Abstract
Diffusely infiltrating gliomas – including glioblastoma (GBM), isocitrate dehydrogenase (IDH) mutant gliomas, and histone 3 (H3) altered gliomas – are primary brain tumors with an invariably fatal outcome. Despite advances in the understanding of their biology, standard, targeted and immune checkpoint inhibitor immunotherapies have proven ineffective in arresting their inexorable progression and associated morbidity and mortality. Recognizing the unique aspects of the immunogenicity of cancer cells, the last decade has seen the development and evaluation of vaccine-based therapies for the treatment of solid tumors, including gliomas. Here we review the current vaccine strategies for the treatment of GBM, IDH-mutant gliomas and diffuse midline glioma H3 K27M-altered. We discuss potential benefits and challenges of vaccine therapies in these specific patient populations.
Keywords: diffuse midline glioma, glioblastoma, glioma, IDH-mutant Glioma, immunotherapy, vaccine
Introduction
The vaccine approach to cancer immunotherapy
The race to develop new immunotherapies has been motivated on the one hand by a series of clinical breakthroughs over the last few years, and on the other by a greater understanding of the interplay between malignant cells, the tumor micro-environment (TME), and the immune system. The main types of immunotherapy that have been deployed or are currently being explored in clinical trials include immune checkpoint inhibitors (ICI), chimeric antigen receptor (CAR) T-cell therapy, cytokine and other immunomodulators, oncolytic viruses and therapeutic cancer vaccines.
Cancer vaccines aim to stimulate a patient’s adaptive immune system by exposing it to a high concentration of tumor antigens. Identifying the right molecular targets is therefore key to designing an effective and specific cancer vaccine. Once target antigens are selected, they are administered in conjunction with immune adjuvants in order to successfully activate the host’s antigen presenting cells (APCs), which must then be capable of inducing sustained CD4+ and CD8+ T-lymphocyte responses. 1 These cells must then be able to reach the tumor and infiltrate it, overcoming any inhibitory signals from the cancer itself or the neighboring TME cells to exert their anti-tumor effect. Finally, this response must be sustained over time despite the immune tolerance and escape mechanisms deployed by tumors. To this day, three therapeutic cancer vaccines have received regulatory approval in the United States and Europe, Sipuleucel-T, for hormone-refractory prostate cancer, 2 and Talimogene Laherparepvec (T-VEC), for metastatic melanoma, 3 and Bacillus Calmette-Guérin, for non-muscle invasive bladder cancer. 4 These three successes, however, have been preceded by a number of negative clinical trials which failed to meet their primary endpoints but have taught us valuable lessons about vaccine design and tumor immunology.
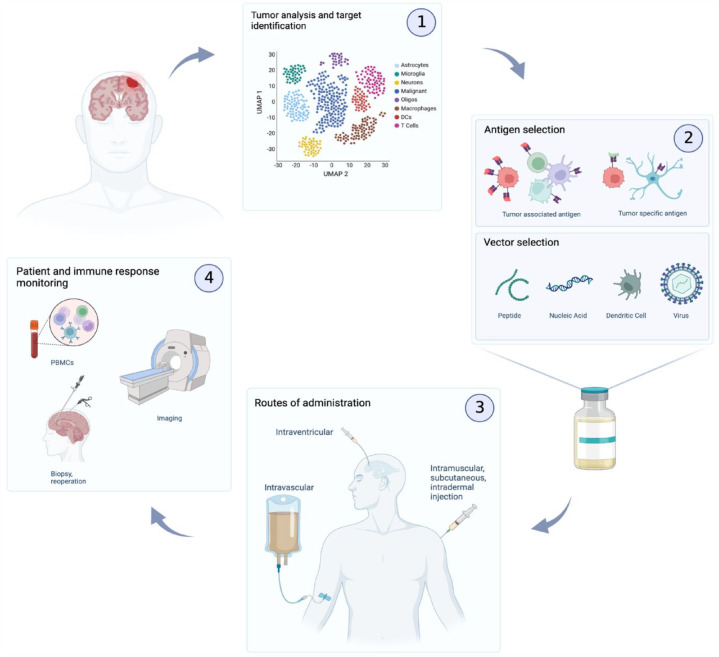
Different strategies exist for antigen selection (Figure 1). Tumor associated antigens (TAAs) are proteins that are present on healthy host cells but are typically overexpressed on cancer cells. Most of the first-generation vaccines that have been tested to date have targeted TAAs. 5 The advantage of TAAs is that they are generally expressed across a large number of patients, and thus represent a convenient ‘off-the-shelf’ treatment solution. Due to their presence on non-tumor cells however, high-affinity T-cells which target them are typically eliminated as part of central tolerance, leading to decreased immunogenicity. 6 To overcome this, strong adjuvants, co-stimulators, and multiple immunizations may be necessary. 7 Tumor specific antigens (TSAs), or neoantigens, on the other hand are found exclusively on malignant cells and arise typically from oncogenic mutations. 8 These are recognized as foreign by the immune system and therefore present a higher immunogenic potential given the lack of central tolerance (second-generation vaccines). Some TSAs are present in multiple patients, most however are patient specific. 5 This leads to the next important question in vaccine design: should the vaccine be pre-manufactured against a shared antigen, or should it be tailored to each patient’s tumor’s unique mutational profile? The latter approach might possibly be more effective, but is limited by the tumor’s mutational burden, and typically requires considerable time and development cost. Finally, multiple antigens may be targeted simultaneously as part of pre-defined or personalized panels, or even using undefined tumor-derived peptides or whole-tumor lysates. 9 Another important consideration when developing a vaccine platform is choosing which vector to use. Tumor antigens of interest can be administered directly as peptides; dendritic cells (DCs) can be pulsed with tumor antigens and then injected either subcutaneously or intra-muscularly to the patient (second- and third-generation vaccines) (Figure 1). Tumor antigens can be encoded in nucleic acid vaccines (either DNA or RNA) and can even be transmitted to the host by oncolytic viruses. Most of these methods have been explored in the context of diffusely infiltrating gliomas and each has its advantages and disadvantages, 10 the discussion of which is beyond the scope of this review.
Figure 1.
Therapeutic cancer vaccines in gliomas.
