Abstract
Background
There is a significant research gap in the field of universal, selective, and indicated prevention interventions for mental health promotion and the prevention of mental disorders. Barriers to closing the research gap include scarcity of skilled human resources, large inequities in resource distribution and utilization, and stigma.
Objectives
To assess the effectiveness of delivery by primary workers of interventions for the promotion of mental health and universal prevention, and for the selective and indicated prevention of mental disorders or symptoms of mental illness in low‐ and middle‐income countries (LMICs). To examine the impact of intervention delivery by primary workers on resource use and costs.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, Global Index Medicus, PsycInfo, WHO ICTRP, and ClinicalTrials.gov from inception to 29 November 2021.
Selection criteria
Randomized controlled trials (RCTs) of primary‐level and/or community health worker interventions for promoting mental health and/or preventing mental disorders versus any control conditions in adults and children in LMICs.
Data collection and analysis
Standardized mean differences (SMD) or mean differences (MD) were used for continuous outcomes, and risk ratios (RR) for dichotomous data, using a random‐effects model. We analyzed data at 0 to 1, 1 to 6, and 7 to 24 months post‐intervention. For SMDs, 0.20 to 0.49 represented small, 0.50 to 0.79 moderate, and ≥ 0.80 large clinical effects. We evaluated the risk of bias (RoB) using Cochrane RoB2.
Main results
Description of studies
We identified 113 studies with 32,992 participants (97 RCTs, 19,570 participants in meta‐analyses) for inclusion. Nineteen RCTs were conducted in low‐income countries, 27 in low‐middle‐income countries, 2 in middle‐income countries, 58 in upper‐middle‐income countries and 7 in mixed settings. Eighty‐three RCTs included adults and 30 RCTs included children. Cadres of primary‐level workers employed primary care health workers (38 studies), community workers (71 studies), both (2 studies), and not reported (2 studies). Interventions were universal prevention/promotion in 22 studies, selective in 36, and indicated prevention in 55 RCTs.
Risk of bias
The most common concerns over risk of bias were performance bias, attrition bias, and reporting bias.
Intervention effects
'Probably', 'may', or 'uncertain' indicates 'moderate‐', 'low‐', or 'very low‐'certainty evidence.
*Certainty of the evidence (using GRADE) was assessed at 0 to 1 month post‐intervention as specified in the review protocol. In the abstract, we did not report results for outcomes for which evidence was missing or very uncertain.
Adults
Promotion/universal prevention, compared to usual care:
‐ probably slightly reduced anxiety symptoms (MD ‐0.14, 95% confidence interval (CI) ‐0.27 to ‐0.01; 1 trial, 158 participants)
‐ may slightly reduce distress/PTSD symptoms (SMD ‐0.24, 95% CI ‐0.41 to ‐0.08; 4 trials, 722 participants)
Selective prevention, compared to usual care:
‐ probably slightly reduced depressive symptoms (SMD ‐0.69, 95% CI ‐1.08 to ‐0.30; 4 trials, 223 participants)
Indicated prevention, compared to usual care:
‐ may reduce adverse events (1 trial, 547 participants)
‐ probably slightly reduced functional impairment (SMD ‐0.12, 95% CI ‐0.39 to ‐0.15; 4 trials, 663 participants)
Children
Promotion/universal prevention, compared to usual care:
‐ may improve the quality of life (SMD ‐0.25, 95% CI ‐0.39 to ‐0.11; 2 trials, 803 participants)
‐ may reduce adverse events (1 trial, 694 participants)
‐ may slightly reduce depressive symptoms (MD ‐3.04, 95% CI ‐6 to ‐0.08; 1 trial, 160 participants)
‐ may slightly reduce anxiety symptoms (MD ‐2.27, 95% CI ‐3.13 to ‐1.41; 1 trial, 183 participants)
Selective prevention, compared to usual care:
‐ probably slightly reduced depressive symptoms (SMD 0, 95% CI ‐0.16 to ‐0.15; 2 trials, 638 participants)
‐ may slightly reduce anxiety symptoms (MD 4.50, 95% CI ‐12.05 to 21.05; 1 trial, 28 participants)
‐ probably slightly reduced distress/PTSD symptoms (MD ‐2.14, 95% CI ‐3.77 to ‐0.51; 1 trial, 159 participants)
Indicated prevention, compared to usual care:
‐ decreased slightly functional impairment (SMD ‐0.29, 95% CI ‐0.47 to ‐0.10; 2 trials, 448 participants)
‐ decreased slightly depressive symptoms (SMD ‐0.18, 95% CI ‐0.32 to ‐0.04; 4 trials, 771 participants)
‐ may slightly reduce distress/PTSD symptoms (SMD 0.24, 95% CI ‐1.28 to 1.76; 2 trials, 448 participants).
Authors' conclusions
The evidence indicated that prevention interventions delivered through primary workers ‐ a form of task‐shifting ‐ may improve mental health outcomes. Certainty in the evidence was influenced by the risk of bias and by substantial levels of heterogeneity. A supportive network of infrastructure and research would enhance and reinforce this delivery modality across LMICs.
Keywords: Humans, Anxiety, Anxiety/diagnosis, Developing Countries, Health Promotion, Mental Disorders, Mental Disorders/prevention & control, Mental Health, Randomized Controlled Trials as Topic
Plain language summary
Primary‐level and community worker interventions for the prevention of mental disorders and the promotion of well‐being in low‐ and middle‐income countries
What is the main aim of this review? The aim of this Cochrane Review was to assess the effects of involving people in primary services and the community, such as nurses, midwives, teachers or caregivers, to promote mental health. The review focused on children and adults living in low‐ and middle‐income countries.
Key messages
The employment use of primary‐level and community workers may improve the mental health of adults and children living in low‐ and middle‐income countries. However, more evidence is needed.
What was studied in this review? Many people who would benefit from mental health support cannot access these services. One reason for this is a lack of specialized mental healthcare staff. This is especially true in low‐ and middle‐income countries. To overcome this barrier, people without a professional background in mental health, such as nurses or teachers, can be trained to deliver some mental health services. In our review, we investigated whether this strategy helps to promote mental health and prevent mental disorders amongst adults and children. We also assessed its costs.
What are the main results of this review?
We included 113 studies from a range of low‐ and middle‐income countries.
The studies assessed the effects of services carried out by primary‐level and community workers on people's mental health, quality of life, and social outcomes.
We grouped interventions depending on their overall objectives. Specifically, we refer to those targeting the whole population as 'promotion/universal prevention', those targeting people at risk for developing a mental disorder as 'selective prevention', and those designed for already presenting some sign of mental disorders as 'indicated prevention'. Below we report evidence of the results of low to moderate‐certainty, directly after the intervention. We did not present results for outcomes for which there was no or very uncertain evidence.
Promotion/universal prevention interventions, compared to usual care:
‐ probably slightly reduced anxiety symptoms in adults
‐ may slightly reduce distress/PTSD symptoms in adults
‐ may improve the quality of life of children
‐ may reduce adverse events in children
‐ may slightly reduce depression symptoms in children
‐ may slightly reduce anxiety symptoms in children
Selective prevention interventions, compared to usual care:
‐ probably slightly reduced depressive symptoms in adults
‐ may slightly reduce functional impairment in children
‐ probably slightly reduced depressive symptoms in children
‐ may slightly reduce anxiety symptoms in children
‐ probably slightly reduced distress/PTSD symptoms in children
Indicated prevention interventions,compared to usual care:
‐ may reduce adverse events in adults
‐ probably slightly reduced functional impairment in adults
‐ decreased slightly functional impairment in children
‐ decreased slightly depressive symptoms in children
‐ may slightly reduce distress/PTSD symptoms in children
Indicated prevention interventions delivered through task‐shifting may improve mental health outcomes.
What are the limitations of the evidence?
The limitations of the evidence in this review stem from the absence of assessments related to the reduction in the incidence of mental disorders in the prevention studies, and the lack of discernible differences in acceptability. Furthermore, the limited number of randomized controlled trials reporting our secondary outcomes, and their low quality, failed to demonstrate clinically significant advantageous effects of the studied prevention interventions for some outcomes in both child and adult populations.
How up‐to‐date is the review?
Review authors searched databases up to November 2021 to find and include all relevant published and unpublished trials.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Promotion/universal prevention interventions compared to control group in preventing mental disorders in adults.
| Promotion/universal prevention interventions compared to control group in preventing mental disorders in adults | ||||||
| Patient or population: preventing mental disorders Setting: low‐and middle‐income countries (China (1 study), Suriname (1 study), Malaysia (1 study), Jamaica (1 study), South Africa (1 study), Pakistan (1 study), Grenada (1 study)) Intervention: promotion/universal prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with promotion/universal prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Quality of life at study endpoint (higher score = better quality of life) | ‐ | SMD 0.23 SD lower (0.51 lower to 0.04 higher) | ‐ | 684 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Scores estimated based on an SMD of ‐0.23 (95% CI ‐0.51 to 0.04). It is uncertain whether promotion/universal prevention interventions have any effect on quality of life among adults without risk factors for mental disorders (at post‐intervention) compared with usual care [there is a small effect according to Cohen 1992].1 |
| Adverse events at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Psychological functioning and impairment at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Depressive symptoms at study endpoint (higher score = higher severity ) | ‐ | SMD 0.31 SD lower (0.78 lower to 0.15 higher) | ‐ | 349 (3 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | Scores estimated based on an SMD of ‐0.31 (95% CI ‐0.78 to 0.15). It is uncertain whether promotion/universal prevention interventions have any effect on depressive symptoms in adults without risk factors for mental disorders (at post‐intervention) compared to usual care [this is a small effect according to Cohen 1992].1 |
| Anxiety symptoms at study endpoint (higher score = higher severity) | The mean anxiety symptoms at study endpoint was 0 | MD 0.14 lower (0.27 lower to 0.01 lower) | ‐ | 158 (1 RCT) | ⊕⊕⊕⊝ Moderateg | Promotion/universal prevention interventions for adults without risk factors for mental disorders probably slightly reduce anxiety symptoms (at post‐intervention) compared to usual care [there is a small effect according to Cohen 1992].1 |
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.24 SD lower (0.41 lower to 0.08 lower) | ‐ | 722 (4 RCTs) | ⊕⊕⊝⊝ Lowh,i | Scores estimated based on an SMD of ‐0.24 (95% CI ‐0.41 to ‐0.08). Promotion/universal prevention interventions may slightly reduce distress/PTSD symptoms in adults without risk factors for mental disorders (at post‐intervention) comparedto usual care [there is a small effect according to Cohen 1992].1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913845297007331. | ||||||
a Downgraded 2 levels owing to study limitations (over 30% of RCTs have overall high risk of bias) b Downgraded 1 level owing to inconsistency (I2 between 50% and 75% (P = 0.05)) c Downgraded 2 levels owing to indirectness (participants with 17‐20 years of age for Duan 2019; outcome measures as proxy of quality of life for Duan 2019 and Hendricks 2019) d Downgraded 1 level owing to study limitations (23% of RCTs had overall high risk of bias. Over 30% of RCTs had overall some concerns.) e Downgraded 2 levels owing to inconsistency (I2 was 75%, point estimates vary across studies) f Downgraded 1 level owing to indirectness (participants with 17‐20 years of age for Duan 2019; unclear age for Yusoff 2015) g Downgraded 1 level owing to imprecision (outcome based on a small number of participants, less than 200) h Downgraded 1 level owing to study limitations (over 30% of studies had some concerns due to deviations from intended interventions and in selection of the reported result) i Downgraded 1 level owing to indirectness (participants with 17‐20 years of age for Duan 2019; outcome measures as proxy of distress for Baker‐Henningham 2019) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Summary of findings 2. Summary of findings table ‐ Selective prevention interventions compared to control group in preventing mental disorders in adults.
| Selective prevention interventions compared to control group in preventing mental disorders in adults | ||||||
| Patient or population: preventing mental disorders Setting: low‐ and middle‐income countries (Thailand (1 study), Lebanon (1 study), Iran (2 studies), Jamaica (1 study), Pakistan (1 study), The Gambia (1 study)) Intervention: selective prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with selective prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Quality of life at study endpoint (higher score = better quality of life) | ‐ | SMD 1.64 lower (2.97 lower to 0.31 lower) | ‐ | 229 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Scores estimated based on an SMD of ‐1.64 (95% CI ‐2.97 to ‐0.31). It is uncertain whether selective prevention interventions have any effect on quality of life among adults with risk factors for mental disorders/lack of protective factors (at post‐intervention) compared with usual care. [There is a large effect according to Cohen 1992]1 |
| Adverse events at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Psychological functioning and impairment at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Depressive symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.69 lower (1.08 lower to 0.3 lower) | ‐ | 223 (4 RCTs) | ⊕⊕⊕⊝ Moderated | Scores estimated based on an SMD of ‐0.69 (95% CI ‐1.08 to ‐0.3). Selective prevention interventions for adults with risk factors for mental disorders/lack of protective factors probably slightly reduce depressive symptoms (at post‐intervention)compared to usual care. [There is a medium effect according to Cohen 1992]1 |
| Anxiety symptoms at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.9 lower (1.44 lower to 0.36 lower) | ‐ | 535 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,e | Scores estimated based on an SMD of ‐0.90 (95% CI ‐1.44 to ‐0.36). It is uncertain whether selective prevention interventions have any effect on distress/PTSD symptoms in adults with risk factors for mental disorders/lack of protective factors (at post‐intervention) compared to usual care. [There is a large effect according to Cohen 1992]1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913907887557578. | ||||||
a Downgraded 2 level owing to inconsistency (I2 was higher than 75%, P < 0.00001) b Downgraded 1 level owing to indirectness (outcome measures as proxy of quality of life) c Downgraded 1 level owing to imprecision (outcome based on a small number of participants) d Downgraded 1 level owing to study limitations (over 20% of RCTs have overall high risk of bias, and all others RCTs have overall some concerns) e Downgraded 1 level owing to indirectness (outcome measures as proxy of distress) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Summary of findings 3. Summary of findings table ‐ Indicated prevention interventions compared to control group in preventing mental disorders in adults.
| Indicated prevention interventions compared to control group in preventing mental disorders in adults | ||||||
| Patient or population: preventing mental disorders Setting: low‐ and middle‐income countries (Turkey, Iran (2 studies), China (4 studies), Malaysia, Guatemala, India (3 studies), Bosnia and Herzegovina, Brazil (3 studies), Vietnam, South Africa (3 studies), Tanzania, Kenya, Nepal, Burundi, Jamaica (2 studies), Ghana, Philippines) Intervention: indicated prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with indicated prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint (RR < 1 denotes lower risk of mental diagnosis) | 170 per 1000 | 51 per 1000 (10 to 267) | RR 0.30 (0.06 to 1.57) | 843 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | It is uncertain whether indicated prevention interventions have any effect on the risk of mental disorders in adults with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. |
| Quality of life at study endpoint (higher score = better quality of life) | ‐ | SMD 0.36 lower (0.61 lower to 0.12 lower) | ‐ | 1136 (8 RCTs) | ⊕⊝⊝⊝ Very lowe,f | Scores estimated based on an SMD of ‐0.36 (95% CI ‐0.61 to ‐0.12). It is uncertain whether indicated prevention interventions have any effect on quality of life among adults with a high vulnerability to develop mental disorders (at post‐intervention) compared with usual care. [There is a small effect according to Cohen 1992]1 |
| Adverse events at study endpoint | Not pooled | Not pooled | Not pooled | (1 RCT) | ⊕⊕⊝⊝ Lowg,h | Indicated prevention interventions may reduce adverse events in adults with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. |
| Psychological functioning and impairment at study endpoint (higher score = higher disability) | ‐ | SMD 0.12 lower (0.39 lower to 0.15 higher) | ‐ | 663 (4 RCTs) | ⊕⊕⊕⊝ Moderatei | Scores estimated based on an SMD of ‐0.12 (95% CI ‐0.39 to 0.15). Indicated prevention interventions for adults with a high vulnerability to develop mental disorders probably slightly reduce functional impairment (at post‐intervention)compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Depressive symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.16 lower (0.3 lower to 0.03 lower) | ‐ | 2341 (18 RCTs) | ⊕⊝⊝⊝ Very lowj,k,l | Scores estimated based on an SMD of ‐0.16 (95% CI ‐0.3 to ‐0.03). It is uncertain whether indicated prevention interventions have any effect on depressive symptoms in adults with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Anxiety symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 1.19 lower (2.02 lower to 0.35 lower) | ‐ | 250 (5 RCTs) | ⊕⊝⊝⊝ Very lowm,n | Scores estimated based on an SMD of ‐1.19 (95% CI ‐2.02 to ‐0.035). It is uncertain whether indicated prevention interventions have any effect on depressive symptoms in adults with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a large effect according to Cohen 1992]1 |
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.54 lower (0.95 lower to 0.14 lower) | ‐ | 2536 (19 RCTs) | ⊕⊝⊝⊝ Very lowl,n | Scores estimated based on an SMD of ‐0.54 (95% CI ‐0.95 to ‐0.14). It is uncertain whether indicated prevention interventions have any effect on distress/PTSD symptoms in adults with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a medium effect according to Cohen 1992]1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913973563280333. | ||||||
a Downgraded 2 levels owing to study limitations (over 30% of RCTs had high risk of bias due to missing outcome data and in selection of the reported result) b Downgraded 2 levels owing to inconsistency (I2 was higher than 75%, point estimates vary widely across studies) c Downgraded 1 level owing to indirectness (outcome measures as proxy of diagnosis of mental disorders) d Downgraded 1 level owing to imprecision (outcome based on wide confidence interval ranged from favouring Indicated Prevention Intervention to no clinical effect) e Downgraded 2 levels owing to inconsistency (I2 was higher than 75%, P = 0.003) f Downgraded 1 level owing to indirectness (outcome measures as proxy of quality of life) g Downgraded 1 level owing to publication bias (only 1 "negative" RCT) h Downgraded 1 level owing to study limitations i Downgraded 1 level owing to indirectness (outcome measures as proxy of psychological functioning and impairment) j Downgraded 1 level owing to study limitations (all RCTs had some concerns in measurement of the outcome; over 10% of studies had high concerns due to deviations from intended interventions) k Downgraded 1 level owing to inconsistency (I2 between 50% and 75%, P = 0.002) l Downgraded 1 level owing to publication bias (funnel plot suggests high asymmetry: RCTs expected in the bottom right quadrant are missing) m Downgraded 1 level owing to study limitations (over 30% of RCTs had some concerns due to deviations from intended interventions and in measurement of the outcome) n Downgraded 2 levels owing to study limitations (over 30% of RCTs had some concerns due to deviations from intended interventions and in measurement of the outcome) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Summary of findings 4. Summary of findings table ‐ Promotion/universal prevention interventions compared to control group in preventing mental disorders in children.
| Promotion/universal prevention interventions compared to control group in preventing mental disorders in children | ||||||
| Patient or population: preventing mental disorders Setting: low‐ and middle‐income countries (Brazil (1 study), Uganda (1 study), Mexico (1 study), Tanzania (1 study), Mauritius (1 study), Iran (1 study)) Intervention: promotion/universal prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with promotion/universal prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Quality of life at study endpoint (higher score = better quality of life) | ‐ | SMD 0.25 SD lower (0.39 lower to 0.11 lower) | ‐ | 803 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Scores estimated based on an SMD of ‐0.25 (95% CI ‐0.39 to ‐0.11). Promotion/universal prevention interventions may improve the quality of life of children without risk factors for mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Adverse events at study endpoint (RR < 1 indicates lower risk of adverse events) | Not pooled | Not pooled | Not pooled | (1 RCT) | ⊕⊕⊝⊝ Lowc,d | Promotion/universal prevention interventions may reduce adverse events in children without risk factors for mental disorders (at post‐intervention) compared to usual care |
| Psychological functioning and impairment at study endpoint (higher score = higher disability) | ‐ | SMD 0.04 higher (0.9 lower to 0.98 higher) | ‐ | 212 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,e,f,g | Scores estimated based on an SMD of 0.04 (95% CI ‐0.9 to 0.98). It is uncertain whether promotion/universal prevention interventions have any effect on functional impairment in children without risk factors for mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Depressive symptoms at study endpoint (higher score = higher severity) | MD 3.04 SD lower (6 lower to 0.08 lower) | ‐ | 160 (1 RCT) | ⊕⊕⊝⊝ Lowc,h | Promotion/universal prevention interventions may slightly reduce depression symptoms in children without risk factors for mental disorders (at post‐intervention) compared to usual care. [There is a large effect according to Cohen 1992]1 | |
| Anxiety symptoms at study endpoint (higher score = higher severity) | MD 2.77 higher (3.13 lower to 1.41 lower) | ‐ | 183 (1 RCT) | ⊕⊕⊝⊝ Lowc,h | Promotion/universal prevention interventions may slightly reduce anxiety symptoms in children without risk factors for mental disorders (at post‐intervention) compared to usual care. [There is a medium effect according to Cohen 1992]1 | |
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.83 SD lower (2.48 lower to 0.82 higher) | ‐ | 800 (2 RCTs) | ⊕⊝⊝⊝ Very lowi,j,k,l | Scores estimated based on an SMD of ‐0.83 (95% CI ‐2.48 to 0.82). It is uncertain whether promotion/universal prevention interventions have any effect on distress/PTSD symptoms in children without risk factors for mental disorders (at post‐intervention) compared to usual care. [There is a large effect according to Cohen 1992]1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913294102284283. | ||||||
a Downgraded 1 level owing to study limitations (all RCTs had some concerns for the deviations from the intended interventions and in measurement of the outcome) b Downgraded 1 level owing to indirectness (outcome measures as proxy of quality of life) c Downgraded 1 level owing to study limitations (RCT had some concerns for the deviations from the intended interventions and in measurement of the outcome) d Downgraded 1 level owing to imprecision (0 total events) e Downgraded 2 levels owing to inconsistency (I2 was higher than 75%, point estimates vary widely across RCTs, and CIs show minimal overlap) f Downgraded 1 level owing to indirectness (outcome measures as proxy of psychological functioning and impairment) g Downgraded 1 level owing to imprecision (outcome based on wide confidence interval that included no effect and appreciable benefit and harm) h Downgraded 1 level owing to imprecision (outcome based on a small number of participants, less than 200) i Downgraded 2 level owing to study limitations (over 30% of RCTs had high risk of bias due to deviations from the intended interventions and missing outcome data) j Downgraded 2 levels owing to inconsistency (I2 was higher than 75%, P < 0.00001, point estimates vary widely across studies, and CIs show no overlap) k Downgraded 1 level owing to indirectness (outcome measures as proxy of distress) l Downgraded 1 level owing to imprecision (wide confidence interval ranged from favouring promotion/prevention intervention to no clinical effect) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Summary of findings 5. Summary of findings table ‐ Selective prevention interventions compared to control group in preventing mental disorders in children.
| Selective prevention interventions compared to control group in preventing mental disorders in children | ||||||
| Patient or population: preventing mental disorders Setting: low‐ and middle‐income countries (Uganda (1 study), Thailand (1 study), Democratic Republic of Congo (1 study), Brazil (1 study)) Intervention: selective prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with selective prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Quality of life at study endpoint (higher score = better quality of life) | MD 1.1 higher (3.32 lower to 1.12 higher) | ‐ | 115 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether selective prevention interventions have any effect on quality of life among children with risk factors for mental disorders/lack of protective factors (at post‐intervention) compared with usual care. [There is a small effect according to Cohen 1992]1 | |
| Adverse events at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Psychological functioning and impairment at study endpoint (higher score = higher disability) | MD 0.02 higher (0.09 lower to 0.05 higher) | ‐ | 479 (1 RCT) | ⊕⊕⊝⊝ Lowc,d | There is no evidence that selective prevention interventions improve functional impairment in children with risk factors for mental disorders/lack of protective factors (at post‐intervention) compared to usual care. 1 | |
| Depressive symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0 SD (0.16 lower to 0.15 higher) | ‐ | 638 (2 RCTs) | ⊕⊕⊕⊝ Moderated | Scores estimated based on an SMD of 0.0 (95% CI ‐0.16 to 0.15). Selective prevention interventions for children with risk factors for mental disorders/lack of protective factors probably slightly reduce depressive symptoms (at post‐intervention)compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Anxiety symptoms at study endpoint (higher score = higher severity) | MD 4.5 higher (12.05 lower to 21.05 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ Lowe | Selective prevention interventions may make little or no difference to anxiety symptoms in children with risk factors for mental disorders/lack of protective factors (at post‐intervention) compared to usual care. 1 | |
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | MD 2.14 lower (3.77 lower to 0.51 lower) | ‐ | 159 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Selective prevention interventions for children with risk factors for mental disorders/ lack of protective factors probably slightly reduce distress/PTSD symptoms (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913697396673091. | ||||||
a Downgraded 2 levels owing to study limitations (over 30% of RCTs had high risk of bias due to deviations from intended interventions and missing outcome data, in measurement of the outcome, and in selection of the reported result) b Downgraded 1 level owing to imprecision (outcome based on a small number of participants, less than 200) c Downgraded 1 level owing to indirectness (outcome measures as proxy of psychological functioning and impairment) d Downgraded 1 level owing to imprecision (confidence interval ranged from favouring selective prevention intervention to no clinical effect) e Downgraded 2 levels owing to imprecision (outcome based on a small number of participants, less than 200, and confidence interval ranged from favouring selective prevention intervention to no clinical effect) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Summary of findings 6. Summary of findings table ‐ Indicated prevention interventions compared to control group in preventing mental disorders in children.
| Indicated prevention interventions compared to control group in preventing mental disorders in children | ||||||
| Patient or population: preventing mental disorders Setting: low‐ and middle‐income countries (China (1 study), Tanzania (1 study), Kenya (1 study), Sri Lanka (1 study), Belize (1 study)) Intervention: indicated prevention interventions Comparison: control group | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control group | Risk with indicated prevention interventions | |||||
| Diagnosis of mental disorders at study endpoint (RR < 1 denotes lower risk of mental diagnosis) | 336 per 1000 | 259 per 1000 (171 to 393) | RR 0.77 (0.51 to 1.17) | 220 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | It is uncertain whether indicated prevention interventions have any effect on the risk of mental disorders in children with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. |
| Quality of life at study endpoint (higher score = better quality of life) | ‐ | SMD 0.65 SD lower (2.09 lower to 0.79 higher) | ‐ | 152 (2 RCTs) | ⊕⊝⊝⊝ Very lowd,e,f | Scores estimated based on an SMD of ‐0.65 (95% CI ‐2.09 to 0.79). It is uncertain whether indicated prevention interventions have any effect on quality of life among children with a high vulnerability to develop mental disorders (at post‐intervention) compared with usual care. [There is a small effect according to Cohen 1992]1 |
| Adverse events at study endpoint | No studies that measured this outcome were identified. | (0 studies) | ‐ | |||
| Psychological functioning and impairment at study endpoint (higher score = higher disability) | ‐ | SMD 0.29 SD lower (0.47 lower to 0.1 lower) | ‐ | 448 (2 RCTs) | ⊕⊕⊕⊕ High | Scores estimated based on an SMD of ‐0.29 (95% CI ‐0.47 to ‐0.1). Indicated prevention interventions decrease slightly functional impairment in children with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is [a small effect according to Cohen 1992]1 |
| Depressive symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.18 SD lower (0.32 lower to 0.04 lower) | ‐ | 771 (4 RCTs) | ⊕⊕⊕⊕ High | Scores estimated based on an SMD of ‐0.18 (95% CI ‐0.32 to ‐0.04). Indicated prevention interventions decrease slightly depressive symptoms in children with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Anxiety symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.09 lower (0.22 lower to 0.04 higher) | ‐ | 888 (3 RCTs) | ⊕⊝⊝⊝ Very lowg,h | Scores estimated based on an SMD of ‐0.09 (95% CI ‐0.22 to 0.04). It is uncertain whether indicated prevention interventions have any effect on anxiety symptoms in children with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| Distress/PTSD symptoms at study endpoint (higher score = higher severity) | ‐ | SMD 0.24 SD higher (1.28 lower to 1.76 higher) | ‐ | 448 (2 RCTs) | ⊕⊕⊝⊝ Lowi,j | Scores estimated based on an SMD of 0.24 (95% CI ‐1.28 to 1.76). Indicated prevention interventions may slightlyreduce distress/PTSD symptoms in children with a high vulnerability to develop mental disorders (at post‐intervention) compared to usual care. [There is a small effect according to Cohen 1992]1 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429913780370754267. | ||||||
a Downgraded 1 level owing to study limitations (RCT did not provided information about allocation concealment, and outcome assessment was not described as masked) b Downgraded 1 level owing to indirectness (outcome measures as proxy of depression) c Downgraded 1 level owing to imprecision (outcome based on wide confidence interval ranging from favouring indicated prevention intervention to no clinical effect) d Downgraded 2 levels owing to study limitations (over 30% of RCTs had high risk in selection of the reported result) e Downgraded 1 level owing to indirectness (outcome measures as proxy of quality of life) f Downgraded 1 level owing to imprecision (outcome based on a small number of participants, less than 200) g Downgraded 2 levels owing to study limitations (over 30% of RCTs had high risk of bias due to deviations from intended interventions and missing outcome data) h Downgraded 1 level owing to indirectness (outcome measures as proxy of anxiety) i Downgraded 1 level owing to inconsistency (point estimates vary widely across studies) j Downgraded 1 level owing to imprecision (outcome based on wide confidence interval that included no effect and appreciable benefit and harm) 1 J, Cohen. A power primer. Psychological Bulletin ; 1992.
Background
Description of the condition
Worldwide, the global burden of mental, neurological, and substance use disorders is high. The latest global burden of disease studies estimated that mental, behavioural, and neuropsychiatric disorders are amongst the top 30 causes of all years lived with disability; the highest contributors are anxiety and depressive disorders, drug use disorders, and alcohol use disorders (Kyu 2018; Murray 2020; Santomauro 2021). Mental health and behavioural disorders contribute 7.4% of the global burden of disease in the world ‐ more than, for example, tuberculosis (2%), HIV/AIDS (3.3%), or malaria (4.6%) (Whiteford 2013). The contribution of major depressive disorders alone to worldwide disability‐adjusted life‐years (DALYs) increased by 37% between 1990 and 2010 and is predicted to rise further (Murray 2012; Prince 2007). In addition, self‐inflicted injuries and alcohol‐related disorders are likely to increase in the ranking of disease burden due to the decline in communicable diseases and because of a predicted increase in war and violence. The disease burden due to Alzheimer’s disease is also increasing; this is linked to the demographic transition towards an ageing population (Vos 2012). Despite these figures, levels of public expenditure on mental health are still low (a global median of 2.1% of government health expenditure) and particularly meagre in lower resource settings. Data from official WHO reports highlight persistent gaps in the availability of mental health resources, significant variation between high‐ and low‐income countries and across regions, and the need for continued country‐level investment in policies, plans, health services, and monitoring systems for mental health care (WHO 2021a).
Political instability, war, and natural disasters disproportionally affect populations living in low‐ and middle‐income countries (LMICs) (Guha‐Sapir 2014). In addition, people living in these contexts, are more prone to experiencing extreme chronic poverty (less than 1.90 international dollars per day), which causes about 385 million of children under the age of 18 years to experience severe deprivation of basic human needs, including food, water, sanitation, health, and education. The World Health Organization (WHO) estimates that the prevalence of post‐traumatic stress disorder (PTSD), depression, and mixed anxiety disorders in conflict settings is at 22% (Charlson 2019), which is, conservatively, around five times higher than the general population. The COVID‐19 pandemic is an additional public health emergency that occurred against an already complex backdrop of psychological suffering. It further affected social determinants of mental health, also rasing concerns about the deprioritization of the psychological needs of people in LMICs. Risk factors for mental disorders and increased symptomatology can include community‐level risk factors (e.g. neighbourhood disadvantages, high levels of community violence, community‐level gender inequitable norms), family‐level risk factors (e.g. intimate partner violence, harsh parenting), or individual‐level risk factors (e.g. low self‐esteem, maladaptive coping strategies). Similarly, protective factors can operate at multiple levels of the social environment. Promotion interventions may target promotive factors (i.e. factors associated with an increased chance of achieving positive mental health states).
These illnesses also imply substantial economic costs. One recent report on the global economic burden of non‐communicable diseases (NCDs) suggests that, by the early 2030s, mental health conditions alone will account for the loss of an additional USD 16.1 trillion, with a dramatic impact on productivity and quality of life (Bloom 2011). Data on the macroeconomic costs for LMIC settings remain uncertain (Hu 2006). However, the economic and social costs for individuals and families are substantial. High direct costs are incurred in countries where health spending is met largely through private, as opposed to public, spending, and where health insurance and employer‐met health payments are insubstantial (Patel 2007b). High indirect costs are incurred as the result of informal caregiving and lost work opportunities, as well as untreated disorders and their associated disabilities (Chisholm 2000).
All over the world, the gap between individuals in need of mental health interventions and those who actually receive such care is very large (WHO 2018). Previous estimates suggest that more than 56% of people with depression (De Silva 2014; Kohn 2004; Lora 2012; Patel 2010), along with 78% of persons with alcohol use disorders (Kohn 2004), do not receive care. A study of 21 countries via the WHO Mental Health Surveys found that 52.6% of persons with depressive disorders in LMICs had received no treatment in the past 12 months, and only 20.5% of persons with depressive disorders had received minimally adequate treatment (Thronicroft 2016). Major barriers to closing the treatment gap include scarcity of skilled human resources, large inequities and inefficiencies in resource distribution and utilization, and the significant stigma associated with mental health conditions (Barber 2019). Recent studies report the existence of a range of cost‐effective interventions in mental health care available that can be implemented in LMICs (Barbui 2020; Purgato 2018a). The global mental health community, therefore, advocates for the scaling‐up of these evidence‐based services, that focus on task‐shifting as a mean to bridge the treatment gap (Patel 2018).
Description of the intervention
A recent Lancet Commission sought to align global mental health efforts with sustainable development goals, and emphasized the importance of efforts to prevent mental health disorders and promote mental health, in addition to scaling‐up treatments (Patel 2018). Prevention and promotion of mental health have previously been advocated as critical by the WHO (WHO 2002), and prevention is part of the WHO Mental Health Action Plan (WHO 2013). Prevention and promotion efforts are an important complementary focus, in addition to the treatment of mental disorders, given that (1) many mental disorders have risk factors in the social environment (e.g. gender‐based violence, poverty) that can be effectively addressed; (2) there are limitations to the extent that treatments alone can reduce the burden associated with mental disorders (Freeman 2016); and (3) cost‐effective prevention and promotion interventions are available (Knapp 2011).
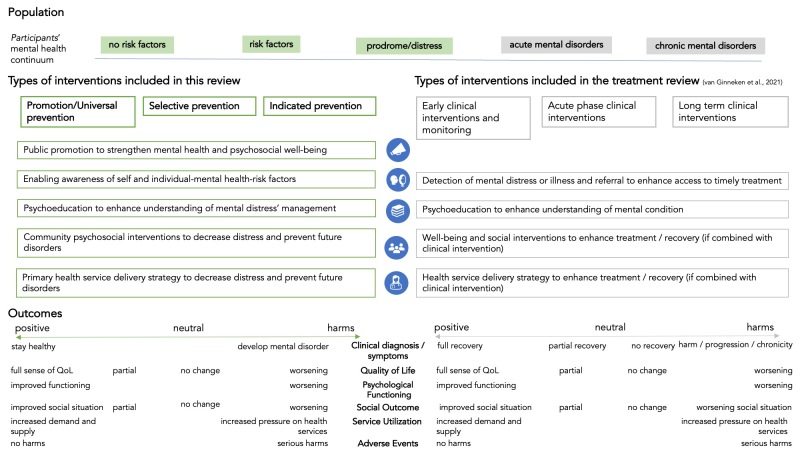
In the present review, we followed the classification of promotion and prevention interventions described by the Institute of Medicine (IOM) report on preventing mental disorders (Institute of Medicine 1994; Institute of Medicine 2009; National Academies of Sciences 2019).
Promotion is an approach aimed at strengthening positive aspects of mental health and psychosocial well‐being; it includes, for example, intervention components that foster pro‐social behaviour, self‐esteem, positive coping with stress, and decision‐making capacity (Table 7) (WHO 2014). The definition of promotion has been recently updated to include a wider set of interventions provided at societal, community, individual, and family levels. These updates reflect important trends in research in the field of public mental health and reveal the enduring importance of a spectrum of key tools for fostering mental health (National Academies of Sciences 2019).
1. Definitions.
| Adults | Participants who were ≥ 18 years old. If some studies had an age range from, for example, 16 years upwards, and a majority of participants (≥ 80%) were over 18 years of age, we included these study participants as adults. |
| Children and adolescents | Children (from birth to 18 years) were considered as a separate group of participants, as they have: • different patterns of psychopathology/mental disorders; and • different help‐seeking behaviours that would, therefore, require different interventions, in different settings (e.g. schools), and a different approach to interventions (e.g. worker interventions such as teacher‐led interventions). |
| Promotion | Promotion is an approach aimed at strengthening positive aspects of mental health and psychosocial well‐being; it includes, for example, components to foster prosocial behaviour, self‐esteem, positive coping with stress, and decision‐making capacity (National Academies of Sciences 2019; WHO 2014). Prevention is an approach aimed at reducing the likelihood of future disorder within the general population or amongst people who are identified as being at risk for developing a full‐blown disorder (Eaton 2012; Tol 2015a). |
| Universal prevention | Universal prevention includes strategies that can be offered to the whole population, based on evidence that prevention strategies are likely to provide some benefit to all (i.e. reduce the probability of a disorder), which clearly outweighs the costs and risks of negative consequences. Examples of common universal prevention interventions include:
|
| Selective prevention | Selective prevention refers to strategies that are targeted to subpopulations identified as being at elevated risk for a disorder; it includes:
|
| Indicated prevention | Indicated prevention includes strategies that are targeted to individuals who are identified (or individually screened) as having increased vulnerability for a disorder based on some individual assessment. These interventions include:
|
| First‐level care, primary care, and community care | First‐level contact with formal health services consists of community‐based interventions or primary care interventions (or both), on their own or attached to hospital settings, provided they had no specialist input apart from supervision (modified from Wiley‐Exley 2007). This would include promotion or prevention programmes in outpatient clinics or primary care practices. This would not include programmes in hospitals unless these programmes were providing prevention interventions to outpatients. Community programmes involve detection of mental disorders in all age groups, often done outside the health facility, for example, through school, training, and other community settings. |
| Low‐ and middle‐income countries (LMICs) | Any country that has ever been an LMIC, as defined by the World Bank lists of LMICs |
| Primary care health workers (PHWs) | Health workers who are not specializing in mental disorders or have not received in‐depth professional specialist training in this clinical area. They work in primary care centres or in the community. These individuals include doctors, nurses, auxiliary nurses, lay health workers, and allied health personnel such as social workers and occupational therapists. This category does not include professional specialist health workers such as psychiatrists, psychiatric nurses, or mental health social workers. For inclusion, PHWs received some training in mental conditions (in the control group or in the intervention group), but this would not constitute a professional category. Study authors made a judgement of what constitutes ‘some training’. Examples of ‘some training’ may include an undergraduate module or a short course in mental health. |
| Community workers (CWs) | People involved as community‐level workers but who are not within the health sector, as many people, particularly adolescents and young adults, have limited contact with health workers. This category includes teachers/trainers/support workers from schools and colleges, along with other volunteers or workers within community‐based networks or nongovernmental organisations. These CWs have an important role, particularly in promotion of mental health and detection of mental disorders (Patel 2007b; Patel 2008). We excluded from this review studies that looked at informal care provided by family members or that extended care only to members of their own family (i.e. who were unavailable to other members of the community). As was previously highlighted in Lewin’s Cochrane Review, “these interventions are qualitatively different from other LHW [lay health worker] interventions included in this review given that parents or spouses have an established close relationship with those receiving care, which could affect the process and effects of the intervention” (Lewin 2010). |
| Primary‐level workers (PWs) | Broad term to encompass both CWs and PHWs |
CW: community worker LMIC: low‐ and middle‐income country PHW: primary‐level health worker PW: primary‐level worker
Prevention is an approach aimed at reducing the likelihood of future mental disorders in the general population or amongst people who are identified as being at risk for developing a full‐blown mental disorder (Eaton 2012; Purgato 2020; Tol 2015a). Prevention is further subdivided on the basis of the targeted population.
-
Universal prevention includes strategies that can be offered to the whole population based on evidence that prevention strategies are likely to provide some benefit to all (i.e. reduce the probability of disorder), which clearly outweighs the costs and risks of negative consequences. Examples of common universal prevention interventions include:
community‐wide provision of information on the negative effects of specific behaviours;
protection against human rights violations in the whole population (e.g. community mobilization to reduce gender‐based violence); and
community‐wide efforts to improve livelihood as a key protective factor for mental health (e.g. working on lifting restrictions on movement and employment for everyone in a refugee camp).
-
Selective prevention refers to strategies that target subpopulations identified as being at elevated risk for a disorder because they have known risk factors or lack protective factors. Examples include:
support for children whose parents have a mental illness;
strengthening of community networks for vulnerable families by activating social networks and supportive communication; and
stress management training in communities affected by chronic poverty.
-
Indicated prevention includes strategies that are targeted to individuals who are identified (or individually screened) as having increased vulnerability for a disorder based on some individual assessment of symptoms experienced but not meeting criteria for a full‐blown mental disorder. These interventions include but are not limited to:
mentoring programmes aimed at teachers and caregivers of children with behavioural problems; and
-
prevention of postnatal depression amongst women with heightened levels of prenatal symptoms (Institute of Medicine 2009) or interventions to prevent intimate partner violence (Turner 2020).
These interventions may be delivered at an individual level or at a group level and include antenatal and postnatal classes, parenthood classes, group interventions for managing distress, and continuity of care (home visits, follow‐ups) (O'Connor 2019; US Preventive Services Task Force 2019).
Primary healthcare workers (PHWs) are first‐level providers who have received general health rather than specialist training in mental health and can be based in a primary care clinic or in the community. It has been suggested that PWs (primary‐level workers) could deliver general and mental health interventions that are at least as effective and acceptable as those delivered by specialist health workers (Chatterjee 2003). In addition, PW interventions often have lower up‐front costs compared with those provided by professional specialist health workers. However, it is possible that these savings may be cancelled out by higher downstream resource use (Chisholm 2000). To address this concern, we aimed to include data on the costs and cost‐effectiveness of PW interventions. PHWs include professionals (mainly nurses, midwives, and other general health professionals) and para‐professionals (such as trained lay health providers, e.g. traditional birth attendants). PHWs do not include those with specialist mental health training, for example, psychiatrists, psychologists, psychiatric nurses, or mental health social workers with formal training. Community workers (CWs) are individuals who work in the community but not within the health sector. These might include teachers, trainers, support workers at schools and colleges, and other volunteers or workers within community‐based networks or non‐governmental organizations (NGOs). CWs are an additional human resource that can be widely deployed in delivering promotion and prevention interventions (Patel 2007a). In this review, both these categories of providers (PHWs and CWs) are referred to under the umbrella heading of primary‐level workers (PWs).
PWs have been deployed for a variety of services, including those delivered in governmental organizations, private clinics, halfway homes, schools, and other community settings. For example, PWs have been involved in supporting and befriending carers and in ensuring intervention adherence (Tol 2020). Nurses, social workers, and CWs may also take on follow‐up or educational/promotive roles (Araya 2003; Chatterjee 2003; Patel 2008). In addition, doctors with general mental health training have been involved in the identification, diagnosis, treatment, and referral of complex cases (Patel 2008).
A summary of the main definitions adopted in this review is provided in Table 7.
How the intervention might work
Prevention interventions commonly target known modifiable risk and protective factors for mental disorders.
Although a vast array of interventions can be implemented to meet a population's psychosocial needs, there are some common elements, especially when interventions target smaller groups or families. Many interventions include techniques from cognitive‐behavioural therapy (CBT) which work at stopping negative cycles, breaking down feelings and problems that generate psychological suffering, anxiety or depression into separate parts. This mechanism facilitates problem management and changes negative thought patterns, improving the way people feel (Hofmann 2017). CBT may comprise, for example, facilitated discussion, strengthening of social networks, space provided for sharing personal experiences and exchange of peer support, and opportunities to practice coping skills to manage adversity. Sessions on problem‐solving skills and emotional support Interventions may work by challenging participants to replace negative or critical self‐talk with compassionate, more constructive self‐talk, and to consider different viewpoints for managing problems (Hofmann 2017). Interventions may also consist of sessions with psychoeducational contents, strategies for stress management, enhanced insight and relationship/rapport building, networking support, communication skills, self‐help interventions, and motivational enhancement (Buntrock 2016; Panter‐Brick 2018).
In many LMICs, training and retaining sufficient numbers of mental health specialists to meet current needs is not feasible. Therefore, it is important in these settings to consider options for expanding access to mental health promotion and disorder prevention services. Given that they are far more numerous and often more accessible than mental health specialists, the deployment of PWs for this purpose is one option that could prove to be of value in LMICs. This review, therefore, focused on the evaluation of a task‐shifting model as a possible implementation modality for prevention and promotive interventions in LMICs.
Why it is important to do this review
This review is limited to LMICs, where the need for PWs is greater than in high‐income settings. Far fewer mental health professionals are present in LMICs (the median number of psychiatrists is 172 times lower in low‐income countries (LICs) than in high‐income countries (HICs)) (Kakuma 2011; WHO 2011b). These differences in the organization of mental health services between LMICs and HICs, with poorer countries having few or no mental health service structures in primary care or in the community, mean that the problem of providing mental health interventions, especially in preventing mental disorders or promoting psychological well‐being, is different in such settings. PWs may need to work with little or no support from specialist mental health services. Consequently, PW interventions might be expected to function differently in LMICs as compared to HICs (Cuijpers 2018; Purgato 2019a).
The paucity of mental health professionals and the abundance of challenges for mental health systems in LMICs make it imperative to focus attention on prevention and promotion strategies via a public health approach (Tol 2015a). To address current shortages of mental health workers, prevention interventions in LMICs have been conceived as short, simple, and mostly delivered through a task‐shifting approach that includes different forms of intervention delivery. Delivery strategies range from informal delivery of simple interventions to more complex and longer strategies, as stepped‐care models. Task‐shifting is increasingly emphasized in global mental health and holds promise for improving access to mental health interventions (Jensen 2018; Patel 2018).
However, reviews on the task‐shifting approach to mental health interventions in LMICs have tended to focus more on treatment interventions (Akhtar 2022; Purgato 2018a; Singla 2017; Van Ginneken 2021). Evidence on the effectiveness of mental health prevention and promotion interventions in LMICs is scarce. Systematic reviews have focused mainly on populations living in high‐income settings, raising applicability concerns related to contextual factors and resource availability, including, for example, the lack of professionals in low‐resource contexts (i.e. psychiatrists, and psychologists) (Barbui 2020). In addition, LMICs differ from HICs with regard to social determinants of mental health, e.g. exposure to conflicts and wars, poverty, and gender‐based violence may be more frequent in LMICs (Lund 2018).
Populations in LMICs can conceptualize and seek assistance for mental health problems and mental health promotion in a wide variety of ways; these approaches may differ from conceptualizations and help‐seeking patterns seen in high‐income industrialized countries. Thus, evidence regarding the effectiveness of interventions implemented in HICs may not directly apply or be relevant to LMICs. For the reasons mentioned above, we expect that interventions might be applied differently in LMICs (Abdulmalik 2019).
The aim of this review is therefore to bridge this gap by assessing the effectiveness of delivery by primary workers of interventions for the promotion of mental health and for prevention of mental disorders or symptoms of mental illness in LMICs. Through this work, we aim to provide a picture of the characteristics and quality of the promotive and preventative research initiatives that have been carried out in lower resource settings. In addition, we strive to provide insights on what type of promotion strategies (universal, selective, and indicated) work best for whom (children and adults). This is overall in line with the WHO principle of integrating mental health into primary care and with the specific prevention objective of the WHO Action Plan for global mental health (WHO 2008; WHO 2021b).
This review has been conducted in parallel with an update of the Cochrane Review focused on treatment interventions in LMICs (Van Ginneken 2013; Van Ginneken 2021).
Objectives
To assess the effectiveness of delivery by primary workers of interventions for the promotion of mental health and for prevention of mental disorders or symptoms of mental illness in LMICs.
To examine the impact of intervention delivery by primary workers on resource use and costs associated with the provision of mental health care in LMICs.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs. We included trials that employed a cross‐over design ‐ whilst we acknowledge that this design is rarely used in intervention studies ‐ and we used data from the first randomized stage only. We excluded quasi‐randomized trials, such as those that allocate participants by using alternate days of the week. We considered both individual and cluster‐randomized trials as eligible for inclusion.
We included economic studies conducted as part of included effectiveness studies. We considered full economic evaluations (cost‐effectiveness analyses, cost‐utility analyses, or cost‐benefit analyses), cost analyses, and comparative resource utilization studies. We planned to report only cost and resource usage outcomes from these studies.
Types of participants
Participants
We included participants of any age, gender, ethnicity, and religion. We conducted two separate meta‐analyses on the different outcomes ‐ one for children and adolescents (younger than 18 years) and one for adults (18 years of age and older). Studies with mixed population groups (children and adolescents; adults) were allocated according to the proportion of participants belonging to the child and adolescent age range, or to the adult age range. When the intervention was designed and directed to a specific population group (i.e. children and adolescents or adults), we entered outcome data on efficacy for the specific target group for which the intervention was designed and delivered. For each of these two populations, we conducted meta‐analyses by different outcomes. We included studies focused on the prevention of any common mental disorder. Carers of study participants were included in meta‐analyses on adults, as some interventions may be directed at carers rather than at participants themselves, and consequently, mental health outcomes were measured in carers.
Settings
We considered studies conducted in LMICs. We used the World Bank criteria for categorizing a country as low‐ or middle‐income (World Bank 2020); these criteria provide a historical date of when countries were LMICs. If a country was an LMIC at some point during the recruitment of study participants, we included the study. We excluded studies undertaken in high‐income countries (at the time of study recruitment).
We included mental health promotion and/or prevention interventions delivered in primary care or community settings, refugee camps, schools, communities, survivors’ homes, and detention facilities. We excluded studies evaluating mental health promotion and/or prevention interventions undertaken outside of primary or community settings.
Diagnoses
Given the focus on interventions for the promotion of mental health and prevention of mental disorders, we restricted this review to participants without any formal diagnosis at the time the trial was undertaken. However, because many studies screened on the basis of a risk factor or heightened symptoms (without excluding people with diagnosed mental disorders), we could not exclude trial participants who might have fulfilled the criteria for an actual psychiatric diagnosis that remained unobserved because it was not investigated when the trial was undertaken. For example, we included populations who left their homes due to a sudden impact, threat, or conflict; populations exposed to political violence/armed conflicts/natural and industrial disasters; those with major losses or in poverty; and those belonging to a group (i.e. discriminated against or marginalized) experiencing political oppression, family separation, disruption of social networks, destruction of community structures and resources and trust, increased gender‐based violence, and undermined community structures or traditional support mechanisms (IASC 2007).
Comorbidities
We included studies that recruited participants with physical disorders and studies that focused on the prevention of multiple mental disorders.
Types of interventions
Included interventions
We included trials of primary‐level and/or community health worker interventions for promoting mental health and/or preventing mental disorders. Included mental health promotion or prevention interventions were delivered by primary‐level and/or community workers. Primary‐level health workers (PHWs) are first‐level providers who have received general health rather than specialist mental health training and can be based in a primary care clinic or in the community. PHWs include professionals (doctors, nurses, midwives, and other general health professionals) and para‐professionals (such as trained lay health providers, e.g. traditional birth attendants). PHWs do not include those with specialist mental health training, for example, psychiatrists, psychologists, psychiatric nurses, or mental health social workers. Community workers (CWs) are individuals who work in the community but not within the health sector. These might include teachers, trainers, support workers from schools and colleges, and other volunteers or workers within community‐based networks or non‐governmental organizations (NGOs). These CWs are not trained health workers but have a mental health role. They represent a further human resource employed in the delivery of promotion and prevention interventions (Patel 2007a). In this review, both of these categories of providers (PHWs and CWs) were referred to under the umbrella heading of 'primary‐level workers' (PWs).
This review aimed to include the following comparisons.
Provision of promotion and/or prevention interventions by primary‐level health workers and/or community workers versus usual care (little prevention or promotion strategy).
Provision of promotion and/or prevention interventions by primary‐level health workers and/or community workers versus no prevention or promotion strategy.
Provision of promotion and/or prevention interventions by primary‐level health workers and/or community workers versus interventions delivered by professionals with specialist mental health training.
We grouped the interventions as follows.
Promotion of mental health (e.g. interventions with a mental health or psychological component aimed at creating living conditions and environments that support mental health and encourage healthy lifestyles). We included any types of promotion interventions with a mental health component, delivered at individual, group, family, community, and/or societal levels (National Academies of Sciences 2019).
Universal prevention (e.g. community‐wide provision of information on positive coping methods to help people feel safe and hopeful; to protect against human rights violations; and to support community‐wide efforts to reduce poverty as a key risk for mental illness) (IASC 2007).
Selective prevention (e.g. psychological first aid for people with heightened levels of psychological distress after exposure to severe stressors, loss, or bereavement). These interventions involve human, supportive, and practical help covering both a social and a psychological dimension. They work through communication (asking about people's needs and concerns; listening to people; and helping them to feel calm), practical support (i.e. providing meals or water); a psychological approach (including teaching stress management skills and helping people cope with problems) (WHO 2011a); facilitation of community support for vulnerable individuals by activating social networks and communication; and structured cultural and recreational activities supporting the development of resilience (Institute of Medicine 2009), such as traditional dancing, artwork, sports, and puppetry (Tol 2011).
Indicated prevention (e.g. mentoring programmes aimed at children with behavioural problems; psychosocial support for school children with subclinical levels of post‐traumatic stress disorder (PTSD), anxiety, depression, or somatic symptoms and related disorders; prevention of postnatal depression in women with heightened levels of prenatal symptoms) (Institute of Medicine 2009).
Interventions were delivered through any means, including, for example, face‐to‐face meetings, digital tools, radio, telephone, or self‐help booklets, between participants and PHWs. Both individual and group interventions were included, with no limit on the number of sessions.
As this review was conducted in parallel with the update of the Cochrane Review on treatment interventions (Van Ginneken 2013; Van Ginneken 2021), we looked at the aim of the study and decided whether the aim was prevention or treatment, and we looked at the inclusion criteria for participants (these criteria must include specific mental distress/prodromal symptoms or a diagnosable disorder). When there was no clear distinction between prevention and treatment groups, we made a pragmatic decision on whether these trials were primarily about well‐being/prevention or about treatment and then allocated them to the appropriate review, or included them in both reviews.
Excluded interventions
We excluded interventions aimed at treating people with a diagnosed mental disorder. We also excluded studies that included participants on the basis of scoring above a cut‐off on a symptom checklist, with the explicit authors' stated intention to identify people with mental disorders. We excluded interventions aimed at treating people with a diagnosed disorder (Van Ginneken 2021).
Types of outcome measures
Primary outcomes
Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders at study endpoint, determined according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV‐TR (APA 2000), DSM‐V (APA 2013), International Classification of Diseases (ICD)‐10 (WHO 1992), or any other standardized criteria.
Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders at 1 to 6 months post‐intervention.
Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders at 7 to 24 months post‐intervention.
Quality of life.
Adverse events experienced during the intervention.
Secondary outcomes
Psychological functioning and impairment.
Changes in service utilisation and contact coverage, including admission rates to hospital whether related to mental disorder or not; attendance rates in regard to utilization of primary or community services; or increased demand and/or referral rates from the primary/community setting to a mental health specialist.
Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms).
Social outcomes (e.g. perception of social inclusion).
Resource use (for health services: personnel time allocated/number of consultations, other opportunity costs of the intervention, or other aspects of the health service; for participants: extra costs of travel, lost productivity, employment status, income, work absenteeism, retention, educational attainment).
Carer mental health.
We grouped primary and secondary outcomes into three sets of time points.
Post‐intervention (0 to 1 month after the intervention) (to detect incidence/symptoms, reduction of the intervention).
1 to 6 months post‐intervention (to detect sustained incidence/symptom reduction).
7 to 24 months post‐intervention (indicating medium‐ to long‐term reduction).
We chose the latest time point within that category if several time points fitted within a category. We however included a time point that correlated best with other studies being compared within each outcome.
We included studies that reported only secondary outcomes of the review.
Search methods for identification of studies
Electronic searches
The EPOC (Effective Practice and Organisation of Care) Information Specialist developed search strategies in consultation with the review authors.
We searched the following databases for primary studies, from inception to date of search on 29 November 2021.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 11) in the Cochrane Library.
MEDLINE and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions: 1946 to 24 November 2021, Ovid.
Embase: 1974 to 24 November 2021, Ovid.
CINAHL (Cumulative Index to Nursing and Allied Health Literature): 1980 to date searched, EBSCO.
Global Index Medicus, World Health Organization (WHO) (www.globalindexmedicus.net/).
APA PsycINFO: 1806 to November Week 3 2021, Ovid.
Search strategies comprised natural language and controlled vocabulary terms. We did not apply any limits on the language of publication, and we searched all databases from inception to the date of search, except for MEDLINE and Embase. MEDLINE and Embase were limited to records from the last few months to find those not yet included in CENTRAL. Searches were limited by the use of study design filters appropriate to the stated inclusion criteria. See Appendix 1 for all strategies used.
For this prevention review, we used the search strategies developed for the complementary Cochrane Review on treatment (Van Ginneken 2013; Van Ginneken 2021), with appropriate adaptation.
Searching other resources
Trial registries
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) (www.who.int/ictrp) (searched on 29 November 2021).
ClinicalTrials.gov, US National Institutes of Health (www.clinicaltrials.gov) (searched on 29 November 2021).
Grey literature
We conducted a grey literature search to identify studies not indexed in the databases listed above.
In particular, we:
reviewed reference lists of all included studies and relevant systematic reviews for additional potentially eligible primary studies;
contacted authors of included studies/reviews to clarify reported published information and to seek unpublished results/data;
contacted researchers with expertise relevant to the review topic/EPOC interventions.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates. Review authors (NvG, MP, EU, EP, CC) independently screened titles and abstracts for inclusion. We (NvG, MP, CC, EU, EP, CCad) retrieved the full‐text study report/publication and independently screened the full text and identified studies for inclusion; we identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author (CB, WT, DP).
We listed in the Characteristics of excluded studies table studies that initially appeared to meet the inclusion criteria but that we later excluded. We collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We also provided any information we were able to obtain about ongoing studies. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We extracted descriptive and outcome data for each study using an adapted version of the EPOC standard data collection form (EPOC 2017a). We piloted the form on at least one study in the review. One review author (MP, DP, CC, EP, CCad, LA) independently extracted descriptive data consecutively, and these were cross‐checked by a second review author (EP, EU, JA). We noted in the Characteristics of included studies table if outcome data were reported in an unusable way. We extracted the following study characteristics from the included studies, and we entered the data into RevMan Web.
Methods: study design; number of study centres and locations; study settings; withdrawals; dates of study; follow‐up.
Participants: number; mean age; age range; gender; clinical conditions; inclusion criteria; exclusion criteria; other relevant characteristics such as ethnicity and socioeconomic status.
Interventions: type and length of intervention; theory of change (hypothesized risk, protective, promotive factors); full description of cadre(s) of primary‐level health and/or community workers including details on supervision, training, and length, frequency, and type of experience; intervention components; comparison; fidelity assessment.
Setting: country; type of health and/or community service (e.g. NGO, government‐funded).
Outcomes: main and other outcomes specified and collected; time points reported.
Notes: funding for the trial; notable conflicts of interest of trial authors; ethical approval.
For economic data, we developed a specific data extraction form based on the format and guidelines used to produce structured abstracts of economic evaluations for inclusion in the National Health Service (NHS) Economic Evaluation Database (NHS EED) (University of York 2002), which we adapted to the specific requirements of this review.
We contacted the authors of included studies via email to collect key unpublished information.
Review authors who were authors of included studies were not involved in the following steps for those studies: study selection, data extraction, risk of bias assessment, and GRADE assessment.
Assessment of risk of bias in included studies
Two review authors (EP, CC) independently assessed risk of bias applying Cochrane Risk of Bias 2 tool and using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 8 (Higgins 2019), along with guidance from the EPOC Group (EPOC 2017b). We resolved disagreements by discussion or by consultation with a third review author (CB, MP). We assessed risk of bias according to the following domains.
Bias arising from the randomization process.
Bias due to deviations from the intended interventions.
Bias due to missing outcome data.
Bias in measurement of the outcome.
Bias in selection of the reported result.
We answered signalling questions as: yes, probably yes, probably no, no, no information. We summarized risk of bias judgements for study results for each of the domains listed. We assigned a risk of bias assessment (low, some concerns, high) to results of the included studies using the approach suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We considered results of studies that had low risk of bias for all key domains, or for which it seemed unlikely for bias to seriously alter the results, to have low risk of bias. We considered results of studies to have some concerns for their risk of bias when risk of bias in at least one domain indicated some concern, or when results were judged to have some bias that could plausibly raise doubts about the conclusions. We considered results of studies to have high risk of bias when they had high risk of bias in at least one domain, or when we judged that they had serious bias that decreased the certainty of the conclusions.
We considered blinding separately for all outcomes (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported rating scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. We did not exclude studies on the grounds of their risk of bias, but we clearly reported the risk of bias when presenting study results.
When considering interventions' effects, we took into account the risk of bias for studies that contributed to that outcome.
We evaluated cluster‐randomized controlled trials using the specific section in the Risk of Bias 2 tool (Higgins 2019).
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We estimated the effect of the intervention by using risk ratio (RR), together with the appropriate associated 95% confidence interval (CI), for dichotomous data; and mean difference (MD) or standardized mean difference (SMD), together with the 95% appropriate associated confidence interval, for continuous data (Higgins 2019). We ensured that an increase in scores for continuous outcomes could be interpreted in the same way for each outcome, and we explained the direction to the reader, and reported when the directions were reversed, if this was necessary. For SMDs, we used the Cochrane Handbook for Systematic Reviews of Interventions to interpret their clinical relevance: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988; Higgins 2011). We attempted to establish minimally important differences per outcome (as suggested in Guyatt 2013).
Unit of analysis issues
We included cluster‐RCTs when primary healthcare facilities, schools, or classes within schools rather than single individuals were the unit of allocation. Because variation in response to psychological or social intervention between clusters may be influenced by cluster membership, we included, when possible, data adjusted with an intra‐cluster correlation coefficient (ICC).
We adjusted the results for clustering by multiplying standard errors of the estimates by the square root of the design effect when the design effect was calculated as DEff = 1 + (M ‐ 1) ICC, where M is the mean cluster size and ICC is the intra‐cluster correlation coefficient. When included studies did not report ICCs for respective outcome measures, we derived ICCs from a different outcome from the same study, or from a different study included in the same meta‐analysis. If the ICC value was not reported or was not available from trial authors directly, we assumed it to be 0.05 (Higgins 2011; Ukoumunne 1999). We combined adjusted measures of effects of cluster‐randomized trials with results of non‐cluster‐randomized trials when it was possible to adjust adequately the results of cluster trials.
Dealing with missing data
We contacted investigators to verify key study characteristics and to obtain missing outcome data when possible (e.g. when a study was identified as abstract only). We tried to compute missing summary data from other reported statistics. We documented all correspondence with trial authors and reported in the Results and Acknowledgements sections details from trial authors who responded. For cluster‐RCTs, we contacted study authors for an ICC value when data were not adjusted and could not be identified from the trial report. As mentioned above, when the ICC was neither available from the trial reports nor directly available from the trial authors, we assumed it to be 0.05 (Ukoumunne 1999).
For continuous data, we applied a looser form of intention‐to‐treat (ITT) analysis, whereby all participants with at least one post‐baseline measurement were represented by their last‐observation‐carried‐forward (LOCF). If the authors of included RCTs stated that they used an LOCF approach, we checked details on LOCF strategy and used data as reported by the study authors. When study authors reported only the standard error (SE) or t statistics or P values, we calculated standard deviations (SDs) according to Altman 1996.
For dichotomous data, we applied a modified ITT analysis without imputation of outcomes where missing, as all analyses with dichotomous outcomes were negative (diagnoses; adverse events).
When it was not possible to obtain data, we reported the level of missingness and considered how that might have impacted the certainty of evidence.
Assessment of heterogeneity
If we found a sufficient number of studies for which we judged participants, interventions, comparisons, and outcomes to be sufficiently similar, we conducted a meta‐analysis (Borenstein 2009). We obtained an initial visual overview of statistical heterogeneity by scrutinizing the forest plots while looking at the overlap between CIs around the estimate for each included study. To quantify the impact of heterogeneity on each meta‐analysis, we used the I² statistic, and we considered the following ranges, according to the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2019).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
The importance of the observed I² statistic was related to the magnitude and direction of intervention effects and the strength of evidence for heterogeneity (Higgins 2011; Purgato 2012). If we identified substantial heterogeneity, we explored this through prespecified subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 trials in a meta‐analysis, we created and examined a funnel plot to explore possible publication biases and interpreted the results with caution (Sterne 2011).
Data synthesis
We undertook meta‐analyses only when this was meaningful (i.e. when the population, intervention, comparison, outcome, and underlying intervention question and the theory of change were similar enough for pooling to make sense) (Borenstein 2009). A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed and considered the implications of this. When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. intervention A versus usual care and intervention B versus usual care) had to be entered into the same meta‐analysis, we halved the control group to avoid double‐counting. Whenever sufficient data were available, we grouped studies for comparison by type of provider (e.g. primary‐health workers‐led, community workers‐led, collaborative), type of intervention (promotion, universal, selective, indicated prevention), and particular risk, protective, or promotive factors targeted (Eaton 2012; Tol 2015a).
Given the potential heterogeneity of mental health promotion and prevention interventions, we used a random‐effects model in all analyses. The random‐effects model has the highest generalisability in empirical examinations of summary effect measures for meta‐analyses (Furukawa 2002). We examined the robustness of this summary measure by checking the results under a fixed‐effect model. We reported material differences between models.
Specifically, for dichotomous data, we used the Mantel‐Haenszel method, as this is preferable in Cochrane Reviews given its better statistical properties when there are few events (Higgins 2011). We adopted the inverse variance method for continuous data: this method minimizes the imprecision of the pooled effect estimate, as the weight given to each study is chosen to be the inverse of the variance of the effect estimate (Higgins 2011).
Economic data
We aimed to conduct all elements of the economics component of this review according to current guidance on the use of economics methods in the preparation and maintenance of Cochrane Reviews (Shemilt 2006). We classified the included economic evaluations based on an established system (Drummond 2005; Trautmann 2016). We summarized the characteristics and results of included economic evaluations by using additional tables. We displayed resource use and cost data in a table, along with unit cost data (when available). A unit cost is defined as the cost of each specific resource input calculated by multiplying the measured number of units (quantities) of an item of resource use (e.g. the number of hours of time provided by a senior teacher) by an applicable unit cost (e.g. the salary cost of one hour of senior teacher time). We reported the currency and price year applicable to measures of costs and unit costs in each original study. Measures of costs are highly likely to vary across and within study settings and over time. This is the product of variations in underlying quantities of resource use and variations in underlying unit costs. This approach is consistent with that used in the parallel review on treatment that is being updated (Van Ginneken 2013).
Subgroup analysis and investigation of heterogeneity
Within each comparison, we planned the following subgroup analyses.
Category of health worker (e.g. professionals, health workers, non‐professional health workers, community workers).
-
Setting of care.
Community settings, camps, schools.
Chronic or acute humanitarian versus non‐humanitarian settings.
Type of prevention intervention (universal, selective, indicated).
Type of promotion intervention (individual, group).
Specific risk, protective, or promotive factor targeted.
We planned to use the following outcomes in subgroup analysis.
Proportion of individuals developing new mental distress or mental disorders (incidence).
Quality of life outcomes.
Harmful outcomes: number of people experiencing harm during the intervention.
Change from baseline in average rating scale scores (e.g. psychological symptoms) for the study population.
If the number of included studies for each comparison was sufficient, we performed subgroup analyses to check whether the intervention effect varied with different population characteristics. When applicable, or when subgroup analysis was not possible, we described subgroup differences narratively in the Results section.
For random‐effects meta‐analyses, we used the formal Chi² test and the I² statistic for subgroup differences in RevMan 5, to detect statistically significant subgroup differences.
Sensitivity analysis
We planned to perform sensitivity analyses defined a priori to assess the robustness of our conclusions and to explore its impact on effect sizes. This involved the following.
Restricting analysis to published studies.
Restricting analysis to studies measuring the incidence of mental disorders (i.e. studies in which all participants at baseline scored below defined symptom thresholds on rating scales).
Restricting analysis to studies with low risk of bias, as specified in incomplete outcome data and selective reporting.
Excluding trials with methodological characteristics that might generate the highest heterogeneity in a meta‐analysis (when a meta‐analysis has I² > 75%).
Stakeholder consultation and involvement
Consultation with stakeholders was conducted by authors Nadja van Ginneken, Simon Lewin, and Paul Garner of the parallel review focused on treatment of mental disorders in LMICs (Van Ginneken 2021). Consultation was organized as follows.
A group face‐to‐face consultation with seven LMIC clinicians who were mature students/master's students or PhD students at the Liverpool School of Tropical Medicine (December 2018).
An online consultation with seven implementers, academics, and policy‐makers from LMICs, and a further four written answers from further stakeholders received by email (February to April 2019).
An updated literature review of mental health terminology and descriptions.
The overall message that emanated from this consultation was that we should consider the spectrum of mental illness as broader than encompassing only diagnostic categories (Patel 2018). According to this framework, the current review complemented the parallel review on treatment interventions in LMICs.
Summary of findings and assessment of the certainty of the evidence
Two review authors (EP, CC) independently assessed the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) (Guyatt 2008). We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2019), and in the EPOC Worksheets (EPOC 2017c), and we used GRADEpro software (GRADEpro GDT). We resolved disagreements on certainty ratings by discussion and planned to provide justification for decisions to downgrade or upgrade ratings by using footnotes in the table and making comments to aid readers' understanding of the review when necessary. We used plain language statements to report these findings in the review (EPOC 2017c).
We summarized review findings in a summary of findings table(s) for the main intervention comparison(s) and included the following outcomes.
Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off of a screening tool) of mental disorders at study endpoint, determined according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV‐TR (APA 2000), DSM‐V (APA 2013), International Classification of Diseases (ICD)‐10 (WHO 1992), or any other standardized criteria.
Quality of life.
Adverse events experienced during the intervention.
Change in mental health symptoms seen on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms).
Resource use (for health services: personnel time allocated/number of consultations, other opportunity costs of the intervention, or other aspects of the health service; for participants: extra cost of travel, lost productivity, employment status, income, work absenteeism, retention, educational attainment).
Psychological functioning and impairment.
If during the review process, we became aware of an important outcome that we failed to list in our planned summary of findings tables, we planned to include the relevant outcome and explain the reasons for this in the section Differences between protocol and review. We considered whether there was any additional outcome information that could not be incorporated into meta‐analyses and noted this in the comments; we stated if this supported or contradicted information derived from meta‐analyses. If it was not possible to meta‐analyze the data, we summarized the results in the text. Only the post‐intervention time point was presented for each outcome in the summary of findings tables.
Results
Description of studies
Detailed descriptions of all studies are found in Characteristics of included studies. This section contains detailed characteristics of each included study.
Results of the search
We screened 11,938 records and included 113 randomized controlled trials with 32,992 participants and 97 in the quantitative synthesis of this review (19,570 participants). We identified 83 ongoing studies, and 25 studies awaiting classification. From our search of all the above databases and trial registries on 20 August 2020, we screened 9437 titles and abstracts (Figure 1). We performed a second search on 29 November 2021, we screened 2501 titles and abstracts and identified 27 studies that are included (Figure 1). We contacted 76 trial authors for additional information and/or data, and 33 of them replied to our requests.
1.

Flow chart of studies
Included studies
Study design
Of the 113 included studies, 67 were randomized trials and 46 were cluster‐randomized trials.
Setting
Of the 113 included studies, 19 were conducted in low‐income countries, 27 in low‐middle‐income countries, 2 studies in middle‐income countries, and 58 studies in upper‐middle‐income countries. Seven studies were conducted in mixed settings, i.e. low to lower‐middle income or upper‐middle to lower‐middle‐income. A map of countries where the RCTs were conducted is available in Figure 2.
2.
Map of countries from included studies
There were 23 studies from rural, 63 studies from urban and 11 studies in both rural and urban contexts. For 16 studies, the setting was not specified. Some interventions were delivered in freestanding primary care facilities (12 studies) or in hospital‐based primary care facilities (17 studies). In 19 studies, the interventions were delivered in community groups, in 26 studies at school, and in one study in the workplace. Interventions were delivered at home or in other settings in 36 studies, while in two studies the setting was not specified.
Participants
There were 83 studies that included adults and 30 that included children (up to 18 years).
Conditions
Most studies (n = 65) targeted the prevention of common mental disorders (39 studies of which 34 were included in meta‐analyses) and/or psychological distress (26 studies of which 21 were included in meta‐analyses). Amongst the other included studies, 18 covered perinatal depression or mental disorders and 30 targeted child mental disorders (24 studies included in meta‐analyses). See Effects of interventions for details on the analyses.
Interventionists
Various cadres of primary‐level workers were employed: primary care health workers (38 studies), community workers (71 studies), or both (2 studies). In two studies, the intervention was delivered online and the specific cadres were not described. The remuneration of interventionists was generally not reported or poorly described. The background of these interventionists is reported in detail under Characteristics of included studies.
Interventions
In 22 studies, the prevention interventions delivered were categorized as universal prevention or promotion of well‐being, targeting the general public or the whole population group, regardless of whether people were at higher risk or not of developing a disorder. For example, we included in this category programmes for all caregiver‐child dyads of students attending primary schools and designed to develop social skills in elementary‐age children using a universal, low‐cost approach. In 36 studies, the delivered interventions were categorized as selective prevention, targeting participants whose risk of developing a mental disorder was significantly higher than that of the rest of the population (e.g. exposed to specific risk factors), for example, support interventions for caregivers of patients with Alzheimers with distress levels below the cut‐off. In 55 studies, interventions were categorized as indicated prevention, as they were directed to smaller groups of participants at high risk for mental disorders and that might already present with some sign of disorder like psychological symptoms, without meeting the criteria for a formal diagnosis according to diagnostic tools or cut‐offs in rating scales. For example, we included in this category the intervention Self‐Help Plus, a pre‐recorded audio course, delivered by trained facilitators in a group setting and complemented with an illustrated self‐help book adapted for the target cultural group. The intervention is based on acceptance and commitment therapy and was delivered to participants with increased psychological distress (GHQ ≥ 3) and without a formal diagnosis according to the MINI. Intervention categorization is summarized in Figure 3.
3.
Economic studies
Of the included studies, three (Chang 2015; Osborn 2020; Tol 2012) reported economic evaluations which are summarized in Table 8 with costs reported in dollars. These studies were not included in meta‐analyses due to the heterogeneity across measures and economic outcomes.
2. Economic analysis.
| Author year | Country | Type of economic analysis | Study population | Interventions | Intervention‐specific costs and cost‐effectiveness | Resources (i.e. costs to health services other than intervention costs; patient/society costs and productivity) |
| Jordans 2010; Tol 2012 | Sri Lanka | Cost analysis | Children (both male and female, 9 to 12 years of age) | Classroom‐based intervention (CBI) vs waiting list | Costs: cost analyses for intervention group demonstrated mean cost per service user was USD 8.85 (56% of which is human resources cost) | Health service costs: ‘costs’ included broader package |
| ‐ | ‐ | Cost data: calculated | ‐ | Delivered by LHWs (paraprofessional interventionists); training: 2 weeks in Sri Lanka | Cost‐effectiveness: cost analyses represented basic calculations. Presented data did not allow for more sophisticated analyses | Patient cost: none reported |
| Chang 2015 | Jamaica | Cost analysis | Adults (female, age not specified) | Parenting intervention with routine primary health care vs usual care | Costs: the cost per child was USD 100.9 for 1 year of intervention | Health service cost: USD 100.9 per child including equipment purchases, materials, training, and wages |
| ‐ | ‐ | ‐ | ‐ | Delivered by community health workers and nurses; training: 3‐day workshops with viewing of films and role plays | Cost‐effectiveness: not reported | Patient cost: none reported |
| Osborn 2020 | Kenya | Cost analysis | Adolescents (both male and females, 13 to 18 years of age) | Shamiri‐Digital Wellness vs active control | Cost: USD 3.57 per student to deliver Shamiri‐Digital | Health service cost: health service costs included equipment (computers, desks, chairs) with an hourly cost of USD 0.97, totalling USD 104.65 for the 9 months of the intervention |
| ‐ | ‐ | ‐ | ‐ | Self‐help digital‐based intervention | Cost‐effectiveness: depending on the definition of clinically meaningful improvement, 7.1 to 9.7 students needed to receive the intervention for 1 student to experience a clinically meaningful improvement, which translated to a cost of USD 25.35 to USD 34.62 per student | Patient cost: none reported |
CBI: classroom‐based intervention LHW: lay health workers USD: US dollar vs: versus
Excluded studies
We excluded 218 studies. These studies were of interest but were excluded due to specific criteria for participants, interventions, comparators, and outcomes (PICOs) not being met (see Characteristics of excluded studies). The most common reason for exclusion was ineligible population. The other reasons for exclusion were, in descending order, the following: ineligible intervention, ineligible study design, ineligible outcome, ineligible setting, and duplicates.
Risk of bias in included studies
We applied the Cochrane Risk of Bias 2 tool to individual and cluster‐randomized trials (Higgins 2019). The most often identified biases across studies were performance bias ('risk of bias due to deviations from the intended interventions' ‐ 'effect of assignment to intervention and effect of adhering to intervention'), attrition bias ('risk of bias due to missing outcome data'), and reporting bias ('risk of bias in the selection of the reported result').
Adults
1. Risk of bias arising from the randomization process (Domain 1‐A)
Two studies (Jewkes 2008; Sanfilippo 2020) had a high risk of bias with regard to Domain 1‐A. In both cluster‐randomized controlled trials, the reported high RoB, was determined by the timing of identification or recruitment of participants. This is because individual participants were not identified and recruited before randomization and, for both, it was likely that the selection of individual participants was affected by knowledge of the intervention assigned to the cluster as assessed by items 1b.1 and 1b.2.
Ten studies presented a risk of bias classified as having 'some concerns'. This is because, in most cases, studies did not provide enough information on allocation concealment, and 62 had a low risk.
2. Risk of bias due to deviations from the intended interventions (effect of assignment to intervention and effect of adhering to intervention) (Domain 2‐B)
A high risk of bias for the Domain 2‐B was reported for 13 outcomes from six studies (Chang 2015; Hirani 2018; Ozcan 2020; Rachasrimuang 2018; Skar 2021; Ward 2020) in the adult population.
For Chang 2015(indicated prevention; depressive symptoms at endpoint), Ward 2020(indicated prevention; depressive symptoms, distress/PTSD symptoms and social outcomes, all at endpoint and 7‐24 months), Hirani 2018(universal prevention; quality of life at endpoint), and Rachasrimuang 2018(selective prevention; quality of life and depressive symptoms, both at 1 to 6 months) this is due to deviations from the intended intervention that arose because of the trial context, as assessed by item 2.3. These deviations were deemed to be likely affecting the outcome and to be not balanced between groups (items 2.4, 2.5). Skar 2021(indicated prevention; diagnosis of mental disorders at 1 to 6 months) and Ozcan 2020(selective prevention; quality of life and social outcomes, both at 1 to 6 months) instead present a high risk of bias related to the analyses used to estimate the effect of assignment to intervention (items 2.6 and 2.7). For Skar 2021, this was because not enough information was provided: a) to evaluate if an appropriate analysis was used to estimate the effect of assignment to intervention, and b) to evaluate the potential for a substantial impact (on the result) of the failure to analyze participants in the group to which they were randomized. For Ozcan 2020 instead, there was a chance that an appropriate analysis was not used to estimate the effect of assignment to intervention, as indicated by the quote: "The exclusion criteria were as follows: (i) being lost to follow‐up (…)".
For Domain 2‐B, 57 out of 175 outcomes from 26 included studies, presented a risk of bias classified as having 'some concerns'. Most of these studies did not provide enough information on whether the analysis used to estimate the effect of adhering was appropriate. Nevertheless, they held no potential for a substantial impact (on the result) of the failure to analyze participants in the group to which they were randomized.
A total of 105 out of the 175 outcomes from 46 included studies had a low risk of bias.
3. Risk of bias due to missing outcome data (Domain 3‐C)
A high risk of bias for the Domain 3‐C was reported for 12 outcomes from five included studies (Dybdahl 2001, indicated prevention; quality of life, distress/PTSD symptoms and social outcomes, all at endpoint; Rachasrimuang 2018, selective prevention; quality of life and depressive symptoms, both at 1‐6 months; Rodriguez 2021, indicated prevention; depressive symptoms, anxiety symptoms and distress/PTSD symptoms, all at endpoint); Rotheram‐Borus 2014a, indicated prevention; diagnosis of mental disorder at endpoint, 1 to 6 months and 7 to 24 months; Skar 2021, indicated prevention; diagnosis of mental disorders at 1 to 6 months) for the adult population. For all, data for this outcome were not available for all randomized participants/clusters that recruited participants/individual participants within clusters, or information on this (item 3.1) was not reported. In addition, there was no evidence that the result was not biassed by missing data (3.2) and it was likely that 'missingness' in the outcome depended on its true value (3.3, 3.4). Alternatively, not enough information was provided to give a judgement on the item.
For Domain‐3‐C, 9 out of 175 outcomes from four included studies, presented a RoB classified as having 'some concerns'. For these outcomes, data were not available for all randomized participants/clusters that recruited participants/individual participants within clusters, and there was no evidence that results were not biassed by missingness.
A total of 154 out of the 175 outcomes from 65 included studies had a low risk of bias.
4. Risk of bias in measurement of the outcome (Domain 4‐D)
A high risk of bias for Domain 4‐D was reported for three outcomes from one included study (Duan 2019; promotion/universal prevention; quality of life, depressive symptoms and distress/PTSD symptoms, all at endpoint). This was due to the fact that a different method for assessment was used at post‐intervention for the control and intervention groups.
For Domain 4‐D, 151 out of 175 outcomes from 72 included studies had a RoB classified as having 'some concerns'. For the great majority of these, outcome assessors (which were participants for self‐reported outcome measures) were most likely aware of intervention allocation across these non‐blinded designs. Across these studies, knowledge of the assigned intervention could have influenced participant‐reported outcomes, but there was no reason to believe that it did.
A total of 21 out of the 175 outcomes from five included studies had a low risk of bias.
5. Risk of bias in selection of the reported result (Domain 5‐E)
A high risk of bias for Domain 5‐E was reported for five outcomes from three included studies (Latina 2019; Rodriguez 2021; Rotheram‐Borus 2014a). For Rotheram‐Borus 2014a(indicated prevention; diagnosis of mental disorder at endpoint, 1 to 6 months and 7 to 24 months) and Latina 2019(promotion/universal prevention; quality of life at endpoint), this was due to the fact that one outcome mentioned in the protocol was not reported in the results, suggesting outcome selection (item 5.2). Rodriguez 2021(indicated prevention; distress/PTSD symptoms at endpoint), instead presented a high risk of bias related to both outcome selection and analyses selection.
For Domain 5‐E, 15 out of 175 outcomes from six included studies, had a RoB classified as having 'some concerns'. Most of these studies did not provide enough information to assess if a trial was analyzed in accordance with a prespecified plan or selection from multiple outcomes or analyses occurred.
A total of 155 out of the 175 outcomes from 67 included studies had a low risk of bias.
6. Overall risk of bias
An overall high risk of bias was reported for 31 outcomes from 13 included studies (Chang 2015; Duan 2019; Dybdahl 2001; Hirani 2018; Jewkes 2008; Latina 2019; Ozcan 2020; Rachasrimuang 2018; Rodriguez 2021; Rotheram‐Borus 2014a; Sanfilippo 2020; Skar 2021; Ward 2020) analyzing promotion/prevention interventions amongst adults. This overall judgement was determined by a high risk of bias reported in domain 1‐A for two studies (Jewkes 2008 and Sanfilippo 2020), domain 2‐B for six studies (Chang 2015; Hirani 2018; Ozcan 2020; Rachasrimuang 2018; Skar 2021; Ward 2020), domain 3‐C for five studies (Dybdahl 2001; Rachasrimuang 2018; Rodriguez 2021; Rotheram‐Borus 2014a; Skar 2021), domain 4‐D for one study (Duan 2019), and finally domain 5‐E for three studies (Latina 2019; Rodriguez 2021; Rotheram‐Borus 2014a). Amongst these, four studies had a high risk of bias for more than one domain (Rachasrimuang 2018; Rodriguez 2021; Rotheram‐Borus 2014a; Skar 2021).
A total of 127 out of the 175 outcomes from 59 included studies were reported to have 'some concerns', 17 outcomes from four studies had instead a low risk of bias.
Children
1. Risk of bias arising from the randomization process (Domain 1‐A)
No study had a high risk of bias with regard to the randomization process. A total of four studies were reported to have 'some concerns' in Domain 1‐A. Amongst these, most did not provide enough information on allocation concealment. A total of 20 studies had a low risk of bias.
2. Risk of bias due to deviations from the intended interventions (effect of assignment to intervention and effect of adhering to intervention) (Domain 2‐B)
A high risk of bias for Domain 2‐B was reported for eight outcomes from four studies (Ager 2011; Hull 2021; Mohamadi 2021; Velásquez 2015).
For Velásquez 2015(promotion/universal prevention; depressive symptoms, anxiety symptoms, and social outcomes, all at 1 to 6 months) and Hull 2021(indicated prevention; anxiety symptoms, and social outcomes, both at endpoint), this was due to deviations from the intended intervention that arose because of the trial context, as assessed by item 2.3. These deviations were deemed to be likely affecting the outcome and to be not balanced between groups (items 2.4, 2.5).
The two aforementioned studies as well as Ager 2011(selective prevention; quality of life at endpoint) and Mohamadi 2021(promotion/universal prevention; distress/PTSD symptoms, at endpoint and 1‐6 months), presented a high risk of bias related to the analyses used to estimate the effect of assignment to intervention (items 2.6 and 2.7). This was because not enough information was provided to evaluate: a) if an appropriate analysis was used to estimate the effect of assignment to intervention, and b) the potential for a substantial impact (on the result) of the failure to analyze participants in the group to which they were randomized.
Nineteen out of 71 outcomes from seven included studies, were reported as having 'some concerns' in Domain 2‐B. Amongst these, most did not provide enough information on whether the analysis used to estimate the effect of adhering was appropriate. Nevertheless, they held no potential for a substantial impact (on the result) of the failure to analyze participants in the group to which they were randomized.
A total of 44 out of the 71 outcomes from 13 included studies had a low risk of bias.
3. Risk of bias due to missing outcome data (Domain 3‐C)
A high risk of bias for Domain 3‐C was reported for five outcomes from three included studies (Ager 2011; Hull 2021; Mohamadi 2021). For Ager 2011(selective prevention; quality of life at endpoint), data were not available for all randomized participants (3.1), there was no evidence that the result was not biassed by missing data (3.2). It was likely that missingness in the outcome data depended on its true value (3.3, 3.4). For Hull 2021(indicated prevention; anxiety symptoms, and social outcomes, both at endpoint) and Mohamadi 2021(promotion/universal prevention; distress/PTSD symptoms, at endpoint and 1 to 6 months), not enough information was provided to provide a judgement on any of these items.
Two out of 71 outcomes from one included study were reported to have 'some concerns' in Domain 3‐C. Amongst these, data were not available for all randomized participants, clusters, or individual participants within a cluster, and there was no evidence that results were not biassed by missingness.
A total of 64 out of the 71 outcomes from 20 included studies had a low risk of bias.
4. Risk of bias in measurement of the outcome (Domain 4‐D)
No study had a high risk of bias due to the measurement of the outcome. All outcomes from all 24 included studies were reported to have 'some concerns' in Domain 4‐D. Outcome assessors (which were participants for self‐reported outcome measures) were most likely aware of intervention allocation across these non‐blinded designs. Across these studies, knowledge of the assigned intervention could have influenced participant's reported outcomes, but there was no reason to believe that it did.
5. Risk of bias in selection of the reported result (Domain 5‐E)
A high risk of bias for the Domain 5‐E was reported for six outcomes from three included studies (Ager 2011; Dhital 2019; Osborn 2020). For Ager 2011(selective prevention; quality of life at endpoint) and Osborn 2020(indicated prevention; quality of life, depressive symptoms and anxiety symptoms, all at endpoint), this was due to the fact that one outcome mentioned in the protocol was not reported in the results, suggesting outcome selection. Dhital 2019(selective prevention; depressive symptoms and distress/PTSD symptoms, both at 1 to 6 months), instead presented a high risk of bias related to the fact that not all planned data collection was reported in the results.
Two out of 71 outcomes from one included study, were reported to have 'some concerns' in Domain 5‐E. This was because not enough information was provided to assess if a trial was analysed in accordance with a prespecified plan or selection from multiple outcomes or analyses that occurred was provided.
A total of 63 out of the 71 outcomes from 21 included studies had a low risk of bias.
6. Other potential sources of bias
We visually inspected funnel plots to identify asymmetry in any of the comparisons between psychological treatments and comparators when we identified 10 or more studies. We did not identify any asymmetry in the distribution of studies (Figure 4; Figure 5).
4.

5.

7. Overall risk of bias
An overall high risk of bias was reported for 13 outcomes from six studies (Ager 2011; Dhital 2019; Hull 2021; Mohamadi 2021; Osborn 2020; Velásquez 2015) analyzing promotion/prevention interventions amongst children. This overall judgement was determined by a high risk of bias reported in three domains (2, 3 and 5) for one study (Ager 2011), in two domains (2 and 3) for two studies (Mohamadi 2021 and Hull 2021), and in one domain for three studies (Velásquez 2015 (2) Dhital 2019 and Osborn 2020 (5)).
For the remaining 58 out of 71 outcomes from 18 included studies, the risk of bias was classified as having 'some concerns'. No outcome or study had an overall low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Results of meta‐analyses (n = 97 studies) of outcomes for the following comparisons were described:
Primary‐level health worker and/or community worker‐led universal prevention or promotion interventions for adults (n = 11 studies, 11.3%);
Primary‐level health worker and/or community worker‐led selective prevention interventions for adults (n = 20 studies, 20.6%);
Primary‐level health worker and/or community worker‐led indicated prevention interventions for adults (n = 42 studies, 43.3%);
Primary‐level health worker and/or community worker‐led universal prevention or promotion interventions for children (n = 8 studies, 8.2%);
Primary‐level health worker and/or community worker‐led selective prevention interventions for children (n = 7 studies, 7.2%);
Primary‐level health worker and/or community worker‐led indicated prevention interventions for children (n = 9 studies, 9.3%).
We were not able to group studies by type of provider (e.g. primary health workers‐led, community workers‐led, collaborative), and by risk, protective, or promotive factors targeted due to the small number of studies for each subgroup, and to the lack of information on risk, protective, and promotive factors. We were not able to report the comparison of promotion or prevention interventions versus interventions delivered by professionals with specialist mental health training, as no data were available for this comparison.
Outcome data on efficacy were extracted for the specific population group for which the intervention was designed and delivered. For example, when the intervention was designed and directed to adults, we entered efficacy data on adults (even though in some RCTs data on children were also available to measure potential indirect benefits). The same applied for interventions directed to children and adolescents.
Comparison 1. Primary‐level health worker and/or community worker‐led universal prevention or promotion interventions for adults (n = 11 studies)
We identified 11 RCTs (6 cluster‐RCTs (54.5%) and five individual RCTs (45.5%)) contributing to meta‐analyses in this comparison. The total sample size was n = 3099 (1567 participants (50.6%) for the intervention group and 1532 participants (49.4%) for the control group).
As for the type of PWs, the majority of interventions (n = 7 studies, 63.6%) were delivered by community workers (CWs) whereas, in four studies (36.4%), interventions were delivered by primary health workers (PWs) (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, trained facilitators, caregivers, coaches, and peer leaders; PWs included social workers, community‐based health workers, medical teachers, and village doctors. For most of the studies, participants in the control group were allocated to usual care (n = 9 studies, 81.8%); for the remaining two studies (18.2%), the control group was guided by a PW without training and/or supervision or was allocated to active control. Two studies (18.2%) had been conducted in schools, two (18.2%) in a community setting, one (9.1%) at home, one (9.1%) at a workplace and the remaining five (45.5%) in other settings, such as universities, or local parishes. From a wider geographical perspective, four studies (36.4%) were conducted in an urban context, four (36.4%) in a rural one and, for the remaining three studies (27.3%), it was not specified. Seven studies (63.6%) were undertaken in upper‐middle income countries, three (27.3%) in low‐middle‐income countries and for one study (9.1%) income level changed from low‐middle to upper‐middle. As for the condition, most studies addressed psychological distress (n = 8 studies, 72.7%), two studies (18.2%) targeted the prevention of common mental disorders, and one (9.1%) addressed perinatal mental disorders.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
1.1. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 1: Diagnosis of mental disorders at 1‐6 months
1.2. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 2: Diagnosis of mental disorders at 7‐24 months
At 0 to 1 month post‐intervention
No studies reported on this outcome.
At 1 to 6 months post‐intervention
One study with 137 participants (George 2020) identified that universal prevention/promotion interventions may lead to a decrease in the incidence of mental disorders at 1 to 6 months, although the actual effect range indicates it may have little or no difference (RR 0.57, 95% CI 0.28 to 1.18; I2 = NA; P = 0.13) (Analysis 1.1).
At 7 to 24 months post‐intervention
One study with 323 participants (Rockers 2018) identified that universal prevention/promotion interventions may lead to a decrease in the incidence of mental disorders at 7 to 24 months, although the actual effect range indicates it may have little or no difference (RR 0.67, 95% CI 0.43 to 1.05; I2 = NA; P = 0.08) (Analysis 1.2).
2. Quality of life
1.3. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 3: Quality of life at 0‐1 months
1.4. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 4: Quality of life at 7‐24 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, it is uncertain whether universal prevention/promotion interventions improve quality of life (SMD ‐0.23, 95% CI ‐0.51 to 0.04; I2 = 61%; P = 0.10; 4 studies, 684 participants; very low‐certainty evidence due to study limitations, inconsistency, and indirectness) (Analysis 1.3).
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
One study (Zhou 2010) identified a positive effect of universal prevention/promotion interventions over the control group (MD ‐7.30, 95% CI ‐12.28 to ‐2.32; I2 = NA; P = 0.004; 222 participants) (Analysis 1.4).
3. Adverse events experienced during the intervention
No studies reported on this outcome.
Secondary outcomes
1. Psychological functioning and impairment
No studies reported on this outcome.
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9)
1.5. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 5: Depressive symptoms at 0‐1 months
1.6. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 6: Depressive symptoms at 1‐6 months
1.7. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 7: Depressive symptoms at 7‐24 months
1.8. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 8: Anxiety symptoms at 0‐1 months
1.9. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 9: Distress/PTSD symptoms at 0‐1 months
Three studies (349 participants) (Duan 2019; Hendriks 2019; Yusoff 2015) reported data on depressive symptoms at 0 to 1 month post‐intervention, indicating uncertainty of beneficial effects for universal prevention/promotion interventions versus control (SMD ‐0.31, 95% CI ‐0.78 to 0.15; I2 = 61%; P = 0.19; 3 studies, 349 participants; very low confidence due to study limitations, inconsistency and indirectness) (Analysis 1.5). At 1 to 6 months, only one study with 153 participants provided data (Yusoff 2015) indicating that a universal prevention/promotion intervention may improve depressive symptoms, although the actual effect range suggests it may have little or no difference (MD ‐1.25, 95% CI ‐3.28 to 0.78; I2 = NA; P = 0.23) (Analysis 1.6). At 7 to 24 months, two studies with 1207 participants (Jewkes 2008; Yusoff 2015) identified a positive effect of universal prevention/promotion interventions over control (SMD ‐0.13, 95% CI ‐0.24 to ‐0.02; I2 = 0%; P = 0.02) (Analysis 1.7).
For anxiety symptoms at 0 to 1 month post‐intervention, one study with 158 participants (Hendriks 2019) found a probable difference in favour of the intervention versus control (MD ‐0.14, 95% CI ‐0.27 to ‐0.01; I2 = NA; P = 0.04; moderate certainty due to imprecision) (Analysis 1.8). At 1 to 6 and 7 to 24 months, no studies reported on this outcome.
For distress/PTSD symptoms, four studies with 722 participants (Baker‐Henningham 2019; Bell 2008; Duan 2019; Hendriks 2019) identified that interventions may have a slightly beneficial effect at 0 to 1 month post‐intervention (SMD ‐0.24, 95% CI ‐0.41 to ‐0.08; I2 = 8 %; P = 0.04; 722 participants; low certainty due to study limitations and indirectness) (Analysis 1.9). At 1 to 6 and 7 to 24 months, no studies reported on this outcome.
4. Social outcomes (e.g. perception of social inclusion)
1.10. Analysis.

Comparison 1: Promotion/universal prevention interventions versus control group in preventing mental disorders in adults, Outcome 10: Social outcomes at 0‐1 months
One study with 158 participants (Hendriks 2019) identified a positive effect of interventions versus control condition in improving social outcomes at 0 to 1 month post‐intervention (MD ‐0.45, 95% CI ‐0.82 to ‐0.08; I2 = NA; P = 0.02; 158 participants). At 1 to 6 and 7 to 24 months, no studies reported on this outcome.
5. Carer mental health
No studies reported on this outcome.
Comparison 2. Primary‐level health worker and/or community worker‐led selective prevention interventions for adults (n = 20 studies)
We identified 20 RCTs (8 cluster‐RCTs (40%) and 12 individual RCTs (60%)) contributing to meta‐analyses in this comparison. The total sample size was 4160 (2087 participants (50.2%) for the intervention group and 2073 participants (49.8%) for the control group).
As for the type of PWs, in nine studies (45%), interventions were delivered by community workers (CWs) whereas in 10 studies (55%) by primary health workers (PWs), and in one study it was a self‐help intervention (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, community health aiders, trained yoga instructors, family volunteers, peer counsellors, and local groups; PWs included physiotherapists, nursing assistants, healthcare assistants, antenatal care staff team, and midwives. For most of the studies, participants in the control group were allocated to usual care (n = 18 studies, 90%); for the remaining two studies (10%), the control group was guided by a PW without training and/or supervision or was allocated to a waiting list. Six studies (30%) had been conducted in PC facilities (hospitals) and two (10%) in PC facilities (freestanding), eight (40%) at home, three (15%) in a community setting, two (10%) in other settings (yoga university and offices). From a wider geographical perspective, 13 studies (65%) were conducted in an urban context, four (20%) in a rural one, two (10%) both in an urban and rural context, and one in a slum. Ten studies (50%) were undertaken in upper‐middle‐income countries, four (20%) in low‐middle income countries, three (15%) in low‐income countries and, for one study (5%), income level changed from low‐middle to upper‐middle whereas for the remaining two studies (10%), income level changed from low to low‐middle. As for the condition, most studies addressed psychological distress (n = 12 studies, 60%) and eight studies (40%) targeted the prevention of perinatal mental disorders.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
At 0 to 1 month post‐intervention
No studies reported on this outcome.
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
One study with 349 participants (Tripathy 2010) identified an uncertain effect of selective prevention interventions at 7 to 24 months post‐intervention (RR 1.81, 95% CI 0.17 to 19.82; I2 = NA; P = 0.63) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 1: Diagnosis of mental disorders at 7‐24 months
2. Quality of life
2.2. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 2: Quality of life at 0‐1 months
2.3. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 3: Quality of life at 1‐6 months
At 0 to 1 month post‐intervention
Three studies (McCann 2015; Miller 2020; Rahimi 2021a) (229 participants) showed that it is uncertain whether selective prevention interventions were more effective than control in improving quality of life at 0 to 1 month post‐intervention (SMD ‐1.64, 95% CI ‐2.97 to ‐0.31; 229 participants, I2 = 92%; P = 0.02; very low‐certainty evidence due to inconsistency, indirectness, and publication bias) (Analysis 2.2).
At 1 to 6 months post‐intervention
Five studies (Hamdani 2021b; McCann 2015; Ozcan 2020; Rachasrimuang 2018; Yang 2022) (798 participants) provided data on this outcome. We identified a positive effect of selective prevention interventions versus control group in improving quality of life (SMD ‐1.05, 95% CI ‐1.84 to ‐0.26; I2 = 96%; P = 0.009; 798 participants) (Analysis 2.3).
At 7 to 24 months post‐intervention
No studies reported on this outcome.
3. Adverse events experienced during the intervention
No studies reported on this outcome.
Secondary outcomes
1. Psychological functioning and impairment
No studies reported on this outcome.
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9)
2.4. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 4: Depressive symptoms at 0‐1 months
2.5. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 5: Depressive symptoms at 1‐6 months
2.6. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 6: Anxiety symptoms at 1‐6 months
2.7. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 7: Distress/PTSD symptoms at 0‐1 months
2.8. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 8: Distress/PTSD symptoms at 1‐6 months
2.9. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 9: Distress/PTSD symptoms at 7‐24 months
For depressive symptoms (Analysis 2.4) at 0 to 1 month post‐intervention, we collected four studies with 223 participants (Baker‐Henningham 2005; Hirani 2010; Ramezani 2017; Sanfilippo 2020). The studies probably identified an important effect of selective preventive interventions versus control in reducing depressive symptoms (SMD ‐0.69, 95% CI ‐1.08 to ‐0.30; I2 = 39%; P = 0.0005; 223 participants; moderate certainty due to study limitations). At 1 to 6 months (Analysis 2.5), three studies (Barnes 2019; Rachasrimuang 2018; Sanfilippo 2020) provided data in favour of selective prevention interventions (SMD ‐0.60, 95% CI ‐1.00 to ‐0.21; I2 = 32%; P = 0.002; 186 participants). At 7 to 24 months, no studies reported on this outcome.
For anxiety symptoms at 1 to 6 months (Analysis 2.6), we collected one study with 2026 participants (Langer 1996) showing that selective prevention interventions may lead to decreased symptoms, although the actual effect range indicates it may have little or no difference (MD ‐0.80, 95% CI ‐1.87 to 0.27; I2 = NA; P = 0.14). At endpoint and 7 to 24 months, no studies reported on this outcome.
For distress/PTSD symptoms at 0 to 1 month post‐intervention (Analysis 2.7), we collected seven studies (Cerquera Córdoba 2021; Chattha 2008; Li 2019; McCann 2015; Miller 2020; Ramezani 2017; Sanfilippo 2020) and found that selective prevention interventions may have a positive effect over controls but the evidence is very uncertain (SMD ‐0.90, 95% CI ‐1.44 to ‐0.36; I2 = 88%; P = 0.001; 535 participants; very low‐certainty evidence due to inconsistency and indirectness). At 1 to 6 months (Analysis 2.8), we collected five studies (Dias 2008; McCann 2015; Rahman 2009; Sanfilippo 2020; Yang 2022) and found a difference in favour of selective prevention interventions (SMD ‐0.67, 95% CI ‐1.21 to ‐0.12; 464 participants, I2 = 86%; P = 0.02). At 7 to 24 months, we did not identify any differences between study arms (Analysis 2.9).
4. Social outcomes (e.g. perception of social inclusion)
(Analysis 2.10; Analysis 2.11; Analysis 2.12)
2.10. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 10: Social outcomes at 0‐1 months
2.11. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 11: Social outcomes at 1‐6 months
2.12. Analysis.

Comparison 2: Selective prevention intervention versus control group in preventing mental disorders in adults, Outcome 12: Social outcomes at 7‐24 months
At 0 to 1 month post‐intervention, we found three studies (Cerquera Córdoba 2021; Rahimi 2021a; Vargas‐Porras 2021). Selective prevention interventions improved social outcomes compared with the control group (SMD ‐9.50, 95% CI ‐15.29 to ‐3.70; I2 = 81%; P = 0.001; 121 participants) (Analysis 2.10). At 1 to 6 months, selective prevention interventions may lead to decrease symptoms, although the actual effect range indicates it may have little or no difference (SMD ‐0.88, 95% CI ‐2.56 to 0.80; 2 studies, 236 participants; I2 = NA; P = 0.30) (Analysis 2.11). At 7 to 24 months, one study (Cerquera Córdoba 2021) with 27 participants showed a positive effect of selective prevention over control in improving social outcomes (MD ‐15.70, 95% CI ‐28.35 to ‐3.05; I2 = NA; P = 0.02; 27 participants) (Analysis 2.12).
5. Resource use
No studies reported on this outcome.
6. Carer mental health
No studies reported on this outcome.
Comparison 3. Primary‐level health worker and/or community worker‐led indicated prevention interventions for adults (n = 42 studies)
We identified 42 RCTs (11 cluster‐RCTs (26.2%) and 31 individual RCTs (73.8%)) contributing to meta‐analyses in this comparison. Total sample size was 6214 (3100 participants (49.9%) for the intervention group and 3114 participants (50.1%) for the control group).
As for the type of PWs, in 25 studies (59.5%), interventions were delivered by community workers (CWs) whereas in 18 studies (42.9%) interventions were delivered by primary health workers (PWs) (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, lay community workers, group leaders, yoga instructors, teachers, and peer counsellors; PWs included midwives, nurses, physicians, and trained general practitioners. For most of the studies, participants in the control group were allocated to usual care (n = 35 studies, 83.3%); for one study (2.4%), the control group was guided by a PW without training and/or supervision and for the remaining six studies (14.3%), the control group was active control or waiting list. Nine studies (21.4%) had been conducted in a hospital and 6 (14.3%) in a freestanding PC facility, 13 (31%) in a community setting, seven (16.7%) at home, two (4.8%) in schools, one at the workplace and the remaining four (9.5%) in other settings, such as churches, mixed settings (homes, social centres). From a wider geographical perspective, 26 studies (61.9%) were conducted in an urban context, seven (16.7%) in a rural one, five (11.9%) both in urban and rural contexts, and for the remaining four studies (9.5%) it was not specified. Twenty‐five studies (59.5%) were undertaken in upper‐middle income countries, 11 (26.2%) in low‐middle income countries, two (4.8%) in middle‐income countries, two (4.8%) in low‐income countries, and for two studies (4.8%) income level changed from low‐middle to upper‐middle. As for the condition, the majority of studies (n = 32, 76.2%) targeted the prevention of common mental disorders, nine studies (21.4%) addressed perinatal mental disorders and the remaining one (2.4%) targeted psychological distress.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
(Analysis 3.1; Analysis 3.2; Analysis 3.3)
3.1. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 1: Diagnosis of mental disorders at 0‐1 months
3.2. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 2: Diagnosis of mental disorders at 1‐6 months
3.3. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 3: Diagnosis of mental disorders at 7‐24 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, we found three studies with 843 participants. It is uncertain whether indicated prevention interventions reduce the incidence of mental health diagnosis (RR 0.30, 95% CI 0.06 to 1.57; I2 = 87%; P = 0.15; 3 studies, 843 participants; very low‐certainty evidence due to study limitations, inconsistency, indirectness, and imprecision) (Analysis 3.1).
At 1 to 6 months post‐intervention
At 1 to 6 months, we found six studies with 1352 participants (Acarturk 2022; Cooper 2009; Rong 2021b; Rotheram‐Borus 2014a; Rotheram‐Borus 2014b; Skar 2021). We found a difference in favour of interventions over controls (RR 0.65, 95% CI 0.50 to 0.84; I2 = 21%; P = 0.001; 1352 participants) (Analysis 3.2).
At 7 to 24 months post‐intervention
At 7 to 24 months, we found two studies with 380 participants. Indicated prevention interventions may lead to a decrease in the incidence of mental disorders at 7 to 24 months, although the actual effect range indicates it may have little or no difference (RR 0.69, 95% CI 0.41 to 1.19; I2 = 0%; P = 0.18) (Analysis 3.3).
2. Quality of life
(Analysis 3.4; Analysis 3.5; Analysis 3.6)
3.4. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 4: Quality of life at 0‐1 months
3.5. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 5: Quality of life at 1‐6 months
3.6. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 6: Quality of life at 7‐24 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, we found eight studies (Acarturk 2022; Bernardi 2020; Cheng 2021; Chomat 2019; Dybdahl 2001; Escolar 2014; Novelli 2018; Song 2019). Indicated prevention interventions may improve quality of life but the evidence is very uncertain (SMD ‐0.36, 95% CI ‐0.61 to ‐0.12; I2 = 68%; P = 0.004; 1136 participants; very low‐certainty evidence due to inconsistency, publication bias, and indirectness) (Analysis 3.4).
At 1 to 6 months post‐intervention
At 1 to 6 months, indicated prevention interventions may have little or no effect on improving quality of life (SMD ‐0.04, 95% CI ‐0.23 to 0.16; I2 = 34%; P = 0.70; 4 studies, 847 participants) (Analysis 3.5).
At 7 to 24 months post‐intervention
At 7 to 24 months, indicated prevention interventions may lead to a decrease in the incidence of mental disorders, although the actual effect range indicates it may have little or no difference (SMD ‐0.80, 95% CI ‐3.53 to 1.93; I2 = NA; P = 0.57; 1 study, 94 participants) (Analysis 3.6).
3. Adverse events experienced during the intervention
3.7. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 7: Adverse events at 0‐1 months
At 0 to 1 month post‐intervention, only Acarturk 2022 reported that no participants experienced adverse events in the intervention and control groups.
Secondary outcomes
1. Psychological functioning and impairment
(Analysis 3.8; Analysis 3.9; Analysis 3.10)
3.8. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 8: Psychological functioning and impairment at 0‐1 months
3.9. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 9: Psychological functioning and impairment at 1‐6 months
3.10. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 10: Psychological functioning and impairment at 7‐24 months
Indicated prevention interventions probably make little or no difference at 0 to 1 month post‐intervention (SMD ‐0.12, 95% CI ‐0.39 to 0.15; I2 = 38%; P = 0.39; 4 studies, 663 participants; moderate certainty due to indirectness) (Analysis 3.8). At 1 to 6 months (Analysis 3.9), the actual effect range indicates it may make little or no difference (SMD ‐0.10, 95% CI ‐0.60 to 0.41; I2 = 77%; P = 0.71; 2 studies, 594 participants), as well as at 7 to 24 months (SMD ‐0.21, 95% CI ‐0.47 to 0.04; I2 = 38%; P = 0.10; 2 studies, 241 participants) (Analysis 3.10).
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 3.11; Analysis 3.12; Analysis 3.13; Analysis 3.17; Analysis 3.18; Analysis 3.19; Analysis 3.14; Analysis 3.15; Analysis 3.16; Analysis 3.17; Analysis 3.18; Analysis 3.19)
3.11. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 11: Depressive symptoms at 0‐1 months
3.12. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 12: Depressive symptoms at 1‐6 months
3.13. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 13: Depressive symptoms at 7‐24 months
3.17. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 17: Distress/PTSD symptoms at 0‐1 months
3.18. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 18: Distress/PTSD symptoms at 1‐6 months
3.19. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 19: Distress/PTSD symptoms at 7‐24 months
3.14. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 14: Anxiety symptoms at 0‐1 months
3.15. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 15: Anxiety symptoms at 1‐6 months
3.16. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 16: Anxiety symptoms at 7‐24 months
At 0 to 1 month post‐intervention, we found 18 studies evaluating depressive symptoms. Indicated prevention interventions may have little to no effect in reducing depressive symptoms compared to control groups but the evidence is very uncertain (SMD ‐0.16, 95% CI ‐0.30 to ‐0.03; I2 = 56%; P = 0.02; 2341 participants; very low‐certainty evidence due to inconsistency, publication bias, and study limitations) (Analysis 3.11). At 1 to 6 months, we collected 11 studies confirming that the effect of the intervention was maintained (SMD ‐0.34, 95% CI ‐0.58 to ‐0.10; I2 = 88%; P = 0.005; 2609 participants) (Analysis 3.12). At 7 to 24 months, we found eight studies that suggested that indicated prevention interventions may lead to slightly decreased depressive symptoms, although the actual effect range indicates it may have little or no difference (SMD ‐0.10, 95% CI ‐0.22 to 0.01; I2 = 39%; P = 0.08; 2149 participants) (Analysis 3.13).
For anxiety symptoms at 0 to 1 month post‐intervention, the collected five studies (Bernardi 2020; Ferreira‐Vorkapic 2018; Hajarian Abhari 2021; Rao 2017; Rodriguez 2021) identified that indicated prevention interventions may have a positive effect over controls but the evidence is very uncertain (SMD ‐1.19, 95% CI ‐2.02 to ‐0.35; I2 = 89%; P = 0.005; 250 participants; very low certainty due to study limitations and inconsistency) (Analysis 3.14). At 1 to 6 months, we collected four studies (Dayhimi 2020; Rong 2021a; Srisuwan 2020; Xu 2021) suggesting that the beneficial effect of the intervention was maintained (SMD ‐0.23, 95% CI ‐0.37 to ‐0.09; I2 = 84%; P = 0.002; 771 participants) (Analysis 3.15). At 7 to 24 months, indicated prevention interventions may slightly decrease anxiety symptoms, although the actual effect range indicates the effect of the intervention may have little or no difference (SMD ‐0.12, 95% CI ‐0.29 to 0.05; I2 = 92%; P = 0.17; 2 studies, 549 participants) (Analysis 3.16).
For distress/PTSD symptoms at 0 to 1 month post‐intervention, indicated prevention interventions may have a positive effect but the evidence is very uncertain (SMD ‐0.54, 95% CI ‐0.95 to ‐0.14; I2 = 95%; P = 0.009; 19 studies, 2536 participants; very low certainty due to inconsistency and publication bias) (Analysis 3.17). At 1 to 6 months, the effect of the intervention was maintained (SMD ‐0.29, 95% CI ‐0.51 to ‐0.07; I2 = 75%; P = 0.008; 9 studies, 1702 participants) (Analysis 3.18). At 7 to 24 months, the actual effect range indicates the effect of the intervention may have little or no difference (SMD ‐0.04, 95% CI ‐0.45 to 0.38; I2 = 91%; P = 0.86; 5 studies, 1081 participants) (Analysis 3.19).
4. Social outcomes (e.g. perception of social inclusion)
(Analysis 3.20; Analysis 3.21)
3.20. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 20: Social outcomes at 0‐1 months
3.21. Analysis.

Comparison 3: Indicated prevention intervention versus control group in preventing mental disorders in adults, Outcome 21: Social outcomes at 7‐24 months
At 0 to 1 month and at 7 to 24 months post‐intervention, the actual effect range indicates the effect of the intervention may make little or no difference on social outcomes. At 1 to 6 months, no studies reported on this outcome.
5. Resource use
No studies reported on this outcome.
6. Carer mental health
No studies reported on this outcome.
Comparison 4. Primary‐level health worker and/or community worker‐led universal prevention or promotion interventions for children (n = 8 studies)
We identified eight RCTs (six cluster‐RCTs (75%) and two individual RCTs (25%)) contributing to meta‐analyses in this comparison. Total sample size was 1496 (760 participants (50.8%) for the intervention group and 736 participants (49.2%) for the control group).
As for the type of PWs, all interventions (n = 8 studies, 100%) were delivered by community workers (CWs) but, in one study (12.5%), the intervention was also delivered by primary health workers (PWs) (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, school teachers, yoga teachers, staff, and student facilitators, whereas PWs included midwives. For most of the studies, participants in the control group were allocated to usual care (n = 5 studies, 62.5%); for one study (12.5%) the control group was guided by a PW without training and/or supervision and, for the remaining two studies (25%), the control group was active control or waiting list. All eight studies (100%) had been conducted in schools. From a wider geographical perspective, seven studies (87.5%) were conducted in an urban context and one in a rural context. Five studies (62.5%) were undertaken in upper‐middle‐income countries and three (37.5%) in low‐income countries. As for the condition, most included studies (n = 5, 62.5%) targeted the prevention of child mental disorders and three studies (37.5%) aimed at improving psychological well‐being.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
At 0 to 1 month post‐intervention
No studies reported on this outcome.
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
No studies reported on this outcome.
2. Quality of life
4.1. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 1: Quality of life at 0‐1 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, we found two studies (Barbosa Filho 2017; Devries 2015) showing that universal prevention/promotion interventions may show a positive effect of the interventions versus control in improving the quality of life (SMD ‐0.25, 95% CI ‐0.39 to ‐0.11; I2 = 0%; P = 0.0003; 2 studies, 803 participants; low certainty due to study limitations and indirectness) (Analysis 4.1).
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
No studies reported on this outcome.
3. Adverse events experienced during the intervention
4.2. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 2: Adverse events at 0‐1 months
One study (Devries 2015) provided information on this outcome at 0 to 1 month reporting that no participants experienced adverse events in either study arm.
Secondary outcomes
1. Psychological functioning and impairment
(Analysis 4.3; Analysis 4.4; Analysis 4.5)
4.3. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 3: Psychological functioning and impairment at 0‐1 months
4.4. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 4: Psychological functioning and impairment at 1‐6 months
4.5. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 5: Psychological functioning and impairment at 7‐24 months
At 0 to 1 month post‐intervention (Analysis 4.3), it is uncertain whether universal prevention/promotion interventions improve psychological functioning (SMD 0.04, 95% CI ‐0.90 to 0.98; I2 = 82%; P = 0.93; 2 studies, 212 participants; low certainty due to study limitations, inconsistency, indirectness and imprecision). At 1 to 6 months follow‐up (Analysis 4.4), universal prevention/promotion interventions may lead to increased psychological functioning, although the actual effect range indicates it may have little or no difference (MD ‐0.29, 95% CI ‐0.93 to 0.35; I2 = NA; P = 0.37; 1 study, 90 participants). At 7 to 24 months (Analysis 4.5), one study (Berger 2018) identified a difference in favour of the intervention over the control condition (MD ‐3.33, 95% CI ‐5.03 to ‐2.63; I2 = NA; P = 0.0001; 183 participants).
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 4.10; Analysis 4.11; Analysis 4.12)
4.6. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 6: Depressive symptoms at 0‐1 months
4.7. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 7: Depressive symptoms at 1‐6 months
4.8. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 8: Anxiety symptoms at 0‐1 months
4.9. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 9: Anxiety symptoms at 1‐6 months
4.10. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 10: Anxiety symptoms at 7‐24 months
4.11. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 11: Distress/PTSD symptoms at 0‐1 months
4.12. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 12: Distress/PTSD symptoms at 1‐6 months
For depressive symptoms, we found one study (Rivet‐Duval 2011) reporting that interventions may improve symptoms at 0 to 1 month post‐intervention (SMD ‐3.04, 95% CI ‐6.00 to ‐0.08; I2 = NA%; P = 0.04; 160 participants; low certainty due to study limitations and imprecision) (Analysis 4.6), but the effect was not maintained at 1 to 6 months (SMD ‐0.00, 95% CI ‐0.20 to 0.20; I2 = 0%; P = 0.98; 3 studies, 385 participants) (Analysis 4.7). At 7 to 24 months, no studies reported on this outcome.
For anxiety symptoms at 0 to 1 month post‐intervention, we found one study (Berger 2018) reporting that universal prevention/promotion interventions may reduce anxiety symptoms (MD ‐2.27, 95% CI ‐3.13 to ‐1.41; I2 = NA; P < 0.00001; 183 participants; low certainty due to study limitations and imprecision) (Analysis 4.8). At 1 to 6 months, the difference was not important (MD ‐0.13, 95% CI ‐0.41 to 0.15; I2 = NA; P = 0.37; 1 study, 125 participants) (Analysis 4.9) while, at 7 to 24 months, the study of Berger 2018 confirmed a positive effect of the intervention (SMD ‐2.27, 95% CI ‐3.10 to ‐1.44; I2 = NA; P < 0.00001; 183 participants) (Analysis 4.10).
For distress/PTSD symptoms at 0 to 1 month post‐intervention, it is uncertain whether interventions improve distress (SMD ‐0.83, 95% CI ‐2.48 to 0.82; I2 = 98%; P = 0.33; 2 studies, 800 participants) (Analysis 4.11) while, at 1 to 6 months, we found one study (Mohamadi 2021) reporting that universal prevention/promotion interventions improved symptoms of distress/PTSD (MD ‐4.51, 95% CI ‐5.86 to ‐3.16; I2 = NA; P < 0.00001; 106 participants) (Analysis 4.12). No data were available on this outcome at 7 to 24 months follow‐up.
4. Social outcomes (e.g. perception of social inclusion)
(Analysis 4.13; Analysis 4.14; Analysis 4.15)
4.13. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 13: Social outcomes at 0‐1 months
4.14. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 14: Social outcomes at 1‐6 months
4.15. Analysis.

Comparison 4: Promotion/universal prevention interventions versus control group in preventing mental disorders in children, Outcome 15: Social outcomes at 7‐24 months
At 0 to 1 month post‐intervention (Analysis 4.13), universal prevention/promotion interventions may lead to increased psychological functioning, although the actual effect range indicates it may have little or no difference (SMD ‐0.32, 95% CI ‐0.76 to 0.12; I2 = 67%; P = 0.15; 3 studies, 321 participants) and at 1 to 6 months (SMD ‐0.27, 95% CI ‐0.82 to 0.28; I2 = 75%; P = 0.34; 2 studies, 215 participants) (Analysis 4.14). At 7 to 24 months, we collected one study (Berger 2018) reporting a positive effect of the intervention over the control group (MD ‐0.70, 95% CI ‐1.07 to ‐0.33; I2 = NA; P = 0.0002; 183 participants) (Analysis 4.15).
5. Resource use
No studies reported data on this outcome.
6. Carer mental health
No studies reported data on this outcome.
Comparison 5. Primary‐level health worker and/or community worker‐led selective prevention interventions for children (n = 7 studies)
We identified seven RCTs (four cluster‐RCTs (57.1%) and three individual RCTs (42.9%)) contributing to meta‐analyses in this comparison. The total sample size was 1572 (800 participants (50.9%) for the intervention group and 772 participants (49.1%) for the control group).
As for the type of PWs, most of the interventions (n = 6 studies, 85.7%) were delivered by community workers (CWs) whereas, in only one study (14.3%), the intervention was delivered by primary health workers (PWs) (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, teachers, facilitators, and coaches, whereas PWs included nursing students. For most of the studies, participants in the control group were allocated to usual care (n = 6 studies, 85.7%); for the remaining study (14.3%), the control group was a waiting‐list condition. Four studies (57.1%) had been conducted in schools, one (14.3%) in hospitals, and the remaining two (28.6%) in other settings, such as local churches and multiple community spaces. From a wider geographical perspective, three studies (42.9%) were conducted in an urban context, one (14.3%) in a rural context, one (14.3%) in both urban and rural contexts and, for the remaining two studies (28.6%), it was not specified. One study (14.3%) was undertaken in an upper‐middle income country, one (14.3%) in a low‐middle income country, and the remaining five studies (71.4%) in low‐income countries. As for the condition, the majority of studies (n = 6 studies, 85.7%) targeted the prevention of child mental disorders and one study (14.3%) aimed at improving psychological well‐being.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
At 0 to 1 month post‐intervention
No studies reported on this outcome.
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
No studies reported on this outcome.
2. Quality of life
5.1. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 1: Quality of life at 0‐1 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, we found one study (Ager 2011) indicating that it is uncertain whether selective prevention interventions improve quality of life (MD ‐1.10, 95% CI ‐3.32 to 1.12; I2 = NA; P = 0.33; 115 participants; very low certainty due to study limitations and imprecision) (Analysis 5.1).
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
No studies reported on this outcome.
3. Adverse events experienced during the intervention
No studies reported data on this outcome.
Secondary outcomes
1. Psychological functioning and impairment
5.2. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 2: Psychological functioning and impairment at 0‐1 months
At 0 to 1 month post‐intervention, there is no evidence that selective prevention interventions improves functional impairment (MD ‐0.02, 95% CI ‐0.09 to 0.05; I2 = NA; P = 0.57; 1 study, 479 participants; low certainty due to indirectness and imprecision). At 1 to 6 and 7 to 24 months, no studies reported on this outcome.
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9)
5.3. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 3: Depressive symptoms at 0‐1 months
5.4. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 4: Depressive symptoms at 1‐6 months
5.5. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 5: Depressive symptoms at 7‐24 months
5.6. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 6: Anxiety symptoms at 0‐1 months
5.7. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 7: Anxiety symptoms at 1‐6 months
5.8. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 8: Distress/PTSD symptoms at 0‐1 months
5.9. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 9: Distress/PTSD symptoms at 1‐6 months
For depressive symptoms, selective prevention interventions probably make little or no difference to symptoms at 0 to 1 month post‐intervention (SMD 0.00, 95% CI ‐0.16 to 0.15; I2 = 0%; P = 0.96; 2 studies, 638 participants; moderate certainty due to imprecision) (Analysis 5.3). We did not identify any important difference between the intervention and control group at 1 to 6 months and 7 to 24 months (Analysis 5.4; Analysis 5.5).
For anxiety symptoms, selective prevention interventions may make little or no difference to symptoms at 0 to 1 month post‐intervention (MD 4.50, 95% CI ‐12.05 to 21.05; I2 = NA; P = 0.59; 1 study, 28 participants; low certainty due to imprecision) (Analysis 5.6). At 1 to 6 months, one study reported a worsening of anxiety symptoms (MD 1.42, 95% CI ‐0.00 to 2.84; I2 = NA; P = 0.05; 1 study, 143 participants) (Analysis 5.7). At 7 to 24 months, no studies provided information on this outcome.
For distress/PTSD symptoms at 0 to 1 month post‐intervention, we found one study (O’Callaghan 2014) reporting that interventions probably improve symptoms of distress/PTSD (MD ‐2.14, 95% CI ‐3.77 to ‐0.51; I2 = NA; P = 0.01; 159 participants; moderate certainty due to imprecision) (Analysis 5.8). Selective prevention interventions have an uncertain effect at 1 to 6 months (SMD ‐0.40, 95% CI ‐2.49 to 1.69; I2 = NA; P = 0.71; 1 study, 213 participants) (Analysis 5.9). No data were available on this outcome at 7 to 24 months follow‐up.
4. Social outcomes (e.g. perception of social inclusion)
5.10. Analysis.

Comparison 5: Selective prevention intervention versus control group in preventing mental disorders in children, Outcome 10: Social outcomes at 0‐1 months
At 0 to 1 month post‐intervention, we did not find any differences between the intervention and control group. No data were available for the other time points.
5. Resource use
No studies reported data on this outcome.
6. Carer mental health
No studies reported data on this outcome.
Comparison 6. Primary‐level health worker and/or community worker‐led indicated prevention interventions for children (n = 9 studies)
We identified nine RCTs (five cluster‐RCTs (55.6%) and four individual RCTs (44.4%)) contributing to meta‐analyses in this comparison. The total sample size was 3029 (1684 participants for the intervention group and 1345 participants for the control group).
As for the type of PWs, most of the interventions (n = 7 studies, 77.8%) were delivered by community workers (CWs) whereas, in one study (11.1%), the intervention was delivered by primary health workers (PHWs) and, in another study (11.1%) there was a self‐help intervention (see Appendix 2 for a complete list of studies regarding the classification of PWs). CWs included, for instance, adult refugee facilitators, teachers, paraprofessional interventionists, lay counsellors, whereas PWs included social workers. For most of the studies, participants in the control group were allocated to usual care (n = 8 studies, 88.9%); for the remaining study (11.1%), the control group was allocated to active control. Eight studies (88.9%) had been conducted in schools and one (11.1%) in a refugee camp. From a wider geographical perspective, one study (11.1%) was conducted in an urban context, three (33.3%) in a rural context, one (11.1%) in both urban and rural contexts and, for the remaining four studies (44.4%), it was not specified. Three studies (33.3%) were undertaken in upper‐middle income countries, four (44.4%) in low‐middle income countries and two studies (22.2%) in low‐income countries. As for the condition, all included studies (n = 9 studies, 100%) targeted the prevention of child mental disorders.
Primary outcomes
1. Diagnosis (or a proxy thereof, as assessed by scoring above a cut‐off for a screening tool) of mental disorders
6.1. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 1: Diagnosis of mental disorders at 0‐1 months
6.2. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 2: Diagnosis of mental disorders at 1 to 6 months
At 0 to 1 month post‐intervention
At 0 to 1 month post‐intervention, we found one study (Yu 2002) with 220 participants. It is uncertain whether indicated prevention interventions reduce the incidence of mental health diagnosis (RR 0.77, 95% CI 0.51 to 1.17; I2 = NA; P = 0.22; 1 study, 220 participants; very low certainty due to study limitations indirectness and imprecision) (Analysis 6.1).
At 1 to 6 months post‐intervention
At 1 to 6 months, we found one study (Yu 2002) that indicated prevention interventions may lead to decreased incidence, although the actual effect range indicates the effect of the intervention may have little or no difference (RR 0.77, 95% CI 0.51 to 1.17; I2 = NA; P = 0.19; 220 participants) (Analysis 6.2).
At 7 to 24 months post‐intervention
No studies reported on this outcome.
2. Quality of life
6.3. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 3: Quality of life at 0‐1 months
At 0 to 1 month post‐intervention
It is uncertain whether indicated prevention interventions improve quality of life at 0 to 1 month post‐intervention (SMD ‐0.65, 95% CI ‐2.09 to 0.79; I2 = 0%; P = 0.38; 2 studies, 152 participants; very low certainty due to study limitations, indirectness, and imprecision) (Analysis 6.3).
At 1 to 6 months post‐intervention
No studies reported on this outcome.
At 7 to 24 months post‐intervention
No studies reported on this outcome.
3. Adverse events experienced during the intervention
No studies reported data on this outcome.
Secondary outcomes
1. Psychological functioning and impairment
6.4. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 4: Psychological functioning and impairment at 0‐1 months
6.5. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 5: Psychological functioning and impairment at 1‐6 months
At 0 to 1 month post‐intervention, we collected two studies (Fine 2021; Tol 2012) reporting a difference in favour of the intervention over the control group (SMD ‐0.29, 95% CI ‐0.47 to ‐0.10; I2 = 0%; P = 0.003; 448 participants; high certainty) (Analysis 6.4). At 1 to 6 months, we failed to identify any difference between intervention and control groups (Analysis 6.5). At 7 to 24 months, no studies reported on this outcome.
2. Changes in service utilization and contact coverage
No studies reported on this outcome.
3. Changes in mental health symptoms captured on rating scales (i.e. depressive symptoms, anxiety symptoms, distress/PTSD symptoms)
(Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9; Analysis 6.10; Analysis 6.11; Analysis 6.12)
6.6. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 6: Depressive symptoms at 0‐1 months
6.7. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 7: Depressive symptoms at 1‐6 months
6.8. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 8: Depressive symptoms at 7‐24 months
6.9. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 9: Anxiety symptoms at 0‐1 months
6.10. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 10: Anxiety symptoms at 1‐6 months
6.11. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 11: Distress/PTSD symptoms at 0‐1 months
6.12. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 12: Distress/PTSD symptoms at 1‐6 months
For depressive symptoms, we found four studies (Fine 2021; Osborn 2020; Tol 2012; Yu 2002) reporting a beneficial effect of the intervention over control at 0 to 1 month post‐intervention (SMD ‐0.18, 95% CI ‐0.32 to ‐0.04; I2 = 0; P = 0.01; 771 participants; high certainty) (Analysis 6.6). At 1 to 6 months, this effect was not maintained while, at 7 to 24 months, we found one study by Shinde 2018 that indicated prevention interventions were more effective than controls in reducing depressive symptoms (MD ‐1.27 95% CI ‐1.90 to ‐0.64; I2 = NA; P < 0.0001; 904 participants) (Analysis 6.8).
For anxiety symptoms, it is uncertain whether indicated prevention interventions improve symptoms at 0 to 1 month post‐intervention (SMD ‐0.09, 95% CI ‐0.22 to 0.04; I2 = 0%; P = 0.19; 3 studies, 888 participants; very low certainty due to study limitations and indirectness) (Analysis 6.9). At 1 to 6 months follow‐up, we did not find any important difference between intervention and control groups (Analysis 6.10). At 7 to 24 months, no studies reported data on this outcome.
For distress/PTSD symptoms at 0 to 1 month post‐intervention, prevention interventions may make little or no difference to symptoms (SMD 0.24, 95% CI ‐1.28 to 1.76; I2 = 0%; P = 0.76; 2 studies, 448 participants; low certainty due to inconsistency and imprecision) (Analysis 6.11). At 1 to 6 months, we failed to identify any important difference between intervention and control groups (Analysis 6.12). No study provided data at 7 to 24 months.
4. Social outcomes (e.g. perception of social inclusion)
(Analysis 6.13; Analysis 6.14)
6.13. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 13: Social outcomes at 0‐1 months
6.14. Analysis.

