Abstract
Introduction:
The significance of hyoid dynamics in OSA pathophysiology remains unclear. Drug-induced sleep endoscopy (DISE) is often used for evaluating patients intolerant to positive airway pressure (PAP) therapy. We performed DISE with concurrent hyoid-focused ultrasonography to quantify hyoid dynamics during obstructive and non-obstructive breathing.
Methods:
A cross-sectional analysis from a prospective cohort of patients undergoing DISE with PAP titration (DISE-PAP) and hyoid-focused ultrasound was conducted. Hyoid ultrasound was performed during obstructive breathing, and non-obstructive breathing after PAP administration. Motion was quantified by generating displacement curves based on echo-tracking hyoid movement. The image analysis protocol for quantifying hyoid displacement was performed independently by two researchers and reliability of measures was assessed. Univariate and multivariate regressions were performed for various clinical data and hyoid displacement during obstructive breathing.
Results:
Twenty patients met inclusion criteria. On average, the cohort was male (75%), elderly (65.9±10 yrs), overweight (29.3±3.99 kg/m2), and with moderate-to-severe OSA (29.3±12.5 events/hr). Mean hyoid displacement during obstructive breathing was 5.81 mm (±3.48). In all patients, hyoid displacement decreased after PAP administration (−3.94mm (95% CI:−5.10, −2.78; p<0.0001). Inter-rater reliability for measures of hyoid displacement was excellent. After multivariate regression, hyoid displacement at baseline was associated with higher AHI (β [95% CI] = 0.18 [0.03, 0.33], p=0.020).
Conclusion:
During DISE, hyoid displacement is greater during obstructive breathing with significant variability amongst patients. Further, these ultrasonographic measurements had excellent intra- and inter-rater reliability. Additional, larger studies are needed to understand contributors to hyoid mobility.
Level of Evidence:
4
Keywords: obstructive sleep apnea, hyoid, ultrasound, sleep endoscopy
Lay Summary
Ultrasound during drug-induced sleep endoscopy is a new application that allows clinicians to measure hyoid bone movement in patients with obstructive sleep apnea. The amount of hyoid motion is variable in OSA patients and more studies are needed to understand this variability.
Introduction
Obstructive sleep apnea (OSA) is a sleep-breathing disorder characterized by inspiratory flow limitation due to upper airway collapse.1 This collapse results in partial (hypopneas) or complete (apneas) airway obstruction, triggering arousals from sleep and subsequent sleep-fragmentation. OSA affects more men than women, with estimated prevalence between 15-30% for men and 10-15% for women.2,3,4 Recently, the prevalence of OSA has been shown to increase, in part reflecting the rising rates of obesity – the primary risk factor for OSA.4 Continuous positive airway pressure (CPAP) has been the mainstay of OSA, but adherence remains low.5 Difficulties with CPAP use prompt some patients to seek alternative therapies, including upper airway surgery.
Patient selection is an important step in evaluating PAP alternative patients, as various phenotypes of OSA have been previously identified.6 Sleep surgeons may employ drug-induced sleep endoscopy (DISE), a method of evaluating upper airway collapse.7,8 DISE enables classification of upper airway collapse patterns via a range of scoring systems such as the VOTE, and is often used to guide surgical decision-making.9,10 Recently, DISE has been modified with the addition of CPAP (termed DISE-PAP) to quantify measures of upper airway collapsibility such as pharyngeal critical pressure (PCRIT) and pharyngeal opening pressure (PhOP).11,12,13 PhOP is the pressure level during DISE-PAP at which inspiratory flow limitation is completely abolished. In addition, DISE-PAP allows for evaluation of both flow-limited and non-flow-limited breathing, representing a dynamic assessment of upper airway collapse.
Upper airway collapse is determined by a complex interplay between anatomical and neuromuscular components.14 One anatomical structure for which the role in OSA pathophysiology and treatment is incompletely understood is the hyoid bone. Increased pharyngeal length as measured by mandibular plane to hyoid distance (MPH) is associated with increased airway collapsibility.15 However, surgical manipulation of the hyoid bone has had mixed success in treating OSA.16,17,18 As hyoid surgery is aimed at stabilizing or anteriorizing the bone, various studies have called for investigation of hyoid dynamics and the impact of its stabilization in OSA patients.15,19 DISE-PAP offers a unique setting of drug-induced sleep which enables investigation of hyoid dynamics during normal and obstructive breathing. Within this context, ultrasonography, a non-invasive and reliable acquisition platform, permits real-time evaluation of hyoid motion.20
In the present study, our primary aim was to use submental ultrasound during DISE-PAP to quantify hyoid dynamics during obstructive and non-obstructive breathing. We hypothesized that hyoid displacement would be greater during obstructive breathing compared to non-obstructive breathing. Our secondary aim was to evaluate the intra- and inter-rater reliability of our ultrasonography analysis protocol. We also explored the relationship between hyoid displacement and demographics, anthropometrics, and measures of OSA severity from polysomnography.
Materials and Methods
Participants
We performed a cross-sectional analysis from a prospective cohort of OSA patients seeking PAP alternatives. All patients underwent DISE-PAP and submental ultrasound at the Hospital of the University of Pennsylvania. The study was approved by Institutional Review Board at the University of Pennsylvania (IRB#: 849542). Inclusion criteria were as follows: age greater than or equal to 18 years and apnea-hypopnea index (AHI) > 5 events/hour. Patients were excluded due to either inadequate quality or quantity of recordings captured.
