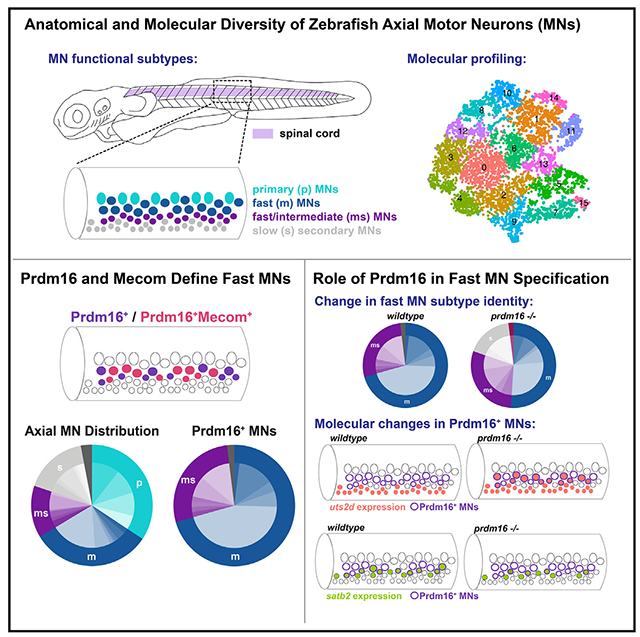
SUMMARY
Locomotion requires precise control of the strength and speed of muscle contraction and is achieved by recruiting functionally distinct subtypes of motor neurons (MNs). MNs are essential to movement and differentially susceptible in disease, but little is known about how MNs acquire functional subtype-specific features during development. Using single-cell RNA profiling in embryonic and larval zebrafish, we identify novel and conserved molecular signatures for MN functional subtypes and identify genes expressed in both early post-mitotic and mature MNs. Assessing MN development in genetic mutants, we define a molecular program essential for MN functional subtype specification. Two evolutionarily conserved transcription factors, Prdm16 and Mecom, are both functional subtype-specific determinants integral for fast MN development. Loss of prdm16 or mecom causes fast MNs to develop transcriptional profiles and innervation similar to slow MNs. These results reveal the molecular diversity of vertebrate axial MNs and demonstrate that functional subtypes are specified through intrinsic transcriptional codes.
Graphical abstract
In brief
D’Elia et al. define the molecular profiles of motor neurons essential for swimming speed control in zebrafish. They find that two transcription factors, Prdm16 and Mecom, are essential to specify motor neurons involved in fast locomotion. These results indicate that motor neuron functional subtypes are specified through intrinsic genetic programs.
INTRODUCTION
To modulate locomotor speed, animals engage functionally specialized motor neurons (MNs) to contract distinct muscle fibers.1,2 Smaller muscle fibers, called slow-twitch fibers, produce slower and sustained movements, while larger fast-twitch fibers are recruited progressively to increase speed or force.3,4 Mammalian MNs innervate either fast- or slow-twitch muscle fibers. These MN “functional subtypes” have diverse attributes matched to their roles in muscle contraction.5 Notably, in diseases such as amyotrophic lateral sclerosis (ALS), fast MNs are more susceptible to degeneration.6 Although MN functional subtypes are essential for locomotor speed control, little is known about the molecular determinants that specify their identity.
Spinal MNs can also be differentiated anatomically as they cluster into motor pools that innervate individual muscles (e.g., biceps or triceps).7 In contrast with functional subtypes, the mechanisms that specify MN anatomical subtypes are well understood.3,7 During embryonic development, transcription factors such as Lim-, Hox-, and Mnx-homeodomain proteins drive cascades of gene expression that determine muscle-specific targeting, somatotopic organization, and integration into premotor locomotor networks.8–12 Mammalian motor pools contain both fast and slow functional subtypes, with no clear anatomic organization, hindering an understanding of how they are specified during development. Recent transcriptional profiling of mature MNs has uncovered differentially expressed genes that mark fast and slow MNs,13–15 underscoring a key question: do transcriptional codes specify functional subtypes of MNs?
The larval zebrafish is an ideal model system to define the molecular determinants of MN functional subtypes. First, the molecular determinants of both appendicular (fin) and axial (trunk) MNs are highly conserved16–22 and regulate muscle targeting.23–25 Second, MN functional subtype identities are mapped in both space (dorsoventral soma position) and time (birth date). Larval zebrafish have three broad classes of axial MNs: primary (fast, dorsal, first born), fast secondary (fast, dorsal, middle born), and slow (ventral, last born) (Figure 1A). These three classes can be further subdivided along characteristic anatomical and physiological lines.26–33 Finally, the exceptional genetic and optical accessibility of zebrafish facilitates in vivo evaluation of functional subtypes after loss of function, the gold standard for identifying molecular determinants.
Figure 1. Zebrafish motor neuron functional subtypes are molecularly distinct.
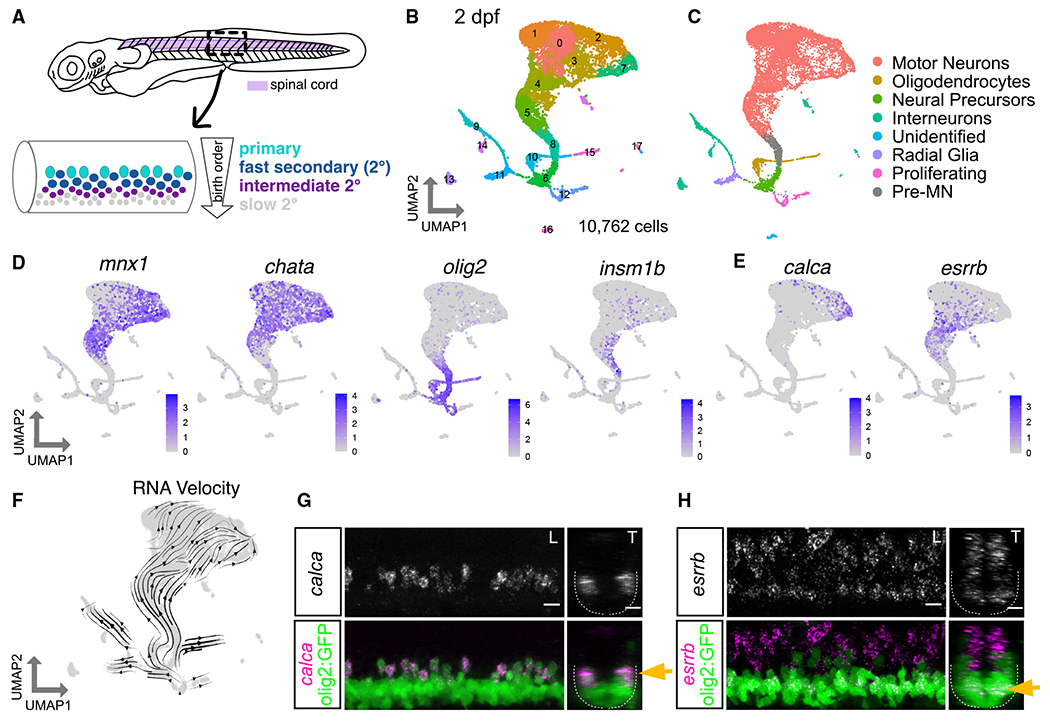
(A) Diagram of 3 dpf zebrafish showing location of spinal cord in purple. Crop of the tail shows the spinal cord at a lateral view. MN functional subtypes indicated by colors are organized along the dorsoventral axis and by birth date.
(B) Uniform manifold approximation and projection (UMAP) representation of 10,762 GFP+ cells at 2 dpf from 90 Tg(olig2:dsRed2) larvae. Each dot represents one cell; 18 cell clusters are represented by coloring.
(C) Major cell classes indicated by colors represented in the UMAP as determined by marker genes.
(D) Feature plots of marker genes for MN maturity. Purple indicates expression.
(E) Feature plots for markers of mammalian fast (calca) and slow (esrrb) MNs.
(F) RNA Velocity streamlines overlaid on UMAP coordinates in (B).
(G) FISH of calca at 2 dpf. Left image (L) is lateral view, and right image (T) is transverse view of the spinal cord. Gold arrow indicates dorsoventral localization of gene expression within MN population. Dotted line indicates ventral spinal cord boundary.
(H) FISH of esrrb at 2 dpf. Scale bars for (G) and (H), 10 μm.
Here we leverage the organization of MNs in zebrafish to (1) uncover the molecular diversity among MN functional subtypes and (2) identify transcriptional determinants of MN functional subtype identity. First, we discovered remarkable molecular diversity among fast and slow secondary MNs, and defined both conserved and novel markers that are maintained between early developmental stages through functional maturity. Next, we focused on two conserved transcription factors expressed in fast secondary MNs: Prdm16 and Mecom. Loss of these genes leads fast secondary MNs to acquire the anatomical projections and molecular features that define slow MNs. Our data provide novel insights into the molecular diversity of vertebrate spinal MNs and establish that MN functional subtypes are determined by intrinsic genetic codes acting early in development.
RESULTS
Molecular diversity of zebrafish MN subtypes
We first set out to evaluate the molecular heterogeneity of zebrafish MNs during development at 2 days post-fertilization (dpf) (Figure S1A). At 2 dpf, the oldest MNs (primary MNs and fast secondary MNs) are largely mature and active during locomotion.34–37 At this time point, slow secondary MNs have been recently born and are in the process of innervating muscle. To isolate MNs, we used a Tg(olig2:dsRed2)vu19 reporter line that labels the pMN progenitor domain and its progeny (which includes MNs, oligodendrocytes, and some interneurons).38,39 After dissection of the body caudal to the hindbrain, dissociation, and isolation of cells by fluorescence-activated cell sorting (FACS), we performed single-cell RNA sequencing (scRNA-seq) (Figures S1B and S1H). We characterized a total of 10,762 cells after filtering (Figures 1 and S1). Analyses using known markers identified several broad classes of cell types among clusters (Figures 1C and 1D), including MNs, oligodendrocytes, and interneurons (Figures S1C and S1D). MN clusters were identified as those expressing chata (Figure 1D).
As birth date and functional subtype are linked in zebrafish MNs, we determined the relative birth order of different MN clusters and their respective putative functional class (Figure 1A). The expression of two genes, olig2 and insm1b, which are progenitor and pro-neural genes, respectively, suggested that clusters 5 and 8 were younger MNs (Figures 1B and 1D). The expression of mature MN marker (chata) was higher in MN clusters 0, 1, 2, 3, 4, and 7, indicating that they are likely more mature MNs. We used RNA Velocity40,41 to assay the progression of gene expression profiles across development. RNA Velocity streamlines diverged from the progenitor population into clusters of four major cell types (MNs, oligodendrocytes, interneurons, and radial glia) (Figures 1C and 1F). We integrated our data with a previously published dataset of olig2+ MN populations at 1 and 1.5 dpf.39 We found that these datasets could be well integrated (Figure S1E). RNA Velocity on the merged datasets appropriately predicted trajectories and pseudotime on the basis of sample age (Figures S1F and S1G). With well-defined MN subtype birth dates, cluster birth order determined by RNA Velocity implies we can potentially differentiate young (slow) from old (fast) functional subtypes.
We investigated whether previously characterized mammalian markers of adult fast and slow MNs could discriminate functional subtypes in embryonic zebrafish MNs. We observed that expression of a fast MN marker, calca, was restricted to putative older MN clusters, while expression of a slow MN marker, estrogen-related receptor beta (esrrb in zebrafish), was enriched in putative younger MN clusters (Figure 1E).42 This segregation is consistent with these markers being conserved in fast and slow zebrafish MN functional subtypes, respectively. We acknowledge the limits of RNA Velocity,43 and therefore confirmed the relative birth dates of these clusters by determining the localization of these putative MN functional subtype markers in vivo by fluorescence in situ hybridization (FISH). This analysis showed dorsal MNs express calca and ventral MNs express esrrb (Figures 1G, 1H, S5A, and S5B). These results also demonstrated the inferred ages of MN clusters from the RNA Velocity and maturity analysis were consistent with marker localization.
To further investigate the molecular diversity among secondary MNs, we performed a deeper analysis of chata+ MN clusters at 2 dpf (6,103 MNs; Figure 2A). A heatmap of marker genes showed clear transcriptional heterogeneity across chata+ clusters (Figure S2E and Table S2). Genes that distinguished clusters included transcription factors, guidance molecules, and signaling and structural proteins. A Gene Ontology analysis of MN cluster markers revealed a particular enrichment for genes involved in neural development and metabolism (Figure S2G). To determine if the detected molecular diversity at 2 dpf was due solely to the birth date differences of the subtypes or if it reflected sustained genetic differences, we assessed MN molecular diversity at 5 dpf when all functional subtypes have innervated and can contract muscle.27 We performed the analogous experiment (Figure S1B) at 5 dpf assessing 14,882 cells after filtering (Figures S2A and S2D). We similarly repeated our analyses on chata+ MN clusters at 5 dpf (8,087 MNs; Figures 2B, S2B, S2C, and S2F and Table S3). Comparing the two datasets (Figures 2A and 2B), 74% of cluster marker genes in the 5 dpf dataset were shared with the 2 dpf dataset, indicating the majority were conserved across time points (Figure 2C). We also performed a correlation analysis between the clusters at the two time points. Assessing intergroup distances among clusters at both 2 and 5 dpf, we found most of the clusters at the earlier stage were closely related to a corresponding 5 dpf cluster (Figure 2D). These results indicate the molecular diversity between subtypes at 2 dpf reflects sustained molecular differences among subtypes.
Figure 2. Heterogeneity of motor neuron functional subtypes is identifiable during development and at functional maturity.
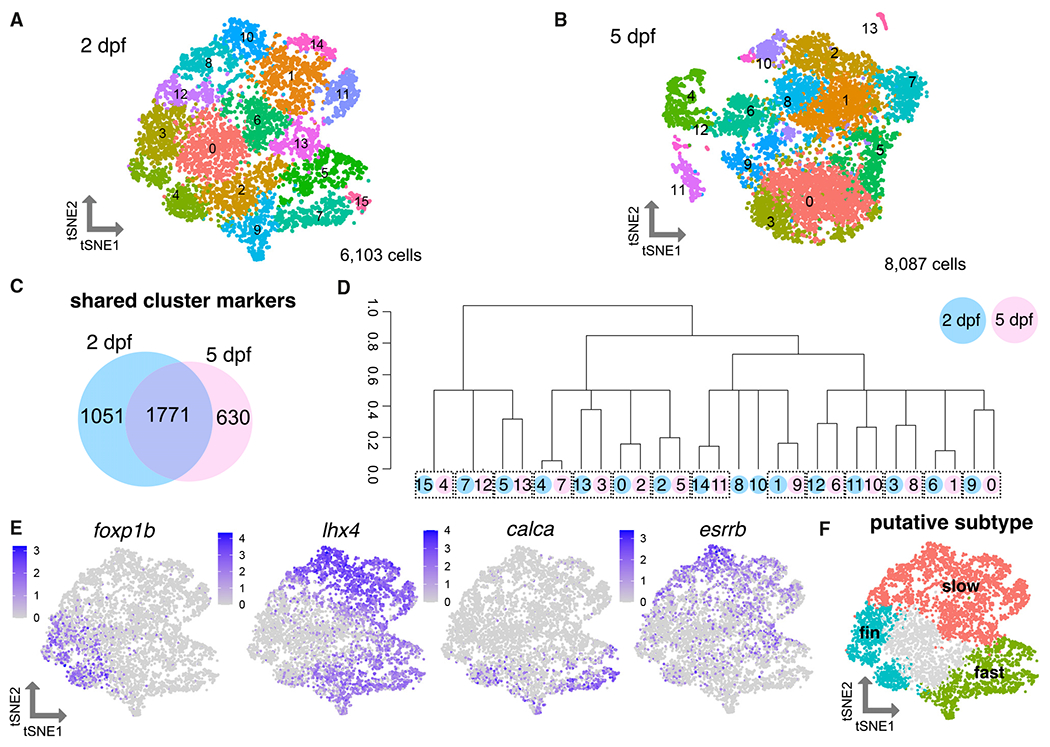
(A) tSNE (t-distributed stochastic neighbor embedding) representing 6,103 MNs at 2 dpf.
