Highlights
-
•
Hearing alteration may occur during Dengue, Chikungunya, and Zika infections.
-
•
Otalgia, hypoacusis, vertigo and tinnitus were the most common symptoms.
-
•
Sensorineural hearing loss was more notiaceable in adults exposed to Zika virus.
-
•
The actual effect of arboviruses on hearing from adults is unknown.
Keywords: Zika virus, Chikungunya virus, Dengue, Hearing disorders, Auditory perceptual disorders
Abstract
Objectives
To identify and understand the evidence regarding hearing changes related to acquired Dengue, Chikungunya, and Zika virus infection in adult individuals.
Methods
A scoping review was performed according to the recommendations of The Joanna Briggs Institute and guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews in the Embase, PubMed/Medline, ScienceDirect, Scopus, and Web of Science databases without restriction on language and year of publication. Case studies, observational studies, and clinical trials reporting hearing loss in adult subjects (>18–60 years of age) of both sexes with DENV, CHIKV, or ZIKV diagnosed by positive molecular/serological examination by RT-PCR or IgM/IgG by ELISA method were included.
Results
Thirteen studies met the inclusion criteria and were selected for review. The occurrence of auditory symptoms caused by arboviroses and the presence of permanent or transient sensorineural hearing loss was variable in adults.
Conclusions
Dengue, Chikungunya, and Zika infections in adults are associated with a variety of auditory symptoms. The frequency of permanent or transient sensorineural hearing loss is low but not negligible.
Introduction
Dengue (DENV), Chikungunya (CHIKV), and Zika (ZIKV) are arboviruses of endemic co-circulation in Brazil.1 They are considered a public health concern worldwide due to their history of resurgence associated with environmental and social factors that favor their occurrence, especially situations of sanitary and economic vulnerability.2, 3
The infection caused by these pathogens may result in immediate or late hearing sequelae that affect different age groups because of damage to the structures or functions of the inner ear.4, 5 In addition, different auditory manifestations have been reported for patients with DENV, such as tinnitus, vertigo, sudden hearing loss, and sound intolerance,6 however, these outcomes were heterogeneous, and the sample size was not representative.
As for CHIKV, one study7 showed that a 31-year-old adult patient who recovered from infection had hearing loss with persistent auditory symptoms; however, the causal mechanisms were unclear. In contrast, ZIKV is highlighted for its high prevalence and a causal link to fetal and congenital neurological abnormalities that include microcephaly and Guillain-Barré Syndrome (GBS), a rare immune-mediated condition affecting peripheral nerves.8
Early evidence9 has shown that prenatal exposure to ZIKV infection is associated with sensory-neural hearing loss. In general, its effect on the infant population has been well studied,10 and in 2019, the Joint Committee on Infant Hearing inserted prenatal exposure to ZIKV as a risk factor for hearing loss.11 This report provided strong evidence of the relationship of ZIKV with early hearing impairment and suggested follow-up beyond the pediatric age group.11
However, in adults, the actions of arboviruses on hearing are still poorly understood.12 In summary, there are still gaps about the main hearing changes found in adult individuals with DENV, CHIKV, and ZIKV, with only a few reports of sudden deafness after infection.5, 7, 13
Therefore, the aim of this scoping review is to identify and understand the evidence regarding hearing changes related to acquired Dengue, Chikungunya, and Zika virus infection in adult individuals.
Methods
The literature review was conducted according to the recommendations of The Joanna Briggs Institute (JBI) for scoping reviews14 and guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020).15 The complete research protocol was registered and previously published in the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42022335879.
Review question
The guiding research question “What hearing characteristics may be altered in adult individuals with confirmed DENV, CHIKV, and/or ZIKV infection?” was designed for the selection and search of the studies through the Population, Concept, and Context strategy. Thus, “P” was defined as adult patients (>18 years), “C” as hearing characteristics, and the last “C” as an infection acquired by the arboviruses of Dengue, Chikungunya, and Zika.
Data search
The literature search was conducted up to October 31, 2022, using Embase, PubMed/Medline, ScienceDirect, Scopus, and Web of Science databases. The search strategy was tailored to each database and included descriptors and keywords related to arboviruses and hearing impairment (Supplementary Table 1). No age range limiters were used to ensure the retrieval of as many relevant studies as possible.