Clinical Data
Demographic and anthropometric information including sex, age, weight, height, and race were obtained from the electronic medical record. Polysomnographic data, including overall, sleep stage-dependent and positional AHI were obtained. In addition, obstructive apnea predominance was calculated by dividing obstructive apnea index (oAI) by total AHI.
DISE-PAP Procedure
The DISE-PAP evaluation was performed on an integrated recording platform designed to acquire clinically relevant anatomic and physiologic characteristics of each patient’s upper airway. A detailed description of the DISE-PAP setup has been previously published.12 In short, the nasolaryngoscope was passed through a custom-fitted mask into the nasal cavity. CPAP (S9 VPAP, ResMed Inc., San Diego CA) was applied and titrated using a nasal or customized oro-nasal mask (Pulmodyne, Indianapolis, IN) attached to a pneumotachometer. A pressure-sensitive catheter (Mikro-cath™, Millar, Houston, TX) was passed to the retro-epiglottic space to measure respiratory effort (Millar, Houston, TX).21 Pulse oximetry was monitored, and a dual lumen oral cannula provided supplemental O2 (2-4 L/min) and measured end-tidal CO2 to detect mouth breathing. Propofol anesthesia was administered to achieve sedation using a probability ramp infusion system as previously described, with a target Bispectral Index (BIS) (Medtronic, Minneapolis, Minnesota) range of 50 – 70.22
Ultrasound Acquisition
Ultrasound recordings were obtained with a Zonare S3 scanner (Mindray North America, Mahwah, NJ, USA) using a curved array C6-2 probe. Once the desired plane of anesthesia was reached, the ultrasound probe was placed submentally and in the midsagittal plane. The probe tip was carefully placed at the symphysis of the mandible with both mandible and hyoid in field of view, and minimal pressure was applied to avoid changing mouth position during DISE (Figure 1). After observation of obstructive hypopneas and apneas, “baseline” was called, and the first ultrasound recording was initiated. Figure 2 demonstrates a side-by-side view of sagittal CT and hyoid-focused ultrasound. Then, PAP was administered starting at ~ 2 cm H2O and increased by 1 cm H2O during respiratory arousals until flow-limited breathing was abolished for >50% of breaths at the same pressure level. This is known as the pharyngeal opening pressure (PhOP). A second ultrasound recording was obtained for analysis. A detailed description of the ultrasound analysis and acquisition protocol is outlined in S1.
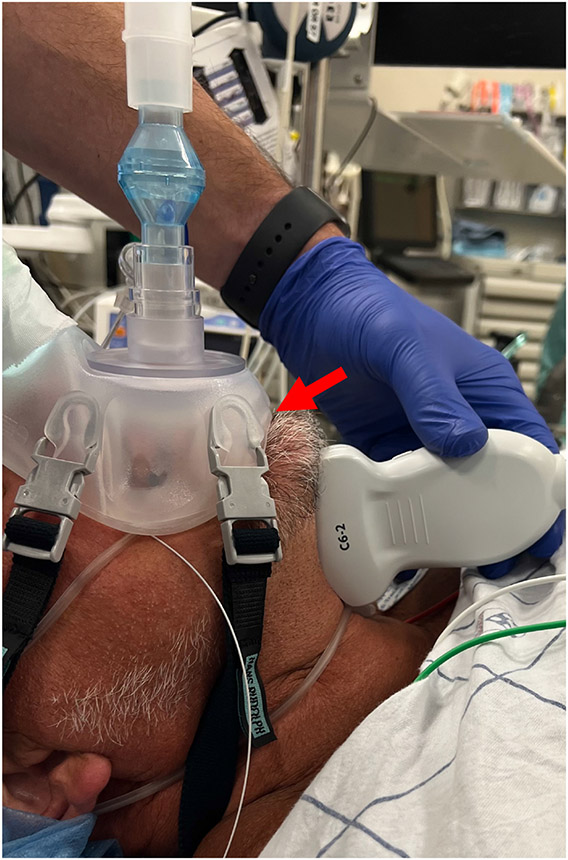
Figure 1.
C6-2 probe positioned sagittal and submentally. Patient is supine with neck neutral. Submental space is free of instruments and facial hair. Red arrow - inferior cusp of oronasal mask has been excised for probe placement.
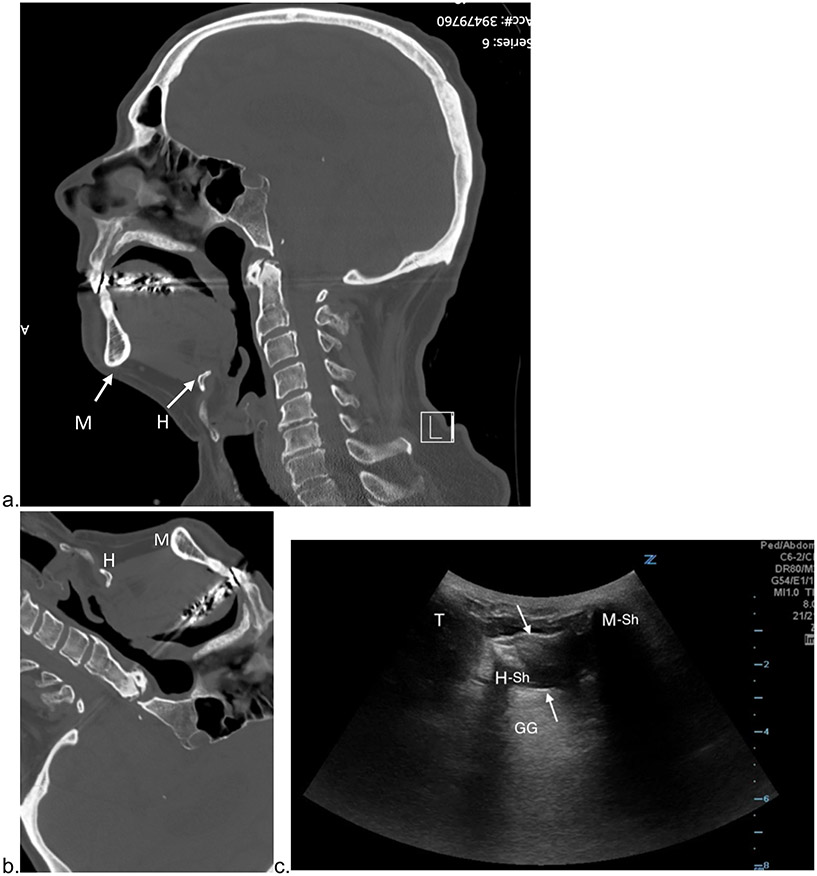
Figure 2: Ultrasound Orientation Using CT Imaging.
