Abstract
The polymorphic microbiome has been defined as one of the “Hallmarks of Cancer”. Extensive studies have now uncovered the role of oral microbiota in cancer development and progression. Bacteria, fungi, archaea, and viruses in the oral cavity interact dynamically with the oral microenvironment to maintain the oral micro-ecological homeostasis. This complex interaction is influenced by many factors, such as maternal transmission, personal factors and environmental factors. Dysbiosis of oral microbiota can disturbed this host–microbiota interaction, leading to systemic diseases. Numerous studies have shown the potential associations between oral microbiota and a variety of cancers. However, the underlying mechanisms and therapeutic insights are still poorly understood. In this review, we mainly focus on the following aspects: (1) the factors affect oral microbiota composition and function; (2) the interaction between microenvironment and oral microbiota; (3) the role of multi-kingdom oral microbiota in human health; (4) the potential underlying mechanisms and therapeutic benefits of oral microbiota against cancer. Finally, we aim to describe the impact of oral microbiota on cancer progression and provide novel therapeutic insights into cancer prevention and treatment by targeting oral microbiota.
Keywords: Oral microbiota, Cancer, Cancer diagnosis, Tumor therapy
Introduction
The human oral cavity represents another giant habitat of microbiota in addition to the gut (Aas et al. 2005). Emerging studies have investigated the role of oral microbiota in human health and its dynamic changes after birth (Nelson-Filho et al. 2013; Aagaard et al. 2014). Besides, the oral microbiota compositions in oral cavities under variable health conditions are also intensively investigated, which gives adequate evidence that oral microbiota is closely related to human health (Griffen et al. 2012; Burne et al. 2012; Wang et al. 2016b; Corrêa et al. 2017).
The oral cavity exhibits complex structures and microenvironments, and the resident microorganisms differ in diverse oral niches (Bowen et al. 2018). In most cases, oral microorganisms form biofilms embedded in an extracellular polymeric substances (EPS) matrix, through which the oral microbiota could interplay with the oral microenvironment (Bowen et al. 2018). Thus, the living microorganisms in certain region can also change the microenvironment. However, many factors, like dietary habits, can affect this regulation. For example, the high-carbohydrate diet is linked to a significant decrease in microbial diversity of the oral microbiota, thus leading to more aciduric-cariogenic bacteria, which further changes the pH and other properties in the oral cavity (Millen et al. 2022).
The multi-kingdom microbiota interaction has attracted significant attention in recent years (Rao et al. 2021; Liu et al. 2022). In general, microorganisms include bacteria, fungi, archaea and viruses, but investigations on the last three kingdoms are still insufficient.
Most recently, the role of the microbiota in cancer progression has received considerable attention, and polymorphic microbiomes have been defined as the new emerging hallmark of cancer (Hanahan 2022). As the second largest and most diverse microbial community in human body, the oral microbiota also plays a potential role in cancer progression, diagnosis and treatment. Previous studies have already uncovered its correlation with oral cancer, lung cancer, and colorectal cancer (Zhang et al. 2019; Hosgood et al. 2021; Rezasoltani et al. 2022). In this review, we will discuss the specific relationship between the oral microbiota and cancer, the potential mechanisms involved in the oral microbiota associated carcinogenesis and provide new insights into clinical therapy.
Microenvironment in the Human Oral Cavity
The distinct microenvironments of oral cavity depend on the exposure to the external environment and continuous encounter with exogenous microbes in a certain degree (Sedghi et al. 2021). The oral microbiota works as the bridge between external environment and host health, attracting major attention these years (Sedghi et al. 2021). Increasing evidence supports an association between the placental microbiota and the oral microbiota (Aagaard et al. 2014; Tuominen et al. 2019). Comparing the placental microbiome to those present in other body site niches, as reported by the Human Microbiome Project (HMP), the placental microbiome showed a high similarity with the oral microbiome (Aagaard et al. 2014). Furthermore, the neonatal oral cavity microbiota shared features mainly with the placental microbiota rather than cervical or maternal oral microbiota, suggesting that the neonatal oral microbiota may have a prenatal origin (Tuominen et al. 2019). The study evaluated the dynamics of oral microbial colonization in 51 healthy newborns from 10 min to 53 h after birth, which showed that Staphylococcus epidermidis (S. epidermidis) was firstly detected in oral microbiota samples from newborns 10 min and 8 h after birth; between 8 h and 16 h, streptococci were then appeared; and between 24 h and 53 h, S. epidermidis and Staphylococcus aureus (S. aureus) predominate in the oral cavity (Nelson-Filho et al. 2013).
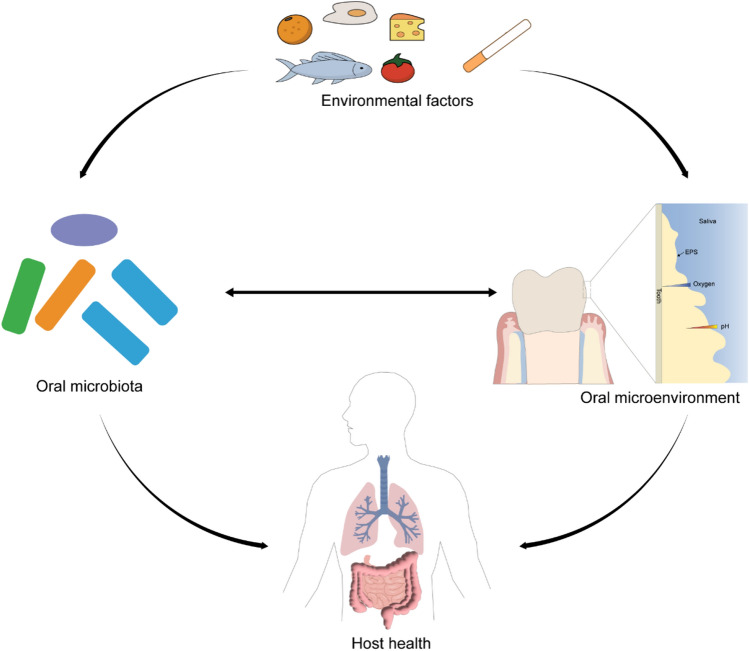
Oral mucosal homeostasis and even human health can be mainly driven by the normal function of the oral microbiota (Lamont et al. 2018). Meanwhile, the oral microbiota's spatial and structural organization depends on the oral cavity microenvironment (Mark Welch et al. 2016). Factors affecting the microbial colonization includes pH, oxygen concentration, redox, etc., and vary with different sites in the oral cavity (Bowen et al. 2018). Mark Welch et al. (2016) proposed a hedgehog structure of oral microorganism distribution, which is driven by environmental and biochemical gradients. Specifically, anaerobic taxa tend to be in the interior, whereas facultative or obligate aerobes tend to be at the periphery based on the oxygen concentration change. Oral surfaces are predominantly colonized by facultative anaerobes, such as Actinomyces species. Within the confines of the subgingival area, reduced oxygen tensions favor population shifts with an increasing abundance of strict anaerobes such as Bacteroidaceae species and Spirochaetes (Lamont et al. 2018). The relationship among environmental factors, oral microbiota, oral microenvironment and host health is depicted in Fig. 1.
Fig. 1.
