Abstract
Body composition is related to cardiometabolic disorders and is a major driver of the growing incidence of type 2 diabetes mellitus (T2DM). Altered fat distribution and decreased muscle mass are related to dysglycemia and impose adverse health-related outcomes in people with T2DM. Hence, improving body composition and maintaining muscle mass is crucial in T2DM. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are novel glucose-lowering medications gaining popularity because of their cardiorenal-protective effects and weight-lowering characteristics. However, reports on myopathy secondary to SGLT2 inhibitor treatment raised a safety concern. The importance of maintaining muscle mass in people with T2DM necessitates further investigation to explore the impact of novel medications on body composition. In this review, we discussed current evidence on the impact of SGLT2 inhibitors on body composition in people with T2DM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01481-7.
Keywords: SGLT2 inhibitors, Type 2 DM, DXA, BIA, Muscle mass
Key Summary Points
| Why carry out this study? |
| Altered body composition plays a significant role in the pathogenesis and prognosis of type 2 diabetes mellitus. |
| Sodium–glucose cotransporter 2 (SGLT2) inhibitors are new glucose-lowering drugs that reduce body weight, but their effect on body composition remains uncertain. |
| This review aimed to investigate the association of SGLT2 inhibitors and body composition in type 2 diabetes mellitus. |
| What was learned from the review? |
| SGLT2 inhibitors reduce total body weight in people with type 2 diabetes mellitus. Reduction of body weight is primarily due to the loss of fat mass. |
| In long-term studies, loss of fat-free mass approximately contributes 35% of body weight reduction. This change is comparable to the body composition changes reported after lifestyle interventions and bariatric surgery. |
| The impact of SGLT2 inhibitors on body composition significantly differs from sulfonylureas and dipeptidyl peptidase 4 inhibitors, but it is similar to glucagon-like peptide 1 receptor agonists. |
Introduction
Type 2 diabetes mellitus (T2DM) is a growing public health burden, affecting more than 400 million individuals worldwide [1]. The condition is associated with long-term adverse outcomes, namely cardiovascular disease (CVD), chronic kidney disease (CKD), as well as increased risk of mortality [2]. Modifiable risk factors including dietary habits and alterations in body composition influence the burden of T2DM [3]. In this context, the significance of body composition extends beyond measures such as body weight (BW) and body mass index (BMI). Other factors such as abnormal fat distribution, particularly visceral adiposity, and reduction of lean mass are more crucial [4, 5]. Thus, understanding the effects of therapeutic interventions on body composition in T2DM is clinically significant.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are a novel class of oral glucose-lowering medications that increase renal glucose excretion while posing little risk of hypoglycemia [6]. They also reduce blood pressure and have been associated with cardiorenal protection [7–9]. SGLT2 inhibitors have been associated with weight reduction through fat mass loss, but concerns have been raised regarding their potential impact on muscle mass [10]. Rare cases of sarcopenia and myopathy have been reported after using SGLT2 inhibitors, particularly in older individuals taking statins [11, 12]. However, recent animal studies revealed opposite trends, i.e., increased skeletal muscle mass and hand grip strength after treatment with SGLT2 inhibitors [13].
Considering the mentioned controversial findings, it is important to evaluate body composition changes associated with SGLT2 inhibitors. In this review we summarized current evidence and the key underlying mechanisms related to the effects of SGLT2 inhibitors on body composition, focusing on fat mass and fat-free mass.
Type 2 Diabetes Mellitus and Body Composition
Changes in body composition influence the risk of developing T2DM through a variety of mechanisms such as insulin resistance, metabolic dysfunction, and de novo lipogenesis in ectopic tissues [14, 15]. There is also a bidirectional interplay between T2DM and loss of muscle mass giving rise to a vicious cycle [16]. Sarcopenia, characterized as progressive age-related muscle mass and function decline, is more common in people with T2DM and is influenced mainly by insulin resistance [17, 18]. Sarcopenia contributes to poor glycemic control and increased risk of adverse outcomes such as frailty, particularly among the aging population [17, 19].
Impaired insulin action in skeletal muscle disrupts multiple pathways, thereby promoting protein degradation and muscle catabolism, while also hampering protein synthesis [20]. A chronic inflammatory state, oxidative stress, increased reactive oxygen species, and accumulation of advanced glycation end-products (AEGs) also exacerbate muscle loss in people with diabetes [21–23].
Effective weight management and improving body composition are crucial in the management of T2DM and achieving better glycemic control while minimizing diabetes-related chronic complications [24, 25]. Current guidelines emphasize on the importance of sustained weight loss and muscle mass restoration through individualized therapeutic approaches, dietary modifications, and regular exercise [14, 26–29].
Mechanism of Action of Sodium–Glucose Cotransporter 2 Inhibitors on Body Composition
SGLT2 inhibitors reduce renal glucose reabsorption, leading to decreased blood glucose levels independent of insulin release [30]. In a healthy adult, almost all of the filtered glucose is reabsorbed, primarily by SGLT2 proteins [31]. These proteins are expressed in the proximal convoluted tubules of the kidneys and actively transport glucose into the interstitium [32]. In individuals with diabetes, SGLT2 proteins are paradoxically upregulated, resulting in excessive glucose reabsorption despite high blood glucose levels [33]. Inhibition of SGLT2 transporters promotes renal glucose excretion and improves glucose homeostasis and insulin sensitivity. Additional metabolic benefits include an early diuretic effect and calorie loss in the urine [34], causing a significant weight reduction [6, 35]. Despite sustained urinary glucose excretion, compensatory adaptation mechanisms attenuate excess water and calorie loss associated with long-term SGLT2 inhibition. These mechanisms include upregulation of the renin–angiotensin–aldosterone system, increased appetite, and changes in energy expenditure/substrate utilization [36, 37].
SGLT2 inhibitors also induce gluconeogenesis while enhancing the uptake, utilization, and catabolism of fatty acids, leading to various effects on lipid metabolism, including glucagon release, ketogenesis, and free fatty acid oxidation and mobilization [38, 39]. Consequently, SGLT2 inhibitors are linked to alterations in body composition even during prolonged use.