(B) tSNE representing 8,087 MNs at 5 dpf.
(C) Venn diagram demonstrating overlap in cluster markers from 2 and 5 dpf MNs.
(D) Dendrogram demonstrating relationships between 2 and 5 dpf MN clusters. Circle color indicates age (blue for 2 dpf, pink for 5 dpf). Circle number indicates cluster from (A) or (B). Dotted boxes group a 2 dpf cluster and its most closely related 5 dpf cluster. Expression of Hox genes may also contribute conserved molecular profiles between the 2 and 5 dpf datasets.
(E) Feature plots showing fin (foxp1b), axial (lhx4), fast (calca), and slow (esrrb) MN marker expression within 2 dpf MN clusters as in (A). Purple indicates expression.
(F) General map within tSNE space of putative MN subpopulations within 2 dpf MN clusters.
Conserved and divergent expression of MN subtype markers
To delineate which MN subtype each cluster represents, we first evaluated gene expression of known determinants of subtype fates. We found previously that a marker of limb-innervating MNs, Foxp1, is expressed by pectoral fin MNs in zebrafish larvae at 3 dpf and these MNs lacked expression of an axial MN determinant, lhx3.44 We marked clusters within the 2 dpf data that expressed foxp1b and lacked expression of lhx3 and lhx4 as pectoral fin MNs (Figures 2E, 2F, S2H, and S3A). Expression of an adductor fin MN subtype marker, lhx1a,45 marked a portion of these clusters (Figure S2H). lhx3/4 were widely expressed within almost all remaining axial MN clusters, in contrast with tetrapod axial MNs where expression of Lhx3 is sustained only by dorsally projecting medial motor column (MMC) axial MNs.
Axial MN clusters could be classified as putative fast or slow axial MNs on the basis of the expression of calca and esrrb (Figures 2E, 2F, and S3A). These broad functional groups include multiple clusters, indicating molecular diversity that could reflect additional subtype divisions. For example, MNs within the fast and slow groups could be distinguished by differences in Hox gene expression, likely reflecting the rostrocaudal domain from which they originated (Figure S2I). On the basis of these marker assessments, we assigned putative broad functional identities of the clusters (Figure 2F).
Molecular signatures of zebrafish MN functional subtypes
In addition to calca and essrb, we identified and characterized several novel markers of axial MN subtypes (Figures 3A and S3). We confirmed the localization of expression of novel markers using FISH in the Et(−0.6hsp70l:GAL4-VP16)s1020t;Tg(UAS:GFP) line, hereafter referred to as Tg(olig2::GFP), which labels the pMN domain and its progeny, including all MNs. We determined general dorsal-ventral soma positioning of mature MNs using Mnx (Hb9) antibody staining (Figure 3B). The most ventral GFP+ cells were Mnx− indicating that they were progenitors or oligodendrocytes. Within the mature population, dorsal MNs were generally classified as fast and the ventral MNs were generally classified as slow. We did not classify intermediate MNs, as their subtype specificity has not been determined at larval stages. We found multiple novel markers expressed in fast MNs, including pcp4a, calb2b, nefma, prdm16, scn4ba, gfra1b, mafba, and rbfox3a (Figures 3A and S5). pcp4a and calb2b labeled the dorsal-most fast axial MNs, including both primary and secondary MNs (Figures 3C, 3D, S5C, and S5D). These markers also maintained their dorsal expression at 5 dpf (Figures S4A and S4B).
Figure 3. Characterization of motor neuron functional subtype-restricted markers.
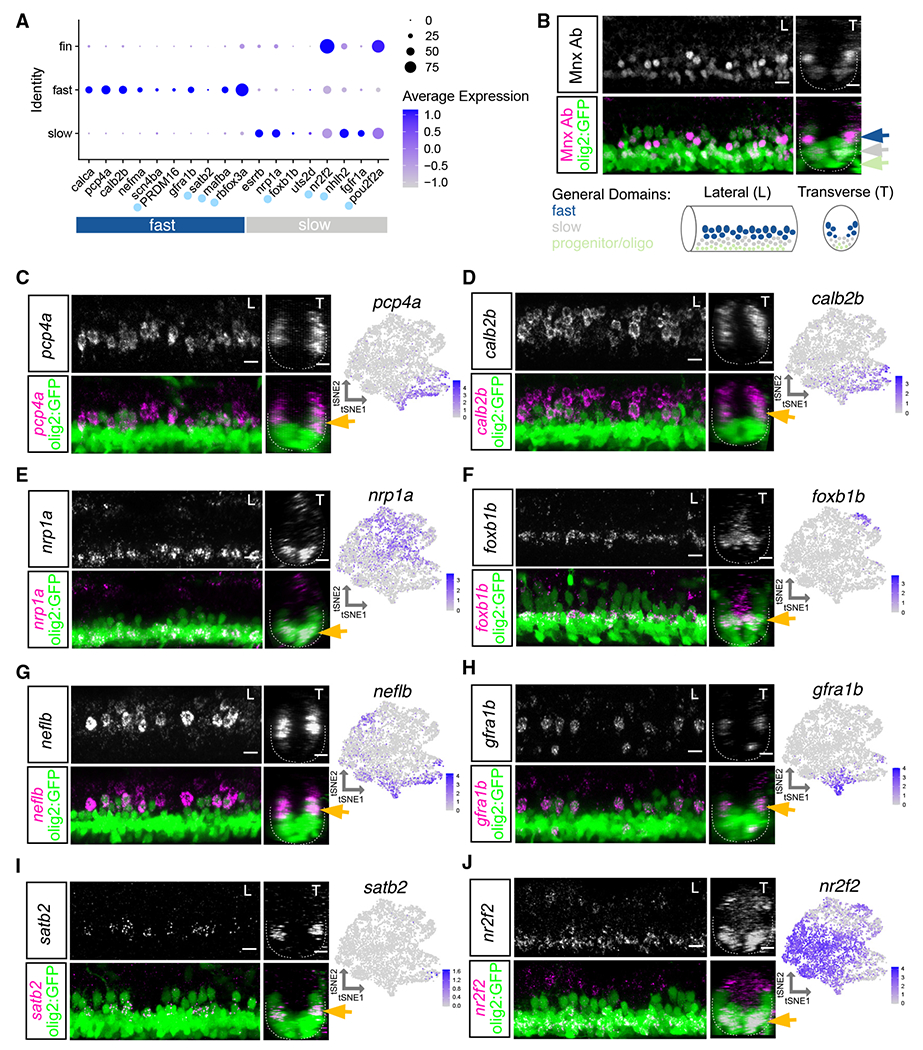
(A) Dot plot indicating expression of general fast and slow MN functional subtype markers. Light blue dot next to gene name indicates a transcription factor. At 2 dpf, uts2d is expressed by a small number of MNs.
(B) Top: antibody stain demonstrating domain of Mnx (Hb9) expression within mature MNs in Tg(olig2::GFP) larvae at 2 dpf. Bottom: schematic indicating general domains of expression considered when verifying functional subtype expression. Dorsal GFP+ cells are putative fast MNs (blue), most ventral are progenitors or oligodendrocytes (green), and those between are putative slow MNs (gray).
(C–J) FISH and feature plots for MN subpopulation markers at 2 dpf. Gold arrow indicates dorsoventral localization of FISH expression within MN population. Dotted line indicates ventral spinal cord boundary. Left image (L) is lateral view, and right image (T) is transverse view. Scale bars, 10 μm.
Although many markers of slow MN clusters were shared with fin MNs (nr2f2, nhlh2, and pou2f2a), we found several that had specific slow MN expression, including nrp1a, foxb1b, uts2d, and fgfr1a (Figure 3A). In addition to transcription factors, cluster-restricted genes encode molecules that are related to axon guidance, growth, and signaling. nrp1a, which has a role in axon guidance,46 was widely expressed within all slow clusters and, accordingly, expressed within ventral MNs (Figures 3E and S5E). Some markers, such as uts2d, were reported to be present in MNs,47 but their subpopulation specificity and function remain unknown. We did not identify any primary MN-specific markers within our dataset but found many of the fast MN markers labeled both primary and secondary dorsal MNs. Some of these markers had higher expression in primary MNs such as neflb (Figures 3G and S5G).
Several novel markers labeled smaller subsets of functional groups, including gfra1b, which labeled two of the fast MN clusters and foxb1b which labeled two slow MN clusters (Figures 3F, 3H, S5F, and S5H). FISH at 2 dpf revealed gfra1b was expressed in only a subset of dorsal fast MNs. We found an array of guidance molecules with cluster-specific expression that could potentially contribute to between- and within-subtype innervation diversity (Figures S4C and S4D). The restricted expression of foxb1b within two slow MN clusters was reflected in its more limited expression within the ventral domain. foxb1b did not label as many of the most ventral MNs as did the slow MN marker nrp1a; foxb1b might therefore label MNs involved in intermediate speed swimming.
We also identified markers of mammalian axial MN subpopulations that are conserved in zebrafish. A recent study48 found subpopulations of tetrapod axial MMC neurons marked by Satb2, Nr2f2, and Bcl11b. We found all three markers expressed within zebrafish axial MNs. satb2 was restricted within one fast MN cluster and confirmed to be in only a few MNs per hemisegment by FISH (Figures 3I and S5I). nr2f2 was strongly expressed within fin MN clusters and a portion of axial MNs. FISH demonstrated nr2f2 to be enriched in ventral axial MNs (Figures 3J and S5J). bcl11ba was detected in both fast and slow axial MN clusters and labeled all spinal cord cell types by FISH (data not shown). These data demonstrate that tetrapod axial MN subtype markers are conserved in zebrafish, though their subtype restrictions likely vary.
Prdm16 and Mecom mark putative fast axial MNs
To identify potential fate-determining genes for MN functional subtypes, we assessed transcription factors that were expressed in either fast or slow clusters (Figure 3A). A transcription factor from the Prdm family, prdm16, was detected in fast MN clusters and is a paralog of a previously described axial MMC-specific transcription factor in mice, Mecom (also known as Evi1 or Prdm3).49 Both prdm16 and mecom were expressed in fast MN clusters at 2 dpf (Figure 4A). In situ hybridization at 30 h post-fertilization (hpf) revealed that both prdm16 and mecom are expressed in a clustered population of cells in the ventral spinal cord (Figure 4B). Using an antibody that recognizes both Prdm16 and Mecom (Figures 4C and 4D, magenta), as well as one that recognizes Mecom alone (Figure 4D, green), we confirmed these transcription factors were expressed in a subset of dorsal MNs at 2 dpf. We also detected prdm16 transcripts in mouse MMC neurons (Figure S6A), as well as Mecom protein in chick and skate axial MNs (Figures S6B and S6C), indicating expression of these factors is evolutionarily conserved.
Figure 4. Prdm16 and Mecom mark dorsally positioned motor neurons.
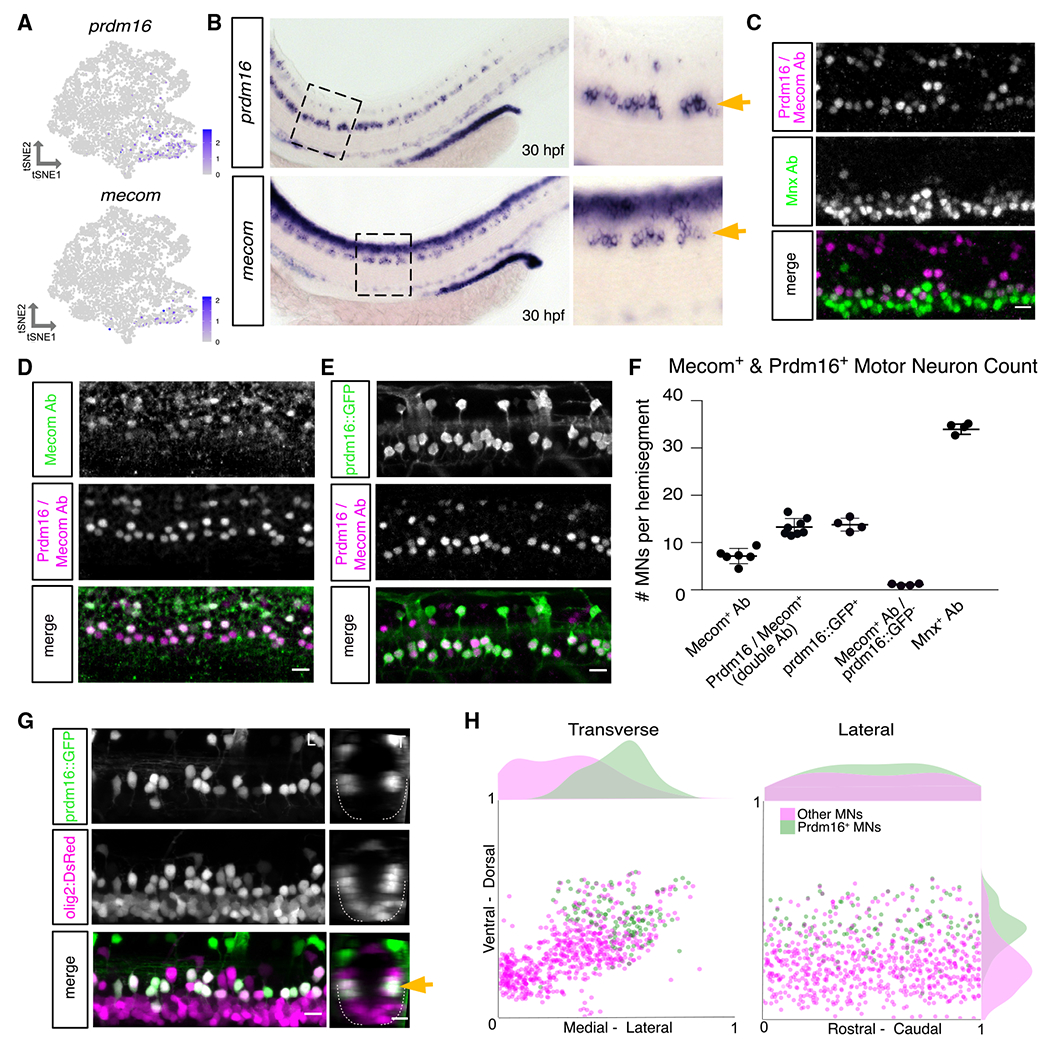
(A) Feature plot of prdm16 and mecom expression in 2 dpf MN tSNE showing localization in fast MN clusters as in Figure 2F.
