Abstract
3-Cyano-7-cyclopropylamino-pyrazolo[1,5-a]pyrimidines, including the chemical probe SGC-CK2-1, are potent and selective inhibitors of CSNK2A in cells but have limited utility in animal models due to their poor pharmacokinetic properties. While developing analogues with reduced intrinsic clearance and the potential for sustained exposure in mice, we discovered that phase II conjugation by GST enzymes was a major metabolic transformation in hepatocytes. A protocol for codosing with ethacrynic acid, a covalent reversible GST inhibitor, was developed to improve the exposure of analogue 2h in mice. A double codosing protocol, using a combination of ethacrynic acid and irreversible P450 inhibitor 1-aminobenzotriazole, increased the blood level of 2h by 40-fold at a 5 h time point.
Introduction
The appearance of a novel coronavirus SARS-CoV-2 and the resulting pandemic has highlighted the need for effective treatments against COVID-19.1 Host-directed therapies that target cellular pathways required for virus replication have emerged as a promising approach to combat viral infections.2 Protein kinases have been proposed as potential targets for development of antiviral drugs.3 Among these kinases, casein kinase 2α (CSNK2A) has been shown to play a role in the replication of β-coronaviruses (β-CoV), including SARS-CoV-2.4−6 Recent studies have demonstrated the efficacy of CSNK2A inhibitors in reducing the replication of β-CoV in vitro.5 However, there has yet to be a demonstration of anti-COVID-19 efficacy in vivo.
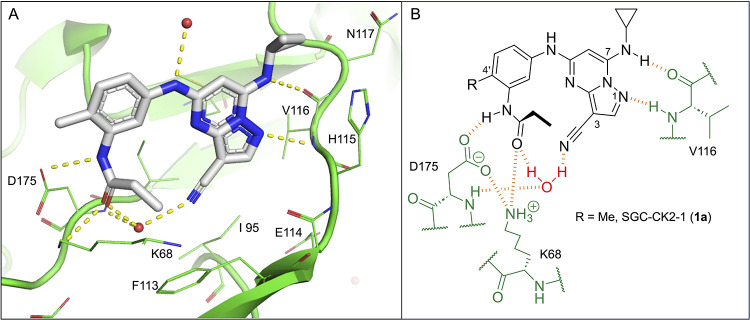
3-Cyano-7-cyclopropylamino-pyrazolo[1,5-a]pyrimidine (PZP) is a promising chemotype of the CSNK2A inhibitor that has demonstrated potent activity in vitro.7 PZPs possess good selectivity for CSNK2A over other kinases and display unique chemical features that contribute to their potent inhibitory activity (Figure 1). Specifically, X-ray crystallography studies (Figure 1A) revealed that the 4′-methyl substituent of the chemical probe SGC-CK2-1 (1a) enforces an s-cis (E)-conformation of the propionamide, allowing it to coordinate with a bound water molecule, which also interacts with the cyano group of the PZP core (Figure 1B). Notably, the alkyl group of the propionamide contributes to the exquisite selectivity of the PZP-based CSNK2A inhibitors over other kinases.8 However, despite their promising in vitro activity, PZPs have shown limited bioavailability in rodents by oral dosing due to their low to moderate aqueous solubility, high first-pass metabolism, and rapid clearance.9,10
Figure 1.
(A) X-ray cocrystal structure of SGC-CK2-1 (1a) bound to CSNK2A1 (PDB: 6Z83). Image created in PyMOL 2.4.0 with the CSNK2A1 protein shown as a green ribbon and 1a shown as sticks colored by the atom type. Bound water molecules are shown as red spheres. Intermolecular hydrogen bonds are shown as yellow dashed lines. (B) Illustration of the key interactions observed in the X-ray structure. The 7-cyclopropylamine and N-1 of the PZP form H-bond interactions with V116 in the hinge region. The 4′-substituent (Me for 1a) causes the amide to adopt an s-cis conformation that allows the carbonyl group to interact with K68 and a bound water molecule. The alkyl group of the amide is placed in a pocket that increases the selectivity over other kinases. The cyano group of the PZP also interacts with the bound water molecule, which is further coordinated through interaction with the backbone NH of D175.
In this paper, we report our initial studies to identify a PZP-based CSNK2A inhibitor with potent antiviral activity and high sustained blood levels for use in a mouse-adapted model of COVID-19 developed for preclinical evaluation of potential drug therapies.11,12 We discovered that the rapid clearance of PZPs in mice was due to pathways of both phase I and phase II metabolism. Codosing with a glutathione S-transferase (GST) enzyme inhibitor and an irreversible cytochrome P450 inhibitor greatly reduced the intrinsic clearance of a PZP analogue in mice and may be a viable approach to increase in vivo exposure for preclinical pharmacology studies.
Results
Antiviral PZPs with Reduced Phase I Metabolism
All compounds were screened for CSNK2A1 and CSNK2A2 activity using a NanoBRET assay in HEK293 cells and for inhibition of mouse β-CoV replication using an MHV-nLuc assay in DBT cells.5 Kinetic solubility was determined by using a miniaturized shake flask method. The stability of compounds to phase I metabolism was determined by incubation with mouse liver microsomes for 30 min. SGC-CK2-1 (1a) demonstrated low aqueous solubility and rapid metabolism in primary mouse liver microsomes (MLM), with only 40% of the parent compound remaining after 30 min (Table 1). Since hepatic metabolism of 1a would likely lead to rapid in vivo clearance, we decided to focus initially on increasing its stability in MLM. SMARTCyp,13,14 a web-based predictor of metabolic hot spots, analyzes sites of metabolism using a ligand-based approach from a library of molecular fragments that can be applied across multiple species and isoforms of CYPs.15 SMARTCyp suggested that the cyclopropylamine and the 4′-methyl group might be sites of P450 oxidation (Figure S1). As modification of the cyclopropylamine, which forms part of the hinge-binding motif (Figure 1B), resulted in a large decrease in CSNK2A potency,5,8 we opted to study analogues with modification of the 4′-methyl group to lower logD, improve aqueous solubility, and possibly reduce P450 metabolism.16 Analogues 1b–g with substituted ethylenediamines replacing the 4′-methyl group demonstrated good CSNK2A potency and retained antiviral activity, as previously reported.5 Analogues 1b–g had around 50- to 60-fold improved aqueous solubility. The ethylenediamines 1b–d also had reduced metabolism in MLM compared to that of 1a (Table 1). In contrast, diamines containing a pyrrolidine (1e) or piperidine (1f) were highly metabolized despite their improved aqueous solubility, possibly due to their increased lipophilicity. The piperazine (1g) had greatly improved metabolic stability but at a cost of lower CSNK2A potency and antiviral IC50 > 1 μM. From this initial series of analogues of 1a, we found that the addition of ethylenediamines at the 4′-position resulted in PZPs with good antiviral activity and reduced P450 metabolism in MLM. Although the piperazine 1g had the best metabolic stability, it showed reduced potency in both CSNK2A and antiviral assays.
Table 1. Modification of the 4′-Position Group of the Propionamide Series.
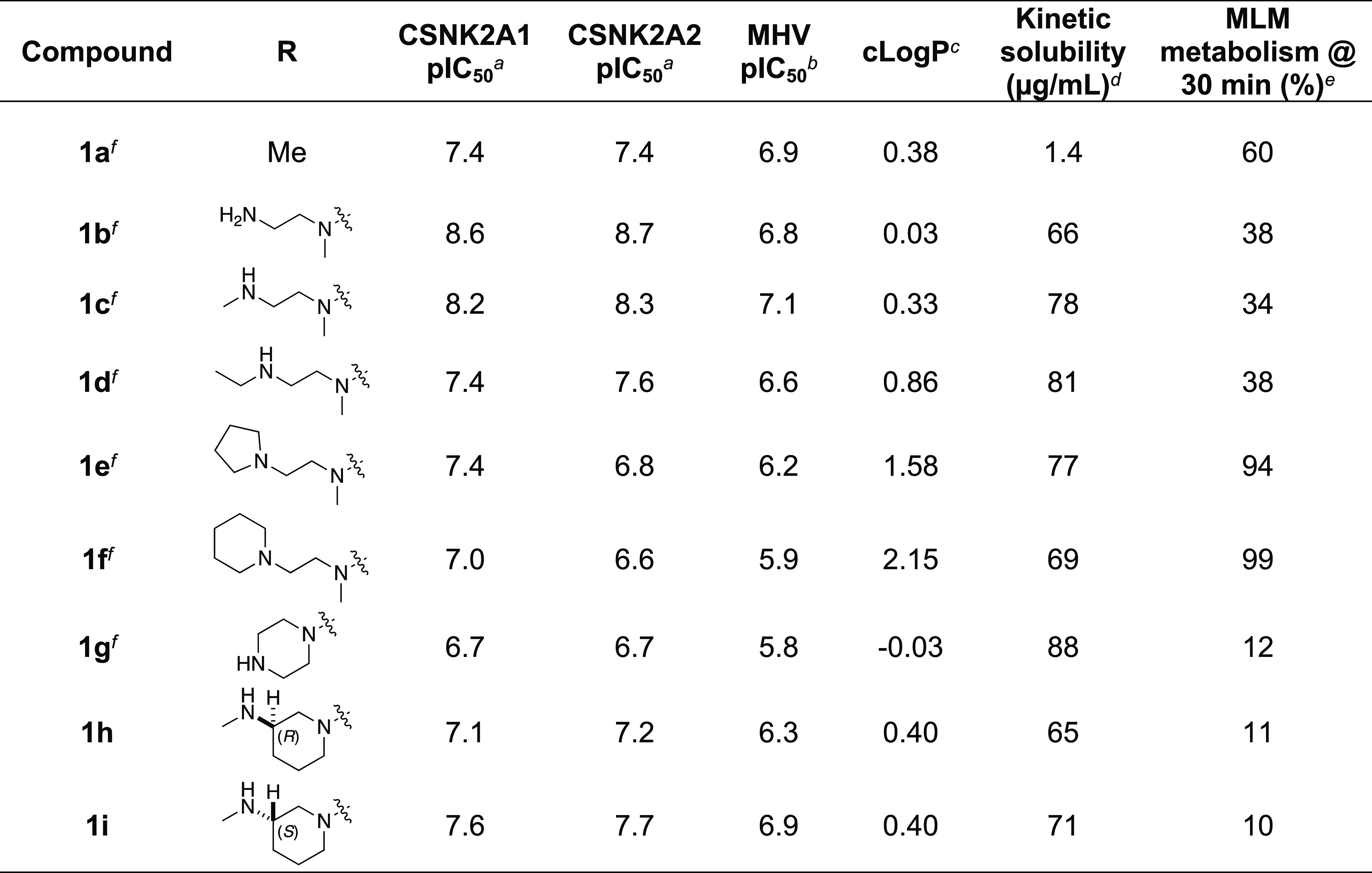
In-cell target engagement by the NanoBRET assay, n = 1.
Inhibition of MHV-nLuc replication, n = 3 with SE ± 0.1.
Values from ChemDraw Professional v16.0.
Miniaturized shake flask using CLND detection, n = 1.
Metabolism of the compound in primary mouse liver microsomes (MLM) calculated as 100 – (% remaining), n = 1.
Compound previously reported in ref (5).
To build on these results, we designed new analogues with 3-aminomethylpiperidine substituents at the 4′-position to combine the features of both the cyclic and linear diamines. The synthesis employed the methods previously developed for analogues 1b–g (Scheme 1). Addition of the (R)- or (S)-enantiomer of the N-Boc-protected 3-aminomethylpiperidine to the acyl-2-fluoro-5-nitroaniline (x, R1 = Et) followed by reduction of the nitro group yielded the aniline intermediate (y, R1 = Et). Palladium-catalyzed Buchwald–Hartwig cross-coupling with the 5-chloro-pyrazolo[1,5-a]pyrimidine core followed by N-Boc deprotection yielded the 3R-isomer (1h) and the 3S-isomer (1i). Both isomers, 1h and 1i, demonstrated good aqueous solubility and low metabolism in mouse liver microsomes compared to 1a (Table 1). The 3S-isomer (1i) was more potent than the 3R-isomer (1h) in both the CSNK2A and antiviral assay. Overall, 1i had a comparable potency to 1a but with improved solubility and metabolic stability.
Scheme 1. Synthesis Previously Developed for Analogues 1b–g.
Reagents and conditions: (i) R2-H, K2CO3, MeCN. (ii) H2, Pd–C, MeOH. (iii) 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile, BINAP, Pd(OAc)2, Cs2CO3, dioxane, μW, 130 °C. (iv) TFA, CH2Cl2.
