Abstract
Objective:
To determine (A) if differences in executive function (EF) and cognition precede weight gain or (B) if weight gain causes changes to EF and cognition.
Methods:
Data were gathered from the Adolescent Brain Cognitive Development Study (4.0 release; 9-to-12-years-old, n=2,794, 100% healthy weight at baseline [i.e., 9/10-years-old], 12.4% unhealthy weight by 11/12-years-old). EF and cognition were assessed across several domains (e.g., impulsivity, inhibitory control, processing speed, memory); body mass index (BMI) was calculated from height and weight. Nested random-effects mixed models examined (A) BMI~EF*Time (variation in EF/cognition precedes weight gain) and (B) EF~BMI*Time (weight gain causes changes to EF/cognition) and controlled for sex, puberty, and caregiver education; random effects were site and subject.
Results:
Variation in impulsivity, memory, learning, and processing speed were associated with greater increases in BMI trajectories from 9-to-12-years-old. Weight gain was associated with a decrease in inhibitory control, but no other associations were observed.
Conclusion:
Underlying variation in EF and cognition may be important for weight gain, but 2-years of weight gain may not be enough to have clinical implications on EF and cognition beyond inhibitory control. These findings suggest that more attention should be paid to including EF programs in obesity prevention efforts.
Keywords: pediatric obesity, executive function, weight gain, adolescence, decision-making
1. Introduction
Prior to the COVID-19 pandemic, 20% of US children had obesity(1) but the disruption of daily routines (e.g., diminished physical activity, increased food access) increased rates dramatically.(2) Childhood obesity is associated with several preventable early onset medical comorbidities like diabetes, cardiovascular disease, cancer, and dementia.(3) Because it is highly likely that children with obesity will become adults with obesity,(4) there is a dire need to understand its causes and create effective interventions. Although the contributing casual factors of obesity are vast and multidimensional,(3) it is undeniable that the brain plays a key role in facilitating and maintaining obesity:(5) internal signals (e.g., neurotransmitters, gut hormones) initiate and terminate food intake to maintain homeostasis. However, external stimuli (e.g., visual stimuli, taste, smell) can override homeostatic mechanisms, and induce hedonic overeating at times when energy reserves are plenty (i.e., eating in the absence of hunger).(6) Hedonic overeating is thought to be controlled by brain processes that are involved in executive function (EF).(7)
The most common definition of EF includes three cores: cognitive flexibility, working memory, and inhibition, which then relate to higher order EFs such as reasoning, planning and problem solving.(8,9) In the current manuscript, we expanded the definition of EF to include domains that do not typically fall within these core functions (e.g., reading comprehension, picture vocabulary), as others have advocated for a broader framework (10,11) that is not limited to traditional definitions. (8,9) For instance, measures of impulsivity are better integrated in hot vs. cold EF models as these arguably could be reflective of failures in top-down regulation (cold system, inhibition).(12) This broader framework was chosen because these processes facilitate other cognitive abilities and regulate behavior; deficits in these processes are thought to have maladaptive consequences (e.g., poor eating habits, smoking).(13) In both adults and children, behavioral deficits in EF have been associated with having obesity,(14-18) increased food intake,(19) and continued weight gain.(12) The Dual Process Model of Overeating postulates that hedonic overeating and subsequent weight gain may be related to an underlying inability to suppress unnecessary food intake (i.e., deficits in EF).(9) However, animal models have shown that overeating has downstream effects on cognition, like EF.(20) Thus, postulating that weight gain has a casual role on EF deficits.
To date, most studies evaluating the relationship between EF and weight status have been cross-sectional, limited in scope, or conducted in small samples. Thus, the mechanism underlying EF’s role in obesity facilitation and maintenance is unknown. Furthermore, little is known about which EFs are most related to unhealthy weight gain. Moreover, childhood through adolescence is a period in which the brain is undergoing rapid changes in several regions associated with EF.(21) Yet, it is unknown if (A) weight gain during this time disrupts or accelerates normative development or its functional consequences; and (B) which EF may be more sensitive to weight gain during this critical time. Understanding how EF and weight gain coincide during development may offer potential insights for behavioral (e.g., neurocognitive) treatment and intervention programs.
In the current report, we evaluated A) if differences in EF and cognition precede weight gain (i.e., the Dual Processes Model of Overeating); or B) if weight gain causes changes to EF and cognition. Weight gain was modeled with respect to changes in body mass index (BMI, where a positive change would be a proxy for weight gain) across a two-year period in pre and early adolescence in a subsample of youth enrolled in the Adolescent Brain Cognitive Development (ABCD) Study® (n=2,794, ages 9/10-to-11/12-years-old, 4.0 release). Importantly, these associations were evaluated in a subsample of youth who were initially of a healthy weight at baseline (i.e., 9/10-years-old). As a number of these youth (n=346) transitioned to unhealthy weights (e.g., overweight/obese) within a two-year period, the current study can evaluate the natural concurrent progressions of changes in EF and cognition and BMI. Here, we report findings across multidimensional aspects of EF and cognition to better understand how differences in EF and cognition may predispose some youth to gain unhealthy weight and how BMI relates to changes in EF and cognition.
2. Methods
2.1. Study design
The ABCD Study® is a 21-site 10-year cohort study that enrolled 11,878 youth aged 9/10-years-old at baseline (08/2016-10/2018). Recruitment was tailored to match the demographic population of the United States Census. Details pertaining to study design, assessments, objectives, and protocols are published in numerous documents and on their website (www.ABCDStudy.org). Assessments are conducted annually but the protocol is varied. Broadly, the ABCD Study® was designed to assess cognitive and health development throughout adolescence. The current manuscript focused on assessments from baseline (9/10-years-old) and the two-year follow-up (11/12-years-old), and analyses were restricted to a subset of youth who were initially of a healthy weight with useable data at each time point (n=2,794, 12.4% had overweight/obesity by the age 11/12-years-old, 51.2% male, 72.8% White). Details pertaining to overall ABCD Study® inclusion as well as exclusion criteria that was applied to obtain a sample optimal for our analyses are available in the Supplemental Materials. Assessments included in this manuscript were limited to anthropometrics, demographics, and task and questionnaire-based assessments of EF and cognition.
2.2. Physical health assessments
Participants were weighed to the nearest 0.1 at each visit by a trained researcher in light clothing using a physician’s scale (Detecto model 439, Webb City, MO.) Height was assessed on the same scale, with the youth’s heels against the height rod. Two measurements were acquired, while a third was taken if measurements varied by more than ¼ of an inch or 0.1lb. Height and weight were converted into BMI (kg/m2) and BMI percentiles according to the CDC’s sex-age-height-weight specific growth charts(22) for clinical interpretations. Unadjusted (raw) BMI values were used in the statistical analyses to evaluate within-subject change. The Pubertal Development Scale(23) was administered yearly to caregivers and youth. Details regarding physical health assessments are published elsewhere,(24) and in the Supplemental Materials.
2.3. Demographics
Caregivers reported on the youth’s race and ethnicity, sex at birth, date of birth, family structure, and socioeconomic status (e.g., education) of the family (see Supplemental Materials for details).