Comparison 6: Indicated prevention intervention versus control group in preventing mental disorders in children, Outcome 14: Social outcomes at 1‐6 months
At 0 to 1 month post‐intervention, we failed to identify any difference between intervention and control groups (Analysis 6.13) while at 1 to 6 months, we collected two studies (Jordans 2010; Thurman 2017) that indicated prevention interventions were more effective than control groups in improving social outcomes (SMD ‐0.22, 95% CI ‐0.41 to ‐0.03; I2 = 0%; P = 0.02; 421 participants) (Analysis 6.14). No study provided data at 7 to 24 months.
5. Resource use
No studies reported data on this outcome.
6. Carer mental health
No studies reported data on this outcome.
Subgroup and sensitivity analyses
We presented all the meta‐analyses according to the type of prevention intervention (universal, selective, indicated).
Due to the low number of studies available for each type of prevention intervention, we were not able to perform all the planned subgroup and sensitivity analyses.
Category of health worker (e.g. primary care professionals, non‐professional health workers, community workers). This analysis was not performed as most interventions were delivered by community and para‐professional workers.
-
Setting of care.
Community settings, camps, schools: this analysis was not performed given the small number of RCTs for each category.
Chronic or acute humanitarian versus non‐humanitarian settings: the number of RCTs conducted in humanitarian crises was very low compared to those in LMICs not exposed to the acute phase of a humanitarian crisis.
Type of promotion intervention (individual, group): it was not possible to conduct this analysis as most of the interventions were delivered in a group setting.
Specific risk, protective, or promotive factor targeted; RCT reports did not provide specific information on the targeted risk, protective, or promotive factors to conduct this analysis.
Subgroup analyses
All the analyses were conducted according to the type of prevention interventions and, for this reason, no subgroup analyses were needed on this variable. We were able to conduct subgroup analyses considering the category of health workers and the setting of care. These analyses did not identify any differences across subgroups in relation to the assessed outcomes (P > 0.05). We were not able to conduct subgroup analyses considering chronic or acute humanitarian versus non‐humanitarian settings, the type of promotion intervention in terms of individual versus group modality, and the risk, protective, or promotive factor targeted. No sufficient information was reported in the included studies to clearly identify the variables listed above.
Sensitivity analyses
We conducted sensitivity analyses including only studies with low risk of bias, as specified in incomplete outcome data and selective reporting. The effect of indicated prevention interventions for adults on psychological functioning and impairment was statistically significant at 0 to 1 month post‐intervention after excluding studies with high risk of bias and those having some concerns. For mental health symptoms, both for depressive and PTSD symptoms, the effect of indicated prevention interventions was no longer statistically significant at 0 to 1 month post‐intervention, after excluding studies with high risk of bias and those having some concerns.
We also conducted analyses excluding trials with methodological characteristics that might generate the highest heterogeneity in meta‐analysis (I² > 75%). The effect of indicated prevention interventions on the diagnosis of mental disorders was statistically significant after excluding studies generating the highest heterogeneity, and I2 was lowered to 0%. For quality of life, no differences were detected at 0 to 1 month post‐intervention study endpoint, while the effect of the intervention was not statistically significant at 1 to 6 months.
We were not able to conduct sensitivity analysis considering only published studies, and sensitivity analysis including only studies measuring the incidence of mental disorders (i.e. studies in which all participants at baseline scored below defined symptom thresholds on rating scales).
Discussion
This review included 113 randomized trials evaluating the effectiveness of promotion and prevention interventions delivered through a task‐shifting approach in 39 LMICs. The majority of studies were focused on adult populations (83 RCTs), while only a minority assessed promotion and prevention interventions for children (30 RCTs).
Summary of main results
This review aimed to assess the effectiveness of promotion and prevention interventions delivered through a task‐shifting approach by primary level and community workers to the child and adolescent, and adult populations in LMICs.
With regard to primary outcomes in adults, promotion/universal prevention and selective prevention interventions were not effective in reducing the likelihood of receiving a diagnosis of mental disorders at any time points. Indicated prevention interventions had a significant effect in reducing the frequency of diagnosed mental disorders at 1 to 6 months while, at other time points, we failed to identify a significant effect. For quality of life, promotion/universal prevention interventions were effective at 7 to 24 months follow‐up, while selective and indicated prevention interventions had a beneficial effect at the study endpoint, that in the selective prevention interventions was maintained at 1 to 6 months. In respect to adverse events, the outcome was poorly reported and no inference could be drawn. With regard to secondary outcomes, we found a small‐to‐moderate effect of interventions in reducing psychological symptoms. Functioning was assessed only in indicated prevention studies without detecting any significant difference at any time point. For social outcomes, promotion/universal prevention and selective interventions were more effective than controls at the study endpoint, and the positive effect was maintained at 7 to 24 months for selective prevention interventions. Indicated prevention interventions did not provide any beneficial effect for this outcome.
In children, only studies on indicated prevention interventions provided data on the diagnosis of mental disorders and failed to identify a beneficial effect of prevention interventions over controls. For the quality of life outcome, promotion/universal prevention interventions significantly improved this outcome at the study endpoint, while selective and indicated prevention studies failed to show a beneficial effect. The outcome of adverse events was reported only in one study in the promotion/universal prevention category without showing any difference against the control condition.
With regard to secondary outcomes, we found that promotion/universal prevention interventions improved functioning at 7 to 24 months, while indicated prevention interventions had a moderate effect in improving functioning and impairment at the study endpoint. Promotion/universal prevention interventions were more effective than controls in improving symptoms of depression and anxiety at the study endpoint, and in improving anxiety and distress at follow‐up (7 to 24 months and 1 to 6 months, respectively). Selective prevention interventions provided a beneficial effect on distress symptoms at the study endpoint. Indicated prevention interventions were more effective than controls in improving depressive symptoms at the study endpoint and at 7 to 24 months while, for anxiety and distress, we failed to identify a significant effect. For social outcomes, promotion/universal prevention interventions were significantly more effective than controls at 7 to 24 months, while indicated prevention was more effective at 1 to 6 months.
Though we could not do subgroup analyses per health worker group, those represented were community and social workers, teachers, nurses, lay counsellors, and facilitators receiving specific training for intervention delivery. This task‐shifting approach allows a broader scope of interventions in primary care when specialists are unavailable, and this review confirms that intervention delivery through PWs may improve many relevant clinical outcomes, as already highlighted in previous systematic reviews on treatment interventions (Purgato 2018a; Van Ginneken 2021).
Overall, these findings indicate the benefit of specific types of prevention interventions for reducing the likelihood of being diagnosed with any mental disorders, and improving the quality of life for adults living in LMICs, but show a lack of effect for specific types of prevention interventions and over longer follow‐up. This lack of effect could be related to different factors. Firstly, to the characteristics of interventions, which were sometimes short in duration and focused not only on mental health but also on other areas (e.g. home‐care practices and health‐seeking behaviours). Secondly, to the social determinants of mental health, like exposure to ongoing and chronic adversities that participants had to manage in low‐resource settings, exposure to traumatic events, and unsecured food, water, and housing (Lund 2018). Available data failed to show a strong and beneficial effect in reducing the likelihood of being diagnosed with any mental disorders or improving the quality of life. There were only a few included studies reporting on this outcome, i.e. diagnosis of mental disorders, as the majority of promotion/universal and selective prevention interventions focused on the reduction of symptoms. Additionally and as expected, we identified heterogeneity in outcome measure tools and population groups. An in‐depth mediation analysis would help to shed light on the mechanisms of action of interventions, and the complementary appraisal of moderators would contribute to optimizing the delivery modality, and would allow matching participants with the most appropriate interventions (Cuijpers 2022; Purgato 2021b).
Based on the review results, we identified a significant gap in the scientific literature on intervention for promoting mental health or preventing mental health conditions, and also a lack of tailored task‐shifted interventions for specific conditions with proven efficacy in treatment settings. Future research trajectories might include the design and implementation of RCTs with a clear prevention design, i.e. aimed at reducing the incidence of mental disorders as a primary outcome. Only a minority of the trials included in this review were primarily designed with this goal. Finally, data suggest that research on children should be expanded as, in comparison with adults, little evidence was available to draw firm conclusions on this population.
Overall completeness and applicability of evidence
Interventions were categorized according to the characteristics of their target population group following a public mental health approach (Purgato 2020; Tol 2015a). Promotion and universal prevention interventions were categorized under the same label, as it was often complex to distinguish multi‐component interventions aimed at strengthening well‐being and improving mental health, which fall into the promotion category (Eaton 2012; Papola 2022; WHO 2014), and interventions to universally prevent mental health conditions (Ceccarelli 2022; Tol 2015a; Tol 2015b; WHO 2019). For example, promotion/universal prevention included social skills programmes for all the students attending selected grades, interventions for building self‐esteem, keeping calm, thinking resourcefully, identifying and accessing support networks, considering the perspective of others, and keeping the peace (Castillo 2019; O'Reilly 2018). Examples of selective prevention interventions included psychosocial structured activities delivered in school settings and designed for trauma recovery, interventions with physical exercises, facilitation of self‐efficacy and decision‐making skills, problem‐solving, and maintaining a balanced lifestyle. Indicated prevention interventions, delivered to those already showing some signs of disorders (i.e. psychological symptoms below cut‐offs of rating scales) without meeting the criteria for a psychiatric diagnosis (Wahlbeck 2015), included counselling based on the principles of cognitive‐behavioural therapy, mindfulness, and yoga‐inspired techniques. Some of the indicated prevention interventions included in this review belong to the range of scalable psychological interventions developed by the WHO for use in settings affected by adversity (Sijbrandij 2017; WHO 2018). Amongst these, we find the WHO Self‐Help Plus intervention, a short strategy based on Acceptance and Commitment Therapy that was tested in a large sample of adult refugees resettled in Turkey (Acarturk 2022; Epping‐Jordan 2016). Overall, all analyzed interventions aimed at enhancing protective factors (e.g. increased social skills, coping strategies to manage stress, and problem‐solving ability; building social connectedness and community relationships; and encouraging family support and positive community network) for the prevention of mental health disorders. This is in line with the promotion and prevention component of the WHO community‐based rehabilitation guidelines (WHO 2010). Almost all included interventions adopted a community‐based approach, were implemented in the life context of the participants, and were undertaken together with a significant person for the participant (i.e. caregiver, parent, partner). This explains the increased number of interventions addressing the everyday social impacts on mental health in LMICs, considering the interaction between the local social environment and psychosocial well‐being. In line with this, the majority of interventions assessed in the included RCTs were designed and delivered for preventing common mental health conditions, like depression, PTSD, and anxiety, which are the most prevalent conditions in low‐resource settings affected by humanitarian crises (Charlson 2019).
Within low‐resource settings, the task‐shifting approach has been developed and implemented for improving the efficacy and effectiveness of mental health services, starting with extending their accessibility (Hoeft 2018; Purgato 2020). The intervention gap between people in need of mental health interventions and those who actually access services is constantly increasing, thus requiring the adoption of innovative strategies, such as task‐shifting (Hoeft 2018; Mendenhall 2014; Soltan 2022). In line with this consideration, a recent systematic review with individual patient data meta‐analysis focused on psychological interventions in low‐ and middle‐income countries, and found an association between the task‐shifting approach and an increased reduction in the severity of depressive symptoms (Karyotaki 2022).
The ways in which the organization of society, social interactions, and relationships affect the risk of, and protection from, mental disorders is called 'social domain'. There is widespread global evidence that mental disorders in populations are strongly socially determined, and our findings suggest a growth focus on the 'social domain' as a crucial social determinant of mental health, as reported in the UN Sustainable Development Goals (Burgess 2020; Lund 2018; Patel 2018).
The included studies employed different types of primary workers, in line with the scientific literature on psychological and social interventions (Shahmalak 2019; Van Ginneken 2021). Some workers were existing cadres within the health sector (i.e. nurses, midwives), while others were additionally trained resources. Resources included for example teachers, peer mentors, lay counsellors, peer educators, social workers, field workers, and caregivers. Primary workers received short training before the intervention delivery and, in most cases, received supervision during the course of the study. The training was usually manualized and focused strictly on the intervention's content and delivery. While many studies presented this basic description of training and supervision procedures, many failed to report detailed information on these aspects. Nevertheless, it would be of great value for authors of RCTs to provide detailed descriptions of training contents and duration, to improve readers' understanding of the interventions (Shahmalak 2019).
Regarding the control conditions, we found that the waiting list, no treatment, and treatment‐as‐usual were the most reported comparators, despite the methodological recommendation of using active psychological control groups in psychotherapy research (Guidi 2018). The use of 'inactive' controls does not allow establishing whether any significant difference is related to some specific treatment ingredients, introduced by the experimental procedure, or to nonspecific factors, such as attention and opportunity for disclosure (Guidi 2018; Mulder 2017; Riello 2021). Regardless, inactive controls remain widely used (Barbui 2020; Faltinsen 2022). As a consequence, we were not able to compare active prevention interventions against each other, although this would be vital to increase our understanding of the mechanism of action of prevention interventions (Purgato 2019b; Purgato 2021a).
Data on children and adolescents were not available for some important outcomes or were not available at medium‐ and long‐term follow‐up. This paucity of research on children and adolescents is in line with what was highlighted in the WHO 2020 guidelines on mental health promotive and preventive interventions for adolescents, which indicated a crucial need for research on the topic, especially in low‐resource or high‐adversity settings (WHO 2020).
Promotion and prevention interventions were delivered in variable conditions of cultural contexts, which were not accounted for in our analyses. Factors that can facilitate or impede the implementation of mental health interventions vary across contexts and cultures and examination prior to, during, and after the process of implementation is relevant (Faregh 2019).
Finally, we were not able to perform all the planned subgroup and sensitivity analyses. This was due to the low number of studies available for each type of prevention intervention.
Quality of the evidence
Even though the RCT is the design of choice for evaluating the efficacy and acceptability of healthcare interventions (Jüni 2001; Purgato 2010), the level of evidence generated by our review is poor as evaluated with the Cochrane risk of bias tool 2. This is consistent with our grading within the summary of findings tables (from very low to moderate) (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
This review included 113 trials, all of which were randomized and covered a wide range of interventions and settings. For studies included in the meta‐analyses, evidence for most outcomes was of low‐ to moderate‐certainty. The most often identified biases across studies in both children and adolescents and adults were risk of bias due to deviations from the intended interventions (effect of assignment to intervention and effect of adhering to intervention). This is due to the fact that participants randomized to psychological interventions typically know whether they have been randomized to the active intervention or to the control group, and the same is true for people delivering the interventions (Cuijpers 2015). These biases are likely to be especially large in studies with waiting lists or treatment‐as‐usual controls (Cuijpers 2016; Mohr 2014). Other identified biases were related to the risk of bias due to missing outcome data, and the risk of bias in the selection of the reported results, which has been associated with the risk of inflation of effect sizes, contributing to the current uncertainties in assessing the outcomes of psychological interventions (Miguel 2021). On the contrary, the domain related to the randomization process was evaluated as being at low risk of bias in the majority of studies on adults, and in all the studies focused on children and adolescents.
For the adult population, only four studies had an overall low risk of bias. The remaining studies had a high risk of bias or some concerns. Although the number of trials available on the child and adolescent population was smaller, the risk of bias identified was consistent with RCTs for adults.
Additional possible sources of bias not included in the risk of bias assessment were those specifically related to the topic of this review: socio‐cultural differences in relation to psychological suffering across countries; transposition of mental health concepts from western to non‐western cultures (Kaiser 2015), with very different understandings and ways of dealing with psychological distress; and social norms and ways of discussing distress with strangers (Barbui 2017). Moreover, researchers did not always report details on language and nationality, social/economic class, education, geography, age, and background of the people delivering the interventions. These characteristics might have an influence on the establishment of relationships and trust, and thus on some study outcomes. Finally, even though we did not explore the risk of bias related to research allegiance, that is a specific intellectual conflict of interest that is consistent with one’s professional or personal commitment to one type of intervention and may consequently distort the outcomes (Leykin 2009), the use of the task‐shifting approach in which those people delivering the interventions were not involved in its development should lower the probability of this risk.
Potential biases in the review process
Although we tried to include any primary level and community worker categories, it is possible that some studies have been missed. Moreover, although it is unlikely that RCTs would be conducted and would not be publicly accessible, not all those conducting research may necessarily value academic publications, so work may be disseminated through other channels. In addition, primary level and community workers do not have standard widely accepted definitions yet, so some readers may disagree with the definitions that we used or with how this review has aggregated interventionists.
In addition, when we were unable to collect data on our primary outcome, in some cases, we used the number of participants with symptom levels below cut‐offs as a proxy of the cases diagnosed with formal diagnostic instruments, such as the MINI Neuropsychiatric Interview (Sheehan 1998). In the present review, we included interventions with broadly similar aims and methods in countries with similar incomes. However, the diversity of prevention approaches and the different sociocultural and healthcare system contexts in which these interventions were delivered might explain the identified heterogeneity in results for some outcomes.
In general, only a small proportion of RCTs focused on children and adolescents, even though it is known that psychological suffering and exposure to traumatic events during childhood and adolescence can negatively impact future achievements (also at academic and work levels) and raise serious risks for health, such as substance abuse, psychological symptoms and suicidal ideation (Betancourt 2020; Fergusson 2005). A Lancet Commission on Adolescent Health and Well‐being suggested investing in child mental health in light of triple dividends of benefits: now, into future adult life, and for the next generation of children (Patton 2016).
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review assessing the effects of prevention interventions delivered through a task‐shifting approach for reducing the frequency of mental disorders and improving psychological outcomes.
Our findings indicated that there is a scarcity of evidence on child and adolescent populations compared to adults, and are consistent with those of a Cochrane Review on prevention interventions in LMICs affected by humanitarian crises that collected seven trials with 2398 participants (Papola 2020). The review of Papola and colleagues is the only review we are aware of with a primary outcome on the incidence of mental disorders. This work did not identify any RCT providing data on this outcome at any time point. In relation to the improvement of psychological symptoms in adults, our findings align with those of a Cochrane Review of psychological therapies in humanitarian settings in LMICs (Purgato 2018a), and with a systematic review carried out by Tol and colleagues in 2011 (Tol 2011), which identified substantial beneficial effects of psychological interventions versus control conditions for adults with symptoms of PTSD. Our results of a positive effect of preventive psychological interventions in reducing depressive symptoms in adults are in line with the results of a systematic review and Individual Participant Data (IPD) meta‐analysis collecting 13 RCTs with IPD from 11 RCTs with 4145 participants. Included interventions were CBT, behavioural activation, problem‐solving therapy, and supportive therapy, and all of them were delivered using a task‐shifting approach (Karyotaki 2022). Authors found that task‐shared psychological interventions were associated with a larger reduction in depressive symptom severity and a greater chance of response and remission than control measures, confirming the results of other reviews conducted in LMIC settings (Cuijpers 2018; Singla 2017).
For children and adolescents, the review by Tol and colleagues detected a non‐substantial trend in favour of interventions versus control conditions for PTSD symptoms, along with a substantial effect for internalizing symptoms. A systematic review with IPD meta‐analysis collected data on 3143 children from 11 trials in humanitarian settings in LMICs. This review collected evidence‐based psychotherapeutic interventions within the focused psychosocial support layer of the IASC pyramid (IASC 2007). These interventions involved techniques inspired by psychotherapeutic approaches such as cognitive‐behavioural therapy, but not following complete standard treatment protocols, and the inclusion of additional techniques aimed at establishing strengths, such as creative expressive techniques (e.g. drama, dance, music, art, and games), social support‐building activities (e.g. cooperative games, trust‐focused activities, sharing difficulties, and coping methods), or mind–body‐oriented skills (e.g. meditation and breathing exercises). Authors identified a beneficial effect of interventions in reducing PTSD symptoms and functional impairment, and in increasing hope, coping, and social support (Purgato 2018b).
Our results on secondary outcomes align also with those of a systematic review on the effectiveness of mental health promotion interventions for young people in LMICs, which collected 22 studies (RCTs and quasi‐randomized studies) on 20 school‐based and community interventions (Barry 2013). The review found significant positive effects on students’ emotional and behavioural well‐being, including reduced depression and anxiety and improved coping skills (Barry 2013). Taking into account primary‐level worker interventions for people with mental disorders and distress in LMICs, Van Ginneken 2021 found a difference in the effectiveness of interventions between adult and child populations. As a matter of fact, for adult populations (adults with depression and anxiety, women with depression related to pregnancy and childbirth, and adults in humanitarian settings with post‐traumatic stress or depression and anxiety), treatments from lay health workers may reduce symptoms of depression, whereas, for children in humanitarian settings with post‐traumatic stress or depression and anxiety, LHW‐led interventions may have little to no effect on post‐traumatic stress and depressive symptoms.
Authors' conclusions
Implications for practice.
Very low‐ to high‐certainty evidence suggests the effectiveness of prevention psychological interventions delivered through a task‐shifting approach across a range of psychological outcomes for people living in LMICs. Prevention interventions were effective in reducing psychological symptoms and improving the quality of life in adults, with a large effect of the interventions. The concept of task‐shifting is highly relevant for improving access to prevention interventions in underserved areas, and several factors could be considered in implementing this approach. Challenges in implementation might be related to psychological distress amongst the workforce delivering the interventions, and acceptance of the added workforce by other healthcare staff (Padmanathan 2013). For this reason, the use of task‐shifting should be complemented by a systematic assessment of the available workforce in diverse settings and its interest and capacity to learn and/or assume relevant tasks. In the present review, we identified a variety of effective task‐sharing strategies employing different workers like teachers, nurses, community workers, and peers. This allows for potentially broad and efficient use of health human resources, especially as health systems worldwide struggle to maintain essential services and particularly while responding to the COVID‐19 pandemic.
Evidence‐based guidelines may facilitate the implementation of promotion and prevention programmes delivered in LMICs through a task‐shifting approach. Guidelines may be developed also considering the needs of specific categories of populations, such as carers of people with Alzheimer's, pregnant women, diabetes‐2 patients, and school students.
Implications for research.
Results from this systematic review show that prevention interventions are effective in decreasing the diagnoses of mental disorders and improving psychological symptoms, functioning, quality of life, and social outcomes in adult and child populations. We found a beneficial effect with a large effect size for some clinically relevant outcomes, such as the decrease in psychological symptoms and quality of life improvement. For children, there was a paucity of evidence for many outcomes. This review revealed a gap in the knowledge base in the longer term and in the child and adolescent population. Future trials should be randomized, should be specifically designed according to a preventative aim (i.e. incidence), using socioculturally appropriate and validated instruments to measure outcomes. Moreover, the following issues should be carefully considered when planning the prevention research agenda in global mental health.
To develop a core outcome set for RCTs investigating prevention and promotion interventions implemented through a task‐shifting approach in LMICs, in order to facilitate the collection of the same important outcomes across studies. The choice of outcome measures should be pragmatic, and meaningful for LMIC settings, with implementation and process outcomes specific to the task‐shifting approach. Moreover, the consensus process should involve LMIC stakeholders to guarantee that the priorities for interventions amongst patients, families, healthcare professionals, and paraprofessionals are appropriately considered (Boehnke 2022).
To identify specific outcome measures that are related to the prevention of mental disorders and promotion of well‐being, overcoming a medicalizing approach to mental health, in which 'mental health' is seen as synonymous with 'mental disorder' (Aho 2008).
To identify critical areas where there is a gap in research, for example, interventions for children and adolescents. This would imply the implementation of interventions across different age ranges using RCT designs.
To perform individual participant data component network meta‐analyses, in order 1) to compare active intervention strategies to each other; 2) to disentangle the efficacy of various intervention components (including delivery format, contents, and techniques), 3) and to understand how interventions work based on their specific ingredients. This methodology has already been used in mental health research (Furukawa 2021; Miklowitz 2021; Pompoli 2018), and its application in the field of prevention interventions in global mental health would contribute to closing a significant research gap on the mechanism of action of psychosocial interventions.
To perform the cost‐effectiveness analyses of interventions while conducting RCTs.
History
Protocol first published: Issue 3, 2021
Notes
This review is based on standard text and guidance provided by Cochrane Effective Practice and Organisation of Care (EPOC).
Risk of bias
Risk of bias for analysis 1.9 Distress/PTSD symptoms at 0‐1 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Baker‐Henningham 2019 | Low risk of bias | 1a.1: PY; 1a.2: Y. Quote: "Schools were randomised to intervention or control group in the summer preceding the intervention
selected child.
year. Randomisation was conducted by an independent statistician who was blind to the identity of
Schools were randomised to intervention or control group in the summer preceding the
the schools." 1a.3: NI. Note: No useful information is reported to evaluate this element. 1b.1: PY; Note: schools and teachers were identified and rectruited before the randomization of clusters, children from the teacher's classes were randomly selected at a later stage (outcome of interest refers to teachers); 1b.2 NA; 1b.3 NA; |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: all 14 clusters, schools, that recruited participants were analyzed; 3.1b: Y. Note:there was 1 loss among teachers (out of 54), no reported loss among children . 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: no evidence to suggest differences in measurement between intervention and control groups. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. Research assistants that were involved in data collection were blind to allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note:No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Bell 2008 | Low risk of bias | 1a.1: Y;1.2: PY. Title mentions RCT design.
Quote: "A school was then assigned an experimental school status on the basis of a random pick of all participating schools. A second pick was done of the remaining schools in the area for a control school." 1.3: NI. Note: No information provided about baseline characterists. 1b.1: Y. Quote: "CHAMPSA staff would explain the CHAMPSA program and chools were then requested to indicate if they wished to participate." Then, the schools were randomized for intervention or control group.Overall comment: Low risk. |
Some concerns | 2.1a: PY; 2.1b: PY. Due to the nature of the intervention, participants were most likely aware that they were in a trial and were aware of their assigned intevrention.Quote: "Active caregiver consent and student assent were obtained before study participation". 2.2: Y. School teachers, health educators and community caregivers were trained as facilitators for the program. 2.3: NI. Note: No information provided. 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analyses used to estimate the effect pf assignment to intervention. 2.7: PN. Note: No evidence to suggest that this had a substantial impact on the results. |
Low risk of bias | 3.1a: NI. No information provided.
3.1b: Y. Quote: "Ninety‐four percent of families who begna the CHAMPSA HIV prevention intervention completed the netire program. Twenty children and 14 adults did not finish." 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note:The selected outcome method (Social Capital) is validated and established. 4.2: PN. Quote: "Measures were obtained before and once after participation in the intervention". No evidence to suggest differences in measurement between intervention and control. 4.3a: Y. The outcome assessors (caregiver) were aware that the trial was taking place. 4.3b: Y. The outcome assessors (caregiver) were aware of the intervention received by study participation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Some concerns | 5.1: PN. Note: The data were collected n additional sessions before and after the program session due the loe‐literacy levels of by reading aloud the items by adult participants. 5.2: PN. Note: no protocol available.No evidence to suggest outcome selection. All outcome mentioned in the methods and results were reported. 5.3: PN. No protocol available. No evdience to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in three domains (2, 4, 5), but not to be at high risk of bias for any domain. |
| Duan 2019 | Some concerns | 1.1: PY; 1.2: NI. Quote: "Randomization
was conducted through computer random number
generators." 1.3: PN. Quote: "no significant difference in the preassessment variables between the two groups" |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of allocation 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. Data was analyzed for participants whose follow‐up data was available (73%) 2.7: PN. No evidence to suggest analysis in the wrong group or exclusion of participants that could have had a substantial impact on the results |
Some concerns | 3.1: PN. Note: Out of the 52 randomized participants, 14 (27%) were lost at follow‐up (1 week after intervention). At post‐intervention, the rate was of 21%. 3.2: PN. Note: No information to suggest that results were not biased by missing outcome data. 3.3: PY. Note: Missing data occurred for reasons that could be related to at least one of the assessed outcomes.The dropouts were balanced across comparison groups. Quote: "the dropout rate was 27% because the data were collected by means of online questionnaire and a few students forgot to complete the measures in time". 3.4: PN. |
High risk of bias | 4.1: N. Note: The selected outcome method (BIT) is established, validated for the local context, and assessed in its psychometric properties for this sample. 4.2: PY. Note: It appears that different data collection methods were used at post‐intervention for the control and intervention group. Quote: "At the end of the lesson, the intervention group was asked to fill out the postintervention test immediately, and the data of control group were obtained through online questionnaires within the same day." 4.3: NA. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: NA. 4.5: NA. |
Some concerns | 5.1: NI. Note: Information provided is insufficient to make a judgment on this item as a specific method section for statistical procedures is missing. 5.2: NI. Note: Information provided is insufficient to make a judgment on this item as a specific method section for statistical procedures is missing. 5.3: NI. Note: Information provided is insufficient to make a judgment on this item as a specific method section for statistical procedures is missing. |
High risk of bias | The study is judged to be at high risk of bias in one domain (4). |
| Hendriks 2019 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Quote: "Participants were randomly assigned to either the intervention
group (n = 87) or the wait‐list control group
(n = 86), using the online program research randomizer
(https://www.randomizer.org)."; "At the end of the session they received a sealed opaque
envelope containing their given group number, which
corresponded to one of the conditions. This procedure
was performed by a different independent party to maximize
allocation concealment." 1.3: PN. Quote: "No significant differences of socio‐demographics between groups were found, except for gender and ethnicity, with an overrepresentation of male participants from Javanese descent in the intervention group." |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Of the 173 randomized participants, 15 did not attend the baseline assessment due to work‐related scheduling conflicts. These absences were unrelated to the randomization itself and were the result of workflow processes, not individual volition. Due to these initial drop‐outs, a modified intention‐to‐treat (mITT) analysis was used" 2.7: NA. |
Low risk of bias | 3.1: PY. Quote: "In total, 158 participants completed the baseline assessment
and started the intervention, 144 (91%) participants
completed the post‐test at seven weeks, and 120
(76%) the three month follow‐up assessment." 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Quote: "Cronbach’s alpha in the present study was
.89 at pretest, .92 at post‐test, and.94 at follow‐up." 4.2: PN. Note: no evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. The investigators in the trial were blinded. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PN. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: no evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 2.1 Diagnosis of mental disorders at 7‐24 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Tripathy 2010 | Low risk of bias | 1a.1: PY; 1a.2: Y. Note: Randomization was obtained through the drawing of folded papers in the presence of extrnal observers in order to convince local communities to participate. 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: N: particpants were recruited after randomization; 1b.2: PN. Note: there is no evidence to suggest that selection of individual participants was affected by knoweldge of the intervention assigned to the cluster; 1b.3:PN. Note: groups were comparable at baseline, the observed differences are compatible with chance in a cluster design. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY. Note: data was available for all clusters; 3.1b: Y. Note: data was available for nearly all participants within clusters, Quote: "All 18 selected clusters
had the intervention. Loss to follow‐up after birth as a result of migration or refusal of interview was 86 (<1%)
of 9770 women in intervention clusters and 173 (2%) of 9260 in control clusters." 3.2: NA. 3.3: NA. |
Some concerns | 4.1: PN. Note: The outcome measure is widely established and validated for use across different contexts, included the one of the trial. 4.2: PN. No evidence to suggest that. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants were most likely aware of the group allocation due to the nature of the intervention 4.4: PY, 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, protocol and final article not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. Further outcomes than those mentioned in the protocol were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 3.11 Depressive symptoms at 0‐1 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Acarturk 2022 | Low risk of bias | 1.1: Y. Quote: "Participants were randomly assigned either to the Self‐Help Plus arm or to ECAU only, in a 1:1 ratio." 1.2: Y. Quote "The randomization schedule was generated by Castor Electronic Data Capture (EDC). (..) Research team members involved in
recruitment were able to access the web‐based software to randomize each newly enrolled participant, but were not able to access the randomization list, and were not aware of the block size." 1.3: N. Note: No significant differences at baseline. |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "We followed an intent‐to‐treat approach for analysis of primary and secondary outcomes" 2.7: NA. |
Low risk of bias | 3.1: PY. Quote: " The distribution of participants lost to follow‐up was similar between the study groups (15.53% vs. 14.06%)" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Asnani 2021 | Low risk of bias | 1.1: Y; 1.2: Y. Note: Quote: "A random numbers
table, computer generated by an independent staff statistician, was kept centrally. Once baseline
measurements were completed, the study coordinator telephoned the central site for the
next assignment based on the random number table and informed the subject of her allocation
group. Th" 1.3: PN. Note: Baseline characteristics were overall comparable across groups, significant differences were reported for 2 variables (compatible with chances) |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention. Some changes were made to the protocol before recruitment. These are reported in the manuscript. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were performed based on intention to treat principles" 2.7: NA. |
Low risk of bias | 3.1: Y. Note: Data was availalble for all 64 randomized participants, there were no losses at the 6 months follow‐up. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The selected outcome method (CES‐D) is widely validated and established and demonstrated good inter‐item reliability in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Staff involved in data collection was blinded to the allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. Authors mention that the CES‐D was not initially planned to be an outcome of interest in the study, this was added in a second moment, but still before the beginning of recruitment. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Baumgartner 2021 | Low risk of bias | 1a.1: PY; 1a.2: PY. Researchers performed stratified constrained randomization. This was carried out through software. Quote: "Constrained randomization was implemented using the cvcrand Stata package by the US‐based team". 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. It appears the randomization was conducted prior to the recruitment of participants: 1b.2: PN. It is unlikely that the selection of individual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. There were some baseline imbalances that were compatible with chance, there is no evidence to indicate differential identification or recruitment of individual participants. | Some concerns | 2.1 a: NI; 2.1b: PY; 2.2: PY. No information was provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. The low literacy of the lay providers could have impacted implementation fidelity. Quote: "This strategy supported the potential sustainability of the intervention but came with literacy challenges for delivering the iMBC content with full fidelity". 2.4: PN. No evidence to suggest deviations from the intended intervention would have influenced the outcomes. 2.5: NA. 2.6: PY. Data were analysed for most randomized participants. 2.7: NA. | Low risk of bias | 3.1a: PY. All included clusters were analyzed. 3.1b: PY. Data were available for nearly all participants within the cluster. Quote: "303 (81%), 313 (83.7%), and 266 (71.1%) participants were followed up at the mini‐survey, imme‐ diate post‐intervention survey (follow‐up 1), and the 8‐month post‐ intervention survey (follow‐up 2) time points, respectively ". 3.2: NA. 3.3: NA. 3.4: NA. | Some concerns | 4.1: N. The outcome measure is well established and reported good psychometric properties in this sample. 4.2: PN. No evidence to suggest differences in measurement between intervention and control. 4.3A: NI; 4.3b: PY. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. | Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. | Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Chaharrahifard 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design; Quote: "the samples were randomly assigned to two groups of received counseling (intervention) and non‐received counseling (control) by four‐sized randomized blocks." 1.2: Y. Quote: "The details of blocks were contained in asset of sealed. 1.3: N. Quote: "There was not significant difference between the demographic characteristics in the control and intervention group." |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.5: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.6: N. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized. |
Low risk of bias | 3.1: PY. Note: Drop‐out rate: 21%. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Chang 2015 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Title mentions RCT design. Quote: "The evaluation was a cluster randomized trial conducted in Jamaica, Antigua, and St Lucia with public health center as the unit of randomization." 1.3: N. Note: Quote: "There were no significant differences between groups in enrollment characteristics" |
High risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on wheter participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. Note: Quote: "Clinics were often noisy and crowded, and some mothers would have had difficulty hearing and seeing the films. It also made it difficult for the health workers to interact with the mothers during the demonstration." 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were by intention to treat" 2.7: NA. |
Low risk of bias | 3.1a: NI. No information on wheter data was available for all clusters.
3.1b: PY. 3.2: PY. Quote: "Loss did not differ by group (16% of children were lost to follow‐up in the control and 14% were lost to follow‐up in the intevrention)" 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome measure (center for Epidemiologic Studies‐depression Scale) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurements between intevrention and control. 4.3a: PY; 4.3b: PY. Note: Interviewers and testers were blind to center assignment but participants (mothers) probably knew that they were in a trial and participants were most likely aware of the group allocation due to the nature of the intervention. Self‐assessment scale. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcome, but there is no reason to believe that it did |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Chew 2018 | Low risk of bias | 1a.1: Y; 1a.2: PY. Note: Title mentions RCT design.
Quote: "In assigning the HCs to the VEMOFIT group (VG) or attention‐control groups (AG), randomisation will be carried out after stratification by cluster size and geographical areas of the 10 HCs." Quote: "A member of the Data Management Services team at the University Medical center, Ultrecht will carry out the randomisation." 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: PY. Seems that participants were recruited after randomization. |
Low risk of bias | 2.1a: PY. 2.1b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes".
2.2.: Y. Nurses were trained to deliver the intervention. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: The analysis was carried out on an intention to treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY. All 10 clusters that recruited participants were analyzed.
3.1b: PN. 92 from intervention group (n = 145) and 79 from control group (n = 150) did not receive allocated intervention. In addition to the 33 people lost to follow up at 12 months, 5 additional missing data were noted with regard to PHQ‐9. 3.2: PN. No evidence that the result was not biased by missing data. 3.3: PN; 3.4: NA. Note: Missingness in the outcome do not depend on its true value. |
Low risk of bias | 4.1: N. Note: The selected outcome method (Malay Brief Illness Perception Quetsionnaire, MBIPQ) is validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control 4.3a: PY.Outcome assessors (participants) were most likeley aware that a trial was taking a place. 4.3b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes" 4.4: NA. 4.5: NA. |
Low risk of bias | 5.1: PY. Note: Protocol is available. 5.2: PN. Note: No evidence to suggest outcome selection. Almost all outcomes mentioned in the methods and protocol were reported (It is not reported "heath‐care utilization hospital" that is a secondary outcome). 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methdis and protocol were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Escolar 2014 | Some concerns | 1.1: Y; 1.2: NI. Quote: "The respondents were then randomly assigned to either the experimental or control group" No information provided about allocation concealment. 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: NI. Note: No information provided. 2.4: NA. 2.5: NA. 2.6: NI. Note: No infomation provided. 2.7: N. Note: No drop‐out. |
Low risk of bias | 3.1: Y. Note: No missing data. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: Y. Note: Only self‐reported measure. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No protocol available. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in three domains (1, 2, 4), but not to be at high risk of bias for any domain. |
| Ferreira‐Vorkapic 2018 | Low risk of bias | 1.1: Y; 1.2: Y. Quote: "(...) volunteers were randomly allocated to one of the three experimental groups. A separate examiner coordinated the group distribution (and all statistical analysis was performed by an expert from another
state), using the random allocation method." 1.3: N. Quote: "the baseline data showing no significant differences between the groups, except for education years, where the relaxation group showed a higher level of education" |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Drop‐out: 0 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (BAI) is widely validated and established across multiple languages.ility in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Gao 2015 | Low risk of bias | 1.1: Y. Quote: "The randomization sequence was generated using a computerized random number generator and the allocation was kept in sealed opaque consecutively numbered envelopes." 1.2.: Y. Quote: "This simple randomization scheme was independently prepared by a research assistant who was not involved in determining eligibility, providing care, or assessing outcome." 1.3: N. Quote: "There were no significant differences between the two groups in their demographic, obstetric and related characteristics (...) There were also no significant differences between the two groups in their baseline measures" |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: BA 2.6: Y. Note: Intention to treat analysis done. 2.7: NA |
Low risk of bias | 3.1: PY. Note: Drop‐out: 14 of 180. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated in Chinese mothers and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4.: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Hinton 2021 | Low risk of bias | 1a.1: PY. 1a.2: Y. Quote: "Randomizationwas at the level of clusters, defined as geographic areas (ie, communes) served by commune health stations which have a population
range of ≈5000‐15,000 people. Randomization was conducted
by one of the study investigators residing in the United States through
the flip of a coin." 1a.3: NI. No useful information is reported to evaluate this element 1b.1: N. Note: particpants were recruited after randomization; 1b.2: PN. Note: there is no evidence to suggest that selection of individual participants was affected by knoweldge of the intervention assigned to the cluster; 1b.3:N. Note: no significant differences at baseline were identified. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: Y. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: no evidence to suggest failure in analyzing any clyster; 3.1b: PY. Note: data was avaiable for 85% of participants at follow‐up. 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: no evidence to suggest that. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: Y. Note: A pre‐determined statistical plan is avaiable and in line with procedures reported in the final manuscript 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Lachman 2017 | Low risk of bias | 1.1: Y; 1.2:Y. Quote: "An external researcher not directly involved in the study conducted the randomization procedures remotely in Oxford, United
Kingdom. Participants were randomly assigned on a 1:1 ratio to an intervention or wait‐list control group after baseline data collection
using a concealed computerized program, SealedEnvelope™."; "Our implementing partner, Clowns Without Borders South Africa, notified participants of their
allocation status via telephone." 1.3:PN. |
Low risk of bias | 2.1:Y; 2.2:Y. Quote: "Although program implementers and participants were aware of their allocation status, researchers
conducting self‐report interviews and observational assessments were blind to allocation." 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4:NA. 2.5:NA. 2.6:Y. Quote: "Data analyses were conducted with an intention‐to‐treat design" 2.7:NA. |
Low risk of bias | 3.1:Y. Note: 3 out of the 68 participants randomized were lost at follow‐up (2.9%). 3.2:NA. 3.3:NA. 3.4:NA. |
Some concerns | 4.1:PN. Note: The selected outcome method is widely validated and established, appropriate for the local context, and assessed in its psychometric properties for this sample. 4.2:PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3:Y. Note: Outcome assessors (participants) were most likely aware of intervention allocation. The research assistants administering the questionnaires were blinded. 4.4: PY; 4.5:PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did |
Some concerns | 5.1:PY. Note: No evidence to suggest otherwise, the protocol and manuscript do not show discrepancies. 5.2: PY. Note: A few of the secondary outcomes mentioned in the protocol (e.g. ASSIST, Grover‐Counter Scale of Cognitive Development) were not reported in this manuscript. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol and methods were reported in the results. |
Some concerns | The study is judged to raise some concerns in two domains (4 and 5), but not to be at high risk of bias for any domain. |
| Lachman 2020 | Low risk of bias | 1a.1: Y; 1a.2: Y. Quote: "Cluster randomisation was conducted at village level prior to baseline assessments, to reduce the likelihood of contamination between arms. An external researcher used concealed computer‐generated codes to randomly allocate eight villages into three treatment arms and a control arm "; "The implementing partner notified the participating families of their allocation status after baseline data collection." 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. Note: randomization was conducted prior to the recruitment of participants 1b.2: PN. Note: groups were comparable at baseline, see Table 1. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were aware of intervention allocation. 2.3: PN. Note: No evidence to indicate deviations from protocol. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a.: Y. Note: all included clusters were analyzed; 3.1b: PY. Note: data were available for nearly all participants within the cluster. Quote:"Study retention was considerably higher than anticipated with 94.8% adults (n=235/248), 87.5% children (n=154/176) and 87.8% in their early childhood (n=122/139) assessments completed at post‐treatment, with no differences between arms " 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The outcome measure is widely established and validated for use across different contexts. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Luoto 2020 | Low risk of bias | 1a.1: PY; 1a.2: PY. Quote: "Using a random
number generator in Stata, two authors (JEL and ILG) randomly assigned villages in a 1:1:1 ratio to one of three study groups, stratified by sub‐county" 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: Y. Note: from protocol it is clear that recruitment of both adults and children as well as baseline assessments, took place before randomization to study arms |
Low risk of bias | 2.1 a: NI; 2.1b:PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. Quote: "Due to the nature of the intervention, masking of
participants and community health volunteers who
delivered the intervention was not possible" 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: all 60 clusters that recruited participants were analyzed; 3.1b: PY. Note: 92 participants out of 1152 were lost at followup (8%). 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. Efforts were made to keep the data collection teams as masked to group status as possible. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note:No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Rodriguez 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design. Quote: "Students who completed the web‐based baseline assessment measures were randomly assigned to a brief, 4‐week internet‐based mindfulness intervention (MIND), or to the intervention plus peer counselor support (MIND+)." 1.2: Y. Quote: "The randomization sequence was sourced through random.org [49], an automated, web‐based randomization service that generates randomness using atmospheric noise. Using random.org, two 25‐person blocks were used to randomize the participants into 2 equally‐sized groups." 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.7: N. Note: There was not a substantial impact of the failure to analyze participants in the group to which they were randomized. |
High risk of bias | 3.1: PN. Note: Drop‐out: 31 of 54. 3.2: PN. Note: Proportions of lost to follow‐up between control and intervention are not comparable (n= 20 and n= 11). 3.3: NI; 3.4: NI. Note: No information about documented reasons that withdrawal are related to the outcome. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. 5.3: PN. Note: No evidence to suggest analyses selection. |
High risk of bias | The study is judged to be at high risk of bias in one domain (3). |
| Sangraula 2020 | Low risk of bias | 1a.1: PY. 1a.2: PY. Quote: "A randomization procedure was used, in which names of the two VDCs were written on cards and placed into a hat. The District Public Health Officer (DPHO) was to draw one card from the hat which would be allocated as the intervention arm VDC and the VDC in the remaining card would be allocated to the control arm. Program staff, including community psychosocial workers (CPSWs) and research assistants (RAs) were only assigned to either VDC after the random drawing to reduce risk of unblinding. 1a.3: PN. There is no evidencde to suggest problems in the randomization process 1b.1: PN. it appears the randomization was conducted prior to the recruitment of participants. 1b.2: PN. It is unlikely that the selection of inidividual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. there is no evidence to indicate imbalances in baseline values to indicate differentail identification or recruitment of individual participants. |
Low risk of bias | 2.1 a:NI. 2.1b: PN. 2.2: PN. Quote: "CPSWs, RAs, trial participants, and local mhGAP trained health workers were blinded to the allocation of the study conditions. The VDCs of the two arms were separated by another VDC which worked as a buffer and physical barrier against contamination between the two arms. CPSWs in both arms were instructed not to disclose the treatment that any participants received except with their clinical supervisors. The trial statistician was blinded to treatment arm during analysis. " 2.3: NA. 2.4: NA. 2.5: NA. 2.6: PY. Data was analysed for most participants randomized. |
Low risk of bias | 3.1a: Y. all included clusters were analyzed; 3.1b: PY. data were available for nearly all participants within the cluster 3.2: NA. 3.3: NA. 3.4: NA. |
Low risk of bias | 4.1: PN. The outcome measure (PHQ‐9) is widely established and used across contexts, included the one of the trial 4.2: PN. No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a:NI. 4.3b: PN. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were blinded |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Song 2019 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Block randomization, computer‐generated random list. Using opaque and sealed envelopes for group allocation code keeping. This was done by a person not involved in data collection. 1.3: N. Quote: "No considerable differences were observed between the intervention and control groups in terms of demographic characteristics, baseline outcome and mediating variables." |
Low risk of bias | 2.1: Y; 2.2: PY. Note: Due to the nature of the intervention participants were most likely aware of their allocation. 2.3: PN. Note: No evidence to suggest issues in recruitment and engagement nor failures to implement intervention 2.4: NA. 2.5: NA. 2.6: Y. Note: An ITT analysis was conducted. 2.7: NA. |
Low risk of bias | 3.1: PY. Note: Data at end line was missing, due to withdrawal or loss to follow‐up, for 29 participants (circa 24%). 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Quote: "GDS is easy to administer and is a valid tool for assessing depressive symptoms in individuals with mild cognitive impairment"; The Cronbach’s alpha in this study was 0.784. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Researchers involved in data collection were blinded to group allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Ward 2020 | Low risk of bias | 1.1: PY; 1.2: PY. Quote: "Randomization was conducted after data collection by an off‐site statistician with no other contact with the trial"." 1.3: PN. Baseline characteristics were overall comparable across groups as demonstrated by Table 1. |
High risk of bias | 2.1: PY; 2.2: PY. Due to the nature of the intervention participants and personnel were moslt likely aware of their assigned intervention. 2.3: PY. There is some evidence to suggest possible contamination between the intervention and control group. Quote: "In addition, in the dense living environments of informal settlements, it is possible that there was contamination between intervention and control groups." 2.4: PY. Contamination could have affected the outcomes. 2.5: PY. Contamination is more liekly to affect the participants in the control group. 2.6: PY. No explicit mention of the analysis used to estimate the effect of assignment to intervention, but data was analyzed for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t". 2.7: NA. |
Low risk of bias | 3.1: PY. Data was availalble for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. The selected outcome method is widely validated and established across contexts and demonstrated good psychometric properties in the study 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did 4.5: PN. |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies.. 5.2: PN. No evidence to suggest outcome selection.. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the methods were reported.. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Yeomans 2010 | Low risk of bias | 1.1: Y; 1.2: NI.Note: Title mentions RCT design. No further information. 1.3: N. Quote: "There were no significant baseline differences between the three treatment groups across age, gender, ethnicity, symptoms, education level, traumatic events experienced, or on prior exposure to trauma discourse" |
Some concerns | 2.1: Y; 2.2: PY. Quote: "Participants (...) were informed of random allocation procedures". Due to the nature of the intervention, the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Lost to follow‐up: 7 of 120 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (HSCL‐25) is widely validated and established across multiple languages. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 4.3 Psychological functioning and impairment at 0‐1 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Amador Buenabad 2020 | Low risk of bias | 1a.1: Y; 1b.2: Y. Note: Title mentions cluster RCT design.
Quote: "The sample was randomized at the school level (...). All the randomization processes used the lottery method. As for the experimental conditions, schools, participants, teaching staf, statisticians, supervisors, and evaluators were blinded throughout the study until the pre‐test
was completed. In order to avoid communication between participants, each school was in
a diferent area in the south of the city. 1.3: PN. Note: Not significant differences at baseline 1b.1: PN. the participants were recruited after school‐randomization. Quote: ". Once schools agreed to engage in the research project, teachers, caregivers and children were invited to information meetings. At these meetings, written informed consent was obtained from all adult participants and parents of all nonadult participants. Written informed assent was obtained from the children (...). Participants were assessed in February and June 2016, before and after the intervention." 1b.2: PN there is no evidence to suggest that selection was affected by knowledge of the intervention assigned to the cluster; 1b.3: PN. Some baseline imbalances are presents but compatible with chance |
Some concerns | 2.1a: PY. Quote: "written informed consent was obtained from all adult participants and parents of all nonadult participants. Written informed assent was obtained from the children"
2.1b: PY. Quote: "As for the experimental conditions, schools, participants, teaching staf, statisticians, supervisors, and evaluators were blinded throughout the study until the pre‐test
was completed" 2.2: PY. People delivering the intervention (teachers) were trained for doing that. They were most likely aware of their assigned intervention. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention taht arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: NI. Note: No information provided. 2.7: PN. Note: There is no reason to believe that it did. |
Low risk of bias | 3.1a: PY. 3.1b: PY. Dropout: 2 for Huellitas intervention, 0 for control group. 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: N. Quote: "The reliability for the present sample was.83" 4.2: PN. Note: no evidence to suggest differences in measurement between intervention and control groups. 4.3a: Y. Outcome assessors (participants) were most likeley aware that a trial was taking a place. 4.3b: PY. Due to the nature of intervention, ouctome assessors (research assistants + participants) are most likely aware of the intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PN. Note: Protocol is available.
No evidence to suggest otherwise, protocol and manuscript do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and protocol were reported 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and protocol were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Berger 2018 | Low risk of bias | 1a.1: Y; 1a.2: PY. Quote: "The principal of this school randomply chose (by tossing a coin) to implement the ESPS (experimental group) in only one of the two classes in each of the 4th‐6th grades, while the other classes participated in Social Studies (SS, control group)." 1.3: N. Quote: "No significant differences were found at baseline (prior to the intervention) between the intervention and control group for any of the outcome measures" 1b.1: Y. Quote: "The ESPS program was presented to all the principals from the primary schools in the Meru district. (...). Since we had limited funds, we randomly chose (by tossic dice) one school for the study." |
Some concerns | 2.1a: PY; 2.1b: PY. Quote: "All the parents or guardians whose children were assigned to the ESPS consented to their children's participation in the study and expressed support for the program"
2.2: Y. The teachers were trained in a workshop by the first author for the experimental exercises" 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: NI. Note: Not enough information provided to give a judgemnet on this item. 2.7: PN. Note: No evidence to suggest that failure to analyzed participants in randomized groups could a substantial impact on the results. |
Low risk of bias | 3.1a:PY. No evidence to suggest cluster loss. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (Spence Anxiety scale for children‐SCAS) is validated and established. Quote: "Cronbach alpha in current sample was 0.74)" 4.2: PN. No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a: NI; 4.3b: PY. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No protocol available. 5.2: PN. Note: No evdience to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: no evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 4.4 Psychological functioning and impairment at 1‐6 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Huang 2017 | Low risk of bias | 1a.1: PY; 1a.2: PY. 1.1 Quote: "the study of effectiveness outcomes applies a cluster randomized wait‐list controlled design in 10 Ugandan schools". 1.2: Randomization was done by a researcher that was unfamiliar with the study schools 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: N: participants were recruited after cluster allocation to intervention or control; 1b.2: NI ‐ No evidence to suggest that selection was influenced by knowledge of the assigned intervention (93% of elegible teachers participated) for teachers, but teachers selected children for inclusion in a non‐random fashion; 1b.3: PN: there were no significant differences for intervention groups. Overall: Some concerns |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY ‐ all clusters were analyzed; 3.1b: PN: data was available for almost all teachers (84/86%), loss at follow‐up was greater for families/children (62‐69%) 3.2: PN. No evidence that the result was not biased by missing data 3.3: PN; 3.4: NA. Quote: "the followed and non‐followed families did not differ by condition or on family demographic characteristics (i.e., food insecurity status, household size), and baseline child effectiveness outcome measures. Therefore, we assumed data were missing completely at random" |
Some concerns | 4.1: PN. Note: The outcome measure is widely established and adapted for use in Nepal. 4.2: PN. Note: No evidence to suggest that. 4.3a: NI; 4.3b: PY. Note: no information provided on whether participants knew that they were in a trial, participants were most likely aware of the group allocation due to the nature of the intervention 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 8.2 Depressive symptoms at 0‐1 months—indicated prevention adults.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chew 2018 | Low risk of bias | 1a.1: Y; 1a.2: PY. Note: Title mentions RCT design.
Quote: "In assigning the HCs to the VEMOFIT group (VG) or attention‐control groups (AG), randomisation will be carried out after stratification by cluster size and geographical areas of the 10 HCs." Quote: "A member of the Data Management Services team at the University Medical center, Ultrecht will carry out the randomisation." 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: PY. Seems that participants were recruited after randomization. |
Low risk of bias | 2.1a: PY. 2.1b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes".
2.2.: Y. Nurses were trained to deliver the intervention. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: The analysis was carried out on an intention to treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY. All 10 clusters that recruited participants were analyzed.
3.1b: PN. 92 from intervention group (n = 145) and 79 from control group (n = 150) did not receive allocated intervention. In addition to the 33 people lost to follow up at 12 months, 5 additional missing data were noted with regard to PHQ‐9. 3.2: PN. No evidence that the result was not biased by missing data. 3.3: PN; 3.4: NA. Note: Missingness in the outcome do not depend on its true value. |
Low risk of bias | 4.1: N. Note: The selected outcome method (Malay Brief Illness Perception Quetsionnaire, MBIPQ) is validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control 4.3a: PY.Outcome assessors (participants) were most likeley aware that a trial was taking a place. 4.3b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes" 4.4: NA. 4.5: NA. |
Low risk of bias | 5.1: PY. Note: Protocol is available. 5.2: PN. Note: No evidence to suggest outcome selection. Almost all outcomes mentioned in the methods and protocol were reported (It is not reported "heath‐care utilization hospital" that is a secondary outcome). 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methdis and protocol were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Sangraula 2020 | Low risk of bias | 1a.1: PY. 1a.2: PY. Quote: "A randomization procedure was used, in which names of the two VDCs were written on cards and placed into a hat. The District Public Health Officer (DPHO) was to draw one card from the hat which would be allocated as the intervention arm VDC and the VDC in the remaining card would be allocated to the control arm. Program staff, including community psychosocial workers (CPSWs) and research assistants (RAs) were only assigned to either VDC after the random drawing to reduce risk of unblinding. 1a.3: PN. There is no evidencde to suggest problems in the randomization process 1b.1: PN. it appears the randomization was conducted prior to the recruitment of participants. 1b.2: PN. It is unlikely that the selection of inidividual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. there is no evidence to indicate imbalances in baseline values to indicate differentail identification or recruitment of individual participants. |
Low risk of bias | 2.1 a:NI. 2.1b: PN. 2.2: PN. Quote: "CPSWs, RAs, trial participants, and local mhGAP trained health workers were blinded to the allocation of the study conditions. The VDCs of the two arms were separated by another VDC which worked as a buffer and physical barrier against contamination between the two arms. CPSWs in both arms were instructed not to disclose the treatment that any participants received except with their clinical supervisors. The trial statistician was blinded to treatment arm during analysis. " 2.3: NA. 2.4: NA. 2.5: NA. 2.6: PY. Data was analysed for most participants randomized. |
Low risk of bias | 3.1a: Y. all included clusters were analyzed; 3.1b: PY. data were available for nearly all participants within the cluster 3.2: NA. 3.3: NA. 3.4: NA. |
Low risk of bias | 4.1: PN. The outcome measure (PHQ‐9) is widely established and used across contexts, included the one of the trial 4.2: PN. No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a:NI. 4.3b: PN. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were blinded |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
Risk of bias for analysis 9.1 Depressive symptoms at 0‐1 months—indicated prevention adults.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 9.1.1 Community workers | ||||||||||||
| Acarturk 2022 | Low risk of bias | 1.1: Y. Quote: "Participants were randomly assigned either to the Self‐Help Plus arm or to ECAU only, in a 1:1 ratio." 1.2: Y. Quote "The randomization schedule was generated by Castor Electronic Data Capture (EDC). (..) Research team members involved in
recruitment were able to access the web‐based software to randomize each newly enrolled participant, but were not able to access the randomization list, and were not aware of the block size." 1.3: N. Note: No significant differences at baseline. |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "We followed an intent‐to‐treat approach for analysis of primary and secondary outcomes" 2.7: NA. |
Low risk of bias | 3.1: PY. Quote: " The distribution of participants lost to follow‐up was similar between the study groups (15.53% vs. 14.06%)" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Baumgartner 2021 | Low risk of bias | 1a.1: PY; 1a.2: PY. Researchers performed stratified constrained randomization. This was carried out through software. Quote: "Constrained randomization was implemented using the cvcrand Stata package by the US‐based team". 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. It appears the randomization was conducted prior to the recruitment of participants: 1b.2: PN. It is unlikely that the selection of individual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. There were some baseline imbalances that were compatible with chance, there is no evidence to indicate differential identification or recruitment of individual participants. | Some concerns | 2.1 a: NI; 2.1b: PY; 2.2: PY. No information was provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. The low literacy of the lay providers could have impacted implementation fidelity. Quote: "This strategy supported the potential sustainability of the intervention but came with literacy challenges for delivering the iMBC content with full fidelity". 2.4: PN. No evidence to suggest deviations from the intended intervention would have influenced the outcomes. 2.5: NA. 2.6: PY. Data were analysed for most randomized participants. 2.7: NA. | Low risk of bias | 3.1a: PY. All included clusters were analyzed. 3.1b: PY. Data were available for nearly all participants within the cluster. Quote: "303 (81%), 313 (83.7%), and 266 (71.1%) participants were followed up at the mini‐survey, imme‐ diate post‐intervention survey (follow‐up 1), and the 8‐month post‐ intervention survey (follow‐up 2) time points, respectively ". 3.2: NA. 3.3: NA. 3.4: NA. | Some concerns | 4.1: N. The outcome measure is well established and reported good psychometric properties in this sample. 4.2: PN. No evidence to suggest differences in measurement between intervention and control. 4.3A: NI; 4.3b: PY. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. | Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. | Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Chang 2015 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Title mentions RCT design. Quote: "The evaluation was a cluster randomized trial conducted in Jamaica, Antigua, and St Lucia with public health center as the unit of randomization." 1.3: N. Note: Quote: "There were no significant differences between groups in enrollment characteristics" |
High risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on wheter participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. Note: Quote: "Clinics were often noisy and crowded, and some mothers would have had difficulty hearing and seeing the films. It also made it difficult for the health workers to interact with the mothers during the demonstration." 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were by intention to treat" 2.7: NA. |
Low risk of bias | 3.1a: NI. No information on wheter data was available for all clusters.
3.1b: PY. 3.2: PY. Quote: "Loss did not differ by group (16% of children were lost to follow‐up in the control and 14% were lost to follow‐up in the intevrention)" 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome measure (center for Epidemiologic Studies‐depression Scale) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurements between intevrention and control. 4.3a: PY; 4.3b: PY. Note: Interviewers and testers were blind to center assignment but participants (mothers) probably knew that they were in a trial and participants were most likely aware of the group allocation due to the nature of the intervention. Self‐assessment scale. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcome, but there is no reason to believe that it did |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Ferreira‐Vorkapic 2018 | Low risk of bias | 1.1: Y; 1.2: Y. Quote: "(...) volunteers were randomly allocated to one of the three experimental groups. A separate examiner coordinated the group distribution (and all statistical analysis was performed by an expert from another
state), using the random allocation method." 1.3: N. Quote: "the baseline data showing no significant differences between the groups, except for education years, where the relaxation group showed a higher level of education" |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Drop‐out: 0 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (BAI) is widely validated and established across multiple languages.ility in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Hinton 2021 | Low risk of bias | 1a.1: PY. 1a.2: Y. Quote: "Randomizationwas at the level of clusters, defined as geographic areas (ie, communes) served by commune health stations which have a population
range of ≈5000‐15,000 people. Randomization was conducted
by one of the study investigators residing in the United States through
the flip of a coin." 1a.3: NI. No useful information is reported to evaluate this element 1b.1: N. Note: particpants were recruited after randomization; 1b.2: PN. Note: there is no evidence to suggest that selection of individual participants was affected by knoweldge of the intervention assigned to the cluster; 1b.3:N. Note: no significant differences at baseline were identified. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: Y. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: no evidence to suggest failure in analyzing any clyster; 3.1b: PY. Note: data was avaiable for 85% of participants at follow‐up. 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: no evidence to suggest that. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: Y. Note: A pre‐determined statistical plan is avaiable and in line with procedures reported in the final manuscript 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Lachman 2017 | Low risk of bias | 1.1: Y; 1.2:Y. Quote: "An external researcher not directly involved in the study conducted the randomization procedures remotely in Oxford, United
Kingdom. Participants were randomly assigned on a 1:1 ratio to an intervention or wait‐list control group after baseline data collection
using a concealed computerized program, SealedEnvelope™."; "Our implementing partner, Clowns Without Borders South Africa, notified participants of their
allocation status via telephone." 1.3:PN. |
Low risk of bias | 2.1:Y; 2.2:Y. Quote: "Although program implementers and participants were aware of their allocation status, researchers
conducting self‐report interviews and observational assessments were blind to allocation." 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4:NA. 2.5:NA. 2.6:Y. Quote: "Data analyses were conducted with an intention‐to‐treat design" 2.7:NA. |
Low risk of bias | 3.1:Y. Note: 3 out of the 68 participants randomized were lost at follow‐up (2.9%). 3.2:NA. 3.3:NA. 3.4:NA. |
Some concerns | 4.1:PN. Note: The selected outcome method is widely validated and established, appropriate for the local context, and assessed in its psychometric properties for this sample. 4.2:PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3:Y. Note: Outcome assessors (participants) were most likely aware of intervention allocation. The research assistants administering the questionnaires were blinded. 4.4: PY; 4.5:PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did |
Some concerns | 5.1:PY. Note: No evidence to suggest otherwise, the protocol and manuscript do not show discrepancies. 5.2: PY. Note: A few of the secondary outcomes mentioned in the protocol (e.g. ASSIST, Grover‐Counter Scale of Cognitive Development) were not reported in this manuscript. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol and methods were reported in the results. |
Some concerns | The study is judged to raise some concerns in two domains (4 and 5), but not to be at high risk of bias for any domain. |
| Lachman 2020 | Low risk of bias | 1a.1: Y; 1a.2: Y. Quote: "Cluster randomisation was conducted at village level prior to baseline assessments, to reduce the likelihood of contamination between arms. An external researcher used concealed computer‐generated codes to randomly allocate eight villages into three treatment arms and a control arm "; "The implementing partner notified the participating families of their allocation status after baseline data collection." 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. Note: randomization was conducted prior to the recruitment of participants 1b.2: PN. Note: groups were comparable at baseline, see Table 1. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were aware of intervention allocation. 2.3: PN. Note: No evidence to indicate deviations from protocol. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a.: Y. Note: all included clusters were analyzed; 3.1b: PY. Note: data were available for nearly all participants within the cluster. Quote:"Study retention was considerably higher than anticipated with 94.8% adults (n=235/248), 87.5% children (n=154/176) and 87.8% in their early childhood (n=122/139) assessments completed at post‐treatment, with no differences between arms " 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The outcome measure is widely established and validated for use across different contexts. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Luoto 2020 | Low risk of bias | 1a.1: PY; 1a.2: PY. Quote: "Using a random
number generator in Stata, two authors (JEL and ILG) randomly assigned villages in a 1:1:1 ratio to one of three study groups, stratified by sub‐county" 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: Y. Note: from protocol it is clear that recruitment of both adults and children as well as baseline assessments, took place before randomization to study arms |
Low risk of bias | 2.1 a: NI; 2.1b:PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. Quote: "Due to the nature of the intervention, masking of
participants and community health volunteers who
delivered the intervention was not possible" 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: all 60 clusters that recruited participants were analyzed; 3.1b: PY. Note: 92 participants out of 1152 were lost at followup (8%). 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. Efforts were made to keep the data collection teams as masked to group status as possible. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note:No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Rodriguez 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design. Quote: "Students who completed the web‐based baseline assessment measures were randomly assigned to a brief, 4‐week internet‐based mindfulness intervention (MIND), or to the intervention plus peer counselor support (MIND+)." 1.2: Y. Quote: "The randomization sequence was sourced through random.org [49], an automated, web‐based randomization service that generates randomness using atmospheric noise. Using random.org, two 25‐person blocks were used to randomize the participants into 2 equally‐sized groups." 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.7: N. Note: There was not a substantial impact of the failure to analyze participants in the group to which they were randomized. |
High risk of bias | 3.1: PN. Note: Drop‐out: 31 of 54. 3.2: PN. Note: Proportions of lost to follow‐up between control and intervention are not comparable (n= 20 and n= 11). 3.3: NI; 3.4: NI. Note: No information about documented reasons that withdrawal are related to the outcome. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. 5.3: PN. Note: No evidence to suggest analyses selection. |
High risk of bias | The study is judged to be at high risk of bias in one domain (3). |
| Sangraula 2020 | Low risk of bias | 1a.1: PY. 1a.2: PY. Quote: "A randomization procedure was used, in which names of the two VDCs were written on cards and placed into a hat. The District Public Health Officer (DPHO) was to draw one card from the hat which would be allocated as the intervention arm VDC and the VDC in the remaining card would be allocated to the control arm. Program staff, including community psychosocial workers (CPSWs) and research assistants (RAs) were only assigned to either VDC after the random drawing to reduce risk of unblinding. 1a.3: PN. There is no evidencde to suggest problems in the randomization process 1b.1: PN. it appears the randomization was conducted prior to the recruitment of participants. 1b.2: PN. It is unlikely that the selection of inidividual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. there is no evidence to indicate imbalances in baseline values to indicate differentail identification or recruitment of individual participants. |
Low risk of bias | 2.1 a:NI. 2.1b: PN. 2.2: PN. Quote: "CPSWs, RAs, trial participants, and local mhGAP trained health workers were blinded to the allocation of the study conditions. The VDCs of the two arms were separated by another VDC which worked as a buffer and physical barrier against contamination between the two arms. CPSWs in both arms were instructed not to disclose the treatment that any participants received except with their clinical supervisors. The trial statistician was blinded to treatment arm during analysis. " 2.3: NA. 2.4: NA. 2.5: NA. 2.6: PY. Data was analysed for most participants randomized. |
Low risk of bias | 3.1a: Y. all included clusters were analyzed; 3.1b: PY. data were available for nearly all participants within the cluster 3.2: NA. 3.3: NA. 3.4: NA. |
Low risk of bias | 4.1: PN. The outcome measure (PHQ‐9) is widely established and used across contexts, included the one of the trial 4.2: PN. No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a:NI. 4.3b: PN. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were blinded |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Ward 2020 | Low risk of bias | 1.1: PY; 1.2: PY. Quote: "Randomization was conducted after data collection by an off‐site statistician with no other contact with the trial"." 1.3: PN. Baseline characteristics were overall comparable across groups as demonstrated by Table 1. |
High risk of bias | 2.1: PY; 2.2: PY. Due to the nature of the intervention participants and personnel were moslt likely aware of their assigned intervention. 2.3: PY. There is some evidence to suggest possible contamination between the intervention and control group. Quote: "In addition, in the dense living environments of informal settlements, it is possible that there was contamination between intervention and control groups." 2.4: PY. Contamination could have affected the outcomes. 2.5: PY. Contamination is more liekly to affect the participants in the control group. 2.6: PY. No explicit mention of the analysis used to estimate the effect of assignment to intervention, but data was analyzed for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t". 2.7: NA. |
Low risk of bias | 3.1: PY. Data was availalble for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. The selected outcome method is widely validated and established across contexts and demonstrated good psychometric properties in the study 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did 4.5: PN. |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies.. 5.2: PN. No evidence to suggest outcome selection.. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the methods were reported.. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Yeomans 2010 | Low risk of bias | 1.1: Y; 1.2: NI.Note: Title mentions RCT design. No further information. 1.3: N. Quote: "There were no significant baseline differences between the three treatment groups across age, gender, ethnicity, symptoms, education level, traumatic events experienced, or on prior exposure to trauma discourse" |
Some concerns | 2.1: Y; 2.2: PY. Quote: "Participants (...) were informed of random allocation procedures". Due to the nature of the intervention, the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Lost to follow‐up: 7 of 120 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (HSCL‐25) is widely validated and established across multiple languages. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Subgroup 9.1.2 Primary healthcare workers | ||||||||||||
| Asnani 2021 | Low risk of bias | 1.1: Y; 1.2: Y. Note: Quote: "A random numbers
table, computer generated by an independent staff statistician, was kept centrally. Once baseline
measurements were completed, the study coordinator telephoned the central site for the
next assignment based on the random number table and informed the subject of her allocation
group. Th" 1.3: PN. Note: Baseline characteristics were overall comparable across groups, significant differences were reported for 2 variables (compatible with chances) |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention. Some changes were made to the protocol before recruitment. These are reported in the manuscript. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were performed based on intention to treat principles" 2.7: NA. |
Low risk of bias | 3.1: Y. Note: Data was availalble for all 64 randomized participants, there were no losses at the 6 months follow‐up. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The selected outcome method (CES‐D) is widely validated and established and demonstrated good inter‐item reliability in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Staff involved in data collection was blinded to the allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. Authors mention that the CES‐D was not initially planned to be an outcome of interest in the study, this was added in a second moment, but still before the beginning of recruitment. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Chaharrahifard 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design; Quote: "the samples were randomly assigned to two groups of received counseling (intervention) and non‐received counseling (control) by four‐sized randomized blocks." 1.2: Y. Quote: "The details of blocks were contained in asset of sealed. 1.3: N. Quote: "There was not significant difference between the demographic characteristics in the control and intervention group." |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.5: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.6: N. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized. |
Low risk of bias | 3.1: PY. Note: Drop‐out rate: 21%. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Chew 2018 | Low risk of bias | 1a.1: Y; 1a.2: PY. Note: Title mentions RCT design.
Quote: "In assigning the HCs to the VEMOFIT group (VG) or attention‐control groups (AG), randomisation will be carried out after stratification by cluster size and geographical areas of the 10 HCs." Quote: "A member of the Data Management Services team at the University Medical center, Ultrecht will carry out the randomisation." 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: PY. Seems that participants were recruited after randomization. |
Low risk of bias | 2.1a: PY. 2.1b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes".
2.2.: Y. Nurses were trained to deliver the intervention. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: The analysis was carried out on an intention to treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY. All 10 clusters that recruited participants were analyzed.
3.1b: PN. 92 from intervention group (n = 145) and 79 from control group (n = 150) did not receive allocated intervention. In addition to the 33 people lost to follow up at 12 months, 5 additional missing data were noted with regard to PHQ‐9. 3.2: PN. No evidence that the result was not biased by missing data. 3.3: PN; 3.4: NA. Note: Missingness in the outcome do not depend on its true value. |
Low risk of bias | 4.1: N. Note: The selected outcome method (Malay Brief Illness Perception Quetsionnaire, MBIPQ) is validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control 4.3a: PY.Outcome assessors (participants) were most likeley aware that a trial was taking a place. 4.3b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes" 4.4: NA. 4.5: NA. |
Low risk of bias | 5.1: PY. Note: Protocol is available. 5.2: PN. Note: No evidence to suggest outcome selection. Almost all outcomes mentioned in the methods and protocol were reported (It is not reported "heath‐care utilization hospital" that is a secondary outcome). 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methdis and protocol were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Escolar 2014 | Some concerns | 1.1: Y; 1.2: NI. Quote: "The respondents were then randomly assigned to either the experimental or control group" No information provided about allocation concealment. 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: NI. Note: No information provided. 2.4: NA. 2.5: NA. 2.6: NI. Note: No infomation provided. 2.7: N. Note: No drop‐out. |
Low risk of bias | 3.1: Y. Note: No missing data. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: Y. Note: Only self‐reported measure. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No protocol available. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in three domains (1, 2, 4), but not to be at high risk of bias for any domain. |
| Gao 2015 | Low risk of bias | 1.1: Y. Quote: "The randomization sequence was generated using a computerized random number generator and the allocation was kept in sealed opaque consecutively numbered envelopes." 1.2.: Y. Quote: "This simple randomization scheme was independently prepared by a research assistant who was not involved in determining eligibility, providing care, or assessing outcome." 1.3: N. Quote: "There were no significant differences between the two groups in their demographic, obstetric and related characteristics (...) There were also no significant differences between the two groups in their baseline measures" |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: BA 2.6: Y. Note: Intention to treat analysis done. 2.7: NA |
Low risk of bias | 3.1: PY. Note: Drop‐out: 14 of 180. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated in Chinese mothers and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4.: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Song 2019 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Block randomization, computer‐generated random list. Using opaque and sealed envelopes for group allocation code keeping. This was done by a person not involved in data collection. 1.3: N. Quote: "No considerable differences were observed between the intervention and control groups in terms of demographic characteristics, baseline outcome and mediating variables." |
Low risk of bias | 2.1: Y; 2.2: PY. Note: Due to the nature of the intervention participants were most likely aware of their allocation. 2.3: PN. Note: No evidence to suggest issues in recruitment and engagement nor failures to implement intervention 2.4: NA. 2.5: NA. 2.6: Y. Note: An ITT analysis was conducted. 2.7: NA. |
Low risk of bias | 3.1: PY. Note: Data at end line was missing, due to withdrawal or loss to follow‐up, for 29 participants (circa 24%). 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Quote: "GDS is easy to administer and is a valid tool for assessing depressive symptoms in individuals with mild cognitive impairment"; The Cronbach’s alpha in this study was 0.784. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Researchers involved in data collection were blinded to group allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
Risk of bias for analysis 10.1 Depressive symptoms at 0‐1 months—indicated prevention adults.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 10.1.1 Community setting | ||||||||||||
| Acarturk 2022 | Low risk of bias | 1.1: Y. Quote: "Participants were randomly assigned either to the Self‐Help Plus arm or to ECAU only, in a 1:1 ratio." 1.2: Y. Quote "The randomization schedule was generated by Castor Electronic Data Capture (EDC). (..) Research team members involved in
recruitment were able to access the web‐based software to randomize each newly enrolled participant, but were not able to access the randomization list, and were not aware of the block size." 1.3: N. Note: No significant differences at baseline. |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "We followed an intent‐to‐treat approach for analysis of primary and secondary outcomes" 2.7: NA. |
Low risk of bias | 3.1: PY. Quote: " The distribution of participants lost to follow‐up was similar between the study groups (15.53% vs. 14.06%)" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Baumgartner 2021 | Low risk of bias | 1a.1: PY; 1a.2: PY. Researchers performed stratified constrained randomization. This was carried out through software. Quote: "Constrained randomization was implemented using the cvcrand Stata package by the US‐based team". 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. It appears the randomization was conducted prior to the recruitment of participants: 1b.2: PN. It is unlikely that the selection of individual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. There were some baseline imbalances that were compatible with chance, there is no evidence to indicate differential identification or recruitment of individual participants. | Some concerns | 2.1 a: NI; 2.1b: PY; 2.2: PY. No information was provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. The low literacy of the lay providers could have impacted implementation fidelity. Quote: "This strategy supported the potential sustainability of the intervention but came with literacy challenges for delivering the iMBC content with full fidelity". 2.4: PN. No evidence to suggest deviations from the intended intervention would have influenced the outcomes. 2.5: NA. 2.6: PY. Data were analysed for most randomized participants. 2.7: NA. | Low risk of bias | 3.1a: PY. All included clusters were analyzed. 3.1b: PY. Data were available for nearly all participants within the cluster. Quote: "303 (81%), 313 (83.7%), and 266 (71.1%) participants were followed up at the mini‐survey, imme‐ diate post‐intervention survey (follow‐up 1), and the 8‐month post‐ intervention survey (follow‐up 2) time points, respectively ". 3.2: NA. 3.3: NA. 3.4: NA. | Some concerns | 4.1: N. The outcome measure is well established and reported good psychometric properties in this sample. 4.2: PN. No evidence to suggest differences in measurement between intervention and control. 4.3A: NI; 4.3b: PY. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. | Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. | Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Escolar 2014 | Some concerns | 1.1: Y; 1.2: NI. Quote: "The respondents were then randomly assigned to either the experimental or control group" No information provided about allocation concealment. 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and the interventionists were most likely aware of the allocation. 2.3: NI. Note: No information provided. 2.4: NA. 2.5: NA. 2.6: NI. Note: No infomation provided. 2.7: N. Note: No drop‐out. |
Low risk of bias | 3.1: Y. Note: No missing data. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: Y. Note: Only self‐reported measure. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No protocol available. No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in three domains (1, 2, 4), but not to be at high risk of bias for any domain. |
| Lachman 2017 | Low risk of bias | 1.1: Y; 1.2:Y. Quote: "An external researcher not directly involved in the study conducted the randomization procedures remotely in Oxford, United
Kingdom. Participants were randomly assigned on a 1:1 ratio to an intervention or wait‐list control group after baseline data collection
using a concealed computerized program, SealedEnvelope™."; "Our implementing partner, Clowns Without Borders South Africa, notified participants of their
allocation status via telephone." 1.3:PN. |
Low risk of bias | 2.1:Y; 2.2:Y. Quote: "Although program implementers and participants were aware of their allocation status, researchers
conducting self‐report interviews and observational assessments were blind to allocation." 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4:NA. 2.5:NA. 2.6:Y. Quote: "Data analyses were conducted with an intention‐to‐treat design" 2.7:NA. |
Low risk of bias | 3.1:Y. Note: 3 out of the 68 participants randomized were lost at follow‐up (2.9%). 3.2:NA. 3.3:NA. 3.4:NA. |
Some concerns | 4.1:PN. Note: The selected outcome method is widely validated and established, appropriate for the local context, and assessed in its psychometric properties for this sample. 4.2:PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3:Y. Note: Outcome assessors (participants) were most likely aware of intervention allocation. The research assistants administering the questionnaires were blinded. 4.4: PY; 4.5:PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did |
Some concerns | 5.1:PY. Note: No evidence to suggest otherwise, the protocol and manuscript do not show discrepancies. 5.2: PY. Note: A few of the secondary outcomes mentioned in the protocol (e.g. ASSIST, Grover‐Counter Scale of Cognitive Development) were not reported in this manuscript. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol and methods were reported in the results. |
Some concerns | The study is judged to raise some concerns in two domains (4 and 5), but not to be at high risk of bias for any domain. |
| Lachman 2020 | Low risk of bias | 1a.1: Y; 1a.2: Y. Quote: "Cluster randomisation was conducted at village level prior to baseline assessments, to reduce the likelihood of contamination between arms. An external researcher used concealed computer‐generated codes to randomly allocate eight villages into three treatment arms and a control arm "; "The implementing partner notified the participating families of their allocation status after baseline data collection." 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: PN. Note: randomization was conducted prior to the recruitment of participants 1b.2: PN. Note: groups were comparable at baseline, see Table 1. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were aware of intervention allocation. 2.3: PN. Note: No evidence to indicate deviations from protocol. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a.: Y. Note: all included clusters were analyzed; 3.1b: PY. Note: data were available for nearly all participants within the cluster. Quote:"Study retention was considerably higher than anticipated with 94.8% adults (n=235/248), 87.5% children (n=154/176) and 87.8% in their early childhood (n=122/139) assessments completed at post‐treatment, with no differences between arms " 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The outcome measure is widely established and validated for use across different contexts. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcomes selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Sangraula 2020 | Low risk of bias | 1a.1: PY. 1a.2: PY. Quote: "A randomization procedure was used, in which names of the two VDCs were written on cards and placed into a hat. The District Public Health Officer (DPHO) was to draw one card from the hat which would be allocated as the intervention arm VDC and the VDC in the remaining card would be allocated to the control arm. Program staff, including community psychosocial workers (CPSWs) and research assistants (RAs) were only assigned to either VDC after the random drawing to reduce risk of unblinding. 1a.3: PN. There is no evidencde to suggest problems in the randomization process 1b.1: PN. it appears the randomization was conducted prior to the recruitment of participants. 1b.2: PN. It is unlikely that the selection of inidividual participants was affected by knowledge of the intervention assigned to the cluster. 1b.3: PN. there is no evidence to indicate imbalances in baseline values to indicate differentail identification or recruitment of individual participants. |
Low risk of bias | 2.1 a:NI. 2.1b: PN. 2.2: PN. Quote: "CPSWs, RAs, trial participants, and local mhGAP trained health workers were blinded to the allocation of the study conditions. The VDCs of the two arms were separated by another VDC which worked as a buffer and physical barrier against contamination between the two arms. CPSWs in both arms were instructed not to disclose the treatment that any participants received except with their clinical supervisors. The trial statistician was blinded to treatment arm during analysis. " 2.3: NA. 2.4: NA. 2.5: NA. 2.6: PY. Data was analysed for most participants randomized. |
Low risk of bias | 3.1a: Y. all included clusters were analyzed; 3.1b: PY. data were available for nearly all participants within the cluster 3.2: NA. 3.3: NA. 3.4: NA. |
Low risk of bias | 4.1: PN. The outcome measure (PHQ‐9) is widely established and used across contexts, included the one of the trial 4.2: PN. No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a:NI. 4.3b: PN. No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were blinded |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Song 2019 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Block randomization, computer‐generated random list. Using opaque and sealed envelopes for group allocation code keeping. This was done by a person not involved in data collection. 1.3: N. Quote: "No considerable differences were observed between the intervention and control groups in terms of demographic characteristics, baseline outcome and mediating variables." |
Low risk of bias | 2.1: Y; 2.2: PY. Note: Due to the nature of the intervention participants were most likely aware of their allocation. 2.3: PN. Note: No evidence to suggest issues in recruitment and engagement nor failures to implement intervention 2.4: NA. 2.5: NA. 2.6: Y. Note: An ITT analysis was conducted. 2.7: NA. |
Low risk of bias | 3.1: PY. Note: Data at end line was missing, due to withdrawal or loss to follow‐up, for 29 participants (circa 24%). 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Quote: "GDS is easy to administer and is a valid tool for assessing depressive symptoms in individuals with mild cognitive impairment"; The Cronbach’s alpha in this study was 0.784. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Researchers involved in data collection were blinded to group allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Ward 2020 | Low risk of bias | 1.1: PY; 1.2: PY. Quote: "Randomization was conducted after data collection by an off‐site statistician with no other contact with the trial"." 1.3: PN. Baseline characteristics were overall comparable across groups as demonstrated by Table 1. |
High risk of bias | 2.1: PY; 2.2: PY. Due to the nature of the intervention participants and personnel were moslt likely aware of their assigned intervention. 2.3: PY. There is some evidence to suggest possible contamination between the intervention and control group. Quote: "In addition, in the dense living environments of informal settlements, it is possible that there was contamination between intervention and control groups." 2.4: PY. Contamination could have affected the outcomes. 2.5: PY. Contamination is more liekly to affect the participants in the control group. 2.6: PY. No explicit mention of the analysis used to estimate the effect of assignment to intervention, but data was analyzed for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t". 2.7: NA. |
Low risk of bias | 3.1: PY. Data was availalble for almost all randomized participants. Quote: "For caregiver self‐report, the follow‐up rate was 97.0% at t1 and 91.9% at t" 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. The selected outcome method is widely validated and established across contexts and demonstrated good psychometric properties in the study 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY. Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did 4.5: PN. |
Low risk of bias | 5.1: PY. No evidence to suggest otherwise, methods and results do not show discrepancies.. 5.2: PN. No evidence to suggest outcome selection.. 5.3: PN. No evidence to suggest analyses selection. All analyses mentioned in the methods were reported.. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Subgroup 10.1.2 Other | ||||||||||||
| Asnani 2021 | Low risk of bias | 1.1: Y; 1.2: Y. Note: Quote: "A random numbers
table, computer generated by an independent staff statistician, was kept centrally. Once baseline
measurements were completed, the study coordinator telephoned the central site for the
next assignment based on the random number table and informed the subject of her allocation
group. Th" 1.3: PN. Note: Baseline characteristics were overall comparable across groups, significant differences were reported for 2 variables (compatible with chances) |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention. Some changes were made to the protocol before recruitment. These are reported in the manuscript. 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were performed based on intention to treat principles" 2.7: NA. |
Low risk of bias | 3.1: Y. Note: Data was availalble for all 64 randomized participants, there were no losses at the 6 months follow‐up. 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: PN. Note: The selected outcome method (CES‐D) is widely validated and established and demonstrated good inter‐item reliability in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. Staff involved in data collection was blinded to the allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. Authors mention that the CES‐D was not initially planned to be an outcome of interest in the study, this was added in a second moment, but still before the beginning of recruitment. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Chaharrahifard 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design; Quote: "the samples were randomly assigned to two groups of received counseling (intervention) and non‐received counseling (control) by four‐sized randomized blocks." 1.2: Y. Quote: "The details of blocks were contained in asset of sealed. 1.3: N. Quote: "There was not significant difference between the demographic characteristics in the control and intervention group." |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.5: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.6: N. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized. |
Low risk of bias | 3.1: PY. Note: Drop‐out rate: 21%. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Chang 2015 | Low risk of bias | 1.1: Y; 1.2: PY. Note: Title mentions RCT design. Quote: "The evaluation was a cluster randomized trial conducted in Jamaica, Antigua, and St Lucia with public health center as the unit of randomization." 1.3: N. Note: Quote: "There were no significant differences between groups in enrollment characteristics" |
High risk of bias | 2.1a: NI; 2.1b: PY; 2.2: PY. Note: No information provided on wheter participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PY. Note: Quote: "Clinics were often noisy and crowded, and some mothers would have had difficulty hearing and seeing the films. It also made it difficult for the health workers to interact with the mothers during the demonstration." 2.4: NA. 2.5: NA. 2.6: Y. Quote: "Analyses were by intention to treat" 2.7: NA. |
Low risk of bias | 3.1a: NI. No information on wheter data was available for all clusters.
3.1b: PY. 3.2: PY. Quote: "Loss did not differ by group (16% of children were lost to follow‐up in the control and 14% were lost to follow‐up in the intevrention)" 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome measure (center for Epidemiologic Studies‐depression Scale) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurements between intevrention and control. 4.3a: PY; 4.3b: PY. Note: Interviewers and testers were blind to center assignment but participants (mothers) probably knew that they were in a trial and participants were most likely aware of the group allocation due to the nature of the intervention. Self‐assessment scale. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcome, but there is no reason to believe that it did |
Low risk of bias | 5.1: PY. Note: no evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods are reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
High risk of bias | The study is judged to be at high risk of bias in one domain (2). |
| Chew 2018 | Low risk of bias | 1a.1: Y; 1a.2: PY. Note: Title mentions RCT design.
Quote: "In assigning the HCs to the VEMOFIT group (VG) or attention‐control groups (AG), randomisation will be carried out after stratification by cluster size and geographical areas of the 10 HCs." Quote: "A member of the Data Management Services team at the University Medical center, Ultrecht will carry out the randomisation." 1.3: NI. Note: No useful information is reported to evaluate this element 1b.1: PY. Seems that participants were recruited after randomization. |
Low risk of bias | 2.1a: PY. 2.1b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes".
2.2.: Y. Nurses were trained to deliver the intervention. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: The analysis was carried out on an intention to treat basis. 2.7: NA. |
Low risk of bias | 3.1a: PY. All 10 clusters that recruited participants were analyzed.
3.1b: PN. 92 from intervention group (n = 145) and 79 from control group (n = 150) did not receive allocated intervention. In addition to the 33 people lost to follow up at 12 months, 5 additional missing data were noted with regard to PHQ‐9. 3.2: PN. No evidence that the result was not biased by missing data. 3.3: PN; 3.4: NA. Note: Missingness in the outcome do not depend on its true value. |
Low risk of bias | 4.1: N. Note: The selected outcome method (Malay Brief Illness Perception Quetsionnaire, MBIPQ) is validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control 4.3a: PY.Outcome assessors (participants) were most likeley aware that a trial was taking a place. 4.3b: PN. Quote: "We use a modified informed consent procedure to ensure that patients are unware of the two different intervention programmes" 4.4: NA. 4.5: NA. |
Low risk of bias | 5.1: PY. Note: Protocol is available. 5.2: PN. Note: No evidence to suggest outcome selection. Almost all outcomes mentioned in the methods and protocol were reported (It is not reported "heath‐care utilization hospital" that is a secondary outcome). 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methdis and protocol were reported. |
Low risk of bias | The study is judged to be at low risk of bias for all domains. |
| Ferreira‐Vorkapic 2018 | Low risk of bias | 1.1: Y; 1.2: Y. Quote: "(...) volunteers were randomly allocated to one of the three experimental groups. A separate examiner coordinated the group distribution (and all statistical analysis was performed by an expert from another
state), using the random allocation method." 1.3: N. Quote: "the baseline data showing no significant differences between the groups, except for education years, where the relaxation group showed a higher level of education" |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention participants and personnel were most likely aware of their assigned intervention 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Drop‐out: 0 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (BAI) is widely validated and established across multiple languages.ility in the study (0.9) 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
| Gao 2015 | Low risk of bias | 1.1: Y. Quote: "The randomization sequence was generated using a computerized random number generator and the allocation was kept in sealed opaque consecutively numbered envelopes." 1.2.: Y. Quote: "This simple randomization scheme was independently prepared by a research assistant who was not involved in determining eligibility, providing care, or assessing outcome." 1.3: N. Quote: "There were no significant differences between the two groups in their demographic, obstetric and related characteristics (...) There were also no significant differences between the two groups in their baseline measures" |
Low risk of bias | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: BA 2.6: Y. Note: Intention to treat analysis done. 2.7: NA |
Low risk of bias | 3.1: PY. Note: Drop‐out: 14 of 180. 3.2: NA 3.3: NA 3.4: NA |
Some concerns | 4.1: N. Note: The selected outcome method (EPDS) is widely validated in Chinese mothers and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4.: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All outcomes mentioned in the methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Hinton 2021 | Low risk of bias | 1a.1: PY. 1a.2: Y. Quote: "Randomizationwas at the level of clusters, defined as geographic areas (ie, communes) served by commune health stations which have a population
range of ≈5000‐15,000 people. Randomization was conducted
by one of the study investigators residing in the United States through
the flip of a coin." 1a.3: NI. No useful information is reported to evaluate this element 1b.1: N. Note: particpants were recruited after randomization; 1b.2: PN. Note: there is no evidence to suggest that selection of individual participants was affected by knoweldge of the intervention assigned to the cluster; 1b.3:N. Note: no significant differences at baseline were identified. |
Low risk of bias | 2.1a: NI; 2.1b: PY; 2.2: Y. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: no evidence to suggest failure in analyzing any clyster; 3.1b: PY. Note: data was avaiable for 85% of participants at follow‐up. 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: no evidence to suggest that. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants were most likely aware of the group allocation due to the nature of the intervention. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: Y. Note: A pre‐determined statistical plan is avaiable and in line with procedures reported in the final manuscript 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Luoto 2020 | Low risk of bias | 1a.1: PY; 1a.2: PY. Quote: "Using a random
number generator in Stata, two authors (JEL and ILG) randomly assigned villages in a 1:1:1 ratio to one of three study groups, stratified by sub‐county" 1a.3: NI. No useful information is reported to evaluate this element. 1b.1: Y. Note: from protocol it is clear that recruitment of both adults and children as well as baseline assessments, took place before randomization to study arms |
Low risk of bias | 2.1 a: NI; 2.1b:PY; 2.2: PY. Note: No information provided on whether participants knew that they were in a trial, due to the nature of the intervention they (as those delivering the intervention) were most likely aware of intervention allocation. Quote: "Due to the nature of the intervention, masking of
participants and community health volunteers who
delivered the intervention was not possible" 2.3: PN. Note: No evidence to suggest deviations from the intended intervention that arose because of the trial context. 2.4: NA. 2.5: NA. 2.6: Y. Note: Data was analysed on an intention‐to‐treat basis. 2.7: NA. |
Low risk of bias | 3.1a PY. Note: all 60 clusters that recruited participants were analyzed; 3.1b: PY. Note: 92 participants out of 1152 were lost at followup (8%). 3.2: NA. 3.3: NA; 3.4: NA. |
Some concerns | 4.1: PN. The outcome measure (CES‐D) is widely established and used across contexts. 4.2: PN. Note: No evidence to suggest that measurement or ascertainment of the outcome differed between intervention groups. 4.3a: NI; 4.3b: PY. Note: No information provided on whether participants knew that they were in a trial, participants (outcome assessors) were most likely aware of the group allocation due to the nature of the intervention. Efforts were made to keep the data collection teams as masked to group status as possible. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned interventio could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note:No evidence to suggest otherwise, protocol and paper do not show discrepancies. 5.2: PN. Note:No evidence to suggest outcome selection. All outcomes mentioned in the protocol, methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the protocol, methods and results were reported. |
Some concerns | The study is judged to raise some concerns in one domain (4), but not to be at high risk of bias for any domain. |
| Rodriguez 2021 | Low risk of bias | 1.1: Y. Note: Title mentions RCT design. Quote: "Students who completed the web‐based baseline assessment measures were randomly assigned to a brief, 4‐week internet‐based mindfulness intervention (MIND), or to the intervention plus peer counselor support (MIND+)." 1.2: Y. Quote: "The randomization sequence was sourced through random.org [49], an automated, web‐based randomization service that generates randomness using atmospheric noise. Using random.org, two 25‐person blocks were used to randomize the participants into 2 equally‐sized groups." 1.3: PN. Note: No significant differences at baseline. |
Some concerns | 2.1: PY; 2.2: PY. Note: Due to the nature of the intervention, participants and the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention. 2.4: NA 2.5: NA 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention. 2.7: N. Note: There was not a substantial impact of the failure to analyze participants in the group to which they were randomized. |
High risk of bias | 3.1: PN. Note: Drop‐out: 31 of 54. 3.2: PN. Note: Proportions of lost to follow‐up between control and intervention are not comparable (n= 20 and n= 11). 3.3: NI; 3.4: NI. Note: No information about documented reasons that withdrawal are related to the outcome. |
Some concerns | 4.1: N. Note: The selected outcome method (PHQ‐9) is widely validated and established. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. 5.3: PN. Note: No evidence to suggest analyses selection. |
High risk of bias | The study is judged to be at high risk of bias in one domain (3). |
| Subgroup 10.1.3 Refugee camp | ||||||||||||
| Yeomans 2010 | Low risk of bias | 1.1: Y; 1.2: NI.Note: Title mentions RCT design. No further information. 1.3: N. Quote: "There were no significant baseline differences between the three treatment groups across age, gender, ethnicity, symptoms, education level, traumatic events experienced, or on prior exposure to trauma discourse" |
Some concerns | 2.1: Y; 2.2: PY. Quote: "Participants (...) were informed of random allocation procedures". Due to the nature of the intervention, the interventionists were most likely aware of the allocation. 2.3: PN. Note: No evidence to suggest issues to implement intervention 2.4: NA. 2.5: NA. 2.6: NI. Note: No explicit mention of the analysis used to estimate the effect of assignment to intervention 2.7: NA. Note: There was not a substantial impact of the failure to analyse participants in the group to which they were randomized |
Low risk of bias | 3.1: Y. Note: Lost to follow‐up: 7 of 120 3.2: NA. 3.3: NA. 3.4: NA. |
Some concerns | 4.1: N. Note: The selected outcome method (HSCL‐25) is widely validated and established across multiple languages. 4.2: PN. Note: No evidence to suggest differences in measurement between intervention and control. 4.3: PY. Note: Outcome assessors (participants) were most likely aware of intervention allocation. 4.4: PY; 4.5: PN. Note: Knowledge of the assigned intervention could influence participant‐reported outcomes, but there is no reason to believe that it did. |
Low risk of bias | 5.1: PY. Note: No evidence to suggest otherwise, methods and results do not show discrepancies. 5.2: PN. Note: No evidence to suggest outcome selection. All outcomes mentioned in the methods and results were reported. 5.3: PN. Note: No evidence to suggest analyses selection. All analyses mentioned in the methods were reported. |
Some concerns | The study is judged to raise some concerns in two domains (2 and 4), but not to be at high risk of bias for any domain. |
Acknowledgements
The Norwegian Satellite of Cochrane Effective Practice and Organisation of Care supported the authors in developing this review. The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Simon Lewin, Norwegian University of Science and Technology;
Contact Editor (provided comments/methods review): Susan Munabi‐Babigumira, Norwegian Institute of Public Health;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Elizabeth Paulsen, Norwegian Institute of Public Health;
Information Specialist (provided support in designing, running and reporting the searches): Marit Johansen, Norwegian Institute of Public Health;
Copy Editor (copy editing and production): Anne Lethaby, Central Production Service; Christina Baswell, J&J Editorial; and Margaret Silvers, Copy Editor, J&J Editorial;
Peer‐reviewers (provided comments and recommended an editorial decision): Andrew Hutchings, London School of Hygiene & Tropical Medicine (statistical review); Ingunn Marie Stadskleiv Engebretsen, University of Bergen (clinical/content review); Gloria Pedersen, Department of Psychiatry, Division of Global Mental Health, The George Washington University (clinical/content review); and Arvin Bhana, Centre for Rural Health, College of Health Sciences, UKZN University of KwaZulu‐Natal (clinical/content review).
We acknowledge the contribution of review authors to the complementary treatment review (Weng Chin, Yen Chian Lim, Rakesh Singh, Hakan Foss, Ambika Thapa‐Pachya, Ujala Shahmalak, Sarah McMullen, Antonio Rojas‐Garcia, Ussif Mohammed Al‐Amin).
We also acknowledge all the researchers and authors whom we consulted to ensure the eligibility of their studies in this review. The following authors provided their written consent to be acknowledged: Hull DM, Skar AMS, Duru Asiret G, Devries K, Rotheram‐Borus MJ, Fabbri C, Lachman JM, Hinton L, Walker SP, Mc Conachie H, Yusoff MSB, Barnes R, Krutikova S, Ansai JH, Khuzwayo N, Hartmann M, Diedrichs P.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (CENTRAL) Issue 11 2021, Cochrane Library, Wiley (searched 29 November 2021) | ||
| ID | Search | Hits |
| #1 | [mh “mental disorders”] | 78191 |
| #2 | [mh “mania”] | 7 |
| #3 | [mh “mental health”] | 1776 |
| #4 | [mh “depression”] | 13361 |
| #5 | [mh "child development"] | 2691 |
| #6 | [mh “mentally disabled persons”] | 61 |
| #7 | [mh "self‐injurious behavior"] | 1590 |
| #8 | (mental next health* or mental* next ill* or mental* next disorder* or mental* next well*):ti,ab,kw | 34039 |
| #9 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) near/2 (disorder? or illness* or dependence or abuse or misuse or use)):ti,ab,kw | 27787 |
| #10 | (depressi* near/2 (sign* or symptom* or disorder*)):ti,ab,kw | 33155 |
| #11 | (depress* near/3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or (in next patient*) or outpatient* or (out next patient*))):ti,ab,kw | 52998 |
| #12 | ((depress* or distress*) near/3 (postnatal* or post natal* or maternal*)):ti,ab,kw | 3778 |
| #13 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or "borderline personality" or (stress near/2 disorder*) or (adjustment next disorder?) or (psychological next/1 trauma*) or schizophrenia or psychoses or psychosis or (stress next syndrome?) or (distress next syndrome?) or (combat next disorder?) or (war next disorder?) or ptsd or dementia):ti,ab,kw | 159131 |
| #14 | (((post next trauma*) or posttrauma*) near/3 (stress* or disorder?)):ti,ab,kw | 6355 |
| #15 | ((psychological next trauma) or psychotrauma*):ti,ab,kw | 348 |
| #16 | (alcoholism or alcoholic? or (drug next addict*) or (drug next abus*) or (drug next misuse) or (drug next user?)):ti,ab,kw | 17448 |
| #17 | ((learning or mental* or intellectual) next (disabled or disabilit* or disorder? or difficult*)):ti,ab,kw | 13315 |
| #18 | ((dissociative near/3 (disorder* or reaction*)) or dissociation):ti,ab,kw | 1607 |
| #19 | ((bipolar or behavioral or beahavioural or obsessive or panic or mood or delusional) near/2 (disorder? or illness* or disease?)):ti,ab,kw | 17060 |
| #20 | (trichotillomani* or OCD or (obsess* next compulsi*) or GAD or (stress next reaction?) or "acute stress" or neurosis or neuroses or neurotic or mania):ti,ab,kw | 11187 |
| #21 | (affective* next (disorder? or disease? or illness* or symptom?)):ti,ab,kw | 2742 |
| #22 | ((mental or psychological or emotional or (psycho next social) or psychosocial) next (stress* or distress*)):ti,ab,kw | 8698 |
| #23 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) next (symptom* or disorder* or condition* or depress* or anxiety)):ti,ab,kw | 1532 |
| #24 | ("mental relapse" or fatigue or (somatic next symptom?) or worry or worries or panic or (low next mood?) or (mood next problem?)):ti,ab,kw | 43191 |
| #25 | ((anxiety next disorder?) or agoraphobi* or (general* next anxi*) or "separation anxiety" or "neurocirculatory asthenia" or (neurotic next disorder?) or (social next phobi*) or (self next harm*) or (self next injur*) or suicid*):ti,ab,kw | 20529 |
| #26 | (slow* next (thought? or think*)):ti,ab,kw | 20 |
| #27 | (mental* next develop*):ti,ab,kw | 522 |
| #28 | {or #1‐#27} | 272553 |
| #29 | [mh “primary health care”] | 7993 |
| #30 | [mh “physicians, family”] | 460 |
| #31 | [mh “physicians, primary care”] | 170 |
| #32 | [mh “general practitioners”] | 313 |
| #33 | [mh “general practice”] | 2487 |
| #34 | [mh “family practice”] | 1978 |
| #35 | [mh “social support”] | 3482 |
| #36 | [mh “community health workers”] | 536 |
| #37 | [mh "allied health personnel"] | 1259 |
| #38 | [mh “community health services”] | 14693 |
| #39 | [mh “schools”] | 3435 |
| #40 | [mh “school health services”] or [mh “school mental health services”] | 1679 |
| #41 | [mh “rural health”] | 541 |
| #42 | [mh “rural population”] | 1865 |
| #43 | [mh “nurses, community health”] | 28 |
| #44 | [mh “nurses, public health”] | 7 |
| #45 | [mh “family nursing”] | 40 |
| #46 | [mh “primary care nursing”] | 33 |
| #47 | [mh “rural nursing”] | 1 |
| #48 | [mh “community health nursing”] | 350 |
| #49 | [mh “school nursing”] | 83 |
| #50 | (primary near/5 (care or health*)):ti,ab,kw | 40026 |
| #51 | ((family next practi*) or (family next doctor*) or (family next physician*) or gp* or (general next practi*)):ti,ab,kw | 24304 |
| #52 | (school* or teacher* or rural* or community):ti,ab,kw | 90378 |
| #53 | ((non next specialist*) or nonspecialist* or (social next worker*) or trainer?):ti,ab,kw | 4085 |
| #54 | (psycho next social or psychosocial):ti,ab,kw | 17252 |
| #55 | (caregiver* or (care next giver?) or layperson*):ti,ab,kw | 15707 |
| #56 | (paraprofessional* or (para next professional*) or (non next physician?) or (non next clinician?)):ti,ab,kw | 436 |
| #57 | (allied near/2 (professional? or person* or staff or worker?)):ti,ab,kw | 494 |
| #58 | (lay near/2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)):ti,ab,kw | 648 |
| #59 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or (practice next nurs*) or (district next nurs*) or (health next visitor?)):ti,ab,kw | 12181 |
| #60 | {or #29‐#59} | 183769 |
| #61 | #28 and #60 | 55512 |
| #62 | (afghanistan OR albania OR algeria OR "american samoa" OR angola OR "antigua and barbuda" OR antigua OR barbuda OR argentina OR armenia OR armenian OR aruba OR azerbaijan OR bahrain OR bangladesh OR barbados OR "republic of belarus" OR belarus OR byelarus OR belorussia OR byelorussian OR belize OR "british honduras" OR benin OR dahomey OR bhutan OR bolivia OR "bosnia and herzegovina" OR bosnia OR herzegovina OR botswana OR bechuanaland OR brazil OR brasil OR bulgaria OR "burkina faso" OR "burkina fasso" OR "upper volta" OR burundi OR urundi OR "cabo verde" OR "cape verde" OR cambodia OR kampuchea OR "khmer republic" OR cameroon OR cameron OR cameroun OR "central african republic" OR "ubangi shari" OR chad OR chile OR china OR colombia OR comoros OR "comoro islands" OR "iles comores" OR mayotte OR "democratic republic of the congo" OR "democratic republic congo" OR congo OR zaire OR "costa rica" OR "cote d’ivoire" OR "cote d’ ivoire" OR "cote divoire" OR "cote d ivoire" OR "ivory coast" OR croatia OR cuba OR cyprus OR "czech republic" OR czechoslovakia OR djibouti OR "french somaliland" OR dominica OR "dominican republic" OR ecuador OR egypt OR "united arab republic" OR "el salvador" OR "equatorial guinea" OR "spanish guinea" OR eritrea OR estonia OR eswatini OR swaziland OR ethiopia OR fiji OR gabon OR "gabonese republic" OR gambia OR "georgia (republic)" OR georgia OR georgian OR ghana OR "gold coast" OR gibraltar OR greece OR grenada OR guam OR guatemala OR guinea OR "guinea bissau" OR guyana OR "british guiana" OR haiti OR hispaniola OR honduras OR hungary OR india OR indonesia OR timor OR iran OR iraq OR "isle of man" OR jamaica OR jordan OR kazakhstan OR kazakh OR kenya OR "democratic people’s republic of korea" OR "republic of korea" OR north korea OR south korea OR korea OR kosovo OR kyrgyzstan OR kirghizia OR kirgizstan OR "kyrgyz republic" OR kirghiz OR laos OR "lao pdr" OR "lao people's democratic republic" OR latvia OR lebanon OR "lebanese republic" OR lesotho OR basutoland OR liberia OR libya OR "libyan arab jamahiriya" OR lithuania OR macau OR macao OR "macedonia (republic)" OR macedonia OR madagascar OR "malagasy republic" OR malawi OR nyasaland OR malaysia OR "malay federation" OR "malaya federation" OR maldives OR "indian ocean islands" OR "indian ocean" OR mali OR malta OR micronesia OR "federated states of micronesia" OR kiribati OR "marshall islands" OR nauru OR "northern mariana islands" OR palau OR tuvalu OR mauritania OR mauritius OR mexico OR moldova OR moldovian OR mongolia OR montenegro OR morocco OR ifni OR mozambique OR "portuguese east africa" OR myanmar OR burma OR namibia OR nepal OR "netherlands antilles" OR nicaragua OR niger OR nigeria OR oman OR muscat OR pakistan OR panama OR "papua new guinea" OR paraguay OR peru OR philippines OR philipines OR phillipines OR phillippines OR poland OR "polish people's republic" OR portugal OR "portuguese republic" OR "puerto rico" OR romania OR russia OR "russian federation" OR ussr OR "soviet union" OR "union of soviet socialist republics" OR rwanda OR ruanda OR samoa OR "pacific islands" OR polynesia OR "samoan islands" OR "navigator island" OR "navigator islands" OR "sao tome and principe" OR "saudi arabia" OR senegal OR serbia OR seychelles OR "sierra leone" OR slovakia OR "slovak republic" OR slovenia OR melanesia OR "solomon island" OR "solomon islands" OR "norfolk island" OR "norfolk islands" OR somalia OR "south africa" OR "south sudan" OR "sri lanka" OR ceylon OR "saint kitts and nevis" OR "st. kitts and nevis" OR "saint lucia" OR "st. lucia" OR "saint vincent and the grenadines" OR "saint vincent" OR "st. vincent" OR grenadines OR sudan OR suriname OR surinam OR "dutch guiana" OR "netherlands guiana" OR syria OR "syrian arab republic" OR tajikistan OR tadjikistan OR tadzhikistan OR tadzhik OR tanzania OR tanganyika OR thailand OR siam OR "timor leste" OR "east timor" OR togo OR "togolese republic" OR tonga OR "trinidad and tobago" OR trinidad OR tobago OR tunisia OR turkey OR "turkey (republic)" OR turkmenistan OR turkmen OR uganda OR ukraine OR uruguay OR uzbekistan OR uzbek OR vanuatu OR "new hebrides" OR venezuela OR vietnam OR "viet nam" OR "middle east" OR "west bank" OR gaza OR palestine OR yemen OR yugoslavia OR zambia OR zimbabwe OR "northern rhodesia" OR "global south" OR "africa south of the sahara" OR "sub saharan africa" OR "subsaharan africa" OR "africa, central" OR "central africa" OR "africa, northern" OR "north africa" OR "northern africa" OR magreb OR maghrib OR sahara OR "africa, southern" OR "southern africa" OR "africa, eastern" OR "east africa" OR "eastern africa" OR "africa, western" OR "west africa" OR "western africa" OR "west indies" OR "indian ocean islands" OR caribbean OR "central america" OR "latin america" OR "south and central america" OR "south america" OR "asia, central" OR "central asia" OR "asia, northern" OR "north asia" OR "northern asia" OR "asia, southeastern" OR "southeastern asia" OR "south eastern asia" OR "southeast asia" OR "south east asia" OR "asia, western" OR "western asia" OR "europe, eastern" OR "east europe" OR "eastern europe" OR "developing country" OR "developing countries" OR "developing nation" OR "developing nations" OR "developing population" OR "developing populations" OR "developing world" OR "less developed country" OR "less developed countries" OR "less developed nation" OR "less developed nations" OR "less developed population" OR "less developed populations" OR "less developed world" OR "lesser developed country" OR "lesser developed countries" OR "lesser developed nation" OR "lesser developed nations" OR "lesser developed population" OR "lesser developed populations" OR "lesser developed world" OR "under developed country" OR "under developed countries" OR "under developed nation" OR "under developed nations" OR "under developed population" OR "under developed populations" OR "under developed world" OR "underdeveloped country" OR "underdeveloped countries" OR "underdeveloped nation" OR "underdeveloped nations" OR "underdeveloped population" OR "underdeveloped populations" OR "underdeveloped world" OR "middle income country" OR "middle income countries" OR "middle income nation" OR "middle income nations" OR "middle income population" OR "middle income populations" OR "low income country" OR "low income countries" OR "low income nation" OR "low income nations" OR "low income population" OR "low income populations" OR "lower income country" OR "lower income countries" OR "lower income nation" OR "lower income nations" OR "lower income population" OR "lower income populations" OR "underserved country" OR "underserved countries" OR "underserved nation" OR "underserved nations" OR "underserved population" OR "underserved populations" OR "underserved world" OR "under served country" OR "under served countries" OR "under served nation" OR "under served nations" OR "under served population" OR "under served populations" OR "under served world" OR "deprived country" OR "deprived countries" OR "deprived nation" OR "deprived nations" OR "deprived population" OR "deprived populations" OR "deprived world" OR "poor country" OR "poor countries" OR "poor nation" OR "poor nations" OR "poor population" OR "poor populations" OR "poor world" OR "poorer country" OR "poorer countries" OR "poorer nation" OR "poorer nations" OR "poorer population" OR "poorer populations" OR "poorer world" OR "developing economy" OR "developing economies" OR "less developed economy" OR "less developed economies" OR "lesser developed economy" OR "lesser developed economies" OR "under developed economy" OR "under developed economies" OR "underdeveloped economy" OR "underdeveloped economies" OR "middle income economy" OR "middle income economies" OR "low income economy" OR "low income economies" OR "lower income economy" OR "lower income economies" OR "low gdp" OR "low gnp" OR "low gross domestic" OR "low gross national" OR "lower gdp" OR "lower gnp" OR "lower gross domestic" OR "lower gross national" OR lmic OR lmics OR "third world" OR "lami country" OR "lami countries" OR "transitional country" OR "transitional countries" OR "emerging economies" OR "emerging nation" OR "emerging nations"):ti,ab,kw | 104511 |
| #63 | #61 and #62 | 5293 |
| #64 | #60 and #62 with 'Dementia and Cognitive Improvement', 'Schizophrenia', 'Common Mental Disorders', 'Drugs and Alcohol', 'Developmental, Psychosocial and Learning Problems' in Cochrane Groups | 759 |
| #65 | #63 or #64 | 5364 |
| MEDLINE and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to November 24, 2021, Ovid (searched 29 November 2021) | |
| Searches | Results |
| exp mental disorders/ | 1334640 |
| mania/ | 180 |
| mental health/ | 48895 |
| depression/ | 134978 |
| child development/ | 48979 |
| mentally disabled persons/ | 3681 |
| exp self‐injurious behavior/ | 76929 |
| (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab,kf. | 251779 |
| ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab,kf. | 161005 |
| (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab,kf. | 129671 |
| (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab,kf. | 191151 |
| ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab,kf. | 9628 |
| (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab,kf. | 930547 |
| ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab,kf. | 39702 |
| (psychological trauma or psychotrauma*).ti,ab,kf. | 2114 |
| (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab,kf. | 141985 |
| ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab,kf. | 87257 |
| ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab,kf. | 116125 |
| ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab,kf. | 84084 |
| (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab,kf. | 59489 |
| (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab,kf. | 20532 |
| ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab,kf. | 57522 |
| ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab,kf. | 7316 |
| (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab,kf. | 145162 |
| (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab,kf. | 133296 |
| (slow* adj (thought? or think*)).ti,ab,kf. | 86 |
| (mental* adj develop*).ti,ab,kf. | 3360 |
| or/1‐27 | 2397718 |
| primary health care/ | 85558 |
| physicians, family/ | 16806 |
| physicians, primary care/ | 4047 |
| general practitioners/ | 9206 |
| general practice/ | 14453 |
| family practice/ | 66174 |
| exp social support/ | 76363 |
| community health workers/ | 5996 |
| allied health personnel/ | 12406 |
| exp community health services/ | 319662 |
| schools/ | 44959 |
| school health services/ or school mental health services/ | 18001 |
| rural health/ | 23755 |
| rural population/ | 65142 |
| nurses, community health/ | 945 |
| nurses, public health/ | 460 |
| family nursing/ | 1546 |
| primary care nursing/ | 544 |
| rural nursing/ | 119 |
| community health nursing/ | 19724 |
| school nursing/ | 5507 |
| (primary adj5 (care or health*)).ti,ab,kf. | 184894 |
| (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab,kf. | 293232 |
| (school* or teacher* or rural* or community).ti,ab,kf. | 979629 |
| (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab,kf. | 24612 |
| (psycho‐social or psychosocial).ti,ab,kf. | 111747 |
| (caregiver* or care giver? or layperson*).ti,ab,kf. | 80304 |
| (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab,kf. | 2385 |
| (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab,kf. | 5747 |
| (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab,kf. | 108541 |
| or/29‐58 | 1943460 |
| 28 and 59 | 372513 |
| (afghanistan or albania or algeria or american samoa or angola or "antigua and barbuda" or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or republic of belarus or belarus or byelarus or belorussia or byelorussian or belize or british honduras or benin or dahomey or bhutan or bolivia or "bosnia and herzegovina" or bosnia or herzegovina or botswana or bechuanaland or brazil or brasil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cabo verde or cape verde or cambodia or kampuchea or khmer republic or cameroon or cameron or cameroun or central african republic or ubangi shari or chad or chile or china or colombia or comoros or comoro islands or iles comores or mayotte or democratic republic of the congo or democratic republic congo or congo or zaire or costa rica or "cote d’ivoire" or "cote d’ ivoire" or cote divoire or cote d ivoire or ivory coast or croatia or cuba or cyprus or czech republic or czechoslovakia or djibouti or french somaliland or dominica or dominican republic or ecuador or egypt or united arab republic or el salvador or equatorial guinea or spanish guinea or eritrea or estonia or eswatini or swaziland or ethiopia or fiji or gabon or gabonese republic or gambia or "georgia (republic)" or georgian or ghana or gold coast or gibraltar or greece or grenada or guam or guatemala or guinea or guinea bissau or guyana or british guiana or haiti or hispaniola or honduras or hungary or india or indonesia or timor or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or "democratic people’s republic of korea" or republic of korea or north korea or south korea or korea or kosovo or kyrgyzstan or kirghizia or kirgizstan or kyrgyz republic or kirghiz or laos or lao pdr or "lao people's democratic republic" or latvia or lebanon or lebanese republic or lesotho or basutoland or liberia or libya or libyan arab jamahiriya or lithuania or macau or macao or "macedonia (republic)" or macedonia or madagascar or malagasy republic or malawi or nyasaland or malaysia or malay federation or malaya federation or maldives or indian ocean islands or indian ocean or mali or malta or micronesia or federated states of micronesia or kiribati or marshall islands or nauru or northern mariana islands or palau or tuvalu or mauritania or mauritius or mexico or moldova or moldovian or mongolia or montenegro or morocco or ifni or mozambique or portuguese east africa or myanmar or burma or namibia or nepal or netherlands antilles or nicaragua or niger or nigeria or oman or muscat or pakistan or panama or papua new guinea or new guinea or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or "polish people's republic" or portugal or portuguese republic or puerto rico or romania or russia or russian federation or ussr or soviet union or union of soviet socialist republics or rwanda or ruanda or samoa or pacific islands or polynesia or samoan islands or navigator island or navigator islands or "sao tome and principe" or saudi arabia or senegal or serbia or seychelles or sierra leone or slovakia or slovak republic or slovenia or melanesia or solomon island or solomon islands or norfolk island or norfolk islands or somalia or south africa or south sudan or sri lanka or ceylon or "saint kitts and nevis" or "st. kitts and nevis" or saint lucia or "st. lucia" or "saint vincent and the grenadines" or saint vincent or "st. vincent" or grenadines or sudan or suriname or surinam or dutch guiana or netherlands guiana or syria or syrian arab republic or tajikistan or tadjikistan or tadzhikistan or tadzhik or tanzania or tanganyika or thailand or siam or timor leste or east timor or togo or togolese republic or tonga or "trinidad and tobago" or trinidad or tobago or tunisia or turkey or "turkey (republic)" or turkmenistan or turkmen or uganda or ukraine or uruguay or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or middle east or west bank or gaza or palestine or yemen or yugoslavia or zambia or zimbabwe or northern rhodesia or global south or africa south of the sahara or sub‐saharan africa or subsaharan africa or africa, central or central africa or africa, northern or north africa or northern africa or magreb or maghrib or sahara or africa, southern or southern africa or africa, eastern or east africa or eastern africa or africa, western or west africa or western africa or west indies or indian ocean islands or caribbean or central america or latin america or "south and central america" or south america or asia, central or central asia or asia, northern or north asia or northern asia or asia, southeastern or southeastern asia or south eastern asia or southeast asia or south east asia or asia, western or western asia or europe, eastern or east europe or eastern europe or developing country or developing countries or developing nation? or developing population? or developing world or less developed countr* or less developed nation? or less developed population? or less developed world or lesser developed countr* or lesser developed nation? or lesser developed population? or lesser developed world or under developed countr* or under developed nation? or under developed population? or under developed world or underdeveloped countr* or underdeveloped nation? or underdeveloped population? or underdeveloped world or middle income countr* or middle income nation? or middle income population? or low income countr* or low income nation? or low income population? or lower income countr* or lower income nation? or lower income population? or underserved countr* or underserved nation? or underserved population? or underserved world or under served countr* or under served nation? or under served population? or under served world or deprived countr* or deprived nation? or deprived population? or deprived world or poor countr* or poor nation? or poor population? or poor world or poorer countr* or poorer nation? or poorer population? or poorer world or developing econom* or less developed econom* or lesser developed econom* or under developed econom* or underdeveloped econom* or middle income econom* or low income econom* or lower income econom* or low gdp or low gnp or low gross domestic or low gross national or lower gdp or lower gnp or lower gross domestic or lower gross national or lmic or lmics or third world or lami countr* or transitional countr* or emerging economies or emerging nation?).ti,ab,sh,kf. | 2140218 |
| exp randomized controlled trial/ | 552423 |
| controlled clinical trial.pt. | 94551 |
| randomi#ed.ti,ab. | 699505 |
| placebo.ab. | 223375 |
| randomly.ti,ab. | 371399 |
| Clinical Trials as topic.sh. | 198195 |
| trial.ti. | 251720 |
| or/62‐68 | 1459730 |
| exp animals/ not humans/ | 4919146 |
| 69 not 70 | 1345624 |
| 60 and 61 and 71 | 4735 |
| Embase 1974 to 2021 November 24, Ovid (searched 29 November 2021) | ||
| # | Searches | Results |
| 1 | exp *mental disease/ | 1443256 |
| 2 | exp *mental health/ | 52566 |
| 3 | *mentally disabled person/ | 381 |
| 4 | *child development/ | 20960 |
| 5 | *automutilation/ | 8656 |
| 6 | (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab,kf. | 300227 |
| 7 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab,kf. | 225247 |
| 8 | (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab,kf. | 175130 |
| 9 | (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab,kf. | 269137 |
| 10 | ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab,kf. | 12480 |
| 11 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab,kf. | 1262187 |
| 12 | ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab,kf. | 48723 |
| 13 | (psychological trauma or psychotrauma*).ti,ab,kf. | 2916 |
| 14 | (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab,kf. | 195376 |
| 15 | ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab,kf. | 105659 |
| 16 | ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab,kf. | 119962 |
| 17 | ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab,kf. | 124558 |
| 18 | (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab,kf. | 74604 |
| 19 | (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab,kf. | 28692 |
| 20 | ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab,kf. | 76667 |
| 21 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab,kf. | 9990 |
| 22 | (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab,kf. | 224981 |
| 23 | (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab,kf. | 172550 |
| 24 | (slow* adj (thought? or think*)).ti,ab,kf. | 139 |
| 25 | (mental* adj develop*).ti,ab,kf. | 4425 |
| 26 | or/1‐25 | 2833351 |
| 27 | exp *primary health care/ | 65619 |
| 28 | *general practitioner/ | 25667 |
| 29 | *general practice/ | 38170 |
| 30 | exp *social support/ | 24656 |
| 31 | exp *health auxiliary/ | 3235 |
| 32 | exp *community care/ | 57213 |
| 33 | exp *paramedical personnel/ | 234710 |
| 34 | *family nursing/ | 884 |
| 35 | *rural health nursing/ | 71 |
| 36 | exp *school/ | 148284 |
| 37 | exp *school health service/ | 11342 |
| 38 | *rural health care/ | 7942 |
| 39 | *rural health/ | 611 |
| 40 | *rural population/ | 13409 |
| 41 | (primary adj5 (care or health*)).ti,ab,kf. | 245616 |
| 42 | (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab,kf. | 378669 |
| 43 | (school* or teacher* or rural* or community).ti,ab,kf. | 1192675 |
| 44 | (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab,kf. | 37052 |
| 45 | (psycho‐social or psychosocial).ti,ab,kf. | 153356 |
| 46 | (caregiver* or care giver? or layperson*).ti,ab,kf. | 112353 |
| 47 | (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab,kf. | 8424 |
| 48 | (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab,kf. | 3013 |
| 49 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab,kf. | 188864 |
| 50 | or/27‐49 | 2395229 |
| 51 | 26 and 50 | 437030 |
| 52 | random*.ti,ab. | 1725541 |
| 53 | factorial*.ti,ab. | 42387 |
| 54 | (crossover* or cross over*).ti,ab. | 115246 |
| 55 | ((doubl* or singl*) adj blind*).ti,ab. | 250954 |
| 56 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 1136190 |
| 57 | crossover procedure/ | 68721 |
| 58 | single blind procedure/ | 44388 |
| 59 | randomized controlled trial/ | 684134 |
| 60 | double blind procedure/ | 189763 |
| 61 | or/52‐60 | 2581973 |
| 62 | exp animal/ not human/ | 5005276 |
| 63 | 61 not 62 | 2328170 |
| 64 | (afghanistan or albania or algeria or american samoa or angola or "antigua and barbuda" or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or republic of belarus or belarus or byelarus or belorussia or byelorussian or belize or british honduras or benin or dahomey or bhutan or bolivia or "bosnia and herzegovina" or bosnia or herzegovina or botswana or bechuanaland or brazil or brasil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cabo verde or cape verde or cambodia or kampuchea or khmer republic or cameroon or cameron or cameroun or central african republic or ubangi shari or chad or chile or china or colombia or comoros or comoro islands or iles comores or mayotte or democratic republic of the congo or democratic republic congo or congo or zaire or costa rica or "cote d’ivoire" or "cote d’ ivoire" or cote divoire or cote d ivoire or ivory coast or croatia or cuba or cyprus or czech republic or czechoslovakia or djibouti or french somaliland or dominica or dominican republic or ecuador or egypt or united arab republic or el salvador or equatorial guinea or spanish guinea or eritrea or estonia or eswatini or swaziland or ethiopia or fiji or gabon or gabonese republic or gambia or "georgia (republic)" or georgian or ghana or gold coast or gibraltar or greece or grenada or guam or guatemala or guinea or guinea bissau or guyana or british guiana or haiti or hispaniola or honduras or hungary or india or indonesia or timor or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or "democratic people’s republic of korea" or republic of korea or north korea or south korea or korea or kosovo or kyrgyzstan or kirghizia or kirgizstan or kyrgyz republic or kirghiz or laos or lao pdr or "lao people's democratic republic" or latvia or lebanon or lebanese republic or lesotho or basutoland or liberia or libya or libyan arab jamahiriya or lithuania or macau or macao or "macedonia (republic)" or macedonia or madagascar or malagasy republic or malawi or nyasaland or malaysia or malay federation or malaya federation or maldives or indian ocean islands or indian ocean or mali or malta or micronesia or federated states of micronesia or kiribati or marshall islands or nauru or northern mariana islands or palau or tuvalu or mauritania or mauritius or mexico or moldova or moldovian or mongolia or montenegro or "montenegro (republic)" or morocco or ifni or mozambique or portuguese east africa or myanmar or burma or namibia or nepal or netherlands antilles or nicaragua or niger or nigeria or oman or muscat or pakistan or panama or papua new guinea or new guinea or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or "polish people's republic" or portugal or portuguese republic or puerto rico or romania or russia or russian federation or ussr or soviet union or union of soviet socialist republics or rwanda or ruanda or samoa or pacific islands or polynesia or samoan islands or navigator island or navigator islands or "sao tome and principe" or saudi arabia or senegal or serbia or seychelles or sierra leone or slovakia or slovak republic or slovenia or melanesia or solomon island or solomon islands or norfolk island or norfolk islands or somalia or south africa or south sudan or sri lanka or ceylon or "saint kitts and nevis" or "st. kitts and nevis" or saint lucia or "st. lucia" or "saint vincent and the grenadines" or saint vincent or "st. vincent" or grenadines or sudan or suriname or surinam or dutch guiana or netherlands guiana or syria or syrian arab republic or tajikistan or tadjikistan or tadzhikistan or tadzhik or tanzania or tanganyika or thailand or siam or timor leste or east timor or togo or togolese republic or tonga or "trinidad and tobago" or trinidad or tobago or tunisia or turkey or "turkey (republic)" or turkmenistan or turkmen or uganda or ukraine or uruguay or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or middle east or west bank or gaza or palestine or yemen or yugoslavia or zambia or zimbabwe or northern rhodesia or global south or africa south of the sahara or "sub saharan africa" or subsaharan africa or africa, central or central africa or africa, northern or north africa or northern africa or magreb or maghrib or sahara or africa, southern or southern africa or africa, eastern or east africa or eastern africa or africa, western or west africa or western africa or west indies or indian ocean islands or caribbean region or caribbean islands or caribbean or central america or latin america or "south and central america" or south america or asia, central or central asia or asia, northern or north asia or northern asia or asia, southeastern or southeastern asia or south eastern asia or southeast asia or south east asia or asia, western or western asia or europe, eastern or east europe or eastern europe or developing country or developing countries or developing nation? or developing population? or developing world or less developed countr* or less developed nation? or less developed population? or less developed world or lesser developed countr* or lesser developed nation? or lesser developed population? or lesser developed world or under developed countr* or under developed nation? or under developed population? or under developed world or underdeveloped countr* or underdeveloped nation? or underdeveloped population? or underdeveloped world or middle income countr* or middle income nation? or middle income population? or low income countr* or low income nation? or low income population? or lower income countr* or lower income nation? or lower income population? or underserved countr* or underserved nation? or underserved population? or underserved world or under served countr* or under served nation? or under served population? or under served world or deprived countr* or deprived nation? or deprived population? or deprived world or poor countr* or poor nation? or poor population? or poor world or poorer countr* or poorer nation? or poorer population? or poorer world or developing econom* or less developed econom* or lesser developed econom* or under developed econom* or underdeveloped econom* or middle income econom* or low income econom* or lower income econom* or low gdp or low gnp or low gross domestic or low gross national or lower gdp or lower gnp or lower gross domestic or lower gross national or lmic or lmics or third world or lami countr* or transitional countr* or emerging economies or emerging nation?).ti,ab,sh,kw. | 2409351 |
| 65 | 51 and 63 and 64 | 8335 |
| 66 | limit 65 to embase | 4674 |
CINAHL (Cumulative Index to Nursing and Allied Health Literature) 1980 to date searched, EBSCO (searched 29 November 2021)
| S67 | S66 [Limiters ‐ Exclude MEDLINE] | 2,333 |
| S66 | S55 AND S65 | 4,981 |
| S65 | S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 | 1,440,060 |
| S64 | TI transitional W0 countr* OR AB transitional W0 countr* | 82 |
| S63 | TI (lmic or lmics or third W0 world or lami W0 countr*) OR AB (lmic or lmics or third W0 world or lami W0 countr*) | 3,356 |
| S62 | TI (low N3 middle N3 countr*) OR AB (low N3 middle N3 countr*) | 10,203 |
| S61 | TI (low* N0 (gdp or gnp or gross W0 domestic or gross W0 national)) OR AB (low* N0 (gdp or gnp or gross W0 domestic or gross W0 national)) | 77 |
| S60 | TI ((developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income) N0 (economy or economies)) OR AB ((developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income) N0 (economy or economies)) | 160 |
| S59 | TI ((developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income or underserved or under W0 served or deprived or poor*) N0 (countr* or nation* or population* or world)) OR AB ((developing or less* W0 developed or under W0 developed or underdeveloped or middle W0 income or low* W0 income or underserved or under W0 served or deprived or poor*) N0 (countr* or nation* or population* or world)) | 37,503 |
| S58 | TX (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or "burkina faso" or "burkina fasso" or "upper volta" or burundi or urundi or cambodia or "khmer republic" or kampuchea or cameroon or cameroons or cameron or camerons or "cape verde" or "central african republic" or chad or chile or china or colombia or comoros or "comoro islands" or comores or mayotte or congo or zaire or "costa rica" or "cote d'ivoire" or "ivory coast" or croatia or cuba or cyprus or czechoslovakia or "czech republic" or slovakia or "slovak republic" or djibouti or "french somaliland" or dominica or "dominican republic" or "east timor" or "east timur" or "timor leste" or ecuador or egypt or "united arab republic" or "el salvador" or eritrea or estonia or ethiopia or fiji or gabon or "gabonese republic" or gambia or gaza or "georgia republic" or "georgian republic" or ghana or "gold coast" or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or "isle of man" or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or "kyrgyz republic" or kirghiz or kirgizstan or "lao pdr" or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or "malagasy republic" or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or "marshall islands" or mauritania or mauritius or "agalega islands" or mexico or micronesia or "middle east" or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or "netherlands antilles" or "new caledonia" or nicaragua or niger or nigeria or "northern mariana islands" or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or "puerto rico" or romania or rumania or roumania or russia or russian or rwanda or ruanda or "saint kitts" or "st kitts" or nevis or "saint lucia" or "st lucia" or "saint vincent" or "st vincent" or grenadines or samoa or "samoan islands" or "navigator island" or "navigator islands" or "sao tome" or "saudi arabia" or senegal or serbia or montenegro or seychelles or "sierra leone" or slovenia or "sri lanka" or ceylon or "solomon islands" or somalia or "south africa" or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tadjikistan or tadzhik or tanzania or thailand or togo or "togolese republic" or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or "soviet union" or "union of soviet socialist republics" or uzbekistan or uzbek or vanuatu or "new hebrides" or venezuela or vietnam or "viet nam" or "west bank" or yemen or yugoslavia or zambia or zimbabwe or rhodesia) | 1,337,490 |
| S57 | TX (africa or asia or caribbean or "west indies" or "south america" or "latin america" or "central america") | 333,213 |
| S56 | (MH "Developing Countries") OR (MH "Low and Middle Income Countries") | 21,605 |
| S55 | S48 AND S54 | 25,349 |
| S54 | S49 OR S50 OR S51 OR S52 OR S53 | 520,819 |
| S53 | (MH "Random Assignment") | 71,422 |
| S52 | (MH "Clinical Trials+") | 328,986 |
| S51 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 346,006 |
| S50 | PT clinical trial | 109,766 |
| S49 | PT randomized controlled trial | 136,876 |
| S48 | S26 AND S47 | 257,105 |
| S47 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 | 1,207,026 |
| S46 | TI (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice W0 nurs* or district W0 nurs* or health W0 visitor*) OR AB (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice W0 nurs* or district W0 nurs* or health W0 visitor*) | 83,623 |
| S45 | TI (paraprofessional* or para‐professional* or allied W0 health* N0 (professional* or person* or staff or worker*) or non‐physician* or non‐clinician*) OR AB (paraprofessional* or para‐professional* or allied W0 health* N0 (professional* or person* or staff or worker*) or non‐physician* or non‐clinician*) | 3,76 |
| S44 | TI (lay N2 (heal* or person* or counsellor* or counselor* or worker* or therapist*)) OR AB (lay N2 (heal* or person* or counsellor* or counselor* or worker* or therapist*)) | 1,514 |
| S43 | TI (caregiver* or care W0 giver* or layperson*) OR AB (caregiver* or care W0 giver* or layperson*) | 57,101 |
| S42 | TI (psycho‐social or psychosocial) OR AB (psycho‐social or psychosocial) | 56,955 |
| S41 | TI (non‐specialist* or nonspecialist* or social W0 worker* or trainer*) OR AB (non‐specialist* or nonspecialist* or social W0 worker* or trainer*) | 20,852 |
| S40 | TI (school* or teacher* or rural* or community) OR AB (school* or teacher* or rural* or community) | 471,133 |
| S39 | TI (family W0 practi* or family W0 doctor* or family W0 physician* or gp* or general W0 practi*) OR AB (family W0 practi* or family W0 doctor* or family W0 physician* or gp* or general W0 practi*) | 63,906 |
| S38 | TI (primary N5 (care or health*)) OR AB (primary N5 (care or health*)) | 106,395 |
| S37 | (MH "Community Health Nursing+") OR (MH "Family Nursing") OR (MH "School Health Nursing") OR (MH "Rural Health Nursing") | 42,825 |
| S36 | (MH "Rural Population") | 12,024 |
| S35 | (MH "Rural Health") | 6,975 |
| S34 | (MH "School Health Services+") | 23,122 |
| S33 | (MH "Schools+") | 78,766 |
| S32 | (MH "Community Health Services+") | 455,88 |
| S31 | (MH "Community Health Workers") | 3,93 |
| S30 | (MH "Support, Psychosocial+") | 93,192 |
| S29 | (MH "Family Practice") | 26,218 |
| S28 | (MH "Physicians, Family") | 21,698 |
| S27 | (MH "Primary Health Care") | 69,109 |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 947,144 |
| S25 | TI (mental* N0 develop*) OR AB (mental* N0 develop*) | 1,379 |
| S24 | TI (slow* N0 (thought* or think*)) OR AB (slow* N0 (thought* or think*)) | 48 |
| S23 | TI (anxiety W0 disorder* or agoraphobi* or general* W0 anxi* or separation W0 anxiety or neurocirculatory W0 asthenia or neurotic W0 disorder* or social W0 phobi* or self‐harm* or self‐injur* or suicid*) OR AB (anxiety W0 disorder* or agoraphobi* or general* W0 anxi* or separation W0 anxiety or neurocirculatory W0 asthenia or neurotic W0 disorder* or social W0 phobi* or self‐harm* or self‐injur* or suicid*) | 53,05 |
| S22 | TI (mental relapse or fatigue or somatic symptom* or worry or worries or panic or low mood* or mood problem*) OR AB (mental relapse or fatigue or somatic symptom* or worry or worries or panic or low mood* or mood problem*) | 59,087 |
| S21 | TI ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) N0 (symptom* or disorder* or condition* or depress* or anxiety)) OR AB ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) N0 (symptom* or disorder* or condition* or depress* or anxiety)) | 2,928 |
| S20 | TI ((mental or psychological or emotional or psycho‐social or psychosocial) N0 (stress* or distress*)) OR AB ((mental or psychological or emotional or psycho‐social or psychosocial) N0 (stress* or distress*)) | 25,362 |
| S19 | TI (affective* N0 (disorder* or disease* or illness* or symptom*)) OR AB (affective* N0 (disorder* or disease* or illness* or symptom*)) | 3,957 |
| S18 | TI (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress W0 reaction* or acute W0 stress or neuros#s or neurotic) OR AB (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress W0 reaction* or acute W0 stress or neuros#s or neurotic) | 11,114 |
| S17 | TI ((bipolar or behavio#ral or obsessive or panic or mood or delusional) N2 (disorder* or illness* or disease*)) OR AB ((bipolar or behavio#ral or obsessive or panic or mood or delusional) N2 (disorder* or illness* or disease*)) | 26,539 |
| S16 | TI ((dissociative N3 (disorder* or reaction*)) or dissociation) OR AB ((dissociative N3 (disorder* or reaction*)) or dissociation) | 5,365 |
| S15 | TI ((learning or mental* or intellectual) N0 (disabled or disabilit* or disorder* or difficult*)) OR AB ((learning or mental* or intellectual) N0 (disabled or disabilit* or disorder* or difficult*)) | 39,787 |
| S14 | TI (alcoholism or alcoholic* or drug addict* or drug abus* or drug misuse or drug user*) OR AB (alcoholism or alcoholic* or drug addict* or drug abus* or drug misuse or drug user*) | 34,602 |
| S13 | TI (psychological trauma or psychotrauma*) OR AB (psychological trauma or psychotrauma*) | 1,568 |
| S12 | TI ((post‐trauma* or posttrauma*) N3 (stress* or disorder*)) OR AB ((post‐trauma* or posttrauma*) N3 (stress* or disorder*)) | 18,072 |
| S11 | TI (depression or anxiety or alzheimer* or schizoaffective or mania or manic or borderline personality or (stress N2 disorder*) or adjustment disorder* or (psychological N1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome* or distress syndrome* or combat disorder* or war disorder* or ptsd or dementia) OR AB (depression or anxiety or alzheimer* or schizoaffective or mania or manic or borderline personality or (stress N2 disorder*) or adjustment disorder* or (psychological N1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome* or distress syndrome* or combat disorder* or war disorder* or ptsd or dementia) | 321,024 |
| S10 | TI ((depress* or distress*) N3 (postnatal* or post natal* or maternal*)) OR AB ((depress* or distress*) N3 (postnatal* or post natal* or maternal*)) | 6,25 |
| S9 | TI (depress* N3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth* or elder* or late* W0 life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)) OR AB (depress* N3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth* or elder* or late* W0 life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)) | 80,079 |
| S8 | TI (depressi* N2 (sign* or symptom* or disorder*)) OR AB (depressi* N2 (sign* or symptom* or disorder*)) | 61,293 |
| S7 | TI ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) N2 (disorder* or illness* or dependence or abuse or misuse or use)) OR AB ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) N2 (disorder* or illness* or dependence or abuse or misuse or "use")) | 88,244 |
| S6 | TI (mental W0 health* or mental* W0 ill* or mental* W0 disorder* or mental* W0 well*) OR AB (mental W0 health* or mental W0 illness* or mental W0 disorder* or mental* W0 well*) | 146,697 |
| S5 | (MH "Injuries, Self‐Inflicted") OR (MH "Self‐Injurious Behavior") OR (MH "Suicide+") OR (MH "Mania") | 39,532 |
| S4 | (MH "Child Development") | 24,674 |
| S3 | (MH "Mentally Disabled Persons") | 5,467 |
| Global Index Medicus, WHO (pesquisa.bvsalud.org/gim/advanced/?lang=en) (searched 29 November 2021) |
| "substance related disorder" OR "substance related disorders" OR "substance abuse" OR "alcohol abuse" OR "alcohol dependence" OR "alcohol related" OR depressi* OR anxiety OR schizophrenia OR psychoses OR psychosis OR "stress syndrome" OR "distress syndrome" OR "combat disorder" OR "war disorder" OR "posttrauma stress" OR "post‐trauma stress" OR "post‐traumatic stress" OR ptsd OR dementia OR alcoholism OR alcoholic OR "drug addict" OR "drug abuse" OR "drug abuser" OR "drug misuse" OR "drug user" OR "drug users" OR "learning disabled" OR "learning disability" OR "learning disabilities" OR "learning disorder" OR "learning disorders" OR "learning difficulty" OR "learning difficulties" OR "mental disabled" OR "mental disability" OR "mental disabilities" OR "mental disorder" OR "mental disorders" OR "mental difficulty" OR "mental difficulties" OR "mentally disabled" OR "intellectual disability" OR "intellectual disabilities" OR "intellectual disorder" OR "intellectual disorders" OR "intellectual difficulty" OR "intellectual difficulties" OR "intellectually disabled" OR "mental health" |
| AND |
| "primary health" OR "primary care" OR "primary healthcare" OR "community" OR school* OR teacher* OR rural OR "psycho‐social" OR psychosocial OR caregiver* OR paraprofessional* OR "lay counsellor" OR "lay counselor" OR "lay worker" OR "lay therapist" OR "lay counsellors" OR "lay counselors" OR "lay workers" OR "lay therapists" OR "general practice" OR "family practice" OR "midwife" OR "midwives" OR "health visitor" OR "social worker" |
| AND: Filter: Type of study = controlled clinical trial |
| APA PsycInfo 1806 to November Week 3 2021, Ovid (searched 29 November 2021) | ||
| 1 | exp mental disorders/ | 908850 |
| 2 | exp mental health/ | 75438 |
| 3 | depression (emotion)/ | 26262 |
| 4 | childhood development/ | 74246 |
| 5 | exp intellectual development disorder/ | 46321 |
| 6 | exp learning disorders/ | 34855 |
| 7 | exp learning disabilities/ | 28224 |
| 8 | exp self‐injurious behavior/ | 6715 |
| 9 | exp suicide/ | 37065 |
| 10 | (mental health* or mental* ill* or mental* disorder* or mental* well*).ti,ab. | 272692 |
| 11 | ((substance or alcohol or opioid or morphine or marijuana or heroin or cocaine) adj2 (disorder? or illness* or dependence or abuse or misuse or "use")).ti,ab. | 130645 |
| 12 | (depressi* adj2 (sign* or symptom* or disorder?)).ti,ab. | 108789 |
| 13 | (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or child* or adolesc* or teen* or youth? or elder* or late* life* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ti,ab. | 157843 |
| 14 | ((depress* or distress*) adj3 (postnatal* or post natal* or maternal*)).ti,ab. | 8135 |
| 15 | (depression or anxiety or alzheimer? or schizoaffective or mania or manic or borderline personality or (stress adj2 disorder*) or adjustment disorder? or (psychological adj1 trauma*) or schizophrenia or psychoses or psychosis or stress syndrome? or distress syndrome? or combat disorder? or war disorder? or ptsd or dementia).ti,ab. | 642133 |
| 16 | ((post‐trauma* or posttrauma*) adj3 (stress* or disorder?)).ti,ab. | 43398 |
| 17 | (psychological trauma or psychotrauma*).ti,ab. | 2437 |
| 18 | (alcoholism or alcoholic? or drug addict* or drug abus* or drug misuse or drug user?).ti,ab. | 62843 |
| 19 | ((learning or mental* or intellectual) adj (disabled or disabilit* or disorder? or difficult*)).ti,ab. | 97811 |
| 20 | ((dissociative adj3 (disorder* or reaction*)) or dissociation).ti,ab. | 20113 |
| 21 | ((bipolar or behavio?ral or obsessive or panic or mood or delusional) adj2 (disorder? or illness* or disease?)).ti,ab. | 78839 |
| 22 | (trichotillomani* or OCD or obsess*‐compulsi* or GAD or stress reaction? or acute stress or neuros#s or neurotic).ti,ab. | 59043 |
| 23 | (affective* adj (disorder? or disease? or illness* or symptom?)).ti,ab. | 20020 |
| 24 | ((mental or psychological or emotional or psycho‐social or psychosocial) adj (stress* or distress*)).ti,ab. | 42403 |
| 25 | ((sub‐syndrom* or sub‐threshold or sub‐clinical or subsyndrom* or subthreshold or subclinical or minor or brief) adj (symptom* or disorder* or condition* or depress* or anxiety)).ti,ab. | 5964 |
| 26 | (mental relapse or fatigue or somatic symptom? or worry or worries or panic or low mood? or mood problem?).ti,ab. | 61878 |
| 27 | (anxiety disorder? or agoraphobi* or general* anxi* or separation anxiety or neurocirculatory asthenia or neurotic disorder? or social phobi* or self‐harm* or self‐injur* or suicid*).ti,ab. | 119411 |
| 28 | (slow* adj (thought? or think*)).ti,ab. | 63 |
| 29 | (mental* adj develop*).ti,ab. | 3157 |
| 30 | or/1‐29 | 1489025 |
| 31 | primary health care/ | 19539 |
| 32 | family physicians/ | 1578 |
| 33 | general practitioners/ | 6103 |
| 34 | exp allied health personnel/ | 6006 |
| 35 | social support/ | 39326 |
| 36 | exp community health/ | 6590 |
| 37 | exp community services/ | 50983 |
| 38 | exp school based intervention/ | 20377 |
| 39 | exp schools/ | 73792 |
| 40 | school nurses/ | 938 |
| 41 | rural environments/ | 19606 |
| 42 | (primary adj5 (care or health*)).ti,ab. | 42571 |
| 43 | (family practi* or family doctor* or family physician* or gp* or general practi*).ti,ab. | 33485 |
| 44 | (school* or teacher* or rural* or community).ti,ab. | 756618 |
| 45 | (non‐specialist* or nonspecialist* or social worker* or trainer?).ti,ab. | 33125 |
| 46 | (psycho‐social or psychosocial).ti,ab. | 89492 |
| 47 | (caregiver* or care giver? or layperson*).ti,ab. | 55205 |
| 48 | paraprofessional*.ti,ab. | 2351 |
| 49 | (lay adj2 (heal* or person* or counsellor? or counselor? or worker? or therapist?)).ti,ab. | 1572 |
| 50 | (paraprofessional? or para‐professional? or (allied health* adj (professional? or person* or staff or worker?)) or non‐physician? or non‐clinician?).ti,ab. | 3715 |
| 51 | (midwife or midwive* or pharmacist* or pharmacy or pharmacies or practice nurs* or district nurs* or health visitor?).ti,ab. | 12546 |
| 52 | or/31‐51 | 1018552 |
| 53 | 30 and 52 | 335446 |
| 54 | exp clinical trial/ | 13018 |
| 55 | random*.ti,ab. | 218898 |
| 56 | ((clinical or control*) adj3 trial*).ti,ab. | 83450 |
| 57 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 27904 |
| 58 | (volunteer* or control group or controls).ti,ab. | 262985 |
| 59 | placebo/ or placebo*.ti,ab. | 42325 |
| 60 | or/54‐59 | 493248 |
| 61 | 53 and 60 | 41334 |
| 62 | developing countries.sh. | 5915 |
| 63 | (africa or asia or caribbean or west indies or south america or latin america or central america).hw,ti,ab. | 37911 |
| 64 | (afghanistan or albania or algeria or angola or antigua or barbuda or argentina or armenia or armenian or aruba or azerbaijan or bahrain or bangladesh or barbados or benin or byelarus or byelorussian or belarus or belorussian or belorussia or belize or bhutan or bolivia or bosnia or herzegovina or hercegovina or botswana or brasil or brazil or bulgaria or burkina faso or burkina fasso or upper volta or burundi or urundi or cambodia or khmer republic or kampuchea or cameroon or cameroons or cameron or camerons or cape verde or central african republic or chad or chile or china or colombia or comoros or comoro islands or comores or mayotte or congo or zaire or costa rica or cote d'ivoire or ivory coast or croatia or cuba or cyprus or czechoslovakia or czech republic or slovakia or slovak republic or djibouti or french somaliland or dominica or dominican republic or east timor or east timur or timor leste or ecuador or egypt or united arab republic or el salvador or eritrea or estonia or ethiopia or fiji or gabon or gabonese republic or gambia or gaza or georgia republic or georgian republic or ghana or gold coast or greece or grenada or guatemala or guinea or guam or guiana or guyana or haiti or honduras or hungary or india or maldives or indonesia or iran or iraq or isle of man or jamaica or jordan or kazakhstan or kazakh or kenya or kiribati or korea or kosovo or kyrgyzstan or kirghizia or kyrgyz republic or kirghiz or kirgizstan or lao pdr or laos or latvia or lebanon or lesotho or basutoland or liberia or libya or lithuania or macedonia or madagascar or malagasy republic or malaysia or malaya or malay or sabah or sarawak or malawi or nyasaland or mali or malta or marshall islands or mauritania or mauritius or agalega islands or mexico or micronesia or middle east or moldova or moldovia or moldovian or mongolia or montenegro or morocco or ifni or mozambique or myanmar or myanma or burma or namibia or nepal or netherlands antilles or new caledonia or nicaragua or niger or nigeria or northern mariana islands or oman or muscat or pakistan or palau or palestine or panama or paraguay or peru or philippines or philipines or phillipines or phillippines or poland or portugal or puerto rico or romania or rumania or roumania or russia or russian or rwanda or ruanda or saint kitts or st kitts or nevis or saint lucia or st lucia or saint vincent or st vincent or grenadines or samoa or samoan islands or navigator island or navigator islands or sao tome or saudi arabia or senegal or serbia or montenegro or seychelles or sierra leone or slovenia or sri lanka or ceylon or solomon islands or somalia or south africa or sudan or suriname or surinam or swaziland or syria or tajikistan or tadzhikistan or tadjikistan or tadzhik or tanzania or thailand or togo or togolese republic or tonga or trinidad or tobago or tunisia or turkey or turkmenistan or turkmen or uganda or ukraine or uruguay or ussr or soviet union or union of soviet socialist republics or uzbekistan or uzbek or vanuatu or new hebrides or venezuela or vietnam or viet nam or west bank or yemen or yugoslavia or zambia or zimbabwe or rhodesia).hw,ti,ab. | 236477 |
| 65 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab. | 19953 |
| 66 | ((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab. | 424 |
| 67 | (low* adj (gdp or gnp or gross domestic or gross national)).ti,ab. | 48 |
| 68 | (low adj3 middle adj3 countr*).ti,ab. | 4038 |
| 69 | (lmic or lmics or third world or lami countr*).ti,ab. | 2215 |
| 70 | transitional countr*.ti,ab. | 66 |
| 71 | or/62‐70 | 262608 |
| 72 | 61 and 71 | 3565 |
| ClinicalTrials.gov, US National Institutes of Health (www.clinicaltrials.gov) (searched 29 November 2021) | |
| Condition | "mental health" OR "mental illness" OR "mental disorder" |
| AND | |
| Intervention | school OR psychosocial OR lay OR non‐specialist OR teacher OR paraprofessional OR community‐based OR "community mental health" OR "community worker" OR "primary care" OR "general practice" OR "family practice" |
| Limited to | Interventional Studies | First posted from 08/20/2020 to 11/26/2021 |
| WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) (www.who.int/ictrp) (searched 29 November 2021) | |
| Advanced search: | |
| CONDITION | (mental health OR mental illness OR mental disorder) |
| AND | |
| INTERVENTION | (non specialist OR non specialists OR community based OR community worker OR ommunity workers OR primary care OR primary health care) |
| Limited to | Recruitment status: All Date of registration between: 01/08/2020 and 29/11/2021 |
Appendix 2. References to studies for the classification of PWs according to comparisons
Appendix. References to studies for the classification of PWs according to comparisons
Comparison 1. Lay health worker or community worker‐led universal prevention or promotion interventions for adults (n = 11 studies)
|
PC health worker (PHW) [n = 4 studies] |
Community worker (CW) [n = 7 studies] |
| Duan 2019; Rockers 2018; Yusoff 2015; Zhou 2010 | Baker‐Henningham 2019; Bell 2008; George 2020; Hendriks 2019; Hirani 2018; Jewkes 2008; Latina 2019 |
Comparison 2. Lay health worker or community worker‐led selective prevention interventions for adults (n = 20 studies)
|
PC health worker (PHW) [n = 10 studies] |
Community worker (CW) [n = 10 studies] |
| Barnes 2019; Cerquera Córdoba 2021; Dias 2008; Langer 1996; Li 2019; Ozcan 2020; Rahman 2009; Ramezani 2017; Vargas‐Porras 2021; Yang 2022 | Baker‐Henningham 2005; Chattha 2008; Hamdani 2021a; Hirani 2010; Miller 2020; Rachasrimuang 2018; Rahimi 2021a; Sanfilippo 2020; Tripathy 2010 |
*For one study (McCann 2015), it was not specified (NA)
Comparison 3. Lay health worker or community worker‐led indicated prevention interventions for adults (n = 42 studies)
|
PC health worker (PHW) [n = 18 studies]* |
Community worker (CW) [n = 25 studies]* |
| Hajarian Abhari 2021; Asnani 2021; Chaharrahifard 2021; Chang 2015; Cheng 2021; Chew 2018; Dayhimi 2020; Duru Aşiret 2021; Escolar 2014; Gao 2015; Gavrilova 2009; Jiang 2021; Novelli 2018; Rajeswari 2020; Rong 2021b; Song 2019; Srisuwan 2020; Xu 2021 | Acarturk 2022; Baumgartner 2021; Bernardi 2020; Chang 2015; Chomat 2019; Cooper 2009; Dias 2019; Dybdahl 2001; Eloff 2014; Ferreira‐Vorkapic 2018; Hinton 2021; Lachman 2017; Lachman 2020; Luoto 2020; Rao 2017; Rodriguez 2021; Rong 2021a; Rotheram‐Borus 2014a; Rotheram‐Borus 2014b; Sangraula 2020; Sherman 2009; Singla 2015; Skar 2021; Ward 2020; Yeomans 2010 |
*For one study (Chang 2015), intervention was delivered by both types of PWs.
Comparison 4. Lay health worker or community worker‐led universal prevention or promotion interventions for children (n = 8 studies)
|
PC health worker (PHW) [n = 1 study]* |
Community worker (CW) [n = 8 studies]* |
| Mohamadi 2021 | Amador Buenabad 2020; Barbosa Filho 2017; Berger 2018; Devries 2015; Huang 2017; Mohamadi 2021; Rivet‐Duval 2011; Velásquez 2015 |
*For one study (Mohamadi 2021), the intervention was delivered by both types of PWs.
Comparison 5. Lay health worker or community worker‐led selective prevention interventions for children (n = 7 studies)
|
PC health worker (PHW) [n = 1 study] |
Community worker (CW) [n = 6 studies] |
| Tobias da Silva 2017 | Ager 2011; Annan 2017; Dhital 2019; Fabbri 2021; O’Callaghan 2014; Richards 2014 |
Comparison 6. Lay health worker or community worker‐led indicated prevention interventions for children (n = 9 studies)
|
PC health worker (PHW) [n = 1 study]* |
Community worker (CW) [n = 7 studies]* |
| Thurman 2017 | Fine 2021; Hull 2021; Jordans 2010; Leventhal 2015; Shinde 2018; Tol 2012; Yu 2002 |
*For one study (Osborn 2020), it was not specified (NA).
CW: community worker PHW: primary‐level health worker PW: primary‐level worker
Data and analyses
Comparison 1. Promotion/universal prevention interventions versus control group in preventing mental disorders in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Diagnosis of mental disorders at 1‐6 months | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.18] |
| 1.2 Diagnosis of mental disorders at 7‐24 months | 1 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.05] |
| 1.3 Quality of life at 0‐1 months | 4 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.51, 0.04] |
| 1.4 Quality of life at 7‐24 months | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐7.30 [‐12.28, ‐2.32] |
| 1.5 Depressive symptoms at 0‐1 months | 3 | 349 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.78, 0.15] |
| 1.6 Depressive symptoms at 1‐6 months | 1 | 153 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐3.28, 0.78] |
| 1.7 Depressive symptoms at 7‐24 months | 2 | 1207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.02] |
| 1.8 Anxiety symptoms at 0‐1 months | 1 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.27, ‐0.01] |
| 1.9 Distress/PTSD symptoms at 0‐1 months | 4 | 722 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.41, ‐0.08] |
| 1.10 Social outcomes at 0‐1 months | 1 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.82, ‐0.08] |
Comparison 2. Selective prevention intervention versus control group in preventing mental disorders in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Diagnosis of mental disorders at 7‐24 months | 1 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.17, 19.82] |
| 2.2 Quality of life at 0‐1 months | 3 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐2.97, ‐0.31] |
| 2.3 Quality of life at 1‐6 months | 5 | 798 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.84, ‐0.26] |
| 2.4 Depressive symptoms at 0‐1 months | 4 | 223 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.08, ‐0.30] |
| 2.5 Depressive symptoms at 1‐6 months | 3 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.00, ‐0.21] |
| 2.6 Anxiety symptoms at 1‐6 months | 1 | 2026 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.87, 0.27] |
| 2.7 Distress/PTSD symptoms at 0‐1 months | 7 | 535 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.44, ‐0.36] |
| 2.8 Distress/PTSD symptoms at 1‐6 months | 5 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.21, ‐0.12] |
| 2.9 Distress/PTSD symptoms at 7‐24 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 8.80 [‐3.55, 21.15] |
| 2.10 Social outcomes at 0‐1 months | 3 | 121 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐15.29, ‐3.70] |
| 2.11 Social outcomes at 1‐6 months | 2 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐2.56, 0.80] |
| 2.12 Social outcomes at 7‐24 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐15.70 [‐28.35, ‐3.05] |
Comparison 3. Indicated prevention intervention versus control group in preventing mental disorders in adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Diagnosis of mental disorders at 0‐1 months | 3 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.57] |
| 3.2 Diagnosis of mental disorders at 1‐6 months | 6 | 1352 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.84] |
| 3.3 Diagnosis of mental disorders at 7‐24 months | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.19] |
| 3.4 Quality of life at 0‐1 months | 8 | 1136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.61, ‐0.12] |
| 3.5 Quality of life at 1‐6 months | 4 | 847 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.23, 0.16] |
| 3.6 Quality of life at 7‐24 months | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐3.53, 1.93] |
| 3.7 Adverse events at 0‐1 months | 1 | 547 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.8 Psychological functioning and impairment at 0‐1 months | 4 | 663 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.39, 0.15] |
| 3.9 Psychological functioning and impairment at 1‐6 months | 2 | 594 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.60, 0.41] |
| 3.10 Psychological functioning and impairment at 7‐24 months | 2 | 241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.47, 0.04] |
| 3.11 Depressive symptoms at 0‐1 months | 18 | 2341 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.03] |
| 3.12 Depressive symptoms at 1‐6 months | 11 | 2609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 3.13 Depressive symptoms at 7‐24 months | 8 | 2149 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.22, 0.01] |
| 3.14 Anxiety symptoms at 0‐1 months | 5 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.02, ‐0.35] |
| 3.15 Anxiety symptoms at 1‐6 months | 4 | 771 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.37, ‐0.09] |
| 3.16 Anxiety symptoms at 7‐24 months | 2 | 549 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 3.17 Distress/PTSD symptoms at 0‐1 months | 19 | 2536 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.95, ‐0.14] |
| 3.18 Distress/PTSD symptoms at 1‐6 months | 9 | 1702 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 3.19 Distress/PTSD symptoms at 7‐24 months | 5 | 1081 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.45, 0.38] |
| 3.20 Social outcomes at 0‐1 months | 5 | 932 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.26, 0.09] |
| 3.21 Social outcomes at 7‐24 months | 1 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.88, 1.50] |
Comparison 4. Promotion/universal prevention interventions versus control group in preventing mental disorders in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Quality of life at 0‐1 months | 2 | 803 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.39, ‐0.11] |
| 4.2 Adverse events at 0‐1 months | 1 | 694 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.3 Psychological functioning and impairment at 0‐1 months | 2 | 212 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.90, 0.98] |
| 4.4 Psychological functioning and impairment at 1‐6 months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.93, 0.35] |
| 4.5 Psychological functioning and impairment at 7‐24 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐5.03, ‐1.63] |
| 4.6 Depressive symptoms at 0‐1 months | 1 | 160 | Mean Difference (IV, Random, 95% CI) | ‐3.04 [‐6.00, ‐0.08] |
| 4.7 Depressive symptoms at 1‐6 months | 3 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.20, 0.20] |
| 4.8 Anxiety symptoms at 0‐1 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.13, ‐1.41] |
| 4.9 Anxiety symptoms at 1‐6 months | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 4.10 Anxiety symptoms at 7‐24 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.10, ‐1.44] |
| 4.11 Distress/PTSD symptoms at 0‐1 months | 2 | 800 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐2.48, 0.82] |
| 4.12 Distress/PTSD symptoms at 1‐6 months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐4.51 [‐5.86, ‐3.16] |
| 4.13 Social outcomes at 0‐1 months | 3 | 321 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.76, 0.12] |
| 4.14 Social outcomes at 1‐6 months | 2 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.82, 0.28] |
| 4.15 Social outcomes at 7‐24 months | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.07, ‐0.33] |
Comparison 5. Selective prevention intervention versus control group in preventing mental disorders in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Quality of life at 0‐1 months | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐3.32, 1.12] |
| 5.2 Psychological functioning and impairment at 0‐1 months | 1 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 5.3 Depressive symptoms at 0‐1 months | 2 | 638 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.16, 0.15] |
| 5.4 Depressive symptoms at 1‐6 months | 3 | 791 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.12, 0.39] |
| 5.5 Depressive symptoms at 7‐24 months | 1 | 435 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.76, 1.20] |
| 5.6 Anxiety symptoms at 0‐1 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐12.05, 21.05] |
| 5.7 Anxiety symptoms at 1‐6 months | 1 | 143 | Mean Difference (IV, Random, 95% CI) | 1.42 [‐0.00, 2.84] |
| 5.8 Distress/PTSD symptoms at 0‐1 months | 1 | 159 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐3.77, ‐0.51] |
| 5.9 Distress/PTSD symptoms at 1‐6 months | 1 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.49, 1.69] |
| 5.10 Social outcomes at 0‐1 months | 2 | 638 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.42, 0.48] |
Comparison 6. Indicated prevention intervention versus control group in preventing mental disorders in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Diagnosis of mental disorders at 0‐1 months | 1 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.17] |
| 6.2 Diagnosis of mental disorders at 1 to 6 months | 1 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |
| 6.3 Quality of life at 0‐1 months | 2 | 152 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.09, 0.79] |
| 6.4 Psychological functioning and impairment at 0‐1 months | 2 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.10] |
| 6.5 Psychological functioning and impairment at 1‐6 months | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.13, 0.27] |
| 6.6 Depressive symptoms at 0‐1 months | 4 | 771 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.32, ‐0.04] |
| 6.7 Depressive symptoms at 1‐6 months | 6 | 2483 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.17, 0.03] |
| 6.8 Depressive symptoms at 7‐24 months | 1 | 904 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐1.90, ‐0.64] |
| 6.9 Anxiety symptoms at 0‐1 months | 3 | 888 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.22, 0.04] |
| 6.10 Anxiety symptoms at 1‐6 months | 3 | 1362 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.15, 0.08] |
| 6.11 Distress/PTSD symptoms at 0‐1 months | 2 | 448 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.28, 1.76] |
| 6.12 Distress/PTSD symptoms at 1‐6 months | 2 | 417 | Mean Difference (IV, Random, 95% CI) | 0.96 [‐0.55, 2.47] |
| 6.13 Social outcomes at 0‐1 months | 2 | 435 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.15, 0.46] |
| 6.14 Social outcomes at 1‐6 months | 2 | 421 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.41, ‐0.03] |
Comparison 7. Sensitivity analysis—excluding studies that might generate high heterogeneity (I² > 75%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Diagnosis of mental disorders at 0‐1 months—indicated prevention adults | 2 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.05, 0.33] |
| 7.2 Quality of life at 0‐1 months—selective prevention adults | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.26, ‐0.06] |
| 7.3 Quality of life at 1‐6 months—selective prevention adults | 3 | 634 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.05] |
7.1. Analysis.

