Abstract
Objective.
To understand how vaginal microbiota composition affects antiretroviral concentrations in the setting of hormonal contraception initiation.
Methods.
Cervicovaginal fluid (CVF) concentrations of tenofovir, lamivudine, and efavirenz from 73 Malawian women living with HIV were compared before and after initiation of depot-medroxyprogesterone acetate (DMPA) or levonorgestrel implant. We evaluated antiretroviral concentrations and vaginal microbiota composition/structure in the context of contraception initiation and predicted genital shedding using multivariable repeated measurements models fit by generalized estimating equations.
Results.
Mean lamivudine CVF concentrations decreased 37% 1 month after contraception initiation. Subgroup analyses revealed a 41% decrease in women 1 month after initiating levonorgestrel implant, but no significant difference was observed in DMPA group alone. Tenofovir, lamivudine, and efavirenz CVF concentrations were positively correlated with anaerobic bacteria associated with non-optimal vaginal microbiota. Risk of genital HIV shedding was not significantly associated with tenofovir or lamivudine CVF concentrations (tenofovir RR: 0.098, p=0.75; lamivudine RR: 0.142, p=0.54). Lack of association between genital HIV shedding and efavirenz CVF concentrations did not change when adjusting for vaginal microbiota composition, and lamivudine/tenofovir CVF concentrations (RR: 1.33, p=0.531).
Conclusion.
No effect of hormone initiation on genital shedding provides confidence that women with HIV on either DMPA or levonorgestrel implant contraception will not have compromised ART efficacy. The unexpected positive correlation between antiretroviral CVF concentrations and certain bacterial taxa relative abundance requires further work to understand the mechanism and clinical relevance.
Keywords: HIV, Microbiome, Progestin contraception, Antiretroviral Concentrations, Genital Shedding
Introduction
The effect of progestin contraceptives on antiretroviral concentrations are unknown in the female genital tract (FGT), which has implications for HIV treatment and prevention. In 2020, approximately 15.4 million women of childbearing age (15 – 49 years old) were living with HIV, with 50% in Sub-Saharan Africa (SSA), and many were on both contraceptives and antiretrovirals [1]. Studies examining female to male HIV transmission in the context of hormonal contraception have been mixed with adjusted hazard ratios from 0.46 to 1.76. [2,3] We have previously shown no association between HIV genital shedding and initiation of two progestin contraceptives, depot medroxyprogesterone acetate (DMPA) or levonorgestrel implant up to 33 months post contraception initiation in Malawian women living with HIV (WLWH), [4,5]
Non-optimal vaginal microbiota have been associated with a decrease in tenofovir efficacy, which may have implications for HIV genital shedding. Identifying vaginal microbiota effect on antiretroviral integrity in the vaginal microenvironment would provide insight for characterizing dose-response relationships.
Here, we sought to characterize associations between CVF antiretroviral exposure and the vaginal microbiota. We also examined correlations between CVF tenofovir and lamivudine concentrations and genital shedding of HIV in the setting of DMPA and levonorgestrel implant contraception initiation.
Methods
Study recruitment, enrollment, and visits
The study procedures for this randomized trial have been previously described [6] and primary results published. [5] Detailed methods can be found in the Supplemental Methods. Under a protocol approved by University of North Carolina and U.S. Centers for Disease Control and Prevention IRB, the Malawi National Health Sciences Research Committee, and the Malawi Pharmacy Medicines and Poisons Board, participants were recruited between May 2014- April 2015 from patients desiring contraception at Bwaila Maternity Hospital in Lilongwe, Malawi. Enrolled participants provided informed consent to be randomized to either DMPA or levonorgestrel implant. Samples were collected during first 14 days of next anticipated menstrual cycle to capture the follicular phase, and then again between day 15 from onset of last menses until start of next menses to capture the luteal phase. Women then initiated DMPA or levonorgestrel implant during first seven days after start of next menses. Women returned for repeat sample collection three days and one month after contraceptive initiation.
Weck-Cell/ vaginal swab collection and cervicovaginal fluid ART analysis
Efavirenz, tenofovir, and lamivudine were quantified in Weck-Cel sponges by the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core (CPAC) using validated HPLC-MS/MS methods (Supplemental Methods and [4]). HIV-1 RNA quantification was performed on the Abbott m2000 System after RNA extraction from TearFlo strips by the UNC Center for AIDS Research HIV/STD Laboratory Core [5].