Eligibility criteria
Case studies, observational studies, and clinical trials reporting hearing loss in adult subjects (>18–60 years of age) of both sexes with DENV, CHIKV, or ZIKV diagnosed by positive molecular/serological examination by RT-PCR or IgM/IgG by ELISA method were included. There was no restriction on the year and language of publication.
Studies that included individuals with hearing loss or complaints prior to infection, a history of exposure to constant noise (80 dBNa for more than 8 h/day), psychiatric disorders, and neurological and genetic syndromes, congenital or acquired prior to infection were excluded. In addition to in-vitro studies, animal studies, editorials, book chapters, reports, commentaries, notes, conference abstracts, and literature reviews.
Study selection
Data analysis occurred in four steps: identification, screening, eligibility, and inclusion. In the identification stage, appropriate studies were selected by individual database searches. The bibliographies of included studies were manually reviewed for additional references.
The reference manager application Rayyan16 was used to store and share studies between reviewers and to remove duplicates. In the screening and eligibility step, the title, abstract, and full text were read by two independent reviewers to rule out studies that did not meet the eligibility criteria. Any discrepancies between them on study eligibility were resolved through discussion or after consultation with a third team member. At the inclusion stage, studies that met all the previous steps were aggregated for data extraction.
Data extraction and analysis
For analysis, information on study identification (author, year, and place), study design, sample characteristics (population, sample size, and age), presence and type of arbovirus, presence of associated neurological manifestation, hearing assessment method and hearing alteration were extracted in a Microsoft Office — Excel® spreadsheet.
The grades of hearing impairment were reclassified to homogenize the results between the studies considering four-frequency Pure-Tone Average (4fPTA) by obtaining the means of the thresholds at 500, 1000, 2000, and 4000 Hz for each ear, and the values suggested by the World Health Organization as follows17: (1) normal, ≤19.50; (2) mild, 19.51–34.5; (3) moderate, 34.51–49.5; (4) moderately severe, 49.51–64.5; (5) severe, 64.51–80.5; and (6) profound, ≥80.51 dB HL.
Results
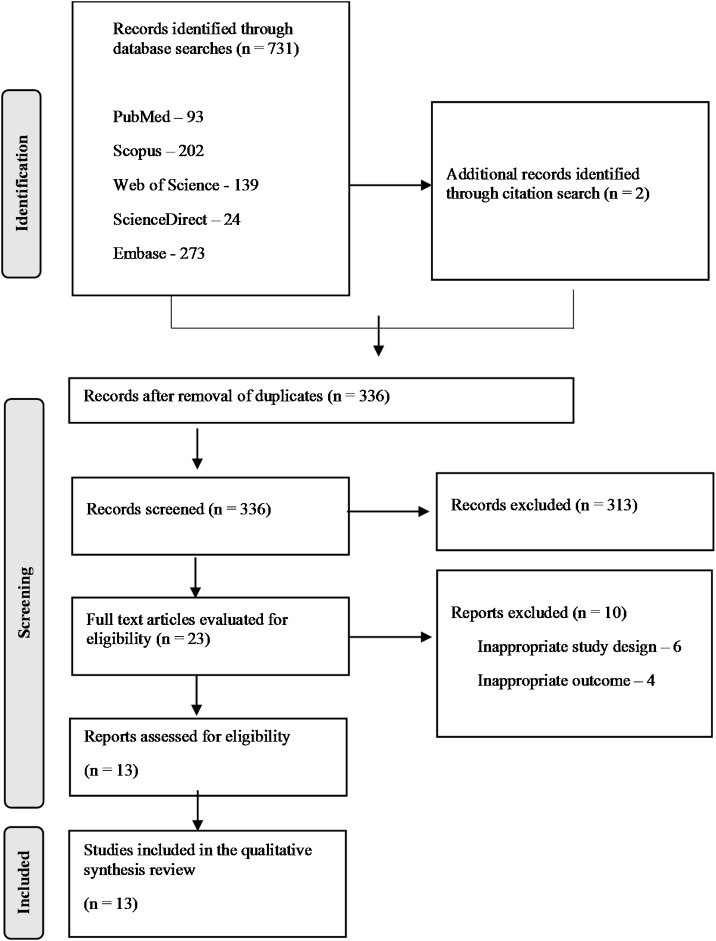
After searching the databases, 731 references of potential studies were identified, plus two references retrieved by manual search in the citations and references. After removing the duplicates, 335 articles were screened by reading the title and abstract, where 310 articles were excluded for not answering the guiding question of this research.
