Abstract
Background:
Persons with dementia (PWD) have high rates of polypharmacy. While previous studies have examined specific types of problematic medication use in PWD, we sought to characterize a broad spectrum of medication misuse and overuse among community-dwelling PWD.
Methods:
We included community-dwelling adults aged ≥66 in the Health and Retirement Study from 2008-2018 linked to Medicare and classified as having dementia using a validated algorithm. Medication usage was ascertained over the 1-year prior to an HRS interview date. Potentially problematic medications were identified by: 1) medication overuse including over-aggressive treatment of diabetes/hypertension (e.g., insulin/sulfonylurea with hemoglobin A1c<7.5%) and medications inappropriate near end of life based on STOPPFrail and 2) medication misuse including medications that negatively affect cognition and medications from 2019 Beers and STOPP Version 2 criteria. To contextualize, we compared medication use to people without dementia through a propensity matched cohort by age, sex, comorbidities, and interview year. We applied survey weights to make our results nationally representative.
Results:
Among 1,441 PWD, median age was 84 (interquartile range=78-89), 67% female, and 14% Black. Overall, 73% of PWD were prescribed ≥1 potentially problematic medication with a mean of 2.09 per individual in the prior year. This was notable across several domains, including 41% prescribed ≥1 medication that negatively affects cognition. Frequently problematic medications included proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), opioids, antihypertensives, and antidiabetic agents. Problematic medication use was higher among PWD compared to those without dementia with 73% vs. 67% prescribed ≥1 problematic medication (p=0.002) and mean of 2.09 vs. 1.62 (p<0.001), respectively.
Conclusion:
Community-dwelling PWD frequently receive problematic medications across multiple domains and at higher frequencies compared to those without dementia. Deprescribing efforts for PWD should focus not only on potentially harmful central nervous system-active medications but also other classes such as PPIs and NSAIDs.
Keywords: cognitive impairment, dementia, polypharmacy, medication overuse, potentially inappropriate medication
INTRODUCTION
An estimated 6.5 million individuals aged 65 years and older were living with Alzheimer’s disease and related dementias in the United States in 2022.1 Persons with dementia (PWD) often have multiple comorbidities and high symptom burdens that contribute to a high prevalence of polypharmacy.2,3 Polypharmacy is associated with several adverse health outcomes, including hospitalizations, emergency department visits, and increased mortality.4,5 Drug-related problems are prevalent among PWD and contribute to unwanted hospitalizations at higher rates than those without dementia.6-9 This is especially prominent in PWD due in part to increased susceptibility to medication side effects, drug-drug interactions, and difficulty with medication management leading to unintentional non-adherence and medication errors.7,8,10
Many medications may be problematic among PWD. For example, guidelines for diabetes management recommend less strict glycemic control for individuals with multimorbidity and limited life expectancy as the risks associated with certain antidiabetic agents, such as insulin and sulfonylureas, may outweigh the benefits of long-term tight glycemic control.11,12 Similarly, many medications may cause net harm when used alone or in combination with other medications. For example, strongly anticholinergic and sedative-hypnotic medications have been associated with adverse outcomes including accelerated cognitive decline which is particularly worrisome in PWD who have decreased cognitive reserve.13,14
While several prior studies have documented a high prevalence of potentially inappropriate medication (PIM) use among PWD in the nursing home, less is known about the prevalence of medication misuse and overuse among the estimated ~70% of PWD living in the community.15,16 Previous work has shown that community-dwelling PWD often receive PIMs at higher frequencies compared to individuals without dementia and receive more medications across different medication classes.17,18 However, less research has focused on a comprehensive approach to capturing a broad spectrum of potentially problematic medication use. Therefore, we sought to characterize the frequency and types of medication misuse and overuse in community-dwelling PWD across several domains, including overaggressive treatment of chronic conditions, medications inappropriate near the end of life, medications that negatively affect cognition, and medications to avoid based on consensus criteria. We additionally compare patterns of medication misuse and overuse among PWD to individuals without dementia.
METHODS
Study cohort
We included participants from the Health and Retirement Study (HRS) between 2008-2018, a nationally representative survey of US adults in which individuals are interviewed every 2 years.19 We included community-dwelling individuals aged ≥66 with at least 12 consecutive months of Medicare Part A/B/D enrollment prior to a specific HRS interview date. Individuals were classified as having dementia based on a validated algorithm.20,21 This algorithm, which includes predictors such as age, sex, cognitive tests, and physical functioning, was developed on HRS participants who underwent neuropsychological evaluations. It has displayed good accuracy in validation studies.21
Once an individual was classified as having dementia, they were classified with dementia for all subsequent waves. For individuals classified as having dementia with only one wave available, we used this wave as the index date to obtain medication information in the previous year. For individuals with multiple HRS interviews having a dementia classification, we selected one wave as the index date prioritizing the wave when the individual was selected for an enhanced face to face (EFTF) interview. Prioritizing EFTF interviews allowed us to collect information on hemoglobin A1c (HbA1c), cystatin C, and systolic blood pressure (SBP). Individuals are assigned to an EFTF interview every other wave (every 4 years).
Ascertainment of medication usage
We used a 1-year look-back period from a specific HRS interview date to ascertain medication usage which has been shown to have high sensitivity/specificity for identifying cross-sectional medication use (Supplementary Figure S1).22 We obtained information on individual prescriptions, prescription dates, and days of supply. We linked Medicare Part D prescriptions to the Medi-Span database to collect information on drug name and drug class based on a Generic Product Identifier classification system. Unless otherwise specified, we considered medication usage as any days supplied (see Supplementary Methods for definitions of chronic medication use and overlapping prescriptions).
Measures of medication overuse and misuse
We defined two categories of potentially problematic medication use: medication overuse and misuse (Table 1). Measures within these domains were selected based on literature review of inappropriate medication use for older adults.15,23,24 Information on how individual criteria were operationalized is provided in the Supplementary Appendix and Methods.
