ABSTRACT
Membranes are a universal barrier to all cells. Phospholipids, essential bacterial membrane components, are composed of a polar head and apolar fatty acid (FA) chains. Most bacterial FAs are synthesized by the Type II FA synthesis pathway (FASII). In Streptococcaceae, Enterococci, and Lactococcus lactis, a unique feedback mechanism controls the FASII gene expression. FabT, encoded in the FASII main locus, is the repressor, and it is activated by long-chain acyl-acyl carrier protein (acyl-ACP). Many Streptococci, Enterococcus faecalis, but not L. lactis, possess two ACPs. The AcpA-encoding gene is within the FASII locus and is coregulated with the FASII genes. Acyl-AcpA is the end product of FASII. The AcpB-encoding gene is in operon with plsX encoding an acyl-ACP:phosphate acyltransferase. The role of acyl-AcpB as FabT corepressor is controversial. Streptococcus pyogenes, which causes a wide variety of diseases ranging from mild non-invasive to severe invasive infections, possesses AcpB. In this study, by comparing the expression of FabT-controlled genes in an acpB-deleted mutant with those in a wild-type and in a fabT mutant strain, grown in the presence or absence of exogenous FAs, we show that AcpB is the S. pyogenes FabT main corepressor. Its deletion impacts membrane FA composition and bacterial adhesion to eucaryotic cells, highlighting the importance of FASII control.
Importance
Membrane composition is crucial for bacterial growth or interaction with the environment. Bacteria synthesize fatty acids (FAs), membrane major constituents, via the Type II FAS (FASII) pathway. Streptococci control the expression of the FASII genes via a transcriptional repressor, FabT, with acyl-acyl carrier proteins (ACPs) as corepressor. Streptococcus pyogenes that causes a wide variety of diseases ranging from mild non-invasive to severe invasive infections possesses two ACPs. acpA, but not acpB, is a FASII gene. In this study, we show that acyl-AcpBs are FabT main corepressors. Also, AcpB deletion has consequences on the membrane FA composition and bacterial adhesion to host cells. In addition to highlighting the importance of FASII control in the presence of exogeneous FAs for the adaptation of bacteria to their environment, our data indicate that FASII gene repression is mediated by a corepressor whose gene expression is not repressed in the presence of exogenous FAs.
KEYWORDS: Streptococcus pyogenes, fatty acid synthesis, regulation, Acyl-ACP, corepressor
INTRODUCTION
Cell membranes commonly comprise a phospholipid bilayer; phospholipids are composed of a polar head and an apolar body constituted of fatty acids (FAs). FA structure and length are decisive for membrane topology and properties such as fluidity, permeability, and integrity. These features are crucial for the adaptation of bacteria to various environments (1). The FAs are synthesized by the Type II FA synthesis pathway (FASII) that is widespread among bacteria. It consists of an initiation phase followed by a recursive elongation cycle, which produces acyl-acyl carrier protein (acyl-ACP). In Streptococci, unsaturated FAs are produced via a shunt of this cycle that is catalyzed by FabM, a trans-2, cis-3-decenoyl-ACP isomerase. The shunted step that leads to saturated FAs is catalyzed by FabK, an enoyl-(acyl-carrier protein) reductase. In contrast to the FASII pathway, the regulation of the FASII genes is not conserved among bacteria. In Streptococci, Enterococci, and Lactococcus lactis, a unique feedback mechanism controls the expression of the FASII genes, involving the transcriptional repressor, FabT [for review (2)]. FabT, a member of the MarR family of regulators, is encoded in the FASII main locus. The role of FabT as a FASII repressor can readily be observed by the repression of the FASII genes in the wild-type strain grown in the presence of exogenous FAs (eFAs) and the loss of this repression in fabT mutant strains (3, 4). Depending on the Streptococcus species, the fabM gene is either not controlled or less repressed by FabT than the fabK gene. As a consequence, there is, in addition to lengthening of the FA chains, a shift in the saturated FAs/unsaturated FAs ratio in the membrane phospholipids of fabT mutant strains (4 – 6).
As initially demonstrated in Streptococcus pneumoniae, FabT possesses an unconventional corepressor, acyl-ACPs [(7) for review (2)]. FabT–acyl-ACP binding to its DNA target varies according to the acyl length, increasing with long-chain acyls (7). As suggested in S. pneumoniae and confirmed in Enterococcus faecalis, it also depends on the saturation state of the FA, the cis unsaturated FA maximizing FabT-DNA affinity (3). In addition to the acyl moiety, the ACP may differ. Indeed, in many Streptococci, as well as in E. faecalis, but not in L. lactis, there are two ACPs. AcpA is encoded within the FASII locus, and acyl-AcpA is the end product of the FASII pathway. The AcpB-encoding gene is found in the same operon as plsX; PlsX, an acyl-ACP-phosphate acyltransferase, is involved in the conversion between acyl-ACP and Acyl-PO4 3- and in the phospholipid biosynthetic pathway (8 – 10). The expression of acpB is, in contrast to that of acpA, not controlled by FabT (3, 6). The role of AcpB in FASII gene control is controversial, being seemingly dependent on the species. In S. pneumoniae, FabT–acyl-ACP DNA binding affinity tests using purified FabT and acyl-AcpA or acyl-AcpB indicated that only AcpB increased DNA binding (8). In E. faecalis, acpB mutant and wild-type strains had similar fabT gene expression, even in the presence of eFAs that are incorporated and induce FabT-mediated repression (3, 11). The role of AcpB as a FabT corepressor thus remains an open question.