The remaining 25 articles were assessed for eligibility by reading the full text. Of these, six articles were excluded for inappropriate study design and 4 for no auditory outcomes. A detailed overview of the study selection process is presented in the flow chart in Fig. 1.
Figure 1.
Flow diagram of study selection.
A description of the identifying characteristics of the 13 included studies is presented in Table 1. Overall, regarding the presence and type of arbovirus, most studied, six studies5, 6, 18, 19, 20, 21 described dengue-related hearing changes in adult subjects.
Table 1.
Characteristics of the studies included in the review in adult subjects with a confirmed infection by Dengue, Chikungunya, and Zika viruses.
| Arbovirus | Author/year | Country | Aim | Study design | Sample (N) | Mean age (years) | Hearing assessment method | Main conclusions |
|---|---|---|---|---|---|---|---|---|
| Dengue | Denis et al., 2003 | Brazil | Evaluate patients with dengue who present with otorhinolaryngological symptoms | Cross-sectional study | 30 | 33.7 | Self-reporting | The clinical suspicion of dengue is essential because of the different otorhinolaryngological manifestations |
| Diniz et al., 2021 | Brazil | To report a case of aseptic meningitis, acute renal failure, and sensorineural hearing loss in a 42-year-old man with severe dengue fever | Case report | 1 | 42 | Audiometry | Six-month follow-up showed persistent deafness, suggesting an association between dengue and hearing loss | |
| Mughal et al., 2022 | Pakistan | To report a case of unilateral sensorineural hearing loss after dengue | Case report | 1 | 46 | Rinne test, Webber test and audiometry | Sensory sensorineural hearing loss is a rare presentation in dengue that doctors need to investigate | |
| Rahme et al., 2020 | Brazil | To describe four patients who presented with serologically confirmed dengue infection and cochleovestibular manifestations | Case report | 4 | 55 | Audiometry, vídeo head impulse test and acufenometry | The cochleovestibular manifestations in dengue are heterogeneous | |
| Ribeiro et al., 2015 | Brazil | To present a case of dengue hemorrhagic fever that evolved with sensorineural hearing loss | Case report | 1 | 60 | Audiometry | No other cause was found for sudden deafness and the correlation with dengue fever was questioned | |
| Soni et al., 2021 | India | To explore the association of dengue with hearing loss | Prospective cohort | 10 | 29 | Audiometry, tympanometry, brainstem auditory evoked potential, and steady state | Hearing loss in dengue, even if mild, is irreversible. The cause of the loss has not yet been found, and further studies are needed | |
| Chikungunya | Couturier et al., 2012 | France | To measure the frequency and risk factors for rheumatic manifestations after chikungunya infection and to assess their impact on quality of life | Prospective cohort | 227 | 50.3 | Questionnaire | Medical follow-up was recommended to support possible associated depression and anxiety |
| Dutta et al., 2011 | India | Finding the prevalence of chikungunya in Assam, northeast India | Cross-sectional study | 10 | NR | Audiometry | Highlights the importance of an epidemiological and entomological investigation for detection of the emergence of chikungunya | |
| Jain et al., 2018 | India | Report two cases of chikungunya encephalitis | Case report | 2 | 32.5 | NR | Neurological complications may occur during the infectious process or after a period of 15–20 days | |
| Zika | Aspahan et al., 2019 | Brazil | To report a case of neuromyelitis optical spectrum disorder associated with Zika virus infection | Case report | 1 | 35 | NR | The pathophysiology of neurological disorders related to arbovirus infections has not yet been established, and further research is needed for this purpose |
| Martins et al., 2017 | Brazil | To characterize the otologic findings in two adult patients, post-infection by Zika virus | Case study series | 2 | 45 | Audiometry, tympanometry, brainstem evoked potentials, transient and distortion product evoked otoacoustic emissions | Audiological findings demonstrate possible neuronal involvement in the complaints presented, associated or not with the peripheral component, in infected patients | |
| Tappe et al., 2015 | Germany | To report an acute Zika infection that presented with bilateral hearing difficulties during illness | Case report | 1 | 45 | Self-reporting | The cause of hearing difficulties remains unclear. Increased clinical and laboratory awareness may help diagnose outside epidemic events | |
| Vinhaes et al., 2016 | Brazil | Report one confirmed and two probable cases of Zika with transient sensorineural hearing loss | Case report | 3 | 23 | Audiometry | An association between Zika infection and transient hearing loss has been suggested |
NR, not reported.
