Abstract
Objective
To develop and validate a risk score based on preoperative clinical-radiological parameters for predicting overall survival (OS) in patients undergoing surgical resection for hepatocellular carcinoma (HCC).
Methods
From July 2010 to December 2021, consecutive patients with surgically-proven HCC who underwent preoperative contrast-enhanced MRI were retrospectively enrolled. A preoperative OS risk score was constructed in the training cohort using a Cox regression model and validated in a propensity score-matched internal validation cohort and an external validation cohort.
Results
A total of 520 patients were enrolled, among whom 210, 210, and 100 patients were from the training, internal validation, and external validation cohorts, respectively. Independent predictors for OS included incomplete tumor “capsule,” mosaic architecture, tumor multiplicity, and serum alpha-fetoprotein, which were incorporated into the “OSASH score.” The C-index the OSASH score was 0.85, 0.81, and 0.62 in the training, internal, and external validation cohorts, respectively. Using 32 as the cutoff point, the OSASH score stratified patients into prognostically distinct low- and high-risk groups among all study cohorts and six subgroups (all p < 0.05). Furthermore, patients with BCLC stage B-C HCC and OSASH-low risk achieved comparable OS to that of patients with BCLC stage 0-A HCC and OSASH-high risk in the internal validation cohort (5-year OS rates, 74.7 vs. 77.8%; p = 0.964).
Conclusion
The OSASH score may help predict OS in HCC patients undergoing hepatectomy and identify potential surgical candidates among those with BCLC stage B-C HCC.
Clinical relevance statement
By incorporating three preoperative MRI features and serum AFP, the OSASH score may help predict postsurgical overall survival in patients with hepatocellular carcinoma and identify potential surgical candidates among those with BCLC stage B and C HCC.
Key Points
• The OSASH score incorporating three MRI features and serum AFP can be used to predict OS in HCC patients who received curative-intent hepatectomy.
• The score stratified patients into prognostically distinct low- and high-risk strata in all study cohorts and six subgroups.
• Among patients with BCLC stage B and C HCC, the score identified a subgroup of low-risk patients who achieved favorable outcomes after surgery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-023-09725-7.
Keywords: Carcinoma, hepatocellular; Prognosis; Mortality; Magnetic resonance imaging; Hepatectomy
Introduction
Liver cancer is the sixth most lethal malignancy globally, and hepatocellular carcinoma (HCC) accounts for ~ 90% of primary liver cancer cases [1, 2]. Individualized prognostication is critical for informing optimal patient care in HCC. To this end, several staging systems have been developed based on tumor burden (e.g., size, number, vascular invasion, and extrahepatic metastasis), liver function, and patient performance status [3–7]. Among them, the most widely used system is the Barcelona Clinic Liver Cancer (BCLC) system, which allows prognostication and subsequent treatment allocation [3]. Nonetheless, there is great survival heterogeneity in each risk subgroup [8, 9], shedding light on the unsatisfactory performance of current systems in profiling the comprehensive landscape of tumor aggressiveness and the unmet need for HCC prognostication refinement.
Hepatectomy is the backbone for curative-intent treatment in early-stage HCC, offering a 5-year survival of 35–70% [2]. Nevertheless, controversy still shrouds the surgical indications for HCC. In line with the BCLC system, Western practice guidelines restrict liver resection to patients with very early- to early-stage HCC [10, 11]. However, growing evidence showed more favorable outcomes for liver resection in selected patients with intermediate- to advanced-stage HCC in comparison to transarterial chemoembolization and systemic therapies [12–16], as incorporated into major Asian guidelines [7, 17–19]. In spite of this, data remain scarce regarding the optimal surgical candidates among patients with intermediate- to advanced-stage HCC.
Overall survival (OS) has been widely accepted as the most important endpoint in oncology and HCC research and is not subject to investigator bias. Encouraging results have been reported on the utility of gadoxetate disodium–enhanced magnetic resonance imaging (EOB-MRI) features for noninvasive prediction of worse OS in HCC patients after treatment [20–23]. Nevertheless, these studies were hampered by a small sample size (e.g., 120–376 patients) and lack of external validation. Additionally, there are limited data on the comparison between the EOB-MRI and extracellular contrast agent-enhanced MRI (ECA-MRI) in HCC prognostication, whilst the latter is a more available, cost-effective, and time-efficient examination with superior arterial phase quality [24]. Furthermore, to our knowledge, few attempts have been made to assess the capacity of MRI-based prognostic tools in informing surgical recommendations for patients with intermediate- to advanced-stage HCC.
Therefore, we aimed to develop and externally validate a risk score based on preoperative clinical-radiological data to predict OS in HCC patients after hepatectomy. Additionally, we sought to investigate whether this score could be used to stratify prognosis and identify patients with intermediate- to advanced-stage HCC who would potentially achieve favorable prognosis after surgery.
Materials and methods
This retrospective dual-institutional study was approved by the institutional review boards of West China Hospital, Sichuan University (Chengdu, Sichuan, China) and Henan Provincial People’s Hospital (Zhengzhou, Henan, China). The requirements for informed consent were waived.
Patients
From July 2015 to November 2020, consecutive patients who underwent preoperative contrast-enhanced EOB-MRI within 2 months before resection for HCC at institution 1 were retrospectively enrolled and constituted the training cohort (termed the “EOB-MRI cohort”). The inclusion criteria were (a) surgically proven HCC; (b) R0 resection (defined as the complete macroscopic removal of tumor with a negative microscopic margin); (c) absence of a previous history of HCC treatment; and (d) absence of any co-malignancy other than HCC. The exclusion criteria were (a) distant metastasis at preoperative work-ups; (b) ruptured HCC; (c) incomplete clinical or pathological data; (d) inadequate MR images for analysis; and (e) without follow-up information.
If the developed risk score incorporated HBP imaging features, internal validation would be performed in the EOB-MRI cohort; otherwise, internal validation would be performed in an independent ECA-MRI cohort to test the model’s generalizability in broader populations. Specifically, from July 2010 to December 2021, consecutive patients who underwent preoperative ECA-MRI within 2 months before resection for HCC at institution 1 following the same eligibility criteria were enrolled and constituted the “ECA-MRI cohort.” The ECA-MRI-based internal validation cohort was created using propensity score matching as detailed in the “Statistical analysis” section. The selection of these two MRI contrast agents was based on the clinicians’ recommendations and patients’ preferences.
From April 2014 to March 2019, consecutive patients who underwent EOB-MRI within 2 months prior to resection for HCC at institution 2 following the same eligibility criteria were retrospectively enrolled and formed the external validation cohort.
In all study cohorts, data including clinical information, laboratory indexes (i.e., aspartate aminotransferase, alanine aminotransferase, total bilirubin, albumin, platelet, prothrombin time [PT], the international normalized ratio [INR], alkaline phosphatase, gamma-glutamyl transferase, and alpha-fetoprotein [AFP]) within 1 month prior to surgery and histopathological results were recorded. Baseline laboratory data with ≤ 5% missingness were imputed by medians, with 0.4% (2/520) of missing values for AFP, 0.2% (1/520) for PT, and 0.5% (2/420) for INR. Cirrhosis was diagnosed by the recommendations in the Clinical Practice Guidelines [25]. The albumin-bilirubin (ALBI) score was calculated using previously described approaches [26].
MRI technique
MRI was performed with six 3.0-T systems and two 1.5-T systems. Liver MRI sequences included: T2-weighted imaging, diffusion-weighted imaging with apparent diffusion coefficient maps, T1-weighted in- and opposed-phase imaging, and T1-weighted dynamic imaging consisting of precontrast phase, late arterial phase, portal venous phase, delayed phase (ECA-MRI) or transitional phase (EOB-MRI), and HBP (EOB-MRI) images. Details on MRI protocols are provided in Supplementary Material 1 and Table S1.
Image analysis
All deidentified MR images were transmitted to institution 1 and reviewed independently by two fellowship-trained abdominal radiologists (readers 1 and 2, with 7 and 10 years of experience in liver MRI, respectively) who were informed that all enrolled patients had HCC, but were unaware of the remaining clinicopathological and follow-up information. Any discrepancy in imaging interpretation was resolved by a senior abdominal radiologist (reader 3, with over 20 years of experience in liver MRI).