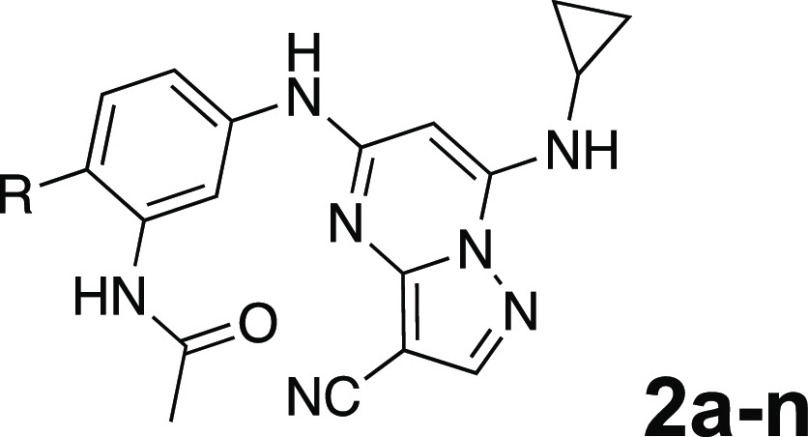
To further improve the potency, we synthesized 4′-substituted analogues in the acetamide series of PZPs (Table 2). The compounds were synthesized by the established route from the acyl-2-fluoro-5-nitroaniline (x, R1 = Me) and the appropriate secondary amine for the R2-substituent (Scheme 1). For those R2-substituents with chiral centers, the enantiomerically pure amine was utilized as a building block. The 4′-methyl analogue (2a) had shown subnanomolar activity on CSNK2A and good activity in the antiviral assay.52a had a lower clogP and slightly improved solubility and metabolic stability compared to the corresponding propionamide (1a) (Table 2). The synthesis of the 3R-isomer (2b) and 3S-isomer (2c) of the 3-aminomethylpiperidine gave analogues with 10-fold improved potency in the CSNK2A and the antiviral assays while maintaining good metabolic stability. The 3S-isomer (2c) was again the more potent of the two enantiomers. Additional 4′-substituted analogues were synthesized in the acetamide series. The 3R-isomer (2d) and 3S-isomer (2e) of the 3-aminomethyl pyrrolidine also combined potency on CSNK2A and antiviral activity with good metabolic stability. Switching the orientation of the 3-aminomethyl pyrrolidine gave analogue 2f with similar potency but slightly poorer metabolic stability. The piperazine (2g) was less potent on CSNK2A and in the antiviral assay, but had good metabolic stability, as had been seen in the corresponding propionamide (1g). We had previously prepared and tested morpholine (2h),5 which had improved antiviral activity compared to 2g. Although morpholine (2h) showed low aqueous solubility, its metabolic stability was much better than the parent analogue 2a (Table 2). The introduction of a hydroxy substituent in 2i maintained activity and improved aqueous solubility but decreased the metabolic stability. The oxo-analogues 2j–n had lower activity in the antiviral assay and poor metabolic stability, despite some analogues (e.g., 2l) having low clogP and good solubility.
Table 2. Modification of the 4′-Position Group of the Acetamide Series.
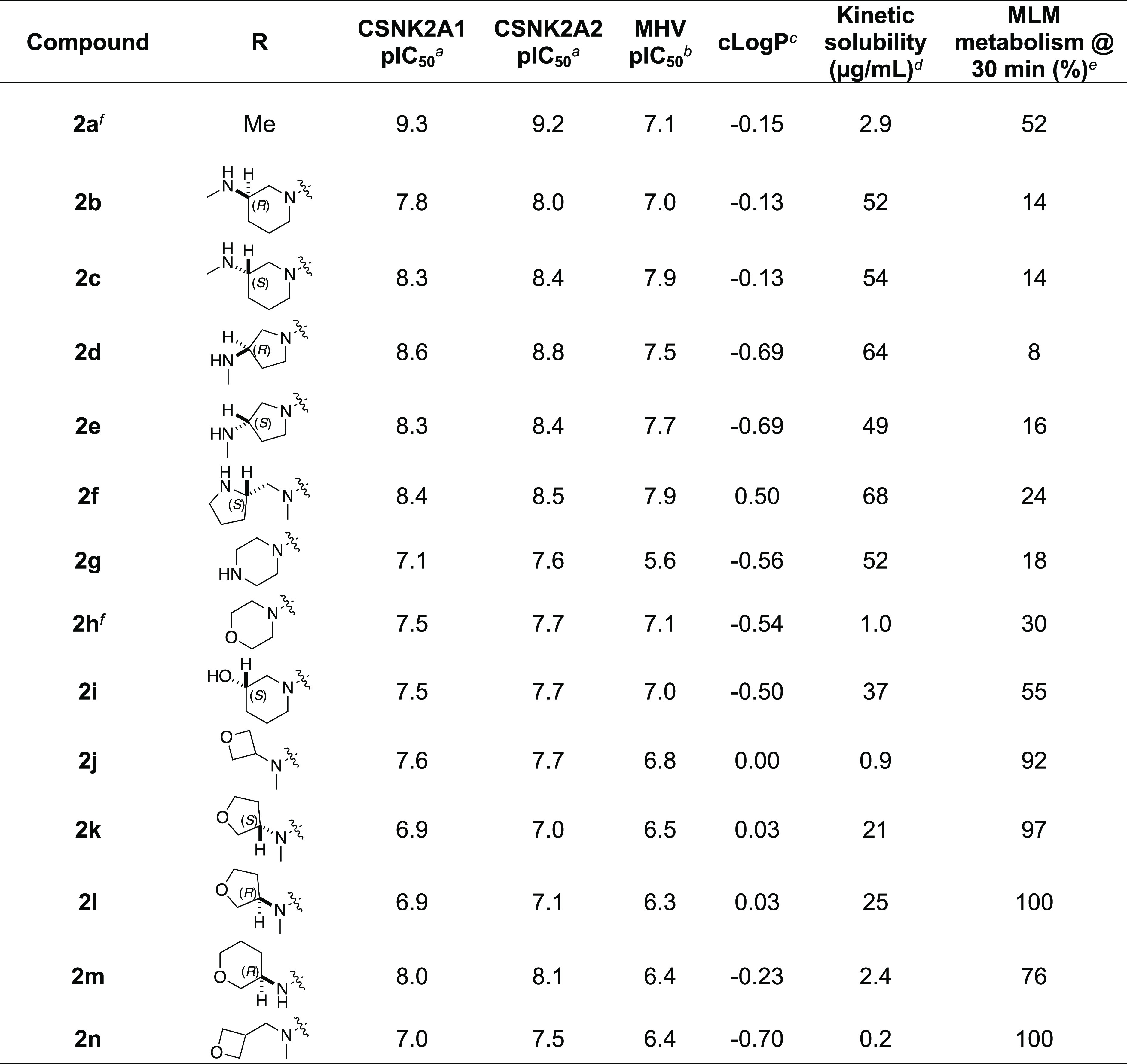
In-cell target engagement by the NanoBRET assay, n = 1.
Inhibition of MHV-nLuc replication, n = 3 with SE ± 0.1.
Values from ChemDraw Professional v16.0.
Miniaturized shake flask using CLND detection, n = 1.
Metabolism of the compound in primary mouse liver microsomes (MLM) calculated as 100 – (% remaining), n = 1.
Compound previously reported in ref (5).
Pharmacokinetic Profiling of PZPs
Having identified several analogues with improved metabolic stability in MLM and potent activity in the CSNK2A and antiviral assays, we selected three compounds (1i, 2c, and 2e) for pharmacokinetic studies. These analogues showed only 10–16% metabolism in the primary MLM after 30 min of incubation. The compounds were dosed at 1.0 mg/kg i.v. and 10 mg/kg i.p. to mice, and the level of the parent compound was monitored over 24 h (Table 3). Despite their stability in MLM, all three compounds demonstrated moderate to high clearance following i.v. dosing, with a t1/2 of 1.2–1.8 h. Similar results were observed following i.p. dosing with a t1/2 of 1.2–1.5 h. Although the bioavailability by i.p. dosing was good, the rapid clearance resulted in low total exposure and blood levels that fell below the IC50 for antiviral activity within 1 h.
Table 3. In Vivo Mouse Pharmacokinetic Parameters of 1i, 2c, and 2ea.
|
i.v.(1 mg/kg) |
i.p.(10 mg/kg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| compound | Cmax (ng/mL) | AUClast (ng·h/mL) | Vdss (L/kg) | t1/2 (h) | CLint (mL/min/kg) | Cmax (ng/mL) | AUClast (ng·h/mL) | t1/2 (h) | F (%) |
| 1i | 1100 | 670 | 2.60 | 1.3 | 36 | 4700 | 7010 | 1.2 | 100 |
| 2c | 350 | 200 | 5.07 | 1.8 | 84 | 670 | 770 | 1.5 | 40 |
| 2e | 810 | 250 | 2.67 | 1.2 | 69 | 1500 | 1300 | 1.4 | 53 |
Mice dosed by i.v. or i.p. route of administration, n = 3. Compounds quantified by LC-MS.
Phase II Metabolism of PZPs
To further explore the reason for the rapid in vivo clearance of the PZPs (1i, 2c, and 2e), we performed time course studies to measure their rate of intrinsic clearance using in vitro systems of hepatic metabolism (Table 4). In mouse primary liver microsomes, the three compounds showed low intrinsic clearance consistent with the single time point measurements (Tables 1 and 2). Repeating the experiment with primary liver microsomes that included the S9 fraction resulted in a decrease in clearance, indicating that metabolic stability was unaffected by the inclusion of the cytosolic enzymes. However, when incubated with primary mouse hepatocytes, all three compounds were rapidly metabolized with high rates of intrinsic clearance (Table 4). The results suggested that a non-P450 metabolic pathway could be responsible for the rapid hepatic clearance of the PZPs in hepatocytes and in mice in vivo.17
Table 4. In Vitro Mouse Metabolism of 1i, 2c, and 2ea.
| compound | liver microsomes CLint (mL/min/kg) | S9 fraction CLint (mL/min/kg) | hepatocytes CLint (mL/min/kg) |
|---|---|---|---|
| 1i | <24 | 0 | 143 |
| 2c | <24 | 1 | 132 |
| 2e | 27 | 4 | 69 |
Compounds quantified by LC-MS at five time points over 5 h, n = 1.
Metabolite ID Study of 1i
To identify the metabolic pathway responsible for the rapid clearance in mouse hepatocytes, we performed a metabolite ID study using 3S-aminomethylpiperidine (1i). Incubation of 1i for 4 h in primary cultures of mouse hepatocytes resulted in the appearance of 12 metabolites with unique retention times by ultrahigh performance liquid chromatography (uHPLC) (Figure 2A). Analysis of the metabolites by mass spectroscopy and quantification by UV absorbance enabled the assignment of the primary pathways of metabolic transformation (Table 4 and Figure 2B and Figure S2). Six metabolites (M1–M5 and M10) were assigned structures that resulted from GSH conjugation of the PZP (Figure 2B), a phase II metabolic transformation that could only occur in whole hepatocytes that contain active GST enzymes. GSH conjugation was observed in the major fraction of the total metabolites, as quantified by UV absorbance (Table 5) and is likely to be an important pathway of clearance of the PZP 1i (Figure 2B). A trio of GSH conjugates, M3, M4, and M5, were observed with the same molecular weight but different uHPLC retention times. They were assigned as constitutional isomers, resulting from GSH conjugation at different positions of the PZP (Figure 2B). Another pair of metabolites, M1 and M2, that arise from an additional oxidative dealkylation of the cyclopropyl group are also constitutional isomers from the conjugation of GSH at different positions on the PZP. M10 was a unique metabolite from cleavage of the bond between the piperidine and the phenyl ring in addition to GSH conjugation. Demethylation of the secondary amine on the piperidine was found in M6 and M11 as the other main site of metabolism (Table 4 and Figure 2B). Several minor pathways of oxidation, amide hydrolysis, and dehydrogenation were also assigned by analysis of the metabolites M7–M9 and M12 (Figure 2B). The initial P450 oxidation of electron-rich aromatic rings to arene oxide18 or quinone–imine19 intermediates are known activation steps for GSH conjugation, making the amino-substituted phenyl ring the most likely site of phase II metabolism (Figure 2C). Although we initially considered the nitrile as a potential site of conjugation, since it can undergo a Pinner reaction with free GSH,20 there is no evidence that the reaction is catalyzed by GST enzymes.21,22 Thus, the metabolite ID study implicated both cytochrome P450 and GST enzymes as likely to be responsible for the rapid clearance of the PZPs in mouse hepatocytes through a combination of demethylation and GSH conjugation (Figure 2C).
Figure 2.