The studies were published between the years 200318 and 2022,20 and most of them were produced in Brazil (n = 7 articles).5, 6, 13, 18,19, 22, 23 The most frequent study design was a case report in eight studies.5, 6, 12, 13,19, 20, 22, 24 The sample size and mean age ranged in the studies from 15, 12, 19, 20,22 to 22725 participants and from 2313 to 605 years of age.
Two studies mentioned neurological alterations associated with the researched arboviruses, one relating CHIKV24 in a case of encephalitis in the brainstem and another pertinent to ZIKV22 in a case of acute myelitis. However, the neurological issue was not assessed and was directly associated with the hearing alterations presented.
Furthermore, the most commonly used hearing assessment method was tonal audiometry (n = 8 studies).5, 6, 7, 13, 19, 20, 21, 23 In contrast, two studies22, 24 did not clarify which hearing assessment methodology was used to confirm hearing difficulties and the occurrence of hearing loss from arbovirus infections.
Main hearing alterations in Dengue, Chikungunya, and Zika
The main hearing alterations in adult individuals with a confirmed infection by DENV, CHIKV, and ZIKV viruses are presented in Table 2.
Table 2.
Main hearing alterations in adult individuals with a confirmed infection by Dengue, Chikungunya, and Zika viruses.
| Arbovirus | Study | Hearing results |
Change in the grade of hearing loss | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal hearing (N) | Symptoms and complaints |
Improvement of symptoms | Hearing loss |
||||||||||
| Hypoacusis | Hyperacusis | Otalgia | Vertigo/tinnitus | Tinnitus | (N) | Type | Grade | Ear | |||||
| Dengue (n = 47) | Denis et al., 2003 | ‒ | ‒ | ‒ | 11 | 6 | 2 | NR | ‒ | ‒ | ‒ | ‒ | ‒ |
| Diniz et al., 2021 | ‒ | ‒ | ‒ | ‒ | 1 | ‒ | NR | 1 | SN | Moderately severe on right and profound on left | Both | Yes | |
| Mughal et al., 2022 | ‒ | 1 | ‒ | ‒ | ‒ | 1 | Yes | 1 | SN | Profound | Left | Yes | |
| Rahme et al., 2020 | 1 | ‒ | 1 | ‒ | 2 | 3 | Yes | 2 | SN | Mild on right and profound on left | Left | Yes | |
| Ribeiro et al., 2015 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1 | SN | Profound | Both | No | |
| Soni et al., 2021 | 7 | 2 | ‒ | ‒ | ‒ | 1 | NR | 3 | SN | Mild (NS) | Both | NS | |
| Chikungunya | Couturier et al., 2012 | ‒ | 13 | ‒ | ‒ | ‒ | ‒ | NR | ‒ | ‒ | ‒ | ‒ | ‒ |
| Dutta et al., 2011 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1 | SN | Mild to severe (NS) | Both | NS | |
| Jain et al., 2018 | ‒ | 1 | ‒ | ‒ | ‒ | ‒ | NR | ‒ | ‒ | ‒ | ‒ | ‒ | |
| Zika | Aspahan et al., 2019 | ‒ | 1 | ‒ | ‒ | 1 | ‒ | NR | ‒ | ‒ | ‒ | ‒ | ‒ |
| Martins et al., 2017 | 1 | ‒ | ‒ | ‒ | 2 | 2 | NR | 1 | SN | Moderate (NS) | Left | NS | |
| Tappe et al., 2015 | ‒ | 1 | ‒ | ‒ | ‒ | ‒ | Yes | ‒ | ‒ | ‒ | ‒ | ‒ | |
| Vinhaes et al., 2016 | 1 | ‒ | ‒ | ‒ | 1 | 2 | NR | 2 | SN | Severe on right and profound on left | Both | Yes | |
NR, not reported; NS, not specified exact hearing thresholds, so the degree of hearing loss was not reclassified; SN, sensorineural; PM, mixed hearing loss.