Comparison 7: Sensitivity analysis—excluding studies that might generate high heterogeneity (I² > 75%), Outcome 1: Diagnosis of mental disorders at 0‐1 months—indicated prevention adults
7.2. Analysis.

Comparison 7: Sensitivity analysis—excluding studies that might generate high heterogeneity (I² > 75%), Outcome 2: Quality of life at 0‐1 months—selective prevention adults
7.3. Analysis.

Comparison 7: Sensitivity analysis—excluding studies that might generate high heterogeneity (I² > 75%), Outcome 3: Quality of life at 1‐6 months—selective prevention adults
Comparison 8. Sensitivity analysis—excluding studies with high risk of bias and some concerns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Psychological functioning and impairment at 0‐1 months—indicated prevention adults | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.77, ‐0.05] |
| 8.2 Depressive symptoms at 0‐1 months—indicated prevention adults | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.62, 0.23] |
| 8.3 Distress/PTSD symptoms at 0‐1 months—indicated prevention adults | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.13, 0.84] |
8.1. Analysis.

Comparison 8: Sensitivity analysis—excluding studies with high risk of bias and some concerns, Outcome 1: Psychological functioning and impairment at 0‐1 months—indicated prevention adults
8.2. Analysis.

Comparison 8: Sensitivity analysis—excluding studies with high risk of bias and some concerns, Outcome 2: Depressive symptoms at 0‐1 months—indicated prevention adults
8.3. Analysis.

Comparison 8: Sensitivity analysis—excluding studies with high risk of bias and some concerns, Outcome 3: Distress/PTSD symptoms at 0‐1 months—indicated prevention adults
Comparison 9. Subgroup analysis—category of health worker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Depressive symptoms at 0‐1 months—indicated prevention adults | 18 | 2341 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.03] |
| 9.1.1 Community workers | 12 | 1775 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.21, 0.08] |
| 9.1.2 Primary healthcare workers | 6 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.62, ‐0.08] |
| 9.2 Depressive symptoms at 1‐6 months—indicated prevention adults | 11 | 2609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 9.2.1 Community workers | 5 | 1463 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.34, ‐0.14] |
| 9.2.2 Primary healthcare workers | 6 | 1146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.95, 0.08] |
| 9.3 Distress/PTSD symptoms at 0‐1 months—indicated prevention adults | 19 | 2536 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.95, ‐0.14] |
| 9.3.1 Community workers | 14 | 1906 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.39, 0.00] |
| 9.3.2 Primary healthcare workers | 5 | 630 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.90, 0.23] |
9.1. Analysis.

Comparison 9: Subgroup analysis—category of health worker, Outcome 1: Depressive symptoms at 0‐1 months—indicated prevention adults
9.2. Analysis.

Comparison 9: Subgroup analysis—category of health worker, Outcome 2: Depressive symptoms at 1‐6 months—indicated prevention adults
9.3. Analysis.

Comparison 9: Subgroup analysis—category of health worker, Outcome 3: Distress/PTSD symptoms at 0‐1 months—indicated prevention adults
Comparison 10. Subgroup analysis—setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Depressive symptoms at 0‐1 months—indicated prevention adults | 18 | 2341 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.03] |
| 10.1.1 Community setting | 8 | 1278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.43, 0.09] |
| 10.1.2 Other | 9 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.31, ‐0.03] |
| 10.1.3 Refugee camp | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.56, 0.34] |
| 10.2 Depressive symptoms at 1‐6 months—indicated prevention adults | 11 | 2609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 10.2.1 Community setting | 4 | 1458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.37, ‐0.16] |
| 10.2.2 Other | 6 | 1005 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.94, 0.14] |
| 10.2.3 School | 1 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.72, ‐0.06] |
| 10.3 Distress/PTSD symptoms at 0‐1 months—indicated prevention adults | 19 | 2536 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.95, ‐0.14] |
| 10.3.1 Community setting | 6 | 1033 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.23, 0.16] |
| 10.3.2 Other | 11 | 1367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.57, ‐0.08] |
| 10.3.3 Refugee camp | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.73, 0.17] |
| 10.3.4 School | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.04, ‐0.01] |
10.1. Analysis.

Comparison 10: Subgroup analysis—setting, Outcome 1: Depressive symptoms at 0‐1 months—indicated prevention adults
10.2. Analysis.

Comparison 10: Subgroup analysis—setting, Outcome 2: Depressive symptoms at 1‐6 months—indicated prevention adults
10.3. Analysis.