Methods for Evaluating Body Composition
Body composition refers to the proportion of different tissues in the human body, such as fat, muscle, bone, and water [40]. Methods for analyzing body composition are categorized as direct (e.g., cadaveric) or indirect methods, with the latter estimating body composition using mathematical equations based on known parameters [41]. Traditionally, body composition is divided into fat mass and fat-free mass, but more detailed models further divide fat-free mass into water, proteins, and minerals [42] (Table 1).
Table 1.
Different compartments of body composition
| Compartment | Definition |
|---|---|
| Fat mass | Mass of adipose tissue |
| Fat-free mass | Total body mass except fat mass |
| Lean body mass | Fat-free mass except for mineral content (bones) |
| Skeletal muscle mass | Lean body mass minus connective tissue, skin, and other organs |
Simple anthropometric measurements such as BMI, waist circumference, and waist-to-height ratio offer quick assessments of body composition but lack detailed information [43, 44]. Computed tomography (CT) scan and magnetic resonance imaging (MRI) are the gold standard methods for evaluating body composition; however, high cost and low accessibility limit their widespread use [45].
Common methods for measuring body composition include bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DXA). BIA rapidly assesses body composition by measuring impedance after passing a small electrical current through the body. It estimates total body water, fat-free mass, and fat mass using single or multiple frequencies [46]. DXA has higher precision in the assessment of body composition. It uses X-ray imaging to measure bone density, fat mass, and lean mass [42]. Both DXA and BIA offer segmental assessments of body composition in regions like arms, legs, and trunk.
When interpreting the findings for clinical decision-making, it is crucial to consider the limitations of each technique. BIA results rely on a constant, population-based level of hydration and body fluid distribution, making them susceptible to other factors such as target population, stage of obesity, fluid overload, dehydration, and recent exercise prior to testing [47, 48]. DXA offers greater precision but requires trained technicians and uses estimation algorithms that may not be applicable to certain populations [49]. Additionally, caution should be exercised when using terms like fat-free mass and lean body mass interchangeably with skeletal muscle mass, as there are distinctions between these compartments (Table 1).
Methods
We searched PubMed/Medline, Scopus, and Web of Science from inception to July 2023 for studies that assessed changes in body composition (including fat mass and fat-free mass) among patients with T2DM treated with SGLT2 inhibitors. The search included Medical Subject Heading (MeSH) terms for “body composition” OR “muscle mass” OR “lean body mass” OR “anthropometric indices” AND “type 2 diabetes mellitus” AND “sodium-glucose cotransporter 2 inhibitors”. The following drug names were also included: “empagliflozin”, “dapagliflozin”, “Farxiga”, “Xigduo”, “canagliflozin”, “Jardiance”, “Glyxambi”, “ertugliflozin”, “Stegaltro”, “Steglujan”, “Qtren”, “ipragliflozin”, “sotagliflozin”, “Zynquista”, “tofogliflozin”, “luseogliflozin”, “licogliflozin”, “Novartis”, and “Phlorizin”. The search was limited to randomized clinical trials (RCT), and additional manual searching was performed.
The search yielded 1100 results, of which 1080 were excluded. Exclusion criteria were animal studies, duplicated studies, studies with unrelated topics, inappropriate study designs, inaccessible full text, and incomplete data. Finally, 20 articles were included for review in this study. Quality assessment of the included articles were conducted using National Institute of Health (NIH) Tool for Quality Assessment of Controlled Intervention Studies (available from www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The quality of studies were rated as “good”, “fair”, and “poor”.
To facilitate report and comparing the findings, we refer to changes in the lean body mass and skeletal muscle mass as the fat-free mass in this review, acknowledging the differences between these definitions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results and Discussion
We identified 20 RCTs investigating body composition change after treatment with SGLT2 inhibitors and details of the reviewed studies are presented in Tables 1 and 2. The method of body composition assessment is unspecified in one study [50]. Eleven studies used DXA [51–61], six studies used BIA [62–67], and two used a combination of DXA, BIA, and MRI [68, 69]. Also, studies had mostly fair to good qualities (supplementary material, Table S1).