The relationship among environmental factors, oral microbiota, oral microenvironment and host health. Environmental factors like diet and smoke could alter oral microbiota composition, abundance and metabolic activity as well as oral microenvironment including pH, oxygen concentration; the oral microbiota interact with oral microenvironment, and they collaboratively impact host health
Factors Affecting the Oral Microbiota
The oral microbiota is a complex entirety, which can be influenced by lots of factors including maternal transmission, gene heterogeneity, and environmental factors, such as geography, dietary habits and smoking (Yu et al. 2017; Tierney et al. 2019; Willis and Gabaldón 2020; Ramadugu et al. 2021; Fregatto et al. 2021; Ogbanga et al. 2023). Here, we summarized the factors which affect the oral microbiota (Table 1).
Table 1.
List of potential factors affecting oral microbiome
| Factors | Effects on oral microbiome | References | |
|---|---|---|---|
| Maternal transmission | Placenta microbiota | Train and tune the developing fetus's immune system for oral microbiome preparation | (Kaan et al. 2021) |
| The delivery mode | Infants born by caesarean section had initially skewed oral bacterial content compared with vaginally delivered infants | (Dzidic et al. 2018) | |
| Breastfeeding | Lead to lower relative abundances of Prevotella and Veillonella in oral cavity | (Ramadugu et al. 2021) | |
| Gene heterogeneity | / | The oral microbiomes contain vast and individual-specific genetic content | (Tierney et al. 2019; Liu et al. 2021a) |
| Environmental factors | Geography | Oral microbes are specialized in different habitats verified in China, Italy, Indonesia | (Widyarman et al. 2021; Zhu et al. 2022; Ogbanga et al. 2023) |
| Diet | Dietary carbohydrate and sucrose intake were inversely associated with subgingival bacteria alpha diversity; Overexposure to sucrose lead to an EPS-rich biofilm matrix, a lower pH and higher abundance of acid-tolerant microorganisms in oral cavity | (Millen et al. 2022) | |
| Cigarette smoking | Cause lower relative abundance of the phylum Proteobacteria, the genera Capnocytophaga, Peptostreptococcus and Leptotrichia and a higher relative abundance of Atopobium and Streptococcus | (Wu et al. 2016) | |
| Oral hygiene | Proper oral care like flossing and toothbrushing decreases the relative abundance of dental pathogens; Rinsing with chlorhexidine mouthwash changes the salivary microbiomes and leads to more acidity in the mouth | (Adams et al. 2017; Brookes et al. 2021) | |
The contribution of maternal microbiota to early oral microbiota formation has been revealed recently (Dzidic et al. 2018; Moossavi et al. 2019; Eshriqui et al. 2020; Kaan et al. 2021). Tuominen et al. (2019) supported the prenatal origin of the neonatal oral microbiota by 16S ribosomal RNA (rRNA) gene sequencing (V3–V4 region) because the neonatal oral microbiota share features mainly with the microbes detected in the placenta. It was also previously thought that the placenta microbiota translocated directly to the fetus (Aagaard et al. 2014). However, in fact the placenta microbiota trained and tuned the developing fetus's immune system to prepare the fetus for postnatal microbial encounters (Kaan et al. 2021). The delivery mode also influenced the infant's oral microbiota mainly in the first 3–8 months of life after born (Lif Holgerson et al. 2011; Nelun Barfod et al. 2011). In addition to delivery mode, breastfeeding duration also influences the oral microbiota. As indicated by the Sweden study, the microbiota species-level profiles were quite different between infants breastfed for 12 months and infants breastfed for less than 6 months when using constrained correspondence analysis (CCA) (Dzidic et al. 2018). And breastfed children had lower relative abundances of Prevotella and Veillonella than infants not breastfed (Dzidic et al. 2018; Ramadugu et al. 2021). Interestingly, breastfeeding could impact infants' oral microbiota, and the babies' oral cavity could affect the breastmilk microbiota in return (Moossavi et al. 2019).
The oral microbiota varies from person to person, mainly due to the host factors (Tierney et al. 2019; Liu et al. 2021a). A meta-analysis covering 3655 samples from two different body sites, the oral cavity and the gut, depicted the genetic landscape in human oral microbiome and found that half of every person's microbial gene is unique (Tierney et al. 2019). Apart from this, another study has characterized the oral metagenome from more than 1915 individuals for both the tongue dorsum and saliva, which indicated significant human genomic associations with the oral metagenome (Liu et al. 2021a).
Alterations in the composition of oral microbiota are associated with geography. For example, the oral microbiota composed of three different Chinese cohorts across various geographic areas (Shenzhen: southern China, Yunnan: southwest China, Beijing: northern China) was distinct (Zhu et al. 2022). Besides, studies carried out in other countries confirmed that oral microbes were specific in different habitats (Pasolli et al. 2019; Widyarman et al. 2021; Ogbanga et al. 2023). For example, the study about the oral microbiomes of individuals living in two regions of Italy found differentially abundant oral taxa between Lombardy and Piedmont, Italy (Ogbanga et al. 2023). Furthermore, the enrolled Indonesian women displayed a remarkable difference in the bacterial community profiles between the urban and rural localities (Widyarman et al. 2021).
The oral microbiota colonization also affected by diet (Maslowski and Mackay 2011; David et al. 2014b). Recent studies have revealed that dietary carbohydrate and sucrose intake were inversely correlated with subgingival bacteria alpha diversity. Higher sucrose intake may result in poor oral or systemic health outcomes in elder women by changing the normal oral microbiome (Millen et al. 2022). Furthermore, during early childhood, the foundation of oral microbiota communities is determined by dietary composition. Sulyanto et al. (2019) discovered that the introduction of solid food changed the oral microbial communities, as indicated by a significant increase in microbial diversity and richness. Oral microorganisms residing within biofilms are embedded in an EPS matrix, through which the oral microbiota could interplay with the oral microenvironment (Bowen et al. 2018). On one hand, the oral microbiota embedded in EPS matrix modulates the oral microenvironment due to their metabolic activity; for example, the acid-producing microorganisms within biofilms result in lower pH (Takahashi and Nyvad 2011). On the other hand, the microenvironment change reset the oral microbiota community. For instance, Streptococcus mutans, one major cause of dental caries, is the leading producer of insoluble glucans among oral bacteria, which could make a suitable microenvironment for other aciduric-cariogenic bacteria to thrive and become established, contributing to human plaque (Gross et al. 2012; Simón-Soro and Mira 2015; Guo et al. 2015). In addition, the EPS matrix also affects the collective microbial behavior, function and virulence (Koo et al. 2013; Lin et al. 2021). EPS production directly mediates microbial adherence ability while forming a polymeric matrix that enhances the mechanical stability of biofilms (Flemming et al. 2016).
Cigarette smoke could produce numerous toxicants that directly come into contact with the human oral cavity and then change the oral microbiota (Macgregor 1989). A cohort study containing 1204 American adults assessed the relationship between smoking and the oral microbiota, explaining how smoking results in oral microbiota change and further causes oral diseases and even systemic diseases (Wu et al. 2016). The result showed that current smokers had a lower relative abundance of the phylum Proteobacteria, the genera Capnocytophaga, Peptostreptococcus and Leptotrichia, while induced a higher relative abundance of Atopobium and Streptococcus compared with never smokers (Wu et al. 2016). In addition, functional analysis showed that bacterial genera depleted by smoking were related to carbohydrate, energy metabolism and xenobiotic metabolism (Wu et al. 2016).