(B) prdm16 and mecom in situ hybridization at 30 hpf in zebrafish. Zoomed image indicated by dashed box. Gold arrows indicate MNs. Additional staining in the dorsal spinal cord is labeling of some interneurons (prdm16) and a progenitor domain (mecom).
(C) Antibody stain of Prdm16/Mecom within Mnx+ MNs in the zebrafish spinal cord at 2 dpf. Dorsal Prdm16/Mecom+; Mnx− cells are a subpopulation of interneurons.
(D) Antibody stain showing overlap in expression between Prdm16/Mecom double antibody and Mecom alone antibody at 2 dpf.
(E) Antibody stain showing overlap in expression between GFP+ cells in Tg(prdm16::GFP) line and Prdm16/Mecom double antibody at 2 dpf.
(F) Quantification of Prdm16 and/or Mecom-expressing MNs from stains in (C)–(E). Line with bars indicates average with SEM.
(G) Image of the spinal cord of a Tg(prdm16::GFP);Tg(olig2:dsRed2) larva at 5 dpf. Gold arrow indicates dorsoventral localization of GFP+ MNs within dsRed2+ population. Left image (L) is lateral view, and right image (T) is transverse view.
(H) Soma position plot for GFP+ MNs and all other MNs quantified from five larvae at 5 dpf as in (G). Left plot shows the transverse view. Right plot shows the lateral view. Histograms on the top and sides of the plots indicate the distribution of cells across the axes. Green indicates GFP+;dsRed2+ cells. Magenta indicates GFP−;dsRed+ cells. Although magenta (other MNs) count could include olig2 progenitors or oligodendrocytes, these were largely excluded on the basis of morphology and localization. Scale bars, 10 μm.
To visualize Prdm16+ MNs, we crossed a GAL4 enhancer trap at the prdm16 locus gSAIzGFFM1116A, hereafter referred to as Tg(prdm16:GAL4), with a Tg(5xUAS:EGFP) line, hereafter called Tg(prdm16::GFP). Staining 2 dpf embryos with the Prdm16/Mecom double antibody demonstrated near complete overlap with GFP+ MNs (~92%; Figure 4E). Cell counting indicated that there are ~14 Prdm16+ positive cells per hemisegment, and about half were also Mecom+ (Figure 4F). We crossed the Tg(prdm16::GFP) line to an Tg(olig2:dsRed2) line and found that the dorsal positioning of GFP+ MNs was maintained at 5 dpf (Figure 4G). There was a reduction of about 3 or 4 GFP+ MNs per hemisegment from 3 to 5 dpf, possibly reflecting a decay in fluorescence in the oldest MNs (Figure S6D). By normalizing the position of Prdm16+ MN soma to landmarks (see STAR Methods), we observed they make up most, but not all, dorsal and lateral MNs at 5 dpf (Figure 4H). The remaining dorsal GFP− MNs are likely primary MNs because of their large size and positioning. Consistent with the scRNA-seq data, expression patterns of Prdm16 and Mecom suggest that they mark secondary MNs involved in fast swimming.
Prdm16 marks secondary MNs that innervate fast muscle
In zebrafish, slow muscle is located superficially and innervated by septal (s) nerves, while deeper fast muscle is innervated by medial (m) nerves (Figure 5A).31 To determine which muscle fiber types are innervated by Prdm16+ MNs, we performed confocal microscopy on Tg(prdm16::GFP);Tg(olig2:dsRed2) fish. The entire MN population, represented by dsRed+ axons, innervated both fast and slow muscle (Figure 5B). By contrast, GFP+ axons primarily innervated fast muscle via the medial nerves, and the majority of dsRed+ axons in slow muscle were GFP−. A detailed quantification of innervation demonstrated Prdm16+ MNs innervated all four quadrants of fast muscle with a slight ventral bias (Figure 5C). These data demonstrate that Prdm16+ MNs predominately innervate medial fast muscles. Notably, unlike in mammals, the Prdm16+ MNs in zebrafish do not exclusively innervate dorsal axial musculature.
Figure 5. Prdm16 and Mecom define secondary fast motor neurons.
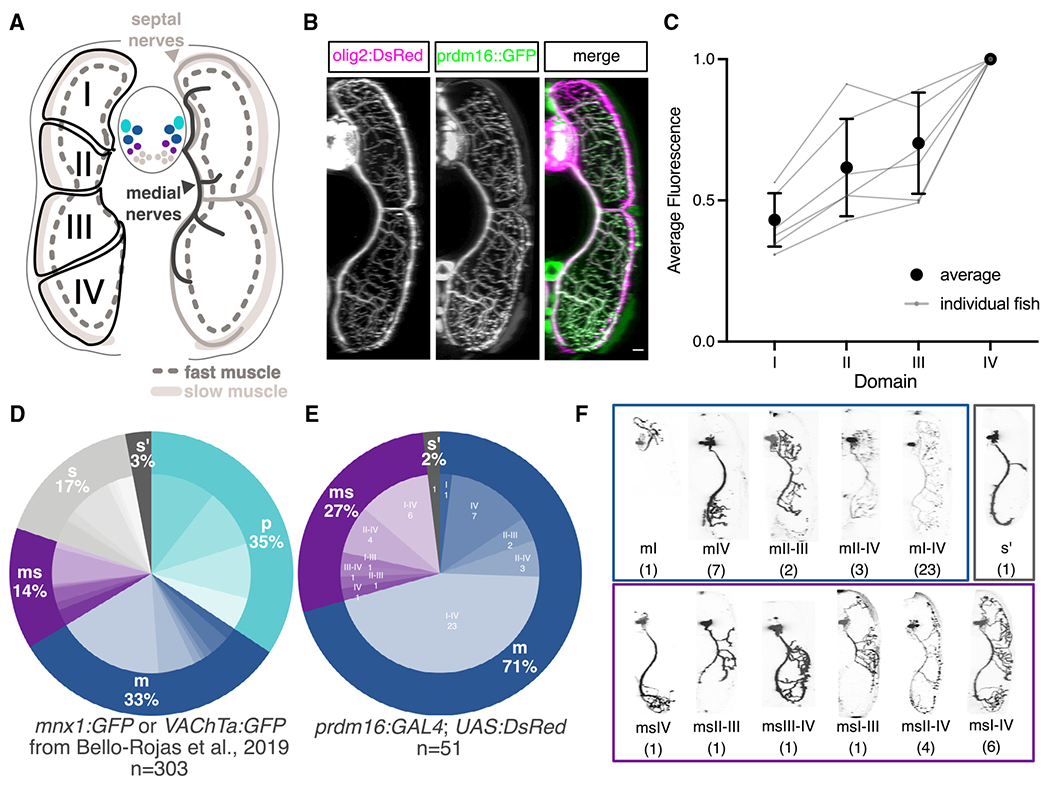
(A) Schematic of a transverse cross section of the zebrafish tail showing (left) the four dorsal-ventral muscle quadrants (black solid line with labels I, II, III, and IV) as defined by primary MN innervation pattern and the different muscle functional fiber types (solid light gray as slow, dashed dark gray outline as fast) and (right) the nerves that innervate them (slow muscle by septal nerves [light gray] and fast muscle by medial nerves [dark gray]). Primary MNs (p-type, light blue), responsible for escape behaviors, project along the medial nerve to innervate one of four fast muscle quadrants. m-type secondary MNs (dark blue) innervate fast muscle via the medial nerves. ms-type secondary MNs (purple) innervate both fast and slow muscle via the medial and septal nerves. s-type secondary MNs (light gray) innervate slow muscle via the septal nerves. Each individual secondary MN can innervate from one to four quadrants (I–IV) of muscle.
(B) Transverse view of the zebrafish tail showing muscle innervation in Tg(prdm16::GFP);Tg(olig2:dsRed2) larvae at 5 dpf. Scale bar, 10 μm.
(C) Average GFP fluorescence from Tg(prdm16::GFP) within each muscle quadrant normalized to the fourth quadrant within each fish at 5 dpf. Gray lines indicate individual fish. Black points indicates average with SEM.
(D) Pie chart showing subtype proportions labeled in pan-MN sparse label experiment.31 Inner subtype distribution numbers detailed in Table S1.
(E) Pie chart showing subtype proportions labeled in Tg(prdm16:GAL4).
(F) Example images of all sparsely labeled subtypes in (E). Number in parentheses indicates number of times subtype was labeled.
To examine the anatomical projections of single, sparsely labeled Prdm16 MNs, we injected a pUAS:dsRed plasmid50 into Tg(prdm16:GAL4) embryos at the single-cell stage. Labeled cells were categorized as either fast primary MNs (p) or secondary MNs that project along the medial (m), septal (s), or both nerves (ms). Secondary MNs projecting along the medial nerve (m-type) innervate fast muscle, s-type MNs innervate slow muscle, while ms-type MNs innervate both fast and slow muscle. Combining the functional class (p, m, ms, or s) and the dorsal-ventral quadrants of muscle innervation (I, II, III, and/or IV) gives a subtype classification to individual MNs in each hemisegment (Figure 5A; Table S1). A previous study31 identified 26 unique types using pan-MN labeling plasmids (Figure 5D; Table S1; n = 303). We found that 98% of our labeled Prdm16+ MNs belonged to either the m (71%) or ms class (27%) (Figures 5E and 5F; n = 51). Within these classes, there was a range of innervation subtypes (Figure 5F; Table S1). We identified 5 of 7 m-type subtypes and 5 of 7 ms-type subtypes identified previously,31 as well as one additional new ms subtype (ms III-IV). We labeled no p-type cells and 1 s′-type cell with an underdeveloped axon. A larger proportion of labeled MNs innervated the ventral quadrants, consistent with the trend detected with total population fluorescence (Figures 5C and S6E). The current limits of our tools do not allow us to identify which of these Prdm16+ subtypes also express Mecom. Hypothesis testing by resampling (STAR Methods) allowed us to reject the null hypothesis that Prdm16 labels all MNs: m (p = 0.0015), p (p = 0), and s (p = 0) counts were all different than expected. These experiments confirm that Prdm16+ selectively labels m-type and ms-type fast MNs.
Prdm16 and Mecom are essential for the specification of fast MNs
To determine if Prdm16 and Mecom are necessary for the specification of fast MNs in zebrafish, we adopted a loss-of-function approach. Using CRISPR-Cas9, we created prdm16 and mecom mutant lines (Figures S6F–S6K). Aside from a failure to inflate their swim bladder, prdm16 and mecom mutants are grossly morphologically indistinguishable from controls through 7 dpf (Figures S6H and S6I).
We first determined if loss of prdm16 and mecom led to a change in MN survival or positioning. Quantification of GFP+ MNs in the Tg(prdm16::GFP);Tg(olig2:dsRed2) line showed no MN loss in either prdm16 or mecom mutants at 5 dpf (Figure S6L). Double-mutant embryos also showed no significant change in the number of GFP+ MNs (Figure S7A). The position of both GFP+ and GFP− MNs were unchanged in either prdm16 or mecom mutants (Figure S6M). These results indicate prdm16 and mecom are not required for fast MN survival or soma positioning.
To assess whether loss of prdm16 or mecom affects fast or slow muscle innervation, we analyzed the Tg(prdm16::GFP);Tg(olig2:dsRed2) line in prdm16 and mecom mutants. We observed no gross denervation of any muscle type or quadrant (Figures 6A and S7G). The innervation of each dorsoventral quadrant shifted away from the ventral bias found in wildtype (WT) innervation, visible as a slope change in both prdm16 and mecom mutants (Figure 6B). Double-mutant larvae, which are overall smaller than their control and single mutant siblings, qualitatively had reduced muscle size and a decrease in fast muscle innervation (Figure S7B). These results suggest that, while fast muscle is still innervated after deletion of prdm16 or mecom, there appears to be a shift in the proportion of MN subtypes and more dramatic losses with loss of both alleles.
Figure 6. prdm16 and mecom are required for normal muscle innervation.
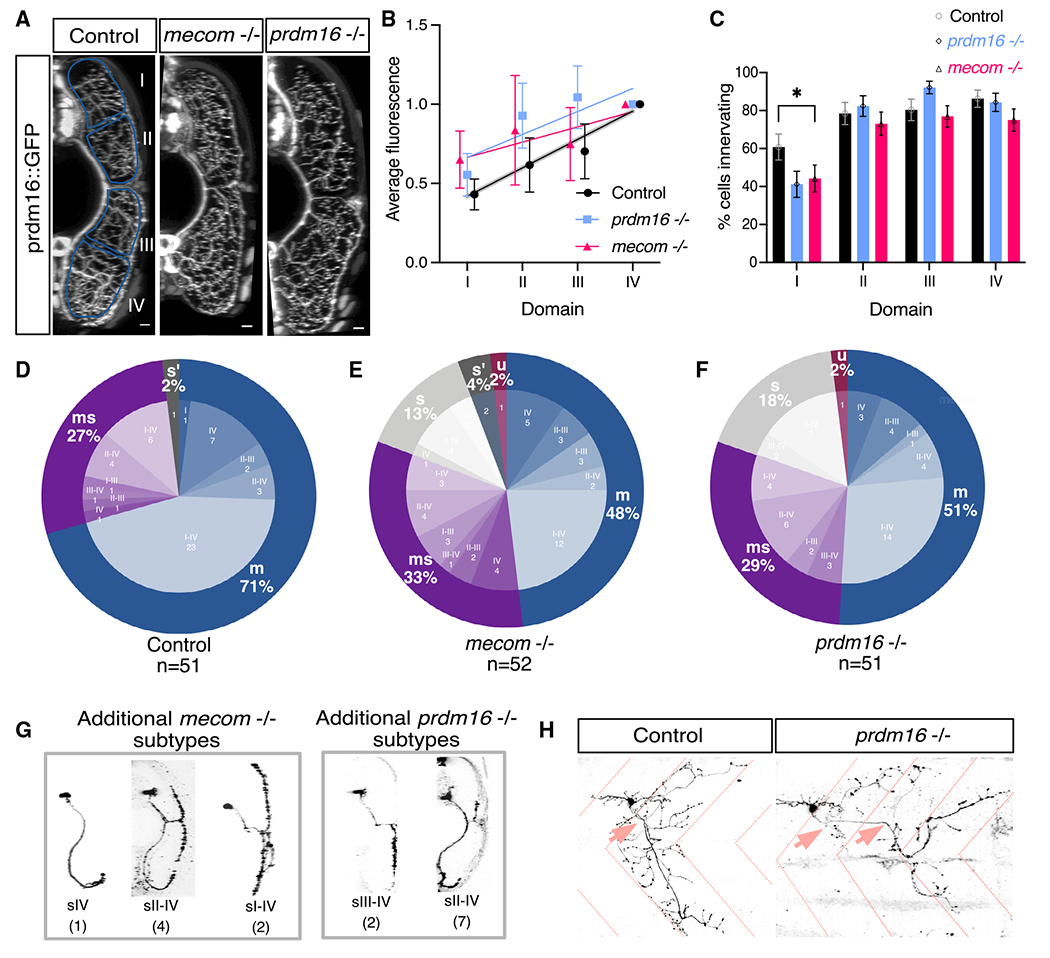
(A) Transverse view of the zebrafish tail showing Prdm16+ MN muscle innervation in Tg(prdm16::GFP) control, mecom mutant, and prdm16 mutant larvae at 5 dpf. Scale bars, 10 μm.