Table 1:
Domains used to assess potentially problematic medication use
| Domain | Measure type | Measure sources | Examples |
|---|---|---|---|
| Medication overuse | Over-aggressive treatment of chronic conditions | Choosing Wisely, clinical guidelines12,25 |
|
| Medications inappropriate near the end of life | STOPPFrail Criteria26 |
|
|
| Medication misuse | Medications that negatively affect cognition | Strongly anticholinergic medications from 2019 Beers criteria Table 7, Sedative Load Model23,28 |
|
| Drugs to avoid criteria | 2019 Beers and STOPP Version 2 criteria23,24 |
|
Abbreviations: CNS, central nervous system; CrCl, creatinine clearance; SBP, systolic blood pressure; STOPP, Screening Tool of Older Persons' Prescriptions; STOPPFrail, Screening Tool of Older Persons' Prescriptions in Frail adults with limited life expectancy
Classification of medication overuse
Within the medication overuse domain, we included two subdomains: over-aggressive treatment of chronic conditions (diabetes/hypertension) and medications inappropriate near the end of life.25,26 For overtreatment of diabetes/hypertension, we used information from the EFTF interview, in which an individual may have his or her SBP collected and/or blood drawn. We defined over-aggressive treatment of diabetes as prescriptions for insulin or sulfonylureas in the 6 months prior to when an individual with diabetes had a HbA1c<7.5%. We defined over-aggressive treatment of hypertension as prescriptions for antihypertensives in the 6 months prior to an average SBP<110 based on 3 measurements during the EFTF interview. As individuals may have indications for specific antihypertensives even if SBP<110 (e.g., beta-blockers in heart failure), we excluded individuals with specific diagnoses (Supplementary Methods, Table S1).
Medications inappropriate near the end of life were assessed using the Screening Tool of Older Persons’ Prescriptions in Frail adults with a limited life expectancy (STOPPFrail).26 STOPPFrail involves medications that may be inappropriate for individuals with limited life expectancy (e.g., statins). We operationalized limited life expectancy as >50% 1-year mortality using the Gagne comorbidity index (score >9).27
Classification of medication misuse
Medication misuse was classified in two sub-domains: medications that negatively affect cognition and medications to avoid based on consensus criteria. Medications that negatively affect cognition included strongly anticholinergic medications (Table 7 of 2019 Beers criteria) and sedative-hypnotics based on previous studies and the Sedative Load Model (Supplementary Tables S2, S3).23,28,29 The second sub-domain involved medications based on the 2019 American Geriatrics Society Beers Criteria for PIM Use in Older Adults and the Screening Tool of Older Persons’ Prescriptions Version 2 (STOPP-V2).23,24 Medications included in the 2019 Beers and STOPP-V2 criteria are categorized broadly as medications to avoid in older adults in general, medications to avoid given comorbidities (e.g., chronic kidney disease, heart failure), and medications that may be harmful when used in combination (e.g., opioids and benzodiazepines). Details are provided in Supplementary Methods. In brief, we used International Classification of Diseases (ICD)-9 and ICD-10 diagnosis codes in the 1-year look-back period from Medicare outpatient, inpatient, and Carrier files to identify comorbidities. For criteria based on creatinine clearance cut-offs, we used diagnosis codes or estimated glomerular filtration rate (eGFR) values obtained from a cystatin C measure from the EFTF interview.30 For criteria involving overlapping medications or chronic use (e.g., >3 months continuous use), we used fill dates and days of supply. Given that we did not have detailed information on the indication for medication usage, our general approach was to omit criterion that were challenging to determine inappropriateness (Supplementary Methods).
Matched cohort
To place our results in context, we compared medication use for individuals with and without dementia. We performed 1:1 greedy nearest neighbor propensity score matching without replacement with a caliper distance of 0.25.31,32 We 1:1 matched individuals with and without dementia by year of assessment, age, sex, and comorbidity count (sum score of self-reported hypertension, diabetes, cancer, heart disease, lung disease, stroke, and arthritis which covers a wide range of common chronic diseases among older adults). We matched on these characteristics, as done in previous studies, to obtain groups with broadly comparable characteristics with the goal of describing differential exposure to these medications rather than a more comprehensive matching strategy which runs the risk of over-controlling for contextual factors that often accompany a dementia diagnosis and are important factors in the lives of PWD.17,18,33
Outcomes
Our primary outcomes were 1) percentage of individuals receiving at least 1 medication flagged by our measures and 2) mean number of flagged medications per individual. We report these metrics overall and across individual domains. As some medications were flagged under multiple domains (e.g., lorazepam under medications that negatively affect cognition, 2019 Beers, and STOPP-V2), we counted each medication only once when reporting the overall measure.
Statistical analysis
Data were analyzed incorporating survey weights to account for the HRS complex survey design. Since some individuals did not have a HbA1c or SBP available (e.g., might not have participated in an EFTF interview), we used multiple imputation (MI) to create 10 datasets with imputed HbA1c and SBP values to ascertain potentially problematic medication use in the over-aggressive treatment of diabetes/hypertension sub-domain (Supplementary Methods). In the matched cohort, given that individuals could be included in the pool of individuals without dementia multiple times across different interview years, we accounted for repeated measures and intra-individual correlation using a generalized estimating equation (GEE) model. To compare percentage of individuals with ≥1 flagged medication, we calculated odds ratios and p-values using GEE models with binomial distribution. To compare mean number of flagged medications, we calculated incidence rate ratios and p-values using GEE models with Poisson distribution.
We performed several sensitivity analyses (Supplementary Methods). First, we created 2 additional cohorts: matched only on year of assessment and matched on several additional factors (e.g., functional impairments, healthcare utilization). Second, we repeated our analysis using inverse probability of treatment weighting (IPTW). Third, we performed an analysis that included and excluded criteria that either only applied to PWD or differed between persons with and without dementia (Supplementary Table S4). Fourth, we performed an analysis in which the non-dementia cohort was not preferentially selected based on an EFTF. Fifth, we conducted an index of local sensitivity to nonignorability (ISNI) analysis to assess the robustness of multiple imputation. Analyses were conducted using SAS 9.4 (SAS Institute Inc.) and STATA 17.0 (Stata Corp). The statistical significance threshold was 2-sided p-value<0.05. The study was reviewed and approved by the University of California, San Francisco Committee on Human Research.