2.4. EF and cognitive assessment:
Biennial assessments of EF and cognition were conducted at the baseline and two-year follow-up. EF trait-based questionnaires that measured impulsivity and (the lack of) inhibition, and reward consisted of the Behavioral Inhibition System / Behavioral Approach System (BIS/BAS) and the Urgency, Premeditation (lack of), Perseverance (lack of), Sensation Seeking, Positive Urgency, Impulsive Behavioral Scale (UPPS-P; [i.e., impulsivity assessment]). EF and cognitive tasks included assessments of inhibitory control, proxies of working memory, processing speed, verbal/visual learning and memory, and vocabulary and were assessed with the National Institutes of Health (NIH) Toolbox, Rey Auditory Verbal Learning Test (RAVLT), and the Little Man Task. Although not all measures fit the common definition of EF, these were close approximations, so we will use the term EF to refer to both classic EF and other cognitive processes that depend on optimal EF. Details for these assessments are available elsewhere,(24) and, in the Supplemental Materials.
3. Analysis
3.1. Linear mixed-effects modeling
Data preprocessing and analyses were conducted using Python (version 3.8.5) on a Mac computer (OS 12.5.1). Prior to analyses, data were checked for skewness, kurtosis, and multicollinearity using the variance inflation factor (statsmodels version 0.12.2). Outliers were removed if values were 3>SD ± mean. Continuous independent variables were transformed using scikit-learn’s standard scaler package (version 0.24.2). Mixed models were conducted using the pymer4 package (version 0.7.7; https://github.com/ejolly/pymer4). Model 1 focused on whether EF*Time related to the rate of change in BMI (see Model 1). Model 2 focused on whether BMI*Time related to the rate of change in EF (see Model 2 below). Time refers to data collection that occurred at baseline (e.g., Time 0, ages 9/10-years-old) and the two-year follow-up (e.g., Time 2, ages 11/12-years-old). Random intercepts (e.g., subject nested within ABCD site) were included to account for variation between sites and within subject. Fixed factors (e.g., sex, education) were effects coded. Age and puberty were colinear, so, we chose to only include puberty as it has robust associations with EF maturation.(25) Models were corrected using the Benjamini-Hochberg method(26) separately for each domain (e.g., UPPS, BIS/BAS, NIH Toolbox) and corrected for the interaction term (i.e., effect of interest).
4. Results
4.1. General results
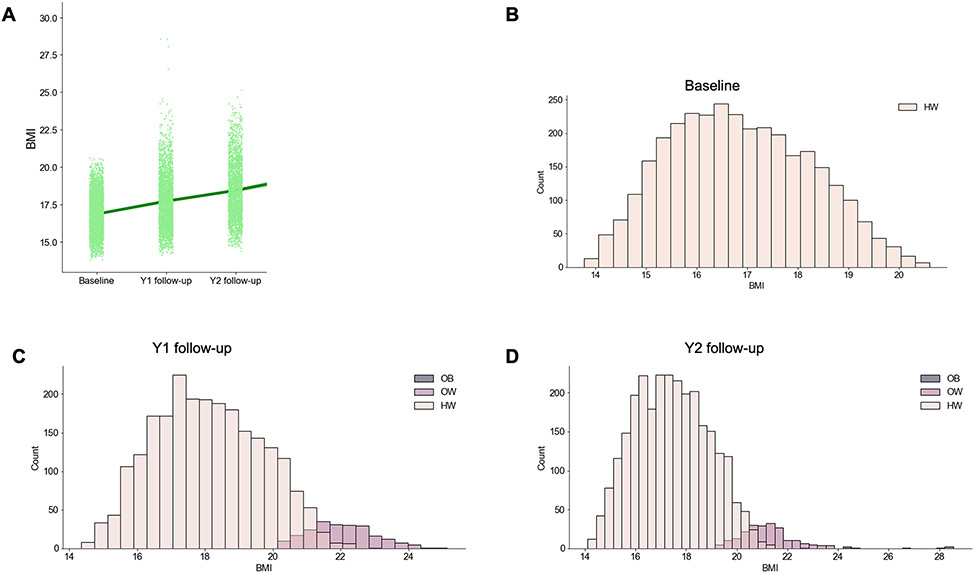
The number of participants included for each analysis differed across tasks (see Supplemental Materials Table 1 for the N’s included per task; Table 1 displays the demographics). At baseline, all youth were of a healthy weight, but, by the two-year follow-up, 323 youth transitioned to have overweight (11.6%), and 23 transitioned to have obesity (0.8%). Additionally, by the two-year-follow-up, 151 youth had extreme weight gain (as defined in Adise et al., (2022) (27)), while 38 youth had extreme weight gain but were still classified as having a healthy weight at the two-year-follow-up. Notably, the demographics of the sample included in the analyses did differ from the entire sample with regard to race, ethnicity, and education. Figure 1 displays the distribution of BMI and weight status for each year of data collection.
Table 1.
Participant characteristics
| Whole sample (n=11,878) |
Subsample (n=2,794) | ||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p |
| Age (months) | |||||
| Baseline | 119 | 7.5 | 119 | 7.0 | 0.741 |
| Y2 | 143.5 | 7.6 | 142.8 | 7.0 | <0.001 |
| Puberty | |||||
| Baseline | 2 | 0.8 | 1.9 | 0.8 | <0.001 |
| Y2 | 2.7 | 1 | 2.6 | 1 | <0.001 |
| BMI | |||||
| Baseline | 18.8 | 3.8 | 17.0 | 1.4 | <0.001 |
| Y2 | 20.6 | 4.6 | 18.7 | 2.1 | <0.001 |
| n | % | n | % | ||
| Sex | |||||
| Male | 6192 | 52.1 | 1425 | 51.0 | 0.390 |
| Female | 5683 | 47.8 | 1369 | 49.0 | |
| Missing | 3 | ||||
| Race | |||||
| White | 7524 | 64.3 | 2032 | 72.7 | <0.001 |
| Black | 1869 | 16 | 277 | 9.9 | |
| Asian | 275 | 2.3 | 75 | 2.7 | |
| AIAN/NHPI | 78 | 0.7 | 22 | 0.8 | |
| Other | 525 | 4.5 | 96 | 3.4 | |
| Multi-race | 1434 | 12.3 | 277 | 10.5 | |
| Ethnicity | |||||
| Hispanic | 2411 | 20.6 | 414 | 14.8 | <0.001 |
| Non-Hispanic | 9312 | 79.4 | 2380 | 85.2 | |
| Caregiver report of education | |||||
| <HS | 568 | 4.8 | 76 | 2.7 | <0.001 |
| HS/GED | 1079 | 9.1 | 143 | 5.1 | |
| Some College | 2978 | 25.1 | 592 | 21.2 | |
| BA degree | 2969 | 25 | 803 | 28.7 | |
| Postgraduate degree | 3987 | 33.6 | 1180 | 42.2 | |
| Missing | 295 | 2.5 | |||
| Baseline Weight Class | |||||
| Underweight | 468 | 3.9 | |||
| Healthy Weight | 7601 | 64.0 | 2794 | 100 | <0.001 |
| Overweight | 1801 | 15.2 | |||
| Obese | 1992 | 16.8 | |||
| Missing | 16 | 0.1 | |||
| Y2 Weight Class | |||||
| Underweight | 286 | 2.7 | |||
| Healthy Weight | 4818 | 46.3 | 2448 | 87.6 | <0.001 |
| Overweight | 1216 | 11.7 | 323 | 11.6 | |
| Obese | 1381 | 13.3 | 23 | 0.8 | |
| Missing | 2714 | 26.1 | |||
Note. Participant characteristics are displayed for the largest possible N. Thus, the participant characteristics may differ slightly for those youth who were included in each analysis. Y2 = year 2 follow-up; BMI = body mass index; AIAN/NHPI = American Indian, Alaska Native/Native Hawaiian, Pacific Islander; HS = high school; GED = Generalized Education Degree; BA = bachelor’s degree. Descriptive statistics are displayed by caregiver self-reported race only for interpretation of sample diversity. P-values reflect chi-squared and t-tests were appropriate.
