Abstract
BACKGROUND:
Increasing HIV testing and treatment coverage among people living with HIV (PLHIV) is essential for achieving global AIDS epidemic control. However, compared to women, cis-gender heterosexual men living with HIV are significantly less likely to know their HIV status, initiate anti-retroviral therapy (ART) and achieve viral suppression. This is particularly true in South Africa, where men are also at increased risk of mortality resulting from AIDS-related illnesses. While there is growing knowledge of Treatment as Prevention or the concept Undetectable=Untransmittable (U=U) among PLHIV in Western and high-income countries, the reach and penetration of the U=U message in sub-Saharan Africa remains limited, and few studies have evaluated the impact of accessible and relatable U=U messages on ART initiation and adherence. To address these gaps, rigorous evaluations of interventions that incorporate U=U messages are needed, especially among men in high prevalence settings.
METHODS:
Building on our U=U messages that we previously developed for men using behavioral economics insights and a human-centered design, we will conduct two sequential hybrid type 1 effectiveness-implementation trials to evaluate the impact of U=U messages on men’s uptake of community-based HIV testing and ART initiation (Trial 1), and retention in care and achievement of viral suppression (Trial 2). A cluster randomized trial will be implemented for Trial 1, with HIV testing service site-days randomized to U=U or standard-of-care (SoC) messages inviting men to test for HIV. An individual-level randomized control trial will be implemented for Trial 2, with men initiating ART at six government clinics randomized to receive U=U counselling or SoC treatment adherence messaging. We will incorporate a multi-method evaluation to inform future implementation of U=U messaging interventions. The study will be conducted in the Buffalo City Metro Health District of the Eastern Cape Province and in the Cape Town Metro Health District in the Western Cape Province in South Africa.
DISCUSSION:
These trials are the first to rigorously evaluate the impact of U=U messaging on HIV testing uptake, ART initiation and achievement of viral suppression among African men. If effective, these messaging interventions can shape global HIV testing, treatment and adherence counselling guidelines and practices.
Keywords: South Africa, HIV/AIDS, Anti-retroviral therapy (ART), HIV Prevention Undetectable Equals Untransmittable (U=U), Treatment as Prevention (TasP)
BACKGROUND
Increasing HIV testing and treatment coverage among people living with HIV (PLHIV) is essential for achieving global AIDS epidemic control (1). Compared to women, cis-gender heterosexual men living with HIV (MLHIV) are significantly less likely to know their HIV status, initiate anti-retroviral therapy (ART) or achieve viral suppression (2, 3). This is particularly true in South Africa, where men are also at increased risk of mortality resulting from AIDS-related illnesses (4–8). Moreover, new HIV infections among women are driven, in part, by gaps in testing and treatment among men (9–11). Ultimately, increasing men’s uptake of HIV testing and treatment initiation must be prioritized to both achieve viral suppression and improve HIV-related health outcomes among men, and to accelerate the decline in HIV incidence among women.
Following landmark studies showing the benefits of Treatment as Prevention (TasP), the Undetectable Equals Untransmittable (U = U) Campaign was launched in 2016 to disseminate scientific evidence that PLHIV who take ART and have an undetectable viral load (< 200 copies/mL) cannot sexually transmit HIV (12–17). This has been further borne out by the recent review which confirms that sexual transmission of HIV is unlikely from an individual whose HIV viral load is < 1000 copies /ml (18, 19). Particularly for men, the U = U message can accelerate progress towards the UNAIDS 95–95-95 targets by reducing anxiety associated with HIV testing (1st 95), encouraging ART initiation (2nd 95), and reducing fear of transmitting HIV to sexual partners by promoting treatment adherence to achieve viral suppression (3rd 95) (20, 21). While there is growing knowledge of TasP/U = U among PLHIV in Western and high-income countries, the reach and penetration of the U = U message in sub-Saharan Africa (SSA) has been limited (22–35) and few studies have evaluated the impact of accessible and relatable U = U messages on HIV testing and ART initiation and adherence in the region (36–38). To address these gaps, rigorous evaluations of interventions that incorporate U = U messages are needed, especially among cis-gendered heterosexual men in high prevalence settings.
Informed by behavioral economics principles (39–42) and using a human-centered design process (42–46), our team developed U = U messaging for men and conducted a pilot randomized trial of these peer-delivered messages on HIV testing uptake and HIV positive yield among men in Cape Town, South Africa (48). The U = U messages sought to reduce fear of testing HIV-positive by emphasizing the various health benefits of ART, including the ability to protect sex partners even during condomless sex. The pilot data showed that when presented as part of community-based HIV testing services (HTS), peer-delivered U = U messaging increased the odds of HIV testing uptake by 60% compared to standard messaging about the availability of free HIV testing (48). We subsequently conducted participatory prototyping to further refine U = U messages to improve ART initiation and early retention in care.
STUDY AIMS
Building upon this work, we will conduct two sequential hybrid type 1 effectiveness-implementation randomized trials (49, 50) to compare the impact of U = U messaging versus standard of care on men’s uptake of testing, initiation of ART, and achievement of viral suppression. Our study aims to 1) evaluate the impact of U = U messaging on men’s uptake of HTS and ART initiation; 2) evaluate the impact of U = U messaging on retention in care and viral suppression among MLHIV who initiate ART; and 3) conduct a multi-method evaluation to inform future implementation of U = U messaging interventions. We hypothesize that, compared to standard of care messaging and counselling, U = U messaging will increase HTS uptake by men, increase the yield of men initiating ART at 30 days, increase viral suppression at 6 months, and increase retention in care at 12 months. We will also characterize intervention implementation outcomes and identify key, multi-level contextual factors that may block or facilitate implementation of a U = U intervention within HIV service delivery.
METHODS
Study Setting
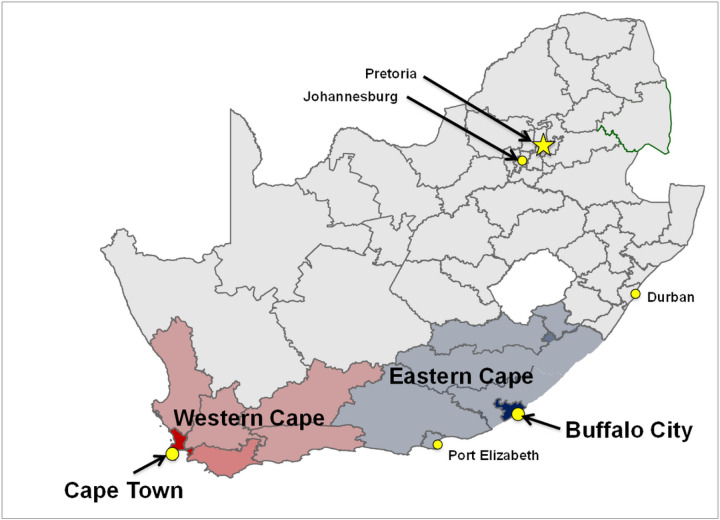
We will conduct the study in the Cape Town Metro (CPT; Western Cape Province) and the Buffalo City Metro (BCM; Eastern Cape Province) health districts, South Africa (Fig. 1) – two settings with a high HIV prevalence and where our team has existing strong community engagement and health facility partnerships. Of note, the Eastern Cape Province (EC) is a historically under-resourced and under-researched province.
Figure 1:
Map of Study Districts: Cape Town Metro (Western Cape Province) and Buffalo City Metro (Eastern Cape Province)
In 2022, the estimated adult HIV prevalence in the Eastern and Western Cape Provinces, respectively, was 19.4% and 11.2% (51). That same year, an estimated 92.6% of individuals living with HIV in the Eastern Cape knew their status, 74.9% were on ART, but only 62.6% were virally suppressed (i.e., < 1000c/ml) (52). In comparison, in the Western Cape, an estimated 93.7% of PLHIV knew their status, 70.3% were on ART, but only 62.4% were virally suppressed (i.e., < 1000c/ml) (51, 52). These statistics are well below the UNAIDS global target for viral suppression of 95%by the year 2025.