RESULTS
Supplementary Figure S2 shows the flow chart to identify participants. The final cohort included 1,475 individuals classified with probable dementia during the study period and 9,199 individuals in the pool of individuals without dementia (Supplementary Table S5). In the matched cohort involving 1,441 PWD, the median age was 83.6 (interquartile range=78.1-89.0), 947 (survey-weighted 66.5%) were female, 289 (survey-weighted 14.4%) identified as non-Hispanic Black, and 246 (survey-weighted 14.1%) identified as Hispanic (Table 2). Compared to individuals without dementia, PWD in the matched cohort had a greater number of functional dependencies. PWD were prescribed a similar number of unique medications with ≥28-day supply in the prior year (median 6.6 vs. 6.5).
Table 2:
Baseline characteristics of community-dwelling older adults with and without dementia enrolled in the Health and Retirement Study from 2008-2018 included in the primary cohort matched on age, sex, comorbidity count, and year of assessment
| Persons with dementia (n = 1,441) |
Persons without dementia (n = 1,441) |
||
|---|---|---|---|
| Characteristic | Number (weighted %)a | Number (weighted %)a | SMD |
| Age in years, median (IQR) | 83.6 (78.1-89.0) | 83.1 (77.2-88.4) | 0.001 |
| Female sex | 947 (66.5%) | 947 (65.4%) | −0.02 |
| Race/Ethnicity | 0.42 | ||
| Non-Hispanic White | 876 (69.2%) | 1160 (86.1%) | |
| Non-Hispanic Black | 289 (14.4%) | 150 (6.5%) | |
| Hispanic | 246 (14.1%) | 105-110 (~6.0%)b | |
| Other | 30 (2.3%) | <25 (<1.5%)b | |
| Marital status (%) | −0.07 | ||
| Married or partnered | 554 (37.5%) | 603 (41.3%) | |
| Single or widowed | 887 (62.5%) | 838 (58.7%) | |
| Lives alone (%) | 448 (34.2%) | 626 (45.8%) | −0.24 |
| Self-reported comorbidities | |||
| Cancer | 338 (23.0%) | 367 (24.3%) | −0.03 |
| Diabetes | 486 (31.0%) | 449 (31.2%) | −0.005 |
| Heart disease | 639 (44.3%) | 686 (48.1%) | −0.08 |
| Hypertension | 1099 (74.3%) | 1121 (76.7%) | −0.05 |
| Lung disease | 218 (16.6%) | 226 (16.1%) | 0.01 |
| Median (IQR) number of IADL dependencies (range 0-5) | 1.8 (0-3.7) | 0 (0-0) | 0.02 |
| Median (IQR) number of ADL dependencies (range 0-6) | 0.8 (0-3.2) | 0 (0-0.1) | 0.01 |
| Median (IQR) number of chronic medicationsc | 6.6 (4.1-10.2) | 6.5 (4.0-10.1) | 0.001 |
| Polypharmacy (prescription for ≥5 chronic medications)c | 1103 (75.9%) | 1100 (74.7%) | 0.03 |
| 1-year predicted mortality risk >50% from Gagne index | 85 (5.9%) | 46 (3.4%) | 0.12 |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; IQR, interquartile range; SMD, standardized mean difference
The numbers in each column represent the raw unweighted number of individuals within each cohort with a particular characteristic. The weighted percentages in each column represent the column percent based on the weighted sample size after using national survey weights from the Health and Retirement Study
Results are presented in this manner due to the Centers for Medicare and Medicaid Services (CMS) cell suppression size policy which sets the minimum threshold for the display of CMS data. This was necessary as the “Other” category involved <25 individuals.
The number of medications was assessed by looking at the number of unique medications with at least a 28-day supply for an individual in the 1-year look back period.
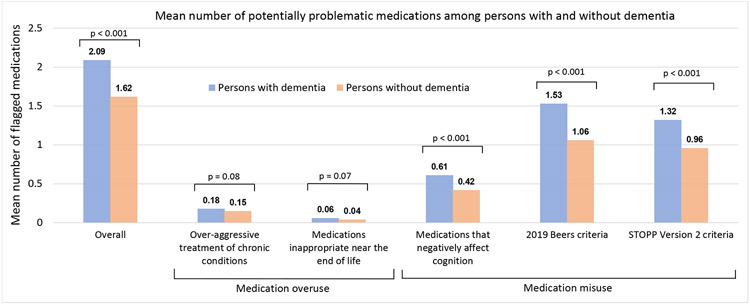
Overall, across all domains, 73% of PWD received ≥1 potentially problematic medication (Table 3, Supplementary Table S6). The overall mean number of flagged medications per PWD was 2.09 (Figure 1). Problematic medication use was prominent across several domains. For example, the percentage of PWD receiving ≥1 flagged medication and mean number of flagged medications was 17% and 0.18 for over-aggressive treatment of diabetes/hypertension, 41% and 0.61 for medications that negatively affect cognition, 60% and 1.53 for 2019 Beers, and 66% and 1.32 for STOPP-V2, respectively. Among the 85 (survey-weighted 5.9%) PWD eligible for the criteria of medications inappropriate near the end of life using STOPPFrail, 18.5% had ≥1 flagged medication with a mean of 0.33 medications. Supplementary Table S7 shows the number and percentage of individuals with diabetes and hypertension who met criteria for over-aggressive treatment of these conditions. Notably, ~40% of individuals with diabetes and A1c<7.5% with and without dementia were receiving insulin/sulfonylureas.