Oral hygiene also affects the microbial balance within oral cavity (Hallang et al. 2021). Proper oral care, like flossing and toothbrushing in the correct manner, can help decrease the relative abundance of dental pathogens, while incorrect oral care may disrupt the oral microbiota homeostasis, thus leading to deterioration of the oral cavity microenvironment and even causing diseases (Corby et al. 2008; David et al. 2014a; Adams et al. 2017). It is reported that rinsing with chlorhexidine mouthwash majorly changed the salivary microbiomes of healthy people, leading to more acidity in the mouth and lower nitrite availability, then causing higher blood pressure (Brookes et al. 2021). However, undesirable oral care behaviors do not attract public attention and remain widespread in dentistry to manage oral diseases, which become a potential risk to people's health (Campbell 2021).
Interaction Between Multi-kingdom Oral Microbiota and Host
A growing body of literature explores the multi-kingdom microbiota interaction, including bacteria, fungi, archaea and viruses. The multi-kingdom microbiota interaction could shape host-associated microbiota, thus deepening our understanding of microbiota ecology (Rao et al. 2021). Besides, multi-kingdom microbiota could be the potential diagnostic tools and therapeutic targets for certain diseases, like colorectal cancer (Liu et al. 2022).
The human oral microbiota is complex and includes interactions between bacteria, fungi, archaea, viruses or phages, and other microorganisms. The oral microbiota's multi-kingdom interaction, which involves both intra- and cross-kingdom interactions, has been recently well-established (Fujinami et al. 2021; Cheung et al. 2022). Cheung et al. (2022) showed that the oral microbiota of Chinese adults consists of multiple bacterial phyla, including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria, as well as fungal phyla, such as Ascomycota and Basidiomycota. They also explored the potential cross-kingdom interactions between bacterial and fungal microbiota of the healthy human oral cavity. A polymicrobial interaction between these dominant taxa could contribute to the homeostatic oral environment. Moreover, these interactions may be influenced by environmental factors, such as dietary habits, hygiene practices and saliva composition. Genetic factors, especially those associated with immune regulation, may also shape the oral microbiota. Fungal species such as Candida albicans (C. albicans) is reported to be required for oral bacteria-fungal interaction. The addition of C. albicans to an in vitro model of early oral biofilm formation significantly reduced the abundance of several bacterial genera, such as Streptococcus, Neisseria, and Veillonella, while increasing the abundance of other genera, such as Granulicatella and Prevotella, implying that C. albicans could alter the bacterial microbiota in vitro oral biofilms, demonstrating its potential to disrupt oral microbiota homeostasis and compromise teeth health (Janus et al. 2017; Du et al. 2021). Nonetheless, it should also be noted that the effects of C. albicans on the bacterial microbiota of early oral biofilms vary depending on the species present in the biofilm (Bertolini and Dongari-Bagtzoglou 2019).
According to recent studies, viruses represent an important component of oral microbiota in human health and disease. The highly complex network of interactions between bacteria and phages has been uncovered by combining whole-genome sequencing of bacterial and phage populations and insights from phenotypic assays (Wang et al. 2016a). On one hand, phage–bacteria interaction networks in the human oral microbiome are beneficial because bacteriophages reduce the number of pathogenic bacteria. On the other hand, it can also be harmful when bacteriophages transfer genetic material that could increase the virulence of certain bacterial species (Wang et al. 2016a).
Oral Microbiota in Cancer Progression
As the second leading cause of death in United States (Siegel et al. 2023), cancer poses a great threat to human health worldwide. According to cancer statistics published in 2023, the 5-year relative survival rate for all cancers combined has increased from 49% during the mid-1970s to 68% for diagnoses from 2012 to 2018 (Siegel et al. 2023). Moreover, based on the exploration of cancer and microbiomes, polymorphic microbiomes have been included as one of the hallmarks of cancer (Hanahan 2022). There is growing evidence that the natural bacterial flora in the oral cavity may play a critical role in cancer development (Mascitti et al. 2019). The bacteria can promote carcinogenesis or immunosuppression or cause chronic inflammation, increasing the risk of cancer development. Furthermore, certain microorganisms, like C. albicans and S. aureus, disrupted oral cavity homeostasis and upregulated oncogenes expression, particularly phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PI3KCA), HRas proto-oncogene (hRAS), mechanistic target of rapamycin kinase (mTOR), B-Raf proto-oncogene (BRAF), further promoting cancer progression (Amaya Arbeláez et al. 2021). In addition, some bacteria in the oral cavity may reduce the efficacy of chemotherapy used for cancer treatment and correlate with chemotherapy-induced oral mucositis (Triarico et al. 2022). Therefore, identifying individual microbial species and their functional activities in the oral cavity may provide new insights into cancer prevention and treatment. Here we will discuss specifically about how oral microbiota influences different types of cancer.
Oral Cancer
The oral microbiota composition is reported to be associated with an increased risk of oral cancer (Zhang et al. 2019). Specifically, several types of bacteria, including Porphyromonas gingivalis (P. gingivalis), Fusobacterium nucleatum (F. nucleatum), Streptococcus species (Streptococcus sp.), have been intensely studied, and the results claim that they are closely related with oral cancer risk (Al-Hebshi et al. 2017; Wen et al. 2020; Rai et al. 2021). Additionally, some fungal species, like C. albicans and viruses, such as human papillomavirus (HPV), are responsible for higher rates of oral cancer (Chaitanya et al. 2016; Chung et al. 2017; Mäkinen et al. 2018). The functional role of these microbes in promoting oral cancer and their potential mechanisms are discussed.
P. gingivalis is an anaerobic gram-negative bacterium which resides in the mouth and is closely associated with periodontal diseases (Tokutomi et al. 2015). P. gingivalis was previously reported to be carcinogenic as it activates inflammatory immune responses in the host (Mustapha et al. 2007). Based on this, Sayehmiri et al. (2015) investigated the significant association between P. gingivalis and oral cancer and found that P. gingivalis increased the chance of cancer development as much as 1.36 times. The underlying mechanisms of P. gingivalis promotion for oral cancer can be briefly summarized as enhancement of cell proliferation, suppression of apoptotic cell death, production of toxic metabolites and modulation of immune response (Lamont et al. 2022). The detail mechanism and pathways have been elaborated below.
Fusobacteria, one of the Gram-negative anaerobic bacteria, is often colonized in the oral cavity. They could modulate oral carcinogenesis and promote cancer progression. Several studies have shown that Fusobacteria are correlated with both the initiation and promotion stages of oral tumorigenesis. For example, certain species of Fusobacterium, such as F. nucleatum have been detected to be enriched in and around oral squamous cell carcinoma (OSCC) lesions compared to the healthy oral mucosa, suggesting that Fusobacterium species are the major pro-tumorigenic oral bacteria (Al-Hebshi et al. 2017). F. nucleatum is known to activate the expression of MYC proto-oncogene (MYC), Janus Kinase 1 (JAK1) and Signal transducer and activator of transcription 3 (STAT3), three oncogenes that have both anti-apoptotic and proliferative effects on cancer cells, increasing the risk of malignancy (Shirogane et al. 1999; Harrandah et al. 2020). In addition, F. nucleatum actives nuclear factor kappa-B (NF-κB) and upregulate microRNA (miR)-21 expression to promote the colorectal cancer cell proliferation (Yang et al. 2017). Besides, the protein F. nucleatum adhesin A (FadA) can bind to E-cadherin to activate the potentially oncogenic E-cadherin-β catenin signaling pathway and Wnt/β-catenin modulator annexin A, leading to carcinogenesis (Rubinstein et al. 2013, 2019). These findings suggest that Fusobacteria can modulate oral carcinogenesis and promote cancer progression (Harrandah et al. 2020; Li et al. 2022b).