On a per-patient basis, the following features were evaluated: (a) imaging features related to tumor burden [10, 11, 27]; (b) presence or absence of major, ancillary, LR-TIV and LR-M features as defined by Liver Imaging Reporting and Data System (LI-RADS) version 2018 [27]; (c) presence or absence of other imaging features related to tumor biology or patient outcomes [28–31]; and (d) presence or absence of imaging features related to underlying liver diseases [32, 33] (Table 1). For multiple tumors, radiologic features of the largest tumor were recorded for analysis.
Table 1.
All evaluated MRI features and the definitions
| Feature | Definition |
|---|---|
| Tumor burden-related features | |
| Tumor multiplicity | Number of definite intrahepatic HCC lesions with characteristic enhancement pattern [10, 11] |
| Tumor size | See page 16–157 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Bilobar involvement | Bilobar involvement of definite HCC on contrast-enhanced MRI |
| LI-RADS major features | |
| Nonrim APHE | See page 16–66 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Nonperipheral "washout" | See page 16–138 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Enhancing "capsule" | See page 16–187 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| LI-RADS ancillary features | |
| Favoring malignancy in general, not HCC in particular | |
| Corona enhancement | See page 16–265 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Fat sparing in solid mass | See page 16–272 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Diffusion restriction | See page 16–278 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Mild-moderate T2 hyperintensity | See page 16–283 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Iron sparing in solid mass | See page 16–289 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| TP hypointensity | See page 16–295 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| HBP hypointensity | See page 16–300 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Favoring HCC in particular | |
| Nonenhancing "capsule" | See page 16–309 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Nodule-in-nodule | See page 16–319 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Mosaic architecture | See page 16–314 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Fat in mass, more than adjacent liver | See page 16–323 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Blood products in mass | See page 16–329 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Favoring benignity | |
| Iron in mass, more than liver | See page 16–355 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Marked T2 hyperintensity | See page 16–362 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| HBP isointensity | See page 16–369 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Tumor in vein | See page 16–243 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| LR-M features | |
| Targetoid appearances | |
| Rim APHE | See page 16–38 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Peripheral "washout" | See page 16–125 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Delayed central enhancement | See page 16–221 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Targetoid restriction | See page 16–234 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Targetoid TP or HBP appearance | See page 16–227 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Nontargetoid features | |
| Infiltrative appearance | See page 16–241 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Marked diffusion restriction | See page 16–241 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Necrosis or severe ischemia | See page 16–241 of Chapter 16 of LI-RADS v2018 CT/MRI Manual [27] |
| Other tumor-related prognostic features | |
| Intratumoral artery | Presence of discrete arterial enhancement within the tumor [28] |
| Incomplete tumor "capsule" | An absence of “capsule” or the presence of a disrupted “capsule” in any imaging plane [29] |
| Nonsmooth tumor margin | Non-nodular tumors or nodular tumors with irregular margin and budding portion at the tumor periphery in any imaging plane [28, 30] |
| Marked HBP hypointensity | Signal intensity of the liver observation in the HBP lower than that of liver and similar to or lower than that of vessels |
| HBP peritumoral hypointensity | Presence of wedge-shaped or flame-like hypointense area adjacent to the tumor border on HBP images [30] |
| HBP hypointense nodule without APHE | HBP hypointensity was defined as unequivocally darker signal intensity in HBP in whole or in part than liver, and APHE was defined as nonrim-like or rim enhancement in AP showing unequivocally higher intensity or attenuation in any part than liver [31] |
| Underlying liver disease-related features | |
| Radiological cirrhosis | Unequivocal morphological alterations of liver, including surface nodularity, small liver volume, expansion of space between liver and anterior abdominal wall and perihilar, gallbladder fossa and ligamentum teres spaces, hypertrophy of caudate and/or lateral left section, atrophy of anterior right section and/or medial left section, anterolateral flattening, notching of the posterior medial right lobe and parenchymal nodules, with or without manifestations of portal hypertension (portal-systemic collaterals, splenomegaly and/or ascites) |
| Diffuse fatty change | Diffuse signal intensity drop of the liver parenchyma on opposed-phase images compared with the in-phase images |
| Diffuse iron overload | Diffuse signal intensity drop of the liver parenchyma on in-phase images compared with the opposed-phase images |
| Width of main portal vein, cm | Diameter of the main portal vein, which is measured at least 1 cm distal to the confluence of splenic and superior mesenteric vein and at least 1 cm proximal to the first branch of the main portal vein, to avoid the effect of convergence/divergence, on coronal images [32] |
| Collateral circulation | Enhancing tortuous channels in esophageal, epigastric, perisplenic, paraumbilical, or retroperitoneal locations [33] |
| Gastroesophageal varices | Discrete enhancing tortuous channel abutting the luminal surface of the esophageal or gastric wall or contacting/protruding into luminal space [33] |
| Splenomegaly | Length > 13 cm [33] |
| Ascites | Presence of free fluid in the abdomen or pelvis [33] |
AP, arterial phase; APHE, arterial phase hyperenhancement; CT, computed tomography; HCC, hepatocellular carcinoma; HBP, hepatobiliary phase; LI-RADS/LR, Liver Imaging Reporting and Data System; MRI, magnetic resonance imaging; TP, transitional phase
Follow-up
OS was defined as the time interval from hepatectomy to death from any cause, and patients who were alive were censored at the date of the last follow-up. Follow-up ended on June 15, 2022, for institution 1 and July 30, 2021, for institution 2.
Statistical analysis
Categorical variables were compared using the chi-square test or Fisher's exact test, while continuous variables were compared by the Student’s t test or Mann–Whitney U test, as appropriate. Interobserver agreement was assessed by computing Cohen’s κ statistics for binary features, weighted κ statistics for categorical features, and intraclass correlation coefficient for continuous variables, respectively.
Propensity score matching
Propensity score matching was performed to minimize the effects of potential confounders and selection bias between the training and internal validation cohorts [34]. The propensity score was estimated by logistic regression, with covariates including sex, age, liver cirrhosis, BCLC stage, and mortality as independent variables, and the type of study cohorts (training vs. internal validation cohorts) as the dependent variable for model fitting. Enrolled patients were matched using 1:1 optimal pair matching. The standardized mean difference was calculated to assess the covariate balance between the two matched cohorts, with a goal-to-achieve value < 0.15. For the matched data, categorical variables were compared using the McNemar test, whilst continuous variables were compared using the paired t test or Wilcoxon signed-rank test, when applicable.
Development and validation of the risk score
Univariable Cox regression analysis was performed to identify potential predictors in the training cohort, whilst adjusting for patients’ sex and age. To develop an easy-to-apply score, continuous variables were transformed into binary variables as per normal ranges of laboratory indexes or clinical relevance. Spearman’s correlation coefficient was calculated to investigate the collinearity between variables; when collinearity was encountered, variables with the largest hazard ratios in the univariable analysis were selected for further analysis.
Variables with a p < 0.01 at univariable analysis were entered into the multivariable Cox regression model; the final model was selected using the backward stepwise approach with Akaike Information Criterion and five-fold cross-validation. A risk score was constructed based on the final multivariable Cox regression model. The effect of the variable with the highest β-coefficient was assigned 20 points, and all scaled β-coefficients were rounded to the nearest integer. Harrell’s concordance index (C-index) was used to measure the discrimination of the risk score [35], and the calibration curve was drawn to assess model calibration [36].
Survival analysis
OS was calculated by the Kaplan-Meier method and compared by the log-rank test, with a false discovery rate-adjusted approach applied [37]. To classify patients into high- and low-risk survival groups, the optimal cutoff value of the risk score was determined by X-tile software (version 3.6.1). Subgroup analyses were performed based on six available clinical-radiological-pathological variables known to affect HCC prognosis, including tumor size, tumor-in-vein, microvascular invasion (MVI), tumor differentiation, liver cirrhosis, and the ALBI grade. The prognostic value of the risk score was also assessed in subgroups of patients undergoing resection within (stage 0-A) and beyond (stage B-C) BCLC criteria. To ensure an adequate number of patients for each subgroup, the training and internal validation cohorts were used for subgroup analyses.