Table 3:
Frequency of potentially problematic medication use among community-dwelling older adults with and without dementia overall and by domain in the primary cohort matched on age, sex, comorbidity count, and year of assessment
| Percent with at least 1 potentially problematic medication | |||
|---|---|---|---|
| Medication domain | Persons with dementia (n = 1,441) |
Persons without dementia (n = 1,441) |
P-value |
| Overall | 73% | 67% | p = 0.002 |
| Medication overuse | |||
| Over-aggressive treatment of diabetes and hypertension | 17% | 14% | p = 0.07 |
| Medications inappropriate near end of life (based on STOPPFrail)a | 4% | 2% | p = 0.02 |
| Medication misuse | |||
| Medications that negatively affect cognition (e.g., strongly anticholinergic, sedative-hypnotic) | 41% | 30% | p < 0.001 |
| 2019 Beers criteria | 60% | 51% | p < 0.001 |
| STOPP Version 2 criteria | 66% | 53% | p < 0.001 |
Abbreviations: STOPP, Screening Tool of Older Persons’ Prescriptions; STOPPFrail, Screening Tool of Older Persons’ Prescriptions in Frail adults with a limited life expectancy
The number of individuals eligible for the criteria of medications inappropriate near the end of life based on Screening Tool of Older Persons’ Prescriptions in Frail adults with a limited life expectancy (STOPPFrail) (i.e., predicted 1-year mortality risk >50% based on the Gagne index) was 85 (5.9%) among PWD and 46 (3.4%) among persons without dementia. Among the 85 PWD eligible for this criteria, the percentage with ≥1 flagged medication was 18.5% for a mean of 0.33 flagged medications. Among the 46 persons without dementia eligible for this criteria, the percentage with ≥1 flagged medication was 21.9% for a mean of 0.29 flagged medications.
Figure 1:
Mean number of medications identified as potentially problematic among community-dwelling older adults with and without dementia overall and by domain in the primary cohort matched on age, sex, comorbidity count, and year of assessmenta
Abbreviations: STOPP-V2, Screening Tool of Older Persons’ Prescriptions Version 2
a As some medications were flagged under multiple domains (e.g., lorazepam under medications that negatively affect cognition, 2019 Beers, and STOPP-V2), we counted each medication only once when reporting the overall summary measure.
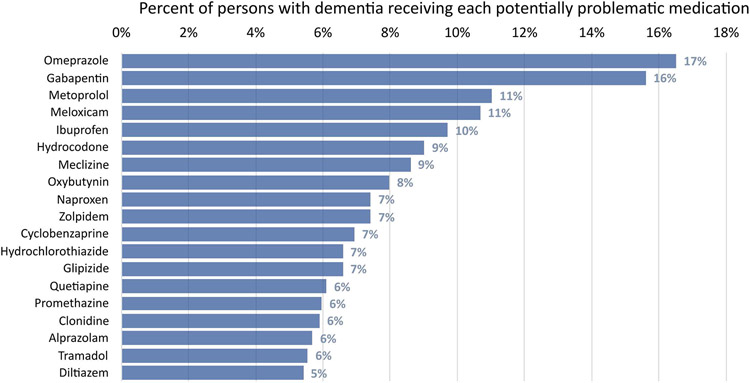
Figure 2 shows the most frequent medications among PWD identified as potentially problematic. This included medications from multiple classes, including proton pump inhibitors (PPIs), gabapentin, non-steroidal anti-inflammatory drugs (NSAIDs), opioids, selected antihypertensives and antidiabetic agents, antihistamines/antiemetics (e.g., meclizine and promethazine), and urinary anticholinergics.
Figure 2:
Medications most frequently identified as potentially problematic among community-dwelling older adults with dementia in the primary cohort (n=1,441)a
a Certain medications could be flagged as potentially problematic in multiple ways. For example, proton pump inhibitors could be flagged based on use for >8 weeks in the absence of reason for continued use (e.g., diagnosis code for Barrett’s esophagus). Gabapentin could be flagged under the “medications that negatively affect cognition” domain (as a sedative-hypnotic) or through the 2019 Beers criteria (overlapping prescription of gabapentin/opioid or combination of ≥3 central nervous system active drugs). Non-steroidal anti-inflammatory drugs like meloxicam and naproxen could be flagged when used for prolonged durations (e.g., consecutive fills for >90 days), when used with certain diagnosis codes (e.g., gastric ulcer), in advanced chronic kidney disease, or when used with anticoagulants. Antihypertensives (e.g., metoprolol, clonidine) and antidiabetic agents (e.g., glipizide) could be flagged under the “over-aggressive treatment of chronic conditions” domain (e.g., systolic blood pressure <110 on antihypertensive without alternative indication), when used in combination with another medication (e.g., beta-blocker and verapamil/diltiazem), or when used in setting of specific conditions (e.g., beta-blocker with history of heart block).
In the matched cohort, the percentage of individuals with ≥1 flagged medication overall was 73% among PWD and 67% among persons without dementia (p=0.002) (Table 3). The mean number of flagged medications overall was 2.09 vs. 1.62 (p<0.001) among those with and without dementia, respectively (Figure 1). In general, PWD received more potentially problematic medications across domains. Supplementary Figure S3 shows the distribution of potentially problematic medications among individuals with and without dementia.
Sensitivity analyses
In the cohort matched on year of assessment, PWD were older and had more comorbidities and functional impairments (Supplementary Table S8). The cohort matched on several factors showed similar characteristics between persons with and without dementia (Supplementary Table S9). Our primary results were essentially unchanged in the cohort matched on year of assessment (Supplementary Table S10). In the cohort matched on several additional factors, persons with and without dementia had a similar mean number of flagged medications (2.09 vs. 2.04, p=0.72) and similar percentage of individuals with ≥1 flagged medication (73% vs. 71%, p=0.37) (Supplementary Table S11).
Our primary results were similar in the sensitivity analysis using IPTW (Supplementary Table S12). Including and excluding criteria that were specific to individuals with dementia did not significantly change the overall results (Supplementary Table S13). One notable difference was in the STOPP-V2 criteria where the mean number of flagged medications changed from 1.32 vs. 0.96 (p<0.001) to 0.98 vs. 0.94 (p=0.49) among persons with and without dementia, respectively. An analysis where individuals were not preferentially selected based on the EFTF interview showed similar overall results (Supplementary Table S14). The ISNI sensitivity analysis suggested that the MI results were reliable for the analysis (Supplementary Methods, Tables S15 & S16, Figure S4).
DISCUSSION
In this nationally representative study, community-dwelling PWD were frequently prescribed medications that may indicate medication misuse and overuse across a variety of domains. Problematic medication use spanned many medication classes with the most prominent including PPIs, NSAIDs, opioids, antihypertensives, and antidiabetic agents. In a matched cohort, PWD received more of these potentially problematic medications and at a higher frequency across most domains compared to persons without dementia. We extend the results of previous studies by taking a more comprehensive approach to show the broad spectrum of potentially problematic medications among community-dwelling PWD.
