Abstract
The ovarian reserve is defined as the quantity of oocytes stored in the ovary or the number of oocytes that can be recruited. Ovarian reserve can be affected by many factors, including hormones, metabolites, initial ovarian reserve, environmental problems, diseases, and medications, among others. With the trend of postponing of pregnancy in modern society, diminished ovarian reserve (DOR) has become one of the most common challenges in current clinical reproductive medicine. Attributed to its unclear mechanism and complex clinical features, it is difficult for physicians to administer targeted treatment. This review focuses on the factors associated with ovarian reserve and discusses the potential influences and pathogenic factors that may explain the possible mechanisms of DOR, which can be improved or built upon by subsequent researchers to verify, replicate, and establish further study findings, as well as for scientists to find new treatments.
Keywords: Diminished ovarian reserve, Hormone, Metabolism, Primordial follicular pool, Environmental factors
Introduction
Ovarian reserve is the quantity of primordial follicles and follicles that can be recruited into the pre-antral and antral stages and are competent for ovulation [1]. Women have inherent and finite number of ovarian follicles that are gradually descend during their reproductive years until poor reproductive outcome occurs [2]. The growth of ovarian follicles composes the basis of female reproduction, and the proliferation of granulosa cells (GCs) is a foundational process required to ensure normal follicular development [3]. Clinicians consider several ovarian reserve tests, including biochemical tests, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), inhibin B, and antimüllerian hormone (AMH), and ultrasound imaging of the ovaries like antral follicle count (AFC) [4, 5]. As a complex clinical sign, ovarian reserve can be influenced by many factors such as age, environment, primordial follicular pool, diseases and drugs, and some unknown elements [1, 6]. Compared with the normal group at a similar age, the number of available follicles or oocytes decreased to a very poor count in women with DOR [5]. With the progress of modern society and the postponement of pregnancy, DOR has become an intractable problem for women eager to become pregnant and also for the societal environment of the next generation [7]. Thus far, the etiology and pathogenesis of DOR are still vague; therefore, this review details about the variable factors that could affect ovarian reserve and hopes to improve our understanding of DOR by reorganizing its effects, putting forward some potential reasons for DOR, and hoping to have some impact on future clinical treatment.
Hormonal influence on ovarian reserve
AMH
As one of the most commonly used clinical markers to estimate ovarian reserve, AMH level is a reliable index that reflects the pool of follicles in the gonadotropin-independent phase and ovarian follicle count [5, 8, 9]. Accumulated AMH plays a key role in facilitating follicular growth from the pre-antral to the antral phase [10]. In antral follicles, AMH secretion gradually decreases from a peak to undetectable levels at 8–10 mm follicle diameter [11]. Furthermore, AMH levels appeared to reflect the number of growing and primordial follicles [12]. Thus, AMH plays an important role in the recruitment of primordial follicles, and the absence of the AMH gene results in more primordial follicles in young mice and fewer in older mice [13], suggesting that AMH deficiency depletes the resting pool, causing successively low AFC in the early years and finally inducing DOR. The local effects of AMH on folliculogenesis may also be stage-dependent. Additionally, the gene of AMH expression in GCs is positively associated with AMH levels and the expression of the FSH receptor (FSHR), androgen receptor (AR) and AMH receptor (AMHR), and negatively associated with the levels of estradiol (E2) and progesterone (P) in the corresponding follicular fluid, suggesting a reflective function of AMH concentration in synchronous follicular growth [14]. The same study also indicated that AMH levels in the circulation reflect the constitution of follicles ranging from 5 to 8 mm in diameter.
FSH and LH
The quantity of small antral follicles at the initiation of each follicle development cycle is a manifestation of the ovarian reserve. Sufficient FSH production is required for the growth of smaller follicles, which is related to follicle recruitment [15, 16]. The presence of FSH is crucial after the pre-antral follicle stage, although the correct level of FSH at the pre-antral stage is associated with greater oocyte quality [17]. Sustained and sufficient stimulation of FSH is key to the action of aromatase enzymes and estradiol-dependent follicular development; therefore, it is initially at the stage of large antral follicles at the onset of ovulation [18]. In vitro experiments have shown an association between FSH and steroid production, substance exchange, and metabolism, which ultimately induces follicle maturation [19].
Adequate LH secretion is necessary for the further maturation of follicles, especially preovulatory follicles [18, 20]. Research has shown that appropriate stimulation of LH is required for follicular growth by regulating the action of steroids on ovarian cells; however, excess LH could lead to the inhibition of the differentiation and proliferation of GCs [18]. Furthermore, the initial number of antral follicles chosen for ovulation is highly dependent on the distribution density and regulatory activity of the FSHR and LHR on the membrane of GCs [16, 21]. Moreover, adequate FSHR and LHR are required for further follicle maturation by activating the conversion of androstenedione to estrogen [15]. The quality of oocytes can be compromised by a low density of FSH and insufficient expression of FSHR and LHR [22].
Inhibin B
Inhibin B is secreted by growing follicles, accumulates in the follicular compartment, and is released into circulation. Its primary function is to selectively inhibit the synthesis and secretion of FSH and activate LH. As it is a GC-specific hormone, its intrafollicular concentration is related to the size of the follicle, and its serum level could reflect follicle synthesis. Inhibin B is more effective in predicting women with AFC ranging from 5 to 7 mm [23]. Inhibin B summits during the follicle growth reaching a diameter of 8–10 mm [11], and the episodic inhibin B secretion pattern shows a peak during the follicular phase and ovulation [24]. Andersen hypothesized that inhibin B participates in the selection of dominant follicles during the natural human menstrual cycle [25]. Inhibin B is secreted in response to follicle-stimulating by FSH and promotes androgen production. Androgens then accumulate in adjacent GCs and upregulate the expression of FSHR and LHR, ultimately leading to increased sensitivity to gonadotropins in the follicle and further follicular growth.
Estrogen, progesterone and androgens
There is E2-dependent, calcium-mediated cytoplasmic maturation in human oocytes [26]. A study found that follicular fluid (FF) obtained from dominant follicles had a higher estrogen/androgen ratio than atretic subordinate follicles, indicating that well-developed follicles require abundant estrogen [16]. Estrogen receptor (ER) plays an important role in follicle development beyond the antral stage by regulating the transcriptional levels of genes involved in the communication between GCs and oocyte, oocyte maturation and gonadotropin-induced follicle development [20, 27].
Additionally, progesterone secretion gradually increases during antral follicle development [11], surges in the preovulatory period, and peaks in the dominant follicles [28]. Abnormally high levels of both P and E2 can negatively regulate follicle development from the primordial to the primary stage and impair mitosis of GCs, suggesting the arrest of primordial follicle growth [29, 30]. Thus, the over secretion of P and E2 at the early stage of follicular development affects the ovarian reserve.
Androgens exert complex effects on follicular development. The expression of AR in GCs is enriched in the pre-antral and early antral stages of follicles and is progressively absent in late preovulatory follicles [31, 32]. The absence of AR results in the apoptosis of GCs and subsequently compromises the ovarian reserve [33, 34].
Spatiotemporal pattern of hormone-mediated folliculogenesis
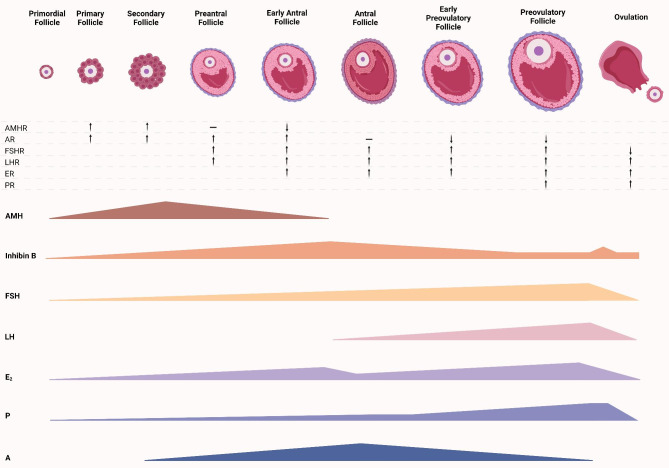
From the perspective of synchronized follicular development, a well-orchestrated spatiotemporal pattern of hormone-mediated folliculogenesis is crucial for ovarian reserve. One of the basic functions of AMH in folliculogenesis is the transition of follicles from the FSH-independent to the FSH-dependent stage, that is, the period from pre-antral to antral follicle stages [10]. FSH-stimulated pre-antral follicle development can be inhibited by AMH, and a lack of FSH induces attenuation of primordial follicle recruitment, suggesting that AMH can regulate the sensitivity of early follicles to FSH [35], and over secretion of AMH can lead to a dramatic drop in the number of follicles after the primary stage [36]. The tight association between AMH and AMHR expression in GCs suggests that cells and receptors are involved in the synchronous mechanisms that may influence follicular development. This is further supported by the significant association between AMHR and FSHR [14]. During dominant follicular selection, the secretion of E2 and P increases rapidly, and the concentration of inhibin B reaches its peak [11]. In contrast, the AMH level drops to its lowest point, indicating the cooperation of these hormones [11]. A high concentration of AMH during the early period of follicular growth also suppresses the secretion of E2, which is related to preovulatory follicle selection, by eliminating its function by acting on the aromatase of FSH [10]. Moreover, an appropriate androgen concentration is beneficial for FSH-dependent pre-antral follicular development by augmenting the expression of FSH at the mRNA level [37]. Therefore, as shown in Fig. 1, an appropriate concentration, opportune action, and synergistic response mechanism of intrafollicular hormones are essential for regulating the development of follicles in relation to the phenotypic ovarian reserve.
Fig. 1.