Table 2.
Characteristics of clinical trials reporting changes in body composition with SGLT2is treatment
| Study | Year, country | Population | Intervention | Sample size | Age (years) | Baseline BMI (kg/m2) | Concurrent medications |
|---|---|---|---|---|---|---|---|
| Bolinder et al. [53] | 2012, multicenter | T2DM | Dapagliflozin 10 mg/day | 91 | 60.6 ± 8.2 | 32.1 ± 3.9 | Exclusive treatment with metformin |
| Placebo | 89 | 60.8 ± 6.9 | 31.7 ± 3.9 | ||||
| Bolinder et al. [52] | 2014, multicenter | T2DM | Dapagliflozin 10 mg/day | 69 | 60.6 ± 8.2 | 32.1 ± 3.9 | Exclusive treatment with metformin |
| Placebo | 71 | 60.8 ± 6.9 | 31.7 ± 3.9 | ||||
| Blonde et al. [51] | 2016, multicenter | T2DM | Canagliflozin 100 mg/day | 63 | 64.3 ± 6.6 | 30.9 ± 4.8 | Antihyperglycemic agents |
| Canagliflozin 300 mg/day | 73 | 63.0 ± 6.0 | 31.6 ± 4.3 | ||||
| Placebo | 75 | 64.2 ± 6.4 | 32.0 ± 5.5 | ||||
| Fadini et al. [62] | 2017, Italy | T2DM | Dapagliflozin 10 mg/day | 15 | 66.3 ± 1.8 | 28.4 ± 1.4 | Oral glucose-lowering drugs or insulin |
| Placebo | 16 | 61.0 ± 1.8 | 32.8 ± 1.4 | ||||
| Inoue et al. [69] | 2019, Japan | T2DM | Ipragliflozin 50 mg/day | 24 | 60.5 ± 9.8 | 27.9 ± 4.0 | Insulin alone or plus oral hypoglycemic agents |
| Placebo | 24 | 60.8 ± 12.1 | 27.7 ± 4.5 | ||||
| Chehregosha et al. [56] | 2021, Iran | T2DM/NAFLD | Empagliflozin 10 mg | 35 | 50.5 ± 8.4 | 30.9 ± 3.3 | NR |
| Pioglitazone | 34 | 52.5 ± 7.9 | 29.4 ± 3.7 | ||||
| Placebo | 37 | 51.8 ± 7.8 | 30.2 ± 4.4 | ||||
| Lauritsen et al. [58] | 2021, Denmark | T2DM | Empagliflozin 25 mg/day vs. placebo (crossover design) | 13 | 62 ± 6 | 31.5 ± 5.0 | Metformin |
| Horibe et al. [68] | 2022, Japan | T2DM | Dapagliflozin 5 mg/day | 26 | 59.7 ± 12.0 | 28.0 ± 4.0 | Oral hypoglycemic agents other than SGLT2i |
| Placebo | 24 | 62.3 ± 6.5 | 27.6 ± 3.8 | ||||
| Brandt-Jacobsen et al. [54] | 2023, Denmark | T2DM | Empagliflozin 25 mg/day | 38 | 65.7 ± 9.1 | 32.8 ± 5.6 | Unspecified glucose-lowering treatment |
| Placebo | 40 | 66.4 ± 8.7 | 30.3 ± 5.9 | ||||
| Nakaguchi et al. [60] | 2020, Japan | T2DM | Empagliflozin 10 mg/day | 31 | 66.3 ± 9.5 | 25.8 ± 4.1 | Insulin |
| Liraglutide | 30 | 67.2 ± 9.0 | 26.4 ± 4.6 | ||||
| McCrimmon et al. [59] | 2020, multicenter | T2DM | Canagliflozin 300 mg/day | 90 | 58.6 ± 10.1 | 32.3 ± 5.5 | Metformin |
| Semaglutide | 88 | 57.8 ± 9.9 | 32.6 ± 6.4 | ||||
| Cefalu et al. [55] | 2013, multicenter | T2DM | Canagliflozin 100 mg/day | 111 | NR | NR | Metformin |
| Canagliflozin 300 mg/day | 102 | ||||||
| Glimepiride | 96 | ||||||
| Kitazawa et al. [64] | 2020, Japan | T2DM | Tofogliflozin 20 mg/day | 33 | 57.3 ± 11.4 | 25.3 ± 3.9 | Metformin and DPP4 inhibitors |
| Glimepiride | 31 | 57.6 ± 9.3 | 25.4 ± 3.8 | ||||
| Wolf et al. [61] | 2021, Brazil | T2DM | Dapagliflozin 10 mg/day | 44 | 58 ± 7 | 30 (7) | Up to two oral hypoglycemic agents |
| Glibenclamide | 45 | 58 ± 7 | 30 (7) | ||||
| Tsurutani et al. [50] | 2018, multicenter | T2DM | Ipragliflozin 50 mg/day | 60 | 53.5 ± 11.72 | 28.8 (6.3) | Patients with prior use of SGLT2is or incretin-related agents were excluded |
| Sitagliptin | 59 | 54.0 ± 10.7 | 28.5 (5.2) | ||||
| Zeng et al. [67] | 2022, Taiwan | T2DM | Empagliflozin 25 mg/day | 46 | 58.9 ± 9.9 | 27.7 ± 5.0 | Premixed insulin with or without OAD |
| Linagliptin | 51 | 58.7 ± 10.2 | 28.0 ± 3.5 | ||||
| Kato et al. [63] | 2017, Japan | T2DM |
Dapagliflozin 5 mg/day vs. control (Cross-over design) |
Preceding group = 27 | 48.7 ± 11.5 | 30.3 ± 5.3 | Insulin or antidiabetic drugs |
| Following group = 29 | 49.4 ± 11.8 | 29.6 ± 4.9 | |||||
| Shimizu et al. [65] | 2018, Japan | T2DM/NAFLD | Dapagliflozin 5 mg/day | 33 | 56.2 ± 11.5 | 27.6 ± 4.7 | Three OAD with or without insulin |
| Control | 24 | 57.1 ± 13.8 | 28.3 ± 3.5 | ||||
| Yamakage et al. [66] | 2020, Japan | T2DM | Dapagliflozin 5 mg/day | 27 | 58.4 ± 13.0 | 31.3 ± 7.6 | Sulfonylureas, biguanides, alpha-glucosidase, DPP4 inhibitors, or their combination |
| Control | 27 | 60.7 ± 11.9 | 30.7 ± 6.2 | ||||
| Han et al. [57] | 2020, Korea | T2DM | Ipragliflozin 50 mg/day | 30 | 52.5 ± 10.3 | 30.4 ± 5.4 | Metformin and pioglitazone combination for at least 8 weeks |
| Control | 15 | 56.7 ± 11.8 | 30.2 ± 2.5 |
T2DM type 2 diabetes, BMI body mass index, NR not reported, SGLT2i sodium–glucose cotransporter 2 inhibitors, DPP4 dipeptidyl peptidase 4, OAD oral antidiabetic drugs
Data are reported as mean ± standard deviation or median (interquartile range)
In the following sections, we present and discuss the findings based on the study duration and the comparators.
