporting cells and cochlear duct floor. Underlined genes are described to be differentially expressed between vestibular and cochlear cell types.
(E) Dot plot displaying standardized expression of known marker genes in cochlear and vestibular cell types.
Coch., cochlear; Vest., vestibular. See also Figure S6.
We used differentially expressed genes and marker genes (Figures S6A–S6D) as well as recently published single-cell datasets of the mouse inner ear24,27,28,55,56 to annotate the populations of the integrated inner ear dataset (Figure 3C). We identified a mesenchymal population, various epithelial populations of both cochlear and vestibular identity, hair cells, neurons, glial cells, cycling cells, endothelial cells, macrophages, and melanocytes (Figures 3C and S6). To confirm the vestibular versus the cochlear identity of the epithelial cell population, we analyzed the differential gene expression between the putative cochlear duct floor and vestibular supporting cells (Figure 3D). ADAMTSL1, MEIS2, EBF3, and ERBB4 were genes highly expressed within vestibular populations, in contrast to GATA3, NR2F1, and SULF1 which are present in cochlear populations, as has been shown on a single-cell level for the developing mouse inner ear.27,56 In addition, the cochlear-specific RORB is expressed in the cochlear duct floor (Figure 3E).56,57 The cochlear cell types could be separated (Figure 3E) into a medial (TECTA, FGF10, and JAG1), prosensory (ISL1, LGR5, SOX2, and FGF20) and lateral domain of the duct floor (BMP4 and GATA3) as well as the cochlear roof (OTX2, FGF9, WNT4, and GSC), based on data from the developing mouse cochlea.56,58–63 The vestibular population is composed of putative supporting cells (OTOGL and USH1C) with a crista-associated ZPLD1+ supporting cell population, roof cells (NTN1, SMOC2, and WNT3) and dark cells (KCNE1, ATP1B2, and SPP1) expressing marker genes that have been described for the developing mouse vestibular organs.27,55 Moreover, our data unveiled the presence of a distinct cluster of epithelial cells exhibiting a vestibular signature. Despite analyses, we were unable to ascertain their precise identity and thus designated them as “epithelial cells.” It is plausible that this population could comprise canal epithelium, which lacks a well-defined signature in existing literature. The remaining epithelial cluster was composed of a mixed population of endolymphatic duct/sac cells (WNT2B) and vestibular transitional cells (OC90 and ALDH1A3).27,64 We validated a subset of differentially expressed genes and marker genes in an FW9 human fetal inner ear (Figure S6E). In the utricle, we confirmed spotty expression of LGR6 specifically in the vestibular sensory domain, which also contains MYO7A+ hair cells. Additionally, we observed OC90+ DACH1− transitional cells and OC90+ DACH1+ dark cell epithelium. In the cochlea, we confirmed the presence of the GATA3+ SOX2+ prosensory domain, as well as TECTA+ expression in the tectorial membrane and apical expression in the medial domain of the duct floor. We also identified GATA3+ SOX2− expression in the lateral duct floor, and OC90+ OTX2+ expression in the roof. Taken together, our IHC findings validate the expression of differentially expressed genes and marker genes in the human fetal inner ear, further supporting the accuracy of our annotations.
Within the mesenchymal population, chondrocytes and a large cluster of POM cells were identified. The chondrocytes were mostly identified in the FW7.5 inner ear, as during preparation of this sample the otic capsule was not removed. Similar to the developing mouse vestibular organs, different cell populations could be identified in the remaining mesenchymal population, with a transcriptomic difference between epithelium-associated mesenchyme and capsule-associated mesenchyme.27 We observed capsule-associated POM (DCN, GPC3, and COL3A1) clustering separate from the epithelium-associated POM (COCH, POU3F4, OTOR, APOD, and TBX18). We were not able to distinguish between the development of vestibular and cochlear mesenchyme, because of the unavailability of reference data. Nonetheless, our dataset represents a single-cell level analysis of early-stage inner ear development in combination with human adult vestibular organs. As such, it is a valuable resource not only for organoid comparative analysis, but also for the broader scientific community seeking to understand the complex mechanisms underlying inner ear development.
POM within the IEOs is associated with the sensory epithelium
To assess how IEOs resemble the human inner ear, we used Symphony, a tool for single-cell reference mapping.65 Using this R package, IEO-derived cell types were mapped as a query to the reference atlas of the human inner ear. We performed these analyses separately for the otic mesenchymal, epithelial, and hair cell populations.
We mapped the putative POM cluster present in the IEO dataset to the human inner ear atlas reference (Figures 4A and 4B). A large overlap was present with the reference POM cluster, more specifically with the epithelium-associated POM. Expression of CRYM, OTOR, COCH, and POU3F4 within the IEO-derived POM confirmed the epithelium-associated identity (Figure 4C). The other region overlapping was composed of POM that was found in the human fetal inner ears (Figure S6B). We did not observe any overlap with the capsule-associated POM from the reference human inner ear atlas. To confirm the presence of the epithelium-associated POM, we performed additional IHC analyses of D75 IEOs. Surrounding inner ear vesicles containing MYO7A+ hair cells, we noticed the presence of OTOR+ POU3F4+ POM (Figure 4D). These OTOR-expressing cells resembled the OTOR+ mesenchymal cells in vivo that directly underlie the fetal vestibular sensory epithelium (Figure 4E).
Figure 4. IEO-derived POM resembles human fetal epithelium-associated POM.
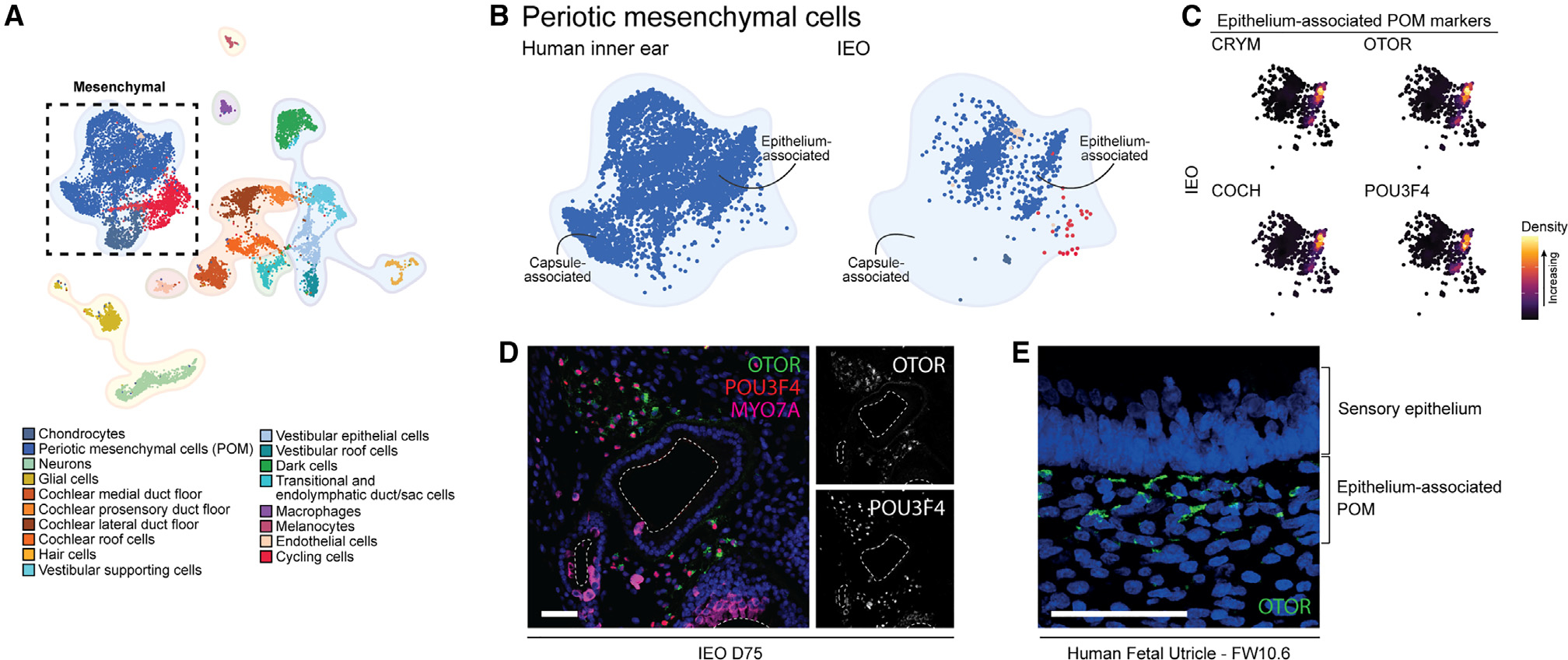
(A) UMAP plot of annotated human inner ear atlas highlighting mesenchymal cell types.
(B) UMAP plot comparing reference POM (left) and mapped IEO-derived POM population (right) using Symphony.
(C) Density plots showing expression of epithelium-associated markers in the IEO-derived POM population.
(D) OTOR+ POU3F4+ mesenchymal cells surrounding IEO-vesicles containing MYO7A+ hair cells at D75. Scale bar, 50 μm.
(E) OTOR+ mesenchymal cells beneath fetal utricle sensory epithelium (FW10.6). Scale bar, 20 μm.
POM, periotic mesenchyme. IHC images are representative of n ≥ 6 aggregates (≥2 experiments) (D) or n ≥ 2 human inner ear tissues of matching fetal age (E).
IEOs contain immature type I and type II vestibular hair cells
IEO-derived hair cells were mapped to hair cells of the human inner ear atlas reference using Symphony (Figures 5A and 5B). We do not expect that any cochlear hair cells were captured in the human inner ear atlas, as these arise only by FW10 of fetal development.66,67 Within the reference hair cells, fetal developing hair cells clustered separately from the adult hair cells (Figures 5B and 6B). Immature fetal hair cells expressed SOX2, ATOH1, and ANXA4 (Figure 5C). Additionally, two distinct adult hair cell populations were present, showing specific expression for markers of type I and type II vestibular hair cells, OCM68 and ANXA4,69 respectively (Figure 5C). The IEO-derived hair cells overlapped with the developing immature hair cells and expressed SOX2 and ATOH1 (Figures 5B and 5C).
Figure 5. Immature type I and type II vestibular hair cells in IEOs.
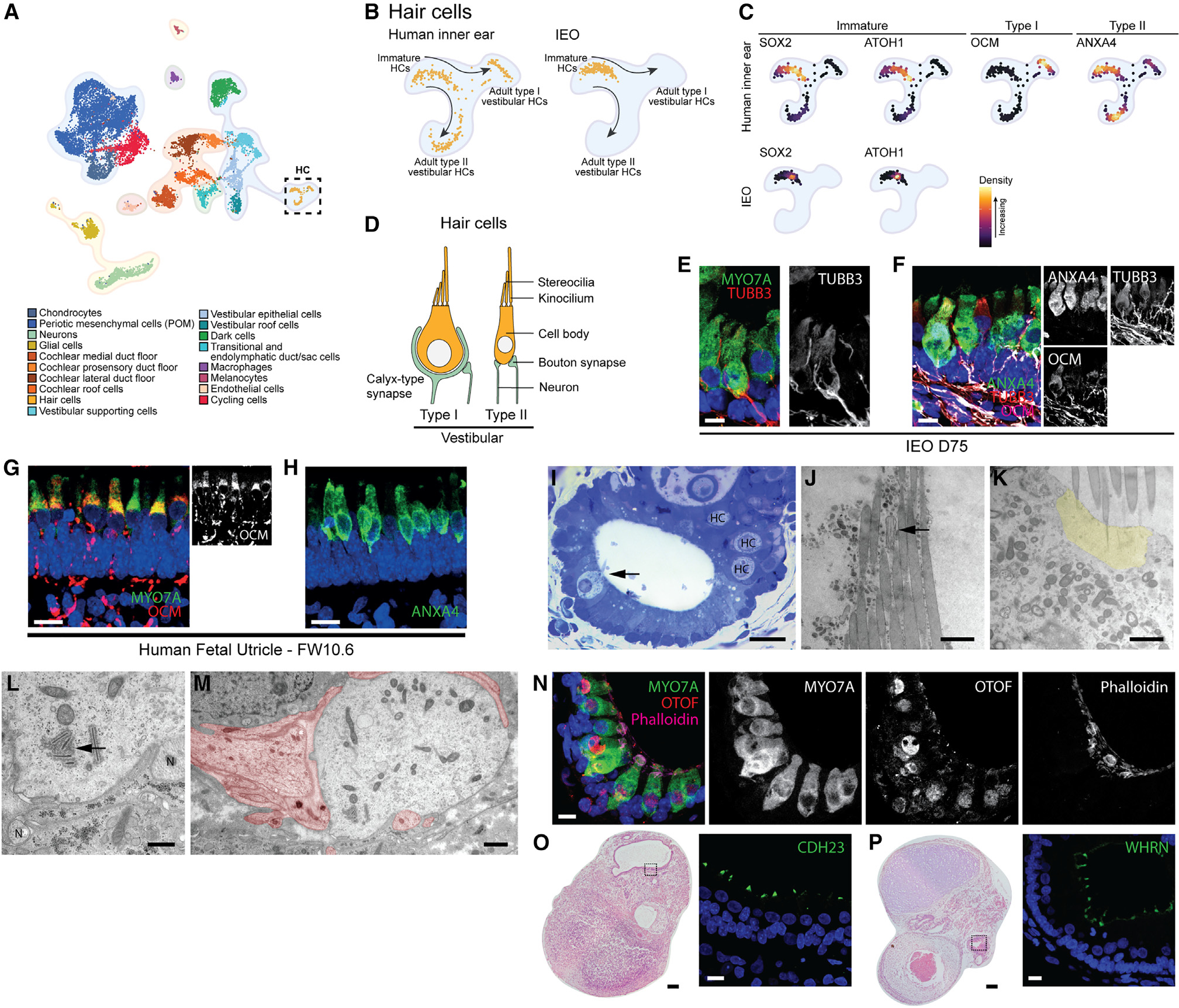
(A) UMAP plot of annotated human inner ear atlas highlighting hair cells (HCs).
(B) UMAP plot comparing reference hair cell population (left) and mapped IEO-derived hair cell population (right) using Symphony.
(C) Density plots of reference hair cell population (left) for immature, type I, and type II vestibular markers, and density plots of mapped IEO-derived hair cells showing expression of immature markers.
(D) Schematic depicting morphological differences between type I and type II vestibular hair cells.
(E) Calyx-type synapse (TUBB3+) surrounding a hair cell (MYO7A+). Scale bar, 10 μm.
(F) Expression of ANXA4 and OCM in hair cells of a D75 IEO, showing subsets with none, either, or both. Scale bar, 10 μm.
(G) OCM expression in hair cells of human fetal utricle (FW10.6). Scale bar, 10 μm.
(H) ANXA4 expression in human fetal utricle (FW10.6). Scale bar, 10 μm.
(I) Methylene blue-azure II staining of a D74 IEO-vesicle containing hair cells (arrow and “HC”). Scale bar, 20 μm.
(J) Hair bundle with stereocilia and kinocilium cross-section (arrow). Scale bar, 1 μm.