Using aggregate South African National Department of Health clinic-level HIV programmatic data, we identified three clinic catchment areas per study district (N = 6) with high HIV prevalence. Study participants for Aim 1 will be recruited from communities within the catchment areas of six large government HIV treatment clinics located in the Kilpfontein and Gugulethu communities of Cape Town (n = 3), and the Mdantsane, Duncan Village and Dimbaza communities of BCM (n = 3). Study participants for Aim 2 will be recruited from within these same six ART clinics. Residents from these communities are primarily black and Xhosa-speaking. Black South Africans living in peri-urban settings (e.g., Kilpfontein, Gugulethu, Mdantsane and Duncan Village) have among the highest rates of HIV infection in South Africa, while those living in rural settings (e.g., Dimbaza) have among the poorest access to health clinics. Our peri-urban selected communities are densely populated, with a significant number of residents living in informal housing; Gugulethu is one of the oldest but fastest-growing township communities in CPT, while Mdantsane is one of the largest townships in the country. For Aim 3, we will gather quantitative implementation process measures from recruitment and refusal logs, participant sociodemographic data, tracking tools, and clinic and testing site characteristics, as well as using validated tools at the mid-point of recruitment for each Aim 1&2 at each site. We will also conduct qualitative interviews with key stakeholders to inform targets and mechanisms for implementation strategies. Recruitment for Aim 3 will occur through long-standing research collaborations and strong networks and relationships in the region; eligible participants will be sought through meeting announcements, flyers, and email outreach.
Trial 1: Uptake of HIV Testing & Linkage-to-Care.
Overview and Study Flow
To evaluate the impact of U = U messaging on men’s uptake of HIV and ART initiation, we will leverage our well-established mobile HIV testing vehicles (i.e., mobile Tutu Tester Trucks) and pop-up tents in the study communities (53–58). We will conduct a hybrid type 1 effectiveness-implementation randomized controlled trial, with randomization at the ‘site-day’ level (Fig. 2) (59). A site-day is defined as one day at one testing site. HTS site-days (N = 320) will be randomized to U = U or Standard-of-Care (SoC) messaging to invite men (N = 28,880) to test for HIV. This study design allows us to evaluate the impact of U = U messaging on HIV testing uptake and on ART initiation.
Figure 2:

Aim 1 Trial Design and Flow
Cluster Randomization and Placement of Testing Services
Based on the clinic catchment area, and in consultation with local community leaders, mobile testing units or temporary testing tents (collectively referred to as community-based HIV testing services; CB-HTS) will be located at high foot-traffic sites (e.g., taxi ranks, entrance/exits to markets, shopping malls and supermarkets) in each clinic catchment area (i.e.,16 unique testing sites). Each site will be assigned a sequence of interventions for each day on which mobile testing units or temporary testing tents are located there, with SoC and U = U frequency equally balanced in blocks of 4 or 6 days. To minimize the potential for contamination (e.g., an individual receiving an intervention card one day and revisiting the site to test the following day), no site will be visited on consecutive calendar days. Randomization sequence will be determined prior to study launch and revealed to the CB-HTS team the night before each site visit.
Participant recruitment and data acquisition
Following procedures developed in our pilot trial, (59) trained male peers will approach, invite and provide invitation cards for free HIV testing services (Fig. 3) to a total of 28,880 men aged ≥ 15 years who happen to be in the immediate vicinity (± 200m) of testing sites. Male peers will deliver the scripted U = U message and U = U invitation card (Fig. 3) on intervention days, and the SoC HIV testing message script along with the SoC invitation card on control days; the SoC invitation script is approximately the same length as the U = U script. Invitation cards will be pre-printed with a unique participant identification code (PID). The male peers will write the date, time of distribution and their initials on each invitation card as they are handed out. All men presenting to a HIV testing site will be asked to provide their invitation card to testing site staff. Eligible men presenting a study invitation card for HIV testing will be invited to join the study and asked to provide written informed consent and personal contact details.
Figure 3:
U = U and Standard messaging invitation cards for HIV testing services
Using a process successfully implemented during the pilot trial, a site receptionist will record the invitation card’s unique PID, and the date and time of presentation. Reasons for non-enrollment will be recorded. The following inclusion criteria will be applied 1) male; 2) aged ≥ 15 years; 3) presentation of a study-issued invitation card; and 4) provision of informed consent. Consenting participants will receive a unique study ID number (UID),and be asked to complete a baseline questionnaire administered by study staff prior to being directed to an HIV testing counsellor for a rapid HIV test. Men passively presenting to a testing site (i.e., not following a testing invitation) will be provided with free HIV testing but will not be enrolled in the study.
Per South African National HIV testing guidelines (59), all individuals seeking HTS (i.e., study and non-study participants) will be consented by a certified HIV testing counsellor to receive standard pre-test counselling and an HIV rapid test. Study participants who receive an HIV negative test result, regardless of treatment assignment, will receive standard post-test counselling; and will be offered HIV prevention services (Tutu Testers in Western Cape) or a referral for HIV prevention services at local government clinics (Eastern Cape). Participants in the U = U intervention arm with an HIV-positive test result will receive U = U post-test counselling (Supplemental Table 1). Participants in the SoC condition who receive an HIV-positive test result will receive standard (guidelines-based) post-test counselling about ART initiation. Community members (male or female) who passively present to any of our testing venues, or men who do not present with an invitation or do not provide consent will receive standard testing per South African national guidelines (60) including standard pre- and post-test counselling.
Outcomes and data analysis plan
The primary outcome will be the intention to treat, which we defined as ART initiation within 30 days of receiving a positive HIV result. ART initiation will be established through participant self-report (via phone calls) and through review of laboratory records from the National Health Laboratory Service or clinic records where available.
Generalized estimating equations (GEE) with a logistic link function and an independent working correlation structure will be used to estimate the odds of ART initiation following U = U versus SoC messaging and will be averaged across sites. A significance level of 0.05 will be employed and calendar quarter will be controlled for to account for seasonal variability in behaviour.
Secondary outcomes will include HIV testing uptake, first-time testing, and test-positivity among those who receive the invitation cards as well as ART initiation among those who test positive for HIV. For this, mixed effects models will be used to estimate: 1) the difference in HIV testing uptake depending on U = U versus SoC messaging exposure, and 2) between-site and within-site HIV-testing uptake variability. Similar as for the primary outcome analyses, GEE and working correlation structure will also be used for the secondary outcomes. To better identify where in the HIV testing-linkage to care sequence U = U may specifically have an impact, we will estimate the odds ratio and 95% CI for U = U versus SoC for each subsequent outcome conditional on results for the previous outcome (i.e., the outcome of testing positive given the behaviour of presenting for testing, and the behaviour of ART initiation given the outcome of an HIV positive result). Participant groups will be characterized at each step of the sequence to assess how U = U messaging might be influencing specific groups of individuals to test or link to care. Causal methods (potential outcomes) will be used to assess if individuals who test positive following U = U versus SoC messaging are more likely to initiate ART.
Sample Size Calculations and Statistical power
A sample size of 28,880 invitation cards distributed (i.e.,14,440 per intervention message) gives us 84% power to detect a risk difference in our primary outcome (yield, or ART initiations as a percent of invitations distributed) of 0.156%, and a risk ratio of 2.57. In other words, we can detect a difference in yield of 0.1% (1 ART initiation per 1000 invitation cards) for SoC versus 0.257% (2–3 ART initiations per 1000 invitation cards) for the U = U intervention. Our sample size calculations are informed by pilot data (59), experience in the field, and recent HIV incidence.
For the U = U intervention arm, we estimated that 15% would present for testing, 3.5% of those who test would test HIV-positive, and 49% of those receiving a positive test result would initiate ART, for a yield of 0.257%. For the SoC arm, we estimated that 10% would present for testing, 2.5% of those who test would test HIV-positive and 40% of those testing positive would initiate ART, for an overall yield of ART initiation among those invited to test of 0.10%. The sample size calculation assumed a within-site-day intraclass correlation (ICC) of 0.01 for subjects sampled on the same day at a single site, and a between-site-day ICC of 0.009 for subjects sampled on two consecutive sampling days at a site. A discrete-time decay correlation structure using an exponential decay function of the time ‘j’ between site days allowed the correlation between subjects sampled at longer intervals apart to decrease over time. Power calculations were conducted by uploading a design matrix generated in R(V4.2.2) to Shiny CRT (61). To achieve this sample size, we will distribute 90 invitation cards per site day for a total of 320 site days (160 for U = U intervention and 160 for SoC).
