Abstract
Background
Prior sexual trauma (ST) is associated with greater risk for posttraumatic stress disorder after a subsequent traumatic event; however, the underlying neurobiological mechanisms remain opaque. We investigated longitudinal posttraumatic dysfunction and amygdala functional dynamics following admission to an emergency department for new primarily nonsexual trauma in participants with and without previous ST.
Methods
Participants (N = 2178) were recruited following acute trauma exposure (primarily motor vehicle collision). A subset (n = 242) completed magnetic resonance imaging that included a fearful faces task and a resting-state scan 2 weeks after the trauma. We investigated associations between prior ST and several dimensions of posttraumatic symptoms over 6 months. We further assessed amygdala activation and connectivity differences between groups with or without prior ST.
Results
Prior ST was associated with greater posttraumatic depression (F1,1120 = 28.35, p = 1.22 × 10−7, ηp2 = 0.06), anxiety (F1,1113 = 17.43, p = 3.21 × 10−5, ηp2 = 0.05), and posttraumatic stress disorder (F1,1027 = 11.34, p = 7.85 × 10−4, ηp2 = 0.04) severity and more maladaptive beliefs about pain (F1,1113 = 8.51, p = .004, ηp2 = 0.02) but was not related to amygdala reactivity to fearful versus neutral faces (all ps > .05). A secondary analysis revealed an interaction between ST and lifetime trauma load on the left amygdala to visual cortex connectivity (peak Z value: −4.41, corrected p < .02).
Conclusions
Findings suggest that prior ST is associated with heightened posttraumatic dysfunction following a new trauma exposure but not increased amygdala activity. In addition, ST may interact with lifetime trauma load to alter neural circuitry in visual processing regions following acute trauma exposure. Further research should probe the relationship between trauma type and visual circuitry in the acute aftermath of trauma.
Keywords: Longitudinal study, Neuroimaging, Posttraumatic stress disorder, Sexual trauma
Approximately 1 in 3 women and 1 in 8 men in the United States have experienced rape, sexual assault, or childhood sexual abuse (CSA) during their lifetime (1, 2, 3, 4, 5, 6). Sexually traumatic events carry the greatest conditional risk for posttraumatic stress disorder (PTSD) development compared with all other types of trauma (7, 8, 9) and are associated with greater general posttraumatic dysfunction (i.e., depression, anxiety) than other types of trauma (2,10, 11, 12). Furthermore, sexual trauma (ST) may compound risk for psychopathology after a new, subsequent trauma [e.g., motor vehicle collision; (13,14)]. Given that 50% to 90% of the population will experience a traumatic event in their lifetime (7,15,16), the likelihood of experiencing a subsequent traumatic event after prior ST is high. Although recent neuroimaging studies conducted in the early aftermath of trauma have revealed several neural predictors of posttraumatic dysfunction, whether and how prior ST may modulate these effects in individuals is still unknown. Understanding the interplay between prior ST and subsequent trauma may contribute to more accurate predictive models of individual susceptibility to PTSD.
The fact that ST is likely to result in PTSD is well established by existing literature. Prior work has found that approximately 94% of female rape survivors met symptomatic criteria for PTSD 2 weeks posttrauma, with approximately 50% continuing to meet criteria 3 months later (17,18). ST is also associated with increased depression and anxiety (2,19,20) as well as symptoms of dissociation (21). Significant associations between CSA and psychological distress in adults have been described, including anxiety, depression, PTSD, and dissociation (22, 23, 24). The apparent preexisting elevated risk for psychopathology in survivors of ST may affect the course of posttraumatic dysfunction after a new trauma and may be better elucidated through the identification of neural signatures. In particular, identifying neural signatures of risk for PTSD through functional neuroimaging may contribute to the ability to implement targeted early interventions for those most at risk.
Prior research suggests that the amygdala, a region critical to threat learning and expression, may be a major neural substrate of PTSD vulnerability (25,26). Earlier functional neuroimaging studies of PTSD conducted in small samples have shown that the amygdala is hyperactive in response to emotional stimuli, leading to symptoms of hyperarousal, heightened fear, and impairments in emotional regulation in individuals with the disorder (27,28). More recent work conducted with larger samples further suggests that reactivity to fearful faces may help to distinguish between biotypes of PTSD risk (29). Studies with large samples have also found neighborhood disadvantage in children to be related to blunted amygdala reactivity (30) and greater PTSD symptoms in a highly trauma-exposed population to be related to faster amygdala habituation to repeated fearful faces (31), suggesting that childhood adversity (e.g., CSA) may be predictive of a more blunted than a hyperactive response. In prospective studies, amygdala reactivity to emotional stimuli has emerged as predictive of PTSD when magnetic resonance imaging (MRI) scans were done both prior to exposure to a traumatic event (32) and in the early aftermath of trauma (33). Alterations in amygdala connectivity to regions including the prefrontal cortex (PFC) and dorsal anterior cingulate cortex have also been demonstrated in individuals with PTSD (34,35). In women, decreased functional connectivity (FC) between the right amygdala and left ventromedial PFC during threat processing was associated with PTSD (36). In the early aftermath of trauma, greater resting-state FC (rsFC) between the dorsal lateral PFC and an arousal network, which encompassed the amygdala, hippocampus, mamillary bodies, midbrain, and pons, was associated with reduced PTSD symptoms 3 months later (37). Furthermore, the interconnections between the amygdala and visual circuitry are particularly relevant for processing of threat-relevant visual stimuli in PTSD, and recent work, including in the AURORA study, has identified the role of the structure of the ventral visual stream in PTSD symptoms over time (38,39). For example, a study comparing visual processing in individuals with and without PTSD found that activity in response to visual scenes was lower in participants with PTSD in the ventral stream of the visual system (40). Thus, prior research suggests that amygdala reactivity and connectivity to other brain regions, which are important for the appraisal and regulation of threat, may play an important role in the development of posttraumatic psychopathology.
Despite the importance of previous imaging work, lack of consideration for potential effects of prior ST is a critical limitation of the current literature. Understanding the modulatory effects of prior ST on future PTSD symptom development and amygdala function in the acute aftermath of a new trauma may help inform more targeted clinical and neuromodulatory interventions. ST has previously been linked to alterations in amygdala function that may be important for understanding PTSD vulnerability. More specifically, prior work found that adolescent girls who had experienced physical or sexual assault and had greater amygdala reactivity to faces showed less symptom improvement after therapy (41). Furthermore, physical and sexual assault victims with greater amygdala reactivity to negative stimuli had more PTSD symptoms (42). However, these studies examined the effects of a single trauma episode and did not consider previous life events or subsequent traumatic events. The lack of neuroimaging studies of ST survivors after later trauma, particularly longitudinal neuroimaging studies, represents a gap in knowledge that must be filled to elucidate potential mechanisms of vulnerability to adverse neural sequelae in ST survivors following other traumatic event.