Resection or biopsy of the tumor provides valuable tissue with which to identify immunogenic targets in gliomas. (1) Tumors are dissociated and are subjected to bulk and single-cell RNA-sequencing, whole exome sequencing (WES), and other analyses in order to identify expressed mutations which may be targeted with a vaccine. (2) These antigens, whether specific to the malignant cells (TSAs) or overexpressed on them (TAAs) are then loaded onto the vaccine as peptides, encoded as nucleic acids, pulsed to APCs such as dendritic cells (DCs), or packaged within oncolytic viruses to be delivered to the patient. (3) This delivery can be performed by various methods such as intravenous, intramuscular, intradermal, subcutaneous, or intraventricular injection. (4) Imaging by MRI can then be leveraged to assess the local inflammation around the tumor as well as the progression of the disease. Peripheral blood mononuclear cell (PBMCs) can be collected to evaluate peripheral immune responses. Post-treatment biopsy offers invaluable insight into the local dynamics between the immune system and the malignant cells within the TME.
Glioma background
Gliomas are the most common type of primary malignant brain tumors. In 2021, the fifth edition of the WHO Classification of Tumors of the Central Nervous System provided the most up-to-date organization of these tumors according to histopathologic features and molecular parameters. 11 The mutational status of isocitrate dehydrogenase type 1 (IDH1) and type 2 (IDH2) is central to the classification of these tumors. 12 In adults, diffuse gliomas represent three entities: IDH-wildtype glioblastoma (GBM, Grade 4), IDH-mutant astrocytoma (Grade 2–4), and IDH-mutant oligodendroglioma (Grade 2–3). GBM harbor histological signs of anaplasia such as microvascular proliferation and necrosis, and contain molecular alterations including epidermal growth factor receptor (EGFR) amplification, telomerase reverse transcriptase promoter mutation, or concurrent chromosome 7 gain/chromosome 10 loss. IDH-mutant astrocytomas are typically associated with mutations in TP53 and loss of ATRX, whereas IDH-mutant oligodendrogliomas are characterized by the co-deletion of 1p/19q. 11 Both carry a slightly better prognosis than the IDH-wildtype tumors, where survival commonly remains under 2 years. 13 Pediatric high-grade gliomas on the other hand can be characterized into four distinct categories using their histone mutation status to delineate categories. 14 The four types are diffuse midline glioma (DMG) H3 K27-altered, diffuse hemispheric glioma H3 G34-mutant, diffuse pediatric-type high-grade glioma H3-wildtype IDH-wildtype, and finally infant-type hemispheric glioma. 11 For all entities mentioned above, treatment revolves around three main pillars: when the tumor is deemed operable, the patient will undergo surgery, followed by chemotherapy with temozolomide (TMZ), and radiotherapy.15,16 Despite these efforts, diffuse gliomas invariably recur, infiltrating additional brain areas leading to increasing disability and death. Novel therapeutic agents are desperately needed, and recent clinical studies offer a glimmer of hope for these terrible diseases.
Unique immunotherapy challenges of diffuse infiltrating gliomas
Although initially considered a relatively homogenous histological group of tumors, recent advances in next-generation sequencing and other molecular profiling methods have vastly improved our understanding of diffuse infiltrating gliomas, resulting in revised classifications reflecting the unique biology and clinical behavior of these tumors.12,17 The heterogeneity of these tumors, both on a population level (inter-tumoral heterogeneity), and within individual tumors themselves (intra-tumoral heterogeneity), has now been well documented. 18 In GBM, the genomic landscape is relatively homogenous compared to other tumors,19–21 whereas transcriptomic studies have highlighted the presence of three bulk gene expression subtypes.22,23 At the same time, single-cell profiling methods have enabled the characterization of the intra-tumoral heterogeneity of gliomas and the composition of their TME.24,25 Unfortunately, these advances have yet to be translated into improved approaches to therapy. Gliomas harbor a unique set of challenges to immunotherapy, including a low mutational burden (TMB), intrinsic immunosuppressive mechanisms, a notoriously immunosuppressive tumor microenvironment, and a high level of inter- and intra-tumoral heterogeneity and cellular plasticity. Despite these obstacles, select small studies have offered encouragement for a role for immunotherapy in the treatment of diffuse gliomas.26,27
It is widely accepted that the central nervous system (CNS) benefits from active immune surveillance. 28 Microglia make up the bulk of resident CNS immune cells in the healthy brain, while border associated macrophages, blood-derived monocytes, dendritic cells (DCs), and neutrophils are also present in a smaller proportion. 29 These leukocytes can travel through the cerebral spinal fluid (CSF) to the deep cervical lymph nodes via dural lymphatic vessels,30–32 which provide an interface between the CSF and the lymphatic system for T cell activation and migration. Furthermore, ICI studies in brain-metastatic melanoma have shown that peripherally activated tumor-specific T cells are able to traffic to the CNS and induce a sustained clinical response in these patients. 33 These immune surveillance and activation mechanisms establish a mechanistic foundation for the development of vaccine therapy in gliomas. We provide here an update on recent cancer vaccine developments and a selection of key lessons that have been learned from previous trials in gliomas. A selection of notable glioma vaccine trials are outlined in Table 1.
Table 1.
Select clinical trials exploring therapeutic vaccine strategies in gliomas.
