Abstract
Aging is one of the major risk factors for many chronic diseases, including diabetes, neuropathy, hypertension, cancer, and neurodegenerative diseases. However, the mechanism behind aging and how aging affects a variety of disease progression remains unknown. Recent research demonstrated the cytochrome P450 (CYP)-epoxide hydrolase (EH) metabolites of polyunsaturated fatty acids (PUFAs) play a critical role in the abovementioned age-associated diseases. Therefore, aging could affect the abovementioned chronic diseases by modulating CYP-EH PUFA metabolism. Unfortunately, investigating how aging affects CYP-EH metabolism in human and mammalian models poses significant challenges.
In this regard, we will use C. elegans as a model organism to investigate the aging effects on CYP-EH metabolism of PUFA, owing to its long history of being used to study aging and its associated benefits of conducting aging research. This project will develop analytical tools to measure the endogenous levels of CYP-EH PUFA metabolites in C. elegans using state-of-the-art ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS). These metabolites are very potent but present in low abundance. The dramatic increase in sensitivity in UPLC-MS/MS allows us to monitor these metabolites over the lifespan of C. elegans with minimum samples. Our results show that C. elegans produces similar CYP PUFA metabolites to mammals and humans using our SPE-UPLC-MS/MS method. We will also show that our method successfully determined the CYP-EH PUFA metabolites profile changes induced by the inhibition of C. elegans EH. The method developed from this project will significantly improve our understanding of the role of dietary PUFAs and associated metabolism on aging and neurodegeneration and will uncover new mechanisms of how aging affects neurodegeneration through the modulation of PUFA metabolic pathways.
Graphical abstract

1. Introduction
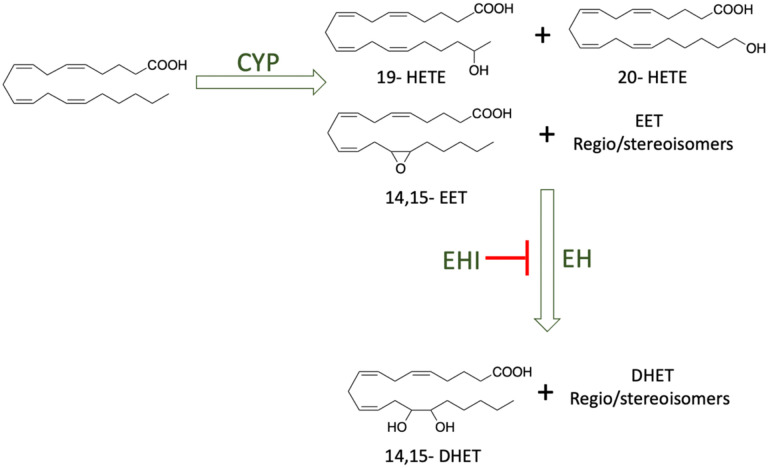
Aging is one of the major risk factors for many diseases, particularly neurodegenerative diseases. For example, it is projected that by 2050, the prevalence of Alzheimer’s Diseases (AD) will double every 5 years after age 65,1 and the number of patients with AD will triple by 2050 without an effective treatment.2 Thus, understanding and modifying the aging process could hugely impact patients with AD and other aging-associated neurodegenerative diseases. Recent research showed that dietary lipids have significant effects on aging and cell senescence,3–5 and their downstream oxidized polyunsaturated fatty acid (PUFA) metabolites (namely oxylipins) are key lipid mediators for many disease states including but not limited to, cancer, lupus, cardiovascular6–8, and neurodegenerative diseases.9–12An increase in the expression of enzymes that produce oxylipins, including, lipoxygenase (LOX)13, Cyclooxygenase (COX)14 soluble epoxide hydrolase (sEH), and microsomal epoxide hydrolase (mEH) is observed in AD compared to healthy individuals.15–17 As a result, endogenous levels of specific oxylipins produced by sEH are upregulated in AD individuals. Moreover, inhibiting the epoxide hydrolase (EH) metabolism of epoxy fatty acids, to their dihydroxy fatty acid metabolites by inhibitors or a genetic knockout is beneficial for neurodegenerative diseases, including Parkinson’s disease (PD) and AD.18,19 (Figure 1) Our recent study also indicated that dihydroxyeicosadienoic acid (DHED), an epoxide hydrolase metabolite of dihomo-gamma-linolenic acid, induces degeneration of dopaminergic neurons through ferroptosis, which could affect aging.11 However, the exact role of oxylipins in neurodegeneration and whether they play any critical role in aging remains elusive.20 Therefore, investigating whether aging has any effects on the oxylipins level and vice versa will help to develop more effective preventative measures and treatments for age-related diseases, including neurodegenerative diseases. However, investigating the molecular interactions between oxylipins and aging poses several challenges. First, the mechanisms of aging are understudied and complex. Therefore, conducting studies on an intact animal will likely be beneficial. In addition, aging is a long process, and the experimental settings are difficult to control throughout the aging studies.21–23 Moreover, oxylipins are largely present at low levels, and there are hundreds of different oxylipins produced in animals. To further complicate the picture, the structures of these oxylipins are very similar, but their functions are very diverse and sometimes in opposition.24 Besides, the endogenous levels for some oxylipins can change up to 3 orders of magnitude depending on the disease status.25 Therefore, it is necessary to monitor the in vivo level of every oxylipin to understand how oxylipin biosynthesis affects the aging process comprehensively. Yet, this creates very difficult analytical challenges.
Figure 1.
Metabolic pathway of polyunsaturated fatty acids. Cytochrome 450 (CYP), epoxide hydrolase (EH), and epoxide hydrolase inhibitor (EHI).
To solve these challenges, this study used Caenorhabditis elegans (C. elegans) as an animal model to investigate the molecular interaction between aging and oxylipin biosynthesis. C. elegans has been used for aging research for decades, owing to its short lifespan, ease of handling and ability to conduct imaging studies, adaptability to high-throughput assays, possessing similar neuronal structures and functions, and the presence of a conserved genome between C. elegans and humans.26 In addition, we developed an analytical method using ultra-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry to monitor and quantify the oxylipin profile in C. elegans. The developed method is a combination of different analytical techniques, including solid phase extraction (SPE), HPLC, electrospray ionization (ESI), and tandem mass spectrometry to tackle all the challenges associated with qualification and quantification of oxylipins in C. elegans. Our results indicate that we successfully developed a fully validated analytical method to quantify the oxylipins in C. elegans. Furthermore, we determined that C. elegans has a conserved cytochrome P450-epoxide hydrolase metabolic pathway for PUFAs. Our method will eventually facilitate an understanding of the biological functions of oxylipins in aging.
2. Results and discussion
2.1. Mass spectrometry optimization
Optimization of the LC and MS parameters was done to improve oxylipin detection because these molecules are present in very low concentrations. Considering this, the mass parameters, including collision and cone voltage, were optimized to increase the signal-to-noise ratio, while simultaneously decreasing the limit of detection (LOD). In a quadrupole-based mass spectrometer, dwell time, which is the amount of time a mass spectrometer spends measuring the intensity of a particular mass-to-charge ratio during a single scan, controls the frequency of data acquisition. Thus, the dwell time could be considered as a trade-off between sensitivity and noise. While the longer dwell time provides a higher number of measurements across a peak, experiencing higher noise is inevitable.27 Thus, to get the maximum number of measurements with minimum noise, the acquisition was divided into ten functions based on the retention time of the compounds. We employed the “auto” feature available in the MassLynx V4.2 software, which automatically determined the dwell time by considering the number of transitions in each function. By utilizing this approach, we ensured sufficient time for acquiring reliable signal-to-noise ratios and an appropriate number of scans per peak. Furthermore, a type I internal standard (IS) was used to normalize the loss of oxylipins due to sample preparation. Type I internal standards are essentially deuterated versions of selected oxylipins that can be easily differentiated by mass spectrometry. The rationale for selecting such internal standards lies in their similarity to the physical properties of the target compounds. This similarity ensures that the loss experienced during extraction is consistent, allowing for the generalization of the loss to other oxylipins, therefore making an accurate normalization. Another advantage of using deuterated oxylipins as internal standards is that their endogenous concentrations in biological samples are negligible. As a result, the signal observed in mass spectrometry analysis is solely derived from the spiked internal standard with known concentration. A list of ISs, their concentration, and more detail is provided in (SI Table 1).
To achieve optimal sensitivity and selectivity in this method, a thorough analysis was conducted to identify specific fragments that occur in the ionized molecules of the sample. The selection of most transitions was based on identifying cleavages near double bonds or functional groups like epoxy or hydroxyl modification. In certain cases, prioritizing selectivity over sensitivity was deemed crucial, leading to the selection of a more distinctive fragmentation ion, even if it wasn’t the most intense. This approach ultimately contributes to a more precise analysis. For instance, in the case of the CYP450 metabolite of EPA, 8,9-EEQ, a fragment with a m/z of 127 was chosen instead of the more sensitive fragment at 255, with the primary aim of enhancing the selectivity of analysis of these regioisomers at the presence of 11,12- EEQ. Selecting m/z of 127 is crucial since these two isomers have very close retention times and the exact same molecular weight (SI Figure 2). The summary of optimized transitions and mass spectrometric parameters is listed in Table 1. By meticulously selecting suitable multiple reaction monitoring (MRM) transitions, optimizing cone and collision energy, and employing the appropriate LC mobile phases and gradient (SI Table 3),28 we achieved successful separation and detection of distinct metabolites of PUFAs and regioisomers (SI Figure 2), With all these considerations, the total number of oxylipins in this method was higher compared to other studies.28,29
Table 1.