Microbiome Sequencing and Community State Type Assignment
16S ribosomal RNA gene amplicon sequencing was performed on DNA extracted from vaginal swabs as previously published. [7] Community state types (CSTs) describe the vaginal bacterial communities present and were determined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical clustering [8].
Statistical Analysis
Cervicovaginal concentrations of tenofovir, lamivudine, and efavirenz were log transformed for all analyses. CVF concentrations of tenofovir, lamivudine, and efavirenz were compared between follicular and luteal phase visits to test antiretroviral differences throughout the menstrual cycle using a Kruskal Wallace test. We compared CVF drug concentrations before and after contraceptive initiation to test changes due to contraception using a multivariable generalized linear model with repeated measurement fits with generalized estimating equations (GEE).
Differences in CVF antiretroviral concentrations between assigned CST groups independent of time were evaluated using a multivariable generalized linear model with GEE. CST I was the reference as it is considered an optimal CST due to high relative abundance of L. crispatus. [8] We also compared changes in mean tenofovir, lamivudine, and efavirenz CVF concentrations between CSTs, dependent and independent of time.
The 20 most prevalent bacterial taxa, each with a relative abundance greater than 0.1%, were included in this analysis. The rest of the present taxa were added into “other” category. Single variable generalized linear model with repeated measurement fits with GEE was performed to examine significant associations between CVF antiretroviral concentrations and individual bacterial taxa.
Associations of detectable (≥200 copies/ml) genital tract HIV-1 RNA with antiretroviral CVF drug concentrations were evaluated using a multivariable log-binomial model with repeated measurements fit with GEE adjusted for CST and relative abundance.
Results
Participant Characteristics
We included all 73 WLWH in the trial (37 randomized to DMPA and 36 to levonorgestrel implant). Sixty-two women had data from all four visits; 11 women had only three visits. Supplemental Figure 1 depicts vaginal bacterial community states and the corresponding CSTs for all 281 samples. Supplemental Table 1 shows the number of women in each CST group at each visit. Supplemental Figure 2 depicts changes in CST classification over time for each participant.
Associations between cervicovaginal fluid antiretroviral concentrations and hormonal contraception initiation
CVF concentrations of tenofovir, lamivudine, and efavirenz were not significantly different between follicular and luteal phases (TFV, p = 0.31; 3TC, p = 0.92; EFV, p = 0.92). Therefore, we pooled pre-initiation visits when comparing changes in antiretroviral concentrations between pre-contraception initiation, three days, and one month post-contraception initiation.
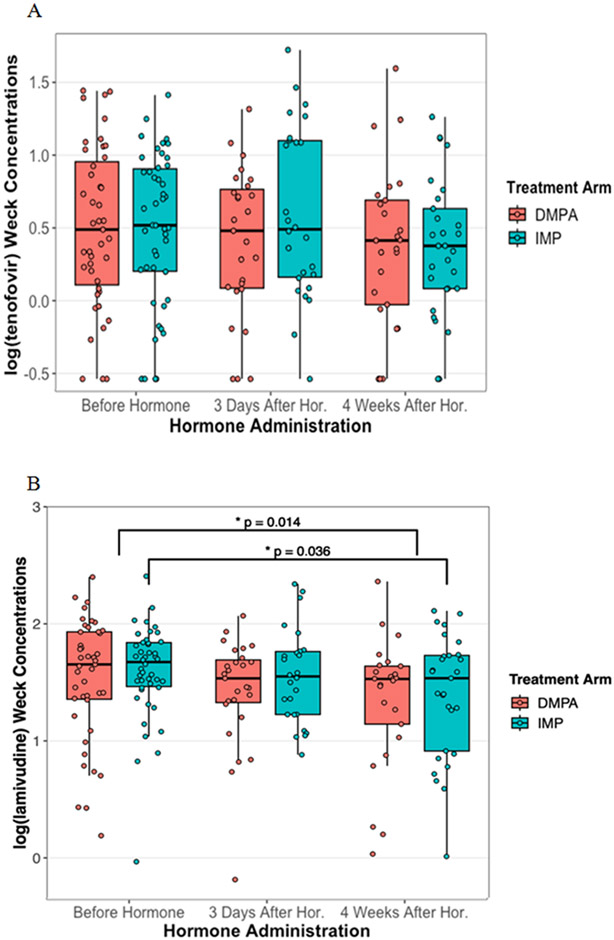
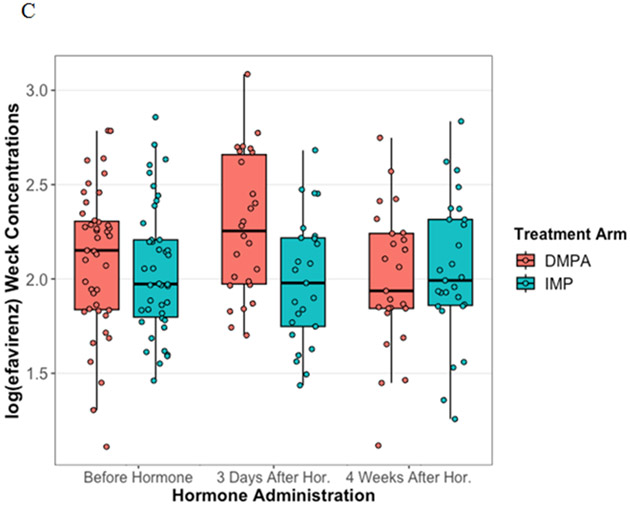
Tenofovir and efavirenz CVF concentrations were not significantly affected by contraception use up to one month after initiation. (Figure 1A, p=0.75; Figure 1C, p=0.23) Lamivudine CVF concentrations decreased by 37% one month after contraception initiation (Figure 1B, p=0.014). Specifically, women who initiated levonorgestrel implant had a 41% decrease in CVF lamivudine concentrations while women on DMPA had no effect (levonorgestrel, p=0.036; DMPA, p=0.085).