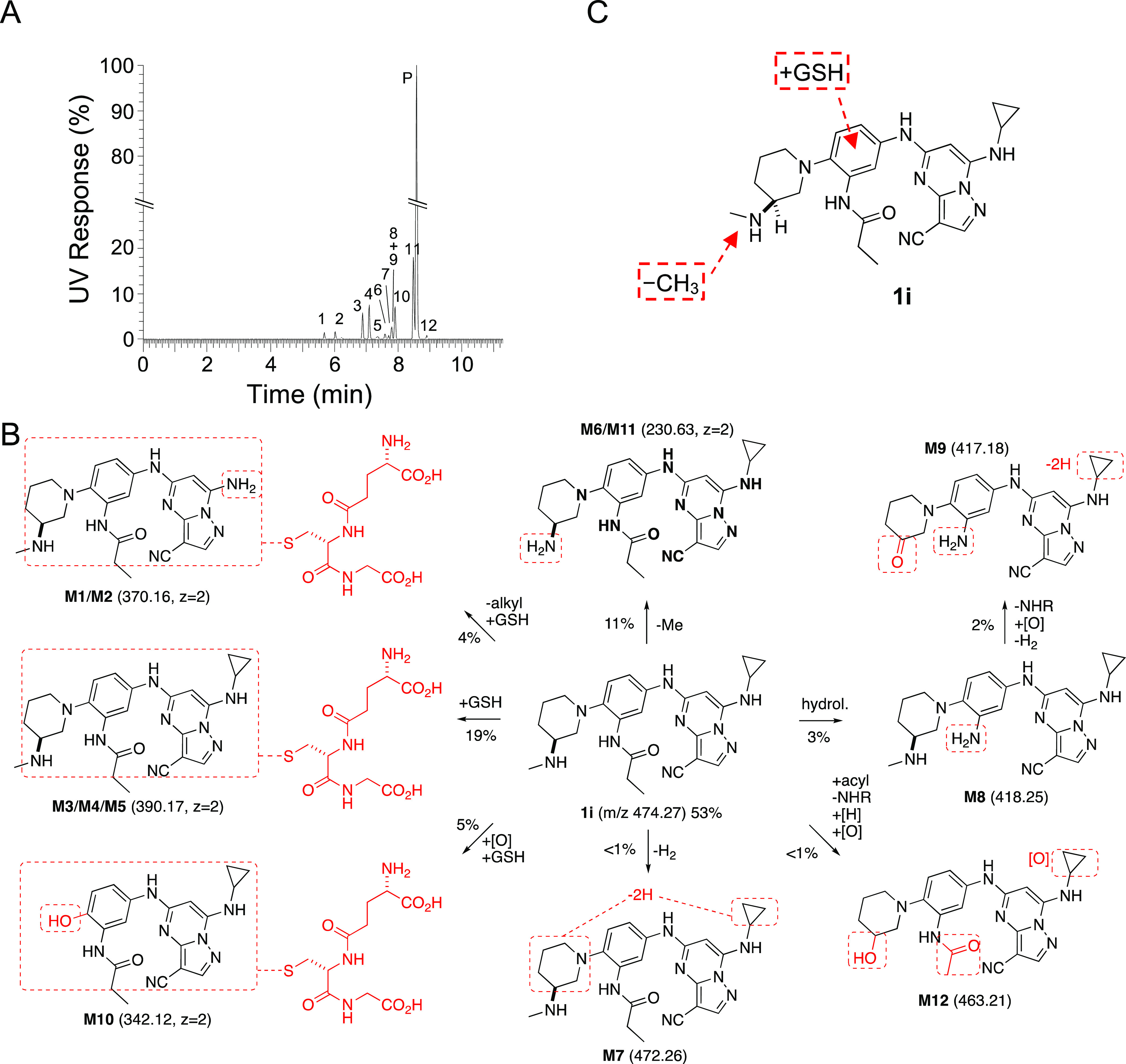
(A) Identification of 12 metabolites of 1i (parent P) after incubation in mouse primary hepatocytes for 4 h. The relative quantity of each metabolite was determined by UV. (B) Structural assignment of the metabolites (M1–M12) from MS fragmentation (see Figure S2). Molecular ions are shown in parentheses. Doubly charged ions are indicated as z = 2. The relative percent contribution of each metabolite taken from Table 5. (C) Summary of the major sites of metabolism of 1i in mouse primary hepatocytes.
Table 5. Metabolites of 1i in Mouse Hepatocytes.
| transformation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| peaka | time (min) | mass (m/z) | –alkyl | +GSH | –CH3 | –H2 | hydrol. | –NH2 | [O] | [H] | acyl. | rel. (%) |
| M1 | 5.67 | 370.16b | X | X | <1 | |||||||
| M2 | 6.03 | 370.16b | X | X | 3 | |||||||
| M3 | 6.89 | 390.17b | X | 9 | ||||||||
| M4 | 7.10 | 390.17b | X | 9 | ||||||||
| M5 | 7.34 | 390.17b | X | <1 | ||||||||
| M6 | 7.59 | 230.63b | X | <1 | ||||||||
| M7 | 7.70 | 472.26 | X | <1 | ||||||||
| M8 | 7.79 | 418.25 | X | <1 | ||||||||
| M9 | 7.80 | 417.18 | X | X | X | X | 2 | |||||
| M10 | 7.91 | 342.12b | X | X | 5 | |||||||
| M11 | 8.47 | 230.63b | X | 10 | ||||||||
| parent | 8.58 | 474.27 | 53 | |||||||||
| M12 | 8.90 | 463.21 | X | X | X | X | X | <1 | ||||
| total rel. (%)c | 4 | 27 | 11 | 3 | 4 | 3 | 8 | 1 | 1 | |||
Hepatocyte Metabolism of Antiviral PZPs
Eight acetamide PZPs (2a–f and 2h–i) with an IC50 ≤ 100 nM in the antiviral assay were selected for time course metabolic stability profiling in mouse and human primary hepatocytes (Table 6). Unfortunately, all acetamide PZPs (2a–f and 2h–i) showed high intrinsic clearance in mouse hepatocytes, independent of their stability in liver microsomes (Table 2), suggesting that they were substrates for phase II GST enzymes. The in vitro metabolism data in mouse hepatocytes predicted that acetamide PZPs with potent antiviral activity would likely have a rapid in vivo clearance in mice. In contrast, the compounds had much lower intrinsic clearance in human primary hepatocytes, with only 2h and 2i showing moderate and high rates of clearance, respectively. These results demonstrated that the metabolism of the PZPs in primary hepatocytes was predominantly a species-specific issue, with intrinsic clearance always much higher in mouse than human hepatocytes. Although this was a promising result for the future development of PZPs as therapeutic drugs for use in humans, it still limited their utility as pharmacological tools for studies in mice.
Table 6. Mouse and Human Hepatocyte Clearancea.
Compounds quantified by LC-MS at five time points over 5 h, n = 1.
Inhibition of GSH Conjugation in Hepatocytes
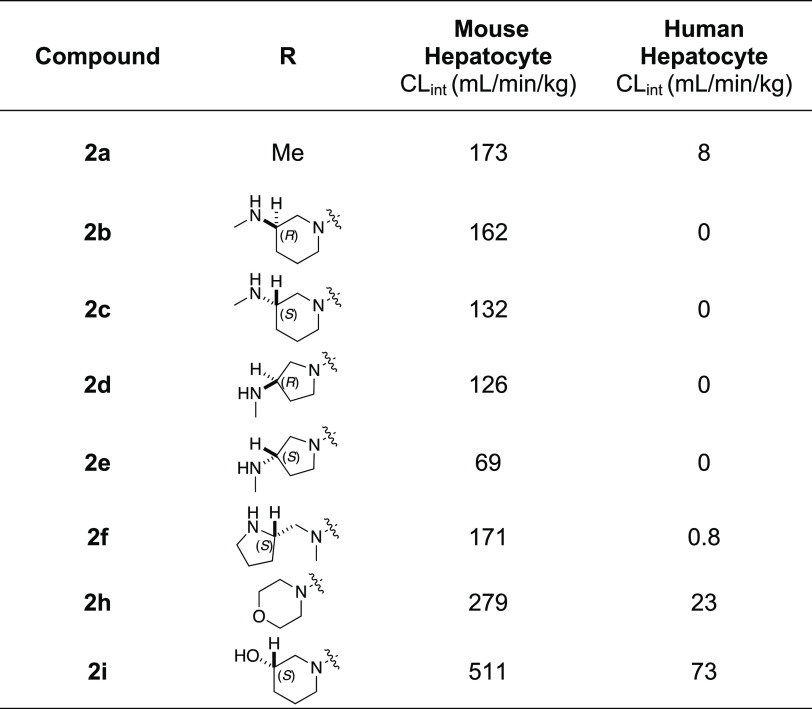
Given our primary objective of identifying a potent CSNK2A inhibitor for use in an in vivo mouse COVID-19 efficacy model to validate the host kinase as a potential antiviral drug target, we opted to explore the effect of inhibition of phase II GST enzymes on intrinsic clearance. Ethacrynic acid (EA) is an FDA-approved loop diuretic that is also a potent covalent, but reversible, inhibitor of GST enzymes.23 EA has demonstrated potent inhibition of GST enzymes in a perfused rat liver and human cancer cell lines,23 and although it has been used in human clinical studies to block GSH conjugation,24 there are only a few reports of its use in rodents as a GST inhibitor.25−27 To determine if EA could improve the metabolic stability of a PZP in primary mouse hepatocytes, we tested the effect of codosing on the stability of 2h, which was one of the analogues with the highest intrinsic clearance (Table 6). The intrinsic clearance of 2h in primary mouse hepatocytes was determined by measuring the decrease in the level of the parent compound over 2 h (Figure 3). Parallel sets of incubations were performed in the presence of increasing doses of EA from 0 to 400 μM. Intrinsic clearance was >250 mL/min/kg in the absence of EA. However, in the presence of doses of EA from 10 to 50 μM, the intrinsic clearance was decreased by >50% (Figure 3). At doses of EA above 100 μM, the intrinsic clearance of 2h was further decreased to <50 mL/min/kg. These codosing experiments in primary mouse hepatocytes demonstrated that EA was effective at increasing the metabolic stability of PZP 2h, presumably by blocking its GSH conjugation.
Figure 3.
Mouse hepatocyte clearance of 2h in the presence of GST inhibitor EA.
Codosing of 2h with Inhibitors of Metabolic Enzymes In Vivo
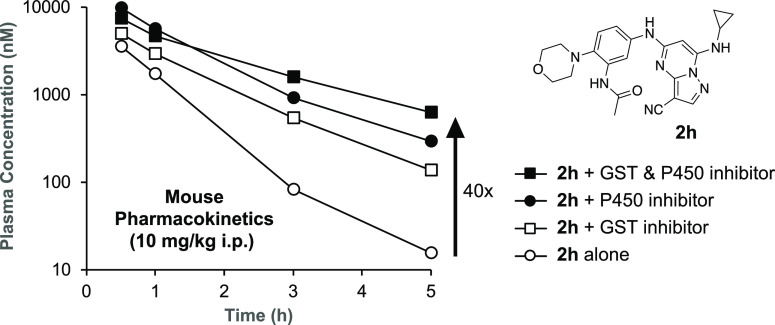
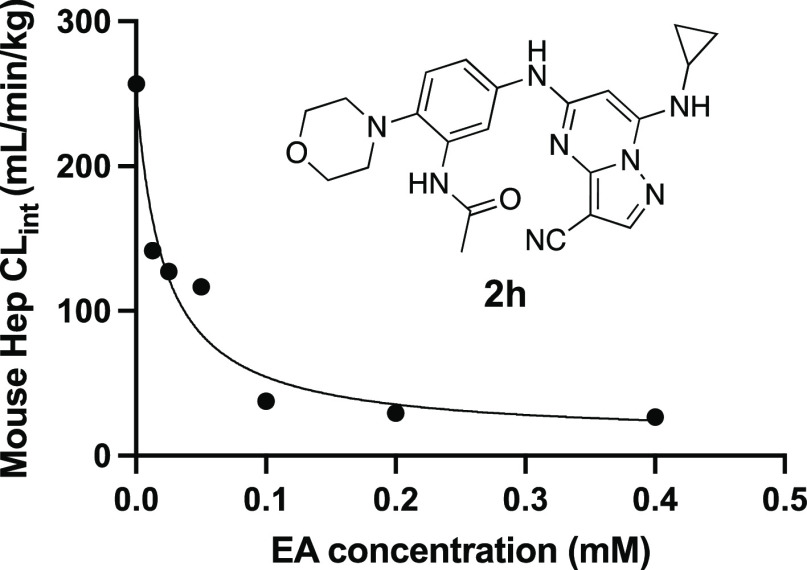
To determine if inhibition of GSH conjugation could be an effective strategy to increase the bioavailability of 2h in vivo, we performed a series of codosing studies in mice of 2h with either EA or 1-aminobenzotriaole (1-ABT), a widely used broad-spectrum inhibitor of mouse cytochrome P450s28 (Figure 4). When dosed alone to mice at 10 mg/kg i.p., 2h was rapidly cleared from the circulation with t1/2 = 35 min and AUC of around 4000 h·nM. A 6 h pretreatment and codosing with 10 mg/kg i.p. EA resulted in a small increase in t1/2 and AUC. Increasing the dose to 30 mg/kg i.p. EA resulted in an additional increase in both parameters, demonstrating that the inhibition of GST enzymes could reduce the intrinsic clearance of 2h. The 30 mg/kg i.p. dose of EA increased the t1/2 by 1.5-fold and AUC by 1.8-fold. Unfortunately, we were unable to further increase the dose of EA to 100 mg/kg i.p. due to the observation of toxicity in mice on repeated dosing (Table S1). Since GSH conjugation of electron-rich aromatic groups requires an initial cytochrome P450 oxidation step, we also evaluated the in vivo pharmacokinetics of 2h in mice pretreated with 1-ABT for 2 h. 1-ABT was able to reduce the CLint of 2h in primary mouse hepatocytes to <32 mL/min/kg (Figure S3). A decrease in the clearance of 2h was also observed in mice treated with 1-ABT at 100 mg/kg p.o. with increases in t1/2 and AUC of 1.6- and 3.5-fold, respectively, compared to the dosing of 2h alone. The larger increase in AUC with 1-ABT compared with EA may be due to the direct inhibition of phase I P450 metabolism and indirect inhibition of phase II GSH conjugation. Importantly, the results demonstrated that it was possible to increase the circulating levels of the PZP CSNK2A inhibitor 2h by codosing with either a GST inhibitor or a P450 inhibitor. Finally, to determine whether combined codosing with EA and 1-ABT could further decrease the clearance of 2h, we performed a study in the presence of both the GST and P450 inhibitors. A combination of 30 mg/kg i.p. EA (6 h pretreatment and codosed) with 100 mg/kg p.o. 1-ABT (2 h pretreatment) resulted in the highest levels of 2h at the 3 and 5 h time points (Figure 4), with the t1/2 increased by 2.4-fold compared to dosing of 2h alone.