Figure 1.
Body mass index (BMI) and weight status classification (according to the CDC age-sex-weight-height growth charts) distributions. HW = healthy weight; OW = overweight; OB = obese. B-D represent the number of youth who transitioned from a healthy weight to having overweight or obesity by the year 1 (Y1) and year 2 (Y2) follow-up visits.
4.2. Does Underlying Differences in EF Relate to Greater Increases in BMI? A test of the Dual Processes Model of Overeating
4.2.1. UPPS
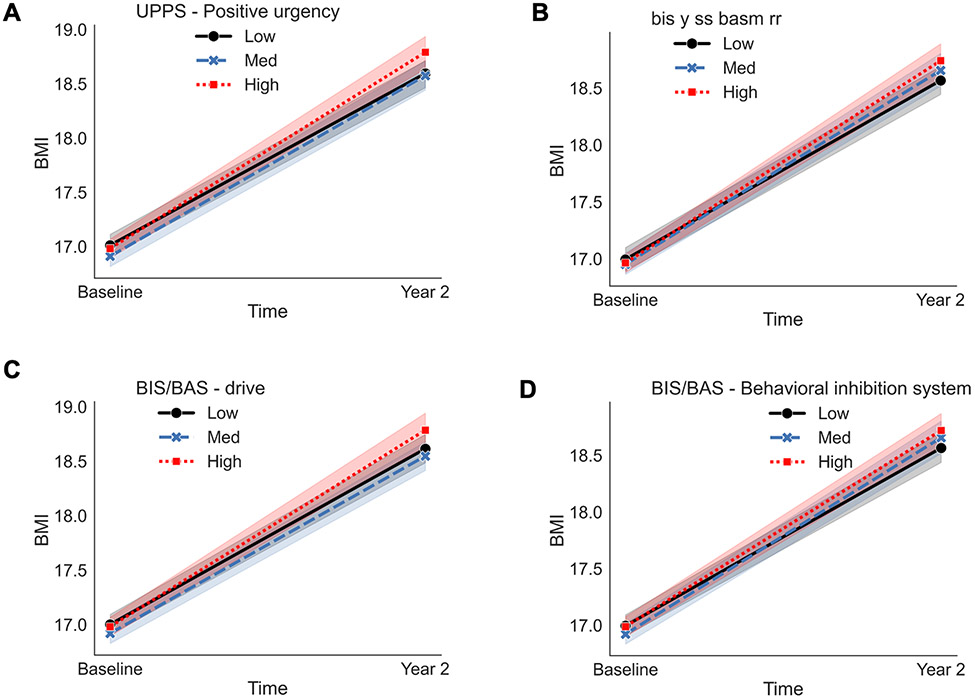
Significant EF*Time interactions on BMI were observed for the Positive Urgency subscale, where greater Positive Urgency scores were associated with greater increases in the rate of change in BMI over the two-years (β=0.12, p<0.001, Figure 2A). No other associations were observed and all results are reported in Table 2.
Figure 2.
Observed data are plotted on the x- and y-axis for each assessment measurement at levels of −1 standard deviation (SD; low, solid black line with circle end points), the mean (e.g., med, blue, large dashed line with x end points), and +1 SD (e.g., high, red mini dashed line with square end points). BMI = body mass index. UPPS = Urgency, Premeditation (lack of) Perseverance (lack of), Sensation Seeking, Positive Urgency, Impulsive Behavior Scale. BIS/BAS = Behavioral Inhibition System/ Behavioral Approach System. Baseline = ages 9/10-years-old; Year 2= ages 11/12-years-old. The mixed model was adjusted for sex, puberty, and caregiver education. Fixed effects were effects coded and random effects were modeled for ABCD Study® site and subject. An interaction term was included for EF*Time (e.g., baseline [ages, 9/10-years-old], Year 2 [ages 11/12-years-old]).
Table 2.
The change in EF by Time on BMI from baseline to the two-year follow-up.