In the case of Streptococcus pyogenes, two ACPs have been identified (10). S. pyogenes and S. pneumoniae AcpBs share 43% identity and 69% similarity (2). S. pyogenes is a Gram-positive strictly human pathogen that yields from mild superficial infections to more invasive infections; S. pyogenes infections also induce post-infectious complications (12). Altogether, S. pyogenes infections are responsible for approximately 517,000 deaths annually worldwide (13).
To determine whether, and to what extent, AcpB is a FabT corepressor in S. pyogenes, we studied the effects of deleting acpB on FASII gene expression in bacteria grown in the presence or absence of eFAs. We also determined the consequences of acpB deletion on FA composition of membrane phospholipids as well as on S. pyogenes–host cell interaction.
RESULTS AND DISCUSSION
S. pyogenes AcpB is a FabT corepressor
A strain expressing a FabTH105Y point mutation, named mFabT, was constructed in the reference strain M28PF1 [an emm28 strain, referred to as wild type (WT)] (10, 14). We initially constructed, in the emm28 background, a fabT-deleted strain. However, it displayed poor growth in laboratory media (THY, THY-Tween 80) [our results to be published elsewhere (4)]. This correlates with the delayed or poor growth of other S. pyogenes fabT-deleted mutants in laboratory medium (6, 15) and with a report describing the impossibility to construct one without deleting other genes (16). The histidine in position 105 was chosen because it was identified in isolates cultured from nonhuman primates and it is contained within the α5 helix that is involved in dimerization and in the interaction with acyl-ACPs (6, 8). The mFabT strain has a weakened capacity to repress fabT expression (14). An acpB-deleted strain, ΔAcpB, was constructed in the WT strain. The back-to-the wild-type (BWT) strain was added as a control: it was obtained during the ΔAcpB strain construction and possesses a wild-type acpB gene as well as the same single surreptitious mutation as the ΔAcpB strain (see Material and Methods section) (Table S1). Growths of WT, BWT, ΔAcpB, and mFabT strains were compared in the laboratory medium THY, supplemented or not with an exogenous source of unsaturated FA (here 0.1% Tween 80, source of oleic acid C18:1Δ9) (Fig. S1). All strains grew similarly.
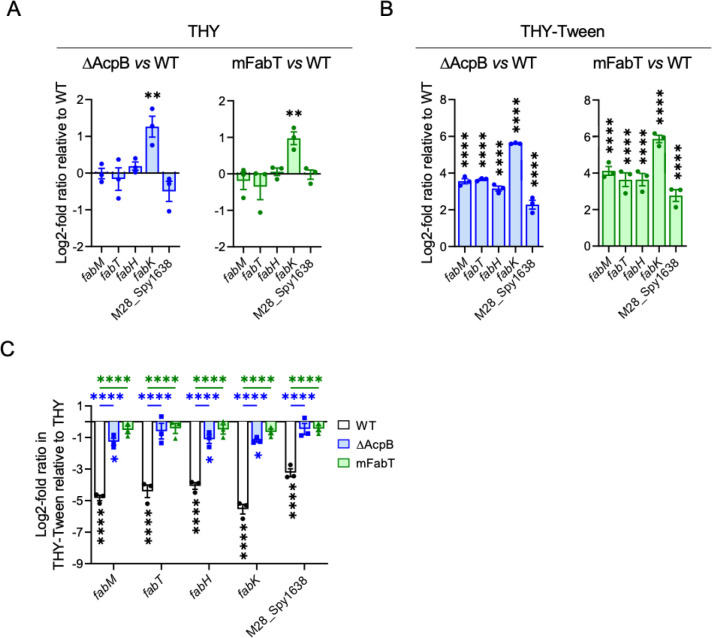
To test the role of AcpB as a FabT corepressor, we analyzed by qRT-PCR the effect of the acpB deletion on the expression of a set of genes controlled by FabT: FASII genes and, in addition, a gene that is coregulated with the FASII genes although it does not belong to the FASII locus, namely, M28_Spy1638 (2, 14). Expression was assayed in the absence or the presence of Tween 80 (Fig. 1). The expression of the set of genes was first compared in the BWT and the WT strains and found to be unchanged (Fig. S2). Data were then analyzed by comparing results pairwise between ΔAcpB and WT or mFabT and WT strains in each medium (Fig. 1A and B). In THY, the expression of fabK is higher in the acpB mutant than in the WT strain (Fig. 1A). In THY-Tween 80, all the tested genes were more expressed in the acpB mutant strain (Fig. 1B). These data suggested that AcpB is involved in the FA-dependent FASII gene repression. Similar results were obtained with the mFabT mutant strain. In Lactobacillales, FASII genes are repressed in the presence of eFAs (17). This is an advantage for bacteria that can use eFAs to synthesize their FAs since this process is energy-consuming (2). Our data show that the H105Y point mutation suffices to limit FabT repression capacity. In both strains, and except for the fabK operon, the derepression is only observed in the presence of eFAs. This suggests that in the absence of eFAs, a steady state of the FASII gene expression rate is reached that does not necessitate repression of all the FASII genes.