Statistical analyses were performed using R software (version 3.5.1; The R Foundation for Statistical Computing) and SPSS Statistics (version 26.0; IBM). A two-tailed p < 0.05 indicated a statistically significant difference.
Results
Patient characteristics
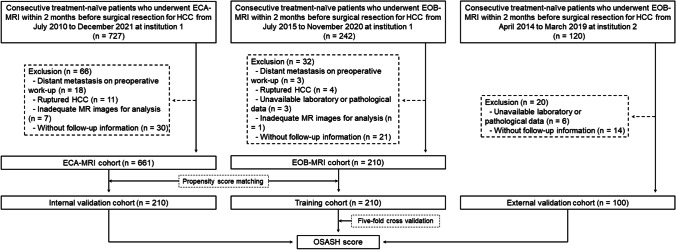
At institution 1, a total of 210 patients (mean age ± standard deviation, 52.1 ± 11.6 years; 173 men) in the “EOB-MRI cohort” constituted the training cohort. Given that no HBP imaging features were included in the final prognostic model, we matched 210 patients (mean age ± standard deviation, 53.5 ± 11.0 years; 178 men) in the “ECA-MRI cohort” for internal validation, with an adequate balance of all matching variables (Table 2 and S2). At institution 2, a total of 100 patients (mean age ± standard deviation, 56.2 ± 10.0 years; 81 men) were included and constituted the external validation cohort (Fig. 1).
Table 2.
Baseline patient characteristics and MRI features
| Characteristic | Training cohort (n = 210) | Internal validation cohort (n = 210) | p valuea | External validation cohort (n = 100) | p valueb |
|---|---|---|---|---|---|
| Age (y)† | 52.1 ± 11.6 | 53.5 ± 11.0 | 0.180# | 56.2 ± 10.0 | 0.002 |
| Sex | 0.598# | 0.768 | |||
| Female | 37 (17.6) | 32 (15.2) | 19 (19.0) | ||
| Male | 173 (82.4) | 178 (84.8) | 81 (81.0) | ||
| Cause of liver disease | 0.040 | 0.208 | |||
| HBV | 194 (92.4) | 181 (86.2) | 88 (88.0) | ||
| Others | 16 (7.6) | 29 (13.8) | 12 (12.0) | ||
| Cirrhosis | 104 (49.5) | 101 (48.1) | 0.845# | 70 (70.0) | 0.001 |
| Child–Pugh class | 0.200 | < 0.001 | |||
| A | 207 (98.6) | 203 (96.7) | 85 (85.0) | ||
| B | 3 (1.4) | 7 (3.3) | 15 (15.0) | ||
| ALBI grade | 0.103 | < 0.001 | |||
| 1 | 170 (81.0) | 155 (73.8) | 30 (30.0) | ||
| 2 | 40 (19.0) | 54 (25.7) | 68 (68.0) | ||
| 3 | 0 (0.0) | 1 (0.5) | 2 (2.0) | ||
| Laboratory index | |||||
| AST, IU/L‡ | 35.0 (27.0–48.0) | 34.0 (26.8–50.0) | 0.966 | 34.0 (26.2–50.7) | 0.980 |
| ALT, IU/L‡ | 37.0 (24.0–53.3) | 35.5 (23.0–57.3) | 0.899 | 41.0 (30.0–72.4) | 0.002 |
| TBIL, umol/L‡ | 13.5 (10.8–17.5) | 13.4 (9.8–17.4) | 0.282 | 14.9 (10.5–20.0) | 0.116 |
| ALB, g/L‡ | 42.9 (40.4–45.9) | 42.7 (39.4–45.2) | 0.230 | 37.6 (34.0–39.9) | < 0.001 |
| PLT, × 10^9/L‡ | 137.5 (98.5–183.0) | 126.5 (94.0–176.3) | 0.182 | 124.0 (89.5–154.3) | 0.034 |
| PT, seconds‡ | 11.9 (11.1–12.6) | 11.8 (11.2–12.5) | 0.786 | 13.5 (12.5–14.9) | < 0.001 |
| INR‡,§ | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.438 | NA (NA–NA) | … |
| ALP, IU/L‡,§ | 90.0 (72.0–115.0) | 88.0 (69.0–115.3) | 0.858 | NA (NA–NA) | … |
| GGT, IU/L‡,§ | 49.5 (28.8–86.3) | 52.5 (30.0–117.0) | 0.103 | NA (NA–NA) | … |
| AFP, ng/mL | 0.601 | 0.001 | |||
| ≤ 400 | 145 (69.0) | 140 (66.7) | 87 (87.0) | ||
| > 400 | 65 (31.0) | 70 (33.3) | 13 (13.0) | ||
| BCLC stage | 0.852# | 0.702 | |||
| 0 | 29 (13.8) | 28 (13.3) | 13 (13.0) | ||
| A | 101 (48.1) | 101 (48.1) | 48 (48.0) | ||
| B | 34 (16.2) | 40 (19.0) | 21 (21.0) | ||
| C | 46 (21.9) | 41 (19.5) | 18 (18.0) | ||
| MVI | 96 (45.7) | 90 (42.9) | 0.556 | 53 (53.0) | 0.230 |
| Tumor differentiation | 0.133 | < 0.001 | |||
| Well or Moderate | 136 (64.8) | 121 (57.6) | 84 (84.0) | ||
| Poor or undifferentiated | 74 (35.2) | 89 (42.4) | 16 (16.0) | ||
| Follow-up, months‡ | 48.1 (24.5–66.7) | 51.3 (35.4–72.8) | < 0.001* | 35.5 (33.3–49.2) | 0.091* |
| No. of death | 42 (20.0) | 41 (19.5) | 1.000# | 20 (20.0) | 1.000 |
| MRI features | |||||
| Tumor burden-related features | |||||
| Tumor multiplicity | 0.080 | 0.557 | |||
| 1 | 134 (63.8) | 147 (70.0) | 58 (58.0) | ||
| 2 or 3 | 43 (20.5) | 45 (21.4) | 22 (22.0) | ||
| ≥ 4 | 33 (15.7) | 18 (8.6) | 20 (20.0) | ||
| Tumor size, cm‡ | 4.1 (2.4–6.9) | 4.2 (2.8–7.1) | 0.411 | 3.4 (2.2–4.9) | 0.022 |
| Bilobar involvement | 29 (13.8) | 20 (9.5) | 0.171 | 28 (28.0) | 0.003 |
| LI-RADS major features | |||||
| Nonrim APHE | 199 (94.8) | 194 (92.4) | 0.320 | 85 (85.0) | 0.004 |
| Nonperipheral "washout" | 194 (92.4) | 148 (70.5) | < 0.001 | 91 (91.0) | 0.676 |
| Enhancing "capsule" | 139 (66.2) | 170 (81.0) | 0.001 | 55 (55.0) | 0.057 |
| LI-RADS ancillary features | |||||
| Favoring malignancy in general, not HCC in particular | |||||
| Corona enhancement | 81 (38.6) | 102 (48.6) | 0.039 | 38 (38.0) | 0.923 |
| Fat sparing in solid mass§§§ | 9 (4.3) | 8 (3.8) | 0.804 | NA (NA) | … |
| Diffusion restriction | 210 (100.0) | 210 (100.0) | … | 100 (100.0) | … |
| Mild-moderate T2 hyperintensity | 206 (98.1) | 209 (99.5) | 0.372 | 93 (93.0) | 0.053 |
| Iron sparing in solid mass | 26 (12.4) | 29 (13.8) | 0.664 | 33 (33.0) | < 0.001 |
| TP hypointensity§§ | 205 (97.6) | NA (NA) | … | 95 (95.0) | 0.381 |
| HBP hypointensity§§ | 204 (97.1) | NA (NA) | … | 98 (98.0) | 0.951 |
| Favoring HCC in particular | |||||
| Nonenhancing "capsule" | 40 (19.0) | 1 (0.5) | < 0.001 | 1 (1.0) | < 0.001 |
| Nodule-in-nodule | 74 (35.2) | 87 (41.4) | 0.192 | 18 (18.0) | 0.002 |
| Mosaic architecture | 92 (43.8) | 70 (33.3) | 0.027 | 29 (29.0) | 0.012 |
| Fat in mass, more than adjacent liver§§§ | 82 (39.0) | 79 (37.6) | 0.763 | NA (NA) | … |
| Blood products in mass | 79 (37.6) | 81 (38.6) | 0.841 | 30 (30.0) | 0.189 |
| Favoring benignity | |||||
| Iron in mass, more than liver | 3 (1.4) | 0 (0.0) | 0.248 | 4 (4.0) | 0.218 |