| Interaction | β | 95% CI | DF | T-stat | p | Sig |
|---|---|---|---|---|---|---|
| UPPS | ||||||
| Negative Urgency * Time | 0.05 | [−0.02, 0.12] | 3192.98 | 1.36 | 0.17 | |
| Lack of Planning * Time | 0 | [−0.06, 0.07] | 3096.1 | 0.06 | 0.95 | |
| Sensation Seeking * Time | 0 | [−0.06, 0.07] | 3007.36 | 0.13 | 0.89 | |
| Positive Urgency * Time | 0.12 | [0.06, 0.19] | 3144.44 | 3.63 | <0.001 | *** a |
| Lack of Perseverance * Time | 0.04 | [−0.02, 0.1] | 3052.97 | 1.19 | 0.23 | |
| BIS/BAS | ||||||
| BIS * Time | 0.08 | [0.02, 0.15] | 3146.54 | 2.42 | 0.02 | * a |
| Drive * Time | 0.08 | [0.01, 0.15] | 3155.46 | 2.38 | 0.02 | * a |
| Reward Response * Time | 0.07 | [0.0, 0.14] | 3233.14 | 1.96 | 0.05 | * |
| Fun Seeking * Time | 0.03 | [−0.04, 0.1] | 3212.09 | 0.84 | 0.4 | |
| Little Man Task | ||||||
| Efficiency * Time | 0.09 | [0.02, 0.164] | 3574.21 | 2.51 | 0.01 | * a |
| NIH Toolbox | ||||||
| Flanker Inhibitory Control * Time | −0.08 | [−0.15, −0.0] | 2981.9 | −2.1 | 0.04 | * a |
| Picture Vocabulary * Time | −0.08 | [−0.14, −0.02] | 2687.8 | −2.4 | 0.02 | * a |
| Pattern Comparison * Time | −0.07 | [−0.15, 0.0] | 2875.43 | −1.94 | 0.05 | |
| Reading Comprehension * Time | −0.08 | [−0.14, −0.01] | 2645.87 | −2.35 | 0.02 | * a |
| Picture Sequence Memory * Time | −0.1 | [−0.16, −0.03] | 2928.79 | −2.7 | <0.01 | ** a |
| RAVLT | ||||||
| Learning * Time | −0.125 | [−0.195, −0.054] | 2286.903 | −3.465 | 0.001 | ***a |
| List B correct * Time | −0.148 | [−0.223, −0.074] | 2475.644 | −3.898 | <0.001 | ***a |
| Immediate Delay * Time | −0.143 | [−0.215, −0.072] | 2325.556 | −3.93 | <0.001 | ***a |
| Long Delay * Time | −0.146 | [−0.217, −0.075] | 2303.462 | −4.016 | <0.001 | ***a |
| Repetition List B * Time | 0.015 | [−0.066, 0.096] | 2781.802 | 0.356 | 0.722 | |
| Repetition Immediate Delay * Time | 0.024 | [−0.056, 0.103] | 2707.237 | 0.582 | 0.561 | |
| Repetition Long Delay * Time | −0.039 | [−0.121, 0.044] | 2694.271 | −0.912 | 0.362 | |
| Total Repetitions * Time | −0.009 | [−0.085, 0.067] | 2490.833 | −0.232 | 0.816 | |
| Intrusions List B * Time | 0.022 | [−0.06, 0.104] | 2826.468 | 0.52 | 0.603 | |
| Intrusions Immediate Delay * Time | −0.059 | [−0.141, 0.022] | 2837.695 | −1.423 | 0.155 | |
| Intrusions Long Delay * Time | −0.005 | [−0.086, 0.076] | 2818.678 | −0.13 | 0.896 |
Note. All effects that were significant survived Benjamini-Hochberg correction. BMI = body mass index. RAVLT = Rey Auditory Verbal Listening Task; NIH = National Institutes of Health; BIS/BAS = Behavioral Inhibition System/Behavioral Approach System; UPPS = Urgency, Premeditation (lack of) Perseverance (lack of), Sensation Seeking, Positive Urgency, Impulsive Behavior Scale. Flanker Inhibitory Control and Attention Task was abbreviated in the table to Flanker Inhibitory Control; Pattern Comparison Processing Speed was abbreviated to Pattern Comparison in the table
survived correction
4.2.2. BIS/BAS
Significant EF*Time interactions on BMI were observed for the Drive and the Behavioral Inhibition System (BIS) subscales. Youth who scored higher on the Drive (e.g., persistent pursuit of desired goals, β=0.08, p=0.016) and BIS subscales (e.g., anticipation of punishment, β=0.08, p=0.017) had greater increases in the rate of change in BMI over the two-years (Figure 2C-D). No other associations were observed. All results are reported in Table 2.
4.2.3. NIH Toolbox
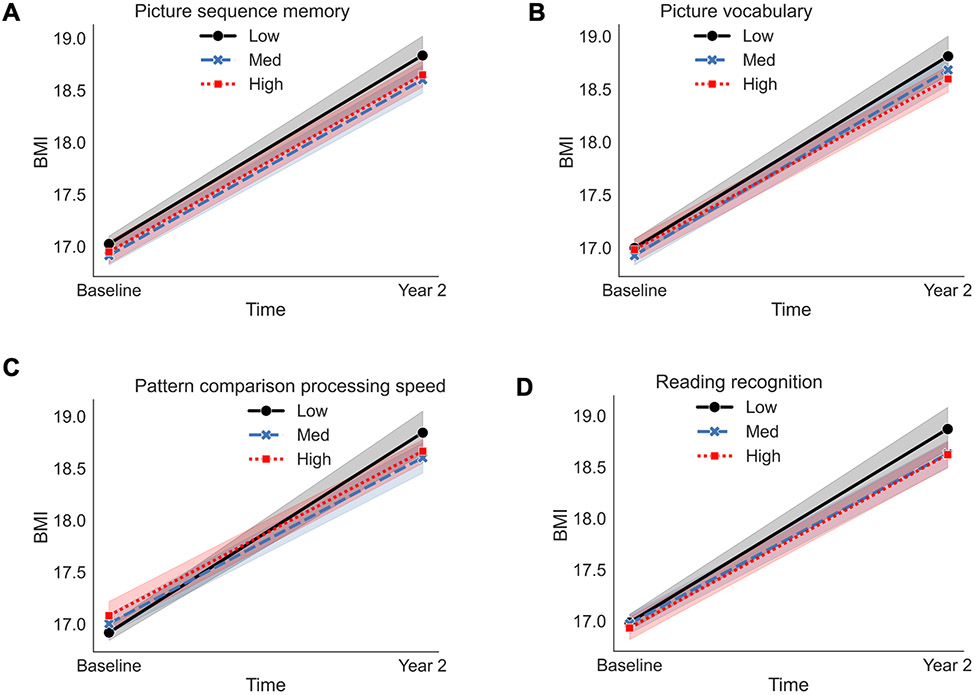
Significant EF*Time interactions on BMI were observed for 4 NIH Toolbox assessments, namely Flanker Inhibitory Control and Attention, Picture Vocabulary, Pattern Comparison Processing Speed, Reading Comprehension, and Picture Sequence Memory. Lower scores on the Flanker Inhibitory Control and Inhibitory Control (β=−0.08, p=0.036), Picture Vocabulary (i.e., verbal IQ, β=−0.08, p=0.017), Reading Comprehension (e.g., language, oral reading, β=−0.08, p=0.019), and Picture Sequence Memory (e.g., episodic memory, β=−0.1, p=0.007) were associated with greater increases in the rate of change in BMI over the two-years (Figure 3A-D). All results are reported in Table 2.
Figure 3.
Visualizations of the two-way interaction effects for the NIH Toolbox results for the Dual Processes Model of Overeating (i.e., underlying differences in EF are related to changes in weight status [dependent variable]). A-D represent observed data are plotted on the x- and y-axis for each assessment measurement at levels of −1 standard deviation (SD; low, solid black line with circle end points), the mean (e.g., med, blue, large dashed line with x end points), and +1 SD (e.g., high, red mini dashed line with square end points). The mixed model was adjusted for sex, puberty, and caregiver education. Fixed effects were effects coded and random effects were modeled for ABCD Study® site and subject. An interaction term was included for EF*Time (e.g., baseline [ages, 9/10-years-old], Year 2 [ages 11/12-years-old]). Low corresponds to lower performance. BMI = body mass index.