Fig 1.
The ΔAcpB strain has lost most of the eFA-related transcriptional repression capacity. WT, ΔAcpB, and mFabT strains were grown in THY (A, C) or THY-Tween (B, C), and RNAs were quantified by qRT-PCR. Expression was normalized to that of gyrA; relative gene expression is expressed as the log2-fold ratio in ΔAcpB vs WT strain (right, A and B) and mFabT vs WT strain (left, A and B). (C) The relative gene expression is expressed as the log2-fold ratio in a given strain grown in THY-Tween vs in THY. White, WT; blue, ΔAcpB; green, mFabT. Significance of differences: stars below bars, in the two media for a given strain; stars above bars, in the two media between WT and ΔAcpB or WT and mFabT. No statistically significant differences were found between ΔAcpB and mFabT. N = 3. Two-way ANOVA, Bonferroni post-test, *P < 0.05, **P < 0.01, and ****P < 0.0001.
To further analyze the mechanistic interaction between FabT and AcpB, we compared the ΔAcpB and mFabT strain repression defects in the presence of eFAs (Fig. 1C, stars below the columns). eFA addition led to a substantial repression between 12- and 25-fold for all the genes tested in the WT strain. Importantly, whereas there was a weaker, around twofold, but significant repression for three out of the five genes tested in the ΔAcpB mutant, there was no repression in the mFabT mutant strain. This indicates that the ΔAcpB strain has lost less of its repression capacity than the mFabT strain. However, whereas the repression defect between each mutant strain and the WT strain was significant, that between the ΔAcpB and the mFabT was not (Fig. 1C, stars above the columns). This suggests that AcpB’s role on the FabT-dependent eFA repression of the FASII gene repression is predominant. Altogether, these results demonstrate that S. pyogenes AcpB is a major player in the transcriptional repression of the FASII genes in the presence of long unsaturated FAs which maximize FabT-DNA affinity (3).
The growth difference between the ΔAcpB and the ΔFabT strains is probably due to the incomplete derepression in the former strain that would enable the ΔAcpB strain to somewhat adapt to its environment. That the mFabT strain grows better than the ΔFabT, in fact like the ΔAcpB strain, suggests that FabT-H105Y protein has not lost all its activity.
Our conclusion on the role of AcpB in S. pyogenes is in sharp contrast with that obtained in E. faecalis, where fabT expression was similar in an acpB mutant and the wild-type strain (3). It is in agreement with the important role described for S. pneumoniae AcpB in FabT-dependent regulation of gene expression as shown by a biochemical approach (8). Hence, our data strongly suggest that in S. pyogenes, acyl-AcpB is a major corepressor of FabT. Yet, in the absence of AcpB, a weak repression exists, indicating a role for another corepressor. FabT corepressors are acyl-ACPs (7). There are only two ACPs in S. pyogenes (2, 10). This suggests that S. pyogenes AcpA may also acts as a FabT corepressor. The AcpB- and FabT-dependent repressions were observed when Tween was added. Tween is mainly composed of C18:1Δ9, and long-chain unsaturated FAs are the acyls maximizing FabT-DNA affinity (3). However, AcpA and AcpB may bind preferentially different types of acyl chains, and consequently, their role in FA synthesis and in FASII gene repression may depend on the eFAs present. The latter vary in the various environments encountered during an infection. The relative role of each Acp in S. pyogenes needs to be further investigated. Altogether, the corepressing role of acyl-AcpBs may be different in various species and warrants further analysis in other organisms.
