Abstract
Shiotani, H, Mizokuchi, T, Yamashita, R, Naito, M, and Kawakami, Y. Influence of body mass on running-induced changes in mechanical properties of plantar fascia. J Strength Cond Res 37(11): e588–e592, 2023—Body mass is a major risk factor for plantar fasciopathy; however, evidence explaining the process between risk factors and injury development is limited. Long-distance running induces transient and site-specific reduction in plantar fascia (PF) stiffness, reflecting mechanical fatigue and microscopic damage within the tissue. As greater mechanical loads can induce greater reduction in tissue stiffness, we hypothesized that the degree of running-induced change in PF stiffness is associated with body mass. Ten long-distance male runners (age: 21 − 23 years, body mass: 55.5 ± 4.2 kg; mean ± SD) and 10 untrained men (age: 20 − 24 years, body mass: 58.4 ± 5.6 kg) ran for 10 km. Before and immediately after running, the shear wave velocity (SWV) of PF at the proximal site, which is an index of tissue stiffness, was measured using ultrasound shear wave elastography. Although the PF SWV significantly decreased after running in runners (−4.0%, p = 0.010) and untrained men (−21.9%, p < 0.001), runners exhibited smaller changes (p < 0.001). The relative changes in SWV significantly correlated with body mass in both runners (r = −0.691, p = 0.027) and untrained individuals (r = −0.723, p = 0.018). These results indicate that a larger body mass is associated with a greater reduction in PF stiffness. Our findings provide in vivo evidence of the biomechanical basis for body mass as a risk factor for plantar fasciopathy. Furthermore, group differences suggest possible factors that reduce the fatigue responses, such as adaptation enhancing the resilience of PF and running mechanics.
Key Words: long-distance running, elasticity, fatigue, plantar fasciopathy, supersonic shear imaging, ultrasound shear wave elastography
Introduction
Plantar fasciopathy is one of the most common running-related injuries (31,33). Although the etiology of plantar fasciopathy is not yet fully understood (39), mechanical overload is widely accepted as a cause of this injury (2,25). Both intrinsic and extrinsic factors may be involved in mechanical load. For instance, body mass is an intrinsic risk factor (24,36). Habitual exercise levels (e.g., frequency, intensity, and duration) have been proposed as extrinsic risk factors (32,33). In support of the latter, long-distance runners, regardless of their performance level, are known to be the most vulnerable to this injury (21,33,35). However, there is limited in vivo evidence explaining the process between the risk factors and the development of plantar fasciopathy.
The plantar fascia (PF), a dense connective tissue that courses along the plantar surface of the foot, is the primary arch-supporting tissue contributing to the spring-like function of the foot arch in humans (13,14). At each foot contact during running, the PF is subjected to sizable stress with the longitudinal strain reaching up to 6% (4,37,40). Previous simulation studies have demonstrated that stress along the PF concentrates at its proximal site (4,5). Repetitive and site-specific stress on the soft tissues can result in reduced stiffness and increased strain upon load (38), reflecting mechanical fatigue as well as microscopic damage within the tissue. Indeed, a previous in vivo study has demonstrated that long-distance running induces a transient reduction in stiffness at the proximal site of the PF (28). Such fatigue and damage to the PF accumulate training by training and may lead to tissue degeneration and eventually to the development of plantar fasciopathy.
Previous mechanical testing studies have demonstrated that greater mechanical loads can induce greater reduction in soft tissue stiffness (15,38). Hence, a greater load on the foot during running may lead to greater reduction in PF stiffness. In the abovementioned study (28), running kinetics and kinematics were not measured; thus, it was not possible to examine the associations between the reduction of PF stiffness and applied loads during running. However, it is conceivable that the magnitude of ground reaction force and impulse is proportional to body mass at constant running velocities (3,34). Thus, we developed a further hypothesis that the degree of running-induced change in PF stiffness is associated with body mass. If our hypothesis is supported, we can provide in vivo evidence of the biomechanical basis for body mass as a risk factor for plantar fasciopathy. Therefore, this study aimed to examine the association between running-induced changes in PF stiffness and body mass.