4.2.4. Little Man Task
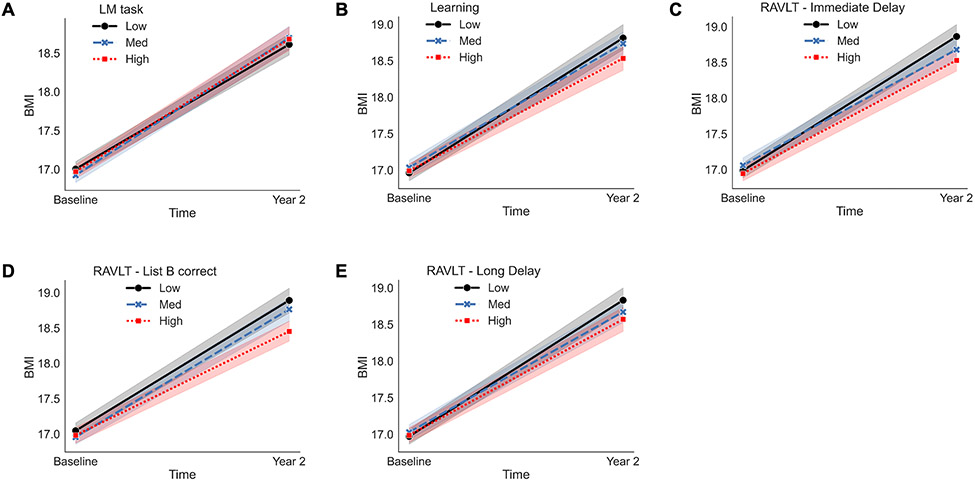
There was a significant EF*Time interaction on BMI for efficiency performance on the Little Man Task. Lower efficiency scores (e.g., visuospatial processing, β=−0.13, p=0.01) were associated with greater increases in the rate of change in BMI over two years (Figure 4A). All results are reported in Table 2.
Figure 4.
Visualizations of the two-way interaction effects for the Little Man Task (LM task) and Rey Auditory Verbal Listening Task (RAVLT) results for the Dual Processes Model of Overeating (i.e., underlying differences in EF are related to changes in weight status [dependent variable]). A-E represent observed data are plotted on the x- and y-axis for each assessment of measurement at levels of −1 standard deviation (SD; low, solid black line with circle end points), the mean (e.g., med, blue, large dashed line with x end points), and +1 SD (e.g., high, red mini dashed line with square end points). The mixed model was adjusted for sex, puberty, and caregiver education. Fixed effects were effects coded and random effects were modeled for ABCD Study® site and subject. An interaction term was included for EF*Time (e.g., baseline [ages, 9/10-years-old], Year 2 [ages 11/12-years-old]). Low corresponds to lower performance. BMI = body mass index.
4.2.5. RAVLT
Significant EF*Time interactions on BMI were observed for 4 outcomes on the RAVLT task. Lower scores on the Learning (β=−0.13, p<0.001), Immediate Recall (β=−0.14, p<0.001), Long Recall (β=−0.15, p<0.001), and Interference List B (β=−0.15, p<0.001) metrics were associated with increases in the rate of BMI over the two years (Figure 4B-E). All results are reported in Table 2.
4.3. Does weight gain lead to variation in EF?
There was a significant BMI*Time interaction on Flanker Inhibitory Control and Attention Task (β=−0.60, p=0.01). No other associations were observed (see Table 3).
Table 3.
The relationship of BMI by time on each EF assessment.
| Outcome variable | Interaction | β | 95% CI | DF | T-stat | p | Sig |
|---|---|---|---|---|---|---|---|
| UPPS | |||||||
| Negative Urgency | BMI * Time | 0.02 | [−0.12, 0.15] | 3363.55 | 0.28 | 0.78 | |
| Lack of Planning | BMI * Time | −0.06 | [−0.17, 0.06] | 3314.81 | −1 | 0.32 | |
| Sensation Seeking | BMI * Time | 0.08 | [−0.06, 0.21] | 3263.58 | 1.09 | 0.28 | |
| Positive Urgency | BMI * Time | 0.13 | [−0.01, 0.28] | 3339.42 | 1.78 | 0.08 | |
| Lack of Perseverance | BMI * Time | 0.05 | [−0.05, 0.16] | 3282.29 | 1.01 | 0.31 | |
| BIS/BAS | |||||||
| BIS | BMI * Time | 0.01 | [−0.19, 0.22] | 3344.46 | 0.13 | 0.89 | |
| Drive | BMI * Time | 0.11 | [−0.04, 0.26] | 3356.24 | 1.39 | 0.16 | |
| Reward Response | BMI * Time | 0.11 | [−0.04, 0.26] | 3356.24 | 1.39 | 0.16 | |
| Fun Seeking | BMI * Time | 0 | [−0.17, 0.16] | 3359.81 | −0.01 | 0.99 | |
| Little Man Task | |||||||
| Efficiency | BMI * Time | 0 | [−0.0, 0.0] | 3542.38 | 1.29 | 0.2 | |
| NIH Toolbox | |||||||
| Flanker Inhibitory Control | BMI * Time | −0.59 | [−0.98, −0.21] | 3212.5 | −3 | <0.01 | **a |
| Picture Vocabulary | BMI * Time | −0.06 | [−0.37, 0.25] | 3049.55 | −0.36 | 0.72 | |
| Pattern Comparison | BMI * Time | −0.33 | [−1.06, 0.41] | 3137.93 | −0.87 | 0.38 | |
| Reading Comprehension | BMI * Time | −0.03 | [−0.25, 0.18] | 2997.16 | −0.3 | 0.76 | |
| Picture Sequence Memory | BMI * Time | 0.36 | [−0.27, 1.0] | 3172.52 | 1.12 | 0.26 | |
| RAVLT | |||||||
| Learning | BMI * Time | −0.45 | [−0.91, 0.01] | 2519.61 | −1.91 | 0.06 | |
| List B correct | BMI * Time | −0.08 | [−0.18, 0.03] | 2637.35 | −1.41 | 0.16 | |
| Immediate Delay | BMI * Time | −0.09 | [−0.24, 0.05] | 2533.63 | −1.25 | 0.21 | |
| Long Delay | BMI * Time | −0.13 | [−0.29, 0.02] | 2529.54 | −1.73 | 0.08 | |
| Repetition List B | BMI * Time | −0.01 | [−0.03, 0.02] | 2831.56 | −0.58 | 0.56 | |
| Repetition Immediate Delay | BMI * Time | 0.04 | [−0.04, 0.11] | 2767.28 | 0.96 | 0.34 | |
| Repetition Long Delay | BMI * Time | 0.02 | [−0.04, 0.08] | 2763.25 | 0.68 | 0.50 | |
| Total Repetitions | BMI * Time | −0.03 | [−0.34, 0.28] | 2653.53 | −0.18 | 0.86 | |
| Intrusions List B | BMI * Time | 0.02 | [−0.02, 0.06] | 2852.87 | 1.15 | 0.25 | |
| Intrusions Immediate Delay | BMI * Time | −0.04 | [−0.07, −0.01] | 2834.83 | −2.12 | 0.04 | * |
| Intrusions Long Delay | BMI * Time | 0 | [−0.04, 0.04] | 2792.81 | 0.02 | 0.99 |
Note. All effects that were significant survived Benjamini-Hochberg correction; BMI = body mass index. RAVLT = Rey Auditory Verbal Listening Task; NIH = National Institutes of Health; BIS/BAS = Behavioral Inhibition System/Behavioral Approach System; UPPS = Urgency, Premeditation (lack of) Perseverance (lack of), Sensation Seeking, Positive Urgency, Impulsive Behavior Scale. Flanker Inhibitory Control and Attention Task was abbreviated in the table to Flanker Inhibitory Control; Pattern Comparison Processing Speed was abbreviated to Pattern Comparison in the table
survived correction.