Trial 2: Retention in Care and Viral Suppression
Overview and Study Flow
To evaluate the impact of U = U messaging on retention in care and viral load (VL) suppression among HIV-positive men who initiate ART, we will conduct a hybrid type 1 effectiveness-implementation randomized controlled trial. Randomization will be stratified by the 6 study clinics, to account for differences in baseline viral loads of the catchment area populations initiating ART within each clinic. Individual-level randomization will be used with random block sizes of 4 to 8. The study staff counsellors will recruit men who are either newly initiating or re-initiating ART at study clinics in Cape Town (n = 3) and BCM (n = 3). Inclusion criteria for trial 2 are as follows: 1) male 2) aged ≥ 15 years 3) newly initiating ART or re-initiating ART after 6 months of being lost-to-care; 4) live in Buffalo City or Cape Town Metro Health Districts; and 5) provision of written informed consent. Participants (N = 1100) will be individually randomized to receive either U = U messaging (intervention group; n = 550) or SoC messaging (control group; n = 550) as part of ART initiation counselling and during routine HIV care and treatment. Our trial design allows evaluation of both shorter-term impact and longer-term durability of the U = U messaging for ART adherence and treatment retention.
Participant recruitment and data acquisition
All participants will receive standard ART initiation and adherence counselling from a DoH clinic nurse per South African National Guidelines (Fig. 4) (60). Thereafter, study staff counsellors will either (1) deliver U = U initiation messaging, or (2) reinforce SoC messaging through a guidelines-based script of comparable length (Fig. 4). Additionally, participants will also receive a small business-sized card either re-emphasizing the U = U or the SoC message (62). At enrolment, participants will be informed that they have the option to receive monthly SMS messages (Supplemental Fig. 1). A mid-month U = U or Attention Matched Control one-way text message will be sent to participants opting to receive SMS messages (Supplemental Fig. 1). Monthly text messaging is not part of the SOC protocol in South Africa, thus our decision to employ an attention matched control message for the SoC participant group. SMS paradata will be collected to track message delivery and receipt. Study staff counsellors will in addition verbally deliver U = U or reinforce SoC adherence messaging (Fig. 4) to participants during routine medication refill visits, which are scheduled monthly. Paradata from SMS messages and number of clinic visits attended will be recorded to enable mediation analysis (63, 64). In accordance with South African ART clinical and adherence guidelines(65, 66), clinic nurses provide patients on ART with feedback based on VL test results. Study participants in the U = U condition with a suppressed VL (< 50 copies/mL) will receive Basic U = U messaging, while those with an unsuppressed VL (≥ 50 copies/mL) will receive Enhanced U = U messaging (Fig. 4). Participants allocated to SoC will receive guidelines-based reinforced SoC adherence messaging based on their VL. (67)
Figure 4:
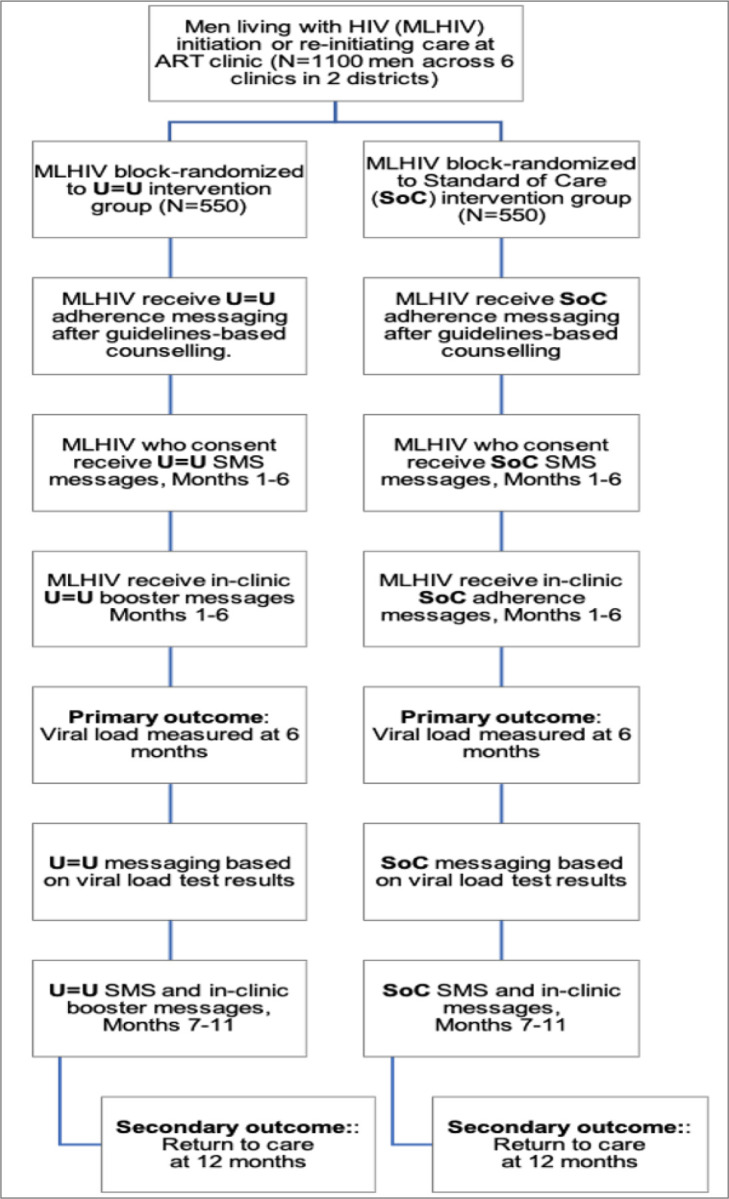
Aim 2 Trial Design and Flow
Outcomes and data analysis plan
The primary outcome will be the proportion of MLHIV who are virally suppressed at 6 months (impact) and will be analysed using logistic regression with clinic fixed effects. The secondary outcome will be the proportion of MLHIV retained care at 12 months (durability) and will be analysed using logistic regression with clinic fixed effects with outcomes as (1) the number of clinic visits attended prior to six months, and (2) presentation for VL testing at 12 months. VL testing is conducted at 6 months, 12 months and annually thereafter by the Department of Health in accordance with South African national HIV care and treatment guidelines. Should a VL not be recorded in a participant’s medical record, we will consult the SA-NHLS laboratory information system to determine if and when VL testing was performed and record the participant’s VL should it be available. At the 6- and 12-month mark, each participant’s clinic-based medical records will be abstracted for: 1) ART refill and clinic attendance history, and 2) VL results.
All participants lost to follow-up at 6 months will be contacted by study staff using the contact information provided at study enrolment to assess self-reported treatment engagement and invited to return to the clinic to complete a study questionnaire. At 12 months, all participants will also be asked to complete an RedCap administered endline questionnaire in the same manner as the baseline questionnaire. A causal mediation analysis will be conducted to explore direct and indirect effects of the U = U intervention on VL suppression with number of visits as the mediating variable. Pre-specified subgroups for the analyses of the primary and secondary outcomes (the proportion of MLHIV retained in care at 12 months) are those participants: (1) initiating ART for the first time vs. re-initiating ART and (2) Eastern Cape versus Western Cape. For secondary analyses the primary analysis will be repeated using as outcomes:
Sample Size Calculations and Statistical Power.
Trial 2 is powered to detect an 8% difference in the primary outcome. For a two-sided Type I error rate of 0.05, and using a two-sample proportions test as the basis of the calculation, we have 80% power to discern between 70% (U = U intervention) versus 62% (SoC intervention) viral suppression at 6 months.
Implementation Assessment
Overview and Study Flow
We will use the RE-AIM and Consolidated Framework for Implementation Research (CFIR) frameworks, and informed by behavioural economics (68–73), and behavioral design (74) to identify implementation barriers and facilitators of the U = U interventions. Perceptions and intervention mechanisms will be explored to inform how the U = U interventions could be implemented and scaled in the future. RE-AIM will inform the identification, collection, and analysis of quantitative indicators related to the U = U interventions over the course of the two sequential trials. CFIR will guide a qualitative exploration of the conditions and contexts shaping successful implementation through interviews with key stakeholders (i.e., participants, research/clinic staff, health department managers, policy makers).