Methods
Experimental Approach to the Problem
Experimental data were obtained from a previous study (28), which examined different topics (site-specificity and group differences in running-induced changes in morphological and mechanical properties of PF, and their relationships with the lowering of the foot arch). Details of the experiment can be found elsewhere (28). We have summarized it here.
The subjects included were 10 long-distance male runners and 10 untrained men, matched for baseline physical characteristics (i.e., age, height, and body mass). Using ultrasound shear wave elastography, shear wave velocity (SWV) of the PF at its proximal site (in proximity to the calcaneus) was measured as an index of tissue stiffness. The PF SWV was measured before (pre) and immediately after (post) 10 km of running, and the relative change was calculated. The correlations between running-induced changes in PF SWV and body mass were examined.
Subjects
Ten long-distance male runners (age 22.0 ± 0.7 years, height 1.68 ± 0.04 m, body mass 55.5 ± 4.2 kg; means ± SD) and 10 untrained men (age 22.5 ± 1.4 years, height 1.70 ± 0.05 m, body mass 58.4 ± 5.6 kg) were included in the study. All subjects were healthy, free from lower-extremity injuries for the past 12 months, and had no subjective symptoms that would impede running at the time of measurement. The runners had maintained habitual running of at least 10 km/week for the past 12 months, and their running experiences ranged between 9 and 16 years. The untrained subjects were either sedentary or lightly active, and none of them had been involved in any structured training program or continuous sports participation for at least 12 months before the measurement. Age, height, and body mass were not significantly different between runners and untrained men (p > 0.05). The subjects were asked not to perform any strenuous exercise for at least 24 hours before the measurement. This study was approved by the Human Research Ethics Committee of Waseda University and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects after the purpose, procedures, and possible risks of this study were explained.
Procedures
The main measurements were conducted in an indoor biomechanical laboratory. Body height was measured with a manual stadiometer (YG-200; Yagami, Aichi, Japan), and body mass was measured with a digital scale (HBF-701; OMRON, Kyoto, Japan), while the subjects wore their own sports clothes and were barefoot. Body mass index (BMI) was calculated as the body mass relative to the square of body height.
To measure PF stiffness, ultrasound shear wave elastography, which is a valid and reliable technique for evaluating the stiffness of skeletal muscles, tendons, and fasciae in vivo (9,11,27,29), was used. This technique uses multiple push pulses to generate shear waves within soft tissues and measures their velocity (SWV). Because SWV is related to the Young's modulus and shear modulus of soft tissues, it can be used as an index of tissue stiffness (9,11). Details of ultrasound measurements and data processing were based on previously published work, showing excellent intratrial and intertrial reliability of PF SWV with intraclass correlation coefficients of >0.9 (27,29). During ultrasound measurements, the subjects were requested to rest in a supine position on an examination bed with the knee fully extended. The right foot was secured to a custom-made fixture, with the ankle and toe digits in the neutral position. The PF at the proximal site (in proximity to the calcaneus) was scanned longitudinally using an Aixplorer ultrasound scanner (version 6.4, Supersonic Imagine, Aix-en-Provence, France) with a linear array probe (SL 15-4, Supersonic Imagine, Aix-en-Provence, France) (Figure 1). The scanning head of the probe was coated with a transmission gel, and an acoustic standoff pad (Gelpad for StatUS, Enraf-Nonius, Rotterdam, Netherlands) was used to avoid excessive compression of the skin surface. B-mode ultrasound images and shear wave data were recorded for 7 seconds at a sampling rate of 12 Hz. Three images separated by 12 frames from the middle of the 7 seconds recording were selected for further analysis. SWV was measured as the mean value within the region of interest (ROI), which was manually traced over the fascial boundaries of the PF using a measurement tool included in the Aixplorer software (Q-box Trace, Supersonic Imagine, Aix-en-Provence, France). Care was taken to exclude any rejection (i.e., the area with pixels having no color) within ROI. SWV was measured for 3 images and averaged to obtain a representative value.
Figure 1.