Streptococcus species have been detected in the oral cavity of individuals with oral cancer and may be associated with oral carcinogenesis in specific cases (Tsai et al. 2022). For example, Streptococcus salivarius is identified to be correlated strongly with OSCC, while Streptococcus species are associated with various cancers, like oropharyngeal cancer (Pushalkar et al. 2012; Yang et al. 2018a). However, the role and mechanism of Streptococcus species in promoting the development and progression of cancers is still not fully understood.
The dominant C. albicans, the opportunistic human fungal pathogen in the oral microbiota, can increase the risk of oral cancers (Ramirez-Garcia et al. 2016). Specifically, C. albicans was reported to modulate oral carcinogenesis through several mechanisms. Firstly, it can elevate matrix metalloproteinase (MMP) expression to promote the invasion and metastasis of cancer cells (Hajari Taheri et al. 2013). It can also induce both inflammatory processes and oxidative stress, which can facilitate the mutation of normal cells, leading to tumor progression (Ramirez-Garcia et al. 2013). In addition, C. albicans can influence various cell signaling pathways and transcription factors, promoting cancer survival, invasion, and metastasis. For instance, the Kirsten rat sarcoma virus (KRAS) signaling pathway and down-stream E2 factor (E2F) target genes may be the main mechanisms underlying OSCC carcinogenesis with C. albicans infection (Hsieh et al. 2022).
Colorectal Cancer (CRC)
Colorectal cancer is the third leading cause of cancer-related deaths in both men and women. More than 1.9 million new colorectal cancer (including anus) cases and 935,000 deaths were estimated to occur in 2020 (Sung et al. 2021).
The microbiota of the oral cavity has a great influence on the progression of colorectal cancer by modulating different pathways, including inflammation, angiogenesis, immune system modulation, epithelial cell proliferation, apoptosis, metabolism and absorption of nutrients.
The clinical strategies used for CRC screening are often limited by the diagnostic miss rate of CRC, especially at the early stage, and high cost (Ladabaum et al. 2020). Moreover, enormous efforts have been made to explore the potential application of gut microbiota as non-invasive biomarkers for CRC (Liu et al. 2022). Increasing attention has been paid to the relationship between oral microbiota and CRC diagnosis. It has been shown that saliva-based biomarkers can predict colorectal cancer at the early stage of pre-cancerous lesions, which can help to improve early detection methods and allow for better outcomes (Rapado-González et al. 2019; Raza et al. 2022; Rezasoltani et al. 2022).
The oral microbiota may also affect treatment outcomes of colorectal cancer. Dong et al. (2021) reported that the change of oral microbiota could alter the intestinal bacterial composition at tumor site in CRC mouse models using 16S rRNA sequencing. In synergy with its intestinal counterparts, the oral microbiota impinges on the efficacy and prognosis of radiotherapy for CRC. Nevertheless, more efforts are required to explore how oral microbiota impacts the efficacy of different therapies towards CRC.
Lung Cancer
Lung cancer is one of the most severe cancer types attracting extensive public attention. It is the leading cause of cancer morbidity and mortality in men. For women, it ranks as the third for incidence, after breast and colorectal cancer, and the second for mortality, after breast cancer (Sung et al. 2021).
Several cohort studies revealed the correlation between oral microbiota and lung cancer, suggesting that lower alpha diversity of oral microbiota composition could increase the risk of lung cancer for never-smokers and eventually lead to malignant lung cancer (Yu et al. 2016; Hosgood et al. 2021). It is in line with another cohort study that identified the salivary microbiome of 75 non-smoking female lung cancer patients and 172 matched healthy individuals using 16S rRNA gene amplicon sequencing, which finally reported that non-smoking female lung cancer patients exhibited significantly lower oral microbial diversity (Yang et al. 2018b). Future investigations should aim to elucidate the exact mechanisms by which bacteria in the saliva influence lung cancer development to develop better diagnostic and therapeutic approaches for this condition. Specifically, certain species like Capnocytophaga, Veillonella and P. gingivalis have been significantly higher in the saliva of lung cancer patients, which can serve as potential biomarkers for the disease detection and classification (Yan et al. 2015; Liu et al. 2021b). Functional analysis revealed that the microbial metabolism was more diverse than the lung cancer group. However, cancer-related pathways, cell motility, and the cyclooxygenase (COX) inhibitor pathways were enriched in the lung cancer microbiome, giving more insights into the diagnosis, treatment, and management of lung cancer by regulating the oral microbiome (Jiang et al. 2022; Vogtmann et al. 2022).
The Potential Mechanism of Oral Microbiota Influencing Cancer
Some microorganisms in the oral cavity can directly activate oncogenes or integrate oncogenes into host genomes to induce amplification of host genes in pathways related to cancer progression, thus promoting tumor growth and metastasis (Garrett 2015).
Oral microbiota metabolic activities can also contribute to carcinogenesis. Some oral bacteria, like P. gingivalis, F. nucleatum, Prevotella intermedia and Aggregatibacter actinomycetemcomitans, can produce volatile sulfur compounds (VSCs), such as hydrogen sulfide (H2S), methyl mercaptan (CH3SH), which has toxicity and can cause genomic instability, further leading to carcinogenesis (Hellmich and Szabo 2015; Milella 2015; Karpiński 2019; Lin et al. 2023). In addition, some species of Lactobacillus, Lactococcus and Streptococcus, which produce lactic acid, cause lower pH levels in the oral cavity (Snel et al. 2011). The acidic microenvironment of tumors induced by the lactic and other acids promotes cancer development by increasing the metastatic efficiency of tumors and suppressing anticancer immunity (Lunt et al. 2009; Boedtkjer and Pedersen 2020; Wang et al. 2020). There also exist other specific metabolites secreted by oral microorganisms which could stimulate cancer progression. For example, FadA, uniquely secreted by F. nucleatum, induces oncogenic and inflammatory responses by modulating E-cadherin/β-catenin signaling to stimulate the growth of CRC cells (Rubinstein et al. 2013).