(B) Average GFP fluorescence of each muscle quadrant normalized to the fourth quadrant for control Tg(prdm16::GFP) fish (n = 6), mecom mutant (n = 9), and prdm16 mutant (n = 10) larvae at 5 dpf. Error bars indicate SEM. Lines illustrate linear regression for each condition and gray area surrounding control indicates SD of the slope, ±0.00555.
(C) Percentage of cells from control, mecom mutant, and prdm16 mutant Tg(prdm16:GAL4) sparse label experiments in (D)–(F) that innervated each dorsoventral muscle quadrant. Asterisk indicates significant difference (prdm16 −/− domain I, p = 0.000891; mecom −/− domain I, p = 0.004121), all other domains were not significantly different (p > 0.00625).
(D) Pie chart showing subtype proportions labeled in Tg(prdm16:GAL4) sparse label for control larvae. Replicated from Figure 5E.
(E) Pie chart showing subtype proportions labeled in Tg(prdm16:GAL4) sparse label for mecom mutant larvae.
(F) Pie chart showing subtype proportions labeled in Tg(prdm16:GAL4) sparse label for prdm16 mutant larvae.
(G) Example images of all sparsely labeled subtypes unique to mutants (E and F). Number in parentheses indicates number of times subtype was labeled.
(H) Example of an unclassifiable cell found in mutant sparse label experiment from the lateral view (right) compared with a control cell (left). Dashed coral lines indicate myotome boundaries. Coral arrows indicate motor exit points. Controls can indicate either WT or prdm16 +/− or mecom +/− animals, which are phenotypically indistinguishable.
To further explore a potential change in muscle innervation, we performed sparse labeling by injecting a pUAS:dsRed plasmid in a Tg(prdm16:GAL4) mutant background. We saw a significant decrease in the proportion of cells innervating quadrant I in mutants (Figure 6C; prdm16 −/− domain I, p = 0.000891; mecom −/− domain I, p = 0.004121; all other domains, p > 0.00625). More strikingly, in contrast to control larvae, we observed that 13% (7 of 52) of labeled cells in mecom mutants and 18% (9 of 51) of labeled cells in prdm16 mutants were s type with innervation of only slow muscle (Figures 6E–6G, S7E, and S7F; Table S1). Hypothesis testing by resampling (STAR Methods) allowed us to reject the null that the cell types we observed in mutants reflect the WT Prdm16+ distribution of cells: there were fewer m types (p = 0.0034, prdm16 mutants; p = 0.0011, mecom mutants); more s types (p = 0, prdm16 mutants; p = 0.0001, mecom mutants); and comparable numbers of ms types (p = 0.392, prdm16 mutants; p = 0.1786, mecom mutants). In addition, in both prdm16 and mecom mutants, we observed MNs with axons exiting from multiple motor exit points and extending beyond the normal myotome boundaries. We considered these “unclassifiable” or “u” types (Figures 6E, 6F, and 6H). Although the high mortality rate of double mutants prohibited sparse labeling, we were able to assess prdm16−/−;mecom+/− larvae. Compared with single mutants, we observed nearly twice as many s-type MNs at 31% (10 of 32) and a higher proportion of unclassifiable MNs at 9% (3 of 32) (Figure S7C; Table S1). Compared with the WT Prdm16+ distribution, there were fewer m types (p = 0), more s types (p = 0), and comparable numbers of ms types (p = 0.5274).
To determine if prdm16 or mecom mutants had swim speed deficits, we used a previously established free-swimming analysis pipeline to compare mutant larvae and swim bladder-less controls.51 mecom mutants were more likely to execute slower and shorter bouts and have an exaggerated nose-up posture compared with swim bladder-less controls (Video S1; Figure S7D). prdm16 mutants had more drastic posture and coordination defects compared with mecom mutants, prohibiting quantitative analyses of swimming behaviors (Video S2). These speed changes and instability could be from a miscoordination of motor programs or abnormal fast MN development and activity, but the global nature of the mutations makes it impossible to attribute these effects specifically to fast MN changes.
Molecular changes in fast MNs with the loss of Prdm16 or Mecom
To investigate molecular changes in fast MNs after loss of prdm16 or mecom, we compared the transcriptional profiles of control and single mutant larvae MNs. We first sorted Tg(Prdm16::GFP);Tg(olig2:dsRed2) mutant embryos at 5 dpf by the absence of a swim bladder (Figure S8A). We isolated MNs by FACS for both GFP and dsRed to attempt to enrich for fast MNs (Figure S8E). The dim green fluorescence at 5 dpf and bleed-through from the red channel limited our ability to separate GFP+ and GFP− MNs. Combined, the two samples for each condition were 14,882 cells for WT, 9,005 cells for mecom mutants, and 12,445 cells for prdm16 mutants. The overall quality of the samples was comparable (Figure S8C). Cells from all samples were pooled for downstream analysis. MN clusters were identified using chata expression (18,848 MNs total; Figures S8B and S8D) and reclustered for further analysis (Figure 7A). As prdm16 and mecom are no longer expressed in fast MNs at 5 dpf, we identified the fast MN cluster (14) in the WT sample using other markers, including calca, calb2b, and pcp4a. The limited representation of fast MNs at 5 dpf is likely due to a decay of fluorescence in older MNs.
Figure 7. Erosion of fast motor neuron transcriptional profiles in prdm16 mutants.
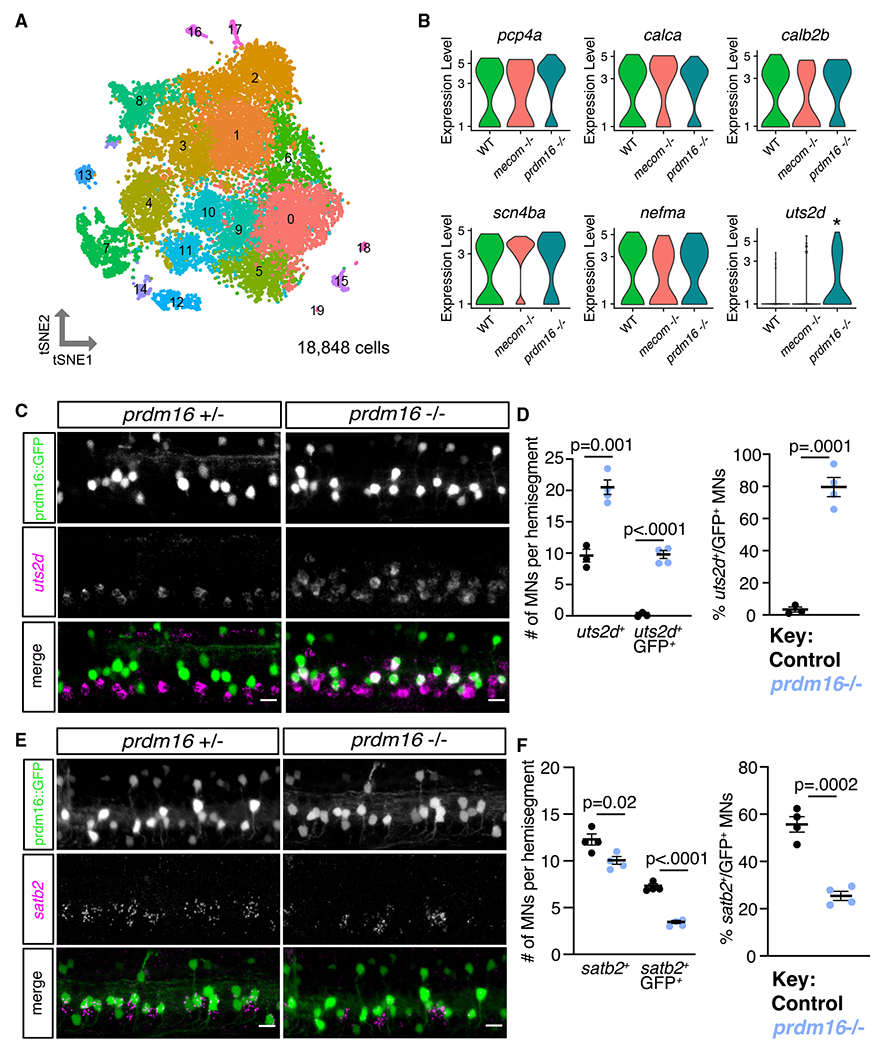
(A) tSNE representing all MNs from WT (8,145), mecom mutant (4,210), and prdm16 mutant (6,493) larvae at 5 dpf. Cluster 14 was determined to be fast MNs (WT, n = 88; mecom mutant, n = 40; and prdm16 mutant, n = 65).
(B) Violin plots representing log fold expression of fast MN markers (pcp4a, calca, calb2b, scn4ba, nefma and slow MN marker (uts2d) in cluster 14 of (A) identified as normally Prdm16+ fast MNs. uts2d showed a 3 log fold change increase in prdm16 mutants (adjusted p = 0.0194).
(C) FISH of uts2d in Tg(prdm16::GFP) control and prdm16 mutant larvae at 5 dpf. Scale bars, 10 μm.
(D) Left: quantification of uts2d+ cells and GFP+ uts2d+ cells in Tg(prdm16::GFP) larvae at 5 dpf as in (C). Right: percentage of total GFP+ cells that express uts2d. Line indicates average with SEM. Unpaired t tests.
(E) FISH of satb2 in Tg(prdm16::GFP) control and prdm16 mutant larvae at 5 dpf. Scale bars, 10 μm.
(F) Left: quantification of satb2+ cells and GFP+; satb2+ cells in Tg(prdm16::GFP) larvae at 5 dpf as in (E). Right: percentage of total GFP+ cells that express satb2. Line indicates average with SEM. Unpaired t tests.
We then performed differential gene expression analysis between each mutant and the WT sample within fast MN cluster 14. The gene with the largest fold change between WT and mutant samples was uts2d which had a three log fold change increase in prdm16 mutants (adjusted p = 0.0194; Figure 7B). Analyses by FISH in control embryos at 5 dpf showed that uts2d is normally expressed within slow MNs ventral to Prdm16+ MNs (Figures 7C, 7D, and S8G). In prdm16 mutants, the majority of Prdm16+ MNs (80%) ectopically expressed uts2d (Figures 7C and 7D). To determine whether fast MN molecular signatures were disrupted within the mutant samples, we also screened additional genes normally expressed within Prdm16+ fast MNs. There was no significant change in expression of pcp4a, calca, calb2b, scn4ba, or nefma within prdm16 or mecom mutant cluster 14 (Figure 7B). Although we confirmed no loss in pcp4a or calb2b expression by FISH, we did find a partial loss of satb2 expression in prdm16 mutant fast MNs (Figures 7E, 7F,and S8F). The limited detection of changed genes in mutants may be due to the small number of fast MNs in the 5 dpf dataset. The gain of expression of uts2d and partial loss of satb2 expression suggests the loss of prdm16 alters the transcriptional profiles of fast MNs to be more slow-like.
DISCUSSION
In this study, we show that axial MN subtypes are remarkably diverse and identify transcriptional determinants of MN functional subtypes. Leveraging the larval zebrafish spinal cord, we defined both conserved and novel markers that define functional subtypes both during early development and at functional maturity. We found that two conserved transcription factors, Prdm16 and Mecom, are expressed in fast secondary MNs. Loss of these genes leads fast secondary MNs to acquire features that normally define slow MNs. These data provide insights into molecular diversity of vertebrate spinal MNs and establish that MN functional subtypes are determined by intrinsic genetic programs acting in early development.
Molecular diversity of axial MNs
Our findings reveal unappreciated transcriptional diversity in axial MNs that offers insight into the molecular underpinnings of MN specification. We observed that zebrafish fin and tetrapod limb MNs (1) have conserved expression of foxp1 and lhx1a and (2) both lack expression of lhx3/lhx4.44,45 Interestingly, although lhx3/4 were excluded from fin MN clusters, they were widely expressed within zebrafish axial MNs. In mammals, the specification of ventrally projecting MN subtypes requires downregulation of lhx3/4, as sustained expression of lhx3 in all MNs blocks the specification of limb, preganglionic, and hypaxial subtypes.52,53 One interpretation of these findings could be that Lhx3+ MNs represent an ancient and more molecularly simple MN subtype fate.54
We show that axial MNs are highly diverse, and this molecular heterogeneity exists across vertebrate species. Two new markers, prdm16 and mecom, as well as two recently identified48 MMC subtype markers, satb2 and nr2f2, mark subpopulations of zebrafish axial MNs. satb2 and nr2f2 appear to mark two populations of secondary MNs with satb2 expressed in a population overlapping with prdm16+ fast MNs and nr2f2 labeling putative slow MNs and progenitors. We conclude that MN subtype markers, including those marking axial motor columns and their diverse subpopulations of pools, are conserved among vertebrates.
Our finding that molecular signatures of MN subtypes persist from early development to functional maturity extends previous work toward a more complete description of zebrafish axial MNs. Previously, relating the well-established anatomical and functional diversity of zebrafish axial MNs to molecular heterogeneity was an open challenge. For example, the developmental timing of expression of Islet, Mnx, and Lhx family members are known to be important for specifying features of primary MNs, but these factors are also dynamically expressed within secondary MN subtypes.16–22 Our findings indicate that both slow and fast secondary MNs express selective markers of subtype identity. Importantly, although we classified dorsally positioned markers as “fast” and ventrally positioned ones as “slow,” prior work further refines these categories, including primary/secondary and intermediate MN distinctions. The absence of intermediate muscle or defined intermediate subtypes in the larval zebrafish underscores the importance of performing similar functional classification with accompanying molecular profiling in juvenile/adult zebrafish.28,30 We hypothesize that MNs located near the boundary of the dorsal/ventral (fast/slow) distinction that project along both the medial and septal nerves, and/or those that express genes that mark a portion of the fast or slow groups are plausible candidates.