| Disease | Phase | Target | Vaccine strategy | Status | Primary endpoint | Results | Toxicities |
|---|---|---|---|---|---|---|---|
| GBM, newly diagnosed | I | NeoVax: Personalized neoantigen | Long peptide vaccine with concurrent RTX TMZ Pembro-lizumab |
Recruiting, est. completion date: 2026 | Safety, biological activity | mPFS 7.6 months, mOS 16.8 months 34 | No DLT |
| GBM, newly diagnosed | I | GAPVAC: Personalized neoantigen | 2 peptide vaccines (APVAC-1 & APVAC-2) with concurrent CRT Maintenance TMZ |
Completed | Safety, biological activity, and feasibility | mPFS 14.2 months, mOS 29 months 35 | Anaphylaxis (n = 2), cerebral edema (n = 1) |
| GBM, newly diagnosed | I | NeoVax: Personalized neoantigen | Peptide vaccine with concurrent nivolumab ipilimumab |
Terminated | Safety, feasibility | N/A | N/A |
| GBM, newly diagnosed | III | Rindo-pepimut: EGFRvIII | Peptide vaccine with concurrent TMZ |
Terminated for futility | OS | mOS 20.1 months 36 | Grade 3 or 4 AEs (treatment versus control): thrombo-cytopenia [32 (9%) versus 23 (6%)], fatigue [6 (2%) versus 19 (5%)], brain edema [8 (2%) versus 11 (3%)], seizure [nine (2%) versus eight (2%)], and headache [6 (2%) versus 10 (3%)]. 16 deaths in the study were caused by AEs [nine (4%) and seven (3%)] |
| GBM, relapsed, EGFRvIII-expr. | II | Rindo-pepimut: EGFRvIII | Peptide vaccine with concurrent bevacizumab |
Completed | PFS rate, objective response rate | PFS6 28% for treatment, versus 16% for control; mDOR of 7.8 months versus 5.6 months; ability to discontinue steroids for 6 months (33% versus 0%) 37 | Grade 3 or 4 events considered related to Rindopepimut: increase in alanine amino-transferase (n = 1), increase in gamma – glutamyl transferase (n = 1) |
| GBM, newly diagnosed | IIa | SurVaxM: Survivin | Peptide vaccine with concurrent TMZ |
Active, not recruiting, est. completion date: 2023 | 6-month PFS | mPFS 11.4 months, mOS 25.9 months 38 | No serious AEs |
| GBM, newly diagnosed | II | UCPVax-Glio: TERT | Peptide vaccine with concurrent TMZ |
Recruiting, est. completion date: 2023 | Immuno-genicity | N/A | N/A |
| GBM, newly diagnosed | I | GNOS-PV01: Personalized neoantigen DNA | DNA vaccine with CELLECTRA®2000 EP Device, plasmid encoded IL-12 and concurrent RTX |
Active, not recruiting, est. completion date: 2023 | Safety, tolerability, and feasibility | N/A | N/A |
| GBM, newly diagnosed | I | Total tumor mRNA, pp65 CMV | RNA loaded lipid particle with concurrent CRT |
Recruiting, est. completion date: 2026 | Feasibility, safety, maximum tolerated dose | N/A | N/A |
| GBM, recurrent | I/II | WT1 | DC vaccine with concurrent TMZ |
Recruiting, est. completion date: 2026 | OS | N/A | N/A |
| GBM, recurrent | I | CD133 | DC vaccine pulsed with peptide antigen | Completed | Safety, tolerability | Safe and well tolerated 39 | N/A |
| GBM, newly diagnosed | I/II | pp65 CMV | DC vaccine with Td toxoid preconditioning and concurrent TMZ |
Active, not recruiting, est. completion date: 2022 | Feasibility, safety | mOS 41.1 months40–42 | N/A |
| GBM, newly diagnosed | II | pp65 CMV | CMV RNA-Pulsed Dendritic Cells with Td toxoid preconditioning | Recruiting, est. completion date: 2024 | Change in mOS | N/A | N/A |
| GBM, recurrent | I | pp65 CMV | DC vaccine with concurrent nivolumab |
Terminated | Safety | N/A | Grade IV: wound infection (n = 2), meningitis (n = 1) 43 |
| GBM, newly diagnosed | II | ICT-107: AIM-2, MAGE-1, TRP-2, gp100, HER-2, IL-13Ra2 | DC vaccine pulsed with immunogenic antigens | Completed | OS | mOS 17.0 months, mPFS 11.2 months44,45 | Overall well tolerated, no difference in toxicity between treatment groups |
| GBM, newly diagnosed | III | ICT-107: AIM-2, MAGE-1, TRP-2, gp100, HER-2, IL-13Ra2 | DC vaccine pulsed with immunogenic antigens | Suspended for lack of funding | OS | N/A | N/A |
| GBM, newly diagnosed | III | DCVax-L: tumor lysate | DC vaccine with concurrent TMZ |
Completed | OS | mOS 23.1 months from surgery 46 | Grade 3 or 4 AEs in 2.1% of ITT patients (n = 7) |
| GBM, newly diagnosed | I | Tumor lysate | DC vaccine with imiquimod pretreatment | Completed | Treatment-related AEs | N/A | N/A |
| GBM, recurrent | I | Tumor lysate | DC vaccine with concurrent Pembro-lizumab |
Recruiting | Cell cycle-related signature, expansion of T cell receptor clones, incidence of AEs | N/A | N/A |
| GBM, newly diagnosed or recurrent | I | GSC cell line lysate | DC vaccine with concurrent TMZ RTX bevacizumab |
Completed | Safety, tolerability, number of serious AEs, treatment-related toxicities | Newly diagnosed group: mPFS 8.75 months, mOS 20.36 months; recurrent group: mPFS 3.23 months, mOS 11.97 months 47 | No grade 3 or 4 toxicity |
| GBM, recurrent or pro-gressive | I/II | gp96 heat shock protein-peptide complex | Peptide vaccine | Completed | Safety, tolerability, time to disease recurrence and pro-gression, OS | mOS 42.6 weeks, mPFS 19.1 weeks 48 | 1 grade 3 event directly attributable to the vaccine (fatigue) |
| GBM, newly diagnosed | II | gp96 heat shock protein-peptide complex | Peptide vaccine | Completed | 1-year OS | N/A | N/A |
| GBM, newly diagnosed | II | pp65 CMV | DC vaccine with Td toxoid preconditioning and concurrent TMZ basiliximab |
Completed | mOS, percentage of 111In-labeled dendritic cells migrating to the inguinal lymph nodes | Migration of vaccinating DCs to draining lymph nodes 41 | N/A |
| GBM, newly diagnosed or recurrent | II | Tumor lysate | DC vaccine | Active, not recruiting, est. completion date: 2025 | Most effective combi-nation of DC vaccine com-ponents | N/A | N/A |
| IDHm glioma | I | IDH1R132H | Peptide vaccine | Completed | Safety and tolerability assessed by regime limiting toxicity, immuno-genicity | 3-year PF rate 0.63, 3-year death-free rate 0.83, 2-year PFS among patients with immune responses 49 | No grade 2–4 vaccine-related events |
| IDHm glioma, pro-gressive | I | IDH1R132H | Peptide vaccine | Recruiting, est. completion date: 2024 | Safety and tolerability assessed by regime limiting toxicity | N/A | N/A |
| IDHm glioma, recurrent, grade II | I | IDH1R132H | Peptide vaccine with Td toxoid preconditioning and concurrent TMZ |
Completed | Percentage of participants with un-acceptable toxicity | Induced specific immune responses 50 | Grade 2 injection site reaction (n = 1) |
| DMG; pediatric HGG; medullo-blastoma | II | pp65 CMV | Peptide vaccine with Td toxoid preconditioning and concurrent TMZ |
Not yet recruiting | PFS and OS | N/A | N/A |
| DMG; pediatric HGG; medullo-blastoma | I | SurVaxM: Survivin | Peptide vaccine | Recruiting, est. completion date: 2028 | Toxicity | N/A | N/A |
| DMG; pediatric HGG | I/II | WT1 | mRNA-loaded DC vaccine with concomitant CRT |
Active, not recruiting, est. completion date: 2027 | Safety, feasibility | N/A | N/A |
| DMG, H3K27M-altered | I | 16 neoepitopes | Neoantigen heat shock protein vaccine with concomitant balstilimab zalifrelimab |
Recruiting, est. completion date: 2025 | Safety and tolerability | N/A | N/A |
| DMG, H3K27M-altered | I/II | K27M peptide | Peptide vaccine with concomitant nivolumab |
Active, not recruiting, est. completion date: 2024 | AEs, OS | mOS 16.1 months for responder 51 | No grade-4 AEs |
| H3-mutated glioma, newly diagnosed | I | K27M peptide | Peptide vaccine with imiquimod pretreatment | Recruiting, est. completion date: 2025 | Safety and immuno-genicity | N/A | N/A |
| DMG | I | Tumor cell-line lysate | DC vaccine | Completed | Number of serious AEs per patient | Specific immune responses in n = 8/9 patients 52 | Intratumoral hemorrhage (n = 1), osteomyelitis grade 3 (n = 1) |
AE, adverse event; CMV, cytomegalovirus; CRT, chemo-radiation therapy; DCs, dendritic cells; DLT, dose-limiting toxicity; DMGs, diffuse midline gliomas; EGFR, epidermal growth factor receptor; EGFRvIII, EGFR variant III; GBM, glioblastoma; HGG, high-grade glioma; IDHm, isocitrate dehydrogenase mutant; ITT, intention-to-treat; mDOR, median duration of response; mOS, median overall-survival; mPFS, median progression-free survival; PFS6, progression-free survival at 6 months; PF rate, progression-free rate; RTX, radiotherapy; TERT, telomerase reverse transcriptase; Td toxoid, tetanus-diphtheria toxoid; TMZ, temozolomide.