AcpB deletion modifies the S. pyogenes membrane FA composition
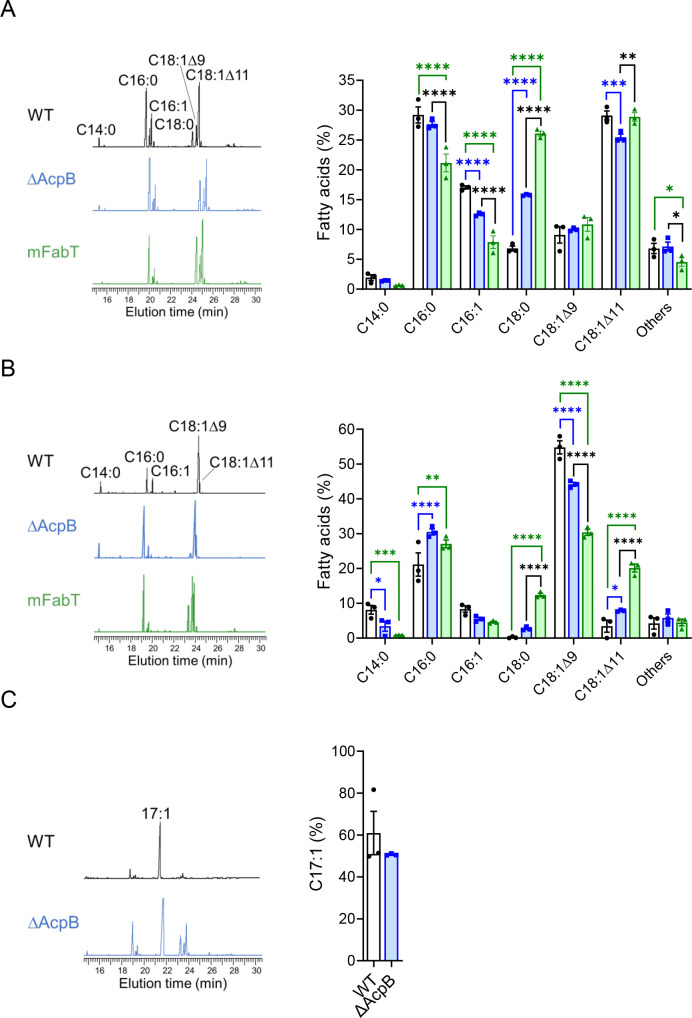
acpB deletion and fabT mutation lead to a derepression of FASII gene expression, and FabT controls the FA chain length and unsaturated/saturated FA ratio (4, 5). We therefore analyzed the consequences of acpB deletion on the FA composition in S. pyogenes phospholipids in the absence or presence of Tween 80 in the medium (Fig. 2A and B; Table S2). As a control, the membrane FA composition of the BWT and the WT strains was compared and found to be similar (Fig. S3A and B; Table S2).
Fig 2.
The FA membrane composition of the ΔAcpB strain differs from that of WT and mFabT strains. FA composition of WT, ΔAcpB, and mFabT strains grown in (A) THY medium and (B) THY-Tween; (C) THY-C17:1. (A, B, and C) Left, FA profiles; right, quantified proportions of major FAs. N = 3 (Table S2). (A and B) Right, two-way ANOVA, Bonferroni post-test; (C) t-test, no statistical difference; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
We compared the membrane FA composition of the ΔAcpB strain and the WT control. In the absence of Tween 80, ΔAcpB phospholipids contained less unsaturated FAs (C16:1 and C18:1Δ11) and more saturated and longer FAs (C18:0) than the WT strain phospholipids (15.78% and 6.82%, respectively) (Fig. 2A). Thus, the deletion of acpB altered the phospholipid composition in favor of more saturated and longer chain FAs. In the presence of Tween 80, source of oleic acid C18:1Δ9, the ΔAcpB membrane contained 1.2-fold less C18:1Δ9 (44.18% vs 54.8%) and twofold more C18:1Δ11 (7.88% vs 3.41%) than the WT strain (Fig. 2B). The weaker C18:1Δ9 percentage in the ΔAcpB membrane than in the WT strain may result from an incorporation defect. We evaluated FA incorporation in medium supplemented with C17:1 (THY-C17:1), which is not synthesized by bacteria (Fig. 2C; Fig. S3C). The ΔAcpB strain was not impacted in its C17:1 incorporation, accounting for the mild C18:1Δ9 difference. The same type of differences was observed in the FA phospholipid composition between the mFabT and the WT strain in both growth conditions, more saturated and longer chain FAs in the mFabT strain (Fig. 2A and B).
The longer acyl chains are a direct consequence of the derepression of the FASII genes in the mutant strains. Also, the higher concentration of saturated FAs is due to the derepression of fabK, but not of fabM, in the absence of Tween, and to a stronger derepression of fabK than of fabM in its presence. Such modifications in a fabT mutant strain have already been described in S. pneumoniae (5).