Participant recruitment and data acquisition
We will recruit and consent HTS providers (n = 20), ART nurses (n = 24), clinic operation staff (n = 12) and clinic service managers (n = 12) representing both the Cape Town and Buffalo City Metro health districts (n = 68 total); this sample size is sufficient to generate stakeholder preferences for provide a signal about implementation outcomes within and across study sites and districts for both trials, without putting undue burden on clinic or testing site staff. We will also purposively recruit, consent and interview 30 individuals from seven key stakeholder groups for each interview round (N = 60 total interviews). Key stakeholder groups will include: 1) MLHIV who attend the testing sites (trial 1) or clinics (trial 2) where our trials are conducted (n = 10); 2) health care providers (i.e., nurses or physicians) at the clinics where the retention and viral suppression trial is conducted (n = 10); 3) lay health workers and counsellors (n = 10); 4) clinic leads (n = 10); 5) district, provincial and national departments of health National Department of Health (NDOH) (n = 10); 6) PEPFAR implementing partner leads (n = 6); and 7) South Africa HIV Task Force members (n = 4). Recruitment will occur through long-standing research collaborations and strong networks and relationships throughout South Africa; eligible participants will be sought through meeting announcements, flyers, and email outreach.
To describe intervention effectiveness and inform future implementation efforts, we will gather quantitative implementation process measures from recruitment and refusal logs, participant sociodemographic data, tracking tools, and clinic and testing site characteristics. These data will be collected throughout the implementation of trials 1 and 2. To identify key, multi-level contextual factors that may block or facilitate implementation of a U = U intervention within HIV service delivery, we will conduct 1) in-depth qualitative interviews with key stakeholders to inform targets and mechanisms for implementation strategies, and 2) validated quantitative implementation measures. Data will be collected at the mid-point of recruitment for each trial at each site.
Data Analysis Plan
Passively and actively collected process indicators informed by the RE-AIM framework will be synthesized and summarized iteratively over the course of the two trials. Descriptive statistics and comparisons by study site, provider type, and trial will be used to assess implementation success, interpret trial results, and identify implementation targets and mechanisms. Verbatim transcripts of semi-structured interviews will be entered into NVivo qualitative analysis software (QSR international, Burlington MA) for data management and analysis. Two separate qualitative analyses will be conducted. First, a hybrid inductive-deductive thematic analysis (75) will be conducted using both a priori codes related to CFIR domains and constructs, as well as de novo codes that emerge from the data.
Two team members will code the first three interviews by consensus, then the next three interviews independently and review them together to ensure consensus. Thereafter, all interview transcripts will be coded by two team members independently, with double-coding of 20% of interviews to ensure inter-rater reliability. Outstanding coding questions and disagreements will be resolved by consensus of the team. We will qualitatively analyze themes across interview rounds and stakeholder types to identify key opportunities and challenges for implementation of the U = U interventions. Second, the NUDGE framework (74, 76, 77) will be employed to uncover specific barriers to implementation of evidence-based interventions through the application of behavioral insights to rich contextual data such as in-depth interviews. Behavioral barriers to intervention adoption, adaptation, fidelity, and maintenance identified through this process will inform future implementation strategies and policy recommendations.
DATA MANAGEMENT
Participant Identification
All eligible study participants that provide informed consent will be assigned a Participant Identification Number (PIN) by REDCap. This PIN composition will allow us to identify participants at the community and clinic level, thus ensuring convenient integration of various datasets to carry-out various level-based analysis approaches. Participants’ allocated PIN will be used for the duration of the study at each data collection point. Results from other data sources such as the South African electronic TB register (ETR.net) and District Health Information Systems (DHIS), as well as laboratory databases and clinic registers will be back captured onto REDCap, therefore making REDCap the primary data collection and storage platform for this study. A secure link-log (linking participant identifiers such as names, date of births, etc. and the PIN) database will be developed and stored in a separate REDCap database accessible only to the Principal Investigators (PIs) and those study staff granted access upon approval by the PIs.
Data Storage
All study databases will be hosted on secure cloud-based platforms. Datasets will be merged using primary identifiers and stored in a secure, password-protected, web-based database which will only be accessible to authorized project staff. Paper records of participants will be kept in lockable filing cabinets. Paper records, excluding informed consent forms, will only contain PINs. Qualitative data including audio files and password-protected transcripts will be stored on a secure, access-controlled cloud-based database (Sharepoint).
Data Quality
Data quality will be assured by automated data quality checks and skip patterns in REDCap, field-based data quality clerks and office-based data administrators. Quality checks will be performed daily, with inconsistencies rectified using our data query resolution platform. Scheduled and unscheduled quality inspections will be performed on a randomly selected 10% of participants.
Communication with participants
No active follow-up activities are required for Aim 1; however, should individuals referred for ART initiation not be identified within one of these three data sources within 31 days of receiving their HIV positive test result, study staff will work to actively contact, trace, and link these individuals to HIV care and treatment.
For Aim 2, to ensure follow-up of participants for the purpose of data collection/verification activities, we will employ two follow-up methods that we have successfully used in previous studies:
Method #1) Participants will be asked to provide detailed personal contact information, including their phone number(s) and home addresses for themselves, a family member, or a friend/neighbour. If a participant has a personal cell phone, the cell number will be verified by calling the phone in front of the participant. Method #2) A green sticker will be discretely placed in a participant’s medical file. This green sticker will alert clinic ART nurses that the patient is a participant in our study. When a study participant returns to the clinic for routine ART refills or VL testing, clinic pharmacists or ART nurses will be asked to refer the study participant to a RC for study activities, including booster and VL result report-back messaging.
Retention: To observe the impact of U = U messaging on viral suppression and retention in care, no additional active follow-up activities or retention planning other than those described will be conducted. If a participant misses a clinic visit at months 6 or 12, RCs will make up to three contact attempts via phone to invite the participant to return to the clinic. If phone follow-up is not successful, research staff will work with clinic-based community tracers to contact the participant. Community tracers will not be told about the participants’ Involvement in our study.
Given that we want to observe the impact of our U = U messaging on ART adherence, viral suppression and retention in care, no additional active follow-up activities, or retention planning, other than those described, will be conducted. Should a participant be missed upon clinic attendance at months 6 or 12, RCs will make up to three attempts to make contact via phone and invite a participant to return to the clinic and complete a study questionnaire. Should follow-up by phone not be successful, research staff will work with clinic-based community tracers to contact the participant. To ensure our participants’ confidentiality, community tracers will never be told of an individual’s participation in our study. Community tracers will only know to communicate that the clinic is trying to follow up with them and to please return to the clinic.
Data and safety monitoring
All research staff will be trained to identify, probe for and report adverse events (AEs) and social harms (SHs). Occurrence of AEs and SHs, will be collected at every visit. All AEs and SHs reported outside of research visits/activities will also be documented and reported. An AE list will be compiled and reviewed by the Data Safety and Monitoring Board (DSMB) which is set up be set up for this study and has ultimate ability to terminate the trial should interventions prove to have unacceptable risk. All DSMB members will have no direct association with this study or the study sponsors.
DISSEMINATION
The results of the study will be analysed for publication in scientific journals and presentation at relevant scientific conferences. All presentations, abstracts, or manuscripts will be submitted to the study PIs for review and approval prior to submission and no professional writers will be used. The study's results be communicated to key stakeholder groups, such as the South African local, provincial and the national health departments. The study's results will also be presented to study participants, community members, and clinic staff through town hall style meetings and 1-pager flyers. Key interim results, (HIV testing, linkage, and retention in care among men), will be shared with provincial and district stakeholders on a bi-annually basis. The lessons learned from implementation will also be shared with them.
DISCUSSION
These trials are the first to rigorously evaluate the impact of U = U messaging on HIV testing uptake, ART initiation and achievement of viral suppression. Our intervention and trial design make three important contributions to the HIV prevention literature. First, we bring human-centered design and behavioral economics insights to the design of U = U messaging for men in South Africa. Previous research on U = U messaging has used conventional health communication and health behavior change theories to inform message development, with limited efforts to develop U = U messages that speak to men in Sub-Saharan Africa in particular. We collaborated with a human-centered design firm in South Africa, Matchboxology,(48, 78) to understand how HIV and ART are perceived by men living with and/or affected by HIV, and to co-design messages that specifically resonated with men’s lived experience, aspirations, and preferences. Following our promising pilot study showing the potential of the U = U message to increase men’s uptake of HIV testing, our proposed study will be among the first to evaluate U = U messages at scale.
Second, our study examines the effect of U = U messages across the HIV care continuum: Most RCTs of behavioral interventions examine one specific behavior or outcome; for behavioral research on HIV, this is typically one step of the HIV care continuum. Our study is novel in testing whether U = U messaging can motivate MLHIV to test for HIV, start ART, adhere to ART, and achieve viral suppression (the most common rationale for U = U messages); as well as whether it can motivate men who don’t know their HIV status to seek HIV testing services. Our study will be among the first to test the effect of U = U messages across the HIV cascade in two sequential RCTs.
