Abstract
Wolbachia bacteria of arthropods are at the forefront of basic and translational research on multipartite host-symbiont-pathogen interactions. These microbes are vertically inherited from mother to offspring via the cytoplasm. They are the most widespread endosymbionts on the planet due to their infamous ability to manipulate the reproduction of their hosts to spread themselves in a population, and to provide a variety of fitness benefits to their hosts. Importantly, some strains of Wolbachia can inhibit viral pathogenesis within and between arthropod hosts. Mosquitoes carrying the wMel Wolbachia strain of Drosophila melanogaster have a greatly reduced capacity to spread viruses like dengue and Zika to humans. Therefore, Wolbachia are the basis of several global vector control initiatives. While significant research efforts have focused on viruses, relatively little attention has been given to Wolbachia-fungal interactions despite the ubiquity of fungal entomopathogens in nature. Here, we demonstrate that Wolbachia increase the longevity of their Drosophila melanogaster hosts when challenged with a spectrum of yeast and filamentous fungal pathogens. We find that this pattern can vary based on host genotype, sex, and fungal species. Further, Wolbachia correlates with higher fertility and reduced pathogen titers during initial fungal infection, indicating a significant fitness benefit. This study demonstrates Wolbachia’s role in diverse fungal pathogen interactions and determines that the phenotype is broad, but with several variables that influence both the presence and strength of the phenotype. These results enhance our knowledge of the strategies Wolbachia uses that likely contribute to such a high global symbiont prevalence.
Introduction:
Microbe-host symbioses are ubiquitous in nature and exhibit a broad range of relationships from facultative parasitism to obligate mutualism1,2. Microbial symbionts of arthropods in particular exhibit a striking array of phenotypes in their hosts2, ranging from provision of nutrients3 to protection from parasitoids4 to death of the host’s offspring5. One microbial symbiont, Wolbachia pipientis, is an exemplary case of a microbe with diverse symbiont-host interactions. Wolbachia are obligate intracellular bacteria found in germline and somatic tissues of diverse arthropods and are almost exclusively inherited vertically through the cytoplasm of infected mothers6. They are found in an estimated 40–52% of all arthropod species on Earth7,8, making them the most widespread endosymbiont and “the world’s greatest pandemic”9,10. There is such genetic diversity that there are 18 recognized Wolbachia supergroups11–13. Some can act as “reproductive parasites” that manipulate host reproduction to facilitate their spread by enhancing the relative fitness of infected female transmitters14. Others are obligate mutualists necessary for host oogenesis or early development15. Depending on context, Wolbachia can use their diverse genetic toolkit to engage in a variety of interactions with their hosts. These interactions have had immense impacts on both basic and applied research in many fields, including utility in fighting human diseases vectored or caused by insects and nematodes and to an understanding of the role of symbionts in shaping host evolutionary processes6,16–18.
Wolbachia’s employment of such diverse host interactions has been critical to its global success, however, these phenotypes do not fully explain how widespread Wolbachia is. Indeed, while some strains are reproductive manipulators (enhancing the fitness of the infected matriline)5,10,19–21 or obligate mutualists (enhancing the fitness of all hosts)12,22–24, but many are not, even among organisms that have been phenotypically assessed25. Some strains also exhibit no reproductive parasitism in and provide no currently known fitness benefit26,27. Further, those that are reproductive manipulators can vary both in the effect size of their phenotype (either weak or strong induction28–31) and in their frequency in the population (high or low32–35). Even when reproductive phenotypes or benefits are known, they are often context-dependent and vary based on factors such as temperature36–39, symbiont density40,41, or host genetic background42. Further, in the wild, vertical transmission fidelity of Wolbachia is not 100%27,43,44, making the basis of the symbiont’s maintenance in populations even less clear. For many years, a question of significant focus in the field has been how it is that Wolbachia is so widespread45, particularly given the fact that we have not identified a clear host fitness benefit of the symbiont for all strains or contexts. Research over the years has identified some contributing factors such as nutritional contributions of the symbiont to the host46,47, as well as rescue of host deficiencies like mutations in the key sex development regulator sex-lethal48,49 and germline stem cell self-renewal and differentiation deficiencies50. Yet these contributing factors do not fully answer the question, and other factors must be involved.
One such crucial and somewhat common beneficial Wolbachia-host interaction was discovered through work on an early theory that Wolbachia’s prevalence could be based on an ability to inhibit pathogens, thereby conferring a significant fitness benefit to the host51–53. The rationale was based partially on the observation that facultative infection (as opposed to obligate mutualism) is relatively common with Wolbachia infections, but with few accompanying known benefits to explain their frequency. It was also partially based on an observation that Wolbachia infection correlated with host resistance to infection with the common Drosophila C virus (DCV)51. Two foundational early studies on this topic demonstrated that Drosophila melanogaster flies with their native Wolbachia strain exhibit greater longevity on the order of days to weeks of increased life when infected with several common arthropod RNA viruses51,54. This coincides with reduced viral load in Wolbachia-viral co-infection, which increases host fitness and survival likelihood though reduced pathogen burden. These and latter studies also demonstrated that the phenotype could be induced by some additional Wolbachia strains or in additional host genetic backgrounds or species, but that the effect was largely restricted to RNA viruses (not DNA viruses)55. Finally, and crucially, some Wolbachia strains are also able to inhibit the transmission of viral (and some other) pathogens to new host individuals, including pathogens spread by mosquitoes to humans51,54,56,57. This ability of the symbiont to protect its host from viruses is considered a major factor contributing to Wolbachia’s success.
Virus pathogen blocking has therefore become an eminent area of Wolbachia research not only for its broad applicability across the symbiont genus and importance to basic biology, but also for its translational potential. For example, Aedes aegypti mosquitoes and other common human disease vectors exhibit significantly reduced capacities to transmit parasites like malaria57 or viruses like Zika56, dengue58,59, yellow fever60, or chikungunya61 to humans when they carry certain strains of Wolbachia. This feature has made Wolbachia central to global efforts to reduce disease through groups like MosquitoMate62 and the World Mosquito Program63. These programs rear Wolbachia-positive mosquitoes on a massive scale and release mosquitoes into the wild. One strategy is to release infected females that then outcompete local Wolbachia-negative counterparts and replace them with a disease-resistant population. Collaborative efforts through this program across four continents have resulted in stable, wild Wolbachia-positive populations in many locations and significant reductions in disease58,64. Arthropod vector-borne diseases are responsible for millions of illnesses, deaths, and contribute to significant inequality around the world65, and the use of Wolbachia-positive mosquitoes is one of our most promising solutions66–68.
In contrast with all of this progress on viruses, comparatively little research has been done on Wolbachia interactions with non-viral pathogens57,69. This is despite the extraordinary genetic and phenotypic diversity of Wolbachia symbioses that indicate the likelihood of broader protective abilities. Early theory predicted that pathogen protection could increase the relative fitness of hosts with Wolbachia compared to those without, contributing to maintenance and spread of the symbiont32, and this was one of the original bases for investigations into viral pathogen blocking, and could apply to many other types of pathogens too51,54. However, one particular gap in the research is the potential for Wolbachia to inhibit fungal pathogens. Fungal pathogens of arthropods are common in the wild70, yet few studies have investigated the interactions between Wolbachia, hosts, and fungal pathogens, and the studies that do present different results. One early study showed no effect of wRi Wolbachia strain infection on survival from topical cuticle infection of the common insect fungal pathogen, Beauveria bassiana, in D. simulans male flies71. Another reported higher survival of D. melanogaster female flies with their native wMel Wolbachia symbiont after immersion in a suspension of B. bassiana72. Conversely, a third study on infection of female spider mites in topical contact with B. bassiana or Metarhizium fungal pathogens indicated that Wolbachia may actually increase mortality of the host with fungal infection73. A fourth investigated the effect of Wolbachia on injection with two Beauveria pathogens on Aedes albopictus and Culex pipiens mosquitoes74. This study found no enhancement in host survival with the symbiont, but reported some putative differences in host immune gene expression and reduced fungal load in some contexts. Finally, a recent study indicates that the wPni strain of Pentalonia aphids may result in increased survival of hosts infected topically with the specialized fungal pathogen, Pandora neoaphidis75. Thus, there have been several investigations, with some prior reports indicating that Wolbachia may interact with fungal pathogens in some contexts.