a) upright sagittal CT of the head at the level of the incisive canal: b) CT rotated and cropped to match ultrasound image orientation. c) Corresponding submental sagittal ultrasound: M = mandible. M-Sh = mandible shadow. H = hyoid bone. H-Sh = hyoid shadow. GG = Genioglossus. T = Thyroid Cartilage. Between the white arrows are suprahyoid muscle fibers.
Ultrasound Analysis
The ultrasound clip was viewed in Noxturnal US v6.3.1 (NoxMedical, Reykjavík, Iceland), and both the direction and timing of hyoid displacement relative to breath cycle was corroborated with endoscopic recordings and physiologic data. This was confirmed by three researchers (M.H.P, R.C.D, and E.T). A video showing the synchrony of channels and images in Noxturnal US is shown in Video S1.
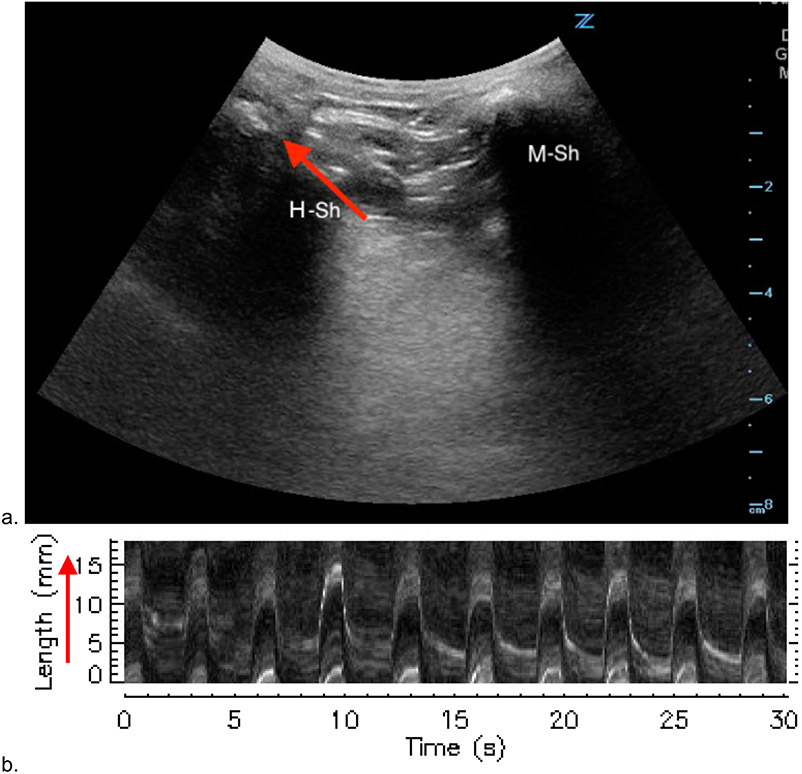
Ultrasound clips were then uploaded to an analysis software Imagelab (L3HarrisGeospatial, Colorado, US). Hyoid displacement was captured via a linear vector which spanned across the hyoid to hyoid-shadow interface from its initial position to its point-of-maximal displacement. Analysis of hyoid bone movement across this vector generated a motiongram. A motiongram is a two-dimensional graphical representation of hyoid displacement over time (seconds). For each subject, a baseline and PhOP motiongram were created. Figure 3 demonstrates the vector of hyoid motion on ultrasound and its corresponding motiongram. A video demonstrating the generation of motiongrams is shown in Video S2.
Figure 3: Generation of Motiongrams with M-mode.
a) Ultrasound with landmarks. Red arrow denoting vector along which motion was analyzed for motiongram. b) Generated motiongram of hyoid displacement at baseline. Red arrow is the translated m-mode vector on ultrasound clip, representing the y axis in millimeters. M-Sh = mandible shadow. H-Sh = hyoid shadow.
Intra-rater and Inter-rater reliability
In a subset of ten patients, two independent raters (M.H.P and V.T.) created and measured motiongrams at baseline and PhOP. Each breath was measured three times, and the average of the three values were compared within and across raters to understand variability and agreement. Reliability was determined by utilizing mixed effects modeling to determine the proportion of total variability attributable to differences among subjects, among breaths within subjects, among breaths within raters, and between raters.