We then compared ΔAcpB and mFabT membrane FA composition in order to estimate the contribution of AcpB on the impact of FabT on phospholipid FAs (Fig. 2A and B, black stars, Table S2). In both media, the differences between each mutant strain and the WT strain resembled one another. However, the FA composition of the ΔAcpB and mFabT strains differed. In THY, the ΔAcpB membrane contained more C16:1 (12.55% vs 7.88%) and less C18:0 (15.78% vs 26.08%) and C18:1Δ11 (25.46% vs 28.88%) than that of the mFabT strain (Fig. 2A). Thus, in THY, the ΔAcpB FAs were shorter than those of the mFabT strain. In the presence of the eFAs, brought by addition of Tween 80, differences were also notable with less C18:0 (2.72% vs 12.39%) and C18:1Δ11 (7.88% vs 20.12%) and more C18:1Δ9 (44.18 vs 30.36) in the ΔAcpB vs the mFabT strain (Fig. 2B). These results indicate that the impact on the FA composition of the acpB deletion, both in the absence and in the presence of eFAs, was not as important as that of fabT mutation. As mentioned above, eFA-induced repression was totally lost in the mFabT strain but not in the ΔAcpB strain (Fig. 1C, stars below the columns). This difference yields a higher FASII enzyme synthesis in the mFabT strain that, in turn, affects more the phospholipid FA composition in the mFabT than in the ΔAcpB strain.
Our data indicate that AcpB, as a FabT corepressor, controls the membrane FA composition. They also support that S. pyogenes acyl-AcpBs are the main but not the sole FabT corepressors. This supports our suggestion that in the absence of AcpB, acyl-AcpAs are corepressors. Indeed, Acyl-AcpAs result both from the interaction of de novo-synthesized acyls and, in the presence of eFAs, from the reverse PlsX reaction that catalyzes the production of acyl-ACP from the FakAB-synthesized acyl-phosphates (2, 9). In consequence, and since the mFabT and ΔAcpB membrane FA compositions are different both in the presence and the absence of eFAs, acyl-AcpAs may operate in both conditions.
The ΔAcpB strain displays a defect in adhesion to eucaryotic cells
In S. pneumoniae, fabT-deleted strain displayed a decreased adhesion capacity (18). We thus wondered whether the ΔAcpB strain would display an adhesion defect. As for all previous phenotypes, the BWT strain had the same adhesion capacity as the WT strain (Fig. S4). We then analyzed the adhesion efficiency of the ΔAcpB strain; it is defective (Fig. 3). Furthermore, no adhesion difference was observed between the ΔAcpB and mFabT strains. In S. pneumoniae, the adhesion defect is a consequence of the indirect activation of the capsule synthesis by FabT (18). The emm28 strains do not produce a capsule (10, 19, 20). They synthesize about twenty adhesins that are covalently anchored to the peptidoglycan via a membrane enzyme named sortase (21, 22). The modification in the membrane FA composition may lead to sortase defective activity that in turn yields a faulty set-up of some adhesins. Our data indicate that although there is a difference in the ΔAcpB and the mFabT strain membrane FA composition, both strains had a similar adhesion defect. This suggests that the FA composition variation between the ΔAcpB and the WT strains is sufficient to impact adhesion.
Fig 3.
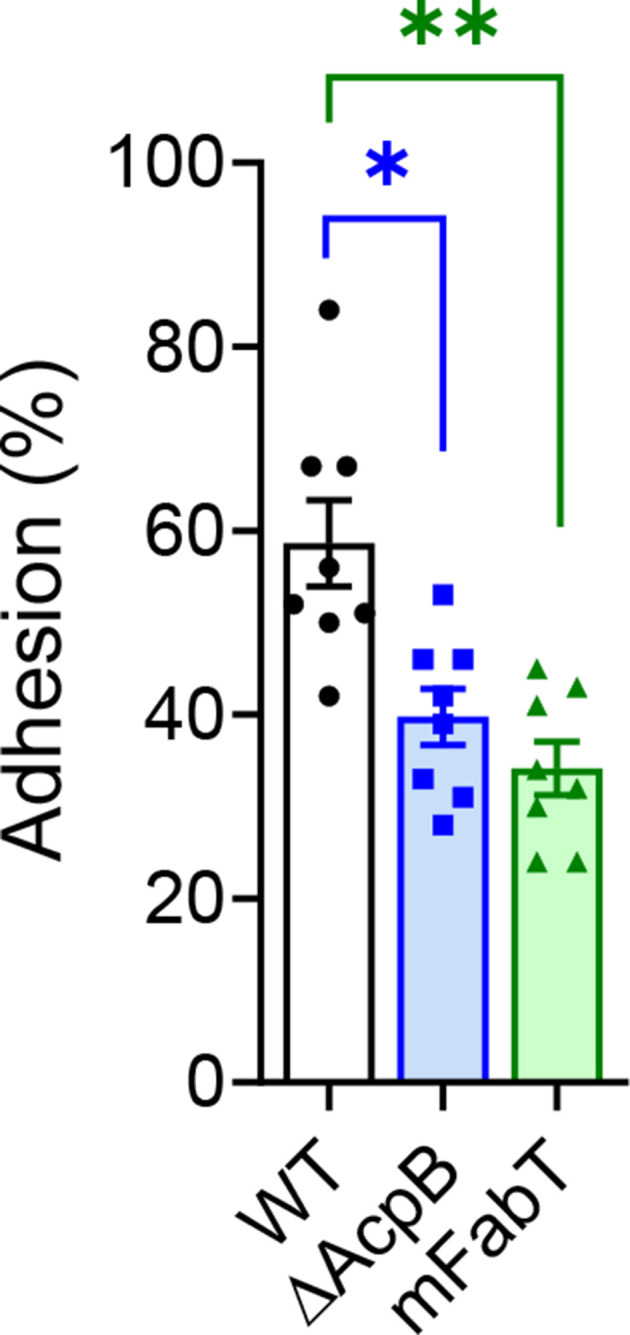
The ΔAcpB mutant strain has an adhesion defect to human cells. Adhesion capacity to human endometrial cells of WT, ΔAcpB, and mFabT strains. Bars and symbols, white, WT strain; blue, ΔAcpB strain; green, mFabT strain. N = 8; one-way ANOVA, Bonferroni post-test, *P < 0.05 and **P < 0.001.