Despite this research, the question of Wolbachia’s ability to interact with fungal pathogens on a larger scale remains unanswered. It is unclear how broad the fungal blocking ability is in terms of host, symbiont, and pathogen factors, and if the phenotype is likely to be common or not. This difficulty is because the studies draw different conclusions from different contexts. These prior reports have used different host species, host sexes, Wolbachia strains, pathogen species, pathogen concentrations, routes of pathogen infection, and been measured by different host fitness and health assays or conducted over different lengths of time71–75. These factors make it difficult to compare across studies, as there are multiple variables between any two publications. Further, due to the small number of studies, limited parameters have been tested thus far. Thus, the breadth of Wolbachia-fungal interactions is unclear, as comparison between studies is difficult and there is limited published data.
To begin to fill this gap in knowledge, we conducted a series of systemic fungal infection assays using D. melanogaster flies with the wMel Wolbachia symbiont in the context of several host and pathogen variables. Notably, wMel is the initial strain that was reported to inhibit viruses and mosquitoes transinfected with this symbiont strain are the basis of many of the global vector control initiatives51,54,58. This approach addresses several outstanding research questions in this area: (i) can Wolbachia inhibition of fungal pathogenesis be confirmed when tested in various contexts, (ii) how broad is this protective phenotype within one Wolbachia strain, and (iii) do factors such as fungal pathogen species, fungal pathogen types (filamentous vs yeast), host sex, and host genetic background contribute to the Wolbachia-fungal pathogen interaction. Here we report that Wolbachia is indeed capable of significantly increasing the longevity and reproductive fitness of flies infected with a wide variety of fungal pathogens, and the phenotype is influenced by several host and pathogen factors.
Results:
Wolbachia’s association with an increase in longevity of flies infected with filamentous fungi is dependent on genetic background and host sex
To test the breadth and ability of Wolbachia to inhibit fungal pathogenesis in flies, a series of systemic infection assays were conducted. Experiments were performed with two different Drosophila melanogaster host background lines infected with their native wMel Wolbachia. The host strains themselves have diverse origins: the w1118 line was collected in California, USA and was reported in 198576, and the wk line was collected in 1960 in Karsnäs, Sweden77. Different collection origins together with Illumina sequencing showing a high number of SNPs between the D. melanogaster lines indicate the lines represent genetically diverse host backgrounds. Each strain has its own natural Wolbachia along with genetically identical counterpart strains that were previously treated with antibiotics to remove the symbiont. Thus, we tested four strains total: w1118 with Wolbachia, w1118 without Wolbachia, wk with Wolbachia, and wk without Wolbachia. Whole genome sequencing of the Wolbachia symbionts of each strain indicates that they are highly similar despite disparate origins, with only a single divergent SNP across the entire genome. This SNP is a silent (synonymous) polymorphism in a membrane transporter of the major facilitator superfamily, which transports small solutes78. Thus, the vast majority of genetic differences between strains can be attributed to the host, and most phenotypic differences are therefore likely due to the host as well.
To determine if Wolbachia can increase the longevity of flies infected with fungi as hypothesized, systemic infections were performed with both sexes of all four strains against a variety of pathogens. We started with several Aspergillus and Fusarium filamentous fungal species that infect both arthropods and humans: Aspergillus fumigatus, Aspergillus flavus, Fusarium oxysporum, and Fusarium graminaerum (Figure 1). Survival was scored daily for three weeks, as differences in survival were broadly apparent across treatment groups for most pathogens by this point. The data revealed several key results. First, Wolbachia was associated with significantly greater survival across the trial period in many contexts. In the wk background, Wolbachia-positive flies had higher survival for all pathogens except Fusarium oxysporum, which was only significant when comparing within just males (Figure 1). Second, genetic backgrounds played a significant role in the infection outcomes. Indeed, Wolbachia was not a significant predictor of increased longevity for any of the pathogens in the w1118 host background, except when considering sex (Figure S1). Third, sex is repeatedly a significant factor in survival outcomes for some pathogens. Males alone had a significant increase in longevity for Aspergillus fumigatus and Fusarium oxysporum for both genetic backgrounds (Figures 1a,c & S1a,c), with a statistically significant Wolbachia × sex interaction for A. fumigatus in the wk background and Fusarium oxysporum in the w1118 background (Figures 1a, S1c). Fourth, the host strains had generally different overall susceptibilities to fungal infection, with wk generally having lower survival than w1118 in both Wolbachia-positive and -negative contexts (Figures 1 & S1, mean 51.1% death for all pathogen infections combined in the w1118 background by day 21, 60.4% death in the wk background). In particular, there is a significant Wolbachia × genotype interaction for Aspergillus flavus (*p=0.043, Table S1).
Figure 1. Wolbachia increases the longevity of flies of the wk background line infected with several filamentous fungal pathogens.
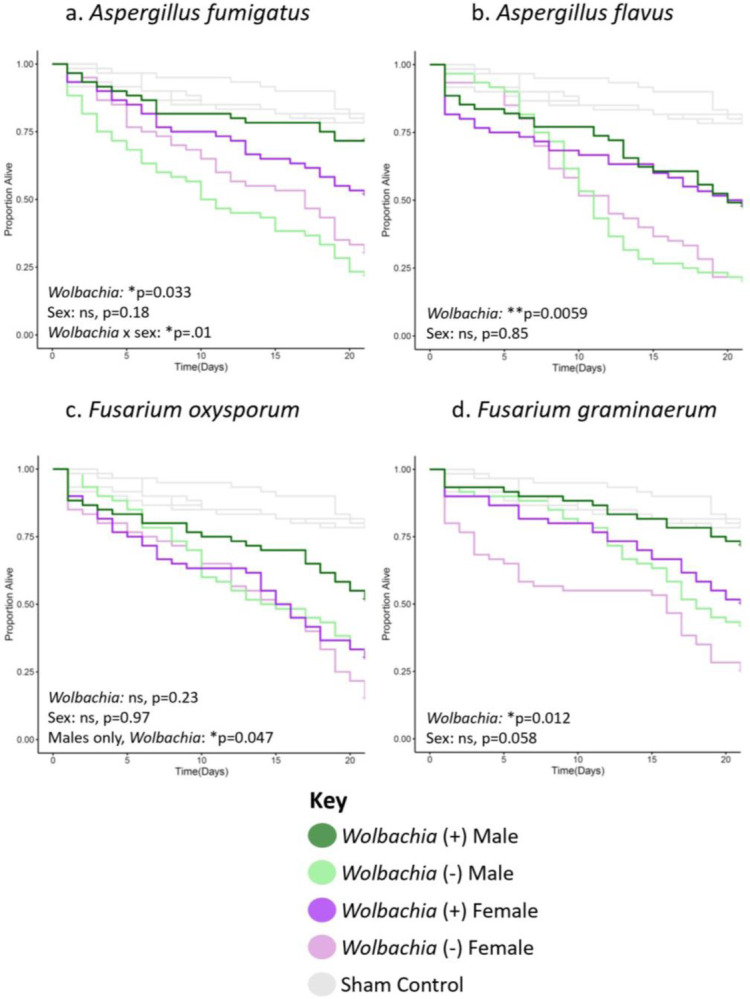
Flies of each given background and sex were systemically infected with the indicated pathogen. Infections were performed with either (a) Aspergillus fumigatus, (b) Aspergillus flavus, (c) Fusarium oxysporum, or (d) Fusarium graminaerum. Infections of all groups were performed side-by-side, along with those of the w1118 background line (Figure S1), with at least two blocks of infections performed on different days. Each line represents a total of 60 flies. Sham controls were performed with sterile 20% glycerol. Full statistics, available in Table S1, were done with a Cox mixed effects model. Controls are the same in all panels and in Figure 2a because they were performed concurrently in the same background.
Wolbachia can increase the longevity of flies infected with filamentous fungal entomopathogens
To determine if Wolbachia could also increase longevity of flies infected with common filamentous fungal insect pathogens (entomopathogens), we performed systemic infections with Beauveria bassiana, Metarhizium anisopliae, Clonostachys rosea, and Trichoderma atroviride. Beauveria and Metarhizium in particular are ubiquitous insect pathogens and are the subject of extensive research in biocontrol of pests in particular79, while Clonostachys and Trichoderma are also globally widespread and have received recent attention in biocontrol as well80–82. The latter two were collected from mosquitoes, and are thus of potential relevance to mosquito biology (Table S2). Similar to the results of the pathogens in Figures 1 & S1, Wolbachia increased longevity in many, but not all fungal infection contexts (Figures 2 & S2). Namely, Wolbachia significantly increased longevity for Beauveria bassiana and Clonostachys rosea in the wk background (Figure 2a,c), and Beauveria bassiana and Metarhizium anisopliae in the w1118 background (Figure S2a,b). Thus, there is some positive longevity effect of the symbiont in either background, not just wk, but the effect depends on the pathogen. Further, sex was also a factor with a significant effect for Beauveria bassiana and Metarhizium anispoliae in the wk background (Figure 2a,b) and Metarhizium anisopliae and Trichoderma atroviride in the w1118 background (Figure S2b,d). Additionally, as with previous infections, wk was broadly more susceptible to infection as flies generally died earlier and at higher rates than their w1118 counterparts (Figures 2 & S2, mean 70.3% death for all entomopathogen infections combined in the w1118 background by day 21, 85.8% death in the wk background).