Model of well-orchestrated spatiotemporal pattern of hormone-mediated folliculogenesis. AMH expressed in the stage from primordial to early antral, and decreased in antral stage. AMH plays an important role in the recruitment of primordial follicles. The decline of AMH can enhance the secretion of FSH, LH, and A. The expression of AMH can be finally inhibited by E2 and P. FSH mainly appears after pre-antral follicle stage caused by the decline of AMH and peaks before ovulation, and its sufficient secretion increased E2 level. LH secretion is induced by the decline of inhibin B at antral follicle stage and peaks before ovulation. LH can enhance the secretion of E2. Inhibin B increases before antral follicle stage, drops to a low level after the same stage, and surges again during ovulation. Its secretion is stimulated by FSH and up-regulates androgens. Progesterone secretion gradually increases during antral follicle development, surges in the preovulatory period, and peaks in the dominant follicles. The variation of different receptor is consistent in the expression of corresponding hormones
Important metabolic part about ovarian reserve
Glucose metabolism
Glucose metabolism is necessary to generate sufficient material for follicle expansion, and an increased glucose requirement is necessary for the proliferation and differentiation of CCs and oocyte development. Because of the hydrophobicity of glucose, GCs absorbed glucose through glucose transporters (GLUTs) [38]. The main pathways for glucose metabolism in cumulus-oocyte complexes (COC) include glycolysis, the pentose phosphate pathway (PPP), the hexosamine biosynthetic pathway (HBP) and the polyol pathway [39]. Glycolysis in human GCs is enhanced from primordial to primary follicle [40]. In the absence of phosphofructokinase, energy production in the oocyte relies on pyruvate provided by GCs, and the oocyte synchronously regulates the high expression of phosphofructokinase in GCs [41]. Prior to follicular maturity, the production of pyruvate and lactate in GCs increases due to high energy consumption [42]. Pyruvate deprivation leads to early follicular dysgenesis [43]. Lactate may play a signaling molecular role in the follicular-luteal transition [44], and a lack of lactate causes follicular dysplasia [45]. There are two main functions of PPP in the COC. The first is the production of NADPH for the operation of the antioxidant system, including the combination of reactive oxygen species (ROS) to reduce cellular oxidation levels [42, 46]. The second function is the generation of phosphoribosyl pyrophosphate (PRPP) to synthesize nucleotides and nucleic acids. In addition, PPP is involved in the meiotic induction mechanism of oocytes [47], and inhibition of PPP can reduce the ratio of MII oocytes, demonstrating that PPP is a key regulator of the nuclear and cytoplasmic maturation of oocytes [48]. Additional glucose enters the HBP to provide a substrate for hyaluronic acid production during extracellular matrix expansion [49, 50]. Finally, a small tiny fraction of the absorbed glucose is used by the GCs to produce sorbitol and fructose via the polyol pathway [51]. Sorbitol, a byproduct of the polyol pathway, can increase superoxide dismutase (SOD) levels, which enhances ROS levels and results in follicular dysmaturity and ovarian aging [52, 53]. However, the presence of sorbitol or fructose in GCs and oocytes has not yet been established.
Lipids and cholesterol
Lipids, which are mainly metabolized in CCs around the oocyte, are potential resources for energy production in ovarian cells [54, 55]. Fatty acid β-oxidation, which is induced by the LH surge, plays an initial role in the metabolism of COCs, and the inhibition of fatty acid β-oxidation results in decreased oocyte quality [56]. The concentration of some fatty acids (i.e. pentadecanoic acid, heptadecanoic acid, cis-11-octadecenoic acid, cis-11-eicosenoic acid, cis,cis-11,14-eicosadienoic acid, and behenic acid, ect) were lower in DOR group with statistical significance, and the decreased transcription of HADHA (hydroxyacyl-coenzyme A dehydrogenase), ACSL (fatty acids β-oxidation related genes) were also decreased in DOR patients [57]. Therefore, further studies on the relationship between fatty acids and ovarian reserve are required.
The adiponectin signaling pathway is involved in ovarian activities, such as the regulation of steroid production in GCs and oocyte growth [58]. One possible mechanism of action of adiponectin is the activation of the hypothalamic-pituitary axis, and the absence of adiponectin interferes with the production of FSH and LH and subsequently influences ovulation [59]. Moreover, AdipoRon, an adiponectin-like synthetic, inhibits folliculogenesis and GCs proliferation by regulating aromatase expression, steroid production, and estrogen secretion by increasing the activity of phosphodiesterase, which causes cyclic adenosine monophosphate (cAMP) production [60].
Alterations in steroid hormones are related to oocyte development, and cholesterol participates in steroidogenesis. Studies have shown that intracellular cholesterol concentration is likely to reflect the developmental competence of the oocyte [61, 62]. Furthermore, cholesterol can be converted to progesterone and estradiol, which can further regulate the function of GCs [63, 64], and the synergistic effect of progesterone and estradiol delays oocytes development at an early stage [65]. Genes involved in steroid biosynthesis are different between the DOR and NOR groups [66], and subsequent research demonstrated that cholesterol-related metabolites, including coprostanone, 11 A-acetoxyprogesterone, 17α-hydroxyprogesterone, and estradiol, are decreased in the GCs of the DOR group [67]. Moreover, the transcriptional levels of SCAP, a key gene involved in cholesterol regulation, and CYP19A1, a key gene related to steroidogenesis, were downregulated in the DOR group. Cholesterol synthesis and transport-related genes such as IDI1, FDFT1, CYP51A1, and STARD1, also decreased significantly.
Mitochondria
Mitochondrial biogenesis is one of the key intracellular pathways involved in programming the ovarian reserve, and the mitochondrial state of GCs directly influences the ovarian reserve [68]. Enhanced ROS levels, reduced mitochondrial membrane potential (MMP, an index of mitochondrial function), and decreased mitochondrial DNA (mtDNA) copy number were observed in women with DOR [69]. Because mitochondria are active, their by-products, such as ROS, can intervene to natural cell function by damaging DNA and/or proteins in cells. Thus, increased ROS levels are associated with adverse follicular growth. In humans, mutated mtDNA is strongly associated with ageing phenotypes and reduced lifespan [70]. Introducing functional mtDNA or increasing the amount of mtDNA produced by resveratrol leads to the restoration of ovarian health [71, 72]. A study focusing on mitochondrial biogenesis via cells in the follicle found that mtDNA copy number is downregulated in both GCs and oocytes of DOR patients, and the expression level of PPARGC-1 A (a transcriptional co-activator regulating mitochondrial biogenesis and activity), POLG (encoding the enzyme synthesizing mtDNA), OPA1 (involved in mitochondrial dynamics) and TFAM (a transcription factor related to mtDNA transcription and replication) is decreased significantly in GCs of human with DOR [73]. Therefore, it is obvious that the dysfunction of mitochondrial biogenesis has a detrimental effect on the ovarian reserve and ultimately induces infertility.
Possible mechanism of incongruous metabolism in DOR
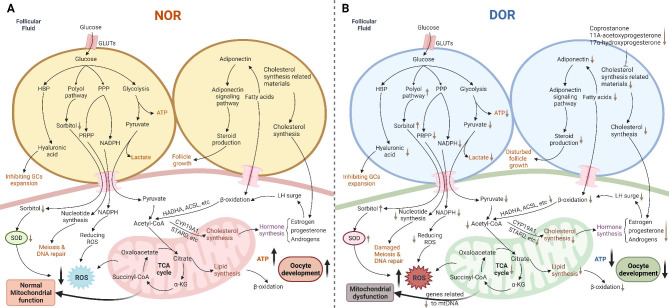
These orchestrated metabolic pathways play an initial role in maintaining the ovarian reserve (Fig. 2A). According to previous studies and the mechanism of metabolism in ovarian function, we hypothesized that the bidirectional communication of glucose, lipids, and cholesterol in the COC may illustrate a possible mechanism of DOR (Fig. 2B): First, during glucose metabolism, the expression of GLUTs in the cytomembrane of GCs may decrease, which may induce a lack of glucose in the GCs. Thus, glycolysis, the PPP, and the HBP were suppressed. This suppression results in deficiencies of pyruvate, NADPH, and hyaluronic acid. However, the polyol pathway is likely to activate by some potential factors which can enhance sorbitol levels. Second, fatty acids have been found in patients with DOR, and a lack of fatty acids reduces the function of β-oxidation, and reduces the cholesterol synthesis in oocyte. Decreased cholesterol synthesis in COC lead to insufficient of steroid hormones, and decreased estrogen cannot stimulate LH surge-induced β-oxidation. Third, mitochondrial dysfunction is an important cause of DOR, as it is the pivot of glucose and lipids metabolism. Furthermore, pyruvate and fatty acids transported to the oocytes are converted to acetyl-CoA, which participates in the TCA cycle to generate sufficient ATP for follicular development. However, this process is abnormal in the DOR. Overall, these dysfunctions are related to a lack of energy, insufficient substances, and rising ROS, and ultimately induce abnormal follicle growth, which is a feature of DOR.
Fig. 2.
The interconnected loop of glucose, lipids cholesterol and mitochondrial metabolism. A Well-function of glucose, lipids, cholesterol and mitochondrial metabolism in maintaining ovarian reserve. B Abnormal metabolism of glucose, fatty acids, and cholesterol in the DOR model. Glycolysis, the PPP, and the HBP were suppressed because of decreased GLUTs expression. Deficiencies of pyruvate, NADPH, and hyaluronic acid will lead to a lack of ATP, increased ROS level, damage of meiosis and DNA repair, and inhibition of GCs expansion. The activation of the polyol pathway can enhance sorbitol levels, leading to up-regulated SOD level and impairs mitochondrial function. A lack of fatty acids reduces the function of β-oxidation and the cholesterol synthesis in oocyte, which lead to insufficient of steroid hormones. Decreased estrogen cannot stimulate LH surge-induced β-oxidation. Insufficient of steroid hormones and ATP caused by these metabolic pathways can disturb follicle growth. Mitochondria is the pivot of glucose and lipids metabolism, and its dysfunction can impair these processes
Maternal influences on primordial follicular pool of the fetus
The embryonic period plays a key role in the establishment of ovarian function. The ovaries of female human newborn babies contain approximately 1–2 million of non-growing follicles, which is a fixed and established number. The count of NFGs or even reserved oocytes later decreases due to follicular atresia and apoptosis with aging, and finally, the decline reaches its culmination in menopause [74–76]. Therefore, the proportion of ovarian reserve achieved before birth determines the later ovarian reserve and limits the reproductive lifespan of a female [77]. This section focusing on the maternal factors that can affect primordial follicular pool of their offspring.