In the current study, we investigated the impact of prior ST on posttraumatic pathology including PTSD, depression, anxiety, and dissociation following an acute traumatic event requiring an emergency department (ED) evaluation in a multisite, longitudinal study. We also assessed differences in amygdala reactivity to fearful compared with neutral faces and amygdala rsFC between those with and without a history of ST. We hypothesized that individuals with a history of ST would show greater symptoms of posttraumatic dysfunction persisting for 6 months following the acute trauma. We also hypothesized that those with ST would have greater activation in the amygdala to fearful compared with neutral faces than the group without ST. We anticipated that ST would modulate the rsFC of the amygdala to other regions involved in threat processing (i.e., PFC, hippocampus, visual cortex). The study findings demonstrate that prior ST exacerbates the psychological impact of subsequent trauma and contributes to variability in the neural circuitry of PTSD in the early aftermath of trauma, making it an important consideration in the evaluation of recent trauma victims.
Methods and Materials
Participants
Participants were recruited from EDs across the United States as part of a longitudinal, multisite parent study of adverse neuropsychiatric sequelae (the AURORA study) (43). Participants who were 18 to 65 years of age were recruited from an ED after experiencing a qualifying traumatic event including a motor vehicle collision, sexual assault, physical assault, a fall of 10 feet or more, or a mass casualty incident. Other traumatic events were also qualifying if the event involved actual or threatened death, serious injury, or sexual violence exposure, either by direct exposure, witnessing, or learning about it, as reported by the participant and agreed upon by the research assistant who was doing the assessment. Full details of the recruitment protocol have been described previously (43).
A total of 2626 participants who were recruited for the AURORA study between September 2017 and July 31, 2020, were included in this investigation (see the Supplement for details on the overall sample). A subset of participants (n = 436) were invited to complete an MRI data collection session. Additional MRI exclusion criteria included a history of seizures, epilepsy, Alzheimer’s disease, dementia, or Parkinson’s disease, and any MRI contraindication (e.g., metal or ferromagnetic implants, pregnancy, claustrophobia). MRI participants were scanned within 2 weeks of their ED visit for acute trauma at 1 of 5 imaging sites. Of the invited participants, 54 participants did not provide task or resting-state functional MRI (fMRI) data and were therefore excluded. We excluded an additional 92 participants for incomplete scan data, motion criteria, or for other behavioral, anatomical, or technical reasons (complete criteria are detailed in the Supplement). Complete data on ST, our primary variable of interest, were missing for an additional 48 participants; therefore, we excluded these participants from analyses (see details below for classification of ST). The final imaging sample consisted of 242 participants (Table 1). Most participants were recruited after a motor vehicle collision (70.3%), and slightly more than half (55.4%) reported having experienced at least 1 instance of prior ST as indexed by the Life Events Checklist for DSM-5 and the Childhood Trauma Questionnaire (see details below). In the final sample, only 2 participants (0.83%) were recruited from the ED following a sexual assault, and index trauma type did not differ between the prior ST group and the group without prior ST (p = .61) (see Table 1). Thus, we were unable to consider the polyvictimization aspect of prior ST in relation to posttraumatic dysfunction. Participants provided written informed consent, and all study procedures were approved by each site’s institutional review board.
Table 1.
Participant Demographics and Sample Characteristics in the Imaging Sample (n = 242)
| Characteristic | Prior Sexual Trauma, n = 134 | No Prior Sexual Trauma, n = 108 | p Value (ANOVA, χ2) |
|---|---|---|---|
| Gender | .001 | ||
| Man | 32 (23.88%) | 48 (44.44%) | |
| Woman | 102 (76.12%) | 60 (55.56%) | |
| Age, Years | 35.79 (12.50) | 33.86 (12.63) | .24 |
| Race/Ethnicity | .35 | ||
| Non-Hispanic Black | 52 (38.81%) | 54 (50.47%) | |
| Non-Hispanic White | 52 (38.81%) | 33 (30.84%) | |
| Hispanic | 24 (17.91%) | 16 (14.95%) | |
| Other | 6 (4.48%) | 4 (3.74%) | |
| Educational Attainment | .17 | ||
| Some high school or less | 10 (7.46%) | 4 (3.70%) | |
| High school graduate or GED | 29 (21.64%) | 36 (33.33%) | |
| Some college or associate degree | 53 (39.55%) | 44 (40.74%) | |
| College graduate | 29 (21.64%) | 17 (15.74%) | |
| Graduate school | 13 (9.70%) | 7 (6.48%) | |
| Income | .37 | ||
| <$19,001 | 36 (28.57%) | 21 (19.44%) | |
| $19,001–$35,000 | 40 (31.75%) | 38 (35.19%) | |
| $35,001–$50,000 | 19 (15.08%) | 16 (14.81%) | |
| $50,001–$75,000 | 11 (8.73%) | 15 (13.89%) | |
| $75,001–$100,000 | 11 (8.73%) | 6 (5.56%) | |
| >$100,000 | 9 (7.14%) | 12 (11.11%) | |
| CTQ Total | 14.94 (11.01) | 4.10 (5.07) | <.001 |
| LEC-5 Total | 12.90 (9.58) | 6.54 (7.15) | <.001 |
| Index Trauma Event Type | .61 | ||
| Motor vehicle collision | 89 (66.42%) | 81 (75.00%) | |
| Physical assault | 17 (12.69%) | 10 (9.26%) | |
| Fall < 10 ft | 8 (5.97%) | 5 (4.63%) | |
| Nonmotorized collision | 7 (5.22%) | 2 (1.85%) | |
| Animal-related | 5 (3.73%) | 2 (1.85%) | |
| Other | 4 (2.99%) | 4 (3.70%) | |
| Fall ≥ 10 ft | 1 (0.75%) | 3 (2.78%) | |
| Sexual assault | 1 (0.75%) | 1 (0.93%) | |
| Mass/public trauma | 1 (0.75%) | 0 (0.00%) | |
| Burns | 1 (0.75%) | 0 (0.00%) | |
| Poisoning | 0 (0.00%) | 0 (0.00%) |
Values are presented as mean (SD) or n (%).
ANOVA, analysis of variance; CTQ, Childhood Trauma Questionnaire; LEC-5, Life Events Checklist for DSM-5.
Measures
Detailed information on the psychometric measures included is provided in the Supplement and in prior AURORA study data (44, 45, 46). In the current study, ST was defined as reporting having personally experienced at least 1 instance of one of the following: 1) CSA, 2) rape or sexual assault, or 3) another unwanted sexual experience. CSA was assessed using an abbreviated form of the Childhood Trauma Questionnaire—Short Form (47). Prior rape, sexual assault, or other unwanted sexual experience was assessed by the Life Events Checklist for DSM-5 (48,49). If a participant answered affirmatively to any 1 of these 3 questions, they were coded as “yes” for ST, regardless of whether they were missing data for either or both of the other questions. However, given the potential for individuals not to report prior ST (50,51), participants had to have denied all 3 experiences to be coded as “no” for ST. Otherwise, they were coded as “missing.” This method achieved good internal reliability (Cronbach’s α = 0.74). In additional analyses, we also calculated total Childhood Trauma Questionnaire—Short Form and Life Events Checklist for DSM-5 scores without the ST items.