Third, we bring behavioral economics insights to both our intervention design and our identification of implementation targets. Building on prior work by our team, we adopt a behavioral economics lens both for the design of our U = U interventions for the two trials, and in our implementation inquiry. We apply the NUDGE framework, (76, 77) an innovative behavioral diagnosis and design approach developed by members of our team, to identify specific barriers to implementation for U = U interventions based on stakeholder interviews. Our approach recognizes that providers, clinic managers, and policy makers are subject to the same biases and heuristic thinking in decision-making and in resource and attention allocation that PLHIV are in their decisions about testing, ART initiation, and retention.
By conducting our study in two different provinces, including a province (Eastern Cape) where very little behavioral research on HIV interventions has been conducted, our study will produce generalizable knowledge about the impact of delivering theory-based U = U messaging at multiple points along the HIV care continuum for men in diverse communities with high burden of HIV. The inclusion of the EC is a strength since community and health system voices from this part of the country are seldom heard. EC’s resource-poor environment (compared to more typical study sites in Cape Town, Johannesburg or Durban) will provide a new and unique environment in which to understand implementation facilitators and barriers. We will also learn about implementation barriers that may limit the reach and adoption of successful interventions in clinical practice. If shown to be effective, our intervention will inform the implementation of HIV testing and ART adherence counselling guidelines in South Africa and globally.
Supplementary Material
Acknowledgements
N/A
Abbreviations
- ART
Anti-retroviral therapy
- BCM
Buffalo City Metro
- CB-HTS
Community-based HIV testing services
- EC
Eastern Cape Province
- HTS
HIV testing services
- MLHIV
Men living with HIV
- PLHIV
People living with HIV
- TasP
Treatment as prevention
- U=U
Undetectable Equals Untransmittable
- VL
Viral load
Funding Statement
This study is funded by the US National Institutes of Health (R01MH129223). The funders had no role in the study design; collection, management, analysis, and interpretation of data; writing of the report; nor the decision to submit the report for publication and will not have ultimate authority over any of these activities.
Footnotes
Declarations
Availability of data and materials
The full study protocol and datasets used in publications will be made freely available in the OSFHOME repository under project name Improving HIV testing, linkage, and retention in care for men through U=U messaging (DOI 10.17605/OSF.IO/CRJX4; https://osf.io/crjx4/).
Ethics approval and consent to participate
The protocol, informed consent form and all translations, participant education and recruitment materials have been approved by the ethical review bodies responsible for oversight of research and have been reviewed and approved by the University of Cape Town, Research Ethics Committee (Approval Number: 556/2021) with respect to scientific content and compliance with applicable research and human subjects’ regulations. IRB approvals have also been granted by University of Pennsylvania via IRB reliance on the University of Cape Town. Eastern Cape Provincial Department of Health approval (Approval Number: EC_202204_002) has been obtained to conduct this study in Buffalo City Metropolitan Health District. City of Cape Town Health approval (Approval Number: 9832) has been obtained to conduct this study in Cape Town. All participants will be required to provide written informed consent prior to being enrolled in the study. Subsequent to initial review and approval, the responsible Ethics Committees will review the protocol for renewal once every 12 months. The protocol, informed consent forms and all participant materials, and amendments thereof were or will be reviewed and approved by the ethical review bodies responsible for oversight of research. The Investigators will submit safety and progress reports to the Ethics Committees at least annually, and within 3 months of study termination or completion. These reports will include the total number of participants enrolled in the study, the number of participants who completed the study, all changes in the research activity, and all unanticipated problems involving risks to human subjects or others.
Competing interests
The authors declare that they have no competing interests.
TRIAL STATUS
This protocol paper reflects protocol version 2.0, dated 31/08/2023. The trial was first registered on ClinicalTrials.gov (NCT05602376) on 27/10/2022 with the last update posted 10/03/2023. Participant recruitment for Trial 1 began on 31 March 2023 and is expected to be completed by February 2024.
Contributor Information
Andrew Medina-Marino, Desmond Tutu HIV Centre, University of Cape Town.
Nkosiyapha Sibanda, Desmond Tutu HIV Centre, University of Cape Town.
Mary Putt, University of Pennsylvania.
Dvora Joseph Davey, University of California, Los Angeles.
Phillip Smith, Desmond Tutu HIV Centre, University of Cape Town.
Harsha Thirumurthy, University of Pennsylvania.
Linda-Gail Bekker, Desmond Tutu HIV Centre, University of Cape Town.
Alison Buttenheim, University of Pennsylvania.
References
- 1.PCBSS_Global_AIDS_Strategy_2021--2026_EN.pdf [Internet]. [cited 2023 Aug 14]. Available from: https://www.unaids.org/sites/default/files/PCBSS_Global_AIDS_Strategy_2021--2026_EN.pdf [Google Scholar]
- 2.Addressing a blind spot in the response to HIV — Reaching out to men and boys | UNAIDS [Internet]. [cited 2023 Aug 14]. Available from: https://www.unaids.org/en/resources/documents/2017/blind_spot [Google Scholar]
- 3.Global AIDS Update — Seizing the moment — Tackling entrenched inequalities to end epidemics | UNAIDS [Internet]. [cited 2023 Aug 14]. Available from: https://www.unaids.org/en/resources/documents/2020/global-aids-report [Google Scholar]
- 4.Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, Tanser F, Pillay D, Bärnighausen T. Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa. PLoS Med. 2015. Nov;12(11):e1001905; discussion e1001905. Tsai AC, editor. PLoS Med. 2015 Nov 24;12(11):e1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Population-wide differentials in HIV service access and outcomes in the Western Cape for men as compared to women, South Africa: 2008 to 2018: a cohort analysis. J Int AIDS Soc. 2020. Jun;23 Suppl 2:e25530. J Intern AIDS Soc [Internet]. 2020 Jun [cited 2023 Aug 14];23(S2). Available from: 10.1002/jia2.25530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study. Celentano DD, editor. PLoS Med. 2012. Sep 4;9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson LF, May MT, Dorrington RE, Cornell M, Boulle A, Egger M, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. Suthar AB, editor. PLoS Med. 2017. Dec 12;14(12):e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, Mabaso M, Ramlagan S, North A, van Zyl J, Mohlabane N, Dietrich C, Naidoo I and the SABSSM V Team (2019) South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. Cape Town: HSRC Press. [Google Scholar]