Figure 2. Wolbachia increases the longevity of flies of the wk background line infected with certain filamentous fungal entomopathogens.
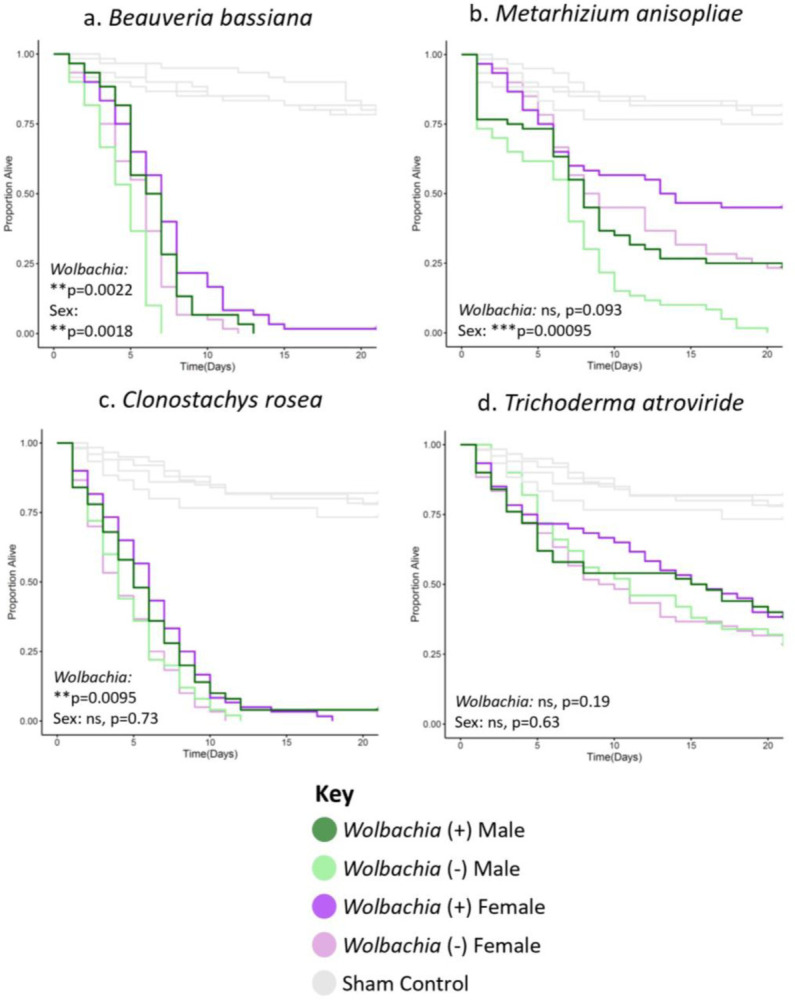
Flies of each given background and sex were systemically infected with the indicated pathogen. Infections were performed with either (a) Beauveria bassiana, (b) Metarhizium anisopliae, (c) Clonostachys rosea, or (d) Trichoderma atroviride. Infections of all groups were performed side-by-side, along with those of the w1118 background line (Figure S2), with at least two blocks of infections performed on different days. Each line represents a total of 60 flies. Sham controls were performed with sterile 20% glycerol. Full statistics, available in Table S1, were done with a Cox mixed effects model. Controls for panel 2a are the same for Figure 1, and the panels in 2b–d are the same because they were performed concurrently in the same background.
Wolbachia can increase the longevity of flies infected with yeasts
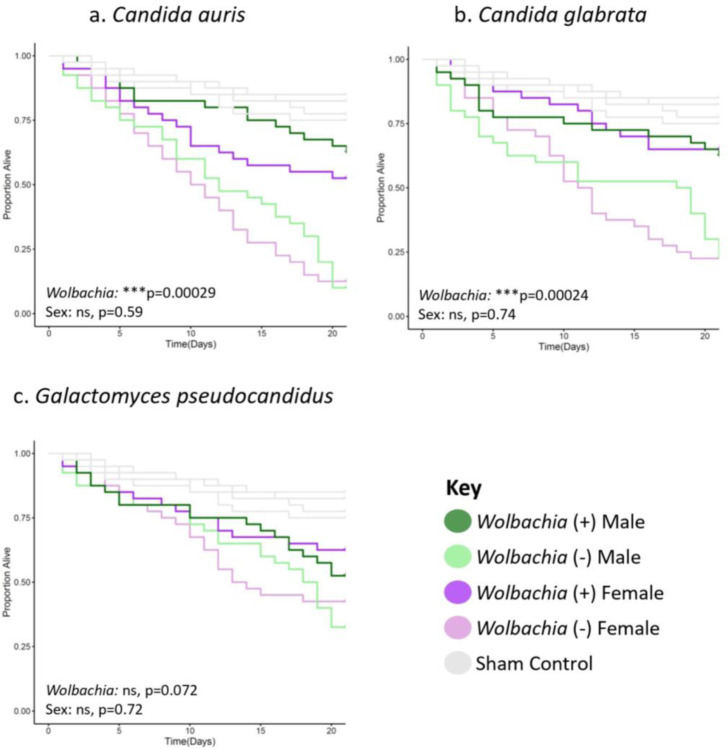
To test if Wolbachia could also increase the longevity of flies infected with yeast, we performed systemic infections using Candida auris, Candida glabrata, and Galactomyces pseudocandidus. For Candida pathogens, Wolbachia significantly increased longevity of wk background flies. In contrast, Wolbachia did not significantly increase longevity for any of the yeast pathogens in the w1118 background. Further, sex was not a significant factor in any of the yeast infections for either background. However, flies of the wk background again were more broadly susceptible to infection based on higher overall mortality (mean 40% death for all yeast infections combined in the w1118 background by day 21, 58.3% death in the wk background).
Wolbachia can partially rescue female fertility reduction after infection
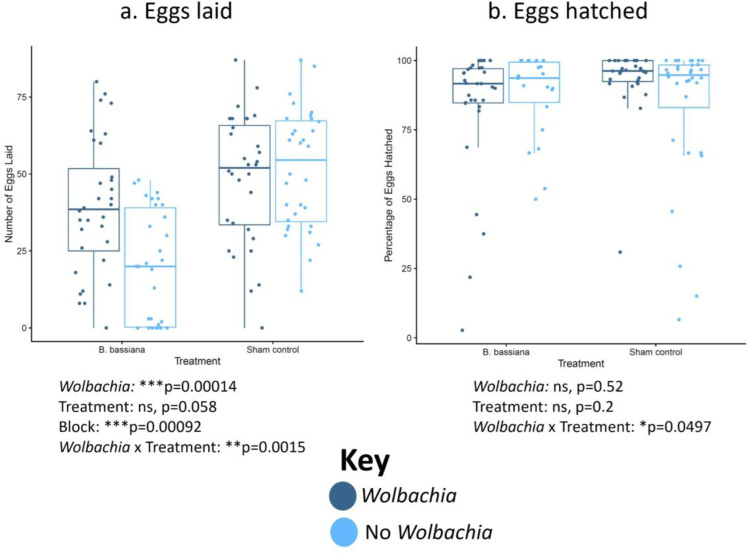
To assess whether Wolbachia impacts fitness of hosts early in fungal infection, female flies were systemically infected with B. bassiana because Wolbachia significantly increased longevity for all treatment groups with this pathogen (Figures 2a, S2a). Egg laying and egg hatching rates were quantified for the first 3 days post infection for flies with either the infection or a sham control (Figures 4, S4). Although both Wolbachia-positive and Wolbachia-negative flies laid similar numbers of eggs in the wk background without treatment, and although the overall egg-laying was lower in B. bassiana-infected flies, Wolbachia significantly increased egg-laying with fungal infection (Figure 4). This was also true in the w1118 background (Figure S4). In contrast, the percentage of eggs hatched was not greatly impacted by either Wolbachia or fungal infection in either background (Figures 4b, S4b).
Figure 4. Wolbachia increases the number of eggs laid but not the percentage of eggs hatched post-B. bassiana infection in the wk background line.