Comparison 10: Subgroup analysis—setting, Outcome 3: Distress/PTSD symptoms at 0‐1 months—indicated prevention adults
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acarturk 2022.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from 2018 to 2020. Country: Turkey Income classification: upper‐middle‐income country in 2018‐2020 Geographical scope: Istanbul and Mardin, Turkey Healthcare setting: community groups |
| Participants | 1. Age: mean (SD) 31.5 (9.0) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: for 61.8% of participants, primary school was the highest level of education, while 14.5% received an academic education. Inclusion criteria a. aged 18 years or older; b. able to speak and understand Arabic; c. being under temporary protection according to Law on Foreigners and International Protection; d. experiencing psychological distress, as shown by a score of 3 or more on the 12‐item dichotomously scored General Health Questionnaire; e. having completed oral and written informed consent to enter the study. Exclusion criteria a. presence of any mental disorder according to the Mini International Neuropsychiatric Interview (MINI); b. evidence of acute medical conditions contraindicating study participation; c. evidence of imminent suicide risk or suicide risk scored as “moderate or high” on the MINI; d. signs of impaired decision‐making capacity emerging from responses during the clinical interview. Refugees who were excluded because of a diagnosis of a mental disorder and/or imminent suicide risk were referred for treatment to a health professional. Note: at baseline, the intervention and control group scores for General Health Questionnaire (GHQ‐12) were, respectively, 17.363 (4.519) and 16.776 (4.299). Stated purpose: to test the effectiveness of a self‐help psychological intervention developed by the World Health Organization, called Self‐Help Plus, in preventing the development of mental disorders among Syrian refugees experiencing psychological distress in Turkey |
| Interventions |
Name: Self Help Plus (SH+) Title/name of PW and number: facilitators (2 for each group session) 1. Selection: nonspecialist facilitators 2. Educational background: no specialist mental health training 3. Training: received training on the SH+ WHO manual 4. Supervision: followed close supervision following the SH+ manual 5. Incentives/remuneration: not specified Prevention type: indicated prevention – participants presented some level of distress as indicated by GHQ scores, but all those who screened positive to the MINI were excluded. Intervention details: the SH+ intervention consists of a pre‐recorded audio course, delivered by trained facilitators in a group setting and complemented with an illustrated self‐help book adapted for the target cultural group. The intervention is based on acceptance and commitment therapy, a form of cognitive behavioural therapy. It is delivered across five 2‐hour sessions. The audio material imparts key information about stress management and guides participants through individual exercises and small group discussions. The self‐help book reviews all essential content and concepts. In this study, a version of the intervention previously adapted for Syrian populations was used. Control: usual care (enhanced) – enhanced care as usual was provided to participants in both groups and consisted of routinely delivered social support and/or care. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1 to 6 months) |
| Notes |
Source of funding: European Commission (grant agreement no. 779255) Notes on validation of instruments (screening and outcomes): all outcome measures were selected as being previously validated in the context of the application. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03587896 |
Ager 2011.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in the 2007‐2009 school year. Country: Uganda Income classification: low‐income country in 2007‐2009 Geographical scope: Gulu and Amuru districts where, during the conflict, internally displaced people (IDP) camps arose Healthcare setting: schools (primary) |
| Participants | 1. Age: 7‐12 years 2. Gender: both 3. Socioeconomic background: disrupted economic and social development due to conflict 4. Educational background: children attending primary schools Inclusion criteria a. all children attending school; b. in selecting the initial grade level for intervention, teachers were encouraged to consider the number of children in that year group who met certain criteria, such as those who isolated themselves, were frequently absent, seemed particularly stressed, had low self‐esteem, and/or had a violent family background. Exclusion criteria not specified Note: considerations on baseline scores not applicable for this study Stated purpose: evaluating the impact of the PSSA intervention as its implementation was scaled up in Gulu and Amuru Districts from 2007 to 2009 |
| Interventions |
Name: Psychosocial Structured Activities (PSSA) intervention Title/name of PW and number: teachers 1. Selection: not specified. Data were available for the intervention group for 176 teachers at baseline and 98 at follow‐up and in the control group for 173 at baseline and 107 at follow‐up. 2. Educational background: not specified 3. Training: Regular school teachers trained in the methodology in a residential workshop before the start of the school year. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective prevention – “all children who had grown up in the context of stress and conflict, not only formerly abducted children or those with identified psychological difficulties”. Intervention details: a school‐based, multiphased approach designed to aid trauma recovery. Composed of a) 15 structured teacher‐led sessions on safety and control, incorporating play therapy, drama, art, and movement; b) a teacher‐facilitated community service component to encourage helping the disadvantaged (e.g. sick and elderly); c) periodic meetings to encourage parental involvement by Save the Children Uganda. Control: waiting list |
| Outcomes |
Participants’ outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: reported but not specifically related to this trial Notes on validation of instruments (screening and outcomes): references for validation not reported Additional information: none Handling the data: no dataset available Prospective trial registration number: not reported |
Amador Buenabad 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2015‐2016. Country: Mexico City, Mexico Income classification: upper‐middle‐income country from 1990 Geographical scope: urban Healthcare setting: 4 urban public schools |
| Participants | 1. Age: caregivers 38.46 ± 8.56; children 8.75 ± 1.34 years 2. Gender: both 3. Socioeconomic background: caregivers had varied employment statuses. 4. Educational background: caregivers had completed, on average, 11.74 years of education (SD = 3.24); children attending 3rd, 4th or 5th year of primary school. Inclusion criteria—children: a. enrolment in 2nd, 4th or 5th grade in the selected elementary school; b. consent for participation by at least one legal guardian; c. assent from the child; d. participation by at least one primary caregiver, and complete pre‐test. Inclusion criteria—caregivers: a. 18 years of age or older; b. spend time with the child on a regular basis; c. agree to participate and complete pre‐test. Exclusion criteria not reported Note: at baseline, the intervention and control group scores for the Externalizing Subscale of the Child Behavior Checklist (CBCL) were, respectively, 57.6 (10.45) and 52.75 (7.90). Stated purpose: to evaluate the effectiveness of the interventions Dejando Huellitas en tu Vida (Leaving Traces on Your Life [Huellitas]) and Criando con Amor, Promoviendo Armonía y Superación en México (Raising Children with Love, Promoting Harmony and Self‐Improvement [CAPAS‐Mx]) |
| Interventions |
Name: Making trails in your life/Huellitas Title/name of PW and number: supervised trained teachers, each group of students having a responsible school teacher 1. Selection: not specified 2. Educational background: not specified 3. Training: the teachers were trained during 14 sessions (30 h) prior to starting the intervention based on an application manual. Three creators of the preventive model Huellitas provided the training (application manual (Gutiérrez, 2009). 4. Supervision: all interaction sessions were videotaped for review and to provide feedback to the teachers by programme managers. 5. Incentives/remuneration: the teachers who implemented the intervention received MX$150 (approximately US$8) at the end of each module. Caregivers were compensated with MX$100 (approximately US$5) for each completed evaluation. Children who completed evaluations received a pencil at pre‐test and a water bottle at post‐test. At the end of the post‐test, all the teachers in the selected groups received a notebook. Prevention type: universal – for all caregiver‐child dyads of students attending the selected grades of the included primary schools, specifically Huellitas “was designed to develop social skills in elementary‐age children using a universal, low‐cost approach”—CAPAS‐Mx “provides universal prevention and selective intervention for externalizing”. Baseline scores of the CBCL are within the normal range for the scale. Intervention details Huellitas intervention: a social skills programme for children, administered in 12 weekly sessions (60 minutes each). The main topics are (1) personal aspects of the child, such as expressing and respecting each other's emotions and opinions; (2) prevention of abuse and maltreatment; (3) their relationships in their school and family environments; (4) equity, nondiscrimination and self acceptance. CAPAS‐Mx: manualized intervention, consisting of 12 sessions that last approximately 1.5 h each. In order to promote skills development, parenting educators use an active teaching methodology (e.g. Role Play and Modelling). After each session, caregivers have an assignment to practice the skill that was learned at home, and between each session, a phone call is made by a parenting educator to follow up on the home practice assignment. When parents or caregivers missed a session, replacement sessions were provided. At the end of each session, participants completed a satisfaction questionnaire. In each school, parenting groups were offered on different days and at different times to maximize caregivers’ ability to attend groups. Multicomponent intervention (Huellitas and CAPAS‐MX): Huellitas and CAPAS‐Mx were delivered simultaneously, optimizing families’ time. Control: waiting list – both interventions were provided 3 months after the interventions were ended in the other experimental conditions. |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data only from the Huellitas intervention. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: this research was conducted with funding from the National Council for Science and Technology (CONACYT) of Mexico, through the PDCPN‐2014‐248428 project. Notes on validation of instruments (screening and outcomes): the CBCL is a widely adopted measure that has been validated across contexts; the social outcome scale was developed for the study. Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN11345846 |
Annan 2017.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: recruitment began in October 2011; data were collected between January 2012‐May 2013. Country: Thailand Income classification: lower‐middle‐income country in 2011‐2013 Geographical scope: in 20 rural, periurban, and urban sites in the northwestern province of Tak, Thailand, on the border with Burma Healthcare setting: schools (primary), multiple community spaces (e.g. schools and community halls) |
| Participants | 1. Age: children 7‐15; caregivers 18+ 2. Gender: both 3. Socioeconomic background: 33‐37% of caregivers unemployed, income: mean 4644‐5401 Thai Baht 4. Educational background: most caregivers had primary education or less; 27% of children were not in school. Inclusion criteria: a. inclusion criteria included being from Burma/Myanmar and a caregiver (biological or nonbiological) to a child between the ages of 8 and 12 years; b. participants could speak either Burmese or Karen. Families also were asked screening questions from the Multiple Indicator Cluster Survey to assess for ongoing severe violence; if responses indicated immediate concerns, IRC staff conducted home visits and coordinated more intensive services as needed but did not exclude them from the study. Exclusion criteria: A few families were excluded from the study analyses for other concerns related to the validity of survey data but remained enrolled and were included in the intervention. Stated purpose: prevention (ClinicalTrials.gov); improvement in mental health outcomes of children affected by forced migration |
| Interventions |
Name: Happy Families Program (HFP) Title/name of PW and number: community workers (IRC team members and community‐based facilitators). The total number is not specified but the delivery was carried out by teams in each site, including 2 facilitators for the caregiver and 2 for the child group. 1. Selection: facilitators recruited from the local communities underwent interviews and reference checks prior to selection. 2. Educational background: not specified 3. Training: facilitators were trained to deliver intervention content using principles of social learning theory; training was 5 days long and took place in 2011 for IRC staff—no specifications on training timeline for community facilitators. 4. Supervision: monitoring visits to programme sites every 2 to 3 weeks to monitor facilitator performance using a standardized checklist and to provide tailored supervision and coaching by IFRC staff 5. Incentives/remuneration: not specified Prevention type: selective prevention – participants were included based upon the presence of a risk factor (children experiencing displacement and poverty). Intervention details: parenting and family skills intervention, consisting of 14 weekly sessions in which caregivers learn parenting skills and children learn social skills in separate groups and join together in the second hour for in vivo practice and feedback from facilitators, as well as positive family interaction through structured and unstructured play Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: USAID displaced children and orphan fund Notes on validation of instruments (screening and outcomes): authors used the CBCL which is widely validated. The Child Psychosocial Protective Factors Scale was developed for the study. Additional information: none Handling the data: no dataset available Prospective trial registration number: NCT01668992 |
Arango 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: not specified Country: Colombia Income classification: upper‐middle‐income country since 2008 Geographical scope: Cali Healthcare setting: community groups |
| Participants | 1. Age: intervention 59.4 + 10.8, control 55.1 + 11.2 years 2. Gender: both 3. Socioeconomic background: most participants were in the higher bound of the income classification. 4. Educational background: not specified Inclusion criteria: Caregivers related to the person with dementia a. were the primary caregiver of that person; b. had been providing care for at least 3 months; c. were knowledgeable about the patient’s family and medical history; and d. had no self‐reported history of neurological and psychiatric disorders or learning disabilities. Exclusion criteria: none specified Note: considerations on baseline scores not applicable for this study Stated purpose: to examine the effectiveness of a group cognitive‐behavioural intervention in improving the mental health of dementia caregivers |
| Interventions |
Name: coping with frustration Title/name of PW and number: local outreach workers (not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective prevention – participants were included based upon the presence of a risk factor (being caregivers of people affected by dementia with no history of psychiatric disorder). Intervention details: the goal of this 8‐week intervention is to introduce family caregivers to a variety of cognitive‐behavioural strategies that they can use to manage negative feelings (e.g. anger and frustration) that arise within the context of caregiving. These cognitive‐behavioural strategies and skills include relaxation, identification and challenging of dysfunctional thoughts, the use of positive self‐statements, and assertiveness, which are taught within a structured classroom format in small groups ranging in size from 6 to 10 participants. Control: active control – an educational programme of equal duration (8 weeks) and time commitment (2 hours/week) as the experimental group. This educational programme was designed by the authors to include an attentional and educational component but not the experimental intervention’s practical application of cognitive‐behavioural stress management skills. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 1 to 6 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the selected outcomes were reported to have been validated for use in Spanish. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Asnani 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2015‐2016. Country: Jamaica Income classification: upper‐middle‐income country in 2015‐2016 Geographical scope: urban (Kingston) Healthcare setting: outpatient, sickle cell unit (SCU) |
| Participants | 1. Age: 28.8 (5.9) years 2. Gender: both 3. Socioeconomic background: 45% in the lowest third of household possession 4. Educational background: 71.4% secondary education Inclusion criteria: a. all mothers of children aged 6‐12 months with severe sickle cell disease (SCD) genotypes; b. attending the clinic at the SCU. Exclusion criteria: none reported Note: at baseline, the intervention and control group scores for the Center for Epidemiologic Studies Depression Scale (CES‐D) were, respectively, 15.9 (13.4) and 17.2 (10). Stated purpose: to assess the feasibility and potential efficacy of a problem‐solving skills training intervention, aimed at improving psychological outcomes in mothers of children with SCD |
| Interventions |
Name: Problem‐Solving Therapy (PST) Title/name of PW and number: SCS clinic nurses 1. Selection: experienced and providing routine clinical care 2. Educational background: no details available. 3. Training: PST training with the study investigators, delivered through 5 sessions conducted over 2 weeks and totalling 9 hours. It included discussions of the intervention, use of the interventionists’ manual, and role‐playing. 4. Supervision: to assess fidelity, a checklist was developed with items that measured the interventionists’ implementation of the various steps of the intervention. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented some level of distress as indicated by CES‐D scores. Intervention details: problem‐solving skills therapy aimed to empower patients or caregivers in attending to daily social and other challenges that might arise, especially with the presence of a chronic illness. The approach is based on cognitive behavioural therapy and was adapted from the “Bright IDEAS” intervention for mothers of children with cancer. The stages of PST are identification of the problems; generating possible solutions; evaluating and implementing the preferred solutions; and evaluating to see if the solutions were successful. The parent/caregiver will be taught a process of problem‐solving with reference to general everyday problems as well as specific problems which may arise while parenting a child with SCD. All mothers in the treatment arm also received an intervention to build their skills in promoting their child’s development that was delivered at the same session. The parenting skills intervention used videos, discussion, demonstration, and practice of activities, and has been used previously in Jamaica. Control: waiting list – attended individual routine clinic visits on days that the intervention groups were not being held. At the end of the study, control dyads were exposed to all intervention materials. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Grand Challenges Canada‐ Saving Brains Programme Notes on validation of instruments (screening and outcomes): none Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02394899 |
Attanasio 2014.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2010‐2011. Country: Colombia Income classification: upper‐middle‐income country in 2010‐2011 Geographical scope: 3 geographical regions proximal to Bogotá Healthcare setting: homes of the participants |
| Participants | 1. Age: children aged 12 to 24 months; mothers aged 27.63 (6.96) years in control, 28.34 (6.95) in simulation group, 27.50 (6.23) in supplementation, 27.92 (6.55) in both intervention groups 2. Gender: both for children, female for caregivers 3. Socioeconomic background: not specified 4. Educational background: mothers’ mean ages of education 7.70 (3.51) in control, 7.21 (3.41) in simulation group, 7.41 (3.53) in supplementation, 7.48 (3.43) in both intervention groups Inclusion criteria: families with children aged 12‐24 months Exclusion criteria: none reported Note: at baseline, the intervention and control group scores for Center for Epidemiologic Studies Depression Scale Short Version (CES‐D‐10) were in the range of 8.38 to 9.61 depending on group allocation. Stated purpose: to assess the effectiveness of an integrated early child development intervention, combining stimulation and micronutrient supplementation |
| Interventions |
Name: stimulation Title/name of PW and number: female community leaders 1. Selection: home visitors were selected from amongst the mother leaders of the communities. 2. Educational background: not specified 3. Training: they received training by 6 trained mentors (with an undergraduate degree in psychology social work or fieldwork experience with families and children). 4. Supervision: they received supervision by 6 trained mentors. 5. Incentives/remuneration: hired on a part‐time basis Prevention type: indicated – participants presented some level of distress as indicated by CES‐D scores. Intervention details Stimulation: the psychosocial stimulation programme was based on the Jamaican home visiting model, adapted for use in Colombia. Home visitors made weekly home visits where they demonstrated play activities using low‐cost or homemade toys, picture books, and form boards. These materials were left in the homes for the week after the visit and were changed weekly. The aims of the visits were to improve the quality of maternal‐child interactions and to assist mothers to participate in developmentally appropriate learning activities, many centred on daily routines. Supplementation: the micronutrient supplementation consisted of Sprinkles (Hexagon Nutrition, Mumbai, India) – encapsulated micronutrients in powder form – developed to treat childhood anaemia. Stimulation + supplementation: psychosocial stimulation and micronutrient supplementation Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (not available) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this study was funded by the Economic and Social Research Council (grant RES‐062‐23‐1548), Inter‐American Development Bank, World Bank, and International Growth Center. Notes on validation of instruments (screening and outcomes): the measure ES‐D is widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN18991160 |
Baker‐Henningham 2005.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: not specified Country: Jamaica Income classification: low‐middle‐income country from 1987 to 2005 Geographical scope: urban (Kingston, St Andrew, and St Catherine) Healthcare setting: homes |
| Participants | 1. Age: intervention group 26.0 (7.1) years, control group 25.7 (7.2) years 2. Gender: caregivers were females. 3. Socioeconomic background: (1) crowding, persons per room: 2.8 (1.3), 2.9 (1.4); (2) sanitation range 0‐12: 7.8 (2.8), 7.9 (2.6); (3) possessions, range 0‐10: 5.1 (1.9), 5.1 (1.7) 4. Educational background: % who completed high school: intervention group 43.8%, control group 37.7% Inclusion criteria—children: a. aged between 9 months and 30 months; b. weight for age currently below –1.5 z‐scores of the National Centre for Health Statistics (NCHS) references 15 and clinic records showing that they had been below –2 z‐scores in the last 3 months; c. birth weight greater than 1.8 kg; d. singleton birth; e. absence of chronic disease and/or obvious disability. Inclusion criteria—mothers: mother of children meeting the aforementioned inclusion criteria Exclusion criteria: none reported Note: at baseline, the intervention and control group scores for the Center for Epidemiological Studies Depression Scale (CES‐D) were, respectively, 5 (2.6) and 4.9 (2.2). Stated purpose: to determine the effect of early childhood stimulation with undernourished children and their mothers on maternal depression |
| Interventions |
Name: childhood stimulation and parenting practices Title/name of PW and number: community health aides (number not specified) 1. Selection: paraprofessionals employed in government health centres 2. Educational background: not specified 3. Training: 4 weeks of pre‐service training on health and nutrition, and we provided an additional 2 weeks of training covering child development, parenting issues, and how to conduct the intervention 4. Supervision: supervisor observed each aide conducting visits once a month and visited the health centre every fortnight to discuss the programme and review the records of each visit. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (being mothers of undernourished children) and presented baseline levels of distress well below the cutoff for the measure. Intervention details: The intervention focused on improving child development by improving mothers’ knowledge and practices of child‐rearing and their parenting self‐esteem. Community health aides visited the homes weekly for half an hour and demonstrated age‐appropriate activities for the child by involving both the mother and child in play. Home‐made toys and books and materials in the home were used to keep the intervention low cost. Parenting issues, including the importance of praise, attention, and responsiveness, appropriate discipline strategies, child nutrition, and how to promote children’s play and learning were also discussed. Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Thrasher Research Fund, USA, with subsidiary grants from the British High Commission–DFID, Jamaica and the University of the West Indies Mona Campus Research and Publication Fund. The Ministry of Health Jamaica supported the Community Health Aides. This work was undertaken in collaboration with Great Ormond Street Hospital for Children NHS Trust which receives a proportion of its funding from the NHS Executive. Notes on validation of instruments (screening and outcomes): the measure CES‐D is widely validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Baker‐Henningham 2019.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: not specified Country: Jamaica Income classification: upper‐middle‐income country between 2014 (year of ethical approval)‐2019 (year of publication) Geographical scope: urban (inner‐city areas of Kingston) Healthcare setting: schools |
| Participants | 1. Age: intervention group 39.5 (10.9), control group 43.2 (10.2) years 2. Gender: 100% of teachers were females. 3. Socioeconomic background: not specified 4. Educational background: 100% of teachers completed high school. Inclusion criteria: Schools were the principal, and all grade 1 teachers consented to participate in the trial. Exclusion criteria: Schools that: a. were attached to a teacher‐training college; b. had competitive entry; and/or c. had teachers who had participated in at least three rounds of a previous study. Note: at baseline, the intervention and control group scores for the Center for Epidemiologic Studies Depression Scale (CES‐D) were, respectively, (median, range) 9.0 (0 to 46) and 8.0 (0 to 48). Stated purpose: to investigate the effect of a school‐based violence prevention programme implemented in grade 1 classrooms in Jamaican primary schools |
| Interventions |
Name: violence prevention Title/name of PW and number: facilitators (2) 1. Selection: the junior facilitator was supervised by the senior facilitator, who supported her in the classroom once a month and met fortnightly to discuss the progress made by each teacher. 2. Educational background: not specified 3. Training: the two facilitators were trained and supported by the Principal Investigator. 4. Supervision: weekly supervision meetings 5. Incentives/remuneration: not specified Prevention type: universal – all participants were eligible for inclusion (all grade 1 teachers in the school), and their baseline scores for CES‐D were well below the cutoff for the measure. Intervention details: the intervention involved training grade 1 teachers in selected core content from the IRIE Classroom Toolbox. The selected content focused primarily on the use of positive and proactive strategies to promote children’s positive behavior and prevent negative behaviour (e.g. use of praise, teaching classroom rules). The key concepts introduced were (1) teaching rules and routines, (2) using praise in the classroom and paying attention to positive behaviour, (3) being proactive to prevent child behaviour problems, (4) promoting children's social‐emotional competence, (5) interactive storybook reading and (6) promoting children’s active participation in teaching and learning activities. Control: usual care |
| Outcomes |
Participants’ outcomes of interest for this review
Note: CES‐D data were not included; it was not yet available in the right format. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: this research was funded by GRAND CHALLENGES CANADA (Global Mental Health Stream), grant number 0592‐04. Notes on validation of instruments (screening and outcomes): Teacher Burnout Scale by Richmond 2001 was assessed through principal component factor analysis in this study. Additional information: none Handling the data: not applicable Prospective trial registration number: ISCTRN94883310 |
Barbosa Filho 2017.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study recruited participants in 2014; the results were first published in 2017. Country: Brazil Income classification: upper‐middle‐income country from 2014 to 2017 Geographical scope: 6 full‐time schools (all schools were in areas with a low Human Development Index—HDI, a composite index ranging from 0 to 1; the closest to 1 were those indicating that the neighbourhood was more developed, based on life expectancy, education level, and standard of living) of Fortaleza, in northeast Brazil Healthcare setting: schools |
| Participants | 1. Age: 11‐13 years (52.9 %; n. 574);14‐18 years (47.1 %; n. 511) 2. Gender: both 3. Socioeconomic background: higher SES (25.5 %; n 277); lower SES (73.9 %; n 802) 4. Educational background: elementary students (grades 7 to 9) Inclusion criteria: a. all 6 full‐time schools with the programme called Programa Saúde na Escola in Fortaleza were eligible; b. students of both sexes; c. students aged 12 to 15 years; d. students who are enrolled in 7 to 9 grade classes; e. all schools in areas with a low Human Development Index (HDI). Exclusion criteria: a. students younger than 12 years old and older than 15 years old; b. students with uncompleted data at baseline or 4‐month follow‐up; c. students absent on the school days with data collection; d. students who dropped out of the school; e. students who refused to participate in data collection or intervention. Note: considerations on baseline scores not applicable for this study Stated purpose: evaluate the effect of Fortaleca sua Saude programme intervention on potential physical activity determinants and whether gender, age, SES, nutritional status, and PA level at baseline were moderators of the intervention effect amongst students |
| Interventions |
Name: Fortaleça sua Saúde programme Title/name of PW and number: teachers + undergraduate Physical Education student + school manager; (total number not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: training for teachers: a 4‐hour training session was conducted at the beginning of the school semester regarding the relationship between health, school, and academic performance. Teachers received a supplemental manual in order to help with classroom activities. The second component included a four‐hour physical education (PE) teacher‐specific training conducted at the beginning of the school semester. A supplemental manual with lesson plans and handouts was also developed and distributed to teachers. Posters and text materials were produced by the students during the classwork or the homework. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: universal – all participants (students of selected schools and grades) were eligible for inclusion. Intervention details: the intervention schools had four main component strategies: (1) all teachers from the three interventions participated in training to perform lessons in the classrooms that discussed active and healthy lifestyles (text production, production, and exposition of videos, posters, and/or booklets (newsletters or flyers) on different health issues); (2) 4‐hour physical education (PE) teacher‐specific training was conducted at the beginning of the school semester; then all PE classes (20 classes with two PE lessons per week) during the semester were supported by an undergraduate PE student. In addition, posters and text materials were produced by the students during the classwork or the homework; (3) supervised 10 to 15‐min sessions called “Gym in School” were performed twice a week. These sessions were composed of activities in small and large groups in order to involve young people in PA during free time at school. Space and equipment were structured and made available for playing games during free time in the school day. All games were supplemented by banners displayed in schools that explained the game rules and how to access equipment; (4) the materials produced in the classroom and PE classes (e.g. posters, newsletters, and flyers on health issues) were available in schools. In addition, pamphlets were directed at students and parents. The pamphlets were delivered to a member of the school administration (co‐ordinator or director), and they were delivered early in the school day, during classes, and parent/teacher meetings in school. Control: no intervention |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: there was no financial funding to perform this study. Notes on validation of instruments (screening and outcomes): the authors used a previously validated instrument to measure determinants. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02439827 |
Barnes 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the protocol was approved in 2013; the study was published in 2016. Country: South Africa Income classification: upper‐middle‐income country in 2013‐2016 Geographical scope: urban, Free State province Healthcare setting: primary health clinic |
| Participants | 1. Age: women between the ages of 40 and 64 years 2. Gender: female 3. Socioeconomic background: control group: n: 14 unemployment (cannot find work, health problems, family care); intervention group: n: 19 unemployment (same reasons as the control group) 4. Educational background: were required to have a minimum literacy level equivalent to 4 years of schooling to be able to complete and understand the workbook Inclusion criteria: a. women screened positive for musculoskeletal conditions; b. understand English and or Sesotho; c. with access to a telephone; d. willing to commit to the intervention. Exclusion criteria: a. participants with depression; b. participants with other chronic diseases including stroke, cancer, cardiovascular diseases (coronary heart disease), and chronic respiratory diseases; c. participants with diagnosed neurological disorders or confined to a wheelchair. Note: considerations on baseline scores not applicable for this study Stated purpose: to assess the benefits of a 6‐week intervention for women with musculoskeletal conditions |
| Interventions |
Name: non‐pharmacological intervention programme Title/name of PW and number: research assistants, physiotherapists (number not specified) 1. Selection: not specified 2. Educational background: physiotherapist 3. Training: training provided by the first authors 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective – participants were included based upon the presence of a risk factor (women affected by musculoskeletal conditions). Intervention details: the intervention included physical exercise in a group format, health education, facilitation of self‐efficacy and self‐management, decision‐making skills, problem‐solving, and maintaining a balanced lifestyle. They were provided with tools, including a workbook, to facilitate the development of self‐management, decision‐making skills, problem‐solving skills, and obtaining and utilizing resources to assist with managing the chronic disease. The workbook included sections on goal‐setting, problem‐solving tasks, and exercise diaries to facilitate skills acquisition. The intervention also included dietary information, as well as safety aspects and practical considerations. The frequency of the programme was once a week with a duration of 2 h (1 h education and 1 h supervised exercises). Control: usual care – the control group, who were continuing with usual care, were requested to return in 6 weeks for follow‐up measures. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: by the National Research Foundation (South Africa) Notes on validation of instruments (screening and outcomes): “the EQ‐5D‐3L instrument has been validated in a variety of settings, including South Africa and Zimbabwe” Additional information: none Handling the data: not applicable Prospective trial registration number: PACTR 201511000689333 |
Baumgartner 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from August 2018 to February 2020. Country: Ghana Income classification: lower‐middle‐income country in 2018 Geographical scope: rural northern Ghana Healthcare setting: community groups (C‐PrES groups, composed of ∼20 to 25 women from the same community) |
| Participants | 1. Age: minimum age 16 years [mean age 26.95 (SD 6.80) years] 2. Gender: female 3. Socioeconomic background: ~20% of participants for each five SES asset index (quintiles) 4. Educational background: 48.7% of participants had no education. Inclusion criteria: Eligible participants had to be: a. registered in a C‐PrES group; b. 16 years or older; c. currently pregnant at baseline; and d. planning to maintain residence in the community for at least 6 months (duration of the programme). Exclusion criteria: not specified Note: at baseline, the intervention and control group scores for Patient Health Questionnaire 9 (PHQ‐9) were, respectively, 5.54 (3.82) and 6.81 (4.21). Stated purpose: to assess the impact of the Integrated Mothers and Babies Course and Early Childhood Development (iMBC/ECD) intervention on (1) the mental health of mothers of children under age 2 living in rural northern Ghana and (2) the socioemotional development of their children |
| Interventions |
Name: Integrated Mothers and Babies Course and Early Childhood Development (iMBC/ECD) programme Title/name of PW and number: ‘model mothers’: women from the communities and supervised by GHS community health officers and CRS REST II field staff; ~32 (one for each community group) but not well specified 1. Selection: “identified by CRS and GHS staff as women who were pregnant or had a child under age 2 and who exhibited what they considered healthy MNCHN‐related behaviors” 2. Educational background: supposed to be literate in the local language 3. Training: 1‐week training in July 2018 and a 3‐day refresher training in November 2018 on iMBC delivered by masters‐level CRS staff who had themselves been trained by one of the iMBC original developers, a clinical psychologist based in the USA and a master iMBC trainer from Kenya 4. Supervision: weekly supervision by GHS nurses 5. Incentives/remuneration: not specified Prevention type: indicated prevention – prevention of mental health of women during pregnancy and the first two years of the life of their children. Participants presented with some level of distress at baseline as indicated by PHQ‐9. Intervention details: the Integrated Mothers and Babies Course (iMBC) is an evidence‐based intervention for preventing postpartum depression that has been adapted for use in low‐resource settings. The iMBC content is based on the principles of cognitive‐behavioural therapy and attachment theory. Control: usual care (routine educational group sessions that promote the adoption of key MNCHN behaviours [e.g. newborn care, exclusive breastfeeding, etc.]) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 7‐24 months) |
| Notes |
Source of funding: Catholic Relief Services and the Leona M. & Harry B. Helmsley Charitable Trust. Notes on validation of instruments (screening and outcomes): the PHQ‐9 has been previously validated and recommended for use in Ghana, though not in the Mampruli or Nabt languages. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03665246 |
Bell 2008.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between May 2003 and April 2006. Country: South Africa Income classification: lower‐middle‐income country in 2003, upper‐middle from 2004 to 2006 Geographical scope: 20 primary schools located within the 4 community areas (Molweni, KwaNyusawa, KwaNgcolosi and Qadi) of KwaDedangendlale, which is 40 km outside of Durban on the eastern seaboard of South Africa Healthcare setting: community groups |
| Participants | 1. Age: 9‐13 years for children and caregivers aged 18 or above 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 19% of caregivers reported having no formal education, 46% had a level of education between grades 1‐5, and 35% reported having a formal education between grades. Inclusion criteria: a. children between the ages of 9‐13 years old; b. being reared by an adult caregiver age > 18 years that fulfils parenting responsibilities; c. enrolled in school; d. indicated agreement to participate in the study via caregiver consent and child assent. Exclusion criteria: not specified Note: considerations on baseline scores not applicable for this study Stated purpose: to test the effectiveness of the CHAMP amongst black South Africans in KwaZulu‐Natal, South Africa |
| Interventions |
Name: CHAMPSA Title/name of PW and number: community caregivers (number not specified) 1. Selection: selected from the community (schools) 2. Educational background: low literacy levels of caregivers (just under 50% had fifth‐grade education) 3. Training: training entailed attending detailed workshops covering the purpose and content of each session and participatory experiential methods, including facilitation skills. Prior to delivery of the intervention, facilitators rehearsed the various sessions. Further, the previous week’s activities were reviewed through observing and evaluating each facilitator. These weekly meetings also included debriefing sessions and workshops on stress management, dealing with grief and bereavement, and the importance of boundaries and containment when working as facilitators. 4. Supervision: weekly meetings for monitoring/managing the progress of the intervention 5. Incentives/remuneration: not specified Prevention type: universal – the intervention was defined as universal: “due to the universal prevention intervention being conducted in a typical randomized control design, the internal validity of our findings are considered strong, as the major difference in variables impinging on the experimental and control subjects was CHAMPSA”. Intervention details: the final adapted CHAMPSA manualized programme [AmaQhawe (Champions) programme] comprises 10 90‐minute sessions delivered over 10 weekends. The sessions were designed to increase HIV knowledge and decrease stigma surrounding HIV infections; increase authoritative parenting, caregiver decision‐making and caregiver monitoring of children; increase family frequency and comfort discussing hard‐to‐discuss subjects (e.g. sexuality and risky behaviours); increase connectedness to caregiver social networks; decrease neighbourhood disorganization, and increase social control and cohesion. The manual introduces these skills through dramatic depiction in a cartoon‐based storyline. Control: usual care (HIV prevention messages) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: the research project was funded by the National Institute of Mental Health (NIMH) grant #2RO1 MH‐01‐004 (principal investigator: Bell, Carl C. $2,179,890). The 2007 Collaborative HIV Adolescent Mental Health Program South Africa (CHAMPSA) service funding comes from a donation from a private nonpharmaceutical source and is earmarked for HIV prevention service delivery ($150,000). Notes on validation of instruments (screening and outcomes): the GHQ is a widely established measure that has been validated across contexts. Additional information: none Handling the data: no dataset available Prospective trial registration number: not reported |
Berger 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from September 2013 to January 2015. Country: Tanzania Income classification: low‐income country in 2013‐2015 Geographical scope: Meru district Healthcare setting: 6 classes of a public primary school in the Meru district of Tanzania |
| Participants | 1. Age: mean 12.46 (SD: 0.91) 2. Gender: both 3. Socioeconomic background: child's residence—122 family, 61 orphanage 4. Educational background: primary school (grade level 4, 5, or 6) Inclusion criteria: all schools in the Meru district interested in participating in the programme Exclusion criteria: not reported Note: at baseline, the intervention and control group scores for the Spence Anxiety scale for children (SCAS) were, respectively, 16.33 (3.87) and 16.08 (3.8) Stated purpose: to evaluate the effectiveness of the adapted ESPS in enhancing resiliency of Tanzanian students and promoting their prosocial orientation |
| Interventions |
Name: ERSAE‐Stress‐Prosocial (ESPS) intervention Title/name of PW and number: homeroom teachers (4‐6) 1. Selection: “Six schools that were interested in the program decided to send between 4‐6 teachers who were trained in delivering the ESPS program to their students.” 2. Educational background: secondary education certificate (known in Tanzania as ‘‘Grade A’’ teachers) with a teaching experience ranging between 4‐12 years 3. Training: the homeroom teachers were trained in a 4‐day workshop (24 hours); they acquired psychoeducational materials, participated in experiential exercises, practised skills, and learned dissemination techniques all based on the ERSAE‐Stress‐Prosocial (ESPS) manual. 4. Supervision: during the implementation in the classes, the two Tanzanian mental health professionals observed and then supervised the teachers on a bi‐monthly basis. They also consulted with the first author via scheduled Skype sessions. 5. Incentives/remuneration: not specified Prevention type: universal – all participants (students of a public primary school) were eligible for inclusion, and their baseline levels of anxiety (scores for the SCAS) were well below the cut‐off for the measure. The intervention was defined as universal, aiming at promoting prosocial behaviours. Intervention details: the ESPS is a universal school‐based programme composed of sixteen 90‐minute sessions divided into two sets of strategies—stress‐reduction interventions and prosocial interventions (i.e. perspective‐taking, empathy training, mindfulness, and compassion‐cultivating practices). Based on the school curriculum’s requirements, the teachers delivered the course content of the original 16‐session manual in two weekly 45‐minute sessions. Each session contained a warm‐up exercise, experimental work, psycho‐educational knowledge, a contemplative practice, a learned skill, and homework assignments. Control: active control. The control classes received 2‐hour social studies classes weekly based on the Ministry of Education curriculum for primary schools in the mainland, which matched the amount of time spent on the ESPS intervention. These lessons were also provided by the homeroom teachers and were delivered in the control classes at the same time as the ESPS intervention. The contents included topics such as the history of the country, political issues, civics, family life, and education for general values. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 7‐24 months) |
| Notes |
Source of funding: the author(s) received no financial support for the research, authorship, and/or publication of this article. Notes on validation of instruments (screening and outcomes): the scales used were previously reported as reliable and tested for validity in the sample (SCAS, Child Diagnostic Interview Schedule, SDQ). Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Bernardi 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from September 2016 to September 2017. Country: Brazil Income classification: upper‐middle‐income country in 2016‐2017 Geographical scope: city of Vitória, state of Espírito Santo, Southeastern Brazil Healthcare setting: Children's Hospital of the Great Vitória area: Hospital Infantil Nossa Senhora da Glória (HINSG) |
| Participants | 1. Age: participants' age group varied between 18 and 50 years, with the majority being aged 31 to 40 years (44%) 2. Gender: both 3. Socioeconomic background: NA 4. Educational background: all the participants had some level of schooling, and 58% attended school for more than 9 years. Inclusion criteria—children: children and adolescents undergoing cancer treatment. Inclusion criteria—caregivers: caregivers of: a. children and adolescents admitted to HINSG as new cases of cancer for treatment older than 18 years; b. accompanied the children or adolescents for a minimum period of 40 hours per week; c. had never had previous contact with yoga, meditation or similar techniques. Exclusion criteria: a. hospitalizations or deaths over very short periods, which prevented us from inviting the caregiver to participate in the study; b. hospitalizations in which the patient's clinical condition was already in an advanced and irreversible state, that is, the patient was on palliative care; c. cases in which the volunteer had previous knowledge of the patient's diagnosis. Note: at baseline, the intervention and control group scores for the State‐Trait Anxiety Inventory (STAI) were, respectively, 54.8 (10.6) and 54.3 (9.4). Stated purpose: to investigate the effects of the Hatha Yoga intervention on levels of anxiety, subjective well‐being, mindfulness, and awareness of caregivers of children and adolescents undergoing cancer treatment |
| Interventions |
Name: Hatha Yoga Title/name of PW and number: qualified Hatha‐Yoga instructor (1) 1. Selection: not specified 2. Educational background: not specified 3. Training: not applicable 4. Supervision: not applicable 5. Incentives/remuneration: not specified Prevention type: indicated prevention – participants of the clinical and control groups presented some levels of anxiety as indicated by STAI scores, and they were included based upon the presence of a risk factor (being caregivers of children and adolescents undergoing cancer treatment). Intervention details: Hatha Yoga, its main branch in the Western world, uses body poses, breathing exercises, relaxation and meditation with the purpose of self‐perception and self‐knowledge, which assists individuals in their physical, psychological, spiritual and social dimensions. Control: usual care (routine of care provision for the children/adolescents without participating in the intervention) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the STAI is validated widely across contexts; translation and validation were not reported for this sample. The EBES and EMEP questionnaires had cultural validation. Additional information: none Handling the data: not applicable Prospective trial registration number: Brazilian Clinical Trials Registry, protocol RBR‐3vz3nd |
Betancourt 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted around 2012‐2014. Country: Rwanda Income classification: low‐income country in 2012‐2014 Geographical scope: urban. Families were recruited through health centres in Kayonza District (located in Eastern Province, Rwanda) where PIH/IMB provides support to the public health system. Healthcare setting: home |
| Participants | 1. Age: (mean, SD) for children, 11.76 (2.88); for caregivers, 41.27 (8.23) 2. Gender: both 3. Socioeconomic background: (mean, SD) SES for families 0.10 (0.08) 4. Educational background: not specified Inclusion criteria: a. being an adult‐headed household; b. at least one caregiver was HIV‐positive; c. at least one child aged 7–17; d. willingness to discuss HIV with school‐age children. Exclusion criteria: a. not living in the catchment area; b. presenting with untreated mental illness; c. active suicidal ideation/attempts in the family also constitutes exclusion criteria; d. HIV‐positive children with non‐disclosed HIV status; e. presence of concerns in caregivers' capacity to participate in the programme in addition to other caretaking duties. Note: at baseline, the intervention and control group scores for the Center for Epidemiological Studies Depression Scale for Children (CES‐DC) were, respectively, 13.58 (10.89) and 12.74 (10.68). Stated purpose: to evaluate the Family Strengthening Intervention (FSI‐HIV), a family home‐visiting intervention to promote mental health and improve parent‐child relationships in families with caregivers living with HIV |
| Interventions |
Name: FSI‐HIV Title/name of PW and number: community workers (6) 1. Selection: Rwandan 2. Educational background: bachelor‐level 3. Training: 2‐week training in the FSI‐HIV focused on role‐playing to build skills in family counselling. 4. Supervision: a Rwanda‐based master‐level project manager randomized families and provided weekly on‐site supervision. Study leaders, including a child psychiatrist and clinical psychologist, provided additional weekly supervision to the intervention team by phone. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (caregivers were HIV‐positive), but those presenting with untreated mental conditions were excluded from the study. Children's baseline levels of distress were well below the cut‐off. Intervention details: the FSI‐HIV is delivered in approximately 90‐minute weekly home‐visiting sessions which span an initial pre‐meeting, the six core modules including a culminating family meeting, and a follow‐up to the family meeting in order to debrief together about what occurred during the family meeting. The FSI‐HIV model blends meetings solely for the caregivers and solely for the children together with a meeting with all family members. Overall, there are at least two combined family meetings, three sessions just with caregivers and at least two sessions just with children. As needed, supplementary psychoeducation on genocide‐related trauma was developed and provided for families where the issue arose. Control: usual care—families received standard social work services through the Ministry of Health, which included sessions at the health centre and/or home facilitated by a social worker. Treatment‐as‐usual (TAU) social workers received no additional training and were asked to provide usual social work services. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (not available) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this study was funded by a grant from the National Institute of Mental Health (R34 MH084679), seed funding from the Harvard Center on the Developing Child, The Peter C. Alderman Foundation, the Bayer Prevention Science fund and the Julie Henry Junior Faculty Development Fund of the Harvard T. H. Chan School of Public Health. Notes on validation of instruments (screening and outcomes): “Measures were adapted and validated for the Rwandan context following mixed methods research on local constructs of family functioning, mental health, and resilience.” Additional information: none Handling the data: not applicable Prospective trial registration number: NCT01509573 |
Cerquera Córdoba 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from February 2019 to February 2020. Country: Colombia Income classification: upper‐middle‐income country in 2019‐2020 Geographical scope: Bucaramanga and its metropolitan area Healthcare setting: patient's homes (home‐care companies with nursing assistants) |
| Participants | 1. Age: average age of caregivers 55.1 years 2. Gender: both 3. Socioeconomic background: median socioeconomic level (64%) 4. Educational background: secondary education level or less (58%) Inclusion criteria: a. being a relative of the patient; b. living in the same home; c. performing the role of caregiver for at least 8 hours a day for more than 3 months; d. receiving no remuneration. Exclusion criteria: ceasing to be the main caregiver, unpaid, for reasons such as prolonged hospitalization (more than 20 days), institutionalization, or death of the patient Note: at baseline, the total sample score for the Zarit Burden Interview (ZBI) at baseline was 45 (16.8). Stated purpose: to evaluate the efficacy of a multicomponent programme with transdisciplinary intervention to reduce the burden and improve social support of caregivers of patients with Alzheimer's |
| Interventions |
Name: respite care group; multicomponent plus respite care group Title/name of PW and number Intervention 1: transdisciplinary work of 6 psychologists, 4 physiotherapists, 4 speech therapists Intervention 2: nursing assistants (for respite group) 1. Selection: home care provider company 2. Educational background: not specified 3. Training: for respite group nursing assistants trained in the proper handling of patients and support in the execution of activities of daily living—8 hours transdisciplinary team (6 psychologists, 4 physiotherapists, 4 speech therapists) 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective prevention – participants were included based upon the presence of a risk factor (being caregivers of patients with Alzheimers) and presented distress levels at baseline well below the cut‐off for the measure. Intervention details Respite care group: relay “breathing”. Respite care was carried out for 8 weeks, once a week with a duration of 4 hours per day, by nursing assistants through a home care provider company. Multicomponent plus respite care group: multicomponent, integrated by the components of psychoeducation, systemic communication, and physiotherapy, with the objectives of favouring the management of body posture, movements, and physical activity, as well as promoting assertive communication where the quality of communication and affectivity was recognized, through the transdisciplinary work of 6 psychologists, 4 physiotherapists, and 4 speech therapists, who had academic sufficiency in the subject. The multicomponent intervention was carried out for 8 weeks. This intervention group also received the “relay breathing” intervention. Control: waiting list – no intervention (delivery of respite intervention at the end of the study) |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the respite care group intervention and control. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month post‐intervention; 7‐24 months post‐intervention) |
| Notes |
Source of funding: Ministry of Science, Technology and Innovation (MINCIENCIAS) Notes on validation of instruments (screening and outcomes): for the ZBI, the version by Martín‐Carrasco and colleagues was used. This tool presents good reliability and validity for the Colombian population. The MOS has a reliability evaluation for Colombia (Cronbach's alpha between 0.921 to 0.736). Additional information: none Handling the data: not applicable Prospective trial registration number: not specified |
Chaharrahifard 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from May to September 2018. Country: Iran Income classification: upper‐middle income country in 2018 Geographical scope: Karaj, Iran Healthcare setting: prenatal ward of Kamali Hospital, a provincial referral centre for mothers with high‐risk pregnancies |
| Participants | 1. Age: adults but age not specified 2. Gender: female 3. Socioeconomic background: moderate economic level in 75.8% for the intervention group and 78.8% for control 4. Educational background: elementary education in 48.5% (intervention group) and 51.5% (control group) Inclusion criteria: a. being nulliparous; b. having the gestational age of 34 weeks and higher based on the last menstrual period or first sonography screening; c. being literate; d. being Iranian; e. being diagnosed with a high‐risk pregnancy; f. staying for at least three days in the prenatal ward of Kamali Hospital. Exclusion criteria: a. woman with lack of attendance to one group session; b. having a history of mental disorders; c. having a baby with physical and mental abnormalities; d. place of delivery outside Kamali Hospital. Note: at baseline, the intervention and control group scores for the Edinburgh Postnatal Depression Scale (EPDS) were, respectively, 10.96 (5.78) and 12.12 (6.39). Stated purpose: to investigate the effect of a midwife‐led psycho‐education intervention on parental stress, competency, and postpartum depression in nulliparous women hospitalized with a high‐risk pregnancy |
| Interventions |
Name: midwife‐led psycho‐education intervention Title/name of PW and number: 1 midwife (trained MSc student of counselling in midwifery‐researcher) 1. Selection: not specified 2. Educational background: MSc student of counselling in midwifery 3. Training: not specified 4. Supervision: feedback from mothers at the end of each training session 5. Incentives/remuneration: not specified Prevention type: indicated prevention – participants were included based upon the presence of a risk factor (women with high‐risk pregnancies) and presented with some level of distress at baseline as indicated by Center for Epidemiologic Studies Depression Scale (CES‐D) scores. Intervention details: midwife‐led psychoeducation in 2 sessions for a period of approximately 60 to 90 minutes in addition to slides, booklets, and group discussions during admission to high‐risk pregnancy ward. They were then followed up by telephone until delivery, alongside two individual training sessions for approximately 60 minutes immediately after the delivery. Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months) |
| Notes |
Source of funding: deputy of Research and Technology of Alborz University of Medical Sciences for financial support Notes on validation of instruments (screening and outcomes): for PSI Form: in Iran, the reliability of this instrument was obtained as 0.84 through test re‐test and internal consistency coefficient for total stress, and the alpha value for total stress index was 0.90. For EPDS: the reliability of the Iranian version of this instrument was determined through test re‐test (0.8), and the Cronbach’s alpha value was 0.77. Additional information: none Handling the data: not applicable Prospective trial registration number: Iranian Clinical Trial Center (IRCT20150119020719N10) |
Chang 2015.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: recruitment was conducted in 2011‐2013. Country: Jamaica (the study included also a location in Antigua and St Lucia, but these were not classified as LMICs in the study period so they were not included) Income classification: upper‐middle‐income country in 2011‐2013 Geographical scope: parishes of Kingston and St Andrew Healthcare setting: primary care facility (free standing); viewing of films, and discussions were conducted in the waiting area in each public healthcare centre while mothers waited to see the nurse. |
| Participants | 1. Age: infants aged ≥ 10 weeks were excluded. 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: mother’s highest school grade level for intervention (n = 251; mean 10.1, SD 1.3), for control (n = 250; mean 10, SD 1.3) Inclusion criteria: mothers and infants attending government (public) primary care health centres for child care Exclusion criteria: a. infants with obvious mental or physical disabilities; b. twins. Note: at baseline, sample scores for Center for Epidemiological Studies Depression Scale (CES‐D) were 14.8 (10.85) for the intervention group, and 15.32 (10.68) for the control group. Stated purpose: to test the effectiveness of a parenting training programme integrated into primary care |
| Interventions |
Name: parenting intervention with routine primary health care Title/name of PW and number: community health workers (CHWs) and nurses (number not specified) 1. Selection: not specified 2. Educational background: CHWs had a minimum of 3 years’ secondary‐level education. 3. Training: PHWs received pre‐service training of up to 20 weeks or in‐service training, with limited information on child development. Training in the intervention comprised 3‐day workshops with viewing of films and role play. CHWs were given manuals that provided the steps and content for each health visit. Before a new set of topics was shown, a supervisor visited the clinic, reviewed the topics with the CHWs, and provided guidance in discussions and practice. 4. Supervision: The supervisor monitored implementation quality every 6 weeks using 3‐point ratings of how well the CHW involved the mothers and acknowledged and praised their efforts. 5. Incentives/remuneration: not specified Prevention type: indicated – mothers attending the primary care facilities that presented some level of distress at baseline as indicated by CES‐D scores Intervention details: short films developed in Jamaica with 5 mother‐child pairs. Nine modules, each ∼3 minutes in duration, covered the following topics: love, responding and comforting, talking to children, praise, using bath time to play and learn, looking at books, simple toys to make, drawing and games, and puzzles. In each centre, CHWs discussed the activities shown with the mothers and demonstrated them. Viewing of films and discussions were conducted in the waiting area while mothers waited to see the nurse. Mothers practised the activities with their children and were encouraged to make them part of their daily routine. The median duration of the discussion sessions was 16 minutes. All children were seen by a nurse at each visit. The nurses gave the mothers message cards that reinforced the topics on the films and reviewed the cards with them. They encouraged the mothers to do the activities and to watch the films if they had not done so. At ages 9 and 12 months, nurses gave the parents a picture book, and at 18 months a 3‐piece puzzle to take home. Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: supported by the Inter‐American Development Bank Notes on validation of instruments (screening and outcomes): the selected outcome is widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN43108304 |
Chattha 2008.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: not specified Country: India Income classification: lower‐income country until the date of publication of the study Geographical scope: participants were recruited from gynaecological outpatient clinics in 14 different areas of Bangalore city (India). Healthcare setting: Swami Vivekananda Yoga Research Foundation (SVYASA), a yoga university |
| Participants | 1. Age: age between 45 and 55 years irrespective of whether they were menstruating regularly 2. Gender: female 3. Socioeconomic background: of the total participants, 87.76% were housewives, and those who worked were either high school teachers or bank officials. 4. Educational background: high school education or more Inclusion criteria: a. age between 45 and 55 years irrespective of whether they were menstruating regularly (symptomatic women who had stopped menstruating more than 3 years ago were also included); b. a serum follicle‐stimulating hormone (FSH) level of 15 mIU/mL or more on the sixth day of the menstrual cycle if the woman was menstruating regularly or at the time of recruitment, if the woman had stopped menstruating or had irregular cycles; c. women who had undergone hysterectomy with retained ovaries were also included. Exclusion criteria: a. having practised yoga for 1 month or more; b. no knowledge of English; c. less than high school education; e. taking psychiatric medication; f. other exclusion criteria based on health status (e.g. gynaecological problems such as endometriosis, diabetes mellitus, hypo‐/hyperthyroidism). Note: at baseline, the intervention and control group scores for the Perceived Stress Scale (PSS) were, respectively, 17.74 (6.15) and 17.3 (6.61). Stated purpose: to study the effect of yoga on the climacteric symptoms, perceived stress, and personality in perimenopausal women |
| Interventions |
Name: Integrated Approach to Yoga Therapy (IAYT) Title/name of PW and number: trained instructors for yoga (number not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—women in the perimenopausal phase whose baseline levels scores indicate that they were well below the cut‐off for high stress as measured by PSS scores Intervention details: IAYT consists of a set of yoga exercises (Patanjali yoga sutras and Mandukaya karika), which were done for 1 hour per day, 5 days per week for 8 weeks by trained instructors. The yoga module highlights the concepts of a holistic approach to health management at physical, mental, emotional, and intellectual levels with techniques to improve mental equilibrium. The list of practices were (1) sun salutation, (2) breathing exercises, (3) cycling meditation, (4) lectures on IAYT, diet, emotion culture, concepts, and management of stress according to yogic principles. Control: active control – the control group practised a set of exercises comprising easy (nonsweating) body movements supervised by physical trainers for 1 hour daily, 5 days per week for 8 weeks. There were also lectures and individual counselling on conventional modern medical concepts about a healthy lifestyle including diet, exercise, and psychology of menopause and stress management techniques. |
| Outcomes |
Participants’outcomes of interest for this review
Carers' outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): validity of the PSS amongst menopausal women (not in the country of trial execution) was reported. Additional information: none Handling the data: no data available Prospective trial registration number: not reported |
Cheng 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from April 2014 to October 2015. Country: China Income classification: upper‐middle income country in 2014‐2015 Geographical scope: Xi'an Healthcare setting: two tertiary teaching hospitals |
| Participants | 1. Age: mean age of participants was 55.0 years (range from 18 to 89 years). 2. Gender: both 3. Socioeconomic background: 58.68% less than 3000 RMB monthly income for the intervention group and 67.77% for the control group 4. Educational background: 43.80% had secondary/high school diplomas for the intervention group and 28.10% for the control group. Inclusion criteria: adult patients with type 2 diabetes with Haemoglobin A1c (HbA1c) over 58 mmol/mol [poorly controlled type 2 diabetes], accessible by telephone at home, and cognitively intact (indicated by Abbreviated Mental Test score of 6 or above). Exclusion criteria: patients with severe medical conditions, hearing or vision impairment, and psychiatric problems. Note: at baseline, the intervention and control group scores for the Diabetes Distress Scale (DDS) were, respectively, 2.67 (0.81) and 2.87 (0.84). Stated purpose: to evaluate the effectiveness of an empowerment‐based intervention on empowerment level, psychological distress, and quality of life amongst patients with poorly controlled type 2 diabetes |
| Interventions |
Name: empowerment‐based intervention group (Empowerment Process Model) Title/name of PW and number: 5 trained nurses 1. Selection: for educational background 2. Educational background: registered nurses with bachelor degree who had a track record in evidence‐based practice and with over 5 years’ experience in diabetes education 3. Training: 2‐day workshop delivered by nurse specialists with expertise in diabetes care and a researcher with expertise in empowerment philosophy 4. Supervision: clinical supervision was maintained throughout the intervention period by trainers and investigators. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented some level of distress as indicated by DDS scores. Intervention details: Empowerment Process Model: setting personally meaningful motivated goals and then inspired through action‐reflection dynamics. Patients were enabled to synthesize personal resources, including self‐management knowledge, skills, and self‐efficacy. Control: usual care (attentional control: twice‐weekly general health education classes delivered by ward nurses; also received postdischarge biweekly telephone social calls within 1 month after hospital to compensate for potential Hawthorne effects due to professional attention and contacts) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 1‐6 months) |
| Notes |
Source of funding: Hong Kong PhD Fellowship Scheme Notes on validation of instruments (screening and outcomes): the Chinese version of the DDS has demonstrated adequate reliability and validity. In this study, the subscales of the DDS demonstrated satisfactory internal consistency. Additional information: none Handling the data: not applicable Prospective trial registration number: ChiCTR‐IPR‐14005492 |
Chew 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from April 2016 to February 2018. Country: Malaysia Income classification: upper‐middle‐income country from 2016 to 2018 Geographical scope: not specified Healthcare setting: 10 public health clinics in Malaysia |
| Participants | 1. Age: mean (SD) age: 55.6 (10.8) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria: a. Malay individuals; b. aged ≥ 18 years; c. diagnosed with type 2 diabetes for at least 2 years and having regular follow‐up; d. presenting with a mean score ≥ 3 on the 17‐item Diabetes Distress Scale (DDS‐17); e. with either HbA1c ≥ 64 mmol/mol (8.0%), blood pressure ≥ 140/90 mmHg or LDL level ≥ 2.6 mmol/L. Exclusion criteria: a. being pregnant or lactating; b. having a known psychiatric/psychological disorder that could impair judgement and memory; c. unable to read or understand English or Malay; d. score ≥ 20 on the 9‐item Patient Health Questionnaire 9 (PHQ‐9). Note: at baseline, the intervention and control group scores for the PHQ‐9 were, respectively, 4.3 (3.3) and 6.0 (4.8). Stated purpose: to evaluate the effectiveness of a brief, value‐based emotion‐cognition‐focused educational program (VEMOFIT) in Malay adults with type 2 diabetes mellitus |
| Interventions |
Name: Value‐Based and Emotion‐Focused Educational Programme (VEMOFIT) Title/name of PW and number: nurse‐coaches and doctors (number not specified) 1. Selection: not specified 2. Educational background: healthcare background (nurses and doctors) 3. Training: nurse‐coaches and doctors were trained to conduct both programmes at their own health clinics, supported by respective intervention materials and presentation slides. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated prevention – intervention aimed at adults with type 2 diabetes mellitus presenting with distress but excluding those with a score on the Patient Health Questionnaire 9 (PHQ‐9) indicative of severe depression Intervention details: the VEMOFIT consisted of four biweekly group sessions exploring personal values and providing diabetes education (session 1), a training on recognizing (session 2) and managing emotions (session 3) in the self and others, providing social support and setting short‐ and long‐term goals (session 4), and a booster session 3 months after the last session of the main intervention, reviewing the patient’s goals and rehearsing the content of the fourth session. Each participant was allowed to bring along one significant other as a co‐participant. Control: active control – participants in the attention control program were offered three sessions over the same period to discuss emotional experiences, social support at home, and health clinic services regarding diabetes. They were not accompanied, and the sessions were not structured according to any module. The nurse‐coaches and doctors leading the attention control programme were trained in active listening. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months, 7‐24 months) |
| Notes |
Source of funding: the trial is funded by the Malaysian MOHNIH Research Grant (MRG). This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. Notes on validation of instruments (screening and outcomes): the Malay versions of the DDS, PHQ‐9, and Summary of Diabetes Self‐Care Activities (SDSCA) were reported to be validated. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02730078 |
Chomat 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between November 2013 to September 2015. Country: Guatemala Income classification: lower‐middle‐income country in 2013‐2015 Geographical scope: five rural Mam communities in San Juan Ostuncalco municipality and three periurban K’iche’ communities in Quetzaltenango city Healthcare setting: sessions took place in settings of participant's choosing (i.e. house, community centre). |
| Participants | 1. Age: 15‐43 years. Maternal age: 26.2 ± 6.5 for control group; 26.2 ± 6.3 for intervention group 2. Gender: female 3. Socioeconomic background: control group: 92.4% housewives; 57.4% economically insecure household. Intervention group: 95.1% housewives; 55.7% economically insecure household 4. Educational background: control group: 20% no formal schooling; 34.5% incomplete primary; 23.6% complete primary; 14.5% incomplete secondary; 5.5% complete secondary; 1.8% higher education Intervention group: 14.1% no formal schooling; 42.3% incomplete primary; 21.1% complete primary; 11.3% incomplete secondary; 5.5% complete secondary; 1.8% higher education Inclusion criteria: a. pregnant or under 2 years postpartum; b. at least one of the following conditions: i) socioeconomic disadvantage, ii) domestic violence, iii) difficult interpersonal relationships, iv) poor social support, v) psychological distress. Exclusion criteria: not specified. Note: at baseline, the total raw scores for Hopkins Symptom Checklist‐25 (HSCL) were, respectively, 35.7 (11.4) and 36.7 (10.7), which translate to sum scores ranging from 1.42 to 1.54. Stated purpose: to test acceptability, feasibility and impact of a co‐designed group psychosocial intervention (Women’s Circles) in a population with significant need but no access to mental health services |
| Interventions |
Name: Women's Circles Title/name of PW and number: community health workers (CHWs), 9 former CHWs, 6 comadronas, and 1 community leader (16) 1. Selection: the 16 circle leaders were identified based on prior collaborations and expressed interest and were invited to co‐design and co‐facilitate the intervention. 2. Educational background: one had no formal schooling, six had incomplete and five completed primary schooling, and four had incomplete secondary schooling. 3. Training: training by our research team lasted 50 hours. After their own researcher‐led 10‐session Women’s Circle, where the 16 leaders acted as participants, they practised session delivery (2/week, over 5 weeks). Additional training included crisis response, counselling, group facilitation, and self‐care skill‐building. All training activities were carried out in the leaders’ homes, on a rotating basis, as per their preference. 4. Supervision: ongoing support included phone debriefing and direct observation of a random sample of sessions, carried out with all leaders by our research team. 5. Incentives/remuneration: the PWs received per diems of 50 quetzals (USD$7 per day). Prevention type: indicated prevention – inclusion criteria based on known risk factors, including psychological distress. All participants presented some level of distress at baseline as indicated by HSCL scores. Intervention details: 10 sessions of a culturally safe psychosocial intervention. Pre‐sessions involved toy‐making of dolls, books, or rattles mothers could use to stimulate and play with their infants. Sessions started with an inclusive participant‐led prayer, followed by a prior session recap. A group game or dinámica served as an icebreaker. Activities that enabled personal and group reflection (drawing, dramatization) led to sharing lessons learned, aspirations, and personal experiences. A closing dinámica released tensions or promoted relaxation, through a guided meditation or deep breathing exercises. Sessions concluded with a collective embrace. Sessions took place every fortnight in settings of participant’s choosing (i.e. house, community centre), and lasted on average 2 hours. The intervention extended over 5 months, with sessions taking place every other week. Control: waiting list. Control women did not receive the intervention but were invited to join a Women’s Circle when the postintervention assessment was complete. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: this study was supported by a Grand Challenges Canada Global Mental Health seed grant (grant number 0333–04). The funding body had no role in the design of the study, in data collection, analysis or interpretation, or in writing the manuscript. Notes on validation of instruments (screening and outcomes): all instruments underwent pilot testing and semantic validation in Spanish. Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN13964819 |
Cooper 2009.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: overall trial start 2000; overall trial end 2006 Country: South Africa Income classification: lower‐middle and upper‐middle‐income country between 2000 and 2006 Geographical scope: area of Khayelitsha (SST and Town II), a periurban settlement in Cape Town; SST is an informal settlement of shacks characterized by particularly high levels of unemployment and poverty; most shacks are without running water, and considerable overcrowding exists. Many of the inhabitants are recent migrants from rural parts of South Africa. Town II, into which SST merges, is characterized by a somewhat better standard of living. Healthcare setting: home of the participants |
| Participants | 1. Age: intervention group (n = 220), years (mean and SD): 25.2 (5.23). Control group (n = 229), years (mean and SD): 26.2 (5.84). 2. Gender: female 3. Socioeconomic background: not specified 4. Educational background: 28% in the intervention and 30% in control had received education for 6 years or less. Inclusion criteria: pregnant women within a defined area of Khayelitsha, a periurban settlement on the outskirts of Cape Town. Exclusion criteria: not specified. Note: at baseline, 16% of participants in the intervention and control group presented with depression as indicated by the Structured Clinical Interview for DSM‐IV diagnoses (SCID). Stated purpose: to assess the efficacy of an intervention designed to improve the mother‐infant relationship and security of infant attachment in a South African periurban settlement with marked adverse socioeconomic circumstances |
| Interventions |
Name: home‐visiting intervention Title/name of PW and number: lay community workers trained in intervention manual (4) 1. Selection: they had been selected with help from the local community council. They were mothers themselves. 2. Educational background: the women had no formal specialist qualifications. Two had completed schooling. 3. Training: they received training over a 4‐month period in basic parenting and counselling skills, as well as in the specific mother‐infant intervention. The home visitors received 3 weeks of training in the intervention over a 4‐month period and weekly group supervision from a community clinical psychologist. 4. Supervision: group supervision throughout the study, on a weekly basis, offering session by session supervision with an experienced community clinical psychologist 5. Incentives/remuneration: “We did not pay the women for their participation in the research, but at each assessment, we provided a small gift for the infant (an item of clothing).” Prevention type: indicated prevention – the study recruited in the last trimester of their pregnancy. At baseline, they presented some levels of distress (16% presenting with depression as indicated by the Structured Clinical Interview for DSM‐IV diagnoses). Intervention details: 16 visits home‐based (visited, ideally, twice antenatally, weekly for the first 8 weeks postpartum, fortnightly for a further 2 months, and then monthly for 2 months, 1‐h visits). The contents of mental health sessions are counselling and psychological secure attachment. The intervention was manualized. Control: usual care – women in the control group received the normal service provided by the local infant clinic. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: this study was supported by a grant (B574100) from the Wellcome Trust. MT was supported by a fellowship from the Vlotman Trust. Notes on validation of instruments (screening and outcomes): the DSM‐IV interview had been translated and then back‐translated; the EPDS is widely adopted and validated across contexts (no specific reference to validation in South Africa is given in the study). Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN25664149 |
Dayhimi 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2010‐2011. Country: Iran Income classification: upper‐middle‐income country in 2010‐2011 Geographical scope: Teheran, Iran Healthcare setting: Sheibani Health Care Center in Tehran |
| Participants | 1. Age: 18‐35 years (mean age 22 years) 2. Gender: female 3. Socioeconomic background: less than adequate (52.3‐67.4%) 4. Educational background: high school graduation educational levels (64.4%) Inclusion criteria: a. 18‐35 years of age primi gravida pregnant women; b. at least elementary school educational level; c. Iranian and spoke Persian; d. with an adverse history of psychological disorders or medical diseases mimicking anxiety symptoms such as hyperthyroidism, as well as lack of severe and pathologic anxiety (based on Spielbergers questionnaire). Exclusion criteria: a. score of 10 or higher in the Beck depression scale; b. participation in other classes about pregnancy; c. disobeying study protocol (more than one session of absence in the counselling classes). Note: at baseline, the intervention and control group scores for State‐Trait Anxiety Inventory (STAI), mode section, were, respectively, 40.89 (7.59) and 40.28 (7.81). Stated purpose: to determine the effect of obstetric counselling on the anxiety of pregnant women |
| Interventions |
Name: counselling Title/name of PW and number: 1 midwife (first author) 1. Selection: not specified 2. Educational background: MSc in midwifery 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated prevention – participants presented with mild and moderate anxiety at baseline, but all those with scores of higher than 10 on the Beck survey were excluded. Intervention details: counselling based on a predefined protocol, for 5 sessions in 5 weeks (a duration of 60 to 90 minutes for each session) Control: usual care (routine prenatal care) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: none Notes on validation of instruments (screening and outcomes): the validity of the questionnaires and STAI was approved by five faculty members of the midwifery faculty of Shahid Beheshti University of Medical Sciences, a statistics consultant, and a psychologist consultant. Additional information: none Handling the data: not applicable Prospective trial registration number: not specified |
Devries 2015.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from January 2012 to September 2014. Country: Uganda Income classification: lower‐income country between 2012 and 2014 Geographical scope: Luwero District Healthcare setting: 42 primary schools (activities with students and staff were conducted in schools), some activities involve creating a better school environment by painting murals on school walls, and hanging codes of conduct in visible places; however, the intervention does not require any physical infrastructure. |
| Participants | 1. Age: most students were aged 11 to 14 years, mean age 13 (1.5) years 2. Gender: both 3. Socioeconomic background: more than half reported eating fewer than three meals in the day before the survey. 4. Educational background: not specified Inclusion criteria—schools: a. schools in Luwero District, Uganda; b. schools with more than 40 students registered in Primary 5. Inclusion criteria—children: a. registered as a Primary 5, 6, or 7 pupil; b. any school staff member. Exclusion criteria—schools: existing programme related to prevention of violence against children or school governance. Exclusion criteria—children: not able to understand consent and study procedures. Note: at baseline, the intervention and control group scores for the Strengths and Difficulties Questionnaire (SDQ) were, respectively, 0.47 (0.26) and 0.46 (0.27). Stated purpose: assess if the Good School Toolkit, a complex behavioural intervention, could reduce physical violence from school staff to Ugandan primary school children. |
| Interventions |
Name: the Good School Toolkit Intervention Title/name of PW and number: 2 staff and 2 student protagonists (4) 1. Selection: from an inception visit of 2 hours where Raising Voices introduces the Toolkit to school staff 2. Educational background: not specified 3. Training: 100 h of training Raising Voices staff with individualized coaching support to understand the ideas and content of the Toolkit. The key protagonists in each school are not required to have any specific background or training, but receive the 3‐day residential workshop and ongoing support. 4. Supervision: during the intervention, Raising Voices staff members to provide direct one‐on‐one support in the form of in‐person visits and telephone calls to staff protagonists, and in‐person visits to student protagonists. Raising Voices staff made in‐person visits to protagonists in each school on a quarterly basis, and telephoned school staff members approximately monthly, although this varied slightly depending on need. 5. Incentives/remuneration: schools did not receive any inducement or incentive for participation (other than receiving the Toolkit intervention). Prevention type: universal prevention – students and school staff of primary schools with no restriction on inclusion criteria. Participants at baseline presented with difficulties scores well below the cut‐off for the measure. Intervention details: the Good School Toolkit is a complex intervention that aims to foster a change of operational culture at the school level, developed by the Ugandan NGO Raising Voices. The Toolkit consists of six steps designed to be implemented in sequence and draws on the Transtheoretical Model of behaviour change. The steps contain more than 60 different activities for staff, students and administration, focused around topics such as improving the school compound and creating a better learning environment, respect and understanding power relationships, improving teaching techniques, creating accountability, and learning nonviolent methods of discipline. These are delivered by two staff and two students “protagonists”, who are chosen at the outset of the intervention to lead processes at each school. Control: waiting list – delivery of the Good Schools Toolkit at the end of the study |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: MRC, DfID, Wellcome Trust, Hewlett Foundation Notes on validation of instruments (screening and outcomes): all outcomes measured with instruments widely used internationally had been validated in a variety of settings as reported in the paper. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT01678846 |
Dhital 2019.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2016 and 2017. Country: Nepal Income classification: lower‐income country in 2016‐2017 Geographical scope: Dhading, an earthquake‐affected district of Nepal Healthcare setting: 15 government secondary schools |
| Participants | 1. Age: intervention group (n = 605): mean 12.9 (SD: 1.3); control group (n = 615): mean 12.9 (SD: 1.4) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: grades 6 to 8 Inclusion criteria: a. adolescents studying in grades 6, 7, and 8 of the selected schools at the time of data collection; b. adolescents with written consent from themselves and their guardian; c. adolescents without any known diagnosis of mental health problems. Exclusion criteria: adolescents who refused to participate. Note: at baseline, the intervention and control group scores for the Child PTSD Symptom Scale (CPSS) were, respectively, 16.4 (7.9) and 17.4 (8.1). Stated purpose: examined the effect of training for school teachers on psychosocial support for adolescents’ mental health and hope in an earthquake‐affected district in Nepal |
| Interventions |
Name: psychosocial support by teachers Title/name of PW and number: teachers (16) 1. Selection: the school principals from each school nominated two teachers to participate in the training. 2. Educational background: not specified 3. Training: the clinical psychologist provided two days of training on psychosocial support for the school teachers. The training comprised eight sessions in total with 1 to 2 hours for each session. The training was aimed at enabling the teachers to apply the different components of psychosocial support in their daily classroom activities. The sessions covered the following topics: key concepts and principles of psychosocial support; how children react to a crisis situation; the role of teachers in promoting psychosocial well‐being; how to discuss a crisis with children; activities for improved learning and recovery; how to manage challenging behaviour in the classroom; identifying and assisting children who may need more advanced support; teachers' well‐being. 4. Supervision: the research team interacted with the teachers at 6 months follow‐up through focus group discussions (FGDs) to understand their perspectives on the usefulness of the training and the activities they conducted after the training. 5. Incentives/remuneration: not specified Prevention type: selective prevention – adolescents from grades 6 to 8 from all the schools located in the districts that were affected by a natural disaster. Participants presented with some levels of distress which were well below the cut‐off for the measure. Intervention details: the intervention in this study was a teacher‐mediated school‐based intervention which falls under the second layer of intervention as outlined in Inter‐Agency Standing Committee (IASC) guidelines. Control: usual care – the schools in the control group did not receive any training on psychosocial support; the teachers for grades 6 to 8 did not receive any training on psychosocial support. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: this work was supported by the Grant‐in‐Aid for Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, and National Center for Global Health and Medicines, and Post‐Disaster Health Promotion Project in Dhading from The Association of Medical Doctors of Asia in Tokyo, Japan. The funding sources had no role in the study design, implementation of the intervention, data collection and analysis, and interpretation of data; in the writing of the report or in the decision to submit the article for publication. Notes on validation of instruments (screening and outcomes): the scales used (CPSS, Depression Self‐Rating Scale) were all validated for use in Nepal. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03387007 |
Dias 2008.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2015 and 2017. Country: India Income classification: lower‐middle‐income country in 2015 to 2017 Geographical scope: 2 semi‐urban administrative areas (Talukas) in Goa Healthcare setting: home |
| Participants | 1. Age: carers around 53 years; patients with dementia around 78 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 40% of patients with dementia and 20% of carers had below primary education. Most (90%) were unable to afford paid help. Inclusion criteria: a. all probable cases will be examined by a trained clinician (AD) to confirm the diagnosis of dementia according to DSM‐IV criteria and graded using the Clinical Dementia Rating (CDR) Scale; b. CDR mild and moderate dementia; c. the principal caregiver, as identified by the family, was enrolled for the trial. The principal caregiver was generally the spouse, although in some instances another family member was the principal caregiver, particularly when the spouse was not in a position to care. Exclusion criteria: CDR severe dementia or severe comorbid physical health conditions. Note: at baseline, the intervention and control group scores for General Health Questionnaire (GHQ‐12) were, respectively, 4 (2.8) and 2.50 (2.3). Stated purpose: to develop and evaluate the effectiveness of a home‐based intervention in reducing caregiver burden, promoting caregiver mental health, and reducing behavioural problems in elderly persons with dementia |
| Interventions |
Name: 10/66 flexible stepped‐care brief carer intervention Title/name of PW and number: healthcare assistants (HCA)s, 2 in each taluk), and 1 lay health counsellor (LC), shared by both taluks (5 in total) 1. Selection: HCA: knowledge of the local language, being literate, motivated to involve in community care of older people. LC: she was part of the intervention team/authors; member of the Dementia Society in Goa. 2. Educational background: HCA: passed the higher secondary school, LC: not specified. 3. Training: HCA: intensive training module over 1 week developed/adapted to local settings. Trained in key skills including listening and counselling skills, bereavement counselling, stress management, and health advice for common health problems. Trained by the author (geriatrician/epidemiologist) and LHC. LHC: not specified. 4. Supervision: meetings every 2 weeks with psychiatrist and LC. The HCA would meet the psychiatrist twice a month to give updates on the person with dementia, especially if they were taking medication. In addition, met with the LC every 2 weeks to share experiences, support one another, and problem‐solve difficult situations. LC: supervised by the psychiatrists. 5. Incentives/remuneration: LC, Rs 5000/month. HCA, not specified. Prevention type: selective – caregivers of people with dementia who presented baseline levels of distress well below the cut‐off used in the literature for the measure Intervention details: home visits at least every 2 weeks for 6 months. HCAs: Intervention for carers: psychoeducation plus follow‐up and some counselling skills. Patients or carers (or both) had a follow‐up with the psychiatrist, and patients may be prescribed medication. Control: waiting list – control arm dyads received only education and information regarding dementia and were then placed on a waiting list to receive the intervention after 6 months. Both intervention and control were free to utilize existing health services during this time. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: World Health Organization Notes on validation of instruments (screening and outcomes): all these instruments were translated into Konkani, the local language of Goa, using standard methods of translation and back‐translation. These instruments have been used in India for the 10/66 caregiver studies. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT00479271 |
Dias 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from 2015 (the first participant entered the trial) to 2017 (the last participant exited). The paper was published in 2019. Country: India Income classification: low‐middle‐income country from 2015 to 2019 Geographical scope: urban and rural, recruited from rural and urban primary health care clinics and the broader community in Goa, India Healthcare setting: the Depression in later life (DIL) intervention was provided at places convenient to participants (usually their home or in social or religious centres). |
| Participants | 1. Age: N. 181; mean 69.6 (SD 7.2) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 22.6% illiterate; 20.4% literate (no formal education); 31.5% primary school; 21.0% secondary school; 3.9% graduate; 0.6% professional/postgraduate Inclusion criteria: a. mildly symptomatic person without current mental illness; b. warranted other active treatment (e.g. antidepressant medication); c. 60 years or older (as determined from government identification cards and medical records); d. scores of 4 or higher on the rater‐administered, 12‐item General Health Questionnaire (GHQ‐12), with scores ranging from 0 to 12 (higher scores indicating greater symptoms of depression and anxiety), and not in a current episode of major depression; e. ability to speak Konkani, Hindi, or English; f. residence in the same locality for the subsequent 12 months. Exclusion criteria: a. persons with major depression or anxiety disorders within the past 12 months as determined by the Mini International Neuropsychiatric Interview (MINI) 6.0; b. moderate‐to‐high suicide risk (i.e. intent or plan to attempt suicide in the near future); c. history of psychiatric disorders other than nonpsychotic unipolar major depression or anxiety disorder; e. low cognitive scores (< 24 on the Hindi Mini‐Mental State Examination [HMMSE]; score range, 0‐30, with higher scores indicating better cognitive functioning); f. currently taking antidepressants; g. living with an unstable or acute medical illness that would interfere with trial participation. Note: at baseline, the intervention and control group scores for the GHQ‐12 were, respectively, 6.29 (1.87) and 6.23 (1.90). Stated purpose: to assess whether an intervention for depression prevention provided by lay counsellors is effective in older adults from low‐ and middle‐income countries |
| Interventions |
Name: the DIL intervention Title/name of PW and number: lay counsellors (4) 1. Selection: member of the local community, 30 years or older 2. Educational background: they were graduates from any non‐health‐related field (including counselling). 3. Training: the LCs were trained in workshops conducted by 2 members of the research team (M.S. and J.Q.M.) and were required to demonstrate proficiency in 2 practice cases. 4. Supervision: weekly supervision locally and biweekly via Skype from the United States was performed for therapy quality assurance. 5. Incentives/remuneration: not specified Prevention type: indicated – the intervention was defined as indicated: “consistent with indicated depression prevention, we enrolled mildly symptomatic persons without current mental illness that warranted other active treatment.” Intervention details: the DIL intervention (a mix of problem‐solving therapy [PSt] and Brief Behavioral Treatment for Insomnia [BBTI] using lay healthy counsellors) thus dealt with 2 potentially modifiable risk factors for major depression: avoidant or passive coping and insomnia. The DIL intervention sessions, 30 to 40 minutes in length, were provided at places convenient to participants (usually their home or in social or religious centres) for 6 sessions that spanned 6 to 10 weeks. We included 2 booster sessions, 1 each at months 7 and 10, to encourage practice and maintenance of skills for dealing adaptively with future problems. Control: usual care – the control group received care as usual (CAU) together with the same schedule of outcome assessments used in the DIL in the intervention. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 1‐6 months; 7‐24 months) |
| Notes |
Source of funding: this study was supported by grants MH R34 96997 and MH P30 90333 from National Institute of Mental Health. Notes on validation of instruments (screening and outcomes): the selected measures are widely used and validated for use across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02145429 |
Duan 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the time in which the study was conducted is unclear. Country: China Income classification: upper‐middle‐income country from 2010 and the date of publication Geographical scope: urban—Wuhan, China Healthcare setting: University (Wuhan) |
| Participants | 1. Age: 17‐20 years of age 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: enrolled freshmen students Inclusion criteria: a. enrolled freshmen students; b. living on campus; c. willing to participate voluntarily; d. could understand and respond to Chinese; e. have not participated in any other similar interventions before. Exclusion criteria: students suffering severe psychotic symptoms or substance abuse (as determined by the psychological evaluation at the beginning of admission). Note: at baseline, the scores for the Depression Anxiety Stress Scale of 21 items (DASS‐21) ranged from 1.33 to 1.84. Stated purpose: to investigate the efficiency of a single‐session character‐strength‐based cognitive intervention on enhancing freshmen’s adaptability |
| Interventions |
Name: character‐strength‐based
cognitive intervention Title/name of PW and number: social worker (1) 1. Selection: had practical experience in running group work and positive psychology intervention 2. Educational background: social work graduate 3. Training: “was trained and certified to deliver the intervention” 4. Supervision: “To ensure the intervention sufficiency, the instructor was required to adhere to the intervention protocol. The intervention was closely supervised.” 5. Incentives/remuneration: not specified Prevention type: universal – all freshmen students were eligible for inclusion, and their baseline scores for the DASS‐21 were well below the cut‐off for the measure. Intervention details: the intervention was offered at the beginning of the first semester of the freshmen year in the form of a 90‐min lesson. The single‐session intervention was designed with two parts (i.e. a cognition section and a behaviour section) based on CBT. The first one contained two activities: (a) identifying character strengths and (b) character strengths 360 focusing on promoting self‐awareness and helping them reconstruct the cognition of their own character strengths. The second one, including (c) signature character strengths and (d) nominate goals, aimed at helping participants use their character strengths to set goals and structure daily activities, so that they can transfer their character strengths into daily life. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: the authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Wuhan University Teaching Reform and Construction Project and Wuhan University Humanities and Social Sciences Academic Development Program for Young Scholars “Sociology of Happiness and Positive Education”. Notes on validation of instruments (screening and outcomes): the DASS‐21 has demonstrated having good psychometric properties in previous investigations amongst college students in its Chinese version. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Duru Aşiret 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from January to November 2020. Country: Turkey Income classification: upper‐middle income country in 2020 Geographical scope: Central Anatolia Region of Turkey Healthcare setting: home (related to home care unit of a training and research hospital) |
| Participants | 1. Age: average age of the caregivers was 46.82 (SD 10.82 years); average age of the patients was 82.06 (SD 6.56 years). 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 79.3% of caregivers were primary school graduates. Inclusion criteria—for family caregivers: a. being 18 years or older; b. taking care of their patient for at least 3 months; c. not having any problems concerning communication; d. being willing to participate in the research. Inclusion criteria—for patients: a. 65 years and older; b. had a diagnosis of intermediate or advanced stage of Alzheimers; c. had a score of 19 or below on the Standardized Mini‐Mental State Exam (MMSE); d. had been using psychotropic medication for at least 2 months if they were taking any; e. were residing within the borders of the city where the research was conducted; f. were receiving service from the home care unit. In addition, patients and caregivers without COVID‐19 were included in the study. Exclusion criteria: a. caregivers who, at the time when the research was carried out, were not residing in the central district of the city where the study was conducted; b. caregivers who refused to participate in the research. Note: at baseline, the intervention and control group scores for the Neuropsychiatric Inventory Burden of Care Score were, respectively, 29.73 (8.25) and 23.85 (9.07). Stated purpose: to determine the effect of the training conducted according to PLST (Progressively Lowered Stress Threshold Model) on the care burden, care satisfaction, and life satisfaction of the caregivers and on the neuropsychiatric symptoms and agitation level of the patients |
| Interventions |
Name: PLST Title/name of PW and number: internal medicine nurse 1. Selection: not specified 2. Educational background: master's and doctoral degrees in internal medicine nursing 3. Training: not audited training (“courses on mental health during master's and doctoral courses in internal medicine nursing” as reported by the author during contact) 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants were caregivers of people affected by Alzheimers. At baseline, they had some level of distress as indicated by the Neuropsychiatric Inventory Burden of Care scores. Intervention details: the PLST model provides a conceptual basis for the effects of stress for a patient with dementia and the accompanying temporary agitation; it was developed by Hall and Buckwalter. PLST interventions basically include training the caregiver by making an appropriate care plan for the patient. Within the scope of the research, three home visits were made to the caregivers in the intervention group. Control: other (caregivers received educational materials prepared by the researchers on the care of Alzheimer’s patients and solving caregivers’ problems during home visits) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: Scientific Research Projects Coordination Unit of Aksaray University Notes on validation of instruments (screening and outcomes): for the Neuropsychiatric Inventory, “the validity and reliability study for the Turkish version was done by Akça Kalem 2005 and the Cronbach’s alpha value was found to be 0.79.23 The Cronbach’s alpha coefficient of the scale in this study was 0.7”. For the LSS, “the scale was adapted to Turkish by Köker in 1991, who reported it demonstrated good test–retest reliability, and had a Cronbach’s alpha value of 0.71.29. The Cronbach’s alpha coefficient of the scale in this study was 0.97”. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Dybdahl 2001.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 1995‐1996. Country: Bosnia and Herzegovina Income classification: middle‐income country in 1995‐1996 Geographical scope: urban (town of Tuzla, a multiethnic industrial town in northeastern Bosnia) Healthcare setting: home |
| Participants | 1. Age: mothers: mean 30.7 years (SD 4.9), range 20‐44 years; children: mean age 5.5 years (SD 0.7) 2. Gender: both 3. Socioeconomic background: mothers: 85% urban origin, married 63%, widowed 36%, divorced 1%, living in private accommodation 60%, refugee camp 40% 4. Educational background: mothers: education 14% illiterate (mean 5.3 years, SD 2.8; range 0‐14 years) Inclusion criteria: internally displaced Bosnian mothers with a child aged 5‐6 years. Exclusion criteria: a. participating in any other intervention programme; b. unlikely to move out of the area before November 1996. Note: at baseline, amongst mothers, the intervention and control group scores for the Impact of Event Scale (IES) were, respectively, 71.2 (26.8) and 62.1 (22.9). Stated purpose: designed to evaluate the effects of a psychosocial intervention programme on children in war‐torn Bosnia and Herzegovina |
| Interventions |
Name: psychosocial intervention Title/name of PW and number: group leaders; 5 preschool teachers trained for the study 1. Selection: not specified 2. Educational background: not specified 3. Training: in a group of 3‐8 group leaders by a mental health professional. Duration: 5‐day workshop. Before arrival, the participants received basic information about the programme, its background and aims. Content: participants introduced themselves to one another, received written material and introductory training in some of the key issues, such as trauma, child development and the importance of interaction and communication (mother‐child), two 3‐hour seminars. Then 3 days of more detailed description of the programme and reinforcing through group work, demonstrations, role‐plays and discussion the above topics (roles of caretaker, trauma and its effects on adults and children, groups and group dynamics, supervision, logbook). 4. Supervision: weekly group meetings (with 6‐8 group leaders with a supervisor (a mental health professional) and later twice a month) 5. Incentives/remuneration: not specified Prevention type: indicated – internally displaced refugees with experiences of traumatic events. They presented with some level of distress as indicated by the IES scores. Intervention details: group work using a manual‐based approach derived from therapeutic discussions with war‐traumatized women at the Psychological Centre in Tuzla (1993‐1996), and the ICDP; semi‐structured group discussions introduced by group leaders dedicated to providing information about trauma and trauma reactions in adults and children, as well as suggestions for how to meet common post‐traumatic needs and problems, with an emphasis on strengthening participants' own coping strategies and reinforcing existing normal basic communication and interaction skills. Direct attention was given to the mothers and their mental health, to their beliefs and knowledge about children, and the reactions and needs of adults and children following traumatic events. Mothers were also visited once at home to establish rapport and express support. Group leader met weekly with 2 groups of mothers (5 per group) for 5 months; 1 additional visit to each mother at her home at start of programme. CONTROL: usual care—participants received free basic medical care. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: UNICEF; University of Tromso Notes on validation of instruments (screening and outcomes): mother's rating of child's concentration and concentration problems; perceived social support – not validated separately. IES scores: not diagnostic of PTSD, but some literature suggests IES score above 33 suggestive of PTSD. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Eloff 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: not specified Country: South Africa Income classification: lower‐middle to upper‐middle‐income country from 1987 to 2014 (year of publication) Geographical scope: two separate communities within Tshwane (formerly Pretoria) in South Africa Healthcare setting: community sessions |
| Participants | 1. Age: children 6‐10 years, caregivers 33.1 (5.9) for intervention group; 33.1 (6.0) for the control group 2. Gender: female 3. Socioeconomic background: 23% employed; 34% employed 4. Educational background: intervention group. Primary 12%, secondary 86%, tertiary 2%. Control group: primary 14%, secondary 83%, tertiary 3%. Inclusion criteria: a. HIV‐positive women attending clinics; b. all participants able to communicate in at least one of five local languages (Sepedi, Setswana, Sesotho, isiZulu, or English); c. the children were aged 6 to 10 years and lived with their mothers at least 5 days per week. Exclusion criteria: families were excluded if the child or others living in the household were known to be HIV‐positive or were reported as having a life‐threatening illness. Note: at baseline, amongst HIV‐positive women, the intervention and control group scores for the Center for Epidemiological Studies‐Depression (CES‐D) were, respectively, 16.51 (0.74) and 15.87 (0.76). Stated purpose: to assess the efficacy of an intervention designed to promote resilience in young children living with their HIV‐positive mothers |
| Interventions |
Name: not specified Title/name of PW and number: community care workers (2) 1. Selection: not specified 2. Educational background: they have good interpersonal skills and had at least 12 years of education. 3. Training: the training included information about HIV and AIDS and skills for facilitating groups, counselling, and identification and management of children's emotional and behavioural problems. It was delivered by a social worker. 4. Supervision: community care workers were supervised by a social worker. 5. Incentives/remuneration: not specified Prevention type: indicated – intervention designed for mothers who were HIV‐positive. Participants presented with some level of distress as indicated by the CES‐D scores, which are still below the cut‐off levels reported in the literature for people living with HIV. Intervention details: the intervention groups were conducted over 24 weekly sessions, each lasting 75 minutes. For the first 14 sessions, mothers and children participated in separate groups occurring concurrently, and thereafter they participated together in 10 interactive sessions. The first 7 sessions focused on the mothers' issues relating to living with HIV; these were followed by sessions addressing parenting. The children's sessions focused on building self‐esteem and enhancing interpersonal and practical life skills. Activities included board games, storytelling and traditional cultural games. The final 10 joint sessions were designed to promote healthy parent‐child interaction and modelling of positive parenting behaviours and included activities such as compiling a family legacy box. The focus was on overarching skills rather than HIV‐specific themes. The study included 12 groups, with approximately 15 participants in each group. The mother and child groups were each facilitated by 2 community care workers. Control: usual care – subjects randomized to the standard care condition were provided with information about local resources available for assistance. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the outcomes scales (Center for Epidemiologic Studies, Brief COPE, Child Depression Inventory, Revised Child Anxiety Manifest scale) were adapted, translated, and assessed in their psychometric properties in the sample. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Escolar 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between November 2011 and March 2012. Country: Philippines Income classification: lower‐middle‐income country in 2011 Geographical scope: suburban community located just outside a large metropolitan city in the Philippines Healthcare setting: community‐based (not reported in detail) |
| Participants | 1. Age: 60‐80 years 2. Gender: both 3. Socioeconomic background: all the participants were retired and were mainly getting their pension from the Social Security System (SSS) (73.7%). Those who did not have any pension were mostly supported by their families (76.2%), and a majority were still living with their families (82.5%). 4. Educational background: 50% elementary; 42.5% high school; 7.5% college graduate Inclusion criteria: a. between 60 to 80 years of age; b. able to read and write in Filipino; c. no mental illness present; d. no history of stroke, cardiac arrest, or chronic pulmonary obstructive disorders that can impair the ability to participate in the study’s activities; e. have good muscle strength and good motor functioning skill; f. not currently involved in any educational programmes being offered in the community. Exclusion criteria: a. dementia or other severe cognitive impairment; b. psychiatric illness, other severe illness; c. inability to walk independently with or without assistive devices, and with fracture. Note: at baseline, the intervention and control group scores for the Geriatric Depression Scale Short Form (GDS‐S) were, respectively, 5.72 (2.07) and 7.26 (3.19). Stated purpose: to assess the effectiveness of community‐based third age learning programmes on the life satisfaction, self‐esteem, and level of depression of a select group of Filipino elderly in a community setting |
| Interventions |
Name: community‐based educational programmes Title/name of PW and number: volunteer nurses, physical education instructors and food technology instructor 1. Selection: not specified 2. Educational background: not specified 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the GDS‐S E scores, but all those with psychiatric illnesses were excluded from the study. Intervention details: the educational programmes were a mix of activities that catered to the needs of the Filipino elderly for physical activity, mental stimulation, social engagement, health promotion, and development of new skills. Three main programmes were implemented: (a) a wellness programme, (b) a physical fitness activity programme, and (c) livelihood training programmes. It was offered over the course of 4 months: the wellness programme, 6 sessions, facilitated by volunteer nurses, provided health instruction to the elderly about proper nutrition, diet, and exercise. The programme also included discussions on conditions common amongst the elderly population. The physical fitness activities were conducted by physical education instructors weekly for 2 months with each session lasting for 30‐40 minutes. The livelihood training programmes provided were facilitated by a food technology instructor. Six sessions were given on food preparation, proper handling and storage of food, and the actual making of food products. Control: no intervention |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): data on internal consistency for the selected outcome measures were reported (GDS‐S, Rosemberg Self‐Esteem Scale, LSITA‐SF). Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Fabbri 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2018‐2021. Country: Tanzania Income classification: low‐income country in 2018 Geographical scope: Nyarugusu refugee camp Healthcare setting: schools |
| Participants | 1. Age: 43% were 11‐14, 30% were younger than 10, 22% were 15‐20, 3% were 21 or older. 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: Most were in primary grades. Inclusion criteria—schools: all 27 primary and secondary schools in Nyarugusu refugee camp in Tanzania. Inclusion criteria—students: all students who can speak Kirundi or Swahili and are capable of providing assent. Inclusion criteria—teachers: all teachers who can speak Kirundi or Swahili and who are capable of providing informed consent. Exclusion criteria: none reported. Note: at baseline, the total sample scores for the Mood and Feelings Questionnaire (MFQ) were 3.8 (4.7). Stated purpose: to assess if EmpaTeach intervention could reduce physical violence from teachers to students in Nyarugusu refugee camp, Tanzania |
| Interventions |
Name: EmpaTeach Title/name of PW and number: teachers 1. Selection: teachers acting as group co‐ordinators were selected based upon suggestions by their collogues, no expression of supportive views towards the use of harsh discipline, with proficient facilitation skills. 2. Educational background: not specified 3. Training: teachers followed the EmpaTeach self‐guided teacher training intervention; group co‐ordinators received a 3‐day training on facilitation skill‐building, the programme content. 4. Supervision: not specified 5. Incentives/remuneration: neither teachers nor group received any form of payment for their participation in the intervention. Prevention type: selective—participants were included if they attended schools in the refugee camp. They presented with some level of distress as indicated by MFQ scores that were well below the cut‐off for the measure. All students attending all schools in the refugee camp were eligible to be participants, 8.8% reported baseline levels of depression above the cut‐off. Intervention details: the EmpaTeach intervention is a behaviourally informed, self‐guided teacher training intervention designed to reduce and prevent teachers’ use of corporal punishment in the classroom. Group sessions were conducted by facilitators and co‐ordinators in a face‐to‐face fashion with a group of 3 to 15 teachers. The content of the intervention focused on empathy‐building exercises and on group work to learn and practice self‐regulation techniques, strategies to promote well‐being, positive disciplinary methods, and classroom management strategies. The intervention was based on a booklet developed specifically to self‐guide teachers through each of the 12 sessions in the programme. The intervention supported teachers in creating action plans for change for responding to students’ positive and negative behaviours and reflecting on future actions when they would encounter problems. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: the research was supported by funding from an anonymous donor to the International Rescue Committee and by a research grant by MRC/DfID/NIHR MR/S023860/1 to KD at the London School of Hygiene and Tropical Medicine. Notes on validation of instruments (screening and outcomes): all instruments adopted had been widely used and validated in international settings. Items were translated to Kiswahili and Kirundi, cognitively tested and adapted when needed. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03745573 |
Ferreira‐Vorkapic 2018.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was published in 2018. Country: Brazil Income classification: upper‐middle‐income country from 2006 Geographical scope: urban Healthcare setting: Federal University of Sergipe and a partner yoga centre |
| Participants | 1. Age: aged between 30 and 55 years 2. Gender: men and women 3. Socioeconomic background: not reported 4. Educational background: professors at the Federal University of Sergipe Inclusion criteria: a. healthy subjects; b. men and women; c. professors at the Federal University of Sergipe, Brazil. Exclusion criteria: individuals with chronic pulmonary disease, making use of psychotropic drugs, and with previous meditation or yoga experience. Note: at baseline, the interventions and control group scores for the Beck Anxiety Inventory (BAI) were, respectively, (mean, SE): 19.9 (1.4); 20.3 (2.3); and 19.6 (1.8). For the Beck Depression Inventory (BDI), they were instead 13.2 (1.9); 11.4 (1.6); and 11.5 (1.5). Stated purpose: observing the impact of Yoga Nidra and seated meditation on the anxiety and depression levels of college professors |
| Interventions |
Name: meditation (mindfulness) and relaxation (Yoga Nidra) Title/name of PW and number: yoga instructor 1. Selection: first author: Camila Ferreira‐Vorkapic 2. Educational background: certified yoga instructor 3. Training: not reported 4. Supervision: not reported 5. Incentives/remuneration: not reported Prevention type: indicated – participants presented with some level of distress as indicated by BAI and BDI scores, but these were below the clinically relevant scores for both measures. Intervention details: during a 3‐month period, volunteers attended seated meditation or relaxation sessions. All sessions lasted for 45 to 50 min and were carried out twice a week at the Federal University of Sergipe and a partner yoga centre. Contemplative practices were executed by the first author (a certified yoga instructor), which utilized a detailed attendance register to check the compliance. Meditation (mindfulness): all mindfulness practices employed here are part of the Mindfulness‐Based Stress Reduction Program. Relaxation (Yoga Nidra): it is also called “psychic sleep”, an old yogic practice that provides deep psychological and physical relaxation while maintaining mental functions to be functional and alert. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the meditation intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the selected measures are widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: 29390514.3.0000.5546 (http://plataformabrasil.saude.gov.br) |
Fine 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from September 2018 to February 2019. Country: Tanzania Income classification: low‐income country in 2018‐2019 Geographical scope: Mtendeli refugee camp in the Kigoma region of northwestern Tanzania Healthcare setting: 6 villages in Zone A of Mtendeli refugee camp, Burundian population |
| Participants | 1. Age: adolescents between the ages of 10 and 14 (mean = 12.3, SD = 1.5); caregivers between the ages of 20 and 60 (mean = 39.5, SD = 10.1) 2. Gender: both 3. Socioeconomic background: not reported 4. Educational background: for adolescents, levels of education ranged from 1 to 8 years, with a larger group (42.7%) reporting 4 to 5 years of schooling; for caregivers, higher on average amongst the ETAU group, with 41.4% reporting 6 or more years in school compared to 14.3% in the EASE group). Inclusion criteria: a. eligible adolescents screened positive for psychological distress based on a score ≥ 8 on the Kirundi version of the Child Psychosocial Distress Screener (CPDS); b. residence in the village for the duration of the study; c. fluency in Kirundi; d. ability to follow and understand verbal instructions. Exclusion criteria: a. severe cognitive or neurological impairment as determined by the caregiver‐reported Ten Questions Screen (Durkin 1990); b. imminent suicide risk; c. lack of caregiver consent. Note: at baseline, the total sample scores for the Child PTSD Symptom Scale (CPSS) were 7.5 (9.3). Stated purpose: to evaluate the feasibility, acceptability, relevance, and safety of the World Health Organization’s Early Adolescent Skills for Emotions (EASE) intervention amongst Burundian refugee adolescents and their caregivers in Tanzania |
| Interventions |
Name: EASE intervention Title/name of PW and number: 9 facilitators from the community—adult refugees (5 EASE facilitators and 4 ETAU facilitators) 1. Selection: based on their experience 2. Educational background: at least a high school education 3. Training: 8‐day training consisted of education on distress in adolescents, group management skills, facilitation skills, and basic counselling skills. The training also included role‐plays to rehearse key strategies of the EASE intervention in a classroom environment, as well as practice cycles in which facilitators administered full EASE sessions to their peers while under observation by a local supervisor. 4. Supervision: fidelity was monitored during intervention delivery, and weekly group supervision was provided to EASE facilitators by local supervisor. 5. Incentives/remuneration: not specified Prevention type: indicated prevention – adolescent refugees screening positive for psychological distress were eligible for inclusion. CPSS scores indicated minimal symptoms related to PTSD. Intervention details: EASE intervention was developed by the WHO to reduce symptoms of internalizing disorders, including depression and anxiety, amongst young adolescents living in contexts of adversity in LMICs. The manualized intervention consists of 7 weekly group sessions for 10‐ to 14‐year‐olds and is designed to be delivered in‐person by nonspecialist facilitators. Control: usual care (ETAU – enhanced treatment‐as‐usual consisting of individual psychoeducation session, which was provided jointly for an eligible adolescent and his or her caregiver, and was conducted in each participating family’s home) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Oak Foundation Notes on validation of instruments (screening and outcomes): “CPSS, SWEMWBS, and CTQ have been validated in a range of cross‐cultural settings.” Additional information: none Handling the data: not applicable Prospective trial registration number: not specified |
Gao 2015.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from September 2012 to February 2013. Country: China Income classification: upper‐middle income country in 2012‐2013 Geographical scope: Guangzhou Healthcare setting: regional teaching hospital in Guangzhou where the birth rate was more than 5000 babies per year |
| Participants | 1. Age: for the intervention group mean age 28.49 (SD 2.73), for the control group mean age 28.67 (SD 2.91) 2. Gender: female 3. Socioeconomic background: monthly household income ≥¥6000 for 64.4‐66.7% of participants 4. Educational background: college or above for 84.4‐86.7% of participants Inclusion criteria: a. first‐time mother who had given birth to a single full‐term health baby (gestation age 37–40 weeks, body weight over 2500 g and Apgar score equal or above 8); b. married and living with their husband. Exclusion criteria: a. women with past or family psychiatric history and major postnatal complications, such as puerperal infection, postpartum haemorrhage and amniotic fluid embolism; b. also women who had received interpersonal psychotherapy (IPT)‐oriented childbirth education programme during pregnancy. Note: at baseline, the intervention and control group scores for the Edinburgh Postnatal Depression Scale (EPDS) were, respectively, 7.38 (3.76) and 7.69 (3.31). Stated purpose: to investigate the effects of an IPT‐oriented postnatal psychoeducation programme on postpartum depressive symptoms, social support and maternal role competence in Chinese first‐time mothers |
| Interventions |
Name: IPT‐oriented postnatal psychoeducation programme Title/name of PW and number: 1 midwife educator (fist author) 1. Selection: not specified 2. Educational background: midwife educator, who had experiences in delivering IPT‐oriented intervention 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the EPDS scores, but all those with past or family psychiatric history were excluded. Intervention details: IPT‐oriented postnatal psychoeducation programme: 1‐hour education session before discharge and one telephone follow‐up within the 2 weeks after discharge from the hospital. Specific IPT techniques, such as information‐giving, use of affect, clarification, signalling what is significant, reviewing relationship and communication patterns, and providing social support were applied throughout the programme. Control: usual care (brief visit from a nurse in the postnatal ward to give them a pamphlet on sources of assistance for mothers on discharge from hospital) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Medical Scientific Research Foundation of Guangdong Province, China Notes on validation of instruments (screening and outcomes): the EPDS has been validated in Chinese mothers (Guo 1993). For the PSSS, the original PSSS has good reliability and validity (Zimet 1988). Additional information: none Handling the data: not applicable Prospective trial registration number: study number A2012164 |
Gavrilova 2009.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2000‐2004. Country: Russia Income classification: middle‐income country in 2000‐2004 Geographical scope: Moscow, South administrative district; patients registered in 3 general practices. Healthcare setting: group community training |
| Participants | 1. Age: patients: > 65 years; carers' mean age 61.5 years (SD 17.6) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria: a. carer of patients > 65 years; b. patients met DSM‐IV criteria for dementia. Exclusion criteria: a. serious current physical illness for the patient; b. no family carer; c. > 1 person with dementia in the same household. Note: at baseline, the intervention and control group scores for the Self‐Reporting Questionnaire (SRQ) were, respectively, 5.8 (3.2) and 6.7 (3.9). Stated purpose: tests the effectiveness of the 10/66 caregiver intervention amongst people with dementia and their carers |
| Interventions |
Name: 10/66 brief carer intervention Title/name of PW and number: newly qualified doctors (number not specified) 1. Selection: not specified 2. Educational background: medical degree 3. Training: 2‐day training, using the 10/66 intervention manual (includes vignettes, role plays, live interviews) 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants were caregivers of people with dementia. They presented with some level of distress as indicated by SRQ‐20 scores. Intervention details: intervention for carers; content (manualized approach): 3 modules: assessment of cognitive and functional impairment, carers' knowledge and understanding, care arrangements (1 session), basic education about dementia illness, what to expect in future, locally available resources (2 sessions), training regarding dealing with specific problem behaviours (2 sessions). Duration/frequency: 5 weekly 30‐minute sessions. Control: waiting list – on a waiting list for the intervention (medical care for both intervention and control was provided during the study period) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: World Health Organization Notes on validation of instruments (screening and outcomes): the scales used (including the Self Report Questionnaire, ZBI, Neuropsychiatric Inventory) are widely validated; no specific information on adaptations for local context. Additional information: “Validation of the Self Reporting Questionnaire 20‐Item (SRQ‐20) for Use in a Low‐ and Middle‐Income Country Emergency Centre Setting” (van der Westhuizen 2016) Handling the data: not applicable Prospective trial registration number: ISRCTN41039907 |
George 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study intervention and assessment period extended from June 2011 to January 2013. Country: India Income classification: lower‐middle‐income country between 2011‐2013 Geographical scope: rural coastal villages in India Healthcare setting: community spaces |
| Participants | 1. Age: 24.96 (3.21) 2. Gender: female 3. Socioeconomic background: 1 to 4% were employed 4. Educational background: education below matric: 28 (43.8%) in intervention, 11 (15.1%) in the positive control, and 19 (29.7%) in the negative control Inclusion criteria: stayed in the village for the period of the intervention. Exclusion criteria: a. were > 32 weeks of pregnancy (as traditionally these women are discouraged from venturing out of their homes); b. had pregnancy‐related complications such as a history of antepartum haemorrhage or had been advised bed rest. Note: 11 to 14% of participants scored above 10 on the Edinburgh Postnatal Depression Scale (EPDS). Stated purpose: assess the effectiveness of a low‐intensity group intervention led by lay workers during the antenatal period in reducing postnatal depression at 6‐12 weeks postpartum |
| Interventions |
Name: problem‐based low‐intensity group intervention Title/name of PW and number: lay health workers (4) 1. Selection: these workers had earlier been trained to deliver simple supportive measures and life skills programmes for 5 years prior to the current investigation. 2. Educational background: not specified 3. Training: the training was conducted over a period of 50 hours and included principles of group work, problem‐solving, simple cognitive strategies, and coping skills both emotional and behavioural. About 20 hours of this was part of ongoing training for other psychosocial interventions. This was conducted by a psychiatrist. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: universal – all participants were eligible for inclusion; less than 15% of them presented with depression levels above the cut‐off for the measure at baseline. Intervention details: the active intervention was delivered over 3 days, with three sessions per day, each 45 to 60 minutes long. The intervention involved a framework provided by risk factors for postnatal depression and common concerns of childbearing women in the community. The intervention involved eliciting common problems, discussing principles of problem‐solving, understanding the importance of interpersonal relationships, illustrating simple cognitive strategies, and a discussion of specific emotional and behavioural strategies for problems elicited. Concerns regarding delivery, breastfeeding, and a session on postnatal depression, specifically common symptoms, consequences, and appropriate help‐seeking behaviour were also included. Structured modules were designed for the delivery of the intervention and handouts in the local language were employed where necessary. Control (2 control groups): 1. active control, which consisted of general sessions on pregnancy, delivery, breastfeeding, immunization, development, nutritional and hygiene needs of the child 2. usual care, which consisted of regular antenatal visits and supplements recommended during pregnancy |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the problem‐based low‐intensity group intervention and active control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: unclear Notes on validation of instruments (screening and outcomes): the instruments were translated to the local languages using standardized procedures (EPDS, Global Assessment of Functioning scale). Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Hajarian Abhari 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2018. Country: Iran Income classification: upper‐income country in 2018 Geographical scope: Mashhad Healthcare setting: health centres |
| Participants | 1. Age: 18‐35 [mean age 23.3 years (SD 3.9) for intervention group and 24.4 (SD 4.4) years for control group] 2. Gender: female 3. Socioeconomic background: 76.7% of the sample reported unsatisfactory income level. 4. Educational background: 40‐43% had a diploma. Inclusion criteria: a. Iranian and residency in Mashhad; b. primiparous women; c. age range of 18‐35 years; d. basic literacy; e. gestational age of 35 weeks; f. singleton pregnancy; g. intentional pregnancies; h. no high‐risk pregnancy; i. absence of foetal anomalies and disorders based on ultrasonography; j. no history of medical and psychological disorders or use of psychiatric medications; k. no drug or alcohol addiction; l. no history of infertility; m. absence of Post‐Traumatic Stress Disorder (PTSD) and severe depression, anxiety, and stress based on Depression, Anxiety and Stress Scale—21 Items (DASS‐21) (score less than 20 for depression, less than 14 for anxiety and less than 25 for stress), and no causes for C‐section. Exclusion criteria: a. absence in the counselling programme for more than one session; b. occurrence of high‐risk complications during pregnancy, during/after delivery; c. occurrence of stressful events during the study. Note: at baseline, the intervention and control group scores for the DASS‐21 were, respectively, 8.7 (1.7) and 8.7 (1.8). Stated purpose: to assess the effect of counselling based on Gamble's approach on postpartum anxiety in primiparous women |
| Interventions |
Name: midwife‐led individual counselling based on Gamble's approach Title/name of PW and number: midwives (1 delivered the intervention but 5 midwife researchers in total) 1. Selection: not specified 2. Educational background: university (department of nursing and midwifery) 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: none Prevention type: indicated prevention – participants presented some level of distress as indicated by DASS‐21 scores, but all those who presented severe levels were excluded. Intervention details: counselling based on Gamble’s approach is designed based on the principles of cognitive‐behavioural therapy. In this counselling, it is believed that people's beliefs, thoughts, and attitudes affect their feelings and behaviour. Counselling based on Gamble’s approach involves 9 strategies, including the development of a midwife‐parturient woman medical relationship, acknowledgement of the maternal feeling about delivery, assisting mothers to reveal their emotions, eliminating mothers' ambiguities, connecting behaviours, emotions, and delivery, revision of the stages of delivery, developing social support, promoting maternal adaptation, encouraging positive maternal perceptions, and finding solutions. Control: usual care (routine prenatal care and training) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the questionnaire was validated in a study carried out by Sahebi 2005, and in the study by Effati‐Daryani 2017 for utilization during pregnancy and postpartum. In the current study, the Cronbach alpha reliability coefficient for the scales of depression, anxiety, and stress was reported as 0.82%, 0.74%, and 0.78%, respectively. Additional information: none Handling the data: not applicable Prospective trial registration number: IRCT20180520020039747N1 |
Hamdani 2021a.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from April 2016 to April 2018. Country: Pakistan Income classification: lower‐middle income country in 2017 Geographical scope: rural subdistrict of Gujar Khan in Rawalpindi district Healthcare setting: village‐based groups |
| Participants | 1. Age: mean (SD) age of children was 6.72 (± 2.76). 2. Gender: both 3. Socioeconomic background: mean monthly income 19,236.65 (SD 17,617.78) Pakistani rupees 4. Educational background: mothers’ literacy, 33% (177/540) of mothers had no formal education, whereas 56% (303/540) of mothers had completed some schooling (7 [± 1.5] years of schooling) Inclusion criteria: a. trial participants were children aged between 2 and 12 years, residing in the study subdistrict for the duration of the study; b. positive score on any of the Ten Questions Screen questionnaire items 1, 4, 5, 7, 8, 9, 10 for neurodevelopmental delay—a cross‐culturally valid instrument to screen children with developmental difficulties; c. clinical assessment of screened‐positive children with developmental delays and disorder(s) according to the WHO mhGAP developmental disorders guidelines for clinical assessment in primary healthcare settings by a trained clinical psychologist. Exclusion criteria: not specified. Note: considerations on baseline scores not applicable for this study Stated purpose: to evaluate the effectiveness of this technology (assisted), delivered by family volunteers; parents’ skills training intervention to improve functioning in children with developmental disorders in a rural community of Rawalpindi, Pakistan |
| Interventions |
Name: WHO mhGAP guidelines‐based intervention Title/name of PW and number: 62 family volunteers (FVs), facilitators from the community 1. Selection: either the parents or others who were related to the children with developmental disorders, had at least eight grades of formal education, and volunteered to be trained and supervised by the trainers for at least 6 months duration of the programme. 2. Educational background: at least eight grades of formal education 3. Training: 9 weekly group sessions followed by competency rating using adapted version of ENACT using tablet‐based tool. Provided by 10 trainers who had completed at least a Masters in Clinical Psychology and had at least 1 year of experience in working with children and families with developmental disorders. Only competent trainers (mean score above 2.5 on all domains of adapted version of ENACT) were allowed to train and supervise the FVs. 4. Supervision: FVs were fortnightly supervised by the trainers during the programme delivery; similarly, trainers were supervised fortnightly by the master trainer. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (caregivers of children with a developmental delay). Intervention details: intervention based on the guidelines of WHO mhGAP‐IG module on developmental disorders. The adapted intervention consists of 9 sessions delivered in a group format to caregivers of children with developmental disorders. Control: usual care (ETAU [enhanced treatment‐as‐usual]), including (a) Community Health Workers (CHWs), in the ETAU arm received training in recognizing signs and symptoms of developmental disorders and making referrals to their primary care physicians for treatment; and (b) the primary care physicians received a half‐day orientation session on identification, management and referral guidelines of WHO mhGAP on developmental disorders. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: Grand Challenges, Canada and the Government of Canada (Grant number 0746‐05‐GMH). Matched funding was provided by Autism Speaks (Grant number 10377), USA and Human Development Research Foundation, Islamabad, Pakistan. Notes on validation of instruments (screening and outcomes): the PedsQL has shown sound psychometric properties in different cultures. Additional information: none Handling the data: not applicable Prospective trial registration number: trial registration clinicaltrials.gov, NCT02792894 |
Hendriks 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2017. Country: Suriname Income classification: upper‐middle‐income country in 2017 Geographical scope: Paramaribo, the capital city of Suriname Healthcare setting: workplace |
| Participants | 1. Age: 36.3 years (SD = 9.6) 2. Gender: both 3. Socioeconomic background: the majority of participants had an income of 1000‐2000 SR$. 4. Educational background: the majority of participants had a lower (36%) or the middle level of educational attainment (45%). Inclusion criteria: a. age between 18 and 60 years; b. sufficiently fluent in the Dutch language to capably fill out questionnaires, read a training manual, and participate in written exercises; c. available to participate in an opening session, followed by six 2‐ to 3‐hour intervention sessions, for 6 consecutive weeks. Exclusion criteria: not specified. Note: at baseline, the intervention and control group scores for the Depression Anxiety Stress Scale—21 items (DASS‐21) were, respectively, 1.78 (0.51) and 1.71 (0.39). Stated purpose: to evaluate the effects of a culturally adapted multicomponent positive psychology intervention (MPPI) on resilience |
| Interventions |
Name: Strong Minds Suriname Title/name of PW and number: coaches (9) 1. Selection: employees with various ethnic backgrounds, thereby representing the ethnic composition of the participants in the study 2. Educational background: not specified 3. Training: received training on the intervention (no further information provided) 4. Supervision: supervisor observed the interaction between the coaches and the participants during each session and debriefed them afterward. 5. Incentives/remuneration: not specified Prevention type: universal – all employees were eligible for inclusion, and their baseline scores for the DASS‐21 were well below the cut‐off for the measure. Intervention details: the programme is based on the Shell Resilience Program and was adapted for the local context. It consists of 6 sessions titled 1) Be grateful; 2) Positivity starts with you; 3) Developing your own strengths; 4) Your goals are attainable; 5) Overcoming your problems; 6) Let it go. Positive activities conducted during the sessions included: relaxation and breathing exercises, psycho‐education, homework assignments. Control: waiting list – at the end of the study the control participants received the intervention. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) Note: data at 3 months were not included in our review given that the waiting‐list control group already received the intervention at this time point. |
| Notes |
Source of funding: the study was funded by the University of Amsterdam and sponsored by the following participating Surinamese companies: Multi Electronic System N.V., Surinaamse Postspaarbank N.V., and the InterMed Group. Except for Wantley Sardjo, the CEO of Multi Electrical System N.V., and co‐author in this study, funders had no role in, or control over the collection, management, analysis, interpretation, and publication of the data. Notes on validation of instruments (screening and outcomes): the Dutch scales were validated amongst other Dutch‐speaking populations (e.g. MHC‐SF, DASS‐21). Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Hinton 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2018. Country: Vietnam Income classification: low‐middle‐income country in 2018 Geographical scope: semirural area outside of Hanoi, Vietnam Healthcare setting: home or other place of choice |
| Participants | 1. Age: control 58.7 years (13.9); intervention 59.0 years (10.4) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: control 7.2 years (2.6); intervention 8.3 years (3.4) of education Inclusion criteria: a. age 18 or older; b. family member most involved in the dementia patient’s day‐to‐day care; c. score on the Zarit Burden Interview (ZBI) (4‐item) of ≥ 6; d. caring for an older adult with a dementia diagnosis and a Clinical Dementia Rating (CDR) score of 1 or above. Exclusion criteria: caregivers identified as having difficulties in the consent process due to cognitive impairment or severe sensory impairment. Note: at baseline, the intervention and control group scores for Patient Health Questionnaire 4 (PHQ‐4) were, respectively, 4.1 (3.0) and 4.6 (2.8). Stated purpose: to assess the efficacy and feasibility of a family caregiving intervention in northern Vietnam |
| Interventions |
Name: REACH VN Title/name of PW and number: community workers/interventionist (number not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: training of interventionists in Vietnam consisted of didactic sessions based on the REACH VA training materials as well as “hands‐on” field experience in a case series prior to the study onset in. The training was carried out by certified by a senior member of the research team (HN) who is bilingual and was herself certified by the REACH VA intervention by the Memphis Caregiver Center affiliated with the University of Tennessee. 4. Supervision: after each session, an observer will complete the Treatment Delivery Form to document the delivery of key components of the intervention. 5. Incentives/remuneration: not specified Prevention type: indicated – participants, caregivers of people with dementia, presented with some level of distress as indicated by the PHQ‐4 scores at baseline. Intervention details: participants in the intervention group will receive a culturally adapted intervention based on the REACH VA intervention. The intervention itself consists of 4 “core” training sessions on problem‐solving, mood management/cognitive restructuring, stress management (e.g. signal breath, mindfulness medication, pleasant event scheduling), and communication, plus up to 2 additional sessions based on the caregiver’s needs and clinical judgement and will be delivered over the course of 2 to 3 months. Each session lasts approximately 1 hour and will be delivered by an interventionist certified to deliver the intervention. The interventionist will deliver face‐to‐face, but there will also be the option of conducting sessions by phone. At the first session, each participant will receive written materials on dementia as well as a caregiver notebook with detailed information on a variety of topics related to dementia caregiving and will serve as a reference during the intervention. Control: enhanced usual care – the enhanced control condition will consist of a single face‐to face session that will occur at the time of enrolment and be held in the caregiver’s home (or another place of their choosing). Caregivers will be educated about the nature of dementia and provided with written educational materials. |
| Outcomes |
Participants’outcomes of interest for this review
Note: data in the adult population, as only caregivers received the intervention (not patients with dementia) Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: National Institute on Aging and Fogarty International Center of the National Institutes of Health under award number R21AG054262 (Hinton and Nguyen MPI) Notes on validation of instruments (screening and outcomes): the selected measures are widely adopted and validated across contexts (PHQ, ZBI). Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03587974 |
Hirani 2010.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2000‐2004. Country: Pakistan Income classification: low‐income country in 2000‐2004 Geographical scope: urban (inner‐city slum area of Karachi [Pakistan], a sprawling metropolis of 18 million residents located on the Arabian Sea) Healthcare setting: adult literacy centers (ACLs) |
| Participants | 1. Age: 25‐35 years 2. Gender: female 3. Socioeconomic background: economically disadvantaged women. Household size was 6‐10 people for most women, and monthly household income averaged US$55.00. 4. Educational background: most women reported < 4 years of formal education, and most women were not employed. Inclusion criteria: a. women in adult literacy programmes in each of the randomly chosen clusters were recruited into the study; with b. age range 25‐35 years. Exclusion criteria: not specified. Note: considerations on baseline scores not applicable for this study Stated purpose: to provide an evidence‐based intervention to address the primary health problems confronting women in Pakistan and worldwide: depression and violence. Specifically, we tested the differential effectiveness of a community‐derived intervention of ESB, developed through community‐based participatory methods against an evidence‐based empirically tested counselling model. |
| Interventions |
Name: group counselling intervention Title/name of PW and number: community health workers (number not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: 21 hours training, included skill‐building on components of the intervention as well as research ethics of privacy and confidentiality 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (economic disadvantage). Intervention details Group counselling: 8 weekly sessions at the ALCs; the key components of the module included stress and anger management, effective communication, active listening, and supportive problem‐solving. ESB intervention: 8 weekly sessions at the ALCs; the key topics covered were skills for employment attainment and retention such as effective communication, balancing personal and work life and time management, conflict resolution, dealing with abuse and harassment, enhancing self‐efficacy, effective parenting, and personal hygiene and grooming. Control: usual care – the control group received no additional services. |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the group counselling intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Aga Khan University Research Council Notes on validation of instruments (screening and outcomes): BDI‐II, GSE and IPV instruments validated internationally but not mentioned if validated in a Pakistani context Additional information: none Handling the data: not applicable Prospective trial registration number: not specified |
Hirani 2018.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between November 2015 and February 2016. Country: Pakistan Income classification: lower‐middle‐income country in 2015‐2016 Geographical scope: Karachi, Pakistan Healthcare setting: family health centre (FHC) in an urban community was selected as a study site. |
| Participants | 1. Age: 20‐45 years; intervention 30.63 (5.67), control 32.25 (6.64) 2. Gender: female 3. Socioeconomic background: low socioeconomic status; “More than 50% of the women in both groups reported a total family monthly income between 10,000 and 24,000 rupees ($150.00–$360.00 USD per month), where 22% of women in the intervention group and 20% of women in the control group were working”. 4. Educational background: 85% of women in the intervention group and 77% of women in the control group had formal schooling; the majority had a secondary education. Inclusion criteria: a. speaking Urdu language; b. did not have diagnosed mental health disorders; c. resided within the catchment area. Exclusion criteria: a. women who did not speak Urdu; or b. who had an active migration plan to leave the study site. Note: considerations on baseline scores not applicable for this study Stated purpose: enhancing resilience and quality of life |
| Interventions |
Name: social support intervention Title/name of PW and number: community workers (3) 1. Selection: local community health workers, at least 18 years of age, with good communication skills in Urdu 2. Educational background: minimum of grade 10 education 3. Training: they received 10–15 hours of one‐to‐one training from the principal author (SH) on topics related to the study’s purpose and methods; ethical responsibilities for respect, privacy, and confidentiality, and the content of the intervention modules. 4. Supervision: CHWs received direct supervision throughout the study by the first author of the study. 5. Incentives/remuneration: not specified Prevention type: universal – all women living in the catchment area with no diagnosis of a mental health disorder were eligible. Intervention details: the intervention was offered to six unique groups, and each group was composed of a maximum of 10 participants (total of 60 women). The intervention was manualized and consisted of 6 weekly sessions lasting 1‐1.5 hours. The primary emphasis of the intervention was to provide participants with a safe environment in which they could learn about and discuss the concept of stress and its impact on their lives; share their feelings and experiences, and give/receive support to/from each other. Each week a topic was identified, alongside learning objectives, information about the topic, and suggested exercises to encourage interaction and discussion. Examples of meeting activities include “Define social support and discuss its importance to health” and “Identify the influence of stress on the body, the mind, relationships, and health”. Control: women in the control group participated in a sham intervention involving a single didactic information session on the significance of mental health. The session was conducted by a nurse (not related to the study) and included a definition of mental health, a discussion of its significance, and identification of factors that contribute to or detract from poor mental health. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: no relevant information reported Notes on validation of instruments (screening and outcomes): the WHOQOL‐BREF is a widely validated outcome measure that was also psychometrically tested in the local population (Urdu language measures pilot‐tested). Additional information: none Handling the data: no dataset available Prospective trial registration number: not reported |
Huang 2017.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2013‐2014. Country: Uganda Income classification: low‐income country in 2013‐2014 Geographical scope: urban, Kampala Healthcare setting: 10 schools in Kampala (5 matched pairs on the number of teachers and students) |
| Participants | 1. Age: between 4 and 8 years of age or nursery to third grade 2. Gender: both 3. Socioeconomic background: 35.1% of caregivers reported food insecurity. 4. Educational background: 46.1% of caregivers had a secondary or higher education. Inclusion criteria: a. early childhood teachers (serving students between 4 and 8 years of age or nursery to third grade) from participating schools interested in participating; b. children and families selected by the teachers from the classes for outcome assessment. Exclusion criteria: not specified. Note: at baseline, the intervention and control group scores for the Pediatric Symptom Checklist (PPSC) ranged from 0.96 to 1.3 (internalizing) and 0.97 to 1.04 (externalizing). Stated purpose: investigated the implementation quality and effectiveness of one component of an evidence‐based intervention from a developed country (USA) in a low‐income country (Uganda) |
| Interventions |
Name: ParentCorps Title/name of PW and number: teachers (number not specified) 1. Selection: early childhood teachers (serving students between 4 and 8 years of age or nursery to third grade) from participating schools 2. Educational background: not specified 3. Training: during the ParentCorps FUNdamentals, which lasted 5 days, teachers were asked to reflect on their assumptions about students and families and to connect those assumptions to their current practices and capacity to help children succeed. Within this context of reflection about values and goals, teachers also learned a set of evidence‐based practices to choose from that are consistent with their own values that will enable them to meet goals for themselves and the students in their classrooms. Additional 13 weekly group sessions, named ParentsCorp Coaching (1 to 1.5 hours), were planned at the school to help teachers use evidence‐based practices effectively in the classroom. This was conducted by trained mental health professionals. 4. Supervision: fidelity was assessed through a number of outcome measures. 5. Incentives/remuneration: because the training was provided during non‐school days and outside the school, each teacher received funds (i.e. US$50) to compensate for travel and time. Prevention type: universal – all students were eligible for inclusion, and their baseline scores for the Pediatric Symptom Checklist (PPSC) were well below the cut‐off for the measure. The intervention as defined as “early childhood mental health promotion” in the study. Intervention details: early school teachers who were trained over 5 days by 10 Ugandan mental health professionals who spent 10 days in the US where they received training at the ParentCorps Academy. Teachers’ training included large‐group experiential training and small‐group coaching to introduce and support a range of evidence‐based practices to create nurturing and predictable classroom experiences. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: this study was funded by the National Institutes of Health (R21MH097115‐01A1). Notes on validation of instruments (screening and outcomes): all outcomes were checked for their psychometric properties during the study. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Hull 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from early September 2011 to late May 2012. Country: Belize Income classification: upper‐middle income country in 2011‐2012 Geographical scope: Belize District Healthcare setting: schools |
| Participants | 1. Age: students aged 7‐9 years (standards 1‐3) and 10‐12 years (standards 4‐6) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: students Inclusion criteria: each school was requested to participate for the duration of an entire school year with the understanding at the outset that there was a 50% probability any school may or may not be selected to implement the Positive Action programme. Exclusion criteria: not specified. Note: at baseline, the intervention and control group scores for the Behaviour Assessment Systems for Children (BASC) were, respectively, 12.73 (2.55) and 12.69 (2.55). Stated purpose: to examine post‐treatment positive youth development competencies (i.e. social‐emotional character development, peer affiliation, substance abuse and violence tendencies, moral beliefs, pro‐social behaviour, and school self‐esteem) in comparison to students in a randomly assigned control group of schools. The research question was: do students exposed to treatment in the form of Positive Action exhibit higher post‐treatment positive youth development competencies than students in a randomly assigned control group of schools? |
| Interventions |
Name: Positive Action programme Title/name of PW and number: school teachers 1. Selection: not specified 2. Educational background: not specified 3. Training: teacher training programme related to social‐emotional/character development or behaviour management 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the BASC. Intervention details: Positive Action programme to address the physical, intellectual, social, and emotional domains that interact with different ecologies of the child: school, family, and community. This is grounded in the Theory of Triadic Influence (TTI). Whole‐school implementation across classrooms and grades [6 units], as well as parent resources so that transformation occurred in more than one setting—school, home, and family. Parent education occurred at the intervened schools on parent night, once per month at each school, led by social workers using instructions and activities from the 42 (30 to 45 minute) lessons in the Positive Action Family Kit. Social workers assigned to each school utilized Positive Action’s Bullying Prevention kit and Conflict Resolution kit for pull‐out instruction with children that met a higher tier of need for intervention. Control: usual care (Health and Family Life Education curriculum) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Inter‐American Development Bank and the Government of Japan Notes on validation of instruments (screening and outcomes): validated questionnaires Additional information: none Handling the data: not applicable Prospective trial registration number: ClinicalTrials.gov NCT03026335 |
Izgu 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from June 2018 to September 2019. Country: Turkey Income classification: upper‐middle income country in 2018‐2019 Geographical scope: Ankara, Turkey Healthcare setting: endocrine outpatient unit of Ankara University Medical Faculty, Ibn‐i Sina Hospital |
| Participants | 1. Age: mean age of participants was 64.2 ± 8.1 years in the relaxation group (RG), 61.6 ± 8.0 years in the meditation group (MG), and 64.1± 6.6 years in the control group (CG). 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: most of participants had at least graduated from primary school (RG = 60%, MG = 78.3%, CG = 73.9%). Inclusion criteria: a. diagnosed with DPNP; b. at least primary school graduates; c. not using any other complementary or integrative therapy during the study period. Exclusion criteria: a. neuropathy history due to any other causes such as megaloblastic anaemia, fibromyalgia, autoimmune diseases, hypothyroidism, and lumbar disc hernia; b. having end‐stage renal failure, chronic obstructive pulmonary disease, advanced cardiac failure, musculoskeletal disorders, or depression; c. having a diabetic foot ulcer or amputation. Note: at baseline, the intervention and control group scores for OUTCOME NAME were, respectively, MEAN (SD) and MEAN (SD). Stated purpose: to examine the effects of progressive muscle relaxation and mindfulness meditation on the severity of diabetic peripheral neuropathic pain (DPNP), fatigue, and quality of life in patients with type 2 diabetes |
| Interventions |
Name: intervention 1—MG intervention 2—RG Title/name of PW and number: 1 trained nurse (third co‐author, research assistant at the Internal Medicine Nursing Department) 1. Selection: not specified 2. Educational background: certified and experienced in progressive muscle relaxation and mindfulness meditation 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (being type 2 diabetes patients). Intervention details Intervention 1—MG: a sitting mindfulness meditation, as a part of the mindfulness‐based stress reduction (MBSR) programme, was used. Each daily session lasted for 20 min, with thoughts focusing on deep breathing and the present moment. Intervention 2—RG: the progressive muscle relaxation sessions were performed following the steps of the progressive muscle relaxation programme designed by Jacobson 1938. Each daily session lasted for 20 min, during which participants were instructed about tensing and relaxing the body muscles, along with deep breathing. The patients practised daily progressive muscle relaxation, beginning with the muscles of the face and head, followed by those of the neck, shoulders, hands, chest, abdomen, legs, and feet. For both MG and RG: face‐to‐face sessions of progressive muscle relaxation and mindfulness meditation training were provided by using training booklets. After these theoretical sessions, which lasted for 30 min, all progressive muscle relaxation and mindfulness meditation steps were practised by the participants under the supervision of the third co‐author for 20 min. Training sessions for the RG and MG were undertaken only once in a silent patient training room of the endocrine outpatient unit. Patients in the RG or MG practised these interventions once daily for 20 min for a total of 12 weeks. Control: usual care (patients in the CG received an attention‐matched control education session only once, in the same patient training room) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): for NEPIQOL, in the Turkish validity and reliability study, the Cronbach’s alpha value was reported as 0.95, while in the current study it was calculated as 0.85. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT04287439 |
Jamali 2016.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was published in 2016. Country: Iran Income classification: upper‐middle‐income country Geographical scope: urban, Mazandaran province (Northern Iran) Healthcare setting: middle schools |
| Participants | 1. Age: 13 to 14 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: middle‐school students Inclusion criteria: a. willingness to complete a full mental health assessment; b. age range between 13 and 14 years. Exclusion criteria: no exclusion criteria reported. Note: considerations on baseline scores not applicable for this study Stated purpose: assess the effects of life skill training in middle‐school students on mental health, attitudes towards addiction, stress |
| Interventions |
Name: life skills training Title/name of PW and number: community worker (CW), qualified teachers/trainers 1. Selection: not specified 2. Educational background: not specified 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: universal – all students aged 13 to 14 in middle school were eligible. Intervention details: the intervention group received life skills training for 1 month in 8 sessions (2 sessions a week for 2 hours). The following skills were taught in two sessions: empathy, problem‐solving, critical thinking, coping skills, self‐regulation, and assertion skills. The training method involved lecture‐style presentations, group activities, role‐plays, and question‐and‐answer opportunities. Control: no intervention – the control group did not participate in any training sessions during the same period of the intervention. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): a tool was developed for this study, based upon previously validated questionnaires. Within this sample, it showed good test‐retest reliability and validity. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
James 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between July 2014 and April 2015. Country: Haiti Income classification: low‐income country in 2014‐2015 Geographical scope: metropolitan Port‐au‐Prince (Haiti) during the acute crisis (mortality is still higher than it was before the crisis) Healthcare setting: intervention groups within communities |
| Participants | 1. Age: from 18 to 65 years 2. Gender: both 3. Socioeconomic background: currently employed, n (%): 27 (5.7%) 4. Educational background: mean education, years (SD): 7.3 (4.5); range: 0 to 20 Inclusion criteria: a. aged from 18 to 65 years; b. either gender; c. available to attend 3‐day intervention training; d. able to understand consent procedures; e. resident of community and present in community during recent hurricane/monsoon season. Exclusion criteria: a. unable to attend the 3‐day intervention training; b. unable to understand consent procedures. Note: at baseline, the total sample scores for the Beck Anxiety Inventory (BAI) were 15.6 (13.8). Stated purpose: to assess the effects of an integrated mental health and disaster preparedness intervention on symptoms associated with depression, post‐traumatic stress disorder, anxiety, and functional impairment in earthquake and flood‐affected communities in Haiti |
| Interventions |
Name: mental health integrated disaster preparedness Title/name of PW and number: lay mental health workers 1. Selection: not specified 2. Educational background: not specified 3. Training: intensive week‐long session consisting of manual review and role‐play provided by principal investigators (both mental health professionals) 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants, exposed to severe stressors. presented with some level of baseline anxiety as indicated by the BAI. Intervention details: the mental health integrated disaster preparedness intervention utilizes an experiential approach, including facilitated discussion, space for sharing personal experiences and exchange of peer‐support, establishing safety and practicing coping skills targeting disaster‐related distress, and hands‐on training in disaster preparedness and response techniques for use by participants in their own lives and to support other community members. Day 1 includes discussion about mental health and psychosocial reactions to disaster‐related stress, and teaching associated coping strategies, including skills to reduce potential avoidance of disaster‐related material (e.g. self‐calming through breathing, grounding, mindfulness, and muscle relaxation exercises). On day 2, the workshop transitions to focus on disaster preparedness, including facilitated discussions regarding links between common attributions for disasters (natural causes, God’s will) and preparedness motivation. Facilitators introduce common scientific explanations for disasters such as earthquakes and floods and share recommended preparedness strategies. This is done without discouraging pre‐existing cultural and religious beliefs which participants are encouraged to maintain alongside new information. At the end of day 2 and moving into day 3, participants practice providing disaster and mental health‐related peer support to one another, including through a “mini‐disaster simulation” in which participants demonstrate skills learned throughout the 3 days. The intervention was manualized. Control: waiting list – following the third data collection time point, waiting‐list control group participants were invited to participate in the intervention. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (not available) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this work was supported by the Research for Health in Humanitarian Crises (R2HC) program, managed by Enhancing Learning and Research for Humanitarian Assistance (ELRHA) (CWM and LJ, ELRHA project number #10944). The £8 million R2HC program is funded equally by the Wellcome Trust and DFID, with ELRHA overseeing the program’s execution and management. The funder had no role in study design, data collection, analysis, interpretation, or writing. Notes on validation of instruments (screening and outcomes): “when possible, culturally‐adapted and validated measures were used”. Additional information: none Handling the data: not applicable Prospective trial registration number: CTRI/2018/02/012002 |
Jewkes 2008.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from 2003 to 2006. Country: South Africa Income classification: upper‐middle‐income country from 2003 to 2006 Geographical scope: rural areas (70 villages) in the Eastern Cape province of South Africa Healthcare setting: schools—the sessions were mainly held on school premises after school hours. |
| Participants | 1. Age: 15‐26 years (more than 50% older than 18) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: most (96%+) currently in school, highest proportions in 9th and 10th grade Inclusion criteria—clusters: eligible locations were: a. about 10 km from the nearest cluster (to minimize contamination of study arms); b. had a senior or junior secondary school; and c. had community willing to participate (established through a process of community mobilization). Inclusion criteria—individual participants: a. aged 16‐23; b. normally resident in the village where they were at school; c. mature enough to understand the study and the consent process. Exclusion criteria: not specified. Note: at baseline, the intervention and control group scores for the Center for Epidemiological Survey for Depression (CES‐D) were, respectively, 11.36 (9.670) and 10.93 (9.989). Stated purpose: to assess the impact of Stepping Stones, an HIV prevention programme, on the incidence of HIV and risky behaviour |
| Interventions |
Name: South African Stepping Stones (second edition) Title/name of PW and number: facilitators (11) 1. Selection: the same sex as the participants and either the same age or a little older; they were selected, in part, for their open‐mindedness and gender sensitivity. 2. Educational background: most had further education or had undergone life skills training. 3. Training: 3‐week‐long training and 2 practice groups 4. Supervision: facilitators were supervised (no further information reported). 5. Incentives/remuneration: not specified Prevention type: universal – all village residents in the age range were eligible for inclusion, and their baseline scores for the CES‐D were well below the cut‐off for the measure. Intervention details: Stepping Stones uses participatory learning approaches, including critical reflection, role‐play, and drama, and draws the everyday reality of participants’ lives into the sessions. It is delivered to single‐sex groups, which are run in parallel, and has 13 three‐hour‐long sessions that are complemented by three meetings of male and female peer groups and a final community meeting. The programme spanned about 50 hours and ran for 6 to 8 weeks. The sessions covered how we act and what shapes our actions; sex and love; conception and contraception; taking risks and sexual problems; unwanted pregnancy; sexually transmitted diseases and HIV; safer sex and condoms; gender‐based violence; motivations for sexual behaviour; dealing with grief and loss; and communication skills. Control: active control – the control intervention was a single 3‐hour session on HIV, safer sex, and condoms. The content was taken from Stepping Stones. This was delivered by facilitators who were trained and supervised separately to reduce contamination. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) |
| Notes |
Source of funding: National Institute of Mental Health grant No MH 64882‐01 and South African Medical Research Council. KD was funded from the Harry F Guggenheim Foundation and by the Emory Center for AIDS Research (P30 AI050409). Notes on validation of instruments (screening and outcomes): no specifications reported in the paper; the AUDIT and CES‐D have been extensively validated and used also in the country of application of the trial. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT00332878 |
Jiang 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from April 2017 to December 2018 (then 12‐month follow‐up). Country: China Income classification: upper‐middle income country in 2017‐2018 Geographical scope: mainland China Healthcare setting: four hospitals in mainland China with levels not above grade III‐B |
| Participants | 1. Age: mean age 56.91 (SD 10.05) years; for intervention group 57.35 (SD 9.09) years and for control group 56.46 (SD 10.95) years 2. Gender: both 3. Socioeconomic background: individual monthly income < 2000 RMB ($296.09) in 53.21% of patients (54.89% [intervention group] and 51.52% [control group]) 4. Educational background: 43.40% only primary school or below; more or equal to junior high school in 57.89% (intervention group) and 55.30% (control group) Inclusion criteria Patients were recruited if they were: a. diagnosed with T2DM; b. aged between 18 and 75 years; c. had their HbA1c in the past 12 weeks no less than 7.5%; d. not on insulin in the past 3 months. Exclusion criteria: a. patients being pregnant or preparing for pregnancy; b. with psychological problems or cognition disorders; c. developing severe diabetes complications; d. participating in other research projects Note: at baseline, the intervention and control group scores for the Diabetes Distress Scale (DDS) were, respectively, 2.43 and 2.33. Stated purpose: to assess the benefits of SSEP amongst type 2 diabetes mellitus patients not on insulin at a 12‐month follow‐up |
| Interventions |