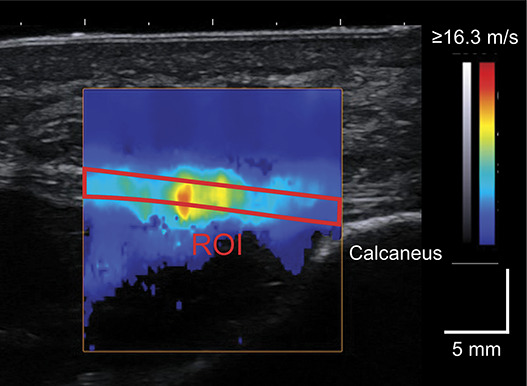
A representative shear wave image of the plantar fascia at the proximal site. The red outline bounds the region of interest (ROI).
After the ultrasound measurements, the subjects were asked to run for 10 km on a 700-m outdoor asphalt-surface circular path adjacent to the laboratory. The subjects were allowed to perform low-intensity exercises, such as walking and jogging, for up to 700 m as a warm-up before the running task. The running velocity was standardized to 10 km/h to ensure the identical loads during running for both the groups. During running, the lap time was recorded under the supervision of the authors, and the running velocity was adjusted by oral instructions. To account for potential left-right differences in applied loads to the feet during curve running (6,30), subjects ran 5 km each in counterclockwise and clockwise directions. Subjects wore their own socks and running shoes. None of the subjects used unsuitable shoes (e.g., minimalist, high cushion, high motion control, or badly deteriorated shoes) or orthotics that could confound the results. All the subjects successfully completed this running task with an average completion time of 0:59:57 (ranging from 0:59:04 to 1:00:57) without interruption, resting, or walking. Body mass and PF SWV were measured again immediately after the completion of the running task. Care was taken to ensure that the subjects were in the same posture for all measurements, before and after running.
Statistical Analyses
The normality of the data was assessed using the Shapiro-Wilk test. After normality was confirmed, a 2 × 2 split-plot analysis of variance (ANOVA) with group (runners and untrained men) as the between-subjects factor and time (prerunning and postrunning) as the within-subject factor was performed to compare the running-induced changes in PF SWV. If a significant interaction was found, comparisons of the PF SWV before and after running in each group were performed using a paired t test. In addition, the relative changes in PF SWV from prerunning to postrunning (%ΔSWV) were calculated and compared between runners and untrained men using an unpaired t test. Partial η2 (for ANOVA) and Cohen's d (for a post hoc test) were calculated as indices of effect size. For the within-subject factor, the dependence between mean values was corrected using the following equation:
| (1) |
where Mdiff is the mean difference between conditions, SDpooled is the pooled SD, and r is the correlation between the mean values (20). Effect size was interpreted as small (0.2 ≤ d < 0.5), medium (0.5 ≤ d < 0.8), or large (d ≥ 0.8) (8). A post hoc power analysis (G*Power v3.1, Heinrich Heine-Universität Dusseldorf, Germany) estimated the effect size needed for 80% statistical power was d ≥ 0.853. The correlations between %ΔSWV and body mass and BMI were examined using Pearson’s product-moment correlation coefficients. Body mass and BMI at baseline were used for correlation analysis. Statistical significance was set at α = 0.05. Statistical analysis was performed using the SPSS software (SPSS Statistics 28, IBM, Armonk, NY).
Results
PF SWV showed a significant group-time interaction (p = 0.001, η2 = 0.455). PF SWV significantly decreased after running in both runners (p = 0.010, d = 1.026) and untrained subjects (p < 0.001, d = 1.541) (Figure 2). Runners showed a significantly smaller %ΔSWV than untrained men (−4.0% and −21.9%, respectively; p < 0.001, d = 1.912). In both runners and untrained subjects, %ΔSWV correlated with body mass (r = −0.691, p = 0.027 and r = −0.759, p = 0.011, respectively) and BMI (r = −0.723, p = 0.018 and r = −0.745, p = 0.013, respectively) (Figure 3).
Figure 2.