| Marked T2 hyperintensity | 3 (1.4) | 3 (1.4) | 1.000 | 5 (5.0) | 0.141 |
| HBP isointensity§§ | 4 (1.9) | NA (NA) | … | 2 (2.0) | 1.000 |
| Tumor in vein | 44 (21.0) | 41 (19.5) | 0.716 | 15 (15.0) | 0.212 |
| LR-M features | |||||
| Targetoid appearances | |||||
| Rim APHE | 10 (4.8) | 11 (5.2) | 0.823 | 12 (12.0) | 0.020 |
| Peripheral "washout" | 1 (0.5) | 0 (0.0) | 1.000 | 2 (2.0) | 0.244 |
| Delayed central enhancement | 2 (1.0) | 11 (5.2) | 0.011 | 6 (6.0) | 0.025 |
| Targetoid restriction | 0 (0.0) | 8 (3.8) | 0.007 | 3 (3.0) | 0.033 |
| Targetoid TP or HBP appearance§§ | 2 (1.0) | NA (NA) | … | 0 (0.0) | 1.000 |
| Nontargetoid features | |||||
| Infiltrative appearance | 42 (20.0) | 42 (20.0) | 1.000 | 22 (22.0) | 0.684 |
| Marked diffusion restriction | 94 (44.8) | 34 (16.2) | < 0.001 | 53 (53.0) | 0.174 |
| Necrosis or severe ischemia | 72 (34.3) | 95 (45.2) | 0.022 | 36 (36.0) | 0.767 |
| LIRADS category | 0.071 | 0.002 | |||
| LR-3 | 1 (0.5) | 0 (0.0) | 2 (2.0) | ||
| LR-4 | 12 (5.7) | 22 (10.5) | 3 (3.0) | ||
| LR-5 | 185 (88.1) | 169 (80.5) | 77 (77.0) | ||
| LR-M | 12 (5.7) | 19 (9.0) | 18 (18.0) | ||
| Other tumor-related prognostic features | |||||
| Intratumoral artery | 71 (33.8) | 77 (36.7) | 0.540 | 25 (25.0) | 0.117 |
| Incomplete tumor "capsule" | 138 (65.7) | 164 (78.1) | 0.005 | 79 (79.0) | 0.017 |
| Nonsmooth tumor margin | 131 (62.4) | 166 (79.0) | < 0.001 | 95 (95.0) | < 0.001 |
| Marked HBP hypointensity§§ | 172 (81.9) | NA (NA) | … | 92 (92.0) | 0.019 |
| HBP peritumoral hypointensity§§ | 79 (37.6) | NA (NA) | … | 20 (20.0) | 0.002 |
| HBP hypointense nodule without APHE§§ | 73 (34.8) | NA (NA) | … | 25 (25.0) | 0.084 |
| Underlying liver disease-related features | |||||
| Radiological cirrhosis | 94 (44.8) | 128 (61.0) | 0.001 | 63 (63.0) | 0.003 |
| Diffuse fatty change§§§ | 19 (9.0) | 13 (6.2) | 0.270 | NA (NA) | < 0.001 |
| Diffuse iron overload | 52 (24.8) | 39 (18.6) | 0.124 | 35 (35.0) | 0.061 |
| Width of main portal vein, cm‡ | 1.5 (1.3–1.6) | 1.5 (1.3–1.6) | 0.207 | 1.5 (1.3–1.6) | 0.570 |
| Collateral circulation | 112 (53.3) | 127 (60.5) | 0.139 | 59 (59.0) | 0.348 |
| Gastroesophageal varices | 52 (24.8) | 113 (53.8) | < 0.001 | 59 (59.0) | < 0.001 |
| Splenomegaly | 85 (40.5) | 94 (44.8) | 0.375 | 43 (43.0) | 0.673 |
| Ascites | 0 (0.0) | 20 (9.5) | < 0.001 | 9 (9.0) | < 0.001 |
Unless indicated otherwise, data are the number of patients, with percentages in parentheses. Group comparisons were performed with the Student’s t test or Mann–Whitney U test for continuous variables and chi-square or Fisher's exact test for categorical variables, as appropriate
#Group comparisons were performed with the paired t test or Wilcoxon signed rank test for continuous variables and McNemar test for categorical variables, as appropriate. *Group comparisons were performed with the log-rank test
aTraining vs. internal validation cohorts. bTraining vs. external validation cohorts
†Data are means ± standard deviations. ‡Data are medians, with interquartile range in parentheses. §Data were unavailable in the external validation cohort. §§Data were unavailable in the internal validation cohort owing to the lack of TP and HBP images on ECA-MRI. §§§Data were unavailable in the external validation cohort owing to the lack of T1-weighted in- and opposed-phase images
AFP, alpha-fetoprotein; ALB, albumin; ALBI, albumin-bilirubin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APHE, arterial phase hyperenhancement; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; GGT, gamma-glutamyl transferase; HBP, hepatobiliary phase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; INR, international normalized ratio; LI-RADS/LR, Liver Imaging Reporting and Data System; MRI, magnetic resonance imaging; MVI, microvascular invasion; NA, not available; PLT, platelet; PT, prothrombin time; TBIL, total bilirubin; TP, transitional phase
Fig. 1.
Flowchart of patient selection. EOB-MRI, gadoxetate disodium-enhanced magnetic resonance imaging; ECA-MRI, extracellular contrast agent-enhanced magnetic resonance imaging; HCC, hepatocellular carcinoma; MR, magnetic resonance
Patients from the training cohort were younger than those from the external validation cohort (mean age, 52.1 vs. 56.2 years; p = 0.002), with less frequent cirrhosis (49.5 vs. 70.0%; p = 0.001) and larger tumors (median size, 4.1 vs. 3.4 cm; p = 0.022). The median follow-up period was 48.1 (interquartile range [IQR]: 24.5–66.7), 51.3 (IQR: 35.4–72.8), and 35.5 (IQR: 33.3–49.2) months for the training, internal validation and external validation cohorts, respectively. Baseline patient characteristics and MRI features are summarized in Table 2.
Development and validation of the OSASH score for predicting OS
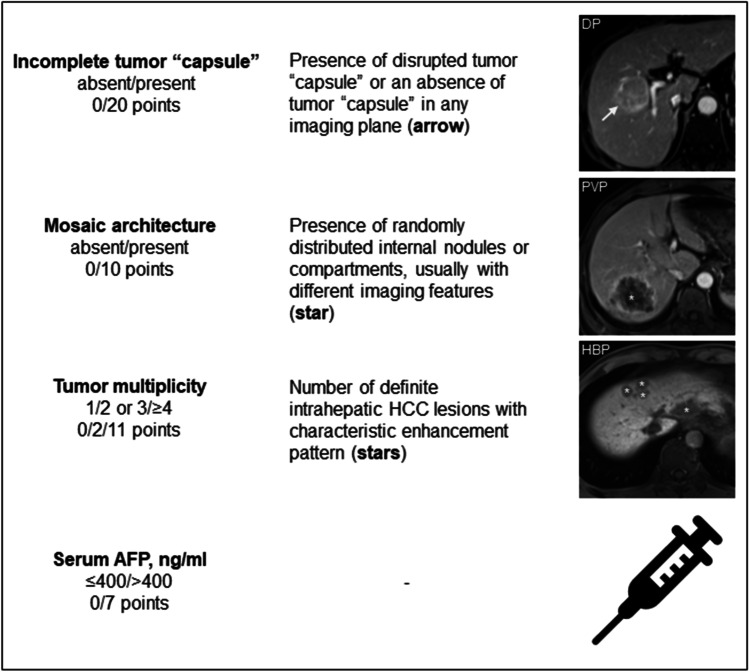
In the training cohort, 11 variables were significantly associated with worse OS at univariable Cox regression analysis, and four of them (i.e., incomplete tumor “capsule”, mosaic architecture, tumor multiplicity, and serum AFP > 400 ng/mL) were included in the final model at multivariable analysis (Table 3). By incorporating these variables, a simplified risk score termed the “OSASH score” (named by incorporating the key letters of overall survival after surgery for HCC) was developed (Fig. 2).