Effect of Treatment Duration on Body Composition
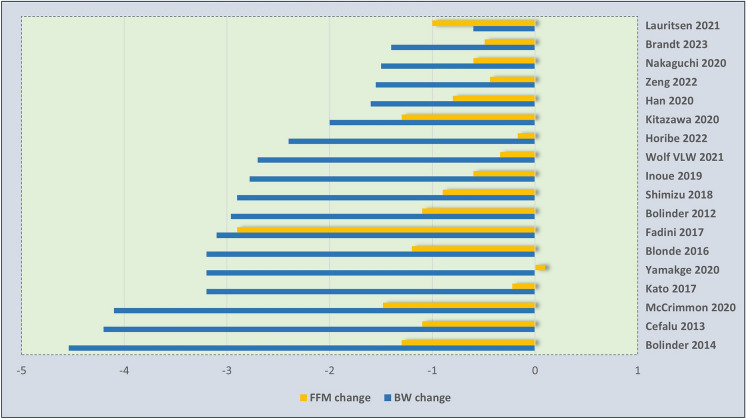
There were four studies with long-term duration (follow-up > 24 weeks). They showed that SGLT2 inhibitors are associated with a reduction of 2.5–4.5 kg in BW, 2–2.6 kg in fat mass, and 0.9–1.5 kg in fat-free mass, compared to the baseline. Notably, loss of fat-free mass comprised 25–36% of the total weight reduction [51, 52, 55, 59] (Table 3). In the remaining studies with shorter duration of follow-up, loss of fat-free mass ranged widely from 0 to 20% of total weight loss in three studies [61, 63, 66], 20–50% in seven studies [53, 54, 60, 65, 67–69], and more than 50% in three studies [57, 62, 64] (Fig. 1). In one study, the reduction in fat-free mass was greater than the overall weight loss, and the fat-free mass loss-to-BW loss ratio exceeded 1 [58].
Table 3.
Body composition changes following SGLT2 inhibitors treatment
| Author, year, country | Assessment method | Duration (weeks) | Body composition indices in SGLT2is groupa (kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline BW | BW change | Baseline fat mass | Fat mass change | Baseline fat-free massb | Fat-free mass change | ||||
| Compared to placebo | |||||||||
| 1 | Bolinder et al. (2012), [53] | DXA | 24 | 92.1 | − 2.96* | 33.6 | − 2.22* | 56.2 | − 1.1* |
| 2 | Bolinder et al. (2014), [52] | DXA | 102 | 92.1 | − 4.54* | 33.7 | − 2.80 | 55.3 | − 1.30 |
| 3 | Blonde et al. [51] | DXA | 26 |
100: 88.9 300: 93.2 |
− 2.5* − 3.2* |
32.2 33.8 |
− 1.9* − 2.4* |
51.2 53.2 |
− 0.9* − 1.2* |
| 4 | Fadini et al. [62] | BIA | 12 | NR | − 3.1* | NR | − 0.1 | NR | − 2.9* |
| 5 | Inoue et al. [69] | DXA/BIA | 24 | 72.34 | − 2.78* |
DXA: 23.29 BIA: 22.14 |
DXA: − 2.07* BIA: − 2.21* |
DXA: 41.63 BIA: 47.14 |
DXA: − 0.6 BIA: − 0.56 |
| 6 | Chehregosha et al. [56] | DXA | 24 | 82.2 | − 2.7* | NR | NR | NR | NR |
| 7 | Lauritsen et al. [58] | DXA | 4 | 95.2 | − 0.6 | 31.4 | − 0.2 | 60.4 | − 1.0* |
| 8 | Horibe et al. [68] |
DXA/ BIA |
24 | 73.29 | − 2.40* |
DXA: 25.43 BIA: 23.27 |
DXA: − 2.32* BIA: − 1.73* |
DXA: 45.86 BIA: 47.92 |
DXA: − 0.17 BIA: − 0.64 |
| 9 | Brandt–Jacobsen et al. [54] | DXA | 13 | 97.1 | − 1.40* | 30.2 | − 0.9* | 67.4 | − 0.49 |
| Compared to GLP-1 receptor agonists | |||||||||
| 10 | Nakaguchi et al. [60] | DXA | 24 | 69.0 | − 1.5 | 19.2 | − 0.7 | 46.1 | − 0.6 |
| 11 | McCrimmon et al. [59] | DXA | 52 | 87.6 | − 4.1 | 32.5 | − 2.62 | 51.3 | − 1.48 |
| Compared to sulfonylureas | |||||||||
| 12 | Cefalu et al. [55] | DXA | 52 |
100 mg: 84.4 300 mg: 85.9 |
− 4.4* − 4.2* |
28.2 29.3 |
NR |
47.7 44.6 |
− 0.9* − 1.1* |
| 13 | Kitazawa et al. [64] | BIA | 24 | 67.0 | − 2.0* | 19.4 | − 0.7* | 47.6 | − 1.3* |
| 14 | Wolf et al. [61] | DXA | 12 | 81.6 | − 2.7* | 29.9 | − 2.0* | 51.3 | − 0.34* |
| Compared to DPP4 inhibitors | |||||||||
| 15 | Tsurutani et al. [50] | NR | 12 | NR | − 2.2* | NR | NR | NR | NR |
| 16 | Zeng et al. [67] | BIA | 24 | 71.4 | − 1.55* | 20.8 | − 1.02 | 46.5 | − 0.44* |
| Compared to conventional treatment | |||||||||
| 17 | Kato et al. [63] | BIA | 12 |
Grp 1: 80.0 Grp 2: 81.7 |
Grp 1: − 1.2 Grp 2: − 3.2 |
NR |
Grp 1: − 1.39 Grp 2: − 1.97 |
NR |
Grp 1: − 0.12 Grp 2: − 0.22 |
| 18 | Shimizu et al. [65] | BIA | 24 | 73.6 | − 2.9* | NR | NR | 27.8 | − 0.9* |
| 19 | Yamakage et al. [66] | BIA | 24 | 80.5 | − 3.2* | NR | NR | 25.9 | 0.1 |
| 20 | Han et al. [57] | DXA | 24 | 84.2 | − 1.6* | 24.7 | − 1.0 | 56.6 | − 0.8 |
SGLT2 sodium–glucose cotransporter 2 inhibitors, BW body weight, T2DM type 2 diabetes mellitus, DXA dual-energy X-ray absorptiometry, BIA bioelectrical impedance analysis, NR not reported, NAFLD nonalcoholic fatty liver disease, Grp group
*Statistically significant between-group P value
aData are presented as mean or median values
bThe label fat-free mass includes muscle mass, lean mass, and fat-free mass
Fig. 1.