In conclusion, in the presence of long-chain unsaturated FA, acyl-AcpBs are FabT main corepressors in S. pyogenes and most probably other Streptococci. Indeed, such a role for AcpB was described in S. pneumoniae using DNA-binding affinity experiments (8 – 10). That it is not the sole FabT corepressor is supported by the fact that, in contrast to the strain deleted for fabT in the same genetic background [our unpublished results (4)], the ΔAcpB strain grows like the WT strain in laboratory media. Also, the ΔAcpB and the mFabT membrane composition differs, the variation to the WT strain being more important in the mFabT strain. In addition, AcpB cannot be the major FabT corepressor in all fabT-harboring species. Indeed, acpB is absent or severely truncated in over half the Streptococcus agalactiae-sequenced strains (2). Moreover, in an E. faecalis ΔAcpB strain, FA synthesis is decreased in the presence of unsaturated eFAs indicating that FabT-dependent transcription repression is retained (3). Furthermore, there is no AcpB in L. lactis. Finally, in S. pyogenes, an acpB deletion, through AcpB contribution to FabT transcription regulation of FASII, weakens bacteria–host cell adhesion, supporting the importance of the FASII regulation for bacterial interaction with its host.
MATERIALS AND METHODS
Bacterial strains and culture conditions
The strains used in this study are described in Table S1. S. pyogenes strains were grown under static condition at 37°C in Todd Hewitt broth supplemented with 0.2% east extract (THY) or on THY agar plates (THYA). To study, the role of eFA addition or eFA incorporation, the medium was supplemented with 0.1% Tween 80 (THY-Tween) (Sigma-Aldrich, P1754), essentially composed of C18:1Δ9 or 100 µM C17:1 (Larodan, Sweden). Overnight cultures were diluted to an OD600nm = 0.05 and grown in the indicated medium to the exponential phase (OD600nm comprising between 0.4 and 0.5).
Strain construction
The primers used for the generation of the plasmids and verifying the different strains are described in Table S3. The ΔAcpB were obtained by homologous recombination of the plasmid pG1AcpB following the same protocol as described previously (23, 24). The DNA fragments encompassing fabT were cloned in BamHI–EcoRI-digested pG1 using the In-Fusion Cloning Kit (Clontech) (25). This led to the deletion of acpB from nucleotides 14 to 203, relative to the translation initiation site. The back-to-the wild type, i.e., reversion of the single cross-over and restoration of a wild-type acpB, was carried out as previously described (26). The whole genomes of the constructed strains were sequenced; one identical surreptitious mutation was found in both the ΔAcpB (accession number, SRR24223284) and the BWT (accession number, SRR24223283) strains in priA; the G in position 1073 was replaced by a T leading to the replacement of the 358th amino acid residue, a tryptophane by a leucine.
RNA isolation and first-strand cDNA synthesis, quantitative PCR (qPCR)
S. pyogenes strains were cultured at 37°C in THY or THY-Tween, and cells were harvested at exponential growth phase (OD600nm comprising between 0.4 and 0.5). RNA isolation, cDNA synthesis, and quantitative PCR were carried out as previously described (4).
Fatty acid analysis
Strains were grown in THY, THY-Tween, or THY-C17:1 until OD600nm = 0.4–0.5. FAs were extracted and analyzed as previously described (10, 26 – 28) . Briefly, analyses were performed in a split-splitless injection mode on an AutoSystem XL Gas Chromatograph (Perkin-Elmer) equipped with a ZB-Wax capillary column (30 m × 0.25 mm × 0.25 mm; Phenomenex, France). Data were recorded and analyzed by TotalChrom Workstation (Perkin-Elmer). FA peaks were detected between 12 and 40 min of elution and identified by comparing with retention times of purified esterified FA standards (Mixture ME100, Larodan, Sweden). Results are shown as percent of the specific FA compared with total peak areas (TotalChrom Workstation; Perkin-Elmer).