5. Discussion
Although several studies have suggested that EF is related to overeating and unhealthy weight gain, the mechanisms driving these processes are unknown. To our knowledge, this was the first study to assess the natural progression of changes in BMI and EF over a two-year period in early adolescence amongst a sample of youth who were initially of a healthy weight at ages 9/10-years-old. To this end, we tested A) if variation in EF preceded weight gain (i.e., Dual Processes Model of Overeating which postulates that underlying differences in EF contribute to poor food intake decisions); and B) if weight gain had negative consequences on EF.(9,20,28) Overall, across several assessments of traditional EF (e.g., impulsivity, inhibitory control) and broader cognitive function (e.g., processing span, word recall), we found stronger evidence that underlying differences in EF and cognition may precede weight gain (i.e., Dual Processes Model of Overeating). As these underlying differences in EF and other cognitive processes showed greater increases in the rate of change in BMI, this may be due to inability to make good food-based decisions. Surprisingly, there was less support for the idea that weight gain causes changes to EF and cognition. It is possible that two-years is not enough time (or that these initially healthy weight youth did not gain enough weight) to observe weight-related effects on EF. Nevertheless, future studies are warranted that follow children over a longer period of time to determine if other changes in EF start to occur at a later time.
5.1. Differences in EF and their relation to increased weight status: evidence for the Dual Processes Model of Overeating
The Dual Processes Model of Overeating suggests that underlying differences in EF affects the ability to resist temptation and stick to longer-term plans (e.g., diet adherence, preventing overeating).(9) Essentially, within this framework, the desire to consume a rewarding food is stronger than the ability to stop behavior and adhere to a healthy diet. In our study, we tested this theory by examining how variation in EF and cognition related to the rate of change in BMI over a two-year period of pre/early adolescence. Of particular interest was examining the relationship between impulsivity and BMI, as higher scores on impulsivity assessments have been associated with weight gain and food intake across several studies in children.(14,15,17,18) Here, impulsivity was indirectly measured with standard and validated trait-based questionnaires (e.g., BIS/BAS, UPPS-P) and tasks (e.g., Flanker Inhibitory Control and Attention - NIH Toolbox). Our results showed that youth who scored higher on trait-based impulsivity measures (e.g., Positive Urgency, Reward Responsiveness, Drive, and BIS) and lower on inhibitory control assessments (e.g., Flanker Inhibitory Control and Attention) had greater increases in the rate of change in BMI from 9-to-12-years-old. This suggests that underlying differences in impulsivity may be facilitating increased weight gain. Although the ABCD Study® did not assess food intake, we interpret increases in BMI (i.e., weight gain) to be a proxy for overeating, as an energy balance model suggests that weight gain occurs when there is an excess of calories.(29) However, future studies are needed to assess the direct mechanism between food intake, unhealthy weight gain, and changes in EF over time. We also acknowledge that there are other factors that may explain these relationships, as environmental factors like socioeconomic status have been associated with both obesity and EF in children.(30-32) Notably, our models controlled for caregiver education (a proxy for socioeconomic status), and, as such, we interpret the relationship between impulsivity and weight gain to be independent of socioeconomic status. However, future studies are needed to confirm this relationship. Importantly, our results corroborate the literature showing an association between increased BMI and sensitivity to rewards and punishment,(18) which are facets of impulsivity.
Although much literature has focused on impulsivity (e.g., reward, inhibitory control) as it relates to food intake and unhealthy weight gain, optimal functioning of other cognitive functions are also integral to making adequate food-based decisions.(33) Several studies have noted correlations between these processes and obesity in animals(34,35) and humans.(33,36,37) Cognitive abilities such as memory interact with basic EFs to create adaptable responses.(38) For example, memories about previously learned associations help to inform consumption due to previous beliefs, such as pleasant or unpleasant experiences, or diet adherence.(33) However, memory can help to prevent overeating, by providing signals related to satiety (e.g., helping us to remember when we ate last and how full we are).(39) Thus, variation in memory may contribute to issues with appetite control, and cause overeating despite not being hungry. As such, this may be one explanation for why steeper increases in the rate of change of BMI over two years were related to lower performance on memory and learning paradigms. For example, youth with variation in memory processing may provide inaccurate information about hunger and satiety, which could lead to overeating. Hence, together with the tendency to act impulsively, underlying differences in memory and learning may contribute to unhealthy weight gain. Although our results do confer with cross-sectional studies,(36,37,40-42) future studies are needed to understand the exact physiologic mechanisms or neurocognitive domains driving the associations between cognitive functions and weight gain.
5.2. Weight gain and its association to changes in EF:
BMI was related to the rate of change in performance on the Flanker Inhibitory Control and Attention Task; as BMI increased from 9/10-to-11/12, performance decreased, providing some evidence that weight gain may cause changes to EF.(20) However, BMI was not related to change in any other assessments of EF or cognition. This was somewhat surprising as animal research has shown evidence that overeating and weight gain are related to cognitive deficits via neuroinflammation during adolescence.(34,35) In children, longitudinal studies suggested that increases in BMI z-scores were related to microstructural changes in the nucleus accumbens (a region involved in reward and motivation), in a circular way, such that alterations in this structure were associated with additional weight gain as well as greater intake of high fat food.(43) On the other hand, two years of extreme weight gain was related to structural changes in brain regions associated with EF but not trait-based impulsivity.(27) Further, in a subsample of healthy weight youth at baseline, BMI at 9/10-years-old was not related to brain structure two-years later.(44) Therefore, it may be that two years of human adolescence may not be enough time (or not enough weight was gained amongst these initially healthy weight youth) to observe consequences of weight gain on EF (as assessed by questionnaires and tasks). From a prevention and intervention perspective, these lack of findings is encouraging, and suggest that two years of weight gain may not lead to long-lasting effects on EF. However, adolescence is also a period of normative development in EF maturation, and it is unknown how weight gain may affect future maturation. Additionally, it is unknown how much weight gain is needed to show effects on EF. As such, future research is needed to understand these associations over A) a longer period of time; B) amongst youth with greater variation in unhealthy weight gain; and C) whether these associations are due to neuroinflammation or other mechanisms (e.g., vascular, hormonal and/or metabolic changes).