(K) Apical region of a hair cell with cuticular plate (yellow) and stereociliary rootlets. Scale bar, 1 μm.
(L) Basal region of a hair cell showing synaptic ribbons (arrow) and bouton-like synapses (“N”). Scale bar, 500 nm.
(M) Transection of type I vestibular hair cell almost entirely surrounded by calyx-type synapse (in red). Scale bar, 500 nm.
(N) OTOF expression in MYO7A+ hair cells. Scale bar, 10 μm.
(O) H&E overview with CDH23 in stereocilia by IHC. Scale bars: H&E, 100 μm; IHC, 20 μm.
(P) H&E overview with WHRN in stereocilia by IHC. Scale bars: H&E, 100 μm; IHC, 20 μm.
H&E and IHC images are representative of n ≥ 6 aggregates (≥2 experiments) (E, F, and N–P) or n ≥ 2 human inner ear tissues of matching fetal age (G and H). TEM images are representative of n R 3.
Figure 6. Endolymphatic duct/sac, cochlear epithelia, and vestibular supporting cells in IEOs.
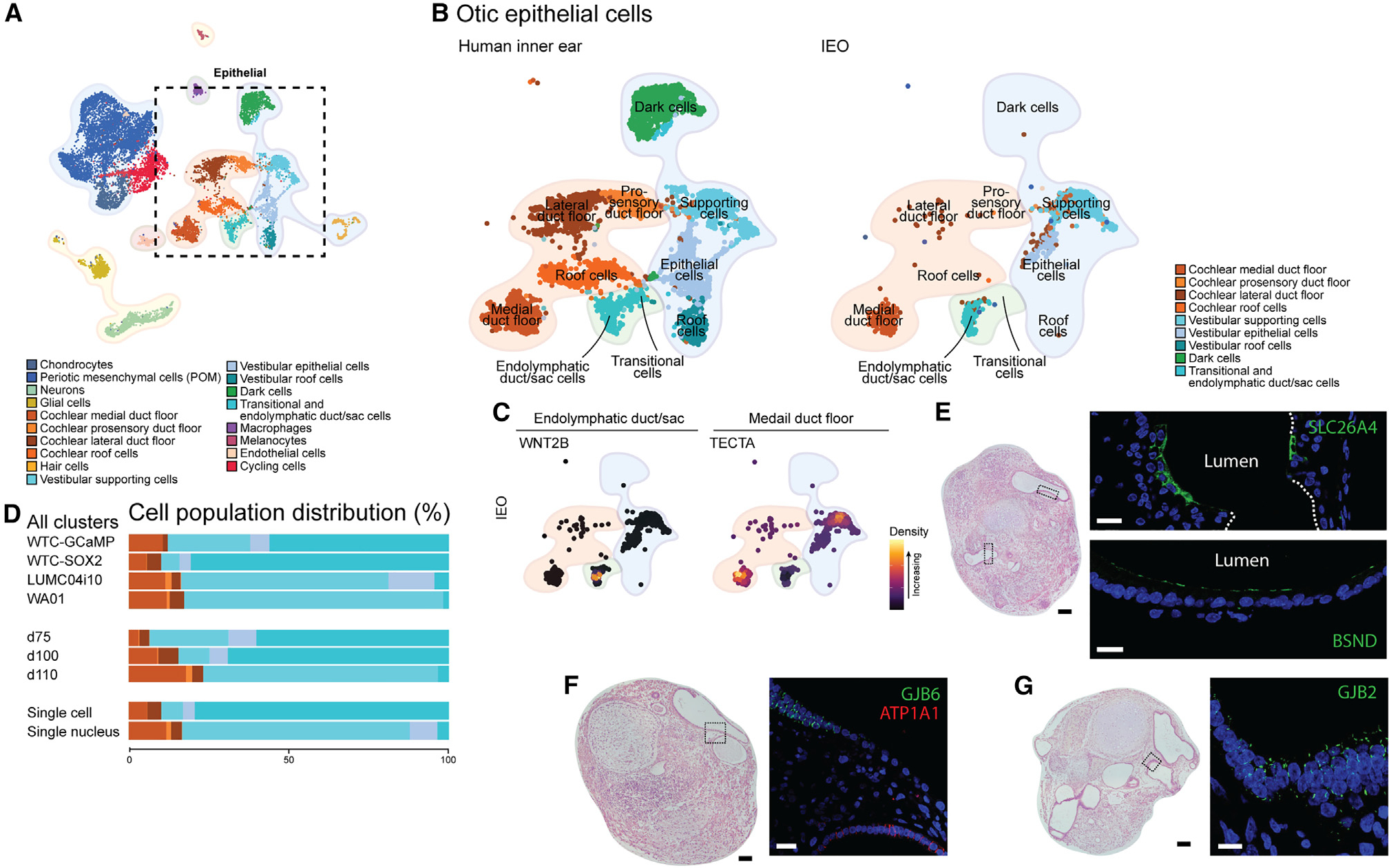
(A) UMAP plot of annotated human inner ear atlas highlighting epithelial cell types.
(B) UMAP plot comparing reference epithelial cell populations (left) and mapped IEO-derived otic epithelium population (right) using Symphony.
(C) Density plots of IEO-derived epithelial populations showing expression of WNT2B (endolymphatic duct/sac marker) and TECTA (medial duct floor marker).
(D) Relative contribution of cell types per cell line, time point, and technique used.
(E) H&E overview with expression of SLC26A4 and BSND in the highlighted area (D75). Scale bars: H&E, 100 μm; IHC, 20 μm.
(F) H&E overview and localized expression of GJB6 and ATP1A1 within the highlighted area (D75 IEO). Scale bars: H&E, 100 μm; IHC, 20 μm.
(G) H&E overview and expression of GJB2 within the highlighted epithelium (D75 IEO). Scale bars: H&E, 100 μm; IHC, 20 μm.
H&E and IHC images are representative of n ≥ 6 aggregates (≥2 experiments) (E–G).
Although the fetal immature vestibular hair cells cluster together (Figure 5B and S6B), a distinction can be made between developing type I and type II vestibular hair cells at FW9.54 Type I and type II vestibular hair cells can be distinguished by their shape and the type of synapse (Figure 5D). We identified calyx-type synapses (TUBB3+) in D75 IEOs (Figure 5E), which are typically associated with type I vestibular hair cells. Although we observed these presumptive type I hair cells in IEOs, they did not exhibit the characteristic “neck” structure, which is a hallmark of more mature type I hair cells and develops after birth in mice.70 It is possible that the IEO-derived hair cells were still immature at the time of our analysis. Additionally, within IEOs we could identify ANXA4+ hair cells, a known marker for developing hair cells and type II vestibular hair cells, with some of these hair cells also expressing OCM, a marker for type I vestibular hair cells (Figure 5F). This expression is similar to the human fetal utricle (Figures 5G and 5H). Additionally, we performed light-microscopical and ultrastructural analyses of the hair cells present in IEOs. The IEOs consisted of an open lumen lined by a layer of columnar cells with small, densely stained nuclei in their basal part (Figure 5I). Interspersed between these epithelial cells are flask-shaped cells with a large, round nucleus and from which a large hair bundle protrudes into the lumen. The morphology of these cells closely resembles that of vestibular hair cells. Using TEM, the flask-like cells contain a large hair bundle, in which the individual stereocilia are of varying length (Figure 5J). These stereocilia are inserted into the cuticular plate in the apical part of the hair cell as is evident by the presence of stereociliary rootlets (Figure 5K). At the opposite pole of these hair cells, frequently seen features were synaptic ribbons which are oriented opposite the bouton-type synapses associated with the basal membranes of the hair cells (Figure 5L). As these synapses did not contain any vesicles it may be surmised that these are afferent neurons. The observation that these cells were associated with bouton-type synapses seemed to indicate that they were type II vestibular hair cells.18 Other hair cells, however, resembled the calyx-type synapse seen with type I vestibular hair cells (Figure 5M).
Further analyses revealed that these immature vestibular hair cells express proteins of genes linked to SNHL. In addition to MYO7A (DFNA11, DFNB2, and Usher syndrome type I), OTOF (DFNB9), CDH23 (DFNB12 and Usher syndrome type I), and WHRN (DFNB31) are present within the hair cell or its stereocilia.
Cochlear and endolymphatic duct/sac epithelium induced in IEOs
On the basis of previous work, the IEOs are assumed to have a vestibular sensory epithelium containing supporting cells.18 In line with this hypothesis, most IEO-epithelial populations were mapped to the vestibular supporting cells and epithelial cells, with a total lack of vestibular dark cells and roof cells (Figures 6A and 6B). However, IEO-derived epithelial populations also overlapped with the endolymphatic duct/sac cells, expressing WNT2B (Figure 6C). Furthermore, a large overlap is seen with the cochlear medial duct floor, to a lesser degree for the cochlear lateral duct floor, where it is almost absent for the prosensory domain and cochlear roof cells. Marker gene expression of TECTA confirmed the medial duct floor-like population within the IEOs (Figure 6C). TECTA expression in the vestibular supporting cells may be explained by its temporal expression in the vestibular organs.71 The presence of these populations was consistent over cell lines and time points, although in varying degrees (Figure 6D).
Using IHC, we were able to show the presence of SLC26A4 and BSND, similar to the developing human inner ear (Figures 6E and S6E). Mutations in SLC26A4 may cause SNHL in DFNB4 and Pendred syndrome, and mutations in BSND can lead to DFNB73. Within the IEO epithelia we were able to show additional expression of ATP1A1, GJB6, and GJB2 (Figures 6F and 6G). Mutations in GJB2 are the most common cause of nonsyndromic SNHL causing DFNA3A and DFNB1A. Within epithelia these connexin-coding genes were expressed together with GJB6 that causes DFNA3B. These proteins are in vivo expressed in a spatiotemporal manner in the inner ear epithelia, including the human medial cochlear duct floor47 and sensory vestibular epithelia.72 Moreover, ATP1A1, implicated in age-related hearing loss,73 has a spatiotemporal expression during the development of the inner ear,46,66 and shows epithelial expression within IEOs (Figure 6F).
Cell-cell communication analysis highlights interactions between endothelial cells and hair cells
To better understand interactions between cell types in the IEO, we performed a cell-to-cell communication analysis using Cell-Chat.74 This R package computes signaling pathway interactions between cell types using a database of known interactions among ligands, receptors, and cofactors. A total of 223 signaling pathways were analyzed, of which CellChat determined 85 pathways significantly engaged within the IEOs (Table S1). These include signaling pathways that are known to be essential for the inner ear and neurosensory development: BMP, FGF, NOTCH, TGFβ and WNT signaling (Figure S7A).75–78 By delving deeper, we identified known ligand-receptor interactions within the IEOs (Figure 7A). For instance, the interaction of NECTIN3 with NECTIN1 and/or NECTIN2 between supporting cells and hair cells contributes to the arrangement of the sensory epithelium.79 Also the interactions between JAG1, JAG2, or DLL1 with NOTCH1, NOTCH2, or NOTCH3 between these same cell types are known mechanisms by which NOTCH signaling contributes to hair cell formation.80–83 Additionally, the POM, a crucial component for hair cell differentiation,84 demonstrates communication with hair cells and supportive cells, highlighting the role of BMP signaling, in interacting to known receptors within the sensory epithelium.85 These ligand-receptor interactions of the NECTIN, NOTCH, and BMP pathways could be validated in the fetal inner ear data (Figure 7A). Within these interactions, we observed consistently higher p values in the fetal inner ear data compared with the IEO data. We hypothesize that this observation may be attributed to the difference in experimental modalities, as the former uses snRNA-seq data while the latter combines snRNA-seq and scRNA-seq. As the CellChat analysis revealed these expected interactions in the IEOs, we were confident that this approach could be used to identify novel interactions. We noted that the vascular endothelial cells showed a high number of interactions with other cell types, including hair cells and the other otic cell types (Figure 7B). By IHC, we found endothelial cells (PECAM1+) throughout the IEO aggregates, including locations near in the mesenchyme adjacent to hair cell-containing (MYO7A+) epithelia, similar to the developing fetal sensory epithelium (Figure 7C). To evaluate the interactions that might contribute to hair cell development, we focused on the interactions between endothelial cells and hair cells and found 52 relevant signaling pathways (Figure S7B). We subsequently analyzed the ligand-receptor interactions within these signaling pathways and validated these by using the ligand-receptor interactions in the fetal data of the human inner ear atlas (Figures S7C and S7D). These validated interactions include interactions in the AGRN, ANGPT, ANGPTL, CD39, HSPG, NT, and NRXN signaling pathways, suggesting that these play a role in formation of specific cell types in the IEOs, including endothelial cells and hair cells (Figure 7D). The close proximity of endothelial cells to hair cells, the number of interactions between these cell types, as well as the validated ligand-receptor interactions, are indicative for a strong association in proper sensory development.
Figure 7. Cell-cell communication analysis of IEOs focusing on hair cells and endothelial cells.
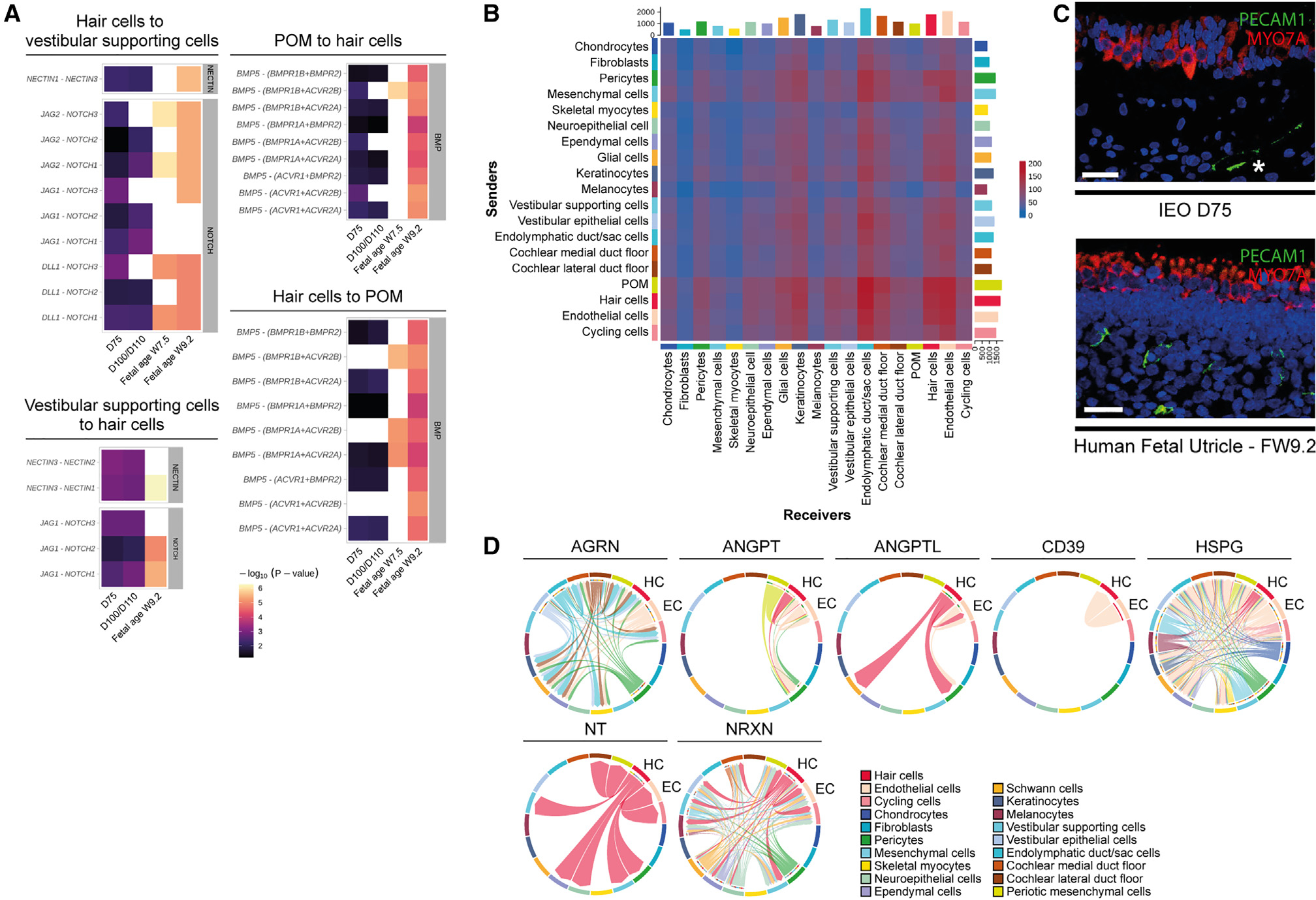
(A) Ligand-receptor interactions in NECTIN, NOTCH, and BMP signaling pathways. Validated in human inner ear atlas (FW7.5 and/or FW9.2).