Figure 1. Antiretroviral concentrations affected by Progestin contraception initiation.
Cervicovaginal fluid concentrations of tenofovir, lamivudine and efavirenz were measured from Wek-Cel sponges using LC-MS/MS. The log transformed tenofovir (A), lamivudine (B), and efavirenz (C) concentrations were compared before, 3 days after, and 4 weeks after contraception initiation using general estimating equations. Concentrations between women on depot medroxyprogesterone acetate injectable (red) and levonorgestrel implant (teal) were compared at each visit. There were no significant statistical differences for tenofovir or efavirenz. Mean lamivudine concentrations significantly decreased 37% 1 month after contraception initiation. Mean lamivudine CVF concentrations decreased 41% in women on the levonorgestrel implant post-contraception initiation compared to pre-contraception initiation, but no significant difference for DMPA.
Antiretroviral concentrations in the cervicovaginal fluid before, 3 days after, and 1 month after contraceptive hormone initiation for A) tenofovir, B) lamivudine, and C) efavirenz.
Associations between cervicovaginal fluid antiretroviral concentrations and structure of the microbiota
Six CSTs were defined for this study: CST I, III-A, III-B, IV-A, IV-B, and IV-C. CST I and III are dominated by L. crispatus and L. iners, respectively, whereas CST IVs comprise diverse sets of anaerobic bacteria and little Lactobacilli. No women with available drug concentrations were in CST II. Drug concentrations within CSTs were not affected by visit (Supplemental Figure 3 A-C), suggesting that contraception initiation did not affect the relationship between CSTs and antiretrovirals. Additionally, Haddad et al. found no effects of DMPA and levonorgestrel on vaginal microbiota in the same cohort of women.[7] Therefore, visits were pooled for analyses.
Cervicovaginal concentrations for all three drugs were associated with specific CSTs which varied by drug (Table 1). Timepoints with vaginal swabs assigned to CST III-B had 60% lower tenofovir CVF concentrations than CST I (p=0.026). Lamivudine CVF concentrations were significantly lower in samples in CSTs III-A, IV-A, and IV-B compared to CST I. (III-A: 41%, p=0.01; IV-A: 46%, p=0.019; IV-B: 48%, p=0.016). Efavirenz CVF concentrations in samples from CST IV-C were 62% lower than CST I (p=0.0094).
Table 1.
Multivariable General Estimating Equation Models Predicting tenofovir, lamivudine, and efavirenz cervicovaginal fluid concentrations with Community State Types.