Cell culture
HEC-1-A (ATCC_ HTB-112TM) endometrial epithelial cells were cultured as recommended, in McCoy’s 5A Medium (Gibco, Ref. 26600080) supplemented with 10% fetal bovine serum at 37°C, 5% of CO2.
Bacterial adhesion capacity
S. pyogenes adhesion capacity was realized as described previously after growing the bacteria in THY to an OD600nm of 0.4 to 0.5 (23). Confluent HEC-1-A cells in 24-well plates were washed three times, bacteria were added at a multiplicity of infection of 1, and plates were centrifuged for 5 min at 1,000 rpm to synchronize bacterial adhesion. After 1 h of incubation at 4°C, the cells were washed three times with PBS and lysed with distilled water. Serial dilutions of cellular lysates were plated on THYA plates, and the number of CFUs was determined after 24 h of growth at 37°C. The values were normalized to the inoculum for each experiment.
Statistical analysis
Data were analyzed with GraphPad Prism version 9.4.1. The tests used are indicated in the figure legends. Statistical significance is indicated by ns (not significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
ACKNOWLEDGMENTS
We thank Alexandra Gruss for her constant interest and support. We thank Tiphaine Pouillon and Maëva Gauduin, undergraduates in the laboratory, for their technical help.
C.L. was supported by Université Paris Cité (BioSPC, n°51809666), FRM (FDT202106012831), and FEMS (FEMS Congress Attendance Grant for poster n°7875 in 2021 and FEMS grant LISSSD 2022 n°LISS-213065). Q.Z. was supported by bourse SSHN jeune chercheur and innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20212700). This work was supported by DIM One Health (RPH17043DJA).
Footnotes
This article was submitted via the Active Contributor Track (ACT). Agnes Fouet, the ACT-eligible author, secured reviews from Stephen B. Melville, Virginia Tech, Gregory Bokinsky, Technische Universiteit Delft, Jesus Eraso, Houston Methodist Academic Institute, and Li Qiong, University of Science and Technology of China Hefei.
Contributor Information
Clara Lambert, Email: clara.lambert@inserm.fr.
Agnes Fouet, Email: agnes.fouet@inserm.fr.
Michael J. Federle, University of Illinois Chicago, Chicago, Illinois, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jb.00274-23.
Supplemental Figures S1 to S4.
Methods used solely for the supplemental figures.
Supplemental Tables S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Zhang YM, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233. doi: 10.1038/nrmicro1839 [DOI] [PubMed] [Google Scholar]
- 2. Lambert C, Poyart C, Gruss A, Fouet A. 2022. FabT, a bacterial transcriptional repressor that limits futile fatty acid biosynthesis. Microbiol Mol Biol Rev 86:e0002922. doi: 10.1128/mmbr.00029-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zou Q, Zhu L, Cronan JE. 2022. The Enterococcus faecalis Fabt transcription factor regulates fatty acid biosynthesis in response to exogeneous fatty acids. Front Microbiol 13:877582. doi: 10.3389/fmicb.2022.877582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambert C, Bachmann C, Gaillard M, Hautcoeur A, Gloux K, Guilbert T, Méhats C, Prost B, Solgadi A, Abreu S, Andrieu M, Poyart C, Gruss A, Fouet A. 2023. The double-edged role of FASII regulator FabT in Streptococcus pyogenes infection. Biorxiv. doi: 10.1101/2023.06.07.543851 [DOI]
- 5. Lu YJ, Rock CO. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol Microbiol 59:551–566. doi: 10.1111/j.1365-2958.2005.04951.x [DOI] [PubMed] [Google Scholar]
- 6. Eraso JM, Olsen RJ, Beres SB, Kachroo P, Porter AR, Nasser W, Bernard PE, DeLeo FR, Musser JM. 2016. Genomic landscape of intrahost variation in group A Streptococcus: repeated and abundant mutational inactivation of the FabT gene encoding a regulator of fatty acid synthesis. Infect Immun 84:3268–3281. doi: 10.1128/IAI.00608-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jerga A, Rock CO. 2009. Acyl-Acyl carrier protein regulates transcription of fatty acid biosynthetic genes via the FabT repressor in Streptococcus pneumoniae. J Biol Chem 284:15364–15368. doi: 10.1074/jbc.C109.002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuo G, Chen ZP, Jiang YL, Zhu Z, Ding C, Zhang Z, Chen Y, Zhou CZ, Li Q. 2019. Structural insights into repression of the pneumococcal fatty acid synthesis pathway by repressor FabT and co-repressor Acyl-ACP. FEBS Lett 593:2730–2741. doi: 10.1002/1873-3468.13534 [DOI] [PubMed] [Google Scholar]