Table 3.
Univariable and multivariable Cox regression analysis of prognostic factors for overall survival in the training cohort
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio (95%CI) | p value | Hazard ratio (95%CI) | β-estimate (95%CI) | p value | Point |
| Age | 0.97 (0.94–0.99) | 0.018 | ||||
| Sex, female vs. male | 0.74 (0.36–1.50) | 0.403 | ||||
| Etiology, HBV vs. non-HBV | 1.06 (0.33–3.43) | 0.923 | ||||
| Cirrhosis, absent vs. present | 0.73 (0.40–1.35) | 0.322 | ||||
| ALBI grade, 1 vs. 2 | 0.65 (0.27–1.55) | 0.331 | ||||
| AST, ≤ 40 vs. > 40 IU/L | 1.95 (1.06–3.57) | 0.031 | ||||
| ALT, ≤ 50 vs. > 50 IU/L | 1.29 (0.67–2.49) | 0.441 | ||||
| TBIL, ≤ 19 vs. > 19 umol/L | 0.65 (0.27–1.55) | 0.333 | ||||
| ALB, ≥ 40 vs. < 40 g/L | 0.73 (0.34–1.57) | 0.414 | ||||
| PLT, ≥ 100 vs. < 100 × 10^9/L | 0.61 (0.27–1.37) | 0.23 | ||||
| PT, ≤ 13 vs. > 13 s | 1.33 (0.61–2.88) | 0.471 | ||||
| INR, ≤ 1.1 vs. > 1.1 | 0.94 (0.43–2.03) | 0.87 | ||||
| ALP, ≤ 160 vs. > 160 IU/L | 1.48 (0.45–4.81) | 0.518 | ||||
| GGT, ≤ 60 vs. > 60 IU/L | 3.10 (1.67–5.75) | < 0.001 | ||||
| AFP, ≤ 400 vs. > 400 ng/mL | 4.10 (2.21–7.60) | < 0.001 | 2.26 (1.18–4.31) | 0.81 (0.17–1.46) | 0.014 | 7 |
| Tumor multiplicity | ||||||
| 1 | Reference | Reference | ||||
| 2 or 3 | 3.24 (1.40–7.47) | 0.006 | 1.26 (0.53–3.04) | 0.23 (–0.64–1.11) | 0.600 | 2 |
| ≥ 4 | 10.11 (4.83–21.13) | < 0.001 | 3.36 (1.51–7.45) | 1.21 (0.41–2.01) | 0.003 | 11 |
| Tumor size† | 1.22 (1.14–1.31) | < 0.001 | ||||
| Enhancing "capsule", absent vs. present | 0.72 (0.39–1.33) | 0.297 | ||||
| Corona enhancement, absent vs. present | 3.67 (1.93–6.97) | < 0.001 | ||||
| Iron sparing in solid mass, absent vs. present | 0.95 (0.37–2.43) | 0.919 | ||||
| Mosaic architecture, absent vs. present† | 6.55 (2.91–14.76) | < 0.001 | 2.99 (1.28–6.95) | 1.09 (0.25–1.94) | 0.011 | 10 |
| Fat in mass, more than adjacent liver, absent vs. present | 0.47 (0.24–0.94) | 0.033 | ||||
| Blood products in mass, absent vs. present | 4.16 (2.16–8.00) | < 0.001 | ||||
| Tumor in vein, absent vs. present | 4.69 (2.56–8.60) | < 0.001 | ||||
| Infiltrative appearance, absent vs. present | 5.69 (3.08–10.53) | < 0.001 | ||||
| Marked diffusion restriction, absent vs. present | 2.05 (1.10–3.82) | 0.024 | ||||
| Necrosis or severe ischemia, absent vs. present | 1.87 (1.02–3.43) | 0.042 | ||||
| LIRADS category, LR-3/4/5 vs. LR-M | 2.45 (0.96–6.25) | 0.060 | ||||
| Bilobar involvement, absent vs. present | 2.23 (1.12–4.43) | 0.023 | ||||
| Intratumoral artery, absent vs. present | 4.15 (2.20–7.80) | < 0.001 | ||||
| Incomplete tumor "capsule", absent vs. present§ | 25.36 (3.49–184.44) | 0.001 | 8.99 (1.16–69.32) | 2.20 (0.15–4.24) | 0.035 | 20 |
| Nonsmooth tumor margin, absent vs. present§ | 13.84 (3.34–57.32) | < 0.001 | ||||
| Marked HBP hypointensity, absent vs. present | 3.11 (0.96–10.08) | 0.058 | ||||
| HBP peritumoral hypointensity, absent vs. present | 3.50 (1.84–6.66) | < 0.001 | ||||
| HBP hypointense nodule without APHE | 1.12 (0.60–2.09) | 0.725 | ||||
| Radiological cirrhosis, absent vs. present | 0.76 (0.41–1.41) | 0.379 | ||||
| Diffuse iron overload, absent vs. present | 0.49 (0.19–1.24) | 0.131 | ||||
| Width of main portal vein, cm | 0.34 (0.08–1.48) | 0.149 | ||||
| Collateral circulation, absent vs. present | 1.33 (0.72–2.46) | 0.368 | ||||
| Gastroesophageal varices, absent vs. present | 1.34 (0.70–2.58) | 0.383 | ||||
| Splenomegaly, absent vs. present | 0.94 (0.50–1.75) | 0.850 | ||||
The OSASH score for an individual patient could be calculated by the following formula:
OSASH score = Incomplete tumor “capsule” (absent = 0; present = 20) + Mosaic architecture (absent = 0; present = 10) + Tumor multiplicity (solitary = 0; two or three = 2; four or more = 11) + AFP (≤ 400 ng/mL = 0; > 400 ng/mL = 7)
†Due to significant collinearity (rs = 0.715, p < 0.001), “mosaic architecture” was entered into the multivariable Cox regression model owing to the largest hazard ratio. §Due to significant collinearity (rs = 0.744, p < 0.001), "incomplete tumor “capsule” was entered into the multivariable Cox regression model owing to the largest hazard ratio
AFP, alpha-fetoprotein; ALB, albumin; ALBI, albumin-bilirubin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APHE, arterial phase hyperenhancement; AST, aspartate aminotransferase; CI, confidence interval; GGT, gamma-glutamyl transferase; HBP, hepatobiliary phase; HBV, hepatitis B virus; INR, international normalized ratio; LI-RADS/LR, Liver Imaging Reporting and Data System; MVI, microvascular invasion; PLT, platelet; PT, prothrombin time; TBIL, total bilirubin
Fig. 2.
Graphical illustration of the OSASH score. AFP, alpha-fetoprotein; DP, delayed phase; HBP, hepatobiliary phase; HCC, hepatocellular carcinoma; PVP, portal venous phase
The C-index of the OSASH score for predicting OS was 0.85 (95% confidence interval [CI]: 0.78–0.91), 0.81 (95% CI: 0.75–0.88), and 0.62 (95% CI: 0.49–0.75) in the training, internal validation and external validation cohorts, respectively. The calibration plots showed an overall good agreement between the score-predicted risk of death and the observed incidence of death in all cohorts (Figure S1). Interobserver agreement for the OSASH score was presented in Table S3.