Female flies were systemically infected with B. bassiana or treated with a sham control. The flies then laid eggs for 3 days post-infection. (a) Numbers of eggs laid. (b) Proportion of eggs hatched. Each dot represents the total offspring of a single female, with an overall mean of 35 eggs laid. The boxes indicate the interquartile range. Outer edges of the box indicate 25th (lower) and 75th (upper) percentiles and the middle line indicates 50th percentile (median). Whiskers represent maximum and minimum ranges of data within 1.5 times the interquartile range of the box. Statistics are based on a logistic regression (Table S1). The entire experiment was performed twice, and graphs represent a combination of data from both blocks.
Wolbachia associates with reduced fungal titer after infection
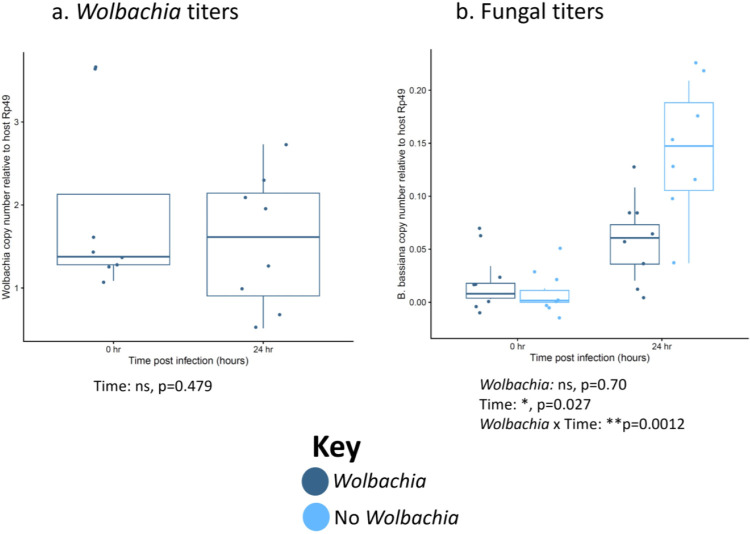
To determine if enhanced longevity is likely based on killing or reduction of pathogen (immune resistance) vs tolerance and maintenance of the pathogen (immune tolerance), and to determine if reproductive benefits with fungal infection in Figures 4 & S4 can be attributed to reduced pathogen load, we measured fungal and Wolbachia titers over time in B. bassiana-infected females (Figure 5). We measured over the first 24 h because this is before flies begin to die and many essential early host molecular responses to pathogen infection begin by this timepoint during infection83,84. We find that Wolbachia titer stays constant over the 24 h period (Figure 5a) and that pathogen load is not significantly different between lines immediately post-infection (Figure 5b). Thus, both Wolbachia-positive and -negative flies are receiving similar starting amounts of pathogen. However, by 24 h post-infection, we see that pathogen load is reduced in the Wolbachia-positive flies compared to those without Wolbachia. This trend holds true in the w1118 background as well.
Figure 5. Wolbachia associates with reduced pathogen titer after infection with no significant change in Wolbachia titer in wk flies.
Female flies were systemically infected with the indicated fungal pathogen and pathogen titers were measured both immediately after infection and 24 h post-infection. Dots represent pools of 3 infected females. (a) Wolbachia titers. (b) B. bassiana titers. The boxes indicate the interquartile range. Outer edges of the box indicate 25th (lower) and 75th (upper) percentiles and the middle line indicates 50th percentile (median). Whiskers represent maximum and minimum ranges of data within 1.5 times the interquartile range of the box. Statistics are based on a logistic regression (Table S1). The entire experiment was performed twice, and graphs represent a combination of data from both blocks.
Discussion:
In the 15 years since the discovery of Wolbachia-based virus inhibition, there has been significant research into the mechanism and translational applications of the phenotype51,54,55,64. However, comparatively little attention has been given to the potential for Wolbachia to interact with other types of pathogens, including fungi. Prior research gave contrasting results either suggesting there was a Wolbachia-fungal infection interaction72,75 or not71,73,74. However, these previous studies were performed in different contexts with many different variables between them. Thus, the breadth of Wolbachia’s ability to interact with fungal pathogens as well as identification of factors that influence the putative phenotype have remained unclear. Given the likely importance of fungal interactions to the basic biology of Wolbachia and potential applications in areas like agriculture, these are important research topics to address. For example, the large field trials that release Wolbachia-positive mosquitoes to combat arthropod-transmitted viruses rely on Wolbachia’s reproductive manipulations of the host to help spread itself in the wild64. The Wolbachia-positive mosquitoes must reach a sometimes unstable equilibrium level to reliably spread85, which could be altered by fitness impacts induced through fungal infection. Further, many agricultural fungal diseases are vectored by arthropods and Wolbachia could be used as a tool to combat disease spread. To begin filling this gap, we sought here to test Wolbachia-fungus interactions by systemically infecting the model host Drosophila melanogaster with a panel of fungal pathogens and measuring host longevity. We included several variables that we hypothesized might be important factors in any potential pathogen-blocking phenotype, including host genotype, host sex, and pathogen species. We then tested the effect of Wolbachia on host fertility and pathogen load when infected or not with fungus.
The main conclusions that can be drawn from the results are that the wMel strain of D. melanogaster has a broad, but variable ability to inhibit fungal pathogenesis and that both host and pathogen variables significantly contribute to infection outcomes. Across the systemic infection assays (Figures 1–3, S1–S3), we found a variety of patterns in the results. There are cases where Wolbachia-positive flies live significantly longer with fungal infection in all tested contexts, such as B. bassiana (Figures 2a, S2a). Notably, this is in agreement with one prior study that showed D. melanogaster females with Wolbachia lived longer when dipped in a suspension of the same pathogen72, suggesting that the phenotype may hold with multiple different infection routes as well. There were also cases where Wolbachia significantly increased host longevity in only one host background, such as the Aspergillus and Fusarium pathogens (Figures 1, S1), C. rosea (Figures 2c, S2c), and Candida pathogens (Figures 3, S3), examples for which Wolbachia was only significant in the wk background. In contrast, Wolbachia was significant in only the w1118 background for M. anisopliae infection (Figures 2b, S2b), so either host genotype can result in a statistically significant outcome while the other does not. However, and on a related note, the effect size of Wolbachia on host survival may be small in a given context and may lead to lower power to detect the differences with our sample sizes, like M. anisopliae in wk (Figure 2b) or F. graminaerum in w1118 (Figure S1d). In contrast, there was one case where the infection outcome was not significant in any context, with the T. atroviride pathogen (Figures 2d, S2d), so there may not be an interaction with all pathogens. Further, there were no cases of increased mortality with Wolbachia-fungal co-infection, as was suggested in a prior study with fungal pathogens in Wolbachia-positive spider mites73. Thus, broadly speaking, both pathogen species and host genetics are factors that significantly associate with Wolbachia-fungus co-infection outcomes. These patterns suggest that the mechanism(s) of protection are likely not universal to fungal infection, and that host factors are likely involved.
Figure 3. Wolbachia increases the longevity of flies of the wk background line infected with yeast pathogens.
Flies of each given background and sex were systemically infected with the indicated pathogen. Infections were performed with either (a) Candida auris, (b) Candida glabrata, or (c) Galactomyces pseudocadidus. Infections of all groups were performed side-by-side, along with those of the w1118 background line (Figure S3), with at least two blocks of infections performed on different days. Each line represents a total of 60 flies. Sham controls were performed with sterile 20% glycerol. Full statistics, available in Table S1, were done with a Cox mixed effects model. Controls are the same in all panels and because they were performed concurrently in the same background.