Six studies5, 6, 18, 19, 20, 21 investigated hearing changes in 47 individuals with DENV. In these individuals, otalgia was the most frequent symptom (23.40%),18 followed by vertigo/tinnitus (19.14%),6, 18, 19 tinnitus (14.89%)6, 18, 20, 21 and hypoacusis (6.38%).20, 21 Symptom improvement was reported in two studies.6, 20 The occurrence of sensorineural hearing loss was 17.02% (n = 8 individuals)5, 6, 19, 20, 21 of profound grade5, 6, 19, 20 and with significant change in hearing thresholds implying improvement in the degree of hearing loss over time in most studies (Supplementary Table 2).6, 19, 20
Three studies7, 24, 25 investigated hearing changes in 239 individuals with CHIKV. Hypoacusis was the only symptom reported (5.85%),24, 25 and mild to severe sensory sensorineural hearing loss was reported only in a single study for one individual,7 with no significant change in hearing thresholds implying an improvement in the degree of hearing loss.7 It is noteworthy that the authors should have specified the tone thresholds at each Frequency.7 Unfortunately, this made it impossible to reclassify the degree of hearing loss for homogeneity of the data.
Three studies12, 22, 23 investigated hearing alterations in 7 individuals with ZIKV. The most frequent symptoms were vertigo/tinnitus (57.14%)13, 22, 23 and tinnitus (57.14%).13, 23 It was not reported whether there was an improvement in these specific symptoms. The occurrence of moderate-grade sensorineural hearing loss was 42.85% (n = 3 individuals)13, 23 with a change in in hearing thresholds implying improvement in the degree of hearing loss.13
Discussion
This scoping review is the first study of its kind to provide systematic and semiquantitative insight that the presence of hearing alterations during infection with DENV, CHIKV, and ZIKV viruses in adult subjects agrees with previously published findings in other viral infections showing that the auditory system can be compromised to varying degrees of severity.26
The high endemic prevalence of dengue, especially in Brazil, where most studies were produced, associated with social and environmental issues, justifies why this has been the most studied arbovirus and is even related to hearing alterations.27, 28
An audiometry may have been the most widely used hearing evaluation method because it reveals the integrity of the peripheral auditory pathways besides accurately estimating hearing thresholds. However, since damage to the central nervous system has already been reported in adult individuals with ZIKV or CHIKV.29, 30 Assessment of central auditory processing was expected; however, no study has applied tests for this purpose, demonstrating that there are gaps in central auditory functioning in the adult population.
The heterogeneity of hearing alterations in arboviruses is a common finding,26 in which sensory sensorineural hearing loss can occur with distinct degrees of severity and have transient or permanent characteristic degrees.5, 7, 13 DENV is not recognized as causing hearing loss,5 and its pathological mechanisms have not been clarified.21 However, the most accepted hypothesis points out that hearing loss occurs by the impairment in vascular permeability of the terminal artery that supplies the cochlea due to the severity and evolution of the disease5 with the possibility of hemorrhagic shock.31
Moreover, unreported pre-existing chronic comorbidities may have propitiated the hearing alterations, as exposed in the study by Diniz et al.,19 that included aseptic meningitis and acute kidney injury. The possibility of symptom remission and improvement in audibility reveals the importance of treatment and auditory monitoring, mainly because hearing alterations can happen late after DENV infection.22
CHIKV has been associated with decreased hearing acuity with persistent auditory symptoms,7 with sensory sensorineural hearing loss being of lower occurrence when compared to the other arboviruses studied here.25 The neurotropic nature of CHIKV affects auditory neurons, similarly to other viral infections, which affect the organ of Corti, vascular stria, and tectory membrane,32 enabling demyelination neuropathy and various auditory disorders in the infected.33, 34
In ZIKV infection, auditory alterations are specific manifestations that can occur during acute infection, having the character of transient sensory-neural impairment of gradual spontaneous resolution.12 Vinhaes and colleagues13 performed serial audiometry and showed that the sensory hearing loss had a transient character with an improvement of the audibility levels in up to 28 days.
The molecular and morphological damage to cochlear structures by ZIKV infection has been explained by multiple complex mechanisms that contribute to hearing loss;35 however, damage to central auditory pathways is questioned due to the neurotropic behavior of the virus.36, 37, 38
Because ZIKV is cytopathic to neurons, it infects microvascular endothelial cells in the brain, allowing viral access by impairing nuclear responses of innate immunity.36 In addition to disrupting the activity of essential proteins involved in developing the neurosensory system, such as ZPR1, the infection plays an evasive role in mediated dysregulation.37
A study38 using mice demonstrated that ZIKV infection in adult neural stem cells leads to cell death and reduced proliferation. These data suggested that adult neural stem cells are vulnerable to ZIKV neuropathology, just as the adult brain can be. This finding has been confirmed by studies,39, 40, 41 in humans demonstrating cranial nerve involvement causing severe encephalitis and other rare neurological disorders, vulnerability observed in adult neural cells to ZIKV neuropathology may generate consequences of exposure in the adult brain of late manifestation.