Changes in total body weight and fat-free mass (kg) following SGLT2 inhibitor treatment
The study of Fadini et al. also demonstrated that dapagliflozin treatment for 12 weeks causes a high proportional loss of fat-free mass [62]. They reported that BIA-derived fat-free mass was reduced by 2.9 kg (accounting for over 90% of BW reduction), with a total body water loss of 2.4 kg. Bioelectrical impedance vector analysis (BIVA) in this study showed that dapagliflozin mainly reduced the fluid content of the body.
Changes in visceral and subcutaneous adipose tissue were investigated in 10 studies [53, 56, 57, 59, 63, 65–69]. SGLT2 inhibitors reduced visceral and subcutaneous adipose tissue greater than placebo; however, this difference was only significant in the study of Bolinder et al. [53]. The difference in changes were not significant when SGLT2 inhibitors were compared to compared to semaglutide and linagliptin [59, 67]. Additional details regarding these changes can be found in supplementary material, Table S2.
Unless contraindicated, glucose-lowering medications are prescribed for a lifetime. As mentioned earlier, the effects of SGLT2 inhibitors on body composition vary on the basis of the duration of treatment. Initial weight reduction is attributed to the loss of fluid and calories. In the long term, BW reduction becomes attenuated as a result of counter-regulatory mechanisms, and studies indicate that it plateaus after about 26 weeks [70]. Consequently, in the long term, SGLT2 inhibitors result in a modest decrease in BW, around 2–3 kg [71].
Body compartments with high water content, namely fat-free mass, are particularly affected by acute fluid loss [62]. In contrast, longer assessments of patients with T2DM who were treated with empagliflozin showed insignificant changes in body water and fat-free mass [67]. These findings support the notion that fat-free mass is potentially more affected early following SGLT2 inhibitor treatment [58].
Two previous meta-analyses have explored the impact of SGLT2 inhibitors on body composition and their overall findings are consistent with those observed in long-term studies [72, 73]. The meta-analysis by Pan and colleagues reported that the mean difference [95% confidence interval] when comparing SGLT2 inhibitors with the control group was − 2.73 kg [− 3.32 to − 2.13] for BW, − 1.16 kg [− 2.01 to − 0.31] for fat mass, − 0.76 kg [− 1.53 to 0.01] for lean mass, and − 1.01 kg [− 1.91 to − 0.11] for skeletal muscle mass [73]. A network meta-analysis by Ida et al. revealed that canagliflozin and dapagliflozin significantly reduced fat-free mass compared to placebo, accounting for 20–30% of the total weight reduction [72].
Weight reduction interventions generally result in both fat mass and fat-free mass losses, albeit to different extents, depending on their mechanism of action, concurrent medications, baseline BMI, and gender [74, 75]. Current evidence suggests that loss of fat-free mass contributes to about one-third of the total reduction in BW following lifestyle interventions and bariatric surgery [76–79] (supplementary material, Table S3). Changes in body composition associated with SGLT2 inhibitor treatment are comparable to other weight reduction interventions. Nonetheless, it is crucial to investigate the impact of acute loss of fat-free mass on patient outcomes, especially among vulnerable individuals.
Sodium–Glucose Cotransporter 2 Inhibitors Versus Other Comparators
In this review, nine placebo-controlled studies were included. The between-group mean difference of changes in total BW and fat-free mass were about − 1.5 to − 3 kg and + 0.27 to − 0.9 kg, respectively [51–54, 56, 58, 62, 68, 69] (supplementary material, Fig. S1). MRI analyses on iliopsoas muscle surface area after 24 weeks of treatment also showed no significant alterations compared to placebo [68, 69]. In a sub-study of the CANTATA-SU trial, the effects of add-on canagliflozin treatment on body composition over 26 weeks were assessed, with both 100 mg and 300 mg per day doses [51]. Both doses caused significant changes in BW, fat mass, and fat-free mass compared to placebo, while the proportional loss of fat-free mass was consistent at approximately 37%.
In three studies, sulfonylureas were the comparator drugs [55, 61, 64]. Our review found that sulfonylureas increased BW, fat mass, and fat-free mass across all three studies, resulting in significant differences when compared to SGLT2 inhibitors. The mean difference was − 3 to − 5 kg for total BW and − 1.2 to − 2.2 kg for fat-free mass. Sulfonylureas are insulin secretagogues agents and are associated with weight gain [80]. Taking note of these differences between sulfonylureas and SGLT2 inhibitors helps us tailor individualized treatment plans. For patients with T2DM who are overweight, SGLT2 inhibitors might be considered as a preferred treatment option as they address glucose control and body composition simultaneously.
Two studies compared the effects of SGLT2 inhibitors to dipeptidyl peptidase 4 (DPP4) inhibitors on body composition [50, 67]. The first involved Asian patients with uncontrolled T2DM despite receiving premixed insulin, and was conducted over 24 weeks [67]. In this study, baseline BMIs were 28.0 ± 3.5 kg and 27.7 ± 5.0 kg/m2 in linagliptin and empagliflozin groups, respectively. As expected, empagliflozin reduced BW by 1.8 kg compared to linagliptin. The authors also report a difference in change of − 1.39 kg in fat-free mass [67]. The other study, which lasted for a shorter duration of 12 weeks, was conducted among Japanese people with T2DM and BMI over 28 kg/m2 [50]. It showed that the changes in BW and skeletal muscle index (SMI) with ipragliflozin compared to sitagliptin were − 1.61 kg and − 0.035 kg/m2, respectively. Further studies are needed to explore the effects of SGLT2 inhibitors vs DPP4 inhibitors on fat-free mass.