Summary of multiple reaction monitoring (MRM)(m/z), collision energy (CE), cone voltage (CV), retention time (RT)(min), the lower limit of quantification (LLOQ) (nM), the limit of detection (LOD)(nM), the upper limit of quantification (ULOQ)(nM) of oxylipin metabolite.
| Analytes | MRM | Cone Volt. | Collision Volt. | RT (min) | LLOQ (nM) | LOD (nM) | ULOQ (nM) |
|---|---|---|---|---|---|---|---|
| 15,16-DiHODE | 311.20 > 223.00 | 44 | 22 | 6.31 | 0.063 | 0.021 | 50 |
| 12,13-DiHODE | 311.20 > 183.00 | 36 | 22 | 6.33 | 1.250 | 0.416 | 1000 |
| 9,10-DiH0DE | 311.20 > 201.00 | 52 | 16 | 6.35 | 0.063 | 0.021 | 25 |
| 17,18-DiHETE | 335.20 > 247.00 | 39 | 16 | 6.63 | 0.139 | 0.046 | 1000 |
| 14,15-DiHETE | 337.20 > 207.00 | 33 | 16 | 6.97 | 0.063 | 0.021 | 25 |
| 11,12-DiHETE | 335.10 > 167.00 | 36 | 16 | 7.15 | 0.063 | 0.021 | 25 |
| 12,13-DiHOME | 313.20 > 183.00 | 20 | 22 | 7.30 | 0.063 | 0.021 | 50 |
| 8,9-DiHETE | 335.20 > 127.00 | 36 | 20 | 7.50 | 0.250 | 0.083 | 100 |
| 9,10-DiHOME | 313.20 > 201.00 | 20 | 22 | 7.70 | 0.063 | 0.021 | 50 |
| 14,15-DiHETrE | 337.20 > 207.00 | 33 | 16 | 8.00 | 0.063 | 0.021 | 50 |
| 11,12-DiHETrE | 337.20 > 167.00 | 51 | 22 | 8.60 | 0.250 | 0.083 | 200 |
| 20-HEPE | 317.20 > 243.00 | 28 | 16 | 8.65 | 0.625 | 0.208 | 500 |
| 14,15 DHED | 339.10 > 209.00 | 28 | 22 | 8.84 | 0.125 | 0.042 | 100 |
| 9-HOTrE | 293.20 > 171.00 | 40 | 16 | 8.84 | 0.312 | 0.104 | 125 |
| 10,11-DiHDPE | 361.20 > 153.00 | 46 | 16 | 8.91 | 0.125 | 0.042 | 100 |
| 13-HOTrE | 293.20 > 195.00 | 36 | 16 | 9.02 | 0.625 | 0.208 | 250 |
| 18-HEPE | 317.10 > 215.00 | 44 | 16 | 9.03 | 0.625 | 0.208 | 500 |
| 8,9-DiHETrE | 337.20 > 127.00 | 15 | 22 | 9.18 | 0.250 | 0.083 | 200 |
| 11,12-DHED | 339.10 > 169.00 | 28 | 22 | 9.25 | 0.083 | 0.028 | 100 |
| 19-HETE | 319.10 > 275.00 | 44 | 16 | 9.44 | 0.625 | 0.208 | 250 |
| 5,6-DiHETE | 335.20 > 145.00 | 21 | 16 | 8.20 | 10.000 | 5.000 | 2000 |
| 15-HEPE | 317.00 > 219.00 | 36 | 10 | 9.52 | 0.312 | 0.035 | 250 |
| 20-HETE | 319.20 > 275.00 | 44 | 16 | 9.58 | 0.312 | 0.104 | 250 |
| 8,9-DHED | 339.10 > 187.00 | 28 | 22 | 9.63 | 0.125 | 0.042 | 100 |
| 7,8-DiHDPE | 361.20 > 113.00 | 44 | 22 | 9.64 | 0.625 | 0.208 | 250 |
| 8-HEPE | 317.10 > 155.00 | 36 | 16 | 9.91 | 0.208 | 0.069 | 100 |
| 12-HEPE | 317.10 > 179.00 | 44 | 10 | 9.91 | 0.312 | 0.104 | 50 |
| 5,6-DiHETrE | 337.20 > 145.00 | 52 | 16 | 10.06 | 0.250 | 0.083 | 100 |
| 13-HODE | 295.20 > 195.00 | 20 | 16 | 10.49 | 1.000 | 0.334 | 400 |
| 5-HEPE | 317.10 > 115.00 | 36 | 16 | 10.58 | 0.625 | 0.069 | 500 |
| 15,16 -EpODE | 293.10 > 235.00 | 34 | 16 | 10.91 | 0.500 | 0.083 | 200 |
| 15-HETE | 319.20 > 219.00 | 45 | 10 | 10.97 | 0.312 | 0.104 | 250 |
| 17,18-EpETE | 317.20 > 215.00 | 20 | 10 | 11.03 | 0.416 | 0.139 | 200 |
| 9,10-EpODE | 293.10 > 171.00 | 28 | 16 | 11.11 | 0.104 | 0.035 | 50 |
| 12,13-EpODE | 293.10 > 183.00 | 34 | 16 | 11.37 | 2.500 | 0.833 | 2000 |
| 11-HETE | 319.20 > 167.00 | 28 | 16 | 11.48 | 0.125 | 0.042 | 100 |
| 14,15-EpETE | 317.20 > 207.00 | 27 | 10 | 11.60 | 0.167 | 0.055 | 200 |
| 11,12-EpETE | 317.20 > 167.00 | 15 | 10 | 11.77 | 0.250 | 0.083 | 100 |
| 12-HETE | 319.10 > 179.00 | 44 | 16 | 11.82 | 0.500 | 0.167 | 400 |
| 8,9-EpETE | 317.20 > 127.00 | 20 | 16 | 11.99 | 1.250 | 0.416 | 200 |
| 8-HETE | 319.10 > 155.00 | 44 | 16 | 11.95 | 0.500 | 0.167 | 400 |
| 9-HETE | 319.00 > 151.00 | 28 | 16 | 12.26 | 1.250 | 0.139 | 500 |
| 5,6-EpETE | 317.20 > 189.00 | 20 | 10 | 12.35 | 0.500 | 0.167 | 400 |
| 5-HETE | 319.20 > 115.00 | 28 | 16 | 12.72 | 0.167 | 0.055 | 200 |
| 19,20-EpDPE | 343.20 > 299.00 | 27 | 10 | 12.80 | 0.625 | 0.208 | 250 |
| 12,13-EpOME | 295.20 > 195.00 | 20 | 16 | 13.02 | 0.312 | 0.104 | 250 |
| 14,15-EpETrE | 319.20 > 219.00 | 33 | 10 | 13.15 | 0.625 | 0.208 | 100 |
| 9,10-EpOME | 295.20 > 171.00 | 20 | 16 | 13.31 | 0.312 | 0.104 | 50 |
| 16,17 EpDPE | 343.20 > 274.00 | 20 | 10 | 13.40 | 1.250 | 0.416 | 500 |
| 13,14-EpDPE | 343.10 > 193.00 | 30 | 10 | 13.56 | 0.208 | 0.069 | 100 |
| 10,11-EpDPE | 343.10 > 153.00 | 36 | 10 | 13.73 | 0.250 | 0.028 | 40 |
| 7,8-EpDPE | 343.10 > 109.00 | 44 | 16 | 14.09 | 1.250 | 0.416 | 1000 |
| 8,9-EpETrE | 319.20 > 155.00 | 27 | 10 | 14.25 | 1.250 | 0.416 | 500 |
| 14,15 -EpEDE | 321.10 > 221.00 | 36 | 16 | 14.58 | 0.104 | 0.035 | 50 |
| 5,6-EpETrE | 319.20 > 191.00 | 20 | 10 | 14.61 | 2.500 | 0.832 | 1000 |
| 11,12-EpETrE | 319.20 > 167.00 | 27 | 10 | 14.93 | 0.208 | 0.069 | 250 |
| 8,9-EpEDE | 321.20 > 157.00 | 28 | 16 | 15.05 | 0.416 | 0.139 | 500 |
| 11,12-EpEDE | 321.20 > 181.00 | 36 | 16 | 15.14 | 0.416 | 0.139 | 500 |
2.2. Method validation
2.2.1. LOQ and linearity
Limit of detection (LOD) and limit of quantification (LOQ) are essential parameters that indicate the lowest analyte concentrations that can be reliably measured by an analytical method. In this study, LOD is defined as the amount of a sample required to produce a signal-to-noise ratio (S/N) of 3 or greater, while the LOQ demands an S/N of 10 or higher to be acceptable. To determine these critical thresholds, four consecutive runs of calibration standards were analyzed within the same day. This approach allowed for establishment of LOQs ranging from 0.063 nM to 10 nM and LODs spanning from 0.021 nM to 5 nM, where the analytes were evaluated at a final volume of 100 μL ethanol (75% v/v with water containing a 10 nM concentration of CUDA which is the type II internal standard) (Table 1). The least favorable LOD and LOQ were observed for 12,13-DiHODE (0.416, 1.25 nM), 5,6-DiHETE (5, 10 nM), and 12,13-EpODE (0.833, 2.5 nM), whereas the best sensitivity or lowest LOD and LOQ belong to these three compounds 15,16-DiHODE, 14,15-DiHETE, and 9,10-DiHOME which both have LOD at 0.021 and LOQ at 0.063 nM) (Table 1). The linearity of the method was three orders of magnitude for most compounds when it is evaluated within the concentration range between the lower limit of quantification (LLOQ) and the upper limit of quantification (ULOQ), as determined by the calibration curves constructed for each analyte (SI Figures 1, and 3).