Figure 4.
In vivo clearance of 2h in mice in the presence of a GST inhibitor (EA) or a P450 inhibitor (1-ABT). (A) Blood levels of 2h over 5 h following a 10 mg/kg dose i.p. All data are the average from three mice. The dose of EA or 1-ABT is indicated in parentheses in mg/kg. EA was administered i.p. as a 6 h pretreatment and again codosed with 2h. 1-ABT was administered p.o. as a 2 h pretreatment. (B) Pharmacokinetic parameters.
Discussion and Conclusions
The 3-cyano-7-cyclopropylamino-pyrazolo[1,5-a]pyrimidine (PZP) is the most potent and selective chemotype of ATP-competitive CSNK2A inhibitors,7 with fewer kinase off-targets than silmitasertib and related compounds.29 SGC-CK2-1 (1a)8 has been established as a high-quality chemical probe for studying CSNK2 signaling in the phenotypic assay and human-derived primary cells.30−32 However, the use of PZP-based CSNK2A inhibitors as chemical probes in rodent pharmacology models has been limited by their generally poor pharmacokinetic properties. Although Dowling and co-workers reported that 2a had modest oral bioavailability in rats (10 mg/kg, Cmax = 300 nM, F = 25%), it showed in vivo activity only at short time points after dosing.9 After extensive work on the series, Dowling and co-workers concluded that an analogue with an ethylenediamine substituent at the 4′-position could be used as a pharmacological tool by i.v. or i.p. dosing, despite its rapid clearance in rats.10 For the studies reported herein, we decided to focus on development of an in vivo pharmacological probe that would maintain high sustained exposure in mice following i.p. dosing since this route of administration was preferred in many academic laboratories for preclinical target validation studies.33
Initially, we examined the potential of the 4′-substitued analogues of SGC-CK2-1 (1a) to improve solubility and reduce its rapid metabolism in mouse liver microsomes. Testing several of the previously prepared analogues of 1a(5,8) confirmed prior observations10 of reduced metabolism of linear and cyclic diamine analogues with clogP < 0.5 (Table 1). After synthesizing many new 4′-substituted PZPs in the acetamide (1) and propionamide (2) series, the chiral 3-methylaminopipiperides 1h–i and 2b–c and 3-methylaminopyrollidines 2d–e were found to have the best balance of reduced MLM metabolism and good cellular potency in the antiviral assay. However, despite their improved stability to phase I metabolism, 1i, 2c, and 2e showed rapid clearance in mice following i.v. or i.p. administration (Table 3).
After discovering that 1i, although stable in MLM, was rapidly metabolized in mouse primary hepatocytes, we performed a metabolic ID study that identified phase II GSH conjugation as a major metabolic transformation (Table 4 and Figure 2). This result suggested that phase II metabolism by GST enzymes was a previously underappreciated metabolic liability of PZPs containing electron-rich di- and tri-aniline rings. Unfortunately, all potent antiviral PZPs showed rapid metabolism in mouse primary hepatocytes (Table 6). Although the metabolic instability may be species-specific, since it was not observed in human primary hepatocytes, the rapid clearance in mouse primary hepatocytes predicted that none of the potent antiviral PZP analogues would be suitable for use in the mouse-adapted COVID-19 model.
To explore ways to reduce the phase II metabolism of a PZP analogue, we investigated codosing with EA, a well-characterized broad-spectrum covalent, but reversible, inhibitor of rodent and human GSTs.23 The studies were performed with the morpholine analogue 2h, which exhibited potent antiviral activity but very high clearance in primary mouse hepatocytes. Codosing of EA with 2h in primary mouse hepatocytes demonstrated that metabolism could be reduced in a dose-dependent manner, with >90% reduction in intrinsic clearance at EA concentrations >100 μM (Figure 3).
Translating the use of EA as a GST inhibitor to an in vivo setting in mice was not straightforward. EA is an inhibitor of NKCC2, a Na–K–Cl transporter, and is approved for use in humans as a loop diuretic for treatment of high blood pressure and edema.34 At its commonly used 50 mg oral dose in humans, EA does not cause significant drug–drug interactions that might result from GST inhibition. However, in rodents, no dose-ranging studies to establish the lowest effective dose for GST inhibition have been reported. Although, doses of 10–20 mg/kg p.o. have been used to boost the activity of oncology drugs in mice.25−27 Our pharmacokinetic studies in mice revealed that the maximum tolerated dose of EA by i.p. dosing was only 30 mg/kg. Doses of 100 mg/kg and higher resulted in clinical symptoms including death (Table S1). Although the reason for the toxicity of EA by i.p. administration is unclear, the rapid onset suggests that it may be unrelated to the inhibition of liver metabolism enzymes. Nevertheless, we observed a reduction in the plasma clearance of 2h in mice at both the 10 and 30 mg/kg i.p. codoses of EA without toxicity. Furthermore, the clearance of 2h was also reduced by a 100 mg/kg p.o. dose of the irreversible cytochrome P450 inhibitor 1-ABT, which would be expected to block directly phase I metabolism as well as indirectly phase II metabolism that proceeds through arene oxide or quinone–imine intermediates. The most effective strategy to decrease the clearance of 2h was a codosing protocol, using a combination of 30 mg/kg i.p. EA and 100 mg/kg p.o. 1-ABT. With this approach, the blood level of 2h was boosted 40-fold at the 5 h time point.
In conclusion, our findings demonstrate that phase II metabolism limits the in vivo exposure of PZP-based CSNK2A inhibitors in mice and severely limits their utility as pharmacological probes despite their remarkable potency and selectivity in cells. Although the use of EA as a GST inhibitor provides an effective method to reduce the intrinsic clearance of the current antiviral PZP molecules in mice, we are continuing to optimize the series to identify new analogues with reduced potential for phase II metabolism. Our goal is to find in vivo chemical probes for CSNK2A that can be dosed in mice without the additional complication of codosing with multiple inhibitors of drug-metabolizing enzymes.
Experimental Section
NanoBRET Assay
Assays were run with a modified version of the previously published protocols.5,8 HEK293 cells were cultured at 37 °C in 5% CO2 in Dulbecco’s modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (VWR/Avantor). A transfection complex of DNA at 10 μg/mL was created, consisting of 9 μg/mL carrier DNA (Promega) and 1 μg/mL CSNK2A-NLuc fusion DNA in Opti-MEM without serum (Gibco). FuGENE HD (Promega) was added at 30 μL/mL to form a lipid:DNA complex. The solution was then mixed and incubated at room temperature for 20 min. The transition complex was mixed with a 20× volume of HEK293 cells at 20,000 cells/mL in DMEM/FBS, and 100 μL/well was added to a 96-well plate that was incubated overnight at 37 °C and 5% CO2. The following day, the media were removed via aspiration and replaced with 85 μL of Opti-MEM without phenol red. A total of 5 μL per well of 20× NanoBRET Tracer K10 (Promega) at 10 μM for CSNK2A1 or 5 μM for CSNK2A2 in Tracer Dilution Buffer (Promega N291B) was added to all wells, except the “no tracer” control wells. Test compounds (10 mM in DMSO) were diluted 100× in Opti-MEM media to prepare stock solutions and evaluated at 11 concentrations. A total of 10 μL per well of the 10-fold test compound stock solutions (final assay concentration of 0.1% DMSO) were added. For “no compound” and “no tracer” control wells, DMSO in Opti-MEM was added for a final concentration of 1.1% across all wells; 96-well plates containing cells with NanoBRET Tracer K10 and test compounds (100 μL total volume per well) were equilibrated (37 °C/5% CO2) for 2 h. The plates were cooled to room temperature for 15 min. The NanoBRET NanoGlo substrate (Promega) at a ratio of 1:166 to Opti-MEM media in combination with an extracellular NLuc Inhibitor (Promega) diluted at 1:500 (10 μL of 30 mM stock per 5 mL of the Opti-MEM plus substrate) was combined to create a 3× stock solution. A total of 50 μL of the 3× substrate/extracellular NL inhibitor was added to each well. The plates were read within 30 min on a GloMax Discover luminometer (Promega) equipped with a 450 nm BP filter (donor) and 600 nm LP filter (acceptor) using 0.3 s of integration time. Raw milliBRET (mBRET) values were obtained by dividing the acceptor emission values (600 nm) by the donor emission values (450 nm) and multiplying by 1000. Averaged control values were used to represent complete inhibition (no tracer control: Opti-MEM + DMSO only) and no inhibition (tracer only control: no compound, Opti-MEM + DMSO + Tracer K10 only) and were plotted alongside the raw mBRET values. The data was first normalized and then fit using the Sigmoidal 4PL binding curve in Prism Software to determine IC50 values.
MHV Assay
DBT cells were cultured at 37 °C in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (Gibco) and penicillin and streptomycin (Sigma-Aldrich). DBT cells were plated in 96-well plates to be 80% confluent at the start of the assay. Test compounds were diluted to 15 μM in DMEM. Serial 4-fold dilutions were made in DMEM, providing a concentration range of 15–0.22 μM. Media were aspirated from the DBT cells, and 100 μL of the diluted test compounds was added to the cells for 1 h at 37 °C. After 1 h, MHV-nLuc5 was added at an MOI of 0.1 in 50 μL of DMEM so that the final concentration of the first dilution of the compound was 10 μM (T = 0). After 10 h, the media were aspirated, and the cells were washed with PBS and lysed with passive lysis buffer (Promega) for 20 min at room temperature. Relative light units (RLUs) were measured by using a luminometer (Promega; GloMax). Triplicate data was analyzed in Prism Graphpad to generate IC50 values.
Kinetic Solubility
Phosphate buffered saline (50 mL, PBS, Fisher, pH 7.4) was added to HPLC grade H2O (450 mL) for a total dilution factor of 1:10 and final PBS concentration of 1×. The test compound (6 μL) as a 10 mM DMSO stock solution was combined with the aqueous PBS solution (294 μL) for 50-fold dilution in a Millipore solubility filter plate with a 0.45 μM polycarbonate filter membrane using a Hamilton Starlet liquid handler. The final DMSO concentration was 2.0%, and the maximum theoretical compound concentration was 200 μM. The filter plate was heat-sealed for the duration of a 24 h incubation period. The sample was placed on a rotary shaker (200 rpm) for 24 h at ambient temperature (21.6–22.8 °C) and then vacuum-filtered. All filtrates were injected into a chemiluminescent nitrogen detector for quantification. The equimolar nitrogen response of the detector was calibrated using standards that span the dynamic range of the instrument from 0.08 to 4500 μg/mL nitrogen. The filtrates were quantified with respect to this calibration curve. The calculated solubility values were corrected for background nitrogen present in DMSO and the media used to prepare the samples.