Two studies compared the effects of SGLT2 inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists on body composition in patients with T2DM, utilizing DXA as the assessment method [59, 60]. Nakaguchi et al. reported results from a 24-week treatment period, indicating a nonsignificant between-group difference of − 0.1 kg for fat-free mass [60]. Another study, conducted over 52 weeks, also reported a nonsignificant mean difference of − 0.78 kg (95% confidence interval − 1.61, 0.04) for fat-free mass. Two reviews exploring current evidence on this issue reported comparable effects of GLP-1 receptor agonists and SGLT2 inhibitors on fat-free mass [81, 82]. Moreover, a network meta-analysis found that semaglutide was associated with a greater reduction in BW and fat-free mass, followed by canagliflozin and dapagliflozin [72]. Although current evidence supports the comparable effects of GLP-1 receptor agonists and SGLT2 inhibitors on body composition, limited head-to-head comparison data warrant caution in making treatment decisions. On the other hand, certain patients with T2DM benefit from combination therapy of GLP-1 receptor agonists and SGLT2 inhibitors, including those with multiple risk factors, atherosclerotic cardiovascular disease, and patients not reaching specific treatment goals (e.g., obesity) [83, 84]. Further research is needed to explore the outcomes of such combination therapies on body composition.
Other studies included in this review, investigated the effects of add-on therapy with SGLT2 inhibitors among patients with T2DM who were taking oral antidiabetic medications and/or insulin [57, 63, 65, 66]. Significant reduction in total BW was reported with SGLT2 inhibitor therapy, without a clinically meaningful effect on fat-free mass [57, 63, 65, 66].
Strengths, Limitations, and Future Direction
In this study, we performed a systematic search, and included published RCTs. Additionally, the overall quality of the included studies was evaluated. These strengths enabled us to comprehensively examine how SGLT2 inhibitors affect body composition in individuals with T2DM. Nevertheless, there were some limitations when interpreting the findings. The major limitation was that data were largely heterogeneous in relation to the study duration, method of body composition assessment, and patients’ characteristics. In addition, investigating the changes in body composition was not the primary outcome in most of the studies. Also, a few studies considered background medications or the effect of multiple treatment regimens. For instance, Nakaguchi et al. compared the effects of treatment with empagliflozin versus liraglutide among patients who were taking insulin as the baseline regimen [60]. This study reported smaller changes in all compartments of body composition compared to McCrimmon et al.’s study, which also reported the effects of SGLT2 inhibitors versus GLP-1 receptor agonists on body composition [59]. These findings further highlight the importance of fractional changes in fat-free mass when assessing the safety of SGLT2 inhibitors.
Current meta-analyses have not fully addressed the heterogeneities in patient characteristics including comparator drugs, method of body composition assessment, and study duration [72, 73]. Pan and colleagues performed subgroup analysis based on different comparators, but the duration of treatment was not considered [73]. The method of body composition assessment was also not considered in the study of Ida and colleagues [72]. A major limitation is the lack of a universally recognized standard method to measure body composition. As mentioned earlier, BIA is affected by fixed assumptions of hydration status. This is highly significant when coexisting comorbidities, mainly CKD, are present [85]. Future trials should prioritize using more precise methods like DXA and diligently account for potential confounding factors.
Conclusion
Current evidence suggests that weight loss associated with SGLT2 inhibitor treatment is mainly due to the reduction of fat mass. Loss of fat-free mass contributes to one-third of total BW loss. This change is comparable to the body composition changes reported after lifestyle interventions, bariatric surgery, and GLP-1 receptor agonist treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Mohammad E. Khamseh contributed to the study conception and design. Soodeh Jahangiri, Mohammad E. Khamseh, and Mojtaba Malek were involved in the drafting of the manuscript. Mohammad E. Khamseh, Mojtaba Malek, and Sanjay Kalra contributed to reviewing and editing the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Soodeh Jahangiri, Mojtaba Malek, Sanjay Kalra, and Mohammad E. Khamseh have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Safiri S, Karamzad N, Kaufman JS, et al. Prevalence, deaths and disability-adjusted-life-years (DALYs) due to type 2 diabetes and its attributable risk factors in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. Front endocrinol. 2022;13:838027. [DOI] [PMC free article] [PubMed]
- 2.Yang JJ, Yu D, Wen W, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open. 2019;2(4):e192696. doi: 10.1001/jamanetworkopen.2019.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haines MS, Leong A, Porneala BC, Meigs JB, Miller KK. Association between muscle mass and diabetes prevalence independent of body fat distribution in adults under 50 years old. Nutr Diabetes. 2022;12(1):29. doi: 10.1038/s41387-022-00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta P, Lanca C, Gan ATL, et al. The association between body composition using dual energy X-ray absorptiometry and type-2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. 2019;9(1):12634. doi: 10.1038/s41598-019-49162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73–79. doi: 10.1097/MED.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai Y, Uneda K, Yamada T, et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2022;183:109146. doi: 10.1016/j.diabres.2021.109146. [DOI] [PubMed] [Google Scholar]
- 8.Scheen AJ. Effects of glucose-lowering agents on surrogate endpoints and hard clinical renal outcomes in patients with type 2 diabetes. Diabetes Metab. 2019;45(2):110–121. doi: 10.1016/j.diabet.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka H, Yokomizo H, Nakamura S, et al. Differential effect of canagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on slow and fast skeletal muscles from nondiabetic mice. Biochem J. 2022;479(3):425–444. doi: 10.1042/BCJ20210700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R, Alcantara R, Popli T, et al. Myopathy associated with statins and SGLT2 – a review of literature. Curr Probl Cardiol. 2021;46(4):100765. doi: 10.1016/j.cpcardiol.2020.100765. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda M, Iizuka K, Kato T, et al. Sodium–glucose cotransporter 2 inhibitor and sarcopenia in a lean elderly adult with type 2 diabetes: a case report. J diabetes Investig. 2020;11(3):745–747. doi: 10.1111/jdi.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamba R, Okamura T, Hashimoto Y, et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J Cachexia Sarcopenia Muscle. 2022;13(1):574–588. doi: 10.1002/jcsm.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki H, Tauchi S, Machann J, et al. Fat distribution patterns and future type 2 diabetes. Diabetes. 2022;71(9):1937–1945. doi: 10.2337/db22-0315. [DOI] [PubMed] [Google Scholar]