2.2.2. Assessing the accuracy and precision of the method
To evaluate the accuracy and precision of the method, we prepared five replicates of quality control samples including the lower limit of quantification (LLOQ), the lower quality control (LQC), middle-quality control (MQC), and higher quality control (HQC). For intra-day accuracy assessment, the percent difference between the mean concentration per analytical run and the expected concentration was determined. Five replicates were injected three times within the same day. This approach allowed for a robust evaluation of the method’s consistency and reliability during a single day of operation. Inter-day accuracy was also evaluated by injecting five replicates once daily for three consecutive days. This assessment provided insights into the method’s performance over multiple days, highlighting its stability and dependability across an extended period. Intra-day precision was evaluated as the relative standard deviation of three rounds of injection on the same day, while inter-day precision represented the relative standard deviation of the measurements (n=5, for each QC concentration) injected on three different days. Both precision values were calculated, and most of them were found to be within the acceptable criteria (≤ 25%) (Table 2). Less than 20 compounds showed accuracy and precision out of the accepted range for at least one of the QCs, except for some of the LLOQ samples. The compounds that exhibited the least favorable accuracy and precision in the analysis were 5,6-DiHETE and 5,6-DiHETrE. This outcome was anticipated due to the compounds’ susceptibility to intramolecular lactonization (SI Figure 4).30 Furthermore, compounds such as 9-HODE, and 13-HODE displayed precision and accuracy values that fell outside the acceptable range. It is worth mentioning that the accuracy and precision of certain prostaglandins and lipoxins were also found to be lower than the acceptable criteria, which explains why these compounds were excluded from our analysis (Table 2). However, this observation does not raise concern, as these compounds are not naturally present in C. elegans. This is further supported by the fact that C. elegans lacks homologs for LOX and COX enzymes, which are responsible for the endogenous production of these compounds.31 As a result, we expected that these compounds might not be produced in significant amounts. Furthermore, our oxylipin analysis with C. elegans also demonstrated that the metabolites from LOX and COX metabolic pathways were not detected in our method (SI Table 6). Therefore, the data related to these metabolites were excluded from this study; a list of these compounds is provided in supporting information (SI Table 5). The two figures of merit were acquired for all oxylipins listed in (Table 2), with the exception of metabolites from COX and LOX enzymes. Moving forward, we will primarily concentrate on CYP450 and EH enzyme metabolites. Despite these findings, in general, intra-day precision was found to be better than inter-day precision. Several factors could contribute to this observation, including instrument stability, reagent and sample stability, calibration curve stability, and instrument drift.
Table 2.
Accuracy and precision of oxylipins, Inter- and Intera-day precision represents the relative standard deviation (RSD) of the measurements (n=5). The intra-day accuracy was determined as the percent difference between the mean concentration per analytical run and the expected concentration. N.D. refers to the concentration below LOQ. Accuracy and precision of eicosanoids metabolites, Interday. precision represents the relative standard deviation of the measurements (n=5). The intra-day accuracy was determined as the percent difference between the mean concentration per analytical run and the expected concentration. The LLOQ, the LQC, MQC, and HQC concentration.
| Analyst | LLQC | LQC | MQC | HQC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| intera-day | Interday | intera-day | Interday | intera-day | Interday | intera-day | Interday | |||||||||
| Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | |
| 9,10-DiH0DE | 27.67 | 15.59 | 17.5 | 17.86 | −15.16 | 8.95 | 3.76 | 14.84 | 32.33 | 11.41 | 23.5 | 20.74 | 30.5 | 7.65 | 17.68 | 18.5 |
| 5,15-DiHETE | −20.42 | 14.98 | −20.83 | 15.12 | −10.11 | 7.82 | 0.89 | 9.92 | 0.54 | 8.77 | −2.17 | 5.98 | 3.3 | 6.54 | 1.577 | 5.63 |
| 17,18-DiHETE | 34 | 10.78 | 36.33 | 11.51 | 26.08 | 7.25 | 26.84 | 6.56 | 34.9 | 13.32 | 19.07 | 13.71 | 24.2 | 10.2 | 18.16 | 5.61 |
| 14,15-DiHETE | 20 | 21.13 | 24.33 | 18.73 | 26.91 | 7.81 | 23.25 | 7.21 | 22.67 | 14.52 | 17.33 | 18.41 | 29.4 | 5.61 | 26.24 | 7.74 |
| 11,12-DiHETE | 6.67 | 16.49 | 16.67 | 20.91 | 26.34 | 7.69 | 25.13 | 7.09 | 20 | 14.86 | 19.33 | 15.67 | 27.2 | 5.21 | 24.96 | 7.67 |
| 12,13-DiHOME | 55 | 83.51 | 65.71 | 68.48 | 28.11 | 8.32 | 38.13 | 13.38 | 3.7 | 15.12 | 11.07 | 17.32 | 9.6 | 6.39 | 14.08 | 6.14 |
| 8,9-DiHETE | 25.83 | 15.24 | 27.5 | 8.45 | 22.52 | 8.74 | 22.21 | 7.39 | 26.83 | 12.72 | 22.08 | 14.29 | 28 | 6.56 | 29.34 | 6.28 |
| 9,10-DiH0ME | 20 | 29.67 | 85.33 | 99.69 | 17.71 | 15.29 | 19.89 | 20.64 | −4.33 | 19.84 | 13 | 16.79 | 5.9 | 7.56 | 12.99 | 3.75 |
| 14,15-DiHETrE | 22.67 | 17.49 | 31 | 18.6 | 26.9 | 6.2 | 26.58 | 10.21 | 27.67 | 16.47 | 18.67 | 19.92 | 28.6 | 7.34 | 27.57 | 7.06 |
| 19,20-DiHDPE | 22.5 | 14.01 | 29.17 | 10.77 | 22.8 | 6.58 | 21.73 | 7.64 | 25.83 | 12.4 | 25.17 | 15.37 | 27.4 | 8.77 | 24.41 | 9.46 |
| 5,6-DiHETE | 300.25 | 49.22 | 267.9 | 53.83 | 108.26 | 31.29 | 75.05 | 38.28 | 463.25 | 86.48 | 442.8 | 87.6 | 246.3 | 13.37 | 223.79 | 16.21 |
| 16,17-DiHDPE | 3.33 | 8.51 | −1.67 | 11.64 | −9.54 | 7.44 | −11.27 | 6.79 | 3.67 | 6.81 | 1.83 | 7.45 | 10.7 | 4.18 | 9.35 | 4.34 |
| 13,14-DiHDPE | 2.5 | 13.2 | −5.83 | 6.85 | −18.05 | 10.17 | −15.34 | 10.65 | −1.5 | 5.35 | −3.42 | 4.9 | 4.1 | 6.04 | 1.62 | 6.53 |
| 11,12-DiHETrE | 15.83 | 17.05 | 20.83 | 17.34 | −16.46 | 9.41 | −13.69 | 9.07 | 1.67 | 5.85 | 1.92 | 6.64 | 7.5 | 6.4 | 3.97 | 6.87 |