Notably, host sex was a significant predictor of infection outcome in several cases as a standalone variable. For example, females had increased longevity compared to males with B. bassiana and M. anisopliae infection in wk hosts (Figures 2a,b) and M. anisopliae infection in w1118 hosts (Figure S2b), regardless of Wolbachia status. In one case, however, male w1118 flies survived at higher rates than females for T. atroviride infection (Figure S2d), so the pattern of higher female survival is not always true. Broadly speaking, sex differences in infection outcomes have long been noted in the literature, and are conserved across diverse host and pathogen species86–88. Some of the results presented here are also in line with observations that males of many species are often more susceptible to infection than females89. Within Drosophila, prior research has shown sex differences in infection are common, can favor either males or females, and depend on many different factors90. Indeed, infectious challenge with a broad spectrum of bacterial pathogens in D. melanogaster demonstrated that females were more broadly susceptible to infection91, while another study showed greater female survival with E. coli challenge92. Those studies identified specific regulators or sensors in both the IMD and Toll pathways that are sexually dimorphic in their expression or activation, contributing to differential immune responses. Sex differences in gut pathology93, sexual antagonism in immune resistance and tolerance mechanisms94, sex chromosome regulation of immune responses95, and sex differences in behavior symptoms96 have all been reported for bacterial or viral infections in Drosophila. Reports on sex differences in fungal infection have shown mixed results. Notably, several studies have examined sex-specific outcomes of B. bassiana infection in D. melanogaster. One study showed no sex differences in D. melanogaster cuticle infection with B. bassiana97, another showed higher male survival with B. bassiana cuticle infection98, and a third also showed higher male survival with B. bassiana infection introduced either by spray method or injection99. In the third case, removal of various Toll and Imd genes ablated the dimorphism, indicating their role in the phenotype99. Notably, the results herein differed, with females showing marginally higher survival with B. bassiana infection in the wk line (Figure 2a), and no sex differences in the w1118 line (Figure S2a). This could be due to differences in the host genetic background strains used in this vs other studies in addition to differences in pathogen infection method or pathogen strain. Thus, sex differences in infection, favoring males or females, are common and the result of many different factors. The fact that we observe sex differences in our results here, but to different extents and in different directions in various contexts, is largely in line with the literature. Future work will be needed to determine basis of these sex differences.
Sex was not only significant predictor of host outcomes alone, but also in combination with Wolbachia presence or absence. One particularly interesting case was the significant Wolbachia × sex interaction with F. oxysporum infection in the w1118 background (Figure S1c). In this case, only Wolbachia-positive males survived significantly longer with fungal infection, not females. A similar trend was seen in the wk background, where statistical significance was evident only when specifically testing within males (Figure 1c). The interaction term of Wolbachia × sex was not significant, but these sorts of interactions also suffer from low power. Thus, the mechanism of Wolbachia protection from fungal pathogenesis may partially depend on host factors that differ between the sexes, at least in F. oxysporum infection. As for why Wolbachia may protect males despite transmission mainly through females, it may be due to the dependency of the symbiont on males to induce reproductive parasitism in this species100. Notably, the literature investigating Wolbachia blocking of viruses and bacteria in arthropods often focuses on one specific sex as opposed to both together, particularly for mosquito research, where viruses are transmitted through female bloodmeals54,56,101–106. However, at least one study reports that female D. melanogaster infections with Drosophila C Virus are similar to males51. Due to few studies comparing the sexes, it is unclear if there are sexually dimorphic outcomes in other cases of Wolbachia pathogen blocking or what the molecular and genetic bases of putative Wolbachia × sex interactions may be. However, some possibilities include sex differences in Wolbachia density, tissue tropism, or dependency on sexually dimorphic host immune responses to inhibit pathogenesis. Future research will be required to investigate this more fully.
Additionally, there was variation in the size of survival differences between Wolbachia-positive and -negative flies. In some cases, the difference was small but significant, as with B. bassiana (Figures 2a, S2a). In others, the difference was large, such as the Candida infections in the wk background, (Figures 3a,b). Further, there were differences in longevity based on host genetic background, with the wk flies often succumbing to death earlier, or with fewer overall survivor by the end of the trial period. These results indicate that Wolbachia’s impact on fly survival during fungal infection can have a wide range, from only a slight increase in longevity to a much larger one, and that host genetics alone (both sex and genetic background) still significantly influence infection outcomes regardless of Wolbachia status. However, even with a modest increase in longevity of a few days for B. bassiana-infected flies with Wolbachia as an example, the fitness benefits in early stages of infection are significant too (Figures 4, S4). Indeed, the observed increase in early fertility is likely due to reduced pathogen load during initial infection (Figures 4, S4, 5b, S5b). Notably, the lower fungal titers are not due to fluctuating Wolbachia titers, as they remain the same during infection (Figures 5a, S5a). This indicates that the symbiont would likely confer a high fitness benefit to a host infected with fungus in the wild due to the combined effects of laying more eggs per day and living more days.
The potential mechanism of fungal pathogen blocking will be the subject of future study. From the reduced pathogen load, it is likely to be an immune resistance mechanism as opposed to tolerance, either of which are known in flies84,94,107. In addition, since factors like host sex and genetic background are significant variables, this suggests that the mechanism is likely at least partially mediated through the host. Importantly, the Wolbachia strains from each background are nearly genetically identical, with only one single identifiable SNP segregating between the two strains. Although this does not rule out the possibility of differences due to factors like different tissue tropism or DNA structural differences not uncovered by Illumina sequencing, it suggests that differences in phenotypes are likely due to the host rather than symbiont. They do appear to have similar whole-body titers (Figures 5a, S5a), so overall titer probably does not explain any differences. However, future research will need to investigate the relative roles of host and symbiont further. Notably, there is likely to be some overlap in the mechanism(s) of viral and fungal pathogen blocking in Drosophila. First, wMel can block both types of pathogens based on the results here and shown elsewhere51,54,72. Second, some of the molecular mechanisms contributing to viral blocking could also ostensibly apply to fungal pathogens, such as immune priming108, increased ROS production109, or competition for resources between symbiont and pathogen110–112.
Based on the results, we draw several main conclusions: 1) wMel can confer broad, but not universal, protection against fungal pathogenesis, 2) fungal pathogen blocking by Wolbachia is highly context-dependent, with host sex, genetics, and pathogen species being significant determinants of host outcomes, and 3) inhibition of fungal pathogenesis can have positive fitness impacts on the host from early during infection, likely due to reduced pathogen load. Many questions remain unanswered and future work will be needed to investigate this further. For example: How broad is the phenotype in terms of symbiont strains, fly species and strains, and pathogen species? How do other host variables like age impact the phenotype? How do symbiont density and tissue tropism impact the phenotype? Are the results applicable to other insect species for potential translational use in agriculture or other fields? What is the mechanism of fungal pathogen blocking, and can it help inform the mechanism of viral pathogen blocking? How prevalent is fungal pathogen blocking in the wild? This and prior studies pave the way to answering these and other important questions.
Materials and Methods:
Fly strains and husbandry
Fly strains include Drosophila melanogaster w1118 (one strain with Wolbachia, one cured of Wolbachia via tetracycline) and D. melanogaster wk (one strain with Wolbachia, one cured of Wolbachia via tetracycline). The wk line was isolated in Karsnäs, Sweden in 1960 (white allele named for location of isolation)77 and the w1118 line was isolated in California and described in 1985 (white allele named for date of isolation)76. Both were maintained in various labs since their isolation. Flies were reared on CMY media: 64.3 g/L cornmeal (Flystuff Genesee Scientific, San Diego CA), 79.7 mL/L molasses (Flystuff Genesee Scientific), 35.9 g/L yeast (Genesee Scientific inactive dry yeast nutritional flakes), 8 g/L agar (Flystuff Genesee Scientific Drosophila type II agar), 15.4 mL of antimicrobial mixture [50 mL phosphoric acid (Thermo Fisher, Waltham MA), 418 mL propionic acid (Thermo Fisher), 532 mL deionized water], and 1g/L tegosept (Genesee Scientific). Flies were kept at 25°C on a 16h light/8 h dark light cycle.
Microbial strains and growth conditions for fly infections
The microorganisms used in this study are summarized in Table S2.
Yeast colonies were grown for 16 h on potato dextrose (PD) agar at 30°C. To grow cultures for fly infections, yeast isolates were grown overnight for 16 h from a single colony in 2 mL PD broth (BD, Sparks MA) with shaking at 225 rpm. Isolates were then prepared as described below. Filamentous fungi were prepared by purifying conidia grown on PD agar at 30°C (Fusarium, Aspergillus, and Beauveria) or 25°C (Metarhizium, Clonostachys, and Trichoderma) for 1–2 weeks. Autoclaved DI water was poured over each plate and the conidia were suspended in the liquid. This was then poured over a filter (Millipore Sigma, Burlington MA, Miracloth 22–25 μm pore size) and the filtrate was placed into a 50 mL falcon tube. This was then centrifuged at 1000 rpm for 5 min and the supernatant was discarded. The conidia were then resuspended in sterile 20% glycerol and were counted using a hemocytometer. The conidia concentrations used in this study were (conidia/mL): Aspergillus fumigatus (1.75×109), Aspergillus flavus (1.18×108), Fusarium oxysporum (9.65×107), Fusarium graminaerum (1.24×108), Beauveria bassiana (4.38×108), Metarhizium anisopliae (1.5×107), Clonostachys rosea (1×108), and Trichoderma atroviride (7.2×107).