Vaccines for glioblastoma
Glioblastoma is the most common and malignant primary brain tumor. 53 Despite considerable research to find novel targets for therapy, the global standard of care continues to comprise surgery followed by concomitant radiotherapy and chemotherapy with the alkylating agent temozolomide (TMZ). 54
In principle, GBM’s notoriously low tumor mutational burden 55 would be an obstacle to the development of a vaccine, but recent studies challenge the notion that TMB is the main determinant of response to immunotherapy by providing evidence that GBMs with higher TMBs do not necessarily respond better to ICI immunotherapy. 56 Another factor to consider, is the high degree of subclonal neoantigens that arise due to the extensive heterogeneity of these tumors. 57 Epidermal growth factor receptor variant III (EGFRvIII) seemed like an ideal target for a vaccine: it is a tumor-specific mutated protein that is not expressed in healthy tissue, and it is present in around 20–25% of GBM cases when evaluating bulk samples.58,59 Preclinical studies showed the protein to be immunogenic60,61 and single arm, early clinical trials (ACTIVATE, ACT II, ACT III) demonstrated encouraging results for the peptide vaccine Rindopepimut with a median overall survival (OS) of 20–22 months.62,63 The double-blinded, phase III trial ACT IV was however discontinued after it disappointingly failed to meet its primary endpoint, with no significant difference in OS in patients with minimal residual disease (MRD). The study enrolled 745 patients who were randomly assigned to receive Rindopepimut with GM-CSF or controlkeyhole limpet hemocyanin via monthly intradermal injection until progression or intolerance, concurrent with standard oral temozolomide. 36 The median OS in the treatment group was 20.1 versus 20.0 months in the control group.
In parallel to the ACT IV trial, a smaller phase II trial named ReACT sought to evaluate the efficacy of Rindopepimut in recurrent GBM in combination with bevacizumab, a vascular endothelial growth factor A inhibitor, versus bevacizumab alone. 37 Amongst the 73 recruited patients, 6-month progression-free survival (PFS) was 28% in the combination therapy group compared to 16% in the control group. Two points relative to EGFRvIII are important to note with both these trials: (i) EGFRvIII is a subclonal antigen,64,65 and (ii) loss of EGFRvIII occurs spontaneously in 50% of cases at recurrence 66 independently of treatment. This reinforces the notion that correct persistent antigen selection is crucial, and that clonality of a given antigen might be more important than overall mutational burden in heterogeneous tumors such as gliomas. 67
Survivin is an anti-apoptotic protein which is strongly expressed in GBM and is associated with an unfavorable prognosis.68–71 SurVaxM is an altered multi-epitope peptide vaccine conjugate currently under investigation in a phase IIa trial (NCT02455557) 38 with encouraging preliminary results. SurVaxM in combination with TMZ was well tolerated with no serious adverse events (AEs) reported, the median PFS was 11.4 months from the time of the first dose, and median OS in the cohort of 63 patients was 25.9 months. Importantly, the study was able to show that SurVaxM is capable of mounting both CD8+ and CD4+ T-cell responses. This is important, as there is emerging evidence that an effective T helper cell response is necessary for an effective anti-tumor response.72–77
Regarding the dendritic cell vaccine approach, the final results of the DCVax-L phase III trial were recently published. 78 The DCVax-L trial recruited 331 patients with newly diagnosed GBM between 2007 and 2015, with 232 patients receiving the vaccine plus TMZ, and 99 patients receiving placebo plus TMZ, with a crossover option to the active treatment arm for those receiving placebo at the time of progression. A first report came out in 2018 showing an acceptable safety profile with AEs similar between the two treatment groups. 46 By the end of the trial, 90% of patients had been treated with the DCVax-L, and the median OS of both treatment groups combined was 23.1 months from the time of surgery. 46 The PFS (the primary endpoint of the study) was not reported. Furthermore, the IDH mutational status of patients was not investigated, possibly accounting for the extended survival observed in this trial. For the second report, the primary outcome was changed to OS and an external control population was introduced to measure the new primary outcome. 78 Concerns have been raised in relation to the matching of this new external population compared to the initial cohort, in particular over prognostic factors such as age, steroid use, performance status, and extent of resection. 79 Thus, conclusive evidence for the clinical use of DCVax-L in GBM is still awaited, highlighting the importance of stringent design of prospective clinical trials.