Name: Self‐efficacy‐focused Structured Education Program (SSEP) Title/name of PW and number: trained registered nurses and physicians; 20 in total (3 physicians and 2 nurses in each research centre) 1. Selection: not specified 2. Educational background: physicians and nurses 3. Training: all the research staff received the training before research began (details in Jiang 2019); they received research guidelines about the study functions and responsibilities from the central researchers. 4. Supervision: monitoring every 3 months by the central researchers 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the DDS scores, but all those with psychological problems were excluded. Intervention details: the programme consisted of four structured curriculums and regular follow‐up. It was delivered in a group format (with 4 to 8 patients in the respective group), once per week and continued for 4 weeks. The programme aimed to promote patients recruited in the learning, motivate patients to change, and develop and sustain self‐management behaviours by primarily stressing the enhancement of patients’ self‐efficacy. Control: usual care (individual face‐to‐face diabetes education presented by physicians during each medical clinic visit, as well as the conventional class education delivered by physicians and nurses per month). In addition, the follow‐up/3 months was offered by nurses via face‐to‐face/telephone). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: Hainan Provincial Natural Science Foundation of China (820RC631, 819QN229), Young Talents’ Science and Technology Innovation Project of Hainan Association for Science and Technology (QCXM202019), the Project of Science Research Project in Hainan University of Higher Education (Hnky2020‐36), and Hainan Health Commission Health Industry Research Project (20A200286) Notes on validation of instruments (screening and outcomes): the internal consistency, reliability and criterion validity of C‐DDS were established (Li 2012). Cronbach’s α was 0.952 in this study. Additional information: none Handling the data: not applicable Prospective trial registration number: ChiCTR‐IOR‐17011007 |
Jordans 2010.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from December 2006 to March 2007. Country: Nepal Income classification: low‐income country in 2006‐2007 Geographical scope: 4 districts of rural south‐western Nepal (Banke, Dang, Bardia, Kailali) Healthcare setting: school |
| Participants | 1. Age: 11‐14 years 2. Gender: both 3. Socioeconomic background: significant differences in groups despite randomization: more Brahmins in the treatment group, more Terai caste in the waiting list (none in the intervention group) 4. Educational background: there was a higher proportion of participants with higher education among the treatment group. Inclusion criteria: a. school‐aged children; b. positive Child Psychosocial Distress Screener score (cut‐off score unspecified). Exclusion criteria: a. psychiatric problems (mutism, mental retardation, dissociative disorders, epilepsy without medication, panic or phobic disorders, and child psychosis); b. schools excluded if they were in Village Development Committees (VDCs) where the intervention was already implemented and schools in adjoining VDCs to avoid contamination. Note: at baseline, the intervention and control group scores for the Child PTSD Symptom Scale (CPSS) were, respectively, 20.15 (5.47); 21.01 (6.46). Stated purpose: to assess the efficacy of a classroom‐based intervention (CBI) among school‐going children in rural Nepal as a psychosocial intervention to address children affected by armed conflict in LMICs |
| Interventions |
Name: CBI Title/name of PW and number: paraprofessional interventionists/facilitators (16) 1. Selection: gender‐balanced group, from targeted communities 2. Educational background: based on previous experience and affinity to work with children 3. Training: 15‐day skills‐oriented course (duration and trainers not specified) 4. Supervision: regular supervision by an experienced counsellor 5. Incentives/remuneration: information from author: the facilitators received a monthly remuneration of 4000 NPR for running the CBI sessions. Prevention type: indicated – participants were positive according to the Child Psychosocial Distress Screener score, but all those with a diagnosed psychiatric problem were excluded. Intervention details: protocolized group intervention; eclectic intervention based on concepts from creative‐expressive and experiential therapy, co‐operative play, and cognitive behavioural therapy. Use of the same manual as for Tol 2008 (Center for Trauma Psychology in Boston); 5 weeks, 15 sessions (about 60‐minute sessions). The CBI was offered as part of a multilayered care system that included activities geared towards strengthening community resilience through parental support groups, recreational activities, community sensitization, and psycho‐education (tier 1), the CBI to target children with elevated psychosocial distress upon primary screening (tier 2), and individual supportive and problem‐solving counselling and referral to psychiatric care (if available) for children, mainly referred on from the group intervention, in need of more individualized or specialised care (tier 3). Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: Save the Children USA (Nepal Office) Notes on validation of instruments (screening and outcomes): measures were translated and validated: “Test–retest reliability of the instruments was determined among 20 participants”; 1 screening measure, the CPDS, was developed for the Nepali context specifically and validated in another study. Additional information: none Handling the data: not applicable Prospective trial registration number: ISRCTN48004304 |
Karmaliani 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between December 2015 and January 2018. Country: Pakistan Income classification: low‐middle‐income country between 2015‐2018 Geographical scope: not specified Healthcare setting: 40 gender‐specific public schools |
| Participants | 1. Age: 12‐13 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 6th grade Inclusion criteria—schools: a. single‐sex, government schools; b. sufficiently large spaces for safe play; c. 35+ students in grade 6 where parental consent could be sought. Inclusion criteria—children: all 6th graders attending the selected schools. Exclusion criteria—schools: campus schools with a single administration responsible for multiple schools in the same area. Exclusion criteria—children: none reported. Note: considerations on baseline scores not applicable for this study Stated purpose: to determine the impact of the intervention on school‐based peer violence (victimization and perpetration) and depression amongst school children |
| Interventions |
Name: The Positive Child and Youth Development Title/name of PW and number: coaches (20 adult coaches + 120 junior leaders) 1. Selection: criteria for coach selection include previous experience in working with children, a passion and willingness to participate in Right To Play’s training, a positive attitude toward child protection, and living in relatively close proximity to the school where the coaches will work. Junior leaders are given leadership training, and they participate as assistants to the coaches, for example, by leading warm‐up exercises. 2. Educational background: intermediate education 3. Training: training was done in accordance with Right To Play’s Junior Leader Facilitation Toolkit. 4. Supervision: the research team verified completion of the intended number of sessions and observed a session in each school each month to ensure compliance with the manual. 5. Incentives/remuneration: not specified Prevention type: universal – all 6th‐grade students were eligible for inclusion. Intervention details: the intervention in Pakistan was based on 103 play‐based learning activities, each with a specific goal as specified in the manual. These structured activities, designed to help children and adolescents improve their confidence, resilience, and critical thinking, were developed by a team of experts including educationists, athletes, teacher‐trainers, and psychologists. Coaches selected an activity for a session from the manual. After the game, they led a three‐step discussion following the formula Reflect‐Connect‐Apply, which involved reflection on the activity and how it made participants feel or what had been learned from it, discussion connecting this to daily life, and application more broadly to other circumstances. Over the 2 years of this study, 120 sessions were conducted in each class, with, on average, two sessions of 35 minutes per week, resulting in 60 sessions in a year. Control: waiting list – regular schooling programme; received intervention for 6 months after study completion. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this is an output from the What Works to Prevent Violence: A Global Programme, funded by the UK Aid from the UK Department for International Development (DFID) for the benefit of developing countries, managed by the South African Medical Research Council; Department for International Development, UK Government. Notes on validation of instruments (screening and outcomes): the CDI is a widely adopted and validated outcome measure. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Khan 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2014 and 2016. Country: Pakistan Income classification: lower‐middle‐income country in 2014‐2016 Geographical scope: urban; “poor urban localities”, “metropolitan districts of Lahore and Rawalpindi” Healthcare setting: primary care facilities, private general practitioner's clinics |
| Participants | 1. Age: at baseline mother mean age: 27.1; child mean age 9.7/15.9 days 2. Gender: female for caregivers, children both 3. Socioeconomic background: not specified 4. Educational background: mean education years in intervention group 8.3 (±3.9), in control 8.6 (±3.8) Inclusion criteria—clinic: a. relative maximum number of years’ establishment; b. higher patient load; c. availability of maternal and child health care. Inclusion criteria—mother/child dyad Each (self or peer‐referred) mother visiting the clinic with a child aged < 40 days was assessed by the clinic assistant for inclusion eligibility based on: a. the child being 2.5 kg at birth; b. free of congenital abnormalities; c. without a history of delayed cry at birth and/or seizures. Exclusion criteria: none reported. Note: considerations on baseline scores not applicable for this study Stated purpose: to assess the effectiveness of delivering a contextualized early child development (ECD) mother‐counselling intervention. |
| Interventions |
Name: Integrated Early Child Development package Title/name of PW and number: clinic assistants (32) and general practitioners (32) 1. Selection: from 32 poor union councils: “Private clinics in the selected union councils were mapped, and two clinics in each union council were shortlisted based on relative maximum number of years’ establishment, higher patient load, and availability of maternal and child health care.” 2. Educational background: general practitioners standard training; clinical assistant “usually a local male, with 10–12 years of schooling but no formal paramedic training”. 3. Training: “Clinic assistants trained on: 1) conduct of structured counselling session using the flip‐book; 2) administration of the first two questions of the Patient Health Questionnaire‐9 (known as PHQ‐2); 3) measurement and recording of child length and weight ‐ Private GPs trained on: 1) clinical management of children with malnutrition and developmental delay in the private clinic setting and specialist referral; 2) diagnosis of maternal depression PHQ‐9”; “Training will last approximately 2 hours, and will include a mixture of explanation by the project field coordinator, and role‐play exercises by participants.” 4. Supervision: from author correspondence: monitoring was done for record‐keeping only. 5. Incentives/remuneration: not specified Prevention type: universal – all mothers with a child aged 40+ days were eligible for inclusion. Intervention details: intervention focused on quarterly counselling of mothers via a specifically designed flip‐book. The tool‐assisted counselling supplements the mother’s ability to promote age‐appropriate activities for ECD and improved child nutrition and to manage her own depression. The contents of each counselling session were designed to take ≤ 10 minutes. The registered mother‐child pairs were required to visit the clinic every 3 months to get assessed and counselled by the clinic assistant, when the child was aged 3, 6, and 9 months. Control: usual care – the current mother‐child care at a private clinic is curative‐oriented (that is, responding to an ailment) rather than health promotion‐oriented. In ECD care, the private clinics do not provide any child development counselling, instead of responding to any complaint that is reported or noted. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: the project was funded by Grand Challenge Canada, Saving Brains, and was implemented by the Association for Social Development, Pakistan (Ref. No. 0585‐03). Notes on validation of instruments (screening and outcomes): the PHQ‐9 is a widely adopted and validated tool. Additional information: none Handling the data: not applicable Prospective trial registration number: not available |
Kuo 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2015‐2017. Country: Cape Town, South Africa Income classification: upper‐middle‐income country from 2004 Geographical scope: urban community outside of Cape Town Healthcare setting: family‐friendly space in a community |
| Participants | 1. Age: for adolescents, the average age was 14; caregivers were older than 18. 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria—adolescents: a. 13‐15 years; b. concurs that the adult identified is their primary caregiver; c. when more than one child in the family falls within the eligible age range, one child will be chosen at random; d. lives in the household at least 4 days a week; e. subclinical thresholds of depressive symptoms. Inclusion criteria—caregivers: a. 18+ years; b. primary caregiver or the person responsible for childcare in the household on a day‐to‐day basis (as identified by the household); c. when more than one primary caregiver exists in the household, one will be chosen at random; lives in the household at least 4 days a week; d. subclinical thresholds of depressive symptoms. Exclusion criteria: a. cognitive impairments that would not allow them to provide informed consent or assent; b. if they participated in qualitative phases of the study; c. report no or low symptoms or clinically significant thresholds of depression. Note: at baseline, total sample scores for the Center for Epidemiologic Studies Depression Scale (CES‐D) were 12.11 (2.05). Stated purpose: to assess the acceptability, feasibility, and preliminary efficacy of Our Family Our Future, a resilience‐oriented intervention engaging families in the prevention of adolescent HIV and depression |
| Interventions |
Name: Our Family Our Future Title/name of PW and number: facilitators (2) 1. Selection: significant prior experience interacting with adolescents and families affected by HIV and poor mental health 2. Educational background: bachelor’s and master’s level training 3. Training: training occurred through an initial two‐week interactive didactic seminar and completion of a mock course of intervention delivery. 4. Supervision: weekly support was provided during the intervention delivery. 5. Incentives/remuneration: not specified Prevention type: indicated – participants were included if they presented subclinical thresholds of depressive symptoms; all those who report no or low symptoms or clinically significant thresholds of depression were excluded. Intervention details: Our Family Our Future is a ‘selective’ behavioural prevention programme, designed to address HIV/STI acquisition, sexual risk behaviour, and depression among adolescents (ages 14‐16) in communities with high HIV prevalence and from families where adolescents and parents already exhibit mild, potentially troublesome, depressive symptoms but do not reach the threshold for further screening for a significant clinical depressive disorder. This intervention involves parent‐child dyads who receive the intervention in a community setting, in a facilitated group format. The intervention is composed of 3‐hour sessions, held weekly for 3 consecutive weeks with an individual family meeting in the third or fourth week depending on family desires. Control: waiting list – participants are offered usual care and delivered the intervention at the end of the study. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the scale (CES‐D) was validated for use in the local population. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02432352 |
Lachman 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2013. Country: South Africa Income classification: upper‐middle‐income country in 2013 Geographical scope: urban, Khayelitsha, a low‐income suburb in Cape Town, South Africa Healthcare setting: intervention delivered by local NGO; when parents missed a session, 1‐to‐1 home consultations were provided. |
| Participants | 1. Age: parents: control 41.09 (13.32), intervention 42.06 (13.16); children: control 5.18 (1.73), intervention 5.62 (1.65) 2. Gender: both 3. Socioeconomic background: 97% of parents were unemployed, 75% lived in informal housing. 4. Educational background: majority (73.5% in control, 90.9% in intervention) had not completed high school. Inclusion criteria: a. isiXhosa‐speaking adults over the age of 18; b. identify themselves as a primary guardian of at least one child aged 3 to 8 years, with elevated child behaviour problems based on a cut‐off of 11 or more problems on the parent‐report form of the Eyberg Child Behavior Inventory Problem scale; c. reside in the same household as their children for at least 4 nights per week, in order to assure adequate time for engagement in parenting skills at home with their children. Exclusion criteria: none reported. Note: at baseline, the intervention and control group scores for the Beck Depression Inventory (BDI) were, respectively, 12.36 and 14.6. Stated purpose: to examine the initial effects of a parenting programme in reducing the risk of child maltreatment in highly‐deprived and vulnerable communities in Cape Town, South Africa |
| Interventions |
Name: Sinovuyo Caring Families Program for Young Children Title/name of PW and number: community‐based workers 1. Selection: not specified 2. Educational background: some basic level of training in early childhood development 3. Training: not specified 4. Supervision: by authors 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the Beck Depression Inventory (BDI) scores, below the cut‐off for clinically significant levels for the measure. Intervention details: 12 weekly sessions. Each session lasted between 2 and 3 hours and included the following activities: (a) opening prayer, (b) mindful physical exercise, (c) children’s song, (d) discussion on home activities from previous session, (e) introduction of core parenting principle, (f) group discussion on the benefits of the principle, (g) working through illustrated stories, (h) practicing parenting skills through role‐plays, (i) assignment of home activities to implement the skills learned during the session, and (j) closing prayer. Facilitators follow a manualized programme protocol designed for low‐resource settings, which requires no equipment beyond homemade toys from recycled materials, paper, and pens. Parenting principles are introduced using traditional stories and illustrated scenarios that mirror typical extended family households in the South African context. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: the research leading to these results received funding from the Ilifa Labantwana Fund (T141/79), the John Fell Fund, University of Oxford (103/757), the South African National Lottery Distribution Trust Fund (43137), and the European Research Council under the European Union's Seventh Framework Programme (FP7/2007‐2013; ERC grant agreement 313421). Notes on validation of instruments (screening and outcomes): the selected measures had been previously used and adapted for use in South Africa. The BDI‐II was validated. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT01802294 |
Lachman 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2015‐2016. Country: Tanzania Income classification: low‐income country in 2015‐2016 Geographical scope: rural, Shinyanga Rural District Healthcare setting: community groups |
| Participants | 1. Age: ages across adults 43.12 (12.71), teens 13.41 (2.01), child 11.18 (3.91), infant 17.94 (9.84) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 26.2% of adults had not completed primary school; 61% of teens enrolled in school; 58.9% of children enrolled in school. Inclusion criteria—caregivers Families who were members of the selected farmer groups in each village and: a. age 18 or older; b. primary caregiver of a child in the household aged 3 to 17; c. lived in household ≥ 4 nights per week; d. registered member of agricultural farmer group and provided consent to participate. Inclusion criteria—children: a. aged 10 to 17 years from all participating families; b. lived in the house ≥ 4 nights per week; c. caregiver participating in study; d. adult and child provided consent; Exclusion criteria: none reported. Note: at baseline, the intervention and control group scores for the Center for Epidemiologic Studies Depression Scale (CES‐D) ranged from 13.80 to 15.12. Stated purpose: to assess the combined and separate effects of parenting and economic strengthening programmes on reducing violence against children |
| Interventions |
Name: Skilful parenting; Agribusiness Title/name of PW and number: professional trainers; trained staff (number not specified) 1. Selection: professional trainers from Investing in Children and Societies, an international nonprofit organization; trained staff from a local economic enterprise initiative working in collaboration with the Ministry of Agriculture 2. Educational background: not specified 3. Training: facilitators were trained using the Skillful Parenting Guide Manual. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – children presented with some level of distress as indicated by the CES‐D scores. Intervention details Skilful parenting: a 12‐session group‐based programme consisting of five sessions on parenting skills, two on child protection, and five on family budgeting. It was delivered to farmer groups by Kiswahili‐speaking professional trainers from Investing in Children and Societies, an international nonprofit organization with local offices in Shinyanga. It reinforces positive parenting practices, empowering parents to address the challenges that they face in bringing up their children. The intervention helps create parent peer groups to share ideas, support, information, and resources in the community. The intervention involves weekly sessions with farmer groups, awareness raising amongst local authorities and communities, and the establishment of parent peer groups. Topics consist of the following issues related to parenting: roles and responsibilities; family relations; communication; values; positive discipline; child protection; and family budgeting. The Agribusiness programme: targets food and income insecurity by providing smallholder farmer groups access to drought‐resistant seeds, credit for farm inputs, advice to improve farming techniques and market connections. The intervention is delivered to farmer group members by trained staff from a local economic enterprise initiative working in collaboration with the Ministry of Agriculture over three intensive workshops during the planting season and ongoing support through harvesting season. Skilful parenting + Agribusiness: a combination of both Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the skilful parenting intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: the Skilful Parenting and Agribusiness Child Abuse Prevention Study was supported by the UBS Optimus Foundation (Grant: 7849.09), the Netherlands Ministry of Foreign Affairs, and the Complexity in Health Improvement Programme of the Medical Research Council MRC UK (Grant: MC_UU_12017/14). Notes on validation of instruments (screening and outcomes): the adopted measures are widely validated and established. Additional information: none Handling the data: NA Prospective trial registration number: NCT02633319 |
Langer 1996.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: not specified Country: Brazil, Cuba, Argentina, Mexico Income classification: Brazil, Argentina, and Mexico upper‐middle‐income, Cuba low‐middle from 1990 to 1996 (year of study publication) Geographical scope: cities of Rosario (Argentina), Pelotas (Brazil), La Habana (Cuba), and Mexico City (Mexico) Healthcare setting: participants’ homes |
| Participants | 1. Age: intervention 24.3 (6.6); control 24.6 (6.6) 2. Gender: female 3. Socioeconomic background: 55.1% in the intervention and 54.9% in control groups had low family incomes. 4. Educational background: schooling years in intervention 8.4 (3.7), in control 8.4 (3.8) Inclusion criteria: Women who were: a. attending the antenatal clinics in the four participating centres between the 15th and the 22nd week of gestation with singleton pregnancies; b. presenting at least one of the following factors: previous low birth weight, stillbirth or infant death; age under 18 years; weight under 50 kg; height under 150 cm; overcrowding; smoking; low family income (according to locally defined cut‐offs); schooling of less than 3 years; lack of husband or partner. Exclusion criteria: presenting chronic conditions that might interfere with pregnancy and require special care. Note: at baseline, the intervention and control group scores for the Spielberger State‐Trait Anxiety‐Inventory (STAI) were, respectively, 39.3 (12.3) and 39.1 (12.5). Stated purpose: to evaluate a psychosocial support intervention during pregnancy aimed at improving perinatal health and mothers' psychosocial conditions |
| Interventions |
Name: psychosocial support Title/name of PW and number: home visitors (not specified) 1. Selection: female social workers or obstetric nurses 2. Educational background: background in social work or obstetric nursing 3. Training: 33 months before the intervention the home visitors received a special training course; they also received an operational manual. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—including all pregnant women with 1+ risk factor. Prevention type: selective—participants were pregnant women included based upon the presence of a risk factor. They also presented with some level of distress as indicated by STAI scores that were below the cut‐off for the measure. Intervention details: the intervention was aimed at increasing social support and reducing stress and anxiety. Its core was four home visits delivered at approximately the 22nd, 26th, 30th, and 34th weeks of gestation. The number of visits could be increased up to six when considered needed. The psychosocial support programme had four main components: (a) reinforcement of social support network; (b) provision of emotional support; (e) improvement of knowledge about pregnancy and delivery; (d) reinforcement of adequate health services utilization. The pregnant woman, the visitor, and a so‐called ‘support person’ participated in the home visits. This ‘support person’ (husband/partner, mother, sister, friend or neighbour) was selected by the patient to share with her intervention activities and was strongly encouraged to be involved throughout the pregnancy, helping the woman to solve problems, promote healthy behaviours and prenatal care attendance. Control: usual care – the control group received the routine antenatal care available at each of the participating institutions. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the STAI Spanish edition had been previously validated in Latin American countries; the scale used for distress evaluation was specifically developed for the study. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Latina 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: not specified Country: Grenada Income classification: upper‐middle‐income country from 1997 Geographical scope: island of Grenada, parishes of St David's, St Andrew's, St George's, St John's, and St Mark's Healthcare setting: local parishes |
| Participants | 1. Age: 18‐95; mean (SD): 51.4 (14.5) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria: a. resident of Grenada; b. one or more vascular risk factors. Exclusion criteria: a. unable/unwilling to provide consent; b. age of under 18 or more than 70 years old; c. inability to self‐monitor; d. pregnant women; e. morbidly ill with the expectation of death within a year. Note: considerations on baseline scores not applicable for this study Stated purpose: to test the effectiveness of peer support strategy for CV risk reduction in the island of Grenada |
| Interventions |
Name: Grenada Heart Project Title/name of PW and number: peer leaders (number not specified) 1. Selection: selected from motivated individuals willing to undergo additional training from the research staff to moderate the peer groups and take attendance at group meetings 2. Educational background: not specified 3. Training: 3‐hour training session on leadership and communication skills in addition to the relevant healthy behaviour promotion 4. Supervision: visits to each group were made by the project administrator in Grenada. 5. Incentives/remuneration: not specified Prevention type: universal – all residents of the community were eligible for inclusion. Intervention details: all participants received a series of educational lectures at time of enrolment. The intervention group was organized into groups of 8–12 individuals in their local parish. The peer group meetings were planned to meet monthly for 1 year, to promote 150 minutes weekly of physical activity, consumption of at least five fruits and vegetables daily, smoking cessation, and blood pressure management. Some of the specific topics discussed included low‐salt diet and hypertension, diabetes prevention, coping strategies for stress, and smoking cessation. Leaders were able to adapt themes for each meeting to their particular interest. Control: usual care – series of educational lectures at the time of enrolment on the themes of motivation to change, physical activity, healthy diet, smoking cessation, blood pressure, and stress management. Upon consenting, participants received a blank notebook and health literacy materials/brochures provided by the American Heart Association. Participants were asked to self manage for 1 year. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: this study was funded by the Louis B Mayer Foundation. V.F. is a recipient of funding from the American Heart Association under grant No 14SFRN20490315. R.F.‐J. is a recipient of funding from the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 707642. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV‐2015‐0505). Notes on validation of instruments (screening and outcomes): not reported Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02428920 |
Leventhal 2015.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study commenced in 2013. Country: India Income classification: low‐middle‐income country from 2013 to date of publication Geographical scope: rural, state of Bihar Healthcare setting: girls' schools |
| Participants | 1. Age: 12.99 (1.17) years 2. Gender: female 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria: all girls attending VII‐VIII Standards (Stds.; equivalent to US 7/8 grades) in the selected schools. Exclusion criteria: none reported. Note: at baseline, the intervention and control group scores for the Patient Health Questionnaire‐9 (PHQ‐9) were, respectively, 7.23 and 8.05. Stated purpose: to examine the effects of Girls First, a combined psychosocial (Girls First Resilience Curriculum [RC]) and adolescent physical health (Girls First Health Curriculum [HC]) intervention (RC, HC) versus its individual components (i.e. RC, HC) and a control group. |
| Interventions |
Name: resilience curriculum; resilience curriculum and physical health curriculum Title/name of PW and number: facilitators (number not specified) 1. Selection: they had to be women, aged 18 years or older. 2. Educational background: at least a Standard X education (equivalent to US 10th grade) 3. Training: PFs were then trained over 5 days to facilitate RC. MTs and PFs received a 3‐day follow‐up training midway through the programme. MTs provided PFs with supervision and refresher trainings approximately twice per month throughout the intervention. This was carried out by Master Trainers with a Master’s level degree in psychology, social work, or a similar discipline. 4. Supervision: supervision and refresher trainings approximately twice per month throughout the intervention by master trainers 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the PHQ‐9 scores. Intervention details Girls First Resilience curriculum: consisted of 23 weekly sessions designed to improve girls’ psychosocial resilience, or their ability to bounce back from challenges. RC includes a strong focus on goals and planning (grouping 1), social support (grouping 3), and identity (grouping 13). Girls First Resilience curriculum + Physical Health curriculum: 2 interventions were tested that together made up Girls First: RC and HC. Both interventions used a facilitated peer‐support pedagogy that included case studies, small group activities, and group discussion. Control: usual care; girls received no intervention beyond attending their middle school academic classes. |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the Girls First Resilience curriculum and Girls First Resilience curriculum + Physical Health curriculum intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): the selected measures are widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Li 2017.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from 2013 to 2017. Country: China Income classification: upper‐middle‐income country from 2010 Geographical scope: poor rural areas Healthcare setting: not specified |
| Participants | 1. Age: adults 18+, children aged 6‐18 2. Gender: both 3. Socioeconomic background: median annual income was 20,000 yuan (3030 USD) 4. Educational background: people living with HIV (PLH): 40% had no education, adult family members: 30% junior high school or above. Inclusion criteria a. PLH: age 18 or over, being a resident of one of the 24 selected villages, who is currently a HIV‐seropositive parent of a child between 6‐18 years in his/her family, and who provides informed consent; b. family members: 18 years and older, being a resident of one of the 24 selected villages, who is aware of PLA's HIV status, who has consent from participating PLH to be invited to join the study, and who provides informed consent. If there are two PLHs in a household, they both will be recruited as PLH participants; c. children: aged 6 to 18 years, being a resident of one of the 24 selected villages, who lives in an HIV‐affected family in which at least one or both parents is HIV‐positive, and who provides parent/guardian permission, child/youth assent forms or informed consent if aged 18. Exclusion criteria: a. those who cannot give informed consent (e.g. intoxicated); b. those who have a permanent disability (e.g. deaf, serious mental illness, mental retardation); c. anyone who does not meet the inclusion criteria. Note: at baseline, the intervention and control group scores for Zung Self‐Rating Depression Scale for PLH were, respectively, 22.3 (5.2) and 22.3 (5.5). Stated purpose: to evaluate the efficacy of an intervention aimed at improving the mental health of PLH and their family members. |
| Interventions |
Name: Together for Empowerment Activities (TEA) Title/name of PW and number: 2‐3 intervention facilitators for each facilitator team 1. Selection: a team of intervention facilitators recruited from a pool of health educators working at various agencies at provincial, country, and township levels 2. Educational background: local health educators 3. Training: they had no systematic training in mental health, but they understand that health includes both physical and mental health and know resources/services for mental health needs. All intervention facilitators went through intensive training and numerous mock sessions. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective prevention – participants are PLH and their family members that reside in poor rural areas. The depressive symptoms at baseline are in the “normal range” (25‐49). Intervention details: the TEA intervention activities were delivered: 1) at the individual level, TEA Gathering—six separate intervention sessions were conducted for PLH and their family members to deal with their specific HIV‐related challenges. Interactive group activities, such as games, pair‐share, role‐plays, and discussions, were delivered to establish a healthy daily routine, improve physical and mental health, improve family and parent‐child relationships, and encourage community integration; 2) at the family level, TEA Time—six types of family activities were conducted at home after each TEA Gathering session. The family activities involved all members to strengthen family interaction and support; and 3) at the community level, TEA Garden—three community events consisting of a health fair, an amusing sports event, and a family talent show were organized by both intervention participants and community leaders to enhance community and social integration. These initial intervention activities took place between 6 and 8 weeks. To maintain the intervention effect, reunions were held every 2 months during the first 12 months and every 4 months during the remainder of the study period (10 total reunion sessions). The intervention was manualized. Control: usual care – PLH in the control group continued to receive the Chinese government’s standard of care for the population. In addition, limited programme activities were added to the control group to tease out intervention effects from the impact of attention. There were three group sessions once a week, with the content areas focused on healthy daily routine, antiretroviral drugs adherence and side effects, nutrition, and personal and family hygiene. These control group sessions were didactic lecture‐based health education, with no interactive activities between facilitators and participants nor between PLH and family members. In addition, village health workers visited the control group families once a week for the initial 3 weeks and once a month for 12 months. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7 to 24 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this work was supported by the National Institute of Child Health & Human Development/National Institutes of Health under Grant [R01HD068165]. Notes on validation of instruments (screening and outcomes): Zung Self‐Rating Depression Scale: the scale has been validated among PLH and their family members in China in 2011 (Cronbach’s alpha = 0.81). Perceived Caregiver Burden Scale: used in the previous studies in Asia (Cronbach’s alpha = 0.88). Additional information: none Handling the data: not available Prospective trial registration number: NCT01762553 |
Li 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2016. Country: China Income classification: upper‐middle‐income country in 2016 Geographical scope: Bengbu, Anhui Province, China Healthcare setting: clinic of a medical college‐affiliated hospital |
| Participants | 1. Age: intervention: 28.50 ± 3.58; control: 28.16 ± 3.15 2. Gender: female 3. Socioeconomic background: family average income 3000‐5000 RMB for approximately half of the participants and above 5000 for the other half 4. Educational background: approximately half had achieved a maximum education level of senior high school and half a university degree or above. Inclusion criteria: a. able to communicate and understand Chinese; b. had no severe medical conditions including heart disease, diabetes, and kidney disease. Exclusion criteria: a. pre‐existing mental illness, such as depression; b. complications of pregnancy, such as incipient abortion. Note: at baseline, the intervention and control group scores for Pregnancy Pressure Scale (PPS) were, respectively, 0.26 (0.17) and 0.23 (0.15). Stated purpose: to examine the effectiveness of cognitive‐behavioural stress management for pregnant women |
| Interventions |
Name: cognitive‐behavioural stress management Title/name of PW and number: facilitator (4) 1. Selection: from the antenatal care staff team, with extensive work experience and highly developed communication skills 2. Educational background: had professional training 3. Training: “received training for the study”, no further information provided 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—including all pregnant women. Participants presented with some baseline level of stress below the cut‐off for the measure. Intervention details: the intervention group received cognitive intervention, relaxation techniques training, and problem‐solving training and obtained a social support strategy for a pregnant woman. The intervention was carried out an extra 7 times during the whole period of pregnancy: 4 times (once every two weeks) between 12 and 28 weeks, 2 times (once every 4 weeks) between 28 and 36 weeks, and 1 time between 37 and 38 weeks. The sessions covered information on (1) the physiological and psychological changes in pregnancy, (2) coping methods for pregnancy stress, (3) relaxation techniques and (4) family support. Following each seminar, the facilitator invited participants to participate in group discussions to share their pregnancy experiences, including their problem‐solving strategies. The cognitive‐behavioural stress management was delivered mainly through face‐to‐face communication in the hospital, but in some cases, it was delivered via phone and email. Control: usual care – the control group received routine antenatal care and pregnancy health education instruction (including advice on diet, foetal monitoring and daily care during pregnancy). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Department of Education of Anhui Province Notes on validation of instruments (screening and outcomes): the scale was developed for use in China, tested, and demonstrated to have good variability. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Luoto 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted in 2018‐2021. Country: Kenya Income classification: lower‐middle‐income country Geographical scope: rural—subcounties of East Rachuonyo, South Rachuonyo, and Sabatia in western Kenya Healthcare setting: group sessions took place in local community centres or churches; these were paired with home visits for one of the intervention arms. |
| Participants | 1. Age: mother's age around 27‐29 2. Gender: both 3. Socioeconomic background: these predominantly rural areas are characterized by high rates of poverty, child mortality, and stunting (31 to 34%). 4. Educational background: around 8.8 years of education for mothers, and 9.4 to 9.7 for fathers Inclusion criteria Mothers or other female primary caregivers: a. aged 15 years or older; b. with a child aged 6–24 months without signs of severe mental or physical impairment; c. if married or in established relationships, fathers or male caregivers aged 18 years or older were also eligible to participate. Exclusion criteria: child with signs of severe mental or physical impairment. Note: at baseline, the intervention (1 and 2) and control group scores for Center for Epidemiologic Studies Depression Scale (CES‐D) were, respectively, 10.3 (6.1); 9.8 (7.1); and 10.2 (7.1). Stated purpose: to test the effectiveness of two group‐based delivery models for an integrated ECD responsive stimulation and nutrition education intervention using Kenya’s network of community health volunteers. |
| Interventions |
Name: group‐based parenting interventions Title/name of PW and number: community health volunteer (CHV) (40) 1. Selection: part‐time volunteers and members of their communities tasked with improving community health and linking individuals to primary health‐care services 2. Educational background: mean 11 years of education and mean 9 years of CHV experience 3. Training: training of sessions 1 to 8 took place at the start of the programme; training of sessions 9 to 16 took place at midline. CHVs received a manual in their language of delivery and in English. Trainings included both classroom time to review each session as well as supervised practice with mothers and children. Monthly 1‐day refresher trainings were also performed in each subcounty to ensure CHVs were prepared ahead of each session. Knowledge was tested with a paper‐and‐pencil test at the end of each training. Research team and later NGO staff were following a train‐the‐trainers model. 4. Supervision: CHVs were rated on skills such as facilitating discussion, coaching parents, answering questions, as well as overall session quality and engagement. CHVs were provided with supervisor feedback immediately after each session. Trained members of local NGO involved in intervention implementation. 5. Incentives/remuneration: the research project paid CHVs a monthly stipend for their duties, according to local policy. Prevention type: indicated – programme tailored for all mother‐child dyads in the selected communities: mothers presented with some level of distress as indicated by the CES‐D scores. Intervention details Msingi Bora (Good Foundation) — group sessions only: the Msingi Bora curriculum focused on five key practices: responsive play, responsive communication, hygiene, nutrition, and love and respect in the family. The sessions emphasized parents learning new practices with their child, spouse, and peers through demonstration and coached practice, group‐based problem‐solving, and peer support. Sessions took place in local community centres or churches. Every fourth session served as a review session, for which households receiving the group‐only intervention continued with group meetings. In half of the villages, fathers were invited to participate in the intervention. They were invited to all 16 sessions, 12 of which were for both mothers and fathers and 4 of which were separate sessions by sex, including the first 2, as a way to try to encourage their participation, practising respectful communication, father involvement in child‐care, and emotional support between spouses. Similar topics were covered in the four corresponding mother‐child sessions so that the curriculum was identical across mothers, regardless of the intervention group. Msingi Bora (Good Foundation) – group sessions + home visits: households receiving the mixed‐delivery intervention received individual home visits. Community health volunteers in mixed‐delivery villages visited each participant household during the same week that a group review session was held in group‐only villages. During these home visits, the community health volunteers delivered review messages identical to those in the group reviews, but the focus was tailored to that family. The same fathers involvement procedures were carried out in this group too. Control: usual care – households in villages assigned to the comparison group did not receive any interventions other than information about child feeding during the baseline survey. |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the Msingi Bora (Good Foundation) group sessions only from intervention and control groups. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health Notes on validation of instruments (screening and outcomes): no specifications given for the scales included in the review; nevertheless, the used tools have been widely validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03548558 |
Masquiller 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from 2007 to 2010. Country: South Africa Income classification: upper‐middle‐income country from 2007 Geographical scope: urban—five districts in the Free State Province of South Africa (i.e. Lejweleputswa; Motheo; Thabo Mofutsanyana; Fezile Dabi; Xhariep) Healthcare setting: 12 public antiretroviral (ART) clinics across the five districts |
| Participants | 1. Age: 38.9 (SD: 9.5) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: most received some secondary education. Inclusion criteria: a. HIV‐positive; b. eligible for public sector ARV treatment (CD4 < 200 and/or WHO stage 4); c. commenced ARV treatment in past 4 weeks; d. patient resident in town/village where ART clinic was located. Exclusion criteria: a. HIV‐negative; b. not eligible for public sector ARV treatment; c. had not commenced ARV treatment; d. commenced ARV treatment longer than 1 month ago; e. patient not resident in town/village where ART clinic was located. Note: considerations on baseline scores not applicable for this study Stated purpose: to analyze the influence of a peer adherence support (PAS) intervention and the family environment on the state of hope in PLWHA |
| Interventions |
Name: PAS Title/name of PW and number: peer adherence supporters (approximately 60) 1. Selection: individuals had to have been on ART therapy for ≥ 12 months and live within walking distance from the relevant clinic. 2. Educational background: at least a grade‐10 certificate 3. Training: peer adherence reporters received 5 days of basic training in ART and adherence support. The training focused on seven main themes: facts about HIV/AIDS, ART, adherence supported needed by an ART client, nutrition, infection control at home, and using a healthcare team approach. On the 5th day of training, peer adherence supporters’ knowledge and practical skills were assessed by the trainers using an oral test and practical exercise. The training was provided by staff at the school. 4. Supervision: supervisors visited each clinic once a month to meet with the relevant peer adherence supporters. During this visit, peer adherence supporters received re‐training on selected topics included in the original main training to refresh and maintain knowledge transfer. In addition, supervisors debriefed PAS, dealt with any logistic problems, and collected completed checklists from PAS. Supervisors completed a standard, short report for each site visit. Following each monthly visit, supervisors met with the PAS co‐ordinator at the Faculty of Medicine, who collated information from their reports and further discussions into a monthly progress report. Information in the monthly report was used to determine PAS payments for the subsequent month. The supervision was delivered by two trained peer adherence support co‐ordinators. 5. Incentives/remuneration: peer adherence supporters were paid a monthly stipend of $100 (ZAR 800), conditional on performance. Prevention type: selective—participants were included based upon the presence of a risk factor (living with HIV/AIDS). Intervention details: the intervention consisted of additional biweekly PAS for a period of 18 months. The peer adherence supporters were people living with HIV/AIDS (PLWHA) who had been on ART for at least 12 months and who had received theoretical and practical training on HIV/AIDS, ART and adherence, nutrition and infection control in the home, based on material developed by the UFS’s School of Nursing. When visiting patients, peer adherence supporters provided support with adherence and discussed matters that can make adherence more difficult (e.g. stigma). They identified possible side effects of ART and acted appropriately. When necessary, they referred patients to a clinic. Other topics (e.g. unemployment or pension grants) were discussed as well. Control: usual care – antiretroviral therapy (ART) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months; 7‐24 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: we are particularly grateful to the following funding agencies: The Research Committee of the World Bank, the Bank‐Netherlands Program Partnership, WB‐DfID Evaluation of the Community Response to HIV and AIDS, the Programme to Support Pro‐Poor Policy Development (PSPPD; a partnership between the Presidency, Republic of South Africa and the European Union), the Health Economics and Aids Research Division (HEARD) at the University of
Kwazulu‐Natal, the University of the Free State (UFS), and South Africa’s National Research Foundation (NRF). Notes on validation of instruments (screening and outcomes): HADS scales are widely validated and established. Additional information: none Handling the data: not available Prospective trial registration number: DOH‐27‐ 0907‐2025; NCT00821366 |
McCann 2015.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2007‐2008. Country: Thailand Income classification: low‐middle‐income country in 2007‐2008 Geographical scope: provinces in northern Thailand Healthcare setting: carers were recruited from the outpatient department of a psychiatric hospital. |
| Participants | 1. Age: 41 ± 9 years 2. Gender: both 3. Socioeconomic background: 74.1% employed 4. Educational background: 51.9% high school or below Inclusion criteria: a. primary carer of an adult receiving treatment at the outpatient department for moderate depression (International Classification of Disease‐10 classification); b. aged 18–60 years; c. capable of writing and reading Thai; d. had a telephone at home. Exclusion criteria: presently receiving treatment for acute mental illness (as advised by outpatient department nurses). Note: at baseline, the intervention and control group scores for Experience of Caregiving Inventory (ECI) total negative score were, respectively, 100.8 (37.4) and 99.5 (33.8). Stated purpose: to examine the efficacy of a cognitive behaviour therapy‐guided self‐help manual in increasing resilience in caregivers of individuals with depression, in comparison to caregivers who receive routine support only. |
| Interventions |
Name: guided self‐help Title/name of PW and number: caregivers, as the intervention is a guided self‐help book (27 caregivers assigned to the intervention arm) 1. Selection: caregivers of patients diagnosed with depression 2. Educational background: described in population section 3. Training: participants were asked to complete one module of the self‐help book per week. 4. Supervision: treatment fidelity was evaluated during the weekly telephone calls, where participants were asked predetermined questions about the content of the module finished that week. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (caregiving) and presented with some level of distress as indicated by the ECI scores that were below the cut‐off for the measure. Intervention details: the manual comprised eight modules: (i) gives an outline of depression and encourages readers to engage in physical activity; (ii) affirms the importance of maintaining social contact and physical exercise; (iii) enables participants to discern the way they feel and think; (iv) emphasizes how to change thought patterns from negative to positive; (v) highlights how healthy living, social support, and problem‐solving enable behaviour change and contribute to overcoming depression; (vi) equips the person to improve sleep pattern and to sustain favourable thoughts, behaviours, and emotions; (vii) illustrates how to practice progressive muscle relaxation to assist them to deal with stress; and (viii) re‐emphasizes earlier learned skills in thought challenging, dealing with difficult events and changing behaviour. Participants were asked to finish one module per week, and each module took approximately 2 hours to complete. The manual was provided for 8 weeks only. Control: usual care – control group participants were given standard support while accompanying the family member with depression to the outpatient department for prescription of antidepressant or antianxiety and antidepressant medications and consultations. Standard support included receiving minimal support and information from mental health nurses about supporting the affected family member. |
| Outcomes |
Participants’outcomes of interest for this review
Note: included as adult outcome given that the intervention was designed for caregivers only Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months post‐intervention) |
| Notes |
Source of funding: self‐funded/unfunded Notes on validation of instruments (screening and outcomes): the measure was translated and adapted for use in Thailand; no specifications were given on its psychometric properties. Additional information: none Handling the data: not applicable Prospective trial registration number: ACTRN12614000774628 |
McConachie 2000.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: from 1993‐1995 (recruitment) to 2000 (publication) Country: Bangladesh Income classification: low‐income country until 2014 Geographical scope: one special school is in central Dhaka; the other community school is near Dhamrai in a rural setting within 50 km. Healthcare setting: clinics at 2 school bases of the Bangladesh Protibondhi Foundation |
| Participants | 1. Age: children 1.5‐5 years; mothers from 24.1 (3.8) to 28.0 (5.3) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 18‐56% of mothers had no education Inclusion criteria: a. children between the ages of 1.5 and 5 years at presentation; b. lived in Dhaka or within 15 km of the rural base; c. assessed by a neurodevelopmental paediatrician to have cerebral palsy; d. mothers of children with cerebral palsy. Exclusion criteria: there were no exclusion criteria. Note: at baseline, the intervention (1 and 2) and control group scores for Self‐Report Questionnaire (SRQ) were, respectively, 4.44 (3.12) for urban and 7.79 (5.77) for rural context; 4.45 (3.75) and 7.08 (4.93). Stated purpose: to compare the efficacy of an outreach programme for young children with cerebral palsy with centre‐based and “minimal intervention” control groups |
| Interventions |
Name Intervention 1: distance training Intervention 2: mother‐child group Title/name of PW and number: “therapist” 1. Selection: from special education teachers 2. Educational background: not specified 3. Training: additional in‐service training in physiotherapy or speech therapy 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (mothers of children affected by developmental disability) and presented with some level of distress as indicated by the SRQ scores that were below the cut‐off for the measure. Intervention details Intervention 1: conducted both in rural and urban locations. Simple outreach way of giving advice to parents of children living far from the schools. Caregivers were given the pictorial manuals, which illustrate positions, activities, and simple home‐made aids adapted for children with impairments, based on well‐established sources, and different manuals cover motor skills, speech and language, and cognitive skills. The suggestions appropriate to the child’s stage of development are practised with parents in a 1‐ to 2‐hour session before they take the manual home. This is combined with monthly visits to “therapists”, for demonstrations and practicing of techniques. Intervention 2: conducted in urban area only. The mother‐child group intervention was held in a part of the urban special school; it was offered daily, led by a “therapist” with extra training in physiotherapy, and involved both the children and their parents. It was thus a centre‐based, regular attendance intervention. Practice in daily living skills such as using a cup and developmental activities such as sorting by colour were included. Control: enhanced usual care—conducted in rural area only. The parents and children in the health advice group were usually seen only at the beginning of the study and then not again until invited to attend the final assessment. The child’s health was discussed as part of the assessment, and then nutritional advice and vitamin supplements were given to the parents as appropriate (this also applied to the other intervention groups on the first assessment). The parents in the health advice group were not given detailed advice on the positioning or other techniques but were given a box of simple local toys and books for their children to play with at home (not given to parents in the other groups). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): the SRQ and FSS are validated and established. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Miller 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2019‐2020. Country: Lebanon Income classification: upper‐middle‐income country from 2018 Geographical scope: urban—city of Tripoli in North Lebanon Healthcare setting: offices of three community‐based organizations (CBOs) with which War Child Holland collaborates in the target communities |
| Participants | 1. Age: children 3 to 12; parents not specified 2. Gender: both 3. Socioeconomic background: most caregivers were not working. 4. Educational background: most parents had at least primary education. Inclusion criteria: a. Syrian refugee or vulnerable host community families with at least one child between the ages of 3 to 12 years; b. both primary caregivers willing to participate in the study and willing to commit to attending all nine sessions of the Caregiver Skills Intervention if randomized to the Caregiver Skills Intervention arm of the study; c. participating caregivers are Arabic speaking. Exclusion criteria: a. prior or current participation by either caregiver in a parenting or stress management intervention; b. family does not have a child aged 3 to 12 years; c. anyone who is unable, even with assistance, to complete the assessment questionnaires; d. unwillingness of either caregiver to give informed consent. Note: at baseline, the intervention and control group scores for Kessler Psychological Distress (K10) were, respectively, 30.27 (9.74) and 29.69 (8.12); for the Warwick‐Edinburgh Mental Wellbeing Scale (WEMWBS), 49.52 (7.27) and 49.21 (6.75). Stated purpose: to test the feasibility of our study methodology prior to conducting a definitive RCT on the Caregiver Skills Intervention |
| Interventions |
Name: Caregiver Support Intervention (CSI) Title/name of PW and number: facilitators (number not specified) 1. Selection: non‐mental health specialist, 24 years or older, with at least 2 years of experience implementing psychosocial interventions, preferably with adults, even more preferably with parents/caregivers, emotionally mature 2. Educational background: high school education required, university education desirable 3. Training: CSI facilitators participate in a 6‐day (42‐hour) training which combines didactic and experiential components and includes extensive practice implementing the intervention within the training group. In addition to covering the specific content of the sessions (e.g. parental stress and well‐being, relaxation exercises, coping with anger and frustration, child development, positive parenting), trainees also learn group facilitation skills through extensive role‐playing and continuous feedback. 4. Supervision: weekly supervision by a trained social worker (supervised by PI and local psychologist) 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (parents with a refugee background) and presented with some level of distress. The WEMWBS scores were not indicative of low mental well‐being. Intervention details: the CSI is a nine‐session weekly group intervention, offered separately to women and men. Sessions one through four focus exclusively on caregiver well‐being (covering topics such as stress and relaxation, lowering stress, and coping with frustration and anger). Sessions five through eight focus on strengthening parenting under conditions of adversity and draw heavily on social learning theory and commonly used methods of training in positive parenting (i.e. increasing awareness of the impact of stress on parenting, increasing positive parent‐child interactions and the use of non‐violent discipline methods, and reducing harsh parenting). Session 9 entails a review and closing of the intervention. In addition, in each session, participants are introduced to a new relaxation or stress management technique. These techniques are also provided to participants in Arabic on mp3 files, which they can either play on their smartphones or on mp3 players provided at the start of the programme. Participants are encouraged to practice any relaxation or stress management activity at least three times each week. Control: waiting list – receiving intervention upon completion of the study |
| Outcomes |
Participants’outcomes of interest for this review
Note: the primary focus of the intervention is promoting caregiver mental health. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: funding by grants from the Bernard van Leer Foundation and Open Society Foundations Notes on validation of instruments (screening and outcomes): the adopted measures were tested for their psychometric properties in the study. Additional information: not applicable Handling the data: not applicable Prospective trial registration number: not reported |
Mohamadi 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted during the academic year of 2018‐2019. Country: Iran Income classification: upper‐middle income country in 2018‐2019 Geographical scope: urban and suburban area of Shahroud Healthcare setting: schools |
| Participants | 1. Age: mean age of students was 14.44 ± 0.51 years. 2. Gender: female 3. Socioeconomic background: not specified 4. Educational background: students Inclusion criteria: eighth grade students with Iranian nationality. Exclusion criteria: a. consumption of psychiatric drugs; b. having family problems such as death or divorce of parents in the past 6 months; c. not attending two consecutive classes and not cooperating with the researcher during the study. Note: at baseline, the intervention (1 and 2) and control group scores for Standard Symptom Checklist‑25, Persian version (SCL‑25) were, respectively, 52.56 (3.05); 50.74 (2.80); and 51.49 (3.61). Stated purpose: to compare the motivational interview and peer groups in promoting mental health and knowledge and performance about puberty health in adolescent girls |
| Interventions |
Name: group counselling Title/name of PW and number Intervention 1: master in consultation in midwifery Intervention 2: peer students 1. Selection Intervention 1: not specified Intervention 2: active volunteers who scored higher on the puberty health questionnaire prior to the start of the study 2. Educational background Intervention 1: master in consultation in midwifery Intervention 2: students 3. Training Intervention 2: in one session, the educational content was explained to peer educators by the researcher. 4. Supervision Intervention 2: peer‐to‐peer educators’ relationship with the researcher continued after the intervention so that educators could ask their questions. 5. Incentives/remuneration: none Prevention type: universal – all adolescent girl students were eligible for inclusion and their baseline scores for the SCL‑25 were well below the cut‐off for the measure. Intervention details Intervention 1—group counselling: motivational interviewing was presented to students (groups of 7‐9 students) by a master in consultation in midwifery during five sessions of 60 to 90 minutes and one session per week. Intervention 2—peers: one formal training session was held by peer educators to other students, and the information was then passed on informally to peers in small groups (5 to 6 students) within 1 month. Each peer educator was responsible for transmitting information to 5 to 6 other students. Control: waiting list – no intervention (after sampling and intervention, two training sessions on puberty health were conducted by the researcher). |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the peers and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months) |
| Notes |
Source of funding: none Notes on validation of instruments (screening and outcomes): Persian version of SCL‑25 questionnaire. Cronbach’s alpha for the short‐form questionnaire of mental health is 0.97. Additional information: none Handling the data: not applicable Prospective trial registration number: IRCT20180209038675N1 |
Novelli 2018.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2013 and 2014. Country: Brazil Income classification: upper‐middle‐income country in 2013‐2014 Geographical scope: urban, Santos, a large seaside city in the Southeast of Brazil Healthcare setting: home of participants |
| Participants | 1. Age: patients: 81.37 ± 7.57; caregivers 65.97 ± 10.13 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: patients: 9.97 (± 5.32) years; caregivers: 12.10 (± 4.44) years; “In addition, caregivers who participated had high levels of education, which do not reflect the Brazilian demographic characteristics.” Inclusion criteria—caregivers Family caregivers had to a. be 18 years of age or older; b. provide at least 4 hours of daily care; and c. be willing to learn to use activities during care. Inclusion criteria—people with dementia a. 60 years of age or older; b. previous diagnosis of dementia according to National Institute on Aging‐Alzheimerʼs Association criteria; c. able to perform at least 2 basic activities of daily living (e.g. bathing, grooming, and dressing); d. presence of ≥ 2 BPSD in the last 30 days; e. being under stable pharmacological treatment for at least 3 months. Exclusion criteria: none reported. Note: at baseline, the intervention and control group scores for Zarit Burden Interview were, respectively, 30.33 (11.44) and 32.47 (11.55). Stated purpose: to evaluate the effects of the Tailored Activity Program—Brazilian version (TAP‐BR) on behavioural symptoms and the quality of life (QOL) in persons with dementia, as well as on their caregivers and on caregiver burden |
| Interventions |
Name: TAP‐BR Title/name of PW and number: occupational therapist (7) 1. Selection: not specified 2. Educational background: not specified 3. Training: 24 hours of training involving lectures and role‐play by a master trainer certified in the TAP programme 4. Supervision: closely supervised by the study co‐ordinator and participated in biweekly meetings to review cases and troubleshoot implementation challenges 5. Incentives/remuneration: not specified Prevention type: indicated – participants, caregivers of people with dementia, presented with some level of distress as indicated by the Zarit Burden Interview scores. Intervention details: intervention focusing on matching activities to the capabilities, habits, and interests of the person with dementia, as well as training of the caregiver in their use as part of daily care. It consists of 8 sessions, delivered over 4 months, divided into 3 treatment phases. 1) Assessment: to identify preserved capabilities; 2) implementation: to find and provide training for potential activities of interest to be implemented by caregivers; 3) generalization: to provide caregiver training to learn how to simplify the activity in preparation for future declines, and generate the strategies learned. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Note: included in the adult population given that the intervention does not have a prevention component for patients with dementia Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): “All measures used in this study with people with dementia and caregivers are widely used in clinical practice and scientific research and have been validated for use in Brazil.” Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Osborn 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2019 and 2020. Country: Kenya Income classification: low‐middle‐income country in 2019 and 2020 Geographical scope: urban, Kiambu County, outskirts of Nairobi Healthcare setting: private secondary school |
| Participants | 1. Age: 13‐18 years 2. Gender: both 3. Socioeconomic background: private boarding school admitting low‐income students 4. Educational background: 1‐3rd form (grades 9‐11) Inclusion criteria: a. participants must be aged between 13 and 18 years; b. all Kenyan high school students; c. male and female. Exclusion criteria: there were no exclusion criteria for any participant who met the inclusion criteria. Note: at baseline, the intervention and control group scores for Patient Health Questionnaire‐8 (PHQ‐8) were, respectively, 10.60 (5.37) and 9.68 (4.75). Stated purpose Psychosocial purpose of the trial: to reduce depressive and anxiety symptoms and to improve overall well‐being in Kenyan high school students. |
| Interventions |
Name: Shamiri‐Digital Wellness Title/name of PW and number: no PWs 1. Selection—self‐help intervention: no PWs 2. Educational background: no PWs 3. Training: the intervention was an internet‐based digital intervention (one of the modules included a “growth testimonial” by a peer; feedback from recent school graduates [peers] was involved in the adaptation to digital form). 4. Supervision: students were informed that they could talk to the study staff should they be distressed and that depending on the kind and severity of the distress, the staff would seek help per local customs and regulations in the school. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the PHQ‐8 scores. Intervention details: the Shamiri‐Digital intervention consists of three modules: growth mindset, gratitude, and value affirmation. In the growth‐mindset module, participants learned about the brain’s ability to grow in response to challenges in various domains (e.g. academic, interpersonal, and personality traits). Then, participants read a growth testimonial written by a Kenyan peer. Afterward, participants wrote their own growth stories about a challenge they faced and overcame. In the gratitude module, participants learned about the importance of practising and expressing gratitude. In a “good things” exercise, participants listed three good things in their lives for which they were grateful. In the value‐affirmation module, participants learned about the importance of affirming personal values (presented as “virtues,” the more common term in Kenya). Participants wrote about a time in which they used their values to guide life decisions. The programme included no audio or multimedia content. Manual: full intervention available online at http://supp.apa.org/psycarticles/supplemental/ccp000050/ccp0000505_supp.html Control: active control—it consisted of two modules, note‐taking skills and effective study habits. In the first module, participants learned a step by‐ step framework for note‐taking. Participants then reflected on how they could use this framework to improve their studying, and they practised by applying the skill to a brief article. In the module on effective study habits, they learned five study habits they could use to optimize the time spent studying. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): PHQ‐8: the PHQ‐8 has also demonstrated adequate internal consistency (α = 0.73) and discriminant validity with Kenyan adolescents. Cronbach’s alpha for the PHQ‐8 in the present study was 0.73. GAD‐7: it has shown adequate internal consistency (α = 0.78) and discriminant validity with Kenyan youths. In the present study, the Cronbach’s alpha for the GAD‐7 was 0.82. SWEMWBS: the Cronbach’s alpha for the SWEMWBS in the present sample was 0.70. Additional information: copyright and permission – those who wish to reuse APA copyrighted material in a non‐APA publication must secure from APA written permission to reproduce a journal article in full or journal text of more than 800 cumulative words or more than 3 tables and/or figures. APA normally grants permission contingent on permission of the author, inclusion of the APA copyright notice on the first page of reproduced material, and payment of a fee of $25 per page. Libraries are permitted to photocopy beyond the limits of U.S. copyright law: (1) post‐1977 articles, provided the per‐copy fee in the code for this journal (0022‐ 006X/20/$12.00) is paid through the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923; (2) pre‐1978 articles, provided that the per‐copy fee stated in the Publishers’ Fee List is paid through the Copyright Clearance Center. For more information along with a permission request form go to: www.apa.org/about/contact/copyright/index.aspx. Handling the data—supplementary materials available: dx.doi.org/10.1037/ccp0000505.supp Prospective trial registration number: PACTR201906810558181 |
Ozcan 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between July 2016 and June 2017. Country: Turkey Income classification: upper‐middle income country in 2016‐2017 Geographical scope: city located in the east of Turkey Healthcare setting: of the eight sessions, the first one was held in the hospital and the others at the participants’ homes. |
| Participants | 1. Age: between the ages of 18 and 35; mean age 25.16 (SD 4.0) in the intervention group and 25.09 (SD 4.5) in the control group 2. Gender: female 3. Socioeconomic background: total range of income $118‐$1354 4. Educational background: high school for 30.9% in intervention group and 32.7% in control group; undergraduate for 29.1% in both groups Inclusion criteria: a. between the ages of 18 and 35; b. primiparae; c. able to speak, read, and write in Turkish; d. full‐term (between weeks 38 and 42) vaginal delivery; e. haemoglobin value of at least 10 mg/dL; f. experienced risky conditions during gestation or delivery; g. undergone mediolateral episiotomy (because episiotomy impairs the integrity of a tissue. Healing such episiotomy incisions as soon as possible is quite important to conserve structural integrity). Exclusion criteria: a. lost to follow‐up; b. unable to communicate; c. withdrawing from the study voluntarily. Note: at baseline, the intervention and control group scores for World Health Organization Quality‐of‐Life Scale, Psychological health domain (WHOQOL), Psychological Health Domain were, respectively, 67.72 (15.24) and 63.48 (16.25). Stated purpose: to identify the application of LCM in the nursing process in women within the postpartum period and to investigate the effect of postpartum care given in line with LCM on primiparae |
| Interventions |
Name: Levine's Conservation Model (LCM) program Title/name of PW and number: nurse (1) 1. Selection: not specified 2. Educational background: nurse training with pilates knowledge background 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (first pregnancy) Intervention details: LCM as theoretical framework. Eight‐session nursing care programme based on LCM was provided to the women in the intervention group within a period of 12 weeks. Each session lasted approximately 60 to 120 minutes. In these trainings, leaflets containing information about breastfeeding, personal hygiene, fatigue, sleep, nutrition and Pilates exercises prepared by the researchers in the light of the literature data were used. Control: usual care (standard nursing care given after birth, solely containing breastfeeding training) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): WHOQOL scale translated into Turkish and revised by Eser 1999. In this study, the Cronbach’s alpha value was 0.89. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
O’Callaghan 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2012. Country: Democratic Republic of Congo Income classification: low‐income country in 2012 Geographical scope: rural—Li‐May and Kiliwa, two small villages in Dungu territory, in Haut Uele Province, with an estimated combined population of less than 1000 inhabitants Healthcare setting: local churches |
| Participants | 1. Age: 7‐18 years old 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria Children ages 7 to 18 and their caregivers: a. living in a war‐affected community; b. facing current risks of attack/abduction by armed groups. “…it was not designed as a mental health intervention to treat specific psychiatric conditions and so no symptom cut‐off points were used for eligibility”. Exclusion criteria none reported Note: at baseline, the intervention and control group scores for the 8‐item Impact of Events Scale (CRIES‐8) were, respectively, 11.80 (5.56) and 11.89 (5.28). Stated purpose: to develop and evaluate a community‐participative psychosocial intervention involving life skills and relaxation training and mobile cinema screenings with this war‐affected population living under current threat |
| Interventions |
Name: family‐focused psychosocial intervention Title/name of PW and number: local lay facilitators (6: 3 males, 3 females) 1. Selection: not specified 2. Educational background: not specified 3. Training: facilitators were given a copy of the manualized intervention in French and met for 3 hours with the lead researcher the day before delivering each module. 4. Supervision: facilitators met for 3 hours with the lead researcher the day before delivering each module in order to review the previous module taught, and prepare for the subsequent intervention session. A translator was hired so that the lead researcher could monitor the teaching components of the intervention, provide on‐site clinical supervision during sessions and ensure that each section in each module of the manual was covered in the intervention. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (living in war‐affected area) and presented with some level of distress as indicated by the CRIES‐8 scores that were below the cut‐off for the measure. Intervention details: a psychosocial intervention based on 3 components: (1) “ChuoChaMaisha”, a youth life skills leadership programme developed and piloted in Tanzania; (2) Mobile Cinema clips—narrative, fictional films, produced and created in the local language to address stigma and discrimination and model how young people, parents and the village community could welcome formerly abducted children back into their communities; and (3) relaxation technique scripts used in trauma‐focused CBT. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: “This project was funding by a donor who wishes to remain anonymous and who financed the costs of the intervention through the NGO, Discover the Journey. The funding organization had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.” Notes on validation of instruments (screening and outcomes): all adopted measures were previously validated for use in DR Congo. Additional information: not applicable Handling the data: not applicable Prospective trial registration number: NCT01542398 |
Panter‐Brick 2018.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2015‐2016. Country: Jordan Income classification: upper‐middle‐income country in 2015, lower‐middle income country in 2016 Geographical scope: urban, “youth residing in Jordanian urban centers into five cycles of program delivery” Healthcare setting: youth centres, designed as ‘Adolescent Friendly Spaces’ in partnership with local community‐based organizations engaged in building civic society or development training, open 9 am to 9 pm. In northern Jordan, the programme was implemented in the urban centers of Irbid, Jarash, Mafraq, Ajloun, and Zarqa governorates. |
| Participants | 1. Age: 12 to 18 years 2. Gender: both 3. Socioeconomic background Across cycle 1 (quasi‐experimental design) and cycle 2 (RCT): household wealth index of mean 7.88 (SD: 2.88) at T2 and 7.67 (2.99) at T3; Syrian refugees were poorer. 4. Educational background Across cycle 1 (quasi‐experimental design) and cycle 2 (RCT): education grade (0‐12) mean 7.04 (SD: 2.15) at T2 and 6.98 (2.16) at T3. Inclusion criteria: “Eligibility is based on vulnerability and need, determined by Mercy Corps staff during screening interviews to assess age, self‐reported mental health difficulties and poor access to local services. Siblings are included when families prefer brothers and sisters to travel and participate together.” Exclusion criteria: a. not being a refugee; b. not having self‐reported mental health difficulties and poor access to local services. Note: at baseline, the intervention and control group scores for Strengths and Difficulties Questionnaires (SDQ) were, respectively, 14.99 (6.29) and 14.85 (5.90). Stated purpose: to test the impacts of an 8‐week programme of structured activities informed by a profound stress attunement (PSA) framework (Advancing Adolescents), delivered in group format to 12‐ to 18‐year‐olds in communities heavily affected by the Syrian crisis |
| Interventions |
Name: Advancing Adolescents Title/name of PW and number: coaches (number not specified) 1. Selection: male and female coaches are lay volunteers (21‐ to 60‐year‐olds) from the local area. 2. Educational background: not specified 3. Training: “they complete a 16‐day training in program delivery, to work as instructors, facilitators, mentors, and animators. The Coaches Foundation Training Programme focuses on emotional and behavioural regulation (‘Hearts and Heads’) and experiential learning (‘Creative Facilitation’). The manual incorporates the following components: practising healthy communication; defining profound stress, its impact on the human brain, and principles of attainment; developing gender equity and adolescent protection; building psychosocial resilience; and enhancing skills of creative facilitation for effective technical training. The coaches develop technical training ‘session plans’ to lead structured activities, with guidance from Mercy Corps.” This was conducted by the Mercy Corps monitoring and evaluation team. 4. Supervision: “A lay coordinator monitors and supports the project plans during their development and implementation. Weekly meetings are scheduled to review progress, share experiences and address issues arising. Refresher training courses are offered to lay coaches before each new cycle of implementation.” 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor. Quote: “Eligibility is based on vulnerability and need, determined by Mercy Corps staff during screening interviews to assess age, self‐reported mental health difficulties and poor access to local services. Siblings are included when families prefer brothers and sisters to travel and participate together.” Participants presented with some level of distress as indicated by SDQ scores that were below the cut‐off for the measure. Intervention details: the Advancing Adolescents (Arabic: Nubader) programme is a structured, 8‐week psychosocial intervention for adolescents in humanitarian crises, based on profound stress attainment processes. It features three elements that are widely viewed as important to support youth adjustment in contexts of complex emergencies: (a) safety – establishment of a ‘safe space’ within the community as a base for activities and site of protection; (b) support – facilitation of social support and self‐expression; and (c) structured, group‐based activities. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: not available Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this research was funded by Elrha’s Research for Health in Humanitarian Crises (R2HC) Programme. The R2HC programme is funded equally by the Wellcome Trust and the UK Government. Notes on validation of instruments (screening and outcomes): “we used regionally/internationally validated scales, chosen on the basis of their simplicity, cultural relevance, psychometric properties and usage in conflict areas.” Additional information: none Handling the data: not applicable Prospective trial registration number: NCT03012451 |
Prabhakaran 2019.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from 2016 to 2017. Country: Haryana (North India) and Karnataka (South India) Income classification: low‐middle‐income country from 2007 Geographical scope: rural Healthcare setting: 20 community health centres |
| Participants | 1. Age: 55.1 ± 11.0 years 2. Gender: both 3. Socioeconomic background: 27.1% were employed. 4. Educational background: 42.9% had higher than primary school education. Inclusion criteria Participants: a. were ≥ 30 years of age; b. intended to reside in the catchment area of CHCs for ≥ 1 year; c. had been diagnosed with hypertension with systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or type 2 diabetes mellitus with fasting blood glucose ≥ 140 mg/dL or postprandial blood glucose ≥ 200 mg/dL. Exclusion criteria: a. pregnant women, patients with type 1 diabetes mellitus, patients requiring immediate referral to tertiary care because of accelerated hypertension or diabetic complications; b. patients with learning difficulties or vision or hearing impairments; c. patients with malignancy or other life‐threatening conditions. Note: considerations on baseline scores not applicable for this study Stated purpose: to assess the impact of the mWellcare system on mental health status in patients with hypertension and diabetes |
| Interventions |
Name: mWellcare system Title/name of PW and number: physicians, nurses 1. Selection: not specified 2. Educational background: not specified 3. Training: it provided centralized training on the current clinical management guidelines to all physicians. For NCD nurses, it provided training in the management of hypertension, diabetes mellitus, depression, and tobacco and alcohol use. In addition, 3 days of training were provided to nurses on using the mWellcare system. 4. Supervision: the training was supplemented by another 2 days of onsite supervision and support. They conducted a rigorous process evaluation in the trial. Trained research staff using a structured observation checklist conducted periodic monitoring visits of trial sites to assess the fidelity of the intervention (by staff). 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (chronic condition: hypertension or diabetes). Intervention details: the mWellcare system was an Android application built on the CommCare platform. The mWellcare system was designed to generate EDS recommendations for the management of hypertension and diabetes mellitus, comorbid depression, and alcohol and tobacco use, tailored to the participant’s profile and risk level. It stored the health records electronically, enabling long‐term monitoring and follow‐up. It was also equipped to send short message service reminders (to take medication and attend follow‐up visits) to patients. In the mWellcare arm, the NCD nurse used a tablet computer installed with the mWellcare system to collect data on patient history, blood pressure, blood glucose, depression, tobacco and alcohol use, and current medications. From this patient‐specific clinical information, the mWellcare system generated a decision support recommendation (DSR) for the physician. The DSR printout summarized information on patient profile, diagnosed condition, comorbid conditions, and previous and current medications and recommended a treatment plan for the 5 chronic conditions based on standard guidelines. The DSR also provided a lifestyle modification advisory and date for the next follow‐up visit. Control: usual care – in the control group, they provided training to physicians on the clinical management guidelines for hypertension and diabetes mellitus. In addition, as with the mWellcare arm, charts on the management of these conditions were displayed prominently at the outpatient clinics. They also conducted the NCD nurses’ training in the management of hypertension and diabetes mellitus. To balance both study arms, they provided the EUC NCD nurses with a tablet computer (without the mWellcare system) for collecting data at the baseline visit. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: this research study was supported by the Wellcome Trust (grant 096735/A/11/Z). The funding source had no role in the design of this study; during the execution,
analyses, and interpretation of data; or in the decision to submit results. Notes on validation of instruments (screening and outcomes): the PHQ‐9 is a widely established and validated tool. Additional information: none Handling the data: the online‐only Data Supplement is available with this article at www.ahajournals.org/doi/suppl/10.1161/circulationaha.118.038192. Prospective trial registration number: NCT02480062 |
Rachasrimuang 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted around 2016 and published in 2018. Country: Thailand Income classification: upper‐middle‐income country in 2016‐2018 Geographical scope: rural, Mainapiang Subdistrict, Wangyai District, Khon Kaen province, Thailand Healthcare setting: homes of the participants, in 9 villages in the area |
| Participants | 1. Age: 60+; mean age 71 to 72 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: most had completed primary school or lower. Inclusion criteria: a. 60 years old and over; b. lived in the study area for more than 6 months. Exclusion criteria: elderly persons who are dependent, severely disabled, and unable to participate in activities and communicate with others. Note: at baseline, the intervention and control group scores for Geriatric Depression Scale, Thai version (TGDS) were, respectively, 70.28 (18.56) and 72.12 (23.88). Stated purpose: to evaluate the effectiveness of home visits programme by a youth volunteer on the health‐related quality of life and depression amongst elderly persons living in a rural community |
| Interventions |
Name: home visits by youth volunteers Title/name of PW and number: youth volunteers (~25) 1. Selection: not specified 2. Educational background: attending grades 6 to 9 in extended primary school 3. Training: training for 3 days and 2 nights on (1) What and who is ageing? Ageing situation in Thailand and its impact; (2) Limitation and changing in older persons; (3) Youth and volunteer spirit to brighten the community future; (4) Sharing, giving and sacrifice; (5) Mission possible—How to take care of older persons; (6) Helping each other work as a team; and (7) Be ready—set a work plan together. This was conducted by the research team. 4. Supervision: youth volunteers were monitored by the research team; no further details. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (being elderly people). Participants were older adults living in the area; they presented with some level of distress as indicated by TGDS scores that were below the cut‐off for the measure. Intervention details: the study intervention was a home visit by youth volunteers for 18 weeks. Each volunteer was assigned to the same 6/7 elderly people. They were trained on (1) What and who is ageing? Ageing situation in Thailand and its impact; (2) Limitation and changing in older persons; (3) Youth and volunteer spirit to brighten the community future; (4) Sharing, giving and sacrifice; (5) Mission possible – How to take care of older persons; (6) Helping each other work as a team; and (7) Be ready – set a work plan together. Control: usual care – conventional care by their family and children |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months postintervention) |
| Notes |
Source of funding: the study was supported by the Tawanchai Foundation for Cleft Lip‐Palate and Craniofacial Deformities and the Center of Cleft Lip‐ Cleft Palate and Craniofacial Deformities, Khon Kaen University, under the Tawanchai Royal Grant Project. Notes on validation of instruments (screening and outcomes): the measures used were validated for use in Thailand and are widely adopted across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Rahimi 2021a.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from November 2018 to April 2019. Country: Iran Income classification: upper‐middle income country in 2018‐2019 Geographical scope: Mashhad, Iran Healthcare setting: home (telecommunication by using telephone and virtual social networks) |
| Participants | 1. Age: intervention and control groups in the mean of age (51.1 ± 8.4 vs 48.5 ± 8.3 years, respectively) 2. Gender: both 3. Socioeconomic background: for control group, 53.4% sufficient income; for intervention group, 60% less than adequate income 4. Educational background: in both groups, most of the patients had nonacademic education. Inclusion criteria: a. willingness to participate, providing written informed consent for participation; b. age at least 18 years; c. having colorectal cancer based on cytological diagnostic findings and confirmed by an oncologist (based on patient files); d. having minimum literacy, auditory and visual health; e. willingness to share their telephone number for calls and the ability to use smartphones. Exclusion criteria: a. unwillingness to continue participation; b. returning incomplete questionnaires; c. not completing the intervention course for any reasons or attention for < 75% of the determined amount; d. not establishing successful telephone and Internet calls; e. having unstable clinical conditions during the research period, e.g. haemodynamic changes, reduced consciousness level, or the occurrence of fistulas. Note: at baseline, the intervention and control group scores for Warwick‐Edinburgh Subjective Wellbeing Scale (WEMWBS)—Subjective well‐being were, respectively, 27.8 (5.4) and 27.6 (6.3). Stated purpose: to evaluate the impact of peer support through telecommunications on the subjective well‐being of colorectal cancer patients |
| Interventions |
Name: peer support programme with telecommunication Title/name of PW and number: 4 (2 men and 2 women) patients (peer counsellors) 1. Selection: previously mentioned criteria for selecting patients, in addition to scoring at least 40 on the WEMWBS and successfully passing the stages of treatment 2. Educational background: having minimum literacy (see inclusion criteria) 3. Training: a workshop session (120 min) was held for familiarizing the volunteers in the peer group with the research. Peer volunteers received explanations on the objectives of the study. They also acquired knowledge and skills for providing their experimental knowledge to the intervention group. In this workshop, the peers were familiarized with subjective well‐being, ways to establish supportive and strong relationships, and subjective well‐being improvement strategies. Training was provided by a psychologist. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (cancer patients); the WEMWBS scores at baseline are not indicative of low mental health. Intervention details: the intervention group received the support programme (for emotional, information, and evaluation dimensions) by the peers. The peer support programme met two times a week by phone and three times a week using virtual social networks (based on patients’ preferences). Control: usual care (routine nursing care programme) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: Deputy for Research Notes on validation of instruments (screening and outcomes): in the present study, the reliability of the instrument (WEMWBS) was determined using internal and external reliability methods. For this purpose, a questionnaire was given to 30 patients. Values of Cronbach’s alpha for optimism construct, energetic structure, and positive relationship with others structure were 0.783, 0.741, and 0.748, respectively. Cronbach’s alpha had high internal reliability (α = 0.875). Also, the reliability of the test‐retest in two weeks was confirmed for the whole questionnaire (r = 0.81). Additional information: none Handling the data: not applicable Prospective trial registration number: IRCT20190123042480N1 |
Rahman 2009.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: not specified Country: Pakistan Income classification: low‐income country until 2008, lower‐middle from 2009 Geographical scope: rural—Kaller Syedan, a rural subdistrict of Rawalpindi, Pakistan Healthcare setting: home of the participants in the 24 villages for intervention |
| Participants | 1. Age: mean age 27.3 2. Gender: female 3. Socioeconomic background: family income mean—3060 Pakistani rupees 4. Educational background: years of education, mean—5.9 to 6.3 Inclusion criteria: a. all women in the 3rd trimester of pregnancy were eligible; b. married; c. in their last trimester of pregnancy that were registered with LHWs. Exclusion criteria: a. women with a complicated pregnancy; b. women with a medical condition. Note: at baseline, the intervention and control group scores for WHO Self‐Reporting Questionnaire‐20 (SRQ) were, respectively, 7.87 (4.88) and 7.13 (4.72). Stated purpose: the purpose of this study was to carry out a more robust assessment of the impact of the ‘Learning Through Play’ programme using a cluster‐randomized design. |
| Interventions |
Name: Learning Through Play Title/name of PW and number: lady health workers (number not specified) 1. Selection: members of local community 2. Educational background: have completed secondary schools 3. Training: full‐day training workshop and refresher session of 1 hour on the second birth month stage of development by trained psychologist. They were given the manual for reference and suggesting teaching guidelines. 4. Supervision: the researchers monitored the training of LHWs and mothers to ensure that the programme was adhered to. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (last trimester of pregnancy) and presented with some level of distress as indicated by SRQ scores that were below the cut‐off for the measure. Intervention details: the ‘Learning Through Play’ programme is intended to stimulate early child development. The central feature of the program is a pictorial calendar devised for parents, depicting eight successive stages of child development, with illustrations of parent–child play and other activities that promote parental involvement, learning and attachment. A key feature of the ‘Learning Through Play’ programme is its emphasis on the quality of the mother–infant interaction and helping the mother read infant cues and develop sensitive responsiveness towards the infant through play, which can be pleasurable for both the mother and the infant. LHWs conducted half‐day workshops with groups of participants (6‐8 mothers). This was followed by fortnightly home visits where LHWs spent 15 to 20 minutes with the mothers to discuss their child’s development; the mothers were encouraged to meet in groups on their own to support each other in the use of the techniques outlined in the calendar. Control: usual care – routine follow‐up visits |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): the outcome is widely adopted and validated across contexts. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Rajeswari 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from May 2015 to June 2017. Country: India Income classification: low‐middle‐income country in 2015‐2017 Geographical scope: urban and rural—63 (50.40%) of the intervention group and 59 (47.20%) of the control group were residing in a suburban area. Healthcare setting: at the Department of Obstetrics and Gynaecology, Sri Ramachandra Institute of Higher Education and Research (outpatient) |
| Participants | 1. Age: majority 25‐29 2. Gender: female 3. Socioeconomic background: 28‐29% had high school education. 4. Educational background: not specified Inclusion criteria: a. low‐risk primigravidae; b. 21‐22 weeks of gestational age; c. planning to undergo delivery and postnatal care; d. having minimal to moderate stress. Exclusion criteria: a. primigravidae associated with medical and obstetrical complications; b. practising any other relaxation technique; c. not willing to participate. Note: considerations on baseline scores not applicable for this study Stated purpose: to assess the efficacy of progressive muscle repose on stress and anxiety amongst primigravidae |
| Interventions |
Name: progressive muscle relaxation Title/name of PW and number: PC health workers (2) 1. Selection: not specified 2. Educational background: doctor of nursing practice and an expert in reproductive medicine 3. Training: not specified 4. Supervision: monitoring only for intervention adherence by phone calls to participants 5. Incentives/remuneration: not specified Prevention type: indicated – participants with minimal to moderate stress were eligible for inclusion. Intervention details: along with the routine care, progressive muscle relaxation was taught by the researcher on a one‐to‐one basis to the primigravidae from 21 to 22 weeks of gestation with the help of a video for two consecutive days, with each session lasting for 20‐25 minutes. The routine was followed by the primigravidae in the following days. In progressive muscle relaxation, each muscle group such as arms, face, shoulder, and upper and lower extremities are tensed for 10 seconds and released, taking a few deep breaths. It begins with the top of the body and goes down. To ensure daily practice, weekly reinforcement was given through phone; direct reinforcement was given during antenatal check, and also, diary of performance was maintained by the primigravidae. It was ensured that every day phone calls were made until the message was delivered. Control: usual care – standard antenatal care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months) |
| Notes |
Source of funding: nil Notes on validation of instruments (screening and outcomes): the selected measures are widely adopted and used across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Ramezani 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: it is unclear when the study was conducted; it was published in 2017. Country: Iran Income classification: upper‐middle‐income country from 2010 to 2017 Geographical scope: urban—Shahroud (northeast of Iran) Healthcare setting: 11 urban healthcare centres that provide services for pregnant women |
| Participants | 1. Age: the mean age of the cognitive‐behavioural counselling, solution‐focused, and control groups were 25.17 ± 5.2, 26.14 ± 4.8, and 26.2 ± 3.9, respectively. 2. Gender: female 3. Socioeconomic background: the majority of participants in the cognitive‐behavioural counselling, solution‐focused, and control groups had a moderate economic status (43.5%; 60.96, 53.1%, respectively). 4. Educational background: at least primary education; the mean number of years of education of the cognitive‐behavioural counselling, solution‐focused, and control groups were 13.2 ± 2.7, 13.3 ± 3.,1 11.8 ± 3, respectively. Inclusion criteria: Nulliparous pregnant women who a. who had at least a primary education; b. did not participate in childbirth preparation classes. Exclusion criteria: a. having a chronic disease; b. addiction, history of mental illnesses such as schizophrenia, depression, anxiety in the past and in this pregnancy. Note: at baseline, the intervention (1 and 2) and control group scores for Austin inventory—Persian version were, respectively, 6.1 (4.6); 4.2 (3.6); and 6.71 (4.9). Stated purpose: to evaluate the effect of cognitive‐behavioural approach and solution‐focused counselling on prevention of postpartum depression in nulliparous pregnant women |
| Interventions |
Name Intervention 1: solution‐focused counselling Intervention 2: cognitive‐behavioural counselling Title/name of PW and number: PC health worker (PHW), midwife (1) 1. Selection: not specified 2. Educational background: not specified 3. Training: “The counseling was conducted by a midwife who had been trained in counseling for two years; the same PHW conducted both interventions.” 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—all nulliparous pregnant women with no history of mental disorders or chronic illness were eligible for inclusion. Intervention details Intervention 1—solution‐focused counselling: 3 times 1.5‐hour counselling sessions (weekly) + routine pregnancy healthcare services; the content of the sessions included explaining the purpose of counselling, introducing maternity blues and postpartum depression, the exact definition of the problem by the client in the form of a sentence and a word, setting objectives and operational goals, reviewing the solutions to the problem, providing and checking homework, finding exceptions. Intervention 2—cognitive‐behavioural: 4 times 1.5‐hour counselling sessions (weekly) + routine pregnancy healthcare services; the content of the sessions included explaining the purpose of counselling, introducing maternity blues and postpartum depression, explaining the role of thoughts on emotions, providing and checking homework to record thoughts and feelings, explaining the thoughts of depressed people, training on depression‐prevention activities (e.g. relaxation, mother and baby skin‐to‐skin contacts). Control: usual care – routine pregnancy healthcare services |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the solution‐focused intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: nil Notes on validation of instruments (screening and outcomes): the selected measures are widely adopted across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Rao 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: no information on when the study was conducted; it was published in 2017. Country: India Income classification: low‐middle‐income country from 2007 Geographical scope: urban—Bangalore City Healthcare setting: schools |
| Participants | 1. Age: 30‐55 2. Gender: female 3. Socioeconomic background: upper‐middle and middle class 4. Educational background: intervention: 17 ± 1.5 years; control: 17.3 ± 1.2 years Inclusion criteria: a. female teachers; b. aged between 30 and 55 years; c. willing to participate in the study; d. had no previous exposure to any form of yoga practice. Exclusion criteria: a. suffered from any psychological disorder; b. had a recent history of a surgical intervention; c. had sleep problems or were on sleep medication; d. had neurological or metabolic disorders; e. had experienced a head injury or stroke; f. were pregnant. Note: at baseline, the intervention and control group scores for Spielberg’s State‐Trait Anxiety Inventory (STAI)—state anxiety were, respectively, 44.30 (12.49) and 43.53 (10.08). Stated purpose: the study intended to examine the effects of a mind sound resonance technique (MSRT) intervention for 1 month on perceived stress, quality of sleep, cognitive function, state and trait anxiety, psychological distress, and fatigue amongst female teachers. |
| Interventions |
Name: MSRT Title/name of PW and number: yoga teacher (1) 1. Selection: certified female yoga teacher 2. Educational background—from author correspondence: “she had studied psychology course in her PG”. 3. Training—from author correspondence: “She (Yoga expert) was trained in delivering yoga protocol (MSRT) in the university by an Assistant Professor of Yoga.” 4. Supervision—from author correspondence: “The study participants received supervised MSRT sessions for the entire duration of the study by the yoga expert and the whole intervention was monitored by a researcher (researcher supervisor ‐ Ph.D. holder).” 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of baseline anxiety as indicated by the Spielberg’s STAI scores, but all those who suffered from any psychological disorder were excluded. Intervention details: “Mind sound resonance technique (MSRT) is a mindfulness‐based, yogic relaxation technique that includes the generation of an internal vibration and resonance all over the body after chanting the Mahamrityunjaya mantra and syllables such as A, U, M, and OM, repeatedly. It can be practised in a sitting or supine position. The intervention was carried out in a silent room from 11:00 AM to 12:00 PM in a supine posture. Regular attendance was assessed by maintaining an attendance register, and participants with 70% attendance were included in the statistical analysis.”; “Participants in the MSRT group participated in MSRT for 30 min/d, 5 d/wk, for the duration of 1 mo. The participants in the control group followed their normal daily routines.” Control: usual care – “the participants in the control group followed their normal daily routines”. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): all the selected measures are widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Richards 2014.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2010. Country: Uganda Income classification: low‐income country in 2010 Geographical scope: Gulu, Uganda (largest urban center in northern Uganda) Healthcare setting: 10 selected primary schools |
| Participants | 1. Age: 11 to 14 2. Gender: male 3. Socioeconomic background: not specified 4. Educational background: enrolled in sixth grade Inclusion criteria—schools: out of 33 primary schools in Gulu municipality, pupils from the 10 most centrally located were selected for assessment. Inclusion criteria—adolescents: all boys enrolled in sixth grade at these schools could take part in the RCT. Exclusion criteria: none reported. Note: at baseline, the intervention and control group scores for Acholi Psychosocial Assessment Instrument—Depression were, respectively, 21.20 (11.61) and 24.79 (13.16). At baseline, the intervention and control group scores for Acholi Psychosocial Assessment Instrument—Anxiety were, respectively, 8.14 (4.50) and 9.01 (5.16). Stated purpose: to examine the effects of a sport‐for‐development programme on adolescent physical fitness and mental health in Gulu, Uganda |
| Interventions |
Name: sport‐for‐development Title/name of PW and number: coaches (6 paid staff and 32 volunteers) 1. Selection: volunteers were selected by paid staff. 2. Educational background: not specified 3. Training: coaches received 2 weeks of training to develop their coaching skills prior to the season commencing. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (living in a post‐conflict area) and presented with some level of distress as indicated by the Acholi Psychosocial Assessment Instrument scores that were below the cut‐off for the measure. Intervention details: community‐based programme lasting 11 weeks. The goal was to use sport as a vehicle to promote physical fitness and mental health. Coaches were encouraged to promote participation and equal game time for all team members. Each coach was provided with equipment to conduct at least one 1.5‐hour training session per week. Each weekend the GMKL participants took part in a 40‐minute game of football. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: this study was funded by the DPhil scholarship at the University of Oxford of the chief investigator and the sponsors of the sport‐for‐development organizations that implemented the intervention (OA Projects, The Kids League). Notes on validation of instruments (screening and outcomes): the selected scale to assess depression and anxiety (Acholi Psychosocial Assessment Instrument) was developed, validated, and its reliability tested in Gulu. Additional information: none Handling the data: not applicable Prospective trial registration number: not reported |
Rivet‐Duval 2011.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from 2003 to 2004. Country: Mauritius Income classification: upper‐middle‐income country in 2003‐2004 Geographical scope: urban Healthcare setting: public schools |
| Participants | 1. Age: from 12 to 16 years 2. Gender: both 3. Socioeconomic background: almost all participants lived with their nuclear families, and the majority had at least one parent employed on a full‐time basis. 4. Educational background: single‐sex secondary public schools in Mauritius Inclusion criteria: all students in Years 7 and 9 at two single‐sex schools were invited to participate. Exclusion criteria: there were no specified exclusion criteria. Note: at baseline, the intervention and control group scores for Reynolds Adolescent Depression Scale‐2 (RADS‐2) were, respectively, 51.81 (9.07) and 50.61 (9.70). Stated purpose: to assess the efficacy of a universal prevention programme for adolescent depression implemented by school teachers in Mauritius. |
| Interventions |
Name: RAP‐A programme Title/name of PW and number: eight programme facilitators (teachers) 1. Selection: experienced teachers (six females, two males), four from each school 2. Educational background: not specified 3. Training: facilitators attended a 2‐day training workshop involving 16 hours of training conducted by one of the research team who was a certified RAP trainer; this trainer also provided ongoing support for the teachers when required. The training workshop involved: 1) information on adolescent mental health, and specifically adolescent depression; 2) theory underlying the programme; 3) programme content and implementation techniques. 4. Supervision: to maintain programme integrity, one half‐day booster training session was organized 6 months following initial training. 5. Incentives/remuneration: not specified Prevention type: universal – all students in years 7‐9 in the selected schools were eligible for inclusion, and their baseline scores for the RADS were well below the cut‐off for the measure. In addition, the authors defined the intervention as “a universal prevention program for adolescent depression”. Intervention details: the RAP‐A programme is a manualized group treatment programme developed by Shochet, Holland, and Whitefield (Shochet 1997a; Shochet 1997b). It involved 11 one‐hour weekly sessions with 8 to 12 participants per group. It included both cognitive‐behavioural and interpersonal approaches covering topics such as building self‐esteem, keeping calm, self‐talk, thinking resourcefully, problem‐solving, identifying and accessing support networks, considering the perspective of others, and keeping the peace. To maintain programme integrity, the programme was delivered in English, and the overall structure and content of the programme were unchanged. The cultural relevance of the programme was discussed with the programme facilitators (teachers), and their feedback indicated that no changes were required to the programme. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 1‐6 months) |
| Notes |
Source of funding: not reported Notes on validation of instruments (screening and outcomes): the reliability and validity of the RADS‐2 are adequate. Additional information: none Handling the data: not reported Prospective trial registration number: ACTRN12608000137392 |
Rockers 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2015 and 2016. Country: Zambia Income classification: low‐middle‐income country in 2015‐2016 Geographical scope: rural area in the catchment areas of 5 health facilities in Choma and Pemba districts, Southern Province, Zambia Healthcare setting: health facilities |
| Participants | 1. Age: caregivers average 27 years; child 6‐12 months 2. Gender: caregivers were female, children both. 3. Socioeconomic background Household wealth quintile, mean (SD)—control: 2.85 (1.42), intervention: 3.13 (1.41). 4. Educational background: fewer than half had completed primary school. Inclusion criteria: to be eligible for the study, a household had to have a child between 6 and 12 months of age at the time of enrolment. Exclusion criteria: caregivers younger than 15 years of age were excluded. Note: At baseline, the intervention and control group scores for Self‐Reporting Questionnaire (SRQ), score > 7 were, respectively, 3.72 (2.89) and 4.57 (3.45). Stated purpose: to evaluate the impact of a community‐based parenting group intervention on child development in Zambia |
| Interventions |
Name: community‐based parenting group intervention Title/name of PW and number: child development agent (CDA) was a community‐based health worker; head mothers were members of the community (number not specified). 1. Selection: CDAs were selected through consultation within communities; head mothers were selected by the members of the group. 2. Educational background: CDAs had previous experience providing community‐based health services. 3. Training: “prior to the start of the study, CDAs were trained on how to support group meetings”; “CDAs trained on the curriculum 3 meeting rounds at a time every 6 weeks”; head mothers were trained by CDAs according to the planned curriculum prior to each round. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: universal – all households with a child in the selected age range were eligible for inclusion, and their baseline scores for the SRQ were well below the cut‐off for the measure. Intervention details Original 1‐year study—2 services: a) fortnightly home visit by a CDA who screened and referred children for infections and acute malnutrition and encouraged caregivers to use routine child health services; b) parent group meetings where they were taught a diverse curriculum that included content on cognitive stimulation and play practices, child nutrition, and cooking practices, and self‐care for good mental health. These were led by a trained “head mother”. During the year 2 study extension, the household visit component of the intervention was dropped, while facilitation of the fortnightly parenting group meetings continued. This change was motivated by the findings from the year 1 assessment, which suggested that the parenting groups were the primary driver of observed positive behaviour change. Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) |
| Notes |
Source of funding: the first year of the study was funded through grants from Grand Challenges Canada (0349‐03) and PATH (DFI.1836‐672968‐GRT). The second year of the study was funded through a grant from the Policy Research Fund at the Department for International Development (DFID), United Kingdom (55204321). At the time of the study, author R.C.H. worked in the DFID Zambia office and was not part of the Policy Research Fund team. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Notes on validation of instruments (screening and outcomes): the WHO SRQ is a widely adopted measure that has been validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02234726 |
Rodriguez 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in May 2018. Country: China Income classification: upper‐middle income country in 2018 Geographical scope: Beijing Healthcare setting: home |
| Participants | 1. Age: mean age was 23.5 years (SD 3.17). 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 29 (54%) were master’s students, 21 (39%) were undergraduate students, and 4 (7%) were doctoral students. Inclusion criteria: a. currently enrolled in a university in China (undergraduate, graduate, or doctoral); b. has a smartphone and regular access to the internet; c. demonstrates the ability to read and understand Mandarin; d. reports passing at least College English Test (level 4); e. experiences at least mild depression and anxiety. Exclusion criteria: a. aged < 18 years; b. does not provide proof of current student status and emergency contact; c. currently experiences manic or psychotic symptoms; d. expresses suicidal or homicidal ideation during the intake phone interview. Note: at baseline, the intervention and control group scores for Patient Health Questionnaire‐9 (PHQ‐9) were, respectively, 11.56 (5.3) and 9.70 (4.7). At baseline, the intervention and control group scores for Generalized Anxiety Disorder‐7 (GAD‐7) were, respectively, 8.96 (4.2) and 8.33 (4.0). At baseline, the intervention and control group scores for Depression Anxiety Stress Scale (DASS‐21) were, respectively, 9.48 (3.3) and 8.31 (3.5). Stated purpose: to examine whether an adjunctive, task‐shifting component (MIND+) enhances treatment engagement in a mindfulness intervention for stress and depression amongst Chinese undergraduate and graduate students |
| Interventions |
Name: MIND+—internet‐based mindfulness intervention (Be Mindful internet‐based and self‐guided course) plus peer counsellor support Title/name of PW and number: 4 volunteer peer counsellors 1. Selection Peer coaches' inclusion criteria: is currently enrolled in a university in Beijing (undergraduate, graduate, or doctoral); has a smartphone and regular access to the internet; demonstrates the ability to read and communicate in Mandarin and English; is willing to provide brief (15‐20 minute) peer‐support chats per week per participant; is willing to participate in web‐based group supervision for 1 hour per week; is willing to complete the internet‐based mindfulness intervention. Exclusion criteria: is aged < 18 years; reports previous or current format training in mindfulness or psychotherapy; reports current treatment (psychotherapy or medication) for a mental health problem; is unable to attend the day‐long, in‐person training in Beijing. We selected 4 individuals as peer counsellors based on their English proficiency, reported level of enthusiasm for the project, and the researchers’ assessment of their nonspecific factors. 2. Educational background: university students 3. Training: in‐person training took place for 8 hours in Beijing; training was didactic and experimental; candidates were given opportunities to practice using the skills in dyads and to receive coaching and feedback; training was provided by the first author and research assistants. 4. Supervision: weekly group supervision with research coordinator (MR), 2 research assistants, and the 4 peer counsellors 5. Incentives/remuneration: participants were compensated for completing the baseline questionnaire packet, post‐treatment questionnaires, and for responding to each daily assessment; the total amount that the participants could make from this course was approximately US $28. Prevention type: indicated – participants were included on the basis of the presence of mild depressive and anxiety symptoms, but all those who presented severe psychiatric symptoms were excluded. Intervention details: 4‐week internet‐based mindfulness intervention plus peer counsellor support. The Be Mindful course is an internet‐based mindfulness training programme produced by Wellmind Media. “Be Mindful delivers all the elements of mindfulness‐based cognitive therapy in an internet‐based course that can be completed in 4 weeks” (self‐guided course). In MIND+ condition, peer counsellors were encouraged to provide a brief (15‐20 minutes) weekly meeting to support and encourage participants in their completion of the internet‐based intervention. Control: other (MIND [only] intervention – Be Mindful internet‐based and self‐guided course) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): for the PHQ‐9, the Cronbach α reliability coefficient in this sample was 0.85. For GAD‐7, the Cronbach α reliability coefficient in this sample was 0.87. For DASS‐21, the Cronbach α reliability coefficients for depression, anxiety, and stress in this sample were 0.82, 0.74, and 0.77, respectively. Additional information: none Handling the data: not applicable Prospective trial registration number: not specified |
Rong 2021a.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from May to December 2019. Country: China Income classification: upper‐middle income country in 2019 Geographical scope: Wuhan, located in central China Healthcare setting: prenatal clinic of a large hospital for the recruitment, but yoga intervention was conducted in a spacious and bright yoga classroom in the same city. |
| Participants | 1. Age: mean age for intervention group 29.00 ± 2.81 years and for control group 28.16 ± 2.78 years 2. Gender: female 3. Socioeconomic background—monthly per capita income (yuan): 6000 to 10,000 (40.6% intervention group, 31.3% control); > 10,000 (37.5% intervention group and 40.6% control) 4. Educational background: mostly college graduate (75% intervention group and 71% control) Inclusion criteria: a. primigravida, 18–27 weeks of gestation; b. over 18 years of age; c. singleton pregnancy; d. deemed suitable for yoga exercise based on a physical exam; e. Chinese‐speaking; f. living in Wuhan until delivery. Exclusion criteria: a. preferred a painless delivery; b. major obstetric or medical‐related complications; c. foetal abnormalities or intrauterine growth retardation (IUGR); d. had experienced major stress‐inducing events in the latest week; e. participation in yoga or an exercise programme of similar intensity during the previous 12 months or simultaneous exercise. Note: at baseline, the intervention and control group scores for Edinburgh Postnatal Depression Scale (EPDS) were, respectively, 9.63 (4.32) and 8.53 (4.34). At baseline, the intervention and control group scores for State Anxiety Inventory (STAI) were, respectively, 38.25 (8.74) and 34.84 (9.93). Stated purpose: to evaluate the efficacy of yoga on physiological and psychological discomforts and delivery outcomes in Chinese primiparas |
| Interventions |
Name: yoga Title/name of PW and number: 2, a professional yoga instructor and the researcher 1. Selection: not specified 2. Educational background: the professional yoga instructor had a degree of Master in Yoga from Zhejiang University. 3. Training: researcher’s specialized training had been provided by the instructor prior to this study, allowing the researcher to assist in guiding and correcting participants’ yoga postures during the exercise. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the EPDS and STAI scores. Intervention details: 12‐week yoga exercise three times per week. Each yoga exercise lesson lasted for 60 minutes, including a 10‐minute warm‐up, 40‐minute yoga posture exercise, and a 10‐minute meditation. Each posture exercise included preparation, practice and repetition, controlled breathing, and mindful awareness. Control: usual care (routine prenatal health care only. Routine prenatal health care was provided by prenatal clinic obstetricians and midwives with more than 10 years of experience in obstetrics. The participants were provided with educational materials on the prenatal period, preparation for delivery, and skills needed during delivery. They were contacted during follow‐up by telephone and WeChat, a popular Chinese social media platform). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: none Notes on validation of instruments (screening and outcomes): “The internal consistency coefficient of the EPDS as previously reported was 0.87. In the current study, the Cronbach’s α coefficient was 0.773.” “The Cronbach’s α coefficient of the S‐AI [sic] as previously reported was 0.90. In the current study, it was 0.925.” Additional information: none Handling the data: not applicable Prospective trial registration number: clinicaltrial.gov (no. ChiCTR1900025307) |
Rong 2021b.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between March and June 2016. Country: China Income classification: upper‐middle income country in 2016 Geographical scope: Guangzhou, in Guangdong, China Healthcare setting: university (Zhongshan School of Medicine, Sun Yat‐sen University) |
| Participants | 1. Age: mean age 21.0 (range 20.0 to 21.0) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: 3rd‐ and 4th‐year medical students Inclusion criteria: a. 3rd‐ and 4th‐year medical students; b. studying major subjects of clinical medicine; and c. lacking a history of mental illness. Exclusion criteria: not specifed. Note: at baseline, the intervention and control group scores for Patient Health Questionnaire‐9 (PHQ‐9) were, respectively, 6.6 (3.7) and 5.9 (3.0). At baseline, the total group prevalence for depression measured with PHQ‐9 was 19.9% (N = 29). Stated purpose: to investigate the efficacy of the intervention courses designed to enhance the mental health and empathy of senior Chinese medical students |
| Interventions |
Name: structural intervention courses Title/name of PW and number: physicians 1. Selection: not specified 2. Educational background: not specified 3. Training: not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by PHQ‐9 scores. Only those who lacked a history of mental illness were eligible for inclusion. Intervention details: intervention courses were delivered every month for 3 months after regular medical courses; three small groups (~25 students/group), and each group was guided by a corresponding lecture. Each course lasted approximately 60 minutes. Three modules—“Establishing a Sense of Achievement”, “Means for Efficient Patient‐Doctor Communication”, and “Strategies to Manage Medical Errors”. Control: other (classes in which students were encouraged to discuss their difficulties in clinical work and school life, guided by a teacher. “This was done in a leisurely, friendly atmosphere. Correspondingly, the teacher shared related experiences or gave suggestions for dealing with these problems. However, there was no structural course form provided and no predefined topics were discussed”). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: MOOC Construction Project of Sun Yat‐sen University in 2018 (number 80000‐18832627) Notes on validation of instruments (screening and outcomes): validated questionnaire (PHQ‐9). A total score of > 9 points or the presence of suicidal tendency indicated the existence of depressive symptoms. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02645643 |
Rotheram‐Borus 2014a.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from 2008 to 2010. Country: South Africa Income classification: upper‐middle‐income country in 2008‐2010 Geographical scope: urban and rural, KwaZulu‐Natal Healthcare setting: 8 clinics in the province were selected for randomization. |
| Participants | 1. Age: women had an average age of 26.5 years (SD = 5.5). 2. Gender: female 3. Socioeconomic background: 44.8% were employed; 59.7% lived in formal housing. 4. Educational background: 79.7% had some secondary education. Inclusion criteria: pregnant women who tested seropositive for HIV. Exclusion criteria: none reported. Note: at baseline, the intervention and control group prevalence for depression measured with General Health Questionnaire (GHQ), scores > 7, was, respectively, 14.7% (N = 80) and 13.6% (N = 89). Stated purpose: to evaluate the effect of clinic‐based support by HIV‐positive peer mentors, in addition to standard clinic care, on maternal and infant well‐being among women living with HIV (WLH) from pregnancy through the infant’s first year of life |
| Interventions |
Name: enhanced intervention Title/name of PW and number: peer mentors (number not specified) 1. Selection: peer mentors were recruited from advertisements placed in the clinics, and WLH who were childbearing and had good social skills were selected as peer mentors. 2. Educational background: not specified 3. Training: “the Peer Mentors were trained for about 2 months prior to implementation and were certified after being observed; in‐person supervision was provided weekly”; “Peer Mentors were trained in cognitive‐behavioural skills, applying knowledge of PMTCT to daily life, building maternal skills, acquiring information in a manual, practicing each session serving as WLH, building skills using vignettes, supporting WLH to cope with their HIV status, and creating a personal statement about how the Peer Mentor adapted to her HIV status. The training was performed by senior collaborators”. 4. Supervision: supervision is conducted by supervisors who rotate to each site every 2 weeks to observe and provide feedback and supervise weekly group sessions; supervisors give support to peer mentors to help them cope with their own feelings about the difficult situations in women’s lives. 5. Incentives/remuneration: not specified Prevention type: indicated – 14.7% of participants scored above the cut‐off of the GHQ at baseline. Intervention details: on the day of their HIV diagnosis, WLH met with a peer mentor and were invited to attend eight meetings with peers, in addition to standard care. The meetings cover: 1) normalizing being a WLH; 2) establishing healthy daily routines without alcohol or smoking; 3) adhering to medications and visits, monitoring of health status, etc.; 4) obtaining a child support grant; 5) using a single feeding method, not using traditional medicines during this time; 6) building and maintaining a social network; 7) consistent condom use, implementing universal precautions; 8) encouraging couple HIV testing and disclosure of HIV serostatus; and 9) bonding with her infant. Control: usual care |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months, 7‐24 months) |
| Notes |
Source of funding: this study was funded by the NIMH grant R01MH077553. In addition, this work was supported by the Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30MH058107; the UCLA AIDS Institute and the UCLA Center for AIDS Research (CFAR) NIH grant P30AI028697; and the National Center for Advancing Translational Sciences through UCLA CSTI grant UL1TR000124. Notes on validation of instruments (screening and outcomes): the GHQ is a widely used instrument that has been validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT00972699 |
Rotheram‐Borus 2014b.
| Study characteristics | |
| Methods |
tudy design: cluster‐RCT Duration of study: the study was conducted from 2009 to 2014. Country: South Africa Income classification: upper‐middle‐income country Geographical scope: urban—townships in Cape Town Healthcare setting: participant's homes |
| Participants | 1. Age: 18+; mean 26.4, SD 5.5 2. Gender: female 3. Socioeconomic background: 46.8% had a monthly household income > 2000 rand. 4. Educational background: mean highest education level (SD)—10.3 (1.8) Inclusion criteria: a. pregnant women and their children; b. age 18 or older; c. informed consent. Exclusion criteria: a. psychosis, neurological damage, inability to communicate with interviewer; b. inability to give consent. Note: at baseline, the intervention and control group prevalence for Edinburgh Postnatal Depression Scale (EPDS) > 18, there were 204 mothers (16.5%; 1238 total). Stated purpose: to test a mother‐to‐mother intervention during pregnancy and after delivery with mothers in South Africa |
| Interventions |
Name: Philani Title/name of PW and number: community health workers (CHWs) 1. Selection: CHWs were selected to have good social/communication skills, problem‐solving skills, and thriving children (positive deviants). 2. Educational background: CHWs were women with 10th‐ to 12th‐grade education around 40 years old (range 34 to 59). 3. Training: CHWs were trained for 1 month in cognitive‐behavioural change strategies and role‐playing using an intervention manual and watching videotapes of common challenging situations that CHWs might face during home visits. Specifically, a CHW has trained in 1) foundational skills in behaviour change; 2) application of key health information about HIV, alcohol use, malnutrition, and general maternal and child health; and 3) coping with their own life challenges. 4. Supervision: CHWs were certified and supervised biweekly with random observations of home visits. 5. Incentives/remuneration: CHWs worked 20 hours weekly and were paid R1250 a month (about 150 USD). Prevention type: indicated prevention – at baseline, there were 204 mothers (16.5%; 1238 total) with EPDS > 18; those affected by psychosis, neurological damage, or inability to communicate with interviewer were excluded. Intervention details: eight health messages were delivered regarding healthy pregnancy, HIV/TB testing and PMTCT, reducing alcohol use and malnutrition, and encouraging breastfeeding, with the aim to deliver these messages in at least four antenatal visits and four postnatal visits within the first two months of life. On average, CHWs made six antenatal visits (SD = 3.8), five postnatal visits between birth and 2 months postbirth (SD = 1.9), and afterward about 1.4 visits/month (range: 0.1 to 6.4 visits/month). Sessions lasted on average 31 minutes each. Control: usual care – standard clinic care in Cape Town is accessible and provides free HIV testing, dual regimen therapies for WLH, consistent access to milk tins (formula), TB and CD4 cell testing, cotrimoxazole for infants until HIV testing, HIV polymerase chain reaction (PCR) testing for infants at six weeks, postnatal visits at one week, treatment for WLH, and HIV testing for partners of WLH. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: this work was supported by NIAAA grant R01 AA017104, the Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30 MH58107; the UCLA Center for AIDS Research (CFAR) grant P30 AI028697; and the National Center for Advancing Translational Sciences through UCLA CSTI Grant UL1 TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. Notes on validation of instruments (screening and outcomes): the EPDS is a widely validated and established tool. Additional information: none Handling the data: they are found in the “analysis details” docx. Prospective trial registration number: NCT00996528 |
Sanfilippo 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from November 2018 to May 2019. Country: The Gambia Income classification: low‐income country in 2018 Geographical scope: rural and urban as well as Wolof‐speaking and Mandinka‐speaking areas in The Gambia Healthcare setting: local antenatal clinics |
| Participants | 1. Age: participants were between the ages of 18 and 40 (M = 26.95, SD = 5.72). 2. Gender: female 3. Socioeconomic background: not specified 4. Educational background: 50% of women received an informal education (Arabic); 31% secondary/tertiary education; 15% primary education; 4% no education. Inclusion criteria: All participants who a. were attending the consented sites during the active study period; b. were 18 or older; c. spoke either Mandinka or Wolof fluently; and d. were 14 to 24 weeks pregnant were invited to take part in the study. e. Participants were not preselected based on their mental health symptoms. Exclusion criteria: a. women with a history of a late‐term miscarriage; b. those who had a current or a history of psychosis. Note: at baseline, the intervention and control group scores for Edinburgh Postnatal Depression Scale (EPDS) were, respectively, 2.90 (3.07) and 5.16 (4.46). At baseline, the intervention and control group scores for Self‐Reporting Questionnaire‐20 (SRQ‐20) were, respectively, 6.22 (3.83) and 7.97 (3.99). Stated purpose: to test the feasibility of a Community Health Intervention through Musical Engagement (CHIME) to help reduce CMD symptoms in pregnant women compared with standard care. The study had five objectives—1) to obtain demographic information on the eligible population; 2) to determine if our measurement tools, the EPDS and the SRQ‐20, are useable; 3) to determine if the intervention is deliverable; 4) to determine if the stepped‐wedge trial design is deliverable and obtain information that will inform the definitive study; and 5) to determine if this type of intervention is culturally appropriate and well received by the community and health workers. |
| Interventions |
Name: CHIME Title/name of PW and number: 4 local Kanyeleng groups (all‐female fertility societies, each composed of approximately 10 women) 1. Selection: each clinic also had an active local Kanyeleng group who could deliver the intervention. 2. Educational background: not specified 3. Training: training workshop held with the Kanyeleng groups before the intervention 4. Supervision: a community health nurse (CHN) at each clinic was present to observe, take attendance data and report any issues of concern to the research team including any potential adverse effects. 5. Incentives/remuneration: all participants were offered a total of 600 Dalasi (about US$12) for their time; 200 Dalasi (about US$4) at each data collection time point. Prevention type: selective—participants were included based upon the presence of a risk factor (pregnancy) and presented with some level of distress as indicated by EPDS scores that were below the cut‐off for the measure. Intervention details: CHIME draws on cognitive behavioural therapy principles from the Towards Parenthood and ACORN interventions. Participants in the CHIME intervention attended six 60‐minute music sessions held once a week over 6 weeks at their local antenatal clinic in addition to receiving standard antenatal care. Control: usual care (standard antenatal clinic care without any additional intervention. Standard care consists of four or more regular visits to the antenatal clinic with little to no mental healthcare). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month; 1‐6 months) |
| Notes |
Source of funding: MRC‐AHRC Global Public Health—Partnership Awards scheme (MR/R024618/1) awarded to Professor Lauren Stewart Notes on validation of instruments (screening and outcomes): “Both [questionnaires] were translated into Mandinka and Wolof. The translation method was based on suggestions from the WHO, Hanlon 2008 and Cox 2014. The EPDS has been validated for perinatal use in other African contexts, and used in The Gambia before, though a validated version could not be obtained.” Additional information: none Handling the data: not applicable Prospective trial registration number: Pan African Clinical Trials Registry (PACTR201901917619299) |
Sangraula 2020.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2017 and 2019. Country: Nepal Income classification: low‐middle‐income country Geographical scope: rural, Sindhuli district, Nepal Healthcare setting: Village Development Committees (VDC) |
| Participants | 1. Age: age PM+‐46.7 (14.0), EUC: 49.3 (13.6) 2. Gender: both 3. Socioeconomic background: the majority (48‐55%) were housewives, followed by 33‐35% being farmers. 4. Educational background: 59‐80% were illiterate; 12‐18% informal education. Inclusion criteria: a. 18 years and older; b. all sexes eligible; c. score > 2 on General Health Questionnaire (dichotomous item scoring method); d. score > 16 on World Health Organization Disability Assessment Scale; Exclusion criteria: a. presence of a severe mental disorder (e.g. psychosis); b. alcohol use disorder (score > 16 on the alcohol use disorders identification test [AUDIT]). Note: at baseline, the intervention and control group scores for the Patient Health Questionnaire‐9 (PHQ‐9) were, respectively, 9.8 (4.9) and 10.7 (4.4). At baseline, the intervention and control group scores for the General Health Questionnaire (GHQ‐12) were, respectively, 24.3 (4.8) and 21.3 (4.7). At baseline, the intervention and control group scores for the World Health Organization Disability Assessment Schedule (WHODAS) were, respectively, 21.8 (5.3) and 20.8 (4.1). Stated purpose: to assess the feasibility and acceptability of locally adapted Group PM+ for women and men in an earthquake‐affected region of rural Nepal |
| Interventions |
Name: Group Problem Management Plus (PM+) Title/name of PW and number: community‐based psychosocial workers/PM+ facilitators (4) 1. Selection: “The requirement for the non‐specialists will be at least 10 years of education, over 25 years of age, and living in either the EUC or Group PM+ VDC.” 2. Educational background: 10+ years of education 3. Training: nonspecialists will receive the standard training to become community psychosocial workers by TPO Nepal. This takes place over 20 days and consists of the teaching of basic psychological skills. Community‐based psychosocial workers (CPSWs) from the intervention were given an additional 10‐day Group PM+ training using the adapted manual and other clinical materials. Intervention training includes education on adversity and its impact upon mental health, basic counselling skills, delivering Group PM+, skills in group facilitation, and facilitator self‐care. Transcultural Psychosocial Organization (TPO) Nepal is a Nepali nongovernmental mental health research and training organization, with specific expertise in humanitarian settings. 4. Supervision: fidelity assessment as part of the feasibility study (adequate: all four Group PM+ facilitators adhered to 75% or more items in each of the group sessions they conducted). This was done by the research team. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by PHQ‐9 scores. All those who presented with a severe mental disorder were excluded. Intervention details: participants in the intervention arm received five sessions of Group PM+, with each session lasting 2.5 to 3 hours. Sessions included: (1) managing stress, (2) behavioural activation, (3) managing problems, (4) strengthening social support and (5) review of techniques. Control: usual care (enhanced) – the group consisted of six to eight people separated by gender and with gender‐matched facilitators. Volunteer local helpers supported facilitators by organising logistics and reminding participants about the sessions. CPSWs were the service providers for the groups and are a cadre of psychosocial workers in Nepal that are trained through and work for NGOs, such as TPO Nepal. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: this study was funded by USAID/OFDA. The trial sponsors had no role in the collection, management, analysis, and interpretation of data; nor the decision to submit the report for publication. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. Notes on validation of instruments (screening and outcomes): the selected outcomes are well established and validated across contexts. Additional information: none Handling the data: the supplementary material for this article can be found at doi.org/10.1017/S2045796020000414. Raw data are available as additional supporting files. Prospective trial registration number: NCT03359486 |
Sherman 2009.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2005 (start of recruitment) and 2009 (publication). Country: Chiang Mai, Thailand Income classification: low‐middle‐income country from 2005 to 2009 Geographical scope: urban Healthcare setting: in the community, done in an ‘unmarked building’, which was a drug treatment centre |
| Participants | 1. Age: 18 to 25 years old 2. Gender: both 3. Socioeconomic background: about one‐third worked, one‐third students, one‐third unemployed; primarily Buddhist (97.1%) and ethnically Thai (99.2%). A majority (63.8%) reported living with their parents. 4. Educational background: participants' education level was low, with only 39% reporting being currently in school and a median of 9 (interquartile range: 9‐11) years of schooling. Inclusion criteria: a. between the ages of 18 and 25 at screening; b. used methamphetamine at least three times; c. had sex at least three times in the past 3 months; d. were able to enrol at least one of their sex or drug network members in the study within 45 days of screening. Exclusion criteria: a. if they refused to have blood drawn or provide urine; b. if they were enrolled in another prevention study; c. if they refused to provide locator information. Note: at baseline, the intervention and control group scores for Center for Epidemiologic Studies Depression Scale (CES‐D) were, respectively, 20.0 (9.7) and 18.3 (9.1). Stated purpose: to examine the effects of a peer network intervention and a life skills intervention on methamphetamine and HIV risk behaviours amongst 18‐ to 25‐year‐olds |
| Interventions |
Name: peer education condition Title/name of PW and number: peer educators (6) 1. Selection: 2 facilitators with 1 back‐up who were in their early 20s and had been a part of the ethnography team in the study's first phase 2. Educational background: not specified 3. Training: intensive 1‐week‐long training session, implemented using a manual. Facilitators were trained by the study's first and third authors. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the CES‐D scores. Intervention details: seven 2‐hour sessions for each group undertaken by the facilitators over 1 month with twice‐weekly sessions. Participants in the peer education condition also attended 2 booster sessions that occurred 3 and 6 months after study entry. The aim was to teach participants to think critically about and reduce their methamphetamine use and sexual risk behaviours. Participants were taught communication skills that they practiced in role‐plays during the sessions and used to convey methamphetamine and risk reduction messages to specific social network members that were identified through a social network inventory administered at baseline. Sessions comprised interactive teaching modules, instructive games, and problem‐solving activities. Sessions ended with assigning peer education homework in which participants would discuss a specific issue with specific peers (MA‐using and/or sexual partners), which were reviewed at the beginning of the next session. Control: usual care – a life‐skills building approach based on a skills‐building approach that was largely derived from cognitive behavioural psychology, which is widely used with youth in drug treatment and juvenile justice settings in Thailand. The sessions focused on the causes and consequences of methamphetamine use at the individual level, with specific attention to stress in the role of drug use. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): authors reported that the CES‐D had been validated among Thai adolescents. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Shinde 2018.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2015 and 2016. Country: India Income classification: low‐middle‐income country in 2015‐2016 Geographical scope: Nalanda district of Bihar state, India Healthcare setting: government‐run secondary schools |
| Participants | 1. Age: 13‐14 2. Gender: both 3. Socioeconomic background: 65‐70% backward caste, 19‐24% scheduled caste*—“In the caste categories, backward or other caste is a collective term used by the Government of India to classify castes which are socially disadvantaged, while the scheduled castes are officially designated groups of historically disadvantaged people in India. General caste is a term used in India to denote groups of people who do not qualify for any of the affirmative action schemes by the Government of India”. 4. Educational background: grade 9 students Inclusion criteria for clusters: a. secondary and higher secondary schools; b. implementation of Tarang‐Adolescence Education Programme; c. 100 or more students enrolled in grade IX; d. 5 or more teachers employed in the school. Inclusion criteria for individual participants: all the students (boys and girls) studying in standard IX in all randomly assigned 75 schools in the academic year of April 2015 to March 2016. Exclusion criteria for clusters: a. upgraded schools (grade I‐XII); b. Tarang‐Adolescence Education Programme not being implemented; c. less than 100 students enrolled in grade IX; d. 4 or fewer teachers employed in the school. Exclusion criteria for participants: none reported. Note: at baseline, the intervention (1 and 2) and control group scores for Patient Health Questionaire‐9 (PHQ‐9) were, respectively, 6.61 (5.3), 6.51 (5.4), and 6.4 (5.2). Stated purpose: to assess the effectiveness of a multicomponent whole‐school health promotion intervention (SEHER) in grade 9 students (aged 13 to 14 years) at government‐run secondary schools |
| Interventions |
Name: school health promotion intervention (SEHER); school health promotion intervention (SEHER) + government‐run Adolescence Education Program (AEP) Title/name of PW and number: lay counsellor (1 per school) for intervention A, a teacher for intervention B (1 per school) 1. Selection: lay counsellors were selected following structured interview, had to speak the local language; teachers were nominated by school principals, were required to have a minimum of 5 years teaching experience in secondary schools, more than 15 years of service remaining, and not teaching the AEP curriculum. 2. Educational background: lay counsellors were required to have a bachelor degree. 3. Training: the teachers and lay counsellors were trained separately in a 1‐week‐long training, with an identical curriculum. This training session was followed up with in‐service training through separate monthly group meetings. 4. Supervision: 3 planned visits per month; 8 supervisors with master’s degree in psychology, sociology, or social work and to have more than 2 years of experience of working with adolescents 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by PHQ‐9 scores. Intervention details School health promotion intervention (SEHER): the school health promotion intervention (SEHER) is a multicomponent intervention emphasizing the importance of a positive school climate. The intervention identifies four priority areas for action: promoting social skills amongst adolescents; engaging the school community (i.e. adolescents, teachers, and parents) in school‐level decision‐making processes; providing access to factual knowledge about health and risk behaviours to the school community; and enhancing problem‐solving skills amongst adolescents. Actions are taken on a whole school level (addressing themes such as hygiene, bullying, mental health, substance use, reproductive and sexual health, gender and violence, rights and responsibilities, and study skills) and individual student level problem‐solving‐based counselling to students who self‐referred or were referred by teachers for health complaints, social difficulties, nutritional problems, and academic difficulties. For those students with serious physical or emotional and behavioural difficulties, referral pathways to specialists were provided. School health promotion intervention (SEHER) + government‐run AEP: in the school health promotion intervention (SEHER) + government‐run AEP group, a trained teacher from each school ran classroom‐based sessions on the process of growing up, establishing positive and responsible relationships, gender and sexuality, prevention of HIV and other sexually transmitted infections, and substance use (16 hours total). Control: usual care – government‐run AEP |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the SEHER + AEP intervention and the control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months; 7‐24 months) |
| Notes |
Source of funding: John D and Catherine T MacArthur Foundation, USA and the United Nations Population Fund India Office Notes on validation of instruments (screening and outcomes): the selected outcome measure is widely adopted and validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT02484014 |
Singla 2015.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 2013 and 2015. Country: Uganda Income classification: low‐income country in 2013‐2015 Geographical scope: rural—Lira, a northern district of Uganda Healthcare setting: unspecified location for group sessions, home for home visit component |
| Participants | 1. Age: child—intervention 22.44 (6.4), control 22.23 (6.2) months; mother—intervention 28.04 (7), control 26.57 (7.2) years 2. Gender: both 3. Socioeconomic background: 94‐95% of mothers were farmers. 4. Educational background: years of education—intervention 3.91 (2.9), control 4.02 (2.8) Inclusion criteria—children: a. 12 months to 36 months (child); b. all sexes. Inclusion criteria—mothers: mothers of children ages 12‐24 months. Exclusion criteria: disabled children. Note: at baseline, the intervention and control group scores for the Center for Epidemiological Studies Depression Scale (CES‐D) were, respectively, 15.13 (9.58) and 12.84 (7.88). Stated purpose: to assess an integrated, community‐based parenting intervention that targeted both child development and maternal well‐being in rural Uganda |
| Interventions |
Name: community‐based parenting intervention Title/name of PW and number: community volunteers (13) 1. Selection: volunteers were selected by the community and Plan Uganda staff on the basis of their reputation in the community, communication and language skills, and a minimum of a sixth‐grade education. 2. Educational background: average 8th grade 3. Training: training (14 days) focused on the programme content and effective communication skills that emphasized both common skills (e.g. a nonjudgemental, empathic stance) and motivational interviewing (e.g. open‐ended questions and rolling with resistance). The training was conducted by the research team and members of Plan Uganda. 4. Supervision: assistance in preparing the sessions, provision of feedback following session attendance (on average 6 sessions were attended) was given by staff members who provided the training. 5. Incentives/remuneration: not specified Prevention type: indicated – mothers presented some level of distress at baseline, as assessed through the CES‐D. Intervention details: 12 sessions (60 to 90 minutes) integrated intervention programme to groups of parents on a fortnightly basis addressing child care (play, talk, diet, hygiene, and love and respect) and maternal well‐being (e.g. increasing father involvement) + one booster session. Parents were encouraged to learn through a series of active and interactive activities (e.g. role‐play, games, parent‐child interactions, and group‐based problem‐solving), and were assigned homework to practice between sessions. Parents also received one or two home visits (40 to 50 minutes) to review the five parenting messages. Two mother‐care sessions were delivered to mothers only, two to fathers only, and two were delivered to mothers and fathers together. The sessions dealt with love and respect in three primary relationships: the mother’s relationship with herself, her child, and her spouse, as in other maternal mental health interventions. Control: waiting list – while on a waiting list, these communities focused on creating preschools for older children supported by Plan Uganda. At the end of the baseline interview, participants also received nutrition information, which included a coloured poster to identify what local foods constitute a diverse diet. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐ 6 months) |
| Notes |
Source of funding: Plan Uganda via Plan Finland (Ministry of Foreign Affairs) and Plan Australia (Australian Aid) Notes on validation of instruments (screening and outcomes): the CES‐D had been previously used in Uganda. Additional information: none Handling the data: not applicable Prospective trial registration number: NCT01906606 |
Skar 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between August 2012 and August 2015. Country: Colombia Income classification: upper‐middle income country in 2012‐2015 Geographical scope: Chocó department Healthcare setting: community‐based child centres |
| Participants | 1. Age: caregivers had an average age of 31.89 years (range 18‐64). 2. Gender: both 3. Socioeconomic background: low‐income families—almost half of the caregivers (48.3%) lived in low‐income households (< US$197 per month), and 40.3% of employed caregivers received a salary of US$33 per month or less. 4. Educational background: nearly half of caregivers (47.1%) had higher education. Inclusion criteria: a. families belonging to six social service child centres run by Instituto Colombiano de Bienestar Familiar (ICBF). Parents attended the health‐promoting entity called Entidades Promotoras de Salud, which offers health services subsidized by the government to low‐income families. b. All registered parents were eligible for inclusion in the study if they had a child within the relevant age group (3 to 4 years). Exclusion criteria: not specified. Note: at baseline, the total group prevalence for scores above clinical cut‐off (> 8) of the Shona Symptom Questionnaire (SSQ) was 19.2% (N = 33). Stated purpose: to investigate whether the International Child Development Programme (ICDP), by focusing on strengthening positive caregiving and familial relationships, is effective as a violence preventive measure and whether a specific violence prevention curriculum (element b and c in Cook 2017) will add to the effect, when compared with participation in regular social programme activities at child care centres. An additional aim of the study is to investigate potential predictors of violence and mental health problems. |
| Interventions |
Name: community activities (CA) + ICDP; violence curriculum (VC) CA + ICDP Title/name of PW and number: local care persons within a society 1. Selection: not specified 2. Educational background: not specified 3. Training: yes, provided by certified ICDP trainers, but not specified 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – 19.2% participants presented with some level of distress as indicated by SSQ scores. Intervention details CA + ICDP: CA + ICDP groups followed the general recommendations of ICDP, in which two ICDP‐trained facilitators were to initiate discussions and activities related to the three dialogues for good caregiver‐child interaction in ICDP, namely emotions, communication, and regulation. ICDP methods include caregiver self‐activation through group discussions, role‐play, home practice between the group meetings, and reporting back to the group. There were 12 ICDP group meetings. VC CA + ICDP: the CA + ICDP + VC groups were run in the same way as the ICDP groups; however, the ICDP part was more intensive, as it was implemented over six group meetings rather than 12. Following that, the caregivers attended six group meetings with a preventive VC through informative workshops where the aims are to (a) sensitize and train community stakeholders on child development, effects of violence, legislation and policy frameworks, and their role in protecting children, and (b) develop formal and informal child protection systems, followed by a plan of action to protect children from violence. VC methods include caregiver self‐activation in form of designing protective strategies and developing monitoring tools to follow‐up on the two aims. Control: usual care (all participants attended the child centres, which had a number of health, nutrition, and educational facilities available. The comparison group received no additional intervention). |
| Outcomes |
Participants’outcomes of interest for this review
Note: we included data from the “VC CA + ICDP” intervention and control group. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: the evaluation project has been supported by the Children and Violence Evaluation Challenge Fund, a joint initiative funded by Bernhard van leer Foundation, Oak Foundation, and UBS Optimus Foundation and hosted by NEF. Notes on validation of instruments (screening and outcomes): SSQ has been validated in low‐income settings in Zimbabwe. It has a satisfactory sensitivity against a diagnosis of depression (84%) and anxiety (73%), and internal reliability ranging from α = 0.74 (Chibanda 2016) to α = 0.85 (Patel 1997). Additional information: none Handling the data: not applicable Prospective trial registration number: Regional Committees for Medical and Health Research Ethics (reference number 2012/1169/REK Sør‐Øst A) |
Song 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from October 2017 to July 2018. Country: China Income classification: upper‐middle‐income country from 2017 to 2018 Geographical scope: urban—Hangzhou City Healthcare setting: 2 community health centres |
| Participants | 1. Age: 75.78 ± 6.28 years old 2. Gender: both 3. Socioeconomic background: 60+% had a monthly income < average value in the local city. 4. Educational background: less than 20% were illiterate. Inclusion criteria: a. community‐dwelling elderly people aged 60 or above; b. identified with mild cognitive impairment by the Montreal Cognitive Assessment (Chinese version, MoCA‐C). Exclusion criteria: a. participants with conditions that were contraindicated for exercise training according to the American College of Sports Medicine (ACSM); b. prescription of antidepressant agents, which may confound the outcome measurement; c. severe neurological disorders (e.g. brain injury, stroke, Parkinson’s disease) which greatly impair cognitive function; d. regular engagement in moderate or vigorous‐intensity aerobic exercises of > 150 min per week. Note: at baseline, the intervention and control group scores for Geriatric Depression Scale (GDS) were, respectively, 5.33 (3.48) and 5.67 (3.7). At baseline, the intervention and control group scores for Quality of Life—Alzheimer’s disease scale (QOL‐AD) were, respectively, 29.80 (3.23) and 29.18 (2.70). Stated purpose: to evaluate the effects of a moderate‐intensity aerobic exercise programme on the cognitive function and health‐related quality of life of Chinese elderly with mild cognitive impairment and to explore the mediating roles of depressive mood and sleep quality in the exercise‐cognition relationship. |
| Interventions |
Name: moderate‐intensity aerobic exercise training Title/name of PW and number: nurses (2) 1. Selection: not specified 2. Educational background: registered nurses 3. Training: 4‐week training programme 4. Supervision: the nurses performed a return demonstration during the training and exhibited good protocol compliance. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by GDS scores. Intervention details: 16‐week aerobic stepping exercise programme with three 60‐minute group training sessions (20 participants per group) per week. Motivational strategies, including goal‐setting, verbal encouragement, and emotional incentives based on the self‐efficacy theory, were incorporated into this exercise intervention to ensure compliance. Control: active control – the control intervention was a 16‐week health education programme that was delivered by a general practitioner in the community healthcare centre. The health education programme included eight biweekly educational classes (45 min/each session). No information relating to brain health and physical exercise was included. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): the selected measures were validated for use in China. Additional information: none Handling the data: none Prospective trial registration number: not reported |
Srisuwan 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted in 2017. Country: Thailand Income classification: upper‐middle‐income country in 2017 Geographical scope: “Central region of Thailand” Healthcare setting: geriatric clinic (outpatient clinic) |
| Participants | 1. Age: 65.7 ± 4.3 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: most had a Bachelor's degree (63% in intervention, 59% in control). Inclusion criteria Participants: a. were aged > 60 years; b. visited the geriatric clinic, Outpatient department, Phramongkutklao Hospital, Bangkok, Thailand. Exclusion criteria: a. Thai version of Hospital Anxiety and Depression Scale (HADS) higher than 11 on anxiety or depression; b. Thai version of Montreal Cognitive Assessment (MoCA) less than 26; c. had any conditions affecting participation in programme activities, e.g. balancing problems, hearing impairment as well as any psychiatric diseases and neurological problems such as stroke. Note: at baseline, the intervention and control group scores for HADS‐Depression were, respectively, 2.92 (2.13) and 2.75 (2.31). At baseline, the intervention and control group scores for HADS‐Anxiety were, respectively, 4.61 (2.45) and 3.53 (2.21). Stated purpose: to assess the effectiveness of a group‐based 8‐week multicomponent CT by using the TEAM‐V Program concerning cognition, mood, and IADL amongst healthy older adults over 1 year |
| Interventions |
Name: TEAM‐V multidomain cognitive training programme Title/name of PW and number: general practitioner nurse and practice nurse (number not specified) 1. Selection: not specified 2. Educational background: not specified 3. Training: 4‐day workshop covering clinical and instrumental assessment of cognitive function and method of delivering the CT programme 4. Supervision: from author correspondence—“Geriatrician monitored the intervention such as contents and tools of training”. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by HADS scores. All those who had a score indicative of severe depression or anxiety were excluded. Intervention details: “Multi‐domain CT program consisting of training of executive function, attention, memory and visuospatial function. The training comprised 5 sessions, with a 2‐week interval between each session and 120 minutes per session. Each session involved training in different domains of cognition. Participants were encouraged to practice their homework during the intervention period. After the intervention, participants were encouraged to continue practice CT as much as possible. Examples of homework include: identifying internal and external distracters in daily living and memory techniques using in real life such techniques to remember shopping lists.” Control: usual care – standard clinical care from usual healthcare professionals |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: the research project was partially supported by The Thai Health Promotion Foundation. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. Notes on validation of instruments (screening and outcomes): the HADS is a widely adopted measure that has been validated across contexts. Additional information: none Handling the data: not applicable Prospective trial registration number: TCTR20190709003 |
Thurman 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: between 30 September 2014 and 5 February 2015, eligible female participants were identified. The intervention ended in 2015. The study was published on 24 April 2017. Country: South Africa Income classification: upper‐middle‐income country Geographical scope: 3 periurban towns of Free State province Healthcare setting: 11 schools |
| Participants | 1. Age: 13‐17 years 2. Gender: female 3. Socioeconomic background: not specified 4. Educational background: enrolled in 9th grade Inclusion criteria: a. 13 years to 18 years; b. female; c. attends one of the participating schools; d. English or Sesotho speaker. Exclusion criteria: previous participation in CWBFN grief counselling group. Note: at baseline, the intervention and control group scores for the Center for Epidemiological Studies—Depression Scale for Children (CES‐DC) were, respectively, 17.2 (10.9) and 16.6 (10.7). At baseline, the intervention and control group scores for Brief Problem Monitor—Parent Form (BPM) were, respectively, 10.2 (7.0) and 8.2 (5.9). At baseline, the intervention and control group scores for the 2‐Way Social Support Scale were, respectively, 15.0 (4.8) and 15.3 (4.4). Stated purpose: to assess the effectiveness of time‐limited adolescent grief counselling peer groups in improving the psychosocial well‐being of bereaved female adolescents. |
| Interventions |
Name: Abangane Title/name of PW and number: social workers or social auxiliary worker 1. Selection: selected by the NGO Child 2. Educational background: not specified 3. Training: 4‐day training, 1 year prior experience in delivering the programme (to participants not included in the study population), and 3‐day refresher training before study initiation 4. Supervision: facilitators attended weekly supervision meetings and provided a written account of each session for supervision and quality assurance purposes by CWBFN programme manager. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the CES‐DC scores. Intervention details: the intervention called Abangane (“friends” in isiZulu) consists of 8 sessions. Abangane support groups include activities guided by cognitive behavioural therapy principles and indigenous games and songs, contextually relevant stories and scenarios, as well as discussions about cultural rituals and traditions surrounding death. Groups met for weekly, interactive 90‐minute sessions that included an average of three structured activities focused on experiences of loss and grief, coping skills, and the links between feelings, thoughts, and behaviour. The panel presents the overarching theme for each session and a brief description of activities. All sessions included an opening and closing ritual and time for reflection. Homework was assigned at the end of each session and discussed at the start of the next one, including identifying sources of support, defining goals, and recognizing and challenging negative thoughts. Each participant was provided with a journal to use for recording their progress and feelings. All participants had access to the standard of care consisting of a school‐based CWBFN counsellor available to serve students based on self‐referral or referral by a teacher. Control: waiting list – wait‐listed adolescents will be able to participate in Abangane at the close of the study. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: US Agency for International Development Southern Africa. The funder of this study reviewed the final protocol, but had no role in the study design, data collection, data analysis, data interpretation, writing of this report, or decision to submit this paper for publication. The first and second authors (TRT and BGL) had full access to the data, and the corresponding first author (TRT) takes responsibility for its integrity and the decision to submit this report. Notes on validation of instruments (screening and outcomes): the 20‐item CES‐DC has been previously applied amongst South African youth. Cronbach’s a = 0.87 at baseline. The BPM is a widely used measure with well‐established cross‐cultural validity and has been used to document the extent of emotional and behavioural problems amongst HIV‐affected children in South Africa. Cronbach’s a = 0.83 at baseline. SSS‐R: Cronbach’s α = 0.86 at baseline. Additional information: none Handling the data: not available Prospective trial registration number: NCT02368808 |
Tobias da Silva 2017.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from May to October 2015. Country: Brazil Income classification: upper‐middle income country in 2015 Geographical scope: urban, São Paulo city Healthcare setting: paediatric unit of the University Hospital, University of São Paulo (HUUSP) and Darcy Vargas Children’s Hospital (HIDV) |
| Participants | 1. Age: range 6‐11 years old; mean age 8.9 ± 1.73 for the intervention group and 10.0 ± 1.2 for the control group 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: school‐age children Inclusion criteria: a. age between 6 and 11 years; b. hospitalized for at least 24 hours, at least one peripheral intravenous puncture; c. accepted to participate in the study; d. authorization of parents or guardians as proposed in informed consent; e. have no confirmed medical diagnosis of neurological and/or cognitive disorder. Exclusion criteria: children who were in isolation. Note: at baseline, the total sample scores for the Child Drawing: Hospital (CD‐H) were, respectively, 76.1 (23.0). Stated purpose: to evaluate the effects of the Dramatic Therapeutic Play (DTP) technique on the degree of anxiety in hospitalized school‐age children |
| Interventions |
Name: DTP Title/name of PW and number: fourth‐year undergraduate nursing students 1. Selection: not specified 2. Educational background: undergraduate nursing students 3. Training: discipline of mental health nursing in the third year of graduation; trained by the professor responsible for the project 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (being hospitalized children) and presented with some distress as indicated by CD‐H scores that were below the cut‐off for the measure. Intervention details: the children in the intervention group, after access to the recreational activities of the toy library and participation in a DTP session applied by the researchers, were asked to draw a picture of a person in the hospital. Children in the intervention group underwent the DTP after being submitted to peripheral intravenous puncture. DTP makes it possible to externalize feelings, as well as experiences that are not verbalized, relieving tensions and expressing fears underlying the stressful situation. The technique consists of allowing the child to dramatize situations that are being experienced during the hospitalization and can assume diverse roles, such as one of the health professionals, or a family member. Control: usual care (patients in the control group, were allowed access to the recreational activities of the toy library and at a random moment were asked to draw a person in the hospital). |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): CD‐H questionnaire not validated in Brazil Additional information: none Handling the data: not applicable Prospective trial registration number: U1111‐1190‐8305 |
Tol 2012.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was started in 2007 and was published in 2012. Country: Tellippalai and Uduvil divisions of the Jaffna district in northern Sri Lanka Income classification: low‐middle‐income country from 2007 to 2012 Geographical scope: not specified Healthcare setting: 12 schools per study condition |
| Participants | 1. Age: 9‐12 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: grade 4 to 7 Inclusion criteria: a. grades 4 through 7 (ages 9‐12); b. the existence of risk factors (i.e. reporting exposure to war‐related events, distress during such exposure, current psychological symptoms, and affected school functioning); c. the absence of protective factors (i.e. reporting a lack of social support and coping capacity); d. screened children for meeting inclusion criteria using the Child Psychosocial Distress Screener (CPDS), a screening instrument with established cross‐cultural construct validity that was developed for use with children affected by armed conflict; e. outcome scores for PTSD‐depressive‐anxiety symptoms not above cut‐off at baseline. Exclusion criteria: a. no children were excluded after meeting inclusion criteria; b. a small group of children reporting severe mental problems during screening was provided individual supportive counselling in addition to being enrolled in the study (N = 19, 4.8%). Note: at baseline, the intervention and control group scores for Child PTSD Symptom Scale (CPSS) were, respectively, 15.03 (8.89) and 15.70 (9.12). At baseline, the intervention and control group scores for Depression Self‐Rating Scale (DSRS) were, respectively, 8.39 (4.54) and 8.56 (4.37). At baseline, the intervention and control group scores for Anxiety Related Emotional Disorders (SCARED‐5) were, respectively, 3.29 (2.13) and 3.17 (2.16). At baseline, the intervention and control group scores for function impairment were, respectively, 3.64 (4.47) and 3.23 (4.37). Stated purpose: to evaluate the outcomes of a school‐based secondary prevention intervention for children affected by the ongoing war in northern Sri Lanka |
| Interventions |
Name: school‐based group intervention Title/name of PW and number: paraprofessionals 1. Selection: selected for their affinity and capacity to work with children demonstrated in role‐plays and interviews 2. Educational background: at least high school diploma 3. Training: trained for 1 year prior to the study 4. Supervision: supervised in implementing the intervention for 1 year prior to the study 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the CPSS, DSRS, and SCARED‐5 scores. Intervention details: the mental health intervention consisted of 15 sessions over 5 weeks of a school‐based group intervention. The manualized intervention consists of cognitive behavioural techniques (psychoeducation, strengthening coping, and guided exposure to past traumatic events through drawing) and creative expressive elements (co‐operative games, structured movement, music, drama, and dance) with groups of around 15 children, aimed at decreasing symptoms of common mental disorders and strengthening protective factors. Each session is divided into four parts, starting and ending with structured movement, songs and dance with the use of a “parachute” (i.e. large circular coloured fabric). The second part is based on a “central activity” focused on the main theme of that week (e.g. a drama exercise to identify social supports in the environment, or drawing of traumatic events), and the third part is a co‐operative game (i.e. a game in which all children have to participate in order to promote group cohesion). The intervention was part of a larger public mental health programme for children affected by war, including primary and tertiary prevention approaches. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Cost analysis Time points: baseline, post‐intervention (< 1 month; 1‐6 months) |
| Notes |
Source of funding: PLAN Netherlands Notes on validation of instruments (screening and outcomes) 1. CPSS: internal reliability (Cronbach alpha) in this sample was 0.84. 2. DSRS: internal reliability in this sample was 0.65. 3. SCARED‐5: internal reliability in this sample was 0.52. 4. Not available (constructed scale) Additional information: none Handling the data: not available Prospective trial registration number: not available |
Tripathy 2010.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted between 31 July 2005 to 30 July 2008. Country: Jharkhand and Orissa Districts, India Income classification: low‐income country from 2005 to 2006; low‐and‐middle income country from 2007 to 2008 Geographical scope: rural Healthcare setting: not reported |
| Participants | 1. Age: 15‐49 years 2. Gender: female 3. Socioeconomic background: majority of households in scheduled tribe, owning less than 2 bighas (< 0.27 hectares) 4. Educational background: most did not have any formal school education (69‐78%); 70‐78% cannot read. Inclusion criteria: a. women; b. aged 15‐49 years; c. residing in the project area; d. had given birth during the study (31 July 2005 to 30 July 2008). Exclusion criteria: symptoms of severe depression. Note: considerations on baseline scores for Kessler‐10 item scale (K‐10) not applicable for this study Stated purpose: a participatory intervention with women's groups to reduce neonatal mortality in underserved tribal communities of eastern India, to improve home‐care practices and health‐seeking behaviour of pregnant and postnatal women and their family members, and to reduce maternal depression in the intervention areas |