Identification of problematic medication use in PWD has primarily focused on individuals with advanced dementia in the nursing home.16 The few studies among community-dwelling PWD have predominantly used populations outside the United States or attending specialized memory care clinics or focused on overall prescribing of medications.16-18,34 A 2020 review of 6 studies reported a pooled prevalence for PIM use among community-dwelling PWD of 31% with estimates ranging from 13.9% to 54.4%.16 Among individual studies, a 2014 Danish study found that PIM use for community-dwelling PWD was 38.1% based on red-yellow-green list and 20.4% based on the German PRISCUS list.18 The most frequent PIMs included NSAIDs (11.4%), quetiapine (8.1%), and nitrofurantoin (7.2%). A retrospective cohort involving 11,175 PWD aged ≥65 in England found a PIM prevalence of 73% using STOPP-V2, with frequent contributing medications including anticholinergics, duplicate drug class prescriptions, and NSAIDs.33
We extend the results of these studies through a more comprehensive assessment of medication misuse and overuse. PIMs only capture a small percentage of inappropriate prescribing. We identified different situations in which medications may be inappropriate, such as over-aggressive treatment of diabetes/hypertension and those inappropriate in the presence of specific diagnosis codes. Additionally, we defined medication inappropriateness based on comorbidities, duration of use, and clinical/laboratory indicators rather than simply flagging its presence. We show that potentially problematic medication use in PWD not only involves central nervous system (CNS)-active medications (e.g., benzodiazepines/antipsychotics) but also other classes of medications, including PPIs, NSAIDs, antihypertensives, and antidiabetic agents.
The high frequency of medication overuse and misuse among PWD is particularly concerning. First, PWD are at high risk for drug-related problems.7,8,35 PWD are more likely to be hospitalized due to adverse drug events and medication errors than persons without dementia with one study identifying that anticoagulants and opioids contributed to the majority of admissions.6 Additionally, many of the potentially problematic medications highlighted in our study, such as strongly anticholinergic medications and sedative-hypnotics, have been linked with accelerated cognitive decline, hospitalizations, and death.13,14,36 Second, PWD may be less likely to benefit from aggressive management of chronic conditions due to limited life expectancy. Clinical guidelines for chronic diseases such as diabetes frequently emphasize the need to tailor treatment goals as shorter-term harms of medications may outweigh longer-term benefits.11,12,37 Third, high rates of medications frequently prescribed for symptom management, such as PPIs, NSAIDs, and antihistamines/antiemetics, can also be problematic among PWD. PPIs are frequently prescribed for long-term use without a compelling indication, contributing to increased medication burden, drug-drug interactions, vitamin deficiencies, and possibly fractures/infections.38 Many antihistamines and anticholinergic antiemetics frequently prescribed in our cohort (e.g., meclizine and promethazine), are not recommended given adverse effects, lack of significant benefit, and/or presence of safer alternatives.
To place our results in context, we compared problematic medication use among PWD to persons without dementia. PWD received more potentially problematic medications and at a higher frequency across most domains. One explanation is more frequent prescribing of sedative-hypnotics and anticholinergic antidepressants/antipsychotics due to behavioral issues and mood disorders in PWD.39 Second, PWD are more likely to have problematic medication combinations despite similar number of overall medications. This was evidenced by a higher number of opioids used in combination with gabapentin/benzodiazepines or the presence of ≥3 CNS-active medications similar to previous studies.40,41 Third, management of the same comorbidity may differ in these two groups. For example, PWD may have more difficulty managing lower urinary tract symptoms with behavioral interventions leading to higher use of urinary anticholinergics.42
Our study indicates that efforts to reduce polypharmacy in PWD should target a wide range of medications contributing to medication misuse and overuse. The majority of PWD are willing to deprescribe medications if a doctor said it was possible.43,44 Given the broad range of medications flagged as potentially inappropriate, clinicians and pharmacists should take a holistic approach to deprescribing efforts.45 On an individual level, patients and families should be asked about which medications they feel are most problematic or burdensome. This can be followed by a comprehensive medication assessment that assesses the efficacy and benefits/harms of each medication considering an individual’s values and remaining life expectancy. From a health systems perspective, potentially problematic medications identified in our study with high frequency such as PPIs, NSAIDs, opioids, gabapentin, and antihypertensives/diabetes medications may be potential common targets for deprescribing. Many websites and educational brochures are available that can assist in this shared decision-making process.46,47 Deprescribing decisions must be individualized based on factors such as values/preferences, careful assessment of benefits/harms, and feasibility of alternative approaches to managing conditions (e.g., chronic pain). Results from deprescribing interventions among PWD highlight the need to identify subgroups of PWD most likely to benefit from these interventions which is a topic for future research.48
The primary strength of our study is the utilization of a nationally representative sample of community-dwelling PWD linked to detailed medication data. Our comprehensive approach to medication classification highlights a broad spectrum of medication inappropriateness. A few limitations should be acknowledged. First, participants were classified as having dementia using a validated algorithm which may be subject to misclassification. However, we feel that our cohort represents the typical phenotype of community-dwelling PWD in which a thorough evaluation at a specialized memory clinic has not been performed and the diagnosis is often based on basic cognitive testing and functional decline. While the dementia classification algorithm has shown good accuracy in validation studies against gold standard dementia diagnoses, it may be less accurate among racial/ethnic minorities and less-educated individuals.21 Second, we omitted several criteria either due to limited information (e.g., lack of data on sodium values) or difficulty in operationalizing the criteria. We chose a conservative approach, omitting criterion that were challenging to determine whether the medication was potentially inappropriate. We acknowledge that some medications identified as potentially problematic may be reasonable choices based on individual circumstances. Our aim was to identify medications often considered misused/overused and frequently represent candidates to consider for discontinuation. Third, we were not able to collect information on medications commonly obtained over the counter, such as aspirin, other NSAIDs (e.g., ibuprofen), iron, and vitamins. Fourth, our measure of over-aggressive treatment of hypertension/diabetes may be an over-estimate given the 6-month look-back period from date of HbA1c/SBP. However, previous studies have highlighted low deintensification rates in similar settings.49 Finally, we included individuals who were enrolled in Medicare Part D and with ≥1 claim in the previous year. We also did not have information on individuals enrolled in Medicare Advantage which is an important area for future research given limited studies on problematic medication use.50
CONCLUSIONS
Community-dwelling PWD frequently receive medications considered potentially misused and overused across several domains, including overaggressive treatment of hypertension/diabetes, medications that negatively affect cognition, and medications to avoid based on consensus criteria. Compared to individuals without dementia, PWD were more likely to receive these medications. Understanding the extent of problematic medication use in PWD is the first step in guiding interventions to areas most likely to reduce problematic prescribing. Our results suggest that deprescribing efforts in this population should focus not just on harmful CNS-active medications but on the frequent use of medications across different classes, such as PPIs and NSAIDs.