- 15.Smith GI, Shankaran M, Yoshino M, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khamseh ME, Malek M, Aghili R, Emami Z. Sarcopenia and diabetes: pathogenesis and consequences. Br J Diabetes Vasc Dis. 2011;11(5):230–234. doi: 10.1177/1474651411413644. [DOI] [Google Scholar]
- 17.Dhar M, Kapoor N, Suastika K, et al. South Asian Working Action Group on SARCOpenia (SWAG-SARCO)—a consensus document. Osteoporosis Sarcopenia. 2022;8(2):35–57. doi: 10.1016/j.afos.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Huang X, Dong M, Wen S, Zhou L, Yuan X. The association between sarcopenia and diabetes: from pathophysiology mechanism to therapeutic strategy. Diabetes Metab Syndr Obes. 2023;16:1541–1554. doi: 10.2147/DMSO.S410834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purnamasari D, Tetrasiwi EN, Kartiko GJ, Astrella C, Husam K, Laksmi PW. Sarcopenia and chronic complications of type 2 diabetes mellitus. Rev Diabet Stud. 2022;18(3):157–165. doi: 10.1900/RDS.2022.18.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry BD, Caldow MK, Brennan-Speranza TC, et al. Muscle atrophy in patients with type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc Immunol Rev. 2016;22:94–109. [PMC free article] [PubMed] [Google Scholar]
- 22.Scicchitano BM, Pelosi L, Sica G, Musarò A. The physiopathologic role of oxidative stress in skeletal muscle. Mech Ageing Dev. 2018;170:37–44. doi: 10.1016/j.mad.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Waqas K, Chen J, Trajanoska K, et al. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with sarcopenia. J Clin Endocrinol Metab. 2022;107(2):e793–e803. doi: 10.1210/clinem/dgab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):911–922. doi: 10.1016/S2213-8587(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Asberry PJ, Shofer JB, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care. 2002;25(9):1504–1510. doi: 10.2337/diacare.25.9.1504. [DOI] [PubMed] [Google Scholar]
- 26.Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Ther. 2021;12(5):1227–1247. doi: 10.1007/s13300-021-01035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng TP, Feng L, Nyunt MS, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128(11):1225–36.e1. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 29.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–754. doi: 10.2337/dci19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao EC. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes. 2014;32(1):4–11. doi: 10.2337/diaclin.32.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis VW, Brazy PC. Phosphate and glucose transport in the proximal convoluted tubule: mutual dependency on sodium. Adv Exp Med Biol. 1978;103:79–80. doi: 10.1007/978-1-4684-7758-0_8. [DOI] [PubMed] [Google Scholar]
- 32.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61(10):2079–2086. doi: 10.1007/s00125-018-4654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Wu N, Sun C, Jin D, Lu H. Effects of SGLT-2 inhibitors on adipose tissue distribution in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2023;15(1):113. doi: 10.1186/s13098-023-01085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad F, Dokmak G, Bader M, Karaman R. A comprehensive review on weight loss associated with anti-diabetic medications. Life (Basel) 2023;13(4):1012. doi: 10.3390/life13041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schork A, Saynisch J, Vosseler A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46. doi: 10.1186/s12933-019-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev. 2019;20(6):816–828. doi: 10.1111/obr.12841. [DOI] [PubMed] [Google Scholar]
- 38.Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022;21(1):83. doi: 10.1186/s12933-022-01512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szekeres Z, Toth K, Szabados E. The effects of SGLT2 inhibitors on lipid metabolism. Metabolites. 2021;11(2):87. doi: 10.3390/metabo11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borga M, West J, Bell JD, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66(5):1–9. doi: 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguado-Henche S, Pellico L. Body composition: evaluation methods. Eur JAnat. 2005;9(2):117–124. [Google Scholar]
- 42.Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148(5):648–658. doi: 10.4103/ijmr.IJMR_1777_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali A-k, Mohammad Javad Z, Ehsan S, et al. Can anthropometric indices predict the chance of hypertension? A multicentre cross-sectional study in Iran. BMJ Open. 2022;12(11):e062328. doi: 10.1136/bmjopen-2022-062328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller MJ, Braun W, Pourhassan M, Geisler C, Bosy-Westphal A. Application of standards and models in body composition analysis. Proc Nutr Soc. 2016;75(2):181–187. doi: 10.1017/S0029665115004206. [DOI] [PubMed] [Google Scholar]
- 46.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19(5):433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 47.Brunani A, Perna S, Soranna D, et al. Body composition assessment using bioelectrical impedance analysis (BIA) in a wide cohort of patients affected with mild to severe obesity. Clin Nutr. 2021;40(6):3973–3981. doi: 10.1016/j.clnu.2021.04.033. [DOI] [PubMed] [Google Scholar]
- 48.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:26. doi: 10.1186/1475-2891-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nana A, Slater GJ, Stewart AD, Burke LM. Methodology review: using dual-energy X-ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int J Sport Nutr Exerc Metab. 2015;25(2):198–215. doi: 10.1123/ijsnem.2013-0228. [DOI] [PubMed] [Google Scholar]
- 50.Tsurutani Y, Nakai K, Inoue K, et al. Comparative study of the effects of ipragliflozin and sitagliptin on multiple metabolic variables in Japanese patients with type 2 diabetes: a multicentre, randomized, prospective, open-label, active-controlled study. Diabetes Obes Metab. 2018;20(11):2675–2679. doi: 10.1111/dom.13421. [DOI] [PubMed] [Google Scholar]
- 51.Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med. 2016;128(4):371–380. doi: 10.1080/00325481.2016.1169894. [DOI] [PubMed] [Google Scholar]