- 9.Vandormael A, Cuadros D, Kim HY, Bärnighausen T, Tanser F. Vandormael A, Cuadros D, Kim HY, Bärnighausen T, Tanser F. The state of the HIV epidemic in rural KwaZulu-Natal, South Africa: a novel application of disease metrics to assess trajectories and highlight areas for intervention. Int J Epidemiol. 2020. Apr 1;49(2):666–675. doi: 10.1093/ije/dyz269. Int J Epidemiol. 2020 Apr 1;49(2):666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandormael A, Akullian A, Siedner M, de Oliveira T, Bärnighausen T, Tanser F. Vandormael A, Akullian A, Siedner M, de Oliveira T, Bärnighausen T, Tanser F. Declines in HIV incidence among men and women in a South African population-based cohort. Nat Commun. 2019. Dec 2;10(1):5482. doi: 10.1038/s41467-019-13473-y. Nat Commun. 2019 Dec 2;10(1):5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakigozi G, Chang L, Reynolds S, Nalugoda F, Kigozi G, Quinn T, Gray R, Kisakye A, Ndyanabo A, Ssekubugu R, Serwadda D, Wawer M, Kagaayi J, Grabowski M. Rapidly declining HIV incidence among men and women in Rakai, Uganda. Conference on Retroviruses and Opportunistic Infections (CROI); 2020. Mar 8; Boston. [Internet]. [cited 2023 Aug 16]. Available from: https://www.healio.com/news/infectious-disease/20200312/hiv-incidence-declines-among-men-women-in-rakai-uganda [Google Scholar]
- 12.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016. Jul 12;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016. Sep 1;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018. Aug;5(8):e438–47. [DOI] [PubMed] [Google Scholar]
- 15.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019. Jun 15;393(10189):2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevention-Access-Campaign-Consensus-Statement.pdf [Internet]. [cited 2023 Aug 15]. Available from: https://www.pwatoronto.org/wp-content/uploads/2017/10/2017-Prevention-Access-Campaign-Consensus-Statement.pdf [Google Scholar]
- 17.Eisinger RW, Dieffenbach CW, Fauci AS. Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019. Feb 5;321(5):451–452. JAMA. 2019 Feb 1;321(5):451–2. [DOI] [PubMed] [Google Scholar]
- 18.Broyles LN, Luo R, Boeras D, Vojnov L. Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet. 2023. Aug 5;402(10400):464–471. doi: 10.1016/S0140-6736(23)00877-2. Epub 2023 Jul 22. Lancet. 2023 Aug 5;402(10400):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker LG, Smith P, Ntusi NAB. Bekker LG, Smith P, Ntusi NAB. HIV is sexually untransmittable when viral load is undetectable. Lancet. 2023. Aug 5;402(10400):428–429. doi: 10.1016/S0140-6736(23)01519-2. Epub 2023 Jul 22. Lancet. 2023 Aug 5;402(10400):428–9. [DOI] [PubMed] [Google Scholar]
- 20.Prevention Access Campaign – The revolution in living and loving with HIV [Internet]. [cited 2023 Aug 15]. Available from: https://preventionaccess.org/ [Google Scholar]
- 21.Calabrese SK, Mayer KH. Calabrese SK, Mayer KH. Providers should discuss U=U with all patients living with HIV. Lancet HIV. 2019. Apr;6(4):e211–e213. Lancet HIV. 2019 Apr;6(4):e211–3. [DOI] [PubMed] [Google Scholar]
- 22.Siegel K, Meunier É. Siegel K, Meunier É. Awareness and Perceived Effectiveness of HIV Treatment as Prevention Among Men Who Have Sex with Men in New York City. AIDS Behav. 2019. Jul;23(7):1974–1983. doi: 10.1007/s10461-019-02405-y. AIDS Behav. 2019 Jul;23(7):1974–83. [DOI] [PubMed] [Google Scholar]
- 23.Card KG, Armstrong HL, Lachowsky NJ, Cui Z, Sereda P, Carter MA, et al. Card KG, Armstrong HL, Lachowsky NJ, Cui Z, Sereda P, Carter A, Montaner JSG, Hogg RS, Roth EA, Moore DM. Belief in Treatment as Prevention and Its Relationship to HIV Status and Behavioral Risk. J Acquir Immune Defic Syndr. 2018. Jan 1;77(1):8–16. doi: 10.1097/QAI.0000000000001557. J Acquir Immune Defic Syndr. 2018 Jan 1;77(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter A, Lachowsky N, Rich A, Forrest JI, Sereda P, Cui Z, et al. Carter A, Lachowsky N, Rich A, Forrest JI, Sereda P, Cui Z, Roth E, Kaida A, Moore D, Montaner JS, Hogg RS. Gay and bisexual men’s awareness and knowledge of treatment as prevention: findings from the Momentum Health Study in Vancouver, Canada. J Int AIDS Soc. 2015. Aug 11;18(1):20039. doi: 10.7448/IAS.18.1.20039. J Int AIDS Soc. 2015;18(1):20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rendina HJ, Cienfuegos-Szalay J, Talan A, Jones SS, Jimenez RH. Rendina HJ, Cienfuegos-Szalay J, Talan A, Jones SS, Jimenez RH. Growing Acceptability of Undetectable = Untransmittable but Widespread Misunderstanding of Transmission Risk: Findings From a Very Large Sample of Sexual Minority Men in the United States. J Acquir Immune Defic Syndr. 2020. Mar 1;83(3):215–222. doi: 10.1097/QAI.0000000000002239. J Acquir Immune Defic Syndr. 2020 Mar 1;83(3):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rendina HJ, Cienfuegos-Szalay J, Talan A, Jones SS, Jimenez RH. Rendina HJ, Cienfuegos-Szalay J, Talan A, Jones SS, Jimenez RH. Growing Acceptability of Undetectable = Untransmittable but Widespread Misunderstanding of Transmission Risk: Findings From a Very Large Sample of Sexual Minority Men in the United States. J Acquir Immune Defic Syndr. 2020. Mar 1;83(3):215–222. doi: 10.1097/QAI.0000000000002239. J Acquir Immune Defic Syndr. 2020 Mar 1;83(3):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paschen-Wolff MM, Campbell ANC, Tross S, Castro M, Berg H, Braunstein S, et al. Paschen-Wolff MM, Campbell ANC, Tross S, Castro M, Berg H, Braunstein S, Borges C, Jarlais DD. HIV Treatment Knowledge in the Context of “Treatment as Prevention” (TasP). AIDS Behav. 2020. Oct;24(10):2984–2994. doi: 10.1007/s10461-020-02849-7. AIDS Behav. 2020 Oct;24(10):2984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngure K, Ongolly F, Dolla A, Awour M, Mugwanya KK, Irungu E, et al. Ngure K, Ongolly F, Dolla A, Awour M, Mugwanya KK, Irungu E, Mugo N, Bukusi EA, Morton J, Odoyo J, Wamoni E, Barnabee G, Peebles K, O’Malley G, Baeten JM; Partners Scale-Up Project Team. “I just believe there is a risk” understanding of undetectable equals untransmissible (U = U) among health providers and HIV-negative partners in serodiscordant relationships in Kenya. J Int AIDS Soc. 2020. Mar;23(3):e25466. doi: 10.1002/jia2.25466. Journal of the International AIDS Society. 2020;23(3):e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holt M, Lea T, Schmidt HM, Murphy D, Rosengarten M, Crawford D, et al. Holt M, Lea T, Schmidt HM, Murphy D, Rosengarten M, Crawford D, Ellard J, Kolstee J, de Wit J. Increasing Belief in the Effectiveness of HIV Treatment as Prevention: Results of Repeated, National Surveys of Australian Gay and Bisexual Men, 2013–15. AIDS Behav. 2016. Jul;20(7):1564–71. doi: 10.1007/s10461-016-1306-0. AIDS and Behavior. 2016 Jul 1;20. [DOI] [PubMed] [Google Scholar]
- 30.Making the invisible, visible: a cross-sectional study of late presentation to HIV/AIDS services among men who have sex with men from a large urban center of Brazil | BMC Public Health | Full Text [Internet]. [cited 2023 Aug 15]. Available from: 10.1186/1471-2458-14-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndyabakira A, Chamie G, Emperador D, Marson K, Kamya M, Havlir D, et al. Ndyabakira A, Chamie G, Emperador D, Marson K, Kamya MR, Havlir DV, Kwarisiima D, Thirumurthy H. Men’s Beliefs About the Likelihood of Serodiscordance in Couples with an HIV-Positive Partner: Survey Evidence from Rural Uganda. AIDS Behav. 2020. Mar;24(3):967–974. doi: 10.1007/s10461-019-02531-7. AIDS and Behavior. 2020 Mar 1;24. [DOI] [PubMed] [Google Scholar]
- 32.Prati G, Zani B, Pietrantoni L, Scudiero D, Perone P, Cosmaro L, et al. Prati G, Zani B, Pietrantoni L, Scudiero D, Perone P, Cosmaro L, Cerioli A, Oldrini M. PEP and TasP Awareness among Italian MSM, PLWHA, and High-Risk Heterosexuals and Demographic, Behavioral, and Social Correlates. PLoS One. 2016. Jun 13;11(6):e0157339. doi: 10.1371/journal.pone.0157339. PLoS One. 2016;11(6):e0157339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couffignal C, Papot E, Etienne A, Legac S, Laouénan C, Beres D, et al. Couffignal C, Papot E, Etienne A, Legac S, Laouénan C, Beres D, Blum L, Khuong-Josses MA, Lepretre A, Papazian P, Yazdanpanah Y, Bouvet E. Treatment as prevention (TasP) and perceived sexual changes in behavior among HIV-positive persons: a French survey in infectious diseases departments in Paris. AIDS Care. 2020. Jul;32(7):811–817. doi: 10.1080/09540121.2019.1653438. Epub 2019 Aug 21. AIDS Care. 2020 Jul 2;32(7):811–7. [DOI] [PubMed] [Google Scholar]