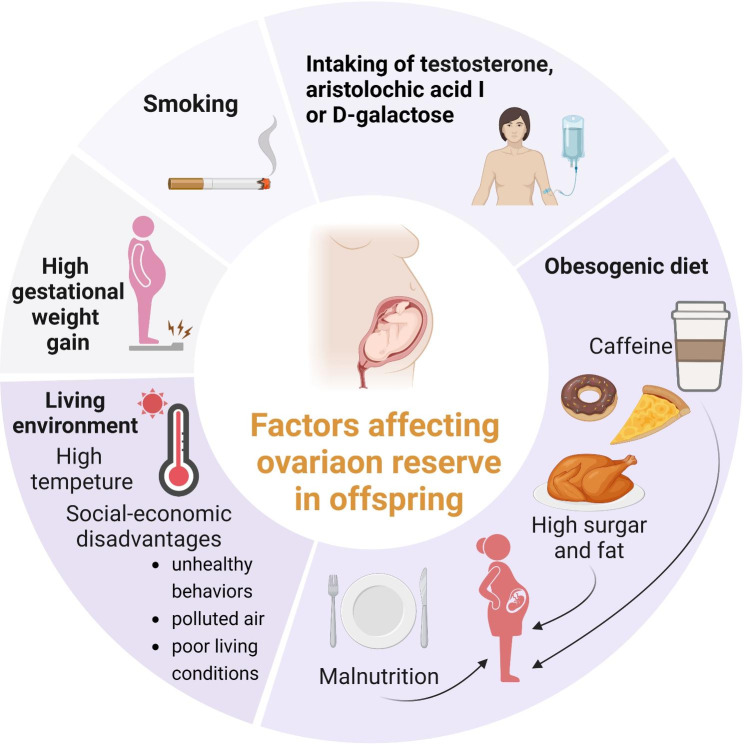
Because the intrauterine environment is closely related to fetal growth and development, an unhealthy intrauterine environment can have a detrimental effect on a newborn’s ovarian reserve. For example, some studies have found that maternal testosterone treatment [78], high gestational weight gain and smoking during pregnancy [79, 80], as well as exposure to aristolochic acid I [81] and D-galactose exposure [82], which are associated with an abnormal intrauterine environment, may interfere with the primordial folliculogenesis of the fetal ovary.
Dietary habits before or during pregnancy can affect the reproductive function of offspring. Studies in animal models have suggested that maternal undernutrition results in a smaller ovary in the fetus and an increased testosterone concentration [83, 84]. In addition, protein restriction in mothers has been shown to impair germ cell and blood vessel development in the fetal ovaries of sheep and the number of primordial follicles in mice [85, 86]. The second study also found that primordial follicles were significantly depleted by 37% at PN21 and 51% at 24-week mice with statistical significance. It has been suggested that the effects of inadequate protein intake may persist for two generations or more, based on evidence that low-protein grandmother diets lead to DOR and a rapid decline in ovarian telomere length [87]. However, if the offspring are exposed to a maternal obesogenic (high-fat or high-sugar) diet, their ovarian reserve will likely be depleted as they grow up [88]. Moreover, maternal caffeine intake during pregnancy is negatively associated with the ovarian reserve in offspring [89].
The ovarian reserve of the offspring is also likely to be affected by the socio-economic environment in which the mothers live [90]. Lower ovarian reserve in child with maternal socio-economic disadvantages may be caused by exposure to unhealthy behaviors, polluted air and poor living conditions in socio-economically disadvantaged areas, resulting in an unsuitable intrauterine growth environment. Evidence from a US farm demonstrated that prenatal exposure to the farm environment (reflecting an uninhabitable living environment) is associated with lower anti-Müllerian hormone concentrations in adulthood, supporting the link between maternal living conditions and the ovarian reserve of their children [91]. Furthermore, exposure to high environmental temperatures impairs the establishment of ovarian reserves in offspring [92]. Chronic inflammation of mother induces intrauterine growth restriction in the fetuses and reduction in the follicles in primordial follicular pool through premature cell apoptosis [93].
All in all, these maternal influences on primordial follicular pool of fetus have been summarized in Fig. 3. Since the promising applications and investigations of artificial intelligence in reproductive medicine [94], scientists can contribute a digital model, which depends on the combination these risk factors and clinical symptoms, to predict how much can maternal factors affects their offspring’s ovarian reserve.
Fig. 3.
Summary of DOR caused by maternal reasons. Maternal risks that impair ovarian reserve of their offspring have been summarized, including high gestational weight gain, smoking, treatments, diet, and living environment
Internal and external factors affecting ovarian reserve
Follicle fluid
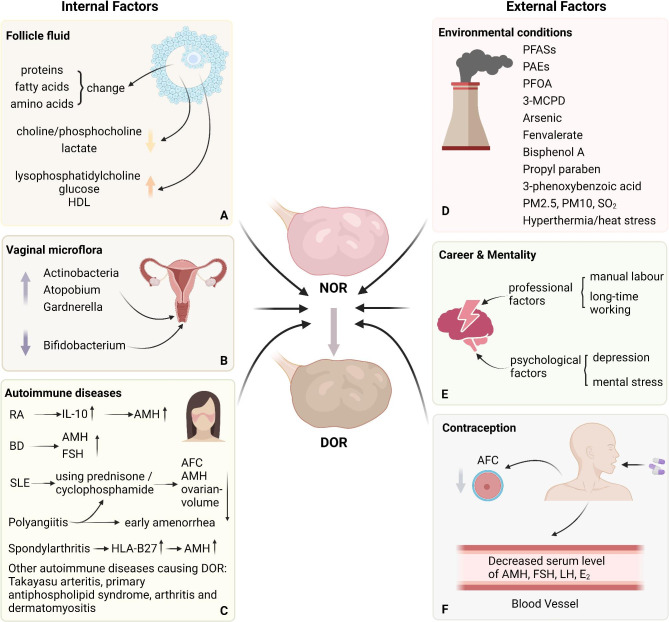
The follicle is a unique microenvironment, and as the immediate contact environment of the follicle, follicular fluid plays an essential role in providing the necessary nutrients and material for follicle maturation and oocyte growth. Previous studies have revealed the presence of various proteins associated with reproductive disorders [95, 96]. Refinement of plasma proteins, which make up a significant proportion of the protein in the follicular fluid, showed that the remaining fractions were mainly classified as inflammation-related proteins, complement factors, and proteins involved in lipid metabolism [96, 97]. Fatty acids are important components of the follicular fluid. For example, higher linolenic acid, lower palmitic acid, the concentration of saturated fatty acids, including arachidic acid, erucic acid, tricosanoic acid, and lignoceric acid, and an unbalanced ratio of n-6: n-3 polyunsaturated fatty acids may negatively affect oocyte quality [98, 99]. Follicular T4 and P4 levels are related to several fatty acids, including arachidonic acid, pentadecanoic acid and heptadecanoic acid [98]. In addition, higher lysophosphatidylcholine levels in follicular fluid are negatively associated with follicle growth [100]. The role of amino acids in the follicular fluid cannot be underestimated. L-alanine, glycine and L-glutamate are positively correlated with oocyte quality, and their deficiency is related to later abnormal blastocyst development [98]. However, in vitro, the high turnover of amino acids, especially valine and isoleucine, in oocytes reflect decreased developmental potential [101]. Moreover, decreasing lactate and choline/phosphocholine concentrations and increasing glucose levels and high-density lipoprotein (HDL) levels in follicular fluid are related to the dysfunction of oocyte development [102]. The concentration of hormones such as AMH and progesterone in the follicular fluid affects oocyte quality [103]. Most importantly, these studies will allow investigation of the relationship between follicular fluid content and ovarian reserve (Fig. 4A).
Fig. 4.
The influence of ovarian reserve caused by internal and external factors. A The internal factors including changes of contents in follicle fluid, vaginal microflora alteration, and autoimmune diseases. In follicular fluids, the concentration of proteins, fatty acids, and amino acids, the decreased choline/phosphocholine and lactate, and increased lysophosphatidylcholine, glucose, and HDL are related to DOR. B The Actinobacteria, Atopobium and Gardnerella are increased in vagina of patients with DOR while Bifidobacterium is decreased. C Some autoimmune diseases can lead to DOR by regulating the level of related hormones. D The external factors including environmental pollution, psychological problems, and taking contraception. Living conditions such as persistent organic pollutants, polluted air, and heat stress can affect ovarian reserve by disturbing follicular development, inducing ovarian fibrosis, enhancing GCs apoptosis, and up-regulating inflammatory factors expression. E Psychological factors are potential influence of ovarian reserve. F Taking contraception can reduce the serum level of AMH, FSH, LH, and E2, and AFC.
Vaginal microflora
The vaginal microbiota is relative to women of reproductive age, and its variation is significantly correlated with the decline in ovarian reserve [104]. Using AMH, inhibin B, FSH, and LH as ovarian reserve biomarkers, Actinobacteria, Atopobium and Gardnerella were found to be negatively associated with AMH and inhibin B and positively associated with FSH and LH. However, Bifidobacterium exhibited the opposite effect. Its levels were positively associated with AMH levels and negatively associated with FSH and LH levels. This finding agrees with the need to focus on the effects of dysbacteriosis of in vaginal environment on oocyte reserves (Fig. 4B).