Prdm16 and Mecom specify fast MN subtypes
Our loss-of-function experiments establish two transcription factors, Prdm16 and Mecom, as indispensable in the development of a subset of fast MNs. Prdm16 and Mecom exclusively mark secondary MNs that innervate fast muscle. Loss of either prdm16 or mecom leads to fast MN developmental deficits, including the acquisition of anatomical and molecular features of slow subtypes. In both prdm16 and mecom mutants, fast MNs retain many of their molecularly defined features, but now express a marker normally restricted to slow MNs. The absence of a complete fast-to-slow fate transformation could be due to functional compensation between these factors, but the high lethality rate of double mutants precludes a detailed molecular characterization. Alternatively, these factors may play more specific roles by acting in fast MN to repress subsets of genes that define slow MN fate.
Both Prdm16 and Mecom have been shown to function as repressors in certain contexts.55 Our findings argue against a role for prdm16 and mecom in neurogenesis as both genes are expressed post-mitotically and presumptive fast MNs are still generated in mutant lines, although they acquire features of slow MNs. We propose that they work to suppress gene programs that define alternate MN subtype fates. Such suppression is consistent with our observation that prdm16 mutants derepress uts2d, a gene of unknown function47 whose homolog uts2b is expressed in mammalian axial MNs.56
Prior to our discovery of Prdm16 and Mecom as a determinant of fast secondary MNs, the mechanisms for functional subtype specification remained unresolved. One proposal was that MN functional subtype identity is plastic and sensitive to retrograde influences from muscle fibers once the targets are reached.57–60 Alternatively, differences between MN functional subtypes might reflect early defined intrinsic programs.42,61–63 Our results provide strong evidence that an intrinsic transcription factor code determines functional subtype and is supported by our finding that expression of Prdm16 and Mecom in MNs precedes secondary MN muscle innervation (data not shown).64 Broadly, functional subtype specification of MNs therefore resembles the transcriptional codes that specify classes of functionally distinct subtypes elsewhere in the nervous system.65–69
Implications for locomotor speed circuit organization and assembly
Our findings establish that functional subtype-restricted molecular codes exist in conjunction with, and likely control, established temporal aspects of locomotor speed circuit maturation. To regulate speed, zebrafish rely on circuits composed of descending neurons, spinal interneurons, and MNs.27,28,70,71 Shared functional properties (fast/slow) among cell types within speed control circuits reflect birth date,27,37,72,73 similar to other sensorimotor circuits.74–78 Spatial and temporal morphogen gradients, as well as sequential expression of transcription factors, have been implicated in generating spinal neuron subtype diversity across species.79,80 Birth date-dependent subtype differences have been linked to temporally regulated post-mitotic transcription factor programs in vertebrates81,82 and invertebrates.83,84 We propose that Prdm16 and Mecom operate as intrinsic determinants acting within a temporal program that defines MN functional subtype fates.
Broadly, our work defines novel molecular signatures for diverse spinal MN subtypes and reveals genetic mechanisms of MN functional subtype specification in the vertebrate nervous system. Every vertebrate muscle is innervated by both slow and fast MN subtypes; the discovery that a transcription factor code establishes functional subtype identity thus speaks broadly to key mechanisms of motor control. Furthermore, these data establish key regulatory nodes in fast and slow MN development, potentially opening new avenues to understand the differential susceptibility of MNs in diseases such as ALS. Finally, identifying the hallmarks of functional subtype determination permits comparative studies of molecular underpinnings of locomotor control and its evolutionary origins.
Limitations of the study
In this study, we identified molecular signatures for fast and slow MN subtypes in larval zebrafish and found two transcription factors important for key features of fast MN development. However, it remains to be determined whether Prdm16 and Mecom contribute to functional differences between MN functional subtypes, such as intrinsic firing properties. Moreover, although the loss of either of these factors causes fast MNs to acquire characteristics of slow MNs, most fast molecular features are still retained in mutant MNs. These results suggest that other intrinsic factors, yet to be identified, allow selective expression of fast molecular features within Prdm16+ and Mecom+ MN populations. Finally, the developmental mechanisms responsible for the emergence of molecular differences between MN functional subtypes and topographical organization are still unclear. Although our studies support a model in which functional subtypes are specified by intrinsic molecular programs, how these programs are established, whether through temporal or mechanisms or spatial cues, remains to be determined.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Jeremy Dasen (jeremy.dasen@nyulangone.org). This study does not report original code.
Materials availability
Mutant fish lines generated in this study will be deposited to the Zebrafish International Resource Center (ZIRC).
Data and code availability
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-Evi1/Mecom | Cell signaling | RRID:AB_2184098 |
| Chicken and rabbit anti-GFP | Invitrogen | RRID:AB_2534023 |
| Guinea-pig anti-zebrafish Prdm16/Mecom antibody | Covance | N/A |
| Rabbit anti-zebrafish Mecom antibody | Covance | N/A |
| Mouse anti-Hb9 antibody | Invitrogen | RRID:AB_2540929 |
| Chemicals, peptides, and recombinant proteins | ||
| Tween | Fisher Scientific | BP337-100 |
| Triton | Sigma Aldrich | T8787-100ML |
| MS-222 | Sigma Aldrich | E10521 |
| 32% PFA | Electron Microscopy Sciences | 15714 |
| Proteinase K | ThermoFisher Scientific | 25530049 |
| BSA | ThermoFisher Scientific | 50-253-966 |
| Papain | Worthington Biochemical | LK003150 |
| HBSS | ThermoFisher Scientific | 14-170-112 |
| EBSS | ThermoFisher Scientific | 24010043 |
| DNase | Worthington Biochemical | LK003172 |
| DAPI | Sigma Aldrich | D9542-10mg |
| Critical commercial assays | ||
| Digoxigenin-dUTP labeling kit | Roche | N/A |
| in situ hybridization chain reaction v3.0 (HCR) | Molecular Instruments | N/A |
| DreamTaq Green PCR Master Mix (2X) | Thermo Scientific | K1082 |
| Deposited data | ||
| RNA-seq of WT zebrafish MNS at 1 dpf and 1.5 dpf | Scott et al.39 | GEO: GSE173350 |
| Raw and analyzed 10x Genomics scRNA-seq datasets | This paper | GEO: GSE240026 |
| Experimental models: Organisms/strains | ||
| Zebrafish: Tg(olig2:dsRed2)vu19 | Kucenas et al.38 | N/A |
| Zebrafish: Tg(prdm16:GAL4) | NBRP | gSAIzGFFM1116A |
| Zebrafish: Tg(olig2:GFP) | Scott et al.85 | N/A |
| Zebrafish: mecomnyc41 | This paper | N/A |
| Zebrafish: prdm16nyc142 | This paper | N/A |
| Zebrafish: Tg(prdm16:GAL4); prdm16nyc584 | This paper | N/A |
| WT Mice: mixed genetic background | New York University Grossman School of Medicine Animal Facilities | N/A |
| Leucoraja erinacea embryos | Marine Resources Department of MBL | N/A |
| Specific Pathogen Free Fertile Chicken Eggs | AVS bio | N/A |
| Oligonucleotides | ||
| Prdm16 d17/d22 forward primer (CCATTAAAGCCATTGCCTCC) |
GeneWiz | N/A |
| Prdm16 d17/d22 reverse primer (GAACATGCCAGGATTGAGTG) |
GeneWiz | N/A |
| Mecom d25 forward primer (CCGCTGGAGGGTTGTTTGC) |
GeneWiz | N/A |
| Mecom d25 reverse primer (CCGATGGTAAAGTCCTGATGG) |
GeneWiz | N/A |
| Prdm16 gal4 forward primer (CCGTGACTGATCGCCTGGCAAG) |
GeneWiz | N/A |
| Prdm16 gal4 reverse primer (GCCGGGCAGCATATCCAGATCG) |
GeneWiz | N/A |
| HCR probes | Molecular Instruments | N/A |
| Recombinant DNA | ||
| 5UAS zfSynaptophysin:GFP-5UAS DsRedExpress | Addgene | RRID:Addgene_74317 |
| Software and algorithms | ||
| ImageJ | Schindelin et al.86 | https://imagej.nih.gov/ij/ |
| Imaris (Bitplane) | Oxford Instruments | https://imaris.oxinst.com/ |
| Python v3 | Python Software Foundation | https://www.python.org |
| Prism v9 | GraphPad | https://www.graphpad.com/features |
| Seurat v4 | Hao et al.87 | https://satijalab.org/seurat/ |
| Velocyto v0.17.17 | Manno et al.40 | https://velocyto.org/velocyto.py/ |
| CellRanger v3.0.2 | Zheng et al.88 | https://support.10xgenomics.com/single-cell-gene-expression/software/overview/welcome |
| LabVIEW | National Instruments Corporation | https://www.ni.com/en-us/shop/software/products/labview.html |
| CHOPCHOP online tool | Labun et al.89 | https://chopchop.cbu.uib.no/ |
| Other | ||
| Glass cuvettes | Starna Cells Inc. | (93/G.10 55 × 55 × 10mm) |
| Digital CMOS cameras | FLIR Systems | Blackfly PGE-23S6M |
| 5W 940nm infrared LED back light | eBay | N/A |
| Infrared filter | Edmund Optics | 43–953 |
| Aspheric condenser lens with diffuser | Thor Labs | ACL5040-DG15-B |
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Fish care
All procedures involving zebrafish larvae (Danio rerio) were approved by the Institutional Animal Care and Use Committee of the New York University Grossman School of Medicine. Fertilized eggs were collected and maintained at 28.5°C on a standard 14/10 h light/dark cycle. Before 5 dpf, larvae were maintained at densities of 20–50 larvae per Petri dish of 10 cm diameter, filled with 25–40 mL E3 with 0.5 ppm methylene blue.
Mouse/chick care
All procedures involving mice or chicks were performed in accordance with the US National Institutes of Health Animal Protection Guidelines and approved by the Institutional Animal Care and Use Committee of the New York University Grossman School of Medicine. Animal ages are indicated in the main text and figure legends. No sex-specific differences in reported phenotypes are expected, but were not formally tested.
Skate care
Leucoraja erinacea embryos of the desired stages were obtained from the Marine Resources Department of Woods Hole Marine Biology Laboratory (MBL). Animals at MBL are maintained in accordance with procedures approved by the MBL Institutional Animal Care and Use Committee following standards established by the National Institutes of Health. Prior to use, egg cases were maintained in reconstituted Instant Ocean (Aquarium Systems) at 16°C with a standard aquarium filtration system. Before manipulation, embryos were anesthetized with MS-222 (0.17 g/L; Sigma-Aldrich) at room temperature for 10 min.90
Fish lines
Experiments were done on the wildtype or the mitfa−/− background to remove pigment. We used Tg(olig2:dsRed2)vu19.38 Tg(olig2::GFP) line is Et(−0.6hsp70L:GAL4-VP16)s1020t86 crossed with Tg(5xUAS:EGFP).91 Tg(prdm16:GAL4) is gSAIzGFFM1116A created by a gene trap method [insertion site CACACTTCAGTGCTTTTGCAATTTTCTAGTCTGCATCAACTGCTCTGAGCT located upstream of the prdm16 locus on Chr 8 in GRCz10].92 Tg(prdm16::GFP) is Tg(prdm16:GAL4) crossed with Tg(5xUAS:EGFP).
Generation of mutants
prdm16 and mecom mutant lines were generated via CRISPR-based mutagenesis. Guide RNAs (gRNAs) were designed using the CHOPCHOP online tool.89 Location of guides was chosen to be in the largest exon based on its presence in multiple described isoforms of each gene. gRNAs were incubated with Cas9 protein before co-injection into embryos at the single cell stage. Injected embryos were raised and their progeny were screened by genotyping to identify germline mutations. Multiple founders were identified and their progeny with variable mutations consistently lacked swim bladders. We focused on one of these alleles for the following experiments. Each mutant had a sizable deletion (mecomnyc41 mutant = 25 bp, prdm16nyc142 mutant = 17 bp, Tg(prdm16:GAL4);prdmnyc587 mutant = 22 bp) detectable by genotyping that created a nonsense mutation and a premature stop codon. Loss of protein in mutants was validated using antibodies.
METHOD DETAILS
Dissection, cell dissociation, and FACS
dsRed positive cells were isolated from 2 dpf or 5 dpf control, mecom mutant, or prdm16 mutant zebrafish embryos on an Tg(olig2:dsRed2) or Tg(prdm16::GFP);Tg(olig2:dsRed2) background. Trunk and tail tissue were separated from cranial tissue. Tissue was then finely chopped using a razor blade, dissociated using papain, filtered, and resuspended for sorting. Cells were sorted using the Sony SH800 FACS Cell Sorter. GFP−, dsRed2−, and single fluorophore control embryos were also included as controls for the FACS setup. DAPI was used for a live/dead marker. Sorted cells were then counted using a hemocytometer and spun down to resuspend at a higher concentration. Cells were then processed using the standard 10x Genomics and CellRanger(v5.0.1) pipeline.88 Raw sequencing reads were mapped to the zebrafish reference genome (build GRCz11).
Single cell analysis
To evaluate the 2 dpf scRNA-seq data, we used Seurat v4.87 To ensure only high-quality cells were retained for downstream analysis, cells with fewer than 200 genes, more than 5,000 genes, over 6% mitochondrial counts, and total RNA counts over 30,000 were removed. Data was normalized using the NormalizeData Seurat function and variable features were identified using FindVariableFeatures. The top 2000 variable genes were used in the PCA. Based on Jack Straw and an elbow plot of 30 principal components,93 21 principal components were used in the downstream analysis. For the 5 dpf data, we used a Tg(prdm16::GFP);Tg(olig2:dsRed2) transgenic line. To ensure only high-quality cells were retained for downstream analysis, cells with fewer than 200 genes, more than 3,500 genes, over 10% mitochondrial counts, and total RNA counts over 30,000 were removed. Since samples were sorted for both dsRed and GFP, we used the top 2000 variable genes to find anchors to integrate the GFP+ and GFP− negative populations using IntegrateData. This was also done to combine the WT, mecom mutant, and prdm16 mutant datasets. There, the top 2000 variable genes were used in the PCA, and 21 principal components were used in the downstream analysis. To isolate MN clusters, we reran the above analyses on data from clusters that expressed chata. After MNs were reclustered, we reevaluated mitochondrial and gene counts within each cluster. In the 2 dpf data, three clusters were identified as having high mitochondrial counts and low gene counts. These were determined as outliers within the data. After their removal, the remaining cells were reclustered. For 2 dpf data, 20 principal components were used for MN clustering. For 5 dpf data, 30 principal components were used for MN clustering. For the 2 dpf v 5 dpf analysis, R package CIDER (v0.99.0)94 was used to assess inter-group similarity between 2 dpf and 5 dpf clusters. Gene Ontology analysis was done using ShinyGO (v0.76.3).95
RNA velocity
The.loom file from velocyto (v0.17.17)40 was used to calculate splicing information for each gene, which was then used to compute RNA velocities for each cell according to standard parameters in scVelo (v0.2.4).41 UMAP coordinates calculated with Seurat were exported and used at the basis for the velocity streamline plot in Figure 1E. Pseudotime was then computed based on RNA velocity information according to suggested parameters in the scVelo package.