Supplementary Material
Supplementary Appendix (see separate file): Details on the characterization of different criteria included in this study
Supplementary Methods: Additional details on cohort construction, definitions of specific criteria, and statistical analysis plan.
Supplementary Table S1: Classification of potentially problematic medication use for over-aggressive treatment of hypertension (i.e., average systolic blood pressure <110 during enhanced face to face interview and on certain antihypertensives without clear alternative reason)
Supplementary Table S2: Classification of strongly anticholinergic medication use based on the 2019 Beers criteria Table 7
Supplementary Table S3: Classification of sedative-hypnotic medications based on previous studies and the Sedative Load Model
Supplementary Table S4: Criteria that were excluded in a sensitivity analysis to compare persons with and without dementia based on only those criteria that were the same in each groups
Supplementary Table S5: Number of individuals classified as having dementia by year
Supplementary Table S6: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the primary cohort matched on age, sex, comorbidity count, and year of assessment
Supplementary Table S7: Number and survey weighted percentage of persons with diabetes and hypertension who met criteria for over-aggressive treatment of these conditions in the primary cohort matched on age, sex, comorbidity count, and year of assessment
Supplementary Table S8: Baseline characteristics of community-dwelling older adults with and without dementia enrolled in the Health and Retirement Study from 2008-2018 in the cohort matched only on year of assessment
Supplementary Table S9: Baseline characteristics of community-dwelling older adults with and without dementia enrolled in the Health and Retirement Study from 2008-2018 in the fully matched cohort
Supplementary Table S10: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the cohort matched only on year of assessment
Supplementary Table S11: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the fully matched cohort
Supplementary Table S12: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains using propensity score matching and inverse probability of treatment weighting
Supplementary Table S13: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the primary matched cohort including and excluding criteria that are specific to individuals with dementia
Supplementary Table S14: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and in the medication overuse domain in the primary matched cohort and a separate matched cohort in which the non-dementia controls were not preferentially selected based on presence of enhanced face to face interview
Supplementary Table S15: Results from the index of local sensitivity to nonignorability (ISNI) method for the linear regression model for systolic blood pressure and hemoglobin A1c
Supplementary Table S16: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and in the over-aggressive treatment of chronic conditions sub-domain for the primary cohort comparing methods using multiple imputation (MI) (primary analysis) and the index of local sensitivity to nonignorability (ISNI) (sensitivity analysis)
Supplementary Figure S1: Example outline of cohort entry and timing of medication assessment for an individual enrolled in the Health and Retirement study
Supplementary Figure S2: Flow chart of individuals aged 66 years and older with and without dementia in the Health and Retirement Study from 2008-2018 included in this study
Supplementary Figure S3: Distribution of the number of potentially problematic medications identified across all criteria among persons with and without dementia in the primary matched cohort
Supplementary Figure S4: Histograms representing the distribution of systolic blood pressure and hemoglobin A1c values under the different methods, including index of local sensitivity to nonignorability (ISNI), multiple imputation, and non-missing observed data
Key Points:
In this national study, community-dwelling persons with dementia frequently received medications classified as potentially misused and overused based on a broad set of criteria such as over-aggressive treatment of diabetes/hypertension, medications that negatively affect cognition, and medications from consensus criteria (2019 Beers and STOPP Version 2).
Medications frequently identified as potentially problematic spanned multiple classes with the most common including proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), opioids, antihypertensives, and antidiabetic agents (e.g., when used in the setting of controlled blood pressure/hemoglobin A1c or for other problematic reasons).
Compared to persons without dementia, persons with dementia were more likely to receive these potentially problematic medications across several domains.
Why does this matter?
Understanding the frequency and types of potentially problematic medication use among community-dwelling older adults with dementia is the first step in guiding interventions to areas most likely to reduce problematic prescribing. Our results suggest that deprescribing efforts in this population should focus not only on potentially harmful central nervous system-active medications but also other classes such as PPIs and NSAIDs.
ACKNOWLEDGMENTS
This work was supported by the following grants from the National Institute on Aging (NIA): Dr. Deardorff (T32AG000212), Dr. Growdon (T32AG000212, R03AG078804), Dr. Yaffe (R35AG071916), Dr. Boscardin (P01AG066605, P30AG044281), Dr. Boockvar (P01AG066605), Dr. Steinman (P01AG066605, P30AG044281, K24AG049057). Dr. Growdon was also supported by the following grant from the National Institutes of Health/Agency for Healthcare Research and Quality: K12HS026383.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
This work was presented during an oral symposium at the Gerontological Society of America Annual Meeting November 2022.
Sponsor’s Role: None.