Autoimmune diseases
Autoimmune diseases are also associated with reduced fertility. The number of AFC, AMH level and determination of ovarian volume were significantly decreased in systemic lupus erythematosus (SLE) patients [105, 106], and the AMH level is related to the duration of the disease and The Systemic Lupus International Collaborating Clinics damage index [107], which is a criterion for the diagnosis and severity of SLE. Another study indicated that disease activity did not seem to affect the ovarian reserve and adrenal gland hormones in adult female SLE patients; the median ovarian volume was significantly lower in SLE patients with current prednisone use [108]. Childhood SLE patients treated with cyclophosphamide had higher median FSH levels and lower median AMH and AFC levels in adulthood than those not treated with cyclophosphamide [109]. Moreover, exposure to and accumulation of cyclophosphamide affect ovarian function and lead to DOR in women with SLE [110] (Fig. 4C). However, as described in an article on disease severity and ovarian reserve tests, it is difficult to determine whether the disease itself or its treatment caused the outcome [111].
A recent systematic review and meta-analysis involving 679 patients with rheumatoid arthritis (RA) and 1,460 controls showed that patients with RA have lower AMH levels [112]. A previous study demonstrated that the AMH level was lower in the RA group, but there was no significance of AMH levels in the activity rheumatoid arthritis, rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), erosions, C-reactive protein (CRP) level or therapeutic schedule [113]. Another study reported a similar result, but found a negative correlation between the levels of AMH and IL-10 [114]. Therefore, we suggest that the decrease in AMH levels in RA patients may not be caused by RF but may be related to a more common inflammatory factor caused by RA, and IL-10 is one of the possibilities (Fig. 4C).
Women with Bechet’s disease (BD) have lower serum AMH levels and higher FSH levels [115], indicating that infertility in these patients may be due to decreased ovarian reserve; however, another study focusing on similar research found no difference [116] (Fig. 4C). As a specific biomarker of spondylarthritis, the expression of HLA-B27 is negatively associated with AMH levels, suggesting an adverse impact on the ovarian reserve in the patients [117] (Fig. 4C). A study involving 42 women with polyangiitis who were receiving oral cyclophosphamide therapy found that these women had significantly lower AMH levels, higher FSH levels, and the possibility of early amenorrhea, which conforms to the DOR standard, indicating that exposure to cyclophosphamide is likely to result in decline in the ovarian reserve [118] (Fig. 4C). Additionally, a tendency for the ovarian reserve to decline has been observed in Takayasu arteritis [119], primary antiphospholipid syndrome [120], arthritis [121], and dermatomyositis [122] (Fig. 4C).
In conclusion, it is still controversial whether infertility due to autoimmune diseases is associated with reduced ovarian reserve function; however, the correlation between autoimmune diseases and DOR cannot be ruled out.
Environmental exposure
Life events can affect organ function at the ovarian reserve level [123, 124] (Fig. 4D). Because environmental pollution and unhealthy lifestyles are becoming global problems, it is advisable to pay more attention to the effects of environmental pollution, changes in ambient temperature, and poor habits on the reproductive capacity of women.
Persistent organic pollutants caused by pervasive contaminants in drinking water, food, everyday packaging materials, and other contact substances can also reduce ovarian reserve. Exposure to perfluoroalkyl and polyfluoroalkyl substances (PFASs) causes the depletion of follicular cells, promoting menopause and infertility [125]. The influence of PFASs is dose-dependent and leads to decreased serum levels of estradiol and progesterone and apoptosis of oocyte [126, 127]. The toxicity of 3-monochloro-1,2-propanediol (3-MCPD) profoundly affects female ovarian function, including a lower ovary/body ratio, regulation of follicular development, and AFC, higher ovarian fibrosis, GCs apoptosis, and increased expression of inflammatory factors [128]. Moreover, corticosterone and cortisol in FF of women expose to phthalates (PAEs), a plasticizer which can release into environment, are decreased significantly [129].
Individuals may unconsciously be exposed to toxic substances. For instance, a high concentration of arsenic has been found in women with DOR, and arsenic inhibits the expression of steroidogenic factor-1 by upregulating the DNA methylation level of its promoter region resulting in decreased expression of relative proteins, including STAR, CYP11A1 and CYP19A1, which are important genes related to lipid metabolism during follicle growth [130]. Chronic exposure to propyl paraben [131], 3-phenoxybenzoic acid [132], or bisphenol A [133] was strongly associated with abnormal laboratory test results, indicating potential toxicity to the ovarian reserve. Fenvalerate, an insecticide widely used in modern agriculture, inhibits follicle expansion by interfering with steroidogenesis by downregulating STAR and P450 side chain cleavage enzyme expression [134]. A recent study has also found that perfluorooctanoic acid (PFOA) is enriched in the FF of patients with DOR and that the metabolic composition of FF is affected by PFOA [135].
Increasing attention is being paid to the effects of the living environment on ovarian reserve. There is an inverse relationship between outdoor air pollution (PM2.5, PM10 and SO2) and the reduction in AFC or AMH [136–139]. Previous studies have illustrated that the development of ovarian follicles and oocyte competence are counteracted by hyperthermia or heat stress [140, 141]. In humans, with an average increase in the ambient temperature of around 1 °C, AFC decreases by 1.6%, indicating that heat is a negative factor in ovarian reserve [142]. As environmental stress, heat stress induces the apoptosis of ovarian cells through several processes, such as enhancing the expression level of BCL2L1(regulator of apoptosis during mammalian ovarian maturation) [141] and miR-33 (factor suppressing VEGF signaling) [143], regulating heat stress proteins (HSP70 and HAPA13) levels [144], and activating the FasL/Fas and TNF-a systems [145]. Heat stress also alters glucose, cholesterol and non-esterified fatty acid levels in FF [146], greatly reducing gonadotropin receptor expression in GCs [147].
Psychological factors
Studies focusing on occupational factors have shown that women working hard and long hours, especially those who have to handle heavy objects and work non-daily shifts, have lower ovarian reserve [148]. Evidence shows that this negative effect may promote a decline in AFC in women [149]. Furthermore, women with a history of depression have significantly higher FSH levels, and psychological stress is negatively related to AMH levels in females with statistical significance [150]. On the other hand, DOR women with higher self-esteem may have less fertility distress and a better effect on reproductive outcome [151]. Therefore, we hypothesized that the working model, as one of the most common daily influences, may be closely related to the mental state of professional women, and poor psychological conditions, such as fatigue, anxiety, despondency or tension, are a vulnerability factor in DOR, or optimism is a protective factor in maintaining the ovarian reserve. Part E of Fig. 4 have shown above results.
Contraception
With the development of pharmaceutical science and female awareness, contraception usage to avoid pregnancy is becoming more common among women aged 15–50 [152]. It is reported that almost 90% of sexually active women who do not want to become pregnant use contraception [153], demonstrating that contraception is an influential element of DOR. An investigation involving 887 women using or not using oral contraception found a significant decrease in AMH levels and a reduced AFC of approximately 5-7 mm and 8-10 mm in diameter, after adjusting for age, BMI, smoking and maternal age at menopause [154]. A study with a large number of recruits suggested a similar result: women who use hormonal contraceptives or combined oral contraceptive pills have a lower mean AMH level than women who do not use contraceptives [155]. Moreover, hormonal contraception can impair AFC, ovarian volume, and ovarian vascular indices and reduce serum FSH, LH, and E2 levels [156, 157] (Fig. 4F).
Possible intervention in the future
Assisted reproduction technique (ART) is a final method for infertilities, whereas, studies have found that these treatments may lead to adverse outcomes for their babies, who are more likely to have neuro-psycho-motor malfunction, high risk of small for gestational age babies, low birthweight, preterm birth (PTB), and congenital heart diseases [158–160]. Previous studies have summarized that cell or gene therapies can resuscitate ovarian function which is affected by internal and external factors [161, 162]. Thus, it is beneficial to find some new methods to intervene in patients themselves rather than blindly rely on ART, and this part put forward possible pointcuts.
Conclusion
This review provides clear and convicting evidence that the mechanism of the DOR is so intricate and ambiguous that it must be rearranged and recognized. These results would be conducive to a better understanding of the mechanisms and influence of follicular development and ovarian reserves. Collectively, subsequent studies may look at aspects of mechanistic studies on how sex hormones and metabolism affect female ovarian reserve, disease, risk factors, and maternal levels of certain hormones during pregnancy; advanced studies on the elimination of risk factors in living conditions; and social studies on how to pay more attention to women’s mental health.
Acknowledgements
Not applicable.
Abbreviations
- DOR
Diminished ovarian reserve
- NOR
Normal ovarian reserve
- GCs
Granulosa cells
- AFC
Antral follicle count
- AMH
Antimüllerian hormone
- FSH
Follicle-stimulating hormone
- LH
Luteinizing hormone
- E2
Estrogen
- P
Progesterone
- AMHR
Antimüllerian hormone receptor
- AR
Androgen receptor
- FSHR
Follicle-stimulating hormone receptor
- LHR
Luteinizing hormone receptor
- ER
Estrogen receptor
- PR
Progesterone receptor
- FF
Follicular fluid
- GLUT
Glucose transporter
- COC
Cumulus-oocyte complexes
- PPP
Pentose phosphate pathway
- HBP
Hexosamine biosynthetic pathway
- ROS
Reactive oxygen species
- PRPP
Phosphoribosyl pyrophosphate
- SOD
Superoxide dismutase
- cAMP
Cyclic adenosine monophosphate
- MMP
Mitochondrial membrane potential
- mtDNA
Mitochondrial DNA
- HDL
High-density lipoprotein
- SLE
Systemic lupus erythematosus
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- anti-CCP
Anti-cyclic citrullinated peptide
- CRP
C-reactive protein
- BD
Bechet’s disease
- PFASs
Polyfluoroalkyl substances
- PFOA
Perfluorooctanoic acid
- ART
Assisted reproduction technique
Authors’ contributions
Zhu Q.Y. contributed to conceptualization of the idea of the review and drafted the manuscript. Li Y. edited all figures. Ma J.H. critically reviewed the manuscript. Ma H. performed some parts of literature search. Liang X.L. approved the final version of the manuscript.