Integration with Scott et al. 2021
Raw.fastq files were downloaded from 1 day to 1.5 day timepoints published in,39 2021 NCBI GEO #GSE173350. For this analysis, the.fastq files for the raw data for both Scott et al. and our datasets were analyzed via CellRanger v3.0.2 as done in their study. We used the.fastq files to produce.loom files (with splicing data for each gene) via velocyto splice calling. We then processed these.loom files for RNA velocity as above, and then we integrated both published datasets with our later timepoints using HarmonyPy (v0.0.5).96
Mouse in situ hybridization
Embryos were fixed in 4% PFA for 1.5–2 h at 4°C. Embryos were washed 5–6 times in cold PBS, 15–30 min for each wash, and incubated overnight in 30% sucrose. Antisense riboprobes were generated using the Digoxigenin-dUTP (SP6/T7) labeling kit (Sigma-Aldrich). RNA was extracted from E12.5 embryos using TRIzol (Invitrogen) and cDNA was generated using SuperScript II Reverse Transcriptase (Invitrogen). Riboprobes were prepared by PCR using cDNA templates, incorporating a T7 polymerase promoter sequence in the antisense oligo. Sections were dried for 10–15 min at room temperature, placed in 4% PFA, and fixed for 10 min at room temperature. Slides were then washed three times for 3 min each in PBS, and then placed in Proteinase K solution (1 mg/mL) for 5 min at room temperature. After an additional PFA fixation and washing step, slides were treated in triethanolamine for 10 min, to block positive charges in tissue. Slides were then washed three times in PBS and blocked for 2–3 h in hybridization solution (50% form-amide, 5X SSC, 5X Denhardt’s solution, 0.2 mg/mL yeast RNA, 0.1 mg/mL salmon sperm DNA). Prehybridization solution was removed, and replaced with 100 mL of hybridization solution containing 100 ng of DIG-labeled antisense probe. Slides were then incubated overnight (12–16 h) at 72°C. After hybridization, slides were transferred to a container with 400 mL of 5X SSC and incubated at 72°C for 20 min. During this step, coverslips were removed using forceps. Slides were then washed in 400 mL of 0.2X SSC for 1 h at 72°C. Slides were transferred to buffer B1 (0.1M Tris pH 7.5, 150mM NaCl) and incubated for 5 min at room temperature. Slides were then transferred to staining trays and blocked in 0.75 mL/slide of B1 containing 10% heat inactivated goat serum. The blocking solution was removed and replaced with antibody solution containing 1% heat inactivated goat serum and a 1:5000 dilution of anti-DIG-AP antibody (Roche). Slides were then incubated overnight at 4°C in a humidified chamber. The following day, slides were washed 3 times, 5 min each, with 0.75 mL/slide of buffer B1. Slides were then transferred to buffer B3 (0.1 M Tris pH 9.5, 100 mM NaCl, 50 mM MgCl2) and incubated for 5 min. Slides were then developed in 0.75 mL/slide of B3 solution containing 3.5 mL/mL BCIP and 3.5 mL/mL NBT for 12–48 h. After color development, slides were washed in ddH20 and coverslipped in Glycergel (Agilent). A more detailed in situ hybridization protocol is available on the Dasen lab website: https://www.dasenlab.com/lab-protocols.
Zebrafish in situ hybridization (ISH)
Preparation of RNA probes was done as described above. DNA templates were made using a zebrafish 30 hpf cDNA library generated from polyA RNA extracted with Trizol. Whole mount in situ hybridization was performed as previously described.97 Embryos were fixed in 4% PFA overnight at room temperature. Following fixation, they were permeabilized in methanol at −20°C, rehydrated, permeabilized with 10 mg/mL proteinase K in PBST for 8 min at room temperature, and post-fixed in 4% PFA for 20 min at room temperature. Embryos were bleached in a 10% H2O2, 10% KOH solution prepared in ddH2O and washed twice for 5 min in PBS with 0.1% Tween (PBST). Blocking and probe hybridization were performed at 68°C overnight. Following probe hybridization and washes, embryos were blocked in 2% BSA in PBST and incubated with anti-DIG-AP antibody overnight at 4°C. Embryos were washed and developed in NBT/BCIP solution overnight at room temperature.
Zebrafish fluorescent in situ hybridization (FISH)
Protocol adapted from98: Hybridization chain reaction (HCR) probes were generated by the HCR 3.0 probe maker99 based on methodology generated by.100 Probe pairs were created based off the sense sequence of the gene cDNA from NCBI. Embryos were previously fixed at the desired age with4% PFA in PBS overnight at 4°C. Following fixation, the embryos were dehydrated in 100% methanol at −20°C until used. When needed, the embryos were rehydrated through a titer of methanol and PBST, washed two times with PBST for 5 min, and permeabilized in pre-chilled acetone at −20°C for 12 min. After acetylation, the samples underwent three PBST washes for 5 min. Further permeabilization was performed by placing the embryos in 10 mg/mL proteinase K in PBST at room temperature. Optimal proteinase K incubation time was determined by embryo age. Two day post fertilization embryos were digested for 20 min and five day post fertilization embryos for 50 min. The samples were then rinsed with PBST three times for 5 min and post-fixed in 4% PFA in PBST for 20 min at room temperature. Embryos were washed 5 times with PBST for 5 min each and incubated in prewarmed whole mount probe hybridization buffer (from Molecular Instruments) at 37°C for 30 min. Incubation with HCR probe mixture solution was performed overnight at 37°C. Four 15 min washes of preheated probe wash buffer (from Molecular Instruments) were performed at 37°C. The embryos were then moved to room temperature, washed two times with 5x SSCT for 5 min each, and placed in the probe amplification buffer for 30 min. As the larvae underwent preamplification, aliquots of amplifier hairpins were snap cooled by heating to 95°C for 90 s and cooling at room temperature in the dark for 30 min. The embryos were incubated in the dark with the amplification buffer and snap cooled hairpins overnight at room temperature. Excess amplifier hairpins were removed by washing with two 5 min washes, two 30 min washes, and one last 5 min wash of 5x SSCT. Finally, they were washed three times with PBST for 5 min each.
Chick/skate immunohistochemistry
For antibody staining of sections, slides were first placed in PBS for 5 min to remove OCT. Sections were then transferred to humidified trays and blocked for 20–30 min in 0.75 mL/slide of PBT (PBS with 0.1% Triton) containing 1% Bovine serum albumin (BSA). The blocking solution was replaced with primary staining solution containing antibodies diluted in PBT with 0.1% BSA. Primary antibody staining was performed overnight at 4°C. Slides were then washed three times for 5 min each in PBT. Fluorophore-conjugated secondary antibodies were diluted 1:500-1:1000 in PBT and filtered through a 0.2 mm syringe filter. Secondary antibody solution was added to slides (0.75 mL/slide) and incubated at room temperature for 1 h. Slides were washed three times in PBT, followed by a final wash in PBS. Coverslips were placed on slides using 110 mL of Vectashield (Vector Laboratories).
Zebrafish immunohistochemistry
All steps were done in a 1.5 mL Eppendorf tube. Dechorionated embryos were fixed in 8% PFA in 2X Fix Buffer (8.0 g sucrose +30 μL 1M CaCl2 + 10 mL 10X PBS in 80 μL ddH2O) at 4°C for 2 h. They were then rinsed once and washed three times for 20 min each in PBS with 0.1% Tween (PBST). Embryos were put in 150 mM Tris for 5 min at RT, then put in a 70° water bath for 15 min. Embryos were then washed twice for 5 min in PBST. Embryos were then put in 0.5% Triton in PBS for 15 min. Embryos were then bleached only if pigmented in a 10% H2O2, 10% KOH solution prepared in ddH2O and washed twice for 5 min in PBS with 0.1% Tween. Embryos were blocked for 1 h in PBT, 2.5% DMSO, and 5% Heat Inactivated Goat Serum. Primary antibodies were diluted in PBT with 1% BSA, and embryos were incubated at 4°C for 3 or 4 nights. Embryos were then rinsed once and washed four times for 30 min each in PBS with 0.1% Tween. Secondary antibodies were diluted in PBT with 1% BSA, and embryos were incubated at 4°C overnight. Embryos were then rinsed once and washed four times for 30 min each in PBST. Embryos were mounted in agarose or on a slide in Vectashield (Vector Laboratories) to be imaged. Protocol adapted from.16
Sparse labeling
For sparse labeling, the GAL4-UAS system was used to drive mosaic expression of a reporter construct with a membrane-targeted fluorophore selectively in Prdm16-expressing neurons. Mosaic expression was obtained by injecting 40 ng/μL of a pUAS:dsRed plasmid with or without Tol2 messenger RNA into one-to two-cell Tg(prdm16:GAL4) embryos using a microinjector. pUAS:dsRed is 5UAS zfSynaptophysin:GFP-5UAS DsRedExpress [RRID:Addgene_74317] originally from.50
Imaging
Live zebrafish were anesthetized in 0.02% MS-222 and mounted on their side in 2% low-melt agarose for image collection. Larvae were imaged between 2 and 5 dpf on a Zeiss LSM800 confocal microscope with a 20× water-immersion objective (Zeiss W Plan-Apochromat 20×/1.0 DIC CG = 0.17 M27 75mm). For single labeled neurons, a z stack was acquired to capture the entire morphology of the cell using the 20× objective with an optical section of 1.5 μm. All images were taken within the mid-trunk of the embryos or larvae (between segments 6 and 20). All lateral images are maximum intensity projections through half of the spinal cord and cropped to two hemisegments. All transverse images were created by rotating and interpolating the z stack 90° in Fiji.86 The spinal cord transverse images are maximum projections of half of a hemisegment. The muscle transverse images are cropped according to the myotome boundary prior to rotation, and a maximum projection created from the entire myotome. Due to the dim fluorophore expression within older MN populations (because of the progenitor promotor), gamma was adjusted to visualize the entire population evenly (≥ 0.45).
Sparse label MN subtype classification
Only hemisegments containing one labeled MN were used for subtype classification based on axon morphology. Three-dimensional images were reconstructed and analyzed using Fiji as described above.86
Antibodies
Antibodies against LIM HD proteins and other proteins were used as described.20,21,101 Additional antibodies were obtained as follows: rabbit anti- Evi1/Mecom (Cell Signaling, RRID: AB_2184098); chicken and rabbit anti-GFP (Invitrogen, RRID: AB_2534023). Guinea pig anti-zebrafish Prdm16/Mecom antibody and Rabbit anti-zebrafish Mecom antibody were made by Covance (Denver, PA) with custom chosen peptides. Serial dilutions were done to test optimal concentrations to use for zebrafish whole mount staining. Prdm16/Mecom antibody was found to also work in chick and mouse spinal cord.
Mapping soma positions
Three-dimensional images were reconstructed and analyzed using Imaris (Bitplane). Neural soma positions were first auto-determined using the Spots function, then verified by eye to exclude any non-soma structures that were detected. Coordinates were then exported from Imaris and plotted using custom MATLAB scripts based on previously published scripts.73,102 To account for variations in spinal cord height and width across different fish, coordinates were normalized relative to the dorsal-most and ventral-most aspects of the spinal cord as well as along mediolateral landmarks marked medially by center of the spinal cord, and laterally by the lateral-most boundary between the spinal cord and the axial musculature. The dorsal and ventral aspects of the spinal cord were determined from differential interference contrast (DIC) images captured during image acquisition on the confocal microscope. Variations in myotome width were also accounted for by normalizing coordinates relative to the most rostral and caudal planes of the segment marked by the ventral root exit point from the hemisegment before as the most rostral and the ventral root of the neuron’s segment as the most caudal. Normalized coordinates from all MNs or interneurons were then either plotted as a scatterplot or pooled to generate separate contour plots that represent the variation in densities. Histograms were also generated to summaries the positional variability on the x and y axes of the scatterplots. Distribution contours were made in MATLAB using the kde2d function (MATLAB File Exchange), which estimates a bivariate kernel density over the set of normalized coordinates using a 32x32 grid.103,104 Contour plots were then generated from the calculated densities using the contour function in MATLAB. Contour lines are displayed in increasing increments of one-sixth of the peak density where the innermost contour represents the highest density of coordinates.
Behavioral measurement
All behavioral experiments were performed at 5 dpf. Control or mutant larvae were put into an air-tight glass cuvettes (93/G/10 55 × 55 × 10 mm, Starna Cells, Inc., Atascadero, CA, USA) at 5 dpf sealed with parafilm and a custom fit SYLGARD cap. All groups of fish were checked for swim bladders following the experiment and validated by genotyping. If any fish inflated their swim bladder or was of the wrong genotype, the entire group’s data was thrown out. Larvae were filmed in groups of 8 siblings in a glass cuvette filled with 24–26 mL E3 and recorded for 24 h as described previously.51,105 The larvae’s normal 14/10 light/dark cycle was maintained within the light-tight box using LED lights on a timer. Video was captured using digital CMOS cameras (Blackfly PGE-23S6M, FLIR Systems, Goleta CA) equipped with close-focusing, manual zoom lenses (18–108 mm Macro Zoom 7000 Lens, Navitar, Inc., Rochester, NY, USA) with f-stop set to 16 to maximize depth of focus. The field-of-view, approximately 2 × 2 cm2, was aligned concentrically with the tank face. A 5W 940nm infrared LED back-light (eBay) was transmitted through an aspheric condenser lens with a diffuser (ACL5040-DG15-B, ThorLabs, NJ). An infrared filter (43–953, Edmund Optics, NJ) was placed in the light path before the imaging lens. Digital video was recorded at 40 Hz with an exposure time of 1 ms. Kinematic data was extracted in real time using the NI-IMAQ vision acquisition environment of LabVIEW (National Instruments Corporation, Austin, TX, USA). Background images were subtracted from live video, intensity thresholding and particle detection were applied, and age-specific exclusion criteria for particle maximum. Feret diameter (the greatest distance between two parallel planes restricting the particle) was used to identify larvae in each image. In each frame, the position of the visual center of mass and posture (body orientation in the pitch, or nose-up/down, axis) was collected. Posture was defined as the orientation, relative to horizontal, of the line passing through the visual centroid that minimizes the visual moment of inertia. A larva with posture zero at any given time has its longitudinal axis horizontal, while +90° is nose-up vertical, and −90° is nose-down vertical.
Behavioral analysis
Data analysis and modeling were performed using Python 3. As previously described51,105 epochs of consecutively saved frames lasting at least 2.5 s were incorporated in subsequent analyses if they contained only one larva. Instantaneous differences of body particle centroid position were used to calculate speed. Inter-bout intervals (IBIs) were calculated from bout onset times (speed positively crossing 4 mm/s) in epochs containing multiple bouts, and consecutively detected bouts faster than 10 Hz were merged into single bouts. Numerous properties of swim bouts were calculated. The maximum speed of a bout was determined from the largest displacement across two frames during the bout. Displacement across each pair of frames at speeds above 4 mm/s was summed to find net bout displacement. Pitch angle distributions were computed pitch angles during IBIs. To calculate probability distributions of parameters, data from all bouts was pooled in each group (1240/1780 bouts and 3105/7392 IBI pitch measurements for mutant/control). Number of bins for plotting probability distributions satisfy Sturges’ rule (13 bins for speed and displacement; 15 bins for posture). Confidence intervals were estimated by resampling data 1000 times with a sample size 1/5 of that of the group (mutant or control) with fewer number of measurements (n = 248 for speed and displacement; n = 621 for IBI posture). To determine statistical significance, two-sample Kolmogorov-Smirnov tests were applied to original measurements (speed, displacement, and posture).