Besides, the oral microbiota modulates the pathogenesis of cancers, including cell cycle regulation, cell proliferation and cell apoptosis (Karpiński 2019). P. gingivalis, commonly found in oral microbiota that has been linked to OSCC, has cell cycle regulation ability and antiapoptotic activity, which could alter intrinsic mitochondrial apoptosis pathways by activating JAK1/AKT/STAT3 signaling (Yilmaz et al. 2004; Mao et al. 2007; Karpiński 2019). Specifically, Kuboniwa et al. (2008) demonstrated that P. gingivalis accelerated S-phase progression of the cell cycle through manipulation of cyclin/cyclin-dependent kinase (CDK) activity and reduced the level of the tumor suppressor protein p53, thereby promoting tumor development. P. gingivalis is able to promote the pro-survival and proliferation of cancer cells by suppressing p53 function (Olsen et al. 2016). And P. gingivalis has been shown to upregulate miR-203 expression in gingival epithelial cells, which increases the activity of STAT3 through downregulation of suppressor of cytokine signaling 3 (SOCS3) to inhibit apoptosis (Moffatt and Lamont 2011). It was shown that phosphorylation of the Bcl-2-associated death promoter (Bad) and caspase-9 caused by JAK1-induced activation of phosphoinositide 3-kinase (PI3K) have inhibited their pro-apoptotic properties (Yao et al. 2010). And the increased ratio of Bcl-2 to Bcl-2-associated X (BAX) caused by P. gingivalis infection could significantly reduce the apoptotic activity of epithelial cells (Nakhjiri et al. 2001; Yao et al. 2010). As for modulation of immune response, P. gingivalis gingipains (cysteine proteases) could regulate T cell anergy by upregulating of B7-H1 receptor in OSCC cells (Groeger et al. 2011). The immune system has been closely associated with microbe-associated molecular patterns (MAMPs), which include microorganism substances like lipopolysaccharide (LPS), peptidoglycan, bacterial DNA or RNA, and so on (Koliarakis et al. 2019). Receptors responsible for this are called pattern recognition receptors (PRRs) and are divided into numerous families, such as the Toll-like receptors (TLRs) (Underhill and Iliev 2014). F. nucleatum, one of the key bacteria in oral microbiota, could affect CRC tumorigenesis through three major components: Fap2, FadA and LPS. LPS can interact with TLRs, activating the MyD88 and NF-κΒ pathway. This interaction leads to reduced caspase activity and increased autophagy, resulting in reduced apoptosis (Chen et al. 2017; Koliarakis et al. 2019). And LPS binds with the T cell immunoglobulin and ITIM domain (TIGIT) receptor of natural killer (NK) and T cells, leading to suppression of anti-tumor immunity (Koliarakis et al. 2019). FadA binds to E-cadherin, causing dephosphorylation and activation of β-catenin (Rubinstein et al. 2013). NF-κΒ and β-catenin increase the synthesis of pro-inflammatory cytokines like interleukin (IL)-1β, IL-6, IL-8, IL-18, tumor necrosis factor (TNF)-α and upregulate oncogenic pathways of Cmyc/CyclinD and miR-21 (Chen et al. 2017). The pro-inflammatory state is further enhanced by the binding of Fap2 to Gal-GalNAc (Abed et al. 2016).
Oral Microbiota as a Biomarker and Therapeutic Target
Flemer et al. (2018) reported that the heterogeneity of CRC was closely related with certain oral microorganism like Lachnospiraceae and suggested that the oral microbiota acted as an alternative screen for detecting CRC by profiling the oral, colonic and fecal microbiota from CRC patients, colorectal polyps patients, and healthy controls, respectively. This study constructed a classification model with oral swab microbiota which could distinguish individuals with CRC or polyps from controls with high sensitivity: 53% (CRC)/67% (polyps) and 96% specificity. Further combination of the data from fecal microbiota can even increase sensitivity of this model to 76% (CRC)/88% (polyps) (Flemer et al. 2018). Consistently, another study, assessing the oral microbiota from CRC patients, colorectal adenoma (CRA) patients, and healthy controls with 16S rRNA sequencing, has constructed a random forest model to distinguish CRC patients and healthy subjects. This study concluded that oral microbiota-based biomarkers could predict the risks for developing CRC (Zhang et al. 2020). In addition to CRC, the oral microbiota also provides a new diagnostic tool for other cancer types like OSCC, throat cancer and so on (Yang et al. 2018a; Wang et al. 2019). Wang et al. (2019) demonstrated that the salivary microbiota of throat cancer patients was significantly different from those with polyps or healthy subjects. Based on putatively important constituent bacteria such as Aggregatibacter, Pseudomonas, Bacteroides, and Ruminiclostridium, they constructed diagnostic models with 87.5% accuracy [area under the curve (AUC) = 0.875, 95% confidence interval (CI): 0.695–1.000] for early diagnosis of throat cancer. Yang et al. (2018a) selected a bacterial marker panel with three bacterial species, F. periodonticum, S. mitis, and P. pasteri, resulting in an AUC of 0.956 (95% CI = 0.925–0.986) in discriminating OSCC stage four with the healthy controls. However, the technology for early detection of malignant tumor by oral microbiota remains at its infancy stage, there is a predilection of particular species of cancer (Zhang et al. 2020).
The oral microbiota can also be a therapeutic target for treating diseases (Willis and Gabaldón 2020). Therapies like application of oral probiotic candidates in shaping the chemistry of the oral microenvironment has been proposed (Campbell 2021). Introducing commercially available probiotics, such as Bifidobacterium, Lactobacillus, and Streptococcus, can increase the alpha diversity of oral microbiota, which is potential to treat diseases by alteration of the oral microbiota composition (Dassi et al. 2018). It is becoming a promising therapeutic strategy against cancer, especially oral cancer, by targeting the oral pathogens such as F. nucleatum, Streptococcus mutans, Neisseria meningitidis, and their virulence factors (Chattopadhyay et al. 2019). However, oral microbiota application remains challenging as the human microbiota is a complex and multi-dimensional interconnected micro-ecological system. In addition, the cancer therapeutic approaches related with microbiota differ in effectiveness from person to person. Thus, the individualized therapy should not be neglected. Current approaches for profiling and therapeutic targeting focus mainly on limited genomic and immune profiling, without enough consideration of the consistent profiling or targeting of microorganisms residing in tissues, tumors, or the gut (Giannakis et al. 2016; Chen et al. 2016). With the emerging role of microbiota in carcinogenesis and anti-cancer therapy, the role of microbiota should be included in the individualized therapy together with diet, stress, and other lifestyle factors (Park et al. 2022). Besides, perturbations of the oral microbiota may have unforeseen effects, which means this novel strategy's long-term implications and side effects are still unclear (Willis and Gabaldón 2020). Therefore, the application of the oral microbiota in cancer prevention and treatment awaits more large-scale and longitudinal studies.
Conclusion
Increasing evidence has demonstrated the crucial role of oral microbiota in human health. The oral microbiota interacts dynamically with oral microenvironment, which was affected with many factors, including maternal transmission, diet and so on, thus influencing host health.
As polymorphic microbiomes are listed as one of the hallmarks of cancer, the correlation of oral microbiota and cancer has emerging (Hanahan 2022). The oral microbiota is found to be associated with various cancer types, like oral cancer, colorectal cancer, lung cancer, which has been elaborated in detail above. There are other cancer types like gastric cancer, pancreatic cancer, breast cancer also have correlations with oral microbiota (Li et al. 2022a). Although current studies have revealed the underlying mechanisms contributing to carcinogenesis and modulation of cancers pathogenesis such as host genetic regulation, oral microbiota metabolic activities and modulation of cancer pathogenesis, including cell cycle regulation, cell proliferation and cell apoptosis (Milella 2015; Garrett 2015; Karpiński 2019). Investigation of the role of oral microbiota associated with the cancer progression remains at its infancy. And these mechanisms cannot explain the tumorigenesis of all cancer types, so more researches are needed to concentrate on this concern.