Participants reported posttraumatic symptoms across several domains retrospectively (past 30 days) in the ED and at 2 weeks (past 14 days), 8 weeks, 3 months, and 6 months (past 30 days) after trauma. PTSD symptoms were assessed using the PTSD Symptom Checklist for DSM-5 (PCL-5) (52). Baseline (i.e., pretrauma) PTSD symptoms or a probable diagnosis of PTSD were not exclusionary criteria. Anxiety symptoms were assessed with the Participant-Reported Outcomes Measurement Information System Anxiety Scale Short Form 7a, and depression symptoms were assessed with the Participant-Reported Outcomes Measurement Information System Depression Short Form 8b scale (53,54). Dissociative symptoms were measured with a modified version of the Brief Dissociative Experiences Scale–Modified (55,56). Symptoms that may be considered consistent within a cognitive domain (i.e., maladaptive beliefs about one’s pain) (57) were assessed with the Rumination subscale of the Pain Catastrophizing Scale (58).
MRI Procedures
MRI data were obtained from participants within approximately 2 weeks after trauma exposure as described in our prior reports (29,37,38,59). Detailed information on acquisition parameters by site and imaging processing are available in the Supplement. Briefly, fMRIPrep was used to preprocess task and resting-state fMRI data. During the fMRI task, participants passively viewed fearful and neutral faces in block presentations with a fixation cross presented between each block. Group-level statistical modeling was completed on the fearful > neutral faces contrast extracted for the amygdala using the CIT168 atlas (60). Additional supplemental analyses were completed on data extracted for the insula (61) and hippocampus (62). The resting-state fMRI data were further processed within the AFNI program 3dTproject to perform linear detrending, censoring of non–steady-state volumes identified by fMRIPrep, bandpass filtering (0.01–0.1 Hz), and regression of the white matter, corticospinal fluid, and global signal to account for potential physiological noise. The mean fMRI signal time course was extracted separately from the left and right medial amygdala as defined by the Brainnetome atlas (63), and z-transformed Pearson correlation coefficients were calculated between each region of interest (ROI) and the rest of the brain (i.e., 2 voxelwise connectivity maps for the left/right amygdala per participant). Group-level statistical modeling was completed in AFNI using the separate voxelwise connectivity maps.
Statistical Analyses
Statistical analyses were completed in R 4.1.1 (https://www.R-project.org/) through R Studio 2021.09.0+351 and AFNI (https://afni.nimh.nih.gov/). Hypotheses related to effects of prior ST on posttraumatic stress, depression, anxiety, dissociation, and maladaptive beliefs about pain symptoms were assessed with repeated-measures analyses of covariance (ANCOVAs) with time point (i.e., pretrauma or week 2, week 8, month 3, or month 6 follow-up sessions) as our within-subjects factor and prior ST (i.e., yes/no) as our between-subjects factor. ANCOVAs were first conducted with demographic covariates (age at time of ED admission, yearly income, education level) and were Bonferroni corrected at a corrected alpha level of 0.05 for significance (5 models per comparison, thus critical p value = .05/5). Secondary ANCOVAs included covariates for trauma variables (Childhood Trauma Questionnaire total score without the sexual abuse variable and Life Events Checklist for DSM-5 standard total score without sexual assault variables) to account for effects of other trauma exposures, and results were also Bonferroni corrected at a corrected alpha level of 0.05 for significance.
Hypotheses related to ROI activation to fearful > neutral faces were assessed with one-way ANCOVAs using the same covariates as in previous models (primary models only included demographic covariates; secondary models included both demographic and trauma-related covariates, Bonferroni corrected at a corrected alpha level of 0.05 for significance, 2 models per comparison, thus critical p value = .05/2). To assess hypotheses related to seed-based rsFC, we used AFNI’s 3dttest++, assessing voxelwise FC for our a priori ROIs (left and right amygdala) as a function of history of ST. For primary models, analyses included covariates for scanner site, age, income level, and education level. We again covaried for overall prior trauma as well as PCL-5 scores at week 2 in secondary models with a corrected alpha level of 0.05 maintained by setting a cluster-forming threshold of p < .005. Gender was not included as a covariate given the high known multicollinearity of sex/gender with prevalence of ST (in the current sample, history of ST for 32 men [23.9%] and 102 women [76.1%]). Exploratory analyses investigated the interaction between other lifetime trauma and prior ST on amygdala reactivity and rsFC.
Results
Participant Demographic and Psychological Characteristics
Sample characteristics including participant demographics and trauma history are presented in Table 1. Childhood trauma (excluding CSA) and lifetime trauma (excluding rape or sexual assault and other unwanted sexual experience) levels were significantly higher among participants who also reported a history of ST. There were significantly more women compared with men among the individuals who experienced ST than among those who did not experience ST (p = .001). The samples did not differ significantly on age, race/ethnicity, income, educational attainment, or index trauma event type.
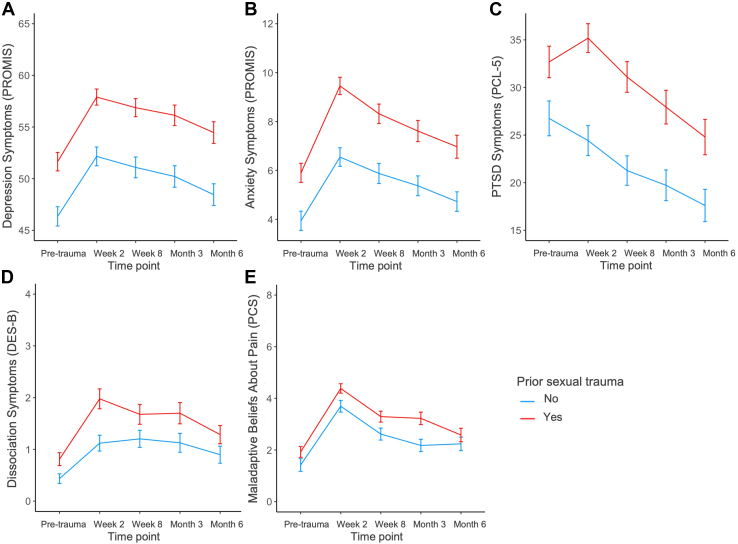
Participants with a history of ST in the imaging subsample had significantly greater posttraumatic dysfunction across time points (Figure 1 and Table 2). Main effects of ST were largest for depression (F1,1068 = 64.11, p = 3.06 × 10−15, ηp2 = 0.06) followed by anxiety (F1,1062 = 50.49, p = 2.20 × 10−12, ηp2 = 0.05), PTSD (F1,981 = 36.17, p = 2.55 × 10−9, ηp2 = 0.04), dissociation (F1,1063 = 17.55, p = 3.03 × 10−5, ηp2 = 0.02), and maladaptive beliefs about pain (F1,1055 = 16.01, p = 6.73 × 10−5, ηp2 = 0.02) after adjusting for demographic covariates (age, income, and education level). After including additional trauma-related covariates to control for all previous lifetime trauma excluding ST, we observed smaller main effects of ST, but these remained significant except for dissociation (p = .14) (Table 2). There was a significant effect of time point (i.e., pretrauma, week 2, week 8, month 3, and month 6) on all symptoms when adjusting for demographic and trauma covariates, such that symptoms generally increased from pretrauma to week 2 before decreasing over time. The interaction between time point and ST was not significant in any of the models. When we assessed effects of ST in the overall sample (N = 2178), effects varied slightly, but overall, the same findings were observed (see Supplemental Results; Tables S1, S3, S4). These data suggest that prior ST exacerbates the severity of posttraumatic symptoms across domains over a period of at least 6 months from the time when a new trauma exposure occurred.