QUANTIFICATION AND STATISTICAL ANALYSIS
Cell counting
Cell counting was performed using Fiji86 or Imaris (Bitplane). Statistical analyses of cell counting were carried out using Prism 9 software with two-tailed Student’s t-tests unless otherwise noted. Differences were considered to be significant when p values were below 0.05. Graphs of quantitative data are plotted as means with standard error of mean (SEM) as error bars.
Muscle domain fluorescence
Domain fluorescence was measured using Fiji.86 Z-stacks of spinal muscle were cropped to one myotome and rotated 90°. A maximum intensity projection of the transverse view of the GFP (Prdm16+) channel was made. An ROI was drawn for each muscle quadrant, as defined in Figure 6A, per.31 The mean pixel intensity was calculated for each domain and normalized to domain IV.
Sparse label analysis
Hypothesis testing was performed by bootstrapping. First, a null distribution of cell types was estimated by resampling with replacement. p values were then computed explicitly as the fraction of times that the number of cells drawn from the null distribution met or exceeded the observed number. Each p value corresponds to n = 10,000 such simulations. The null distributions were either the distributions of all motor neurons, derived from counts in31 (26 types, n = 303 cells), or the distribution of prdm16+ motor neurons (n = 51 cells).
Supplementary Material
Highlights.
Larval zebrafish axial motor neurons have extensive molecular diversity
Fast motor neuron subtypes express the transcription factors Prdm16 and Mecom
Prdm16 and Mecom are required for fast motor neuron development
Motor neuron functional subtypes arise through intrinsic molecular programs
ACKNOWLEDGMENTS
Research was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under awards R00DC012775, R56DC016316, and R01DC017489 to D.S. and F31DC019554 to K.R.H. and the National Institute of Neurological Disorders and Stroke under awards F31NS110235 to K.P.D., F99NS129179 to D.G., T32NS086750 to K.P.D. and D.G., and R35NS116858 to J.S.D., by the Leon Levy Foundation to Y.Z., by the Irma T. Hirschl/Monique Weill-Caulier Trust, by the Brain Research Foundation, and by the Dana Foundation to D.S., and by Cancer Center Support grant P30CA016087 to the NYU Langone Health Genome Technology Center. Transgenic support came from the NIG Scientist and the National BioResource Project from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank members of the Knaut, Kucenas, McLean, and Liddelow labs for technical assistance with in situ, antibody staining, MN classification, and especially Philip Hasel for single-cell sequencing analysis. We thank members of the Dasen, Liddelow, Nagel, and Schoppik labs for support and advice.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.113049.
DECLARATION OF INTERESTS
J.D. is a member of the advisory board at Cell Reports.
REFERENCES
- 1.Schiaffino S, and Reggiani C (2011). Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev 91, 1447–1531. 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 2.Nijssen J, Comley LH, and Hedlund E (2017). Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 133, 863–885. 10.1007/s00401-017-1708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stifani N (2014). Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci 8, 293. 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thébault MT, Izem L, Leroy JP, Gobin E, Charrier G, and Raffin JP (2005). AMP-deaminase in elasmobranch fish: A comparative histochemical and enzymatic study. Comp. Biochem. Physiol. B Biochem. Mol. Biol 141, 472–479. 10.1016/j.cbpc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Zengel JE, Reid SA, Sypert GW, and Munson JB (1985). Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J. Neurophysiol 53, 1323–1344. 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- 6.Kanning KC, Kaplan A, and Henderson CE (2010). Motor Neuron Diversity in Development and Disease. Annu. Rev. Neurosci 33, 409–440. 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 7.Dasen JS(2022). Establishing the Molecular and Functional Diversity of Spinal Motoneurons. In Advances in Neurobiology(Springer International Publishing), pp. 3–44. 10.1007/978-3-031-07167-6_1. [DOI] [PubMed] [Google Scholar]
- 8.Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, and Sockanathan S (1999). Requirement for the Homeobox Gene Hb9 in the Consolidation of Motor Neuron Identity. Neuron 23, 659–674. 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- 9.Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, and Pfaff SL (1999). Active Suppression of Interneuron Programs within Developing Motor Neurons Revealed by Analysis of Homeodomain Factor HB9. Neuron 23, 675–687. 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- 10.Arber S (2012). Motor Circuits in Action: Specification, Connectivity, and Function. Neuron 74, 975–989. 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ, Lewallen KA, and Pfaff SL (2012). Spatial organization of cortical and spinal neurons controlling motor behavior. Curr. Opin. Neurobiol 22, 812–821. 10.1016/j.conb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippidou P, and Dasen JS (2013). Hox Genes: Choreographers in Neural Development, Architects of Circuit Organization. Neuron 80, 12–34. 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum JA, Klemm S, Shadrach JL, Guttenplan KA, Nakayama L, Kathiria A, Hoang PT, Gautier O, Kaltschmidt JA, Greenleaf WJ, and Gitler AD (2021). Single-cell transcriptomic analysis of the adult mouse spinal cord reveals molecular diversity of autonomic and skeletal motor neurons. Nat. Neurosci 24, 572–583. 10.1038/s41593-020-00795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alkaslasi MR, Piccus ZE, Hareendran S, Silberberg H, Chen L, Zhang Y, Petros TJ, and Le Pichon CE (2021). Single nucleus RNA-sequencing defines unexpected diversity of cholinergic neuron types in the adult mouse spinal cord. Nat. Commun 12, 2471. 10.1038/s41467-021-22691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russ DE, Cross RBP, Li L, Koch SC, Matson KJE, Yadav A, Alkaslasi MR, Lee DI, Le Pichon CE, Menon V, and Levine AJ (2021). A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat. Commun 12, 5722. 10.1038/s41467-021-25125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seredick SD, Van Ryswyk L, Hutchinson SA, and Eisen JS (2012). Zebrafish Mnx proteins specify one motoneuron subtype and suppress acquisition of interneuron characteristics. Neural Dev. 7, 35. 10.1186/1749-8104-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seredick S, Hutchinson SA, Van Ryswyk L, Talbot JC, and Eisen JS (2014). Lhx3 and Lhx4 suppress Kolmer–Agduhr interneuron characteristics within zebrafish axial motoneurons. Development 141, 3900–3909. 10.1242/dev.105718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, and Eisen JS (1995). Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development 121, 4117–4125. 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson SA, and Eisen JS (2006). Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development 133, 2137–2147. 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- 20.Jung H, Mazzoni EO, Soshnikova N, Hanley O, Venkatesh B, Duboule D, and Dasen JS (2014). Evolving Hox Activity Profiles Govern Diversity in Locomotor Systems. Dev. Cell 20, 171–187. 10.1016/j.devcel.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, and Pfaff SL (1994). Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79, 957–970. 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 22.D’Elia KP, and Dasen JS (2018). Development, functional organization, and evolution of vertebrate axial motor circuits. Neural Dev. 13, 10. 10.1186/s13064-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beattie CE (2000). Control of motor axon guidance in the zebrafish embryo. Brain Res. Bull 53, 489–500. 10.1016/s0361-9230(00)00382-8. [DOI] [PubMed] [Google Scholar]
- 24.Rodino-Klapac LR, and Beattie CE (2004). Zebrafish topped is required for ventral motor axon guidance. Dev. Biol 273, 308–320. 10.1016/j.ydbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Hilario JD, Rodino-Klapac LR, Wang C, and Beattie CE (2009). Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Dev. Biol 326, 190–200. 10.1016/j.ydbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Menelaou E, and McLean DL (2012). A Gradient in Endogenous Rhythmicity and Oscillatory Drive Matches Recruitment Order in an Axial Motor Pool. J. Neurosci 32, 10925–10939. 10.1523/jneurosci.1809-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean DL, Fan J, Higashijima S.i., Hale ME, and Fetcho JR (2007). A topographic map of recruitment in spinal cord. Nature 446, 71–75. 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- 28.Ampatzis K, Song J, Ausborn J, and El Manira A (2013). Pattern of Innervation and Recruitment of Different Classes of Motoneurons in Adult Zebrafish. J. Neurosci 33, 10875–10886. 10.1523/jneurosci.0896-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WC, and Brehm P (2017). A Gradient in Synaptic Strength and Plasticity among Motoneurons Provides a Peripheral Mechanism for Locomotor Control. Curr. Biol 27, 415–422. 10.1016/j.cub.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel JP, Ausborn J, Ampatzis K, Mahmood R, Eklöf-Ljunggren E, and El Manira A (2011). Principles governing recruitment of motoneurons during swimming in zebrafish. Nat. Neurosci 14, 93–99. 10.1038/nn.2704. [DOI] [PubMed] [Google Scholar]
- 31.Bello-Rojas S, Istrate AE, Kishore S, and McLean DL (2019). Central and peripheral innervation patterns of defined axial motor units in larval zebrafish. J. Comp. Neurol 527, 2557–2572. 10.1002/cne.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen H, Eckenstein K, Weihrauch V, Stigloher C, and Brehm P (2020). Primary and secondary motoneurons use different calcium channel types to control escape and swimming behaviors in zebrafish. Proc. Natl. Acad. Sci. USA 117, 26429–26437. 10.1073/pnas.2015866117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naranjo D, Wen H, and Brehm P (2015). Zebrafish CaV2.1 Calcium Channels Are Tailored for Fast Synchronous Neuromuscular Transmission. Biophys. J 108, 578–584. 10.1016/j.bpj.2014.11.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers PZ, Eisen JS, and Westerfield M (1986). Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci 6, 2278–2289. 10.1523/jneurosci.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmel CB, Warga RM, and Kane DA (1994). Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276. 10.1242/dev.120.2.265. [DOI] [PubMed] [Google Scholar]
- 36.Saint-Amant L, and Drapeau P (1998). Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol 37, 622–632. https://doi.org/10.1002/(sici)1097-4695(199812)37:4<622::aid-%neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.McLean DL, and Fetcho JR (2009). Spinal Interneurons Differentiate Sequentially from Those Driving the Fastest Swimming Movements in Larval Zebrafish to Those Driving the Slowest Ones. J. Neurosci 29, 13566–13577. 10.1523/jneurosci.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, and Appel B (2008). CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci 11, 143–151. 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott K, O’Rourke R, Winkler CC, Kearns CA, and Appel B (2021). Temporal single-cell transcriptomes of zebrafish spinal cord pMN progenitors reveal distinct neuronal and glial progenitor populations. Dev. Biol 479, 37–50. 10.1016/j.ydbio.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lönnerberg P, Furlan A, et al. (2018). RNA velocity of single cells. Nature 560, 494–498. 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergen V, Lange M, Peidli S, Wolf FA, and Theis FJ (2020). Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol 38, 1408–1414. 10.1038/s41587-020-0591-3. [DOI] [PubMed] [Google Scholar]
- 42.Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Memic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, et al. (2010). Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as markers for fast motor neurons and partition cells. J. Comp. Neurol 518, 2284–2304. 10.1002/cne.22332. [DOI] [PubMed] [Google Scholar]
- 43.Bergen V, Soldatov RA, Kharchenko PV, and Theis FJ (2021). RNA velocity–current challenges and future perspectives. Mol. Syst. Biol 17, e10282. 10.15252/msb.202110282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung H, Baek M, D’Elia KP, Boisvert C, Currie PD, Tay BH, Venkatesh B, Brown SM, Heguy A, Schoppik D, and Dasen JS (2018). The Ancient Origins of Neural Substrates for Land Walking. Cell 172, 667–682.e15. 10.1016/j.cell.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uemura Y, Kato K, Kawakami K, Kimura Y, Oda Y, and Higashijima SI (2020). Neuronal Circuits That Control Rhythmic Pectoral Fin Movements in Zebrafish. J. Neurosci 40, 6678–6690. 10.1523/jneurosci.1484-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato-Maeda M, Tawarayama H, Obinata M, Kuwada JY, and Shoji W (2006). Sema3a1 guides spinal motor axons in a cell- and stage-specific manner in zebrafish. Development 133, 937–947. 10.1242/dev.02268. [DOI] [PubMed] [Google Scholar]
- 47.Quan FB, Gaillard AL, Alejevski F, Pézeron G, and Tostivint H (2021). Urotensin II-related peptide (Urp) is expressed in motoneurons in zebrafish, but is dispensable for locomotion in larva. Peptides 146, 170675. 10.1016/j.peptides.2021.170675. [DOI] [PubMed] [Google Scholar]
- 48.Liau ES, Jin S, Chen YC, Liu WS, Yong LW, Tsai CT, Calon M, Yu JK, Su YH, Nedelec S, et al. (2021). Single-cell transcriptomic analysis unveils spinal motor neuron subtype diversity underpinning the water-to-land transition in vertebrates. Preprint at bioRxiv. 10.101101/2021.09.29.462340. [DOI] [Google Scholar]
- 49.Hanley O, Zewdu R, Cohen LJ, Jung H, Lacombe J, Philippidou P, Lee DH, Selleri L, and Dasen JS (2016). Parallel Pbx -Dependent Pathways Govern the Coalescence and Fate of Motor Columns. Neuron 91, 1005–1020. 10.1016/j.neuron.2016.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer MP, and Smith SJ (2006). Evidence from In Vivo Imaging That Synaptogenesis Guides the Growth and Branching of Axonal Arbors by Two Distinct Mechanisms. J. Neurosci 26, 3604–3614. 10.1523/jneurosci.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrlich DE, and Schoppik D (2017). Control of Movement Initiation Underlies the Development of Balance. Curr. Biol 27, 334–344. 10.1016/j.cub.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma K, Leonard AE, Lettieri K, and Pfaff SL (2000). Genetic and epigenetic mechanisms contribute to motor neuron pathfinding. Nature 406, 515–519. 10.1038/35020078. [DOI] [PubMed] [Google Scholar]
- 53.Dasen JS, De Camilli A, Wang B, Tucker PW, and Jessell TM (2008). Hox Repertoires for Motor Neuron Diversity and Connectivity Gated by a Single Accessory Factor. FoxP1. Cell 134, 304–316. 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Dasen JS, and Jessell TM (2009). Chapter Six Hox Networks and the Origins of Motor Neuron Diversity. In Current Topics in Developmental Biology (Elsevier; ), pp. 169–200. 10.1016/s0070-2153(09)88006-x. [DOI] [PubMed] [Google Scholar]