With the deepening research on the correlation between oral microbiota and cancer, researchers are gradually focusing their attention to the application of oral microbiota in cancer diagnosis and treatment. Cancer diagnosis, especially early-stage diagnosis, remains a major challenge. At present, many studies have found that certain key oral microorganisms play a role in specific cancer types, and by utilizing these key microorganisms we can establish new diagnosis models for early-stage cancer. For instance, the diagnostic model combining Aggregatibacter, Pseudomonas, Bacteroides, and Ruminiclostridium, which are key oral microorganisms in throat cancer, has 87.5% accuracy for early diagnosis of throat cancer (Wang et al. 2019). Oral microbiota has also become a promising therapeutic strategy for cancer. Oral probiotics and oral microbiota transplantation have been proposed as new cancer therapies (Mohammed et al. 2018; Campbell 2021). But more efforts are required to identify novel biomarkers in oral microbiota communities and therapeutic strategies related with oral microbiota for cancer diagnosis and treatment.
Now most researches on the relationship between oral microbiota and the occurrence and development of cancer are correlational studies. More studies should focus on the molecular mechanisms to find the causality between oral microbiota and tumorigenesis. In addition, the application of oral microbiota in cancer diagnosis and treatment is still in the testing stage, that needs more researchers committed to transforming research results into medical technology, bringing hope to more cancer patients.
Acknowledgements
This work was supported by MOST Key R&D Program of China (2022YFC2304703), National Natural Science Foundation of China (32270202), Innovative research team of high-level local universities in Shanghai. The authors are thankful for the support from all lab members in Liu Lab (Fun Guy Group) in Shanghai Jiao Tong University School of Medicine.
Abbreviations
- Bad
Bcl-2-associated death promoter
- BAX
Bcl-2-associated X
- BRAF
B-Raf proto-oncogene
- C. albicans
Candida albicans
- CCA
Constrained correspondence analysis
- CDK
Cyclin-dependent kinase
- CH3SH
Methyl mercaptan
- COX
Cyclooxygenase
- CRA
Colorectal adenoma
- CRC
Colorectal cancer
- E2F
E2 factor
- EPS
Extracellular polymeric substances
- FadA
Fusobacterium nucleatum Adhesin A
- F. nucleatum
Fusobacterium nucleatum
- HMP
Human Microbiome Project
- HPV
Human papillomavirus
- hRAS
HRas proto-oncogene
- H2S
Hydrogen sulfide
- JAK1
Janus kinase 1
- KRAS
Kirsten rat sarcoma virus
- LPS
Lipopolysaccharide
- MAMPs
Microbe-associated molecular patterns
- MMP
Matrix metalloproteinase
- mTOR
Mechanistic target of rapamycin kinase
- MYC
MYC proto-oncogene
- NK
Natural killer
- OSCC
Oral squamous cell carcinoma
- P. gingivalis
Porphyromonas gingivalis
- PI3K
Phosphoinositide 3-kinase
- PI3KCA
Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha
- PRRs
Pattern recognition receptors
- rRNA
Ribosomal RNA
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- STAT3
Signal transducer and activator of transcription 3
- Streptococcus sp.
Streptococcus Species
- TIGIT
T cell immunoglobulin and ITIM domain
- TLRs
Toll-like receptors
- VSCs
Volatile sulfur compounds
Authors' Contributions
NL conceived and designed the manuscript. XW and HX wrote the manuscript and drafted the figures and tables. NL revised the manuscript. All authors have read and approved the final manuscript.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Code availability
Not applicable.
Declarations
Conflict of interest
No conflicts of interest to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Aagaard K, Ma J, Antony KM et al (2014) The placenta harbors a unique microbiome. Sci Transl Med 6. 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed]
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abed J, Emgård JEM, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215–225. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SE, Arnold D, Murphy B, et al. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci Rep. 2017;7:43344. doi: 10.1038/srep43344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7:1834. doi: 10.1038/s41598-017-02079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya Arbeláez MI, de Paula E, Silva ACA, Navegante G, et al. Proto-oncogenes and cell cycle gene expression in normal and neoplastic oral epithelial cells stimulated with soluble factors from single and dual biofilms of Candida albicans and Staphylococcus aureus. Front Cell Infect Microbiol. 2021;11:627043. doi: 10.3389/fcimb.2021.627043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M, Dongari-Bagtzoglou A. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. Adv Exp Med Biol. 2019;1197:69–78. doi: 10.1007/978-3-030-28524-1_6. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes ZLS, Belfield LA, Ashworth A, et al. Effects of chlorhexidine mouthwash on the oral microbiome. J Dent. 2021;113:103768. doi: 10.1016/j.jdent.2021.103768. [DOI] [PubMed] [Google Scholar]
- Burne RA, Zeng L, Ahn SJ, et al. Progress dissecting the oral microbiome in caries and health. Adv Dent Res. 2012;24:77–80. doi: 10.1177/0022034512449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K (2021) Oral microbiome findings challenge dentistry dogma. Nature. 10.1038/d41586-021-02920-w [DOI] [PubMed]
- Chaitanya NCSK, Allam NSJ, Gandhi Babu DB, et al. Systematic meta-analysis on association of human papilloma virus and oral cancer. J Cancer Res Ther. 2016;12:969–974. doi: 10.4103/0973-1482.179098. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat. 2019;18:153303381986735. doi: 10.1177/1533033819867354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Peng Y, Yu J et al (2017) Invasive Fusobacteriumnucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 8:31802–31814. 10.18632/oncotarget.15992 [DOI] [PMC free article] [PubMed]
- Cheung MK, Chan JYK, Wong MCS, et al. Determinants and interactions of oral bacterial and fungal microbiota in healthy Chinese adults. Microbiol Spectr. 2022;10:e0241021. doi: 10.1128/spectrum.02410-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung LM, Liang JA, Lin CL, et al. Cancer risk in patients with candidiasis: a nationwide population-based cohort study. Oncotarget. 2017;8:63562–63573. doi: 10.18632/oncotarget.18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby PMA, Biesbrock A, Bartizek R, et al. Treatment outcomes of dental flossing in twins: molecular analysis of the interproximal microflora. J Periodontol. 2008;79:1426–1433. doi: 10.1902/jop.2008.070585. [DOI] [PubMed] [Google Scholar]
- Corrêa JD, Calderaro DC, Ferreira GA, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. 2017;5:34. doi: 10.1186/s40168-017-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassi E, Ferretti P, Covello G, et al. The short-term impact of probiotic consumption on the oral cavity microbiome. Sci Rep. 2018;8:10476. doi: 10.1038/s41598-018-28491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Materna AC, Friedman J, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Li Y, Xiao H, et al. Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep. 2021;37:109886. doi: 10.1016/j.celrep.2021.109886. [DOI] [PubMed] [Google Scholar]