Fly infections
Yeast cultures were grown overnight in the conditions described above. Yeasts C. glabrata, C. auris, and G. pseudocandidus were diluted in PD broth to an optical density (OD) value of A600= 200 +/− 5 for Candida auris and Galactomyces pseudocandidus, and an OD value of A600=220 +/− 5 for Candida glabrata. Filamentous fungi were prepared as described above. Mated males or females 4–6 days old of a given genotype were pierced in the thorax just beneath the wing using a 0.15 mm dissecting pin (Entosphinx, Czech Republic, No. 15 Minuten pins 12 mm long 0.15 mm diameter) dipped into the diluted culture or control. Controls were the growth broth for yeasts (PD broth) or sterile 20% glycerol for the filamentous fungi. Flies were then placed in groups of 10 per food vial. 20–30 individuals of each treatment × sex × genotype group were infected in each block, and at least two blocks of infections were performed on separate days for every experiment.Flies were counted for survival daily for 21 days.
Fertility assay
To measure fertility post-infection, 32 virgin 3–5 day old females were collected from each fly strain (w1118 and wk, with or without Wolbachia). Half of the samples of each strain was infected with B. bassiana, as described above. The other half was given 20% glycerol control treatments, also as described above. They were then immediately crossed to 2–4 day old males of the same genotype. Eggs were collected by placing single male-female pairs into a 6 oz. square bottom Drosophila bottle (Fisher Scientific, Hampton NH) covered with a grape juice agar plate [100% concord grape juice (Welch’s, MA), tegosept (Genesee Scientific, San Diego CA), 200-proof ethanol (Decon Laboratories Inc, PA), agar (Teknova, Hollister CA), DI water] with yeast paste (Fleischmann’s Active Dry Yeast, Heilsbronn Germany, mixed 1:1 volume with water). These bottles were placed at 25°C incubator overnight. Grape plates were swapped the next morning (16 hr later) with fresh plates and yeast. The bottles were placed back in the incubator and flies were allowed to lay eggs for 72 h. Plates were then removed and eggs were counted immediately. Plates were then kept covered for 24 h and egg hatching was recorded.
DNA Extractions
DNA extractions were performed with a modified protocol using reagents from the Qiagen Puregene Cell Core Kit (cat. #158046). Cells from samples were lysed by adding 100 μL chilled Cell Lysis Solution to each tube, homogenizing the sample with a pestle, incubating at 65°C for 15 min, then cooling on ice. To precipitate protein, 33 μL Protein Precipitation Solution was added to each sample followed by vortexing for 10 s. Samples were cooled on ice for 5 minutes, and then centrifuged at 14,000 rpm for 3 min. To precipitate DNA, the supernatant was removed and mixed with 100 μL pure isopropanol per sample and each sample was inverted 50 times to mix. The samples were centrifuged 5 min at 14,000 rpm, and supernatant was discarded. Then, 100 μL 70% ethanol was added to each sample and tubes were inverted several times to wash the DNA pellet. Samples were centrifuged 1 min at 14,000 rpm and supernatant was discarded. Tubes were inverted over a paper towel for 10 minutes to dry. DNA was then resuspended with 30 μL DNA Hydration Solution per sample, left at room temperature overnight to allow resuspension, and then frozen and kept at −20°C the next day until use.
Wolbachia and fungal titers
To measure microbial titers post-infection, virgin 3–5 day old females were collected from each fly strain. Flies were then given the indicated treatment, either B. bassiana or 20% glycerol sham control. They were then collected at 0 and 24 hr post infection. Samples were flash frozen at their given time point. This led to 10 samples of 3 flies per treatment × time group. This was done for each of the four fly strains.
qPCR was then performed using the Bio-Rad SsoAdvanced Universal SYBR Green Supermix (cat. #1725270) according to manufacturer instructions. Primers are listed in Table S3. qPCR was then performed using a Bio-Rad CFX Connect System with the following conditions: 50°C 10 min, 95°C 5 min, 40x (95°C 10 s, 55°C 30 s), 95°C 30 s. Differences in gene expression were done by calculating 2−Δct.
Drosophila and Wolbachia sequencing and analysis
For the comparison of the Wolbachia from the w1118 and wk strains, DNA from 3 female flies each of each strain with Wolbachia was extracted as described above. Samples were prepared for whole genome sequencing with the xGen™ DNA Library Prep EZ Kit (Integrated DNA Technologies, #10009821) with a protocol modified to 1/4 reaction volumes. Briefly, 100 ng of DNA from each sample was buffer exchanged via Ampure XP bead purification (Beckman Coulter Life Sciences product number A63881) into the low EDTA TE buffer needed for the xGen™ kit, resulting in a starting input volume of 5 μL. Genomic DNA was enzymatically fragmented to an expected 350 bp insert size, end repaired, and A-tailed in one reaction step. Stubby Y adapters were then ligated onto the fragmented DNA, and reactions were bead-purified following adapter ligation. Unique dual indexes were added to each sample with eight cycles of PCR amplification of the program provided in the xGen™ DNA Library Prep EZ Kit protocol. The libraries were then bead-purified twice, first by a 0.6X purification ratio, followed by a 1.2X purification ratio to provide adapter and primer dimer free libraries. Library quantity was determined with the broad range dsDNA Qubit Assay on the Qubit 1 Fluorometer (Thermofisher Scientific), and the library quality and median library size was assessed with a D1000 screen tape on the TapeStation 4150 (Agilent Technologies). Nanomolar concentrations were determined for each library based on their Qubit concentration in ng/μL and an averaged 442 bp library size. Libraries were pooled at 3 nM concentration along with another set of libraries for a different project. The libraries were sequenced at the University of Kansas Medical Center Genome Sequencing Facility on a NovaSeq 6000 S2 150PE flowcell (Illumina Technologies).
Raw reads were trimmed and filtered using fastp113 with default parameters and removing the first and last 5 bases from each sequence. Reads were then mapped to a chimeric assembly of D. melanogaster (Release 6 plus ISO1 MT from NCBI) and wMel Wolbachia (ASM1658442v1 from NCBI) using bwa114 and samtools115 with default parameters. SNPs were called using Freebayes116 with ploidy set to 1 since the host was inbred and Wolbachia is haploid, and filtered with vcffilter117 with depth greater than 10 and quality greater than 30.
Data visualization and statistical analyses
Data analysis and figure generation were performed in R118 version 4.2.2, using several packages: coxme119 (version 2.2.18.1), ggplot2120 (version 3.4.0), cowplot121 (version 1.1.1), car (version 3.1.1)122, SurvMiner123 (version 0.4.9), and SurvMisc124 (version 0.5.6). Dot plots were analyzed with a logistic regression. Longevity plots with infection were analyzed using a Cox proportional hazard model with no Wolbachia as the reference.
Supplementary Material
Importance:
Wolbachia bacteria of arthropods are at the forefront of global initiatives to fight arthropod-borne viruses. Despite great success in using the symbiont to fight viruses, little research has focused on Wolbachia-fungal interactions. Here, we find that Wolbachia of Drosophila melanogaster, the same strain widely used in antiviral initiatives, can also increase the longevity of flies systemically infected with a panel of yeast and filamentous fungal pathogens. The symbiont also partially increases host fertility and reduces fungal titers during early infection, indicating a significant fitness benefit. This represents a major step forward in Wolbachia research since its pathogen blocking abilities can now be extended to a broad diversity of another major branch of microbial life. This discovery may inform basic research on pathogen blocking and has potential translational applications in areas including biocontrol in agriculture.
Acknowledgments:
We would like to thank P. Shahrestani and K. Michel for providing certain microbial strains, as well as J. Blumenstiel for providing fly lines. This work was supported by two National Institutes of Health (NIH) K-INBRE P20 GM103418 postdoctoral awards (to JIP), National Science Foundation (NSF) Postdoctoral Fellowship in Biology (PRFB) DBI 2109772 to JIP, NIH K-INBRE P20 GM103418 student award to AA, and NIH grant R01 AI139154 to RLU.
Data Availability:
All data will be deposited in Dryad upon publication of this manuscript.
References:
- 1.Buchner P. Endosymbiosis of animals with plant microorganisms. (1965).