| Community State Type* | Estimate | Standard Error | P-value |
|---|---|---|---|
| Tenofovir | |||
| I | REF | REF | REF |
| III-A | −0.21 | 0.12 | 0.078 |
| III-B | −0.34 | 0.15 | 0.026* |
| IV-A | −0.18 | 0.13 | 0.15 |
| IV-B | −0.18 | 0.14 | 0.19 |
| IV-C | 0.0287 | 0.13 | 0.83 |
| Lamivudine | |||
| I | REF | REF | REF |
| III-A | −0.25 | 0.09 | 0.010* |
| III-B | −0.33 | 0.17 | 0.052 |
| IV-A | −0.29 | 0.12 | 0.019* |
| IV-B | −0.28 | 0.11 | 0.016* |
| IV-C | −0.11 | 0.11 | 0.34 |
| Efavirenz | |||
| I | REF | REF | REF |
| III-A | −0.25 | 0.15 | 0.10 |
| III-B | −0.050 | 0.16 | 0.75 |
| IV-A | −0.23 | 0.14 | 0.10 |
| IV-B | −0.27 | 0.17 | 0.11 |
| IV-C | −0.42 | 0.16 | 0.0094* |
Community state types describe the different vaginal bacterial communities present in the participants of the current study. They were defined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical clustering. CST I and III are dominated by L. crispatus and L. iners respectively, whereas CST IVs comprise of diverse sets of anaerobic bacteria and low to no lactobacilli.
For all three drugs, there was a positive correlation between CVF drug concentrations and relative abundance of anaerobic taxa (Supplemental Table 2). CVF tenofovir concentrations were positively correlated with Prevotella bivia, L. crispatus, and the phylum Proteobacteria. CVF lamivudine concentrations were positively correlated with phylum Proteobacteria, genus Megasphaera, L. crispatus, and P. bivia. CVF efavirenz was positively correlated with Atopobium vaginae. CVF efavirenz concentrations were negatively correlated with “other” bacteria (all bacteria with relative abundance <0.1%) but had a high standard error (SE = 32.23). G. vaginalis was not significantly associated with CVF drug concentrations (TFV: p=0.98; 3TC: p=0.66; EFV: p=0.48).
Risk of genital shedding
In 12 out of the 73 women, genital HIV shedding was detectable at one or more of visits included in this analysis. Neither tenofovir nor lamivudine CVF concentrations were associated with genital HIV shedding, even after adjusting for CST and bacterial load (RR: 0.35, p=0.31; RR: 0.34, p=0.48). Here, we found no significant improvement over our previously published analysis with CVF efavirenz [4] for the prediction of risk of genital HIV shedding after adjusting for vaginal microbiota with efavirenz, tenofovir, and lamivudine. (EFV, RR: 1.35, p=0.064; TFV, RR: 0.22, p=0.93: 3TC, RR: 0.13, p=0.73).
Discussion
Hormonal contraception and vaginal microbiota may affect antiretroviral disposition and efficacy, such as genital HIV shedding, in the FGT. While we found no significant changes in tenofovir or efavirenz CVF concentrations due to the initiation of contraception, lamivudine concentrations decreased one month after levonorgestrel implant initiation, with no significant changes in women on DMPA. Notably, contraception did not influence viral shedding. Further decreases in lamivudine concentrations more than a month after contraceptive initiation are possible and may warrant further study.
While CST III-B was associated with lower tenofovir concentrations compared to CST I, tenofovir concentrations were not correlated with L. iners relative abundance, dominating species in CST III-B. This pattern was also seen with lamivudine and efavirenz, suggesting not one bacterial taxon is responsible for changes in CVF antiretroviral concentration, and differences may be more driven by functional profiles. Functional differences exist between strains of the same species and these cannot be accounted for in our analyses. [9]
Findings of no correlation between any drug in CVF and G. vaginalis contrasts with Klatt et al. who showed a decrease in tenofovir concentrations in a co-culture with G. vaginalis and P. bivia in vitro, attributed to bacterial metabolism of tenofovir to adenine. [10] However, our findings are consistent with two Phase 1 studies of a tenofovir/levonorgestrel intravaginal ring showing no long term correlations between tenofovir CVF concentrations and G. vaginalis. [11,12]
There are two possible mechanisms behind the unexpected positive correlations between CVF antiretroviral concentrations and anaerobic bacteria taxa associated with non-optimal vaginal microbiota: 1) Lactobacillus species sequester these drugs, and 2) changes in pH with anaerobic bacteria affecting drug partitioning. Tavena et al. 2018 showed a decrease in tenofovir being transported and metabolized by L. crispatus in vitro and showed as pH increased, tenofovir uptake decreased. [13] It is unclear how these findings would affect HIV transmission risk, and further studies are needed.
We previously reported no association between genital shedding and CVF concentrations of efavirenz, but tenofovir and lamivudine CVF concentrations were not included. Here we confirmed that adjusting for the composition or structure of the vaginal microbiota when evaluating associations between efavirenz, tenofovir, lamivudine and genital HIV shedding did not improve our prediction of risk of genital HIV shedding. These findings further support previous evidence that progestin contraception does not impact the risk of HIV transmission from women on effective ART to their partners. [4,5,14-16]
The results of our study are limited by small sample size and the nature of sub-group analyses. Furthermore, we were only able to measure CVF antiretroviral concentrations, not genital tissue concentrations, though tissue concentrations may be a better efficacy surrogate.