| 20-HEPE | −19 | 16.03 | −11 | 8 | −29.5 | 8.39 | −28.25 | 9.68 | −22.57 | 13.58 | −10.5 | 7.23 | −12.5 | 11.28 | −1.21 | 4.92 |
| 9-HOTrE | −14.67 | 21.18 | −3.33 | 29.43 | −24.16 | 12.58 | −18.51 | 7.14 | −23.27 | 10.08 | −17.07 | 8.62 | −19.7 | 14.52 | −4.18 | 7.75 |
| 14,15 DHED | 5 | 13.35 | 6.67 | 13.91 | −22.09 | 5.39 | −18.47 | 6.87 | 0.5 | 7.88 | −1.17 | 6.19 | 19.3 | 6.79 | 13.21 | 9.30 |
| 18-HEPE | −15 | 20.74 | −0.67 | 5.34 | −22.95 | 6.02 | −20.47 | 6.96 | −21.2 | 16.97 | −4.7 | 4.98 | −4.7 | 9.92 | 6.21 | 3.40 |
| 8,9-DiHETrE | −15 | 26.77 | 6.67 | 13.19 | −20.26 | 6.93 | −16.9 | 7.62 | −20.58 | 12.38 | −7.17 | 6.43 | −8.9 | 10.21 | 1.41 | 5.75 |
| 11,12 DHED | −8.42 | 24.84 | −7.08 | 15.87 | −12.21 | 5.68 | −8.11 | 4.81 | −15.21 | 15.21 | −13.13 | 11.18 | −13.2 | 9.57 | −5.05 | 14.76 |
| 19-HETE | −5.4 | 13.74 | 11.43 | 13.51 | −9 | 4.38 | −12.75 | 5.24 | −8.45 | 8.38 | −0.47 | 9.81 | 1.1 | 4.79 | 10.55 | 8.86 |
| 15-HEPE | 2.93 | 14.39 | 9.53 | 13.99 | −5.75 | 6.97 | −4.67 | 5.81 | −11.53 | 11.63 | −5.24 | 10.78 | −6 | 5.96 | 5.03 | 11.59 |
| 20-HETE | 0.53 | 18.78 | 5.07 | 14.75 | −7.25 | 7.13 | −6 | 7.51 | −9.57 | 9.02 | 1.14 | 11.92 | −0.1 | 4.76 | 7.04 | 7.86 |
| 8,9 DHED | −5.5 | 8.95 | 0.83 | 9.17 | −8.75 | 5.78 | −3.75 | 6.62 | 2.75 | 9.35 | 7.83 | 8.38 | 4.3 | 4.39 | 10.47 | 6.04 |
| 7,8-DiHDPE | 26.25 | 8.62 | 18.13 | 7.74 | 3.5 | 5.88 | 3.08 | 4.68 | 6.36 | 30.35 | 14.92 | 9.35 | 18.4 | 6.95 | 17.47 | 7.62 |
| 12-HEPE | −2.4 | 16.17 | −5.27 | 9.65 | −15.75 | 5.78 | −11.33 | 5.99 | −16.19 | 12.35 | −16.01 | 7.01 | −9.5 | 6.08 | −4.07 | 8.78 |
| 8-HEPE | −3.77 | 11.82 | −7.73 | 6.72 | −17 | 5.55 | −17.5 | 4.69 | −11.78 | 6.01 | −15.01 | 4.81 | −3.5 | 3.41 | −2.39 | 4.57 |
| 5,6-DiHETrE | 65.75 | 11.5 | 41.5 | 14.62 | 4.06 | 4.02 | −3.54 | 5.66 | 84.13 | 39.89 | 29.26 | 20.82 | 56.3 | 16.09 | 29.28 | 8.47 |
| 22-HDHA | 0.6 | 11.01 | 7.27 | 13.96 | −16 | 5.69 | −17.58 | 5.5 | −8.73 | 7.83 | −8.78 | 5.65 | −0.2 | 5.13 | 2.36 | 7.35 |
| 13-HODE | 302.67 | 21.8 | 289.5 | 24.68 | 26.41 | 6.76 | 39.58 | 17.24 | 24.5 | 7.54 | 21.27 | 7.56 | 20 | 4.73 | 17.11 | 6.96 |
| 5-HEPE | −1.3 | 16.21 | −1.43 | 8.2 | −16.63 | 5.74 | −17.92 | 4.74 | −10.3 | 8.84 | −8.91 | 8.16 | −5.4 | 4.7 | 1.17 | 8.36 |
| 20-HDHA | 22.77 | 19.39 | 10.97 | 7.77 | −11.13 | 4.8 | −9.58 | 5.29 | 17.5 | 14.28 | 3.79 | 8.87 | 26.2 | 8.9 | 13.68 | 9.17 |
| 9-HODE | 335.17 | 11.78 | 52.8 | 85.59 | 82.5 | 4.36 | 80.83 | 10.46 | 25.75 | 10.18 | 32.81 | 10.25 | 21.2 | 4.25 | 18.62 | 5.64 |
| 15,16-EpODE | N.D. | N.D. | 13.63 | 26.63 | −23.44 | 17.77 | −24.79 | 15.43 | −3.79 | 12.87 | −1.71 | 9.73 | 17.1 | 4.79 | 12.41 | 6.31 |
| 15-HETE | 5.93 | 11.59 | −2 | 9.29 | −8.25 | 8.72 | −5.83 | 5.79 | 15.71 | 6.6 | 9.03 | 7.82 | 24.3 | 4.95 | 16.52 | 3.08 |
| 17,18-EpETE | −8.4 | 19.25 | −7.7 | 15.03 | −26.75 | 18.8 | −23.88 | 14 | −1.45 | 12.52 | −0.63 | 10.96 | 19.6 | 3.93 | 9.34 | 8.47 |
| 13-oxo-ODE | 89.87 | 24.35 | 81 | 28.83 | 20 | 7.61 | 20 | 6.54 | 8.51 | 11.7 | 11.05 | 9.15 | 9.6 | 4.41 | 0.87 | 6.95 |
| 9,10-EpODE | N.D. | N.D.! | −2.5 | 11.26 | −24.25 | 19.31 | −23.67 | 14.24 | 9.35 | 10.67 | 4.03 | 11.36 | 27.4 | 3.4 | 10.81 | 10.46 |
| 17-HDoHE | 14.92 | 17.5 | −2.95 | 6.46 | −11.69 | 6.07 | −8.5 | 5.57 | 4.64 | 7.53 | 4.28 | 8.05 | 9.2 | 6.87 | 8.92 | 5.12 |
| 12,13-EpODE | ND | ND | 2.64 | 9.8 | −28.25 | 13.89 | −25.75 | 11.48 | 1.59 | 9.63 | −0.15 | 7.98 | 21.1 | 4.33 | 12.27 | 7.69 |
| 15-oxo-ETE | −1.92 | 14.31 | −4.92 | 6.35 | −12.81 | 7.45 | −15.21 | 6.98 | −1.57 | 8.77 | 0.85 | 8.41 | 6.4 | 4.09 | 5.19 | 3.63 |
| 11-HETE | −13.33 | 12.27 | −10.17 | 8.38 | −24.84 | 13.2 | −24.58 | 14.93 | 0.62 | 4.46 | −1.93 | 5.61 | 9.7 | 4.01 | 3.34 | 3.89 |
| 14,15-EpETE | −6.29 | 19.61 | −0.75 | 8.8 | −14.38 | 8.46 | −11.67 | 6.77 | 6.67 | 10.19 | 1.94 | 10.2 | 23.3 | 4.16 | 10.20 | 10.22 |
| 9-oxo-ODE | 74.83 | 19.76 | 84.92 | 15.97 | −2.34 | 6.84 | −1.04 | 6.23 | 5.57 | 9.96 | 7.24 | 8.9 | 11.8 | 4.4 | 7.50 | 5.52 |
| 11,12-EpETE | −16.92 | 17.81 | −4.83 | 9.07 | −25.94 | 14.21 | −20.21 | 16.42 | −20.13 | 13.18 | −3.28 | 9.54 | −2 | 6.27 | 12.46 | 6.48 |
| 12-HETE | −12 | 11.52 | −9.46 | 15.94 | −9.53 | 5.55 | −1.88 | 6.58 | 5.62 | 6.08 | 9.76 | 6.86 | 16.7 | 3.51 | 17.56 | 2.42 |
| 8-HETE | −13.25 | 17.58 | −10.88 | 14.72 | −7.34 | 8.07 | 3.85 | 9.67 | 5.52 | 7.79 | 8.88 | 5.11 | 20.4 | 5.65 | 19.39 | 5.42 |
| 8,9-EpETE | −18.73 | 28.26 | 1.95 | 11.84 | −18.25 | 13.1 | −7.29 | 15.85 | −16.45 | 14.46 | 1.9 | 9.3 | −1.9 | 6.54 | 7.60 | 4.22 |
| 15(S)-HETrE | 2.33 | 12.16 | 2.5 | 11.06 | −21.25 | 7.67 | −14.58 | 9.13 | 7.07 | 7.33 | 3.53 | 9.63 | 27.4 | 5.57 | 20.06 | 9.65 |
| 12-oxo-ETE | −36.38 | 16.66 | −24.37 | 11.81 | −28 | 8.43 | −28.04 | 7.51 | −27.59 | 11.89 | −28.76 | 14.99 | −44.1 | 17.3 | −28.37 | 14.46 |
| 9-HETE | −21.3 | 13.22 | −16.58 | 11.66 | −13.56 | 7.64 | −1.92 | 10.74 | −2.8 | 9.08 | 8.26 | 7.08 | 14 | 4.99 | 20.92 | 3.87 |
| 5,6-EpETE | 16.75 | 26.48 | 34.13 | 49.61 | 24.84 | 25.12 | 134.06 | 48.69 | −12.98 | 64.8 | 6.33 | 90.58 | −62.1 | 13.49 | −74.47 | 23.12 |
| 5-HETE | 0.25 | 11.98 | 0.42 | 11.22 | −18.44 | 5.33 | −7.08 | 8.68 | 1.08 | 7.67 | 3.6 | 5.89 | 17.2 | 4.54 | 14.35 | 5.33 |
| 19,20-EpDPE | −0.1 | 21.81 | −0.83 | 12.15 | −31.25 | 12.42 | −25.25 | 11.36 | −17.43 | 14.79 | −4.34 | 10.05 | 5.9 | 7.76 | 13.04 | 6.47 |
| 12,13-EpOME | 111.13 | 23.92 | 242.8 | 48.38 | 14.75 | 25.16 | 15.5 | 17.11 | −5.86 | 15.22 | 10.13 | 10.53 | 3.4 | 6.28 | 11.80 | 4.97 |
| 14,15-EpETrE | −1.73 | 10.84 | −2.77 | 9.81 | −25.25 | 10.09 | −26.67 | 9.93 | −10.25 | 6.62 | −13.78 | 7.85 | 4.7 | 3.84 | 4.31 | 4.90 |
| 9,10-EpOME | 171.07 | 18.1 | 426.8 | 119.45 | 15.5 | 8.85 | 18.33 | 16.89 | 8.65 | 5.13 | 11.11 | 5.86 | 5.6 | 3.66 | 9.47 | 5.55 |
| 16,17-EpDPE | −5.28 | 7.25 | −2.7 | 8.65 | −18.94 | 6.48 | −15.63 | 6.81 | −7.21 | 5.66 | −2.85 | 5.98 | 0.9 | 2.25 | 7.30 | 5.84 |
| 13,14-EpDPE | −8.53 | 10.5 | −9.17 | 6.57 | −21.25 | 8.76 | −24.25 | 9.27 | −13.36 | 6.39 | −14.69 | 6.09 | −0.4 | 3.01 | 1.65 | 4.59 |
| 10,11 -EpDPE | 0.17 | 10.94 | −8.08 | 5.36 | −22.19 | 6.68 | −23.75 | 7.15 | −12.73 | 8.26 | −15.23 | 8.35 | −4.3 | 1.79 | −1.33 | 4.47 |