- 2.Perlmutter J. I. & Bordenstein S. R. Microorganisms in the reproductive tissues of arthropods. Nature Reviews Microbiology, doi: 10.1038/s41579-019-0309-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas A. E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annual Review of Entomology 43, 17–37, doi: 10.1146/annurev.ento.43.1.17 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Xie J., Butler S., Sanchez G. & Mateos M. Male killing Spiroplasma protects Drosophila melanogaster against two parasitoid wasps. Heredity 112, 399 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen J. H. & Barr A. R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232, 657–658, doi: 10.1038/232657a0 (1971). [DOI] [PubMed] [Google Scholar]
- 6.Kaur R. et al. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host & Microbe (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinert L. A., Araujo-Jnr E. V., Ahmed M. Z. & Welch J. J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B: Biological Sciences 282, 20150249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zug R. & Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7, e38544, doi: 10.1371/journal.pone.0038544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LePage D. & Bordenstein S. R. Wolbachia: Can we save lives with a great pandemic? Trends in Parasitology 29, 385–393, doi: 10.1016/j.pt.2013.06.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werren J. H., Baldo L. & Clark M. E. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology 6, 741–751, doi: 10.1038/nrmicro1969 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Wang G.-H., Jia L.-Y., Xiao J.-H. & Huang D.-W. Discovery of a new Wolbachia supergroup in cave spider species and the lateral transfer of phage WO among distant hosts. Infection, Genetics and Evolution 41, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Taylor M., Bordenstein S. & Slatko B. Microbe Profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes. Microbiology 164, 1345–1347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slatko B. et al. Pseudoscorpion Wolbachia symbionts: Diversity and evidence for a new supergroup S. (2020). [DOI] [PMC free article] [PubMed]
- 14.Hurst G. D. & Frost C. L. Reproductive parasitism: maternally inherited symbionts in a biparental world. Cold Spring Harbor Perspectives in Biology 7, a017699 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dedeine F., Bouletreau M. & Vavre F. Wolbachia requirement for oogenesis: occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida. Heredity 95, 394 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Manoj R. R. S., Latrofa M. S., Epis S. & Otranto D. Wolbachia: endosymbiont of onchocercid nematodes and their vectors. Parasites & Vectors 14, 1–24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong J.-T., Li T.-P., Wang M.-K. & Hong X.-Y. Wolbachia-based strategies for control of agricultural pests. Current Opinion in Insect Science, 101039 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Ant T. H., Mancini M. V., McNamara C. J., Rainey S. M. & Sinkins S. P. Wolbachia-virus interactions and arbovirus control through population replacement in mosquitoes. Pathogens and Global Health 117, 245–258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst G. D. D. et al. Male–killing Wolbachia in two species of insect. Proceedings of the Royal Society of London. Series B: Biological Sciences 266, 735–740, doi: 10.1098/rspb.1999.0698 (1999). [DOI] [Google Scholar]
- 20.Schilthuizen M. O. & Stouthamer R. Horizontal transmission of parthenogenesis–inducing microbes in Trichogramma wasps. Proceedings of the Royal Society of London. Series B: Biological Sciences 264, 361–366 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchon D., Rigaud T. & Juchault P. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proceedings of the Royal Society of London. Series B: Biological Sciences 265, 1081–1090 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosokawa T. et al. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nature Microbiology 1, 15011 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Taylor M. J., Bandi C. & Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Advances in Parasitology 60, 245–284, doi: 10.1016/s0065308x(05)60004-8 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Dedeine F. et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proceedings of the National Academy of Sciences 98, 6247–6252 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann A. A., Clancy D. & Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76, 1–8 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Hamm C. A. et al. Wolbachia do not live by reproductive manipulation alone: infection polymorphism in Drosophila suzukii and D. subpulchrella. Molecular Ecology 23, 4871–4885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meany M. K. et al. Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana. Evolution 73, 1278–1295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds K. T. & Hoffmann A. A. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genetics Research 80, 79–87 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Yamada R., Floate K. D., Riegler M. & O’Neill S. L. Male development time influences the strength of Wolbachia-induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics 177, 801–808, doi: 10.1534/genetics.106.068486 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hague M. T., Mavengere H., Matute D. R. & Cooper B. S. Environmental and genetic contributions to imperfect wMel-like Wolbachia transmission and frequency variation. Genetics 215, 1117–1132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narita S., Shimajiri Y. & Nomura M. Strong cytoplasmic incompatibility and high vertical transmission rate can explain the high frequencies of Wolbachia infection in Japanese populations of Colias erate poliographus (Lepidoptera: Pieridae). Bulletin of Entomological Research 99, 385–391 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann A. A., Hercus M. & Dagher H. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148, 221–231 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyer K. A. & Jaenike J. Evolutionary dynamics of a spatially structured host-parasite association: Drosophila innubila and male-killing Wolbachia. Evolution 59, 1518–1528 (2005). [PubMed] [Google Scholar]
- 34.Kittayapong P., Baimai V. & O’Neill S. L. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. American Journal of Tropical Medicine and Hygiene 66, 108–111 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Jiggins F. M., Bentley J. K., Majerus M. E. & Hurst G. D. How many species are infected with Wolbachia? Cryptic sex ratio distorters revealed to be common by intensive sampling. Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 1123–1126 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurst G. D., Johnson A. P., Schulenburg J. H. & Fuyama Y. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156, 699–709 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murdock C. C., Blanford S., Hughes G. L., Rasgon J. L. & Thomas M. B. Temperature alters Plasmodium blocking by Wolbachia. Scientific Reports 4, 3932 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bordenstein S. R. & Bordenstein S. R. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS One 6, e29106, doi: 10.1371/journal.pone.0029106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chrostek E., Martins N., Marialva M. S. & Teixeira L. Wolbachia-conferred antiviral protection is determined by developmental temperature. mBio 12, 10.1128/mbio.02923-02920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborne S. E., Iturbe-Ormaetxe I. a., Brownlie J. C., O’Neill S. L. & Johnson K. N. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Applied and Environmental Microbiology 78, 6922–6929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda T., Ishikawa H. & Sasaki T. Infection density of Wolbachia and level of cytoplasmic incompatibility in the Mediterranean flour moth, Ephestia kuehniella. Journal of Invertebrate Pathology 84, 1–5 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Hughes G. L. & Rasgon J. L. Transinfection: a method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Molecular Biology 23, 141–151, doi: 10.1111/imb.12066 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann A. A., Turelli M. & Harshman L. G. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126, 933–948 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unckless R. L., Boelio L. M., Herren J. K. & Jaenike J. Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proceedings of the Royal Society B: Biological Sciences 276, 2805–2811 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiggins F. M., Randerson J. P., Hurst G. D. & Majerus M. E. How can sex ratio distorters reach extreme prevalences? Male-killing Wolbachia are not suppressed and have near-perfect vertical transmission efficiency in Acraea encedon. Evolution 56, 2290–2295 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Lindsey A. R. et al. Wolbachia is a nutritional symbiont in Drosophila melanogaster. bioRxiv, 2023.2001. 2020.524972 (2023). [Google Scholar]
- 47.Hosokawa T., Koga R., Kikuchi Y., Meng X.-Y. & Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proceedings of the National Academy of Sciences 107, 769–774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starr D. J. & Cline T. W. A host–parasite interaction rescues Drosophila oogenesis defects. Nature 418, 76–79 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Ote M., Ueyama M. & Yamamoto D. Wolbachia protein TomO targets nanos mRNA and restores germ stem cells in Drosophila sex-lethal mutants. Current Biology 26, 2223–2232 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Russell S. L., Castillo J. R. & Sullivan W. T. Wolbachia endosymbionts manipulate GSC self-renewal and differentiation to enhance host fertility. bioRxiv, 2022.2012. 2015.520626 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teixeira L., Ferreira A. & Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6, e2, doi: 10.1371/journal.pbio.1000002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipsitch M., Nowak M. A., Ebert D. & May R. M. The population dynamics of vertically and horizontally transmitted parasites. Proceedings of the Royal Society of London. Series B: Biological Sciences 260, 321–327 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Harcombe W. & Hoffmann A. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. Journal of Invertebrate Pathology 87, 45–50 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Hedges L. M., Brownlie J. C., O’neill S. L. & Johnson K. N. Wolbachia and virus protection in insects. Science (New York, N.Y.) 322, 702–702 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Lindsey A. R., Bhattacharya T., Newton I. L. & Hardy R. W. Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses 10, 141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutra H. L. et al. Wolbachia blocks currently circulating Zika Virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host & Microbe 19, 771–774, doi: 10.1016/j.chom.2016.04.021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes G. L., Koga R., Xue P., Fukatsu T. & Rasgon J. L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathogens 7, e1002043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann A. A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457, doi: 10.1038/nature10356 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Walker T. et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453, doi: 10.1038/nature10355 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Van den Hurk A. F. et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Neglected Tropical Diseases 6, e1892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aliota M. T. et al. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Neglected Tropical Diseases 10, e0004677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MosquitoMate I. MosquitoMate: Environmentally friendly innovative mosquito control, <https://mosquitomate.com> (2022).