- 34.Newman CE, de Wit J, Persson A, Holt M, Slavin S, Kidd MR, et al. Newman CE, de Wit J, Persson A, Holt M, Slavin S, Kidd MR, Post JJ, Wright E, Mao L. Understanding Concerns About Treatment-as-Prevention Among People with HIV who are not Using Antiretroviral Therapy. AIDS Behav. 2015. May;19(5):821–31. doi: 10.1007/s10461-014-0959-9. AIDS Behav. 2015 May;19(5):821–31. [DOI] [PubMed] [Google Scholar]
- 35.Bavinton BR, Holt M, Grulich AE, Brown G, Zablotska IB, Prestage GP. Bavinton BR, Holt M, Grulich AE, Brown G, Zablotska IB, Prestage GP. Willingness to Act upon Beliefs about “Treatment as Prevention” among Australian Gay and Bisexual Men. PLoS One. 2016. Jan 7;11(1):e0145847. doi: 10.1371/journal.pone.0145847. PLOS ONE. 2016 Jan 7;11(1):e0145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bor J, Fischer C, Modi M, Richman B, Kinker C, King R, et al. Bor J, Fischer C, Modi M, Richman B, Kinker C, King R, Calabrese SK, Mokhele I, Sineke T, Zuma T, Rosen S, Bärnighausen T, Mayer KH, Onoya D. Changing Knowledge and Attitudes Towards HIV Treatment-as-Prevention and “Undetectable = Untransmittable”: A Systematic Review. AIDS Behav. 2021. Dec;25(12):4209–4224. doi: 10.1007/s10461-021-03296-8. Epub 2021 May 25. AIDS Behav. 2021;25(12):4209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalichman SC, Cherry C, Kalichman MO, Amaral CM, White D, Pope H, et al. Kalichman SC, Cherry C, Kalichman MO, Amaral CM, White D, Pope H, Swetzes C, Eaton L, Macy R, Cain D. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health. 2011. Mar;101(3):531–8. doi: 10.2105/AJPH.2010.197608. Epub 2011 Jan 13. Am J Public Health. 2011 Mar;101(3):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalichman SC, Cherry C, Kalichman MO, Eaton LA, Kohler J, Montero C, et al. Kalichman SC, Cherry C, Kalichman MO, Eaton LA, Kohler JJ, Montero C, Schinazi RF. Mobile Health Intervention to Reduce HIV Transmission: A Randomized Trial of Behaviorally Enhanced HIV Treatment as Prevention (B-TasP). J Acquir Immune Defic Syndr. 2018. May 1;78(1):34–42. doi: 10.1097/QAI.0000000000001637. J Acquir Immune Defic Syndr. 2018 May 1;78(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NUDGE: Improving Decisions About Health, Wealth, and Happiness [Internet]. [cited 2023 Aug 15]. Available from: https://www.researchgate.net/publication/235413094_NUDGE_Improving_Decisions_About_Health_Wealth_and_Happiness [Google Scholar]
- 40.Millar MG, Millar K. Millar MG, Millar K. Negative affective consequences of thinking about disease detection behaviors. Health Psychol. 1995. Mar;14(2):141–6. doi: 10.1037//0278-6133.14.2.141. Health Psychol. 1995 Mar;14(2):141–6. [DOI] [PubMed] [Google Scholar]
- 41.Rice T. Rice T. The behavioral economics of health and health care. Annu Rev Public Health. 2013;34:431–47. doi: 10.1146/annurev-publhealth-031912-114353. Epub 2013 Jan 7. Annu Rev Public Health. 2013;34:431–47. [DOI] [PubMed] [Google Scholar]
- 42.Arndt J, Goldenberg JL. Arndt J, Goldenberg JL. Where Health and Death Intersect: Insights from a Terror Management Health Model. Curr Dir Psychol Sci. 2017;26(2):126–131. doi: 10.1177/0963721416689563. Epub 2017 Apr 6. Curr Dir Psychol Sci. 2017;26(2):126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catalani C, Green E, Owiti P, Keny A, Diero L, Yeung A, et al. Catalani C, Green E, Owiti P, Keny A, Diero L, Yeung A, Israelski D, Biondich P. A clinical decision support system for integrating tuberculosis and HIV care in Kenya: a human-centered design approach. PLoS One. 2014. Aug 29;9(8):e103205. doi: 10.1371/journal.pone.0103205. PLoS One. 2014;9(8):e103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harte R, Glynn L, Rodríguez-Molinero A, Baker PM, Scharf T, Quinlan LR, et al. Harte R, Glynn L, Rodríguez-Molinero A, Baker PM, Scharf T, Quinlan LR, ÓLaighin G. A Human-Centered Design Methodology to Enhance the Usability, Human Factors, and User Experience of Connected Health Systems: A Three-Phase Methodology. JMIR Hum Factors. 2017. Mar 16;4(1):e8. doi: 10.2196/humanfactors.5443. JMIR Hum Factors. 2017 Mar 16;4(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beres LK, Simbeza S, Holmes CB, Mwamba C, Mukamba N, Sharma A, et al. Beres LK, Simbeza S, Holmes CB, Mwamba C, Mukamba N, Sharma A, Munamunungu V, Mwachande M, Sikombe K, Bolton Moore C, Mody A, Koyuncu A, Christopoulos K, Jere L, Pry J, Ehrenkranz PD, Budden A, Geng E, Sikazwe I. Human-Centered Design Lessons for Implementation Science: Improving the Implementation of a Patient-Centered Care Intervention. J Acquir Immune Defic Syndr. 2019. Dec;82 Suppl 3(3):S230–S243. doi: 10.1097/QAI.0000000000002216. Erratum in: J Acquir Immune Defic Syndr. 2020 May 1;84(1):e10. J Acquir Immune Defic Syndr. 2019 Dec;82 Suppl 3(3):S230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazzano AN, Martin J, Hicks E, Faughnan M, Murphy L. Bazzano AN, Martin J, Hicks E, Faughnan M, Murphy L. Human-centred design in global health: A scoping review of applications and contexts. PLoS One. 2017. Nov 1;12(11):e0186744. doi: 10.1371/journal.pone.0186744. PLoS One. 2017;12(11):e0186744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matheson GO, Pacione C, Shultz RK, Klügl M. Matheson GO, Pacione C, Shultz RK, Klügl M. Leveraging human-centered design in chronic disease prevention. Am J Prev Med. 2015. Apr;48(4):472–9. doi: 10.1016/j.amepre.2014.10.014. Epub 2015 Feb 18. Am J Prev Med. 2015 Apr;48(4):472–9. [DOI] [PubMed] [Google Scholar]
- 48.Smith PJ, Joseph Davey DL, Schmucker L, Bruns C, Bekker LG, Medina-Marino A, et al. Smith PJ, Joseph Davey DL, Schmucker L, Bruns C, Bekker LG, Medina-Marino A, Thirumurthy H, Buttenheim AM. Participatory Prototyping of a Tailored Undetectable Equals Untransmittable Message to Increase HIV Testing Among Men in Western Cape, South Africa. AIDS Patient Care STDS. 2021. Nov;35(11):428–434. doi: 10.1089/apc.2021.0101. AIDS Patient Care STDS. 2021 Nov;35(11):428–34. [DOI] [PubMed] [Google Scholar]
- 49.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012. Mar;50(3):217–26. doi: 10.1097/MLR.0b013e3182408812. Med Care. 2012 Mar;50(3):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curran GM, Landes SJ, McBain SA, Pyne JM, Smith JD, Fernandez ME, et al. Curran GM, Landes SJ, McBain SA, Pyne JM, Smith JD, Fernandez ME, Chambers DA, Mittman BS. Reflections on 10 years of effectiveness-implementation hybrid studies. Front Health Serv. 2022. Dec 8;2:1053496. doi: 10.3389/frhs.2022.1053496. Front Health Serv. 2022;2:1053496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson LF, Dorrington RE, Moolla H. Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med. 2017. Jul 27;18(1):694. doi: 10.4102/sajhivmed.v18i1.694. South Afr J HIV Med. 2017 Jul 27;18(1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson L, Dorrington R. Thembisa version 4.6: A model for evaluating the impact of HIV/AIDS in South Africa. [Google Scholar]
- 53.Medina-Marino A, Bezuidenhout D, Ngwepe P, Bezuidenhout C, Facente SN, Mabandla S, et al. Medina-Marino A, Bezuidenhout D, Ngwepe P, Bezuidenhout C, Facente SN, Mabandla S, Hosek S, Little F, Celum CL, Bekker LG. Acceptability and feasibility of leveraging community-based HIV counselling and testing platforms for same-day oral PrEP initiation among adolescent girls and young women in Eastern Cape, South Africa. J Int AIDS Soc. 2022. Jul;25(7):e25968. doi: 10.1002/jia2.25968. J Int AIDS Soc. 2022 Jul;25(7):e25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Schaik N, Kranzer K, Wood R, Bekker LG. van Schaik N, Kranzer K, Wood R, Bekker LG. Earlier HIV diagnosis--are mobile services the answer? S Afr Med J. 2010. Oct 1;100(10):671–4. doi: 10.7196/samj.4162. S Afr Med J. 2010 Oct 1;100(10):671–4. [DOI] [PubMed] [Google Scholar]