Figure 1.
Posttraumatic dysfunction and prior sexual trauma in the 6 months following acute trauma in the imaging sample (n = 242). (A) Total depression symptoms (Participant-Reported Outcomes Measurement Information System [PROMIS] Short Form). (B) Total anxiety symptoms (PROMIS Short Form). (C) Total posttraumatic stress disorder (PTSD) symptoms (PTSD Symptom Checklist for DSM-5 [PCL-5]). (D) Total dissociation symptoms (Brief Dissociative Experiences Scale–Modified [DES-B]). (E) Total symptoms of maladaptive beliefs about pain (Pain Catastrophizing Scale [PCS] Rumination subscale). For all symptom types, the group with history of sexual trauma presented with higher symptoms when adjusting for demographic covariates. When trauma-related covariates were included in models, only the effect of sexual trauma on dissociation symptoms became nonsignificant (p = .14). Plots are presented without adjustment for covariates. Lines indicate mean scores, and error bars indicate ±1 standard error.
Table 2.
Prior Sexual Trauma and Posttraumatic Dysfunction in First 6 Months Following Trauma in the Imaging Sample (n = 242)
| Symptom Type | Demographic Covariates |
Trauma and Demographic Covariates |
||
|---|---|---|---|---|
| F Value | p Value | F Value | p Value | |
| Depression, PROMIS | ||||
| Time point | 14.77 | 9.48 × 10−12 | 14.75 | 1.00 × 10−11 |
| Sexual trauma | 64.11 | 3.06 × 10−15 | 23.41 | 1.51 × 10−6 |
| Time point × sexual trauma | 0.09 | .99 | 0.10 | .98 |
| Anxiety, PROMIS | ||||
| Time point | 19.68 | 1.22 × 10−15 | 19.98 | 7.43 × 10−16 |
| Sexual trauma | 50.49 | 2.20 × 10−12 | 11.07 | 9.09 × 10−4 |
| Time point × sexual trauma | 0.67 | .61 | 0.65 | .62 |
| PTSD, PCL-5 | ||||
| Time point | 10.13 | 4.92 × 10−8 | 9.22 | 2.59 × 10−7 |
| Sexual trauma | 36.17 | 2.55 × 10−9 | 6.05 | .01 |
| Time point × sexual trauma | 0.97 | .42 | 1.16 | .33 |
| Dissociation, DES-B | ||||
| Time point | 11.53 | 3.65 × 10−9 | 11.13 | 7.76 × 10−9 |
| Sexual trauma | 17.55 | 3.03 × 10−5 | 2.15 | .14 |
| Time point × sexual trauma | 0.85 | .49 | 0.54 | .71 |
| Maladaptive Beliefs About Pain, PCS | ||||
| Time point | 34.84 | 2.35 × 10−27 | 33.96 | 1.23 × 10−26 |
| Sexual trauma | 16.01 | 6.73 × 10−5 | 6.24 | .01 |
| Time point × sexual trauma | 0.92 | .45 | 0.81 | .52 |
DES-B, Brief Dissociative Experiences Scale–Modified; PCL-5, PTSD Checklist for DSM-5; PCS, Pain Catastrophizing Scale (Rumination subscale); PROMIS, Participant-Reported Outcomes Measurement Information System (Anxiety Scale Short Form 7a and Depression Short Form 8b); PTSD, posttraumatic stress disorder.
Given the observed main effect of prior ST on total PCL-5 scores, we further explored effects of ST on PCL-5 symptom clusters, with previously used covariates (primary models included only demographic covariates, whereas secondary models included both demographic and trauma-related covariates). In primary models, the effect of ST was significant on each symptom cluster (Table 3), with the largest effect observed for criterion D (negative alterations in cognition and mood). In secondary models controlling for overall lifetime trauma, the effect of ST remained significant only for criterion D. These follow-up results suggest that the effect of prior ST on posttraumatic stress may be driven by symptoms of negative thoughts and feelings.
Table 3.
Prior Sexual Trauma and Types of PCL-5 Symptom Clusters in the Imaging Sample (n = 242)
| Symptom Cluster | Demographic Covariates |
Trauma and Demographic Covariates |
||
|---|---|---|---|---|
| F Value | p Value | F Value | p Value | |
| Intrusion | 9.00 | .003 | 1.59 | .21 |
| Avoidance | 6.19 | .01 | 0.68 | .41 |
| Negative Alterations in Cognition and Mood | 21.30 | 6.65 × 10–6 | 4.47 | .04 |
| Hyperarousal | 10.38 | .001 | 1.79 | .18 |
PCL-5, PTSD Checklist for DSM-5.
Reactivity to Fearful and Neutral Faces and ST
We did not observe statistically significant effects of prior ST on neural reactivity to fearful faces within the left (F1,218 = 1.61, p = .21) or right (F1,218 = 1.91, p = .17) amygdala in primary or secondary models (F1,205 = 0.55, p = .46 and F1,205 = 1.28, p = .26, respectively). Additional ROIs were explored and are detailed in Table S5. These findings suggest that prior ST does not affect neural reactivity to fearful faces within these brain regions after a recent trauma.
Seed-based rsFC and ST
We did not observe a significant effect of prior ST on amygdala connectivity to other brain regions (all ps > .05). However, in exploratory post hoc tests, we found that there was an interaction between ST and lifetime trauma on amygdala-to-visual cortex connectivity (peak Z value: −4.41, corrected p < .01, X = −6.5, Y = −104.5, Z = 13.5) (Figure 2). More specifically, the non-ST group showed a positive relationship between lifetime trauma and amygdala-to-visual cortex connectivity (r100 = 0.20, p = .04), while the ST group showed a negative relationship (r110 = −0.25, p = .01). Of note, the prior models of reactivity to fearful faces did not show a significant interaction between lifetime trauma and ST on left or right amygdala reactivity (p = .73 and p = .41, respectively). These data suggest that the past occurrence of ST alters the influence of prior traumatic experiences on neural connectivity patterns after a recent trauma.
Figure 2.
Left amygdala to visual cortex connectivity and prior sexual trauma (ST) in imaging sample (n = 242). (A) After accounting for both demographic and trauma-related covariates, we found that the effect of prior ST on left amygdala-to-visual cortex connectivity in a resting state had an interaction with overall lifetime trauma load. (B) In the group with ST, stronger connectivity was negatively associated with overall lifetime trauma load, and in the group without ST, the opposite pattern was found. LEC-5, Life Events Checklist for DSM-5.
Discussion
ST is a known risk factor for the development of adverse neuropsychiatric sequelae, but how it may modulate the impact of a subsequent trauma is unclear. In this investigation, symptoms of PTSD, depression, anxiety, dissociation, and maladaptive beliefs about pain in the early aftermath of acute trauma were significantly higher in individuals with a history of ST than in individuals without a history of ST. Contrary to our hypotheses, history of ST was not associated with differences in amygdala reactivity to fearful faces. However, there was an interaction between history of ST and exposure to other traumas on amygdala-to-visual cortex rsFC. The current findings suggest a need to consider history of ST in the development of predictive models of adverse neuropsychiatric sequelae and treatment in the early aftermath of trauma.