Neurological complications related to CHIKV and ZIKV arboviruses have been reported only in the most severe cases of arboviral infection or coinfection, demonstrating a rare viral neuropathic effect that is still poorly understood.29 CHIKV encephalitis presented as a brainstem syndrome and boomerang sign24 and ZIKV-associated neuromyelitis optical disorder,22 illustrate this scenario and show that both may be one of the causes of demyelination or tissue alteration post- or parainfectious.22, 24
Inflammatory processes with demyelinating lesions in the cochlear nerve may cause hearing impairment22; however, no study has directly evaluated this possibility. An essential factor to be considered is the effect of antibiotics used in some studies to ameliorate the acute symptoms of arboviral infection,19 as they are notoriously known to induce multiple adverse reactions in the body, such as resistance to bacteria and irreversible or long-lasting hearing alterations.42 Therefore, it cannot be ruled out that part of the hearing difficulties presented are consequences of iatrogenic drug treatment43 added to the neurotropic activity of viruses.
The main limitation of this research concerns the small amount of data that exists to characterize the hearing alterations in each arbovirus. Secondarily, the lack of homogenization between samples and the lack of objective data for measuring hearing function in some studies12, 18, 22, 24,25 added to the low follow-up time of the studies21, 25 probably underestimates the true prevalence and impairs the quality of the data by the risk of bias. Furthermore, no study has directly examined the relationship between other complications, such as neurological and hearing impairment.22, 24 Thus, future epidemiological studies with representative populations and careful methodology when evaluating hearing loss may promote the confirmation and generalization of the results presented.
Conclusion
Preliminary evidence supports those hearing alterations may occur during Dengue, Chikungunya, and Zika infections, with the variable occurrence of auditory symptoms and the presence of sensorineural hearing loss that may be permanent or transient. Otalgia, hypoacusis, vertigo/tinnitus, and tinnitus were the most common symptoms, and sensorineural hearing loss was more remarkable for patients exposed to the Zika virus.
Audiological follow-up and treatment are suggested to reduce the severity of long-term auditory viral sequelae. However, due to the presence of limitations related to study designs, mainly by the high number of case reports and methodological limitations, the actual effect of arboviruses on hearing may be substantially different from the estimation in this review.
Therefore, future studies should aim to establish the causal relationship between auditory alterations in larger samples affected by these arboviruses in order to define a clear mechanism that explains the auditory symptoms.
Funding
No funding was received.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.bjorl.2023.101342.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Morales I., Rosenberger K.D., Magalhaes T., Morais C.N.L., Braga C., Marques E.T.A., et al. Diagnostic performance of anti-Zika virus IgM, IgAM and IgG ELISAs during co-circulation of Zika, dengue, and chikungunya viruses in Brazil and Venezuela. PLoS Negl Trop Dis. 2021;15:e0009336. doi: 10.1371/journal.pntd.0009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musso D., Gubler D.J. Zika virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Wang J., Cheng X., Hu H., Guo C., Huang J., et al. The worldwide seroprevalence of Denv, CHIKV and ZIKV infection: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15:e0009337. doi: 10.1371/journal.pntd.0009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thawani A., Sammudin N.H., Reygaerts H.S., Wozniak A.N., Munnamalai V., Kuhn R.J., et al. Zika virus can directly infect and damage the auditory and vestibular components of the embryonic chicken inner ear. Dev Dyn. 2020;249:867–883. doi: 10.1002/dvdy.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro B.N.F., Guimarães A.C., Yazawa F., Takara T.F.M., de Carvalho G.M., Zappelini C.E.M. Sensorineural hearing loss in hemorrhagic dengue? Int J Surg Case Rep. 2015;8:38–41. doi: 10.1016/j.ijscr.