| 5-oxo-ETE | −8.78 | 10.03 | −7.07 | 8.45 | −3.81 | 12.06 | −3.37 | 10.8 | −13.81 | 13.07 | −9.02 | 13.55 | −12.4 | 2.82 | −3.64 | 7.98 |
| 11,12-EpETrE | 4.6 | 7.58 | 6.37 | 5 | −20.13 | 6.19 | −19.25 | 6.29 | −1.38 | 5.82 | 0.02 | 5.56 | 8.3 | 2.76 | 11.35 | 3.74 |
| 7,8-EpDPE | N.D. | N.D. | −3 | 4.25 | −19.5 | 5.82 | −18.92 | 5.58 | −6.09 | 6.3 | −5.77 | 6.79 | 1.7 | 2.44 | 4.95 | 4.62 |
| 8,9-EpETrE | −11.72 | 12.27 | −3.77 | 8.06 | −12.81 | 6.18 | −15.17 | 8.79 | −1.46 | 5.64 | −2.26 | 5.45 | 6.7 | 3.86 | 8.36 | 5.66 |
| 14,15EED | 4.67 | 10.73 | −4.33 | 7.05 | −25.25 | 13.04 | −27.67 | 8.6 | −3.43 | 7.08 | −9.73 | 6.61 | 10.3 | 3.32 | 8.35 | 3.11 |
| 8,9EED | −1.87 | 10.76 | −2.78 | 7.53 | −23.63 | 6.56 | −24.75 | 7.69 | 2.95 | 5.91 | −0.17 | 5.46 | 11 | 3.33 | 8.83 | 4.49 |
| 11,12EED | 6.42 | 6.8 | −9.67 | 16.34 | −19.94 | 5.38 | −23.92 | 5.18 | 3.28 | 6.99 | −3.18 | 6.11 | 9.7 | 4.15 | 6.74 | 4.95 |
| 15,16-DiHODE | 13.33 | 20.19 | 18.33 | 20.31 | −6.56 | 5.87 | 9.62 | 11.93 | 10.33 | 6.51 | 12.67 | 6.47 | 18.1 | 3.05 | 15.7 | 3.12 |
| 12,13-DiHODE | 12.67 | 8.73 | 9.67 | 9.27 | −9.47 | 6.38 | 6.76 | 11.07 | 10.18 | 5.35 | 7.07 | 6.67 | 17.7 | 3.91 | 11.37 | 6.45 |
| 10,11-DiHDPE | 6.67 | 10.73 | −10 | 14.09 | −10.64 | 8.37 | −6.25 | 7.03 | 5 | 7.14 | 3 | 6.43 | 9.1 | 4.1 | 8.44 | 4.56 |
| 13-HOTrE | −19.33 | 28.39 | −1.33 | 6.49 | −23.84 | 5.82 | −19.96 | 7.13 | −20.73 | 16.74 | −5.47 | 6.4 | −10.8 | 12.61 | 2.29 | 6.77 |
In this study, it is noteworthy to highlight that certain compound, including metabolites derived from DGLA, three distinct isomeric forms of EpEDE, and DHED, were successfully synthesized and comprehensively characterized for the very first time. As a result, the incorporation of these oxylipins in our method enables us to determine these important lipids in C. elegans, like DHED which induces neurodegeneration and ferroptosis,11 thereby contributing valuable insights to the quantitative lipidomics and targeted metabolomics field.
2.2.3. Evaluating recoveries and extraction efficiencies
Recoveries were calculated for QC samples, with results ranging from 72%–108% (n=5). These values indicate that most of the analytes were successfully extracted and recovered during the sample preparation process, suggesting a reliable and efficient methodology. The precision was observed to be satisfactory, with most RSDs falling below 12%. Our results demonstrate that the method provides consistent results across replicates, highlighting its suitability for accurate quantification of analytes. Moreover, the extraction efficiencies exhibited stability across different analytes, further supporting the method’s robustness and applicability to various compounds, the recovery result is comparable with other oxylipin studies in human plasma and serum (SI Table 4).32
2.3. C. elegance sample analysis
To evaluate our developed method, we analyzed the oxylipins in C. elegans samples, where age-synchronized worms were grown on OP50, and collected on day 1 of adulthood (SI Figure 5).
2.3.1. Optimizing the homogenization solvent
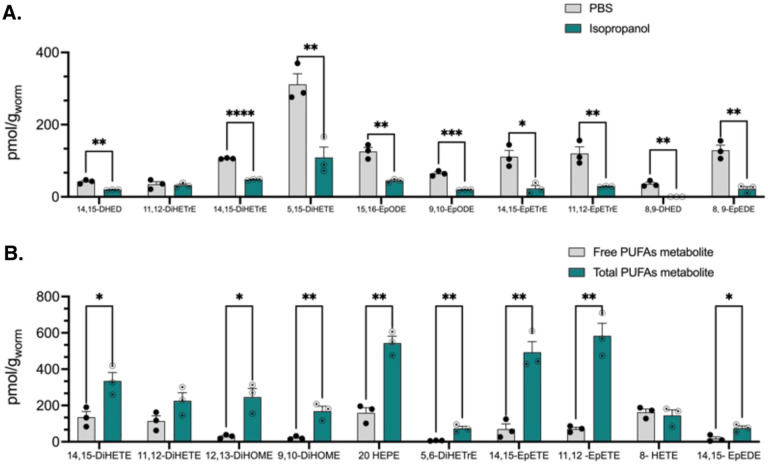
Tissue and animal samples, such as C. elegans, require homogenization prior to sample preparation to ensure efficient extraction of oxylipins. To achieve accurate quantification of PUFA metabolites in worm homogenates, it is essential to assess the solvent composition for optimal oxylipin recovery.33 Considering this, two different solvents, phosphate-buffered saline (PBS) as a water-based solvent and isopropanol as an organic solvent, were tested in conjunction with bead-based homogenization for preparing C. elegans homogenates. A comparison of the two solvents revealed that a few metabolites demonstrated similar results for both PBS and isopropanol, with no statistically significant differences between them, such as 11,12 DiHETrE (Figure 2A). One potential reason for the lower efficiency of isopropanol as an organic solvent could be the formation of protein precipitates, which may interfere with the extraction process. Thus, the choice of homogenization solvent plays a critical role in the overall success of oxylipin extraction and quantification.
Figure 2.
A) Optimization of solvent for C. elegans homogenization step, using two different solvents PBS and isopropanol. Oxylipin metabolites concentrations (pMol/g), mean ± SEM (n=3). Statistical differences between two different solvent groups were evaluated by multiple unpaired t-tests with *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001, non-significant is not shown). B) Oxylipin composition before and after hydrolysis in C. elegans samples. Control represents free fatty acids metabolites, whereas hydrolysis shows the total amount of oxylipin metabolites. Concentrations are mean ± SEM (n=3). Statistical differences between control and hydrolysis groups were evaluated by multiple unpaired t-tests with *P ≤ 0.05, **P ≤ 0.01, non-significant is not shown).
2.3.2. Assessing oxylipin composition before and after hydrolysis in C. elegans sample
To gain a comprehensive understanding of the function of PUFA metabolites,34 we needed to analyze both free and esterified oxylipins because studies have revealed that several oxylipins also initiate their effects through their phospholipid-esterified product.35 In addition, recent studies revealed that the cell membrane can act as a reservoir for free epoxy-FAs, and an external stimulus can trigger the release of these esterified epoxy-FAs, triggering subsequent biological effects.36
To determine the total oxylipin levels, a well-established KOH/methanol method was used based on a published paper37 (the detailed hydrolysis method is described in SI). After the hydrolysis process, certain oxylipins exhibited significantly different concentrations when compared to the nonhydrolyzed samples. These included both CYP450 metabolites, namely 11,12- EpETE, and 14,15- EpEDE, as well as epoxide hydrolase metabolites, specifically 9,10- DiHOME and 14,15- DiHETE.