Statistical Analysis
Continuous variables are summarized as means and standard deviations or medians and ranges, as appropriate. Categorical variables are summarized using frequencies and percentages. A two-sided paired t-test was used to determine if the amount of hyoid displacement significantly changed from baseline to PhOP, with statistical significance based on a p<0.05. Linear regression analysis was used to explore the relationship between amplitude of hyoid displacement and both clinical (e.g., age, sex, race, BMI, and height) and disease severity (e.g., overall, positional, and sleep-stage-specific AHI) characteristics. Multivariate regression was performed adjusting for age, sex, and BMI when examining the association between hyoid displacement at baseline and clinical variables. Results are reported as observed and standardized β-coefficients and associated 95% confidence intervals (CIs). Complementary analyses using Pearson’s linear correlations were also performed.
Statistical significance was based on a p<0.05 for comparisons of hyoid displacement at baseline and during PhOP. Given the descriptive nature of association analyses, a p<0.05 was also used for determining statistical significance for association analyses. Intra-rater and inter-rater reliability were examined by calculating the variance due to differences between patients, breaths within patients, raters, and residual factors and determining the proportion of total variability that was due to biological differences. Analyses were performed using Stata/SE v14.3 (StataCorp, College Station, TX) and SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Participant Characteristics
Between 12/1/2021 and 10/15/2022, 32 patients underwent DISE-PAP with concurrent hyoid-focused ultrasound. Of these patients, 20 met our inclusion criteria and 12 were excluded: 8 (25.0%) patients had poor ultrasound technique and probe motion, 3 (9.4%) had less than three breaths available per clip, and 1 (3.1%) had excessive facial hair precluding ultrasound probe contact with the neck. Table 1 shows the baseline demographics of our study sample. On average, patients were older (65.9±10.0 years), male (75%), overweight (29.3±3.99 kg/m2), and with moderate-to-severe OSA (29.3±12.5 events/hr).
Table 1:
Sample Characteristics (n=20).
| Variable | Mean ± SD | Minimum | Maximum | Median |
|---|---|---|---|---|
| Age | 65.9 ± 10.0 | 48 | 80 | 68 |
| Male | 75% | - | - | - |
| Body Mass Index (kg/m2) | 29.3 ± 3.99 | 20 | 35 | 29.5 |
| Apnea Hypopnea Index (AHI) | 29.3 ± 12.5 | 15 | 59 | 26.5 |
Reduced Hyoid Displacement from Baseline to PhOP (Primary Outcome)
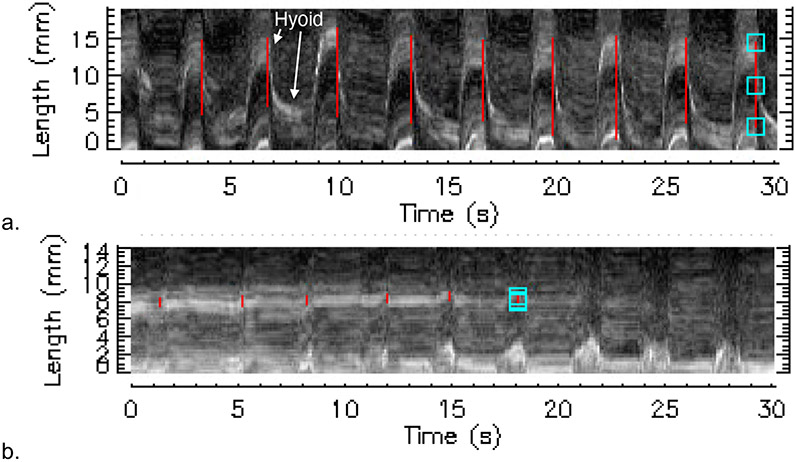
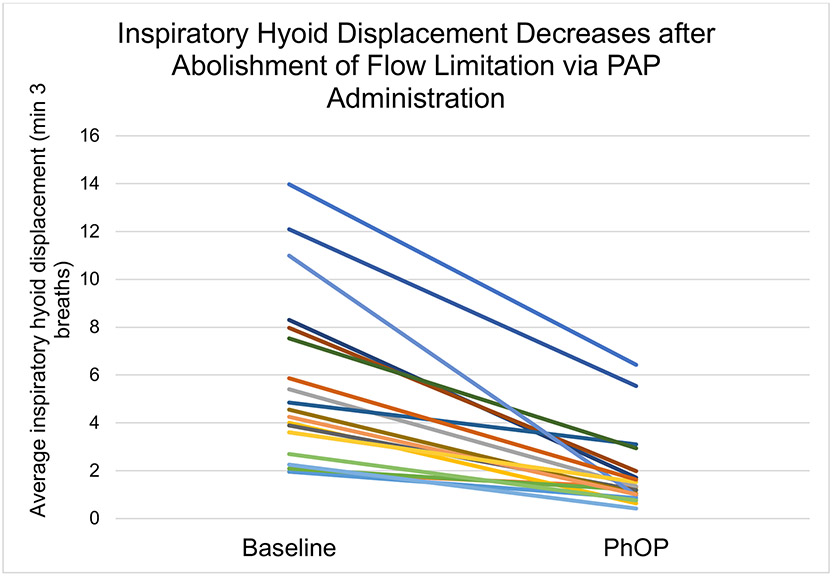
A total of 291 breaths were analyzed across obstructive (53.2% of breaths with 7.9±3.3 breaths per patient) and non-obstructive breathing (46.8% of breaths with 6.8±1.8 breaths per patient). The mean of patient-level average amplitude of hyoid displacement during baseline was 5.8±3.5 mm, with a median (interquartile range [IQR]) of 4.7 (3.1 to 7.8) mm. Mean subject-level average amplitude of hyoid displacement at PhOP was 1.9±1.6 mm, with a median (IQR) of 1.3 (1.0 to 2.0) mm, (Figure 4). There was a statistically significant change in average hyoid displacement from baseline to PhOP of −3.9 mm (95% CI: −5.1, −2.8; p<0.0001) (see Figure 5). Of note, all patients had a reduction in average hyoid displacement after abolishment of flow limitation from baseline to PhOP (range: −10.0 to −0.7 mm), reflecting qualitatively similar but variable reduction in displacement after PAP administration.