Survival risk stratification based on the OSASH score
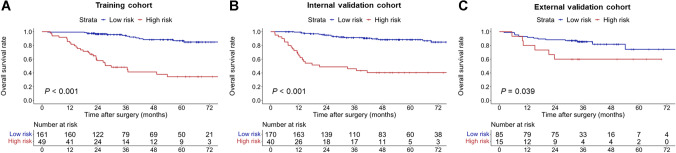
Using 32 as the cutoff point for the OSASH score derived from the training cohort (Table 4; Fig. 3A), patients in the internal validation cohort were divided into prognostically distinct low and high-risk groups (5-year OS rates, 88.5% vs. 40.5%; p < 0.001) (Table 4; Fig. 3B). Similar results were also obtained in the external validation cohort (5-year OS rates, 74.3% vs. 60.0%; p = 0.039) (Table 4; Fig. 3C).
Table 4.
Median OS, 3- and 5-year OS rates, and hazard ratios for OSASH score risk subclasses in all cohorts
| Cohort and risk group | No | Median OS, months (95%CI) | 3-year OS rate, % (95%CI) | 5-year OS rate, % (95%CI) | Hazard Ratio (95%CI) | p value |
|---|---|---|---|---|---|---|
| Training cohort | < 0.001 | |||||
| Low risk | 161 | NA (NA–NA) | 94.6 (90.6–98.7) | 86.9 (80.1–94.2) | Reference | |
| High risk | 49 | 29.7 (23.3–NA) | 48.3 (35.7–65.4) | 34.5 (22.0–54.0) | 9.16 (4.81–17.45) | |
| Internal validation cohort | < 0.001 | |||||
| Low risk | 170 | NA (NA–NA) | 91.4 (87.0–96.0) | 88.5 (83.2–94.1) | Reference | |
| High risk | 40 | 21.0 (12.6–NA) | 46.1 (32.7–64.9) | 40.5 (27.5–59.6) | 8.36 (4.49–15.56) | |
| External validation cohort | 0.039 | |||||
| Low risk | 85 | NA (NA–NA) | 85.5 (78.2–93.5) | 74.3 (59.4–93.1) | Reference | |
| High risk | 15 | NA (21.3–NA) | 60.0 (39.7–90.7) | 60.0 (39.7–90.7) | 2.64 (1.01–6.90) | |
Captions:
CI, confidence interval; NA, not available; OS, overall survival
Fig. 3.
Kaplan-Meier curves demonstrating differences in OS between the OSASH-low and OSASH-high risk patients with HCC in the (A) training, (B) internal validation, and (C) external validation cohorts. HCC, hepatocellular carcinoma; OS, overall survival
The OSASH score predicted OS in six subgroups
In each subgroup, as mentioned above, OSASH-low-risk patients had significantly longer OS than that of OSASH-high risk patients in both the training (Table 5; Figure S2) and internal validation (Table 5; Figure S3) cohorts (all p < 0.05).
Table 5.
5-year OS rates and hazard ratios for OSASH score risk subclasses in six subgroups in the training and internal validation cohorts
| Variable and risk group | Training cohort (n = 210) | Internal validation cohort (n = 210) | ||||||
|---|---|---|---|---|---|---|---|---|
| No | 5-year OS rate, % (95%CI) | Hazard ratio (95%CI) | p value | No | 5-year OS rate, % (95%CI) | Hazard Ratio (95%CI) | p value | |
| Tumor diameter, ≤ 5 cm | < 0.001 | < 0.001 | ||||||
| Low risk | 114 | 88.4 (80.0–97.7) | Reference | 119 | 93.5 (89.0–98.3) | Reference | ||
| High risk | 9 | 44.4 (17.6–100.0) | 8.94 (2.60–30.65) | 5 | 40.0 (13.7–100.0) | 12.98 (3.35–50.31) | ||
| Tumor diameter, > 5 cm | < 0.001 | < 0.001 | ||||||
| Low risk | 47 | 83.7 (72.4–96.8) | Reference | 51 | 74.5 (60.9–91.2) | Reference | ||
| High risk | 40 | 33.0 (19.9–54.6) | 6.16 (2.64–14.38) | 35 | 40.9 (27.2–61.6) | 4.13 (1.92–8.86) | ||
| Tumor-in-vein, absent | < 0.001 | 0.013 | ||||||
| Low risk | 147 | 89.5 (83.1–96.5) | Reference | 151 | 93.4 (89.0–98.0) | Reference | ||
| High risk | 19 | 34.6 (17.5–68.6) | 9.54 (4.05–22.49) | 18 | 76.2 (58.2–99.7) | 3.93 (1.23–12.62) | ||
| Tumor-in-vein, present | 0.040 | < 0.001 | ||||||
| Low risk | 14 | 67.5 (45.4–100.0) | Reference | 19 | 52.5 (32.8–84.3) | Reference | ||
| High risk | 30 | 38.3 (23.5–62.5) | 2.99 (1.00–8.96) | 22 | 13.6 (4.8–39.0) | 4.16 (1.80–9.62) | ||
| MVI, absent | 0.002 | < 0.001 | ||||||
| Low risk | 108 | 92.7 (86.6–99.3) | Reference | 113 | 95.0 (90.7–99.4) | Reference | ||
| High risk | 6 | 62.5 (32.0–100.0) | 8.86 (1.71–45.96) | 7 | 57.1 (30.1–100.0) | 9.95 (2.47–40.12) | ||
| MVI, present | < 0.001 | < 0.001 | ||||||
| Low risk | 53 | 74.3 (59.4–92.9) | Reference | 57 | 73.1 (59.9–89.3) | Reference | ||
| High risk | 43 | 31.0 (18.4–52.2) | 4.84 (2.26–10.34) | 33 | 36.8 (23.1–58.6) | 4.18 (2.03–8.62) | ||
| Well or moderate tumor differentiation | < 0.001 | < 0.001 | ||||||
| Low risk | 116 | 91.5 (84.9–98.6) | Reference | 108 | 89.8 (83.6–96.5) | Reference | ||
| High risk | 20 | 36.3 (17.5–75.1) | 11.55 (4.18–31.87) | 13 | 44.0 (23.3–83.0) | 8.75 (3.37–22.72) | ||
| Poor tumor differentiation | < 0.001 | < 0.001 | ||||||
| Low risk | 45 | 76.8 (62.4–94.5) | Reference | 62 | 86.3 (77.3–96.5) | Reference | ||
| High risk | 29 | 32.5 (18.3–57.9) | 5.39 (2.33–12.48) | 27 | 39.1 (24.1–63.4) | 7.42 (3.04–18.10) | ||
| Cirrhosis, absent | < 0.001 | < 0.001 | ||||||
| Low risk | 75 | 83.5 (73.2–95.2) | Reference | 94 | 89.0 (82.0–96.7) | Reference | ||
| High risk | 31 | 36.6 (20.2–66.2) | 6.83 (2.90–16.09) | 15 | 59.3 (38.7–90.7) | 5.31 (1.88–14.97) | ||
| Cirrhosis, present | < 0.001 | < 0.001 | ||||||
| Low risk | 86 | 89.8 (81.2–99.3) | Reference | 76 | 88.0 (80.6–96.2) | Reference | ||
| High risk | 18 | 30.9 15.1–63.1) | 12.33 (4.62–32.96) | 25 | 29.5 (15.7–55.5) | 10.01 (4.30–23.30) | ||
| ALBI grade, 1 | < 0.001 | < 0.001 | ||||||
| Low risk | 130 | 85.5 (77.4–94.4) | Reference | 128 | 89.8 (84.2–95.9) | Reference | ||
| High risk | 40 | 32.2 (19.1–54.0) | 8.79 (4.39–17.62) | 27 | 51.2 (35.2–74.3) | 7.46 (3.34–16.69) | ||
| ALBI grade, 2 or 3 | 0.001 | < 0.001 | ||||||
| Low risk | 31 | 91.4 (80.3–100.0) | Reference | 42 | 84.6 (72.9–98.2) | Reference | ||
| High risk | 9 | 46.7 (21.0–100.0) | 10.38 (1.86–57.76) | 13 | 17.3 (4.9–60.6) | 12.77 (4.27–38.20) | ||
ALBI, albumin-bilirubin; CI, confidence interval; HCC, hepatocellular carcinoma; MVI, microvascular invasion; NA, not available; OS, overall survival
Prognostic impact of the OSASH score in patients across different BCLC stages
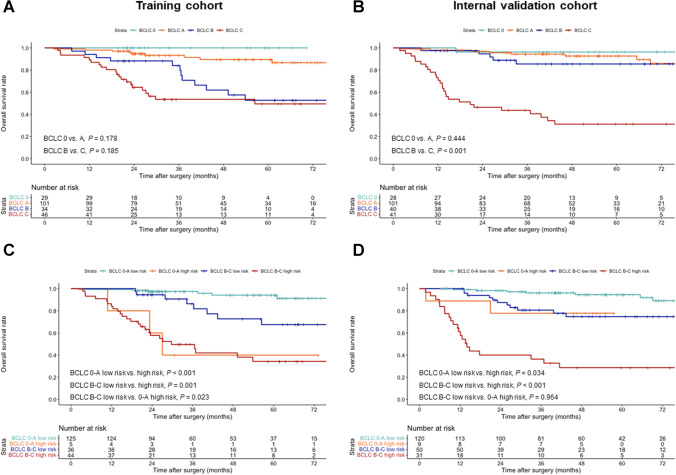
Survival risk stratification based on the BCLC algorithm
In the training cohort, comparable outcomes were obtained for patients with BCLC stage 0 and A HCC (5-year OS rates, 100.0% vs. 89.6%; p = 0.178) and for patients with BCLC stage B and C HCC (5-year OS rates, 52.7% vs. 49.4%; p = 0.185) (Table S4; Fig. 4A). In the internal validation cohort, patients with BCLC stage 0 and A HCC had similar outcomes (5-year OS rates, 96.3% vs. 92.7%; p = 0.444), whereas patients with BCLC stage B HCC had significantly longer OS than that of those with BCLC stage C HCC (5-year OS rates, 85.5% vs. 31.2%; p < 0.001) (Table S4; Fig. 4B).