| Interventions |
Name: Participatory Women’s Groups and Health committee Title/name of PW and number: female facilitators 1. Selection: local female facilitator identified by community members 2. Educational background: not specified 3. Training: 7‐day residential training course to practice participatory communication techniques 4. Supervision: fortnightly meetings by district co‐ordinators 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (women who just gave birth). Those who were identified by interviewers as having symptoms of severe depression were referred to the nearest tertiary mental health centre. Intervention details: 20 (monthly sessions) focusing on social support, problem‐solving skills, discussion of mental health challenges. Identifying and prioritizing maternal and newborn health problems; identifying strategies to address these problems and discussing their effects; and health‐service input meetings for village representatives to discuss maternal and newborn health entitlement issues. Control: usual care + health‐service input meetings for village representatives to discuss maternal and newborn health entitlement issues |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Yes Time points: baseline, post‐intervention (7‐24 months) |
| Notes |
Source of funding: Health Foundation, UK Department for International Development, Wellcome Trust, and the Big Lottery Fund (UK) Notes on validation of instruments (screening and outcomes): K‐10, a questionnaire for the detection of common mental disorders in community settings, that has been used in India and World Mental Health Surveys Additional information: none Handling the data: not available Prospective trial registration number: ISRCTN21817853 |
Vargas‐Porras 2021.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from January to September 2019. Country: Colombia Income classification: upper‐middle income country in 2019 Geographical scope: Bucaramanga, Floridablanca, Girón, or Piedecuesta Healthcare setting: maternal‐child health care centre that is a national and regional referral centre for maternal‐child care and application of national healthcare standards; intervention delivered through home‐based and telephone‐based sessions. |
| Participants | 1. Age: over 24 years in 66.67% (intervention group) and 48.48% control group; 24 years or under in 33.33% (intervention group) and 51.52% (control group) 2. Gender: female 3. Socioeconomic background: low in 48.48% (intervention group) and 72.73% (control); medium in 51.52% (intervention) and 27.27 (control) 4. Educational background: more than secondary school in 96.97% (intervention) and 69.70% (control) Inclusion criteria: a. living in Bucaramanga, Floridablanca, Girón, or Piedecuesta (Colombians who are culturally similar regarding beliefs about the care of mothers and infants, religion, mestizo ethnic group, and Spanish language); b. age ≥ 18 years; c. postpartum; d. first‐time mothers of healthy term infants; e. self‐reported partner support; f. owning a smartphone with internet access. Exclusion criteria: a. illiteracy; b. multiple pregnancies; c. postpartum depression; d. mental disorders; e. behavioural disorders; f. mother being admitted to the hospital or having her newborn admitted to an intensive care unit during the postpartum. Note: at baseline, the intervention and control group scores for Functional Social Support Subscale were, respectively, 76.12 (8.67) and 74.00 (10.58). Stated purpose: to test the efficacy of a multimodal nursing intervention (AMACOMPRI), based on Mercer's Becoming a Mother Theory, in supporting the process of becoming a mother in first‐time mothers of term infants |
| Interventions |
Name: multimodal nursing intervention “Maternal Support for Becoming a First‐time Mother” (AMACOMPRI), in addition to usual postnatal healthcare Title/name of PW and number: 1 maternal and perinatal nursing expert with a master's degree and 15 years professional experience in postpartum education (first author) 1. Selection: not specified 2. Educational background: master's degree in nursing 3. Training: training occurred over 1 month by thoroughly rehearsing the protocol for each session following the intervention manual. 4. Supervision: two supervisors provided feedback to audio records to ensure consistent and compliant delivery. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (first‐time mothers) and presented with some level of distress as indicated by BaM and EPDS scores that were below the cut‐off for the measures. Intervention details: eight nurse‐delivered home‐based and telephone‐based sessions. The intervention was delivered by alternating four 90‐minute in‐person visits and four 15‐minute telephone calls. The first two sessions focused on functional social support, the second two on the mother‐infant bond, the third two on perceived maternal self‐efficacy, and the last two on becoming a mother. Control: usual care (usual postnatal care including (a) predischarge nursing guidance on postpartum care, newborn care, and breastfeeding; (b) an obstetric follow‐up appointment (at day 8 postpartum for vaginal delivery and day 10 after caesarean section) focused on detection and control of potential puerperal complications (e.g. wound inspection, monitoring for postpartum hypertension, or infection); (c) newborn follow‐up appointment (3 to 5 days postpartum) for assessment of adaptation to extrauterine life, nutritional state, and neonatal abnormalities or infection as well as providing breastfeeding advice) |
| Outcomes |
Participants’outcomes of interest for this review
Note: maternal stress (BaM‐13 scale) and depression (EPDS) outcomes were only available for baseline. Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not specified Notes on validation of instruments (screening and outcomes): this instrument was translated into Spanish, culturally adapted, and validated in Colombian first‐time mothers of term infants (Vargas‐Porras 2020). Cronbach's α in the present study was 0.92. Additional information: none Handling the data: not applicable Prospective trial registration number: ClinicalTrials.gov (registration number NCT03594526) |
Vazir 2013.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was published in 2013. Country: India Income classification: low‐middle‐income country from 2007 Geographical scope: rural—60 villages in Andhra Pradesh Healthcare setting: home |
| Participants | 1. Age: mean maternal age (years)—control group (CG) 21.9 (SD 3.1); complementary feeding group (CFG) 22.3 (SD 3.5); responsive complementary feeding and playgroup (RCF&PG) 22.3 (SD 3.4) 2. Gender: female 3. Socioeconomic background: not reported 4. Educational background: % illiterate—37.4 CG, 38.9 CFG, 38.2 RCF&PG; % primary school—34.9 CG, 35.8 CFG, 29.8 RCF&PG; % secondary or high school—27.2 CG, 25.3 CFG, 32 RCF&PG; % of mothers working—52.5% CG; 56.4 CFG; 55.3 RCF&PG Inclusion criteria—cluster: villages that provided the required sample of pregnant women in their third trimester of pregnancy, over a period of 6 months. Exclusion criteria—cluster: no explicit exclusion criteria. Note: at baseline, the intervention (1 and 2) and control group scores for Center for Epidemiological Survey—Depression scale (CES‐D) were, respectively, 30.4 (8.08); 30.1 (8.35); and 30.8 (8.48). Stated purpose: to determine whether a cluster‐randomized educational intervention focusing on responsive feeding and mother‐child interaction, in combination with messages about appropriate breastfeeding and complementary feeding from 3 to 15 months of age, would improve adequacy of dietary intake, iron status, growth and development |
| Interventions |
Intervention name: complementary feeding group (CFG); responsive complementary feeding and play group (RCF&PG) Title/name of PW and number: village‐level workers (VWs) 1. Selection: local women who were mothers (one from each village) 2. Educational background: minimum high school‐level education 3. Training: the VWs received supervised training on how to counsel mothers/caregivers using the pictorial flip carts. The intervention teams (60 VWs) were trained to have focused ‘conversations’ with mothers for the various intervention topics. 4. Supervision: trained graduates in nutrition supervised the VWs, examined their records of visits and asked mothers independently what they were told in the VWs’ last visit. They also held periodic reinforcement training sessions with VWs. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by CES‐D scores. Intervention details CFG: in addition to the ICDS services, mothers in this group received nutrition education messages on sustained breastfeeding and complementary feeding through twice‐monthly or four times a month (depending on the age of the infant) home visits over 12 months by the trained village women (VWs) using flip charts, other visual material, demonstrations and counselling sessions. Age‐appropriate intervention messages and materials used for complementary feeding followed the Pan American Health Organization (PAHO)/World Health Organization (WHO) Guidelines (PAHO/WHO 2003). RCF&PG: In addition to the ICDS services, mothers in this group received education on complementary feeding as in the CFG (11 messages), eight messages and skills on responsive feeding, and eight developmental stimulation messages using five simple toys. These age‐appropriate messages and skills on how to understand and respond to infants’ cues of hunger/appetite or satiation comprised the responsive feeding intervention, consistent with some of the responsive feeding messages developed in Guideline #3 of the PAHO/WHO Guidelines (PAHO/WHO 2003) and messages on play and stimulation. This group of mothers also received developmentally appropriate toys five times during the intervention with instructions on how to use them to engage and play with their children. Control: usual care – mothers and infants in this group received only the routine ICDS services. It is the only major national programme in India that provides young children and mothers supplementary nutrition, healthcare, and pre‐school education. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: Indian Council of Medical Research, India and the NIH/NICHD (5 R01 HD042219‐S1); additional funding from UNICEF, New York Notes on validation of instruments (screening and outcomes): the CES‐D is a widely validated and established tool. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Velásquez 2015.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from June 2012 to November 2012. Country: Bogotá, Colombia Income classification: upper‐middle‐income country in 2012 Geographical scope: urban Healthcare setting: a public school operated by private educational institution |
| Participants | 1. Age: students from grades 5, 8, and 9 2. Gender: both 3. Socioeconomic background: students from a public school located in a socioeconomically disadvantaged area in the city of Bogotá 4. Educational background: grades 5, 8, and 9 Inclusion criteria: a. students from a public school (operated by a private educational institution) in Bogotà; b. grades 5, 8, and 9; c. were given consent forms for their parents; d. outcome scores for depressive and anxiety symptoms not above cut‐off at baseline. Exclusion criteria: no exclusion criteria; all students attending the school were eligible. Note: at baseline, the intervention and control group scores for Strengths and Difficulties Questionnaire (SDQ‐3)‐Anxiety were, respectively, 1.76 (0.96) and 1.60 (0.87). At baseline, the intervention and control group scores for SDQ‐3‐Depression were, respectively, 1.43 (1.03) and 0.96 (0.73). At baseline, the intervention and control group scores for prosocial behaviour were, respectively, 0.12 (0.06) and 0.11 (0.05). Stated purpose: to examine the efficacy of a yoga programme implemented in a low‐socioeconomic status school for prevention of depression, anxiety, and aggression |
| Interventions |
Name: yoga Title/name of PW and number: yoga teacher 1. Selection: school outsider and who was experienced in the conduction of yoga training 2. Educational background: not specified 3. Training: experienced yoga teachers trained to manage large classes and children with behavioural problems implemented the intervention. 4. Supervision: not specified 5. Incentives/remuneration: participants (children) were given a small snack after the end of each session. Prevention type: universal – all students attending the school were eligible for inclusion, and their baseline scores for the SDQ‐3 were well below the cut‐off for the measure. Intervention details: 24 2‐hour sessions focusing on all aspects of the individual: physical, energetic, mental, emotional, psychic, and spiritual. Overall, the protocol consisted of a number of postures (asanas), breathing exercises (pranayamas), relaxation (yoga nidra), and meditation techniques that were chosen for each of the 24 sessions. The activities in each session included postures practiced in a dynamic and active way. The selection of the postures was made based on previous empirical findings that showed that they could be used specifically to stimulate self‐confidence and self‐worth, as well as self‐regulation, relaxation, and consciousness. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: funding for this study was provided by the support to Corporacion Dunna—alternativas creativas para la paz—from RAMO. Notes on validation of instruments (screening and outcomes): SDQ—items were selected and adapted from this scale to evaluate depression (Cronbach’s alpha = 0.71) and anxiety (Cronbach’s alpha = 0.70). Additional information: none Handling the data: not available Prospective trial registration number: not available |
Ward 2020.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between February 2014 and March 2016. Country: South Africa Income classification: upper‐middle income country in 2014‐2016 Geographical scope: two historically black African peri‐urban settlements, amongst the most deprived in Cape Town, with high levels of HIV and community and family violence Healthcare setting: community |
| Participants | 1. Age: the majority of caregivers' age/range – 25‐38 years (58.78% for control group and 62.16% for intervention group) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: not specified Inclusion criteria: a. age 18+ years; b. primary caregiver of child aged 2 to 9 years, regardless of status as biological parent; c. co‐residing with child 4+ nights per week; d. reporting 15+ problem behaviours on the ECBI problem scale. Exclusion criteria: not specified. Note: at baseline, the intervention and control group scores for Beck Depression Inventory (BDI) were, respectively, 15.74 (10.90) and 15.39 (12.12). At baseline, the intervention and control group scores for Parenting Stress Index (PSI) were, respectively, 114.59 (19.33) and 111.92 (21.53). At baseline, the intervention and control group scores for Medical Outcomes Study Social Support Survey were, respectively, 21.03 (5.98) and 20.58 (6.29). Stated purpose: to explore whether a programme designed for the conditions of LMICs could be delivered with fidelity, acceptable to caregivers, and effective in increasing positive parenting and decreasing harsh parenting, thereby reducing child conduct problems. We aimed to target families at elevated risk for harsh parenting by screening for the presence of parental concern about child conduct problems. |
| Interventions |
Name: Parenting for Lifelong Health (PLH) programme Title/name of PW and number: paraprofessional community members 1. Selection: high school‐level education 2. Educational background: high school‐level education 3. Training: facilitators hired and trained during the first pilot study to conduct the programme (Lachman 2017) 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the BDI scores. Intervention details: PLH for Young Children, a low‐cost 12‐session programme designed to increase positive parenting and reduce harsh parenting and conduct problems in children aged 2 to 9. The first half of the programme focused on positive relationship building through dedicated one‐on‐one time and positive reinforcement of desirable behaviour. Subsequent sessions taught limit‐setting through instruction giving, household rules, and daily routines and nonviolent discipline strategies using redirect, ignore, time‐out, and consequences for decreasing undesirable behaviour. Caregivers practiced new skills in role‐play during each of the 12 three‐hour sessions and at home with their children. Control: usual care (services as usual) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 7‐24 months) |
| Notes |
Source of funding: funded largely by Ilifa Labantwana, a South African NGO and in part by the European Research Council (ERC) [FP7/2007‐2013/ERC grant agreement n°313421], the John Fell and Clarendon Funds, Rand Merchant Bank Fund, and the ApexHi Charitable Trust. Notes on validation of instruments (screening and outcomes): validated questionnaires. All measures were translated into isiXhosa (the local language) by consensus forward translation and checked by back translation. Additional information: none Handling the data: not applicable Prospective trial registration number: ClinicalTrials.gov (NCT02165371); Pan African Clinical Trial Registry (PACTR201402000755243); Violence Prevention Trials Register (www.preventviolence. info/Trials?ID=24). |
Willis 2019.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between December 2014 and November 2015. Country: Midlands province, Zimbabwe Income classification: low‐income country from 2014 to 2015 Geographical scope: rural Healthcare setting: primary care facility (3 clinics: 2 in the intervention group and 1 in the control group) |
| Participants | 1. Age: 10‐15 years; majority aged 10 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: among those attending, most were in primary school. Inclusion criteria: a. HIV‐positive adolescents; b. 10‐15 years old; c. ART—aware of their HIV status; d. accessing ART at the study clinic; e. consent from caregiver; f. assent from adolescent. Exclusion criteria: a. unaware of HIV status; b. nonconsent from caregiver; c. nonassent from adolescent. Note: at baseline, the intervention and control group scores for quality of life were, respectively, 3.16 and 3.61. Stated purpose: to determine the effectiveness of Community Adolescent Treatment Supporters (CATS) services on improving linkage to services and retention in care, adherence and psychosocial well‐being among 100 adolescents living with HIV (ALHIV) in a rural district of Zimbabwe |
| Interventions |
Name: CATS services Title/name of PW and number: 9 CATS 1. Selection: 18‐24 years old living with HIV 2. Educational background: not specified 3. Training: PWs trained by Ministry of Health and Child Care (MoHCC) 4. Supervision: all nine CATS attended a weekly feedback meeting at the clinic by CATS mentor. 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (living with HIV). Intervention details: participants in the intervention arm received the same standard of care but were also allocated to one CATS for additional support for 12 months. This included a weekly home visit during which the allocated CATS provided HIV and ART information and counselling as well as monitored the participants’ adherence and general well‐being. In the event that the participant was unwell or faced difficulties with adherence, the CATS would refer the participant to the CATS mentor in their district. The mentor would then liaise with the participants’ clinic for follow‐up. CATS additionally supported caregivers with information and counselling. Control: usual care – standard of care provided by the MoHCC, including monthly clinic reviews, ART, adherence counselling, CD4 monitoring and management of opportunistic infections. Treatment and care was led by a nurse and/or a primary counsellor. |
| Outcomes |
Participants’outcomes of interest for this review Nil Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) Note: data were not included in the meta‐analysis because they were not provided in the right format or were not available even after attempted author contact. |
| Notes |
Source of funding: Bristol‐Myers Squibb Foundation (BMSF). P.M. from BMSF also contributed as an author of the final manuscript. Notes on validation of instruments (screening and outcomes): the questionnaire was created ad hoc for the study. Additional information: none Handling the data: the data are not publicly available but can be obtained from the corresponding author (NW). No permissions are needed to obtain the data. Prospective trial registration number: PACTR201711002755428 |
Xu 2021.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was conducted from July 2017 to October 2018. Country: China Income classification: upper‐middle income country in 2017‐2018 Geographical scope: Xuzhou City in north of Jiangsu Province, eastern China Healthcare setting: community health service stations |
| Participants | 1. Age: mean age for the intervention group 63.81 ± 9.94 years and for the control group 62.91 ± 9.59 years 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: mostly (548 participants completers) high school and above Inclusion criteria: a. type 2 diabetes mellitus diagnosed at least 6 months before recruitment; b. Patient Health Questionnaire‐9 (PHQ‐9) scores ≥ 5 or Generalized Anxiety Disorder‐7 (GAD‐7) scores ≥ 5, and normal cognitive function at entry. Exclusion criteria: a. GAD‐7 scores < 5 or PHQ‐9 scores < 5; b. psychiatric history or cognitive impairment; c. severe diabetic complications; d. active suicidal ideation or a history of attempted suicide; e. taken antipsychotic drugs; f. history of panic disorder or bipolar depression; g. harmful or hazardous alcohol use; h. participation in any other interventional clinical trial; i. pregnancy; j. lactation and medically unstable condition (e.g. cancer, stroke, cardiovascular disease, chronic obstructive pulmonary disease, severe psychosis); k. participants with life expectancy less than 1 year; l. unwillingness to participate in the study; m. inability to regularly attend sessions (being absent for more than two sessions); n. experience of severe crisis and stress before the study; o. patients diagnosed as type 2 diabetes mellitus in the preceding 6 months. Note: at baseline, the intervention and control group scores for GAD‐7 were, respectively, 7.60 (5.08) and 7.80 (4.84). At baseline, the intervention and control group scores for PHQ‐9 were, respectively, 7.09 (4.37) and 7.00 (4.70). Stated purpose: to assess whether group cognitive behavioural therapy (GCBT) delivered by general practitioners reduces anxiety and depression and improves glycaemic levels in adults with type 2 diabetes mellitus |
| Interventions |
Name: GCBT Title/name of PW and number: 75 trained general practitioners 1. Selection: general practitioners selected from 24 healthcare centres 2. Educational background: not specified 3. Training: 3 days of training on eight GCBT modules 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by the PHQ‐9 and GAD‐7 scores. All those with psychiatric history or cognitive impairment were excluded. Intervention details: participants received 10 GCBT sessions in 10 consecutive days. Each session lasted 40 to 50 minutes and was followed by a 10‐ to 15‐minute discussion. After class, participants were asked to practice diaphragmatic breathing and progressive muscle relaxation daily before bedtime, to practice Baduanjin (a kind of traditional Chinese activity) every morning and evening, and to record their practice with a smartphone and send a short video to general practitioners for evaluation. Participants were also asked to record the timing and resolution of negative emotions, to narrate their problems and the needs of diabetes education. Control: usual care (UC) by general practitioners of primary healthcare services. The UC included conventional face‐to‐face follow‐ups for adults with type 2 diabetes mellitus every 3 months and recording patient health status, monitoring blood glucose and blood pressure, according to the NBPHS requirement. General practitioners should also give advice to diet, exercise, glycaemic control, prevention of complications, side effects of drugs and medication for each patient during follow‐up. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months, 7‐24 months) |
| Notes |
Source of funding: Preventive Medicine Research Projects of Jiangsu Province Health Department in 2015 and 2018 (Y2015010 and Y2018016), the Science and Technology projects of Xuzhou city in 2015 (KC15SM046), and the Youth Medical Talent Project of ‘Ke Jiao Qiang Wei Projects' in Jiangsu Province (QNRC2016375) Notes on validation of instruments (screening and outcomes): validated questionnaires Additional information: none Handling the data: not applicable Prospective trial registration number: Chinese Clinical Trials Registry (reference: ChiCTRIOP‐ 16008045) |
Yang 2022.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted from June to December 2019. Country: China Income classification: upper‐middle income country in 2019 Geographical scope: Zhejiang province Healthcare setting: nursing home in Zhejiang province |
| Participants | 1. Age: patients aged 65 years or more (average age: 85.36 years); caregivers not specified 2. Gender: both 3. Socioeconomic background: 68.85% blue‐collar workers, 71.31% living alone 4. Educational background: 31.71% illiterate, 30.89% primary education level (1‐6 years), 17.07% middle education level (7‐12 years), 20.33% high education level (> 13 years) Inclusion criteria (Alzheimer's patients): a. aged 65 years or more; b. diagnosed as AD by NIA‐AA criteria; c. lived in the selected nursing homes; d. agreed with participating in the project. Exclusion criteria (Alzheimer's patients): a. consciousness disorders; b. serious suicidal tendency; c. deaf; d. unable to communicate; e. serious disability leaving the patient unable to participate in activities. Participation suspension if: f. unwilling to continue; g. severe physical diseases after enrolment; h. leaving nursing homes (or other violations of inclusion criteria). Note: at baseline, the intervention and control group scores for Degree of Distress were, respectively, 1.72 (2.87) and 1.23 (2.30). Stated purpose: to investigate if a multisectoral comprehensive intervention would improve the life quality of elderly patients with Alzheimer Disease and their caregivers |
| Interventions |
Name: comprehensive intervention (multisectoral Cooperative Care Model) Title/name of PW and number: a multisectoral cooperative medical team including neurologists, nurses, patients’ caregivers, family members, rehabilitation therapists and social workers 1. Selection: not specified 2. Educational background: not specified 3. Training: only caregivers received a specific training (36 hours of systematic training) by the nursing staff. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: selective—participants were included based upon the presence of a risk factor (caregivers of Alzheimer's patients) and presented with some level of distress as indicated by the Degree of Distress scores that were below the cut‐off for the measure. Intervention details: multisectoral co‐operative comprehensive intervention (in addition to routine intervention) was mainly divided into five parts: (a) self‐care training to slow down the functional degradation—activities including dressing up, folding clothes, making food, led by caregivers through daily life; (b) exercise rehabilitation—stretching, walking and other exercises conducted by physical therapists twice a week; (c) cognitive memory—games, conducted by rehabilitation therapists two to four times a week; (d) social interest—activities including writing calligraphy, finger painting, reading newspapers and so on were conducted by nursing staff four to five times a week; (e) nursing training (for the patients’ caregivers, rehabilitation therapists and nurses)—gave them systematic training about self‐health knowledge and problem behaviour processing skills, combined with mental behaviour problems treatment, in order to reduce the pressure on caregivers and improve their nursing ability. Control: usual care (the control group was given routine intervention treatment in accordance with medical advice, which mainly included drug intervention and health education, and their caregivers were given basic health consultation services) |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (1‐6 months) |
| Notes |
Source of funding: National Science Foundation for Young Scientists of China (81803314), General project of Medical Science and Technology in Zhejiang province (2019KY001 and 2018KY193) Notes on validation of instruments (screening and outcomes): for QOL‐AD – Zhang Huimin and colleagues translated QOL‐AD scale into a Chinese version with Cronbach's α coefficient of 0.835, and it was 0.814 and 0.717 in this study for patients and caregivers, respectively (Liu 2012). Additional information: none Handling the data: not applicable Prospective trial registration number: ChiCTR1900023777 (China Clinical Trial Registration Center (CciCTR)) |
Yeomans 2010.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 2007 and 2010. Country: Burundi Income classification: low‐middle‐income country between 2007 and 2010 Geographical scope: two internally displaced persons camps in rural Burundi Healthcare setting: camps in rural, north‐central Burundi |
| Participants | 1. Age: mean age 36.6 years (SD = 12.8) 2. Gender: 44.4% female 3. Socioeconomic background: 48.3% lived in the camps. 4. Educational background: only 5% of the sample had completed more than 6 years of education. Inclusion criteria a. Almost all participants had been directly victimized by violence during or since the conflict onset in 1993, and many as much as 14 years prior to the time of the study. b. Baseline scores of Harvard Trauma Questionnaire Part IV (HTQ) (mean, SD: 2.14, 0.49 for intervention group 1; 2.25, 0.62 for intervention group 2; 2.04, 0.50 for the control group) and The Hopkins Symptom Checklist‐25 (HSCL) (mean, SD: 2.15, 0.57 for intervention group 1; 2.10, 0.65 for intervention group 2; 2.02, 0.60 for the control group) are within the standard cut‐off score (2.5 for HTQ and 2.25 for HSCL). Exclusion criteria: not reported. Note: at baseline, the intervention (1 and 2) and control group scores for HSCL‐25, total score were, respectively, 2.15 (0.57); 2.10 (0.65); and 2.02 (0.60). At baseline, the intervention (1 and 2) and control group scores for HTQ were, respectively, 2.14 (0.49); 2.25 (0.62); and 2.04 (0.50). Stated purpose: to evaluate the effects of PTSD psychoeducation within a larger trauma healing and reconciliation intervention in a rural region of Burundi |
| Interventions |
Name: workshop with psychoeducation Title/name of PW and number: Burundian facilitators 1. Selection: chosen by the nonprofit 2. Educational background: extensive experience with trauma workshop facilitation and for having demographics comparable to the participants (rural, poor, many without substantial formal education, and balanced in gender and ethnicity) 3. Training: full day of training dedicated to the modification of the standard workshop to accomodate planned differences in condition 4. Supervision: not reported 5. Incentives/remuneration: not reported Prevention type: indicated – participants presented with some level of distress as indicated by the HSCL‐25 and HTQ scores. Intervention details Intervention 1: workshop with psychoeducation (WP): six groups of approximately 20 participants gathered for 3 days, and 1 month later each workshop group reconvened for a full day follow‐up session during which major workshop components were reinforced. The 3‐day workshop used discussion, experimental exercises aimed at fostering interpersonal exchange, and games to explore themes of trauma, loss, anger, trust, and the roots of violence. Psychoeducational content on the first day of the workshop included a 90‐minute presentation and discussion of the 17 specific symptoms of PTSD. Coping trauma was addressed in terms of teaching relaxation skills. The Healing and Reconciling Our Communities workshop manual emphasized that recovery from trauma lies in the restoration of the relations between community members and individuals. Intervention 2: workshop with no psychoeducation (WPN): the active workshop condition with no psychoeducation was identical to the standard intervention, but it did not include the introduction of PTSD psychoeducational content and additional time was devoted to an exercise in which participants formed pairs and answered questions provided to them. Control: waiting‐list control condition – they received the workshops after the second assessment period. |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (2 weeks after intervention's completion) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): all instruments were translated and, prior to their use, the measures were checked for content and semantic equivalence by the three‐person Burundian advisory team. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Yu 2002.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was conducted between 1998 (training) and 2002 (publishing). Country: Beijing, China Income classification: low‐income country in 1998, low‐and‐middle‐income country from 1999 to 2002 Geographical scope: not specified Healthcare setting: schools affiliated to Peking university |
| Participants | 1. Age: 11.8 ± 1.69 years 2. Gender: both 3. Socioeconomic background: most living in a family with monthly income of 1001‐2000 yuan 4. Educational background: 4th/5th/6th grade in primary school or 1st or 2nd grade in high school Inclusion criteria: a. 4th, 5th, and 6th grade children in the Affiliated Elementary School of Peking University and 1st and 2nd grade children in the Affiliated High School of Peking University; b. presence of depressive symptoms, screened with Children's Depression Inventory (CDI); c. family conflict, screened with conflict subscales of FES. Exclusion criteria: no exclusion criteria. Note: at baseline, the intervention and control group scores for CDI were, respectively, 17.44 (9.47) and 16.72 (9.29). Stated purpose: the Penn Optimism Program (POP) developed at the University of Pennsylvania was designed to prevent future depressive symptoms in children and young adolescents at risk. The intervention goals were to enhance participants’ resilience in the face of negative life events by training them to challenge pessimistic causal explanations and teaching them other coping strategies. |
| Interventions |
Name: the POP Title/name of PW and number: 8 teachers 1. Selection: four facilitators were recruited from the two schools as leaders of the treatment groups. Although these teachers taught other courses, the possibility of multiple‐role interference was eliminated by arranging that children taught by a teacher in school courses would not participate in groups led by that teacher. 2. Educational background: not specified 3. Training: the teachers received a total of 40 hours training in summer 1998 by David Lei Yu (first author). A manual was used during the training. 4. Supervision: weekly supervision meetings were scheduled to ensure teaching quality and adherence to the manual, especially at the beginning of the intervention. 5. Incentives/remuneration: not specified Prevention type: indicated – participants presented with some level of distress as indicated by CDI scores. Quote: “The two screening questionnaire [Children’s Depression Inventory (CDI) and the Cohesion and Conflict subscales of FES] scores were converted to z scores and summed. Three hundred fifty‐five children whose overall risk scores were in the top 25% of their age group were invited to participate. The opportunity to participate in the study was offered in descending order based on the risk scores”. Intervention details: the POP is designed to prevent future depressive symptoms in children and young adolescents at risk. The intervention goals are to enhance participants' resilience in the face of negative life events by training them to challenge pessimistic causal explanations and teaching them other coping strategies. It comprises 10 sessions covering topics such as the ABC mode for thoughts and feelings, thinking styles, de‐catastrophizing, family conflict, assertiveness and negotiation, coping with conflict, dealing with procrastination, social‐skills training, decision‐making and problem‐solving. Control: no intervention |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): the CDI has demonstrated satisfactory levels of reliability and validity in the United States as well as in mainland China. They found the internal consistency of the CDI (Cronbach's alpha) to be from 0.81 to 0.89 in various studies in China. Additional information: none Handling the data: not available Prospective trial registration number: not available |
Yusoff 2015.
| Study characteristics | |
| Methods |
Study design: RCT Duration of study: the study was published in 2014. Country: Malaysia Income classification: upper‐middle‐income country from 1992 Geographical scope: not specified Healthcare setting: university (medical school) |
| Participants | 1. Age: not specified (adults) 2. Gender: both 3. Socioeconomic background: not specified 4. Educational background: medical students from each year of study (mainly in first academic year accepted) Inclusion criteria: a. medical students from each year of study; b. attend a 3‐h briefing session on the study protocol; c. signed informed consent form. Exclusion criteria: no exclusion criteria. Note: at baseline, the intervention and control group scores for Beck’s Depression Inventory (BDI) were, respectively, 5.55 (4.98) and 4.56 (4.66). Stated purpose: to evaluate the effectiveness of a DEAL‐based based intervention on medical students' depression symptoms, coping strategies, and perceived stressors |
| Interventions |
Name: DEAL‐based intervention Title/name of PW and number: medical teachers 1. Selection: not specified 2. Educational background: not specified 3. Training: it did not require any special training. 4. Supervision: not specified 5. Incentives/remuneration: not specified Prevention type: universal prevention – all participants were eligible for inclusion, and their baseline scores for the BDI were well below the cut‐off for the measure. Intervention details: the intervention is a 4‐hour educational workshop based on the DEAL model. It comprises four sections; the first section focuses on an introduction to the workshop and delivering information about stress, stressors, and coping strategies that are relevant to medical students (Section 1.0); the second section focuses on the practical aspects of self‐evaluation‐related stress, stressors and coping strategies (Section 2.0); the third section focuses on group work on dealing with stressful situations based on video clips (Section 3.0); the fourth section focuses on sharing experience, feedback, and conclusion about the whole activities (Section 4.0). Participants completed the intervention within 240 minutes (4 hours) over a half‐day. Control: waiting list |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month, 1‐6 months, 7‐24 months) |
| Notes |
Source of funding: University Sains Malaysia funding this study under the Research University Grant 1001/PPSP/812086 Notes on validation of instruments (screening and outcomes): BDI has been validated across regions, and the reliability coefficients (Cronbach’s alpha) have ranged from 0.76 to 0.95, with a mean of 0.86. Its uses have been validated in a nonpsychiatric sample. The measurement tool has been validated by previous studies in the Malaysian context. Additional information: additional dataset available after request to the corresponding author Handling the data: none Prospective trial registration number: not available |
Zhou 2010.
| Study characteristics | |
| Methods |
Study design: cluster‐RCT Duration of study: the study was started in 2006 and published in 2010. Country: China Income classification: low‐middle‐income country from 2006 to 2009; upper‐middle‐income country in 2010 Geographical scope: rural Healthcare setting: home |
| Participants | 1. Age: local residents more than 60 years old (intervention 71.5 ± 7.4; control 71.8 ± 7.4) 2. Gender: both 3. Socioeconomic background: most participants' annual income < 5000 RMB (1 yuan RMB = 0.142857 U.S. dollars [June, 2008 rate]) annually 4. Educational background: most participants illiterate Inclusion criteria: a. elderly individuals 60 years old or older; b. local residents in Baimu or Wangzhai town. Exclusion criteria: subjects with dementia, mental illness, limitations on cognition, and hospitalization at the time of contact were excluded. Note: at baseline, the intervention and control group scores for Short Form Health Survey 36‐Mental combined scale (SF‐36) were, respectively, 68.0 (17.6) and 65.3 (18.8). Stated purpose: to spread the knowledge of healthy lifestyles and to provide health‐related information to the older adult population |
| Interventions |
Name: individualized health intervention Title/name of PW and number: village doctors 1. Selection: village doctors dispatched by community health services, speaking the local dialect and having good relationships with the local residents 2. Educational background: not specified 3. Training: not specified 4. Supervision: workshop on the 10th of each month for feedback on the intervention and advice on its implementation by the research team 5. Incentives/remuneration: not specified Prevention type: universal – all participants were eligible for inclusion, and their baseline scores for the SF‐36 were well below the cut‐off for the measure. Intervention details: tailored programme involving three components: (a) main health problems, (b) health behavioural prescriptions, and (c) intervention record and effect assessment schedule. The ordinary main health problems in this study were hypertension, coronary heart disease, chronic bronchitis, cigarette smoking, alcohol drinking, heavy salt intake, poor diet, and lack of regular exercise. Participants were given a health behavioural prescription (a prompt card) and then assessed on their state of change (i.e. pre‐contemplation, contemplation, preparation, action, and maintenance). Each participant received at least a home visit (lasting 20 to 30 minutes) per month over the intervention period (9 months) for encouragement and support. Motivational interviewing techniques were used, including consciousness‐raising, dramatic relief, self‐liberation, self‐revaluation, helping relationships and stimulus control + Community Health Campaign (CHC). Control: usual care – CHC: basic health promotion measure conducted in Wuyi County since 2005 to spread knowledge of a healthy lifestyle to the older population |
| Outcomes |
Participants’outcomes of interest for this review
Carers’ outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention (7‐24 months) |
| Notes |
Source of funding: not available Notes on validation of instruments (screening and outcomes): the SF‐36 was widely used to assess the quality of life because of its comprehensiveness, brevity, and high standard of reliability and validity. Thus, it has been used to assess the health status of older adults. Additional information: none Handling the data: not available Prospective trial registration number: not available |
ABC: antecedent (A), behaviour (B), consequence (C) ACL: adult literacy centre ACORN: a brief intervention to reduce maternal anxiety during pregnancy ACSM: American College of Sports Medicine ACT: adolescent coordinated transition ADDQOL: Audit of Diabetes Dependent Quality of Life AEP: Adolescence Education Program ALHIV: adolescents living with HIV AMACOMPRI: multimodal nursing intervention “Maternal Support for Becoming a First‐time Mother” APAI: Acholi Psychosocial Assessment Instrument ART: anti‐retroviral therapy ARV: anti‐retroviral AUDIT: Alcohol Use Disorders Identification Test AYPA: African Youth Psychosocial Assessment BAI: Beck Anxiety Inventory BaM(‐13): Being a Mother Scale BASC: Behaviour Assessment System for Children BBTI: Brief Behavioral Treatment for Insomnia BDI: Beck's Depression Inventory BIT: Brief Inventory of Thriving BPM(‐P): Brief Problem Monitor(‐parent form) BPSD: behavioral psychological symptoms of dementia CA: community activities CAPAS‐Mx: Criando con Amor, Promoviendo Armonía y Superación en México (Raising Children with Love, Promoting Harmony and Self‐Improvement [CAPAS‐Mx]) CATS: community adolescent treatment supporters CAU: care as usual CBCL: Child Behaviour Checklist CBI: classroom‐based intervention CBO: community‐based organisations CBT: cognitive‐ behavioural therapy CD4: CD4 T lymphocytes or 'helper T cells' CDA: child development agent CD‐H: Child Drawing: Hospital CDI: Children’s Depression Inventory CDR: Clinical Dementia Rating CES‐D(‐CC): Center for Epidemiologic Studies Depression Scale (‐children) CFG: complementary feeding group CFI: Children’s Function Impairment CG: control group CH: community health CHAMP(SA): Collaborative HIV Adolescent Mental Health Program South Africa CHC: community health campaign CHIME: community psychosocial music intervention CHN: community health nurse CHV: community health volunteer CHW: community health workers CMD: common mental disorder C‐PrES: Community Pregnancy Surveillance and Targeted Education Sessions CPDS: Child Psychosocial Distress Screener CPSS: Child PTSD Symptom Scale CPSW: community‐based psychosocial workers CRIES‐8: Children's Revised Impact of Events Scale‐8 CRS: Catholic Relief Services CSI: caregiver support intervention CT: cognitive training CV: cardiovascular CW: community worker CWBFN: Child Welfare Bloemfontein & Childline Free State DASS‐21: Depression Anxiety Stress Scale‐21 DDS: Diabetes Distress Scale DEAL: medical student well‐being workshop, Family Environment Scale model DIL: depression in later life DPNP: diabetic peripheral neuropathic pain DSI: Daily Stress Index DSM: Diagnostic and Statistical Manual of Mental Disorders DSR: decision support recommendation DSRS: Depression Self‐Rating Scale DTP: Dramatic Therapeutic Play EASE: World Health Organization’s Early Adolescent Skills for Emotions EBES: Life of the Subjective Well‐being Scale ECBI: Eyberg Child Behavior Inventory ECD: Early Childhood Development ECI: Experience of Caregiving Inventory EDS: electronic decision support EMEP: Ways of Coping Scale ENACT: ENhancing Assessment of Common Therapeutic factors EPDS: Edinburgh Postnatal Depression Scale EPNDS: Edinburgh Postnatal Depression scale EQ‐5D‐3/5L: EuroQoL 5‐Dimension‐3/5‐Level ERSAE: universal teacher‐delivered program ESB: Economic Skill Building ESPS: ERSAE‐Stress‐Prosocial ETAU: enhanced treatment as usual EUC: enhanced usual care FES: Family Environment Scale FGD: focus group discussions FHC: family health centre FSH: follicle‐stimulating hormone FSI: family strengthening intervention FSS: Family Support Scale FV: family volunteers GAD‐7: Generalized Anxiety Disorder‐7 GCBT: group cognitive behavioural therapy GDS(‐S): Geriatric Depression Scale (‐Short Form) GHQ(‐12): General Health Questionnaire(‐ 12) GHS: Ghana Health Service GMKL: Gum Marom Kids League GP: general practitioner GSE: General Self‐Efficacy Scale HADS: Hospital Anxiety and Depression Scale HbA1c: hemoglobin A1c HC: health curriculum HCA: healthcare assistants HCQ: Huellitas Caregivers Questionnaire HDI: Human Development Index HE: health education HFP: Happy Families Program HIDV: Darcy Vargas Children’s Hospital HINSG: Hospital Infantil Nossa Senhora da Glória HIV: human immunodeficiency virus (H)MMSE: (Hindi) Mini‐Mental State Examination HSCL(‐25): Depression Subscale of the Hopkins Symptom Checklist‐25 HTQ: Harvard Trauma Questionnaire Huellitas: Dejando Huellitas en tu Vida (Leaving Traces on Your Life [Huellitas]) intervention HUUSP: University Hospital, University of São Paulo IADL: instrumental activities of daily living IASC: Inter‐Agency Standing Committee IAYT: Integrated Approach to Yoga Therapy ICDP: International Child Development Programme ICDS: Integrated Child Development Services ICBF: Instituto Colombiano de Bienestar Familiar IDP: internally displaced people IES: Impact of Event Scale IMB: Inshuti Mu Buzima iMBC: Integrated Mothers and Babies Course IPT: interpersonal psychotherapy IPV: intimate partner violence IRC: International Rescue Committee IRIE: IRIE Classroom Toolbox ISSL: Lipp's Stress Symptoms Inventory for Adults IUGR: intrauterine growth retardation K‐10: Kessler‐10 Item Scale LC: lay health counselor LCM: Levine's Conservation Model LDL: low‐density lipoprotein LHW: lay health worker LMIC: low‐ and middle‐ income country LSITA‐SF: Life Satisfaction Index for the Third Age‐Short Form LSNS: Lubben Social Network Scale LSS: Life Satisfaction Scale MA: methamphetamine MBSR: mindfulness‐based stress reduction MFQ: Mood and Feelings Questionnaire MHC‐SF: Mental Health Continuum Short Form MG: meditation group mhGAP: WHO Mental Health Gap Action ProgrammE MIND+: Be Mindful internet‐based and self‐guided course MINI: Mini International Neuropsychiatric Interview MNCHN: Maternal, Newborn, Child Health, and Nutrition MoCA‐C: Montreal Cognitive Assessment‐Chinese version MoHCC: Ministry of Health and Child Care MOS: Social Support Medical Outcomes Study Questionnaire MPPI: multi‐component positive psychology intervention MPSS: Modified PTSD Symptom Scale MSRT: mind sound resonance technique MT: master trainer MWellcare: mHealth‐Based Electronic Decision Support System NA: not assessed NBPHS: national basic public health services NCD: noncommunicable diseases NCHS: National Centre for Health Statistics NEPIQOL: Neuropathic Pain Impact on Quality of Life Questionnaire NGO: non‐governmental organisation NIA‐AA: NIA‐AA Revised Diagnostic Criteria for Alzheimer's Disease PA: physical activity PAHO: Pan American Health Organization PAS: peer adherence support PC: primary care PCR: polymerase chain reaction PE: physical education PedsQL: Pediatric Quality of Life Inventory PF: programme facilitator PG: playgroup or postgraduate education PHQ(‐2/4/8/9): Patient Health Questionnaire (‐2/4/8/9) PHW: primary healthcare worker PI: principal investigator PIH: Partners In Health PLH: people living with HIV or Parenting for Lifelong Health program PLST: Progressively Lowered Stress Threshold Model PLWHA: people living with HIV/AIDS PM+: Problem Management Plus PMTCT: Prevent Mother‐to‐Child Transmission POP: Penn Optimism Program PPS: Pregnancy Pressure Scale PPSC: Pediatric Symptom Checklist PS: psychosocial stimulation PSA: profound stress attunement PSI(‐SF): Parenting Stress Index (‐short form) PSS: Perceived Stress Scale PSSA: Psychosocial Structured Activities PSSS: Perceived Social Support Scale PST: Problem‐Solving Therapy PTSD: post‐traumatic stress disorder PW: primary‐level workers QOL: quality of life QOL(‐AD): Quality of Life in Alzheimer's Disease Scale RADS‐2: Reynolds Adolescent Depression Scale‐2 RAP‐A: Resourceful Adolescent Program‐adolescent version RC: resilience curriculum RCF&PG: responsive complementary feeding and playgroup RCT: randomized controlled trial REACH VN/VA: Resources for Advancing Alzheimer’s Caregiver Health in Vietnam REST: Rural Emergency Health Service and Transport RG: relaxation group RMB: renmimbi ('the people's currency') is the currency of China SBP: systolic blood pressure SCARED‐5: Screen for Anxiety Related Emotional Disorders SCAS: Spence Children's Anxiety Scale SCD: sickle cell disease SCID: Structured Clinical Interview for the DSM‐IV Diagnoses SCL(‐25): Symptom Check List(‐25) SCU: sickle cell unit SD: standard deviation SDSCA: Summary of Diabetes Self‐Care Activities SDQ(‐3): Strengths and Difficulties Questionnaire (‐3) SE: standard error SEHER: school health promotion intervention SES: socioeconomic status SF‐36: Short Form Health Survey SH+: Self Help Plus SRQ: Self‐Reporting Questionnaire SSEP: self‐efficacy‐focused structured education program SSQ: Shona Symptom Questionnaire SSRS: Social Support Rating Scale SSS(‐R): Social Support Scale (‐Revised) SSS: Social Security System STAI: State‐Trait Anxiety Inventory STI: sexually transmitted infection SWEMWBS: Short Warwick‐Edinburgh Mental Well‐being Scale SWLS: Satisfaction with Life Scale T2DM: type 2 diabetes TAP‐BR: Tailored Activity Program—Brazilian version TAU: treatment as usual TB: tuberculosis TEA: together for empowerment activities TGDS: Geriatric Depression Scale Thai version TPO: Transcultural Psychosocial Organization TTI: Theory of Triadic Influence UC: usual care UCT: unconditional cash transfer VC: violence curriculum VDC: Village Development Committees VEMOFIT: Value‐Based and Emotion‐Focused Educational Program VW: village‐level workers WEBWBS: Warwick‐Edinburg Mental Well‐being Scale WHO: World Health Organization WHO‐5: World Health Organization‐5 Well‐being Index WHODAS: WHO Disability Assessment Schedule WHOQOL‐BREF: World Health Organization ‐ Quality of Life ‐ short version WLH: women living with HIV WP: workshop with psychoeducation WPN: workshop with no psychoeducation ZBI: Zarit Burden Interview ZLDSI: Zanmi Lasante Depression Symptom Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdullahzadeh 2021 | Ineligible population |
| Ackan 2019 | Ineligible study design |
| Ahmadi 2021 | Ineligible intervention |
| Al‐Ameri 2021 | Ineligible population |
| Al‐Faris 1997 | Ineligible intervention |
| Alampay 2019 | Ineligible population |
| Alvi 2021 | Ineligible intervention |
| Ansai 2015 | Ineligible intervention |
| Anusuya 2021 | Ineligible population |
| Araya 2013 | Ineligible intervention |
| Araújo 2016 | Ineligible population |
| Asampong 2021 | Ineligible population |
| Attanasio 2019 | Ineligible population and outcome |
| Atukunda 2019 | Ineligible study design |
| Azizi 2010 | Ineligible population |
| Baek 2013 | Ineligible setting |
| Baker‐Henningham 2009 | Ineligible intervention |
| Bakhtavar 2007 | Ineligible intervention |
| Barnhart 2020 | Ineligible population |
| Baruah 2021 | Ineligible population |
| Betancourt 2014 | Ineligible population |
| Bhana 2014 | Ineligible intervention |
| Bliznashka 2021 | Ineligible population |
| Bogdanov 2021 | Ineligible population |
| Bolton 2014 | Ineligible population |
| Bose 2015 | Ineligible population |
| Burmaoğlu 2021 | Ineligible study design |
| Chandra 2018 | Ineligible study design |
| Chatterjee 2014 | Ineligible outcomes |
| Chen 2018 | Ineligible population |
| Cheung 2020 | Ineligible setting |
| Chibanda 2014 | Ineligible population |
| Christofides 2020 | Ineligible population |
| Clarke 2014 | Ineligible population |
| Cluver 2020 | Ineligible population |
| Conde 2018 | Ineligible intervention |
| Çankaya 2021 | Ineligible population |
| de Miranda 1999 | Ineligible intervention |
| Decker 2020 | Ineligible population |
| Dike 2021 | Ineligible intervention |
| Dirani 2021 | Ineligible intervention |
| do Nascimento 2015 | Ineligible outcomes |
| Dow 2020 | Ineligible population |
| DRKS00000214 | Ineligible study design |
| Efendi 2020 | Ineligible population |
| Egbegi 2021 | Ineligible intervention |
| Ekrami 2019 | Ineligible intervention |
| Esala 2017 | Ineligible population |
| Estebari 2018 | Ineligible intervention |
| Francis 2021 | Ineligible outcome |
| Fuhr 2019 | Ineligible population |
| Gallegos 2013 | Ineligible study design |
| Getanda 2022 | Ineligible population |
| Ghasemzadeh 2020 | Ineligible intervention |
| Ghawadra 2020 | Ineligible population |
| Ghodsbin 2015 | Ineligible intervention |
| Gok Ugur 2019 | Ineligible population |
| Griffiths 2020 | Ineligible outcomes |
| Gul 2021 | Ineligible intervention |
| Gureje 2019 | Ineligible population |
| Haack 2021 | Ineligible population |
| Haghighat 2018 | Ineligible population |
| Hajisadeghian 2021 | Ineligible intervention |
| Hamdani 2020 | Ineligible population |
| Hamdani 2021b | Duplicate |
| Hamdi 2020 | Ineligible population |
| Hamester 2016 | Ineligible study design |
| Hartmann 2020 | Ineligible outcomes |
| Heizomi 2020 | Ineligible study design |
| Hill 2021 | Ineligible setting |
| Hinsberger 2017 | Ineligible intervention |
| Hortense 2020 | Ineligible intervention |
| Huis in ‘t Veld 2019 | Ineligible population |
| Husain 2021a | Ineligible intervention |
| Husain 2021b | Ineligible population |
| Husain 2021c | Ineligible population |
| Häfele 2021 | Ineligible intervention |
| Iremeka 2021 | Ineligible intervention and outcomes |
| Ismayilova 2018 | Ineligible population |
| ISRCTN15986016 | Ineligible population |
| Jackson 2021 | Ineligible population |
| Jamshidi 2020 | Ineligible population |
| Jaywant 2020 | Ineligible population |
| Jordans 2021 | Ineligible population |
| Kagawa 2017 | Ineligible outcomes |
| Kane 2022 | Ineligible population |
| Karimi 2019 | Ineligible intervention |
| Karimli 2019 | Ineligible intervention |
| Kazazi 2021 | Ineligible intervention |
| Khan 2017 | Ineligible population |
| Khuzwayo 2020 | Ineligible outcomes |
| Kidder 2013 | Ineligible outcomes |
| Kivumbi 2019 | Ineligible intervention |
| Koebach 2021 | Ineligible population |
| Kohli 2017 | Ineligible intervention |
| Kor 2019 | Ineligible setting |
| Kulis 2019 | Ineligible outcomes |
| Lai 2019 | Ineligible setting |
| Langoni 2019 | Ineligible intervention |
| Latifi 2020 | Ineligible intervention |
| Lawrence 2020 | Ineligible study design |
| Li 2015 | Ineligible population |
| Li 2016 | Ineligible intervention |
| Li 2020a | Ineligible population |
| Luo 2019 | Ineligible outcomes |
| Ma 2018 | Ineligible intervention |
| Maarefvand 2015 | Ineligible population |
| Madhombiro 2020 | Ineligible population |
| Mahdavi 2017 | Ineligible intervention |
| Mahmoudi 2004 | Ineligible population |
| Manyaapelo 2019 | Ineligible outcomes |
| Marais 2011 | Ineligible outcomes |
| Markkula 2019 | Ineligible population |
| Masoumi 2020 | Ineligible intervention |
| McBain 2015 | Ineligible study design |
| McLaughlin 2012 | Ineligible outcomes |
| Mecdi Kaydirac 2021 | Ineligible population |
| Mehri 2020 | Duplicate |
| Michelson 2020 | Ineligible population |
| Milani 2017 | Ineligible study design |
| Miller‐Matero 2021 | Ineligible setting |
| Mirmahmoodi 2020 | Ineligible population |
| Mohammadi 2011 | Ineligible intervention |
| Mohd‐Sidik 2018 | Ineligible population |
| Moshki 2014 | Ineligible intervention |
| Murray 2016 | Ineligible outcomes |
| Mutyambizi‐Mafunda 2019 | Ineligible population |
| Nabunya 2014 | Ineligible intervention |
| Nabunya 2020 | Ineligible population |
| Nahar 2015 | Ineligible population |
| NCT02059863 | Ineligible population |
| NCT04638101 | Ineligible setting |
| Nestadt 2019 | Ineligible intervention |
| Nguyen 2020 | Ineligible outcomes |
| Nunez Pumariega 2020 | Ineligible population |
| Nyamathi 2012 | Ineligible population |
| Obiweluozo 2021 | Ineligible intervention |
| Ogum Alangea 2020 | Ineligible population |
| Owais 2020 | Ineligible study design |
| Oz 2020 | Ineligible intervention |
| O’Farrelly 2021 | Ineligible setting |
| Pan 2019 | Ineligible population |
| Pandya 2021 | Ineligible population |
| Papas 2011 | Ineligible population |
| Papas 2021 | Ineligible population |
| Paranthaman 2010 | Ineligible population |
| Park 2019 | Ineligible setting |
| Parvin 2008 | Ineligible population |
| Patel 2017 | Ineligible population |
| Pelegrini 2020 | Ineligible population |
| Peltonen 2012 | Ineligible population |
| Peltzer 2012 | Ineligible intervention |
| Peltzer 2013 | Ineligible population |
| Peltzer 2020 | Ineligible population |
| Perry 1989 | Ineligible outcomes |
| Petersen 2014 | Ineligible population |
| Rabiei 2020 | Ineligible intervention |
| Rahimi 2021b | Ineligible population |
| Rajai 2021 | Ineligible intervention |
| Rivero 2021 | Ineligible intervention |
| Rossow 2021 | Ineligible study design |
| Rotheram‐Borus 2018 | Ineligible population |
| Sanchez 2017 | Ineligible outcomes |
| Sanchez 2019 | Ineligible population |
| Sanchez 2021 | Ineligible outcome |
| Sapkota 2020 | Ineligible intervention |
| Saw 2020 | Ineligible population |
| Scharf 2021 | Ineligible setting |
| Schwitters 2015 | Ineligible population |
| Seyedrasooli 2020 | Ineligible intervention |
| Shaw 2021 | Ineligible population |
| Sherlee 2020 | Ineligible population |
| Shin 2009 | Ineligible population |
| Shirazi 2008 | Ineligible outcomes |
| Sikander 2019 | Ineligible population |
| Siriwardhana 2013 | Ineligible study design |
| Sokolovic 2022 | Ineligible study design |
| Sousa 2020 | Ineligible setting |
| Ssewamala 2009 | Ineligible population |
| Stansert Katzen 2021 | Ineligible study design |
| Suihami 2020 | Ineligible intervention |
| Suleman 2021 | Ineligible population |
| Sun 2021 | Ineligible intervention |
| Suteerangkul 2021 | Ineligible intervention |
| Taghizadeh 2008 | Ineligible population |
| Tam 2019 | Ineligible intervention |
| Taneja 2020 | Ineligible intervention |
| Tawfik 2021 | Ineligible population |
| TCTR20200610001 | Ineligible study design |
| Tekur 2012 | Ineligible intervention |
| Tiwari 2010 | Ineligible setting |
| Tol 2014 | Ineligible population |
| Toulabi 2012 | Ineligible intervention |
| Ural 2021 | Ineligible study design |
| Uzdavines 2021 | Ineligible population |
| Vakilian 2019 | Ineligible population |
| Van't Hof 2021 | Ineligible population |
| Vancampfort 2021 | Ineligible study design |
| Villar 1992 | Ineligible outcomes |
| Waechter 2021 | Ineligible setting |
| Wainberg 2021 | Ineligible population |
| Walker 2006 | Ineligible outcomes |
| Wasil 2021 | Duplicate |
| Wolf 2019 | Ineligible outcomes |
| Wolmer 2005 | Ineligible study design |
| Won 2020 | Ineligible setting |
| Wong 2020 | Ineligible setting |
| Xiao 2021 | Ineligible study design |
| Xie 2019 | Ineligible population |
| Xu J 2022 | Ineligible study design |
| Yamada 2021 | Ineligible intervention |
| Ye 2020 | Ineligible intervention |
| Zeng 2016 | Ineligible study design |
| Zeziulin 2021 | Ineligible intervention |
| Zhang 2019 | Ineligible intervention |
| Zhang 2021 | Ineligible population |
| Zheng 2021a | Ineligible intervention |
| Zhou 2020 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Ajh 2006.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Pregnant women Inclusion criteria: a. pregnant women Exclusion criteria: not specified Stated purpose: prevention of depression after delivery |
| Interventions |
Intervention: supportive activities during pregnancy Control: not specified |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Akbarzadeh 2012.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Primigravida women Inclusion criteria: a. primigravida women referred to Hafiz and Shushtari hospitals in Shiraz Exclusion criteria: not specified Stated purpose: to determine the effect of relaxation and attachment behaviours training on anxiety in first‐time mothers |
| Interventions |
Intervention: relaxation and attachment behaviors training In addition to routine pregnancy care, four 90‐minute sessions of attachment behaviours and relaxation training courses were held during 4 weeks (once a week) Control: usual care (routine pregnancy care) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Andaroon 2018.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Pregnant women Inclusion criteria: a. primiparous women with the gestational ages of 28 to 30 weeks referred to healthcare centres of Mashhad Exclusion criteria: not reported Stated purpose: to evaluate the effect of individual counselling program by a midwife on anxiety during pregnancy in nulliparous women. |
| Interventions |
Intervention: individual counselling programme The subjects in the intervention group received three sessions (once every two weeks) of the individual counselling programme based on the content of the consultation model in the Gamble's study. Control: usual care |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Baba Nazari 2014.
| Methods | Abstract, full‐text is not available Study design: RCT Country: Iran |
| Participants | Pregnant women Inclusion criteria: a. pregnant women who attended antenatal care between August to October 2013 Exclusion criteria: a. women who had gone to psychological doctor due to some mental illnesses; b. those with a background of using drugs; c. those who left their job because of pregnancy. Stated purpose: to investigate the effect of spiritual intelligence training on decreasing pregnancy anxiety in Shiraz |
| Interventions |
Intervention: spiritual intelligence training spiritual intelligence training based on Tiri Noklain and Obani's models. It consists of 10 90‐minute sessions. Control: no treatment |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (not specified) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Bazrafshan 2010.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Pregnant women Inclusion criteria: a. primigravid women aged 15‐35 who were in the 3rd trimester of pregnancy Exclusion criteria: not specified Stated purpose: to assess the effect of slow stroke back massages on anxiety level amongst primigravid women in two clinics in Shiraz in 2007 |
| Interventions |
Intervention: massage The intervention group received slow stroke back massage for 10 minutes on three consecutive mornings. Control: control group (not specified) |
| Outcomes |
Participantsoutcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediately post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Bernardi 2013.
| Methods | No information on interventionists' background Study design: RCT Country: Brazil |
| Participants | Women with mastectomy Inclusion criteria: a. over 21 years of age b. mastectomized in several stages of treatment c. without any previous engagement in the Rehabilitation Program for Mastectomised Women Exclusion criteria: a. distant metastasis and recurrence of the illness b. any kind of apparent psychosis, disability, mental problems, dementia, deficit hearing, and/or speech that could compromise the interview or surgery Stated purpose: evaluate the effects of the Hatha‐Yoga intervention on anxiety, signs, and symptoms of stress on women with mastectomy |
| Interventions |
Intervention: Hatha‐Yoga intervention The intervention was applied individually to volunteers, in a quiet place with the use of mats and pillows‐like support material. The intervention was composed of six Hatha‐Yoga practices that last for 45 minutes, the script of which is based on a protocol consisting of a moment of welcome and internalization of volunteer women, awareness of diaphragmatic breathing, performing body postures, performing breathing exercises, relaxation, exercises of concentration preparatory for meditation. Additionally, these women received guidance to practise Hatha‐Yoga and were encouraged to practise the intervention in their homes. After the closing of the intervention, participants had the opportunity to ask for clarification of doubts and to interact with testimonials and with the instructor. Control: waiting list |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (< 1 month) |
| Notes |
Source of funding: not available Prospective trial registration number: not available |
Foo 2020.
| Methods | No information on baseline data for depressive, anxiety symptoms and distress Study design: RCT Country: Malaysia |
| Participants | Knee osteoarthritis patients Inclusion criteria: a. diagnosed with primary knee OA on the basis of medical evaluation (knee pain for most days of the month before and bony enlargement of the knee) and radiographic examination showing Kellgren–Lawrence (K‐L) classification of grade 2 or higher b. had an average pain intensity of 40 or higher on a 100 mm visual analogue scale in the 7 days before baseline assessment Exclusion criteria: a. knee pain caused by conditions other than knee OA b. had knee replacement surgery of the affected knee in the past year c. were currently receiving or had undergone cognitive behavioural‐based therapy or other psychotherapy (including counselling) d. had participated in any other clinical study in the past 12 months e. had been diagnosed with mental disorder, pregnancy or were breastfeeding Stated purpose: to develop a cognitive behavioural‐based therapy intervention module for physiotherapists and nurses and to evaluate its effectiveness in treating pain, functional disability and the psychological outcomes of knee OA patients in Malaysian tertiary hospitals |
| Interventions |
Intervention: health‐led cognitive behavioural‐based group therapy Participants in the intervention group, besides being given The Knee Book, also received three sessions of group cognitive behavioural‐based therapy. The two‐and‐a‐half‐hour sessions were conducted bi‐weekly in groups of eight to twelve participants. The session ended with exercises, diaphragmatic breathing, knee muscle relaxation and a six‐minute walk test. Each session began with an introduction and lecture, a problem‐solving task, skills‐training, homework assignments and feedback of the session which took approximately 45 min to complete. Control: usual care (participants attended clinic and physiotherapy sessions as usual on their fixed appointment dates. All participants received advice on symptom management and standard exercises to remain active. The participants in the “passive” control group received no further intervention and were each provided with The Knee Book). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 months) |
| Notes |
Source of funding: Universiti of Putra Malaysia, Research Medical Centre grants (04‐02‐12‐1746RU) Prospective trial registration number: NMRR‐15‐74‐24008 |
Heidari 2014.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Family caregivers of elderly individuals with dementia Inclusion criteria: a. family caregivers in referral centres for elderly with dementia Exclusion criteria: not specified Stated purpose: to investigate the effect of family education programme on depression, anxiety and stress in family caregivers of elderly individuals with dementia |
| Interventions |
Intervention: family education programme Control: control group (not specified) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Heidari 2020.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Caregivers of Alzheimer's patients Inclusion criteria: a. being a member of the family b. taking care of patients at home c. having the ability to read and write d. patient care for a year or more without having cognitive disease e. no previous training in the ways of coping Exclusion criteria: a. Alzheimer's patient dies b. non‐co‐operation c. severe stressful event such as a divorce or the death of loved ones in the last three months Stated purpose: to determine the effect of problem‐oriented coping strategies training on perceived stress in the family caregivers of the elderly with Alzheimers |
| Interventions |
Intervention: problem‐oriented coping strategies training eight training programme sessions of problem‐based coping strategies in the form of weekly sessions with each session lasting 45 minutes based on the specified content Control: other (two 45‐minute sessions of training in relation to Alzheimers) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (2 weeks post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: IRCT2016050327736N1 |
Hemmat Makan 2021.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Diabetic adolescents Inclusion criteria: a. adolescent diabetic member of the Tehran Diabetes Association in 2019 Exclusion criteria: not specified Stated purpose: to determine the effect of self‐care education on blood glucose, diabetic quality of life, and emotional/behavioural disorders in adolescents with diabetes |
| Interventions |
Intervention: self‐care education The experimental group received self‐care education for 10 sessions of 45 minutes (two sessions per week) Control: no education |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (not specified) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Jingna 2012.
| Methods | The paper is in Chinese; translation is not available. Study design: RCT Country: China |
| Participants | Old people (> 80 years old) Inclusion criteria: a. old people at home whose cognition was clear and whose age was over 80 years old Exclusion criteria: not specified Stated purpose: to probe into the influence of community nurse‐led multidisciplinary team home visiting service on psychological states such as depression and loneliness of community with old people of advanced age at home. |
| Interventions |
Intervention: community nurse‐led multidisciplinary team home visiting service The intervention group patients received the nurse‐led multidisciplinary team home visiting. Control: usual care (the control group cases received routine community health service). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months and 6 months after intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Khalili 2019.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Pregnant women subjected to domestic violence Inclusion criteria: a. pregnant women subjected to domestic violence, referred to comprehensive health centres in Zahedan for receiving prenatal care in 2018 Exclusion criteria: not specified Stated purpose: to determine the effect of supportive‐educational interventions on psychological distress amongst pregnant women subjected to domestic violence |
| Interventions |
Intervention: supportive‐educational intervention The intervention group received four supportive‐educational individual sessions during two weeks. Control: usual care (the control group received routine care during this period). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4 weeks after intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Khosravi 2022.
| Methods | No information on interventionists' background Study design: RCT Country: Iran |
| Participants | Caregivers of patients with mental disorders Inclusion criteria: a. having consented to participate in the study b. having the ability of communication c. non‐addiction of patient’s family d. lack of physical or cognitive impairments impeding them from attending meetings e. age more than 18 years old f. more than 6 months having passed from the definitive diagnosis of the patient’s psychiatric disorder according to the psychiatrist and DSM‐IV criteria g. no experience of a traumatic or stressful incidence over the past 6 months in the family caregivers Exclusion criteria: a. unwillingness to continue b. absence in more than two sessions c. severe psychological problems or stress during the sessions Stated purpose: to investigate the effect of a spirituality‐based programme on stress, anxiety, and depression of caregivers of patients with mental disorders |
| Interventions |
Intervention: spirituality‐based programme The intervention lasted for a two‐month duration. The intervention group underwent 6 sessions of spirituality programme training during 60 min and two training classes per week. The experimental group members were also encouraged to practise some spiritual skills to manage their stress, anxiety, and depression outside the sessions as homework. The content was compiled based on the authentic sources of Quranic verses, hadiths, and the narration of the infallibles and through consultation with both seminary and university professors. Control: no intervention (control group’s subjects received no spirituality‐based educational intervention in this study and they only participated in two standard group training sessions related to general mental disorders). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediately after intervention and 1 month post‐intervention). |
| Notes |
Source of funding: none Prospective trial registration number: not specified |
Küçük 2020.
| Methods | No information on interventionists' background Study design: RCT (quasi‐experimental) Country:Turkey |
| Participants | Children/adolescents (12‐18 years) with parental psychiatric disorders Inclusion criteria: a. being between the ages of 12–18 b. having a parent with a psychiatric problem c. knowing that the parent has a diagnosis of mental illness d. being able to read and write in Turkish e. not having any problems that prevent understanding and responding to questions f. being open to communication and agreeing to participate in the research g. having written permission from his/her family Exclusion criteria: not specified Stated purpose: to determine the effectiveness of psychoeducation programme which was developed to improve the coping skills and to increase the psychological resistance of 12–18 years children/adolescents whose parents have psychiatric disorders |
| Interventions |
Intervention: psychoeducation programme The psychoeducation programme developed for children/adolescents with a parent with a psychiatric disorder aimed to teach the children basic concepts of mental well‐being, to help them practice these concepts in their lives, to help them develop skills to cope with difficulties that can arise as a result of living with parents with psychiatric disorders and to increase their psychological resilience levels. The programme was prepared by the researchers in accordance with the literature and consisted of eight consecutive sessions. Each session was 45–60 minutes and took the form of individual interviews with the child/adolescent. Role‐playing, tale writing/completion, story‐poetry‐letter writing, questions and answers, straight narration, homework and slide‐show training methods and other techniques were used in the sessions. Control: no intervention (for the 20 children/adolescents included in the control group, two sessions were conducted individually. In addition, at the end of the first session, children/adolescents in the control group watched the trailer for the animated film Inside Out, which was produced by Pixar Animation Studios in 2015, and the DVD was given to them). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Notes |
Source of funding: TUBITAK‐3001 (Number: 217S263) project by the Scientific and Technological Research Council of Turkey Prospective trial registration number: not specified |
Li 2020b.
| Methods | No information on baseline data for depressive symptoms Study design: cluster‐RCT Country: Vietnam |
| Participants | Family members of people who use drugs Inclusion criteria: a. be at least 18 years of age b. be a family member of the PWUD participant in the study c. know the PWUD participant’s drug‐using status d. live with the PWUD in the selected study commune Exclusion criteria: not specified Stated purpose: to assess the intervention effect by comparing family members’ mental health and family functioning between the intervention condition and the control condition |
| Interventions |
Intervention: name and description The intervention was delivered in two consecutive steps. The first step was to provide intervention group CHW with training in basic behavioural change theories and skills to communicate with PWUD and their family members effectively. The second step was to have the trained CHW deliver group intervention sessions to family members of PWUD in the communes. Each session was about 1 h in length and was held in a private room in the local commune health centre. The contents of the family member’s group sessions focused on developing a healthy family routine, coping with caregiver burden, shifting perspectives to manage negative emotions, forming coalitions amongst family members, and facilitating a positive behavioural change of PWUD. The group sessions also served to address societal stigma facing family members of PWUD and develop social support links for the families to integrate into their community. Control: control condition (not specified) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (12‐month follow‐up) |
| Notes |
Source of funding: National Institute on Drug Abuse of the National Institutes of Health under award number [R01DA033609] and National Institute of Mental Health of the National Institutes of Health under award number [P30MH058107]. Prospective trial registration number: not specified |
Mohammadi‐Yeganeh 2008.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Oral contraceptive pill users Inclusion criteria: a. women who were suitable candidates to use OCPs Exclusion criteria: not specified Stated purpose: to determine whether stress management education could influence mood and perceived stress in oral contraceptive users |
| Interventions |
Intervention: stress management education The experimental group used OCPs for three cycles with routine contraception counselling and concurrently exposed to one session of stress management education, and 3 times telephone counselling. Control: usual care (the control group received only routine contraception counselling during OCP use for three months). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Moradi 2010.
| Methods | The paper is in Persian; translation is not available. Study design: RCT Country: Iran |
| Participants | Workers of petrochemical company Inclusion criteria: a. workers of petrochemical company Exclusion criteria: not specified Stated purpose: to survey effects of TTM in substance abuse prevention amongst petrochemical workers in Iran |
| Interventions |
Intervention: educational programme for drug abuse prevention Education programme was prepared according to resistance skills, decisional balance, processes of change (cognitive & behaviour), self‐esteem, self‐efficacy and life skills. The intervention group received an educational programme by lecture and group discussion about the principles of the transtheoretical model (TTM). The intervention group received an educational package, including posters and pamphlets, within 5 months. Control: waiting list, usual care, etc. |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (5 months post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Nisar 2021.
| Methods | Abstract; full‐text is not available. Study design: RCT Country: Pakistan |
| Participants | Patients of end‐stage renal disease Inclusion criteria: a. patients of end‐stage renal disease Exclusion criteria: not specified Stated purpose: to find out the effect of supportive‐expressive group therapy in end‐stage kidney disease patients on the outcome of better health‐related quality of life and survival |
| Interventions |
Intervention: supportive‐expressive group therapy Control: control group (not specified) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (not specified) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
PACTR202009845194666.
| Methods | Conference abstract; full‐text is not available. Study design: cluster‐RCT Country: Zambia |
| Participants | School children grades 4‐6 (ages ~10‐13 years) Inclusion criteria: a. in grades 4, 5, or 6 b. able to read and understand English at a 3rd grade level as verified by their schoolteacher c. has average or higher academic standing as verified by their schoolteacher d. able to participate in all three data collection time points over the course of approximately 12 months e. has written parental permission to participate f. student provides written assent to participate Exclusion criteria: a. is unable to read and understand English at a 3rd grade level as verified by their schoolteacher b. has a neurodevelopmental disability that limits their ability to provide informed assent for participation in the programme, as verified by their caregiver and teacher Stated purpose: to adapt and evaluate the impact of a spiritually‐based youth character and resilience training curriculum called GROW (Global Resilience Oral Workshops) on resilience, hope, a sense of meaning in life, and the use of alcohol and drugs amongst 600 Zambian school children grades 4‐6 (ages ~10‐13 years) recruited from 30 schools |
| Interventions |
Intervention: Global Resilience Oral Workshops (GROW) Global Resilience Oral Workshops (GROW) is a novel youth resilience curriculum rooted in positive psychology which incorporates a number of innovative aspects designed to promote cross‐cultural implementation. Instruction centres around 24 character strengths which were identified through a study of worldwide cultures and religious traditions, then field tested and found to have widespread cultural uptake. Content is taught through storytelling, drama, and interactive exercises, rather than didactic lectures. The curriculum also incorporates a major focus on spirituality. The intervention is delivered in weekly after‐school classes of 2 hours each. Control: waiting list (delayed receipt of GROW curriculum after initial start group completed their classes) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (specific time if provided |
| Notes |
Source of funding: John Templeton Foundation grant 60857; AIRO International & On Track Ministries; LEAH training grant #T71MC00009, MCHB, HRSA Prospective trial registration number: PACTR202009845194666 |
Puffer 2016.
| Methods | No information on the type of control group Study design: RCT Country: Kenya |
| Participants | Adolescents (10‐16 years old) and their caregivers Inclusion criteria: a. All 56 churches in the division were enumerated and mapped and represented multiple types of churches, including several Protestant denominations, churches based on indigenous beliefs, and a few Catholic congregations. b. All families from these congregations with at least one adolescent living at home between the ages of 10 and 16 were eligible to participate. Exclusion criteria: a. Youth living away from home the majority of the time (e.g. at boarding school) were not eligible to participate. Stated purpose: to evaluate a family‐ and church‐based intervention for adolescents and caregivers in rural Kenya to improve family relationships, reduce HIV risk, and promote mental health |
| Interventions |
Intervention: READY READY was developed in collaboration with a local Community Advisory Committee (CAC) using community‐based participatory methods. The central objective of the 9‐session READY intervention was to improve family relationships as a protective factor against risky sexual behaviour and mental health symptoms. The intervention incorporated evidence‐based strategies from behavioural family communication skills training, skills‐based HIV prevention interventions, behavioural parent training, and cognitive behavioural therapies. The 2‐hour sessions were divided into three modules: Economic Empowerment, Emotional Support, and HIV Education and Prevention. Control: usual care (stepped wedge trial with usual care – no intervention) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (1 month and 3 months post‐intervention) |
| Notes |
Source of funding: the NIH Fogerty International Center, Johnson & Johnson, the Duke Global Health Institute, and the Duke Center for AIDS Research Prospective trial registration number: not specified |
Sharma 2021.
| Methods | No information on interventionists' background Study design: RCT (quasi‐experimental design) Country: India |
| Participants | Caregivers of patients with schizophrenia Inclusion criteria: a. family members of any age who are living with the patient of age range between 25 and 45 years from the past 2 years Exclusion criteria: not specified Stated purpose: to understand the role of family psychoeducation (FPE) in the management of schizophrenia and the well‐being of the caregiver |
| Interventions |
Intervention: family psychoeducation (FPE) The Psychoeducation Intervention Package was divided into five sessions in which parents were first educated about schizophrenia then the therapist applied the tactics to improve communication skills and normalised negatively expressed emotions of the caregivers. The caregivers were empowered to prioritise their needs and social interests in their own mental health and taught to divide time between caregiving and recreational space. The researcher was trained and conducted 5 psychoeducation sessions with caregivers, on 7–10 days’ intervals in the treatment group. The intervention continued for over 1.5 months. Control: no intervention |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Notes |
Source of funding: none Prospective trial registration number: not specified |
Shuzhen 2015.
| Methods | The paper is in Chinese; translation is not available. Study design: RCT Country: China |
| Participants | Community patients with coronary heart disease Inclusion criteria: a. patients with coronary heart disease Exclusion criteria: not specified Stated purpose: to probe into the effectiveness of multidisciplinary team self‐management interventions for community patients with coronary heart disease |
| Interventions |
Intervention: multidisciplinary team self‐management intervention Control: usual care (routine health education in community) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3‐months post‐intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Xia 2017.
| Methods | The paper is in Chinese; translation is not available. Study design: RCT Country: China |
| Participants | Family caregivers of elderly with dementia Inclusion criteria: a. family caregivers of elderly with dementia Exclusion criteria: not specified Stated purpose: to explore the effect of a family visit of community nurses on family caregivers' burden of care and quality of life of the elderly with dementia |
| Interventions |
Intervention: nursing intervention community nurse family visit programme, including the caregiver training‐issued "dementia caregiver manual" and telephone follow‐up for a period of 3 months Control: usual care (routine community management). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
Zhao 2021.
| Methods | No information on baseline data for depressive symptoms Study design: RCT Country: China |
| Participants | Pregnant women Inclusion criteria: a. primiparous with a single foetus; b. had an Edinburgh Postnatal Depression Scale (EPDS) score ≥ 9; c. were in the third trimester (≥ 28 weeks and < 35 weeks); d. planned to give birth and undergo follow‐up at 42 days postpartum in the research hospital. Exclusion criteria: a. intellectual disabilities, severe mental diseases, or obstetric complications associated with breastfeeding cessation Stated purpose: to determine the effects of an individualized mixed management combined lactation education and psychoeducation intervention on breastfeeding outcomes and postpartum depression (PPD) at 3 and 42 days postpartum |
| Interventions |
Intervention: individualized mixed management intervention Participants in the intervention group received the individualized mixed management intervention, which consisted of four face‐to‐ face sessions in the prenatal clinic. Each intervention session was separate from routine clinic examinations. Each session lasted approximately 60 min and consisted of (1) a 10‐min discussion on a predesignated topic, such as depression, fear during pregnancy, birth, and the postpartum period, and breastfeeding satisfaction; (2) a 40‐min psychoeducation or lactation education intervention presented using PowerPoint and teaching tools, including short breastfeeding videos, a breast model, and a breastfeeding simulation; and (3) 10 mins of feedback on the content of the intervention or counselling related to perinatal depression or breastfeeding. Control: usual care (the control group received usual perinatal care and completed routine group education courses in the hospital about maternal health and childcare during the perinatal period. If a participant experienced depressive symptoms during follow‐up or had suicidal ideation, she was referred to the psychiatry department of our affiliated general hospital for further treatment). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3‐days and 42‐days postpartum) |
| Notes |
Source of funding: Fudan University Nursing Research Funding, protocol No. FNF201605 Prospective trial registration number: not specified |
Zheng 2021b.
| Methods | The paper is in Chinese; translation is not available. Study design: RCT Country: China |
| Participants | Patients with acute myocardial infarction (AMI) Inclusion criteria: a. patients with acute myocardial infarction (AMI) who received percutaneous coronary intervention (PCI) Exclusion criteria: not specified Stated purpose: to explore the application effect of peer support interventions in the nursing of patients with acute myocardial infarction (AMI) after percutaneous coronary intervention (PCI) |
| Interventions |
Intervention: Peer support intervention Peer support intervention in addition to routine health education guidance and follow⁃up management Control: Usual care (routine health education guidance and follow⁃up management) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post 6‐month intervention) |
| Notes |
Source of funding: not specified Prospective trial registration number: not specified |
AMI: acute myocardial infarction APRS: Adolescent Psychological Resilience Scale BAI: Beck Anxiety Inventory CAC: Community Advisory Committee CDI: Children’s Depression Inventory CHW: community health workers DASS‐21: Depression Anxiety Stress Scale‐21 DVD: digital video disc EBDS: Emotional Behavioral Disorders Scale EPDS: Edinburgh Postnatal Depression Scale EQ‐5D‐3L: European Quality of Life 5‐ Dimension 3‐Level ESR: emotional self‐rating scale FPE: family psychoeducation GROW: Global Resilience Oral Workshops HADS: Hospital Anxiety and Depression Scale HIV: human immunodeficiency virus K‐10: Kessler‐10 Item Scale LSS: Life Satisfaction Scale OA: osteoarthritis OCP: oral contraceptive pill PANAS: Positive and Negative Affect Schedule PCI: percutaneous coronary intervention PPD: postpartum depression PSS: Perceived Stress Scale PTSD: post‐traumatic stress disorder PWB: Ryff Psychological Well‐being scale PWUD: people who use drugs RCT: randomized controlled trial SDQ: Strengths and Difficulties Questionnaire SDS: Zung Self‐Rating Depression Scale ‐ short version SF‐36: Short Form Health Survey STAI: State‐Trait Anxiety Inventory TTM: trans‐theoretical model VAS: Visual Analogue Scale WPAQ: Wendenburg Pregnancy Anxiety Questionnaire
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620001014943.
| Study name | The Mbereko + Men Model: evaluating the effect of a community‐based, gender‐synchronized parenting intervention with mothers and fathers on maternal mental health, care‐seeking for maternal, newborn and child health services, and care and support in the home for mothers and infants in Manicaland, Zimbabwe |
| Methods |
Study design: RCT Country: Zimbabwe |
| Participants | Women who are pregnant or have a child aged up to 2 years and men resident in the same community Inclusion criteria: Women participating in the intervention: a. currently resident in study site; b. pregnant and/or has a child aged 0‐2 years. Men participating in the intervention: a. currently resident in the study site. Women participating in the repeat cross‐sectional community based surveys: a. has given birth within the previous 0‐6 months; b. resident in study site for a minimum of 12 months; c. aged 16 years and older; and d. able to give informed consent. Men participating in the repeat cross‐sectional community based surveys: a. has a child aged 0‐6 months, or female partner has given birth within the previous 0‐6 months; b. resident in study site for a minimum of 12 months; c. aged 16 years and older; d. able to give informed consent; and e. female partner or mother of his child has provided consent for the man to be surveyed. Married and unmarried women and men will be eligible to participate. Women and men who are no longer in an ongoing relationship with the father or mother of their child will be eligible to participate. Exclusion criteria: Not specified Stated purpose: to evaluate the effect of a community‐based, gender‐synchronized parenting intervention with mothers and fathers on maternal mental health, care‐seeking for maternal, newborn and child health services, and care and support in the home for mothers and infants |
| Interventions |
Intervention: Mbereko + Men intervention The Mbereko + Men intervention is delivered through two gender‐synchronized components. The first component (Mbereko), delivered with women who are pregnant or have a child aged up to 2 years, is monthly facilitated participatory learning and action (PLA) cycles, complemented by saving and lending clubs formed by PLA group members. The second component (+ Men), delivered with men resident in the same community, is monthly education and dialogue forums, complemented by a participatory charter developed by men through the dialogue forums that focuses on how men can support family health through their actions. The intervention is delivered primarily through facilitated group discussion. Control: Usual care (free antenatal and maternal and child health services) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Service utilization (proportion of women accessing predefined essential maternal, newborn and child health (MNCH) services) Time points: baseline, post‐intervention (18 months) |
| Starting date | 14 July 2016 Recruitment: completed |
| Contact information | Ms Liz Comrie‐Thomson, liz.comriethomson@burnet.edu.au |
| Notes |
Source of funding: Australian Government Department of Foreign Affairs and Trade Prospective trial registration number: ACTRN12620001014943 |
ACTRN12621000189820.
| Study name | Randomised controlled trial of a stepped care intervention comprising Self‐Help Plus and Problem Management Plus versus Self‐Help Plus on anxiety and depression in Jordanians experiencing distress |
| Methods |
Study design: RCT Country: Jordan |
| Participants | Participants will be adults indicating moderate distress Inclusion criteria: a. Syrian refugees; b. Aged at least 18 years; c. K6 score of at least 6; and d. WHODAS score of at least 16. Exclusion criteria: a. imminent plans of suicide; b. psychotic disorders; c. severe cognitive impairment; d. identification of risk of the person’s safety (e.g. partner violence); and e. plans to return to Syria in next 12 months. Stated purpose: test how a stepped care programme can (a) provide scalable programs to all people who require assistance, and (b) offer much‐needed programmes to those who do not benefit from initial intervention |
| Interventions |
Intervention: Stepped care (SH+ and PM+ or monitoring) Therapy commences with Self‐Help Plus (SH+), which involves once‐weekly 120‐minute sessions delivered by a facilitator over 5 weeks, delivered and based on audiobooks to groups of 20‐30 people at a time. Participants who are not distressed following SH+ will continue to be assessed but will receive no further assistance. Participants who are distressed at the end of SH+ will be provided with Problem Management Plus (PM+). PM+ is a programme developed by the World Health Organization. PM+ involves once‐weekly 120‐minute sessions delivered by a facilitator over 5 weeks to groups of 8‐10 people at a time. SH+ will teach participants strategies in mindfulness and acceptance strategies. Intervention timeline: 10 weeks of intervention (5 weeks of SH+ and 5 weeks of PM+ or 5 weeks of SH+ and 5 weeks of monitoring). Control: Single intervention (SH+ and monitoring). The Single Intervention commences with Self‐Help Plus (SH+), which involves once‐weekly 120‐minute sessions delivered by a facilitator over 5 weeks, delivered and based on audiobooks to groups of 20‐30 people at a time. Timeline: 5 weeks of SH+ and 5 weeks of monitoring |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (specific time if provided) |
| Starting date | 1 April 2021 (first participant enrolment) |
| Contact information | Prof Richard Bryant, r.bryant@unsw.edu.au |
| Notes |
Source of funding: ELRHA Prospective trial registration number: ACTRN12621000189820 |
Al Azdi 2021.
| Study name | Effectiveness of an integrated care package for refugee mothers and children: protocol for a cluster randomized controlled trial |
| Methods |
Study design: cluster‐RCT Country: Bangladesh |
| Participants | Refugee mothers and their children Inclusion criteria (for mother‐child pairs): a. Child should be less than or equal to 6 weeks old, live with their biological mother, had a gestational period of at least 36 weeks, and weighed at least 2.5 kilograms at birth. Exclusion criteria: a. children with congenital abnormalities; b. mothers that have to move out of the area during the study period. Stated purpose: to evaluate the effectiveness of an integrated care package in reducing the prevalence of developmental delays amongst children aged 1 year and improving their mothers’ mental health status |
| Interventions |
Intervention: Integrated care package A total of four counselling sessions will be delivered to mother‐child dyads by CHWs in the intervention arm to promote early child development and maternal mental health. The counselling sessions will focus on the child’s cognitive and physical development, and the mother’s mental health based on a few key messages. The counselling contents are developed in consultation with technical experts, supported by a pictorial flip book that has been modified according to the local context and translated into Burmese to be consistent in delivering the messages. Each session will take at least 10‐15 minutes. Control: No intervention (the control arm will be strengthened by providing a 2‐day training to CHWs on recruitment of the mother‐child dyads, administration of outcome measures, record‐keeping, log maintenance, compliance, and communication. They will also be trained on taking anthropometric measurements to record the children’s height, weight, and mid‐upper arm circumference (MUAC) every quarter. These inputs will be the same for the control and intervention arms). |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (endpoint) |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: Grand Challenges Canada, Saving Brains (grant number SB‐1810‐19890) Prospective trial registration number: ISRCTN10892553 |
Amudhan 2021.
| Study name | Project SUMS (Scaling Up of Mental Health in Schools): design and methods for a pragmatic, cluster‐randomized waiting‐list‐controlled trial on integrated school mental health intervention for adolescents |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Students studying in 6–8 grade Inclusion criteria: a. all children enrolled in class 6th and 7th grade of the participating higher‐primary public schools Exclusion criteria: a. children who did not give informed assent; b. children whose parents/guardians did not provide written informed consent; c. children who were absent for the baseline assessment even after two attempted visits; d. children aged > 15 years at enrolment. Stated purpose: to assess the effectiveness of evidence‐informed integrated school mental health intervention (SUMS) in promoting mental health knowledge, positive attitudes towards mental illness, and behaviours for First Aid in Mental Health amongst adolescent school children |
| Interventions |
Intervention: SUMS intervention (MHP + MHL + MH‐FA) The Scaling Up of Mental Health in Schools intervention is a classroom‐based teacher‐led integrated school mental health intervention that will promote positive mental health, mental health literacy and behaviours for First Aid in Mental Health amongst school‐going adolescent school children. The SUMS intervention takes the form of a classroom‐ready resource (the curriculum resource guide) designed to be delivered by usual classroom teachers to students in grades 6 and 7 (ages 12 to 15 years). The curriculum resource guide contains 10 modules categorized under 3 units (Mental Health Promotion, Mental Health Literacy and First Aid in Mental Health). Control: Usual care (control‐schools will continue to provide regular teaching as usual of the existing course to their students. The control group will receive the interventions at the end of the 12‐month follow‐up assessment in intervention‐schools). |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (12‐month follow‐up) |
| Starting date | Recruitment after March 2022 |
| Contact information | Senthil Amudhan, sam_mmc1999@yahoo.co.in |
| Notes |
Source of funding: Indian Council of Medical Research, New Delhi, India under “Call for concept proposals for identifying Young and Middle level Faculty to participate in Research Methodology Workshop” Prospective trial registration number: Clinical Trials Registry‐India: CTRI/2019/07/020394 |
Aung 2021.
| Study name | Community‐integrated intermediary care (CIIC) service model to enhance family‐based, long‐term care for older people: protocol for a cluster‐randomized controlled trial in Thailand |
| Methods |
Study design: cluster‐RCT Country: Thailand |
| Participants | Older adults (> 60 years) and their caregivers Inclusion criteria: a. > 60 years of age; b. has a family caregiver(s); c. either male or female; d. resident in study site districts. Exclusion criteria: a. lack of informed consent by those > 60 years old or their family caregivers; b. cannot understand the explanation for informed consent despite being provided with language support; c. in a household without an older person > 60 years old; d. cognitive impairment or severe impairment of decision‐making abilities. Stated purpose: to assess the effectiveness of a CIIC facility and its functions to assist families providing LTC for older adults in terms of reducing caregivers’ burden as the primary outcome. Another objective is to evaluate the effectiveness of the CIIC model in terms of the following secondary outcomes: impact on ADL, depression, and QOL of older people. |
| Interventions |
Intervention: Care capacity building for family caregivers based on community‐integrated intermediary care (CIIC) service model Caregiving capacity building educational programme will be provided to the family caregivers of dependent older adults. Training delivered at home or can be group training, depending on the individual situation and specific care need. Control: Usual care (i.e. the current system of LTC common to all provinces in Thailand), consisting principally of a volunteer‐assisted home care service in addition to traditional family‐based home care. |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 months) |
| Starting date | May 2019 |
| Contact information | Myo Nyein Aung, dr.myonyeinaung@gmail.com |
| Notes |
Source of funding: World Health Organization Centre for Health Development (WHO Kobe Centre – WKC: K18020) Prospective trial registration number: Thai Clinical Trials Registry TCTR20190412004 |
Bello 2021.
| Study name | Reducing anxiety and depression in infertility among Nigerian women: an exploratory psycho‐educational intervention trial (RADIANT) |
| Methods |
Study design: RCT Country: Nigeria |
| Participants | Women attending gynaecology clinics in UCH or AMH on account of infertility Inclusion criteria: a. women aged 18 years and above; b. who have been trying to conceive for at least one year; c. who do not have any children. Exclusion criteria: not specified Stated purpose: to develop content for a culturally relevant and cost‐effective psychoeducational intervention package and to evaluate its effectiveness for reducing symptoms of anxiety and depression |
| Interventions |
Intervention: Psychoeducational video Culturally‐relevant and cost‐effective psychoeducational intervention package aimed at reducing social and emotional problems amongst women with infertility in Ibadan, Nigeria. The developed content will then be translated into an audiovisual drama production as a user‐friendly tool for the target audience. Control: Usual care (usual treatment offered all attendees at the gynaecology clinics ‐ comprising health talks by public health nurses and explanations and counselling provided by the managing physician) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 and 6 weeks follow‐up) |
| Starting date | NA |
| Contact information | Folasade Adenike Bello, dr.nikebello@yahoo.com |
| Notes |
Source of funding: Translational Research and Fellowship Grant (grant number: CTR16A00‐1) from the College Research and Innovation Management (CRIM) Unit of the College of Medicine, University of Ibadan, Ibadan, Nigeria Prospective trial registration number: Pan African Clinical Trial Registry [PACTR201901892865101] |
Benfer 2018.
| Study name | Community‐based parent‐delivered early detection and intervention programme for infants at high risk of cerebral palsy in a low‐resource country (Learning through Everyday Activities with Parents (LEAP‐CP)) |
| Methods |
Study design: RCT Country: India |
| Participants | Infants at high risk of cerebral palsy and their parents (caregivers) Inclusion criteria: a. Infants must live in one of the study geographical areas and be 12–40 weeks CA. b. Infants must also have one or more risk factors: maternal infection (antenatal); low birth weight (< 2.5 kg); preterm delivery (< 37 weeks); hypoxic ischaemic encephalopathy; perinatal asphyxia; neonatal jaundice requiring treatment; prolonged hypoglycaemia; seizures after birth; admission to neonatal intensive care unit or special newborn care unit; post‐neonatal complications in infant (infection, head injury, near drowning), altered tone or delayed motor milestones for the infant. To be eligible for the intervention substudy, infants must be assessed in the detection substudy to be at ‘high risk’ of CP based on the GMs or HINE, as follows: at 12–17 weeks CA infants with absent/abnormal fidgety movements on GMs assessment are considered high risk; at 18–40 weeks, CA infants scoring below the established HINE cut‐points will be considered high risk of CP (< 56 points at 3 months (SE 96%; sp 85%), < 59 points at 6 months (SE 90%; sp 89%), < 62 points at 9 months (SE 90%; sp 91%) Exclusion criteria: a. Infants with known or suspected congenital or chromosomal abnormalities which are likely to affect their neurodevelopmental outcome; those diagnosed with neurodegenerative conditions and those that are considered medically fragile Infants who have been screened as ‘low risk’ on the GMs and HINE will not be eligible to participate in the intervention substudy. Stated purpose: 1) to determine the effectiveness of a community‐based parent‐delivered intervention on infant’s developmental outcomes for those at high risk of CP; 2) to determine the effectiveness of a community‐based parent‐delivered intervention on caregiver’s mental health outcomes; 3) to determine the predictive validity of GMs assessment administered at 12–17 weeks for detecting CP at 18 months in high‐risk infants in West Bengal; 4) To determine the predictive validity of the HINE when administered from 18 to 40 weeks for detecting CP at 18 months in high‐risk infants in West Bengal. |
| Interventions |
Intervention: LEAP‐CP: Learning through Everyday Activities with Parents for infants at high risk of Cerebral Palsy The LEAP‐CP intervention is a multi‐domain family‐centred best practice intervention consisting of infant goal‐directed therapeutic strategies and learning games and caregiver educational modules. The components shown necessary for effective interventions for infants with CP include (1) goal‐directed tasks; (2) home‐based delivery and include (3) active motor learning and (4) strategies to enrich the home environment. LEAP‐CP is based on principles of parent coaching which promote caregiver problem‐solving and self‐determination. Specifically, LEAP‐CP includes: a) activity‐based motor and cognitive skills training, based on goals identified by parents; b) enrichment, which facilitates enhanced cognitive, motor and multisensory learning; c) the parent educational modules, evidence‐based discussion topics which cover three broad areas: ‘learn’, ‘grow’ and ‘love’. The LEAP intervention will commence at 3–9 months CA at a dose of 20 min per day for 5 days per week (1.6 hours) up to 6 months CA (total dose 19.2 hours); then graduate to 30 min per day for 5 days per week (2.5 hours per week) from 6 to 9 months CA (total 30 hours); then 40 min per day for 5 days per week (3.3 hours per week) from 9 to 12 months CA (total 40 hours). In addition, there will be approximately 15 hours of direct intervention administered during home visits by either the parent or CDW. The overall dose will be 104.2 hours for the entire intervention up to 18 months corrected age (CA). Control: Usual care: Heath Advice (the Health Advice is based on the WHO’s Integrated Management of Childhood Illness Key Family Practices. This includes counselling on breastfeeding and introduction of complementary nutrition, hygiene practices, vaccination counselling and management of the sick child. It also includes clinical signs indicating the need for referral to existing health services. The same service delivery model and visiting schedule will be used as for the intervention arm (a fortnightly home visit for 15 visits), with a different CDW visiting standard care group families to avoid contamination. There will not be a direct intervention dose delivered to infants in this study arm) Notes: concurrent therapies (care‐as‐usual): Infants from both study arms are able to continue to access medical and therapy support as per their family’s preference. |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention and at 18 months CA) |
| Starting date | March 2017 (Field work will be conducted from March 2017 to March 2019) |
| Contact information | Katherine A Benfer, katherine.benfer@uqconnect. edu.au |
| Notes |
Source of funding: Cerebral Palsy Alliance Project Grant (PG6916); Queen Elizabeth II Diamond Jubilee Postdoctoral Scholarship, Endeavour (KB), Australian Commonwealth Government; NHMRC Fellowship (RB); NHMRC Centre for Research Excellence (Australasian Cerebral Palsy Clinical Trials Network) Prospective trial registration number: ANZCTR 12616000653460p |
Burchert 2019.
| Study name | Randomized controlled trial to test the (cost‐)effectiveness of Step‐by‐Step, a smartphone‐based self‐help programme for Syrian refugees in Egypt |
| Methods |
Study design: RCT Country: Egypt |
| Participants | Adult Syrian refugees with symptoms of psychological distress and problems in daily life Inclusion criteria: a. Syrian displaced person living in Egypt; b. Arabic‐speaking; c. minimum age 18 years; d. K10 score > 15 and WHODAS 2.0 score > 16; e. access to a smartphone (iOS or Android) or web‐browser; f. internet access. Exclusion criteria: a. serious suicidal thoughts or plan Stated purpose: to evaluate the effects of Step‐by‐Step on mental health and the use of health services |
| Interventions |
Intervention: TAU + Step‐by‐Step Step‐by‐Step is a smartphone‐ and internet‐based self‐help programme with an introduction session (15 minutes) and 5 weekly sessions (each 30 minutes), a digital mood diary, contact‐on‐demand by trained and supervised non‐specialist, Syrian research assistants (“e‐helpers”) and information on treatment‐as‐usual (TAU) in Egypt. Participants in this group can make use of other care services in parallel. Control: TAU + information (access to information about their symptoms, and on how to get help when experiencing difficult emotions or problems. Participants receive basic psychoeducation and information on treatment‐as‐usual (TAU) in Egypt. Participants in this group can make use of other care services in parallel). |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Access to health services Time points: baseline, post‐intervention (3 months after randomization) |
| Starting date | 1 December 2020 (first enrolment) |
| Contact information | Mr. Sebastian Burchert; s.burchert at fu‐berlin.de |
| Notes |
Source of funding: European Union’s Horizon 2020 Research and Innovation Program Societal Challenges under grant agreement No 733337 Prospective trial registration number: DRKS00023505 |
ChiCTR1800015602.
| Study name | Development of a chronic disease self‐management support programme for spouse caregivers of persons with dementia in China: a single‐centre non‐blinded randomized control trial |
| Methods |
Study design: RCT Country: China |
| Participants | Caregivers of persons with dementia Inclusion criteria: a. the principal caregiver who is the spouse of person with the diagnosed dementia based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) Exclusion criteria: a. caregivers who were not spouses of relatives with dementia; b. spouse caregivers who did not get involved in major healthcare, decision‐making and care of relatives with dementia; c. spouse caregiver who currently had serious mental and/or physical illness(es) that was expected to be unable to complete the trial and/or to undertake care as the principle caregiver; d. spouse caregiver who simultaneously provided major care for another family member with a chronic medical condition or for a grandchild. Stated purpose: to evaluate the effect of a Chronic Disease Self‐Management Support Programme for dementia carers |
| Interventions |
Intervention: Chronic Disease Self‐Management Support Programme Six 2‐weekly group sessions of the programme Control: Usual care (monthly educational presentations) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (post‐test and follow‐up stages) |
| Starting date | 1 September 2018 |
| Contact information | Shu Ying Zhang, zhangsy@tongji.edu.cn |
| Notes |
Source of funding: Nature Science Foundation of China (No. 81571364), Shanghai Municipal Committee of Health and Family Planning (No.201540041) Prospective trial registration number: ChiCTR1800015602 |
ChiCTR1900023145.
| Study name | Evaluation of a prevention program for depression among high school adolescent in mainland China: a cluster‐randomized controlled trial |
| Methods |
Study design: cluster‐RCT Country: China |
| Participants | High school adolescents Inclusion criteria: a. Chinese high school students Exclusion criteria: No exclusion criteria because this is a universal prevention. If there is a crisis, conduct the crisis intervention first, then receive our course. Stated purpose: to examine the effectiveness of a prevention programme based on cognitive behavioural therapy for depressive symptoms amongst high school students |
| Interventions |
Intervention: Course based on cognitive behavioural therapy Control: Other (course that teaches career development) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention and 6‐months follow‐up) |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: self‐funded Prospective trial registration number: ChiCTR1900023145 |
ChiCTR2000028878.
| Study name | Intervention for children's dental anxiety and dental behavior management problems: a cluster‐randomized trial |
| Methods |
Study design: cluster‐RCT Country: China |
| Participants | Children in primary school (7‐8 years old) Inclusion criteria: a. children at the second grade of primary schools in September 2018, at the Nanshan District of the city of Shenzhen, China; b. informed consents signed by their parents. Exclusion criteria: a. children who already had a dental visit; b. refused to participate; c. mental illness. Stated purpose: to evaluate whether the EL intervention can reduce children's dental anxiety and dental behaviour management problems |
| Interventions |
Intervention: Experiential learning Control: Health education |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: Grant for Scientific and Technology Research (No. JCYJ20170306155909623) from the Bureau of Science and Technology Innovation Commission of Shenzhen City Prospective trial registration number: ChiCTR2000028878 |
ChiCTR2000039133.
| Study name | Randomized controlled trial of Enhancing Contact Model on reducing stigma of mental illness in family caregivers of persons with schizophrenia in rural China |
| Methods |
Study design: RCT Country: China |
| Participants | Family caregivers of persons with schizophrenia Inclusion criteria: For family caregivers: a. being the father or mother of a person with schizophrenia identified in 2015; b. aged 38‐75 years; c. living together with and caring for the patient; d. not participants in our prior PFI studies; e. able to provide written informed consent. For person with schizophrenia: a. identified in the 2015 survey; b. aged ≥ 18 years; c. not participants in our prior PFI studies. Exclusion criteria: For the family caregivers: a. likely to engage in a risk behaviour (e.g. suicide or violence) imminently; b. identified by a trained health professional as unsuitable to join the study (e.g. with mental illness). Stated purpose: to test the short (post‐ intervention) and long‐term (9‐month follow‐up) effectiveness of a newly developed Enhancing Contact Model (ECM) Intervention for reducing self‐stigma in family caregivers of persons with schizophrenia, and to examine whether ECM is more effective in reducing self‐stigma than psychoeducational family intervention (PFI) |
| Interventions |
Intervention: 1. Enhancing Contact Model Programme Group 2. Psychoeducational Family Intervention Programme Group Control: Control group (not specified) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (specific time if provided) |
| Starting date | From 1 September 2018 to 28 February 2021 |
| Contact information | Ran Maosheng, msran@hku.hk |
| Notes |
Source of funding: Research Grants Council, University Grants Committee, General Research Fund Prospective trial registration number: ChiCTR2000039133 |
CTRI/2017/04/008385.
| Study name | Interventions for stigma for persons with epilepsy in India |
| Methods |
Study design: RCT Country: India |
| Participants | Adult patients with epilepsy Inclusion criteria: a. consenting; b. persons diagnosed as having epilepsy [As per definition of ILAE, at least two or more unprovoked or reflex seizures > 24 h apart or one unprovoked or reflex seizure with a probability of recurrence of at least 60% or diagnosis of an epilepsy syndrome]; c. persons with epilepsy who screen positive on Kilifi Stigma Scale for epilepsy. Exclusion criteria: a. non‐consenting; b. persons with epilepsy having any other clinically significant confounding physical/cognitive disability or stigmatizing condition (as determined by the attending physician); c. practising mediation/yoga regularly or sporadically for a period of three months or more prior to the recruitment period. Stated purpose: to evaluate the efficacy of an integrated yoga module as an intervention for reducing epilepsy‐related stigma in persons with epilepsy in India |
| Interventions |
Intervention: Integrated Yoga Module for Persons with Epilepsy (IYMPE) In addition to usual medical care, patients will be administered an Integrated yoga module for persons with epilepsy consisting of loosening practices (5 minutes), breathing exercises (10 minutes) and meditation (15 minutes). The intervention consists of 7 supervised sessions covered over a duration of 12 weeks. These sessions will be conducted by a trained yoga instructor. This module shall be supplemented with a structured session of psychoeducation regarding epilepsy and its psychosocial aspects. Control: Active control: sham yoga module (in addition to treatment‐as‐usual and psychoeducation module, patients shall be administered a sham yoga module consisting of 7 sessions covered over a duration of 12 weeks by a trained instructor. This group would be given exercises that mimic the yoga practices, but they would be not be given instructions on the corresponding breath modulation and synchronization, body awareness and focus, and chanting techniques that are the key components of yoga) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months and 6 months follow‐up) |
| Starting date | 24 April 2017 (first enrolment) |
| Contact information | Manjari Tripathi, mtripathiaiims@gmail.com |
| Notes |
Source of funding: All India Institute of Medical Sciences, Ansari Nagar East, Gautam Nagar, New Delhi‐110029 Prospective trial registration number: CTRI/2017/04/008385 |
CTRI/2020/11/028808.
| Study name | A factorial randomized trial of structured physical activity training and brief cognitive behavioural therapy for prevention of repeat hospital admission and death among acute decompensated heart failure patients |
| Methods |
Study design: RCT Country: India |
| Participants | Acute decompensated heart failure patients Inclusion criteria: a. age more than or equal to 18 years; b. citizen of India, who is a permanent resident of Kerala; c. heart failure patients as defined in ESC 2016. Exclusion criteria: a. refused to provide consent; b. enrolled in other cardiac rehabilitation programmes; c. bedridden. Stated purpose: to see if rehabilitation strategies of structured physical activity and brief cognitive behavioural therapy for depression and self‐management reduces the risk of repeat hospitalization and death in heart failure patients |
| Interventions |
Intervention: PACT‐HF. A 2‐by‐2 factorial trial for structured physical activity and brief cognitive behaviour therapy for heart failure patients A structured exercise‐training programme and a brief cognitive behaviour therapy will be delivered by a non‐physician health worker. The health worker will be trained to deliver exercise and face‐to‐face CBT sessions for people reporting in outpatient settings of the hospital. The intervention will be given three times during the first three months and thereafter once in every three months. Control: No intervention |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (24 months) |
| Starting date | 1 December 2020 (first enrolment) |
| Contact information | Dr Harikrishnan S, drharikrishnan@outlook.com |
| Notes |
Source of funding: Indian Council of Medical Research, Ansari Nagar, New Delhi, India ‐ 110029 Prospective trial registration number: CTRI/2020/11/028808 |
CTRI/2021/01/030403.
| Study name | Moving pictures: using digital media to improve dementia care in India |
| Methods |
Study design: RCT Country: India |
| Participants | Family caregivers of persons with dementia Inclusion criteria: a. age above 18 years; b. proficiency in English and/or Hindi/Kannada; c. family carer of a person with dementia; d. capacity to consent; e. own a smartphone. Exclusion criteria: a. participants involved in the development phase of the study |
| Interventions |
Intervention: Moving pictures resources Usual clinical care plus access and use of the Moving pictures digital resources on dementia care that will be developed in the initial phases of the study Control: Usual care (usual clinical care and access to non‐dementia resources (e.g. on healthy living)) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months and 6 months post‐intervention) |
| Starting date | 1 April 2021 (first enrolment) |
| Contact information | Dr Santosh Loganathan, dr.santosh32@gmail.com |
| Notes |
Source of funding: Alzheimer’s Association (US) as part of the 2020 January Alzheimer’s Association Research Grant (AARG) program Prospective trial registration number: CTRI/2021/01/030403 |
Daruwalla 2019.
| Study name | Community interventions to prevent violence against women and girls in informal settlements in Mumbai: the SNEHA‐TARA pragmatic cluster‐randomized controlled trial |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Women, men, and adolescents Inclusion criteria: a. any resident of an intervention cluster may participate in the intervention; b. women, men, and adolescents who will be eligible to participate in group activities, and women who will be eligible to volunteer as sanginis. Exclusion criteria: not reported Stated purpose: testing the effects of community mobilisation through groups and volunteers in a parallel‐group, phased, cluster‐randomized controlled pragmatic superiority trial, with 1:1 allocation to intervention and control in a total 48 urban informal settlement clusters |
| Interventions |
Intervention: SNEHA (Society for Nutrition, Education and Health Action) programme on Prevention of Violence against Women and Children Unrestricted access to services provided by the implementing organization: crisis intervention, counselling, police liaison, medical attention, mental health intervention, family interventions, and legal recourse. In addition, participants will receive community mobilization activities with groups of women, men and adolescents, and with individual women volunteers. Control: Unrestricted access to services provided by the implementing organisation: crisis intervention, counselling, police liaison, medical attention, mental health intervention, family interventions, and legal recourse |
| Outcomes |
Participants'outcomes of interest for this review
Carers' outcomes of interest for this review Nil Economic outcomes Nil Time points: baseline, post‐intervention |
| Starting date | 2017/2018 |
| Contact information | David Osrin, email: d.osrin@ucl.ac.uk |
| Notes |
Source of funding: Wellcome Trust funded the study (206417/Z/17/Z) Prospective trial registration number: Controlled Trials Registry of India, CTRI/2018/02/012047; ISRCTN, ISRCTN84502355 |
Desrosiers 2021.
| Study name | mHealth‐supported delivery of an evidence‐based family home‐visiting intervention in Sierra Leone: protocol for a pilot randomized controlled trial |
| Methods |
Study design: RCT Country: Sierra Leone |
| Participants | Families with a child aged 6‐36 months Inclusion criteria: a. Sierra Leonean household with cohabitating caregivers (e.g. father/mother, mother/grandmother, mother/partner) and child (aged 6‐36 months) with both caregivers aged 18 or older; b. 1 caregiver scoring at least 62.5 on the Difficulties in Emotion Regulation Scale (DERS). Both caregivers must agree to attend FSI‐ECD sessions; however, if 1 caregiver decides to withdraw, the family can still continue to participate. If enrolled families have more than 1 child aged 6‐36 months, we will include all eligible children as study participants. Exclusion criteria: a. families who do not meet all inclusion criteria; b. families who experience active family crises (e.g. current suicidality or psychosis, serious medical condition, or cognitive impairment as assessed by a study social worker). Stated purpose: to (1) apply a user‐centred design to develop and test mHealth tools to improve supervision and fidelity monitoring of community health workers (CHWs) delivering the FSI‐ECD and (2) conduct a pilot randomized controlled trial of the FSI‐ECD to assess feasibility, acceptability, and preliminary effects on caregiver mental health, emotion regulation, caregiving behaviours, and family violence in high‐risk families with children aged 6‐36 months in comparison with control families receiving standard care |
| Interventions |
Intervention: Family Strengthening Intervention for Early Childhood Development (FSI‐ECD/called Sugira Muryango in Rwanda) The FSI‐ECD is composed of 4 core components: (1) developing problem‐solving, stress management, and emotion regulation skills; (2) cultivating positive parenting skills and fostering father/male co‐caregiver engagement; (3) developing communication and conflict resolution skills; and (4) exploring alternatives to harsh punishment and practising nonviolent child discipline. The FSI‐ECD is delivered in 12 modules in the home via coaching by CHWs. Sessions are delivered once per week and last approximately 90 minutes. Control: Usual care (standard CHW care involves 3 home‐visiting, educational sessions delivered to families following childbirth, with weekly supervision via phone or face‐to‐face. Topics of home‐visiting sessions include skilled postnatal care for mothers, early initiation of breastfeeding, nutrition, immunization services, handwashing and hygiene practices, building the capacity of family members to take care of newborns and children under age 5) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Preliminary cost‐effectiveness analysis to assess the economic value of the mHealth‐supported delivery of the FSI‐ECD versus standard care with standard supervision Time points: baseline, post‐intervention (immediate post‐intervention and 3 months follow‐up) |
| Starting date | 15 December 2020 |
| Contact information | Alethea Desrosiers, alethea.desrosiers@bc.edu |
| Notes |
Source of funding: National Institutes of Mental Health Prospective trial registration number: NCT04481399 |
Devassy 2021.
| Study name | Resiliency Engagement and Care in Health (REaCH): a telephone befriending intervention for upskilled rural youth in the context of COVID‐19 pandemic |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Participants aged 18–35 years who have recently completed their course out of the DDU‐GKY initiative Inclusion criteria: a. alumni students of the DDU‐GKY programme who are either working or are in search of a job; b. own a smartphone. Exclusion criteria: a. do not possess a smartphone; b. unable to operate a smartphone; c. receipt of treatment for pre‐existing mental health conditions in the last year. Stated purpose: to promote mental well‐being and reduce depressive symptoms by assisting participants to mobilize social support from family, friends and significant others by using the telephonic befriending intervention. |
| Interventions |
Intervention: Telephone befriending intervention Participants receiving befriending from trained DDU‐GKY staff, through one‐to‐one phone calls which they will receive in their homes, at a convenient time. The telephonic befriending intervention will be conducted in three phases: (i) proactive engagement and crisis intervention, (ii) problem‐solving oriented support therapy and (iii) assertive linkage with community resources (see Table 2). The three phases will spread across four phone calls for 30 min to 1‐h duration. Control: No intervention: general enquiry phone calls (participate in a baseline and follow‐up assessment using the same instruments. They will receive four general enquiry phone calls lasting 5 to 30 min. It will be a general enquiry about the precautions that are necessary to protect themselves from the infection, and how the family is coping with the lockdown‐related issues. The main focus will be given on psychoeducation‐based enquiries on COVID‐19). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate follow‐up ‐ 4 weeks) |
| Starting date | 7 August 2020 |
| Contact information | Saju Madavanakadu Devassy, saju@rajagiri.edu; sajumadavan@gmail.com |
| Notes | Prospective trial registration number: Clinical Trial Registry India (ICMR‐NIMS) CTRICTRI/2020/07/026834 |
Dominiquez‐Rodriguez 2021.
| Study name | A self‐applied multi‐component psychological online intervention based on UX, for the prevention of complicated grief disorder in the Mexican population during the COVID‐19 outbreak |
| Methods |
Study design: RCT Country: México |
| Participants | Spanish‐speaking male and/or female users meeting the inclusion criteria recruited via the online Grief COVID platform Inclusion criteria: a. to have a communication device with access to the internet (computer, tablet, or mobile); b. to have a valid e‐mail address; c. to have basic digital skills in the use of an operational system and Internet browsing; d. to be fluent in Spanish, since the complete intervention is in such language; e. to have symptoms of depression, state anxiety and/or acute stress disorder grief symptom. Exclusion criteria: a. to have a diagnosis of psychotic disorder; b. to have more than 6 months passed since the death of the loved person; c. to receive psychological and/or pharmacological treatment during the study; d. to have a moderate to a high score in the suicide scale; e. to have a recent attempt of suicide (3 months); f. to have a diagnosis of post‐traumatic stress disorder. Stated purpose: to design and implement a self‐applied intervention composed of 12 modules focused on the decrease of the risk of developing CGD, and increasing the life quality, and as a secondary objective to reduce the symptomatology of anxiety, depression, and increase of sleep quality |
| Interventions |
Intervention: Grief Covid intervention 12 sessions of a multi‐component psychological intervention focused on the decrease of the risk of developing CGD, increasing the life quality, reduction of symptoms of anxiety/depression and increase of sleep quality. Each session will be administered every third day to give time to do the tasks, and not too long to reduce the chance of abandoning the treatment. Control: Waiting‐list (participants in this group will not receive the treatment immediately; they will receive the intervention 1.5–2 months after the pre‐measurements were taken) |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months and 6 months follow‐up) |
| Starting date | 22 December 2020 |
| Contact information | Alejandro Dominguez‐Rodriguez, alejandro.dominguez.r@campusviu.es |
| Notes |
Source of funding: Autonomous University of Ciudad Juárez Prospective trial registration number: NCT04638842 |
Dowdall 2017.
| Study name | The benefits of early book sharing (BEBS) for child cognitive and socioemotional development in South Africa |
| Methods |
Study design: RCT Country: South Africa |
| Participants | Caregivers of children aged between 23 and 27 months Inclusion criteria: a. families with children aged 23–27 months at the time of baseline assessment; b. requiring an adult primary caregiver who is at least 18 years old, lives in the household with the child for at least four nights per week, and who consents to participate in the study. Exclusion criteria: a. chronic illness or disability in the child or the adult that would prevent them from fully participating in the intervention Stated purpose: to evaluate a book‐sharing intervention for caregivers of children aged between 23 and 27 months designed to promote child cognitive and socioemotional development |
| Interventions |
Intervention: Early book sharing (BEBS) The intervention is a group‐based, dialogic book‐sharing programme based on our previous programme. The intervention consists of 60–90‐min sessions run weekly for eight consecutive weeks. The programme is delivered to groups of three to six caregivers and their children. Each session focuses on different and incremental techniques for caregivers to apply during book‐sharing. For the first six sessions, there is a ‘book of the week’ that the carers take home to share with their child, and that they bring back the following week. In session 7 all the key principles are reviewed, and the child chooses which of the six books they want to take home for that week. During the final, eighth session, there is a group discussion where caregivers are guided in reflecting on the programme and they discuss plans for continuing with their book‐sharing – such as registering at a nearby children’s library or continuing to meet as a group. For the 6‐month period following session 8, the facilitator visits each participant bi‐monthly to deliver a new picture book and have a short encouraging conversation with the caregiver about their book‐sharing. Control: Waiting‐list control (being offered the intervention once the three waves of assessment have been completed) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention and 6‐months follow‐up) |
| Starting date | February 2016 (recruitment start) |
| Contact information | Peter J. Cooper, p.j.cooper@rdg.ac.uk |
| Notes |
Source of funding: Sexual Violence Research Initiative (SVRI) at the South African Medical Research Council (MRC) Prospective trial registration number: ISRCTN71109104 |
Draper 2020.
| Study name | Pilot implementation of Bukhali: a preconception health trial in South Africa |
| Methods |
Study design: cluster‐RCT Country: South Africa |
| Participants | 18‐ to 25‐year‐old women Inclusion criteria: a. women aged 18‐25 years at baseline measurement Exclusion criteria: a. women with type‐I diabetes, cancer or epilepsy; b. intellectual disability; c. not able or willing to provide consent. Stated purpose: to describe the findings and learnings from the pilot implementation of the HeLTI trial in SA, including a description of intervention strategies and adaptations to the trial design |
| Interventions |
Intervention: Bukhali The intervention arm was designed to be delivered by CHWs (26–40 years old) who would (1) dispense multiple‐micronutrient supplements and resource material, (2) provide health feedback (body mass index (BMI), blood pressure, anaemia and lifestyle) and free services (HIV and pregnancy testing) through monthly individual sessions and (3) facilitate monthly peer sessions on Saturdays. The materials were designed for use in monthly peer sessions with approximately 15 women, facilitated by a Health Helper, over 18 months. Peer sessions were structured to encourage group discussion and also had take‐home activities. Control: Standard of care plus (standard access to healthcare plus additional input on life skills: materials are developed for a call centre intervention that covers practical life skills. Information will be provided via email, SMS and telephonic conversation. The call centre team will offer centre‐based HIV and pregnancy testing and counselling). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (2 years postpartum) |
| Starting date | 1 April 2019 |
| Contact information | CE Draper, catherine.draper@wits.ac.za |
| Notes |
Source of funding: South African Medical Research Council and the Canadian Institutes of Health Research Prospective trial registration number: PACTR201903750173871 |
Fan 2020.
| Study name | Evaluation of smartphone APP‐based case‐ management services amongst antiretroviral treatment‐naïve HIV‐positive men who have sex with men |
| Methods |
Study design: RCT Country: China |
| Participants | Men who have sex with men (MSM) patients Inclusion criteria: a. aged 18 years or older; b. HIV‐positive; c. ART‐naïve, and planning to initiate ART on the day of recruitment; d. self‐reported to be HIV‐infected through homosexual transmission; e. access to the internet on a smartphone; f. having a WeChat account and using it in daily communication; g. willing to provide written informed consent. Exclusion criteria: a. hospitalized due to severe opportunistic infections Stated purpose: to design and evaluate the efficacy of an APP‐based case‐management intervention amongst men who have sex with men who are newly initiating ART, using the IMB model as the theoretical framework |
| Interventions |
Intervention: APP‐based case‐management intervention The Self‐Help Plus intervention consists of a pre‐recorded audio course, delivered by trained facilitators in a group setting and complemented with an illustrated self‐help book adapted for the target cultural group. The intervention is based on acceptance and commitment therapy, a form of cognitive‐behavioural therapy. It is delivered across five 2‐hour sessions. The audio material imparts key information about stress management and guides participants through individual exercises and small group discussions. The self‐help book reviews all essential content and concepts. In this study, a version of the intervention previously adapted for Syrian populations was used. Control: The control group receives SOC service at the hospital, which commences with a 20‐min ART education session for MSM patients newly initiating ART. |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (months 3, 6, and 12) |
| Starting date | NA |
| Contact information | Xiaoyang Fan, Jing Gu, gujing5@mail.sysu.edu.cn |
| Notes |
Source of funding: National Natural Science Foundation of China (Grant #71774178); Science and Technology Planning Project of Guangdong (Grant #2017A020212006); the Guangzhou Science and Technology Project (Grant #201607010368); and National Major Science and Technology Projects of China: Infectious Disease Prevention and Control (Grant #2018ZX10715004) Prospective trial registration number: NCT03860116 |
Fisher 2018.
| Study name | Addressing multiple modifiable risks through structured community‐based Learning Clubs to improve maternal and infant health and infant development in rural Vietnam |
| Methods |
Study design: cluster‐RCT Country: Vietnam |
| Participants | Pregnant women Inclusion criteria: a. all women who are aged at least 18 years, pregnant and less than 20 weeks’ gestation (determined on the basis of the first day of the last menstrual period) living in the selected communes Exclusion criteria: a. women who have a cognitive disability (determined by the local commune health station staff) or other serious physical disabilities which prohibit attendance Stated purpose: to assess the effectiveness of an 18‐month, 20‐module, structured, facilitated women’s health and early childhood development Learning Clubs intervention in reducing deficient cognitive development amongst 2‐year‐olds in rural Vietnam |
| Interventions |
Intervention: Learning Clubs intervention The Learning Clubs intervention is a structured programme that combines perinatal stage‐specific essential information, learning activities and social support in accessible facilitated community‐based groups of women. The intervention comprises 20 educational modules, delivered in face‐to‐face groups at a community centre and in one home visit. The evidence‐informed content has been drawn from interventions to address individual risks: maternal nutrition, mental health or parenting capabilities, infant health and/or development or gender‐based violence and empowerment: Thinking Healthy, What Were We Thinking, Sisters for Life and Care for Child Development programmes and WHO guidelines for nutrition and breastfeeding. The programme will be implemented in facilitated small groups of women meeting every 2 weeks in community centres from mid‐pregnancy and every 4 weeks after childbirth, until the end of the first postpartum year (a total of 19 facilitated group sessions) and one home visit during the first eight postpartum weeks. Control: Usual care (usual standard of pregnancy and postpartum healthcare, including free antenatal checks, birth in a medical facility and access to the National Growth Monitoring and Expanded Immunisation Programmes) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness (calculate the direct and indirect costs of implementing the Learning Clubs programme compared with the cost of the usual standard of care; benefits of the intervention; incremental cost‐effectiveness ratio (ICER); economic and social return on investment (ROI). See Nguyen 2019) Time points: baseline, post‐intervention (F1: late pregnancy (32 weeks of gestation); F2: when infant is 1 year old; F3: when toddler is 2 years old) |
| Starting date | May 2018 (trial recruitment) |
| Contact information | Jane Fisher, jane.fisher@monash.edu |
| Notes |
Source of funding: Australian National Health and Medical Research Council Project Grant (GNT1100147) and seed funding from Grand Challenges Canada under the Saving Brains Initiative (2014‐2015) Prospective trial registration number: ACTRN12617000442303 |
Guo 2018.
| Study name | The effect of Imaginary Working Qigong on the psychological well‐being of college students |
| Methods |
Study design: RCT Country: China |
| Participants | College students from BUCM who are in the first or second year aged 18 to 25 years Inclusion criteria: a. college students, aged between 18 and 25 years, a full‐time freshman or sophomore; b. right‐handed; c. voluntarily willing to participate in this study; d. agreeing to participate in this trial after receiving a thorough explanation of the purposes and characteristics of the trial and willing to sign the written informed consent form, available and willing to complete all IWQ training sessions and relevant psychological outcomes assessment on time; e. truthfully filled out the training and testing record forms and cooperate with the relevant psychological outcomes measure. Exclusion criteria: a. being or having been engaged in a long‐term regular exercising meditation Qigong or other forms of Qigong or athletic sports; b. a member of the Martial Arts Association, Yoga Association, Dance Association, Aerobics Association, Sanda Association, or Taekwondo Association, and so forth; c. a family history of psychosis, neurasthenia, stress disorder, personality disturbance, mental sickness induced by taking psychoactive substances; d. suffered from malignant tumour, severe consumptive disease, cerebrovascular disease, communicable disease, mental illness, and severe cardiovascular, liver, kidney, gastrointestinal and haematological diseases, musculoskeletal system diseases, or other contraindication to mild‐to‐moderate physical exertion; e. high risk of suicide as elicited by interview or have head trauma within the past 6 months; f. who used antianxietics, antidepressants, antipsychotic drugs or anti‐insomnia drugs at a month before the start of the study; g. those with a metal or heart pacemaker implanted in the body; h. women who are pregnant, lactating, or planning to become pregnant; i. being or having participated in other similar clinical trials that affect the relevant psychological outcomes of this study; j. inability to comprehend and complete the study assessments or to be likely to encounter difficulties in adhering to this study instructions; k. participants whom the research investigators judge to be inappropriate. Stated purpose: to examine the feasibility and acceptability of IWQ program amongst college students |
| Interventions |
Intervention: Imaginary Working Qigong (IWQ) The IWQ training includes supervised training and independence training. Supervised training will be performed lasting 4 weeks at the Qigong training classroom of the university and then 4 weeks of independence training according to own situation to decide the time and place. Training will be performed for 40 minutes per day, and each session will include a warm‐up of relaxation for 10 minutes; specified IWQ was performed and refined for 30 minutes. The training scheme originated from the IWQ recorded in Traditional Chinese Medicine Qigong and Chinese Medicine Qigong Training Guidance. An initial workshop conducted over 10 consecutive half‐days by 2 qualified coaches will be designed before IWQ training. After finishing the training of 8 weeks, follow‐up will be done on all participants for 4 weeks. During the 4‐week unsupervised follow‐up period, all of the participants will return to their original lifestyles, but be required to record their mind and body health condition, daily physical activities, or sport information. Control: Waiting list (No specific exercise training will be administered on the participants in the control group. They will be informed to keep their original daily lifestyle in the intervention period and requested not to commence any relaxation techniques, mindfulness meditation, or any other mindfulness‐based training or participate in other regular mind‐body exercises, such as yoga or other forms of Qigong. At the completion of the study, they will be given the same 8 weeks of IWQ training after the 13 weeks so as to increase involvement compliance rate). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4 weeks after intervention: immediately after 4‐week unsupervised follow‐up period) |
| Starting date | NA |
| Contact information | Yulong Wei, wylbucm@163.com |
| Notes |
Source of funding: National Natural Science Foundation of China (No.81473746) Prospective trial registration number: ChiCTR‐BON‐17010848 |
Hossain 2019.
| Study name | Effects of adding psychosocial stimulation for children of lactating mothers using an unconditional cash transfer platform on neurocognitive behaviour of children in rural Bangladesh |
| Methods |
Study design: cluster‐RCT Country: Bangladesh |
| Participants | Mothers with a child aged 6–16 months Inclusion criteria: a. mothers with a child aged 6–16 months; b. eligible to receive UCT (1. Psychosocial stimulation and UCT with HE arm: receiving unconditional cash from government. 2. UCT with HE arm: receiving unconditional cash from government. 3. Comparison arm: eligible to receive UCT but not under UCT programme); c. not expected to leave the study site for more than 2 months; d. has a legally acceptable representative capable of understanding the informed consent document and providing consent on the participant’s behalf. Exclusion criteria: a. legal guardian unwilling or unable to provide written informed consent; b. known congenital anomaly, developmental disorder or severe developmental delay; c. if the child cannot be tested due to physical or behavioural problems; d. children of multiple birth e.g. twin, triplets. Stated purpose: to measure effects of adding PS for children of lactating mothers enrolled to receive UCT with health education (HE) on neurocognitive behaviour of children in rural Bangladesh |
| Interventions |
Interventions: Intervention 1: PS + UCT‐HE (psychosocial stimulation and UCT (maternity allowance) with HE awareness programme) Psychosocial stimulation: the participants will receive fortnightly sessions of psychosocial stimulation at home by community female play leaders for one year. The curriculum is based on improving the mother‐child interaction and providing developmentally appropriate activities for the child. Unconditional cash transfer (maternity allowance) with health education awareness programme: MOWCA provides maternity allowance of taka 500 ($6.25) for each targeted poor pregnant mother under safety net programme of the GOB. The mothers receive the cash every six months for two years through a banking channel. Intervention 2: UCT‐HE only Unconditional cash transfer (maternity allowance) with health education awareness programme: MOWCA provides maternity allowance of taka 500 ($6.25) for each targeted poor pregnant mother under safety net programme of the GOB. The mothers receive the cash every six months for two years through a banking channel. Control: Other: participants who are eligible to receive UCT but do not receive it due to resource constraints of the government |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness analysis: direct and indirect costs Time points: baseline, post‐intervention (follow‐up 12 months) |
| Starting date | 20 August 2017 |
| Contact information | Sheikh Jamal Hossain, sheikh.jamal@icddrb.org or Jamal_jeweldu@yahoo.com |
| Notes |
Source of funding: Grand Challenges Canada under Saving Brains Programme Prospective trial registration number: NCT03281980 |
Imran 2018.
| Study name | World Health Organization 'School Mental Health Manual'‐based training for school teachers in Urban Lahore, Pakistan |
| Methods |
Study design: RCT Country: Pakistan |
| Participants | Schools teachers of grades 1–10 Inclusion criteria: a. all teachers in the participating schools of grades 1–10 of both genders; b. students of 11–16 years old studying in the participating schools whose parents will give informed consent. Exclusion criteria: a. lack of informed consent; b. teachers who are leaving/not planning to be in the school in 3 months’ time; c. teachers who have not been involved in active teaching in last 6 months. Stated purpose: to demonstrate the effectiveness of a teacher training programme based on the WHO‐EMRO Manual of School Mental Health in improving teacher’s mental health literacy as compared to a waiting‐list control group. The secondary objective is to evaluate the effect of the WHO‐EMRO School Mental Health Manual‐based intervention in improving self‐efficacy amongst school teachers. |
| Interventions |
Intervention: World Health Organization, Eastern Mediterranean Region (WHO‐EMRO) School Mental Health Manual The intervention group will receive a training workshop based on a local adaptation of the WHO‐EMRO School Mental Health Manual. The training will be three 6‐h sessions delivered face‐to‐face over a 2‐week period. Topics to be covered in training workshop are as follows: module 1) Social‐Emotional Childhood Development; module 2) Mental Health Promoting Schools (Promotion and Prevention); module 3) Addressing Student Mental Health Problems in Your Classroom (and when to refer for additional help). Control: Waiting‐list control group (not receiving training during the study period) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months follow‐up) |
| Starting date | December 2016 Completed (30 November 2018) |
| Contact information | Nazish Imran, nazishimrandr@gmail.com |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT02937714 |
ISRCTN11059214.
| Study name | Measuring the benefits of the Reach Up early childhood parenting programme in Jamaica |
| Methods |
Study design: RCT Country: Jamaica |
| Participants | Mothers of children from poor circumstances between the ages of 5‐24 months Inclusion criteria: a. child between 5‐24 months; At least one of the following: b. child referred to the nutrition clinic for under‐nutrition; c. child whose last recorded height‐for‐age measurement was below ‐1 SD of the WHO reference standards; d. families where the child or mother is registered with the conditional cash transfer programme (PATH); e. mothers who are currently pregnant with a child under 2 years of age; f. children who live in low socioeconomic environments; g. adolescent mothers 16‐19 years. Exclusion criteria: a. child in daycare or has no consistent caregiver; b. baby has a major disability likely to affect their development; c. mother is aged under 16 years. Stated purpose: to evaluate the implementation process to inform the continued roll‐out of the programme and the impact of the intervention on child development and parenting when implemented at a larger scale. The main research questions are 1. What are the benefits to child development and behaviour when the Reach Up programme is delivered through government primary health care services in Jamaica? 2. What are the factors associated with a successful implementation of the Reach Up programme as delivered through routine health services across Jamaica? |
| Interventions |
Intervention: Reach Up curriculum Each family will receive the usual healthcare provided by the health centre along with fortnightly (every 2 weeks) visits at home from a community health aide. At this visit, she will demonstrate play activities to the mother and encourage her to practice these activities during the 2‐week interval. These visits will be conducted over 10 months. At the end of this period, families will be contacted by the research team and an impact evaluation conducted. Control: Usual care (each family will receive the usual healthcare provided by the health centre. After 10 months families will be contacted and an impact evaluation conducted). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Starting date | 13 May 2019 |
| Contact information | Susan Walker, susan.walker@uwimona.edu.jm |
| Notes |
Source of funding: Ministry of Health Prospective trial registration number: ISRCTN11059214 |
ISRCTN12021015.
| Study name | REACH UP: pilot of a cluster‐randomized controlled trial of an early childhood parenting programme for children 12‐30 months in a rural district in Zimbabwe |
| Methods |
Study design: cluster‐RCT Country: Zimbawe |
| Participants | Caregivers with a child aged 12–30 months Inclusion criteria: a. caregivers with a child aged 12–30 months, residing in the catchment area served by the selected ECD centres Exclusion criteria: a. children with congenital abnormalities or other known disabilities that could affect development Stated purpose: not specified |
| Interventions |
Intervention: REACH UP curriculum Two home visits per month are conducted in the children’s homes and one group session is conducted monthly at the ECD centre. The home visits utilize age and developmentally appropriate activities from the REACH UP curriculum. Toys are left in the home and exchanged at subsequent visits. The monthly group session includes discussions on topics of interest to the caregivers such as child development, child abuse and nutrition. Each group session lasts approximately 1‐1.5 h. Control: Usual care (mother‐child pairs enrolled in the control group receive the usual care provided by the JF Kapnek Trust team when they attend the ECD centres). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate follow‐up) |
| Starting date | 8 June 2016 (first enrolment) Completed: 03/11/2018 |
| Contact information | Joanne Smith, joanne.smith02@uwimona.edu.jm |
| Notes |
Source of funding: Open Society Foundations, Open Society Institute Budapest Foundation Prospective trial registration number: ISRCTN12021015 |
ISRCTN14396374.
| Study name | Adapting DIALOG+ and building capacity in schools to support mental well‐being and resilience in post‐conflict Colombia during the COVID‐19 pandemic |
| Methods |
Study design: RCT Country: Colombia |
| Participants | Adolescent students (aged 12‐18 years) Inclusion criteria: a. Mental Health Survey: adolescents (aged 12‐18 years) in eight public schools who are willing to participate and whose parents provide informed consent; b. DIALOG+ Adaptation: adolescents (aged 12‐18 years) who request advice from a teacher or school counsellor. Exclusion criteria: unwilling or unable to provide informed consent or assent Stated purpose: to build capacity in school mental health and to adapt an intervention called DIALOG+ for use in adolescents in post‐ conflict Colombia during the COVID‐19 pandemic |
| Interventions |
Intervention: DIALOG+ Adaptation 8 teachers/40 adolescents; to have twice‐monthly DIALOG+ meetings for 6 months Control: Usual care control group (4 teachers/20 adolescents; meetings as requested, without DIALOG) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 months) |
| Starting date | 15 April 2021 (first enrolment) |
| Contact information | Francois van Loggerenberg, f.vanloggerenberg@qmul.ac.uk |
| Notes |
Source of funding: Newton Fund, Ministry of Science, Technology and Innovation (MInCiencias) Prospective trial registration number: ISRCTN14396374 |
ISRCTN16205138.
| Study name | Effectiveness of short‐term audio mindfulness in the Chinese community: a pilot study |
| Methods |
Study design: RCT Country: China |
| Participants | Community‐dwelling adults in mainland China will be recruited online Inclusion criteria: a. adults over 18 years old; b. can understand and read Mandarin; c. have a smartphone with consistent internet access and can receive audio from the researcher every day; d. have spare time to listen to audio for 10‐15 minutes every day for 21 consecutive days. Exclusion criteria: a. practised mindfulness mediation before; b. receive any medication or psychotherapy currently; c. been diagnosed with depression, anxiety, or other mental illness. Stated purpose: to test the effectiveness of a short‐term audio mindfulness meditation (SAM) programme for reducing signs of negative emotions in Chinese community‐dwelling people during the period of COVID‐19 |
| Interventions |
Intervention: Online audio‐mindfulness meditation programme Participants will spend 10 to 20 minutes listening to the audio contents and practice daily mindfulness exercises throughout 3‐week with a total of 21 sessions. Control: Waiting list (participants will receive the same audio‐mindfulness programme for self‐practice after all data collection procedures in the mindfulness group will be completed). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (1 week, 2 weeks, and 3 weeks) |
| Starting date | 3 October 2020 (15 March2021 recruitment start date) Completed trial |
| Contact information | Dr Joshua Nan, joshuanan@hkbu.edu.hk |
| Notes |
Source of funding: investigator‐initiated and funded Prospective trial registration number: ISRCTN16205138 |
ISRCTN20474555.
| Study name | Community‐based sociotherapy adapted for Refugees: the COSTAR study |
| Methods |
Study design: cluster‐RCT Country: Uganda and Rwanda |
| Participants | Adult Congolese refugees Inclusion criteria: a. adult > 18 years; b. living in Kyangwali (updated 22/07/2019, previously: Nakivale) settlement, Uganda and Gihembe refugee camp, Rwanda; c. identify as Congolese refugees; d. have a self‐reported good level of fluency in the languages that aCBS will be delivered in (Kinyarwanda in Rwanda/Kiswahili in Uganda). Exclusion criteria: a. current diagnosis of a complex mental disorder (e.g. psychotic disorders, PTSD, substance dependence); b. severe cognitive impairment (e.g. severe intellectual disability, dementia); c. actively expresses suicidal intent. Individuals that are excluded because of a diagnosis of a mental disorder or imminent risk of suicide will be referred for urgent local mental health support. Stated purpose: study hypothesis: 1. Community‐Based Sociotherapy (CBS) intervention will be superior to enhanced care as usual (ECU) in lowering the levels of depressive symptomatology at 16‐ (primary endpoint) and 32‐week (secondary endpoint) follow‐up. 2. CBS will be superior to enhanced care as usual (ECU) in improving the levels of well‐being and quality of life. 3. Refugees in the CBS intervention arm will incur lower health care costs compared to ECU group. |
| Interventions |
Intervention: Adapted Community Based Sociotherapy (aCBS) intervention The Adapted Community Based Sociotherapy (aCBS) intervention is delivered in groups of 10 to 15 people living in the same geographic area (e.g. villages in camps). The aCBS intervention does not target specific diagnoses or symptoms. Rather attention is placed on being inclusive of all people in the community. Thus, aCBS minimises the potential stigma associated with disorder‐focused interventions. Control: Enhanced care‐as‐usual (ECU) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness (healthcare costs) Time points: baseline, post‐intervention (16 weeks and 32 weeks post‐baseline) |
| Starting date | 01 June 2019 |
| Contact information | Ross White, ross.white@liverpool.ac.uk |
| Notes |
Source of funding: Economic & Social Research Council (ES/S000976/1) Prospective trial registration number: ISRCTN20474555 |
ISRCTN49881357.
| Study name | Cluster‐randomized trial of community‐based trauma healing on sexual and physical violence victimization of women and perpetration by men in the Democratic Republic of Congo |
| Methods |
Study design: cluster‐RCT Country: Democratic Republic of Congo |
| Participants | Adults residents of selected villages Inclusion criteria: a. all residents from selected villages Exclusion criteria: a. residents outside randomly selected 80 villages Stated purpose: to evaluate the impact of a Community‐Based Trauma Healing (CBTH) implemented in Eastern Democratic Republic of the Congo (DRC) by Search for Common Ground (SFGC) as part of a wider programme, that aims to reduce gender‐based violence |
| Interventions |
Intervention: Community‐Based Trauma Healing (CBTH) Community‐Based Trauma Healing (CBTH), a programme implemented in Eastern DRC, as a core strategy to help strengthen community capacity to support the trauma healing process and increase individual mental health, and reduce GBV in a (post)‐conflict context CBTH‐facilitated sessions: trauma healing (TH) sessions about each month for a total of 12 sessions. Each TH session brought together 20‐25 interested persons from the community, each session spanning across three days, meeting for about 2‐3 hours each day. Separate sessions were organized for men and women. Using the facilitation guide, THCs facilitate each session to enable participants to identify incidents that affect their personality and cause them trauma. In addition, people were invited to participate in village celebrations to share experiences. Control: No CBTH intervention |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Service uptake (bespoke questionnaire) Time points: baseline, post‐intervention (time not specified) |
| Starting date | 12 September 2018 (1 January 2019 recruitment start date) Completed trial |
| Contact information | Dr Maarten Voors, maarten.voors@wur.nl |
| Notes |
Source of funding: United States Agency for International Development Prospective trial registration number: ISRCTN49881357 |
ISRCTN77689525.
| Study name | Sharing stories: A digital intervention for caregivers with young children in the COVID‐19 era to promote child social, emotional and language development, responsive parenting and parental mental well‐being in Zambia, Tanzania, and Uganda |
| Methods |
Study design: RCT Country: Zambia, Tanzania, and Uganda |
| Participants | Caregivers of young children aged between 9 and 32 months Inclusion criteria: a. primary caregivers of children between the ages of 9‐32 months at enrolment; b. living in selected areas; c. have access to a working smartphone in their household. Exclusion criteria: 1. caregivers aged under 18 years Stated purpose: a digital intervention for caregivers with young children in the COVID‐19 era to promote child social, emotional and language development, responsive parenting and parental mental well‐being |
| Interventions |
Intervention: Sharing stories The intervention is based on the World Health Organization’s Parenting for Lifelong Health shared reading programme and the WHO Thinking Healthy programme. The Parenting for Lifelong Health shared reading programme will be combined with specific content on caregiver mental health and well‐being, adapted for digital delivery via WhatsApp. Caregivers in the intervention group will receive all intervention content on WhatsApp over a six‐week period, using a combination of text and audio messages, photos, infographics and short video clips. A weekly WhatsApp group chat session is used to deliver the intervention content, complimented by recap messages throughout the week. In addition, caregivers receive two digital picture books a week. Each group consists of 30 to 40 participants, moderated by two trained intervention facilitators, fluent in the local languages. Control: Waiting list (caregivers in the control condition will receive no intervention during the duration of the trial). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: Baseline, post‐intervention (6 weeks) |
| Starting date | 25 August 2020 (first enrolment) Completed trial (31 May 2021) |
| Contact information | Sarah Skeen, skeen@sun.ac.za |
| Notes |
Source of funding: The LEGO Foundation Prospective trial registration number: ISRCTN77689525 |
ISRCTN80690743.
| Study name | Project HASHTAG: testing a school‐based intervention to improve adolescent mental health in Nepal and South Africa |
| Methods |
Study design: cluster‐RCT Country: Nepal and South Africa |
| Participants | School‐going adolescents in South Africa (grade 8) and Nepal (grade 7‐9) Inclusion criteria: a. school‐going adolescents in South Africa (grade 8) and Nepal (grade 7‐9) Exclusion criteria: not specified Stated purpose: as this is a feasibility trial, we will evaluate the feasibility of the intervention and trial procedures. |
| Interventions |
Intervention: HASHTAG HASHTAG is a multi‐level intervention for young people in grade 8 or equivalent that aims to promote positive mental health and prevent mental health conditions (specifically, depression and anxiety) and reduce risk behaviours. The HASHTAG intervention comprises two modules: 1) Thriving Environment in Schools (TES), a whole‐school intervention, and 2) Thriving Together (TT), a group‐based intervention delivered directly to young adolescents. TES is a school climate improvement strategy that seeks to modify adolescents’ social and emotional environment through a whole‐school approach to create a school culture of connectedness and supportive relationships. It will include 1) School Action Groups, 2) teacher‐focused workshops, and 3) mental health awareness raising activities. TT will be delivered to students in grade 8 in South Africa and grade 7‐9 in Nepal. It will include six 90‐minute weekly sessions focused on emotional regulation, stress management, problem‐solving, interpersonal skills and relationships, and assertiveness training. Control: Enhanced treatment as usual (eTAU: control arm schools will receive a shortened version of the student sessions after follow‐up interviews are complete). |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediately post‐intervention) |
| Starting date | 18 July 2019 (recruitment start date: 1 March 2021) |
| Contact information | Prof Mark Tomlinson, markt@sun.ac.za |
| Notes |
Source of funding: Medical Research Council (UK) Prospective trial registration number: ISRCTN80690743 |
ISRCTN83649248.
| Study name | Improving the mental health and quality of life of people affected by leprosy or Buruli ulcer in Southern Nigeria |
| Methods |
Study design: RCT Country: Nigeria |
| Participants | People affected by leprosy or Buruli ulcer Inclusion criteria: a. affected by leprosy or Buruli ulcer; b. affected by leprosy or Buruli ulcer registered for treatment from 2014 up to 1 year before the end of the intervention; c. aged between 15 to 65 years registered for treatment. Exclusion criteria: a. refusal to give consent; b. pregnant women; c. patients who need urgent medical attention; d. patients unable to communicate clearly. Stated purpose: the study proposes a holistic, community‐oriented approach for improving access and utilization of mental health services through interventions using holistic approach work synergistically to reduce mental disorders and improve quality of life amongst persons affected by leprosy or Buruli ulcer. |
| Interventions |
Intervention: Holistic, community‐oriented approach 1. Engaging selected community members as lay counsellors to provide psychotherapy and counselling services for persons affected by leprosy or Buruli ulcer 2. Formation of self‐help groups (SHG) amongst persons affected by leprosy or Buruli ulcer for peer support and improving self‐esteem 3. Training of healthcare workers to provide pharmacological treatment or referral services to experts where necessary The intervention phase will take about 1‐2 years with quarterly community sensitization during supervisory visits by the research team to promote social inclusion, raise awareness on mental health problems and availability of services to create demand and enhance utilization Control: No intervention |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (after 2 years of intervention) |
| Starting date | 01 June 2021 (first enrolment) |
| Contact information | Ngozi Ekeke, ngozi.ekeke@dahw.org |
| Notes |
Source of funding: Leprosy Research Initiative (LRI) Prospective trial registration number: ISRCTN83649248 |
Keynejad 2020.
| Study name | Problem‐solving therapy (PST) tailored for intimate partner violence (IPV) versus standard PST and enhanced usual care for pregnant women experiencing IPV in rural Ethiopia |
| Methods |
Study design: RCT Country: Ethiopia |
| Participants | Pregnant women experiencing intimate partner violence (IPV), with depressive symptoms and functional impact Inclusion criteria: a. speak Amharic (the official regional language); b. aged 16 years or over; c. between 12 and 34 weeks gestation of pregnancy; d. intending to reside in the study area for the duration of the study; e. score 5 or more on PHQ‐9 with functional impairment (tenth question); f. report IPV in the past year (in a current or previous relationship) during screening; g. consent to participate, including to accept enhanced usual care or to attend four sessions of PST‐IPV or PST (if randomised to a treatment arm). Exclusion criteria: a. acutely unwell; b. require emergency treatment; c. identified by the ANC provider during pre‐screening as having possible psychotic symptoms; d. unable to understand the interview (e.g. diagnosed with severe intellectual disability or dementia, or unable to speak Amharic); e. expect to move away from the study area before the study is completed. Stated purpose: to determine the feasibility and acceptability of the intervention and study design to inform a future fully powered RCT |
| Interventions |
Intervention: Intervention 1: PST‐IPV Intervention 2: standard PST Both PST‐IPV and standard PST intervention arms follow the same structure of four sessions: session 1 focuses on basic psychoeducation, introduction to PST; session 2 focuses on revising session 1, coping strategies for ‘group A’ problems and the six‐step problem‐solving method for ‘group C’ problems; session 3 focuses on revising session 2, coping strategies for ‘group B’ problems and psychoeducation about the phases of coping with bereavement and loss; session 4 focuses on revising session 3, using problem‐solving skills in everyday life and reviewing how the coping strategies worked in practice. PST‐IPV content and materials are adapted to address the needs and experiences of women affected by IPV, whilst standard PST content and materials are generic. Adaptations for women experiencing IPV include training staff using content and materials from the new WHO curriculum on caring for women subjected to violence, attention to safety and sensitivity where women list IPV‐related problems during sessions (including training with worked examples of problem‐solving focused on IPV), and adaptation of PST case studies to reflect common problems associated with IPV. Control: Enhanced usual care (standard clinical care and information only about sources of support) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (9 weeks after baseline: > 1 month post‐intervention) |
| Starting date | Recruitment of participants has not yet commenced. |
| Contact information | Charlotte Hanlon, charlotte.hanlon@kcl.ac.uk |
| Notes |
Source of funding: National Institute of Health Research (NIHR) Global Health Research Unit on Health System Strengthening in sub‐Saharan Africa (ASSET), King’s College London (GHRU 16/136/54) using UK aid from the UK Government and other sources of support specified for all authors Prospective trial registration number: PACTR202002513482084 |
Lokuge 2018.
| Study name | Protocol for a cluster‐randomized controlled trial evaluating the impact of a preschool‐based capacity building intervention on intimate partner violence and substance misuse in Sri Lanka |
| Methods |
Study design: cluster‐RCT Country: Sri Lanka |
| Participants | Families (parents) utilizing government preschools Inclusion criteria: a. Families utilizing government preschools in two urban areas in Sri Lanka. All parents of children attending these preschools will be eligible for inclusion in the study and will be included if consenting. Exclusion criteria: not specified Stated purpose: to determine whether such preschool‐based capacity building programmes reduce the prevalence of IPV in preschool‐attending families. Secondary aims include assessing the impact of such programmes on awareness and uptake of services, and on the prevalence of alcohol and drug misuse in these families. |
| Interventions |
Intervention: Capacity‐building intervention A community‐based support programme delivered by preschool teachers and volunteer parents that will increase awareness, knowledge and uptake of available services for IPV and substance misuse, and of the link between these issues and poorer education outcomes in children. The following specific activities and interventions will be implemented in the intervention preschools: capacity building, training, and support to selected mothers on the provision of safe, confidential and relevant community‐based referral and support services to women affected by IPV; capacity building, training and support to selected fathers on the provision of safe, confidential and relevant community‐based referral and support to men seeking support for substance misuse problems; capacity building and on‐the‐job training and support to preschool teachers on provision of IPV and substance misuse prevention educational messages (including the links between these issues and poor child development), and referral pathways to services for these issues Control: No intervention in control preschools (if the intervention is demonstrated as being effective, these control preschools will receive the intervention at the conclusion of the study) |
| Outcomes |
Participants'outcomes of interest for this review Nil Economic outcomes Uptake of services for IPV and substance misuse ‐ locally‐relevant tools developed during initial project workshops Time points: baseline, post‐intervention (10 months post‐intervention) |
| Starting date | 23 January 2018 Completed (28 February 2019) |
| Contact information | Polly Wallace, Polly.Wallace@anu.edu.au |
| Notes |
Source of funding: The Commonwealth Department of Foreign Affairs and Trade, Australia Prospective trial registration number: NCT03341455 |
Mapurunga 2020.
| Study name | The MBHP‐Elderly Study |
| Methods |
Study design: RCT Country: Brazil |
| Participants | Older adults assisted in primary care (≥ 60 years) Inclusion criteria: a. men and women 60 years or older; b. elderly classified as cognitively normal (CDR = 0) and/or with mild cognitive impairment (CDR = 0.5); c. literate; d. not have an advanced computer level; e. good hearing to follow the practices. Exclusion criteria: a. participants performing contemplative practices such as yoga, tai‐chi‐chuan, vipassana, Zen Buddhism, mindfulness, and other meditative practices in the last 6 months; b. patients with acute phase of depression evaluated by the Geriatric Depression Scale (GDS‐15); c. patients with psychotic diagnosis; d. taking drugs that cause cognitive impairment; e. score 1.0 or more at the CDR applied during the recruitment by a trained gerontologist in order to diagnose cognitive impairment. Stated purpose: to evaluate the feasibility and preliminary efficacy of the MBHP programme on quality of life (primary outcome) of older adults assisted in PC comparing to a cognitive stimulation active control group |
| Interventions |
Intervention: Mindfulness‐Based Health Promotion (MBHP) programme It is originally an 8‐session (2 or 1.5 h each) programme based on the MBSR model created by Kabat‐Zinn and colleagues. The MBHP is adapted to the context of PC with a framework that supports the learning process to individuals from different cultures and education backgrounds. In this study participants will have a weekly meeting of an hour and 30 min for 8 weeks to perform the MBI (MBHP protocol) accompanied by extra four 1‐h meetings through a maintenance group with all participants after the eighth week (each maintenance session took place fortnightly), totalling a 4‐month (16‐week) intervention. Control: Active control group: cognitive stimulation control group (This training is a computer class workshop based on a model proposed by Xavier and colleagues, which uses the staging methodology considering cognitive and functional resources accordingly to the level of complexity and difficulties from the executive functions theory. The abilities learned at the workshop will be basic computer functions. Participants will take computer‐based cognitive stimulation classes for 4 months, once‐a‐week 1.5‐h meetings without a maintenance phase, totalling 16 weeks). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention and 1‐year follow‐up) |
| Starting date | June 2018 |
| Contact information | Marcelo Demarzo, demarzo@unifesp.br |
| Notes |
Source of funding: Coordenação de Aperfeiçoamento de Pessoas de Nível Superior—Brasil (CAPES)—Finance Code 001 (Master fellowship) and by Mente Aberta ‐ Brazilian Center for Mindfulness and Health Promotion through subsidizing the materials needed for this research Prospective trial registration number: NCT03048708 |
Mariano 2021.
| Study name | Effectiveness of the Elos 2.0 prevention programme for the reduction of problem behaviours and promotion of social skills in schoolchildren |
| Methods |
Study design: cluster‐RCT Country: Brazil |
| Participants | Students aged 6 to 10 years Inclusion criteria: School: a. public school; b. at least one class of each grade (first to fourth grade) and all children in these grades will be included in the trial. Exclusion criteria: not specified Stated purpose: to evaluate the effectiveness of the Elos 2.0 Programme, which is a version adapted to Brazil for proposed implementation by the Ministry of Health, in the reduction of problem behaviours and promotion of prosocial behaviours in children in public schools |
| Interventions |
Intervention: Elos Game 2.0 The Elos Programme is based on the Good Behaviour Game, which is widely used and prevents and/or reduces students’ disruptive behaviours. By changing interactions that are considered maladaptive in the classroom context, the programme intends to promote mental health and prevent and/or reduce problem behaviours (e.g. disruptiveness, aggressivity and shyness). Teachers will present four rules to be followed by the student teams during the Elos Game 2.0: (1) follow the instructions for the activities, (2) adhere to the voice levels (silence, whispering, group voice, presentation or street voice), (3) comply with assigned positions (remain seated, stand up and walk as arranged or stand up and walk freely) and (4) be kind. To play the Elos Game, teachers should select pedagogical activities that are consistent with the school curriculum and can be performed autonomously. Control: Usual care (treatment‐as‐usual in Brazil, i.e. no behavioural intervention at the school) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (after trial completion) |