(B) Heatmap showing total interactions between cell types. x axis, receivers (with receptors); y axis, senders (with ligands).
(C) PECAM1+ endothelial cells underlying sensory epithelium with MYO7A+ hair cells in D75 IEO (upper) and FW9.2 fetal utricle (lower). Images are representative of n ≥ 6 aggregates (≥2 experiments) or n ≥ 2 human inner ear tissue with matching fetal age. Scale bars, 50 μm.
(D) Chord diagrams displaying interactions between cell types in IEOs. Arrows indicate sender to receiver cell types. POM, periotic mesenchyme.
IEOs express SNHL genes and proteins
IEOs are valuable for disease modeling, including syndromic and nonsyndromic SNHL which has been associated with more than 150 genes. We examined whether these genes are expressed in the appropriate cell types of the IEO model. Most SNHL genes are linked to specific inner ear cell types.86,87 We created a comprehensive summary table of gene/protein expression for 158 genes (Table S2). Using heatmaps and protein expression, we developed a scoring system to determine the suitability of studying a gene of interest using IEOs (Table S2; Figure S8). Sixty-seven of 158 genes showed expression in IEOs in the correct cell populations. Furthermore, because the IEOs display predominantly vestibular-like features, we identified whether a given gene had been linked to vestibular phenotypes. With modified induction techniques, the list of target genes in IEOs is expected to expand in future studies, including cochlear supporting cells and hair cells. Together, our data provide a powerful resource for the community to design and plan SNHL modeling studies using IEOs.
DISCUSSION
In this study, we differentiate multiple hPSC lines to induce inner ear cell types and used single-cell transcriptomics to evaluate aggregates up to 110 days in culture. We also created a human inner ear single-cell atlas containing data from fetal and adult inner ear tissue. Our data show that differentiation efficiency of IEOs is largely dependent on the initial BMP-4 concentration and can be determined by epithelial thickness at D3. The change of this epithelial thickness following BMP-4 treatment resembles the ectoderm thickness at the gastrula stage which is controlled by BMP activity (Figure S1B).76,88,89 Using efficiently differentiated aggregates, we discovered the presences of additional off-target cell types (cranial skeletal myocytes, ependymal cells, vascular endothelial cells) and show that most of the cell types have fetal gene expression signatures. These cell types could potentially be used by adaption of the current protocol, similar to the generation of hair-bearing skin organoids.38 Likewise, the on-target formation of otic epithelium and POM within the IEOs show overlap with the fetal transcriptomic data.
Using the human inner ear atlas, we determined that IEOs primarily consist of vestibular hair cells, vestibular supporting and epithelial cells, and a relatively smaller contribution of endolymphatic duct/sac cells and cochlear cell types. Interestingly, other nonsensory and cochlear prosensory cells are lacking, indicating that specific inner ear cell types are induced. The vestibular epithelia and endolymphatic duct/sac cells are derived from the dorsal otic vesicle, with the formation of vestibulocochlear ganglion-like neurons and cochlear epithelium from the anterior-ventral part of the otic vesicle.76,90,91 Although cochlear-like epithelium is present in the IEOs, the prosensory development is lacking, possibly because of insufficient BMP activity63 which is not required for proper vestibular sensory formation.60 Manipulating BMP and retinoic acid, to induce nonsensory epithelia,92 may lead to more patterned IEOs with a clinically relevant cell type heterogeneity. Additionally, the IEOs up to D110 resemble the human inner ear at approximately FW9: displaying immature vestibular hair cells, development of specific cochlear domains and vestibular cell types, and the presence of melanocytes surrounding the otic epithelia.46 Using the endothelial population and media flow could enhance maturation similar to kidney organoids.93 Alternatively, prolonged (>D110) cell cultures in which off-target induction is addressed, could result in a more mature phenotype. Currently, off-target cell types pose a challenge for their use in pre-clinical studies, as it hampers prolonged culture and inhibits inner ear epithelia maintenance.
In addition to the IEO single-cell data, we present the human inner ear atlas containing vestibular and cochlear tissue from the first trimester as well as adult vestibular tissue. No single-cell reference of the human inner ear was available, as recent work using the transcriptomics approaches is focused on mouse inner ears24,27,55,94,95 or limited to human cochlear tissues.28 Future research efforts aim to expand on the human inner ear atlas in order to generate additional resources for understanding inner ear development and interrogating development in the context of genetic SNHL. Current knowledge of these pathophysiological mechanisms is often missing, biased toward cochlear neurosensory epithelium, and disregards the spatiotemporal expression of genes during inner ear development. Additionally, there is increasing awareness for vestibular dysfunction associated with SNHL.96 This paper motivates the importance of human inner ear atlases containing the whole inner ear throughout different stages of development.
Using both the IEO and human inner ear single-cell data, we observed known cell-cell interactions within the IEOs that contribute to neurosensory development. Additional computed interactions highlighted the potential role of endothelial cells in hair cell development. Endothelial cells have been implicated in neurosensory development in zebrafish97 and influence organoid formation of kidney and intestine.93,98,99 We identified signaling pathways, such as AGRN and NT signaling, that are involved in otic vesicle development100–103 and hair cell development,104,105 respectively. However, their interaction with endothelial cells in the inner ear has not been previously described. For other signaling pathways (ANGPT, ANGPTL, CD39, HSPG, and NRXN), limited literature exists on their involvement in hair cells,106–110 but these have functions in endothelial homeostasis, blood-brain barrier function, or renal glomerular integrity.111–117 One might surmise their interaction in inner ear endothelial integrity with the interaction between hair cells and endothelial cells being important for their development and subsequent function. These results show how IEOs are a promising model system to further investigate developmental inner ear signaling pathways.
Although diversity or maturity of the human inner ear is not fully captured, IEOs could potentially be used for disease modeling. Based on protein and gene expression, a promising SNHL cause to model is, for instance, congenital Usher syndrome in which the function of hair cells, in both the cochlea and vestibular organs is affected. Within the epithelium also DFNA3A and DFNB1A (GJB2) as well as DFNA3B (GJB6) can be modeled. Additionally, DFNA9 (COCH), DFNA40 (CRYM), and DFNX2 (POU3F4), all of which affect the function of POM, can potentially be modeled within the IEOs. These are examples of numerous other gene targets that could be evaluated in IEOs. The ability to use the IEOs for disease modeling provides a broadly applicable framework to develop cell or gene therapies for genetic syndromic and nonsyndromic SNHL.
Limitations of the study
There are several limitations to our study. First, we could not perform a thorough analysis of cell line differences or batch-to-batch analysis, because of the methods used to analyze the IEOs (scRNA-seq or snRNA-seq). Second, minor cell populations might not be detected in samples of <10,000 cells or nuclei captured per experiment. Third, merging the single-cell and single-nucleus data required correction for mitochondrial and ribosomal content between methods. Furthermore, our human inner ear atlas is based on a limited number of samples, and further developmental stages need to be included to create a more comprehensive and accurate atlas. However, our integration approach can be used as new datasets are published. Additionally, different expression patterns between the single-cell and single-nucleus data were observed when analyzing gene expression. Last, because of shallow sequencing depth, we could not provide a thorough insight into transcription factors as cell identity drivers. Future datasets combining single-cell transcriptomics with spatial biology or chromatin state would allow more insight into inner ear development. We encourage fellow researchers to use our dataset and human inner ear atlas for additional discoveries.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wouter H. van der Valk (w.h.van_der_valk@lumc.nl).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Single-cell and single-nucleus RNA-seq data have been deposited the Gene Expression Omnibus and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. The data can also be accessed through a data exploration platform hosted by the Expression Analysis Resource,124 available at https://umgear.org/p?l=hIEOandInnerEar.
This paper does not report original code. Scripts used for scRNAseq and snRNAseq of IEOs analysis are available at https://github.com/Koehler-Lab/, scripts used for the human inner ear atlas are available at https://github.com/OtoBiologyLeiden/.
Any additional information required to reanalyze data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-ACTC1 (1:50) | R&D systems | Cat#: MAB9308-100 |
| Mouse monoclonal anti-ANXA4 (1:200) | Sigma-Aldrich | Cat#: SAB4200121; RRID: AB_10621934 |
| Mouse monoclonal anti-ATP1A1 (1:200) | Novus Biologicals | Cat#: NB300-146; RRID: AB_2060981 |
| Rabbit polyclonal anti-BSND (1:750) | Novus Biologicals | Cat#: NBP2-49101 |
| Mouse monoclonal anti-CDH1 (1:50) | BD Transduction Laboratories | Cat#: 610182; RRID: AB_397581 |
| Rabbit polyclonal anti-CDH23 (1:50) | Invitrogen | Cat#: PA5-53564; RRID: AB_2639592 |
| Rabbit polyclonal anit-DACH1 (1:50) | Invitrogen | Cat#: PA5-52968; RRID: AB_2640365 |
| Mouse monoclonal anti-GATA3 (1:500) | BD Transduction Laboratories | Cat#: 558686; RRID: AB_2108590 |
| Rabbit polyclonal anti-GJB2 (1:100) | Alomone Labs | Cat#: ACC-212; RRID: AB_11124274 |
| Rabbit polyclonal anti-GJB6 (1:100) | Invitrogen | Cat#: PA5-11640; RRID: AB_2111053 |
| Rabbit monoclonal anti-LGR6 (1:100) | Abcam | Cat#: ab126747; RRID: AB_11132458 |
| Mouse monoclonal anti-MLANA (1:50) | Abcam | Cat#: ab731; RRID: AB_305836 |
| Rabbit polyclonal anti-MYO7A (1:100) | Proteus Biosciences | Cat#: 25-6790; RRID: AB_10015251 |
| Rabbit monoclonal anti-MYO7A (1:50) | Novus Biologicals | Cat#: NBP1-84266; RRID: AB_11016173 |
| Mouse monoclonal anti-MYO7A (1:30) | DSHB | Cat#: 138-1s; RRID: AB_2282417 |
| Mouse monoclonal anti-MYOG (1:10) | DSHB | Cat#: F5D; RRID: AB_2146602 |
| Rabbit polyclonal anti-OC90 (1:100) | Invitrogen | Cat#: PA5-71564; RRID: AB_2717418 |
| Rabbit polyclonal anti-OCM (1:200) | Abcam | Cat#: ab150947 |
| Rabbit polyclonal anti-OTOF (1:100) | Novus Biologicals | Cat#: NBP1-46574; RRID: AB_10009850 |
| Rabbit polyclonal anti-OTOR (1:100) | Invitrogen | Cat#: PA5-55053; RRID: AB_2645111 |
| Goat polyclonal anti-OTX2 (1:20) | R&D systems | Cat#: AF1979; RRID: AB_2157172 |
| Goat polyclonal anti-PDGFRB (1:15) | R&D systems | Cat#: AF385; RRID: AB_355339 |
| Mouse monoclonal anti-PECAM1 (1:50) | Dako | Cat#: M082329-2 |
| Alexa Fluor™ 568 phalloidin | Invitrogen | Cat#: A12380 |
| Chicken polyclonal anti-POU3F4 (1:10000) | Aves Labs | Cat#: AB_2814704; RRID: AB_2814704 |
| Rabbit polyclonal anti-SIX1 (1:100) | Invitrogen | Cat#: PA5-51654; RRID: AB_2647319 |
| Rabbit polyclonal anti-SLC26A4 (1:100) | Novus Biologicals | Cat#: NBP1-85237; RRID: AB_11032075 |
| Mouse monoclonal anti-SOX2 (1:100) | BD Transduction Laboratories | Cat#: 561469; RRID: AB_10694256 |
| Goat polyclonal anti-SOX10 (1:50) | Invitrogen | Cat#: PA5-47001; RRID: AB_2608449 |
| Rabbit polyclonal anti-TECTA (1:200) | Invitrogen | Cat#: PA5-80102; RRID: AB_2747217 |
| Rabbit monoclonal anti-TFAP2A (1:50) | Abcam | Cat#: ab108311; RRID: AB_10861200 |
| Rabbit polyclonal anti-TNNT1 (1:500) | R&D systems | Cat#: NBP2-38855 |
| Rabbit monoclonal anti-TTR (1:50) | Invitrogen | Cat#: MA5-32634; RRID: AB_2809911 |
| Mouse monoclonal anti-TUBB3A (1:100) | Biolegend | Cat#: 801202; RRID: AB_10063408 |
| Mouse monoclonal anti-TUBB3A (1:100) | R&D Systems | Cat#: MAB1195; RRID: AB_357520 |
| Mouse monoclonal anti-WRHN (1:50) | Abnova | Cat#: H00025861-M05; RRID: AB_10629381 |
| Donkey anti-mouse Alexa Fluor™ 488 (1:1000–2000) | Invitrogen | Cat#: A21202; RRID: AB_141607 |
| Donkey anti-mouse Alexa Fluor™ 568 (1:1000–2000) | Invitrogen | Cat#: A10037; RRID: AB_2534013 |
| Donkey anti-mouse Alexa Fluor™ 594 (1:1000–2000) | Invitrogen | Cat#: A21203; RRID: AB_141633 |
| Donkey anti-mouse Alexa Fluor™ 647 (1:1000–2000) | Invitrogen | Cat#: A31571; RRID: AB_162542 |
| Donkey anti-mouse Alexa Fluor™ 680 (1:1000–2000) | Invitrogen | Cat#: A10038; RRID: AB_2534014 |
| Goat anti-mouse IgG1 Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21121; RRID: AB_2535764 |
| Goat anti-mouse IgG1 Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21124; RRID: AB_2535766 |
| Goat anti-mouse IgG1 Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21240; RRID: AB_2535809 |
| Goat anti-mouse IgG2a Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21131; RRID: AB_2535771 |