Mouse Liver Microsomal Stability
Compounds as 10 mM DMSO stock solutions were diluted to 2.5 mM with DMSO and again to 0.5 mM with MeCN to give a final solution containing a 0.5 mM compound in 1:4 DMSO/MeCN. Liver microsomes from male CD-1 mice were sourced from Xenotech (Kansas City, KS), lot no. 1710069. A reaction plate was prepared by adding 691.25 μL and prewarmed (37 °C) microsomal solution (0.63 mg/mL protein in 100 mM KPO4 with 1.3 mM EDTA) to an empty well of a 96-well plate and maintained at 37 °C. The diluted 0.5 mM compound (8.75 μL) was added to the microsomal solution in the reaction plate and mixed thoroughly by repeated pipetting to give a final assay concentration of the 5.0 μM compound. The resulting solutions were preincubated for 5 min at 37 °C and then dispensed into T = 0 and incubation plates. For the T = 0 plates, an aliquot (160 μL) of each reaction solution was added to an empty well of a 96-well plate as an exact replicate of the reaction plate. Cold (4 °C) MeOH (400 μL) was added to each well and mixed thoroughly by repeated pipetting. NADPH regeneration solution (40 μL) was added to each well and mixed thoroughly by repeated pipetting. For the T = 30 min incubation plate, NADPH (95 μL) was added to the remaining solution (microsomes + test compound) in each well in the previously prepared reaction plate to initiate the reaction. The plate was sealed and incubated at 37 °C for 30 min. An aliquot (100 μL) was removed from each well at the desired time point and dispensed into a well of a 96-well plate. Cold (4 °C, 200 μL) MeOH was added to quench the reaction. All plates were sealed, vortexed, and centrifuged at 3000 rpm, 4 °C for 15 min, and the supernatants were transferred for analysis by LC-TOFMS. The supernatant (20 μL) was injected onto an AQUASIL C18 column and eluted using a fast-generic gradient program. TOFMS data was acquired using Agilent 6538 Ultra High Accuracy TOF MS in extended dynamic range (m/z 100–1000) using generic MS conditions in positive mode. Following data acquisition, exact mass extraction and peak integration were performed using MassHunter Software (Agilent Technologies). The stability of the compound was calculated as the percent remaining of the unchanged parent at T = 30 min relative to the peak area at T = 0 min.
To determine CLint, aliquots of 50 μL were taken from the reaction solution at 0, 15, 30, 45, and 60 min. The reaction was stopped by the addition of four volumes of cold MeCN with IS (100 nM alprazolam, 200 nM imipramine, 200 nM labetalol, and 2 μM ketoprofen). Samples were centrifuged at 3,220g for 40 min, and 90 μL of the supernatant was mixed with 90 μL of ultrapure H2O and then used for LC-MS/MS analysis. Peak areas were determined from extracted ion chromatograms, and the slope value, k, was determined by linear regression of the natural logarithm of the remaining percentage of the parent drug vs incubation time curve. The intrinsic clearance (CLint in μL/min/mg) was calculated using the relationship CLint = kV/N where V is the incubation volume and N is the amount of protein per well. To measure CLint in the presence of microsomal and cytosolic enzymes, the experiment was repeated using liver S9 fraction microsomes from male CD-1 mice sourced from Xenotech (Kansas City, KS), lot no. 1510255.
Hepatocyte Stability
Human cryopreserved hepatocytes were supplied by BioIVT (lot QZW, 10 pooled donors). Mouse cryopreserved hepatocytes were supplied by BioIVT (lot ZPG, pooled male CD-1). Vials of cryopreserved hepatocytes were removed from storage and thawed in a 37 °C water bath with gentle shaking, and then, the contents were poured into a 50 mL thawing medium conical tube. Vials were centrifuged at 100g for 10 min at room temperature. The thawing medium was aspirated, and hepatocytes were resuspended with a serum-free incubation medium to yield ∼1.5 × 106 cells/mL. Cell viability and density were counted using AO/PI fluorescence staining, and then, cells were diluted with a serum-free incubation medium to a working cell density of 0.5 × 106 viable cells/mL. Aliquots of 198 μL of hepatocytes were dispensed into each well of a 96-well noncoated plate. The plate was placed in an incubator for approximately 10 min. Aliquots of 2 μL of the 100 μM test compound in duplicate and positive control were added into the respective wells of the noncoated 96-well plate to start the reaction. The final concentration of the test compound was 1 μM. The plate was placed in an incubator for the designed time points. Contents (25 μL) were transferred and mixed with six volumes (150 μL) of cold MeCN with IS (100 nM alprazolam, 200 nM labetalol, 200 nM caffeine, and 200 nM diclofenac) to terminate the reaction at time points of 0, 15, 30, 60, 90, and 120 min. Samples were centrifuged for 45 min at 3,220g, an aliquot of 100 μL of the supernatant was diluted with 100 μL of ultrapure H2O, and the mixture was used for LC-MS/MS analysis. Peak areas were determined from extracted ion chromatograms, and the slope value, k, was determined by linear regression of the natural logarithm of the remaining percentage of the parent drug vs incubation time curve. The intrinsic clearance (CLint in μL/min/106 cells) was calculated using the relationship CLint = kV/N where V is the incubation volume (0.2 mL) and N is the number of hepatocytes per well (0.1 × 106 cells). Scaling factors to convert CLint from μL/min/106 cells to mL/min/kg were 2540 (human hepatocytes) and 11,800 (mouse hepatocytes).
Pharmacokinetics (PK)
Male CD-1 mice (6–8 weeks, 20–30 g) were dosed by intravenous (i.v.) or interperitoneal (i.p.) administration. For i.v. administration, a single dose of the compound (1 mg/kg) was administered as a 5 mL/kg volume of a 0.2 mg/mL solution in NMP/solutol/PEG-400/normal saline (v/v/v/v, 10:5:30:55) to three mice. For i.p. administration, a single dose of the compound (10 mg/kg) was administered as a 10 mL/kg volume of a 1.0 mg/mL solution in 0.5% HPMC/0.2% Tween 80/99.3% H2O or DMSO/PEG-400/saline (v/v/v/v, 10:30:60) to three mice. The mice had free access to water and food. Blood (0.03 mL) was collected from the dorsal metatarsal vein at 0.5, 1, 3, and 5 h time points (snapshot PK) or at 0.08, 0.25, 0.5, 1, 2, 4, 8, and 24 h (full PK). Blood from each sample was transferred into plastic microcentrifuge tubes containing EDTA-K2, mixed well, and then placed in a cold box prior to centrifugation. Blood samples were centrifuged at 4000g for 5 min at 4 °C to obtain plasma and then stored at −75 °C prior to analysis. Concentrations of the test compound in the plasma samples were determined using a Prominence LC-30AD, AB Sciex Triple Quan 5500 LC-MS/MS instrument fitted with a HALO 160A C18 column (2.7 μm, 2.1 × 50 mm) using a mobile phase of 5–95% MeCN in H2O with 0.1% formic acid. PK parameters were calculated from the mean plasma concentration versus time by a noncompartmental model using WinNonlin 8.3 (Phoenix) to determine Cmax, AUClast, t1/2, CLint, and F.
For codosing studies, 2h (10 mg/kg) was administered i.p. as a 10 mL/kg volume of a 1.0 mg/mL solution in DMSO/PEG-400/saline (v/v/v/v, 10:30:60). EA (10 or 30 mg/kg) was administered i.p. as a 1 mL/kg volume of a 30 mg/mL solution in NMP/PEG-400/water (v/v/v, 10:60:30) as a 6 h pretreatment and again at the time of dosing of 2h. 1-ABT (100 mg/kg) was administered p.o. as a 10 mL/kg volume of a 10 mg/mL solution in saline as a 2 h pretreatment. Blood (0.03 mL) was collected from the dorsal metatarsal vein at 0.5, 1, 3, and 5 h time points and the level of 2h in plasma determined by LC-MS/MS as described above. With this dosing protocol, no adverse clinical observations were recorded during the study.
General Synthetic Methods
All chemical reagents were commercially available except those whose synthesis is described below. All reaction mixtures and column eluents were monitored via analytical thin-layer chromatography (TLC) performed on precoated fluorescent silica gel plates, 200 μm with an F254 indicator; visualization was accomplished by UV light (254/365 nm). NMR spectra were obtained on a Bruker/AVANCE NEO 400 MHz instrument. LC-MS measurements were determined on Shimadzu LC-AB + LCMS-2020, Shimadzu LC-AD + LCMS-2020, Shimadzu LC-AD xR + LCMS-2020, or Agilent 1200 + Infinitylab LC/MSD instruments. Purity was determined by HPLC measurement using a Shimadzu LC-20 + LCMS-2020 instrument fitted with an Agilent PoroShell 120 EC-C18 column (45 °C, 2.7 μm, 3.0 × 50 mm); 8 min chromatography ran 0.037% TFA in water/MeCN (19:1) (solvent A), 0.018% TFA/MeCN (solvent B), and gradient 0–60% (solvent B) over 6.0 min, held at 60% for 1.0 min, and returned to 0% (solvent B) for 1.0 min at a flow rate of 1.0 mL/min; 4 min chromatography ran 0.037% TFA in water/MeCN (19:1) (solvent A), 0.018% TFA/MeCN (solvent B), and gradient 10–80% (solvent B) over 3.0 min, held at 80% for 0.5 min, and returned to 0% (solvent B) for 0.5 min at a flow rate of 1.0 mL/min, which indicated that all final compounds were >95% pure.
General Procedure A
Synthesis of Intermediate y
To a solution of N-(2-fluoro-5-nitrophenyl)propionamide (800 mg, 3.77 mmol) or N-(2-fluoro-5-nitrophenyl)acetamide (747 mg, 3.77 mmol) and an amine (3.77 mmol) in MeCN (8 mL) was added K2CO3 (1.56 g, 11.3 mmol) at 25 °C. The mixture was stirred at 100 °C for 10 h. The reaction mixture was concentrated in vacuo. Water (200 mL) was added, and the mixture was extracted with EtOAc (2× 250 mL). The combined organic phase was washed with brine (250 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. To the resulting nitrobenzene (4.67 mmol) in MeOH (3 mL) was added 10% Pd/C (900 mg) under a N2 atmosphere. The suspension was degassed, purged with H2 three times, and then stirred under H2 (15 psi) at 25 °C for 10 h. The reaction mixture was filtered through a celite pad. The pad was washed with MeOH (2× 8 mL), and the combined filtrate was concentrated in vacuo. The residue was purified by flash silica gel chromatography (eluent of 0–8%, MeOH/CH2Cl2) to yield intermediate y.