| Starting date | March 2019 (recruitment start date) |
| Contact information | Marília Mariano, mariano.mrl@gmail.com |
| Notes |
Source of funding: Research and Innovation Grant for the Prevention of Mental Disorders and Use of Alcohol and other Drugs (“Pesquisas e Inovações em Prevenção de Transtornos Mentais e Uso de Álcool e Outras Drogas”), funded by the Brazilian Ministry of Health (TED #176/2017) Prospective trial registration number: RBR‐86c6j |
Mutedzi 2019.
| Study name | Improving bereavement outcomes in Zimbabwe: protocol for a feasibility cluster trial of the 9‐cell bereavement tool |
| Methods |
Study design: cluster‐RCT Country: Zimbabwe |
| Participants | Bereaved community members Inclusion criteria: a. at least 18 years old; b. resident within their neighbourhoods; c. someone with whom they interact with on a daily basis; d. who they know to have been bereaved in the past 6 months; e. ability to either verbally consent or be able to consent in writing; f. able to read and write; g. expected to attend and participate in the study. Exclusion criteria: not specified Stated purpose: to determine the feasibility of implementing the 9‐cell bereavement tool and recruitment to experimental evaluation. |
| Interventions |
Intervention: The 9‐cell bereavement tool The 9‐cell bereavement tool is administered in a 7‐h (1‐day) session. Community lay caregivers are an appropriate target as social support can improve grief and bereavement outcomes. It assesses personal feelings in relation to bereavement, identifies judgemental attitudes, inappropriate religious tenets, lack of understanding, effects of family, and community support. Control: Waiting‐list (participants will receive the intervention at the end of the study, following the final end‐line data collection point) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months post‐baseline and 3 months post‐midline) |
| Starting date | July 2017 Completed trial |
| Contact information | Barbara Mutedzi, bmutedzi@gmail.com |
| Notes |
Source of funding: King’s College London, BuildCARE (Building Capacity, Access, Rights and Empowerment) Africa Prospective trial registration number: ISRCTN16484746 |
Müller 2019.
| Study name | Effects of a school‐based health intervention programme in Port Elizabeth, South Africa: the KaziBantu project |
| Methods |
Study design: cluster‐RCT Country: South Africa |
| Participants | Children in grades 1‐6 aged (between 6 and 16 years) and school teachers Inclusion criteria: Children: a. grade 1‐6; b. aged 6‐14 years; c. written informed consent by parent/guardian; d. not participating in other clinical trials; e. not suffering from medical conditions that prevent participation in physical activity. Teachers: a. involved in implementation of the school‐based health promotion programme; b. ticked all questions with "yes" in the Physical Activity Readiness Questionnaire to be able to take part in the cardiorespiratory fitness test. Exclusion criteria: Children: a. suffering severe malnourishment (as diagnosed by a study nurse following national guidelines. In this case, children will be referred to local clinics). Teachers: a. acute or chronic medical conditions that prevent participation in a submaximal fitness test (if uncertain, participant will be asked to consult a general practitioner and provide a doctor's certification before he/she is included in the study); b. temporary illness such as a cold or fever (to participate in cardiorespiratory fitness test); c. minimum 50% employment rate for at least 6 months. Stated purpose: to assess how effective school‐based intervention programmes are on communicable diseases, risk factors for non‐communicable diseases, health behaviours (beliefs and actions relating to health and well‐being) and psychosocial health in school‐aged children in disadvantaged neighbourhoods in Port Elizabeth, South Africa. Additionally, study aims to develop and pilot‐test a workplace health intervention for primary school teachers. |
| Interventions |
Intervention: School‐based health promotion programme For children: children will take part in a school‐based health promotion programme that lasts for 32 school weeks. This involves one 40‐minute long physical education lesson per week, one 40‐minute moving‐to‐music lesson per week, and 3 health‐education and 3 nutrition‐education lessons (all 40 minutes long) across the study period. Children will also undergo deworming (helminths) using a single dose of albendazole or mebendazole. For teachers: teachers will take part in a 6‐month workplace health promotion programme. There will be a baseline assessment including measurements relating to perceived health, disease history, blood tests, body measurements, physical activity, mental health and stress and quality of life. Following this, teachers will receive a personal health profile providing an overview of cardiovascular and mental health that is used to estimate their health risks, along with brief information on how to interpret this. The workplace health programme will last for 20 weeks and involve individually tailored lifestyle coaching workshops. Control: Control group (not specified) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (after 12 months) |
| Starting date | 1 July 2018 Completed (31 December 2019) |
| Contact information | Uwe Pühse, uwe.puehse@unibas.ch |
| Notes |
Source of funding: Novartis Foundation, Basel, Switzerland Prospective trial registration number: ISRCTN18485542 |
NCT03231358.
| Study name | Our Family Our Future: a resilience‐oriented family intervention to prevent adolescent HIV/STI infection and depression in South Africa |
| Methods |
Study design: RCT Country: South Africa |
| Participants | Adolescents (14‐16 years) and their parents Inclusion criteria: a. 14‐16 years; b. adolescent concurs that the adult identified is their parent (to also include primary caregivers in the parental role); c. when more than one child in the family falls within the eligible age range, one child will be chosen at random; d. lives in the household at least 4 days a week. Exclusion criteria: a. no or low symptoms (< 6) or clinically significant thresholds of depression (16+) Stated purpose: to test the efficacy of Our Family Our Future, an integrated intervention for preventing HIV and depression onset amongst adolescents |
| Interventions |
Intervention: Our Family Our Future Our Family Our Future is a 'selective' behavioural prevention programme, designed to address HIV/STI acquisition, sexual risk behaviour, and depression amongst adolescents (ages 14‐16) in communities with high HIV prevalence and from families where adolescents and parents already exhibit mild, potentially troublesome, depressive symptoms but do not reach the threshold for further screening for a significant clinical depressive disorder. This intervention involves parent‐child dyads who receive the intervention in a community setting, in a facilitated group format. The intervention consists of 3‐hour sessions, held weekly for 3 consecutive weeks with an individual family meeting in the third or fourth week depending on family desires. Control: Usual care (consisting of a packet of existing available brochures on HIV, STIs, mental health including places to access care) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (12 months) |
| Starting date | 21 November 2018 |
| Contact information | Caroline Kuo, caroline_kuo@brown.edu |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT03231358 |
NCT03243396.
| Study name | Building and Sustaining Interventions for Children: task‐sharing mental health care in low‐resource settings (BASIC) |
| Methods |
Study design: cluster‐RCT Country: Kenya |
| Participants | Children and adolescents (11 to 14 years) Inclusion criteria: a. child or young adolescent between the ages of 11 and 14 at the time of enrolment; b. child lost one or both parents to death at least 6 months ago or later, and when the child was 4 years old or older; c. lives in the community with at least one adult guardian (18 years old or older); d. experiencing borderline or clinically significant levels of post‐traumatic stress or childhood traumatic grief (as indicated by a score of 18 or higher on the Child Post‐traumatic Stress Scale, or a score of 35 or higher on the Inventory of Complicated Grief). Exclusion criteria: a. known developmental or cognitive disability; b. attends private school; c. child and family are about to move; d. children who lost a parent less than 6 months ago (since they may be experiencing a normal grief reaction and may not necessarily be in need of the treatment for CTG); e. caregiver of the child refuses to participate; f. lay counsellor is not literate; g. lay counsellor does not have a mobile phone; h. lay counsellor refuses to serve as a counsellor; i. site leader refuses to allow their site to participate in the study. Stated purpose: 1) identify actionable IPPs that predict adoption (delivery) and fidelity (high‐quality delivery) after 10 sites in each sector implement TF‐CBT (sequence 1). Use identified IPPs to (Aim 1a) guide implementation planning support for subsequent sites and to (Aim 1b) generate testable hypotheses about IPPs as causal mechanisms. 2) Test mechanisms of implementation success in both sectors across all 7 sequences. 3) Test TF‐CBT effectiveness (i.e. mental health outcomes; functioning) and cost in both sectors |
| Interventions |
Intervention: Trauma‐focused cognitive behavioural therapy (Pamoja Tunaweza) ‐ Health (community health volunteer) sector These child/adolescent participants and one of their guardians will receive Pamoja Tunaweza, the locally adapted version of trauma‐focused cognitive behavioural therapy, in a community setting from community health volunteers. TF‐CBT intervention is composed of eight small‐group sessions, including eight children and one guardian for each child, and will meet separately, with joint activities in the final three sessions. TF‐CBT will be delivered via community health volunteers in the community setting, and via selected teachers in the school setting – with two lay counsellors leading the child group, and one leading the guardian group. Most TF‐CBT components (psychoeducation, parenting, relaxation, cognitive coping, grief specific skills) will be delivered in groups, but 2‐3 individual sessions mid‐group will be used for imaginal exposure (i.e. talking about/processing traumatic events). Control: Trauma‐focused cognitive behavioural therapy (Pamoja Tunaweza) ‐ Education (teacher) sector These child/adolescent participants and one of their guardians will receive Pamoja Tunaweza, the locally adapted version of trauma‐focused cognitive behavioural therapy, in their school setting from teachers employed by their school. |
| Outcomes |
Participants' outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (end of 8‐session treatment, assessed up to 18 weeks) |
| Starting date | 1 February 2018 |
| Contact information | Kathryn Whetten and Shannon Dorsey |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT03243396 |
NCT03887312.
| Study name | Phone‐delivered psychological intervention (t‐CETA) for mental health problems in 8‐17 year‐old Syrian refugee children (t‐CETA) |
| Methods |
Study design: RCT Country: Lebanon |
| Participants | Syrian refugee children and adolescents (8‐17 year‐olds) Inclusion criteria: a. aged 8‐17 years, male or female; b. llve with a parent or other legal guardian; c. child and/or parent identifies that the child has mental health difficulties and requests services; d. at high risk of having a mental disorder as indexed by falling in the top 40% of the distribution in any one of the following child‐report questionnaires: (i) Screen for Child Anxiety Related Emotional Disorders (SCARED), (ii) Center for Epidemiological Studies Depression Scale for Children (CES‐DC), (iii) Child PTSD Symptom Scale (CPSS); AND falling in the top 40% of the distribution in the following parent report questionnaire: Strengths and Difficulties Questionnaire (SDQ) total difficulties [criterion 4 is only applicable to children for whom these data are available from participation in the BIOPATH study; criterion 5 takes precedence over criterion 4 where both are available]; e. confirmation of significant level of symptoms and functional impairment on clinical interview (MINI KID) as indicated by (i) meeting full or probable diagnostic criteria for ANY of the following: any category of mood disorder, any category of anxiety disorder, PTSD, conduct disorder, or oppositional defiant disorder; AND (ii) Clinical Global Impression severity (CGI‐s) score of > 3; f. parent/legal guardian gives informed consent and child gives assent to take part. Exclusion criteria: a. problem for which t‐CETA would not be appropriate, including psychiatric disorders for which CETA treatment is not recommended (e.g. bipolar disorder, psychosis), severe distress (e.g. acute suicidal ideation), or problems that would preclude delivery over the telephone (e.g. selective mutism); b. parent or legal guardian is not able to provide consent; c. child protection issues (e.g. acute maltreatment) that are judged by clinician to make trial inclusion inappropriate; d. any inclusion criteria not met. Stated purpose: evaluates the effectiveness of t‐CETA, a version of Common Elements Treatment Approach (CETA) adapted to be delivered over the telephone, in treating common mental health problems in 8‐17 year old Syrian refugee children living in Lebanon |
| Interventions |
Intervention: Telephone‐delivered Common Elements Treatment Approach (t‐CETA) Cognitive behavioural therapy (CBT)‐based approach delivered over the telephone. Components are available for common problems, including anxiety, depression, PTSD, conduct problems, substance abuse, and safety issues (including self‐harm or suicidal ideation), and a tailored treatment package is produced for each child based on the presenting problem(s) and response to treatment. There are components for use with both child and caregiver. t‐CETA sessions of up to 30 minutes will be delivered 1‐2 times per week for approximately 8‐12 weeks. The number and content of sessions will be tailored to each child, thus there will be some variation. Control: Usual care (Médecins du Monde treatment‐as‐usual: case manager‐led care, with referral to a psychotherapist or psychiatrist as necessary. Médecins du Monde's approach is based on a joint collaboration between mental health trained case managers (who undergo extensive training by experts in the field on topics including Psychological First Aid, Child Protection, Gender Based Violence, etc.) and psychotherapists from different schools (providing eye movement desensitization and reprocessing [EMDR] for trauma, interpersonal therapy [IPT] for depression, cognitive behavioural therapy [CBT], motivational counselling, familial or systemic therapy, and integrative approaches). Thus, the number and content of sessions, and the person delivering treatment (case manager, psychotherapist, psychiatrist) vary). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediately after intervention and 3 months post‐intervention) |
| Starting date | 1 May 2019 Completed (31 January 2020) |
| Contact information | Michael Pluess, m.pluess@qmul.ac.uk |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT03887312 |
NCT03960944.
| Study name | An experimental study of psychological effect of school based‐yoga on low‐income school children |
| Methods |
Study design: RCT Country: India |
| Participants | School children aged 8‐13 years Inclusion criteria: a. low‐income group; b. parental consent to participate in study. Exclusion criteria: a. disabilities; b. substance abuse; c. living outside the school areas. Stated purpose: to investigate the effects of a structured yoga programme on psychological well‐being in low‐income school children |
| Interventions |
Intervention: Yoga 30‐min yoga sessions were conducted in schools for 8 weeks. The 30‐min yoga session included physical posture, breathing exercises, yoga games and relaxation. Control: Usual care (usual activities in school) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediately) |
| Starting date | 21 May 2018 Completed (12 March 2019) |
| Contact information | Rajubhai P Odedra (principal investigator) |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT03960944 |
NCT04234815.
| Study name | Evaluating impacts of CSS for veterans and their families in Ukraine |
| Methods |
Study design: cluster‐RCT Country: Ukraine |
| Participants | Adult veterans in Ukraine or an adult family member of a veteran Inclusion criteria: a. Ukrainian adults (age 18 or older) who are veterans of the anti‐terrorist operations (ATO) in Ukraine or an adult family member of a veteran; b. meets a minimum total problem score on the locally validated screener indicative of at least moderate symptoms of distress. Exclusion criteria: a. identify as ATO veterans but are still active (i.e. active duty) in the Ukrainian military; b. arrive late to the workshop, operationalized as not having time to participate in the self‐assessment review, will be excluded. In online workshops, late‐arriving participants will be redirected to a waiting room, where they will be encouraged to attend a future session instead (and if so, could be included in the study). For in‐person workshops, due to travel and other efforts required for attendance, late arriving participants will be allowed to join the workshop, but excluded from the study due to lacking sufficient exposure. They can also choose to attend a future workshop, but if attending a portion of an initial workshop would remain study ineligible because they could potentially be exposed to both arms of the study; c. identified by service providers as needing higher‐level care rather than outpatient psychotherapy will be immediately referred to that care and excluded from the study. This exclusion criterion refers to imminent danger to self or others that requires urgent safety procedures and institution‐based mental health care. Stated purpose: to evaluate the effectiveness of a brief, single‐session psychosocial workshop, "CETA Short Session" (CSS), for reducing symptoms of distress and functional impairment and increasing treatment engagement amongst conflict veterans and their families in Ukraine |
| Interventions |
Intervention: CETA Short Session (CSS) CETA Short Session (CSS) is a brief, low‐intensity psychosocial intervention incorporating foundational components of the Common Elements Treatment Approach (CETA). CETA is an 8‐12 session, transdiagnostic psychotherapy for common mental disorders. CSS was designed to serve as both a prevention/support approach for a broader range of needs, and an engagement/outreach strategy to identify and refer individuals to treatment. For this study, a single‐session, 1.5‐2 hour group workshop that includes psychoeducation, self‐assessment, safety screening, and training in cognitive coping was applied. Participants with at least moderate symptoms of distress will also receive a follow‐up phone call to discuss next steps occurring within one week after workshop attendance. On this phone call, they will also be asked about their use of the cognitive coping skill over the last week, and provided feedback if using it incorrectly. Control: Enhanced Treatment‐as‐Usual (eTAU) A single‐session, approximately 1‐hour group workshop that includes the same psychoeducation, self‐assessment, and safety screening as the experimental condition, but no training in cognitive coping. This intervention is the same as CSS with the exception that the cognitive coping skill training is removed. Participants with at least moderate symptoms of distress will also receive a follow‐up phone call to discuss next steps occurring within one week after workshop attendance. |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (1‐month follow‐up) |
| Starting date | 28 February 2020 |
| Contact information | Sergiy Bogdanov, s.bogdanov@ukma.edu.ua |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04234815 |
NCT04252807.
| Study name | A common elements‐based intervention to improve maternal psychological well‐being and mother‐infant interaction |
| Methods |
Study design: RCT Country: Pakistan |
| Participants | Distressed pregnant women Inclusion criteria: a. pregnant with third trimester (28 gestational weeks); b. aged 18‐40 years; c. intent to reside in the study areas until the completion of the study; d. score ≥ 9 on the SRQ. Exclusion criteria: a. women who require immediate or ongoing medical or psychiatric care reported; b. severe previous or current obstetric morbidity including eclampsia and antepartum haemorrhage; c. medical disorders that require inpatient management (e.g. diabetes, hypertension, thromboembolism, cardiac disease). Stated purpose: to develop an online training curriculum to train lay health workers in common elements based intervention to improve maternal psychological well‐being and improve mother‐infant interaction amongst distressed mothers in low‐resource rural community settings of Pakistan. The impact of intervention on maternal well‐being, infant growth, nutrition and development will be evaluated at 12 months' postpartum. |
| Interventions |
Intervention: Common elements based integrated intervention In addition to the routine care delivered by Lady Health Workers (LHWs), the participants in the intervention arm will receive common elements‐based integrated intervention that combines evidence‐based elements from packages of care addressing early stimulation, responsive feeding and perinatal depression. The participants will receive 15 monthly sessions at home by lay health workers. First three sessions will be delivered to the participants in the third trimester of pregnancy, followed by 12 monthly sessions afterwards. The intervention consists of three modules including 1) mothers' well‐being, 2) infant nutrition, early stimulation and breastfeeding and 3) mother‐infant interaction. Control: Usual care (the participants in the control arm will receive the routine monthly visits by the trained lady health workers (LHWs) of their respective areas. The LHWs are trained to provide antenatal care and referral, immunization services and support to community mobilization, provision of family planning and basic curative care via door‐to‐door visits to the households of their allocated areas). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (12 months postpartum; for depression also 6 months postpartum) |
| Starting date | 7 February 2020 |
| Contact information | Syed Usman Hamdani |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT04252807 |
NCT04255472.
| Study name | Effectiveness of the WHO Caregivers Skills Training (CST) Program for children with developmental disorders and delays in rural community settings in Pakistan |
| Methods |
Study design: RCT Country: Pakistan |
| Participants | Children (2‐9 years) with developmental disorders Inclusion criteria: a. aged 2‐9 years old, with developmental disorders and delays as screened by TQS; b. screened positive on communication problems as identified by Communication and Symbolic Behavior Scale (CSBS) score < 41; c. developmental Disability‐Children's Global Assessment Scale (DD‐CGAS) score ≥ 51 as assessed by clinician. Exclusion criteria: a. epilepsy with seizures in the previous 6 months; b. cerebral palsy as assessed by the clinician; c. comorbid physical and mental conditions in the child that require inpatient hospitalization; d. significant uncorrected hearing and visual impairment in child or parent; e. any severe psychiatric or physical illness in primary caregiver requiring inpatient hospitalization. Stated purpose: to evaluate the effectiveness of the WHO CST program plus treatment‐as‐usual (TAU) vs. TAU to improve caregiver‐child interaction in children with developmental disorders and delays, when implemented by non‐specialist health care facilitators in low‐resource rural community settings of Rawalpindi, Pakistan |
| Interventions |
Intervention: WHO Caregivers Skills Training (CST) Program Caregivers are provided with tangible strategies to appropriately respond to their children's emotional regulation, engagement, and communication. Further, the programme focuses on helping caregivers to develop their children's communication and adaptive skills while reducing challenging behaviour by focusing on identifying the function of the behaviour and learning to teach developmentally appropriate replacement skills. The WHO CST program includes nine group sessions delivered at a community venue (e.g. school, home) and three home visits: the first at entry prior to session 1, the second after session 4, and the third after the final group session. Participants will receive 3‐hour group training sessions of WHO CST programme once every week for 9 weeks and 3 individual home sessions delivered via non‐specialist health care facilitators over a duration of 3 months. Training for programme facilitators will be included prior to the delivery of the intervention. Control: Usual care (TAU in primary healthcare centres for childhood developmental disorders and delays usually consists of no treatment, or a range of alternate treatment regimens, such as multi‐vitamin syrups and tablets. Evidence‐based mental health care is currently not available in primary healthcare centres. A complete record of services availed by the trial participants at tertiary mental healthcare centres will be maintained by using an adapted Client Services Receipt Inventory (CSRI) for children with developmental disorders and delays at baseline and end point). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Health services utilization ‐ Client Services Receipt Inventory, adapted (CSRI) Time points: baseline, post‐intervention (9 months post‐intervention) |
| Starting date | 11 February 2020 |
| Contact information | Syed Usman Hamdani, usman.hamdani@hdrfoundation.org |
| Notes |
Source of funding: Human Development Research Foundation, Pakistan Prospective trial registration number: NCT04255472 |
NCT04289272.
| Study name | Evaluating Dove Confident Me in India |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | School students (11‐14 years) Inclusion criteria: a. co‐educational secondary schools in New Delhi; b. middle‐income schools or private schools; c. sufficient proficiency in speaking, reading and writing in Hinglish. Exclusion criteria: a. single‐sex schools; b. low‐income schools; c. not sufficient proficiency in speaking, reading or writing in Hinglish. Stated purpose: 1) to conduct a small‐scale acceptability study of a 'Confident Me', a body image intervention, amongst 11‐13‐year olds in New Delhi, India, to understand its acceptability, feasibility, and preliminary efficacy in a metropolitan area of India; 2) to refine 'Confident Me' based on the acceptability study, and to conduct a randomised controlled trial to evaluate its efficacy at improving body image and related outcomes amongst 11‐13‐year olds in New Delhi, India |
| Interventions |
Intervention: Dove Confident Me Dove Confident Me is a school‐based intervention co‐created by researchers at La Trobe University (Australia), the Centre for Appearance Research UWE, teachers, students, and education experts, and the Dove Self‐Esteem Project (the social mission for personal care brand Dove). The five‐session intervention (1 lesson per week for 5 weeks, 5 x 45‐minute lessons) is aimed at adolescents aged between 11‐13 years, and targets recognized risk factors for body dissatisfaction, by addressing societal appearance ideals (Session 1), media literacy (Session 2), appearance comparisons (Session 3), appearance‐related conversations and teasing (Session 4), and promoting 'body activism' (Session 5). The intervention consists of classroom‐based discussion and small group activities, and uses audiovisual materials and worksheets to facilitate learning. Control: Usual care (students receive lessons‐as‐usual) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (post‐intervention and 10‐weeks follow‐up) |
| Starting date | 1 February 2018 Completed (1 December 2019) |
| Contact information | Phillippa C Diedrich, Phillippa.Diedrichs@uwe.ac.uk |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04289272 |
NCT04307849.
| Study name | Youth‐friendly sexual and reproductive healthcare pilot in Mumbai, India |
| Methods |
Study design: RCT Country: India |
| Participants | Adolescent girls and young women (15‐25 years) (AGYW) Inclusion criteria: a. AGYW between the ages of 15‐25 years; b. provides consent or assent; c. living in the study area for one year or more; d. if unmarried and under the age of 18, have parental consent to participate. Married AGYW and/or those age 18 or over will be recruited if they meet inclusion criteria and provide informed consent. Exclusion criteria: a. AGYW who are unable to give consent due to psychological or mental limitations; b. if unmarried and under the age of 18, do not have parental consent to participate. Stated purpose: to systematically adapt, pilot test, and evaluate an integrated community/facility intervention to improve the uptake of adolescent‐friendly services for married and unmarried adolescent girls and young women (AGYW; ages 15‐25) in a low‐income area |
| Interventions |
Intervention: Adolescent health club A systematic approach for the adaptation of sexual health/HIV‐related evidence‐based interventions Control: Waiting list (the waiting‐listed group will begin the intervention after the intervention group). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Clinic service uptake/use Time points: baseline, post‐intervention (10 weeks) |
| Starting date | September 2021 (estimated study start date) |
| Contact information | Marie Brault, marie.brault@yale.edu |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04307849 |
NCT04383327.
| Study name | School‐based mental health effectiveness study |
| Methods |
Study design: cluster‐RCT Country: Uganda |
| Participants | School teachers and parents Inclusion criteria: a. for the school staff (teachers, head teachers): they must be in the recruited study schools and teaching in pre‐primary to primary 4 classrooms or holding the head teachers/administration leadership position in school. The inclusion criteria for Parent Leaders are: they must be at least 18 years old and have served as a Parent‐Teacher‐Association member or Parent Leader in the school for at least 1 year. b. for the PD/PDT programme implementers: they must have current employment with eligible partners (i.e. medical/mental health institutions, teacher training colleges), with professional experiences in teacher training or mental health training. c. for parents: caregivers must be at least 18 years old, their children must be enrolled in pre‐primary or primary 1 to 4 classes (or between 3 and 10 years old) in the recruited schools, and willing to have their child assessed by research staff. Parents and children will have diverse characteristics (e.g. randomly selected from school student lists). About 10% of families will be randomly selected from the student lists. The proposed study will be open to both men and women caregivers. Exclusion criteria: a. evidence of psychopathology or cognitive impairment severe enough to preclude giving consent, or completing the survey instruments or the focus group of the study; b. minors (aged < 18) will also be excluded. Additional criteria should be included as appropriate for the study design and risk. Stated purpose: to address EBI effectiveness and implementation knowledge gaps by providing a preventive EBI (ParentCorps‐ Professional Development; PD) that utilizes a task‐shifting and a scalable implementation model to promote early childhood students' mental health in a LMIC – Uganda |
| Interventions |
Interventions: Intervention 1: ParentCorps‐Professional Development (PD) + T‐Wellness ParentCorps‐Professional Development (PD): multi‐component school‐based intervention that promotes early childhood mental health and development. PD is a school‐based EBI and preventive mental health service provision model that supports teachers and school personnel to apply EBI strategies to promote young children's mental health. Teachers and PTAs (pre‐primary to 4th grade) will participate in a 3‐day ParentCorps‐PD training before the 1st school term. They will also receive 8 sessions (12 hours) of face‐to‐face group‐based coaching during the 1st and 2nd terms. Coaching sessions are to help teachers apply EBI strategies in their classrooms, engage families, and develop competencies. T‐Wellness: A brief teacher stress management psychoeducation package, adapted from EBIs including a half‐day workshop for common stress management and a half‐day for burnout management, and three follow‐up group support sessions (3 additional monthly 1‐hr wellness sessions for teachers as a group in each school, a total 15 hours of coaching). Intervention 2: ParentCorps‐Professional Development (PD) (only) Control: No intervention |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 months and 18 months) |
| Starting date | January 2021 (estimated study start date) |
| Contact information | Keng‐Yen Huang, Keng‐Yen.Huang@nyulangone.org |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04383327 |
NCT04542317.
| Study name | Efficacy of a dementia family caregiver support intervention in Vietnam |
| Methods |
Study design: cluster‐RCT Country: Vietnam |
| Participants | Family caregivers of persons with dementia Inclusion criteria: a. family member will need to be the identified adult (age 18 and above) primary caregiver (i.e. the person who provides the most time day‐to‐day providing care) to an older adult with dementia who is living in the community. In the event that the primary caregiver is not available to participate, an alternate family member who provides substantial care (i.e. at least 4 hours/week) to the older adult with dementia will be eligible. b. caregivers will need to score ≥ 6 on the Zarit Burden Inventory 4‐item version. All participants will be living in designated clusters in Hai Duong, Vietnam. c. clusters will have a minimum of 5 participants and a maximum of 15 participants. Clusters will be defined as geographic areas serving local health stations. Exclusion criteria: a. significant cognitive impairment or sensory deficit Stated purpose: to test the efficacy of a psychosocial intervention to support Alzheimer's family caregivers in Vietnam |
| Interventions |
Intervention: REACH VN REACH VN, a culturally adapted version of REACH VA, a multi‐component dementia family caregiver support intervention that includes psychoeducation, stress reduction, problem‐solving, and skill‐building. Participants will receive 4‐6 sessions in‐person or by phone over the course of 2‐3 months. Control: Enhanced control (a single session focused on education about the nature of dementia) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (specific time if provided) |
| Starting date | 15 October 2020 |
| Contact information | Ladson Hinton, lwhinton@ucdavis.edu |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04542317 |
NCT04693585.
| Study name | Development and pilot test of a postnatal mHealth intervention ‐ phase 2 (MESSAGE) |
| Methods |
Study design: RCT Country: India |
| Participants | Women in postpartum Inclusion criteria: a. postnatal (within 2 weeks); b. 18+ years old. Exclusion criteria: a. women below 18 years of age; b. women with high‐risk pregnancies; c. women who delivered preterm, suffer severe maternal complications or they or their baby are otherwise sick in the first week. Stated purpose: to test the optimized intervention to understand the feasibility, acceptability and preliminary effectiveness of several intervention modalities amongst postnatal women in rural India |
| Interventions |
Interventions: Intervention 1: MESSSSAGE ‐ live Real‐time live voice call (mHealth education and social support intervention) plus standard of postnatal care Intervention 2: MESSSSAGE ‐ asynchronous Text‐based, asynchronous, on‐demand social support (mHealth education and social support intervention) plus standard of postnatal care Intervention 3: MESSSSAGE ‐ both live and asynchronous support Real‐time live voice call plus standard of postnatal care; text‐based, asynchronous, on‐ demand social support plus standard of postnatal care Control: Usual care (standard postnatal care: 3 visits in first 7 days) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 weeks, 3 months, 6 months) |
| Starting date | 30 July 2021 |
| Contact information | Alison M El Ayadi, alison.elayadi@ucsf.edu |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT04693585 |
NCT04723277.
| Study name | Efficacy of teacher‐delivered child mental healthcare in primary schools of India (TeaLeaF) |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Children in rural primary schools (class I‐IV) Inclusion criteria: Schools: a. does not receive government aid (i.e. not subject to the rules and regulations of government or government‐aided schools; b. at least 3 full‐time classroom teachers on staff; c. annual student fees $180/11,500 Indian rupee (INR) or less. Teachers: a. employed at a participating school; b. primary teaching responsibility in the primary grade level; c. 18 years or older. Children: a. enrolled in class I‐IV; b. enrolled in the classroom of a participating teacher. Exclusion criteria: Schools: a. not located in the rural Darjeeling Himalayas (defined as the Mirik, Kurseong, and Darjeeling Sadar subdivisions of the Darjeeling District and outside the statutory towns of Darjeeling, Kurseon, and Mirik) Teachers: a. have been convicted and/or are under investigation for any child‐related misconduct or maltreatment Children: a. do not have a parent or guardian who can provide informed consent Stated purpose: to evaluate the efficacy of teacher‐delivered transdiagnostic mental healthcare for children in rural primary schools of India. Implementation process and context will also be examined. |
| Interventions |
Intervention: Tealeaf: Mansik Swastha Tealeaf is a task‐shifting intervention in which teachers deliver transdiagnostic mental health care. Mental health challenges are understood through basic functional behaviour assessments, providing a framework for the analysis of observable behaviours. Teachers deliver care primarily through the incorporation of basic therapeutic interactions into classroom instruction time, supplemented by one‐on‐one interactions with the child and family. Control: Enhanced Usual Care (EUC) is a less intensive version of the Tealeaf intervention. The EUC service package has been designed to be the most intensive form of care that could be envisioned as viable in the study setting in the foreseeable future without a significant increase in resource investment. |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (8 months from baseline) |
| Starting date | 1 January 2019 |
| Contact information | Christina Cruz |
| Notes |
Source of funding: not specified (only list of Sponsors and Collaborators) Prospective trial registration number: NCT04723277 |
NCT04782882.
| Study name | The effect of progressive muscle relaxation and laughter therapy on women undergoing in vitro fertilization |
| Methods |
Study design: RCT Country: Turkey |
| Participants | Women undergoing in vitro fertilization Inclusion criteria: a. women between the ages of 20 and 46; b. literate in Turkish language; c. diagnosed with infertility by an obstetrician or medical doctor; d. primary or secondary infertility. Exclusion criteria: a. diagnosed psychiatric disorder; b. prior experience in relaxation intervention; c. lack of ovarian response in previous treatments; d. undergoing frozen embryo transfer. Stated purpose: to evaluate the effect of progressive muscle relaxation exercises and laughter therapy on the mental health and treatment outcomes of women receiving IVF treatment |
| Interventions |
Intervention: Progressive muscle relaxation + laughter therapy Laughter therapy: laughter therapy is a technique that changes the mood by breathing correctly and by combining childish exercises with laughter exercises to turn into real laughter for no reason. Laughter therapy was applied for 15‐20 min. Progressive muscle relaxation procedure: the participants were asked to tighten and relax large muscle groups in the body with deep breathing exercises. Progressive muscle relaxation was applied to the muscles of the head and face, neck, chest, back, arms, hands, abdomen, hips, legs and feet. The procedure was applied in a dark environment and accompanied by candlelight and relaxing music. Progressive muscle relaxation exercises were performed for 15‐20 min under candlelight and accompanied by music. The procedures were received as a group (2‐6 people) in 3‐4 face‐to‐face. Control: Usual care |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention, within 15 days) |
| Starting date | 3 August 2020 Completed (29 December 2020) |
| Contact information | Sibel Kıyak |
| Notes |
Source of funding: not specified (Necmettin Erbakan University as sponsor) Prospective trial registration number: NCT04782882 |
NCT04819711.
| Study name | Addressing perinatal depression in deprived areas of Istanbul, Turkey |
| Methods |
Study design: RCT Country: Turkey |
| Participants | Pregnant women between 12‐30 weeks' gestation Inclusion criteria: a. pregnant women over 18 years between 12‐30 weeks' gestation; b. access to the internet: c. intend to attend all 5 sessions of the antenatal classes. Exclusion criteria: a. currently receiving any form of counselling or mental health care; b. report suicidal ideation (women); c. if miscarriage, stillbirth or preterm birth, will also be excluded from the follow‐up assessment. Stated purpose: to pilot this adapted on‐line group intervention in selected hospitals' pregnancy schools |
| Interventions |
Intervention: Thinking Healthy Programme The Thinking Healthy Programme (THP) is an evidence‐based intervention that is based on principles of cognitive behavioural therapy. It includes strategies that incorporate behavioural activation, active listening, collaboration with the family, guided discovery, and homework. The adapted THP consists of 5 integrated sessions. Session 1 introduces the programme and focuses on engagement of participants, introduction of THP, and breathing exercises. Session 2 focuses on psychoeducation and problem‐solving. Session 3 focuses on the mother's well‐being and introduces activities such as physical exercises, good sleep practices and ensuring a balanced diet. Session 4 focuses on the mother's relationship with the unborn baby. Session 5 focuses on activating social support from family, friends and peers and provides closure of therapy. Control: Usual care (antenatal pregnancy school classes: 5 sessions of the routine group antenatal pregnancy school classes. The class provides education about pregnancy, birth and newborn care and offers support to women. The women will also be able to access all usual care and support offered by the participating hospitals) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4‐6 weeks after intervention) |
| Starting date | April 2021 |
| Contact information | Perran Boran, drperran@yahoo.com |
| Notes |
Source of funding: not specified (only list of sponsors and collaborators) Prospective trial registration number: NCT04819711 |
NCT04890665.
| Study name | Online multi‐component psychological intervention for healthcare workers during COVID‐19 pandemic |
| Methods |
Study design: RCT Country: México |
| Participants | Healthcare workers Inclusion criteria: a. access to a communication device with access to the internet (computer, tablet, and mobile); b. a valid email address; c. basic digital skills in the use of an operational system and internet browsing; d. understand Spanish since all the contents are in this language; e. symptoms of anxiety, depression, burnout, and fatigue compassion. Exclusion criteria: a. diagnosis of psychotic disorder; b. receiving psychological and/or pharmacological treatment during the study; c. moderate‐to‐high score on the suicide scale; d. recent attempt of suicide (3 months); e. refuse to accept participation. Stated purpose: to carry out a randomized clinical trial with healthcare workers in Mexico through a web platform |
| Interventions |
Intervention: Online psychological intervention for healthcare workers The intervention is based on Cognitive Behavioural Therapy, Mindfulness, Behavioural Activation Therapy, Acceptance and Commitment Therapy and Positive Psychology, aimed at psychoeducation, regarding the manifestations of anxiety, depression, burnout, fatigue compassion, post‐traumatic stress disorder, and affectations in sleep quality and perception of life quality in healthcare workers. The participants will have the option to do 3 extra modules that are complimentary for the intervention and that, according to the scientific literature, could affect the mental health of healthcare workers related to how to deliver bad health news, psychological first aid and how beliefs could influence physical and emotional self‐care in the face of the COVID‐19 pandemic. Control: Other (The participants in this group will receive exactly the same intervention but delivered through a therapist in a weekly session through an online video call, through Zoom, Skype, or Microsoft Teams. The participants will be informed also about the 3 extra modules and briefly what are the contents of these modules so they can accept or not receive these extra contents). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: no clear specification for funding Prospective trial registration number: NCT04890665 |
NCT04949061.
| Study name | The effectiveness of culturally adapted cognitive behavioral intervention among COVID‐19 survivors |
| Methods |
Study design: RCT Country: Turkey |
| Participants | Individuals infected with and recovered from coronavirus disease (COVID‐19) Inclusion criteria: a. 18 years or above; b. infected with COVID‐19 and currently recovered; c. scoring 16 or above on Kessler Psychological Distress Scale (K10). Exclusion criteria: a. imminent suicidal risk; b. severe psychiatric disorder (psychotic disorders, acute mania, substance/alcohol addiction, cluster B personality disorders). Stated purpose: to implement culturally adapted cognitive behavioural intervention (CA‐CBI) to COVID‐19 survivors and evaluate the effectiveness of the intervention in reducing the psychological distress for this particular group |
| Interventions |
Intervention: Culturally adapted cognitive behavioural intervention (CA‐CBI) CA‐CBI is an intervention based on culturally adapted cognitive behavioural therapy (CA‐CBT) which was developed by Devon Hinton. This transdiagnostic intervention has a structured manual which can be culturally adapted and will be used to decrease psychological distress and increase quality of life by targeting cognitive and behavioural changes. The experimental group will receive an 8‐session CA‐CBI in an online group format. Control: Enhanced Treatment‐as‐Usual, ETA‐U (the control group will receive the information about freely available psychological support options. Also, they will receive brief psychoeducation about the mental health problems and psychological distress via online leaflets. After all the measurements are completed, the control group will be able to receive the CA‐CBI). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (one week and five weeks after the intervention) |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT04949061 |
NCT04958707.
| Study name | Evaluating the feasibility of a mobile phone‐based intervention on depression, anxiety, and stress of the dementia patients' caregivers |
| Methods |
Study design: RCT Country: Vietnam |
| Participants | Caregivers of patients with dementia Inclusion criteria: a. primary caregivers of patients with dementia for at least the past 6 months and will continue to be for the next 6 months of the intervention. Dementia patients are those who have been diagnosed with dementia for at least 6 months and are living in the community. b. able to read and understand Vietnamese (at least primary education), and willing to participate in the study; c. having a smartphone that has the Zalo app or willing to have this Zalo app be installed (there will be short training of using Zalo for new users). Exclusion criteria: a. any acute diseases or cognitive impairment (screening by Mini‐Cog); b. vision or hearing impairment. Stated purpose: (1) understanding the information and skills required by informal carers to populate the content of the intervention and (2) test the clinical feasibility of the intervention and feasibility of a fully‐power randomized controlled trial |
| Interventions |
Intervention: Informational support group The participants in the intervention group will be added to a chat group in the Zalo app, named the Dementia Caregiver Support group. Weekly, the investigator will post one of the eight topics identified in phase 1. The posted information will be based on evidence‐based resources and consulted by geriatricians, the neurologist, and the psychologist who specialized in dementia. Immediately after posting the topic, one investigator will call the participants to ensure they read and understand the post. The chat group monitor will also collect the questions or comments from the carers who are encouraged to share their feelings or experiences with relevant questions. Then the monitor will post the answers after consulting with the experts. Control: Usual care (participants are introduced to the website Alzheimer.org to search for eligible information. They will be interviewed by a questionnaire at baseline, post‐intervention and 3 months post‐ intervention) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (9 months) |
| Starting date | 1 September 2021 |
| Contact information | Nguyen Tran To Tran |
| Notes |
Source of funding: Prospective trial registration number: NCT04958707 |
NCT05053178.
| Study name | The effect of mindfulness‐based mandala activity on anxiety and spiritual well‐being levels of senior nursing students |
| Methods |
Study design: RCT Country: Turkey |
| Participants | Senior nursing students Inclusion criteria: a. registering the theoretical and practical courses within the scope of Nursing Vocational Courses Application II; b. with internet access; c. agree to participate in the study. Exclusion criteria: a. receiving psychiatric treatment (pharmacological and/or psychotherapy); b. substance addiction; c. participated in any meditation‐based therapy programme before. Stated purpose: to determine the effect of mindfulness‐based mandala activity on the spiritual well‐being and anxiety levels of senior nursing students in a parallel‐group pretest‐post‐test randomized controlled study design |
| Interventions |
Intervention: Mindfulness‐based mandala activity Mindfulness‐based mandala activity was applied to the intervention group via the Zoom online program. Students will be divided into groups of 6‐10 and mandala activities will be carried out. With three weeks of mandala activity, breathing exercises, affirmations, etc., a therapeutic application programme was created and implemented. Control: Usual care (standard support programme given by the school administration and course instructors will be applied for clinical problems. At the end of the study, it is planned to apply mandala activities amongst the students in the control group). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 weeks after) |
| Starting date | 10 May 2021 Completed trial (30 June 2021) |
| Contact information | Çiğdem Sarı Öztürk |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT05053178 |
NCT05130944.
| Study name | Feasibility of community psychosocial intervention for women |
| Methods |
Study design: cluster‐RCT Country: Ecuador and Panamá |
| Participants | Migrant and host community women Inclusion criteria: a. adult (18+ years) women residing in the study community who speak and understand Spanish; b. displaced or host community members. Exclusion criteria: a. severe psychological distress (Kessler‐6 ≥ 13); b. moderate or high risk of suicide; c. cognitive impairment. Stated purpose: 1) Explore the relevance, acceptability, and feasibility of integrating a stress management intervention into community‐based participatory women's group. 2) Examine the feasibility of conducting a fully‐powered cluster‐randomized controlled trial evaluating the effectiveness and implementation of integrating a stress management intervention into a community‐based participatory women's group as compared to community‐based participatory women's groups alone |
| Interventions |
Intervention: Entre Nosotras (group psychosocial intervention) + stress management intervention Entre Nosotras is a community‐and strengths‐based intervention designed to mobilize social support, strengthen community connectedness, and stimulate collective action to promote the safety and well‐being of women. A series of five 2‐hour group sessions that are administered weekly by two trained female peer facilitators, who are selected by HIAS outreach workers and/or community leaders. The content of the sessions is based on the principles of Psychological First Aid and the Participatory Action Cycle, which is intended to generate community‐led problem‐solving around priority issues affecting their well‐being. Doing What Matters in Times of Stress: The stress management components are derived from the World Health Organization Doing What Matters in Times of Stress illustrated guide for coping with adversity (World Health Organization, 2020). The illustrated guide and accompanying audio files are publicly available: https://www.who.int/publications/i/item/9789240003927. During five sessions, the intervention involves 15‐20 minute exercises covering a range of stress management and coping skills. Control: Active control: Entre Nosostras (group psychosocial intervention only) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (5 weeks post‐intervention) |
| Starting date | 9 September 2021 |
| Contact information | Claire Greene, mg4069@cumc.columbia.edu |
| Notes |
Source of funding: not specified Prospective trial registration number: NCT05130944 |
Newman 2021.
| Study name | An eHealth intervention for promoting COVID‐19 knowledge and protective behaviors and reducing pandemic distress among sexual and gender minorities: protocol for a randomized controlled trial (#SafeHandsSafeHearts) |
| Methods |
Study design: RCT Country: Thailand and India |
| Participants | LGBT+ people Inclusion criteria: a. aged 18 years and older; b. self‐identify as LGBT+ using local, culturally appropriate self‐identifications; c. reside in one of the two cities (Bangkok and Mumbai); d. able to understand and willing to provide informed consent; e. able to understand primary language(s) at the site (Thai, Hindi/Marathi, or English). Exclusion criteria: a. not use exclusion criteria based on mental health. The Patient Health Questionnaire‐2 (PHQ‐2) will be administered in the baseline survey; those with scores indicative of clinical depression (≥ 3 on the depression scale) will be referred by peer counsellors to in‐house mental health professionals on call at the site. Stated purpose: to evaluate the effectiveness of a brief, peer‐delivered eHealth intervention to increase COVID‐19 knowledge and public health–recommended protective behaviours, and reduce psychological distress amongst LGBT+ people residing in Bangkok, Thailand, and Mumbai, India. |
| Interventions |
Intervention: eHealth intervention 3‐session, peer counsellor–delivered eHealth intervention based on motivational interviewing (MI) and psychoeducation (45 minutes to 1‐hour weekly individual sessions). MI is a client‐centred counselling approach that elicits and strengthens intrinsic motivation for change; psychoeducation integrates education and counselling to promote mental health. Control: Waiting list (the waiting‐list control group will receive brief reminders by mobile phone to support retention. Governments and public health ministries in India and Thailand provide almost daily briefings about COVID‐19 via multiple sources: TV, newspapers, Facebook, WhatsApp, LineChat, Instagram, and SMS text messages. Online messenger platforms (e.g. WhatsApp, Line) provide LGBT‐targeted information, with additional government mobile apps developed for general populations.) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (2 weeks after post‐intervention and two months follow‐up) |
| Starting date | 1 August 2021 (start recruitment date) |
| Contact information | Peter A Newman, p.newman@utoronto.ca |
| Notes |
Source of funding: not reported Prospective trial registration number: NCT04870723 |
PACTR202006601935462.
| Study name | Promoting adherence to anti‐retroviral therapy and viral suppression among HIV positive young people in Uganda through group support psychotherapy: study protocol for a pilot randomized controlled trial |
| Methods |
Study design: RCT Country: Uganda |
| Participants | Dyads of HIV seropositive young persons (10‐18 years) and a caregiver Inclusion criteria: a. participant dyads must consist of an HIV seropositive young person (10‐18 years) with ≥ 1000 viral copies/mL 6 months after initiating first‐line ART and a caregiver aged 19 years and older. Exclusion criteria: a. participant dyads will be excluded if they have visual or hearing impairments, active untreated major mental illness (untreated psychosis or mania or high suicide risk) or severe medical conditions (active tuberculosis or pneumonia) that would interfere with participation in interventions. Stated purpose: to assess the feasibility, acceptability and preliminary effectiveness and cost‐effectiveness of GSP in promoting ART adherence and viral suppression amongst HIV‐positive young people with non‐viral suppression 6 months after initiating first‐line ART at the Kitgum Hospital HIV clinic (for pilot randomized controlled trial) |
| Interventions |
Intervention: Group support psychotherapy 8 weekly sessions of group support psychotherapy. GSP sessions for caregivers will proceed as previously described in the SEEK‐GSP trial. GSP sessions for the young people will follow the same format but with a focus on challenges faced by young people living with HIV. Besides being gender‐specific, they will also be age‐specific with participants being grouped into the following age categories: 13‐14; 15‐16 and 17‐18). Control: Usual care: intensive adherence counselling (4 individual adherence counselling sessions of intensive adherence counselling (IAC) for 3–6 months and repeat VL testing before switching to second‐line therapy) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness Time points: baseline, post‐intervention (6 months and 12 months) |
| Starting date | 1 August 2020 |
| Contact information | Etheldreda Nakimuli Mpungu, ethelmpungu@gmail.com |
| Notes |
Source of funding: CRI Foundation Prospective trial registration number: PACTR202006601935462 |
PACTR202101590919131.
| Study name | Impact of brief psychoeducation intervention on caregiver burden of caregivers of children and adolescents with mental retardation attending the outpatient clinic in the Federal Neuropsychiatric Hospital Benin city, Nigeria |
| Methods |
Study design: RCT Country: Nigeria |
| Participants | Caregivers of children and adolescents with mental retardation Inclusion criteria: a. family caregiver who is at least 18 years old and offers at least 6 hours of care daily; b. caregivers who give their consent to participate in the study. Exclusion criteria: a. caregiver who is paid to care for the patient; b. caregiver who is too ill to participate in the study (chronic/acute physical or psychological illness). Stated purpose: to determine the prevalence and the sociodemographic correlates of burden as well as the impact of a brief psychoeducation intervention on caregiver burden amongst family caregivers of children and adolescents with mental retardation, receiving outpatient care at the Federal Neuropsychiatric Hospital (FNPH) Benin City. |
| Interventions |
Intervention: Brief psychoeducation Received both routine care (comprised pharmacological and psychosocial management of clinic attendees and supportive counselling for clinic attendees and caregivers) and a brief psychoeducation intervention consist of a 45‐minute group session every week for a month. The intervention had four parts: week 1. Introduction and education on mental retardation, week 2. Skills to improve coexistence in the family, week 3. Relative’s self‐care, week 4. Feedback and evaluation. Apart from the first session, subsequent sessions began with a recap of the previous session and ended with a summary of the session for that day. Control: Usual care (routine care comprised pharmacological and psychosocial management of clinic attendees and supportive counselling for clinic attendees and caregivers) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4th week and 8th week) |
| Starting date | 2 September 2019 Completed (20 April 2020 last follow‐up date) |
| Contact information | Atim Archibong, eka_56@yahoo.com |
| Notes |
Source of funding: Atim Archibong Prospective trial registration number: PACTR202101590919131 |
Pallitto 2016.
| Study name | Testing a counselling intervention in antenatal care for women experiencing partner violence |
| Methods |
Study design: RCT Country: South Africa |
| Participants | Pregnant women Inclusion criteria: a. pregnant women who are at least 18 years old and less than 33 weeks gestation (to allow enough time for the second intervention session); b. able to communicate in one of the most common local languages (English, Sotho, or Zulu); c. reporting that they have experienced physical or sexual violence by their current or most recent partner in the past 12 months. Exclusion criteria: a. reported risk of imminent lethal violence by a partner; b. fear that a child in the home is at immediate risk of lethal violence by the partner; c. at suicidal risk (as determined by having ideation with a plan to commit suicide). Stated purpose: 1) to test a nurse‐led counselling intervention for abused pregnant women receiving antenatal care in South Africa that could be incorporated into the routine antenatal care package; 2) To determine whether the counselling intervention being tested during pregnancy can reduce the recurrence of intimate partner violence and the frequency and severity of this violence; 3) To determine whether the counselling intervention being tested is effective in improving safety, empowerment, mental health, and help‐ and health‐seeking behaviour of abused pregnant women; 4) To assess whether, at baseline, women experiencing intimate partner violence differ from women who do not experience intimate partner violence in regard to mental health, self‐efficacy, and HIV risk behaviours; 5) To assess whether HIV‐positive women who have experienced intimate partner violence differ from HIV‐positive women who have not experienced intimate partner violence with regard to PMTCT uptake and adherence as measured at follow‐up |
| Interventions |
Intervention: Safe & Sound intervention The Safe & Sound intervention is based on a nurse‐led "empowerment counselling model"; and it consists of two 30‐min counselling sessions. This model promoted by Dutton includes two complementary components – (a) improving women's safety and protection, including negotiating safer sex with a partner, while (b) enhancing her decision‐making and problem‐solving ability in her relationship. Safe & Sound covers a combination of the following elements that will be individually tailored depending on women’s experience and her readiness to change: empathetic listening, cyclical nature of partner violence, evaluating danger and discussing option, developing safety strategies, pregnancy changes, legal steps, available resources. Control: Enhanced usual care (a referral list to local resources) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 weeks postpartum) |
| Starting date | 1 March 2014 Completed (29 July 2016) |
| Contact information | Christina Pallitto, pallittoc@who.int |
| Notes |
Source of funding: grant from Flanders International Cooperation Agency, WHO Initiative for Health Pregnancy in Southern Africa, 2010–2016, and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Re‐ search, Development and Research Training in Human Reproduction (HRP) Prospective trial registration number: ISRCTN35969343 and DOH‐27‐0414‐4720 |
Pathare 2020.
| Study name | Evaluation of the SPIRIT integrated suicide prevention programme |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Adolescent students (14‐16 years) and adult community members Inclusion criteria: For primary sampling units (villages): a. Village Council has agreed to participate in the study; b. there is a high school in the village with more than 35 students and the school has agreed to participate in the study; c. total population of the village is ≤ 6000 people (the population size was determined considering the distance travelled by villagers to access the central storage facility (CSF)); d. primary occupation of the villagers is farming or agriculture‐related work involving use of pesticides. For secondary sampling units (participants): a. adolescents in grade 9 in public high schools (14–16 years of age) and adult community members (18 years and older) residing in rural villages in Mehsana district Exclusion criteria: not specified Stated purpose: to evaluate the public health impact of an integrated suicide prevention programme implemented in 62 villages and compared to 62 control villages in Mehsana district of Gujarat, India, by evaluating: 1) the reach and adoption of the SPIRIT interventions in the target villages; 2) the effectiveness of the prevention programme in reducing suicide rates and suicidal behaviours in target populations; 3) the economic costs of delivering the suicide prevention programme. |
| Interventions |
Interventions: Intervention 1: school‐based intervention (Youth Aware of Mental health (YAM)) The school‐based intervention consists of a universal mental‐health promotion programme in schools within intervention villages aimed at preventing depression, reducing suicidal ideation, and promoting mental health amongst students in grade 9 who are between 14 and 16 years of age. The school‐based suicide prevention programme consists of a locally adapted version of the Youth Aware of Mental Health Programme (YAM). YAM is an interactive programme for adolescents delivered within a teacher‐free space, aiming to promote discussion and increase knowledge about mental health and the development of problem‐solving skills and emotional intelligence. YAM is a manualized 5‐h programme broken down into 3 h of role‐play sessions and 2 h of interactive lectures and discussions about mental health at the beginning and end of the intervention. In addition, students receive a booklet on mental health issues and strategies to deal with difficult life events. Intervention 2: community storage of pesticides This intervention consists of setting‐up community storage boxes placed in the Village Council office premises or at a central location. Each farming household is offered a locker at this facility free of charge and the family is encouraged to store all pesticides in this box. A trained attendant (facility manager) will be stationed at the community storage facility and will document use of the boxes on a day‐to‐day basis. Intervention 3: community health worker (CHW) training in identification, support and referral of persons with suicidal risk and behaviour This intervention consists of training and a follow‐up programme for CHWs in the intervention villages to identify people at risk of self‐harm and suicide and to support and refer such persons to appropriate local services. The training is based on the World Health Organization Mental Health Action Programme (mhGAP), Self‐harm/Suicide module. The training module includes information on how to identify and act in cases of persons at risk of self‐harm and suicide, when and to whom to refer, and type of psychosocial support to provide at different stages. Control: Usual care (not receive any intervention to prevent suicide other than enhanced usual care, which involves provision of brochures with information on available mental health services and other resources for seeking help, such as emergency helpline numbers and contact details of public and non‐governmental healthcare services) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Intervention costs (costs of implementation and intervention, and the intervention impact) Time points: baseline, post‐intervention (3 months and 12 months follow‐up) |
| Starting date | 1 August 2018 (first enrolment) |
| Contact information | Soumitra Pathare, spathare@cmhlp.org |
| Notes |
Source of funding: National Institutes of Mental Health (NIMH) through grant number 5U19MH113174–03 REVISED awarded to SP, LV and LSZ (PIs) Prospective trial registration number: CTRI/2017/04/008313 |
Paula 2021.
| Study name | Brief interventions for older adults (BIO) delivered by non‐specialist community health workers to reduce at‐risk drinking in primary care: a study protocol for a randomised controlled trial |
| Methods |
Study design: RCT Country: Brazil |
| Participants | Older individuals (≥ 60 years) considered at‐risk drinkers Inclusion criteria: a. 60 years or older; b. registered at PCUs in São José dos Campos; c. identified as at‐risk drinkers based on Alcohol Use Disorders Identification Test‐Consumption (AUDIT‐C) score ≥ 4. Exclusion criteria: a. received previous treatment for substance use disorders, except tobacco, in the last 90 days; b. severe mental or physical illness that may impair the acquisition of alcohol consumption data; c. requiring hospitalisation; d. unable to communicate clearly and/or who are intoxicated at the time of screening. Stated purpose: to address two main research questions: 1) What is the efficacy of BIO compared with usual care (waiting list) for reducing alcohol consumption in primary care? 2) What are the effects of BIO on physical activity, cognition, quality of life (QoL) and depression? |
| Interventions |
Intervention: Brief Intervention for Older adults (BIO) The BIO is a technique with a duration 10–15 min that is designed to change behaviour and includes seven components: (1) feedback; (2) identification; (3) information; (4) reflection; (5) normative; (6) construction; (7) booklet. The intervention will be delivered by CHWs, who will receive specific training in the methodology. This BIO was based on the intervention ‘Brief Advice’ (https://www.sips.iop.kcl.ac.uk) and adapted for the older adult and the Brazilian population. The participants will receive the same usual care as the control group plus the BIO. Control: Usual care (waiting list: the participants allocated to this group will receive usual care during the period of study. For this group, we will offer the intervention immediately after the 6‐month follow‐up.) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Use of health services Time points: baseline, post‐intervention (6 months) |
| Starting date | January 2022 |
| Contact information | Tassiane Cristine Santos Paula, tassiane.psi@gmail.com |
| Notes |
Source of funding: grant from the FAPESP (São Paulo Research Foundation) Thematic Project: ref. number 2015/19472‐5 Prospective trial registration number: RBR‐8rcxkk |
Rath 2020.
| Study name | Community youth teams facilitating participatory adolescent groups, youth leadership activities and livelihood promotion to improve school attendance, dietary diversity and mental health among adolescent girls in rural eastern India |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Adolescent girls (and boys) aged 10–19 years Inclusion criteria: a. adolescent girls aged 10–19 years living in the 38 study clusters during the baseline and/or endline surveys are eligible to participate in study interviews. b. adolescent boys and girls, aged 10–19 years, within the study area (whether living there or not). Exclusion criteria: a. girls who decline to be interviewed or who are living outside the study clusters Note: Although, for financial and logistical reasons, our trial outcomes relate only to girls, we decided to include both boys and girls in the intervention because the intervention activities were relevant and potentially beneficial to boys and because some health‐related problems [... ] may be more effectively addressed by engaging with both boys and girls. Stated purpose: to assess whether an intervention involving a community youth team facilitating participatory peer‐led adolescent groups, youth leadership activities and livelihood promotion can improve school attendance, nutrition and mental health amongst adolescent girls in rural India |
| Interventions |
Intervention: Jharkhand Initiative for Adolescent Health (JIAH) The intervention is a community youth team that delivers participatory adolescent groups, youth leadership activities, and livelihood promotion. Each cluster has a community youth team delivering parallel intervention activities. The team comprises a peer facilitator (yuva saathi, meaning “friend of youth”) aged 20–25 years, a youth leadership facilitator and a livelihood promoter. Activities are open to all girls and boys in the community and include sports events such as football tournaments, archery, and run‐a‐thons, as well as problem‐solving sessions and nature walks. Both intervention and control clusters have livelihood promoters; livelihood promotion activities aim to provide adolescents with practical skills which they can use in later life and that improve food security for families. In each cluster, yuva saathis facilitate meetings which are mainly held in community meeting spaces. The first five meetings aim to introduce adolescents to the intervention. After the first five meetings, the groups work through four consecutive Participatory Learning and Action (PLA) cycles. Each PLA cycle comprises five to seven meetings and has four distinct phases: (1) identifying problems affecting adolescents in the community (meeting 1), (2) identifying and deciding on strategies to address these problems (meetings 2–3), (3) implementing the strategies (meetings 4–6), and (4) evaluating the process (meeting 7). Control: Waiting list, usual care, etc. |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention) |
| Starting date | 1 December 2015 (recruitment start date: 1 March 2016) |
| Contact information | Kelly Rose‐Clarke, kelly.rose‐clarke@kcl.ac.uk |
| Notes |
Source of funding: programme grant from the Children’s Investment Fund Foundation to Ekjut and University College London Prospective trial registration number: ISRCTN17206016 |
Rotheram‐Borus 2017.
| Study name | To evaluate if increased supervision and support of South African government health workers’ home visits improves maternal and child outcomes |
| Methods |
Study design: cluster‐RCT Country: South Africa |
| Participants | Pregnant women Inclusion criteria: a. living in the catchment area; b. not identified as psychotic or delusional based on the interviewer’s judgement. Exclusion criteria: a. inability to give informed consent; b. inability to converse with the interviewer or the CHW; c. death of the mother or infant. Stated purpose: to examine whether the benefits of ongoing accountability and supervision within an existing government funded and implemented community health workers (CHW) home visiting programme ensure the effectiveness of home visiting |
| Interventions |
Intervention: Accountable Care Condition (AC) In the Accountable Care Condition, additional monitoring and accountability systems that Philani routinely uses are implemented. All CHWs will receive a mobile phone and initial Philani training for conducting home visits. The CHWs’ implementation will be consistently monitored and an accountability system will be established. CHWs in the AC will log their home visits on their mobile phones, including a rating of the content and skills addressed on the visit, children’s height and weight, and report on achievement of outcomes such as receiving the child grant, immunizations, breastfeeding, and retention and adherence to HIV care. Supervision will facilitate CHW skill improvement over time. In addition to gathering real‐time data on the health of the household, the mobile phone monitoring system automatically lists all follow‐up visits needed for each week. Control: Usual care (Standard Care Condition of initial Philani training, but with supervision and monitoring being delivered by local government structures and systems. The CHW will visit the mothers twice monthly during pregnancy until children are 6 months and then monthly until the children reach 2 years of age. After the first 6 months, the CHW focuses on encouraging mothers to stimulate and support their children daily. Households in the target areas will be visited by government‐funded CHWs. The existing government‐implemented training, monitoring, and supervision structures will remain in place with the only addition being an initial Philani training for conducting home visits) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (3 months, 6 months, 15 months and 24 months postpartum) |
| Starting date | 1 June 2017 |
| Contact information | Mary Jane Rotheram‐Borus, mrotheram@mednet.ucla.edu |
| Notes |
Source of funding: National Institute of Mental Health (NIMH; R01MH111391), the Center for HIV Identification, Prevention and Treatment Services (CHIPTS; P30MH058107), the UCLA Center for AIDS Research (CFAR; P30AI028697), the National Center for Advancing Translational Sciences through UCLA Clinical and Translational Science Institute (CTSI; UL1TR001881), and the Postdoctoral HIV Research Training Program for HIV Combination Prevention (T32; T32MH109205) Prospective trial registration number: NCT02957799 |
Saju 2021.
| Study name | Swāsthya, an integrated chronic condition management programme for families of patients with hypertension and diabetes mellitus |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Families of patients with hypertension and diabetes mellitus Inclusion criteria: a. Eligible families will be those with at least one family member with a confirmed diagnosis of HTN and/or DM. b. Family members must be either first‐degree blood relatives or spouses of the patient. Exclusion criteria: a. bedridden and terminally ill patients Stated purpose: to improve treatment adherence and medical compliance by addressing social, behavioural, and cognitive barriers to ensure the control of chronic conditions such as HTN and DM |
| Interventions |
Intervention: Swāsthya intervention (social, behavioural, and cognitive or multiple interventions) The package comprising five separate modules on general, behavioural, social, cognitive, and multiple interventions prepared by the research team. General interventions will be delivered to all participants included in the intervention arm. Behavioural, social, and cognitive interventions will be separately administered to participants based on the group they belong to (behavioural risk, social risk and cognitive risk groups). For the fourth group, that is, the multiple risk groups, interventions will be customised based on their priority needs. All the interventions are developed targeting the entire family. Control: Usual care (patients with HTN and/or DM in the control arm will be referred to PHCs or encouraged to access their preferred health care facility) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 months and 12 months post‐baseline) |
| Starting date | 1 February 2021 (recruitment start date) |
| Contact information | M D Saju, saju@rajagiri.edu or sajumadavan@gmail.com |
| Notes |
Source of funding: Rajagiri College of Social Sciences (Autonomous) and IMPRESS, ICSSR under the grant number IMPRESS/P798/2045/2018‐19/ICSSR Prospective trial registration number: CTRI/2020/12/029474 |
Sam‐Agudu 2017.
| Study name | Adolescent Coordinated Transition (ACT) to improve health outcomes among young people living with HIV in Nigeria |
| Methods |
Study design: cluster‐RCT Country: Nigeria |
| Participants | Adolescents living with HIV (ALHIV), 13‐ to 17‐year‐olds Inclusion criteria: For study sites: a. amongst secondary‐ and tertiary level PEPFAR supported healthcare facilities with at least 21 months’ experience in providing comprehensive HIV care and treatment services; b. at least 20 ALHIV enrolled in care as of 31 July 1206; c. separate paediatric and adult HIV care teams and clinics; d. at least one dedicated physician and one dedicated nurse for each paediatric and adult HIV clinic. For participants: a. documented HIV infection; b. aware of HIV diagnosis; c. currently on ART. Exclusion criteria: a. medically unstable patients Stated purpose: to measure the comparative effectiveness of the ACT intervention versus usual care on post‐transfer retention in care amongst ALHIV at 21 and 24 months post‐transfer |
| Interventions |
Intervention: Adolescent Coordinated Transition (ACT) The ACT intervention is adapted and modified from the model described by Maturo 2011. ACT has three main components: 1) Paediatric Adult Pediatric Adult (PAPA) model: alternating paediatric adult visits during the 21‐month transition period that allows both adult and paediatric clinicians and the transitioning adolescents to address difficulties related to transition and adapt to the termination of the paediatric provider‐patient relationship. 2) A monthly peer‐led organized support group (OS) facilitated by trained young adults living with HIV and guided by a standardized six‐module curriculum covering HIV basics, treatment and adherence, support networks, adolescent rights, living positively, and member choice. The OSG curriculum will address topics that will enhance the adolescent's knowledge of HIV disease and how to self‐manage their medical care. 3) A case management team consisting of a physician, a nurse, and a trained patient advocate Control: Usual care (The usual “transition” of care for ALHIV in Nigeria is abrupt transfer to adult care, defined as immediate handover of the ALHIV from paediatric to adult care, without a transition period, and no formal or documented communication between paediatric and adult providers prior to, or after transfer. There is also no structured pre‐transfer education and/or counselling provided to ALHIV and their caregivers with respect to procedures and expectations in adult care. In CG clinics, ALHIV will stop accessing paediatric care and start accessing the adult HIV clinic at the routine age of transfer at the facility. Adolescents will have access to all available services at the adult clinic including any adult support groups that may be available). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (12‐months, 24‐months and 36‐months post‐baseline) |
| Starting date | 28 June 2017 |
| Contact information | Echezona E. Ezeanolue, eezeanolue@gmail.com or echezona.ezeanolue@gmail.com |
| Notes |
Source of funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD089871 to EEE and NASA Prospective trial registration number: NCT03152006 |
SLCTR/2020/015.
| Study name | Effectiveness of a mindfulness‐based intervention on physiological and psychological parameters in pregnant women in Anuradhapura district, Sri Lanka |
| Methods |
Study design: cluster‐RCT Country: Sri Lanka |
| Participants | Pregnant women Inclusion criteria: a. pregnant women of all ages; b. registered in ‘pregnant mothers register’ of public health midwives and visiting field antenatal clinics in Anuradhapura district; c. plan to reside in Anuradhapura district throughout their pregnancy; d. in their second trimester (POA/gestational age 13–28 weeks). Exclusion criteria: a. pregnant women who are planning to leave the study area prior to delivery; b. unable to read or understand spoken Sinhala language; c. history of or current psychotic disease; d. severe physical illnesses limiting mobility. Stated purpose: to test the effectiveness of a mindfulness‐based intervention on physiological and psychological parameters in pregnant women in Anuradhapura district, Sri Lanka |
| Interventions |
Intervention: Mindfulness intervention Weekly mindfulness intervention which will be carried out for a duration of 6 weeks. Each session will be 1.5 hours to 2 hours long and will be carried out in a Sinhala medium. Each session will comprise a talk on mindfulness principles, a session on simple mindful movements (Tai Chi introductory exercises) carried out in the seated position and simple meditation sessions, such as awareness of the breath, loving kindness meditation and awareness of the present moment. Control: Usual care (routine antenatal care: at the field level is the care provided to pregnant mothers by the public health staff mainly including the area medical officer of health (MOH) and the area public health midwife (PHM) according to the maternal care package guidelines given by the Family Health Bureau. The services are provided via antenatal clinics, domiciliary visits by the public health staff). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (after the 6th week of intervention) |
| Starting date | 1 June 2021 (first enrolment) |
| Contact information | Dr. Sujanthi Priyanka Wickramage, sujanthi.wickramage@gmail.com |
| Notes |
Source of funding: World Bank AHEAD project Prospective trial registration number: SLCTR/2020/015 |
SLCTR/2020/022.
| Study name | Impact of mindfulness‐based trimodal prehabilitation on functional recovery and selected surgical outcomes of patients with colorectal cancer admitted to surgical professorial unit of Colombo South Teaching Hospital Sri Lanka; a randomised control trial |
| Methods |
Study design: RCT Country: Sri Lanka |
| Participants | Colorectal cancer patients Inclusion criteria: a. male and female; b. above 18 years; c. diagnosed with colorectal cancer and awaiting surgery; d. fit enough to perform planned physical exercise. Exclusion criteria: a. awaiting palliative colorectal surgery; b. undergoing emergency colorectal surgery; c. not fit enough to perform planned physical activities; d. unable to perform the planned physical exercise (e.g.amputees, comorbidities which prevent planned exercise); e. already participating in mindfulness, yoga, or exercises for more than 30 minutes per day. Stated purpose: to study what impact would mindfulness have on functional recovery, nutritional status, biochemical markers and psychological markers of patients with colorectal cancer receiving trimodal prehabilitation admitted to Surgical Professorial unit of Colombo South Teaching Hospital Sri Lanka |
| Interventions |
Intervention: Mindfulness‐based trimodal prehabilitation programme The multidisciplinary mindfulness‐based trimodal prehabilitation programme is composed of four elements: 1. exercise programme, 2. nutritional intervention, 3. psychological coping, 4. mindfulness protocol. For Mindfulness Protocol: the mindfulness‐based 4‐week programme will be delivered during the prehabilitation process for the intervention group. A trained mindfulness practitioner will explain the basic concepts and significance of mindfulness practice for patient well‐being, guide patients on mindful walking and mindful sitting. An instruction CD with every session, which they can use for practice of mindfulness at home will be given. For psychological support, the intervention group will be contacted by the investigator over the telephone. The intervention will be provided for a period of four weeks only once during the study cycle. Control: Usual care (standard therapy/practice (without mindfulness): the same intervention trimodal prehabilitation (exercise programme, nutritional intervention and psychological coping) will be offered during the prehabilitation (4 weeks) with no component of mindfulness‐based procedure). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4 weeks after prehabilitation or before the surgery, 4 weeks postoperatively, 8 weeks postoperatively) |
| Starting date | 1 November 2020 |
| Contact information | Prof. Bawantha Gamage, bawantha@sjp.ac.lk |
| Notes |
Source of funding: not specified Prospective trial registration number: SLCTR/2020/022 |
Sorsdahl 2021.
| Study name | Addressing the mental health needs of adolescents in South African communities |
| Methods |
Study design: RCT Country: South Africa |
| Participants | Adolescents at risk for depression and alcohol use disorders Inclusion criteria: a. aged between 15 and 18 years old; b. rovide written informed assent/consent to participate in the study; c. written informed parental consent to participate if younger than 18 years of age; d. screen at risk for depression with a score ≥ 10 on the Center for Epidemiology Studies Depression Scale short form (CES‐D‐10) and/or screen at moderate or severe risk for alcohol‐related health problems, with a score ≥ 5 on the Alcohol, Smoking and Substance Use Involvement Test‐Youth (ASSIST‐Y); and report at least 2 episodes of heavy drinking (≥ 5 standard drinks on a single occasion) in the last month. Exclusion criteria: a. currently receiving any form of treatment for a mental or substance use disorder Stated purpose: to (i) test the feasibility and acceptability of the ASPIRE intervention as well as (ii) the feasibility of all study procedures and performance of outcome measures to inform a fully powered future trial. A second objective is to explore the initial effect of this intervention on days of heavy drinking and symptoms of depression. |
| Interventions |
Intervention: ASPIRE intervention ASPIRE intervention, a four‐session blended multi‐component counselling intervention adapted for South African adolescents at risk for depression and alcohol use disorders. Participants will receive session one as the control group (screening for heavy alcohol use and depression, feedback, psychoeducation and motivational interviewing), plus an additional three sessions of a blended multi‐component counselling intervention. The intervention is largely based on Lazarus and Folkman’s coping theory, teaches problem‐focused coping skills for mutable problems and emotion‐focused coping strategies (acceptance and seeking support) for immutable problems. All sessions have a motivational component, a psychoeducation component (in which participants are taught problem‐solving skills and how to apply them) and include an opportunity to apply newly learned skills through exercises and homework. From enrolment, participants in the intervention arm will have 6 weeks to receive all four sessions of the intervention. Each counselling session should be spaced at least 5 days apart, with the first session occurring immediately after the baseline assessment. The duration of each counselling session is approximately 45–60 min. Control: Active control: session 1 and referral to care (participants assigned to this arm will have received a single counselling session (~1 h in duration) prior to allocation. This is session one of the ASPIRE intervention, and the content and delivery of this session will be identical to that received by participants assigned to the intervention condition. The session will conclude with the participant setting personal goals and developing a plan for change, guided by the counsellor. Participants will also be given referrals to usual care providers for further follow‐up if required) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 weeks, and 3 months post‐randomization) |
| Starting date | 4 November 2019 (recruitment start date) Due to COVID‐19, all non‐essential community‐based research was put on hold from 17 March 2020. |
| Contact information | K. Sorsdahl, Katherine.sorsdahl@uct.ac.za |
| Notes |
Source of funding: Joint Global Health Trials Initiative with joint funding from the Medical Research Council, Wellcome Trust and United Kingdom’s National Institutes for Health Research and Department for International Development (MR/R018464/1). BM is supported by the South African Medical Research Council. Prospective trial registration number: PACTR20200352214510 |
Srivastav 2021.
| Study name | Structured, multifactorial randomized controlled intervention to investigate physical activity levels, body composition and diet in obese and overweight adolescents |
| Methods |
Study design: cluster‐RCT Country: India |
| Participants | Overweight and obese male and female adolescents aged 11–16 years Inclusion criteria: a. male and female; b. 11‐15 years; c. attending schools; d. BMI > 85th percentile on standard CDC control BMI for age growth; e. clear screening using PAR‐Q+. Exclusion criteria: a. diagnosed obesity due to metabolic, endocrine or medications; b. unable to attend weekly sessions in schools; c. diagnosed unsafe for participation by paediatrician or GP; d. involved in another structured exercise programme. Stated purpose: to compare the effectiveness of an exercise and dietary intervention that is culturally appropriate and school‐based to that of an exercise‐only intervention |
| Interventions |
Intervention: Intervention 1: multifactorial intervention (MI) The multifactorial (MI) group A will include PA, diet education for the adolescent and parent education about healthy eating and PA/exercise. A qualified dietitian and physiotherapists will develop a manual educating student and their parents about healthy diet and optimum PA for the adolescent age group. The adolescents in the MI group will be given exercise, according to ACSM guidelines, for 60 min for 2 days a week during the physical therapy (PT) classes under the supervision of a physiotherapist and physical education teacher. Participants will also be educated about the importance of regular PA and healthy eating pattern verbally in the educational session at the beginning of the intervention of the programme. Educational awareness programmes will be conducted for the parents, and after the programme. Intervention 2: exercise (EX) Group B (EX) participants will be given exercise only for 45 min during PT classes under the supervision of a physiotherapist and physical education teacher. The same procedures as for group A will be followed but with no dietary education or behaviour change advice. Control: No intervention: (group C control (CON) participants will be encouraged to continue their regular daily activities and dietary pattern. They will also be reassessed post‐study and at follow‐up points. No nutritional education will be provided to the parents in group C). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention, 3, 6 and 12 months follow‐up) |
| Starting date | 1 May 2019 (first enrolment) |
| Contact information | Prateek Srivastav, prateek.srivastav@manipal.edu |
| Notes |
Source of funding: not declared Prospective trial registration number: CTRI/2019/04/018834 |
TCTR20200601002.
| Study name | A randomized controlled trial on effectiveness of Mental Health & Psychosocial Support Module (MHPSS) in reduction of depression, anxiety and stress among healthcare workers during COVID‐19 pandemic in Jerantut, Pahang |
| Methods |
Study design: RCT Country: Malaysia |
| Participants | Healthcare workers Inclusion criteria: a. Malaysian citizen; b. HCWs working in Klinik Kesihatan Bandar Jerantut; c. on‐duty since 3 February 2020 (1st COVID case detected in Malaysia). Exclusion criteria: a. HCWs on medical/maternity/study leave; b. HCWs with underlying psychiatric problems under psychiatry follow‐up. Stated purpose: to test the effectiveness of Mental Health & Psychosocial Support Module (MHPSS) in reduction of depression, anxiety and stress amongst healthcare workers during COVID‐19 pandemic in Jerantut, Pahang |
| Interventions |
Intervention: Mental Health & Psychosocial Support Module (MHPSS) The duration of the intervention is 1 month, consists of a 15‐minute daily session that will be done at 4.45 pm to 5.00 pm daily during weekdays, 1‐hour group session from 11.00 am to 12.00 pm twice weekly and 1‐hour individual session if needed by the participants. The framework for the module includes giving information, safety, basic needs, extended services and identification of persons who are emotionally overwhelmed. The intervention focuses on relaxation technique, leisure activities, art therapy and coping skills. The contents of the module are tailored to individual participants' needs. Control: Waiting‐list and active control group (normal counselling and will be given MHPSS module after the intervention group completed the post‐intervention evaluations) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention |
| Starting date | 1 June 2020 (first enrolment) |
| Contact information | Amir Faisal, amirfaisal_30@yahoo.com |
| Notes |
Source of funding: self‐sponsored Prospective trial registration number: TCTR20200601002 |
Tran 2020.
| Study name | School‐based, two‐arm, parallel, controlled trial of a culturally adapted resilience intervention to improve adolescent mental health in Vietnam |
| Methods |
Study design: RCT Country: Vietnam |
| Participants | Adolescent students (grade 10, 15‐16 years) Inclusion criteria: a. in grade 10 (usually aged 15–16 years) studying in selected classes Exclusion criteria: a. students whose parents do not give permission for them to participate; b. students who do not wish to participate. Stated purpose: to translate, culturally verify and adapt the RAP for Vietnamese adolescents, combined with the strength‐focused psychoeducational resources for teachers (Happy House); and establish the effectiveness of the Happy House intervention in improving the mental health of adolescents in Vietnam |
| Interventions |
Intervention: Happy House Happy House will consist of materials incorporating the principles of RAP, which include workshop‐style exercises for adolescents focusing on the six core components of RAP: personal strengths, managing stress, cognitive style, problem‐solving, support networks and interpersonal relationships. Students will receive six weekly 90‐min school‐based group sessions. Students will receive text messages between the sessions (two times per week) via a social network (Zalo/Facebook messenger). The text messages will be short (under 60 characters), include graphics and remind students about key messages of each session. Control: Usual care (students and teachers will receive only the usual curriculum, which does not include any mental health programmes) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (2 weeks post‐intervention and after six months) |
| Starting date | 6 October 2020 (first enrolment) Completed (22 May 2021 last data collection) |
| Contact information | Jane Fisher, jane.fisher@monash.edu |
| Notes |
Source of funding: Australian National Health and Medical Research Council—NAFOSTED Joint Call for Collaborative Research Projects (GNT1158429) Prospective trial registration number: ACTRN12620000088943 |
Tăut 2021.
| Study name | Prevention of child mental health problems through parenting interventions in Southeastern Europe (RISE) |
| Methods |
Study design: RCT Country: North Macedonia, Republic of Moldova and Romania |
| Participants | Primary caregivers of children aged 2 to 9 years old Inclusion criteria: a. aged 18 years or older; b. responsible for the care of a child between the ages of 2 and 9; c. report at least subclinical levels of child’s behavioural problems as assessed with the oppositional defiant disorder subscale (ODD) of the Child and Adolescent Behavior Inventory (CABI, scores ≥ 10 will be included); d. spent at least four nights a week with the child in the same household during the previous month and will continue to do so; e. agree to being randomised to one of the conditions; f. consent to participate in the full study; g. adequate language skills to participate in the group/lecture, either in the primary language of the group or with additional language support provided. Exclusion criteria: a. primary caregivers whose children have been removed from their custody Stated purpose: to test the efficacy and cost‐effectiveness of an optimized version of the promising Parenting for Lifelong Health Programme for Young Children (PLH‐YC, 5 sessions), against a standard lecture on parenting issues (control group, 1 session) |
| Interventions |
Intervention: Parenting for Lifelong Health for Young Children (PLH‐YC) programme, optimized version The optimized version of PLH‐YC will be delivered over five weekly sessions using a participatory, non‐didactic approach to engage parents in learning positive parenting and child behaviour management skills. PHL‐YC programme focuses on consolidating parenting skills involved in relationship building (spending one‐to‐one time with children and emotional coaching), positive reinforcement of children’s adaptive behaviours (praising and rewarding, providing positive instructions, setting household rules, and routines) and teaching positive discipline strategies (ignoring negative attention‐seeking and unreasonable demands, time‐out, and establishing reasonable consequences for inappropriate behaviours). Programme activities include illustrated comics modelling how to implement key parenting skills, home activity assignments to apply these skills with their children, and group discussions addressing challenges experienced when applying home activities. The programme also includes simple mindfulness stress reduction exercises. Control: Active control (parents will receive a structured PowerPoint presentation on parenting and child development issues, called “Raising Healthy Children” (duration: 1 to 1.5 hours). Four topics will be covered: (1) stages of child development; (2) potential risk factors for child internalising problems; (3) resources and protective factors; (4) tips: what parents can do to promote children’s development). |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness Time points: baseline, post‐intervention (4 months and 12 months after pre‐assessment) |
| Starting date | December 2020 |
| Contact information | Diana Tăut, dianataut@psychology.ro |
| Notes |
Source of funding: European Union’s Horizon 2020 research and innovation programme under grant agreement No 779318 Prospective trial registration number: NCT04721730 |
UMIN000042807.
| Study name | Developing an adaptive intervention for the mental health and psychosocial support of health workers in the Philippines under COVID‐19 pandemic using the Sequential, Multiple Assignment, Randomized Trial (SMART) |
| Methods |
Study design: cluster‐RCT Country: Philippines |
| Participants | Healthcare workers Inclusion criteria: a. healthcare workers at the institution (from the WHO definition, healthcare workers here are everyone who works at the facility) Exclusion criteria: a. do not wish to participate in this study; b. currently undergoing treatment for mental illness. Stated purpose: to assess the mental health problems of healthcare workers responding to the COVID‐19 pandemic in the Philippines and to explore suitable online mental health and psychosocial support using Sequential Multiple Assignment Randomized Trial (SMART) |
| Interventions |
Intervention: Intervention 1: psychological counselling and stress management training (SMT) for the high‐risk group In the first intervention phase, participants are randomly assigned to either two PCs or a combination of two PCs and STM. This initial intervention phase lasts 2‐3 weeks, after which a second assessment is conducted. Those who respond will have completed the treatment. Those who do not respond will be randomly assigned to receive either two additional PC sessions or two additional sessions of PC combined with STM. The third assessment is done 6 weeks after the first assessment. Intervention 2: Psychological First Aid (PFA) and stress management training for the low‐risk group In the first intervention phase, participants will be randomized into three groups: the first group will receive PFA, the second group will receive SMT, and the third group will be the control. Two to three weeks later, a second assessment will be conducted. Those who respond will have completed the treatment; those who do not respond to both PFA and SMT will be randomly assigned to receive the treatment they did not receive or a combination of both for 2‐3 weeks. The third assessment is done 6 weeks after the first assessment. Control: No treatment (the control group will also be assessed 2‐3 weeks after the intervention group and 4‐6 weeks after the intervention group) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (6 weeks after first assessment) |
| Starting date | NA |
| Contact information | NA |
| Notes |
Source of funding: Japan Agency for Medical Research and Development (AMED) Prospective trial registration number: UMIN000042807 |
Venturo‐Conerly 2021.
| Study name | Testing the effects of the Shamiri Intervention and its components on anxiety, depression, well‐being, and academic functioning in Kenyan adolescents |
| Methods |
Study design: RCT Country: Kenya |
| Participants | Adolescent students (12‐21 years) Inclusion criteria: a. student in a participating school between the ages of 12 and 21 Exclusion criteria: a. fail attention checks during baseline measures No participant who meets these criteria will be excluded for any other reasons other than the capacity of the groups; should more students than we can have in the group be interested in participation, we will randomly select students to join the groups. Stated purpose: 1) to compare the effects of each intervention and control condition on the primary outcomes (depressive symptoms, anxiety symptoms, academic performance, and wellness); 2) to compare the effects of each group on secondary outcomes; 3) to identify for whom these interventions are most effective and their mechanisms of change |
| Interventions |
Interventions: Intervention 1: growth intervention (only) The growth mindset intervention consists of four small group (8–15 students) sessions delivered by Kenyan lay providers, lasting 1 h each. It is designed to strengthen individuals’ belief that the brain can adaptively respond to obstacles in various aspects of life (e.g. academic, interpersonal, and personality traits). The intervention includes didactics, activities, and group discussion. Intervention 2: gratitude intervention (only) The gratitude‐only intervention focuses on promoting intentionally noticing, communicating, and appreciating feelings of thankfulness. Throughout the four 1‐h small group (8–15 students) sessions, participants learn the concept of gratitude and apply the skills necessary for practising gratitude in their interpersonal relations, academics, and other aspects of life. Intervention 3: value affirmation intervention (only) The value affirmation intervention, lasting for four 1‐h sessions in a small group of 8–15 students, aims to teach the concept of noticing and living according to personal core values, thus contributing to a sense of purpose. Intervention 4: Shamiri intervention The Shamiri Intervention consists of four small group (8–15 students) sessions lasting for 4 weeks. The first two sessions focus on the concept of growth mindset and strategies for growth, the third session focuses on gratitude, and the fourth and final session focuses on value affirmation. Control: Active control: study skills (The study skills control intervention, lasting for 4 h spread across 4 weeks, is an active control intervention designed to control for all the nonspecific aspects of group psychotherapy including meeting in a group of students with a trained lay provider once a week, having discussions, completing activities in session, and completing homework assignments.) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (4‐week endpoint, and 1‐month, 3‐month, 6‐month, and 9‐month follow‐up) |
| Starting date | 1 May 2021 |
| Contact information | Katherine E. Venturo‐Conerly, katherinevc16@gmail.com |
| Notes |
Source of funding: Templeton World Charity Foundation, grant # TWCF0509. Prospective trial registration number: PACTR202104716135752 |
Wang 2021.
| Study name | An individualized telephone‐based care support program for rural family caregivers of people with dementia |
| Methods |
Study design: cluster‐RCT Country: China |
| Participants | Family caregivers of people with dementia Inclusion criteria: a. primary family caregivers of people diagnosed with dementia living at home; b. aged 18 years or above; c. access to a reliable telephone; d. having cared for people with dementia for no less than 6 months; e. able to communicate in Mandarin; f. able to provide informed consent to participate in this study. Exclusion criteria: a. hired caregivers by the family; b. with diseases that are unable to provide informed consent (e.g. severe mental health problems or cognitive impairment). Stated purpose: to adapt and evaluate an evidence‐based and culturally‐tailored individualized telephone‐based care support (ITBCS) programme for family caregivers of people with dementia in rural China |
| Interventions |
Intervention: Individualized Telephone‐based Care Support (ITBCS) programme The ITBCS program includes two major components: intensive ITBCS intervention and telephone consultations at the maintenance phase. The intensive ITBCS intervention consists of four steps: step 1 ‐ orientation talk; step 2 ‐ education booklet delivery; step 3 ‐ structured care support sessions; step 4 ‐ structured evaluation of each care support session. Each family caregiver will receive 12 telephone contacts distributed over 3 months that focus on five care support themes. After the intensive ITBCS intervention, participants will receive monthly maintenance telephone consultation sessions for 6 months. Control: Usual care (participants in the control group will not receive the ITBCS programme during the intervention phase. They will continue with usual services available for them during this period without any restriction) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Quality‐adjusted life‐years (QALYs) Healthcare utilization (direct and indirect costs) Time points: baseline, post‐intervention (9 months and 15 months after baseline) |
| Starting date | End of December 2021 (participants recruitment start) |
| Contact information | Yao Wang, yaowang0428@163.com |
| Notes |
Source of funding: Humanities and Social Science Foundation of the Ministry of Education, People’s Republic of China [Grant number: 19YJCZH177], the Open Competition Research Project of China Medical Board [Grant number: #19‐344], and the Flinders/Central South University Collaborative Research Project [Grant number: 2021xyhlzzzjj001] Prospective trial registration number: ChiCTR2000038821 |
Xu 2020.
| Study name | Group‐based intervention to improve developmental status among children age 6–18 months in rural Shanxi province, China |
| Methods |
Study design: cluster‐RCT Country: China |
| Participants | Children aged 6–18 months and their primary caregivers Inclusion criteria: a. aged 4–16 months at the time of recruitment; b. each child has at least one stable primary caregiver; c. children and their primary caregivers have no plans to migrate within the next 1 year. Exclusion criteria: a. refusal to participate in the study Stated purpose: to (1) evaluate the effectiveness of an integrated group‐based intervention, the Care Group Intervention, in enhancing ECD amongst children aged 6–18 months and (2) conduct a cost‐effectiveness analysis |
| Interventions |
Intervention: Care Group Intervention The intervention focuses on five key components, which include good health, adequate nutrition, responsive caregiving, security and safety, and opportunities for early learning under the framework of nurturing care promoted by WHO and UNICEF. The intervention comprises small groups of 3–10 children within a certain age range and their primary caregivers that are led by well‐trained facilitators in the same town. Frequencies are two times per month for children aged 6–23 months and once a month for children aged 24–30 months. The intervention emphasises early learning, responsive caring, security and safety, healthcare, feeding and nutrition, and child protection. Control: Usual care (National Public Health Service (NPHS): participants receive free vaccination and health management services for children aged 0–6 years) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Cost‐effectiveness analysis to examine both the costs and outcomes of the Care Group Intervention Time points: baseline, post‐intervention (specific time if provided) |
| Starting date | July 2018 (research design and pilot study), April 2019 (sample recruitment and baseline survey) |
| Contact information | Dr Hongyan Guan, cip_ghy@yeah.net |
| Notes |
Source of funding: Hong Kong Committee for UNICEF China (Programme No: 0860/A0/05/502) Prospective trial registration number: ChiCTR1900022894 |
Zheng 2020.
| Study name | Internet‐based support program on parenting outcomes for Chinese primiparous women |
| Methods |
Study design: RCT Country: China |
| Participants | Primiparous women Inclusion criteria: a. first‐time mothers with healthy babies; b. aged 18 years or above; c. married; d. able to response the questionnaires. Exclusion criteria: a. women or their children have severe physical and mental diseases Stated purpose: to evaluate the effects of internet‐based support programme for primiparous women in terms of improving the levels of maternal self‐efficacy, social support, and satisfaction; and reducing their postpartum depression symptoms |
| Interventions |
Intervention: Internet‐based support programme (ISP) Based on the self‐efficacy theory and the social exchange theory, the internet‐based support programme has five modules: (a) learning forum of parenting knowledge and skills; (b) communication forum; (c) ask‐the‐expert forum; (d) baby home forum; and (e) reminder forum. The ISP intervention has a fixed structure and lasts approximately 3 months. Control: Usual care (routine care consists of supports from the obstetricians and obstetric nurses during the 3–5 days hospitalization; and home visits from the community doctors on the 3rd, 7th, 14th, and 28th days postpartum. The women in the study group will have access to the ISP and receive routine care in the postpartum period) |
| Outcomes |
Participants'outcomes of interest for this review
Economic outcomes Nil Time points: baseline, post‐intervention (immediate post‐intervention and 3 months follow‐up) |
| Starting date | May 2020 (recruitment start date) |
| Contact information | Qun Wang, qunwang@szu.edu.cn |
| Notes |
Source of funding: Natural Science Foundation of China Youth project (grant no. 81703234), Medical Science and Technology Research fund project of Guangdong Province (grant no. A2018335), Basic Research Free Exploration Project of Shenzhen City (grant no. JCYJ20170818094440661, JCYJ20180305163459491) Prospective trial registration number: ChiCTR2000033154 |
AC: accountable care condition (a)CBS: (adapted) community‐based socio‐therapy ACSM: American College of Sports Medicine ACT: Adolescent Coordinated Transition ADL: activities of daily living AGYW: adolescent girls and young women ALHIV: adolescents living with HIV AMH: Adeoyo Maternity Hospital ANC: antenatal care APP: application ART: anti‐retroviral therapy ASPIRE: ASPIRE intervention ASSIST‐Y: Alcohol, Smoking and Substance Use Involvement Test‐Youth ATO: Anti‐Terrorist Operations AUDIT‐C: Alcohol Use Disorders Identification Test‐Consumption BASIC: Building and Sustaining Interventions for Children BDI: Beck Depression Inventory BEPS: Early Book Sharing BIO: brief interventions for older adults BMI: body mass index BPEI: burden ‐ patient enablement instrument BPM‐Y: Brief Problem Monitor–Youth Brief‐COPE: Coping Orientation to Problems Experienced Inventory ‐ short version BUCM: Beijing University of Chinese Medicine CA: corrected age CABI: Child and Adolescent Behavior Inventory CA‐CBT: Culturally Adapted Cognitive Behavioral Intervention CASP‐16: Control, Autonomy, Self‐realisation and Pleasure‐16 CBCL: Child Behavior Checklist CBT: cognitive‐behavioural therapy CBTH: Community Based Trauma Healing CD: compact disc CDR: Clinical Dementia Rating CDW: community disability workers CESD(‐R): Center for Epidemiologic Studies Depression Scale(‐revised) CETA: Common Elements Treatment Approach CGD: Complicated Grief Disorder CGI‐s: Clinical Global Impression severity CHU9D: Child Health Utility 9D CHW: community health workers CON: group C control COSTAR: COSTAR study COVID: Coronavirus Disease CP: cerebral palsy CIIC: Community‐Integrated Intermediary Care CPSS: Perceived Stress Scale, Chinese version CSBS: Communication and Symbolic Behavior Scale CSF: central storage facility or Coping Self‐efficacy Scale CSRI: Client Services Receipt Inventory CSS: CETA Short Session CST: caregivers skills training DASS(‐21): Depression, Anxiety, Stress Scale(‐21) DD‐CGAS: Developmental Disability‐Children's Global Assessment Scale DDU‐GKY: Deen Dayal Upadhyaya Grameen Kaushalya Yojana DERS: Difficulties in Emotion Regulation Scale DIALOG+: DIALOG+ intervention DM: diabetes mellitus DRC: Democratic Republic of the Congo DSM‐5: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition EBI: evidence‐based intervention ECD: early childhood development ECM: Enhancing Contact Model ECU/EUC: enhanced care as usual/enhanced usual care EL: EL intervention EPDS: Edinburgh Postnatal Depression Scale ESC: European Society of Cardiology ETAU: enhanced treatment as usual EX: exercise FNPH: Federal Neuropsychiatric Hospital FSI‐ECD: Family Strengthening Intervention for Early Childhood Development FSS: Functional Social Support Questionnaire GAD‐7: Generalized Anxiety Disorder Assessment‐7 GDS: Geriatric Depression Scale GHQ‐12: General Health Questionnaire GM: general movement GSP: group support psychotherapy HADS: Hospital Anxiety and Depression Scale HASTAG: HASTAG project HE: health education HIAS: Hebrew Immigrant Aid Society HINE: Hammersmith Infant Neurological Examination HIV: human immunodeficiency virus HSCL: Hopkins Symptom Checklist HTN: hypertension IAC: Intensive Adherence Counseling ICER: incremental cost‐effectiveness ratio IDSS: International Depression Symptom Scale ILAE: International League Against Epilepsy IMB: Information‐motivation‐behavioral Skills INR: Indian rupee iOS: operating system for mobile devices manufactured by Apple IPP: implementation policies and practices IPV: intimate partner violence ISP: internet‐based support programme ITBCS: Individualized Telephone‐based Care Support IVF: in vitro fertilization IWQ: Imaginary Working Qigong IYMPE: Integrated Yoga Module for Persons with Epilepsy JIAH: Jharkhand Initiative for Adolescent Health K6/10: Kessler‐6/10 Item Scale LEAP‐CP: Learning through Everyday Activities with Parents for infants at high risk of Cerebral Palsy LGBT+: lesbian, gay, bisexual, transgender+ LTC: long‐term care LHW: lady health workers MANSA: Manchester Short assessment MBHP: mindfulness‐based health promotion MBI: mindfulness‐based intervention MBSR: mindfulness‐based stress reduction MCSF: Mental Health Continuum‐Short Form Questionnaire MESSSSAGE: mHealth Interactive Education and Social Support Intervention for Improving Postnatal Health MFQ: Mood and Feelings Questionnaire MH‐FA: First Aid in Mental Health mh‐GAP: World Health Organization Mental Health Action Programme MHL: mental health literacy MHP: mental health promotion MHPSS: Mental Health and Psychosocial Support Module MI: multifactorial intervention MINI: Mini International Neuropsychiatric Interview Mini‐Cog: Mental Status Assessment of Older Adults MNCH: maternal, newborn and child health MOH: medical officer of health MOS: Medical Outcomes Study MOWCA: maternity allowance with health education awareness programme MSM: men who have sex with men MSPSS: Multidimensional Scale of Perceived Social Support MSSS: Modified Social Support Survey MUAC: mid‐upper arm circumference NA: not available ODD: oppositional defiant disorder OS(G): organized support (group) OSSS: Oslo Social Support Scale PA: physical activity PACT‐HF: physical activity and brief cognitive behaviour therapy for heart failure patients PAPA: Pediatric Adult Pediatric Adult PAR‐Q+: Physical Activity Readiness for Everyone PATH: conditional cash transfer programme PC: primary care PCL(‐5): Post Traumatic Stress Disorder Checklist(‐5) PD: ParentCorps‐ Professional Development PDT: ParentCorps‐Professional Development (PD) + T‐Wellness PedsQL: Pediatric Quality of Life Inventory PFA: Psychological First Aid PFI: Psychoeducational Family Intervention PHC: primary health centre PHQ‐9/A: Patient Health Questionnaire‐9/adolescent PHM: public health midwife PLA: participatory learning and action PLH‐YC: Parenting for Lifelong Health for Young Children PMTCT: prevention of mother‐to‐child transmission POA: pregnant women in their second trimester PROMIS: Pictorial Pediatric Symptom Checklist‐17 ProQOL: Professional Quality of Life Measure PS: psychosocial stimulation PSI‐SF: Parenting Stress Index ‐ short form PST: problem‐solving therapy PSYCHLOPS: Psychological Outcome Profiles Instrument PSS: Post‐traumatic Stress Disorder Symptom Scale PSS: Perceived Stress Scale PSSS: Postpartum Social Support Scale PT: physical therapy PTSD: post‐traumatic stress disorder PM+: Problem Management Plus QALYs: quality‐adjusted life‐years QOL: quality of life QoLIE‐10: Quality of Life in Epilepsy‐10 RAP: Resourceful Adolescent Programme RCT: randomized controlled trial REaCH: Resiliency Engagement and Care in Health RISE: Prevention of Child Mental Health Problems in Southeastern Europe ‐ Adapt, Optimise, Test, and Extend Parenting for Lifelong Health ROI: return on investment SAM: short‐term audio mindfulness meditation SCARED: Screen for Child Anxiety Related Emotional Disorders SCS: Social Connectedness Scale SDQ: Strengths and Difficulties Questionnaire SDS: Social Distance Scale SE: standard error SF‐12: Short‐Form Health Survey 12‐item SFGC: Search for Common Ground SH+: Self‐Help Plus SHG: self‐help groups SIX: Objective Social Outcomes Index SMART: Sequential Multiple Assignment Randomized Trial SMBM: Shirom‐Melamed Burnout Measure SMFQ: Short Moods and Feeling Questionnaire SMS: short message (or messaging) service SMT: stress management training SNEHA: Society for Nutrition, Education and Health Action SPIRIT: Integrated Suicide Prevention Programme SPSI: Social Problem‐Solving Inventory ‐ Adolescent version SRQ‐20: Self Reporting Questionnaire SSQ: Shona Symptoms Questionnaire SSRS: Social Support Rating Scale~ STAI: State‐Trait Anxiety Inventory STI: sexually transmitted infection SUMS: scaling up of mental health in schools SWEMWBS: Short Warwick‐Edinburgh Mental Well‐being Scale TAU: treatment as usual t‐CETA: Phone‐Delivered Psychological Intervention TeaLeaF: Teacher‐delivered Child Mental Healthcare in Primary Schools of India TES: Thriving Environment in Schools TF‐CBT: Trauma‐Focused Cognitive Behavioral Therapy TH: trauma healing THP: Thinking Healthy Programme TOCA‐C: Teacher Observation of Classroom Adaptation‐Checklist TQS: Ten Questions Screen TT: Thriving Together UCH: University College Hospital UCT: unconditional cash transfer UWE: Centre for Appearance Research UX: user experience VL: viral load WHO: World Health Organization WHO‐EMRO: World Health Organization, Eastern Mediterranean Region WHO‐5: World Health Organisation‐5 Well‐being IndexWHODAS: WHO Disability Assessment Scale WHOQOL‐BREF: World Health Organization Quality of Life Instruments YAM: Youth Aware of Mental health ZBI(‐12): Zarit Burden Interview(‐12)
Differences between protocol and review
In the protocol phase, we planned to include interventions for preventing substance misuse and neuropsychiatric conditions, but we ultimately did not include these conditions as most RCTs failed to collect outcomes related to mental health. Moreover, it was difficult to distinguish prevention and treatment interventions due to extremely high heterogeneity with the consequent risk of misclassification.
Promotion interventions and universal prevention interventions were merged as a unique category in the meta‐analyses given the overlap of interventions' ingredients, potential mechanism of action, and target population groups. In the analyses of dichotomous data, available case analysis was used in place of imputing negative outcomes for missing dichotomous outcomes because all the outcomes measures were negative. This is a conservative approach as this way we increase the resulting percentage of participants with a negative outcome, therefore diluting the estimation of the outcome and the beneficial effect of the intervention.
Contributions of authors
Conceiving the review: MP, CB, WT, NvG.
Designing the review: MP, CB.
Co‐ordinating the review: MP, CB.
Designing search strategies: MJ, MP, NvG.
Writing the review: MP, CB, WT, EP, CCad, CL, MJ, NvG, JA, RC, DP, EU, CC, EP, LA, FA.
Providing general advice on the protocol: CB, CL, FA, MJ, WT.
Securing funding for the review: CB.
Performing previous work that was the foundation of the current study: MP, CB, EP, WT, CL, MJ, DP, NvG, JA, RC.
Sources of support
Internal sources
-
University of Verona, Italy
N/A
External sources
-
None, Other
N/A
Declarations of interest
Conflicts of interest: MP, EP, CC, CCad, JA, FA, LA, RC, MJ, CL, DP, EU, NvG, WT, CB: none known.
Financial conflicts of interest: MP, EP, CC, CCad, JA, FA, LA, RC, MJ, CL, DP, EU, NvG, WT, CB: none known.
Review authors involved in the conduct, analysis, and publication of a study that could be included in the review were not involved in study eligibility decisions, data extraction, risk of bias or GRADE assessments for that study.
New
References
References to studies included in this review
Acarturk 2022 {published data only}
- Acarturk C, Uygun E, Ilkkursun Z, Carswell K, Tedeschi F, Batu M, et al. Effectiveness of a WHO self-help psychological intervention for preventing mental disorders among Syrian refugees in Turkey: a randomized controlled trial. World Psychiatry 2022;21(1):88-95. [DOI: 10.1002/wps.20939] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03587896. Implementation of Self Help Plus in adult Syrian refugees in Turkey (RE-DEFINE) (RE-DEFINE) [Refugee emergency: defining and implementing novel evidence-based psychosocial interventions - the Turkey site]. clinicaltrials.gov/ct2/show/NCT03587896 (first received 16 July 2018).
- Purgato M, Carswell K, Acarturk C, Au T, Akbai S, Anttila M, et al. Effectiveness and cost-effectiveness of Self-Help Plus (SH+) for preventing mental disorders in refugees and asylum seekers in Europe and Turkey: study protocols for two randomised controlled trials. BMJ Open 2019;9(5):e030259. [DOI: 10.1136/bmjopen-2019-030259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ager 2011 {published data only}
- Ager A, Akesson B, Stark L, Flouri E, Okot B, McCollister F, et al. The impact of the school-based psychosocial structured activities (PSSA) program on conflict-affected children in northern Uganda: psychosocial structured activities with conflict-affected children in northern Uganda. Journal of Child Psychology and Psychiatry 2011;52(11):1124-33. [DOI: 10.1111/j.1469-7610.2011.02407.x] [DOI] [PubMed] [Google Scholar]
Amador Buenabad 2020 {published data only}
- Amador Buenabad NG, Sánchez Ramos R, Schwartz S, Gutiérrez López ML, Díaz Juárez AD, Ortiz Gallegos AB, et al. Cluster randomized trial of a multicomponent school-based program in Mexico to prevent behavioral problems and develop social skills in children. Child & Youth Care Forum 2020;49:343-64. [DOI: 10.1007/s10566-019-09535-3] [DOI] [Google Scholar]
- ISRCTN11345846. Effectiveness of a school model for the integral prevention of risk behaviors: a cluster-randomised trial [A four-arm, cluster-randomised study to evaluate the effectiveness of a multicomponent intervention to prevent risk behaviors in Mexican students through the development of social skills in children and positive parenting in their caregivers]. www.isrctn.com/ISRCTN11345846 (first received 31 October 2017). [DOI: 10.1186/ISRCTN11345846] [DOI]
Annan 2017 {published data only}
- Annan J, Sim A, Puffer ES, Salhi C, Betancourt TS. Improving mental health outcomes of Burmese migrant and displaced children in Thailand: a community-based randomized controlled trial of a parenting and family skills intervention. Prevention Science 2017;18(7):793-803. [DOI: 10.1007/s11121-016-0728-2] [DOI] [PubMed] [Google Scholar]
- International Rescue Committee. Building Happy Families: impact evaluation of a parenting and family skills intervention for migrant and displaced Burmese families in Thailand. New York (NY): International Rescue Committee; 2014 November, available from www.researchgate.net/publication/306268893_Building_Happy_Families_Impact_evaluation_of_a_parenting_and_family_skills_intervention_for_migrant_and_displaced_Burmese_families_in_Thailand.
- NCT01668992. Impact evaluation of a family-based intervention with Burmese migrant and displaced children and families in Tak Province, Thailand. clinicaltrials.gov/ct2/show/NCT01668992 (first received 20 August 2012).
- Puffer ES, Annan J, Sim AL, Salhi C, Betancourt TS. The impact of a family skills training intervention among Burmese migrant families in Thailand: a randomized controlled trial. PLOS One 2017;12(3):e0172611. [DOI: 10.1371/journal.pone.0172611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arango 2014 {published data only (unpublished sought but not used)}
- Arango-Lasprilla JC, Panyavin I, Merchán EJ, Perrin PB, Arroyo-Anlló EM, Snipes DJ, et al. Evaluation of a group cognitive–behavioral dementia caregiver intervention in Latin America. American Journal of Alzheimer's Disease & Other Dementias 2014;29(6):548-55. [DOI: 10.1177/1533317514523668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asnani 2021 {published data only}
- Asnani MR, Francis D, Knight-Madden J, Chang-Lopez S, King L, Walker S. Integrating a problem-solving intervention with routine care to improve psychosocial functioning among mothers of children with sickle cell disease: a randomized controlled trial. PLOS One 2021;16(6):e0252513. [DOI: 10.1371/journal.pone.0252513] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02394899. Improving parental psychosocial functioning and early developmental outcomes in children with sickle cell disease [Integrating a parenting intervention with routine care to improve parental psychosocial functioning and early developmental outcomes in children with sickle cell disease]. clinicaltrials.gov/ct2/show/NCT02394899 (first received 20 March 2015).
Attanasio 2014 {published data only (unpublished sought but not used)}
- Attanasio OP, Fernández C, Fitzsimons EO, Grantham-McGregor SM, Meghir C, Rubio-Codina M. Using the infrastructure of a conditional cash transfer program to deliver a scalable integrated early child development program in Colombia: cluster randomized controlled trial. BMJ 2014;349:g5785. [DOI: 10.1136/bmj.g5785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN18991160. Early childhood development: identifying successful interventions and the mechanisms behind them [Early childhood development: a cluster-randomised controlled trial to identify successful interventions and the mechanisms behind them]. www.isrctn.com/ISRCTN18991160 (first received 9 December 2009). [DOI: 10.1186/ISRCTN18991160] [DOI]
Baker‐Henningham 2005 {published data only}
- Baker-Henningham H, Powell C, Walker S, Grantham-McGregor S. The effect of early stimulation on maternal depression: a cluster randomised controlled trial. Archives of Disease in Childhood 2005;90(12):1230-4. [DOI: 10.1136/adc.2005.073015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker‐Henningham 2019 {published data only}
- Baker-Henningham H, Scott Y, Bowers M, Francis T. Evaluation of a violence-prevention programme with Jamaican primary school teachers: a cluster randomised trial. International Journal of Environmental Research and Public Health 2019;16(15):2797. [DOI: 10.3390/ijerph16152797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Henningham H, Vera-Hernández M, Alderman H, Walker S. Irie Classroom Toolbox: a study protocol for a cluster-randomised trial of a universal violence prevention programme in Jamaican preschools. BMJ Open 2016;6(5):e012166. [DOI: 10.1136/bmjopen-2016-012166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbosa Filho 2017 {published data only}
- Barbosa Filho VC, Bandeira A, Minatto G, Linard J, Silva J, Costa R, et al. Effect of a Multicomponent Intervention on Lifestyle Factors among Brazilian Adolescents from Low Human Development Index Areas: A Cluster-Randomized Controlled Trial. IJERPH 2019;16(2):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Filho VC, Samara da Silva K, Mota J, Vieira NFC, do Amaral Gubert F, da Silva Lopes A. “For whom was it effective?” Moderators of the effect of a school-based intervention on potential physical activity determinants among Brazilian students. Preventive Medicine 2017;97:80-85. [DOI: 10.1016/j.ypmed.2017.01.007] [DOI] [PubMed] [Google Scholar]
- Barbosa Filho VC, da Silva Lopes A, Barroso Lima A, Souza EA, do Amaral Gubert F, Silva KS, et al. Rationale and methods of a cluster-randomized controlled trial to promote active and healthy lifestyles among Brazilian students: the "Fortaleça sua Saúde" program. BMC Public Health 2015;15:1212. [DOI: 10.1186/s12889-015-2543-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Filho VC, da Silva KS, Mota J, Beck C, da Silva Lopes A. A Physical Activity Intervention for Brazilian Students From Low Human Development Index Areas: A Cluster-Randomized Controlled Trial. Journal of Physical Activity & Health 2016;13(11):1174-1182. [DOI: 10.1123/jpah.2016-0113] [DOI] [PubMed] [Google Scholar]
- NCT02439827. The "Fortaleça Sua Saúde" program for active and healthy lifestyle among Brazilian students [The "Fortaleça Sua Saúde" program: a cluster-randomized controlled trial to promote active and healthy lifestyle among Brazilian students]. clinicaltrials.gov/ct2/show/NCT02439827 (first received 12 May 2015). [DOI] [PMC free article] [PubMed]
Barnes 2019 {published and unpublished data}
- Barnes RY, Jelsma J, Parker R. Improvements in health-related quality of life and function in middle-aged women with chronic diseases of lifestyle after participating in a non-pharmacological intervention programme: a pragmatic randomised controlled trial. African Journal of Disability 2019;8:a428. [DOI: 10.4102/ajod.v8i0.428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumgartner 2021 {published data only}
- Baumgartner JN, Ali M, Gallis JA, Lillie M, Owusu R, Abubakr-Bibilazu S, et al. Effect of a lay counselor-delivered integrated maternal mental health and early childhood development group-based intervention in Northern Ghana: a cluster-randomized controlled trial. Global Mental Health 2021;8:e18. [DOI: 10.1017/gmh.2021.15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Gallis JA, Ali M, Lillie M, Abubakr-Bibilazu S, Adam H, et al. The impact of a maternal mental health intervention on intimate partner violence in Northern Ghana and the mediating roles of social support and couple communication: secondary analysis of a cluster randomized controlled trial. BMC Public Health 2021;21(1):2010. [DOI: 10.1186/s12889-021-12121-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03665246. Evaluation of the iMBC/ECD model in Ghana [Evaluation of the iMBC/ECD model on maternal mental health and child development in Ghana]. clinicaltrials.gov/ct2/show/NCT03665246 (first received 11 September 2018).
Bell 2008 {published data only}
- Bell CC, Bhana A, Petersen I, McKay MM, Bannon W, Gibbons R, et al. Building protective factors to offset sexually risky behaviors among black youths: a randomized control trial. Journal of the National Medical Association 2008;100(8):936-44. [DOI: 10.1016/s0027-9684(15)31408-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2018 {published data only}
- Berger R, Benatov J, Cuadros R, VanNattan J, Gelkopf M. Enhancing resiliency and promoting prosocial behavior among Tanzanian primary-school students: a school-based intervention. Transcultural Psychiatry 2018;55(6):821-45. [DOI: 10.1177/1363461518793749] [DOI] [PubMed] [Google Scholar]
Bernardi 2020 {published data only}
- Bernardi ML, Amorim MH, Salaroli LB, Zandonade E. Effects of Hatha Yoga on caregivers of children and adolescents with cancer: a randomized controlled trial [Efectos del Hatha-Yoga en cuidadores de niños y adolescentes con cáncer: un estudio aleatorizado y controlado]. Escola Anna Nery 2020;24(1):e20190133. [DOI: 10.1590/2177-9465-EAN-2019-0133] [DOI] [Google Scholar]
Betancourt 2017 {published data only (unpublished sought but not used)}
- Betancourt TS, Ng LC, Kirk CM, Brennan RT, Beardslee WR, Stulac S, et al. Family-based promotion of mental health in children affected by HIV: a pilot randomized controlled trial. Journal of Child Psychology and Psychiatry 2017;58(8):922-30. [DOI: 10.1111/jcpp.12729] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01509573. Pilot feasibility trial of the family strengthening intervention in Rwanda (FSI-R) [Family-based prevention of mental health problems in HIV/AIDS-affected children (R34MH084679-01A1)]. clinicaltrials.gov/ct2/show/NCT01509573 (first received 13 January 2012).
Cerquera Córdoba 2021 {published data only}
- Cerquera Córdoba AM, Tiga-Loza DC, Álvarez Anaya WA, Peña ED, Jaimes Espíndola LR, Plata Osma LJ. Randomized controlled trial of a multicomponent program for informal caregivers of Alzheimer’s patients [Ensayo controlado aleatorizado de un programa multicomponente para cuidadores informales de pacientes con Alzheimer]. Revista Cuidarte 2021;12(2):e2002. [DOI: 10.15649/cuidarte.2002] [DOI] [Google Scholar]
Chaharrahifard 2021 {published data only}
- Chaharrahifard L, Motlagh AJ, Akbari-Kamrani M, Ataee M, Esmaelzadeh-Saeieh S. The effect of midwife-led psycho-education on parental stress, postpartum depression and parental competency in high risk pregnancy women: a randomized controlled trial. Journal of Caring Sciences 2021;10(2):70-6. [DOI: 10.34172/jcs.2021.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2015 {published and unpublished data}
- Chang SM, Grantham-McGregor SM, Powell CA, Vera-Hernández M, Lopez-Boo F, Baker-Henningham H, et al. Integrating a parenting intervention with routine primary health care: a cluster randomized trial. Pediatrics 2015;136(2):272-80. [DOI: 10.1542/peds.2015-0119] [DOI] [PubMed] [Google Scholar]
- ISRCTN43108304. Evaluation of parenting interventions to promote child development through home visits or a health centre based approach in three Caribbean countries [Home and health centre delivery of early childhood stimulation: cluster randomised trial of the impact of interventions to improve parenting of children aged 2-18 months on child development in the Caribbean countries]. www.isrctn.com/ISRCTN43108304 (first received 6 May 2011).
- Walker SP, Baker-Henningham H, Chang SM, Powell CA, Lopez-Boo F, Grantham-Mcgregor S. Implementation of parenting interventions through health services in Jamaica. Vulnerable Children and Youth Studies 2017;13(2):127-41. [DOI: 10.1080/17450128.2017.1395100] [DOI] [Google Scholar]
- Walker SP, Powell C, Chang-Lopez SM, Baker-Henningham H, Grantham-McGregor S, Vera-Hernández M, et al. Delivering parenting interventions through health services in the Caribbean: impact, acceptability and costs. Washington (DC): Inter-American Development Bank, Social Protection and Health Division; 2015 November. Report No. IDB-WP-642. [LINK: publications.iadb.org/en/publication/17041/delivering-parenting-interventions-through-health-services-caribbean]
Chattha 2008 {published data only}
- Chattha R, Raghuram N, Venkatram P, Hongasandra NR. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: a randomized control study. Menopause 2008;15(5):862-70. [DOI: 10.1097/gme.0b013e318167b902] [DOI] [PubMed] [Google Scholar]
Cheng 2021 {published data only}
- Cheng L, Sit JW, Choi K-C, Chair S-Y, Li X, Wu Y, et al. Effectiveness of a patient-centred, empowerment-based intervention programme among patients with poorly controlled type 2 diabetes: a randomised controlled trial. International Journal of Nursing Studies 2018;79:43-51. [DOI: 10.1016/j.ijnurstu.2017.10.021] [DOI] [PubMed] [Google Scholar]
- Cheng L, Sit JW, Choi K-C, Chair S-Y, Li X, Wu Y, et al. The effects of an empowerment-based self-management intervention on empowerment level, psychological distress, and quality of life in patients with poorly controlled type 2 diabetes: a randomized controlled trial. International Journal of Nursing Studies 2021;116:103407. [DOI: 10.1016/j.ijnurstu.2019.103407] [DOI] [PubMed] [Google Scholar]
Chew 2018 {published data only}
- Chew B-H, Vos RC, Fernandez A, Ghazali SS, Shamsuddin NH, Ismail M, et al. The effectiveness of an emotion-focused educational programme in reducing diabetes distress in adults with type 2 diabetes mellitus at 12-month follow-up: a cluster randomized controlled trial. Therapeutic Advances in Endocrinology and Metabolism 2019;10:2042018819853761. [DOI: 10.1177/2042018819853761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew B-H, Vos RC, Stellato RK, Ismail M, Rutten GE. The effectiveness of an emotion-focused educational programme in reducing diabetes distress in adults with type 2 diabetes mellitus (VEMOFIT): a cluster randomized controlled trial. Diabetic Medicine 2018;35(6):750-9. [DOI: 10.1111/dme.13615] [DOI] [PubMed] [Google Scholar]
- Chew B-H, Vos RC, Ghazali SS, Shamsuddin NH, Fernandez A, Mukhtar F, et al. The effectiveness of a value-based emotion-cognition-focused educational programme to reduce diabetes-related distress in Malay adults with type 2 diabetes (VEMOFIT): study protocol for a cluster randomised controlled trial. BMC Endocrine Disorders 2017;17(1):22. [DOI: 10.1186/s12902-017-0172-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02730078. Value-based emotion-focused educational programme to reduce diabetes-related distress (VEMOFIT) [The effectiveness of a value-based emotion-focused educational programme to reduce diabetes-related distress in Malay adults with type 2 diabetes (VEMOFIT): a cluster randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT02730078 (first received 6 April 2016).
Chomat 2019 {published data only}
- Chomat AM, Menchú AI, Andersson N, Ramirez-Zea M, Pedersen D, Bleile A, et al. Women’s circles as a culturally safe psychosocial intervention in Guatemalan indigenous communities: a community-led pilot randomised trial. BMC Women's Health 2019;19(1):53. [DOI: 10.1186/s12905-019-0744-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN13964819. Co-design of group psychosocial health intervention for indigenous women in Guatemala [Tackling maternal psychosocial distress among marginalized women in Guatemala: a community-based approach]. www.isrctn.com/ISRCTN13964819 (first received 20 June 2018).
Cooper 2009 {published data only}
- Cooper PJ, Tomlinson M, Swartz L, Landman M, Molteno C, Stein A, et al. Improving quality of mother-infant relationship and infant attachment in socioeconomically deprived community in South Africa: randomised controlled trial. BMJ 2009;338:b974. [DOI: 10.1136/bmj.b974] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper P, Arteche A, Stein A, Tomlinson M. Randomized controlled trial of a home-visiting intervention on infant cognitive development in peri-urban South Africa. Developmental Medicine & Child Neurology 2016;58(3):270-6. [DOI: 10.1111/dmcn.12873] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN25664149. A controlled trial of a community based mother-infant intervention in a South African peri-urban settlement. www.isrctn.com/ISRCTN25664149 (first received 6 July 2006).
Dayhimi 2020 {published data only}
- Dayhimi M, Kariman N, Shams J, Baghban AA. Evaluation of group consulting on pregnancy anxiety: a randomized clinical trial. Advances in Nursing & Midwifery 2020;29(1):16-21. [LINK: journals.sbmu.ac.ir/en-jnm/article/view/27792] [Google Scholar]
Devries 2015 {published data only}
- Devries KM, Allen E, Child JC, Walakira E, Parkes J, Elbourne D, et al. The Good Schools Toolkit to prevent violence against children in Ugandan primary schools: study protocol for a cluster randomised controlled trial. Trials 2013;14:232. [DOI: 10.1186/1745-6215-14-232] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries KM, Knight L, Allen E, Parkes J, Kyegombe N, Naker D. Does the Good Schools Toolkit reduce physical, sexual and emotional violence, and injuries, in girls and boys equally? A cluster-randomised controlled trial. Prevention Science 2017;18(7):839-53. [DOI: 10.1007/s11121-017-0775-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries KM, Knight L, Child JC, Mirembe A, Nakuti J, Jones R, et al. The Good School Toolkit for reducing physical violence from school staff to primary school students: a cluster-randomised controlled trial in Uganda. Lancet Global Health 2015;3(7):e378-86. [DOI: 10.1016/S2214-109X(15)00060-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayiwa J, Clarke K, Knight L, Allen E, Walakira E, Namy S, et al. Effect of the Good School Toolkit on school staff mental health, sense of job satisfaction and perceptions of school climate: Secondary analysis of a cluster randomised trial. Preventive Medicine 2017;101:84-90. [DOI: 10.1016/j.ypmed.2017.05.022] [DOI] [PubMed] [Google Scholar]
- Knight L, Allen E, Mirembe A, Nakuti J, Namy S, Child JC, et al. Implementation of the Good School Toolkit in Uganda: a quantitative process evaluation of a successful violence prevention program. BMC Public Health 2018;18(1):608. [DOI: 10.1186/s12889-018-5462-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01678846. Good schools study (GSS) [The good schools study: cluster randomised controlled trial of a program to prevent violence against children in schools]. clinicaltrials.gov/ct2/show/NCT01678846 (first received 5 September 2012).
Dhital 2019 {published data only}
- Dhital R, Shibanuma A, Miyaguchi M, Kiriya J, Jimba M. Effect of psycho-social support by teachers on improving mental health and hope of adolescents in an earthquake-affected district in Nepal: a cluster randomized controlled trial. PLOS One 2019;14(10):e0223046. [DOI: 10.1371/journal.pone.0223046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03387007. Psycho-social support on mental health and hope of adolescents affected by earthquake in Nepal [Effect of psycho-social support training by school teachers on improving mental health and hope of school going adolescents in earthquake affected districts in Nepal]. clinicaltrials.gov/ct2/show/NCT03387007 (first received 29 December 2017).
Dias 2008 {published data only}
- Dias A, Dewey ME, D'Souza J, Dhume R, Motghare DD, Shaji KS, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLOS One 2008;3(6):e2333. [DOI: 10.1371/journal.pone.0002333] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00479271. Evaluating the effectiveness of a community based intervention for persons with dementia and their caregivers in a developing country [The dementia home care project: a randomised controlled trial to evaluate the effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India]. clinicaltrials.gov/ct2/show/NCT00479271 (first received 28 May 2007).
Dias 2019 {published data only}
- Dias A, Azariah F, Anderson SJ, Sequeira M, Cohen A, Morse JQ, et al. Effect of a lay counselor intervention on prevention of major depression in older adults living in low- and middle-income countries: a randomized clinical trial. JAMA Psychiatry 2019;76(1):13-20. [DOI: 10.1001/jamapsychiatry.2018.3048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A, Azariah F, Sequeira M, Krishna R, Morse JQ, Cohen A, et al. Adaptation of problem-solving therapy for primary care to prevent late-life depression in Goa, India: the ‘DIL’ intervention. Global Health Action 2019;12(1):1420300. [DOI: 10.1080/16549716.2017.1420300] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02145429. Preventing depression in late life: a model for low and middle income countries. clinicaltrials.gov/ct2/show/NCT02145429 (first received 22 May 2014).
Duan 2019 {published data only}
- Duan W, Bu H. Randomized trial investigating of a single-session character-strength-based cognitive intervention on freshman’s adaptability. Research on Social Work Practice 2019;29(1):82-92. [DOI: 10.1177/1049731517699525] [DOI] [Google Scholar]
Duru Aşiret 2021 {published data only}
- Duru Aşiret G, Kütmeç Yılmaz C, Sayın Kasar K. Investigation of the effects of interventions made according to the Progressively Lowered Stress Threshold Model on the care outcomes of Alzheimer patients and their families: a randomized clinical trial. Psychogeriatrics 2021;21(5):738-48. [DOI: 10.1111/psyg.12734] [DOI] [PubMed] [Google Scholar]
Dybdahl 2001 {published data only}
- Dybdahl R. Children and mothers in war: an outcome study of a psychosocial intervention program. Child Development 2001;72(4):1214-30. [DOI: 10.1111/1467-8624.00343] [DOI] [PubMed] [Google Scholar]
Eloff 2014 {published data only}
- Eloff I, Finestone M, Makin JD, Boeving-Allen A, Visser M, Ebersöhn L, et al. A randomized clinical trial of an intervention to promote resilience in young children of HIV-positive mothers in South Africa. AIDS 2014;28 Suppl 3:S347-57. [DOI: 10.1097/QAD.0000000000000335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Escolar 2014 {published data only}
- Escolar Chua RL, Guzman AB. Effects of third age learning programs on the life satisfaction, self-esteem, and depression level among a select group of community dwelling Filipino elderly. Educational Gerontology 2014;40(2):77-90. [DOI: 10.1080/03601277.2012.701157] [DOI] [Google Scholar]
Fabbri 2021 {published and unpublished data}
- Devries KM, Fabbri C, Allen E, Barongo V, Shayo E, Greco G, et al. Preventing violence against children in schools (PVACS): protocol for a cluster randomised controlled trial of the EmpaTeach behavioural intervention in Nyarugusu Refugee Camp. BMC Public Health 2019;19(1):1295. [DOI: 10.1186/s12889-019-7627-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri C, Rodrigues K, Leurent B, Allen E, Qiu M, Zuakulu M, et al. The EmpaTeach intervention for reducing physical violence from teachers to students in Nyarugusu Refugee Camp: a cluster-randomised controlled trial. PLOS Medicine 2021;18(10):e1003808. [DOI: 10.1371/journal. pmed.1003808] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03745573. Preventing violence against children in schools study (PVACS) [Preventing violence against children in schools: protocol for a cluster randomised controlled trial of the EmpaTeach behavioural intervention in Nyarugusu Refugee Camp]. clinicaltrials.gov/ct2/show/NCT03745573 (first received 19 November 2018). [DOI] [PMC free article] [PubMed]
Ferreira‐Vorkapic 2018 {published data only}
- Ferreira-Vorkapic C, Borba-Pinheiro CJ, Marchioro M, Santana D. The impact of Yoga Nidra and seated meditation on the mental health of college professors. International Journal of Yoga 2018;11(3):215-23. [DOI: 10.4103/ijoy.IJOY_57_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fine 2021 {published data only}
- Fine SL, Malik A, Guimond M-F, Nemiro A, Temu G, Likindikoki S, et al. Improving mental health in low-resource settings: a feasibility randomized controlled trial of a transdiagnostic psychological intervention among Burundian refugee adolescents and their caregivers. Behaviour Research and Therapy 2021;145:103944. [DOI: 10.1016/j.brat.2021.103944] [DOI] [PubMed] [Google Scholar]
Gao 2015 {published data only}
- Gao L, Xie W, Yang X, Chan SW. Effects of an interpersonal-psychotherapy-oriented postnatal programme for Chinese first-time mothers: a randomized controlled trial. International Journal of Nursing Studies 2015;52(1):22-9. [DOI: 10.1016/j.ijnurstu.2014.06.006] [DOI] [PubMed] [Google Scholar]
Gavrilova 2009 {published data only}
- Gavrilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care—the 10/66 Dementia Research Group’s randomized control trial of a caregiver intervention in Russia. International Journal of Geriatric Psychiatry 2009;24(4):347–54. [DOI: 10.1002/gps.2126] [DOI] [PubMed] [Google Scholar]
George 2020 {published data only}
- George C, Kumar AV, Girish N. Effectiveness of a group intervention led by lay health workers in reducing the incidence of postpartum depression in South India. Asian Journal of Psychiatry 2020;47:101864. [DOI: 10.1016/j.ajp.2019.101864] [DOI] [PubMed] [Google Scholar]
Hajarian Abhari 2021 {published data only}
- Hajarian Abhari Z, Karimi FZ, Taghizdeh Z, Mazloum SR, Asghari Nekah SM. Effect of counseling based on Gamble's approach on postpartum anxiety in primiparous women. Journal of Midwifery and Reproductive Health 2021;9(1):2530-40. [DOI: 10.22038/jmrh.2019.44596.1535] [DOI] [Google Scholar]
Hamdani 2021a {published data only}
- Hamdani SU, Huma ZE, Suleman N, Akhtar P, Nazir H, Masood A, et al. Effectiveness of a technology-assisted, family volunteers delivered, brief, multicomponent parents’ skills training intervention for children with developmental disorders in rural Pakistan: a cluster randomized controlled trial. International Journal of Mental Health Systems 2021;15(1):53. [DOI: 10.1186/s13033-021-00476-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendriks 2019 {published data only}
- Hendriks T, Schotanus-Dijkstra M, Hassankhan A, Sardjo W, Graafsma T, Bohlmeijer E, et al. Resilience and well-being in the Caribbean: Findings from a randomized controlled trial of a culturally adapted multi-component positive psychology intervention. The Journal of Positive Psychology 2019;15(2):238-253. [DOI: 10.1080/17439760.2019.1590624] [DOI] [Google Scholar]
Hinton 2021 {published data only}
- Hinton L, Nguyen H, Nguyen HT, Harvey DJ, Nichols L, Martindale‐Adams J, et al. Advancing family dementia caregiver interventions in low- and middle-income countries: a pilot cluster randomized controlled trial of resources for advancing Alzheimer’s caregiver health in Vietnam (REACH VN). Alzheimer's & Dementia: Translational Research & Clinical Interventions 2021;6(1):e12063. [DOI: 10.1002/trc2.12063] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03587974. Randomized controlled trial to test an Alzheimer's family caregiver intervention in Vietnam [Cluster randomized controlled trial to test the feasibility, acceptability and preliminary effectiveness of a psychosocial intervention to support Alzheimer's family caregivers in Vietnam]. clinicaltrials.gov/ct2/show/NCT03587974 (first received 16 July 2018).
- Nguyen TA, Nguyen H, Pham T, Nguyen TH, Hinton L. A cluster randomized controlled trial to test the feasibility and preliminary effectiveness of a family dementia caregiver intervention in Vietnam: the REACH VN study protocol. Medicine 2018;97(42):e12553. [DOI: 10.1097/MD.0000000000012553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirani 2010 {published data only}
- Hirani SS, Karmaliani R, McFarlane J, Asad N, Madhani F, Shehzad S. Testing a community derived intervention to promote women’s health: preliminary results of a 3-arm randomized controlled trial in Karachi, Pakistan. Southern Online Journal of Nursing Research 2010;10(3):1-10. [LINK: ecommons.aku.edu/pakistan_fhs_son/112/] [Google Scholar]
Hirani 2018 {published data only}
- Hirani SS, Norris CM, Vliet KJ, Zanten SV, Karmaliani R, Lasiuk G. Social support intervention to promote resilience and quality of life in women living in Karachi, Pakistan: a randomized controlled trial. International Journal of Public Health 2018;63(6):693-702. [DOI: 10.1007/s00038-018-1101-y] [DOI] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang K-Y, Nakigudde J, Calzada E, Boivin MJ, Ogedegbe G, Brotman LM. Implementing an early childhood school-based mental health promotion intervention in low-resource Ugandan schools: study protocol for a cluster randomized controlled trial. Trials 2014;15:471. [DOI: 10.1186/1745-6215-15-471] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K-Y, Nakigudde J, Rhule D, Gumikiriza-Onoria JL, Abura G, Kolawole B, et al. Transportability of an evidence-based early childhood intervention in a low-income African country: results of a cluster randomized controlled study. Prevention Science 2017;18(8):964-75. [DOI: 10.1007/s11121-017-0822-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01857388. School-based mental health program implementation in Uganda [Implementing a school-based mental health prevention program in Ugandan schools]. clinicaltrials.gov/ct2/show/NCT01857388 (first received 20 May 2013).
Hull 2021 {published data only}
- Hull DM, Hinerman KM, Fagan MA, Powell MG, Näslund-Hadley EI, Hayes D. Positive youth development in Belize: a cluster-randomised trial of Positive Action. Educational Psychology 2021;41(8):1003-23. [DOI: 10.1080/01443410.2021.1922611] [DOI] [Google Scholar]
Izgu 2020 {published data only}
- Izgu N, Gok Metin Z, Karadas C, Ozdemir L, Metinarikan N, Corapcıoglu D. Progressive muscle relaxation and mindfulness meditation on neuropathic pain, fatigue, and quality of life in patients with type 2 diabetes: a randomized clinical trial. Journal of Nursing Scholarship 2020;52(5):476-87. [DOI: 10.1111/jnu.12580] [DOI] [PubMed] [Google Scholar]
Jamali 2016 {published data only (unpublished sought but not used)}
- Jamali S, Sekineh S, Nia HS, Goudarzian AH, Beik S, Allen K-A. The effect of life skills training on mental health of Iranian middle school students: a preliminary study. Iranian Journal of Psychiatry 2016;11(4):269-72. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
James 2020 {published data only (unpublished sought but not used)}
- CTRI/2018/02/012002. Disaster mental health in Nepal and Haiti [Enhancing community resilience in the acute aftermath of disaster: evaluation of a disaster mental health intervention]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=23011&EncHid=&userName=CTRI/2018/02/012002 (first received 20 February 2018).
- James LE, Welton-Mitchell C, Noel JR, James AS. Integrating mental health and disaster preparedness in intervention: a randomized controlled trial with earthquake and flood-affected communities in Haiti. Psychological Medicine 2020;50(2):342-52. [DOI: 10.1017/S0033291719000163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jewkes 2008 {published and unpublished data}
- Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of Stepping Stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ 2008;337:a506. [DOI: 10.1136/bmj.a506] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00332878. Stepping Stones program for preventing HIV infection in residents of rural South African communities [RCT of Stepping Stones behavioural intervention for HIV]. clinicaltrials.gov/ct2/show/NCT00332878 (first received 2 June 2006).
Jiang 2021 {published data only}
- Jiang X-J, Jiang H, Chen Y, Wu X-A, Yu X-L, Liu L, et al. The effectiveness of a self-efficacy-focused structured education program (SSEP) in improving metabolic control and psychological outcomes of type 2 diabetes patients: a 12-month follow-up of a multicenter randomized controlled trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2021;14:305-13. [DOI: 10.2147/DMSO.S290029] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-J, Jiang H, Lu YH, Liu SL, Wang JP, Tang RS, et al. The effectiveness of a self‐efficacy‐focused structured education programme on adults with type 2 diabetes: a multicentre randomised controlled trial. Journal of Clinical Nursing 2019;28(17-18):3299-3309. [DOI: 10.1111/jocn.14908] [DOI] [PubMed] [Google Scholar]
Jordans 2010 {published data only}
- Jordans MJ, Komproe IH, Tol WA, Kohrt BA, Luitel NP, Macy RD, et al. Evaluation of a classroom-based psychosocial intervention in conflict-affected Nepal: a cluster randomized controlled trial. Journal of Child Psychology and Psychiatry 2010;51(7):818-26. [DOI: 10.1111/j.1469-7610.2010.02209.x] [DOI] [PubMed] [Google Scholar]
Karmaliani 2020 {published data only (unpublished sought but not used)}
- Karmaliani R, McFarlane J, Khuwaja HM, Somani Y, Bhamani SS, Ali TS. Right To Play’s intervention to reduce peer violence among children in public schools in Pakistan: a cluster-randomized controlled trial. Global Health Action 2020;13(1):1836604. [DOI: 10.1080/16549716.2020.1836604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane J, Karmaliani R, Khuwaja HM, Gulzar S, Somani R, Saeed AT, et al. Preventing peer violence against children: methods and baseline data of a cluster randomized controlled trial in Pakistan. Global Health, Science and Practice 2017;5(1):115-37. [DOI: 10.9745/GHSP-D-16-00215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2018 {published data only (unpublished sought but not used)}
- ISRCTN48032200. Employing general practitioners for delivering a child development package in Pakistan [A sustainable public-private partnership for delivering integrated child development care in Pakistan: a clustered randomized controlled trial]. https://www.isrctn.com/ISRCTN48032200 (first received 18 November 2014). [DOI: 10.1186/ISRCTN48032200] [DOI]
- Khan MA, Owais SS, Blacklock C, Anil S, Ishaq S, Maqbool S, et al. Delivering integrated child development care in Pakistan: protocol for a clustered randomised trial. BJGP Open 2017;1(1):bjgpopen17X100677. [DOI: 10.3399/bjgpopen17X100677] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Owais SS, Maqbool S, Ishaq S, Khan HJ, Minhas FA, et al. Is integrated private-clinic based early child development care effective? A clustered randomised trial in Pakistan. BJGP Open 2018;2(2):bjgpopen18X101593. [DOI: 10.3399/bjgpopen18X101593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuo 2020 {published data only (unpublished sought but not used)}
- Kuo C, Mathews C, Giovenco D, Atujuna M, Beardslee W, Hoare J, et al. Acceptability, feasibility, and preliminary efficacy of a resilience-oriented family intervention to prevent adolescent HIV and depression: a pilot randomized controlled trial. AIDS Education and Prevention 2020;32(1):67-81. [DOI: 10.1521/aeap.2020.32.1.67] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02432352. Our family our future: acceptability and feasibility study of a family prevention program for HIV risk and depression [Our family our future: family prevention of HIV risk and depression in HIV-endemic South Africa]. clinicaltrials.gov/ct2/show/NCT02432352 (first received 4 May 2015).
Lachman 2017 {published data only}
- Lachman JM, Cluver L, Ward CL, Hutchings J, Mlotshwa S, Wessels I, et al. Randomized controlled trial of a parenting program to reduce the risk of child maltreatment in South Africa. Child Abuse & Neglect 2017;72:338-51. [DOI: 10.1016/j.chiabu.2017.08.014] [DOI] [PubMed] [Google Scholar]
- Lachman JM, Kelly J, Cluver L, Ward CL, Hutchings J, Gardner F. Process evaluation of a parenting program for low-income families in South Africa. Research on Social Work Practice 2018;28(2):188-202. [DOI: 10.1177/1049731516645665] [DOI] [Google Scholar]
- NCT01802294. Sinovuyo caring families project - pilot trial (SCFP) [Sinovuyo caring families project - pilot randomised controlled trial of a parenting program to reduce child behavior problems in Xhosa children ages 3 to 8 in South Africa]. clinicaltrials.gov/ct2/show/NCT01802294 (first received 1 March 2013).
Lachman 2020 {published data only}
- Lachman J, Wamoyi J, Spreckelsen T, Wight D, Maganga J, Gardner F. Combining parenting and economic strengthening programmes to reduce violence against children: a cluster randomised controlled trial with predominantly male caregivers in rural Tanzania. BMJ Global Health 2020;5(7):e002349. [DOI: 10.1136/bmjgh-2020-002349] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02633319. Malezi na kilimo bora - skilful parenting and agribusiness child abuse prevention study (SPACAPS) [Malezi ne kilimo bora (good parenting and farming) - skilful parenting and agribusiness child abuse prevention study (SPACAPS)]. clinicaltrials.gov/ct2/show/NCT02633319 (first received 17 December 2015).
Langer 1996 {published data only}
- Langer A, Farnot U, Garcia C, Barros F, Victora C, Belizan JM, et al. The Latin American trial of psychosocial support during pregnancy: effects on mother's wellbeing and satisfaction. Social Science & Medicine 1996;42(11):1589-97. [DOI: 10.1016/0277-9536(95)00262-6] [DOI] [PubMed] [Google Scholar]
Latina 2019 {published data only}
- Latina J, Fernandez-Jimenez R, Bansilal S, Sartori S, Vedanthan R, Lewis M, et al. Grenada Heart Project–community health action to encourage healthy behaviors (GHP-CHANGE): a randomized control peer group–based lifestyle intervention. American Heart Journal 2020;220:20-8. [DOI: 10.1016/j.ahj.2019.08.022] [DOI] [PubMed] [Google Scholar]
- NCT02428920. Grenada heart project - community health action to encourage healthy behaviors (GHP CHANGE). clinicaltrials.gov/ct2/show/NCT02428920 (first received 29 April 2015).
Leventhal 2015 {published data only}
- Leventhal KS, DeMaria LM, Gillham JE, Andrew G, Peabody J, Leventhal SM. A psychosocial resilience curriculum provides the “missing piece” to boost adolescent physical health: a randomized controlled trial of Girls First in India. Social Science & Medicine 2016;161:37-46. [DOI: 10.1016/j.socscimed.2016.05.004] [DOI] [PubMed] [Google Scholar]
- Leventhal KS, Gillham J, DeMaria L, Andrew G, Peabody J, Leventhal S. Building psychosocial assets and wellbeing among adolescent girls: a randomized controlled trial. Journal of Adolescence 2015;45:284-95. [DOI: 10.1016/j.adolescence.2015.09.011] [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li L, Ji G, Liang L-J, Ding Y, Tian J, Xiao Y. A multilevel intervention for HIV-affected families in China: Together for Empowerment Activities (TEA). Social Science & Medicine 2011;73(8):1214-21. [DOI: 10.1016/j.socscimed.2011.07.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ji G, Liang L-J, Lin C, Hsieh J, Lan C-W, et al. Efficacy of a multilevel intervention on the mental health of people living with HIV and their family members in rural China. Health Psychology 2017;36(9):863-71. [DOI: 10.1037/hea0000503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liang L-J, Lin C, Lan C-W, Ji G, Xiao Y. Changes in behavioral outcomes among children affected by HIV: results of a randomized controlled trial in China. Journal of Health Psychology 2019;24(11):1581-94. [DOI: 10.1177/1359105317746479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01762553. TEA for families and children: a randomized intervention trial [TEA (Together for Empowerment Activities) for families and children: a randomized intervention trial]. clinicaltrials.gov/ct2/show/NCT01762553 (first received 7 January 2013).
Li 2019 {published data only}
- ISRCTN54477888. Cognitive behavioural stress managementduring pregnancy [Effective cognitive behavioural stress management to reduce pregnancy stress among pregnant women: a randomised controlled trial]. www.isrctn.com/ISRCTN54477888 (first received 7 October 2018). [DOI: 10.1186/ISRCTN54477888] [DOI]
- Li J, Shao D, Xu X, Zhang Y, Jiang Y, Hall J. Cognitive behavior stress management during pregnancy: a randomized controlled trial. Contemporary Nurse 2019;55(6):543-53. [DOI: 10.1080/10376178.2020.1729827] [DOI] [PubMed] [Google Scholar]
Luoto 2020 {published data only}
- Luoto JE, Lopez Garcia I, Aboud FE, Fernald LC, Singla DR. Testing means to scale early childhood development interventions in rural Kenya: the Msingi Bora cluster randomized controlled trial study design and protocol. BMC Public Health 2019;19(1):259. [DOI: 10.1186/s12889-019-6584-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto JE, Lopez Garcia I, Aboud FE, Singla DR, Fernald LC, Pitchik HO, et al. Group-based parenting interventions to promote child development in rural Kenya: a multi-arm, cluster-randomised community effectiveness trial. Lancet Global Health 2020;9(3):e309-19. [DOI: 10.1016/ S2214-109X(20)30469-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03548558. Testing means to scale early childhood development interventions in rural Kenya. clinicaltrials.gov/ct2/show/NCT03548558 (first received 7 June 2018).
Masquiller 2014 {published data only (unpublished sought but not used)}
- Bhargava A, Booysen FL, Walsh CM. Health status, food insecurity, and time allocation patterns of patients with AIDS receiving antiretroviral treatment in South Africa. AIDS Care 2017;30(3):361-8. [DOI: 10.1080/09540121.2017.1371665] [DOI] [PubMed] [Google Scholar]
- Booysen F, Walque D, Over M, Hashimoto S, Reuck C. A randomized control trial of a peer adherence and nutritional support program for public sector antiretroviral patients. Washington (DC): World Bank Group, Development Research Group, Human Development and Public Services Team; 2016 July. Report No. 7760.
- Masquillier C, Wouters E, Mortelmans D, Booysen FL. Families as catalysts for peer adherence support in enhancing hope for people living with HIV/AIDS in South Africa. Journal of the International AIDS Society 2014;17(1):18802. [DOI: 10.7448/IAS.17.1.18802] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00821366. Effective AIDS treatment and support in The Free State (FEATS) [Effective AIDS treatment and support in The Free State (FEATS): adherence and nutritional support for effective and sustainable antiretroviral treatment in resource constrained settings]. clinicaltrials.gov/ct2/show/NCT00821366 (first received 13 January 2009).
- Pappin M, Wouters E, Booysen FL. Anxiety and depression amongst patients enrolled in a public sector antiretroviral treatment programme in South Africa: a cross- sectional study. BMC Public Health 2012;12:244. [DOI: 10.1186/1471-2458-12-244] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2015 {published data only}
- ACTRN12614000774628. Evaluation of a self-help manual for supporting family carers of clients with depression in Thailand: a randomised controlled trial [Effect of a self-help manual for supporting family carers of clients with depression in Thailand, on resilience, expressed emotion and experience of caregiving: a randomised controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366639 (first received 2 July 2014).
- McCann TV, Songprakun W, Stephenson J. A randomized controlled trial of guided self-help for improving the experience of caring for carers of clients with depression. Journal of Advanced Nursing 2015;71(7):1600-10. [DOI: 10.1111/jan.12624] [PMID: ] [DOI] [PubMed] [Google Scholar]
- McCann TV, Songprakun W, Stephenson J. Effectiveness of guided self-help in decreasing expressed emotion in family caregivers of people diagnosed with depression in Thailand: a randomised controlled trial. BMC Psychiatry 2015;15:258. [DOI: 10.1186/s12888-015-0654-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann TV, Songprakun W, Stephenson J. Efficacy of a self‐help manual in increasing resilience in carers of adults with depression in Thailand. International Journal of Mental Health Nursing 2016;25(1):62-70. [DOI: 10.1111/inm.12178] [DOI] [PubMed] [Google Scholar]
McConachie 2000 {published data only (unpublished sought but not used)}
- McConachie H, Huq S, Munir S, Ferdous S, Zaman S, Khan NZ. A randomized controlled trial of alternative modes of service provision to young children with cerebral palsy in Bangladesh. Journal of Pediatrics 2000;137(6):769-76. [DOI: 10.1067/mpd.2000.110135] [DOI] [PubMed] [Google Scholar]
Miller 2020 {published data only}
- ISRCTN22321773. Helping refugee parents thrive: an evaluation of the caregiver support intervention with Syrian refugees in Lebanon [A randomized controlled trial of the caregiver support intervention with Syrian refugees in Lebanon]. www.isrctn.com/ISRCTN22321773 (first received 31 July 2019).
- Miller KE, Arnous M, Tossyeh F, Chen A, Bakolis I, Koppenol-Gonzalez GV, et al. Protocol for a randomized control trial of the caregiver support intervention with Syrian refugees in Lebanon. Trials 2020;21(1):277. [DOI: 10.1186/s13063-020-4175-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Koppenol-Gonzalez GV, Arnous M, Tossyeh F, Chen A, Nahas N, et al. Supporting Syrian families displaced by armed conflict: a pilot randomized controlled trial of the Caregiver Support Intervention. Child Abuse & Neglect 2020;106:104512. [DOI: 10.1016/j.chiabu.2020.104512] [DOI] [PubMed] [Google Scholar]
Mohamadi 2021 {published data only}
- Mohamadi S, Paryab S, Mousavi SA, Keramat A, Motaghi Z, Garkaz O. Comparison of the effect of motivational interview and peer group education on knowledge and performance about puberty and mental health in adolescent girls. Journal of Nursing and Midwifery Sciences 2021;8(3):178-85. [DOI: 10.4103/JNMS.JNMS_65_20] [DOI] [Google Scholar]
Novelli 2018 {published data only}
- Novelli MM, Machado SC, Lima GB, Cantatore L, Sena BP, Rodrigues RS, et al. Effects of the Tailored Activity Program in Brazil (TAP-BR) for persons with dementia: a randomized pilot trial. Alzheimer Disease & Associated Disorders 2018;32(4):339-45. [DOI: 10.1097/WAD.0000000000000256] [DOI] [PubMed] [Google Scholar]
Osborn 2020 {published data only}
- Osborn TL, Rodriguez M, Wasil AR, Venturo-Conerly KE, Gan J, Alemu RG, et al. Single-session digital intervention for adolescent depression, anxiety, and well-being: outcomes of a randomized controlled trial with Kenyan adolescents. Journal of Consulting and Clinical Psychology 2020;88(7):657-68. [DOI: 10.1037/ccp0000505] [DOI] [PubMed] [Google Scholar]
- PACTR201906810558181. Internet-based Shamiri single session intervention [The Shamiri single session digital intervention for adolescent anxiety and depression: a randomized control trial of an internet-based intervention in a low-resourced school in Kenya]. trialsearch.who.int/?TrialID=PACTR201906810558181 (first received 13 June 2019).
- Wasil AR, Kacmarek CN, Osborn TL, Palermo EH, DeRubeis RJ, Weisz JR, et al. Economic evaluation of an online single-session intervention for depression in Kenyan adolescents. Journal of Consulting and Clinical Psychology 2021;89(8):657-67. [DOI: 10.1037/ccp0000669] [DOI] [PubMed] [Google Scholar]
Ozcan 2020 {published data only}
- Ozcan S, Eryilmaz G. Using Levine’s conservation model in postpartum care: a randomized controlled trial. Health Care for Women International 2020;42(4-6):794-814. [DOI: 10.1080/07399332.2020.1797038] [DOI] [PubMed] [Google Scholar]
O’Callaghan 2014 {published data only}
- NCT01542398. A family-based, resilience-focused intervention for war-affected communities in north-eastern Democratic Republic of Congo [Is a family-based, life skills focused intervention effective in reducing psychological distress and stigma and improving inter-personal relations and functioning among former LRA abductees and other war-affected children in their community in Dungu, the Democratic Republic of Congo?]. www.clinicaltrials.gov/ct2/show/NCT01542398 (first received 2 March 2012).
- O’Callaghan P, Branham L, Shannon C, Betancourt TS, Dempster M, McMullen J. A pilot study of a family focused, psychosocial intervention with war-exposed youth at risk of attack and abduction in north-eastern Democratic Republic of Congo. Child Abuse & Neglect 2014;38(7):1197-1207. [DOI: 10.1016/j.chiabu.2014.02.004] [DOI] [PubMed] [Google Scholar]
Panter‐Brick 2018 {published data only (unpublished sought but not used)}
- NCT03012451. A psychosocial program impact evaluation in Jordan [Measuring the health and wellbeing impacts of a scalable program of psychosocial intervention for refugee youth]. clinicaltrials.gov/ct2/show/NCT03012451 (first received 6 January 2017).
- Panter-Brick C, Dajani R, Eggerman M, Hermosilla S, Sancilio A, Ager A. Insecurity, distress and mental health: experimental and randomized controlled trials of a psychosocial intervention for youth affected by the Syrian crisis. Journal of Child Psychology and Psychiatry 2018;59(5):523-41. [DOI: 10.1111/jcpp.12832] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prabhakaran 2019 {published data only}
- Jha D, Gupta P, Ajay VS, Jindal D, Perel P, Prieto-Merino D, et al. Protocol for the mWellcare trial: a multicentre, cluster randomised, 12-month, controlled trial to compare the effectiveness of mWellcare, an mHealth system for an integrated management of patients with hypertension and diabetes, versus enhanced usual care in India. BMJ Open 2017;7(8):e014851. [DOI: 10.1136/bmjopen-2016-014851] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02480062. mWELLCARE: an integrated mHealth system for the prevention and care of chronic disease (mWELLCARE). www.clinicaltrials.gov/ct2/show/NCT02480062 (first received 24 Jun3 2015).
- Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation 2019;139(3):380-91. [DOI: 10.1161/CIRCULATIONAHA.118.038192] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rachasrimuang 2018 {published data only}
- Rachasrimuang S, Kuhirunyaratn P, Bumrerraj S. Effectiveness of a home visit programme by youth volunteers on health-related quality of life and depression among elderly persons: results from a cluster randomized controlled trial in rural Thailand. Journal of the Medical Association of Thailand 2018;101(Suppl 5):s189-95. [LINK: www.jmatonline.com/index.php/jmat/article/view/9866] [Google Scholar]
Rahimi 2021a {published data only}
- Rahimi M, Mahdizadeh M, Chamanzari H, Mahdizadeh S-M. The effect of peer support with telecommunication on subjective well-being in colorectal patients: a randomized controlled clinical trial. International Journal of Community Based Nursing and Midwifery 2021;9(2):127-38. [DOI: 10.30476/ijcbnm.2021.88061.1484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2009 {published data only}
- Rahman A, Iqbal Z, Roberts C, Husain N. Cluster randomized trial of a parent-based intervention to support early development of children in a low-income country. Child: Care, Health and Development 2009;35(1):56-62. [DOI: 10.1111/j.1365-2214.2008.00897.x] [DOI] [PubMed] [Google Scholar]
Rajeswari 2020 {published data only}
- Rajeswari S, SanjeevaReddy N. Efficacy of progressive muscle relaxation on pregnancy outcome among anxious Indian primi mothers. Iranian Journal of Nursing and Midwifery Research 2020;25(1):23-30. [LINK: ijnmr.mui.ac.ir/index.php/ijnmr/article/view/1682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramezani 2017 {published data only}
- Ramezani S, Khosravi A, Motaghi Z, Hamidzadeh A, Mousavi SA. The effect of cognitive-behavioural and solution-focused counselling on prevention of postpartum depression in nulliparous pregnant women. Journal of Reproductive and Infant Psychology 2017;35(2):172-82. [DOI: 10.1080/02646838.2016.1266470] [DOI] [PubMed] [Google Scholar]
Rao 2017 {published data only}
- Rao M, Metri KG, Raghuram N, Hongasandra NR. Effects of mind sound resonance technique (yogic relaxation) on psychological states, sleep quality, and cognitive functions in female teachers: a randomized, controlled trial. Advances in Mind-Body Medicine 2017;31(1):4-9. [PMID: ] [PubMed] [Google Scholar]
Richards 2014 {published data only}
- Richards J, Foster C, Townsend N, Bauman A. Physical fitness and mental health impact of a sport-for-development intervention in a post-conflict setting: randomised controlled trial nested within an observational study of adolescents in Gulu, Uganda. BMC Public Health 2014;14:619. [DOI: 10.1186/1471-2458-14-619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rivet‐Duval 2011 {published data only}
- ACTRN12608000137392. Preventing adolescent depression in Mauritius [Preventing adolescent depression in Mauritius: a randomized controlled trial of a universal school-based program]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82641 (first received 27 February 2008).
- Rivet-Duval E, Heriot S, Hunt C. Preventing adolescent depression in Mauritius: a universal school-based program. Child and Adolescent Mental Health 2011;16(2):86-91. [DOI: 10.1111/j.1475-3588.2010.00584.x] [DOI] [PubMed] [Google Scholar]
Rockers 2018 {published data only}
- NCT02234726. Improving early childhood development in Zambia (IECDZ). clinicaltrials.gov/ct2/show/NCT02234726 (first received 9 September 2014).
- Rockers PC, Fink G, Zanolini A, Banda B, Biemba G, Sullivan C, et al. Impact of a community-based package of interventions on child development in Zambia: a cluster-randomised controlled trial. BMJ Global Health 2016;1(3):e000104. [DOI: 10.1136/bmjgh-2016-000104] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockers PC, Zanolini A, Banda B, Chipili MM, Hughes RC, Hamer DH, et al. Two-year impact of community-based health screening and parenting groups on child development in Zambia: follow-up to a cluster-randomized controlled trial. PLOS Medicine 2018;15(4):e1002555. [DOI: 10.1371/journal.pmed.1002555] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2021 {published data only}
- Rodriguez M, Eisenlohr-Moul TA, Weisman J, Rosenthal MZ. The use of task shifting to improve treatment engagement in an internet-based mindfulness intervention among Chinese university students: randomized controlled trial. JMIR Formative Research 2021;5(10):e25772. [DOI: 10.2196/25772] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rong 2021a {published data only}
- Rong L, Wang R, Ouyang Y-Q, Redding SR. Efficacy of yoga on physiological and psychological discomforts and delivery outcomes in Chinese primiparas. Complementary Therapies in Clinical Practice 2021;44:101434. [DOI: 10.1016/j.ctcp.2021.101434] [DOI] [PubMed] [Google Scholar]
Rong 2021b {published data only}
- NCT02645643. Medical education intervention for medical students [Intervention to promote medical students empathy, well-being and study emotion-a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT02645643 (first received 5 January 2016).
- Rong R, Chen W, Dai Z, Gu J, Chen W, Zhou Y, et al. Improvement of the management of mental well-being and empathy in Chinese medical students: a randomized controlled study. BMC Medical Education 2021;21(1):378. [DOI: 10.1186/s12909-021-02813-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2014a {published data only}
- NCT00972699. Mentor mothers: a sustainable family intervention in South African townships. clinicaltrials.gov/ct2/show/NCT00972699 (first received 7 September 2009).
- Richter L, Rotheram-Borus MJ, Van Heerden A, Stein A, Tomlinson M, Harwood JM, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS and Behavior 2014;18(4):706-15. [DOI: 10.1007/s10461-014-0694-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Richter L, Rooyen H, Heerden A, Tomlinson M, Stein A, et al. Project Masihambisane: a cluster randomised controlled trial with peer mentors to improve outcomes for pregnant mothers living with HIV. Trials 2011;12:2. [DOI: 10.1186/1745-6215-12-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Richter LM, Heerden A, Rooyen H, Tomlinson M, Harwood JM, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLOS ONE 2014;9(1):e84867. [DOI: 10.1371/journal.pone.0084867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2014b {published data only}
- NCT00996528. Neighborhood alcohol & HIV prevention in South African townships (Philani). clinicaltrials.gov/ct2/show/NCT00996528 (first received 16 October 2009).
- Rotheram-Borus MJ, Tomlinson M, le Roux IM, Harwood JM, Comulada S, O'Connor MJ, et al. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLOS ONE 2014;9(10):e105934. [DOI: 10.1371/journal.pone.0105934] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, Rotheram-Borus MJ, le Roux IM, Youssef M, Nelson SH, Scheffler A, et al. Thirty-six-month outcomes of a generalist paraprofessional perinatal home visiting intervention in South Africa on maternal health and child health and development. Prevention Science 2016;17(8):937-48. [DOI: 10.1007/s11121-016-0676-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux IM, Tomlinson M, Harwood JM, O’Connor MJ, Worthman CM, Mbewu N, et al. Outcomes of home visits for pregnant mothers and their infants: a cluster randomised controlled trial. AIDS 2013;27(9):1461-71. [DOI: 10.1097/QAD.0b013e3283601b53] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanfilippo 2020 {published data only}
- PACTR201901917619299. Community singing-based intervention for perinatal mental health in The Gambia [Testing the feasibility of a community singing-based intervention for perinatal mental health in The Gambia]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=5878 (first received 24 January 2019).
- Sanfilippo KR, McConnell B, Cornelius V, Darboe B, Huma HB, Gaye M, et al. A study protocol for testing the feasibility of a randomised stepped wedge cluster design to investigate a community health intervention through musical engagement (CHIME) for perinatal mental health in The Gambia. Pilot and Feasibility Studies 2019;5:124. [DOI: 10.1186/s40814-019-0515-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo KR, McConnell B, Cornelius V, Darboe B, Huma HB, Gaye M, et al. Community psychosocial music intervention (CHIME) to reduce antenatal common mental disorder symptoms in The Gambia: a feasibility trial. BMJ Open 2020;10(11):e040287. [DOI: 10.1136/bmjopen-2020-040287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangraula 2020 {published data only}