5.3. Implications
EF training has been shown to increase adherence to treatment programs,(28) but results are largely short-lived as only a few studies showed 10-year effects; while EF training for weight loss is promising, many behavioral programs do not obtain long-term success. One explanation for this could be that we lack a refined understanding of the exact mechanisms of interaction between EF and weight gain and the impact of pubertal development. Therefore, our results do have relevance for treatment programs that may wish to target youth in earlier stages of weight gain, prior to large variability in puberty (although our analyses did control for pubertal effects). Long-term treatment studies are still needed to examine the effects of behavioral treatment programs. Within this vein, healthy EF positively contributes to other health-fostering behaviors that go beyond dietary choices, and thus, training may yield to crossed benefits with respect to several other quality-of-life determinants (e.g., physical activity, good sleep habits, greater social interactions, academic success).(13,45) Lastly, since children with obesity are more likely to become adults with obesity,(4)(48) early interventions may be necessary to prevent the cascade of adverse events often attributed to having obesity. In addition to EF training, behavioral lifestyle interventions may also benefit from our findings, as EF training is not usually incorporated into one’s daily activities, are short-term, and time-consuming. Therefore, early life educational programs may benefit to incorporate EF exercises into the core curriculum, to strengthen EF early in childhood.
5.4. Strengths and limitations
Our study has many strengths, such as evaluating theoretical cause versus consequence relationships between EF and cognition and BMI over a 2-year period in a sample of youth who were initially of healthy weight. Notably, the sample utilized in this study was pooled from a large and geographically diverse group of participants enrolled in the ABCD Study®. However, we do recognize the study’s limitations. First, the ABCD Study® did not collect markers of peripheral (i.e., systemic) or neural inflammation, which effectively limits the insight into the exact causal mechanisms of these relationships apart from the theoretical models.(9,20) Additionally, there were no markers of insulin sensitivity or hormonal and vascular change, and together this limits the interpretation of our results as to what mechanisms may be driving the association between weight gain and changes to inhibition. Second, the ABCD Study® did not collect objective food intake assessments, so it is unknown how our findings relate to actual food intake decisions. Third, it is possible that the EF assessments in the ABCD Study® examined general cognitive function, and performance on these tasks may not translate to food-based decision-making. Fourth, ABCD Study® did not assess all components of the NIH Toolbox beyond baseline.(47) Fifth, the sample demographics included in the manuscript differed from the larger consortium participant pool (e.g., higher percentage of youth identifying of white, higher percentage of youth living in families with higher income, lower percentage of youth identifying as Latino/a/x), with the general ABCD Study® sample also being relatively homogenous. Relatedly, excluding youth who had overweight or obesity at baseline limits the generalizability of the findings due to potential exclusion of groups who have higher rates of obesity risk (e.g., Blacks, Latinos). Additionally, we note the limitations of only including individuals with complete data, as this introduces another bias into the dataset and dampens the generalizability of the findings. To circumvent bias of a complete case analyses, we ran additional analyses to account for all available data (see Supplemental Materials), but the results remained unchanged. However, our results may not be generalizable to diverse groups, and we note there may have been bias that excluded individuals from participation due to restricting the analyses to only healthy weight youth. Lastly, BMI is a proxy for adiposity and the metabolic consequences of an increase in BMI for children are unknown. The ABCD Study® did not collect other markers of adiposity or markers of metabolic health.
5.5. Conclusions
In youth who were initially of a healthy weight at baseline, we found evidence that variation amongst several EF and cognitive domains was related to a significant increase in the rate of change in BMI over two years. This evidence supports the Dual Processes Model of Overeating hypothesis which postulates that underlying differences in EF contribute to poor food intake decisions and subsequent weight gain.(9) Specifically, underlying differences in impulsivity and inhibitory control, as well as memory, learning and processing speed were all predictive of weight gain. That said, there was less support for the idea that weight gain has negative consequences on EF as only greater increases in BMI were related to decreases in inhibitory control, but not to other EF assessments. (e.g., reward, impulsivity, working memory, processing speed). Thus, it may be that two years of weight gain affects inhibitory control (i.e., a stopping mechanism), but the amount of weight gain (or duration) may not be enough to have substantial impacts on other EFs. Moreover, our findings may have implications for prevention programs that may wish to target these cognitive functions to prevent further unhealthy eating and excess weight gain. Additionally, treatment programs may wish to enhance their programs to focus more on strengthening inhibitory control for weight loss success.
Supplementary Material
STUDY IMPORTANCE.
What is already known about this subject?
Several studies show that variation in executive functioning (EF) and cognition is related to overeating and obesity. However, the mechanisms are unknown as to what comes first: do underlying differences in EF “cause” obesity (via poor food choices) or does weight gain have negative “consequences” on EF?
What are the new findings of this manuscript?
The current manuscript evaluated the natural progression of weight gain and EF and cognitive development while testing two theoretical cause vs. consequence models in a sample of youth who at baseline (i.e., ages 9/10 years) were initially of a healthy weight (12% had overweight by ages 11/12-years-old). We found support for the notion that underlying variation in EF and cognition relates to weight gain, but less support for the idea that weight gain is related to changes in EF and cognition.
How might your results change the direction of research or the focus of clinical practice?
Understanding the natural trajectory of weight gain and EF and cognitive development has clinical implications for treatment programs. Importantly, our results showed that two-years may not be enough time (or not enough weight was gained) to observe clinical effects on EF or cognition during development. Thus, suggesting that if intervention occurs early, it may be able to prevent long-term negative cognitive outcomes.
Acknowledgements:
The authors would like to thank the following individuals for their expertise and assistance throughout all aspects of the study: Drs. Stephanie Bodison and Panteha Hayati Rezvan, and Ms. Trinh Luu. The authors would also like to thank all of the participants in the ABCD Study®.
Funding acknowledgements:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM Study® (https://abcdstudy.org/), held in the NIMH Data Archive (NDA). The ABCD Study® is supported by the National Institutes of Health and National Institute on Drug Abuse and additional federal partners under award numbers U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA050987, U01DA050988, U01DA050989, U01DA051016, U01DA051018, U01DA051037, U01DA051038, and U01DA051039. A full list of supporters is available at https://abcdstudy.org/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators/. The ABCD Study® consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or other ABCD Study® consortium investigators. The ABCD Study® data repository grows and changes over time. The ABCD Study® data used in this report came from https://doi.org/10.15154/1503209. Additional funding from the NIH NIDDK provided support of SA (K01 DK135847).
Footnotes
Conflicts of Interest:
MIG receives book royalties from Penguin Random House for his book “Sugarproof”. ESR received NIH funding. KER received funding from the USDA, NIH and California Initiative for the Advancement of Personalized Medicine.