In conclusion, we found hormone initiation did not affect genital shedding, providing confidence that WLWH on either DMPA or levonorgestrel implant contraception will not have compromised ART efficacy. Tenofovir, lamivudine, and efavirenz CVF concentrations had a positive correlation with several anaerobic bacteria taxa. Identification of a mechanism would increase our understanding of the complex environment of the FGT providing insights into improving ART and viral suppression in this compartment.
Supplementary Material
Supplemental Figure 3. Drug concentrations by CST were not affected by Progestin contraception initiation. Differences in CVF antiretroviral concentrations or A) Tenofovir, B) lamivudine, and C) Efavirenz between assigned CST groups independent of time were evaluated using a multivariable generalized linear model with GEE. TFV = tenofovir; 3TC = lamivudine; EFV = efavirenz; CST = community state types. There were no women with CST III-B during the luteal phase.
Supplemental Figure 1. Heatmap defining Community State Types. DNA was extracted from vaginal swabs and sequenced using 16S microbiome sequencing. Community state types describe the different vaginal bacterial communities present in the participants of the current study and were defined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical cluster. CST I and III are dominated by L. crispatus and L. iners respectively, whereas CST IVs comprise of diverse sets of anaerobic bacteria and low to no lactobacilli. The 20 most prevalent bacterial taxa, which all had a relative abundance greater than 0.1%, were included in analyses. The rest of the bacterial taxa present were added into “other” category.
Supplemental Figure 2. CST status for each participant and changes over time, by contraception treatment arm. DNA was extracted from vaginal swabs and sequenced using 16S microbiome sequencing. Community state types describe the different vaginal bacterial communities present in the participants of the current study and were defined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical cluster. CST I and III are dominated by L. crispatus and L. iners respectively, whereas CST IVs comprise of diverse sets of anaerobic bacteria and low to no lactobacilli. The 20 most prevalent bacterial taxa, which all had a relative abundance greater than 0.1%, were included in analyses. The rest of the bacterial taxa present were added into “other” category.
Acknowledgments
We would like to thank the Lilongwe District Management Team for their support of our study and The Lighthouse Trust and Area 25 Health Centre for allowing us to inform potential participants about the study at their clinics. A.M.L and M.R.N drafted the manuscript. A.M.L performed data analysis. M.C. oversaw drug quantification from cervicovaginal fluid. J.N. completed genital viral load testing. J.R. and P.G. performed the microbiome analysis and determined community state types. J.T. led study implementation. S.H. assisted with protocol development and project management. L.H. and A.K. designed the study and oversaw the conduct of the study and data analysis. All authors contributed to and reviewed the manuscript.
This work was supported by the National Institutes of Health [grant numbers K01TW009657 (JHT), K23-HD078153 (LBH), P30-AI50410 (UNC CFAR), TL1R002493, K08 AI134262) and UL1TR002494 (NCATS)], the US Centers for Disease Control and Prevention [grant numbers U48DP001944, 200-2015-M-63021], the Bill & Melinda Gates Foundation [grant number OPP1090837], and USAID [grant number AID-OAA-A-15-00045]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This work was supported by the National Institutes of Health [grant numbers K01-TW009657 (JHT), K23-HD078153 (LBH), P30-AI50410 (UNC CFAR), K08 AI134262 (MRN), TL1R002493 and UL1TR002494 (AML)], the US Centers for Disease Control and Prevention [grant numbers U48DP001944, 200-2015-M-63021], the Bill & Melinda Gates Foundation [grant number OPP1090837], and USAID [grant number AID-OAA-A-15-00045]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.UNAIDS. Epidemiological Estimates, 2021. ; 2021. https://www.unaids.org/en/resources/documents/2021/global-commitments-local-action
- 2.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS 2013; 27:493–505. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SJ, Polis CB, Curtis KM. The safety of hormonal contraceptives for women living with HIV and their sexual partners. Contraception 2016; 93:11–16. [DOI] [PubMed] [Google Scholar]