- 9. Zhu L, Zou Q, Cao X, Cronan JE. 2019. Enterococcus faecalis encodes an atypical auxiliary Acyl carrier protein required for efficient regulation of fatty acid synthesis by exogenous fatty acids. mBio 10:e00577-19. doi: 10.1128/mBio.00577-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longo M, De Jode M, Plainvert C, Weckel A, Hua A, Château A, Glaser P, Poyart C, Fouet A. 2015. Complete genome sequence of Streptococcus pyogenes emm28 clinical isolate M28Pf1, responsible for a puerperal fever. Genome Announc 3. doi: 10.1128/genomeA.00750-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for gram-positive pathogens. Nat 458:83–86. doi: 10.1038/nature07772 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham MW. 2008. Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol 609:29–42. doi: 10.1007/978-0-387-73960-1_3 [DOI] [PubMed] [Google Scholar]
- 13. Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. doi: 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 14. Lambert C, Poullion T, Zhang Q, Schmitt A, Masse JM, Gloux K, Poyart C, Fouet A. 2023. A Streptococcus pyogenes DegV protein regulates the membrane lipid content and limits the formation of extracellular vesicles. PLoS One 18:e0284402. doi: 10.1371/journal.pone.0284402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tatsuno I, Okada R, Matsumoto M, Hata N, Matsui H, Zhang Y, Isaka M, Hasegawa T. 2016. Relevance of spontaneous FabT mutations to a streptococcal toxic shock syndrome to non-streptococcal toxic shock syndrome transition in the novel-type Streptococcus pyogenes isolates that lost a salRK. APMIS 124:414–424. doi: 10.1111/apm.12521 [DOI] [PubMed] [Google Scholar]
- 16. Port GC, Vega LA, Nylander AB, Caparon MG. 2014. Streptococcus pyogenes polymyxin B-resistant mutants display enhanced exportal integrity. J Bacteriol 196:2563–2577. doi: 10.1128/JB.01596-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao J, Rock CO. 2017. Exogenous fatty acid metabolism in bacteria. Biochimie 141:30–39. doi: 10.1016/j.biochi.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Ye W, Wu K, Xiao S, Zheng Y, Shu Z, Yin Y, Zhang X. 2021. Inactivation of transcriptional regulator FabT influences colony phase variation of Streptococcus pneumoniae. mBio 12:e0130421. doi: 10.1128/mBio.01304-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flores AR, Chase McNeil J, Shah B, Van Beneden C, Shelburne SA. 2019. Capsule-negative emm types are an increasing cause of pediatric group A streptococcal infections at a large pediatric hospital in Texas. J Pediatric Infect Dis Soc 8:244–250. doi: 10.1093/jpids/piy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, Beres SB, LeFebvre RB, Musser JM. 2005. Genome sequence of a serotype M28 strain of group a streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J Infect Dis 192:760–770. doi: 10.1086/430618 [DOI] [PubMed] [Google Scholar]
- 21. Dramsi S, Bierne H. 2017. Spatial organization of cell wall-anchored proteins at the surface of gram-positive bacteria. Curr Top Microbiol Immunol 404:177–201. doi: 10.1007/82_2016_4 [DOI] [PubMed] [Google Scholar]
- 22. Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73:407–450. doi: 10.1128/MMBR.00014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weckel A, Ahamada D, Bellais S, Méhats C, Plainvert C, Longo M, Poyart C, Fouet A. 2018. The N-terminal domain of the R28 protein promotes emm28 group A Streptococcus adhesion to host cells via direct binding to three integrins. J Biol Chem 293:16006–16018. doi: 10.1074/jbc.RA118.004134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Six A, Bellais S, Bouaboud A, Fouet A, Gabriel C, Tazi A, Dramsi S, Trieu-Cuot P, Poyart C. 2015. Srr2, a multifaceted adhesin expressed by ST-17 hypervirulent group B Streptococcus involved in binding to both fibrinogen and plasminogen. Mol Microbiol 97:1209–1222. doi: 10.1111/mmi.13097 [DOI] [PubMed] [Google Scholar]
- 25. Biswas I, Gruss A, Ehrlich SD, Maguin E. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175:3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hays C, Lambert C, Brinster S, Lamberet G, du Merle L, Gloux K, Gruss A, Poyart C, Fouet A. 2021. Type II fatty acid synthesis pathway and cyclopropane ring formation are dispensable during Enterococcus faecalis systemic infection. J Bacteriol 203:e0022121. doi: 10.1128/JB.00221-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for gram-positive pathogens. Nat 458:83–86. doi: 10.1038/nature07772 [DOI] [PubMed] [Google Scholar]
- 28. Kénanian G, Morvan C, Weckel A, Pathania A, Anba-Mondoloni J, Halpern D, Gaillard M, Solgadi A, Dupont L, Henry C, Poyart C, Fouet A, Lamberet G, Gloux K, Gruss A. 2019. Permissive fatty acid incorporation promotes staphylococcal adaptation to FASII antibiotics in host environments. Cell Rep 29:3974–3982. doi: 10.1016/j.celrep.2019.11.071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures S1 to S4.
Methods used solely for the supplemental figures.
Supplemental Tables S1 to S3.