Another innovative approach to vaccine design consists in taking multiple epitopes of neoantigens and combining them in personalized vaccines to maximize the immunogenicity of the treatment. Keskin et al. showed that such an approach is technically feasible and can generate a response with specific T cells, which are capable of migrating from the periphery into the GBM microenvironment. 34 In this small (eight patients), proof-of-principle study, 59 coding mutations were discovered on average in each patient using WES, a subset of which was shown to be expressed in each tumor using RNA-seq. Patients received a personalized vaccine 19.9 weeks on average after surgery, three of which received two follow up boosters, the other five having to withdraw due to disease progression. Median PFS and OS were 7.6 months and 16.8 months respectively. Reactivity of PBMCs to specific peptides was leveraged to analyze levels of immunization. The two patients in the cohort who did not receive dexamethasone generated immune responses against multiple neoantigens as opposed to their steroid-treated counterparts. This response comprised both CD8+ and CD4+ T cells despite using major histocompatibility complex (MHC) class I binding prediction algorithms and included antigen-specific memory T cells. Five patients including both steroid-naïve individuals underwent surgery post-vaccination, and tissue analyses showed an increase in infiltrating CD8+ T cells in the two patients who did not receive dexamethasone. Single-cell TT-cell receptor (TCR) sequencing was able to show that a subset of these infiltrating cytotoxic T lymphocytes (CTLs) were specific to vaccine neoantigens, however these cells exhibited a profound exhaustion phenotype with expression of multiple co-inhibitory receptors. This study showed that despite the generation of systemic and local specific immune responses, important barriers remain in terms of tumor-intrinsic immune evasion mechanisms and TME immunosuppression.80–82 In addition, this study illustrates the negative effect of steroids on any immunotherapy efforts in GBM and provides another evidence in support of the combination of vaccines with ICIs.
This multiple-antigen vaccine approach was also used by Hilf et al. for the GAPVAC101 trial, 35 where 15 patients received two successive vaccines, APVAC1 which targeted unmutated TAAs and APVAC2 which targeted specific neoantigens, both in combination with GM-CSF and poly-ICLC as well as standard of care (SOC). Personalization of each vaccine was based on mutations and analysis of the immunopeptidome and transcriptome of each patient by mass spectrometry and microarray analyses, respectively. APVAC1 was individually composed of the 10 highest ranking peptides drawn from a library of MHC class I and II binding peptides overrepresented in GBM. APVAC2 was comprised of 1–2 neoepitopes that are specific to each patient. A total of 15 patients received APVAC1 and 11 received APVAC2. The former was shown to induce sustained responses of central memory CD8+ T cells, whereas the latter generated Th1 CD4+ T cell responses. Two patients experienced anaphylaxis post-vaccination and one patient required high-dose steroid treatment for potentially immune-mediated grade 3 cerebral edema. In contrast with the NeoVax trial above, 34 the increase in PD-1 expression was shown to be low to moderate. Importantly, the authors note that pre-vaccination tumor-infiltrating lymphocytes (TILs) showed no reactivity to any of the identified APVAC antigens, suggesting that spontaneous T cell immunity is rare in GBM. Median OS and PFS was 29.0 months and 14.2 months, respectively. These studies highlight the advantage of concurrently using multiple mutations, especially considering (i) the current difficulty at predicting which peptides will be particularly successful at mounting an immune response, and (ii) the relatively low number of mutations in GBM. Two trials are currently testing the combination of NeoVax with pembrolizumab (NCT02287428), and NeoVax with nivolumab and ipilimumab (NCT03422094).
Vaccines for IDH-mutant glioma
Mutations in the IDH gene are a defining oncogenic event in the development of a group of diffuse gliomas that range from low- to high-grade, affect predominantly a younger population, and are molecularly distinct from GBM.83,84 The most frequent mutation involves the heterozygous replacement of an arginine to a histidine at the amino acid position 132 (R132H) in IDH1. 85 This neomorphic mutation results in the enzymatic overproduction of 2-hydroxyglutarate, 86 an oncometabolite that plays a central role in the malignant transformation of these tumors via epigenetic mechanisms and exerts an immunosuppressive effect on the TME.87,88 Importantly, this mutation is expressed ubiquitously across all cells of the tumor and is typically conserved over time.89–91 For these reasons, the IDH1 R132H mutation is an attractive target for immunotherapy.
IDH1 R132H was shown to contain an immunogenic epitope which can be presented on MHC class II and induce CD4+ T-helper one responses that are specific to the mutated peptide and able to discriminate between the wildtype protein. 92 These encouraging results formed the basis of the NOA16 trial (NCT02454634) which sought to evaluate the safety of an IDH1 R132H vaccine (IDH1-vac) in 33 patients with grade 3 and 4 IDH1 R132H+ astrocytomas in combination with radiotherapy and TMZ. 49 The trial provided evidence of safety, with adverse vaccine-related reactions being common (90.6% of patients affected) but restricted to grade 1. Furthermore, 93.3% of patients presented vaccine-induced immune responses. Interestingly, these responses were observed across multiple human leukocyte antigen (HLA) alleles and did not correspond to previous in vitro predictions of HLA binding affinities to the IDH1 R132H peptide. 49 Beyond meeting its safety endpoint, follow up analyses showed the 3-year progression-free rate and death-free rate to be 0.63 and 0.84 respectively. Patients with demonstrable immune responses had a 2-year progression-free rate of 0.82, whereas the two patients who were not able to mount an immune response both showed progression within the first 2 years. Finally, the authors noted a high frequency of radiographic pseudoprogression in patients taking part in the trial compared to a molecularly matched control group, possibly due to vaccine-related intratumoral inflammation. 93 Single-cell analyses of the TILs within the areas of pseudoprogression showed the presence of regulatory T cells, activated CD40LG + CD4 T cells, and CXCL13 + CD4+ T cells, the last two dominated by a single IDH1 R132H-reactive T-cell receptor.
IDH1-vac is currently being investigated in a multicenter phase I trial (AMPLIFY-NEOVAC, NCT03893903) in combination with the anti-PD-L1 antibody avelumab in 48 patients with recurrent astrocytoma or oligodendroglioma. 94 This provides an important opportunity to test the combination between a vaccine, to promote antitumor responses, and an ICI, to counteract tumoral and microenvironmental immunosuppression. A critical part of this study involves the regular monitoring of T- and B-cell responses in the blood, single-cell analyses of both tumor tissue and TILs, and proteomic and transcriptional analyses of liquid and fecal biopsies. 94 This will hopefully provide a comprehensive insight into the determinants of response and resistance to vaccine treatment in IDH-mutant gliomas, questions of major clinical relevance for which clear answers are lacking.