However, for metabolites such as 8-HETE, and 11,12-DiHETE, the concentration difference between hydrolyzed samples and control samples was not statistically meaningful (Figure 2B). This observation underscores the importance of evaluating oxylipin composition both before and after hydrolysis in C. elegans samples. The variations in concentration might be attributed to the different susceptibilities of esterified oxylipins to hydrolysis or their varying affinities for enzymes involved in their metabolism. Further studies could provide insights into the specific factors contributing to these differences and improve our understanding of oxylipin metabolism in C. elegans.
To determine whether the alterations in oxylipin profile before and after hydrolysis were due to the breakdown of specific lipid metabolism or an alternation of the esterified oxylipin profiles within a membrane, a separate experiment was conducted by the identical hydrolysis using Type I IS only. During this experiment, we noticed that the relative abundance of all different internal standards under hydrolysis conditions is the same, which shows that the relative stability of all oxylipins under the hydrolysis condition is the same. Therefore, the significant increase in concentration of specific oxylipins after hydrolysis can be attributed to their relatively high level in the cell membrane as an esterified form.
2.3.3. Matrix effect in C. elegans and ionization efficiency
Matrix effects (ME) pose significant challenges to quantitative LC-MS analysis by compromising the precision, sensitivity, and accuracy of the methodology. It is essential to thoroughly assess the presence of matrix effects during method development to ensure trustworthy analytical results38. The matrix effect plays a significant role in oxylipin analysis in a variety of biological samples. Unlike other cases, it is not feasible to construct a calibration curve using the same matrix due to the endogenous oxylipins in most biological samples. Therefore, we were compelled to rely on 75% EtOH as the solvent to create our calibration curve. To quantitatively evaluate matrix effects in the post-extraction addition method, we will compare the concentration of the analyte in a standard solution with that of a post-extraction spiked with the analyte at an equal concentration. Our results indicated that compounds like PGB2-d4, 15(S)-HETE-d8, 9-HODE-d4, and 8,9-EET-d11 showed a significant matrix effect of more than 20%. This change indicates that the worm matrix decreases ionization efficiency in ESI, the calibration curve concentration, spiked concentration, and calculated matrix effect for each compound are shown in Table 3. An extracted ion chromatogram (XIC) of oxylipin in the calibration curve and day one worm samples is provided (SI Figure 1). Two common ways to tackle the ME are reducing the injection volume and diluting the sample before injection. None of these two strategies are applicable for oxylipin due to the extremely low concentration of these compounds in biological samples. We decided to normalize our result by the type I internal standard, this approach is the most appropriate technique available to decrease the ME.
Table 3.
The matrix effect can be quantitatively evaluated by comparing the concentration of the analyte in standard solution (A) to that of a post-extract spiked with the analyte at the same concentration (B).
| Compound | Calibration curve (A) | Spiked (B) | Matrix effect % |
|---|---|---|---|
| PGB2-d4 | 15.63 | 19.73 | −26.25±5.56 |
| LTB4-d4 | 10.08 | 9.83 | 2.45± 8.28 |
| 8,9-DiHETrE-d11 | 15.70 | 18.83 | −19.96 ± 2.21 |
| 9-HODE-d4 | 16.70 | 20.63 | −23.55±9.22 |
| 15(S)-HETE-d8 | 20.50 | 23.30 | −13.66±5.22 |
| 5-HETE-d8 | 42.00 | 56.97 | −35.63± 7.49 |
| 8,9-EET-d11 | 42.10 | 56.50 | −34.19± 1.87 |
2.3.4. Preparation of Age-synchronized worm
Traditional techniques for preventing progeny overgrowth during C. elegans lifespan and aging often employ 5-fluoro-2′-deoxyuridine (FUDR) for sterilization. While FUDR has been popularly employed to inhibit DNA and RNA synthesis, thereby eliminating progeny contamination, there are significant concerns tied to its use.39,40 For example, the effects of FUDR have been primarily studied in wild-type (N2) worms. Notably, the interactions between FUDR and specific mutant strains have raised questions about its efficacy and reliability41,42. Studies like those by Aitalhaj et al. and the gas-1 strain underscore potential interferences, possibly skewing results and influencing longevity43,44. Furthermore, the idea that the impact of FUDR sterilization is linear could be an oversimplification. External signals from the reproductive system have the potential to alter nematode lifespan45, hinting at potential unexplored dimensions of FUDR effects. Additionally, our previous study raised concerns over the possible interference of FUDR with CYP-EH metabolites
Amid the aforementioned challenges with FUDR, our approach transitioned to a filtration method to achieve age synchronization.46 This method was meticulously designed to draw a clear boundary between adult worms and their progeny, resulting in a striking separation efficiency of over 99% (SI Figure 5). It is noteworthy to mention that while there was a minor loss of adults during filtration, primarily because live animals occasionally adhered to the filter pores or passed through during the washing process, this loss was quantified to be less than 15% (SI Figure 5). The high efficacy of this method became even more pronounced as the worms aged. Initial stages required daily filtrations, but as the adult worms advanced in their life cycle and stopped producing progeny, the filtration frequency diminished. After the initial week, the predominant aim shifted from rigorous progeny segregation to primarily transferring worms to plates with fresh food.
2.3.5. Analysis of C. elegans with and without EHI
To determine whether our method can quantify the oxylipin changes in C. elegans, we quantified the oxylipin profiles in C. elegans with and without the treatment with an epoxide hydrolase inhibitor (EHI). Harris et al. showed that 12-(3-((3s,5s,7s)-adamantan-1-yl) ureido) dodecanoic acid (AUDA), blocks the conversion of epoxyoctadecenoic acids to dihydroxyoctadecenoic acid by inhibiting C. elegans epoxide hydrolases.47
The oxylipin profile of AUDA-treated worm as compared with vehicle control revealed an overall increase in the levels of epoxy-FA metabolites, and a decrease in dihydroxy-FA metabolites, with the most pronounced change observed in (i) 12,13-DiHOME among LA metabolites, (ii) 8,9-DHED and 14,15-DHED for DGLA metabolites, (iii)11,12-EpETrE, and 14, 15-DiHETrE for AA metabolites, and (iv) 17,18-EpETE, 11,12-DiHETE, and 14,15-DiHETE for EPA metabolites (Figure 3). This observation is intriguing, as it implies that the connection between epoxide hydrolase activity and overall oxylipin levels could be more complex than initially thought. In particular, the presumption is that inhibiting epoxide hydrolase activity would stabilize or increase the endogenous level of epoxy fatty acids and decrease the corresponding epoxide hydrolase products (dihydroxy fatty acids). This observation could be attributed to the presence of alternative pathways involved in the metabolism of epoxy- and dihydroxy-FA metabolites, as well as potential feedback regulation of other enzymes that play a part in this process. In addition, the diverse effects caused by the supplementation of AUDA on a variety of epoxy-FA-to-dihydroxyPUFA ratios could also be attributed to the selectivity of the C. elegans EHs. Moreover, we found a higher concentration of different EPA metabolites, and the lower concentration of the ALA metabolites compared to the other PUFA metabolites. This pattern aligns with the levels of their respective parent PUFAs found in the worm.48
Figure 3.
PUFA metabolites of C. elegans in the first day of adulthood, with and without epoxide hydrolase inhibitor treatment. The concentration of each compound (pmol/g) represents a replicate of independent populations of whole worm lysate that were 10 mg or greater, mean ± SEM (n=3). Statistical differences between two different groups were evaluated by multiple unpaired t-tests with *P ≤ 0.05, **P ≤ 0.01, and non-significant is not shown).
It is worth mentioning that interpreting the effect of EH inhibitors could not be done correctly by solely tracking the Ep-PUFAs or dihydroxy-FA levels independently, as there is a physiological balance between these different oxylipins (Figure 1). Therefore, to further explore the main effect of EH inhibition, the epoxy-to-dihydroxy ratio of different PUFA oxylipin metabolites was studied, which is generally considered a marker of EH inhibition in vivo.49,50 Our results show that wildtype worms treated with AUDA exhibit an overall increase in the epoxy-to-dihydroxy fatty acid ratio, with the most significant increase related to the 11,12-(EED/DHED), 14,15-(EED/DHED), 11,12-(EEQ/DiHETE), and 17,18-(EEQ/DiHETE). This corroborates with previous studies that reported an increase in the epoxy-to-dihydroxy fatty acid ratio upon administration of EHI (Figure 4A–D).
Figure 4.
(A–D) The ratio of epoxy to dihydroxy PUFAs metabolite of C. elegans in the first day of adulthood, with and without epoxide hydrolase inhibitor treatment. The concentration of each compound (pMol/g) represents a replicate of independent populations of whole worm lysate that were 10 mg or greater, mean ± SEM (n=3). Statistical differences between two different groups were evaluated by multiple unpaired t-tests with *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and non-significant is not shown). (E -G). Represent the comparison of the total, ω−6, and ω−3 PUFA epoxy, dihydroxy metabolites and hydroxy metabolites in C. elegans on the first day of adulthood, with and without epoxide hydrolase inhibitor treatment. The concentration of each compound (pmol/g) represents a triplicate of independent populations of whole worm lysate that were 10 mg or greater, mean ± SEM (n=3). Statistical differences between two different groups were evaluated by multiple unpaired t-tests with *P ≤ 0.05, **P ≤ 0.01, and non-significant is not shown).