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahme I.M.P., Pereira G.M., Sanchez T.G. Different cochleovestibular manifestations and outcomes in patients diagnosed with dengue. Braz J Otorhinolaryngol. 2020;86:55–60. doi: 10.1016/j.bjorl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta P., Khan S.A., Khan A.M., Borah J., Chowdhury P., Mahanta J. First evidence of chikungunya virus infection in Assam, Northeast India. Trans R Soc Trop Med Hyg. 2011;105:355–357. doi: 10.1016/j.trstmh.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Mier-y-Teran-Romero L., Delorey M.J., Sejvar J.J., Johansson M.A. Guillain–Barré syndrome risk among individuals infected with Zika virus: a multi-country assessment. BMC Med. 2018;16:67. doi: 10.1186/s12916-018-1052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leal M.C., Muniz L.F., Ferreira T.S.A., Santos C.M., Almeida L.C., Van Der Linden V., et al. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection — Brazil, November 2015–May 2016. MMWR Morb Mortal Wkly Rep. 2016;65:917–919. doi: 10.15585/mmwr.mm6534e3. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa M.H.M., Garcia C.F.D., Magalhães Barbosa M.C., Robaina J.R., Prata-Barbosa A., Lima M.A.M.T., et al. Normal hearing function in children prenatally exposed to Zika virus. Int Arch Otorhinolaryngol. 2020;24:e299–e307. doi: 10.1055/s-0039-3399539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hora L.C., Muniz L.F., Griz S.M., Silva J.D., Britto D.B.L.A., Venâncio L.G.A., et al. Frequency-following response and auditory behavior in children with prenatal exposure to the Zika virus. Int Arch Otorhinolaryngol. 2021;26:380–389. doi: 10.1055/s-0041-1726048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tappe D., Nachtigall S., Kapaun A., Schnitzler P., Günther S., Schmidt-Chanasit J. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis. 2015;21:911–913. doi: 10.3201/eid2105.141960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinhaes E.S., Santos L.A., Dias L., Andrade T.N.A., Bezerra V.H., Carvalho A.T., et al. Transient hearing loss in adults associated with Zika virus infection. Clin Infect Dis. 2017;64:675–677. doi: 10.1093/cid/ciw770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters M.D.J., Godfrey C.M., Khalil H., McInerney P., Parker D., Soares C.B. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humes L.E. The World Health Organization’s hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int J Audiol. 2019;58:12–20. doi: 10.1080/14992027.2018.1518598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis C.K., Cavalcanti K.M., Meirelles R.C., Martinelli B., Valença D.C. Manifestações otorrinolaringológicas em pacientes com dengue. Rev Bras Otorrinolaringol. 2003;69:644–647. [Google Scholar]
- 19.Diniz R., dos Santos E., Chagas G., Daher E. Severe dengue associated with aseptic meningitis, acute kidney injury, and sudden sensorineural hearing loss: a case report. Asian Pac J Trop Med. 2021;14:187. [Google Scholar]
- 20.Mughal A., Wasif M., Abbas S.A., Ghaloo S.K., Vardag A.B.S., Awan M.O. Sudden sensorineural hearing loss: a rare presentation of dengue fever. J Pak Med Assoc. 2022;72:1862–1864. doi: 10.47391/JPMA.3847. [DOI] [PubMed] [Google Scholar]
- 21.Soni K., Bohra G.K., Nair N.P., Kaushal D., Patro S.K., Goyal A. Sensorineural hearing loss in dengue: a pilot study. Iran J Otorhinolaryngol. 2021;33:157–161. doi: 10.22038/ijorl.2020.39874.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspahan M.C., Leonhard S.E., Gomez R.S., Rocha E.D.S., Vilela M.R.D.S., Alvarenga P.P.M., et al. Neuromyelitis optica spectrum disorder associated with Zika virus infection. Neurol Clin Pract. 2019;9:e1–e3. doi: 10.1212/CPJ.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins O.R., Rodrigues P.A.L., Santos A.C.M., Ribeiro E.Z., Nery A.F., Lima J.B., et al. Achados otológicos em pacientes pós-infecção pelo zika vírus: estudos de caso. Audiol Commun Res. 2017;22:e1850. [Google Scholar]
- 24.Jain R., Khan I., Khandelwal K., Saini P., Chaudhary R. Chikungunya encephalitis presenting as a brainstem syndrome and “boomerang” sign. Neurol India. 2018;66:578. doi: 10.4103/0028-3886.227269. [DOI] [PubMed] [Google Scholar]
- 25.Couturier E., Guillemin F., Mura M., Léon L., Virion J.-M., Letort M.-J., et al. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatology (Oxford) 2012;51:1315–1322. doi: 10.1093/rheumatology/kes015. [DOI] [PubMed] [Google Scholar]