Vaccines for DMG, H3 K27M-altered
The dismal prognosis associated with DMGs, H3 K27M-altered, combined with their relative insensitivity to current treatment modalities make them a high priority target for the development of immunotherapies. These aggressive pediatric tumors are refractory to conventional chemotherapy, and typically present in the thalamus, brainstem, or spinal cord which prevents or severely limits surgical resection. Focal radiation remains the standard of care, with concurrent and adjuvant chemotherapy being of equivocal benefit. According to the 2021 WHO classification of CNS tumors, 80–85% of DMGs harbor a lysine to methionine mutation on the 27th position of histone H3.1 or H3.3 (H3.1 K27M and H3.3 K27M respectively). 17 The presence of this mutation establishes a WHO grade 4 grading. Beyond playing a role in the pathogenesis of the disease, these mutations represent a possible target for immunotherapy 95 with high disease specificity 96 and low probability for antigen loss-mediated escape. 97 These tumors however possess unique challenges to the development of immune therapies, the first of which is inherent to their location – involving vital structures along the midline of the neuroaxis – where immunological activation and its accompanying inflammation and edema may cause serious side effects. Furthermore, DMGs are extremely heterogeneous tumors with complex karyotypes exhibiting gains of chromosome 1q along with losses of 11p, 13q, and 14q, and numerous genetic alterations within cancer-relevant pathways such as PDGFRA, TP53, MYC, PVT-1/MYC, RB1, and PTEN. 98 Lastly, DMGs have been shown to be particularly immunosuppressive with low levels of microglia, infiltrating CD3+ T-lymphocytes, and inflammatory markers with simultaneously upregulated TGFβ1 signaling.99–101
The H3.3 K27M mutation was shown to be contained in a neoantigen epitope capable of stimulating a specific T-cell response. 95 A peptide vaccine was developed targeting this sequence and 19 H3.3 K27M+ patients between the ages of 3 and 21 were enrolled in a trial (NCT02960230) which showed the vaccine to be safe with no grade 4 treatment-related AEs. 51 Cytometry by time-of-flight was leveraged to conduct immune analyses on PBMCs. The authors were thus able to show that despite the treatment not improving the overall outcome for H3.3 K27M+ patients, there was a trend toward survival benefit in the subgroup of patients who showed an expansion of H3.3 K27M-reactive CD8+ T cells, with a median OS of 16.3 months and a prolonged median PFS compared to their immune non-responder counterparts. Additionally, the study highlighted two distinct myeloid-derived suppressor cell populations, labeled MDSC-high and MDSC-low, the latter of which had statistically significant correlation increased OS and PFS compared to the MDSC-high group. One patient showed a partial radiographic response at weeks 12 and 24 after 9 vaccinations. The administration of steroids was also shown to be correlated with lower rates of vaccine-specific CD8+ T cells and higher proportion of MDSCs. The authors of this study argue in favor of obtaining post-treatment biopsies as such tissue was not available and therefore no conclusions were reached concerning T cell infiltration of the tumor, antigen loss or HLA downregulation. 102
Another multicenter phase I trial is currently exploring a long peptide vaccine containing a K27M-mutated histone-3 sequence in combination with the human anti-PD-L1 antibody atezolizumab (INTERCEPT-H3, NCT04808245 103 ). Combinations with ICIs are also being explored in the rHSC-DIPGVax trial (NCT04943848) which seeks to evaluate the safety and tolerability of an off-the-shelf, neo-antigen heat shock protein containing 16 common peptide neo-epitopes with the anti-PD-L1 antibody balstilimab and the anti-CTLA-4 antibody zalifrelimab. An autologous dendritic cell vaccine pulsed with an allogenic tumoral cell line lysate was also shown to be safe and feasible (NCT02840123), with immune responses detected in PBMCs and the CSF. 52 Further results are eagerly anticipated, in particular in light of initial promising results with GD2-Car T cells against this type of tumor. 27
Discussion
There is considerable interest and therapeutic development regarding cancer vaccines for the treatment of infiltrating gliomas. Completed pre-clinical studies and clinical trials are outlining the landscape of challenges that need to be overcome to see these treatments enter the clinic. On a molecular level, identifying the correct antigens remains the single most important step in the design of a vaccine, and new algorithms are being developed in order to better predict which peptides will be most suited to being presented on MHC I & II and eliciting immune responses.104–107 Future vaccine efforts should aim to include both MHC I- and MHC II-presenting peptides, as CD4+ activation has been consistently shown to improve cytotoxic responses76,108 and extensive APC stimulation might be necessary to induce robust cancer immunity.109,110 A better understanding cellular interactions between malignant cells and the microenvironment in brain tumors will no doubt continue to be developed by spatial and single-cell profiling studies. 111 Future preclinical studies will need to be able to rely on robust experimental models which recapitulate the complexity of the brain microenvironment and the interplay between malignant and immune cells.
The design of clinical trials themselves will need to be rethought; OS and PFS, hitherto the foundations of trial metrics, fail to acknowledge the subtleties of immune activation, and further granularity will be needed to dissect whether a vaccine is effective or not. For example, when OS was deemed equivalent between treatment arms of ACT IV, the trial was terminated and Rindopepimut was considered a failure. 36 This ignores the fact that the vaccine was shown to induce a notable humoral response, and further analyses about the activation of T cell populations and tumor infiltration are sorely missed, leaving us with more questions than answers. The importance of histological characterization of the tumor after treatment is becoming increasingly apparent, and an ever-greater number of clinicians are calling for the reevaluation of the necessity of biopsies for recurrent gliomas. 112 The identification of specific biomarkers will also permit in the future to select patient populations who are most likely to benefit from vaccines and other immunotherapy interventions. 113 Finally, new standardized criteria for evaluating response and side effects in neuro-oncology immunotherapy trials are being developed such as the Immunotherapy Reponse Assessment in Neuro-Oncology criteria, which provide a framework for treatment evaluation and caution against premature assumptions of inefficacy in early phase trials. 114
The groundwork has been laid showing that vaccines are an effective treatment modality in diffuse gliomas, but they are unlikely to be sufficient as a monotherapy. Increasingly, clinical trials are exploring combinations with other small molecule inhibitors and immunotherapies such as ICIs, CAR T and bi-specific T-cell engager therapies. Current staples of treatment will also need to be reevaluated. Dexamethasone is currently given to most patients with CNS malignancies to control edema and relieve symptoms. This has well known immunosuppressive effects on T cell populations, has been shown to upregulate immune checkpoint inhibition, 115 and limit the clinical benefit of immune checkpoint blockade in GBM. 116 Alternatives to steroids such bevacizumab are currently being explored.117–119 The timing of inoculation in relation to both residual disease and concomitant treatments is also worth careful planning. Currently, both FDA approved vaccines are used in metastatic, treatment-resistant disease, implying that the cancer and immune system have been exposed to each other for a long time and have grown accustomed to each other’s presence. Treatment initiation as early as possible during immunological priming might be more efficient than during the chronic immune homeostasis phase. Results from ACT IV showed that patients with residual disease performed better than those without, contradicting the commonly held belief that MRD is instrumental for the success of immunotherapy. 36 Further investigation is required into determining the optimal window of treatment for different tumors.