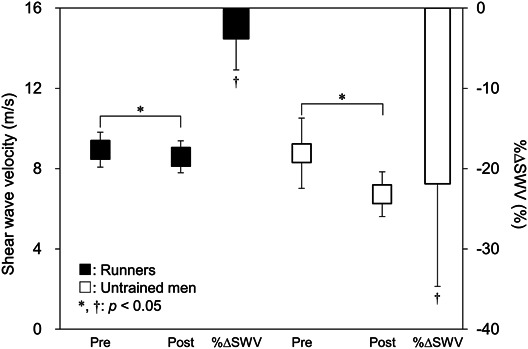
Box plots comparing shear wave velocity at prerunning and postrunning in runners (closed box) and untrained men (opened box) and bar charts comparing the relative changes in shear wave velocity (%ΔSWV). *Significant difference between prerunning and postrunning (p < 0.05). †Significant difference between runners and untrained men (p < 0.05).
Figure 3.
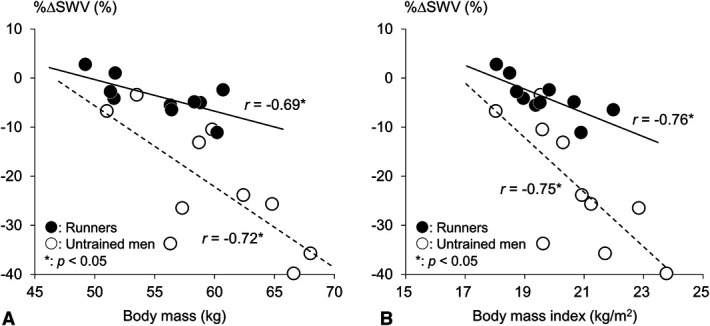
Correlations of the relative changes in shear wave velocity (%ΔSWV) with body mass (A) and body mass index (B) in runners (closed circle) and untrained men (opened circle). The regression lines are shown with correlation coefficients in runners (bold line) and untrained men (dotted line). *Significant correlation between the variables (p < 0.05).
Discussion
Running-induced changes in PF SWV correlated with body mass in both the groups, which supports our hypothesis. These findings indicate that a larger body mass is associated with a greater reduction in PF stiffness, thus highlighting the impact of body mass on PF stiffness. It has been suggested that the magnitude of ground reaction force and impulse during running is proportional to body mass (3,34) and that greater mechanical stress can induce a greater reduction in tissue stiffness (38). Simulation studies have demonstrated that the stress applied to the PF concentrates at the proximal site (4,5), where SWV was measured in this study. The reduction in PF stiffness may reflect mechanical fatigue and microscopic damage within the tissue owing to body mass-dependent and site-specific stresses. Thus, with greater body mass, PF can be more fatigued and more damaged by greater stress. This, repeated during each training session, may lead to tissue degeneration and eventually to the development of plantar fasciopathy. Our findings provide in vivo evidence of the biomechanical basis for body mass as a risk factor for plantar fasciopathy.
In this study, runners and untrained men with comparable body masses demonstrated different fatigue responses to PF SWV. This can be attributed to several potential factors. First, individual differences in running mechanics are involved in the amount of mechanical stress applied to the PF (19,41). Untrained individuals and novice runners exhibit larger joint angular excursions with higher individual variability as compared with well-trained runners (12,18). This can result in application of diverse mechanical stresses on the foot, leading to different fatigue responses. Second, a previous animal study reported that daily external loads affect the stiffness, collagen content, stress relaxation, and hysteresis of connective tissue (16). Because the runners in this study had no history of plantar fasciopathy, they might be successful cases, already optimally adapted to running-specific training. Thus, such adaptive changes in the viscoelasticity of PF in runners toward being more resilient might also be a factor in the different fatigue responses observed between the groups.