REFERENCES:
- 1.2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 2.Bunn F, Burn AM, Goodman C, et al. Comorbidity and dementia: a scoping review of the literature. BMC Medicine. 2014;12(1):192. doi: 10.1186/s12916-014-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amjad H, Snyder SH, Wolff JL, Oh E, Samus QM. Before Hospice: Symptom Burden, Dementia, and Social Participation in the Last Year of Life. J Palliat Med. 2019;22(9):1106–1114. doi: 10.1089/jpm.2018.0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006 [DOI] [PubMed] [Google Scholar]
- 5.Chang TI, Park H, Kim DW, et al. Polypharmacy, hospitalization, and mortality risk: a nationwide cohort study. Sci Rep. 2020;10(1):18964. doi: 10.1038/s41598-020-75888-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullan J, Burns P, Mohanan L, Lago L, Jordan M, Potter J. Hospitalisation for medication misadventures among older adults with and without dementia: A 5-year retrospective study. Australasian Journal on Ageing. 2019;38(4):e135–e141. doi: 10.1111/ajag.12712 [DOI] [PubMed] [Google Scholar]
- 7.Lau CYE, Wojt I, Jeon YH, Hilmer SN, Tan ECK. Prevalence and Risk Factors for Drug-Related Problems in People With Dementia Living in the Community: A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association. Published online March 8, 2022. doi: 10.1016/j.jamda.2022.01.083 [DOI] [PubMed] [Google Scholar]
- 8.Xue Qin QN, Ming LC, Abd Wahab MS, Tan CS, Yuda A, Hermansyah A. Drug-related problems among older people with dementia: A systematic review. Research in Social and Administrative Pharmacy. Published online March 1, 2023. doi: 10.1016/j.sapharm.2023.02.015 [DOI] [PubMed] [Google Scholar]
- 9.Nair NP, Chalmers L, Connolly M, et al. Prediction of Hospitalization due to Adverse Drug Reactions in Elderly Community-Dwelling Patients (The PADR-EC Score). PLOS ONE. 2016;11(10):e0165757. doi: 10.1371/journal.pone.0165757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott RA, Goeman D, Beanland C, Koch S. Ability of older people with dementia or cognitive impairment to manage medicine regimens: a narrative review. Curr Clin Pharmacol. 2015;10(3):213–221. doi: 10.2174/1574884710666150812141525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2019;104(5):1520–1574. doi: 10.1210/jc.2019-00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes—2023. Diabetes Care. 2022;46(Supplement_1):S97–S110. doi: 10.2337/dc23-S006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalisch Ellett LM, Pratt NL, Ramsay EN, Barratt JD, Roughead EE. Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J Am Geriatr Soc. 2014;62(10):1916–1922. doi: 10.1111/jgs.13054 [DOI] [PubMed] [Google Scholar]
- 14.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA Internal Medicine. 2019;179(8):1084–1093. doi: 10.1001/jamainternmed.2019.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46. doi: 10.1177/2042098616670798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado J, Bowman K, Clare L. Potentially inappropriate prescribing in dementia: a state-of-the-art review since 2007. BMJ Open. 2020;10(1):e029172. doi: 10.1136/bmjopen-2019-029172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Growdon ME, Gan S, Yaffe K, Steinman MA. Polypharmacy among older adults with dementia compared with those without dementia in the United States. Journal of the American Geriatrics Society. 2021;69(9):2464–2475. doi: 10.1111/jgs.17291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristensen RU, Nørgaard A, Jensen-Dahm C, Gasse C, Wimberley T, Waldemar G. Polypharmacy and Potentially Inappropriate Medication in People with Dementia: A Nationwide Study. Journal of Alzheimer’s Disease. 2018;63(1):383–394. doi: 10.3233/JAD-170905 [DOI] [PubMed] [Google Scholar]
- 19.Fisher GG, Ryan LH. Overview of the Health and Retirement Study and Introduction to the Special Issue. Work Aging Retire. 2018;4(1):1–9. doi: 10.1093/workar/wax032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary Costs of Dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianattasio KZ, Wu Q, Glymour MM, Power MC. Comparison of Methods for Algorithmic Classification of Dementia Status in the Health and Retirement Study. Epidemiology. 2019;30(2):291–302. doi: 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson TS, Jing B, Wray CM, et al. Comparison of pharmacy database methods for determining prevalent chronic medication use. Med Care. 2019;57(10):836–842. doi: 10.1097/MLR.0000000000001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 24.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Geriatrics Society ∣ Choosing Wisely. Published February 24, 2015. Accessed October 14, 2022. https://www.choosingwisely.org/societies/american-geriatrics-society/
- 26.Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age and Ageing. 2017;46(4):600–607. doi: 10.1093/ageing/afx005 [DOI] [PubMed] [Google Scholar]
- 27.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. Journal of Clinical Epidemiology. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Rihani SB, Deodhar M, Darakjian LI, et al. Quantifying Anticholinergic Burden and Sedative Load in Older Adults with Polypharmacy: A Systematic Review of Risk Scales and Models. Drugs Aging. 2021;38(11):977–994. doi: 10.1007/s40266-021-00895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloane P, Ivey J, Roth M, Roederer M, Williams CS. Accounting for the sedative and analgesic effects of medication changes during patient participation in clinical research studies: measurement development and application to a sample of institutionalized geriatric patients. Contemp Clin Trials. 2008;29(2):140–148. doi: 10.1016/j.cct.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado J, Jones L, Bradley MC, et al. Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing. 2021;50(2):457–464. doi: 10.1093/ageing/afaa147 [DOI] [PubMed] [Google Scholar]
- 34.Cross AJ, George J, Woodward MC, et al. Potentially Inappropriate Medications and Anticholinergic Burden in Older People Attending Memory Clinics in Australia. Drugs Aging. 2016;33(1):37–44. doi: 10.1007/s40266-015-0332-3 [DOI] [PubMed] [Google Scholar]
- 35.Shepherd H, Livingston G, Chan J, Sommerlad A. Hospitalisation rates and predictors in people with dementia: a systematic review and meta-analysis. BMC Medicine. 2019;17(1):130. doi: 10.1186/s12916-019-1369-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnjidic D, Hilmer SN, Hartikainen S, et al. Impact of High Risk Drug Use on Hospitalization and Mortality in Older People with and without Alzheimer’s Disease: A National Population Cohort Study. PLoS One. 2014;9(1):e83224. doi: 10.1371/journal.pone.0083224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: A systematic review of approaches, rates and outcomes. Diabetes Obes Metab. 2019;21(7):1668–1679. doi: 10.1111/dom.13724 [DOI] [PubMed] [Google Scholar]
- 38.Thurber KM, Otto AO, Stricker SL. Proton pump inhibitors: Understanding the associated risks and benefits of long-term use. American Journal of Health-System Pharmacy. Published online January 11, 2023:zxad009. doi: 10.1093/ajhp/zxad009 [DOI] [PubMed] [Google Scholar]