Fig. 4.
Kaplan-Meier curves according to (A, B) BCLC stage and (C, D) the OSASH score combined with BCLC stage subgroups in the training and internal validation cohorts. BCLC, Barcelona Clinic Liver Cancer
Incremental prognostic value of the OSASH score to the BCLC algorithm
To further assess the incremental value of the OSASH score to the BCLC algorithm, subgroup analyses were performed in patients with BCLC stage 0-A and B-C HCC. In both the training and internal validation cohorts, OSASH-low-risk patients achieved significantly longer OS than that of OSASH-high-risk patients in either patients with BCLC stage 0-A HCC (internal validation cohort: 5-year OS rates, 94.6% vs. 77.8%; p = 0.034) or with BCLC stage B-C HCC (internal validation cohort: 5-year OS rates, 74.7% vs. 28.7%; p < 0.001) (Table S4; Fig. 4C and D).
Furthermore, in the training cohort, patients with BCLC stage B-C HCC and OSASH-low risk had significantly longer OS than those with BCLC stage 0-A HCC and OSASH-high risk (5-year OS rates, 67.5% vs. 40.0%; p = 0.023) (Fig. 4C). In the internal validation cohort, patients with BCLC stage B-C HCC and OSASH-low risk achieved comparable outcomes to that of those with BCLC stage 0-A HCC and OSASH-high risk (5-year OS rates, 74.7% vs. 77.8%; p = 0.964) (Fig. 4D and S4).
Discussion
In this large dual-institutional cohort study, by integrating three MRI features and serum AFP, we developed and validated a preoperative risk score for the prediction of OS in HCC patients undergoing hepatectomy. The score displayed similarly good prognostic performance in the training and internal validation cohorts but a reduced performance in the external validation cohort. It was capable of stratifying patients into 2 prognostically distinct risk strata among all study cohorts and six subgroups. This was clinically relevant because it may allow the identification of a small portion of patients at high risk of death, for whom more intensive surveillance could be considered, and adjuvant therapies might provide survival benefit. Furthermore, among patients with BCLC stage B and C HCC without extrahepatic metastasis, this score identified a subgroup of low-risk patients who achieved favorable prognoses after resection, suggesting its potential value to complement traditional staging systems for OS prediction.
In the present study, 38.5% (200/520) of surgical patients had intermediate- to advanced-stage HCC. Despite representing a marked deviation from the current BCLC recommendations [3], our study population captured the context of real-world clinical practice of high-volume tertiary care centers in China, where a proportion of patients underwent hepatectomy for intermediate- to advanced-stage HCC according to the multidisciplinary team recommendations and patients’ preferences [7]. However, patients with intermediate- to advanced-stage HCC harbor substantial tumor heterogeneity, hence a preoperative patient selection is critical to identify the optimal surgical candidates. In our study, patients with BCLC stage B-C HCC and OSASH-low risk might be potential surgical candidates because these patients showed 5-year OS rates approaching 70%, which were similar to patients with BCLC 0-A HCC and OSASH-high risk. However, future prospective multi-institutional studies are required to test the reliability and reproducibility of our findings.
To date, EOB-MRI has been more commonly used in published prognostic researches, mainly because it can additionally provide HBP imaging features for analysis, of which some (e.g., HBP peritumoral hypointensity) have been closely linked to HCC prognosis [20, 23, 38]. Therefore, in the current study, to comprehensively explore the prognostic value of all available imaging features, the risk score was initially developed in an EOB-MRI cohort (the training cohort). However, our results showed that no HBP imaging features were independently associated with OS, which motivated us to test the reproducibility of our findings in a propensity score-matched ECA-MRI cohort. It turned out that the ECA-MRI cohort showed comparably discriminatory power as the EOB-MRI cohort. These preliminary observations suggest that the risk score was applicable for both EOB-MRI and ECA-MRI to help predict OS for HCC patients undergoing surgical resection.
The OSASH score was constructed with 4 variables profiling the tumor burden (tumor multiplicity) and biology (incomplete tumor “capsule,” mosaic architecture, and AFP). The mechanisms underlying these clinic-radiological alterations are still in research. The presence of an incomplete tumor “capsule” often indicates infiltrative tumor growth and poorer survival [39]. Previous studies have identified incomplete tumor “capsule” as an imaging marker for predicting MVI [40], postoperative extrahepatic metastasis [41], and high BRAF and RAF1 expression in HCC [42], and the latter could accelerate tumor proliferation and differentiation and promote tumor invasion and metastasis. Mosaic architecture refers to the presence of randomly distributed internal nodules or components, usually with different imaging features in terms of enhancement, intensity, shape, and size [27]. Histopathologically, it corresponds to the appearance of different foci of clonal expansion at various stages of hepatocarcinogenesis, of which some may comprise fat metamorphosis, necrosis, blood products, cystic degeneration, and fibrosis septa [43]. Therefore, mosaic architecture is regarded as an imaging marker of tumor heterogeneity at the histological level, while the latter is a critical prognostic element. Serum AFP > 400 ng/mL also denoted a worse OS in our study, keeping in line with previous reports [44, 45]. AFP can promote tumor growth partly by the inhibition of apoptosis; besides, it was also associated with the upregulation of vascular endothelial growth factor signaling, thereby promoting tumor angiogenesis and metastasis [46].
This study had several limitations. Firstly, there might have been unavoidable selection bias owing to the retrospective design. Particularly, our patients were enrolled over a time span of 11 years, which could be a potential source of bias given the evolutions in MRI and surgical techniques. Apart from that, there was a time difference of 1 year in the last follow-up time between the two institutions, which might have impacted our results. Thus, further prospective multicenter studies are warranted to validate the presented findings. Secondly, the OSASH score did not achieve a good prognostic performance in the external validation cohort, which might have been due to the relatively small external sample size and the substantial heterogeneities in the study population, MRI parameters, and surgical techniques between the two institutions. Therefore, future large-scale studies are needed to verify the generalizability of the OSASH score in different populations. Thirdly, due to the smaller sample size in the external validation cohort, it was impossible to conduct further survival analyses to examine whether OSASH-low-risk patients had better outcomes than OSASH-high-risk patients in different subgroups, as shown in the internal validation cohort. Hence, the predictive ability of the OSASH score in various subgroups requires to be further externally validated using another larger cohort.