| Goat anti-mouse IgG2a Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21134; RRID: AB_2535773 |
| Goat anti-mouse IgG2a Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21241; RRID: AB_2535810 |
| Goat anti-mouse IgG2b Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21141; RRID: AB_2535778 |
| Goat anti-mouse IgG2b Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21144; RRID: AB_2535780 |
| Goat anti-mouse IgG2b Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21242; RRID: AB_2535811 |
| Donkey anti-rabbit Alexa Fluor™ 488 (1:1000) | Abcam | Cat#: ab150061; RRID: AB_2571722 |
| Donkey anti-rabbit Alexa Fluor™ 488 (1:1000–2000) | Invitrogen | Cat#: A21206; RRID: AB_2535792 |
| Donkey anti-rabbit Alexa Fluor™ 568 (1:1000–2000) | Invitrogen | Cat#: A10042; RRID: AB_2534017 |
| Donkey anti-rabbit Alexa Fluor™ 594 (1:1000–2000) | Invitrogen | Cat#: A21207; RRID: AB_141637 |
| Donkey anti-rabbit Alexa Fluor™ 647 (1:1000–2000) | Invitrogen | Cat#: A31573; RRID: AB_2536183 |
| Donkey anti-rabbit Alexa Fluor™ 680 (1:1000–2000) | Invitrogen | Cat#: A10043; RRID: AB_2534018 |
| Donkey anti-goat Alexa Fluor™ 488 (1:1000) | Abcam | Cat#: ab150133; RRID: AB_2832252 |
| Goat anti-chicken Alexa Fluor™ 594 (1:1000) | Invitrogen | Cat#: A11042; RRID: AB_2534099 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Stempro Accutase Cell Dissociation Reagent | Gibco | Cat#: A1110501 |
| Y27632 | Stemgent | Cat#: 04-0012-02 |
| Essential 8 Flex medium | Gibco | Cat#: A2858501 |
| Vitronectin | Gibco | Cat#: A14700 |
| Normocin | Invivogen | Cat#: ant-nr-1 |
| EDTA | Gibco | Cat#: 15575020 |
| E6 medium | Gibco | Cat#: A1516401 |
| Matrigel Growth Factor Reduced | Corning | Cat#: 354230 |
| SB431542 | Stemgent | Cat#: 04-0010-05 |
| basic FGF | Peprotech | Cat#: 100-18B |
| BMP-4 | Peprotech | Cat#: 120-05 |
| BMP-4 | Stemgent | Cat#: 03-0007 |
| BMP-4 | R&D | Cat#: 314-BP |
| BMP-4 | R&D | Cat#: 314-BPE |
| LDN | Stemgent | Cat#: 04-0074-02 |
| CHIR99021 | Stemgent | Cat#: 04-0004-02 |
| Advanced DMEM:F12 medium | Gibco | Cat#: 12634010 |
| Neurobasal medium | Gibco | Cat#: 21103049 |
| B27 Supplement without vitamin A | Gibco | Cat#: 12587010 |
| N2 Supplement | Gibco | Cat#: 17502048 |
| Glutamax | Gibco | Cat#: 35050061 |
| β-Mercaptoethanol | Gibco | Cat#: 21985023 |
| Low Melting Point Agarose | Invitrogen | Cat#: 16520050 |
| PBS | Gibco | Cat#: 14190144 |
| TrypLE | Gibco | Cat#: 12605036 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#: A7030 |
| Trypan Blue | Gibco | Cat#: 15250061 |
| Tris-HCl | Millipore-Sigma | Cat#: T2194 |
| NaCl | Millipore-Sigma | Cat#: 59222C |
| MgCl2 | Millipore-Sigma | Cat#: M1028 |
| Nonidet P40 Substitute | Millipore-Sigma | Cat#: 74385 |
| DEPC-treated water | Invitrogen | Cat#: 750024 |
| Rnase inhibitor | Millipore-Sigma | Cat#: 3335402001 |
| RNAlater | Invitrogen | Cat#: AM7020 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Chromium Next GEM Single Cell 3' | 10X Genomics | Cat#: PN-1000128 |
| Library & Gel Bead Kit v3.1 | ||
| Chromium Next GEM Single Cell 3' | 10X Genomics | Cat#: PN-120267 |
| Library & Gel Bead Kit v2 | ||
| Anti-Nuclear Pore Complex Proteins | Merck | Cat#: MAb414 |
| Fc-blocking reagent | Biolegend | Cat#: 422302 |
|
| ||
| Deposited data | ||
|
| ||
| RNA sequencing data IEO | This manuscript | GEO: GSE214099 |
| RNA sequencing data human inner ear | This manuscript | GEO: GSE213796 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| WA01 ESC line | WiCell | N/A |
| AICS-0074 iPSC line (WTC-SOX2) | Allen Institute | N/A |
| WTC-GCaMP iPSC line | Bruce Conklin at the Gladstone Institutes/UCSF | N/A |
| LUMC0004iCtrl10 iPSC line (LUMC04i10) | LUMC iPSC Hotel | N/A |
| LUMC044iCtrl44 iPSC line (LUMC44i44) | LUMC iPSC Hotel | N/A |
| SAH0047-02 iPSC line | BCH Human Neuron Core | N/A |
| GON0515-03 iPSC line | BCH Human Neuron Core | N/A |
| GON0926-02 iPSC line | BCH Human Neuron Core | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| CellChat 1.4.0 | Jin et al.74 | https://github.com/sqjin/CellChat |
| CellRanger 6.1.0 | 10× Genomics | https://www.10xgenomics.com/support |
| Rstudio version 1.4.1106 | Rstudio | https://www.rstudio.com/ |
| Seurat version 4.1.0 | Satija et al.118 | https://satijalab.org/seurat/ |
| ggplot2 version 3.3.6 | Wickham119 | https://ggplot2.tidyverse.org/ |
| Nebulosa version 1.0.2 | Alquicira-Hernandez et al.120 | www.github.com/powellgenomicslab/Nebulosa |
| Symphony version 0.1.0 | Kang et al.65 | https://github.com/immunogenomics/symphony |
| ImageJ-Fiji | ImageJ | https://imagej.net/software/fiji/ |
| R version 4.0.5 | R-project | https://www.r-project.org/ |
| SCTransform version 0.3.3 | Hafemeister et al.121 | https://github.com/satijalab/sctransform |
| LAS X Life Science | Leica | https://www.leica-microsystems.com/ |
| Adobe Illustrator 2022 | Adobe | https://www.adobe.com/ |
| Adoble Photoshop 2022 | Adobe | https://www.adobe.com/ |
| Leica Application Suite (LAS) | Leica | https://www.leica-microsystems.com/ |
| SerialEM version 4.0 | Mastronarde122 | https://bio3d.colorado.edu/SerialEM/ |
| IMOD version 4.11 | Kremer et al.123 | https://bio3d.colorado.edu/imod/ |
|
| ||
| Other | ||
|
| ||
| 96-well U-bottom plates with a super-low cell attachment surface | Thermo Scientific | Cat#: 174925 |
| 24-well plates with a super-low cell attachment surface | Thermo Scientific | Cat#: 174930 |
| Dounce tissue grinder | Kimble | Cat#: 885300-0002 |
| 70 μm MACS SmartStrainer | Miltenyi Biotec | Cat#: 130-098-462 |
| 40 μm Flowmi cell strainer | Bel-Art | Cat#: H13680-0040 |
| 10 μm Pluristrainer | PluriSelect | Cat#: 43-10020-40 |
| 20 μm Pluristrainer | PluriSelect | Cat#: 43-10010-40 |
| Cryomolds | Andwin Scientific | Cat#: 25608 |
| Ultra 45° diamond knife | Diatome | Cat#: Ultra 45° |
| Histo diamond knife | Diatome | Cat#: Histo |
| Single-slot copper grids | Veco | Cat#: 12563-CU |
| Aminoalkylsilane-coated glass slides | VWR | Cat#: 631-1165 |
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Pluripotent stem cell lines
Experiments were performed with eight apparently healthy hPSC lines. The WA01 human embryonic stem cell line (male, ethnicity unknown, passages 47–52) was purchased from WiCell Research Institute, provided by Dr. James Thomson at the University of Wisconsin. The two commercially available hiPSC lines that were used are from the same parental male background line (ethnicity Japanese, GM25256 from the Coriell Institute): AICS-0074 with the SRY-Box Transcription Factor 2-mEGFP (SOX2-GFP) reporter (hereafter WTC-SOX2, passages 34–46) acquired from the Allen Institute and WTC-GCaMP with a version of the genetically engineered calcium indicator (passages 36–44) provided by Bruce Conklin at the Gladstone Institutes/UCSF.125 We used additional hiPSC lines that were generated in-house. Generated by the Human iPSC Hotel (Leiden University Medical Center) are LUMC0004iCtrl10 (hereafter LUMC04i10, male, ethnicity unknown, passages 15–36)126 and LUMC0044iCtrl44 (hereafter LUMC44i44, female, ethnicity unknown, passages 20–43).127 Generated by the Human Neuron Core (Boston Children’s Hospital) are SAH0047–02 (female, ethnicity unknown, passages 23–25),128 GON0515–03 (male, ethnicity unknown, passages 22–24)129 and GON0926–02 (female, ethnicity unknown, passages 19–21). Cell lines were evaluated for pluripotency, normal karyotype, and tested negative for mycoplasma prior to experimentation. Cells were cultured on 6-well plates coated with vitronectin recombinant human protein (A14700, Gibco, concentration 0.5 μg/cm) and maintained in Essential 8 Flex medium (A2858501, Gibco) with Normocin (ant-nr-1, Invivogen, concentration 100 μg/mL), hereafter E8 medium. The E8 medium was replenished every other day or every day depending on cell confluency. At approximately 80% confluence (every 4–5 days on average), WTC-SOX2 cells were passaged as single cells using Stempro Accutase Cell Dissociation Reagent (hereafter Accutase; A1110501, Gibco) using 10 mM Y27632 (04–0012-02, Stemgent). The Y27632 was removed within 24 h after passaging by a medium replenishment. All other lines were passaged at approximately 80% confluency (every 4–5 days on average) as tiny clusters (3–5 cells on average) using 0.5 mM EDTA (15575020, Gibco, concentration 10 mM).
Human inner ear tissue
Collection of human fetal inner ear tissue was performed according to the Dutch legislation (Fetal Tissue Act, 2001) and the WMA Declaration of Helsinki guidelines. For this project, we obtained approval from the Medical Research Ethics Committee of Leiden University Medical Center (protocol registration number B19.070) as well as written informed consent of the donor following the Guidelines on the Provision of Fetal Tissue set by the Dutch Ministry of Health, Welfare, and Sport (revised version, 2018). Apparently healthy inner ear tissue was collected after elective termination of pregnancy by vacuum aspiration as previously described.130 Embryonic or fetal age was determined by obstetric ultrasonography prior to termination, i.e., gestational age minus two weeks, with a standard error of two days. No other characteristics were documented in line with legislation and medical ethical approval. Intact inner ears were either stored in RNAlater (AM7020, Invitrogen) when processed for snRNAseq experiments or processed as previously described.66 In summary, inner ears were obtained from vacuum-aspirated tissue collected in phosphate-buffered saline (PBS) with a pH of 7.4. The tissue was then transferred to 4% formaldehyde, prepared from paraformaldehyde, in 0.1 M Na+/K + -phosphate buffer with a pH of 7.4, and fixed for at least one night at 4°C.
Adult vestibular tissue from a 47-year-old male was collected during translabyrinthine vestibular schwannoma surgery in PBS with a pH of 7.4 similar to previous work by our group.130 No other characteristics were documented in line with legislation and medical ethical approval. Ampullae and the utricle were obtained separately and evaluated under a dissection microscope before being experimentally processed. This project was approved by the Medical Research Ethics Committee of Leiden University Medical Center (protocol registration number B18.028) and tissue was obtained after written informed consent of the donor.
METHOD DETAILS
BMP-4 preparation
BMP-4 from different vendors were used for these experiments. BMP-4 from Peprotech (120–05) was reconstituted in sterile 5 mM citric acid containing 0.2% (wt/vol) of human serum albumin to a concentration of 100 μg/mL. BMP-4 from Stemgent (03–0007) and R&D Systems (314-BPE) were reconstituted in sterile 4 mM HCl to a concentration of 100 μg/mL. BMP-4 from R&D Systems (314-BP) was reconstituted in sterile 4 mM HCl containing 0.1% (wt/vol) human serum albumin to a concentration of 100 μg/mL. Reconstituted BMP-4 was stored as 2 μL aliquots at −80°C and replaced every six months.
Differentiation of hPSCs
Inner ear organoid induction followed the protocol recently published with minor alterations.18,21 In brief, colonies of human pluripotent stem cells were detached and dissociated using Accutase and collected as a single-cell suspension in E8 medium containing 20 μM Y27632. 3,500 cells in 100 μL suspension were distributed per well of 96-well U-bottom plates with a super-low cell attachment surface (174925, Thermo Scientific). After centrifugation at 110×g for 6 min to aid aggregation, the cell aggregates were incubated in a 37°C incubator under 5% CO2 for 48 h. After 24 h, 100 μL of fresh E8 medium was added per well to dilute out Y27632.
At differentiation day 0 (D0), all cell aggregates were collected and individually transferred to a new 96-well U-bottom plate with a super-low cell attachment surface in 100 μL of E6 medium (A1516401, Gibco) with Normocin (concentration 100 μg/mL), hereafter E6 medium, containing 2% Matrigel Growth Factor Reduced (hereafter Matrigel; 354230, Corning), 10 μM SB431542 (04–0010-05, Stemgent), 4 ng/mL basic FGF (hereafter bFGF; 100–18B, PeproTech) and 0–40 ng/mL BMP-4 (listed in the key resources table). On D3, 25 μL of E6 per well was added in with an end-concentration of 200 ng/mL LDN (04–0074-02, Stemgent) and 50 μg/mL bFGF, making the total volume 125 μL per well. This treatment induces cranial neural crest formation. On D6, the total volume is increased to 200 μL by adding 75 μL of fresh E6 medium. On D8, 100 μL of the medium was changed for E6 containing 6 μM CHIR99021 (hereafter CHIR; 04–0004-02, Stemgent) to induce otic placode formation. On D10, 100 μL of the medium was changed for fresh E6 containing 3 μM CHIR.
On D12, the aggregates were transferred to Organoid Maturation Medium (OMM) containing 1% Matrigel and 3 μM CHIR in a 24-well plate with a super-low cell attachment surface (174930, Thermo Scientific) on an in-incubator orbital shaker. OMM is a 1:1 mixture of advanced DMEM:F12 (12634010, Gibco) and Neurobasal Medium (21103049, Gibco), supplemented with 0.5x B27 without vitamin A (12587010, Gibco), 0.5x N2 Supplement (17502048, Gibco), 1x Glutamax (35050061, Gibco), 0.1 mM β-mercaptoethanol (21985023, Gibco), and Normocin. On D15, a half medium change was performed with OMM containing 1% Matrigel and 3 μM CHIR. On D18, a half medium change with OMM was performed, diluting out the Matrigel and CHIR. On D21, a full medium change with OMM was performed. Half of the medium was replenished every 3 days, with a full medium change every 9 days. The volume was gradually increased starting from 500 μL at D12, to 1 mL on approximately D45, to 1.5 mL on approximately D60 and later.