Of particular note in this study is the interaction effect between ST and lifetime trauma exposure in predicting amygdala-to-visual cortex rsFC. Emerging work has begun to highlight the importance of visual circuitry and extrinsic connectivity of visual systems in relation to trauma and stress-related disorders (37, 38, 39,64). Given that visual cortex activity is modulated by the amygdala, the visual symptoms of PTSD may be related to dysregulated visual cortex activity (65,66). Altered connectivity between the amygdala and visual cortex has also been observed in individuals with PTSD (67,68). Specifically, hyperactivity of the amygdala and associated enhancement of visual perceptual processing of trauma-related stimuli may be related to visual re-experiencing and intrusive symptoms such as flashbacks and may partially support hypervigilance and attention to threat-related cues (66,67,69). A study of patients with mood and anxiety disorders found that blood oxygen level–dependent activity in the amygdala and ventral visual cortex covaried significantly with trauma such that individuals with high trauma showed the smallest blood oxygen level–dependent changes in response to emotional versus neutral scenes in these regions (70). Given that highly traumatized participants with prior ST in this study showed reduced amygdala–visual cortex rsFC, this pattern may reflect a posttraumatic outcome that is tied more to negative cognition/depression. Prior research has found decreased rsFC of the amygdala to the visual cortex in major depressive disorder (71). Furthermore, the largest effects of prior ST were on criterion D components of PTSD (i.e., negative cognitions/mood) and depression symptoms. These results may also be interpreted in light of other findings of lower activity in the ventral visual stream during a picture-viewing task in individuals with PTSD versus trauma-exposed control subjects without PTSD (40) given that the group with ST in our sample was significantly more likely to meet criteria for a probable diagnosis of PTSD. One speculative hypothesis in light of these findings is that high polytrauma that includes ST promotes avoidance behaviors to minimize exposure to trauma-related cues, which in turn inhibits extinction memory processes and promotes more frequent negative thoughts about the trauma. Future research that involves specifically testing visual threat memory processes in trauma survivors with ST is needed to fully elucidate this potential mechanism.
These findings are consistent with a large body of previous literature that demonstrated that ST is a risk factor for later mental health disorders. As in prior work (72, 73, 74, 75), the current study found that individuals with a history of ST have increased posttraumatic symptom severity across a number of domains (e.g., PTSD, depression, and anxiety). The group with prior ST had higher symptoms prior to the new trauma as well as elevated symptoms after the acute traumatic event, suggesting that the acute phase after a new trauma may be an especially vulnerable period for this population and that ST may thus have especially deleterious effects on mental health. In general, interpersonal traumas result in higher rates of PTSD than noninterpersonal traumas such as motor vehicle collisions or natural disasters (9,76, 77, 78). Furthermore, prior literature suggests that sexual assault is by its nature unique even when compared with other types of interpersonal trauma because it is a crime that violates and “disrupts the sense of autonomy, control, and mastery over one’s body” (79). This unique nature of ST may explain why it is a singularly important risk factor for later psychopathology, and taken together with prior literature, the current results suggest that history of ST exacerbates posttraumatic dysfunction following a new trauma. Thus, care providers should be especially sensitive to the possibility of heightened distress both in the short- and long-term following events such as motor vehicle collisions in individuals with prior ST.
We did not observe differences in amygdala reactivity to fearful faces between individuals with and without a history of ST. We initially hypothesized that ST would be associated with greater amygdala reactivity given that a large body of prior work has found amygdala hyperactivity in people with PTSD (e.g., 28,80). Furthermore, in previous analyses of AURORA study data, amygdala reactivity to fearful faces was related to PTSD symptom presentations (29). However, there is also evidence that repeated trauma and childhood adversity may be related to blunted rather than elevated amygdala reactivity (30,81). In the current study, although some participants were exposed to prior ST and others were not, all participants in the current study had been recently exposed to a traumatic event. It is possible that trauma-exposed individuals, regardless of prior ST, exhibit comparably high levels of reactivity to threat-related/emotional stimuli due to the recency of their traumatic event. It may also be true that prior ST modulates amygdala reactivity at a later time scale than was assessed in the current study. Additional longitudinal studies are required to assess whether prior ST may modulate amygdala reactivity to threat in the months after trauma exposure.
Several limitations of the current study should be noted. First, we are unable to determine whether observed neurobiological alterations are preexisting risk factors or the result of the acute trauma because MRIs could not be obtained prior to the ED visit. Although our study design allows for greater understanding of the possible neurobiological mechanisms present in the early aftermath of acute trauma, it remains impractical and expensive to implement a fully prospective longitudinal study design of acute trauma. Without pre-existing knowledge of who will go on to experience a traumatic event that brings them to the ED, scanning individuals as early as 2 weeks after such a trauma is likely to be one of the most practical ways of studying the progression of posttraumatic symptoms and possible biological alterations during the acute phase of trauma exposure. Second, we applied stringent exclusion criteria for categorizing ST that might have excluded certain individuals who experienced prior ST but chose to decline to answer these questions due to distress or other concerns. Third, the available measure assessing dissociation was likely insufficient to comprehensively assess the range of symptoms of dissociation that have been linked to traumatic sexual experiences (82,83). As noted in the Methods and Materials, only 2 of the 8 items of the Brief Dissociative Experiences Scale–Modified were included, and both items assessed derealization. It is possible that a more extensive measure that also assesses depersonalization could have revealed the hypothesized effects of ST on dissociation more strongly. Fourth, although we observed effects of prior ST on neural connectivity and posttraumatic symptoms, many of the effect sizes were small to moderate in size. Thus, although the differences are significant, it may be important to consider how these factors interact with one another as opposed to focusing on individual effects. Fifth, our connectivity analyses used resting-state data, and our inferences are thus limited by a lack of concurrent behavioral/task-specific data. Future research should leverage task-related connectivity approaches to probe the relationship between the amygdala and visual circuitry in participants with different trauma backgrounds. Finally, it is possible that the developmental timing (e.g., in childhood, adolescence, or adulthood) of prior ST might have affected the current findings. Information on the timing of prior trauma exposure was not available, and thus, we are unable to fully delineate the impact of ST timing on posttraumatic reactions.
In conclusion, the current study investigated the impact of prior ST on posttraumatic dysfunction and alterations in neural circuitry following an acute trauma. We found that prior ST was related to greater symptoms of depression, PTSD, anxiety, dissociation, and maladaptive beliefs about pain that persisted during the 6 months following acute trauma. In addition, we found that there was an interaction between ST and other lifetime trauma on amygdala-to-visual cortex FC during a resting-state MRI scan. These findings suggest that prior ST is an important determinant in the progression of symptoms following a later trauma and that it may also be related to alterations in visual circuitry modulated by amygdala activity. Particular care should be taken in the treatment of individuals with prior ST following traumatic events. Furthermore, increased understanding of interactions between threat and visual processing brain regions after trauma may open new avenues for neuroscience-based interventions in the early aftermath of trauma.
Acknowledgments and Disclosures
This project was supported by the National Institute of Mental Health (NIMH) (Grant Nos. K00MH119603 and U01MH110925), the U.S. Army MRMC, One Mind, and The Mayday Fund.