Long-distance running has become an increasingly popular exercise to achieve physical fitness, a healthier lifestyle, and weight loss in particular (22). Our results suggest that the condition at the beginning of habitual running to lose weight may have the greatest impact on the mechanical properties of PF and thus the highest risk of plantar fasciopathy. Although only male subjects were included in this study, body mass is a risk factor for plantar fasciopathy in both male and female subjects (24,36). Thus, the association between running-induced changes in PF stiffness and body mass would not be sex specific. Besides, overweight or obese individuals were not included in this study because the BMI of the subjects ranged from 18.0 to 23.8 kg/m2. To fully understand the etiology of plantar fasciopathy and adaptability of PF, the acute and chronic effects of running in a wider range of population in terms of age, sex, body mass, body composition, and sporting background are worth examining in future studies.
One of the limitations of this study is that the running kinematics and kinetics could not be assessed. As the present results suggest the existence of other factors that reduce stress accumulation, investigations into the effect of running mechanics on the mechanical properties of PF would lead to a better understanding of the nature of PF adaptability as well as the etiology of plantar fasciopathy. Furthermore, the runners in this study can be categorized as recreational runners (7). Runners at different performance levels exhibit different running mechanics (1,42) and fatigue responses (26,43). Thus, competitive and elite runners may exhibit different signs of adaptation in PF. Comparisons between runners with different performance levels should be incorporated in future studies.
In summary, this study demonstrated that the degree of reduction in PF stiffness after long-distance running correlates with body mass, indicating that a larger body mass is associated with greater mechanical fatigue and microscopic damage within the PF. Our findings provide in vivo evidence of the biomechanical basis for body mass as a risk factor for plantar fasciopathy. Nevertheless, runners exhibited a smaller reduction in PF stiffness as compared with untrained individuals, while possessing comparable body mass, suggesting possible factors to reduce the fatigue responses, such as adaptation enhancing the resilience of PF and running mechanics.
Practical Applications
In team or group training, it is common to impose the same running conditions such as duration, time, and velocity on a group of runners. Our results demonstrated that long-distance runners had a more resilient PF, which could minimize the risk of running-related injuries, including plantar fasciopathy. However, even in well-trained runners, larger body mass can lead to a greater reduction in PF stiffness after running. A larger body mass is considered one of the major risk factors for plantar fasciopathy (36). In addition, previous epidemiological studies have suggested that long-distance runners, regardless of their performance levels, demonstrate the highest prevalence of plantar fasciopathy (21,33,35). Based on these findings, runners and their coaches should keep in mind that greater mechanical stress can accumulate in the PF of heavier runners even at comparable performance levels and training volumes. Toward minimizing the risk of plantar fasciopathy while maintaining fitness and performance level in heavier runners, replacing some runs with nonimpact or low-impact aerobic cross-training methods, such as cycling or outdoor elliptical bike, can be effective (17,23). The reduction in PF stiffness after 10-km running at 10 km/h has previously been demonstrated to be transient because it was observed to recover in approximately 60 minutes (28). However, a previous study reported that flattening of the foot arch height, which can be a simple indicator of foot condition as it has also been reported to be associated with a reduction in PF stiffness (28), persists for more than 1 week after a full marathon (10). Thus, the persistence of PF stiffness reduction may depend on running duration and intensity. In addition, patients with plantar fasciopathy exhibit a lower PF SWV than healthy individuals (44). It is speculated that PF stiffness will not fully recover and will persist at lower levels as tissue degeneration and injury develop because of excessive accumulation of mechanical fatigue and microscopic damage within the PF. Continuous monitoring of the PF SWV (or foot arch height) may help assess tissue conditions and prevent injury.
Acknowledgments
This work was part of the research activities of the Human Performance Laboratory, Comprehensive Research Organization, Waseda University. This work was supported by JSPS KAKENHI Grant Numbers 19J14912 and 16H01870. The authors express their gratitude to Drs. Pavlos E. Evangelidis, Takaki Yamagishi, and Natsuki Sado, and Mr. Hidetaka Hayashi for their advice and grammatical corrections on the manuscript. No conflict of interest, financial or otherwise, is declared by the authors.
Footnotes
This work was part of the research activities of the Human Performance Laboratory, Comprehensive Research Organization, Waseda University. This work was supported by JSPS KAKENHI Grant Numbers 19J14912 and 16H01870.