In vitro measurements of aggregate morphology
Aggregates were monitored during the differentiation period with a Nikon TS2R microscope or an EVOS M5000 microscope using either bright-field or phase-contrast visualization. Images collected were analyzed using ImageJ measuring epithelial thickness at three different locations, circularity, and area.
Vibratome-slicing of aggregates
Aggregates were embedded in low 2% low melting point agarose (16520050, Invitrogen) and 200 mm thick slices were obtained using a motorized vibratome (VT1200, Leica Microsystems). Sections were obtained in ice-cold PBS (14190144, Gibco) and bright-field evaluation was performed using a Nikon TS2R microscope. Selected sections were processed for scRNAseq as described below.
Aggregate dissociation to single cells for scRNAseq
Vibratome sections of randomly selected aggregates were pooled at specified time points. Three to four number of aggregates were placed in warm TrypLE solution (12605036, Gibco) and incubated in a 37°C incubator under 5% CO2 on an orbital shaker set to 65 rpm. The mixture was triturated with a pipette using wide bore p1000 tips every 10 min. Dissociation to single cells was complete after 60 min and confirmed by bright-field visualization using a Nikon TS2R. TrypLE activity was stopped by adding cold 3% bovine serum albumin (BSA; A7030, Sigma-Aldrich) in PBS. To remove residual cell aggregates and debris, the suspension was filtered with a 40 μm Flowmi cell strainer (H13680–0040, Bel-Art). After washing three times with 3% BSA in PBS, cells were resuspended in ice-cold 3% BSA in PBS and filtered again with a 40 μm Flowmi cell strainer. Cell viability was determined by using Trypan Blue (15250061, Gibco) and an automated cell counter (Countess II, Thermo Fisher). Cell suspensions were diluted with cold 3% BSA in PBS to reach a final concentration of 1,000 cells/μl.
Aggregate dissociation to single nuclei for snRNAseq
Two to four randomly selected aggregates were processed at specified time points. Aggregates were pooled, washed once with ice-cold PBS, and resuspended in lysis buffer that contained 10 mM Tris-HCl (T2194, Millipore-Sigma), 10 mM NaCl (59222C, Millipore-Sigma), 3 mM MgCl2 (M1028, Millipore-Sigma), 0.1% Nonidet P40 Substitute (74385, Millipore-Sigma) in DEPC-treated water (750024, Invitrogen). The suspension was transferred to a Dounce tissue grinder (885300–0002, Kimble) and ground every 5 min. After 20 min, dissociation to single nuclei was confirmed by bright-field visualization (EVOS M5000, Thermo Scientific). After trituration using p1000 and p200 tips, the suspension was filtered through a 70 mm MACS SmartStrainer (130–098-462, Miltenyi Biotec), centrifuged at 500×g for 5 min at 4°C and resuspended in 1% BSA in PBS (AM2616, Invitrogen) containing 200 U/μl RNase inhibitor (3335402001, Millipore-Sigma). After filtration using a 40 μm Flowmi cell strainer and centrifugation at 500g for 5 min at 4°C, the nuclei suspension was resuspended to a final concentration of 1,000 cells/μL.
For one experiment of D110 aggregates generated using the LUMC04i10 hiPSC line, we performed nuclear hashtagging of two separate aggregates using single nuclei hashing antibodies (MAb414, Merck). To this end, the nuclei suspension was blocked using Fc-blocking reagent (422302, Biolegend) for 5 min on ice followed by incubation with the hashing antibody (1 μg per 100 μL) for 10 min on ice. Subsequently, nuclei were washed three times using 1% BSA in PBS containing 200 U/μl RNase inhibitor, pooled together 1:1, and resuspended to a final concentration of 1,000 cells/μl.
Human inner ear tissue dissociation for snRNAseq
Human fetal inner ear tissue was stored in RNAlater at 4°C and processed to a single nuclei suspension within 16 h after collection. The adult human tissue, comprising one ampulla (from the anterior or lateral semicircular canal) and one utricle, was processed within 20 min of collection. The inner ear tissue was assessed under a dissection microscope (M205C, Leica). As for the fetal inner ears, residual tissue was removed using the same microscope. The FW7.5 fetal inner ear was processed with the otic capsule intact, while the otic capsule was manually removed for the FW9.2 fetal inner ear tissue. For the FW9.2 specimen, our dissection strategy resulted in isolating the membranous labyrinth of the cochlea and vestibular system, which was then processed for nuclei isolation. The human inner ear tissue followed a similar protocol of the aggregate dissociation to single nuclei as described above. Minor alterations include the lysis buffer that contains 0.005% Nonidet P40 substitute and additional filtration steps following the 40 μm Flowmi cell strainer: the suspension was filtered through a 20 μm and 10 μm Pluristrainer (43–10020-40 and 43–10010-40, PluriSelect) to remove debris.
RNA-seq cDNA library preparation and sequencing
Single cell and nucleus gene expression libraries were generated on the 10x Genomics Chromium platform using the Chromium Next GEM Single Cell 3′ Library & Gel Bead Kit v2 (scRNAseq) or v3.1 (snRNAseq) and Chromium Next GEM Chip G Single Cell Kit (10x Genomics) according to the manufacturer’s protocol. Hashtag libraries were amplified and barcoded as in Stoeckius et al.131 Gene expression and hashtag libraries were sequenced on a NextSeq500 or NovaSeq 6000 S4 flow cell using v1 Chemistry (Illumina) and FASTQ files were generated using Cell Ranger mkfastq (10x Genomics).
Analysis of scRNAseq and snRNAseq data
The 10x Genomics Cell Ranger 6.1.0 pipeline (http://support.10xgenomics.com/) was used to generate demultiplexed FASTQ files from the raw sequencing reads. The reads were aligned to the human reference GRCh38 genome. For snRNAseq reads, reads were mapped to introns as wells as exons, resulting in a greater number of genes detected per nucleus. Levels of gene expression were analyzed using the number of UMIs (unique molecular indices) detected in each cell. Cell Ranger was used to generate filtered gene expression matrices.
Low-quality cells were removed by quality control on UMI number, number of genes, and percentage of transcripts aligned to mitochondrial genes as well as ribosomal genes. The parameters used were different between the single-cell and single-nucleus data. Cell line-unique single nucleotide polymorphisms (SNPs) were used to demultiplex the single-nucleus data, by which we could assign a cell line identity to 97.3% of the nuclei. Using Seurat,118 and after normalization and scaling of the data, identification of variable features was performed using SCTransform121 regressing out the effect of cell cycle, mitochondrial content and/or ribosomal content. We computed PCA using selected number of highly variable genes. Harmony correction was performed to integrate the datasets into a uniform low-dimensional representation.132 These integrated datasets were used to visualize clustering by Uniform Manifold Approximation and Projection (UMAP) plotting techniques. Cell identity assignation was performed using differential expression analysis and manually by using cell type-specific marker genes.
R packages ggplot2119 and Nebulosa120 were used to plot quantitative gene expression and specific gene expression patterns. Symphony was used to map the integrated IEO scRNAseq and snRNAseq data to the human inner ear atlas.65 Cell-to-cell communication analyses were performed through CellChat (version 1.4.0) and the provided human ligands and receptor pairs database. We computed the probability of communication at the ligand-receptor pair and pathway levels as described in the original manuscript.74 Communication between cell types observed in less than 10 cells and with adjusted p-values greater than 0.1 were removed.
Aggregate processing and immunohistochemistry
Samples were washed twice with PBS and fixed using 4% formaldehyde (1.04005.1000, Merck) in 0.1 M Na+/K+-phosphate buffer (pH 7.4) overnight at 4°C. Fixed samples were either paraffin embedded or processed for cryosectioning. For paraffin embedding, aggregates were dehydrated in an ascending ethanol (70%–99%; 84050068.2500, Boom) series, cleared in xylene (534056, Honeywell), and embedded in paraffin wax (2079, Klinipath). Sections of 4–5 μm were cut using a rotary microtome (HM355S, Thermo Scientific). Sections were deparaffinized in xylene and rehydrated in a descending series of ethanol (96%–50%) and several rinses in deionized water. At approximately every 10 sections, one section was selected for routine staining with hematoxylin (40859001, Klinipath) and eosin (40829002, Klinipath). Before immunostaining, antigen unmasking was performed either in 10 mM sodium citrate buffer (pH 6.0; S1804–500G, Sigma-Aldrich) for 12 min at 97°C. For cryosectioning, aggregates were cryoprotected with graded treatments of 15% and 30% sucrose solutions in PBS and embedded in tissue-freezing medium (1518313, General Data Healthcare) using cryomolds (25608, Andwin Scientific). Cryosections of 12 μm were cut using a cryostat (CM-1860, Leica Microsystems).
For immunostaining, sections were rinsed in washing buffer consisting of 0.05% Tween-20 (H5152, Promega) and incubated for 30 min with blocking solution consisting of 5% bovine serum albumin (BSA; A7030, Sigma-Aldrich) and 0.05% Tween-20 in PBS, followed by overnight incubation at 4°C with the primary antibodies listed in the key resources table. Next, sections were incubated at ambient temperature (RT) with the Alexa Fluor-conjugated secondary antibodies for 2 h, listed in the key resources table. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1,000; D1306, Invitrogen). Sections were mounted with ProLong Gold Antifade Mountant (P36934, Invitrogen). Negative controls were carried out by matching isotype controls and/or omitting primary antibodies. Positive controls were carried out by staining sections of known positive human tissue samples based upon their protein expression profiles, including human inner ear tissue. At least two separate immunostaining experiments were performed with each primary antibody.
Human fetal inner ear tissue processing and immunohistochemistry
Fixed fetal inner ear tissue was dehydrated in an ascending series of ethanol (70%–99%), cleared in xylene, and embedded in paraffin wax. After sectioning, deparaffinization, and rehydration as previously outlined, HE staining was conducted on approximately every 10th slide for quality assessment and identification of inner ear morphology and specific inner ear structures.
Sample processing for transmission electron microscopy
At D74, LUMC04i10 IEOs (n = 6) were collected and fixed overnight with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) at 4°C. Fixed specimens were rinsed (3 × 20 min) in 0.1 M sodium cacodylate buffer (pH 7.4) at RT prior to post-fixation in a solution containing 1% OsO4 and 1% K4Ru(CN)6 (potassium hexacyanoruthenate (II) hydrate) in 0.1 M sodium cacodylate buffer (pH 7.4) for 2 h at 4°C followed by several washes in distilled water. Dehydration was performed in an ascending ethanol series (70%−100) and 100% 1,2-propylene oxide followed by infiltration with a mixture of Spurr’s low-viscosity resin and 1,2-propylene oxide (1:1) for 1 h and fresh pure resin under continuous agitation for 2 h at RT. Finally, each specimen was separately embedded in fresh pure resin in a polyethylene embedding mold and polymerized overnight at 70°C.
Light microscopy
For light-microscopical assessment, semi-thin (1 μm) plastic sections were cut with a diamond knife (Histo, DiATOME) on a microtome (RM2265, Leica Microsystems), collected on aminoalkylsilane-coated glass slides, stained with a warm aqueous solution containing 1% methylene blue, 1% azure II and 1% sodium tetraborate, and mounted in Entellan (1079610100, Merck) under a glass coverslip.
Image acquisition and processing
Sections stained with either HE or methylene blue-azure II were examined with a Leica DM5500B microscope equipped with a Leica DFC450C digital color camera. Digital images were acquired and stored using Leica Application Suite software (LAS version 4.5; Leica Microsystems GmbH). Images were adjusted for optimal contrast and brightness and assembled into figures using Adobe Illustrator 2021 or Adobe Photoshop 2021 (Adobe Systems Software Ltd).
Immunostained sections were acquired with a Leica SP8 confocal laser scanning microscope using Leica objectives (20×/0.7 dry HC PL Apo, 40×/1.3 oil HC PL Apo CS2, 63×/1.4 oil HC PL Apo or 100×/1.3 oil HC PL Fluotar), operating under Leica Application Suite X microscope software (LAS X, Leica Microsystems). Maximal projections were obtained from image stacks with optimized z-step size. Brightness and contrast adjustments were performed with Fiji (ImageJ version 1.52p).
Transmission electron microscopy
For ultrastructural examination, representative or interesting areas were selected based on light-microscopical assessment. Ultrathin (60–90 nm) sections were cut with a diamond knife (Ultra 45°, DiATOME) on an Ultrotome III (LKB) and collected upon Pioloform-coated, single-slot copper grids. The sections were contrast-stained with 7% uranyl acetate in 70% methanol and Reynolds’ lead citrate solution and examined in a FEI Tecnai 12 transmission electron microscope (operating at 80 kV) equipped with a Veleta 4-megapixel side-mount CCD camera (Olympus Soft Imaging Solutions GmbH).
Digital images were acquired with TIA software (TEM Imaging & Analysis, version 4.7 SP3; FEI). Alternatively, sets of serial digital images with 10% overlap (at an original magnification of ×30,000) were acquired with the automated EM data acquisition software package SerialEM133 (https://bio3d.colorado.edu/SerialEM). Final alignment (stitching) of the sets of overlapping images and montage blending were done using the reconstruction and modeling software package IMOD123 (https://bio3d.colorado.edu/imod) operating under the Unix toolkit Cygwin. Montages or single images were finally saved as TIFF and imported into Fiji ImageJ for further image processing, examination and selection of interesting areas. Images were assembled into figures using Adobe Photoshop.
QUANTIFICATION AND STATISTICAL ANALYSIS
For analyzing the in vitro measurements of aggregate morphology, statistical significance was analyzed with two-way ANOVA with a Sidak correction for multiple comparisons. Data was considered statistically significant if p < 0.05.
The immunohistochemical images are representative for inner ear tissue obtained from individual samples of the same fetal week, limited to a minimum of 2 replicates due to the paucity of the tissue. Immunohistochemical images of the inner ear organoids are representative for at least 2 individual experiments with n = 6–10 aggregates per experiment. Electron microscopical images are representative for at least n = 3 aggregates.
Supplementary Material
Highlights.