- 55.Govindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, et al. Govindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, Freedberg KA, Bassett Bekker LG IV. Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One. 2013. Nov 13;8(11):e80017. doi: 10.1371/journal.pone.0080017. PLoS One. 2013 Nov 13;8(11):e80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medina-Marino A, Daniels J, Bezuidenhout D, Peters R, Farirai T, Slabbert J, et al. Medina-Marino A, Daniels J, Bezuidenhout D, Peters R, Farirai T, Slabbert J, Guloba G, Johnson S, Bekker LG, Nkhwashu N. Outcomes from a multimodal, at-scale community-based HIV counselling and testing programme in twelve high HIV burden districts in South Africa. J Int AIDS Soc. 2021. Mar;24(3):e25678. doi: 10.1002/jia2.25678. J Int AIDS Soc. 2021 Mar;24(3):e25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina-Marino A, Bezuidenhout D, Hosek S, Barnabas RV, Atujuna M, Bezuidenhout C, et al. Medina-Marino A, Bezuidenhout D, Hosek S, Barnabas RV, Atujuna M, Bezuidenhout C, Ngwepe P, Peters RPH, Little F, Celum CL, Daniels J, Bekker LG. The Community PrEP Study: a randomized control trial leveraging community-based platforms to improve access and adherence to pre-exposure prophylaxis to prevent HIV among adolescent girls and young women in South Africa-study protocol. Trials. 2021. Jul 26;22(1):489. doi: 10.1186/s13063-021-05402-6. Trials. 2021 Jul 26;22(1):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith PJ, Daniels J, Bekker LG, Medina-Marino A. Smith PJ, Daniels J, Bekker LG, Medina-Marino A. What motivated men to start PrEP? A cross-section of men starting PrEP in Buffalo city municipality, South Africa. BMC Public Health. 2023. Mar 2;23(1):418. doi: 10.1186/s12889-023-15306-6. BMC Public Health. 2023 Mar 2;23:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith P, Buttenheim A, Schmucker L, Bekker LG, Thirumurthy H, Davey DLJ. Smith P, Buttenheim A, Schmucker L, Bekker LG, Thirumurthy H, Davey DLJ. Undetectable = Untransmittable (U = U) Messaging Increases Uptake of HIV Testing Among Men: Results from a Pilot Cluster Randomized Trial. AIDS Behav. 2021. Oct;25(10):3128–3136. doi: 10.1007/s10461-021-03284-y. Epub 2021 May 31. AIDS Behav. 2021;25(10):3128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.South African National Department of Health. HIV Testing Services: Policy 2016 [Internet]. Pretoria, South Africa; 2016 Jul [cited 2020 Dec 29]. Available from: https://sahivsoc.org/Files/HTS%20Policy%2028%20July%20final%20copy.pdf [Google Scholar]
- 61.Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol. 2020. Jun 1;49(3):979–995. doi: 10.1093/ije/dyz237. Int J Epidemiol. 2020 Jun 1;49(3):979–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein A, Ramaseshan B. Towards the identification of customer experience touch point elements. Journal of Retailing and Consumer Services. 2016. May 1;30:8–19. [Google Scholar]
- 63.Bauermeister JA, Golinkoff JM, Muessig KE, Horvath KJ, Hightow-Weidman LB. Bauermeister JA, Golinkoff JM, Muessig KE, Horvath KJ, Hightow-Weidman LB. Addressing engagement in technology-based behavioural HIV interventions through paradata metrics. Curr Opin HIV AIDS. 2017. Sep;12(5):442–446. doi: 10.1097/COH.0000000000000396. Curr Opin HIV AIDS. 2017 Sep;12(5):442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch KG, Cary M, Gallop R, Ten Have TR. Lynch KG, Cary M, Gallop R, Ten Have TR. Causal Mediation Analyses for Randomized Trials. Health Serv Outcomes Res Methodol. 2008;8(2):57–76. doi: 10.1007/s10742-008-0028-9. Health Serv Outcomes Res Methodol. 2008;8(2):57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.South African National Department of Health. HIV Testing Services: Policy 2016 [Internet]. Pretoria, South Africa; 2016 Jul [cited 2023 Aug 15]. Available from: https://sahivsoc.org/Files/HTS%20Policy%2028%20July%20final%20copy.pdf. [Google Scholar]
- 66.National Consolidated Guidelines 30062020 signed PRINT v7.pdf [Internet]. [cited 2023 Aug 15]. Available from: https://knowledgehub.health.gov.za/system/files/elibdownloads/2020-07/National%20Consolidated%20Guidelines%2030062020%20signed%20PRINT%20v7.pdf [Google Scholar]
- 67.National Department of Health. Adherence Guidelines for HIV, TB and NCDs [Internet]. Republic of South Africa National Department of Health; 2016 [cited 2021 Apr 17]. Available from: https://www.nacosa.org.za/wp-content/uploads/2018/05/SOP-Adherence-counselling-A5-booklet-19-03-2017.pdf [Google Scholar]
- 68.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009. Aug 7;4:50. doi: 10.1186/1748-5908-4-50. Implement Sci. 2009 Aug 7;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.King DK, Shoup JA, Raebel MA, Anderson CB, Wagner NM, Ritzwoller DP, et al. King DK, Shoup JA, Raebel MA, Anderson CB, Wagner NM, Ritzwoller DP, Bender BG. Planning for Implementation Success Using RE-AIM and CFIR Frameworks: A Qualitative Study. Front Public Health. 2020. Mar 3;8:59. doi: 10.3389/fpubh.2020.00059. Front Public Health. 2020;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glasgow RE, Estabrooks PE. Glasgow RE, Estabrooks PE. Pragmatic Applications of RE-AIM for Health Care Initiatives in Community and Clinical Settings. Prev Chronic Dis. 2018. Jan 4;15:E02. doi: 10.5888/pcd15.170271. Prev Chronic Dis. 2018 Jan 4;15:E02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, Ory MG, Estabrooks PA. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front Public Health. 2019. Mar 29;7:64. doi: 10.3389/fpubh.2019.00064. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022. Oct 29;17(1):75. doi: 10.1186/s13012-022-01245-0. Implementation Science. 2022 Oct 29;17(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beidas RS, Buttenheim AM, Mandell DS. Beidas RS, Buttenheim AM, Mandell DS. Transforming Mental Health Care Delivery Through Implementation Science and Behavioral Economics. JAMA Psychiatry. 2021. Sep 1;78(9):941–942. doi: 10.1001/jamapsychiatry.2021.1120. JAMA Psychiatry. 2021 Sep 1;78(9):941–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandimika C, Bokolo S, ChettyMakkan C, Long L, Mistri P, Schmucker L, Buttenheim A. The NUDGE Handbook: A practical guide to behavioural design based on the NUDGE framework. Version 2. June 2023. Indlela Behavioural Insights Unit. Available at https://indlela.org/nudge-handbookTimelines. [Google Scholar]
- 75.Swain J. A Hybrid Approach to Thematic Analysis in Qualitative Research: Using a Practical Example. 2018. [Google Scholar]
- 76.Brewer JD, Shinnick J, Román K, Santos MP, Paz-Soldan VA, Buttenheim AM. Brewer JD, Shinnick J, Román K, Santos MP, Paz-Soldan VA, Buttenheim AM. Behavioral Insights Into Micronutrient Powder Use for Childhood Anemia in Arequipa, Peru. Glob Health Sci Pract. 2020. Dec 23;8(4):721–731. doi: 10.9745/GHSP-D-20-00078. Glob Health Sci Pract. 2020 Dec 23;8(4):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stewart RE, Beidas RS, Last BS, Hoskins K, Byeon YV, Williams NJ, et al. Stewart RE, Beidas RS, Last BS, Hoskins K, Byeon YV, Williams NJ, Buttenheim AM. Applying NUDGE to Inform Design of EBP Implementation Strategies in Community Mental Health Settings. Adm Policy Ment Health. 2021. Jan;48(1):131–142. doi: 10.1007/s10488-020-01052-z. Adm Policy Ment Health. 2021 Jan;48(1):131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matchboxology [Internet]. [cited 2023 Aug 15]. Available from: https://matchboxology.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.