This experiment was conducted in the biomechanical laboratory at the Waseda University (Tokorozawa Campus) and on an outdoor asphalt-surface circular path adjacent to the laboratory.
Contributor Information
Tomohiro Mizokuchi, Email: mizo-tomo.88@akane.waseda.jp.
Ryo Yamashita, Email: janne.yama@akane.waseda.jp.
Munekazu Naito, Email: munekazunaito@gmail.com.
Yasuo Kawakami, Email: ykawa@waseda.jp.
References
- 1.Anderson T. Biomechanics and running economy. Sports Med 22: 76–89, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Bolgla LA, Malone TR. Plantar fasciitis and the windlass mechanism: A biomechanical link to clinical practice. J Athl Train 39: 77–82, 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.Chang YH, Huang HW, Hamerski CM, Kram R. The independent effects of gravity and inertia on running mechanics. J Exp Biol 203: 229–238, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Chen TLW, Wong DW, Wang Y, Lin J, Zhang M. Foot arch deformation and plantar fascia loading during running with rearfoot strike and forefoot strike: A dynamic finite element analysis. J Biomech 83: 260–272, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Chen YN, Chang CW, Li CT, Chang CH, Lin CF. Finite element analysis of plantar fascia during walking: A quasi-static simulation. Foot Ankle Int 36: 90–97, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Churchill SM, Trewartha G, Bezodis IN, Salo AIT. Force production during maximal effort bend sprinting: Theory vs reality. Scand J Med Sci Sports 26: 1171–1179, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Clermont CA, Benson LC, Osis ST, Kobsar D, Ferber R. Running patterns for male and female competitive and recreational runners based on accelerometer data. J Sports Sci 37: 204–211, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. A power primer. Psychol Bull 112: 155–159, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Eby SF, Song P, Chen S, et al. Validation of shear wave elastography in skeletal muscle. J Biomech 46: 2381–2387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukano M, Inami T, Nakagawa K, Narita T, Iso S. Foot posture alteration and recovery following a full marathon run. Eur J Sport Sci 18: 1338–1345, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Helfenstein-Didier C, Andrade RJ, Brum J, et al. In vivo quantification of the shear modulus of the human achilles tendon during passive loading using shear wave dispersion analysis. Phys Med Biol 61: 2485–2496, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Hobara H, Kimura K, Omuro K, et al. Differences in lower extremity stiffness between endurance-trained athletes and untrained subjects. J Sci Med Sport 13: 106–111, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Huang CK, Kitaoka HB, An KN, Chao EY. Biomechanical evaluation of longitudinal arch stability. Foot Ankle 14: 353–357, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. The spring in the arch of the human foot. Nature 325: 147–149, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Ker RF, Wang XT, Pike AVL. Fatigue quality of mammalian tendons. J Exp Biol 203: 1317–1327, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Khayyeri H, Blomgran P, Hammerman M, et al. Achilles tendon compositional and structural properties are altered after unloading by botox. Sci Rep 7: 13067, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein IE, White JB, Rana SR. Comparison of physiological variables between the elliptical bicycle and run training in experienced runners. J Strength Cond Res 30: 2998–3006, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Maas E, De Bie J, Vanfleteren R, Hoogkamer W, Vanwanseele B. Novice runners show greater changes in kinematics with fatigue compared with competitive runners. Sports Biomech 17: 350–360, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Morin JB, Dalleau G, Kyrolainen H, Jeannin T, Belli A. A simple method for measuring stiffness during running. J Appl Biomech 21: 167–180, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 7: 105–125, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen RO, Ronnow L, Rasmussen S, Lind M. A prospective study on time to recovery in 254 injured novice runners. PLoS One 9: e99877, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen RO, Videbaek S, Hansen M, et al. Does running with or without diet changes reduce fat mass in novice runners? A 1-year prospective study. J Sports Med Phys Fitness 56: 105–113, 2016. [PubMed] [Google Scholar]