The content is sole responsibility of the authors and does not necessarily represent the official views of any of the funders. This article reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health (NIH) or of the submitters submitting original data to the NIMH Data Archive (NDA).
The investigators thank the trauma survivors participating in the AURORA Study. Their time and effort during a challenging period of their lives make our efforts to improve recovery for future trauma survivors possible.
Data and/or research tools used in the preparation of this manuscript were obtained from the NDA. The NDA is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): NIMH Data Archive Digital Object Identifier 10.15154/1528118.
LAML reports an unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation (ISSTD), grant support from the NIMH, K01 MH118467, and the Julia Kasparian Fund for Neuroscience Research. LAML also reports spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the current work. TCN has received research support from the NIH, the Veterans Administration, and the Rainwater Charitable Foundation and consulting income from Jazz Pharmaceuticals. In the last 3 years, GDC has received research funding from the National Science Foundation, NIH, and LifeBell AI and unrestricted donations from AliveCor Inc., Amazon Research, the Center for Discovery, the Gates Foundation, Google, the Gordon and Betty Moore Foundation, MathWorks, Microsoft Research, Nextsense Inc., One Mind Foundation, the Rett Research Foundation, and Samsung Research. GDC has financial interest in AliveCor Inc. and Nextsense Inc. He also is the CTO of MindChild Medical and CSO of LifeBell AI and has ownership in both companies. These relationships are unconnected to the current work. SLR reports grants from NIH during the conduct of the study; personal fees from Society of Biological Psychiatry (SOBP) paid role as secretary, other from Oxford University Press royalties, other from American Psychiatric Publishing Inc. royalties, other from Veterans Administration per diem for oversight committee, and other from Community Psychiatry/Mindpath Health paid board service, including equity outside the submitted work; other from National Association of Behavioral Healthcare for paid Board service; and Leadership roles on Board or Council for SOBP, Anxiety and Depression Association of America, and National Network of Depression Centers. SS has received funding from the Florida Medical Malpractice Joint Underwriter’s Association AES Safety of Healthcare Services Grant; Allergan Foundation; the NIH/National Investigation Agency-Funded Jacksonville Aging Studies Center (JAX-ASCENT; R33AG05654); and the Substance Abuse and Mental Health Services Administration (1H79TI083101-01); and the Florida Blue Foundation. CWJ has no competing interests related to this work, though he has been an investigator on studies funded by Astra Zeneca, Vapotherm, Abbott, and Ophirex. JJ receives consulting payments from Janssen Pharmaceuticals. Over the past 3 years, DAP has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Concert Pharmaceuticals, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, and Takeda Pharmaceuticals; honoraria from the Psychonomic Society and the American Psychological Association (for editorial work) and Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. In addition, he has received stock options from Neumora Therapeutics (former BlackThorn Therapeutics), Compass Pathways, Engrail Therapeutics, and Neuroscience Software. SEH has no competing interests related to this work, though in the last 3 years he has received research funding from Aptinyx and Arbor Medical Innovations, and consulting payments from Aptinyx, Heron Therapeutics, and Eli Lilly. JME reports support from the NIH through Grant Nos. R01HD079076 & R03HD094577: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research. He also reports funding from New South Wales Health, Spinal Cord Injury Award (2020–2025) and consulting fees (<$15,000 per annum) from Orofacial Therapeutics, LLC. In the past 3 years, RCK was a consultant for Cambridge Health Alliance, Canandaigua VA Medical Center, Holmusk, Partners Healthcare, Inc., RallyPoint Networks, Inc., and Sage Therapeutics. He has stock options in Cerebral Inc., Mirah, PYM, and Roga Sciences. KCK research has been supported by the Robert Wood Johnson Foundation, the Kaiser Family Foundation, the Harvard Center on the Developing Child, Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard, the NIH, One Mind, the Anonymous Foundation, and Cohen Veterans Bioscience. She has been a paid consultant for Baker Hostetler, Discovery Vitality, and the Department of Justice. She has been a paid external reviewer for the Chan Zuckerberg Foundation, the University of Cape Town, and Capita Ireland. She has had paid speaking engagements in the last 3 years with the American Psychological Association, European Central Bank. Sigmund Freud University—Milan, Cambridge Health Alliance, and Coverys. She receives royalties from Guilford Press and Oxford University Press. SAM served as a consultant for Walter Reed and for Arbor Medical Innovations. KJR has performed scientific consultation for Bioxcel, Bionomics, Acer, Takeda, and Jazz Pharma; serves on Scientific Advisory Boards for Sage and the Brain Research Foundation, and he has received sponsored research support from Takeda, BrainsWay, and Alto Neuroscience. NGH reports grant support from the NIMH, K00 MH119603. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.02.004.
Contributor Information
Jennifer S. Stevens, Email: jennifer.stevens@emory.edu.
Nathaniel G. Harnett, Email: nharnett@mclean.harvard.edu.
Supplementary Material
References
- 1.Briere J., Elliott D.M. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27:1205–1222. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin E.R., Menon S.V., Bystrynski J., Allen N.E. Sexual assault victimization and psychopathology: A review and meta-analysis. Clin Psychol Rev. 2017;56:65–81. doi: 10.1016/j.cpr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott D.M., Mok D.S., Briere J. Adult sexual assault: Prevalence, symptomatology, and sex differences in the general population. J Trauma Stress. 2004;17:203–211. doi: 10.1023/B:JOTS.0000029263.11104.23. [DOI] [PubMed] [Google Scholar]
- 4.Finkelhor D., Shattuck A., Turner H.A., Hamby S.L. The lifetime prevalence of child sexual abuse and sexual assault assessed in late adolescence. J Adolesc Health. 2014;55:329–333. doi: 10.1016/j.jadohealth.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Mellins C.A., Walsh K., Sarvet A.L., Wall M., Gilbert L., Santelli J.S., et al. Sexual assault incidents among college undergraduates: Prevalence and factors associated with risk [published correction appears in PLoS One 2018;13:e0192129] PLoS One. 2017;12 doi: 10.1371/journal.pone.0186471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogeltanz N.D., Wilsnack S.C., Harris T.R., Wilsnack R.W., Wonderlich S.A., Kristjanson A.F. Prevalence and risk factors for childhood sexual abuse in women: National survey findings. Child Abuse Negl. 1999;23:579–592. doi: 10.1016/s0145-2134(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 7.Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 8.Ozer E.J., Best S.R., Lipsey T.L., Weiss D.S. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 9.Resnick H.S., Kilpatrick D.G., Dansky B.S., Saunders B.E., Best C.L. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 10.Molnar B.E., Buka S.L., Kessler R.C. Child sexual abuse and subsequent psychopathology: Results from the National comorbidity Survey. Am J Public Health. 2001;91:753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paolucci E.O., Genuis M.L., Violato C. A meta-analysis of the published research on the effects of child sexual abuse. J Psychol. 2001;135:17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- 12.Thompson K.M., Crosby R.D., Wonderlich S.A., Mitchell J.E., Redlin J., Demuth G., et al. Psychopathology and sexual trauma in childhood and adulthood. J Trauma Stress. 2003;16:35–38. doi: 10.1023/A:1022007327077. [DOI] [PubMed] [Google Scholar]