Funding
National Natural Science Foundation of China (No.81960278).
Science and Technology Plans Project of Lanzhou City (2021-1-45).
Excellence Program Project for students of the First Clinical Medical School of Lanzhou University (20220060008).
Data Availability
The datasets generated and/or analyzed during the current study are available in the MEDLINE repository. https://pubmed.ncbi.nlm.nih.gov/.
All figures were depicted by BioRender. https://www.biorender.com/.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20:353–69. doi: 10.1093/humupd/dmt057. [DOI] [PubMed] [Google Scholar]
- 2.Mark-Kappeler CJ, Hoyer PB, Devine PJ. Xenobiotic effects on ovarian preantral follicles. Biol Reprod. 2011;85:871–83. doi: 10.1095/biolreprod.111.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Chang Y, Wei L, Chen J, Li J, Goldsmith S, Silber S, Liang X. Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J Assist Reprod Genet. 2019;36:1225–35. doi: 10.1007/s10815-019-01446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Testing Interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151–7. doi: 10.1016/j.fertnstert.2020.09.134. [DOI] [PubMed] [Google Scholar]
- 5.Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217:129–40. doi: 10.1016/j.ajog.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Tal R, Seifer DB. Potential mechanisms for racial and ethnic differences in antimüllerian hormone and ovarian reserve. Int J Endocrinol. 2013;2013:818912. doi: 10.1155/2013/818912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2021;70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Alviggi C, Esteves SC, Conforti A. Ovarian reserve tests: are they only a quantitative measure? Fertil Steril. 2020;113:761–2. doi: 10.1016/j.fertnstert.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Assens M, Dyre L, Henriksen LS, Brocks V, Sundberg K, Jensen LN, Pedersen AT, Main KM. Menstrual pattern, Reproductive hormones, and transabdominal 3D Ultrasound in 317 adolescent girls. J Clin Endocrinol Metab 2020, 105. [DOI] [PubMed]
- 10.Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31:1522–30. doi: 10.1093/humrep/dew100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25:1282–7. doi: 10.1093/humrep/deq019. [DOI] [PubMed] [Google Scholar]
- 12.Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–34. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 13.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 14.Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C. Which follicles make the most anti-mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–27. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 15.Loumaye E, Engrand P, Shoham Z, Hillier SG, Baird DT. Clinical evidence for an LH ceiling? Hum Reprod. 2003;18:2719–20. doi: 10.1093/humrep/deg493. [DOI] [PubMed] [Google Scholar]
- 16.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- 17.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- 18.Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online. 2007;15:326–37. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- 19.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–50. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarthi VP, Ratri A, Masumi S, Borosha S, Ghosh S, Christenson LK, Roby KF, Wolfe MW, Rumi MAK. Granulosa cell genes that regulate ovarian follicle development beyond the antral stage: the role of estrogen receptor β. Mol Cell Endocrinol. 2021;528:111212. doi: 10.1016/j.mce.2021.111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baird DT. A model for follicular selection and ovulation: lessons from superovulation. J Steroid Biochem. 1987;27:15–23. doi: 10.1016/0022-4731(87)90289-5. [DOI] [PubMed] [Google Scholar]
- 22.Regan SL, Knight PG, Yovich JL, Stanger JD, Leung Y, Arfuso F, Dharmarajan A, Almahbobi G. Infertility and ovarian follicle reserve depletion are associated with dysregulation of the FSH and LH receptor density in human antral follicles. Mol Cell Endocrinol. 2017;446:40–51. doi: 10.1016/j.mce.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Wen J, Huang K, Du X, Zhang H, Ding T, Zhang C, Ma W, Zhong Y, Qu W, Liu Y, et al. Can Inhibin B reflect Ovarian Reserve of Healthy Reproductive Age women effectively? Front Endocrinol (Lausanne) 2021;12:626534. doi: 10.3389/fendo.2021.626534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89:927–33. doi: 10.1016/j.fertnstert.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 25.Yding Andersen C. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol Hum Reprod. 2017;23:16–24. doi: 10.1093/molehr/gaw070. [DOI] [PubMed] [Google Scholar]
- 26.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Khristi V, Chakravarthi VP, Singh P, Ghosh S, Pramanik A, Ratri A, Borosha S, Roby KF, Wolfe MW, Rumi MAK. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Mol Cell Endocrinol. 2018;474:214–26. doi: 10.1016/j.mce.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 28.van Dessel HJ, Schipper I, Pache TD, van Geldorp H, de Jong FH, Fauser BC. Normal human follicle development: an evaluation of correlations with oestradiol, androstenedione and progesterone levels in individual follicles. Clin Endocrinol (Oxf) 1996;44:191–8. doi: 10.1046/j.1365-2265.1996.662483.x. [DOI] [PubMed] [Google Scholar]
- 29.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–37. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 30.Yuan XH, Yang CR, Wang XN, Zhang LL, Gao XR, Shi ZY. Progesterone maintains the status of granulosa cells and slows follicle development partly through PGRMC1. J Cell Physiol. 2018;234:709–20. doi: 10.1002/jcp.26869. [DOI] [PubMed] [Google Scholar]
- 31.Lenie S, Smitz J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol Reprod. 2009;80:685–95. doi: 10.1095/biolreprod.107.067280. [DOI] [PubMed] [Google Scholar]
- 32.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–61. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 33.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101:11209–14. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103:224–9. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–9. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- 36.Pankhurst MW, Kelley RL, Sanders RL, Woodcock SR, Oorschot DE, Batchelor NJ. Anti-Müllerian hormone overexpression restricts preantral ovarian follicle survival. J Endocrinol. 2018;237:153–63. doi: 10.1530/JOE-18-0005. [DOI] [PubMed] [Google Scholar]
- 37.Fujibe Y, Baba T, Nagao S, Adachi S, Ikeda K, Morishita M, Kuno Y, Suzuki M, Mizuuchi M, Honnma H, et al. Androgen potentiates the expression of FSH receptor and supports preantral follicle development in mice. J Ovarian Res. 2019;12:31. doi: 10.1186/s13048-019-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warzych E, Lipinska P. Energy metabolism of follicular environment during oocyte growth and maturation. J Reprod Dev. 2020;66:1–7. doi: 10.1262/jrd.2019-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27:27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Zhang W, Wang Z, Zheng N, Yuan F, Li B, Li X, Deng L, Lin M, Chen X, Zhang M. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mTOR signaling. Cell Death Dis. 2022;13:87. doi: 10.1038/s41419-022-04541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cetica P, Pintos L, Dalvit G, Beconi M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction. 2002;124:675–81. [PubMed] [Google Scholar]
- 42.Herta AC, von Mengden L, Akin N, Billooye K, Coucke W, van Leersum J, Cava-Cami B, Saucedo-Cuevas L, Klamt F, Smitz J, Anckaert E. Characterization of carbohydrate metabolism in in vivo- and in vitro-grown and matured mouse antral follicles†. Biol Reprod. 2022;107:998–1013. doi: 10.1093/biolre/ioac124. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Hayashi Y, Takehara A, Ito-Matsuoka Y, Tachibana M, Yaegashi N, Matsui Y. Abnormal early folliculogenesis due to impeded pyruvate metabolism in mouse oocytes†. Biol Reprod. 2021;105:64–75. doi: 10.1093/biolre/ioab064. [DOI] [PubMed] [Google Scholar]
- 44.Baufeld A, Vanselow J. Lactate-induced effects on bovine granulosa cells are mediated via PKA signaling. Cell Tissue Res. 2022;388:471–7. doi: 10.1007/s00441-021-03569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J, Huo P, Cui K, Wei H, Cao J, Wang J, Liu Q, Lei X, Zhang S. Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome. Cell Commun Signal. 2022;20:61. doi: 10.1186/s12964-022-00876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoque SAM, Umehara T, Kawai T, Shimada M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic Biol Med. 2021;163:344–55. doi: 10.1016/j.freeradbiomed.2020.12.434. [DOI] [PubMed] [Google Scholar]
- 47.Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod. 1998;58:1084–94. doi: 10.1095/biolreprod58.4.1084. [DOI] [PubMed] [Google Scholar]
- 48.Herrick JR, Brad AM, Krisher RL. Chemical manipulation of glucose metabolism in porcine oocytes: effects on nuclear and cytoplasmic maturation in vitro. Reproduction. 2006;131:289–98. doi: 10.1530/rep.1.00835. [DOI] [PubMed] [Google Scholar]
- 49.Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139:685–95. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- 50.Frank LA, Sutton-McDowall ML, Brown HM, Russell DL, Gilchrist RB, Thompson JG. Hyperglycaemic conditions perturb mouse oocyte in vitro developmental competence via beta-O-linked glycosylation of heat shock protein 90. Hum Reprod. 2014;29:1292–303. doi: 10.1093/humrep/deu066. [DOI] [PubMed] [Google Scholar]