Acknowledgments
None.
Footnotes
ORCID iD: Luis Nicolas Gonzalez Castro  https://orcid.org/0000-0001-7699-5188
https://orcid.org/0000-0001-7699-5188
Contributor Information
Alexander Jucht, University of Lausanne, Lausanne, Vaud, Switzerland.
Sydney Dumont, Massachusetts General Hospital, Boston, MA, USA.
Channing Pooley, Massachusetts General Hospital, Boston, MA, USA.
Luis Nicolas Gonzalez Castro, Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02115, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Alexander Jucht: Data curation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Sydney Dumont: Data curation; Writing – review & editing.
Channing Pooley: Data curation; Writing – review & editing.
Luis Nicolas Gonzalez Castro: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: building a bridge over troubled waters. Cell 2022; 185: 2770–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med 2010; 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 3. Rehman H, Silk AW, Kane MP, et al. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer 2016; 4: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus calmette-guerin immunotherapy for recurrent ta, t1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized southwest oncology group study. J Urol 2000; 163: 1124–1129. [PubMed] [Google Scholar]
- 5. Saxena M, van der Burg SH, Melief CJM, et al. Therapeutic cancer vaccines. Nat Rev Cancer 2021; 21: 360–378. [DOI] [PubMed] [Google Scholar]
- 6. Bluestone JA, Anderson M. Tolerance in the age of Immunotherapy. New Engl J Med 2020; 383: 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh M, Vianden C, Cantwell MJ, et al. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat Commun 2017; 8: 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer 2019; 18: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morse MA, Gwin Wr, 3rd, Mitchell DA. Vaccine therapies for cancer: then and now. Target Oncol 2021; 16: 121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frederico SC, Hancock JC, Brettschneider EES, et al. Making a cold tumor hot: the role of vaccines in the treatment of glioblastoma. Front Oncol 2021; 11: 672508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 12. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022; 128: 47–58. [DOI] [PubMed] [Google Scholar]
- 13. Ostrom QT, Cioffi G, Waite K, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol 2021; 23: iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aggarwal P, Luo W, Pehlivan KC, et al. Pediatric versus adult high grade glioma: immunotherapeutic and genomic considerations. Front Immunol 2022; 13: 1038096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berger TR, Wen PY, Lang-Orsini M, et al. World Health Organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol 2022; 8: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 16. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2022; 19: 357–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 2021; 23: 1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becker AP, Sells BE, Haque SJ, et al. Tumor heterogeneity in glioblastomas: from light microscopy to molecular pathology. Cancers 2021; 13: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017; 168: 613–628. [DOI] [PubMed] [Google Scholar]
- 21. Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014; 505: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Q, Hu X, Muller F, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 2017; 32: 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neftel C, Laffy J, Filbin MG, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 2019; 178: 835–849.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaligne R, Gaiti F, Silverbush D, et al. Epigenetic encoding, heritability and plasticity of glioma transcriptional cell states. Nat Genet 2021; 53: 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019; 25: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022; 603: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci 2012; 15: 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 2018; 48: 380–395.e6. [DOI] [PubMed] [Google Scholar]
- 30. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 2018; 21: 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (checkmate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol 2021; 22: 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019; 565: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019; 565: 240–245. [DOI] [PubMed] [Google Scholar]
- 36. Weller M, Butowski N, Tran D, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 2017; 18: 1373–1385. [DOI] [PubMed] [Google Scholar]
- 37. Reardon DA, Desjardins A, Vredenburgh JJ, et al. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clin Cancer Res 2020; 26: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 38. Ahluwalia MS, Reardon DA, Abad AP, et al. Phase IIa study of SurVaxM Plus adjuvant temozolomide for newly diagnosed glioblastoma. J Clin Oncol 2023; 41: 1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rudnick JD, Fink KL, Landolfi JC, et al. Immunological targeting of CD133 in recurrent glioblastoma: a multi-center phase I translational and clinical study of autologous CD133 dendritic cell immunotherapy. J Clin Oncol 2017; 35: 2059. [Google Scholar]
- 40. Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015; 519: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Batich KA, Mitchell DA, Healy P, et al. Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin Cancer Res 2020; 26: 5297–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sampson JH, Batich KA, Mitchell DA, et al. Reproducibility of outcomes in sequential trials using CMV-targeted dendritic cell vaccination for glioblastoma. J Clin Oncol 2022; 40: 2005. [Google Scholar]
- 43. Peters KB, Archer GE, Norberg P, et al. Safety of nivolumab in combination with dendritic cell vaccines in recurrent high-grade glioma. J Clin Oncol 2019; 37: e13526. [Google Scholar]
- 44. Armstrong TS, Wen PY, Reardon DA, et al. Comparative impact of treatment on clinical benefit in patients with glioblastoma (GBM) enrolled in the phase II trial of ICT-107. J Clin Oncol 2015; 33: 2036. [Google Scholar]
- 45. Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res 2019; 25: 5799–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med 2018; 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu JL, Omofoye OA, Rudnick JD, et al. A phase I study of autologous dendritic cell vaccine pulsed with allogeneic stem-like cell line lysate in patients with newly diagnosed or recurrent glioblastoma. Clin Cancer Res 2022; 28: 689–696. [DOI] [PubMed] [Google Scholar]
- 48. Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol 2014; 16: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Platten M, Bunse L, Wick A, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 2021; 592: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohan A, Peters K, Hotchkiss K, et al. Immu-06. Targeting Idh1 Mutant Grade ii recurrent gliomas using a peptide vaccination strategy. Neurooncol Adv 2021; 3: iv5– iv6. [Google Scholar]
- 51. Mueller S, Taitt JM, Villanueva-Meyer JE, et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Investig 2020; 130: 6325–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benitez-Ribas D, Cabezón R, Flórez-Grau G, et al. Corrigendum: Immune Response generated with the administration of autologous dendritic cells pulsed with an allogenic tumoral cell-lines lysate in patients with newly diagnosed diffuse intrinsic pontine glioma. Front Oncol 2018; 8: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System [Internet], https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Central-Nervous-System-2007 (2007, accessed 19 January 2021). [DOI] [PMC free article] [PubMed]
- 54. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 55. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Merchant M, Ranjan A, Pang Y, et al. Tumor mutational burden and immunotherapy in gliomas. Trends Cancer 2021; 7: 1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020; 580: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weller M, Kaulich K, Hentschel B, et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer 2014; 134: 2437–2447. [DOI] [PubMed] [Google Scholar]