- 13.DiGangi J.A., Gomez D., Mendoza L., Jason L.A., Keys C.B., Koenen K.C. Pretrauma risk factors for posttraumatic stress disorder: A systematic review of the literature. Clin Psychol Rev. 2013;33:728–744. doi: 10.1016/j.cpr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kessler R.C., Aguilar-Gaxiola S., Alonso J., Bromet E.J., Gureje O., Karam E.G., et al. The associations of earlier trauma exposures and history of mental disorders with PTSD after subsequent traumas. Mol Psychiatry. 2018;23:1892–1899. doi: 10.1038/mp.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication [published correction appears in Arch Gen Psychiatry 2005;62:768. Merikangas, Kathleen R [added]] Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nöthling J., Abrahams N., Jewkes R., Mhlongo S., Lombard C., Hemmings S.M.J., Seedat S. Risk and protective factors affecting the symptom trajectory of posttraumatic stress disorder post-rape. J Affect Disord. 2022;309:151–164. doi: 10.1016/j.jad.2022.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Rothbaum B.O., Foa E.B., Riggs D.S., Murdock T., Walsh W. A prospective examination of post-traumatic stress disorder in rape victims. J Trauma Stress. 1992;5:455–475. [Google Scholar]
- 19.Au T.M., Dickstein B.D., Comer J.S., Salters-Pedneault K., Litz B.T. Co-occurring posttraumatic stress and depression symptoms after sexual assault: A latent profile analysis. J Affect Disord. 2013;149:209–216. doi: 10.1016/j.jad.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Campbell R., Dworkin E., Cabral G. An ecological model of the impact of sexual assault on women’s mental health. Trauma Violence Abuse. 2009;10:225–246. doi: 10.1177/1524838009334456. [DOI] [PubMed] [Google Scholar]
- 21.Dancu C.V., Riggs D.S., Hearst-Ikeda D., Shoyer B.G., Foa E.B. Dissociative experiences and posttraumatic stress disorder among female victims of criminal assault and rape. J Trauma Stress. 1996;9:253–267. doi: 10.1007/BF02110659. [DOI] [PubMed] [Google Scholar]
- 22.Amado B.G., Arce R., Herraiz A. Psychological injury in victims of child sexual abuse: A meta-analytic review. Psychosoc Interv. 2015;24:49–62. [Google Scholar]
- 23.Lev-Wiesel R. Child sexual abuse: A critical review of intervention and treatment modalities. Child Youth Serv Rev. 2008;30:665–673. [Google Scholar]
- 24.Neumann D.A., Houskamp B.M., Pollock V.E., Briere J. The long-term sequelae of childhood sexual abuse in women: A meta-analytic review. Child Maltreat. 1996;1:6–16. [Google Scholar]
- 25.Harnett N.G., Goodman A.M., Knight D.C. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp Neurol. 2020;330 doi: 10.1016/j.expneurol.2020.113331. [DOI] [PubMed] [Google Scholar]
- 26.Admon R., Milad M.R., Hendler T. A causal model of post-traumatic stress disorder: Disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 29.Stevens J.S., Harnett N.G., Lebois L.A.M., van Rooij S.J.H., Ely T.D., Roeckner A., et al. Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. Am J Psychiatry. 2021;178:1037–1049. doi: 10.1176/appi.ajp.2021.20101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huggins A.A., McTeague L.M., Davis M.M., Bustos N., Crum K.I., Polcyn R., et al. Neighborhood disadvantage associated with blunted amygdala reactivity to predictable and unpredictable threat in a community sample of youth. Biol Psychiatry Glob Open Sci. 2022;2:242–252. doi: 10.1016/j.bpsgos.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y.J., van Rooij S.J.H., Ely T.D., Fani N., Ressler K.J., Jovanovic T., Stevens J.S. Association between posttraumatic stress disorder severity and amygdala habituation to fearful stimuli. Depress Anxiety. 2019;36:647–658. doi: 10.1002/da.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin K.A., Busso D.S., Duys A., Green J.G., Alves S., Way M., Sheridan M.A. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens J.S., Kim Y.J., Galatzer-Levy I.R., Reddy R., Ely T.D., Nemeroff C.B., et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81:1023–1029. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sripada R.K., King A.P., Garfinkel S.N., Wang X., Sripada C.S., Welsh R.C., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Wingen G.A., Geuze E., Vermetten E., Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens J.S., Jovanovic T., Fani N., Ely T.D., Glover E.M., Bradley B., Ressler K.J. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harnett N.G., van Rooij S.J.H., Ely T.D., Lebois L.A.M., Murty V.P., Jovanovic T., et al. Prognostic neuroimaging biomarkers of trauma-related psychopathology: Resting-state fMRI shortly after trauma predicts future PTSD and depression symptoms in the Aurora study. Neuropsychopharmacology. 2021;46:1263–1271. doi: 10.1038/s41386-020-00946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harnett N.G., Finegold K.E., Lebois L.A.M., van Rooij S.J.H., Ely T.D., Murty V.P., et al. Structural covariance of the ventral visual stream predicts posttraumatic intrusion and nightmare symptoms: A multivariate data fusion analysis. Transl Psychiatry. 2022;12:321. doi: 10.1038/s41398-022-02085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harnett N.G., Stevens J.S., Fani N., van Rooij S.J.H., Ely T.D., Michopoulos V., et al. Acute posttraumatic symptoms are associated with multimodal neuroimaging structural covariance patterns: A possible role for the neural substrates of visual processing in posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:129–138. doi: 10.1016/j.bpsc.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller-Pfeiffer C., Schick M., Schulte-Vels T., O’Gorman R., Michels L., Martin-Soelch C., et al. Atypical visual processing in posttraumatic stress disorder. Neuroimage Clin. 2013;3:531–538. doi: 10.1016/j.nicl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cisler J.M., Sigel B.A., Kramer T.L., Smitherman S., Vanderzee K., Pemberton J., Kilts C.D. Amygdala response predicts trajectory of symptom reduction during Trauma-Focused cognitive-behavioral therapy among adolescent girls with PTSD. J Psychiatr Res. 2015;71:33–40. doi: 10.1016/j.jpsychires.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Protopopescu X., Pan H., Tuescher O., Cloitre M., Goldstein M., Engelien W., et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 43.McLean S.A., Ressler K., Koenen K.C., Neylan T., Germine L., Jovanovic T., et al. The Aurora Study: A longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry. 2020;25:283–296. doi: 10.1038/s41380-019-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harnett N.G., Dumornay N.M., Delity M., Sanchez L.D., Mohiuddin K., Musey P.I., et al. Prior differences in previous trauma exposure primarily drive the observed racial/ethnic differences in posttrauma depression and anxiety following a recent trauma [published online Jan 31] Psychol Med. 2022 doi: 10.1017/S0033291721004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joormann J., McLean S.A., Beaudoin F.L., An X., Stevens J.S., Zeng D., et al. Socio-demographic and trauma-related predictors of depression within eight weeks of motor vehicle collision in the Aurora study. Psychol Med. 2022;52:1934–1947. doi: 10.1017/S0033291720003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler R.C., Ressler K.J., House S.L., Beaudoin F.L., An X., Stevens J.S., et al. Socio-demographic and trauma-related predictors of PTSD within 8 weeks of a motor vehicle collision in the Aurora study. Mol Psychiatry. 2021;26:3108–3121. doi: 10.1038/s41380-020-00911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 48.Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. Psychometric properties of the life events checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 49.Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. The Life Events Checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD. https://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp Available at:
- 50.Kelly T.C., Stermac L. Underreporting in sexual assault: A review of explanatory factors. Balt J Psychol. 2008;9:30–45. [Google Scholar]
- 51.Panel on Measuring Rape and Sexual Assault in Bureau of Justice Statistics Household Surveys. Committee on National Statistics; Division on Behavioral and Social Sciences and Education; National Research Council. Kruttschnitt C., Kalsbeek W.D., House C.C. National Academies Press; Washington: 2014. Estimating the Incidence of Rape and Sexual Assault. [PubMed] [Google Scholar]
- 52.Weathers F.W., Litz B.T., Keane T.M., Palmieri P.A., Marx B.P., Schnurr P.P. PTSD Checklist for DSM-5 (PCL-5). Instrument available from the National Center for PTSD. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp Available at:
- 53.Pilkonis P.A., Choi S.W., Reise S.P., Stover A.M., Riley W.T., Cella D., PROMIS Cooperative Group Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schalet B.D., Pilkonis P.A., Yu L., Dodds N., Johnston K.L., Yount S., et al. Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–127. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson E.B., Putnam F.W. An update on the Dissociative Experiences Scale. Dissociation Progr Dissociative Disord. 1993;6:16–27. [Google Scholar]
- 56.Dalenberg C., Carlson E.B. Severity of Dissociative Symptoms - Adult (Brief Dissociative Experiences Scale (DES-B) – Modified). Instrument available from the American Psychiatric Association: Online Assessment Measures. https://www.psychiatry.org/psychiatrists/practice/dsm/educational-resources/assessment-measures Available at:
- 57.Walton D.M., Elliott J.M. A new clinical model for facilitating the development of pattern recognition skills in clinical pain assessment. Musculoskelet Sci Pract. 2018;36:17–24. doi: 10.1016/j.msksp.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan M.J.L., Bishop S.R., Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 59.Steuber E.R., Seligowski A.V., Roeckner A.R., Reda M., Lebois L.A.M., van Rooij S.J.H., et al. Thalamic volume and fear extinction interact to predict acute posttraumatic stress severity. J Psychiatr Res. 2021;141:325–332. doi: 10.1016/j.jpsychires.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyszka J.M., Pauli W.M. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum Brain Mapp. 2016;37:3979–3998. doi: 10.1002/hbm.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 62.Hammers A., Allom R., Koepp M.J., Free S.L., Myers R., Lemieux L., et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L., et al. The human Brainnetome atlas: A new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morey R.A., Dunsmoor J.E., Haswell C.C., Brown V.M., Vora A., Weiner J., et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry. 2015;5 doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hendler T., Rotshtein P., Yeshurun Y., Weizmann T., Kahn I., Ben-Bashat D., et al. Sensing the invisible: Differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 66.Weston C.S. Posttraumatic stress disorder: A theoretical model of the hyperarousal subtype. Front Psychiatry. 2014;5:37. doi: 10.3389/fpsyt.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilboa A., Shalev A.Y., Laor L., Lester H., Louzoun Y., Chisin R., Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Nilsen A.S., Blix I., Leknes S., Ekeberg Ø., Skogstad L., Endestad T., et al. Brain activity in response to trauma-specific, negative, and neutral stimuli. A fMRI study of recent road traffic accident survivors. Front Psychol. 2016;7:1173. doi: 10.3389/fpsyg.2016.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleim B., Ehring T., Ehlers A. Perceptual processing advantages for trauma-related visual cues in post-traumatic stress disorder. Psychol Med. 2012;42:173–181. doi: 10.1017/S0033291711001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sambuco N., Bradley M., Herring D., Hillbrandt K., Lang P.J. Transdiagnostic trauma severity in anxiety and mood disorders: Functional brain activity during emotional scene processing. Psychophysiology. 2020;57 doi: 10.1111/psyp.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramasubbu R., Konduru N., Cortese F., Bray S., Gaxiola-Valdez I., Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Resick P.A. The psychological impact of rape. J Interpers Violence. 1993;8:223–255. [Google Scholar]
- 73.Rowland G.E., Mekawi Y., Michopoulos V., Powers A., Fani N., Bradley B., et al. Distinctive impacts of sexual trauma versus non-sexual trauma on PTSD profiles in highly trauma-exposed, Black women. J Affect Disord. 2022;317:329–338. doi: 10.1016/j.jad.2022.08.099. [DOI] [PubMed] [Google Scholar]
- 74.Saunders B.E., Villeponteaux L.A., Lipovsky J.A., Kilpatrick D.G., Veronen L.J. Child sexual assault as a risk factor for mental disorders among women: A community survey. J Interpers Violence. 1992;7:189–204. [Google Scholar]
- 75.Zinzow H.M., Resnick H.S., McCauley J.L., Amstadter A.B., Ruggiero K.J., Kilpatrick D.G. Prevalence and risk of psychiatric disorders as a function of variant rape histories: Results from a national survey of women. Soc Psychiatry Psychiatr Epidemiol. 2012;47:893–902. doi: 10.1007/s00127-011-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breslau N. The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J Clin Psychiatry. 2001;62(Suppl 17):16–22. [PubMed] [Google Scholar]
- 77.Forbes D., Fletcher S., Parslow R., Phelps A., O’Donnell M., Bryant R.A., et al. Trauma at the hands of another: Longitudinal study of differences in the posttraumatic stress disorder symptom profile following interpersonal compared with noninterpersonal trauma. J Clin Psychiatry. 2012;73:372–376. doi: 10.4088/JCP.10m06640. [DOI] [PubMed] [Google Scholar]
- 78.Forbes D., Lockwood E., Phelps A., Wade D., Creamer M., Bryant R.A., et al. Trauma at the hands of another: Distinguishing PTSD patterns following intimate and nonintimate interpersonal and noninterpersonal trauma in a nationally representative sample. J Clin Psychiatry. 2014;75:147–153. doi: 10.4088/JCP.13m08374. [DOI] [PubMed] [Google Scholar]
- 79.Rose D.S. “Worse than death”: Psychodynamics of rape victims and the need for psychotherapy. Am J Psychiatry. 1986;143:817–824. doi: 10.1176/ajp.143.7.817. [DOI] [PubMed] [Google Scholar]
- 80.Koenigs M., Grafman J. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McTeague L.M., Lang P.J., Laplante M.C., Cuthbert B.N., Shumen J.R., Bradley M.M. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sar V. Epidemiology of dissociative disorders: An overview. Epidemiol Res Int. 2011;2011:1–8. [Google Scholar]
- 83.Wolf E.J., Miller M.W., Reardon A.F., Ryabchenko K.A., Castillo D., Freund R. A latent class analysis of dissociation and posttraumatic stress disorder: Evidence for a dissociative subtype. Arch Gen Psychiatry. 2012;69:698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.