In conclusion, by incorporating three preoperative MRI features and serum AFP, we developed and validated the OSASH score for the prediction of postsurgical OS in HCC patients, which identified a subgroup of low-risk patients with BCLC stage B and C HCC who achieved favorable prognosis after resection. Future multicenter prospective studies with rigorous design are needed to validate our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Jeong Hee Yoon from the Department of Radiology of Seoul National University Hospital and Seoul National University College of Medicine for assistance with the manuscript editing, and the Clinical Research Management Department of West China Hospital for assistance with the study design and implementation.
Abbreviations
- AFP
Alpha-fetoprotein
- ALBI
Albumin-bilirubin
- BCLC
Barcelona Clinic Liver Cancer
- C-index
Concordance index
- CI
Confidence interval
- ECA-MRI
Extracellular contrast agent-enhanced magnetic resonance imaging
- EOB-MRI
Gadoxetate disodium-enhanced magnetic resonance imaging
- HBP
Hepatobiliary phase
- HCC
Hepatocellular carcinoma
- INR
International normalized ratio
- IQR
Interquartile range
- LI-RADS
Liver Imaging Reporting and Data System
- MR
Magnetic resonance
- MRI
Magnetic resonance imaging
- MVI
Microvascular invasion
- OS
Overall survival
- PT
Prothrombin time
Funding
This study has received funding from the National Natural Science Foundation of China (No. 81971571, 82101997), the Science and Technology Support Program of Sichuan Province (No. 2021YFS0141, 2021YFS0021), and the Henan provincial science and technology research project (No. 212102310689).
Declarations
Guarantor
The scientific guarantor of this publication is Bin Song, M.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One co-author (Yuanan Wu, Big Data Research Center, University of Electronic Science and Technology of China, Chengdu, Sichuan, China) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects (35%, 184/520) have been previously reported in another study. The prior work proposed an MRI-based risk score for prediction of HCC recurrence after hepatectomy, while the current work aimed to develop a risk score based on preoperative clinical-radiological features for prediction of overall survival and to investigate its utility in identifying patients with intermediate- to advanced-stage HCC who would potentially benefit from liver resection.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong Wei, Fangfang Fu, and Hanyu Jiang contributed equally to this work.
Contributor Information
Meiyun Wang, Email: mywang@ha.edu.cn.
Bin Song, Email: songlab_radiology@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A et al (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7:6 [DOI] [PubMed]
- 3.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–847. doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 6.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700.e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Sun H, Wang Z et al (2020) guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer 9:682–720 [DOI] [PMC free article] [PubMed]
- 8.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 9.Lee DW, Yim HJ, Seo YS, et al. Prognostic assessment using a new substaging system for Barcelona clinic liver cancer stage C hepatocellular carcinoma: a nationwide study. Liver Int. 2019;39:1109–1119. doi: 10.1111/liv.14117. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182-236. [DOI] [PubMed]
- 11.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 12.Famularo S, Donadon M, Cipriani F, et al. Hepatectomy versus sorafenib in advanced nonmetastatic hepatocellular carcinoma: a real-life multicentric weighted comparison. Ann Surg. 2022;275:743–752. doi: 10.1097/SLA.0000000000005373. [DOI] [PubMed] [Google Scholar]
- 13.Labgaa I, Taffé P, Martin D, et al. Comparison of partial hepatectomy and transarterial chemoembolization in intermediate-stage hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2020;9:138–147. doi: 10.1159/000505093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68:977–993. doi: 10.1002/hep.29883. [DOI] [PubMed] [Google Scholar]
- 15.Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 17.Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea (2019) 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol 20:1042-1113 [DOI] [PMC free article] [PubMed]
- 18.Kudo M, Matsui O, Izumi N, et al. jsh consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334–351. doi: 10.1016/j.annonc.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Öcal O, Ingrisch M, Ümütlü MR, et al. Prognostic value of baseline imaging and clinical features in patients with advanced hepatocellular carcinoma. Br J Cancer. 2022;126:211–218. doi: 10.1038/s41416-021-01577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HJ, Kim H, Lee DH, et al. Gadoxetate-enhanced MRI features of proliferative hepatocellular carcinoma are prognostic after surgery. Radiology. 2021;300:572–582. doi: 10.1148/radiol.2021204352. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Chen J, Jiang H, et al. Gadoxetic acid-enhanced MRI radiomics signature: prediction of clinical outcome in hepatocellular carcinoma after surgical resection. Ann Transl Med. 2020;8:870. doi: 10.21037/atm-20-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae JS, Kim JH, Lee DH, Kim JH, Han JK. Hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with HCC: prognostic features before resection, ablation, or TACE. Eur Radiol. 2021;31:3627–3637. doi: 10.1007/s00330-020-07499-w. [DOI] [PubMed] [Google Scholar]
- 24.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266:452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593–619. doi: 10.1007/s00535-021-01788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Radiology (2018) CT/MRI liver imaging reporting and data system v2018 core. American College of Radiology, America. Available via https://www.acr.org/-/media/ACR/Files/Clinical-Resources/LIRADS/Chapter-16-Imaging-features.pdf. Accessed 1 Feb 2022
- 28.Renzulli M, Brocchi S, Cucchetti A, et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology. 2016;279:432–442. doi: 10.1148/radiol.2015150998. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Wei J, Fu F, et al. Predicting microvascular invasion in hepatocellular carcinoma: a dual-institution study on gadoxetate disodium-enhanced MRI. Liver Int. 2022;42:1158–1172. doi: 10.1111/liv.15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Joo I, Kim SY, Kang TW, et al. Radiologic-pathologic correlation of hepatobiliary phase hypointense nodules without arterial phase hyperenhancement at gadoxetic acid-enhanced MRI: a multicenter study. Radiology. 2020;296:335–345. doi: 10.1148/radiol.2020192275. [DOI] [PubMed] [Google Scholar]
- 32.Stamm ER, Meier JM, Pokharel SS, et al. Normal main portal vein diameter measured on CT is larger than the widely referenced upper limit of 13 mm. Abdom Radiol (NY) 2016;41:1931–1936. doi: 10.1007/s00261-016-0785-9. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh SK, Yin M, Takahashi N, Glockner JF, Talwalkar JA, Ehman RL. Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging. 2015;40:766–775. doi: 10.1007/s00261-015-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald RJ, McDonald JS, Kallmes DF, Carter RE. Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology. 2013;269:640–645. doi: 10.1148/radiol.13131465. [DOI] [PubMed] [Google Scholar]
- 35.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57:289–300. [Google Scholar]
- 38.Wei H, Jiang H, Zheng T, et al. LI-RADS category 5 hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MRI for early recurrence risk stratification after curative resection. Eur Radiol. 2021;31:2289–2302. doi: 10.1007/s00330-020-07303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu DS, Siripongsakun S, Kyong Lee J, et al. Complete tumor encapsulation on magnetic resonance imaging: a potentially useful imaging biomarker for better survival in solitary large hepatocellular carcinoma. Liver Transpl. 2013;19:283–291. doi: 10.1002/lt.23597. [DOI] [PubMed] [Google Scholar]
- 40.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the Milan Criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 41.Jun L, Zhenlin Y, Renyan G, et al. Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist. 2012;17:963–969. doi: 10.1634/theoncologist.2011-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong Z, Huang K, Liao B, et al. Prediction of sorafenib treatment-related gene expression for hepatocellular carcinoma: preoperative MRI and histopathological correlation. Eur Radiol. 2019;29:2272–2282. doi: 10.1007/s00330-018-5882-4. [DOI] [PubMed] [Google Scholar]
- 43.Cerny M, Chernyak V, Olivié D, et al. LI-RADS version 2018 ancillary features at MRI. Radiographics. 2018;38:1973–2001. doi: 10.1148/rg.2018180052. [DOI] [PubMed] [Google Scholar]
- 44.Wang JC, Hou JY, Chen JC, et al. Development and validation of prognostic nomograms for single large and huge hepatocellular carcinoma after curative resection. Eur J Cancer. 2021;155:85–96. doi: 10.1016/j.ejca.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XP, Gao YZ, Chen ZH, et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology. 2019;69:2076–2090. doi: 10.1002/hep.30490. [DOI] [PubMed] [Google Scholar]
- 46.Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214–2229. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.