- Du Q, Ren B, He J, et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021;15:894–908. doi: 10.1038/s41396-020-00823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzidic M, Collado MC, Abrahamsson T, et al. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018;12:2292–2306. doi: 10.1038/s41396-018-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshriqui I, Viljakainen HT, Ferreira SRG, et al. Breastfeeding may have a long-term effect on oral microbiota: results from the Fin-HIT cohort. Int Breastfeed J. 2020;15:42. doi: 10.1186/s13006-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemer B, Warren RD, Barrett MP, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67:1454–1463. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Fregatto LF, Costa IB, De Bortoli TD, et al. Oral hygiene and oral microbiota in children and young people with neurological impairment and oropharyngeal dysphagia. Sci Rep. 2021;11:18090. doi: 10.1038/s41598-021-97425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami W, Nishikawa K, Ozawa S, et al. Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J Oral Biosci. 2021;63:175–183. doi: 10.1016/j.job.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger S, Domann E, Gonzales JR, et al. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology. 2011;216:1302–1310. doi: 10.1016/j.imbio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Gross EL, Beall CJ, Kutsch SR, et al. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, McLean JS, Lux R, et al. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep. 2015;5:18015. doi: 10.1038/srep18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajari Taheri F, Seyedolmohadesin M, Bayat M, et al. The effect of Candida albicans systemic infection on matrix metalloproteinases in breast cancer bearing BALB/c mice. Iran J Allergy Asthma Immunol. 2013;12:81–85. [PubMed] [Google Scholar]
- Hallang S, Esberg A, Haworth S, Johansson I. Healthy oral lifestyle behaviours are associated with favourable composition and function of the oral microbiota. Microorganisms. 2021;9:1674. doi: 10.3390/microorganisms9081674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- Harrandah AM, Chukkapalli SS, Bhattacharyya I, et al. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol. 2020;13:1849493. doi: 10.1080/20002297.2020.1849493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich MR, Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosgood HD, Cai Q, Hua X, et al. Variation in oral microbiome is associated with future risk of lung cancer among never-smokers. Thorax. 2021;76:256–263. doi: 10.1136/thoraxjnl-2020-215542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y-P, Wu Y-H, Cheng S-M, et al. Single-cell RNA sequencing analysis for oncogenic mechanisms underlying oral squamous cell carcinoma carcinogenesis with Candida albicans infection. Int J Mol Sci. 2022;23:4833. doi: 10.3390/ijms23094833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus MM, Crielaard W, Volgenant CMC, et al. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9:1270613. doi: 10.1080/20002297.2016.1270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang Y, Wang H et al (2022) In-depth metaproteomics analysis of oral microbiome for lung cancer. Research (Wash DC) 2022:9781578. 10.34133/2022/9781578 [DOI] [PMC free article] [PubMed]
- Kaan AMM, Kahharova D, Zaura E (2021) Acquisition and establishment of the oral microbiota. Periodontology 2000 86:123–141. 10.1111/prd.12366 [DOI] [PMC free article] [PubMed]
- Karpiński TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7:20. doi: 10.3390/microorganisms7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliarakis I, Messaritakis I, Nikolouzakis TK, et al. Oral bacteria and intestinal dysbiosis in colorectal cancer. IJMS. 2019;20:4146. doi: 10.3390/ijms20174146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92:1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Hasegawa Y, Mao S, et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158:418–432. doi: 10.1053/j.gastro.2019.06.043. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Fitzsimonds ZR, Wang H, Gao S (2022) Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 89:154–165. 10.1111/prd.12425 [DOI] [PMC free article] [PubMed]
- Li S, He M, Lei Y, et al. Oral microbiota and tumor—a new perspective of tumor pathogenesis. Microorganisms. 2022;10:2206. doi: 10.3390/microorganisms10112206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu Y, Zhang L (2022b) Role of the microbiome in oral cancer occurrence, progression and therapy. Microb Pathog 169:105638. 10.1016/j.micpath.2022.105638 [DOI] [PubMed]
- Lif Holgerson P, Harnevik L, Hernell O, et al. Mode of birth delivery affects oral microbiota in infants. J Dent Res. 2011;90:1183–1188. doi: 10.1177/0022034511418973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Chen J, Zhou X, Li Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit Rev Microbiol. 2021;47:667–677. doi: 10.1080/1040841X.2021.1915959. [DOI] [PubMed] [Google Scholar]
- Lin H, Yu Y, Zhu L et al (2023) Implications of hydrogen sulfide in colorectal cancer: mechanistic insights and diagnostic and therapeutic strategies. Redox Biol 59:102601. 10.1016/j.redox.2023.102601 [DOI] [PMC free article] [PubMed]
- Liu X, Tong X, Zhu J, et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021;7:117. doi: 10.1038/s41421-021-00356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NN, Jiao N, Tan JC, et al. Multi-kingdom microbiota analyses identify bacterial–fungal interactions and biomarkers of colorectal cancer across cohorts. Nat Microbiol. 2022;7:238–250. doi: 10.1038/s41564-021-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yuan X, Chen K et al (2021b) Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl Oncol 14:100972. 10.1016/j.tranon.2020.100972 [DOI] [PMC free article] [PubMed]
- Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- Macgregor ID. Effects of smoking on oral ecology. A review of the literature. Clin Prev Dent. 1989;11:3–7. [PubMed] [Google Scholar]
- Mäkinen A, Nawaz A, Mäkitie A, Meurman JH. Role of non-Albicans Candida and Candida albicans in oral squamous cell cancer patients. J Oral Maxillofac Surg. 2018;76:2564–2571. doi: 10.1016/j.joms.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark Welch JL, Rossetti BJ, Rieken CW, et al. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascitti M, Togni L, Troiano G, et al. Beyond head and neck cancer: the relationship between oral microbiota and tumour development in distant organs. Front Cell Infect Microbiol. 2019;9:232. doi: 10.3389/fcimb.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- Milella L. The negative effects of volatile sulphur compounds. J Vet Dent. 2015;32:99–102. doi: 10.1177/089875641503200203. [DOI] [PubMed] [Google Scholar]
- Millen AE, Dahhan R, Freudenheim JL, et al. Dietary carbohydrate intake is associated with the subgingival plaque oral microbiome abundance and diversity in a cohort of postmenopausal women. Sci Rep. 2022;12:2643. doi: 10.1038/s41598-022-06421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed H, Varoni E, Cochis A, et al. Oral dysbiosis in pancreatic cancer and liver cirrhosis: a review of the literature. Biomedicines. 2018;6:115. doi: 10.3390/biomedicines6040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moossavi S, Sepehri S, Robertson B, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe. 2019;25:324–335.e4. doi: 10.1016/j.chom.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol. 2007;78:2289–2302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Nelson-Filho P, Borba IG, de Mesquita KSF, et al. Dynamics of microbial colonization of the oral cavity in newborns. Braz Dent J. 2013;24:415–419. doi: 10.1590/0103-6440201302266. [DOI] [PubMed] [Google Scholar]
- Nelun Barfod M, Magnusson K, Lexner MO, et al. Oral microflora in infants delivered vaginally and by caesarean section: delivery mode and microbial profile. Int J Pediatr Dent. 2011;21:401–406. doi: 10.1111/j.1365-263X.2011.01136.x. [DOI] [PubMed] [Google Scholar]
- Ogbanga N, Nelson A, Ghignone S et al (2023) The oral microbiome for geographic origin: an Italian study. Forensic Sci Int Genet 64:102841. 10.1016/j.fsigen.2023.102841 [DOI] [PubMed]