Figure 4:
Motiongram at Baseline vs Motiongram at PhOP. 4a: measured motiongram at baseline (patient-mean = 10.98 mm). 4b: measured motiongram at PhOP (patient-mean = 0.97 mm). Hyoid hyperechoic line is labeled.
Figure 5: Inspiratory Hyoid Displacement Decreases after Abolishment of Flow Limitation via PAP Administration.
X axis shows clips taken during either obstructive (baseline) or non-obstructive (PhOP) breathing. Y axis shows hyoid displacement during inspiration in millimeters. (−3.94 mm, 95% CI: −5.10, −2.78; *** = p < 0.0001)
Reliability of Hyoid Displacement Measurements (Secondary Outcome)
The intra-rater reliability for measures of hyoid displacement at baseline obtained by primary rater (M.H.P.) was excellent (ICC = 0.980). The inter-rater reliability for measures of hyoid displacement at baseline was also excellent (ICC = 0.940). A detailed table demonstrating factors of variability is shown (S2).
Associations with Hyoid Displacement (Exploratory Outcomes)
In our exploratory analyses, we sought to determine the relationship between hyoid displacement during obstructive breathing and various demographics, anthropometrics, and clinical factors (S3). In bivariate, unadjusted regression analysis, AHI was significantly associated with hyoid displacement (β [95% CI] = 0.19 [0.05, 0.32], Std. β = 0.56 [0.15, 0.97], p = 0.032), whereas age, sex, BMI, and race were not significantly associated with hyoid displacement at baseline. Stage dependent AHI (REM, NREM), positional AHI (non-supine vs supine), and obstructive apnea percentage (oAI%) were not correlated with hyoid displacement at baseline. After adjusting for age, sex, and BMI, AHI remained significantly correlated with severity of hyoid displacement at baseline (β [95% CI] = 0.18 [0.03, 0.33], Std. β = 0.55 [0.10, 1.01], p = 0.020). Thus, for every 1 unit increase in AHI, hyoid displacement at baseline obstructive breathing during DISE increased by 0.2 mm.
Discussion
This is the first study utilizing ultrasonography during DISE to quantify hyoid displacement in OSA patients. Our results inform three features of hyoid displacement during drug-induced sleep. First, increased hyoid displacement occurs during inspiration. Second, all patients had a reduction in inspiratory hyoid displacement after flow limitation was abolished with PAP therapy. Third, patients exhibited varying degrees of hyoid displacement at baseline. Additionally, our use of m-mode analysis protocol for quantification of hyoid displacement was found to have strong intra- and inter-rater reliability. Finally, hyoid displacement amplitude was significantly associated with total AHI but not demographics, anthropometrics, and other PSG data.
Two prior studies have examined hyoid dynamics in OSA patients. Kohno et al examined hyoid displacement via lateral neck radiographs of 50 patients (30 patients with OSA) undergoing general anesthesia. Similar to our patients’ hyoid motion during inspiration, increasing lung volumes resulted in caudal displacement of the hyoid.23 Comparisons of values of hyoid displacement between these studies are challenging as this study did not examine the influence of obstructive breathing and muscle paralysis differs from drug-induced sleep.24 Hofauer et al investigated hyoid and tongue protrusion in response to hypoglossal nerve stimulation (HGNS) using ultrasonography in 16 patients.25 The authors showed mean hyoid displacement during stimulation to be 5.7±3.9mm. Despite differences in hyoid directionality, our data share two features with this work: magnitude and variability of hyoid motion. Of note, Hofauer and colleagues did not specify the sleep-wake state of the patients during ultrasound acquisition, making difficult additional comparisons to our dataset.
In our study, hyoid displacement amplitude during obstructive breathing varied significantly amongst patients. Given the hyoid’s position between the trachea and mandible, we examined literature to better inform our understanding of hyoid motion. Tong et al used dynamic awake MRI to evaluate tracheal displacement in normal and OSA individuals and found caudal tracheal displacement (TD) on inspiration.26 Although their study was not conducted during obstructive breathing, they found increased TD magnitude to associate with AHI (r=0.47) as well. As the hyoid and trachea are linked by ligaments and muscles, these findings support the findings in the current investigation regarding the directionality and clinical associations of hyoid displacement. Pepin et al evaluated mandibular motion and epiglottic pressure signals in patients undergoing PSG and found a strong relationship between mandibular motion and respiratory effort (r>0.9).27 Given the relationship between respiratory effort and these adjacent structures, we are currently investigating the relationship between respiratory effort and hyoid displacement in OSA patients during DISE.