- 51.Brownlee M. Biochemistry and molecular cell biology of diabetic Complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 52.Wang YC, Ma YD, Liu H, Cui ZH, Zhao D, Zhang XQ, Zhang LX, Guo WJ, Long Y, Tu SS, et al. Hyperandrogen-induced polyol pathway flux increase affects ovarian function in polycystic ovary syndrome via excessive oxidative stress. Life Sci. 2023;313:121224. doi: 10.1016/j.lfs.2022.121224. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Yan Z, Liu H, Li L, Yuan C, Qin L, Cai L, Liu J, Hu Y, Cui Y. Sorbitol accumulation decreases oocyte quality in aged mice by altering the intracellular redox balance. Aging. 2021;13:25291–303. doi: 10.18632/aging.203747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auclair S, Uzbekov R, Elis S, Sanchez L, Kireev I, Lardic L, Dalbies-Tran R, Uzbekova S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am J Physiol Endocrinol Metab. 2013;304:E599–613. doi: 10.1152/ajpendo.00469.2012. [DOI] [PubMed] [Google Scholar]
- 55.de Andrade Melo-Sterza F, Poehland R. Lipid metabolism in bovine oocytes and early embryos under in vivo, in Vitro, and stress conditions. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed]
- 56.Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014;148:R15–27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z, Fan Q, Zhu Q, He R, Li Y, Liu C, Wang J, Liang X. Decreased fatty acids induced granulosa cell apoptosis in patients with diminished ovarian reserve. J Assist Reprod Genet. 2022;39:1105–14. doi: 10.1007/s10815-022-02462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbe A, Bongrani A, Mellouk N, Estienne A, Kurowska P, Grandhaye J, Elfassy Y, Levy R, Rak A, Froment P, Dupont J. Mechanisms of Adiponectin Action in Fertility: an overview from Gametogenesis to Gestation in humans and animal models in normal and pathological conditions. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed]
- 59.Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y, Lin Y, Jin Y, Roy G, Zhao A, Li F. Adiponectin Deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology. 2016;157:4875–87. doi: 10.1210/en.2015-2080. [DOI] [PubMed] [Google Scholar]
- 60.Grandhaye J, Hmadeh S, Plotton I, Levasseur F, Estienne A, LeGuevel R, Levern Y, Ramé C, Jeanpierre E, Guerif F, et al. The adiponectin agonist, AdipoRon, inhibits steroidogenesis and cell proliferation in human luteinized granulosa cells. Mol Cell Endocrinol. 2021;520:111080. doi: 10.1016/j.mce.2020.111080. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe H, Hirai S, Tateno H, Fukui Y. Variation of cholesterol contents in porcine cumulus-oocyte complexes is a key factor in regulation of fertilizing capacity. Theriogenology. 2013;79:680–6. doi: 10.1016/j.theriogenology.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 62.Comiskey M, Warner CM. Spatio-temporal localization of membrane lipid rafts in mouse oocytes and cleaving preimplantation embryos. Dev Biol. 2007;303:727–39. doi: 10.1016/j.ydbio.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ke FC, Chuang LC, Lee MT, Chen YJ, Lin SW, Wang PS, Stocco DM, Hwang JJ. The modulatory role of transforming growth factor beta1 and androstenedione on follicle-stimulating hormone-induced gelatinase secretion and steroidogenesis in rat granulosa cells. Biol Reprod. 2004;70:1292–8. doi: 10.1095/biolreprod.103.023531. [DOI] [PubMed] [Google Scholar]
- 64.Sahmi M, Nicola ES, Silva JM, Price CA. Expression of 17beta- and 3beta-hydroxysteroid dehydrogenases and steroidogenic acute regulatory protein in non-luteinizing bovine granulosa cells in vitro. Mol Cell Endocrinol. 2004;223:43–54. doi: 10.1016/j.mce.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Burks DM, McCoy MR, Dutta S, Mark-Kappeler CJ, Hoyer PB, Pepling ME. Molecular analysis of the effects of steroid hormones on mouse meiotic prophase I progression. Reprod Biol Endocrinol. 2019;17:105. doi: 10.1186/s12958-019-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He R, Zhao Z, Yang Y, Liang X. Using bioinformatics and metabolomics to identify altered granulosa cells in patients with diminished ovarian reserve. PeerJ. 2020;8:e9812. doi: 10.7717/peerj.9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang X, Zhao Z, Fan Q, Li H, Zhao L, Liu C, Liang X. Cholesterol metabolism is decreased in patients with diminished ovarian reserve. Reprod Biomed Online. 2022;44:185–92. doi: 10.1016/j.rbmo.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16:715–25. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Z, Shi C, Han H, Wang Y, Liang R, Chen X, Shen H. Mitochondria-related changes and metabolic dysfunction in low prognosis patients under the POSEIDON classification. Hum Reprod. 2021;36:2904–15. doi: 10.1093/humrep/deab203. [DOI] [PubMed] [Google Scholar]
- 70.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlöf S, Oldfors A, Wibom R, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 71.Ross JM, Stewart JB, Hagström E, Brené S, Mourier A, Coppotelli G, Freyer C, Lagouge M, Hoffer BJ, Olson L, Larsson NG. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature. 2013;501:412–5. doi: 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ragonese F, Monarca L, De Luca A, Mancinelli L, Mariani M, Corbucci C, Gerli S, Iannitti RG, Leonardi L, Fioretti B. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertil Steril. 2021;115:1063–73. doi: 10.1016/j.fertnstert.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Boucret L, Chao de la Barca JM, Morinière C, Desquiret V, Ferré-L’Hôtellier V, Descamps P, Marcaillou C, Reynier P, Procaccio V, May-Panloup P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod. 2015;30:1653–64. doi: 10.1093/humrep/dev114. [DOI] [PubMed] [Google Scholar]
- 74.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS ONE. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 76.Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M, Tapanainen JS. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86:3421–9. doi: 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- 77.Mazaud S, Guigon CJ, Lozach A, Coudouel N, Forest MG, Coffigny H, Magre S. Establishment of the reproductive function and transient fertility of female rats lacking primordial follicle stock after fetal gamma-irradiation. Endocrinology. 2002;143:4775–87. doi: 10.1210/en.2002-220464. [DOI] [PubMed] [Google Scholar]
- 78.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–93. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 79.Fraser A, McNally W, Sattar N, Anderson EL, Lashen H, Fleming R, Lawlor DA, Nelson SM. Prenatal exposures and anti-mullerian hormone in female adolescents: the Avon Longitudinal Study of parents and children. Am J Epidemiol. 2013;178:1414–23. doi: 10.1093/aje/kwt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strohsnitter WC, Hatch EE, Hyer M, Troisi R, Kaufman RH, Robboy SJ, Palmer JR, Titus-Ernstoff L, Anderson D, Hoover RN, Noller KL. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol. 2008;167:727–33. doi: 10.1093/aje/kwm351. [DOI] [PubMed] [Google Scholar]
- 81.Qiqi L, Junlin H, Xuemei C, Yi H, Fangfang L, Yanqing G, Yan Z, Lamptey J, Zhuxiu C, Fangfei L, et al. Fetal exposure of aristolochic acid I undermines ovarian reserve by disturbing primordial folliculogenesis. Ecotoxicol Environ Saf. 2022;236:113480. doi: 10.1016/j.ecoenv.2022.113480. [DOI] [PubMed] [Google Scholar]
- 82.Rostami Dovom M, Noroozzadeh M, Mosaffa N, Piryaei A, Zadeh-Vakili A, Aabdollahifar MA, Rahmati M, Farhadi-Azar M, Ramezani Tehrani F. Maternal exposure to D-galactose reduces Ovarian Reserve in female rat offspring later in Life. Int J Endocrinol Metab. 2022;20:e123206. doi: 10.5812/ijem-123206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rae MT, Palassio S, Kyle CE, Brooks AN, Lea RG, Miller DW, Rhind SM. Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction. 2001;122:915–22. [PubMed] [Google Scholar]
- 84.Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JL, Hildebrandt TB, Lonergan P, Ireland JJ, Evans AC. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol Reprod. 2013;88:92. doi: 10.1095/biolreprod.112.107235. [DOI] [PubMed] [Google Scholar]
- 85.Nwachukwu CU, Woad KJ, Barnes N, Gardner DS, Robinson RS. Maternal protein restriction affects fetal ovary development in sheep. Reprod Fertil. 2021;2:161–71. doi: 10.1530/RAF-20-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winship AL, Gazzard SE, Cullen-McEwen LA, Bertram JF, Hutt KJ. Maternal low-protein diet programmes low ovarian reserve in offspring. Reproduction. 2018;156:299–311. doi: 10.1530/REP-18-0247. [DOI] [PubMed] [Google Scholar]
- 87.Aiken CE, Tarry-Adkins JL, Ozanne SE. Transgenerational Developmental Programming of Ovarian Reserve. Sci Rep. 2015;5:16175. doi: 10.1038/srep16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aiken CE, Tarry-Adkins JL, Penfold NC, Dearden L, Ozanne SE. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. Faseb j. 2016;30:1548–56. doi: 10.1096/fj.15-280800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eubanks AA, Nobles CJ, Hill MJ, DeCherney AH, Kim K, Sjaarda LA, Perkins NJ, Ye A, Zolton JR, Silver RM, et al. Recalled maternal lifestyle behaviors associated with anti-müllerian hormone of adult female offspring. Reprod Toxicol. 2020;98:75–81. doi: 10.1016/j.reprotox.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bleil ME, English P, Valle J, Woods NF, Crowder KD, Gregorich SE, Cedars MI. Is in utero exposure to maternal socioeconomic disadvantage related to offspring ovarian reserve in adulthood? Womens Midlife Health. 2018;4:5. doi: 10.1186/s40695-018-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upson K, Weinberg CR, Nichols HB, Dinse GE, D’Aloisio AA, Sandler DP, Baird DD. Early-life farm exposure and Ovarian Reserve in a US Cohort of women. Epidemiology. 2021;32:672–80. doi: 10.1097/EDE.0000000000001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Succu S, Sale S, Ghirello G, Ireland JJ, Evans ACO, Atzori AS, Mossa F. Exposure of dairy cows to high environmental temperatures and their lactation status impairs establishment of the ovarian reserve in their offspring. J Dairy Sci. 2020;103:11957–69. doi: 10.3168/jds.2020-18678. [DOI] [PubMed] [Google Scholar]