- NCT03359486. Pilot feasibility study of psychosocial support to improve well-being of adults in humanitarian crises in Nepal (PM+) [Pilot feasibility study of focused psychosocial support to improve the psychosocial well-being and functioning of adults affected by humanitarian crisis in Nepal]. clinicaltrials.gov/ct2/show/NCT03359486 (first received 2 December 2017).
- Sangraula M, Turner EL, Luitel NP, van‘t Hof E, Shrestha P, Ghimire R, et al. Feasibility of Group Problem Management Plus (PM+) to improve mental health and functioning of adults in earthquake-affected communities in Nepal. Epidemiology and Psychiatric Sciences 2020;29:e130. [DOI: 10.1017/S2045796020000414] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangraula M, van’t Hof E, Luitel NP, Turner EL, Marahatta K, Nakao JH, et al. Protocol for a feasibility study of group-based focused psychosocial support to improve the psychosocial well-being and functioning of adults affected by humanitarian crises in Nepal: Group Problem Management Plus (PM+). Pilot and Feasibility Studies 2018;4:126. [DOI: 10.1186/s40814-018-0315-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2009 {published data only}
- German D, Sutcliffe CG, Sirirojn B, Sherman SG, Latkin CA, Aramrattana A, et al. Unanticipated effect of a randomized peer network intervention on depressive symptoms among young methamphetamine users in Thailand. Journal of Community Psychology 2009;40(7):799-813. [DOI: 10.1002/jcop.21488] [DOI] [Google Scholar]
- Sherman SG, Sutcliffe C, Srirojn B, Latkin CA, Aramratanna A, Celentano DD. Evaluation of a peer network intervention trial among young methamphetamine users in Chiang Mai, Thailand. Social Science & Medicine 2009;68(1):69-79. [DOI: 10.1016/j.socscimed.2008.09.061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinde 2018 {published data only}
- NCT02484014. Evaluation of school-based health promotion programmes in Bihar, India (SEHER) [Strengthening the evidence base on effective school based interventions for promoting adolescent health (SEHER)]. clinicaltrials.gov/ct2/show/NCT02484014 (first received 29 June 2015).
- Shinde S, Pereira B, Khandeparkar P, Sharma A, Patton G, Ross DA, et al. The development and pilot testing of a multicomponent health promotion intervention (SEHER) for secondary schools in Bihar, India. Global Health Action 2017;10(1):1385284. [DOI: 10.1080/16549716.2017.1385284] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Weiss HA, Khandeparkar P, Pereira B, Sharma A, Gupta R, et al. A multicomponent secondary school health promotion intervention and adolescent health: An extension of the SEHER cluster randomised controlled trial in Bihar, India. PLOS Medicine 2020;17(2):e1003021. [DOI: 10.1371/journal. pmed.1003021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Weiss HA, Varghese B, Khandeparkar P, Pereira B, Sharma A, et al. Promoting school climate and health outcomes with the SEHER multi-component secondary school intervention in Bihar, India: a cluster-randomised controlled trial. Lancet 2018;392(10163):2465-77. [DOI: 10.1016/S0140-6736(18)31615-5] [DOI] [PubMed] [Google Scholar]
- Singla DR, Shinde S, Patton G, Patel V. The mediating effect of school climate on adolescent mental health: findings from a randomized controlled trial of a school-wide intervention. Journal of Adolescent Health 2021;69(1):90-9. [DOI: 10.1016/j.jadohealth.2020.09.030] [DOI] [PubMed] [Google Scholar]
Singla 2015 {published data only}
- NCT01906606. Effectiveness of parenting programs on child development and maternal well-being in rural Uganda. clinicaltrials.gov/ct2/show/NCT01906606 (first received 24 July 2013).
- Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster-randomised trial. Lancet Global Health 2015;3(8):e458-69. [DOI: 10.1016/S2214-109X(15)00099-6] [DOI] [PubMed] [Google Scholar]
- Singla DR, Kumbakumba E. The development and implementation of a theory-informed, integrated mother-child intervention in rural Uganda. Social Science & Medicine 2015;147:242-51. [DOI: 10.1016/j.socscimed.2015.10.069] [DOI] [PubMed] [Google Scholar]
Skar 2021 {published data only}
- Skar A-M, Sherr L, Macedo A, Tetzchner S, Fostervold KI. Evaluation of parenting interventions to prevent violence against children in Colombia: a randomized controlled trial. Journal of Interpersonal Violence 2021;36(1-2):NP1098-1126. [DOI: 10.1177/0886260517736881] [DOI] [PubMed] [Google Scholar]
Song 2019 {published data only}
- Song D, Yu DS. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. International Journal of Nursing Studies 2019;93:97-105. [DOI: 10.1016/j.ijnurstu.2019.02.019] [DOI] [PubMed] [Google Scholar]
Srisuwan 2020 {published data only}
- Srisuwan P, Nakawiro D, Chansirikarnjana S, Kuha O, Chaikongthong P, Suwannagoot T. Effects of a group-based 8-week multicomponent cognitive training on cognition, mood and activities of daily living among healthy older adults: a one-year follow-up of a randomized controlled trial. Journal of Prevention of Alzheimer's Disease 2020;7(2):112-21. [DOI: 10.14283/jpad.2019.42] [DOI] [PubMed] [Google Scholar]
Thurman 2017 {published data only}
- NCT02368808. Adolescent bereavement initiative in The Free State, South Africa [Coping with loss: a bereavement initiative for adolescent girls in The Free State, South Africa]. clinicaltrials.gov/ct2/show/NCT02368808 (first received 23 February 2013).
- Thurman TR, Luckett BG, Nice J, Spyrelis A, Taylor TM. Effect of a bereavement support group on female adolescents' psychological health: a randomised controlled trial in South Africa. Lancet Global Health 2017;5(6):e604-14. [DOI: 10.1016/S2214-109X(17)30146-8] [DOI] [PubMed] [Google Scholar]
Tobias da Silva 2017 {published and unpublished data}
- Tobias da Silva SG, Santos MA, Floriano CM, Damião EB, Campos FV, Rossato LM. Influence of Therapeutic Play on the anxiety of hospitalized school-age children: clinical trial [Influencia del Juguete Terapéutico en la ansiedad de niños en edad escolar hospitalizados: ensayo clínico]. Revista Brasileira de Enfermagem 2017;70(6):1244-9. [DOI: 10.1590/0034-7167-2016-0353] [DOI] [PubMed] [Google Scholar]
Tol 2012 {published data only}
- Jordans MJ, Komproe IH, Tol WA, Susanty D, Vallipuram A, Ntamatumba P, et al. Practice-driven evaluation of a multi-layered psychosocial care package for children in areas of armed conflict. Community Mental Health Journal 2011;47:267–77. [DOI: 10.1007/s10597-010-9301-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol WA, Komproe IH, Jordans MJ, Vallipuram A, Sipsma H, Sivayokan S, et al. Outcomes and moderators of a preventive school-based mental health intervention for children affected by war in Sri Lanka: a cluster randomized trial. World Psychiatry 2012;11(2):114-22. [DOI: 10.1016/j.wpsyc.2012.05.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathy 2010 {published data only}
- Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 2010;375(9721):1182-92. [DOI: 10.1016/S0140-6736(09)62042-0] [DOI] [PubMed] [Google Scholar]
Vargas‐Porras 2021 {published data only}
- Vargas‐Porras C, Roa‐Díaz ZM, Hernández‐Hincapié HG, Ferré‐Grau C, Molina‐Fernández MI. Efficacy of a multimodal nursing intervention strategy in the process of becoming a mother: a randomized controlled trial. Research in Nursing & Health 2021;44(3):424-37. [DOI: 10.1002/nur.22123] [DOI] [PubMed] [Google Scholar]
Vazir 2013 {published data only (unpublished sought but not used)}
- Fernandez Rao S, Bentley ME, Balakrishna N, Griffiths P, Creed‐Kanashiro H, Vazir S, et al. A complementary feeding and play intervention improves the home environment and mental development among toddlers in rural India. Maternal & Child Nutrition 2020;16(Suppl 3):e13066. [DOI: 10.1111/mcn.13066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazir S, Engle P, Balakrishna N, Griffiths PL, Johnson SL, Creed‐Kanashiro H, et al. Cluster-randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal & Child Nutrition 2013;9(1):99-117. [DOI: 10.1111/j.1740-8709.2012.00413.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Velásquez 2015 {published data only}
- Velásquez AM, López MA, Quiñonez N, Paba DP. Yoga for the prevention of depression, anxiety, and aggression and the promotion of socio-emotional competencies in school-aged children. Educational Research and Evaluation 2015;21(5-6):407-21. [DOI: 10.1080/13803611.2015.1111804] [DOI] [Google Scholar]
Ward 2020 {published data only}
- NCT02165371. The Sinovuyo caring families project: a randomized controlled trial of a parenting programme [Sinovuyo caring families project]. clinicaltrials.gov/ct2/show/NCT02165371 (first received 17 June 2014).
- PACTR201402000755243. Sinovuyo caring families project. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=755 (first received 30 January 2014).
- Ward CL, Wessels IM, Lachman JM, Hutchings J, Cluver LD, Kassanjee R, et al. Parenting for lifelong health for young children: a randomized controlled trial of a parenting program in South Africa to prevent harsh parenting and child conduct problems. Journal of Child Psychology and Psychiatry 2020;61(4):503-12. [DOI: 10.1111/jcpp.13129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willis 2019 {published data only}
- PACTR201711002755428. The impact of community adolescent treatment supporters on linkage, retention, adherence and psychosocial wellbeing among adolescents with HIV [The effectiveness of community adolescent treatment supporters (CATS) in improving linkage and retention in care, adherence to ART and psychosocial well-being: a longitudinal study among adolescents with HIV in rural Zimbabwe]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=2755 (first received 11 November 2017).
- Willis N, Milanzi A, Mawodzeke M, Dziwa C, Armstrong A, Yekeye I, et al. Effectiveness of community adolescent treatment supporters (CATS) interventions in improving linkage and retention in care, adherence to ART and psychosocial well-being: a randomised trial among adolescents living with HIV in rural Zimbabwe. BMC Public Health 2019;19(1):117. [DOI: 10.1186/s12889-019-6447-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2021 {published data only}
- Xu C, Dong Z, Zhang P, Chang G, Xiang Q, Zhang M, et al. Effect of group cognitive behavioural therapy on psychological stress and blood glucose in people with type 2 diabetes mellitus: a community‐based cluster randomized controlled trial in China. Diabetic Medicine 2021;38(2):e14491. [DOI: 10.1111/dme.14491] [DOI] [PubMed] [Google Scholar]
Yang 2022 {published data only}
- Yang L, Xuan C, Yu C, Jin X, Zheng P, Yan J. Effects of comprehensive intervention on life quality among the elderly with Alzheimer disease and their caregivers based on mixed models. Nursing Open 2022;9(2):1412-22. [DOI: 10.1002/nop2.917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeomans 2010 {published data only}
- Yeomans PD, Forman EM, Herbert JD, Yuen E. A randomized trial of a reconciliation workshop with and without PTSD psychoeducation in Burundian sample. Journal of Traumatic Stress 2010;23(3):305-12. [DOI: 10.1002/jts.20531] [DOI] [PubMed] [Google Scholar]
Yu 2002 {published data only}
- Yu DL, Seligman ME. Preventing depressive symptoms in Chinese children. Prevention & Treatment 2002;5(1):9. [DOI: 10.1037/1522-3736.5.1.59a] [DOI] [Google Scholar]
Yusoff 2015 {published and unpublished data}
- Yusoff MS, Esa AR. A DEAL-based intervention for the reduction of depression, denial, self-blame and academic stress: a randomized controlled trial. Journal of Taibah University Medical Sciences 2015;10(1):82-92. [DOI: 10.1016/j.jtumed.2014.08.003] [DOI] [Google Scholar]
Zhou 2010 {published data only}
- Zhou B, Chen K, Yu Y, Wang H, Zhang S, Zheng W. Individualized health intervention: behavioral change and quality of life in an older rural Chinese population. Educational Gerontology 2010;36(10-11):919-39. [DOI: 10.1080/03601271003689514] [DOI] [Google Scholar]
References to studies excluded from this review
Abdullahzadeh 2021 {published data only}
- Abdullahzadeh M, Khosravi N. Designing a need‑based program for relieving psychological distress of family caregivers of leukemia patients: a randomized controlled trial. Supportive Care in Cancer 2021;29(12):7601-10. [DOI: 10.1007/s00520-021-06353-z] [DOI] [PubMed] [Google Scholar]
Ackan 2019 {published data only}
- Akcan A, Ergun A. The effect of an aggressive behavior prevention program on kindergarten students. Public Health Nursing 2019;36(3):330-40. [DOI: 10.1111/phn.12575] [DOI] [PubMed] [Google Scholar]
Ahmadi 2021 {published data only}
- Ahmadi Z, Robat AA, Teshnizi SH, Jamhiry R, Faghih A. Comparison of the effect of thought distraction by music therapy and the presence of caregiver on anxiety level of patients undergoing endoscopy. Journal of HAYAT 2021;27(2):161-75. [LINK: hayat.tums.ac.ir/article-1-4092-en.html] [Google Scholar]
Al‐Ameri 2021 {published data only}
- Al-Ameri MH. Effectiveness of a Psychosocial Rehabilitation Programme for Iraqi Repatriated Prisoners of the Iran-Iraq War, 1980-1988 [Doctoral Thesis]. Liverpool (UK): Liverpool John Moores University, 2012. [LINK: researchonline.ljmu.ac.uk/id/eprint/10831/] [Google Scholar]
Alampay 2019 {published data only}
- Alampay LP, Galvez Tan LJ, Tuliao AP, Baranek P, Ofreneo MA, Lopez GD, et al. A pilot randomized controlled trial of a mindfulness program for Filipino children. Mindfulness 2019;11:303-16. [DOI: 10.1007/s12671-019-01124-8] [DOI] [Google Scholar]
- Galvez Tan LJ, Alampay LP. Exploring moderators of intervention effects of a mindfulness program for Filipino children. International Journal of School & Educational Psychology 2022;10(3):368-82. [DOI: 10.1080/21683603.2020.1856741] [DOI] [Google Scholar]
Al‐Faris 1997 {published data only}
- Al-Faris E, Al-Subaie A, Khoja T, Al-Ansary L, Abdul-Raheem F, Al-Hamdan N, et al. Training primary health care physicians in Saudi Arabia to recognize psychiatric illness. Acta Psychiatrica Scandinavica 1997;96(6):439-44. [DOI: 10.1111/j.1600-0447.1997.tb09945.x] [DOI] [PubMed] [Google Scholar]
Alvi 2021 {published data only}
- Alvi MH, Shiri T, Iqbal N, Husain MO, Chaudhry I, Shakoor S, et al. Cost-effectiveness of a culturally adapted manual-assisted brief psychological intervention for self-harm in Pakistan: a secondary analysis of the culturally adapted manual-assisted brief psychological randomized controlled trial. Value in Health Regional Issues 2021;27:65-71. [DOI: 10.1016/j.vhri.2021.08.005] [DOI] [PubMed] [Google Scholar]
Ansai 2015 {published data only}
- Ansai JH, Rebelatto JR. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: a randomized controlled trial. Geriatrics & Gerontology International 2015;15(9):1127-34. [DOI: 10.1111/ggi.12411] [DOI] [PubMed] [Google Scholar]
- NCT01983397. Effects of two physical exercise protocols on cognition and physical performances in elderly over 80 years. www.clinicaltrials.gov/ct2/show/NCT01983397 (first received 14 November 2013).
Anusuya 2021 {published data only}
- Anusuya US, Mohanty S, Saoji AA. Effect of mind sound resonance technique (MSRT–Ayoga-based relaxation technique) on psychological variables and cognition in school children: a randomized controlled trial. Complementary Therapies in Medicine 2021;56:102606. [DOI: 10.1016/j.ctim.2020.102606] [DOI] [PubMed] [Google Scholar]
Araújo 2016 {published data only}
- Araújo WS, Garcia Romero W, Zandonade E, Costa Amorim ME. Effects of relaxation on depression levels in women with high-risk pregnancies: a randomised clinical trial. Revista Latino-Americana de Enfermagem 2016;24:e2806. [DOI: 10.1590/1518-8345.1249.2806] [DOI] [PMC free article] [PubMed] [Google Scholar]
Araya 2013 {published data only}
- Araya R, Fritsch R, Spears M, Rojas G, Martinez V, Barroilhet S, et al. School intervention to improve mental health of students in Santiago, Chile: a randomized clinical trial. JAMA Pediatrics 2013;167(11):1004. [DOI: 10.1001/jamapediatrics.2013.2361] [DOI] [PubMed] [Google Scholar]
Asampong 2021 {published data only}
- Asampong E, Ibrahim A, Sensoy-Bahar O, Kumbelim K, Yaro PB, McKay MM, et al. Adaption and implementation of the multiple-family group intervention in Ghana. Psychiatric Services 2021;72(5):571-7. [DOI: 10.1176/appi.ps.201900626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attanasio 2019 {published data only}
- Attanasio O, Caeyers B, Cattan S, Sosa LC, Krutikova S, Leighton P, et al. Improving early childhood development in rural Ghana through scalable community-run play schemes: programme impact evaluation report. London (UK): Institute for Fiscal Studies Reports; 2019 July. [LINK: ifs.org.uk/publications/improving-early-childhood-development-rural-ghana-through-scalable-community-run-play]
Atukunda 2019 {published data only}
- Atukunda P, Muhoozi GK, Westerberg AC, Iversen PO. Nutrition, hygiene and stimulation education for impoverished mothers in rural Uganda: effect on maternal depression symptoms and their associations to child development outcomes. Nutrients 2019;11(7):1561. [DOI: 10.3390/nu11071561] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atukunda P, Muhoozi GK, den Broek TJ, Kort R, Diep LM, Kaaya AN, et al. Child development, growth and microbiota: follow-up of a randomized education trial in Uganda. Journal of Global Health 2019;9(1):010431. [DOI: 10.7189/jogh.09.010431] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhoozi GK, Atukunda P, Diep LM, Mwadime R, Kaaya AN, Skaare AB, et al. Nutrition, hygiene, and stimulation education to improve growth, cognitive, language, and motor development among infants in Uganda: a cluster-randomized trial. Maternal & Child Nutrition 2018;14(2):e12527. [DOI: 10.1111/mcn.12527] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azizi 2010 {published data only}
- Azizi M, Lamiyan M, Faqihzadeh S, Nematollahzadeh M. The effect of counseling on the rate of traumatic postpartum anxiety in nulliparous women: a single-blind randomized clinical trial. Journal of Kermanshah University of Medical Sciences 2010;14(3):219-27. [LINK: brieflands.com/articles/jkums-79475.html] [Google Scholar]
Baek 2013 {published data only}
- Baek ES, Park HJ. Effects of a case management program on self-efficacy, depression and anxiety in pregnant women with gestational diabetes mellitus. Korean Journal of Women Health Nursing 2013;19(2):88-98. [DOI: 10.4069/kjwhn.2013.19.2.88] [DOI] [PubMed] [Google Scholar]
Baker‐Henningham 2009 {published data only}
- Baker-Henningham H, Walker S, Powell C, Meeks Gardner J. A pilot study of the Incredible Years Teacher Training programme and a curriculum unit on social and emotional skills in community pre-schools in Jamaica. Child: Care, Health and Development 2009;35(5):624-31. [DOI: 10.1111/j.1365-2214.2009.00964.x] [DOI] [PubMed] [Google Scholar]
Bakhtavar 2007 {published data only}
- Bakhtavar I, Neshat-Doost HR, Molavi H, Bahrami F. Efficacy of meta cognitive behavioral therapy in reducing self punishment in patients with post traumatic stress disorder [اثربخشي درمان رفتاري- فراشناختي بر كاهش ميزان خودتنبيهي در بيماران مبتلا بهاختلال استرس پس از سانحه]. Journal of Research in Behavioral Sciences 2007;5(2):93-8. [LINK: rbs.mui.ac.ir/article-1-124-en.html] [Google Scholar]
Barnhart 2020 {published data only}
- Barnhart DA, Farrar J, Murray SM, Brennan RT, Antonaccio CM, Sezibera V, et al. Lay-worker delivered home visiting promotes early childhood development and reduces violence in Rwanda: a randomized pilot. Journal of Child and Family Studies 2020;29:1804-17. [DOI: 10.1007/s10826-020-01709-1] [DOI] [Google Scholar]
Baruah 2021 {published data only}
- Baruah U, Varghese M, Loganathan S, Mehta KM, Gallagher‐Thompson D, Zandi D, et al. Feasibility and preliminary effectiveness of an online training and support program for caregivers of people with dementia in India: a randomized controlled trial. International Journal of Geriatric Psychiatry 2021;36(4):606-17. [DOI: 10.1002/gps.5502] [DOI] [PubMed] [Google Scholar]
Betancourt 2014 {published data only}
- Betancourt TS, McBain R, Newnham EA, Akinsulure-Smith AM, Brennan RT, Weisz JR, et al. A behavioral intervention for war-affected youth in Sierra Leone: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(12):1288–97. [DOI: 10.1016/j.jaac. 2014.09.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhana 2014 {published data only}
- Bhana A, Mellins CA, Petersen I, Alicea S, Myeza N, Holst H, et al. The VUKA family program: piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care 2014;26(1):1-11. [DOI: 10.1080/09540121.2013.806770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bliznashka 2021 {published data only}
- Bliznashka L, Yousafzai AK, Asheri G, Masanja H, Sudfeld CR. Effects of a community health worker delivered intervention on maternal depressive symptoms in rural Tanzania. Health Policy and Planning 2021;36(4):473-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogdanov 2021 {published data only}
- Bogdanov S, Augustinavicius J, Bass JK, Metz K, Skavenski S, Singh NS, et al. A randomized-controlled trial of community-based transdiagnostic psychotherapy for veterans and internally displaced persons in Ukraine. Global Mental Health 2021;8:e32. [DOI: 10.1017/gmh.2021.27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2014 {published data only}
- Bolton P, Lee C, Haroz EE, Murray L, Dorsey S, Robinson C, et al. A transdiagnostic community-based mental health treatment for comorbid disorders: development and outcomes of a randomized controlled trial among Burmese refugees in Thailand. PLOS Medicine 2014;11(11):e1001757. [DOI: 10.1371/journal.pmed.1001757] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bose 2015 {published data only}
- Bose GN. Changes in depression status in low socioeconomic perinatal subjects in rural India after supervised physical exercise: a randomized controlled study. Indian Journal of Psychiatry 2015;57(4):412-3. [DOI: 10.4103/0019-5545.171832] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burmaoğlu 2021 {published data only}
- Burmaoğlu GE. Comparison of cognitive-spiritual hope therapy and dialectical behavior therapy on reducing job stress in exceptional school teachers. Pakistan Journal of Medical & Health Sciences 2021;15(2):771-7. [LINK: pjmhsonline.com/2021/feb/771.pdf] [Google Scholar]
Çankaya 2021 {published data only}
- Çankaya S, Şimşek B. Effects of antenatal education on fear of birth, depression, anxiety, childbirth self-efficacy, and mode of delivery in primiparous pregnant women: a prospective randomized controlled study. Clinical Nursing Research 2021;30(6):818-29. [DOI: 10.1177/1054773820916984] [DOI] [PubMed] [Google Scholar]
Chandra 2018 {published data only}
- Chandra PS, Parameshwaran S, Satyanarayana VA, Varghese M, Liberti L, Duggal M, et al. I have no peace of mind—psychosocial distress expressed by rural women living with HIV in India as part of a mobile health intervention—a qualitative study. Archives of Women's Mental Health 2018;21(5):525-31. [DOI: 10.1007/s00737-018-0827-0] [DOI] [PubMed] [Google Scholar]
Chatterjee 2014 {published data only}
- Chatterjee S, Naik S, John S, Dabholkar H, Balaji M, Koschorke M, et al. Effectiveness of a community-based intervention for people with schizophrenia and their caregivers in India (COPSI): a randomised controlled trial. Lancet 2014;383(9926):1385-94. [DOI: 10.1016/S0140-6736(13)62629-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen W-T, Shiu C, Yang JP, Wang K, Zhang L, Zhang J, et al. Quality of life in HIV-infected Chinese women and their family caregivers: an intervention study. AIDS Care 2018;30(12):1572-9. [DOI: 10.1080/09540121.2018.1510095] [LINK: escholarship.org/uc/item/64j9s5m1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2020 {published data only}
- Cheung DS, Kor PP, Jones C, Davies N, Moyle W, Chien WT, et al. The use of modified mindfulness-based stress reduction and mindfulness-based cognitive therapy program for family caregivers of people living with dementia: a feasibility study. Asian Nursing Research 2020;14(4):221-30. [DOI: 10.1016/j.anr.2020.08.009] [DOI] [PubMed] [Google Scholar]
Chibanda 2014 {published data only}
- Chibanda D, Shetty AK, Tshimanga M, Woelk G, Stranix-Chibanda L, Rusakaniko S. Group problem-solving therapy for postnatal depression among HIV-positive and HIV-negative mothers in Zimbabwe. Journal of the International Association of Providers of AIDS Care 2014;13(4):335-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Christofides 2020 {published data only}
- Christofides NJ, Hatcher AM, Rebombo D, McBride R-S, Munshi S, Pino A, et al. Effectiveness of a multi-level intervention to reduce men’s perpetration of intimate partner violence: a cluster randomised controlled trial. Trials 2020;21:359. [DOI: 10.1186/s13063-020-4185-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2014 {published data only}
- Clarke K, Azad K, Kuddus A, Shaha S, Nahar T, Aumon BH, et al. Impact of a participatory intervention with women’s groups on psychological distress among mothers in rural Bangladesh: secondary analysis of a cluster-randomised controlled trial. PLOS One 2014;9(10):e110697. [DOI: 10.1371/journal.pone.0110697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cluver 2020 {published data only}
- Cluver L, Shenderovich Y, Meinck F, Berezin MN, Doubt J, Ward CL, et al. Parenting, mental health and economic pathways to prevention of violence against children in South Africa. Social Science & Medicine 2020;262:113194. [DOI: 10.1016/j.socscimed.2020.113194] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwi AA, Cluver L, Meinck F, Doubt J, Lachman JM, Shenderovich Y, et al. Mediation pathways for reduced substance use among parents in South Africa: a randomized controlled trial. BMC Public Health 2021;21(1):1656. [DOI: 10.1186/s12889-021-11651-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conde 2018 {published data only}
- Conde K, Brandariz RA, Lichtenberger A, Cremonte M. The effectiveness of a brief intervention for reducing adolescent alcohol consumption [Efectividad de una intervención breve para reducir el consumo de alcohol en adolescentes]. Revista Ciencias de la Salud 2018;16(3):393-407. [DOI: 10.12804/revistas.urosario.edu.co/revsalud/a.7261] [DOI] [Google Scholar]
Decker 2020 {published data only}
- Decker MR, Wood SN, Hameeduddin Z, Kennedy SR, Perrin N, Tallam C, et al. Safety decision-making and planning mobile app for intimate partner violence prevention and response: randomised controlled trial in Kenya. BMJ Global Health 2020;5(7):e002091. [DOI: 10.1136/bmjgh-2019-002091] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MR, Wood SN, Kennedy SR, Hameeduddin Z, Tallam C, Akumu I, et al. Adapting the myPlan safety app to respond to intimate partner violence for women in low and middle income country settings: app tailoring and randomized controlled trial protocol. BMC Public Health 2020;20(1):808. [DOI: 10.1186/s12889-020-08901-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Miranda 1999 {published data only}
- Miranda CT, Paula CS, Palma D, da Silva EM, Martin D, Nóbrega FJ. Impact of the application of neurolinguistic programming to mothers of children enrolled in a day care center of a shantytown. Sao Paulo Medical Journal 1999;117(2):63-71. [DOI: 10.1590/s1516-31801999000200004] [DOI] [PubMed] [Google Scholar]
Dike 2021 {published data only}
- Dike IC, Onyishi CN, Adimora DE, Ugodulunwa CA, Adama GN, Ugwu GC, et al. Yoga complemented cognitive behavioral therapy on job burnout among teachers of children with autism spectrum disorders. Medicine 2021;100(22):e25801. [DOI: 10.1097/MD.0000000000025801] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dirani 2021 {published data only}
- Dirani LA, Shamseddeen W, Ali LB, Elbejjani M, Raad H, Fadlallah N, et al. Effectiveness of a preventive parenting program combining attachment and behavioral approaches in an Arab context: a cluster-based randomized control trial. Prevention Science 2021;23(2):248-59. [DOI: 10.1007/s11121-021-01311-x] [DOI] [PubMed] [Google Scholar]
do Nascimento 2015 {published data only}
- do Nascimento MO, De Micheli D. Evaluation of different school-based preventive interventions for reducing the use of psychotropic substances among students: a randomized study [Avaliação de diferentes modalidades de ações preventivas na redução do consumo de substâncias psicotrópicas em estudantes no ambiente escolar: um estudo randomizado]. Ciência & Saúde Coletiva 2015;20(8):2499-2510. [DOI: 10.1590/1413-81232015208.15152014] [DOI] [PubMed] [Google Scholar]
Dow 2020 {published data only}
- Dow DE, Mmbaga BT, Gallis JA, Turner EL, Gandhi M, Cunningham CK, et al. A group-based mental health intervention for young people living with HIV in Tanzania: results of a pilot individually randomized group treatment trial. BMC Public Health 2020;20(1):1358. [DOI: 10.1186/s12889-020-09380-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00000214 {published data only}
- DRKS00000214. Saving and empowering young lives in Europe (SEYLE) [Saving and empowering young lives in Europe: promoting health through the prevention of risk-taking and self-destructive behaviours]. drks.de/search/en/trial/DRKS00000214 (first received 27 October 2009).
Efendi 2020 {published data only}
- Efendi F, Indarwati R, Aurizki GE. Effect of trauma-focused cognitive behavior therapy on depression and the quality of life of the elderly in Indonesia. Working with Older People 2020;24(3):149-57. [DOI: 10.1108/WWOP-02-2020-0004] [DOI] [Google Scholar]
Egbegi 2021 {published data only}
- Egbegi DR, Bella-Awusah T, Omigbodun O, Ani C. A controlled trial of cognitive behavioural therapy-based strategies for insomnia among in-school adolescents in southern Nigeria. Child and Adolescent Psychiatry and Mental Health 2021;15:52. [DOI: 10.1186/s13034-021-00406-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekrami 2019 {published data only}
- Ekrami F, Charandabi SM, Kheiroddin JB, Mirghafourvand M. The effect of counselling on depression and anxiety of women with unplanned pregnancy: a randomized controlled trial. Community Mental Health Journal 2019;55:1047–56. [DOI: 10.1007/s10597-019-00428-2] [DOI] [PubMed] [Google Scholar]
Esala 2017 {published data only}
- Esala JJ, Taing S. Testimony therapy with ritual: a pilot randomized controlled trial. Journal of Traumatic Stress 2017;30(1):94-8. [DOI: 10.1002/jts.22163] [DOI] [PubMed] [Google Scholar]
Estebari 2018 {published data only}
- Estebsari F, Dastoorpoor M, Mostafaei D, Khanjani N, Khalifehkandi ZR, Foroushani AR, et al. Design and implementation of an empowerment model to prevent elder abuse: a randomised controlled trial. Clinical Interventions in Aging 2018;13:669-79. [DOI: 10.2147/CIA.S158097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2021 {published data only}
- Francis T, Baker-Henningham H. Design and Implementation of the Irie Homes Toolbox: a violence prevention, early childhood, parenting program. Frontiers in Public Health 2020;8:582961. [DOI: 10.3389/fpubh.2020.582961] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T, Baker-Henningham H. The Irie Homes Toolbox: a cluster randomized controlled trial of an early childhood parenting program to prevent violence against children in Jamaica. Children and Youth Services Review 2021;126:106060. [DOI: 10.1016/j.childyouth.2021.106060] [DOI] [Google Scholar]
Fuhr 2019 {published data only}
- Fuhr DC, Weobong B, Lazarus A, Vanobberghen F, Weiss HA, Singla DR, et al. Delivering the Thinking Healthy Programme for perinatal depression through peers: an individually randomised controlled trial in India. Lancet Psychiatry 2019;6(2):115-27. [DOI: 10.1016/S2215-0366(18)30466-8] [DOI] [PubMed] [Google Scholar]
Gallegos 2013 {published data only}
- Gallegos J, Linan-Thompson S, Stark K, Ruvalcaba N. Preventing childhood anxiety and depression: testing the effectiveness of a school based program in Mexico [La prevención de la ansiedad y de la depresión en la infancia: estudio de la eficacia de un programa escolar en México]. Psicología Educativa 2013;19(1):37-44. [DOI: 10.5093/ed2013a6] [DOI] [Google Scholar]
Getanda 2022 {published data only}
- Getanda EM, Vostanis P. Feasibility evaluation of psychosocial intervention for internally displaced youth in Kenya. Journal of Mental Health 2022;31(6):774-82. [DOI: 10.1080/09638237.2020.1818702] [DOI] [PubMed] [Google Scholar]
Ghasemzadeh 2020 {published data only}
- Ghasemzadeh S, Naghsh Z. Effectiveness of unified protocols for transdiagnostic treatment in emotion regulation of mothers and anxiety of children with type I diabetes. Iranian Red Crescent Medical Journal 2020;22(11):e145. [DOI: 10.32592/ircmj.2020.22.11.145] [DOI] [Google Scholar]
Ghawadra 2020 {published data only}
- Ghawadra SF, Lim Abdullah K, Choo WY, Danaee M, Phang CK. The effect of mindfulness‐based training on stress, anxiety, depression and job satisfaction among ward nurses: a randomized control trial. Journal of Nursing Management 2020;28(5):1088-97. [DOI: 10.1111/jonm.13049] [DOI] [PubMed] [Google Scholar]
Ghodsbin 2015 {published data only}
- Ghodsbin F, Ahmadi ZS, Jahanbin I, Sharif F. The effects of laughter therapy on general health of elderly people referring to Jahandidegan Community Center in Shiraz, Iran, 2014: a randomized controlled trial. International Journal of Community Based Nursing and Midwifery 2015;3(1):31-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Gok Ugur 2019 {published data only}
- Gok Ugur H, Orak OS, Aktas YY, Enginyurt O, Saglambilen O. Effects of music therapy on the care burden of in-home caregivers and physiological parameters of their in-home dementia patients: a randomized controlled trial. Complementary Medicine Research 2019;26(1):22-30. [DOI: 10.1159/000490348] [DOI] [PubMed] [Google Scholar]
Griffiths 2020 {published data only}
- Griffiths J, Thaikruea L, Wongpakaran N, Munkhetvit P, Kittisares A, Varnado P. Effects of combined physical movement activity and multifaceted cognitive training in older people with mild neurocognitive disorder in a rural community: a randomized control trial. Dementia and Geriatric Cognitive Disorders 2020;49(2):194-201. [DOI: 10.1159/000507922] [DOI] [PubMed] [Google Scholar]
Gul 2021 {published data only}
- Gul M, Aqeel M. Acceptance and commitment therapy for treatment of stigma and shame in substance use disorders: a double-blind, parallel-group, randomized controlled trial. Journal of Substance Use 2021;26(4):413-19. [DOI: 10.1080/14659891.2020.1846803] [DOI] [Google Scholar]
Gureje 2019 {published data only}
- Gureje O, Oladeji BD, Montgomery AA, Araya R, Bello T, Chisholm D, et al. High- versus low-intensity interventions for perinatal depression delivered by non-specialist primary maternal care providers in Nigeria: cluster randomised controlled trial (the EXPONATE trial). British Journal of Psychiatry 2019;215(3):528-35. [DOI: 10.1192/bjp.2019.4] [DOI] [PubMed] [Google Scholar]
Haack 2021 {published data only}
- Haack LM, Araujo EA, Meza J, Friedman LM, Spiess M, Alcaraz Beltrán DK, et al. Can school mental health providers deliver psychosocial treatment improving youth attention and behavior in Mexico? A pilot randomized controlled trial of CLS-FUERTE. Journal of Attention Disorders 2021;25(14):2083-97. [DOI: 10.1177/1087054720959698] [DOI] [PubMed] [Google Scholar]
Häfele 2021 {published data only}
- Häfele CA, Rombaldi AJ, Feter N, Häfele V, Gervini BL, Domingues MR, et al. Effects of an exercise program on health of people with epilepsy: a randomized clinical trial. Epilepsy & Behavior 2021;117:107904. [DOI: 10.1016/j.yebeh.2021.107904] [DOI] [PubMed] [Google Scholar]
Haghighat 2018 {published data only}
- Haghighat M, Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Malakouti J, Erfani M. The effect of spiritual counseling on stress and anxiety in pregnancy: a randomized controlled clinical trial. Iranian Red Crescent Medical Journal 2018;20(4):e64094. [DOI: 10.5812/ircmj.64094] [DOI] [Google Scholar]
Hajisadeghian 2021 {published data only}
- Hajisadeghian R, Ghezelbash S, Mehrabi T. The effects of a psychosocial support program on perceived stress of family caregivers of patients with mental disorders. Iranian Journal of Nursing and Midwifery Research 2021;26(1):47-53. [DOI: 10.4103/ijnmr.IJNMR_36_20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamdani 2020 {published data only}
- Hamdani SU, Rahman A, Wang D, Chen T, Ommeren M, Chisholm D, et al. Cost-effectiveness of WHO problem management plus for adults with mood and anxiety disorders in a post-conflict area of Pakistan: randomised controlled trial. British Journal of Psychiatry 2020;217(5):623-29. [DOI: 10.1192/bjp.2020.138] [DOI] [PubMed] [Google Scholar]
Hamdani 2021b {published data only}
- Hamdani SU, Akhtar P, E-Huma Z, Nazir H, Minhas FA, Sikander S, et al. WHO Parents Skills Training (PST) programme for children with developmental disorders and delays delivered by Family Volunteers in rural Pakistan: study protocol for effectiveness implementation hybrid cluster randomized controlled trial. Global Mental Health 2017;4:e11. [DOI: 10.1017/gmh.2017.7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani SU, Huma ZE, Suleman N, Akhtar P, Nazir H, Masood A, et al. Effectiveness of a technology‑assisted, family volunteers delivered, brief, multicomponent parents’ skills training intervention for children with developmental disorders in rural Pakistan: a cluster randomized controlled trial. International Journal of Mental Health Systems 2021;15(1):53. [DOI: 10.1186/s13033-021-00476-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02792894. Family networks (FaNs) for children with developmental disorders and delays (FaNs) [Evaluation of family networks (FaNs) for children with developmental disorders and delays program: a cluster randomized control trial]. clinicaltrials.gov/ct2/show/NCT02792894 (first received 8 June 2016).
Hamdi 2020 {published data only}
- Hamidi F, Javadnoori M, Hosseinifard SM, Nikbakht R. The effectiveness of mindfulness-based training on anxiety in pregnant women with gestational diabetes. Family Medicine & Primary Care Review 2020;22(4):279–83. [DOI: 10.5114/fmpcr.2020.100430] [DOI] [Google Scholar]
Hamester 2016 {published data only}
- Hamester L, Souza EN, Cielo C, Moraes MA, Pellanda LC. Effectiveness of a nursing intervention in decreasing the anxiety levels of family members of patients undergoing cardiac surgery: a randomized clinical trial. Revista Latino-Americana de Enfermagem 2016;24:e2729. [DOI: 10.1590/1518-8345.0208.2729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2020 {published data only}
- Hartmann M, Datta S, Browne EN, Appiah P, Banay R, Caetano V, et al. A combined behavioral economics and cognitive behavioral therapy intervention to reduce alcohol use and intimate partner violence among couples in Bengaluru, India: results of a pilot study. Journal of Interpersonal Violence 2020;36(23-4):NP12456-80. [DOI: 10.1177/0886260519898431] [DOI] [PubMed] [Google Scholar]
Heizomi 2020 {published data only}
- Heizomi H, Allahverdipour H, Jafarabadi MA, Bhalla D, Nadrian H. Effects of a mental health promotion intervention on mental health of Iranian female adolescents: a school-based study. Child and Adolescent Psychiatry and Mental Health 2020;14:36. [DOI: 10.1186/s13034-020-00342-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2021 {published data only}
- Hill A-M, Moorin R, Slatyer S, Bryant C, Hill K, Waldron N, et al. Evaluating the provision of Further Enabling Care at Home (FECH+) for informal caregivers of older adults discharged home from hospital: protocol for a multicentre randomised controlled trial. BMJ Open 2021;11(6):e046600. [DOI: 10.1136/ bmjopen-2020-046600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinsberger 2017 {published data only}
- Hinsberger M, Holtzhausen L, Sommer J, Kaminer D, Elbert T, Seedat S, et al. Feasibility and effectiveness of narrative exposure therapy and cognitive behavioral therapy in a context of ongoing violence in South Africa. Psychological Trauma: Theory, Research, Practice, and Policy 2017;9(3):282-91. [DOI: 10.1037/tra0000197] [DOI] [PubMed] [Google Scholar]
Hortense 2020 {published data only}
- Hortense FT, Bergerot CD, De Domenico EB. Quality of life, anxiety and depression in head and neck cancer patients: a randomized clinical trial [Calidad de vida, ansiedad y depresión de pacientes con cáncer de cabeza y cuello: estudio clínico randomizado]. Revista da Escola de Enfermagem da USP 2020;54:e03546. [DOI: 10.1590/S1980-220X2018040103546] [DOI] [PubMed] [Google Scholar]
Huis in ‘t Veld 2019 {published data only}
- Huis in ‘t Veld D, Ensoy-Musoro C, Pengpid S, Peltzer K, Colebunders R. The efficacy of a brief intervention to reduce alcohol use in persons with HIV in South Africa, a randomized clinical trial. PLOS One 2019;14(1):e0220799. [DOI: 10.1371/journal.pone.0220799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husain 2021a {published data only}
- Husain MO, Khoso AB, Renwick L, Kiran T, Saeed S, Lane S, et al. Culturally adapted family intervention for schizophrenia in Pakistan: a feasibility study. International Journal of Psychiatry in Clinical Practice 2021;25(3):258-67. [DOI: 10.1080/13651501.2020.1819332] [DOI] [PubMed] [Google Scholar]
Husain 2021b {published data only}
- Husain N, Kiran T, Shah S, Rahman A, Ur-Rehman R, Saeed Q, et al. Efficacy of learning through play plus intervention to reduce maternal depression in women with malnourished children: a randomized controlled trial from Pakistan. Journal of Affective Disorders 2021;278:78-84. [DOI: 10.1016/j.jad.2020.09.001] [DOI] [PubMed] [Google Scholar]
Husain 2021c {published data only}
- Husain N, Kiran T, Fatima B, Chaudhry IB, Husain M, Shah S, et al. An integrated parenting intervention for maternal depression and child development in a low-resource setting: Cluster randomized controlled trial. Depression and Anxiety 2021;38(9):925-39. [DOI: 10.1002/da.23169] [DOI] [PubMed] [Google Scholar]
Iremeka 2021 {published data only}
- Iremeka FU, Ede MO, Amaeze FE, Okeke CI, Ilechukwu LC, Ukaigwe PC, et al. Improving work-life balance among administrative officers in Catholic primary schools: assessing the effect of a Christian religious rational emotive behavior therapy. Medicine 2021;100(24):e26361. [DOI: 10.1097/MD.0000000000026361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismayilova 2018 {published data only}
- Ismayilova L, Karimli L, Sanson J, Gaveras E, Nanema R, Tô-Camier A, et al. Improving mental health among ultra-poor children: two-year outcomes of a cluster-randomized trial in Burkina Faso. Social Science & Medicine 2018;208:180-9. [DOI: 10.1016/j.socscimed.2018.04.022] [DOI] [PubMed] [Google Scholar]
ISRCTN15986016 {published data only}
- ISRCTN15986016. Prevention of dementia using mobile phone applications [Prevention of dementia using mobile phone applications: a randomised controlled trial]. www.isrctn.com/ISRCTN15986016 (first received 19 June 2019).
Jackson 2021 {published data only}
- Jackson K, Wadley AL, Parker R. Managing pain in HIV/AIDS: a therapeutic relationship is as effective as an exercise and education intervention for rural amaXhosa women in South Africa. BMC Public Health 2021;21(1):302. [DOI: 10.1186/s12889-021-10309-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamshidi 2020 {published data only}
- Jamshidi F, Rajabi S, Dehghani Y. How to heal their psychological wounds? Effectiveness of EMDR therapy on post‐traumatic stress symptoms, mind‐wandering and suicidal ideation in Iranian child abuse victims. Counselling and Psychotherapy Research 2021;21(2):412-21. [DOI: 10.1002/capr.12339] [DOI] [Google Scholar]
Jaywant 2020 {published data only}
- Jaywant SS, Patil SS, Shrivastav DS. To analyze the effect of person-environment-occupation intervention model on stress and breast-feeding efficacy on mothers of preterm neonates: a randomized controlled study. Indian Journal of Occupational Therapy 2020;52(1):24-9. [DOI: 10.4103/ijoth.ijoth_1_20] [DOI] [Google Scholar]
Jordans 2021 {published data only}
- Jordans MJ, Kohrt BA, Sangraula M, Turner EL, Wang X, Shrestha P, et al. Effectiveness of Group Problem Management Plus, a brief psychological intervention for adults affected by humanitarian disasters in Nepal: a cluster randomized controlled trial. PLOS Medicine 2021;18(6):e1003621. [DOI: 10.1371/journal.pmed.1003621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kagawa 2017 {published data only}
- Kagawa RM, Deardorff J, García-Guerra A, Knauer HA, Schnaas L, Neufeld LM, et al. Effects of a parenting program among women who began childbearing as adolescents and young adults. Journal of Adolescent Health 2017;61(5):634-41. [DOI: 10.1016/j.jadohealth.2017.05.023] [DOI] [PubMed] [Google Scholar]
Kane 2022 {published data only}
- Kane JC, Sharma A, Murray LK, Chander G, Kanguya T, Skavenski S, et al. Efficacy of the common elements treatment approach (CETA) for unhealthy alcohol use among adults with HIV in Zambia: results from a pilot randomized controlled trial. AIDS and Behavior 2022;26(2):523-36. [DOI: 10.1007/s10461-021-03408-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karimi 2019 {published data only}
- Karimi Z, Rezaee N, Shakiba M, Navidian A. The effect of group counseling based on quality of life therapy on stress and life satisfaction in family caregivers of individuals with substance use problem: a randomized controlled trial. Issues in Mental Health Nursing 2019;40(12):1012-18. [DOI: 10.1080/01612840.2019.1609635] [DOI] [PubMed] [Google Scholar]
Karimli 2019 {published data only}
- Karimli L, Ssewamala FM, Neilands TB, Wells CR, Bermudez LG. Poverty, economic strengthening, and mental health among AIDS orphaned children in Uganda: Mediation model in a randomized clinical trial. Social Science & Medicine 2019;228:17-24. [DOI: 10.1016/j.socscimed.2019.03.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazazi 2021 {published data only}
- Kazazi L, Shati M, Mortazavi SS, Nejati V, Foroughan M. The impact of computer-based cognitive training intervention on the quality of life among elderly people: a randomized clinical trial. Trials 2021;22(1):51. [DOI: 10.1186/s13063-020-05008-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017 {published data only}
- Khan MN, Dherani M, Chiumento A, Atif N, Bristow K, Sikander S, et al. Evaluating feasibility and acceptability of a local psycho-educational intervention for pregnant women with common mental problems affected by armed conflict in Swat, Pakistan: A parallel randomized controlled feasibility trial. International Journal of Social Psychiatry 2017;63(8):724-35. [DOI: 10.1177/0020764017734001] [DOI] [PubMed] [Google Scholar]
Khuzwayo 2020 {published data only}
- Khuzwayo N, Taylor M, Connolly C. Changing youth behaviour in South Africa. Health SA Gesondheid 2020;25(1):1031. [DOI: 10.4102/hsag.v25i0.1031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidder 2013 {published data only}
- Kidder DP, Bachanas P, Medley A, Pals S, Nuwagaba-Biribonwoha H, Ackers M, et al. HIV prevention in care and treatment settings: baseline risk behaviors among HIV patients in Kenya, Namibia, and Tanzania. PLOS One 2013;8(2):e57215. [DOI: 10.1371/journal.pone.0057215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kivumbi 2019 {published data only}
- Kivumbi A, Byansi W, Ssewamala FM, Proscovia N, Damulira C, Namatovu P. Utilizing a family-based economic strengthening intervention to improve mental health wellbeing among female adolescent orphans in Uganda. Child and Adolescent Psychiatry and Mental Health 2019;13:14. [DOI: 10.1186/s13034-019-0273-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koebach 2021 {published data only}
- Koebach A, Carleial S, Elbert T, Schmitt S, Robjant K. Treating trauma and aggression with narrative exposure therapy in former child and adult soldiers: a randomized controlled trial in Eastern DR Congo. Journal of Consulting and Clinical Psychology 2021;89(3):143-55. [DOI: 10.1037/ccp0000632] [DOI] [PubMed] [Google Scholar]
Kohli 2017 {published data only}
- Kohli A, Perrin NA, Remy MM, Alfred MB, Arsene KB, Nadine MB, et al. Adult and adolescent livestock productive asset transfer programmes to improve mental health, economic stability and family and community relationships in rural South Kivu Province, Democratic Republic of Congo: a protocol of a randomised controlled trial. BMJ Open 2017;7(3):e013612. [DOI: 10.1136/bmjopen-2016-013612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kor 2019 {published data only}
- Kor PP, Liu JY, Chien WT. Effects of a modified mindfulness-based cognitive therapy for family caregivers of people with dementia: a pilot randomized controlled trial. International Journal of Nursing Studies 2019;98:107-17. [DOI: 10.1016/j.ijnurstu.2019.02.020] [DOI] [PubMed] [Google Scholar]
Kulis 2019 {published data only}
- Kulis SS, Marsiglia FF, Porta M, Arévalo Avalos MR, Ayers SL. Testing the Keepin’ it REAL substance use prevention curriculum among early adolescents in Guatemala City. Prevention Science 2019;20(4):532-43. [DOI: 10.1007/s11121-018-0956-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2019 {published data only}
- Lai CK, Chin KC, Zhang Y, Chan EA. Psychological outcomes of life story work for community‐dwelling seniors: a randomised controlled trial. International Journal of Older People Nursing 2019;14(3):e12238. [DOI: 10.1111/opn.12238] [DOI] [PubMed] [Google Scholar]
Langoni 2019 {published data only}
- Langoni CS, Resende TL, Barcellos AB, Cecchele B, da Rosa JN, Knob MS, et al. The effect of group exercises on balance, mobility, and depressive symptoms in older adults with mild cognitive impairment: a randomized controlled trial. Clinical Rehabilitation 2019;33(3):439-49. [DOI: 10.1177/0269215518815218] [DOI] [PubMed] [Google Scholar]
Latifi 2020 {published data only}
- Latifi A, Saeedinezhad F, Keikhaei A, Aliabad GM. The effect of cognitive-emotional intervention on psychological distress in mothers of children with cancer. Medical-Surgical Nursing Journal 2020;9(4):e113171. [DOI: 10.5812/msnj.113171] [DOI] [Google Scholar]
Lawrence 2020 {published data only}
- Lawrence KC, Falaye AO. Trauma‐focused counselling and social effectiveness skills training interventions on impaired psychological functioning of internally displaced adolescents in Nigeria. Journal of Community & Applied Social Psychology 2020;30(6):616-27. [DOI: 10.1002/casp.2477] [DOI] [Google Scholar]
Li 2015 {published data only}
- Li J. A Randomized Controlled Study to Evaluate the Efficacy of a Positive Psychology and Social Networking Intervention in Reducing Depressive Symptoms Among HIV-infected Men Who Have Sex With Men in China [Doctoral thesis]. Hong Kong (China): Chinese University of Hong Kong, 2015. [Google Scholar]
Li 2016 {published data only}
- Li F, Yao P, Hsue C, Xu J, Lou Q. Impact of “conversation maps” on diabetes distress and self-efficacy of Chinese adult patients with type 2 diabetes: a pilot study. Patient Preference and Adherence 2016;10:901-8. [DOI: 10.2147/PPA.S95449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020a {published data only}
- Li Y-H, Mu T-Y, Zhang L, Zhang C-L, Wu D, Chen J-J, et al. Internet‐based intervention for postpartum depression in China (“Mommy go”): protocol for a randomized controlled trial [中国产后抑郁症网络干预(“Mommy go”):随机对照试验方案]. Journal of Advanced Nursing 2020;76(9):2416-25. [DOI: 10.1111/jan.14436] [DOI] [PubMed] [Google Scholar]
Luo 2019 {published data only}
- Luo R, Emmers D, Warrinnier N, Rozelle S, Sylvia S. Using community health workers to deliver a scalable integrated parenting program in rural China: a cluster-randomized controlled trial. Social Science & Medicine 2019;239:112545. [DOI: 10.1016/j.socscimed.2019.112545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2018 {published data only}
- Ma C, Zhou W, Tang Q, Huang S. The impact of group-based Tai chi on health-status outcomes among community-dwelling older adults with hypertension. Heart & Lung 2018;47(4):337-44. [DOI: 10.1016/j.hrtlng.2018.04.007] [DOI] [PubMed] [Google Scholar]
Maarefvand 2015 {published data only}
- Maarefvand M, Eghlima M, Rafiey H, Rahgozar M, Tadayyon N, Deilamizadeh A, et al. Community-based relapse prevention for opiate dependents: a randomized community controlled trial. Community Mental Health Journal 2015;51(1):21-9. [DOI: 10.1007/s10597-014-9772-1] [DOI] [PubMed] [Google Scholar]
Madhombiro 2020 {published data only}
- Madhombiro M, Kidd M, Dube B, Dube M, Mutsvuke W, Muronzie T, et al. Effectiveness of a psychological intervention delivered by general nurses for alcohol use disorders in people living with HIV in Zimbabwe: a cluster randomized controlled trial. Journal of the International AIDS Society 2020;23(12):e25641. [DOI: 10.1002/jia2.25641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahdavi 2017 {published data only}
- Mahdavi B, Fallahi-Khoshknab M, Mohammadi F, Hosseini MA, Haghi M. Effects of spiritual group therapy on caregiver strain in home caregivers of the elderly with Alzheimer's disease. Archives of Psychiatric Nursing 2017;31(3):269-73. [DOI: 10.1016/j.apnu.2016.12.003] [DOI] [PubMed] [Google Scholar]
Mahmoudi 2004 {published data only}
- Mahmoudi Q, Azimi H, Zarghami M. The effect of assertiveness training on nursing students' anxiety and courage [تاثير آموزش قاطعيت بر ميزان اضطراب تحصيلي دانش آموزان]. Scientific Journal of Gorgan University of Medical Sciences 2004;6(2):66-72. [LINK: goums.ac.ir/journal/article-1-205-en.html] [Google Scholar]
Manyaapelo 2019 {published data only}
- Manyaapelo T, Van den Borne B, Ruiter RA, Sifunda S, Reddy P. Effectiveness of a health behavioural intervention aimed at reduction of risky sexual behaviours among young men in the KwaZulu-Natal Province, South Africa. International Journal of Environmental Research and Public Health 2019;16(11):1938. [DOI: 10.3390/ijerph16111938] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2011 {published data only}
- Marais S, Jordaan E, Viljoen D, Olivier L, Waal J, Poole C. The effect of brief interventions on the drinking behaviour of pregnant women in a high‐risk rural South African community: a cluster randomised trial. Early Child Development and Care 2011;181(4):463-74. [DOI: 10.1080/03004430903450392] [DOI] [Google Scholar]
Markkula 2019 {published data only}
- Markkula N, Lehti V, Adhikari P, Peña S, Heliste J, Mikkonen E, et al. Effectiveness of non-medical health worker-led counselling on psychological distress: a randomized controlled trial in rural Nepal. Global Mental Health 2019;6:e15. [DOI: 10.1017/gmh.2019.15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masoumi 2020 {published data only}
- Masoumi Z, Abdoli F, Esmaeilzadeh S, Sadeghi T. The effect of supportive-training intervention on the burnout of mothers with disabled child: a randomized clinical trial. Journal of Caring Sciences 2020;9(3):133-9. [DOI: 10.34172/jcs.2020.020] [DOI] [PMC free article] [PubMed] [Google Scholar]
McBain 2015 {published data only}
- McBain RK, Salhi C, Hann K, Kellie J, Kamara A, Salomon JA, et al. Improving outcomes for caregivers through treatment of young people affected by war: a randomized controlled trial in Sierra Leone. Bulletin of the World Health Organization 2015;93(12):834-41. [DOI: 10.2471/BLT.14.139105] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLaughlin 2012 {published data only}
- McLaughlin KA, Zeanah CH, Fox NA, Nelson CA. Attachment security as a mechanism linking foster care placement to improved mental health outcomes in previously institutionalized children: Institutionalization, attachment and internalizing disorders. Journal of Child Psychology and Psychiatry 2012;53(1):46-55. [DOI: 10.1111/j.1469-7610.2011.02437.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mecdi Kaydirac 2021 {published data only}
- Mecdi Kaydirak M, Aslan E. Efficacy of nursing support in the pre- and postmedical termination of pregnancy phases: a randomized study. Omega 2021;84(1):51-68. [DOI: 10.1177/0030222819877791] [DOI] [PubMed] [Google Scholar]
Mehri 2020 {published data only}
- Mehri M, Chehrzad MM, Maleki M, Kousha M, Akhlaghi E, Mardani A. The effect of behavioral parent training of children with attention deficit hyperactivity disorder on parents’ mental health. Neurology, Psychiatry and Brain Research 2020;37:53-9. [DOI: 10.1016/j.npbr.2020.06.003] [DOI] [Google Scholar]
Michelson 2020 {published data only}
- Michelson D, Malik K, Parikh R, Weiss HA, Doyle AM, Bhat B, et al. Effectiveness of a brief lay counsellor-delivered, problem-solving intervention for adolescent mental health problems in urban, low-income schools in India: a randomised controlled trial. Lancet Child & Adolescent Health 2020;4(8):571-82. [DOI: 10.1016/S2352-4642(20)30173-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milani 2017 {published data only}
- Milani HS, Amiri P, Mohseny M, Abadi A, Vaziri SM, Vejdani M. Postpartum home care and its effects on mothers’ health: a clinical trial. Journal of Research in Medical Sciences 2017;22:96. [DOI: 10.4103/jrms.JRMS_319_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller‐Matero 2021 {published data only}
- Miller-Matero LR, Hecht LM, Miller MK, Autio K, Pester BD, Tobin ET, et al. A brief psychological intervention for chronic pain in primary care: a pilot randomized controlled trial. Pain Medicine 2021;22(7):1603-11. [DOI: 10.1093/pm/pnaa444] [DOI] [PubMed] [Google Scholar]
Mirmahmoodi 2020 {published data only}
- Mirmahmoodi M, Mangalian P, Ahmadi A, Dehghan M. The effect of mindfulness-based stress reduction group counseling on psychological and inflammatory responses of the women with breast cancer. Integrative Cancer Therapies 2020;19:1534735420946819. [DOI: 10.1177/1534735420946819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2011 {published data only}
- Mohammadi MR, Akbari AA, Hatami N, Mokri A, Kaviani H, Salmanian M, et al. Effectiveness of grouped spiritual psychotherapy on patients with opium using disorder. Hakim Research Journal 2011;14(3):144-50. [LINK: www.sid.ir/paper/29581/en] [Google Scholar]
Mohd‐Sidik 2018 {published data only}
- Mohd-Sidik S, Akhtari-Zavare M, Periasamy U, Rampal L, Fadhilah SI, Mahmud R. Effectiveness of chemotherapy counselling on self-esteem and psychological affects among cancer patients in Malaysia: randomized controlled trial. Patient Education and Counseling 2018;101(5):862-71. [DOI: 10.1016/j.pec.2018.01.004] [DOI] [PubMed] [Google Scholar]
Moshki 2014 {published data only}
- Moshki M, Baloochi Beydokhti T, Cheravi K. The effect of educational intervention on prevention of postpartum depression: an application of health locus of control. Journal of Clinical Nursing 2014;23(15-16):2256-63. [DOI: 10.1111/jocn.12505] [DOI] [PubMed] [Google Scholar]
Murray 2016 {published data only}
- Murray L, De Pascalis L, Tomlinson M, Vally Z, Dadomo H, MacLachlan B, et al. Randomized controlled trial of a book-sharing intervention in a deprived South African community: effects on carer-infant interactions, and their relation to infant cognitive and socioemotional outcome. Journal of Child Psychology and Psychiatry 2016;57(12):1370-9. [DOI: 10.1111/jcpp.12605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutyambizi‐Mafunda 2019 {published data only}
- Mutyambizi-Mafunda V, Myers B, Sorsdahl K, Lund C, Naledi T, Cleary S. Integrating a brief mental health intervention into primary care services for patients with HIV and diabetes inSouth Africa: study protocol for a trial-based economic evaluation. BMJ Open 2019;9(5):e026973. [DOI: 10.1136/bmjopen-2018-026973] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Lund C, Lombard C, Joska J, Levitt N, Butler C, et al. Comparing dedicated and designated models of integrating mental health into chronic disease care: study protocol for a cluster randomized controlled trial. Trials 2018;19(1):185. [DOI: 10.1186/s13063-018-2568-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Petersen-Williams P, Westhuizen C, Lund C, Lombard C, Joska JA, et al. Community health worker-delivered counselling for common mental disorders among chronic disease patients in South Africa: a feasibility study. BMJ Open 2019;9(1):e024277. [DOI: 10.1136/bmjopen-2018-024277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabunya 2014 {published data only}
- Nabunya P, Ssewamala FM, Ilic V. Family economic strengthening and parenting stress among caregivers of AIDS-orphaned children: results from a cluster randomized clinical trial in Uganda. Children and Youth Services Review 2014;44:417-21. [DOI: 10.1016/j.childyouth.2014.07.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabunya 2020 {published and unpublished data}
- NCT03307226. Suubi4Her: a combination intervention addressing HIV risk behaviors among older adolescent girls transitioning into adulthood in Uganda. clinicaltrials.gov/ct2/show/NCT03307226 (first received 11 October 2017).
- Nabunya P, Damulira C, Byansi W, Muwanga J, Bahar OS, Namuwonge F, et al. Prevalence and correlates of depressive symptoms among high school adolescent girls in southern Uganda. BMC Public Health 2020;20(1):1792. [DOI: 10.1186/s12889-020-09937-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssewamala FM, Bermudez LG, Neilands TB, Mellins CA, McKay MM, Garfinkel I, et al. Suubi4Her: a study protocol to examine the impact and cost associated with a combination intervention to prevent HIV risk behavior and improve mental health functioning among adolescent girls in Uganda. BMC Public Health 2018;18(1):693. [DOI: 10.1186/s12889-018-5604-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahar 2015 {published data only}
- Nahar B, Hossain I, Hamadani JD, Ahmed T, Grantham-McGregor S, Persson L-A. Effect of a food supplementation and psychosocial stimulation trial for severely malnourished children on the level of maternal depressive symptoms in Bangladesh: community-based trial and maternal depressive symptoms. Child: Care, Health and Development 2015;41(3):483-93. [DOI: 10.1111/cch.12176] [DOI] [PubMed] [Google Scholar]
NCT02059863 {published data only}
- Bhopal S, Roy R, Verma D, Kumar D, Avan B, Khan B, et al. Impact of adversity on early childhood growth & development in rural India: findings from the early life stress sub-study of the SPRING cluster randomised controlled trial (SPRING-ELS). PLOS One 2019;14(1):e0209122. [DOI: 10.1371/journal.pone.0209122] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal S, Verma D, Roy R, Soremekun S, Kumar D, Bristow M, et al. The contribution of childhood adversity to cortisol measures of early life stress amongst infants in rural India: findings from the early life stress sub-study of the SPRING cluster randomised controlled trial (SPRING-ELS). Psychoneuroendocrinology 2019;107:241-50. [DOI: 10.1016/j.psyneuen.2019.05.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02059863. SPRING cluster randomised controlled trial (SPRING) [SPRING (for the millennium development goals): sustainable programme incorporating nutrition and games (for maximising development, growth and survival)]. clinicaltrials.gov/ct2/show/NCT02059863 (first received 11 February 2014).
NCT04638101 {published data only}
- NCT04638101. Building the path to resilience in preterm infants: mindfulness-based intervention [Building the path to resilience in preterm infants: a neuroimaging investigation of the impact of multisensory and neurocognitive interventions concern: mindfulness-based intervention]. clinicaltrials.gov/ct2/show/NCT04638101 (first received 20 November 2020).
Nestadt 2019 {published data only}
- Nestadt DF, Saisaengjan C, McKay MM, Bunupuradah T, Pardo G, Lakhonpon S, et al. CHAMP+ Thailand: pilot randomized control trial of a family-based psychosocial intervention for perinatally HIV-infected early adolescents. AIDS Patient Care and STDs 2019;33(5):227-36. [DOI: 10.1089/apc.2019.0021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2020 {published data only}
- Nguyen AJ, Dang H-M, Bui D, Phoeun B, Weiss B. Experimental evaluation of a school-based mental health literacy program in two Southeast Asian nations. School Mental Health 2020;12(4):716-31. [DOI: 10.1007/s12310-020-09379-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nunez Pumariega 2020 {published data only}
- Nunez Pumariega Y, Vitória Calheiros PR, Nunez Cardenas R, Farias ES, Pumariega Torres CD. Relapse prevention based on mindfulness in the treatment of tobacco dependence - Brazil [Prevenção de recaídas baseada em mindfulness no tratamento do tabagismo- Brasil]. CES Psicología 2020;13(2):129-43. [DOI: 10.21615/cesp.13.2.9] [DOI] [Google Scholar]
Nyamathi 2012 {published data only (unpublished sought but not used)}
- Nyamathi A, Salem BE, Meyer V, Ganguly KK, Sinha S, Ramakrishnan P. Impact of an Asha intervention on depressive symptoms among rural women living with AIDS in India: comparison of the Asha-Life and Usual Care program. AIDS Education and Prevention 2012;24(3):280-93. [DOI: 10.1521/aeap.2012.24.3.280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obiweluozo 2021 {published data only}
- Obiweluozo PE, Ede MO, Onwurah CN, Uzodinma UE, Dike IC, Ejiofor JN. Impact of cognitive behavioural play therapy on social anxiety among school children with stuttering deficit: a cluster randomised trial with three months follow-up. Medicine 2021;100(19):e24350. [DOI: 10.1097/MD.0000000000024350] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogum Alangea 2020 {published data only}
- Addo-Lartey AA, Ogum Alangea D, Sikweyiya Y, Chirwa ED, Coker-Appiah D, Jewkes R, et al. Rural response system to prevent violence against women: methodology for a community randomised controlled trial in the central region of Ghana. Global Health Action 2019;12(1):1612604. [DOI: 10.1080/16549716.2019.1612604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03237585. Impact assessment of the rural response system to reduce violence against women in Ghana (GhanaCHiPS). clinicaltrials.gov/ct2/show/NCT03237585 (first received 2 August 2017).
- Ogum Alangea D, Addo-Lartey AA, Chirwa ED, Sikweyiya Y, Coker-Appiah D, Jewkes R, et al. Evaluation of the rural response system intervention to prevent violence against women: findings from a community- randomised controlled trial in the central region of Ghana. Global Health Action 2020;13(1):1711336. [DOI: 10.1080/16549716.2019.1711336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Owais 2020 {published data only}
- Owais SS. Effectiveness and Experience of an Integrated Maternal Mental Healthcare Intervention in Private Clinics and Public Health Facilities in Pakistan [Doctoral dissertation]. Columbia (SC): University of South Carolina, 2020. [LINK: scholarcommons.sc.edu/etd/5977] [Google Scholar]
Oz 2020 {published data only}
- Oz H, Oz F. A psychoeducation program for stress management and psychosocial problems in multiple sclerosis. Nigerian Journal of Clinical Practice 2020;23(11):1598-606. [DOI: 10.4103/njcp.njcp_462_19] [DOI] [PubMed] [Google Scholar]
O’Farrelly 2021 {published data only}
- O’Farrelly C, Watt H, Babalis D, Bakermans-Kranenburg MJ, Barker B, Byford S, et al. A brief home-based parenting intervention to reduce behavior problems in young children: a pragmatic randomized clinical trial. JAMA Pediatrics 2021;175(6):567-76. [DOI: 10.1001/jamapediatrics.2020.6834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2019 {published data only}
- Pan Y, Chen R. The effect of a nurse-led cognitive behavioral protocol on depressive symptoms and coping strategies of dementia caregivers. Journal of Nursing Research 2019;27(6):e55. [DOI: 10.1097/jnr.0000000000000327] [DOI] [PubMed] [Google Scholar]
Pandya 2021 {published data only}
- Pandya SP. Intervention outcomes, anxiety, self-esteem, and self-efficacy with DHH students in universities. Journal of Deaf Studies and Deaf Education 2021;26(1):58-69. [DOI: 10.1093/deafed/enaa027] [DOI] [PubMed] [Google Scholar]
Papas 2011 {published data only}
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya: RCT alcohol outcomes in Kenya. Addiction 2011;106(12):2156-66. [DOI: 10.1111/j.1360-0443.2011.03518.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papas 2021 {published data only}
- Papas RK, Gakinya BN, Mwaniki MM, Lee H, Keter AK, Martino S, et al. A randomized clinical trial of a group cognitive–behavioral therapy to reduce alcohol use among human immunodeficiency virus‐infected outpatients in western Kenya. Addiction 2021;116(2):305-18. [DOI: 10.1111/add.15112] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01503255. A stage 2 cognitive-behavioral trial: reduce alcohol first in Kenya intervention (RAFIKI). clinicaltrials.gov/ct2/show/NCT01503255 (first received 4 January 2012).
Paranthaman 2010 {published data only}
- Paranthaman V, Satnam K, Lim JL, Amar-Singh HS, Sararaks S, Nafiza MN, et al. Effective implementation of a structured psychoeducation programme among caregivers of patients with schizophrenia in the community. Asian Journal of Psychiatry 2010;3(4):206-12. [DOI: 10.1016/j.ajp.2010.07.002] [DOI] [PubMed] [Google Scholar]
Park 2019 {published data only}
- Park YR, Sohng K-Y. Effects of a customized health promotion program on depression, cognitive functioning, and physical health of elderly women living alone in community: a cluster randomized controlled trial [맞춤형 건강증진 프로그램이 여성 독거노인의 우울과 인지기능 및 신체 건강에 미치는 효과: 무작위 집락 배정 설계]. Journal of Korean Academy of Nursing 2019;49(5):515-25. [DOI: 10.4040/jkan.2019.49.5.515] [DOI] [PubMed] [Google Scholar]
Parvin 2008 {published data only}
- Parvin N, Alavi A, Alidost E, Forozandeh N, Hosseinzadeh S, Kamkhah AF, et al. The effects of group therapy on mental health condition in mothers of thalassemic patients. Journal of Shahrekord University of Medical Sciences 2008;10(3):37-43. [LINK: journal.skums.ac.ir/article-1-465-en.html] [Google Scholar]
Patel 2017 {published data only}
- Patel V, Weobong B, Weiss HA, Anand A, Bhat B, Katti B, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet 2017;389(10065):176-85. [DOI: 10.1016/S0140-6736(16)31589-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelegrini 2020 {published data only}
- Pelegrini LN, Araújo LS, Say KG, Avelar MP, Say KG. Effects of meditation associated with education in neurosciences of pain in adults with fibromyalgia: a randomized controlled trial [Efeitos da meditação associada a educação em neurociências da dor em adultos com fibromialgia: ensaio clínico controlado e randomizado]. SMAD: Revista Eletrônica Saúde Mental Álcool e Drogas 2020;16(3):3-13. [DOI: 10.11606/issn.1806-6976.smad.2020.167602] [DOI] [Google Scholar]
Peltonen 2012 {published data only}
- Peltonen K, Qouta S, El Sarraj E, Punamäki R-L. Effectiveness of school-based intervention in enhancing mental health and social functioning among war-affected children. Traumatology 2012;18(4):37-46. [DOI: 10.1177/1534765612437380] [DOI] [Google Scholar]
Peltzer 2012 {published data only}
- Peltzer K, Ramlagan S, Jones D, Weiss SM, Fomundam H, Chanetsa L. Efficacy of a lay health worker led group antiretroviral medication adherence training among non-adherent HIV-positive patients in KwaZulu-Natal, South Africa: Results from a randomized trial. SAHARA-J: Journal of Social Aspects of HIV/AIDS 2012;9(4):218-26. [DOI: 10.1080/17290376.2012.745640] [DOI] [PubMed] [Google Scholar]
Peltzer 2013 {published data only}
- Peltzer K, Naidoo P, Louw J, Matseke G, Zuma K, Mchunu G, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health 2013;13:699. [DOI: 10.1186/1471-2458-13-699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltzer 2020 {published data only}
- Peltzer K, Abbamonte JM, Mandell LN, Rodriguez VJ, Lee TK, Weiss SM, et al. The effect of male involvement and a prevention of mother-to-child transmission (PMTCT) intervention on depressive symptoms in perinatal HIV-infected rural South African women. Archives of Women's Mental Health 2020;23(1):101-11. [DOI: 10.1007/s00737-019-00955-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 1989 {published data only}
- Perry CL, Grant M, Ernberg G, Florenzano RU, Langdon MC, Myeni AD, et al. WHO collaborative study on alcohol education and young people: outcomes of a four-country pilot study. International Journal of the Addictions 1989;24(12):1145-71. [DOI: 10.3109/10826088909048710] [DOI] [PubMed] [Google Scholar]
Petersen 2014 {published data only}
- Petersen I, Hanass Hancock J, Bhana A, Govender K. A group-based counselling intervention for depression comorbid with HIV/AIDS using a task shifting approach in South Africa: a randomized controlled pilot study. Journal of Affective Disorders 2014;158:78-84. [DOI: 10.1016/j.jad.2014.02.013] [DOI] [PubMed] [Google Scholar]
Rabiei 2020 {published data only}
- Rabiei L, Eslami AA, Abbasi M, Afzali SM, Hosseini SM, Masoudi R. Evaluating the effect of family-centered intervention program on care burden and self-efficacy of hemodialysis patient caregivers based on social cognitive theory: a randomized clinical trial study. Korean Journal of Family Medicine 2020;41(2):84. [DOI: 10.4082/kjfm.18.0079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahimi 2021b {published data only}
- Rahimi R, Hasanpour S, Mirghafourvand M, Esmaeilpour K. Effect of Hope-oriented group counseling on mental health of infertile women with failed IVF cycles: a randomized controlled trial. BMC Psychiatry 2021;21(1):286. [DOI: 10.1186/s12888-021-03280-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajai 2021 {published data only}
- Rajai N, Lami B, Pishgooie AH, Habibi H, Alavizerang F. Evaluating the effect of peer-assisted education on the functioning in family caregivers of patients with schizophrenia: a clinical trial study. Korean Journal of Family Medicine 2021;42(5):356-62. [DOI: 10.4082/kjfm.20.0098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rivero 2021 {published data only}
- Rivero LM, Andrade AL, Figueredo LZ, Pinheiro BD, De Micheli D. Evaluation of FunFRIENDS program in prevention of anxiety in Brazilian children: a randomized controlled pilot trial. Ciência & Saúde Coletiva 2020;25:4497-508. [DOI: 10.1590/1413-812320202511.33072018] [DOI] [PubMed] [Google Scholar]
Rossow 2021 {published data only}
- Rossouw L, Burger RP, Burger R. Testing an incentive-based and community health worker package intervention to improve maternal health and nutrition outcomes: a pilot randomized controlled trial. Maternal and Child Health Journal 2021;25(12):1913-22. [DOI: 10.1007/s10995-021-03229-w] [DOI] [PubMed] [Google Scholar]
Rotheram‐Borus 2018 {published data only}
- NCT02358226. HIV & drug abuse prevention for South African men. clinicaltrials.gov/ct2/show/NCT02358226 (first received 6 February 2015).
- Rabie S, Bantjes J, Gordon S, Almirol E, Stewart J, Tomlinson M, et al. Who can we reach and who can we keep? Predictors of intervention engagement and adherence in a cluster randomized controlled trial in South Africa. BMC Public Health 2020;20(1):275. [DOI: 10.1186/s12889-020-8357-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Tomlinson M, Mayekiso A, Bantjes J2, Harris DM, Stewart J, et al. Gender-specific HIV and substance abuse prevention strategies for South African men: study protocol for a randomised controlled trial. Trials 2018;19:417. [DOI: 10.1186/s13063-018-2804-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2017 {published data only}
- Sanchez ZM, Valente JY, Sanudo A, Pereira AP, Cruz JI, Schneider D, et al. The #Tamojunto drug prevention program in Brazilian schools: a randomized controlled trial. Prevention Science 2017;18(7):772-82. [DOI: 10.1007/s11121-017-0770-8] [DOI] [PubMed] [Google Scholar]
Sanchez 2019 {published data only}
- Sanchez MG. Mindfulness-Based Intervention for Caregivers of Elderly People With Dementia [Intervenção Baseada em Mindfulness Para Cuidadores de Idosos com Demência] [Masters thesis]. São Carlos (Brazil): Federal University of São Carlos, 2019. [LINK: repositorio.ufscar.br/handle/ufscar/11165] [Google Scholar]
Sanchez 2021 {published data only}
- Sanchez ZM, Valente JY, Galvão PP, Gubert FA, Melo MH, Caetano SC, et al. A cluster randomized controlled trial evaluating the effectiveness of the school‐based drug prevention program #Tamojunto2.0. Addiction 2021;116(6):1580-92. [DOI: 10.1111/add.15358] [DOI] [PubMed] [Google Scholar]
Sapkota 2020 {published data only}
- Sapkota D, Baird K, Saito A, Rijal P, Anderson D. Antenatal-based pilot psychosocial intervention to enhance mental health of pregnant women experiencing domestic and family violence in Nepal. Journal of Interpersonal Violence 2020;37(5-6):NP3605-27. [DOI: 10.1177/0886260520948151] [DOI] [PubMed] [Google Scholar]
Saw 2020 {published data only}
- Saw JA, Tam CL, Thanzami V, Bonn G. Contextualized school-based cognitive behavioral therapy (CBT) intervention for Malaysian secondary school students. Frontiers in Psychiatry 2020;11:565896. [DOI: 10.3389/fpsyt.2020.565896] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scharf 2021 {published data only}
- Scharf B, Zhu S, Tomlin S, Cheon J, Mooney-Doyle K, Baggs JG, et al. Feasibility of an intervention study to support families when their loved one has life-sustaining therapy withdrawn. Journal of Hospice and Palliative Nursing 2021;23(1):89-97. [DOI: 10.1097/NJH.0000000000000717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwitters 2015 {published data only}
- Schwitters A, Sabatier J, Seth P, Glenshaw M, Remmert D, Pathak S, et al. HIV and alcohol knowledge, self-perceived risk for HIV, and risky sexual behavior among young HIV-negative men identified as harmful or hazardous drinkers in Katutura, Namibia. BMC Public Health 2015;15:1182. [DOI: 10.1186/s12889-015-2516-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seyedrasooli 2020 {published data only}
- Seyedrasooli A, Malakouti J, Jabraeili M, Heykalabadi S, Ghogazadeh M. The effect of the supportive program on the anxiety of mothers of infants with gastrointestinal anomalies. Nursing Practice Today 2020;7(2):106-13. [DOI: 10.18502/npt.v7i2.2732] [DOI] [Google Scholar]
Shaw 2021 {published data only}
- Shaw SA, Ward KP, Pillai V, Ali LM, Karim H. A randomized clinical trial testing a parenting intervention among Afghan and Rohingya refugees in Malaysia. Family Process 2021;60(3):788-805. [DOI: 10.1111/famp.12592] [DOI] [PubMed] [Google Scholar]
Sherlee 2020 {published data only}
- Sherlee JI, David A. Effectiveness of yogic visual concentration (Trataka) on cognitive performance and anxiety among adolescents. Journal of Complementary & Integrative Medicine 2020;17(3):20190055. [DOI: 10.1515/jcim-2019-0055] [DOI] [PubMed] [Google Scholar]
Shin 2009 {published data only}
- Shin JY, Nhan NV, Lee S-B, Crittenden KS, Flory M, Hong HT. The effects of a home-based intervention for young children with intellectual disabilities in Vietnam. Journal of Intellectual Disability Research 2009;53(4):339-52. [DOI: 10.1111/j.1365-2788.2008.01151.x] [DOI] [PubMed] [Google Scholar]
Shirazi 2008 {published data only}
- Shirazi M, Zeinaloo AA, Parikh SV, Sadeghi M, Taghva A, Arbabi M, et al. Effects on readiness to change of an educational intervention on depressive disorders for general physicians in primary care based on a modified Prochaska model—a randomized controlled study. Family Practice 2008;25(2):98-104. [DOI: 10.1093/fampra/cmn008] [DOI] [PubMed] [Google Scholar]
Sikander 2019 {published data only}
- Sikander S, Ahmad I, Atif N, Zaidi A, Vanobberghen F, Weiss HA, et al. Delivering the Thinking Healthy Programme for perinatal depression through volunteer peers: a cluster randomised controlled trial in Pakistan. Lancet Psychiatry 2019;6(2):128-39. [DOI: 10.1016/S2215-0366(18)30467-X] [DOI] [PubMed] [Google Scholar]
- NCT02111915. Thinking healthy program - peer delivered (Pakistan) (THPP-P) [Thinking healthy program - peer delivered in Pakistan]. clinicaltrials.gov/ct2/show/NCT02111915 (first received 11 April 2014).
Siriwardhana 2013 {published data only}
- Siriwardhana C, Adikari A, Van Bortel T, McCrone P, Sumathipala A. An intervention to improve mental health care for conflict-affected forced migrants in low-resource primary care settings: a WHO MhGAP-based pilot study in Sri Lanka (COM-GAP study). Trials 2013;14:423. [DOI: 10.1186/1745-6215-14-423] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sokolovic 2022 {published data only}
- Sokolovic N, Grujic S, Pajic S. Evaluation of the Support, not Perfection parenting program in Serbia. European Journal of Developmental Psychology 2022;19(3):365-82. [DOI: 10.1080/17405629.2021.1911800] [DOI] [Google Scholar]
Sousa 2020 {published data only}
- Sousa L, Sequeira C, Ferré‐Grau C, Graça L. ‘Living Together With Dementia’: preliminary results of a training programme for family caregivers. Scandinavian Journal of Caring Sciences 2021;35(1):86-95. [DOI: 10.1111/scs.12821] [DOI] [PubMed] [Google Scholar]
Ssewamala 2009 {published data only}
- Ssewamala FM, Han C-K, Neilands TB. Asset ownership and health and mental health functioning among AIDS-orphaned adolescents: findings from a randomized clinical trial in rural Uganda. Social Science & Medicine 2009;69(2):191-8. [DOI: 10.1016/j.socscimed.2009.05.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stansert Katzen 2021 {published data only}
- Stansert Katzen L, le Roux KW, Almirol E, Rezvan PH, le Roux IM, Mbewu N, et al. Community health worker home visiting in deeply rural South Africa: 12-month outcomes. Global Public Health 2021;16(11):1757-70. [DOI: 10.1080/17441692.2020.1833960] [DOI] [PubMed] [Google Scholar]
Suihami 2020 {published data only}
- NCT03601884. Effectiveness of OHP in improving self-efficacy in patients with diabetes mellitus (OHP) [Effectiveness of optimal health program in improving self-efficacy in patients with diabetes mellitus in Putrajaya, Malaysia]. clinicaltrials.gov/ct2/show/NCT03601884 (first received 26 July 2018).
- Suhaimi AF, Ibrahim N, Tan K-A, Silim UA, Moore G, Ryan B, et al. Effectiveness of a culturally adapted biopsychosocial intervention (POHON SIHAT) in improving self-efficacy in patients with diabetes attending primary healthcare clinics in Putrajaya, Malaysia: study protocol of a randomised controlled trial. BMJ Open 2020;10(2):e033920. [DOI: 10.1136/bmjopen-2019-033920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suleman 2021 {published data only}
- Suleman A, Mootz JJ, Feliciano P, Nicholson T, O’Grady MA, Wall M, et al. Scale-up study protocol of the implementation of a mobile health SBIRT approach for alcohol use reduction in Mozambique. Psychiatric Services 2021;72(10):1199-208. [DOI: 10.1176/appi.ps.202000086] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2021 {published data only}
- Sun Y, Wang X, Li N, Bian J. Influence of psychological nursing and health education on depression, anxiety and life quality of elderly patients with lung cancer. Psychogeriatrics 2021;21(4):521-7. [DOI: 10.1111/psyg.12700] [DOI] [PubMed] [Google Scholar]
Suteerangkul 2021 {published data only}
- Suteerangkul P, Lagampan S, Kalampakorn S, Auemaneekul N. The effects of community participation program on smoke-free homes in a suburban community of Thailand. Tobacco Induced Diseases 2021;19:35. [DOI: 10.18332/tid/133876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taghizadeh 2008 {published data only}
- Taghizadeh Z, Jafarbegloo M, Arbabi M, Faghihzadeh S. The effect of counseling on post traumatic stress disorder after a traumatic childbirth. Journal of HAYAT 2008;13(4):23-31. [LINK: hayat.tums.ac.ir/article-1-160-en.html] [Google Scholar]
Tam 2019 {published data only}
- Tam CC, Li X, Benotsch EG, Lin D. A resilience‐based intervention programme to enhance psychological well‐being and protective factors for rural‐to‐urban migrant children in China. Applied Psychology: Health and Well‐Being 2020;12(1):53-76. [DOI: 10.1111/aphw.12173] [DOI] [PubMed] [Google Scholar]
Taneja 2020 {published data only}
- Mazumder S, Taneja S, Dube B, Bhatia K, Ghosh R, Shekhar M, et al. Effect of community-initiated kangaroo mother care on survival of infants with low birthweight: a randomised controlled trial. Lancet 2019;394(10210):1724-36. [DOI: 10.1016/S0140-6736(19)32223-8] [DOI] [PubMed] [Google Scholar]
- NCT02631343. Community kangaroo mother care for improving child survival and brain development in low birth weight newborns (CKMC-DEV) [A community-based model of delivery of kangaroo mother care for improving child survival and brain development in low birth weight newborns]. clinicaltrials.gov/ct2/show/NCT02631343 (first received 16 December 2015).
- Taneja S, Sinha B, Upadhyay RP, Mazumder S, Sommerfelt H, Martines J, et al. Community initiated kangaroo mother care and early child development in low birth weight infants in India-a randomized controlled trial. BMC Pediatrics 2020;20(1):150. [DOI: 10.1186/s12887-020-02046-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tawfik 2021 {published data only}
- Tawfik NM, Sabry NA, Darwish H, Mowafy M, Soliman SS. Psychoeducational program for the family member caregivers of people with dementia to reduce perceived burden and increase patient’s quality of life: a randomized controlled trial. Journal of Primary Care & Community Health 2021;12:21501327211014088. [DOI: 10.1177/21501327211014088] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20200610001 {published data only}
- TCTR20200610001. Effectiveness of the family-management intervention on improving quality of life and reducing burden of family with autistic children in Vietnam. thaiclinicaltrials.org/show/TCTR20200610001 (first received 1 June 2020).
Tekur 2012 {published data only}
- Tekur P, Nagarathna R, Chametcha S, Hankey A, Nagendra HR. A comprehensive yoga programs improves pain, anxiety and depression in chronic low back pain patients more than exercise: An RCT. Complementary Therapies in Medicine 2012;20(3):107-18. [DOI: 10.1016/j.ctim.2011.12.009] [DOI] [PubMed] [Google Scholar]
Tiwari 2010 {published data only}
- NCT01054898. Effectiveness of a telephone intervention to improve the mental health of abused women [A RCT to test the effectiveness of a telephone intervention to improve the mental health of community dwelling women abused by their intimate partners]. clinicaltrials.gov/ct2/show/NCT01054898 (first received 22 January 2010).
- Tiwari A, Fong DY, Yuen KH, Yuk H, Pang P, et al. Effect of an advocacy intervention on mental health in Chinese women survivors of intimate partner violence: a randomized controlled trial. JAMA 2010;304(5):536-43. [DOI: 10.1001/jama.2010.1052] [DOI] [PubMed] [Google Scholar]
Tol 2014 {published data only}
- ISRCTN42284825. Efficacy of a school-based psychosocial intervention to deal with the psychosocial impact of armed conflict on school-aged children in Burundi. isrctn.com/ISRCTN42284825 (first received 24 February 2006).
- Tol WA, Komproe IH, Jordans MJ, Ndayisaba A, Ntamutumba P, Sipsma H, et al. School-based mental health intervention for children in war-affected Burundi: a cluster randomized trial. BMC Medicine 2014;12:56. [DOI: 10.1186/1741-7015-12-56] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toulabi 2012 {published data only}
- Toulabi T, Khosh Niyat Nikoo M, Amini F, Nazari H, Mardani M. The influence of a behavior modification interventional program on body mass index in obese adolescents. Journal of the Formosan Medical Association 2012;111(3):153-9. [DOI: 10.1016/j.jfma.2011.05.007] [DOI] [PubMed] [Google Scholar]
Ural 2021 {published data only}
- Ural A, Beji NK. The effect of health-promoting lifestyle education program provided to women with gestational diabetes mellitus on maternal and neonatal health: a randomized controlled trial. Psychology, Health & Medicine 2021;26(6):657-70. [DOI: 10.1080/13548506.2020.1856390] [DOI] [PubMed] [Google Scholar]
Uzdavines 2021 {published data only}
- Uzdavines A, Gonzalez RD, Price A, Broadway D, Smith TL, Rodrigues M, et al. Acceptance and commitment training for veterans with polytrauma: a randomized controlled trial protocol. Contemporary Clinical Trials 2021;111:106601. [DOI: 10.1016/j.cct.2021.106601] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakilian 2019 {published data only}
- Vakilian K, Zarei F, Majidi A. Effect of acceptance and commitment therapy (ACT) on anxiety and quality of life during pregnancy: a mental health clinical trial study. Iranian Red Crescent Medical Journal 2019;21(8):e89489. [DOI: 10.5812/ircmj.89489] [DOI] [Google Scholar]
Van't Hof 2021 {published data only}
- Van't Hof E, Heim E, Abi Ramia J, Burchert S, Cornelisz I, Cuijpers P, et al. Evaluating the effectiveness of an e-mental health intervention for people living in Lebanon: protocol for two randomized controlled trials. JMIR Research Protocols 2021;10(1):e21585. [DOI: 10.2196/21585] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vancampfort 2021 {published data only}
- Vancampfort D, Byansi PK, Namutebi H, Kinyanda E, Bbosa RS, Ward PB, et al. The efficacy of a lay health workers – led physical activity counselling program in patients with HIV and mental health problems: a real-world intervention from Uganda. AIDS Care 2021;33(9):1189-95. [DOI: 10.1080/09540121.2021.1874268] [DOI] [PubMed] [Google Scholar]
Villar 1992 {published data only}
- Villar J, Farnot U, Barros F, Victora C, Langer A, Belizan JM. A randomized trial of psychosocial support during high-risk pregnancies. The Latin American Network for Perinatal and Reproductive Research. New England Journal of Medicine 1992;327(18):1266-71. [DOI: 10.1056/NEJM199210293271803] [DOI] [PubMed] [Google Scholar]
Waechter 2021 {published data only}
- Waechter R, Stahl G, Rabie S, Colak B, Johnson- Rais D, Landon B, et al. Mitigating medical student stress and anxiety: should schools mandate participation in wellness intervention programs? Medical Teacher 2021;43(8):945-55. [DOI: 10.1080/0142159X.2021.1902966] [DOI] [PubMed] [Google Scholar]
Wainberg 2021 {published data only}
- Wainberg ML, Lovero KL, Duarte CS, Fiks Salem A, Mello M, Bezuidenhout C, et al. Partnerships in research to implement and disseminate sustainable and scalable evidence-based practices (PRIDE) in Mozambique. Psychiatric Services 2021;72(7):802-11. [DOI: 10.1176/appi.ps.202000090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2006 {published data only}
- Walker SP, Chang SM, Powell CA, Simonoff E, Grantham-McGregor SM. Effects of psychosocial stimulation and dietary supplementation in early childhood on psychosocial functioning in late adolescence: follow-up of randomised controlled trial. BMJ 2006;333(7566):472. [DOI: 10.1136/bmj.38897.555208.2F] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasil 2021 {published data only}
- PACTR201906810558181. Internet -based Shamiri single session intervention [The Shamiri single session digital intervention for adolescent anxiety and depression: a randomized control trial of an internet-based intervention in a low-resourced school in Kenya]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=8189 (first received 21 June 2019).
- Wasil AR, Kacmarek CN, Osborn TL, Palermo EH, DeRubeis RJ, Weisz JR, et al. Economic evaluation of an online single-session intervention for depression in Kenyan adolescents. Journal of Consulting and Clinical Psychology 2021;89(8):657-67. [DOI: 10.1037/ccp0000669] [DOI] [PubMed] [Google Scholar]
Wolf 2019 {published data only}
- Wolf S, Aber JL, Behrman JR, Peele M. Longitudinal causal impacts of preschool teacher training on Ghanaian children's school readiness: evidence for persistence and fade-out. Developmental Science 2019;22(5):e12878. [DOI: 10.1111/desc.12878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolmer 2005 {published data only}
- Wolmer L, Laor N, Dedeoglu C, Siev J, Yazgan Y. Teacher-mediated intervention after disaster: a controlled three-year follow-up of children's functioning. Journal of Child Psychology and Psychiatry 2005;46(11):1161-8. [DOI: 10.1111/j.1469-7610.2005.00416.x] [DOI] [PubMed] [Google Scholar]
Won 2020 {published data only}
- Won GH, Lee JH, Choi TY, Yoon S, Kim SY, Park JH. The effect of a mental health promotion program on Korean firefighters. International Journal of Social Psychiatry 2020;66(7):675-81. [DOI: 10.1177/0020764020920918] [DOI] [PubMed] [Google Scholar]
Wong 2020 {published data only}
- Wong AK, Wong FK. The psychological impact of a nurse-led proactive self-care program on independent, non-frail community-dwelling older adults: A randomized controlled trial. International Journal of Nursing Studies 2020;110:103724. [DOI: 10.1016/j.ijnurstu.2020.103724] [DOI] [PubMed] [Google Scholar]
Xiao 2021 {published data only}
- Xiao H, Zhao Z, Zhang C, Wang J. Influence of standardized nursing intervention combined with mindfulness stress reduction training on the curative effect, negative emotion, and quality of life in patients with chronic gastritis and gastric ulcer. Hindawi Evidence-Based Complementary and Alternative Medicine 2021;2021:2131405. [DOI: 10.1155/2021/2131405] [LINK: www.hindawi.com/journals/ecam/2021/2131405/] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Xie 2019 {published data only}
- Xie J, He G, Ding S, Pan C, Zhang X, Zhou J, et al. A randomized study on the effect of modified behavioral activation treatment for depressive symptoms in rural left-behind elderly. Psychotherapy Research 2019;29(3):372-82. [DOI: 10.1080/10503307.2017.1364444] [DOI] [PubMed] [Google Scholar]
Xu J 2022 {published data only}
- Xu J, Yang F, Si L, Qian D. Do integrated health care interventions improve well‑being among older adults with hypertension? evidence from rural China. Social Indicators Research 2022;160:825-43. [DOI: 10.1007/s11205-020-02482-w] [DOI] [Google Scholar]
Yamada 2021 {published data only}
- Yamada C, Siste K, Hanafi E, Ophinni Y, Beatrice E, Rafelia V, et al. Relapse prevention group therapy via video-conferencing for substance use disorder: protocol for a multicentre randomised controlled trial in Indonesia. BMJ Open 2021;11(9):e050259. [DOI: 10.1136/bmjopen-2021-050259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2020 {published data only}
- Ye J, Xiao A, Wang C, Xia Z, Yu L, Li S, et al. Evaluating the effectiveness of a CRSCE-based de-escalation training program among psychiatric nurses: a study protocol for a cluster randomized controlled trial. BMC Health Services Research 2020;20(1):642. [DOI: 10.1186/s12913-020-05506-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2016 {published data only}
- Zeng Q, He Y, Shi Z, Liu W, Tao H, Bu S, et al. A community-based controlled trial of a comprehensive psychological intervention for community residents with diabetes or hypertension. Shanghai Archives of Psychiatry 2016;28(2):72-85. [DOI: 10.11919/j.issn.1002-0829.216016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeziulin 2021 {published data only}
- Zeziulin O, Mollan KR, Shook-Sa BE, Hanscom B, Lancaster KE, Dumchev K, et al. Depressive symptoms and use of HIV care and medication-assisted treatment among people with HIV who inject drugs. AIDS 2021;35(3):495-501. [DOI: 10.1097/QAD.0000000000002774] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang C, Zhao H, Zhu R, Lu J, Hou L, Yang XY, et al. Improvement of social support in empty-nest elderly: results from an intervention study based on the Self-Mutual-Group model. Journal of Public Health 2019;41(4):830-9. [DOI: 10.1093/pubmed/fdy185] [DOI] [PubMed] [Google Scholar]
Zhang 2021 {published data only}
- Zhang X, Lin JL, Gao R, Chen N, Huang GF, Wang L, et al. Application of the hospital‐family holistic care model in caregivers of patients with permanent enterostomy: a randomized controlled trial. Journal of Advanced Nursing 2021;77(4):2033-49. [DOI: 10.1111/jan.14691] [DOI] [PubMed] [Google Scholar]
Zheng 2021a {published data only}
- Zheng Y, Wang W, Zhong Y, Wu F, Zhu Z, Tham Y-C, et al. A peer-to-peer live-streaming intervention for children during COVID-19 homeschooling to promote physical activity and reduce anxiety and eye strain: cluster randomized controlled trial. Journal of Medical Internet Research 2021;23(4):e24316. [DOI: 10.2196/24316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2020 {published data only}
- Zhou Y, Jiang M. Intervention effect of physical education on mental health of college students. Revista Argentina de Clínica Psicológica 2020;29(2):765-71. [DOI: 10.24205/03276716.2020.308] [DOI] [Google Scholar]
References to studies awaiting assessment
Ajh 2006 {published data only}
- Ajh N, Unesian M, Fili A, Abasi Motejaded A. The study of supportive activities during pregnancy on postpartum depression [تأثیر†اقدامات†حمایتی†در†دوران†بارداری†بر†پیشگیری†از†افسردگی†پس†از†زایمان]. Journal of HAYAT 2006;12(3):73-80. [LINK: iranjournals.nlai.ir/handle/123456789/635927] [Google Scholar]
Akbarzadeh 2012 {published data only}
- Akbarzadeh M, Toosi M, Zare N, Sharif F. Effect of relaxation and attachment behaviors training on anxiety in first-time mothers in Shiraz City, 2010: a randomized clinical trial [تاثير آموزش تن آرامي و رفتارهاي دلبستگي بر ميزان اضطراب مادران نخست باردار شهر شيراز: کارآزمايي باليني تصادفي شده]. Journal of Qom University of Medical Sciences 2012;6(4):14-23. [LINK: journal.muq.ac.ir/article-1-125-en.html] [Google Scholar]
Andaroon 2018 {published data only}
- Andaroon N, Kordi M, Kimiaee SA, Esmaily H. Effect of individual counseling program by a midwife on anxiety during pregnancy in nulliparous women. Iranian Journal of Obstetrics, Gynecology and Infertility [Majallah-i Zanān, Māmā̓ī va Nāzā̓ī-i Īrān] 2018;20(12):86-95. [DOI: 10.22038/ijogi.2017.10434] [DOI] [Google Scholar]
Baba Nazari 2014 {published data only}
- Baba Nazari L, Zamani P. Effect of spiritual intelligence on decreasing pregnant women's anxiety during pregnancy in Shiraz City. Journal of Jahrom University of Medical Sciences 2014;11:244. [LINK: web.s.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=20087993&AN=99118942&h=rKjAsTnVvqG%2fhYYXITafI%2fZSl%2fc95dqEcTEpShKI3bI18n4uiIqsYQ5X0pppFpDMoUIO8n3ljSAHYUiabTifrg%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d20087993%26AN%3d99118942] [Google Scholar]
Bazrafshan 2010 {published data only}
- Bazrafshan MR, Ghorbani Z. The effect of back surface stroke (SSBM) massage on anxiety in nulliparous pregnant women [تاثير ماساژ به روش استروك سطحي پشت (SSBM) بر اضطراب زنان باردار نخست زا]. Journal of HAYAT 2010;16(1):34-40. [LINK: hayat.tums.ac.ir/article-1-95-fa.html] [Google Scholar]
Bernardi 2013 {published data only}
- Bernardi ML, Amorim MH, Zandonade E, Santaella DF, Barbosa JD. The effects of Hatha yoga exercises on stress and anxiety levels in mastectomized women [Efeitos da intervenção Hatha-Yoga nos níveis de estresse e ansiedade em mulheres mastectomizadas]. Ciência & Saúde Coletiva 2013;18(12):3621-32. [DOI: 10.1590/s1413-81232013001200018] [DOI] [PubMed] [Google Scholar]
Foo 2020 {published data only}
- Foo CN, Arumugam M, Lekhraj R, Lye M-S, Mohd-Sidik S, Osman ZJ. Effectiveness of health-led cognitive behavioral-based group therapy on pain, functional disability and psychological outcomes among knee osteoarthritis patients in Malaysia. International Journal of Environmental Research and Public Health 2020;17(17):6179. [DOI: 10.3390/ijerph17176179] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heidari 2014 {published data only}
- Ghaedi Heydari F, Pahlava Nazadeh S, Maghsoudi J, Ghazavi Z. The effect of family education program on caregivers' depression, anxiety and stress in families of the elderly with dementia [تأثير برنامه آموزش خانواده بر ميزان افسردگي، اضطراب و استرس مراقبينخانوادگي سالمندان مبتلا به دمانس]. Nursing Education 2014;3(1):12-20. [LINK: jne.ir/article-1-287-fa.html] [Google Scholar]
Heidari 2020 {published data only}
- Heidari S, Mirzaei T, Heydarinezad Chatrodi M, Heidarzadeh A. Effect of problem-oriented coping strategies training on perceived stress in the family caregivers of the elderly with Alzheimer. Journal of HAYAT 2021;26(4):384-95. [LINK: hayat.tums.ac.ir/article-1-3818-en.html] [Google Scholar]
Hemmat Makan 2021 {published data only}
- Hemmat Makan N, Golshani F, Baghdasarians A, Emamipour S. The effect of self-care education on blood glucose, diabetic quality of life, and emotional behavioral disorders in adolescents with diabetes. Journal of Diabetes Nursing 2021;9(1):1274-86. [LINK: jdn.zbmu.ac.ir/article-1-456-en.html] [Google Scholar]
Jingna 2012 {published data only}
- Jingna J, Liqun C, Shoumei J. Influence of community nurse-led multidisciplinary team home visiting services on psychological state of old age of home elderly [社区护士主导的全科团队家访服务对高龄居家老人心理状况的影响]. Chinese Nursing Research [护理研究] 2012;2012(11):975-8. [LINK: caod.oriprobe.com/articles/29338126/Influence_of_community_nurse_led_multidisciplinary_team_home_visiting_.htm] [Google Scholar]
Khalili 2019 {published data only}
- Khalili Z, Navai M, Shakiba M, Navidian A. The effect of supportive-educational intervention on psychological distress in pregnant women subjected to domestic violence: a randomized controlled trial [بررسی تأثیر مداخله آموزشی - حمایتی بر پریشانی روانشناختی زنان باردار تحتخشونت خانگی: یک مطالعه کارآزمایی بالینی]. Journal of HAYAT 2019;25(2):151-67. [LINK: hayat.tums.ac.ir/article-1-2970-en.html] [Google Scholar]
Khosravi 2022 {published data only}
- Khosravi F, Fereidooni‑Moghadam M, Mehrabi T, Moosavizade SR. The effect of a spirituality‑based program on stress, anxiety, and depression of caregivers of patients with mental disorders in Iran. Journal of Religion and Health 2022;61:93-108. [DOI: 10.1007/s10943-021-01372-w] [DOI] [PubMed] [Google Scholar]
Küçük 2020 {published data only}
- Küçük L, Bilgin H, Ikican TC, Kaçar S, Kadak MT, Demirel ÖF, et al. Evaluation of the effectiveness of psychoeducation given to children with parental psychiatric disorders. Issues in Mental Health Nursing 2020;41(11):985-94. [DOI: 10.1080/01612840.2020.1756010] [LINK: www.tandfonline.com/doi/full/10.1080/01612840.2020.1756010] [DOI] [PubMed] [Google Scholar]
Li 2020b {published data only}
- Li L, Lin C, Liang L-J, Feng N, Pham L, Hien NT. Evaluating an intervention for family members of people who use drugs in Vietnam. Social Science & Medicine 2020;261:113238. [DOI: 10.1016/j.socscimed.2020.113238] [LINK: www.sciencedirect.com/science/article/abs/pii/S0277953620304573?via%3Dihub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi‐Yeganeh 2008 {published data only}
- Mohammadi-Yeganeh L, Bastani F, Feizi Z, Agilar-Vafaie M, Haghani H. Effect of stress management education on mood and perceived stress among oral contraceptive pill users. Iran Journal of Nursing 2008;21(53):63-73. [LINK: ijn.iums.ac.ir/article-1-410-en.html] [Google Scholar]
Moradi 2010 {published data only}
- Moradi MH, Heydarnia A, Rochi GB, Jahangiri M. Investigating the effect of education based on the model of stages of change in the prevention of substance use in petrochemical workers [بررسي تاثيرآموزش مبتني بر مدل مراحل تغيير در پيشگيري از مصرف موادمخدر در كارگران پتروشيمي]. Journal of Islamic Azad University 2010;19(4 Seq 58):246-55. [LINK: www.sid.ir/fa/journal/ViewPaper.aspx?id=104176] [Google Scholar]
Nisar 2021 {published data only}
- Nisar Z, Sajjad H, Anwar N. Effect of group psycho social support on quality of life and mortality with end stage kidney disease. Nephrology Dialysis Transplantation 2020;35(Suppl 3):P0843. [DOI: 10.1093/ndt/gfaa142.P0843] [LINK: academic.oup.com/ndt/article/35/Supplement_3/gfaa142.P0843/5853740] [DOI] [Google Scholar]
PACTR202009845194666 {published data only}
- PACTR202009845194666. Global Resilience Oral Workshops (GROW) Zambia: a storytelling approach to hope and resilience through character training and spiritual practices [A delayed-intervention control group design 1:1 cluster-randomized trial of Global Resilience Oral Workshops (GROW) Zambia, a storytelling approach to youth hope and resilience through character training and spiritual practices]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=12331 (first received 3 September 2020).
Puffer 2016 {published data only}
- Puffer ES, Green EP, Sikkema KJ, Broverman SA, Ogwang-Odhiambo RA, Pian J. A church-based intervention for families to promote mental health and prevent HIV among adolescents in rural Kenya: results of a randomized trial. Journal of Consulting and Clinical Psychology 2016;84(6):511-25. [DOI: 10.1037/ccp0000076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2021 {published data only}
- Sharma M, Srivastava S, Pathak A. Family psychoeducation as an intervention tool in the management of schizophrenia and the psychological wellbeing of caregivers. Indian Journal of Community Medicine 2021;46(2):304-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuzhen 2015 {published data only}
- Shuzhen K, Wenhui J, Weizhi L. Application research on multidisciplinary team self-management in intervention for patients with community coronary heart disease [多学科团队自我管理在社区冠心病病人干预中的应用研究]. Chinese Nursing Research [护理研究] 2015;2015(20):2456-9. [LINK: caod.oriprobe.com/articles/45402328/Application_research_on_multidisciplinary_team_sel.htm] [Google Scholar]
Xia 2017 {published data only}
- Xia L, Liqun C, Changying J. Influence of family visit of community nurses for the elderly with dementia family caregivers burden of care and quality of life of the elderly with dementia [社区护士家庭访视对失智老人居家照顾者照顾负荷及生活质量的影响]. Chinese Nursing Research [护理研究] 2017;2017(33):4206-9. [LINK: https://caod.oriprobe.com/articles/53043375/Influence_of_family_visit_of_community_nurses_for_.htm] [Google Scholar]
Zhao 2021 {published data only}
- Zhao Y, Lin Q, Wang J. An evaluation of a prenatal individualised mixed management intervention addressing breastfeeding outcomes and postpartum depression: a randomised controlled trial. Journal of Clinical Nursing 2021;30(9-10):1347-59. [DOI: 10.1111/jocn.15684] [DOI] [PubMed] [Google Scholar]
Zheng 2021b {published data only}
- Zheng F, Qiao L, Zhong Z, Duan Y, Ding S. Application of peer support intervention in nursing care of patients with acute myocardial infarction after PCI [同伴支持干预在急性心肌梗死病人PCI术后护理中的应用]. Chinese Nursing Research [护理研究] 2021;2021(20):3581-7. [LINK: caod.oriprobe.com/articles/62194382/tong_ban_zhi_chi_gan_yu_zai_ji_xing_xin_ji_geng_si.htm] [LINK: search.ebscohost.com/login.aspx? direct=true&db=cin20&AN=153302825& site=ehost - live] [Google Scholar]
References to ongoing studies
ACTRN12620001014943 {published data only}
- ACTRN12620001014943. The Mbereko+Men model: evaluating the effect of a community based, gender synchronised parenting intervention with mothers and fathers on maternal mental health, care-seeking for maternal, newborn and child health services, and care and support in the home for mothers and infants in Manicaland, Zimbabwe. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620001014943 (first received 12 June 2020).
ACTRN12621000189820 {published data only}
- ACTRN12621000189820p. Effect of a stepped care intervention on anxiety and depression in distressed people in Jordan [Randomised controlled trial of a stepped care intervention comprising self-help plus and problem management plus versus self-help plus on anxiety and depression in Jordanians experiencing distress]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12621000189820 (first received 14 November 2020).
Al Azdi 2021 {published data only}
- Al Azdi Z, Islam K, Khan MA, Khan N, Ejaz A, Khan MA, et al. Effectiveness of an integrated care package for refugee mothers and children: protocol for a cluster randomized controlled trial. JMIR Research Protocols 2021;10(5):e25047. [DOI: 10.2196/25047] [LINK: www.researchprotocols.org/2021/5/e25047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amudhan 2021 {published data only}
- Amudhan S, Jangam K, Mani K, Murugappan NP, Sharma E, Mahapatra P, et al. Project SUMS (scaling up of mental health in schools): design and methods for a pragmatic, cluster randomised waitlist-controlled trial on integrated school mental health intervention for adolescents. BMC Public Health 2021;21(1):2034. [DOI: 10.1186/s12889-021-12086-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aung 2021 {published data only}
- Aung MN, Moolphate S, Yuasa M, Aung TN, Koyanagi Y, Supakankunti S, et al. Community-integrated intermediary care (CIIC) service model to enhance family-based, long-term care for older people: protocol for a cluster randomized controlled trial in Thailand. JMIR Research Protocols 2021;10(3):e20196. [DOI: 10.2196/20196] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bello 2021 {published data only}
- Bello FA, Adeolu JO, Abdulmalik JO, Abdus-salam RA, Egbokhare OA, Otekunrin OA, et al. Reducing anxiety and depression in infertility among Nigerian women: an exploratory psycho-educational intervention trial (RADIANT) study protocol. Pan African Medical Journal 2021;39:43. [LINK: www.panafrican-med-journal.com/content/article/39/43/full/] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benfer 2018 {published data only}
- Benfer KA, Novak I, Morgan C, Whittingham K, Khan NZ, Ware RS, et al. Community-based parent-delivered early detection and intervention programme for infants at high risk of cerebral palsy in a low-resource country (Learning through Everyday Activities with Parents (LEAP-CP): protocol for a randomised controlled trial. BMJ Open 2018;8(6):e021186. [DOI: 10.1136/bmjopen-2017-021186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burchert 2019 {published data only}
- Burchert S, Alkneme MS, Bird M, Carswell K, Cuijpers P, Hansen P, et al. User-centered app adaptation of a low-intensity e-mental health intervention for Syrian refugees. Frontiers in Psychiatry 2019;9:663. [DOI: 10.3389/fpsyt.2018.00663] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRKS00023505. STRENGTHS SBS RCT Egypt [Randomized controlled trial to test the (cost-)effectiveness of Step-by-Step, a smartphone-based self-help program for Syrian refugees in Egypt]. drks.de/search/en/trial/DRKS00023505 (first received 3 November 2020).
ChiCTR1800015602 {published data only}
- ChiCTR1800015602. Chronic disease self-management support programme for spouse caregivers of persons with dementia in China [Development of a chronic disease self-management support programme for spouse caregivers of persons with dementia in China: a single-centre non-blinded randomised control trial]. chictr.org.cn/showprojEN.html?proj=26228 (first received 11 April 2018).
ChiCTR1900023145 {published data only}
- ChiCTR1900023145. Evaluation of a prevention program for depression among high school adolescent in mainland China: a cluster randomized controlled trial [Development and evaluation of a prevention program for depression among high school adolescent in mainland China: a cluster randomized controlled trial]. chictr.org.cn/showprojEN.html?proj=39002 (first received 13 May 2019).
ChiCTR2000028878 {published data only}
- ChiCTR2000028878. Intervention for children's dental anxiety and dental behavior management problems: a cluster randomized trial [Experiential learning intervention for children's dental anxiety: a cluster randomized trial]. chictr.org.cn/showprojEN.html?proj=47970 (first received 5 January 2020).
ChiCTR2000039133 {published data only}
- ChiCTR2000039133. Randomized controlled trial of Enhancing Contact Model on reducing stigma of mental illness in family caregivers of persons with schizophrenia in rural China. chictr.org.cn/showprojEN.html?proj=62815 (first received 18 October 2020).
CTRI/2017/04/008385 {published data only}
- CTRI/2017/04/008385. To evaluate the efficacy of an integrated yoga module as an intervention for reducing epilepsy related stigma in persons with epilepsy in India [Interventions for stigma for persons with epilepsy in India]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=18654&EncHid=&modid=&compid=%27,%2718654det%27 (first received 20 April 2017).
CTRI/2020/11/028808 {published data only}
- CTRI/2020/11/028808. A factorial trial of structured physical activity and brief cognitive behavior therapy among heart failure patients [A factorial randomised trial of structured physical activity training and brief cognitive behavioural therapy for prevention of repeat hospital admission and death among acute decompensated heart failure patients]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=47002&EncHid=&userName=CTRI/2020/11/028808 (first received 2 November 2020).
CTRI/2021/01/030403 {published data only}
- CTRI/2021/01/030403. A study to develop and use digital media like videos for caregivers to improve dementia care in India [Moving pictures: using digital media to improve dementia care in India]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=50794&EncHid=&userName=CTRI/2021/01/030403 (first received 12 January 2021).
Daruwalla 2019 {published data only}
- Daruwalla N, Machchhar U, Pantvaidya S, D'Souza V, Gram L, Copas A, et al. Community interventions to prevent violence against women and girls in informal settlements in Mumbai: the SNEHA-TARA pragmatic cluster randomised controlled trial. Trials 2019;20(1):743. [DOI: 10.1186/s13063-019-3817-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN84502355. Community interventions to prevent violence against women and girls in informal settlements in Mumbai: the SNEHA-TARA trial. isrctn.com/ISRCTN84502355 (first received 19 February 2018). [DOI] [PMC free article] [PubMed]
Desrosiers 2021 {published data only}
- Desrosiers A, Schafer C, Esliker R, Jambai M, Betancourt T. mHealth-supported delivery of an evidence-based family home-visiting intervention in Sierra Leone: protocol for a pilot randomized controlled trial. JMIR Research Protocols 2021;10(2):e25443. [DOI: 10.2196/25443] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04481399. Mobile health (mHealth) tools to improve delivery quality of a family home visiting intervention [mHealth tools to improve service delivery quality of an evidence-based family home visiting intervention to prevent family violence among high risk families in Sierra Leone]. clinicaltrials.gov/ct2/show/NCT04481399 (first received 22 July 2020).
Devassy 2021 {published data only}
- Devassy SM, Allagh KP, Benny AM, Scaria L, Cheguvera N, Sunirose IP. Resiliency engagement and care in health (REaCH): a telephone befriending intervention for upskilled rural youth in the context of COVID-19 pandemic—study protocol for a multi-centre cluster randomised controlled trial. Trials 2021;22(1):500. [DOI: 10.1186/s13063-021-05465-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominiquez‐Rodriguez 2021 {published data only}
- Dominguez-Rodriguez A, Martínez-Luna SC, Hernández Jiménez MJ, De La Rosa-Gómez A, Arenas-Landgrave P, Esquivel Santoveña EE, et al. A self-applied multi-component psychological online intervention based on UX, for the prevention of complicated grief disorder in the Mexican population during the COVID-19 outbreak: protocol of a randomized clinical trial. Frontiers in Psychology 2021;12:644782. [DOI: 10.3389/fpsyg.2021.644782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowdall 2017 {published data only}
- Dowdall N, Cooper PJ, Tomlinson M, Skeen S, Gardner F, Murray L. The benefits of early book sharing (BEBS) for child cognitive and socio-emotional development in South Africa: study protocol for a randomised controlled trial. Trials 2017;18(1):118. [DOI: 10.1186/s13063-017-1790-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Draper 2020 {published data only}
- Draper CE, Prioreschi A, Ware LJ, Lye S, Norris SA. Pilot implementation of Bukhali: a preconception health trial inSouth Africa. SAGE Open Medicine 2020;8:1-12. [DOI: 10.1177/2050312120940542] [LINK: journals.sagepub.com/doi/10.1177/2050312120940542] [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACTR201903750173871. Bukhali: building knowledge, optimising physical and mental health, and setting up healthier life trajectories in South African women [A preconception cluster randomised controlled trial, part of the Healthy Life Trajectories Initiative (HeLTI)]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=6015 (first received 27 March 2019).
Fan 2020 {published data only}
- Fan X, She R, Liu C, Zhong H, Lau JT, Hao C, et al. Evaluation of smartphone APP-based case-management services among antiretroviral treatment-naïve HIV-positive men who have sex with men: a randomized controlled trial protocol. BMC Public Health 2020;20(1):85. [DOI: 10.1186/s12889-020-8171-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2018 {published data only}
- Fisher J, Tran T, Luchters S, Tran TD, Hipgrave DB, Hanieh S, et al. Addressing multiple modifiable risks through structured community-based Learning Clubs to improve maternal and infant health and infant development in rural Vietnam: protocol for a parallel group cluster randomised controlled trial. BMJ Open 2018;8(7):e023539. [DOI: 10.1136/bmjopen-2018-023539] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Sweeny K, Tran T, Luchters S, Hipgrave DB, Hanieh S, et al. Protocol for an economic evaluation alongside a cluster randomised controlled trial: cost-effectiveness of Learning Clubs, a multicomponent intervention to improve women's health and infant's health and development in Vietnam. BMJ Open 2019;9(12):e031721. [DOI: 10.1136/bmjopen-2019-031721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2018 {published data only}
- Guo Y, Xu M, Ji M, Wei Z, Zhang J, Hu Q, et al. The effect of Imaginary Working Qigong on the psychological well-being of college students: study protocol for a randomized controlled trial. Medicine 2018;97(44):e13043. [DOI: 10.1097/MD.0000000000013043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hossain 2019 {published data only}
- Hossain SJ, Roy BR, Salveen N-E, Hasan MI, Tipu SM, Shiraji S, et al. Effects of adding psychosocial stimulation for children of lactating mothers using an unconditional cash transfer platform on neurocognitive behavior of children in rural Bangladesh: protocol for a cluster randomized controlled trial. BMC Psychology 2019;7(1):13. [DOI: 10.1186/s40359-019-0289-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imran 2018 {published data only}
- Imran N, Rahman A, Chaudhry N, Asif A. World Health Organization "School Mental Health Manual"-based training for school teachers in Urban Lahore, Pakistan: study protocol for a randomized controlled trial. Trials 2018;19(1):290. [DOI: 10.1186/s13063-018-2679-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN11059214 {published data only}
- ISRCTN11059214. Measuring the benefits of the Reach Up early childhood parenting programme in Jamaica [Reach Up early childhood parenting programme: evaluation of implementation and benefits in Jamaica]. isrctn.com/ISRCTN11059214 (first received 17 May 2019).
ISRCTN12021015 {published data only}
- ISRCTN12021015. REACH UP: pilot of a cluster randomized controlled trial of an early childhood parenting programme for children 12 - 30 months in a rural district in Zimbabwe. isrctn.com/ISRCTN12021015 (first received 30 July 2020).
ISRCTN14396374 {published data only}
- ISRCTN14396374. A research study in Colombia, to build capacity in school mental health and to adapt an intervention called DIALOG+ for use in adolescents in post-conflict Colombia during the COVID-19 pandemic [Adapting DIALOG+ and building capacity in schools to support mental wellbeing and resilience in post-conflict Colombia during the COVID-19 pandemic]. isrctn.com/ISRCTN14396374 (first received 9 April 2021).
ISRCTN16205138 {published data only}
- ISRCTN16205138. Effectiveness of short-term audio mindfulness in the Chinese community: a pilot study [Effectiveness and moderated mediation of short-term audio mindfulness for Chinese community-dwelling people: a randomized controlled trial]. isrctn.com/ISRCTN16205138 (first received 23 February 2021).
ISRCTN20474555 {published data only}
- ISRCTN20474555. Community-based socio-therapy adapted for refugees: the COSTAR study [Treating depressive symptomatology in Congolese refugees in Uganda and Rwanda: adapting and evaluating community-based socio-therapy]. isrctn.com/ISRCTN20474555 (first received 15 March 2019).
ISRCTN49881357 {published data only}
- ISRCTN49881357. Cluster randomized trial of community based trauma healing on sexual and physical violence victimization of women and perpetration by men in the Democratic Republic of Congo [Impact evaluation of community based trauma healing in the Democratic Republic of Congo]. isrctn.com/ISRCTN49881357 (first received 2 September 2021).
ISRCTN77689525 {published data only}
- ISRCTN77689525. Sharing Stories: a digital intervention for caregivers with young children in the COVID-19 era to promote child social, emotional and language development, responsive parenting and parental mental wellbeing in Zambia, Tanzania, and Uganda [Does a digital intervention, delivered to caregivers with young children in Zambia, Tanzania and Uganda, promote child social, emotional and language development, responsive parenting and parental mental wellbeing when compared to a wait-list control group?]. isrctn.com/ISRCTN77689525 (first received 25 November 2020).
ISRCTN80690743 {published data only}
- ISRCTN80690743. Project HASHTAG: testing a school-based intervention to improve adolescent mental health in Nepal and South Africa [Feasibility trial of a school-based intervention to promote positive mental health, prevent mental health disorders, and reduce risk behaviours in young adolescents in Nepal and South Africa]. isrctn.com/ISRCTN80690743 (first received 27 July 2021).
ISRCTN83649248 {published data only}
- ISRCTN83649248. Improving the mental health and wellbeing of people affected by leprosy or Buruli ulcer in Nigeria. isrctn.com/ISRCTN83649248 (first received 2 March 2021). [LINK: trialsearch.who.int/?TrialID=ISRCTN83649248]
Keynejad 2020 {published data only}
- Keynejad RC, Bitew T, Sorsdahl K, Myers B, Honikman S, Medhin G, et al. Problem solving therapy (PST) tailored for intimate partner violence (IPV) versus standard PST and enhanced usual care for pregnant women experiencing IPV in rural Ethiopia: protocol for a randomised controlled feasibility trial. Trials 2020;21(1):454. [DOI: 10.1186/s13063-020-04331-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lokuge 2018 {published data only}
- Lokuge K, Wallace P, Subasinghe K, Thurber K, De Silva T, Clarke N, et al. Protocol for a cluster-randomised controlled trial evaluating the impact of a preschool-based capacity building intervention on intimate partner violence and substance misuse in Sri Lanka. BMC Public Health 2018;18(1):572. [DOI: 10.1186/s12889-018-5423-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03341455. Family violence and alcohol and drug misuse in Sri Lanka [Developing, implementing and evaluating preschool based interventions to address family violence and alcohol and drug misuse in Sri Lanka]. clinicaltrials.gov/ct2/show/NCT03341455 (first received 14 November 2017).
Mapurunga 2020 {published data only}
- Mapurunga MV, Andreoni S, Oliveira DR, Sarubbi V Jr, Bonilha AC, D'Almeida V, et al. Protocol for a nested randomized controlled trial to evaluate the feasibility and preliminary efficacy of the mindfulness based health promotion program on the quality of life of older adults assisted in primary care-"the MBHP-Elderly study". Frontiers in Medicine 2020;7:563099. [DOI: 10.3389/fmed.2020.563099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariano 2021 {published data only}
- Mariano M, da Silva AR, Lima JL, Pinho NT, Cogo-Moreira H, Melo MH, et al. Effectiveness of the Elos 2.0 prevention programme for the reduction of problem behaviours and promotion of social skills in schoolchildren: study protocol for a cluster-randomized controlled trial. Trials 2021;22(1):468. [DOI: 10.1186/s13063-021-05408-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 2019 {published data only}
- ISRCTN18485542. Effects of a school-based health intervention programme in Port Elizabeth, South Africa: the KaziBantu project [Effects of a school-based health intervention programme in marginalized neighbourhoods of Port Elizabeth, South Africa: the KaziBantu project]. isrctn.com/ISRCTN18485542 (first received 9 July 2018).
- Müller I, Smith D, Adams L, Aerts A, Damons BP, Degen J, et al. Effects of a school-based health intervention program in marginalized communities of Port Elizabeth, South Africa (the KaziBantu study): protocol for a randomized controlled trial. JMIR Research Protocols 2019;8(7):14097. [DOI: 10.2196/14097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutedzi 2019 {published data only}
- Mutedzi B, Langhaug L, Hunt J, Nkhoma K, Harding R. Improving bereavement outcomes in Zimbabwe: protocol for a feasibility cluster trial of the 9-cell bereavement tool. Pilot and Feasibility Studies 2019;5:66. [DOI: 10.1186/s40814-019-0450-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03231358 {published data only}
- NCT03231358. Our Family Our Future: a resilience-oriented family intervention to prevent adolescent HIV/STI Infection and depression in South Africa. clinicaltrials.gov/ct2/show/NCT03231358 (first received 27 July 2017).
NCT03243396 {published data only}
- NCT03243396. Building and sustaining interventions for children: task-sharing mental health care in low-resource settings (BASIC) [Building and sustaining interventions for children (BASIC): task-sharing mental health care in low-resource settings]. clinicaltrials.gov/ct2/show/NCT03243396 (first received 9 August 2017).
NCT03887312 {published data only}
- NCT03887312. Phone-delivered psychological intervention (t-CETA) for mental health problems in 8-17 year-old Syrian refugee children (t-CETA) [Development, piloting and evaluation of a phone-delivered psychological intervention (t-CETA) for Syrian refugee children in Lebanon: phase II]. clinicaltrials.gov/ct2/show/NCT03887312 (first received 22 March 2019).
NCT03960944 {published data only}
- NCT03960944. An experimental study of psychological effect of school based-yoga on low-income school children [Psychological effect of school based-yoga on low-income school children: randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT03960944 (first received 23 May 2019).
NCT04234815 {published data only}
- NCT04234815. Evaluating impacts of CSS for veterans and their families in Ukraine [Evaluating impacts of a brief psychosocial workshop for veterans and their families in Ukraine]. clinicaltrials.gov/ct2/show/NCT04234815 (first received 21 January 2020).
NCT04252807 {published data only}
- Huma ZE, Gillani A, Shafique F, Rashid A, Mahjabeen B, Javed H, et al. Evaluating the impact of a common elements-based intervention to improve maternal psychological well-being and mother-infant interaction in rural Pakistan: study protocol for a randomised controlled trial. BMJ Open 2021;11(7):e047609. [DOI: 10.1136/bmjopen-2020-047609] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04252807. A common elements-based intervention to improve maternal psychological well-being and mother-infant interaction [Evaluating the impact of a common elements-based intervention to improve maternal psychological well-being and mother-infant interaction in a low resource community setting of rural Pakistan]. clinicaltrials.gov/ct2/show/NCT04252807 (first received 5 February 2020).
NCT04255472 {published data only}
- NCT04255472. Effectiveness of the WHO caregivers skills training program [Effectiveness of the WHO caregivers skills training (CST) program for children with developmental disorders and delays in rural community settings in Pakistan: an individual randomized controlled trial (RCT)]. https://clinicaltrials.gov/ct2/show/study/NCT04255472 (first received 5 February 2020).
NCT04289272 {published data only}
- NCT04289272. Evaluating Dove Confident Me in India [A pilot randomised control trial to examine the acceptability, feasibility, and efficacy of 'Confident Me' school workshops for body confidence in India]. clinicaltrials.gov/ct2/show/NCT04289272 (first received 28 February 2020).
NCT04307849 {published data only}
- NCT04307849. Youth-friendly sexual and reproductive healthcare pilot in Mumbai, India [Engaging providers, community members, and young women to adapt and pilot a youth-friendly sexual and reproductive health package in low-income communities in India]. clinicaltrials.gov/ct2/show/NCT04307849 (first received 13 March 2020).
NCT04383327 {published data only}
- NCT04383327. School-based mental health effectiveness study [Effectiveness and implementation of an early childhood school-based mental health intervention in low-resource communities]. clinicaltrials.gov/ct2/show/NCT04383327 (first received 12 May 2020).
NCT04542317 {published data only}
- NCT04542317. Efficacy of a dementia family caregiver support intervention in Vietnam [Cluster randomized controlled trial to test the efficacy of a psychosocial intervention (REACH VN) to support Alzheimer's family caregivers in Vietnam]. clinicaltrials.gov/ct2/show/NCT04542317 (first received 9 September 2020).
NCT04693585 {published data only}
- NCT04693585. Development and pilot test of a postnatal mHealth intervention - phase 2 (MESSAGE) [Development and pilot test of an mHealth interactive education and social support intervention for improving postnatal health - phase 2]. clinicaltrials.gov/ct2/show/NCT04693585 (first received 5 January 2021).
NCT04723277 {published data only}
- NCT04723277. Efficacy of teacher-delivered child mental healthcare in primary schools of India (TeaLeaF) [TeaLeaF: teachers leading the frontlines]. clinicaltrials.gov/ct2/show/NCT04723277.
NCT04782882 {published data only}
- NCT04782882. The effect of progressive muscle relaxation and laughter therapy on women undergoing in vitro fertilization [Effectiveness of progressive muscle relaxation and laughter therapy on mental health and treatment outcomes in women undergoing in vitro fertilization: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04782882 (first received 4 March 2021). [DOI] [PubMed]
NCT04819711 {published data only}
- NCT04819711. Addressing perinatal depression in deprived areas of Istanbul, Turkey [Addressing perinatal depression in deprived areas of Istanbul, Turkey: a pilot randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT04819711 (first received 29 March 2021).
NCT04890665 {published data only}
- NCT04890665. Online multi-component psychological intervention for healthcare workers during COVID-19 pandemic [Effectiveness of a self-applied multi-component psychological online intervention based on user experience, for anxiety, depression, burnout, fatigue compassion on healthcare workers during the COVID-19 outbreak: a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT04890665 (first received 18 May 2021).
NCT04949061 {published data only}
- NCT04949061. The effectiveness of culturally adapted cognitive behavioral intervention among COVID-19 survivors [Culturally adapted cognitive behavioral intervention to reduce psychological distress among COVID-19 survivors: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04949061 (first received 2 July 2021).
NCT04958707 {published data only}
- NCT04958707. A mobile phone-based intervention on dementia patients' caregivers in Vietnam [Evaluating the feasibility of a mobile phone-based intervention (Zalo app) on depression, anxiety, and stress of family caregivers of people with dementia]. clinicaltrials.gov/ct2/show/NCT04958707 (first received 12 July 2021).
NCT05053178 {published data only}
- NCT05053178. The effect of mindfulness-based mandala activity on anxiety and spiritual well-being levels of senior nursing students [The effect of mindfulness-based mandala activity on anxiety and spiritual well-being]. clinicaltrials.gov/ct2/show/NCT05053178 (first received 22 September 2021).
NCT05130944 {published data only}
- NCT05130944. Feasibility of community psychosocial intervention for women [Integrating psychosocial support into protection and community programs for women in Ecuador and Panama]. clinicaltrials.gov/ct2/show/NCT05130944 (first received 23 November 2021).
Newman 2021 {published data only}
- Newman P, Chakrapani V, Williams C, Massaquoi N, Tepjan S, Roungprakhon S, et al. An eHealth intervention for promoting COVID-19 knowledge and protective behaviors and reducing pandemic distress among sexual and gender minorities: protocol for a randomized controlled trial (#SafeHandsSafeHearts). JMIR Research Protocols 2021;10(12):e34381. [DOI: 10.2196/34381] [DOI] [PMC free article] [PubMed] [Google Scholar]
PACTR202006601935462 {published data only}
- PACTR202006601935462. Promoting adherence to anti-retroviral therapy and viral suppression among HIV positive young people in Uganda through group support psychotherapy [Promoting adherence to anti-retroviral therapy and viral suppression among HIV positive young people in Uganda through group support psychotherapy: study protocol for a pilot randomized controlled trial]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=11111 (first received 18 June 2020).
PACTR202101590919131 {published data only}
- PACTR202101590919131. Impact of brief psycho-education intervention on caregiver burden of caregivers of children and adolescents with mental retardation attending the outpatient clinic in the Federal Neuropsychiatric Hospital Benin City, Nigeria. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=14619 (first received 27 January 2021).
Pallitto 2016 {published data only}
- ISRCTN35969343. Addressing violence against pregnant women in antenatal care: testing an intervention in South Africa. isrctn.com/ISRCTN35969343 (first received 11 May 2016).
- Pallitto C, García-Moreno C, Stöeckl H, Hatcher A, MacPhail C, Mokoatle K, et al. Testing a counselling intervention in antenatal care for women experiencing partner violence: a study protocol for a randomized controlled trial in Johannesburg, South Africa. BMC Health Services Research 2016;16(1):630. [DOI: 10.1186/s12913-016-1872-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pathare 2020 {published data only}
- CTRI/2017/04/008313. SPIRIT: a suicide prevention project [SPIRIT - suicide prevention and implementation research initiative]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=18256&EncHid=&userName=CTRI/2017/04/008313 (first received 7 April 2017).
- Pathare S, Shields-Zeeman L, Vijayakumar L, Pandit D, Nardodkar R, Chatterjee S, et al. Evaluation of the SPIRIT integrated suicide prevention programme: study protocol for a cluster-randomised controlled trial in rural Gujarat, India. Trials 2020;21(1):572. [DOI: 10.1186/s13063-020-04472-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paula 2021 {published data only}
- Paula TC, Chagas C, Noto AR, Formigoni ML, Pereira TV, Ferri CP. Brief interventions for older adults (BIO) delivered by non-specialist community health workers to reduce at-risk drinking in primary care: a study protocol for a randomised controlled trial. BMJ Open 2021;11(5):e043918. [DOI: 10.1136/bmjopen-2020-043918] [DOI] [PMC free article] [PubMed] [Google Scholar]
- RBR-8rcxkk. Evaluation of an intervention to reduce alcohol consumption among older adults [Evaluation of the effectiveness of strategies to reduce at-risk drinking among older adults]. ensaiosclinicos.gov.br/rg/RBR-8rcxkk (first received 9 March 2020).
Rath 2020 {published data only}
- ISRCTN17206016. Participatory adolescent groups, youth leadership training and livelihood promotion to improve school attendance, dietary diversity and mental health among adolescent girls in rural eastern India [Community youth teams facilitating participatory adolescent groups, youth leadership training and livelihood promotion activities to improve school attendance, dietary diversity and mental health among adolescent girls aged 10-19 years in rural eastern India: a cluster-randomised controlled trial]. www.isrctn.com/ISRCTN17206016 (first received 25 June 2018).
- Rath S, Prost A, Samal S, Pradhan H, Copas A, Gagrai S, et al. Community youth teams facilitating participatory adolescent groups, youth leadership activities and livelihood promotion to improve school attendance, dietary diversity and mental health among adolescent girls in rural eastern India: protocol for a cluster-randomised controlled trial. Trials 2020;21(1):52. [DOI: 10.1186/s13063-019-3984-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2017 {published data only}
- NCT02957799. Improving South African government workers' capacities to deliver HIV interventions [A randomized controlled trial to improve the South African government's community health workers' capacities to deliver evidence-based interventions for optimizing HIV outcomes and reducing its comorbidities]. clinicaltrials.gov/ct2/show/NCT02957799 (first received 8 November 2016).
- Rotheram-Borus MJ, Le Roux K, Le Roux IM, Christodoulou J, Laurenzi C, Mbewu N, et al. To evaluate if increased supervision and support of South African government health workers' home visits improves maternal and child outcomes: study protocol for a randomized control trial. Trials 2017;18(1):368. [DOI: 10.1186/s13063-017-2074-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saju 2021 {published data only}
- Saju MD, Varghese BM, Scaria L, Benny AM, Yohannan SV, Cheguvera N, et al. Swāsthya, an integrated chronic condition management programme for families of patients with hypertension and diabetes mellitus: a study protocol for a randomised controlled trial. BMC Family Practice 2021;22(1):15. [DOI: 10.1186/s12875-020-01357-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sam‐Agudu 2017 {published data only}
- NCT03152006. Adolescent coordinated transition Nigerian HIV+ youth (ACT) [Adolescent coordinated transition (ACT) to improve health outcomes among Nigerian HIV+ youth]. clinicaltrials.gov/ct2/show/NCT03152006 (first received 12 May 2017).
- Sam-Agudu NA, Pharr JR, Bruno T, Cross CL, Cornelius LJ, Okonkwo P, et al. Adolescent coordinated transition (ACT) to improve health outcomes among young people living with HIV in Nigeria: study protocol for a randomized controlled trial. Trials 2017;18(1):595. [DOI: 10.1186/s13063-017-2347-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
SLCTR/2020/015 {published data only}
- SLCTR/2020/015. Effectiveness of a mindfulness-based intervention on physiological and psychological parameters in pregnant women in Anuradhapura district, Sri Lanka – a randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=SLCTR/2020/015 (first received 9 June 2020).
SLCTR/2020/022 {published data only}
- SLCTR/2020/022. Impact of mindfulness-based trimodal prehabilitation on functional recovery and selected surgical outcomes of patients with colorectal cancer admitted to surgical professorial unit of Colombo South Teaching Hospital Sri Lanka; a randomised control trial. https://slctr.lk/trials/slctr-2020-022 (first received 23 October 2020).
Sorsdahl 2021 {published data only}
- Sorsdahl K, Westhuizen C, Neuman M, Weiss HA, Myers B. Addressing the mental health needs of adolescents in South African communities: a protocol for a feasibility randomized controlled trial. Pilot and Feasibility Studies 2021;7(1):69. [DOI: 10.1186/s40814-021-00803-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srivastav 2021 {published data only}
- Srivastav P, Vaishali K, Bhat VH, Broadbent S. Structured, multifactorial randomised controlled intervention to investigate physical activity levels, body composition and diet in obese and overweight adolescents. BMJ Open 2021;11(3):e044895. [DOI: 10.1136/bmjopen-2020-044895] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tăut 2021 {published data only}
- Frantz I, Foran HM, Lachman JM, Jansen E, Hutchings J, Băban A, et al. Prevention of child mental health problems in Southeastern Europe: a multicentre sequential study to adapt, optimise and test the parenting programme 'Parenting for Lifelong Health for Young Children', protocol for stage 1, the feasibility study. BMJ Open 2019;9(1):e026684. [DOI: 10.1136/bmjopen-2018-026684] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmana JM, Heinrichs N, Jansen E, Brühlc A, Taut D, Fang X, et al. Preventing child mental health problems through parenting interventions in Southeastern Europe (RISE): protocol for a multi-country cluster randomized factorial study. Contemporary Clinical Trials 2019;86:105855. [DOI: 10.1016/j.cct.2019.105855] [DOI] [PubMed] [Google Scholar]
- NCT03552250. Prevention of child mental health problems in southeastern Europe [Prevention of child mental health problems in southeastern Europe - adapt, optimize, test and extend parenting for lifelong health]. clinicaltrials.gov/ct2/show/NCT03552250 (first received 11 June 2018).
- NCT03865485. Prevention of child mental health problems in southeastern Europe (RISE) - a factorial study (phase 2 of MOST) (RISE) [RISE- prevention of child mental health problems in southeastern Europe - adapt, optimize, test and extend parenting for lifelong health - a factorial study (phase 2 of MOST)]. clinicaltrials.gov/ct2/show/NCT03865485 (first received 7 March 2019).
- NCT04721730. Prevention of child mental health problems in southeastern Europe - phase 3 (RISE) [Prevention of child mental health problems in southeastern Europe - adapt, optimize, test, and extend parenting for lifelong health - 'RISE' - the randomized controlled trial (phase 3 of MOST)]. clinicaltrials.gov/ct2/show/NCT04721730 (first received 25 January 2021).
- Tăut D, Băban A, Frantz I, Dănilă I, Lachman JM, Heinrichs N, et al. Prevention of child mental health problems through parenting interventions in Southeastern Europe (RISE): study protocol for a multi-site randomised controlled trial. Trials 2021;22(1):960. [DOI: 10.1186/s13063-021-05817-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20200601002 {published data only}
- TCTR20200601002. A randomized controlled trial on effectiveness of mental health & psychosocial support module (MHPSS) in reduction of depression, anxiety and stress among healthcare workers during COVID-19 pandemic in Jerantut, Pahang. thaiclinicaltrials.org/show/TCTR20200601002 (first received 28 May 2020).
Tran 2020 {published data only}
- ACTRN12620000088943. Culturally-adapted resourceful adolescent program to improve the mental health of adolescents in Vietnam [Addressing an unrecognised public health problem in Vietnam: a clustered randomised controlled trial of the culturally adapted Resourceful Adolescent Program to improve adolescent mental health]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000088943 (first received 14 January 2020).
- Tran T, Nguyen HT, Shochet I, Wurfl A, Orr J, Nguyen N, et al. School-based, two-arm, parallel, controlled trial of a culturally adapted resilience intervention to improve adolescent mental health in Vietnam: study protocol. BMJ Open 2020;10(10):e039343. [DOI: 10.1136/bmjopen-2020-039343] [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000042807 {published data only}
- UMIN000042807. Developing an adaptive intervention for the mental health and psychosocial support of health workers in the Philippines under COVID-19 pandemic using the Sequential, Multiple Assignment, Randomized Trial (SMART). center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000048854 (first received 23 December 2020).
Venturo‐Conerly 2021 {published data only}
- PACTR202104716135752. Five-arm Shamiri trial [Dismantling the Shamiri intervention for anxiety, depression, wellbeing, and academic functioning in Kenyan adolescents: study protocol for a five-arm randomized controlled trial]. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=14677 (first received 19 April 2021). [DOI] [PMC free article] [PubMed]
- Venturo-Conerly KE, Osborn TL, Wasil AR, Le H, Corrigan E, Wasanga C, et al. Testing the effects of the Shamiri Intervention and its components on anxiety, depression, wellbeing, and academic functioning in Kenyan adolescents: study protocol for a five-arm randomized controlled trial. Trials 2021;22(1):829. [DOI: 10.1186/s13063-021-05736-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2021 {published data only}
- Wang Y, Xiao LD, Yu Y, Huang R, You H, Liu M. An individualized telephone-based care support program for rural family caregivers of people with dementia: study protocol for a cluster randomized controlled trial. BMC Geriatrics 2021;21(1):629. [DOI: 10.1186/s12877-021-02575-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2020 {published data only}
- Xu M, Liu A, Zhao C, Fang H, Huang X, Berman S, et al. Group-based intervention to improve developmental status among children age 6-18 months in rural Shanxi province, China: a study protocol for a cluster randomised controlled trial. BMJ Open 2020;10(10):e037156. [DOI: 10.1136/bmjopen-2020-037156] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2020 {published data only}
- Zheng X, Huang L, Fang Q, Zhang Y, Zhang Y, Li X, et al. Internet-based support program on parenting outcomes for Chinese primiparous women: study protocol for a randomized controlled trial. Journal of Advanced Nursing 2020;76(11):3155-63. [DOI: 10.1111/jan.14517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdulmalik 2019
- Abdulmalik J. Optimal Mental Health: An Everyday Guide. Ibadan (Nigeria): Inspiration House Publishing, 2019. [Google Scholar]
Aho 2008
- Aho K. Medicalizing mental health: a phenomenological alternative. Journal of Medical Humanities 2008;29(4):243-59. [DOI: 10.1007/s10912-008-9065-1] [DOI] [PubMed] [Google Scholar]
Akhtar 2022
- Akhtar A, Koyiet P, Rahman A, Schafer A, Hamdani SU, Cuijpers P, et al. Residual posttraumatic stress disorder symptoms after provision of brief behavioral intervention in low- and middle-income countries: an individual-patient data meta-analysis. Depression and Anxiety 2022;39(1):71-82. [DOI: 10.1002/da.23221] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akça Kalem 2005
- Akça Kalem S, Hanagası H, Kertesz A, Gürvit H. Validation study of the Turkish translation of the frontal behavioral inventory (FBI). In: 21st International Conference of Alzheimer’s Disease International; 2005 Sept 28-Oct 1; Istanbul, Turkey. 2005.
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI: 10.1136/bmj.313.7066.1200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 1976
- Andrews FM, Withey SB. Social Indicators of Well-Being. New York City: Plenum Press, 1976. [DOI: 10.1007/978-1-4684-2253-5] [DOI] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): The Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd revised edition. Washington (DC): The Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th revised edition. Washington (DC): The Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V). 5th edition. Washington (DC): The Association, 2013. [Google Scholar]
Araya 2003
- Araya R, Rojas G, Fritsch R, Gaete J, Rojas M, Simon G, et al. Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 2003;361(9362):995-1000. [DOI: 10.1016/S0140-6736(03)12825-5] [DOI] [PubMed] [Google Scholar]
Barber 2019
- Barber S, Gronholm PC, Ahuja S, Rüsch N, Thornicroft G. Microaggressions towards people affected by mental health problems: a scoping review. Epidemiology and Psychiatric Sciences 2019;29:e82. [DOI: 10.1017/S2045796019000763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbui 2017
- Barbui C, Purgato M, Churchill R, Adams C, Amato L, Macdonald G, et al. Cochrane for global mental health. Lancet Psychiatry 2017;4(4):e6. [DOI: 10.1016/S2215-0366(17)30090-1] [DOI] [PubMed] [Google Scholar]
Barbui 2020
- Barbui C, Purgato M, Abdulmalik J, Acarturk C, Eaton J, Gastaldon C, et al. Efficacy of psychosocial interventions for mental health outcomes in low-income and middle-income countries: an umbrella review. Lancet Psychiatry 2020;7(2):162-72. [DOI: 10.1016/S2215-0366(19)30511-5] [DOI] [PubMed] [Google Scholar]
Barry 2013
- Barry MM, Clarke AM, Jenkins R, Patel V. A systematic review of the effectiveness of mental health promotion interventions for young people in low and middle income countries. BMC Public Health 2013;13:835. [DOI: 10.1186/1471-2458-13-835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Betancourt 2020
- Betancourt TS, Thomson DL, Brennan RT, Antonaccio CM, Gilman SE, VanderWeele TJ. Stigma and acceptance of Sierra Leone's child soldiers: a prospective longitudinal study of adult mental health and social functioning. Journal of the American Academy of Child and Adolescent Psychiatry 2020;59(6):715-26. [DOI: 10.1016/j.jaac.2019.05.026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2011
- Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The Global Economic Burden of Non-Communicable Diseases. Geneva (Switzerland): World Economic Forum, 2011. [Google Scholar]
Boehnke 2022
- Boehnke JR, Rana RZ, Kirkham JJ, Rose L, Agarwal G, Barbui C, et al. Development of a core outcome set for multimorbidity trials in low/middle-income countries (COSMOS): study protocol. BMJ Open 2022;12(2):e051810. [DOI: 10.1136/bmjopen-2021-051810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. When does it make sense to perform a meta-analysis? In: Introduction to Meta-Analysis. 1st edition. Chichester (UK): John Wiley & Sons, 2009:357-64. [DOI: 10.1002/9780470743386.ch40] [DOI] [Google Scholar]
Buntrock 2016
- Buntrock C, Ebert DD, Lehr D, Smit F, Riper H, Berking M, et al. Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA 2016;315(17):1854-63. [DOI: 10.1001/jama.2016.4326] [DOI] [PubMed] [Google Scholar]
Burgess 2020
- Burgess RA, Jain S, Petersen I, Lund C. Social interventions: a new era for global mental health? Lancet Psychiatry 2020;7(2):118-9. [DOI: 10.1016/S2215-0366(19)30397-9] [DOI] [PubMed] [Google Scholar]
Castillo 2019
- Castillo EG, Ijadi-Maghsoodi R, Shadravan S, Moore E, Mensah MO III, Docherty M, et al. Community interventions to promote mental health and social equity. Current Psychiatry Reports 2019;21(5):35. [DOI: 10.1007/s11920-019-1017-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ceccarelli 2022
- Ceccarelli C, Prina E, Muneghina O, Jordans M, Barker E, Miller K, et al. Adverse childhood experiences and global mental health: avenues to reduce the burden of child and adolescent mental disorders. Epidemiology and Psychiatric Sciences 2022;31:e75. [DOI: 10.1017/S2045796022000580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlson 2019
- Charlson F, Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet 2019;394(10194):240-8. [DOI: 10.1016/S0140-6736(19)30934-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatterjee 2003
- Chatterjee S, Patel V, Chatterjee A, Weiss HA. Evaluation of a community-based rehabilitation model for chronic schizophrenia in rural India. British Journal of Psychiatry 2003;182:57-62. [DOI: 10.1192/bjp.182.1.57] [DOI] [PubMed] [Google Scholar]
Chibanda 2016
- Chibanda D, Verhey R, Gibson LJ, Munetsi E, Machando D, Rusakaniko S, et al. Validation of screening tools for depression and anxiety disorders in a primary care population with high HIV prevalence in Zimbabwe. Journal of Affective Disorders 2016;1998:50-5. [DOI: 10.1016/j.jad.2016.03.006] [DOI] [PubMed] [Google Scholar]
Chisholm 2000
- Chisholm D, Sekar K, Kumar KK, Saeed K, James S, Mubbashar M, et al. Integration of mental health care into primary care. British Journal of Psychiatry 2000;176:581-8. [DOI: 10.1192/bjp.176.6.581] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Cook 2017
- Cook P, Mack E, Manrique M. Protecting young children from violence in Colombia: linking caregiver empathy with community child rights indicators as a pathway for peace in Medellin’s Comuna. Peace and Conflict: Journal of Peace Psychology 2017;23:38-45. [DOI: 10.1037/pac0000194] [DOI] [Google Scholar]
Cox 2014
- Cox JL, Holden J, Henshaw C. Perinatal Mental Health: the Edinburgh Postnatal Depression Scale (EPDS) Manual. London: The Royal College of Psyciatrists Publications, 2014. [Google Scholar]
Cuijpers 2015
- Cuijpers P, Karyotaki E, Andersson G, Li J, Mergl R, Hegerl U. The effects of blinding on the outcomes of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. European Psychiatry 2015;30(6):685-93. [DOI: 10.1016/j.eurpsy.2015.06.005] [DOI] [PubMed] [Google Scholar]
Cuijpers 2016
- Cuijpers P, Cristea IA. How to prove that your therapy is effective, even when it is not: a guideline. Epidemiology and Psychiatric Sciences 2016;25(5):428-35. [DOI: 10.1017/S2045796015000864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2018
- Cuijpers P, Karyotaki E, Reijnders M, Purgato M, Barbui C. Psychotherapies for depression in low- and middle-income countries: a meta-analysis. World Psychiatry 2018;17(1):90-101. [DOI: 10.1002/wps.20493] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2022
- Cuijpers P, Ciharova M, Quero S, Miguel C, Driessen E, Harrer M, et al. The contribution of "individual participant data" meta-analyses of psychotherapies for depression to the development of personalized treatments: a systematic review. Journal of Personalized Medicine 2022;12(1):93. [DOI: 10.3390/jpm12010093] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Silva 2014
- De Silva MJ, Lee L, Fuhr DC, Rathod S, Chisholm D, Schellenberg J, et al. Estimating the coverage of mental health programmes: a systematic review. International Journal of Epidemiology 2014;43(2):341-53. [DOI: 10.1093/ije/dyt191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2005
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd edition. Oxford (UK): Oxford University Press, 2005. [Google Scholar]
Durkin 1990
- Durkin M, Davidson L, Hasan M, Khan N, Thorburn MJ, Zaman S. Screening for childhood disabilities in community settings. In: Thorbum MJ, Marfo K, editors(s). Practical Approaches to Childhood Disability in Developing Countries. Spanish Town, Jamaica: 3D Projects, 1990:179-97. [Google Scholar]
Eaton 2012
- Eaton WW, editor(s). Public Mental Health. 1st edition. New York (NY): Oxford University Press, 2012. [Google Scholar]
Effati‐Daryani 2017
- Effati‐Daryani F, Mirghafourvand M, Mohammad‐Alizadeh‐Charandabi S, Shiri‐Sarand F, Zarei S. Sleep quality and its relationship with quality of life in Iranian pregnant women. International Journal of Nursing Practice 2017;23(2):e12518. [DOI] [PubMed] [Google Scholar]
EPOC 2017a
- Cochrane Effective Practice and Organisation of Care (EPOC). Data collection form. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 23 May 2023).
EPOC 2017b
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 23 May 2023).
EPOC 2017c
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 23 May 2023).
Epping‐Jordan 2016
- Epping‐Jordan JE, Harris R, Brown FL, Carswell K, Foley C, García‐Moreno C, et al. Self‐Help Plus (SH+): a new WHO stress management package. World Psychiatry 2016;15(3):295-6. [DOI: 10.1002/wps.20355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eser 1999
- Eser SY, Fidaner H, Fidaner C, Elbi H, Eser E, Goker, E. The validity and reliability of quality of life WHOQOL-Bref. Psychiatry Journal of Psychopharmacology 1999;7:5-13. [Google Scholar]
Faltinsen 2022
- Faltinsen E, Todorovac A, Bruun LS, Hróbjartsson A, Gluud C, Kongerslev MT, et al. Control interventions in randomised trials among people with mental health disorders. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: MR000050. [DOI: 10.1002/14651858.MR000050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faregh 2019
- Faregh N, Lencucha R, Ventevogel P, Dubale BW, Kirmayer LJ. Considering culture, context and community in mhGAP implementation and training: challenges and recommendations from the field. International Journal of Mental Health Systems 2019;13:58. [DOI: 10.1186/s13033-019-0312-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fergusson 2005
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Suicidal behaviour in adolescence and subsequent mental health outcomes in young adulthood. Psychological Medicine 2005;35(7):983–93. [DOI: 10.1017/s0033291704004167] [DOI] [PubMed] [Google Scholar]
Flannery 1990
- Flannery RB. Social support and psychological trauma: a methodological review. Journal of Traumatic Stress 1990;3:593-611. [Google Scholar]
Freeman 2016
- Freeman M. Global mental health in low and middle income, especially African countries. Epidemiology and Psychiatric Sciences 2016;25(6):503-5. [DOI: 10.1017/S2045796016000482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002;31(1):72-6. [DOI: 10.1093/ije/31.1.72] [DOI] [PubMed] [Google Scholar]
Furukawa 2021
- Furukawa TA, Suganuma A, Ostinelli EG, Andersson G, Beevers CG, Shumake J, et al. Dismantling, optimising, and personalising Internet cognitive behavioural therapy for depression: a systematic review and component network meta-analysis using individual participant data. Lancet Psychiatry 2021;8(6):500-11. [DOI: 10.1016/S2215-0366(21)00077-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 2 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guha‐Sapir 2014
- Guha-Sapir D, Hoyois P, Below R. Annual disaster statistical review 2013: the numbers and trends. Brussels (Belgium): Centre for Research on the Epidemiology of Disasters; 2014. [LINK: reliefweb.int/sites/reliefweb.int/files/resources/ADSR_2013.pdf]
Guidi 2018
- Guidi J, Brakemeier E-L, Bockting CL, Cosci F, Cuijpers P, Jarrett RB, et al. Methodological recommendations for trials of psychological interventions. Psychotherapy and Psychosomatics 2018;87(5):276-84. [DOI: 10.1159/000490574] [DOI] [PubMed] [Google Scholar]
Guo 1993
- Guo S, Chen L, Bao Y. The prevalence and risk factors of postpartum depression. Chinese Journal of Clinical Obstetrics and Gynaecology 1993;28:532-53. [Google Scholar]
Gutiérrez, 2009
- Gutiérrez ML, Villatoro JA, Gaytán L, Álamo A. Infancia, Adicciones y Salud Mental: Manual del Programa de Prevención “Dejando Huellitas en tu Vida”. México: Instituto Nacional de Psiquiatría Ramón de la Fuente, 2009. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles for continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):e173-83. [DOI: 10.1016/j.jclinepi.2012.08.001] [DOI] [PubMed] [Google Scholar]
Hanlon 2008
- Hanlon C, Medhin G, Alem A, Araya M, Abdulahi A, Hughes M, et al. Detecting perinatal common mental disorders in Ethiopia: validation of the self-reporting questionnaire and Edinburgh postnatal depression scale. Journal of Affective Disorders 2008;108(3):251-62. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hoeft 2018
- Hoeft TJ, Fortney JC, Patel V, Unützer J. Task-sharing approaches to improve mental health care in rural and other low-resource settings: a systematic review. Journal of Rural Health 2018;34(1):48-62. [DOI: 10.1111/jrh.12229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2017
- Hofmann SG, Asmundson GJ, editor(s). The Science of Cognitive Behavioral Therapy. 1st edition. Cambridge (MA): Academic Press, 2017. [Google Scholar]
Hu 2006
- Hu T-W. Perspectives: an international review of the national cost estimates of mental illness, 1990-2003. Journal of Mental Health Policy and Economics 2006;9(1):3-13. [PMID: ] [PubMed] [Google Scholar]
IASC 2007
- Inter-Agency Standing Committee. IASC Guidelines on Mental Health and Psychosocial Support in Emergency Settings. Geneva (Switzerland): The Committee, 2007. [LINK: interagencystandingcommittee.org/system/files/2020-11/IASC%20Guidelines%20on%20Mental%20Health%20and%20Psychosocial%20Support%20in%20Emergency%20Settings%20%28English%29.pdf] [Google Scholar]
Institute of Medicine 1994
- Institute of Medicine (US) Committee on Prevention of Mental Disorders, Mrazek PJ, Haggerty RJ, editor(s). Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington (DC): National Academies Press, 1994. [PubMed] [Google Scholar]
Institute of Medicine 2009
- National Research Council (US) and Institute of Medicine (US) Committee on the Prevention of Mental Disorders and Substance Abuse Among Children, Youth, and Young Adults: Research Advances and Promising Interventions, O'Connell E, Boat T, Warner KE, editor(s). Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities. Washington (DC): National Academies Press, 2009. [PubMed] [Google Scholar]
Jacobson 1938
- Jacobson E. Progressive Relaxation. Chicago: University of Chicago Press, 1938. [Google Scholar]
Jensen 2018
- Jensen JK, Ciolino JD, Diebold A, Segovia M, Degillio A, Solano-Martinez J, et al. Comparing the effectiveness of clinicians and paraprofessionals to reduce disparities in perinatal depression via the mothers and babies course: protocol for a cluster-randomized controlled trial. JMIR Research Protocols 2018;7(11):e11624. [DOI: 10.2196/11624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42-6. [DOI: 10.1136/bmj.323.7303.42] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaiser 2013
- Kaiser B, Kohrt BA, Keys H, Khoury NM, Brewster A. Strategies for assessing mental health in Haiti: local instrument development and transcultural translation. Transcultural Psychiatry 2013;50:532-58. [DOI] [PubMed] [Google Scholar]
Kaiser 2015
- Kaiser BN, Haroz EE, Kohrt BA, Bolton PA, Bass JK, Hinton DE. “Thinking too much”: a systematic review of a common idiom of distress. Social Science and Medicine 2015;147:170–83. [DOI: 10.1016/j.socscimed.2015.10.044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakuma 2011
- Kakuma R, Minas H, Ginneken N, Dal Poz MR, Desiraju K, Morris JE, et al. Human resources for mental healthcare: current situation and strategies for action. Lancet 2011;378(9803):1654-63. [DOI: 10.1016/S0140-6736(11)61093-3] [DOI] [PubMed] [Google Scholar]
Karyotaki 2022
- Karyotaki E, Araya R, Kessler RC, Waqas A, Bhana A, Rahman A, et al. Association of task-shared psychological interventions with depression outcomes in low- and middle-income countries: a systematic review and individual patient data meta-analysis. JAMA Psychiatry 2022;79(5):430-43. [DOI: 10.1001/jamapsychiatry.2022.0301] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knapp 2011
- Knapp H, Fletcher M, Taylor A, Chan K, Goetz MB. No clinic left behind: providing cost-effective in-services via distance learning. Journal of Healthcare Quality 2011;33(5):17-24. [DOI: 10.1111/j.1945-1474.2011.00117.x] [DOI] [PubMed] [Google Scholar]
Kohn 2004
- Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bulletin of the World Health Organization 2004;82(11):858-66. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Kyu 2018
- Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al, GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1859-922. [DOI: 10.1016/S0140-6736(18)32335-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lachman 2017
- Lachman J, Cluver L, Ward C, Hutchings J, Wessels I, Mlotshwa S, et al. Randomized controlled trial of a parenting program to reduce the risk of child maltreatment in South Africa. Child Abuse and Neglect 2017;72:338-51. [DOI] [PubMed] [Google Scholar]
Lewin 2010
- Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No: CD004015. [DOI: 10.1002/14651858.CD004015.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leykin 2009
- Leykin Y, DeRubeis RJ. Allegiance in psychotherapy outcome research: separating association from bias. Clinical Psychology: Science and Practice 2009;16(1):54-65. [DOI: 10.1111/j.1468-2850.2009.01143.x] [DOI] [Google Scholar]
Li 2012
- Li MZ. Study and Application of Depression Screening Methods in Patients with Diabetes. Peking University, 2012. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2012
- Liu FL, Wang SH, Feng XM, et al. The present situation and countermeasures of quality of life in patients with mild cognitive impairment (MCI). Chinese Journal of Modern Nursing 2012;18(6):742-4. [Google Scholar]
Lora 2012
- Lora A, Kohn R, Levav I, McBain R, Morris J, Saxena S. Service availability and utilization and treatment gap for schizophrenic disorders: a survey in 50 low- and middle-income countries. Bulletin of the World Health Organization 2012;90(1):47-54. [DOI: 10.2471/BLT.11.089284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lund 2018
- Lund C, Brooke-Sumner C, Baingana F, Baron EC, Breuer E, Chandra P, et al. Social determinants of mental disorders and the sustainable development goals: a systematic review of reviews. Lancet Psychiatry 2018;5(4):357-69. [DOI: 10.1016/S2215-0366(18)30060-9] [DOI] [PubMed] [Google Scholar]
Maturo 2011
- Maturo D, Powell A, Major-Wilson H, Sanchez K, De Santis JP, Friedman LB. Development of a protocol for transitioning adolescents with HIV infection to adult care. Journal of Pediatric Health Care 2011;25(1):16-23. [DOI] [PubMed] [Google Scholar]
Mendenhall 2014
- Mendenhall E, De Silva MJ, Hanlon C, Petersen I, Shidhaye R, Jordans M, et al. Acceptability and feasibility of using non-specialist health workers to deliver mental health care: stakeholder perceptions from the PRIME district sites in Ethiopia, India, Nepal, South Africa, and Uganda. Social Science & Medicine 2014;118:33-42. [DOI: 10.1016/j.socscimed.2014.07.057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miguel 2021
- Miguel C, Karyotaki E, Cuijpers P, Cristea IA. Selective outcome reporting and the effectiveness of psychotherapies for depression. World Psychiatry 2021;20(3):444-5. [DOI: 10.1002/wps.20900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miklowitz 2021
- Miklowitz DJ, Efthimiou O, Furukawa TA, Scott J, McLaren R, Geddes JR, et al. Adjunctive psychotherapy for bipolar disorder: a systematic review and component network meta-analysis. JAMA Psychiatry 2021;78(2):141-50. [DOI: 10.1001/jamapsychiatry.2020.2993] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohr 2014
- Mohr DC, Ho J, Hart TL, Baron KG, Berendsen M, Beckner V, et al. Control condition design and implementation features in controlled trials: a meta-analysis of trials evaluating psychotherapy for depression. Translational Behavioral Medicine 2014;4(4):407-23. [DOI: 10.1007/s13142-014-0262-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulder 2017
- Mulder R, Murray G, Rucklidge J. Common versus specific factors in psychotherapy: opening the black box. Lancet Psychiatry 2017;4(12):953-62. [DOI: 10.1016/S2215-0366(17)30100-1] [DOI] [PubMed] [Google Scholar]
Murray 2012
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197-223. [DOI: 10.1016/S0140-6736(12)61689-4] [DOI] [PubMed] [Google Scholar]
Murray 2020
- Murray CJ, Abbafati C, Abbas KM, Abbasi M, Abbasi-Kangevari M, Abd-Allah F, et al, GBD 2019 Viewpoint Collaborators. Five insights from the Global Burden of Disease Study 2019. Lancet 2020;396(10258):1135-59. [DOI: 10.1016/S0140-6736(20)31404-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Academies of Sciences 2019
- National Academies of Sciences, Engineering, and Medicine. Fostering Healthy Mental, Emotional, and Behavioral Development in Children and Youth: A National Agenda. Washington (DC): National Academies Press, 2019. [DOI: 10.17226/25201] [DOI] [PubMed] [Google Scholar]
O'Connor 2019
- O'Connor E, Senger CA, Henninger ML, Coppola E, Gaynes BN. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019;321(6):588-601. [DOI: 10.1001/jama.2018.20865] [DOI] [PubMed] [Google Scholar]
O'Reilly 2018
- O'Reilly M, Svirydzenka N, Adams S, Dogra N. Review of mental health promotion interventions in schools. Social Psychiatry and Psychiatric Epidemiology 2018;53(7):647-62. [DOI: 10.1007/s00127-018-1530-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padmanathan 2013
- Padmanathan P, De Silva MJ. The acceptability and feasibility of task-sharing for mental healthcare in low and middle income countries: a systematic review. Social Sciences & Medicine 2013;97:82-6. [DOI: 10.1016/j.socscimed.2013.08.004] [DOI] [PubMed] [Google Scholar]
PAHO/WHO 2003
- PAHO/WHO. Guiding Principles for Complementary Feeding of the Breastfed Child. Pan American Health Organization/World Health Organization, 2003. [Google Scholar]
Panter‐Brick 2018
- Panter-Brick C, Dajani R, Eggerman M, Hermosilla S, Sancilio A, Ager A. Insecurity, distress and mental health: experimental and randomized controlled trials of a psychosocial intervention for youth affected by the Syrian crisis. Journal of Child Psychology and Psychiatry 2018;59(5):523-41. [DOI: 10.1111/jcpp.12832] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papola 2020
- Papola D, Purgato M, Gastaldon C, Bovo C, Ommeren M, Barbui C, et al. Psychological and social interventions for the prevention of mental disorders in people living in low- and middle-income countries affected by humanitarian crises. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD012417. [DOI: 10.1002/14651858.CD012417.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papola 2022
- Papola D, Prina E, Ceccarelli C, Gastaldon C, Tol WA, Ommeren M, et al. Psychological and social interventions for the promotion of mental health in people living in low- and middle-income countries affected by humanitarian crises. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD014300. [DOI: 10.1002/14651858.CD014300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 1997
- Patel V, Simunyu E, Gwanzura F, Lewis G, Mann A. The Shona Symptom Questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psychiatrica Scandinavica 1997;95:469-75. [DOI: 10.1111/j.1600-0447.1997.tb10134.x] [DOI] [PubMed] [Google Scholar]
Patel 2007a
- Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, et al. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet 2007;370(9591):991-1005. [DOI: 10.1016/S0140-6736(07)61240-9] [DOI] [PubMed] [Google Scholar]
Patel 2007b
- Patel V, Chisholm D, Kirkwood BR, Mabey D. Prioritizing health problems in women in developing countries: comparing the financial burden of reproductive tract infections, anaemia and depressive disorders in a community survey in India. Tropical Medicine & International Health 2007;12(1):130-9. [DOI: 10.1111/j.1365-3156.2006.01756.x] [DOI] [PubMed] [Google Scholar]
Patel 2008
- Patel VH, Kirkwood BR, Pednekar S, Araya R, King M, Chisholm D, et al. Improving the outcomes of primary care attenders with common mental disorders in developing countries: a cluster randomized controlled trial of a collaborative stepped care intervention in Goa, India. Trials 2008;9:4. [DOI: 10.1186/1745-6215-9-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2010
- Patel V, Maj M, Flisher AJ, De Silva MJ, Koschorke M, Prince M, WPA Zonal and Member Society Representatives. Reducing the treatment gap for mental disorders: a WPA survey. World Psychiatry 2010;9(3):169–76. [DOI: 10.1002/j.2051-5545.2010.tb00305.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2018
- Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018;392(10157):1553-98. [DOI: 10.1016/S0140-6736(18)31612-X] [DOI] [PubMed] [Google Scholar]
Patton 2016
- Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet 2016;387(10036):2423-78. [DOI: 10.1016/S0140-6736(16)00579-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pompoli 2018
- Pompoli A, Furukawa TA, Efthimiou O, Imai H, Tajika A, Salanti G. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychological Medicine 2018;48(12):1945-53. [DOI: 10.1017/S0033291717003919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2007
- Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet 2007;370(9590):859-77. [DOI: 10.1016/S0140-6736(07)61238-0] [DOI] [PubMed] [Google Scholar]
Purgato 2010
- Purgato M, Barbui C, Cipriani A. Assessing risk of bias in randomized controlled trials. Epidemiology and Psychiatric Sciences 2010;19(4):296-7. [DOI: 10.1017/S1121189X00000622] [DOI] [PubMed] [Google Scholar]
Purgato 2012
- Purgato M, Adams CE. Heterogeneity: the issue of apples, oranges and fruit pie. Epidemiology and Psychiatric Sciences 2012;21(1):27-9. [DOI: 10.1017/s2045796011000643] [DOI] [PubMed] [Google Scholar]
Purgato 2018a
- Purgato M, Gastaldon C, Papola D, Ommeren M, Barbui C, Tol WA. Psychological therapies for the treatment of mental disorders in low- and middle-income countries affected by humanitarian crises. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD011849. [DOI: 10.1002/14651858.CD011849.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purgato 2018b
- Purgato M, Gross AL, Betancourt T, Bolton P, Bonetto C, Gastaldon C, et al. Focused psychosocial interventions for children in low-resource humanitarian settings: a systematic review and individual participant data meta-analysis. Lancet Global Health 2018;6(4):e390-400. [DOI: 10.1016/S2214-109X(18)30046-9] [DOI] [PubMed] [Google Scholar]
Purgato 2019a
- Purgato M, Jayaram G, Surkan PJ, Bass J, Bolton P. Encompassing a global mental health perspective into psychotherapy research: a critique of approaches to measuring the efficacy of psychotherapy for depression. Epidemiology and Psychiatric Sciences 2019;28(3):275-7. [DOI: 10.1017/S2045796018000781] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purgato 2019b
- Purgato M, Carswell K, Acarturk C, Au T, Akbai S, Anttila M, et al. Effectiveness and cost-effectiveness of Self-Help Plus (SH+) for preventing mental disorders in refugees and asylum seekers in Europe and Turkey: study protocols for two randomised controlled trials. BMJ Open 2019;9(5):e030259. [DOI: 10.1136/bmjopen-2019-030259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purgato 2020
- Purgato M, Uphoff E, Singh R, Thapa Pachya A, Abdulmalik J, Ginneken N. Promotion, prevention and treatment interventions for mental health in low-and middle-income countries through a task-shifting approach. Epidemiology and Psychiatric Sciences 2020;29:e150. [DOI: 10.1017/S204579602000061X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purgato 2021a
- Purgato M, Carswell K, Tedeschi F, Acarturk C, Anttila M, Au T, et al. Effectiveness of self-help plus in preventing mental disorders in refugees and asylum seekers in Western Europe: a multinational randomized controlled trial. Psychotherapy and Psychosomatics 2021;90(6):403-14. [DOI: 10.1159/000517504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purgato 2021b
- Purgato M, Singh R, Acarturk C, Cuijpers P. Moving beyond a 'one-size-fits-all' rationale in global mental health: prospects of a precision psychology paradigm. Epidemiology and Psychiatric Sciences 2021;30:e63. [DOI: 10.1017/S2045796021000500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riello 2021
- Riello M, Purgato M, Bove C, Tedeschi F, MacTaggart D, Barbui C, et al. Effectiveness of Self-Help Plus (SH+) in reducing anxiety and post-traumatic symptomatology among care home workers during the COVID-19 pandemic: a randomized controlled trial. Royal Society Open Science 2021;8(11):210219. [DOI: 10.1098/rsos.210219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahebi 2005
- Sahebi A, Asghari MJ, Salari RS. Validation of depression anxiety and stress scale (DASS-21) for an Iranian population. Journal of Iranian Psychologists 2005;1(4):1384. [Google Scholar]
Sampson 1997
- Sampson RJ, Raudenbush S, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 1997;277:918-24. [DOI] [PubMed] [Google Scholar]
Santomauro 2021
- Santomauro DF, Mantilla Herrera AM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al, COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021;398(10312):1700-12. [DOI: 10.1016/S0140-6736(21)02143-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahmalak 2019
- Shahmalak U, Blakemore A, Waheed MW, Waheed W. The experiences of lay health workers trained in task-shifting psychological interventions: a qualitative systematic review. International Journal of Mental Health Systems 2019;13:64. [DOI: 10.1186/s13033-019-0320-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheehan 1998
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 1998;59 Suppl 20:22-33, 34-57. [PubMed] [Google Scholar]
Shemilt 2006
- Shemilt I, Mugford M, Drummond M, Eisenstein E, Mallender J, McDaid D, et al, Campbell & Cochrane Economics Methods Group (CCEMG). Economics methods in Cochrane systematic reviews of health promotion and public health related interventions. BMC Medical Research Methodology 2006;6:55. [DOI: 10.1186/1471-2288-6-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shochet 1997a
- Shochet IM, Holland D, Whitefield K. Resourceful Adolescent Program: Group Leader's Manual. Brisbane, Australia: Griffith University, 1997. [Google Scholar]
Shochet 1997b
- Shochet IM, Holland D, Whitefield K. Resourceful Adolescent Program: Participant Workbook. Brisbane, Australia: Griffith University, 1997. [Google Scholar]
Sijbrandij 2017
- Sijbrandij M, Acarturk C, Bird M, Bryant RA, Burchert S, Carswell K, et al. Strengthening mental health care systems for Syrian refugees in Europe and the Middle East: integrating scalable psychological interventions in eight countries. European Journal of Psychotraumatology 2017;8(Suppl 2):1388102. [DOI: 10.1080/20008198.2017.1388102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singla 2017
- Singla DR, Kohrt BA, Murray LK, Anand A, Chorpita BF, Patel V. Psychological treatments for the world: lessons from low- and middle-income countries. Annual Review of Clinical Psychology 2017;13:149-81. [DOI: 10.1146/annurev-clinpsy-032816-045217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soltan 2022
- Soltan F, Cristofalo D, Marshall D, Purgato M, Taddese H, Vanderbloemen L, et al. Community-based interventions for improving mental health in refugee children and adolescents in high-income countries. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD013657. [DOI: 10.1002/14651858.CD013657.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Thronicroft 2016
- Thornicroft G, Chatterji S, Evans-Lacko S, Gruber M, Sampson N, Aguilar-Gaxiola S, et al. Undertreatment of people with major depressive disorder in 21 countries. British Journal of Psychiatry 2017;210(2):119-24. [DOI: 10.1192/bjp.bp.116.188078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2011
- Tol WA, Barbui C, Galappatti A, Silove D, Betancourt TS, Souza R, et al. Mental health and psychosocial support in humanitarian settings: linking practice and research. Lancet 2011;378(9802):1581-91. [DOI: 10.1016/S0140-6736(11)61094-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2015a
- Tol WA, Purgato M, Bass JK, Galappatti A, Eaton W. Mental health and psychosocial support in humanitarian settings: a public mental health perspective. Epidemiology and Psychiatric Sciences 2015;24(6):484-94. [DOI: 10.1017/S2045796015000827] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2015b
- Tol WA. Stemming the tide: promoting mental health and preventing mental disorders in low- and middle-income countries. Global Mental Health 2015;8(2):e11. [DOI: 10.1017/gmh.2015.9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tol 2020
- Tol WA, Leku MR, Lakin DP, Carswell K, Augustinavicius J, Adaku A, et al. Guided self-help to reduce psychological distress in South Sudanese female refugees in Uganda: a cluster randomised trial. Lancet Global Health 2020;8(2):e254-63. [DOI: 10.1016/S2214-109X(19)30504-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trautmann 2016
- Trautmann S, Rehm J, Wittchen HU. The economic costs of mental disorders: do our societies react appropriately to the burden of mental disorders? EMBO Reports 2016;17(9):1245-9. [DOI: 10.15252/embr.201642951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2020
- Turner DT, Riedel E, Kobeissi LH, Karyotaki E, Garcia-Moreno C, Say L, et al. Psychosocial interventions for intimate partner violence in low and middle income countries: a meta-analysis of randomised controlled trials. Global Health 2020;10(1):010409. [DOI: 10.7189/jogh.10.010409] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii-92. [PMID: ] [PubMed] [Google Scholar]
University of York 2002
- NHS Centre for Reviews and Dissemination, University of York. The NHS Economic Evaluation Database (NHS EED). Cost-Effectiveness Matters 2002;6(1):1-4. [LINK: www.york.ac.uk/media/crd/em61.pdf]
US Preventive Services Task Force 2019
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force Recommendation Statement. JAMA 2019;321(6):580-7. [DOI: 10.1001/jama.2019.0007] [DOI] [PubMed] [Google Scholar]
van der Westhuizen 2016
- Westhuizen C, Wyatt G, Williams JK, Stein DJ, Sorsdahl K. Validation of the Self Reporting Questionnaire 20-Item (SRQ-20) for use in a low- and middle-income country emergency centre setting. International Journal of Mental Health and Addiction 2016;14(1):37-48. [DOI: 10.1007/s11469-015-9566-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Ginneken 2013
- Van Ginneken N, Tharyan P, Lewin S, Rao GN, Meera SM, Pian J, et al. Non-specialist health worker interventions for the care of mental, neurological and substance-abuse disorders in low- and middle-income countries. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD009149. [DOI: 10.1002/14651858.CD009149.pub2] [DOI] [PubMed] [Google Scholar]
Van Ginneken 2021
- Van Ginneken N, Chin WY, Lim YC, Ussif A, Singh R, Shahmalak U, et al. Primary‐level worker interventions for the care of people living with mental disorders and distress in low‐and middle‐income countries. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD009149. [DOI: 10.1002/14651858.CD009149.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vargas‐Porras 2020
- Vargas‐Porras C, Roa‐Díaz ZM, Ferré‐Grau C, De Molina‐Fernández MI. Psychometric properties of the functional social support domain of perinatal infant care social support. Investigación y Educación en Enfermería 2020;38(2):e04. [DOI: 10.17533/udea.iee.v38n2e04] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman A, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahlbeck 2015
- Wahlbeck K. Public mental health: the time is ripe for translation of evidence into practice. World Psychiatry 2015;14(1):36-42. [DOI: 10.1002/wps.20178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteford 2013
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382(9904):1575-86. [DOI: 10.1016/S0140-6736(13)61611-6] [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD-10). Geneva (Switzerland): World Health Organization, 1992. [Google Scholar]
WHO 2002
- Department of Mental Health and Substance Dependence, World Health Organization. Prevention and Promotion in Mental Health. Geneva (Switzerland): World Health Organization, 2002. [LINK: apps.who.int/iris/bitstream/handle/10665/42539/9241562161.pdf] [Google Scholar]
WHO 2008
- World Health Organization, World Organization of Family Doctors (WONCA). Integrating Mental Health Into Primary Care: A Global Perspective. Geneva (Switzerland): World Health Organization, 2008. [LINK: apps.who.int/iris/bitstream/handle/10665/43935/9789241563680_eng.pdf?sequence=1&isAllowed=y] [Google Scholar]
WHO 2010
- World Health Organization. Community Based Rehabilitation: CBR Guidelines. Geneva (Switzerland): The Organization, 2010. [LINK: www.who.int/publications/i/item/9789241548052] [PubMed] [Google Scholar]
WHO 2011a
- World Health Organization, War Trauma Foundation, World Vision International. Psychological First Aid: Guide for Field Workers. Geneva (Switzerland): World Health Organization, 2011. [LINK: www.who.int/publications/i/item/9789241548205] [Google Scholar]
WHO 2011b
- World Health Organization. Mental Health Atlas 2011. Geneva (Switzerland): The Organization, 2011. [LINK: www.who.int/publications/i/item/9799241564359] [Google Scholar]
WHO 2013
- World Health Organization. Mental Health Action Plan 2013-2020. Geneva (Switzerland): The Organization, 2013. [LINK: https://www.who.int/mental_health/publications/action_plan/en/] [Google Scholar]
WHO 2014
- World Health Organization. Mental health: strengthening our response. www.who.int/mediacentre/factsheets/fs220/en/ (accessed prior to 24 May 2023).
WHO 2018
- World Health Organization. mhGAP Operations Manual: Mental Health Gap Action Programme (mhGAP). Geneva (Switzerland): The Organization, 2018. [LINK: apps.who.int/iris/bitstream/handle/10665/275386/9789241514811-eng.pdf?ua=1] [Google Scholar]
WHO 2019
- World Health Organization. Mental health. www.who.int/features/factfiles/mental_health/en/ (accessed 2019).
WHO 2020
- World Health Organization. Guidelines on Mental Health Promotive and Preventive Interventions for Adolescents: Helping Adolescents Thrive. Geneva (Switzerland): The Organization, 2020. [LINK: apps.who.int/iris/bitstream/handle/10665/336864/9789240011854-eng.pdf] [PubMed] [Google Scholar]
WHO 2021a
- World Health Organization. Mental Health Atlas 2020. Geneva (Switzerland): The Organization, 2021. [LINK: www.who.int/publications/i/item/9789240036703] [Google Scholar]
WHO 2021b
- World Health Organization. Comprehensive Mental Health Action Plan 2013-2030. Geneva (Switzerland): The Organization, 2021. [LINK: www.who.int/publications/i/item/9789240031029] [Google Scholar]
Wiley‐Exley 2007
- Wiley-Exley E. Evaluations of community mental health care in low- and middle-income countries: a 10-year review of the literature. Social Science & Medicine 2007;64(6):1231-41. [DOI: 10.1016/j.socscimed.2006.11.009] [DOI] [PubMed] [Google Scholar]
World Bank 2020
- The World Bank. World Bank country and lending groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 2020).
Zimet 1988
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment 1988;52(1):30-41. [DOI: 10.1207/s15327752jpa5201_2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Purgato 2021
- Purgato M, Abdulmalik JO, Prina E, Ceccarelli C, Tol WA, Ginneken N, et al. Primary‐level and community worker interventions for the prevention of mental disorders and the promotion of well‐being in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014722. [DOI: 10.1002/14651858.CD014722] [DOI] [PMC free article] [PubMed] [Google Scholar]