Static hyoid position in the upper airway is associated with OSA severity.15,28 Our results suggest increased dynamic hyoid movement in humans during DISE is also related to OSA severity. The mechanisms which determine static hyoid position and dynamic hyoid motion may be different altogether, though their association has not been studied.29 Previous studies suggest increased soft tissue volume within a given cranial dimension dictates inferior hyoid position.28,30 Other investigations suggested negative intrathoracic pressure generation to be a driver for dynamic tracheal and hyoid displacement, and suggested caudal displacement of these structures may be compensatory to stabilize the airway.26,29,31 However, as this compensatory role has not yet been demonstrated in human OSA patients, the possibility of hyoid movement as simply a symptom of greater respiratory effort remains. Thus, we aim to investigate the relationship and associated mechanism between static hyoid position and dynamic hyoid motion.
Stabilization procedures targeting the hyoid bone have demonstrated mixed OSA outcomes.17,18,32 Two common approaches for hyoid suspension, hyomandibular suspension and hyothyroidopexy, aim to move the hyoid anteriorly and either superiorly (to mandible) or inferiorly (to thyroid cartilage).33,34 Studies have suggested this anterior movement of the hyoid to be critical for improved airway resistance and decreased collapsibility.35,36 However, Amatoury et al showed caudal hyoid displacement to improve airway patency and reduce extraluminal peri-pharyngeal pressure, indicating the compensatory value of caudal hyoid displacement.29 Thus, although hyoid suspension procedures move the hyoid anteriorly, they may also reduce hyoid mobility precluding a key compensatory mechanism in maintaining airway patency. Therefore, evaluating caudal hyoid mobility before and after surgery may help understand the mechanism of action and related outcomes of these procedures.
Our study has some notable limitations. With regards to our patient population, our study sample was both small and without control patients. With regards to DISE, instability of sedation plane can impact respiratory function and potentially confound measures of hyoid displacement.10 Additionally, the secondary function of CPAP as a pneumatic stent has been described, thus it may contribute to the attenuation of hyoid displacement at PhOP. Further, PAP administration may have altered the position of the hyoid in the upper airway, influencing its mobility. Our ultrasound acquisition and analysis protocol also has limitations. Due to our acquisition learning curve, initial patients had non-standardized data which failed to capture the hyoid during our events of interest and were thereby excluded. The C6-2 curvilinear ultrasound probe did not fit some patients either due to body habitus or excessive facial hair. Although care was taken to minimize the pressure of the ultrasound probe on the submental space, this potentially could have influenced free motion of the hyoid. Additionally, while ultrasound itself is an accessible device, the analysis software to generate motiongrams is proprietary, limiting access. In quantification of hyoid displacement, we did not account for mandibular motion, which may artificially augment our measures of hyoid displacement. Finally, due to both craniocaudal and anteroposterior variability in hyoid position and mandibular motion on inspiration, quantification of hyoid displacement in the craniocaudal and AP axes was not feasible. Future work will consider visualizing static anatomic reference points (e.g. cervical spine) to enable vector analysis of hyoid movement.
However, our study bears several strengths. Our DISE platform allows for synchronous view of physiologic, ultrasonographic, and endoscopic data (Video S2). This integration of signals is paramount for accurate analysis across data streams. In addition, our application of PAP enables both measures of upper airway collapsibility and observation of the non-flow limited state. Ultrasonography is also non-invasive, non-radiating, portable, and relatively inexpensive. Finally, our secondary outcomes showed excellent reliability, similar to other non-OSA studies.20
Conclusion
Ultrasonography during DISE-PAP is a novel, reliable method to quantify hyoid displacement during DISE. We observed a large reduction in hyoid displacement between obstructive and non-obstructive breathing with significant variability among patients. While the clinical significance of static hyoid position in OSA has been reported, this investigation provides a methodologic framework for further study of dynamic hyoid movement in OSA patients.
Supplementary Material
S1: Hyoid Displacement Quantification Methodology.
A detailed ultrasound imaging acquisition and analysis protocol for quantification of hyoid motion during drug-induced sleep endoscopy.
S2: ICC values for measures of hyoid displacement captured at baseline and PhOP by two raters (M.H.P. and V.T.)
S3: Associations between Hyoid Displacement and Demographics, Anthropometrics, and Polysomnographic Data
* Predicted change in hyoid displacement (mm) for 1 unit increase in variable; **Effect size = Expected SD change in hyoid displacement for 1 SD increase in variable
Video S2: Generation of Motiongrams using Imagelab
Hyoid displacement is contained within the drawn m-mode vector. Motiongram animates in as breaths proceed, demonstrating the motion curve represents hyoid displacement across the specified vector. The ultrasound clip was originally 30 seconds long, and was sped up 6x using Lumafusion (LumaTouch, Seattle, WA, USA) for purposes of synchrony with motiongram animation. The motiongram demonstrates time in seconds on the x axis and distance in millimeters on the y axis.
Video S1: Hyoid Motion during DISE-PAP Synchronized View
This video demonstrates the synchronicity between the endoscopic view and submental ultrasound during DISE-PAP. This clip was taken during an ultrasound clip recording at baseline with obstructive breathing. The top-most channel is the PAP level, here indicating there was no positive pressure being applied. Second is the “Diff Pressure” channel, which represents the airflow captured by the pneumotachometer. The third channel is our retro-epiglottic catheter, which is a measure of respiratory effort during DISE. The fourth channel is SpO2%, our pulse oximetry recording. This recording occurs during flow limited breathing. Additionally, on endoscopy, there is a view of the tongue base and epiglottis. Ultrasonography shows hyoid motion away from the mandible during inspiration.