- 55.Zannino DA, and Sagerström CG (2015). An emerging role for prdm family genes in dorsoventral patterning of the vertebrate nervous system. Neural Dev. 10, 24. 10.1186/s13064-015-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawai A, Pfennig S, Bulajić M, Miller A, Khodadadi-Jamayran A, Mazzoni EO, and Dasen JS (2022). PRC1 sustains the integrity of neural fate in the absence of PRC2 function. Elife 11, e72769. 10.7554/elife.72769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foehring RC, Sypert GW, and Munson JB (1987). Motor-unit properties following cross-reinnervation of cat lateral gastrocnemius and soleus muscles with medial gastrocnemius nerve. I. Influence of motoneurons on muscle. J. Neurophysiol 57, 1210–1226. 10.1152/jn.1987.57.4.1210. [DOI] [PubMed] [Google Scholar]
- 58.Mendell LM, Collins WF, and Munson JB (1994). Retrograde determination of motoneuron properties and their synaptic input. J. Neurobiol 25, 707–721. 10.1002/neu.480250610. [DOI] [PubMed] [Google Scholar]
- 59.Munson JB, Foehring RC, Mendell LM, and Gordon T (1997). Fast-to-Slow Conversion Following Chronic Low-Frequency Activation of Medial Gastrocnemius Muscle in Cats. II. Motoneuron Properties. J. Neurophysiol 77, 2605–2615. 10.1152/jn.1997.77.5.2605. [DOI] [PubMed] [Google Scholar]
- 60.Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, and Sanes JR (2010). Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development 137, 3489–3499. 10.1242/dev.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rafuse VF, Milner LD, and Landmesser LT (1996). Selective Innervation of Fast and Slow Muscle Regions during Early Chick Neuromuscular Development. J. Neurosci 16, 6864–6877. 10.1523/jneurosci.16-21-06864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forsgren S, Bergh A, Carlsson E, and Thornell LE (1993). Calcitonin gene-related peptide expression at endplates of different fibre types in muscles in rat hind limbs. Cell Tissue Res. 274, 439–446. 10.1007/bf00314540. [DOI] [PubMed] [Google Scholar]
- 63.Müller D, Cherukuri P, Henningfeld K, Poh CH, Wittler L, Grote P, Schlüter O, Schmidt J, Laborda J, Bauer SR, et al. (2014). Dlk1 Promotes a Fast Motor Neuron Biophysical Signature Required for Peak Force Execution. Science 343, 1264–1266. 10.1126/science.1246448. [DOI] [PubMed] [Google Scholar]
- 64.Brustein E, Saint-Amant L, Buss RR, Chong M, McDearmid JR, and Drapeau P (2003). Steps during the development of the zebrafish locomotor network. J. Physiol. Paris 97, 77–86. 10.1016/j.jphysparis.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Okaty BW, Miller MN, Sugino K, Hempel CM, and Nelson SB (2009). Transcriptional and Electrophysiological Maturation of Neocortical Fast-Spiking GABAergic Interneurons. J. Neurosci 29, 7040–7052. 10.1523/jneurosci.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tremblay R, Lee S, and Rudy B (2016). GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292. 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelsom C, and Lu W (2013). Development and specification of GABAergic cortical interneurons. Cell Biosci. 3, 19. 10.1186/2045-3701-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, and Rubenstein JLR (2010). Dlx5 and Dlx6 Regulate the Development of Parvalbumin-Expressing Cortical Interneurons. J. Neurosci 30, 5334–5345. 10.1523/jneurosci.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diego M, Gelman OM, and Rubenstein JLR (2012). The Generation of Cortical Interneurons. In Jasper’s Basic Mechanisms of the Epilepsies [Internet], J.L. Noebels, M. Avolia, M.A. Rogawski, R.W. Olsen, and A.V. Delgado-Escueta, eds. (National Center for Biotechnology Information (US)). [PubMed] [Google Scholar]
- 70.Berg EM, Mrowka L, Bertuzzi M, Madrid D, Picton LD, and El Manira A (2022). Brainstem circuits encoding start, speed, and duration of swimming in adult zebrafish. Neuron 111, 372–386.e4. [DOI] [PubMed] [Google Scholar]
- 71.Song J, Cohen B, Zachariah P, Liu J, Larson EL, Fontanel P, Picton LD, and Manira AE (2020). Multiple Rhythm-Generating Circuits Act in Tandem with Pacemaker Properties to Control the Start and Speed of Locomotion. Neuron 41, 1048–1057. 10.1016/j.neuron.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 72.Pujala A, and Koyama M (2019). Chronology-based architecture of descending circuits that underlie the development of locomotor repertoire after birth. Elife 8, e42135. 10.107554/elife.42135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kishore S, Cadoff EB, Agha MA, and McLean DL (2020). Orderly compartmental mapping of premotor inhibition in the developing zebrafish spinal cord. Science 370, 431–436. 10.1126/science.abb4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greaney MR, Privorotskiy AE, D’Elia KP, and Schoppik D (2017). Extraocular motoneuron pools develop along a dorsoventral axis in zebrafish, Danio rerio. J. Comp. Neurol 525, 65–78. 10.1002/cne.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barsh GR, Isabella AJ, and Moens CB (2017). Vagus Motor Neuron Topographic Map Determined by Parallel Mechanisms of hox5 Expression and Time of Axon Initiation. Curr. Biol 27, 3812–3825.e3. 10.1016/j.cub.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isabella AJ, Stonick JA, Dubrulle J, and Moens CB (2021). Intrinsic Positional Memory Guides Target-specific Axon Regeneration in the Zebrafish Vagus Nerve. Development 148. 10.1242/dev.199706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, Hildebrand DGC, Morgan JL, Jia Y, Slimmon N, and Bagnall MW (2022). Organization of the gravity-sensing system in zebrafish. Nat. Commun 13, 5060. 10.1038/s41467-022-32824-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldblatt D, Huang S, Greaney MR, Hamling KR, Voleti V, Perez-Campos C, Patel KB, Li W, Hillman EMC, Bagnall MW, et al. (2022). Neuronal birthdate reveals topography in a vestibular brainstem circuit for gaze stabilization. Preprint at bioRxiv. 10.1001/2022.10.21.513243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agalliu D, Takada S, Agalliu I, McMahon AP, and Jessell TM (2009). Motor Neurons with Axial Muscle Projections Specified by Wnt4/5 Signaling. Neuron 61, 708–720. 10.1016/j.neuron.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delile J, Rayon T, Melchionda M, Edwards A, Briscoe J, and Sagner A (2019). Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development (Cambridge, U. K.) 146, dev173807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Khindi T, Sherman MB, Kodama T, Gopal P, Pan Z, Kiraly JK, Zhang H, Goff LA, du Lac S, and Kolodkin AL (2022). The transcription factor Tbx5 regulates direction-selective retinal ganglion cell development and image stabilization. Curr. Biol 32, 4286–4298.e5. 10.1016/j.cub.2022.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng YR, James RE, Yan W, Kay JN, Kolodkin AL, and Sanes JR (2020). Binary Fate Choice between Closely Related Interneuronal Types Is Determined by a Fezf1-Dependent Postmitotic Transcriptional Switch. Neuron 105, 464–474.e6. 10.1016/j.neuron.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voortman L, Anderson C, Urban E, Yuan L, Tran S, Neuhaus-Follini A, Derrick J, Gregor T, and Johnston RJ (2022). Temporally dynamic antagonism between transcription and chromatin compaction controls stochastic photoreceptor specification in flies. Dev. Cell 57, 1817–1832.e5. 10.1016/j.devcel.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Özel MN, Gibbs CS, Holguera I, Soliman M, Bonneau R, and Desplan C (2022). Coordinated control of neuronal differentiation and wiring by sustained transcription factors. Science. 10.101126/science.add1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, and Baier H (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323–326. 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- 86.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, et al. (2017). Massively parallel digital transcriptional profiling of single cells. Nat. Commun 8, 14049. 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Labun K, Montague TG, Gagnon JA, Thyme SB, and Valen E (2016). CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44, W272–W276. 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dahn RD, Davis MC, Pappano WN, and Shubin NH (2007). Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature 445, 311–314. 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- 91.Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, and Kawakami K (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255–1260. 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawakami K, Asakawa K, Hibi M, Itoh M, Muto A, and Wada H (2016). Gal4 driver transgenic zebrafish: Powerful tools to study developmental biology, organogenesis, and neuroscience. Advances in Genetics 95, 65–87. 10.1016/bs.adgen.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. (2015). Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214. 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, Ahmed AA, and Yau C (2021). CIDER: an interpretable meta-clustering framework for single-cell RNA-seq data integration and evaluation. Genome Biol. 22, 337. 10.1186/s13059-021-02561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ge SX, Jung D, and Yao R (2020). ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629. 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, and Raychaudhuri S (2019). Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296. 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thisse C, and Thisse B (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc 3, 59–69. 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 98.Ibarra-García-Padilla R, Howard AGA, Singleton EW, and Uribe RA (2021). A protocol for whole-mount immuno-coupled hybridization chain reaction (WICHCR) in zebrafish embryos and larvae. STAR Protoc. 2, 100709. 10.1016/j.xpro.2021.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuehn E, Clausen DS, Null RW, Metzger BM, Willis AD, and Özpolat BD (2022). Segment number threshold determines juvenile onset of germline cluster expansion in Platynereis dumerilii. J. Exp. Zool. B Mol. Dev. Evol 338, 225–240. 10.1002/jez.b.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi HMT, Schwarzkopf M, Fornace ME, Acharya A, Artavanis G, Stegmaier J, Cunha A, and Pierce NA (2018). Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753. 10.1242/dev.165753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dasen JS, Tice BC, Brenner-Morton S, and Jessell TM (2005). A Hox Regulatory Network Establishes Motor Neuron Pool Identity and Target-Muscle Connectivity. Cell 123, 477–491. 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Kishore S, and Fetcho JR (2013). Homeostatic regulation of dendritic dynamics in a motor map in vivo. Nat. Commun 4, 2086. 10.1038/ncomms3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, et al. (2016). Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell 165, 207–219. 10.1016/j.cell.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Botev ZI, Grotowski JF, and Kroese DP (2010). Kernel density estimation via diffusion. Ann. Stat 38. 10.1214/10-aos799. [DOI] [Google Scholar]
- 105.Ehrlich DE, and Schoppik D (2019). A primal role for the vestibular sense in the development of coordinated locomotion. Elife 8, e45839. 10.7554/elife.45839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-Evi1/Mecom | Cell signaling | RRID:AB_2184098 |
| Chicken and rabbit anti-GFP | Invitrogen | RRID:AB_2534023 |
| Guinea-pig anti-zebrafish Prdm16/Mecom antibody | Covance | N/A |
| Rabbit anti-zebrafish Mecom antibody | Covance | N/A |
| Mouse anti-Hb9 antibody | Invitrogen | RRID:AB_2540929 |
| Chemicals, peptides, and recombinant proteins | ||
| Tween | Fisher Scientific | BP337-100 |
| Triton | Sigma Aldrich | T8787-100ML |
| MS-222 | Sigma Aldrich | E10521 |
| 32% PFA | Electron Microscopy Sciences | 15714 |
| Proteinase K | ThermoFisher Scientific | 25530049 |
| BSA | ThermoFisher Scientific | 50-253-966 |
| Papain | Worthington Biochemical | LK003150 |
| HBSS | ThermoFisher Scientific | 14-170-112 |
| EBSS | ThermoFisher Scientific | 24010043 |
| DNase | Worthington Biochemical | LK003172 |
| DAPI | Sigma Aldrich | D9542-10mg |
| Critical commercial assays | ||
| Digoxigenin-dUTP labeling kit | Roche | N/A |
| in situ hybridization chain reaction v3.0 (HCR) | Molecular Instruments | N/A |
| DreamTaq Green PCR Master Mix (2X) | Thermo Scientific | K1082 |
| Deposited data | ||
| RNA-seq of WT zebrafish MNS at 1 dpf and 1.5 dpf | Scott et al.39 | GEO: GSE173350 |
| Raw and analyzed 10x Genomics scRNA-seq datasets | This paper | GEO: GSE240026 |
| Experimental models: Organisms/strains | ||
| Zebrafish: Tg(olig2:dsRed2)vu19 | Kucenas et al.38 | N/A |
| Zebrafish: Tg(prdm16:GAL4) | NBRP | gSAIzGFFM1116A |
| Zebrafish: Tg(olig2:GFP) | Scott et al.85 | N/A |
| Zebrafish: mecomnyc41 | This paper | N/A |
| Zebrafish: prdm16nyc142 | This paper | N/A |
| Zebrafish: Tg(prdm16:GAL4); prdm16nyc584 | This paper | N/A |
| WT Mice: mixed genetic background | New York University Grossman School of Medicine Animal Facilities | N/A |
| Leucoraja erinacea embryos | Marine Resources Department of MBL | N/A |
| Specific Pathogen Free Fertile Chicken Eggs | AVS bio | N/A |
| Oligonucleotides | ||
| Prdm16 d17/d22 forward primer (CCATTAAAGCCATTGCCTCC) |
GeneWiz | N/A |
| Prdm16 d17/d22 reverse primer (GAACATGCCAGGATTGAGTG) |
GeneWiz | N/A |
| Mecom d25 forward primer (CCGCTGGAGGGTTGTTTGC) |
GeneWiz | N/A |
| Mecom d25 reverse primer (CCGATGGTAAAGTCCTGATGG) |
GeneWiz | N/A |
| Prdm16 gal4 forward primer (CCGTGACTGATCGCCTGGCAAG) |
GeneWiz | N/A |
| Prdm16 gal4 reverse primer (GCCGGGCAGCATATCCAGATCG) |
GeneWiz | N/A |
| HCR probes | Molecular Instruments | N/A |
| Recombinant DNA | ||
| 5UAS zfSynaptophysin:GFP-5UAS DsRedExpress | Addgene | RRID:Addgene_74317 |
| Software and algorithms | ||
| ImageJ | Schindelin et al.86 | https://imagej.nih.gov/ij/ |
| Imaris (Bitplane) | Oxford Instruments | https://imaris.oxinst.com/ |
| Python v3 | Python Software Foundation | https://www.python.org |
| Prism v9 | GraphPad | https://www.graphpad.com/features |
| Seurat v4 | Hao et al.87 | https://satijalab.org/seurat/ |
| Velocyto v0.17.17 | Manno et al.40 | https://velocyto.org/velocyto.py/ |
| CellRanger v3.0.2 | Zheng et al.88 | https://support.10xgenomics.com/single-cell-gene-expression/software/overview/welcome |
| LabVIEW | National Instruments Corporation | https://www.ni.com/en-us/shop/software/products/labview.html |
| CHOPCHOP online tool | Labun et al.89 | https://chopchop.cbu.uib.no/ |
| Other | ||
| Glass cuvettes | Starna Cells Inc. | (93/G.10 55 × 55 × 10mm) |
| Digital CMOS cameras | FLIR Systems | Blackfly PGE-23S6M |
| 5W 940nm infrared LED back light | eBay | N/A |
| Infrared filter | Edmund Optics | 43–953 |
| Aspheric condenser lens with diffuser | Thor Labs | ACL5040-DG15-B |