- 59. Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol 2007; 25: 2288–2294. [DOI] [PubMed] [Google Scholar]
- 60. Choi BD, Archer GE, Mitchell DA, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol 2009; 19: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 2003; 9: 4247–4254. [PubMed] [Google Scholar]
- 62. Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol 2011; 13: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol 2015; 17: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Francis JM, Zhang CZ, Maire CL, et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov 2014; 4: 956–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Platten M. EGFRvIII vaccine in glioblastoma-InACT-IVe or not ReACTive enough? Neuro Oncol 2017; 19: 1425–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol 2015; 17: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Platten M, Grassl N. Vaccines targeting gliomas: antigens matter. J Clin Oncol 2023; 41: 1466–1469. [DOI] [PubMed] [Google Scholar]
- 68. Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998; 58: 5315–5320. [PubMed] [Google Scholar]
- 69. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003; 3: 46–54. [DOI] [PubMed] [Google Scholar]
- 70. Chakravarti A, Zhai GG, Zhang M, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene 2004; 23: 7494–7506. [DOI] [PubMed] [Google Scholar]
- 71. Xie D, Zeng YX, Wang HJ, et al. Expression of cytoplasmic and nuclear survivin in primary and secondary human glioblastoma. Br J Cancer 2006; 94: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Accolla RS, Lombardo L, Abdallah R, et al. Boosting the MHC class II-restricted tumor antigen presentation to CD4+ T helper cells: a critical issue for triggering protective immunity and re-orienting the tumor microenvironment toward an anti-tumor state. Front Oncol 2014; 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015; 520: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marty Pyke R, Thompson WK, Salem RM, et al. Evolutionary pressure against MHC class II binding cancer mutations. Cell 2018; 175: 416–428.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccin 2019; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 2021; 28: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20: 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol 2023; 9: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Preusser M, van den Bent MJ. Autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L) in glioblastoma: breakthrough or fata morgana? Neuro Oncol 2023; 25: 631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol 2015; 17(Suppl 7): vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mangani D, Weller M, Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol 2017; 130: 1–9. [DOI] [PubMed] [Google Scholar]
- 82. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res 2018; 24: 4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. New Engl J Med 2009; 360: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller JJ, Gonzalez Castro LN, McBrayer S, et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol 2023; 25: 4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009; 118: 469–474. [DOI] [PubMed] [Google Scholar]
- 86. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009; 462: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018; 24: 1192–1203. [DOI] [PubMed] [Google Scholar]
- 88. Richardson LG, Nieman LT, Stemmer-Rachamimov AO, et al. IDH-mutant gliomas harbor fewer regulatory T cells in humans and mice. OncoImmunology 2020; 9: 1806662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 2009; 174: 1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol 2011; 29: 4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia 2014; 28: 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014; 512: 324–327. [DOI] [PubMed] [Google Scholar]
- 93. Agnihotri S, Yang K, Mitchell DA, et al. A vaccine for glioma. Nat Cancer 2021; 2: 584–586. [DOI] [PubMed] [Google Scholar]
- 94. Bunse L, Rupp AK, Poschke I, et al. AMPLIFY-NEOVAC: a randomized, 3-arm multicenter phase I trial to assess safety, tolerability and immunogenicity of IDH1-vac combined with an immune checkpoint inhibitor targeting programmed death-ligand 1 in isocitrate dehydrogenase 1 mutant gliomas. Neurol Res Pract 2022; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chheda ZS, Kohanbash G, Okada K, et al. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med 2018; 215: 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 2012; 124: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 2016; 26: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bernstock JD, Hoffman SE, Kappel AD, et al. Immunotherapy approaches for the treatment of diffuse midline gliomas. OncoImmunology 2022; 11: 2124058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lin GL, Wilson KM, Ceribelli M, et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci Transl Med 2019; 11: eaaw0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ross JL, Chen Z, Herting CJ, et al. Platelet-derived growth factor beta is a potent inflammatory driver in paediatric high-grade glioma. Brain 2021; 144: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Findlay IJ, De Iuliis GN, Duchatel RJ, et al. Pharmaco-proteogenomic profiling of pediatric diffuse midline glioma to inform future treatment strategies. Oncogene 2022; 41: 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gupta N, Goumnerova LC, Manley P, et al. Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol 2018; 20: 1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. German Cancer Research Center. A MultIceNTER Phase I Peptide VaCcine Trial to Exploit NeoePitope-Specific T Cells for the Treatment of H3-Mutated Gliomas - (INTERCEPT-H3) [Internet]. Report No.: NCT04808245, https://clinicaltrials.gov/ct2/show/NCT04808245 (2023, accessed 9 February 2023).
- 104. Dutoit V, Herold-Mende C, Hilf N, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain J Neurol 2012; 135: 1042–1054. [DOI] [PubMed] [Google Scholar]
- 105. Nielsen M, Andreatta M. Netmhcpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med 2016; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jensen KK, Andreatta M, Marcatili P, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018; 154: 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gfeller D, Bassani-Sternberg M. Predicting antigen presentation—what could we learn from a million peptides? Front Immunol 2018; 9: 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science 2018; 359: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 109. Lin MJ, Svensson-Arvelund J, Lubitz GS, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer 2022; 3: 911–926. [DOI] [PubMed] [Google Scholar]
- 110. Suek N, Campesato LF, Merghoub T, et al. Targeted APC activation in cancer immunotherapy to enhance the abscopal effect. Front Immunol [Internet] 2019; 10: 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Karimi E, Yu MW, Maritan SM, et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 2023; 614: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nduom EK, Gephart MH, Chheda MG, et al. Re-evaluating biopsy for recurrent glioblastoma: a position statement by the Christopher Davidson forum investigators. Neurosurgery 2021; 89: 129–132. [DOI] [PubMed] [Google Scholar]
- 113. Lynes JP, Nwankwo AK, Sur HP, et al. Biomarkers for immunotherapy for treatment of glioblastoma. J Immunother Cancer 2020; 8: e000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015; 16: e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 2018; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Iorgulescu B, Gokhale P, Speranza M, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res 2021; 27: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gerstner ER, Duda DG, di Tomaso E, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol 2009; 6: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Meng X, Zhao R, Shen G, et al. Efficacy and safety of bevacizumab treatment for refractory brain edema: case report. Medicine 2017; 96: e8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abrams DA, Hanson JA, Brown JM, et al. Timing of surgery and bevacizumab therapy in neurosurgical patients with recurrent high grade glioma. J Clin Neurosci 2015; 22: 35–39. [DOI] [PubMed] [Google Scholar]