Acknowledgements:
Kendra Troske, BA, assisted with study coordination and data organization. Theodore Cary, MS, developed Imagelab analysis program on IDL programming language.
Funding:
This study was made possible by a grant from the National Institute of Health (1R01HL144859-04).
Footnotes
Conflicts of Interest
MHP: None
ET: grant research funding by NIH, Inspire
VT: None
ES: Consultant for Inspire Medical
CS: None
SS: None
BTK: Salary for consulting work with Biomedical Statistical Consulting
ARS: Consultant for Inspire Medical, ZOLL, Respicardia, MERZ Pharmaceutical, and invited talks to Intersect Inc
RCD: grant research funding by NIH, Inspire, Nyxoah Medical
This manuscript was presented at the International Society of Sleep Surgeons 2022 Annual Meeting in Philadelphia, PA, September 9-10th, 2022
References
- 1.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931 [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108(5):246–249. [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea – New pathways for targeted therapy. Sleep Medicine Reviews. 2018;37:45–59. doi: 10.1016/j.smrv.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Blumen M, Bequignon E, Chabolle F. Drug-induced sleep endoscopy: A new gold standard for evaluating OSAS? Part II: Results. European Annals of Otorhinolaryngology, Head and Neck Diseases. 2017;134(2):109–115. doi: 10.1016/j.anorl.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 8.Aktas O, Erdur O, Cirik AA, Kayhan FT. The role of drug-induced sleep endoscopy in surgical planning for obstructive sleep apnea syndrome. European Archives of Oto-Rhino-Laryngology. 2015;272(8):2039–2043. doi: 10.1007/s00405-014-3162-8 [DOI] [PubMed] [Google Scholar]
- 9.Kezirian EJ, Hohenhorst W, De Vries N. Drug-induced sleep endoscopy: The VOTE classification. European Archives of Oto-Rhino-Laryngology. 2011;268(8):1233–1236. doi: 10.1007/s00405-011-1633-8 [DOI] [PubMed] [Google Scholar]
- 10.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2013;9(5):433–438. doi: 10.5664/jcsm.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold AR, Schwartz AR. The Pharyngeal Critical Pressure. Chest. 1996;110(4):1077–1088. doi: 10.1378/chest.110.4.1077 [DOI] [PubMed] [Google Scholar]
- 12.Dedhia RC, Seay EG, Schwartz AR. Beyond VOTE: The New Frontier of Drug-Induced Sleep Endoscopy. ORL. Published online September 27, 2021:1–6. doi: 10.1159/000518660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seay EG, Keenan BT, Schwartz AR, Dedhia RC. Evaluation of Therapeutic Positive Airway Pressure as a Hypoglossal Nerve Stimulation Predictor in Patients With Obstructive Sleep Apnea. JAMA Otolaryngol Head Neck Surg. 2020;146(8):691. doi: 10.1001/jamaoto.2020.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol (1985). 2007;102(2):547–556. doi: 10.1152/japplphysiol.00282.2006 [DOI] [PubMed] [Google Scholar]
- 15.Genta PR, Schorr F, Eckert DJ, et al. Upper Airway Collapsibility is Associated with Obesity and Hyoid Position. Sleep. 2014;37(10):1673–1678. doi: 10.5665/sleep.4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilici S, Yigit O, Celebi OO, Yasak AG, Yardimci AH. Relations Between Hyoid-Related Cephalometric Measurements and Severity of Obstructive Sleep Apnea. Journal of Craniofacial Surgery. 2018;29(5). https://journals.lww.com/jcraniofacialsurgery/Fulltext/2018/07000/Relations_Between_Hyoid_Related_Cephalometric.37.aspx [DOI] [PubMed] [Google Scholar]
- 17.Bowden MT, Kezirian EJ, Utley D, Goode RL. Outcomes of hyoid suspension for the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2005;131(5):440–445. doi: 10.1001/archotol.131.5.440 [DOI] [PubMed] [Google Scholar]
- 18.Song SA, Wei JM, Buttram J, et al. Hyoid surgery alone for obstructive sleep apnea: A systematic review and meta-analysis. The Laryngoscope. 2016;126(7):1702–1708. doi: 10.1002/lary.25847 [DOI] [PubMed] [Google Scholar]
- 19.Schwab RJ, Wang SH, Verbraecken J, et al. Anatomic predictors of response and mechanism of action of upper airway stimulation therapy in patients with obstructive sleep apnea. Sleep. 2018;41(4):1–12. doi: 10.1093/sleep/zsy021 [DOI] [PubMed] [Google Scholar]
- 20.Chen YC, Hsiao MY, Wang YC, Fu CP, Wang TG. Reliability of Ultrasonography in Evaluating Hyoid Bone Movement. J Med Ultrasound. 2017;25(2):90–95. doi: 10.1016/j.jmu.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter SG, Carberry JC, Grunstein RR, Eckert DJ. Polysomnography with an epiglottic pressure catheter does not alter obstructive sleep apnea severity or sleep efficiency. J Sleep Res. 2019;28(5):e12773. doi: 10.1111/jsr.12773 [DOI] [PubMed] [Google Scholar]
- 22.Atkins JH, Mandel JE, Rosanova G. Safety and Efficacy of Drug-Induced Sleep Endoscopy Using a Probability Ramp Propofol Infusion System in Patients with Severe Obstructive Sleep Apnea. Anesthesia & Analgesia. 2014;119(4):805–810. doi: 10.1213/ANE.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 23.Kohno A, Kitamura Y, Kato S, et al. Displacement of the hyoid bone by muscle paralysis and lung volume increase: the effects of obesity and obstructive sleep apnea. Sleep. 2019;42(1). doi: 10.1093/sleep/zsy198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehsan Z, Mahmoud M, Shott SR, Amin RS, Ishman SL. The effects of Anesthesia and opioids on the upper airway: A systematic review: Anesthesia and Opioids Effects on the Upper Airway. The Laryngoscope. 2016;126(1):270–284. doi: 10.1002/lary.25399 [DOI] [PubMed] [Google Scholar]