(R)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)piperidin-1-yl)phenyl)propionamide (1h)
Intermediate y was synthesized from tert-butyl (R)-methyl(piperidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)propionamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (100 mg, 0.43 mmol) and intermediate y (170 mg, 0.43 mmol) in dioxane (3 mL) was added Cs2CO3 (418 mg, 1.28 mmol), BINAP (40 mg, 0.06 mmol), and Pd(OAc)2 (14.0 mg, 0.06 mmol) at 25 °C. The mixture was degassed, purged with N2, and then heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–4%, MeOH/CH2Cl2). To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 150 × 40 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 15–45%, 10 min). Compound 1h (66.3 mg, 11.3%) was obtained as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.65 (s, 1H), 9.33 (br s, 1H), 8.44 (s, 1H), 8.33 (s, 1H), 8.19 (s, 1H), 8.04 (d, J = 2.4 Hz, 1H), 7.82 (d, J = 7.2 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 6.03 (s, 1H), 3.13 (br s, 1H), 3.05 (d, J = 10.4 Hz, 1H), 2.89–2.80 (m, 2H), 2.60–2.58 (m, 2H), 2.52–2.51 (m, 1H), 2.48–2.44 (m, 4H), 1.92–1.91 (m, 1H), 1.75 (s, 2H), 1.62–1.60 (m, 1H), 1.12 (t, J = 7.6 Hz, 3H), 0.80–0.79 (m, 2H), 0.72–0.70 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 172.04, 165.85, 156.77, 150.78, 148.07, 144.88, 137.77, 136.07, 132.78, 119.97, 114.74, 76.13, 54.06, 52.95, 38.69, 30.66, 29.41, 23.17, 9.79, 6.39. HPLC Rt = 3.656 min in 8 min chromatography, purity 99.6%. LC-MS Rt = 1.925 min in 4 min chromatography, purity 98.0%, MS ESI calcd. for 473.27 [M + H]+ 474.27, found 474.4.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)piperidin-1-yl)phenyl)propionamide (1i)
Intermediate y was synthesized from tert-butyl (S)-methyl(piperidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)propionamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (112 mg, 0.48 mmol) and intermediate y (200 mg, 0.53 mmol) in dioxane (3 mL) was added Cs2CO3 (502 mg, 1.53 mmol), BINAP (48 mg, 0.07 mmol), and Pd(OAc)2 (16.8 mg, 0.07 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–4%, MeOH/CH2Cl2). To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 150 × 40 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 0–38%, 36 min). Compound 1i (115 mg, 37.0%) was obtained as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.65 (s, 1H), 9.09 (s, 1H), 8.43–8.36 (m, 1H), 8.34 (s, 1H), 8.20 (s, 1H), 8.07–8.01 (m, 1H), 7.89–7.78 (m, 1H), 7.12 (d, J = 8.8 Hz, 1H), 6.03 (s, 1H), 3.24 (s, 1H), 3.12–3.05 (m, 1H), 2.93–2.84 (m, 1H), 2.83–2.75 (m, 1H), 2.68–2.54 (m, 5H), 2.48–2.41 (m, 2H), 1.95–1.60 (m, 4H), 1.13 (t, J = 7.6 Hz, 3H), 0.84–0.66 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ: 172.16, 165.58, 156.97, 150.97, 148.26, 145.06, 137.96, 136.25, 132.97, 120.21, 115.33, 114.91, 76.32, 54.47, 53.83, 53.02, 31.24, 29.65, 23.36, 21.77, 9.95, 6.57. HPLC Rt = 3.612 min in 8 min chromatography, purity 99.6%. LC-MS Rt = 1.567 min in 4 min chromatography, purity 100.0%, MS ESI calcd. for 473.3 [M + H]+ 474.3, found 474.5.
(R)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)piperidin-1-yl)phenyl)acetamide (2b)
Intermediate y was synthesized from tert-butyl (R)-methyl(piperidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (112 mg, 0.48 mmol) and intermediate y (200 mg, 0.55 mmol) in dioxane (3 mL) was added Cs2CO3 (541 mg, 1.65 mmol), BINAP (51 mg, 0.08 mmol), and Pd(OAc)2 (17.9 mg, 0.08 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–32%, EtOAc/petroleum ether) to give the product. To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Phenomenex C18 75 × 30 mm × 3 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 2–32%, 24 min). Compound 2b (32.8 mg, 27.4%) was obtained as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.63 (s, 1H), 9.41 (s, 1H), 8.41 (s, 1H), 8.32 (s, 1H), 8.16 (s, 1H), 7.99 (d, J = 1.6 Hz, 1H), 7.83 (d, J = 6.4 Hz, 1H), 7.10 (d, J = 8.8 Hz, 1H), 6.03 (s, 1H), 3.22 (s, 1H), 3.06 (d, J = 11.2 Hz, 1H), 2.93 (d, J = 8.4 Hz, 1H), 2.84 (d, J = 11.2 Hz, 1H), 2.58 (s, 2H), 2.53 (s, 3H), 2.18 (s, 3H), 1.95 (s, 1H), 1.79 (s, 2H), 1.67–1.56 (m, 1H), 0.80 (d, J = 6.8 Hz, 2H), 0.70 (d, J = 2.8 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.62, 163.65, 156.97, 150.98, 148.26, 145.08, 137.91, 136.26, 133.03, 120.20, 115.42, 114.96, 76.32, 54.08, 53.19, 40.15, 39.99, 39.78, 24.09, 23.36, 6.59. HPLC Rt = 3.321 min in 8 min chromatography, purity 99.9%. LC-MS Rt = 1.551 min in 4 min chromatography, purity 99.8%, MS ESI calcd. for 459.25 [M + H]+ 460.25, found 460.4.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)piperidin-1-yl)phenyl)acetamide (2c)
Intermediate y was synthesized from tert-butyl (S)-methyl(piperidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (237 mg, 1.02 mmol) and intermediate y (460 mg, 1.27 mmol) in dioxane (6 mL) was added Cs2CO3 (1.0 g, 1.65 mmol), BINAP (94.8 mg, 0.14 mmol), and Pd(OAc)2 (33.2 mg, 0.14 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–3%, MeOH/CH2Cl2). To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 100 × 30 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 30–50%, 10 min). Compound 2c (58 mg, 27.4%) was obtained as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.64 (s, 1H), 9.38–9.21 (m, 1H), 8.39–8.30 (m, 1H), 8.17 (s, 1H), 7.97 (s, 1H), 7.89–7.77 (m, 1H), 7.10 (d, J = 8.8 Hz, 1H), 6.03 (s, 1H), 3.11–3.03 (m, 2H), 3.02–2.90 (m, 2H), 2.89–2.82 (m, 1H), 2.62–2.53 (m, 5H), 2.20 (s, 3H), 2.06–1.94 (m, 1H), 1.89–1.72 (m, 2H), 1.67–1.58 (m, 1H), 0.84–0.76 (m, 2H), 0.73–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.66, 162.94, 156.95, 150.96, 148.25, 145.07, 137.91, 136.26, 132.85, 120.17, 115.48, 114.94, 113.94, 76.32, 54.13, 53.14, 39.99, 30.59, 24.20, 23.36, 6.58. HPLC Rt = 3.324 min in 8 min chromatography, purity 99.2%. LC-MS Rt = 1.549 min in 4 min chromatography, purity 99.6%, MS ESI calcd. for 459.25 [M + H]+ 460.25, found 460.4.
(R)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)pyrrolidin-1-yl)phenyl)acetamide (2d)
Intermediate y was synthesized from tert-butyl (R)-methyl(pyrrolidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (134 mg, 0.57 mmol) and intermediate y (250 mg, 0.72 mmol) in dioxane (3 mL) was added Cs2CO3 (0.5 g, 0.82 mmol), BINAP (47.4 mg, 0.07 mmol), and Pd(OAc)2 (16.6 mg, 0.07 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Waters Torus 2-PIC 150 mm × 19 mm × 5 μm; mobile phase: [Heptane-EtOH (0.1% NH3/H2O)]; B%: 10–50%, 5 min) to give the product as a yellow solid. To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 100 mm × 30 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 25–45%, 10 min). Compound 2d (20 mg, 30.2%) was obtained as a white solid. 1H NMR (400 MHz, MeOD-d4) δ 9.56 (s, 1H), 9.30 (s, 1H), 8.46–8.28 (m, 2H), 8.16 (s, 1H), 7.93–7.60 (m, 2H), 7.02 (d, J = 8.4 Hz, 1H), 5.99 (s, 1H), 3.55–3.50 (m, 1H), 3.34–3.09 (m, 4H), 2.95–2.84 (m, 1H), 2.61–2.51 (m, 4H), 2.26–2.06 (m, 4H), 1.9–1.86 (m, 1H), 0.86–0.76 (m, 2H), 0.75–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.49, 157.01, 151.02, 148.19, 145.00, 134.54, 130.86, 118.11, 116.30, 114.95, 76.17, 57.99, 54.44, 49.20, 39.99, 23.89, 23.32, 6.55. HPLC Rt = 3.063 min in 8 min chromatography, purity 99.8%. LC-MS Rt = 1.422 min in 4 min chromatography, purity 99.9%, MS ESI calcd. for 445.23 [M + H]+ 446.23, found 446.4.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-(methylamino)pyrrolidin-1-yl)phenyl)acetamide (2e)
Intermediate y was synthesized from tert-butyl (S)-methyl(pyrrolidin-3-yl)carbamate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (520 mg, 2.23 mmol) and intermediate y (930 mg, 2.67 mmol) in dioxane (15 mL) was added Cs2CO3 (2.2 g, 6.7 mmol), BINAP (207 mg, 0.31 mmol), and Pd(OAc)2 (72.7 mg, 0.31 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–51%, EtOAc/petroleum ether) to give the product as a gray solid. To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Welch Xtimate C18 150 × 30 mm × 5 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 0–90%, 14 min). Compound 2e (30.6 mg, 16.2%) was obtained as a light-yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.59 (s, 1H), 9.42 (s, 1H), 8.41 (s, 1H), 8.33 (s, 1H), 8.18 (s, 1H), 7.86 (s, 1H), 7.76 (s, 1H), 7.03 (d, J = 8.8 Hz, 1H), 6.00 (s, 1H), 3.56 (s, 1H), 3.30 (d, J = 4.4 Hz, 1H), 3.21–3.16 (m, 1H), 3.16–3.11 (m, 1H), 2.82 (q, J = 8.0 Hz, 1H), 2.57 (s, 1H), 2.52–2.51 (m, 3H), 2.26–2.17 (m, 1H), 2.14 (s, 3H), 1.97 (s, 1H), 0.84–0.76 (m, 2H), 0.72–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.51, 165.64, 157.00, 151.01, 148.19, 145.00, 134.62, 131.13, 118.19, 116.20, 115.48, 114.95, 76.16, 76.04, 57.88, 54.30, 49.20, 31.64, 28.59, 23.90, 23.32, 6.55. HPLC Rt = 3.080 min in 8 min chromatography, purity 99.4%. LC-MS Rt = 1.420 min in 4 min chromatography, purity 99.7%, MS ESI calcd. for 445.23 [M + H]+ 446.23, found 446.4.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(methyl(pyrrolidin-2-ylmethyl)amino)phenyl)acetamide (2f)
Intermediate y was synthesized from tert-butyl (S)-2-((methylamino)methyl)pyrrolidine-1-carboxylate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (52.0 mg, 0.220 mmol) and intermediate y (100 mg, 0.280 mmol) in dioxane (2 mL) was added Cs2CO3 (209 mg, 0.64 mmol), BINAP (20 mg, 0.03 mmol), and Pd(OAc)2 (7.0 mg, 0.03 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 0–2%, MeOH/CH2Cl2) to give the product as a brown solid. To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 150 mm × 40 mm × 10 μm; mobile phase: [water (NH4HCO3)-MeCN]; B%: 22–62%, 25 min). Compound 2f (25.0 mg, 30.3%) was obtained as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 10.76–10.36 (m, 1H), 9.61 (s, 1H), 8.37–8.30 (m, 2H), 8.16 (s, 1H), 7.89–7.77 (m, 1H), 7.17 (d, J = 8.8 Hz, 1H), 6.05 (s, 1H), 3.48–3.39 (m, 1H), 3.36–3.33 (m, 2H), 3.01–2.91 (m, 1H), 2.85–2.74 (m, 1H), 2.66 (s, 3H), 2.61–2.54 (m, 1H), 2.14 (s, 3H), 1.92–1.58 (m, 3H), 1.30–1.17 (m, 1H), 0.83–0.75 (m, 2H), 0.74–0.67 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.17, 156.98, 150.95, 148.20, 145.02, 137.56, 135.98, 132.98, 120.22, 114.90, 76.25, 65.21, 59.77, 51.82, 31.69, 24.21, 23.32, 22.38, 6.55. HPLC Rt= 3.426 min in 8 min chromatography, purity 99.4%. LC-MS Rt= 2.058 min in 4 min chromatography, purity 95.5%, MS ESI calcd. for 445.23 [M + H]+ 446.23, found 460.3.