We also compared the overall level of CYP-EH metabolites related to ω−3 and ω−6 PUFAs (Figure 4 E, and F). The wildtype worms show a higher level of ω−3 Ep-PUFAs (600–800 pmol/g) compared to ω−6 Ep-PUFAs (90–120 pmol/g), while the dihydroxy-FA level was almost similar for both ω−3 and ω−6-PUFAs (150–200 pmol/g). Intriguingly, administration of AUDA resulted in a significant increase in ω−3 Ep-PUFAs and a significant decrease in ω−3 dihydroxy-FAa. On the other hand, we only observed a decrease in ω−6 Dihydroxy-FAs, while the ω−6 Ep-PUFAs remained unchanged.
Besides, we could measure several hydroxy-PUFA in wild-type worms (Figure 4G). It should be noted that the hydroxy-PUFAs detected in this research are predominantly generated via three main pathways in mammals: (i) LOX enzymes, (ii) non-enzymatic oxidation, and (iii) cytochrome P450 (CYP450) enzymes. Given the absence of LOX homologs in C. elegans, the production of hydroxy-PUFAs in this organism is likely primarily facilitated by non-enzymatic oxidation, CYP450 pathways, or an undiscovered enzyme with monohydroxylation activity.51–53 This observation highlights the unique metabolic processes in C. elegans and the potential for further study of these mechanisms. We also found that AUDA treatment could change the hydroxy-PUFA levels in worms, with a statistically significant decrease in 5-HETE, 12-HETE, and 15-HETE. Whereas for the hydroxy metabolites like 19-HETE and 20-HEPE, the difference caused by AUDA treatment was not statistically significant. These findings emphasize the complex effects of AUDA treatment on hydroxy-PUFA levels and point to possible indirect influences on other enzymatic pathways or feedback regulation mechanisms. Further research is necessary to determine the potential roles of these changes and to elucidate the precise mechanisms underlying the observed alterations in hydroxy-PUFA levels.
3. Conclusion
Overall, these findings highlight the efficacy of our method for quantifying the oxylipin profile in C. elegans and offer valuable insights into the effects of epoxide hydrolase inhibition on PUFA metabolites. The method could be used to further reveal intricate relationships between enzymatic activity, oxylipin levels, and the roles of different metabolic pathways in C. elegans, pointing to the possibility of complex regulatory mechanisms at play. By providing a comprehensive picture of alterations in oxylipin profiles after AUDA treatment, our work lays the foundation for future investigations into the underlying mechanisms and potential therapeutic applications of epoxide hydrolase inhibitors.
This study aimed to develop a reliable and accurate LC-MS/MS method for quantifying the oxylipin profile in the C. elegans animal model. The developed method can be employed in future studies to investigate the effects of various diseases, treatments, or genetic manipulations on the oxylipin profile and to assess potential therapeutic interventions using C. elegans as an animal model. Ultimately, the knowledge gained from these studies may contribute to a better understanding of the role of oxylipins in health and disease and may potentially lead to the development of novel therapeutic strategies. By further refining the method and expanding the range of oxylipins analyzed, researchers can continue to build upon the foundation established in this study and enhance our understanding of the complex interplay between oxylipin metabolism and various biological processes.
Supplementary Material
Acknowledgment
This study was supported, by the National Institute of Aging R03 AG075465, the Pearl Aldrich Endowment for Aging Research, and startup funding from Michigan State University. The Peal Aldrich Endowment for Aging Research Student award partially supported . We acknowledge the MSU RTSF Mass Spectrometry and Metabolomics Core Facilities for support with oxylipin and lipidomic analysis. We would like to thank Prof. Daniel Jones for his advice help and assistance. Prism and Biorender partly generate figures.
Reference:
- (1).Hou Y.; Dan X.; Babbar M.; Wei Y.; Hasselbalch S. G.; Croteau D. L.; Bohr V. A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15 (10), 565–581. [DOI] [PubMed] [Google Scholar]
- (2).Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimers. Dement. 2019, 15 (3), 321–387. [DOI] [PubMed] [Google Scholar]
- (3).Hamsanathan S.; Gurkar A. U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Johnson A. A.; Stolzing A. The Role of Lipid Metabolism in Aging, Lifespan Regulation, and Age-Related Disease. Aging Cell 2019, 18 (6), e13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Vierck J. L.; Dodson M. V. Interpretation of Cell Culture Phenomena. Methods Cell Sci. 2000, 22 (1), 79–81. [DOI] [PubMed] [Google Scholar]
- (6).Zhang J.; Yang J.; Duval C.; Edin M. L.; Williams A.; Lei L.; Tu M.; Pourmand E.; Song R.; Graves J. P.; DeGraff L. M.; Wong J. J.-L.; Wang Y.; Sun Q.; Sanidad K. Z.; Wong S.; Han Y.; Zhang Z.; Lee K. S. S.; Park Y.; Xiao H.; Liu Z.; Decker E. A.; Cui W.; Zeldin D. C.; Zhang G. CYP Eicosanoid Pathway Mediates Colon Cancer-Promoting Effects of Dietary Linoleic Acid. FASEB J. 2023, 37 (7), e23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Favor O. K.; Chauhan P. S.; Pourmand E.; Edwards A. M.; Wagner J. G.; Lewandowski R. P.; Heine L. K.; Harkema J. R.; Lee K. S. S.; Pestka J. J. Lipidome Modulation by Dietary Omega-3 Polyunsaturated Fatty Acid Supplementation or Selective Soluble Epoxide Hydrolase Inhibition Suppresses Rough LPS-Accelerated Glomerulonephritis in Lupus-Prone Mice. Front. Immunol. 2023, 14, 1124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cambiaggi L.; Chakravarty A.; Noureddine N.; Hersberger M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24 (7), 6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Eriksdotter M.; Vedin I.; Falahati F.; Freund-Levi Y.; Hjorth E.; Faxen-Irving G.; Wahlund L.-O.; Schultzberg M.; Basun H.; Cederholm T.; Palmblad J. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients during Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers. Dis. 2015, 48 (3), 805–812. [DOI] [PubMed] [Google Scholar]
- (10).Jernerén F.; Cederholm T.; Refsum H.; Smith A. D.; Turner C.; Palmblad J.; Eriksdotter M.; Hjorth E.; Faxen-Irving G.; Wahlund L.-O.; Schultzberg M.; Basun H.; Freund-Levi Y. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J. Alzheimers. Dis. 2019, 69 (1), 189–197. [DOI] [PubMed] [Google Scholar]
- (11).Sarparast M.; Pourmand E.; Hinman J.; Vonarx D.; Reason T.; Zhang F.; Paithankar S.; Chen B.; Borhan B.; Watts J. L.; Alan J.; Lee K. S.S. Dihydroxy-Metabolites of Dihomoγ-Linolenic Acid Drive Ferroptosis-Mediated Neurodegeneration. ACS Cent. Sci. 2023, 9 (5), 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Camunas-Alberca S. M.; Moran-Garrido M.; Sáiz J.; Villaseñor A.; Taha A. Y.; Barbas C. The Role of Oxylipins and Their Validation as Biomarkers in the Clinical Context. Trends Analyt. Chem. 2023, 164 (117065), 117065. [Google Scholar]
- (13).Chu J.; Giannopoulos P. F.; Ceballos-Diaz C.; Golde T. E.; Pratico D. Adeno-Associated Virus-Mediated Brain Delivery of 5-Lipoxygenase Modulates the AD-like Phenotype of APP Mice. Mol. Neurodegener. 2012, 7 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yermakova A. V.; Rollins J.; Callahan L. M.; Rogers J.; O’Banion M. K. Cyclooxygenase-1 in Human Alzheimer and Control Brain: Quantitative Analysis of Expression by Microglia and CA3 Hippocampal Neurons. J. Neuropathol. Exp. Neurol. 1999, 58 (11), 1135–1146. [DOI] [PubMed] [Google Scholar]
- (15).Ghosh A.; Comerota M. M.; Wan D.; Chen F.; Propson N. E.; Hwang S. H.; Hammock B. D.; Zheng H. An Epoxide Hydrolase Inhibitor Reduces Neuroinflammation in a Mouse Model of Alzheimer’s Disease. Sci. Transl. Med. 2020, 12 (573), eabb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Marowsky A.; Burgener J.; Falck J. R.; Fritschy J.-M.; Arand M. Distribution of Soluble and Microsomal Epoxide Hydrolase in the Mouse Brain and Its Contribution to Cerebral Epoxyeicosatrienoic Acid Metabolism. Neuroscience 2009, 163 (2), 646–661. [DOI] [PubMed] [Google Scholar]