Targeted hiPSC differentiation efficiency is dependent on initial BMP signaling
Transcriptomics defines periotic mesenchymal cells, vestibular hair cells, and more
Genes linked to congenital inner ear dysfunction are expressed in the organoid model
Cell communication analysis reveals a role of endothelium in sensory development
ACKNOWLEDGMENTS
This work was supported by the Novo Nordisk Foundation (NNF21CC0073729 to H.L.), the Hoogenboom-Beckfonds foundation, Erasmus+ (to W.H.v.d.V.), ZonMw (446001025 to W.H.v.d.V.), and the NIH (R01AR075018, R01DC 017461, and R03DC015624 to K.R.K.). We thank the Human iPSC Hotel (Leiden University Medical Center) as well as Dr. M. Sahin and Dr. J. Gonzalez-Heydrich (Boston Children’s Hospital) for sharing hiPSC lines. We thank Dr. H. Versnel, F.G.J. Hendriksen, and the Cell Microscopy Core (University Medical Center Utrecht, the Netherlands) for use of their laboratory facilities. We acknowledge the Mildred Clinics (Arnhem and Eindhoven, the Netherlands) for supplying inner ear samples and the Light and Electron Microscopy Facility (Leiden University Medical Center) for their expertise and microscopes. We also thank T.O. Verhagen, MD, and L.C.M. Grijpink, MD (Leiden University Medical Center), as well as Dr. J. Lee and S.A. Serdy (Boston Children’s Hospital) for their support in preparation of this paper. Additionally, we thank Dr. T. Coate (Georgetown University) for the POU3F4 antibody. The antibodies developed by Dr. D.J. Orten and Dr. W.E. Wright were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology. Last, we acknowledge the Harvard Single Cell Core, the Harvard Chan Bioinformatics Core, the Research Computing Group (Harvard University), the BCH HPC Cluster Enkefalos 2 (Boston Children’s Hospital), and the Leiden Genome Technology Center (Leiden University Medical Center) for their support and services related to RNA sequencing data, as well as Dr. S.M. Kielbasa (Leiden University Medical Center) for demultiplexing the snRNA-seq data.
Footnotes
DECLARATION OF INTERESTS
K.R.K. is an inventor on patents relating to the IEO model.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112623.
REFERENCES
- 1.WHO (2021). Deafness and Hearing Loss. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- 2.Hülse R, Biesdorf A, Hörmann K, Stuck B, Erhart M, Hülse M, and Wenzel A (2019). Peripheral vestibular disorders: an epidemiologic survey in 70 million individuals. Otol. Neurotol. 40, 88–95. 10.1097/MAO.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 3.Butcher E, Dezateux C, Cortina-Borja M, and Knowles RL (2019). Prevalence of permanent childhood hearing loss detected at the universal newborn hearing screen: systematic review and meta-analysis. PLoS One 14, e0219600. 10.1371/journal.pone.0219600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sontag MK, Yusuf C, Grosse SD, Edelman S, Miller JI, McKasson S, Kellar-Guenther Y, Gaffney M, Hinton CF, Cuthbert C, et al. (2020). Infants with congenital disorders identified through newborn screening - United States, 2015–2017. MMWR Morb. Mortal. Wkly. Rep. 69, 1265–1268. 10.15585/mmwr.mm6936a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shearer AE, Hildebrand MS, and Smith RJH (1993). Hereditary hearing loss and deafness overview. In GeneReviews, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, and Amemiya Aeds. (University of Washington, Seattle). [PubMed] [Google Scholar]
- 6.Van Beeck Calkoen EA, Engel MSD, Van de Kamp JM, Yntema HG, Goverts ST, Mulder MF, Merkus P, and Hensen EF (2019). The etiological evaluation of sensorineural hearing loss in children. Eur. J. Pediatr. 178, 1195–1205. 10.1007/s00431-019-03379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei C, Dong H, Nisenbaum E, Thielhelm T, Nourbakhsh A, Yan D, Smeal M, Lundberg Y, Hoffer ME, Angeli S, et al. (2021). Genetics and the individualized therapy of vestibular disorders. Front. Neurol. 12, 633207. 10.3389/fneur.2021.633207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taiber S, and Avraham KB (2019). Genetic therapies for hearing loss: accomplishments and remaining challenges. Neurosci. Lett. 713, 134527. 10.1016/j.neulet.2019.134527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang PC, Hashino E, and Nelson RF (2020). Progress in modeling and targeting inner ear disorders with pluripotent stem cells. Stem Cell Rep. 14, 996–1008. 10.1016/j.stemcr.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roccio M (2021). Directed differentiation and direct reprogramming: applying stem cell technologies to hearing research. Stem Cell. 39, 375–388. 10.1002/stem.3315. [DOI] [PubMed] [Google Scholar]
- 11.Roccio M, and Edge ASB (2019). Inner Ear Organoids: New Tools to Understand Neurosensory Cell Development, Degeneration and Regeneration. Development 146. 10.1242/dev.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roccio M, Senn P, and Heller S (2020). Novel insights into inner ear development and regeneration for targeted hearing loss therapies. Hear. Res. 397, 107859. 10.1016/j.heares.2019.107859. [DOI] [PubMed] [Google Scholar]
- 13.Czajkowski A, Mounier A, Delacroix L, and Malgrange B (2019). Pluripotent stem cell-derived cochlear cells: a challenge in constant progress. Cell. Mol. Life Sci. 76, 627–635. 10.1007/s00018-018-2950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stojkovic M, Han D, Jeong M, Stojkovic P, and Stankovic KM(2021). Human induced pluripotent stem cells and CRISPR/Cas-mediated targeted genome editing: platforms to tackle sensorineural hearing loss. Stem Cell. 39, 673–696. 10.1002/stem.3353. [DOI] [PubMed] [Google Scholar]
- 15.Zine A, Messat Y, and Fritzsch B (2021). A human induced pluripotent stem cell-based modular platform to challenge sensorineural hearing loss. Stem Cell. 39, 697–706. 10.1002/stem.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durán-Alonso MB (2020). Stem cell-based approaches: possible route to hearing restoration? World J. Stem Cell. 12, 422–437. 10.4252/wjsc.v12.i6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Valk WH, Steinhart MR, Zhang J, and Koehler KR (2021). Building inner ears: recent advances and future challenges for in vitro organoid systems. Cell Death Differ. 28, 24–34. 10.1038/s41418-020-00678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler KR, Nie J, Longworth-Mills E, Liu XP, Lee J, Holt JR, and Hashino E (2017). Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 35, 583–589. 10.1038/nbt.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie J, Ueda Y, Solivais AJ, and Hashino E (2022). CHD7 regulates otic lineage specification and hair cell differentiation in human inner ear organoids. Nat. Commun. 13, 7053. 10.1038/s41467-022-34759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda Y, Moore ST, and Hashino E (2022). Directed differentiation of human pluripotent stem cells into inner ear organoids. Methods Mol. Biol. 2520, 135–150. 10.1007/7651_2021_448. [DOI] [PubMed] [Google Scholar]
- 21.Steinhart MR, Serdy SA, van der Valk WH, Zhang J, Kim J, Lee J, and Koehler KR (2022). Defining inner ear cell type specification at single-cell resolution in a model of human cranial development. SSRN Electronic J. 10, 3974124. [Google Scholar]
- 22.Betancur P, Bronner-Fraser M, and Sauka-Spengler T (2010). Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl. Acad. Sci. USA 107, 3570–3575. 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slyper M, Porter CBM, Ashenberg O, Waldman J, Drokhlyansky E, Wakiro I, Smillie C, Smith-Rosario G, Wu J, Dionne D, et al. (2020). A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors. Nat. Med. 26, 792–802. 10.1038/s41591-020-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korrapati S, Taukulis I, Olszewski R, Pyle M, Gu S, Singh R, Griffiths C, Martin D, Boger E, Morell RJ, and Hoa M (2019). Single cell and single nucleus RNA-seq reveal cellular heterogeneity and homeostatic regulatory networks in adult mouse stria vascularis. Front. Mol. Neurosci. 12, 316. 10.3389/fnmol.2019.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakken TE, Hodge RD, Miller JA, Yao Z, Nguyen TN, Aevermann B, Barkan E, Bertagnolli D, Casper T, Dee N, et al. (2018). Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS One 13, e0209648. 10.1371/journal.pone.0209648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding J, Adiconis X, Simmons SK, Kowalczyk MS, Hession CC, Marjanovic ND, Hughes TK, Wadsworth MH, Burks T, Nguyen LT, et al. (2020). Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 38, 737–746. 10.1038/s41587-020-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkerson BA, Zebroski HL, Finkbeiner CR, Chitsazan AD, Beach KE, Sen N, Zhang RC, and Bermingham-McDonogh O (2021). Novel cell types and developmental lineages revealed by single-cell RNA-seq analysis of the mouse crista ampullaris. Elife 10, e60108. 10.7554/eLife.60108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu KS, Frumm SM, Park JS, Lee K, Wong DM, Byrnes L, Knox SM, Sneddon JB, and Tward AD (2019). Development of the mouse and human cochlea at single cell resolution. Preprint at bioRxiv, 739680. 10.1101/739680. [DOI] [Google Scholar]
- 29.Lindskog C, Linné J, Fagerberg L, Hallström BM, Sundberg CJ, Lindholm M, Huss M, Kampf C, Choi H, Liem DA, et al. (2015). The human cardiac and skeletal muscle proteomes defined by transcriptomics and antibody-based profiling. BMC Genom. 16, 475. 10.1186/s12864-015-1686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M, and Jørgensen HF (2018). Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 9, 4567. 10.1038/s41467-018-06891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whalen RG, Butler-Browne GS, and Gros F (1978). Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J. Mol. Biol. 126, 415–431. 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- 32.Vandekerckhove J, Bugaisky G, and Buckingham M (1986). Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J. Biol. Chem. 261, 1838–1843. [PubMed] [Google Scholar]
- 33.Anderson PA, Malouf NN, Oakeley AE, Pagani ED, and Allen PD(1991). Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ. Res. 69, 1226–1233. 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- 34.Choi IY, Lim H, Cho HJ, Oh Y, Chou BK, Bai H, Cheng L, Kim YJ, Hyun S, Kim H, et al. (2020). Transcriptional landscape of myogenesis from human pluripotent stem cells reveals a key role of TWIST1 in maintenance of skeletal muscle progenitors. Elife 9, e46981. 10.7554/eLife.46981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimaldi A, and Tajbakhsh S (2021). Diversity in cranial muscles: origins and developmental programs. Curr. Opin. Cell Biol. 73, 110–116. 10.1016/j.ceb.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Kasahara H, Bartunkova S, Schinke M, Tanaka M, and Izumo S(1998). Cardiac and extracardiac expression of Csx/Nkx2.5 homeodomain protein. Circ. Res. 82, 936–946. 10.1161/01.res.82.9.936. [DOI] [PubMed] [Google Scholar]
- 37.Wu CL, Dicks A, Steward N, Tang R, Katz DB, Choi YR, and Guilak F (2021). Single cell transcriptomic analysis of human pluripotent stem cell chondrogenesis. Nat. Commun. 12, 362. 10.1038/s41467-020-20598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ, and Koehler KR (2020). Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404. 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong K, Ahmad I, Jason Clark J, Seline A, Bertroche T, Mostaert B, Van Daele DJ, and Hansen MR (2018). Nf2 mutation in Schwann cells delays functional neural recovery following injury. Neuroscience 374, 205–213. 10.1016/j.neuroscience.2018.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, Van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, et al. (2018). Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22. 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wangzhou A, Paige C, Ray PR, Dussor G, and Price TJ (2021). Diversity of receptor expression in central and peripheral mouse neurons estimated from single cell RNA sequencing. Neuroscience 463, 86–96. 10.1016/j.neuroscience.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber D, Pereira JA, Gerber J, Tan G, Dimitrieva S, Yángüez E, and Suter U (2021). Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT). Elife 10, e58591. 10.7554/eLife.58591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald A, Lu B, Caron M, Caporicci-Dinucci N, Hatrock D, Petrecca K, Bourque G, and Stratton JA (2021). Single cell transcriptomics of ependymal cells across age, region and species reveals cilia-related and metal ion regulatory roles as major conserved ependymal cell functions. Front. Cell. Neurosci. 15, 703951. 10.3389/fncel.2021.703951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White RM, and Zon LI (2008). Melanocytes in development, regeneration, and cancer. Cell Stem Cell 3, 242–252. 10.1016/j.stem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Belote RL, Le D, Maynard A, Lang UE, Sinclair A, Lohman BK, Planells-Palop V, Baskin L, Tward AD, Darmanis S, and Judson-Torres RL (2021). Human melanocyte development and melanoma dedifferentiation at single-cell resolution. Nat. Cell Biol. 23, 1035–1047. 10.1038/s41556-021-00740-8. [DOI] [PubMed] [Google Scholar]
- 46.Van Beelen ESA, Van der Valk WH, de Groot JCMJ, Hensen EF, Locher H, and Van Benthem PPG (2020). Migration and fate of vestibular melanocytes during the development of the human inner ear. Dev. Neurobiol. 80, 411–432. 10.1002/dneu.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Locher H, de Groot JCMJ, Van Iperen L, Huisman MA, Frijns JHM, and Chuva de Sousa Lopes SM (2015). Development of the stria vascularis and potassium regulation in the human fetal cochlea: insights into hereditary sensorineural hearing loss. Dev. Neurobiol. 75, 1219–1240. 10.1002/dneu.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schupp JC, Adams TS, Cosme C Jr., Raredon MSB, Yuan Y, Omote N, Poli S, Chioccioli M, Rose KA, Manning EP, et al. (2021). Integrated single-cell atlas of endothelial cells of the human lung. Circulation 144, 286–302. 10.1161/circulationaha.120.052318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berendam SJ, Koeppel AF, Godfrey NR, Rouhani SJ, Woods AN, Rodriguez AB, Peske JD, Cummings KL, Turner SD, and Engelhard VH (2019). Comparative transcriptomic analysis identifies a range of immunologically related functional elaborations of lymph node associated lymphatic and blood endothelial cells. Front. Immunol. 10, 816. 10.3389/fimmu.2019.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalucka J, De Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, et al. (2020). Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e20. 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Gordon EJ, Gale NW, and Harvey NL (2008). Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dynam. 237, 1901–1909. 10.1002/dvdy.21605. [DOI] [PubMed] [Google Scholar]
- 52.Kaipainen A, Korhonen J, Mustonen T, Van Hinsbergh VW, Fang GH, Dumont D, Breitman M, and Alitalo K (1995). Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 92, 3566–3570. 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Driver EC, and Kelley MW (2020). Development of the Cochlea. Development 147. 10.1242/dev.162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson Chacko L, Pechriggl EJ, Fritsch H, Rask-Andersen H, Blumer MJF, Schrott-Fischer A, and Glueckert R (2016). Neurosensory differentiation and innervation patterning in the human fetal vestibular end organs between the gestational weeks 8–12. Front. Neuroanat. 10, 111. 10.3389/fnana.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jan TA, Eltawil Y, Ling AH, Chen L, Ellwanger DC, Heller S, and Cheng AG (2021). Spatiotemporal dynamics of inner ear sensory and non-sensory cells revealed by single-cell transcriptomics. Cell Rep. 36, 109358. 10.1016/j.celrep.2021.109358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto R, Ohnishi H, Omori K, and Yamamoto N (2021). In silico analysis of inner ear development using public whole embryonic body single-cell RNA-sequencing data. Dev. Biol. 469, 160–171. 10.1016/j.ydbio.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Li C, Wang Y, Wang G, Lu Y, He S, Sun Y, and Liu Z (2020). Fate-mapping analysis using Rorb-IRES-Cre reveals apical-to-basal gradient of Rorb expression in mouse cochlea. Dev. Dynam. 249, 173–186. 10.1002/dvdy.111. [DOI] [PubMed] [Google Scholar]