- 63.Program W. M. The World Mosquito Program, <https://www.worldmosquitoprogram.org> (2022).
- 64.O’Neill S. L. et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Research 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization. A global brief on vector-borne diseases. (WHO, 2014). [Google Scholar]
- 66.O’Neill S. L. The use of Wolbachia by the World Mosquito Program to interrupt transmission of Aedes aegypti transmitted viruses. Dengue and Zika: Control and Antiviral Treatment Strategies, 355–360 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Mains J. W., Kelly P. H., Dobson K. L., Petrie W. D. & Dobson S. L. Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes. Journal of Medical Entomology (2019). [DOI] [PubMed] [Google Scholar]
- 68.Beebe N. W. et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proceedings of the National Academy of Sciences 118, e2106828118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Y. H., Woolfit M., Rancès E., O’Neill S. L. & McGraw E. A. Wolbachia-associated bacterial protection in the mosquito Aedes aegypti. PLoS Neglected Tropical Diseases 7, e2362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vega F. E., Meyling N. V., Luangsa-ard J. J. & Blackwell M. Fungal entomopathogens. Insect Pathology, 171–220 (2012). [Google Scholar]
- 71.Fytrou A., Schofield P. G., Kraaijeveld A. R. & Hubbard S. F. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proceedings. Biological Sciences 273, 791–796, doi: 10.1098/rspb.2005.3383 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panteleev D. et al. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika 43, 1277–1280 (2007). [PubMed] [Google Scholar]
- 73.Zélé F., Altıntaş M., Santos I., Cakmak I. & Magalhães S. Population‐specific effect of Wolbachia on the cost of fungal infection in spider mites. Ecology and Evolution 10, 3868–3880 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramirez J. L., Schumacher M. K., Ower G., Palmquist D. E. & Juliano S. A. Impacts of fungal entomopathogens on survival and immune responses of Aedes albopictus and Culex pipiens mosquitoes in the context of native Wolbachia infections. PLoS Neglected Tropical Diseases 15, e0009984 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Higashi C. et al. ANOTHER TOOL IN THE TOOLBOX: Wolbachia-mediated protection against a specialized fungal pathogen of aphids. bioRxiv, 2023.2007. 2024.550390 (2023). [Google Scholar]
- 76.Levis R., Hazelrigg T. & Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science (New York, N.Y.) 229, 558–561 (1985). [DOI] [PubMed] [Google Scholar]
- 77.Luning K. Genetics of inbred Drosophila melanogaster. Hereditas 95, 181–188 (1981). [Google Scholar]
- 78.Law C. J., Maloney P. C. & Wang D.-N. Ins and outs of major facilitator superfamily antiporters. Annual Reviews in Microbiology 62, 289–305 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Islam W. et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microbial Pathogenesis 159, 105122 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Poveda J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biological Control 159, 104634 (2021). [Google Scholar]
- 81.Peng Y. et al. Research progress on phytopathogenic fungi and their role as biocontrol agents. Frontiers in Microbiology 12, 670135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Z.-B. et al. Biology and applications of Clonostachys rosea. Journal of Applied Microbiology 129, 486–495 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Schlamp F. et al. Dense time-course gene expression profiling of the Drosophila melanogaster innate immune response. BMC Genomics 22, 1–22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duneau D. et al. Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. eLife 6, e28298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turelli M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64, 232–241 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Klein S. L. & Flanagan K. L. Sex differences in immune responses. Nature Reviews Immunology 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Lotter H. & Altfeld M. Sex differences in immunity. in Seminars in Immunopathology. 133–135 (Springer; ). [DOI] [PubMed] [Google Scholar]
- 88.vom Steeg L. G. & Klein S. L. SeXX matters in infectious disease pathogenesis. PLoS Pathogens 12, e1005374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuk M. The sicker sex. PLoS Pathogens 5, e1000267 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belmonte R. L., Corbally M.-K., Duneau D. F. & Regan J. C. Sexual dimorphisms in innate immunity and responses to infection in Drosophila melanogaster. Frontiers in Immunology 10, 3075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duneau D. F. et al. The Toll pathway underlies host sexual dimorphism in resistance to both Gram-negative and Gram-positive bacteria in mated Drosophila. BMC Biology 15, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vincent C. M. & Dionne M. S. Disparate regulation of IMD signaling drives sex differences in infection pathology in Drosophila melanogaster. Proceedings of the National Academy of Sciences 118, e2026554118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Regan J. C. et al. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 5, e10956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vincent C. M. & Sharp N. P. Sexual antagonism for resistance and tolerance to infection in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences 281, 20140987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kutch I. C. & Fedorka K. M. Y-linked variation for autosomal immune gene regulation has the potential to shape sexually dimorphic immunity. Proceedings of the Royal Society B: Biological Sciences 282, 20151301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vale P. F. & Jardine M. D. Sex-specific behavioural symptoms of viral gut infection and Wolbachia in Drosophila melanogaster. Journal of Insect Physiology 82, 28–32 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Kraaijeveld A. R., Barker C. L. & Godfray H. C. J. Stage-specific sex differences in Drosophila immunity to parasites and pathogens. Evolutionary Ecology 22, 217–228 (2008). [Google Scholar]
- 98.Taylor K. & Kimbrell D. Host immune response and differential survival of the sexes in Drosophila. Fly 1, 197–204 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Shahrestani P. et al. Sexual dimorphism in Drosophila melanogaster survival of Beauveria bassiana infection depends on core immune signaling. Scientific Reports 8, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffmann A. A., Clancy D. J. & Merton E. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136, 993–999 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mancini M. V., Herd C. S., Ant T. H., Murdochy S. M. & Sinkins S. P. Wolbachia strain wAu efficiently blocks arbovirus transmission in Aedes albopictus. Plos Neglected Tropical Diseases 14, e0007926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreira L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009). [DOI] [PubMed] [Google Scholar]
- 103.Mousson L. et al. Wolbachia modulates Chikungunya replication in Aedes albopictus. Molecular Ecology 19, 1953–1964 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Cogni R., Ding S. D., Pimentel A. C., Day J. P. & Jiggins F. M. Wolbachia reduces virus infection in a natural population of Drosophila. Communications Biology 4, 1327 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osborne S. E., Leong Y. S., O’Neill S. L. & Johnson K. N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathogens 5, e1000656 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong Z. S., Hedges L. M., Brownlie J. C. & Johnson K. N. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PloS One 6, e25430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chambers M. C., Jacobson E., Khalil S. & Lazzaro B. P. Consequences of chronic bacterial infection in Drosophila melanogaster. PloS One 14, e0224440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rancès E., Ye Y. H., Woolfit M., McGraw E. A. & O’Neill S. L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathogens 8, e1002548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan X. et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences 109, E23–E31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caragata E. P. et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathogens 9, e1003459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caragata E. P., Rancès E., O’Neill S. L. & McGraw E. A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microbial Ecology 67, 205–218 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Molloy J. C., Sommer U., Viant M. R. & Sinkins S. P. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Applied and Environmental Microbiology 82, 3109–3120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen S., Zhou Y., Chen Y. & Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics (Oxford, England) 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li H. & Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics (Oxford, England) 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Danecek P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garrison E. & Marth G. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207.3907 (2012). [Google Scholar]
- 117.Garrison E., Kronenberg Z. N., Dawson E. T., Pedersen B. S. & Prins P. Vcflib and tools for processing the VCF variant call format. bioRxiv, 2021.2005.2021.445151, doi: 10.1101/2021.05.21.445151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2020). [Google Scholar]
- 119.Therneau T. Package ‘coxme’: Mixed Effects Cox Models. (R Foundation for Statistical Computing, Vienna, Austria, 2022). [Google Scholar]
- 120.Villanueva R. A. M. & Chen Z. J. ggplot2: elegant graphics for data analysis. (Taylor & Francis, 2019). [Google Scholar]
- 121.Wilke C. O., Wickham H. & Wilke M. C. O. Package ‘cowplot’. Streamlined Plot Theme and Plot Annotations for ‘ggplot2 (2019). [Google Scholar]
- 122.Fox J. & Weisberg S. An R Companion to Applied Regression. Third edn, (Sage, Thousand Oaks CA, 2019). [Google Scholar]
- 123.Kassambara A., Kosinski M., Biecek P. & Fabian S. Package ‘survminer’. Drawing Survival Curves using ‘ggplot2’(R package version 03 1) (2017). [Google Scholar]
- 124.Dardis C. & Dardis M. C. Package ‘survMisc’. (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be deposited in Dryad upon publication of this manuscript.