- 26.Campos G.S., Bandeira A.C., Sardi S.I. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrioli D.C., Busato M.A., Lutinski J.A. Spatial and temporal distribution of dengue in Brazil, 1990–2017. PLoS One. 2020;15:e0228346. doi: 10.1371/journal.pone.0228346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brito A.F., Machado L.C., Oidtman R.J., Siconelli M.J.L., Tran Q.M., Fauver J.R., et al. Lying in wait: the resurgence of dengue virus after the Zika epidemic in Brazil. Nat Commun. 2021;12:2619. doi: 10.1038/s41467-021-22921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brito Ferreira M.L., Militão de Albuquerque M. de F.P., de Brito C.A.A., França de R.F.O., Moreira A.J.P., Machado de M.I.M., et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study. Lancet Neurol. 2020;19:826–839. doi: 10.1016/S1474-4422(20)30232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muñoz L.S., Parra B., Pardo C.A. Neurological implications of Zika virus infection in adults. J Infect Dis. 2017;216:S897–S905. doi: 10.1093/infdis/jix511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witayathawornwong P., Jirachanchai O., Kasemsut P., Mahawijit N., Srisakkwa R. Severe perinatal dengue hemorrhagic fever in a low-birth-weight infant. Southeast Asian J Trop Med Public Health. 2012;43:62–67. [PubMed] [Google Scholar]
- 32.Prabhu P., Gafoor S.A. Human viruses: diseases, treatments and vaccines. Springer International Publishing; 2021. Effect of chikungunya viral infection on the auditory system; pp. 187–192. [Google Scholar]
- 33.Agarwal A., Vibha D., Srivastava A.K., Shukla G., Prasad K. Guillain-Barre syndrome complicating chikungunya virus infection. J Neurovirol. 2017;23:504–507. doi: 10.1007/s13365-017-0516-1. [DOI] [PubMed] [Google Scholar]
- 34.Prabhu P. Acquired auditory neuropathy spectrum disorder after an attack of chikungunya: case study. Eur Arch Otorhinolaryngol. 2016;273:257–261. doi: 10.1007/s00405-015-3578-9. [DOI] [PubMed] [Google Scholar]
- 35.Yee K.T., Neupane B., Bai F., Vetter D.E. Zika virus infection causes widespread damage to the inner ear. Hear Res. 2020;395:108000. doi: 10.1016/j.heares.2020.108000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conde J.N., Schutt W.R., Mladinich M., Sohn S.Y., Hearing P., Mackow E.R. NS5 sumoylation directs nuclear responses that permit Zika virus to persistently infect human brain microvascular endothelial cells. J Virol. 2020;94:e01086–e01120. doi: 10.1128/JVI.01086-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glover K.K.M., Zahedi-Amiri A., Lao Y., Spicer V., Klonisch T., Coombs K.M. Zika infection disrupts proteins involved in the neurosensory system. Front Cell Dev Biol. 2020;8:571. doi: 10.3389/fcell.2020.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Saucedo-Cuevas L., Regla-Nava J.A., Chai G., Sheets N., Tang W., et al. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016;19:593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azevedo R.S.S., Araujo M.T., Martins Filho A.J., Oliveira C.S., Nunes B.T.D., Cruz A.C.R., et al. Zika virus epidemic in Brazil. I. Fatal disease in adults: clinical and laboratorial aspects. J Clin Virol. 2016;85:56–64. doi: 10.1016/j.jcv.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajahram G.S., Hale G., Bhatnagar J., Hiu J., Thayan R., William T., et al. Postmortem evidence of disseminated Zika virus infection in an adult patient. Int J Infect Dis. 2019;83:163–166. doi: 10.1016/j.ijid.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Soares C.N., Brasil P., Carrera R.M., Sequeira P., Filippis A.B., Borges V.A., et al. Fatal encephalitis associated with Zika virus infection in an adult. J Clin Virol. 2016;83:63–65. doi: 10.1016/j.jcv.2016.08.297. [DOI] [PubMed] [Google Scholar]
- 42.Ferraro S., Convertino I., Leonardi L., Blandizzi C., Tuccori M. Unresolved gustatory, olfactory and auditory adverse drug reactions to antibiotic drugs: a survey of spontaneous reporting to eudravigilance. Expert Opin Drug Saf. 2019;18:1245–1253. doi: 10.1080/14740338.2019.1676724. [DOI] [PubMed] [Google Scholar]
- 43.Nazer L.H., Brown A.R.T., Awad W. Iatrogenic toxicities in the Intensive Care Unit. Crit Care Clin. 2021;37:625–641. doi: 10.1016/j.ccc.2021.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.