- 58.Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, and Wu DK (1999). Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development 126, 2335–2343. 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi T, Ray CA, and Bermingham-McDonogh O (2008). Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28, 5991–5999. 10.1523/jneurosci.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, and Groves AK (2010). BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 30, 15044–15051. 10.1523/jneurosci.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDo-nogh O, and Cheng AGL (2011). Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455–469. 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo XJ, Deng M, Xie X, Huang L, Wang H, Jiang L, Liang G, Hu F, Tieu R, Chen R, and Gan L (2013). GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Hum. Mol. Genet. 22, 3609–3623. 10.1093/hmg/ddt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munnamalai V, and Fekete DM (2020). The acquisition of positional information across the radial axis of the cochlea. Dev. Dynam. 249, 281–297. 10.1002/dvdy.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honda K, Kim SH, Kelly MC, Burns JC, Constance L, Li X, Zhou F, Hoa M, Kelley MW, Wangemann P, et al. (2017). Molecular architecture underlying fluid absorption by the developing inner ear. Elife 6, e26851. 10.7554/eLife.26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang JB, Nathan A, Weinand K, Zhang F, Millard N, Rumker L, Moody DB, Korsunsky I, and Raychaudhuri S (2021). Efficient and precise single-cell reference atlas mapping with Symphony. Nat. Commun. 12, 5890. 10.1038/s41467-021-25957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Locher H, Frijns JHM, Van Iperen L, de Groot JCMJ, Huisman MA, and Chuva de Sousa Lopes, S.M. (2013). Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 8, 20. 10.1186/1749-8104-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pujol R, and Lavigne-Rebillard M (1985). Early stages of innervation and sensory cell differentiation in the human fetal organ of Corti. Acta Otolaryngol. Suppl. 423, 43–50. 10.3109/00016488509122911. [DOI] [PubMed] [Google Scholar]
- 68.Simmons DD, Tong B, Schrader AD, and Hornak AJ (2010). Oncomodulin identifies different hair cell types in the mammalian inner ear. J. Comp. Neurol. 518, 3785–3802. 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McInturff S, Burns JC, and Kelley MW (2018). Characterization of spatial and temporal development of type I and type II hair cells in the mouse utricle using new cell-type-specific markers. Biol. Open 7, bio038083. 10.1242/bio.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rüsch A, Lysakowski A, and Eatock RA (1998). Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J. Neurosci. 18, 7487–7501. 10.1523/jneurosci.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rau A, Legan PK, and Richardson GP (1999). Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J. Comp. Neurol. 405, 271–280. [PubMed] [Google Scholar]
- 72.Forge A, Becker D, Casalotti S, Edwards J, Marziano N, and Nevill G (2003). Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J. Comp. Neurol. 467, 207–231. 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 73.Ding B, Walton JP, Zhu X, and Frisina RD (2018). Age-related changes in Na, K-ATPase expression, subunit isoform selection and assembly in the stria vascularis lateral wall of mouse cochlea. Hear. Res. 367, 59–73. 10.1016/j.heares.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan C-H, Myung P, Plikus MV, and Nie Q (2021). Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088. 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebeid M, and Huh SH (2017). FGF signaling: diverse roles during cochlear development. BMB Rep. 50, 487–495. 10.5483/bmbrep.2017.50.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groves AK, and Fekete DM (2012). Shaping sound in space: the regulation of inner ear patterning. Development 139, 245–257. 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kakuki T, Kohno T, Nishida S, Konno T, Kikuchi S, Ohwada K, Nakano M, Tezuka M, Takano K, and Kojima T (2022). FOXO3/TGF-β signal-dependent ciliogenesis and cell functions during differentiation of temperature-sensitive mouse cochlear precursor hair cells. Histochem. Cell Biol. 157, 415–426. 10.1007/s00418-021-02068-8. [DOI] [PubMed] [Google Scholar]
- 78.Munnamalai V, and Fekete DM (2016). Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 143, 4003–4015. 10.1242/dev.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda T, Kominami K, Wang S, Togashi H, Hirata K.i., Mizoguchi A, Rikitake Y, and Takai Y (2014). Aberrant cochlear hair cell attachments caused by Nectin-3 deficiency result in hair bundle abnormalities. Development 141, 399–409. 10.1242/dev.094995. [DOI] [PubMed] [Google Scholar]
- 80.Kiernan AE, Xu J, and Gridley T (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4. 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown RM 2nd, Nelson JC, Zhang H, Kiernan AE, and Groves AK (2020). Notch-mediated lateral induction is necessary to maintain vestibular prosensory identity during inner ear development. Dev. Biol. 462, 74–84. 10.1016/j.ydbio.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hao J, Koesters R, Bouchard M, Gridley T, Pfannenstiel S, Plinkert PK, Zhang L, and Praetorius M (2012). Jagged1-mediated Notch signaling regulates mammalian inner ear development independent of lateral inhibition. Acta Otolaryngol. 132, 1028–1035. 10.3109/00016489.2012.690533. [DOI] [PubMed] [Google Scholar]
- 83.Brooker R, Hozumi K, and Lewis J (2006). Notch ligands with contrasting functions: jagged1 and Delta1 in the mouse inner ear. Development 133, 1277–1286. 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 84.Doetzlhofer A, White PM, Johnson JE, Segil N, and Groves AK(2004). In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev. Biol. 272, 432–447. 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Lewis RM, Keller JJ, Wan L, and Stone JS (2018). Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium. Hear. Res. 364, 1–11. 10.1016/j.heares.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delmaghani S, and El-Amraoui A (2020). Inner ear gene therapies takeoff: current promises and future challenges. J. Clin. Med. 9, 2309. 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nishio SY, Hattori M, Moteki H, Tsukada K, Miyagawa M, Naito T, Yoshimura H, Iwasa YI, Mori K, Shima Y, et al. (2015). Gene expression profiles of the cochlea and vestibular endorgans: localization and function of genes causing deafness. Ann. Otol. Rhinol. Laryngol. 124 (Suppl 1), 6s–48s. 10.1177/0003489415575549. [DOI] [PubMed] [Google Scholar]
- 88.Wilson PA, and Hemmati-Brivanlou A (1995). Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331–333. 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 89.Mayordomo R, Rodríguez-Gallardo L, and Alvarez IS (1998). Morphological and quantitative studies in the otic region of the neural tube in chick embryos suggest a neuroectodermal origin for the otic placode. J. Anat. 193, 35–48. 10.1046/j.1469-7580.1998.19310035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohta S, and Schoenwolf GC (2018). Hearing Crosstalk: The Molecular Conversation Orchestrating Inner Ear Dorsoventral Patterning, 7 (Wiley Interdiscip Rev Dev Biol). 10.1002/wdev.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koundakjian EJ, Appler JL, and Goodrich LV (2007). Auditory neurons make stereotyped wiring decisions before maturation of their targets. J. Neurosci. 27, 14078–14088. 10.1523/jneurosci.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bok J, Raft S, Kong K-A, Koo SK, Dräger UC, and Wu DK (2011). Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc. Natl. Acad. Sci. USA 108, 161–166. 10.1073/pnas.1010547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, et al. (2019). Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262. 10.1038/s41592019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolla L, Kelly MC, Mann ZF, Anaya-Rocha A, Ellis K, Lemons A, Palermo AT, So KS, Mays JC, Orvis J, et al. (2020). Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 11, 2389. 10.1038/s41467-020-16113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burns JC, Kelly MC, Hoa M, Morell RJ, and Kelley MW (2015). Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat. Commun. 6, 8557. 10.1038/ncomms9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang A, Shearer AE, Zhou GW, Kenna M, Poe D, Licameli GR, and Brodsky JR (2021). Peripheral vestibular dysfunction is a common occurrence in children with non-syndromic and syndromic genetic hearing loss. Front. Neurol. 12, 714543. 10.3389/fneur.2021.714543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taberner L, Bañón A, and Alsina B (2020). Sensory neuroblast quiescence depends on vascular cytoneme contacts and sensory neuronal differentiation requires initiation of blood flow. Cell Rep. 32, 107903. 10.1016/j.celrep.2020.107903. [DOI] [PubMed] [Google Scholar]
- 98.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175. 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palikuqi B, Nguyen DHT, Li G, Schreiner R, Pellegata AF, Liu Y, Redmond D, Geng F, Lin Y, Gó mez-Salinero JM, et al. (2020). Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585, 426–432. 10.1038/s41586-020-2712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heaney DL, and Schulte BA (2003). Dystroglycan expression in the mouse cochlea. Hear. Res. 177, 12–20. 10.1016/s0378-5955(02)00769-4. [DOI] [PubMed] [Google Scholar]
- 101.Kim MJ, Liu IH, Song Y, Lee J-A, Halfter W, Balice-Gordon RJ, Linney E, and Cole GJ (2007). Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology 17, 231–247. 10.1093/glycob/cwl069. [DOI] [PubMed] [Google Scholar]
- 102.Lewis MA, Schulte BA, Dubno JR, and Steel KP (2022). Investigating the characteristics of genes and variants associated with self-reported hearing difficulty in older adults in the UK Biobank. BMC Biol. 20, 150. 10.1186/s12915-022-01349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talenti G, Robson C, Severino MS, Alves CA, Chitayat D, Dahmoush H, Smith L, Muntoni F, Blaser SI, and D’Arco F (2021). Characteristic cochlear hypoplasia in patients with walker-warburg syndrome: a radiologic study of the inner ear in a-dystroglycan-related muscular disorders. AJNR. Am. J. Neuroradiol. 42, 167–172. 10.3174/ajnr.A6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aragona M, Porcino C, Guerrera MC, Montalbano G, Laurà R, Cometa M, Levanti M, Abbate F, Cobo T, Capitelli G, et al. (2022). The BDNF/TrkB neurotrophin system in the sensory organs of zebrafish. Int. J. Mol. Sci. 23, 2621. 10.3390/ijms23052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Zhang X, Zhang Q, Wang R, Ma J, Bai X, and Wang D(2022). Loxhd1b inhibits the hair cell development in zebrafish: possible relation to the BDNF/TrkB/ERK pathway. Front. Cell. Neurosci. 16, 1065309. 10.3389/fncel.2022.1065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matilainen T, Haugas M, Kreidberg JA, and Salminen M (2007). Analysis of Netrin 1 receptors during inner ear development. Int. J. Dev. Biol. 51, 409–413. 10.1387/ijdb.072273tm. [DOI] [PubMed] [Google Scholar]
- 107.O’Keeffe MG, Thorne PR, Housley GD, Robson SC, and Vlajkovic SM (2010). Developmentally regulated expression of ectonucleotidases NTPDase5 and NTPDase6 and UDP-responsive P2Y receptors in the rat cochlea. Histochem. Cell Biol. 133, 425–436. 10.1007/s00418-010-0682-1. [DOI] [PubMed] [Google Scholar]
- 108.O’Keeffe MG, Thorne PR, Housley GD, Robson SC, and Vlajkovic SM (2012). Hair cell specific NTPDase6 immunolocalisation in vestibular end organs: potential role of purinergic signaling in vestibular sensory transduction. J. Vestib. Res. 22, 213–219. 10.3233/ves-2012-00461. [DOI] [PubMed] [Google Scholar]
- 109.Trautman MS, Kimelman J, and Bernfield M (1991). Developmental expression of syndecan, an integral membrane proteoglycan, correlates with cell differentiation. Development 111, 213–220. 10.1242/dev.111.1.213. [DOI] [PubMed] [Google Scholar]
- 110.Vlajkovic SM, Thorne PR, Sévigny J, Robson SC, and Housley GD (2002). Distribution of ectonucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Hear. Res. 170, 127–138. 10.1016/s0378-5955(02)00460-4. [DOI] [PubMed] [Google Scholar]
- 111.Fedele G, Cazzaniga A, Castiglioni S, Locatelli L, Tosoni A, Nebuloni M, and Maier JAM (2022). The presence of BBB hastens neuronal differentiation of cerebral organoids – the potential role of endothelial derived BDNF. Biochem. Biophys. Res. Commun. 626, 30–37. 10.1016/j.bbrc.2022.07.112. [DOI] [PubMed] [Google Scholar]
- 112.Graziano S, Marchiò S, Bussolino F, and Arese M (2013). A peptide from the extracellular region of the synaptic protein a Neurexin stimulates angiogenesis and the vascular specific tyrosine kinase Tie2. Biochem. Biophys. Res. Commun. 432, 574–579. 10.1016/j.bbrc.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 113.Kanthi YM, Sutton NR, and Pinsky DJ (2014). CD39: interface between vascular thrombosis and inflammation. Curr. Atherosclerosis Rep. 16, 425. 10.1007/s11883-014-0425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liebner S, Czupalla CJ, and Wolburg H (2011). Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 55, 467–476. 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 115.Miner JH (2011). Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis 7, 75–82. 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morisada T, Kubota Y, Urano T, Suda T, and Oike Y (2006). Angiopoietins and angiopoietin-like proteins in angiogenesis. Endothelium 13, 71–79. 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- 117.Pretorius D, Richter RP, Anand T, Cardenas JC, and Richter JR(2022). Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol. 16, 100121. 10.1016/j.mbplus.2022.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Satija R, Farrell JA, Gennert D, Schier AF, and Regev A (2015). Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502. 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag; ). [Google Scholar]
- 120.Alquicira-Hernandez J, and Powell JE (2021). Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics 37, 2485–2487. 10.1093/bioinformatics/btab003. [DOI] [PubMed] [Google Scholar]
- 121.Hafemeister C, and Satija R (2019). Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296. 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mastronarde D (2003). SerialEM: A Program for Automated Tilt Series Acquisition on Tecnai Microscopes Using Prediction of Specimen Position. Microsc. Microanal. 9, 1182–1183. 10.1017/S1431927603445911. [DOI] [Google Scholar]
- 123.Kremer JR, Mastronarde DN, and McIntosh JR (1996). Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76. 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 124.Orvis J, Gottfried B, Kancherla J, Adkins RS, Song Y, Dror AA, Olley D, Rose K, Chrysostomou E, Kelly MC, et al. (2021). gEAR: gene Expression Analysis Resource portal for community-driven, multiomic data exploration. Nat. Methods 18, 843–844. 10.1038/s41592-021-01200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, et al. (2015). Automated video-based analysis of contractility and calcium flux in human-induced pluripotent stem cell-derived cardiomyocytes cultured over different spatial scales. Tissue Eng. C Methods 21, 467–479. 10.1089/ten.TEC.2014.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dambrot C, Braam SR, Tertoolen LGJ, Birket M, Atsma DE, and Mummery CL (2014). Serum supplemented culture medium masks hypertrophic phenotypes in human pluripotent stem cell derived cardiomyocytes. J. Cell Mol. Med. 18, 1509–1518. 10.1111/jcmm.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen X, Janssen JM, Liu J, Maggio IT, Jong AEJ, Mikkers HMM, and Gonçalves MAFV (2017). In trans paired nicking triggers seamless genome editing without double-stranded DNA cutting. Nat. Commun. 8, 657. 10.1038/s41467-017-00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ebrahimi-Fakhari D, Saffari A, Wahlster L, Di Nardo A, Turner D, Lewis TL Jr., Conrad C, Rothberg JM, Lipton JO, Kölker S, et al. (2016). Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 17, 1053–1070. 10.1016/j.celrep.2016.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shlevkov E, Basu H, Bray MA, Sun Z, Wei W, Apaydin K, Karhohs K, Chen PF, Smith JLM, Wiskow O, et al. (2019). A high-content screen identifies TPP1 and aurora B as regulators of axonal mitochondrial transport. Cell Rep. 28, 3224–3237.e5. 10.1016/j.celrep.2019.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Beelen ESA, van der Valk WH, Verhagen TO, de Groot JCMJ, Madison MA, Shadmanfar W, Hensen EF, Jansen JC, van Benthem PPG, Holt JR, and Locher H (2022). Efficient viral transduction in fetal and adult human inner ear explants with AAV9-PHP.B vectors. Biomolecules 12, 816. 10.3390/biom12060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stoeckius M, Zheng S, Houck-Loomis B, Hao S, Yeung BZ, Mauck WM, Smibert P, and Satija R (2018). Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224. 10.1186/s1305-9018-1603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, and Raychaudhuri S (2019). Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296. 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51. 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell and single-nucleus RNA-seq data have been deposited the Gene Expression Omnibus and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. The data can also be accessed through a data exploration platform hosted by the Expression Analysis Resource,124 available at https://umgear.org/p?l=hIEOandInnerEar.