- 52.Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 53.Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 54.Brandt-Jacobsen NH, Jürgens M, Hasbak P, et al. Reduction of cardiac adipose tissue volume with short-term empagliflozin treatment in patients with type 2 diabetes: a substudy from the SIMPLE randomized clinical trial. Diabetes Obes Metab. 2023;25(3):844–855. doi: 10.1111/dom.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 56.Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, et al. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Ther. 2021;12(3):843–861. doi: 10.1007/s13300-021-01011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han E, Lee YH, Lee BW, Kang ES, Cha BS. Ipragliflozin additively ameliorates non-alcoholic fatty liver disease in patients with type 2 diabetes controlled with metformin and pioglitazone: a 24-week randomized controlled trial. J Clin Med. 2020;9(1):259. doi: 10.3390/jcm9010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauritsen KM, Nielsen BRR, Tolbod LP, et al. SGLT2 inhibition does not affect myocardial fatty acid oxidation or uptake, but reduces myocardial glucose uptake and blood flow in individuals with type 2 diabetes: a randomized double-blind, placebo-controlled crossover trial. Diabetes. 2021;70(3):800–808. doi: 10.2337/db20-0921. [DOI] [PubMed] [Google Scholar]
- 59.McCrimmon RJ, Catarig AM, Frias JP, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63(3):473–485. doi: 10.1007/s00125-019-05065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakaguchi H, Kondo Y, Kyohara M, Konishi H, Oiwa K, Terauchi Y. Effects of liraglutide and empagliflozin added to insulin therapy in patients with type 2 diabetes: a randomized controlled study. J Diabetes Investig. 2020;11(6):1542–1550. doi: 10.1111/jdi.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolf VLW, Breder I, de Carvalho LSF, et al. Dapagliflozin increases the lean-to total mass ratio in type 2 diabetes mellitus. Nutr Diabetes. 2021;11(1):17. doi: 10.1038/s41387-021-00160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16(1):42. doi: 10.1186/s12933-017-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato K, Suzuki K, Aoki C, et al. The effects of intermittent use of the SGLT-2 inhibitor, dapagliflozin, in overweight patients with type 2 diabetes in Japan: a randomized, crossover, controlled clinical trial. Expert Opin Pharmacother. 2017;18(8):743–751. doi: 10.1080/14656566.2017.1317748. [DOI] [PubMed] [Google Scholar]
- 64.Kitazawa T, Seino H, Ohashi H, et al. Comparison of tofogliflozin versus glimepiride as the third oral agent added to metformin plus a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes: a randomized, 24-week, open-label, controlled trial (STOP-OB) Diabetes Obes Metab. 2020;22(9):1659–1663. doi: 10.1111/dom.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21(2):285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 66.Yamakage H, Tanaka M, Inoue T, Odori S, Kusakabe T, Satoh-Asahara N. Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: a randomized, controlled trial. J Diabetes Investig. 2020;11(3):653–661. doi: 10.1111/jdi.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng YH, Liu SC, Lee CC, Sun FJ, Liu JJ. Effect of empagliflozin versus linagliptin on body composition in Asian patients with type 2 diabetes treated with premixed insulin. Sci Rep. 2022;12(1):17065. doi: 10.1038/s41598-022-21486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horibe K, Morino K, Miyazawa I, et al. Metabolic changes induced by dapagliflozin, an SGLT2 inhibitor, in Japanese patients with type 2 diabetes treated by oral anti-diabetic agents: a randomized, clinical trial. Diabetes Res Clin Pract. 2022;186:109781. doi: 10.1016/j.diabres.2022.109781. [DOI] [PubMed] [Google Scholar]
- 69.Inoue H, Morino K, Ugi S, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig. 2019;10(4):1012–1021. doi: 10.1111/jdi.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribola FA, Cançado FB, Schoueri JH, De Toni VF, Medeiros VH, Feder D. Effects of SGLT2 inhibitors on weight loss in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21(1):199–211. [PubMed] [Google Scholar]
- 71.Perseghin G, Solini A. The EMPA-REG outcome study: critical appraisal and potential clinical implications. Cardiovasc Diabetol. 2016;15(1):85. doi: 10.1186/s12933-016-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ida S, Kaneko R, Imataka K, et al. Effects of antidiabetic drugs on muscle mass in type 2 diabetes mellitus. Curr Diabetes Rev. 2021;17(3):293–303. doi: 10.2174/1573399816666200705210006. [DOI] [PubMed] [Google Scholar]
- 73.Pan R, Zhang Y, Wang R, Xu Y, Ji H, Zhao Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. PLoS ONE. 2022;17(12):e0279889. doi: 10.1371/journal.pone.0279889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511–519. doi: 10.3945/an.116.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31(5):743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 77.Turicchi J, O'Driscoll R, Finlayson G, et al. Associations between the proportion of fat-free mass loss during weight loss, changes in appetite, and subsequent weight change: results from a randomized 2-stage dietary intervention trial. Am J Clin Nutr. 2020;111(3):536–544. doi: 10.1093/ajcn/nqz331. [DOI] [PubMed] [Google Scholar]
- 78.Prentice AM, Goldberg GR, Jebb SA, Black AE, Murgatroyd PR, Diaz EO. Physiological responses to slimming. Proc Nutr Soc. 1991;50(2):441–458. doi: 10.1079/PNS19910055. [DOI] [PubMed] [Google Scholar]
- 79.Nuijten MAH, Eijsvogels TMH, Monpellier VM, Janssen IMC, Hazebroek EJ, Hopman MTE. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: a systematic review and meta-analysis. Obes Rev. 2022;23(1):e13370. doi: 10.1111/obr.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Apovian CM, Okemah J, O'Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36(1):44–58. doi: 10.1007/s12325-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Massimino E, Izzo A, Riccardi G, Della PG. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: current evidence and underlying mechanisms. Cells. 2021;10(8):1958. doi: 10.3390/cells10081958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJ. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul) 2019;34(3):247–262. doi: 10.3803/EnM.2019.34.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gourdy P, Darmon P, Dievart F, Halimi J-M, Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM) Cardiovasc Diabetol. 2023;22(1):79. doi: 10.1186/s12933-023-01798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castellana M, Cignarelli A, Brescia F, et al. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: a meta-analysis. Sci Rep. 2019;9(1):19351. doi: 10.1038/s41598-019-55524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duren DL, Sherwood RJ, Czerwinski SA, et al. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol. 2008;2(6):1139–1146. doi: 10.1177/193229680800200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.