- 93.Shalom-Paz E, Weill S, Ginzberg Y, Khatib N, Anabusi S, Klorin G, Sabo E, Beloosesky R. IUGR induced by maternal chronic inflammation: long-term effect on offspring’s ovaries in rat model-a preliminary report. J Endocrinol Invest. 2017;40:1125–31. doi: 10.1007/s40618-017-0681-3. [DOI] [PubMed] [Google Scholar]
- 94.Medenica S, Zivanovic D, Batkoska L, Marinelli S, Basile G, Perino A, Cucinella G, Gullo G, Zaami S. The future is coming: Artificial Intelligence in the treatment of Infertility could improve assisted Reproduction outcomes-the Value of Regulatory Frameworks. Diagnostics (Basel) 2022, 12. [DOI] [PMC free article] [PubMed]
- 95.Schweigert FJ, Gericke B, Wolfram W, Kaisers U, Dudenhausen JW. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Hum Reprod. 2006;21:2960–8. doi: 10.1093/humrep/del257. [DOI] [PubMed] [Google Scholar]
- 96.Hanrieder J, Nyakas A, Naessén T, Bergquist J. Proteomic analysis of human follicular fluid using an alternative bottom-up approach. J Proteome Res. 2008;7:443–9. doi: 10.1021/pr070277z. [DOI] [PubMed] [Google Scholar]
- 97.Zakerkish F, Brännström M, Carlsohn E, Sihlbom C, van der Post S, Thoroddsen A. Proteomic analysis of follicular fluid during human ovulation. Acta Obstet Gynecol Scand. 2020;99:917–24. doi: 10.1111/aogs.13805. [DOI] [PubMed] [Google Scholar]
- 98.Matoba S, Bender K, Fahey AG, Mamo S, Brennan L, Lonergan P, Fair T. Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod Fertil Dev. 2014;26:337–45. doi: 10.1071/RD13007. [DOI] [PubMed] [Google Scholar]
- 99.O’Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction. 2013;146:389–95. doi: 10.1530/REP-13-0184. [DOI] [PubMed] [Google Scholar]
- 100.Yang J, Li Y, Li S, Zhang Y, Feng R, Huang R, Chen M, Qian Y. Metabolic signatures in human follicular fluid identify lysophosphatidylcholine as a predictor of follicular development. Commun Biol. 2022;5:763. doi: 10.1038/s42003-022-03710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hemmings KE, Maruthini D, Vyjayanthi S, Hogg JE, Balen AH, Campbell BK, Leese HJ, Picton HM. Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotrophin treatment. Hum Reprod. 2013;28:1031–44. doi: 10.1093/humrep/des458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril. 2012;97:1078–1084e1071. doi: 10.1016/j.fertnstert.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 103.O’Brien Y, Wingfield M, O’Shea LC. Anti-Müllerian hormone and progesterone levels in human follicular fluid are predictors of embryonic development. Reprod Biol Endocrinol. 2019;17:47. doi: 10.1186/s12958-019-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen J, Feng Y, Yan W, Yuan S, Zhang J, Luo A, Wang S. Vaginal microbiota changes in patients with premature ovarian insufficiency and its correlation with ovarian function. Front Endocrinol (Lausanne) 2022;13:824282. doi: 10.3389/fendo.2022.824282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morales-Martínez FA, Salas-Castro C, García-Garza MR, Valdés-Martínez O, García-Luna SM, Garza-Elizondo M, Vidal-Gutiérrez O. Saldívar-Rodríguez D, Sordia-Hernández LH: evaluation of the Ovarian Reserve in Women with systemic Lupus Erythematosus. J Family Reprod Health. 2021;15:38–44. doi: 10.18502/jfrh.v15i1.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulug P, Oner G, Kasap B, Akbas EM, Ozcicek F. Evaluation of ovarian reserve tests in women with systemic Lupus Erythematosus. Am J Reprod Immunol. 2014;72:85–8. doi: 10.1111/aji.12249. [DOI] [PubMed] [Google Scholar]
- 107.Martins NFE, Seixas MI, Pereira JP, Costa MM, Fonseca JE. Anti-müllerian hormone and ovarian reserve in systemic Lupus Erythematosus. Clin Rheumatol. 2017;36:2853–4. doi: 10.1007/s10067-017-3797-0. [DOI] [PubMed] [Google Scholar]
- 108.Lourenço DMR, Araújo DB, Aikawa NE, Yamakami LYS, Borba EF, Maciel GAR, Soares-Junior JM, Baracat EC, Pereira RMR, Bonfa E, Silva CA. Adrenal steroidogenesis and ovarian reserve in adult childhood-onset systemic lupus erytematosus patients. Clin Rheumatol. 2021;40:3651–8. doi: 10.1007/s10067-021-05677-9. [DOI] [PubMed] [Google Scholar]
- 109.de Araujo DB, Yamakami LY, Aikawa NE, Bonfá E, Viana VS, Pasoto SG, Pereira RM, Serafin PC, Borba EF, Silva CA. Ovarian reserve in adult patients with childhood-onset lupus: a possible deleterious effect of methotrexate? Scand J Rheumatol. 2014;43:503–11. doi: 10.3109/03009742.2014.908237. [DOI] [PubMed] [Google Scholar]
- 110.Giambalvo S, Garaffoni C, Silvagni E, Furini F, Rizzo R, Govoni M, Bortoluzzi A. Factors associated with fertility abnormalities in women with systemic Lupus Erythematosus: a systematic review and meta-analysis. Autoimmun Rev. 2022;21:103038. doi: 10.1016/j.autrev.2022.103038. [DOI] [PubMed] [Google Scholar]
- 111.Di Mario C, Petricca L, Gigante MR, Barini A, Barini A, Varriano V, Paglionico A, Cattani P, Ferraccioli G, Tolusso B, Gremese E. Anti-Müllerian hormone serum levels in systemic Lupus Erythematosus patients: influence of the Disease severity and therapy on the ovarian reserve. Endocrine. 2019;63:369–75. doi: 10.1007/s12020-018-1783-1. [DOI] [PubMed] [Google Scholar]
- 112.Zhang XH, Zhang YA, Chen X, Qiao PY, Zhang LY. Assessment of the Ovarian Reserve by serum Anti-Müllerian hormone in rheumatoid arthritis patients: a systematic review and Meta-analysis. Int Arch Allergy Immunol. 2022;183:462–9. doi: 10.1159/000520133. [DOI] [PubMed] [Google Scholar]
- 113.Brouwer J, Laven JS, Hazes JM, Schipper I, Dolhain RJ. Levels of serum anti-Müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1534–8. doi: 10.1002/acr.22013. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Corbeto M, Martínez-Mateu S, Pluma A, Ferrer R, López-Lasanta M, De Agustín JJ, Barceló M, Julià A, Marsal S. The ovarian reserve as measured by the anti-Müllerian hormone is not diminished in patients with rheumatoid arthritis compared to the healthy population. Clin Exp Rheumatol. 2021;39:337–43. [PubMed] [Google Scholar]
- 115.Mont’Alverne AR, Yamakami LY, Gonçalves CR, Baracat EC, Bonfá E, Silva CA. Diminished ovarian reserve in Behçet’s Disease patients. Clin Rheumatol. 2015;34:179–83. doi: 10.1007/s10067-014-2680-5. [DOI] [PubMed] [Google Scholar]
- 116.şahİn A, Karakuş S, Durmaz Y, Yildiz Ç, Aydin H, Cengİz AK. Ovarian reserve is preserved in Behçet’s Disease. Int J Rheum Dis. 2017;20:2070–6. doi: 10.1111/1756-185X.12693. [DOI] [PubMed] [Google Scholar]
- 117.Henes M, Froeschlin J, Taran FA, Brucker S, Rall KK, Xenitidis T, Igney-Oertel A, Lawrenz B, Henes JC. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic Diseases: impact of rheumatoid arthritis, Behçet’s Disease and spondyloarthritis on anti-Müllerian hormone levels. Rheumatology (Oxford) 2015;54:1709–12. doi: 10.1093/rheumatology/kev124. [DOI] [PubMed] [Google Scholar]
- 118.Clowse ME, Copland SC, Hsieh TC, Chow SC, Hoffman GS, Merkel PA, Spiera RF, Davis JC, Jr, McCune WJ, Ytterberg SR, et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (Wegener’s) Arthritis Care Res (Hoboken) 2011;63:1777–81. doi: 10.1002/acr.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mont’Alverne AR, Pereira RM, Yamakami LY, Viana VS, Baracat EC, Bonfá E, Silva CA. Reduced ovarian reserve in patients with Takayasu arteritis. J Rheumatol. 2014;41:2055–9. doi: 10.3899/jrheum.131360. [DOI] [PubMed] [Google Scholar]
- 120.Yamakami LY, Serafini PC, de Araujo DB, Bonfá E, Leon EP, Baracat EC, Silva CA. Ovarian reserve in women with primary antiphospholipid syndrome. Lupus. 2014;23:862–7. doi: 10.1177/0961203314529468. [DOI] [PubMed] [Google Scholar]
- 121.Alexander VM, Ashley-Martin J, Riley JK, Cooper AR, Ratts VS, Jungheim ES. Association between arthritis treatments and ovarian reserve: a prospective study. Reprod Biomed Online. 2021;42:1203–10. doi: 10.1016/j.rbmo.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Souza FH, Shinjo SK, Yamakami LY, Viana VS, Baracat EC, Bonfá E, Silva CA. Reduction of ovarian reserve in adult patients with dermatomyositis. Clin Exp Rheumatol. 2015;33:44–9. [PubMed] [Google Scholar]
- 123.Yarde F, Broekmans FJ, van der Pal-de Bruin KM, Schönbeck Y, te Velde ER, Stein AD, Lumey LH. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum Reprod. 2013;28:3328–36. doi: 10.1093/humrep/det331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Björvang RD, Hassan J, Stefopoulou M, Gemzell-Danielsson K, Pedrelli M, Kiviranta H, Rantakokko P, Ruokojärvi P, Lindh CH, Acharya G, Damdimopoulou P. Persistent organic pollutants and the size of ovarian reserve in reproductive-aged women. Environ Int. 2021;155:106589. doi: 10.1016/j.envint.2021.106589. [DOI] [PubMed] [Google Scholar]