- Olsen I, Taubman MA, Singhrao SK. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J Oral Microbiol. 2016;8:33029. doi: 10.3402/jom.v8.33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EM, Chelvanambi M, Bhutiani N, et al. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- Pasolli E, Asnicar F, Manara S, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176:649–662.e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AK, Panda M, Das AK, et al. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch Microbiol. 2021;203:137–152. doi: 10.1007/s00203-020-02011-w. [DOI] [PubMed] [Google Scholar]
- Ramadugu K, Bhaumik D, Luo T, et al. Maternal oral health influences infant salivary microbiome. J Dent Res. 2021;100:58–65. doi: 10.1177/0022034520947665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Garcia A, Rementeria A, Aguirre-Urizar JM, et al. Candida albicans and cancer: can this yeast induce cancer development or progression? Crit Rev Microbiol. 2016;42:181–193. doi: 10.3109/1040841X.2014.913004. [DOI] [PubMed] [Google Scholar]
- Ramirez-Garcia A, Arteta B, Abad-Diaz-de-Cerio A et al (2013) Candida albicans increases tumor cell adhesion to endothelial cells in vitro: intraspecific differences and importance of the mannose receptor. PLoS One 8:e53584. 10.1371/journal.pone.0053584 [DOI] [PMC free article] [PubMed]
- Rao C, Coyte KZ, Bainter W, et al. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature. 2021;591:633–638. doi: 10.1038/s41586-021-03241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapado-González Ó, Majem B, Álvarez-Castro A, et al. A novel saliva-based miRNA signature for colorectal cancer diagnosis. J Clin Med. 2019;8:2029. doi: 10.3390/jcm8122029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Khan AQ, Inchakalody VP, et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J Exp Clin Cancer Res. 2022;41:99. doi: 10.1186/s13046-022-02318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezasoltani S, Aghdaei HA, Jasemi S, et al. Oral microbiota as novel biomarkers for colorectal cancer screening. Cancers (Basel) 2022;15:192. doi: 10.3390/cancers15010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MR, Baik JE, Lagana SM et al (2019) Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep 20:e47638. 10.15252/embr.201847638 [DOI] [PMC free article] [PubMed]
- Sayehmiri F, Sayehmiri K, Asadollahi K, et al. The prevalence rate of Porphyromonas gingivalis and its association with cancer: a systematic review and meta-analysis. Int J Immunopathol Pharmacol. 2015;28:160–167. doi: 10.1177/0394632015586144. [DOI] [PubMed] [Google Scholar]
- Sedghi L, DiMassa V, Harrington A et al (2021) The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontology 2000 87:107–131. 10.1111/prd.12393 [DOI] [PMC free article] [PubMed]
- Shirogane T, Fukada T, Muller JM, et al. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11:709–719. doi: 10.1016/s1074-7613(00)80145-4. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Snel J, Marco ML, Kingma F, et al. Competitive selection of lactic acid bacteria that persist in the human oral cavity. Appl Environ Microbiol. 2011;77:8445–8450. doi: 10.1128/AEM.06043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulyanto RM, Thompson ZA, Beall CJ, et al. The predominant oral microbiota is acquired early in an organized pattern. Sci Rep. 2019;9:10550. doi: 10.1038/s41598-019-46923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- Tierney BT, Yang Z, Luber JM, et al. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe. 2019;26:283–295.e8. doi: 10.1016/j.chom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutomi F, Wada-Takahashi S, Sugiyama S, et al. Porphyromonas gingivalis-induced alveolar bone loss is accelerated in the stroke-prone spontaneously hypertensive rat. Arch Oral Biol. 2015;60:911–918. doi: 10.1016/j.archoralbio.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Triarico S, Agresti P, Rinninella E, et al. Oral microbiota during childhood and its role in chemotherapy-induced oral mucositis in children with cancer. Pathogens. 2022;11:448. doi: 10.3390/pathogens11040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Chen YY, Chen WC, Chen MF. Streptococcus mutans promotes tumor progression in oral squamous cell carcinoma. J Cancer. 2022;13:3358–3367. doi: 10.7150/jca.73310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Collado MC, Rautava J, et al. Composition and maternal origin of the neonatal oral cavity microbiota. J Oral Microbiol. 2019;11:1663084. doi: 10.1080/20002297.2019.1663084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtmann E, Hua X, Yu G, et al. The oral microbiome and lung cancer risk: an analysis of 3 prospective cohort studies. J Natl Cancer Inst. 2022;114:1501–1510. doi: 10.1093/jnci/djac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gao Y, Zhao F. Phage–bacteria interaction network in human oral microbiome. Environ Microbiol. 2016;18:2143–2158. doi: 10.1111/1462-2920.12923. [DOI] [PubMed] [Google Scholar]
- Wang K, Lu W, Tu Q, et al. Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus. Sci Rep. 2016;6:22943. doi: 10.1038/srep22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yin G, Guo Y, et al. Variations in oral microbiota composition are associated with a risk of throat cancer. Front Cell Infect Microbiol. 2019;9:205. doi: 10.3389/fcimb.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Choi SYC, Niu X, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21:8363. doi: 10.3390/ijms21218363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Mu W, Lu H, et al. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99:666–675. doi: 10.1177/0022034520909312. [DOI] [PubMed] [Google Scholar]
- Widyarman AS, Theodorea CF, Udawatte NS et al (2021) Diversity of oral microbiome of women from urban and rural areas of Indonesia: a pilot study. Front Oral Health 2:738306. 10.3389/froh.2021.738306 [DOI] [PMC free article] [PubMed]
- Willis JR, Gabaldón T. The human oral microbiome in health and disease: from sequences to ecosystems. Microorganisms. 2020;8:308. doi: 10.3390/microorganisms8020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Peters BA, Dominianni C, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016;10:2435–2446. doi: 10.1038/ismej.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Yang M, Liu J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5:3111–3122. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152:851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Yeh YM, Yu HY, et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9:862. doi: 10.3389/fmicb.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mu X, Wang Y, et al. Dysbiosis of the salivary microbiome is associated with non-smoking female lung cancer and correlated with immunocytochemistry markers. Front Oncol. 2018;8:520. doi: 10.3389/fonc.2018.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Jermanus C, Barbetta B, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Gail MH, Consonni D, et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016;17:163. doi: 10.1186/s13059-016-1021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Phillips S, Gail MH, et al. The effect of cigarette smoking on the oral and nasal microbiota. Microbiome. 2017;5:3. doi: 10.1186/s40168-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu Y, Zheng HJ, Zhang CP. The oral microbiota may have influence on oral cancer. Front Cell Infect Microbiol. 2019;9:476. doi: 10.3389/fcimb.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kong C, Yang Y, et al. Human oral microbiome dysbiosis as a novel non-invasive biomarker in detection of colorectal cancer. Theranostics. 2020;10:11595–11606. doi: 10.7150/thno.49515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Tian L, Chen P, et al. Over 50,000 metagenomically assembled draft genomes for the human oral microbiome reveal new taxa. Genom Proteom Bioinform. 2022;20:246–259. doi: 10.1016/j.gpb.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Not applicable.