- 39.Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and Psychological Symptoms of Dementia. Front Neurol. 2012;3:73. doi: 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei YJJ, Schmidt S, Chen C, et al. Quality of opioid prescribing in older adults with or without Alzheimer disease and related dementia. Alzheimer’s Research & Therapy. 2021;13(1):78. doi: 10.1186/s13195-021-00818-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maust DT, Strominger J, Kim HM, et al. Prevalence of Central Nervous System–Active Polypharmacy Among Older Adults With Dementia in the US. JAMA. 2021;325(10):952–961. doi: 10.1001/jama.2021.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green AR, Segal J, Tian J, et al. Use of Bladder Antimuscarinics in Older Adults with Impaired Cognition. J Am Geriatr Soc. 2017;65(2):390–394. doi: 10.1111/jgs.14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Growdon ME, Espejo E, Jing B, et al. Attitudes toward deprescribing among older adults with dementia in the United States. Journal of the American Geriatrics Society. 2022;70(6):1764–1773. doi: 10.1111/jgs.17730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeve E, Bayliss EA, Shetterly S, et al. Willingness of older people living with dementia and mild cognitive impairment and their caregivers to have medications deprescribed. Age and Ageing. 2023;52(1):afac335. doi: 10.1093/ageing/afac335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todd A, Jansen J, Colvin J, McLachlan AJ. The deprescribing rainbow: a conceptual framework highlighting the importance of patient context when stopping medication in older people. BMC Geriatrics. 2018;18(1):295. doi: 10.1186/s12877-018-0978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deprescribing Guidelines and Algorithms. Deprescribing.org. Accessed October 14, 2022. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- 47.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of Inappropriate Benzodiazepine Prescriptions Among Older Adults Through Direct Patient Education: The EMPOWER Cluster Randomized Trial. JAMA Internal Medicine. 2014;174(6):890–898. doi: 10.1001/jamainternmed.2014.949 [DOI] [PubMed] [Google Scholar]
- 48.Bayliss EA, Shetterly SM, Drace ML, et al. Deprescribing Education vs Usual Care for Patients With Cognitive Impairment and Primary Care Clinicians: The OPTIMIZE Pragmatic Cluster Randomized Trial. JAMA Internal Medicine. 2022;182(5):534–542. doi: 10.1001/jamainternmed.2022.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussman JB, Kerr EA, Saini SD, et al. Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients With Diabetes Mellitus. JAMA Internal Medicine. 2015;175(12):1942–1949. doi: 10.1001/jamainternmed.2015.5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei L, Bynum JP, Maust DT. Opioid and CNS-Depressant Medication Prescribing among Older Adults Enrolled in Medicare Advantage Versus Fee-for-Service Medicare. The American Journal of Geriatric Psychiatry. 2022;30(2):249–255. doi: 10.1016/j.jagp.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix (see separate file): Details on the characterization of different criteria included in this study
Supplementary Methods: Additional details on cohort construction, definitions of specific criteria, and statistical analysis plan.
Supplementary Table S1: Classification of potentially problematic medication use for over-aggressive treatment of hypertension (i.e., average systolic blood pressure <110 during enhanced face to face interview and on certain antihypertensives without clear alternative reason)
Supplementary Table S2: Classification of strongly anticholinergic medication use based on the 2019 Beers criteria Table 7
Supplementary Table S3: Classification of sedative-hypnotic medications based on previous studies and the Sedative Load Model
Supplementary Table S4: Criteria that were excluded in a sensitivity analysis to compare persons with and without dementia based on only those criteria that were the same in each groups
Supplementary Table S5: Number of individuals classified as having dementia by year
Supplementary Table S6: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the primary cohort matched on age, sex, comorbidity count, and year of assessment
Supplementary Table S7: Number and survey weighted percentage of persons with diabetes and hypertension who met criteria for over-aggressive treatment of these conditions in the primary cohort matched on age, sex, comorbidity count, and year of assessment
Supplementary Table S8: Baseline characteristics of community-dwelling older adults with and without dementia enrolled in the Health and Retirement Study from 2008-2018 in the cohort matched only on year of assessment
Supplementary Table S9: Baseline characteristics of community-dwelling older adults with and without dementia enrolled in the Health and Retirement Study from 2008-2018 in the fully matched cohort
Supplementary Table S10: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the cohort matched only on year of assessment
Supplementary Table S11: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the fully matched cohort
Supplementary Table S12: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains using propensity score matching and inverse probability of treatment weighting
Supplementary Table S13: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and across the different domains in the primary matched cohort including and excluding criteria that are specific to individuals with dementia
Supplementary Table S14: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and in the medication overuse domain in the primary matched cohort and a separate matched cohort in which the non-dementia controls were not preferentially selected based on presence of enhanced face to face interview
Supplementary Table S15: Results from the index of local sensitivity to nonignorability (ISNI) method for the linear regression model for systolic blood pressure and hemoglobin A1c
Supplementary Table S16: Frequency and mean number of potentially problematic medications among community-dwelling older adults with and without dementia overall and in the over-aggressive treatment of chronic conditions sub-domain for the primary cohort comparing methods using multiple imputation (MI) (primary analysis) and the index of local sensitivity to nonignorability (ISNI) (sensitivity analysis)
Supplementary Figure S1: Example outline of cohort entry and timing of medication assessment for an individual enrolled in the Health and Retirement study
Supplementary Figure S2: Flow chart of individuals aged 66 years and older with and without dementia in the Health and Retirement Study from 2008-2018 included in this study
Supplementary Figure S3: Distribution of the number of potentially problematic medications identified across all criteria among persons with and without dementia in the primary matched cohort
Supplementary Figure S4: Histograms representing the distribution of systolic blood pressure and hemoglobin A1c values under the different methods, including index of local sensitivity to nonignorability (ISNI), multiple imputation, and non-missing observed data