- 25.Hofauer B, Strohl K, Knopf A, et al. Sonographic evaluation of tongue motions during upper airway stimulation for obstructive sleep apnea—a pilot study. Sleep and Breathing. 2017;21(1):101–107. doi: 10.1007/s11325-016-1383-3 [DOI] [PubMed] [Google Scholar]
- 26.Tong J, Jugé L, Burke PG, et al. Respiratory-related displacement of the trachea in obstructive sleep apnea. J Appl Physiol (1985). 2019;127(5):1307–1316. doi: 10.1152/japplphysiol.00660.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepin JL, Le-Dong NN, Cuthbert V, et al. Mandibular Movements are a Reliable Noninvasive Alternative to Esophageal Pressure for Measuring Respiratory Effort in Patients with Sleep Apnea Syndrome. Nat Sci Sleep. 2022;14:635–644. doi: 10.2147/NSS.S346229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. European Respiratory Journal. 2011;38(2):348–358. doi: 10.1183/09031936.00119210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amatoury J, Kairaitis K, Wheatley JR, Bilston LE, Amis TC. Peripharyngeal tissue deformation and stress distributions in response to caudal tracheal displacement: pivotal influence of the hyoid bone? Journal of Applied Physiology. 2014;116(7):746–756. doi: 10.1152/japplphysiol.01245.2013 [DOI] [PubMed] [Google Scholar]
- 30.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):522–530. doi: 10.1164/rccm.200208-866OC [DOI] [PubMed] [Google Scholar]
- 31.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. Journal of Applied Physiology. 1991;70(3):1328–1336. doi: 10.1152/jappl.1991.70.3.1328 [DOI] [PubMed] [Google Scholar]
- 32.Sher AE, Schechtman KB, Piccirillo JF. The Efficacy of Surgical Modifications of the Upper Airway in Adults With Obstructive Sleep Apnea Syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156 [DOI] [PubMed] [Google Scholar]
- 33.Mickelson S Hyoid advancement to the mandible (hyo-mandibular advancement). Operative Techniques in Otolaryngology-Head and Neck Surgery. 2012;23:56–59. doi: 10.1016/j.otot.2011.07.002 [DOI] [Google Scholar]
- 34.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea and the hyoid: a revised surgical procedure. Otolaryngol Head Neck Surg. 1994;111(6):717–721. doi: 10.1177/019459989411100604 [DOI] [PubMed] [Google Scholar]
- 35.Samaha CJ, Tannous HJ, Salman D, Ghafari JG, Amatoury J. Role of surgical hyoid bone repositioning in modifying upper airway collapsibility. Front Physiol. 2022;13:1089606. doi: 10.3389/fphys.2022.1089606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbluth KH, Kwiat DA, Harrison MR, Kezirian EJ. Hyoid Bone Advancement for Improving Airway Patency. Otolaryngology–Head and Neck Surgery. 2012;146(3):491–496. doi: 10.1177/0194599811429522 [DOI] [PubMed] [Google Scholar]
- 37.Adliff M, Ngato D, Keshavjee S, Brenaman S, Granton JT. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997;112(6):1701–1704. doi: 10.1378/chest.112.6.1701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: Hyoid Displacement Quantification Methodology.
A detailed ultrasound imaging acquisition and analysis protocol for quantification of hyoid motion during drug-induced sleep endoscopy.
S2: ICC values for measures of hyoid displacement captured at baseline and PhOP by two raters (M.H.P. and V.T.)
S3: Associations between Hyoid Displacement and Demographics, Anthropometrics, and Polysomnographic Data
* Predicted change in hyoid displacement (mm) for 1 unit increase in variable; **Effect size = Expected SD change in hyoid displacement for 1 SD increase in variable
Video S2: Generation of Motiongrams using Imagelab
Hyoid displacement is contained within the drawn m-mode vector. Motiongram animates in as breaths proceed, demonstrating the motion curve represents hyoid displacement across the specified vector. The ultrasound clip was originally 30 seconds long, and was sped up 6x using Lumafusion (LumaTouch, Seattle, WA, USA) for purposes of synchrony with motiongram animation. The motiongram demonstrates time in seconds on the x axis and distance in millimeters on the y axis.
Video S1: Hyoid Motion during DISE-PAP Synchronized View
This video demonstrates the synchronicity between the endoscopic view and submental ultrasound during DISE-PAP. This clip was taken during an ultrasound clip recording at baseline with obstructive breathing. The top-most channel is the PAP level, here indicating there was no positive pressure being applied. Second is the “Diff Pressure” channel, which represents the airflow captured by the pneumotachometer. The third channel is our retro-epiglottic catheter, which is a measure of respiratory effort during DISE. The fourth channel is SpO2%, our pulse oximetry recording. This recording occurs during flow limited breathing. Additionally, on endoscopy, there is a view of the tongue base and epiglottis. Ultrasonography shows hyoid motion away from the mandible during inspiration.