- 23.Paquette MR, Peel SA, Smith RE, Temme M, Dwyer JN. The impact of different cross-training modalities on performance and injury-related variables in high school cross country runners. J Strength Cond Res 32: 1745–1753, 2018. [DOI] [PubMed] [Google Scholar]
- 24.Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for plantar fasciitis: A matched case-control study. J Bone Joint Surg 85: 872–877, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Rome K, Howe T, Haslock I. Risk factors associated with the development of plantar heel pain in athletes. Foot 11: 119–125, 2001. [Google Scholar]
- 26.Sanno M, Willwacher S, Epro G, Bruggemann GP. Positive work contribution shifts from distal to proximal joints during a prolonged run. Med Sci Sports Exerc 50: 2507–2517, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Shiotani H, Maruyama N, Kurumisawa K, Yamagishi T, Kawakami Y. Human plantar fascial dimensions and shear wave velocity change in vivo as a function of ankle and metatarsophalangeal joint positions. J Appl Physiol 130: 390–399, 2021. [DOI] [PubMed] [Google Scholar]
- 28.Shiotani H, Mizokuchi T, Yamashita R, Naito M, Kawakami Y. Acute effects of long-distance running on mechanical and morphological properties of the human plantar fascia. Scand J Med Sci Sports 30: 1360–1368, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiotani H, Yamashita R, Mizokuchi T, Naito M, Kawakami Y. Site- and sex-differences in morphological and mechanical properties of the plantar fascia: A supersonic shear imaging study. J Biomech 85: 198–203, 2019. [DOI] [PubMed] [Google Scholar]
- 30.Shiotani H, Yamashita R, Mizokuchi T, et al. Track distance runners exhibit bilateral differences in the plantar fascia stiffness. Sci Rep 11: 9260, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobhani S, Dekker R, Postema K, Dijkstra PU. Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand J Med Sci Sports 23: 669–686, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Taunton JE, Clement DB, McNicol K. Plantar fasciitis in runners. Can J Appl Sport Sci 7: 41–44, 1982. [PubMed] [Google Scholar]
- 33.Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med 36: 95–101, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teunissen LPJ, Grabowski A, Kram R. Effects of independently altering body weight and body mass on the metabolic cost of running. J Exp Biol 210: 4418–4427, 2007. [DOI] [PubMed] [Google Scholar]
- 35.van Gent RN, Siem D, van Middelkoop M, et al. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br J Sports Med 41: 469–480, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Leeuwen KD, Rogers J, Winzenberg T, van Middelkoop M. Higher body mass index is associated with plantar fasciopathy/‘plantar fasciitis': Systematic review and meta-analysis of various clinical and imaging risk factors. Br J Sports Med 50: 972–981, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Wager JC, Challis JH. Elastic energy within the human plantar aponeurosis contributes to arch shortening during the push-off phase of running. J Biomech 49: 704–709, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. J Exp Biol 198: 847–852, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Wearing SC, Smeathers JE, Urry SR, Hennig EM, Hills AP. The pathomechanics of plantar fasciitis. Sports Med 36: 585–611, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Welte L, Kelly LA, Kessler SE, et al. The extensibility of the plantar fascia influences the windlass mechanism during human running. Proc Biol Sci 288: 20202095, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wille CM, Lenhart RL, Wang S, Thelen DG, Heiderscheit BC. Ability of sagittal kinematic variables to estimate ground reaction forces and joint kinetics in running. J Orthop Sports Phys Ther 44: 825–830, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams KR, Cavanagh PR. Relationship between distance running mechanics, running economy, and performance. J Appl Physiol 63: 1236–1245, 1987. [DOI] [PubMed] [Google Scholar]
- 43.Willwacher S, Sanno M, Bruggemann GP. Fatigue matters: An intense 10 km run alters frontal and transverse plane joint kinematics in competitive and recreational adult runners. Gait Posture 76: 277–283, 2019. [DOI] [PubMed] [Google Scholar]
- 44.Wu CH, Chiu YH, Chang KV, Wu WT, Ozcakar L. Ultrasound elastography for the evaluation of plantar fasciitis: A systematic review and meta-analysis. Eur J Radiol 155: 110495, 2022. [DOI] [PubMed] [Google Scholar]