- 4.Kourtis AP, Wiener J, Hurst S, Nelson JAE, Cottrell ML, Corbett A, et al. Brief Report: HIV Shedding in the Female Genital Tract of Women on ART and Progestin Contraception: Extended Follow-up Results of a Randomized Clinical Trial. J Acquir Immune Defic Syndr 2019; 81:163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinula L, Nelson JAE, Wiener J, Tang JH, Hurst S, Tegha G, et al. Effect of the depot medroxyprogesterone acetate injectable and levonorgestrel implant on HIV genital shedding: a randomized trial. Contraception 2018; 98:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourtis AP, Haddad L, Tang J, Chinula L, Hurst S, Wiener J, et al. A randomized clinical trial on the effects of progestin contraception in the genital tract of HIV-infected and uninfected women in Lilongwe, Malawi: Addressing evolving research priorities HHS Public Access. Contemp Clin Trials 2017; 52:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad LB, Tang JH, Davis NL, Kourtis AP, Chinula L, Msika A, et al. Influence of Hormonal Contraceptive Use and HIV on Cervicovaginal Cytokines and Microbiota in Malawi. mSphere Published Online First: 21 February 2023. doi: 10.1128/MSPHERE.00585-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, Mcculle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci 2011; 108 Suppl:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma B, France MT, Crabtree J, Holm JB, Humphrys MS, Brotman RM, et al. A comprehensive non-redundant gene catalog reveals extensive within-community intraspecies diversity in the human vagina. Nat Commun 2020; 11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noël-romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science (80- ) 2017; 945:938–945. [DOI] [PubMed] [Google Scholar]
- 11.Thurman A, Schwartz JL, Ravel J, Gajer P, Marzinke MA, Yousefieh N, et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/ levonorgestrel vaginal rings. PLoS One 2019; 14:e0217229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurman AR, Ravel J, Gajer P, Marzinke MA, Ouattara LA, Jacot T, et al. Vaginal Microbiota and Mucosal Pharmacokinetics of Tenofovir in Healthy Women Using a 90-Day Tenofovir/Levonorgestrel Vaginal Ring. Front Cell Infect Microbiol 2022; 12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taneva E, Sinclair S, Mesquita PM, Weinrick B, Cameron SA, Cheshenko N, et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI insight 2018; 3. doi: 10.1172/JCI.INSIGHT.99545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low AJ, Konate I, Nagot N, Weiss HA, Kania D, Vickerman P, et al. Cervicovaginal HIV-1 Shedding in Women Taking Antiretroviral Therapy in Burkina Faso: A Longitudinal Study. J Acquir Immune Defic Syndr 2014; 65.www.jaids.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day S, Graham SM, Masese LN, Richardson BA, Kiarie JN, Jaoko W, et al. A prospective cohort study of the effect of depot medroxyprogesterone acetate on detection of plasma and cervical HIV-1 in women initiating and continuing antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 66:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CC, Ellington SR, Davis NL, Coombs RW, Pyra M, Hong T, et al. Prevalence, Magnitude, and Correlates of HIV-1 Genital Shedding in Women on Antiretroviral Therapy. J Infect Dis J Infect Dis ® 2017; 216:1534–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 3. Drug concentrations by CST were not affected by Progestin contraception initiation. Differences in CVF antiretroviral concentrations or A) Tenofovir, B) lamivudine, and C) Efavirenz between assigned CST groups independent of time were evaluated using a multivariable generalized linear model with GEE. TFV = tenofovir; 3TC = lamivudine; EFV = efavirenz; CST = community state types. There were no women with CST III-B during the luteal phase.
Supplemental Figure 1. Heatmap defining Community State Types. DNA was extracted from vaginal swabs and sequenced using 16S microbiome sequencing. Community state types describe the different vaginal bacterial communities present in the participants of the current study and were defined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical cluster. CST I and III are dominated by L. crispatus and L. iners respectively, whereas CST IVs comprise of diverse sets of anaerobic bacteria and low to no lactobacilli. The 20 most prevalent bacterial taxa, which all had a relative abundance greater than 0.1%, were included in analyses. The rest of the bacterial taxa present were added into “other” category.
Supplemental Figure 2. CST status for each participant and changes over time, by contraception treatment arm. DNA was extracted from vaginal swabs and sequenced using 16S microbiome sequencing. Community state types describe the different vaginal bacterial communities present in the participants of the current study and were defined using Bray-Curtis dissimilarity measure and Ward linkage hierarchical cluster. CST I and III are dominated by L. crispatus and L. iners respectively, whereas CST IVs comprise of diverse sets of anaerobic bacteria and low to no lactobacilli. The 20 most prevalent bacterial taxa, which all had a relative abundance greater than 0.1%, were included in analyses. The rest of the bacterial taxa present were added into “other” category.