N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(piperazin-1-yl)phenyl)acetamide (2g)
Intermediate y was synthesized from tert-butyl piperazine-1-carboxylate and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (302 mg, 1.29 mmol) and intermediate y (433 mg, 1.29 mmol) in dioxane (9 mL) was added Cs2CO3 (1.25 g, 3.84 mmol), BINAP (120 mg, 0.18 mmol), and Pd(OAc)2 (42.0 mg, 0.18 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by flash silica gel chromatography (eluent of 30–70%, EtOAc/petroleum ether) to give the product as a brown solid. To the product in CH2Cl2 (5 mL) was added TFA (7 mL), and the mixture was stirred at 25 °C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 100 mm × 30 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 5–96%, 10 min; B%: 4–34%, 10 min). Compound 2g (178.4 mg, 27.4%) was obtained as a light-yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.68 (s, 1H), 8.92 (s, 1H), 8.33 (m, 1H), 8.48–8.31 (m, 1H), 8.12–7.99 (m, 1H), 7.89 (d, J = 6.8 Hz, 1H), 7.15 (d, J = 9.2 Hz, 1H), 6.03 (s, 1H), 3.35–3.22 (m, 4H), 3.02–2.88 (m, 4H), 2.63–2.55 (m, 1H), 2.16 (s, 3H), 0.87–0.76 (m, 2H), 0.73–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.53, 156.91, 150.90, 148.24, 145.06, 136.92, 136.49, 133.12, 120.45, 115.14, 114.90, 112.84, 76.38, 49.29, 43.46, 39.99, 24.25, 23.34, 6.56. HPLC Rt= 1.599 min in 6 min chromatography, purity 95.6%. LC-MS Rt= 1.003 min in 4 min chromatography, purity 95.9%, MS ESI calcd. for 431.22 [M + H]+ 432.22, found 432.2.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(3-hydroxypiperidin-1-yl)phenyl)acetamide (2i)
Intermediate y was synthesized from (S)-piperidin-3-ol hydrochloride and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (75 mg, 0.32 mmol) and intermediate y (100 mg, 0.40 mmol) in dioxane (3 mL) was added Cs2CO3 (388 mg, 1.19 mmol), BINAP (37.2 mg, 0.05 mmol), and Pd(OAc)2 (12.7 mg, 0.05 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 150 × 30 mm × 5 μm; mobile phase: [water (NH4HCO3)-MeCN]; B%: 20–60%, 25 min) to give compound 2i (25.0 mg, 13.8%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.61 (s, 1H), 9.04 (s, 1H), 8.33 (s, 1H), 8.19–8.04 (m, 2H), 7.88–7.83 (m, 1H), 7.08 (d, J = 8.4 Hz, 1H), 6.03 (s, 1H), 5.09–4.87 (m, 1H), 3.80 (s, 1H), 2.86–2.76 (m, 2H), 2.75–2.67 (m, 1H), 2.65–2.54 (m, 2H), 2.13 (s, 3H), 1.95–1.81 (m, 1H), 1.77–1.55 (m, 2H), 1.54–1.42 (m, 1H), 0.83–0.75 (m, 2H), 0.74–0.67 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 168.74, 157.10, 151.04, 148.24, 145.08, 137.49, 136.04, 134.09, 120.57, 115.02, 114.57, 111.52, 76.29, 48.67, 45.67, 28.83, 24.17, 23.38, 6.61. HPLC Rt= 3.771 min in 8 min chromatography, purity 99.0%. LC-MS Rt= 2.050 min in 4 min chromatography, purity 98.3%, MS ESI calcd. for 446.22 [M + H]+ 447.22, found 447.3.
N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(methyl(oxetan-3-yl)amino)phenyl)acetamide (2j)
Intermediate y was synthesized from N-methyloxetan-3-amine and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (96.0 mg, 0.410 mmol) and intermediate y (120 mg, 0.510 mmol) in dioxane (3 mL) was added Cs2CO3 (398 mg, 1.21 mmol), BINAP (38.1 mg, 0.06 mmol), and Pd(OAc)2 (13.3 mg, 0.06 mmol) at 25 °C. The mixture was degassed, purged with N2, and heated in a microwave reactor at 130 °C for 0.5 h. The reaction mixture was concentrated in vacuo, and the residue was purified by prep-HPLC (column: Xtimate C18 150 × 40 mm × 10 μm; mobile phase: [water (NH4HCO3)-MeCN]; B%: 18–58%, 25 min). Compound 2j (4.70 mg, 5.88%) was obtained as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.65 (s, 1H), 9.06 (s, 1H), 8.34 (s, 1H), 8.19 (s, 1H), 8.10 (s, 1H), 7.86 (d, J = 8.0 Hz, 1H), 6.97 (d, J = 8.8 Hz, 1H), 6.03 (s, 1H), 4.64–4.56 (m, 2H), 4.44–4.37 (m, 2H), 4.38–4.30 (m, 1H), 2.62–2.54 (m, 1H), 2.46 (s, 3H), 2.18 (s, 3H), 0.85–0.76 (m, 2H), 0.75–0.67 (m, 2H). HPLC Rt = 4.106 min in 8 min chromatography, purity 99.9%. LC-MS Rt = 2.487 min in 4 min chromatography, purity 97.9%, MS ESI calcd. for 432.2, [M + H]+ 433.2, found 433.2.
(S)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(methyl(tetrahydrofuran-3-yl)amino)phenyl)acetamide (2k)
Intermediate y was synthesized from (S)-N-methyltetrahydrofuran-3-amine hydrochloride and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (84.3 mg, 0.361 mmol) and intermediate y (90.0 mg, 0.361 mmol) in dioxane (2 mL) was added Cs2CO3 (470 mg, 1.44 mmol), BINAP (33.7 mg, 0.054 mmol), and Pd(OAc)2 (12.2 mg, 0.054 mmol) 25 °C. The mixture was degassed, purged with N2 three times, and then heated in a microwave reactor at 120 °C for 2 h. The mixture was filtered and concentrated to give a residue that was purified by prep-HPLC purification (column: Phenomenex luna C18 150 × 25 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 30–60%, 10 min) to give product 2k (19.3 mg, 11.6% yield) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.68 (s, 1H), 9.06 (s, 1H), 8.35 (s, 1H), 8.24–8.15 (m, 2H), 7.99–7.88 (m, 1H), 7.30 (d, J = 8.8 Hz, 1H), 6.06 (s, 1H), 3.89–3.75 (m, 1H), 3.71–3.62 (m, 3H), 3.57–3.48 (m, 1H), 2.60–2.52 (m, 1H), 2.53 (s, 3H), 2.14 (s, 3H), 2.00–1.88 (m, 1H), 1.86–1.74 (m, 1H), 0.86–0.78 (m, 2H), 0.76–0.68 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 171.96, 155.87, 149.91, 149.11, 145.60, 139.08, 138.85, 129.13, 119.45, 117.37, 114.13, 78.81, 77.86, 39.99, 39.00, 23.50, 6.58. LC-MS: Rt= 0.393 min in 0.8 min chromatography, purity 100.0%, MS ESI calcd. for C23H27N8O2 [M + H]+ 447.22, found 447.1. HPLC: Rt= 1.850 min in 4 min chromatography, purity 97.4%.
(R)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(methyl(tetrahydrofuran-3-yl)amino)phenyl)acetamide (2l)
Intermediate y was synthesized from (R)-N-methyltetrahydrofuran-3-amine hydrochloride and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (98 mg, 0.421 mmol) and intermediate y (105 mg, 0.421 mmol) in dioxane (5 mL) was added Cs2CO3 (411 mg, 1.26 mmol), BINAP (39.3 mg, 0.063 mmol), and Pd(OAc)2 (14.2 mg, 0.063 mmol) 25 °C. The mixture was degassed, purged with N2 three times, and then heated in a microwave reactor at 120 °C for 2 h. The reaction mixture was filtered and concentrated under reduced pressure to give a residue that was purified by prep-TLC (SiO2, CH2Cl2/MeOH = 20/1), and the crude product was purified by prep-HPLC (column: Phenomenex Luna C18 150 × 25 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 29–59%, 8 min). Compound 2l (18.2 mg, 9.71% yield) was obtained as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.67 (s, 1H), 8.98 (s, 1H), 8.34 (s, 1H), 8.17 (d, J = 12.8 Hz, 2H), 7.92 (br d, J = 5.2 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 6.05 (s, 1H), 3.88–3.79 (m, 1H), 3.71–3.62 (m, 3H), 3.56–3.47 (m, 1H), 2.62–2.53 (m, 1H), 2.52 (s, 3H), 2.14 (s, 3H), 1.99–1.86 (m, 1H), 1.84–1.76 (m, 1H), 0.83–0.77 (m, 2H), 0.73–0.68 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 172.48, 156.90, 150.87, 148.20, 145.05, 138.50, 137.18, 134.98, 123.04, 114.87, 76.44, 70.96, 67.04, 63.59, 41.65, 30.73, 23.31, 6.53. LC-MS Rt = 0.395 min in 0.8 min chromatography, purity 44.5%, MS ESI calcd. for 446.50 [M + H]+ 447.50, found 447.1. HPLC: Rt = 1.011 min in 4 min chromatography, purity 96.7%.
(R)-N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-((tetrahydro-2H-pyran-3-yl)amino)phenyl)acetamide (2m)
Intermediate y was synthesized from (R)-tetrahydro-2H-pyran-3-amine hydrochloride and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (220 mg, 0.943 mmol) and intermediate y (235 mg, 0.943 mmol) in dioxane (6 mL) was added Cs2CO3 (915 mg, 2,83 mmol), BINAP (87.6 mg, 0.13 mmol), and Pd(OAc)2 (30.2 mg, 0.13 mmol) 25 °C. The mixture was degassed, purged with N2 three times, and then heated in a microwave reactor at 120 °C for 2 h. The mixture was filtered and concentrated to give a residue that was purified by prep-HPLC purification (column: Phenomenex Luna C18 150 mm × 25 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 26–56%, 10 min, and column: Phenomenex C18 150 × 25 mm × 10 μm; mobile phase: [water (NH4HCO3)-MeCN]; B%: 22–52%, 14 min) to give compound 2m (10 mg, 22.40 μmol, 2.38% yield) as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ 9.33 (s, 1H), 9.19 (s, 1H), 8.29 (s, 1H), 8.09 (s, 1H), 7.45 (s, 1H), 7.39 (s, 1H), 6.73 (d, J = 8.8 Hz, 1H), 5.90 (s, 1H), 4.54 (d, J = 8.0 Hz, 1H), 3.89–3.82 (m, 1H), 3.75–3.67 (m, 1H), 3.42–3.38 (m, 2H), 3.27–3.11 (m, 1H), 2.59–2.52 (m, 1H), 2.06 (s, 3H), 2.01–1.93 (m, 1H), 1.75–1.67 (m, 1H), 1.63–1.45 (m, 2H), 0.82–0.74 (m, 2H), 0.72–0.65 (m, 2H). HPLC: Rt = 1.670 min in 4 min chromatography, purity 97.6%. LC-MS Rt = 0.365 min in 0.8 min chromatography, purity 99.1%, MS ESI calcd. for 446.22 [M + H]+ 447.22, we found 447.2.
N-(5-((3-Cyano-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidin-5-yl)amino)-2-(methyl(oxetan-3-ylmethyl)amino)phenyl)acetamide (2n)
Intermediate y was synthesized from N-methyl-1-(oxetan-3-yl)methanamine hydrochloride and N-(2-fluoro-5-nitrophenyl)acetamide by procedure A. To a solution of 5-chloro-7-(cyclopropylamino)pyrazolo[1,5-a]pyrimidine-3-carbonitrile5 (293 mg, 1.26 mmol) and intermediate y (313 mg, 1.26 mmol) in dioxane (10 mL) was added Cs2CO3 (1.64 g, 5.02 mmol), BINAP (117 mg, 0.188 mmol), and Pd(OAc)2 (42.2 mg, 0.188 mmol) at 25 °C. The mixture was degassed, purged with N2, and then heated in a microwave reactor at 120 °C for 2 h. The reaction mixture was diluted with CH2Cl2/MeOH (10/1, 30 mL) and then filtered, and the filtrate was concentrated to give a crude product that was purified by flash silica gel chromatography (eluent of 30–80% EtOAc/petroleum ether). The crude product was further purified by prep-HPLC (column: Phenomenex Luna C18 150 × 25 mm × 10 μm; mobile phase: [water (HCO2H)-MeCN]; B%: 22–52%, 10 min) to give compound 2n (150.6 mg, 32.9%) as a light-yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.56 (s, 1H), 8.93 (s, 1H), 8.33 (s, 1H), 8.17 (s, 1H), 8.10 (s, 1H), 7.88 (d, J = 7.2 Hz, 1H), 7.24 (d, J = 8.8 Hz, 1H), 6.04 (s, 1H), 4.53 (t, J = 7.2 Hz, 2H), 4.22 (t, J = 6.0 Hz, 2H), 3.15–3.09 (m, 2H), 3.08–3.01 (m, 1H), 2.56 (s, 3H), 2.55–2.51 (m, 1H), 2.12 (s, 3H), 0.84–0.76 (m, 2H), 0.75–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 168.25, 156.93, 150.89, 148.20, 145.01, 136.87, 136.41, 134.17, 122.04, 114.96, 114.85, 111.72, 76.34, 74.77, 59.67, 42.84, 32.92, 24.24, 23.30, 6.52. HPLC Rt= 2.093 min in 4 min chromatography, purity 96.6%. LC-MS Rt= 1.136 min in 3 min chromatography, purity 100%, MS ESI calcd. for 446.22 [M + H]+ 447.22, found 447.2.
Acknowledgments
Constructs for NanoBRET measurements of CSNK2A1 and CSNK2A2 were provided by Promega Corporation (Madison, WI). We thank K. Saikatendu Singh (Takeda, San Diego, CA) for facilitating collaborative interactions and constructive criticism throughout the project. WuXi AppTec (Shanghai, China) provided the chemical synthesis support. Analiza, Inc. (Cleveland, OH) performed kinetic solubility and mouse liver microsome studies. Pharmaron (Beijing, China) provided the in vitro and in vivo pharmacokinetic assay support.