- 125.Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, Leandri RD. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health. 2017;16:37. doi: 10.1186/s12940-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding N, Harlow SD, Randolph JF, Jr, Loch-Caruso R, Park SK. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update. 2020;26:724–52. doi: 10.1093/humupd/dmaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Du G, Huang H, Hu J, Qin Y, Wu D, Song L, Xia Y, Wang X. Endocrine-related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis and receptor reporter gene assays. Chemosphere. 2013;91:1099–106. doi: 10.1016/j.chemosphere.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 128.He QK, Li YP, Xu ZR, Wei WB, Qiao FX, Sun MX, Liu YC, Chen YZ, Wang HL, Qi ZQ, Liu Y. 3-MCPD exposure enhances ovarian fibrosis and reduces oocyte quality in mice. Environ Pollut. 2022;316:120662. doi: 10.1016/j.envpol.2022.120662. [DOI] [PubMed] [Google Scholar]
- 129.Li Y, Xiao N, Liu M, Liu Y, He A, Wang L, Luo H, Yao Y, Sun H. Dysregulation of steroid metabolome in follicular fluid links phthalate exposure to diminished ovarian reserve of childbearing-age women. Environ Pollut. 2023;330:121730. doi: 10.1016/j.envpol.2023.121730. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y, Sun Y, Zhao A, Cai X, Yu A, Xu Q, Wang P, Yao J, Wang Q, Wang W. Arsenic exposure diminishes ovarian follicular reserve and induces abnormal steroidogenesis by DNA methylation. Ecotoxicol Environ Saf. 2022;241:113816. doi: 10.1016/j.ecoenv.2022.113816. [DOI] [PubMed] [Google Scholar]
- 131.Jurewicz J, Radwan M, Wielgomas B, Karwacka A, Klimowska A, Kałużny P, Radwan P, Hanke W. Parameters of ovarian reserve in relation to urinary concentrations of parabens. Environ Health. 2020;19:26. doi: 10.1186/s12940-020-00580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jurewicz J, Radwan P, Wielgomas B, Radwan M, Karwacka A, Kałużny P, Piskunowicz M, Dziewirska E, Hanke W. Exposure to pyrethroid pesticides and ovarian reserve. Environ Int. 2020;144:106028. doi: 10.1016/j.envint.2020.106028. [DOI] [PubMed] [Google Scholar]
- 133.Czubacka E, Wielgomas B, Klimowska A, Radwan M, Radwan P, Karwacka A, Kałużny P, Jurewicz J. Urinary bisphenol A concentrations and Parameters of Ovarian Reserve among women from a fertility clinic. Int J Environ Res Public Health 2021, 18. [DOI] [PMC free article] [PubMed]
- 134.Fei J, Qu JH, Ding XL, Xue K, Lu CC, Chen JF, Song L, Xia YK, Wang SL, Wang XR. Fenvalerate inhibits the growth of primary cultured rat preantral ovarian follicles. Toxicology. 2010;267:1–6. doi: 10.1016/j.tox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 135.Shen H, Gao M, Li Q, Sun H, Jiang Y, Liu L, Wu J, Yu X, Jia T, Xin Y, et al. Effect of PFOA exposure on diminished ovarian reserve and its metabolism. Reprod Biol Endocrinol. 2023;21:16. doi: 10.1186/s12958-023-01056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gaskins AJ, Mínguez-Alarcón L, Fong KC, Abdelmessih S, Coull BA, Chavarro JE, Schwartz J, Kloog I, Souter I, Hauser R, Laden F. Exposure to fine particulate matter and Ovarian Reserve among women from a fertility clinic. Epidemiology. 2019;30:486–91. doi: 10.1097/EDE.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim H, Choe SA, Kim OJ, Kim SY, Kim S, Im C, Kim YS, Yoon TK. Outdoor air pollution and diminished ovarian reserve among infertile Korean women. Environ Health Prev Med. 2021;26:20. doi: 10.1186/s12199-021-00942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hood RB, James P, Fong KC, Mínguez-Alarcón L, Coull BA, Schwartz J, Kloog I, Laden F, Gaskins AJ. The influence of fine particulate matter on the association between residential greenness and ovarian reserve. Environ Res. 2021;197:111162. doi: 10.1016/j.envres.2021.111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feng X, Luo J, Wang X, Xie W, Jiao J, Wu X, Fan L, Qin G. Association of exposure to ambient air pollution with ovarian reserve among women in Shanxi province of north China. Environ Pollut. 2021;278:116868. doi: 10.1016/j.envpol.2021.116868. [DOI] [PubMed] [Google Scholar]
- 140.de Sá ST-JJR, de Viana WF, Camargo JH, Ramos LS, Folhadella AA, Polisseni IM, de Freitas J. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology. 2008;69:155–66. doi: 10.1016/j.theriogenology.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 141.Hale BJ, Hager CL, Seibert JT, Selsby JT, Baumgard LH, Keating AF, Ross JW. Heat stress induces autophagy in pig ovaries during follicular development. Biol Reprod. 2017;97:426–37. doi: 10.1093/biolre/iox097. [DOI] [PubMed] [Google Scholar]
- 142.Gaskins AJ, Mínguez-Alarcón L, VoPham T, Hart JE, Chavarro JE, Schwartz J, Souter I, Laden F. Impact of ambient temperature on ovarian reserve. Fertil Steril. 2021;116:1052–60. doi: 10.1016/j.fertnstert.2021.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiang J, Tao FY, Lu QS, He J, Xu P. Upregulation of miR-33 exacerbates heat-stress-Induced apoptosis in Granulosa Cell and Follicular Atresia of Nile Tilapia (Oreochromis niloticus) by targeting TGFβ1I1. Genes (Basel) 2022, 13. [DOI] [PMC free article] [PubMed]
- 144.Khan A, Dou J, Wang Y, Jiang X, Khan MZ, Luo H, Usman T, Zhu H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J Anim Sci Biotechnol. 2020;11:25. doi: 10.1186/s40104-019-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li GM, Liu LP, Yin B, Liu YY, Dong WW, Gong S, Zhang J, Tan JH. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems. Poult Sci. 2020;99:6084–93. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shehab-El-Deen MA, Leroy JL, Fadel MS, Saleh SY, Maes D, Van Soom A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim Reprod Sci. 2010;117:189–200. doi: 10.1016/j.anireprosci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 147.Shimizu T, Ohshima I, Ozawa M, Takahashi S, Tajima A, Shiota M, Miyazaki H, Kanai Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction. 2005;129:463–72. doi: 10.1530/rep.1.00502. [DOI] [PubMed] [Google Scholar]
- 148.Mínguez-Alarcón L, Souter I, Williams PL, Ford JB, Hauser R, Chavarro JE, Gaskins AJ. Occupational factors and markers of ovarian reserve and response among women at a fertility centre. Occup Environ Med. 2017;74:426–31. doi: 10.1136/oemed-2016-103953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bleil ME, Adler NE, Pasch LA, Sternfeld B, Gregorich SE, Rosen MP, Cedars MI. Depressive symptomatology, psychological stress, and ovarian reserve: a role for psychological factors in ovarian aging? Menopause. 2012;19:1176–85. doi: 10.1097/gme.0b013e31825540d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong YZ, Zhou FJ, Sun YP. Psychological stress is related to a decrease of serum anti-müllerian hormone level in infertile women. Reprod Biol Endocrinol. 2017;15:51. doi: 10.1186/s12958-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cizmeli C, Lobel M, Franasiak J, Pastore LM. Levels and associations among self-esteem, fertility distress, coping, and reaction to potentially being a genetic carrier in women with diminished ovarian reserve. Fertil Steril. 2013;99:2037–44. doi: 10.1016/j.fertnstert.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teal S, Edelman A. Contraception Selection, Effectiveness, and adverse effects: a review. JAMA. 2021;326:2507–18. doi: 10.1001/jama.2021.21392. [DOI] [PubMed] [Google Scholar]
- 153.Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83–93. doi: 10.1016/j.xfre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Birch Petersen K, Hvidman HW, Forman JL, Pinborg A, Larsen EC, Macklon KT, Sylvest R, Andersen AN. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod. 2015;30:2364–75. doi: 10.1093/humrep/dev197. [DOI] [PubMed] [Google Scholar]
- 155.Hariton E, Shirazi TN, Douglas NC, Hershlag A, Briggs SF. Anti-Müllerian hormone levels among contraceptive users: evidence from a cross-sectional cohort of 27,125 individuals. Am J Obstet Gynecol 2021, 225:515.e511-515.e510. [DOI] [PubMed]
- 156.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39:574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 157.Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, Johannsen TH, Nyboe Andersen A. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9. doi: 10.1016/j.rbmo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 158.Gullo G, Scaglione M, Cucinella G, Perino A, Chiantera V, D’Anna R, Laganà AS, Buzzaccarini G. Impact of assisted reproduction techniques on the neuro-psycho-motor outcome of newborns: a critical appraisal. J Obstet Gynaecol. 2022;42:2583–7. doi: 10.1080/01443615.2022.2109953. [DOI] [PubMed] [Google Scholar]
- 159.Berntsen S, Söderström-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A. The health of children conceived by ART: ‘the chicken or the egg?‘. Hum Reprod Update. 2019;25:137–58. doi: 10.1093/humupd/dmz001. [DOI] [PubMed] [Google Scholar]
- 160.Gullo G, Scaglione M, Laganà AS, Perino A, Andrisani A, Chiantera V, Cucinella G, Gitas G, Barra F, Riemma G. Assisted Reproductive techniques and risk of congenital Heart Diseases in children: a systematic review and Meta-analysis. Reprod Sci 2023. [DOI] [PMC free article] [PubMed]
- 161.Medenica S, Abazovic D, Ljubić A, Vukovic J, Begovic A, Cucinella G, Zaami S, Gullo G. The role of cell and Gene Therapies in the treatment of infertility in patients with thyroid autoimmunity. Int J Endocrinol. 2022;2022:4842316. doi: 10.1155/2022/4842316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther. 2019;10:348. doi: 10.1186/s13287-019-1425-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the MEDLINE repository. https://pubmed.ncbi.nlm.nih.gov/.
All figures were depicted by BioRender. https://www.biorender.com/.