- (17).Hung T.-H.; Shyue S.-K.; Wu C.-H.; Chen C.-C.; Lin C.-C.; Chang C.-F.; Chen S.-F. Deletion or Inhibition of Soluble Epoxide Hydrolase Protects against Brain Damage and Reduces Microglia-Mediated Neuroinflammation in Traumatic Brain Injury. Oncotarget 2017, 8 (61), 103236–103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Griñán-Ferré C.; Codony S.; Pujol E.; Yang J.; Leiva R.; Escolano C.; Puigoriol-Illamola D.; Companys-Alemany J.; Corpas R.; Sanfeliu C.; Pérez B.; Loza M. I.; Brea J.; Morisseau C.; Hammock B. D.; Vázquez S.; Pallàs M.; Galdeano C. Pharmacological Inhibition of Soluble Epoxide Hydrolase as a New Therapy for Alzheimer’s Disease. Neurotherapeutics 2020, 17 (4), 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lee H.-T.; Lee K.-I.; Chen C.-H.; Lee T.-S. Genetic Deletion of Soluble Epoxide Hydrolase Delays the Progression of Alzheimer’s Disease. J. Neuroinflammation 2019, 16 (1), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gan L.; Cookson M. R.; Petrucelli L.; La Spada A. R. Converging Pathways in Neurodegeneration, from Genetics to Mechanisms. Nat. Neurosci. 2018, 21 (10), 1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Colman R. J. Non-Human Primates as a Model for Aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 (9), 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mitchell S. J.; Scheibye-Knudsen M.; Longo D. L.; de Cabo R. Animal Models of Aging Research: Implications for Human Aging and Age-Related Diseases. Annu. Rev. Anim. Biosci. 2015, 3 (1), 283–303. [DOI] [PubMed] [Google Scholar]
- (23).Johnson I. P. Response: Commentary: Age-Related Neurodegenerative Disease Research Needs Aging Models. Front. Aging Neurosci. 2016, 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kell D. B. Metabolomic Biomarkers: Search, Discovery and Validation. Expert Rev. Mol. Diagn. 2007, 7 (4), 329–333. [DOI] [PubMed] [Google Scholar]
- (25).Schuchardt J. P.; Schmidt S.; Kressel G.; Dong H.; Willenberg I.; Hammock B. D.; Hahn A.; Schebb N. H. Comparison of Free Serum Oxylipin Concentrations in Hyper- vs. Normolipidemic Men. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89 (1), 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Watts J. L.; Browse J. Genetic Dissection of Polyunsaturated Fatty Acid Synthesis in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (9), 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Thomas S. N.; French D.; Jannetto P. J.; Rappold B. A.; Clarke W. A. Liquid Chromatography-Tandem Mass Spectrometry for Clinical Diagnostics. Nat. Rev. Methods Primers 2022, 2 (1), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yang J.; Schmelzer K.; Georgi K.; Hammock B. D. Quantitative Profiling Method for Oxylipin Metabolome by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2009, 81 (19), 8085–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Simultaneous Quantitation of Lipid Biomarkers for Inflammatory Bowel Disease Using LCMS/MS Chhonker 1 Yashpal S. and Murry 1,4,* Daryl J., Kanvinde 2. 2 Shrey. [DOI] [PMC free article] [PubMed]
- (30).Fulton D.; Falck J. R.; McGiff J. C.; Carroll M. A.; Quilley J. A Method for the Determination of 5,6-EET Using the Lactone as an Intermediate in the Formation of the Diol. J. Lipid Res. 1998, 39 (8), 1713–1721. [PubMed] [Google Scholar]
- (31).Vrablik T. L.; Watts J. L. Polyunsaturated Fatty Acid Derived Signaling in Reproduction and Development: Insights from Caenorhabditis Elegans and Drosophila Melanogaster. Mol. Reprod. Dev. 2013, 80 (4), 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pedersen T. L.; Gray I. J.; Newman J. W. Plasma and Serum Oxylipin, Endocannabinoid, Bile Acid, Steroid, Fatty Acid and Nonsteroidal Anti-Inflammatory Drug Quantification in a 96-Well Plate Format. Anal. Chim. Acta 2021, 1143, 189–200. [DOI] [PubMed] [Google Scholar]
- (33).Höring M.; Krautbauer S.; Hiltl L.; Babl V.; Sigruener A.; Burkhardt R.; Liebisch G. Accurate Lipid Quantification of Tissue Homogenates Requires Suitable Sample Concentration, Solvent Composition, and Homogenization Procedure-A Case Study in Murine Liver. Metabolites 2021, 11 (6), 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Salimon J.; Abdullah B. M.; Salih N. Hydrolysis Optimization and Characterization Study of Preparing Fatty Acids from Jatropha Curcas Seed Oil. Chem. Cent. J. 2011, 5 (1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Annevelink C. E.; Walker R. E.; Shearer G. C. Esterified Oxylipins: Do They Matter? Metabolites 2022, 12 (11), 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ray S.; Kassan A.; Busija A. R.; Rangamani P.; Patel H. H. The Plasma Membrane as a Capacitor for Energy and Metabolism. Am. J. Physiol. Cell Physiol. 2016, 310 (3), C181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Rund K. M.; Heylmann D.; Seiwert N.; Wecklein S.; Oger C.; Galano J.-M.; Durand T.; Chen R.; Gueler F.; Fahrer J.; Bornhorst J.; Schebb N. H. Formation of Trans-Epoxy Fatty Acids Correlates with Formation of Isoprostanes and Could Serve as Biomarker of Oxidative Stress. Prostaglandins Other Lipid Mediat. 2019, 144 (106334), 106334. [DOI] [PubMed] [Google Scholar]
- (38).Matuszewski B.; Constanzer M.; Chavez-Eng C. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem 2003, 75 (13), 3019–3030. [DOI] [PubMed] [Google Scholar]
- (39).Gandhi S.; Santelli J.; Mitchell D. H.; Wesley Stiles J.; Rao Sanadi D. A Simple Method for Maintaining Large, Aging Populations of Caenorhabditis Elegans. Mech. Ageing Dev. 1980, 12 (2), 137–150. [DOI] [PubMed] [Google Scholar]
- (40).Mitchell D. H.; Stiles J. W.; Santelli J.; Sanadi D. R. Synchronous Growth and Aging of Caenorhabditis Elegans in the Presence of Fluorodeoxyuridine. J. Gerontol. 1979, 34 (1), 28– [DOI] [PubMed] [Google Scholar]
- (41).Hosono R.; Mitsui Y.; Sato Y.; Aizawa S.; Miwa J. Life Span of the Wild and Mutant Nematode Caenorhabditis Elegans. Effects of Sex, Sterilization, and Temperature. Exp. Gerontol. 1982, 17 (2), 163–172. [DOI] [PubMed] [Google Scholar]
- (42).Hosono R. Sterilization and Growth Inhibition of Caenorhabditis Elegans by 5Fluorodeoxyuridine. Exp. Gerontol. 1978, 13 (5), 369–374. [DOI] [PubMed] [Google Scholar]
- (43).Aitlhadj L.; Stürzenbaum S. R. The Use of FUdR Can Cause Prolonged Longevity in Mutant Nematodes. Mech. Ageing Dev. 2010, 131 (5), 364–365. [DOI] [PubMed] [Google Scholar]
- (44).Van Raamsdonk J. M.; Hekimi S. FUdR Causes a Twofold Increase in the Lifespan of the Mitochondrial Mutant Gas-1. Mech. Ageing Dev. 2011, 132 (10), 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Hsin H.; Kenyon C. Signals from the Reproductive System Regulate the Lifespan of C. Elegans. Nature 1999, 399 (6734), 362–366. [DOI] [PubMed] [Google Scholar]
- (46).Hunter S.; Maulik M.; Scerbak C.; Vayndorf E.; Taylor B. E. Caenorhabditis Sieve: A Low-Tech Instrument and Methodology for Sorting Small Multicellular Organisms. J. Vis. Exp. 2018, No. 137. 10.3791/58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Harris T. R.; Aronov P. A.; Jones P. D.; Tanaka H.; Arand M.; Hammock B. D. Identification of Two Epoxide Hydrolases in Caenorhabditis Elegans That Metabolize Mammalian Lipid Signaling Molecules. Arch. Biochem. Biophys. 2008, 472 (2), 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Watts J. L. Using Caenorhabditis Elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. J. Clin. Med. Res. 2016, 5 (2). 10.3390/jcm5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Inceoglu B.; Wagner K.; Schebb N. H.; Morisseau C.; Jinks S. L.; Ulu A.; Hegedus C.; Rose T.; Brosnan R.; Hammock B. D. Analgesia Mediated by Soluble Epoxide Hydrolase Inhibitors Is Dependent on CAMP. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (12), 5093–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Inceoglu B.; Zolkowska D.; Yoo H. J.; Wagner K. M.; Yang J.; Hackett E.; Hwang S. H.; Lee K. S. S.; Rogawski M. A.; Morisseau C.; Hammock B. D. Epoxy Fatty Acids and Inhibition of the Soluble Epoxide Hydrolase Selectively Modulate GABA Mediated Neurotransmission to Delay Onset of Seizures. PLoS One 2013, 8 (12), e80922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sarparast M.; Dattmore D.; Alan J.; Lee K. S. S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12 (11), 3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Bazinet R. P.; Layé S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15 (12), 771–785. [DOI] [PubMed] [Google Scholar]
- (53).Czapski G. A.; Czubowicz K.; Strosznajder J. B.; Strosznajder R. P. The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem. Res. 2016, 41 (1–2), 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.