This paper does not report original code. Scripts used for scRNAseq and snRNAseq of IEOs analysis are available at https://github.com/Koehler-Lab/, scripts used for the human inner ear atlas are available at https://github.com/OtoBiologyLeiden/.
Any additional information required to reanalyze data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-ACTC1 (1:50) | R&D systems | Cat#: MAB9308-100 |
| Mouse monoclonal anti-ANXA4 (1:200) | Sigma-Aldrich | Cat#: SAB4200121; RRID: AB_10621934 |
| Mouse monoclonal anti-ATP1A1 (1:200) | Novus Biologicals | Cat#: NB300-146; RRID: AB_2060981 |
| Rabbit polyclonal anti-BSND (1:750) | Novus Biologicals | Cat#: NBP2-49101 |
| Mouse monoclonal anti-CDH1 (1:50) | BD Transduction Laboratories | Cat#: 610182; RRID: AB_397581 |
| Rabbit polyclonal anti-CDH23 (1:50) | Invitrogen | Cat#: PA5-53564; RRID: AB_2639592 |
| Rabbit polyclonal anit-DACH1 (1:50) | Invitrogen | Cat#: PA5-52968; RRID: AB_2640365 |
| Mouse monoclonal anti-GATA3 (1:500) | BD Transduction Laboratories | Cat#: 558686; RRID: AB_2108590 |
| Rabbit polyclonal anti-GJB2 (1:100) | Alomone Labs | Cat#: ACC-212; RRID: AB_11124274 |
| Rabbit polyclonal anti-GJB6 (1:100) | Invitrogen | Cat#: PA5-11640; RRID: AB_2111053 |
| Rabbit monoclonal anti-LGR6 (1:100) | Abcam | Cat#: ab126747; RRID: AB_11132458 |
| Mouse monoclonal anti-MLANA (1:50) | Abcam | Cat#: ab731; RRID: AB_305836 |
| Rabbit polyclonal anti-MYO7A (1:100) | Proteus Biosciences | Cat#: 25-6790; RRID: AB_10015251 |
| Rabbit monoclonal anti-MYO7A (1:50) | Novus Biologicals | Cat#: NBP1-84266; RRID: AB_11016173 |
| Mouse monoclonal anti-MYO7A (1:30) | DSHB | Cat#: 138-1s; RRID: AB_2282417 |
| Mouse monoclonal anti-MYOG (1:10) | DSHB | Cat#: F5D; RRID: AB_2146602 |
| Rabbit polyclonal anti-OC90 (1:100) | Invitrogen | Cat#: PA5-71564; RRID: AB_2717418 |
| Rabbit polyclonal anti-OCM (1:200) | Abcam | Cat#: ab150947 |
| Rabbit polyclonal anti-OTOF (1:100) | Novus Biologicals | Cat#: NBP1-46574; RRID: AB_10009850 |
| Rabbit polyclonal anti-OTOR (1:100) | Invitrogen | Cat#: PA5-55053; RRID: AB_2645111 |
| Goat polyclonal anti-OTX2 (1:20) | R&D systems | Cat#: AF1979; RRID: AB_2157172 |
| Goat polyclonal anti-PDGFRB (1:15) | R&D systems | Cat#: AF385; RRID: AB_355339 |
| Mouse monoclonal anti-PECAM1 (1:50) | Dako | Cat#: M082329-2 |
| Alexa Fluor™ 568 phalloidin | Invitrogen | Cat#: A12380 |
| Chicken polyclonal anti-POU3F4 (1:10000) | Aves Labs | Cat#: AB_2814704; RRID: AB_2814704 |
| Rabbit polyclonal anti-SIX1 (1:100) | Invitrogen | Cat#: PA5-51654; RRID: AB_2647319 |
| Rabbit polyclonal anti-SLC26A4 (1:100) | Novus Biologicals | Cat#: NBP1-85237; RRID: AB_11032075 |
| Mouse monoclonal anti-SOX2 (1:100) | BD Transduction Laboratories | Cat#: 561469; RRID: AB_10694256 |
| Goat polyclonal anti-SOX10 (1:50) | Invitrogen | Cat#: PA5-47001; RRID: AB_2608449 |
| Rabbit polyclonal anti-TECTA (1:200) | Invitrogen | Cat#: PA5-80102; RRID: AB_2747217 |
| Rabbit monoclonal anti-TFAP2A (1:50) | Abcam | Cat#: ab108311; RRID: AB_10861200 |
| Rabbit polyclonal anti-TNNT1 (1:500) | R&D systems | Cat#: NBP2-38855 |
| Rabbit monoclonal anti-TTR (1:50) | Invitrogen | Cat#: MA5-32634; RRID: AB_2809911 |
| Mouse monoclonal anti-TUBB3A (1:100) | Biolegend | Cat#: 801202; RRID: AB_10063408 |
| Mouse monoclonal anti-TUBB3A (1:100) | R&D Systems | Cat#: MAB1195; RRID: AB_357520 |
| Mouse monoclonal anti-WRHN (1:50) | Abnova | Cat#: H00025861-M05; RRID: AB_10629381 |
| Donkey anti-mouse Alexa Fluor™ 488 (1:1000–2000) | Invitrogen | Cat#: A21202; RRID: AB_141607 |
| Donkey anti-mouse Alexa Fluor™ 568 (1:1000–2000) | Invitrogen | Cat#: A10037; RRID: AB_2534013 |
| Donkey anti-mouse Alexa Fluor™ 594 (1:1000–2000) | Invitrogen | Cat#: A21203; RRID: AB_141633 |
| Donkey anti-mouse Alexa Fluor™ 647 (1:1000–2000) | Invitrogen | Cat#: A31571; RRID: AB_162542 |
| Donkey anti-mouse Alexa Fluor™ 680 (1:1000–2000) | Invitrogen | Cat#: A10038; RRID: AB_2534014 |
| Goat anti-mouse IgG1 Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21121; RRID: AB_2535764 |
| Goat anti-mouse IgG1 Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21124; RRID: AB_2535766 |
| Goat anti-mouse IgG1 Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21240; RRID: AB_2535809 |
| Goat anti-mouse IgG2a Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21131; RRID: AB_2535771 |
| Goat anti-mouse IgG2a Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21134; RRID: AB_2535773 |
| Goat anti-mouse IgG2a Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21241; RRID: AB_2535810 |
| Goat anti-mouse IgG2b Alexa Fluor™ 488 (1:2000) | Invitrogen | Cat#: A21141; RRID: AB_2535778 |
| Goat anti-mouse IgG2b Alexa Fluor™ 568 (1:2000) | Invitrogen | Cat#: A21144; RRID: AB_2535780 |
| Goat anti-mouse IgG2b Alexa Fluor™ 647 (1:2000) | Invitrogen | Cat#: A21242; RRID: AB_2535811 |
| Donkey anti-rabbit Alexa Fluor™ 488 (1:1000) | Abcam | Cat#: ab150061; RRID: AB_2571722 |
| Donkey anti-rabbit Alexa Fluor™ 488 (1:1000–2000) | Invitrogen | Cat#: A21206; RRID: AB_2535792 |
| Donkey anti-rabbit Alexa Fluor™ 568 (1:1000–2000) | Invitrogen | Cat#: A10042; RRID: AB_2534017 |
| Donkey anti-rabbit Alexa Fluor™ 594 (1:1000–2000) | Invitrogen | Cat#: A21207; RRID: AB_141637 |
| Donkey anti-rabbit Alexa Fluor™ 647 (1:1000–2000) | Invitrogen | Cat#: A31573; RRID: AB_2536183 |
| Donkey anti-rabbit Alexa Fluor™ 680 (1:1000–2000) | Invitrogen | Cat#: A10043; RRID: AB_2534018 |
| Donkey anti-goat Alexa Fluor™ 488 (1:1000) | Abcam | Cat#: ab150133; RRID: AB_2832252 |
| Goat anti-chicken Alexa Fluor™ 594 (1:1000) | Invitrogen | Cat#: A11042; RRID: AB_2534099 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Stempro Accutase Cell Dissociation Reagent | Gibco | Cat#: A1110501 |
| Y27632 | Stemgent | Cat#: 04-0012-02 |
| Essential 8 Flex medium | Gibco | Cat#: A2858501 |
| Vitronectin | Gibco | Cat#: A14700 |
| Normocin | Invivogen | Cat#: ant-nr-1 |
| EDTA | Gibco | Cat#: 15575020 |
| E6 medium | Gibco | Cat#: A1516401 |
| Matrigel Growth Factor Reduced | Corning | Cat#: 354230 |
| SB431542 | Stemgent | Cat#: 04-0010-05 |
| basic FGF | Peprotech | Cat#: 100-18B |
| BMP-4 | Peprotech | Cat#: 120-05 |
| BMP-4 | Stemgent | Cat#: 03-0007 |
| BMP-4 | R&D | Cat#: 314-BP |
| BMP-4 | R&D | Cat#: 314-BPE |
| LDN | Stemgent | Cat#: 04-0074-02 |
| CHIR99021 | Stemgent | Cat#: 04-0004-02 |
| Advanced DMEM:F12 medium | Gibco | Cat#: 12634010 |
| Neurobasal medium | Gibco | Cat#: 21103049 |
| B27 Supplement without vitamin A | Gibco | Cat#: 12587010 |
| N2 Supplement | Gibco | Cat#: 17502048 |
| Glutamax | Gibco | Cat#: 35050061 |
| β-Mercaptoethanol | Gibco | Cat#: 21985023 |
| Low Melting Point Agarose | Invitrogen | Cat#: 16520050 |
| PBS | Gibco | Cat#: 14190144 |
| TrypLE | Gibco | Cat#: 12605036 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#: A7030 |
| Trypan Blue | Gibco | Cat#: 15250061 |
| Tris-HCl | Millipore-Sigma | Cat#: T2194 |
| NaCl | Millipore-Sigma | Cat#: 59222C |
| MgCl2 | Millipore-Sigma | Cat#: M1028 |
| Nonidet P40 Substitute | Millipore-Sigma | Cat#: 74385 |
| DEPC-treated water | Invitrogen | Cat#: 750024 |
| Rnase inhibitor | Millipore-Sigma | Cat#: 3335402001 |
| RNAlater | Invitrogen | Cat#: AM7020 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Chromium Next GEM Single Cell 3' | 10X Genomics | Cat#: PN-1000128 |
| Library & Gel Bead Kit v3.1 | ||
| Chromium Next GEM Single Cell 3' | 10X Genomics | Cat#: PN-120267 |
| Library & Gel Bead Kit v2 | ||
| Anti-Nuclear Pore Complex Proteins | Merck | Cat#: MAb414 |
| Fc-blocking reagent | Biolegend | Cat#: 422302 |
|
| ||
| Deposited data | ||
|
| ||
| RNA sequencing data IEO | This manuscript | GEO: GSE214099 |
| RNA sequencing data human inner ear | This manuscript | GEO: GSE213796 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| WA01 ESC line | WiCell | N/A |
| AICS-0074 iPSC line (WTC-SOX2) | Allen Institute | N/A |
| WTC-GCaMP iPSC line | Bruce Conklin at the Gladstone Institutes/UCSF | N/A |
| LUMC0004iCtrl10 iPSC line (LUMC04i10) | LUMC iPSC Hotel | N/A |
| LUMC044iCtrl44 iPSC line (LUMC44i44) | LUMC iPSC Hotel | N/A |
| SAH0047-02 iPSC line | BCH Human Neuron Core | N/A |
| GON0515-03 iPSC line | BCH Human Neuron Core | N/A |
| GON0926-02 iPSC line | BCH Human Neuron Core | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| CellChat 1.4.0 | Jin et al.74 | https://github.com/sqjin/CellChat |
| CellRanger 6.1.0 | 10× Genomics | https://www.10xgenomics.com/support |
| Rstudio version 1.4.1106 | Rstudio | https://www.rstudio.com/ |
| Seurat version 4.1.0 | Satija et al.118 | https://satijalab.org/seurat/ |
| ggplot2 version 3.3.6 | Wickham119 | https://ggplot2.tidyverse.org/ |
| Nebulosa version 1.0.2 | Alquicira-Hernandez et al.120 | www.github.com/powellgenomicslab/Nebulosa |
| Symphony version 0.1.0 | Kang et al.65 | https://github.com/immunogenomics/symphony |
| ImageJ-Fiji | ImageJ | https://imagej.net/software/fiji/ |
| R version 4.0.5 | R-project | https://www.r-project.org/ |
| SCTransform version 0.3.3 | Hafemeister et al.121 | https://github.com/satijalab/sctransform |
| LAS X Life Science | Leica | https://www.leica-microsystems.com/ |
| Adobe Illustrator 2022 | Adobe | https://www.adobe.com/ |
| Adoble Photoshop 2022 | Adobe | https://www.adobe.com/ |
| Leica Application Suite (LAS) | Leica | https://www.leica-microsystems.com/ |
| SerialEM version 4.0 | Mastronarde122 | https://bio3d.colorado.edu/SerialEM/ |
| IMOD version 4.11 | Kremer et al.123 | https://bio3d.colorado.edu/imod/ |
|
| ||
| Other | ||
|
| ||
| 96-well U-bottom plates with a super-low cell attachment surface | Thermo Scientific | Cat#: 174925 |
| 24-well plates with a super-low cell attachment surface | Thermo Scientific | Cat#: 174930 |
| Dounce tissue grinder | Kimble | Cat#: 885300-0002 |
| 70 μm MACS SmartStrainer | Miltenyi Biotec | Cat#: 130-098-462 |
| 40 μm Flowmi cell strainer | Bel-Art | Cat#: H13680-0040 |
| 10 μm Pluristrainer | PluriSelect | Cat#: 43-10020-40 |
| 20 μm Pluristrainer | PluriSelect | Cat#: 43-10010-40 |
| Cryomolds | Andwin Scientific | Cat#: 25608 |
| Ultra 45° diamond knife | Diatome | Cat#: Ultra 45° |
| Histo diamond knife | Diatome | Cat#: Histo |
| Single-slot copper grids | Veco | Cat#: 12563-CU |
| Aminoalkylsilane-coated glass slides | VWR | Cat#: 631-1165 |