Glossary
Abbreviations Used:
- CSNK2A
casein kinase 2a
- PZP
3-cyano-7-cyclopropylamino-pyrazolo[1,5-a]pyrimidine
- GST
glutathione S-transferase
- MHV
mouse hepatitis virus
- MLM
mouse liver microsome
- i.v.
intravenous
- i.p.
intraperitoneal
- Cmax
maximum concentration
- AUC
area under the curve
- CLint
intrinsic clearance
- EA
ethacrynic acid
- 1-ABT
1-aminobenzotriazole
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05377.
SMARTCyp prediction of P450 metabolism of SGC-CK2-1 (1a), inhibition of 2h metabolism by 1-ABT, dose-ranging study of EA tolerability in mice by i.p. dosing, and NMR spectra (PDF)
Author Contributions
T.M.W., A.D.A., and N.J.M. conceived the study. X.Y., H.W.O., J.W.B., W.T., E.C., and A.D.A. designed the experiments and compounds. R.J.D. and J.L.S. performed the biological studies. T.M.W. wrote the manuscript. All authors read and approved the manuscript.
The Structural Genomics Consortium (SGC) is a registered charity (no. 1097737) that receives funds from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA (aka EMD in Canada and United States), Pfizer, and Takeda. Research reported in this publication was supported in part by the NC Biotech Center Institutional support grant 2018-IDG-1030, by the NIH Illuminating the Druggable Genome 1U24DK116204-01, and Department of Defense ALSRP award AL190107. This project was supported by the Rapidly Emerging Antiviral Drug Development Initiative (READDI) at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus State and Local Fiscal Recovery Funds program, appropriated by the North Carolina General Assembly. Additional funding was provided by a grant from Millennium Pharmaceuticals (Takeda).
The authors declare no competing financial interest.
Supplementary Material
References
- Li G.; Hilgenfeld R.; Whitley R.; De Clercq E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discovery 2023, 22, 1–27. 10.1038/s41573-023-00672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N.; Sharma S.; Kumar R.; Tripathi B. N.; Barua S.; Ly H.; Rouse B. T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33 (3), e00168. 10.1128/CMR.00168-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cárceles J.; Caballero E.; Gil C.; Martínez A. Kinase Inhibitors as Underexplored Antiviral Agents. J. Med. Chem. 2022, 65 (2), 935–954. 10.1021/acs.jmedchem.1c00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón A. C.; Pérez G. V.; Caballero E.; Rosales M.; Aguilar D.; Vázquez-Blomquist D.; Ramos Y.; Rodríguez-Ulloa A.; Falcón V.; Rodríguez-Moltó M. P.; Yang K.; Perera Y.; Perea S. E. Targeting of Protein Kinase CK2 Elicits Antiviral Activity on Bovine Coronavirus Infection. Viruses 2022, 14 (3), 552. 10.3390/v14030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Dickmander R. J.; Bayati A.; Taft-Benz S. A.; Smith J. L.; Wells C. I.; Madden E. A.; Brown J. W.; Lenarcic E. M.; Yount B. L. Jr.; Chang E.; Axtman A. D.; Baric R. S.; Heise M. T.; McPherson P. S.; Moorman N. J.; Willson T. M. Host Kinase CSNK2 is a Target for Inhibition of Pathogenic SARS-like β-Coronaviruses. ACS Chem. Biol. 2022, 17 (7), 1937–1950. 10.1021/acschembio.2c00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada Meza C. P.; Ruzzene M. Protein Kinase CK2 and SARS-CoV-2: An Expected Interplay Story. Kinases Phosphatases 2023, 1 (2), 141–150. 10.3390/kinasesphosphatases1020009. [DOI] [Google Scholar]
- Dowling J. E.; Chuaqui C.; Pontz T. W.; Lyne P. D.; Larsen N. A.; Block M. H.; Chen H.; Su N.; Wu A.; Russell D.; Pollard H.; Lee J. W.; Peng B.; Thakur K.; Ye Q.; Zhang T.; Brassil P.; Racicot V.; Bao L.; Denz C. R.; Cooke E. Potent and Selective Inhibitors of CK2 Kinase Identified through Structure-Guided Hybridization. ACS Med. Chem. Lett. 2012, 3 (4), 278–283. 10.1021/ml200257n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. I.; Drewry D. H.; Pickett J. E.; Tjaden A.; Krämer A.; Müller S.; Gyenis L.; Menyhart D.; Litchfield D. W.; Knapp S.; Axtman A. D. Development of a Potent and Selective Chemical Probe for the Pleiotropic Kinase CK2. Cell Chem. Biol. 2021, 28 (4), 546–558. 10.1016/j.chembiol.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E.; Alimzhanov M.; Bao L.; Block M. H.; Chuaqui C.; Cooke E. L.; Denz C. R.; Hird A.; Huang S.; Larsen N. A.; Peng B.; Pontz T. W.; Rivard-Costa C.; Saeh J. C.; Thakur K.; Ye Q.; Zhang T.; Lyne P. D. Structure and Property Based Design of Pyrazolo[1,5-a]pyrimidine Inhibitors of CK2 Kinase with Activity in Vivo. ACS Med. Chem. Lett. 2013, 4 (8), 800–805. 10.1021/ml400197u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E.; Alimzhanov M.; Bao L.; Chuaqui C.; Denz C. R.; Jenkins E.; Larsen N. A.; Lyne P. D.; Pontz T.; Ye Q.; Holdgate G. A.; Snow L.; O’Connell N.; Ferguson A. D. Potent and Selective CK2 Kinase Inhibitors with Effects on Wnt Pathway Signaling in Vivo. ACS Med. Chem. Lett. 2016, 7 (3), 300–305. 10.1021/acsmedchemlett.5b00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnon K. H. 3rd; Leist S. R.; Schäfer A.; Edwards C. E.; Martinez D. R.; Montgomery S. A.; West A.; Yount B. L. Jr.; Hou Y. J.; Adams L. E.; Gully K. L.; Brown A. J.; Huang E.; Bryant M. D.; Choong I. C.; Glenn J. S.; Gralinski L. E.; Sheahan T. P.; Baric R. S. A Mouse-adapted Model of SARS-CoV-2 to Test COVID-19 Countermeasures. Nature 2020, 586 (7830), 560–566. 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist S. R.; Dinnon K. H. 3rd; Schäfer A.; Tse L. V.; Okuda K.; Hou Y. J.; West A.; Edwards C. E.; Sanders W.; Fritch E. J.; Gully K. L.; Scobey T.; Brown A. J.; Sheahan T. P.; Moorman N. J.; Boucher R. C.; Gralinski L. E.; Montgomery S. A.; Baric R. S. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 2020, 183 (4), 1070–1085. 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg P.; Gloriam D. E.; Olsen L. The SMARTCyp Cytochrome P450 Metabolism Prediction Server. Bioinformatics 2010, 26 (23), 2988–2989. 10.1093/bioinformatics/btq584. [DOI] [PubMed] [Google Scholar]
- Rydberg P.; Gloriam D. E.; Zaretzki J.; Breneman C.; Olsen L. SMARTCyp: A 2D Method for Prediction of Cytochrome P450-Mediated Drug Metabolism. ACS Med. Chem. Lett. 2010, 1 (3), 96–100. 10.1021/ml100016x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzack J. D.; Kirchmair J. Computational Methods and Tools to Predict Cytochrome P450 Metabolism for Drug Discovery. Chem. Biol. Drug Des. 2019, 93 (4), 377–386. 10.1111/cbdd.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. F. V.; Dickins M. Baseline Lipophilicity Relationships in Human Cytochromes P450 Associated with Drug Metabolism. Drug Metab. Rev. 2003, 35 (1), 1–18. 10.1081/DMR-120018245. [DOI] [PubMed] [Google Scholar]
- Di L.; Keefer C.; Scott D. O.; Strelevitz T. J.; Chang G.; Bi Y. A.; Lai Y.; Duckworth J.; Fenner K.; Troutman M. D.; Obach R. S. Mechanistic Insights from Comparing Intrinsic Clearance Values Between Human Liver Microsomes and Hepatocytes to Guide Drug Design. Eur. J. Med. Chem. 2012, 57, 441–448. 10.1016/j.ejmech.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Potęga A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules 2022, 27 (16), 5252. 10.3390/molecules27165252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll J. P.; Yadav A. S.; Shah N. R. Role of Glucuronidation and P450 Oxidation in the Bioactivation of Bromfenac. Chem. Res. Toxicol. 2018, 31 (4), 223–230. 10.1021/acs.chemrestox.7b00293. [DOI] [PubMed] [Google Scholar]
- MacFaul P. A.; Morley A. D.; Crawford J. J. A Simple In Vitro Assay for Assessing the Reactivity of Nitrile Containing Compounds. Bioorg. Med. Chem. Lett. 2009, 19 (4), 1136. 10.1016/j.bmcl.2008.12.105. [DOI] [PubMed] [Google Scholar]
- Oakley A. Glutathione Transferases: a Structural Perspective. Drug Metab. Rev. 2011, 43 (2), 138. 10.3109/03602532.2011.558093. [DOI] [PubMed] [Google Scholar]
- Allocati N.; Masulli M.; Di Ilio C.; Federici L. Glutathione Transferases: Substrates, Inihibitors and Pro-drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7 (1), 8. 10.1038/s41389-017-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemen J. H. T. M.; Ommen B. V.; Bogaards J. J. P.; Van Bladeren P. J. Ethacrynic Acid and its Glutathione Conjugate as Inhibitors of Glutathione S-transferases. Xenobiotica 1993, 23 (8), 913–923. 10.3109/00498259309059418. [DOI] [PubMed] [Google Scholar]
- Townsend D. M.; Tew K. D. The Role of Glutathione-S-transferase in Anti-cancer Drug Resistance. Oncogene 2003, 22 (47), 7369–7375. 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Gast S. M.; Endo T.; Lu D.; Carson D.; Schmidt-Wolf I. G. H. In Vivo Efficacy of the Diuretic Agent Ethacrynic Acid Against Multiple Myeloma. Leuk. Res. 2012, 36 (5), 598–600. 10.1016/j.leukres.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Liu B.; Huang X.; Hu Y.; Chen T.; Peng B.; Gao N.; Jin Z.; Jia T.; Zhang N.; Wang Z.; Jin G. Ethacrynic Acid Improves the Antitumor Effects of Irreversible Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Breast Cancer. Oncotarget 2016, 7 (36), 58038–58050. 10.18632/oncotarget.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.; Kim Y.; Gast S. M.; Endo T.; Lu D.; Carson D.; Schmidt-Wolf I. G. H. Increased In Vivo Efficacy of Lenalidomide and Thalidomide by Addition of Ethacrynic Acid. In Vivo 2011, 25 (3), 325–333. [PubMed] [Google Scholar]
- de Montellano P. R. O. 1-Aminobenzotriazole: A Mechanism-Based Cytochrome P450 Inhibitor and Probe of Cytochrome P450 Biology. Med. Chem. 2018, 8 (3), 038–065. 10.4172/2161-0444.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi M.; Borgo C.; Pinna L. A.; Ruzzene M. Targeting CK2 in Cancer: a Valuable Strategy or a Waste of Time?. Cell Death Discovery 2021, 7 (1), 325. 10.1038/s41420-021-00717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack M.; Gotz C.; Wrublewsky S.; Montenarh M. SGC-CK2-1 Is an Efficient Inducer of Insulin Production and Secretion in Pancreatic β-Cells. Pharmaceutics 2021, 14 (1), 19. 10.3390/pharmaceutics14010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boewe A. S.; Wemmert S.; Kulas P.; Schick B.; Gotz C.; Wrublewsky S.; Montenarh M.; Menger M. D.; Laschke M. W.; Ampofo E. Inhibition of CK2 Reduces NG2 Expression in Juvenile Angiofibroma. Biomedicines 2022, 10 (5), 966. 10.3390/biomedicines10050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.; Kinoshita C.; Axtman A. D.; Young J. E. Evaluation of a Selective Chemical Probe Validates That CK2Mediates Neuroinflammation in a Human Induced Pluripotent Stem Cell-Derived Mircroglial Model. Front. Mol. Neurosci. 2022, 15, 824956 10.3389/fnmol.2022.824956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Shoyaib A.; Archie S. R.; Karamyan V. T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies?. Pharm. Res. 2019, 37 (1), 12. 10.1007/s11095-019-2745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon P. J.; Heinemann H. O.; Stason W. B.; Laragh J. H. Ethacrynic Acid: Effectiveness and Mode of Diuretic Action in Man. Circulation 1965, 31, 5–18. 10.1161/01.CIR.31.1.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.