References
- 1.Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, et al. National health and nutrition examination survey 2017–march 2020 prepandemic data files-development of files and prevalence estimates for selected health outcomes. Natl Health Stat Report. 2021;2021(158). [Google Scholar]
- 2.Browne NT, Snethen JA, Greenberg CS, Frenn M, Kilanowski JF, Gance-Cleveland B, et al. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. Vol. 56, Journal of Pediatric Nursing. 2021. p. 90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurnani M, Birken C, Hamilton J. Childhood Obesity: Causes, Consequences, and Management. Pediatr Clin North Am [Internet]. 2015;62(4):821–40. Available from: 10.1016/j.pcl.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obesity Reviews. 2016;17(2):95–107. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR, Münzberg H, Morrison CD. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology. 2017. May 1;152(7):1728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Anders Friberg P, Jerlhag E, et al. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord. 2011;12(3):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proceedings of the Nutrition Society [Internet]. 2012. Nov 17 [cited 2014 May 28];71(04):478–87. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3617987&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond A. Executive Functions. Annu Rev Psychol [Internet]. 2013. Jan 3;64(1):135–68. Available from: http://www.annualreviews.org/doi/abs/10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci [Internet]. 2012. Mar [cited 2014 Jul 8];16(3):174–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22336729 [DOI] [PubMed] [Google Scholar]
- 10.RepovŠ G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience [Internet]. 2006;139(1):5–21. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306452205013989 [DOI] [PubMed] [Google Scholar]
- 11.Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex [Internet]. 2017. Jan;86:186–204. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0010945216301071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favieri F, Forte G, Casagrande M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front Psychol. 2019;10(SEP). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray-Burrows K, Taylor N, O’Connor D, Sutherland E, Stoet G, Conner M. A systematic review and meta-analysis of the executive function-health behaviour relationship. Health Psychol Behav Med [Internet]. 2019;7(1):253–68. Available from: 10.1080/21642850.2019.1637740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy [Internet]. 2007. May [cited 2015 Jan 5];45(5):1071–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16828053 [DOI] [PubMed] [Google Scholar]
- 15.Thamotharan S, Lange K, Zale E, Huffhines L, Fields S. The role of impulsivity in pediatric obesity and weight status: A meta-analytic review. Clin Psychol Rev. 2013;33:253–62. [DOI] [PubMed] [Google Scholar]
- 16.Mamrot P, Hanć T. The association of the executive functions with overweight and obesity indicators in children and adolescents: A literature review. Neurosci Biobehav Rev [Internet]. 2019;107(November 2018):59–68. Available from: 10.1016/j.neubiorev.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 17.van den Berg L, Pieterse K, Malik JA, Luman M, Willems van Dijk K, Oosterlaan J, et al. Association between impulsivity, reward responsiveness and body mass index in children. Int J Obes [Internet]. 2011;35(10):1301–7. Available from: http://www.nature.com/doifinder/10.1038/ijo.2011.116 [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Rico E, Río-Valle JS, González-Jiménez E, Campoy C, Verdejo-García A. BMI predicts emotion-driven impulsivity and cognitive inflexibility in adolescents with excess weight. Obesity. 2012;20(8):1604–10. [DOI] [PubMed] [Google Scholar]
- 19.Adise S, White CN, Roberts NJ, Geier CF, Keller KL. Children’s inhibitory control abilities in the presence of rewards are related to weight status and eating in the absence of hunger. Appetite [Internet]. 2021;167(July):105610. Available from: 10.1016/j.appet.2021.105610 [DOI] [PubMed] [Google Scholar]
- 20.Shields GS, Moons WG, Slavich GM. Inflammation, Self-Regulation, and Health: An Immunologic Model of Self-Regulatory Failure. Perspectives on Psychological Science. 2017;12(4):588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci [Internet]. 2016;17:103–17. Available from: 10.1016/j.dcn.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS. 2000 CDC Growth Charts for the United States: Methods and Development. Vol. 11, National Center for Health Statistics. Vital Health Stat 2002. 1–178 p. [PubMed] [Google Scholar]
- 23.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc [Internet]. 1988. Apr;17(2):117–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24277579 [DOI] [PubMed] [Google Scholar]
- 24.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci [Internet]. 2018. Aug;32:55–66. Available from: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaku N, Hoyt LT. Developmental Trajectories of Executive Functioning and Puberty in Boys and Girls. J Youth Adolesc [Internet]. 2019;48(7):1365–78. Available from: 10.1007/s10964-019-01021-2 [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57(1):289–300. [Google Scholar]
- 27.Adise S, Marshall AT, Hahn S, Zhao S, Kan E, Rhee KE, et al. Longitudinal assessment of brain structure and behaviour in youth with rapid weight gain: Potential contributing causes and consequences. Pediatr Obes [Internet]. 2022. Oct 17;(August):1–13. Available from: 10.15154/1503209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes JF, Eichen DM, Barch DM, Wilfley DE. Executive function in childhood obesity: Promising intervention strategies to optimize treatment outcomes. Appetite. 2018;124:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin Sci [Internet]. 2016. Sep 1;130(18):1615–28. Available from: http://clinsci.org/cgi/doi/10.1042/CS20160006 [DOI] [PubMed] [Google Scholar]
- 30.Ursache A, Noble KG. Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016;6(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson GM, Hook CJ, Farah MJ. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev Sci. 2018;21(2):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adise S, Marshall AT, Kan E, Sowell ER. Access to quality health resources and environmental toxins affect the relationship between brain structure and BMI in a sample of pre and early adolescents. Front Public Health [Internet]. 2022. Dec 15;10. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1061049/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson TL, Tracy AL, Schier LA, Swithers SE. A view of obesity as a learning and memory disorder. J Exp Psychol Anim Behav Process. 2014;40(3):261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsan L, Décarie-Spain L, Noble EE, Kanoski SE. Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Vol. 15, Frontiers in Neuroscience. Frontiers Media S.A; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, et al. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2015. Feb 1;25(2):227–39. [DOI] [PubMed] [Google Scholar]
- 36.Loprinzi PD, Frith E. Obesity and episodic memory function. Journal of Physiological Sciences [Internet]. 2018;68(4):321–31. Available from: 10.1007/s12576-018-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai CL, Chen FC, Pan CY, Tseng YT. The neurocognitive performance of visuospatial attention in children with obesity. Front Psychol. 2016;7(JUL):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Bolin J, Lu Z, Carr M. Visuospatial working memory mediates the relationship between executive functioning and spatial ability. Front Psychol. 2018;9(DEC):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiol Behav [Internet]. 2014. Sep;136(1):185–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0031938414001322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu N, Chen Y, Yang J, Li F. Childhood obesity and academic performance: The role of working memory. Front Psychol. 2017;8(APR):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendt S de K, Constantino B, Wendt EA, Mastroeni MF. Influence of weight status at 2 years on memory performance at 4–5 years of age. Ann Hum Biol [Internet]. 2019;46(3):196–204. Available from: 10.1080/03014460.2019.1632928 [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes. 2014;38(4):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapuano KM, Berrian N, Baskin-Sommers A, Décarie-Spain L, Sharma S, Fulton S, et al. Longitudinal Evidence of a Vicious Cycle Between Nucleus Accumbens Microstructure and Childhood Weight Gain. Journal of Adolescent Health [Internet]. 2022. Jun;70(6):961–9. Available from: 10.1016/j.jadohealth.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adise S, Marshall AT, Kan E, Gonzalez MR, Sowell ER. Relating neighborhood deprivation to childhood obesity in the ABCD study: Evidence for theories of neuroinflammation and neuronal stress. Health Psychol. 2022. Dec; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry RE, Braren SH, Rincón-Cortés M, Brandes-Aitken AN, Chopra D, Opendak M, et al. Enhancing Executive Functions Through Social Interactions: Causal Evidence Using a Cross-Species Model. Front Psychol. 2019;10(November):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.